Introduction
The only curative treatment for chronic myeloid leukemia (CML) was previously allogenous hemopoietic cell transplantation (HCT) [1]. With the introduction of the tyrosine kinase inhibitor (TKI) imatinib into CML management 15 years ago and the stunning response and survival results, treatment strategy of CML has profoundly changed. TKI became the first line treatment of choice for CML.
Long term survival
Meanwhile several long-term observational and randomized studies have matured and 10-year survival outcomes are available. An overview is shown in Table 1. 5-year survival ranges around 90%, 10-year survival around 83% and 10-year relative survival compared to the general population is more than 90% [2, 3]. Similar results have been observed in population based registries [4, 5, 6]. More patients died of comorbidities than of CML [7].
Deep molecular responses are achieved in up to 80% aft er 5 to 10 years (Figure 1) suggesting that treatment discontinuation should be possible in these patients [8].


First-line treatment
Current fi rst-line options with TKI are shown in Table 2. Whereas imatinib has been proven to be safe both at 400 and the faster acting 800 mg daily even aft er prolonged periods of time, the also faster acting 2nd generation (2G-)TKI require risk assessment due to rare but serious, potentially life threatening adverse drug reactions. As seen from Fig. 2, no survival advantage has been observed with any treatment option [3, 16, 17]. The lower progression rate to blast crisis observed with 2G-TKI is off set by more deaths due to adverse drug reactions. Variables to be considered in choosing first-line therapy are:
– Risk score;
– Cytogenetics (major-route ACA at diagnosis, high-risk
ACA in the course of CML);
– Comorbidities;
– Costs.
The impact of karyotype at diagnosis was demonstrated by Fabarius et al. [18]. Patients with major route ACA which occur in 1-2% of cases at diagnosis have a much poorer prognosis. Comorbidities do not infl uence progression of CML, but impact survival more than CML. Generic imatinib has become available recently. It decreases treatment costs at equal effi cacy and adds to the advantages of matinib over 2G-TKI.

Second-line therapy
2nd-line therapy is needed in cases of refractoriness to imatinib. Table 3 summarizes comparative efficacy and safety of 2nd-line treatment options. The variables to be considered for second line therapy are:
– Response milestones (Table 4);
– Adherence to therapy;
– Resistance mutations (Table 5);
– Clonal evolution;
– Intolerance;
– Drug safety;
– Health care setting.
The criteria for assessing TKI-response were proposed by the European LeukemiaNet (ELN) for newly diagnosed CML [13] and are depicted in Table 4. Before changing treatment due to resistance, non-adherence to drug-treatment has to be excluded. Non-adherence has been reported as the most frequent reason for treatment failure [19].
When changing treatment due to confi rmed resistance a mutation analysis should be initiated. This can be done simultaneously with changing to the new drug. If the new drug still does not work, the mutation analysis will give a rational basis for selecting the right drug. Table 5 lists the most important mutations and there sensitivity to the currently available TKI.
Adverse TKI reactions have recently been reviewed on behalf of ELN by [20]. Table 6 gives an overview over the most frequently observed adverse TKI reactions.





CML Studies IIIA and IV
CML study IIIA is a geneticly randomized study comparing allogenous HCT with best available drug treatment. It recruited 662 patients, randomized 427 eligible patients (family donor available vs not available) and was published after a median observation time of 12.1 years [15]. Th e key result was equivalence of outcome for low risk patients after transplantion, if performed within one year of diagnosis, and imatinib.
CML study IV is a randomized 5-arm treatment optimization study to explore whether treatment with imatinib 400mg can be improved by doubling the dose, combining imatinib with cytarabine or interferon α (IFN) or applying imatinib aft er IFN failure. 1551 newly diagnosed patients in chronic phase where recruited and the study published after a median observation time of 9.5 years [3]. The key outcome was no superiority of survival of any treatment option (Fig. 2) in spite of signifi cantly faster responses with imatinib 800 mg and the recognition of determinants of survival independent of treatment by multivariate analysis (Table 7).

A comparison of long-term survival aft er HCT or drug treatment showed that low risk patients had similar survival with both options (Fig. 3) [22, 23].

After progression to blast crisis HCT did not provide a significant survival advantage (Fig. 4), although long-term observations of 699 blast crises from the German CML studies showed that most long-term survivors (72%) were patients who received a transplant [24].

Conclusion
• Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients.
• Survival is independent of time to response.
• Outcome of CML is currently more determined by disease and patients’ factors e.g. comorbidities and smoking, and by center effects than by initial treatment selection.
• In low risk patients survival aft er imatinib and transplantation may be similar.
• Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival.
• The 10-year deep molecular remission rates of 70%–80% indicate that the majority of imatinib treated patients are candidates for treatment discontinuation.
Conflict of interest
The author has no conflicts of interest to declare.
References
1. Goldman JM. Chronic myeloid leukemia: reversing the chronic phase. J Clin Oncol. 2010;28(3):363-365.
2. Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, Hoff mann VS, Castagnetti F, Hasford J, Hehlmann R, Simonsson B. Prognosis of long-term survival considering disease-specifi c death in patients with chronic myeloid leukemia. Leukemia. 2016;30(1):48-56.
3. Hehlmann R, Lauseker M, Saußele S, Pfi rrmann M, Krause S, Kolb HJ, Neubauer A, Hossfeld DK, Nerl C, Gratwohl A, Baerlocher GM, Heim D, Brümmendorf TH, Fabarius A, Haferlach C, Schlegelberger B, Müller MC, Jeromin S, Proetel U, Kohlbrenner K, Voskanyan A, Rinaldetti S, Seifarth W, Spieß B, Balleisen L, Goebeler MC, Hänel M, Ho A, Dengler J, Falge C, Kanz L, Kremers S, Burchert A, Kneba M, Stegelmann F, Köhne CA, Lindemann HW, Waller CF, Pfreundschuh M, Spiekermann K, Berdel WE, Müller L, Edinger M, Mayer J, Beelen DW, Bentz M, Link H, Hertenstein B, Fuchs R, Wernli M, Schlegel F, Schlag R, de Wit M, Trümper L, Hebart H, Hahn M, Thomalla J, Scheid C, Schafhausen P, Verbeek W, Eckart MJ, Gassmann W, Pezzutto A, Schenk M, Brossart P, Geer T, Bildat S, Schäfer E, Hochhaus A, Hasford J. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31(11):2398-2406.
4. Höglund M, Sandin F, Hellström K, Björeman M, Björkholm M, Brune M, Dreimane A, Ekblom M, Lehmann S, Ljungman P, Malm C, Markevärn B, Myhr-Eriksson K, Ohm L, Olsson-Strömberg U, Själander A, Wadenvik H, Simonsson B, Stenke L, Richter J. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood. 2013;122(7):1284-1292.
5. Thielen N, Visser O, Ossenkoppele G, Janssen J. Chronic myeloid leukemia in the Netherlands: a population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur J Haematol. 2016;97(2):145-154. 6. Bower H, Björkholm M, Dickmann P, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with CML approaches the life expectancy of the general population. J Clin Oncol 2016;34(24): 2851-2858.
7. Saussele S, Krauss MP, Hehlmann R, Lauseker M, Proetel U, Kalmanti L, Hanfstein B, Fabarius A, Kraemer D, Berdel WE, Bentz M, Staib P, de Wit M, Wernli M, Zettl F, Hebart HF, Hahn M, Heymanns J, Schmidt-Wolf I, Schmitz N, Eckart MJ, Gassmann W, Bartholomäus A, Pezzutto A, Leibundgut EO, Heim D, Krause SW, Burchert A, Hofmann WK, Hasford J, Hochhaus A, Pfirrmann M, Müller MC; Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung and the German CML Study Group. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood 2015;126(1):42-49.
8. Kalmanti L, Saussele S, Lauseker M, Müller MC, Dietz CT, Heinrich L, Hanfstein B, Proetel U, Fabarius A, Krause SW, Rinaldetti S, Dengler J, Falge C, Oppliger-Leibundgut E, Burchert A, Neubauer A, Kanz L, Stegelmann F, Pfreundschuh M, Spiekermann K, Scheid C, Pfi rrmann M, Hochhaus A, Hasford J, Hehlmann R. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV.Leukemia. 2015; 29(5):1123-1132.
9. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O’Brien SG, Druker BJ; IRIS Investigators. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376(10):917-927.
10. Palandri F, Castagnetti F, Alimena G, Testoni N, Breccia M, Luatti S, Rege-Cambrin G, Stagno F, Specchia G, Martino B, Levato L, Merante S, Liberati AM, Pane F, Saglio G, Alberti D, Martinelli G, Baccarani M, Rosti G. Th e long-term durability of cytogenetic responses in patients with accelerated phase chronic myeloid leukemia treated with imatinib 600 mg: the GIMEMA CML Working Party experience after a 7-year follow-up. Haematologica. 2009;94(2):205-12.
11. de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, Szydlo R, Olavarria E, Kaeda J, Goldman JM, Marin D. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358-3363.
12. Cervantes F, López-Garrido P, Montero MI, Jonte F, Martínez J, Hernández-Boluda JC, Calbacho M, Sureda A, Pérez-Rus G, Nieto JB, Pérez-López C, Román-Gómez J, González M, Pereira A, Colomer D. Early intervention during imatinib therapy in patients with newly diagnosed chronic- phase chronic myeloid leukemia: a study of the Spanish PETHEMA group. Haematologica. 2010 Aug;95(8):1317- 1324.
13. Baccarani M, Druker BJ, Branford S, Kim DW, Pane F, Mongay L, Mone M, Ortmann CE, Kantarjian HM, Radich JP, Hughes TP, Cortes JE, Guilhot F. Long-term response to imatinib is not aff ected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: fi nal update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99(5):616-624.
14. Jain P, Kantarjian H, Alattar ML, Jabbour E, Sasaki K, Nogueras Gonzalez G, Dellasala S, Pierce S, Verstovsek S, Wierda W, Borthakur G, Ravandi F, O’Brien S, Cortes J. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from fi ve clinical trials. Lancet Haematol. 2015;2(3):e118-28.
15. Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, Nagler A, Della Casa CM, Morra E, Abruzzese E, D’Emilio A, Stagno F, le Coutre P, Hurtado- Monroy R, Santini V, Martino B, Pane F, Piccin A, Giraldo P, Assouline S, Durosinmi MA, Leeksma O, Pogliani EM, Puttini M, Jang E, Reiff ers J, Piazza, Valsecchi MG, Kim DW. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553-561.
16. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre PD, Etienne G, Dorlhiac-Llacer PE, Clark RE, Flinn IW, Nakamae H, Donohue B, Deng W, Dalal D, Menssen HD, Kantarjian HM. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016 May;30(5):1044-1054.
17. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L, Bradley-Garelik B, Manos G, Hochhaus A.Final 5-Year Study Results of DASISION: Th e Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34(20):2333-2340.
18. Fabarius A, Kalmanti L, Dietz CT, Lauseker M, Rinaldetti S, Haferlach C, Göhring G, Schlegelberger B, Jotterand M, Hanfstein B, Seifarth W, Hänel M, Köhne CH, Lindemann HW, Berdel WE, Staib P, Müller MC, Proetel U, Balleisen L, Goebeler ME, Dengler J, Falge C, Kanz L, Burchert A, Kneba M, Stegelmann F, Pfreundschuh M, Waller CF, Spiekermann K, Brümmendorf TH, Edinger M, Hofmann WK, Pfi rrmann M, Hasford J, Krause S, Hochhaus A, Saußele S, Hehlmann R; SAKK and the German CML Study Group. Impact of unbalanced minor route versus major route karyotypes at diagnosis on prognosis of CML. Ann Hematol. 2015;94(12):2015-2024.
19. Noens L, Hensen M, Kucmin-Bemelmans I, Lofgren C, Gilloteau I, Vrijens B. Measurement of adherence to BCRABL inhibitor therapy in chronic myeloid leukemia: current situation and future challenges. Haematologica. 2014; 99(3):437-447.
20. Steegmann JL, Baccarani M, Breccia M, Casado LF, García-Gutiérrez V, Hochhaus A, Kim DW, Kim TD, Khoury HJ, Le Coutre P, Mayer J, Milojkovic D, Porkka K, Rea D, Rosti G, Saussele S, Hehlmann R, Clark RE. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia.Leukemia. 2016;30(8):1648-1671.
21. Eiring AM, Deininger MW. Individualizing kinase-targeted cancer therapy: the paradigm of chronic myeloid leukemia. Genome Biol. 2014 Sep 17;15(9):461. doi: 10.1186/ s13059-014-0461-8.
22. Gratwohl A, Pfi rrmann M, Zander A, Kröger N, Beelen D, Novotny J, Nerl C, Scheid C, Spiekermann K, Mayer J, Sayer HG, Falge C, Bunjes D, Döhner H, Ganser A, Schmidt-Wolf I, Schwerdtfeger R, Baurmann H, Kuse R, Schmitz N, Wehmeier A, Fischer JT, Ho AD, Wilhelm M, Goebeler ME, Lindemann HW, Bormann M, Hertenstein B, Schlimok G, Baerlocher GM, Aul C, Pfreundschuh M, Fabian M, Staib P, Edinger M, Schatz M, Fauser A, Arnold R, Kindler T, Wulf G, Rosselet A, Hellmann A, Schäfer E, Prümmer O, Schenk M, Hasford J, Heimpel H, Hossfeld DK, Kolb HJ, Büsche G, Haferlach C, Schnittger S, Müller MC, Reiter A, Berger U, Saußele S, Hochhaus A, Hehlmann R; SAKK; German CML Study Group. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016 Mar;30(3):562-569.
23. Saussele S, Lauseker M, Müller MC, Gratwohl A, Beelen D, Bunjes DW et al. Allogeneic hematopoietic stem cell transplantation (HSCT) in the imatinib-era: update on the survival outcome following allogeneic HSCT after imatinib failure; results of the German CML study IV. Blood. 2014;124 (ASH Meeting abstract No. 2567).
24. Hehlmann R, Saussele S. Treatment of chronic myeloid leukemia in blast crisis. Haematologica 2008; 93:1765-1769.
Introduction
The only curative treatment for chronic myeloid leukemia (CML) was previously allogenous hemopoietic cell transplantation (HCT) [1]. With the introduction of the tyrosine kinase inhibitor (TKI) imatinib into CML management 15 years ago and the stunning response and survival results, treatment strategy of CML has profoundly changed. TKI became the first line treatment of choice for CML.
Long term survival
Meanwhile several long-term observational and randomized studies have matured and 10-year survival outcomes are available. An overview is shown in Table 1. 5-year survival ranges around 90%, 10-year survival around 83% and 10-year relative survival compared to the general population is more than 90% [2, 3]. Similar results have been observed in population based registries [4, 5, 6]. More patients died of comorbidities than of CML [7].
Deep molecular responses are achieved in up to 80% aft er 5 to 10 years (Figure 1) suggesting that treatment discontinuation should be possible in these patients [8].


First-line treatment
Current fi rst-line options with TKI are shown in Table 2. Whereas imatinib has been proven to be safe both at 400 and the faster acting 800 mg daily even aft er prolonged periods of time, the also faster acting 2nd generation (2G-)TKI require risk assessment due to rare but serious, potentially life threatening adverse drug reactions. As seen from Fig. 2, no survival advantage has been observed with any treatment option [3, 16, 17]. The lower progression rate to blast crisis observed with 2G-TKI is off set by more deaths due to adverse drug reactions. Variables to be considered in choosing first-line therapy are:
– Risk score;
– Cytogenetics (major-route ACA at diagnosis, high-risk
ACA in the course of CML);
– Comorbidities;
– Costs.
The impact of karyotype at diagnosis was demonstrated by Fabarius et al. [18]. Patients with major route ACA which occur in 1-2% of cases at diagnosis have a much poorer prognosis. Comorbidities do not infl uence progression of CML, but impact survival more than CML. Generic imatinib has become available recently. It decreases treatment costs at equal effi cacy and adds to the advantages of matinib over 2G-TKI.

Second-line therapy
2nd-line therapy is needed in cases of refractoriness to imatinib. Table 3 summarizes comparative efficacy and safety of 2nd-line treatment options. The variables to be considered for second line therapy are:
– Response milestones (Table 4);
– Adherence to therapy;
– Resistance mutations (Table 5);
– Clonal evolution;
– Intolerance;
– Drug safety;
– Health care setting.
The criteria for assessing TKI-response were proposed by the European LeukemiaNet (ELN) for newly diagnosed CML [13] and are depicted in Table 4. Before changing treatment due to resistance, non-adherence to drug-treatment has to be excluded. Non-adherence has been reported as the most frequent reason for treatment failure [19].
When changing treatment due to confi rmed resistance a mutation analysis should be initiated. This can be done simultaneously with changing to the new drug. If the new drug still does not work, the mutation analysis will give a rational basis for selecting the right drug. Table 5 lists the most important mutations and there sensitivity to the currently available TKI.
Adverse TKI reactions have recently been reviewed on behalf of ELN by [20]. Table 6 gives an overview over the most frequently observed adverse TKI reactions.





CML Studies IIIA and IV
CML study IIIA is a geneticly randomized study comparing allogenous HCT with best available drug treatment. It recruited 662 patients, randomized 427 eligible patients (family donor available vs not available) and was published after a median observation time of 12.1 years [15]. Th e key result was equivalence of outcome for low risk patients after transplantion, if performed within one year of diagnosis, and imatinib.
CML study IV is a randomized 5-arm treatment optimization study to explore whether treatment with imatinib 400mg can be improved by doubling the dose, combining imatinib with cytarabine or interferon α (IFN) or applying imatinib aft er IFN failure. 1551 newly diagnosed patients in chronic phase where recruited and the study published after a median observation time of 9.5 years [3]. The key outcome was no superiority of survival of any treatment option (Fig. 2) in spite of signifi cantly faster responses with imatinib 800 mg and the recognition of determinants of survival independent of treatment by multivariate analysis (Table 7).

A comparison of long-term survival aft er HCT or drug treatment showed that low risk patients had similar survival with both options (Fig. 3) [22, 23].

After progression to blast crisis HCT did not provide a significant survival advantage (Fig. 4), although long-term observations of 699 blast crises from the German CML studies showed that most long-term survivors (72%) were patients who received a transplant [24].

Conclusion
• Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients.
• Survival is independent of time to response.
• Outcome of CML is currently more determined by disease and patients’ factors e.g. comorbidities and smoking, and by center effects than by initial treatment selection.
• In low risk patients survival aft er imatinib and transplantation may be similar.
• Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival.
• The 10-year deep molecular remission rates of 70%–80% indicate that the majority of imatinib treated patients are candidates for treatment discontinuation.
Conflict of interest
The author has no conflicts of interest to declare.
References
1. Goldman JM. Chronic myeloid leukemia: reversing the chronic phase. J Clin Oncol. 2010;28(3):363-365.
2. Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, Hoff mann VS, Castagnetti F, Hasford J, Hehlmann R, Simonsson B. Prognosis of long-term survival considering disease-specifi c death in patients with chronic myeloid leukemia. Leukemia. 2016;30(1):48-56.
3. Hehlmann R, Lauseker M, Saußele S, Pfi rrmann M, Krause S, Kolb HJ, Neubauer A, Hossfeld DK, Nerl C, Gratwohl A, Baerlocher GM, Heim D, Brümmendorf TH, Fabarius A, Haferlach C, Schlegelberger B, Müller MC, Jeromin S, Proetel U, Kohlbrenner K, Voskanyan A, Rinaldetti S, Seifarth W, Spieß B, Balleisen L, Goebeler MC, Hänel M, Ho A, Dengler J, Falge C, Kanz L, Kremers S, Burchert A, Kneba M, Stegelmann F, Köhne CA, Lindemann HW, Waller CF, Pfreundschuh M, Spiekermann K, Berdel WE, Müller L, Edinger M, Mayer J, Beelen DW, Bentz M, Link H, Hertenstein B, Fuchs R, Wernli M, Schlegel F, Schlag R, de Wit M, Trümper L, Hebart H, Hahn M, Thomalla J, Scheid C, Schafhausen P, Verbeek W, Eckart MJ, Gassmann W, Pezzutto A, Schenk M, Brossart P, Geer T, Bildat S, Schäfer E, Hochhaus A, Hasford J. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31(11):2398-2406.
4. Höglund M, Sandin F, Hellström K, Björeman M, Björkholm M, Brune M, Dreimane A, Ekblom M, Lehmann S, Ljungman P, Malm C, Markevärn B, Myhr-Eriksson K, Ohm L, Olsson-Strömberg U, Själander A, Wadenvik H, Simonsson B, Stenke L, Richter J. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood. 2013;122(7):1284-1292.
5. Thielen N, Visser O, Ossenkoppele G, Janssen J. Chronic myeloid leukemia in the Netherlands: a population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur J Haematol. 2016;97(2):145-154. 6. Bower H, Björkholm M, Dickmann P, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with CML approaches the life expectancy of the general population. J Clin Oncol 2016;34(24): 2851-2858.
7. Saussele S, Krauss MP, Hehlmann R, Lauseker M, Proetel U, Kalmanti L, Hanfstein B, Fabarius A, Kraemer D, Berdel WE, Bentz M, Staib P, de Wit M, Wernli M, Zettl F, Hebart HF, Hahn M, Heymanns J, Schmidt-Wolf I, Schmitz N, Eckart MJ, Gassmann W, Bartholomäus A, Pezzutto A, Leibundgut EO, Heim D, Krause SW, Burchert A, Hofmann WK, Hasford J, Hochhaus A, Pfirrmann M, Müller MC; Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung and the German CML Study Group. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood 2015;126(1):42-49.
8. Kalmanti L, Saussele S, Lauseker M, Müller MC, Dietz CT, Heinrich L, Hanfstein B, Proetel U, Fabarius A, Krause SW, Rinaldetti S, Dengler J, Falge C, Oppliger-Leibundgut E, Burchert A, Neubauer A, Kanz L, Stegelmann F, Pfreundschuh M, Spiekermann K, Scheid C, Pfi rrmann M, Hochhaus A, Hasford J, Hehlmann R. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV.Leukemia. 2015; 29(5):1123-1132.
9. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O’Brien SG, Druker BJ; IRIS Investigators. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376(10):917-927.
10. Palandri F, Castagnetti F, Alimena G, Testoni N, Breccia M, Luatti S, Rege-Cambrin G, Stagno F, Specchia G, Martino B, Levato L, Merante S, Liberati AM, Pane F, Saglio G, Alberti D, Martinelli G, Baccarani M, Rosti G. Th e long-term durability of cytogenetic responses in patients with accelerated phase chronic myeloid leukemia treated with imatinib 600 mg: the GIMEMA CML Working Party experience after a 7-year follow-up. Haematologica. 2009;94(2):205-12.
11. de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, Szydlo R, Olavarria E, Kaeda J, Goldman JM, Marin D. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358-3363.
12. Cervantes F, López-Garrido P, Montero MI, Jonte F, Martínez J, Hernández-Boluda JC, Calbacho M, Sureda A, Pérez-Rus G, Nieto JB, Pérez-López C, Román-Gómez J, González M, Pereira A, Colomer D. Early intervention during imatinib therapy in patients with newly diagnosed chronic- phase chronic myeloid leukemia: a study of the Spanish PETHEMA group. Haematologica. 2010 Aug;95(8):1317- 1324.
13. Baccarani M, Druker BJ, Branford S, Kim DW, Pane F, Mongay L, Mone M, Ortmann CE, Kantarjian HM, Radich JP, Hughes TP, Cortes JE, Guilhot F. Long-term response to imatinib is not aff ected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: fi nal update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99(5):616-624.
14. Jain P, Kantarjian H, Alattar ML, Jabbour E, Sasaki K, Nogueras Gonzalez G, Dellasala S, Pierce S, Verstovsek S, Wierda W, Borthakur G, Ravandi F, O’Brien S, Cortes J. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from fi ve clinical trials. Lancet Haematol. 2015;2(3):e118-28.
15. Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C, Nagler A, Della Casa CM, Morra E, Abruzzese E, D’Emilio A, Stagno F, le Coutre P, Hurtado- Monroy R, Santini V, Martino B, Pane F, Piccin A, Giraldo P, Assouline S, Durosinmi MA, Leeksma O, Pogliani EM, Puttini M, Jang E, Reiff ers J, Piazza, Valsecchi MG, Kim DW. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553-561.
16. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre PD, Etienne G, Dorlhiac-Llacer PE, Clark RE, Flinn IW, Nakamae H, Donohue B, Deng W, Dalal D, Menssen HD, Kantarjian HM. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016 May;30(5):1044-1054.
17. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L, Bradley-Garelik B, Manos G, Hochhaus A.Final 5-Year Study Results of DASISION: Th e Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J Clin Oncol. 2016;34(20):2333-2340.
18. Fabarius A, Kalmanti L, Dietz CT, Lauseker M, Rinaldetti S, Haferlach C, Göhring G, Schlegelberger B, Jotterand M, Hanfstein B, Seifarth W, Hänel M, Köhne CH, Lindemann HW, Berdel WE, Staib P, Müller MC, Proetel U, Balleisen L, Goebeler ME, Dengler J, Falge C, Kanz L, Burchert A, Kneba M, Stegelmann F, Pfreundschuh M, Waller CF, Spiekermann K, Brümmendorf TH, Edinger M, Hofmann WK, Pfi rrmann M, Hasford J, Krause S, Hochhaus A, Saußele S, Hehlmann R; SAKK and the German CML Study Group. Impact of unbalanced minor route versus major route karyotypes at diagnosis on prognosis of CML. Ann Hematol. 2015;94(12):2015-2024.
19. Noens L, Hensen M, Kucmin-Bemelmans I, Lofgren C, Gilloteau I, Vrijens B. Measurement of adherence to BCRABL inhibitor therapy in chronic myeloid leukemia: current situation and future challenges. Haematologica. 2014; 99(3):437-447.
20. Steegmann JL, Baccarani M, Breccia M, Casado LF, García-Gutiérrez V, Hochhaus A, Kim DW, Kim TD, Khoury HJ, Le Coutre P, Mayer J, Milojkovic D, Porkka K, Rea D, Rosti G, Saussele S, Hehlmann R, Clark RE. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia.Leukemia. 2016;30(8):1648-1671.
21. Eiring AM, Deininger MW. Individualizing kinase-targeted cancer therapy: the paradigm of chronic myeloid leukemia. Genome Biol. 2014 Sep 17;15(9):461. doi: 10.1186/ s13059-014-0461-8.
22. Gratwohl A, Pfi rrmann M, Zander A, Kröger N, Beelen D, Novotny J, Nerl C, Scheid C, Spiekermann K, Mayer J, Sayer HG, Falge C, Bunjes D, Döhner H, Ganser A, Schmidt-Wolf I, Schwerdtfeger R, Baurmann H, Kuse R, Schmitz N, Wehmeier A, Fischer JT, Ho AD, Wilhelm M, Goebeler ME, Lindemann HW, Bormann M, Hertenstein B, Schlimok G, Baerlocher GM, Aul C, Pfreundschuh M, Fabian M, Staib P, Edinger M, Schatz M, Fauser A, Arnold R, Kindler T, Wulf G, Rosselet A, Hellmann A, Schäfer E, Prümmer O, Schenk M, Hasford J, Heimpel H, Hossfeld DK, Kolb HJ, Büsche G, Haferlach C, Schnittger S, Müller MC, Reiter A, Berger U, Saußele S, Hochhaus A, Hehlmann R; SAKK; German CML Study Group. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016 Mar;30(3):562-569.
23. Saussele S, Lauseker M, Müller MC, Gratwohl A, Beelen D, Bunjes DW et al. Allogeneic hematopoietic stem cell transplantation (HSCT) in the imatinib-era: update on the survival outcome following allogeneic HSCT after imatinib failure; results of the German CML study IV. Blood. 2014;124 (ASH Meeting abstract No. 2567).
24. Hehlmann R, Saussele S. Treatment of chronic myeloid leukemia in blast crisis. Haematologica 2008; 93:1765-1769.
Стратегия лечения хронического миелолейкоза (ХМЛ) сильно изменилась после внедрения иматиниба – ингибитора тирозинкиназы (ИТК). Эти препараты стали часто применяться для первой линии лечения при ХМЛ вместо аллогенной трансплантации гемопоэтических стволовых клеток (ТГСК). При выборе ИТК в качестве терапии первой линии учитывают следующие факторы: оценка риска для больного по принятой шкале, цитогенетические маркеры со значимыми дополнительными хромосомными аномалиями (ДХА) при постановке диагноза, а также ДХА высокого риска в процессе развития ХМЛ, сопутствующие заболевания, расходы на лечение. В случаях отсутствия ответа на иматиниб, рассматриваются возможности 2-й линии терапии, с учетом показателей клинического ответа на лечение, соблюдения режима лечения, мутаций, ведущих к лекарственной устойчивости, клональной эволюции лейкоза, непереносимости данного лечения, типа лечебного учреждения.
В программе CML IV, рандомизированном исследовании, направленном на оптимизацию дозы иматиниба и эффектов сочетанной терапии иматиниба с цитарабином или интерфероном α, участвовали 1551 свежевыявленных пациентов в хронической фазе ХМЛ. Основным результатом было отсутствие какого-либо преимущества любого из применявшихся методов лечения. Иматиниб в дозе 400 мг обеспечивает близкую к норме ожидаемую продолжительность жизни у больных с ХМЛ в хронической фазе. Выживаемость пациентов независима от времениответа. Клинические исходы ХМЛ теперь определяются скорее факторами заболевания и особенностями пациентов, например – сопутствующими заболеваниями и курением, а также подходом конкретных медицинских центров, нежели выбором тактики первичного лечения. Сравнение долгосрочной выживаемости после ТГСК или лечения иматинибом показало, что больные из группы низкого риска имели сходную выживаемость при обоих вариантах лечения. Попытки улучшения терапии, например, с применением ТГСК должны быть сосредоточены на группах рефрактерных пациентов, а также на показателях выживаемости, не связанных с ХМЛ. После прогрессии в бластный криз ТГСК не обеспечивает существенного преимущества в выживаемости, хотя специальное исследование показало, что наиболее долгоживущие пациенты (72%) были леченными посредством ТГСК. 70% – 80% частота 10-летней глубокой молекулярной ремиссии указывает на то, что большинство больных, леченых иматинибом, являются кандидатами на прекращение терапии.
Ключевые слова
Хронический миелоидный лейкоз, ингибиторы тирозинкиназы, иматиниб, стратегия лечения, трансплантация гемопоэтических клеток, выживаемость.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19979" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-13-20" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-13-20" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19981" ["VALUE"]=> array(2) { ["TEXT"]=> string(17) "Rüdiger Hehlmann" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(17) "Rüdiger Hehlmann" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19982" ["VALUE"]=> array(2) { ["TEXT"]=> string(47) "Mannheim Medical Faculty, Heidelberg University" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(47) "Mannheim Medical Faculty, Heidelberg University" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19983" ["VALUE"]=> array(2) { ["TEXT"]=> string(2303) "<p style="text-align: justify;"> With introduction of the tyrosine kinase inhibitor (TKI) imatinib, the treatment strategy of CML has profoundly changed. TKIs became the fi rst-line treatment of choice for CML competing with allogeneic hematopoietic stem cell transplantation (HCT). Variables to be considered in choosing TKIs for fi rst-line therapy are as follows: conventional risk score; cytogenetic fi ndings with majorroute additional chromosomal aberrations (ACA) at diagnosis, and high-risk ACA in the course of CML; comorbidities; treatment costs. In cases of refractoriness to imatinib, the 2nd line treatment options are: clinical response milestones; adherence to therapy; resistance mutations; clonal evolution; therapy intolerance; drug safety; health care setting.<br> CML Study IV, a randomized treatment study concerning imatinib dose optimization and combined therapy with imatinib and cytarabine or interferon α included 1551 newly diagnosed patients in chronic phase. The key outcome was no superiority of survival of any treatment option. Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients. Survival is independent of time to response. Outcome of CML is currently more determined by disease and patients’ factors, e.g., comorbidities and smoking, and by center eff ects than by initial treatment selection. A comparison of long-term survival aft er HCT or imatinib treatment showed that low risk patients had similar survival with both options. Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival. After progression to blast crisis, HCT did not provide a signifi cant survival advantage, although a special study showed that most long-term survivors (72%) were patients who received a transplant. The 10-year deep molecular remission rates of 70%-80% indicate that the majority of imatinib-treated patients are candidates for treatment discontinuation. </p> <h2 style="text-align: justify;">Keywords</h2> <p style="text-align: justify;"> Chronic myeloid leukemia, tyrosine kinase inhibitors, imatinib, treatment strategy, hematopoietic stem cell transplantation, survival. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2231) "
With introduction of the tyrosine kinase inhibitor (TKI) imatinib, the treatment strategy of CML has profoundly changed. TKIs became the fi rst-line treatment of choice for CML competing with allogeneic hematopoietic stem cell transplantation (HCT). Variables to be considered in choosing TKIs for fi rst-line therapy are as follows: conventional risk score; cytogenetic fi ndings with majorroute additional chromosomal aberrations (ACA) at diagnosis, and high-risk ACA in the course of CML; comorbidities; treatment costs. In cases of refractoriness to imatinib, the 2nd line treatment options are: clinical response milestones; adherence to therapy; resistance mutations; clonal evolution; therapy intolerance; drug safety; health care setting.
CML Study IV, a randomized treatment study concerning imatinib dose optimization and combined therapy with imatinib and cytarabine or interferon α included 1551 newly diagnosed patients in chronic phase. The key outcome was no superiority of survival of any treatment option. Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients. Survival is independent of time to response. Outcome of CML is currently more determined by disease and patients’ factors, e.g., comorbidities and smoking, and by center eff ects than by initial treatment selection. A comparison of long-term survival aft er HCT or imatinib treatment showed that low risk patients had similar survival with both options. Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival. After progression to blast crisis, HCT did not provide a signifi cant survival advantage, although a special study showed that most long-term survivors (72%) were patients who received a transplant. The 10-year deep molecular remission rates of 70%-80% indicate that the majority of imatinib-treated patients are candidates for treatment discontinuation.
Keywords
Chronic myeloid leukemia, tyrosine kinase inhibitors, imatinib, treatment strategy, hematopoietic stem cell transplantation, survival.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19980" ["VALUE"]=> string(137) "Long-term survival with CML with imatinib or transplantation as first-line treatment: Comparison of outcomes from CML Studies IIIA and IV" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(137) "Long-term survival with CML with imatinib or transplantation as first-line treatment: Comparison of outcomes from CML Studies IIIA and IV" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> &array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19984" ["VALUE"]=> string(4) "1065" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1065" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19985" ["VALUE"]=> string(4) "1066" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1066" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19981" ["VALUE"]=> array(2) { ["TEXT"]=> string(17) "Rüdiger Hehlmann" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(17) "Rüdiger Hehlmann" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(17) "Rüdiger Hehlmann" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19983" ["VALUE"]=> array(2) { ["TEXT"]=> string(2303) "<p style="text-align: justify;"> With introduction of the tyrosine kinase inhibitor (TKI) imatinib, the treatment strategy of CML has profoundly changed. TKIs became the fi rst-line treatment of choice for CML competing with allogeneic hematopoietic stem cell transplantation (HCT). Variables to be considered in choosing TKIs for fi rst-line therapy are as follows: conventional risk score; cytogenetic fi ndings with majorroute additional chromosomal aberrations (ACA) at diagnosis, and high-risk ACA in the course of CML; comorbidities; treatment costs. In cases of refractoriness to imatinib, the 2nd line treatment options are: clinical response milestones; adherence to therapy; resistance mutations; clonal evolution; therapy intolerance; drug safety; health care setting.<br> CML Study IV, a randomized treatment study concerning imatinib dose optimization and combined therapy with imatinib and cytarabine or interferon α included 1551 newly diagnosed patients in chronic phase. The key outcome was no superiority of survival of any treatment option. Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients. Survival is independent of time to response. Outcome of CML is currently more determined by disease and patients’ factors, e.g., comorbidities and smoking, and by center eff ects than by initial treatment selection. A comparison of long-term survival aft er HCT or imatinib treatment showed that low risk patients had similar survival with both options. Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival. After progression to blast crisis, HCT did not provide a signifi cant survival advantage, although a special study showed that most long-term survivors (72%) were patients who received a transplant. The 10-year deep molecular remission rates of 70%-80% indicate that the majority of imatinib-treated patients are candidates for treatment discontinuation. </p> <h2 style="text-align: justify;">Keywords</h2> <p style="text-align: justify;"> Chronic myeloid leukemia, tyrosine kinase inhibitors, imatinib, treatment strategy, hematopoietic stem cell transplantation, survival. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2231) "
With introduction of the tyrosine kinase inhibitor (TKI) imatinib, the treatment strategy of CML has profoundly changed. TKIs became the fi rst-line treatment of choice for CML competing with allogeneic hematopoietic stem cell transplantation (HCT). Variables to be considered in choosing TKIs for fi rst-line therapy are as follows: conventional risk score; cytogenetic fi ndings with majorroute additional chromosomal aberrations (ACA) at diagnosis, and high-risk ACA in the course of CML; comorbidities; treatment costs. In cases of refractoriness to imatinib, the 2nd line treatment options are: clinical response milestones; adherence to therapy; resistance mutations; clonal evolution; therapy intolerance; drug safety; health care setting.
CML Study IV, a randomized treatment study concerning imatinib dose optimization and combined therapy with imatinib and cytarabine or interferon α included 1551 newly diagnosed patients in chronic phase. The key outcome was no superiority of survival of any treatment option. Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients. Survival is independent of time to response. Outcome of CML is currently more determined by disease and patients’ factors, e.g., comorbidities and smoking, and by center eff ects than by initial treatment selection. A comparison of long-term survival aft er HCT or imatinib treatment showed that low risk patients had similar survival with both options. Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival. After progression to blast crisis, HCT did not provide a signifi cant survival advantage, although a special study showed that most long-term survivors (72%) were patients who received a transplant. The 10-year deep molecular remission rates of 70%-80% indicate that the majority of imatinib-treated patients are candidates for treatment discontinuation.
Keywords
Chronic myeloid leukemia, tyrosine kinase inhibitors, imatinib, treatment strategy, hematopoietic stem cell transplantation, survival.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2231) "
With introduction of the tyrosine kinase inhibitor (TKI) imatinib, the treatment strategy of CML has profoundly changed. TKIs became the fi rst-line treatment of choice for CML competing with allogeneic hematopoietic stem cell transplantation (HCT). Variables to be considered in choosing TKIs for fi rst-line therapy are as follows: conventional risk score; cytogenetic fi ndings with majorroute additional chromosomal aberrations (ACA) at diagnosis, and high-risk ACA in the course of CML; comorbidities; treatment costs. In cases of refractoriness to imatinib, the 2nd line treatment options are: clinical response milestones; adherence to therapy; resistance mutations; clonal evolution; therapy intolerance; drug safety; health care setting.
CML Study IV, a randomized treatment study concerning imatinib dose optimization and combined therapy with imatinib and cytarabine or interferon α included 1551 newly diagnosed patients in chronic phase. The key outcome was no superiority of survival of any treatment option. Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients. Survival is independent of time to response. Outcome of CML is currently more determined by disease and patients’ factors, e.g., comorbidities and smoking, and by center eff ects than by initial treatment selection. A comparison of long-term survival aft er HCT or imatinib treatment showed that low risk patients had similar survival with both options. Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival. After progression to blast crisis, HCT did not provide a signifi cant survival advantage, although a special study showed that most long-term survivors (72%) were patients who received a transplant. The 10-year deep molecular remission rates of 70%-80% indicate that the majority of imatinib-treated patients are candidates for treatment discontinuation.
Keywords
Chronic myeloid leukemia, tyrosine kinase inhibitors, imatinib, treatment strategy, hematopoietic stem cell transplantation, survival.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19979" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-13-20" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-13-20" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-13-20" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19980" ["VALUE"]=> string(137) "Long-term survival with CML with imatinib or transplantation as first-line treatment: Comparison of outcomes from CML Studies IIIA and IV" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(137) "Long-term survival with CML with imatinib or transplantation as first-line treatment: Comparison of outcomes from CML Studies IIIA and IV" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(137) "Long-term survival with CML with imatinib or transplantation as first-line treatment: Comparison of outcomes from CML Studies IIIA and IV" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19982" ["VALUE"]=> array(2) { ["TEXT"]=> string(47) "Mannheim Medical Faculty, Heidelberg University" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(47) "Mannheim Medical Faculty, Heidelberg University" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(47) "Mannheim Medical Faculty, Heidelberg University" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19976" ["VALUE"]=> array(2) { ["TEXT"]=> string(31) "Рюдигер Хельманн" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(31) "Рюдигер Хельманн" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(31) "Рюдигер Хельманн" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19978" ["VALUE"]=> array(2) { ["TEXT"]=> string(4891) "<p style="text-align: justify;"> Стратегия лечения хронического миелолейкоза (ХМЛ) сильно изменилась после внедрения иматиниба – ингибитора тирозинкиназы (ИТК). Эти препараты стали часто применяться для первой линии лечения при ХМЛ вместо аллогенной трансплантации гемопоэтических стволовых клеток (ТГСК). При выборе ИТК в качестве терапии первой линии учитывают следующие факторы: оценка риска для больного по принятой шкале, цитогенетические маркеры со значимыми дополнительными хромосомными аномалиями (ДХА) при постановке диагноза, а также ДХА высокого риска в процессе развития ХМЛ, сопутствующие заболевания, расходы на лечение. В случаях отсутствия ответа на иматиниб, рассматриваются возможности 2-й линии терапии, с учетом показателей клинического ответа на лечение, соблюдения режима лечения, мутаций, ведущих к лекарственной устойчивости, клональной эволюции лейкоза, непереносимости данного лечения, типа лечебного учреждения.<br> В программе CML IV, рандомизированном исследовании, направленном на оптимизацию дозы иматиниба и эффектов сочетанной терапии иматиниба с цитарабином или интерфероном α, участвовали 1551 свежевыявленных пациентов в хронической фазе ХМЛ. Основным результатом было отсутствие какого-либо преимущества любого из применявшихся методов лечения. Иматиниб в дозе 400 мг обеспечивает близкую к норме ожидаемую продолжительность жизни у больных с ХМЛ в хронической фазе. Выживаемость пациентов независима от времениответа. Клинические исходы ХМЛ теперь определяются скорее факторами заболевания и особенностями пациентов, например – сопутствующими заболеваниями и курением, а также подходом конкретных медицинских центров, нежели выбором тактики первичного лечения. Сравнение долгосрочной выживаемости после ТГСК или лечения иматинибом показало, что больные из группы низкого риска имели сходную выживаемость при обоих вариантах лечения. Попытки улучшения терапии, например, с применением ТГСК должны быть сосредоточены на группах рефрактерных пациентов, а также на показателях выживаемости, не связанных с ХМЛ. После прогрессии в бластный криз ТГСК не обеспечивает существенного преимущества в выживаемости, хотя специальное исследование показало, что наиболее долгоживущие пациенты (72%) были леченными посредством ТГСК. 70% – 80% частота 10-летней глубокой молекулярной ремиссии указывает на то, что большинство больных, леченых иматинибом, являются кандидатами на прекращение терапии. </p> <h2 style="text-align: justify;">Ключевые слова</h2> <p style="text-align: justify;"> Хронический миелоидный лейкоз, ингибиторы тирозинкиназы, иматиниб, стратегия лечения, трансплантация гемопоэтических клеток, выживаемость. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4819) "
Стратегия лечения хронического миелолейкоза (ХМЛ) сильно изменилась после внедрения иматиниба – ингибитора тирозинкиназы (ИТК). Эти препараты стали часто применяться для первой линии лечения при ХМЛ вместо аллогенной трансплантации гемопоэтических стволовых клеток (ТГСК). При выборе ИТК в качестве терапии первой линии учитывают следующие факторы: оценка риска для больного по принятой шкале, цитогенетические маркеры со значимыми дополнительными хромосомными аномалиями (ДХА) при постановке диагноза, а также ДХА высокого риска в процессе развития ХМЛ, сопутствующие заболевания, расходы на лечение. В случаях отсутствия ответа на иматиниб, рассматриваются возможности 2-й линии терапии, с учетом показателей клинического ответа на лечение, соблюдения режима лечения, мутаций, ведущих к лекарственной устойчивости, клональной эволюции лейкоза, непереносимости данного лечения, типа лечебного учреждения.
В программе CML IV, рандомизированном исследовании, направленном на оптимизацию дозы иматиниба и эффектов сочетанной терапии иматиниба с цитарабином или интерфероном α, участвовали 1551 свежевыявленных пациентов в хронической фазе ХМЛ. Основным результатом было отсутствие какого-либо преимущества любого из применявшихся методов лечения. Иматиниб в дозе 400 мг обеспечивает близкую к норме ожидаемую продолжительность жизни у больных с ХМЛ в хронической фазе. Выживаемость пациентов независима от времениответа. Клинические исходы ХМЛ теперь определяются скорее факторами заболевания и особенностями пациентов, например – сопутствующими заболеваниями и курением, а также подходом конкретных медицинских центров, нежели выбором тактики первичного лечения. Сравнение долгосрочной выживаемости после ТГСК или лечения иматинибом показало, что больные из группы низкого риска имели сходную выживаемость при обоих вариантах лечения. Попытки улучшения терапии, например, с применением ТГСК должны быть сосредоточены на группах рефрактерных пациентов, а также на показателях выживаемости, не связанных с ХМЛ. После прогрессии в бластный криз ТГСК не обеспечивает существенного преимущества в выживаемости, хотя специальное исследование показало, что наиболее долгоживущие пациенты (72%) были леченными посредством ТГСК. 70% – 80% частота 10-летней глубокой молекулярной ремиссии указывает на то, что большинство больных, леченых иматинибом, являются кандидатами на прекращение терапии.
Ключевые слова
Хронический миелоидный лейкоз, ингибиторы тирозинкиназы, иматиниб, стратегия лечения, трансплантация гемопоэтических клеток, выживаемость.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4819) "
Стратегия лечения хронического миелолейкоза (ХМЛ) сильно изменилась после внедрения иматиниба – ингибитора тирозинкиназы (ИТК). Эти препараты стали часто применяться для первой линии лечения при ХМЛ вместо аллогенной трансплантации гемопоэтических стволовых клеток (ТГСК). При выборе ИТК в качестве терапии первой линии учитывают следующие факторы: оценка риска для больного по принятой шкале, цитогенетические маркеры со значимыми дополнительными хромосомными аномалиями (ДХА) при постановке диагноза, а также ДХА высокого риска в процессе развития ХМЛ, сопутствующие заболевания, расходы на лечение. В случаях отсутствия ответа на иматиниб, рассматриваются возможности 2-й линии терапии, с учетом показателей клинического ответа на лечение, соблюдения режима лечения, мутаций, ведущих к лекарственной устойчивости, клональной эволюции лейкоза, непереносимости данного лечения, типа лечебного учреждения.
В программе CML IV, рандомизированном исследовании, направленном на оптимизацию дозы иматиниба и эффектов сочетанной терапии иматиниба с цитарабином или интерфероном α, участвовали 1551 свежевыявленных пациентов в хронической фазе ХМЛ. Основным результатом было отсутствие какого-либо преимущества любого из применявшихся методов лечения. Иматиниб в дозе 400 мг обеспечивает близкую к норме ожидаемую продолжительность жизни у больных с ХМЛ в хронической фазе. Выживаемость пациентов независима от времениответа. Клинические исходы ХМЛ теперь определяются скорее факторами заболевания и особенностями пациентов, например – сопутствующими заболеваниями и курением, а также подходом конкретных медицинских центров, нежели выбором тактики первичного лечения. Сравнение долгосрочной выживаемости после ТГСК или лечения иматинибом показало, что больные из группы низкого риска имели сходную выживаемость при обоих вариантах лечения. Попытки улучшения терапии, например, с применением ТГСК должны быть сосредоточены на группах рефрактерных пациентов, а также на показателях выживаемости, не связанных с ХМЛ. После прогрессии в бластный криз ТГСК не обеспечивает существенного преимущества в выживаемости, хотя специальное исследование показало, что наиболее долгоживущие пациенты (72%) были леченными посредством ТГСК. 70% – 80% частота 10-летней глубокой молекулярной ремиссии указывает на то, что большинство больных, леченых иматинибом, являются кандидатами на прекращение терапии.
Ключевые слова
Хронический миелоидный лейкоз, ингибиторы тирозинкиназы, иматиниб, стратегия лечения, трансплантация гемопоэтических клеток, выживаемость.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19977" ["VALUE"]=> array(2) { ["TEXT"]=> string(133) "Медицинский факультет Маннгейма, Гейдельбергский университет, Германия" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(133) "Медицинский факультет Маннгейма, Гейдельбергский университет, Германия" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(133) "Медицинский факультет Маннгейма, Гейдельбергский университет, Германия" } } } [1]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(2) "91" ["~IBLOCK_SECTION_ID"]=> string(2) "91" ["ID"]=> string(4) "1519" ["~ID"]=> string(4) "1519" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(183) "Трансплантация стволовых клеток при миелопролиферативных заболеваниях в эру молекулярной терапии" ["~NAME"]=> string(183) "Трансплантация стволовых клеток при миелопролиферативных заболеваниях в эру молекулярной терапии" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(19) "21.02.2018 01:35:52" ["~TIMESTAMP_X"]=> string(19) "21.02.2018 01:35:52" ["DETAIL_PAGE_URL"]=> string(142) "/ru/archive/tom-6-nomer-4/obzornye-stati/transplantatsiya-stvolovykh-kletok-pri-mieloproliferativnykh-zabolevaniyakh-v-eru-molekulyarnoy-tera/" ["~DETAIL_PAGE_URL"]=> string(142) "/ru/archive/tom-6-nomer-4/obzornye-stati/transplantatsiya-stvolovykh-kletok-pri-mieloproliferativnykh-zabolevaniyakh-v-eru-molekulyarnoy-tera/" ["LIST_PAGE_URL"]=> string(12) "/ru/archive/" ["~LIST_PAGE_URL"]=> string(12) "/ru/archive/" ["DETAIL_TEXT"]=> string(24304) "Introduction
At the present time, WHO Classifi cation of Myeloid Neoplasms includes a range of myeloproliferative Neoplasms (MPN). Of them, most common are: (1) chronic myeloid leukemia (CML, BCR-ABL1+); (2) chronic neutrophilic leukemia (CNL); (3) polycythemia vera (PV); (4) primary myelofi brosis (PMF); (5) essential thrombocythemia (ET), mastocytosis. A group of myelodysplastic/myeloproliferative
Neoplasms (MDS/MPN) includes chronic myelomonocytic leukemia (CMML); atypical CML (aCML, BCR-ABL1-); juvenile myelomonocytic leukemia (JMML). Some MDS/MPN cases remain unclassifiable.
According to EBMT statistics 2015, 39% of allo HSCTs are performed in acute myeloid leukemia (AML), and 15%, for MDS/MPN (8000 transplants per year) [1]. Only 2% of BMTs are made in chronic myeloid leukemia (CML), mostly, in advanced clinical forms, thus showing increasing efficiency of tyrosine kinase inhibitors (TKI) therapy in chronic phase CML. Th erefore, current challenges for allogeneic SCT in CML in the era of TK inhibitors are worth of discussion. The present review summarizes current strategies for treatment of different myeloid neoplasias.
Current strategies of CML treatment
Previously, allo-HSCT was the only curative treatment for CML, and CML was the most frequent indication for Allo SCT. With Imatinib, TKI became the frontline therapy for newly diagnosed CML. E.g. the IRIS Program “International Randomized Study of Interferon and STI571”, has demonstrated superiority of Imatinib versus interferon treatment in CML therapy [2]. Th erefore, transplant activities in this field dropped quickly, and allo-SCT moved from frontline to second, third-line therapy. In some special situations, the reasons for allo-SCT are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis, BC).
Efficiency of allogeneic HSCT (Allo SCT) in chronic myeloid leukemia in the Imatinib era was evaluated in a subgroup of the Randomized German CML Study IV. Appropriate survival probability was evaluated showing that the patients with elective allo-SCT in fi rst CP (n=20; group I) and those who underwent transplantation aft er Imatinib failure in first CP (n=36; group II) had a comparable 3-year survival probability of 88% and 94%, respectively (CI: 69.3-98.7 and 83.9-99.4). The patients who underwent transplantation in advanced disease (n=28; group III) had a 3-year survival probability of only 59% (CI: 38.6-77.5). Matched pair analysis for 53 patients who underwent transplantation compared to 106 matched Imatinib-treated patients also did not show any differences thus suggesting similar effi ciency of the both treatment strategies [3].
Management of CML blast crisis and TKI usage could be dependent on some proven facts [4]:
• TKI moderately improves OS median survival for <1 year.
• Choice of TKI should be directed by mutational profi le.
• Best prognosis is suggested in patients who achieve 2nd CP.
• In general, allo-SCT improved survival.
As shown by Hehlmann et al [4], the CML IV study with Imatinib treatment in patients with blast crisis CML has shown a survival of ca. 20% over 10 years, as compared to <5% survival in pre-Imatinib era. Distinct improvement in OS and event-free survival (EFS) was shown in BC CML patients when using combination of allo-SCT and TKIs compared to only TKIs. E.g., the median OS in the TKI+allo-HSCT group (20 months, 95% CI 1-74 months) was signifi cantly longer than that in the TKIs group (4.5 months; 95% CI 3.5-5.5 months). Similarly, the 4-year OS rates in the TKIs+allo-HSCT group were signifi cantly higher than those in the TKI group (50.0 vs 10.0%, P=0.016). (b) Th e median EFS in the TKIs+allo-HSCT group (18 months, 95% CI 1-72.7 months) was also signifi cantly longer than in the TKIs group (3 months; 95% CI 1.9-4.0 months). The 4-year EFS rates in the TKIs+allo-HSCT group were signifi cantly higher than those in the TKIs group (50.0 vs 10.0%, P=0.002) (Jiang H 2014). It should be noted that 2/3 of the donors were haploidentical.
Improved progression-free survival (up to 60%) was registered in the patients with advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors [5]. Th us, the donor type may infl uence survival for transplanted patients with advanced CML.
Primary Myelofibrosis
To take into account variable clinical course of a myeloproliferative disorder, the Lille scoring system provides some prognostic criteria, as mentioned:
• The “low” group (Hb-level > 10 g/dL and WBC between 4 and 30 /nL (median OS 93 months);
• The “intermediate” group (Hb-level < 10 g/dL or WBC > 30/< 4 nL (median OS 26 months);
• The “high” group (Hb-level < 10 g/dL and WBC < 4 /nL or > 30 /nL (median OS 13 months).
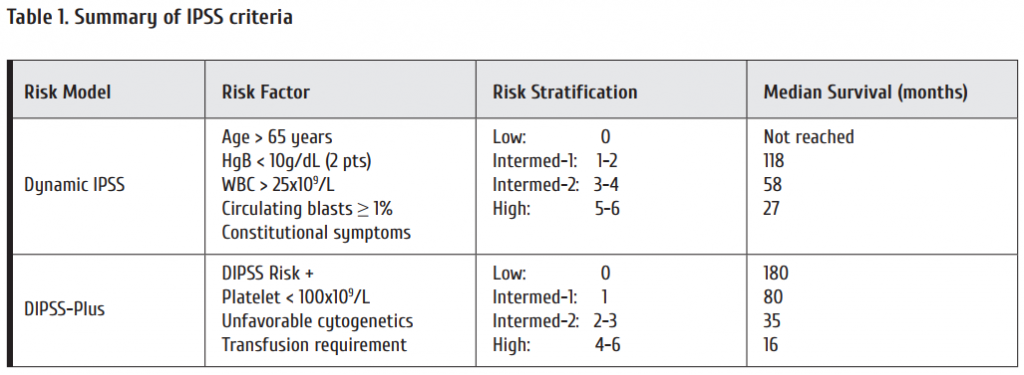
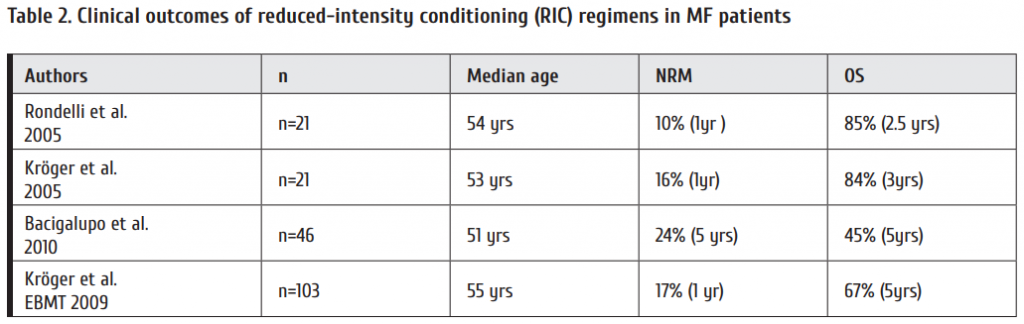
Reduced conditioning regimen when transplanting the MF patients yielded good results in terms of non-relapse-related mortality (NRM) and OS levels (Table 2).
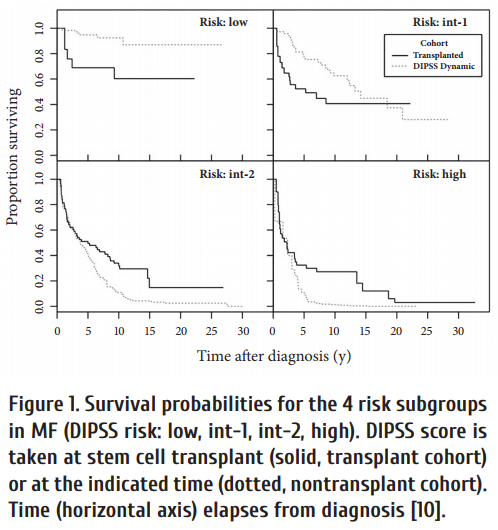
Efficiency of SCT proved to depend on initial risk score, e.g., assessed by DIPSS. As seen from Fig. 1, SCT in advanced disease prolongs survival in high-risk patients. Allogeneic SCT option for myelofi brosis with leukemic transformation was evaluated in EBMT study. Th is involved 1048 cases in allo SCT; 46 patients, with leukemic transformation [11]. The cumulative incidence of treatment-related mortality at 1 year was 28%, and of relapse at 3 years was 47%. The 3-year progression-free (PFS) and overall survival (OS) rates were 26% and 33%, respectively. Hence, allogeneic SCT can cure myelofi brosis patients transformed to leukemia.
Molecular genetic markers, i.e., multiple gene expression profile of leukemic cells may be predictive for the outcome in myelofi brosis patients aft er allogeneic transplantation, especially overexpression of JAK2 and ASXL1 gene, as shown by Kröger et al. [12].
Our previous studies concerned treatment of the myelofibrosis patients who relapsed aft er fi rst allo-SCT [13]. The following treatment consisted of either donor lymphocyte infusions, or second allo-SCT from an alternative donor. A group of 30 patients with relapsed myelofi brosis treated by donor lymphocyte infusions and/or second allo-SCT showed good 5-year overall survival rates. I.e., 2nd SCT caused complete remission in 14 out of 17 patients (82%).
Ruxolitinib, a JAK2 inhibitor, is a promising therapeutic option for MF patients during peritransplant period [14]. Feasibility and safety of Ruxolitinib treatment was tested in Hamburg-Eppendorf clinic and Ruxolitinib was used in 12 patients at a dose of 10+10 mg. Severe GVHD was observed only in 1 case of 12; overall survival was 100% after short follow-up (a mean of 163 days) [15].
Chronic Myelomonocytic Leukemia (CMML)
CMML is another chronic myeloproliferative disorder. Different prognostic scores for CMML were developed in MD Anderson, Mayo Clinics, a Düsseldorf scoring etc. CMML-specifi c prognostic scoring system was the subject to external validation being tested in 578 patients, as seen from Table 3.
Progression-free survival (PFS) in CMML was assessed by a group from the MD Anderson Cancer Center [17]. Th e study was performed in 83 CMML patients. In 78 cases a pre-transplant induction treatment was applied, with 37 patients receiving hypomethylating agents and cytotoxic chemotherapy in 41 cases. Patients treated with a hypomethylating agent had a lower cumulative incidence of relapse at 3 years post-transplant (22%) than those treated with other agents (35%; P=.03), improved progression-free survival (PFS), with no detectable diff erence in treatment-related mortality at 1 year post-HST. In summary, their data support the value of hypomethylating agents administered before allo-SCT, in order to achieve morphologic remission.
Results in CMML patients treated with allo-HSCT depend on the pre-transplant risk scores (HCT-specifi c CPSS), as shown by IBMTR group in 209 patients [16]. I.e., adjusted disease-free survival, starting at the time of transplant, at a median follow-up of 51 months. CPSS score, Karnofsky performance status, and graft source proved to be signifi cant predictors of survival. Th e patients with intermediate-2/high risk had a nearly 2-fold increased risk of death aft er relapse compared to those with low/intermediate-1 CPSS scores.
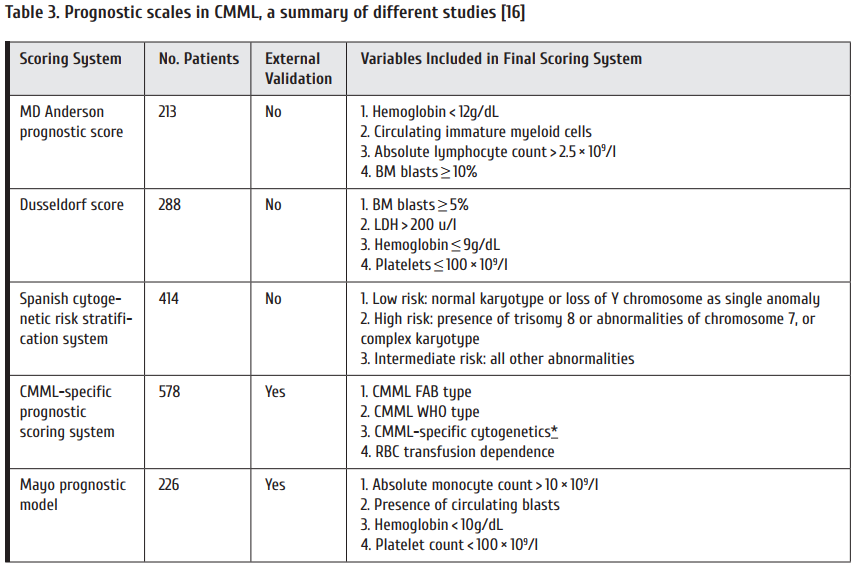
An EBMT study of relapse-free survival (left ) and overall survival (right) according to disease stage (CR versus no-CR) at transplantation was performed in a group of 513 CMML patients The subjects transplanted in CR had lower probability for non-relapse death (P=0·002) and longer relapse-free and OS (P=0·001 and P=0·005, respectively). In multivariate analysis the only signifi cant prognostic factor for survival was the presence of CR at transplantation (P= 0·005). Hence, patients transplanted in CR had signifi cantly longer relapse-free survival and significantly longer overall survival [18].
Moreover, both RFS and OS diff ered according to transplantation within 12 months or aft er 12 months aft er diagnosis of CMML. Early transplant was associated with significantly higher survival rates.
Systemic mastocytosis
Systemic mastocytosis (SM) is a myeloproliferative disorder with clonal expansion of mast cell precursors in various organs [19]. It is a heterogeneous group by the clinical course and malignant precursor biology: it may indolent do not shorten life expectancy. Advanced SM may proceed as Mast Cell Leukemia (MCL), SM with associated hematologic nonmast cell lineage diseases, or aggressive systemic mastocytosis (ASM). Survival rates from months to a few years despite cytoreductive therapy were not too high.
I.e., the 3-year OS and PFS in the total group of mastocytic malignancies are about 50-60% etc. [20]. Allo-SCT is a method of choice in advanced systemic mastocytosis. However, some somatic mutations predispose for inferior clinical outcome in this disorder, i.e., ASXL1 or CBL mutations (Fig. 2). Th ese molecular markers comprise a distinct risk factor when treating ASM [21].
Atypical chronic myeloid leukemia (aCML) with BCR-ABL1-negativity is considered a special clinical entity characterized by leukocytosis, aff ected granulocyte myeloid precursors, dysgranulopoiesis, PH-negativity, SETBP1 expression. Mutated RAS was found in some cases (7/20 [35%] vs 4/29 [14%]) and less JAK2p.V617F (3/42 [7%] vs 10/52 [19%]). Compared with unclassifi ed MDS/MPN-U, patients with aCML showed a significant inferior OS (12.4 months, 95% CI [9.0-16.1] vs 21.8 months, 95% CI [17.6-28.8]) and ACL-free survival (11.2 months, 95% CI [7.0-13.5] vs 18.9 months, 95% CI [12.3-26.3]), as reported by Wang et al. [22].
Age- and risk-score dependent survival in atypical CML were assessed as fi ve-year overall survival following allogeneic transplantation in 42 patients from an EBMT study [23]. As expected, the older age of aCML patients (<45 years) was associated with suffi ciently lower 5-year survival (<40%) as compared to younger patients.
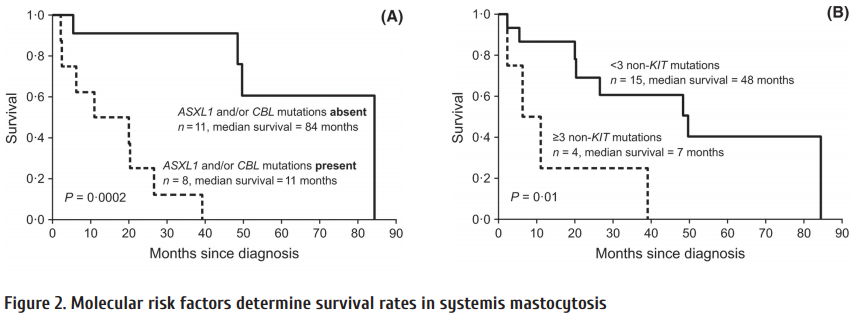
Summary
As based on clinical experience and current multicentric studies, some recommendations may be given on WHOM and WHEN to transplant, as follows:
In CML:
HSCT: in 1st complete remission – TKI failure, TKI intolerance, acceleration phase, blast crisis: TKI ± chemoinduction is performed
In Myelofi brosis:
HSCT at DIPPS INTERMED 2 and high risk scores at INTERMED 1 and with high risk features
In CMML:
Early HST, pre-treatment with hypomethylating agents
In Mastocytosis:
HSCT: in systemic mastocytosis, AHNMD (SM with associated non-mast cell)
in ASM (Aggressive Systemic Mastocytosis)
in MCL (Mast Cell Leukemia)
Conflict of interest
No conflict of interest is declared.
References
1. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, Gennery A, Kröger N, Kuball J, Lanza F, Montoto S, Nagler A, Snowden JA, Styczynski J, Mohty M. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017; 52(6):811-817.
2. Hughes TP, Hochhaus A, Branford S, Müller MC, Kaeda JS, Foroni L, Druker BJ, Guilhot F, Larson RA, O’Brien SG, Rudoltz MS, Mone M, Wehrle E, Modur V, Goldman JM, Radich JP; IRIS investigators. Long-term prognostic signifi cance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116(19):3758-3765.
3. Saussele S, Lauseker M, Gratwohl A, Beelen D W, Bujes D, Schwerdtfeger R, Kolb H-J, Ho A D, Falge C, Holler E, Schlimok G, Zander A, Arnold R, Kanz L, Dengler R, Haferlach C, Schlegelberger B, Pfi rrmann M, Müller M C, Schnittger S, Leitner A, Pletsch N, Hochhaus A, Hasford J, Hehlmann R. Blood. 2010; 115(10): 1880-1885.
4. Hehlmann R, Saußele S, Voskanyan A, Silver RT. Management of CML-blast crisis. Best Pract Res Clin Haematol. 2016; 29(3):295-307.
5. Kongtim P, Adekola K, Milton DR, Ramlal R, Jimenez A, Chen J, Rondon G, Ahmed S, Kebriaei P, Betul O, Hosing CM, Popat U, Khouri I, Jabbour E, Cortes JE, Kantarjian HM, Champlin RE, Ciurea SO. Donor type, in addition to transplantation in chronic phase and myeloablative conditioning, infl uence transplant survival for patients with advanced chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Leukemia. 2017; 31(7):1654-1657.
6. Rondelli D, Barosi G, Bacigalupo A, Prchal JT, Popat U, Alessandrino EP, Spivak JL, Smith BD, Klingemann HG, Fruchtman S, Hoff man R; Myeloproliferative Diseases-Research Consortium. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate-or high-risk patients with myelofi brosis with myeloid metaplasia. Blood. 2005 May 15; 105(10):4115-4119.
7. Kröger N, Zabelina T, Schieder H, Panse J, Ayuk F, Stute N, Fehse N, Waschke O, Fehse B, Kvasnicka HM, Th iele J, Zander A. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofi brosis. Br J Haematol. 2005; 128(5):690-697.
8. Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, Ibatici A, Raiola AM, Frassoni F, De Stefano F, Verdiani S, Casarino L, Barosi G. Allogeneic hemopoietic SCT for patients with primary myelofi brosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010; 45(3):458-463.
9. Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H, Nagler A, Bethge W, Stelljes M, Uharek L, Wandt H, Burchert A, Corradini P, Schubert J, Kaufmann M, Dreger P, Wulf GG, Einsele H, Zabelina T, Kvasnicka HM, Thiele J, Brand R, Zander AR, Niederwieser D, de Witte TM. Allogeneic stem cell transplantation aft er reduced-intensity conditioning in patients with myelofi brosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009; 114(26):5264-5270.
10. Kröger N, Giorgino T, Scott BL, Ditschkowski M, Alchalby H, Cervantes F, Vannucchi A, Cazzola M, Morra E, Zabelina T, Maffi oli M, Pereira A, Beelen D, Deeg HJ, Passamonti F. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofi brosis. Blood. 2015; 125(21):3347-3350.
11. Alchalby H, Zabelina T, Stübig T, van Biezen A, Bornhäuser M, Di Bartolomeo P, Beelen D, Cahn JY, Dreger P, Schroyens W, de Witte T, Olavarria E, Kröger N;Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Allogeneic stem cell transplantation for myelofi brosis with leukemic transformation: a study from the Myeloproliferative Neoplasm Subcommittee of the CMWP of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014; 20(2):279-281.
12. Kröger N, Panagiota V, Badbaran A, Zabelina T, Triviai I, Araujo Cruz MM, Shahswar R, Ayuk F, Gehlhaar M, Wolschke C, Bollin R, Walter C, Dugas M, Wiehlmann L, Lehmann U, Koenecke C, Chaturvedi A, Alchalby H, Stadler M, Eder M, Christopeit M, Göhring G, Koenigsmann M, Schlegelberger B, Kreipe HH, Ganser A, Stocking C, Fehse B, Th ol F, Heuser M. Impact of Molecular Genetics on Outcome in Myelofi brosis Patients aft er Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017; 23(7):1095-1101.
13. Klyuchnikov E, Holler E, Bornhäuser M, Kobbe G, Nagler A, Shimoni A, Könecke C, Wolschke C, Bacher U, Zander AR, Kröger N. Donor lymphocyte infusions and second transplantation as salvage treatment for relapsed myelofibrosis aft er reduced-intensity allograft ing. Br J Haematol. 2012; 159(2):172-181.
14. Gupta V, Gotlib J, Radich JP, Kröger NM, Rondelli D, Verstovsek S, Deeg HJ. Janus kinase inhibitors and allogeneic stem cell transplantation for myelofi brosis. Biol Blood Marrow Transplant. 2014; 20(9):1274-1281.
15. Kröger N, Kadir S S S A, Zabelina T, Wolschke C, Ayuk F A, Badbaran A, Christopeit M. Ruxolitinib during peritransplant period for myelofrbrosis patients undergoing allogeneic stem cell transplantation reduces acute graft -versushost disease. Blood. 2016; 128: 2242.
16. Liu HD, Ahn KW, Hu ZH, Hamadani M, Nishihori T, Wirk B, Beitinjaneh A, Rizzieri D, Grunwald MR, Sabloff M, Olsson RF, Bajel A, Bredeson C, Daly A, Inamoto Y, Majhail N, Saad A, Gupta V, Gerds A, Malone A, Tallman M, Reshef R, Marks DI, Copelan E, Gergis U, Savoie ML, Ustun C, Litzow MR, Cahn JY, Kindwall-Keller T, Akpek G, Savani BN, Aljurf M, Rowe JM, Wiernik PH, Hsu JW, Cortes J, Kalaycio M, Maziarz R, Sobecks R, Popat U, Alyea E, Saber W. Allogeneic Hematopoietic Cell Transplantation for Adult Chronic Myelomonocytic Leukemia. Biol Blood Marrow Transplant. 2017; 23(5):767-775.
17. Kongtim P, Popat U, Jimenez A, Gaballa S, El Fakih R, Rondon G, Chen J, Bueso-Ramos C, Borthakur G, Pemmaraju N, Garcia-Manero G, Kantarjian H, Alousi A, Hosing C, Anderlini P, Khouri IF, Kebriaei P, Andersson BS, Oran B, Rezvani K, Marin D, Shpall EJ, Champlin RE, Ciurea SO. Treatment with hypomethylating agents before allogeneic stem cell transplant improves progression-free survival for patients with chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2016; 22(1):47-53.
18. Symeonidis A, van Biezen A, de Wreede L, Piciocchi A, Finke J, Beelen D, Bornhäuser M, Cornelissen J, Volin L, Muft i G, Chalandon Y, Ganser A, Bruno B, Niederwieser D, Kobbe G, Schwerdtfeger R, de Witte T, Robin M, Kröger N; Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Br J Haematol. 2015 Jul 26. doi: 10.1111/bjh.13576.
19. Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, George TI, Kluin-Nelemans HC, Hermine O, Butterfi eld JH, Hägglund H, Ustun C, Hornick JL, Triggiani M, Radia D, Akin C, Hartmann K, Gotlib J, Schwartz LB, Verstovsek S, Orfao A, Metcalfe DD, Arock M, Horny HP. Refi ned diagnostic criteria and classifi cation of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014; 25(9):1691-700.
20. Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, Shanley R, Hogan WJ, Perales MA, Shore T, Baurmann H, Stuart R, Gruhn B, Doubek M, Hsu JW, Th olouli E, Gromke T, Godley LA, Pagano L, Gilman A, Wagner EM, Shwayder T, Bornhäuser M, Papadopoulos EB, Böhm A, Vercellotti G, Van Lint MT, Schmid C, Rabitsch W, Pullarkat V, Legrand F, Yakoub-Agha I, Saber W, Barrett J, Hermine O, Hagglund H, Sperr WR, Popat U, Alyea EP, Devine S, Deeg HJ, Weisdorf D, Akin C, Valent P. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014; 32(29):3264-3274.
21. Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfi rrmann M, Sotlar K, Horny H-P, Metzgeroth G, Kluger S, Naumann N, Haferlach C, Haferlach T, Valent P, Hofmann W-K, Fabarius A, Cross N C P, Reiter A. Chronic myleproliferative neoplasms, Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816v+ advanced systemic Mastocytosis. Leukemia. 2016; 30: 136-143.
22. Wang SA, Hasserjian RP, Fox PS, Rogers HJ, Geyer JT, Chabot-Richards D, Weinzierl E, Hatem J, Jaso J, Kanagal-Shamanna R, Stingo FC, Patel KP, Mehrotra M, Bueso-Ramos C, Young KH, Dinardo CD, Verstovsek S, Tiu RV, Bagg A, Hsi ED, Arber DA, Foucar K, Luthra R, Orazi A. Atypical chronic myeloid leukemia is clinically distinct from unclassifi able myelodysplastic/myeloproliferative neoplasms. Blood. 2014; 123(17): 2645-2651.
23. Onida F, de Wreede LC, van Biezen A, Eikema DJ, Byrne JL, Iori AP, Schots R, Jungova A, Schetelig J, Finke J, Veelken H, Johansson JE, Craddock C, Stelljes M, Theobald M, Holler E, Schanz U, Schaap N, Bittenbring J, Olavarria E, Chalandon Y, Kröger N. Allogeneic stem cell transplantation in patients with atypical chronic myeloid leukaemia: a retrospective study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol. 2017; 177(5):759-765.
Introduction
At the present time, WHO Classifi cation of Myeloid Neoplasms includes a range of myeloproliferative Neoplasms (MPN). Of them, most common are: (1) chronic myeloid leukemia (CML, BCR-ABL1+); (2) chronic neutrophilic leukemia (CNL); (3) polycythemia vera (PV); (4) primary myelofi brosis (PMF); (5) essential thrombocythemia (ET), mastocytosis. A group of myelodysplastic/myeloproliferative
Neoplasms (MDS/MPN) includes chronic myelomonocytic leukemia (CMML); atypical CML (aCML, BCR-ABL1-); juvenile myelomonocytic leukemia (JMML). Some MDS/MPN cases remain unclassifiable.
According to EBMT statistics 2015, 39% of allo HSCTs are performed in acute myeloid leukemia (AML), and 15%, for MDS/MPN (8000 transplants per year) [1]. Only 2% of BMTs are made in chronic myeloid leukemia (CML), mostly, in advanced clinical forms, thus showing increasing efficiency of tyrosine kinase inhibitors (TKI) therapy in chronic phase CML. Th erefore, current challenges for allogeneic SCT in CML in the era of TK inhibitors are worth of discussion. The present review summarizes current strategies for treatment of different myeloid neoplasias.
Current strategies of CML treatment
Previously, allo-HSCT was the only curative treatment for CML, and CML was the most frequent indication for Allo SCT. With Imatinib, TKI became the frontline therapy for newly diagnosed CML. E.g. the IRIS Program “International Randomized Study of Interferon and STI571”, has demonstrated superiority of Imatinib versus interferon treatment in CML therapy [2]. Th erefore, transplant activities in this field dropped quickly, and allo-SCT moved from frontline to second, third-line therapy. In some special situations, the reasons for allo-SCT are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis, BC).
Efficiency of allogeneic HSCT (Allo SCT) in chronic myeloid leukemia in the Imatinib era was evaluated in a subgroup of the Randomized German CML Study IV. Appropriate survival probability was evaluated showing that the patients with elective allo-SCT in fi rst CP (n=20; group I) and those who underwent transplantation aft er Imatinib failure in first CP (n=36; group II) had a comparable 3-year survival probability of 88% and 94%, respectively (CI: 69.3-98.7 and 83.9-99.4). The patients who underwent transplantation in advanced disease (n=28; group III) had a 3-year survival probability of only 59% (CI: 38.6-77.5). Matched pair analysis for 53 patients who underwent transplantation compared to 106 matched Imatinib-treated patients also did not show any differences thus suggesting similar effi ciency of the both treatment strategies [3].
Management of CML blast crisis and TKI usage could be dependent on some proven facts [4]:
• TKI moderately improves OS median survival for <1 year.
• Choice of TKI should be directed by mutational profi le.
• Best prognosis is suggested in patients who achieve 2nd CP.
• In general, allo-SCT improved survival.
As shown by Hehlmann et al [4], the CML IV study with Imatinib treatment in patients with blast crisis CML has shown a survival of ca. 20% over 10 years, as compared to <5% survival in pre-Imatinib era. Distinct improvement in OS and event-free survival (EFS) was shown in BC CML patients when using combination of allo-SCT and TKIs compared to only TKIs. E.g., the median OS in the TKI+allo-HSCT group (20 months, 95% CI 1-74 months) was signifi cantly longer than that in the TKIs group (4.5 months; 95% CI 3.5-5.5 months). Similarly, the 4-year OS rates in the TKIs+allo-HSCT group were signifi cantly higher than those in the TKI group (50.0 vs 10.0%, P=0.016). (b) Th e median EFS in the TKIs+allo-HSCT group (18 months, 95% CI 1-72.7 months) was also signifi cantly longer than in the TKIs group (3 months; 95% CI 1.9-4.0 months). The 4-year EFS rates in the TKIs+allo-HSCT group were signifi cantly higher than those in the TKIs group (50.0 vs 10.0%, P=0.002) (Jiang H 2014). It should be noted that 2/3 of the donors were haploidentical.
Improved progression-free survival (up to 60%) was registered in the patients with advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors [5]. Th us, the donor type may infl uence survival for transplanted patients with advanced CML.
Primary Myelofibrosis
To take into account variable clinical course of a myeloproliferative disorder, the Lille scoring system provides some prognostic criteria, as mentioned:
• The “low” group (Hb-level > 10 g/dL and WBC between 4 and 30 /nL (median OS 93 months);
• The “intermediate” group (Hb-level < 10 g/dL or WBC > 30/< 4 nL (median OS 26 months);
• The “high” group (Hb-level < 10 g/dL and WBC < 4 /nL or > 30 /nL (median OS 13 months).


Reduced conditioning regimen when transplanting the MF patients yielded good results in terms of non-relapse-related mortality (NRM) and OS levels (Table 2).

Efficiency of SCT proved to depend on initial risk score, e.g., assessed by DIPSS. As seen from Fig. 1, SCT in advanced disease prolongs survival in high-risk patients. Allogeneic SCT option for myelofi brosis with leukemic transformation was evaluated in EBMT study. Th is involved 1048 cases in allo SCT; 46 patients, with leukemic transformation [11]. The cumulative incidence of treatment-related mortality at 1 year was 28%, and of relapse at 3 years was 47%. The 3-year progression-free (PFS) and overall survival (OS) rates were 26% and 33%, respectively. Hence, allogeneic SCT can cure myelofi brosis patients transformed to leukemia.
Molecular genetic markers, i.e., multiple gene expression profile of leukemic cells may be predictive for the outcome in myelofi brosis patients aft er allogeneic transplantation, especially overexpression of JAK2 and ASXL1 gene, as shown by Kröger et al. [12].
Our previous studies concerned treatment of the myelofibrosis patients who relapsed aft er fi rst allo-SCT [13]. The following treatment consisted of either donor lymphocyte infusions, or second allo-SCT from an alternative donor. A group of 30 patients with relapsed myelofi brosis treated by donor lymphocyte infusions and/or second allo-SCT showed good 5-year overall survival rates. I.e., 2nd SCT caused complete remission in 14 out of 17 patients (82%).
Ruxolitinib, a JAK2 inhibitor, is a promising therapeutic option for MF patients during peritransplant period [14]. Feasibility and safety of Ruxolitinib treatment was tested in Hamburg-Eppendorf clinic and Ruxolitinib was used in 12 patients at a dose of 10+10 mg. Severe GVHD was observed only in 1 case of 12; overall survival was 100% after short follow-up (a mean of 163 days) [15].
Chronic Myelomonocytic Leukemia (CMML)
CMML is another chronic myeloproliferative disorder. Different prognostic scores for CMML were developed in MD Anderson, Mayo Clinics, a Düsseldorf scoring etc. CMML-specifi c prognostic scoring system was the subject to external validation being tested in 578 patients, as seen from Table 3.
Progression-free survival (PFS) in CMML was assessed by a group from the MD Anderson Cancer Center [17]. Th e study was performed in 83 CMML patients. In 78 cases a pre-transplant induction treatment was applied, with 37 patients receiving hypomethylating agents and cytotoxic chemotherapy in 41 cases. Patients treated with a hypomethylating agent had a lower cumulative incidence of relapse at 3 years post-transplant (22%) than those treated with other agents (35%; P=.03), improved progression-free survival (PFS), with no detectable diff erence in treatment-related mortality at 1 year post-HST. In summary, their data support the value of hypomethylating agents administered before allo-SCT, in order to achieve morphologic remission.
Results in CMML patients treated with allo-HSCT depend on the pre-transplant risk scores (HCT-specifi c CPSS), as shown by IBMTR group in 209 patients [16]. I.e., adjusted disease-free survival, starting at the time of transplant, at a median follow-up of 51 months. CPSS score, Karnofsky performance status, and graft source proved to be signifi cant predictors of survival. Th e patients with intermediate-2/high risk had a nearly 2-fold increased risk of death aft er relapse compared to those with low/intermediate-1 CPSS scores.

An EBMT study of relapse-free survival (left ) and overall survival (right) according to disease stage (CR versus no-CR) at transplantation was performed in a group of 513 CMML patients The subjects transplanted in CR had lower probability for non-relapse death (P=0·002) and longer relapse-free and OS (P=0·001 and P=0·005, respectively). In multivariate analysis the only signifi cant prognostic factor for survival was the presence of CR at transplantation (P= 0·005). Hence, patients transplanted in CR had signifi cantly longer relapse-free survival and significantly longer overall survival [18].
Moreover, both RFS and OS diff ered according to transplantation within 12 months or aft er 12 months aft er diagnosis of CMML. Early transplant was associated with significantly higher survival rates.
Systemic mastocytosis
Systemic mastocytosis (SM) is a myeloproliferative disorder with clonal expansion of mast cell precursors in various organs [19]. It is a heterogeneous group by the clinical course and malignant precursor biology: it may indolent do not shorten life expectancy. Advanced SM may proceed as Mast Cell Leukemia (MCL), SM with associated hematologic nonmast cell lineage diseases, or aggressive systemic mastocytosis (ASM). Survival rates from months to a few years despite cytoreductive therapy were not too high.
I.e., the 3-year OS and PFS in the total group of mastocytic malignancies are about 50-60% etc. [20]. Allo-SCT is a method of choice in advanced systemic mastocytosis. However, some somatic mutations predispose for inferior clinical outcome in this disorder, i.e., ASXL1 or CBL mutations (Fig. 2). Th ese molecular markers comprise a distinct risk factor when treating ASM [21].
Atypical chronic myeloid leukemia (aCML) with BCR-ABL1-negativity is considered a special clinical entity characterized by leukocytosis, aff ected granulocyte myeloid precursors, dysgranulopoiesis, PH-negativity, SETBP1 expression. Mutated RAS was found in some cases (7/20 [35%] vs 4/29 [14%]) and less JAK2p.V617F (3/42 [7%] vs 10/52 [19%]). Compared with unclassifi ed MDS/MPN-U, patients with aCML showed a significant inferior OS (12.4 months, 95% CI [9.0-16.1] vs 21.8 months, 95% CI [17.6-28.8]) and ACL-free survival (11.2 months, 95% CI [7.0-13.5] vs 18.9 months, 95% CI [12.3-26.3]), as reported by Wang et al. [22].
Age- and risk-score dependent survival in atypical CML were assessed as fi ve-year overall survival following allogeneic transplantation in 42 patients from an EBMT study [23]. As expected, the older age of aCML patients (<45 years) was associated with suffi ciently lower 5-year survival (<40%) as compared to younger patients.

Summary
As based on clinical experience and current multicentric studies, some recommendations may be given on WHOM and WHEN to transplant, as follows:
In CML:
HSCT: in 1st complete remission – TKI failure, TKI intolerance, acceleration phase, blast crisis: TKI ± chemoinduction is performed
In Myelofi brosis:
HSCT at DIPPS INTERMED 2 and high risk scores at INTERMED 1 and with high risk features
In CMML:
Early HST, pre-treatment with hypomethylating agents
In Mastocytosis:
HSCT: in systemic mastocytosis, AHNMD (SM with associated non-mast cell)
in ASM (Aggressive Systemic Mastocytosis)
in MCL (Mast Cell Leukemia)
Conflict of interest
No conflict of interest is declared.
References
1. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, Gennery A, Kröger N, Kuball J, Lanza F, Montoto S, Nagler A, Snowden JA, Styczynski J, Mohty M. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017; 52(6):811-817.
2. Hughes TP, Hochhaus A, Branford S, Müller MC, Kaeda JS, Foroni L, Druker BJ, Guilhot F, Larson RA, O’Brien SG, Rudoltz MS, Mone M, Wehrle E, Modur V, Goldman JM, Radich JP; IRIS investigators. Long-term prognostic signifi cance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116(19):3758-3765.
3. Saussele S, Lauseker M, Gratwohl A, Beelen D W, Bujes D, Schwerdtfeger R, Kolb H-J, Ho A D, Falge C, Holler E, Schlimok G, Zander A, Arnold R, Kanz L, Dengler R, Haferlach C, Schlegelberger B, Pfi rrmann M, Müller M C, Schnittger S, Leitner A, Pletsch N, Hochhaus A, Hasford J, Hehlmann R. Blood. 2010; 115(10): 1880-1885.
4. Hehlmann R, Saußele S, Voskanyan A, Silver RT. Management of CML-blast crisis. Best Pract Res Clin Haematol. 2016; 29(3):295-307.
5. Kongtim P, Adekola K, Milton DR, Ramlal R, Jimenez A, Chen J, Rondon G, Ahmed S, Kebriaei P, Betul O, Hosing CM, Popat U, Khouri I, Jabbour E, Cortes JE, Kantarjian HM, Champlin RE, Ciurea SO. Donor type, in addition to transplantation in chronic phase and myeloablative conditioning, infl uence transplant survival for patients with advanced chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Leukemia. 2017; 31(7):1654-1657.
6. Rondelli D, Barosi G, Bacigalupo A, Prchal JT, Popat U, Alessandrino EP, Spivak JL, Smith BD, Klingemann HG, Fruchtman S, Hoff man R; Myeloproliferative Diseases-Research Consortium. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate-or high-risk patients with myelofi brosis with myeloid metaplasia. Blood. 2005 May 15; 105(10):4115-4119.
7. Kröger N, Zabelina T, Schieder H, Panse J, Ayuk F, Stute N, Fehse N, Waschke O, Fehse B, Kvasnicka HM, Th iele J, Zander A. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofi brosis. Br J Haematol. 2005; 128(5):690-697.
8. Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, Ibatici A, Raiola AM, Frassoni F, De Stefano F, Verdiani S, Casarino L, Barosi G. Allogeneic hemopoietic SCT for patients with primary myelofi brosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010; 45(3):458-463.
9. Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H, Nagler A, Bethge W, Stelljes M, Uharek L, Wandt H, Burchert A, Corradini P, Schubert J, Kaufmann M, Dreger P, Wulf GG, Einsele H, Zabelina T, Kvasnicka HM, Thiele J, Brand R, Zander AR, Niederwieser D, de Witte TM. Allogeneic stem cell transplantation aft er reduced-intensity conditioning in patients with myelofi brosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009; 114(26):5264-5270.
10. Kröger N, Giorgino T, Scott BL, Ditschkowski M, Alchalby H, Cervantes F, Vannucchi A, Cazzola M, Morra E, Zabelina T, Maffi oli M, Pereira A, Beelen D, Deeg HJ, Passamonti F. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofi brosis. Blood. 2015; 125(21):3347-3350.
11. Alchalby H, Zabelina T, Stübig T, van Biezen A, Bornhäuser M, Di Bartolomeo P, Beelen D, Cahn JY, Dreger P, Schroyens W, de Witte T, Olavarria E, Kröger N;Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Allogeneic stem cell transplantation for myelofi brosis with leukemic transformation: a study from the Myeloproliferative Neoplasm Subcommittee of the CMWP of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014; 20(2):279-281.
12. Kröger N, Panagiota V, Badbaran A, Zabelina T, Triviai I, Araujo Cruz MM, Shahswar R, Ayuk F, Gehlhaar M, Wolschke C, Bollin R, Walter C, Dugas M, Wiehlmann L, Lehmann U, Koenecke C, Chaturvedi A, Alchalby H, Stadler M, Eder M, Christopeit M, Göhring G, Koenigsmann M, Schlegelberger B, Kreipe HH, Ganser A, Stocking C, Fehse B, Th ol F, Heuser M. Impact of Molecular Genetics on Outcome in Myelofi brosis Patients aft er Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017; 23(7):1095-1101.
13. Klyuchnikov E, Holler E, Bornhäuser M, Kobbe G, Nagler A, Shimoni A, Könecke C, Wolschke C, Bacher U, Zander AR, Kröger N. Donor lymphocyte infusions and second transplantation as salvage treatment for relapsed myelofibrosis aft er reduced-intensity allograft ing. Br J Haematol. 2012; 159(2):172-181.
14. Gupta V, Gotlib J, Radich JP, Kröger NM, Rondelli D, Verstovsek S, Deeg HJ. Janus kinase inhibitors and allogeneic stem cell transplantation for myelofi brosis. Biol Blood Marrow Transplant. 2014; 20(9):1274-1281.
15. Kröger N, Kadir S S S A, Zabelina T, Wolschke C, Ayuk F A, Badbaran A, Christopeit M. Ruxolitinib during peritransplant period for myelofrbrosis patients undergoing allogeneic stem cell transplantation reduces acute graft -versushost disease. Blood. 2016; 128: 2242.
16. Liu HD, Ahn KW, Hu ZH, Hamadani M, Nishihori T, Wirk B, Beitinjaneh A, Rizzieri D, Grunwald MR, Sabloff M, Olsson RF, Bajel A, Bredeson C, Daly A, Inamoto Y, Majhail N, Saad A, Gupta V, Gerds A, Malone A, Tallman M, Reshef R, Marks DI, Copelan E, Gergis U, Savoie ML, Ustun C, Litzow MR, Cahn JY, Kindwall-Keller T, Akpek G, Savani BN, Aljurf M, Rowe JM, Wiernik PH, Hsu JW, Cortes J, Kalaycio M, Maziarz R, Sobecks R, Popat U, Alyea E, Saber W. Allogeneic Hematopoietic Cell Transplantation for Adult Chronic Myelomonocytic Leukemia. Biol Blood Marrow Transplant. 2017; 23(5):767-775.
17. Kongtim P, Popat U, Jimenez A, Gaballa S, El Fakih R, Rondon G, Chen J, Bueso-Ramos C, Borthakur G, Pemmaraju N, Garcia-Manero G, Kantarjian H, Alousi A, Hosing C, Anderlini P, Khouri IF, Kebriaei P, Andersson BS, Oran B, Rezvani K, Marin D, Shpall EJ, Champlin RE, Ciurea SO. Treatment with hypomethylating agents before allogeneic stem cell transplant improves progression-free survival for patients with chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2016; 22(1):47-53.
18. Symeonidis A, van Biezen A, de Wreede L, Piciocchi A, Finke J, Beelen D, Bornhäuser M, Cornelissen J, Volin L, Muft i G, Chalandon Y, Ganser A, Bruno B, Niederwieser D, Kobbe G, Schwerdtfeger R, de Witte T, Robin M, Kröger N; Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Achievement of complete remission predicts outcome of allogeneic haematopoietic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Br J Haematol. 2015 Jul 26. doi: 10.1111/bjh.13576.
19. Valent P, Sotlar K, Sperr WR, Escribano L, Yavuz S, Reiter A, George TI, Kluin-Nelemans HC, Hermine O, Butterfi eld JH, Hägglund H, Ustun C, Hornick JL, Triggiani M, Radia D, Akin C, Hartmann K, Gotlib J, Schwartz LB, Verstovsek S, Orfao A, Metcalfe DD, Arock M, Horny HP. Refi ned diagnostic criteria and classifi cation of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014; 25(9):1691-700.
20. Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, Shanley R, Hogan WJ, Perales MA, Shore T, Baurmann H, Stuart R, Gruhn B, Doubek M, Hsu JW, Th olouli E, Gromke T, Godley LA, Pagano L, Gilman A, Wagner EM, Shwayder T, Bornhäuser M, Papadopoulos EB, Böhm A, Vercellotti G, Van Lint MT, Schmid C, Rabitsch W, Pullarkat V, Legrand F, Yakoub-Agha I, Saber W, Barrett J, Hermine O, Hagglund H, Sperr WR, Popat U, Alyea EP, Devine S, Deeg HJ, Weisdorf D, Akin C, Valent P. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014; 32(29):3264-3274.
21. Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfi rrmann M, Sotlar K, Horny H-P, Metzgeroth G, Kluger S, Naumann N, Haferlach C, Haferlach T, Valent P, Hofmann W-K, Fabarius A, Cross N C P, Reiter A. Chronic myleproliferative neoplasms, Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816v+ advanced systemic Mastocytosis. Leukemia. 2016; 30: 136-143.
22. Wang SA, Hasserjian RP, Fox PS, Rogers HJ, Geyer JT, Chabot-Richards D, Weinzierl E, Hatem J, Jaso J, Kanagal-Shamanna R, Stingo FC, Patel KP, Mehrotra M, Bueso-Ramos C, Young KH, Dinardo CD, Verstovsek S, Tiu RV, Bagg A, Hsi ED, Arber DA, Foucar K, Luthra R, Orazi A. Atypical chronic myeloid leukemia is clinically distinct from unclassifi able myelodysplastic/myeloproliferative neoplasms. Blood. 2014; 123(17): 2645-2651.
23. Onida F, de Wreede LC, van Biezen A, Eikema DJ, Byrne JL, Iori AP, Schots R, Jungova A, Schetelig J, Finke J, Veelken H, Johansson JE, Craddock C, Stelljes M, Theobald M, Holler E, Schanz U, Schaap N, Bittenbring J, Olavarria E, Chalandon Y, Kröger N. Allogeneic stem cell transplantation in patients with atypical chronic myeloid leukaemia: a retrospective study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol. 2017; 177(5):759-765.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19988" ["VALUE"]=> array(2) { ["TEXT"]=> string(108) "Онкологический Центр Хантсмана, Университет штата Юта, США" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(108) "Онкологический Центр Хантсмана, Университет штата Юта, США" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19989" ["VALUE"]=> array(2) { ["TEXT"]=> string(4153) "<p style="text-align: justify;"> Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.<br> При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.<br> Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза. </p> <h2 style="text-align: justify;">Ключевые слова</h2> <p style="text-align: justify;"> Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4075) "
Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.
При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.
Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза.
Ключевые слова
Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19990" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-21-27" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-21-27" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19991" ["VALUE"]=> array(2) { ["TEXT"]=> string(14) "Axel R. Zander" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(14) "Axel R. Zander" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19992" ["VALUE"]=> array(2) { ["TEXT"]=> string(50) "Huntsman Cancer Institute, University of Utah, USA" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(50) "Huntsman Cancer Institute, University of Utah, USA" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19997" ["VALUE"]=> array(2) { ["TEXT"]=> string(2079) "<p style="text-align: justify;"> A sufficient part of allo-HSCT is now performed for myelodysplastic syndromes and myeloproliferative neoplasms (MPN), whereas chronic myeloid leukemia is mostly treated by tyrosine kinase inhibitors (TKI). In some special situations, the reasons for allo-SCT in CML are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis). However, an improvement in progression-free survival may be obtained in advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors.<br> When planning therapy of primary myelofi brosis, one should take into account variable clinical course of the disease using, e.g., Lille scoring system which provides some prognostic criteria, molecular genetic markers, especially, overexpression of JAK2 gene thus allowing usage of ruxolitinib. Allo-SCT can cure myelofi brosis patients transformed to leukemia. In cases of relapse, a 2nd allo-HSCT or donor lymphocyte infusion may result into prolonged survival of the patients.<br> Results in chronic myelomonocytic leukemia patients treated with allo-HSCT depend on the pre-transplant risk scores. Patients transplanted in CR had signifi cantly longer relapse-free survival and signifi cantly longer overall survival. Early transplants were associated with higher survival rates. In cases of atypical CML, early allogeneic transplant should be performed. Allo-SCT is a method of choice in advanced systemic mastocytosis. It is performed in cases associated with non-mast cell involvement; in aggressive systemic mastocytosis, and in mast cell leukemia. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Myeloproliferative disorders, allogeneic hematopoietic stem cell transplantation, tyrosine kinase inhibitors, chronic myeloid leukemia, primary myelofi brosis, chronic myelomyelocytic leukemia, systemic mastocytosis. </p> <h2 style="text-align: justify;"></h2>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1989) "
A sufficient part of allo-HSCT is now performed for myelodysplastic syndromes and myeloproliferative neoplasms (MPN), whereas chronic myeloid leukemia is mostly treated by tyrosine kinase inhibitors (TKI). In some special situations, the reasons for allo-SCT in CML are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis). However, an improvement in progression-free survival may be obtained in advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors.
When planning therapy of primary myelofi brosis, one should take into account variable clinical course of the disease using, e.g., Lille scoring system which provides some prognostic criteria, molecular genetic markers, especially, overexpression of JAK2 gene thus allowing usage of ruxolitinib. Allo-SCT can cure myelofi brosis patients transformed to leukemia. In cases of relapse, a 2nd allo-HSCT or donor lymphocyte infusion may result into prolonged survival of the patients.
Results in chronic myelomonocytic leukemia patients treated with allo-HSCT depend on the pre-transplant risk scores. Patients transplanted in CR had signifi cantly longer relapse-free survival and signifi cantly longer overall survival. Early transplants were associated with higher survival rates. In cases of atypical CML, early allogeneic transplant should be performed. Allo-SCT is a method of choice in advanced systemic mastocytosis. It is performed in cases associated with non-mast cell involvement; in aggressive systemic mastocytosis, and in mast cell leukemia.
Keywords
Myeloproliferative disorders, allogeneic hematopoietic stem cell transplantation, tyrosine kinase inhibitors, chronic myeloid leukemia, primary myelofi brosis, chronic myelomyelocytic leukemia, systemic mastocytosis.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19993" ["VALUE"]=> string(89) "Stem cell transplantation for myeloproliferative diseases in the era of molecular therapy" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(89) "Stem cell transplantation for myeloproliferative diseases in the era of molecular therapy" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19994" ["VALUE"]=> string(4) "1067" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1067" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19995" ["VALUE"]=> string(4) "1068" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1068" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(9) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19991" ["VALUE"]=> array(2) { ["TEXT"]=> string(14) "Axel R. Zander" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(14) "Axel R. Zander" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(14) "Axel R. Zander" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19997" ["VALUE"]=> array(2) { ["TEXT"]=> string(2079) "<p style="text-align: justify;"> A sufficient part of allo-HSCT is now performed for myelodysplastic syndromes and myeloproliferative neoplasms (MPN), whereas chronic myeloid leukemia is mostly treated by tyrosine kinase inhibitors (TKI). In some special situations, the reasons for allo-SCT in CML are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis). However, an improvement in progression-free survival may be obtained in advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors.<br> When planning therapy of primary myelofi brosis, one should take into account variable clinical course of the disease using, e.g., Lille scoring system which provides some prognostic criteria, molecular genetic markers, especially, overexpression of JAK2 gene thus allowing usage of ruxolitinib. Allo-SCT can cure myelofi brosis patients transformed to leukemia. In cases of relapse, a 2nd allo-HSCT or donor lymphocyte infusion may result into prolonged survival of the patients.<br> Results in chronic myelomonocytic leukemia patients treated with allo-HSCT depend on the pre-transplant risk scores. Patients transplanted in CR had signifi cantly longer relapse-free survival and signifi cantly longer overall survival. Early transplants were associated with higher survival rates. In cases of atypical CML, early allogeneic transplant should be performed. Allo-SCT is a method of choice in advanced systemic mastocytosis. It is performed in cases associated with non-mast cell involvement; in aggressive systemic mastocytosis, and in mast cell leukemia. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Myeloproliferative disorders, allogeneic hematopoietic stem cell transplantation, tyrosine kinase inhibitors, chronic myeloid leukemia, primary myelofi brosis, chronic myelomyelocytic leukemia, systemic mastocytosis. </p> <h2 style="text-align: justify;"></h2>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1989) "
A sufficient part of allo-HSCT is now performed for myelodysplastic syndromes and myeloproliferative neoplasms (MPN), whereas chronic myeloid leukemia is mostly treated by tyrosine kinase inhibitors (TKI). In some special situations, the reasons for allo-SCT in CML are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis). However, an improvement in progression-free survival may be obtained in advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors.
When planning therapy of primary myelofi brosis, one should take into account variable clinical course of the disease using, e.g., Lille scoring system which provides some prognostic criteria, molecular genetic markers, especially, overexpression of JAK2 gene thus allowing usage of ruxolitinib. Allo-SCT can cure myelofi brosis patients transformed to leukemia. In cases of relapse, a 2nd allo-HSCT or donor lymphocyte infusion may result into prolonged survival of the patients.
Results in chronic myelomonocytic leukemia patients treated with allo-HSCT depend on the pre-transplant risk scores. Patients transplanted in CR had signifi cantly longer relapse-free survival and signifi cantly longer overall survival. Early transplants were associated with higher survival rates. In cases of atypical CML, early allogeneic transplant should be performed. Allo-SCT is a method of choice in advanced systemic mastocytosis. It is performed in cases associated with non-mast cell involvement; in aggressive systemic mastocytosis, and in mast cell leukemia.
Keywords
Myeloproliferative disorders, allogeneic hematopoietic stem cell transplantation, tyrosine kinase inhibitors, chronic myeloid leukemia, primary myelofi brosis, chronic myelomyelocytic leukemia, systemic mastocytosis.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1989) "
A sufficient part of allo-HSCT is now performed for myelodysplastic syndromes and myeloproliferative neoplasms (MPN), whereas chronic myeloid leukemia is mostly treated by tyrosine kinase inhibitors (TKI). In some special situations, the reasons for allo-SCT in CML are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis). However, an improvement in progression-free survival may be obtained in advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors.
When planning therapy of primary myelofi brosis, one should take into account variable clinical course of the disease using, e.g., Lille scoring system which provides some prognostic criteria, molecular genetic markers, especially, overexpression of JAK2 gene thus allowing usage of ruxolitinib. Allo-SCT can cure myelofi brosis patients transformed to leukemia. In cases of relapse, a 2nd allo-HSCT or donor lymphocyte infusion may result into prolonged survival of the patients.
Results in chronic myelomonocytic leukemia patients treated with allo-HSCT depend on the pre-transplant risk scores. Patients transplanted in CR had signifi cantly longer relapse-free survival and signifi cantly longer overall survival. Early transplants were associated with higher survival rates. In cases of atypical CML, early allogeneic transplant should be performed. Allo-SCT is a method of choice in advanced systemic mastocytosis. It is performed in cases associated with non-mast cell involvement; in aggressive systemic mastocytosis, and in mast cell leukemia.
Keywords
Myeloproliferative disorders, allogeneic hematopoietic stem cell transplantation, tyrosine kinase inhibitors, chronic myeloid leukemia, primary myelofi brosis, chronic myelomyelocytic leukemia, systemic mastocytosis.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19990" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-21-27" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-21-27" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-21-27" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19993" ["VALUE"]=> string(89) "Stem cell transplantation for myeloproliferative diseases in the era of molecular therapy" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(89) "Stem cell transplantation for myeloproliferative diseases in the era of molecular therapy" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(89) "Stem cell transplantation for myeloproliferative diseases in the era of molecular therapy" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19992" ["VALUE"]=> array(2) { ["TEXT"]=> string(50) "Huntsman Cancer Institute, University of Utah, USA" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(50) "Huntsman Cancer Institute, University of Utah, USA" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(50) "Huntsman Cancer Institute, University of Utah, USA" } ["AUTHORS"]=> array(38) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(1) { [0]=> string(5) "20020" } ["VALUE"]=> array(1) { [0]=> string(2) "11" } ["DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(1) { [0]=> string(2) "11" } ["~DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(56) "Axel R. Zander" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19987" ["VALUE"]=> array(2) { ["TEXT"]=> string(39) "Аксель Р. Цандер<br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(33) "Аксель Р. Цандер" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(33) "Аксель Р. Цандер
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19989" ["VALUE"]=> array(2) { ["TEXT"]=> string(4153) "<p style="text-align: justify;"> Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.<br> При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.<br> Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза. </p> <h2 style="text-align: justify;">Ключевые слова</h2> <p style="text-align: justify;"> Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4075) "
Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.
При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.
Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза.
Ключевые слова
Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4075) "
Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.
При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.
Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза.
Ключевые слова
Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19988" ["VALUE"]=> array(2) { ["TEXT"]=> string(108) "Онкологический Центр Хантсмана, Университет штата Юта, США" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(108) "Онкологический Центр Хантсмана, Университет штата Юта, США" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(108) "Онкологический Центр Хантсмана, Университет штата Юта, США" } } } [2]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(2) "91" ["~IBLOCK_SECTION_ID"]=> string(2) "91" ["ID"]=> string(4) "1520" ["~ID"]=> string(4) "1520" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(225) "Оппортунистическая микрофлора в необычных местах: маркерные патогены при тяжелом посттрансплантационном иммунодефиците" ["~NAME"]=> string(225) "Оппортунистическая микрофлора в необычных местах: маркерные патогены при тяжелом посттрансплантационном иммунодефиците" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(19) "21.02.2018 01:36:04" ["~TIMESTAMP_X"]=> string(19) "21.02.2018 01:36:04" ["DETAIL_PAGE_URL"]=> string(142) "/ru/archive/tom-6-nomer-4/obzornye-stati/opportunisticheskaya-mikroflora-v-neobychnykh-mestakh-markernye-patogeny-pri-tyazhelom-posttransplan/" ["~DETAIL_PAGE_URL"]=> string(142) "/ru/archive/tom-6-nomer-4/obzornye-stati/opportunisticheskaya-mikroflora-v-neobychnykh-mestakh-markernye-patogeny-pri-tyazhelom-posttransplan/" ["LIST_PAGE_URL"]=> string(12) "/ru/archive/" ["~LIST_PAGE_URL"]=> string(12) "/ru/archive/" ["DETAIL_TEXT"]=> string(54740) "Introduction
Early infections caused by opportunistic microorganisms are quite common aft er allo-HSCT, thus requiring massive and expensive anti-infectious therapy. Massive cytoreductive therapy in cancer is followed by pronounced neutropenia and exhaustion of most lymphoid cell populations [1]. Hence, a total defi ciency of immune response is evident since 1-2 weeks aft er conditioning therapy and HSCT.
The immune failure is characterized by exhaustion of specific humoral factors, e.g., serum immunoglobulins, and decreased counts of immune subpopulations (granulocytes, monocytes, T- and B cell subsets, NKs). Th ese immune indexes reflect general drop and recovery of immune system which may continue for months and years [1]. However, these cellular and humoral markers alone cannot accurately predict clinical risk of local infections at epithelial and mucosal surfaces (pneumonia, cystitis, stomatitis et.). The most hazardous terms for development of bacterial, fungal and viral infections are shown in Fig. 1. Post-transplant deficiencies of certain leukocyte populations seem to correlate with viral, bacterial and fungal infections [2]. Hence, the immune failure may, in principle, assessed via detection of multiple microorganisms which normally live and grow on skin and external mucous surfaces (i.e., oral cavity, pharynx, urethral ways etc). These opportunistic pathogens are normally controlled by the host immune surveillance. However, within weeks aft er HSCT, they tend to colonize deeper sites and areas (e.g., respiratory ways, urogenital tract etc.) causing different aff ections of internal organs. Hence, posttransplant detection of microfl ora in bloodstream and, especially, at local inflammation sites refl ects the degree of immune failure. Appropriate clinical experience was translated into numerous clinical recommendations based on empiric antibiotic treatment during early hematopoietic recovery and febrile neutropenia [3-6]. However, despite routine protection of the post-HSCT patients by broad-spectrum antibiotics, and antiviral treatment for a period of myelopoiesis reconstitution (up to 4 weeks), the rates of local post-transplant infections (e.g., pneumonia) may reach 25% of the HSCT patients [7].
Moreover, unusual sites of infection (typhlitis, perirectal infections and atypical forms of cellulitis) in neutropenic patients were noted decades ago [8]. About 60% of febrile neutropenia in early posttransplant period are classified as fever of unknown origin without distinct infectious pathogen, yet most respond to antibacterial therapy. Unusual sites of infection include typhlitis, perirectal infections and atypical forms of cellulitis.
Later on, a number of immunosuppressive drugs are routinely used after allogeneic HSCT (allo-HSCT) in order to prevent graft -versus-host disease (GvHD). In fact, some
immune populations, especially, adaptive immunity cells, may recover at several months and years post-transplant [1,9]. Hence, a long-term immune defi ciency after SCT is a chronic condition predisposing for opportunistic infections, fungal invasions and activation of endogenous viruses. Such infectious markers could be registered, and the co-infection rates may be used for assessing the grade of immune deficiency in the patient.

Severity and clinical signifi cance of the post-HSCT immune deficiency depends on dosage and expected immunotoxic effects of drugs used in the conditioning protocol, i.e., myeloablative or nonmyeloablative regimen. However, individual damage to the patient’s immune system is modified by the following factors:
• Intensity and number of preceding cytoreductive treatments exhausting the myelo-and lymphopoiesis system;
• Pharmacokinetics of cytotoxic drugs which strongly depends on its administration route, altered liver and kidney functions, and genetic variants aff ecting activation or inactivation of the given drug(s);
• Recipient/donor immune conflict, i.e., partial HLA mismatch, severe graft -versus-host disease [11]. Therefore, personalized evaluation of selected pathogen-specific markers, their multiplicity and biodiversity seems to be an efficient tool for specifying degree of immune defi ciency observed at early and later terms post-transplant.
Hence, the aim of our review is to propose the sets (panels) of latent/opportunistic marker microorganisms as potential markers of immune defi ciency, especially, in post-transplant patients. A number of species- and genus-specifi c multiplex PCR kits, as well as some integral microbial markers could be used for severity evaluation of immune defi ciency when testing peripheral blood samples and locally infected sites.

Incidence and sites of opportunistic infections
The list of opportunistic infections in immunocompromised patients is rather broad, however, limited by distinct microbial, fungal and viral species (see Table 1).
Most bacterial species revealed in immunocompromised patients proved to be opportunistic microorganisms, i.e., those which are present in normal human skin or inhabit normal gut mucosa. The main prerequisites for growth and cytopathic/ infl ammatory effects of opportunistic fl ora are as follows: (1) Penetrating skin barrier; (2) Passing mucosal surfaces; (3) Remote dissemination via bloodstream to lymph nodes, spleen, liver, kidney, lungs; (4) Passing blood/brain barrier. As a result, posttransplant patients develop opportunistic infections of lower respiratory ways, brain, urinary tract, as well as deep abscesses caused by skin or intestinal microorganisms. Moreover, the skin microorganisms may colonize catheter lumen, then proceeding into a bloodstream infection.
Incidence of bacterial pathogens in blood cultures from immunocompromised patients
Detection of bacterial pathogens is normally performed in blood cultures, now using automatic incubation/detection systems. Blood is normally regarded as a sterile medium. However, some episodes of septicemia may occur due to occasional microbial contamination, e.g., major purulent surgery, dental extraction etc. Fungal pathogens may be detected in blood both by beta-galactomannan immunoassay, or by DNA diagnostics. Viral infection/reactivation is routinely detected in whole blood or plasma by means of PCR.
Blood screening for bacterial infections may be an efficient tool of infection monitoring. Blood culture done at a regular interval of at least 5 days. Studies on surveillance blood cultures in HSCT are routinely practicized at many centers. E.g., this approach was tested by for 205 patients admitted for auto- or allo-HSCTs [12]. Bloodstream infection was detected in 14.1% of these patients, which is at lower limits of previously reported rates (12.5-40%). Suprisingly, none of the patients who developed bloodstream infection were diagnosed by surveillance blood cultures. Blood cultures taken in presence of clinical infectious symptoms were much more informative. The most common organisms isolated were coagulasenegative staphylococci, 41/84 (49%) in pure culture and an additional 10 (12%) in mixed cultures. Klebsiella spp. and Enterococci each accounted for 5 episodes (6%) of positive cultures; Viridans group streptococci accounted for 2 episodes (2.4%); and the remaining 25% were caused by different organisms.
In some other countries, the spectrum of cultured bloodborne bacteria is shift ed towards Gram-negative microbes, i.e., Pseudomonas aeruginosa, Klebsiella pneumonia, Esherichia coli, Acinetobacter spp, Enterobacter spp. as reported by an Indian team who analyzed data from 653 pediatric cancer patients undergoing chemotherapy [13].
In a Belarus study, the incidence (top-10) of cultivable bacterial pathogens during the early period aft er HSCT, their culture and identifi cation was assessed with BacT/ALERT-Vitec technique [14]. Th e following rating of microbial detection was obtained (Table 2), with Klebsiella pneumonia and Escherichia coli. making >40% of all clinical microbial isolates.
In immunocompromised patients, herpesviruses, fungal infections,
hepatitis B and C are taken into scope.

A comprehensive randomized analysis of posttransplant infections which occured during 2 years aft er HSCT was reported by Young et al. [15] being performed in the patients from USA BMT centers. A total of 499 patients were under study exhibiting 1347 infection episodes of severe or life-threatening grade documented in 384 (77%) patients. 249 (81%) of these patients had received a BM graft and 183/250 (73%) had received a PBSC graft , at a cumulative two-year incidence of infections of 80-85%. Th e majority of these episodes, 810 (60%), were due to bacteria, with a twoyear cumulative incidence of 72.1% and 62.9% in BM versus PBSC recipients, respectively (P = .003). The cumulative incidence of bacterial infections during the first 100 days was 44.8% for BM vs. 35.0% for PBSC (P= 0.027). The infection rates were slightly more often after BM transplantation than following peripheral PBSCT (Table 3).
Local microbiotes as a source of opportunistic flora
Skin surface
Normal skin harbors hundreds of opportunistic or commensal microorganisms including bacteria, fungi and viruses that compose skin microbiome [16]. T e skin cell populations (keratinocytes, dermal cells, fi broblast, macrophages etc.) release a lot of protective factors and molecules (antimicrobial peptides etc.), to limit excessive microbial growth and colonization [17]. Therefore, survival of the microorganisms on the skin surface suffi ciently depends on their ability to resist the host defense mechanisms [18].
The major fraction is represented by coagulase-negative staphylococci such as S.epidermidis [19, 20] as well as more pathogenic S.aureus [21]. Sebaceous glands are colonized by Propionibacterium acne [22]. Candida yeasts also colonize skin surface, being, however, controlled by appropriate defense mechanisms [23]. In immunocompetent organism, these microflora interact with keratinocytes and local immune cells, thus supporting limiting local growth of skin microflora. However, in immunocompromised and elderly patients, the commensal skin staphylococci, Candida yeasts etc. may be isolated from the sites of extradermal opportunistic infections, like catheter- or pacemaker-associated infl ammatory conditions [24]. Clinical studies show that all the mentioned skin microfl ora could be revealed in blood of the immunocompromised patients (see tables 2, 3).
Oral mucosa
Hundreds microbial and viral species, of them – only up to 5-10 marker microbes, Candida yeasts and viruses (EBV). Dental flora could be revealed in bloodstream, and, especially, in heart valves [25], thus demonstrating a wide opportunity of a distant microbial contamination with opportunistic oral microflora.
Oral mucosa is protected by innate immunity factors, playing a crucial role in the regulation of oral health. E.g., epithelial cells lining oral mucosal surfaces provide not only a physical barrier but also produce diff erent antimicrobial peptides, including human β-defensins (hBDs), lactoferrin, secretory leukocyte protease inhibitor (SLPI), and various cytokines [26]. Specific IgA’s provide specifi c immune response. There are a lot of oral co-infections revealed in HIV-infected patients, such as human herpesvirus 6 and 8 (HHV-6, HHV-8), herpes simplex virus 1/2 (HSV-1/2), EBV, and human papilloma virus (HPV). Colonization with Candida species is also quite common in these patients. Oral hairy leukoplakia caused by Epstein Barr virus is a typical infection associated with immune suppression [27].
Non-tuberculosis mycobacteria (non-MBT) are also common among oral microfl ora [28]. When examining healthy persons, the non-MBT DNA was detected in the nostrils of all 10 subjects, in buccal mucosa of 8 subjects, in the oropharynx of 7 subjects, and in the dental plaques of 5 subjects.

Oropharyngeal mucosa
In a group of 82 patients with head-and-neck cancer treated by chemo/radiotherapy, the authors studied subgingival biofilm samples, oral lavages and whole saliva samples obtained to microbiologically analyze the eff ects of cancer treatments (1-year follow-up). As compared to surgically treated patients, an increased prevalence of P. gingivalis, T. forsythia, S. mutans and Candida species was observed in the treated areas, thus demonstrating immunosuppressive action of the cytotoxic therapy [29]. Signifi cance of these fi ndings in oncohematology was evaluated by Schuurhuis et al. [30] after cytostatic treatment of 28 leukaemic and 35 auto-HSCT patients. Acute oral foci of infection were found in 2 leukaemic (7%) and 2 ASCT patients (6%), and chronic oral foci of infection in 24 leukaemic (86%) and 22 ASCT patients (63%). Positive blood cultures with microorganisms potentially originating from the oral cavity occurred in 7 patients during treatment, but did not correlate with development of infectious complications.
Nasopharyngeal area is characterized by interactions between adenoid microorganisms [31]. Adenoid microbiota plays an important role in the development of various infectious and non-infectious diseases of the upper airways, such as otitis media, adenotonsillitis, rhinosinusitis and adenoid hypertrophy. Studies have suggested that adenoids could act as a potential reservoir of opportunistic pathogens. However, previous bacterial surveys of adenoids were mainly culture based and therefore might only provide an incomplete and potentially biased assessment of the microbial diversity. To develop an in-depth and comprehensive understanding of the adenoid microbial communities and test the ‘pathogen reservoir hypothesis’, we carried out a 16S rRNA based, culture-independent survey of bacterial communities on 67 human adenoids removed by surgery. Our survey revealed highly diverse adenoid bacterial communities distinct from those of other body habitats. Despite large interpersonal variations, adenoid microbiota shared a core set of taxa and can be classified into at least five major types based on its bacterial species composition.
Broncho-alveolar mucosa
Colonization of mucosal surfaces with autofl ora (cocci, yeast fungi and molds, latent viruses), has an important stimulatory effect on whole-body development of immune responses [32]. However, the opportunistic local microfl ora in immunocompromised patients may extensively grow on the mucous surfaces in association with more or less severe clinical symptoms, e.g., opportunistic gramnegative bacilli (Enterobacteriaceae, Pseudomonas, Acinetobacter spp.). Moreover, relatively virulent serovars of M. avium complex, like as other non-MBT bacteria, may colonize the bronchial and intestinal mucosal surfaces of healthy individuals in immunocompromised conditions [33]. The same could be found with fungal outgrowth and Toxoplasma activation.
Gastrointestinal tract (GIT)
Extensive gut microbiota contains hundreds of microbial species of which only few are able to grow on media used in clinical laboratories. Th e most detectable opportunistic infectious pathogens are: E.coli, C. diffi cile, Enterococcus fecalis/faecium, etc. Meanwhile, most microorganisms (up to 70%) are fastidious or non-culturable species which may be revealed only by DNA or protein diagnostics.
As reviewed by Callejas-Díaz and Gea-Banacloche [34], the C. difficile infection (CDI) is very frequent at early terms aft er hematopoietic stem cell transplantation (HSCT) – between 6 % and 20% of HSCT recipients during the first year mostly aft er allo (allo-HSCT), thus being a promising bacterial marker of intestinal dysbiosis. For diagnostics, molecular testing for the toxin genes by (PCR) is performed, along enzyme immunoassays (EIA). It correlates with worsening of bowel graft versus host disease (GVHD).
Early after cytoreductive therapy one may observe severe damage of chemosensitive intestinal mucosa [35]. Intestinal microflora undergoes sufficient changes within several days aft er intensive chemotherapy and/or HSCT. At present time, these shift s in microfl ora subsets are eff ectively detected by means of multiplex high-throughput PCR followed by sequencing of the obtained genome fragments and computer-assisted differentiation of these genome segments. The mentioned mucosal damage and infl ammation is associated with a reduced biodiversity of intestinal microfl ora developing aft er HSCT (see a review by [36] Kucher et al., 2017). In turn, altered intestinal microbiota aft er HSCT is shown to be an important risk factor of severe GVHD [37]. Hence, the interactions between gut microbial antigens and immune cells are enhanced early aft er cytotoxic chemotherapy.
Gut bacterial translocation: lessons from HIV infection
A gradually developing immune defi ciency in HIV infection, while bearing other pathogenetic features, is known to be associated with a number of concomitant infectious complications (HCV, tuberculosis etc.) and a variety of somatic comorbidities [38] (Currier, Havlir 2005). Opportunistic infections are commonly associated with HIV infection. E.g., some malignant lymphomas in HIV patients may occur due to activation of latent Epstein-Barr virus and, probably, HHV8 [39], like as secondary lymphomas in post-transplant immunocompromised patients. Bacterial opportunistic infections, such as Pneumocystis jiroveci, Toxoplasma gondii, and Mycobacterium avium threaten the patients at CD4+ T cell counts below 200 cells/mcL [40]. However, exact critical levels for CD4+ cells are quite varying. Th erefore, a multiplex detection of opportunistic pathogens would be performed, in order to assess real individual degree of clinical immunodeficiency.
In HIV-infection, the intestinal damage and microflora changes are followed by activation of monocytes and residual T cells, due to the so-called bacterial translocation, i.e., penetration of gut bacterial cell components from injured intestinal wall to bloodstream [41]. Th e bacterial translocation is registered as endotoxin or soluble monocyte (CD14) antigen detected in blood serum. Th is “subseptic” condition is recognizable in HIV-infected patients, especially, those receiving suboptimal antiretroviral therapy. According to this opinion, the damaged gut epithelium allows diff usion of bacterial antigens which stimulate monocytes, IL-17 and IL-22-secreting cells.
The mentioned markers of microbial translocation in HIV infection showed some pathogenetic links with quantitative shift s in gut microfl ora, as shown by next-generation sequencing of bacterial 16S rRNAs which identifies hundreds of intestinal microbial species [42]. The analysis of gut microbial diversity showed a more restricted microbiota spectrum, both at baseline and aft er antiretroviral therapy (ART). Th e skewed biodiversity of gut flora correlated with decreased CD4 T-cell counts and increased markers of microbial translocation and monocyte activation. Moreover, bacterial lipopolysaccharide (LPS) and soluble CD14 (monocyte antigen) concentrations in blood plasma are shown to predict mortality and disease progression in subjects with HIV infection [41]. Intestinal epithelial damage and altered gut microfl ora in allogeneic HSCT are also well proven [36]. Hence, the markers of bacterial translocation may be potentially eff ective in diagnostics of any immune deficiency, aiming to evaluate possible damage to the gut mucosa and altered immune response aft er HSCT.
Urine and urogenital ways
The urine is sterile in healthy children. Normally, the urinary epithelium is protected by specifi c antibacterial factors, e.g., Tamm-Horsfall protein, lactoferrin, defensins etc [43]. With age, however, the urinary system is colonized in ascending manner with microfl ora from skin (Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococci, Candida) and gut (Escherichia coli, Enterobacter, Enterococcus, Proteus mirabilis). However, hematogenic infections are also possible due to Staph. aureus, Haemophilus infl uenza etc., thus causing chronic cystitis and pyelitis oft en complicated by infectious kidney stone formation [44].
Protozoic infections, e.g.., Toxoplasma gondii, may be also
revealed in urogenital ways over 1st month aft er HSCT [45], with rapid disappearance from urine cells upon hematopoiesis reconstitution.
Early after hematopoietic transplantation, hemorrhagic cystitis is developing in most cases, mainly, due to urothelial damage by toxic cytostatic treatment. Meanwhile, activation of BK and JC viruses (latent polyomaviruses in urothelial cells) are additional markers of hemorrhagic cystitis at the terms of 1-2 months which are found in urine at early terms post HSCT [46].
Catheter-associated urinary infection is a special issue in immunodeficient patients. It may be caused by P.mirabilis migrating from gut [47], or other urease-positive microbes migrating, mainly, from skin. Multiple bacterial species co-exist in microbial biofilms growing on the bladder epithelium and in catheter lumen [48].
Mixed infections in immunocompromised patients
Bacterial/fungal co-infections
Combined infections with bacterial and/or fungal agents after cytotoxic treatment in post-transplant patients were described in many works. However, the mechanisms of their simultaneous emergence are not clear yet. Acute failure of protective immune mechanisms could be the most plausible explanation for these posttransplant conditions. Moreover, multiple occurence of several pathogens in a single biological sample (i.e., blood, urine, or saliva) in HSCT patients may be promoted by a symbiotic microbial growth, thus causing a co-infection. Polymicrobial complexes grown as biofilms are regularly observed in local infections, such as dental caries and parodontitis, otitis media, diabetic foot wounds etc. [49]. Similar growth of bacterial associations is typical to blood catheter-associated infections, or urinary catheter
contamination. Hence, bacterial co-infections are not rare and should be detected in HSCT setting.
The incidence of bacterial and/or fungal infections was assessed in a group of 901 stem cell transplant recipients [50]. Of them, 237 patients (27%) had microbiologically documented microorganisms isolated, including 34 patients (14%) simultaneously infected with multiple microorganisms. Th e co-infected patients seem to be at an increased risk for aGvHD and delayed graft functioning.
The same workers evaluated infection by Clostridium difficile, a common opportunistic microbe, and its association with other infectious agents [51]. Among 822 consecutive autologous and allogeneic HCST recipients, 85 cases (10.3%) of C.diffi cile-associated disease were identified. Significant associations were found between C.diffi cile detection, and neutropenic fever, as well as with bacterial coinfection, and presence of vancomycin-resistant Enterococcus faecium (VRE) colonization. Allo-HSCT patients with C.diffi cile-associated disease showed higher rates of severe gastrointestinal aGvHD.
Viral co-infections
Combined post-transplant viral co-infections are also quite common, thus suggesting their treatment by injection of the virus-specifi c immune lymphocytes [52]. Simultaneous activation of several common endogenous viruses was shown by a multiplex PCR system for 13 common viral species in some patients before allo-HSCT, while being increased within 1st month aft er transplant [53].
Simultaneous co-activation of herpesviruses is a regular finding. E.g., in a Polish study [54] 142 allo-HSCT recipients were tested for 3 herpesviruses (CMV, HHV-6 and HHV-7) for the fi rst 3 months. Reactivation of more than one virus was identifi ed in 31% of analysed patients, with CMV being most common, thus increasing risk of specifi c complications posttransplant.
In a recent study by Koskenvuo et al. [55] the authors studied 23 viruses in 53 paediatric patients up to 3 months aft er allo- HSCT. Interestingly, polyomaviruses predominated over herpesviruses thus showing their common incidence in the immunodefi cient cohort. Of them, 13 patients (25%) had viraemias by multiple viruses. Presence of viral co-infections was signifi cantly associated with aGvHD and steroid use, the factors known to suppress immune functions.
There are specific combinations of certain viral species in post-HSCT patients. According to results obtained by Jeulin et al. [56], HHV6 infection did not correlate with CMV, HSV, EBV. Meanwhile, adenovirus infection and HHV-6 activation seemed to be related in their posttransplant replication.
Mixed infectious pathogens
A large meta-analysis was performed by Klein et al. [57] based on a systematic search of different medical databases on infl uenza virus-associated infections. It covered 27 studies including 3215 participants, with widely varying coinfection rates ranging from 2% to 65%. Most common coinfecting species were S. pneumoniae and Staph. aureus, which accounted for 35% and 28% of infections followed by P.aeruginosa, S. pyogenes, H. infl uenzae,.K. pneumoniae, and M. pneumoniae.
A study by Chebotkevitch et al. [58] has revealed that cytostatic treatment was associated with higher CMV and EBV activation rates if the patients had concomitant bacterial infections, thus confi rming a potentially common reason for mixed-type infections in this cohort (Table 5).
To confirm a need for multiplex studies of infectious agents in local samples, we would like to refer our previous study concerning incidence of diff erent opportunistic pathogens in the tissues of heart valves surgically obtained from cardiosurgery patients subjected to valve replacement (Table 4). Of course, this group comprised patients with a story of septic episodes before the valve disorder had developed. However, a number of DNA species (both microbes and viruses) was revealed in 72% of the heart valves from the patients with septic endocarditis thus showing their high rates of exposure to infectious agents. Moreover, a number of the samples tested has shown positivity for both bacterial and herpesvirus DNA, thus suggesting high probability of mixed valves contamination from the circulating blood. Th e enlisted microbial species may originate from skin (Staphylococci, Streptococci, Candida spp), gut (E.Coli), oral cavity (P.Gingivalis, Actinobacillus actinomycetemcomitans, T.Forsythus), then bacteremia.
Similar data on heart valve contamination by multiple bacteria were obtained by Miller et al. [59] using broad-range 16S rDNA PCR and Sanger sequencing on a specifi c fragment of ribosomal DNA extracted from HV tissues. Hence, the existing diagnostic approach to detection of mixed microbial and viral infections in post-transplant patients seems to be rational and feasible. Multiplicity of infectious agents should then correlate with grade of anti-infectious response and risk of severe GvHD. Th is issue may be resolved by detection of group-specifi c microbial and viral markers.
Multiple infections and post-transplant mortality risk
Some previous results suggest increased mortality of HSCT patients with diagnosed multiple bacterial infections. E.g., Trifi lio et al. [50] have reviewed clinical and laboratory data of 901 HSCT recipients (675 auto- and 226, allo-HSCTs). When studying diff erent biomaterials, the authors have identified 179 patients with proven monomicrobial infection and 59 patients (24%) with multiple microorganisms, of which 34 (14%) were classifi ed as polymicrobial infection (PI), and 25 (10%) as multiple distinct episodes of infection (MDE).


There were no significant connections between the infection multiplicity, and age, gender, diagnosis, time to engraft ment, response to therapy, etc. However, overall mortality at day +100 post transplant was higher in patients with multiple infectious episodes (P =0.02 in the Kaplan–Meier analysis). These patients also proved to be at an increased risk for acute GVHD and graft failure. In particular, early and frequently presenting Gram-negative infections, and fungal (mostly, Candida) infections were associated with high mortality rates.
Common endogenous viruses (4 herpesviruses, BK polyomavirus, and adenovirus) were studied in allo-HSCT setting by Hill et al. (2017) [60]. Th e workers performed weekly quantitative PCR of blood plasma for viral DNA from 404 allo-HSCT patients. CMV was the most common virus (65% of patients), followed by BKV (54%), HHV-6B (46%), AdV (10%), and EBV (9%). CMV, BKV, and HHV-6B were the viruses most frequently seen in combination. Detection of multiple viruses until day +100 was quite common: 90% had ≥1, 62% had ≥2, 28% had ≥3, and 5% had 4 or 5 viruses. Acute GVHD grade 3-4 was associated with detection of ≥2 viruses. Myeloablative conditioning was associated with a signifi cantly higher risk for ≥3 and ≥4 viruses. Age ≤21 was associated with detection of ≥4 viruses (aHR 2.65). In general, activation of multiple dsDNA viruses had a dose-dependent association with increased mortality aft er allo-HSCT, being independent on immune reconstitution rates and acute GVHD. These data suggest opportunities to improve outcomes with better preventive antiviral strategies.
Prototype test systems for detection of multiple marker pathogens
1. Rapid blood cultures performed by several approved systems, i.e., BD BACTEC, BacT/ALERT 3D, VersaTREK and similar automated devices. They use standard aerobic and anaerobic broth media for samples and provide results within few days and even hours. The data are read by colorimetry, fl uorimetry, or redox changes of the media [61]. Th is approach allows to obtain clinical isolates for the most opportunistic bacteria listed in Table 1.
2. Manual and automated multiplex PCR was proposed by several firms, e.g., Roche, Interlabservice. To systhematize this search, a number of diagnostic panels are developed for detection of commensal/opportunistic infectious pathogens. An example of such multiplex diagnostics was developed by Roche (SeptiFast), as shown in Table 6. Appropriate systemic review and meta-analysis of SeptiFast diagnostic kits in septic patients was performed by Chang et al. (2013) [62]. A total of 34 studies enrolling 6012 patients of suspected sepsis were included. In general, sensitivity and specifi city estimates for combined bacteremia and fungemia outcome were 0.75, and 0.92, respectively. However, the mentioned multiplex PCR system covers only some opportunistic bacteria and fungi, thus missing viruses which are prone for reactivation post-HSCT. Mixed bacterial/ viral PCR assays are still under development. E.g., such multiplex real-time PCR was recently used in order to evaluate CNS infection in infants [63].
Moreover, a diagnostic panel for combined opportunistic infections should include multiplex system for detection of infectious agents in the given local sample (urine, BAL, spinal liquor etc. Such panel should detect the most common bacterial, fungal and viral pathogens (up to 15-20), in order to detect immune defi ciency and its degree (by the number of positive fi ndings per sample). It may be based either on multiplex PCR, or on microarray detection mode.
3. A more general, genus-specifi c PCR assays have been developed, e.g., in Russia. Th ese multiplex real-time PCR systems are aimed for diagnostics of vaginal or gut dysbiosis (resp., Femofl or, and ColonoFlor) detecting up to 16 microbial classes in appropriate biological samples. The ratios of diff erent microbiota members in complex biological materials are calculated, thus allowing quantify the balance of typical microbiota species in, urogenital or intestinal specimens.
4. Looking for endotoxin and surrogate markers in blood plasma, a search for microbial translocation via bloodstream (endotoxin, sCD14) may be applied, using the strategy previously used for studies of HIV infection. 
5. Detection of total microbial contamination in blood and other normally sterile media. One may use currently developed methods for detection of total microbial DNA, or specific genes for detecting Gram+ and Gram-negative microorganisms [64]. However, most workers use in-house systems for these studies, thus being allowed for research only.
6. Moreover, some antigenic tests are commonly applied in order to detect opportunistic fungal invasions (betagalactomannan) in blood and bronchoalveolar lavage. A search for mixed infections in posttransplant patients should make diagnostics of immune defi ciency more adapted for the needs of medical practice. Detection of severe-grade immune failure may justify usage of some known treatments in order to enhance innate and adaptive immunity in the patient. E.g., detection of polymicrobial infection may be a indication for usage of immunostimulatory cytokines and hemopoietic growth factors in oncohematological patients [65]. Moreover, fi nding of mixed infection in the patient could be a proven indication for G-SF-primed granulocyte transfusions, at least during posttransplant febrile neutropenia [66].
Conclusion
1) A variety of microbes and viruses normally exists on skin and mucosal surfaces of gastrointestinal and urogenital tract.
2) In cases of immune defi ciency of either type, some of the microorganisms extend or activate at other body sites, thus causing opportunistic infections, e.g., interstitial skin abscesses, pneumonias, septicemias, catheter-associated infections. Activation of endogenous viruses (e.g. CMV) is shown to cause local lesions of diff erent organs and tissues.
3) Hence, multiplicity assessment of common infectious biomarkers (bacteria, fungi and viruses) in blood and other unusual body sites may be used to assess grade of immune deficiency in posttransplant patients at early (cytopenic) and late time periods (chronic immune defi ciency).
4) In view of low culture effi ciency for many microorganisms in humans, the panels for common marker infections are developed which will reflect grade of immune defi ciency. It could be based on express bacterial diagnostics, but, mostly, on multiplex real-time PCR of distinct pathogenic species. SeptiFast could be applied to these purposes, with addition of some multiplex PCR tests for detection of viruses, in order to detect combined viral infections. Some antigenic markers (e.g., endotoxin, beta-galactomannan) could be also screened in the HSCT patients, being complementary to the battery of microbiological tests for specifying grade of the patients’ immunodeficiency.
References
1. Pankratova OS, Chukhlovin AB. Time course of immune recovery and viral reactivation following hematopoietic stem cell transplantation. Cell Th er Transplant. 2016; 5(4):32-43.
2. Podgorny PJ, Pratt LM, Liu Y, Dharmani-Khan P, Luider J, Auer-Grzesiak I, Mansoor A, Williamson TS, Ugarte-Torres A, Hoegh-Petersen M, Khan FM, Larratt L, Jimenez-Zepeda VH, Stewart DA, Russell JA, Daly A, Storek J. Low counts of B cells, natural killer cells, monocytes, dendritic cells, basophils, and eosinophils are associated with postengraft ment infections aft er allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(1):37-46.
3. Maschmeyer G, Ljungman P. Infections in Hematopoietic Stem Cell Transplant Recipients. In: A. Safdar (ed.), Principles and Practice of Cancer Infectious Diseases, Current Clinical Oncology, 17, Springer Science+Business Media, LLC 2011. P.17-25.
4. Hu B, Mohty M, Savani BN. Common non-seasonal viral infections aft er hematopoietic stem cell transplantation. Hematologie 2013; 19: 268-278.
5. Ullmann AJ, Schmidt-Hieber M, Bertz H, Heinz WJ, Kiehl M, Krüger W, Mousset S, Neuburger S, Neumann S, Penack O, Silling G, Vehreschild JJ, Einsele H, Maschmeyer G on behalf of the Infectious DiseasesWorking Party of the German Society for Hematology and Medical Oncology (AGIHO/DGHO) and the DAG-KBT (German Working Group for Blood and Marrow Transplantation). Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines. Ann Hematol. 2016;95(9):1435-1455.
6. Robinson PD, Lehrnbecher T, Phillips R, Dupuis LL, Sung L. Strategies for Empiric Management of Pediatric Fever and Neutropenia in Patients With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: A Systematic Review of Randomized Trials. J Clin Oncol. 2016;34(17):2054-2060. 7. Chen C-S, Boeckh M, Seidel K, Clark JG, Kansu E, Madtes DK, Wagner JL, Witherspoon RP, Anasetti C, Appelbaum FR, Bensinger WI, Deeg HJ, Martin PJ, Sanders JE, Storb R, Storek J, Wade J, Siadak M, Flowers MED, Sullivan KM. Incidence, risk factors, and mortality from pneumonia developing late aft er hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2003;32:515–522.
8. Bodey GP. Unusual presentations of infection in neutropenic patients. Int J Antimicrob Agents. 2000;16(2):93-95.
9. Avigan D, Pirofski L-A, Lazarus HM.Vaccination against infectious disease following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:171-183.
10. Marty FM, Rubin RH. Th e prevention of infection posttransplant: the role of prophylaxis, preemptive and empiric therapy. Transplant Int. 2006;19(1):2-11.
11. Averyanova M.Yu., Vavilov V.N., Bondarenko S.N., Uspenskaya O.S., Stancheva N.V., Semenova E.V., Volkova A.G., Smirnov B.I., Baranova I.B., Zubarovskaya L.S., Klimko N.N., Afanasyev B.V. Bacterial infections in pediatric and adolescent recipients in allogeneic hematopoietic stem cell transplantation: etiology, structure, risk factors. Journal of Infectology, 2013, 5(1): 35-43 (In Russian).
12. Ghazal SS, Stevens MP, Bearman GM, Edmond MB. Utility of surveillance blood cultures in patients undergoing hematopoietic stem celltransplantation. Antimicrobial Resistance and Infection Control 2014, 3:20.
13. Reddy R, Pathania S, Kapil A, Bakhshi S. Review of spectrum and sensitivity of bacterial bloodstream isolates in children with malignancy: A retrospective analysis from a single center. Ind J Cancer. 2014; 51(4): 425-427.
14. Stoma I, Karpov I, Milanovich N, Uss A, Iskrovi. Risk factors for mortality in patients with bloodstream infections during the pre-engraft ment period aft er hematopoietic stem cell transplantation. Blood Res. 2016; 51(2):102-106.
15. Young JAH, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, Knust K, Horowitz MM, Confer DL, Dubberke ER, Pergam SA, Marty FM, Strasfeld LM, Brown JM, Langston AA, Schuster MG, Kaul DR, Martin SI, Anasetti C for the BMT CTN Trial 0201. Infections following transplantation of bone marrow or peripheral-blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016; 22(2):359–370.
16. Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21(12):660-668.
17. Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131(10):1974-1980.
18. Roth RR, James WD. Microbiology of the skin: resident flora, ecology, infection. J Am Acad Dermatol. 1989;20(3):367-390.
19. Widerström M, Wiström J, Sjöstedt A, Monsen T. Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. Eur J Clin Microbiol Infect Dis. 2012;31(1):7-20.
20. Nguyen TH, Park MD, Otto M. Host response to Staphylococcus epidermidis colonization and infections. Front Cell Infect Microbiol. 2017 Mar 21;7:90. doi: 10.3389/ fcimb.2017.00090. eCollection 2017.
21. Guerra FE, Borgogna TR, Patel DM, Sward EW, Voyich JM. Epic immune battles of history: neutrophils vs. Staphylococcus aureus. Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017.
22. Christensen GJ, Brüggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes. 2014;5(2):201-215.
23. Kühbacher A, Burger-Kentischer A, Rupp S. Interaction of Candida Species with the Skin. Microorganisms. 2017 Jun 7;5(2). pii: E32. doi: 10.3390/microorganisms5020032.
24. Da Costa A, Lelièvre H, Kirkorian G, Célard M, Chevalier P, Vandenesch F, Etienne J, Touboul P. Role of the preaxillary flora in pacemaker infections: a prospective study. Circulation. 1998 ;97(18):1791-1795.
25. Zhirekhina OV, Chukhlovin AB, Sysoev KA, Gritsenko VV, Totolian AA. Detection of infectious agents in heart valves during endocarditis using PCR technique. Zh Mikrobiol Epidemiol Immunobiol. 2008; 4:96-98 (In Russian).
26. Nittayananta W, Tao R, Jiang L, Peng Y, Huang Y. Oral innate immunity in HIV infection in HAART era. J Oral Pathol Med. 2016; 45(1):3-8.
27. Reichart PA. Infections of the oral mucosa II. Bacterial, mycotic and viral infections. Mund Kiefer Gesichtschir. 1999 Nov;3(6):298-308. (In German)
28. Macovei L, McCaff erty J, Chen T, Teles F, Hasturk H, Paster BJ, Campos-Neto A.. Th e hidden ‘mycobacteriome’ of the human healthy oral cavity and upper respiratory tract. J Oral Microbiol. 2015; 7: 10.3402/jom.v7.26094.
29. Schuurhuis JM, Stokman MA, Witjes MJ, Langendijk JA, van Winkelhoff AJ, Vissink A, Spijkervet FK. Head and neck intensity modulated radiation therapy leads to an increase of opportunistic oral pathogens. Oral Oncol. 2016;58:32-40.
30. Schuurhuis JM, Span LF, Stokman MA, van Winkelhoff AJ, Vissink A, Spijkervet FK. Eff ect of leaving chronic oral fociuntreated on infectious complications during intensive chemotherapy. Br J Cancer. 2016;114(9):972-978.
31. Ren T, Glatt DU, Nguyen TN, Allen EK, Early SV, Sale M, Winther B, Wu M. 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids. Environ Microbiol. 2013;15(2):535-547.
32. Tlaskalová-Hogenová H, Tucková L, Lodinová-Zádniková R, Stepánková R, Cukrowska B, Funda DP, Striz I, Kozáková H, Trebichavský I, Sokol D, Reháková Z, Sinkora J, Fundová P, Horáková D, Jelínková L, Sánchez D. Mucosal immunity: its role in defense and allergy. Int Arch Allergy Immunol. 2002;128(2):77-89.
33. Collins FM. Mycobacterial disease, immunosuppression, and acquired immunodefi ciency syndrome. Clin Microbiol Rev. 1989; 2(4):360-377.
34. Callejas-Díaz A, Gea-Banacloche JC. Clostridium diffi cile: deleterious impact on hematopoietic stem cell transplantation. Curr Hematol Malig Rep. 2014;9(1):85-90.
35. Peterson DE, Cariello A. Mucosal damage: a major risk factor for severe complications aft er cytotoxic therapy. Semin Oncol. 2004;31(3 Suppl 8):35-44.
36. Kucher MA, Goloschapov OV, Moiseev IS, Afanasyev BV. Fecal microbiota transplantation as a method to treat complications aft er hematopoietic stem cell transplantation. Cell Ther Transplant. 2017, 6(1): 20-29.
37. Shono Y, Docampo MD, Peled JU, Perobelli SM, Jenq RR. Intestinal microbiota-related eff ects on graft -versus-host disease. Int J Hematol. 2015;101(5):428-437.
38. Currier JS, Havlir DV. Complications of HIV disease and antiretroviral therapy. Top HIV Med. 2005;13(1):16-23.
39. Navarro WH, Kaplan LD. AIDS-related lymphoproliferative disease. Blood. 2006; 107(1): 13-20.
40. Hammer SM. Clinical practice. Management of newly diagnosed HIV infection. N Engl J Med. 2005;353(16):1702-1710.
41. Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Th er. 2016 Apr 11;13:19. doi: 10.1186/s12981-016-0103-1. eCollection 2016.
42. Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, Hov JR, Noyan K, Vesterbacka J, Svärd J, Rudi K, Sönnerborg A. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS. 2015; 29(18):2409-18.
43. Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol 2007; 18(11): 2810-2816.
44. Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007;79 [Suppl 1]:32-36.
45. Chukhlovin AB, Zubarovskaya LS, Bondarenko SN, Eismont YuA, Semenov AV, Vladovskaya MD, Totolyan Areg A, Afanasyev BV. Activation of Toxoplasma gondii infection aft er allogeneic transplantation of haematopoietic stem cells: dependence on time of transplantation and serological status of the patients. Infect Immunity. 2014, 4(4):382-386 (In Russian).
46. Chukhlovin AB, Eismont YuA, Vavilov VN, Zubarovskaya LS, Afanasyev BV. Time- and sample-dependent diff erences in polyomavirus incidence following hematopoietic stem cell transplantation. Cell Th er Transpl 2016; 5(1):26-30.
47. Drzewiecka D. Signifi cance and roles of Proteus spp. bacteria in natural environments. Microb Ecol. 2016;72:741-758.
48. Musk DJ Jr, Hergenrother PJ. Chemical countermeasures for the control of bacterial biofi lms: eff ective compounds and promising targets. Curr Med Chem 2006; 13(18): 2163-2177
49. Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev. 2012;25(1):193-213.
50. Trifi lio SM, Pi J, Mehta J. Changing epidemiology of Clostridium diffi cile-associated disease during stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(3):405-409.
51. Trifi lio S, Zhou Z, Fong JL, Zomas A, Liu D, Zhao C, Zhang J, Mehta J. Polymicrobial bacterial or fungal infections: incidence, spectrum of infection, risk factors, and clinical outcomes from a large hematopoietic stem cell transplant center. Transpl Infect Dis. 2015;17(2):267-274.
52. Sutrave G, Blyth E, Gottlieb DJ. Cellular therapy for multiple pathogen infections aft er hematopoietic stem cell transplant. Cytotherapy. 2017. pii: S1465-3249(17)30657-6. doi:10.1016/j.jcyt.2017.07.012.
53. Inazawa N, Hori T, Hatakeyama N, Yamamoto M, Yoto Y, Nojima M, Suzuki N, Shimizu N, Tsutsumi H. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol. 2015;87(8):1427-1435.
54. Dzieciatkowski T, Tomaszewska A, Przybylski M, Rusicka P, Basak GW, Jedrzejczak WW, Wroblewska M, Halaburda K. Analysis of the shedding of three β-herpesviruses DNA in Polish patients subjected to allogeneic hematopoietic stem cell transplantation: Six-year follow up. J Clin Virol. 2016;76:30-35.
55. Koskenvuo M, Rahiala J, Sadeghi M, Waris M, Vuorinen T, Lappalainen M, Norja P, Toppinen M, Saarinen-Pihkala U, Allander T, Söderlund-Venermo M, Hedman K, Ruuskanen O, Vettenranta K. Viremic co-infections in children with allogeneic haematopoietic stem cell transplantation are predominated by human polyomaviruses. Infect Dis (Lond). 2017;49(1):35-41.
56. Jeulin H, Agrinier N, Guery M, Salmon A, Clement L, Bordigoni P, Venard V. Human herpesvirus 6 infection aft er allogeneic stem cell transplantation: incidence, outcome, and factors associated with HHV-6 reactivation. Transplantation 2013;95: 1292-1298.
57. Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, Dugas A. Th e frequency of infl uenza and bacterial coinfection: a systematic review and meta-analysis. Infl uenza Other Respir Viruses. 2016;10(5):394-403.
58. Chebotkevich VN, Bessmeltsev SS, Kiseleva EE, Stizhak NP, Kaytandzhan EI, Burylev VV. Bloodstream infections and herpesvirus activation following intensive chemotherapy of adult oncohematological patients. Cell Ther Transplant. 2016; 5(4):21-31.
59. Miller RGH, Chow B, Pillai D, Church D. Development and evaluation of a novel fast broad-range 16S ribosomal DNA PCR and sequencing assay for diagnosis of bacterial infective endocarditis: multi-year experience in a large Canadian healthcare zone and a literature review. BMC Infectious Diseases. 2016. 16:146
60. Hill JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, Milano F, Delaney C, Sorror ML, Sandmaier BM, Nichols G, Zerr DM, Jerome KR, Schiff er JT, Boeckh M. The cumulative burden of double-stranded DNA virus detection aft er allogeneic HCT is associated with increased mortality. Blood. 2017;129(16):2316-2325.
61. Opota O, Croxatto A, Prod’hom G, Greub G. Blood culture-based diagnosis of bacteraemia: state of the art. Clin Microbiol Infect 2015; 21: 313–322.
62. Chang S-S, Hsieh W-H, Liu T-S, Lee S-H, Wang C-H, et al. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis – a systemic review and meta-analysis. PLoSONE (2013) 8(5): e62323. doi:10.1371/journal.pone.0062323
63. Tann CJ, Nkurunziza P, Nakakeeto M, Oweka J, Kurinczuk JJ, Were J, Nyombi N, Hughes P, Willey BA, Elliott AM, Robertson NJ, Klein N, Harris KA.Prevalence of
bloodstream pathogens is higher in neonatal encephalopathy cases vs. controls using a novel panel of real-time PCR assays. PLoSONE. 2014;9(5):e97259. doi: 10.1371/journal. pone.0097259.
64. Wu YD, Chen LH, Wu XJ, Shang SQ, Lou JT, Du LZ, Zhao ZY. Gram stain-specifi c-probe-based real-time PCR for diagnosis and discrimination of bacterial neonatal sepsis. J Clin Microbiol. 2008;46(8):2613-9. 65. Heuser M, Ganser A, Hoelzer D. Th e hematopoietic growth factors in acute leukemia: a European perspective. Cancer Treat Res. 2011;157:339-362.
66. Rutella S, Pierelli L, Sica S, Serafi ni R, Chiusolo P, Paladini U, Leone F, Zini G, D’Onofrio G, Leone G, Piccirillo N. Efficacy of granulocyte transfusions for eutropenia-related infections: retrospective analysis of predictive factors. Cytotherapy. 2003;5(1):19-30.
Introduction
Early infections caused by opportunistic microorganisms are quite common aft er allo-HSCT, thus requiring massive and expensive anti-infectious therapy. Massive cytoreductive therapy in cancer is followed by pronounced neutropenia and exhaustion of most lymphoid cell populations [1]. Hence, a total defi ciency of immune response is evident since 1-2 weeks aft er conditioning therapy and HSCT.
The immune failure is characterized by exhaustion of specific humoral factors, e.g., serum immunoglobulins, and decreased counts of immune subpopulations (granulocytes, monocytes, T- and B cell subsets, NKs). Th ese immune indexes reflect general drop and recovery of immune system which may continue for months and years [1]. However, these cellular and humoral markers alone cannot accurately predict clinical risk of local infections at epithelial and mucosal surfaces (pneumonia, cystitis, stomatitis et.). The most hazardous terms for development of bacterial, fungal and viral infections are shown in Fig. 1. Post-transplant deficiencies of certain leukocyte populations seem to correlate with viral, bacterial and fungal infections [2]. Hence, the immune failure may, in principle, assessed via detection of multiple microorganisms which normally live and grow on skin and external mucous surfaces (i.e., oral cavity, pharynx, urethral ways etc). These opportunistic pathogens are normally controlled by the host immune surveillance. However, within weeks aft er HSCT, they tend to colonize deeper sites and areas (e.g., respiratory ways, urogenital tract etc.) causing different aff ections of internal organs. Hence, posttransplant detection of microfl ora in bloodstream and, especially, at local inflammation sites refl ects the degree of immune failure. Appropriate clinical experience was translated into numerous clinical recommendations based on empiric antibiotic treatment during early hematopoietic recovery and febrile neutropenia [3-6]. However, despite routine protection of the post-HSCT patients by broad-spectrum antibiotics, and antiviral treatment for a period of myelopoiesis reconstitution (up to 4 weeks), the rates of local post-transplant infections (e.g., pneumonia) may reach 25% of the HSCT patients [7].
Moreover, unusual sites of infection (typhlitis, perirectal infections and atypical forms of cellulitis) in neutropenic patients were noted decades ago [8]. About 60% of febrile neutropenia in early posttransplant period are classified as fever of unknown origin without distinct infectious pathogen, yet most respond to antibacterial therapy. Unusual sites of infection include typhlitis, perirectal infections and atypical forms of cellulitis.
Later on, a number of immunosuppressive drugs are routinely used after allogeneic HSCT (allo-HSCT) in order to prevent graft -versus-host disease (GvHD). In fact, some
immune populations, especially, adaptive immunity cells, may recover at several months and years post-transplant [1,9]. Hence, a long-term immune defi ciency after SCT is a chronic condition predisposing for opportunistic infections, fungal invasions and activation of endogenous viruses. Such infectious markers could be registered, and the co-infection rates may be used for assessing the grade of immune deficiency in the patient.

Severity and clinical signifi cance of the post-HSCT immune deficiency depends on dosage and expected immunotoxic effects of drugs used in the conditioning protocol, i.e., myeloablative or nonmyeloablative regimen. However, individual damage to the patient’s immune system is modified by the following factors:
• Intensity and number of preceding cytoreductive treatments exhausting the myelo-and lymphopoiesis system;
• Pharmacokinetics of cytotoxic drugs which strongly depends on its administration route, altered liver and kidney functions, and genetic variants aff ecting activation or inactivation of the given drug(s);
• Recipient/donor immune conflict, i.e., partial HLA mismatch, severe graft -versus-host disease [11]. Therefore, personalized evaluation of selected pathogen-specific markers, their multiplicity and biodiversity seems to be an efficient tool for specifying degree of immune defi ciency observed at early and later terms post-transplant.
Hence, the aim of our review is to propose the sets (panels) of latent/opportunistic marker microorganisms as potential markers of immune defi ciency, especially, in post-transplant patients. A number of species- and genus-specifi c multiplex PCR kits, as well as some integral microbial markers could be used for severity evaluation of immune defi ciency when testing peripheral blood samples and locally infected sites.

Incidence and sites of opportunistic infections
The list of opportunistic infections in immunocompromised patients is rather broad, however, limited by distinct microbial, fungal and viral species (see Table 1).
Most bacterial species revealed in immunocompromised patients proved to be opportunistic microorganisms, i.e., those which are present in normal human skin or inhabit normal gut mucosa. The main prerequisites for growth and cytopathic/ infl ammatory effects of opportunistic fl ora are as follows: (1) Penetrating skin barrier; (2) Passing mucosal surfaces; (3) Remote dissemination via bloodstream to lymph nodes, spleen, liver, kidney, lungs; (4) Passing blood/brain barrier. As a result, posttransplant patients develop opportunistic infections of lower respiratory ways, brain, urinary tract, as well as deep abscesses caused by skin or intestinal microorganisms. Moreover, the skin microorganisms may colonize catheter lumen, then proceeding into a bloodstream infection.
Incidence of bacterial pathogens in blood cultures from immunocompromised patients
Detection of bacterial pathogens is normally performed in blood cultures, now using automatic incubation/detection systems. Blood is normally regarded as a sterile medium. However, some episodes of septicemia may occur due to occasional microbial contamination, e.g., major purulent surgery, dental extraction etc. Fungal pathogens may be detected in blood both by beta-galactomannan immunoassay, or by DNA diagnostics. Viral infection/reactivation is routinely detected in whole blood or plasma by means of PCR.
Blood screening for bacterial infections may be an efficient tool of infection monitoring. Blood culture done at a regular interval of at least 5 days. Studies on surveillance blood cultures in HSCT are routinely practicized at many centers. E.g., this approach was tested by for 205 patients admitted for auto- or allo-HSCTs [12]. Bloodstream infection was detected in 14.1% of these patients, which is at lower limits of previously reported rates (12.5-40%). Suprisingly, none of the patients who developed bloodstream infection were diagnosed by surveillance blood cultures. Blood cultures taken in presence of clinical infectious symptoms were much more informative. The most common organisms isolated were coagulasenegative staphylococci, 41/84 (49%) in pure culture and an additional 10 (12%) in mixed cultures. Klebsiella spp. and Enterococci each accounted for 5 episodes (6%) of positive cultures; Viridans group streptococci accounted for 2 episodes (2.4%); and the remaining 25% were caused by different organisms.
In some other countries, the spectrum of cultured bloodborne bacteria is shift ed towards Gram-negative microbes, i.e., Pseudomonas aeruginosa, Klebsiella pneumonia, Esherichia coli, Acinetobacter spp, Enterobacter spp. as reported by an Indian team who analyzed data from 653 pediatric cancer patients undergoing chemotherapy [13].
In a Belarus study, the incidence (top-10) of cultivable bacterial pathogens during the early period aft er HSCT, their culture and identifi cation was assessed with BacT/ALERT-Vitec technique [14]. Th e following rating of microbial detection was obtained (Table 2), with Klebsiella pneumonia and Escherichia coli. making >40% of all clinical microbial isolates.
In immunocompromised patients, herpesviruses, fungal infections,
hepatitis B and C are taken into scope.

A comprehensive randomized analysis of posttransplant infections which occured during 2 years aft er HSCT was reported by Young et al. [15] being performed in the patients from USA BMT centers. A total of 499 patients were under study exhibiting 1347 infection episodes of severe or life-threatening grade documented in 384 (77%) patients. 249 (81%) of these patients had received a BM graft and 183/250 (73%) had received a PBSC graft , at a cumulative two-year incidence of infections of 80-85%. Th e majority of these episodes, 810 (60%), were due to bacteria, with a twoyear cumulative incidence of 72.1% and 62.9% in BM versus PBSC recipients, respectively (P = .003). The cumulative incidence of bacterial infections during the first 100 days was 44.8% for BM vs. 35.0% for PBSC (P= 0.027). The infection rates were slightly more often after BM transplantation than following peripheral PBSCT (Table 3).
Local microbiotes as a source of opportunistic flora
Skin surface
Normal skin harbors hundreds of opportunistic or commensal microorganisms including bacteria, fungi and viruses that compose skin microbiome [16]. T e skin cell populations (keratinocytes, dermal cells, fi broblast, macrophages etc.) release a lot of protective factors and molecules (antimicrobial peptides etc.), to limit excessive microbial growth and colonization [17]. Therefore, survival of the microorganisms on the skin surface suffi ciently depends on their ability to resist the host defense mechanisms [18].
The major fraction is represented by coagulase-negative staphylococci such as S.epidermidis [19, 20] as well as more pathogenic S.aureus [21]. Sebaceous glands are colonized by Propionibacterium acne [22]. Candida yeasts also colonize skin surface, being, however, controlled by appropriate defense mechanisms [23]. In immunocompetent organism, these microflora interact with keratinocytes and local immune cells, thus supporting limiting local growth of skin microflora. However, in immunocompromised and elderly patients, the commensal skin staphylococci, Candida yeasts etc. may be isolated from the sites of extradermal opportunistic infections, like catheter- or pacemaker-associated infl ammatory conditions [24]. Clinical studies show that all the mentioned skin microfl ora could be revealed in blood of the immunocompromised patients (see tables 2, 3).
Oral mucosa
Hundreds microbial and viral species, of them – only up to 5-10 marker microbes, Candida yeasts and viruses (EBV). Dental flora could be revealed in bloodstream, and, especially, in heart valves [25], thus demonstrating a wide opportunity of a distant microbial contamination with opportunistic oral microflora.
Oral mucosa is protected by innate immunity factors, playing a crucial role in the regulation of oral health. E.g., epithelial cells lining oral mucosal surfaces provide not only a physical barrier but also produce diff erent antimicrobial peptides, including human β-defensins (hBDs), lactoferrin, secretory leukocyte protease inhibitor (SLPI), and various cytokines [26]. Specific IgA’s provide specifi c immune response. There are a lot of oral co-infections revealed in HIV-infected patients, such as human herpesvirus 6 and 8 (HHV-6, HHV-8), herpes simplex virus 1/2 (HSV-1/2), EBV, and human papilloma virus (HPV). Colonization with Candida species is also quite common in these patients. Oral hairy leukoplakia caused by Epstein Barr virus is a typical infection associated with immune suppression [27].
Non-tuberculosis mycobacteria (non-MBT) are also common among oral microfl ora [28]. When examining healthy persons, the non-MBT DNA was detected in the nostrils of all 10 subjects, in buccal mucosa of 8 subjects, in the oropharynx of 7 subjects, and in the dental plaques of 5 subjects.

Oropharyngeal mucosa
In a group of 82 patients with head-and-neck cancer treated by chemo/radiotherapy, the authors studied subgingival biofilm samples, oral lavages and whole saliva samples obtained to microbiologically analyze the eff ects of cancer treatments (1-year follow-up). As compared to surgically treated patients, an increased prevalence of P. gingivalis, T. forsythia, S. mutans and Candida species was observed in the treated areas, thus demonstrating immunosuppressive action of the cytotoxic therapy [29]. Signifi cance of these fi ndings in oncohematology was evaluated by Schuurhuis et al. [30] after cytostatic treatment of 28 leukaemic and 35 auto-HSCT patients. Acute oral foci of infection were found in 2 leukaemic (7%) and 2 ASCT patients (6%), and chronic oral foci of infection in 24 leukaemic (86%) and 22 ASCT patients (63%). Positive blood cultures with microorganisms potentially originating from the oral cavity occurred in 7 patients during treatment, but did not correlate with development of infectious complications.
Nasopharyngeal area is characterized by interactions between adenoid microorganisms [31]. Adenoid microbiota plays an important role in the development of various infectious and non-infectious diseases of the upper airways, such as otitis media, adenotonsillitis, rhinosinusitis and adenoid hypertrophy. Studies have suggested that adenoids could act as a potential reservoir of opportunistic pathogens. However, previous bacterial surveys of adenoids were mainly culture based and therefore might only provide an incomplete and potentially biased assessment of the microbial diversity. To develop an in-depth and comprehensive understanding of the adenoid microbial communities and test the ‘pathogen reservoir hypothesis’, we carried out a 16S rRNA based, culture-independent survey of bacterial communities on 67 human adenoids removed by surgery. Our survey revealed highly diverse adenoid bacterial communities distinct from those of other body habitats. Despite large interpersonal variations, adenoid microbiota shared a core set of taxa and can be classified into at least five major types based on its bacterial species composition.
Broncho-alveolar mucosa
Colonization of mucosal surfaces with autofl ora (cocci, yeast fungi and molds, latent viruses), has an important stimulatory effect on whole-body development of immune responses [32]. However, the opportunistic local microfl ora in immunocompromised patients may extensively grow on the mucous surfaces in association with more or less severe clinical symptoms, e.g., opportunistic gramnegative bacilli (Enterobacteriaceae, Pseudomonas, Acinetobacter spp.). Moreover, relatively virulent serovars of M. avium complex, like as other non-MBT bacteria, may colonize the bronchial and intestinal mucosal surfaces of healthy individuals in immunocompromised conditions [33]. The same could be found with fungal outgrowth and Toxoplasma activation.
Gastrointestinal tract (GIT)
Extensive gut microbiota contains hundreds of microbial species of which only few are able to grow on media used in clinical laboratories. Th e most detectable opportunistic infectious pathogens are: E.coli, C. diffi cile, Enterococcus fecalis/faecium, etc. Meanwhile, most microorganisms (up to 70%) are fastidious or non-culturable species which may be revealed only by DNA or protein diagnostics.
As reviewed by Callejas-Díaz and Gea-Banacloche [34], the C. difficile infection (CDI) is very frequent at early terms aft er hematopoietic stem cell transplantation (HSCT) – between 6 % and 20% of HSCT recipients during the first year mostly aft er allo (allo-HSCT), thus being a promising bacterial marker of intestinal dysbiosis. For diagnostics, molecular testing for the toxin genes by (PCR) is performed, along enzyme immunoassays (EIA). It correlates with worsening of bowel graft versus host disease (GVHD).
Early after cytoreductive therapy one may observe severe damage of chemosensitive intestinal mucosa [35]. Intestinal microflora undergoes sufficient changes within several days aft er intensive chemotherapy and/or HSCT. At present time, these shift s in microfl ora subsets are eff ectively detected by means of multiplex high-throughput PCR followed by sequencing of the obtained genome fragments and computer-assisted differentiation of these genome segments. The mentioned mucosal damage and infl ammation is associated with a reduced biodiversity of intestinal microfl ora developing aft er HSCT (see a review by [36] Kucher et al., 2017). In turn, altered intestinal microbiota aft er HSCT is shown to be an important risk factor of severe GVHD [37]. Hence, the interactions between gut microbial antigens and immune cells are enhanced early aft er cytotoxic chemotherapy.
Gut bacterial translocation: lessons from HIV infection
A gradually developing immune defi ciency in HIV infection, while bearing other pathogenetic features, is known to be associated with a number of concomitant infectious complications (HCV, tuberculosis etc.) and a variety of somatic comorbidities [38] (Currier, Havlir 2005). Opportunistic infections are commonly associated with HIV infection. E.g., some malignant lymphomas in HIV patients may occur due to activation of latent Epstein-Barr virus and, probably, HHV8 [39], like as secondary lymphomas in post-transplant immunocompromised patients. Bacterial opportunistic infections, such as Pneumocystis jiroveci, Toxoplasma gondii, and Mycobacterium avium threaten the patients at CD4+ T cell counts below 200 cells/mcL [40]. However, exact critical levels for CD4+ cells are quite varying. Th erefore, a multiplex detection of opportunistic pathogens would be performed, in order to assess real individual degree of clinical immunodeficiency.
In HIV-infection, the intestinal damage and microflora changes are followed by activation of monocytes and residual T cells, due to the so-called bacterial translocation, i.e., penetration of gut bacterial cell components from injured intestinal wall to bloodstream [41]. Th e bacterial translocation is registered as endotoxin or soluble monocyte (CD14) antigen detected in blood serum. Th is “subseptic” condition is recognizable in HIV-infected patients, especially, those receiving suboptimal antiretroviral therapy. According to this opinion, the damaged gut epithelium allows diff usion of bacterial antigens which stimulate monocytes, IL-17 and IL-22-secreting cells.
The mentioned markers of microbial translocation in HIV infection showed some pathogenetic links with quantitative shift s in gut microfl ora, as shown by next-generation sequencing of bacterial 16S rRNAs which identifies hundreds of intestinal microbial species [42]. The analysis of gut microbial diversity showed a more restricted microbiota spectrum, both at baseline and aft er antiretroviral therapy (ART). Th e skewed biodiversity of gut flora correlated with decreased CD4 T-cell counts and increased markers of microbial translocation and monocyte activation. Moreover, bacterial lipopolysaccharide (LPS) and soluble CD14 (monocyte antigen) concentrations in blood plasma are shown to predict mortality and disease progression in subjects with HIV infection [41]. Intestinal epithelial damage and altered gut microfl ora in allogeneic HSCT are also well proven [36]. Hence, the markers of bacterial translocation may be potentially eff ective in diagnostics of any immune deficiency, aiming to evaluate possible damage to the gut mucosa and altered immune response aft er HSCT.
Urine and urogenital ways
The urine is sterile in healthy children. Normally, the urinary epithelium is protected by specifi c antibacterial factors, e.g., Tamm-Horsfall protein, lactoferrin, defensins etc [43]. With age, however, the urinary system is colonized in ascending manner with microfl ora from skin (Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococci, Candida) and gut (Escherichia coli, Enterobacter, Enterococcus, Proteus mirabilis). However, hematogenic infections are also possible due to Staph. aureus, Haemophilus infl uenza etc., thus causing chronic cystitis and pyelitis oft en complicated by infectious kidney stone formation [44].
Protozoic infections, e.g.., Toxoplasma gondii, may be also
revealed in urogenital ways over 1st month aft er HSCT [45], with rapid disappearance from urine cells upon hematopoiesis reconstitution.
Early after hematopoietic transplantation, hemorrhagic cystitis is developing in most cases, mainly, due to urothelial damage by toxic cytostatic treatment. Meanwhile, activation of BK and JC viruses (latent polyomaviruses in urothelial cells) are additional markers of hemorrhagic cystitis at the terms of 1-2 months which are found in urine at early terms post HSCT [46].
Catheter-associated urinary infection is a special issue in immunodeficient patients. It may be caused by P.mirabilis migrating from gut [47], or other urease-positive microbes migrating, mainly, from skin. Multiple bacterial species co-exist in microbial biofilms growing on the bladder epithelium and in catheter lumen [48].
Mixed infections in immunocompromised patients
Bacterial/fungal co-infections
Combined infections with bacterial and/or fungal agents after cytotoxic treatment in post-transplant patients were described in many works. However, the mechanisms of their simultaneous emergence are not clear yet. Acute failure of protective immune mechanisms could be the most plausible explanation for these posttransplant conditions. Moreover, multiple occurence of several pathogens in a single biological sample (i.e., blood, urine, or saliva) in HSCT patients may be promoted by a symbiotic microbial growth, thus causing a co-infection. Polymicrobial complexes grown as biofilms are regularly observed in local infections, such as dental caries and parodontitis, otitis media, diabetic foot wounds etc. [49]. Similar growth of bacterial associations is typical to blood catheter-associated infections, or urinary catheter
contamination. Hence, bacterial co-infections are not rare and should be detected in HSCT setting.
The incidence of bacterial and/or fungal infections was assessed in a group of 901 stem cell transplant recipients [50]. Of them, 237 patients (27%) had microbiologically documented microorganisms isolated, including 34 patients (14%) simultaneously infected with multiple microorganisms. Th e co-infected patients seem to be at an increased risk for aGvHD and delayed graft functioning.
The same workers evaluated infection by Clostridium difficile, a common opportunistic microbe, and its association with other infectious agents [51]. Among 822 consecutive autologous and allogeneic HCST recipients, 85 cases (10.3%) of C.diffi cile-associated disease were identified. Significant associations were found between C.diffi cile detection, and neutropenic fever, as well as with bacterial coinfection, and presence of vancomycin-resistant Enterococcus faecium (VRE) colonization. Allo-HSCT patients with C.diffi cile-associated disease showed higher rates of severe gastrointestinal aGvHD.
Viral co-infections
Combined post-transplant viral co-infections are also quite common, thus suggesting their treatment by injection of the virus-specifi c immune lymphocytes [52]. Simultaneous activation of several common endogenous viruses was shown by a multiplex PCR system for 13 common viral species in some patients before allo-HSCT, while being increased within 1st month aft er transplant [53].
Simultaneous co-activation of herpesviruses is a regular finding. E.g., in a Polish study [54] 142 allo-HSCT recipients were tested for 3 herpesviruses (CMV, HHV-6 and HHV-7) for the fi rst 3 months. Reactivation of more than one virus was identifi ed in 31% of analysed patients, with CMV being most common, thus increasing risk of specifi c complications posttransplant.
In a recent study by Koskenvuo et al. [55] the authors studied 23 viruses in 53 paediatric patients up to 3 months aft er allo- HSCT. Interestingly, polyomaviruses predominated over herpesviruses thus showing their common incidence in the immunodefi cient cohort. Of them, 13 patients (25%) had viraemias by multiple viruses. Presence of viral co-infections was signifi cantly associated with aGvHD and steroid use, the factors known to suppress immune functions.
There are specific combinations of certain viral species in post-HSCT patients. According to results obtained by Jeulin et al. [56], HHV6 infection did not correlate with CMV, HSV, EBV. Meanwhile, adenovirus infection and HHV-6 activation seemed to be related in their posttransplant replication.
Mixed infectious pathogens
A large meta-analysis was performed by Klein et al. [57] based on a systematic search of different medical databases on infl uenza virus-associated infections. It covered 27 studies including 3215 participants, with widely varying coinfection rates ranging from 2% to 65%. Most common coinfecting species were S. pneumoniae and Staph. aureus, which accounted for 35% and 28% of infections followed by P.aeruginosa, S. pyogenes, H. infl uenzae,.K. pneumoniae, and M. pneumoniae.
A study by Chebotkevitch et al. [58] has revealed that cytostatic treatment was associated with higher CMV and EBV activation rates if the patients had concomitant bacterial infections, thus confi rming a potentially common reason for mixed-type infections in this cohort (Table 5).
To confirm a need for multiplex studies of infectious agents in local samples, we would like to refer our previous study concerning incidence of diff erent opportunistic pathogens in the tissues of heart valves surgically obtained from cardiosurgery patients subjected to valve replacement (Table 4). Of course, this group comprised patients with a story of septic episodes before the valve disorder had developed. However, a number of DNA species (both microbes and viruses) was revealed in 72% of the heart valves from the patients with septic endocarditis thus showing their high rates of exposure to infectious agents. Moreover, a number of the samples tested has shown positivity for both bacterial and herpesvirus DNA, thus suggesting high probability of mixed valves contamination from the circulating blood. Th e enlisted microbial species may originate from skin (Staphylococci, Streptococci, Candida spp), gut (E.Coli), oral cavity (P.Gingivalis, Actinobacillus actinomycetemcomitans, T.Forsythus), then bacteremia.
Similar data on heart valve contamination by multiple bacteria were obtained by Miller et al. [59] using broad-range 16S rDNA PCR and Sanger sequencing on a specifi c fragment of ribosomal DNA extracted from HV tissues. Hence, the existing diagnostic approach to detection of mixed microbial and viral infections in post-transplant patients seems to be rational and feasible. Multiplicity of infectious agents should then correlate with grade of anti-infectious response and risk of severe GvHD. Th is issue may be resolved by detection of group-specifi c microbial and viral markers.
Multiple infections and post-transplant mortality risk
Some previous results suggest increased mortality of HSCT patients with diagnosed multiple bacterial infections. E.g., Trifi lio et al. [50] have reviewed clinical and laboratory data of 901 HSCT recipients (675 auto- and 226, allo-HSCTs). When studying diff erent biomaterials, the authors have identified 179 patients with proven monomicrobial infection and 59 patients (24%) with multiple microorganisms, of which 34 (14%) were classifi ed as polymicrobial infection (PI), and 25 (10%) as multiple distinct episodes of infection (MDE).


There were no significant connections between the infection multiplicity, and age, gender, diagnosis, time to engraft ment, response to therapy, etc. However, overall mortality at day +100 post transplant was higher in patients with multiple infectious episodes (P =0.02 in the Kaplan–Meier analysis). These patients also proved to be at an increased risk for acute GVHD and graft failure. In particular, early and frequently presenting Gram-negative infections, and fungal (mostly, Candida) infections were associated with high mortality rates.
Common endogenous viruses (4 herpesviruses, BK polyomavirus, and adenovirus) were studied in allo-HSCT setting by Hill et al. (2017) [60]. Th e workers performed weekly quantitative PCR of blood plasma for viral DNA from 404 allo-HSCT patients. CMV was the most common virus (65% of patients), followed by BKV (54%), HHV-6B (46%), AdV (10%), and EBV (9%). CMV, BKV, and HHV-6B were the viruses most frequently seen in combination. Detection of multiple viruses until day +100 was quite common: 90% had ≥1, 62% had ≥2, 28% had ≥3, and 5% had 4 or 5 viruses. Acute GVHD grade 3-4 was associated with detection of ≥2 viruses. Myeloablative conditioning was associated with a signifi cantly higher risk for ≥3 and ≥4 viruses. Age ≤21 was associated with detection of ≥4 viruses (aHR 2.65). In general, activation of multiple dsDNA viruses had a dose-dependent association with increased mortality aft er allo-HSCT, being independent on immune reconstitution rates and acute GVHD. These data suggest opportunities to improve outcomes with better preventive antiviral strategies.
Prototype test systems for detection of multiple marker pathogens
1. Rapid blood cultures performed by several approved systems, i.e., BD BACTEC, BacT/ALERT 3D, VersaTREK and similar automated devices. They use standard aerobic and anaerobic broth media for samples and provide results within few days and even hours. The data are read by colorimetry, fl uorimetry, or redox changes of the media [61]. Th is approach allows to obtain clinical isolates for the most opportunistic bacteria listed in Table 1.
2. Manual and automated multiplex PCR was proposed by several firms, e.g., Roche, Interlabservice. To systhematize this search, a number of diagnostic panels are developed for detection of commensal/opportunistic infectious pathogens. An example of such multiplex diagnostics was developed by Roche (SeptiFast), as shown in Table 6. Appropriate systemic review and meta-analysis of SeptiFast diagnostic kits in septic patients was performed by Chang et al. (2013) [62]. A total of 34 studies enrolling 6012 patients of suspected sepsis were included. In general, sensitivity and specifi city estimates for combined bacteremia and fungemia outcome were 0.75, and 0.92, respectively. However, the mentioned multiplex PCR system covers only some opportunistic bacteria and fungi, thus missing viruses which are prone for reactivation post-HSCT. Mixed bacterial/ viral PCR assays are still under development. E.g., such multiplex real-time PCR was recently used in order to evaluate CNS infection in infants [63].
Moreover, a diagnostic panel for combined opportunistic infections should include multiplex system for detection of infectious agents in the given local sample (urine, BAL, spinal liquor etc. Such panel should detect the most common bacterial, fungal and viral pathogens (up to 15-20), in order to detect immune defi ciency and its degree (by the number of positive fi ndings per sample). It may be based either on multiplex PCR, or on microarray detection mode.
3. A more general, genus-specifi c PCR assays have been developed, e.g., in Russia. Th ese multiplex real-time PCR systems are aimed for diagnostics of vaginal or gut dysbiosis (resp., Femofl or, and ColonoFlor) detecting up to 16 microbial classes in appropriate biological samples. The ratios of diff erent microbiota members in complex biological materials are calculated, thus allowing quantify the balance of typical microbiota species in, urogenital or intestinal specimens.
4. Looking for endotoxin and surrogate markers in blood plasma, a search for microbial translocation via bloodstream (endotoxin, sCD14) may be applied, using the strategy previously used for studies of HIV infection. 
5. Detection of total microbial contamination in blood and other normally sterile media. One may use currently developed methods for detection of total microbial DNA, or specific genes for detecting Gram+ and Gram-negative microorganisms [64]. However, most workers use in-house systems for these studies, thus being allowed for research only.
6. Moreover, some antigenic tests are commonly applied in order to detect opportunistic fungal invasions (betagalactomannan) in blood and bronchoalveolar lavage. A search for mixed infections in posttransplant patients should make diagnostics of immune defi ciency more adapted for the needs of medical practice. Detection of severe-grade immune failure may justify usage of some known treatments in order to enhance innate and adaptive immunity in the patient. E.g., detection of polymicrobial infection may be a indication for usage of immunostimulatory cytokines and hemopoietic growth factors in oncohematological patients [65]. Moreover, fi nding of mixed infection in the patient could be a proven indication for G-SF-primed granulocyte transfusions, at least during posttransplant febrile neutropenia [66].
Conclusion
1) A variety of microbes and viruses normally exists on skin and mucosal surfaces of gastrointestinal and urogenital tract.
2) In cases of immune defi ciency of either type, some of the microorganisms extend or activate at other body sites, thus causing opportunistic infections, e.g., interstitial skin abscesses, pneumonias, septicemias, catheter-associated infections. Activation of endogenous viruses (e.g. CMV) is shown to cause local lesions of diff erent organs and tissues.
3) Hence, multiplicity assessment of common infectious biomarkers (bacteria, fungi and viruses) in blood and other unusual body sites may be used to assess grade of immune deficiency in posttransplant patients at early (cytopenic) and late time periods (chronic immune defi ciency).
4) In view of low culture effi ciency for many microorganisms in humans, the panels for common marker infections are developed which will reflect grade of immune defi ciency. It could be based on express bacterial diagnostics, but, mostly, on multiplex real-time PCR of distinct pathogenic species. SeptiFast could be applied to these purposes, with addition of some multiplex PCR tests for detection of viruses, in order to detect combined viral infections. Some antigenic markers (e.g., endotoxin, beta-galactomannan) could be also screened in the HSCT patients, being complementary to the battery of microbiological tests for specifying grade of the patients’ immunodeficiency.
References
1. Pankratova OS, Chukhlovin AB. Time course of immune recovery and viral reactivation following hematopoietic stem cell transplantation. Cell Th er Transplant. 2016; 5(4):32-43.
2. Podgorny PJ, Pratt LM, Liu Y, Dharmani-Khan P, Luider J, Auer-Grzesiak I, Mansoor A, Williamson TS, Ugarte-Torres A, Hoegh-Petersen M, Khan FM, Larratt L, Jimenez-Zepeda VH, Stewart DA, Russell JA, Daly A, Storek J. Low counts of B cells, natural killer cells, monocytes, dendritic cells, basophils, and eosinophils are associated with postengraft ment infections aft er allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(1):37-46.
3. Maschmeyer G, Ljungman P. Infections in Hematopoietic Stem Cell Transplant Recipients. In: A. Safdar (ed.), Principles and Practice of Cancer Infectious Diseases, Current Clinical Oncology, 17, Springer Science+Business Media, LLC 2011. P.17-25.
4. Hu B, Mohty M, Savani BN. Common non-seasonal viral infections aft er hematopoietic stem cell transplantation. Hematologie 2013; 19: 268-278.
5. Ullmann AJ, Schmidt-Hieber M, Bertz H, Heinz WJ, Kiehl M, Krüger W, Mousset S, Neuburger S, Neumann S, Penack O, Silling G, Vehreschild JJ, Einsele H, Maschmeyer G on behalf of the Infectious DiseasesWorking Party of the German Society for Hematology and Medical Oncology (AGIHO/DGHO) and the DAG-KBT (German Working Group for Blood and Marrow Transplantation). Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines. Ann Hematol. 2016;95(9):1435-1455.
6. Robinson PD, Lehrnbecher T, Phillips R, Dupuis LL, Sung L. Strategies for Empiric Management of Pediatric Fever and Neutropenia in Patients With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: A Systematic Review of Randomized Trials. J Clin Oncol. 2016;34(17):2054-2060. 7. Chen C-S, Boeckh M, Seidel K, Clark JG, Kansu E, Madtes DK, Wagner JL, Witherspoon RP, Anasetti C, Appelbaum FR, Bensinger WI, Deeg HJ, Martin PJ, Sanders JE, Storb R, Storek J, Wade J, Siadak M, Flowers MED, Sullivan KM. Incidence, risk factors, and mortality from pneumonia developing late aft er hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2003;32:515–522.
8. Bodey GP. Unusual presentations of infection in neutropenic patients. Int J Antimicrob Agents. 2000;16(2):93-95.
9. Avigan D, Pirofski L-A, Lazarus HM.Vaccination against infectious disease following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:171-183.
10. Marty FM, Rubin RH. Th e prevention of infection posttransplant: the role of prophylaxis, preemptive and empiric therapy. Transplant Int. 2006;19(1):2-11.
11. Averyanova M.Yu., Vavilov V.N., Bondarenko S.N., Uspenskaya O.S., Stancheva N.V., Semenova E.V., Volkova A.G., Smirnov B.I., Baranova I.B., Zubarovskaya L.S., Klimko N.N., Afanasyev B.V. Bacterial infections in pediatric and adolescent recipients in allogeneic hematopoietic stem cell transplantation: etiology, structure, risk factors. Journal of Infectology, 2013, 5(1): 35-43 (In Russian).
12. Ghazal SS, Stevens MP, Bearman GM, Edmond MB. Utility of surveillance blood cultures in patients undergoing hematopoietic stem celltransplantation. Antimicrobial Resistance and Infection Control 2014, 3:20.
13. Reddy R, Pathania S, Kapil A, Bakhshi S. Review of spectrum and sensitivity of bacterial bloodstream isolates in children with malignancy: A retrospective analysis from a single center. Ind J Cancer. 2014; 51(4): 425-427.
14. Stoma I, Karpov I, Milanovich N, Uss A, Iskrovi. Risk factors for mortality in patients with bloodstream infections during the pre-engraft ment period aft er hematopoietic stem cell transplantation. Blood Res. 2016; 51(2):102-106.
15. Young JAH, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, Knust K, Horowitz MM, Confer DL, Dubberke ER, Pergam SA, Marty FM, Strasfeld LM, Brown JM, Langston AA, Schuster MG, Kaul DR, Martin SI, Anasetti C for the BMT CTN Trial 0201. Infections following transplantation of bone marrow or peripheral-blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016; 22(2):359–370.
16. Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21(12):660-668.
17. Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131(10):1974-1980.
18. Roth RR, James WD. Microbiology of the skin: resident flora, ecology, infection. J Am Acad Dermatol. 1989;20(3):367-390.
19. Widerström M, Wiström J, Sjöstedt A, Monsen T. Coagulase-negative staphylococci: update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. Eur J Clin Microbiol Infect Dis. 2012;31(1):7-20.
20. Nguyen TH, Park MD, Otto M. Host response to Staphylococcus epidermidis colonization and infections. Front Cell Infect Microbiol. 2017 Mar 21;7:90. doi: 10.3389/ fcimb.2017.00090. eCollection 2017.
21. Guerra FE, Borgogna TR, Patel DM, Sward EW, Voyich JM. Epic immune battles of history: neutrophils vs. Staphylococcus aureus. Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017.
22. Christensen GJ, Brüggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes. 2014;5(2):201-215.
23. Kühbacher A, Burger-Kentischer A, Rupp S. Interaction of Candida Species with the Skin. Microorganisms. 2017 Jun 7;5(2). pii: E32. doi: 10.3390/microorganisms5020032.
24. Da Costa A, Lelièvre H, Kirkorian G, Célard M, Chevalier P, Vandenesch F, Etienne J, Touboul P. Role of the preaxillary flora in pacemaker infections: a prospective study. Circulation. 1998 ;97(18):1791-1795.
25. Zhirekhina OV, Chukhlovin AB, Sysoev KA, Gritsenko VV, Totolian AA. Detection of infectious agents in heart valves during endocarditis using PCR technique. Zh Mikrobiol Epidemiol Immunobiol. 2008; 4:96-98 (In Russian).
26. Nittayananta W, Tao R, Jiang L, Peng Y, Huang Y. Oral innate immunity in HIV infection in HAART era. J Oral Pathol Med. 2016; 45(1):3-8.
27. Reichart PA. Infections of the oral mucosa II. Bacterial, mycotic and viral infections. Mund Kiefer Gesichtschir. 1999 Nov;3(6):298-308. (In German)
28. Macovei L, McCaff erty J, Chen T, Teles F, Hasturk H, Paster BJ, Campos-Neto A.. Th e hidden ‘mycobacteriome’ of the human healthy oral cavity and upper respiratory tract. J Oral Microbiol. 2015; 7: 10.3402/jom.v7.26094.
29. Schuurhuis JM, Stokman MA, Witjes MJ, Langendijk JA, van Winkelhoff AJ, Vissink A, Spijkervet FK. Head and neck intensity modulated radiation therapy leads to an increase of opportunistic oral pathogens. Oral Oncol. 2016;58:32-40.
30. Schuurhuis JM, Span LF, Stokman MA, van Winkelhoff AJ, Vissink A, Spijkervet FK. Eff ect of leaving chronic oral fociuntreated on infectious complications during intensive chemotherapy. Br J Cancer. 2016;114(9):972-978.
31. Ren T, Glatt DU, Nguyen TN, Allen EK, Early SV, Sale M, Winther B, Wu M. 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids. Environ Microbiol. 2013;15(2):535-547.
32. Tlaskalová-Hogenová H, Tucková L, Lodinová-Zádniková R, Stepánková R, Cukrowska B, Funda DP, Striz I, Kozáková H, Trebichavský I, Sokol D, Reháková Z, Sinkora J, Fundová P, Horáková D, Jelínková L, Sánchez D. Mucosal immunity: its role in defense and allergy. Int Arch Allergy Immunol. 2002;128(2):77-89.
33. Collins FM. Mycobacterial disease, immunosuppression, and acquired immunodefi ciency syndrome. Clin Microbiol Rev. 1989; 2(4):360-377.
34. Callejas-Díaz A, Gea-Banacloche JC. Clostridium diffi cile: deleterious impact on hematopoietic stem cell transplantation. Curr Hematol Malig Rep. 2014;9(1):85-90.
35. Peterson DE, Cariello A. Mucosal damage: a major risk factor for severe complications aft er cytotoxic therapy. Semin Oncol. 2004;31(3 Suppl 8):35-44.
36. Kucher MA, Goloschapov OV, Moiseev IS, Afanasyev BV. Fecal microbiota transplantation as a method to treat complications aft er hematopoietic stem cell transplantation. Cell Ther Transplant. 2017, 6(1): 20-29.
37. Shono Y, Docampo MD, Peled JU, Perobelli SM, Jenq RR. Intestinal microbiota-related eff ects on graft -versus-host disease. Int J Hematol. 2015;101(5):428-437.
38. Currier JS, Havlir DV. Complications of HIV disease and antiretroviral therapy. Top HIV Med. 2005;13(1):16-23.
39. Navarro WH, Kaplan LD. AIDS-related lymphoproliferative disease. Blood. 2006; 107(1): 13-20.
40. Hammer SM. Clinical practice. Management of newly diagnosed HIV infection. N Engl J Med. 2005;353(16):1702-1710.
41. Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Th er. 2016 Apr 11;13:19. doi: 10.1186/s12981-016-0103-1. eCollection 2016.
42. Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, Hov JR, Noyan K, Vesterbacka J, Svärd J, Rudi K, Sönnerborg A. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS. 2015; 29(18):2409-18.
43. Zasloff M. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J Am Soc Nephrol 2007; 18(11): 2810-2816.
44. Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 2007;79 [Suppl 1]:32-36.
45. Chukhlovin AB, Zubarovskaya LS, Bondarenko SN, Eismont YuA, Semenov AV, Vladovskaya MD, Totolyan Areg A, Afanasyev BV. Activation of Toxoplasma gondii infection aft er allogeneic transplantation of haematopoietic stem cells: dependence on time of transplantation and serological status of the patients. Infect Immunity. 2014, 4(4):382-386 (In Russian).
46. Chukhlovin AB, Eismont YuA, Vavilov VN, Zubarovskaya LS, Afanasyev BV. Time- and sample-dependent diff erences in polyomavirus incidence following hematopoietic stem cell transplantation. Cell Th er Transpl 2016; 5(1):26-30.
47. Drzewiecka D. Signifi cance and roles of Proteus spp. bacteria in natural environments. Microb Ecol. 2016;72:741-758.
48. Musk DJ Jr, Hergenrother PJ. Chemical countermeasures for the control of bacterial biofi lms: eff ective compounds and promising targets. Curr Med Chem 2006; 13(18): 2163-2177
49. Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev. 2012;25(1):193-213.
50. Trifi lio SM, Pi J, Mehta J. Changing epidemiology of Clostridium diffi cile-associated disease during stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(3):405-409.
51. Trifi lio S, Zhou Z, Fong JL, Zomas A, Liu D, Zhao C, Zhang J, Mehta J. Polymicrobial bacterial or fungal infections: incidence, spectrum of infection, risk factors, and clinical outcomes from a large hematopoietic stem cell transplant center. Transpl Infect Dis. 2015;17(2):267-274.
52. Sutrave G, Blyth E, Gottlieb DJ. Cellular therapy for multiple pathogen infections aft er hematopoietic stem cell transplant. Cytotherapy. 2017. pii: S1465-3249(17)30657-6. doi:10.1016/j.jcyt.2017.07.012.
53. Inazawa N, Hori T, Hatakeyama N, Yamamoto M, Yoto Y, Nojima M, Suzuki N, Shimizu N, Tsutsumi H. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol. 2015;87(8):1427-1435.
54. Dzieciatkowski T, Tomaszewska A, Przybylski M, Rusicka P, Basak GW, Jedrzejczak WW, Wroblewska M, Halaburda K. Analysis of the shedding of three β-herpesviruses DNA in Polish patients subjected to allogeneic hematopoietic stem cell transplantation: Six-year follow up. J Clin Virol. 2016;76:30-35.
55. Koskenvuo M, Rahiala J, Sadeghi M, Waris M, Vuorinen T, Lappalainen M, Norja P, Toppinen M, Saarinen-Pihkala U, Allander T, Söderlund-Venermo M, Hedman K, Ruuskanen O, Vettenranta K. Viremic co-infections in children with allogeneic haematopoietic stem cell transplantation are predominated by human polyomaviruses. Infect Dis (Lond). 2017;49(1):35-41.
56. Jeulin H, Agrinier N, Guery M, Salmon A, Clement L, Bordigoni P, Venard V. Human herpesvirus 6 infection aft er allogeneic stem cell transplantation: incidence, outcome, and factors associated with HHV-6 reactivation. Transplantation 2013;95: 1292-1298.
57. Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, Dugas A. Th e frequency of infl uenza and bacterial coinfection: a systematic review and meta-analysis. Infl uenza Other Respir Viruses. 2016;10(5):394-403.
58. Chebotkevich VN, Bessmeltsev SS, Kiseleva EE, Stizhak NP, Kaytandzhan EI, Burylev VV. Bloodstream infections and herpesvirus activation following intensive chemotherapy of adult oncohematological patients. Cell Ther Transplant. 2016; 5(4):21-31.
59. Miller RGH, Chow B, Pillai D, Church D. Development and evaluation of a novel fast broad-range 16S ribosomal DNA PCR and sequencing assay for diagnosis of bacterial infective endocarditis: multi-year experience in a large Canadian healthcare zone and a literature review. BMC Infectious Diseases. 2016. 16:146
60. Hill JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, Milano F, Delaney C, Sorror ML, Sandmaier BM, Nichols G, Zerr DM, Jerome KR, Schiff er JT, Boeckh M. The cumulative burden of double-stranded DNA virus detection aft er allogeneic HCT is associated with increased mortality. Blood. 2017;129(16):2316-2325.
61. Opota O, Croxatto A, Prod’hom G, Greub G. Blood culture-based diagnosis of bacteraemia: state of the art. Clin Microbiol Infect 2015; 21: 313–322.
62. Chang S-S, Hsieh W-H, Liu T-S, Lee S-H, Wang C-H, et al. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis – a systemic review and meta-analysis. PLoSONE (2013) 8(5): e62323. doi:10.1371/journal.pone.0062323
63. Tann CJ, Nkurunziza P, Nakakeeto M, Oweka J, Kurinczuk JJ, Were J, Nyombi N, Hughes P, Willey BA, Elliott AM, Robertson NJ, Klein N, Harris KA.Prevalence of
bloodstream pathogens is higher in neonatal encephalopathy cases vs. controls using a novel panel of real-time PCR assays. PLoSONE. 2014;9(5):e97259. doi: 10.1371/journal. pone.0097259.
64. Wu YD, Chen LH, Wu XJ, Shang SQ, Lou JT, Du LZ, Zhao ZY. Gram stain-specifi c-probe-based real-time PCR for diagnosis and discrimination of bacterial neonatal sepsis. J Clin Microbiol. 2008;46(8):2613-9. 65. Heuser M, Ganser A, Hoelzer D. Th e hematopoietic growth factors in acute leukemia: a European perspective. Cancer Treat Res. 2011;157:339-362.
66. Rutella S, Pierelli L, Sica S, Serafi ni R, Chiusolo P, Paladini U, Leone F, Zini G, D’Onofrio G, Leone G, Piccirillo N. Efficacy of granulocyte transfusions for eutropenia-related infections: retrospective analysis of predictive factors. Cytotherapy. 2003;5(1):19-30.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20000" ["VALUE"]=> array(2) { ["TEXT"]=> string(495) "<sup>1</sup>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский<br> государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup>Университетский госпиталь Тампере, Финляндия" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(459) "1НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский
государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
2Университетский госпиталь Тампере, Финляндия" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20001" ["VALUE"]=> array(2) { ["TEXT"]=> string(4147) "<p style="text-align: justify;"> Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.<br> В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита. </p> <h2 style="text-align: justify;">Ключевые слова</h2> <p style="text-align: justify;"> Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4075) "
Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.
В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита.
Ключевые слова
Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20002" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-28-41" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-28-41" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20003" ["VALUE"]=> array(2) { ["TEXT"]=> string(98) "Alexey B. Chukhlovin<sup>1</sup>, Olga S. Pankratova<sup>2</sup><br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(68) "Alexey B. Chukhlovin1, Olga S. Pankratova2" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20004" ["VALUE"]=> array(2) { ["TEXT"]=> string(286) "<sup>1</sup>R. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology, Th e First St. Petersburg State I. Pavlov Medical University, St. Petersburg, Russia<br> <sup>2</sup>Tampere University Hospital, Tampere, Finland" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(256) "1R. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology, Th e First St. Petersburg State I. Pavlov Medical University, St. Petersburg, Russia
2Tampere University Hospital, Tampere, Finland" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20005" ["VALUE"]=> array(2) { ["TEXT"]=> string(2111) "<p style="text-align: justify;"> Early severe immune suppression occurs aft er cytoreductive cancer treatment, especially, following conditioning therapy and hematopoietic stem cell transplantation (HSCT). Posttransplant recovery of immune response proceeds slowly, in particular, for the lymphocyte subsets. At later terms, immunosuppressive drugs promote the immune defi ciency state. Therefore, HSCT is associated with activation of diff erent endogenous infections. In most instances, the infectious complications are caused by opportunistic microorganisms (bacteria, fungi and viruses) which normally inhabit skin, mucosae etc. Th eir post-treatment proliferation and migration may occur to normally non-involved body areas (blood flow, bronchoalveolar areas, urinary pathways, skin dermal layers, lymph nodes, etc.). Hence, the early posttransplant activation of latent pathogens may be detected in peripheral blood, cerebrospinal fluid, ronchoalveolar lavage, saliva, urine and other samples being normally protected by immune system. The number of infectious species found in the same patient also correlates with higher posttransplant mortality.<br> Therefore, diagnostics of common immune defi ciency markers aft er intensive cytoreductive chemotherapy could be combined with a search for opportunistic infections at the normally non-infected sites (peripheral blood, saliva, urine) as well as aff ected mucosal surfaces (sputum, bronchial secretions, cerebrospinal fl uid). Several validated diagnostic panels (mostly multiplex PCR) were developed, in order to detect and assess number of infectious markers (viral, fungal and bacterial) in the patient. Th ey could be applied for more specific evaluation of immune defi ciency grade. </p> <h2 style="text-align: justify;">Keywords</h2> <p style="text-align: justify;"> Cytoreductive therapy, immune defi ciency, bacteria, viruses, activation, expansion, multiple infections, marker microorganisms. </p> <h2 style="text-align: justify;"></h2>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2017) "
Early severe immune suppression occurs aft er cytoreductive cancer treatment, especially, following conditioning therapy and hematopoietic stem cell transplantation (HSCT). Posttransplant recovery of immune response proceeds slowly, in particular, for the lymphocyte subsets. At later terms, immunosuppressive drugs promote the immune defi ciency state. Therefore, HSCT is associated with activation of diff erent endogenous infections. In most instances, the infectious complications are caused by opportunistic microorganisms (bacteria, fungi and viruses) which normally inhabit skin, mucosae etc. Th eir post-treatment proliferation and migration may occur to normally non-involved body areas (blood flow, bronchoalveolar areas, urinary pathways, skin dermal layers, lymph nodes, etc.). Hence, the early posttransplant activation of latent pathogens may be detected in peripheral blood, cerebrospinal fluid, ronchoalveolar lavage, saliva, urine and other samples being normally protected by immune system. The number of infectious species found in the same patient also correlates with higher posttransplant mortality.
Therefore, diagnostics of common immune defi ciency markers aft er intensive cytoreductive chemotherapy could be combined with a search for opportunistic infections at the normally non-infected sites (peripheral blood, saliva, urine) as well as aff ected mucosal surfaces (sputum, bronchial secretions, cerebrospinal fl uid). Several validated diagnostic panels (mostly multiplex PCR) were developed, in order to detect and assess number of infectious markers (viral, fungal and bacterial) in the patient. Th ey could be applied for more specific evaluation of immune defi ciency grade.
Keywords
Cytoreductive therapy, immune defi ciency, bacteria, viruses, activation, expansion, multiple infections, marker microorganisms.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20006" ["VALUE"]=> string(102) "Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(102) "Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20007" ["VALUE"]=> string(4) "1093" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1093" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20008" ["VALUE"]=> string(4) "1094" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1094" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20003" ["VALUE"]=> array(2) { ["TEXT"]=> string(98) "Alexey B. Chukhlovin<sup>1</sup>, Olga S. Pankratova<sup>2</sup><br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(68) "Alexey B. Chukhlovin1, Olga S. Pankratova2" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(68) "Alexey B. Chukhlovin1, Olga S. Pankratova2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20005" ["VALUE"]=> array(2) { ["TEXT"]=> string(2111) "<p style="text-align: justify;"> Early severe immune suppression occurs aft er cytoreductive cancer treatment, especially, following conditioning therapy and hematopoietic stem cell transplantation (HSCT). Posttransplant recovery of immune response proceeds slowly, in particular, for the lymphocyte subsets. At later terms, immunosuppressive drugs promote the immune defi ciency state. Therefore, HSCT is associated with activation of diff erent endogenous infections. In most instances, the infectious complications are caused by opportunistic microorganisms (bacteria, fungi and viruses) which normally inhabit skin, mucosae etc. Th eir post-treatment proliferation and migration may occur to normally non-involved body areas (blood flow, bronchoalveolar areas, urinary pathways, skin dermal layers, lymph nodes, etc.). Hence, the early posttransplant activation of latent pathogens may be detected in peripheral blood, cerebrospinal fluid, ronchoalveolar lavage, saliva, urine and other samples being normally protected by immune system. The number of infectious species found in the same patient also correlates with higher posttransplant mortality.<br> Therefore, diagnostics of common immune defi ciency markers aft er intensive cytoreductive chemotherapy could be combined with a search for opportunistic infections at the normally non-infected sites (peripheral blood, saliva, urine) as well as aff ected mucosal surfaces (sputum, bronchial secretions, cerebrospinal fl uid). Several validated diagnostic panels (mostly multiplex PCR) were developed, in order to detect and assess number of infectious markers (viral, fungal and bacterial) in the patient. Th ey could be applied for more specific evaluation of immune defi ciency grade. </p> <h2 style="text-align: justify;">Keywords</h2> <p style="text-align: justify;"> Cytoreductive therapy, immune defi ciency, bacteria, viruses, activation, expansion, multiple infections, marker microorganisms. </p> <h2 style="text-align: justify;"></h2>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2017) "
Early severe immune suppression occurs aft er cytoreductive cancer treatment, especially, following conditioning therapy and hematopoietic stem cell transplantation (HSCT). Posttransplant recovery of immune response proceeds slowly, in particular, for the lymphocyte subsets. At later terms, immunosuppressive drugs promote the immune defi ciency state. Therefore, HSCT is associated with activation of diff erent endogenous infections. In most instances, the infectious complications are caused by opportunistic microorganisms (bacteria, fungi and viruses) which normally inhabit skin, mucosae etc. Th eir post-treatment proliferation and migration may occur to normally non-involved body areas (blood flow, bronchoalveolar areas, urinary pathways, skin dermal layers, lymph nodes, etc.). Hence, the early posttransplant activation of latent pathogens may be detected in peripheral blood, cerebrospinal fluid, ronchoalveolar lavage, saliva, urine and other samples being normally protected by immune system. The number of infectious species found in the same patient also correlates with higher posttransplant mortality.
Therefore, diagnostics of common immune defi ciency markers aft er intensive cytoreductive chemotherapy could be combined with a search for opportunistic infections at the normally non-infected sites (peripheral blood, saliva, urine) as well as aff ected mucosal surfaces (sputum, bronchial secretions, cerebrospinal fl uid). Several validated diagnostic panels (mostly multiplex PCR) were developed, in order to detect and assess number of infectious markers (viral, fungal and bacterial) in the patient. Th ey could be applied for more specific evaluation of immune defi ciency grade.
Keywords
Cytoreductive therapy, immune defi ciency, bacteria, viruses, activation, expansion, multiple infections, marker microorganisms.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2017) "
Early severe immune suppression occurs aft er cytoreductive cancer treatment, especially, following conditioning therapy and hematopoietic stem cell transplantation (HSCT). Posttransplant recovery of immune response proceeds slowly, in particular, for the lymphocyte subsets. At later terms, immunosuppressive drugs promote the immune defi ciency state. Therefore, HSCT is associated with activation of diff erent endogenous infections. In most instances, the infectious complications are caused by opportunistic microorganisms (bacteria, fungi and viruses) which normally inhabit skin, mucosae etc. Th eir post-treatment proliferation and migration may occur to normally non-involved body areas (blood flow, bronchoalveolar areas, urinary pathways, skin dermal layers, lymph nodes, etc.). Hence, the early posttransplant activation of latent pathogens may be detected in peripheral blood, cerebrospinal fluid, ronchoalveolar lavage, saliva, urine and other samples being normally protected by immune system. The number of infectious species found in the same patient also correlates with higher posttransplant mortality.
Therefore, diagnostics of common immune defi ciency markers aft er intensive cytoreductive chemotherapy could be combined with a search for opportunistic infections at the normally non-infected sites (peripheral blood, saliva, urine) as well as aff ected mucosal surfaces (sputum, bronchial secretions, cerebrospinal fl uid). Several validated diagnostic panels (mostly multiplex PCR) were developed, in order to detect and assess number of infectious markers (viral, fungal and bacterial) in the patient. Th ey could be applied for more specific evaluation of immune defi ciency grade.
Keywords
Cytoreductive therapy, immune defi ciency, bacteria, viruses, activation, expansion, multiple infections, marker microorganisms.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20002" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-28-41" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-28-41" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2017-6-4-28-41" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20006" ["VALUE"]=> string(102) "Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(102) "Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(102) "Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20004" ["VALUE"]=> array(2) { ["TEXT"]=> string(286) "<sup>1</sup>R. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology, Th e First St. Petersburg State I. Pavlov Medical University, St. Petersburg, Russia<br> <sup>2</sup>Tampere University Hospital, Tampere, Finland" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(256) "1R. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology, Th e First St. Petersburg State I. Pavlov Medical University, St. Petersburg, Russia2Tampere University Hospital, Tampere, Finland" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(256) "1R. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology, Th e First St. Petersburg State I. Pavlov Medical University, St. Petersburg, Russia
2Tampere University Hospital, Tampere, Finland" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "19999" ["VALUE"]=> array(2) { ["TEXT"]=> string(130) "Алексей Б. Чухловин<sup>1</sup>, Ольга С. Панкратова<sup>2</sup><br>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(100) "Алексей Б. Чухловин1, Ольга С. Панкратова2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(100) "Алексей Б. Чухловин1, Ольга С. Панкратова2
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20001" ["VALUE"]=> array(2) { ["TEXT"]=> string(4147) "<p style="text-align: justify;"> Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.<br> В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита. </p> <h2 style="text-align: justify;">Ключевые слова</h2> <p style="text-align: justify;"> Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4075) "
Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.
В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита.
Ключевые слова
Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4075) "
Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.
В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита.
Ключевые слова
Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "20000" ["VALUE"]=> array(2) { ["TEXT"]=> string(495) "<sup>1</sup>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский<br> государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup>Университетский госпиталь Тампере, Финляндия" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(459) "1НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургскийгосударственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
2Университетский госпиталь Тампере, Финляндия" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(459) "1НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский
государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
2Университетский госпиталь Тампере, Финляндия" } } } }
Обзорные статьи
Обзорные статьи
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19976
[VALUE] => Array
(
[TEXT] => Рюдигер Хельманн
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Рюдигер Хельманн
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19977
[VALUE] => Array
(
[TEXT] => Медицинский факультет Маннгейма, Гейдельбергский университет, Германия
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Медицинский факультет Маннгейма, Гейдельбергский университет, Германия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19978
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Стратегия лечения хронического миелолейкоза (ХМЛ) сильно изменилась после внедрения иматиниба – ингибитора тирозинкиназы (ИТК). Эти препараты стали часто применяться для первой линии лечения при ХМЛ вместо аллогенной трансплантации гемопоэтических стволовых клеток (ТГСК). При выборе ИТК в качестве терапии первой линии учитывают следующие факторы: оценка риска для больного по принятой шкале, цитогенетические маркеры со значимыми дополнительными хромосомными аномалиями (ДХА) при постановке диагноза, а также ДХА высокого риска в процессе развития ХМЛ, сопутствующие заболевания, расходы на лечение. В случаях отсутствия ответа на иматиниб, рассматриваются возможности 2-й линии терапии, с учетом показателей клинического ответа на лечение, соблюдения режима лечения, мутаций, ведущих к лекарственной устойчивости, клональной эволюции лейкоза, непереносимости данного лечения, типа лечебного учреждения.<br>
В программе CML IV, рандомизированном исследовании, направленном на оптимизацию дозы иматиниба и эффектов сочетанной терапии иматиниба с цитарабином или интерфероном α, участвовали 1551 свежевыявленных пациентов в хронической фазе ХМЛ. Основным результатом было отсутствие какого-либо преимущества любого из применявшихся методов лечения. Иматиниб в дозе 400 мг обеспечивает близкую к норме ожидаемую продолжительность жизни у больных с ХМЛ в хронической фазе. Выживаемость пациентов независима от времениответа. Клинические исходы ХМЛ теперь определяются скорее факторами заболевания и особенностями пациентов, например – сопутствующими заболеваниями и курением, а также подходом конкретных медицинских центров, нежели выбором тактики первичного лечения. Сравнение долгосрочной выживаемости после ТГСК или лечения иматинибом показало, что больные из группы низкого риска имели сходную выживаемость при обоих вариантах лечения. Попытки улучшения терапии, например, с применением ТГСК должны быть сосредоточены на группах рефрактерных пациентов, а также на показателях выживаемости, не связанных с ХМЛ. После прогрессии в бластный криз ТГСК не обеспечивает существенного преимущества в выживаемости, хотя специальное исследование показало, что наиболее долгоживущие пациенты (72%) были леченными посредством ТГСК. 70% – 80% частота 10-летней глубокой молекулярной ремиссии указывает на то, что большинство больных, леченых иматинибом, являются кандидатами на прекращение терапии.
</p>
<h2 style="text-align: justify;">Ключевые слова</h2>
<p style="text-align: justify;">
Хронический миелоидный лейкоз, ингибиторы тирозинкиназы, иматиниб, стратегия лечения, трансплантация гемопоэтических клеток, выживаемость.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Стратегия лечения хронического миелолейкоза (ХМЛ) сильно изменилась после внедрения иматиниба – ингибитора тирозинкиназы (ИТК). Эти препараты стали часто применяться для первой линии лечения при ХМЛ вместо аллогенной трансплантации гемопоэтических стволовых клеток (ТГСК). При выборе ИТК в качестве терапии первой линии учитывают следующие факторы: оценка риска для больного по принятой шкале, цитогенетические маркеры со значимыми дополнительными хромосомными аномалиями (ДХА) при постановке диагноза, а также ДХА высокого риска в процессе развития ХМЛ, сопутствующие заболевания, расходы на лечение. В случаях отсутствия ответа на иматиниб, рассматриваются возможности 2-й линии терапии, с учетом показателей клинического ответа на лечение, соблюдения режима лечения, мутаций, ведущих к лекарственной устойчивости, клональной эволюции лейкоза, непереносимости данного лечения, типа лечебного учреждения.
В программе CML IV, рандомизированном исследовании, направленном на оптимизацию дозы иматиниба и эффектов сочетанной терапии иматиниба с цитарабином или интерфероном α, участвовали 1551 свежевыявленных пациентов в хронической фазе ХМЛ. Основным результатом было отсутствие какого-либо преимущества любого из применявшихся методов лечения. Иматиниб в дозе 400 мг обеспечивает близкую к норме ожидаемую продолжительность жизни у больных с ХМЛ в хронической фазе. Выживаемость пациентов независима от времениответа. Клинические исходы ХМЛ теперь определяются скорее факторами заболевания и особенностями пациентов, например – сопутствующими заболеваниями и курением, а также подходом конкретных медицинских центров, нежели выбором тактики первичного лечения. Сравнение долгосрочной выживаемости после ТГСК или лечения иматинибом показало, что больные из группы низкого риска имели сходную выживаемость при обоих вариантах лечения. Попытки улучшения терапии, например, с применением ТГСК должны быть сосредоточены на группах рефрактерных пациентов, а также на показателях выживаемости, не связанных с ХМЛ. После прогрессии в бластный криз ТГСК не обеспечивает существенного преимущества в выживаемости, хотя специальное исследование показало, что наиболее долгоживущие пациенты (72%) были леченными посредством ТГСК. 70% – 80% частота 10-летней глубокой молекулярной ремиссии указывает на то, что большинство больных, леченых иматинибом, являются кандидатами на прекращение терапии.
Ключевые слова
Хронический миелоидный лейкоз, ингибиторы тирозинкиназы, иматиниб, стратегия лечения, трансплантация гемопоэтических клеток, выживаемость.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19979
[VALUE] => 10.18620/ctt-1866-8836-2017-6-4-13-20
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2017-6-4-13-20
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19981
[VALUE] => Array
(
[TEXT] => Rüdiger Hehlmann
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Rüdiger Hehlmann
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19982
[VALUE] => Array
(
[TEXT] => Mannheim Medical Faculty, Heidelberg University
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Mannheim Medical Faculty, Heidelberg University
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19983
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
With introduction of the tyrosine kinase inhibitor (TKI) imatinib, the treatment strategy of CML has profoundly changed. TKIs became the fi rst-line treatment of choice for CML competing with allogeneic hematopoietic stem cell transplantation (HCT). Variables to be considered in choosing TKIs for fi rst-line therapy are as follows: conventional risk score; cytogenetic fi ndings with majorroute additional chromosomal aberrations (ACA) at diagnosis, and high-risk ACA in the course of CML; comorbidities; treatment costs. In cases of refractoriness to imatinib, the 2nd line treatment options are: clinical response milestones; adherence to therapy; resistance mutations; clonal evolution; therapy intolerance; drug safety; health care setting.<br>
CML Study IV, a randomized treatment study concerning imatinib dose optimization and combined therapy with imatinib and cytarabine or interferon α included 1551 newly diagnosed patients in chronic phase. The key outcome was no superiority of survival of any treatment option. Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients. Survival is independent of time to response. Outcome of CML is currently more determined by disease and patients’ factors, e.g., comorbidities and smoking, and by center eff ects than by initial treatment selection. A comparison of long-term survival aft er HCT or imatinib treatment showed that low risk patients had similar survival with both options. Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival. After progression to blast crisis, HCT did not provide a signifi cant survival advantage, although a special study showed that most long-term survivors (72%) were patients who received a transplant. The 10-year deep molecular remission rates of 70%-80% indicate that the majority of imatinib-treated patients are candidates for treatment discontinuation.
</p>
<h2 style="text-align: justify;">Keywords</h2>
<p style="text-align: justify;">
Chronic myeloid leukemia, tyrosine kinase inhibitors, imatinib, treatment strategy, hematopoietic stem cell transplantation, survival.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
With introduction of the tyrosine kinase inhibitor (TKI) imatinib, the treatment strategy of CML has profoundly changed. TKIs became the fi rst-line treatment of choice for CML competing with allogeneic hematopoietic stem cell transplantation (HCT). Variables to be considered in choosing TKIs for fi rst-line therapy are as follows: conventional risk score; cytogenetic fi ndings with majorroute additional chromosomal aberrations (ACA) at diagnosis, and high-risk ACA in the course of CML; comorbidities; treatment costs. In cases of refractoriness to imatinib, the 2nd line treatment options are: clinical response milestones; adherence to therapy; resistance mutations; clonal evolution; therapy intolerance; drug safety; health care setting.
CML Study IV, a randomized treatment study concerning imatinib dose optimization and combined therapy with imatinib and cytarabine or interferon α included 1551 newly diagnosed patients in chronic phase. The key outcome was no superiority of survival of any treatment option. Imatinib 400 mg provides close to normal life expectancy in chronic-phase CML patients. Survival is independent of time to response. Outcome of CML is currently more determined by disease and patients’ factors, e.g., comorbidities and smoking, and by center eff ects than by initial treatment selection. A comparison of long-term survival aft er HCT or imatinib treatment showed that low risk patients had similar survival with both options. Attempts at improving treatment should focus on subgroups of refractory disease e.g. by HCT, and on non-CML determinants of survival. After progression to blast crisis, HCT did not provide a signifi cant survival advantage, although a special study showed that most long-term survivors (72%) were patients who received a transplant. The 10-year deep molecular remission rates of 70%-80% indicate that the majority of imatinib-treated patients are candidates for treatment discontinuation.
Keywords
Chronic myeloid leukemia, tyrosine kinase inhibitors, imatinib, treatment strategy, hematopoietic stem cell transplantation, survival.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19980
[VALUE] => Long-term survival with CML with imatinib or transplantation as first-line treatment: Comparison of outcomes from CML Studies IIIA and IV
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Long-term survival with CML with imatinib or transplantation as first-line treatment: Comparison of outcomes from CML Studies IIIA and IV
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19984
[VALUE] => 1065
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1065
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19985
[VALUE] => 1066
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1066
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Рюдигер Хельманн
Стратегия лечения хронического миелолейкоза (ХМЛ) сильно изменилась после внедрения иматиниба – ингибитора тирозинкиназы (ИТК). Эти препараты стали часто применяться для первой линии лечения при ХМЛ вместо аллогенной трансплантации гемопоэтических стволовых клеток (ТГСК). При выборе ИТК в качестве терапии первой линии учитывают следующие факторы: оценка риска для больного по принятой шкале, цитогенетические маркеры со значимыми дополнительными хромосомными аномалиями (ДХА) при постановке диагноза, а также ДХА высокого риска в процессе развития ХМЛ, сопутствующие заболевания, расходы на лечение. В случаях отсутствия ответа на иматиниб, рассматриваются возможности 2-й линии терапии, с учетом показателей клинического ответа на лечение, соблюдения режима лечения, мутаций, ведущих к лекарственной устойчивости, клональной эволюции лейкоза, непереносимости данного лечения, типа лечебного учреждения.
В программе CML IV, рандомизированном исследовании, направленном на оптимизацию дозы иматиниба и эффектов сочетанной терапии иматиниба с цитарабином или интерфероном α, участвовали 1551 свежевыявленных пациентов в хронической фазе ХМЛ. Основным результатом было отсутствие какого-либо преимущества любого из применявшихся методов лечения. Иматиниб в дозе 400 мг обеспечивает близкую к норме ожидаемую продолжительность жизни у больных с ХМЛ в хронической фазе. Выживаемость пациентов независима от времениответа. Клинические исходы ХМЛ теперь определяются скорее факторами заболевания и особенностями пациентов, например – сопутствующими заболеваниями и курением, а также подходом конкретных медицинских центров, нежели выбором тактики первичного лечения. Сравнение долгосрочной выживаемости после ТГСК или лечения иматинибом показало, что больные из группы низкого риска имели сходную выживаемость при обоих вариантах лечения. Попытки улучшения терапии, например, с применением ТГСК должны быть сосредоточены на группах рефрактерных пациентов, а также на показателях выживаемости, не связанных с ХМЛ. После прогрессии в бластный криз ТГСК не обеспечивает существенного преимущества в выживаемости, хотя специальное исследование показало, что наиболее долгоживущие пациенты (72%) были леченными посредством ТГСК. 70% – 80% частота 10-летней глубокой молекулярной ремиссии указывает на то, что большинство больных, леченых иматинибом, являются кандидатами на прекращение терапии.
Ключевые слова
Хронический миелоидный лейкоз, ингибиторы тирозинкиназы, иматиниб, стратегия лечения, трансплантация гемопоэтических клеток, выживаемость.
Обзорные статьи
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 20020
)
[VALUE] => Array
(
[0] => 11
)
[DESCRIPTION] => Array
(
[0] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 11
)
[~DESCRIPTION] => Array
(
[0] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19987
[VALUE] => Array
(
[TEXT] => Аксель Р. Цандер<br>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Аксель Р. Цандер
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19988
[VALUE] => Array
(
[TEXT] => Онкологический Центр Хантсмана, Университет штата Юта, США
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Онкологический Центр Хантсмана, Университет штата Юта, США
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19989
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.<br>
При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.<br>
Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза.
</p>
<h2 style="text-align: justify;">Ключевые слова</h2>
<p style="text-align: justify;">
Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.
При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.
Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза.
Ключевые слова
Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19990
[VALUE] => 10.18620/ctt-1866-8836-2017-6-4-21-27
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2017-6-4-21-27
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19991
[VALUE] => Array
(
[TEXT] => Axel R. Zander
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Axel R. Zander
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19992
[VALUE] => Array
(
[TEXT] => Huntsman Cancer Institute, University of Utah, USA
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Huntsman Cancer Institute, University of Utah, USA
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19997
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
A sufficient part of allo-HSCT is now performed for myelodysplastic syndromes and myeloproliferative neoplasms (MPN), whereas chronic myeloid leukemia is mostly treated by tyrosine kinase inhibitors (TKI). In some special situations, the reasons for allo-SCT in CML are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis). However, an improvement in progression-free survival may be obtained in advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors.<br>
When planning therapy of primary myelofi brosis, one should take into account variable clinical course of the disease using, e.g., Lille scoring system which provides some prognostic criteria, molecular genetic markers, especially, overexpression of JAK2 gene thus allowing usage of ruxolitinib. Allo-SCT can cure myelofi brosis patients transformed to leukemia. In cases of relapse, a 2nd allo-HSCT or donor lymphocyte infusion may result into prolonged survival of the patients.<br>
Results in chronic myelomonocytic leukemia patients treated with allo-HSCT depend on the pre-transplant risk scores. Patients transplanted in CR had signifi cantly longer relapse-free survival and signifi cantly longer overall survival. Early transplants were associated with higher survival rates. In cases of atypical CML, early allogeneic transplant should be performed. Allo-SCT is a method of choice in advanced systemic mastocytosis. It is performed in cases associated with non-mast cell involvement; in aggressive systemic mastocytosis, and in mast cell leukemia.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Myeloproliferative disorders, allogeneic hematopoietic stem cell transplantation, tyrosine kinase inhibitors, chronic myeloid leukemia, primary myelofi brosis, chronic myelomyelocytic leukemia, systemic mastocytosis.
</p>
<h2 style="text-align: justify;"></h2>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
A sufficient part of allo-HSCT is now performed for myelodysplastic syndromes and myeloproliferative neoplasms (MPN), whereas chronic myeloid leukemia is mostly treated by tyrosine kinase inhibitors (TKI). In some special situations, the reasons for allo-SCT in CML are as follows: TKI toxicity; resistance to common drug therapy, and advanced disease (accelerated phase or blast crisis). However, an improvement in progression-free survival may be obtained in advanced CML aft er haploidentical allo-HSCT versus graft ing from HLA-matched related/unrelated donors.
When planning therapy of primary myelofi brosis, one should take into account variable clinical course of the disease using, e.g., Lille scoring system which provides some prognostic criteria, molecular genetic markers, especially, overexpression of JAK2 gene thus allowing usage of ruxolitinib. Allo-SCT can cure myelofi brosis patients transformed to leukemia. In cases of relapse, a 2nd allo-HSCT or donor lymphocyte infusion may result into prolonged survival of the patients.
Results in chronic myelomonocytic leukemia patients treated with allo-HSCT depend on the pre-transplant risk scores. Patients transplanted in CR had signifi cantly longer relapse-free survival and signifi cantly longer overall survival. Early transplants were associated with higher survival rates. In cases of atypical CML, early allogeneic transplant should be performed. Allo-SCT is a method of choice in advanced systemic mastocytosis. It is performed in cases associated with non-mast cell involvement; in aggressive systemic mastocytosis, and in mast cell leukemia.
Keywords
Myeloproliferative disorders, allogeneic hematopoietic stem cell transplantation, tyrosine kinase inhibitors, chronic myeloid leukemia, primary myelofi brosis, chronic myelomyelocytic leukemia, systemic mastocytosis.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19993
[VALUE] => Stem cell transplantation for myeloproliferative diseases in the era of molecular therapy
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Stem cell transplantation for myeloproliferative diseases in the era of molecular therapy
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19994
[VALUE] => 1067
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1067
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 19995
[VALUE] => 1068
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1068
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Аксель Р. Цандер
Значительная часть аллогенных трансплантаций гемопоэтических стволовых клеток (алло-ТГСК) сейчас проводится по поводу миелодиспластического синдрома или миелопролиферативных заболеваний, тогда как хронический миелоидный лейкоз (ХМЛ) лечат, в основном, ингибиторами тирозинкиназ (ИТК). В некоторых особых ситуациях, есть следующие причины для алло-ТГСК при ХМЛ: токсичность препаратов ИТК, резистентность к обычной лекарственной терапии и прогрессия болезни (фаза акселерации или бластный криз). Однако повышения выживаемости без прогрессии можно добиться в продвинутой фазе ХМЛ после гаплоидентичной ТГСК, по сравнению с пересадкой от HLA-совместимого родственного или неродственного донора.
При планировании терапии первичного миелофиброза (ПМФ) следует учитывать различия в клиническом течении заболевания, применяя, например, Лилльскую систему оценок, которая дает прогностические критерии, а также молекулярно-генетические маркеры, в особенности, гиперэкспрессию гена JAK2, что дает основания к применению рук солитиниба. Алло-ТГСК может излечивать больных с ПМФ при трансформации его в лейкоз. В случаях рецидива, вторая алло-ТГСК или инфузия лимфоцитов донора может привести к удлинению продолжительности жизни пациентов.
Результаты терапии больных с хроническим миеломоноцитарным лейкозом, леченных алло-ТГСК, зависят от оценок их клинического риска до пересадки. Пациенты, трансплантированные в полной ремиссии, имеют большую длительность общей и безрецидивной выживаемости. Раннее выполнение трансплантации коррелирует с более высокими цифрами выживаемости пациентов. В случаях атипичного ХМЛ следует проводить раннюю трансплантацию. Алло-ТГСК является методом выбора при обширном системном мастоцитозе. Она проводится в случаях, ассоциированных с вовлечением других клеток, кроме мастоцитов, при агрессивном системном мастоцитозе и при мастоцитарной форме лейкоза.
Ключевые слова
Миелопролиферативные заболевания, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназ, хронический миелоидный лейкоз, первичный миелофиброз, хронический миеломоноцитарный лейкоз, системный мастоцитоз.
Обзорные статьи
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 19999
[VALUE] => Array
(
[TEXT] => Алексей Б. Чухловин<sup>1</sup>, Ольга С. Панкратова<sup>2</sup><br>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Алексей Б. Чухловин1, Ольга С. Панкратова2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 20000
[VALUE] => Array
(
[TEXT] => <sup>1</sup>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский<br>
государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>2</sup>Университетский госпиталь Тампере, Финляндия
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский
государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
2Университетский госпиталь Тампере, Финляндия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 20001
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.<br>
В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита.
</p>
<h2 style="text-align: justify;">Ключевые слова</h2>
<p style="text-align: justify;">
Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.
В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита.
Ключевые слова
Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 20002
[VALUE] => 10.18620/ctt-1866-8836-2017-6-4-28-41
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2017-6-4-28-41
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 20003
[VALUE] => Array
(
[TEXT] => Alexey B. Chukhlovin<sup>1</sup>, Olga S. Pankratova<sup>2</sup><br>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Alexey B. Chukhlovin1, Olga S. Pankratova2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 20004
[VALUE] => Array
(
[TEXT] => <sup>1</sup>R. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology, Th e First St. Petersburg State I. Pavlov Medical University, St. Petersburg, Russia<br>
<sup>2</sup>Tampere University Hospital, Tampere, Finland
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1R. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantology, Th e First St. Petersburg State I. Pavlov Medical University, St. Petersburg, Russia
2Tampere University Hospital, Tampere, Finland
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 20005
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Early severe immune suppression occurs aft er cytoreductive cancer treatment, especially, following conditioning therapy and hematopoietic stem cell transplantation (HSCT). Posttransplant recovery of immune response proceeds slowly, in particular, for the lymphocyte subsets. At later terms, immunosuppressive drugs promote the immune defi ciency state. Therefore, HSCT is associated with activation of diff erent endogenous infections. In most instances, the infectious complications are caused by opportunistic microorganisms (bacteria, fungi and viruses) which normally inhabit skin, mucosae etc. Th eir post-treatment proliferation and migration may occur to normally non-involved body areas (blood flow, bronchoalveolar areas, urinary pathways, skin dermal layers, lymph nodes, etc.). Hence, the early posttransplant activation of latent pathogens may be detected in peripheral blood, cerebrospinal fluid, ronchoalveolar lavage, saliva, urine and other samples being normally protected by immune system. The number of infectious species found in the same patient also correlates with higher posttransplant mortality.<br>
Therefore, diagnostics of common immune defi ciency markers aft er intensive cytoreductive chemotherapy could be combined with a search for opportunistic infections at the normally non-infected sites (peripheral blood, saliva, urine) as well as aff ected mucosal surfaces (sputum, bronchial secretions, cerebrospinal fl uid). Several validated diagnostic panels (mostly multiplex PCR) were developed, in order to detect and assess number of infectious markers (viral, fungal and bacterial) in the patient. Th ey could be applied for more specific evaluation of immune defi ciency grade.
</p>
<h2 style="text-align: justify;">Keywords</h2>
<p style="text-align: justify;">
Cytoreductive therapy, immune defi ciency, bacteria, viruses, activation, expansion, multiple infections, marker microorganisms.
</p>
<h2 style="text-align: justify;"></h2>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Early severe immune suppression occurs aft er cytoreductive cancer treatment, especially, following conditioning therapy and hematopoietic stem cell transplantation (HSCT). Posttransplant recovery of immune response proceeds slowly, in particular, for the lymphocyte subsets. At later terms, immunosuppressive drugs promote the immune defi ciency state. Therefore, HSCT is associated with activation of diff erent endogenous infections. In most instances, the infectious complications are caused by opportunistic microorganisms (bacteria, fungi and viruses) which normally inhabit skin, mucosae etc. Th eir post-treatment proliferation and migration may occur to normally non-involved body areas (blood flow, bronchoalveolar areas, urinary pathways, skin dermal layers, lymph nodes, etc.). Hence, the early posttransplant activation of latent pathogens may be detected in peripheral blood, cerebrospinal fluid, ronchoalveolar lavage, saliva, urine and other samples being normally protected by immune system. The number of infectious species found in the same patient also correlates with higher posttransplant mortality.
Therefore, diagnostics of common immune defi ciency markers aft er intensive cytoreductive chemotherapy could be combined with a search for opportunistic infections at the normally non-infected sites (peripheral blood, saliva, urine) as well as aff ected mucosal surfaces (sputum, bronchial secretions, cerebrospinal fl uid). Several validated diagnostic panels (mostly multiplex PCR) were developed, in order to detect and assess number of infectious markers (viral, fungal and bacterial) in the patient. Th ey could be applied for more specific evaluation of immune defi ciency grade.
Keywords
Cytoreductive therapy, immune defi ciency, bacteria, viruses, activation, expansion, multiple infections, marker microorganisms.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 20006
[VALUE] => Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 20007
[VALUE] => 1093
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1093
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 20008
[VALUE] => 1094
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1094
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Алексей Б. Чухловин1, Ольга С. Панкратова2
государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
2Университетский госпиталь Тампере, Финляндия
Ранний и тяжелый иммунодефицит развивается после циторедуктивной терапии злокачественных новообразований, особенно после кондиционирующей терапии и трансплантации гемопоэтических стволовых клеток (ТГСК). Восстановление иммунного ответа после трансплантации происходит медленно, особенно в субпопуляциях лимфоцитов. В более поздние сроки иммуносупрессивные препараты способствуют поддержанию состояния иммунного дефицита. Поэтому после ТГСК отмечается активация различных эндогенных инфекций. В большинстве случаев инфекционные осложнения здесь вызываются оппортунистическими микроорганизмами (бактериями, грибами или вирусами), которые в норме заселяют кожные покровы, слизистые и др.). После трансплантации, их пролиферация и миграция может возникать в зонах, которые в норме не вовлечены в инфекционный процесс (сосудистый кровоток, бронхоальвеолярные зоны, мочевые пути, дермальные структуры кожи, лимфоузлы и др.). Таким образом, ранняя посттрансплантационная активация латентных патогенов может определяться в периферической крови, спинно-мозговой жидкости, бронхоальвеолярных смывах, ротовой жидкости, моче и других биологических материалах, которые в норме защищены иммунными факторами. Число видов инфекционных агентов у данного пациента также коррелирует c более высокой смертностью трансплантационных больных.
В связи с этим, диагностика обычно применяемых маркеров иммунодефицита после интенсивной циторедуктивной терапии может быть дополнена выявлением оппортунистических инфекций в тех биоматериалах, которые обычно не инфицированы ими (периферическая кровь, ротовая жидкость, моча) а также на пораженных слизистых (мокрота, бронхиальные смывы, спинномозговая жидкость). Разработаны несколько валидированных диагностических панелей, основанные, главным образом, на мультиплексной ПЦР для того, чтобы выявить и определить число маркерных микроорганизмов (вирусов, грибов и бактерий) у пациента. Их можно применять для более точной оценки глубины иммунного дефицита.
Ключевые слова
Циторедуктивная терапия, иммунодефицит, бактерии, вирусы, активация, экспансия, множественные инфекции, маркерные микроорганизмы.

