Introduction
Liver transplantation (LT) for hepatocellular carcinoma (HCC) has witnessed a historic change after the milestone article published by Mazzaferro et al. [1], who confirmed favorable outcomes for HCC patients similar to outcomes of LT for non-HCC indications. Since then, LT and its main option, the deceased-donor liver transplantation (DDLT) has become a well-established line of treatment with a highly curative profile for cirrhotic patients with HCC, but if only a liver graft is available.
The living-donor liver transplantation (LDLT) was introduced in order to reduce the organ shortage, due to expanding waiting lists for the patients awaiting liver transplant. In some countries, where the number of deceased donor is extremely small, the LDLT has been developed as an alternative treatment for the end-stage liver diseases, thus becoming an established treatment in HCC cases [2].
Nevertheless, recurrent HCC (rHCC) is still reported in up to 15% of cases following LT. HCC relapse is associated with dismal prognosis and, subsequently, LT failure in most instances [3]. Currently, LT for HCC has increased four-fold since early 2000s [4]. The impact of recurrent HCC is further enhanced by scarcity of liver grafts and fast expansion of LT waiting lists, whereas hepatic resection and other ablative modalities can offer up to 50% patient survival for HCC relapse [5].
Patient survival was shown to be better following LDLT for HCC compared to those transplanted from Deceased Donor (DD) [6, 7]. Many factors were held responsible for such results, including shorter time to transplant, better graft quality with shorter ischemia period.
Moreover, with the accumulation of cases, the impact of LDLT on the rHCC rates after LT has drawn attention of transplant community, since some reports showed higher incidence of rHCC following LDLT [8].
Many pathogenetic mechanisms were considered to explain these reports, including higher cytokine production in regenerating liver grafts and reduced time to transplant that could conceal aggressive tumor biology promoting the HCC recurrence [9-11]. These data were further analyzed to compare rHCC for patients transplanted from LDLT and DDLT source [12-15].
Hence, the aim of our single-center study was to compare overall and tumor-free survival among HCC patients subjected to liver transplantation from living donor (LDLT) versus liver transplant from deceased donor (DDLT).
Materials and methods
The study was started after obtaining the approval of Research Ethics Committee at our institution. All the patients were consented for the use of their clinical and pathological data for research purposes in anonymous manner, the patients were also informed that their decision to approve or disapprove the research consent will not influence their clinical management.
The cases of pediatric LT or liver retransplantation were excluded from this study. Finally, 136 patients diagnosed with HCC approved the research consent, being subsequently included in the study. Medical charts of the included patients were retrospectively reviewed. HCC was diagnosed by contrast-enhanced computed tomography (CT) and/or abdominal magnetic resonance imaging (MRI). The disease staging was based on chest CT, cranial CT, and technetium-99m bone scintigraphy, to exclude extra-hepatic disease. The following exclusion criteria were considered absolute contraindications for LT at our center:
• Failed or unfeasible downstaging of HCC originally beyond Milan, or UCSF transplant criteria, i.e., stable disease (SD), progressive disease (PD) according to modified RECIST criteria (16);
• Active extra-hepatic malignancies (excluding non-melanoma skin cancers);
• Untreatable advanced cardiopulmonary disease;
• Active infections e.g., active tuberculosis, uncontrollable sepsis;
• Unstable major psychiatric disorders;
• Disabling extensive intracranial neurological deficit;
• Substance abuse or active alcohol abuse over last 6 months;
• Documented medical non-compliance.
A total of 115 adult patients underwent LT for HCC at our institution between August 2006 and December 2019. LT was performed using either cadaveric, or living donor liver transplantation (LDLT). In LDLT setting, the donors were first- and second-degree relatives of respective patients.
Seventy-three patients conformed with Milan liver transplant criteria (MC) and, for the sake of comparison, they were divided into two groups as follows: Group A included forty-four HCC patients transplanted from living donors (LDLT); Group B consisted of twenty-nine HCC patients who received DDLT.
Fig. 1 illustrates the distribution of HCC patients with respect to decision for LT, and if the transplant was performed as LDLT or DDLT. The patients beyond Milan criteria, or those subjected to downstaging locoregional therapy were excluded from the study.

Figure 1. Flowchart of HCC patients enrolled during the study period
Details of the transplant procedure were documented; tumor characteristics were based on the pathological findings. HCC size, number of foci, tumor grade, and lymphatic invasion were diagnosed by an experienced pathologist. Demographic data, pre-transplant features, procedure-related variables, pathological findings were retrieved from the patient charts. Triple immunosuppression protocol was applied for the LT recipients including calcineurin inhibitor (CNI), glucocorticosteroids, and mycophenolate mofetil. Clinical and laboratory data were analyzed with t-test and Chi-square test. P-value of <0.05 was considered statistically significant. Kaplan-Meier curves were used to express survival outcomes, and statistical significance was determined by log-rank test. Overall and tumor-free survival were determined during the control contacts and examination of the patients.
Patient survival was calculated from the date of LT to the date of death or the date of the last follow-up for surviving patients. Graft survival was estimated as a death-censored graft survival. It was calculated from the date of transplantation to the date of irreversible graft failure signified by relisting of the patient on liver transplant waiting list, this method was used to avoid estimation of death with a functioning graft as a graft failure. Tumor-free survival was estimated from the date of transplant to the date of confirmed pathological evidence of recurrent HCC.
Results
Seventy-three patients underwent LT between August 2006 and December 2019 at our center for HCC which met MC. Table 1 is summarizing pre-transplant and pathological variables for these patients.
Table 1. Comparison of pre-transplant and pathological findings in LDLT and DDLT groups of the patients with hepatocellular carcinoma

Time to transplant ranged from 10 to 185 days, patients’ age, and time to transplant differed significantly between group A (living donors) and B (deceased donors), (P value, 0.002 and <0.001 respectively). On the other hand, pathological variables did not show significant difference between both groups.
The mean post-transplant follow-up for the studied patients was 46±33.3 months, ranging from 24.3 to 149.9 months. The overall 5-year patient survival, graft survival and tumor-free survival were 78.6%, 90.1% and 86.3% respectively. During the follow-up, a total of six patients manifested with rHCC (8.2%), i.e., four patients (9.1%) developed rHCC in group A, compared to two-cases (6.9%) of rHCC in group B patients, p value was 0.99. With respect to survival outcomes, LDLT group slightly differed from those who received DDLT. The overall patient survival was higher in Group A, at P=0.09 (log-rank test).
Conversely, tumor-free survival was only marginally better in group B, but it did not reach the significance level (P=0.6). On the other hand, graft survival was almost the same in both groups (P=0.99), as seen in Fig. 2 (A, B, C).

Figure 2. Comparisons for overall survival (A), tumor-free survival (B) and graft survival (C) between the groups A (LDLT) and B (DDLT) using the Kaplan-Meier approach
Discussion
There is a convincing evidence, that LT offers an excellent chance for cure of patients presenting with HCC, especially when their tumor burden is still within Milan criteria. The main concern regarding LT for HCC patients is the issue of liver graft availability and ethical costs to obtain it. Ethical concerns always overshadow the excellent results for LT in HCC patients, especially when allocation system favors HCC patients by the addition of 22 points [3, 4]. Consequently, more liver grafts are being allocated to HCC patients on the expense of patient suffering from liver insufficiency only. Furthermore, the use of LDLT to partially alleviate graft shortage is accompanied by inherent risk for healthy donor. Thus, outcomes of LT for HCC must be at optimal levels, to justify the ethical concerns encountered either in DDLT or LDLT settings. Hence, evaluation of clinical outcomes after LT for HCC in general, and comparative assessment of either LDLT or DDLT advantages over other options is quite important, especially in transplant centers where both LT modes are available.
DDLT is associated with long waiting times and consequently increased mortality and delisting rate while awaiting liver transplant. Hogen et al. [17], reported significantly higher waiting list mortality in UNOS regions with long waiting times compared to regions with short waiting times.
One of the main LDLT benefits is its ability to reduce the time to transplant and decrease the waiting list dropout. This is especially vital to the patients with HCC, to avoid tumor progression. Sandhu et al. [18], described a significantly shorter waiting time for LDLT compared to DDLT (3.1 vs 5.3 months respectively). Similarly, the current study reported significant data from the current study which showed 15% dropout rate for the patients originally presenting with HCC within Milan criteria. More than one third of this dropout rate was due to tumor progression. These patients underwent downstaging protocol using different locoregional neoadjuvant techniques.
The dropout rates vary considerably according to graft allocation policies. In the USA, there is a considerable variability of dropout rate based on the transplant waiting times. Mehta et al. [18], compared the dropout risk for HCC patients who received MELD exception points from 2005 and 2014. They described a significantly higher risk for dropout in UNOS regions with long transplant waiting times, the difference was three times higher than in the areas with short transplant waiting times (24% vs 8% dropout rate, respectively).
Few studies addressed the question of whether LDLT is associated with higher recurrence rate for HCC compared to DDLT, or not. One of the earliest reports on this issue (Lo et al.) described a significantly more common HCC recurrence at their LDLT arm (43 patients), compared to DDLT arm which included 17 patients. The study included heterogenous patient groups, i.e., corresponding the Milan or UCSF criteria, and beyond them. The cases subjected to downstaging locoregional therapy were not excluded from the study [12]. Similar findings were reported by Fischer et al. in their large cohort of patients, and they referred to different tumor characteristics, pretransplant locoregional therapies and short time to transplant as the key factors that explain the higher reported rHCC rates following LDLT [13].
On the other hand, Di Sandro et al., reported that LDLT had the same tumor-free survival compared to DDLT in the patients presenting within Milan transplant criteria, when tumor characteristics were kept unified during the study, and MC was recommended as the selection tool for further comparison of both LT modes [14]. Hence, this study reports similar findings, since LDLT was found to be insignificantly different from DDLT regarding tumor-free survival.
Such results were again confirmed by Sandhu et al., where the similar tumor-free survival rates were described in both LDLT and DDLT in a well-matched cohort of patients, especially for the tumor characteristics [15]. Ogawa et al., and Akamatsu et al., described comparable outcomes for both LDLT and DLT [19, 20]. Furthermore, Zhu et al., and Zhang et al. in their recently reported meta-analysis concluded that LDLT was not inferior to DDLT regarding overall or tumor-free survival [21, 22].
Conclusion
The results of the current study conclude that LDLT – while offering a slightly better overall survival – has insignificantly shorter tumor-free survival compared to DDLT. This is especially true, when the bias from different tumor characteristics was eliminated by studying a perfectly matched cohort of patients presenting with HCC within MC. Furthermore, LDLT group had a significantly shorter time to transplant compared to DDLT group. Consequently, the resulting decrease in dropout rate should be considered when comparing LLDT and DDLT options.
Conflict of interestsWith respect to the current study, the authors declare neither any conflict of interest, nor financial issues to be disclosed.
References
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. doi: 10.1056/NEJM199603143341104
- Akamatsu N, Sugawara Y, Kokudo N. Living donor liver transplantation for patients with hepatocellular carcinoma. Liver Cancer. 2014; 3: 108-118. doi: 10.1159/000343866
- Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008; 134:1342-1351. doi: 10.1053/j.gastro.2008.02.013
- Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11:2362-2371. doi: 10.1111/j.1600-6143.2011.03735.x
- Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266:118-125. doi: 10.1097/SLA.0000000000001894
- Gondolesi GE, Roayaie S, Muñoz L, Kim-Schluger L, Schiano T, Fishbein TM, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004; 239:142-149. doi: 10.1097/01.sla.0000109022.32391.eb
- Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004; 127:S277-S282.
doi: 10.1053/j.gastro.2004.09.042 - Vakili K, Pomposelli JJ, Cheah YL, Akoad M, Lewis WD, Khettry U, et al. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl. 2009; 15:1861-1866. doi: 10.1002/lt.21940
- Yang ZF, Poon RT, Luo Y, Cheung CK, Ho DW, Lo CM, et al. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol. 2004; 173:2507-2515.
doi: 10.4049/jimmunol.173.4.2507 - Shi JH, Huitfeldt HS, Suo ZH, Line PD. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl. 2011;17:866-874.
doi: 10.1002/lt.22325 - Efimova EA, Glanemann M, Liu L, Schumacher G, Settmacher U, Jonas S, et al. Effects of human hepatocyte growth factor on the proliferation of human hepatocytes and hepatocellular carcinoma cell lines. Eur Surg Res. 2004; 36:300-307. doi: 10.1159/000079915
- Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007; 94:78-86. doi: 10.1002/bjs.5528
- Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS Jr, et al, for A2ALL Study Group. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007; 7: 1601-1608.
doi: 10.1111/j.1600-6143.2007.01802.x - Di Sandro S, Slim AO, Giacomoni A, Lauterio A, Mangoni I, Aseni P, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc. 2009; 41: 1283-1285.
doi: 10.1016/j.transproceed.2009.03.022 - Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl. 2012; 18(3):315-22.
doi: 10.1002/lt.22477 - Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60.
doi: 10.1055/s-0030-1247132 - Hogen R, Dinorcia J, Lo M, Nguyen B, Genyk Y, Sher L, Dhanireddy K. Long wait time regions for liver transplantation for HCC have a higher cumulative incidence of death. [abstract]. Am J Transplant. 2017; 17 (suppl 3).
- Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Increasing liver transplantation wait-list dropout for hepatocellular carcinoma with widening geographical disparities: implications for organ allocation. Liver Transpl. 2018; 24(10):1346-1356. doi: 10.1002/lt.25317
- Ogawa K, Takada Y. Living vs. deceased-donor liver transplantation for patients with hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2016;1:35. doi: 10.21037/tgh.2016.04.03. eCollection 2016
- Akamatsu N, Sugawara Y, Kokudo N. Living-donor vs deceased-donor liver transplantation for patients with hepatocellular carcinoma. World J Hepatol. 2014; 6(9):626-631. doi: 10.4254/wjh.v6.i9.626
- Zhu B, Wang J, Li H, Chen X, Zeng Y. Living or deceased organ donors in liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2019; 21(2):133-147. doi: 10.1016/j.hpb.2018.11.004
- Zhang HM, Shi YX, Sun LY, Zhu ZJ. Hepatocellular carcinoma recurrence in living and deceased donor liver transplantation: a systematic review and meta-analysis. Chin Med J. 2019; 132(13):1599-1609. doi: 10.1097/CM9.0000000000000287
" ["~DETAIL_TEXT"]=> string(22008) "
Introduction
Liver transplantation (LT) for hepatocellular carcinoma (HCC) has witnessed a historic change after the milestone article published by Mazzaferro et al. [1], who confirmed favorable outcomes for HCC patients similar to outcomes of LT for non-HCC indications. Since then, LT and its main option, the deceased-donor liver transplantation (DDLT) has become a well-established line of treatment with a highly curative profile for cirrhotic patients with HCC, but if only a liver graft is available.
The living-donor liver transplantation (LDLT) was introduced in order to reduce the organ shortage, due to expanding waiting lists for the patients awaiting liver transplant. In some countries, where the number of deceased donor is extremely small, the LDLT has been developed as an alternative treatment for the end-stage liver diseases, thus becoming an established treatment in HCC cases [2].
Nevertheless, recurrent HCC (rHCC) is still reported in up to 15% of cases following LT. HCC relapse is associated with dismal prognosis and, subsequently, LT failure in most instances [3]. Currently, LT for HCC has increased four-fold since early 2000s [4]. The impact of recurrent HCC is further enhanced by scarcity of liver grafts and fast expansion of LT waiting lists, whereas hepatic resection and other ablative modalities can offer up to 50% patient survival for HCC relapse [5].
Patient survival was shown to be better following LDLT for HCC compared to those transplanted from Deceased Donor (DD) [6, 7]. Many factors were held responsible for such results, including shorter time to transplant, better graft quality with shorter ischemia period.
Moreover, with the accumulation of cases, the impact of LDLT on the rHCC rates after LT has drawn attention of transplant community, since some reports showed higher incidence of rHCC following LDLT [8].
Many pathogenetic mechanisms were considered to explain these reports, including higher cytokine production in regenerating liver grafts and reduced time to transplant that could conceal aggressive tumor biology promoting the HCC recurrence [9-11]. These data were further analyzed to compare rHCC for patients transplanted from LDLT and DDLT source [12-15].
Hence, the aim of our single-center study was to compare overall and tumor-free survival among HCC patients subjected to liver transplantation from living donor (LDLT) versus liver transplant from deceased donor (DDLT).
Materials and methods
The study was started after obtaining the approval of Research Ethics Committee at our institution. All the patients were consented for the use of their clinical and pathological data for research purposes in anonymous manner, the patients were also informed that their decision to approve or disapprove the research consent will not influence their clinical management.
The cases of pediatric LT or liver retransplantation were excluded from this study. Finally, 136 patients diagnosed with HCC approved the research consent, being subsequently included in the study. Medical charts of the included patients were retrospectively reviewed. HCC was diagnosed by contrast-enhanced computed tomography (CT) and/or abdominal magnetic resonance imaging (MRI). The disease staging was based on chest CT, cranial CT, and technetium-99m bone scintigraphy, to exclude extra-hepatic disease. The following exclusion criteria were considered absolute contraindications for LT at our center:
• Failed or unfeasible downstaging of HCC originally beyond Milan, or UCSF transplant criteria, i.e., stable disease (SD), progressive disease (PD) according to modified RECIST criteria (16);
• Active extra-hepatic malignancies (excluding non-melanoma skin cancers);
• Untreatable advanced cardiopulmonary disease;
• Active infections e.g., active tuberculosis, uncontrollable sepsis;
• Unstable major psychiatric disorders;
• Disabling extensive intracranial neurological deficit;
• Substance abuse or active alcohol abuse over last 6 months;
• Documented medical non-compliance.
A total of 115 adult patients underwent LT for HCC at our institution between August 2006 and December 2019. LT was performed using either cadaveric, or living donor liver transplantation (LDLT). In LDLT setting, the donors were first- and second-degree relatives of respective patients.
Seventy-three patients conformed with Milan liver transplant criteria (MC) and, for the sake of comparison, they were divided into two groups as follows: Group A included forty-four HCC patients transplanted from living donors (LDLT); Group B consisted of twenty-nine HCC patients who received DDLT.
Fig. 1 illustrates the distribution of HCC patients with respect to decision for LT, and if the transplant was performed as LDLT or DDLT. The patients beyond Milan criteria, or those subjected to downstaging locoregional therapy were excluded from the study.

Figure 1. Flowchart of HCC patients enrolled during the study period
Details of the transplant procedure were documented; tumor characteristics were based on the pathological findings. HCC size, number of foci, tumor grade, and lymphatic invasion were diagnosed by an experienced pathologist. Demographic data, pre-transplant features, procedure-related variables, pathological findings were retrieved from the patient charts. Triple immunosuppression protocol was applied for the LT recipients including calcineurin inhibitor (CNI), glucocorticosteroids, and mycophenolate mofetil. Clinical and laboratory data were analyzed with t-test and Chi-square test. P-value of <0.05 was considered statistically significant. Kaplan-Meier curves were used to express survival outcomes, and statistical significance was determined by log-rank test. Overall and tumor-free survival were determined during the control contacts and examination of the patients.
Patient survival was calculated from the date of LT to the date of death or the date of the last follow-up for surviving patients. Graft survival was estimated as a death-censored graft survival. It was calculated from the date of transplantation to the date of irreversible graft failure signified by relisting of the patient on liver transplant waiting list, this method was used to avoid estimation of death with a functioning graft as a graft failure. Tumor-free survival was estimated from the date of transplant to the date of confirmed pathological evidence of recurrent HCC.
Results
Seventy-three patients underwent LT between August 2006 and December 2019 at our center for HCC which met MC. Table 1 is summarizing pre-transplant and pathological variables for these patients.
Table 1. Comparison of pre-transplant and pathological findings in LDLT and DDLT groups of the patients with hepatocellular carcinoma

Time to transplant ranged from 10 to 185 days, patients’ age, and time to transplant differed significantly between group A (living donors) and B (deceased donors), (P value, 0.002 and <0.001 respectively). On the other hand, pathological variables did not show significant difference between both groups.
The mean post-transplant follow-up for the studied patients was 46±33.3 months, ranging from 24.3 to 149.9 months. The overall 5-year patient survival, graft survival and tumor-free survival were 78.6%, 90.1% and 86.3% respectively. During the follow-up, a total of six patients manifested with rHCC (8.2%), i.e., four patients (9.1%) developed rHCC in group A, compared to two-cases (6.9%) of rHCC in group B patients, p value was 0.99. With respect to survival outcomes, LDLT group slightly differed from those who received DDLT. The overall patient survival was higher in Group A, at P=0.09 (log-rank test).
Conversely, tumor-free survival was only marginally better in group B, but it did not reach the significance level (P=0.6). On the other hand, graft survival was almost the same in both groups (P=0.99), as seen in Fig. 2 (A, B, C).

Figure 2. Comparisons for overall survival (A), tumor-free survival (B) and graft survival (C) between the groups A (LDLT) and B (DDLT) using the Kaplan-Meier approach
Discussion
There is a convincing evidence, that LT offers an excellent chance for cure of patients presenting with HCC, especially when their tumor burden is still within Milan criteria. The main concern regarding LT for HCC patients is the issue of liver graft availability and ethical costs to obtain it. Ethical concerns always overshadow the excellent results for LT in HCC patients, especially when allocation system favors HCC patients by the addition of 22 points [3, 4]. Consequently, more liver grafts are being allocated to HCC patients on the expense of patient suffering from liver insufficiency only. Furthermore, the use of LDLT to partially alleviate graft shortage is accompanied by inherent risk for healthy donor. Thus, outcomes of LT for HCC must be at optimal levels, to justify the ethical concerns encountered either in DDLT or LDLT settings. Hence, evaluation of clinical outcomes after LT for HCC in general, and comparative assessment of either LDLT or DDLT advantages over other options is quite important, especially in transplant centers where both LT modes are available.
DDLT is associated with long waiting times and consequently increased mortality and delisting rate while awaiting liver transplant. Hogen et al. [17], reported significantly higher waiting list mortality in UNOS regions with long waiting times compared to regions with short waiting times.
One of the main LDLT benefits is its ability to reduce the time to transplant and decrease the waiting list dropout. This is especially vital to the patients with HCC, to avoid tumor progression. Sandhu et al. [18], described a significantly shorter waiting time for LDLT compared to DDLT (3.1 vs 5.3 months respectively). Similarly, the current study reported significant data from the current study which showed 15% dropout rate for the patients originally presenting with HCC within Milan criteria. More than one third of this dropout rate was due to tumor progression. These patients underwent downstaging protocol using different locoregional neoadjuvant techniques.
The dropout rates vary considerably according to graft allocation policies. In the USA, there is a considerable variability of dropout rate based on the transplant waiting times. Mehta et al. [18], compared the dropout risk for HCC patients who received MELD exception points from 2005 and 2014. They described a significantly higher risk for dropout in UNOS regions with long transplant waiting times, the difference was three times higher than in the areas with short transplant waiting times (24% vs 8% dropout rate, respectively).
Few studies addressed the question of whether LDLT is associated with higher recurrence rate for HCC compared to DDLT, or not. One of the earliest reports on this issue (Lo et al.) described a significantly more common HCC recurrence at their LDLT arm (43 patients), compared to DDLT arm which included 17 patients. The study included heterogenous patient groups, i.e., corresponding the Milan or UCSF criteria, and beyond them. The cases subjected to downstaging locoregional therapy were not excluded from the study [12]. Similar findings were reported by Fischer et al. in their large cohort of patients, and they referred to different tumor characteristics, pretransplant locoregional therapies and short time to transplant as the key factors that explain the higher reported rHCC rates following LDLT [13].
On the other hand, Di Sandro et al., reported that LDLT had the same tumor-free survival compared to DDLT in the patients presenting within Milan transplant criteria, when tumor characteristics were kept unified during the study, and MC was recommended as the selection tool for further comparison of both LT modes [14]. Hence, this study reports similar findings, since LDLT was found to be insignificantly different from DDLT regarding tumor-free survival.
Such results were again confirmed by Sandhu et al., where the similar tumor-free survival rates were described in both LDLT and DDLT in a well-matched cohort of patients, especially for the tumor characteristics [15]. Ogawa et al., and Akamatsu et al., described comparable outcomes for both LDLT and DLT [19, 20]. Furthermore, Zhu et al., and Zhang et al. in their recently reported meta-analysis concluded that LDLT was not inferior to DDLT regarding overall or tumor-free survival [21, 22].
Conclusion
The results of the current study conclude that LDLT – while offering a slightly better overall survival – has insignificantly shorter tumor-free survival compared to DDLT. This is especially true, when the bias from different tumor characteristics was eliminated by studying a perfectly matched cohort of patients presenting with HCC within MC. Furthermore, LDLT group had a significantly shorter time to transplant compared to DDLT group. Consequently, the resulting decrease in dropout rate should be considered when comparing LLDT and DDLT options.
Conflict of interestsWith respect to the current study, the authors declare neither any conflict of interest, nor financial issues to be disclosed.
References
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. doi: 10.1056/NEJM199603143341104
- Akamatsu N, Sugawara Y, Kokudo N. Living donor liver transplantation for patients with hepatocellular carcinoma. Liver Cancer. 2014; 3: 108-118. doi: 10.1159/000343866
- Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008; 134:1342-1351. doi: 10.1053/j.gastro.2008.02.013
- Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11:2362-2371. doi: 10.1111/j.1600-6143.2011.03735.x
- Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266:118-125. doi: 10.1097/SLA.0000000000001894
- Gondolesi GE, Roayaie S, Muñoz L, Kim-Schluger L, Schiano T, Fishbein TM, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004; 239:142-149. doi: 10.1097/01.sla.0000109022.32391.eb
- Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004; 127:S277-S282.
doi: 10.1053/j.gastro.2004.09.042 - Vakili K, Pomposelli JJ, Cheah YL, Akoad M, Lewis WD, Khettry U, et al. Living donor liver transplantation for hepatocellular carcinoma: increased recurrence but improved survival. Liver Transpl. 2009; 15:1861-1866. doi: 10.1002/lt.21940
- Yang ZF, Poon RT, Luo Y, Cheung CK, Ho DW, Lo CM, et al. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol. 2004; 173:2507-2515.
doi: 10.4049/jimmunol.173.4.2507 - Shi JH, Huitfeldt HS, Suo ZH, Line PD. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl. 2011;17:866-874.
doi: 10.1002/lt.22325 - Efimova EA, Glanemann M, Liu L, Schumacher G, Settmacher U, Jonas S, et al. Effects of human hepatocyte growth factor on the proliferation of human hepatocytes and hepatocellular carcinoma cell lines. Eur Surg Res. 2004; 36:300-307. doi: 10.1159/000079915
- Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007; 94:78-86. doi: 10.1002/bjs.5528
- Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS Jr, et al, for A2ALL Study Group. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007; 7: 1601-1608.
doi: 10.1111/j.1600-6143.2007.01802.x - Di Sandro S, Slim AO, Giacomoni A, Lauterio A, Mangoni I, Aseni P, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc. 2009; 41: 1283-1285.
doi: 10.1016/j.transproceed.2009.03.022 - Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl. 2012; 18(3):315-22.
doi: 10.1002/lt.22477 - Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60.
doi: 10.1055/s-0030-1247132 - Hogen R, Dinorcia J, Lo M, Nguyen B, Genyk Y, Sher L, Dhanireddy K. Long wait time regions for liver transplantation for HCC have a higher cumulative incidence of death. [abstract]. Am J Transplant. 2017; 17 (suppl 3).
- Mehta N, Dodge JL, Hirose R, Roberts JP, Yao FY. Increasing liver transplantation wait-list dropout for hepatocellular carcinoma with widening geographical disparities: implications for organ allocation. Liver Transpl. 2018; 24(10):1346-1356. doi: 10.1002/lt.25317
- Ogawa K, Takada Y. Living vs. deceased-donor liver transplantation for patients with hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2016;1:35. doi: 10.21037/tgh.2016.04.03. eCollection 2016
- Akamatsu N, Sugawara Y, Kokudo N. Living-donor vs deceased-donor liver transplantation for patients with hepatocellular carcinoma. World J Hepatol. 2014; 6(9):626-631. doi: 10.4254/wjh.v6.i9.626
- Zhu B, Wang J, Li H, Chen X, Zeng Y. Living or deceased organ donors in liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2019; 21(2):133-147. doi: 10.1016/j.hpb.2018.11.004
- Zhang HM, Shi YX, Sun LY, Zhu ZJ. Hepatocellular carcinoma recurrence in living and deceased donor liver transplantation: a systematic review and meta-analysis. Chin Med J. 2019; 132(13):1599-1609. doi: 10.1097/CM9.0000000000000287
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "40" ["~SORT"]=> string(2) "40" ["CODE"]=> string(100) "iskhody-transplantatsii-pecheni-u-patsientov-s-gepatotsellyulyarnoy-kartsinomoy-ot-zhivykh-donorov-p" ["~CODE"]=> string(100) "iskhody-transplantatsii-pecheni-u-patsientov-s-gepatotsellyulyarnoy-kartsinomoy-ot-zhivykh-donorov-p" ["EXTERNAL_ID"]=> string(4) "2054" ["~EXTERNAL_ID"]=> string(4) "2054" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(399) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноровOutcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(3396) "<p style="text-align: justify;">Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).</p> <h3?Пациенты и методы</h3> <p style="text-align: justify;">73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.</p> <h3>Результаты</h3> <p style="text-align: justify;">5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6). </p> <h3>Выводы</h3> <p style="text-align: justify;">Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_META_TITLE"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_META_KEYWORDS"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_META_DESCRIPTION"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_PICTURE_FILE_ALT"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_PICTURE_FILE_TITLE"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "iskhody-transplantatsii-pecheni-u-patsientov-s-gepatotsellyulyarnoy-kartsinomoy-ot-zhivykh-donorov-p" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(261) "Исходы трансплантации печени у пациентов с гепатоцеллюлярной карциномой от живых доноров по сравнению с трансплантацией от погибших доноров" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "iskhody-transplantatsii-pecheni-u-patsientov-s-gepatotsellyulyarnoy-kartsinomoy-ot-zhivykh-donorov-p" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "iskhody-transplantatsii-pecheni-u-patsientov-s-gepatotsellyulyarnoy-kartsinomoy-ot-zhivykh-donorov-p" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "iskhody-transplantatsii-pecheni-u-patsientov-s-gepatotsellyulyarnoy-kartsinomoy-ot-zhivykh-donorov-p" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "207" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28554" ["VALUE"]=> string(10) "16.11.2021" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "16.11.2021" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28555" ["VALUE"]=> string(10) "15.12.2021" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "15.12.2021" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28558" ["VALUE"]=> array(2) { ["TEXT"]=> string(95) "<p>Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(83) "
Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28559" ["VALUE"]=> array(2) { ["TEXT"]=> string(169) "<p>Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(157) "Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28560" ["VALUE"]=> array(2) { ["TEXT"]=> string(3396) "<p style="text-align: justify;">Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).</p> <h3?Пациенты и методы</h3> <p style="text-align: justify;">73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.</p> <h3>Результаты</h3> <p style="text-align: justify;">5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6). </p> <h3>Выводы</h3> <p style="text-align: justify;">Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3241) "Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).
73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.
Результаты
5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6).
Выводы
Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.
Ключевые слова
Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28556" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-43-49" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-43-49" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28561" ["VALUE"]=> array(2) { ["TEXT"]=> string(58) "<p>Mohamed R. Abdelfattah, Hussein Elsiesy</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(46) "Mohamed R. Abdelfattah, Hussein Elsiesy
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28562" ["VALUE"]=> array(2) { ["TEXT"]=> string(736) "<p style="text-align: justify;">Department of Surgery, Faculty of Medicine, University of Alexandria, Alexandria, Egypt</p><br> <p><b>Correspondence:</b><br> Prof. Dr. Mohamed Rabei Abdelfattah, Associate Professor, Department of Surgery, Faculty of Medicine, University of Alexandria, Azzaritta, Alexandria, Egypt, PO BOX 21131<br> Phone: 002010 2306 1111<br> Email: mohamad.rabie@gmail.com</p><br> <p><b>Citation:</b> Abdelfattah MR, Elsiesy H. Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors. Cell Ther Transplant 2022; 11(1): 43-49.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(636) "Department of Surgery, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah, Associate Professor, Department of Surgery, Faculty of Medicine, University of Alexandria, Azzaritta, Alexandria, Egypt, PO BOX 21131
Phone: 002010 2306 1111
Email: mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Elsiesy H. Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors. Cell Ther Transplant 2022; 11(1): 43-49.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28563" ["VALUE"]=> array(2) { ["TEXT"]=> string(1985) "<p style="text-align: justify;"> Our objective was to compare overall and tumor-free survival for the hepatocellular carcinoma (HCC) patients subjected to liver transplantation from living donor (LDLT) <i>versus </i>liver transplantation from deceased donor (DDLT) treated at our center. </p> <h3>Patients and methods</h3> <p style="text-align: justify;"> Seventy-three patients underwent liver transplantation for HCC staged according to Milan criteria (MC). The cases have been divided into two groups: (a) forty-four patients transplanted by means of LDLT, and (b) twenty-nine patients underwent DDLT. The patients beyond MC, or those who underwent downstaging locoregional therapy were excluded from the study. </p> <h3>Results</h3> <p style="text-align: justify;"> Overall survival outcomes at 5 years were, respectively, 80.3% <i>vs </i>70.4%, in LDLT and DDLT groups whereas tumor-free survival was 79.1% <i>vs</i> 76% for LDLT and DDLT cases. LT from living donors showed slightly better patients’ survival compared to liver transplantation from deceased donors DDLT (P=0.09). However, the difference in tumor-free survival between both groups was virtually absent (P=0.6). <h3>Conclusion</h3> <p style="text-align: justify;">The present study confirmed that LDLT, while offering a slightly better overall survival, is associated with similar terms of tumor-free survival compared to transplants from deceased donors. This similarity is especially clear when avoiding biases caused by different tumor features and analyzing a perfectly matched cohort of patients presenting with HCC classified according to the Milan criteria.</p> <h2>Keywords</h2> <p style="text-align: justify;">Hepatocellular carcinoma, liver transplant, living donor, deceased donor, survival. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1797) "Our objective was to compare overall and tumor-free survival for the hepatocellular carcinoma (HCC) patients subjected to liver transplantation from living donor (LDLT) versus liver transplantation from deceased donor (DDLT) treated at our center.
Patients and methods
Seventy-three patients underwent liver transplantation for HCC staged according to Milan criteria (MC). The cases have been divided into two groups: (a) forty-four patients transplanted by means of LDLT, and (b) twenty-nine patients underwent DDLT. The patients beyond MC, or those who underwent downstaging locoregional therapy were excluded from the study.
Results
Overall survival outcomes at 5 years were, respectively, 80.3% vs 70.4%, in LDLT and DDLT groups whereas tumor-free survival was 79.1% vs 76% for LDLT and DDLT cases. LT from living donors showed slightly better patients’ survival compared to liver transplantation from deceased donors DDLT (P=0.09). However, the difference in tumor-free survival between both groups was virtually absent (P=0.6).
Conclusion
The present study confirmed that LDLT, while offering a slightly better overall survival, is associated with similar terms of tumor-free survival compared to transplants from deceased donors. This similarity is especially clear when avoiding biases caused by different tumor features and analyzing a perfectly matched cohort of patients presenting with HCC classified according to the Milan criteria.
Keywords
Hepatocellular carcinoma, liver transplant, living donor, deceased donor, survival.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28557" ["VALUE"]=> string(138) "Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(138) "Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> &array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28564" ["VALUE"]=> string(4) "2802" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2802" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28565" ["VALUE"]=> string(4) "2803" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2803" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28561" ["VALUE"]=> array(2) { ["TEXT"]=> string(58) "<p>Mohamed R. Abdelfattah, Hussein Elsiesy</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(46) "Mohamed R. Abdelfattah, Hussein Elsiesy
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(46) "Mohamed R. Abdelfattah, Hussein Elsiesy
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28563" ["VALUE"]=> array(2) { ["TEXT"]=> string(1985) "<p style="text-align: justify;"> Our objective was to compare overall and tumor-free survival for the hepatocellular carcinoma (HCC) patients subjected to liver transplantation from living donor (LDLT) <i>versus </i>liver transplantation from deceased donor (DDLT) treated at our center. </p> <h3>Patients and methods</h3> <p style="text-align: justify;"> Seventy-three patients underwent liver transplantation for HCC staged according to Milan criteria (MC). The cases have been divided into two groups: (a) forty-four patients transplanted by means of LDLT, and (b) twenty-nine patients underwent DDLT. The patients beyond MC, or those who underwent downstaging locoregional therapy were excluded from the study. </p> <h3>Results</h3> <p style="text-align: justify;"> Overall survival outcomes at 5 years were, respectively, 80.3% <i>vs </i>70.4%, in LDLT and DDLT groups whereas tumor-free survival was 79.1% <i>vs</i> 76% for LDLT and DDLT cases. LT from living donors showed slightly better patients’ survival compared to liver transplantation from deceased donors DDLT (P=0.09). However, the difference in tumor-free survival between both groups was virtually absent (P=0.6). <h3>Conclusion</h3> <p style="text-align: justify;">The present study confirmed that LDLT, while offering a slightly better overall survival, is associated with similar terms of tumor-free survival compared to transplants from deceased donors. This similarity is especially clear when avoiding biases caused by different tumor features and analyzing a perfectly matched cohort of patients presenting with HCC classified according to the Milan criteria.</p> <h2>Keywords</h2> <p style="text-align: justify;">Hepatocellular carcinoma, liver transplant, living donor, deceased donor, survival. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1797) "Our objective was to compare overall and tumor-free survival for the hepatocellular carcinoma (HCC) patients subjected to liver transplantation from living donor (LDLT) versus liver transplantation from deceased donor (DDLT) treated at our center.
Patients and methods
Seventy-three patients underwent liver transplantation for HCC staged according to Milan criteria (MC). The cases have been divided into two groups: (a) forty-four patients transplanted by means of LDLT, and (b) twenty-nine patients underwent DDLT. The patients beyond MC, or those who underwent downstaging locoregional therapy were excluded from the study.
Results
Overall survival outcomes at 5 years were, respectively, 80.3% vs 70.4%, in LDLT and DDLT groups whereas tumor-free survival was 79.1% vs 76% for LDLT and DDLT cases. LT from living donors showed slightly better patients’ survival compared to liver transplantation from deceased donors DDLT (P=0.09). However, the difference in tumor-free survival between both groups was virtually absent (P=0.6).
Conclusion
The present study confirmed that LDLT, while offering a slightly better overall survival, is associated with similar terms of tumor-free survival compared to transplants from deceased donors. This similarity is especially clear when avoiding biases caused by different tumor features and analyzing a perfectly matched cohort of patients presenting with HCC classified according to the Milan criteria.
Keywords
Hepatocellular carcinoma, liver transplant, living donor, deceased donor, survival.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1797) "Our objective was to compare overall and tumor-free survival for the hepatocellular carcinoma (HCC) patients subjected to liver transplantation from living donor (LDLT) versus liver transplantation from deceased donor (DDLT) treated at our center.
Patients and methods
Seventy-three patients underwent liver transplantation for HCC staged according to Milan criteria (MC). The cases have been divided into two groups: (a) forty-four patients transplanted by means of LDLT, and (b) twenty-nine patients underwent DDLT. The patients beyond MC, or those who underwent downstaging locoregional therapy were excluded from the study.
Results
Overall survival outcomes at 5 years were, respectively, 80.3% vs 70.4%, in LDLT and DDLT groups whereas tumor-free survival was 79.1% vs 76% for LDLT and DDLT cases. LT from living donors showed slightly better patients’ survival compared to liver transplantation from deceased donors DDLT (P=0.09). However, the difference in tumor-free survival between both groups was virtually absent (P=0.6).
Conclusion
The present study confirmed that LDLT, while offering a slightly better overall survival, is associated with similar terms of tumor-free survival compared to transplants from deceased donors. This similarity is especially clear when avoiding biases caused by different tumor features and analyzing a perfectly matched cohort of patients presenting with HCC classified according to the Milan criteria.
Keywords
Hepatocellular carcinoma, liver transplant, living donor, deceased donor, survival.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28556" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-43-49" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-43-49" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-43-49" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28557" ["VALUE"]=> string(138) "Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(138) "Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(138) "Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28562" ["VALUE"]=> array(2) { ["TEXT"]=> string(736) "<p style="text-align: justify;">Department of Surgery, Faculty of Medicine, University of Alexandria, Alexandria, Egypt</p><br> <p><b>Correspondence:</b><br> Prof. Dr. Mohamed Rabei Abdelfattah, Associate Professor, Department of Surgery, Faculty of Medicine, University of Alexandria, Azzaritta, Alexandria, Egypt, PO BOX 21131<br> Phone: 002010 2306 1111<br> Email: mohamad.rabie@gmail.com</p><br> <p><b>Citation:</b> Abdelfattah MR, Elsiesy H. Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors. Cell Ther Transplant 2022; 11(1): 43-49.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(636) "Department of Surgery, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah, Associate Professor, Department of Surgery, Faculty of Medicine, University of Alexandria, Azzaritta, Alexandria, Egypt, PO BOX 21131
Phone: 002010 2306 1111
Email: mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Elsiesy H. Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors. Cell Ther Transplant 2022; 11(1): 43-49.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(636) "Department of Surgery, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah, Associate Professor, Department of Surgery, Faculty of Medicine, University of Alexandria, Azzaritta, Alexandria, Egypt, PO BOX 21131
Phone: 002010 2306 1111
Email: mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Elsiesy H. Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors. Cell Ther Transplant 2022; 11(1): 43-49.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28558" ["VALUE"]=> array(2) { ["TEXT"]=> string(95) "<p>Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(83) "Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(83) "Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28554" ["VALUE"]=> string(10) "16.11.2021" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "16.11.2021" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "16.11.2021" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28555" ["VALUE"]=> string(10) "15.12.2021" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "15.12.2021" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "15.12.2021" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28560" ["VALUE"]=> array(2) { ["TEXT"]=> string(3396) "<p style="text-align: justify;">Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).</p> <h3?Пациенты и методы</h3> <p style="text-align: justify;">73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.</p> <h3>Результаты</h3> <p style="text-align: justify;">5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6). </p> <h3>Выводы</h3> <p style="text-align: justify;">Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3241) "Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).
73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.
Результаты
5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6).
Выводы
Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.
Ключевые слова
Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3241) "Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).
73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.
Результаты
5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6).
Выводы
Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.
Ключевые слова
Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28559" ["VALUE"]=> array(2) { ["TEXT"]=> string(169) "<p>Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(157) "Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(157) "Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет
" } } } [1]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "207" ["~IBLOCK_SECTION_ID"]=> string(3) "207" ["ID"]=> string(4) "2053" ["~ID"]=> string(4) "2053" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["~NAME"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(19) "18.04.2022 14:46:14" ["~TIMESTAMP_X"]=> string(19) "18.04.2022 14:46:14" ["DETAIL_PAGE_URL"]=> string(144) "/ru/archive/tom-11-nomer-1/klinicheskie-raboty/mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok/" ["~DETAIL_PAGE_URL"]=> string(144) "/ru/archive/tom-11-nomer-1/klinicheskie-raboty/mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok/" ["LIST_PAGE_URL"]=> string(12) "/ru/archive/" ["~LIST_PAGE_URL"]=> string(12) "/ru/archive/" ["DETAIL_TEXT"]=> string(22443) "Introduction
Hematopoietic stem cell transplantation (HSCT) is used as an efficient therapeutic approach for treatment of oncohematological diseases. This mode of treatment causes profound immunosuppression affecting non-specific and specific immunity. This temporary disorder results into frequent infectious complications over post-transplant period, which have been sufficiently studied. However, bacterial and fungal paranasal sinusitis, which is common in general population, has been scarcely studied in immunocompromised patients after HSCT [1].
According to numerous studies, sinusitis affects approximately 5-44% of all the patients in post-transplant period, mainly, at early terms after HSCT. In this regard, searching for bacteria which colonize nasal cavity and paranasal sinuses during the sinusitis seems to be an urgent task. In general excessive microbiota at the surface of nasal mucosa, paranasal sinuses and upper respiratory tract is of similar composition. Bacteriological studies of cultivable microbiota from the nasal cavity and aspirates of maxillary sinuses in chronic rhinosinusitis were carried out in different clinics of Russian Federation and revealed 154 isolates of aerobic bacteria belonging to 32 species, and 90 anaerobic lines, with predominance of Streptococci, Prevotella in aspirates, less often S.pneumoniae, H.influenza, and S.aureus [2]. Although many other microbial species were isolated from these samples, the authors did not reveal their clear relations to pathogenesis of chronic rhinosinusitis.
Over the last decade, the microbiota of paranasal sinuses has also been studied by molecular biology techniques (quantitative PCR, microarray methods, like as by NGS studies of polymorphisms in 16S rRNA gene which is universal to bacterial microbiota), thus making it possible to identify a lot of non-cultivable and previously unknown types of bacteria [3]. The authors have shown that, along with S.epidermidis, S.aureus, Corynebacteria spp, maxillar sinuses in the patients may harbor, e.g., Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, as well as Stenotrophomonas maltophilia, Enterobacter which may be associated with chronic rhinosinusitis. Significant differences in the composition and diversity of the microbiota of the paranasal sinuses largely depend on the methods used in distinct studies. E.g., analysis of the microbiota at these sites by means of next-generation sequencing (NGS) showed persistence of Staphylococci, Streptococci, Neisseria, Corynebacteria and reduced biodiversity of local microbiota in chronic rhinosinusitis [4]. In general, however, bacteriological evaluation of biological samples retains its diagnostic value because of its ability to assess antibiotic resistance of microbial isolates. The aim of this study was a comparative assessment of aerobic and facultative anaerobic microbiota components of nasal and paranasal cavities in sinusitis, which is often observed in immunocompromised patients after intensive chemotherapy, antibiotic therapy and subsequent HSCT.
Patients and methods
The study involved 194 patients with various myelo- and lymphoproliferative diseases aged from 1 to 62 years treated at the R. M. Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation under the protocols of intensive chemotherapy and allogeneic HSCT over the period of 2016 to 2021 as described in our previous work [5]. During HSCT, patients received an antibiotic prophylaxis regimen that included intravenous administration of fluoroquinolones (sometimes switching to oral administration) from D+1 to D+60. To this purpose, amoxicillin was also prescribed, in particular to pediatric patients. In febrile neutropenia, broad-spectrum antibiotics were empirically administered. Later on, upon occurrence of resistant microbial strains, the patients were treated with antibiotics, orally or systemically, as guided by the in vitro sensitivity testing of the microbial isolates.
As indicated by consulting specialist (ORL clinician), according to clinical indications, the biomaterial was taken from patients (nasal swabs or washings from the paranasal sinuses) at the terms of -100 to +180 days after the day of allogeneic HSCT. Of these specimens, we have examined 124 samples of the maxillary sinus punctures from 97 patients, and 973 scrapings from nasal cavity of the patients from general HSCT group.
Seeding and isolation of bacteria from the biological samples were made by classical bacteriological techniques, The isolated microorganisms were identified by means of commercial biochemical test systems (BBL Crystal), as well as with MALDI-TOF mass spectrometry using VITEK MS instrument. The sensitivity of clinical isolates to antibiotics was determined by means of disk diffusion test systems. The results of microbial sensitivity tests were interpreted according to the Guidelines of European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria. For statistical analysis, the groups of patients were also divided by age: 0-5 years (group 1); 6-14 children (2); 15-21 years old (3); >22 years (4). Moreover, the results of bacteriological examination were classified by terms post-transplant, starting from <100 days before HSCT (point 0); during the 1st month (point 1); 2nd month (point 2); 3rd month (point 3), etc., up to 6 months after HSCT (point 6). Statistical analysis of the data was carried out by means of parametric and nonparametric statistics, concerning individual types of inoculated microorganisms, and for distinct microbial associations at different times after HSCT using the STATISTICA 10 program.
Results
The detection frequency of cultured bacteria from nasal cavity and maxillary sinuses in the oncohematological patients with ORL disorders was as follows: S.epidermidis, 34.7% of biological samples (377/1097); S.viridans, 2.2% (24/1097); S.aureus, 1.91% (21/1097); Klebsiella spp., 1% (11/1097); Corynebacteria spp., 0.9% (10/1097); Pseudomonas spp., 0.54% (6/1097); E. coli, 0.36% (4/1097); Neisseria spp., 0.36% (4/1097); E.faecalis, 0.18% (2/1097). Meanwhile, Proteus, M.luteus, Citrobacter isolates were not revealed. Seeding rates for the most common bacterial species by the age groups are shown in Fig. 1A and 1B.

Figure 1. Age dependence of bacterial seeding rates for the dominant bacterial species in oncohematological patients (group 1: 0-5 years; group 2: 6-14 years; group 3: 15-21 years; group 4: >22 years)
As seen from Fig. 1, the frequency of S.epidermidis detection was minimal in younger age group and increases at the age of >15 years (1A). Also, the occurrence of S.viridans is minimal among younger patients, with a maximum in the older group (>15 years). Moreover, we assessed the dynamics for these species at various times after HSCT (Fig. 2A and 2B).

Figure 2. Dynamics of detection for the main types of bacteria at different terms after HSCT
As seen from Fig. 2A, the detection rate of S.epidermidis is sharply reduced over the first 2 months after HSCT, thus confirming significant depletion of this microbial population due to antibiotic prophylaxis and anti-infectious therapy at the early stages after HSCT. At the same time, frequency of S.viridans did not change significantly during the post-transplant period (up to 6 months).
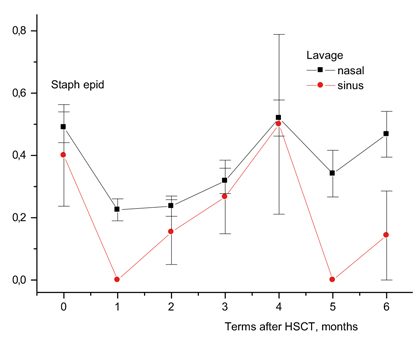
Figure 3. Frequency of S.epidermidis detection in nasal swabs (squares) and maxillary sinuses (circles) at various terms after HSCT
Separate analysis in adjacent infection loci (nasal and maxillar cavities) showed that the most profound suppression of S.epidermidis growth was observed in paranasal sinuses, especially, within 1 month after HSCT (Fig. 3). Of interest, the frequency of S.epidermidis isolation in the presence of clinically sound sinusitis was even more reduced during the 1st month after HSCT, as well as in later periods (>4 months after HSCT), as shown in Fig. 4.
High frequency of Klebsiella spp. isolation in the samples from maxillar sinuses proved to be the most pronounced feature of pathogenic microbiota in the patients at the late terms after HSCT (an average of 16.3% (20/123) versus 2.1% (18/864), p=2×10^14), along with low frequency detection in nasal swabs (Fig. 5). Similarly, high seeding rates from sinus washes were shown for Pseudomonas spp. (8.1%, 10/123 versus 0.7%, 5/864, p=1.5×10^10).

As can be seen from Table 1, S.epidermidis, the most common microflora for the nasopharynx, is found in material from the nose much more often than in swabs from the maxillary sinuses (Fig. 4). Meanwhile, inoculation with S.epidermidis from the maxillary sinuses is minimal during 1 and 5 months after HSCT. At the same time, opportunistic pathogens (E. coli, Klebsiella spp., Pseudomonas spp.) in the lavage from maxillary sinuses of the patients with sinusitis were detected more frequently than in nasal cavity lavage.
Table 1. Comparative detection frequency of main bacterial species in the samples from nasal cavity and paranasal sinuses in the patients with ORL disorders after HSCT

This confirms the diagnostic significance of the bacteriological analysis of the exudate obtained by sinus puncture and, possibly, the protective function of S.epidermidis on the skin and mucous membranes.
Next, we determined the isolation frequency of distinct microbes from the general data massive (nasal cavity + maxillary sinuses) and found that Klebsiella spp. with symptoms of sinusitis occurs significantly more often than in patients free of these symptoms, respectively, 6/102 (5.9%) versus 8/501 (1.6%), p=0.009. A similar increase was shown for Pseudomonas spp. and E.coli (see Table 2). A higher frequency of detection of Klebsiella spp. in the punctures from maxillary sinuses was noted in cases of clinical sinusitis at the 1st, 2nd month, as well as from the 4th month and later post-HSCT.
Table 2. Incidence of different bacterial species in the samples from nasal cavity and/or maxillary sinuses in the patients with sinusitis compared with sinusitis-free patients subjected to HSCT

Moreover, we estimated the frequency of bacterial findings within +30 days since clinical diagnosis of sinusitis. Higher frequency of Pseudomonas spp. (1/378 vs 7/217) was revealed in the samples from paranasal sinuses in 5 patients within 3 weeks after clinical diagnosis of sinusitis.
Antibiotic resistance
Several resistant bacterial strains were isolated from nasal cavity and paranasal sinuses in the patients with sinusitis, mostly, at later terms (>2 months post-transplant).
Over the entire period of time (2019 to 2021), the clinical staphylococcal isolates were tested for their sensitivity to cefoxitin, erythromycin, clindamycin, gentamycin, norfloxacin, tetracycline, tigecycline, and linezolid. According to the expert Guidelines of European Committee on Antimicrobial Susceptibility Testing (EUCAST). Cefoxitin is considered a screening drug in order to discriminate between the categories of methicillin-resistant staphylococci (MRS) and methicillin-sensitive strains (MSS). Throughout the observation terms, the methicillin-resistant S.aureus (MRSA) were isolated in 4% of lavage and puncture samples obtained from maxillar sinuses in the patients with oncohematological disorders. Over this period of time, we did not reveal any S. aureus strains resistant to linezolid or tigecycline. 31% of S.aureus isolates were resistant to erythromycin. The revealed category of erythromycin sensitivity, in accordance with EUCAST rules, is also extended to clarithromycin and roxytromycin. Norfloxacin resistance was shown for 5% of S.aureus strains. The norfloxacin-sensitive staphylococci are regarded as sensitive to moxifloxacin and, at higher doses (or with prolonged infusion), to levofloxacin and ciprofloxacin. Tetracycline resistance of S. aureus strains was shown in 10%. The tetracycline-sensitive staphylococci are also considered sensitive to doxycycline and minocycline. Maximal percentage of antibiotic resistance for staphylococcal strains was found with gentamycin (24%). Gradient diffusion tests for vancomycin resistance were applied to methycillin-resistant staphylococci (E-test). All the MRSA strains proved to be vancomycin-sensitive.
All the E.faecalis isolates obtained from nasal cavity of the patients treated for oncohematological disorders who suffered from sinusitis have shown good sensitivity level with antibacterial drugs, i.e., 100% to ampicillin, linezolid, vancomycin and tigecycline.
100% of S.pneumoniae strains isolated in this study were sensitive to oxacillin, and therefore, to all β-lactam antibiotics. Among them, 90% showed sensitivity to norfloxacin and, hence, to moxifloxacin. Over the study period, we have not revealed any Pneumococcus strains resistant to vancomycin and linezolid. Resistance to tetracycline and erythromycin was detected for 70% of S.pneumoniae strains.
The species of Enterobacterales exhibited different patterns of antibiotic resistance. E.g., all E. coli strains were sensitive to amikacine, 3rd-generation cephalosporins (cefotaxim, ceftazidim, ceftriaxone), protected aminopenicillines, and meropenem. Meanwhile, resistance of Klebsiella pneumoniae ssp pneumoniae, the actual nosocomial pathogen, proved to be increased over this observation period, being as high as 85% to cephalosporins (III generation) and to meropenem (60%).
Discussion
Acute sinusitis is a common complication following HSCT, and several studies addressed this issue [6, 7]. Immunosuppressive drugs, chemotherapy, radiation treatment, prolonged antibiotic therapy, autoaggressive graft-versus-host disease (GVHD), and long periods of hospitalization are predisposing factors for the upper respiratory tract infections. Current clinical diagnostics of sinusitis, both before and after HSCT, is often based on computed tomography (CT) findings, which in many cases correlate with history and clinical examination data [8]. The authors have shown that the severity of pre-transplant sinus lesions revealed on CT scans correlated with clinical and radiological signs of sinusitis later post-transplant, having been associated with a trend for reduced survival. Therefore, the clinical background before HSCT is also important for assessing further risks of developing ORL disorders. Our results of bacteriological testing are based on the clinical cohort treated at the R. M. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantation [5]. Infectious complications in allogeneic HSCT are significantly more common than in autologous HSCT, with respect to cytopenic period and rates of hematopoietic recovery. Our data refer to the patients who received allogeneic HSCT. Acute symptoms of rhinosinusitis may be registered at any term after HSCT, reaching 5.3% (95% CI 5.0%-5.6%) at the stage before transplantation; 3.01% (95% CI 2.8%-3.2%) in the course of engraftment, and 8.13% (95% CI 7.67%-8.60%), post-engraftment. From this comparison, one may suggest that massive antibiotic therapy after HSCT appears to prevent some of infectious conditions at the early stages post-transplant. However, later recolonization of pathogenic microorganisms is possible, including Klebsiella spp., S. aureus, S.pneumoniae, at a high risk of resistant strain selection, which was confirmed by us in the present work. One should note, however, that these 3 types of pathogenic bacteria were detected in a total of 13% of patients with sinusitis, i.e. the pathogen remained unknown in most cases. For additional diagnostics, along with search for pathogenic fungi and viruses, the extended diagnostics, e.g., of strictly anaerobic microbiota, are needed. In this aspect, implementation of advanced sequencing (NGS technique) will be of great importance, thus making it possible to assess biological diversity and the ratio of main microbiota classes in complex clinical samples, e.g., from mucosal surfaces.
Conclusions
1. The studies of relationships between the presence of facultative anaerobic, opportunistic microbiota in maxillar punctures and nasal cavities, and clinical course of sinusitis in the patients undergoing allogeneic HSCT did not reveal any significant correlations with severity of the disease (mild versus moderate grade).
2. Standard bacteriological testing aimed for detection of facultative anaerobic microorganisms in the maxillary sinus punctures after HSCT is of limited value within 1 month after HSCT, due to massive antibiotic therapy and suppressed growth of antibiotic-sensitive bacteria.
3. Massive antimicrobial therapy leads to the selection of resistant strains of Klebsiella spp., Pseudomonas spp., E.coli, S.aureus, mainly within 2 or more months after HSCT.
4. Low frequency of cultivable potentially pathogenic microorganisms in sino-nasal exudates cultures suggests reduced detection efficiency for the etiologically important bacteria which may cause sinusitis.
5. More sensitive and specific methods for detecting bacteria on the oropharyngeal and nasopharyngeal mucosa could be based on DNA diagnostics and multiplex PCR techniques, especially due to possible contamination of these sites with anaerobic bacteria of intestinal origin.
Conflict of interest
None declared.
References
- Bento LR, Ortiz E, Nicola EM, Vigorito AC, Sakano E. Sinonasal disorders in hematopoietic stem cell transplantation. Braz J Otorhinolaryngol. 2014; 80: 285-289. doi: 10.1016/j.bjorl.2014.05.009
- Ivanchenko OA, Karpishchenko SA, Kozlov RS, Krechikova OI, Otvagin IV, Sopko ON, Piskunov GZ, Lopatin AS. The microbiome of the maxillary sinus and middle nasal meatus in chronic rhinosinusitis. Rhinology. 2016; 54: 68-74. doi: 10.4193/Rhino15.018
- Lee JT, Frank DN, Ramakrishnan V. Microbiome of the paranasal sinuses: Update and literature review. Am J Rhinol Allergy 2016; 30: 3-16. doi: 10.2500/ajra.2016.30.4255
- De Boeck I, Wittouck S, Martens K, Claes J, Jorissen M, Steelant B, van den Broek, MFL, Seys SF, Hellings PW, Vanderveken OM, Lebeer S. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. mSphere 2019; 4:e00532-19. doi: 10.1128/mSphere.00532-19
- Dolgov OI, Karpishchenko SA, Rodneva YA, Utimisheva ES, Moiseev IS, Zubarovskaya LS, Kulagin AD. Prevalence of acute inflammatory otorhinolaryngological diseases in hematopoietic stem cell transplant recipients. Russian Otorhinolaryngology. 2021;20(3):20-26 (In Russian.). doi: 10.18692/1810-4800-2021-3-20-26
- Drozd-Sokolowska JE, Sokolowski J, Wiktor-Jedrzejczak W, Niemczyk K. Sinusitis in patients undergoing allogeneic bone marrow transplantation – a review. Braz J Otorhinolaryngol. 2017 Jan-Feb;83(1):105-111. doi: 10.1016/j.bjorl.2016.02.012
- Utimisheva ES, Dolgov OI, Paina OV, Ekushov KA, Vitrischak AA, Smirnov BI, Zubarovskaya LS, Karpischenko SA, Afanasyev BV. Incidence, diagnosis and treatment of sinusitis in children and adolescents after hematopoietic stem cell transplantation. Cell Ther Transplant. 2019; 8(1):46-53.
- Billings KR, Lowe LH, AquinoVM, Biavati MJ. Screening sinus CT scans in pediatric bone marrow transplant patients. Otorhinolaryngology. 2000; 52(3): 253-260.
" ["~DETAIL_TEXT"]=> string(22443) "
Introduction
Hematopoietic stem cell transplantation (HSCT) is used as an efficient therapeutic approach for treatment of oncohematological diseases. This mode of treatment causes profound immunosuppression affecting non-specific and specific immunity. This temporary disorder results into frequent infectious complications over post-transplant period, which have been sufficiently studied. However, bacterial and fungal paranasal sinusitis, which is common in general population, has been scarcely studied in immunocompromised patients after HSCT [1].
According to numerous studies, sinusitis affects approximately 5-44% of all the patients in post-transplant period, mainly, at early terms after HSCT. In this regard, searching for bacteria which colonize nasal cavity and paranasal sinuses during the sinusitis seems to be an urgent task. In general excessive microbiota at the surface of nasal mucosa, paranasal sinuses and upper respiratory tract is of similar composition. Bacteriological studies of cultivable microbiota from the nasal cavity and aspirates of maxillary sinuses in chronic rhinosinusitis were carried out in different clinics of Russian Federation and revealed 154 isolates of aerobic bacteria belonging to 32 species, and 90 anaerobic lines, with predominance of Streptococci, Prevotella in aspirates, less often S.pneumoniae, H.influenza, and S.aureus [2]. Although many other microbial species were isolated from these samples, the authors did not reveal their clear relations to pathogenesis of chronic rhinosinusitis.
Over the last decade, the microbiota of paranasal sinuses has also been studied by molecular biology techniques (quantitative PCR, microarray methods, like as by NGS studies of polymorphisms in 16S rRNA gene which is universal to bacterial microbiota), thus making it possible to identify a lot of non-cultivable and previously unknown types of bacteria [3]. The authors have shown that, along with S.epidermidis, S.aureus, Corynebacteria spp, maxillar sinuses in the patients may harbor, e.g., Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, as well as Stenotrophomonas maltophilia, Enterobacter which may be associated with chronic rhinosinusitis. Significant differences in the composition and diversity of the microbiota of the paranasal sinuses largely depend on the methods used in distinct studies. E.g., analysis of the microbiota at these sites by means of next-generation sequencing (NGS) showed persistence of Staphylococci, Streptococci, Neisseria, Corynebacteria and reduced biodiversity of local microbiota in chronic rhinosinusitis [4]. In general, however, bacteriological evaluation of biological samples retains its diagnostic value because of its ability to assess antibiotic resistance of microbial isolates. The aim of this study was a comparative assessment of aerobic and facultative anaerobic microbiota components of nasal and paranasal cavities in sinusitis, which is often observed in immunocompromised patients after intensive chemotherapy, antibiotic therapy and subsequent HSCT.
Patients and methods
The study involved 194 patients with various myelo- and lymphoproliferative diseases aged from 1 to 62 years treated at the R. M. Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation under the protocols of intensive chemotherapy and allogeneic HSCT over the period of 2016 to 2021 as described in our previous work [5]. During HSCT, patients received an antibiotic prophylaxis regimen that included intravenous administration of fluoroquinolones (sometimes switching to oral administration) from D+1 to D+60. To this purpose, amoxicillin was also prescribed, in particular to pediatric patients. In febrile neutropenia, broad-spectrum antibiotics were empirically administered. Later on, upon occurrence of resistant microbial strains, the patients were treated with antibiotics, orally or systemically, as guided by the in vitro sensitivity testing of the microbial isolates.
As indicated by consulting specialist (ORL clinician), according to clinical indications, the biomaterial was taken from patients (nasal swabs or washings from the paranasal sinuses) at the terms of -100 to +180 days after the day of allogeneic HSCT. Of these specimens, we have examined 124 samples of the maxillary sinus punctures from 97 patients, and 973 scrapings from nasal cavity of the patients from general HSCT group.
Seeding and isolation of bacteria from the biological samples were made by classical bacteriological techniques, The isolated microorganisms were identified by means of commercial biochemical test systems (BBL Crystal), as well as with MALDI-TOF mass spectrometry using VITEK MS instrument. The sensitivity of clinical isolates to antibiotics was determined by means of disk diffusion test systems. The results of microbial sensitivity tests were interpreted according to the Guidelines of European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria. For statistical analysis, the groups of patients were also divided by age: 0-5 years (group 1); 6-14 children (2); 15-21 years old (3); >22 years (4). Moreover, the results of bacteriological examination were classified by terms post-transplant, starting from <100 days before HSCT (point 0); during the 1st month (point 1); 2nd month (point 2); 3rd month (point 3), etc., up to 6 months after HSCT (point 6). Statistical analysis of the data was carried out by means of parametric and nonparametric statistics, concerning individual types of inoculated microorganisms, and for distinct microbial associations at different times after HSCT using the STATISTICA 10 program.
Results
The detection frequency of cultured bacteria from nasal cavity and maxillary sinuses in the oncohematological patients with ORL disorders was as follows: S.epidermidis, 34.7% of biological samples (377/1097); S.viridans, 2.2% (24/1097); S.aureus, 1.91% (21/1097); Klebsiella spp., 1% (11/1097); Corynebacteria spp., 0.9% (10/1097); Pseudomonas spp., 0.54% (6/1097); E. coli, 0.36% (4/1097); Neisseria spp., 0.36% (4/1097); E.faecalis, 0.18% (2/1097). Meanwhile, Proteus, M.luteus, Citrobacter isolates were not revealed. Seeding rates for the most common bacterial species by the age groups are shown in Fig. 1A and 1B.

Figure 1. Age dependence of bacterial seeding rates for the dominant bacterial species in oncohematological patients (group 1: 0-5 years; group 2: 6-14 years; group 3: 15-21 years; group 4: >22 years)
As seen from Fig. 1, the frequency of S.epidermidis detection was minimal in younger age group and increases at the age of >15 years (1A). Also, the occurrence of S.viridans is minimal among younger patients, with a maximum in the older group (>15 years). Moreover, we assessed the dynamics for these species at various times after HSCT (Fig. 2A and 2B).

Figure 2. Dynamics of detection for the main types of bacteria at different terms after HSCT
As seen from Fig. 2A, the detection rate of S.epidermidis is sharply reduced over the first 2 months after HSCT, thus confirming significant depletion of this microbial population due to antibiotic prophylaxis and anti-infectious therapy at the early stages after HSCT. At the same time, frequency of S.viridans did not change significantly during the post-transplant period (up to 6 months).

Figure 3. Frequency of S.epidermidis detection in nasal swabs (squares) and maxillary sinuses (circles) at various terms after HSCT
Separate analysis in adjacent infection loci (nasal and maxillar cavities) showed that the most profound suppression of S.epidermidis growth was observed in paranasal sinuses, especially, within 1 month after HSCT (Fig. 3). Of interest, the frequency of S.epidermidis isolation in the presence of clinically sound sinusitis was even more reduced during the 1st month after HSCT, as well as in later periods (>4 months after HSCT), as shown in Fig. 4.
High frequency of Klebsiella spp. isolation in the samples from maxillar sinuses proved to be the most pronounced feature of pathogenic microbiota in the patients at the late terms after HSCT (an average of 16.3% (20/123) versus 2.1% (18/864), p=2×10^14), along with low frequency detection in nasal swabs (Fig. 5). Similarly, high seeding rates from sinus washes were shown for Pseudomonas spp. (8.1%, 10/123 versus 0.7%, 5/864, p=1.5×10^10).

As can be seen from Table 1, S.epidermidis, the most common microflora for the nasopharynx, is found in material from the nose much more often than in swabs from the maxillary sinuses (Fig. 4). Meanwhile, inoculation with S.epidermidis from the maxillary sinuses is minimal during 1 and 5 months after HSCT. At the same time, opportunistic pathogens (E. coli, Klebsiella spp., Pseudomonas spp.) in the lavage from maxillary sinuses of the patients with sinusitis were detected more frequently than in nasal cavity lavage.
Table 1. Comparative detection frequency of main bacterial species in the samples from nasal cavity and paranasal sinuses in the patients with ORL disorders after HSCT

This confirms the diagnostic significance of the bacteriological analysis of the exudate obtained by sinus puncture and, possibly, the protective function of S.epidermidis on the skin and mucous membranes.
Next, we determined the isolation frequency of distinct microbes from the general data massive (nasal cavity + maxillary sinuses) and found that Klebsiella spp. with symptoms of sinusitis occurs significantly more often than in patients free of these symptoms, respectively, 6/102 (5.9%) versus 8/501 (1.6%), p=0.009. A similar increase was shown for Pseudomonas spp. and E.coli (see Table 2). A higher frequency of detection of Klebsiella spp. in the punctures from maxillary sinuses was noted in cases of clinical sinusitis at the 1st, 2nd month, as well as from the 4th month and later post-HSCT.
Table 2. Incidence of different bacterial species in the samples from nasal cavity and/or maxillary sinuses in the patients with sinusitis compared with sinusitis-free patients subjected to HSCT

Moreover, we estimated the frequency of bacterial findings within +30 days since clinical diagnosis of sinusitis. Higher frequency of Pseudomonas spp. (1/378 vs 7/217) was revealed in the samples from paranasal sinuses in 5 patients within 3 weeks after clinical diagnosis of sinusitis.
Antibiotic resistance
Several resistant bacterial strains were isolated from nasal cavity and paranasal sinuses in the patients with sinusitis, mostly, at later terms (>2 months post-transplant).
Over the entire period of time (2019 to 2021), the clinical staphylococcal isolates were tested for their sensitivity to cefoxitin, erythromycin, clindamycin, gentamycin, norfloxacin, tetracycline, tigecycline, and linezolid. According to the expert Guidelines of European Committee on Antimicrobial Susceptibility Testing (EUCAST). Cefoxitin is considered a screening drug in order to discriminate between the categories of methicillin-resistant staphylococci (MRS) and methicillin-sensitive strains (MSS). Throughout the observation terms, the methicillin-resistant S.aureus (MRSA) were isolated in 4% of lavage and puncture samples obtained from maxillar sinuses in the patients with oncohematological disorders. Over this period of time, we did not reveal any S. aureus strains resistant to linezolid or tigecycline. 31% of S.aureus isolates were resistant to erythromycin. The revealed category of erythromycin sensitivity, in accordance with EUCAST rules, is also extended to clarithromycin and roxytromycin. Norfloxacin resistance was shown for 5% of S.aureus strains. The norfloxacin-sensitive staphylococci are regarded as sensitive to moxifloxacin and, at higher doses (or with prolonged infusion), to levofloxacin and ciprofloxacin. Tetracycline resistance of S. aureus strains was shown in 10%. The tetracycline-sensitive staphylococci are also considered sensitive to doxycycline and minocycline. Maximal percentage of antibiotic resistance for staphylococcal strains was found with gentamycin (24%). Gradient diffusion tests for vancomycin resistance were applied to methycillin-resistant staphylococci (E-test). All the MRSA strains proved to be vancomycin-sensitive.
All the E.faecalis isolates obtained from nasal cavity of the patients treated for oncohematological disorders who suffered from sinusitis have shown good sensitivity level with antibacterial drugs, i.e., 100% to ampicillin, linezolid, vancomycin and tigecycline.
100% of S.pneumoniae strains isolated in this study were sensitive to oxacillin, and therefore, to all β-lactam antibiotics. Among them, 90% showed sensitivity to norfloxacin and, hence, to moxifloxacin. Over the study period, we have not revealed any Pneumococcus strains resistant to vancomycin and linezolid. Resistance to tetracycline and erythromycin was detected for 70% of S.pneumoniae strains.
The species of Enterobacterales exhibited different patterns of antibiotic resistance. E.g., all E. coli strains were sensitive to amikacine, 3rd-generation cephalosporins (cefotaxim, ceftazidim, ceftriaxone), protected aminopenicillines, and meropenem. Meanwhile, resistance of Klebsiella pneumoniae ssp pneumoniae, the actual nosocomial pathogen, proved to be increased over this observation period, being as high as 85% to cephalosporins (III generation) and to meropenem (60%).
Discussion
Acute sinusitis is a common complication following HSCT, and several studies addressed this issue [6, 7]. Immunosuppressive drugs, chemotherapy, radiation treatment, prolonged antibiotic therapy, autoaggressive graft-versus-host disease (GVHD), and long periods of hospitalization are predisposing factors for the upper respiratory tract infections. Current clinical diagnostics of sinusitis, both before and after HSCT, is often based on computed tomography (CT) findings, which in many cases correlate with history and clinical examination data [8]. The authors have shown that the severity of pre-transplant sinus lesions revealed on CT scans correlated with clinical and radiological signs of sinusitis later post-transplant, having been associated with a trend for reduced survival. Therefore, the clinical background before HSCT is also important for assessing further risks of developing ORL disorders. Our results of bacteriological testing are based on the clinical cohort treated at the R. M. Gorbacheva Memorial Research Institute of Children Oncology, Hematology and Transplantation [5]. Infectious complications in allogeneic HSCT are significantly more common than in autologous HSCT, with respect to cytopenic period and rates of hematopoietic recovery. Our data refer to the patients who received allogeneic HSCT. Acute symptoms of rhinosinusitis may be registered at any term after HSCT, reaching 5.3% (95% CI 5.0%-5.6%) at the stage before transplantation; 3.01% (95% CI 2.8%-3.2%) in the course of engraftment, and 8.13% (95% CI 7.67%-8.60%), post-engraftment. From this comparison, one may suggest that massive antibiotic therapy after HSCT appears to prevent some of infectious conditions at the early stages post-transplant. However, later recolonization of pathogenic microorganisms is possible, including Klebsiella spp., S. aureus, S.pneumoniae, at a high risk of resistant strain selection, which was confirmed by us in the present work. One should note, however, that these 3 types of pathogenic bacteria were detected in a total of 13% of patients with sinusitis, i.e. the pathogen remained unknown in most cases. For additional diagnostics, along with search for pathogenic fungi and viruses, the extended diagnostics, e.g., of strictly anaerobic microbiota, are needed. In this aspect, implementation of advanced sequencing (NGS technique) will be of great importance, thus making it possible to assess biological diversity and the ratio of main microbiota classes in complex clinical samples, e.g., from mucosal surfaces.
Conclusions
1. The studies of relationships between the presence of facultative anaerobic, opportunistic microbiota in maxillar punctures and nasal cavities, and clinical course of sinusitis in the patients undergoing allogeneic HSCT did not reveal any significant correlations with severity of the disease (mild versus moderate grade).
2. Standard bacteriological testing aimed for detection of facultative anaerobic microorganisms in the maxillary sinus punctures after HSCT is of limited value within 1 month after HSCT, due to massive antibiotic therapy and suppressed growth of antibiotic-sensitive bacteria.
3. Massive antimicrobial therapy leads to the selection of resistant strains of Klebsiella spp., Pseudomonas spp., E.coli, S.aureus, mainly within 2 or more months after HSCT.
4. Low frequency of cultivable potentially pathogenic microorganisms in sino-nasal exudates cultures suggests reduced detection efficiency for the etiologically important bacteria which may cause sinusitis.
5. More sensitive and specific methods for detecting bacteria on the oropharyngeal and nasopharyngeal mucosa could be based on DNA diagnostics and multiplex PCR techniques, especially due to possible contamination of these sites with anaerobic bacteria of intestinal origin.
Conflict of interest
None declared.
References
- Bento LR, Ortiz E, Nicola EM, Vigorito AC, Sakano E. Sinonasal disorders in hematopoietic stem cell transplantation. Braz J Otorhinolaryngol. 2014; 80: 285-289. doi: 10.1016/j.bjorl.2014.05.009
- Ivanchenko OA, Karpishchenko SA, Kozlov RS, Krechikova OI, Otvagin IV, Sopko ON, Piskunov GZ, Lopatin AS. The microbiome of the maxillary sinus and middle nasal meatus in chronic rhinosinusitis. Rhinology. 2016; 54: 68-74. doi: 10.4193/Rhino15.018
- Lee JT, Frank DN, Ramakrishnan V. Microbiome of the paranasal sinuses: Update and literature review. Am J Rhinol Allergy 2016; 30: 3-16. doi: 10.2500/ajra.2016.30.4255
- De Boeck I, Wittouck S, Martens K, Claes J, Jorissen M, Steelant B, van den Broek, MFL, Seys SF, Hellings PW, Vanderveken OM, Lebeer S. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. mSphere 2019; 4:e00532-19. doi: 10.1128/mSphere.00532-19
- Dolgov OI, Karpishchenko SA, Rodneva YA, Utimisheva ES, Moiseev IS, Zubarovskaya LS, Kulagin AD. Prevalence of acute inflammatory otorhinolaryngological diseases in hematopoietic stem cell transplant recipients. Russian Otorhinolaryngology. 2021;20(3):20-26 (In Russian.). doi: 10.18692/1810-4800-2021-3-20-26
- Drozd-Sokolowska JE, Sokolowski J, Wiktor-Jedrzejczak W, Niemczyk K. Sinusitis in patients undergoing allogeneic bone marrow transplantation – a review. Braz J Otorhinolaryngol. 2017 Jan-Feb;83(1):105-111. doi: 10.1016/j.bjorl.2016.02.012
- Utimisheva ES, Dolgov OI, Paina OV, Ekushov KA, Vitrischak AA, Smirnov BI, Zubarovskaya LS, Karpischenko SA, Afanasyev BV. Incidence, diagnosis and treatment of sinusitis in children and adolescents after hematopoietic stem cell transplantation. Cell Ther Transplant. 2019; 8(1):46-53.
- Billings KR, Lowe LH, AquinoVM, Biavati MJ. Screening sinus CT scans in pediatric bone marrow transplant patients. Otorhinolaryngology. 2000; 52(3): 253-260.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "30" ["~SORT"]=> string(2) "30" ["CODE"]=> string(96) "mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok" ["~CODE"]=> string(96) "mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok" ["EXTERNAL_ID"]=> string(4) "2053" ["~EXTERNAL_ID"]=> string(4) "2053" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(262) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клетокMicrobiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(5126) "<p style="text-align: justify;"> Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК). </p> <h3>Материалы и методы</h3> <p style="text-align: justify;"> В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST. </p> <h3>Результаты</h3> <p style="text-align: justify;"> В биоматериале из полости носа и околоносовых пазух наиболее часто высевали <i>S.epidermidis</i> – 34,7% (377/1097); <i>S.viridans</i> – 2,2% (24/1097); <i>S. aureus</i> – 1,91% (21/1097); <i>Klebsiella spp</i> – 1% (11/1097). Частота выявления <i>S.epidermidis</i> и <i>S.viridans</i> была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста <i>S.epidermidis</i> отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления <i>Klebsiella spp</i> в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения <i>Pseudomonas spp</i> (1/378 <i>vs </i>7/217) в материале из придаточных пазух. </p> <h3>Заключение</h3> <p style="text-align: justify;"> Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов <i>Klebsiella spp, Pseudomonas spp, E.coli, S.aureus</i>, главным образом – в сроки 2 и более мес. после ТГСК. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_META_TITLE"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_META_KEYWORDS"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_META_DESCRIPTION"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_ALT"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_TITLE"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(173) "Микробиота полости носа при синуситах после трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "mikrobiota-polosti-nosa-pri-sinusitakh-posle-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "207" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28542" ["VALUE"]=> string(10) "06.02.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "06.02.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28543" ["VALUE"]=> string(10) "18.03.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "18.03.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28544" ["VALUE"]=> array(2) { ["TEXT"]=> string(566) "<p>Олег И. Долгов<sup>1</sup>, Сергей А. Карпищенко<sup>1</sup>, Екатерина С. Утимишева<sup>1</sup>, Диана А. Григорьянц<sup>1</sup>, Анна А. Спиридонова<sup>1,2</sup>, Иван С. Моисеев<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup>, Алексей Б. Чухловин<sup>1</sup>, Александр Д. Кулагин<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(446) "
Олег И. Долгов1, Сергей А. Карпищенко1, Екатерина С. Утимишева1, Диана А. Григорьянц1, Анна А. Спиридонова1,2, Иван С. Моисеев1, Людмила С. Зубаровская1, Алексей Б. Чухловин1, Александр Д. Кулагин1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28545" ["VALUE"]=> array(2) { ["TEXT"]=> string(435) "<p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(393) "1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия
Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК).
Материалы и методы
В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST.
Результаты
В биоматериале из полости носа и околоносовых пазух наиболее часто высевали S.epidermidis – 34,7% (377/1097); S.viridans – 2,2% (24/1097); S. aureus – 1,91% (21/1097); Klebsiella spp – 1% (11/1097). Частота выявления S.epidermidis и S.viridans была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста S.epidermidis отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления Klebsiella spp в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения Pseudomonas spp (1/378 vs 7/217) в материале из придаточных пазух.
Заключение
Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов Klebsiella spp, Pseudomonas spp, E.coli, S.aureus, главным образом – в сроки 2 и более мес. после ТГСК.
Ключевые слова
Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28547" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-36-42" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-36-42" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28550" ["VALUE"]=> array(2) { ["TEXT"]=> string(429) "<p>Oleg I. Dolgov<sup>1</sup>, Sergey A. Karpishchenko<sup>1</sup>, Ekaterina S. Utimisheva<sup>1</sup>, Diana A. Grigoryanz<sup>1</sup>, Anna A. Spiridonova<sup>1,2</sup>, Ivan S. Moiseev<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Alexei B. Chukhlovin<sup>1</sup>, Alexander D. Kulagin<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(309) "Oleg I. Dolgov1, Sergey A. Karpishchenko1, Ekaterina S. Utimisheva1, Diana A. Grigoryanz1, Anna A. Spiridonova1,2, Ivan S. Moiseev1, Ludmila S. Zubarovskaya1, Alexei B. Chukhlovin1, Alexander D. Kulagin1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28551" ["VALUE"]=> array(2) { ["TEXT"]=> string(693) "<p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> St. Petersburg Pasteur Institute, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Oleg I. Dolgov, Department of Otorhinolaryngology, Pavlov University, 6-8 Tolstoy St, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 845-03-51<br> E-mail: oidolgov@yandex.ru</p><br> <p><b>Citation:</b> Dolgov OI, Karpishchenko SA, Utimisheva ES, et al. Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation. Cell Ther Transplant 2022; 11(1): 36-42.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(573) "1 Pavlov University, St. Petersburg, Russia
2 St. Petersburg Pasteur Institute, St. Petersburg, Russia
Correspondence:
Dr. Oleg I. Dolgov, Department of Otorhinolaryngology,
Pavlov University, 6-8 Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 845-03-51
E-mail: oidolgov@yandex.ru
Citation: Dolgov OI, Karpishchenko SA, Utimisheva ES, et al. Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation. Cell Ther Transplant 2022; 11(1): 36-42.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28552" ["VALUE"]=> array(2) { ["TEXT"]=> string(3275) "<p style="text-align: justify;"> Hematopoietic stem cell transplantation (HSCT) is often accompanied by infectious complications. The aim of this study was a comparative evaluation of facultative anaerobic microbiota members of nasal and paranasal cavity in sinusitis, which often develops in immunocompromised patients, due to intensive chemotherapy and massive antibiotic treatment followed by hematopoietic cell transplantation (HSCT). </p> <h3>Materials and methods</h3> <p style="text-align: justify;"> The study involved 194 patients with various myelo- and lymphoproliferative diseases aged 1 to 62 years who underwent intensive chemotherapy and allogeneic HSCT. As based on appropriate clinical indications, the biomaterial was taken from patients (washings from the paranasal sinuses and/or nasal swabs) within the time period of +100 to +180 days after allogeneic HSCT. We studied 124 samples from maxillary sinus punctures of 97 patients and 973 scrapings from the nasal cavity. Seeding of biological material and isolation of the microorganisms were performed by classical bacteriological techniques. Antibiotic susceptibility of clinical isolates was determined by disk diffusion methods. The data on microbial sensitivity were interpreted by the EUCAST criteria. </p> <h3>Results</h3> <p style="text-align: justify;"> In the samples from nasal cavity and paranasal sinuses, S. epidermidis was most often detected (34.7%, 377/1097); <i>S.viridans</i> (2.2%, 24/1097); <i>S. aureus</i> (1.91%, 21/1097); <i>Klebsiella spp</i> (1%, 11/1097). Detection frequency of <i>S.epidermidis</i> and <i>S.viridans</i> was minimal in the younger age group (up to 5 years), and increased in older groups (>15 years old). Profound suppression of <i>S.epidermidis</i> growth was noted, especially in paranasal sinuses, within 1 month after HSCT in presence of massive antibiotic therapy. High frequency of <i>Klebsiella spp</i> detection was noted in the samples from maxillar sinuses at later terms (2-3 months) after HSCT, at low detection frequency of the pathogen in the specimens from nasal cavity (average 16.3% <i>vs</i> 2.1%, p=2×10^14). In addition, we have estimated frequency of bacterial inoculation within +30 days upon the diagnosis of sinusitis. At the same time, an increased frequency of <i>Pseudomonas spp</i> isolation (1/378 <i>vs </i>7/217) was revealed in the material from paranasal sinuses. </p> <h3>Conclusion</h3> <p style="text-align: justify;"> Bacteriological study of biological samples from maxillary sinuses is of limited value during the 1st month after HSCT accompanied by massive antibiotic therapy which was followed by selection of resistant strains of <i>Klebsiella spp, Pseudomonas spp, E. coli, S. aureus</i>, mainly at the terms of >2 months after HSCT. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Hematopoietic stem cell transplantation, paranasal sinuses, microbiota, antibiotic resistance. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2977) "Hematopoietic stem cell transplantation (HSCT) is often accompanied by infectious complications. The aim of this study was a comparative evaluation of facultative anaerobic microbiota members of nasal and paranasal cavity in sinusitis, which often develops in immunocompromised patients, due to intensive chemotherapy and massive antibiotic treatment followed by hematopoietic cell transplantation (HSCT).
Materials and methods
The study involved 194 patients with various myelo- and lymphoproliferative diseases aged 1 to 62 years who underwent intensive chemotherapy and allogeneic HSCT. As based on appropriate clinical indications, the biomaterial was taken from patients (washings from the paranasal sinuses and/or nasal swabs) within the time period of +100 to +180 days after allogeneic HSCT. We studied 124 samples from maxillary sinus punctures of 97 patients and 973 scrapings from the nasal cavity. Seeding of biological material and isolation of the microorganisms were performed by classical bacteriological techniques. Antibiotic susceptibility of clinical isolates was determined by disk diffusion methods. The data on microbial sensitivity were interpreted by the EUCAST criteria.
Results
In the samples from nasal cavity and paranasal sinuses, S. epidermidis was most often detected (34.7%, 377/1097); S.viridans (2.2%, 24/1097); S. aureus (1.91%, 21/1097); Klebsiella spp (1%, 11/1097). Detection frequency of S.epidermidis and S.viridans was minimal in the younger age group (up to 5 years), and increased in older groups (>15 years old). Profound suppression of S.epidermidis growth was noted, especially in paranasal sinuses, within 1 month after HSCT in presence of massive antibiotic therapy. High frequency of Klebsiella spp detection was noted in the samples from maxillar sinuses at later terms (2-3 months) after HSCT, at low detection frequency of the pathogen in the specimens from nasal cavity (average 16.3% vs 2.1%, p=2×10^14). In addition, we have estimated frequency of bacterial inoculation within +30 days upon the diagnosis of sinusitis. At the same time, an increased frequency of Pseudomonas spp isolation (1/378 vs 7/217) was revealed in the material from paranasal sinuses.
Conclusion
Bacteriological study of biological samples from maxillary sinuses is of limited value during the 1st month after HSCT accompanied by massive antibiotic therapy which was followed by selection of resistant strains of Klebsiella spp, Pseudomonas spp, E. coli, S. aureus, mainly at the terms of >2 months after HSCT.
Keywords
Hematopoietic stem cell transplantation, paranasal sinuses, microbiota, antibiotic resistance.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28548" ["VALUE"]=> string(89) "Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(89) "Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28549" ["VALUE"]=> string(4) "2788" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2788" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28553" ["VALUE"]=> string(4) "2796" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2796" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28550" ["VALUE"]=> array(2) { ["TEXT"]=> string(429) "<p>Oleg I. Dolgov<sup>1</sup>, Sergey A. Karpishchenko<sup>1</sup>, Ekaterina S. Utimisheva<sup>1</sup>, Diana A. Grigoryanz<sup>1</sup>, Anna A. Spiridonova<sup>1,2</sup>, Ivan S. Moiseev<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Alexei B. Chukhlovin<sup>1</sup>, Alexander D. Kulagin<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(309) "Oleg I. Dolgov1, Sergey A. Karpishchenko1, Ekaterina S. Utimisheva1, Diana A. Grigoryanz1, Anna A. Spiridonova1,2, Ivan S. Moiseev1, Ludmila S. Zubarovskaya1, Alexei B. Chukhlovin1, Alexander D. Kulagin1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(309) "Oleg I. Dolgov1, Sergey A. Karpishchenko1, Ekaterina S. Utimisheva1, Diana A. Grigoryanz1, Anna A. Spiridonova1,2, Ivan S. Moiseev1, Ludmila S. Zubarovskaya1, Alexei B. Chukhlovin1, Alexander D. Kulagin1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28552" ["VALUE"]=> array(2) { ["TEXT"]=> string(3275) "<p style="text-align: justify;"> Hematopoietic stem cell transplantation (HSCT) is often accompanied by infectious complications. The aim of this study was a comparative evaluation of facultative anaerobic microbiota members of nasal and paranasal cavity in sinusitis, which often develops in immunocompromised patients, due to intensive chemotherapy and massive antibiotic treatment followed by hematopoietic cell transplantation (HSCT). </p> <h3>Materials and methods</h3> <p style="text-align: justify;"> The study involved 194 patients with various myelo- and lymphoproliferative diseases aged 1 to 62 years who underwent intensive chemotherapy and allogeneic HSCT. As based on appropriate clinical indications, the biomaterial was taken from patients (washings from the paranasal sinuses and/or nasal swabs) within the time period of +100 to +180 days after allogeneic HSCT. We studied 124 samples from maxillary sinus punctures of 97 patients and 973 scrapings from the nasal cavity. Seeding of biological material and isolation of the microorganisms were performed by classical bacteriological techniques. Antibiotic susceptibility of clinical isolates was determined by disk diffusion methods. The data on microbial sensitivity were interpreted by the EUCAST criteria. </p> <h3>Results</h3> <p style="text-align: justify;"> In the samples from nasal cavity and paranasal sinuses, S. epidermidis was most often detected (34.7%, 377/1097); <i>S.viridans</i> (2.2%, 24/1097); <i>S. aureus</i> (1.91%, 21/1097); <i>Klebsiella spp</i> (1%, 11/1097). Detection frequency of <i>S.epidermidis</i> and <i>S.viridans</i> was minimal in the younger age group (up to 5 years), and increased in older groups (>15 years old). Profound suppression of <i>S.epidermidis</i> growth was noted, especially in paranasal sinuses, within 1 month after HSCT in presence of massive antibiotic therapy. High frequency of <i>Klebsiella spp</i> detection was noted in the samples from maxillar sinuses at later terms (2-3 months) after HSCT, at low detection frequency of the pathogen in the specimens from nasal cavity (average 16.3% <i>vs</i> 2.1%, p=2×10^14). In addition, we have estimated frequency of bacterial inoculation within +30 days upon the diagnosis of sinusitis. At the same time, an increased frequency of <i>Pseudomonas spp</i> isolation (1/378 <i>vs </i>7/217) was revealed in the material from paranasal sinuses. </p> <h3>Conclusion</h3> <p style="text-align: justify;"> Bacteriological study of biological samples from maxillary sinuses is of limited value during the 1st month after HSCT accompanied by massive antibiotic therapy which was followed by selection of resistant strains of <i>Klebsiella spp, Pseudomonas spp, E. coli, S. aureus</i>, mainly at the terms of >2 months after HSCT. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Hematopoietic stem cell transplantation, paranasal sinuses, microbiota, antibiotic resistance. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2977) "Hematopoietic stem cell transplantation (HSCT) is often accompanied by infectious complications. The aim of this study was a comparative evaluation of facultative anaerobic microbiota members of nasal and paranasal cavity in sinusitis, which often develops in immunocompromised patients, due to intensive chemotherapy and massive antibiotic treatment followed by hematopoietic cell transplantation (HSCT).
Materials and methods
The study involved 194 patients with various myelo- and lymphoproliferative diseases aged 1 to 62 years who underwent intensive chemotherapy and allogeneic HSCT. As based on appropriate clinical indications, the biomaterial was taken from patients (washings from the paranasal sinuses and/or nasal swabs) within the time period of +100 to +180 days after allogeneic HSCT. We studied 124 samples from maxillary sinus punctures of 97 patients and 973 scrapings from the nasal cavity. Seeding of biological material and isolation of the microorganisms were performed by classical bacteriological techniques. Antibiotic susceptibility of clinical isolates was determined by disk diffusion methods. The data on microbial sensitivity were interpreted by the EUCAST criteria.
Results
In the samples from nasal cavity and paranasal sinuses, S. epidermidis was most often detected (34.7%, 377/1097); S.viridans (2.2%, 24/1097); S. aureus (1.91%, 21/1097); Klebsiella spp (1%, 11/1097). Detection frequency of S.epidermidis and S.viridans was minimal in the younger age group (up to 5 years), and increased in older groups (>15 years old). Profound suppression of S.epidermidis growth was noted, especially in paranasal sinuses, within 1 month after HSCT in presence of massive antibiotic therapy. High frequency of Klebsiella spp detection was noted in the samples from maxillar sinuses at later terms (2-3 months) after HSCT, at low detection frequency of the pathogen in the specimens from nasal cavity (average 16.3% vs 2.1%, p=2×10^14). In addition, we have estimated frequency of bacterial inoculation within +30 days upon the diagnosis of sinusitis. At the same time, an increased frequency of Pseudomonas spp isolation (1/378 vs 7/217) was revealed in the material from paranasal sinuses.
Conclusion
Bacteriological study of biological samples from maxillary sinuses is of limited value during the 1st month after HSCT accompanied by massive antibiotic therapy which was followed by selection of resistant strains of Klebsiella spp, Pseudomonas spp, E. coli, S. aureus, mainly at the terms of >2 months after HSCT.
Keywords
Hematopoietic stem cell transplantation, paranasal sinuses, microbiota, antibiotic resistance.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2977) "Hematopoietic stem cell transplantation (HSCT) is often accompanied by infectious complications. The aim of this study was a comparative evaluation of facultative anaerobic microbiota members of nasal and paranasal cavity in sinusitis, which often develops in immunocompromised patients, due to intensive chemotherapy and massive antibiotic treatment followed by hematopoietic cell transplantation (HSCT).
Materials and methods
The study involved 194 patients with various myelo- and lymphoproliferative diseases aged 1 to 62 years who underwent intensive chemotherapy and allogeneic HSCT. As based on appropriate clinical indications, the biomaterial was taken from patients (washings from the paranasal sinuses and/or nasal swabs) within the time period of +100 to +180 days after allogeneic HSCT. We studied 124 samples from maxillary sinus punctures of 97 patients and 973 scrapings from the nasal cavity. Seeding of biological material and isolation of the microorganisms were performed by classical bacteriological techniques. Antibiotic susceptibility of clinical isolates was determined by disk diffusion methods. The data on microbial sensitivity were interpreted by the EUCAST criteria.
Results
In the samples from nasal cavity and paranasal sinuses, S. epidermidis was most often detected (34.7%, 377/1097); S.viridans (2.2%, 24/1097); S. aureus (1.91%, 21/1097); Klebsiella spp (1%, 11/1097). Detection frequency of S.epidermidis and S.viridans was minimal in the younger age group (up to 5 years), and increased in older groups (>15 years old). Profound suppression of S.epidermidis growth was noted, especially in paranasal sinuses, within 1 month after HSCT in presence of massive antibiotic therapy. High frequency of Klebsiella spp detection was noted in the samples from maxillar sinuses at later terms (2-3 months) after HSCT, at low detection frequency of the pathogen in the specimens from nasal cavity (average 16.3% vs 2.1%, p=2×10^14). In addition, we have estimated frequency of bacterial inoculation within +30 days upon the diagnosis of sinusitis. At the same time, an increased frequency of Pseudomonas spp isolation (1/378 vs 7/217) was revealed in the material from paranasal sinuses.
Conclusion
Bacteriological study of biological samples from maxillary sinuses is of limited value during the 1st month after HSCT accompanied by massive antibiotic therapy which was followed by selection of resistant strains of Klebsiella spp, Pseudomonas spp, E. coli, S. aureus, mainly at the terms of >2 months after HSCT.
Keywords
Hematopoietic stem cell transplantation, paranasal sinuses, microbiota, antibiotic resistance.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28547" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-36-42" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-36-42" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-36-42" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28548" ["VALUE"]=> string(89) "Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(89) "Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(89) "Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28551" ["VALUE"]=> array(2) { ["TEXT"]=> string(693) "<p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> St. Petersburg Pasteur Institute, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Oleg I. Dolgov, Department of Otorhinolaryngology, Pavlov University, 6-8 Tolstoy St, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 845-03-51<br> E-mail: oidolgov@yandex.ru</p><br> <p><b>Citation:</b> Dolgov OI, Karpishchenko SA, Utimisheva ES, et al. Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation. Cell Ther Transplant 2022; 11(1): 36-42.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(573) "1 Pavlov University, St. Petersburg, Russia
2 St. Petersburg Pasteur Institute, St. Petersburg, Russia
Correspondence:
Dr. Oleg I. Dolgov, Department of Otorhinolaryngology,
Pavlov University, 6-8 Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 845-03-51
E-mail: oidolgov@yandex.ru
Citation: Dolgov OI, Karpishchenko SA, Utimisheva ES, et al. Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation. Cell Ther Transplant 2022; 11(1): 36-42.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(573) "1 Pavlov University, St. Petersburg, Russia
2 St. Petersburg Pasteur Institute, St. Petersburg, Russia
Correspondence:
Dr. Oleg I. Dolgov, Department of Otorhinolaryngology,
Pavlov University, 6-8 Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 845-03-51
E-mail: oidolgov@yandex.ru
Citation: Dolgov OI, Karpishchenko SA, Utimisheva ES, et al. Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation. Cell Ther Transplant 2022; 11(1): 36-42.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28544" ["VALUE"]=> array(2) { ["TEXT"]=> string(566) "<p>Олег И. Долгов<sup>1</sup>, Сергей А. Карпищенко<sup>1</sup>, Екатерина С. Утимишева<sup>1</sup>, Диана А. Григорьянц<sup>1</sup>, Анна А. Спиридонова<sup>1,2</sup>, Иван С. Моисеев<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup>, Алексей Б. Чухловин<sup>1</sup>, Александр Д. Кулагин<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(446) "Олег И. Долгов1, Сергей А. Карпищенко1, Екатерина С. Утимишева1, Диана А. Григорьянц1, Анна А. Спиридонова1,2, Иван С. Моисеев1, Людмила С. Зубаровская1, Алексей Б. Чухловин1, Александр Д. Кулагин1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(446) "Олег И. Долгов1, Сергей А. Карпищенко1, Екатерина С. Утимишева1, Диана А. Григорьянц1, Анна А. Спиридонова1,2, Иван С. Моисеев1, Людмила С. Зубаровская1, Алексей Б. Чухловин1, Александр Д. Кулагин1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28542" ["VALUE"]=> string(10) "06.02.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "06.02.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "06.02.2022" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28543" ["VALUE"]=> string(10) "18.03.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "18.03.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "18.03.2022" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28546" ["VALUE"]=> array(2) { ["TEXT"]=> string(5126) "<p style="text-align: justify;"> Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК). </p> <h3>Материалы и методы</h3> <p style="text-align: justify;"> В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST. </p> <h3>Результаты</h3> <p style="text-align: justify;"> В биоматериале из полости носа и околоносовых пазух наиболее часто высевали <i>S.epidermidis</i> – 34,7% (377/1097); <i>S.viridans</i> – 2,2% (24/1097); <i>S. aureus</i> – 1,91% (21/1097); <i>Klebsiella spp</i> – 1% (11/1097). Частота выявления <i>S.epidermidis</i> и <i>S.viridans</i> была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста <i>S.epidermidis</i> отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления <i>Klebsiella spp</i> в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения <i>Pseudomonas spp</i> (1/378 <i>vs </i>7/217) в материале из придаточных пазух. </p> <h3>Заключение</h3> <p style="text-align: justify;"> Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов <i>Klebsiella spp, Pseudomonas spp, E.coli, S.aureus</i>, главным образом – в сроки 2 и более мес. после ТГСК. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4836) "Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК).
Материалы и методы
В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST.
Результаты
В биоматериале из полости носа и околоносовых пазух наиболее часто высевали S.epidermidis – 34,7% (377/1097); S.viridans – 2,2% (24/1097); S. aureus – 1,91% (21/1097); Klebsiella spp – 1% (11/1097). Частота выявления S.epidermidis и S.viridans была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста S.epidermidis отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления Klebsiella spp в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения Pseudomonas spp (1/378 vs 7/217) в материале из придаточных пазух.
Заключение
Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов Klebsiella spp, Pseudomonas spp, E.coli, S.aureus, главным образом – в сроки 2 и более мес. после ТГСК.
Ключевые слова
Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4836) "Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК).
Материалы и методы
В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST.
Результаты
В биоматериале из полости носа и околоносовых пазух наиболее часто высевали S.epidermidis – 34,7% (377/1097); S.viridans – 2,2% (24/1097); S. aureus – 1,91% (21/1097); Klebsiella spp – 1% (11/1097). Частота выявления S.epidermidis и S.viridans была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста S.epidermidis отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления Klebsiella spp в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения Pseudomonas spp (1/378 vs 7/217) в материале из придаточных пазух.
Заключение
Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов Klebsiella spp, Pseudomonas spp, E.coli, S.aureus, главным образом – в сроки 2 и более мес. после ТГСК.
Ключевые слова
Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28545" ["VALUE"]=> array(2) { ["TEXT"]=> string(435) "<p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(393) "1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия
1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only available curative option for acute leukemia nowadays. Many different parameters have significant impact on the final results of HSCT, especially on the more recently defined graft-versus-host disease (GvHD)-free/relapse-free survival (GFRFS) rate, including the pre-HSCT characteristics, such as disease profile at diagnosis and the disease status at the time of transplant, as well as the peri-HSCT factors, i.e. donor type, stem cell source, implemented conditioning regimen and potential post-transplant complications. Aiming to reduce the relapse rates after HSCT, myeloablative conditioning (MAC) regimens at higher dose intensity using busulfan or total body irradiation (TBI) has shown promising results [1]. However, due to higher vulnerability of younger patients to adverse effects of therapeutic irradiation, MAC regimens without TBI are preferred [2, 3]. Moreover, in view of relative complexity for bone marrow collection procedure, along with potentially enhanced graft-versus-leukemia (GvL) effect, peripheral blood (PB) is the preferred source of stem cells for allogeneic HSCT ever more. On the other hand, the increasing number of transplants from human leukocyte antigen (HLA)-haploidentical donors in the patients with acute leukemia is performed due to the absence of suitable related or unrelated HLA-matched donors, thus raising the necessity of understanding, whether HSCT outcomes with this approach are similar to those of more common modes. Over last years, several reports have shown comparable outcomes between HSCT from haploidentical donors and historical HLA-matched related or unrelated donors [4-6]. Hence, additional reports regarding the comparison of different donor types could be a guide to the upcoming therapeutic strategies. To address this issue, we carried out a single-center study, using HSCT with radiation-free MAC regimen, in order to evaluate the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) performed from matched and mismatched related and unrelated donors compared with haploidentical donors in children, adolescents and young adults (CAYA) affected by acute leukemia.
Patients and methods
Patient characteristics
Our study included 180 patients who underwent first allogeneic HSCT for acute leukemia in the CAYA HSCT Department of the Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Tehran, Iran, between January 2014 and January 2021. All data were retrieved retrospectively from clinical records according to the policy approved by the Committee for Medical Ethics of Tehran University of Medical Sciences (TUMS) and after obtaining informed consent from the patients, or their legal guardians.
HSCT parameters
In all patients and their donors, high-resolution HLA molecular typing for HLA-A, -B, -C, -DRB1, and -DQB1 loci was performed. The first donor preference was a 10/10 HLA-matched related donor (MRD), or a 9/10 HLA-mismatched related donor (MMRD). In absence of related donors, an alternative donor including 10/10 HLA-matched unrelated donors (MUD), or 9/10 HLA-mismatched unrelated donors (MMUD), or a related haploidentical donor (Haplo) was chosen, depending on their availability and accessibility.
We proceeded to HSCT if the result of a pre-HSCT bone marrow examination pointed to morphologically complete remission (CR), regardless of the minimal residual disease status. The HSCT procedure was based on irradiation-free MAC regimen including busulfan (a total dose of 3.2-4.8 mg/kg/day, according to patients' ideal body weight, from day -6 to -3), and cyclophosphamide (60 mg/kg/day, day -2 to -1). The GvHD prophylaxis was based on administration of cyclosporine A (CsA) in all the patients, and a short course of methotrexate (10 mg/m2 on day +1, 6 mg/m2 on day +3, +6, and +11) in HSCT from matched and mismatched related and unrelated donors, plus rabbit anti-human thymocytes globulins (ATG-Thymoglobuline, Sanofi, 2.5 mg/kg/day from days -3 to -1) in MMRD, MUD/MMUD and haplo-HSCT groups, and high-dose Pt-Cy treatment (40 mg/kg/day on days +3 and +4) in the Haplo group. We only included patients who received unmanipulated peripheral blood hematopoietic stem cells as graft source.
Considering hazards of CMV reactivation after HSCT, the patients were classified, according to their serological status, into low-risk (donor [D]-/recipient [R]-), intermediate-risk (D+/R-), or high-risk groups (D-/R+ or D+/R+) [7].
Definitions and endpoints
The main purpose of this study was to compare the survival rates of acute leukemia patients who had undergone allogeneic HSCT from different donor types. Overall survival (OS) was defined as the probability of survival, irrespective of the disease state at any point in time. GvHD-free/relapse-free survival (GFRFS) which is regarded as an endpoint more precisely reflective of health status and quality of life post-transplant, was defined as the probability of survival at complete remission of the disease, with sustained donor cell engraftment and absence of either grade III–IV acute GvHD, or chronic GvHD requiring immunosuppressive treatment [8]. Non-relapse mortality (NRM) was defined as probability of death without a relapse after HSCT. The relapse incidence (RI) was defined as the probability to develop a disease relapse.
Donor chimerism was determined on day +15, +30, +60 and +90 after HSCT, and then, if clinically indicated, in whole bone marrow mononuclear cells by means of quantitative PCR of informative short tandem repeats in the donor and recipient [9]. Sustained donor cell engraftment was defined at >0.5×109/L neutrophils and >20×109/L platelets for three consecutive days without blood transfusion support. Graft rejection was defined as a lack of initial engraftment of donor cells (primary), or loss of donor cell engraftment (secondary graft failure), regardless of peripheral cell blood counts. Acute GvHD (aGvHD) and chronic GvHD (cGvHD) were diagnosed and graded according to the published criteria [10, 11]. The mentioned HSCT outcomes were compared between the three categorized groups of different donor types, i.e., the patients transplanted from HLA-matched related (10/10), HLA-mismatched related (9/10) donors (MRD/MMRD), HLA-matched unrelated (10/10), HLA-mismatched (9/10) unrelated donors (MUD/MMUD), and HLA-haploidentical (Haplo) donors.
Statistical evaluation
The patients followed-up beyond 36 months were censored, for better comparison between the groups because some sub-groups had shorter follow-up periods than the other sub-groups. Homogeneity within treatment pairs was evaluated using the Chi-square test or Fisher exact test for qualitative variables and Student's T-test, or Wilcoxon rank-sum test for continuous variables. The endpoints were as follows: OS, GFRFS, relapse-associated, and non-relapse mortality incidence. Kaplan-Meier curves were derived to determine OS and GFRFS, having been compared with log-rank test.
Median follow-up time was established by means of reverse Kaplan-Meier method. After selection of baseline characteristics and clinical variables based on univariable Cox proportional hazards models, multivariable Cox proportional hazards models were fitted.
Variables in the multivariable OS and GFRFS were determined, as based on the P-values of <0.2 in the univariable Cox proportional hazards models. The proportionality of hazards assumption was checked using the global proportionality of hazard test based on Schoenfeld residuals in each of the three multivariable models. There were no deviations from the proportionality of hazards assumption in all multivariable models (results not shown). To account for informative censoring in presence of multiple endpoints, the competing risks in survival analysis were evaluated with nonparametric methods using the cumulative incidence competing risk method. CI for relapses and NRM were calculated by Gray's method. Death beyond relapses was considered a competing event for relapse, and the relapse was considered a competing event for NRM. The Fine-Gray proportional hazard regression model was used to assess the effects of covariates on the relapse frequency and NRM incidence. Like multivariate Cox proportional hazard regression, all the variables at P values of <0.2 in the univariate Fine-Gray proportional hazard regression were included in appropriate multivariate analyses. A two-sided P-value of <0.05 was considered to be statistically significant. The data evaluation was done with STATA version 16 and the packages "survival" and "cmprsk" in R software version 3.3.1.
Results
Patients
The study included 180 patients (120 males and 60 females) at a median age of 12 years (4 months to 24 years) at the time of HSCT, and 123 patients (68.3%) were transplanted at the age of ≤15 years. The donor types were as follows: matched (n=103) and mismatched (n=2) relatives including siblings (n=94) and other relatives (n=11) for a total of 105 cases (58.3%); matched (n=20) and mismatched (n=10) unrelated donors (a total of 30 patients, 16.7%), and haploidentical donors for 45 patients (25%). The patients’ characteristics are summarized in Table 1.
Table 1. Characteristics of the patients and transplant procedure

Notes: ALL: acute lymphoblastic leukemia, AML: acute myeloblastic leukemia, BM: bone marrow, BM+: involvement of bone marrow together with other sites, CR: complete remission, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUD/MMUD: HLA-matched unrelated and HLA-mismatched unrelated donors, R/D: recipient/donor, WBC: white blood cell.
The median follow-up time was 28.7 months for the patients enrolled into the study who were still alive at the end of the study (range: 21.9-34.9). A total of 96 patients presented with B-cell lineage acute lymphoblastic leukemia (ALL); 22 cases, with T-lineage ALL, and 62 patients had acute myeloblastic leukemia (AML). A total of 12 patients suffered from Ph chromosome-positive ALL. All the patients were in complete hematological remission before HSCT, including 93 patients (51.7%) transplanted in their first complete remission (CR1), 67 patients (37.2%) in the second complete remission (CR2), and 20 patients (11.1%) had experienced more than 2 relapses before HSCT. A pre-HSCT cytomegalovirus (CMV) serology showed that more than 90% of the patients were at high risk (recipient [R]+, donor [D]+) for CMV reactivation after HSCT.
Donor cell engraftment
All the patients (180/180) achieved neutrophil counts over 0.5×109/L at a median time of 11 days (range: 7-16). A total of 178 patients achieved platelet counts above 20×109/L, with a median time of 11 days (range: 0-130), and 4 patients died before the platelet engraftment (Table 2). The median time for neutrophil and platelet engraftment in Haplo vs MUD/MMUD vs MRD/MMRD was 12.20 and 14.67 days vs 12.17 and 16.21 days vs 10.73 and 14.30 days, respectively. Two patients from the Haplo group experienced secondary graft failure following CMV reactivation with high viral load after HSCT; one patient was successfully rescued by the second haploidentical HSCT from the same sibling donor, whereas other patient received a second allograft from other parent followed by sustained engraftment and hematopoietic recovery.
Table 2. Engraftment terms and GVHD incidence for the different donor types
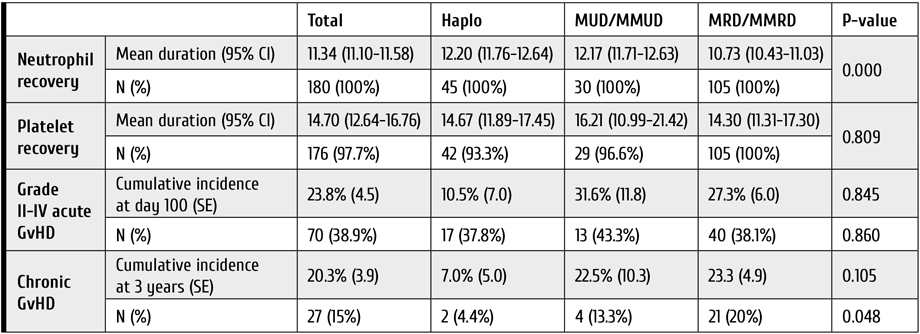
Notes: GvHD: graft-versus-host disease, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUD/MMUD: HLA-matched unrelated and HLA-mismatched unrelated donors.
Acute and chronic GVHD
Grade II to IV of aGvHD was diagnosed in 70 patients (38.9%), being developed at the median term of 15 days after HSCT. Cumulative aGVHD incidence at day 100 was highest in the MUD/MMUD group compared to Haplo and MRD/MMRD, but this difference was not statistically significant [31.6% (±11.8) versus 10.5% (±7.0) versus 27.3% (±6.0), respectively (P=0.845)].
Among 165 patients who survived more than 100 days after HSCT, 27 patients (15%) developed cGvHD, and we observed lower incidence of 3-year cGVHD in the haploidentical group compared to the MUD/MMUD group [7.0% (±5.0) versus 22.5% (±10.3), respectively]. Table 2 represents the comparison for GvHD incidence in the 3 donor types.
Relapse incidence (RI)
The 1-year and 3-year RI of the entire study population was 20.47% (95% CI 14.66-26.97) and 33.85% (95% CI 25.81-41.98), respectively. The 3-year RI in patients of the Haplo group was higher when compared to MUD/MMUD and MRD/MMRD: 40.95% (95% CI 18.41-62.44) versus 32.94% (95% CI 11.92-56.01) versus 33.17% (95% CI 23.64-42.99), respectively (Table 3). This difference was not statistically significant (P=0.902). In the Cox analysis, using both univariate and multivariate approaches, RI was not significantly different among the three donor type groups. In adjusted multivariable RI modeling, the hazard of relapse in the patients from MUD/MMUD group was only 10% lower than for the patients from Haplo group [HR=0.90 (95% CI 0.37-2.19), P=0.826].
Table 3. One- and three-year relapse incidence (RI) and non-relapse mortality (NRM) following HSCT
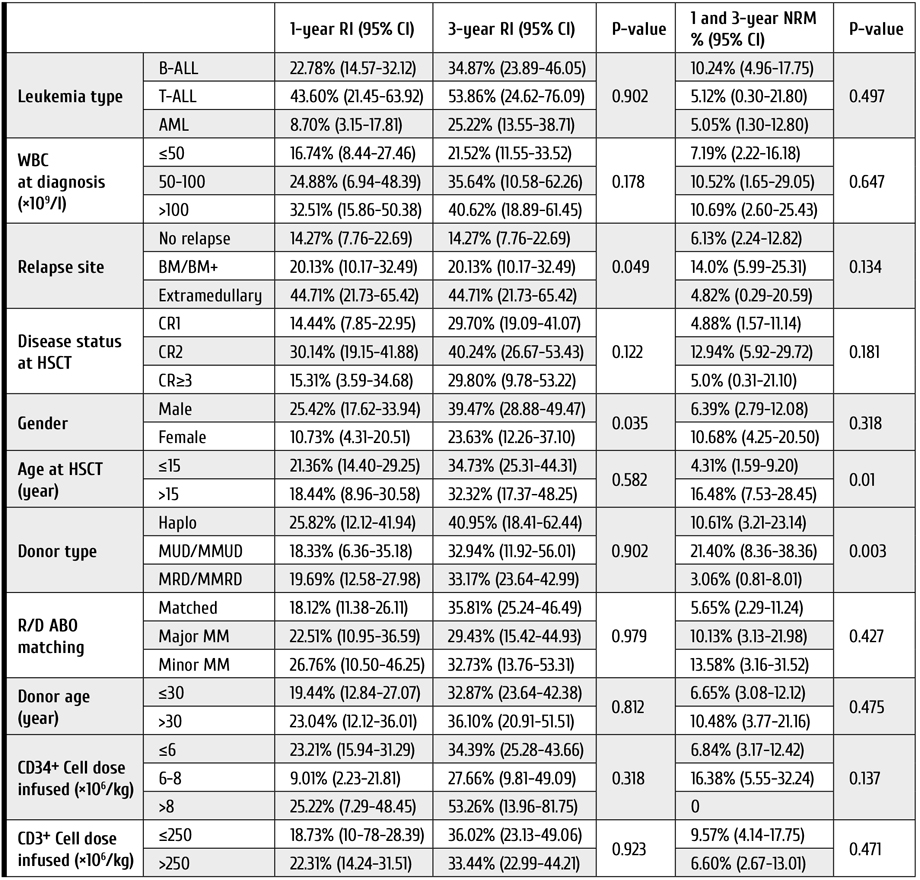
Notes: ALL: acute lymphoblastic leukemia, AML: acute myeloblastic leukemia, BM: bone marrow, BM+: involvement of bone marrow together with other sites, CR: complete remission, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUDMMUD: HLA-matched unrelated and HLA-mismatched unrelated donors, NRM: non-relapse mortality, R/D: recipient/donor, RI: relapse incidence, WBC: white blood cell.
Survival rates and post-HSCT complications
The 3-year OS and GFRFS rates for the entire study cohort were 68.81% (95% CI 60.08-76.01), and 44.19% (95% CI 35.52-54.49), respectively (Fig. 1). Patients in the MUD/MMUD group had the lowest OS and GFRFS compared to other donor types (Table 4).

Figure 1. Clinical outcomes in the cohort of young patients subjected to HSCT from different types of donors. A. Overall survival, B. GvHD-free, relapse-free survival, C. Relapse incidence, D. Non-relapse mortality of patients included in the study. Abscissa, observation terms
Note: Haplo: HLA-haploidentical donors, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUDMMUD: HLA-matched unrelated and HLA-mismatched unrelated donors.
Table 4. One- and three-year overall survival (OS) and GFRFS rates following HSCT in young patients
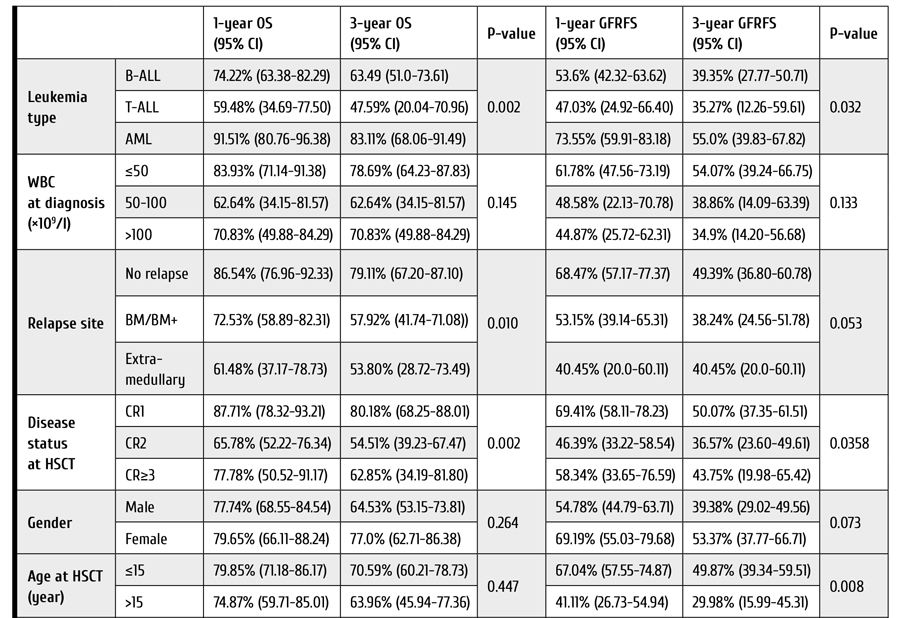

Notes: ALL: acute lymphoblastic leukemia, AML: acute myeloblastic leukemia, BM: bone marrow, BM+: involvement of bone marrow together with other sites, CR: complete remission, GFRFS: GvHD-free/relapse-free survival, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUD/MMUD: HLA-matched unrelated and HLA-mismatched.
The 3-year OS rates were 73.58% (95% CI 62.98-81.59), 54.21% (95% CI 29.61-73.49), and 64.18% (95% CI 39.76-80.79) for MRD/MMRD, MUD/MMUD, and Haplo groups, respectively (P=0.08); The 3-year GFRFS rates were 47.11% (95% CI 36.48-57.02), 30.89% (95% CI 10.70-53.80), and 42.46% (95% CI 20.41-63.01) for MRD/MMRD, MUD/MMUD, and Haplo groups, respectively (P=0.26). In the Cox analysis, using both univariate and multivariate approaches, OS and GFRFS were not significantly different among the 3 donor type groups. Adjusted multivariable modeling of OS based on the variables selected in unadjusted univariate models (see Patients and methods) showed that hazard of death in the patients who received HSCT from MUD/MMUD was about 3.6 times higher than in cases of HSCT from haploidentical donors, and this difference was statistically significant (P=0.05). Moreover, in those patients who received HSCT from MRD/MMRD, the hazard of death was 12 percent higher than for those who received HSCT from haploidentical donors [HR=1.12, (95% CI 0.34-3.67), P=0.84].
The 3-year NRM in all patients was 7.84% (95% CI 4.36-12.62). The patients who underwent MUD/MMUD HSCT showed significantly higher NRM compared to the patients who received Haplo and MRD/MMRD transplants (Table 3): 21.40% (95% CI 8.36-38.36) versus 10.61% (95% CI 3.21-23.14) versus 3.06% (95% CI 0.81-8.01), respectively (P=0.003).
Considering the causes of NRM among patients from MUD/MMUD group who died in the disease remission, we observed six cases of infection and one case of heart failure. In the Haplo group, one patient deceased from NRM had aGvHD, and four others developed infection. In the MRD/MMRD group, one patient was lost due to aGvHD, three patients died with infectious complications, and one case, due to unknown reason.
Adjusted multivariable modeling of NRM showed that hazard of death in the patients who received HSCT from MUD/MMUD was 6 times higher than the hazard of death for the patients who received HSCT from haploidentical donors. This difference was statistically significant (P=0.002). In those patients who received HSCT from MRD/MMRD, the hazard of death was not higher than in those who received HSCT from haploidentical donors (P=0.23).
Although the estimated risk of CMV reactivation prior to HSCT was high in most patients, CMV reactivation after HSCT was detected in a total of 61 cases (33.9%). CMV reactivation after HSCT occurred significantly more often in Haplo and MUD/MMUD group compared with the MRD/MMRD group (55.6% and 43.3% versus 21.9%, respectively, P=0.001). Worth of note, the CMV reactivation post-HSCT was associated with decreased OS and GFRFS in all three groups, being, however, statistically non-significant (P=0.09).
Hemorrhagic cystitis (HC) was another documented complication post-HSCT which occurred in 36 patients (20%), and it mostly affected the patients from Haplo and MUD/MMUD groups compared with MRD/MMRD group (35.6% and 33.3% versus 9.5%, respectively, P=0.000). Sinusoidal obstruction syndrome (SOS) was documented in only 5 patients, i.e. one case from Haplo group, two patients transplanted with MUD/MMUD grafts, and two, from the MRD/MMRD group.
Discussion
Allogeneic HSCT has augmented the potential of cure in patients with acute leukemia [12-15]. Although HLA-compatible related and unrelated donors have been traditionally used for treating acute leukemia patients requiring an allograft, there remains a significant proportion of patients for whom HLA-identical acceptable donor is not available. For these patients, the use of a haploidentical donor combined with alloreactive T cell elimination by Pt-Cy is the most widely adopted strategy [16]. Our study has shown that, for children, adolescents and young adults (CAYA) affected by acute leukemia, haploidentical HSCT followed by Pt-Cy may offer a better and more accessible chance of cure in terms of NRM and survival rates when compared with HSCT from unrelated donors who are hardly available, especially in the COVID-19 pandemic era.
Different studies reported that haploidentical HSCT could provide similar results to those of MUD and MMUD [17-19]. Several reports have shown, at least, comparable outcomes between Haplo and historical MRD, MUD, and MMUD series [20-23]. In our work, in consistence with most studies, the MRD/MMRD group had the best survival rates within the three donor types. Nevertheless, surprisingly, the survival rates were higher in the Haplo group compared to MUD/MMUD group.
Saglio et al., using a TBI-based conditioning regimen, have reported similar OS rates for Haplo and MUD/MMUD in CAYA patients [24]. In our study, OS rates were much higher in Haplo group compared to the MUD/MMUD group. Likewise, in our patients who had undergone haploidentical HSCT, GFRFS was higher and NRM was much lower than the results attained after HSCT from MUD/MMUD.
In terms of GvHD, it has been emphasized that Pt-Cy is able to significantly eliminate alloreactive T cells and, therefore, to reduce the incidence of GvHD, especially its acute form [25]. In addition, ATG has been shown to reduce the rates of severe acute and chronic GvHD in cases of matched or mismatched, unrelated allogeneic HSCT [26, 27]. Chronic GvHD is the leading cause of late complications and death after allogeneic HSCT. Usage of peripheral blood stem cells as a graft source presents a sufficient risk factor for its development, since the T-cell levels in allografts are higher than those in bone marrow [28-30]. Low incidence of GvHD, particularly chronic GvHD, in our patients, as compared to other reports in the literature, despite application of MAC regimen, along with usage of peripheral blood stem cells, could be attributed to high doses of ATG in the conditioning regimen for HSCT in the patients undergoing Haplo and MUD/MMUD HSCT. In our study, the rates of acute and chronic GvHD were even lower in the Haplo group than among the patients in MUD/MMUD group. This could be ascribed to dual in vivo T-cell depletion caused by ATG and Pt-Cy in the Haplo group. However, adoption of the highly effective GvHD prophylaxis may potentially lead to increased risk of relapse. It seems to be true in our study, as we had the highest relapse incidence (RI) in the Haplo group. However, one should note that the difference in RI among our three donor types was not statistically significant. It is presumed that HLA disparity could be considered a contributing factor to allo-reactivity and GvL [31]. In the matched donor transplant setting, the frequency of donor T-cell precursors directed against leukemia-specific antigens mediating GvL may be more limited [32]. Other studies with less rigorous GvHD prophylaxis strategies compared to our approaches, have reported similar RI in Haplo and MUD/MMUD HSCT [24, 34].
With respect to transplant toxicity, our data confirm that the patients undergoing Haplo HSCT have much lower NRM rates compared to patients undergoing MUD/MMUD HSCT, and the rates of complications, such as hemorrhagic cystitis and sinusoidal obstruction syndrome, seem to be comparable within the two groups. Previous studies comparing NRM rates in Haplo (with Pt-Cy) with MRD and MUD transplants (with standard GvHD prophylaxis) have reported inconsistent results. Meanwhile, some studies reported a higher NRM rates in Haplo HSCT [17, 35, 36].
This study was limited by its retrospective design, inability to adjust for unknown factors, the heterogeneity for conditioning regimens and supportive therapy that could affect the study outcomes.
Conclusions
Our study shows that inclusion of ATG into the myeloablative conditioning regimen before transplantation of peripheral blood stem cells from MUD/MMUD and Haplo donors is associated with reduced rates of chronic GvHD and graft failure, concomitantly. The rates of OS and GFRFS were higher in the Haplo group compared to MUD/MMUD, hence, our data supports the view that haploidentical HSCT with peripheral blood stem cells is a practical and valuable clinical option that offers CAYA patients with acute leukemia requiring HSCT and lacking matched available donors, a reasonable opportunity for the disease control. However, further progress is necessary to decrease the relapse rate in these patients.
Declarations
The study was approved by the Committee on Medical Ethics of Tehran University of Medical Sciences (TUMS) and informed consent was obtained from patients or their legal guardians. Authors provide a consent for publication. Primary data and materials are available on request.
Authors' contributions: TR designed and coordinated the study, and managed the patients. AK, MR and NA participated in the management of patients. AK carried out statistical evaluation. SA conceived of the study. All the authors read and approved the final manuscript.
Acknowledgements
We would like to thank Ashraf Sadat Hoseini and other nursing staff for their undeniable assistance in care for our patients.
Competing Interests
None of the authors have any relevant conflict of interest to disclaim about the present article. No funding support for the study is declared.
References
- Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015; 21(7):1299-1307. doi: 10.1016/j.bbmt.2015.03.003
- Friebert SE, Shepardson LB, Shurin SB, Rosenthal GE, Rosenthal NS. Pediatric bone marrow cellularity: are we expecting too much?
J Pediat Hematol/Oncol. 1998; 20(5):439-43. doi: 10.1097/00043426-199809000-00006 - Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001; 19(1):117-125. doi: 10.1016/S0736-0266(00)00010-3
- Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014; 20(10):1573-1579.
doi: 10.1016/j.bbmt.2014.05.029 - Bashey A, Zhang XU, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013; 31(10):1310-1316. doi: 10.1200/JCO.2012.44.3523
- Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014; 20(12):1975-1981. doi: 10.1016/j.bbmt.2014.08.013
- George B, Pati N, Gilroy N, Ratnamohan M, Huang G, Kerridge I, Hertzberg M, Gottlieb D, Bradstock K. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transplant Infect Dis. 2010; 12(4):322-9.
doi: 10.1111/j.1399-3062.2010.00504.x - Balavarca Y, Pearce K, Norden J, Collin M, Jackson G, Holler E, et al. Predicting survival using clinical risk scores and non-HLA immunogenetics. Bone Marrow Transpl. 2015; 50(11):1445-1452. doi: 10.1038/bmt.2015.305
- Thiede C, Florek M, Bornhäuser M, Ritter M, Mohr B, Brendel C, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transpl. 1999; 23:1055-1060.
doi: 10.1038/sj.bmt.1701779 - Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014; 123:3664-3671. doi: 10.1182/blood-2014-01-552984
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18:295-304.
- Afify Z, Hunt L, Green A, Guttridge M, Cornish J, Oakhill A. Factors affecting the outcome of stem cell transplantation from unrelated donors for childhood acute lymphoblastic leukemia in third remission. Bone Marrow Transpl. 2005; 35:1041-1047.
doi: 10.1038/sj.bmt.1704958 - Balduzzi A, Valsecchi MG, Uderzo C, De Lorenzo P, Klingebiel T, Peters C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet. 2005; 366:635-642. doi: 10.1016/S0140-6736(05)66998-X
- Cornish J, Oakhill A. The management of relapsed acute lymphoblastic leukaemia. Bone Marrow Transpl. 2001; 28(1):S9. https://doi.org/10.1038/sj.bmt.1703167
- Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R, et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010; 115:3437-3446. doi: 10.1182/blood-2009-03-207001
- Nagler A, Ruggeri A. Haploidentical stem cell transplantation (HaploSCT) for patients with acute leukemia – an update on behalf of the ALWP of the EBMT. Bone Marrow Transplant. 2019; 54(2): 713-718. doi: 10.1038/s41409-019-0610-5
- Piemontese S, Ciceri F, Labopin M, Arcese W, Kyrcz-Krzemien S, Santarone S, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017; 10(1):1-8.
doi: 10.1186/s13045-017-0394-2 - Sun Y, Beohou E, Labopin M, Volin L, Milpied N, Yakoub-Agha I, et al. Unmanipulated haploidentical versus matched unrelated donor allogeneic stem cell transplantation in adult patients with acute myelogenous leukemia in first remission: a retrospective pair-matched comparative study of the Beijing approach with the EBMT database. Haematologica. 2016; 101:e352-4.
doi: 10.3324/haematol.2015.140509 - Lorentino F, Labopin M, Bernardi M, Ciceri F, Socié G, Cornelissen JJ, et al. Comparable outcomes of haploidentical, 10/10 and 9/10unrelated donor transplantation in adverse karyotype AML in first complete remission. Am J Hematol. 2018; 93:1236-1244.
doi: 10.1002/ajh.25231 - Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014; 20(10):1573-1579.
doi: 10.1016/j.bbmt.2014.05.029 - Bashey A, Zhang XU, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013; 31(10):1310-1316.
doi: 10.1200/JCO.2012.44.3523 - Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014; 20(12):1975-1981. doi: 10.1016/j.bbmt.2014.08.013
- Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014; 124(17):2735-2743. doi: 10.1182/blood-2014-04-571570
- Saglio F, Berger M, Spadea M, Pessolano R, Carraro F, Barone M, et al. Haploidentical HSCT with post transplantation cyclophosphamide versus unrelated donor HSCT in pediatric patients affected by acute leukemia. Bone Marrow Transpl. 2021; 56(3): 586-595. doi: 10.1038/s41409-020-01063-2
- Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Posttransplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019; 129:2357–2373.
doi: 10.1172/JCI124218 - Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009; 10(9): 855-864. doi: 10.1016/S1470-2045(09)70225-6
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, Di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001; 98(10):2942-2947. doi: 10.1182/blood.V98.10.2942
- Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011; 29(16):2230. doi: 10.1200/JCO.2010.33.7212
- Martin PJ, Counts Jr GW, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010; 28(6):1011. doi: 10.1200/JCO.2009.25.6693
- Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015; 21(2):266-274. doi: 10.1016/j.bbmt.2014.10.021
- Shimoni A, Labopin M, Finke J, Ciceri F, Deconinck E, Kröger N, et al. Donor selection for a second allogeneic stem cell transplantation in AML patients relapsing after a first transplant: a study of the Acute Leukemia Working Party of EBMT. Blood Cancer Journal. 2019 ; 9(12):1-9. doi: 10.1038/s41408-019-0251-3
- Distler E, Bloetz A, Albrecht J, Asdufan S, Hohberger A, Frey M, et al. Alloreactive and leukemia-reactive T cells are preferentially derived from naive precursors in healthy donors: implications for immunotherapy with memory T cells. Haematologica. 2011; 96(7):1024. doi: 10.3324/haematol.2010.037481
- Bertaina A, Zecca M, Buldini B, Sacchi N, Algeri M, Saglio F, et al. Unrelated donor vs HLA-haploidentical α/β T-cell- and Bcell-depleted HSCT in children with acute leukemia. Blood. 2018; 132:2594-2607. doi: 10.1182/blood-2018-07-861575
- Versluis J, Labopin M, Ruggeri A, Socie G, Wu D, Volin L, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 2017; 1(7):477-485. doi: 10.1182/bloodadvances.2016002386
- Baron F, Labopin M, Ruggeri A, Cornelissen JJ, Meijer E, Sengeloev H, et al. Impact of donor type in patients with AML given allogeneic hematopoietic cell transplantation after low-dose TBI-based regimen. Clin Cancer Res. 2018; 24(12):2794-2803.
doi: 10.1158/1078-0432.CCR-17-3622 - Rashidi A, Hamadani M, Zhang MJ, Wang HL, Abdel-Azim H, Aljurf M, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019; 3(12):1826-1836.
https://doi.org/10.1182/bloodadvances.2019000050
" ["~DETAIL_TEXT"]=> string(40906) "
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only available curative option for acute leukemia nowadays. Many different parameters have significant impact on the final results of HSCT, especially on the more recently defined graft-versus-host disease (GvHD)-free/relapse-free survival (GFRFS) rate, including the pre-HSCT characteristics, such as disease profile at diagnosis and the disease status at the time of transplant, as well as the peri-HSCT factors, i.e. donor type, stem cell source, implemented conditioning regimen and potential post-transplant complications. Aiming to reduce the relapse rates after HSCT, myeloablative conditioning (MAC) regimens at higher dose intensity using busulfan or total body irradiation (TBI) has shown promising results [1]. However, due to higher vulnerability of younger patients to adverse effects of therapeutic irradiation, MAC regimens without TBI are preferred [2, 3]. Moreover, in view of relative complexity for bone marrow collection procedure, along with potentially enhanced graft-versus-leukemia (GvL) effect, peripheral blood (PB) is the preferred source of stem cells for allogeneic HSCT ever more. On the other hand, the increasing number of transplants from human leukocyte antigen (HLA)-haploidentical donors in the patients with acute leukemia is performed due to the absence of suitable related or unrelated HLA-matched donors, thus raising the necessity of understanding, whether HSCT outcomes with this approach are similar to those of more common modes. Over last years, several reports have shown comparable outcomes between HSCT from haploidentical donors and historical HLA-matched related or unrelated donors [4-6]. Hence, additional reports regarding the comparison of different donor types could be a guide to the upcoming therapeutic strategies. To address this issue, we carried out a single-center study, using HSCT with radiation-free MAC regimen, in order to evaluate the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) performed from matched and mismatched related and unrelated donors compared with haploidentical donors in children, adolescents and young adults (CAYA) affected by acute leukemia.
Patients and methods
Patient characteristics
Our study included 180 patients who underwent first allogeneic HSCT for acute leukemia in the CAYA HSCT Department of the Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Tehran, Iran, between January 2014 and January 2021. All data were retrieved retrospectively from clinical records according to the policy approved by the Committee for Medical Ethics of Tehran University of Medical Sciences (TUMS) and after obtaining informed consent from the patients, or their legal guardians.
HSCT parameters
In all patients and their donors, high-resolution HLA molecular typing for HLA-A, -B, -C, -DRB1, and -DQB1 loci was performed. The first donor preference was a 10/10 HLA-matched related donor (MRD), or a 9/10 HLA-mismatched related donor (MMRD). In absence of related donors, an alternative donor including 10/10 HLA-matched unrelated donors (MUD), or 9/10 HLA-mismatched unrelated donors (MMUD), or a related haploidentical donor (Haplo) was chosen, depending on their availability and accessibility.
We proceeded to HSCT if the result of a pre-HSCT bone marrow examination pointed to morphologically complete remission (CR), regardless of the minimal residual disease status. The HSCT procedure was based on irradiation-free MAC regimen including busulfan (a total dose of 3.2-4.8 mg/kg/day, according to patients' ideal body weight, from day -6 to -3), and cyclophosphamide (60 mg/kg/day, day -2 to -1). The GvHD prophylaxis was based on administration of cyclosporine A (CsA) in all the patients, and a short course of methotrexate (10 mg/m2 on day +1, 6 mg/m2 on day +3, +6, and +11) in HSCT from matched and mismatched related and unrelated donors, plus rabbit anti-human thymocytes globulins (ATG-Thymoglobuline, Sanofi, 2.5 mg/kg/day from days -3 to -1) in MMRD, MUD/MMUD and haplo-HSCT groups, and high-dose Pt-Cy treatment (40 mg/kg/day on days +3 and +4) in the Haplo group. We only included patients who received unmanipulated peripheral blood hematopoietic stem cells as graft source.
Considering hazards of CMV reactivation after HSCT, the patients were classified, according to their serological status, into low-risk (donor [D]-/recipient [R]-), intermediate-risk (D+/R-), or high-risk groups (D-/R+ or D+/R+) [7].
Definitions and endpoints
The main purpose of this study was to compare the survival rates of acute leukemia patients who had undergone allogeneic HSCT from different donor types. Overall survival (OS) was defined as the probability of survival, irrespective of the disease state at any point in time. GvHD-free/relapse-free survival (GFRFS) which is regarded as an endpoint more precisely reflective of health status and quality of life post-transplant, was defined as the probability of survival at complete remission of the disease, with sustained donor cell engraftment and absence of either grade III–IV acute GvHD, or chronic GvHD requiring immunosuppressive treatment [8]. Non-relapse mortality (NRM) was defined as probability of death without a relapse after HSCT. The relapse incidence (RI) was defined as the probability to develop a disease relapse.
Donor chimerism was determined on day +15, +30, +60 and +90 after HSCT, and then, if clinically indicated, in whole bone marrow mononuclear cells by means of quantitative PCR of informative short tandem repeats in the donor and recipient [9]. Sustained donor cell engraftment was defined at >0.5×109/L neutrophils and >20×109/L platelets for three consecutive days without blood transfusion support. Graft rejection was defined as a lack of initial engraftment of donor cells (primary), or loss of donor cell engraftment (secondary graft failure), regardless of peripheral cell blood counts. Acute GvHD (aGvHD) and chronic GvHD (cGvHD) were diagnosed and graded according to the published criteria [10, 11]. The mentioned HSCT outcomes were compared between the three categorized groups of different donor types, i.e., the patients transplanted from HLA-matched related (10/10), HLA-mismatched related (9/10) donors (MRD/MMRD), HLA-matched unrelated (10/10), HLA-mismatched (9/10) unrelated donors (MUD/MMUD), and HLA-haploidentical (Haplo) donors.
Statistical evaluation
The patients followed-up beyond 36 months were censored, for better comparison between the groups because some sub-groups had shorter follow-up periods than the other sub-groups. Homogeneity within treatment pairs was evaluated using the Chi-square test or Fisher exact test for qualitative variables and Student's T-test, or Wilcoxon rank-sum test for continuous variables. The endpoints were as follows: OS, GFRFS, relapse-associated, and non-relapse mortality incidence. Kaplan-Meier curves were derived to determine OS and GFRFS, having been compared with log-rank test.
Median follow-up time was established by means of reverse Kaplan-Meier method. After selection of baseline characteristics and clinical variables based on univariable Cox proportional hazards models, multivariable Cox proportional hazards models were fitted.
Variables in the multivariable OS and GFRFS were determined, as based on the P-values of <0.2 in the univariable Cox proportional hazards models. The proportionality of hazards assumption was checked using the global proportionality of hazard test based on Schoenfeld residuals in each of the three multivariable models. There were no deviations from the proportionality of hazards assumption in all multivariable models (results not shown). To account for informative censoring in presence of multiple endpoints, the competing risks in survival analysis were evaluated with nonparametric methods using the cumulative incidence competing risk method. CI for relapses and NRM were calculated by Gray's method. Death beyond relapses was considered a competing event for relapse, and the relapse was considered a competing event for NRM. The Fine-Gray proportional hazard regression model was used to assess the effects of covariates on the relapse frequency and NRM incidence. Like multivariate Cox proportional hazard regression, all the variables at P values of <0.2 in the univariate Fine-Gray proportional hazard regression were included in appropriate multivariate analyses. A two-sided P-value of <0.05 was considered to be statistically significant. The data evaluation was done with STATA version 16 and the packages "survival" and "cmprsk" in R software version 3.3.1.
Results
Patients
The study included 180 patients (120 males and 60 females) at a median age of 12 years (4 months to 24 years) at the time of HSCT, and 123 patients (68.3%) were transplanted at the age of ≤15 years. The donor types were as follows: matched (n=103) and mismatched (n=2) relatives including siblings (n=94) and other relatives (n=11) for a total of 105 cases (58.3%); matched (n=20) and mismatched (n=10) unrelated donors (a total of 30 patients, 16.7%), and haploidentical donors for 45 patients (25%). The patients’ characteristics are summarized in Table 1.
Table 1. Characteristics of the patients and transplant procedure

Notes: ALL: acute lymphoblastic leukemia, AML: acute myeloblastic leukemia, BM: bone marrow, BM+: involvement of bone marrow together with other sites, CR: complete remission, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUD/MMUD: HLA-matched unrelated and HLA-mismatched unrelated donors, R/D: recipient/donor, WBC: white blood cell.
The median follow-up time was 28.7 months for the patients enrolled into the study who were still alive at the end of the study (range: 21.9-34.9). A total of 96 patients presented with B-cell lineage acute lymphoblastic leukemia (ALL); 22 cases, with T-lineage ALL, and 62 patients had acute myeloblastic leukemia (AML). A total of 12 patients suffered from Ph chromosome-positive ALL. All the patients were in complete hematological remission before HSCT, including 93 patients (51.7%) transplanted in their first complete remission (CR1), 67 patients (37.2%) in the second complete remission (CR2), and 20 patients (11.1%) had experienced more than 2 relapses before HSCT. A pre-HSCT cytomegalovirus (CMV) serology showed that more than 90% of the patients were at high risk (recipient [R]+, donor [D]+) for CMV reactivation after HSCT.
Donor cell engraftment
All the patients (180/180) achieved neutrophil counts over 0.5×109/L at a median time of 11 days (range: 7-16). A total of 178 patients achieved platelet counts above 20×109/L, with a median time of 11 days (range: 0-130), and 4 patients died before the platelet engraftment (Table 2). The median time for neutrophil and platelet engraftment in Haplo vs MUD/MMUD vs MRD/MMRD was 12.20 and 14.67 days vs 12.17 and 16.21 days vs 10.73 and 14.30 days, respectively. Two patients from the Haplo group experienced secondary graft failure following CMV reactivation with high viral load after HSCT; one patient was successfully rescued by the second haploidentical HSCT from the same sibling donor, whereas other patient received a second allograft from other parent followed by sustained engraftment and hematopoietic recovery.
Table 2. Engraftment terms and GVHD incidence for the different donor types

Notes: GvHD: graft-versus-host disease, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUD/MMUD: HLA-matched unrelated and HLA-mismatched unrelated donors.
Acute and chronic GVHD
Grade II to IV of aGvHD was diagnosed in 70 patients (38.9%), being developed at the median term of 15 days after HSCT. Cumulative aGVHD incidence at day 100 was highest in the MUD/MMUD group compared to Haplo and MRD/MMRD, but this difference was not statistically significant [31.6% (±11.8) versus 10.5% (±7.0) versus 27.3% (±6.0), respectively (P=0.845)].
Among 165 patients who survived more than 100 days after HSCT, 27 patients (15%) developed cGvHD, and we observed lower incidence of 3-year cGVHD in the haploidentical group compared to the MUD/MMUD group [7.0% (±5.0) versus 22.5% (±10.3), respectively]. Table 2 represents the comparison for GvHD incidence in the 3 donor types.
Relapse incidence (RI)
The 1-year and 3-year RI of the entire study population was 20.47% (95% CI 14.66-26.97) and 33.85% (95% CI 25.81-41.98), respectively. The 3-year RI in patients of the Haplo group was higher when compared to MUD/MMUD and MRD/MMRD: 40.95% (95% CI 18.41-62.44) versus 32.94% (95% CI 11.92-56.01) versus 33.17% (95% CI 23.64-42.99), respectively (Table 3). This difference was not statistically significant (P=0.902). In the Cox analysis, using both univariate and multivariate approaches, RI was not significantly different among the three donor type groups. In adjusted multivariable RI modeling, the hazard of relapse in the patients from MUD/MMUD group was only 10% lower than for the patients from Haplo group [HR=0.90 (95% CI 0.37-2.19), P=0.826].
Table 3. One- and three-year relapse incidence (RI) and non-relapse mortality (NRM) following HSCT

Notes: ALL: acute lymphoblastic leukemia, AML: acute myeloblastic leukemia, BM: bone marrow, BM+: involvement of bone marrow together with other sites, CR: complete remission, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUDMMUD: HLA-matched unrelated and HLA-mismatched unrelated donors, NRM: non-relapse mortality, R/D: recipient/donor, RI: relapse incidence, WBC: white blood cell.
Survival rates and post-HSCT complications
The 3-year OS and GFRFS rates for the entire study cohort were 68.81% (95% CI 60.08-76.01), and 44.19% (95% CI 35.52-54.49), respectively (Fig. 1). Patients in the MUD/MMUD group had the lowest OS and GFRFS compared to other donor types (Table 4).

Figure 1. Clinical outcomes in the cohort of young patients subjected to HSCT from different types of donors. A. Overall survival, B. GvHD-free, relapse-free survival, C. Relapse incidence, D. Non-relapse mortality of patients included in the study. Abscissa, observation terms
Note: Haplo: HLA-haploidentical donors, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUDMMUD: HLA-matched unrelated and HLA-mismatched unrelated donors.
Table 4. One- and three-year overall survival (OS) and GFRFS rates following HSCT in young patients


Notes: ALL: acute lymphoblastic leukemia, AML: acute myeloblastic leukemia, BM: bone marrow, BM+: involvement of bone marrow together with other sites, CR: complete remission, GFRFS: GvHD-free/relapse-free survival, Haplo: HLA-haploidentical donors, MM: mismatched, MRD/MMRD: HLA-matched related and HLA-mismatched related donors, MUD/MMUD: HLA-matched unrelated and HLA-mismatched.
The 3-year OS rates were 73.58% (95% CI 62.98-81.59), 54.21% (95% CI 29.61-73.49), and 64.18% (95% CI 39.76-80.79) for MRD/MMRD, MUD/MMUD, and Haplo groups, respectively (P=0.08); The 3-year GFRFS rates were 47.11% (95% CI 36.48-57.02), 30.89% (95% CI 10.70-53.80), and 42.46% (95% CI 20.41-63.01) for MRD/MMRD, MUD/MMUD, and Haplo groups, respectively (P=0.26). In the Cox analysis, using both univariate and multivariate approaches, OS and GFRFS were not significantly different among the 3 donor type groups. Adjusted multivariable modeling of OS based on the variables selected in unadjusted univariate models (see Patients and methods) showed that hazard of death in the patients who received HSCT from MUD/MMUD was about 3.6 times higher than in cases of HSCT from haploidentical donors, and this difference was statistically significant (P=0.05). Moreover, in those patients who received HSCT from MRD/MMRD, the hazard of death was 12 percent higher than for those who received HSCT from haploidentical donors [HR=1.12, (95% CI 0.34-3.67), P=0.84].
The 3-year NRM in all patients was 7.84% (95% CI 4.36-12.62). The patients who underwent MUD/MMUD HSCT showed significantly higher NRM compared to the patients who received Haplo and MRD/MMRD transplants (Table 3): 21.40% (95% CI 8.36-38.36) versus 10.61% (95% CI 3.21-23.14) versus 3.06% (95% CI 0.81-8.01), respectively (P=0.003).
Considering the causes of NRM among patients from MUD/MMUD group who died in the disease remission, we observed six cases of infection and one case of heart failure. In the Haplo group, one patient deceased from NRM had aGvHD, and four others developed infection. In the MRD/MMRD group, one patient was lost due to aGvHD, three patients died with infectious complications, and one case, due to unknown reason.
Adjusted multivariable modeling of NRM showed that hazard of death in the patients who received HSCT from MUD/MMUD was 6 times higher than the hazard of death for the patients who received HSCT from haploidentical donors. This difference was statistically significant (P=0.002). In those patients who received HSCT from MRD/MMRD, the hazard of death was not higher than in those who received HSCT from haploidentical donors (P=0.23).
Although the estimated risk of CMV reactivation prior to HSCT was high in most patients, CMV reactivation after HSCT was detected in a total of 61 cases (33.9%). CMV reactivation after HSCT occurred significantly more often in Haplo and MUD/MMUD group compared with the MRD/MMRD group (55.6% and 43.3% versus 21.9%, respectively, P=0.001). Worth of note, the CMV reactivation post-HSCT was associated with decreased OS and GFRFS in all three groups, being, however, statistically non-significant (P=0.09).
Hemorrhagic cystitis (HC) was another documented complication post-HSCT which occurred in 36 patients (20%), and it mostly affected the patients from Haplo and MUD/MMUD groups compared with MRD/MMRD group (35.6% and 33.3% versus 9.5%, respectively, P=0.000). Sinusoidal obstruction syndrome (SOS) was documented in only 5 patients, i.e. one case from Haplo group, two patients transplanted with MUD/MMUD grafts, and two, from the MRD/MMRD group.
Discussion
Allogeneic HSCT has augmented the potential of cure in patients with acute leukemia [12-15]. Although HLA-compatible related and unrelated donors have been traditionally used for treating acute leukemia patients requiring an allograft, there remains a significant proportion of patients for whom HLA-identical acceptable donor is not available. For these patients, the use of a haploidentical donor combined with alloreactive T cell elimination by Pt-Cy is the most widely adopted strategy [16]. Our study has shown that, for children, adolescents and young adults (CAYA) affected by acute leukemia, haploidentical HSCT followed by Pt-Cy may offer a better and more accessible chance of cure in terms of NRM and survival rates when compared with HSCT from unrelated donors who are hardly available, especially in the COVID-19 pandemic era.
Different studies reported that haploidentical HSCT could provide similar results to those of MUD and MMUD [17-19]. Several reports have shown, at least, comparable outcomes between Haplo and historical MRD, MUD, and MMUD series [20-23]. In our work, in consistence with most studies, the MRD/MMRD group had the best survival rates within the three donor types. Nevertheless, surprisingly, the survival rates were higher in the Haplo group compared to MUD/MMUD group.
Saglio et al., using a TBI-based conditioning regimen, have reported similar OS rates for Haplo and MUD/MMUD in CAYA patients [24]. In our study, OS rates were much higher in Haplo group compared to the MUD/MMUD group. Likewise, in our patients who had undergone haploidentical HSCT, GFRFS was higher and NRM was much lower than the results attained after HSCT from MUD/MMUD.
In terms of GvHD, it has been emphasized that Pt-Cy is able to significantly eliminate alloreactive T cells and, therefore, to reduce the incidence of GvHD, especially its acute form [25]. In addition, ATG has been shown to reduce the rates of severe acute and chronic GvHD in cases of matched or mismatched, unrelated allogeneic HSCT [26, 27]. Chronic GvHD is the leading cause of late complications and death after allogeneic HSCT. Usage of peripheral blood stem cells as a graft source presents a sufficient risk factor for its development, since the T-cell levels in allografts are higher than those in bone marrow [28-30]. Low incidence of GvHD, particularly chronic GvHD, in our patients, as compared to other reports in the literature, despite application of MAC regimen, along with usage of peripheral blood stem cells, could be attributed to high doses of ATG in the conditioning regimen for HSCT in the patients undergoing Haplo and MUD/MMUD HSCT. In our study, the rates of acute and chronic GvHD were even lower in the Haplo group than among the patients in MUD/MMUD group. This could be ascribed to dual in vivo T-cell depletion caused by ATG and Pt-Cy in the Haplo group. However, adoption of the highly effective GvHD prophylaxis may potentially lead to increased risk of relapse. It seems to be true in our study, as we had the highest relapse incidence (RI) in the Haplo group. However, one should note that the difference in RI among our three donor types was not statistically significant. It is presumed that HLA disparity could be considered a contributing factor to allo-reactivity and GvL [31]. In the matched donor transplant setting, the frequency of donor T-cell precursors directed against leukemia-specific antigens mediating GvL may be more limited [32]. Other studies with less rigorous GvHD prophylaxis strategies compared to our approaches, have reported similar RI in Haplo and MUD/MMUD HSCT [24, 34].
With respect to transplant toxicity, our data confirm that the patients undergoing Haplo HSCT have much lower NRM rates compared to patients undergoing MUD/MMUD HSCT, and the rates of complications, such as hemorrhagic cystitis and sinusoidal obstruction syndrome, seem to be comparable within the two groups. Previous studies comparing NRM rates in Haplo (with Pt-Cy) with MRD and MUD transplants (with standard GvHD prophylaxis) have reported inconsistent results. Meanwhile, some studies reported a higher NRM rates in Haplo HSCT [17, 35, 36].
This study was limited by its retrospective design, inability to adjust for unknown factors, the heterogeneity for conditioning regimens and supportive therapy that could affect the study outcomes.
Conclusions
Our study shows that inclusion of ATG into the myeloablative conditioning regimen before transplantation of peripheral blood stem cells from MUD/MMUD and Haplo donors is associated with reduced rates of chronic GvHD and graft failure, concomitantly. The rates of OS and GFRFS were higher in the Haplo group compared to MUD/MMUD, hence, our data supports the view that haploidentical HSCT with peripheral blood stem cells is a practical and valuable clinical option that offers CAYA patients with acute leukemia requiring HSCT and lacking matched available donors, a reasonable opportunity for the disease control. However, further progress is necessary to decrease the relapse rate in these patients.
Declarations
The study was approved by the Committee on Medical Ethics of Tehran University of Medical Sciences (TUMS) and informed consent was obtained from patients or their legal guardians. Authors provide a consent for publication. Primary data and materials are available on request.
Authors' contributions: TR designed and coordinated the study, and managed the patients. AK, MR and NA participated in the management of patients. AK carried out statistical evaluation. SA conceived of the study. All the authors read and approved the final manuscript.
Acknowledgements
We would like to thank Ashraf Sadat Hoseini and other nursing staff for their undeniable assistance in care for our patients.
Competing Interests
None of the authors have any relevant conflict of interest to disclaim about the present article. No funding support for the study is declared.
References
- Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Total body irradiation-based myeloablative haploidentical stem cell transplantation is a safe and effective alternative to unrelated donor transplantation in patients without matched sibling donors. Biol Blood Marrow Transplant. 2015; 21(7):1299-1307. doi: 10.1016/j.bbmt.2015.03.003
- Friebert SE, Shepardson LB, Shurin SB, Rosenthal GE, Rosenthal NS. Pediatric bone marrow cellularity: are we expecting too much?
J Pediat Hematol/Oncol. 1998; 20(5):439-43. doi: 10.1097/00043426-199809000-00006 - Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001; 19(1):117-125. doi: 10.1016/S0736-0266(00)00010-3
- Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014; 20(10):1573-1579.
doi: 10.1016/j.bbmt.2014.05.029 - Bashey A, Zhang XU, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013; 31(10):1310-1316. doi: 10.1200/JCO.2012.44.3523
- Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014; 20(12):1975-1981. doi: 10.1016/j.bbmt.2014.08.013
- George B, Pati N, Gilroy N, Ratnamohan M, Huang G, Kerridge I, Hertzberg M, Gottlieb D, Bradstock K. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transplant Infect Dis. 2010; 12(4):322-9.
doi: 10.1111/j.1399-3062.2010.00504.x - Balavarca Y, Pearce K, Norden J, Collin M, Jackson G, Holler E, et al. Predicting survival using clinical risk scores and non-HLA immunogenetics. Bone Marrow Transpl. 2015; 50(11):1445-1452. doi: 10.1038/bmt.2015.305
- Thiede C, Florek M, Bornhäuser M, Ritter M, Mohr B, Brendel C, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transpl. 1999; 23:1055-1060.
doi: 10.1038/sj.bmt.1701779 - Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014; 123:3664-3671. doi: 10.1182/blood-2014-01-552984
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18:295-304.
- Afify Z, Hunt L, Green A, Guttridge M, Cornish J, Oakhill A. Factors affecting the outcome of stem cell transplantation from unrelated donors for childhood acute lymphoblastic leukemia in third remission. Bone Marrow Transpl. 2005; 35:1041-1047.
doi: 10.1038/sj.bmt.1704958 - Balduzzi A, Valsecchi MG, Uderzo C, De Lorenzo P, Klingebiel T, Peters C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet. 2005; 366:635-642. doi: 10.1016/S0140-6736(05)66998-X
- Cornish J, Oakhill A. The management of relapsed acute lymphoblastic leukaemia. Bone Marrow Transpl. 2001; 28(1):S9. https://doi.org/10.1038/sj.bmt.1703167
- Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R, et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010; 115:3437-3446. doi: 10.1182/blood-2009-03-207001
- Nagler A, Ruggeri A. Haploidentical stem cell transplantation (HaploSCT) for patients with acute leukemia – an update on behalf of the ALWP of the EBMT. Bone Marrow Transplant. 2019; 54(2): 713-718. doi: 10.1038/s41409-019-0610-5
- Piemontese S, Ciceri F, Labopin M, Arcese W, Kyrcz-Krzemien S, Santarone S, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017; 10(1):1-8.
doi: 10.1186/s13045-017-0394-2 - Sun Y, Beohou E, Labopin M, Volin L, Milpied N, Yakoub-Agha I, et al. Unmanipulated haploidentical versus matched unrelated donor allogeneic stem cell transplantation in adult patients with acute myelogenous leukemia in first remission: a retrospective pair-matched comparative study of the Beijing approach with the EBMT database. Haematologica. 2016; 101:e352-4.
doi: 10.3324/haematol.2015.140509 - Lorentino F, Labopin M, Bernardi M, Ciceri F, Socié G, Cornelissen JJ, et al. Comparable outcomes of haploidentical, 10/10 and 9/10unrelated donor transplantation in adverse karyotype AML in first complete remission. Am J Hematol. 2018; 93:1236-1244.
doi: 10.1002/ajh.25231 - Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014; 20(10):1573-1579.
doi: 10.1016/j.bbmt.2014.05.029 - Bashey A, Zhang XU, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013; 31(10):1310-1316.
doi: 10.1200/JCO.2012.44.3523 - Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014; 20(12):1975-1981. doi: 10.1016/j.bbmt.2014.08.013
- Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014; 124(17):2735-2743. doi: 10.1182/blood-2014-04-571570
- Saglio F, Berger M, Spadea M, Pessolano R, Carraro F, Barone M, et al. Haploidentical HSCT with post transplantation cyclophosphamide versus unrelated donor HSCT in pediatric patients affected by acute leukemia. Bone Marrow Transpl. 2021; 56(3): 586-595. doi: 10.1038/s41409-020-01063-2
- Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Posttransplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019; 129:2357–2373.
doi: 10.1172/JCI124218 - Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009; 10(9): 855-864. doi: 10.1016/S1470-2045(09)70225-6
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, Di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001; 98(10):2942-2947. doi: 10.1182/blood.V98.10.2942
- Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011; 29(16):2230. doi: 10.1200/JCO.2010.33.7212
- Martin PJ, Counts Jr GW, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010; 28(6):1011. doi: 10.1200/JCO.2009.25.6693
- Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015; 21(2):266-274. doi: 10.1016/j.bbmt.2014.10.021
- Shimoni A, Labopin M, Finke J, Ciceri F, Deconinck E, Kröger N, et al. Donor selection for a second allogeneic stem cell transplantation in AML patients relapsing after a first transplant: a study of the Acute Leukemia Working Party of EBMT. Blood Cancer Journal. 2019 ; 9(12):1-9. doi: 10.1038/s41408-019-0251-3
- Distler E, Bloetz A, Albrecht J, Asdufan S, Hohberger A, Frey M, et al. Alloreactive and leukemia-reactive T cells are preferentially derived from naive precursors in healthy donors: implications for immunotherapy with memory T cells. Haematologica. 2011; 96(7):1024. doi: 10.3324/haematol.2010.037481
- Bertaina A, Zecca M, Buldini B, Sacchi N, Algeri M, Saglio F, et al. Unrelated donor vs HLA-haploidentical α/β T-cell- and Bcell-depleted HSCT in children with acute leukemia. Blood. 2018; 132:2594-2607. doi: 10.1182/blood-2018-07-861575
- Versluis J, Labopin M, Ruggeri A, Socie G, Wu D, Volin L, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 2017; 1(7):477-485. doi: 10.1182/bloodadvances.2016002386
- Baron F, Labopin M, Ruggeri A, Cornelissen JJ, Meijer E, Sengeloev H, et al. Impact of donor type in patients with AML given allogeneic hematopoietic cell transplantation after low-dose TBI-based regimen. Clin Cancer Res. 2018; 24(12):2794-2803.
doi: 10.1158/1078-0432.CCR-17-3622 - Rashidi A, Hamadani M, Zhang MJ, Wang HL, Abdel-Azim H, Aljurf M, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019; 3(12):1826-1836.
https://doi.org/10.1182/bloodadvances.2019000050
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(101) "transplantatsiya-gemopoeticheskikh-kletok-perifericheskoy-krovi-ot-gaploidentichnykh-i-nerodstvennykh" ["~CODE"]=> string(101) "transplantatsiya-gemopoeticheskikh-kletok-perifericheskoy-krovi-ot-gaploidentichnykh-i-nerodstvennykh" ["EXTERNAL_ID"]=> string(4) "2052" ["~EXTERNAL_ID"]=> string(4) "2052" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(546) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного рискаPeripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(6200) "<p style="text-align: justify;">Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.</p> <h3>Результаты</h3> <p style="text-align: justify;">Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.</p> <h3>Выводы</h3> <p style="text-align: justify;">В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_META_TITLE"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_META_KEYWORDS"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_META_DESCRIPTION"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_PICTURE_FILE_ALT"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_PICTURE_FILE_TITLE"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_PICTURE_FILE_NAME"]=> string(101) "transplantatsiya-gemopoeticheskikh-kletok-perifericheskoy-krovi-ot-gaploidentichnykh-i-nerodstvennykh" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(353) "Трансплантация гемопоэтических клеток периферической крови от гаплоидентичных и неродственных доноров при острых лейкозах у детей, подростков и молодых взрослых: анализ конкурентного риска" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(101) "transplantatsiya-gemopoeticheskikh-kletok-perifericheskoy-krovi-ot-gaploidentichnykh-i-nerodstvennykh" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(101) "transplantatsiya-gemopoeticheskikh-kletok-perifericheskoy-krovi-ot-gaploidentichnykh-i-nerodstvennykh" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(101) "transplantatsiya-gemopoeticheskikh-kletok-perifericheskoy-krovi-ot-gaploidentichnykh-i-nerodstvennykh" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "207" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28530" ["VALUE"]=> string(10) "24.01.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "24.01.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28531" ["VALUE"]=> string(10) "18.02.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "18.02.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28532" ["VALUE"]=> array(2) { ["TEXT"]=> string(714) "<p>Тахерех Ростами<sup>1</sup>, Мохамад Р. Ростами<sup>2</sup>, Азадех Кьюмарси<sup>1</sup>, Амир Казаэйян<sup>3</sup>, Неда Алиджани<sup>4</sup>, Хосейн К. Фумани<sup>2</sup>, Соруш Рад<sup>2</sup>, Давуд Бабахани<sup>2</sup>, Таназ Бахри<sup>2</sup>, Мохаммад Ваези<sup>2</sup>, Мариам Бахордар<sup>2</sup>, Сейед А. Мирхоссейни<sup>2</sup>, Сейед А. Моусави<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(546) "
Тахерех Ростами1, Мохамад Р. Ростами2, Азадех Кьюмарси1, Амир Казаэйян3, Неда Алиджани4, Хосейн К. Фумани2, Соруш Рад2, Давуд Бабахани2, Таназ Бахри2, Мохаммад Ваези2, Мариам Бахордар2, Сейед А. Мирхоссейни2, Сейед А. Моусави2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28533" ["VALUE"]=> array(2) { ["TEXT"]=> string(1215) "<p><sup>1</sup> Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br> <sup>2</sup> НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br> <sup>3</sup> Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br> <sup>4</sup> Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1137) "1 Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
2 НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
3 Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
4 Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.
Пациенты и методы
В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.
Результаты
Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.
Выводы
В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.
Ключевые слова
Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28535" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-24-35" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-24-35" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28538" ["VALUE"]=> array(2) { ["TEXT"]=> string(550) "<p>Tahereh Rostami<sup>1</sup>, Mohammad R. Rostami<sup>2</sup>, Azadeh Kiumarsi<sup>1</sup>, Amir Kasaeian<sup>3</sup>, Neda Alijani<sup>4</sup>, Hosein K. Fumani<sup>2</sup>, Soroush Rad<sup>2</sup>, Davood Babakhani<sup>2</sup>, Tanaz Bahri<sup>2</sup>, Mohammad Vaezi<sup>2</sup>, Maryam Barkhordar<sup>2</sup>, Seied A. Mirhosseini<sup>2</sup>, Seied A. Mousavi<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(382) "Tahereh Rostami1, Mohammad R. Rostami2, Azadeh Kiumarsi1, Amir Kasaeian3, Neda Alijani4, Hosein K. Fumani2, Soroush Rad2, Davood Babakhani2, Tanaz Bahri2, Mohammad Vaezi2, Maryam Barkhordar2, Seied A. Mirhosseini2, Seied A. Mousavi2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28539" ["VALUE"]=> array(2) { ["TEXT"]=> string(1607) "<p><sup>1</sup> Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br> <sup>2</sup> Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br> <sup>3</sup> Department of Biostatistics and Epidemiology, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br> <sup>4</sup> Department of lnfectious Diseases, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran</p><br> <p><b>Correspondence:</b><br> Dr. Azadeh Kiumarsi, MD, Assistant Professor, Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Kargar Shomali Street, 1411713131, Tehran, Iran<br> Phone: +98 9121037104<br> Fax: +98 (21) 8802 9397<br> Email: akiumarsi@sina.tums.ac.ir</p><br> <p><b>Citation:</b> Tahereh Rostami, Mohammad R. Rostami, Azadeh Kiumarsi et al. Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis. Cell Ther Transplant 2022; 11(1): 24-35. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1445) "1 Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
2 Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
3 Department of Biostatistics and Epidemiology, Research Institute for Oncology, Hematology and Cell Therapy, Shariati
Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
4 Department of lnfectious Diseases, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Correspondence:
Dr. Azadeh Kiumarsi, MD, Assistant Professor, Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Kargar Shomali Street, 1411713131, Tehran, Iran
Phone: +98 9121037104
Fax: +98 (21) 8802 9397
Email: akiumarsi@sina.tums.ac.ir
Citation: Tahereh Rostami, Mohammad R. Rostami, Azadeh Kiumarsi et al. Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis. Cell Ther Transplant 2022; 11(1): 24-35.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28540" ["VALUE"]=> array(2) { ["TEXT"]=> string(3600) "<p style="text-align: justify;">Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for acute leukemia. Various parameters have significant impact on the final results of HSCT, such as donor type, stem cell source, and the applied conditioning regimen. In the absence of HLA-matched related or unrelated donors, haploidentical donors present a possible alternative for the patients with indications for HSCT. The present single-center study compared the outcomes of HSCT from different donor types using a radiation-free MAC regimen. We compared the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) from matched, or mismatched related, and unrelated donors with those from haploidentical donors in the children, adolescents and young adults (CAYA) treated for acute leukemia.</p> <h3>Patients and methods</h3> <p style="text-align: justify;">In this retrospective study performed since 2014 to 2021, we have evaluated the clinical outcomes among CAYA patients with acute leukemia who underwent peripheral blood T cell-replete HSCT from haploidentical donors <i>versus</i> unrelated donors (including 10/10 or 9/10 HLA-matched), and <i>versus</i> related donors (including 10/10 or 9/10 HLA-matched). The myeloablative conditioning for HSCT was performed as irradiation-free regimen including busulfan and cyclophosphamide. GvHD prophylaxis was based on administration of cyclosporine A in all the patients, accomplished by rabbit anti-human thymocyte globulin in HSCT from unrelated and haploidentical donors, and post-transplant cyclophosphamide in cases of haploidentical donors. For statistical evaluation, an adjusted multivariable proportional hazard Cox and competing risk analyses were used.</p> <h3>Results</h3> <p style="text-align: justify;">Median follow-up time period was 28.7 months (95% CI: 21.9-34.9). Three-year overall survival rate (OS) and GvHD-free/relapse-free survival (GFRFS) rate were 68.81% (95% CI: 60.08%-76.01%) and 44.19% (95% CI: 35.52%-52.49%), respectively. The patients who underwent HSCT from unrelated HLA-matched donors had the lowest OS and GFRFS compared to other donor types. The 3-year non-relapse mortality (NRM) in all patients was 7.84% (95% CI 4.36-12.62). Adjusted multivariable modeling of OS showed that the hazard of death in patients who had undergone HSCT from an unrelated donor, was 3.6 times more than for the patients who underwent HSCT from their haploidentical donors (P=0.05). Likewise, the hazard of NRM after HSCT from unrelated donors was 6 times more than with haploidentical donors (P=0.002). However, the relapse incidence was not significantly different between the two mentioned groups.</p> <h3>Conclusions</h3> <p style="text-align: justify;">In this study, HSCT from haploidentical donors was associated with superior survival rates compared to HSCT from unrelated HLA-matched donors. Hence, haploidentical transplantation with peripheral blood stem cells could be a practical and valuable clinical option that offers a reasonable opportunity for the disease control in CAYA patients with acute leukemia requiring HSCT and lacking matched available donors.</p> <h2>Keywords</h2> <p style="text-align: justify;">Acute leukemia, allogeneic hematopoietic stem cell transplantation, matched related donors, unrelated donors, haploidentical donors, clinical outcomes.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3418) "Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for acute leukemia. Various parameters have significant impact on the final results of HSCT, such as donor type, stem cell source, and the applied conditioning regimen. In the absence of HLA-matched related or unrelated donors, haploidentical donors present a possible alternative for the patients with indications for HSCT. The present single-center study compared the outcomes of HSCT from different donor types using a radiation-free MAC regimen. We compared the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) from matched, or mismatched related, and unrelated donors with those from haploidentical donors in the children, adolescents and young adults (CAYA) treated for acute leukemia.
Patients and methods
In this retrospective study performed since 2014 to 2021, we have evaluated the clinical outcomes among CAYA patients with acute leukemia who underwent peripheral blood T cell-replete HSCT from haploidentical donors versus unrelated donors (including 10/10 or 9/10 HLA-matched), and versus related donors (including 10/10 or 9/10 HLA-matched). The myeloablative conditioning for HSCT was performed as irradiation-free regimen including busulfan and cyclophosphamide. GvHD prophylaxis was based on administration of cyclosporine A in all the patients, accomplished by rabbit anti-human thymocyte globulin in HSCT from unrelated and haploidentical donors, and post-transplant cyclophosphamide in cases of haploidentical donors. For statistical evaluation, an adjusted multivariable proportional hazard Cox and competing risk analyses were used.
Results
Median follow-up time period was 28.7 months (95% CI: 21.9-34.9). Three-year overall survival rate (OS) and GvHD-free/relapse-free survival (GFRFS) rate were 68.81% (95% CI: 60.08%-76.01%) and 44.19% (95% CI: 35.52%-52.49%), respectively. The patients who underwent HSCT from unrelated HLA-matched donors had the lowest OS and GFRFS compared to other donor types. The 3-year non-relapse mortality (NRM) in all patients was 7.84% (95% CI 4.36-12.62). Adjusted multivariable modeling of OS showed that the hazard of death in patients who had undergone HSCT from an unrelated donor, was 3.6 times more than for the patients who underwent HSCT from their haploidentical donors (P=0.05). Likewise, the hazard of NRM after HSCT from unrelated donors was 6 times more than with haploidentical donors (P=0.002). However, the relapse incidence was not significantly different between the two mentioned groups.
Conclusions
In this study, HSCT from haploidentical donors was associated with superior survival rates compared to HSCT from unrelated HLA-matched donors. Hence, haploidentical transplantation with peripheral blood stem cells could be a practical and valuable clinical option that offers a reasonable opportunity for the disease control in CAYA patients with acute leukemia requiring HSCT and lacking matched available donors.
Keywords
Acute leukemia, allogeneic hematopoietic stem cell transplantation, matched related donors, unrelated donors, haploidentical donors, clinical outcomes.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28536" ["VALUE"]=> string(193) "Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(193) "Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28537" ["VALUE"]=> string(4) "2786" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2786" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28541" ["VALUE"]=> string(4) "2787" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2787" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28538" ["VALUE"]=> array(2) { ["TEXT"]=> string(550) "<p>Tahereh Rostami<sup>1</sup>, Mohammad R. Rostami<sup>2</sup>, Azadeh Kiumarsi<sup>1</sup>, Amir Kasaeian<sup>3</sup>, Neda Alijani<sup>4</sup>, Hosein K. Fumani<sup>2</sup>, Soroush Rad<sup>2</sup>, Davood Babakhani<sup>2</sup>, Tanaz Bahri<sup>2</sup>, Mohammad Vaezi<sup>2</sup>, Maryam Barkhordar<sup>2</sup>, Seied A. Mirhosseini<sup>2</sup>, Seied A. Mousavi<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(382) "Tahereh Rostami1, Mohammad R. Rostami2, Azadeh Kiumarsi1, Amir Kasaeian3, Neda Alijani4, Hosein K. Fumani2, Soroush Rad2, Davood Babakhani2, Tanaz Bahri2, Mohammad Vaezi2, Maryam Barkhordar2, Seied A. Mirhosseini2, Seied A. Mousavi2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(382) "Tahereh Rostami1, Mohammad R. Rostami2, Azadeh Kiumarsi1, Amir Kasaeian3, Neda Alijani4, Hosein K. Fumani2, Soroush Rad2, Davood Babakhani2, Tanaz Bahri2, Mohammad Vaezi2, Maryam Barkhordar2, Seied A. Mirhosseini2, Seied A. Mousavi2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28540" ["VALUE"]=> array(2) { ["TEXT"]=> string(3600) "<p style="text-align: justify;">Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for acute leukemia. Various parameters have significant impact on the final results of HSCT, such as donor type, stem cell source, and the applied conditioning regimen. In the absence of HLA-matched related or unrelated donors, haploidentical donors present a possible alternative for the patients with indications for HSCT. The present single-center study compared the outcomes of HSCT from different donor types using a radiation-free MAC regimen. We compared the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) from matched, or mismatched related, and unrelated donors with those from haploidentical donors in the children, adolescents and young adults (CAYA) treated for acute leukemia.</p> <h3>Patients and methods</h3> <p style="text-align: justify;">In this retrospective study performed since 2014 to 2021, we have evaluated the clinical outcomes among CAYA patients with acute leukemia who underwent peripheral blood T cell-replete HSCT from haploidentical donors <i>versus</i> unrelated donors (including 10/10 or 9/10 HLA-matched), and <i>versus</i> related donors (including 10/10 or 9/10 HLA-matched). The myeloablative conditioning for HSCT was performed as irradiation-free regimen including busulfan and cyclophosphamide. GvHD prophylaxis was based on administration of cyclosporine A in all the patients, accomplished by rabbit anti-human thymocyte globulin in HSCT from unrelated and haploidentical donors, and post-transplant cyclophosphamide in cases of haploidentical donors. For statistical evaluation, an adjusted multivariable proportional hazard Cox and competing risk analyses were used.</p> <h3>Results</h3> <p style="text-align: justify;">Median follow-up time period was 28.7 months (95% CI: 21.9-34.9). Three-year overall survival rate (OS) and GvHD-free/relapse-free survival (GFRFS) rate were 68.81% (95% CI: 60.08%-76.01%) and 44.19% (95% CI: 35.52%-52.49%), respectively. The patients who underwent HSCT from unrelated HLA-matched donors had the lowest OS and GFRFS compared to other donor types. The 3-year non-relapse mortality (NRM) in all patients was 7.84% (95% CI 4.36-12.62). Adjusted multivariable modeling of OS showed that the hazard of death in patients who had undergone HSCT from an unrelated donor, was 3.6 times more than for the patients who underwent HSCT from their haploidentical donors (P=0.05). Likewise, the hazard of NRM after HSCT from unrelated donors was 6 times more than with haploidentical donors (P=0.002). However, the relapse incidence was not significantly different between the two mentioned groups.</p> <h3>Conclusions</h3> <p style="text-align: justify;">In this study, HSCT from haploidentical donors was associated with superior survival rates compared to HSCT from unrelated HLA-matched donors. Hence, haploidentical transplantation with peripheral blood stem cells could be a practical and valuable clinical option that offers a reasonable opportunity for the disease control in CAYA patients with acute leukemia requiring HSCT and lacking matched available donors.</p> <h2>Keywords</h2> <p style="text-align: justify;">Acute leukemia, allogeneic hematopoietic stem cell transplantation, matched related donors, unrelated donors, haploidentical donors, clinical outcomes.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3418) "Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for acute leukemia. Various parameters have significant impact on the final results of HSCT, such as donor type, stem cell source, and the applied conditioning regimen. In the absence of HLA-matched related or unrelated donors, haploidentical donors present a possible alternative for the patients with indications for HSCT. The present single-center study compared the outcomes of HSCT from different donor types using a radiation-free MAC regimen. We compared the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) from matched, or mismatched related, and unrelated donors with those from haploidentical donors in the children, adolescents and young adults (CAYA) treated for acute leukemia.
Patients and methods
In this retrospective study performed since 2014 to 2021, we have evaluated the clinical outcomes among CAYA patients with acute leukemia who underwent peripheral blood T cell-replete HSCT from haploidentical donors versus unrelated donors (including 10/10 or 9/10 HLA-matched), and versus related donors (including 10/10 or 9/10 HLA-matched). The myeloablative conditioning for HSCT was performed as irradiation-free regimen including busulfan and cyclophosphamide. GvHD prophylaxis was based on administration of cyclosporine A in all the patients, accomplished by rabbit anti-human thymocyte globulin in HSCT from unrelated and haploidentical donors, and post-transplant cyclophosphamide in cases of haploidentical donors. For statistical evaluation, an adjusted multivariable proportional hazard Cox and competing risk analyses were used.
Results
Median follow-up time period was 28.7 months (95% CI: 21.9-34.9). Three-year overall survival rate (OS) and GvHD-free/relapse-free survival (GFRFS) rate were 68.81% (95% CI: 60.08%-76.01%) and 44.19% (95% CI: 35.52%-52.49%), respectively. The patients who underwent HSCT from unrelated HLA-matched donors had the lowest OS and GFRFS compared to other donor types. The 3-year non-relapse mortality (NRM) in all patients was 7.84% (95% CI 4.36-12.62). Adjusted multivariable modeling of OS showed that the hazard of death in patients who had undergone HSCT from an unrelated donor, was 3.6 times more than for the patients who underwent HSCT from their haploidentical donors (P=0.05). Likewise, the hazard of NRM after HSCT from unrelated donors was 6 times more than with haploidentical donors (P=0.002). However, the relapse incidence was not significantly different between the two mentioned groups.
Conclusions
In this study, HSCT from haploidentical donors was associated with superior survival rates compared to HSCT from unrelated HLA-matched donors. Hence, haploidentical transplantation with peripheral blood stem cells could be a practical and valuable clinical option that offers a reasonable opportunity for the disease control in CAYA patients with acute leukemia requiring HSCT and lacking matched available donors.
Keywords
Acute leukemia, allogeneic hematopoietic stem cell transplantation, matched related donors, unrelated donors, haploidentical donors, clinical outcomes.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3418) "Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for acute leukemia. Various parameters have significant impact on the final results of HSCT, such as donor type, stem cell source, and the applied conditioning regimen. In the absence of HLA-matched related or unrelated donors, haploidentical donors present a possible alternative for the patients with indications for HSCT. The present single-center study compared the outcomes of HSCT from different donor types using a radiation-free MAC regimen. We compared the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) from matched, or mismatched related, and unrelated donors with those from haploidentical donors in the children, adolescents and young adults (CAYA) treated for acute leukemia.
Patients and methods
In this retrospective study performed since 2014 to 2021, we have evaluated the clinical outcomes among CAYA patients with acute leukemia who underwent peripheral blood T cell-replete HSCT from haploidentical donors versus unrelated donors (including 10/10 or 9/10 HLA-matched), and versus related donors (including 10/10 or 9/10 HLA-matched). The myeloablative conditioning for HSCT was performed as irradiation-free regimen including busulfan and cyclophosphamide. GvHD prophylaxis was based on administration of cyclosporine A in all the patients, accomplished by rabbit anti-human thymocyte globulin in HSCT from unrelated and haploidentical donors, and post-transplant cyclophosphamide in cases of haploidentical donors. For statistical evaluation, an adjusted multivariable proportional hazard Cox and competing risk analyses were used.
Results
Median follow-up time period was 28.7 months (95% CI: 21.9-34.9). Three-year overall survival rate (OS) and GvHD-free/relapse-free survival (GFRFS) rate were 68.81% (95% CI: 60.08%-76.01%) and 44.19% (95% CI: 35.52%-52.49%), respectively. The patients who underwent HSCT from unrelated HLA-matched donors had the lowest OS and GFRFS compared to other donor types. The 3-year non-relapse mortality (NRM) in all patients was 7.84% (95% CI 4.36-12.62). Adjusted multivariable modeling of OS showed that the hazard of death in patients who had undergone HSCT from an unrelated donor, was 3.6 times more than for the patients who underwent HSCT from their haploidentical donors (P=0.05). Likewise, the hazard of NRM after HSCT from unrelated donors was 6 times more than with haploidentical donors (P=0.002). However, the relapse incidence was not significantly different between the two mentioned groups.
Conclusions
In this study, HSCT from haploidentical donors was associated with superior survival rates compared to HSCT from unrelated HLA-matched donors. Hence, haploidentical transplantation with peripheral blood stem cells could be a practical and valuable clinical option that offers a reasonable opportunity for the disease control in CAYA patients with acute leukemia requiring HSCT and lacking matched available donors.
Keywords
Acute leukemia, allogeneic hematopoietic stem cell transplantation, matched related donors, unrelated donors, haploidentical donors, clinical outcomes.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28535" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-24-35" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-24-35" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-24-35" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28536" ["VALUE"]=> string(193) "Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(193) "Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(193) "Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28539" ["VALUE"]=> array(2) { ["TEXT"]=> string(1607) "<p><sup>1</sup> Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br> <sup>2</sup> Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br> <sup>3</sup> Department of Biostatistics and Epidemiology, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br> <sup>4</sup> Department of lnfectious Diseases, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran</p><br> <p><b>Correspondence:</b><br> Dr. Azadeh Kiumarsi, MD, Assistant Professor, Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Kargar Shomali Street, 1411713131, Tehran, Iran<br> Phone: +98 9121037104<br> Fax: +98 (21) 8802 9397<br> Email: akiumarsi@sina.tums.ac.ir</p><br> <p><b>Citation:</b> Tahereh Rostami, Mohammad R. Rostami, Azadeh Kiumarsi et al. Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis. Cell Ther Transplant 2022; 11(1): 24-35. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1445) "1 Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
2 Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
3 Department of Biostatistics and Epidemiology, Research Institute for Oncology, Hematology and Cell Therapy, Shariati
Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
4 Department of lnfectious Diseases, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Correspondence:
Dr. Azadeh Kiumarsi, MD, Assistant Professor, Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Kargar Shomali Street, 1411713131, Tehran, Iran
Phone: +98 9121037104
Fax: +98 (21) 8802 9397
Email: akiumarsi@sina.tums.ac.ir
Citation: Tahereh Rostami, Mohammad R. Rostami, Azadeh Kiumarsi et al. Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis. Cell Ther Transplant 2022; 11(1): 24-35.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1445) "1 Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
2 Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
3 Department of Biostatistics and Epidemiology, Research Institute for Oncology, Hematology and Cell Therapy, Shariati
Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
4 Department of lnfectious Diseases, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Correspondence:
Dr. Azadeh Kiumarsi, MD, Assistant Professor, Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Kargar Shomali Street, 1411713131, Tehran, Iran
Phone: +98 9121037104
Fax: +98 (21) 8802 9397
Email: akiumarsi@sina.tums.ac.ir
Citation: Tahereh Rostami, Mohammad R. Rostami, Azadeh Kiumarsi et al. Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis. Cell Ther Transplant 2022; 11(1): 24-35.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28532" ["VALUE"]=> array(2) { ["TEXT"]=> string(714) "<p>Тахерех Ростами<sup>1</sup>, Мохамад Р. Ростами<sup>2</sup>, Азадех Кьюмарси<sup>1</sup>, Амир Казаэйян<sup>3</sup>, Неда Алиджани<sup>4</sup>, Хосейн К. Фумани<sup>2</sup>, Соруш Рад<sup>2</sup>, Давуд Бабахани<sup>2</sup>, Таназ Бахри<sup>2</sup>, Мохаммад Ваези<sup>2</sup>, Мариам Бахордар<sup>2</sup>, Сейед А. Мирхоссейни<sup>2</sup>, Сейед А. Моусави<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(546) "Тахерех Ростами1, Мохамад Р. Ростами2, Азадех Кьюмарси1, Амир Казаэйян3, Неда Алиджани4, Хосейн К. Фумани2, Соруш Рад2, Давуд Бабахани2, Таназ Бахри2, Мохаммад Ваези2, Мариам Бахордар2, Сейед А. Мирхоссейни2, Сейед А. Моусави2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(546) "Тахерех Ростами1, Мохамад Р. Ростами2, Азадех Кьюмарси1, Амир Казаэйян3, Неда Алиджани4, Хосейн К. Фумани2, Соруш Рад2, Давуд Бабахани2, Таназ Бахри2, Мохаммад Ваези2, Мариам Бахордар2, Сейед А. Мирхоссейни2, Сейед А. Моусави2
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28530" ["VALUE"]=> string(10) "24.01.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "24.01.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "24.01.2022" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28531" ["VALUE"]=> string(10) "18.02.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "18.02.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "18.02.2022" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28534" ["VALUE"]=> array(2) { ["TEXT"]=> string(6200) "<p style="text-align: justify;">Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.</p> <h3>Результаты</h3> <p style="text-align: justify;">Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.</p> <h3>Выводы</h3> <p style="text-align: justify;">В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(6042) "Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.
Пациенты и методы
В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.
Результаты
Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.
Выводы
В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.
Ключевые слова
Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(6042) "Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.
Пациенты и методы
В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.
Результаты
Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.
Выводы
В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.
Ключевые слова
Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28533" ["VALUE"]=> array(2) { ["TEXT"]=> string(1215) "<p><sup>1</sup> Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br> <sup>2</sup> НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br> <sup>3</sup> Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br> <sup>4</sup> Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1137) "1 Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
2 НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
3 Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
4 Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
1 Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
2 НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
3 Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
4 Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
Introduction
Over the past decades, allogeneic stem cell transplantation (allo-HSCT) has been used worldwide as a technology aimed both the long-term management and cure of malignant hematological diseases [1]. Allo-HSCT remains a valuable option for treatment of chronic myeloid leukemia (CML) in era of tyrosine kinase inhibitors (TKI) [2-6]. The transplant-eligible population consists of the CML patients with predicted poor outcome if treated with TKIs alone. Despite the superiority of drug treatment, the development of transplant technology, i.e., usage of reduced intensity conditioning regimens, increased donor availability, led to improvement in the results of allo-HSCT in these patients [7,8]. Thus, transplantation is still a potentially curative therapeutic mode in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with 2 TKIs, and patients with advanced-phase CML.
Unfavorable results are mostly associated with impaired graft function, which is manifested in the lack of control over the underlying disease and subsequent relapse, as well as in primary graft failure (PrGF) and poor graft function (PoGF) [9-12]. Several risk factors of post-transplant graft failure were revealed, e.g., patient’s age, donor-recipient blood mismatch and CMV infection (13]. Treatment options for poor graft functioning are still limited. In addition to reinfusion of stem cell, some recent studies report, e.g., positive effects of Eltrombopag, a thrombopoietin mimetic [14].
The aim of the study was to assess the incidence and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Materials and methods
Patients and data collection
We carried out retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in R. M. Gorbacheva Research Institute at the Pavlov University between 1995 and 2020. Information on the disease stage at diagnosis, time to allo-HSCT, transplantation procedure, relapse, and treatment following allo-HSCT was gathered via systematic reviews of the patient records. General approaches to evaluation of HSCT patients at our clinic were described elsewhere [15].
Definitions
CML was diagnosed on the basis of clinical and laboratory data, the detection of Philadelphia (Ph) chromosome and/or the chimeric BCR-ABL gene. Disease phase was defined according to the WHO classification [16]. The first chronic phase (CP1) was recognized in the absence of accelerated phase (AP) and/or blast crisis (BC) in the patient’s history, otherwise CP≥2 was registered. Hematological, cytogenetic and molecular response to the treatment prior to allo-HSCT was defined using ELN criteria [17].
Indications for HSCT
Indications for HSCT were as follows: 1) AP/BC at diagnosis or progression to AP/BC; 2) treatment failure in pre-TKI era; 3) treatment failure due to TKI resistance/intolerance; 4) T315I mutation. TKI resistance and TKI intolerance were defined according to ELN criteria [17].
Laboratory studies
For cytogenetic evaluation, conventional synchronized culture was performed for 48 hours with at least 20 metaphases analyzed per a sample (GTG method). Leukemia cell karyotype was evaluated according to International System for Human Cytogenetic Nomenclature (ISCN) [18]. In cases when the standard cytogenetic investigation was not available (i.e., insufficient material), the bone marrow was assessed with fluorescence in situ hybridization (FISH) probes aimed for detection of (9;22) variants (LSI BCR-ABL, Dual Color, Dual Fusion, "Vysis").
Additional chromosomal abnormalities (ACA) were defined as any structural and numerical chromosomal aberrations other than t(9;22)(q34;q11) (detected by cytogenetic or molecular assays for cryptic abnormalities).
Molecular response after allo-HSCT was evaluated according to the National Comprehensive Cancer Network (NCCN) criteria (2021). PCR monitoring of BCR/ABL was carried out according to NCCN Guidelines once in 3 months for 2 years, then once in every 3 to 6 months. The relative BCR-ABL1 expression level was evaluated according to method described by Gabert et al [19]. This technique includes the following stages: 1) total RNA extraction from peripheral blood of patients with CML, 2) reverse transcription with random hexameric primers, 3) real-time PCR with primers and probes specific to р210, р190 control ABL gene sequences.
Assessment of relative expression levels was based on evaluation of BCR-ABL1/ABL1 ratios in the studied cDNA samples. The ABL1 gene was used for normalization of the results. In order to determine copy numbers of the BCR-ABL1 and ABL1 transcripts, and to assess the reaction effectiveness, standard dilution curves were plotted using a plasmid with inserts of known target gene sequences (Invitrogen, USA), at a standard concentration ranges of 102-106 copies/mcl, according to 2020 European LeukemiaNet (ELN) Recommendations [17]. ABL kinase domain mutations were determined by Sanger direct sequencing.
Post-transplant monitoring
Post-transplant engraftment was defined as absolute neutrophil count (ANC) of >0.5×109/L without administration of colony-stimulating factor within 3 days. Primary graft failure (PrGF) was diagnosed in absence of donor cells in recipient’s bone marrow by the day +30. Donor chimerism was checked at the time of myelopoiesis recovery, i.e. ANC> 0.5×109/L, and by the days +30, 60, +100, +200, and in case of any cytopenia, or signs of relapse. Post-transplant relapse was diagnosed in cases of clinical progression to AP/BC, cytogenetic relapse, or molecular relapse defined as two consecutive positive PCR tests, or, at least, 1-log persistent increase of BCR/ABL transcript level.
The criteria for severe poor graft function (sPGF) were as follows: cytopenia in two or more hematopoietic lineages (platelets <20×109/l, ANC <0.5×109/l, hemoglobin <70 g/l) any time after documented engraftment in presence of full or stable mixed donor chimerism >90% without signs of relapse of underlying disease, rejection or acute graft-versus-host disease (GVHD) grade III-IV.
Secondary graft failure was defined as loss of donor hematopoiesis to <5% and/or ANC counts to <0.5×109/L after initial engraftment being not related to relapse, infection, or drug toxicity [20].
Statistical evaluation
Descriptive characteristics of the cohort included number of cases, proportions for discrete factors, medians and range for continuous values. Individual pre-transplant risk for the HSCT patients was evaluated according to Gratwohl [21]. Overall survival (OS) was assessed using the Kaplan-Meier method from the time of allo-HSCT to the date of last contact or the date of death. Death from any cause was considered as an event.
Survival analysis was performed using log-rank test. Relapse and non-relapse mortality (NRM) rates were summarized using cumulative incidence estimates, with NRM as competing risk for relapse, and relapse regarded as competing risk for NRM.
The event-free survival (EFS) was estimated as a period from allo-HSCT until last contact date, death, or any of the following events: any kind of post-transplant relapse, graft-versus-host disease (GVHD) grade III-IV, severe poor graft function, or secondary graft failure. PrGF and sPGF were estimated as a proportion of cases in the total cohort. Cumulative incidence of sPGF was calculated with respect to competing risks (death before day +30, any type of relapse, GVHD grade III-IV).
The differences between groups were assessed using Fisher's exact test, Pearson χ2 test, and Mann-Whitney U-test for categorical and quantitative characteristics respectively, and Gray’s test for cumulative incidences. All the tests were two-sided, and P-values <0.05 were assessed as indicating for significant associations. Statistical analysis was performed using SPSS, IBM Statistics and EZR free statistical environment, version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
General characteristics of the patients and HSCT procedure
A total of 121 patients diagnosed with CML had undergone allo-HSCT. The median patients’ age was 37 years (range: 18-66). Other baseline characteristics for these patients are presented in Table 1.
The median time between CML diagnosis and allo-HSCT was 31 months (4.5-260). A total of 80 (66%) patients were transplanted in chronic phase (CP), the remaining 41 (34%) patients were in the active phase (AP or BC) at the time of HSCT. The median follow-up from allo-HSCT to the time of the last contact was 15 months (0.5-294).
HLA-matched or partially mismatched unrelated donors were used in 78 cases (65%), while matched related donors were employed in 34 cases (28%), and 9 (7%) patients received haploidentical allo-HSCT. The proportion of bone marrow (BM) and peripheral blood stem cells (PBSC) as the graft sources was almost equal: 49% (n=59) versus 51% (n=62).
Conditioning regimen included oral busulfan 8-12 mg/kg or melphalan 140 mg/m2 in combination with fludarabine 180 mg/m2 or cyclophosphamide. GVHD prophylaxis included calcineurin inhibitor (tacrolimus or cyclosporine A) and mycophenolate mofetil (30 mg/kg), or short course of posttransplant metotrexate, with or without antithymocyte globulin (horse, 60 mg/kg, or rabbit preparations, 5 mg/kg on day -3, -2, or high-dose), post-transplant cyclophosphamide (50 mg/kg, day +3 and +4 after allo-HSCT)/Alemtuzumab was used in two cases (Table 2).


Figure 1. Cumulative incidence of poor graft function post-HSCT in CML patients
Survival and relapse rates
Engraftment was documented in 106 (88%) patients, with median time to neutrophil recovery of 22 (8-58) days. Early death with no signs of engraftment before day +30 occurred in two cases. Thirty-one patients developed post-transplant relapse of any type with median time after allo-HSCT of 106 days (range: 15-333), included early CML relapse/progression before day +30 in 5 cases.
PrGF was documented in 8 (7%) cases. Two patients developed secondary graft failure, both in about two months after initial engraftment with lethal outcome due to severe bacterial infection. Severe poor graft function was diagnosed in 11 (9% of engrafted patients) with cumulative incidence of 10% (95% CI, 5-19) within 1 year after allo-HSCT (Fig. 1). Median time from HSCT to sPGF diagnosis was 43 (18-114) days.
The 1-year cumulative incidence rates of relapse and NRM comprised 35% (95% CI, 26-46) and 26% (95% CI, 18-35), respectively (Fig. 2).
A total of 57 (47%) patients died, the 1-year OS was 60% (95% CI, 51-69). Median OS was not reached. One-year EFS was 41% (95% CI, 32-50), median EFS was 271 (95% CI, 96-365) days (Fig. 3).

Figure 2. One-year cumulative incidence of relapses and NRM after allo-HSCT in CML patients

Figure 3. One-year overall and event-free survival after allogeneic HSCT in CML patients
Factors associated with primary graft failure and severe poor graft function
Among various pre-transplant characteristics, the presence of additional chromosomal abnormalities (ACA) was associated with cumulative incidence of PrGF and sPGF after HSCT. Thus, PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA, p=0.09. HLA-matched allo-HSCTs were beneficial for engraftment: 96% for HLA-matched transplantations vs 89% for allo-HSCT from HLA-mismatched donors, and 78% for haploidentical donors (p=0.05). Other pre-transplant factors didn’t show any statistical correlation with graft failure syndromes after HSCT (Table 3).
Table 3. Potential risk factors for the graft failure after allo-HSCT in CML patients
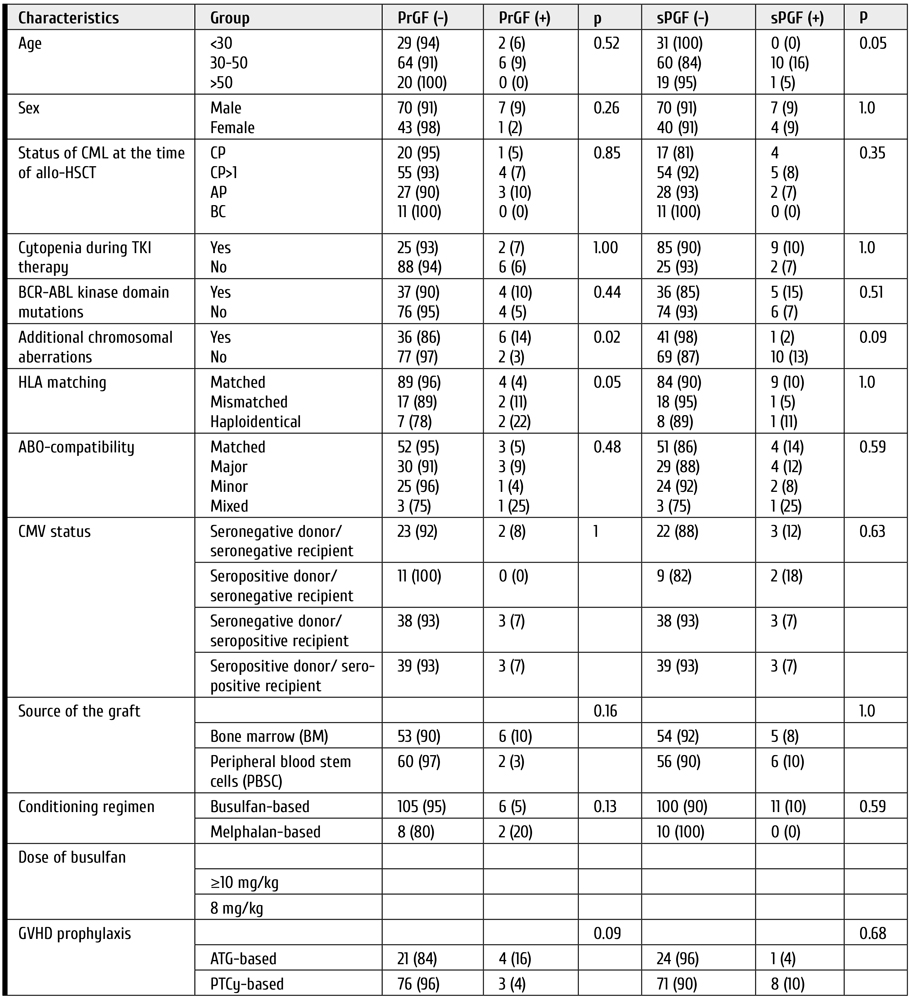
Clinical features and outcomes of graft failure and severe poor graft function
The median follow-up time after allo-HSCT was 68 days (range: 43-1792). All the patients with primary graft failure (PrGF) (n=8) were administered G-CSF, antimicrobial therapy and transfusion support. Two patients received donor lymphocyte infusions without any effect. The second allo-HSCT was performed in 4 cases. A total of 7 patients had lethal outcome (6, of infectious complications; 1, of relapse), whereas one patient is alive after the 2nd allo-HSCT (Fig. 4).

Figure 4. Treatment and outcomes of primary graft failure (PrGF) in CML patients after HSCT
Eleven patients exhibited severe poor graft function (sPGF) within median time of 21 (0-92) days after engraftment. Median length of sPGF was 52 days (range: 14-215). The median time of follow-up after allo-HSCT was 977 days (range: 45-2712).
Early sPGF with criterial cytopenia persisting after engraftment was diagnosed in 4 cases (36%), the remaining patients developed cytopenia after a period of normal graft function. A total of 3 cases of sPGF (27%) developed within 30 days after acute GVHD 2-3 grade (Fig. 5).

Figure 5. Timeline of severe poor graft function (SPGF) in a group of CML patients
All the patients with sPGF received antimicrobial therapy, transfusion support, and G-CSF in case of neutropenia. Other therapeutic options for sPGF therapy were: rituximab (n=4), the second allo-HSCT, or boost stem cell infusion (n=3); eltrombopag (n=1); supportive care (n=5), as seen from Fig. 4. Normal graft function was restored in 8 patients.
A total of 4 patients died. The causes of death were infectious complications (n=3) and late post-transplant relapse (n=1) (Fig. 6).
Figure 6. Treatment and outcomes of severe poor graft function (sPGF) in CML patients post-HSCT
Two cases of secondary graft failure occurred in about 3 months after allo-HSCT. Both patients died due to severe infection.
The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF as compared with those, who were free of these complications. Cumulative incidence of leukemia relapses was 31% (95% CI, 23-42), and 25% (95% CI, 4-87) in the patients with PrGF and engraftment (p=0.97), compared with 22% (95% CI, 3-54) and 32% (95% CI, 24-43) in the patients with sPGF and without sPGF (p=0.52), respectively.
Primary graft failure (PrGF) but not severe poor graft failure (sPGF) significantly increased non-relapse mortality during the first year after allo-HSCT. One-year NRM was 23% (95% CI, 15-32) in engrafted patients versus 71% (95% CI, 39-96) in the patients with PrGF (p<0.0001). Patients with and without sPGF had similar NRM: 20% (95% CI, 5-59) versus 26% (95% CI, 18-36) (p=0.74).
One-year OS was significantly lower in patients with PrGF: 13% (95% CI, 0.7-42) versus 64% (95% CI, 54-72) (p<0.0001) (Fig. 7). On the contrary, sPGF had no statistically significant influence on OS: 73% (95% CI, 37-90) versus 59% (95% CI, 49-69) (p=0.47).

Figure 7. Impact of primary graft failure (PrGF, A), and severe poor graft function (sPGF, B) on overall survival post-HSCT in CML patients
Discussion
In context of TKI therapy progress, the indications for allo-HSCT in CML are becoming more stringent, with respect both to selective TKI choice, relapse diagnostics, and improved transplant technologies [22]. In this regard, it becomes relevant to investigate the causes of allo-HSCT failure and to determine the risk factors for PrGF and sPGF in CML patients. While the factor of post-transplant relapse is discussed in most publications, the issues of PrGF and sPGF remain poorly reflected. Only few authors provided clear definitions and data on the incidence of these complications (mostly PrGF) in the patients with CML. At the same time, most studies of posttransplant graft failure syndromes show that the diagnosis of CML may be among risk factors of this complication. However, most previous studies concerned a heterogeneous range of diagnoses, e.g., acute leukemia, chronic myelo- and lymphoproliferative and non-malignant diseases. To our knowledge, the present work evaluates for the first time the incidence of both PrGF and PGF in a homogeneous cohort of CML patients.
According to our results, PrGF occurred in 7% of cases, thus being higher than in patients with acute leukemia as confirmed by other publications [10]. On the contrary, cumulative incidence of sPGF during the first year after allo-HSCT was 10%. This level is less than in general population of patients after allo-HSCT [15]. A prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT also showed increased rate of graft dysfunction in CML patients after allo-HSCT. Impact of pre-transplant treatment with tyrosine kinase inhibitors of second generation on the allograft function due to myelotoxicity is still under discussion [23]. Presumably, the similar factors may contribute to the development of both PrGF and sPoGF.
We analyzed the data associated with characteristics of patients, donors, and the HSCT procedure. Due to small number of cases in the target groups, only univariate analysis was performed. In contrast to many studies, conditioning regimen, the source and cellularity of the graft, CMV status of the donor and the patient, ABO incompatibility did not show any statistical significance of the disease status, although it is proved an important characteristic for the prognosis of primary graft failure and poor graft function [10, 11, 24, 25, 26, 27, 28, 29, 30].
Nevertheless, it was the presence of ACA that showed statistical significance for PrGF. This may suggest insufficient control of the underlying disease to be among the main causes of any type of graft dysfunction. However, no association between post-transplant relapse and sPGF was noted in our study. The disease recurrence after resolution of graft failure remains an important cause of treatment failure. Contribution of the underlying disease to development of PrGF and sPGF needs to be investigated in future.
HLA incompatibility was another factor for PrGF in univariate analysis. The importance of this characteristic for HSC engraftment is well known [13, 15, 30]. Haploidentical HSCTs in this analysis showed larger proportion of PrGF and sPGF, but this result needs further proofs, as our group was small and mostly retrospective.
The question still exists if the intensity of conditioning regimen may contribute to insufficient hematopoietic reconstitution after allo-HSCT. Impairment of bone marrow microenvironment exposed to high doses of alkylating agents may be one of the possible pathogenic pathways [13, 31]. Nevertheless, our study did not show significant influence of conditioning intensity upon the clinical outcomes.
Natural history of the patients who developed graft failure and poor graft function was of particular interest in this retrospective study. Despite various interventions, primary graft failure is still associated with poor outcomes and death, mostly, due to infectious complications.
Survival and NRM analysis showed that, despite the rare occurrence, PrGF and sPGF are life-threatening and resource-consuming problems. Both PrGF and sPGF need aggressive approach in order to improve outcomes of allo- HSCT. Intensive interventions might be a rescue for, at least, a part of the patients and lead to prolonged survival. Stimulation of residual HSCs by TPO agonists using in the setting of persistent cytopenia after HSCT by several groups might be a promising strategy, although influence of TPO agonists on the leukemic stem cells and risk of relapse is debated. Early employment of a CD34+-selected stem cell boost, or a second allogeneic HSCT to restore an effective haematopoiesis might also be a life-saving option. Identification of patients at high risk for these complications and development strategies for early intervention might be in scope of further investigation.
Conclusion
Both PrGF and sPGF are significant life-threatening problems in allo-HSCT. Specifically, PrGF but not severe poor graft failure (sPGF) significantly increased non-relapse mortality during the first year after allo-HSCT. Meanwhile, the incidence of post-transplant relapses did not differ in the CML patients exhibiting primary graft failure or severe poor graft function. Identification of risk factors for these complications can improve the results of this treatment, by planning HSCT technology, to minimize them and modify approaches to post-transplant therapy.
Conflict of interest
None declared.
References
- Afanasyev BV, Zubarovskaya LS. Role of hematopoietic stem cell transplantation in therapy of adult patients with acute leukemias. Oncohematology. 2006;1(1-2): 70-85. (In Russian).
- Lübking A, Dreimane A, Sandin F et al. Allogeneic stem cell transplantation for chronic myeloid leukemia in the TKI era: population-based data from the Swedish CML registry. Bone Marrow Transplant. 2019;54, 1764-1774. doi: 10.1038/s41409-019-0513-5
- Barrett AJ, Ito S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood. 2015;125(21):3230-3235. doi: 10.1182/blood-2014-10-567784
- Lyubimova LS, Kuzmina LA, E.S.Urnova ES, Zhelnova ES, Anukhina MV, Mendeleyeva LP, et al. Early HLA-identical bone marrow transplantation during chronic myeloid leukemia chronic phase vs. long-term tyrosine kinase inhibitor therapy? Gematologyia i Transfusiologiya. 2012; 57 (3): 6-10. (In Russian).
- Morozova EV, Vlasova YI, Barabanshikova MV, Afanaseva KS, Iurovskaia KS, Gindina TL, Barchatov IM, Alyanskiy AL, Bakin EA, Bondarenko SN, Moiseev IS, Zubarovskaya LS, Afanasyev BV. Allogeneic haematopoietic stem cell transplantation with reduced-intensity conditioning in chronic myeloid leukaemia. Russian Journal of Hematology and Transfusiology. 2020; 65(4): 386-402. (In Russian.). doi: 10.35754/0234-5730-2020-65-4-386-402
- Boehm A, Walcherberger B, Sperr WR, Woehrer S, Dieckmann K, Rosenmayr A, et al. Improved outcome in patients with chronic myelogenous leukemia after allogeneic hematopoietic stem cell transplantation over the past 25 years: A single-center experience. Biol Blood Marrow Transplant. 2011; 17: 133-140. doi: 10.1016/j.bbmt.2010.06.019
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008; 112 (12): 4371-4383.
doi: 10.1182/blood-2008-03-077974 - Klyuchnikov E, Kröger N, Brummendorf TH et al. Current status and perspectives of tyrosine kinase inhibitor treatment in the posttransplant period in patients with chronic myelogenous leukemia (CML). Biol Blood Marrow Transplant. 2010; 16 (3): 301-310.
doi: 10.1016/j.bbmt.2009.08.019 - Bonifacio M, Stagno F, Scaffidi L, Krampera M, Raimondo FD. Management of chronic myeloid leukemia in advanced phase. Front Oncol. 2019; Oct 25; 9:1132. doi: 10.3389/fonc.2019.01132
- Olsson RF, Logan BR, Chaudhury S, Zhu X, Akpek G, Bolwell BJ, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015; 29(8), 1754-1762. doi: 10.1038/leu.2015.75
- Olsson RF, Berggren DM, Ringden O, Mattsson J, Remberger M. Graft failure in reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Blood. 2013; 122 (21): 4559. doi: 10.1182/blood.V122.21.4559.4559
- Locatelli F, Lucarelli B, Merli P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opinion Pharmacother. 2014;15(1): 23-36. doi: 10.1517/14656566.2014.852537
- Xiao Y, Song J, Jiang Z, Li Y, Gao Y, Xu W, Lu Z, Wang Y, Xiao H. Risk-factor analysis of poor graft function after allogeneic hematopoietic stem cell transplantation. Int J Med Sci. 2014; 11(6):652-657. doi: 10.7150/ijms.6337
- Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, Trastulli F, Vitiello S, Cardano F, Pane F, Risitano AM. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019; 54(8):1346-1353. doi: 10.1038/s41409-019-0442-3
- Rudakova TA, Kulagin AD, Klimova OU, Golubovskaya IK, Darskaya EI, Bykova TA, Smirnova AG, Morozova EV, Bondarenko SN, Moiseev IS, et al. Severe poor graft function after allogeneic hematopoietic stem cell transplantation in adult patients: Incidence, risk factors, and outcomes. Clinical Oncohematology. 2019;12(3):309-318 (In Russ). doi: 10.21320/2500-2139-2019-12-3-309-318
- Turkina AG, Zaritsky AYu, Chelysheva EYu. et al. Clinical recommendations for the diagnosis and treatment of chronic myelogenous leukemia. Clinical Oncohematology. 2017;10(3):294-316. (In Russian). doi: 10.21320/2500-2139-2017-10-3-294-316
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
doi: 10.1182/blood-2013-05-501569 - McGowan-Jordan J, Simons A, Schmid M. International Standing Committee on Human Cytogenomic Nomenclature, 2016.
doi: 10.1159/isbn.978-3-318-06861-0 - Gabert J, Beillard E, van der Velden V., et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia- A Europe Against Cancer Program. Leukemia. 2003.17: 2318-2357. doi: 10.1038/sj.leu.2403135
- Valcárcel D, Sureda A. Graft Failure. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. 7th ed. Cham (CH): Springer; 2019. Chapter 41. PMID: 32091792. https://www.ncbi.nlm.nih.gov/books/NBK553942/. doi: 10.1007/978-3-030-02278-5
- Gratwohl A. The EBMT risk score. Bone Marrow Transplantation. 2012;47:749-56. doi: 10.1038/bmt.2011.110
- Craddock CF. We do still transplant CML, don’t we? Hematology Am Soc Hematol Educ Program. 2018 (1): 177-184.
https://doi.org/10.1182/asheducation-2018.1.177 - Masouridi-Levrat S, Olavarria E, Iacobelli S, Aljurf M, Morozova E, Niittyvuopio R, Sengeloev H, Reményi P, Helbig G, Browne P, et al. Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: a prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2022; 57(1):23-30. doi: 10.1038/s41409-021-01472-x
- Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, Ringden O. Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013 Apr; 48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. doi: 10.1038/bmt.2012.239
- Moiseev IS, Morozova EV, Vlasova YY, et al. Interim results of the randomized trial of post-transplanation cyclophosphamide versus rabbit ATG for graft-versus-host disease prophylaxis in chronic myeloproliferative neoplasms and myelodysplastic syndrome. Cell Ther Transplant. 2016; 5(3):62-63. doi: 10.18620/ctt-1866-8836-2016-5-3-49-50
- Hallemeier C. Letter to the editor. Re: High rate of graft failure in 25 patients with chronic myelogenous leukemia conditioned with a reduced-intensity regimen of 550 cGy total body irradiation and cyclophosphamide for unrelated donor transplantation. Biol Blood Marrow Transplant. 2004; 10:726-727. doi: 10.1016/j.bbmt.2004.07.001
- Khoury H, Adkins, Brown RH, Pence, Vij R, Goodnough LT, et al. Low incidence of transplantation-related acute complications in patients with chronic myeloid leukemia undergoing aiiogeneic stem cell transplantation with a low-dose (550 cGy) total body irradiation conditioning regimen. Biol Blood Marrow Transplant. 2001; 7(6):352-358. doi: 10.1016/S1083-8791(01)80006-9
- Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012; 47, 810-816. doi: 10.1038/bmt.2011.194
- Radujkovic A, Dietrich S, Blok HJ, Nagler A, Ayuk F, Finke J, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: A retrospective study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2019; 25(10): 2008-2016. doi: 10.1016/j.bbmt.2019.06.028
- Kuzmich YV, Alyanskiy AI, Ivanova NY, Vitrischak AA, Vladovskaya MD, Morozova YV, Bondarenko SN, Semenova YV, Zubarovskaya LS, Afanasyev BV. Analysis of the results of allogeneic hematopoietic stem cell transplantation depending on HLA matching of the unrelated donor/recipient pair. Oncohematology. 2014; 9(3):25-31. (In Russian). doi: 10.17650/1818-8346-2014-9-3-25-31
- Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014; 20(9): 1440-1443. doi: 10.1016/j.bbmt.2014.05.016
" ["~DETAIL_TEXT"]=> string(35387) "
Introduction
Over the past decades, allogeneic stem cell transplantation (allo-HSCT) has been used worldwide as a technology aimed both the long-term management and cure of malignant hematological diseases [1]. Allo-HSCT remains a valuable option for treatment of chronic myeloid leukemia (CML) in era of tyrosine kinase inhibitors (TKI) [2-6]. The transplant-eligible population consists of the CML patients with predicted poor outcome if treated with TKIs alone. Despite the superiority of drug treatment, the development of transplant technology, i.e., usage of reduced intensity conditioning regimens, increased donor availability, led to improvement in the results of allo-HSCT in these patients [7,8]. Thus, transplantation is still a potentially curative therapeutic mode in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with 2 TKIs, and patients with advanced-phase CML.
Unfavorable results are mostly associated with impaired graft function, which is manifested in the lack of control over the underlying disease and subsequent relapse, as well as in primary graft failure (PrGF) and poor graft function (PoGF) [9-12]. Several risk factors of post-transplant graft failure were revealed, e.g., patient’s age, donor-recipient blood mismatch and CMV infection (13]. Treatment options for poor graft functioning are still limited. In addition to reinfusion of stem cell, some recent studies report, e.g., positive effects of Eltrombopag, a thrombopoietin mimetic [14].
The aim of the study was to assess the incidence and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Materials and methods
Patients and data collection
We carried out retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in R. M. Gorbacheva Research Institute at the Pavlov University between 1995 and 2020. Information on the disease stage at diagnosis, time to allo-HSCT, transplantation procedure, relapse, and treatment following allo-HSCT was gathered via systematic reviews of the patient records. General approaches to evaluation of HSCT patients at our clinic were described elsewhere [15].
Definitions
CML was diagnosed on the basis of clinical and laboratory data, the detection of Philadelphia (Ph) chromosome and/or the chimeric BCR-ABL gene. Disease phase was defined according to the WHO classification [16]. The first chronic phase (CP1) was recognized in the absence of accelerated phase (AP) and/or blast crisis (BC) in the patient’s history, otherwise CP≥2 was registered. Hematological, cytogenetic and molecular response to the treatment prior to allo-HSCT was defined using ELN criteria [17].
Indications for HSCT
Indications for HSCT were as follows: 1) AP/BC at diagnosis or progression to AP/BC; 2) treatment failure in pre-TKI era; 3) treatment failure due to TKI resistance/intolerance; 4) T315I mutation. TKI resistance and TKI intolerance were defined according to ELN criteria [17].
Laboratory studies
For cytogenetic evaluation, conventional synchronized culture was performed for 48 hours with at least 20 metaphases analyzed per a sample (GTG method). Leukemia cell karyotype was evaluated according to International System for Human Cytogenetic Nomenclature (ISCN) [18]. In cases when the standard cytogenetic investigation was not available (i.e., insufficient material), the bone marrow was assessed with fluorescence in situ hybridization (FISH) probes aimed for detection of (9;22) variants (LSI BCR-ABL, Dual Color, Dual Fusion, "Vysis").
Additional chromosomal abnormalities (ACA) were defined as any structural and numerical chromosomal aberrations other than t(9;22)(q34;q11) (detected by cytogenetic or molecular assays for cryptic abnormalities).
Molecular response after allo-HSCT was evaluated according to the National Comprehensive Cancer Network (NCCN) criteria (2021). PCR monitoring of BCR/ABL was carried out according to NCCN Guidelines once in 3 months for 2 years, then once in every 3 to 6 months. The relative BCR-ABL1 expression level was evaluated according to method described by Gabert et al [19]. This technique includes the following stages: 1) total RNA extraction from peripheral blood of patients with CML, 2) reverse transcription with random hexameric primers, 3) real-time PCR with primers and probes specific to р210, р190 control ABL gene sequences.
Assessment of relative expression levels was based on evaluation of BCR-ABL1/ABL1 ratios in the studied cDNA samples. The ABL1 gene was used for normalization of the results. In order to determine copy numbers of the BCR-ABL1 and ABL1 transcripts, and to assess the reaction effectiveness, standard dilution curves were plotted using a plasmid with inserts of known target gene sequences (Invitrogen, USA), at a standard concentration ranges of 102-106 copies/mcl, according to 2020 European LeukemiaNet (ELN) Recommendations [17]. ABL kinase domain mutations were determined by Sanger direct sequencing.
Post-transplant monitoring
Post-transplant engraftment was defined as absolute neutrophil count (ANC) of >0.5×109/L without administration of colony-stimulating factor within 3 days. Primary graft failure (PrGF) was diagnosed in absence of donor cells in recipient’s bone marrow by the day +30. Donor chimerism was checked at the time of myelopoiesis recovery, i.e. ANC> 0.5×109/L, and by the days +30, 60, +100, +200, and in case of any cytopenia, or signs of relapse. Post-transplant relapse was diagnosed in cases of clinical progression to AP/BC, cytogenetic relapse, or molecular relapse defined as two consecutive positive PCR tests, or, at least, 1-log persistent increase of BCR/ABL transcript level.
The criteria for severe poor graft function (sPGF) were as follows: cytopenia in two or more hematopoietic lineages (platelets <20×109/l, ANC <0.5×109/l, hemoglobin <70 g/l) any time after documented engraftment in presence of full or stable mixed donor chimerism >90% without signs of relapse of underlying disease, rejection or acute graft-versus-host disease (GVHD) grade III-IV.
Secondary graft failure was defined as loss of donor hematopoiesis to <5% and/or ANC counts to <0.5×109/L after initial engraftment being not related to relapse, infection, or drug toxicity [20].
Statistical evaluation
Descriptive characteristics of the cohort included number of cases, proportions for discrete factors, medians and range for continuous values. Individual pre-transplant risk for the HSCT patients was evaluated according to Gratwohl [21]. Overall survival (OS) was assessed using the Kaplan-Meier method from the time of allo-HSCT to the date of last contact or the date of death. Death from any cause was considered as an event.
Survival analysis was performed using log-rank test. Relapse and non-relapse mortality (NRM) rates were summarized using cumulative incidence estimates, with NRM as competing risk for relapse, and relapse regarded as competing risk for NRM.
The event-free survival (EFS) was estimated as a period from allo-HSCT until last contact date, death, or any of the following events: any kind of post-transplant relapse, graft-versus-host disease (GVHD) grade III-IV, severe poor graft function, or secondary graft failure. PrGF and sPGF were estimated as a proportion of cases in the total cohort. Cumulative incidence of sPGF was calculated with respect to competing risks (death before day +30, any type of relapse, GVHD grade III-IV).
The differences between groups were assessed using Fisher's exact test, Pearson χ2 test, and Mann-Whitney U-test for categorical and quantitative characteristics respectively, and Gray’s test for cumulative incidences. All the tests were two-sided, and P-values <0.05 were assessed as indicating for significant associations. Statistical analysis was performed using SPSS, IBM Statistics and EZR free statistical environment, version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
General characteristics of the patients and HSCT procedure
A total of 121 patients diagnosed with CML had undergone allo-HSCT. The median patients’ age was 37 years (range: 18-66). Other baseline characteristics for these patients are presented in Table 1.
The median time between CML diagnosis and allo-HSCT was 31 months (4.5-260). A total of 80 (66%) patients were transplanted in chronic phase (CP), the remaining 41 (34%) patients were in the active phase (AP or BC) at the time of HSCT. The median follow-up from allo-HSCT to the time of the last contact was 15 months (0.5-294).
HLA-matched or partially mismatched unrelated donors were used in 78 cases (65%), while matched related donors were employed in 34 cases (28%), and 9 (7%) patients received haploidentical allo-HSCT. The proportion of bone marrow (BM) and peripheral blood stem cells (PBSC) as the graft sources was almost equal: 49% (n=59) versus 51% (n=62).
Conditioning regimen included oral busulfan 8-12 mg/kg or melphalan 140 mg/m2 in combination with fludarabine 180 mg/m2 or cyclophosphamide. GVHD prophylaxis included calcineurin inhibitor (tacrolimus or cyclosporine A) and mycophenolate mofetil (30 mg/kg), or short course of posttransplant metotrexate, with or without antithymocyte globulin (horse, 60 mg/kg, or rabbit preparations, 5 mg/kg on day -3, -2, or high-dose), post-transplant cyclophosphamide (50 mg/kg, day +3 and +4 after allo-HSCT)/Alemtuzumab was used in two cases (Table 2).


Figure 1. Cumulative incidence of poor graft function post-HSCT in CML patients
Survival and relapse rates
Engraftment was documented in 106 (88%) patients, with median time to neutrophil recovery of 22 (8-58) days. Early death with no signs of engraftment before day +30 occurred in two cases. Thirty-one patients developed post-transplant relapse of any type with median time after allo-HSCT of 106 days (range: 15-333), included early CML relapse/progression before day +30 in 5 cases.
PrGF was documented in 8 (7%) cases. Two patients developed secondary graft failure, both in about two months after initial engraftment with lethal outcome due to severe bacterial infection. Severe poor graft function was diagnosed in 11 (9% of engrafted patients) with cumulative incidence of 10% (95% CI, 5-19) within 1 year after allo-HSCT (Fig. 1). Median time from HSCT to sPGF diagnosis was 43 (18-114) days.
The 1-year cumulative incidence rates of relapse and NRM comprised 35% (95% CI, 26-46) and 26% (95% CI, 18-35), respectively (Fig. 2).
A total of 57 (47%) patients died, the 1-year OS was 60% (95% CI, 51-69). Median OS was not reached. One-year EFS was 41% (95% CI, 32-50), median EFS was 271 (95% CI, 96-365) days (Fig. 3).

Figure 2. One-year cumulative incidence of relapses and NRM after allo-HSCT in CML patients

Figure 3. One-year overall and event-free survival after allogeneic HSCT in CML patients
Factors associated with primary graft failure and severe poor graft function
Among various pre-transplant characteristics, the presence of additional chromosomal abnormalities (ACA) was associated with cumulative incidence of PrGF and sPGF after HSCT. Thus, PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA, p=0.09. HLA-matched allo-HSCTs were beneficial for engraftment: 96% for HLA-matched transplantations vs 89% for allo-HSCT from HLA-mismatched donors, and 78% for haploidentical donors (p=0.05). Other pre-transplant factors didn’t show any statistical correlation with graft failure syndromes after HSCT (Table 3).
Table 3. Potential risk factors for the graft failure after allo-HSCT in CML patients

Clinical features and outcomes of graft failure and severe poor graft function
The median follow-up time after allo-HSCT was 68 days (range: 43-1792). All the patients with primary graft failure (PrGF) (n=8) were administered G-CSF, antimicrobial therapy and transfusion support. Two patients received donor lymphocyte infusions without any effect. The second allo-HSCT was performed in 4 cases. A total of 7 patients had lethal outcome (6, of infectious complications; 1, of relapse), whereas one patient is alive after the 2nd allo-HSCT (Fig. 4).

Figure 4. Treatment and outcomes of primary graft failure (PrGF) in CML patients after HSCT
Eleven patients exhibited severe poor graft function (sPGF) within median time of 21 (0-92) days after engraftment. Median length of sPGF was 52 days (range: 14-215). The median time of follow-up after allo-HSCT was 977 days (range: 45-2712).
Early sPGF with criterial cytopenia persisting after engraftment was diagnosed in 4 cases (36%), the remaining patients developed cytopenia after a period of normal graft function. A total of 3 cases of sPGF (27%) developed within 30 days after acute GVHD 2-3 grade (Fig. 5).

Figure 5. Timeline of severe poor graft function (SPGF) in a group of CML patients
All the patients with sPGF received antimicrobial therapy, transfusion support, and G-CSF in case of neutropenia. Other therapeutic options for sPGF therapy were: rituximab (n=4), the second allo-HSCT, or boost stem cell infusion (n=3); eltrombopag (n=1); supportive care (n=5), as seen from Fig. 4. Normal graft function was restored in 8 patients.
A total of 4 patients died. The causes of death were infectious complications (n=3) and late post-transplant relapse (n=1) (Fig. 6).

Figure 6. Treatment and outcomes of severe poor graft function (sPGF) in CML patients post-HSCT
Two cases of secondary graft failure occurred in about 3 months after allo-HSCT. Both patients died due to severe infection.
The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF as compared with those, who were free of these complications. Cumulative incidence of leukemia relapses was 31% (95% CI, 23-42), and 25% (95% CI, 4-87) in the patients with PrGF and engraftment (p=0.97), compared with 22% (95% CI, 3-54) and 32% (95% CI, 24-43) in the patients with sPGF and without sPGF (p=0.52), respectively.
Primary graft failure (PrGF) but not severe poor graft failure (sPGF) significantly increased non-relapse mortality during the first year after allo-HSCT. One-year NRM was 23% (95% CI, 15-32) in engrafted patients versus 71% (95% CI, 39-96) in the patients with PrGF (p<0.0001). Patients with and without sPGF had similar NRM: 20% (95% CI, 5-59) versus 26% (95% CI, 18-36) (p=0.74).
One-year OS was significantly lower in patients with PrGF: 13% (95% CI, 0.7-42) versus 64% (95% CI, 54-72) (p<0.0001) (Fig. 7). On the contrary, sPGF had no statistically significant influence on OS: 73% (95% CI, 37-90) versus 59% (95% CI, 49-69) (p=0.47).

Figure 7. Impact of primary graft failure (PrGF, A), and severe poor graft function (sPGF, B) on overall survival post-HSCT in CML patients
Discussion
In context of TKI therapy progress, the indications for allo-HSCT in CML are becoming more stringent, with respect both to selective TKI choice, relapse diagnostics, and improved transplant technologies [22]. In this regard, it becomes relevant to investigate the causes of allo-HSCT failure and to determine the risk factors for PrGF and sPGF in CML patients. While the factor of post-transplant relapse is discussed in most publications, the issues of PrGF and sPGF remain poorly reflected. Only few authors provided clear definitions and data on the incidence of these complications (mostly PrGF) in the patients with CML. At the same time, most studies of posttransplant graft failure syndromes show that the diagnosis of CML may be among risk factors of this complication. However, most previous studies concerned a heterogeneous range of diagnoses, e.g., acute leukemia, chronic myelo- and lymphoproliferative and non-malignant diseases. To our knowledge, the present work evaluates for the first time the incidence of both PrGF and PGF in a homogeneous cohort of CML patients.
According to our results, PrGF occurred in 7% of cases, thus being higher than in patients with acute leukemia as confirmed by other publications [10]. On the contrary, cumulative incidence of sPGF during the first year after allo-HSCT was 10%. This level is less than in general population of patients after allo-HSCT [15]. A prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT also showed increased rate of graft dysfunction in CML patients after allo-HSCT. Impact of pre-transplant treatment with tyrosine kinase inhibitors of second generation on the allograft function due to myelotoxicity is still under discussion [23]. Presumably, the similar factors may contribute to the development of both PrGF and sPoGF.
We analyzed the data associated with characteristics of patients, donors, and the HSCT procedure. Due to small number of cases in the target groups, only univariate analysis was performed. In contrast to many studies, conditioning regimen, the source and cellularity of the graft, CMV status of the donor and the patient, ABO incompatibility did not show any statistical significance of the disease status, although it is proved an important characteristic for the prognosis of primary graft failure and poor graft function [10, 11, 24, 25, 26, 27, 28, 29, 30].
Nevertheless, it was the presence of ACA that showed statistical significance for PrGF. This may suggest insufficient control of the underlying disease to be among the main causes of any type of graft dysfunction. However, no association between post-transplant relapse and sPGF was noted in our study. The disease recurrence after resolution of graft failure remains an important cause of treatment failure. Contribution of the underlying disease to development of PrGF and sPGF needs to be investigated in future.
HLA incompatibility was another factor for PrGF in univariate analysis. The importance of this characteristic for HSC engraftment is well known [13, 15, 30]. Haploidentical HSCTs in this analysis showed larger proportion of PrGF and sPGF, but this result needs further proofs, as our group was small and mostly retrospective.
The question still exists if the intensity of conditioning regimen may contribute to insufficient hematopoietic reconstitution after allo-HSCT. Impairment of bone marrow microenvironment exposed to high doses of alkylating agents may be one of the possible pathogenic pathways [13, 31]. Nevertheless, our study did not show significant influence of conditioning intensity upon the clinical outcomes.
Natural history of the patients who developed graft failure and poor graft function was of particular interest in this retrospective study. Despite various interventions, primary graft failure is still associated with poor outcomes and death, mostly, due to infectious complications.
Survival and NRM analysis showed that, despite the rare occurrence, PrGF and sPGF are life-threatening and resource-consuming problems. Both PrGF and sPGF need aggressive approach in order to improve outcomes of allo- HSCT. Intensive interventions might be a rescue for, at least, a part of the patients and lead to prolonged survival. Stimulation of residual HSCs by TPO agonists using in the setting of persistent cytopenia after HSCT by several groups might be a promising strategy, although influence of TPO agonists on the leukemic stem cells and risk of relapse is debated. Early employment of a CD34+-selected stem cell boost, or a second allogeneic HSCT to restore an effective haematopoiesis might also be a life-saving option. Identification of patients at high risk for these complications and development strategies for early intervention might be in scope of further investigation.
Conclusion
Both PrGF and sPGF are significant life-threatening problems in allo-HSCT. Specifically, PrGF but not severe poor graft failure (sPGF) significantly increased non-relapse mortality during the first year after allo-HSCT. Meanwhile, the incidence of post-transplant relapses did not differ in the CML patients exhibiting primary graft failure or severe poor graft function. Identification of risk factors for these complications can improve the results of this treatment, by planning HSCT technology, to minimize them and modify approaches to post-transplant therapy.
Conflict of interest
None declared.
References
- Afanasyev BV, Zubarovskaya LS. Role of hematopoietic stem cell transplantation in therapy of adult patients with acute leukemias. Oncohematology. 2006;1(1-2): 70-85. (In Russian).
- Lübking A, Dreimane A, Sandin F et al. Allogeneic stem cell transplantation for chronic myeloid leukemia in the TKI era: population-based data from the Swedish CML registry. Bone Marrow Transplant. 2019;54, 1764-1774. doi: 10.1038/s41409-019-0513-5
- Barrett AJ, Ito S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood. 2015;125(21):3230-3235. doi: 10.1182/blood-2014-10-567784
- Lyubimova LS, Kuzmina LA, E.S.Urnova ES, Zhelnova ES, Anukhina MV, Mendeleyeva LP, et al. Early HLA-identical bone marrow transplantation during chronic myeloid leukemia chronic phase vs. long-term tyrosine kinase inhibitor therapy? Gematologyia i Transfusiologiya. 2012; 57 (3): 6-10. (In Russian).
- Morozova EV, Vlasova YI, Barabanshikova MV, Afanaseva KS, Iurovskaia KS, Gindina TL, Barchatov IM, Alyanskiy AL, Bakin EA, Bondarenko SN, Moiseev IS, Zubarovskaya LS, Afanasyev BV. Allogeneic haematopoietic stem cell transplantation with reduced-intensity conditioning in chronic myeloid leukaemia. Russian Journal of Hematology and Transfusiology. 2020; 65(4): 386-402. (In Russian.). doi: 10.35754/0234-5730-2020-65-4-386-402
- Boehm A, Walcherberger B, Sperr WR, Woehrer S, Dieckmann K, Rosenmayr A, et al. Improved outcome in patients with chronic myelogenous leukemia after allogeneic hematopoietic stem cell transplantation over the past 25 years: A single-center experience. Biol Blood Marrow Transplant. 2011; 17: 133-140. doi: 10.1016/j.bbmt.2010.06.019
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008; 112 (12): 4371-4383.
doi: 10.1182/blood-2008-03-077974 - Klyuchnikov E, Kröger N, Brummendorf TH et al. Current status and perspectives of tyrosine kinase inhibitor treatment in the posttransplant period in patients with chronic myelogenous leukemia (CML). Biol Blood Marrow Transplant. 2010; 16 (3): 301-310.
doi: 10.1016/j.bbmt.2009.08.019 - Bonifacio M, Stagno F, Scaffidi L, Krampera M, Raimondo FD. Management of chronic myeloid leukemia in advanced phase. Front Oncol. 2019; Oct 25; 9:1132. doi: 10.3389/fonc.2019.01132
- Olsson RF, Logan BR, Chaudhury S, Zhu X, Akpek G, Bolwell BJ, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015; 29(8), 1754-1762. doi: 10.1038/leu.2015.75
- Olsson RF, Berggren DM, Ringden O, Mattsson J, Remberger M. Graft failure in reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Blood. 2013; 122 (21): 4559. doi: 10.1182/blood.V122.21.4559.4559
- Locatelli F, Lucarelli B, Merli P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opinion Pharmacother. 2014;15(1): 23-36. doi: 10.1517/14656566.2014.852537
- Xiao Y, Song J, Jiang Z, Li Y, Gao Y, Xu W, Lu Z, Wang Y, Xiao H. Risk-factor analysis of poor graft function after allogeneic hematopoietic stem cell transplantation. Int J Med Sci. 2014; 11(6):652-657. doi: 10.7150/ijms.6337
- Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, Trastulli F, Vitiello S, Cardano F, Pane F, Risitano AM. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019; 54(8):1346-1353. doi: 10.1038/s41409-019-0442-3
- Rudakova TA, Kulagin AD, Klimova OU, Golubovskaya IK, Darskaya EI, Bykova TA, Smirnova AG, Morozova EV, Bondarenko SN, Moiseev IS, et al. Severe poor graft function after allogeneic hematopoietic stem cell transplantation in adult patients: Incidence, risk factors, and outcomes. Clinical Oncohematology. 2019;12(3):309-318 (In Russ). doi: 10.21320/2500-2139-2019-12-3-309-318
- Turkina AG, Zaritsky AYu, Chelysheva EYu. et al. Clinical recommendations for the diagnosis and treatment of chronic myelogenous leukemia. Clinical Oncohematology. 2017;10(3):294-316. (In Russian). doi: 10.21320/2500-2139-2017-10-3-294-316
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
doi: 10.1182/blood-2013-05-501569 - McGowan-Jordan J, Simons A, Schmid M. International Standing Committee on Human Cytogenomic Nomenclature, 2016.
doi: 10.1159/isbn.978-3-318-06861-0 - Gabert J, Beillard E, van der Velden V., et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia- A Europe Against Cancer Program. Leukemia. 2003.17: 2318-2357. doi: 10.1038/sj.leu.2403135
- Valcárcel D, Sureda A. Graft Failure. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. 7th ed. Cham (CH): Springer; 2019. Chapter 41. PMID: 32091792. https://www.ncbi.nlm.nih.gov/books/NBK553942/. doi: 10.1007/978-3-030-02278-5
- Gratwohl A. The EBMT risk score. Bone Marrow Transplantation. 2012;47:749-56. doi: 10.1038/bmt.2011.110
- Craddock CF. We do still transplant CML, don’t we? Hematology Am Soc Hematol Educ Program. 2018 (1): 177-184.
https://doi.org/10.1182/asheducation-2018.1.177 - Masouridi-Levrat S, Olavarria E, Iacobelli S, Aljurf M, Morozova E, Niittyvuopio R, Sengeloev H, Reményi P, Helbig G, Browne P, et al. Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: a prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2022; 57(1):23-30. doi: 10.1038/s41409-021-01472-x
- Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, Ringden O. Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013 Apr; 48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. doi: 10.1038/bmt.2012.239
- Moiseev IS, Morozova EV, Vlasova YY, et al. Interim results of the randomized trial of post-transplanation cyclophosphamide versus rabbit ATG for graft-versus-host disease prophylaxis in chronic myeloproliferative neoplasms and myelodysplastic syndrome. Cell Ther Transplant. 2016; 5(3):62-63. doi: 10.18620/ctt-1866-8836-2016-5-3-49-50
- Hallemeier C. Letter to the editor. Re: High rate of graft failure in 25 patients with chronic myelogenous leukemia conditioned with a reduced-intensity regimen of 550 cGy total body irradiation and cyclophosphamide for unrelated donor transplantation. Biol Blood Marrow Transplant. 2004; 10:726-727. doi: 10.1016/j.bbmt.2004.07.001
- Khoury H, Adkins, Brown RH, Pence, Vij R, Goodnough LT, et al. Low incidence of transplantation-related acute complications in patients with chronic myeloid leukemia undergoing aiiogeneic stem cell transplantation with a low-dose (550 cGy) total body irradiation conditioning regimen. Biol Blood Marrow Transplant. 2001; 7(6):352-358. doi: 10.1016/S1083-8791(01)80006-9
- Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012; 47, 810-816. doi: 10.1038/bmt.2011.194
- Radujkovic A, Dietrich S, Blok HJ, Nagler A, Ayuk F, Finke J, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: A retrospective study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2019; 25(10): 2008-2016. doi: 10.1016/j.bbmt.2019.06.028
- Kuzmich YV, Alyanskiy AI, Ivanova NY, Vitrischak AA, Vladovskaya MD, Morozova YV, Bondarenko SN, Semenova YV, Zubarovskaya LS, Afanasyev BV. Analysis of the results of allogeneic hematopoietic stem cell transplantation depending on HLA matching of the unrelated donor/recipient pair. Oncohematology. 2014; 9(3):25-31. (In Russian). doi: 10.17650/1818-8346-2014-9-3-25-31
- Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014; 20(9): 1440-1443. doi: 10.1016/j.bbmt.2014.05.016
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(100) "pre-i-posttransplantatsionnye-faktory-assotsiirovannye-s-pervichnoy-nedostatochnostyu-i-tyazheloy-di" ["~CODE"]=> string(100) "pre-i-posttransplantatsionnye-faktory-assotsiirovannye-s-pervichnoy-nedostatochnostyu-i-tyazheloy-di" ["EXTERNAL_ID"]=> string(4) "2051" ["~EXTERNAL_ID"]=> string(4) "2051" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(607) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозеPre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(6014) "<p style="text-align: justify;">Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев. </p> <h3>Результаты</h3> <p style="text-align: justify;">Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.</p> <h3>Выводы</h3> <p style="text-align: justify;">Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_META_TITLE"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_META_KEYWORDS"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_META_DESCRIPTION"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_PICTURE_FILE_ALT"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_PICTURE_FILE_TITLE"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "pre-i-posttransplantatsionnye-faktory-assotsiirovannye-s-pervichnoy-nedostatochnostyu-i-tyazheloy-di" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(415) "Пре- и посттрансплантационные факторы, ассоциированные с первичной недостаточностью и тяжелой дисфункцией трансплантата после аллогенной трансплантации гемопоэтических стволовых клеток при хроническом миелоидном лейкозе" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "pre-i-posttransplantatsionnye-faktory-assotsiirovannye-s-pervichnoy-nedostatochnostyu-i-tyazheloy-di" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "pre-i-posttransplantatsionnye-faktory-assotsiirovannye-s-pervichnoy-nedostatochnostyu-i-tyazheloy-di" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "pre-i-posttransplantatsionnye-faktory-assotsiirovannye-s-pervichnoy-nedostatochnostyu-i-tyazheloy-di" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "207" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28521" ["VALUE"]=> string(10) "09.03.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "09.03.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28522" ["VALUE"]=> string(10) "18.03.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "18.03.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28523" ["VALUE"]=> array(2) { ["TEXT"]=> string(384) "<p>Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина, Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(372) "
Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина, Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28524" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28525" ["VALUE"]=> array(2) { ["TEXT"]=> string(6014) "<p style="text-align: justify;">Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев. </p> <h3>Результаты</h3> <p style="text-align: justify;">Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.</p> <h3>Выводы</h3> <p style="text-align: justify;">Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(5856) "Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.
Пациенты и методы
Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев.
Результаты
Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.
Выводы
Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.
Ключевые слова
Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28518" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-13-23" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-13-23" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28526" ["VALUE"]=> array(2) { ["TEXT"]=> string(234) "<p>Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina, Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(222) "Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina, Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28527" ["VALUE"]=> array(2) { ["TEXT"]=> string(817) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia<br> Phone: +7 (911) 927-82-29<br> E-mail: dr_morozova@mail.ru</p><br> <p><b>Citation:</b> Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(727) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (911) 927-82-29
E-mail: dr_morozova@mail.ru
Citation: Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28528" ["VALUE"]=> array(2) { ["TEXT"]=> string(3228) "<p style="text-align: justify;"> Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients. </p> <h3>Patients and methods</h3> <p style="text-align: justify;"> We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases. </p> <h3>Results</h3> <p style="text-align: justify;"> Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA <i>versus </i>3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% <i>versus</i> 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF. </p> <h3>Conclusions</h3> <p style="text-align: justify;"> Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3046) "Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Patients and methods
We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases.
Results
Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF.
Conclusions
Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies.
Keywords
Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28519" ["VALUE"]=> string(192) "Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(192) "Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28520" ["VALUE"]=> string(4) "2765" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2765" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28529" ["VALUE"]=> string(4) "2768" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2768" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28526" ["VALUE"]=> array(2) { ["TEXT"]=> string(234) "<p>Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina, Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(222) "Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina, Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(222) "Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina, Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28528" ["VALUE"]=> array(2) { ["TEXT"]=> string(3228) "<p style="text-align: justify;"> Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients. </p> <h3>Patients and methods</h3> <p style="text-align: justify;"> We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases. </p> <h3>Results</h3> <p style="text-align: justify;"> Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA <i>versus </i>3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% <i>versus</i> 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF. </p> <h3>Conclusions</h3> <p style="text-align: justify;"> Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3046) "Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Patients and methods
We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases.
Results
Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF.
Conclusions
Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies.
Keywords
Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3046) "Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Patients and methods
We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases.
Results
Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF.
Conclusions
Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies.
Keywords
Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28518" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-13-23" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-13-23" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2022-11-1-13-23" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28519" ["VALUE"]=> string(192) "Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(192) "Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(192) "Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28527" ["VALUE"]=> array(2) { ["TEXT"]=> string(817) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia<br> Phone: +7 (911) 927-82-29<br> E-mail: dr_morozova@mail.ru</p><br> <p><b>Citation:</b> Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(727) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (911) 927-82-29
E-mail: dr_morozova@mail.ru
Citation: Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(727) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (911) 927-82-29
E-mail: dr_morozova@mail.ru
Citation: Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28523" ["VALUE"]=> array(2) { ["TEXT"]=> string(384) "<p>Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина, Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(372) "Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина, Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(372) "Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина, Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28521" ["VALUE"]=> string(10) "09.03.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "09.03.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "09.03.2022" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28522" ["VALUE"]=> string(10) "18.03.2022" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(10) "18.03.2022" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(10) "18.03.2022" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28525" ["VALUE"]=> array(2) { ["TEXT"]=> string(6014) "<p style="text-align: justify;">Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев. </p> <h3>Результаты</h3> <p style="text-align: justify;">Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.</p> <h3>Выводы</h3> <p style="text-align: justify;">Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(5856) "Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.
Пациенты и методы
Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев.
Результаты
Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.
Выводы
Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.
Ключевые слова
Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(5856) "Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.
Пациенты и методы
Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев.
Результаты
Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.
Выводы
Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.
Ключевые слова
Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28524" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } }Клинические работы
Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси
Олег И. Долгов1, Сергей А. Карпищенко1, Екатерина С. Утимишева1, Диана А. Григорьянц1, Анна А. Спиридонова1,2, Иван С. Моисеев1, Людмила С. Зубаровская1, Алексей Б. Чухловин1, Александр Д. Кулагин1
Тахерех Ростами1, Мохамад Р. Ростами2, Азадех Кьюмарси1, Амир Казаэйян3, Неда Алиджани4, Хосейн К. Фумани2, Соруш Рад2, Давуд Бабахани2, Таназ Бахри2, Мохаммад Ваези2, Мариам Бахордар2, Сейед А. Мирхоссейни2, Сейед А. Моусави2
Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина, Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин
Клинические работы
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28554
[VALUE] => 16.11.2021
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 16.11.2021
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28555
[VALUE] => 15.12.2021
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 15.12.2021
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28558
[VALUE] => Array
(
[TEXT] => <p>Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28559
[VALUE] => Array
(
[TEXT] => <p>Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28560
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).</p>
<h3?Пациенты и методы</h3>
<p style="text-align: justify;">73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6). </p>
<h3>Выводы</h3>
<p style="text-align: justify;">Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).
73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.
Результаты
5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6).
Выводы
Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.
Ключевые слова
Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28556
[VALUE] => 10.18620/ctt-1866-8836-2022-11-1-43-49
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2022-11-1-43-49
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28561
[VALUE] => Array
(
[TEXT] => <p>Mohamed R. Abdelfattah, Hussein Elsiesy</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Mohamed R. Abdelfattah, Hussein Elsiesy
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28562
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Department of Surgery, Faculty of Medicine, University of Alexandria, Alexandria, Egypt</p><br>
<p><b>Correspondence:</b><br>
Prof. Dr. Mohamed Rabei Abdelfattah, Associate Professor, Department of Surgery, Faculty of Medicine, University of Alexandria, Azzaritta, Alexandria, Egypt, PO BOX 21131<br>
Phone: 002010 2306 1111<br>
Email: mohamad.rabie@gmail.com</p><br>
<p><b>Citation:</b> Abdelfattah MR, Elsiesy H. Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors. Cell Ther Transplant 2022; 11(1): 43-49.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Department of Surgery, Faculty of Medicine, University of Alexandria, Alexandria, Egypt
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah, Associate Professor, Department of Surgery, Faculty of Medicine, University of Alexandria, Azzaritta, Alexandria, Egypt, PO BOX 21131
Phone: 002010 2306 1111
Email: mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Elsiesy H. Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors. Cell Ther Transplant 2022; 11(1): 43-49.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28563
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Our objective was to compare overall and tumor-free survival for the hepatocellular carcinoma (HCC) patients subjected to liver transplantation from living donor (LDLT) <i>versus </i>liver transplantation from deceased donor (DDLT) treated at our center.
</p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">
Seventy-three patients underwent liver transplantation for HCC staged according to Milan criteria (MC). The cases have been divided into two groups: (a) forty-four patients transplanted by means of LDLT, and (b) twenty-nine patients underwent DDLT. The patients beyond MC, or those who underwent downstaging locoregional therapy were excluded from the study.
</p>
<h3>Results</h3>
<p style="text-align: justify;">
Overall survival outcomes at 5 years were, respectively, 80.3% <i>vs </i>70.4%, in LDLT and DDLT groups whereas tumor-free survival was 79.1% <i>vs</i> 76% for LDLT and DDLT cases. LT from living donors showed slightly better patients’ survival compared to liver transplantation from deceased donors DDLT (P=0.09). However, the difference in tumor-free survival between both groups was virtually absent (P=0.6).
<h3>Conclusion</h3>
<p style="text-align: justify;">The present study confirmed that LDLT, while offering a slightly better overall survival, is associated with similar terms of tumor-free survival compared to transplants from deceased donors. This similarity is especially clear when avoiding biases caused by different tumor features and analyzing a perfectly matched cohort of patients presenting with HCC classified according to the Milan criteria.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Hepatocellular carcinoma, liver transplant, living donor, deceased donor, survival.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Our objective was to compare overall and tumor-free survival for the hepatocellular carcinoma (HCC) patients subjected to liver transplantation from living donor (LDLT) versus liver transplantation from deceased donor (DDLT) treated at our center.
Patients and methods
Seventy-three patients underwent liver transplantation for HCC staged according to Milan criteria (MC). The cases have been divided into two groups: (a) forty-four patients transplanted by means of LDLT, and (b) twenty-nine patients underwent DDLT. The patients beyond MC, or those who underwent downstaging locoregional therapy were excluded from the study.
Results
Overall survival outcomes at 5 years were, respectively, 80.3% vs 70.4%, in LDLT and DDLT groups whereas tumor-free survival was 79.1% vs 76% for LDLT and DDLT cases. LT from living donors showed slightly better patients’ survival compared to liver transplantation from deceased donors DDLT (P=0.09). However, the difference in tumor-free survival between both groups was virtually absent (P=0.6).
Conclusion
The present study confirmed that LDLT, while offering a slightly better overall survival, is associated with similar terms of tumor-free survival compared to transplants from deceased donors. This similarity is especially clear when avoiding biases caused by different tumor features and analyzing a perfectly matched cohort of patients presenting with HCC classified according to the Milan criteria.
Keywords
Hepatocellular carcinoma, liver transplant, living donor, deceased donor, survival.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28557
[VALUE] => Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Outcomes of liver transplantation to the patients with hepatocellular carcinoma from living donors versus transplants from deceased donors
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28564
[VALUE] => 2802
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2802
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28565
[VALUE] => 2803
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2803
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Мохамед Р. Абдельфаттах, Хуссейн Эльсиеси
Отдел хирургии, Факультет медицины, Университет Александрии, Александрия, Египет
Целью нашей работы было сравнение общей и безопухолевой заболеваемости у пациентов с гепатоцеллюлярной карциномой (ГЦК), леченных в нашем центре, после трансплантации печени от живых доноров (ЖД) и погибших доноров (ПД).
73 пациентам была выполнена трансплантация печени по поводу ГЦК, стадированной по Миланским критериям (MК). Эти пациенты были распределены по 2 группам: (a) 44 больных трансплантированных от ЖД, и (б) 29 пациентов получидли трансплантаты от ПД. Из исследования были искючены больные, не соответствующие Миланским критериям или пациенты после циторедуктивной локорегионарной терапии.
Результаты
5-летняя выживаемость составила, соответственно, 80,3% и 70,4% среди реципиентов печени от ЖД и ПД, тогда как безопухолевая выживаемость составляла 79,1% и 76%, соответственно, в группах больных, трансплантированных от живых и погибших доноров. Трансплантация печени от живых доноров показала несколько лучшие результаты, нежели трансплантация от погибшиз доноров (P=0.09). Однако разница по безопухолевой выживаемости между этими двумя группами не выявлена (P=0.6).
Выводы
Данное исследование подтвердило, что трансплантация печени от живых доноров, при несколько лучшей общей выживаемости, ассоциирована со сходными сроками безопухолевой выживаемости по сравнению с трансплантацией от погибших доноров. Это сходство особенно выражено при устранении возможных факторов, связанных с особенностями опухолей и анализом данных в хорошо сравнимых группах пациентов с ГЦК, классифицированных по Миланским критериям.
Ключевые слова
Гепатоцеллюлярная карцинома, трансплантация печени, живые доноры, погибшие доноры, выживаемость.
Клинические работы
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28542
[VALUE] => 06.02.2022
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 06.02.2022
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28543
[VALUE] => 18.03.2022
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 18.03.2022
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28544
[VALUE] => Array
(
[TEXT] => <p>Олег И. Долгов<sup>1</sup>, Сергей А. Карпищенко<sup>1</sup>, Екатерина С. Утимишева<sup>1</sup>, Диана А. Григорьянц<sup>1</sup>, Анна А. Спиридонова<sup>1,2</sup>, Иван С. Моисеев<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup>, Алексей Б. Чухловин<sup>1</sup>, Александр Д. Кулагин<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Олег И. Долгов1, Сергей А. Карпищенко1, Екатерина С. Утимишева1, Диана А. Григорьянц1, Анна А. Спиридонова1,2, Иван С. Моисеев1, Людмила С. Зубаровская1, Алексей Б. Чухловин1, Александр Д. Кулагин1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28545
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>2</sup> ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28546
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК).
</p>
<h3>Материалы и методы</h3>
<p style="text-align: justify;">
В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST.
</p>
<h3>Результаты</h3>
<p style="text-align: justify;">
В биоматериале из полости носа и околоносовых пазух наиболее часто высевали <i>S.epidermidis</i> – 34,7% (377/1097); <i>S.viridans</i> – 2,2% (24/1097); <i>S. aureus</i> – 1,91% (21/1097); <i>Klebsiella spp</i> – 1% (11/1097). Частота выявления <i>S.epidermidis</i> и <i>S.viridans</i> была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста <i>S.epidermidis</i> отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления <i>Klebsiella spp</i> в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения <i>Pseudomonas spp</i> (1/378 <i>vs </i>7/217) в материале из придаточных пазух.
</p>
<h3>Заключение</h3>
<p style="text-align: justify;">
Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов <i>Klebsiella spp, Pseudomonas spp, E.coli, S.aureus</i>, главным образом – в сроки 2 и более мес. после ТГСК.
</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК).
Материалы и методы
В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST.
Результаты
В биоматериале из полости носа и околоносовых пазух наиболее часто высевали S.epidermidis – 34,7% (377/1097); S.viridans – 2,2% (24/1097); S. aureus – 1,91% (21/1097); Klebsiella spp – 1% (11/1097). Частота выявления S.epidermidis и S.viridans была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста S.epidermidis отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления Klebsiella spp в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения Pseudomonas spp (1/378 vs 7/217) в материале из придаточных пазух.
Заключение
Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов Klebsiella spp, Pseudomonas spp, E.coli, S.aureus, главным образом – в сроки 2 и более мес. после ТГСК.
Ключевые слова
Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28547
[VALUE] => 10.18620/ctt-1866-8836-2022-11-1-36-42
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2022-11-1-36-42
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28550
[VALUE] => Array
(
[TEXT] => <p>Oleg I. Dolgov<sup>1</sup>, Sergey A. Karpishchenko<sup>1</sup>, Ekaterina S. Utimisheva<sup>1</sup>, Diana A. Grigoryanz<sup>1</sup>, Anna A. Spiridonova<sup>1,2</sup>, Ivan S. Moiseev<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Alexei B. Chukhlovin<sup>1</sup>, Alexander D. Kulagin<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Oleg I. Dolgov1, Sergey A. Karpishchenko1, Ekaterina S. Utimisheva1, Diana A. Grigoryanz1, Anna A. Spiridonova1,2, Ivan S. Moiseev1, Ludmila S. Zubarovskaya1, Alexei B. Chukhlovin1, Alexander D. Kulagin1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28551
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br>
<sup>2</sup> St. Petersburg Pasteur Institute, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Oleg I. Dolgov, Department of Otorhinolaryngology,
Pavlov University, 6-8 Tolstoy St, 197022, St. Petersburg, Russia<br>
Phone: +7 (921) 845-03-51<br>
E-mail: oidolgov@yandex.ru</p><br>
<p><b>Citation:</b> Dolgov OI, Karpishchenko SA, Utimisheva ES, et al. Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation. Cell Ther Transplant 2022; 11(1): 36-42.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Pavlov University, St. Petersburg, Russia
2 St. Petersburg Pasteur Institute, St. Petersburg, Russia
Correspondence:
Dr. Oleg I. Dolgov, Department of Otorhinolaryngology,
Pavlov University, 6-8 Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 845-03-51
E-mail: oidolgov@yandex.ru
Citation: Dolgov OI, Karpishchenko SA, Utimisheva ES, et al. Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation. Cell Ther Transplant 2022; 11(1): 36-42.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28552
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Hematopoietic stem cell transplantation (HSCT) is often accompanied by infectious complications. The aim of this study was a comparative evaluation of facultative anaerobic microbiota members of nasal and paranasal cavity in sinusitis, which often develops in immunocompromised patients, due to intensive chemotherapy and massive antibiotic treatment followed by hematopoietic cell transplantation (HSCT).
</p>
<h3>Materials and methods</h3>
<p style="text-align: justify;">
The study involved 194 patients with various myelo- and lymphoproliferative diseases aged 1 to 62 years who underwent intensive chemotherapy and allogeneic HSCT. As based on appropriate clinical indications, the biomaterial was taken from patients (washings from the paranasal sinuses and/or nasal swabs) within the time period of +100 to +180 days after allogeneic HSCT. We studied 124 samples from maxillary sinus punctures of 97 patients and 973 scrapings from the nasal cavity. Seeding of biological material and isolation of the microorganisms were performed by classical bacteriological techniques. Antibiotic susceptibility of clinical isolates was determined by disk diffusion methods. The data on microbial sensitivity were interpreted by the EUCAST criteria.
</p>
<h3>Results</h3>
<p style="text-align: justify;">
In the samples from nasal cavity and paranasal sinuses, S. epidermidis was most often detected (34.7%, 377/1097); <i>S.viridans</i> (2.2%, 24/1097); <i>S. aureus</i> (1.91%, 21/1097); <i>Klebsiella spp</i> (1%, 11/1097). Detection frequency of <i>S.epidermidis</i> and <i>S.viridans</i> was minimal in the younger age group (up to 5 years), and increased in older groups (>15 years old). Profound suppression of <i>S.epidermidis</i> growth was noted, especially in paranasal sinuses, within 1 month after HSCT in presence of massive antibiotic therapy. High frequency of <i>Klebsiella spp</i> detection was noted in the samples from maxillar sinuses at later terms (2-3 months) after HSCT, at low detection frequency of the pathogen in the specimens from nasal cavity (average 16.3% <i>vs</i> 2.1%, p=2×10^14). In addition, we have estimated frequency of bacterial inoculation within +30 days upon the diagnosis of sinusitis. At the same time, an increased frequency of <i>Pseudomonas spp</i> isolation (1/378 <i>vs </i>7/217) was revealed in the material from paranasal sinuses.
</p>
<h3>Conclusion</h3>
<p style="text-align: justify;">
Bacteriological study of biological samples from maxillary sinuses is of limited value during the 1st month after HSCT accompanied by massive antibiotic therapy which was followed by selection of resistant strains of <i>Klebsiella spp, Pseudomonas spp, E. coli, S. aureus</i>, mainly at the terms of >2 months after HSCT.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Hematopoietic stem cell transplantation, paranasal sinuses, microbiota, antibiotic resistance.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Hematopoietic stem cell transplantation (HSCT) is often accompanied by infectious complications. The aim of this study was a comparative evaluation of facultative anaerobic microbiota members of nasal and paranasal cavity in sinusitis, which often develops in immunocompromised patients, due to intensive chemotherapy and massive antibiotic treatment followed by hematopoietic cell transplantation (HSCT).
Materials and methods
The study involved 194 patients with various myelo- and lymphoproliferative diseases aged 1 to 62 years who underwent intensive chemotherapy and allogeneic HSCT. As based on appropriate clinical indications, the biomaterial was taken from patients (washings from the paranasal sinuses and/or nasal swabs) within the time period of +100 to +180 days after allogeneic HSCT. We studied 124 samples from maxillary sinus punctures of 97 patients and 973 scrapings from the nasal cavity. Seeding of biological material and isolation of the microorganisms were performed by classical bacteriological techniques. Antibiotic susceptibility of clinical isolates was determined by disk diffusion methods. The data on microbial sensitivity were interpreted by the EUCAST criteria.
Results
In the samples from nasal cavity and paranasal sinuses, S. epidermidis was most often detected (34.7%, 377/1097); S.viridans (2.2%, 24/1097); S. aureus (1.91%, 21/1097); Klebsiella spp (1%, 11/1097). Detection frequency of S.epidermidis and S.viridans was minimal in the younger age group (up to 5 years), and increased in older groups (>15 years old). Profound suppression of S.epidermidis growth was noted, especially in paranasal sinuses, within 1 month after HSCT in presence of massive antibiotic therapy. High frequency of Klebsiella spp detection was noted in the samples from maxillar sinuses at later terms (2-3 months) after HSCT, at low detection frequency of the pathogen in the specimens from nasal cavity (average 16.3% vs 2.1%, p=2×10^14). In addition, we have estimated frequency of bacterial inoculation within +30 days upon the diagnosis of sinusitis. At the same time, an increased frequency of Pseudomonas spp isolation (1/378 vs 7/217) was revealed in the material from paranasal sinuses.
Conclusion
Bacteriological study of biological samples from maxillary sinuses is of limited value during the 1st month after HSCT accompanied by massive antibiotic therapy which was followed by selection of resistant strains of Klebsiella spp, Pseudomonas spp, E. coli, S. aureus, mainly at the terms of >2 months after HSCT.
Keywords
Hematopoietic stem cell transplantation, paranasal sinuses, microbiota, antibiotic resistance.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28548
[VALUE] => Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Microbiota of nasal cavity in sinusitis following hematopoietic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28549
[VALUE] => 2788
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2788
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28553
[VALUE] => 2796
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2796
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Олег И. Долгов1, Сергей А. Карпищенко1, Екатерина С. Утимишева1, Диана А. Григорьянц1, Анна А. Спиридонова1,2, Иван С. Моисеев1, Людмила С. Зубаровская1, Алексей Б. Чухловин1, Александр Д. Кулагин1
1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ФБУН НИИ эпидемиологии и микробиологии имени Пастера, Санкт-Петербург, Россия
Трансплантация гемопоэтических стволовых клеток (ТГСК) часто сопровождается инфекционными осложнениями. Целью настоящего исследования была сравнительная оценка состава факультативно-анаэробных представителей микробиоты полости носа и его придаточных пазух при синусите, который нередко развивается у иммунокомпромиссных пациентов после интенсивной химио- и антибиотикотерапии и трансплантации гемопоэтических клеток (ТГСК).
Материалы и методы
В исследовании участвовали 194 пациента с различными миело- и лимфопролиферативными заболеваниями в возрасте от 1 до 62 лет, проходившие интенсивную химиотерапию и аллогенную ТГСК. При наличии клинических показаний у пациентов забирали биоматериал (смывы из околоносовых пазух и/или назальные мазки) в сроки от -100 до +180 суток после аллогенной ТГСК. Исследовано 124 образца пунктатов верхнечелюстных пазух от 97 пациентов и 973 мазка-соскоба из полости носа. Посев биоматериала и выделение микроорганизмов проводили классическими бактериологическими методами. Чувствительность клинических изолятов к антибиотикам определяли диско-диффузионным методом. Интерпретацию результатов чувствительности осуществляли согласно критериям EUCAST.
Результаты
В биоматериале из полости носа и околоносовых пазух наиболее часто высевали S.epidermidis – 34,7% (377/1097); S.viridans – 2,2% (24/1097); S. aureus – 1,91% (21/1097); Klebsiella spp – 1% (11/1097). Частота выявления S.epidermidis и S.viridans была минимальной в младшей возрастной группе (до 5 лет) и возрастала в группах от 15 лет и выше. Глубокое подавление роста S.epidermidis отмечалось (в особенности – в околоносовых синусах) в течение 1-го мес. после ТГСК на фоне массивной антибиотикотерапии. Отмечена высокая частота выявления Klebsiella spp в материале из синусов в поздние сроки (2-3 мес.) после ТГСК при малой частоте выявления в материале из полости носа (в среднем 16,3% против 2.1%, р=2×10^14). Кроме того, мы оценили частоту высеваемости бактерий в сроки +30 сут. от постановки диагноза синусита. При этом была выявлена повышенная частота выделения Pseudomonas spp (1/378 vs 7/217) в материале из придаточных пазух.
Заключение
Бактериологические исследование материала из гайморовых пазух имеет ограниченную ценность в течение 1-го мес. после ТГСК в связи с массивной антибиотикотерапией, которая сопровождается селекцией резистентных штаммов Klebsiella spp, Pseudomonas spp, E.coli, S.aureus, главным образом – в сроки 2 и более мес. после ТГСК.
Ключевые слова
Tрансплантация гемопоэтических стволовых клеток, околоносовые пазухи, микробиота, антибиотикорезистентность.
Клинические работы
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28530
[VALUE] => 24.01.2022
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 24.01.2022
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28531
[VALUE] => 18.02.2022
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 18.02.2022
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28532
[VALUE] => Array
(
[TEXT] => <p>Тахерех Ростами<sup>1</sup>, Мохамад Р. Ростами<sup>2</sup>, Азадех Кьюмарси<sup>1</sup>, Амир Казаэйян<sup>3</sup>, Неда Алиджани<sup>4</sup>,
Хосейн К. Фумани<sup>2</sup>, Соруш Рад<sup>2</sup>, Давуд Бабахани<sup>2</sup>, Таназ Бахри<sup>2</sup>, Мохаммад Ваези<sup>2</sup>, Мариам Бахордар<sup>2</sup>,
Сейед А. Мирхоссейни<sup>2</sup>, Сейед А. Моусави<sup>2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Тахерех Ростами1, Мохамад Р. Ростами2, Азадех Кьюмарси1, Амир Казаэйян3, Неда Алиджани4,
Хосейн К. Фумани2, Соруш Рад2, Давуд Бабахани2, Таназ Бахри2, Мохаммад Ваези2, Мариам Бахордар2,
Сейед А. Мирхоссейни2, Сейед А. Моусави2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28533
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br>
<sup>2</sup> НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br>
<sup>3</sup> Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран<br>
<sup>4</sup> Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
2 НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
3 Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
4 Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28534
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.</p>
<h3>Пациенты и методы</h3>
<p style="text-align: justify;">В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных
доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.</p>
<h3>Выводы</h3>
<p style="text-align: justify;">В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.
Пациенты и методы
В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных
доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.
Результаты
Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.
Выводы
В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.
Ключевые слова
Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28535
[VALUE] => 10.18620/ctt-1866-8836-2022-11-1-24-35
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2022-11-1-24-35
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28538
[VALUE] => Array
(
[TEXT] => <p>Tahereh Rostami<sup>1</sup>, Mohammad R. Rostami<sup>2</sup>, Azadeh Kiumarsi<sup>1</sup>, Amir Kasaeian<sup>3</sup>, Neda Alijani<sup>4</sup>, Hosein K. Fumani<sup>2</sup>, Soroush Rad<sup>2</sup>, Davood Babakhani<sup>2</sup>, Tanaz Bahri<sup>2</sup>, Mohammad Vaezi<sup>2</sup>, Maryam Barkhordar<sup>2</sup>, Seied A. Mirhosseini<sup>2</sup>, Seied A. Mousavi<sup>2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Tahereh Rostami1, Mohammad R. Rostami2, Azadeh Kiumarsi1, Amir Kasaeian3, Neda Alijani4, Hosein K. Fumani2, Soroush Rad2, Davood Babakhani2, Tanaz Bahri2, Mohammad Vaezi2, Maryam Barkhordar2, Seied A. Mirhosseini2, Seied A. Mousavi2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28539
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br>
<sup>2</sup> Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br>
<sup>3</sup> Department of Biostatistics and Epidemiology, Research Institute for Oncology, Hematology and Cell Therapy, Shariati
Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran<br>
<sup>4</sup> Department of lnfectious Diseases, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran</p><br>
<p><b>Correspondence:</b><br>
Dr. Azadeh Kiumarsi, MD, Assistant Professor, Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Kargar Shomali Street, 1411713131, Tehran, Iran<br>
Phone: +98 9121037104<br>
Fax: +98 (21) 8802 9397<br>
Email: akiumarsi@sina.tums.ac.ir</p><br>
<p><b>Citation:</b> Tahereh Rostami, Mohammad R. Rostami, Azadeh Kiumarsi et al. Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis. Cell Ther Transplant 2022; 11(1): 24-35. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
2 Research Institute for Oncology, Hematology and Cell Therapy (RIOHCT), Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
3 Department of Biostatistics and Epidemiology, Research Institute for Oncology, Hematology and Cell Therapy, Shariati
Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
4 Department of lnfectious Diseases, Shariati Hospital, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Correspondence:
Dr. Azadeh Kiumarsi, MD, Assistant Professor, Pediatric Hematology, Oncology and Stem Cell Transplantation, Department of Pediatric Cell Therapy, Research Institute for Oncology, Hematology and Cell Therapy, Shariati Hospital, Kargar Shomali Street, 1411713131, Tehran, Iran
Phone: +98 9121037104
Fax: +98 (21) 8802 9397
Email: akiumarsi@sina.tums.ac.ir
Citation: Tahereh Rostami, Mohammad R. Rostami, Azadeh Kiumarsi et al. Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis. Cell Ther Transplant 2022; 11(1): 24-35.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28540
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for acute leukemia. Various parameters have significant impact on the final results of HSCT, such as donor type, stem cell source, and the applied conditioning regimen. In the absence of HLA-matched related or unrelated donors, haploidentical donors present a possible alternative for the patients with indications for HSCT. The present single-center study compared the outcomes of HSCT from different donor types using a radiation-free MAC regimen. We compared the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) from matched, or mismatched related, and unrelated donors with those from haploidentical donors in the children, adolescents and young adults (CAYA) treated for acute leukemia.</p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">In this retrospective study performed since 2014 to 2021, we have evaluated the clinical outcomes among CAYA patients with acute leukemia who underwent peripheral blood T cell-replete HSCT from haploidentical donors <i>versus</i> unrelated donors (including 10/10 or 9/10 HLA-matched), and <i>versus</i> related donors (including 10/10 or 9/10 HLA-matched). The myeloablative conditioning for HSCT was performed as irradiation-free regimen including busulfan and cyclophosphamide. GvHD prophylaxis was based on administration of cyclosporine A in all the patients, accomplished by rabbit anti-human thymocyte globulin in HSCT from unrelated and haploidentical donors, and post-transplant cyclophosphamide in cases of haploidentical donors. For statistical evaluation, an adjusted multivariable proportional hazard Cox and competing risk analyses were used.</p>
<h3>Results</h3>
<p style="text-align: justify;">Median follow-up time period was 28.7 months (95% CI: 21.9-34.9). Three-year overall survival rate (OS) and GvHD-free/relapse-free survival (GFRFS) rate were 68.81% (95% CI: 60.08%-76.01%) and 44.19% (95% CI: 35.52%-52.49%), respectively. The patients who underwent HSCT from unrelated HLA-matched donors had the lowest OS and GFRFS compared to other donor types. The 3-year non-relapse mortality (NRM) in all patients was 7.84% (95% CI 4.36-12.62). Adjusted multivariable modeling of OS showed that the hazard of death in patients who had undergone HSCT from an unrelated donor, was 3.6 times more than for the patients who underwent HSCT from their haploidentical donors (P=0.05). Likewise, the hazard of NRM after HSCT from unrelated donors was 6 times more than with haploidentical donors (P=0.002). However, the relapse incidence was not significantly different between the two mentioned groups.</p>
<h3>Conclusions</h3>
<p style="text-align: justify;">In this study, HSCT from haploidentical donors was associated with superior survival rates compared to HSCT from unrelated HLA-matched donors. Hence, haploidentical transplantation with peripheral blood stem cells could be a practical and valuable clinical option that offers a reasonable opportunity for the disease control in CAYA patients with acute leukemia requiring HSCT and lacking matched available donors.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Acute leukemia, allogeneic hematopoietic stem cell transplantation, matched related donors, unrelated donors, haploidentical donors, clinical outcomes.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for acute leukemia. Various parameters have significant impact on the final results of HSCT, such as donor type, stem cell source, and the applied conditioning regimen. In the absence of HLA-matched related or unrelated donors, haploidentical donors present a possible alternative for the patients with indications for HSCT. The present single-center study compared the outcomes of HSCT from different donor types using a radiation-free MAC regimen. We compared the results of unmanipulated peripheral blood stem cell transplantation (PBSCT) from matched, or mismatched related, and unrelated donors with those from haploidentical donors in the children, adolescents and young adults (CAYA) treated for acute leukemia.
Patients and methods
In this retrospective study performed since 2014 to 2021, we have evaluated the clinical outcomes among CAYA patients with acute leukemia who underwent peripheral blood T cell-replete HSCT from haploidentical donors versus unrelated donors (including 10/10 or 9/10 HLA-matched), and versus related donors (including 10/10 or 9/10 HLA-matched). The myeloablative conditioning for HSCT was performed as irradiation-free regimen including busulfan and cyclophosphamide. GvHD prophylaxis was based on administration of cyclosporine A in all the patients, accomplished by rabbit anti-human thymocyte globulin in HSCT from unrelated and haploidentical donors, and post-transplant cyclophosphamide in cases of haploidentical donors. For statistical evaluation, an adjusted multivariable proportional hazard Cox and competing risk analyses were used.
Results
Median follow-up time period was 28.7 months (95% CI: 21.9-34.9). Three-year overall survival rate (OS) and GvHD-free/relapse-free survival (GFRFS) rate were 68.81% (95% CI: 60.08%-76.01%) and 44.19% (95% CI: 35.52%-52.49%), respectively. The patients who underwent HSCT from unrelated HLA-matched donors had the lowest OS and GFRFS compared to other donor types. The 3-year non-relapse mortality (NRM) in all patients was 7.84% (95% CI 4.36-12.62). Adjusted multivariable modeling of OS showed that the hazard of death in patients who had undergone HSCT from an unrelated donor, was 3.6 times more than for the patients who underwent HSCT from their haploidentical donors (P=0.05). Likewise, the hazard of NRM after HSCT from unrelated donors was 6 times more than with haploidentical donors (P=0.002). However, the relapse incidence was not significantly different between the two mentioned groups.
Conclusions
In this study, HSCT from haploidentical donors was associated with superior survival rates compared to HSCT from unrelated HLA-matched donors. Hence, haploidentical transplantation with peripheral blood stem cells could be a practical and valuable clinical option that offers a reasonable opportunity for the disease control in CAYA patients with acute leukemia requiring HSCT and lacking matched available donors.
Keywords
Acute leukemia, allogeneic hematopoietic stem cell transplantation, matched related donors, unrelated donors, haploidentical donors, clinical outcomes.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28536
[VALUE] => Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Peripheral blood stem cell transplantation from haploidentical and unrelated versus related donors for acute leukemia in children, adolescents and young adults (CAYA): A competing risk analysis
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28537
[VALUE] => 2786
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2786
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28541
[VALUE] => 2787
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2787
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Тахерех Ростами1, Мохамад Р. Ростами2, Азадех Кьюмарси1, Амир Казаэйян3, Неда Алиджани4, Хосейн К. Фумани2, Соруш Рад2, Давуд Бабахани2, Таназ Бахри2, Мохаммад Ваези2, Мариам Бахордар2, Сейед А. Мирхоссейни2, Сейед А. Моусави2
1 Отдел клеточной терапии у детей, НИИ онкологии, гематологии и клеточной терапии (RIOHCT), Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
2 НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
3 Отдел биостатистики и эпидемиологии, НИИ онкологии, гематологии и клеточной терапии, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
4 Отдел инфекционных болезней, Шариатский госпиталь, Тегеранский университет медицинских наук (TUMS), Тегеран, Иран
Трансплантация аллогенных гематопоэтических клеток (алло-ТГСК) является единственной потенциальной возможностью излечения острого лейкоза. Многие параметры существенно влияют на конечный исход ТГСК, в т.ч. тип донора, источник стволовых клеток и применяемый режим кондиционирования. При отсутствии HLA-совместимого родственного донора, возможными кандидатами могут быть неродственные совместимые или гаплоидентичные доноры для пациентов с показаниями к ТГСК. Для того, чтобы сопоставить исходы ТГСК от доноров различного типа с кондиционированием без облучения, мы сравнили в рамках одноцентрового исследования результаты трансплантации интактных ГСК периферической крови от совместимых и несовместимых, родственных и неродственных доноров, и гаплоидентичных доноров реципиентам детского, подросткового возрастов и молодым взрослым с острыми лейкозами.
Пациенты и методы
В данном ретроспективном исследовании, проводившемся с 2014 по 2021 г., мы оценивали исходы ТГСК с реплецией Т-лимфоцитов от гаплоидентичных доноров или неродственных доноров (совместимость – 10/10 или 9/10), а также в сравнении с неродственными донорами у пациентов с острыми лейкозами этих возрастных групп. Кондиционирование при ТГСК проводили с применением миелоаблативного режима с бусульфаном и циклофосфамидом и без ионизирующего облучения. Профилактика РТПХ включала назначение циклоспорина А всем пациентам, кроличий антитимоцитарный глобулин для неродственных и гаплоидентичных доноров, и циклофосфамид при ТГСК от гаплоидентичных доноров. Статистическую обработку проводили с помощью многовариантного пропорционального анализа рисков по Коксу и анализ конкурирующих рисков.
Результаты
Средний срок наблюдения составлял 28,7 мес. (95% CI: 21,9-34,9). Трехлетняя общая выживаемость (ОВ) и выживаемости без РТПХ и рецидивов были, соответственно, 68,81% (95% CI: 60,08%-76,01%) и 44.19% (95% CI: 35,52%-52,49%). Пациенты после ТГСК от неродственных совместимых доноров имели более низкие уровни ОВ и выживаемости без РТПХ и рецидивов по сравнению с другими типами доноров. Трехлетние показатели безрецидивной летальности (NRM) среди всех пациентов составляли 7,84% (95% CI 4,36-12,62). Адаптированное многовариантное моделирование общей выживаемости показало, что риск гибели пациентов после ТГСК от неродственного донора был в 3,6 раза выше, чем у пациентов, получивших ТГСК от гаплоидентичных доноров (P=0.05). Аналогично, риск безрецидивной смертности (NRM) после ТГСК от неродственных доноров был в 6 раз выше, чем при ТГСК от гаплоидентичных доноров (P=0.002). Однако частота рецидивов не различалась существенно между двумя указанными группами.
Выводы
В данном исследовании показано, что ТГСК от гаплоидентичных доноров была ассоциирована с более высокими уровнями выживаемости, по сравнению с ТГСК от неродственных совместимых доноров. Таким образом, ТГСК от гаплоидентичных доноров может быть предложена в качестве практичной и ценной клинической опции, для пациентов молодых возрастов с острыми лейкозами в случае отсутствия совместимых доноров.
Ключевые слова
Острый лейкоз, аллогенная трансплантация гемопоэтических клеток, совместимые родственные доноры, неродственные доноры, гаплоидентичные доноры, клинические исходы.
Клинические работы
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28521
[VALUE] => 09.03.2022
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 09.03.2022
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28522
[VALUE] => 18.03.2022
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 18.03.2022
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28523
[VALUE] => Array
(
[TEXT] => <p>Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина,
Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина,
Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28524
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28525
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.</p>
<h3>Пациенты и методы</h3>
<p style="text-align: justify;">Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев. </p>
<h3>Результаты</h3>
<p style="text-align: justify;">Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.</p>
<h3>Выводы</h3>
<p style="text-align: justify;">Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.
Пациенты и методы
Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев.
Результаты
Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.
Выводы
Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.
Ключевые слова
Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28518
[VALUE] => 10.18620/ctt-1866-8836-2022-11-1-13-23
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2022-11-1-13-23
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28526
[VALUE] => Array
(
[TEXT] => <p>Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina,
Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina,
Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28527
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia<br>
Phone: +7 (911) 927-82-29<br>
E-mail: dr_morozova@mail.ru</p><br>
<p><b>Citation:</b> Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (911) 927-82-29
E-mail: dr_morozova@mail.ru
Citation: Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28528
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
</p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">
We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases.
</p>
<h3>Results</h3>
<p style="text-align: justify;">
Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA <i>versus </i>3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% <i>versus</i> 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF.
</p>
<h3>Conclusions</h3>
<p style="text-align: justify;">
Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Patients and methods
We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases.
Results
Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF.
Conclusions
Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies.
Keywords
Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28519
[VALUE] => Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28520
[VALUE] => 2765
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2765
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28529
[VALUE] => 2768
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2768
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Елена В. Морозова, Татьяна А. Рудакова, Юлия Ю. Власова, Мария В. Барабанщикова, Татьяна Л. Гиндина, Александр Л. Алянский, Мария Д. Владовская, Иван С. Моисеев, Людмила С. Зубаровская, Александр Д. Кулагин
НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
Аллогенная трансплантация стволовых клеток (алло-ТГСК) используется во всем мире для долгосрочного контроля и лечения злокачественных новообразований системы крови, по-прежнему оставаясь методом выбора в лечении хронического миелоидного лейкоза (ХМЛ) у пациентов, которые не могут достичь длительного полного цитогенетического ответа после лечения ингибиторами тирозинкиназы (ИТК), а также на поздних стадиях заболевания. Наряду с риском рецидива, неблагоприятные результаты ТГСК могут быть связаны с первичной недостаточностью (ПНТ) или плохой функцией трансплантата (ПФТ). Следовательно, целью нашего исследования было оценить частоту и исход ПНТ и тяжелой плохой функции трансплантата (ПФТ) после алло-ТГСК у пациентов с ХМЛ.
Пациенты и методы
Мы провели ретроспективный анализ 121 случая ХМЛ, которым проводилась алло-ТГСК в НИИ им. Р. М. Горбачевой ПСПбГМУ им. И. Павлова за последние 25 лет. ТГСК была показана в случаях продвинутой фазы заболевания или резистентности/непереносимости ИТК у пациентов с ХМЛ. Уровни транскриптов BCR/ABL и дополнительные хромосомные аномалии использовались в качестве лабораторных маркеров персистирующего заболевания. 80 пациентам (66%) трансплантацию проводили в хронической фазе заболевания (ХФ); 41 пациент (34%) находился в фазе акселерации (ФА) или бластном кризе (БК) на момент ТГСК. Трансплантацию от совместимых неродственных доноров выполняли в 65% случаев; от совместимых родственных доноров – в 28%, и от гаплоидентичных доноров – в 7% случаев.
Результаты
Приживление трансплантата отмечено у 106 пациентов (88%). Посттрансплантационные рецидивы зарегистрированы у 31 пациента в сроки от 15 до 333 дней после ТГСК. ПНТ была зарегистрирована в 8 случаях (7%). У двух пациентов развилась вторичная недостаточность трансплантата в течение двух месяцев после первичного приживления с летальными инфекционными осложнениями. Тяжелое нарушение функции трансплантата (ПФТ) диагностировано в 11 случаях (9%), при кумулятивной частоте 10% в течение 1-го года после трансплантации. Среди различных предтрансплантационных характеристик, фактор возраста и, особенно – наличие дополнительных хромосомных аномалий (ДХА) были связаны с кумулятивной частотой первичной и тяжелой вторичной недостаточности трансплантата после ТГСК. Т.е. ПНТ составил 14% в группе с выявленными ДХА по сравнению с 3% в группе без ДХА, (p=0,02), тогда как частота вторичной недостаточности трансплантата у пациентов с ДХА составила 2% против 12% в группе без АЦА (p=0,09). Частота посттрансплантационных рецидивов у пациентов с ПНТ и ПФТ не различалась.
Выводы
Первичная недостаточность трансплантата (ПНТ) способствует безрецидивной смертности в течение первого года после алло-ТГСК у пациентов с ХМЛ. Возникновение посттрансплантационных рецидивов не было связано с ПНТ И ПФТ при ХМЛ. Для улучшения эффективности технологий ТГСК необходима дальнейшая оценка факторов риска несостоятельности или плохой функции трансплантата.
Ключевые слова
Хронический миелоидный лейкоз, трансплантация гемопоэтических стволовых клеток, показания, несостоятельность трансплантата, факторы риска.

