Introduction
Management of patients subjected to extensive body irradiation as a part of conditioning therapy still remains a major challenge. Survival of radiation-induced bone marrow failure depends on the dose of radiation received and the intensity of supportive care which can protect from otherwise lethal infection and give surviving stem cells a chance to replenish blood cell populations. Since radiation effects on blood stem cells occur at doses generally lower than those on other critical organs, the rapidly emerging changes in the peripheral blood cell lineages determine the treatment options. In fact, total body irradiation (TBI) at doses more than 7-8 Gy in humans corresponds to medullar eradication. Under this threshold, spontaneous recovery from residual hematopoietic stem and progenitor cells may be expected within 30–50 days, however, preceded by cytopenic phases of granulocytic, megakaryocytic and erythrocytic lineages. Interestingly, even after TBI, intrinsically radioresistant stem cells have been detected in distinct bone marrow (BM) areas comprising a residual hematopoietic stem and progenitor cell pool [10]. Acute irradiation does not only imply damage to the bone marrow. In a dose-dependent matter, it can also emerge as gastrointestinal and cerebrovascular syndromes leading to development of multiple organ dysfunction (1). Damage to the whole organism is related to a systemic inflammatory response. Different target organs are affected due to activation of innate immune system, resulting in a significant release of inflammatory cytokines [4]. The pathophysiology of such tissue damage appears comparable to that of acute graftversus- host disease (GvHD) following allogeneic stem cell transplantation where a similar ”cytokine storm” has been observed [6]. In absence of appropriate treatment, oxidative stress after high dose ionizing radiation has been involved in delayed morbidity [4]. Management of acute radiation syndrome relies, therefore, on tissue damage repair processes that might be supported by therapies aimed for mitigation of inflammation [4].
Efforts to improve outcome after irradiation focus on the stem cell niche. Therefore, prospective therapies should augment the hematopoietic niche activity to accelerate the in vivo recovery of blood cell populations. Several studies have demonstrated that BM osteoblasts regulate the HSC pool size in vivo via the Jagged1-Notch signaling pathway [7]. For example, parathyroid hormone receptor activation can increase the number of osteoblastic cells, thus resulting in Notch1-mediated expansion of HSC [2]. Mesenchymal stromal cells (MSC) comprise an integrative part of the BM stroma, being also described as osteoblastic progenitors [8]. MSC are multipotential nonhematopoietic progenitor cells capable of differentiating into multiple lineages of the mesenchyme. In bone marrow, the local stromal cells surround HSC and their progeny. The hematopoietic niche provides a sheltering microenvironment that provides maintenance and self-renewal of HSC by shielding them from differentiation and apoptotic stimuli that would otherwise challenge stem cell reserves. Moreover, the hematopoietic niche also controls proliferation and differentiation of HSC and release of mature progeny into peripheral blood flow. Regulation of HSC quiescence, by maintenance of resting HSC in endosteal niche, control of HSC proliferation, differentiation and recruitment in the vascular niche can be ascribed to bone-marrow stromal cells [27]. Thus, physiological role of MSCs is not a mere replacement of mesenchymal tissues such as bone. Moreover, their primary and most important function is to inhibit immunosurveillance and to establish a protective and regenerative microenvironment for HSC.
Clinically, MSCs have been proven to intervene with acute organ impairment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy significantly decreasing the time to full hematopoietic and particularly platelet reconstitution [12]. Additionally, there is evidence for MSC effectiveness in the treatment of steroid resistant GvHD without any side effects, even when obtained from BM of third-party donors [18]. No HLA-match is needed between donor and recipient because MSCs have been shown to be hypoimmunogenic and are not recognized by the recipient immune system even after repeated injections [18]. Finally, MSCs secrete a variety of bioactive molecules [22]. Among those, some essential hematopoietic growth factors including IL-6, IL-11, leukemia inhibitory factor (LIF), stem cell factor (SCF) and Flt3 ligand are produced, as well as factors with immunomodulatory effects, e.g. transforming growth factor-β1 (TGF-β1), prostaglandin E2, indoleamine 2,3-dioxygenase, and others [21]. Additionally, vascular endothelial growth factor (VEGF) secreted by MSCs in abundance might interfere with early apoptotic cell death after irradiation [10]. Therefore, MSCs might be a good candidate for modulation of the hematopoietic niche activity. In summary, MSCs have emerged as a promising therapeutic tool for tissue regeneration and repair. Further clinical interest has been raised by the observation that MSCs are immunoprivileged and might be transplanted from unrelated, i.e. allogeneic donors [21,27]. Altogether, we assumed that MSCs, with their comprehensive trophic potential, could serve as a readily available treatment option after severe radiation exposure. The aim of our study was to evaluate essential biological parameters of MSC, with respect to their lineage- specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts.
Methods and Results
In vitro differentiation of human MSC (hMSC)
As first experiments, we investigated the capability of human BM-derived MSCs (hMSCs) to differentiate into progenitors for hematopoietic (HSC) and endothelial cells (EC). The human MSCs were thoroughly characterized according to the ISCT (International Society for Cellular Therapy) criteria [5], including flow cytometry and their capability to differentiate into three mesodermal lineages [16]. To avoid any contamination of MSCs with HSC, cloned cells were used exclusively. Cloned human MSCs were subjected to differentiation into (i) hematopoietic cells using serum-containing or serum-depleted growth conditions and (ii) endothelial cells (for technical details see ref. 14). Fibroblastoid MSCs (Fig. 1a) formed blast-like cells with noticeably decreased diameter from originally 28.9 ± 6.6 to 15.7 ± 3.5 μm during the differentiation into hematopoietic (Fig. 1b) and endothelial (Fig. 1c) lineages. The in vitro conditions led to cluster formation appearing as an in vitro equivalent of stromal structures from which differentiation proceeded. The cells committed to hematopoietic lineage changed their gene expression towards appropriate profiles of blood cell progenitors (CD117, CD133, CD45) and mature (CD14, CD16, glycophorinA GlyA, CD31, podoplanin PDPN) hematopoietic cells (Fig. 2a). Interestingly, the erythropoietin receptor (EPOR) was upregulated in almost all clones and under all conditions suggesting a definite role for EPO in proliferation and differentiation of mesodermal progenitors. Additionally,
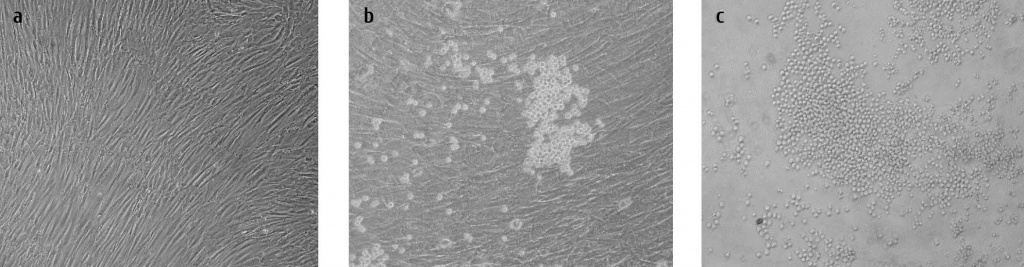
Figure 1: Human MSC display a fibroblastoid morphology during in vitro expansion but form blast-like cells after induction of differentiation. One clonal hMSC culture is shown during expansion (a), differentiation into hematopoietic (b) or endothelial (c) cells.
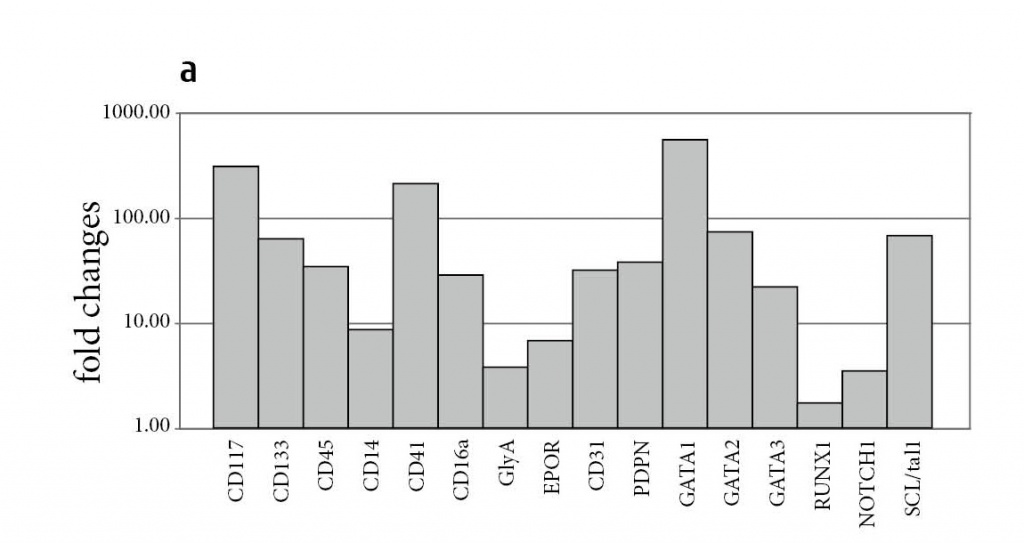
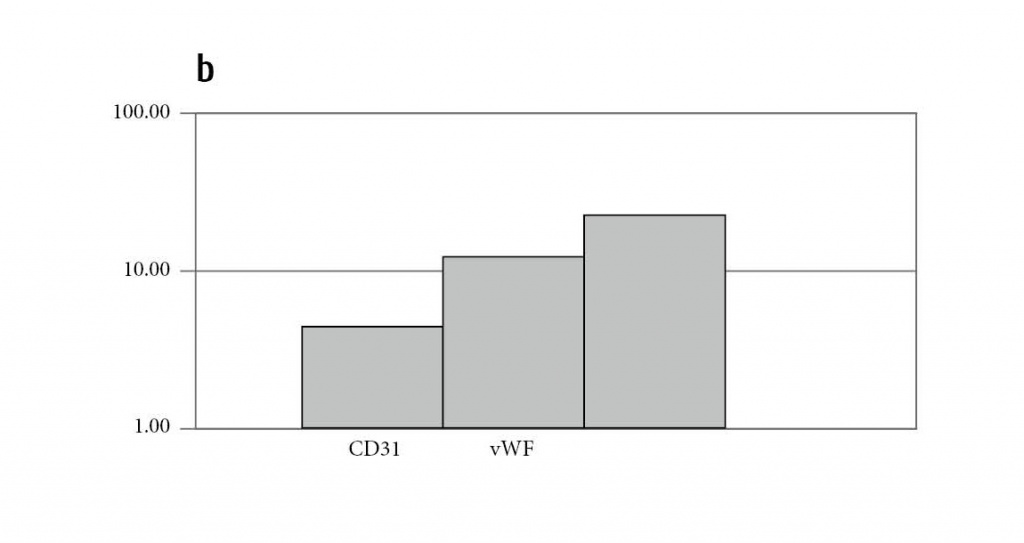
Figure 2: Human MSC significantly upregulate expressions of hematopoietic and endothelial genes after induction of differentiation. Shown are the fold changes of gene expressions of indicated hematopoietic (a) and endothelial (b) genes after differentiation compared to undifferentiated hMSC. GlyA, Glycophorin A; vWF, von Willebrand factor; VEGFR, vascular endothelial growth factor receptor.
a variety of transcription factors responsible for erythropoiesis (SCL/tal1), erythro-megakaryopoiesis (GATA1, GATA2), lymphopoiesis (GATA3), and myelopoiesis (NOTCH1, RUNX1) were upregulated upon serum-containing differentiation. As SCL and RUNX1 are transcription factors essential for HSC formation by instructing lineage specification (9), we suggested an efficient induction of this differentiation pathway in MSCs. Using immunofluorescence, a subpopulation of antigen-positive cells with small round or polymorphic nuclei was detected, showing expression of hematopoietic progenitor and mature antigen expression (not shown, refer to ref. 14), albeit to a rather low degree. In parallel, the same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors (Fig. 2b). At the protein level, single double positive cells for CD31/vWF (von Willebrand factor) and VEGFR-2/CD34 were detected [14].
Hematopoietic and endothelial progenitors share expression of a number of genes, including VEGFR-2, CD34, SCL, GATA2, RUNX1, and CD31, suggesting that investigated hMSCs possess in vitro hemangioblastic capacity, and might act as extrinsic differentiation factors and lineage-inducing regulators. Most potent differentiation was achieved in cultures where the majority of hMSCs adopted stromal function, thus inducing a minor part for differentiation. We concluded from the in vitro results, that MSCs might reconstitute the hematopoietic system. Hypothetically, one pluripotent stem cell would suffice to rescue lethally irradiated hosts. In reality, however, approx. 6 cells are needed [13], i.e. six pluripotent MSCs with the respective potential might suffice to restore hematopoiesis in vivo.
MSCs promote hematopoietic recovery after lethal irradiation
To test in vivo ability of murine MSCs to replenish the hematopoiesis after eradication, lethally irradiated (9.5 Gy) female recipients of the C57Bl/6J-CD45.1 strain were subjected to i.v. transplantation with 106 eGFP-marked male bulk-culture C57Bl/6J mouse MSCs (mMSCs). Mouse MSC were cultured in DMEM/Ham´s F12 + 20% preselected FCS + Glutamin + ß-mercaptoethanol and cells after 9-12 passages used for transplantation. Leukocyte and thrombocyte recovery was similar to recipients transplanted with HSCs (Fig. 3) reaching normalization of white blood cell counts after 4 weeks. Seven months later, the recipients were hematologically well, with a normal distribution of peripheral cell populations (Table 1). Similar experiments were carried out with clonal mMSCs showing one clone (IXH8) with superior survival promoting properties (Table 2). Noteworthy, the IXH8 clone was different from all other cultures showing long-stretched morphology and increased CD34 and CD45, however, without CD105 expression (Table 2).
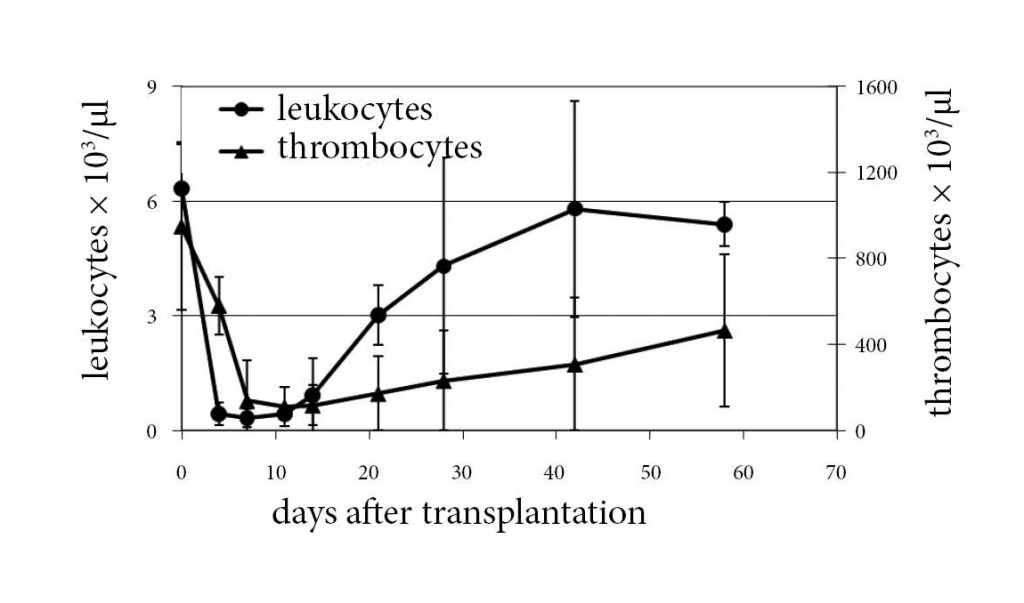
Figure 3: Mouse MSC rescue mice after total body irradiation. Transplantation of bulk mMSC led to a normalization of the peripheral white blood cell count within 4 weeks. Thrombocyte recovery needed approx. 8 weeks for normalization.

Table 1: Peripheral blood cell populations in mMSC transplanted animals. Shown is the distribution of white blood cells 5 months after bulk mMSC transplantation estimated using Pappenheim-stained blood smears.
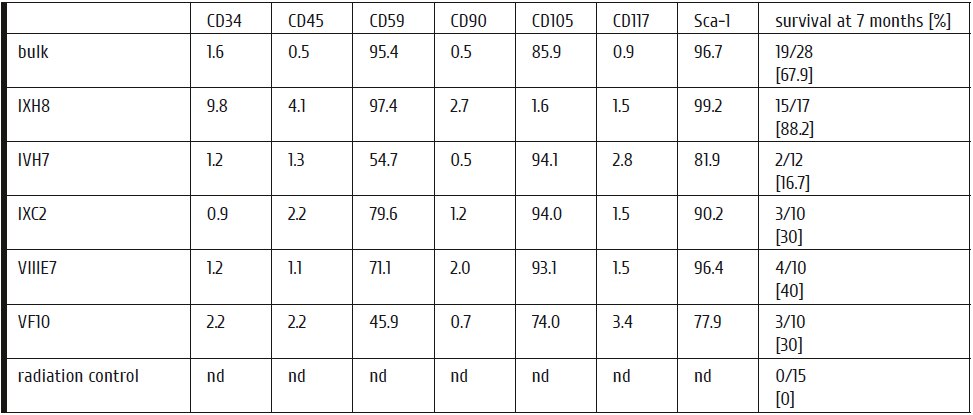
Table 2: Phenotypical characterization of mMSC and recipients’ survival rates after transplantation.
Cultures of eGFP-transduced bulk and cloned mMSC after extended expansion were positive for CD59, CD105 and Sca-1 but negative for the hematopoietic markers CD34, CD45, CD117 and for CD90 by flow cytometry. Clone IXH8 was different from all other cultures in its expression of CD34/CD45 and negativity of CD105 (shown in bold italic). Transplantation with this clone resulted in the highest survival rate of the irradiated recipients, suggesting elevated CD34 and CD45 and no CD105 expressions might be a prerequisite of the high rescue capability. nd, not done.
Transplanted donor cells are detectable short- but not long-term
To trace donor chimerism in recipients, we stained recipient peripheral blood (PB), BM and thymus cells with CD45.2 antibodies and carried out flow cytometry. Interestingly, no CD45.2-positive cells were found at any time point, thus not showing regeneration through donor cells. Y-chromosome-based chimerism analysis in female recipients using specific Y-chromosome primers for quantitative PCR could not detect donor cells in any of investigated tissues including PB and BM (not shown), although animals survived up to the final evaluation after 7 months. Spectral karyotyping of clonal mMSC revealed loss of Y-chromosome (Fig. 4), whereas bulk cultures were still Y-positive at passage13 (not shown).

Figure 4: Spectral karyotyping of mMSC. Shown is the SKY analysis of clone IXH8. SKY analysis of a representative diploid metaphase revealed the loss of the Y-chromosome and this has been observed in all metaphases analyzed.
Next, we used eGFP-specific primers for quantitative PCRbased donor cell detection. Primers for stably integrated eGFP-sequences, however, also failed to detect any donor cells, and no eGFP-positive cells were found in blood, BM or thymus by flow cytometry. Although we cannot completely rule out single donor cells below the detection limit, hematopoietic recovery in recipients is unlikely due to replacement with donor cells. This conclusion contradicts earlier results of hematopoietic recovery after myeloablative TBI with blood-derived mMSCs [11, 15] showing donor characteristics in blood and BM. One fundamental difference between both cell sources is potential in vitro immortalization, altering BM seeding capability of MSC. Therefore, our results support the concept of impaired transplantability of expanded MSC [24] but also challenge the hypothesis of high plasticity of MSC [1].
The distribution kinetics of eGFP+ donor cells after i.v. transplantation identified fast disappearance from PB, reaching ca. 2% after 8 hours and no cells at d10 (Fig. 5a). In contrast, mMSC trapped in lungs quickly (Fig. 5b), however without long-term residence and embolization as shown by lack of donor signals after d+10. Accordingly, no donor cells were detectable evident in the spleen, liver, BM (Fig. 5b), aorta, kidney, intestine, fat, thymus or lymph nodes (not shown). Although we did not find donor derived MSC in the BM, the morphology of this organ was preserved by MSC transplantation showing a normal distribution between different compartments (Fig. 6). Without MSCs, adipocytes are shown to dominate within short time, thus destroying the marrow structure.
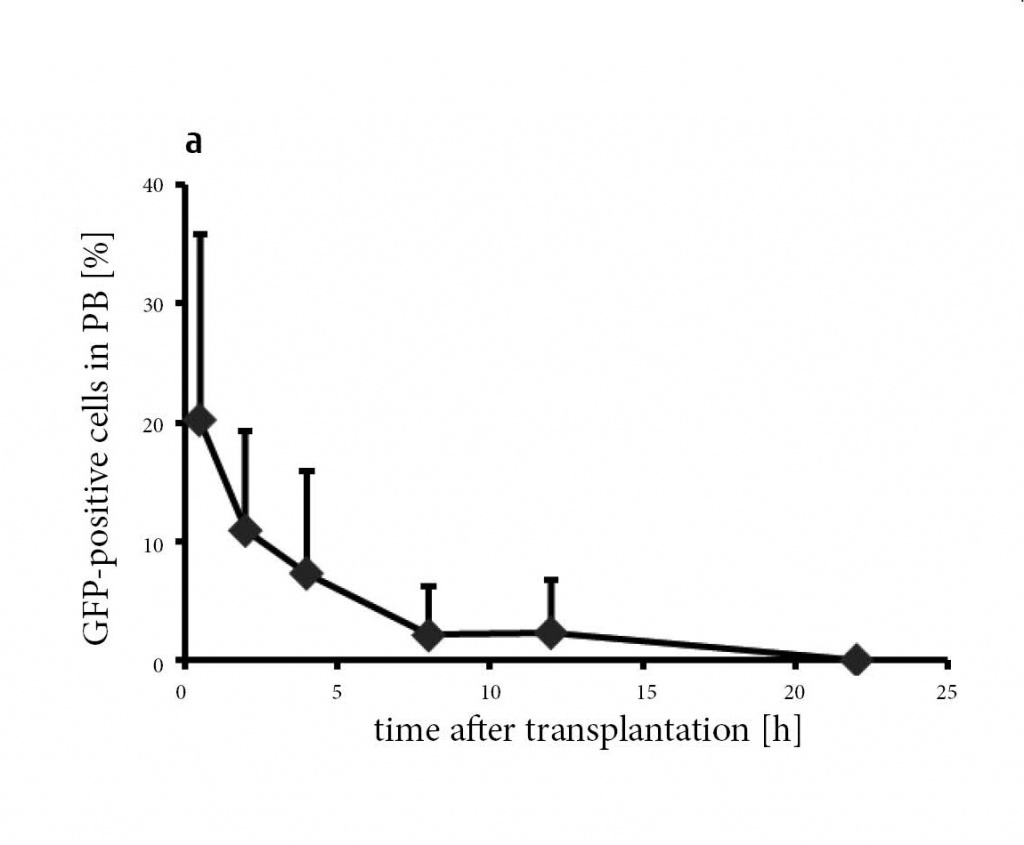
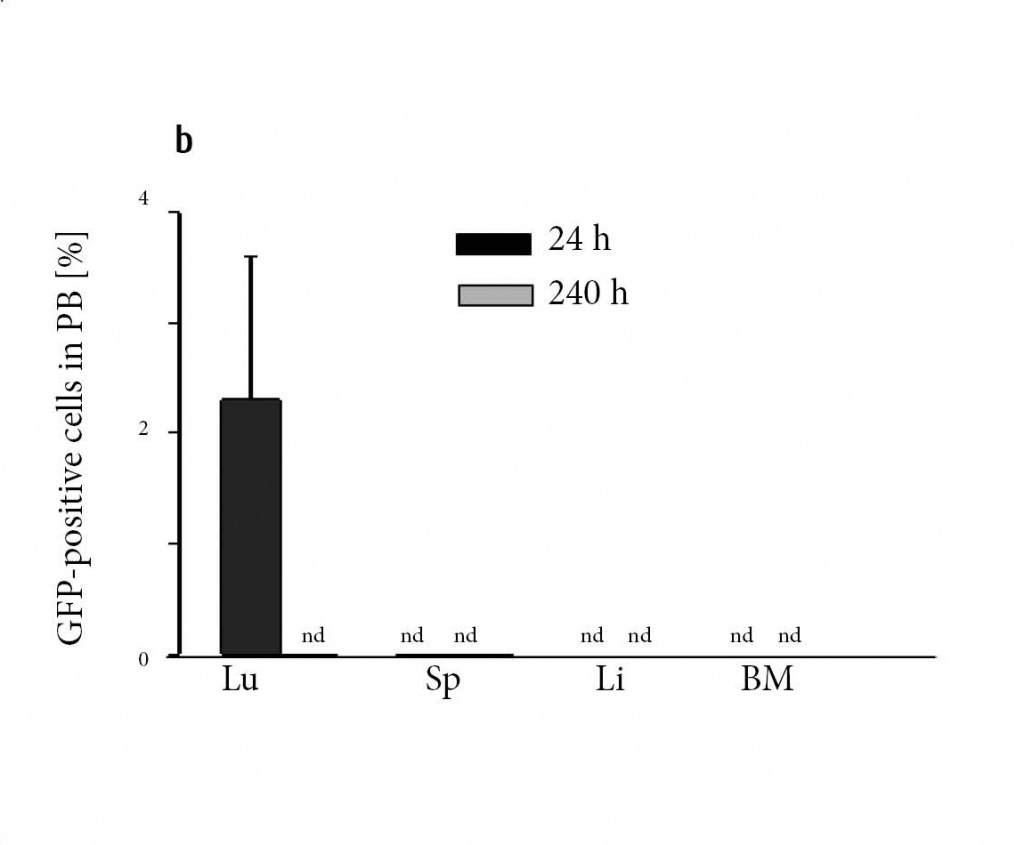
Figure 5: Donor mMSC are not detectable at longer terms.
(a) Tracking of eGFP-labeled clonal IXH8 donor mMSC after transplantation revealed a fast decrease in peripheral blood (PB). Within 8 hours, approx. 2% were quantified in PB and none after 10 days (n = 8 for each time point). (b) mMSC accumulated in lungs (Lu) within 24 h and disappeared within 10 days (240 h). Spleen (Sp), liver (Li) and BM were negative at d1 and d10. nd, not detected.
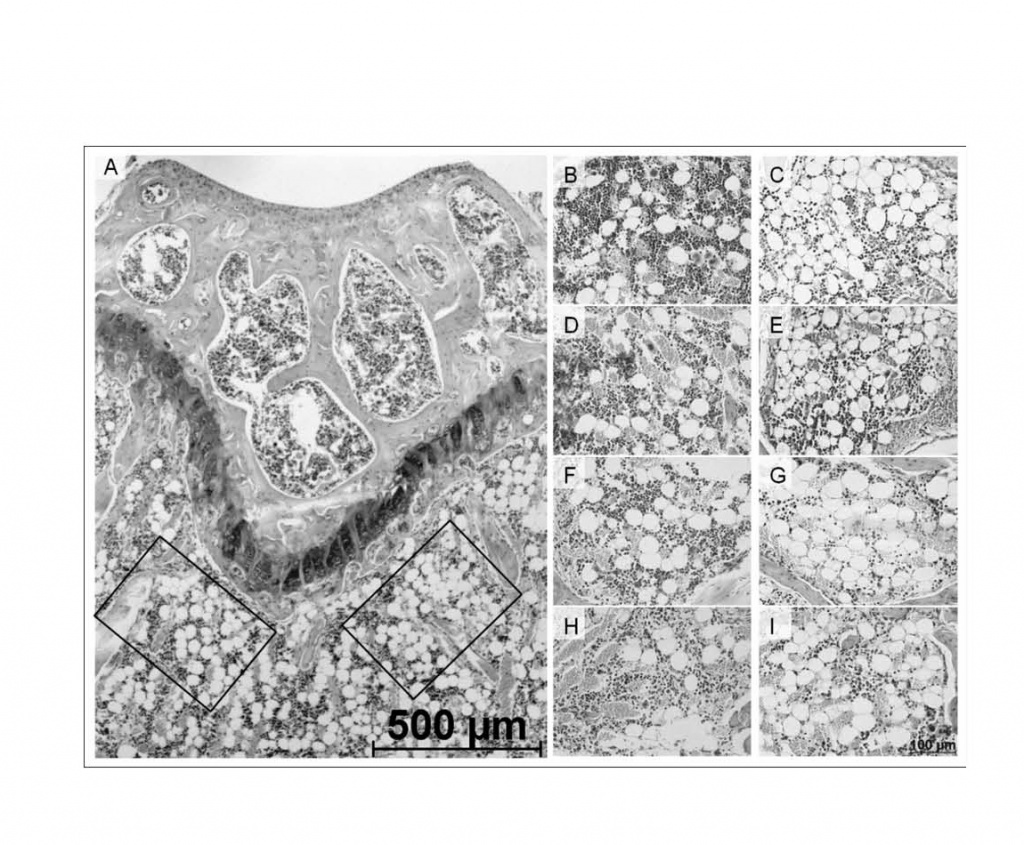
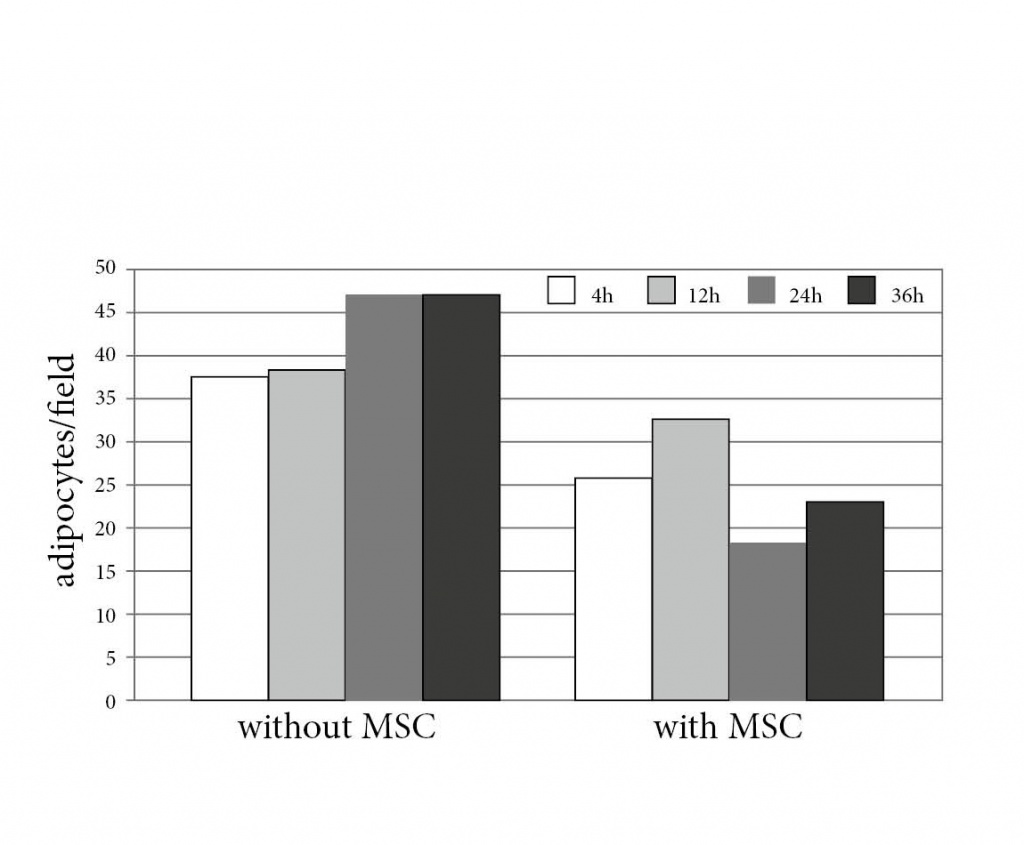
Figure 6. Histomorphology of BM with and without MSC transplantation.
Paraffin embedded long bones from MSC-transplanted or control animals were cut and the number of adipocytes counted in 2 designated
areas (A) per bone from mice with MSC transplantation after 4 (B), 12 (D), 24 (F) and 36 (H) hours or without MSC transplantation
(C, E, G, and I respectively). The lower figure shows the number of adipocytes at each time point.
MSCs change the BM gene expression
While donor mMSC did not home to the BM, we observed a long term recipients´ survival and assumed an influence of MSCs on the BM function. Therefore we carried out microarray analysis of bone marrow cells from MSC-transplanted animals, and compared their gene expression profiles to that of HSC-transplanted animals and age-matched controls [14]. The gene expression profile in BM changed significantly, clustering into separate group as compared to untreated BM or HSC-transplanted mice. Validation of selected genes with high variance proved a beneficial role of MSC in endogenous hematopoietic reconstitution. MSCs caused upregulated protection from oxidative stress, cell cycle, anti-inflammatory and detoxication events (e.g. BRPK, Cdkn1a, Thbs2, Gstm5 gene expression) in a complex way, along with downregulation of lymphoid development, pro-inflammatory events, protein degradation and adhesion/matrix formation for improved cell motility (e.g. gene expressions of Vpreb1, Rag2, Klk6, Klk1b5, Uchl1, Sykb, Gpam, Col5a3, Emid1) [14]. Upon summarising the microarray expression data, we have shown upregulation of the genes which are beneficial to BM reconstitution, whereas the genes with supposed radiation- related BM deterioration were downregulated (Fig. 7).
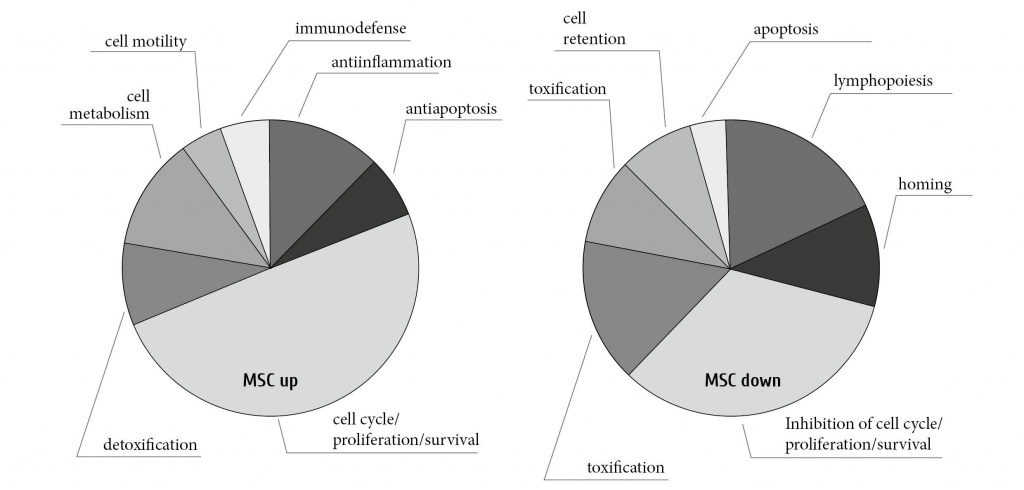
Figure 7. MSC transplantation into lethally irradiated animals changes the gene expression in the bone marrow.
Gene expression data were generated using microarray analysis and significantly regulated genes clustered into functional groups.
Shown are upregulated functional gene groups (MSC up) or downregulated (MSC down) in MSC-transplanted animals.
Potential paracrine mechanism of MSC
Potential mechanisms mediating bone marrow protection by MSCs entrapped in the lung, still remain unclear. Recently, we could show that injection of MSC-derived microvesicles to lethally irradiated animals provided similar protective effects, as transplantation of MSCs per se (Fig. 8). The microvesicles represent a fraction of ultra-small lipid bilayer particles of 30 to 1000 nm size (including exosome fraction) which are known to shuttle proteins, lipids, mRNA and microRNA [25]. Any of these components could participate in radiation protection and recovery of the bone marrow. Interestingly, the microvesicle-associated reconstitution of platelet scores occurred at a faster time frames, as compared to MSCs injections. Further work should reveal a more precise mechanism conferring radiation protection associated with MSC microvesicles.
Discussion
In this study we present an evidence that donor MSCs do not directly reconstitute the hematopoietic system following radiation insult. However, these cells may provide salvage for the surviving HSCs. Acute irradiation produces excessive inflammatory responses (23) which contribute to HSC death if untreated. Along with other organs, the lung is also heavily affected by radiation damage and might retard MSCs. Mesenchymal cells interfere with inflammation by changing overall gene expression profile, both in lungs where they are captured, and in bone marrow compartments. Assuming this, a direct MSC homing to the bone marrow is not necessary for changed gene expression patterns. This mechanism has been described in murine model of myocardial infarction where hMSCs have been shown to produce antiapoptotic TSG6 without significant engraftment [19]. A paracrine, differentiation-independent effect of MSCs did also ameliorate kidney injury [17, 26].
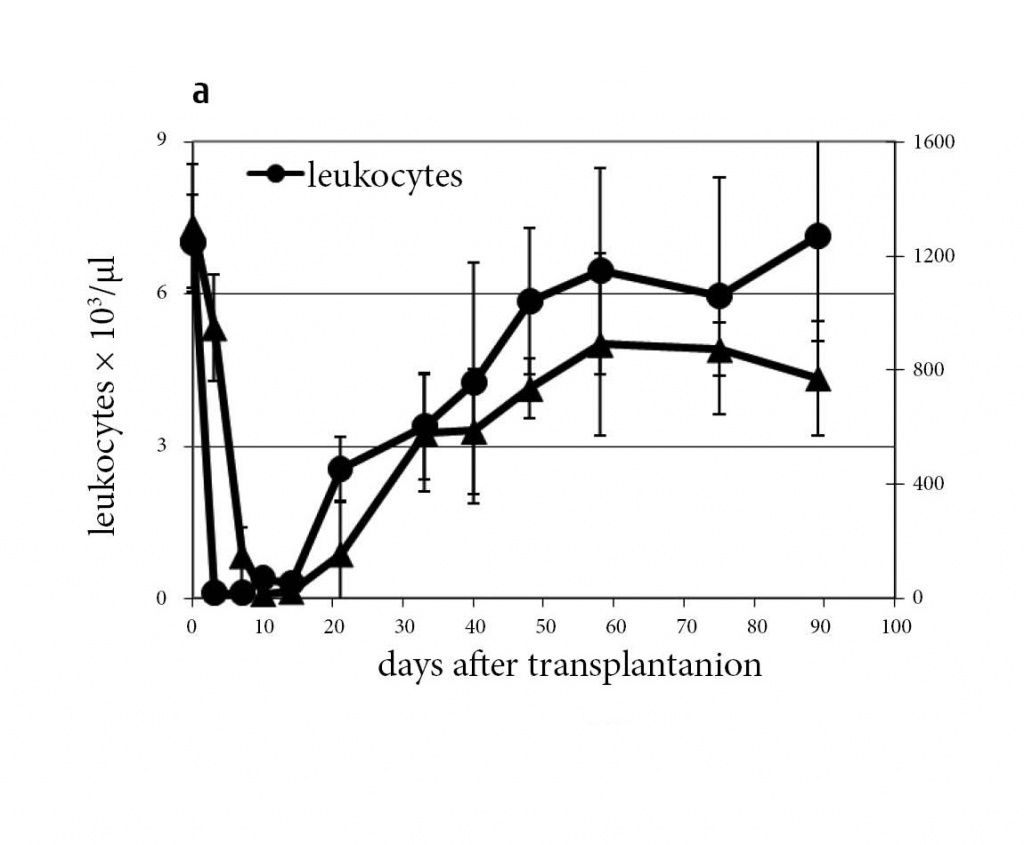
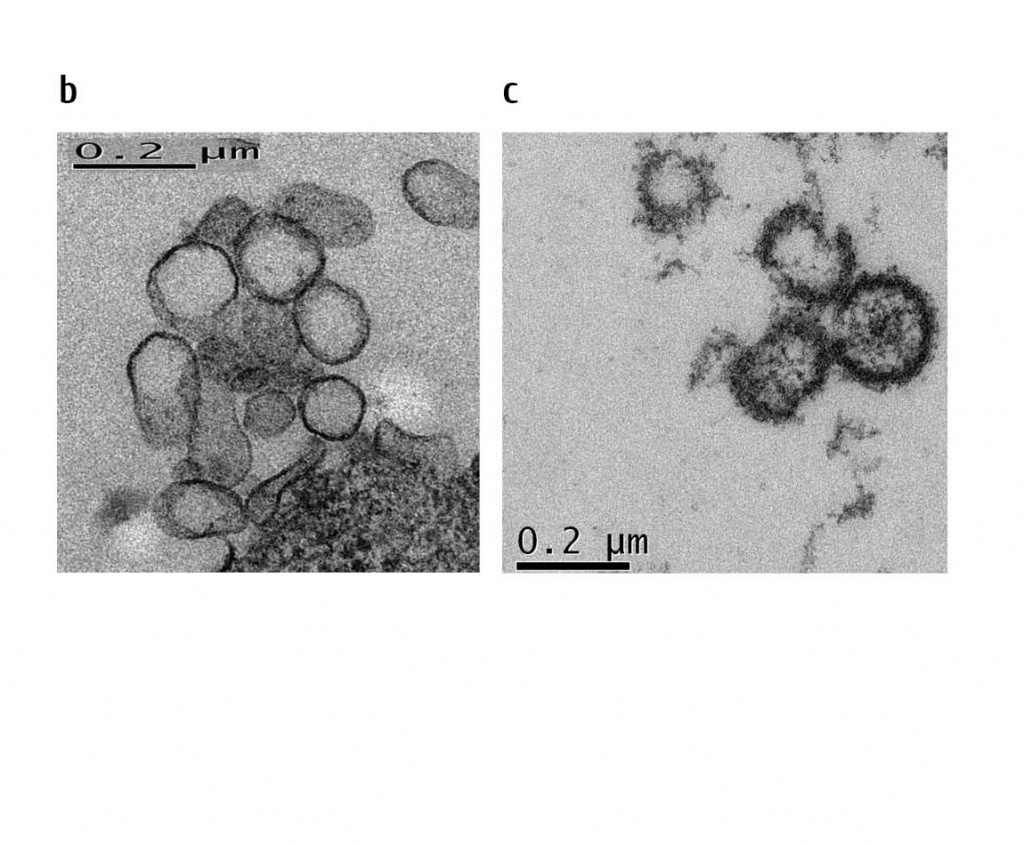
Figure 8: Transplantation of mMSC-derived microvesicles rescues lethally irradiated animals.
Leukocyte counts after mMSC-derived microvesicle injection normalized with similar kinetics as with mMSC, whereas thrombocyte
counts showed a much faster normalization (a)(n=15). Electron microscopy of microvesicles released from mMSC (b) and purified by
ultracentrifugation (c).
What could be expected from MSC as a potential therapeutic tool? Secretion of broad-range bioactive molecules is now believed to be the main mechanism by which the therapeutic effects of MSCs are achieved [20]. MSCs may secrete active factors that (a) inhibit apoptosis and limit the extent of cellular damage; (b) inhibit fibrosis or scarring at the injured sites; (c) protect microvasculature and stimulate angiogenesis, thus improving perfusion rates; and (d) promote proliferation of tissue- specific progenitor cells, as shown for cardiac-, neural- and kidney-specific stem cells [26,27]. In parallel, we have shown in a model with acute irradiation that MSCs boosted anti-inflammatory, anti-apoptotic, detoxifying, cell cycle and anti-oxidative stress control, whereas proinflammatory effects, extracellular matrix formation, and adhesion properties were decreased. In general, MSC injections may result into systemic improvements counteracting deleterious effects of myelosuppression [14].
In conclusion, transplanted MSC might export their inherent trophic effect to unorthodox sites [3], e.g. to lungs. Our results present another piece of evidence for this highly effective paracrine mechanism which may work, e.g., in BM populations, suggesting MSC-infusion to be an efficient treatment option following acute irradiation. Despite some limitations in our existing knowledge, a capacity of MSCs, or MSC-derived microvesicles, to exert hematopoietic support via a bystander mechanisms, might indicate that persistent engraftment at the site of damage is not a mandatory prerequisite. Importantly, a very short-term residence of MSCs in lung and/or the entire organism might critically contribute to the safety of this cell-based therapy, by avoiding potential side effects as tumor formation or maldifferentiation.
Acknowledgements
There are no commercial associations that might create a conflict of interest in connection with this paper.
This work was supported by the Federal Ministry of Education and Research, Germany, grant number 13N8904 and by the “Deutsche José Carreras Leukämie-Stiftung e.V.”, grant number DJCLS R 12/30.
References
- Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nature Med 2001; 7:393–395.
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 23: 841-846.
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98: 1076-1084.
- Chao NJ. Accidental or intentional exposure to ionizing radiation: biodosimetry and treatment options. Exp Hematol 2007; 35: 24-27.
- Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315-317.
- Ferrara JLM, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant 1999; 5:347–356.
- Fliedner TM, Chao NJ, Bader JL, Boettger A, Case C Jr et al. Stem cells, multiorgan failure in radiation emergency medical preparedness: a U.S./European Consultation Workshop. Stem Cells 2009; 27:1205-1211.
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974; 17: 331–340.
- Graf T, Enver T. Forcing cells to change lineages. Nature 2009, 462:587-594.
- Hérodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol 2005; 33:1071-1080.
- Huss R, Lange C, Weissinger EM, Kolb HJ, Thalmeier K. Evidence of peripheral blood derived, plasticadherent CD34(-/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells 2000; 18:252–260.
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18:307–316.
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S et al. Multi-organ, multilineage engraftment by a single bone marrow-derived stem cell. Cell 2001; 105:369–377.
- Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One 2011; 5: 6(1):e14486. doi: 10.1371/journal.pone.0014486.
- Lange C, Kaltz C, Thalmeier K, Kolb HJ, Huss R. Hematopoietic reconstitution of syngeneic mice with a peripheral blood-derived, monoclonal CD34-, Sca-1+, Thy-1(low), c-kit+ stem cell line. J Hematother Stem Cell Res 1999; 8:335–342.
- Lange C, Schroeder J, Lioznov MV, Zander AR. High-potential human mesenchymal stem cells. Stem Cells Dev 2005; 14:70-80.
- Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells are renoprotective in ischemia/reperfusion acute renal failures in rats. Kidney Int 2005; 68:1613-1617.
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H et al. Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versushost disease: a phase II study. Lancet 2008; 371:1579–1586.
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J et al. Intravenous hMSC improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009; 5:54-63.
- Meirelles L da S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009; 20:419-427.
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110:3499–3506.
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/ multipotent stromal cells: the state of transdifferentiation and modes of tissue repair current views. Stem Cells 2007; 25:2896–2902.
- Remberger M, Sundberg B. Cytokine production during myeloablative and reduced intensity therapy before allogeneic stem cell transplantation. Haematologica 2004; 89:710- 716.
- Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003; 17:160-170.
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9:581-593.
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005; 289:F31-42.
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008; 8:726-736.
Introduction
Management of patients subjected to extensive body irradiation as a part of conditioning therapy still remains a major challenge. Survival of radiation-induced bone marrow failure depends on the dose of radiation received and the intensity of supportive care which can protect from otherwise lethal infection and give surviving stem cells a chance to replenish blood cell populations. Since radiation effects on blood stem cells occur at doses generally lower than those on other critical organs, the rapidly emerging changes in the peripheral blood cell lineages determine the treatment options. In fact, total body irradiation (TBI) at doses more than 7-8 Gy in humans corresponds to medullar eradication. Under this threshold, spontaneous recovery from residual hematopoietic stem and progenitor cells may be expected within 30–50 days, however, preceded by cytopenic phases of granulocytic, megakaryocytic and erythrocytic lineages. Interestingly, even after TBI, intrinsically radioresistant stem cells have been detected in distinct bone marrow (BM) areas comprising a residual hematopoietic stem and progenitor cell pool [10]. Acute irradiation does not only imply damage to the bone marrow. In a dose-dependent matter, it can also emerge as gastrointestinal and cerebrovascular syndromes leading to development of multiple organ dysfunction (1). Damage to the whole organism is related to a systemic inflammatory response. Different target organs are affected due to activation of innate immune system, resulting in a significant release of inflammatory cytokines [4]. The pathophysiology of such tissue damage appears comparable to that of acute graftversus- host disease (GvHD) following allogeneic stem cell transplantation where a similar ”cytokine storm” has been observed [6]. In absence of appropriate treatment, oxidative stress after high dose ionizing radiation has been involved in delayed morbidity [4]. Management of acute radiation syndrome relies, therefore, on tissue damage repair processes that might be supported by therapies aimed for mitigation of inflammation [4].
Efforts to improve outcome after irradiation focus on the stem cell niche. Therefore, prospective therapies should augment the hematopoietic niche activity to accelerate the in vivo recovery of blood cell populations. Several studies have demonstrated that BM osteoblasts regulate the HSC pool size in vivo via the Jagged1-Notch signaling pathway [7]. For example, parathyroid hormone receptor activation can increase the number of osteoblastic cells, thus resulting in Notch1-mediated expansion of HSC [2]. Mesenchymal stromal cells (MSC) comprise an integrative part of the BM stroma, being also described as osteoblastic progenitors [8]. MSC are multipotential nonhematopoietic progenitor cells capable of differentiating into multiple lineages of the mesenchyme. In bone marrow, the local stromal cells surround HSC and their progeny. The hematopoietic niche provides a sheltering microenvironment that provides maintenance and self-renewal of HSC by shielding them from differentiation and apoptotic stimuli that would otherwise challenge stem cell reserves. Moreover, the hematopoietic niche also controls proliferation and differentiation of HSC and release of mature progeny into peripheral blood flow. Regulation of HSC quiescence, by maintenance of resting HSC in endosteal niche, control of HSC proliferation, differentiation and recruitment in the vascular niche can be ascribed to bone-marrow stromal cells [27]. Thus, physiological role of MSCs is not a mere replacement of mesenchymal tissues such as bone. Moreover, their primary and most important function is to inhibit immunosurveillance and to establish a protective and regenerative microenvironment for HSC.
Clinically, MSCs have been proven to intervene with acute organ impairment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy significantly decreasing the time to full hematopoietic and particularly platelet reconstitution [12]. Additionally, there is evidence for MSC effectiveness in the treatment of steroid resistant GvHD without any side effects, even when obtained from BM of third-party donors [18]. No HLA-match is needed between donor and recipient because MSCs have been shown to be hypoimmunogenic and are not recognized by the recipient immune system even after repeated injections [18]. Finally, MSCs secrete a variety of bioactive molecules [22]. Among those, some essential hematopoietic growth factors including IL-6, IL-11, leukemia inhibitory factor (LIF), stem cell factor (SCF) and Flt3 ligand are produced, as well as factors with immunomodulatory effects, e.g. transforming growth factor-β1 (TGF-β1), prostaglandin E2, indoleamine 2,3-dioxygenase, and others [21]. Additionally, vascular endothelial growth factor (VEGF) secreted by MSCs in abundance might interfere with early apoptotic cell death after irradiation [10]. Therefore, MSCs might be a good candidate for modulation of the hematopoietic niche activity. In summary, MSCs have emerged as a promising therapeutic tool for tissue regeneration and repair. Further clinical interest has been raised by the observation that MSCs are immunoprivileged and might be transplanted from unrelated, i.e. allogeneic donors [21,27]. Altogether, we assumed that MSCs, with their comprehensive trophic potential, could serve as a readily available treatment option after severe radiation exposure. The aim of our study was to evaluate essential biological parameters of MSC, with respect to their lineage- specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts.
Methods and Results
In vitro differentiation of human MSC (hMSC)
As first experiments, we investigated the capability of human BM-derived MSCs (hMSCs) to differentiate into progenitors for hematopoietic (HSC) and endothelial cells (EC). The human MSCs were thoroughly characterized according to the ISCT (International Society for Cellular Therapy) criteria [5], including flow cytometry and their capability to differentiate into three mesodermal lineages [16]. To avoid any contamination of MSCs with HSC, cloned cells were used exclusively. Cloned human MSCs were subjected to differentiation into (i) hematopoietic cells using serum-containing or serum-depleted growth conditions and (ii) endothelial cells (for technical details see ref. 14). Fibroblastoid MSCs (Fig. 1a) formed blast-like cells with noticeably decreased diameter from originally 28.9 ± 6.6 to 15.7 ± 3.5 μm during the differentiation into hematopoietic (Fig. 1b) and endothelial (Fig. 1c) lineages. The in vitro conditions led to cluster formation appearing as an in vitro equivalent of stromal structures from which differentiation proceeded. The cells committed to hematopoietic lineage changed their gene expression towards appropriate profiles of blood cell progenitors (CD117, CD133, CD45) and mature (CD14, CD16, glycophorinA GlyA, CD31, podoplanin PDPN) hematopoietic cells (Fig. 2a). Interestingly, the erythropoietin receptor (EPOR) was upregulated in almost all clones and under all conditions suggesting a definite role for EPO in proliferation and differentiation of mesodermal progenitors. Additionally,

Figure 1: Human MSC display a fibroblastoid morphology during in vitro expansion but form blast-like cells after induction of differentiation. One clonal hMSC culture is shown during expansion (a), differentiation into hematopoietic (b) or endothelial (c) cells.


Figure 2: Human MSC significantly upregulate expressions of hematopoietic and endothelial genes after induction of differentiation. Shown are the fold changes of gene expressions of indicated hematopoietic (a) and endothelial (b) genes after differentiation compared to undifferentiated hMSC. GlyA, Glycophorin A; vWF, von Willebrand factor; VEGFR, vascular endothelial growth factor receptor.
a variety of transcription factors responsible for erythropoiesis (SCL/tal1), erythro-megakaryopoiesis (GATA1, GATA2), lymphopoiesis (GATA3), and myelopoiesis (NOTCH1, RUNX1) were upregulated upon serum-containing differentiation. As SCL and RUNX1 are transcription factors essential for HSC formation by instructing lineage specification (9), we suggested an efficient induction of this differentiation pathway in MSCs. Using immunofluorescence, a subpopulation of antigen-positive cells with small round or polymorphic nuclei was detected, showing expression of hematopoietic progenitor and mature antigen expression (not shown, refer to ref. 14), albeit to a rather low degree. In parallel, the same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors (Fig. 2b). At the protein level, single double positive cells for CD31/vWF (von Willebrand factor) and VEGFR-2/CD34 were detected [14].
Hematopoietic and endothelial progenitors share expression of a number of genes, including VEGFR-2, CD34, SCL, GATA2, RUNX1, and CD31, suggesting that investigated hMSCs possess in vitro hemangioblastic capacity, and might act as extrinsic differentiation factors and lineage-inducing regulators. Most potent differentiation was achieved in cultures where the majority of hMSCs adopted stromal function, thus inducing a minor part for differentiation. We concluded from the in vitro results, that MSCs might reconstitute the hematopoietic system. Hypothetically, one pluripotent stem cell would suffice to rescue lethally irradiated hosts. In reality, however, approx. 6 cells are needed [13], i.e. six pluripotent MSCs with the respective potential might suffice to restore hematopoiesis in vivo.
MSCs promote hematopoietic recovery after lethal irradiation
To test in vivo ability of murine MSCs to replenish the hematopoiesis after eradication, lethally irradiated (9.5 Gy) female recipients of the C57Bl/6J-CD45.1 strain were subjected to i.v. transplantation with 106 eGFP-marked male bulk-culture C57Bl/6J mouse MSCs (mMSCs). Mouse MSC were cultured in DMEM/Ham´s F12 + 20% preselected FCS + Glutamin + ß-mercaptoethanol and cells after 9-12 passages used for transplantation. Leukocyte and thrombocyte recovery was similar to recipients transplanted with HSCs (Fig. 3) reaching normalization of white blood cell counts after 4 weeks. Seven months later, the recipients were hematologically well, with a normal distribution of peripheral cell populations (Table 1). Similar experiments were carried out with clonal mMSCs showing one clone (IXH8) with superior survival promoting properties (Table 2). Noteworthy, the IXH8 clone was different from all other cultures showing long-stretched morphology and increased CD34 and CD45, however, without CD105 expression (Table 2).

Figure 3: Mouse MSC rescue mice after total body irradiation. Transplantation of bulk mMSC led to a normalization of the peripheral white blood cell count within 4 weeks. Thrombocyte recovery needed approx. 8 weeks for normalization.

Table 1: Peripheral blood cell populations in mMSC transplanted animals. Shown is the distribution of white blood cells 5 months after bulk mMSC transplantation estimated using Pappenheim-stained blood smears.

Table 2: Phenotypical characterization of mMSC and recipients’ survival rates after transplantation.
Cultures of eGFP-transduced bulk and cloned mMSC after extended expansion were positive for CD59, CD105 and Sca-1 but negative for the hematopoietic markers CD34, CD45, CD117 and for CD90 by flow cytometry. Clone IXH8 was different from all other cultures in its expression of CD34/CD45 and negativity of CD105 (shown in bold italic). Transplantation with this clone resulted in the highest survival rate of the irradiated recipients, suggesting elevated CD34 and CD45 and no CD105 expressions might be a prerequisite of the high rescue capability. nd, not done.
Transplanted donor cells are detectable short- but not long-term
To trace donor chimerism in recipients, we stained recipient peripheral blood (PB), BM and thymus cells with CD45.2 antibodies and carried out flow cytometry. Interestingly, no CD45.2-positive cells were found at any time point, thus not showing regeneration through donor cells. Y-chromosome-based chimerism analysis in female recipients using specific Y-chromosome primers for quantitative PCR could not detect donor cells in any of investigated tissues including PB and BM (not shown), although animals survived up to the final evaluation after 7 months. Spectral karyotyping of clonal mMSC revealed loss of Y-chromosome (Fig. 4), whereas bulk cultures were still Y-positive at passage13 (not shown).

Figure 4: Spectral karyotyping of mMSC. Shown is the SKY analysis of clone IXH8. SKY analysis of a representative diploid metaphase revealed the loss of the Y-chromosome and this has been observed in all metaphases analyzed.
Next, we used eGFP-specific primers for quantitative PCRbased donor cell detection. Primers for stably integrated eGFP-sequences, however, also failed to detect any donor cells, and no eGFP-positive cells were found in blood, BM or thymus by flow cytometry. Although we cannot completely rule out single donor cells below the detection limit, hematopoietic recovery in recipients is unlikely due to replacement with donor cells. This conclusion contradicts earlier results of hematopoietic recovery after myeloablative TBI with blood-derived mMSCs [11, 15] showing donor characteristics in blood and BM. One fundamental difference between both cell sources is potential in vitro immortalization, altering BM seeding capability of MSC. Therefore, our results support the concept of impaired transplantability of expanded MSC [24] but also challenge the hypothesis of high plasticity of MSC [1].
The distribution kinetics of eGFP+ donor cells after i.v. transplantation identified fast disappearance from PB, reaching ca. 2% after 8 hours and no cells at d10 (Fig. 5a). In contrast, mMSC trapped in lungs quickly (Fig. 5b), however without long-term residence and embolization as shown by lack of donor signals after d+10. Accordingly, no donor cells were detectable evident in the spleen, liver, BM (Fig. 5b), aorta, kidney, intestine, fat, thymus or lymph nodes (not shown). Although we did not find donor derived MSC in the BM, the morphology of this organ was preserved by MSC transplantation showing a normal distribution between different compartments (Fig. 6). Without MSCs, adipocytes are shown to dominate within short time, thus destroying the marrow structure.


Figure 5: Donor mMSC are not detectable at longer terms.
(a) Tracking of eGFP-labeled clonal IXH8 donor mMSC after transplantation revealed a fast decrease in peripheral blood (PB). Within 8 hours, approx. 2% were quantified in PB and none after 10 days (n = 8 for each time point). (b) mMSC accumulated in lungs (Lu) within 24 h and disappeared within 10 days (240 h). Spleen (Sp), liver (Li) and BM were negative at d1 and d10. nd, not detected.


Figure 6. Histomorphology of BM with and without MSC transplantation.
Paraffin embedded long bones from MSC-transplanted or control animals were cut and the number of adipocytes counted in 2 designated
areas (A) per bone from mice with MSC transplantation after 4 (B), 12 (D), 24 (F) and 36 (H) hours or without MSC transplantation
(C, E, G, and I respectively). The lower figure shows the number of adipocytes at each time point.
MSCs change the BM gene expression
While donor mMSC did not home to the BM, we observed a long term recipients´ survival and assumed an influence of MSCs on the BM function. Therefore we carried out microarray analysis of bone marrow cells from MSC-transplanted animals, and compared their gene expression profiles to that of HSC-transplanted animals and age-matched controls [14]. The gene expression profile in BM changed significantly, clustering into separate group as compared to untreated BM or HSC-transplanted mice. Validation of selected genes with high variance proved a beneficial role of MSC in endogenous hematopoietic reconstitution. MSCs caused upregulated protection from oxidative stress, cell cycle, anti-inflammatory and detoxication events (e.g. BRPK, Cdkn1a, Thbs2, Gstm5 gene expression) in a complex way, along with downregulation of lymphoid development, pro-inflammatory events, protein degradation and adhesion/matrix formation for improved cell motility (e.g. gene expressions of Vpreb1, Rag2, Klk6, Klk1b5, Uchl1, Sykb, Gpam, Col5a3, Emid1) [14]. Upon summarising the microarray expression data, we have shown upregulation of the genes which are beneficial to BM reconstitution, whereas the genes with supposed radiation- related BM deterioration were downregulated (Fig. 7).

Figure 7. MSC transplantation into lethally irradiated animals changes the gene expression in the bone marrow.
Gene expression data were generated using microarray analysis and significantly regulated genes clustered into functional groups.
Shown are upregulated functional gene groups (MSC up) or downregulated (MSC down) in MSC-transplanted animals.
Potential paracrine mechanism of MSC
Potential mechanisms mediating bone marrow protection by MSCs entrapped in the lung, still remain unclear. Recently, we could show that injection of MSC-derived microvesicles to lethally irradiated animals provided similar protective effects, as transplantation of MSCs per se (Fig. 8). The microvesicles represent a fraction of ultra-small lipid bilayer particles of 30 to 1000 nm size (including exosome fraction) which are known to shuttle proteins, lipids, mRNA and microRNA [25]. Any of these components could participate in radiation protection and recovery of the bone marrow. Interestingly, the microvesicle-associated reconstitution of platelet scores occurred at a faster time frames, as compared to MSCs injections. Further work should reveal a more precise mechanism conferring radiation protection associated with MSC microvesicles.
Discussion
In this study we present an evidence that donor MSCs do not directly reconstitute the hematopoietic system following radiation insult. However, these cells may provide salvage for the surviving HSCs. Acute irradiation produces excessive inflammatory responses (23) which contribute to HSC death if untreated. Along with other organs, the lung is also heavily affected by radiation damage and might retard MSCs. Mesenchymal cells interfere with inflammation by changing overall gene expression profile, both in lungs where they are captured, and in bone marrow compartments. Assuming this, a direct MSC homing to the bone marrow is not necessary for changed gene expression patterns. This mechanism has been described in murine model of myocardial infarction where hMSCs have been shown to produce antiapoptotic TSG6 without significant engraftment [19]. A paracrine, differentiation-independent effect of MSCs did also ameliorate kidney injury [17, 26].


Figure 8: Transplantation of mMSC-derived microvesicles rescues lethally irradiated animals.
Leukocyte counts after mMSC-derived microvesicle injection normalized with similar kinetics as with mMSC, whereas thrombocyte
counts showed a much faster normalization (a)(n=15). Electron microscopy of microvesicles released from mMSC (b) and purified by
ultracentrifugation (c).
What could be expected from MSC as a potential therapeutic tool? Secretion of broad-range bioactive molecules is now believed to be the main mechanism by which the therapeutic effects of MSCs are achieved [20]. MSCs may secrete active factors that (a) inhibit apoptosis and limit the extent of cellular damage; (b) inhibit fibrosis or scarring at the injured sites; (c) protect microvasculature and stimulate angiogenesis, thus improving perfusion rates; and (d) promote proliferation of tissue- specific progenitor cells, as shown for cardiac-, neural- and kidney-specific stem cells [26,27]. In parallel, we have shown in a model with acute irradiation that MSCs boosted anti-inflammatory, anti-apoptotic, detoxifying, cell cycle and anti-oxidative stress control, whereas proinflammatory effects, extracellular matrix formation, and adhesion properties were decreased. In general, MSC injections may result into systemic improvements counteracting deleterious effects of myelosuppression [14].
In conclusion, transplanted MSC might export their inherent trophic effect to unorthodox sites [3], e.g. to lungs. Our results present another piece of evidence for this highly effective paracrine mechanism which may work, e.g., in BM populations, suggesting MSC-infusion to be an efficient treatment option following acute irradiation. Despite some limitations in our existing knowledge, a capacity of MSCs, or MSC-derived microvesicles, to exert hematopoietic support via a bystander mechanisms, might indicate that persistent engraftment at the site of damage is not a mandatory prerequisite. Importantly, a very short-term residence of MSCs in lung and/or the entire organism might critically contribute to the safety of this cell-based therapy, by avoiding potential side effects as tumor formation or maldifferentiation.
Acknowledgements
There are no commercial associations that might create a conflict of interest in connection with this paper.
This work was supported by the Federal Ministry of Education and Research, Germany, grant number 13N8904 and by the “Deutsche José Carreras Leukämie-Stiftung e.V.”, grant number DJCLS R 12/30.
References
- Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nature Med 2001; 7:393–395.
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 23: 841-846.
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98: 1076-1084.
- Chao NJ. Accidental or intentional exposure to ionizing radiation: biodosimetry and treatment options. Exp Hematol 2007; 35: 24-27.
- Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315-317.
- Ferrara JLM, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant 1999; 5:347–356.
- Fliedner TM, Chao NJ, Bader JL, Boettger A, Case C Jr et al. Stem cells, multiorgan failure in radiation emergency medical preparedness: a U.S./European Consultation Workshop. Stem Cells 2009; 27:1205-1211.
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974; 17: 331–340.
- Graf T, Enver T. Forcing cells to change lineages. Nature 2009, 462:587-594.
- Hérodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol 2005; 33:1071-1080.
- Huss R, Lange C, Weissinger EM, Kolb HJ, Thalmeier K. Evidence of peripheral blood derived, plasticadherent CD34(-/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells 2000; 18:252–260.
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18:307–316.
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S et al. Multi-organ, multilineage engraftment by a single bone marrow-derived stem cell. Cell 2001; 105:369–377.
- Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One 2011; 5: 6(1):e14486. doi: 10.1371/journal.pone.0014486.
- Lange C, Kaltz C, Thalmeier K, Kolb HJ, Huss R. Hematopoietic reconstitution of syngeneic mice with a peripheral blood-derived, monoclonal CD34-, Sca-1+, Thy-1(low), c-kit+ stem cell line. J Hematother Stem Cell Res 1999; 8:335–342.
- Lange C, Schroeder J, Lioznov MV, Zander AR. High-potential human mesenchymal stem cells. Stem Cells Dev 2005; 14:70-80.
- Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells are renoprotective in ischemia/reperfusion acute renal failures in rats. Kidney Int 2005; 68:1613-1617.
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H et al. Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versushost disease: a phase II study. Lancet 2008; 371:1579–1586.
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J et al. Intravenous hMSC improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009; 5:54-63.
- Meirelles L da S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009; 20:419-427.
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110:3499–3506.
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/ multipotent stromal cells: the state of transdifferentiation and modes of tissue repair current views. Stem Cells 2007; 25:2896–2902.
- Remberger M, Sundberg B. Cytokine production during myeloablative and reduced intensity therapy before allogeneic stem cell transplantation. Haematologica 2004; 89:710- 716.
- Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 2003; 17:160-170.
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9:581-593.
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005; 289:F31-42.
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008; 8:726-736.
Клаудиа Ланге,1 Рудольф Раймер,2 Йозеф Зустин,3 Бербель Брунсвиг-Шпикенхайер1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6699" ["VALUE"]=> array(2) { ["TEXT"]=> string(606) "1. Клиника трансплантации стволовых клеток, Департамент клеточной и генной терапии, Университетский медицинский<br> центр Гамбург-Эппендорф<br> 2. Технологическая платформа микроскопии и анализа изображений, Институт Хайнриха Петте, Гамбург,<br> 3. Институт патологии, Университетский медицинский центр Гамбург Эппендорф" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(588) "1. Клиника трансплантации стволовых клеток, Департамент клеточной и генной терапии, Университетский медицинскийцентр Гамбург-Эппендорф
2. Технологическая платформа микроскопии и анализа изображений, Институт Хайнриха Петте, Гамбург,
3. Институт патологии, Университетский медицинский центр Гамбург Эппендорф" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6700" ["VALUE"]=> array(2) { ["TEXT"]=> string(6841) "<p> Ионизирующее излучение широко применяется в качестве кондиционирующей терапии при транс- плантации костного мозга. Высокодозная радиаци- онная терапия вызывает тяжелое повреждение, в особенности – гемопоэтических стволовых клеток и клеток-предшественников. Попытки улучшения кли- нических исходов после облучения сосредоточены на гемопоэтической нише. Мезенхимные стромальные клетки (МСК) представляют собой интегральную часть стромального микроокружения. При совмест- ной трансплантации с гемопоэтическими стволовыми клетками (ГСК), МСК способны усиливать восстанов- ление кроветворения после химио- и радиационной терапии. Целью нашего исследования была оценка основных биологических параметров МСК, в плане их способности к специфической линейной диффе- ренцировке, выживаемости организма, а также их способности к радиопротекции летально облученных реципиентов. Материалы и методы. Дифференциров- ку in vitro МСК человека в направлении гемопоэтиче- ских (ГСК) или эндотелиальных клеток изучали путем RT-qPCR поверхностных маркеров и других белков. Для тестирования in vivo способности мышиных МСК защищать летально облученных (9.5 Гр) мышей, животных трансплантировали мышиными МСК, ме- чеными eGFP. Длительность донорского химеризма определяли в крови, костном мозге и тимусе по марке- рам CD45.2 и Y-хромосомы. Анализ профилей генной экспрессии в клетках костного мозга проводили по сравнению с соответствующими контролями посред- ством биочипов. Результаты. При дифференцировке гемопоэтических стволовых клеток человека in vitro отмечалась изменения генной экспрессии со спектром экспрессии, типичным для кроветворных предше- ственников и зрелых клеток. Во время дифференци- ровки в средах с сывороткой наблюдалась повышен- ная экспрессия множества факторов, ответственных за эритропоэз, мегакариоцитопоэз, лимфо- и мииело- поэз. Была выявлена популяция клеток с небольшими круглыми или полиморфными ядрами, которые экс- прессировали антигенные маркеры, характерные для клеток-предшественников или зрелых форм, хотя и небольшой степени. Те же клетки приобретали мор- фологические черты эндотелия и экспрессировали гены, специфичные для эндотелиальных клеток при культивировании со специфическими факторами дифференцировки. </p> <p> После введения МСК, летально облученные мыши выживали с нормальным восстановлением кроветво- рения, как при введении кроветворных клеток. Через 7 мес. после облучения реципиенты МСК имели нор- мальное соотношение популяций периферической крови. Не было указаний на наличие сколько-нибудь длительного донорского химеризма после трансплан- тации. Оценка распределения eGFP+ донорских кле- ток после внутривенной трансфузии показала бы- строе исчезновение МСК из периферической крови, до 2% через 8 часов после введения с задержкой клеток в легких, однако без их длительной персистенции и эмболизации сосудов. Исследование экспрессии ге- нов в клетках костного мозга у животных, леченных МСК, показало повышение активности генов, способ- ствующих восстановлению кроветворения, наряду со снижением активности генов, ассоциированных с ра- диационным повреждением костного мозга. Инъек- ции летально облученныи животным микровезикул, происходящих из МСК, приводили к тем же протек- тивным эффектам, что и трансплантация МСКС как таковых. Заключение. Наши результаты представляют дополнительные данные о возможных механизмах вы- сокоэффективного паракринного механизма, который актуален, в частности, для популяций костного мозга, что указывает на то, что инфузии МСК являются эф- фективным средством лечения последствий острого облучения. Кроме того, трансплантация МСК может оказывать свой трофический эффект в необычных ме- стах, в точм числе – легочной ткани реципиента. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(6817) "
Ионизирующее излучение широко применяется в качестве кондиционирующей терапии при транс- плантации костного мозга. Высокодозная радиаци- онная терапия вызывает тяжелое повреждение, в особенности – гемопоэтических стволовых клеток и клеток-предшественников. Попытки улучшения кли- нических исходов после облучения сосредоточены на гемопоэтической нише. Мезенхимные стромальные клетки (МСК) представляют собой интегральную часть стромального микроокружения. При совмест- ной трансплантации с гемопоэтическими стволовыми клетками (ГСК), МСК способны усиливать восстанов- ление кроветворения после химио- и радиационной терапии. Целью нашего исследования была оценка основных биологических параметров МСК, в плане их способности к специфической линейной диффе- ренцировке, выживаемости организма, а также их способности к радиопротекции летально облученных реципиентов. Материалы и методы. Дифференциров- ку in vitro МСК человека в направлении гемопоэтиче- ских (ГСК) или эндотелиальных клеток изучали путем RT-qPCR поверхностных маркеров и других белков. Для тестирования in vivo способности мышиных МСК защищать летально облученных (9.5 Гр) мышей, животных трансплантировали мышиными МСК, ме- чеными eGFP. Длительность донорского химеризма определяли в крови, костном мозге и тимусе по марке- рам CD45.2 и Y-хромосомы. Анализ профилей генной экспрессии в клетках костного мозга проводили по сравнению с соответствующими контролями посред- ством биочипов. Результаты. При дифференцировке гемопоэтических стволовых клеток человека in vitro отмечалась изменения генной экспрессии со спектром экспрессии, типичным для кроветворных предше- ственников и зрелых клеток. Во время дифференци- ровки в средах с сывороткой наблюдалась повышен- ная экспрессия множества факторов, ответственных за эритропоэз, мегакариоцитопоэз, лимфо- и мииело- поэз. Была выявлена популяция клеток с небольшими круглыми или полиморфными ядрами, которые экс- прессировали антигенные маркеры, характерные для клеток-предшественников или зрелых форм, хотя и небольшой степени. Те же клетки приобретали мор- фологические черты эндотелия и экспрессировали гены, специфичные для эндотелиальных клеток при культивировании со специфическими факторами дифференцировки.
После введения МСК, летально облученные мыши выживали с нормальным восстановлением кроветво- рения, как при введении кроветворных клеток. Через 7 мес. после облучения реципиенты МСК имели нор- мальное соотношение популяций периферической крови. Не было указаний на наличие сколько-нибудь длительного донорского химеризма после трансплан- тации. Оценка распределения eGFP+ донорских кле- ток после внутривенной трансфузии показала бы- строе исчезновение МСК из периферической крови, до 2% через 8 часов после введения с задержкой клеток в легких, однако без их длительной персистенции и эмболизации сосудов. Исследование экспрессии ге- нов в клетках костного мозга у животных, леченных МСК, показало повышение активности генов, способ- ствующих восстановлению кроветворения, наряду со снижением активности генов, ассоциированных с ра- диационным повреждением костного мозга. Инъек- ции летально облученныи животным микровезикул, происходящих из МСК, приводили к тем же протек- тивным эффектам, что и трансплантация МСКС как таковых. Заключение. Наши результаты представляют дополнительные данные о возможных механизмах вы- сокоэффективного паракринного механизма, который актуален, в частности, для популяций костного мозга, что указывает на то, что инфузии МСК являются эф- фективным средством лечения последствий острого облучения. Кроме того, трансплантация МСК может оказывать свой трофический эффект в необычных ме- стах, в точм числе – легочной ткани реципиента.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6701" ["VALUE"]=> string(33) "10.18620/1866-8836-2016-5-2-50-59" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(33) "10.18620/1866-8836-2016-5-2-50-59" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6702" ["VALUE"]=> array(2) { ["TEXT"]=> string(221) "<p class="Autor"> Claudia Lange,<sup>1</sup> Rudolph Reimer,<sup>2</sup> Jozef Zustin,<sup>3</sup> Bärbel Brunswig-Spickenheier<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(151) "Claudia Lange,1 Rudolph Reimer,2 Jozef Zustin,3 Bärbel Brunswig-Spickenheier1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6703" ["VALUE"]=> array(2) { ["TEXT"]=> string(302) "1. Clinic for Stem Cell Transplantation, Dept. Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf,<br> 2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,<br> 3. Institute of Pathology, University Medical Center Hamburg-Eppendorf," ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(286) "1. Clinic for Stem Cell Transplantation, Dept. Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf,2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,
3. Institute of Pathology, University Medical Center Hamburg-Eppendorf," ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6704" ["VALUE"]=> array(2) { ["TEXT"]=> string(2624) "<h2> Abstract <br> </h2> <p> Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls. </p> <p> <span style="font-family: Cuprum, sans-serif; font-size: 26px;">Results</span> </p> <p> Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2548) "
Abstract
Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls.
Results
Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6705" ["VALUE"]=> string(134) "Mesenchymal stromal cells protect from consequences of HSCT-transplantation preparatory irradiation: insights into possible mechanisms" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(134) "Mesenchymal stromal cells protect from consequences of HSCT-transplantation preparatory irradiation: insights into possible mechanisms" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> &array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6787" ["VALUE"]=> string(3) "200" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "200" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6788" ["VALUE"]=> string(3) "218" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "218" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(13) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6702" ["VALUE"]=> array(2) { ["TEXT"]=> string(221) "<p class="Autor"> Claudia Lange,<sup>1</sup> Rudolph Reimer,<sup>2</sup> Jozef Zustin,<sup>3</sup> Bärbel Brunswig-Spickenheier<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(151) "Claudia Lange,1 Rudolph Reimer,2 Jozef Zustin,3 Bärbel Brunswig-Spickenheier1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(151) "Claudia Lange,1 Rudolph Reimer,2 Jozef Zustin,3 Bärbel Brunswig-Spickenheier1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6704" ["VALUE"]=> array(2) { ["TEXT"]=> string(2624) "<h2> Abstract <br> </h2> <p> Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls. </p> <p> <span style="font-family: Cuprum, sans-serif; font-size: 26px;">Results</span> </p> <p> Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2548) "
Abstract
Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls.
Results
Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2548) "
Abstract
Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls.
Results
Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6701" ["VALUE"]=> string(33) "10.18620/1866-8836-2016-5-2-50-59" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(33) "10.18620/1866-8836-2016-5-2-50-59" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(33) "10.18620/1866-8836-2016-5-2-50-59" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6705" ["VALUE"]=> string(134) "Mesenchymal stromal cells protect from consequences of HSCT-transplantation preparatory irradiation: insights into possible mechanisms" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(134) "Mesenchymal stromal cells protect from consequences of HSCT-transplantation preparatory irradiation: insights into possible mechanisms" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(134) "Mesenchymal stromal cells protect from consequences of HSCT-transplantation preparatory irradiation: insights into possible mechanisms" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6703" ["VALUE"]=> array(2) { ["TEXT"]=> string(302) "1. Clinic for Stem Cell Transplantation, Dept. Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf,<br> 2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,<br> 3. Institute of Pathology, University Medical Center Hamburg-Eppendorf," ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(286) "1. Clinic for Stem Cell Transplantation, Dept. Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf,2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,
3. Institute of Pathology, University Medical Center Hamburg-Eppendorf," ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(286) "1. Clinic for Stem Cell Transplantation, Dept. Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf,
2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,
3. Institute of Pathology, University Medical Center Hamburg-Eppendorf," } ["AUTHORS"]=> array(38) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(4) { [0]=> string(4) "6961" [1]=> string(4) "6962" [2]=> string(4) "6963" [3]=> string(4) "6964" } ["VALUE"]=> array(4) { [0]=> string(3) "515" [1]=> string(3) "520" [2]=> string(3) "521" [3]=> string(3) "522" } ["DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(4) { [0]=> string(3) "515" [1]=> string(3) "520" [2]=> string(3) "521" [3]=> string(3) "522" } ["~DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(4) { [0]=> string(56) "Claudia Lange" [1]=> string(57) "Rudolph Reimer" [2]=> string(55) "Jozef Zustin" [3]=> string(72) "Bärbel Brunswig-Spickenheier" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6698" ["VALUE"]=> array(2) { ["TEXT"]=> string(282) "<p class="Autor"> Клаудиа Ланге,<sup>1</sup> Рудольф Раймер,<sup>2</sup> Йозеф Зустин,<sup>3</sup> Бербель Брунсвиг-Шпикенхайер<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(212) "
Клаудиа Ланге,1 Рудольф Раймер,2 Йозеф Зустин,3 Бербель Брунсвиг-Шпикенхайер1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(212) "Клаудиа Ланге,1 Рудольф Раймер,2 Йозеф Зустин,3 Бербель Брунсвиг-Шпикенхайер1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6714" ["VALUE"]=> string(22) "05/10/2016 04:41:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "05/10/2016 04:41:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "05/10/2016 04:41:00 pm" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6715" ["VALUE"]=> string(22) "05/28/2016 04:41:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "05/28/2016 04:41:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "05/28/2016 04:41:00 pm" } ["KEYWORDS"]=> array(38) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(4) { [0]=> string(4) "6957" [1]=> string(4) "6958" [2]=> string(4) "6959" [3]=> string(4) "6960" } ["VALUE"]=> array(4) { [0]=> string(3) "516" [1]=> string(3) "517" [2]=> string(3) "518" [3]=> string(3) "519" } ["DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(4) { [0]=> string(3) "516" [1]=> string(3) "517" [2]=> string(3) "518" [3]=> string(3) "519" } ["~DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(4) { [0]=> string(100) "острое ионизирующее излучение" [1]=> string(91) "восстановление гемопоэза" [2]=> string(102) "мезенхимные стромальные клетки" [3]=> string(83) "протективные эффекты" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["CONTACT"]=> array(38) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6716" ["VALUE"]=> string(3) "515" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "515" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(56) "Claudia Lange" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6700" ["VALUE"]=> array(2) { ["TEXT"]=> string(6841) "<p> Ионизирующее излучение широко применяется в качестве кондиционирующей терапии при транс- плантации костного мозга. Высокодозная радиаци- онная терапия вызывает тяжелое повреждение, в особенности – гемопоэтических стволовых клеток и клеток-предшественников. Попытки улучшения кли- нических исходов после облучения сосредоточены на гемопоэтической нише. Мезенхимные стромальные клетки (МСК) представляют собой интегральную часть стромального микроокружения. При совмест- ной трансплантации с гемопоэтическими стволовыми клетками (ГСК), МСК способны усиливать восстанов- ление кроветворения после химио- и радиационной терапии. Целью нашего исследования была оценка основных биологических параметров МСК, в плане их способности к специфической линейной диффе- ренцировке, выживаемости организма, а также их способности к радиопротекции летально облученных реципиентов. Материалы и методы. Дифференциров- ку in vitro МСК человека в направлении гемопоэтиче- ских (ГСК) или эндотелиальных клеток изучали путем RT-qPCR поверхностных маркеров и других белков. Для тестирования in vivo способности мышиных МСК защищать летально облученных (9.5 Гр) мышей, животных трансплантировали мышиными МСК, ме- чеными eGFP. Длительность донорского химеризма определяли в крови, костном мозге и тимусе по марке- рам CD45.2 и Y-хромосомы. Анализ профилей генной экспрессии в клетках костного мозга проводили по сравнению с соответствующими контролями посред- ством биочипов. Результаты. При дифференцировке гемопоэтических стволовых клеток человека in vitro отмечалась изменения генной экспрессии со спектром экспрессии, типичным для кроветворных предше- ственников и зрелых клеток. Во время дифференци- ровки в средах с сывороткой наблюдалась повышен- ная экспрессия множества факторов, ответственных за эритропоэз, мегакариоцитопоэз, лимфо- и мииело- поэз. Была выявлена популяция клеток с небольшими круглыми или полиморфными ядрами, которые экс- прессировали антигенные маркеры, характерные для клеток-предшественников или зрелых форм, хотя и небольшой степени. Те же клетки приобретали мор- фологические черты эндотелия и экспрессировали гены, специфичные для эндотелиальных клеток при культивировании со специфическими факторами дифференцировки. </p> <p> После введения МСК, летально облученные мыши выживали с нормальным восстановлением кроветво- рения, как при введении кроветворных клеток. Через 7 мес. после облучения реципиенты МСК имели нор- мальное соотношение популяций периферической крови. Не было указаний на наличие сколько-нибудь длительного донорского химеризма после трансплан- тации. Оценка распределения eGFP+ донорских кле- ток после внутривенной трансфузии показала бы- строе исчезновение МСК из периферической крови, до 2% через 8 часов после введения с задержкой клеток в легких, однако без их длительной персистенции и эмболизации сосудов. Исследование экспрессии ге- нов в клетках костного мозга у животных, леченных МСК, показало повышение активности генов, способ- ствующих восстановлению кроветворения, наряду со снижением активности генов, ассоциированных с ра- диационным повреждением костного мозга. Инъек- ции летально облученныи животным микровезикул, происходящих из МСК, приводили к тем же протек- тивным эффектам, что и трансплантация МСКС как таковых. Заключение. Наши результаты представляют дополнительные данные о возможных механизмах вы- сокоэффективного паракринного механизма, который актуален, в частности, для популяций костного мозга, что указывает на то, что инфузии МСК являются эф- фективным средством лечения последствий острого облучения. Кроме того, трансплантация МСК может оказывать свой трофический эффект в необычных ме- стах, в точм числе – легочной ткани реципиента. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(6817) "Ионизирующее излучение широко применяется в качестве кондиционирующей терапии при транс- плантации костного мозга. Высокодозная радиаци- онная терапия вызывает тяжелое повреждение, в особенности – гемопоэтических стволовых клеток и клеток-предшественников. Попытки улучшения кли- нических исходов после облучения сосредоточены на гемопоэтической нише. Мезенхимные стромальные клетки (МСК) представляют собой интегральную часть стромального микроокружения. При совмест- ной трансплантации с гемопоэтическими стволовыми клетками (ГСК), МСК способны усиливать восстанов- ление кроветворения после химио- и радиационной терапии. Целью нашего исследования была оценка основных биологических параметров МСК, в плане их способности к специфической линейной диффе- ренцировке, выживаемости организма, а также их способности к радиопротекции летально облученных реципиентов. Материалы и методы. Дифференциров- ку in vitro МСК человека в направлении гемопоэтиче- ских (ГСК) или эндотелиальных клеток изучали путем RT-qPCR поверхностных маркеров и других белков. Для тестирования in vivo способности мышиных МСК защищать летально облученных (9.5 Гр) мышей, животных трансплантировали мышиными МСК, ме- чеными eGFP. Длительность донорского химеризма определяли в крови, костном мозге и тимусе по марке- рам CD45.2 и Y-хромосомы. Анализ профилей генной экспрессии в клетках костного мозга проводили по сравнению с соответствующими контролями посред- ством биочипов. Результаты. При дифференцировке гемопоэтических стволовых клеток человека in vitro отмечалась изменения генной экспрессии со спектром экспрессии, типичным для кроветворных предше- ственников и зрелых клеток. Во время дифференци- ровки в средах с сывороткой наблюдалась повышен- ная экспрессия множества факторов, ответственных за эритропоэз, мегакариоцитопоэз, лимфо- и мииело- поэз. Была выявлена популяция клеток с небольшими круглыми или полиморфными ядрами, которые экс- прессировали антигенные маркеры, характерные для клеток-предшественников или зрелых форм, хотя и небольшой степени. Те же клетки приобретали мор- фологические черты эндотелия и экспрессировали гены, специфичные для эндотелиальных клеток при культивировании со специфическими факторами дифференцировки.
После введения МСК, летально облученные мыши выживали с нормальным восстановлением кроветво- рения, как при введении кроветворных клеток. Через 7 мес. после облучения реципиенты МСК имели нор- мальное соотношение популяций периферической крови. Не было указаний на наличие сколько-нибудь длительного донорского химеризма после трансплан- тации. Оценка распределения eGFP+ донорских кле- ток после внутривенной трансфузии показала бы- строе исчезновение МСК из периферической крови, до 2% через 8 часов после введения с задержкой клеток в легких, однако без их длительной персистенции и эмболизации сосудов. Исследование экспрессии ге- нов в клетках костного мозга у животных, леченных МСК, показало повышение активности генов, способ- ствующих восстановлению кроветворения, наряду со снижением активности генов, ассоциированных с ра- диационным повреждением костного мозга. Инъек- ции летально облученныи животным микровезикул, происходящих из МСК, приводили к тем же протек- тивным эффектам, что и трансплантация МСКС как таковых. Заключение. Наши результаты представляют дополнительные данные о возможных механизмах вы- сокоэффективного паракринного механизма, который актуален, в частности, для популяций костного мозга, что указывает на то, что инфузии МСК являются эф- фективным средством лечения последствий острого облучения. Кроме того, трансплантация МСК может оказывать свой трофический эффект в необычных ме- стах, в точм числе – легочной ткани реципиента.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(6817) "Ионизирующее излучение широко применяется в качестве кондиционирующей терапии при транс- плантации костного мозга. Высокодозная радиаци- онная терапия вызывает тяжелое повреждение, в особенности – гемопоэтических стволовых клеток и клеток-предшественников. Попытки улучшения кли- нических исходов после облучения сосредоточены на гемопоэтической нише. Мезенхимные стромальные клетки (МСК) представляют собой интегральную часть стромального микроокружения. При совмест- ной трансплантации с гемопоэтическими стволовыми клетками (ГСК), МСК способны усиливать восстанов- ление кроветворения после химио- и радиационной терапии. Целью нашего исследования была оценка основных биологических параметров МСК, в плане их способности к специфической линейной диффе- ренцировке, выживаемости организма, а также их способности к радиопротекции летально облученных реципиентов. Материалы и методы. Дифференциров- ку in vitro МСК человека в направлении гемопоэтиче- ских (ГСК) или эндотелиальных клеток изучали путем RT-qPCR поверхностных маркеров и других белков. Для тестирования in vivo способности мышиных МСК защищать летально облученных (9.5 Гр) мышей, животных трансплантировали мышиными МСК, ме- чеными eGFP. Длительность донорского химеризма определяли в крови, костном мозге и тимусе по марке- рам CD45.2 и Y-хромосомы. Анализ профилей генной экспрессии в клетках костного мозга проводили по сравнению с соответствующими контролями посред- ством биочипов. Результаты. При дифференцировке гемопоэтических стволовых клеток человека in vitro отмечалась изменения генной экспрессии со спектром экспрессии, типичным для кроветворных предше- ственников и зрелых клеток. Во время дифференци- ровки в средах с сывороткой наблюдалась повышен- ная экспрессия множества факторов, ответственных за эритропоэз, мегакариоцитопоэз, лимфо- и мииело- поэз. Была выявлена популяция клеток с небольшими круглыми или полиморфными ядрами, которые экс- прессировали антигенные маркеры, характерные для клеток-предшественников или зрелых форм, хотя и небольшой степени. Те же клетки приобретали мор- фологические черты эндотелия и экспрессировали гены, специфичные для эндотелиальных клеток при культивировании со специфическими факторами дифференцировки.
После введения МСК, летально облученные мыши выживали с нормальным восстановлением кроветво- рения, как при введении кроветворных клеток. Через 7 мес. после облучения реципиенты МСК имели нор- мальное соотношение популяций периферической крови. Не было указаний на наличие сколько-нибудь длительного донорского химеризма после трансплан- тации. Оценка распределения eGFP+ донорских кле- ток после внутривенной трансфузии показала бы- строе исчезновение МСК из периферической крови, до 2% через 8 часов после введения с задержкой клеток в легких, однако без их длительной персистенции и эмболизации сосудов. Исследование экспрессии ге- нов в клетках костного мозга у животных, леченных МСК, показало повышение активности генов, способ- ствующих восстановлению кроветворения, наряду со снижением активности генов, ассоциированных с ра- диационным повреждением костного мозга. Инъек- ции летально облученныи животным микровезикул, происходящих из МСК, приводили к тем же протек- тивным эффектам, что и трансплантация МСКС как таковых. Заключение. Наши результаты представляют дополнительные данные о возможных механизмах вы- сокоэффективного паракринного механизма, который актуален, в частности, для популяций костного мозга, что указывает на то, что инфузии МСК являются эф- фективным средством лечения последствий острого облучения. Кроме того, трансплантация МСК может оказывать свой трофический эффект в необычных ме- стах, в точм числе – легочной ткани реципиента.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6699" ["VALUE"]=> array(2) { ["TEXT"]=> string(606) "1. Клиника трансплантации стволовых клеток, Департамент клеточной и генной терапии, Университетский медицинский<br> центр Гамбург-Эппендорф<br> 2. Технологическая платформа микроскопии и анализа изображений, Институт Хайнриха Петте, Гамбург,<br> 3. Институт патологии, Университетский медицинский центр Гамбург Эппендорф" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(588) "1. Клиника трансплантации стволовых клеток, Департамент клеточной и генной терапии, Университетский медицинскийцентр Гамбург-Эппендорф
2. Технологическая платформа микроскопии и анализа изображений, Институт Хайнриха Петте, Гамбург,
3. Институт патологии, Университетский медицинский центр Гамбург Эппендорф" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(588) "1. Клиника трансплантации стволовых клеток, Департамент клеточной и генной терапии, Университетский медицинский
центр Гамбург-Эппендорф
2. Технологическая платформа микроскопии и анализа изображений, Институт Хайнриха Петте, Гамбург,
3. Институт патологии, Университетский медицинский центр Гамбург Эппендорф" } } } [1]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(2) "15" ["~IBLOCK_SECTION_ID"]=> string(2) "15" ["ID"]=> string(3) "523" ["~ID"]=> string(3) "523" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(248) "Оценка энергетического потенциала свежих и хранимых клеток костного мозга с помощью флуоресцентного потенциал-чувствительного зонда" ["~NAME"]=> string(248) "Оценка энергетического потенциала свежих и хранимых клеток костного мозга с помощью флуоресцентного потенциал-чувствительного зонда" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "09/22/2016 11:28:49 am" ["~TIMESTAMP_X"]=> string(22) "09/22/2016 11:28:49 am" ["DETAIL_PAGE_URL"]=> string(153) "/en/archive/vypusk-5-nomer-2/eksperimentalnye-stati/otsenka-energeticheskogo-potentsiala-svezhikh-i-khranimykh-kletok-kostnogo-mozga-s-pomoshchyu-fluore/" ["~DETAIL_PAGE_URL"]=> string(153) "/en/archive/vypusk-5-nomer-2/eksperimentalnye-stati/otsenka-energeticheskogo-potentsiala-svezhikh-i-khranimykh-kletok-kostnogo-mozga-s-pomoshchyu-fluore/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(23621) "
Introduction
Bone marrow transplantation (BMT) is widely used for treatment of different malignant and non-malignant disorders. Quality of hematopoietic cells the graft is a key factor of their successful in vivo expansion.
A classic method to determine colony-forming ability BMC is based on the cultures in semi-solid nutrient media with addition of growth factor cocktails [3,11,17], however, requiring up to 3 weeks for evaluation. CD34+ cell count, a clinically recognized stem cell marker, is ascribed to a heterogenous population of committed and differentiating stem cell progenitors [16]. A more practical, however, less specific test is based on evaluation of aldehyde dehydrogenase (ALDH) activity [5] which is detected in viable hematopoietic stem cells and progenitors. Fluorescence intensity of reaction product is the measureable parameter, thus enabling ALDH-positive cell counts. Therefore, this method makes it possible to estimate in suspension proportion of intact stem cells and their progenies.
Meanwhile, the entire bone marrow cell (BMC) population is highly heterogeneous and contains a large number of mature blood cells and progenitors at different maturation stages. Therefore, energy state and metabolic activity of bone marrow cells are an important parameter of graft quality, especially, upon strict storage conditions. According to a current concept, the functional state of living cells is closely linked to their integrated energy-coupled characteristics (number of active mitochondria in the cytoplasm, transmembrane potentials of plasma and mitochondrial membranes). Changes in these parameters can be monitored in different cell populations using electric potential-sensitive fluorescent probes [13, 18]. Transmembrane electric potential is a universal product of the systems of energy coupling. Application of methods using potential sensitive fluorescent probes allows to investigate the response of cells after exposure to various physical and chemical factors [1, 4, 10, 14], and to assess mitochondrial functions and redox potential of hematopoietic cells [8]. Earlier, we have revealed that intensity of fluorescent signal from cationic probe, iodide 2 [p-(dimethylamino) styryl]-1-methylpyridinium (2-Di-1-ASP) is sensitive to changes of energy potential in living cells, including BMC [12]. Moreover, this energy-dependent fluorescent probe is intended for evaluation of viable cells with intact outer membranes. Hence, appropriate fluorescence values reflect energetic state and general viability of the cell population under study.
Hence, the aim of our work was to test a method of rapid semi-quantitative assessment of bone marrow cell viability by means of a potential-sensitive vital 2-Di-1-ASP probe. A relationship was revealed between fluorescence intensity of the probe, cell viability and residual mitotic ability of marrow cell population during long-term storage.
Materials and methods
We have studied bone marrow cell samples (BMC) of healthy donors harvested for allogeneic transplantations at the R.M.Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology. Harvesting of the bone marrow cell (BMC) suspensions was generally performed according to standard procedure [8]. The native cells were placed in a standard stabilizing solution («Terumo Corporation, Tokyo, Japan» ) containing 100 ml of distilled water: of 2.63 g of sodium citrate; 0,327 g of citric acid; 0,251 g of dihydrophosphate sodium; 2.9 g dextrose anhydrous; 0,0275 g of adenine). The myelokaryocyte numbers were determined manually with a haemocytometer. The cell counts after incubation were expressed as percentages of initial cell numbers. Cell viability was determined by Trypan Blue exclusion test.
To determine mitotic indexes, the cell suspension was stored at room temperature (control). In parallel samples, the specimens were treated with colchicine (Acros Organics, Belgium) at a final concentration of 4.3 μg/ml added after 1 hour of storage, and incubated at 37o C for 5-24 hours. Suspension aliquots were taken at 5, 6, and 24 hours of storage, the cells were fixed according to May-Grünwald, and stained with Giemsa dye. Light microscopy was carried out with Leica DM 750 microscope (Germany), at a 1000x magnification. The pictures were obtained with ICC50 camera (Leica, Germany).
To evaluate energy potential of the cell samples, we used a fluorescent potential-sensitive cationic vital probe (iodide 2[p-(dimethylamino)styryl]-1-methylpyridine, 2-Di-1-ASP, Molecular Probes, Inc., Eugene, OR., USA). The cell aliquots of 0.9 mL, were stored in standard stabilizing solution at a concentration of ~2-3×107 cells/ml in Eppendorf tubes of 1.5 ml at room temperature (20.-.22oC). At defined time points (1 to 72 hours), the cell suspensions were mixed, and 30-μl aliquots were taken and brought to the 0.6-mL Eppendorf tubes, supplied with 2-Di-1-ASP at a final concentration of 40 μm, and incubated at 37oC for 60 min. The cell suspensions (3 μl) were then evaluated in a Lumam-I2 luminescence microscope (Russia). at a 900x magnification. A FMEL-1 photometric device with interference filter was used, at maximal transmittance of 585 nm. Excitation and emission (registration) wavelengths were, respectively, 470 and 560 nm. Fluorescence intensity signals were recorded from single cells. Seventy to hundred cells per sample were tested manually, and the mean cell fluorescence intensity (F̃, arbitrary units) was calculated for each specimen. The studied cells were graded into four classes by their mean F̃ values (resp., 10-30; 30-70; 70-100, and >100 arbitrary units). Photographic pictures of luminescent objects were performed with TCA-5.0 camera, using “Micro-Analysis View” software (LLC “LOMO- Microsystems”, Russia). Statistical analysis of F̃ and cell number changes was performed by ANOVA Repeated Measures analysis for variance-dependent samples [2]. The donor samples were classified by cluster analysis (Euclidean metric), the nearest mean strategy). The significance of differences was evaluated by Wilcoxon-Mann-Whitney criterion. The differences between the groups were considered significant at P<0.05.
Results
Bone marrow cell suspensions stored in a preserving medium showed some initial decrease in cell survival, i.e., 8 to 20% of the cells were Trypan Blue-positive. By the end of storage (72 hours) the mean numbers of Trypan Blue-positive cells increased to 25-30%. Meanwhile, subsequent cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, NF>100,%) D (ratio of NF>100 at 3h storage to NF>100 initial) proved to be the most informative parameter and allows to predict sample-specific differences for F̃ values at later terms. We assumed the D value to be the main parameter reflecting energetic potential of BMC. A cluster analysis was performed, thus allowing us to classify the BMC samples into 3 sub-groups, according to significant inter-group differences in D values (P<0.0001) as shown in Fig.1. Cell fluorescence values in groups 1, 2 and 3 showed, respectively, minimal, maximal and intermediate increase rates in F̃ и NF>100 after 3-h incubation. (Table 1).
Cell counts and F̃ values for later terms (since 5 hours of incubation) were evaluated by means of ANOVA dispersion analysis for repeated measurements. Significant intergroup differences were revealed for both F̃ values (P=0.005) (Fig. 2) and nucleated cell numbers (P<0.0001) (Fig. 3). Hence, a total group of BMC samples could be divided into 3 subgroups, according to sustained increment of potential-dependent fluorescence and cell number changes.

Fig. 1. Distribution by dependence D (ratio of NF>100 at 3h storage to NF>100 initial) on initial fluorescence values (F̃). Abscissa: Mean F̃ values for individual donors; Ordinate: ratio of NF>100 at 3h to NF>100 initial. Groups 1, 2, and 3 showed, respectively, minimal, maximal and intermediate increase rates in F̃ and NF>100 at 3h as compared to initial fluorescence levels.

Fig. 2. Changes of mean fluorescence values in stored BMC samples. Abscissa, storage time (hours), ordinate, mean fluorescence values (F̃) for each time point. Groups 1, 2 and 3 showed, respectively, minimal, maximal and intermediate shifts in F̃ and NF>100 following 3 hours of storage.

Figure 3. Changes in nucleated cell numbers (%) during BMC storage in standard stabilizing solution. Abscissa, storage time (hours), ordinate, nucleated cell numbers (%) for each time point. Groups 1, 2 and 3 showed, respectively, minimal, maximal and intermediate shifts in F̃ and NF>100 following 3 hours of storage.
 Table 1. Mean values for BMC counts (NC), mean fluorescence (F̃) and proportion of highly fluorescent subpopulation (NF>100) at initial terms and following 3 hour-incubation for the 3 groups of BM samples
Table 1. Mean values for BMC counts (NC), mean fluorescence (F̃) and proportion of highly fluorescent subpopulation (NF>100) at initial terms and following 3 hour-incubation for the 3 groups of BM samples
As seen from Table 1, the D (ratio of NF>100 at 3h to NF>100 initial) values show a 1.3- to 2.4-fold increase, depending on the BM sample group, especially, for groups 2 and 3.
Hence, all the BMC samples exhibited increased F̃ over 3 hours of incubation, mostly, due to “bright” cell subpopulation (NF>100),. This increase correlated with elevation of cell counts in suspension.
Preservation of dividing cells under these storage conditions was confirmed by means of standard karyological methods. In particular, a number of mitotic cells were detected in BM suspensions over 5 to 24 hours of incubation, being stainable with the 2-Di-1-ASP probe (Fig. 4). Frequency of mitotic patterns in BMC aliquotes taken at different time points was studied in a special series of BM samples (n=5) The mitotic figures were of typical appearance (Fig. 4). All the samples were analyzed in triplicate. In summary, we have found 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6- colchicine treatment, the mitotic index increased to: 1.8±0.1%. By 24 hours the mitotic indices did not significantly differ from the 6-hour values.
Hence, a sufficient portion of pre-mitotic BM cells showed their ability to divide even after several hours of storage in rather simple medium devoid of growth factors.


Fig.4. Mitotic figures in preparations of stored BMC (2-Di-1-ASP probe staining) (A) - 5-h incubation, 250 arb.units;(B)- 6-h incubation; 110 arb.units.


Fig 5. Mitotic figures in preparations of stored BMC, (A) -6 h incubation (colchicine-free control); (B) - mitosis of granulosytic precursor (6-h with colchicine).
Discussion
Synthetic fluorescent probes showing affinity for mitochondrial membranes are increasingly studied over last decades [15]. Vital cationic probes are used to follow the changes of transmembrane potential which may be registered by microscopy and, potentially, by flow cytometry in different cell populations. Modern experimental protocols with microwell incubation under defined conditions and supplements have been proposed for assessment of bioenergetic changes in cell cultures [7]. In this respect, our general approach well fits current trends in searching bioenergetic parameters of stored cell samples.
Our data presume good preserving properties of a simple marrow conservant containing dextrose, adenine, dihydrophosphate sodium and citrate ions at optimal concentrations. Previous studies were performed with cells stored under physiological conditions. E.g., Lioznov et.al., [9] have studied stability of bone marrow and peripheral blood stem cells at different temperatures. Indeed, the hematopoietic stem cells remained relatively intact for several days at +40С, without significant decrease of proliferative capacity. By contrary, incubation of peripheral stem cells at room temperature is followed by decreased numbers of GM-CFUs to <20% of initial levels, whereas GM-CFU numbers in bone marrow transplants was retained for, at least, 72 hours. We have shown increased cell numbers in BMC samples after 5…24 hours of incubation in the stabilizing solution, further remaining near-constant until 72 hours at room temperature. One may suggest that the BM cells retained their viability (as Trypan Blue-negative forms) and energetic potential after 72-h incubation, even under suboptimal physiological conditions. Our previous studies have shown that the stabilizing solution well preserves BMC as compared to storage in physiological saline, or Hanks’ medium. [12]
More detailed data concerning possible associations between the energy potential determined with fluorescent probe, and other criteria of cell viability should be obtained in future studies, employing advanced experimental protocols.
Conclusion
We have proposed a method for evaluation of storage conditions for bone marrow cells using a supravital fluorescent potential-sensitive 2-Di-1-ASP probe, upon incubation at room temperature in a standard hemoconservant solution. This method may be applied, e.g., for comparative studies of different storage media and treatment regimens for bone marrow samples. Such biophysical approach allows quantitative evaluation of energetic activation, by increasing fluorescence in total cell population at early terms of storage. A correlation was revealed between higher fluorescence rates, increased proportion of “bright” (energetically active) subpopulation, and higher cell counts. Finally, our results have shown maintenance of mitotic cell fraction under the applied incubation conditions. Next step of our studies will include development of high-throughput flow cytometry technique for this fluorescence test. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance should be performed in future.
Acknowledgements
The authors are much appreciated to Lyudmila A.Belyakova (Waldman Institute of Pharmacology) for skilful data analysis, Valentina M.Kravtsova, and Tatyana L Gindina (R.Gorbacheva Research Institute of Children Oncology, Hemaptology and Transplantation) for expert evaluation of bone marrow smears.
Conflict of interests
The authors have no conflicts of interests to declare
References
- Artuhov VG, Putinzetva OV, Bragina VA, Pashkov MV, Vasilenko DV. Fluorescent methods in the research induced by UV radiation changes of structural and functional state of human blood lymphocytes. Bull Exp Biol Med 2012;153(6):891-895.
- Afifi AA, Azen SP. Statistical Analysis, Second Edition: A Computer Oriented Approach Academic Press: N.-Y.-London, 1979, 366 p.
- Dygai AM, Skurihin EG, Pershina OV, Andreeva TV, Hmelevskaya ES, Minakova MY. The role of hemopoietic precursors of various classes in the mechanisms of action of granulocyte colony-stimulating factor on hematopoiesis in cytostatic myelosuppression. Bull Exp Biol Med 2010; 149(4):400-404.
- Ito S; Kusuhara H; Yokochi M, Toyoshima J, Inoue K; Yuasa H, Sugiyama Y. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther 2012;340 (2):393-403.
- Jones RJ, Barber JP, Vala MS, Collector MI, Kaufmann SH, Ludeman SM, Colvin OM, Hilton J. Assessment of aldehyde dehydrogenase in viable cells. Blood 1995; 85(10):2742-2746.
- Kaur A, Jankowska K, Pilgrim C, Fraser ST, New EJ. Studies of Hematopoietic Cell Differentiation with a Ratiometric and Reversible Sensor of Mitochondrial Reactive Oxygen Species. Antioxid Redox Signal. 2016; 24(13): 667-679.
- Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J Vis Exp 2014; (85). doi: 10.3791/51301.
- Lannert H, Able T, Becker S, Sommer M, Braun M, Stadtherr P, Ho A. Optimizing BM harvesting from normal adult donors. Bone Marrow Transplantation 2008; 42:443–447.
- Lioznov M.V., Freiberger P., Kroger N., Zander A.R., Fehse B. Aldehyde dehydrogenase activity as a marker for the quality of hematopoietic stem cell transplants. Bone Marrow Transplant 2005, 35,(9):909-914.
- Morozova GI, Parkhomenko TV, Klitsenko OA, Tomson VV. Stimulating effect of erythropoietin on thymocyte energetics established in vitro with a potential-sensitive fluorescent probe in the mitochondria. Biochem. Suppl. Series A: Membr Cell Biology 2007;1(4):325-330.
- Nissen-Druey C, Tichelli A, Meyer-Monard S. Human hematopoietic colonies in health and disease. Acta Haematol 2005;113(1):5-96.
- Parkhomenko TV, Galibin OV, Mikhailova NB, Babenko EV, Tomson VV. A method for determining the activity of the bone marrow cells. 2013. Russian patent RU №2488826, Bull. № 21. (In Russian)
- Parkhomenko TV, Klytsenko OA, Tomson VV. Erythropoietin stimulates aerobic and anaerobic processes in rat cardiomyocytes. Focus Uni-Lübeck. Suppl. 2012, P. 37.
- Parkhomenko TV, Morozova GI, Klytsenko OA, Tomson VV. Evaluation of restoring Erythropoietin (EPO) effect on rat T-lymphocytes after their treatment with some inhibitors in vitro. Ann Hematol 2003;82(6):S114.
- Solaini G, Sgarbi G, Lenaz G, Baracca A. Evaluating mitochondrial membrane potential in cells. Biosci Rep 2007; 27(1-3):11-21.
- Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I The ISHAGE guidelines for СD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J. Hematother 1996; (5):213-226.
- Ventura GJ, Hester JP, Buescher ES, Vadhan-Raj S, Durrett A, Reading CL Hematopoiesis in limiting dilution cultures: influence of cytokines on human hematopoietic progenitor cells. Exp Hematol 1990; 18(8):878-882.
- Vida TA, Emr SD. A new vital stain visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 1995; 228(5): 779-792.
Introduction
Bone marrow transplantation (BMT) is widely used for treatment of different malignant and non-malignant disorders. Quality of hematopoietic cells the graft is a key factor of their successful in vivo expansion.
A classic method to determine colony-forming ability BMC is based on the cultures in semi-solid nutrient media with addition of growth factor cocktails [3,11,17], however, requiring up to 3 weeks for evaluation. CD34+ cell count, a clinically recognized stem cell marker, is ascribed to a heterogenous population of committed and differentiating stem cell progenitors [16]. A more practical, however, less specific test is based on evaluation of aldehyde dehydrogenase (ALDH) activity [5] which is detected in viable hematopoietic stem cells and progenitors. Fluorescence intensity of reaction product is the measureable parameter, thus enabling ALDH-positive cell counts. Therefore, this method makes it possible to estimate in suspension proportion of intact stem cells and their progenies.
Meanwhile, the entire bone marrow cell (BMC) population is highly heterogeneous and contains a large number of mature blood cells and progenitors at different maturation stages. Therefore, energy state and metabolic activity of bone marrow cells are an important parameter of graft quality, especially, upon strict storage conditions. According to a current concept, the functional state of living cells is closely linked to their integrated energy-coupled characteristics (number of active mitochondria in the cytoplasm, transmembrane potentials of plasma and mitochondrial membranes). Changes in these parameters can be monitored in different cell populations using electric potential-sensitive fluorescent probes [13, 18]. Transmembrane electric potential is a universal product of the systems of energy coupling. Application of methods using potential sensitive fluorescent probes allows to investigate the response of cells after exposure to various physical and chemical factors [1, 4, 10, 14], and to assess mitochondrial functions and redox potential of hematopoietic cells [8]. Earlier, we have revealed that intensity of fluorescent signal from cationic probe, iodide 2 [p-(dimethylamino) styryl]-1-methylpyridinium (2-Di-1-ASP) is sensitive to changes of energy potential in living cells, including BMC [12]. Moreover, this energy-dependent fluorescent probe is intended for evaluation of viable cells with intact outer membranes. Hence, appropriate fluorescence values reflect energetic state and general viability of the cell population under study.
Hence, the aim of our work was to test a method of rapid semi-quantitative assessment of bone marrow cell viability by means of a potential-sensitive vital 2-Di-1-ASP probe. A relationship was revealed between fluorescence intensity of the probe, cell viability and residual mitotic ability of marrow cell population during long-term storage.
Materials and methods
We have studied bone marrow cell samples (BMC) of healthy donors harvested for allogeneic transplantations at the R.M.Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology. Harvesting of the bone marrow cell (BMC) suspensions was generally performed according to standard procedure [8]. The native cells were placed in a standard stabilizing solution («Terumo Corporation, Tokyo, Japan» ) containing 100 ml of distilled water: of 2.63 g of sodium citrate; 0,327 g of citric acid; 0,251 g of dihydrophosphate sodium; 2.9 g dextrose anhydrous; 0,0275 g of adenine). The myelokaryocyte numbers were determined manually with a haemocytometer. The cell counts after incubation were expressed as percentages of initial cell numbers. Cell viability was determined by Trypan Blue exclusion test.
To determine mitotic indexes, the cell suspension was stored at room temperature (control). In parallel samples, the specimens were treated with colchicine (Acros Organics, Belgium) at a final concentration of 4.3 μg/ml added after 1 hour of storage, and incubated at 37o C for 5-24 hours. Suspension aliquots were taken at 5, 6, and 24 hours of storage, the cells were fixed according to May-Grünwald, and stained with Giemsa dye. Light microscopy was carried out with Leica DM 750 microscope (Germany), at a 1000x magnification. The pictures were obtained with ICC50 camera (Leica, Germany).
To evaluate energy potential of the cell samples, we used a fluorescent potential-sensitive cationic vital probe (iodide 2[p-(dimethylamino)styryl]-1-methylpyridine, 2-Di-1-ASP, Molecular Probes, Inc., Eugene, OR., USA). The cell aliquots of 0.9 mL, were stored in standard stabilizing solution at a concentration of ~2-3×107 cells/ml in Eppendorf tubes of 1.5 ml at room temperature (20.-.22oC). At defined time points (1 to 72 hours), the cell suspensions were mixed, and 30-μl aliquots were taken and brought to the 0.6-mL Eppendorf tubes, supplied with 2-Di-1-ASP at a final concentration of 40 μm, and incubated at 37oC for 60 min. The cell suspensions (3 μl) were then evaluated in a Lumam-I2 luminescence microscope (Russia). at a 900x magnification. A FMEL-1 photometric device with interference filter was used, at maximal transmittance of 585 nm. Excitation and emission (registration) wavelengths were, respectively, 470 and 560 nm. Fluorescence intensity signals were recorded from single cells. Seventy to hundred cells per sample were tested manually, and the mean cell fluorescence intensity (F̃, arbitrary units) was calculated for each specimen. The studied cells were graded into four classes by their mean F̃ values (resp., 10-30; 30-70; 70-100, and >100 arbitrary units). Photographic pictures of luminescent objects were performed with TCA-5.0 camera, using “Micro-Analysis View” software (LLC “LOMO- Microsystems”, Russia). Statistical analysis of F̃ and cell number changes was performed by ANOVA Repeated Measures analysis for variance-dependent samples [2]. The donor samples were classified by cluster analysis (Euclidean metric), the nearest mean strategy). The significance of differences was evaluated by Wilcoxon-Mann-Whitney criterion. The differences between the groups were considered significant at P<0.05.
Results
Bone marrow cell suspensions stored in a preserving medium showed some initial decrease in cell survival, i.e., 8 to 20% of the cells were Trypan Blue-positive. By the end of storage (72 hours) the mean numbers of Trypan Blue-positive cells increased to 25-30%. Meanwhile, subsequent cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, NF>100,%) D (ratio of NF>100 at 3h storage to NF>100 initial) proved to be the most informative parameter and allows to predict sample-specific differences for F̃ values at later terms. We assumed the D value to be the main parameter reflecting energetic potential of BMC. A cluster analysis was performed, thus allowing us to classify the BMC samples into 3 sub-groups, according to significant inter-group differences in D values (P<0.0001) as shown in Fig.1. Cell fluorescence values in groups 1, 2 and 3 showed, respectively, minimal, maximal and intermediate increase rates in F̃ и NF>100 after 3-h incubation. (Table 1).
Cell counts and F̃ values for later terms (since 5 hours of incubation) were evaluated by means of ANOVA dispersion analysis for repeated measurements. Significant intergroup differences were revealed for both F̃ values (P=0.005) (Fig. 2) and nucleated cell numbers (P<0.0001) (Fig. 3). Hence, a total group of BMC samples could be divided into 3 subgroups, according to sustained increment of potential-dependent fluorescence and cell number changes.

Fig. 1. Distribution by dependence D (ratio of NF>100 at 3h storage to NF>100 initial) on initial fluorescence values (F̃). Abscissa: Mean F̃ values for individual donors; Ordinate: ratio of NF>100 at 3h to NF>100 initial. Groups 1, 2, and 3 showed, respectively, minimal, maximal and intermediate increase rates in F̃ and NF>100 at 3h as compared to initial fluorescence levels.

Fig. 2. Changes of mean fluorescence values in stored BMC samples. Abscissa, storage time (hours), ordinate, mean fluorescence values (F̃) for each time point. Groups 1, 2 and 3 showed, respectively, minimal, maximal and intermediate shifts in F̃ and NF>100 following 3 hours of storage.

Figure 3. Changes in nucleated cell numbers (%) during BMC storage in standard stabilizing solution. Abscissa, storage time (hours), ordinate, nucleated cell numbers (%) for each time point. Groups 1, 2 and 3 showed, respectively, minimal, maximal and intermediate shifts in F̃ and NF>100 following 3 hours of storage.
 Table 1. Mean values for BMC counts (NC), mean fluorescence (F̃) and proportion of highly fluorescent subpopulation (NF>100) at initial terms and following 3 hour-incubation for the 3 groups of BM samples
Table 1. Mean values for BMC counts (NC), mean fluorescence (F̃) and proportion of highly fluorescent subpopulation (NF>100) at initial terms and following 3 hour-incubation for the 3 groups of BM samples
As seen from Table 1, the D (ratio of NF>100 at 3h to NF>100 initial) values show a 1.3- to 2.4-fold increase, depending on the BM sample group, especially, for groups 2 and 3.
Hence, all the BMC samples exhibited increased F̃ over 3 hours of incubation, mostly, due to “bright” cell subpopulation (NF>100),. This increase correlated with elevation of cell counts in suspension.
Preservation of dividing cells under these storage conditions was confirmed by means of standard karyological methods. In particular, a number of mitotic cells were detected in BM suspensions over 5 to 24 hours of incubation, being stainable with the 2-Di-1-ASP probe (Fig. 4). Frequency of mitotic patterns in BMC aliquotes taken at different time points was studied in a special series of BM samples (n=5) The mitotic figures were of typical appearance (Fig. 4). All the samples were analyzed in triplicate. In summary, we have found 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6- colchicine treatment, the mitotic index increased to: 1.8±0.1%. By 24 hours the mitotic indices did not significantly differ from the 6-hour values.
Hence, a sufficient portion of pre-mitotic BM cells showed their ability to divide even after several hours of storage in rather simple medium devoid of growth factors.


Fig.4. Mitotic figures in preparations of stored BMC (2-Di-1-ASP probe staining) (A) - 5-h incubation, 250 arb.units;(B)- 6-h incubation; 110 arb.units.


Fig 5. Mitotic figures in preparations of stored BMC, (A) -6 h incubation (colchicine-free control); (B) - mitosis of granulosytic precursor (6-h with colchicine).
Discussion
Synthetic fluorescent probes showing affinity for mitochondrial membranes are increasingly studied over last decades [15]. Vital cationic probes are used to follow the changes of transmembrane potential which may be registered by microscopy and, potentially, by flow cytometry in different cell populations. Modern experimental protocols with microwell incubation under defined conditions and supplements have been proposed for assessment of bioenergetic changes in cell cultures [7]. In this respect, our general approach well fits current trends in searching bioenergetic parameters of stored cell samples.
Our data presume good preserving properties of a simple marrow conservant containing dextrose, adenine, dihydrophosphate sodium and citrate ions at optimal concentrations. Previous studies were performed with cells stored under physiological conditions. E.g., Lioznov et.al., [9] have studied stability of bone marrow and peripheral blood stem cells at different temperatures. Indeed, the hematopoietic stem cells remained relatively intact for several days at +40С, without significant decrease of proliferative capacity. By contrary, incubation of peripheral stem cells at room temperature is followed by decreased numbers of GM-CFUs to <20% of initial levels, whereas GM-CFU numbers in bone marrow transplants was retained for, at least, 72 hours. We have shown increased cell numbers in BMC samples after 5…24 hours of incubation in the stabilizing solution, further remaining near-constant until 72 hours at room temperature. One may suggest that the BM cells retained their viability (as Trypan Blue-negative forms) and energetic potential after 72-h incubation, even under suboptimal physiological conditions. Our previous studies have shown that the stabilizing solution well preserves BMC as compared to storage in physiological saline, or Hanks’ medium. [12]
More detailed data concerning possible associations between the energy potential determined with fluorescent probe, and other criteria of cell viability should be obtained in future studies, employing advanced experimental protocols.
Conclusion
We have proposed a method for evaluation of storage conditions for bone marrow cells using a supravital fluorescent potential-sensitive 2-Di-1-ASP probe, upon incubation at room temperature in a standard hemoconservant solution. This method may be applied, e.g., for comparative studies of different storage media and treatment regimens for bone marrow samples. Such biophysical approach allows quantitative evaluation of energetic activation, by increasing fluorescence in total cell population at early terms of storage. A correlation was revealed between higher fluorescence rates, increased proportion of “bright” (energetically active) subpopulation, and higher cell counts. Finally, our results have shown maintenance of mitotic cell fraction under the applied incubation conditions. Next step of our studies will include development of high-throughput flow cytometry technique for this fluorescence test. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance should be performed in future.
Acknowledgements
The authors are much appreciated to Lyudmila A.Belyakova (Waldman Institute of Pharmacology) for skilful data analysis, Valentina M.Kravtsova, and Tatyana L Gindina (R.Gorbacheva Research Institute of Children Oncology, Hemaptology and Transplantation) for expert evaluation of bone marrow smears.
Conflict of interests
The authors have no conflicts of interests to declare
References
- Artuhov VG, Putinzetva OV, Bragina VA, Pashkov MV, Vasilenko DV. Fluorescent methods in the research induced by UV radiation changes of structural and functional state of human blood lymphocytes. Bull Exp Biol Med 2012;153(6):891-895.
- Afifi AA, Azen SP. Statistical Analysis, Second Edition: A Computer Oriented Approach Academic Press: N.-Y.-London, 1979, 366 p.
- Dygai AM, Skurihin EG, Pershina OV, Andreeva TV, Hmelevskaya ES, Minakova MY. The role of hemopoietic precursors of various classes in the mechanisms of action of granulocyte colony-stimulating factor on hematopoiesis in cytostatic myelosuppression. Bull Exp Biol Med 2010; 149(4):400-404.
- Ito S; Kusuhara H; Yokochi M, Toyoshima J, Inoue K; Yuasa H, Sugiyama Y. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther 2012;340 (2):393-403.
- Jones RJ, Barber JP, Vala MS, Collector MI, Kaufmann SH, Ludeman SM, Colvin OM, Hilton J. Assessment of aldehyde dehydrogenase in viable cells. Blood 1995; 85(10):2742-2746.
- Kaur A, Jankowska K, Pilgrim C, Fraser ST, New EJ. Studies of Hematopoietic Cell Differentiation with a Ratiometric and Reversible Sensor of Mitochondrial Reactive Oxygen Species. Antioxid Redox Signal. 2016; 24(13): 667-679.
- Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J Vis Exp 2014; (85). doi: 10.3791/51301.
- Lannert H, Able T, Becker S, Sommer M, Braun M, Stadtherr P, Ho A. Optimizing BM harvesting from normal adult donors. Bone Marrow Transplantation 2008; 42:443–447.
- Lioznov M.V., Freiberger P., Kroger N., Zander A.R., Fehse B. Aldehyde dehydrogenase activity as a marker for the quality of hematopoietic stem cell transplants. Bone Marrow Transplant 2005, 35,(9):909-914.
- Morozova GI, Parkhomenko TV, Klitsenko OA, Tomson VV. Stimulating effect of erythropoietin on thymocyte energetics established in vitro with a potential-sensitive fluorescent probe in the mitochondria. Biochem. Suppl. Series A: Membr Cell Biology 2007;1(4):325-330.
- Nissen-Druey C, Tichelli A, Meyer-Monard S. Human hematopoietic colonies in health and disease. Acta Haematol 2005;113(1):5-96.
- Parkhomenko TV, Galibin OV, Mikhailova NB, Babenko EV, Tomson VV. A method for determining the activity of the bone marrow cells. 2013. Russian patent RU №2488826, Bull. № 21. (In Russian)
- Parkhomenko TV, Klytsenko OA, Tomson VV. Erythropoietin stimulates aerobic and anaerobic processes in rat cardiomyocytes. Focus Uni-Lübeck. Suppl. 2012, P. 37.
- Parkhomenko TV, Morozova GI, Klytsenko OA, Tomson VV. Evaluation of restoring Erythropoietin (EPO) effect on rat T-lymphocytes after their treatment with some inhibitors in vitro. Ann Hematol 2003;82(6):S114.
- Solaini G, Sgarbi G, Lenaz G, Baracca A. Evaluating mitochondrial membrane potential in cells. Biosci Rep 2007; 27(1-3):11-21.
- Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I The ISHAGE guidelines for СD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J. Hematother 1996; (5):213-226.
- Ventura GJ, Hester JP, Buescher ES, Vadhan-Raj S, Durrett A, Reading CL Hematopoiesis in limiting dilution cultures: influence of cytokines on human hematopoietic progenitor cells. Exp Hematol 1990; 18(8):878-882.
- Vida TA, Emr SD. A new vital stain visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 1995; 228(5): 779-792.
Татьяна В. Пархоменко, Олег В. Галибин, Елена В. Вербицкая, Владимир В. Томсон
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6792" ["VALUE"]=> array(2) { ["TEXT"]=> string(456) "НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой, Научно-исследовательский центр,<br> Первый Санкт-Петербургский государственный медицинский Университет им. И.П. Павлова Минздрава РФ, Санкт-<br> Петербург, Россия.<br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(438) "НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой, Научно-исследовательский центр,Первый Санкт-Петербургский государственный медицинский Университет им. И.П. Павлова Минздрава РФ, Санкт-
Петербург, Россия.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6793" ["VALUE"]=> array(2) { ["TEXT"]=> string(5339) "<p> Костный мозг является основным источником гемопоэтических стволовых клеток при клинической трансплантации. Качество трансплантатов костного мозга – ключевой фактор их успешного приживления. Целью нашей работы была проверка полуколичественной методики определения жизнеспособности при хранении в «голодной» среде с помощью флуоресцентного потенциал-чувствительного зонда 2-Di-1-ASP. Мы исследовали 20 образцов клеток костного мозга (ККМ), которые помещали в стандартный стабилизирующий раствор с цитратом натрия, лимонной кислотой, солями фосфатов, декстрозой и аденином. Подсчет клеток и тесты на выживаемость проводили на протяжении 72 часов хранения. Образцы клеток метили зондом 2-Di-1-ASP в определенные сроки. Интенсивность флуоресценции измеряли по отдельным клеткам, рассчитывали средние значения флуоресценции и число миелокариоцитов на образец. Митотические индексы определяли как в препаратах, окрашенных по Романовскому-Гимза, так и после окраски флуоресцентным зондом. Для статистической обработки использовали методы кластерного анализа и стандартные непараметрические тесты. Результаты: Выживаемость ККМ в течение 0-5 часов составляла 80-92% (по тесту с трипановым синим), затем постепенно снижалась до 70-75% к концу сроков хранения. Далее, 3-часовая инкубация ККМ сопровождалась повышением интенсивности флуоресценции (F̃) с данным зондом, главным образом, из-за нарастания доли ярко светящейся субпопуляции (>100 усл.ед., N<sub>F>100</sub>,%). Повышение значений D (отношения N<sub>F>100</sub>,% через 3 часа хранения к N<sub>F>100</sub>,% в исходных пробах) оказалось наиболее информативным, что позволяет прогнозировать специфические величины F̃ на более поздних сроках. Повышение F̃ по сравнению с исходными значениями в этот срок отмечалось во всех образцах ККМ за счет ярко светящихся форм (N<sub>F>100</sub>), что коррелировало с ростом числа клеток. Дополнительный кластерный анализ позволил классифицировать образцы ККМ на 3 подгруппы в связи сдостоверными различиями по значениям D и изменениям клеточности. В частности, значительное число митотических форм наблюдалось в популяциях ККМ псле 5-24 часов хранения, и они ярко окрашивались зондом 2-Di-1-ASP probe. В целом, мы выявили 0,60±0,10% метафазных клеток в исходный срок наблюдения. По мере хранения, частота митотических фигур повышалась до 1,4±0,1%, а, после 6-часовой инкубации с колхицином, митотический индекс возрастал до 1,8±0,1%, что указывало на хорошую степень сохранности пролиферирующей фракции клеток.<br> <br> В заключение, наши результаты показывают сохранение и даже усиление энергетической активности ККМ, выявленное с помощью потенциал-чувствительного зонда и хорошую выживаемость фракции пролиферирующих клеток при жестких условиях инкубации. В дальнейшем следует провести исследования соответствующих механизмов сохранения клеток костного мозга и их энергетического баланса в данных условиях хранения. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(5247) "
Костный мозг является основным источником гемопоэтических стволовых клеток при клинической трансплантации. Качество трансплантатов костного мозга – ключевой фактор их успешного приживления. Целью нашей работы была проверка полуколичественной методики определения жизнеспособности при хранении в «голодной» среде с помощью флуоресцентного потенциал-чувствительного зонда 2-Di-1-ASP. Мы исследовали 20 образцов клеток костного мозга (ККМ), которые помещали в стандартный стабилизирующий раствор с цитратом натрия, лимонной кислотой, солями фосфатов, декстрозой и аденином. Подсчет клеток и тесты на выживаемость проводили на протяжении 72 часов хранения. Образцы клеток метили зондом 2-Di-1-ASP в определенные сроки. Интенсивность флуоресценции измеряли по отдельным клеткам, рассчитывали средние значения флуоресценции и число миелокариоцитов на образец. Митотические индексы определяли как в препаратах, окрашенных по Романовскому-Гимза, так и после окраски флуоресцентным зондом. Для статистической обработки использовали методы кластерного анализа и стандартные непараметрические тесты. Результаты: Выживаемость ККМ в течение 0-5 часов составляла 80-92% (по тесту с трипановым синим), затем постепенно снижалась до 70-75% к концу сроков хранения. Далее, 3-часовая инкубация ККМ сопровождалась повышением интенсивности флуоресценции (F̃) с данным зондом, главным образом, из-за нарастания доли ярко светящейся субпопуляции (>100 усл.ед., NF>100,%). Повышение значений D (отношения NF>100,% через 3 часа хранения к NF>100,% в исходных пробах) оказалось наиболее информативным, что позволяет прогнозировать специфические величины F̃ на более поздних сроках. Повышение F̃ по сравнению с исходными значениями в этот срок отмечалось во всех образцах ККМ за счет ярко светящихся форм (NF>100), что коррелировало с ростом числа клеток. Дополнительный кластерный анализ позволил классифицировать образцы ККМ на 3 подгруппы в связи сдостоверными различиями по значениям D и изменениям клеточности. В частности, значительное число митотических форм наблюдалось в популяциях ККМ псле 5-24 часов хранения, и они ярко окрашивались зондом 2-Di-1-ASP probe. В целом, мы выявили 0,60±0,10% метафазных клеток в исходный срок наблюдения. По мере хранения, частота митотических фигур повышалась до 1,4±0,1%, а, после 6-часовой инкубации с колхицином, митотический индекс возрастал до 1,8±0,1%, что указывало на хорошую степень сохранности пролиферирующей фракции клеток.
В заключение, наши результаты показывают сохранение и даже усиление энергетической активности ККМ, выявленное с помощью потенциал-чувствительного зонда и хорошую выживаемость фракции пролиферирующих клеток при жестких условиях инкубации. В дальнейшем следует провести исследования соответствующих механизмов сохранения клеток костного мозга и их энергетического баланса в данных условиях хранения.
Tatyana V.Parkhomenko, Oleg V.Galibin, Elena V.Verbitskaya, Vladimir V. Tomson
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6796" ["VALUE"]=> array(2) { ["TEXT"]=> string(238) "R.M. Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantion, Research Center, First St.Petersburg<br> State I.P.Pavlov Medical University, Russian Ministry of Health Care, St.Petersburg, Russia<br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(226) "R.M. Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantion, Research Center, First St.PetersburgState I.P.Pavlov Medical University, Russian Ministry of Health Care, St.Petersburg, Russia
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6797" ["VALUE"]=> array(2) { ["TEXT"]=> string(2917) "<p> Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.<br> We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation. </p> <h2>Results</h2> <p> Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, N<sub>F>100</sub>,%). D (ratio of N<sub>F>100</sub> at 3h storage to N<sub>F>100</sub> initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (N<sub>F>100</sub>), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.<br> In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2801) "
Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.
We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation.
Results
Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, NF>100,%). D (ratio of NF>100 at 3h storage to NF>100 initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (NF>100), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.
In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future.
Tatyana V.Parkhomenko, Oleg V.Galibin, Elena V.Verbitskaya, Vladimir V. Tomson
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(108) "Tatyana V.Parkhomenko, Oleg V.Galibin, Elena V.Verbitskaya, Vladimir V. Tomson
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6797" ["VALUE"]=> array(2) { ["TEXT"]=> string(2917) "<p> Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.<br> We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation. </p> <h2>Results</h2> <p> Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, N<sub>F>100</sub>,%). D (ratio of N<sub>F>100</sub> at 3h storage to N<sub>F>100</sub> initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (N<sub>F>100</sub>), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.<br> In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2801) "
Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.
We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation.
Results
Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, NF>100,%). D (ratio of NF>100 at 3h storage to NF>100 initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (NF>100), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.
In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future.
Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.
We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation.
Results
Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, NF>100,%). D (ratio of NF>100 at 3h storage to NF>100 initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (NF>100), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.
In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future.
State I.P.Pavlov Medical University, Russian Ministry of Health Care, St.Petersburg, Russia
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(226) "R.M. Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantion, Research Center, First St.Petersburg
State I.P.Pavlov Medical University, Russian Ministry of Health Care, St.Petersburg, Russia
" } ["AUTHORS"]=> array(38) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(4) { [0]=> string(4) "6953" [1]=> string(4) "6954" [2]=> string(4) "6955" [3]=> string(4) "6956" } ["VALUE"]=> array(4) { [0]=> string(3) "524" [1]=> string(3) "525" [2]=> string(3) "526" [3]=> string(3) "527" } ["DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(4) { [0]=> string(3) "524" [1]=> string(3) "525" [2]=> string(3) "526" [3]=> string(3) "527" } ["~DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(4) { [0]=> string(64) "Tatyana V.Parkhomenko" [1]=> string(58) "Oleg V. Galibin" [2]=> string(63) "Elena V. Verbitskaya" [3]=> string(61) "Vladimir V. Tomson" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6791" ["VALUE"]=> array(2) { ["TEXT"]=> string(190) "<p class="Autor"> Татьяна В. Пархоменко, Олег В. Галибин, Елена В. Вербицкая, Владимир В. Томсон </p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(168) "
Татьяна В. Пархоменко, Олег В. Галибин, Елена В. Вербицкая, Владимир В. Томсон
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(168) "Татьяна В. Пархоменко, Олег В. Галибин, Елена В. Вербицкая, Владимир В. Томсон
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6789" ["VALUE"]=> string(22) "05/12/2016 05:29:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "05/12/2016 05:29:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "05/12/2016 05:29:00 pm" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6790" ["VALUE"]=> string(22) "06/24/2016 05:29:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "06/24/2016 05:29:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "06/24/2016 05:29:00 pm" } ["KEYWORDS"]=> array(38) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(6) { [0]=> string(4) "6947" [1]=> string(4) "6948" [2]=> string(4) "6949" [3]=> string(4) "6950" [4]=> string(4) "6951" [5]=> string(4) "6952" } ["VALUE"]=> array(6) { [0]=> string(3) "488" [1]=> string(3) "528" [2]=> string(3) "529" [3]=> string(3) "530" [4]=> string(3) "531" [5]=> string(3) "532" } ["DESCRIPTION"]=> array(6) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(6) { [0]=> string(3) "488" [1]=> string(3) "528" [2]=> string(3) "529" [3]=> string(3) "530" [4]=> string(3) "531" [5]=> string(3) "532" } ["~DESCRIPTION"]=> array(6) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(6) { [0]=> string(84) "клетки костного мозга" [1]=> string(78) "среда для хранения" [2]=> string(81) "выживаемость клеток" [3]=> string(91) "энергетический потенциал" [4]=> string(89) "митотическая активность" [5]=> string(100) "потенциал-чувствительный зонд" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["CONTACT"]=> array(38) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6831" ["VALUE"]=> string(3) "524" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "524" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(64) "Tatyana V.Parkhomenko" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6793" ["VALUE"]=> array(2) { ["TEXT"]=> string(5339) "<p> Костный мозг является основным источником гемопоэтических стволовых клеток при клинической трансплантации. Качество трансплантатов костного мозга – ключевой фактор их успешного приживления. Целью нашей работы была проверка полуколичественной методики определения жизнеспособности при хранении в «голодной» среде с помощью флуоресцентного потенциал-чувствительного зонда 2-Di-1-ASP. Мы исследовали 20 образцов клеток костного мозга (ККМ), которые помещали в стандартный стабилизирующий раствор с цитратом натрия, лимонной кислотой, солями фосфатов, декстрозой и аденином. Подсчет клеток и тесты на выживаемость проводили на протяжении 72 часов хранения. Образцы клеток метили зондом 2-Di-1-ASP в определенные сроки. Интенсивность флуоресценции измеряли по отдельным клеткам, рассчитывали средние значения флуоресценции и число миелокариоцитов на образец. Митотические индексы определяли как в препаратах, окрашенных по Романовскому-Гимза, так и после окраски флуоресцентным зондом. Для статистической обработки использовали методы кластерного анализа и стандартные непараметрические тесты. Результаты: Выживаемость ККМ в течение 0-5 часов составляла 80-92% (по тесту с трипановым синим), затем постепенно снижалась до 70-75% к концу сроков хранения. Далее, 3-часовая инкубация ККМ сопровождалась повышением интенсивности флуоресценции (F̃) с данным зондом, главным образом, из-за нарастания доли ярко светящейся субпопуляции (>100 усл.ед., N<sub>F>100</sub>,%). Повышение значений D (отношения N<sub>F>100</sub>,% через 3 часа хранения к N<sub>F>100</sub>,% в исходных пробах) оказалось наиболее информативным, что позволяет прогнозировать специфические величины F̃ на более поздних сроках. Повышение F̃ по сравнению с исходными значениями в этот срок отмечалось во всех образцах ККМ за счет ярко светящихся форм (N<sub>F>100</sub>), что коррелировало с ростом числа клеток. Дополнительный кластерный анализ позволил классифицировать образцы ККМ на 3 подгруппы в связи сдостоверными различиями по значениям D и изменениям клеточности. В частности, значительное число митотических форм наблюдалось в популяциях ККМ псле 5-24 часов хранения, и они ярко окрашивались зондом 2-Di-1-ASP probe. В целом, мы выявили 0,60±0,10% метафазных клеток в исходный срок наблюдения. По мере хранения, частота митотических фигур повышалась до 1,4±0,1%, а, после 6-часовой инкубации с колхицином, митотический индекс возрастал до 1,8±0,1%, что указывало на хорошую степень сохранности пролиферирующей фракции клеток.<br> <br> В заключение, наши результаты показывают сохранение и даже усиление энергетической активности ККМ, выявленное с помощью потенциал-чувствительного зонда и хорошую выживаемость фракции пролиферирующих клеток при жестких условиях инкубации. В дальнейшем следует провести исследования соответствующих механизмов сохранения клеток костного мозга и их энергетического баланса в данных условиях хранения. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(5247) "
Костный мозг является основным источником гемопоэтических стволовых клеток при клинической трансплантации. Качество трансплантатов костного мозга – ключевой фактор их успешного приживления. Целью нашей работы была проверка полуколичественной методики определения жизнеспособности при хранении в «голодной» среде с помощью флуоресцентного потенциал-чувствительного зонда 2-Di-1-ASP. Мы исследовали 20 образцов клеток костного мозга (ККМ), которые помещали в стандартный стабилизирующий раствор с цитратом натрия, лимонной кислотой, солями фосфатов, декстрозой и аденином. Подсчет клеток и тесты на выживаемость проводили на протяжении 72 часов хранения. Образцы клеток метили зондом 2-Di-1-ASP в определенные сроки. Интенсивность флуоресценции измеряли по отдельным клеткам, рассчитывали средние значения флуоресценции и число миелокариоцитов на образец. Митотические индексы определяли как в препаратах, окрашенных по Романовскому-Гимза, так и после окраски флуоресцентным зондом. Для статистической обработки использовали методы кластерного анализа и стандартные непараметрические тесты. Результаты: Выживаемость ККМ в течение 0-5 часов составляла 80-92% (по тесту с трипановым синим), затем постепенно снижалась до 70-75% к концу сроков хранения. Далее, 3-часовая инкубация ККМ сопровождалась повышением интенсивности флуоресценции (F̃) с данным зондом, главным образом, из-за нарастания доли ярко светящейся субпопуляции (>100 усл.ед., NF>100,%). Повышение значений D (отношения NF>100,% через 3 часа хранения к NF>100,% в исходных пробах) оказалось наиболее информативным, что позволяет прогнозировать специфические величины F̃ на более поздних сроках. Повышение F̃ по сравнению с исходными значениями в этот срок отмечалось во всех образцах ККМ за счет ярко светящихся форм (NF>100), что коррелировало с ростом числа клеток. Дополнительный кластерный анализ позволил классифицировать образцы ККМ на 3 подгруппы в связи сдостоверными различиями по значениям D и изменениям клеточности. В частности, значительное число митотических форм наблюдалось в популяциях ККМ псле 5-24 часов хранения, и они ярко окрашивались зондом 2-Di-1-ASP probe. В целом, мы выявили 0,60±0,10% метафазных клеток в исходный срок наблюдения. По мере хранения, частота митотических фигур повышалась до 1,4±0,1%, а, после 6-часовой инкубации с колхицином, митотический индекс возрастал до 1,8±0,1%, что указывало на хорошую степень сохранности пролиферирующей фракции клеток.
В заключение, наши результаты показывают сохранение и даже усиление энергетической активности ККМ, выявленное с помощью потенциал-чувствительного зонда и хорошую выживаемость фракции пролиферирующих клеток при жестких условиях инкубации. В дальнейшем следует провести исследования соответствующих механизмов сохранения клеток костного мозга и их энергетического баланса в данных условиях хранения.
Костный мозг является основным источником гемопоэтических стволовых клеток при клинической трансплантации. Качество трансплантатов костного мозга – ключевой фактор их успешного приживления. Целью нашей работы была проверка полуколичественной методики определения жизнеспособности при хранении в «голодной» среде с помощью флуоресцентного потенциал-чувствительного зонда 2-Di-1-ASP. Мы исследовали 20 образцов клеток костного мозга (ККМ), которые помещали в стандартный стабилизирующий раствор с цитратом натрия, лимонной кислотой, солями фосфатов, декстрозой и аденином. Подсчет клеток и тесты на выживаемость проводили на протяжении 72 часов хранения. Образцы клеток метили зондом 2-Di-1-ASP в определенные сроки. Интенсивность флуоресценции измеряли по отдельным клеткам, рассчитывали средние значения флуоресценции и число миелокариоцитов на образец. Митотические индексы определяли как в препаратах, окрашенных по Романовскому-Гимза, так и после окраски флуоресцентным зондом. Для статистической обработки использовали методы кластерного анализа и стандартные непараметрические тесты. Результаты: Выживаемость ККМ в течение 0-5 часов составляла 80-92% (по тесту с трипановым синим), затем постепенно снижалась до 70-75% к концу сроков хранения. Далее, 3-часовая инкубация ККМ сопровождалась повышением интенсивности флуоресценции (F̃) с данным зондом, главным образом, из-за нарастания доли ярко светящейся субпопуляции (>100 усл.ед., NF>100,%). Повышение значений D (отношения NF>100,% через 3 часа хранения к NF>100,% в исходных пробах) оказалось наиболее информативным, что позволяет прогнозировать специфические величины F̃ на более поздних сроках. Повышение F̃ по сравнению с исходными значениями в этот срок отмечалось во всех образцах ККМ за счет ярко светящихся форм (NF>100), что коррелировало с ростом числа клеток. Дополнительный кластерный анализ позволил классифицировать образцы ККМ на 3 подгруппы в связи сдостоверными различиями по значениям D и изменениям клеточности. В частности, значительное число митотических форм наблюдалось в популяциях ККМ псле 5-24 часов хранения, и они ярко окрашивались зондом 2-Di-1-ASP probe. В целом, мы выявили 0,60±0,10% метафазных клеток в исходный срок наблюдения. По мере хранения, частота митотических фигур повышалась до 1,4±0,1%, а, после 6-часовой инкубации с колхицином, митотический индекс возрастал до 1,8±0,1%, что указывало на хорошую степень сохранности пролиферирующей фракции клеток.
В заключение, наши результаты показывают сохранение и даже усиление энергетической активности ККМ, выявленное с помощью потенциал-чувствительного зонда и хорошую выживаемость фракции пролиферирующих клеток при жестких условиях инкубации. В дальнейшем следует провести исследования соответствующих механизмов сохранения клеток костного мозга и их энергетического баланса в данных условиях хранения.
Первый Санкт-Петербургский государственный медицинский Университет им. И.П. Павлова Минздрава РФ, Санкт-
Петербург, Россия.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(438) "НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой, Научно-исследовательский центр,
Первый Санкт-Петербургский государственный медицинский Университет им. И.П. Павлова Минздрава РФ, Санкт-
Петербург, Россия.
" } } } [2]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(2) "15" ["~IBLOCK_SECTION_ID"]=> string(2) "15" ["ID"]=> string(3) "474" ["~ID"]=> string(3) "474" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["~NAME"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "09/22/2016 11:28:33 am" ["~TIMESTAMP_X"]=> string(22) "09/22/2016 11:28:33 am" ["DETAIL_PAGE_URL"]=> string(147) "/en/archive/vypusk-5-nomer-2/eksperimentalnye-stati/primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy/" ["~DETAIL_PAGE_URL"]=> string(147) "/en/archive/vypusk-5-nomer-2/eksperimentalnye-stati/primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(9000) "
Introduction
In 2009, over 150 000 people in the US were waiting for an organ transplant, and only 18% of them received one. Nearly 9000 people died while on the waiting list. The lack of available donors is a major driving force for developing the concept of artificial organs. Strategies for construction or reconstruction of new tissues and organs include: 1) Creating biocompatible templates onto which stem cells and their derivative cells can be seeded; 2) Creating natural templates by decellularization, and seeding stem cells or stem cell-derived cells onto these; and 3) Generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing.
Culturing cells under 3D conditions provides key advantages that make this strategy imperative in approaching the problem of organ replacement. In conventional 2D cultures, primary cells rapidly lose their function, in large part due to perturbed cell-cell contacts. This can lead to rapid loss of polarity and differentiation, as is seen for example in hepatocytes obtained from biopsies. Dissociated hepatocytes in 2D monoculture revert to expression of alpha fetoprotein (AFP) and experience a downregulation of integrins and P450 activity. By contrast, primary hepatocytes cultured under 3D conditions in a microgravity vessel remain fully functional for at least 6 weeks with respect to albumin secretion, integrin beta 6 expression, P450 responsiveness and downregulation of AFP. In short, a liver-like functional status is retained. Thus, 2D monocultures of animal primary cells or of human immortalized cell lines are not representative of normal human tissue. 3D microenvironments provide more complex and physiological cell-cell and cell-matrix interactions, and provide a more reliable platform for physiologically relevant tissue models, disease models and drug testing.
![Figure 1. An example of a 3D-printed tissue scaffold [3]. Figure 1. An example of a 3D-printed tissue scaffold [3].](/upload/medialibrary/780/780d6f070a42245d2890b974c9280343.jpg)
Figure 1. An example of a 3D-printed tissue scaffold [3].
In designing 3D bioprinting strategies, several important
technical issues need to be considered, including:
- Choice of printing technology (microextrusion, laser induced forward transfer – LIFT)
- Choice of biomatrix/hydrogel (alginate, chytosan, etc.)
- Printing parameters (temperature, spatial resolution, cell density, etc.)
- Interactions between multiple cell types
- Use of multiple biomatrices
- The need for associated microfluidics
The choice of printing technology is especially important.
Microextrusion technology is essentially the same as used in thermal inkjet printing, and can attain a spatial resolution down to about 100 um using biogels. Currently available printing platforms provide multiple printing heads and differential temperature control for utilization of diverse materials. Printing parameters must be optimized for each material applied, and material-specific shear forces are relevant when working with labile cells.
Another promising technology is laser-induced forward transfer (LIFT) printing, which uses laser light to force cells from a fluid interface onto a surface, rather than extruding cells through a printing head. This ensures high spatial resolution (<100 um), corresponding essentially to single-cell seeding, and high-speed cell placement (1000 droplets/sec). LIFT is not dependent on using a biomaterial as vehicle, and thus shear forces are less relevant. However, laser power is a significant factor.
3-D bioprinting
One promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds and constructs that reproduce some of the complex interactions occuring among different cell types within a tissue (Figure 1). Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures (Figure 2).

Figure 2. First 3D printed kidney tissue (http://ir.organovo.com/news/press-releases/press-releases-details/2015/Organovo-Describes-First-Full....

Figure 3. An example of a modern bioprinter.
Natural templates
An alternative, and also still experimental, approach to organ manufacture is represented by decellularization and subsequent recellularization of native organs, exemplified by attempts using animal organs, including rat heart [4, 6], rat kidney [1] and monkey lung [2]. As an example of this approach, bone marrow derived mesenchymal stem cells or lung-derived microvascular endothelial cells have been seeded into decellularized lung scaffolds and have generated epithelia that histologically resemble natural respiratory airways [2].
An important challenge when designing tissue scaffolds is the adequate incorporation of vascularization. One potential approach, termed “nano-origami”, involves complex nanostructures that are constructed as topographically patterned 2D substrates that can be seeded with cells and then rolled or folded into a 3D shape [5], see also http://www.materialsviews.com/advanced-origami-nanostructures-from-flowers-to-boxes/http://nextbig future.com/2012/04/logic-gated-nanorobot-for-targeted.html).
Relevance for hematology
A main area of potential relevance of 3D bioprinting for hematologists is in modeling hematopoiesis in a more natural tissue microenvironment. A variety of differentiating blood cells, accessory cells and immune cells exist within the structural microenvironment of bone marrow, creating intimate functional and regulatory relationships. Thus, generating appropriately patterned 3D tissue models could create promising opportunities for investigations of normal and altered hematopoiesis in a realistic biological niche.
Conclusions
3D bioprinting is a field in rapid development. Increasing numbers of studies are highlighting some of the potential applications, including more physiological and penetrating investigation of tissue and organ development and function, the generation of personalized drug testing and disease models, and scaffold and tissue printing for clinical use. 3D bioprinting is likely to contribute substantially to tissue engineering efforts related to organ replacement.
Conflict of interest
None declared
References
- Bonandrini B, Figliuzzi M, Papadimou E, Morigi M, Perico N, Casiraghi F, Dipl C, Sangalli F, Conti S, Benigni A, Remuzzi A, Remuzzi G. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng Part A 2014;20 (9-10):1486-1498.
- Bonvillain RW, Scarritt ME, Pashos NC, Mayeaux JP, Meshberger CL, Betancourt AM, Sullivan DE, Bunnell BA. Nonhuman primate lung decellularization and recellularization using a specialized large-organ bioreactor. J Vis Exp 2013;82:e50825.
- Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 2014;26(19):3124- 3130.
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 2008;14(2):213-221.
- Sun Y, Weng S, Fu J. Microengineered synthetic cellular microenvironment for stem cells. Nanomed Nanobiotech 2012; 4(4):414–427.
- Vunjak-Novakovic G, Lui KO, Tandon N, Chien KR. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng 2011;13:245-267.
" ["~DETAIL_TEXT"]=> string(9000) "
Introduction
In 2009, over 150 000 people in the US were waiting for an organ transplant, and only 18% of them received one. Nearly 9000 people died while on the waiting list. The lack of available donors is a major driving force for developing the concept of artificial organs. Strategies for construction or reconstruction of new tissues and organs include: 1) Creating biocompatible templates onto which stem cells and their derivative cells can be seeded; 2) Creating natural templates by decellularization, and seeding stem cells or stem cell-derived cells onto these; and 3) Generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing.
Culturing cells under 3D conditions provides key advantages that make this strategy imperative in approaching the problem of organ replacement. In conventional 2D cultures, primary cells rapidly lose their function, in large part due to perturbed cell-cell contacts. This can lead to rapid loss of polarity and differentiation, as is seen for example in hepatocytes obtained from biopsies. Dissociated hepatocytes in 2D monoculture revert to expression of alpha fetoprotein (AFP) and experience a downregulation of integrins and P450 activity. By contrast, primary hepatocytes cultured under 3D conditions in a microgravity vessel remain fully functional for at least 6 weeks with respect to albumin secretion, integrin beta 6 expression, P450 responsiveness and downregulation of AFP. In short, a liver-like functional status is retained. Thus, 2D monocultures of animal primary cells or of human immortalized cell lines are not representative of normal human tissue. 3D microenvironments provide more complex and physiological cell-cell and cell-matrix interactions, and provide a more reliable platform for physiologically relevant tissue models, disease models and drug testing.
![Figure 1. An example of a 3D-printed tissue scaffold [3]. Figure 1. An example of a 3D-printed tissue scaffold [3].](/upload/medialibrary/780/780d6f070a42245d2890b974c9280343.jpg)
Figure 1. An example of a 3D-printed tissue scaffold [3].
In designing 3D bioprinting strategies, several important
technical issues need to be considered, including:
- Choice of printing technology (microextrusion, laser induced forward transfer – LIFT)
- Choice of biomatrix/hydrogel (alginate, chytosan, etc.)
- Printing parameters (temperature, spatial resolution, cell density, etc.)
- Interactions between multiple cell types
- Use of multiple biomatrices
- The need for associated microfluidics
The choice of printing technology is especially important.
Microextrusion technology is essentially the same as used in thermal inkjet printing, and can attain a spatial resolution down to about 100 um using biogels. Currently available printing platforms provide multiple printing heads and differential temperature control for utilization of diverse materials. Printing parameters must be optimized for each material applied, and material-specific shear forces are relevant when working with labile cells.
Another promising technology is laser-induced forward transfer (LIFT) printing, which uses laser light to force cells from a fluid interface onto a surface, rather than extruding cells through a printing head. This ensures high spatial resolution (<100 um), corresponding essentially to single-cell seeding, and high-speed cell placement (1000 droplets/sec). LIFT is not dependent on using a biomaterial as vehicle, and thus shear forces are less relevant. However, laser power is a significant factor.
3-D bioprinting
One promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds and constructs that reproduce some of the complex interactions occuring among different cell types within a tissue (Figure 1). Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures (Figure 2).

Figure 2. First 3D printed kidney tissue (http://ir.organovo.com/news/press-releases/press-releases-details/2015/Organovo-Describes-First-Full....

Figure 3. An example of a modern bioprinter.
Natural templates
An alternative, and also still experimental, approach to organ manufacture is represented by decellularization and subsequent recellularization of native organs, exemplified by attempts using animal organs, including rat heart [4, 6], rat kidney [1] and monkey lung [2]. As an example of this approach, bone marrow derived mesenchymal stem cells or lung-derived microvascular endothelial cells have been seeded into decellularized lung scaffolds and have generated epithelia that histologically resemble natural respiratory airways [2].
An important challenge when designing tissue scaffolds is the adequate incorporation of vascularization. One potential approach, termed “nano-origami”, involves complex nanostructures that are constructed as topographically patterned 2D substrates that can be seeded with cells and then rolled or folded into a 3D shape [5], see also http://www.materialsviews.com/advanced-origami-nanostructures-from-flowers-to-boxes/http://nextbig future.com/2012/04/logic-gated-nanorobot-for-targeted.html).
Relevance for hematology
A main area of potential relevance of 3D bioprinting for hematologists is in modeling hematopoiesis in a more natural tissue microenvironment. A variety of differentiating blood cells, accessory cells and immune cells exist within the structural microenvironment of bone marrow, creating intimate functional and regulatory relationships. Thus, generating appropriately patterned 3D tissue models could create promising opportunities for investigations of normal and altered hematopoiesis in a realistic biological niche.
Conclusions
3D bioprinting is a field in rapid development. Increasing numbers of studies are highlighting some of the potential applications, including more physiological and penetrating investigation of tissue and organ development and function, the generation of personalized drug testing and disease models, and scaffold and tissue printing for clinical use. 3D bioprinting is likely to contribute substantially to tissue engineering efforts related to organ replacement.
Conflict of interest
None declared
References
- Bonandrini B, Figliuzzi M, Papadimou E, Morigi M, Perico N, Casiraghi F, Dipl C, Sangalli F, Conti S, Benigni A, Remuzzi A, Remuzzi G. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng Part A 2014;20 (9-10):1486-1498.
- Bonvillain RW, Scarritt ME, Pashos NC, Mayeaux JP, Meshberger CL, Betancourt AM, Sullivan DE, Bunnell BA. Nonhuman primate lung decellularization and recellularization using a specialized large-organ bioreactor. J Vis Exp 2013;82:e50825.
- Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 2014;26(19):3124- 3130.
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 2008;14(2):213-221.
- Sun Y, Weng S, Fu J. Microengineered synthetic cellular microenvironment for stem cells. Nanomed Nanobiotech 2012; 4(4):414–427.
- Vunjak-Novakovic G, Lui KO, Tandon N, Chien KR. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng 2011;13:245-267.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(3) "500" ["~SORT"]=> string(3) "500" ["CODE"]=> string(94) "primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy" ["~CODE"]=> string(94) "primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy" ["EXTERNAL_ID"]=> string(3) "474" ["~EXTERNAL_ID"]=> string(3) "474" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["ELEMENT_META_KEYWORDS"]=> string(156) "стволовые клетки тканевые матрицы 3D-принтинг гемопоэз микроокружение моделирование" ["ELEMENT_META_DESCRIPTION"]=> string(245) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий3D bioprinting applications for in vitro modeling of cellular interactions and tissues" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2502) "Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении." ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_META_TITLE"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_META_KEYWORDS"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_META_DESCRIPTION"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_PICTURE_FILE_ALT"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_PICTURE_FILE_TITLE"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_PICTURE_FILE_NAME"]=> string(98) "primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(159) "Применение 3D-биопринтинга для моделирования in vitro клеточных и тканевых взаимодействий" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(98) "primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(98) "primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(98) "primenenie-3d-bioprintinga-dlya-modelirovaniya-in-vitro-kletochnykh-i-tkanevykh-vzaimodeystviy-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(2) "15" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(6) { [0]=> string(4) "6940" [1]=> string(4) "6941" [2]=> string(4) "6942" [3]=> string(4) "6943" [4]=> string(4) "6944" [5]=> string(4) "6945" } ["VALUE"]=> array(6) { [0]=> string(2) "98" [1]=> string(3) "473" [2]=> string(3) "475" [3]=> string(3) "477" [4]=> string(3) "478" [5]=> string(3) "476" } ["DESCRIPTION"]=> array(6) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(6) { [0]=> string(2) "98" [1]=> string(3) "473" [2]=> string(3) "475" [3]=> string(3) "477" [4]=> string(3) "478" [5]=> string(3) "476" } ["~DESCRIPTION"]=> array(6) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5822" ["VALUE"]=> string(22) "06/15/2016 04:21:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "06/15/2016 04:21:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5823" ["VALUE"]=> string(22) "06/24/2016 04:21:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "06/24/2016 04:21:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5826" ["VALUE"]=> string(2) "97" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(2) "97" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(1) { [0]=> string(4) "6946" } ["VALUE"]=> array(1) { [0]=> string(2) "97" } ["DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(1) { [0]=> string(2) "97" } ["~DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5960" ["VALUE"]=> array(2) { ["TEXT"]=> string(70) "<p class="Autor">Джоэл К. Гловер</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(48) "
Джоэл К. Гловер
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5961" ["VALUE"]=> array(2) { ["TEXT"]=> string(352) "Отдел молекулярной медицины, Институт фундаментальных медицинских наук, Университет Осло, Норвегия Норвежский центр исследования стволовых клеток, Университетский госпиталь Осло, Норвегия" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(352) "Отдел молекулярной медицины, Институт фундаментальных медицинских наук, Университет Осло, Норвегия Норвежский центр исследования стволовых клеток, Университетский госпиталь Осло, Норвегия" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5835" ["VALUE"]=> array(2) { ["TEXT"]=> string(2502) "Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении." ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2502) "Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении." ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5824" ["VALUE"]=> string(32) "10.18620/1866-8836-2016-5-2-8-11" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(32) "10.18620/1866-8836-2016-5-2-8-11" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5828" ["VALUE"]=> array(2) { ["TEXT"]=> string(57) "<p class="Autor">Joel C. Glover</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(35) "Joel C. Glover
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5829" ["VALUE"]=> array(2) { ["TEXT"]=> string(238) "Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo Norwegian Center for Stem Cell Research, Oslo University Hospital Professor, Institute of Basic Medical Sciences, University of Oslo Medical Faculty" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(238) "Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo Norwegian Center for Stem Cell Research, Oslo University Hospital Professor, Institute of Basic Medical Sciences, University of Oslo Medical Faculty" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5903" ["VALUE"]=> array(2) { ["TEXT"]=> string(1340) "The article considers different strategies for seeding stem cells and their progeny and construction of new tissues and organs, i.e., artificial biocompatible templates, natural decellularized templates, and generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing. Generally, culturing cells under 3D conditions allows to retain fully morphological and functional integrity of specialized cells as shown with hepatocytes. In particular, a promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds. Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures. There are some technical issues which should be considered in any single case, including type of printing technology, choice of biomatrix type, printing parameters, etc.<br> <br> Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.<br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1322) "The article considers different strategies for seeding stem cells and their progeny and construction of new tissues and organs, i.e., artificial biocompatible templates, natural decellularized templates, and generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing. Generally, culturing cells under 3D conditions allows to retain fully morphological and functional integrity of specialized cells as shown with hepatocytes. In particular, a promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds. Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures. There are some technical issues which should be considered in any single case, including type of printing technology, choice of biomatrix type, printing parameters, etc.Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5825" ["VALUE"]=> string(86) "3D bioprinting applications for in vitro modeling of cellular interactions and tissues" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(86) "3D bioprinting applications for in vitro modeling of cellular interactions and tissues" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6268" ["VALUE"]=> string(3) "161" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "161" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "6260" ["VALUE"]=> string(3) "213" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "213" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(13) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5828" ["VALUE"]=> array(2) { ["TEXT"]=> string(57) "<p class="Autor">Joel C. Glover</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(35) "
Joel C. Glover
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(35) "Joel C. Glover
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5903" ["VALUE"]=> array(2) { ["TEXT"]=> string(1340) "The article considers different strategies for seeding stem cells and their progeny and construction of new tissues and organs, i.e., artificial biocompatible templates, natural decellularized templates, and generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing. Generally, culturing cells under 3D conditions allows to retain fully morphological and functional integrity of specialized cells as shown with hepatocytes. In particular, a promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds. Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures. There are some technical issues which should be considered in any single case, including type of printing technology, choice of biomatrix type, printing parameters, etc.<br> <br> Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.<br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1322) "The article considers different strategies for seeding stem cells and their progeny and construction of new tissues and organs, i.e., artificial biocompatible templates, natural decellularized templates, and generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing. Generally, culturing cells under 3D conditions allows to retain fully morphological and functional integrity of specialized cells as shown with hepatocytes. In particular, a promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds. Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures. There are some technical issues which should be considered in any single case, including type of printing technology, choice of biomatrix type, printing parameters, etc.Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1322) "The article considers different strategies for seeding stem cells and their progeny and construction of new tissues and organs, i.e., artificial biocompatible templates, natural decellularized templates, and generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing. Generally, culturing cells under 3D conditions allows to retain fully morphological and functional integrity of specialized cells as shown with hepatocytes. In particular, a promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds. Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures. There are some technical issues which should be considered in any single case, including type of printing technology, choice of biomatrix type, printing parameters, etc.
Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5824" ["VALUE"]=> string(32) "10.18620/1866-8836-2016-5-2-8-11" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(32) "10.18620/1866-8836-2016-5-2-8-11" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(32) "10.18620/1866-8836-2016-5-2-8-11" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5825" ["VALUE"]=> string(86) "3D bioprinting applications for in vitro modeling of cellular interactions and tissues" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(86) "3D bioprinting applications for in vitro modeling of cellular interactions and tissues" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(86) "3D bioprinting applications for in vitro modeling of cellular interactions and tissues" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5829" ["VALUE"]=> array(2) { ["TEXT"]=> string(238) "Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo Norwegian Center for Stem Cell Research, Oslo University Hospital Professor, Institute of Basic Medical Sciences, University of Oslo Medical Faculty" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(238) "Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo Norwegian Center for Stem Cell Research, Oslo University Hospital Professor, Institute of Basic Medical Sciences, University of Oslo Medical Faculty" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(238) "Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo Norwegian Center for Stem Cell Research, Oslo University Hospital Professor, Institute of Basic Medical Sciences, University of Oslo Medical Faculty" } ["AUTHORS"]=> array(38) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(1) { [0]=> string(4) "6946" } ["VALUE"]=> array(1) { [0]=> string(2) "97" } ["DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(1) { [0]=> string(2) "97" } ["~DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(56) "Joel C. Glover" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5960" ["VALUE"]=> array(2) { ["TEXT"]=> string(70) "<p class="Autor">Джоэл К. Гловер</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(48) "
Джоэл К. Гловер
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(48) "Джоэл К. Гловер
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5822" ["VALUE"]=> string(22) "06/15/2016 04:21:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "06/15/2016 04:21:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "06/15/2016 04:21:00 pm" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5823" ["VALUE"]=> string(22) "06/24/2016 04:21:00 pm" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "06/24/2016 04:21:00 pm" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "06/24/2016 04:21:00 pm" } ["KEYWORDS"]=> array(38) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(6) { [0]=> string(4) "6940" [1]=> string(4) "6941" [2]=> string(4) "6942" [3]=> string(4) "6943" [4]=> string(4) "6944" [5]=> string(4) "6945" } ["VALUE"]=> array(6) { [0]=> string(2) "98" [1]=> string(3) "473" [2]=> string(3) "475" [3]=> string(3) "477" [4]=> string(3) "478" [5]=> string(3) "476" } ["DESCRIPTION"]=> array(6) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(6) { [0]=> string(2) "98" [1]=> string(3) "473" [2]=> string(3) "475" [3]=> string(3) "477" [4]=> string(3) "478" [5]=> string(3) "476" } ["~DESCRIPTION"]=> array(6) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(6) { [0]=> string(74) "стволовые клетки" [1]=> string(75) "тканевые матрицы" [2]=> string(63) "3D-принтинг" [3]=> string(60) "гемопоэз" [4]=> string(72) "микроокружение" [5]=> string(70) "моделирование" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["CONTACT"]=> array(38) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5826" ["VALUE"]=> string(2) "97" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(2) "97" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(56) "Joel C. Glover" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5835" ["VALUE"]=> array(2) { ["TEXT"]=> string(2502) "Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении." ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2502) "Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении." ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2502) "Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении." } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(4) "5961" ["VALUE"]=> array(2) { ["TEXT"]=> string(352) "Отдел молекулярной медицины, Институт фундаментальных медицинских наук, Университет Осло, Норвегия Норвежский центр исследования стволовых клеток, Университетский госпиталь Осло, Норвегия" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(352) "Отдел молекулярной медицины, Институт фундаментальных медицинских наук, Университет Осло, Норвегия Норвежский центр исследования стволовых клеток, Университетский госпиталь Осло, Норвегия" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(352) "Отдел молекулярной медицины, Институт фундаментальных медицинских наук, Университет Осло, Норвегия Норвежский центр исследования стволовых клеток, Университетский госпиталь Осло, Норвегия" } } } }Experimental article
Claudia Lange,1 Rudolph Reimer,2 Jozef Zustin,3 Bärbel Brunswig-Spickenheier1
Tatyana V.Parkhomenko, Oleg V.Galibin, Elena V.Verbitskaya, Vladimir V. Tomson
Experimental article
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 6957
[1] => 6958
[2] => 6959
[3] => 6960
)
[VALUE] => Array
(
[0] => 516
[1] => 517
[2] => 518
[3] => 519
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 516
[1] => 517
[2] => 518
[3] => 519
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6714
[VALUE] => 05/10/2016 04:41:00 pm
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 05/10/2016 04:41:00 pm
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6715
[VALUE] => 05/28/2016 04:41:00 pm
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 05/28/2016 04:41:00 pm
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6716
[VALUE] => 515
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 515
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 6961
[1] => 6962
[2] => 6963
[3] => 6964
)
[VALUE] => Array
(
[0] => 515
[1] => 520
[2] => 521
[3] => 522
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 515
[1] => 520
[2] => 521
[3] => 522
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6698
[VALUE] => Array
(
[TEXT] => <p class="Autor">
Клаудиа Ланге,<sup>1</sup> Рудольф Раймер,<sup>2</sup>
Йозеф Зустин,<sup>3</sup> Бербель Брунсвиг-Шпикенхайер<sup>1</sup>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Клаудиа Ланге,1 Рудольф Раймер,2
Йозеф Зустин,3 Бербель Брунсвиг-Шпикенхайер1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6699
[VALUE] => Array
(
[TEXT] => 1. Клиника трансплантации стволовых клеток, Департамент клеточной и генной терапии, Университетский медицинский<br>
центр Гамбург-Эппендорф<br>
2. Технологическая платформа микроскопии и анализа изображений, Институт Хайнриха Петте, Гамбург,<br>
3. Институт патологии, Университетский медицинский центр Гамбург Эппендорф
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1. Клиника трансплантации стволовых клеток, Департамент клеточной и генной терапии, Университетский медицинский
центр Гамбург-Эппендорф
2. Технологическая платформа микроскопии и анализа изображений, Институт Хайнриха Петте, Гамбург,
3. Институт патологии, Университетский медицинский центр Гамбург Эппендорф
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6700
[VALUE] => Array
(
[TEXT] => <p>
Ионизирующее излучение широко применяется в качестве кондиционирующей терапии при транс- плантации костного мозга. Высокодозная радиаци- онная терапия вызывает тяжелое повреждение, в особенности – гемопоэтических стволовых клеток и клеток-предшественников. Попытки улучшения кли- нических исходов после облучения сосредоточены на гемопоэтической нише. Мезенхимные стромальные клетки (МСК) представляют собой интегральную часть стромального микроокружения. При совмест- ной трансплантации с гемопоэтическими стволовыми клетками (ГСК), МСК способны усиливать восстанов- ление кроветворения после химио- и радиационной терапии. Целью нашего исследования была оценка основных биологических параметров МСК, в плане их способности к специфической линейной диффе- ренцировке, выживаемости организма, а также их способности к радиопротекции летально облученных реципиентов. Материалы и методы. Дифференциров- ку in vitro МСК человека в направлении гемопоэтиче- ских (ГСК) или эндотелиальных клеток изучали путем RT-qPCR поверхностных маркеров и других белков. Для тестирования in vivo способности мышиных МСК защищать летально облученных (9.5 Гр) мышей, животных трансплантировали мышиными МСК, ме- чеными eGFP. Длительность донорского химеризма определяли в крови, костном мозге и тимусе по марке- рам CD45.2 и Y-хромосомы. Анализ профилей генной экспрессии в клетках костного мозга проводили по сравнению с соответствующими контролями посред- ством биочипов. Результаты. При дифференцировке гемопоэтических стволовых клеток человека in vitro отмечалась изменения генной экспрессии со спектром экспрессии, типичным для кроветворных предше- ственников и зрелых клеток. Во время дифференци- ровки в средах с сывороткой наблюдалась повышен- ная экспрессия множества факторов, ответственных за эритропоэз, мегакариоцитопоэз, лимфо- и мииело- поэз. Была выявлена популяция клеток с небольшими круглыми или полиморфными ядрами, которые экс- прессировали антигенные маркеры, характерные для клеток-предшественников или зрелых форм, хотя и небольшой степени. Те же клетки приобретали мор- фологические черты эндотелия и экспрессировали гены, специфичные для эндотелиальных клеток при культивировании со специфическими факторами дифференцировки.
</p>
<p>
После введения МСК, летально облученные мыши выживали с нормальным восстановлением кроветво- рения, как при введении кроветворных клеток. Через 7 мес. после облучения реципиенты МСК имели нор- мальное соотношение популяций периферической крови. Не было указаний на наличие сколько-нибудь длительного донорского химеризма после трансплан- тации. Оценка распределения eGFP+ донорских кле- ток после внутривенной трансфузии показала бы- строе исчезновение МСК из периферической крови, до 2% через 8 часов после введения с задержкой клеток в легких, однако без их длительной персистенции и эмболизации сосудов. Исследование экспрессии ге- нов в клетках костного мозга у животных, леченных МСК, показало повышение активности генов, способ- ствующих восстановлению кроветворения, наряду со снижением активности генов, ассоциированных с ра- диационным повреждением костного мозга. Инъек- ции летально облученныи животным микровезикул, происходящих из МСК, приводили к тем же протек- тивным эффектам, что и трансплантация МСКС как таковых. Заключение. Наши результаты представляют дополнительные данные о возможных механизмах вы- сокоэффективного паракринного механизма, который актуален, в частности, для популяций костного мозга, что указывает на то, что инфузии МСК являются эф- фективным средством лечения последствий острого облучения. Кроме того, трансплантация МСК может оказывать свой трофический эффект в необычных ме- стах, в точм числе – легочной ткани реципиента.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Ионизирующее излучение широко применяется в качестве кондиционирующей терапии при транс- плантации костного мозга. Высокодозная радиаци- онная терапия вызывает тяжелое повреждение, в особенности – гемопоэтических стволовых клеток и клеток-предшественников. Попытки улучшения кли- нических исходов после облучения сосредоточены на гемопоэтической нише. Мезенхимные стромальные клетки (МСК) представляют собой интегральную часть стромального микроокружения. При совмест- ной трансплантации с гемопоэтическими стволовыми клетками (ГСК), МСК способны усиливать восстанов- ление кроветворения после химио- и радиационной терапии. Целью нашего исследования была оценка основных биологических параметров МСК, в плане их способности к специфической линейной диффе- ренцировке, выживаемости организма, а также их способности к радиопротекции летально облученных реципиентов. Материалы и методы. Дифференциров- ку in vitro МСК человека в направлении гемопоэтиче- ских (ГСК) или эндотелиальных клеток изучали путем RT-qPCR поверхностных маркеров и других белков. Для тестирования in vivo способности мышиных МСК защищать летально облученных (9.5 Гр) мышей, животных трансплантировали мышиными МСК, ме- чеными eGFP. Длительность донорского химеризма определяли в крови, костном мозге и тимусе по марке- рам CD45.2 и Y-хромосомы. Анализ профилей генной экспрессии в клетках костного мозга проводили по сравнению с соответствующими контролями посред- ством биочипов. Результаты. При дифференцировке гемопоэтических стволовых клеток человека in vitro отмечалась изменения генной экспрессии со спектром экспрессии, типичным для кроветворных предше- ственников и зрелых клеток. Во время дифференци- ровки в средах с сывороткой наблюдалась повышен- ная экспрессия множества факторов, ответственных за эритропоэз, мегакариоцитопоэз, лимфо- и мииело- поэз. Была выявлена популяция клеток с небольшими круглыми или полиморфными ядрами, которые экс- прессировали антигенные маркеры, характерные для клеток-предшественников или зрелых форм, хотя и небольшой степени. Те же клетки приобретали мор- фологические черты эндотелия и экспрессировали гены, специфичные для эндотелиальных клеток при культивировании со специфическими факторами дифференцировки.
После введения МСК, летально облученные мыши выживали с нормальным восстановлением кроветво- рения, как при введении кроветворных клеток. Через 7 мес. после облучения реципиенты МСК имели нор- мальное соотношение популяций периферической крови. Не было указаний на наличие сколько-нибудь длительного донорского химеризма после трансплан- тации. Оценка распределения eGFP+ донорских кле- ток после внутривенной трансфузии показала бы- строе исчезновение МСК из периферической крови, до 2% через 8 часов после введения с задержкой клеток в легких, однако без их длительной персистенции и эмболизации сосудов. Исследование экспрессии ге- нов в клетках костного мозга у животных, леченных МСК, показало повышение активности генов, способ- ствующих восстановлению кроветворения, наряду со снижением активности генов, ассоциированных с ра- диационным повреждением костного мозга. Инъек- ции летально облученныи животным микровезикул, происходящих из МСК, приводили к тем же протек- тивным эффектам, что и трансплантация МСКС как таковых. Заключение. Наши результаты представляют дополнительные данные о возможных механизмах вы- сокоэффективного паракринного механизма, который актуален, в частности, для популяций костного мозга, что указывает на то, что инфузии МСК являются эф- фективным средством лечения последствий острого облучения. Кроме того, трансплантация МСК может оказывать свой трофический эффект в необычных ме- стах, в точм числе – легочной ткани реципиента.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6701
[VALUE] => 10.18620/1866-8836-2016-5-2-50-59
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/1866-8836-2016-5-2-50-59
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6702
[VALUE] => Array
(
[TEXT] => <p class="Autor">
Claudia Lange,<sup>1</sup> Rudolph Reimer,<sup>2</sup>
Jozef Zustin,<sup>3</sup> Bärbel Brunswig-Spickenheier<sup>1</sup>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Claudia Lange,1 Rudolph Reimer,2
Jozef Zustin,3 Bärbel Brunswig-Spickenheier1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6703
[VALUE] => Array
(
[TEXT] => 1. Clinic for Stem Cell Transplantation, Dept. Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf,<br>
2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,<br>
3. Institute of Pathology, University Medical Center Hamburg-Eppendorf,
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1. Clinic for Stem Cell Transplantation, Dept. Cell and Gene Therapy, University Medical Center Hamburg-Eppendorf,
2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,
3. Institute of Pathology, University Medical Center Hamburg-Eppendorf,
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6704
[VALUE] => Array
(
[TEXT] => <h2>
Abstract <br>
</h2>
<p>
Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls.
</p>
<p>
<span style="font-family: Cuprum, sans-serif; font-size: 26px;">Results</span>
</p>
<p>
Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Abstract
Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls.
Results
Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6705
[VALUE] => Mesenchymal stromal cells protect from consequences of HSCT-transplantation preparatory irradiation: insights into possible mechanisms
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Mesenchymal stromal cells protect from consequences of HSCT-transplantation preparatory irradiation: insights into possible mechanisms
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6787
[VALUE] => 200
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 200
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6788
[VALUE] => 218
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 218
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Claudia Lange,1 Rudolph Reimer,2 Jozef Zustin,3 Bärbel Brunswig-Spickenheier1
2. Technology Platform Microscopy & Image Analysis, Heinrich-Pette-Institut Hamburg,
3. Institute of Pathology, University Medical Center Hamburg-Eppendorf,
Abstract
Ionizing irradiation is widely used as conditioning therapy in bone marrow (BM) transplantation. High-dose radiation treatment induces profound tissue damage, especially, of hematopoietic stem cells and progenitor cells. Efforts to improve clinical outcomes post- irradiation are focused on the hematopoietic stem cell niche. Mesenchymal stromal cells (MSCs) represent an integrative part of the BM stromal microenvironment. When co-transplanted with HSC, MSCs augment hematopoietic recovery after chemo- or radiotherapy. The aim of our study was to evaluate essential biological parameters of MSCs, with respect to their lineage-specific differentiation capacity, in vivo survival rates, as well as their ability to rescue lethally irradiated hosts. Materials and Methods. In vitro differentiation of human BM-derived MSCs (hMSCs) for hematopoietic (HSC) and endothelial cells (EC) was studied by reverse transcription- quantitative PCR (RT-qPCR) of lineage-specific surface markers and other proteins. To test in vivo ability of murine MSCs to rescue lethally irradiated (9.5 Gy) mice, the animals were transplanted with eGFP-marked murine MSCs (mMSCs). Long-term donor chimerism was assessed in blood, BM and thymus using CD45.2 and Y chromosome markers. A microarray analysis of bone marrow cells from MSC-transplanted animals was also performed, in order to compare their gene expression profiles to appropriate controls.
Results
Upon in vitro differentiation of hMSCs, the hematopoietically differentiated cells changed their gene expression towards a typical profile of progenitor and mature hematopoietic cells. A variety of transcription factors responsible for erythropoiesis, megakaryopoiesis, lympho- and myelopoiesis were up-regulated during differentiation in serum-containing media. A population of cells with small round or polymorphic nuclei was detected which expressed hematopoietic progenitor and mature antigen markers, albeit to a rather low degree. The same cells were able to acquire endothelial morphology and expressed endothelial genes upon cultivation with endothelial promoting factors. Following MSCs transplantation, the lethally irradiated mice showed normal hematopoietic recovery comparable to effects of HSC infusions. Seven months later, the recipients had normal distribution of peripheral blood cell populations. No evidence of donor chimerism was shown at any time
Experimental article
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 6947
[1] => 6948
[2] => 6949
[3] => 6950
[4] => 6951
[5] => 6952
)
[VALUE] => Array
(
[0] => 488
[1] => 528
[2] => 529
[3] => 530
[4] => 531
[5] => 532
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 488
[1] => 528
[2] => 529
[3] => 530
[4] => 531
[5] => 532
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
)
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6789
[VALUE] => 05/12/2016 05:29:00 pm
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 05/12/2016 05:29:00 pm
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6790
[VALUE] => 06/24/2016 05:29:00 pm
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 06/24/2016 05:29:00 pm
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6831
[VALUE] => 524
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 524
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 6953
[1] => 6954
[2] => 6955
[3] => 6956
)
[VALUE] => Array
(
[0] => 524
[1] => 525
[2] => 526
[3] => 527
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 524
[1] => 525
[2] => 526
[3] => 527
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6791
[VALUE] => Array
(
[TEXT] => <p class="Autor">
Татьяна В. Пархоменко,
Олег В. Галибин,
Елена В. Вербицкая,
Владимир В. Томсон
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Татьяна В. Пархоменко,
Олег В. Галибин,
Елена В. Вербицкая,
Владимир В. Томсон
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6792
[VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой, Научно-исследовательский центр,<br>
Первый Санкт-Петербургский государственный медицинский Университет им. И.П. Павлова Минздрава РФ, Санкт-<br>
Петербург, Россия.<br>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой, Научно-исследовательский центр,
Первый Санкт-Петербургский государственный медицинский Университет им. И.П. Павлова Минздрава РФ, Санкт-
Петербург, Россия.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6793
[VALUE] => Array
(
[TEXT] => <p>
Костный мозг является основным источником гемопоэтических стволовых клеток при клинической трансплантации. Качество трансплантатов костного мозга – ключевой фактор их успешного приживления. Целью нашей работы была проверка полуколичественной методики определения жизнеспособности при хранении в «голодной» среде с помощью флуоресцентного потенциал-чувствительного зонда 2-Di-1-ASP. Мы исследовали 20 образцов клеток костного мозга (ККМ), которые помещали в стандартный стабилизирующий раствор с цитратом натрия, лимонной кислотой, солями фосфатов, декстрозой и аденином. Подсчет клеток и тесты на выживаемость проводили на протяжении 72 часов хранения. Образцы клеток метили зондом 2-Di-1-ASP в определенные сроки. Интенсивность флуоресценции измеряли по отдельным клеткам, рассчитывали средние значения флуоресценции и число миелокариоцитов на образец. Митотические индексы определяли как в препаратах, окрашенных по Романовскому-Гимза, так и после окраски флуоресцентным зондом. Для статистической обработки использовали методы кластерного анализа и стандартные непараметрические тесты. Результаты: Выживаемость ККМ в течение 0-5 часов составляла 80-92% (по тесту с трипановым синим), затем постепенно снижалась до 70-75% к концу сроков хранения. Далее, 3-часовая инкубация ККМ сопровождалась повышением интенсивности флуоресценции (F̃) с данным зондом, главным образом, из-за нарастания доли ярко светящейся субпопуляции (>100 усл.ед., N<sub>F>100</sub>,%). Повышение значений D (отношения N<sub>F>100</sub>,% через 3 часа хранения к N<sub>F>100</sub>,% в исходных пробах) оказалось наиболее информативным, что позволяет прогнозировать специфические величины F̃ на более поздних сроках. Повышение F̃ по сравнению с исходными значениями в этот срок отмечалось во всех образцах ККМ за счет ярко светящихся форм (N<sub>F>100</sub>), что коррелировало с ростом числа клеток. Дополнительный кластерный анализ позволил классифицировать образцы ККМ на 3 подгруппы в связи сдостоверными различиями по значениям D и изменениям клеточности. В частности, значительное число митотических форм наблюдалось в популяциях ККМ псле 5-24 часов хранения, и они ярко окрашивались зондом 2-Di-1-ASP probe. В целом, мы выявили 0,60±0,10% метафазных клеток в исходный срок наблюдения. По мере хранения, частота митотических фигур повышалась до 1,4±0,1%, а, после 6-часовой инкубации с колхицином, митотический индекс возрастал до 1,8±0,1%, что указывало на хорошую степень сохранности пролиферирующей фракции клеток.<br>
<br>
В заключение, наши результаты показывают сохранение и даже усиление энергетической активности ККМ, выявленное с помощью потенциал-чувствительного зонда и хорошую выживаемость фракции пролиферирующих клеток при жестких условиях инкубации. В дальнейшем следует провести исследования соответствующих механизмов сохранения клеток костного мозга и их энергетического баланса в данных условиях хранения.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Костный мозг является основным источником гемопоэтических стволовых клеток при клинической трансплантации. Качество трансплантатов костного мозга – ключевой фактор их успешного приживления. Целью нашей работы была проверка полуколичественной методики определения жизнеспособности при хранении в «голодной» среде с помощью флуоресцентного потенциал-чувствительного зонда 2-Di-1-ASP. Мы исследовали 20 образцов клеток костного мозга (ККМ), которые помещали в стандартный стабилизирующий раствор с цитратом натрия, лимонной кислотой, солями фосфатов, декстрозой и аденином. Подсчет клеток и тесты на выживаемость проводили на протяжении 72 часов хранения. Образцы клеток метили зондом 2-Di-1-ASP в определенные сроки. Интенсивность флуоресценции измеряли по отдельным клеткам, рассчитывали средние значения флуоресценции и число миелокариоцитов на образец. Митотические индексы определяли как в препаратах, окрашенных по Романовскому-Гимза, так и после окраски флуоресцентным зондом. Для статистической обработки использовали методы кластерного анализа и стандартные непараметрические тесты. Результаты: Выживаемость ККМ в течение 0-5 часов составляла 80-92% (по тесту с трипановым синим), затем постепенно снижалась до 70-75% к концу сроков хранения. Далее, 3-часовая инкубация ККМ сопровождалась повышением интенсивности флуоресценции (F̃) с данным зондом, главным образом, из-за нарастания доли ярко светящейся субпопуляции (>100 усл.ед., NF>100,%). Повышение значений D (отношения NF>100,% через 3 часа хранения к NF>100,% в исходных пробах) оказалось наиболее информативным, что позволяет прогнозировать специфические величины F̃ на более поздних сроках. Повышение F̃ по сравнению с исходными значениями в этот срок отмечалось во всех образцах ККМ за счет ярко светящихся форм (NF>100), что коррелировало с ростом числа клеток. Дополнительный кластерный анализ позволил классифицировать образцы ККМ на 3 подгруппы в связи сдостоверными различиями по значениям D и изменениям клеточности. В частности, значительное число митотических форм наблюдалось в популяциях ККМ псле 5-24 часов хранения, и они ярко окрашивались зондом 2-Di-1-ASP probe. В целом, мы выявили 0,60±0,10% метафазных клеток в исходный срок наблюдения. По мере хранения, частота митотических фигур повышалась до 1,4±0,1%, а, после 6-часовой инкубации с колхицином, митотический индекс возрастал до 1,8±0,1%, что указывало на хорошую степень сохранности пролиферирующей фракции клеток.
В заключение, наши результаты показывают сохранение и даже усиление энергетической активности ККМ, выявленное с помощью потенциал-чувствительного зонда и хорошую выживаемость фракции пролиферирующих клеток при жестких условиях инкубации. В дальнейшем следует провести исследования соответствующих механизмов сохранения клеток костного мозга и их энергетического баланса в данных условиях хранения.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6794
[VALUE] => 10.18620/1866-8836-2016-5-2-60-66
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/1866-8836-2016-5-2-60-66
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6795
[VALUE] => Array
(
[TEXT] => <p class="Autor">
Tatyana V.Parkhomenko,
Oleg V.Galibin,
Elena V.Verbitskaya,
Vladimir V. Tomson
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Tatyana V.Parkhomenko,
Oleg V.Galibin,
Elena V.Verbitskaya,
Vladimir V. Tomson
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6796
[VALUE] => Array
(
[TEXT] => R.M. Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantion, Research Center, First St.Petersburg<br>
State I.P.Pavlov Medical University, Russian Ministry of Health Care, St.Petersburg, Russia<br>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => R.M. Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantion, Research Center, First St.Petersburg
State I.P.Pavlov Medical University, Russian Ministry of Health Care, St.Petersburg, Russia
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 6797
[VALUE] => Array
(
[TEXT] => <p>
Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.<br>
We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation.
</p>
<h2>Results</h2>
<p>
Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, N<sub>F>100</sub>,%). D (ratio of N<sub>F>100</sub> at 3h storage to N<sub>F>100</sub> initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (N<sub>F>100</sub>), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.<br>
In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.
We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation.
Results
Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, NF>100,%). D (ratio of NF>100 at 3h storage to NF>100 initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (NF>100), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.
In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6798
[VALUE] => Evaluation of energy potential of fresh and stored bone marrow cells using a fluorescent potential-sensitive probe
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Evaluation of energy potential of fresh and stored bone marrow cells using a fluorescent potential-sensitive probe
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6938
[VALUE] => 211
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 211
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6939
[VALUE] => 219
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 219
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Tatyana V.Parkhomenko, Oleg V.Galibin, Elena V.Verbitskaya, Vladimir V. Tomson
State I.P.Pavlov Medical University, Russian Ministry of Health Care, St.Petersburg, Russia
Bone marrow is a primary source of hematopoietic stem cells in clinical transplantation. Quality of bone marrow grafts is a key factor of their successful in vivo expansion. The aim of our work was to test a semi-quantitative technique for assessment of bone marrow cell viability under strict storage conditions, by means of a fluorescent membrane potential-sensitive 2-Di-1-ASP probe.
We have studied 20 samples of normal bone marrow cells (BMC). The cells were placed in a standard storage solution with sodium citrate, citric acid; phosphate salts, dextrose and adenine. Cell counts and viability tests were performed up to 72 hours of incubation. The samples were labeled with 2-Di-1-ASP probe at specified terms. Fluorescence intensity was measured for single nucleated cells, followed by calculating mean fluorescence values and myelokaryocyte numbers. Mitotic indexes were determined both in Giemsa-stained and fluorescent probe-stained cells. Cluster analysis and non-parametric tests were used for statistical evaluation.
Results
Initial cell survival of 80-92% was shown at 3…5 hours of storage, then decreased to 70-75% by the end of incubation. Meanwhile, cell incubation for 3 hours was accompanied by increased fluorescence, in terms of F̃ values, mainly, due to higher proportion of “bright” cell population (>100 arb.units, NF>100,%). D (ratio of NF>100 at 3h storage to NF>100 initial) proved to be the most informative parameter, thus enabling us to predict sample- specific differences for the F̃ values at later terms. All BMC samples exhibited increased F̃, on the account of brighter cell population (NF>100), over 3 hours of incubation. This increase correlated with increase in myelokaryocyte counts. An additional cluster analysis allowed us to classify the BMC samples into 3 sub-groups, by their significant inter-group differences for D values and cell number changes. In particular, a number of mitotic cells were detected in BMC populations at 5 to 24 hours of incubation, showing bright stainability with 2-Di-1- ASP probe. We revealed 0.60±0.10% of metaphase cells at initial time point. After 5-h storage, the frequency of mitotic cells increased to 1.4±0.1%; and, after 6-h colchicine treatment, the mitotic index increased to: 1.8±0.1%, thus showing good preservation of dividing cell fraction.
In summary, our results have shown sustained, and even increased energy activity using a potential-sensitive probe, and good survival of mitotic cell fraction under strict incubation conditions. Appropriate mechanistic studies of the bone marrow cell preservation and energy balance under the given storage conditions should be performed in future.
Experimental article
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 6940
[1] => 6941
[2] => 6942
[3] => 6943
[4] => 6944
[5] => 6945
)
[VALUE] => Array
(
[0] => 98
[1] => 473
[2] => 475
[3] => 477
[4] => 478
[5] => 476
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 98
[1] => 473
[2] => 475
[3] => 477
[4] => 478
[5] => 476
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
)
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 5822
[VALUE] => 06/15/2016 04:21:00 pm
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 06/15/2016 04:21:00 pm
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 5823
[VALUE] => 06/24/2016 04:21:00 pm
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 06/24/2016 04:21:00 pm
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => 5826
[VALUE] => 97
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 97
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 6946
)
[VALUE] => Array
(
[0] => 97
)
[DESCRIPTION] => Array
(
[0] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 97
)
[~DESCRIPTION] => Array
(
[0] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 5960
[VALUE] => Array
(
[TEXT] => <p class="Autor">Джоэл К. Гловер</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Джоэл К. Гловер
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 5961
[VALUE] => Array
(
[TEXT] => Отдел молекулярной медицины, Институт фундаментальных медицинских наук, Университет Осло, Норвегия
Норвежский центр исследования стволовых клеток, Университетский госпиталь Осло, Норвегия
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Отдел молекулярной медицины, Институт фундаментальных медицинских наук, Университет Осло, Норвегия
Норвежский центр исследования стволовых клеток, Университетский госпиталь Осло, Норвегия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 5835
[VALUE] => Array
(
[TEXT] => Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении.
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Статья касается различных стратегий размножения стволовых клеток и их потомства в ходе моделирования новых органов и тканей, в т.ч. – искусственных биосовместимых матриц, естественных бескле- точных матриц, а также создания сложных тканей непосредственно из стволовых клеток и элементов матрикса с применением биореактора или трехмерного печатания (принтинга). В целом, культивирование клеток в 3D-системе позволяет поддерживать полноценные морфологические и функциональные свойства специализированных клеток, как, например, гепатоцитов. В частности, обещающим подходом к созданию трехмерных органных структур с помощью специальных биопринтеров для приготовления основы тканей. Соответствующие исследования привели к производству органоподобных клеточных комплексов, как, например, почечных структур. Есть некоторые технические проблемы, которые следует рассматривать для каждого отдельного случая, включая тип принтера, выбор типа биоматрикса, параметры печатания и т.д. Технологии микроэкструзии и лазер-индуцированного переноса считаются перспективными в этом плане. Естественные субстраты для тканевых и органных «костяков» можно получить путем удаления клеток и последующего посева клеток, как уже показано в экспериментах на животных. Производство 3D-моделей может создать условия для исследовании гемопоэза в его естественном микроокружении.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 5824
[VALUE] => 10.18620/1866-8836-2016-5-2-8-11
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/1866-8836-2016-5-2-8-11
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 5828
[VALUE] => Array
(
[TEXT] => <p class="Autor">Joel C. Glover</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Joel C. Glover
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 5829
[VALUE] => Array
(
[TEXT] => Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo Norwegian Center for Stem Cell Research, Oslo University Hospital Professor, Institute of Basic Medical Sciences, University of Oslo Medical Faculty
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo Norwegian Center for Stem Cell Research, Oslo University Hospital Professor, Institute of Basic Medical Sciences, University of Oslo Medical Faculty
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 5903
[VALUE] => Array
(
[TEXT] => The article considers different strategies for seeding stem cells and their progeny and construction of new tissues and organs, i.e., artificial biocompatible templates, natural decellularized templates, and generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing. Generally, culturing cells under 3D conditions allows to retain fully morphological and functional integrity of specialized cells as shown with hepatocytes. In particular, a promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds. Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures. There are some technical issues which should be considered in any single case, including type of printing technology, choice of biomatrix type, printing parameters, etc.<br>
<br>
Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.<br>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => The article considers different strategies for seeding stem cells and their progeny and construction of new tissues and organs, i.e., artificial biocompatible templates, natural decellularized templates, and generating complex tissues directly from stem cells and matrix materials using bioreactors or 3D-printing. Generally, culturing cells under 3D conditions allows to retain fully morphological and functional integrity of specialized cells as shown with hepatocytes. In particular, a promising approach to 3D organ fabrication is to use special bioprinters to prepare tissue scaffolds. Relevant studies have resulted in the production of organ-like cellular complexes, for example tubular/glomerular kidney structures. There are some technical issues which should be considered in any single case, including type of printing technology, choice of biomatrix type, printing parameters, etc.
Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 5825
[VALUE] => 3D bioprinting applications for in vitro modeling of cellular interactions and tissues
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 3D bioprinting applications for in vitro modeling of cellular interactions and tissues
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6268
[VALUE] => 161
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 161
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 6260
[VALUE] => 213
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 213
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Joel C. Glover
Microextrusion tenchique and laser-induced forward transfer (LIFT) approach are considered as prospective printing technologies. Natural substrates for tissue and organ scaffolds could be obtained by decellularization and subsequent cell seeding, as already shown in animal experiments. Generating 3D tissue models could create promising opportunities for hematopoiesis research in its natural microenvironment.

