Cellular Therapy and Transplantation, 2015
Legal Notice
Terms of use
info@cttjournal.com
array(5) {
[0]=>
array(49) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
["~IBLOCK_SECTION_ID"]=>
string(2) "10"
["ID"]=>
string(3) "458"
["~ID"]=>
string(3) "458"
["IBLOCK_ID"]=>
string(1) "2"
["~IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["~NAME"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["ACTIVE_FROM"]=>
NULL
["~ACTIVE_FROM"]=>
NULL
["TIMESTAMP_X"]=>
string(19) "13.06.2017 12:33:54"
["~TIMESTAMP_X"]=>
string(19) "13.06.2017 12:33:54"
["DETAIL_PAGE_URL"]=>
string(118) "/ru/archive/Volume-1/korotkie-soobshcheniya/transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze/"
["~DETAIL_PAGE_URL"]=>
string(118) "/ru/archive/Volume-1/korotkie-soobshcheniya/transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze/"
["LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["~LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["DETAIL_TEXT"]=>
string(40975) "
Introduction
Multiple sclerosis is a chronic inflammatory disorder of the central nervous system (CNS) caused by autoimmune reactivity of T cells towards CNS myelin components. MS progression inevitably leads to the loss of motor function, sensitive disturbances and cognitive impairment because of the immune-mediated demyelination and axon degeneration [1].
MS is one of the most common neurological disorders, which mainly affects young adults, and causes gradual decrease of their quality of life (QoL). The clinical course of the disease is very heterogeneous. However, it typically presents with a relapsing-remitting course (RRMS; 80% of patients), which is followed after 5–15 years in about 70% of patients by a so-called secondary progressive phase (SPMS) [2]. 10–20% of patients have a primary progressive course, which is characterized by a steady progression from the onset with or without any acute exacerbations (progressive relapsing MS or PRMS, and primary progressive MS or PPMS, respectively).
Conventional therapies do not provide satisfactory control of MS due to their inability to eradicate self-specific T cell clones. Recently, HDCT+auto-HSCT was proposed as a new and promising therapy for MS patients [3,4]. HDCT+auto-HSCT leads to the elimination of autoreactive T cells and, subsequently, to the restoration of a normal immune system.
Since 1995, several clinical studies have addressed the issue of feasibility and efficacy of HDCT+auto-HSCT in MS [3-15]. However, the information about long-term effects of HDCT+auto-HSCT in this patient population is scanty. In addition, the majority of patients included in the above-mentioned studies had SPMS, and were severely disabled with an average EDSS score of 6.5. Unfortunately, even complete suppression of autoimmune inflammation does not lead to a significant improvement of QoL in these patients. Therefore, the patient selection criteria for HDCT+auto-HSCT are still unclear and the proper selection of patients for transplantation remains the key issue.
Another important consideration is the selection of appropriate criteria for the assessment of treatment outcomes for MS patients. Both disease-free period and improvement of patient’s QoL are recognized as important outcome parameters. With this in mind, evaluation of both clinical and patient-reported outcomes in MS patients after HDCT+auto-HSCT is worthwhile. However, neurologists traditionally evaluate the clinical response only and rarely use QoL data in the outcome analysis. This may be partly explained by the fact that QoL questionnaires used for MS patients – both generic and specific – are multidimensional, and the interpretation of changes in several QoL scales/domains might be difficult for physicians. Recently, we have developed an approach to obtain an Integral QoL Index (IQLI) for profile questionnaires (both generic and specific). IQLI is a standardized value based on the properties of a geometric profile formed by the scales of a questionnaire, which is assessed by the method of integral profiles; the index has been validated in different patients’ populations [16]. The advantages of IQLI are its ease of use and the possibility of obtaining one index based on several QoL scales. The use of IQLI makes it possible to overcome the difficulties in the interpretation of QoL data and allows the assessment of patient-reported outcomes, namely the QoL response.
To date, some limited information exists on the clinical response of MS patients to HDCT+auto-SCT at long-term follow-up, whereas the data on QoL response is lacking. In addition, the clinical experience in the application of HDCT+auto-HSCT to various types and stages of MS is very limited. Moreover, the timing for transplantation is still unclear.
We report the follow-up results of a prospective Phase II multicenter trial, which was started in 1999 and has since then been conducted by the Russian Cooperative Group for Cellular Therapy. This study is focused on the efficacy of HDCT+auto-HSCT in terms of clinical and quality of life responses in patients with different types and stages of MS.
Patients and Methods
One hundred and nine patients were enrolled in the study. Patient characteristics are shown in Table 1.
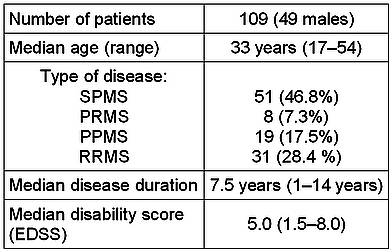
Table 1. Demographic and clinical profile of the patient population.
All patients were refractory to conventional therapy, which included IFNβ and mitoxantrone, as well as steroids, azathiopine, intravenous immunoglobulin and plasmapheresis in some patients. The mean follow-up was 19 months (range, 6–108 months).
The trial was conducted according to the principles of the Helsinki Declaration, and approved by the IRB and Ethics Committees of all of the participating centers before initiation. All patients gave written informed consent.
The neurological disability of MS patients is quantified according to the Expanded disability status scale (EDSS) [17]. The EDSS scores range from 0 (no disability) to 10 (death related to neurological progression) in 0.5-step increments. EDSS scores from 1.0 to 4.5 refer to the fully ambulatory MS patients, while patients with EDSS scores of 7.0 are essentially restricted to a wheelchair.
Patient Eligibility
Criteria for patient selection were: age between 18 and 55 years; diagnosis of multiple sclerosis verified by clinical and laboratory findings; EDSS score 1.5–8.0; normal mental status; absence of severe concomitant diseases.
The disease activity was determined either by magnetic resonance imaging scans displaying active lesions in the CNS (i.e., gadolinium-enhancing lesions, new or enlarging lesions on serial scans) or by clinical assessment showing rapid neurological deterioration, e.g., 0.5-point increase on the EDSS during the 6-months preceding enrollment.
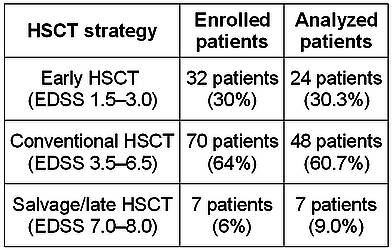
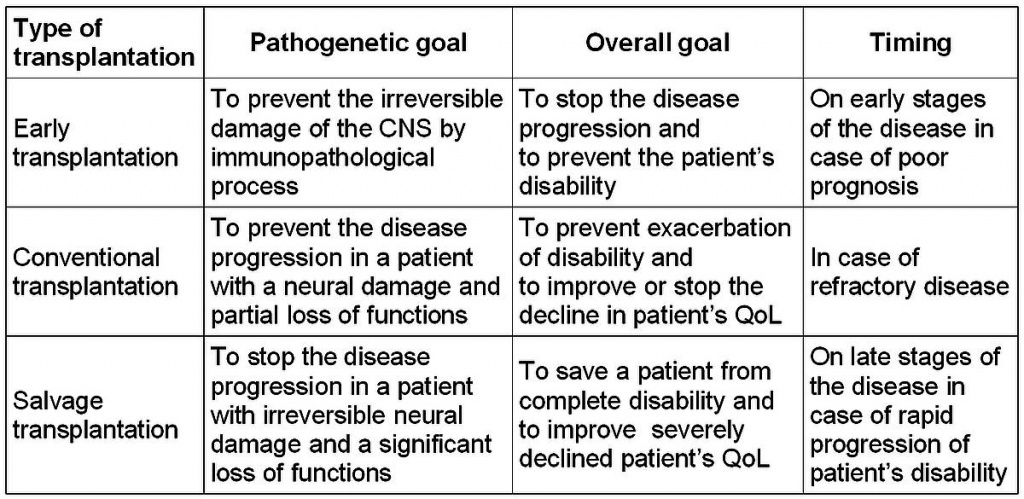
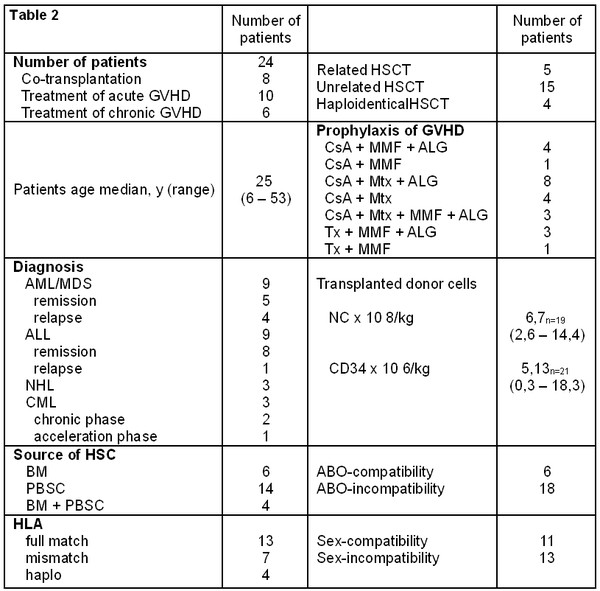
According to our concept there are 3 strategies of HDCT+auto-HSCT [18]. Early HSCT (in MS patients with EDSS 1.5–3.0) is performed soon after diagnosis in case of primary refractory disease or poor prognosis. Conventional HSCT (EDSS 3.5–6.5) is performed in patients with secondary refractory disease. Salvage HSCT (EDSS 7.0–8.0) is an option in case of high disease activity and rapid neurological deterioration in late stages of the disease. All three strategies were applied in this study (Table 2).
Table 2. HSCT timing in the studied patient population
Stem Cell Mobilization and Transplant Procedure
Hematopoietic stem cells were mobilized with G-CSF at 10 μg/kg +/- cyclophosphamide at 4 g/m2 according to EBMT/EULAR guidelines [19]. The grafts were not manipulated. BEAM or BEAM-modified conditioning was used. The BEAM conditioning regimen included BCNU (300 mg/m2) on day -6, etoposide (200 mg/m2) from day -5 to day -2, cytarabine (200 mg/m2 bd) from day -5 to day -2 and melphalan (140 mg/m2) on day -1. It was followed by autologous hematopoietic stem cell transplantation (day 0). In vivo T cell-depletion was achieved through infusion of 30 mg/kg of horse anti-thymocyte globulin (ATG) on days 1 and 2. Five μg/kg s.c. of G-CSF were administered from day 3 post-infusion until granulocyte recovery. For infection prophylaxis oral ciprofloxacin, fluconazole, acyclovir, and IV human Ig were given.
Neurological and QoL assessments
Clinical and QoL assessments were performed at baseline, at discharge, at 3, 6, 9, and 12 months after transplantation, every 6 months thereafter up to 48 months, and then at yearly intervals. Neurological assessment included EDSS score and MRI examinations. QoL was assessed by the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) questionnaire and the Functional Assessment of Multiple Sclerosis (FAMS) questionnaire. The FACT-BMT is a self-administered instrument designed to assess multidimensional aspects of QoL in BMT patients [20]. It consists of the 27-item FACT-General and the 23-item Bone Marrow Transplantation Subscale (BMTS). The FAMS is a disease-specific questionnaire for QoL assessment in MS patients [21]. It consists of 58 questions and contains 7 scales: mobility, symptoms, emotional well-being, general contentment, thinking and fatigue, family/social well-being, and additional concerns.
Definition of response to treatment
According to the EBMT criteria of response, patients with either steady EDSS scores representing a halt of disease progression, or with improved EDSS scores representing subsidence of inflammation in the CNS were regarded as responding to treatment [4,8]. Clinical improvement was defined as a ≥0.5 point decrease in EDSS score as compared to the baseline. Progression was defined as an increase of at least 0.5 points. Both had to be confirmed after 6 months. Clinical relapse was defined as the appearance of new symptoms or worsening of old symptoms of at least 24-hour duration, in the absence of fever in a previously (4 weeks) stable patient.
QoL was assessed by calculating the Integral QoL Index (IQLI) value at different time points on the basis of FACT-BMT questionnaire scores, as described previously [14]. Less than 25% improvement in IQLI compared to the baseline value was considered a minimal QoL response; 25–50% improvement a moderate QoL response; 51–75% improvement a good QoL response; and more than 75% improvement a maximal QoL response.
Results
Adverse events
No toxic deaths were reported among the 109 MS patients , irrespective of their clinical condition at the time of transplant. The transplantation procedure was well tolerated by the patients. Mobilization was successful in all cases, with a median number of 2.1 x106/kg (range 1.5–5.5 x106/kg) collected CD34+ cells. and no major clinical adverse events were observed during this phase. Unmanipulated grafts were infused without complications. Engraftment was uneventful, and no signs of an engraftment syndrome were reported. Median days with PMN< 0.5x109 and Plt < 50x109 were 8 (range from 5 to 11) and 10 days (from 2 to 26), respectively.
Common adverse effects following the immunoablative regimen were thrombocytopenia (100%), neutropenia (100%), fatigue (100%), anemia (80%), alopecia (80%), neutropenic fever (51.6%), hepatic toxicity grade I and II (48.1%), transient neurological dysfunction (22.2%), enteropathy (18.5%). Documented sepsis was registered in one patient.
Clinical outcomes
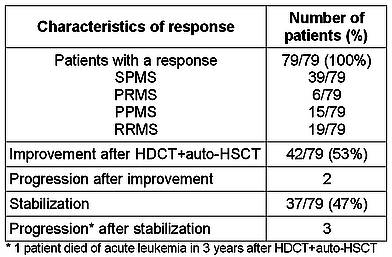
Seventy-nine patients with the follow-up period of at least 9 months or longer were included in the clinical outcome analysis (Table 3).
Table 3. Efficacy of HDCT+auto-HSCT in MS patients
All patients responded to the treatment. At 6 months post-transplant the following distribution of patients according to clinical response was observed: 42 patients (53%) achieved an objective improvement of neurological symptoms (defined as a ≥0.5 point decrease in the EDSS score as compared to the baseline and confirmed over 3 months): 20 SPMS; 11 RRMS; 4 PRMS, and 7 PPMS. Thirty-seven patients (47%) had disease stabilization (steady EDSS level as compared to the baseline and confirmed over 3 months): 19 SPMS; 8 RRMS; 2 PRMS, and 8 PPMS. Among the patients with improvement there were 25 patients after conventional HDCT+auto-HSCT, 15 after early HDCT+auto-HSCT, and 2 after salvage HDCT+auto-HSCT. Among the patients with stabilization there were 23 patients after conventional HDCT+auto-HSCT, 9 after early HDCT+auto-HSCT, and 5 after salvage HDCT+auto-HSCT. At long-term follow-up, the clinical response in 40 patients (50.6%) was classified as an improvement; 34 patients (43.1%) remained stable. Two patients deteriorated to a worse score after 18 months of stabilization (SPMS and PPMS; conventional auto-HSCT), and one patient after 6 months of stabilization (SPMS, conventional auto-HSCT); 2 others progressed after 12 and 30 months of improvement (RRMS, early auto-HSCT and SPMM, conventional auto-HSCT, respectively). No active, new or enlarging lesions were registered in patients without disease progression.
Remarkably, nine patients improved dramatically (≥1.5 point by EDSS). Patients with different types of MS were observed in this group. As an illustration, in a SPMS patient with the baseline EDSS value of 6.0 we observed a 2.0 point decrease on the EDSS scale at 1 month post-transplant, an additional 1.5 point decrease at 6 months and stabilization with EDSS score of 1.5 at 18 months post-transplant. In another case, a RRMS patient with a base-line EDSS score of 4.5 experienced a decrease in EDSS to 2.0 at 1 month post-transplant with a further decrease to 1.0 at 3 months. The latter EDSS level remained stable throughout the entire follow-up period of 1.5 years. The PRMS patient with baseline EDSS value of 6.0 improved at 3 months to EDSS of 4.5, and then showed further improvement at 30 months post-transplant to the EDSS score of 4.0. The EDSS score at the end of follow-up (6.5 years post-transplant) was 3.5. Finally, the PPMS patient with severe disease (EDSS score of 7.5) had a 1.5-point EDSS decrease and maintained this score during the 3.5 years of follow-up.
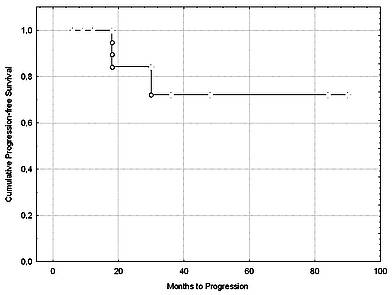
The progression-free survival at 6 years after HDCT+auto-HSCT was 72% (Figure 1). Remarkably, all patients who did not have disease progression were off therapy throughout the post-transplant period.
Figure 1. Progression-free survival after HDCT+auto-HSCT. Probability of progression-free survival in 42 MS patients.
Estimated progression-free survival is 72% at 6 years.
QoL outcomes
QoL monitoring and assessment of QoL response were performed in 44 patients. Forty patients exhibited improved QoL at 6 months post-transplant. An increase in QoL parameters was observed according to both FACT-BMT and FAMS questionnaires. Notably, the patient who progressed 18 months after transplantation (SPMS) exhibited no QoL response. Despite clinical disease stabilization, his QoL gradually deteriorated.
In another case, a QoL deterioration was observed in a PPMS patient at 6 months post-transplant in spite of clinical stabilization during the 18 months after transplantation. It is worth mentioning that the patient (RRMS) who experienced relapse at 2.5 years post-transplant experienced a significant QoL decrease 2 years after transplantation.
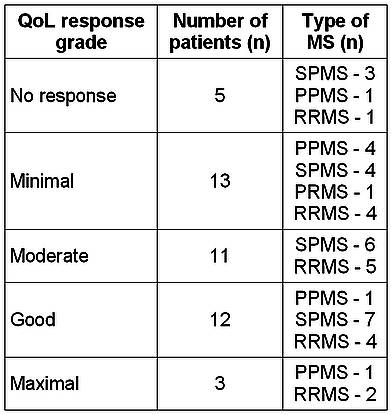
The distribution of patients according to the grades of QoL response one year after HDCT+auto-HSCT is presented in Table 4.
Table 4. Distribution of MS patients according to the grades of QoL response at 1 year after HDCT+auto-HSCT(n=44). As is seen in the table, 3 patients exhibited a maximal QoL response, 12 patients a good QoL response, 11 patients a moderate QoL response, 13 patients a minimal QoL response, and 5 patients no QoL response. Remarkably, patients with a longer follow-up experienced further QoL improvement.
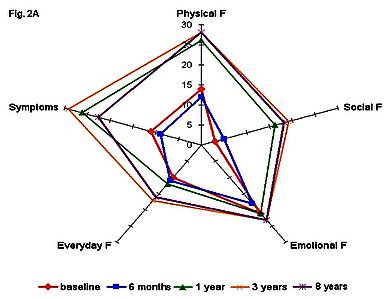
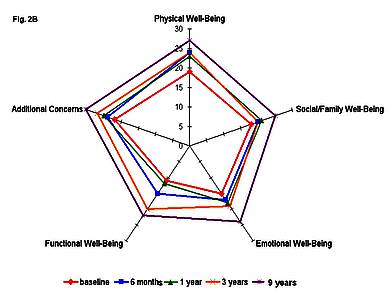
Figure 2 demonstrates the QoL profiles of two MS patients (patient A, a 21-year-old female, PRMS, base-line EDSS 6.0; patient B, a 35-year-old female, SPMS, base-line EDSS 5.0) with the long-term follow-up after HDCT+auto-HSCT.
Figure 2. Quality of life profiles of patient A(a) and patient B (b) at different time points after HDCT+auto-HSCT.
In both patients the QoL parameters improved dramatically at 1 year after HDCT+auto-HSCT.
Improved QoL profiles were preserved throughout the 8-year QoL monitoring of patient A, and the 9-year QoL monitoring of patient B.
Of special interest is the dynamics of QoL profiles of patient A (Figure 2a).
Six months after transplantation an improvement was observed in this patient, which led to the formation of a significantly less compressed and less deformed QoL profile as compared to the baseline.
Further QoL improvement took place at different time points during the 8-year follow-up.
Discussion
Since 1995, HDCT+auto-HSCT has been performed in more than 600 MS patients all over the world. Published clinical results demonstrate that this approach can stop the disease progression in a majority of patients. A comprehensive analysis of the EBMT registry data of 85 patients from 20 centers published by EBMT ADWP in 2002, showed no disease progression for 3 years in 74% of patients [4]. Similar results were obtained in five US studies that included 66 patients in total [6,11]. Remarkably, transplant-related mortality in MS patients does not exceed transplant-related mortality in hematological patients (0–4%).
The results our study have also demonstrated the benefits of HDCT+auto-HSCT in MS. We have included 109 patients with various types of MS from 6 centers affiliated with the Russian Cooperative Group for Cellular Therapy. The transplantation procedure was well tolerated by patients with no transplant-related deaths at all. The efficacy analysis was performed in 79 patients monitored for more than 1 year. All the patients responded to treatment: in 42 patients the EDSS score decreased after HDCT+auto-HSCT as compared to the base-line and was confirmed over 6 months, while disease stabilization (stable EDSS after transplantation confirmed over 6 months) was registered in 37 patients. The majority of patients had either moderate or good QoL responses as well. This data strongly supports the use of HDCT+auto-HSCT as the therapy of choice in autoimmune diseases with imminent patient debilitation, such as MS.
It is worth mentioning that the issues surrounding the patient selection criteria for HDCT+auto-HSCT are still unclear. The advantage of our study is that we included patients with different types of MS. In spite of some evidence that PPMS patients are less responsive to HDCT+auto-HSCT as compared to both SPMS and RRMS [8], the information about the outcomes of HSCT in patients with various types of MS is limited. The results of our study confirm that transplantation is effective in PPMS patients, and patients with different types of MS might benefit from HDCT+auto-HSCT.
Another advantage of our study is the performance of early, conventional or salvage transplantation, while most patients in the previous studies had late stages of MS.
Our data supports the idea that HDCT+auto-HSCT is more effective in young patients with early stages of rapidly progressing disease. In these patients, autoreactive T cells play a pivotal role in MS pathogenesis. HDCT ablates the patient's immune system and eradicates autoimmune T cells. It is followed by HSCT to restore the immune system, which is expected to become tolerant to autoantigens. Such "resetting" of the immune system is only effective at early stages of MS, particularly in relapsing-remitting MS. Later in the clinical course of the disease, processes of axonal degeneration prevail, and the damage to CNS tissue is too significant to expect a neurological recovery after HDCT+auto-HSCT. Indeed, the failure of HDCT+auto-HSCT to prevent progression of the disease when performed in the late stages has been demonstrated in both animal models [22] and in clinical studies [1,11]. Considering the clinical heterogeneity of MS patients, we propose a classification of transplantation approaches based on the concept of HDCT+auto-HSCT in MS (Table 5). The concept focuses on the goals of MS treatment. There are two goals in the treatment of MS patients. The first is pathogenetic, which is to stop the disease progression and prevent the appearance of new lesions in the nervous tissue. The second is to improve or maintain a patient’s QoL. Since MS is incurable and does not shorten the patient’s life span, the QoL improvement should be considered the ultimate goal of MS treatment. Therefore, QoL assessment is the key criterion for the assessment of efficacy of MS treatment in addition to traditional diagnostic tests, which describe the dynamics of the immunopathological process. It is of special importance in patients with late stages of MS.
Table 5. Classification of HDCT+auto-HSCT in MS patients
In conclusion, our study has demonstrated that HDCT+auto-HSCT may be an effective treatment for various types of MS in terms of clinical and patient-reported outcomes at long-term follow-up. The data obtained points to the feasibility of early, conventional, and salvage HDCT+auto-HSCT in MS patients. Further studies should be done to investigate clinical and QoL response in MS patients receiving early, conventional, and salvage transplantation to better define treatment success. The concept of HDCT+auto-HSCT opens a new window of opportunities for MS treatment.
Acknowledgements
We would like to acknowledge Sergei V. Shamanski (Moscow), Andrei D. Kulagin (Novosibirsk), Nikolay I. Baziy (Moscow), Nina E. Osipova (St.Petersburg), Andrei E. Zdorov (Petrozavodsk), and Anton V. Kishtovich (St.Petersburg) for their contribution to the study.
References
1. Burt RK, Cohen B, Lobck L, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: importance of disease stage on outcome. Neurology. 2003;40 Suppl:A150.
2. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938 -952.
3. Burt RK, Cohen B, Rose J, et al. Hematopoietic stem cell transplantation for multiple sclerosis. Arch Neurol. 2005;62:860-864.
4. Fassas A, Anagnostopoulos A, Kazis A, et al. Autologous stem cell transplantation in progressive multiple sclerosis – an interim analysis of efficacy. J Clin Immunol. 2000;20(1):24-30.
5. Brenner MK. Haematopoietic stem cell transplantation for autoimmune disease: limits and future potential. Best Pract Res Clin Haematol. 2004;17:359-374.
6. Burt RK, Cohen BA, Russell E, et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis; failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102:2373-2378.
7. Fassas A, Nash R. Multiple sclerosis. Best Pract Res Clin Hematol. 2004;17:247-262.
8. Fassas A, Passweg JR, Anagnostopoulos A, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol. 2002;249:1088-1097.
9. Kozak T, Havrdova E, Pit’ha J, et al. High-dose immunosuppressive therapy with PBPC support in the treatment of poor risk multiple sclerosis. Bone Marrow Transplant. 2000;25:525-531.
10. Muraro PA, McFarland HF, Martin R. Immunological aspects of multiple sclerosis with emphasis on the potential use of autologous hemopoietic stem cell transplantation. In: Burt RK, Marmont AM, eds. Stem Cell Therapy for Autoimmune Disease. Georgetown, TX: Landes Bioscience. 2004;277-283.
11. Nash RA, Bowen JD, McSweeney PA, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102:2364-2372.
12. Openshaw H, Lund B, Kashyap A, et al. Peripheral blood stem cell transplantation in multiple sclerosis with busulfan and cyclophosphamide conditioning report of toxicity and immunological monitoring. Biology of Blood and Marrow Transplant. 2000;25:525-575.
13. Saccardi R, Mancardi GL, Solari A, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105:2601-2607.
14. Shevchenko Y, Novik A, Ionova T et al. Clinical and quality of life outcomes in patients with multiple sclerosis after high-dose chemotherapy + autologous stem cell transplantation [abstract no. 1875]. Blood. 2004;104:519a.
15. Shevchenko Y, Novik A, Kuznetsov A et al High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Experimental Hematology. 2008;36(8):922-929.
16. Novik A, Ionova T, Bisaga G, et.al. Clinical and Quality of Life Responses to High-Dose Chemotherapy plus Autologous Stem Cell Transplantation in Patients with Multiple Sclerosis: two case reports. Cytotherapy. 2005;7(4):363–367.
17. Kurtzke JF. Rating neurologic impairment in multiple sclerosis; an expanded disability status scale (EDSS). Neurology. 1983;33:1444-52.
18. Shevchenko YL, Novik AA, Ionova TI, et al. Three strategies of high dose chemotherapy + autologous stem cell transplantation in autoimmune diseases. Bone Marrow Transplant. 2004;33 Suppl 1: 346.
19. Tindall A, Gratwohl A. Blood and marrow stem cell transplants in autoimmune disease: A consensus report written on behalf of the European League against Rheumatism (EULAR) and the European Group for Blood and Marrow transplantation (EBMT). Bone Marrow Transplant. 1997;19:643-645.
20. McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation; development of the functional assessment of cancer therapy-bone marrow transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357-368.
21. Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47:129-139.
22. Burt RK, Padilla J, Begolka WS, et al. Effect of disease stage on clinical outcome after syngeneic bone marrow transplantation for relapsing experimental autoimmune encephalomyelitis. Blood. 1998;91:2609-2616.
"
["~DETAIL_TEXT"]=>
string(40975) "
Introduction
Multiple sclerosis is a chronic inflammatory disorder of the central nervous system (CNS) caused by autoimmune reactivity of T cells towards CNS myelin components. MS progression inevitably leads to the loss of motor function, sensitive disturbances and cognitive impairment because of the immune-mediated demyelination and axon degeneration [1].
MS is one of the most common neurological disorders, which mainly affects young adults, and causes gradual decrease of their quality of life (QoL). The clinical course of the disease is very heterogeneous. However, it typically presents with a relapsing-remitting course (RRMS; 80% of patients), which is followed after 5–15 years in about 70% of patients by a so-called secondary progressive phase (SPMS) [2]. 10–20% of patients have a primary progressive course, which is characterized by a steady progression from the onset with or without any acute exacerbations (progressive relapsing MS or PRMS, and primary progressive MS or PPMS, respectively).
Conventional therapies do not provide satisfactory control of MS due to their inability to eradicate self-specific T cell clones. Recently, HDCT+auto-HSCT was proposed as a new and promising therapy for MS patients [3,4]. HDCT+auto-HSCT leads to the elimination of autoreactive T cells and, subsequently, to the restoration of a normal immune system.
Since 1995, several clinical studies have addressed the issue of feasibility and efficacy of HDCT+auto-HSCT in MS [3-15]. However, the information about long-term effects of HDCT+auto-HSCT in this patient population is scanty. In addition, the majority of patients included in the above-mentioned studies had SPMS, and were severely disabled with an average EDSS score of 6.5. Unfortunately, even complete suppression of autoimmune inflammation does not lead to a significant improvement of QoL in these patients. Therefore, the patient selection criteria for HDCT+auto-HSCT are still unclear and the proper selection of patients for transplantation remains the key issue.
Another important consideration is the selection of appropriate criteria for the assessment of treatment outcomes for MS patients. Both disease-free period and improvement of patient’s QoL are recognized as important outcome parameters. With this in mind, evaluation of both clinical and patient-reported outcomes in MS patients after HDCT+auto-HSCT is worthwhile. However, neurologists traditionally evaluate the clinical response only and rarely use QoL data in the outcome analysis. This may be partly explained by the fact that QoL questionnaires used for MS patients – both generic and specific – are multidimensional, and the interpretation of changes in several QoL scales/domains might be difficult for physicians. Recently, we have developed an approach to obtain an Integral QoL Index (IQLI) for profile questionnaires (both generic and specific). IQLI is a standardized value based on the properties of a geometric profile formed by the scales of a questionnaire, which is assessed by the method of integral profiles; the index has been validated in different patients’ populations [16]. The advantages of IQLI are its ease of use and the possibility of obtaining one index based on several QoL scales. The use of IQLI makes it possible to overcome the difficulties in the interpretation of QoL data and allows the assessment of patient-reported outcomes, namely the QoL response.
To date, some limited information exists on the clinical response of MS patients to HDCT+auto-SCT at long-term follow-up, whereas the data on QoL response is lacking. In addition, the clinical experience in the application of HDCT+auto-HSCT to various types and stages of MS is very limited. Moreover, the timing for transplantation is still unclear.
We report the follow-up results of a prospective Phase II multicenter trial, which was started in 1999 and has since then been conducted by the Russian Cooperative Group for Cellular Therapy. This study is focused on the efficacy of HDCT+auto-HSCT in terms of clinical and quality of life responses in patients with different types and stages of MS.
Patients and Methods
One hundred and nine patients were enrolled in the study. Patient characteristics are shown in Table 1.

Table 1. Demographic and clinical profile of the patient population.
All patients were refractory to conventional therapy, which included IFNβ and mitoxantrone, as well as steroids, azathiopine, intravenous immunoglobulin and plasmapheresis in some patients. The mean follow-up was 19 months (range, 6–108 months).
The trial was conducted according to the principles of the Helsinki Declaration, and approved by the IRB and Ethics Committees of all of the participating centers before initiation. All patients gave written informed consent.
The neurological disability of MS patients is quantified according to the Expanded disability status scale (EDSS) [17]. The EDSS scores range from 0 (no disability) to 10 (death related to neurological progression) in 0.5-step increments. EDSS scores from 1.0 to 4.5 refer to the fully ambulatory MS patients, while patients with EDSS scores of 7.0 are essentially restricted to a wheelchair.
Patient Eligibility
Criteria for patient selection were: age between 18 and 55 years; diagnosis of multiple sclerosis verified by clinical and laboratory findings; EDSS score 1.5–8.0; normal mental status; absence of severe concomitant diseases.
The disease activity was determined either by magnetic resonance imaging scans displaying active lesions in the CNS (i.e., gadolinium-enhancing lesions, new or enlarging lesions on serial scans) or by clinical assessment showing rapid neurological deterioration, e.g., 0.5-point increase on the EDSS during the 6-months preceding enrollment.
According to our concept there are 3 strategies of HDCT+auto-HSCT [18]. Early HSCT (in MS patients with EDSS 1.5–3.0) is performed soon after diagnosis in case of primary refractory disease or poor prognosis. Conventional HSCT (EDSS 3.5–6.5) is performed in patients with secondary refractory disease. Salvage HSCT (EDSS 7.0–8.0) is an option in case of high disease activity and rapid neurological deterioration in late stages of the disease. All three strategies were applied in this study (Table 2).
Table 2. HSCT timing in the studied patient population
Stem Cell Mobilization and Transplant Procedure
Hematopoietic stem cells were mobilized with G-CSF at 10 μg/kg +/- cyclophosphamide at 4 g/m2 according to EBMT/EULAR guidelines [19]. The grafts were not manipulated. BEAM or BEAM-modified conditioning was used. The BEAM conditioning regimen included BCNU (300 mg/m2) on day -6, etoposide (200 mg/m2) from day -5 to day -2, cytarabine (200 mg/m2 bd) from day -5 to day -2 and melphalan (140 mg/m2) on day -1. It was followed by autologous hematopoietic stem cell transplantation (day 0). In vivo T cell-depletion was achieved through infusion of 30 mg/kg of horse anti-thymocyte globulin (ATG) on days 1 and 2. Five μg/kg s.c. of G-CSF were administered from day 3 post-infusion until granulocyte recovery. For infection prophylaxis oral ciprofloxacin, fluconazole, acyclovir, and IV human Ig were given.
Neurological and QoL assessments
Clinical and QoL assessments were performed at baseline, at discharge, at 3, 6, 9, and 12 months after transplantation, every 6 months thereafter up to 48 months, and then at yearly intervals. Neurological assessment included EDSS score and MRI examinations. QoL was assessed by the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) questionnaire and the Functional Assessment of Multiple Sclerosis (FAMS) questionnaire. The FACT-BMT is a self-administered instrument designed to assess multidimensional aspects of QoL in BMT patients [20]. It consists of the 27-item FACT-General and the 23-item Bone Marrow Transplantation Subscale (BMTS). The FAMS is a disease-specific questionnaire for QoL assessment in MS patients [21]. It consists of 58 questions and contains 7 scales: mobility, symptoms, emotional well-being, general contentment, thinking and fatigue, family/social well-being, and additional concerns.
Definition of response to treatment
According to the EBMT criteria of response, patients with either steady EDSS scores representing a halt of disease progression, or with improved EDSS scores representing subsidence of inflammation in the CNS were regarded as responding to treatment [4,8]. Clinical improvement was defined as a ≥0.5 point decrease in EDSS score as compared to the baseline. Progression was defined as an increase of at least 0.5 points. Both had to be confirmed after 6 months. Clinical relapse was defined as the appearance of new symptoms or worsening of old symptoms of at least 24-hour duration, in the absence of fever in a previously (4 weeks) stable patient.
QoL was assessed by calculating the Integral QoL Index (IQLI) value at different time points on the basis of FACT-BMT questionnaire scores, as described previously [14]. Less than 25% improvement in IQLI compared to the baseline value was considered a minimal QoL response; 25–50% improvement a moderate QoL response; 51–75% improvement a good QoL response; and more than 75% improvement a maximal QoL response.
Results
Adverse events
No toxic deaths were reported among the 109 MS patients , irrespective of their clinical condition at the time of transplant. The transplantation procedure was well tolerated by the patients. Mobilization was successful in all cases, with a median number of 2.1 x106/kg (range 1.5–5.5 x106/kg) collected CD34+ cells. and no major clinical adverse events were observed during this phase. Unmanipulated grafts were infused without complications. Engraftment was uneventful, and no signs of an engraftment syndrome were reported. Median days with PMN< 0.5x109 and Plt < 50x109 were 8 (range from 5 to 11) and 10 days (from 2 to 26), respectively.
Common adverse effects following the immunoablative regimen were thrombocytopenia (100%), neutropenia (100%), fatigue (100%), anemia (80%), alopecia (80%), neutropenic fever (51.6%), hepatic toxicity grade I and II (48.1%), transient neurological dysfunction (22.2%), enteropathy (18.5%). Documented sepsis was registered in one patient.
Clinical outcomes
Seventy-nine patients with the follow-up period of at least 9 months or longer were included in the clinical outcome analysis (Table 3).
Table 3. Efficacy of HDCT+auto-HSCT in MS patients
All patients responded to the treatment. At 6 months post-transplant the following distribution of patients according to clinical response was observed: 42 patients (53%) achieved an objective improvement of neurological symptoms (defined as a ≥0.5 point decrease in the EDSS score as compared to the baseline and confirmed over 3 months): 20 SPMS; 11 RRMS; 4 PRMS, and 7 PPMS. Thirty-seven patients (47%) had disease stabilization (steady EDSS level as compared to the baseline and confirmed over 3 months): 19 SPMS; 8 RRMS; 2 PRMS, and 8 PPMS. Among the patients with improvement there were 25 patients after conventional HDCT+auto-HSCT, 15 after early HDCT+auto-HSCT, and 2 after salvage HDCT+auto-HSCT. Among the patients with stabilization there were 23 patients after conventional HDCT+auto-HSCT, 9 after early HDCT+auto-HSCT, and 5 after salvage HDCT+auto-HSCT. At long-term follow-up, the clinical response in 40 patients (50.6%) was classified as an improvement; 34 patients (43.1%) remained stable. Two patients deteriorated to a worse score after 18 months of stabilization (SPMS and PPMS; conventional auto-HSCT), and one patient after 6 months of stabilization (SPMS, conventional auto-HSCT); 2 others progressed after 12 and 30 months of improvement (RRMS, early auto-HSCT and SPMM, conventional auto-HSCT, respectively). No active, new or enlarging lesions were registered in patients without disease progression.
Remarkably, nine patients improved dramatically (≥1.5 point by EDSS). Patients with different types of MS were observed in this group. As an illustration, in a SPMS patient with the baseline EDSS value of 6.0 we observed a 2.0 point decrease on the EDSS scale at 1 month post-transplant, an additional 1.5 point decrease at 6 months and stabilization with EDSS score of 1.5 at 18 months post-transplant. In another case, a RRMS patient with a base-line EDSS score of 4.5 experienced a decrease in EDSS to 2.0 at 1 month post-transplant with a further decrease to 1.0 at 3 months. The latter EDSS level remained stable throughout the entire follow-up period of 1.5 years. The PRMS patient with baseline EDSS value of 6.0 improved at 3 months to EDSS of 4.5, and then showed further improvement at 30 months post-transplant to the EDSS score of 4.0. The EDSS score at the end of follow-up (6.5 years post-transplant) was 3.5. Finally, the PPMS patient with severe disease (EDSS score of 7.5) had a 1.5-point EDSS decrease and maintained this score during the 3.5 years of follow-up.
The progression-free survival at 6 years after HDCT+auto-HSCT was 72% (Figure 1). Remarkably, all patients who did not have disease progression were off therapy throughout the post-transplant period.
Figure 1. Progression-free survival after HDCT+auto-HSCT. Probability of progression-free survival in 42 MS patients.
Estimated progression-free survival is 72% at 6 years.
QoL outcomes
QoL monitoring and assessment of QoL response were performed in 44 patients. Forty patients exhibited improved QoL at 6 months post-transplant. An increase in QoL parameters was observed according to both FACT-BMT and FAMS questionnaires. Notably, the patient who progressed 18 months after transplantation (SPMS) exhibited no QoL response. Despite clinical disease stabilization, his QoL gradually deteriorated.
In another case, a QoL deterioration was observed in a PPMS patient at 6 months post-transplant in spite of clinical stabilization during the 18 months after transplantation. It is worth mentioning that the patient (RRMS) who experienced relapse at 2.5 years post-transplant experienced a significant QoL decrease 2 years after transplantation.
The distribution of patients according to the grades of QoL response one year after HDCT+auto-HSCT is presented in Table 4.
Table 4. Distribution of MS patients according to the grades of QoL response at 1 year after HDCT+auto-HSCT(n=44). As is seen in the table, 3 patients exhibited a maximal QoL response, 12 patients a good QoL response, 11 patients a moderate QoL response, 13 patients a minimal QoL response, and 5 patients no QoL response. Remarkably, patients with a longer follow-up experienced further QoL improvement.
Figure 2 demonstrates the QoL profiles of two MS patients (patient A, a 21-year-old female, PRMS, base-line EDSS 6.0; patient B, a 35-year-old female, SPMS, base-line EDSS 5.0) with the long-term follow-up after HDCT+auto-HSCT.
Figure 2. Quality of life profiles of patient A(a) and patient B (b) at different time points after HDCT+auto-HSCT.
In both patients the QoL parameters improved dramatically at 1 year after HDCT+auto-HSCT.
Improved QoL profiles were preserved throughout the 8-year QoL monitoring of patient A, and the 9-year QoL monitoring of patient B.
Of special interest is the dynamics of QoL profiles of patient A (Figure 2a).
Six months after transplantation an improvement was observed in this patient, which led to the formation of a significantly less compressed and less deformed QoL profile as compared to the baseline.
Further QoL improvement took place at different time points during the 8-year follow-up.
Discussion
Since 1995, HDCT+auto-HSCT has been performed in more than 600 MS patients all over the world. Published clinical results demonstrate that this approach can stop the disease progression in a majority of patients. A comprehensive analysis of the EBMT registry data of 85 patients from 20 centers published by EBMT ADWP in 2002, showed no disease progression for 3 years in 74% of patients [4]. Similar results were obtained in five US studies that included 66 patients in total [6,11]. Remarkably, transplant-related mortality in MS patients does not exceed transplant-related mortality in hematological patients (0–4%).
The results our study have also demonstrated the benefits of HDCT+auto-HSCT in MS. We have included 109 patients with various types of MS from 6 centers affiliated with the Russian Cooperative Group for Cellular Therapy. The transplantation procedure was well tolerated by patients with no transplant-related deaths at all. The efficacy analysis was performed in 79 patients monitored for more than 1 year. All the patients responded to treatment: in 42 patients the EDSS score decreased after HDCT+auto-HSCT as compared to the base-line and was confirmed over 6 months, while disease stabilization (stable EDSS after transplantation confirmed over 6 months) was registered in 37 patients. The majority of patients had either moderate or good QoL responses as well. This data strongly supports the use of HDCT+auto-HSCT as the therapy of choice in autoimmune diseases with imminent patient debilitation, such as MS.
It is worth mentioning that the issues surrounding the patient selection criteria for HDCT+auto-HSCT are still unclear. The advantage of our study is that we included patients with different types of MS. In spite of some evidence that PPMS patients are less responsive to HDCT+auto-HSCT as compared to both SPMS and RRMS [8], the information about the outcomes of HSCT in patients with various types of MS is limited. The results of our study confirm that transplantation is effective in PPMS patients, and patients with different types of MS might benefit from HDCT+auto-HSCT.
Another advantage of our study is the performance of early, conventional or salvage transplantation, while most patients in the previous studies had late stages of MS.
Our data supports the idea that HDCT+auto-HSCT is more effective in young patients with early stages of rapidly progressing disease. In these patients, autoreactive T cells play a pivotal role in MS pathogenesis. HDCT ablates the patient's immune system and eradicates autoimmune T cells. It is followed by HSCT to restore the immune system, which is expected to become tolerant to autoantigens. Such "resetting" of the immune system is only effective at early stages of MS, particularly in relapsing-remitting MS. Later in the clinical course of the disease, processes of axonal degeneration prevail, and the damage to CNS tissue is too significant to expect a neurological recovery after HDCT+auto-HSCT. Indeed, the failure of HDCT+auto-HSCT to prevent progression of the disease when performed in the late stages has been demonstrated in both animal models [22] and in clinical studies [1,11]. Considering the clinical heterogeneity of MS patients, we propose a classification of transplantation approaches based on the concept of HDCT+auto-HSCT in MS (Table 5). The concept focuses on the goals of MS treatment. There are two goals in the treatment of MS patients. The first is pathogenetic, which is to stop the disease progression and prevent the appearance of new lesions in the nervous tissue. The second is to improve or maintain a patient’s QoL. Since MS is incurable and does not shorten the patient’s life span, the QoL improvement should be considered the ultimate goal of MS treatment. Therefore, QoL assessment is the key criterion for the assessment of efficacy of MS treatment in addition to traditional diagnostic tests, which describe the dynamics of the immunopathological process. It is of special importance in patients with late stages of MS.
Table 5. Classification of HDCT+auto-HSCT in MS patients
In conclusion, our study has demonstrated that HDCT+auto-HSCT may be an effective treatment for various types of MS in terms of clinical and patient-reported outcomes at long-term follow-up. The data obtained points to the feasibility of early, conventional, and salvage HDCT+auto-HSCT in MS patients. Further studies should be done to investigate clinical and QoL response in MS patients receiving early, conventional, and salvage transplantation to better define treatment success. The concept of HDCT+auto-HSCT opens a new window of opportunities for MS treatment.
Acknowledgements
We would like to acknowledge Sergei V. Shamanski (Moscow), Andrei D. Kulagin (Novosibirsk), Nikolay I. Baziy (Moscow), Nina E. Osipova (St.Petersburg), Andrei E. Zdorov (Petrozavodsk), and Anton V. Kishtovich (St.Petersburg) for their contribution to the study.
References
1. Burt RK, Cohen B, Lobck L, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: importance of disease stage on outcome. Neurology. 2003;40 Suppl:A150.
2. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938 -952.
3. Burt RK, Cohen B, Rose J, et al. Hematopoietic stem cell transplantation for multiple sclerosis. Arch Neurol. 2005;62:860-864.
4. Fassas A, Anagnostopoulos A, Kazis A, et al. Autologous stem cell transplantation in progressive multiple sclerosis – an interim analysis of efficacy. J Clin Immunol. 2000;20(1):24-30.
5. Brenner MK. Haematopoietic stem cell transplantation for autoimmune disease: limits and future potential. Best Pract Res Clin Haematol. 2004;17:359-374.
6. Burt RK, Cohen BA, Russell E, et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis; failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102:2373-2378.
7. Fassas A, Nash R. Multiple sclerosis. Best Pract Res Clin Hematol. 2004;17:247-262.
8. Fassas A, Passweg JR, Anagnostopoulos A, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol. 2002;249:1088-1097.
9. Kozak T, Havrdova E, Pit’ha J, et al. High-dose immunosuppressive therapy with PBPC support in the treatment of poor risk multiple sclerosis. Bone Marrow Transplant. 2000;25:525-531.
10. Muraro PA, McFarland HF, Martin R. Immunological aspects of multiple sclerosis with emphasis on the potential use of autologous hemopoietic stem cell transplantation. In: Burt RK, Marmont AM, eds. Stem Cell Therapy for Autoimmune Disease. Georgetown, TX: Landes Bioscience. 2004;277-283.
11. Nash RA, Bowen JD, McSweeney PA, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102:2364-2372.
12. Openshaw H, Lund B, Kashyap A, et al. Peripheral blood stem cell transplantation in multiple sclerosis with busulfan and cyclophosphamide conditioning report of toxicity and immunological monitoring. Biology of Blood and Marrow Transplant. 2000;25:525-575.
13. Saccardi R, Mancardi GL, Solari A, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105:2601-2607.
14. Shevchenko Y, Novik A, Ionova T et al. Clinical and quality of life outcomes in patients with multiple sclerosis after high-dose chemotherapy + autologous stem cell transplantation [abstract no. 1875]. Blood. 2004;104:519a.
15. Shevchenko Y, Novik A, Kuznetsov A et al High-dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation as a treatment option in multiple sclerosis. Experimental Hematology. 2008;36(8):922-929.
16. Novik A, Ionova T, Bisaga G, et.al. Clinical and Quality of Life Responses to High-Dose Chemotherapy plus Autologous Stem Cell Transplantation in Patients with Multiple Sclerosis: two case reports. Cytotherapy. 2005;7(4):363–367.
17. Kurtzke JF. Rating neurologic impairment in multiple sclerosis; an expanded disability status scale (EDSS). Neurology. 1983;33:1444-52.
18. Shevchenko YL, Novik AA, Ionova TI, et al. Three strategies of high dose chemotherapy + autologous stem cell transplantation in autoimmune diseases. Bone Marrow Transplant. 2004;33 Suppl 1: 346.
19. Tindall A, Gratwohl A. Blood and marrow stem cell transplants in autoimmune disease: A consensus report written on behalf of the European League against Rheumatism (EULAR) and the European Group for Blood and Marrow transplantation (EBMT). Bone Marrow Transplant. 1997;19:643-645.
20. McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation; development of the functional assessment of cancer therapy-bone marrow transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357-368.
21. Cella DF, Dineen K, Arnason B, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47:129-139.
22. Burt RK, Padilla J, Begolka WS, et al. Effect of disease stage on clinical outcome after syngeneic bone marrow transplantation for relapsing experimental autoimmune encephalomyelitis. Blood. 1998;91:2609-2616.
"
["DETAIL_TEXT_TYPE"]=>
string(4) "html"
["~DETAIL_TEXT_TYPE"]=>
string(4) "html"
["PREVIEW_TEXT"]=>
string(0) ""
["~PREVIEW_TEXT"]=>
string(0) ""
["PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["~PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["PREVIEW_PICTURE"]=>
NULL
["~PREVIEW_PICTURE"]=>
NULL
["LANG_DIR"]=>
string(4) "/ru/"
["~LANG_DIR"]=>
string(4) "/ru/"
["SORT"]=>
string(3) "500"
["~SORT"]=>
string(3) "500"
["CODE"]=>
string(73) "transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze"
["~CODE"]=>
string(73) "transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze"
["EXTERNAL_ID"]=>
string(3) "458"
["~EXTERNAL_ID"]=>
string(3) "458"
["IBLOCK_TYPE_ID"]=>
string(7) "journal"
["~IBLOCK_TYPE_ID"]=>
string(7) "journal"
["IBLOCK_CODE"]=>
string(7) "volumes"
["~IBLOCK_CODE"]=>
string(7) "volumes"
["IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["~IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["LID"]=>
string(2) "s2"
["~LID"]=>
string(2) "s2"
["EDIT_LINK"]=>
NULL
["DELETE_LINK"]=>
NULL
["DISPLAY_ACTIVE_FROM"]=>
string(0) ""
["IPROPERTY_VALUES"]=>
array(18) {
["ELEMENT_META_TITLE"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["ELEMENT_META_KEYWORDS"]=>
string(281) "трансплантация стволовых кроветворных клеток рассеянный склероз качество жизни ранняя трансплантация этапная трансплантация трансплантация спасения"
["ELEMENT_META_DESCRIPTION"]=>
string(202) "Трансплантация кроветворных стволовых клеток при рассеянном склерозеAutologous hematopoietic stem cell transplantation in multiple sclerosis"
["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=>
string(4131) " <h3>Введение</h3>
<p>Рассеянный склероз (РС) – хроническое прогрессирующее заболевание центральной нервной системы, которое клинически проявляется мультисистемной неврологической симптоматикой, а патоморфологически характеризуется образованием множественных очагов демиелинизации в белом веществе головного и спинного мозга. Основным механизмом, приводящим к повреждению миелина, является опосредованная Т-лимфоцитами реакция гиперчувствительности замедленного типа, а непосредственными клетками-эффекторами иммунопатологического процесса – макрофаги.</p>
<p>Существующие методы лечения не позволяют достичь устойчивого терапевтического эффекта при рассеянном склерозе. Выдвигалась гипотеза, основанная на доклинических данных, о высокой эффективности аллогенной транплантации стволовых кроветворных клеток (ТСКК). Однако высокая посттрансплантационная летальность не позволила приступить к клиническим исследованиям данного вида терапии РС. По мнению большинства экспертов одним из наиболее перспективных методов лечения РС на сегодняшний день является высокодозная химиотерапия (ВДТ) с аутологичной трансплантацией стволовых кроветворных клеток (АуТСКК). Начиная с 1995 года, безопасность ВДТ+AyТКСК при РС была изучена в ряде клинических исследований. Тем не менее, объем информации о клинической эффективности данного метода и, особенно, о его влиянии на качество жизни больных РС, остается недостаточным. Кроме того, большинство пациентов, включенных в вышеупомянутые исследования, имели вторично-прогрессирующую форму РС и значительную степень инвалидизации со значением шкалы EDSS 4.5-8.5 баллов. К сожалению, даже полное прекращение активности иммунопатологического процесса у таких больных не может привести к значительному улучшению качества жизни. Поэтому вопрос об оптимальных сроках проведения трансплантации по-прежнему остается открытым.</p>
<p>В статье приведены результаты проспективного многоцентрового исследования безопасности и эффективности ВДТ+АуТКСК при РС, которое было начато в 1999 году и в настоящее время объединяет 5 крупных российских медицинских центров. Изучали влияние ВДТ+АуТКСК на клиническое течение и показатели качества жизни больных с разными формами и стадиями РС.</p>"
["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_META_TITLE"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_META_KEYWORDS"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_META_DESCRIPTION"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_PICTURE_FILE_ALT"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_PICTURE_FILE_TITLE"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_PICTURE_FILE_NAME"]=>
string(77) "transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze-img"
["SECTION_DETAIL_PICTURE_FILE_ALT"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_DETAIL_PICTURE_FILE_TITLE"]=>
string(130) "Трансплантация кроветворных стволовых клеток при рассеянном склерозе"
["SECTION_DETAIL_PICTURE_FILE_NAME"]=>
string(77) "transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze-img"
["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=>
string(77) "transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze-img"
["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=>
string(77) "transplantatsiya-krovetvornykh-stvolovykh-kletok-pri-rasseyannom-skleroze-img"
}
["FIELDS"]=>
array(1) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
}
["PROPERTIES"]=>
array(18) {
["KEYWORDS"]=>
array(36) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(6) {
[0]=>
string(5) "11238"
[1]=>
string(5) "11239"
[2]=>
string(5) "11240"
[3]=>
string(5) "11241"
[4]=>
string(5) "11242"
[5]=>
string(5) "11243"
}
["VALUE"]=>
array(6) {
[0]=>
string(3) "441"
[1]=>
string(3) "442"
[2]=>
string(3) "443"
[3]=>
string(3) "444"
[4]=>
string(3) "445"
[5]=>
string(3) "446"
}
["DESCRIPTION"]=>
array(6) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(6) {
[0]=>
string(3) "441"
[1]=>
string(3) "442"
[2]=>
string(3) "443"
[3]=>
string(3) "444"
[4]=>
string(3) "445"
[5]=>
string(3) "446"
}
["~DESCRIPTION"]=>
array(6) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["SUBMITTED"]=>
array(36) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3638"
["VALUE"]=>
string(19) "05.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "05.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
}
["ACCEPTED"]=>
array(36) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3639"
["VALUE"]=>
string(19) "17.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "17.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
}
["PUBLISHED"]=>
array(36) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3640"
["VALUE"]=>
string(19) "03.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "03.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
}
["CONTACT"]=>
array(36) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3641"
["VALUE"]=>
string(3) "448"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "448"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHORS"]=>
array(36) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(12) {
[0]=>
string(5) "11244"
[1]=>
string(5) "11245"
[2]=>
string(5) "11246"
[3]=>
string(5) "11247"
[4]=>
string(5) "11248"
[5]=>
string(5) "11249"
[6]=>
string(5) "11250"
[7]=>
string(5) "11251"
[8]=>
string(5) "11252"
[9]=>
string(5) "11253"
[10]=>
string(5) "11254"
[11]=>
string(5) "11255"
}
["VALUE"]=>
array(12) {
[0]=>
string(3) "447"
[1]=>
string(3) "448"
[2]=>
string(3) "449"
[3]=>
string(2) "34"
[4]=>
string(3) "450"
[5]=>
string(3) "451"
[6]=>
string(3) "452"
[7]=>
string(3) "453"
[8]=>
string(3) "454"
[9]=>
string(3) "455"
[10]=>
string(3) "456"
[11]=>
string(3) "457"
}
["DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(12) {
[0]=>
string(3) "447"
[1]=>
string(3) "448"
[2]=>
string(3) "449"
[3]=>
string(2) "34"
[4]=>
string(3) "450"
[5]=>
string(3) "451"
[6]=>
string(3) "452"
[7]=>
string(3) "453"
[8]=>
string(3) "454"
[9]=>
string(3) "455"
[10]=>
string(3) "456"
[11]=>
string(3) "457"
}
["~DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_RU"]=>
array(36) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3654"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(361) "<p class="Autor">
Ю. Л. Шевченко, А. А. Новик, А. Н. Кузнецов, Б. В. Афанасьев, И. А. Лисуков, O. А. Рукавицын, А. А. Мясников, <br> В. Я. Мельниченко, Д. А. Федоренко, T. И. Ионова, Р. А. Иванов, Г. Городокин
</p>"
["TYPE"]=>
string(4) "TEXT"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(333) "
Ю. Л. Шевченко, А. А. Новик, А. Н. Кузнецов, Б. В. Афанасьев, И. А. Лисуков, O. А. Рукавицын, А. А. Мясников,
В. Я. Мельниченко, Д. А. Федоренко, T. И. Ионова, Р. А. Иванов, Г. Городокин
"
["TYPE"]=>
string(4) "TEXT"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_RU"]=>
array(36) {
["ID"]=>
string(2) "26"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(22) "Организации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "26"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(22) "Организации"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_RU"]=>
array(36) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4024"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(4131) " <h3>Введение</h3>
<p>Рассеянный склероз (РС) – хроническое прогрессирующее заболевание центральной нервной системы, которое клинически проявляется мультисистемной неврологической симптоматикой, а патоморфологически характеризуется образованием множественных очагов демиелинизации в белом веществе головного и спинного мозга. Основным механизмом, приводящим к повреждению миелина, является опосредованная Т-лимфоцитами реакция гиперчувствительности замедленного типа, а непосредственными клетками-эффекторами иммунопатологического процесса – макрофаги.</p>
<p>Существующие методы лечения не позволяют достичь устойчивого терапевтического эффекта при рассеянном склерозе. Выдвигалась гипотеза, основанная на доклинических данных, о высокой эффективности аллогенной транплантации стволовых кроветворных клеток (ТСКК). Однако высокая посттрансплантационная летальность не позволила приступить к клиническим исследованиям данного вида терапии РС. По мнению большинства экспертов одним из наиболее перспективных методов лечения РС на сегодняшний день является высокодозная химиотерапия (ВДТ) с аутологичной трансплантацией стволовых кроветворных клеток (АуТСКК). Начиная с 1995 года, безопасность ВДТ+AyТКСК при РС была изучена в ряде клинических исследований. Тем не менее, объем информации о клинической эффективности данного метода и, особенно, о его влиянии на качество жизни больных РС, остается недостаточным. Кроме того, большинство пациентов, включенных в вышеупомянутые исследования, имели вторично-прогрессирующую форму РС и значительную степень инвалидизации со значением шкалы EDSS 4.5-8.5 баллов. К сожалению, даже полное прекращение активности иммунопатологического процесса у таких больных не может привести к значительному улучшению качества жизни. Поэтому вопрос об оптимальных сроках проведения трансплантации по-прежнему остается открытым.</p>
<p>В статье приведены результаты проспективного многоцентрового исследования безопасности и эффективности ВДТ+АуТКСК при РС, которое было начато в 1999 году и в настоящее время объединяет 5 крупных российских медицинских центров. Изучали влияние ВДТ+АуТКСК на клиническое течение и показатели качества жизни больных с разными формами и стадиями РС.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(4083) "
Введение
Рассеянный склероз (РС) – хроническое прогрессирующее заболевание центральной нервной системы, которое клинически проявляется мультисистемной неврологической симптоматикой, а патоморфологически характеризуется образованием множественных очагов демиелинизации в белом веществе головного и спинного мозга. Основным механизмом, приводящим к повреждению миелина, является опосредованная Т-лимфоцитами реакция гиперчувствительности замедленного типа, а непосредственными клетками-эффекторами иммунопатологического процесса – макрофаги.
Существующие методы лечения не позволяют достичь устойчивого терапевтического эффекта при рассеянном склерозе. Выдвигалась гипотеза, основанная на доклинических данных, о высокой эффективности аллогенной транплантации стволовых кроветворных клеток (ТСКК). Однако высокая посттрансплантационная летальность не позволила приступить к клиническим исследованиям данного вида терапии РС. По мнению большинства экспертов одним из наиболее перспективных методов лечения РС на сегодняшний день является высокодозная химиотерапия (ВДТ) с аутологичной трансплантацией стволовых кроветворных клеток (АуТСКК). Начиная с 1995 года, безопасность ВДТ+AyТКСК при РС была изучена в ряде клинических исследований. Тем не менее, объем информации о клинической эффективности данного метода и, особенно, о его влиянии на качество жизни больных РС, остается недостаточным. Кроме того, большинство пациентов, включенных в вышеупомянутые исследования, имели вторично-прогрессирующую форму РС и значительную степень инвалидизации со значением шкалы EDSS 4.5-8.5 баллов. К сожалению, даже полное прекращение активности иммунопатологического процесса у таких больных не может привести к значительному улучшению качества жизни. Поэтому вопрос об оптимальных сроках проведения трансплантации по-прежнему остается открытым.
В статье приведены результаты проспективного многоцентрового исследования безопасности и эффективности ВДТ+АуТКСК при РС, которое было начато в 1999 году и в настоящее время объединяет 5 крупных российских медицинских центров. Изучали влияние ВДТ+АуТКСК на клиническое течение и показатели качества жизни больных с разными формами и стадиями РС.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["DOI"]=>
array(36) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3655"
["VALUE"]=>
string(29) "10.3205/ctt-2008-ru-000025.02"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-ru-000025.02"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_EN"]=>
array(36) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3656"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(633) "<p class="Autor">Yury L. Shevchenko<sup>1</sup>, Andrei A. Novik<sup>1</sup>, Alexey N. Kuznetsov<sup>1</sup>, Boris V. Afanasiev<sup>2</sup>,
Igor A. Lisukov<sup>3</sup>, Oleg A. Rykavicin<sup>4</sup>, Аlexandr A. Myasnikov<sup>5</sup>, Vladimir Y. Melnichenko<sup>1</sup>, Denis A. Fedorenko<sup>1</sup>, Tatyana I. Ionova<sup>6</sup>, Roman A. Ivanov<sup>1</sup>, and Gary Gorodokin<sup>7</sup> on behalf of the Russian Cooperative Group for Cellular Therapy</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(467) "
Yury L. Shevchenko1, Andrei A. Novik1, Alexey N. Kuznetsov1, Boris V. Afanasiev2,
Igor A. Lisukov3, Oleg A. Rykavicin4, Аlexandr A. Myasnikov5, Vladimir Y. Melnichenko1, Denis A. Fedorenko1, Tatyana I. Ionova6, Roman A. Ivanov1, and Gary Gorodokin7 on behalf of the Russian Cooperative Group for Cellular Therapy
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_EN"]=>
array(36) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4116"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(707) "<p><sup>1</sup>Pirogov National Medical Surgical Center, Moscow, Russia;<br> <sup>2</sup>Pavlov State Medical University, St. Petersburg, Russia; <br><sup>3</sup>Institute of Clinical Immunology, Siberian Branch of Russian Academy of Science, Novosibirsk, Russia;<br><sup> 4</sup>Burdenko Central Military Hospital, Moscow, Russia; <br><sup>5</sup>Republic Hospital, Petrozavodsk, Russia;<br>
<sup>6</sup>Multinational Center of Quality of Life Research, St. Petersburg, Russia;<br>
<sup>7</sup>New Jersey Center for Quality of Life and Health Outcome Research, NJ, USA</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(575) "
1Pirogov National Medical Surgical Center, Moscow, Russia;
2Pavlov State Medical University, St. Petersburg, Russia;
3Institute of Clinical Immunology, Siberian Branch of Russian Academy of Science, Novosibirsk, Russia;
4Burdenko Central Military Hospital, Moscow, Russia;
5Republic Hospital, Petrozavodsk, Russia;
6Multinational Center of Quality of Life Research, St. Petersburg, Russia;
7New Jersey Center for Quality of Life and Health Outcome Research, NJ, USA
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_EN"]=>
array(36) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4117"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1480) "<p>Although there is no effective cure for this disease, high-dose chemotherapy (HDCT), together with autologous hematopoietic stem cell transplantation (auto-HSCT) offers promising results in the treatment of multiple sclerosis (MS) patients.</p>
<h3>Methods</h3>
<p>In this paper we present results of a prospective clinical study of safety and efficacy of HDCT+auto-HSCT in MS patients. One hundred and nine patients with various types of MS were included in this study. The patients underwent early, conventional, or salvage/late transplantation.<br>
<h3>Results</h3>
<p>The transplantation procedure was well tolerated by MS patients, with no transplant-related deaths at all. The efficacy analysis was performed in 79 patients. Forty-two achieved an objective improvement of neurological symptoms (defined as a ≥0.5 point decrease in EDSS score as compared to the baseline and confirmed over 6 months), and 37 patients had disease stabilization (steady EDSS level as compared to the baseline and confirmed over 6 months). Quality of life (QoL) was assessed in 44 patients. Thirty-nine patients exhibited a QoL response 1 year after transplantation.</p>
<h3>Conclusions</h3>
<p> This study provides ample evidence in support of HDCT+auto-HSCT efficacy in MS patients. The results obtained show that transplantation appears to be effective in patients with various types of MS.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1396) "
Although there is no effective cure for this disease, high-dose chemotherapy (HDCT), together with autologous hematopoietic stem cell transplantation (auto-HSCT) offers promising results in the treatment of multiple sclerosis (MS) patients.
Methods
In this paper we present results of a prospective clinical study of safety and efficacy of HDCT+auto-HSCT in MS patients. One hundred and nine patients with various types of MS were included in this study. The patients underwent early, conventional, or salvage/late transplantation.
Results
The transplantation procedure was well tolerated by MS patients, with no transplant-related deaths at all. The efficacy analysis was performed in 79 patients. Forty-two achieved an objective improvement of neurological symptoms (defined as a ≥0.5 point decrease in EDSS score as compared to the baseline and confirmed over 6 months), and 37 patients had disease stabilization (steady EDSS level as compared to the baseline and confirmed over 6 months). Quality of life (QoL) was assessed in 44 patients. Thirty-nine patients exhibited a QoL response 1 year after transplantation.
Conclusions
This study provides ample evidence in support of HDCT+auto-HSCT efficacy in MS patients. The results obtained show that transplantation appears to be effective in patients with various types of MS.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["NAME_EN"]=>
array(36) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3657"
["VALUE"]=>
string(72) "Autologous hematopoietic stem cell transplantation in multiple sclerosis"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(72) "Autologous hematopoietic stem cell transplantation in multiple sclerosis"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["FULL_TEXT_RU"]=>
&array(36) {
["ID"]=>
string(2) "42"
["TIMESTAMP_X"]=>
string(19) "2015-09-07 20:29:18"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(23) "Полный текст"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(12) "FULL_TEXT_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "42"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3658"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(11741) " <h2>Материалы и методы</h2>
<p>В исследование было включено 109 больных РС (49 мужчин, 60 женщин; средний возраст – 33 года; диапазон – 17-54): 51 с вторично-прогрессирующим течением, 19 с первично-прогрессирующим, 8 с прогрессирующе-рецидивирующим и 31 с рецидивирующе-ремиттирующим течением. Значение индекса EDSS до трансплантации колебалось от 1.5 до 8.0 баллов (в среднем было равно 5.0 баллам). Длительность наблюдения составила в среднем 19 месяцев (от 6 до 108 месяцев). Активность заболевания определяли с помощью неврологического осмотра и данных магнитно-резонансной томографии.<br>
Больным были выполнены следующие виды ВДТ+АуТКСК:<br>
<b> - Ранняя трансплантация</b> проводилась в дебюте заболевания при наличии неблагоприятных прогностических факторов в отношении химиорезистентности или возможности тяжелой инвалидизации больного.<br>
<b> - Этапная трансплантация</b> проводилась при выходе заболевания из-под контроля традиционных методов лечения и формировании вторичной химиорезистентности.<br>
<b> - Трансплантация спасения</b> проводилась в далеко зашедшей стадии заболевания при высокой активности иммунопатологического процесса и быстром прогрессировании инвалидизации больного.<br>
Тридцати двум больным была выполнена ранняя трансплантация (EDSS 1.5-3.0); 70 больным - этапная трансплантация (EDSS 3.5-6.5) и 7 больным - трансплантация спасения (EDSS 7.0-8.0).</p>
<p> Оценку клинического ответа и ответа, связанного с качеством жизни, проводили до трансплантации, при выписке из стационара, через 3, 6, 9 и 12 месяцев после трансплантации, затем – каждые 6 месяцев в течение первых 4 лет и ежегодно впоследствии. Изучение неврологического статуса включало определение выраженности неврологического дефицита по шкале EDSS и магнитно-резонансную томографию. Качество жизни больных
16 www.ctt-journal.com 2008;1(2)
оценивали с использованием опросников FACT-BMT (функциональная оценка состояния больных после трансплантации костного мозга) и FAMS (функциональная оценка больных с рассеянным склерозом).
Клиническим улучшением считали уменьшение выраженности неврологической симптоматики, по меньшей мере, на 0.5 балла по шкале EDSS по сравнению с исходным уровнем, при условии, что это улучшение было подтверждено через 6 месяцев на следующем визите. Любое увеличение выраженности неврологической симптоматики по шкале EDSS считали прогрессированием заболевания. Рецидив констатировали при появлении новых симптомов или нарастании выраженности прежних симптомов, по меньшей мере, в течение 24 часов в отсутствие лихорадки у пациента, который был стабилен в течение 4 предшествующих недель.
Ответ, связанный с качеством жизни, характеризовался как минимальный, умеренный, хороший или максимальный. Для определения ответа, связанного с качеством жизни, рассчитывали различия в значении интегрального показателя качества жизни до проведения трансплантации и в различные периоды времени после нее.</p>
<h2>Результаты</h2>
<p>У всех 79 больных со сроком наблюдения ≥9 месяцев отмечено клиническое улучшение или стабилизация в течении заболевания. Во время проведения трансплантации не было зарегистрировано ни одного смертельного исхода и тяжелых неконтролируемых побочных эффектов. Через 6 месяцев после трансплантации распределение пациентов согласно клиническому ответу было следующим: улучшение – 42 (53%) больных, стабилизация – 37 (47%) больных. Среди больных с улучшением у 20 было вторично-прогрессирующее течение, у 7 – первично-прогрессирующее, у 4 – прогрессирующе-рецидивирующее и у 11 – рецидивирующе-ремиттирующее течение. В этой группе 25 больных проведена этапная трансплантация, 15 – ранняя и 2 – трансплантация спасения. Из 37 больных, у которых зарегистрирована стабилизация заболевания, у 19 было вторично-прогрессирующее течение, у 8 – первично-прогрессирующее, у 2 – прогрессирующе-рецидивирующее и у 8 – рецидивирующе-ремиттирующее течение. В этой группе 23 больным проведена этапная трансплантация, 9 – ранняя и 5 – трансплантация спасения.</p>
<p> В более длительные сроки наблюдения у 40 больных (50.6%) сохранялось улучшение, у 34 (43.1%) – стабилизация. У одного больного после 6 месяцев и двух больных после 18 месяцев стабилизации произошло повышение индекса инвалидизации. У двух пациентов прогрессирование заболевания наступило после 12 и 30 месяцев клинического улучшения.
По данным МРТ у всех больных без прогрессирования заболевания после трансплантации отсутствовали активные или новые очаги поражения. В целом, 6-летняя выживаемость без прогрессии после ВДТ+АуТКСК составила 72%. Больные, у которых не было зарегистрировано признаков прогрессирования заболевания, не получали иммуномодулирующую или иммуносупрессивную терапию после трансплантации.
Мониторинг качества жизни проводился у 44 пациентов, включенных в исследование. У 40 из них наблюдали улучшение показателей качества жизни через 6 месяцев после трансплантации. Улучшение параметров качества жизни установлено с помощью опросников – FACT-BMT и FAMS. Через 1 год после ВДТ+АуТКСК зарегистрировано следующее распределение пациентов в соответствии со степенью ответа, связанного с качеством жизни: у 3 больных наблюдали максимальный ответ (более чем 75% улучшение интегрального показателя качества жизни в сравнении с исходным уровнем); у 12 больных – хороший ответ (улучшение на 50-75%); у 11 больных – умеренный ответ (на 25-50%); у 13 – минимальный ответ (улучшение менее чем на 25%) и у 5 больных ответ, связанный с качеством жизни, отсутствовал. Следует отметить, что у пациентов с более длительным сроком наблюдения было отмечено дальнейшее улучшение показателей качества жизни.
В статье представлена классификация типов трансплантации при рассеянном склерозе, основанная на концепции ВДТ+ТКСК при аутоиммунных заболеваниях.</p>
<h2>Заключение</h2>
<p>Высокодозная иммуносупрессивная терапия с аутологичной трансплантацией кроветворных стволовых клеток является эффективным методом лечения больных рассеянным склерозом: у большинства больных после ВДТ+АуТКСК зарегистрировано клиническое улучшение или стабилизация заболевания; ВДТ+АуТКСК сопровождается существенным улучшением качества жизни больных. Результаты свидетельствуют о целесообразности изучения результатов ранней трансплантации, этапной трансплантации и трансплантации спасения. Необходимы дальнейшие исследования для определения оптимальных сроков проведения трансплан-тации и уточнения режимов кондиционирования.
</p>"
["TYPE"]=>
string(4) "TEXT"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(11579) "
Материалы и методы
В исследование было включено 109 больных РС (49 мужчин, 60 женщин; средний возраст – 33 года; диапазон – 17-54): 51 с вторично-прогрессирующим течением, 19 с первично-прогрессирующим, 8 с прогрессирующе-рецидивирующим и 31 с рецидивирующе-ремиттирующим течением. Значение индекса EDSS до трансплантации колебалось от 1.5 до 8.0 баллов (в среднем было равно 5.0 баллам). Длительность наблюдения составила в среднем 19 месяцев (от 6 до 108 месяцев). Активность заболевания определяли с помощью неврологического осмотра и данных магнитно-резонансной томографии.
Больным были выполнены следующие виды ВДТ+АуТКСК:
- Ранняя трансплантация проводилась в дебюте заболевания при наличии неблагоприятных прогностических факторов в отношении химиорезистентности или возможности тяжелой инвалидизации больного.
- Этапная трансплантация проводилась при выходе заболевания из-под контроля традиционных методов лечения и формировании вторичной химиорезистентности.
- Трансплантация спасения проводилась в далеко зашедшей стадии заболевания при высокой активности иммунопатологического процесса и быстром прогрессировании инвалидизации больного.
Тридцати двум больным была выполнена ранняя трансплантация (EDSS 1.5-3.0); 70 больным - этапная трансплантация (EDSS 3.5-6.5) и 7 больным - трансплантация спасения (EDSS 7.0-8.0).
Оценку клинического ответа и ответа, связанного с качеством жизни, проводили до трансплантации, при выписке из стационара, через 3, 6, 9 и 12 месяцев после трансплантации, затем – каждые 6 месяцев в течение первых 4 лет и ежегодно впоследствии. Изучение неврологического статуса включало определение выраженности неврологического дефицита по шкале EDSS и магнитно-резонансную томографию. Качество жизни больных
16 www.ctt-journal.com 2008;1(2)
оценивали с использованием опросников FACT-BMT (функциональная оценка состояния больных после трансплантации костного мозга) и FAMS (функциональная оценка больных с рассеянным склерозом).
Клиническим улучшением считали уменьшение выраженности неврологической симптоматики, по меньшей мере, на 0.5 балла по шкале EDSS по сравнению с исходным уровнем, при условии, что это улучшение было подтверждено через 6 месяцев на следующем визите. Любое увеличение выраженности неврологической симптоматики по шкале EDSS считали прогрессированием заболевания. Рецидив констатировали при появлении новых симптомов или нарастании выраженности прежних симптомов, по меньшей мере, в течение 24 часов в отсутствие лихорадки у пациента, который был стабилен в течение 4 предшествующих недель.
Ответ, связанный с качеством жизни, характеризовался как минимальный, умеренный, хороший или максимальный. Для определения ответа, связанного с качеством жизни, рассчитывали различия в значении интегрального показателя качества жизни до проведения трансплантации и в различные периоды времени после нее.
Результаты
У всех 79 больных со сроком наблюдения ≥9 месяцев отмечено клиническое улучшение или стабилизация в течении заболевания. Во время проведения трансплантации не было зарегистрировано ни одного смертельного исхода и тяжелых неконтролируемых побочных эффектов. Через 6 месяцев после трансплантации распределение пациентов согласно клиническому ответу было следующим: улучшение – 42 (53%) больных, стабилизация – 37 (47%) больных. Среди больных с улучшением у 20 было вторично-прогрессирующее течение, у 7 – первично-прогрессирующее, у 4 – прогрессирующе-рецидивирующее и у 11 – рецидивирующе-ремиттирующее течение. В этой группе 25 больных проведена этапная трансплантация, 15 – ранняя и 2 – трансплантация спасения. Из 37 больных, у которых зарегистрирована стабилизация заболевания, у 19 было вторично-прогрессирующее течение, у 8 – первично-прогрессирующее, у 2 – прогрессирующе-рецидивирующее и у 8 – рецидивирующе-ремиттирующее течение. В этой группе 23 больным проведена этапная трансплантация, 9 – ранняя и 5 – трансплантация спасения.
В более длительные сроки наблюдения у 40 больных (50.6%) сохранялось улучшение, у 34 (43.1%) – стабилизация. У одного больного после 6 месяцев и двух больных после 18 месяцев стабилизации произошло повышение индекса инвалидизации. У двух пациентов прогрессирование заболевания наступило после 12 и 30 месяцев клинического улучшения.
По данным МРТ у всех больных без прогрессирования заболевания после трансплантации отсутствовали активные или новые очаги поражения. В целом, 6-летняя выживаемость без прогрессии после ВДТ+АуТКСК составила 72%. Больные, у которых не было зарегистрировано признаков прогрессирования заболевания, не получали иммуномодулирующую или иммуносупрессивную терапию после трансплантации.
Мониторинг качества жизни проводился у 44 пациентов, включенных в исследование. У 40 из них наблюдали улучшение показателей качества жизни через 6 месяцев после трансплантации. Улучшение параметров качества жизни установлено с помощью опросников – FACT-BMT и FAMS. Через 1 год после ВДТ+АуТКСК зарегистрировано следующее распределение пациентов в соответствии со степенью ответа, связанного с качеством жизни: у 3 больных наблюдали максимальный ответ (более чем 75% улучшение интегрального показателя качества жизни в сравнении с исходным уровнем); у 12 больных – хороший ответ (улучшение на 50-75%); у 11 больных – умеренный ответ (на 25-50%); у 13 – минимальный ответ (улучшение менее чем на 25%) и у 5 больных ответ, связанный с качеством жизни, отсутствовал. Следует отметить, что у пациентов с более длительным сроком наблюдения было отмечено дальнейшее улучшение показателей качества жизни.
В статье представлена классификация типов трансплантации при рассеянном склерозе, основанная на концепции ВДТ+ТКСК при аутоиммунных заболеваниях.
Заключение
Высокодозная иммуносупрессивная терапия с аутологичной трансплантацией кроветворных стволовых клеток является эффективным методом лечения больных рассеянным склерозом: у большинства больных после ВДТ+АуТКСК зарегистрировано клиническое улучшение или стабилизация заболевания; ВДТ+АуТКСК сопровождается существенным улучшением качества жизни больных. Результаты свидетельствуют о целесообразности изучения результатов ранней трансплантации, этапной трансплантации и трансплантации спасения. Необходимы дальнейшие исследования для определения оптимальных сроков проведения трансплан-тации и уточнения режимов кондиционирования.
"
["TYPE"]=>
string(4) "TEXT"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(23) "Полный текст"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["PDF_RU"]=>
array(36) {
["ID"]=>
string(2) "43"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF RUS"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_RU"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "43"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4227"
["VALUE"]=>
string(2) "99"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(2) "99"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF RUS"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["PDF_EN"]=>
array(36) {
["ID"]=>
string(2) "44"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF ENG"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "44"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4208"
["VALUE"]=>
string(2) "98"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(2) "98"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF ENG"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["NAME_LONG"]=>
array(36) {
["ID"]=>
string(2) "45"
["TIMESTAMP_X"]=>
string(19) "2023-04-13 00:55:00"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "NAME_LONG"
["DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "45"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(80)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["~DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
}
}
["DISPLAY_PROPERTIES"]=>
array(14) {
["AUTHOR_EN"]=>
array(37) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3656"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(633) "<p class="Autor">Yury L. Shevchenko<sup>1</sup>, Andrei A. Novik<sup>1</sup>, Alexey N. Kuznetsov<sup>1</sup>, Boris V. Afanasiev<sup>2</sup>,
Igor A. Lisukov<sup>3</sup>, Oleg A. Rykavicin<sup>4</sup>, Аlexandr A. Myasnikov<sup>5</sup>, Vladimir Y. Melnichenko<sup>1</sup>, Denis A. Fedorenko<sup>1</sup>, Tatyana I. Ionova<sup>6</sup>, Roman A. Ivanov<sup>1</sup>, and Gary Gorodokin<sup>7</sup> on behalf of the Russian Cooperative Group for Cellular Therapy</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(467) "
Yury L. Shevchenko1, Andrei A. Novik1, Alexey N. Kuznetsov1, Boris V. Afanasiev2,
Igor A. Lisukov3, Oleg A. Rykavicin4, Аlexandr A. Myasnikov5, Vladimir Y. Melnichenko1, Denis A. Fedorenko1, Tatyana I. Ionova6, Roman A. Ivanov1, and Gary Gorodokin7 on behalf of the Russian Cooperative Group for Cellular Therapy
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(467) "
Yury L. Shevchenko1, Andrei A. Novik1, Alexey N. Kuznetsov1, Boris V. Afanasiev2,
Igor A. Lisukov3, Oleg A. Rykavicin4, Аlexandr A. Myasnikov5, Vladimir Y. Melnichenko1, Denis A. Fedorenko1, Tatyana I. Ionova6, Roman A. Ivanov1, and Gary Gorodokin7 on behalf of the Russian Cooperative Group for Cellular Therapy
"
}
["SUMMARY_EN"]=>
array(37) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4117"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1480) "<p>Although there is no effective cure for this disease, high-dose chemotherapy (HDCT), together with autologous hematopoietic stem cell transplantation (auto-HSCT) offers promising results in the treatment of multiple sclerosis (MS) patients.</p>
<h3>Methods</h3>
<p>In this paper we present results of a prospective clinical study of safety and efficacy of HDCT+auto-HSCT in MS patients. One hundred and nine patients with various types of MS were included in this study. The patients underwent early, conventional, or salvage/late transplantation.<br>
<h3>Results</h3>
<p>The transplantation procedure was well tolerated by MS patients, with no transplant-related deaths at all. The efficacy analysis was performed in 79 patients. Forty-two achieved an objective improvement of neurological symptoms (defined as a ≥0.5 point decrease in EDSS score as compared to the baseline and confirmed over 6 months), and 37 patients had disease stabilization (steady EDSS level as compared to the baseline and confirmed over 6 months). Quality of life (QoL) was assessed in 44 patients. Thirty-nine patients exhibited a QoL response 1 year after transplantation.</p>
<h3>Conclusions</h3>
<p> This study provides ample evidence in support of HDCT+auto-HSCT efficacy in MS patients. The results obtained show that transplantation appears to be effective in patients with various types of MS.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1396) "
Although there is no effective cure for this disease, high-dose chemotherapy (HDCT), together with autologous hematopoietic stem cell transplantation (auto-HSCT) offers promising results in the treatment of multiple sclerosis (MS) patients.
Methods
In this paper we present results of a prospective clinical study of safety and efficacy of HDCT+auto-HSCT in MS patients. One hundred and nine patients with various types of MS were included in this study. The patients underwent early, conventional, or salvage/late transplantation.
Results
The transplantation procedure was well tolerated by MS patients, with no transplant-related deaths at all. The efficacy analysis was performed in 79 patients. Forty-two achieved an objective improvement of neurological symptoms (defined as a ≥0.5 point decrease in EDSS score as compared to the baseline and confirmed over 6 months), and 37 patients had disease stabilization (steady EDSS level as compared to the baseline and confirmed over 6 months). Quality of life (QoL) was assessed in 44 patients. Thirty-nine patients exhibited a QoL response 1 year after transplantation.
Conclusions
This study provides ample evidence in support of HDCT+auto-HSCT efficacy in MS patients. The results obtained show that transplantation appears to be effective in patients with various types of MS.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(1396) "
Although there is no effective cure for this disease, high-dose chemotherapy (HDCT), together with autologous hematopoietic stem cell transplantation (auto-HSCT) offers promising results in the treatment of multiple sclerosis (MS) patients.
Methods
In this paper we present results of a prospective clinical study of safety and efficacy of HDCT+auto-HSCT in MS patients. One hundred and nine patients with various types of MS were included in this study. The patients underwent early, conventional, or salvage/late transplantation.
Results
The transplantation procedure was well tolerated by MS patients, with no transplant-related deaths at all. The efficacy analysis was performed in 79 patients. Forty-two achieved an objective improvement of neurological symptoms (defined as a ≥0.5 point decrease in EDSS score as compared to the baseline and confirmed over 6 months), and 37 patients had disease stabilization (steady EDSS level as compared to the baseline and confirmed over 6 months). Quality of life (QoL) was assessed in 44 patients. Thirty-nine patients exhibited a QoL response 1 year after transplantation.
Conclusions
This study provides ample evidence in support of HDCT+auto-HSCT efficacy in MS patients. The results obtained show that transplantation appears to be effective in patients with various types of MS.
"
}
["DOI"]=>
array(37) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3655"
["VALUE"]=>
string(29) "10.3205/ctt-2008-ru-000025.02"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-ru-000025.02"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(29) "10.3205/ctt-2008-ru-000025.02"
}
["NAME_EN"]=>
array(37) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3657"
["VALUE"]=>
string(72) "Autologous hematopoietic stem cell transplantation in multiple sclerosis"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(72) "Autologous hematopoietic stem cell transplantation in multiple sclerosis"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(72) "Autologous hematopoietic stem cell transplantation in multiple sclerosis"
}
["ORGANIZATION_EN"]=>
array(37) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4116"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(707) "<p><sup>1</sup>Pirogov National Medical Surgical Center, Moscow, Russia;<br> <sup>2</sup>Pavlov State Medical University, St. Petersburg, Russia; <br><sup>3</sup>Institute of Clinical Immunology, Siberian Branch of Russian Academy of Science, Novosibirsk, Russia;<br><sup> 4</sup>Burdenko Central Military Hospital, Moscow, Russia; <br><sup>5</sup>Republic Hospital, Petrozavodsk, Russia;<br>
<sup>6</sup>Multinational Center of Quality of Life Research, St. Petersburg, Russia;<br>
<sup>7</sup>New Jersey Center for Quality of Life and Health Outcome Research, NJ, USA</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(575) "
1Pirogov National Medical Surgical Center, Moscow, Russia;
2Pavlov State Medical University, St. Petersburg, Russia;
3Institute of Clinical Immunology, Siberian Branch of Russian Academy of Science, Novosibirsk, Russia;
4Burdenko Central Military Hospital, Moscow, Russia;
5Republic Hospital, Petrozavodsk, Russia;
6Multinational Center of Quality of Life Research, St. Petersburg, Russia;
7New Jersey Center for Quality of Life and Health Outcome Research, NJ, USA
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(575) "
1Pirogov National Medical Surgical Center, Moscow, Russia;
2Pavlov State Medical University, St. Petersburg, Russia;
3Institute of Clinical Immunology, Siberian Branch of Russian Academy of Science, Novosibirsk, Russia;
4Burdenko Central Military Hospital, Moscow, Russia;
5Republic Hospital, Petrozavodsk, Russia;
6Multinational Center of Quality of Life Research, St. Petersburg, Russia;
7New Jersey Center for Quality of Life and Health Outcome Research, NJ, USA
"
}
["AUTHORS"]=>
array(38) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(12) {
[0]=>
string(5) "11244"
[1]=>
string(5) "11245"
[2]=>
string(5) "11246"
[3]=>
string(5) "11247"
[4]=>
string(5) "11248"
[5]=>
string(5) "11249"
[6]=>
string(5) "11250"
[7]=>
string(5) "11251"
[8]=>
string(5) "11252"
[9]=>
string(5) "11253"
[10]=>
string(5) "11254"
[11]=>
string(5) "11255"
}
["VALUE"]=>
array(12) {
[0]=>
string(3) "447"
[1]=>
string(3) "448"
[2]=>
string(3) "449"
[3]=>
string(2) "34"
[4]=>
string(3) "450"
[5]=>
string(3) "451"
[6]=>
string(3) "452"
[7]=>
string(3) "453"
[8]=>
string(3) "454"
[9]=>
string(3) "455"
[10]=>
string(3) "456"
[11]=>
string(3) "457"
}
["DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(12) {
[0]=>
string(3) "447"
[1]=>
string(3) "448"
[2]=>
string(3) "449"
[3]=>
string(2) "34"
[4]=>
string(3) "450"
[5]=>
string(3) "451"
[6]=>
string(3) "452"
[7]=>
string(3) "453"
[8]=>
string(3) "454"
[9]=>
string(3) "455"
[10]=>
string(3) "456"
[11]=>
string(3) "457"
}
["~DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(12) {
[0]=>
string(72) "
Юрий Л. Шевченко"
[1]=>
string(66) "
Андрей Новик"
[2]=>
string(63) "
Aleksey N. Kuznetsov"
[3]=>
string(60) "
Boris V. Afanasyev"
[4]=>
string(58) "
Igor A. Lisukov"
[5]=>
string(74) "
Олег А. Рукавицын"
[6]=>
string(65) "
Аlexandr A. Myasnikov"
[7]=>
string(66) "
Vladimir Y. Melnichenko"
[8]=>
string(61) "
Denis A. Fedorenko"
[9]=>
string(60) "
Tatyana I. Ionova"
[10]=>
string(58) "
Roman A. Ivanov"
[11]=>
string(57) "
Gary Gorodokin"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["AUTHOR_RU"]=>
array(37) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3654"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(361) "<p class="Autor">
Ю. Л. Шевченко, А. А. Новик, А. Н. Кузнецов, Б. В. Афанасьев, И. А. Лисуков, O. А. Рукавицын, А. А. Мясников, <br> В. Я. Мельниченко, Д. А. Федоренко, T. И. Ионова, Р. А. Иванов, Г. Городокин
</p>"
["TYPE"]=>
string(4) "TEXT"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(333) "
Ю. Л. Шевченко, А. А. Новик, А. Н. Кузнецов, Б. В. Афанасьев, И. А. Лисуков, O. А. Рукавицын, А. А. Мясников,
В. Я. Мельниченко, Д. А. Федоренко, T. И. Ионова, Р. А. Иванов, Г. Городокин
"
["TYPE"]=>
string(4) "TEXT"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(394) "<p class="Autor">
Ю. Л. Шевченко, А. А. Новик, А. Н. Кузнецов, Б. В. Афанасьев, И. А. Лисуков, O. А. Рукавицын, А. А. Мясников, <br> В. Я. Мельниченко, Д. А. Федоренко, T. И. Ионова, Р. А. Иванов, Г. Городокин
</p>"
}
["SUBMITTED"]=>
array(37) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3638"
["VALUE"]=>
string(19) "05.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "05.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "05.11.2008 00:01:00"
}
["ACCEPTED"]=>
array(37) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3639"
["VALUE"]=>
string(19) "17.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "17.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "17.11.2008 00:01:00"
}
["PUBLISHED"]=>
array(37) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3640"
["VALUE"]=>
string(19) "03.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "03.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "03.12.2008 00:01:00"
}
["KEYWORDS"]=>
array(38) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(6) {
[0]=>
string(5) "11238"
[1]=>
string(5) "11239"
[2]=>
string(5) "11240"
[3]=>
string(5) "11241"
[4]=>
string(5) "11242"
[5]=>
string(5) "11243"
}
["VALUE"]=>
array(6) {
[0]=>
string(3) "441"
[1]=>
string(3) "442"
[2]=>
string(3) "443"
[3]=>
string(3) "444"
[4]=>
string(3) "445"
[5]=>
string(3) "446"
}
["DESCRIPTION"]=>
array(6) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(6) {
[0]=>
string(3) "441"
[1]=>
string(3) "442"
[2]=>
string(3) "443"
[3]=>
string(3) "444"
[4]=>
string(3) "445"
[5]=>
string(3) "446"
}
["~DESCRIPTION"]=>
array(6) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(6) {
[0]=>
string(129) "
трансплантация стволовых кроветворных клеток"
[1]=>
string(79) "
рассеянный склероз"
[2]=>
string(71) "
качество жизни"
[3]=>
string(85) "
ранняя трансплантация"
[4]=>
string(87) "
этапная трансплантация"
[5]=>
string(89) "
трансплантация спасения"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["CONTACT"]=>
array(38) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3641"
["VALUE"]=>
string(3) "448"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "448"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(66) "
Андрей Новик"
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["SUMMARY_RU"]=>
array(37) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "4024"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(4131) " <h3>Введение</h3>
<p>Рассеянный склероз (РС) – хроническое прогрессирующее заболевание центральной нервной системы, которое клинически проявляется мультисистемной неврологической симптоматикой, а патоморфологически характеризуется образованием множественных очагов демиелинизации в белом веществе головного и спинного мозга. Основным механизмом, приводящим к повреждению миелина, является опосредованная Т-лимфоцитами реакция гиперчувствительности замедленного типа, а непосредственными клетками-эффекторами иммунопатологического процесса – макрофаги.</p>
<p>Существующие методы лечения не позволяют достичь устойчивого терапевтического эффекта при рассеянном склерозе. Выдвигалась гипотеза, основанная на доклинических данных, о высокой эффективности аллогенной транплантации стволовых кроветворных клеток (ТСКК). Однако высокая посттрансплантационная летальность не позволила приступить к клиническим исследованиям данного вида терапии РС. По мнению большинства экспертов одним из наиболее перспективных методов лечения РС на сегодняшний день является высокодозная химиотерапия (ВДТ) с аутологичной трансплантацией стволовых кроветворных клеток (АуТСКК). Начиная с 1995 года, безопасность ВДТ+AyТКСК при РС была изучена в ряде клинических исследований. Тем не менее, объем информации о клинической эффективности данного метода и, особенно, о его влиянии на качество жизни больных РС, остается недостаточным. Кроме того, большинство пациентов, включенных в вышеупомянутые исследования, имели вторично-прогрессирующую форму РС и значительную степень инвалидизации со значением шкалы EDSS 4.5-8.5 баллов. К сожалению, даже полное прекращение активности иммунопатологического процесса у таких больных не может привести к значительному улучшению качества жизни. Поэтому вопрос об оптимальных сроках проведения трансплантации по-прежнему остается открытым.</p>
<p>В статье приведены результаты проспективного многоцентрового исследования безопасности и эффективности ВДТ+АуТКСК при РС, которое было начато в 1999 году и в настоящее время объединяет 5 крупных российских медицинских центров. Изучали влияние ВДТ+АуТКСК на клиническое течение и показатели качества жизни больных с разными формами и стадиями РС.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(4083) "
Введение
Рассеянный склероз (РС) – хроническое прогрессирующее заболевание центральной нервной системы, которое клинически проявляется мультисистемной неврологической симптоматикой, а патоморфологически характеризуется образованием множественных очагов демиелинизации в белом веществе головного и спинного мозга. Основным механизмом, приводящим к повреждению миелина, является опосредованная Т-лимфоцитами реакция гиперчувствительности замедленного типа, а непосредственными клетками-эффекторами иммунопатологического процесса – макрофаги.
Существующие методы лечения не позволяют достичь устойчивого терапевтического эффекта при рассеянном склерозе. Выдвигалась гипотеза, основанная на доклинических данных, о высокой эффективности аллогенной транплантации стволовых кроветворных клеток (ТСКК). Однако высокая посттрансплантационная летальность не позволила приступить к клиническим исследованиям данного вида терапии РС. По мнению большинства экспертов одним из наиболее перспективных методов лечения РС на сегодняшний день является высокодозная химиотерапия (ВДТ) с аутологичной трансплантацией стволовых кроветворных клеток (АуТСКК). Начиная с 1995 года, безопасность ВДТ+AyТКСК при РС была изучена в ряде клинических исследований. Тем не менее, объем информации о клинической эффективности данного метода и, особенно, о его влиянии на качество жизни больных РС, остается недостаточным. Кроме того, большинство пациентов, включенных в вышеупомянутые исследования, имели вторично-прогрессирующую форму РС и значительную степень инвалидизации со значением шкалы EDSS 4.5-8.5 баллов. К сожалению, даже полное прекращение активности иммунопатологического процесса у таких больных не может привести к значительному улучшению качества жизни. Поэтому вопрос об оптимальных сроках проведения трансплантации по-прежнему остается открытым.
В статье приведены результаты проспективного многоцентрового исследования безопасности и эффективности ВДТ+АуТКСК при РС, которое было начато в 1999 году и в настоящее время объединяет 5 крупных российских медицинских центров. Изучали влияние ВДТ+АуТКСК на клиническое течение и показатели качества жизни больных с разными формами и стадиями РС.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(4083) "
Введение
Рассеянный склероз (РС) – хроническое прогрессирующее заболевание центральной нервной системы, которое клинически проявляется мультисистемной неврологической симптоматикой, а патоморфологически характеризуется образованием множественных очагов демиелинизации в белом веществе головного и спинного мозга. Основным механизмом, приводящим к повреждению миелина, является опосредованная Т-лимфоцитами реакция гиперчувствительности замедленного типа, а непосредственными клетками-эффекторами иммунопатологического процесса – макрофаги.
Существующие методы лечения не позволяют достичь устойчивого терапевтического эффекта при рассеянном склерозе. Выдвигалась гипотеза, основанная на доклинических данных, о высокой эффективности аллогенной транплантации стволовых кроветворных клеток (ТСКК). Однако высокая посттрансплантационная летальность не позволила приступить к клиническим исследованиям данного вида терапии РС. По мнению большинства экспертов одним из наиболее перспективных методов лечения РС на сегодняшний день является высокодозная химиотерапия (ВДТ) с аутологичной трансплантацией стволовых кроветворных клеток (АуТСКК). Начиная с 1995 года, безопасность ВДТ+AyТКСК при РС была изучена в ряде клинических исследований. Тем не менее, объем информации о клинической эффективности данного метода и, особенно, о его влиянии на качество жизни больных РС, остается недостаточным. Кроме того, большинство пациентов, включенных в вышеупомянутые исследования, имели вторично-прогрессирующую форму РС и значительную степень инвалидизации со значением шкалы EDSS 4.5-8.5 баллов. К сожалению, даже полное прекращение активности иммунопатологического процесса у таких больных не может привести к значительному улучшению качества жизни. Поэтому вопрос об оптимальных сроках проведения трансплантации по-прежнему остается открытым.
В статье приведены результаты проспективного многоцентрового исследования безопасности и эффективности ВДТ+АуТКСК при РС, которое было начато в 1999 году и в настоящее время объединяет 5 крупных российских медицинских центров. Изучали влияние ВДТ+АуТКСК на клиническое течение и показатели качества жизни больных с разными формами и стадиями РС.
"
}
["FULL_TEXT_RU"]=>
array(37) {
["ID"]=>
string(2) "42"
["TIMESTAMP_X"]=>
string(19) "2015-09-07 20:29:18"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(23) "Полный текст"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(12) "FULL_TEXT_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "42"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "3658"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(11741) " <h2>Материалы и методы</h2>
<p>В исследование было включено 109 больных РС (49 мужчин, 60 женщин; средний возраст – 33 года; диапазон – 17-54): 51 с вторично-прогрессирующим течением, 19 с первично-прогрессирующим, 8 с прогрессирующе-рецидивирующим и 31 с рецидивирующе-ремиттирующим течением. Значение индекса EDSS до трансплантации колебалось от 1.5 до 8.0 баллов (в среднем было равно 5.0 баллам). Длительность наблюдения составила в среднем 19 месяцев (от 6 до 108 месяцев). Активность заболевания определяли с помощью неврологического осмотра и данных магнитно-резонансной томографии.<br>
Больным были выполнены следующие виды ВДТ+АуТКСК:<br>
<b> - Ранняя трансплантация</b> проводилась в дебюте заболевания при наличии неблагоприятных прогностических факторов в отношении химиорезистентности или возможности тяжелой инвалидизации больного.<br>
<b> - Этапная трансплантация</b> проводилась при выходе заболевания из-под контроля традиционных методов лечения и формировании вторичной химиорезистентности.<br>
<b> - Трансплантация спасения</b> проводилась в далеко зашедшей стадии заболевания при высокой активности иммунопатологического процесса и быстром прогрессировании инвалидизации больного.<br>
Тридцати двум больным была выполнена ранняя трансплантация (EDSS 1.5-3.0); 70 больным - этапная трансплантация (EDSS 3.5-6.5) и 7 больным - трансплантация спасения (EDSS 7.0-8.0).</p>
<p> Оценку клинического ответа и ответа, связанного с качеством жизни, проводили до трансплантации, при выписке из стационара, через 3, 6, 9 и 12 месяцев после трансплантации, затем – каждые 6 месяцев в течение первых 4 лет и ежегодно впоследствии. Изучение неврологического статуса включало определение выраженности неврологического дефицита по шкале EDSS и магнитно-резонансную томографию. Качество жизни больных
16 www.ctt-journal.com 2008;1(2)
оценивали с использованием опросников FACT-BMT (функциональная оценка состояния больных после трансплантации костного мозга) и FAMS (функциональная оценка больных с рассеянным склерозом).
Клиническим улучшением считали уменьшение выраженности неврологической симптоматики, по меньшей мере, на 0.5 балла по шкале EDSS по сравнению с исходным уровнем, при условии, что это улучшение было подтверждено через 6 месяцев на следующем визите. Любое увеличение выраженности неврологической симптоматики по шкале EDSS считали прогрессированием заболевания. Рецидив констатировали при появлении новых симптомов или нарастании выраженности прежних симптомов, по меньшей мере, в течение 24 часов в отсутствие лихорадки у пациента, который был стабилен в течение 4 предшествующих недель.
Ответ, связанный с качеством жизни, характеризовался как минимальный, умеренный, хороший или максимальный. Для определения ответа, связанного с качеством жизни, рассчитывали различия в значении интегрального показателя качества жизни до проведения трансплантации и в различные периоды времени после нее.</p>
<h2>Результаты</h2>
<p>У всех 79 больных со сроком наблюдения ≥9 месяцев отмечено клиническое улучшение или стабилизация в течении заболевания. Во время проведения трансплантации не было зарегистрировано ни одного смертельного исхода и тяжелых неконтролируемых побочных эффектов. Через 6 месяцев после трансплантации распределение пациентов согласно клиническому ответу было следующим: улучшение – 42 (53%) больных, стабилизация – 37 (47%) больных. Среди больных с улучшением у 20 было вторично-прогрессирующее течение, у 7 – первично-прогрессирующее, у 4 – прогрессирующе-рецидивирующее и у 11 – рецидивирующе-ремиттирующее течение. В этой группе 25 больных проведена этапная трансплантация, 15 – ранняя и 2 – трансплантация спасения. Из 37 больных, у которых зарегистрирована стабилизация заболевания, у 19 было вторично-прогрессирующее течение, у 8 – первично-прогрессирующее, у 2 – прогрессирующе-рецидивирующее и у 8 – рецидивирующе-ремиттирующее течение. В этой группе 23 больным проведена этапная трансплантация, 9 – ранняя и 5 – трансплантация спасения.</p>
<p> В более длительные сроки наблюдения у 40 больных (50.6%) сохранялось улучшение, у 34 (43.1%) – стабилизация. У одного больного после 6 месяцев и двух больных после 18 месяцев стабилизации произошло повышение индекса инвалидизации. У двух пациентов прогрессирование заболевания наступило после 12 и 30 месяцев клинического улучшения.
По данным МРТ у всех больных без прогрессирования заболевания после трансплантации отсутствовали активные или новые очаги поражения. В целом, 6-летняя выживаемость без прогрессии после ВДТ+АуТКСК составила 72%. Больные, у которых не было зарегистрировано признаков прогрессирования заболевания, не получали иммуномодулирующую или иммуносупрессивную терапию после трансплантации.
Мониторинг качества жизни проводился у 44 пациентов, включенных в исследование. У 40 из них наблюдали улучшение показателей качества жизни через 6 месяцев после трансплантации. Улучшение параметров качества жизни установлено с помощью опросников – FACT-BMT и FAMS. Через 1 год после ВДТ+АуТКСК зарегистрировано следующее распределение пациентов в соответствии со степенью ответа, связанного с качеством жизни: у 3 больных наблюдали максимальный ответ (более чем 75% улучшение интегрального показателя качества жизни в сравнении с исходным уровнем); у 12 больных – хороший ответ (улучшение на 50-75%); у 11 больных – умеренный ответ (на 25-50%); у 13 – минимальный ответ (улучшение менее чем на 25%) и у 5 больных ответ, связанный с качеством жизни, отсутствовал. Следует отметить, что у пациентов с более длительным сроком наблюдения было отмечено дальнейшее улучшение показателей качества жизни.
В статье представлена классификация типов трансплантации при рассеянном склерозе, основанная на концепции ВДТ+ТКСК при аутоиммунных заболеваниях.</p>
<h2>Заключение</h2>
<p>Высокодозная иммуносупрессивная терапия с аутологичной трансплантацией кроветворных стволовых клеток является эффективным методом лечения больных рассеянным склерозом: у большинства больных после ВДТ+АуТКСК зарегистрировано клиническое улучшение или стабилизация заболевания; ВДТ+АуТКСК сопровождается существенным улучшением качества жизни больных. Результаты свидетельствуют о целесообразности изучения результатов ранней трансплантации, этапной трансплантации и трансплантации спасения. Необходимы дальнейшие исследования для определения оптимальных сроков проведения трансплан-тации и уточнения режимов кондиционирования.
</p>"
["TYPE"]=>
string(4) "TEXT"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(11579) "
Материалы и методы
В исследование было включено 109 больных РС (49 мужчин, 60 женщин; средний возраст – 33 года; диапазон – 17-54): 51 с вторично-прогрессирующим течением, 19 с первично-прогрессирующим, 8 с прогрессирующе-рецидивирующим и 31 с рецидивирующе-ремиттирующим течением. Значение индекса EDSS до трансплантации колебалось от 1.5 до 8.0 баллов (в среднем было равно 5.0 баллам). Длительность наблюдения составила в среднем 19 месяцев (от 6 до 108 месяцев). Активность заболевания определяли с помощью неврологического осмотра и данных магнитно-резонансной томографии.
Больным были выполнены следующие виды ВДТ+АуТКСК:
- Ранняя трансплантация проводилась в дебюте заболевания при наличии неблагоприятных прогностических факторов в отношении химиорезистентности или возможности тяжелой инвалидизации больного.
- Этапная трансплантация проводилась при выходе заболевания из-под контроля традиционных методов лечения и формировании вторичной химиорезистентности.
- Трансплантация спасения проводилась в далеко зашедшей стадии заболевания при высокой активности иммунопатологического процесса и быстром прогрессировании инвалидизации больного.
Тридцати двум больным была выполнена ранняя трансплантация (EDSS 1.5-3.0); 70 больным - этапная трансплантация (EDSS 3.5-6.5) и 7 больным - трансплантация спасения (EDSS 7.0-8.0).
Оценку клинического ответа и ответа, связанного с качеством жизни, проводили до трансплантации, при выписке из стационара, через 3, 6, 9 и 12 месяцев после трансплантации, затем – каждые 6 месяцев в течение первых 4 лет и ежегодно впоследствии. Изучение неврологического статуса включало определение выраженности неврологического дефицита по шкале EDSS и магнитно-резонансную томографию. Качество жизни больных
16 www.ctt-journal.com 2008;1(2)
оценивали с использованием опросников FACT-BMT (функциональная оценка состояния больных после трансплантации костного мозга) и FAMS (функциональная оценка больных с рассеянным склерозом).
Клиническим улучшением считали уменьшение выраженности неврологической симптоматики, по меньшей мере, на 0.5 балла по шкале EDSS по сравнению с исходным уровнем, при условии, что это улучшение было подтверждено через 6 месяцев на следующем визите. Любое увеличение выраженности неврологической симптоматики по шкале EDSS считали прогрессированием заболевания. Рецидив констатировали при появлении новых симптомов или нарастании выраженности прежних симптомов, по меньшей мере, в течение 24 часов в отсутствие лихорадки у пациента, который был стабилен в течение 4 предшествующих недель.
Ответ, связанный с качеством жизни, характеризовался как минимальный, умеренный, хороший или максимальный. Для определения ответа, связанного с качеством жизни, рассчитывали различия в значении интегрального показателя качества жизни до проведения трансплантации и в различные периоды времени после нее.
Результаты
У всех 79 больных со сроком наблюдения ≥9 месяцев отмечено клиническое улучшение или стабилизация в течении заболевания. Во время проведения трансплантации не было зарегистрировано ни одного смертельного исхода и тяжелых неконтролируемых побочных эффектов. Через 6 месяцев после трансплантации распределение пациентов согласно клиническому ответу было следующим: улучшение – 42 (53%) больных, стабилизация – 37 (47%) больных. Среди больных с улучшением у 20 было вторично-прогрессирующее течение, у 7 – первично-прогрессирующее, у 4 – прогрессирующе-рецидивирующее и у 11 – рецидивирующе-ремиттирующее течение. В этой группе 25 больных проведена этапная трансплантация, 15 – ранняя и 2 – трансплантация спасения. Из 37 больных, у которых зарегистрирована стабилизация заболевания, у 19 было вторично-прогрессирующее течение, у 8 – первично-прогрессирующее, у 2 – прогрессирующе-рецидивирующее и у 8 – рецидивирующе-ремиттирующее течение. В этой группе 23 больным проведена этапная трансплантация, 9 – ранняя и 5 – трансплантация спасения.
В более длительные сроки наблюдения у 40 больных (50.6%) сохранялось улучшение, у 34 (43.1%) – стабилизация. У одного больного после 6 месяцев и двух больных после 18 месяцев стабилизации произошло повышение индекса инвалидизации. У двух пациентов прогрессирование заболевания наступило после 12 и 30 месяцев клинического улучшения.
По данным МРТ у всех больных без прогрессирования заболевания после трансплантации отсутствовали активные или новые очаги поражения. В целом, 6-летняя выживаемость без прогрессии после ВДТ+АуТКСК составила 72%. Больные, у которых не было зарегистрировано признаков прогрессирования заболевания, не получали иммуномодулирующую или иммуносупрессивную терапию после трансплантации.
Мониторинг качества жизни проводился у 44 пациентов, включенных в исследование. У 40 из них наблюдали улучшение показателей качества жизни через 6 месяцев после трансплантации. Улучшение параметров качества жизни установлено с помощью опросников – FACT-BMT и FAMS. Через 1 год после ВДТ+АуТКСК зарегистрировано следующее распределение пациентов в соответствии со степенью ответа, связанного с качеством жизни: у 3 больных наблюдали максимальный ответ (более чем 75% улучшение интегрального показателя качества жизни в сравнении с исходным уровнем); у 12 больных – хороший ответ (улучшение на 50-75%); у 11 больных – умеренный ответ (на 25-50%); у 13 – минимальный ответ (улучшение менее чем на 25%) и у 5 больных ответ, связанный с качеством жизни, отсутствовал. Следует отметить, что у пациентов с более длительным сроком наблюдения было отмечено дальнейшее улучшение показателей качества жизни.
В статье представлена классификация типов трансплантации при рассеянном склерозе, основанная на концепции ВДТ+ТКСК при аутоиммунных заболеваниях.
Заключение
Высокодозная иммуносупрессивная терапия с аутологичной трансплантацией кроветворных стволовых клеток является эффективным методом лечения больных рассеянным склерозом: у большинства больных после ВДТ+АуТКСК зарегистрировано клиническое улучшение или стабилизация заболевания; ВДТ+АуТКСК сопровождается существенным улучшением качества жизни больных. Результаты свидетельствуют о целесообразности изучения результатов ранней трансплантации, этапной трансплантации и трансплантации спасения. Необходимы дальнейшие исследования для определения оптимальных сроков проведения трансплан-тации и уточнения режимов кондиционирования.
"
["TYPE"]=>
string(4) "TEXT"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(23) "Полный текст"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(12433) " <h2>Материалы и методы</h2>
<p>В исследование было включено 109 больных РС (49 мужчин, 60 женщин; средний возраст – 33 года; диапазон – 17-54): 51 с вторично-прогрессирующим течением, 19 с первично-прогрессирующим, 8 с прогрессирующе-рецидивирующим и 31 с рецидивирующе-ремиттирующим течением. Значение индекса EDSS до трансплантации колебалось от 1.5 до 8.0 баллов (в среднем было равно 5.0 баллам). Длительность наблюдения составила в среднем 19 месяцев (от 6 до 108 месяцев). Активность заболевания определяли с помощью неврологического осмотра и данных магнитно-резонансной томографии.<br>
Больным были выполнены следующие виды ВДТ+АуТКСК:<br>
<b> - Ранняя трансплантация</b> проводилась в дебюте заболевания при наличии неблагоприятных прогностических факторов в отношении химиорезистентности или возможности тяжелой инвалидизации больного.<br>
<b> - Этапная трансплантация</b> проводилась при выходе заболевания из-под контроля традиционных методов лечения и формировании вторичной химиорезистентности.<br>
<b> - Трансплантация спасения</b> проводилась в далеко зашедшей стадии заболевания при высокой активности иммунопатологического процесса и быстром прогрессировании инвалидизации больного.<br>
Тридцати двум больным была выполнена ранняя трансплантация (EDSS 1.5-3.0); 70 больным - этапная трансплантация (EDSS 3.5-6.5) и 7 больным - трансплантация спасения (EDSS 7.0-8.0).</p>
<p> Оценку клинического ответа и ответа, связанного с качеством жизни, проводили до трансплантации, при выписке из стационара, через 3, 6, 9 и 12 месяцев после трансплантации, затем – каждые 6 месяцев в течение первых 4 лет и ежегодно впоследствии. Изучение неврологического статуса включало определение выраженности неврологического дефицита по шкале EDSS и магнитно-резонансную томографию. Качество жизни больных
16 www.ctt-journal.com 2008;1(2)
оценивали с использованием опросников FACT-BMT (функциональная оценка состояния больных после трансплантации костного мозга) и FAMS (функциональная оценка больных с рассеянным склерозом).
Клиническим улучшением считали уменьшение выраженности неврологической симптоматики, по меньшей мере, на 0.5 балла по шкале EDSS по сравнению с исходным уровнем, при условии, что это улучшение было подтверждено через 6 месяцев на следующем визите. Любое увеличение выраженности неврологической симптоматики по шкале EDSS считали прогрессированием заболевания. Рецидив констатировали при появлении новых симптомов или нарастании выраженности прежних симптомов, по меньшей мере, в течение 24 часов в отсутствие лихорадки у пациента, который был стабилен в течение 4 предшествующих недель.
Ответ, связанный с качеством жизни, характеризовался как минимальный, умеренный, хороший или максимальный. Для определения ответа, связанного с качеством жизни, рассчитывали различия в значении интегрального показателя качества жизни до проведения трансплантации и в различные периоды времени после нее.</p>
<h2>Результаты</h2>
<p>У всех 79 больных со сроком наблюдения ≥9 месяцев отмечено клиническое улучшение или стабилизация в течении заболевания. Во время проведения трансплантации не было зарегистрировано ни одного смертельного исхода и тяжелых неконтролируемых побочных эффектов. Через 6 месяцев после трансплантации распределение пациентов согласно клиническому ответу было следующим: улучшение – 42 (53%) больных, стабилизация – 37 (47%) больных. Среди больных с улучшением у 20 было вторично-прогрессирующее течение, у 7 – первично-прогрессирующее, у 4 – прогрессирующе-рецидивирующее и у 11 – рецидивирующе-ремиттирующее течение. В этой группе 25 больных проведена этапная трансплантация, 15 – ранняя и 2 – трансплантация спасения. Из 37 больных, у которых зарегистрирована стабилизация заболевания, у 19 было вторично-прогрессирующее течение, у 8 – первично-прогрессирующее, у 2 – прогрессирующе-рецидивирующее и у 8 – рецидивирующе-ремиттирующее течение. В этой группе 23 больным проведена этапная трансплантация, 9 – ранняя и 5 – трансплантация спасения.</p>
<p> В более длительные сроки наблюдения у 40 больных (50.6%) сохранялось улучшение, у 34 (43.1%) – стабилизация. У одного больного после 6 месяцев и двух больных после 18 месяцев стабилизации произошло повышение индекса инвалидизации. У двух пациентов прогрессирование заболевания наступило после 12 и 30 месяцев клинического улучшения.
По данным МРТ у всех больных без прогрессирования заболевания после трансплантации отсутствовали активные или новые очаги поражения. В целом, 6-летняя выживаемость без прогрессии после ВДТ+АуТКСК составила 72%. Больные, у которых не было зарегистрировано признаков прогрессирования заболевания, не получали иммуномодулирующую или иммуносупрессивную терапию после трансплантации.
Мониторинг качества жизни проводился у 44 пациентов, включенных в исследование. У 40 из них наблюдали улучшение показателей качества жизни через 6 месяцев после трансплантации. Улучшение параметров качества жизни установлено с помощью опросников – FACT-BMT и FAMS. Через 1 год после ВДТ+АуТКСК зарегистрировано следующее распределение пациентов в соответствии со степенью ответа, связанного с качеством жизни: у 3 больных наблюдали максимальный ответ (более чем 75% улучшение интегрального показателя качества жизни в сравнении с исходным уровнем); у 12 больных – хороший ответ (улучшение на 50-75%); у 11 больных – умеренный ответ (на 25-50%); у 13 – минимальный ответ (улучшение менее чем на 25%) и у 5 больных ответ, связанный с качеством жизни, отсутствовал. Следует отметить, что у пациентов с более длительным сроком наблюдения было отмечено дальнейшее улучшение показателей качества жизни.
В статье представлена классификация типов трансплантации при рассеянном склерозе, основанная на концепции ВДТ+ТКСК при аутоиммунных заболеваниях.</p>
<h2>Заключение</h2>
<p>Высокодозная иммуносупрессивная терапия с аутологичной трансплантацией кроветворных стволовых клеток является эффективным методом лечения больных рассеянным склерозом: у большинства больных после ВДТ+АуТКСК зарегистрировано клиническое улучшение или стабилизация заболевания; ВДТ+АуТКСК сопровождается существенным улучшением качества жизни больных. Результаты свидетельствуют о целесообразности изучения результатов ранней трансплантации, этапной трансплантации и трансплантации спасения. Необходимы дальнейшие исследования для определения оптимальных сроков проведения трансплан-тации и уточнения режимов кондиционирования.
</p>"
}
}
}
[1]=>
array(49) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
["~IBLOCK_SECTION_ID"]=>
string(2) "10"
["ID"]=>
string(3) "466"
["~ID"]=>
string(3) "466"
["IBLOCK_ID"]=>
string(1) "2"
["~IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["~NAME"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["ACTIVE_FROM"]=>
NULL
["~ACTIVE_FROM"]=>
NULL
["TIMESTAMP_X"]=>
string(19) "02.06.2017 18:48:33"
["~TIMESTAMP_X"]=>
string(19) "02.06.2017 18:48:33"
["DETAIL_PAGE_URL"]=>
string(145) "/ru/archive/Volume-1/korotkie-soobshcheniya/predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-/"
["~DETAIL_PAGE_URL"]=>
string(145) "/ru/archive/Volume-1/korotkie-soobshcheniya/predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-/"
["LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["~LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["DETAIL_TEXT"]=>
string(31628) "
Introduction
Graft-versus-host disease (GVHD) remains a major obstacle to successful allogeneic hematopoietic stem cell transplantation (HSCT), causing significant morbidity and mortality, especially in a case of allogeneic unrelated and haploidentical settings. The ability to prevent and treat GVHD is a key to success. A calcineurin inhibitor in combination with methotrexate is still the basic regimen for prophylaxis of both acute GVHD (aGVHD) and chronic GVHD (chGVHD). Steroid therapy still represents the first-line treatment for established GVHD, with a response rate of 30 to 50 %. However, the outcome for patients with severe, steroid-resistant, acute GVHD is poor, and overall survival is low, despite of steady increasing repertoire of available drugs. Improved knowledge of GVHD pathophysiology has led to rational approaches to both prophylaxis and therapy.
Within bone marrow (BM) stroma, there exist subsets of non-hematopoietic cells referred to as mesenchymal stem cells, or mesenchymal stromal cells [1]. MSCs comprise a population of nonhematopoietic bone marrow cells that possess an extensive proliferative potential and ability to differentiate into various cell types [2]. Therefore, it may be used to improve rate and quality of haematopoietic engraftment by regenerating the marrow microenvironment [1,3,5].
MSCs play a significant role in bone marrow microenvironment. The major function of these cells is to provide mechanical support to hematopoietic cells. MCSs express a large number of adhesion molecules, extracellular matrix proteins, cytokines and growth factor receptors, associated with their function and cell interactions within bone marrow stroma [2]. Moreover, MSCs are known to produce a variety of cytokines that are involved in homing (stromal derived factor-1, SDF-1), or proliferation and differentiation of hematopoietic cells (GM-CSF, SCF, IL-6). E.g., the engrafted MSCs may support human hematopoiesis via secreted factors and by physical interactions with hematopoietic cells [7,13].
Moreover, MSCs are able of modifying cellular immune response by multiple mechanisms, suppressing various T cell, B cell and NK cell functions [4,6,8,9], thus suggesting their possible use for treatment of immune-mediated disorders, like as GVHD [11,12]. Thus, MSC are currently under investigation for their potential reparative and immunosuppressive effects.
An opportunity of tolerance induction to allogeneic or xenogeneic grafts following incompatible bone marrow stem cell transplantation into a mismatched recipient was proposed since 1984 [14]. However, only in 2002 it has been clearly demonstrated that human MSCs may inhibit proliferation of T cells [6,8,9]. MSCs are generally considered to be poorly immunogenic cells, since they do not express neither HLA MHC class II antigens, FAS ligand, nor costimulatory molecules, such as В7-1, В7-2, CD40, CD40L on their surface [10]. In addition, MSCs are able to suppress a variety of T-, B-, and NK cell functions, and may affect also dendritic cell activities [9]. However, little is known about probable molecular mechanism(s) responsible for these effects.
Hence, potential applications of MSCs for prophylaxis and treatment of both acute and chronic severe GVHD seem to be quite reasonable [15,16,17]. Co-transplantation of allogeneic MSC and allogeneic HSCs could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution and suppression of GVHD in HSCT.
The aim of our present study was to test a hypothesis that co-transplantations of MSCs could be used either for GVHD prophylaxis, or treatment of severe acute or chronic GVHD following allogeneic HSCT.
Patients and methods
Eligible for current study were children and adults (their age ranged from 6 to 53) with different hematological malignancies, such as acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), non-Hodgkin lymphoma (NHL), myelodisplastic syndrome (MDS), chronic myeloid leukemia (CML).
From October 2005 to May 2008, eight patients received co-transplantation of HSC and MSCs for speeding up engraftment and prophylaxis of GVHD.
Sixteen pts received isolated infusions of MSCs for treatment of steroid-resistant GVHD. Patients or/and their caregivers were fully informed about all aspects of their participation in the study. A signed informed consent form was obtained in all cases.
When performing MSC co-transplantation, related allo-HSCTs were performed in five patients, unrelated allo-HSCTs, in two cases, and haploidentical HCST in one patient. The source of HSC was BM (six cases), peripheral blood stem cells (PBSC) in one patient, and a combination of BM and PBSC in one case.
Seven patients received nonmyeloablative conditioning regimen (fludarabine+melphalan in five pts, fludarabine+busulfan in two pts), and one patient was subject to a myeloablative treatment (busulfane+cyclophosphamide).
Acute GVHD prophylaxis was cyclosporineA (CsA) and methotrexate (Mtx) (short course) in seven patients and CsA and mycophenotate mofetil (MMF) in one case. Patients and transplant characteristics are presented in Table 2 and 3.
Table 2. Patient’s and transplant’s characteristics
Table 3. Number of MSC’s infusion and dosage of MSCs
MSCs were harvested from the BM of HLA-identical sibling in cases of related allo-HSCT (n=5), or from the BM of haploidentical donors in cases of unrelated allo-HSCT (n=3).
Before starting the treatment, BM was aspirated from MSC donor, MSCs been collected and selected. Bone marrow-derived MSCs for transplantation were produced by “Trans-Technology” Ltd Company (license № 99-01-002224 dd. 14.07.2005). Generally, in vitro MSC processing included their specific selection and expansion in culture during 21-28 days, until achieving sufficient therapeutical dose MSC for co-transplantation into HSC recipient (2.0x10^6 cells/kg body weight). (Figure 1 and Table 3). This study used PCR-based testing of common infectious pathogens in bone marrow.
The patients were given in vitro expanded MSCs intravenously 24 hours before HSC infusion. Design of use MSCs shown in Fig.1.
Figure 1. Design of usage of MSC for allogeneic hematopoietic stem cell transplantation
Isolated infusions of MSCs was performed in cases of steroid-resistant acute or chronic GVHD in patients after unrelated HSCT. Two patients had a mismatch in C locus, one, in DRB1 locus, one in B and C locus, one in DRB1 and C locus and three patients were haploidentical to their donors.
Ten patients received single MSC doses, five recipients - 2 doses, and one patient received three MSC doses (Table 2).
For thirteen patients, PBSCs were used as an HSC source. In cases of haplo-HSCT, HSCs represented a combination of G-CSF-primed marrow cells and T-cell depleted PBSCs (CliniMacs technique, Miltenyi Biotec).
MSCs were harvested from the BM of third-party donors in all cases before transplantation, and were cryopreserved until their use.
Results and discussion
A total of thirty-one infusions of mesenchymal stem cells were performed. Eight patients received co-transplantation of HSC and MSCs aiming to improve engraftment, and for GVHD prophylaxis. Sixteen patients received isolated infusions of MSCs for treatment of acute or chronic steroid-resistant GVHD.
Among them, ten patients received single MSC doses, five patients were treated with double MSC infusions, and one patient has got three doses (Table 3). MSC infusion was well tolerated, safe, without immediate infusion-related or late MSC-associated toxicities. Due to rather different indications for MSC infusions in cases of MSC co-transplantation versus isolated infusions, their results will be reviewed and discussed separately.
Results of MSCs use for speeding up engraftment and prevention of GVHD (co-transplantation of MSC and HSC)
According to peripheral leukocyte recovery, HSC engraftment was observed in seven pts (D+16 to +38), whereas platelet reconstitution proceeded by D+14 to +45 post-transplant. Hence, infusion of MSCs before HSCs did not improve engraftment rates as compared to HSCT without co-infusion of MSCs during conditioning (Table 4). After co-transplantation, six patients remained alive between 3 and 25 months. Severe aGVHD (grade III to IV) was not observed in MSC group. Six patients had aGVHD stage 0-I, and one patient exhibited stage II aGVHD. Chronic GVHD was not registered.
Table 4. Result of usage of MSC after co-transplantation
Additional infusions of MSCs for treatment of GVHD were not required due to the absence of severe GVHD. Two patients of this group died. In first case, graft failure was observed by D+16, complicated with disseminated intravenous coagulation and cerebral stroke. The second patient had disease progression and died at D+186. No treatment-related toxicities could be immediately ascribed to infusions of MSCs.
Overall 2.5 years relapse-free survival was 71%. No clinical complications were detected that could be attributed to MSC treatment.
Since non-myelоablative conditioning was used for 88% of co-transplanted patients, we have also compared the outcomes in these cases with general group after HSCT with reduced conditioning regimen.
Overall survival in MSC-treated group proved to be significantly higher, i.e., 71% after co-transplantation versus 34% after HSCT without MSCs (P=0,05). However, these data are rather preliminary and need further confirmation in larger series.
Mean incidence of infections in co-transplanted group was lower (25% against 48% in HSCT group). Two of eight patients developed respiratory, severe CMV and Aspergillus infection.
Result of MSC use for treatment of GVHD
Acute GVHD with involvement of skin was diagnosed in all patients (n=16), isolated involvement of skin (stage II-III) was detectable in eight patients. Combined aGVHD grade II with involvement of skin and liver was registered in one case, liver and gut GVHD, grade II-III was evident in four patients, and gut GVHD grade II-IV was found in three cases. One, two, or three MSC doses were administered, respectively, to ten, five, and one patient. Therefore, a total of twenty-three MSC infusions were performed. In ten patients, MSCs were used for treatment of acute GVHD, and in six cases they were applied for therapy of chronic GVHD.
After isolated infusions of MSCs in steroid-resistant aGVHD, a partial response (PR) was observed in five cases, complete response in two cases, without improvement in three cases. (Tab.5). Hence, after infusion of MSCs in the patients with aGVHD, a detectable response was observed in seven pts of ten (overall response rate 70%). Positive results of MSC administration for treatment of chGVHD were observed in 67%.
Table 5. Result of use MSC for treatment of GVHD
The median MSC dose did not differ for those patients who responded to the therapy, as compared with nonresponder group (2.0 x 10^6/kg b.w.of recipient). Six patients who responded to the first infusion were given a second infusion, to prevent GVHD recurrence upon reduction of immunosuppressive drug treatment. Two patients had complete response but received several (two or three) doses of MSCs because of GVHD recurrence. Four patients had partial responses and were given multiple (two or three) doses. Six of ten patients with involvement of one or two organs in aGVHD did respond to the therapy, as compared to four (of ten) patients with involvement of three organs.
Median time interval from onset of aGVHD to the start of treatment with MSC was 36 days (range 3-116).
Five patients were alive at the time of data analysis (May of 2008), with a median follow-up of 6,3 months (3-14,5 months) after infusion of MSCs.
Three patients had recurrences of their basic diseases, one with NHL, one with acute lymphoblastic leukaemia, and one with acute myeloid leukaemia. Fatal outcome was registered in all these cases. Acute GVHD was the most common cause of death in other cases (six patients of ten), with or without concomitant infection. One patient died with multiorgan failure. Infections in patients who died with acute or chronic GVHD included cytomegalovirus, aspergillosis and an unidentified pathogen.
In more than a half of patients with both acute and chGVHD, a single MSC dose produced a response, whereas in a few patients with partial response or with recurrence of acute or chronic GVHD, several doses were needed to induce a lasting response.
We have analyzed dependence of the response to MSCs infusion of various factors, such as conditioning regimen, GVHD prophylaxis, transplant type, donor and recipient characteristics. However, no significant differences were found, probably because of small and variable group of patients. There was relation between the treatment given before infusion of MSCs and response. In case of MSC infusion in the patients (n=7) after myeloablative HSCT, immunosupression and result of treatment of acute GVHD were better than after non-myeloablative regimen (Figure 2). Usage of ALG (Atgam by Pfizer) in GVHD prophylaxis did worsen the response to GVHD treatment with MSCs (Figure 3). In cases of HLA mismatch, HSCT response to MSCs infusion was more significant than after full-match HSCs. Patients transplanted from donor of opposite sex exhibited a more pronounced response to MSCs. ABO-incompatibility between donor and recipient did not influence response to MSCs. There are no differences in reactions to MSCs after GVHD prophylaxis with cyclosporine A versus tacrolimus.
Efficiency of MSCs therapy of GVHD with depending on:
Figure 2. Conditioning regimen
Figure 3. Usage of ALG
Conclusions
1. MSCs infusion in patients, who underwent allo-HSCT, is well-tolerated, safe, without immediate infusion-related or late MSC-associated toxicities.
2. Infusion of MSCs before HSCs transplantation did not influence duration of engraftment.
3. Infusion of MSCs during conditioning therapy before HSCT may prevent severe acute and chronic GVHD.
4. Infusion of MSCs for treatment-resistant both acute and chronic GVHD lead to reduction of GVHD grade in some patients.
5. Usage MSCs before HSC transplantation did not increase frequency of malignancy relapses.
6. Usage of MSCs seems to be more effective in patients after HSCT with myeloablative regimen and GVHD prophylaxis without ATG.
7. Large randomized clinical trials are necessary for evaluation of therapeutic effect of MSCs in allo-HSCT patients.
References
1. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow: analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-47.
2. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81-88.
3. Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307-16.
4. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11-20.
5. Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34 (+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870-78.
6. Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48.
7. Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733-38.
8. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-22.
9. Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, and Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplantation. 2004;33:597-604.
10. Eliopoulos N, Stagg J, Lejeune L, Pommey S, and Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005 Dec 15;106(13).
11. Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557-64.
12. Fibbe WE and Noort WA. Mesenchymal stem cell and hematopoietic stem cell transplantation. Ann N Y Acad Sci. 2003;996:235-244.
13. Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764-67.
14. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263-72.
15. Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390-97.
16. Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-41.
17. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo M, Remberger M, Dini G, Egeler R, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008 May 10;371.
"
["~DETAIL_TEXT"]=>
string(31628) "
Introduction
Graft-versus-host disease (GVHD) remains a major obstacle to successful allogeneic hematopoietic stem cell transplantation (HSCT), causing significant morbidity and mortality, especially in a case of allogeneic unrelated and haploidentical settings. The ability to prevent and treat GVHD is a key to success. A calcineurin inhibitor in combination with methotrexate is still the basic regimen for prophylaxis of both acute GVHD (aGVHD) and chronic GVHD (chGVHD). Steroid therapy still represents the first-line treatment for established GVHD, with a response rate of 30 to 50 %. However, the outcome for patients with severe, steroid-resistant, acute GVHD is poor, and overall survival is low, despite of steady increasing repertoire of available drugs. Improved knowledge of GVHD pathophysiology has led to rational approaches to both prophylaxis and therapy.
Within bone marrow (BM) stroma, there exist subsets of non-hematopoietic cells referred to as mesenchymal stem cells, or mesenchymal stromal cells [1]. MSCs comprise a population of nonhematopoietic bone marrow cells that possess an extensive proliferative potential and ability to differentiate into various cell types [2]. Therefore, it may be used to improve rate and quality of haematopoietic engraftment by regenerating the marrow microenvironment [1,3,5].
MSCs play a significant role in bone marrow microenvironment. The major function of these cells is to provide mechanical support to hematopoietic cells. MCSs express a large number of adhesion molecules, extracellular matrix proteins, cytokines and growth factor receptors, associated with their function and cell interactions within bone marrow stroma [2]. Moreover, MSCs are known to produce a variety of cytokines that are involved in homing (stromal derived factor-1, SDF-1), or proliferation and differentiation of hematopoietic cells (GM-CSF, SCF, IL-6). E.g., the engrafted MSCs may support human hematopoiesis via secreted factors and by physical interactions with hematopoietic cells [7,13].
Moreover, MSCs are able of modifying cellular immune response by multiple mechanisms, suppressing various T cell, B cell and NK cell functions [4,6,8,9], thus suggesting their possible use for treatment of immune-mediated disorders, like as GVHD [11,12]. Thus, MSC are currently under investigation for their potential reparative and immunosuppressive effects.
An opportunity of tolerance induction to allogeneic or xenogeneic grafts following incompatible bone marrow stem cell transplantation into a mismatched recipient was proposed since 1984 [14]. However, only in 2002 it has been clearly demonstrated that human MSCs may inhibit proliferation of T cells [6,8,9]. MSCs are generally considered to be poorly immunogenic cells, since they do not express neither HLA MHC class II antigens, FAS ligand, nor costimulatory molecules, such as В7-1, В7-2, CD40, CD40L on their surface [10]. In addition, MSCs are able to suppress a variety of T-, B-, and NK cell functions, and may affect also dendritic cell activities [9]. However, little is known about probable molecular mechanism(s) responsible for these effects.
Hence, potential applications of MSCs for prophylaxis and treatment of both acute and chronic severe GVHD seem to be quite reasonable [15,16,17]. Co-transplantation of allogeneic MSC and allogeneic HSCs could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution and suppression of GVHD in HSCT.
The aim of our present study was to test a hypothesis that co-transplantations of MSCs could be used either for GVHD prophylaxis, or treatment of severe acute or chronic GVHD following allogeneic HSCT.
Patients and methods
Eligible for current study were children and adults (their age ranged from 6 to 53) with different hematological malignancies, such as acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), non-Hodgkin lymphoma (NHL), myelodisplastic syndrome (MDS), chronic myeloid leukemia (CML).
From October 2005 to May 2008, eight patients received co-transplantation of HSC and MSCs for speeding up engraftment and prophylaxis of GVHD.
Sixteen pts received isolated infusions of MSCs for treatment of steroid-resistant GVHD. Patients or/and their caregivers were fully informed about all aspects of their participation in the study. A signed informed consent form was obtained in all cases.
When performing MSC co-transplantation, related allo-HSCTs were performed in five patients, unrelated allo-HSCTs, in two cases, and haploidentical HCST in one patient. The source of HSC was BM (six cases), peripheral blood stem cells (PBSC) in one patient, and a combination of BM and PBSC in one case.
Seven patients received nonmyeloablative conditioning regimen (fludarabine+melphalan in five pts, fludarabine+busulfan in two pts), and one patient was subject to a myeloablative treatment (busulfane+cyclophosphamide).
Acute GVHD prophylaxis was cyclosporineA (CsA) and methotrexate (Mtx) (short course) in seven patients and CsA and mycophenotate mofetil (MMF) in one case. Patients and transplant characteristics are presented in Table 2 and 3.
Table 2. Patient’s and transplant’s characteristics
Table 3. Number of MSC’s infusion and dosage of MSCs
MSCs were harvested from the BM of HLA-identical sibling in cases of related allo-HSCT (n=5), or from the BM of haploidentical donors in cases of unrelated allo-HSCT (n=3).
Before starting the treatment, BM was aspirated from MSC donor, MSCs been collected and selected. Bone marrow-derived MSCs for transplantation were produced by “Trans-Technology” Ltd Company (license № 99-01-002224 dd. 14.07.2005). Generally, in vitro MSC processing included their specific selection and expansion in culture during 21-28 days, until achieving sufficient therapeutical dose MSC for co-transplantation into HSC recipient (2.0x10^6 cells/kg body weight). (Figure 1 and Table 3). This study used PCR-based testing of common infectious pathogens in bone marrow.
The patients were given in vitro expanded MSCs intravenously 24 hours before HSC infusion. Design of use MSCs shown in Fig.1.
Figure 1. Design of usage of MSC for allogeneic hematopoietic stem cell transplantation
Isolated infusions of MSCs was performed in cases of steroid-resistant acute or chronic GVHD in patients after unrelated HSCT. Two patients had a mismatch in C locus, one, in DRB1 locus, one in B and C locus, one in DRB1 and C locus and three patients were haploidentical to their donors.
Ten patients received single MSC doses, five recipients - 2 doses, and one patient received three MSC doses (Table 2).
For thirteen patients, PBSCs were used as an HSC source. In cases of haplo-HSCT, HSCs represented a combination of G-CSF-primed marrow cells and T-cell depleted PBSCs (CliniMacs technique, Miltenyi Biotec).
MSCs were harvested from the BM of third-party donors in all cases before transplantation, and were cryopreserved until their use.
Results and discussion
A total of thirty-one infusions of mesenchymal stem cells were performed. Eight patients received co-transplantation of HSC and MSCs aiming to improve engraftment, and for GVHD prophylaxis. Sixteen patients received isolated infusions of MSCs for treatment of acute or chronic steroid-resistant GVHD.
Among them, ten patients received single MSC doses, five patients were treated with double MSC infusions, and one patient has got three doses (Table 3). MSC infusion was well tolerated, safe, without immediate infusion-related or late MSC-associated toxicities. Due to rather different indications for MSC infusions in cases of MSC co-transplantation versus isolated infusions, their results will be reviewed and discussed separately.
Results of MSCs use for speeding up engraftment and prevention of GVHD (co-transplantation of MSC and HSC)
According to peripheral leukocyte recovery, HSC engraftment was observed in seven pts (D+16 to +38), whereas platelet reconstitution proceeded by D+14 to +45 post-transplant. Hence, infusion of MSCs before HSCs did not improve engraftment rates as compared to HSCT without co-infusion of MSCs during conditioning (Table 4). After co-transplantation, six patients remained alive between 3 and 25 months. Severe aGVHD (grade III to IV) was not observed in MSC group. Six patients had aGVHD stage 0-I, and one patient exhibited stage II aGVHD. Chronic GVHD was not registered.

Table 4. Result of usage of MSC after co-transplantation
Additional infusions of MSCs for treatment of GVHD were not required due to the absence of severe GVHD. Two patients of this group died. In first case, graft failure was observed by D+16, complicated with disseminated intravenous coagulation and cerebral stroke. The second patient had disease progression and died at D+186. No treatment-related toxicities could be immediately ascribed to infusions of MSCs.
Overall 2.5 years relapse-free survival was 71%. No clinical complications were detected that could be attributed to MSC treatment.
Since non-myelоablative conditioning was used for 88% of co-transplanted patients, we have also compared the outcomes in these cases with general group after HSCT with reduced conditioning regimen.
Overall survival in MSC-treated group proved to be significantly higher, i.e., 71% after co-transplantation versus 34% after HSCT without MSCs (P=0,05). However, these data are rather preliminary and need further confirmation in larger series.
Mean incidence of infections in co-transplanted group was lower (25% against 48% in HSCT group). Two of eight patients developed respiratory, severe CMV and Aspergillus infection.
Result of MSC use for treatment of GVHD
Acute GVHD with involvement of skin was diagnosed in all patients (n=16), isolated involvement of skin (stage II-III) was detectable in eight patients. Combined aGVHD grade II with involvement of skin and liver was registered in one case, liver and gut GVHD, grade II-III was evident in four patients, and gut GVHD grade II-IV was found in three cases. One, two, or three MSC doses were administered, respectively, to ten, five, and one patient. Therefore, a total of twenty-three MSC infusions were performed. In ten patients, MSCs were used for treatment of acute GVHD, and in six cases they were applied for therapy of chronic GVHD.
After isolated infusions of MSCs in steroid-resistant aGVHD, a partial response (PR) was observed in five cases, complete response in two cases, without improvement in three cases. (Tab.5). Hence, after infusion of MSCs in the patients with aGVHD, a detectable response was observed in seven pts of ten (overall response rate 70%). Positive results of MSC administration for treatment of chGVHD were observed in 67%.
Table 5. Result of use MSC for treatment of GVHD
The median MSC dose did not differ for those patients who responded to the therapy, as compared with nonresponder group (2.0 x 10^6/kg b.w.of recipient). Six patients who responded to the first infusion were given a second infusion, to prevent GVHD recurrence upon reduction of immunosuppressive drug treatment. Two patients had complete response but received several (two or three) doses of MSCs because of GVHD recurrence. Four patients had partial responses and were given multiple (two or three) doses. Six of ten patients with involvement of one or two organs in aGVHD did respond to the therapy, as compared to four (of ten) patients with involvement of three organs.
Median time interval from onset of aGVHD to the start of treatment with MSC was 36 days (range 3-116).
Five patients were alive at the time of data analysis (May of 2008), with a median follow-up of 6,3 months (3-14,5 months) after infusion of MSCs.
Three patients had recurrences of their basic diseases, one with NHL, one with acute lymphoblastic leukaemia, and one with acute myeloid leukaemia. Fatal outcome was registered in all these cases. Acute GVHD was the most common cause of death in other cases (six patients of ten), with or without concomitant infection. One patient died with multiorgan failure. Infections in patients who died with acute or chronic GVHD included cytomegalovirus, aspergillosis and an unidentified pathogen.
In more than a half of patients with both acute and chGVHD, a single MSC dose produced a response, whereas in a few patients with partial response or with recurrence of acute or chronic GVHD, several doses were needed to induce a lasting response.
We have analyzed dependence of the response to MSCs infusion of various factors, such as conditioning regimen, GVHD prophylaxis, transplant type, donor and recipient characteristics. However, no significant differences were found, probably because of small and variable group of patients. There was relation between the treatment given before infusion of MSCs and response. In case of MSC infusion in the patients (n=7) after myeloablative HSCT, immunosupression and result of treatment of acute GVHD were better than after non-myeloablative regimen (Figure 2). Usage of ALG (Atgam by Pfizer) in GVHD prophylaxis did worsen the response to GVHD treatment with MSCs (Figure 3). In cases of HLA mismatch, HSCT response to MSCs infusion was more significant than after full-match HSCs. Patients transplanted from donor of opposite sex exhibited a more pronounced response to MSCs. ABO-incompatibility between donor and recipient did not influence response to MSCs. There are no differences in reactions to MSCs after GVHD prophylaxis with cyclosporine A versus tacrolimus.
Efficiency of MSCs therapy of GVHD with depending on:

Figure 2. Conditioning regimen
Figure 3. Usage of ALG
Conclusions
1. MSCs infusion in patients, who underwent allo-HSCT, is well-tolerated, safe, without immediate infusion-related or late MSC-associated toxicities.
2. Infusion of MSCs before HSCs transplantation did not influence duration of engraftment.
3. Infusion of MSCs during conditioning therapy before HSCT may prevent severe acute and chronic GVHD.
4. Infusion of MSCs for treatment-resistant both acute and chronic GVHD lead to reduction of GVHD grade in some patients.
5. Usage MSCs before HSC transplantation did not increase frequency of malignancy relapses.
6. Usage of MSCs seems to be more effective in patients after HSCT with myeloablative regimen and GVHD prophylaxis without ATG.
7. Large randomized clinical trials are necessary for evaluation of therapeutic effect of MSCs in allo-HSCT patients.
References
1. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow: analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-47.
2. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81-88.
3. Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307-16.
4. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11-20.
5. Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34 (+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870-78.
6. Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48.
7. Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733-38.
8. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-22.
9. Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, and Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplantation. 2004;33:597-604.
10. Eliopoulos N, Stagg J, Lejeune L, Pommey S, and Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005 Dec 15;106(13).
11. Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557-64.
12. Fibbe WE and Noort WA. Mesenchymal stem cell and hematopoietic stem cell transplantation. Ann N Y Acad Sci. 2003;996:235-244.
13. Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764-67.
14. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263-72.
15. Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390-97.
16. Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-41.
17. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo M, Remberger M, Dini G, Egeler R, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008 May 10;371.
"
["DETAIL_TEXT_TYPE"]=>
string(4) "html"
["~DETAIL_TEXT_TYPE"]=>
string(4) "html"
["PREVIEW_TEXT"]=>
string(0) ""
["~PREVIEW_TEXT"]=>
string(0) ""
["PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["~PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["PREVIEW_PICTURE"]=>
NULL
["~PREVIEW_PICTURE"]=>
NULL
["LANG_DIR"]=>
string(4) "/ru/"
["~LANG_DIR"]=>
string(4) "/ru/"
["SORT"]=>
string(3) "500"
["~SORT"]=>
string(3) "500"
["CODE"]=>
string(100) "predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-"
["~CODE"]=>
string(100) "predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-"
["EXTERNAL_ID"]=>
string(3) "466"
["~EXTERNAL_ID"]=>
string(3) "466"
["IBLOCK_TYPE_ID"]=>
string(7) "journal"
["~IBLOCK_TYPE_ID"]=>
string(7) "journal"
["IBLOCK_CODE"]=>
string(7) "volumes"
["~IBLOCK_CODE"]=>
string(7) "volumes"
["IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["~IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["LID"]=>
string(2) "s2"
["~LID"]=>
string(2) "s2"
["EDIT_LINK"]=>
NULL
["DELETE_LINK"]=>
NULL
["DISPLAY_ACTIVE_FROM"]=>
string(0) ""
["IPROPERTY_VALUES"]=>
array(18) {
["ELEMENT_META_TITLE"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["ELEMENT_META_KEYWORDS"]=>
string(200) "трансплантация гемопоэтических стволовых клеток острая РТПХ хроническая РТПХ мезенхимные стволовые клетки"
["ELEMENT_META_DESCRIPTION"]=>
string(398) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСКExperience of clinical mesenchymal stem cells (MSCs) usage for prophylaxis and GVHD treatment in patients undergoing allo-HSCT"
["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=>
string(6560) "<h2>Резюме</h2>
<h2>Введение </h2>
<p class="bodytext">
Костный мозг человека содержит гемопоэтические стволовые клетки (ГСК) и негемопоэтические стволовые клетки, называемые мезенхимными стволовыми клетками (МСК). Эти клетки улучшают приживление ГСК после аллогенной ТГСК и способствуют репарации тканей мезенхимного происхождения, а также способны модулировать иммунный ответ <em>in vitro</em> и <em>in vivo</em>. В результате, ко-трансплантация аллогенных МСК с аллогенными ГСК гипотетически обладает такими положительными эффектами, как улучшение приживления трансплантата и восстановление баланса внутри иммунной системы. Это обстоятельство может быть использовано как для профилактики РТПХ, так и для лечения острой стероид-резистентной РТПХ или хронической РТПХ. В данном исследовании показано, что на терапию МСК отвечают более половины пациентов со стероид-резистентной острой РТПХ.
</p>
<h2>Пациенты и методы</h2>
<p class="bodytext">
В исследование включены пациенты от 6 до 53 лет с ОЛЛ (n=9), ОМЛ (n=7), НХЛ (n=3), МДС (n=2) и ХМЛ (n=3), которым в период с октября 2005 по май 2008 была выполнена аллогенная ТГСК от родственного (n=5) или неродственного доноров (n=19). Для приживления ГСК и профилактики острой РТПХ 8 пациентам проведена ко-трансплантация МСК и ГСК. Шестнадцать пациентов получили изолированное введение МСК для лечения стероид-резистентной РТПХ. Десяти пациентам осуществлено одно введение МСК, пять пациентов два введения и один пациент получил три введения МСК. Процесс выделения и культивирования МСК осуществляли в компании «Транс Технологии» (лицензия № 99-01-002224 от 14.07.2005).
</p>
<h2>Результаты</h2>
<p class="bodytext">
В случае выполнения ко-трансплантации приживление лейкоцитов зарегистрировано на 21 день (от 16 до 38), тромбоцитов на 24 день (от 14 до 45). Острую РТПХ 0-I степеней наблюдали в 85,8% ко-трансплантаций, что не требовало дополнительной терапии, острая РТПХ II-IV развилась у 14,2 % пациентов. У всех пациентов хронической РТПХ не было. Инфекционные осложнения зарегистрированы у 2 пациентов (25%). Общая безрецидивная 2,5-летняя выживаемость составила 71%.<br>
Результаты применения МСК для терапии РТПХ представлены в таблице 1.
</p>
<div class="csc-textpic csc-textpic-intext-left-nowrap">
<div class="csc-textpic-imagewrap">
<dl class="csc-textpic-image csc-textpic-firstcol csc-textpic-lastcol" style="width:600px;">
<img width="420" alt="974b56410a.jpg" src="/upload/medialibrary/98f/98f24ae7195f0030c84d9bbad4190557.jpg" height="163" title="974b56410a.jpg">
</dl>
</div>
</div>
<span style="font-size: 17px; font-family: Cuprum, sans-serif; font-weight: bold; line-height: 24px;">Таблица 1. Результаты применения МСК для терапии РТПХ. </span>
<h2>Выводы</h2>
<p class="bodytext">
1. Инфузии МСК были безопасны, не сопровождались немедленными реакциями во время введения или отсроченными МСК-ассоциированными токсичностями.<br>
2. Инфузия МСК перед аллоТГСК не влияли на приживление трансплантата ГСК. <br>
3. Инфузия МСК при ко-трансплантации в режиме кондиционирования может предотвратить развитие тяжелых форм острой или хронической РТПХ.<br>
4. Инфузия МСК для лечения резистентной острой РТПХ может быть эффективным у ряда пациентов. <br>
5. Использование МСК перед аллоТГСК не увеличивало частоту рецидивов основного заболевания.<br>
6. Использование МСК более эффективно у пациентов, получивших миелоаблативный режим кондиционирования и профилактику острой РТПХ с применением АЛГ.<br>
7. Необходимо проведение дальнейших рандомизированных клинических исследований для оценки терапевтического эффекта МСК у пациентов после аллоТГСК и определения факторов, оказывающих влияние на эффективность МСК терапии.
</p>
"
["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_META_TITLE"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_META_KEYWORDS"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_META_DESCRIPTION"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_PICTURE_FILE_ALT"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_PICTURE_FILE_TITLE"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_PICTURE_FILE_NAME"]=>
string(100) "predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-"
["SECTION_DETAIL_PICTURE_FILE_ALT"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_DETAIL_PICTURE_FILE_TITLE"]=>
string(272) "Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК"
["SECTION_DETAIL_PICTURE_FILE_NAME"]=>
string(100) "predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-"
["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=>
string(100) "predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-"
["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=>
string(100) "predvaritelnye-dannye-klinicheskogo-ispolzovaniya-mezenkhimnykh-stvolovykh-kletok-dlya-profilaktiki-"
}
["FIELDS"]=>
array(1) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
}
["PROPERTIES"]=>
array(18) {
["KEYWORDS"]=>
array(36) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(5) "10703"
[1]=>
string(5) "10704"
[2]=>
string(5) "10705"
[3]=>
string(5) "10706"
}
["VALUE"]=>
array(4) {
[0]=>
string(2) "15"
[1]=>
string(3) "464"
[2]=>
string(3) "465"
[3]=>
string(2) "83"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(2) "15"
[1]=>
string(3) "464"
[2]=>
string(3) "465"
[3]=>
string(2) "83"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["SUBMITTED"]=>
array(36) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5237"
["VALUE"]=>
string(19) "07.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "07.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
}
["ACCEPTED"]=>
array(36) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5238"
["VALUE"]=>
string(19) "09.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "09.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
}
["PUBLISHED"]=>
array(36) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5239"
["VALUE"]=>
string(19) "23.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "23.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
}
["CONTACT"]=>
array(36) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5240"
["VALUE"]=>
string(3) "459"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "459"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHORS"]=>
array(36) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(10) {
[0]=>
string(5) "10707"
[1]=>
string(5) "10708"
[2]=>
string(5) "10709"
[3]=>
string(5) "10710"
[4]=>
string(5) "10711"
[5]=>
string(5) "10712"
[6]=>
string(5) "10713"
[7]=>
string(5) "10714"
[8]=>
string(5) "10715"
[9]=>
string(5) "10716"
}
["VALUE"]=>
array(10) {
[0]=>
string(3) "459"
[1]=>
string(3) "460"
[2]=>
string(2) "37"
[3]=>
string(2) "38"
[4]=>
string(3) "130"
[5]=>
string(2) "42"
[6]=>
string(3) "461"
[7]=>
string(3) "462"
[8]=>
string(3) "463"
[9]=>
string(2) "34"
}
["DESCRIPTION"]=>
array(10) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(10) {
[0]=>
string(3) "459"
[1]=>
string(3) "460"
[2]=>
string(2) "37"
[3]=>
string(2) "38"
[4]=>
string(3) "130"
[5]=>
string(2) "42"
[6]=>
string(3) "461"
[7]=>
string(3) "462"
[8]=>
string(3) "463"
[9]=>
string(2) "34"
}
["~DESCRIPTION"]=>
array(10) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_RU"]=>
array(36) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5251"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(308) "<p class="Autor">Станкевич Ю. А., Головачева А. А., Бабенко Е. В., Алянский А. Л., Паина О. В., Зубаровская Л. С., Семенова E. В., Полынцев Д. Г., Кругляков П. В., Афанасьев Б. В.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(286) "
Станкевич Ю. А., Головачева А. А., Бабенко Е. В., Алянский А. Л., Паина О. В., Зубаровская Л. С., Семенова E. В., Полынцев Д. Г., Кругляков П. В., Афанасьев Б. В.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_RU"]=>
array(36) {
["ID"]=>
string(2) "26"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(22) "Организации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "26"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(22) "Организации"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_RU"]=>
array(36) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5252"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(6560) "<h2>Резюме</h2>
<h2>Введение </h2>
<p class="bodytext">
Костный мозг человека содержит гемопоэтические стволовые клетки (ГСК) и негемопоэтические стволовые клетки, называемые мезенхимными стволовыми клетками (МСК). Эти клетки улучшают приживление ГСК после аллогенной ТГСК и способствуют репарации тканей мезенхимного происхождения, а также способны модулировать иммунный ответ <em>in vitro</em> и <em>in vivo</em>. В результате, ко-трансплантация аллогенных МСК с аллогенными ГСК гипотетически обладает такими положительными эффектами, как улучшение приживления трансплантата и восстановление баланса внутри иммунной системы. Это обстоятельство может быть использовано как для профилактики РТПХ, так и для лечения острой стероид-резистентной РТПХ или хронической РТПХ. В данном исследовании показано, что на терапию МСК отвечают более половины пациентов со стероид-резистентной острой РТПХ.
</p>
<h2>Пациенты и методы</h2>
<p class="bodytext">
В исследование включены пациенты от 6 до 53 лет с ОЛЛ (n=9), ОМЛ (n=7), НХЛ (n=3), МДС (n=2) и ХМЛ (n=3), которым в период с октября 2005 по май 2008 была выполнена аллогенная ТГСК от родственного (n=5) или неродственного доноров (n=19). Для приживления ГСК и профилактики острой РТПХ 8 пациентам проведена ко-трансплантация МСК и ГСК. Шестнадцать пациентов получили изолированное введение МСК для лечения стероид-резистентной РТПХ. Десяти пациентам осуществлено одно введение МСК, пять пациентов два введения и один пациент получил три введения МСК. Процесс выделения и культивирования МСК осуществляли в компании «Транс Технологии» (лицензия № 99-01-002224 от 14.07.2005).
</p>
<h2>Результаты</h2>
<p class="bodytext">
В случае выполнения ко-трансплантации приживление лейкоцитов зарегистрировано на 21 день (от 16 до 38), тромбоцитов на 24 день (от 14 до 45). Острую РТПХ 0-I степеней наблюдали в 85,8% ко-трансплантаций, что не требовало дополнительной терапии, острая РТПХ II-IV развилась у 14,2 % пациентов. У всех пациентов хронической РТПХ не было. Инфекционные осложнения зарегистрированы у 2 пациентов (25%). Общая безрецидивная 2,5-летняя выживаемость составила 71%.<br>
Результаты применения МСК для терапии РТПХ представлены в таблице 1.
</p>
<div class="csc-textpic csc-textpic-intext-left-nowrap">
<div class="csc-textpic-imagewrap">
<dl class="csc-textpic-image csc-textpic-firstcol csc-textpic-lastcol" style="width:600px;">
<img width="420" alt="974b56410a.jpg" src="/upload/medialibrary/98f/98f24ae7195f0030c84d9bbad4190557.jpg" height="163" title="974b56410a.jpg">
</dl>
</div>
</div>
<span style="font-size: 17px; font-family: Cuprum, sans-serif; font-weight: bold; line-height: 24px;">Таблица 1. Результаты применения МСК для терапии РТПХ. </span>
<h2>Выводы</h2>
<p class="bodytext">
1. Инфузии МСК были безопасны, не сопровождались немедленными реакциями во время введения или отсроченными МСК-ассоциированными токсичностями.<br>
2. Инфузия МСК перед аллоТГСК не влияли на приживление трансплантата ГСК. <br>
3. Инфузия МСК при ко-трансплантации в режиме кондиционирования может предотвратить развитие тяжелых форм острой или хронической РТПХ.<br>
4. Инфузия МСК для лечения резистентной острой РТПХ может быть эффективным у ряда пациентов. <br>
5. Использование МСК перед аллоТГСК не увеличивало частоту рецидивов основного заболевания.<br>
6. Использование МСК более эффективно у пациентов, получивших миелоаблативный режим кондиционирования и профилактику острой РТПХ с применением АЛГ.<br>
7. Необходимо проведение дальнейших рандомизированных клинических исследований для оценки терапевтического эффекта МСК у пациентов после аллоТГСК и определения факторов, оказывающих влияние на эффективность МСК терапии.
</p>
"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(6192) "
Резюме
Введение
Костный мозг человека содержит гемопоэтические стволовые клетки (ГСК) и негемопоэтические стволовые клетки, называемые мезенхимными стволовыми клетками (МСК). Эти клетки улучшают приживление ГСК после аллогенной ТГСК и способствуют репарации тканей мезенхимного происхождения, а также способны модулировать иммунный ответ in vitro и in vivo. В результате, ко-трансплантация аллогенных МСК с аллогенными ГСК гипотетически обладает такими положительными эффектами, как улучшение приживления трансплантата и восстановление баланса внутри иммунной системы. Это обстоятельство может быть использовано как для профилактики РТПХ, так и для лечения острой стероид-резистентной РТПХ или хронической РТПХ. В данном исследовании показано, что на терапию МСК отвечают более половины пациентов со стероид-резистентной острой РТПХ.
Пациенты и методы
В исследование включены пациенты от 6 до 53 лет с ОЛЛ (n=9), ОМЛ (n=7), НХЛ (n=3), МДС (n=2) и ХМЛ (n=3), которым в период с октября 2005 по май 2008 была выполнена аллогенная ТГСК от родственного (n=5) или неродственного доноров (n=19). Для приживления ГСК и профилактики острой РТПХ 8 пациентам проведена ко-трансплантация МСК и ГСК. Шестнадцать пациентов получили изолированное введение МСК для лечения стероид-резистентной РТПХ. Десяти пациентам осуществлено одно введение МСК, пять пациентов два введения и один пациент получил три введения МСК. Процесс выделения и культивирования МСК осуществляли в компании «Транс Технологии» (лицензия № 99-01-002224 от 14.07.2005).
Результаты
В случае выполнения ко-трансплантации приживление лейкоцитов зарегистрировано на 21 день (от 16 до 38), тромбоцитов на 24 день (от 14 до 45). Острую РТПХ 0-I степеней наблюдали в 85,8% ко-трансплантаций, что не требовало дополнительной терапии, острая РТПХ II-IV развилась у 14,2 % пациентов. У всех пациентов хронической РТПХ не было. Инфекционные осложнения зарегистрированы у 2 пациентов (25%). Общая безрецидивная 2,5-летняя выживаемость составила 71%.
Результаты применения МСК для терапии РТПХ представлены в таблице 1.
Таблица 1. Результаты применения МСК для терапии РТПХ.
Выводы
1. Инфузии МСК были безопасны, не сопровождались немедленными реакциями во время введения или отсроченными МСК-ассоциированными токсичностями.
2. Инфузия МСК перед аллоТГСК не влияли на приживление трансплантата ГСК.
3. Инфузия МСК при ко-трансплантации в режиме кондиционирования может предотвратить развитие тяжелых форм острой или хронической РТПХ.
4. Инфузия МСК для лечения резистентной острой РТПХ может быть эффективным у ряда пациентов.
5. Использование МСК перед аллоТГСК не увеличивало частоту рецидивов основного заболевания.
6. Использование МСК более эффективно у пациентов, получивших миелоаблативный режим кондиционирования и профилактику острой РТПХ с применением АЛГ.
7. Необходимо проведение дальнейших рандомизированных клинических исследований для оценки терапевтического эффекта МСК у пациентов после аллоТГСК и определения факторов, оказывающих влияние на эффективность МСК терапии.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["DOI"]=>
array(36) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5253"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000024.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000024.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_EN"]=>
array(36) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5254"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(432) "<p class="Autor">Stankevich Y.<sup>1</sup>, Golovacheva A.<sup>1</sup>, Babenko E.<sup>1</sup>, Alyansky A.<sup>1</sup>, Paina O.<sup>1</sup>, Zubarovskaya L.<sup>1</sup>, Semenova E.<sup>1</sup>, Polintsev D.<sup>2</sup>,<br>
Kruglyakov P.<sup>2</sup>, Afanasyev B.<sup>1</sup></p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(284) "
Stankevich Y.1, Golovacheva A.1, Babenko E.1, Alyansky A.1, Paina O.1, Zubarovskaya L.1, Semenova E.1, Polintsev D.2,
Kruglyakov P.2, Afanasyev B.1
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_EN"]=>
array(36) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5355"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(190) "<p><sup>1</sup>Pavlov State Medical University, St. Petersburg, Russia;<br>
<sup>2</sup>"TransTechnology" LtD, St. Petersburg, Russia</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(138) "
1Pavlov State Medical University, St. Petersburg, Russia;
2"TransTechnology" LtD, St. Petersburg, Russia
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_EN"]=>
array(36) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5385"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1415) "<h2>Summary</h2>
<p class="bodytext">
Within bone marrow stroma, there exist subsets of nonhematopoietic cells referred to as mesenchymal stem cells (MSCs), or mesenchymal stromal cells [<a href="#c1192" class="internal-link">1</a>]. These cells may not only improve HSC engraftment and regeneration of damaged tissues after allogeneic transplantation [<a href="#c1199" class="internal-link">7</a>], but also modulate immune responses <em>in vitro</em> and <em>in vivo</em> [<a href="#c1200" class="internal-link">8</a>]. Hence, co-transplantation of allogeneic HSC together with allogeneic MSC hypothetically could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution [<a href="#c1195" class="internal-link">4</a>], GVHD suppression, and it may be used for GVHD prophylaxis, like as for treatment of severe acute or chronic GVHD. This study shows that more than a half of the patients with steroid-refractory acute GVHD responded to treatment with MSCs. However, further randomized clinical trials are necessary for estimation of therapeutic effect of MSCs in allo-HSCT patients and definition of important and significant factors influenced upon MSCs infusion.<br>
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1223) "
Summary
Within bone marrow stroma, there exist subsets of nonhematopoietic cells referred to as mesenchymal stem cells (MSCs), or mesenchymal stromal cells [1]. These cells may not only improve HSC engraftment and regeneration of damaged tissues after allogeneic transplantation [7], but also modulate immune responses in vitro and in vivo [8]. Hence, co-transplantation of allogeneic HSC together with allogeneic MSC hypothetically could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution [4], GVHD suppression, and it may be used for GVHD prophylaxis, like as for treatment of severe acute or chronic GVHD. This study shows that more than a half of the patients with steroid-refractory acute GVHD responded to treatment with MSCs. However, further randomized clinical trials are necessary for estimation of therapeutic effect of MSCs in allo-HSCT patients and definition of important and significant factors influenced upon MSCs infusion.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["NAME_EN"]=>
array(36) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5255"
["VALUE"]=>
string(126) "Experience of clinical mesenchymal stem cells (MSCs) usage for prophylaxis and GVHD treatment in patients undergoing allo-HSCT"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(126) "Experience of clinical mesenchymal stem cells (MSCs) usage for prophylaxis and GVHD treatment in patients undergoing allo-HSCT"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["FULL_TEXT_RU"]=>
array(36) {
["ID"]=>
string(2) "42"
["TIMESTAMP_X"]=>
string(19) "2015-09-07 20:29:18"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(23) "Полный текст"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(12) "FULL_TEXT_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "42"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(23) "Полный текст"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["PDF_RU"]=>
array(36) {
["ID"]=>
string(2) "43"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF RUS"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_RU"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "43"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5256"
["VALUE"]=>
string(3) "109"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "109"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF RUS"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["PDF_EN"]=>
array(36) {
["ID"]=>
string(2) "44"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF ENG"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "44"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5356"
["VALUE"]=>
string(3) "111"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "111"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF ENG"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["NAME_LONG"]=>
array(36) {
["ID"]=>
string(2) "45"
["TIMESTAMP_X"]=>
string(19) "2023-04-13 00:55:00"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "NAME_LONG"
["DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "45"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(80)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["~DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
}
}
["DISPLAY_PROPERTIES"]=>
array(13) {
["AUTHOR_EN"]=>
array(37) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5254"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(432) "<p class="Autor">Stankevich Y.<sup>1</sup>, Golovacheva A.<sup>1</sup>, Babenko E.<sup>1</sup>, Alyansky A.<sup>1</sup>, Paina O.<sup>1</sup>, Zubarovskaya L.<sup>1</sup>, Semenova E.<sup>1</sup>, Polintsev D.<sup>2</sup>,<br>
Kruglyakov P.<sup>2</sup>, Afanasyev B.<sup>1</sup></p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(284) "
Stankevich Y.1, Golovacheva A.1, Babenko E.1, Alyansky A.1, Paina O.1, Zubarovskaya L.1, Semenova E.1, Polintsev D.2,
Kruglyakov P.2, Afanasyev B.1
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(284) "
Stankevich Y.1, Golovacheva A.1, Babenko E.1, Alyansky A.1, Paina O.1, Zubarovskaya L.1, Semenova E.1, Polintsev D.2,
Kruglyakov P.2, Afanasyev B.1
"
}
["SUMMARY_EN"]=>
array(37) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5385"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1415) "<h2>Summary</h2>
<p class="bodytext">
Within bone marrow stroma, there exist subsets of nonhematopoietic cells referred to as mesenchymal stem cells (MSCs), or mesenchymal stromal cells [<a href="#c1192" class="internal-link">1</a>]. These cells may not only improve HSC engraftment and regeneration of damaged tissues after allogeneic transplantation [<a href="#c1199" class="internal-link">7</a>], but also modulate immune responses <em>in vitro</em> and <em>in vivo</em> [<a href="#c1200" class="internal-link">8</a>]. Hence, co-transplantation of allogeneic HSC together with allogeneic MSC hypothetically could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution [<a href="#c1195" class="internal-link">4</a>], GVHD suppression, and it may be used for GVHD prophylaxis, like as for treatment of severe acute or chronic GVHD. This study shows that more than a half of the patients with steroid-refractory acute GVHD responded to treatment with MSCs. However, further randomized clinical trials are necessary for estimation of therapeutic effect of MSCs in allo-HSCT patients and definition of important and significant factors influenced upon MSCs infusion.<br>
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1223) "
Summary
Within bone marrow stroma, there exist subsets of nonhematopoietic cells referred to as mesenchymal stem cells (MSCs), or mesenchymal stromal cells [1]. These cells may not only improve HSC engraftment and regeneration of damaged tissues after allogeneic transplantation [7], but also modulate immune responses in vitro and in vivo [8]. Hence, co-transplantation of allogeneic HSC together with allogeneic MSC hypothetically could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution [4], GVHD suppression, and it may be used for GVHD prophylaxis, like as for treatment of severe acute or chronic GVHD. This study shows that more than a half of the patients with steroid-refractory acute GVHD responded to treatment with MSCs. However, further randomized clinical trials are necessary for estimation of therapeutic effect of MSCs in allo-HSCT patients and definition of important and significant factors influenced upon MSCs infusion.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(1223) "
Summary
Within bone marrow stroma, there exist subsets of nonhematopoietic cells referred to as mesenchymal stem cells (MSCs), or mesenchymal stromal cells [1]. These cells may not only improve HSC engraftment and regeneration of damaged tissues after allogeneic transplantation [7], but also modulate immune responses in vitro and in vivo [8]. Hence, co-transplantation of allogeneic HSC together with allogeneic MSC hypothetically could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution [4], GVHD suppression, and it may be used for GVHD prophylaxis, like as for treatment of severe acute or chronic GVHD. This study shows that more than a half of the patients with steroid-refractory acute GVHD responded to treatment with MSCs. However, further randomized clinical trials are necessary for estimation of therapeutic effect of MSCs in allo-HSCT patients and definition of important and significant factors influenced upon MSCs infusion.
"
}
["DOI"]=>
array(37) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5253"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000024.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000024.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(29) "10.3205/ctt-2008-en-000024.01"
}
["NAME_EN"]=>
array(37) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5255"
["VALUE"]=>
string(126) "Experience of clinical mesenchymal stem cells (MSCs) usage for prophylaxis and GVHD treatment in patients undergoing allo-HSCT"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(126) "Experience of clinical mesenchymal stem cells (MSCs) usage for prophylaxis and GVHD treatment in patients undergoing allo-HSCT"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(126) "Experience of clinical mesenchymal stem cells (MSCs) usage for prophylaxis and GVHD treatment in patients undergoing allo-HSCT"
}
["ORGANIZATION_EN"]=>
array(37) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5355"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(190) "<p><sup>1</sup>Pavlov State Medical University, St. Petersburg, Russia;<br>
<sup>2</sup>"TransTechnology" LtD, St. Petersburg, Russia</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(138) "
1Pavlov State Medical University, St. Petersburg, Russia;
2"TransTechnology" LtD, St. Petersburg, Russia
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(138) "
1Pavlov State Medical University, St. Petersburg, Russia;
2"TransTechnology" LtD, St. Petersburg, Russia
"
}
["AUTHORS"]=>
array(38) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(10) {
[0]=>
string(5) "10707"
[1]=>
string(5) "10708"
[2]=>
string(5) "10709"
[3]=>
string(5) "10710"
[4]=>
string(5) "10711"
[5]=>
string(5) "10712"
[6]=>
string(5) "10713"
[7]=>
string(5) "10714"
[8]=>
string(5) "10715"
[9]=>
string(5) "10716"
}
["VALUE"]=>
array(10) {
[0]=>
string(3) "459"
[1]=>
string(3) "460"
[2]=>
string(2) "37"
[3]=>
string(2) "38"
[4]=>
string(3) "130"
[5]=>
string(2) "42"
[6]=>
string(3) "461"
[7]=>
string(3) "462"
[8]=>
string(3) "463"
[9]=>
string(2) "34"
}
["DESCRIPTION"]=>
array(10) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(10) {
[0]=>
string(3) "459"
[1]=>
string(3) "460"
[2]=>
string(2) "37"
[3]=>
string(2) "38"
[4]=>
string(3) "130"
[5]=>
string(2) "42"
[6]=>
string(3) "461"
[7]=>
string(3) "462"
[8]=>
string(3) "463"
[9]=>
string(2) "34"
}
["~DESCRIPTION"]=>
array(10) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(10) {
[0]=>
string(56) "
Stankevich Y."
[1]=>
string(57) "
Golovacheva A."
[2]=>
string(55) "
Elena Babenko"
[3]=>
string(62) "
Aleksander Alyanskiy"
[4]=>
string(58) "
Olesya V. Paina"
[5]=>
string(65) "
Ludmila S. Zubarovskaya"
[6]=>
string(54) "
Semenova E."
[7]=>
string(55) "
Polintsev D."
[8]=>
string(56) "
Kruglyakov P."
[9]=>
string(60) "
Boris V. Afanasyev"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["AUTHOR_RU"]=>
array(37) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5251"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(308) "<p class="Autor">Станкевич Ю. А., Головачева А. А., Бабенко Е. В., Алянский А. Л., Паина О. В., Зубаровская Л. С., Семенова E. В., Полынцев Д. Г., Кругляков П. В., Афанасьев Б. В.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(286) "
Станкевич Ю. А., Головачева А. А., Бабенко Е. В., Алянский А. Л., Паина О. В., Зубаровская Л. С., Семенова E. В., Полынцев Д. Г., Кругляков П. В., Афанасьев Б. В.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(286) "
Станкевич Ю. А., Головачева А. А., Бабенко Е. В., Алянский А. Л., Паина О. В., Зубаровская Л. С., Семенова E. В., Полынцев Д. Г., Кругляков П. В., Афанасьев Б. В.
"
}
["SUBMITTED"]=>
array(37) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5237"
["VALUE"]=>
string(19) "07.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "07.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "07.11.2008 00:01:00"
}
["ACCEPTED"]=>
array(37) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5238"
["VALUE"]=>
string(19) "09.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "09.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "09.12.2008 00:01:00"
}
["PUBLISHED"]=>
array(37) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5239"
["VALUE"]=>
string(19) "23.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "23.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "23.12.2008 00:01:00"
}
["KEYWORDS"]=>
array(38) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(5) "10703"
[1]=>
string(5) "10704"
[2]=>
string(5) "10705"
[3]=>
string(5) "10706"
}
["VALUE"]=>
array(4) {
[0]=>
string(2) "15"
[1]=>
string(3) "464"
[2]=>
string(3) "465"
[3]=>
string(2) "83"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(2) "15"
[1]=>
string(3) "464"
[2]=>
string(3) "465"
[3]=>
string(2) "83"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(4) {
[0]=>
string(134) "
трансплантация гемопоэтических стволовых клеток"
[1]=>
string(65) "
острая РТПХ"
[2]=>
string(75) "
хроническая РТПХ"
[3]=>
string(97) "
мезенхимные стволовые клетки"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["CONTACT"]=>
array(38) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5240"
["VALUE"]=>
string(3) "459"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "459"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(56) "
Stankevich Y."
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["SUMMARY_RU"]=>
array(37) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5252"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(6560) "<h2>Резюме</h2>
<h2>Введение </h2>
<p class="bodytext">
Костный мозг человека содержит гемопоэтические стволовые клетки (ГСК) и негемопоэтические стволовые клетки, называемые мезенхимными стволовыми клетками (МСК). Эти клетки улучшают приживление ГСК после аллогенной ТГСК и способствуют репарации тканей мезенхимного происхождения, а также способны модулировать иммунный ответ <em>in vitro</em> и <em>in vivo</em>. В результате, ко-трансплантация аллогенных МСК с аллогенными ГСК гипотетически обладает такими положительными эффектами, как улучшение приживления трансплантата и восстановление баланса внутри иммунной системы. Это обстоятельство может быть использовано как для профилактики РТПХ, так и для лечения острой стероид-резистентной РТПХ или хронической РТПХ. В данном исследовании показано, что на терапию МСК отвечают более половины пациентов со стероид-резистентной острой РТПХ.
</p>
<h2>Пациенты и методы</h2>
<p class="bodytext">
В исследование включены пациенты от 6 до 53 лет с ОЛЛ (n=9), ОМЛ (n=7), НХЛ (n=3), МДС (n=2) и ХМЛ (n=3), которым в период с октября 2005 по май 2008 была выполнена аллогенная ТГСК от родственного (n=5) или неродственного доноров (n=19). Для приживления ГСК и профилактики острой РТПХ 8 пациентам проведена ко-трансплантация МСК и ГСК. Шестнадцать пациентов получили изолированное введение МСК для лечения стероид-резистентной РТПХ. Десяти пациентам осуществлено одно введение МСК, пять пациентов два введения и один пациент получил три введения МСК. Процесс выделения и культивирования МСК осуществляли в компании «Транс Технологии» (лицензия № 99-01-002224 от 14.07.2005).
</p>
<h2>Результаты</h2>
<p class="bodytext">
В случае выполнения ко-трансплантации приживление лейкоцитов зарегистрировано на 21 день (от 16 до 38), тромбоцитов на 24 день (от 14 до 45). Острую РТПХ 0-I степеней наблюдали в 85,8% ко-трансплантаций, что не требовало дополнительной терапии, острая РТПХ II-IV развилась у 14,2 % пациентов. У всех пациентов хронической РТПХ не было. Инфекционные осложнения зарегистрированы у 2 пациентов (25%). Общая безрецидивная 2,5-летняя выживаемость составила 71%.<br>
Результаты применения МСК для терапии РТПХ представлены в таблице 1.
</p>
<div class="csc-textpic csc-textpic-intext-left-nowrap">
<div class="csc-textpic-imagewrap">
<dl class="csc-textpic-image csc-textpic-firstcol csc-textpic-lastcol" style="width:600px;">
<img width="420" alt="974b56410a.jpg" src="/upload/medialibrary/98f/98f24ae7195f0030c84d9bbad4190557.jpg" height="163" title="974b56410a.jpg">
</dl>
</div>
</div>
<span style="font-size: 17px; font-family: Cuprum, sans-serif; font-weight: bold; line-height: 24px;">Таблица 1. Результаты применения МСК для терапии РТПХ. </span>
<h2>Выводы</h2>
<p class="bodytext">
1. Инфузии МСК были безопасны, не сопровождались немедленными реакциями во время введения или отсроченными МСК-ассоциированными токсичностями.<br>
2. Инфузия МСК перед аллоТГСК не влияли на приживление трансплантата ГСК. <br>
3. Инфузия МСК при ко-трансплантации в режиме кондиционирования может предотвратить развитие тяжелых форм острой или хронической РТПХ.<br>
4. Инфузия МСК для лечения резистентной острой РТПХ может быть эффективным у ряда пациентов. <br>
5. Использование МСК перед аллоТГСК не увеличивало частоту рецидивов основного заболевания.<br>
6. Использование МСК более эффективно у пациентов, получивших миелоаблативный режим кондиционирования и профилактику острой РТПХ с применением АЛГ.<br>
7. Необходимо проведение дальнейших рандомизированных клинических исследований для оценки терапевтического эффекта МСК у пациентов после аллоТГСК и определения факторов, оказывающих влияние на эффективность МСК терапии.
</p>
"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(6192) "
Резюме
Введение
Костный мозг человека содержит гемопоэтические стволовые клетки (ГСК) и негемопоэтические стволовые клетки, называемые мезенхимными стволовыми клетками (МСК). Эти клетки улучшают приживление ГСК после аллогенной ТГСК и способствуют репарации тканей мезенхимного происхождения, а также способны модулировать иммунный ответ in vitro и in vivo. В результате, ко-трансплантация аллогенных МСК с аллогенными ГСК гипотетически обладает такими положительными эффектами, как улучшение приживления трансплантата и восстановление баланса внутри иммунной системы. Это обстоятельство может быть использовано как для профилактики РТПХ, так и для лечения острой стероид-резистентной РТПХ или хронической РТПХ. В данном исследовании показано, что на терапию МСК отвечают более половины пациентов со стероид-резистентной острой РТПХ.
Пациенты и методы
В исследование включены пациенты от 6 до 53 лет с ОЛЛ (n=9), ОМЛ (n=7), НХЛ (n=3), МДС (n=2) и ХМЛ (n=3), которым в период с октября 2005 по май 2008 была выполнена аллогенная ТГСК от родственного (n=5) или неродственного доноров (n=19). Для приживления ГСК и профилактики острой РТПХ 8 пациентам проведена ко-трансплантация МСК и ГСК. Шестнадцать пациентов получили изолированное введение МСК для лечения стероид-резистентной РТПХ. Десяти пациентам осуществлено одно введение МСК, пять пациентов два введения и один пациент получил три введения МСК. Процесс выделения и культивирования МСК осуществляли в компании «Транс Технологии» (лицензия № 99-01-002224 от 14.07.2005).
Результаты
В случае выполнения ко-трансплантации приживление лейкоцитов зарегистрировано на 21 день (от 16 до 38), тромбоцитов на 24 день (от 14 до 45). Острую РТПХ 0-I степеней наблюдали в 85,8% ко-трансплантаций, что не требовало дополнительной терапии, острая РТПХ II-IV развилась у 14,2 % пациентов. У всех пациентов хронической РТПХ не было. Инфекционные осложнения зарегистрированы у 2 пациентов (25%). Общая безрецидивная 2,5-летняя выживаемость составила 71%.
Результаты применения МСК для терапии РТПХ представлены в таблице 1.
Таблица 1. Результаты применения МСК для терапии РТПХ.
Выводы
1. Инфузии МСК были безопасны, не сопровождались немедленными реакциями во время введения или отсроченными МСК-ассоциированными токсичностями.
2. Инфузия МСК перед аллоТГСК не влияли на приживление трансплантата ГСК.
3. Инфузия МСК при ко-трансплантации в режиме кондиционирования может предотвратить развитие тяжелых форм острой или хронической РТПХ.
4. Инфузия МСК для лечения резистентной острой РТПХ может быть эффективным у ряда пациентов.
5. Использование МСК перед аллоТГСК не увеличивало частоту рецидивов основного заболевания.
6. Использование МСК более эффективно у пациентов, получивших миелоаблативный режим кондиционирования и профилактику острой РТПХ с применением АЛГ.
7. Необходимо проведение дальнейших рандомизированных клинических исследований для оценки терапевтического эффекта МСК у пациентов после аллоТГСК и определения факторов, оказывающих влияние на эффективность МСК терапии.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(6192) "
Резюме
Введение
Костный мозг человека содержит гемопоэтические стволовые клетки (ГСК) и негемопоэтические стволовые клетки, называемые мезенхимными стволовыми клетками (МСК). Эти клетки улучшают приживление ГСК после аллогенной ТГСК и способствуют репарации тканей мезенхимного происхождения, а также способны модулировать иммунный ответ in vitro и in vivo. В результате, ко-трансплантация аллогенных МСК с аллогенными ГСК гипотетически обладает такими положительными эффектами, как улучшение приживления трансплантата и восстановление баланса внутри иммунной системы. Это обстоятельство может быть использовано как для профилактики РТПХ, так и для лечения острой стероид-резистентной РТПХ или хронической РТПХ. В данном исследовании показано, что на терапию МСК отвечают более половины пациентов со стероид-резистентной острой РТПХ.
Пациенты и методы
В исследование включены пациенты от 6 до 53 лет с ОЛЛ (n=9), ОМЛ (n=7), НХЛ (n=3), МДС (n=2) и ХМЛ (n=3), которым в период с октября 2005 по май 2008 была выполнена аллогенная ТГСК от родственного (n=5) или неродственного доноров (n=19). Для приживления ГСК и профилактики острой РТПХ 8 пациентам проведена ко-трансплантация МСК и ГСК. Шестнадцать пациентов получили изолированное введение МСК для лечения стероид-резистентной РТПХ. Десяти пациентам осуществлено одно введение МСК, пять пациентов два введения и один пациент получил три введения МСК. Процесс выделения и культивирования МСК осуществляли в компании «Транс Технологии» (лицензия № 99-01-002224 от 14.07.2005).
Результаты
В случае выполнения ко-трансплантации приживление лейкоцитов зарегистрировано на 21 день (от 16 до 38), тромбоцитов на 24 день (от 14 до 45). Острую РТПХ 0-I степеней наблюдали в 85,8% ко-трансплантаций, что не требовало дополнительной терапии, острая РТПХ II-IV развилась у 14,2 % пациентов. У всех пациентов хронической РТПХ не было. Инфекционные осложнения зарегистрированы у 2 пациентов (25%). Общая безрецидивная 2,5-летняя выживаемость составила 71%.
Результаты применения МСК для терапии РТПХ представлены в таблице 1.
Таблица 1. Результаты применения МСК для терапии РТПХ.
Выводы
1. Инфузии МСК были безопасны, не сопровождались немедленными реакциями во время введения или отсроченными МСК-ассоциированными токсичностями.
2. Инфузия МСК перед аллоТГСК не влияли на приживление трансплантата ГСК.
3. Инфузия МСК при ко-трансплантации в режиме кондиционирования может предотвратить развитие тяжелых форм острой или хронической РТПХ.
4. Инфузия МСК для лечения резистентной острой РТПХ может быть эффективным у ряда пациентов.
5. Использование МСК перед аллоТГСК не увеличивало частоту рецидивов основного заболевания.
6. Использование МСК более эффективно у пациентов, получивших миелоаблативный режим кондиционирования и профилактику острой РТПХ с применением АЛГ.
7. Необходимо проведение дальнейших рандомизированных клинических исследований для оценки терапевтического эффекта МСК у пациентов после аллоТГСК и определения факторов, оказывающих влияние на эффективность МСК терапии.
"
}
}
}
[2]=>
array(49) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
["~IBLOCK_SECTION_ID"]=>
string(2) "10"
["ID"]=>
string(3) "472"
["~ID"]=>
string(3) "472"
["IBLOCK_ID"]=>
string(1) "2"
["~IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["~NAME"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["ACTIVE_FROM"]=>
NULL
["~ACTIVE_FROM"]=>
NULL
["TIMESTAMP_X"]=>
string(19) "17.06.2016 15:08:36"
["~TIMESTAMP_X"]=>
string(19) "17.06.2016 15:08:36"
["DETAIL_PAGE_URL"]=>
string(127) "/ru/archive/Volume-1/korotkie-soobshcheniya/sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka/"
["~DETAIL_PAGE_URL"]=>
string(127) "/ru/archive/Volume-1/korotkie-soobshcheniya/sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka/"
["LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["~LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["DETAIL_TEXT"]=>
string(48905) "
Introduction
Mesenchymal stem cells (MSC) constitute a rare population of multipotent progenitors capable of both supporting hematopoiesis and differentiating into at least osteogenic, adipogenic and chondrogenic lineages [1-3]. Moreover, they exhibit immunomodulatory activity, induce immunotolerance in case of allogenic transplantation [4], and posess the ability to expand in relatively simple in vitro systems [5-7]. These characteristics make MSCs very promising candidates in the development of new cell-based therapeutic strategies, such as the treatment of tissue injuries or the supportive application by hematopoietic stem cell transplantation. Taking into consideration that the number of MSCs and their differentiation capacity decline with age [8], most relevant is the search for alternative sources of these cells.
The advantages of umbilical cord blood (UCB), as a source of stem cells, include their accessibility, non-invasive sampling and, thus, their safety for potential donors [9-11]. Another reason for this study was due to some controversial data about amounts of mesenchymal precursors in UCB [12-17]. Mesenchymal precursors (CFU-F) are found in foetal blood in the early gestational period at concentrations equal to their amounts in the bone-marrow of newborns [18]. However, after the second trimester, the number of MSCs decreases considerably, and, at birth, their frequency in UCB is quite accidental [12,19]. The need for testing of some standards for culturing adhesive cell fractions from UCB, as well as assessment of their contents and functional characteristics provided other motivations for this study.
Materials and Methods
Collection of UCB
UCB samples (mean volume of 60 ml) from full-term deliveries were collected from the unborn placenta after obtaining the mothers' informed consent. A sterile bag system containing citrate phosphate dextrose (CPD) anticoagulant within the collection bag (manufactured by Terumo, Japan) was used. The units were stored at room temperature before processing (up to 33 hours).
Isolation and Culture of Adherent Cells from UCB
To isolate mononuclear cells (MNCs), each UCB unit was diluted 1:1 with phosphate-buffered saline (PBS)/2 mM EDTA (Biolot, Russia), and carefully loaded onto Ficoll-Hypaque solution (Lympho separation medium, ICN, USA, d=1.077). Following a density gradient centrifugation at 435 g for 30 minutes at room temperature, MNCs were removed from the interphase layer and washed two to three times with PBS/EDTA. UCB-derived MNCs were set at a density of 1 x 106/cm2 into six-well culture plates (Corning, USA) containing DMEM low-glucose medium (Gibco, USA) with 20% fetal calf serum (FCS) from selected lots, penicillin 100 UI/ml, and streptomycin 0.1 mg/ml (Gibco, USA).
After overnight incubation at 37°C in a humidified atmosphere containing 5% carbon dioxide, nonadherent cells were removed and fresh medium was added to the wells. Cell cultures were maintained, and remaining nonadherent cells were discarded via the complete exchange of culture medium every 7 days. Culture plates were screened continuously to detect developing colonies of adherent cells. The number of fibroblast colony forming units (CFU-F) was calculated by counting the number of colonies per 108 MNCs.
Fibroblast-like cells were detached between days 16 through 20 after initial plating using 0.04% Trypsin/0.03% EDTA (Gibco, USA). The recovered cells were replated at a density of 4,000 to 5,000 cells/cm2.
Collection and Isolation of Control MSCs from Bone Marrow
MSCs from bone marrow (BM) were obtained by bone marrow puncture. BM cells were aspirated into a 5-ml syringe containing CPD anticoagulant. A total of six samples were obtained, with the donor age ranging from 24 to 56 years. To isolate MSCs from BM, the aspirate was diluted 1:5 and processed as described above. In contrast to MNCs from UCB samples, BM-derived MNCs were cultured at a density of 1 x 106 cells/cm2 in T75 culture flasks (Corning, USA), and the first change of medium was performed 3 days after initial plating. Two weeks later, at reaching 80%–90% confluence, MSCs were detached using trypsin and replated as described for the UCB-derived adherent cells.
Primary Fibroblasts as Controls
Primary cultures of normal human dermal fibroblasts served as negative control in differentiation and comparative gene expression studies. Culture conditions were comparable to BM MSC’s expansion: DMEM low glucose medium (Gibco, USA) containing 20% fetal calf serum (FCS), penicillin 100 UI/ml, and streptomycin 0.1 mg/ml (Gibco, USA).
Immune Phenotypic Analysis
To analyze the cell-surface expression of typical marker proteins in UCB- and BM-derived adherent cells, each from primary culture and second passage, these were labeled with the following anti-human antibodies: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR,DP,DQ FITC; CD 90 PE (Becton Dickinson, USA). Murine isotype antibodies (Becton Dickinson, USA) served as respective controls. Ten thousand labeled cell aliquotes were analyzed using a FACScan flow cytometer running CellQuest software (Becton Dickinson, USA).
Aiming at a subset analysis of the population, we employed the following indices:
• hematopoietic cells index – CD45+ to CD45- cells
• myeloid cell maturation index – CD14+ to CD45+ cells
• hematopoietic progenitor cell index – CD34+CD45+ to CD45+ cells
• endothelial cell-precursors index – CD34+CD45- to CD45- cells
• mesenchymal precursors index – CD90+CD31- to CD45- cells
In vitro Osteogenic and Adipogenic Differentiation Studies
To induce osteogenic differentiation, the cells were seeded at a density of 3.1 x 103 cells/cm2 and cultured in six-well microplates (Costar, USA), until they reached approximately 80% confluence. Additional culture was performed in osteogenic differentiation medium supplemented with 0.1 µM dexamethasone, 10 mM ß-glycerophosphate, 0.05 mM ascorbate, and 10% FCS. The onset of osteoblast formation was evaluated after 3 weeks via calcium accumulation. Accumulation of mineralized calcium phosphate was assessed with von Kossa staining after the protocol from Cheng et al. [20], with a few modifications. The cells were fixed for 15 minutes in 10% formalin (Sigma-Aldrich), and, after washing, they were incubated with 5% silver nitrate (Sigma-Aldrich) for 15 to 30 minutes. Pyrogallol 1% (Merck, Canada) and sodium thiosulfate 5% (Sigma-Aldrich) were used to develop and register a resulting image. In addition, the mineralized matrix was also evaluated by Alizarin-red S staining using 4% formaldehyde for fixation and 1% aqueous Alizarin-Red S (Sigma-Aldrich) solution.
Adipogenic differentiation was induced according to the protocol of Pittenger et al. [2]. Special induction medium, containing DMEM (high glucose), 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methyl-xanthine, 10 µg/ml recombinant human (rh) insulin, 0.2 mM indomethacin, and 20% FCS, was added for 2 to 3 days to the culture microplates. It was then replaced by maintenance medium containing only rh-insulin and 20% FCS. Induction of adipogenic differentiation was apparent via the intracellular accumulation of lipid-rich vacuoles that stained with Oil Red O (Sigma-Aldrich). The cells were fixed with 10% formalin, washed, and stained with a working solution of 0.18% Oil Red O for 5 minutes.
In vitro Model for Studying Colony-stimulating Activity of UCB cells
An essential property of mesenchymal stem cells is their capacity to support hematopoiesis. In order to assess the hemostimulating capacity of the UCB monolayer culture, a modified technique proposed by Afanasyev [21,22] was used (Figure 1). This method determines the clonogenic capacity of granulocytic-macrophage colony forming units (CFU-GM) in “agar drop-liquid medium” culture.
Figure 1. Agar drop-liquid culture system[22]
A-medium; B-feeder cells; C,D,E-colonies; F-clusters; G-agar
The monolayer culture of UCB-derived adherent cells (confluence rate: 70–80%) was used as a source of colony-stimulating activity of MSC from UCB. The cells (CFU-GM) from the UCB mononuclear fraction were targeted in this assay, providing clonal growth in agar cultures. Semisolid agar drops containing target cells were prepared on dry surfaces of sterile Petri dishes. The resulting semisolid drops were transferred to the dishes, and co-incubated for 7 days in the following four versions: 1) with complete culture medium only; 2) with medium containing standard-type leukocyte feeder; 3) with UCB confluent monolayer culture; 4) with both UCB confluent monolayer culture and standard-type leukocyte feeder. Each experiment was performed at least twice.
Colony-forming ability (CFA) and cluster-forming ability (ClFA) were classified according to the number of cells in the colonies (small colonies: containing 20–40 cells; medium-size colonies: 41–100 cells; and large colonies: more than 100 cells) and clusters (large clusters: 10–19 cells; small clusters: 5–9 cells). Depending on the total number of colonies and clusters, the cloning efficiency (CE) per 1х105 explanted mononuclear cells was assessed. A “colony-to-cluster” ratio (Co-Cl) and percentage of large colonies (LC) were supplemental parameters assessed in these cell cultures. Using such parameters, the proliferative potential of the target progenitor cells was tested.
Total RNA Isolation and RT-PCR
Total RNA was extracted from 3 to 30 x105 MSCs using Trizol Reagents (Invitrogen, USA), according to the manufacturer’s instructions. mRNA was subject to reverse transcription (RT) using Superscript II Kit (Invitrogen, USA), again using the manufacturer’s instructions. The resulting cDNA was amplified using an ABI GeneAmp PCR System 2400 (Perkin Elmer Applied Biosystems, Boston, MA) at 94°C for 40 seconds, 56°C for 50 seconds, and 72°C for 60 seconds for 35 cycles, after initial denaturation at 94°C for 5 minutes. Primers used for PCR are listed in Table 1. Fifteen microliters of PCR reaction were fractionated by agarose gel electrophoresis.
Table 1. Primers used for RT-PCR
Statistical Analysis
The statistical significance of the inter-group differences was evaluated with the Mann-Whitney test. The degrees of correlations between the parameters were evaluated by the Spearman test. The differences were considered significant by p values <0.05. The Statistica 6.0 software package was used for all statistical analyses.
Results and Discussion
In most cases, the cultures of plastic-adherent cells proved to be heterogenous. Two main morphological cell types were discernable: spindle-shaped cells that are presumably regarded as mesenchymal stem cells (MSC), and polygonal cells that are most likely of hematopoietic origin (Figure 2).
Figure 2. Heterogeneous UCB culture. No colony formation was observed. UCB samples produced a minimal, non-confluent adherent layer of heterogeneous cells.
In some UCB samples, clonal growth was observed, however the mean cell number per colony did not increase over 100 cells (Figure 3). Large colonies were detectable in 3 of the 40 UCB samples under study. These colonies (>1000 cells per colony) consisted of closely packed, spindle-shaped cells, typical of fibroblast morphology cells.
Figure 3. CFU-F in UCB culture. Adherently growing cells of fibroblastic morphology formed big colonies in 3 UCB samples.
The Phenotypic Composition of UCB Monolayer Cultures
Our efforts to define a distinct phenotype characteristic for MSC have been confounded by the fact that these cells can express a range of cell lineage-specific antigens [2,23].
During analysis of the predominant cellular types, it was discovered that the major fraction (median 60.17%) of plastic-adherent cells from UCB were of hematopoietic origin (CD45+) (Table 2). One-third of the CD45-positive cells belonged to the CD14-positive population fraction (median 14.81%). Cells in the monolayer with an osteoclast-like phenotype (CD45+CD61+) constituted approximately 1.5%. The cells phenotypically comparable with hematopoietic stem cells (HSC-like cells: CD34+CD45+, CD34+HLA DR-, CD117+) were present at a low concentration in the UCB monolayer culture - less than 1.5%.
Table 2. The composition of the UCB monolayer culture.
The numbers of endothelial-like cells in the monolayer cultures were comparable to HSC-like cells. With this background, a heterogenous population of CD45-CD31+ phenotype was the most prominent one.
Despite a lack of specific markers that could characterize a population of MSC’s, we attempted to determine the quantity of these cells in the culture, based upon the fact that mesenchymal stem cells of the bone marrow do not express common leucocytic antigen CD45 and HLA class II antigens but do express class I HLA antigens and the CD90 marker. In this connection, we studied the following populations of phenotypically MSC-like cells: CD45-HLA ABC+ and CD90+CD31-.
Aiming for further validation of available cultural conditions for expansion of MSCs and endothelial precursors, we assessed the following factors influencing the terms of cultivation period in a primary culture under the conditions of initial culture and subsequent passages. During long term cultures (Table 3), the index of cells with hematopoietic markers decreased progressively, and the index of myeloid cell maturation decreased as well, providing evidence of the elimination of hematopoietic cells during the cultivation. We have revealed that the index of HSC-like cells (hematopoietic progenitor cell index) increases during prolonged cultivation, i.e. an index, reflecting a ratio of cells, phenotypically similar to hematopoietic stem cells, to the total number of СD45+-cells (CD34+CD45+, CD117+). This fact implies that this population is eliminated slower than other hematopoietic cells recovered in the UCB monolayer culture.
Table 3. Influence of cultivation period on composition of primary UCB culture
Moreover, the percentage of CD34+HLA DR- cells increases (37.33 to 81.98, p=0.001) in relation to general CD34-positive population, thus supposing that these cells undergo selective proliferation under the given cultural conditions. This result could indirectly confirm a theory that this population is presented via stromal component [24,25]. In the course of long-term cultivation, the index of endothelial cells is shown to be increased (0.013 to 0.025, p=0.02). This fact proves these cultural conditions are favorable for endothelial precursors' maintenance. When assessing the fractions that include mesenchymal precursors, we have found a tendency for an increase in the relative amounts of the CD45-HLA ABC+ subpopulation. However, when analyzing these populations with regard of all non-hematopoietic cells, we did not observe such a tendency. This fact may reflect the persistance of a steady phenotype of these cell populations upon durable cultivation.
During the process of culture passage, we could reveal a decrease of the hematopoietic cell index (3.19 to 1.6, p<0.016) (Table 4). This data may show the inability of the majority of hematopoietic cells for repeated adhesion, thus resulting in their elimination from the monolayer culture. At the same time, the cells phenotypically similar to HSC-like cells (CD34+CD45+) and lymphocytes (CD3+), were still able to adhere recurrently, when compared with other hematopoietic cells. Among cell populations containing mesenchymal precursors, it was noticed that the cells expressed class I HLA antigens, possessed a reduced adhesive ability, and were subject to elimination with sequential passing.
Table 4. Influence of culture passage on the structure of primary UCB culture
When comparing the phenotypical composition of mononuclear fraction and monolayer culture from UCB (Table 5) we discovered that the percentage of cells with CD34+CD45+ phenotype in the culture decreased considerably, along with an increased percentage of cells with СD34+HLA DR- phenotype. Therefore, we can suggest that these cultural conditions may be applied for isolation of the given cell type.
Table 5. The phenotypic composition of the mononuclear fraction and monolayer culture of UCB
This may be also proven by the data from semi-solid methylcellulose culture. The cells from monolayer cultures under study were incapable of forming hematopoietic colonies in the presence of standard hematopoietic growth factors (SCF, GM-CSF, IL-3, IL-6, G-CSF, EPO). This may result from a deficiency of clonogenic precursors in the monolayer, as well as an absence of clonogenic potential among hematopoietic stem-like cells.
The fraction of endothelial cells was higher in the monolayer culture. The percentage of hematopoietic-stem-like-cells and endothelial precursors decreased considerably during the initiation of the culture (38.87 to 1.34, p<0,02).
Analogous to Bieback's paper [12], when assessing the time parameters of sampling and storage of umbilical blood specimens we have revealed a tendency towards a decreased concentration of mesenchymal like stem-cells in the monolayer culture, when a prolonged time-period prior to processing umbilical blood sample with subsequent cultivation occurs (data not shown).
In vitro differentiation of UCB-derived MSC-like cells into adipocytes and osteocytes
Taking the lack of specific markers for identification of mesenchymal precursors into consideration, we tried to reveal the functional characteristics of the given types of cells and to assess the differential potentials in the framework of orthodox plasticity. It is shown that the cells of the UCB monolayer culture are capable of dividing and differentiating to adipocytes and osteoblasts, as was proven by their specific staining (Figure 4). In this figure (upper picture), red lipid inclusions are readily seen in differentiated adipocytes. In the lower picture, calcium insertions in the osteocytes are stainable red or black. In some cultures, however, induction of differentiation initiated detachment of most cells from plastic surface. As a result, the present conditions for differentiation of mesenchymal stem cells from BM are not quite appropriate for induced differentiation of UCB-derived mesenchymal precursors.
Figure 4. Differentiation assay of BM and UCB monolayer culture. Formation of mineralized matrix by Alizarin Red and von Kossa staining evidenced osteogenic differentiation. Adipogenic differentiation was evidenced by the formation of lipid vacuoles in phase-contrast photograph and by oil-red O staining.
Ability of UCB monolayer culture to support ex vivo expansion of CFU-GM
Concerning efficiency of CFU-GM cloning, the UCB monolayer culture does not differ from standard leucocytic feeder. However, when using umbilical cord blood culture as a feeder, UCB-generated growth-promoting capacity and percentage of large colonies were higher (data not shown). Additional analysis (Table 6) leads us to suggest that hematopoietic cells from a UCB monolayer culture have certain advantage over non-hematopoietic cells in terms of colony-stimulation activity (r=0.71, p=0.035), at least in this in vitro model. With respect to non-hematopoietic cells, growth of cellular elements bearing MSC-markers (CD90+CD31-) was accompanied by significant increase in CFU-GM proliferative activity (r=0.82, p=0.007). Higher percentages of monocytes/macrophages, as among hematopoietic elements, is accompanied by growing numbers of large colonies (r=0.67, p=0.045), without producing any significant impact upon cloning efficiency of the progenitors.
Table 6. Hemostimulating capacity of UCB-culture
Some references in the literature contain similar data obtained with another model, i.e., target cells were incubated with UCB cells in suspension cultures, followed by methyl cellulose cultures of non-attached cell populations in presence of standard growth factors, such as SCF, GM-CSF, G-CSF, IL3, IL6 and EPO [26,27]. Within our model, only umbilical cord blood cells of a monolayer culture were used as the colony stimulation source.
Comparative gene expression studies
When assessing the expression of some genes, we have found that the mRNA profile of bone marrow culture did not differ from the cells of UCB culture. Thrombopoietin was an exception, since specific mRNA was not detectable in the 1st passage culture of UCB cells (Table 7).
Table 7. mRNA profile of MSC-like cells from different sources
Conclusions
• Increased cultivation time of UCB mononuclear fraction (over 23 days) leads to a gradual elimination of hematopoietic cells from the culture and an increase in mesenchymal stem cells and endothelial progenitor cells.
• Cellular phenotype in the culture changes during passages, i.e., the quantity of hematopoietic cells is considerably decreased. This results in increased concentration of non-hematopoietic components the of umbilical blood monolayer culture during serial passaging.
• An increased time period of UCB sampling is associated with a decrease in the relative quantity of mesenchymal-like stem cells, along with an increase in the concentration of endothelial precursors in the culture.
• Mesenсhymal stem-like-cells of UCB have the capacity to differentiate into adipogenic and osteogenic lineages, thus suggesting their functional consistency.
• The adhesive fraction of the primary monolayer culture exerts a stimulatory effect upon the colony formation of GM precursors, being similar in type and degree of influence to a standard peripheral blood feeder. The primary effect upon their proliferative capacity may be produced by cellular elements with MSC (CD90+CD31-) markers.
• An increased time interval during sampling and storage of UCB leads to a decrease in the hemostimulating capacity.
• The variable contents of MSC-like cells and CFU-F in umbilical cord blood and/or their reduced repopulation ability may limit their application as an alternative source of MSCs.
References
1. Minguell JJ. Mesenchymal stem cells. Exp Biol Med. 2001;226:507-520.
2. Pittenger M, Mackay A, Beck S, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;84:143-147.
3. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74.
4. Le Blanc K, Ringden O. Mesenchymal stem cells, properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586-591.
5. Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the haematopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340.
6. Friedenstein AJ, Deriglasova UF, Kulagina, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974;2:83-92.
7. Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414-425.
8. Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. О Cell Biochem. 2001;82:583-590.
9. Vladimiskaya EB, Mayorova OA, Roumiantsev SA, Roumiantsev AG. Biological bases and therapy prospects with the stem cells. Moscow: Medpractica; 2005. 391 p.
10. Broxmeyer HE. Proliferation, self-renewal, and survival characteristics of cord blood hematopoietic stem and progenitor cells. In: Broxmeyer HE, ed. Cord Blood: Biology, Immunology, Banking, and Clinical Transplantation. Bethesda, MD: American Association of Blood Banking. 2004:1-21.
11. Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. 2005;7(3):209-218.
12. Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-634.
13. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242.
14. Koegler G, Sensken S, Airey J, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123-135.
15. Lee MW, Choi J, Yang MS, et al. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Comm. 2004;320:273-278.
16. Mareschi K, Biasin E, Piacibello W, et al. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-1100.
17. Wexler S, Donaldson C, Denning-Kendall P, et al. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368-374.
18. Campagnoli C, Roberts I, Kumar S, Bennett P, Bellantuono I, Fisk N. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow Blood. 2001;98:2396-2402.
19. Goodwin HS, Bicknese AR, Chien SN, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat and neural markers. Biol Blood Marrow Transpl. 2001;7:581-588.
20. Cheng SL, Yang JW, Rifas L, et al. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277-286.
22. Afanasiev ВV, Almazov VA. Human hematopoietic progenitor cells. Leningrad: Nauka; 1985. 204 p.
23. Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69-80.
24. Huang S, Terstappen LW. Formation of haematopoietic microenvironment and haematopoietic stem cell from single human bone marrow stem cells. Nature. 1992;360:745-749.
25. Islam A. Hematopoietic stem cells: A new concept. Leuk Res. 1985;9:1415.
26. Ye ZQ, Burkholder JK, Qiu P, Schultz JC, Shahidi NT, Yang NS. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci USA. 1994;91:12140-12144.
27. Lu-Lu L, Liu Y-J, Yang S-G, Zhao Q-J, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017-1026.
"
["~DETAIL_TEXT"]=>
string(48905) "
Introduction
Mesenchymal stem cells (MSC) constitute a rare population of multipotent progenitors capable of both supporting hematopoiesis and differentiating into at least osteogenic, adipogenic and chondrogenic lineages [1-3]. Moreover, they exhibit immunomodulatory activity, induce immunotolerance in case of allogenic transplantation [4], and posess the ability to expand in relatively simple in vitro systems [5-7]. These characteristics make MSCs very promising candidates in the development of new cell-based therapeutic strategies, such as the treatment of tissue injuries or the supportive application by hematopoietic stem cell transplantation. Taking into consideration that the number of MSCs and their differentiation capacity decline with age [8], most relevant is the search for alternative sources of these cells.
The advantages of umbilical cord blood (UCB), as a source of stem cells, include their accessibility, non-invasive sampling and, thus, their safety for potential donors [9-11]. Another reason for this study was due to some controversial data about amounts of mesenchymal precursors in UCB [12-17]. Mesenchymal precursors (CFU-F) are found in foetal blood in the early gestational period at concentrations equal to their amounts in the bone-marrow of newborns [18]. However, after the second trimester, the number of MSCs decreases considerably, and, at birth, their frequency in UCB is quite accidental [12,19]. The need for testing of some standards for culturing adhesive cell fractions from UCB, as well as assessment of their contents and functional characteristics provided other motivations for this study.
Materials and Methods
Collection of UCB
UCB samples (mean volume of 60 ml) from full-term deliveries were collected from the unborn placenta after obtaining the mothers' informed consent. A sterile bag system containing citrate phosphate dextrose (CPD) anticoagulant within the collection bag (manufactured by Terumo, Japan) was used. The units were stored at room temperature before processing (up to 33 hours).
Isolation and Culture of Adherent Cells from UCB
To isolate mononuclear cells (MNCs), each UCB unit was diluted 1:1 with phosphate-buffered saline (PBS)/2 mM EDTA (Biolot, Russia), and carefully loaded onto Ficoll-Hypaque solution (Lympho separation medium, ICN, USA, d=1.077). Following a density gradient centrifugation at 435 g for 30 minutes at room temperature, MNCs were removed from the interphase layer and washed two to three times with PBS/EDTA. UCB-derived MNCs were set at a density of 1 x 106/cm2 into six-well culture plates (Corning, USA) containing DMEM low-glucose medium (Gibco, USA) with 20% fetal calf serum (FCS) from selected lots, penicillin 100 UI/ml, and streptomycin 0.1 mg/ml (Gibco, USA).
After overnight incubation at 37°C in a humidified atmosphere containing 5% carbon dioxide, nonadherent cells were removed and fresh medium was added to the wells. Cell cultures were maintained, and remaining nonadherent cells were discarded via the complete exchange of culture medium every 7 days. Culture plates were screened continuously to detect developing colonies of adherent cells. The number of fibroblast colony forming units (CFU-F) was calculated by counting the number of colonies per 108 MNCs.
Fibroblast-like cells were detached between days 16 through 20 after initial plating using 0.04% Trypsin/0.03% EDTA (Gibco, USA). The recovered cells were replated at a density of 4,000 to 5,000 cells/cm2.
Collection and Isolation of Control MSCs from Bone Marrow
MSCs from bone marrow (BM) were obtained by bone marrow puncture. BM cells were aspirated into a 5-ml syringe containing CPD anticoagulant. A total of six samples were obtained, with the donor age ranging from 24 to 56 years. To isolate MSCs from BM, the aspirate was diluted 1:5 and processed as described above. In contrast to MNCs from UCB samples, BM-derived MNCs were cultured at a density of 1 x 106 cells/cm2 in T75 culture flasks (Corning, USA), and the first change of medium was performed 3 days after initial plating. Two weeks later, at reaching 80%–90% confluence, MSCs were detached using trypsin and replated as described for the UCB-derived adherent cells.
Primary Fibroblasts as Controls
Primary cultures of normal human dermal fibroblasts served as negative control in differentiation and comparative gene expression studies. Culture conditions were comparable to BM MSC’s expansion: DMEM low glucose medium (Gibco, USA) containing 20% fetal calf serum (FCS), penicillin 100 UI/ml, and streptomycin 0.1 mg/ml (Gibco, USA).
Immune Phenotypic Analysis
To analyze the cell-surface expression of typical marker proteins in UCB- and BM-derived adherent cells, each from primary culture and second passage, these were labeled with the following anti-human antibodies: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR,DP,DQ FITC; CD 90 PE (Becton Dickinson, USA). Murine isotype antibodies (Becton Dickinson, USA) served as respective controls. Ten thousand labeled cell aliquotes were analyzed using a FACScan flow cytometer running CellQuest software (Becton Dickinson, USA).
Aiming at a subset analysis of the population, we employed the following indices:
• hematopoietic cells index – CD45+ to CD45- cells
• myeloid cell maturation index – CD14+ to CD45+ cells
• hematopoietic progenitor cell index – CD34+CD45+ to CD45+ cells
• endothelial cell-precursors index – CD34+CD45- to CD45- cells
• mesenchymal precursors index – CD90+CD31- to CD45- cells
In vitro Osteogenic and Adipogenic Differentiation Studies
To induce osteogenic differentiation, the cells were seeded at a density of 3.1 x 103 cells/cm2 and cultured in six-well microplates (Costar, USA), until they reached approximately 80% confluence. Additional culture was performed in osteogenic differentiation medium supplemented with 0.1 µM dexamethasone, 10 mM ß-glycerophosphate, 0.05 mM ascorbate, and 10% FCS. The onset of osteoblast formation was evaluated after 3 weeks via calcium accumulation. Accumulation of mineralized calcium phosphate was assessed with von Kossa staining after the protocol from Cheng et al. [20], with a few modifications. The cells were fixed for 15 minutes in 10% formalin (Sigma-Aldrich), and, after washing, they were incubated with 5% silver nitrate (Sigma-Aldrich) for 15 to 30 minutes. Pyrogallol 1% (Merck, Canada) and sodium thiosulfate 5% (Sigma-Aldrich) were used to develop and register a resulting image. In addition, the mineralized matrix was also evaluated by Alizarin-red S staining using 4% formaldehyde for fixation and 1% aqueous Alizarin-Red S (Sigma-Aldrich) solution.
Adipogenic differentiation was induced according to the protocol of Pittenger et al. [2]. Special induction medium, containing DMEM (high glucose), 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methyl-xanthine, 10 µg/ml recombinant human (rh) insulin, 0.2 mM indomethacin, and 20% FCS, was added for 2 to 3 days to the culture microplates. It was then replaced by maintenance medium containing only rh-insulin and 20% FCS. Induction of adipogenic differentiation was apparent via the intracellular accumulation of lipid-rich vacuoles that stained with Oil Red O (Sigma-Aldrich). The cells were fixed with 10% formalin, washed, and stained with a working solution of 0.18% Oil Red O for 5 minutes.
In vitro Model for Studying Colony-stimulating Activity of UCB cells
An essential property of mesenchymal stem cells is their capacity to support hematopoiesis. In order to assess the hemostimulating capacity of the UCB monolayer culture, a modified technique proposed by Afanasyev [21,22] was used (Figure 1). This method determines the clonogenic capacity of granulocytic-macrophage colony forming units (CFU-GM) in “agar drop-liquid medium” culture.
Figure 1. Agar drop-liquid culture system[22]
A-medium; B-feeder cells; C,D,E-colonies; F-clusters; G-agar
The monolayer culture of UCB-derived adherent cells (confluence rate: 70–80%) was used as a source of colony-stimulating activity of MSC from UCB. The cells (CFU-GM) from the UCB mononuclear fraction were targeted in this assay, providing clonal growth in agar cultures. Semisolid agar drops containing target cells were prepared on dry surfaces of sterile Petri dishes. The resulting semisolid drops were transferred to the dishes, and co-incubated for 7 days in the following four versions: 1) with complete culture medium only; 2) with medium containing standard-type leukocyte feeder; 3) with UCB confluent monolayer culture; 4) with both UCB confluent monolayer culture and standard-type leukocyte feeder. Each experiment was performed at least twice.
Colony-forming ability (CFA) and cluster-forming ability (ClFA) were classified according to the number of cells in the colonies (small colonies: containing 20–40 cells; medium-size colonies: 41–100 cells; and large colonies: more than 100 cells) and clusters (large clusters: 10–19 cells; small clusters: 5–9 cells). Depending on the total number of colonies and clusters, the cloning efficiency (CE) per 1х105 explanted mononuclear cells was assessed. A “colony-to-cluster” ratio (Co-Cl) and percentage of large colonies (LC) were supplemental parameters assessed in these cell cultures. Using such parameters, the proliferative potential of the target progenitor cells was tested.
Total RNA Isolation and RT-PCR
Total RNA was extracted from 3 to 30 x105 MSCs using Trizol Reagents (Invitrogen, USA), according to the manufacturer’s instructions. mRNA was subject to reverse transcription (RT) using Superscript II Kit (Invitrogen, USA), again using the manufacturer’s instructions. The resulting cDNA was amplified using an ABI GeneAmp PCR System 2400 (Perkin Elmer Applied Biosystems, Boston, MA) at 94°C for 40 seconds, 56°C for 50 seconds, and 72°C for 60 seconds for 35 cycles, after initial denaturation at 94°C for 5 minutes. Primers used for PCR are listed in Table 1. Fifteen microliters of PCR reaction were fractionated by agarose gel electrophoresis.
Table 1. Primers used for RT-PCR
Statistical Analysis
The statistical significance of the inter-group differences was evaluated with the Mann-Whitney test. The degrees of correlations between the parameters were evaluated by the Spearman test. The differences were considered significant by p values <0.05. The Statistica 6.0 software package was used for all statistical analyses.
Results and Discussion
In most cases, the cultures of plastic-adherent cells proved to be heterogenous. Two main morphological cell types were discernable: spindle-shaped cells that are presumably regarded as mesenchymal stem cells (MSC), and polygonal cells that are most likely of hematopoietic origin (Figure 2).
Figure 2. Heterogeneous UCB culture. No colony formation was observed. UCB samples produced a minimal, non-confluent adherent layer of heterogeneous cells.
In some UCB samples, clonal growth was observed, however the mean cell number per colony did not increase over 100 cells (Figure 3). Large colonies were detectable in 3 of the 40 UCB samples under study. These colonies (>1000 cells per colony) consisted of closely packed, spindle-shaped cells, typical of fibroblast morphology cells.
Figure 3. CFU-F in UCB culture. Adherently growing cells of fibroblastic morphology formed big colonies in 3 UCB samples.
The Phenotypic Composition of UCB Monolayer Cultures
Our efforts to define a distinct phenotype characteristic for MSC have been confounded by the fact that these cells can express a range of cell lineage-specific antigens [2,23].
During analysis of the predominant cellular types, it was discovered that the major fraction (median 60.17%) of plastic-adherent cells from UCB were of hematopoietic origin (CD45+) (Table 2). One-third of the CD45-positive cells belonged to the CD14-positive population fraction (median 14.81%). Cells in the monolayer with an osteoclast-like phenotype (CD45+CD61+) constituted approximately 1.5%. The cells phenotypically comparable with hematopoietic stem cells (HSC-like cells: CD34+CD45+, CD34+HLA DR-, CD117+) were present at a low concentration in the UCB monolayer culture - less than 1.5%.
Table 2. The composition of the UCB monolayer culture.
The numbers of endothelial-like cells in the monolayer cultures were comparable to HSC-like cells. With this background, a heterogenous population of CD45-CD31+ phenotype was the most prominent one.
Despite a lack of specific markers that could characterize a population of MSC’s, we attempted to determine the quantity of these cells in the culture, based upon the fact that mesenchymal stem cells of the bone marrow do not express common leucocytic antigen CD45 and HLA class II antigens but do express class I HLA antigens and the CD90 marker. In this connection, we studied the following populations of phenotypically MSC-like cells: CD45-HLA ABC+ and CD90+CD31-.
Aiming for further validation of available cultural conditions for expansion of MSCs and endothelial precursors, we assessed the following factors influencing the terms of cultivation period in a primary culture under the conditions of initial culture and subsequent passages. During long term cultures (Table 3), the index of cells with hematopoietic markers decreased progressively, and the index of myeloid cell maturation decreased as well, providing evidence of the elimination of hematopoietic cells during the cultivation. We have revealed that the index of HSC-like cells (hematopoietic progenitor cell index) increases during prolonged cultivation, i.e. an index, reflecting a ratio of cells, phenotypically similar to hematopoietic stem cells, to the total number of СD45+-cells (CD34+CD45+, CD117+). This fact implies that this population is eliminated slower than other hematopoietic cells recovered in the UCB monolayer culture.
Table 3. Influence of cultivation period on composition of primary UCB culture
Moreover, the percentage of CD34+HLA DR- cells increases (37.33 to 81.98, p=0.001) in relation to general CD34-positive population, thus supposing that these cells undergo selective proliferation under the given cultural conditions. This result could indirectly confirm a theory that this population is presented via stromal component [24,25]. In the course of long-term cultivation, the index of endothelial cells is shown to be increased (0.013 to 0.025, p=0.02). This fact proves these cultural conditions are favorable for endothelial precursors' maintenance. When assessing the fractions that include mesenchymal precursors, we have found a tendency for an increase in the relative amounts of the CD45-HLA ABC+ subpopulation. However, when analyzing these populations with regard of all non-hematopoietic cells, we did not observe such a tendency. This fact may reflect the persistance of a steady phenotype of these cell populations upon durable cultivation.
During the process of culture passage, we could reveal a decrease of the hematopoietic cell index (3.19 to 1.6, p<0.016) (Table 4). This data may show the inability of the majority of hematopoietic cells for repeated adhesion, thus resulting in their elimination from the monolayer culture. At the same time, the cells phenotypically similar to HSC-like cells (CD34+CD45+) and lymphocytes (CD3+), were still able to adhere recurrently, when compared with other hematopoietic cells. Among cell populations containing mesenchymal precursors, it was noticed that the cells expressed class I HLA antigens, possessed a reduced adhesive ability, and were subject to elimination with sequential passing.
Table 4. Influence of culture passage on the structure of primary UCB culture
When comparing the phenotypical composition of mononuclear fraction and monolayer culture from UCB (Table 5) we discovered that the percentage of cells with CD34+CD45+ phenotype in the culture decreased considerably, along with an increased percentage of cells with СD34+HLA DR- phenotype. Therefore, we can suggest that these cultural conditions may be applied for isolation of the given cell type.
Table 5. The phenotypic composition of the mononuclear fraction and monolayer culture of UCB
This may be also proven by the data from semi-solid methylcellulose culture. The cells from monolayer cultures under study were incapable of forming hematopoietic colonies in the presence of standard hematopoietic growth factors (SCF, GM-CSF, IL-3, IL-6, G-CSF, EPO). This may result from a deficiency of clonogenic precursors in the monolayer, as well as an absence of clonogenic potential among hematopoietic stem-like cells.
The fraction of endothelial cells was higher in the monolayer culture. The percentage of hematopoietic-stem-like-cells and endothelial precursors decreased considerably during the initiation of the culture (38.87 to 1.34, p<0,02).
Analogous to Bieback's paper [12], when assessing the time parameters of sampling and storage of umbilical blood specimens we have revealed a tendency towards a decreased concentration of mesenchymal like stem-cells in the monolayer culture, when a prolonged time-period prior to processing umbilical blood sample with subsequent cultivation occurs (data not shown).
In vitro differentiation of UCB-derived MSC-like cells into adipocytes and osteocytes
Taking the lack of specific markers for identification of mesenchymal precursors into consideration, we tried to reveal the functional characteristics of the given types of cells and to assess the differential potentials in the framework of orthodox plasticity. It is shown that the cells of the UCB monolayer culture are capable of dividing and differentiating to adipocytes and osteoblasts, as was proven by their specific staining (Figure 4). In this figure (upper picture), red lipid inclusions are readily seen in differentiated adipocytes. In the lower picture, calcium insertions in the osteocytes are stainable red or black. In some cultures, however, induction of differentiation initiated detachment of most cells from plastic surface. As a result, the present conditions for differentiation of mesenchymal stem cells from BM are not quite appropriate for induced differentiation of UCB-derived mesenchymal precursors.
Figure 4. Differentiation assay of BM and UCB monolayer culture. Formation of mineralized matrix by Alizarin Red and von Kossa staining evidenced osteogenic differentiation. Adipogenic differentiation was evidenced by the formation of lipid vacuoles in phase-contrast photograph and by oil-red O staining.
Ability of UCB monolayer culture to support ex vivo expansion of CFU-GM
Concerning efficiency of CFU-GM cloning, the UCB monolayer culture does not differ from standard leucocytic feeder. However, when using umbilical cord blood culture as a feeder, UCB-generated growth-promoting capacity and percentage of large colonies were higher (data not shown). Additional analysis (Table 6) leads us to suggest that hematopoietic cells from a UCB monolayer culture have certain advantage over non-hematopoietic cells in terms of colony-stimulation activity (r=0.71, p=0.035), at least in this in vitro model. With respect to non-hematopoietic cells, growth of cellular elements bearing MSC-markers (CD90+CD31-) was accompanied by significant increase in CFU-GM proliferative activity (r=0.82, p=0.007). Higher percentages of monocytes/macrophages, as among hematopoietic elements, is accompanied by growing numbers of large colonies (r=0.67, p=0.045), without producing any significant impact upon cloning efficiency of the progenitors.
Table 6. Hemostimulating capacity of UCB-culture
Some references in the literature contain similar data obtained with another model, i.e., target cells were incubated with UCB cells in suspension cultures, followed by methyl cellulose cultures of non-attached cell populations in presence of standard growth factors, such as SCF, GM-CSF, G-CSF, IL3, IL6 and EPO [26,27]. Within our model, only umbilical cord blood cells of a monolayer culture were used as the colony stimulation source.
Comparative gene expression studies
When assessing the expression of some genes, we have found that the mRNA profile of bone marrow culture did not differ from the cells of UCB culture. Thrombopoietin was an exception, since specific mRNA was not detectable in the 1st passage culture of UCB cells (Table 7).
Table 7. mRNA profile of MSC-like cells from different sources
Conclusions
• Increased cultivation time of UCB mononuclear fraction (over 23 days) leads to a gradual elimination of hematopoietic cells from the culture and an increase in mesenchymal stem cells and endothelial progenitor cells.
• Cellular phenotype in the culture changes during passages, i.e., the quantity of hematopoietic cells is considerably decreased. This results in increased concentration of non-hematopoietic components the of umbilical blood monolayer culture during serial passaging.
• An increased time period of UCB sampling is associated with a decrease in the relative quantity of mesenchymal-like stem cells, along with an increase in the concentration of endothelial precursors in the culture.
• Mesenсhymal stem-like-cells of UCB have the capacity to differentiate into adipogenic and osteogenic lineages, thus suggesting their functional consistency.
• The adhesive fraction of the primary monolayer culture exerts a stimulatory effect upon the colony formation of GM precursors, being similar in type and degree of influence to a standard peripheral blood feeder. The primary effect upon their proliferative capacity may be produced by cellular elements with MSC (CD90+CD31-) markers.
• An increased time interval during sampling and storage of UCB leads to a decrease in the hemostimulating capacity.
• The variable contents of MSC-like cells and CFU-F in umbilical cord blood and/or their reduced repopulation ability may limit their application as an alternative source of MSCs.
References
1. Minguell JJ. Mesenchymal stem cells. Exp Biol Med. 2001;226:507-520.
2. Pittenger M, Mackay A, Beck S, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;84:143-147.
3. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74.
4. Le Blanc K, Ringden O. Mesenchymal stem cells, properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586-591.
5. Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the haematopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340.
6. Friedenstein AJ, Deriglasova UF, Kulagina, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974;2:83-92.
7. Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414-425.
8. Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. О Cell Biochem. 2001;82:583-590.
9. Vladimiskaya EB, Mayorova OA, Roumiantsev SA, Roumiantsev AG. Biological bases and therapy prospects with the stem cells. Moscow: Medpractica; 2005. 391 p.
10. Broxmeyer HE. Proliferation, self-renewal, and survival characteristics of cord blood hematopoietic stem and progenitor cells. In: Broxmeyer HE, ed. Cord Blood: Biology, Immunology, Banking, and Clinical Transplantation. Bethesda, MD: American Association of Blood Banking. 2004:1-21.
11. Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. 2005;7(3):209-218.
12. Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-634.
13. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242.
14. Koegler G, Sensken S, Airey J, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123-135.
15. Lee MW, Choi J, Yang MS, et al. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Comm. 2004;320:273-278.
16. Mareschi K, Biasin E, Piacibello W, et al. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-1100.
17. Wexler S, Donaldson C, Denning-Kendall P, et al. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368-374.
18. Campagnoli C, Roberts I, Kumar S, Bennett P, Bellantuono I, Fisk N. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow Blood. 2001;98:2396-2402.
19. Goodwin HS, Bicknese AR, Chien SN, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat and neural markers. Biol Blood Marrow Transpl. 2001;7:581-588.
20. Cheng SL, Yang JW, Rifas L, et al. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277-286.
22. Afanasiev ВV, Almazov VA. Human hematopoietic progenitor cells. Leningrad: Nauka; 1985. 204 p.
23. Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69-80.
24. Huang S, Terstappen LW. Formation of haematopoietic microenvironment and haematopoietic stem cell from single human bone marrow stem cells. Nature. 1992;360:745-749.
25. Islam A. Hematopoietic stem cells: A new concept. Leuk Res. 1985;9:1415.
26. Ye ZQ, Burkholder JK, Qiu P, Schultz JC, Shahidi NT, Yang NS. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci USA. 1994;91:12140-12144.
27. Lu-Lu L, Liu Y-J, Yang S-G, Zhao Q-J, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017-1026.
"
["DETAIL_TEXT_TYPE"]=>
string(4) "html"
["~DETAIL_TEXT_TYPE"]=>
string(4) "html"
["PREVIEW_TEXT"]=>
string(0) ""
["~PREVIEW_TEXT"]=>
string(0) ""
["PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["~PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["PREVIEW_PICTURE"]=>
NULL
["~PREVIEW_PICTURE"]=>
NULL
["LANG_DIR"]=>
string(4) "/ru/"
["~LANG_DIR"]=>
string(4) "/ru/"
["SORT"]=>
string(3) "500"
["~SORT"]=>
string(3) "500"
["CODE"]=>
string(82) "sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka"
["~CODE"]=>
string(82) "sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka"
["EXTERNAL_ID"]=>
string(3) "472"
["~EXTERNAL_ID"]=>
string(3) "472"
["IBLOCK_TYPE_ID"]=>
string(7) "journal"
["~IBLOCK_TYPE_ID"]=>
string(7) "journal"
["IBLOCK_CODE"]=>
string(7) "volumes"
["~IBLOCK_CODE"]=>
string(7) "volumes"
["IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["~IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["LID"]=>
string(2) "s2"
["~LID"]=>
string(2) "s2"
["EDIT_LINK"]=>
NULL
["DELETE_LINK"]=>
NULL
["DISPLAY_ACTIVE_FROM"]=>
string(0) ""
["IPROPERTY_VALUES"]=>
array(18) {
["ELEMENT_META_TITLE"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["ELEMENT_META_KEYWORDS"]=>
string(176) "пуповинная кровь человека мезенхимные стволовые клетки иммунофенотипирование культура клеток"
["ELEMENT_META_DESCRIPTION"]=>
string(251) "Состав и функциональные особенности монослойной культуры пуповинной крови человекаComposition and functional properties of monolayer cell culture from human umbilical cord blood"
["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=>
string(8344) "<h2>Резюме</h2>
<h2>Введение </h2>
<p class="bodytext">
В условиях монослойной культуры клетки пуповинной крови способны прикрепляться к пластику и по своей морфологии напоминают культивируемые в сходных условиях мезенхимальные стволовые клетки (МСК) костного мозга. Однако присутствие в прилипающей фракции пуповинной крови МСК до сих пор не является очевидным. Данное исследование выполнено с целью определения состава и ряда функциональных свойств МСК-подобных клеток в монослойной культуре пуповинной крови (МКПК) человека.
</p>
<h2>Материалы и методы</h2>
<p>
Исследовали сорок три образца пуповинной крови, полученые в срочных родах на фоне неосложненной беременности у рожениц при атравматичном заборе. Исследования проводили после 19-31 часов хранения образца. Ядросодержащие клетки выделяли на градиенте плотности фиколла (1,077 г/мл), затем помещали в полную культуральную среду, содержащую среду DMEM LG, эмбриональную телячью сыворотку - 30%, пенициллин (100 Ед/мл), стрептомицин (0,1 мг/мл). Анализ фенотипа монослойной культуры ПК и ее мононуклеарной фракции проводили на проточном цитофлюориметре FACScan. Были использованы следующие конъюгированные флюорохромами антитела: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR. С целью определения гемостимулирующих свойств монослойной культуры ПК проводили клонирование гранулоцитарно-макрофагальных предшественников (КОЕ-ГМ) в культуральной системе «агаровая капля-жидкая среда». В качестве источника колониестимулирующей активности ПК использовали МКПК. Клетками-мишенями были КОЕ-ГМ мононуклеарной фракции ПК, дающие клональный рост в агаровой культуре. Для индукции дифференцировки МСК-подобных клеток ПК в адипогенном и остеогенном направлении клетки помещали в полную среду с добавлением дексаметазона (1х10<sup>-7</sup> М); инсулина (1х10<sup>-9</sup> М) или β-глицерофосфата (7х10<sup>-3</sup> М); дексаметазона (1х10<sup>-8</sup> М); аскорбиновой кислоты (2х10<sup>-4</sup> М) соответственно. Оценка экспрессии генов (CDH11,VCAM1, ITGB1, IL6ST, TFRC, ALCAM, MPL, TPO, ENG, NT5E, IL6R, BGLAP, COL1A2, AFP, LPL, ACTA1, TNNI3, TPM1) проводилось методом RT-PCR (амплификация продуктов обратной транскрипции).
</p>
<h2>Результаты</h2>
<p>
В большинстве случаев культура клеток, прилипших к пластику была гетерогенна: наблюдали узкие веретенообразные клетки и большие полигональные. В ряде образцов обнаруживали небольшие колонии (до 100 клеток). В 3 из 43 исследованных образцов ПК наблюдали крупные колонии, численностью более 1000 плотноупакованных, имеющих типичную для фибробластов веретенообразную форму клеток. При анализе преобладающих клеточных типов было выявлено, что большую часть прикрепленных к пластику клеток составляли гемопоэтические клетки (медиана 60,17%). Около трети от всей СD45-положительной популяции составляли СD14-положительные клетки. Остальные негемопоэтические клетки представляли собой фенотипически гетерогенную популяцию. На фоне длительного культивирования и последовательного пассирования фенотип культуры меняется – отмечается элиминация из культуры гемопоэтических клеток и увеличение доли МСК и ЭКП. При инициации культуры значительно изменяется соотношение ГСК- и ЭКП-подобных клеток среди CD34-положительной популяции в пользу ЭКП. МСК-подобные клетки МКПК способны к дифференцировке в адипоциты и остеобласты, что подтверждается специфической окраской и свидетельствует в пользу их функциональной состоятельности. В ряде культур индукция дифференцировки инициировала открепление большей части клеток. Прилипающая фракция первичной монослойной культуры оказывает стимулирующее влияние на колониеобразование КОЕ-ГМ, по характеру и силе воздействия близкое стандартному лейкоцитарному фидеру. Преимущественное влияние на их пролиферативную активность оказывают клеточные элементы с маркерами МСК (CD90<sup>+</sup>CD31<sup>-</sup>). Удлинение временных параметров получения и хранения образцов ПК приводят к снижению гемостимулирующей активности. При сравнении экспрессии ряда генов выявлено, что профиль экспрессии МСК костного мозга и клеток МКПК идентичен за исключением тромбопоэтина, экспрессия гена которого не отмечалась в МКПК.
</p>
<h2>Заключение</h2>
<p>
Пуповинная кровь содержит субпопуляции клеток негемопоэтического происхождения, фенотипически и функционально сходных с МСК костного мозга. Однако их низкая концентрация, а также сниженная репопулирующая активность в стандартных культуральных условиях, ставят под сомнение возможное использование ПК в качестве альтернативного источника МСК.
</p>"
["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_META_TITLE"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_META_KEYWORDS"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_META_DESCRIPTION"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_PICTURE_FILE_ALT"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_PICTURE_FILE_TITLE"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_PICTURE_FILE_NAME"]=>
string(86) "sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka-img"
["SECTION_DETAIL_PICTURE_FILE_ALT"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_DETAIL_PICTURE_FILE_TITLE"]=>
string(156) "Состав и функциональные особенности монослойной культуры пуповинной крови человека"
["SECTION_DETAIL_PICTURE_FILE_NAME"]=>
string(86) "sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka-img"
["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=>
string(86) "sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka-img"
["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=>
string(86) "sostav-i-funktsionalnye-osobennosti-monosloynoy-kultury-pupovinnoy-krovi-cheloveka-img"
}
["FIELDS"]=>
array(1) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
}
["PROPERTIES"]=>
array(18) {
["KEYWORDS"]=>
array(36) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(4) "5813"
[1]=>
string(4) "5814"
[2]=>
string(4) "5815"
[3]=>
string(4) "5816"
}
["VALUE"]=>
array(4) {
[0]=>
string(3) "469"
[1]=>
string(2) "83"
[2]=>
string(3) "470"
[3]=>
string(3) "471"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(3) "469"
[1]=>
string(2) "83"
[2]=>
string(3) "470"
[3]=>
string(3) "471"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["SUBMITTED"]=>
array(36) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5617"
["VALUE"]=>
string(19) "14.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "14.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
}
["ACCEPTED"]=>
array(36) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5618"
["VALUE"]=>
string(19) "10.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "10.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
}
["PUBLISHED"]=>
array(36) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5619"
["VALUE"]=>
string(19) "26.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "26.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
}
["CONTACT"]=>
array(36) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5620"
["VALUE"]=>
string(3) "148"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "148"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHORS"]=>
array(36) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(4) "5817"
[1]=>
string(4) "5818"
[2]=>
string(4) "5819"
[3]=>
string(4) "5820"
}
["VALUE"]=>
array(4) {
[0]=>
string(3) "148"
[1]=>
string(3) "467"
[2]=>
string(3) "468"
[3]=>
string(2) "34"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(3) "148"
[1]=>
string(3) "467"
[2]=>
string(3) "468"
[3]=>
string(2) "34"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_RU"]=>
array(36) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5625"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(155) "<p class="Autor">Бархатов И. М., Румянцев С. А., Владимирская Е. Б., Афанасьев Б. В.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(133) "
Бархатов И. М., Румянцев С. А., Владимирская Е. Б., Афанасьев Б. В.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_RU"]=>
array(36) {
["ID"]=>
string(2) "26"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(22) "Организации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "26"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(22) "Организации"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_RU"]=>
array(36) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5626"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(8344) "<h2>Резюме</h2>
<h2>Введение </h2>
<p class="bodytext">
В условиях монослойной культуры клетки пуповинной крови способны прикрепляться к пластику и по своей морфологии напоминают культивируемые в сходных условиях мезенхимальные стволовые клетки (МСК) костного мозга. Однако присутствие в прилипающей фракции пуповинной крови МСК до сих пор не является очевидным. Данное исследование выполнено с целью определения состава и ряда функциональных свойств МСК-подобных клеток в монослойной культуре пуповинной крови (МКПК) человека.
</p>
<h2>Материалы и методы</h2>
<p>
Исследовали сорок три образца пуповинной крови, полученые в срочных родах на фоне неосложненной беременности у рожениц при атравматичном заборе. Исследования проводили после 19-31 часов хранения образца. Ядросодержащие клетки выделяли на градиенте плотности фиколла (1,077 г/мл), затем помещали в полную культуральную среду, содержащую среду DMEM LG, эмбриональную телячью сыворотку - 30%, пенициллин (100 Ед/мл), стрептомицин (0,1 мг/мл). Анализ фенотипа монослойной культуры ПК и ее мононуклеарной фракции проводили на проточном цитофлюориметре FACScan. Были использованы следующие конъюгированные флюорохромами антитела: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR. С целью определения гемостимулирующих свойств монослойной культуры ПК проводили клонирование гранулоцитарно-макрофагальных предшественников (КОЕ-ГМ) в культуральной системе «агаровая капля-жидкая среда». В качестве источника колониестимулирующей активности ПК использовали МКПК. Клетками-мишенями были КОЕ-ГМ мононуклеарной фракции ПК, дающие клональный рост в агаровой культуре. Для индукции дифференцировки МСК-подобных клеток ПК в адипогенном и остеогенном направлении клетки помещали в полную среду с добавлением дексаметазона (1х10<sup>-7</sup> М); инсулина (1х10<sup>-9</sup> М) или β-глицерофосфата (7х10<sup>-3</sup> М); дексаметазона (1х10<sup>-8</sup> М); аскорбиновой кислоты (2х10<sup>-4</sup> М) соответственно. Оценка экспрессии генов (CDH11,VCAM1, ITGB1, IL6ST, TFRC, ALCAM, MPL, TPO, ENG, NT5E, IL6R, BGLAP, COL1A2, AFP, LPL, ACTA1, TNNI3, TPM1) проводилось методом RT-PCR (амплификация продуктов обратной транскрипции).
</p>
<h2>Результаты</h2>
<p>
В большинстве случаев культура клеток, прилипших к пластику была гетерогенна: наблюдали узкие веретенообразные клетки и большие полигональные. В ряде образцов обнаруживали небольшие колонии (до 100 клеток). В 3 из 43 исследованных образцов ПК наблюдали крупные колонии, численностью более 1000 плотноупакованных, имеющих типичную для фибробластов веретенообразную форму клеток. При анализе преобладающих клеточных типов было выявлено, что большую часть прикрепленных к пластику клеток составляли гемопоэтические клетки (медиана 60,17%). Около трети от всей СD45-положительной популяции составляли СD14-положительные клетки. Остальные негемопоэтические клетки представляли собой фенотипически гетерогенную популяцию. На фоне длительного культивирования и последовательного пассирования фенотип культуры меняется – отмечается элиминация из культуры гемопоэтических клеток и увеличение доли МСК и ЭКП. При инициации культуры значительно изменяется соотношение ГСК- и ЭКП-подобных клеток среди CD34-положительной популяции в пользу ЭКП. МСК-подобные клетки МКПК способны к дифференцировке в адипоциты и остеобласты, что подтверждается специфической окраской и свидетельствует в пользу их функциональной состоятельности. В ряде культур индукция дифференцировки инициировала открепление большей части клеток. Прилипающая фракция первичной монослойной культуры оказывает стимулирующее влияние на колониеобразование КОЕ-ГМ, по характеру и силе воздействия близкое стандартному лейкоцитарному фидеру. Преимущественное влияние на их пролиферативную активность оказывают клеточные элементы с маркерами МСК (CD90<sup>+</sup>CD31<sup>-</sup>). Удлинение временных параметров получения и хранения образцов ПК приводят к снижению гемостимулирующей активности. При сравнении экспрессии ряда генов выявлено, что профиль экспрессии МСК костного мозга и клеток МКПК идентичен за исключением тромбопоэтина, экспрессия гена которого не отмечалась в МКПК.
</p>
<h2>Заключение</h2>
<p>
Пуповинная кровь содержит субпопуляции клеток негемопоэтического происхождения, фенотипически и функционально сходных с МСК костного мозга. Однако их низкая концентрация, а также сниженная репопулирующая активность в стандартных культуральных условиях, ставят под сомнение возможное использование ПК в качестве альтернативного источника МСК.
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(8142) "
Резюме
Введение
В условиях монослойной культуры клетки пуповинной крови способны прикрепляться к пластику и по своей морфологии напоминают культивируемые в сходных условиях мезенхимальные стволовые клетки (МСК) костного мозга. Однако присутствие в прилипающей фракции пуповинной крови МСК до сих пор не является очевидным. Данное исследование выполнено с целью определения состава и ряда функциональных свойств МСК-подобных клеток в монослойной культуре пуповинной крови (МКПК) человека.
Материалы и методы
Исследовали сорок три образца пуповинной крови, полученые в срочных родах на фоне неосложненной беременности у рожениц при атравматичном заборе. Исследования проводили после 19-31 часов хранения образца. Ядросодержащие клетки выделяли на градиенте плотности фиколла (1,077 г/мл), затем помещали в полную культуральную среду, содержащую среду DMEM LG, эмбриональную телячью сыворотку - 30%, пенициллин (100 Ед/мл), стрептомицин (0,1 мг/мл). Анализ фенотипа монослойной культуры ПК и ее мононуклеарной фракции проводили на проточном цитофлюориметре FACScan. Были использованы следующие конъюгированные флюорохромами антитела: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR. С целью определения гемостимулирующих свойств монослойной культуры ПК проводили клонирование гранулоцитарно-макрофагальных предшественников (КОЕ-ГМ) в культуральной системе «агаровая капля-жидкая среда». В качестве источника колониестимулирующей активности ПК использовали МКПК. Клетками-мишенями были КОЕ-ГМ мононуклеарной фракции ПК, дающие клональный рост в агаровой культуре. Для индукции дифференцировки МСК-подобных клеток ПК в адипогенном и остеогенном направлении клетки помещали в полную среду с добавлением дексаметазона (1х10-7 М); инсулина (1х10-9 М) или β-глицерофосфата (7х10-3 М); дексаметазона (1х10-8 М); аскорбиновой кислоты (2х10-4 М) соответственно. Оценка экспрессии генов (CDH11,VCAM1, ITGB1, IL6ST, TFRC, ALCAM, MPL, TPO, ENG, NT5E, IL6R, BGLAP, COL1A2, AFP, LPL, ACTA1, TNNI3, TPM1) проводилось методом RT-PCR (амплификация продуктов обратной транскрипции).
Результаты
В большинстве случаев культура клеток, прилипших к пластику была гетерогенна: наблюдали узкие веретенообразные клетки и большие полигональные. В ряде образцов обнаруживали небольшие колонии (до 100 клеток). В 3 из 43 исследованных образцов ПК наблюдали крупные колонии, численностью более 1000 плотноупакованных, имеющих типичную для фибробластов веретенообразную форму клеток. При анализе преобладающих клеточных типов было выявлено, что большую часть прикрепленных к пластику клеток составляли гемопоэтические клетки (медиана 60,17%). Около трети от всей СD45-положительной популяции составляли СD14-положительные клетки. Остальные негемопоэтические клетки представляли собой фенотипически гетерогенную популяцию. На фоне длительного культивирования и последовательного пассирования фенотип культуры меняется – отмечается элиминация из культуры гемопоэтических клеток и увеличение доли МСК и ЭКП. При инициации культуры значительно изменяется соотношение ГСК- и ЭКП-подобных клеток среди CD34-положительной популяции в пользу ЭКП. МСК-подобные клетки МКПК способны к дифференцировке в адипоциты и остеобласты, что подтверждается специфической окраской и свидетельствует в пользу их функциональной состоятельности. В ряде культур индукция дифференцировки инициировала открепление большей части клеток. Прилипающая фракция первичной монослойной культуры оказывает стимулирующее влияние на колониеобразование КОЕ-ГМ, по характеру и силе воздействия близкое стандартному лейкоцитарному фидеру. Преимущественное влияние на их пролиферативную активность оказывают клеточные элементы с маркерами МСК (CD90+CD31-). Удлинение временных параметров получения и хранения образцов ПК приводят к снижению гемостимулирующей активности. При сравнении экспрессии ряда генов выявлено, что профиль экспрессии МСК костного мозга и клеток МКПК идентичен за исключением тромбопоэтина, экспрессия гена которого не отмечалась в МКПК.
Заключение
Пуповинная кровь содержит субпопуляции клеток негемопоэтического происхождения, фенотипически и функционально сходных с МСК костного мозга. Однако их низкая концентрация, а также сниженная репопулирующая активность в стандартных культуральных условиях, ставят под сомнение возможное использование ПК в качестве альтернативного источника МСК.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["DOI"]=>
array(36) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5627"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000026.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000026.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_EN"]=>
array(36) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5628"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(211) "<p class="Autor">Barkhatov I. M.<sup>1</sup>, Roumiantsev S. A.<sup>2</sup>, Vladimirskaya E. B.<sup>2</sup>, Afanasyev B. V.<sup>1</sup></p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(141) "
Barkhatov I. M.1, Roumiantsev S. A.2, Vladimirskaya E. B.2, Afanasyev B. V.1
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_EN"]=>
array(36) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5647"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(230) "<p>
<sup>1</sup>Saint-Petersburg Pavlov State Medical University, Russia;<br>
<sup>2</sup>Russian Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(188) "
1Saint-Petersburg Pavlov State Medical University, Russia;
2Russian Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_EN"]=>
array(36) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5666"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(3090) "<h2>Summary</h2>
<h2>Objectives</h2>
<p>
It’s known that during cultivation, adherent cells of umbilical cord blood (UCB) form a monolayer reminiscent, in its composition, of the stromal monolayer of bone marrow (BM) culture. However, the presence of mesenchymal stem cells (MSCs) in UCB still remains uncertain. This study was performed to investigate the composition and some functional characteristics of MSC-like cell populations revealed in the cord blood monolayer culture.
</p>
<h2>Materials and methods</h2>
<p>
Forty-three human UCB samples were under study. All the samples were obtained during full-term deliveries. To produce monolayer cultures, mononuclear cell fractions from UCB were cultivated in a culture medium containing DMEM with 20% FCS, supplied with 1% Pen/Strep. Phenotypic patterns of UCB culture were assessed with a panel of monoclonal antibodies specific for CD34; CD117; CD45; CD14; CD3; CD19; CD31; CD90; HLA DR; and HLA ABC. To determine the functional characteristics of MSCs derived from UCB culture, their differentiation ability and stimulation of hematopoietic colony formation activity were evaluated.
</p>
<h2>Results</h2>
<p>
In most cases, the cultures of plastic-adherent cells proved to be heterogeneous. Both spindle-shaped and polygonal cells were observed. In some samples, clonal growth could be detected. However, the number of fibroblastoid cells did not increase 100 cells per colony. Large colonies were registered in three UCB samples of the 43 under study. As evidenced by immune phenotyping, the monolayer UCB cultures were rather polymorphic and dissimilar in each sample. Most of the cells present in the cultures were macrophages (CD45<sup>+</sup>). However, we also found different amounts of presumably mesenchymal cells, including cells with an endothelial phenotype (CD34<sup>+</sup>CD31<sup>+</sup>).
</p>
<p>
Specific staining showed that the cells from a UCB monolayer culture have the capacity to differentiate into adipocytes and osteoblasts. In some cultures, however, induction of differentiation lead to the detachment of a major cell fraction. Hemostimulatory ability of UCB monolayer cultures depended on the phenotype composition of the monolayer culture. CD45<sup>+</sup> and CD14<sup>+</sup> cells, evidently, are stimulatory for granulocyte-macrophage colony formation. Moreover, levels of non-hematopoietic subpopulations (CD90<sup>+</sup>CD31<sup>-</sup>) in UCB cultures showed a direct correlation with the numbers of CFU-GM colonies produced.
</p>
<h2>Conclusion</h2>
<p>
UCB contains a subpopulation of non-hematopoietic cells possessing phenotypic and some functional characteristics of bone marrow derived mesenchymal stem cells. However, the low content and variable numbers of such cells provide some doubts on the viability of UCB as an alternative source for MSC.
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(2886) "
Summary
Objectives
It’s known that during cultivation, adherent cells of umbilical cord blood (UCB) form a monolayer reminiscent, in its composition, of the stromal monolayer of bone marrow (BM) culture. However, the presence of mesenchymal stem cells (MSCs) in UCB still remains uncertain. This study was performed to investigate the composition and some functional characteristics of MSC-like cell populations revealed in the cord blood monolayer culture.
Materials and methods
Forty-three human UCB samples were under study. All the samples were obtained during full-term deliveries. To produce monolayer cultures, mononuclear cell fractions from UCB were cultivated in a culture medium containing DMEM with 20% FCS, supplied with 1% Pen/Strep. Phenotypic patterns of UCB culture were assessed with a panel of monoclonal antibodies specific for CD34; CD117; CD45; CD14; CD3; CD19; CD31; CD90; HLA DR; and HLA ABC. To determine the functional characteristics of MSCs derived from UCB culture, their differentiation ability and stimulation of hematopoietic colony formation activity were evaluated.
Results
In most cases, the cultures of plastic-adherent cells proved to be heterogeneous. Both spindle-shaped and polygonal cells were observed. In some samples, clonal growth could be detected. However, the number of fibroblastoid cells did not increase 100 cells per colony. Large colonies were registered in three UCB samples of the 43 under study. As evidenced by immune phenotyping, the monolayer UCB cultures were rather polymorphic and dissimilar in each sample. Most of the cells present in the cultures were macrophages (CD45+). However, we also found different amounts of presumably mesenchymal cells, including cells with an endothelial phenotype (CD34+CD31+).
Specific staining showed that the cells from a UCB monolayer culture have the capacity to differentiate into adipocytes and osteoblasts. In some cultures, however, induction of differentiation lead to the detachment of a major cell fraction. Hemostimulatory ability of UCB monolayer cultures depended on the phenotype composition of the monolayer culture. CD45+ and CD14+ cells, evidently, are stimulatory for granulocyte-macrophage colony formation. Moreover, levels of non-hematopoietic subpopulations (CD90+CD31-) in UCB cultures showed a direct correlation with the numbers of CFU-GM colonies produced.
Conclusion
UCB contains a subpopulation of non-hematopoietic cells possessing phenotypic and some functional characteristics of bone marrow derived mesenchymal stem cells. However, the low content and variable numbers of such cells provide some doubts on the viability of UCB as an alternative source for MSC.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["NAME_EN"]=>
array(36) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5629"
["VALUE"]=>
string(95) "Composition and functional properties of monolayer cell culture from human umbilical cord blood"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(95) "Composition and functional properties of monolayer cell culture from human umbilical cord blood"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["FULL_TEXT_RU"]=>
array(36) {
["ID"]=>
string(2) "42"
["TIMESTAMP_X"]=>
string(19) "2015-09-07 20:29:18"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(23) "Полный текст"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(12) "FULL_TEXT_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "42"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(23) "Полный текст"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["PDF_RU"]=>
array(36) {
["ID"]=>
string(2) "43"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF RUS"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_RU"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "43"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5630"
["VALUE"]=>
string(3) "120"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "120"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF RUS"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["PDF_EN"]=>
array(36) {
["ID"]=>
string(2) "44"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF ENG"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "44"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5648"
["VALUE"]=>
string(3) "121"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "121"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF ENG"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["NAME_LONG"]=>
array(36) {
["ID"]=>
string(2) "45"
["TIMESTAMP_X"]=>
string(19) "2023-04-13 00:55:00"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "NAME_LONG"
["DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "45"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(80)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["~DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
}
}
["DISPLAY_PROPERTIES"]=>
array(13) {
["AUTHOR_EN"]=>
array(37) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5628"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(211) "<p class="Autor">Barkhatov I. M.<sup>1</sup>, Roumiantsev S. A.<sup>2</sup>, Vladimirskaya E. B.<sup>2</sup>, Afanasyev B. V.<sup>1</sup></p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(141) "
Barkhatov I. M.1, Roumiantsev S. A.2, Vladimirskaya E. B.2, Afanasyev B. V.1
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(141) "
Barkhatov I. M.1, Roumiantsev S. A.2, Vladimirskaya E. B.2, Afanasyev B. V.1
"
}
["SUMMARY_EN"]=>
array(37) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5666"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(3090) "<h2>Summary</h2>
<h2>Objectives</h2>
<p>
It’s known that during cultivation, adherent cells of umbilical cord blood (UCB) form a monolayer reminiscent, in its composition, of the stromal monolayer of bone marrow (BM) culture. However, the presence of mesenchymal stem cells (MSCs) in UCB still remains uncertain. This study was performed to investigate the composition and some functional characteristics of MSC-like cell populations revealed in the cord blood monolayer culture.
</p>
<h2>Materials and methods</h2>
<p>
Forty-three human UCB samples were under study. All the samples were obtained during full-term deliveries. To produce monolayer cultures, mononuclear cell fractions from UCB were cultivated in a culture medium containing DMEM with 20% FCS, supplied with 1% Pen/Strep. Phenotypic patterns of UCB culture were assessed with a panel of monoclonal antibodies specific for CD34; CD117; CD45; CD14; CD3; CD19; CD31; CD90; HLA DR; and HLA ABC. To determine the functional characteristics of MSCs derived from UCB culture, their differentiation ability and stimulation of hematopoietic colony formation activity were evaluated.
</p>
<h2>Results</h2>
<p>
In most cases, the cultures of plastic-adherent cells proved to be heterogeneous. Both spindle-shaped and polygonal cells were observed. In some samples, clonal growth could be detected. However, the number of fibroblastoid cells did not increase 100 cells per colony. Large colonies were registered in three UCB samples of the 43 under study. As evidenced by immune phenotyping, the monolayer UCB cultures were rather polymorphic and dissimilar in each sample. Most of the cells present in the cultures were macrophages (CD45<sup>+</sup>). However, we also found different amounts of presumably mesenchymal cells, including cells with an endothelial phenotype (CD34<sup>+</sup>CD31<sup>+</sup>).
</p>
<p>
Specific staining showed that the cells from a UCB monolayer culture have the capacity to differentiate into adipocytes and osteoblasts. In some cultures, however, induction of differentiation lead to the detachment of a major cell fraction. Hemostimulatory ability of UCB monolayer cultures depended on the phenotype composition of the monolayer culture. CD45<sup>+</sup> and CD14<sup>+</sup> cells, evidently, are stimulatory for granulocyte-macrophage colony formation. Moreover, levels of non-hematopoietic subpopulations (CD90<sup>+</sup>CD31<sup>-</sup>) in UCB cultures showed a direct correlation with the numbers of CFU-GM colonies produced.
</p>
<h2>Conclusion</h2>
<p>
UCB contains a subpopulation of non-hematopoietic cells possessing phenotypic and some functional characteristics of bone marrow derived mesenchymal stem cells. However, the low content and variable numbers of such cells provide some doubts on the viability of UCB as an alternative source for MSC.
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(2886) "
Summary
Objectives
It’s known that during cultivation, adherent cells of umbilical cord blood (UCB) form a monolayer reminiscent, in its composition, of the stromal monolayer of bone marrow (BM) culture. However, the presence of mesenchymal stem cells (MSCs) in UCB still remains uncertain. This study was performed to investigate the composition and some functional characteristics of MSC-like cell populations revealed in the cord blood monolayer culture.
Materials and methods
Forty-three human UCB samples were under study. All the samples were obtained during full-term deliveries. To produce monolayer cultures, mononuclear cell fractions from UCB were cultivated in a culture medium containing DMEM with 20% FCS, supplied with 1% Pen/Strep. Phenotypic patterns of UCB culture were assessed with a panel of monoclonal antibodies specific for CD34; CD117; CD45; CD14; CD3; CD19; CD31; CD90; HLA DR; and HLA ABC. To determine the functional characteristics of MSCs derived from UCB culture, their differentiation ability and stimulation of hematopoietic colony formation activity were evaluated.
Results
In most cases, the cultures of plastic-adherent cells proved to be heterogeneous. Both spindle-shaped and polygonal cells were observed. In some samples, clonal growth could be detected. However, the number of fibroblastoid cells did not increase 100 cells per colony. Large colonies were registered in three UCB samples of the 43 under study. As evidenced by immune phenotyping, the monolayer UCB cultures were rather polymorphic and dissimilar in each sample. Most of the cells present in the cultures were macrophages (CD45+). However, we also found different amounts of presumably mesenchymal cells, including cells with an endothelial phenotype (CD34+CD31+).
Specific staining showed that the cells from a UCB monolayer culture have the capacity to differentiate into adipocytes and osteoblasts. In some cultures, however, induction of differentiation lead to the detachment of a major cell fraction. Hemostimulatory ability of UCB monolayer cultures depended on the phenotype composition of the monolayer culture. CD45+ and CD14+ cells, evidently, are stimulatory for granulocyte-macrophage colony formation. Moreover, levels of non-hematopoietic subpopulations (CD90+CD31-) in UCB cultures showed a direct correlation with the numbers of CFU-GM colonies produced.
Conclusion
UCB contains a subpopulation of non-hematopoietic cells possessing phenotypic and some functional characteristics of bone marrow derived mesenchymal stem cells. However, the low content and variable numbers of such cells provide some doubts on the viability of UCB as an alternative source for MSC.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(2886) "
Summary
Objectives
It’s known that during cultivation, adherent cells of umbilical cord blood (UCB) form a monolayer reminiscent, in its composition, of the stromal monolayer of bone marrow (BM) culture. However, the presence of mesenchymal stem cells (MSCs) in UCB still remains uncertain. This study was performed to investigate the composition and some functional characteristics of MSC-like cell populations revealed in the cord blood monolayer culture.
Materials and methods
Forty-three human UCB samples were under study. All the samples were obtained during full-term deliveries. To produce monolayer cultures, mononuclear cell fractions from UCB were cultivated in a culture medium containing DMEM with 20% FCS, supplied with 1% Pen/Strep. Phenotypic patterns of UCB culture were assessed with a panel of monoclonal antibodies specific for CD34; CD117; CD45; CD14; CD3; CD19; CD31; CD90; HLA DR; and HLA ABC. To determine the functional characteristics of MSCs derived from UCB culture, their differentiation ability and stimulation of hematopoietic colony formation activity were evaluated.
Results
In most cases, the cultures of plastic-adherent cells proved to be heterogeneous. Both spindle-shaped and polygonal cells were observed. In some samples, clonal growth could be detected. However, the number of fibroblastoid cells did not increase 100 cells per colony. Large colonies were registered in three UCB samples of the 43 under study. As evidenced by immune phenotyping, the monolayer UCB cultures were rather polymorphic and dissimilar in each sample. Most of the cells present in the cultures were macrophages (CD45+). However, we also found different amounts of presumably mesenchymal cells, including cells with an endothelial phenotype (CD34+CD31+).
Specific staining showed that the cells from a UCB monolayer culture have the capacity to differentiate into adipocytes and osteoblasts. In some cultures, however, induction of differentiation lead to the detachment of a major cell fraction. Hemostimulatory ability of UCB monolayer cultures depended on the phenotype composition of the monolayer culture. CD45+ and CD14+ cells, evidently, are stimulatory for granulocyte-macrophage colony formation. Moreover, levels of non-hematopoietic subpopulations (CD90+CD31-) in UCB cultures showed a direct correlation with the numbers of CFU-GM colonies produced.
Conclusion
UCB contains a subpopulation of non-hematopoietic cells possessing phenotypic and some functional characteristics of bone marrow derived mesenchymal stem cells. However, the low content and variable numbers of such cells provide some doubts on the viability of UCB as an alternative source for MSC.
"
}
["DOI"]=>
array(37) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5627"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000026.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000026.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(29) "10.3205/ctt-2008-en-000026.01"
}
["NAME_EN"]=>
array(37) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5629"
["VALUE"]=>
string(95) "Composition and functional properties of monolayer cell culture from human umbilical cord blood"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(95) "Composition and functional properties of monolayer cell culture from human umbilical cord blood"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(95) "Composition and functional properties of monolayer cell culture from human umbilical cord blood"
}
["ORGANIZATION_EN"]=>
array(37) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5647"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(230) "<p>
<sup>1</sup>Saint-Petersburg Pavlov State Medical University, Russia;<br>
<sup>2</sup>Russian Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(188) "
1Saint-Petersburg Pavlov State Medical University, Russia;
2Russian Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(188) "
1Saint-Petersburg Pavlov State Medical University, Russia;
2Russian Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia
"
}
["AUTHORS"]=>
array(38) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(4) "5817"
[1]=>
string(4) "5818"
[2]=>
string(4) "5819"
[3]=>
string(4) "5820"
}
["VALUE"]=>
array(4) {
[0]=>
string(3) "148"
[1]=>
string(3) "467"
[2]=>
string(3) "468"
[3]=>
string(2) "34"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(3) "148"
[1]=>
string(3) "467"
[2]=>
string(3) "468"
[3]=>
string(2) "34"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(4) {
[0]=>
string(61) "
Ildar M. Barkhatov"
[1]=>
string(60) "
Roumiantsev S. A."
[2]=>
string(62) "
Vladimirskaya E. B."
[3]=>
string(60) "
Boris V. Afanasyev"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["AUTHOR_RU"]=>
array(37) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5625"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(155) "<p class="Autor">Бархатов И. М., Румянцев С. А., Владимирская Е. Б., Афанасьев Б. В.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(133) "
Бархатов И. М., Румянцев С. А., Владимирская Е. Б., Афанасьев Б. В.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(133) "
Бархатов И. М., Румянцев С. А., Владимирская Е. Б., Афанасьев Б. В.
"
}
["SUBMITTED"]=>
array(37) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5617"
["VALUE"]=>
string(19) "14.11.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "14.11.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "14.11.2008 00:01:00"
}
["ACCEPTED"]=>
array(37) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5618"
["VALUE"]=>
string(19) "10.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "10.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "10.12.2008 00:01:00"
}
["PUBLISHED"]=>
array(37) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5619"
["VALUE"]=>
string(19) "26.12.2008 00:01:00"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(19) "26.12.2008 00:01:00"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(24) "26.12.2008 00:01:00"
}
["KEYWORDS"]=>
array(38) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(4) "5813"
[1]=>
string(4) "5814"
[2]=>
string(4) "5815"
[3]=>
string(4) "5816"
}
["VALUE"]=>
array(4) {
[0]=>
string(3) "469"
[1]=>
string(2) "83"
[2]=>
string(3) "470"
[3]=>
string(3) "471"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(3) "469"
[1]=>
string(2) "83"
[2]=>
string(3) "470"
[3]=>
string(3) "471"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(4) {
[0]=>
string(92) "
пуповинная кровь человека"
[1]=>
string(97) "
мезенхимные стволовые клетки"
[2]=>
string(86) "
иммунофенотипирование"
[3]=>
string(73) "
культура клеток"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["CONTACT"]=>
array(38) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5620"
["VALUE"]=>
string(3) "148"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "148"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(61) "
Ildar M. Barkhatov"
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["SUMMARY_RU"]=>
array(37) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(4) "5626"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(8344) "<h2>Резюме</h2>
<h2>Введение </h2>
<p class="bodytext">
В условиях монослойной культуры клетки пуповинной крови способны прикрепляться к пластику и по своей морфологии напоминают культивируемые в сходных условиях мезенхимальные стволовые клетки (МСК) костного мозга. Однако присутствие в прилипающей фракции пуповинной крови МСК до сих пор не является очевидным. Данное исследование выполнено с целью определения состава и ряда функциональных свойств МСК-подобных клеток в монослойной культуре пуповинной крови (МКПК) человека.
</p>
<h2>Материалы и методы</h2>
<p>
Исследовали сорок три образца пуповинной крови, полученые в срочных родах на фоне неосложненной беременности у рожениц при атравматичном заборе. Исследования проводили после 19-31 часов хранения образца. Ядросодержащие клетки выделяли на градиенте плотности фиколла (1,077 г/мл), затем помещали в полную культуральную среду, содержащую среду DMEM LG, эмбриональную телячью сыворотку - 30%, пенициллин (100 Ед/мл), стрептомицин (0,1 мг/мл). Анализ фенотипа монослойной культуры ПК и ее мононуклеарной фракции проводили на проточном цитофлюориметре FACScan. Были использованы следующие конъюгированные флюорохромами антитела: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR. С целью определения гемостимулирующих свойств монослойной культуры ПК проводили клонирование гранулоцитарно-макрофагальных предшественников (КОЕ-ГМ) в культуральной системе «агаровая капля-жидкая среда». В качестве источника колониестимулирующей активности ПК использовали МКПК. Клетками-мишенями были КОЕ-ГМ мононуклеарной фракции ПК, дающие клональный рост в агаровой культуре. Для индукции дифференцировки МСК-подобных клеток ПК в адипогенном и остеогенном направлении клетки помещали в полную среду с добавлением дексаметазона (1х10<sup>-7</sup> М); инсулина (1х10<sup>-9</sup> М) или β-глицерофосфата (7х10<sup>-3</sup> М); дексаметазона (1х10<sup>-8</sup> М); аскорбиновой кислоты (2х10<sup>-4</sup> М) соответственно. Оценка экспрессии генов (CDH11,VCAM1, ITGB1, IL6ST, TFRC, ALCAM, MPL, TPO, ENG, NT5E, IL6R, BGLAP, COL1A2, AFP, LPL, ACTA1, TNNI3, TPM1) проводилось методом RT-PCR (амплификация продуктов обратной транскрипции).
</p>
<h2>Результаты</h2>
<p>
В большинстве случаев культура клеток, прилипших к пластику была гетерогенна: наблюдали узкие веретенообразные клетки и большие полигональные. В ряде образцов обнаруживали небольшие колонии (до 100 клеток). В 3 из 43 исследованных образцов ПК наблюдали крупные колонии, численностью более 1000 плотноупакованных, имеющих типичную для фибробластов веретенообразную форму клеток. При анализе преобладающих клеточных типов было выявлено, что большую часть прикрепленных к пластику клеток составляли гемопоэтические клетки (медиана 60,17%). Около трети от всей СD45-положительной популяции составляли СD14-положительные клетки. Остальные негемопоэтические клетки представляли собой фенотипически гетерогенную популяцию. На фоне длительного культивирования и последовательного пассирования фенотип культуры меняется – отмечается элиминация из культуры гемопоэтических клеток и увеличение доли МСК и ЭКП. При инициации культуры значительно изменяется соотношение ГСК- и ЭКП-подобных клеток среди CD34-положительной популяции в пользу ЭКП. МСК-подобные клетки МКПК способны к дифференцировке в адипоциты и остеобласты, что подтверждается специфической окраской и свидетельствует в пользу их функциональной состоятельности. В ряде культур индукция дифференцировки инициировала открепление большей части клеток. Прилипающая фракция первичной монослойной культуры оказывает стимулирующее влияние на колониеобразование КОЕ-ГМ, по характеру и силе воздействия близкое стандартному лейкоцитарному фидеру. Преимущественное влияние на их пролиферативную активность оказывают клеточные элементы с маркерами МСК (CD90<sup>+</sup>CD31<sup>-</sup>). Удлинение временных параметров получения и хранения образцов ПК приводят к снижению гемостимулирующей активности. При сравнении экспрессии ряда генов выявлено, что профиль экспрессии МСК костного мозга и клеток МКПК идентичен за исключением тромбопоэтина, экспрессия гена которого не отмечалась в МКПК.
</p>
<h2>Заключение</h2>
<p>
Пуповинная кровь содержит субпопуляции клеток негемопоэтического происхождения, фенотипически и функционально сходных с МСК костного мозга. Однако их низкая концентрация, а также сниженная репопулирующая активность в стандартных культуральных условиях, ставят под сомнение возможное использование ПК в качестве альтернативного источника МСК.
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(8142) "
Резюме
Введение
В условиях монослойной культуры клетки пуповинной крови способны прикрепляться к пластику и по своей морфологии напоминают культивируемые в сходных условиях мезенхимальные стволовые клетки (МСК) костного мозга. Однако присутствие в прилипающей фракции пуповинной крови МСК до сих пор не является очевидным. Данное исследование выполнено с целью определения состава и ряда функциональных свойств МСК-подобных клеток в монослойной культуре пуповинной крови (МКПК) человека.
Материалы и методы
Исследовали сорок три образца пуповинной крови, полученые в срочных родах на фоне неосложненной беременности у рожениц при атравматичном заборе. Исследования проводили после 19-31 часов хранения образца. Ядросодержащие клетки выделяли на градиенте плотности фиколла (1,077 г/мл), затем помещали в полную культуральную среду, содержащую среду DMEM LG, эмбриональную телячью сыворотку - 30%, пенициллин (100 Ед/мл), стрептомицин (0,1 мг/мл). Анализ фенотипа монослойной культуры ПК и ее мононуклеарной фракции проводили на проточном цитофлюориметре FACScan. Были использованы следующие конъюгированные флюорохромами антитела: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR. С целью определения гемостимулирующих свойств монослойной культуры ПК проводили клонирование гранулоцитарно-макрофагальных предшественников (КОЕ-ГМ) в культуральной системе «агаровая капля-жидкая среда». В качестве источника колониестимулирующей активности ПК использовали МКПК. Клетками-мишенями были КОЕ-ГМ мононуклеарной фракции ПК, дающие клональный рост в агаровой культуре. Для индукции дифференцировки МСК-подобных клеток ПК в адипогенном и остеогенном направлении клетки помещали в полную среду с добавлением дексаметазона (1х10-7 М); инсулина (1х10-9 М) или β-глицерофосфата (7х10-3 М); дексаметазона (1х10-8 М); аскорбиновой кислоты (2х10-4 М) соответственно. Оценка экспрессии генов (CDH11,VCAM1, ITGB1, IL6ST, TFRC, ALCAM, MPL, TPO, ENG, NT5E, IL6R, BGLAP, COL1A2, AFP, LPL, ACTA1, TNNI3, TPM1) проводилось методом RT-PCR (амплификация продуктов обратной транскрипции).
Результаты
В большинстве случаев культура клеток, прилипших к пластику была гетерогенна: наблюдали узкие веретенообразные клетки и большие полигональные. В ряде образцов обнаруживали небольшие колонии (до 100 клеток). В 3 из 43 исследованных образцов ПК наблюдали крупные колонии, численностью более 1000 плотноупакованных, имеющих типичную для фибробластов веретенообразную форму клеток. При анализе преобладающих клеточных типов было выявлено, что большую часть прикрепленных к пластику клеток составляли гемопоэтические клетки (медиана 60,17%). Около трети от всей СD45-положительной популяции составляли СD14-положительные клетки. Остальные негемопоэтические клетки представляли собой фенотипически гетерогенную популяцию. На фоне длительного культивирования и последовательного пассирования фенотип культуры меняется – отмечается элиминация из культуры гемопоэтических клеток и увеличение доли МСК и ЭКП. При инициации культуры значительно изменяется соотношение ГСК- и ЭКП-подобных клеток среди CD34-положительной популяции в пользу ЭКП. МСК-подобные клетки МКПК способны к дифференцировке в адипоциты и остеобласты, что подтверждается специфической окраской и свидетельствует в пользу их функциональной состоятельности. В ряде культур индукция дифференцировки инициировала открепление большей части клеток. Прилипающая фракция первичной монослойной культуры оказывает стимулирующее влияние на колониеобразование КОЕ-ГМ, по характеру и силе воздействия близкое стандартному лейкоцитарному фидеру. Преимущественное влияние на их пролиферативную активность оказывают клеточные элементы с маркерами МСК (CD90+CD31-). Удлинение временных параметров получения и хранения образцов ПК приводят к снижению гемостимулирующей активности. При сравнении экспрессии ряда генов выявлено, что профиль экспрессии МСК костного мозга и клеток МКПК идентичен за исключением тромбопоэтина, экспрессия гена которого не отмечалась в МКПК.
Заключение
Пуповинная кровь содержит субпопуляции клеток негемопоэтического происхождения, фенотипически и функционально сходных с МСК костного мозга. Однако их низкая концентрация, а также сниженная репопулирующая активность в стандартных культуральных условиях, ставят под сомнение возможное использование ПК в качестве альтернативного источника МСК.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(8142) "
Резюме
Введение
В условиях монослойной культуры клетки пуповинной крови способны прикрепляться к пластику и по своей морфологии напоминают культивируемые в сходных условиях мезенхимальные стволовые клетки (МСК) костного мозга. Однако присутствие в прилипающей фракции пуповинной крови МСК до сих пор не является очевидным. Данное исследование выполнено с целью определения состава и ряда функциональных свойств МСК-подобных клеток в монослойной культуре пуповинной крови (МКПК) человека.
Материалы и методы
Исследовали сорок три образца пуповинной крови, полученые в срочных родах на фоне неосложненной беременности у рожениц при атравматичном заборе. Исследования проводили после 19-31 часов хранения образца. Ядросодержащие клетки выделяли на градиенте плотности фиколла (1,077 г/мл), затем помещали в полную культуральную среду, содержащую среду DMEM LG, эмбриональную телячью сыворотку - 30%, пенициллин (100 Ед/мл), стрептомицин (0,1 мг/мл). Анализ фенотипа монослойной культуры ПК и ее мононуклеарной фракции проводили на проточном цитофлюориметре FACScan. Были использованы следующие конъюгированные флюорохромами антитела: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR. С целью определения гемостимулирующих свойств монослойной культуры ПК проводили клонирование гранулоцитарно-макрофагальных предшественников (КОЕ-ГМ) в культуральной системе «агаровая капля-жидкая среда». В качестве источника колониестимулирующей активности ПК использовали МКПК. Клетками-мишенями были КОЕ-ГМ мононуклеарной фракции ПК, дающие клональный рост в агаровой культуре. Для индукции дифференцировки МСК-подобных клеток ПК в адипогенном и остеогенном направлении клетки помещали в полную среду с добавлением дексаметазона (1х10-7 М); инсулина (1х10-9 М) или β-глицерофосфата (7х10-3 М); дексаметазона (1х10-8 М); аскорбиновой кислоты (2х10-4 М) соответственно. Оценка экспрессии генов (CDH11,VCAM1, ITGB1, IL6ST, TFRC, ALCAM, MPL, TPO, ENG, NT5E, IL6R, BGLAP, COL1A2, AFP, LPL, ACTA1, TNNI3, TPM1) проводилось методом RT-PCR (амплификация продуктов обратной транскрипции).
Результаты
В большинстве случаев культура клеток, прилипших к пластику была гетерогенна: наблюдали узкие веретенообразные клетки и большие полигональные. В ряде образцов обнаруживали небольшие колонии (до 100 клеток). В 3 из 43 исследованных образцов ПК наблюдали крупные колонии, численностью более 1000 плотноупакованных, имеющих типичную для фибробластов веретенообразную форму клеток. При анализе преобладающих клеточных типов было выявлено, что большую часть прикрепленных к пластику клеток составляли гемопоэтические клетки (медиана 60,17%). Около трети от всей СD45-положительной популяции составляли СD14-положительные клетки. Остальные негемопоэтические клетки представляли собой фенотипически гетерогенную популяцию. На фоне длительного культивирования и последовательного пассирования фенотип культуры меняется – отмечается элиминация из культуры гемопоэтических клеток и увеличение доли МСК и ЭКП. При инициации культуры значительно изменяется соотношение ГСК- и ЭКП-подобных клеток среди CD34-положительной популяции в пользу ЭКП. МСК-подобные клетки МКПК способны к дифференцировке в адипоциты и остеобласты, что подтверждается специфической окраской и свидетельствует в пользу их функциональной состоятельности. В ряде культур индукция дифференцировки инициировала открепление большей части клеток. Прилипающая фракция первичной монослойной культуры оказывает стимулирующее влияние на колониеобразование КОЕ-ГМ, по характеру и силе воздействия близкое стандартному лейкоцитарному фидеру. Преимущественное влияние на их пролиферативную активность оказывают клеточные элементы с маркерами МСК (CD90+CD31-). Удлинение временных параметров получения и хранения образцов ПК приводят к снижению гемостимулирующей активности. При сравнении экспрессии ряда генов выявлено, что профиль экспрессии МСК костного мозга и клеток МКПК идентичен за исключением тромбопоэтина, экспрессия гена которого не отмечалась в МКПК.
Заключение
Пуповинная кровь содержит субпопуляции клеток негемопоэтического происхождения, фенотипически и функционально сходных с МСК костного мозга. Однако их низкая концентрация, а также сниженная репопулирующая активность в стандартных культуральных условиях, ставят под сомнение возможное использование ПК в качестве альтернативного источника МСК.
"
}
}
}
[3]=>
array(49) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
["~IBLOCK_SECTION_ID"]=>
string(2) "10"
["ID"]=>
string(3) "805"
["~ID"]=>
string(3) "805"
["IBLOCK_ID"]=>
string(1) "2"
["~IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["~NAME"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["ACTIVE_FROM"]=>
NULL
["~ACTIVE_FROM"]=>
NULL
["TIMESTAMP_X"]=>
string(19) "07.06.2017 16:09:34"
["~TIMESTAMP_X"]=>
string(19) "07.06.2017 16:09:34"
["DETAIL_PAGE_URL"]=>
string(145) "/ru/archive/Volume-1/korotkie-soobshcheniya/pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno/"
["~DETAIL_PAGE_URL"]=>
string(145) "/ru/archive/Volume-1/korotkie-soobshcheniya/pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno/"
["LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["~LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["DETAIL_TEXT"]=>
string(18786) "
Introduction
AKI remains a common, serious, and essentially treatment resistant syndrome of rapidly declining renal function. The mortality rates from AKI range from 15% in the general community to 80% for patients with multi-organ failure or for those who develop it post-operatively [1]. Even when renal function appears to fully recover after AKI, it is now recognized that a significant proportion of patients develop end stage renal disease (ESRD) as a consequence of undiagnosed, incompletely resolved AKI, characterized by continued inflammatory and fibrotic processes, and microvascular rarefaction [2]. Consequently, those patients who seemingly recover from AKI frequently go on to develop chronic kidney disease (CKD), eventually requiring chronic hemodialysis or a renal transplant [3].
AKI is most frequently seen in patients with shock, sepsis, trauma, and after major surgery. Patients undergoing cardiac surgery are at particularly high risk with up to 30% of all cardiac surgery patients developing AKI [4]. Many studies of cardiac patients have consistently found certain factors to be associated with increased risk of developing AKI following surgery. These risks include but are not limited to: the type of procedure performed (valve procedures are found to be of particularly high risk); patient age greater than 65; female patient gender; pre-operative serum creatinine value above 1.2 mg/dL, or underlying renal disease; pre-operative capillary glucose above 140 mg/dL; congestive heart failure; combined surgeries; on-pump vs. off pump surgery; and cardiopulmonary bypass surgery time greater than two hours [4-6]. The treatment resistant nature of AKI, combined with high morbidity and mortality, as well as the now recognized frequent progression of AKI to chronic kidney disease (CKD) underscores the urgent need for advances in treatment modalities.
Recent studies from our laboratory have led to the development of a novel approach to AKI treatment. This treatment administers allogeneic or syngeneic MSC to prevent further damage and to facilitate repair of acutely injured kidneys [7-9]. We observed that immediate (post reflow) or delayed (24 hrs post reflow) treatment of I/R AKI in rats with either autologous or allogeneic MSC significantly protects renal function, improves survival and hastens renal repair, mediated by complex paracrine mechanisms (anti-apoptotic, mitogenic, anti-inflammatory, vasculoprotective, angiogenic, anti-fibrotic) 7-10]. The striking hypoimmunogenic and immune modulating properties of MSC make their therapeutic use in allogeneic protocols possible and safe, as has been demonstrated in numerous clinical (www.clinicaltrials.gov) and pre-clinical trials [11, 12].
Compared to vehicle treated animals with I/R AKI, early allogeneic MSC therapy has important late benefits (3 months post AKI) such as maintained creatinine clearance, decreased interstitial fibrosis, and down regulation of pro-fibrotic gene expression levels in the kidney (TGFβ, PAI-1, TIMP-1). In addition, MSC therapy for AKI results in well maintained microvascular density in the kidney, while there is significant micorvascular rarefaction in vehicle treated animals [7]. In AKI, administered MSC do not engraft and disappear from the kidney and other organs within 1 to 3 days.
The aforementioned preclinical studies indicate that effective and specific treatment of AKI with MSC is an intervention that also prevents progressive loss of renal function, a complication that is increasingly recognized to result in ESRD in patients in whom AKI was not diagnosed and treated early after a renal insult [13]. Accordingly, a Phase I Clinical Trial employing this treatment is currently underway (www.clinicaltrials.gov; NCT00733876). This safety trial involves administration of MSC to fifteen patients divided into three cohorts of five patients each. Each cohort receives a defined dose of MSC, low, intermediate or high. As of this writing, dosing of the first cohort is complete, and we report here the outcomes of the first cohort of five patients.
Study Design and Methods
The FDA and the Institutional Review Board of Intermountain Medical Center, Murray, Utah, the site where the trial is carried out, approved the design and conduct of this Phase I Clinical Safety Trial. In addition, prior to initiation of the trial an independent Data Safety and Monitoring Board (DSMB) was appointed, consisting of a general nephrologists, a nephrologist/medical epidemiologist, and a cardiovascular surgeon. This DSMB reviewed the trial protocol and approved the trial, and continues to monitor the clinical data from all enrolled and treated subjects.
The study design is a Phase 1 Safety Trial. The primary objective is to test whether infusion of allogeneic MSC into the suprarenal aorta of patients who have undergone on-pump cardiac surgery (Coronary Artery Bypass Grafting and/or valve surgery) and who are at high risk for AKI following surgery is safe. This is assessed by monitoring patients post operatively for the occurrence of adverse events (AEs) and serious adverse events (SAEs) that are related to the MSC therapy. Specifically, detailed, monthly examinations for six months regarding the development of AEs or SAEs are carried out, and the study subjects are reassessed annually for another three years.
The major endpoint to be measured is safety, as documented by the comparative incidence of Adverse Events, Severe Adverse Events and complications in patients receiving cell-based therapies vs. historical controls for this patient population. AEs will be recorded throughout the course of the study and classified as immediate, postoperative, or delayed. Both common, expected and unusual AEs are listed below.
Potential immediate or early AEs related to the infusion of MSC via a femoral catheter into the suprarenal aorta include femoral catheter related complications such as bleeding at the catheter insertion site, infections, cholesterol plaque dislodgement and secondary visceral or peripheral embolic events.
Immediate AEs and SAEs occurring at the time of operation and immediately post-op (up to 24 hours post-op) include the following: post-operative compromise of heart function due to an unexpected ischemic event; post-operative marked impairment of renal function due to an unexpected ischemic coronary or other event (bleeding, hypotension, heart failure); perioperative complications that will require additional time in order to address these.
Later, post-operative complications (1-30 days post-op) include delayed deterioration in renal function post-op, requiring or not requiring dialysis; bleeding requiring >6 units of blood transfusion; arrhythmia requiring cardioversion; mediastinitis; cerebral vascular accident; prolonged ventilator support (> 24 hours postoperatively); reintubation; acute myocardial infarction; wound infection or hematoma; pericarditis; pneumonia; pulmonary embolism; bacteremia, sepsis, shock; multiorgan failure; death.
Delayed (more than 30 days after operation) AEs and SAEs include: dialysis dependency due to irreversible loss of kidney function; arrhythmia requiring cardioversion; mediastinitis; cerebral vascular accident; acute myocardial infarction; wound infection or hematoma; pneumonia; pulmonary embolism; malignancy; ectopic differentiation of MSC into mesodermal cells (bone, cartilage, fat); death.
The secondary objective of this trial is preliminary efficacy of MSC administration for the potential prevention and treatment of post-operative AKI. Although a priori underpowered, preliminary efficacy in this trial is nevertheless assessed by determining the comparative frequency and severity of post-operative AKI using standard and novel biomarkers of AKI (serum creatinine, BUN, urine output, creatinine clearance, electrolyte, acid-base balance, serum cystatin C, IL-18 and NGAL levels), need for temporary or permanent dialysis, length of hospital stay, and associated 30 day mortality. Study data are compared to published historical data that are collected and available for analysis from the Society of Thoracic Surgeons (www.STS.org). Historical data from this data base are collected and analyzed from all participating centers in the USA, and sub-analyzed for a reporting institution, such as IMC, and comparable institutions.
The trial is currently conducted in one center, IMC in Murray, Utah. Allogeneic MSC, derived from healthy, screened donors, using FDA approved protocols, are culture expanded under cGMP conditions at the University of Utah Cell Therapy Facility, Salt Lake City, Utah. MSC are administered in a dose escalation protocol to a total of 15 patients who have undergone elective, on-pump cardiac surgery (CABG and/or valve replacement). Five patients each receive low, medium or high dose of allogeneic MSC via a femoral catheter into the suprarenal aorta immediately after the patient comes off pump and is hemodynamically stable.
Low, Intermediate and High Doses of allogeneic MSC are defined per FDA approved protocol, and are infused into the suprarenal aorta in 50 ml of normal saline via a femoral catheter.
The enrollment and exclusion criteria for the trial are summarized in Table 1, below.
Table 1.
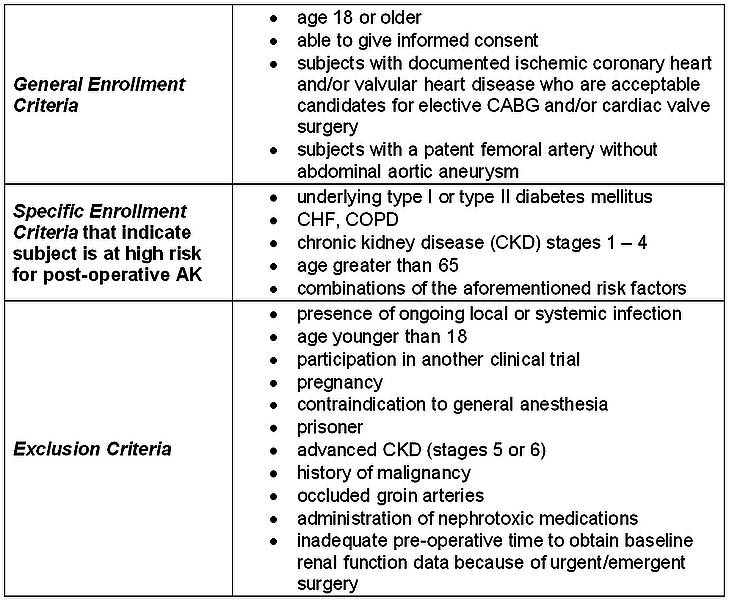
Results
Five eligible patients were enrolled for treatment with the lowest MSC dose. The clinical data on these study subjects are reported with their consent and approval of the IRB. The patients’ pre-operative AKI risk factors and surgical procedures are listed in Table 2. All patients underwent on-pump cardiac surgery for CABG and/or valve repair. All patients had at least one risk factor for post-operative development of AKI.
Table 2.

As stated in the introduction, several cardiac surgery associated factors have been identified as increasing the risk of post-operative AKI. These include the type of surgical procedure being performed, with multiple and/or valve procedures specifically being associated with higher risk; and the length of time on the bypass pump, with a bypass pump time of greater than 2 hours being associated with higher risk [4-6]. Table 3 lists the intra-operative risk factors for each of the five subjects.
Table 3.

Serum creatinine values for each of the five subjects, as markers of renal function, prior to and following surgery up to the present are shown on Figure 1.
Figure 1.

These data demonstrate that none of the first five study subjects developed significant AKI in the immediate postoperative time in the hospital, nor did patients 001-004 after discharge. Subject 005’s post-discharge data are pending. Significantly, no patient required dialysis immediately or later after surgery, and no expected or therapy-specific AEs or SAEs were observed. However, subject 004 died suddenly at home at 26 days after surgery and MSC administration. Both the principal investigator and the members of the DSMB determined that the patient’s death was not related to the study drug or its mode of administration. This SAE was immediately reported to the FDA, IRB and DSMB. The remaining four subjects are doing well as of the time of this report.
Discussion
This report summarizes the clinical course of the first five subjects in this first clinical safety trial world wide in which study subjects received allogeneic MSC after completion of on-pump cardiac surgery. It demonstrates that up to this point after surgery and discharge from the hospital infusion of allogeneic MSC at this low dose is safe, as no AEs or SAEs related to this novel therapy have been observed. Specifically, renal function was well preserved postoperatively, and none of the patients required hemodialysis. The sudden death of patient 004 at 26 days after surgery and MSC administration was judged by both the principal investigator and the members of the DSMB as being unrelated to the administration of allogeneic MSC.
Since close follow-up of each patient is continued for six months, and annual follow-up is conducted for another three years, it is possible that late AEs or SAEs may develop. This may include cardiovascular and pulmonary AEs detailed above, as well as the remote possibility of ectopic differentiation (e.g., in lungs or kidneys) of residual MSC into bone, fat or cartilage cells or oncogenic transformation. However, our detailed pre-clinical studies in experimental animals as well as numerous ongoing clinical trails with MSC (www.clinicaltrials.gov) make the latter AEs unlikely, since we have demonstrated that administered allogeneic MSC do not remain in the animal for more than three days, and that they do not differentiate into target cells and engraft in the kidney that is injured by experimental ischemia and reperfusion, the model that most closely resembles human ischemic AKI.
In the next groups of subjects, the acute and late safety of higher doses of allogeneic MSC will be assessed. At this point, the safety of the higher doses is not predictable and will have to be investigated. However, both our animal data and all reported clinical trials in which similar MSC doses were administered did not result in AEs or SAEs [7, 8, 10];(www.clinicaltrials.gov). It will finally be of interest to determine whether the obtained data from all 15 study subjects will allow an assessment of the preliminary efficacy of allogeneic MSC therapy in this cohort of high risk patients. If demonstrated, using relevant historical controls, it would be the basis for the conduct of a Phase II trial, in which the efficacy of this novel cell-based therapy is tested.
Acknowledgements
We greatly appreciate the excellent work of the University of Utah Cell Therapy facility and its director Dr. Linda Kelley. In this facility, all allogeneic MSC that were administered were characterized, culture expanded and release tested under cGMP conditions. The constructive contributions of the members of the DSMB (Drs. Carl Kablitz, Srinivasan Beddhu and David Affleck) are greatly valued. The conduct of this trial is funded and sponsored by AlloCure, Inc., and partially supported by the National Kidney Foundation (UT, ID), and the Western Institute for Biomedical Research.
References
1. Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810-9.
2. Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis. 2008;15(3):297-307.
3. Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+ - H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294(2):F414-22.
4. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19-32.
5. Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101-7.
6. Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72(5):624-31.
7. Togel F, Cohen A, Zhang P, Yang Y, Hu Z, et al. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2008.
8. Togel F, Weiss K, Yang Y, Hu Z, Zhang P, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292(5):F1626-35.
9. Togel F, Westenfelder C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev Dyn. 2007;236(12):3321-31.
10. Togel F, Hu Z, Weiss K, Isaac J, Lange C, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31-42.
11. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579-86.
12. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11-20.
13. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813-8.
"
["~DETAIL_TEXT"]=>
string(18786) "
Introduction
AKI remains a common, serious, and essentially treatment resistant syndrome of rapidly declining renal function. The mortality rates from AKI range from 15% in the general community to 80% for patients with multi-organ failure or for those who develop it post-operatively [1]. Even when renal function appears to fully recover after AKI, it is now recognized that a significant proportion of patients develop end stage renal disease (ESRD) as a consequence of undiagnosed, incompletely resolved AKI, characterized by continued inflammatory and fibrotic processes, and microvascular rarefaction [2]. Consequently, those patients who seemingly recover from AKI frequently go on to develop chronic kidney disease (CKD), eventually requiring chronic hemodialysis or a renal transplant [3].
AKI is most frequently seen in patients with shock, sepsis, trauma, and after major surgery. Patients undergoing cardiac surgery are at particularly high risk with up to 30% of all cardiac surgery patients developing AKI [4]. Many studies of cardiac patients have consistently found certain factors to be associated with increased risk of developing AKI following surgery. These risks include but are not limited to: the type of procedure performed (valve procedures are found to be of particularly high risk); patient age greater than 65; female patient gender; pre-operative serum creatinine value above 1.2 mg/dL, or underlying renal disease; pre-operative capillary glucose above 140 mg/dL; congestive heart failure; combined surgeries; on-pump vs. off pump surgery; and cardiopulmonary bypass surgery time greater than two hours [4-6]. The treatment resistant nature of AKI, combined with high morbidity and mortality, as well as the now recognized frequent progression of AKI to chronic kidney disease (CKD) underscores the urgent need for advances in treatment modalities.
Recent studies from our laboratory have led to the development of a novel approach to AKI treatment. This treatment administers allogeneic or syngeneic MSC to prevent further damage and to facilitate repair of acutely injured kidneys [7-9]. We observed that immediate (post reflow) or delayed (24 hrs post reflow) treatment of I/R AKI in rats with either autologous or allogeneic MSC significantly protects renal function, improves survival and hastens renal repair, mediated by complex paracrine mechanisms (anti-apoptotic, mitogenic, anti-inflammatory, vasculoprotective, angiogenic, anti-fibrotic) 7-10]. The striking hypoimmunogenic and immune modulating properties of MSC make their therapeutic use in allogeneic protocols possible and safe, as has been demonstrated in numerous clinical (www.clinicaltrials.gov) and pre-clinical trials [11, 12].
Compared to vehicle treated animals with I/R AKI, early allogeneic MSC therapy has important late benefits (3 months post AKI) such as maintained creatinine clearance, decreased interstitial fibrosis, and down regulation of pro-fibrotic gene expression levels in the kidney (TGFβ, PAI-1, TIMP-1). In addition, MSC therapy for AKI results in well maintained microvascular density in the kidney, while there is significant micorvascular rarefaction in vehicle treated animals [7]. In AKI, administered MSC do not engraft and disappear from the kidney and other organs within 1 to 3 days.
The aforementioned preclinical studies indicate that effective and specific treatment of AKI with MSC is an intervention that also prevents progressive loss of renal function, a complication that is increasingly recognized to result in ESRD in patients in whom AKI was not diagnosed and treated early after a renal insult [13]. Accordingly, a Phase I Clinical Trial employing this treatment is currently underway (www.clinicaltrials.gov; NCT00733876). This safety trial involves administration of MSC to fifteen patients divided into three cohorts of five patients each. Each cohort receives a defined dose of MSC, low, intermediate or high. As of this writing, dosing of the first cohort is complete, and we report here the outcomes of the first cohort of five patients.
Study Design and Methods
The FDA and the Institutional Review Board of Intermountain Medical Center, Murray, Utah, the site where the trial is carried out, approved the design and conduct of this Phase I Clinical Safety Trial. In addition, prior to initiation of the trial an independent Data Safety and Monitoring Board (DSMB) was appointed, consisting of a general nephrologists, a nephrologist/medical epidemiologist, and a cardiovascular surgeon. This DSMB reviewed the trial protocol and approved the trial, and continues to monitor the clinical data from all enrolled and treated subjects.
The study design is a Phase 1 Safety Trial. The primary objective is to test whether infusion of allogeneic MSC into the suprarenal aorta of patients who have undergone on-pump cardiac surgery (Coronary Artery Bypass Grafting and/or valve surgery) and who are at high risk for AKI following surgery is safe. This is assessed by monitoring patients post operatively for the occurrence of adverse events (AEs) and serious adverse events (SAEs) that are related to the MSC therapy. Specifically, detailed, monthly examinations for six months regarding the development of AEs or SAEs are carried out, and the study subjects are reassessed annually for another three years.
The major endpoint to be measured is safety, as documented by the comparative incidence of Adverse Events, Severe Adverse Events and complications in patients receiving cell-based therapies vs. historical controls for this patient population. AEs will be recorded throughout the course of the study and classified as immediate, postoperative, or delayed. Both common, expected and unusual AEs are listed below.
Potential immediate or early AEs related to the infusion of MSC via a femoral catheter into the suprarenal aorta include femoral catheter related complications such as bleeding at the catheter insertion site, infections, cholesterol plaque dislodgement and secondary visceral or peripheral embolic events.
Immediate AEs and SAEs occurring at the time of operation and immediately post-op (up to 24 hours post-op) include the following: post-operative compromise of heart function due to an unexpected ischemic event; post-operative marked impairment of renal function due to an unexpected ischemic coronary or other event (bleeding, hypotension, heart failure); perioperative complications that will require additional time in order to address these.
Later, post-operative complications (1-30 days post-op) include delayed deterioration in renal function post-op, requiring or not requiring dialysis; bleeding requiring >6 units of blood transfusion; arrhythmia requiring cardioversion; mediastinitis; cerebral vascular accident; prolonged ventilator support (> 24 hours postoperatively); reintubation; acute myocardial infarction; wound infection or hematoma; pericarditis; pneumonia; pulmonary embolism; bacteremia, sepsis, shock; multiorgan failure; death.
Delayed (more than 30 days after operation) AEs and SAEs include: dialysis dependency due to irreversible loss of kidney function; arrhythmia requiring cardioversion; mediastinitis; cerebral vascular accident; acute myocardial infarction; wound infection or hematoma; pneumonia; pulmonary embolism; malignancy; ectopic differentiation of MSC into mesodermal cells (bone, cartilage, fat); death.
The secondary objective of this trial is preliminary efficacy of MSC administration for the potential prevention and treatment of post-operative AKI. Although a priori underpowered, preliminary efficacy in this trial is nevertheless assessed by determining the comparative frequency and severity of post-operative AKI using standard and novel biomarkers of AKI (serum creatinine, BUN, urine output, creatinine clearance, electrolyte, acid-base balance, serum cystatin C, IL-18 and NGAL levels), need for temporary or permanent dialysis, length of hospital stay, and associated 30 day mortality. Study data are compared to published historical data that are collected and available for analysis from the Society of Thoracic Surgeons (www.STS.org). Historical data from this data base are collected and analyzed from all participating centers in the USA, and sub-analyzed for a reporting institution, such as IMC, and comparable institutions.
The trial is currently conducted in one center, IMC in Murray, Utah. Allogeneic MSC, derived from healthy, screened donors, using FDA approved protocols, are culture expanded under cGMP conditions at the University of Utah Cell Therapy Facility, Salt Lake City, Utah. MSC are administered in a dose escalation protocol to a total of 15 patients who have undergone elective, on-pump cardiac surgery (CABG and/or valve replacement). Five patients each receive low, medium or high dose of allogeneic MSC via a femoral catheter into the suprarenal aorta immediately after the patient comes off pump and is hemodynamically stable.
Low, Intermediate and High Doses of allogeneic MSC are defined per FDA approved protocol, and are infused into the suprarenal aorta in 50 ml of normal saline via a femoral catheter.
The enrollment and exclusion criteria for the trial are summarized in Table 1, below.
Table 1.

Results
Five eligible patients were enrolled for treatment with the lowest MSC dose. The clinical data on these study subjects are reported with their consent and approval of the IRB. The patients’ pre-operative AKI risk factors and surgical procedures are listed in Table 2. All patients underwent on-pump cardiac surgery for CABG and/or valve repair. All patients had at least one risk factor for post-operative development of AKI.
Table 2.

As stated in the introduction, several cardiac surgery associated factors have been identified as increasing the risk of post-operative AKI. These include the type of surgical procedure being performed, with multiple and/or valve procedures specifically being associated with higher risk; and the length of time on the bypass pump, with a bypass pump time of greater than 2 hours being associated with higher risk [4-6]. Table 3 lists the intra-operative risk factors for each of the five subjects.
Table 3.

Serum creatinine values for each of the five subjects, as markers of renal function, prior to and following surgery up to the present are shown on Figure 1.
Figure 1.

These data demonstrate that none of the first five study subjects developed significant AKI in the immediate postoperative time in the hospital, nor did patients 001-004 after discharge. Subject 005’s post-discharge data are pending. Significantly, no patient required dialysis immediately or later after surgery, and no expected or therapy-specific AEs or SAEs were observed. However, subject 004 died suddenly at home at 26 days after surgery and MSC administration. Both the principal investigator and the members of the DSMB determined that the patient’s death was not related to the study drug or its mode of administration. This SAE was immediately reported to the FDA, IRB and DSMB. The remaining four subjects are doing well as of the time of this report.
Discussion
This report summarizes the clinical course of the first five subjects in this first clinical safety trial world wide in which study subjects received allogeneic MSC after completion of on-pump cardiac surgery. It demonstrates that up to this point after surgery and discharge from the hospital infusion of allogeneic MSC at this low dose is safe, as no AEs or SAEs related to this novel therapy have been observed. Specifically, renal function was well preserved postoperatively, and none of the patients required hemodialysis. The sudden death of patient 004 at 26 days after surgery and MSC administration was judged by both the principal investigator and the members of the DSMB as being unrelated to the administration of allogeneic MSC.
Since close follow-up of each patient is continued for six months, and annual follow-up is conducted for another three years, it is possible that late AEs or SAEs may develop. This may include cardiovascular and pulmonary AEs detailed above, as well as the remote possibility of ectopic differentiation (e.g., in lungs or kidneys) of residual MSC into bone, fat or cartilage cells or oncogenic transformation. However, our detailed pre-clinical studies in experimental animals as well as numerous ongoing clinical trails with MSC (www.clinicaltrials.gov) make the latter AEs unlikely, since we have demonstrated that administered allogeneic MSC do not remain in the animal for more than three days, and that they do not differentiate into target cells and engraft in the kidney that is injured by experimental ischemia and reperfusion, the model that most closely resembles human ischemic AKI.
In the next groups of subjects, the acute and late safety of higher doses of allogeneic MSC will be assessed. At this point, the safety of the higher doses is not predictable and will have to be investigated. However, both our animal data and all reported clinical trials in which similar MSC doses were administered did not result in AEs or SAEs [7, 8, 10];(www.clinicaltrials.gov). It will finally be of interest to determine whether the obtained data from all 15 study subjects will allow an assessment of the preliminary efficacy of allogeneic MSC therapy in this cohort of high risk patients. If demonstrated, using relevant historical controls, it would be the basis for the conduct of a Phase II trial, in which the efficacy of this novel cell-based therapy is tested.
Acknowledgements
We greatly appreciate the excellent work of the University of Utah Cell Therapy facility and its director Dr. Linda Kelley. In this facility, all allogeneic MSC that were administered were characterized, culture expanded and release tested under cGMP conditions. The constructive contributions of the members of the DSMB (Drs. Carl Kablitz, Srinivasan Beddhu and David Affleck) are greatly valued. The conduct of this trial is funded and sponsored by AlloCure, Inc., and partially supported by the National Kidney Foundation (UT, ID), and the Western Institute for Biomedical Research.
References
1. Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810-9.
2. Goldberg R, Dennen P. Long-term outcomes of acute kidney injury. Adv Chronic Kidney Dis. 2008;15(3):297-307.
3. Girardi AC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+ - H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294(2):F414-22.
4. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19-32.
5. Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101-7.
6. Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72(5):624-31.
7. Togel F, Cohen A, Zhang P, Yang Y, Hu Z, et al. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2008.
8. Togel F, Weiss K, Yang Y, Hu Z, Zhang P, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292(5):F1626-35.
9. Togel F, Westenfelder C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev Dyn. 2007;236(12):3321-31.
10. Togel F, Hu Z, Weiss K, Isaac J, Lange C, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31-42.
11. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579-86.
12. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11-20.
13. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813-8.
"
["DETAIL_TEXT_TYPE"]=>
string(4) "html"
["~DETAIL_TEXT_TYPE"]=>
string(4) "html"
["PREVIEW_TEXT"]=>
string(0) ""
["~PREVIEW_TEXT"]=>
string(0) ""
["PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["~PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["PREVIEW_PICTURE"]=>
NULL
["~PREVIEW_PICTURE"]=>
NULL
["LANG_DIR"]=>
string(4) "/ru/"
["~LANG_DIR"]=>
string(4) "/ru/"
["SORT"]=>
string(3) "500"
["~SORT"]=>
string(3) "500"
["CODE"]=>
string(100) "pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno"
["~CODE"]=>
string(100) "pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno"
["EXTERNAL_ID"]=>
string(3) "805"
["~EXTERNAL_ID"]=>
string(3) "805"
["IBLOCK_TYPE_ID"]=>
string(7) "journal"
["~IBLOCK_TYPE_ID"]=>
string(7) "journal"
["IBLOCK_CODE"]=>
string(7) "volumes"
["~IBLOCK_CODE"]=>
string(7) "volumes"
["IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["~IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["LID"]=>
string(2) "s2"
["~LID"]=>
string(2) "s2"
["EDIT_LINK"]=>
NULL
["DELETE_LINK"]=>
NULL
["DISPLAY_ACTIVE_FROM"]=>
string(0) ""
["IPROPERTY_VALUES"]=>
array(18) {
["ELEMENT_META_TITLE"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["ELEMENT_META_KEYWORDS"]=>
string(177) "мезенхимные стволовые клетки Острое повреждение почек клеточная терапия клинические испытания"
["ELEMENT_META_DESCRIPTION"]=>
string(612) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердцеInitial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery"
["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=>
string(4416) "<p class="bodytext">Наши обширные данные доклинического исследования, показывают, что острое повреждение почек (ОПП), индуцированное ишемией/реперфузией – резистентное к лечению осложнение у больных - может эффективно лечиться путем назначения аллогенных мезенхимных стволовых клеток (МСК). На этом основании в настоящее время проводится одобренная FDA I фаза клинических испытаний (<a href="http://www.clinicaltrials.gov" target="_blank">www.clinicaltrials.gov</a>; NCT00733876) больных, которые имели высокий риск развития тяжелой ОПП после хирургии на открытом сердце. В рамках испытаний безопасности метода, инфузии аллогенных МСК проводили больным после завершения хирургического вмешательства при аорто-коронарном шунтировании или хирургии клапанов сердца. В исследовании участвовали лица старше 65 лет с наличием почечных заболеваний, сахарного диабета, артериальной гипертензии, коронарной болезни сердца, тяжелой сердечной недостаточности и/или хронической обструктивной болезни легких. Введение МСК проводили по возрастающей, причем первым пяти больным проводилась инфузия клеток в определенной низкой дозе на кг массы тела через бедренный катетер, помещенный в надпочечную часть аорты. Данное сообщение содержит обобщенные сведения о клиническом течении у этих пяти больных, которых лечили по этому протоколу. Почечная функция не нарушалась после операции ни у одного из больных, и на текущий момент не выявлено побочных эффектов или тяжелых негативных явлений. Однако один из больных внезапно скончался через 26 суток после выписки по причинам, которые были расценены главным исследователем и членами Совета по мониторингу данных и безопасности, как не относящиеся к препарату и способу его применения. Следующая группа из пяти больных получит MСК в средней дозе на кг массы тела, и, если при этой дозе не возникнут проблемы с безопасностью, то еще пять больных будут пролечены при высокой дозе МСК на кг массы тела. Предварительная эффективность терапии МСК для профилактики и лечения послеоперационного ОПП в этом контингенте высокого риска (кардиохирургических больных) будет определяться по сравнению исходов у испытуемых лиц (частоты, тяжести и длительности послеоперационного ОПП, временной или постоянной зависимости от диализа, длительности госпитализации или гибели до 30 сут.), и в большой группе больных исторического контроля (база данных на <a href="http://www.STS.org" target="_blank">www.STS.org</a>).<br />
"
["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_META_TITLE"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_META_KEYWORDS"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_META_DESCRIPTION"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_PICTURE_FILE_ALT"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_PICTURE_FILE_TITLE"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_PICTURE_FILE_NAME"]=>
string(100) "pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno"
["SECTION_DETAIL_PICTURE_FILE_ALT"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_DETAIL_PICTURE_FILE_TITLE"]=>
string(419) "Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце"
["SECTION_DETAIL_PICTURE_FILE_NAME"]=>
string(100) "pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno"
["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=>
string(100) "pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno"
["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=>
string(100) "pervichnyy-otchet-o-faze-i-klinicheskikh-ispytaniy-profilaktika-i-lechenie-ostrogo-posleoperatsionno"
}
["FIELDS"]=>
array(1) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
}
["PROPERTIES"]=>
array(18) {
["KEYWORDS"]=>
array(36) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(5) "10963"
[1]=>
string(5) "10964"
[2]=>
string(5) "10965"
[3]=>
string(5) "10966"
}
["VALUE"]=>
array(4) {
[0]=>
string(2) "83"
[1]=>
string(3) "655"
[2]=>
string(3) "490"
[3]=>
string(3) "804"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(2) "83"
[1]=>
string(3) "655"
[2]=>
string(3) "490"
[3]=>
string(3) "804"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["SUBMITTED"]=>
array(36) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10826"
["VALUE"]=>
string(10) "11.11.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "11.11.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
}
["ACCEPTED"]=>
array(36) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10827"
["VALUE"]=>
string(10) "15.12.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "15.12.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
}
["PUBLISHED"]=>
array(36) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10828"
["VALUE"]=>
string(10) "24.12.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "24.12.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
}
["CONTACT"]=>
array(36) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10829"
["VALUE"]=>
string(3) "791"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "791"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHORS"]=>
array(36) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(12) {
[0]=>
string(5) "10967"
[1]=>
string(5) "10968"
[2]=>
string(5) "10969"
[3]=>
string(5) "10970"
[4]=>
string(5) "10971"
[5]=>
string(5) "10972"
[6]=>
string(5) "10973"
[7]=>
string(5) "10974"
[8]=>
string(5) "10975"
[9]=>
string(5) "10976"
[10]=>
string(5) "10977"
[11]=>
string(5) "10978"
}
["VALUE"]=>
array(12) {
[0]=>
string(3) "796"
[1]=>
string(3) "797"
[2]=>
string(3) "798"
[3]=>
string(3) "799"
[4]=>
string(3) "790"
[5]=>
string(3) "800"
[6]=>
string(3) "515"
[7]=>
string(2) "11"
[8]=>
string(3) "801"
[9]=>
string(3) "802"
[10]=>
string(3) "803"
[11]=>
string(3) "791"
}
["DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(12) {
[0]=>
string(3) "796"
[1]=>
string(3) "797"
[2]=>
string(3) "798"
[3]=>
string(3) "799"
[4]=>
string(3) "790"
[5]=>
string(3) "800"
[6]=>
string(3) "515"
[7]=>
string(2) "11"
[8]=>
string(3) "801"
[9]=>
string(3) "802"
[10]=>
string(3) "803"
[11]=>
string(3) "791"
}
["~DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_RU"]=>
array(36) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10842"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(255) "<p class="Autor">Гуч А., Доти Дж., Флорес Дж., Свенсон Л., Тегель Ф., Райсс Р. Г., Ланге К., Цандер А. Р., Ху Дж., Пул С., Жанг П., Вестенвельдер К.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(233) "
Гуч А., Доти Дж., Флорес Дж., Свенсон Л., Тегель Ф., Райсс Р. Г., Ланге К., Цандер А. Р., Ху Дж., Пул С., Жанг П., Вестенвельдер К.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_RU"]=>
array(36) {
["ID"]=>
string(2) "26"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(22) "Организации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "26"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(22) "Организации"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_RU"]=>
array(36) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10881"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(4416) "<p class="bodytext">Наши обширные данные доклинического исследования, показывают, что острое повреждение почек (ОПП), индуцированное ишемией/реперфузией – резистентное к лечению осложнение у больных - может эффективно лечиться путем назначения аллогенных мезенхимных стволовых клеток (МСК). На этом основании в настоящее время проводится одобренная FDA I фаза клинических испытаний (<a href="http://www.clinicaltrials.gov" target="_blank">www.clinicaltrials.gov</a>; NCT00733876) больных, которые имели высокий риск развития тяжелой ОПП после хирургии на открытом сердце. В рамках испытаний безопасности метода, инфузии аллогенных МСК проводили больным после завершения хирургического вмешательства при аорто-коронарном шунтировании или хирургии клапанов сердца. В исследовании участвовали лица старше 65 лет с наличием почечных заболеваний, сахарного диабета, артериальной гипертензии, коронарной болезни сердца, тяжелой сердечной недостаточности и/или хронической обструктивной болезни легких. Введение МСК проводили по возрастающей, причем первым пяти больным проводилась инфузия клеток в определенной низкой дозе на кг массы тела через бедренный катетер, помещенный в надпочечную часть аорты. Данное сообщение содержит обобщенные сведения о клиническом течении у этих пяти больных, которых лечили по этому протоколу. Почечная функция не нарушалась после операции ни у одного из больных, и на текущий момент не выявлено побочных эффектов или тяжелых негативных явлений. Однако один из больных внезапно скончался через 26 суток после выписки по причинам, которые были расценены главным исследователем и членами Совета по мониторингу данных и безопасности, как не относящиеся к препарату и способу его применения. Следующая группа из пяти больных получит MСК в средней дозе на кг массы тела, и, если при этой дозе не возникнут проблемы с безопасностью, то еще пять больных будут пролечены при высокой дозе МСК на кг массы тела. Предварительная эффективность терапии МСК для профилактики и лечения послеоперационного ОПП в этом контингенте высокого риска (кардиохирургических больных) будет определяться по сравнению исходов у испытуемых лиц (частоты, тяжести и длительности послеоперационного ОПП, временной или постоянной зависимости от диализа, длительности госпитализации или гибели до 30 сут.), и в большой группе больных исторического контроля (база данных на <a href="http://www.STS.org" target="_blank">www.STS.org</a>).<br />
"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(4330) "
Наши обширные данные доклинического исследования, показывают, что острое повреждение почек (ОПП), индуцированное ишемией/реперфузией – резистентное к лечению осложнение у больных - может эффективно лечиться путем назначения аллогенных мезенхимных стволовых клеток (МСК). На этом основании в настоящее время проводится одобренная FDA I фаза клинических испытаний (www.clinicaltrials.gov; NCT00733876) больных, которые имели высокий риск развития тяжелой ОПП после хирургии на открытом сердце. В рамках испытаний безопасности метода, инфузии аллогенных МСК проводили больным после завершения хирургического вмешательства при аорто-коронарном шунтировании или хирургии клапанов сердца. В исследовании участвовали лица старше 65 лет с наличием почечных заболеваний, сахарного диабета, артериальной гипертензии, коронарной болезни сердца, тяжелой сердечной недостаточности и/или хронической обструктивной болезни легких. Введение МСК проводили по возрастающей, причем первым пяти больным проводилась инфузия клеток в определенной низкой дозе на кг массы тела через бедренный катетер, помещенный в надпочечную часть аорты. Данное сообщение содержит обобщенные сведения о клиническом течении у этих пяти больных, которых лечили по этому протоколу. Почечная функция не нарушалась после операции ни у одного из больных, и на текущий момент не выявлено побочных эффектов или тяжелых негативных явлений. Однако один из больных внезапно скончался через 26 суток после выписки по причинам, которые были расценены главным исследователем и членами Совета по мониторингу данных и безопасности, как не относящиеся к препарату и способу его применения. Следующая группа из пяти больных получит MСК в средней дозе на кг массы тела, и, если при этой дозе не возникнут проблемы с безопасностью, то еще пять больных будут пролечены при высокой дозе МСК на кг массы тела. Предварительная эффективность терапии МСК для профилактики и лечения послеоперационного ОПП в этом контингенте высокого риска (кардиохирургических больных) будет определяться по сравнению исходов у испытуемых лиц (частоты, тяжести и длительности послеоперационного ОПП, временной или постоянной зависимости от диализа, длительности госпитализации или гибели до 30 сут.), и в большой группе больных исторического контроля (база данных на www.STS.org).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["DOI"]=>
array(36) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10843"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000028.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000028.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_EN"]=>
array(36) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10863"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(516) "<p class="Autor">Anna Gooch<sup>1</sup>, John Doty<sup>2</sup>, Jean Flores<sup>2</sup>, LeAnne Swenson<sup>2</sup>, Florian E Toegel<sup>1,3</sup>, George R Reiss<sup>4</sup>, Claudia Lange<sup>5</sup>, Axel R Zander<sup>5</sup>, Zhuma Hu<sup>1</sup>, Scott Poole<sup>1</sup>, Ping Zhang<sup>1</sup> and Christof Westenfelder<sup>1,6</sup> </p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(350) "
Anna Gooch1, John Doty2, Jean Flores2, LeAnne Swenson2, Florian E Toegel1,3, George R Reiss4, Claudia Lange5, Axel R Zander5, Zhuma Hu1, Scott Poole1, Ping Zhang1 and Christof Westenfelder1,6
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_EN"]=>
array(36) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10864"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1352) "<p class="bodytext"><sup>1</sup>Division of Nephrology, Department of Medicine, University of Utah Health Sciences Center and George E. Wahlen VA HCS, Salt Lake City, Utah, USA; <sup>2</sup>Division of Cardiovascular Surgery, Intermountain Medical Center, Murray, Utah, USA; <sup>3</sup>Jacobi Hospital, Albert Einstein College of Medicine affiliated Medical Center, Bronx, New York, USA; <sup>4</sup>Division of Cardiovascular Surgery, Department of Surgery, University of Utah Health Sciences Center, and Research Service, George E. Wahlen VA HCS, Salt Lake City, Utah, USA; <sup>5</sup>Bone Marrow Transplantation Center, University of Hamburg, Germany; <sup>6</sup>Department of Physiology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA <br /> <br />
<b>Correspondence: </b><br>
Christof Westenfelder, MD, Section of Nephrology (111 N), George E. Wahlen VA Health Sciences Center, 500 Foothill Blvd., Salt Lake City, UT 84148, USA<br> E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.glvmwxsj2aiwxirjiphivDlwg2yxel2ihy');" class="mail">christof.westenfelder@<span style="display:none;">spam is bad</span>hsc.utah.edu</a>
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1168) "
1Division of Nephrology, Department of Medicine, University of Utah Health Sciences Center and George E. Wahlen VA HCS, Salt Lake City, Utah, USA; 2Division of Cardiovascular Surgery, Intermountain Medical Center, Murray, Utah, USA; 3Jacobi Hospital, Albert Einstein College of Medicine affiliated Medical Center, Bronx, New York, USA; 4Division of Cardiovascular Surgery, Department of Surgery, University of Utah Health Sciences Center, and Research Service, George E. Wahlen VA HCS, Salt Lake City, Utah, USA; 5Bone Marrow Transplantation Center, University of Hamburg, Germany; 6Department of Physiology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA
Correspondence:
Christof Westenfelder, MD, Section of Nephrology (111 N), George E. Wahlen VA Health Sciences Center, 500 Foothill Blvd., Salt Lake City, UT 84148, USA
E-mail: christof.westenfelder@spam is badhsc.utah.edu
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_EN"]=>
array(36) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10914"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(2409) "<p class="bodytext">Based on our extensive pre-clinical data that show that ischemia/reperfusion-induced Acute Kidney Injury (AKI), an essentially treatment resistant complication in patients, can be effectively treated by the administration of allogeneic Mesenchymal Stem Cells (MSC), an FDA approved, Phase I Clinical Trial (<a href="http://www.clinicaltrials.gov" target="_blank">www.clinicaltrials.gov</a>; NCT00733876) in patients who are at high risk of developing severe AKI post open heart surgery is currently being conducted. In this safety trial, patients who are undergoing on-pump coronary artery bypass surgery or cardiac valve repair, who are older than 65 years, with underlying renal disease, diabetes mellitus, hypertension, coronary artery disease, congestive heart failure and/or chronic obstructive pulmonary disease will be infused with allogeneic MSC following completion of surgery. The MSC are dosed in an escalating fashion, the initial five patients being infused via a femoral catheter that is placed into the suprarenal aorta with a defined low dose of MSC/kg body weight. This report summarizes the clinical course of the first five patients that have been treated according to this protocol. The renal function did not deteriorate post operatively in any of these patients, nor were adverse (AE) or severe adverse events (SAE) observed to date. However, one patient died suddenly 26 days after discharge from causes that both the principal investigator and the members of the Data and Safety Monitoring Board judged as being unrelated to the study drug and its route of administration. The next group of five study subjects will receive an intermediate dose of MSC/kg body weight, and if no safety concerns arise with this dose, the final five patients will be treated with a high dose of MSC/kg body weight. Preliminary efficacy of MSC therapy in the prevention and treatment of post-operative AKI in this high risk cohort of cardiac surgery patients will be assessed by comparing outcomes in study subjects (frequency, severity and duration of post-operative AKI, dialysis dependency [temporary, permanent], length of stay, and death at 30 days) to those in a large number of historical controls (data base at <a href="http://www.STS.org" target="_blank">www.STS.org</a>).<br /><br />"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(2317) "
Based on our extensive pre-clinical data that show that ischemia/reperfusion-induced Acute Kidney Injury (AKI), an essentially treatment resistant complication in patients, can be effectively treated by the administration of allogeneic Mesenchymal Stem Cells (MSC), an FDA approved, Phase I Clinical Trial (www.clinicaltrials.gov; NCT00733876) in patients who are at high risk of developing severe AKI post open heart surgery is currently being conducted. In this safety trial, patients who are undergoing on-pump coronary artery bypass surgery or cardiac valve repair, who are older than 65 years, with underlying renal disease, diabetes mellitus, hypertension, coronary artery disease, congestive heart failure and/or chronic obstructive pulmonary disease will be infused with allogeneic MSC following completion of surgery. The MSC are dosed in an escalating fashion, the initial five patients being infused via a femoral catheter that is placed into the suprarenal aorta with a defined low dose of MSC/kg body weight. This report summarizes the clinical course of the first five patients that have been treated according to this protocol. The renal function did not deteriorate post operatively in any of these patients, nor were adverse (AE) or severe adverse events (SAE) observed to date. However, one patient died suddenly 26 days after discharge from causes that both the principal investigator and the members of the Data and Safety Monitoring Board judged as being unrelated to the study drug and its route of administration. The next group of five study subjects will receive an intermediate dose of MSC/kg body weight, and if no safety concerns arise with this dose, the final five patients will be treated with a high dose of MSC/kg body weight. Preliminary efficacy of MSC therapy in the prevention and treatment of post-operative AKI in this high risk cohort of cardiac surgery patients will be assessed by comparing outcomes in study subjects (frequency, severity and duration of post-operative AKI, dialysis dependency [temporary, permanent], length of stay, and death at 30 days) to those in a large number of historical controls (data base at www.STS.org).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["NAME_EN"]=>
array(36) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10844"
["VALUE"]=>
string(193) "Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(193) "Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["FULL_TEXT_RU"]=>
array(36) {
["ID"]=>
string(2) "42"
["TIMESTAMP_X"]=>
string(19) "2015-09-07 20:29:18"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(23) "Полный текст"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(12) "FULL_TEXT_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "42"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(23) "Полный текст"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["PDF_RU"]=>
array(36) {
["ID"]=>
string(2) "43"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF RUS"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_RU"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "43"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10845"
["VALUE"]=>
string(3) "453"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "453"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF RUS"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["PDF_EN"]=>
array(36) {
["ID"]=>
string(2) "44"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF ENG"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "44"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10846"
["VALUE"]=>
string(3) "454"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "454"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF ENG"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["NAME_LONG"]=>
array(36) {
["ID"]=>
string(2) "45"
["TIMESTAMP_X"]=>
string(19) "2023-04-13 00:55:00"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "NAME_LONG"
["DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "45"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(80)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["~DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
}
}
["DISPLAY_PROPERTIES"]=>
array(13) {
["AUTHOR_EN"]=>
array(37) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10863"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(516) "<p class="Autor">Anna Gooch<sup>1</sup>, John Doty<sup>2</sup>, Jean Flores<sup>2</sup>, LeAnne Swenson<sup>2</sup>, Florian E Toegel<sup>1,3</sup>, George R Reiss<sup>4</sup>, Claudia Lange<sup>5</sup>, Axel R Zander<sup>5</sup>, Zhuma Hu<sup>1</sup>, Scott Poole<sup>1</sup>, Ping Zhang<sup>1</sup> and Christof Westenfelder<sup>1,6</sup> </p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(350) "
Anna Gooch1, John Doty2, Jean Flores2, LeAnne Swenson2, Florian E Toegel1,3, George R Reiss4, Claudia Lange5, Axel R Zander5, Zhuma Hu1, Scott Poole1, Ping Zhang1 and Christof Westenfelder1,6
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(350) "
Anna Gooch1, John Doty2, Jean Flores2, LeAnne Swenson2, Florian E Toegel1,3, George R Reiss4, Claudia Lange5, Axel R Zander5, Zhuma Hu1, Scott Poole1, Ping Zhang1 and Christof Westenfelder1,6
"
}
["SUMMARY_EN"]=>
array(37) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10914"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(2409) "<p class="bodytext">Based on our extensive pre-clinical data that show that ischemia/reperfusion-induced Acute Kidney Injury (AKI), an essentially treatment resistant complication in patients, can be effectively treated by the administration of allogeneic Mesenchymal Stem Cells (MSC), an FDA approved, Phase I Clinical Trial (<a href="http://www.clinicaltrials.gov" target="_blank">www.clinicaltrials.gov</a>; NCT00733876) in patients who are at high risk of developing severe AKI post open heart surgery is currently being conducted. In this safety trial, patients who are undergoing on-pump coronary artery bypass surgery or cardiac valve repair, who are older than 65 years, with underlying renal disease, diabetes mellitus, hypertension, coronary artery disease, congestive heart failure and/or chronic obstructive pulmonary disease will be infused with allogeneic MSC following completion of surgery. The MSC are dosed in an escalating fashion, the initial five patients being infused via a femoral catheter that is placed into the suprarenal aorta with a defined low dose of MSC/kg body weight. This report summarizes the clinical course of the first five patients that have been treated according to this protocol. The renal function did not deteriorate post operatively in any of these patients, nor were adverse (AE) or severe adverse events (SAE) observed to date. However, one patient died suddenly 26 days after discharge from causes that both the principal investigator and the members of the Data and Safety Monitoring Board judged as being unrelated to the study drug and its route of administration. The next group of five study subjects will receive an intermediate dose of MSC/kg body weight, and if no safety concerns arise with this dose, the final five patients will be treated with a high dose of MSC/kg body weight. Preliminary efficacy of MSC therapy in the prevention and treatment of post-operative AKI in this high risk cohort of cardiac surgery patients will be assessed by comparing outcomes in study subjects (frequency, severity and duration of post-operative AKI, dialysis dependency [temporary, permanent], length of stay, and death at 30 days) to those in a large number of historical controls (data base at <a href="http://www.STS.org" target="_blank">www.STS.org</a>).<br /><br />"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(2317) "
Based on our extensive pre-clinical data that show that ischemia/reperfusion-induced Acute Kidney Injury (AKI), an essentially treatment resistant complication in patients, can be effectively treated by the administration of allogeneic Mesenchymal Stem Cells (MSC), an FDA approved, Phase I Clinical Trial (www.clinicaltrials.gov; NCT00733876) in patients who are at high risk of developing severe AKI post open heart surgery is currently being conducted. In this safety trial, patients who are undergoing on-pump coronary artery bypass surgery or cardiac valve repair, who are older than 65 years, with underlying renal disease, diabetes mellitus, hypertension, coronary artery disease, congestive heart failure and/or chronic obstructive pulmonary disease will be infused with allogeneic MSC following completion of surgery. The MSC are dosed in an escalating fashion, the initial five patients being infused via a femoral catheter that is placed into the suprarenal aorta with a defined low dose of MSC/kg body weight. This report summarizes the clinical course of the first five patients that have been treated according to this protocol. The renal function did not deteriorate post operatively in any of these patients, nor were adverse (AE) or severe adverse events (SAE) observed to date. However, one patient died suddenly 26 days after discharge from causes that both the principal investigator and the members of the Data and Safety Monitoring Board judged as being unrelated to the study drug and its route of administration. The next group of five study subjects will receive an intermediate dose of MSC/kg body weight, and if no safety concerns arise with this dose, the final five patients will be treated with a high dose of MSC/kg body weight. Preliminary efficacy of MSC therapy in the prevention and treatment of post-operative AKI in this high risk cohort of cardiac surgery patients will be assessed by comparing outcomes in study subjects (frequency, severity and duration of post-operative AKI, dialysis dependency [temporary, permanent], length of stay, and death at 30 days) to those in a large number of historical controls (data base at www.STS.org).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(2317) "
Based on our extensive pre-clinical data that show that ischemia/reperfusion-induced Acute Kidney Injury (AKI), an essentially treatment resistant complication in patients, can be effectively treated by the administration of allogeneic Mesenchymal Stem Cells (MSC), an FDA approved, Phase I Clinical Trial (www.clinicaltrials.gov; NCT00733876) in patients who are at high risk of developing severe AKI post open heart surgery is currently being conducted. In this safety trial, patients who are undergoing on-pump coronary artery bypass surgery or cardiac valve repair, who are older than 65 years, with underlying renal disease, diabetes mellitus, hypertension, coronary artery disease, congestive heart failure and/or chronic obstructive pulmonary disease will be infused with allogeneic MSC following completion of surgery. The MSC are dosed in an escalating fashion, the initial five patients being infused via a femoral catheter that is placed into the suprarenal aorta with a defined low dose of MSC/kg body weight. This report summarizes the clinical course of the first five patients that have been treated according to this protocol. The renal function did not deteriorate post operatively in any of these patients, nor were adverse (AE) or severe adverse events (SAE) observed to date. However, one patient died suddenly 26 days after discharge from causes that both the principal investigator and the members of the Data and Safety Monitoring Board judged as being unrelated to the study drug and its route of administration. The next group of five study subjects will receive an intermediate dose of MSC/kg body weight, and if no safety concerns arise with this dose, the final five patients will be treated with a high dose of MSC/kg body weight. Preliminary efficacy of MSC therapy in the prevention and treatment of post-operative AKI in this high risk cohort of cardiac surgery patients will be assessed by comparing outcomes in study subjects (frequency, severity and duration of post-operative AKI, dialysis dependency [temporary, permanent], length of stay, and death at 30 days) to those in a large number of historical controls (data base at www.STS.org).
"
}
["DOI"]=>
array(37) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10843"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000028.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000028.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(29) "10.3205/ctt-2008-en-000028.01"
}
["NAME_EN"]=>
array(37) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10844"
["VALUE"]=>
string(193) "Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(193) "Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(193) "Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery"
}
["ORGANIZATION_EN"]=>
array(37) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10864"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1352) "<p class="bodytext"><sup>1</sup>Division of Nephrology, Department of Medicine, University of Utah Health Sciences Center and George E. Wahlen VA HCS, Salt Lake City, Utah, USA; <sup>2</sup>Division of Cardiovascular Surgery, Intermountain Medical Center, Murray, Utah, USA; <sup>3</sup>Jacobi Hospital, Albert Einstein College of Medicine affiliated Medical Center, Bronx, New York, USA; <sup>4</sup>Division of Cardiovascular Surgery, Department of Surgery, University of Utah Health Sciences Center, and Research Service, George E. Wahlen VA HCS, Salt Lake City, Utah, USA; <sup>5</sup>Bone Marrow Transplantation Center, University of Hamburg, Germany; <sup>6</sup>Department of Physiology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA <br /> <br />
<b>Correspondence: </b><br>
Christof Westenfelder, MD, Section of Nephrology (111 N), George E. Wahlen VA Health Sciences Center, 500 Foothill Blvd., Salt Lake City, UT 84148, USA<br> E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.glvmwxsj2aiwxirjiphivDlwg2yxel2ihy');" class="mail">christof.westenfelder@<span style="display:none;">spam is bad</span>hsc.utah.edu</a>
</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1168) "
1Division of Nephrology, Department of Medicine, University of Utah Health Sciences Center and George E. Wahlen VA HCS, Salt Lake City, Utah, USA; 2Division of Cardiovascular Surgery, Intermountain Medical Center, Murray, Utah, USA; 3Jacobi Hospital, Albert Einstein College of Medicine affiliated Medical Center, Bronx, New York, USA; 4Division of Cardiovascular Surgery, Department of Surgery, University of Utah Health Sciences Center, and Research Service, George E. Wahlen VA HCS, Salt Lake City, Utah, USA; 5Bone Marrow Transplantation Center, University of Hamburg, Germany; 6Department of Physiology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA
Correspondence:
Christof Westenfelder, MD, Section of Nephrology (111 N), George E. Wahlen VA Health Sciences Center, 500 Foothill Blvd., Salt Lake City, UT 84148, USA
E-mail: christof.westenfelder@spam is badhsc.utah.edu
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(1168) "
1Division of Nephrology, Department of Medicine, University of Utah Health Sciences Center and George E. Wahlen VA HCS, Salt Lake City, Utah, USA; 2Division of Cardiovascular Surgery, Intermountain Medical Center, Murray, Utah, USA; 3Jacobi Hospital, Albert Einstein College of Medicine affiliated Medical Center, Bronx, New York, USA; 4Division of Cardiovascular Surgery, Department of Surgery, University of Utah Health Sciences Center, and Research Service, George E. Wahlen VA HCS, Salt Lake City, Utah, USA; 5Bone Marrow Transplantation Center, University of Hamburg, Germany; 6Department of Physiology, University of Utah Health Sciences Center, Salt Lake City, Utah, USA
Correspondence:
Christof Westenfelder, MD, Section of Nephrology (111 N), George E. Wahlen VA Health Sciences Center, 500 Foothill Blvd., Salt Lake City, UT 84148, USA
E-mail: christof.westenfelder@spam is badhsc.utah.edu
"
}
["AUTHORS"]=>
array(38) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(12) {
[0]=>
string(5) "10967"
[1]=>
string(5) "10968"
[2]=>
string(5) "10969"
[3]=>
string(5) "10970"
[4]=>
string(5) "10971"
[5]=>
string(5) "10972"
[6]=>
string(5) "10973"
[7]=>
string(5) "10974"
[8]=>
string(5) "10975"
[9]=>
string(5) "10976"
[10]=>
string(5) "10977"
[11]=>
string(5) "10978"
}
["VALUE"]=>
array(12) {
[0]=>
string(3) "796"
[1]=>
string(3) "797"
[2]=>
string(3) "798"
[3]=>
string(3) "799"
[4]=>
string(3) "790"
[5]=>
string(3) "800"
[6]=>
string(3) "515"
[7]=>
string(2) "11"
[8]=>
string(3) "801"
[9]=>
string(3) "802"
[10]=>
string(3) "803"
[11]=>
string(3) "791"
}
["DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(12) {
[0]=>
string(3) "796"
[1]=>
string(3) "797"
[2]=>
string(3) "798"
[3]=>
string(3) "799"
[4]=>
string(3) "790"
[5]=>
string(3) "800"
[6]=>
string(3) "515"
[7]=>
string(2) "11"
[8]=>
string(3) "801"
[9]=>
string(3) "802"
[10]=>
string(3) "803"
[11]=>
string(3) "791"
}
["~DESCRIPTION"]=>
array(12) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
[8]=>
string(0) ""
[9]=>
string(0) ""
[10]=>
string(0) ""
[11]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(12) {
[0]=>
string(53) "
Anna Gooch"
[1]=>
string(52) "
John Doty"
[2]=>
string(54) "
Jean Flores"
[3]=>
string(57) "
LeAnne Swenson"
[4]=>
string(57) "
Florian Tögel"
[5]=>
string(58) "
George R. Reiss"
[6]=>
string(56) "
Claudia Lange"
[7]=>
string(56) "
Axel R. Zander"
[8]=>
string(51) "
Zhuma Hu"
[9]=>
string(54) "
Scott Poole"
[10]=>
string(53) "
Ping Zhang"
[11]=>
string(64) "
Christof Westenfelder"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["AUTHOR_RU"]=>
array(37) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10842"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(255) "<p class="Autor">Гуч А., Доти Дж., Флорес Дж., Свенсон Л., Тегель Ф., Райсс Р. Г., Ланге К., Цандер А. Р., Ху Дж., Пул С., Жанг П., Вестенвельдер К.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(233) "
Гуч А., Доти Дж., Флорес Дж., Свенсон Л., Тегель Ф., Райсс Р. Г., Ланге К., Цандер А. Р., Ху Дж., Пул С., Жанг П., Вестенвельдер К.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(233) "
Гуч А., Доти Дж., Флорес Дж., Свенсон Л., Тегель Ф., Райсс Р. Г., Ланге К., Цандер А. Р., Ху Дж., Пул С., Жанг П., Вестенвельдер К.
"
}
["SUBMITTED"]=>
array(37) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10826"
["VALUE"]=>
string(10) "11.11.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "11.11.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(10) "11.11.2008"
}
["ACCEPTED"]=>
array(37) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10827"
["VALUE"]=>
string(10) "15.12.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "15.12.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(10) "15.12.2008"
}
["PUBLISHED"]=>
array(37) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10828"
["VALUE"]=>
string(10) "24.12.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "24.12.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(10) "24.12.2008"
}
["KEYWORDS"]=>
array(38) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(4) {
[0]=>
string(5) "10963"
[1]=>
string(5) "10964"
[2]=>
string(5) "10965"
[3]=>
string(5) "10966"
}
["VALUE"]=>
array(4) {
[0]=>
string(2) "83"
[1]=>
string(3) "655"
[2]=>
string(3) "490"
[3]=>
string(3) "804"
}
["DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(4) {
[0]=>
string(2) "83"
[1]=>
string(3) "655"
[2]=>
string(3) "490"
[3]=>
string(3) "804"
}
["~DESCRIPTION"]=>
array(4) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(4) {
[0]=>
string(97) "
мезенхимные стволовые клетки"
[1]=>
string(90) "
Острое повреждение почек"
[2]=>
string(77) "
клеточная терапия"
[3]=>
string(85) "
клинические испытания"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["CONTACT"]=>
array(38) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10829"
["VALUE"]=>
string(3) "791"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "791"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(64) "
Christof Westenfelder"
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["SUMMARY_RU"]=>
array(37) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "10881"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(4416) "<p class="bodytext">Наши обширные данные доклинического исследования, показывают, что острое повреждение почек (ОПП), индуцированное ишемией/реперфузией – резистентное к лечению осложнение у больных - может эффективно лечиться путем назначения аллогенных мезенхимных стволовых клеток (МСК). На этом основании в настоящее время проводится одобренная FDA I фаза клинических испытаний (<a href="http://www.clinicaltrials.gov" target="_blank">www.clinicaltrials.gov</a>; NCT00733876) больных, которые имели высокий риск развития тяжелой ОПП после хирургии на открытом сердце. В рамках испытаний безопасности метода, инфузии аллогенных МСК проводили больным после завершения хирургического вмешательства при аорто-коронарном шунтировании или хирургии клапанов сердца. В исследовании участвовали лица старше 65 лет с наличием почечных заболеваний, сахарного диабета, артериальной гипертензии, коронарной болезни сердца, тяжелой сердечной недостаточности и/или хронической обструктивной болезни легких. Введение МСК проводили по возрастающей, причем первым пяти больным проводилась инфузия клеток в определенной низкой дозе на кг массы тела через бедренный катетер, помещенный в надпочечную часть аорты. Данное сообщение содержит обобщенные сведения о клиническом течении у этих пяти больных, которых лечили по этому протоколу. Почечная функция не нарушалась после операции ни у одного из больных, и на текущий момент не выявлено побочных эффектов или тяжелых негативных явлений. Однако один из больных внезапно скончался через 26 суток после выписки по причинам, которые были расценены главным исследователем и членами Совета по мониторингу данных и безопасности, как не относящиеся к препарату и способу его применения. Следующая группа из пяти больных получит MСК в средней дозе на кг массы тела, и, если при этой дозе не возникнут проблемы с безопасностью, то еще пять больных будут пролечены при высокой дозе МСК на кг массы тела. Предварительная эффективность терапии МСК для профилактики и лечения послеоперационного ОПП в этом контингенте высокого риска (кардиохирургических больных) будет определяться по сравнению исходов у испытуемых лиц (частоты, тяжести и длительности послеоперационного ОПП, временной или постоянной зависимости от диализа, длительности госпитализации или гибели до 30 сут.), и в большой группе больных исторического контроля (база данных на <a href="http://www.STS.org" target="_blank">www.STS.org</a>).<br />
"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(4330) "
Наши обширные данные доклинического исследования, показывают, что острое повреждение почек (ОПП), индуцированное ишемией/реперфузией – резистентное к лечению осложнение у больных - может эффективно лечиться путем назначения аллогенных мезенхимных стволовых клеток (МСК). На этом основании в настоящее время проводится одобренная FDA I фаза клинических испытаний (www.clinicaltrials.gov; NCT00733876) больных, которые имели высокий риск развития тяжелой ОПП после хирургии на открытом сердце. В рамках испытаний безопасности метода, инфузии аллогенных МСК проводили больным после завершения хирургического вмешательства при аорто-коронарном шунтировании или хирургии клапанов сердца. В исследовании участвовали лица старше 65 лет с наличием почечных заболеваний, сахарного диабета, артериальной гипертензии, коронарной болезни сердца, тяжелой сердечной недостаточности и/или хронической обструктивной болезни легких. Введение МСК проводили по возрастающей, причем первым пяти больным проводилась инфузия клеток в определенной низкой дозе на кг массы тела через бедренный катетер, помещенный в надпочечную часть аорты. Данное сообщение содержит обобщенные сведения о клиническом течении у этих пяти больных, которых лечили по этому протоколу. Почечная функция не нарушалась после операции ни у одного из больных, и на текущий момент не выявлено побочных эффектов или тяжелых негативных явлений. Однако один из больных внезапно скончался через 26 суток после выписки по причинам, которые были расценены главным исследователем и членами Совета по мониторингу данных и безопасности, как не относящиеся к препарату и способу его применения. Следующая группа из пяти больных получит MСК в средней дозе на кг массы тела, и, если при этой дозе не возникнут проблемы с безопасностью, то еще пять больных будут пролечены при высокой дозе МСК на кг массы тела. Предварительная эффективность терапии МСК для профилактики и лечения послеоперационного ОПП в этом контингенте высокого риска (кардиохирургических больных) будет определяться по сравнению исходов у испытуемых лиц (частоты, тяжести и длительности послеоперационного ОПП, временной или постоянной зависимости от диализа, длительности госпитализации или гибели до 30 сут.), и в большой группе больных исторического контроля (база данных на www.STS.org).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(4330) "
Наши обширные данные доклинического исследования, показывают, что острое повреждение почек (ОПП), индуцированное ишемией/реперфузией – резистентное к лечению осложнение у больных - может эффективно лечиться путем назначения аллогенных мезенхимных стволовых клеток (МСК). На этом основании в настоящее время проводится одобренная FDA I фаза клинических испытаний (www.clinicaltrials.gov; NCT00733876) больных, которые имели высокий риск развития тяжелой ОПП после хирургии на открытом сердце. В рамках испытаний безопасности метода, инфузии аллогенных МСК проводили больным после завершения хирургического вмешательства при аорто-коронарном шунтировании или хирургии клапанов сердца. В исследовании участвовали лица старше 65 лет с наличием почечных заболеваний, сахарного диабета, артериальной гипертензии, коронарной болезни сердца, тяжелой сердечной недостаточности и/или хронической обструктивной болезни легких. Введение МСК проводили по возрастающей, причем первым пяти больным проводилась инфузия клеток в определенной низкой дозе на кг массы тела через бедренный катетер, помещенный в надпочечную часть аорты. Данное сообщение содержит обобщенные сведения о клиническом течении у этих пяти больных, которых лечили по этому протоколу. Почечная функция не нарушалась после операции ни у одного из больных, и на текущий момент не выявлено побочных эффектов или тяжелых негативных явлений. Однако один из больных внезапно скончался через 26 суток после выписки по причинам, которые были расценены главным исследователем и членами Совета по мониторингу данных и безопасности, как не относящиеся к препарату и способу его применения. Следующая группа из пяти больных получит MСК в средней дозе на кг массы тела, и, если при этой дозе не возникнут проблемы с безопасностью, то еще пять больных будут пролечены при высокой дозе МСК на кг массы тела. Предварительная эффективность терапии МСК для профилактики и лечения послеоперационного ОПП в этом контингенте высокого риска (кардиохирургических больных) будет определяться по сравнению исходов у испытуемых лиц (частоты, тяжести и длительности послеоперационного ОПП, временной или постоянной зависимости от диализа, длительности госпитализации или гибели до 30 сут.), и в большой группе больных исторического контроля (база данных на www.STS.org).
"
}
}
}
[4]=>
array(49) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
["~IBLOCK_SECTION_ID"]=>
string(2) "10"
["ID"]=>
string(3) "825"
["~ID"]=>
string(3) "825"
["IBLOCK_ID"]=>
string(1) "2"
["~IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["~NAME"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["ACTIVE_FROM"]=>
NULL
["~ACTIVE_FROM"]=>
NULL
["TIMESTAMP_X"]=>
string(19) "13.06.2017 13:08:47"
["~TIMESTAMP_X"]=>
string(19) "13.06.2017 13:08:47"
["DETAIL_PAGE_URL"]=>
string(145) "/ru/archive/Volume-1/korotkie-soobshcheniya/unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya/"
["~DETAIL_PAGE_URL"]=>
string(145) "/ru/archive/Volume-1/korotkie-soobshcheniya/unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya/"
["LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["~LIST_PAGE_URL"]=>
string(12) "/ru/archive/"
["DETAIL_TEXT"]=>
string(45132) "
Introduction
An adult organism develops from the most primitive stem cell (SC) called a zygote, which is an oocyte that is fertilized by a sperm cell. This totipotent zygote, the “mother of all stem cells” in the developing body, first gives rise to pluripotent (P)SCs that form morula and, subsequently, to the SCs committed to trophoblasts that will give rise to the placenta and the pluripotent SC population that forms the inner cell mass of the blastocyst. The cells from the inner cell mass of the blastocyst will give rise to the epiblast, a part of the developing embryo, which is the origin of SCs committed to all the three germ layers (meso-, ecto-, and endoderm) [1-5].
Thus, the epiblast could be considered the origin for the SCs committed for all the organs and tissues in developing the embryo proper. PSCs in the epiblast undergo a sequence of specification events, first into multipotent and subsequently into versatile tissue-committed SCs, which play a role in the formation and rejuvenation of various organs [5-7]. The most important questions emerge of whether some of these primitive epiblast-forming PSCs can “escape” specification into more differentiated populations of SCs and retain their pluripotential character, thus surviving among differentiated daughter tissue-committed SCs. Conversely, would all of them undergo tissue/organ specific differentiation and then “disappear” after embryogenesis, and not be found in the adult body (Figure 1)?
-
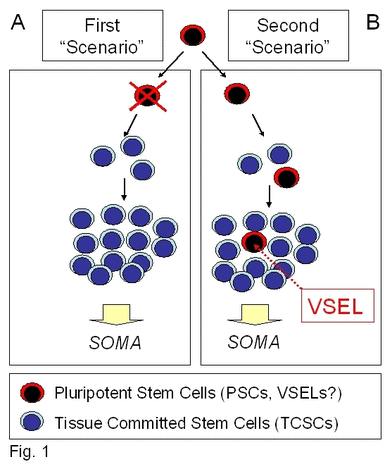
Figure 1. Potential VSELs contribution to tissue rejuvenation.
Panel A: VSELs deposited in adult tissues during embryogenesis/gastrulation may become eliminated after giving rise to TCSCs. Panel B: Conversely, they may survive among TCSCs and serve as a potential back-up/reserve source of TCSCs.
Recently, our group obtained several pieces of evidence that may lend some support for the first possibility. Accordingly, we have identified a population of very primitive SCs in adult tissues that express many markers characteristic for epiblast Scs [8]. Based on this we named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that they are deposited during early gastrulation in developing tissues/organs, survive into adulthood, and play an important role as a back-up population of PSCs in the turnover of tissue-specific/committed SCs (TCSCs) [5, 7, 8].
The presence of pluripotent VSELs in adult tissues may reconcile all previously published data stating that adult tissues may contain a population of PSCs [9, 10]. The existence of such cells had been postulated by several investigators [11, 12]. However, such cells were never purified and identified at the single cell level. Their presence was accepted mainly based on experiments showing that some populations of cells were enriched with adherent cell populations isolated from the bone marrow (BM) or that certain solid organs contain some primitive cells that may differentiate into various tissues.
Accordingly, several populations of non-hematopoietic primitive SCs have been described in the BM and other adult tissues, including: i) mesenchymal (M)SCs [13]; ii) multipotent adult progenitor cells (MAPCs) [14]; iii) marrow-isolated adult multilineage inducible (MIAMI) cells [15]; iv) multipotent adult (MA)SCs [16]; and v) Omnicytes [17, 18]. It is conceivable that all these cells are closely related, overlapping populations of SCs described by different investigators and given various names according to circumstance. Furthermore, the potential relationship between these cells and VSELs is not clear. Since MSCs, MAPCs, MIAMIs, and MASCs are largely derived from the adherent fraction of BM- or adult organ-derived cells, these cells could potentially contain some VSELs attached to or associated with them due to emperipolesis. This requires further investigation.
“Of germ line and soma”: germ line as origin and skeleton of the SC system in the adult body
From a developmental point of view, cells that are “immortal” in mammals are those that belong to the germ line. Accordingly, the germ line passes genomic and mitochondrial DNA to the next generations and creates “mortal soma”, which helps the germ line to fulfill this reproductive mission [19-21]. The most primitive cell in the germ line is the above-mentioned zygote, which is a result of fusion of two gametes (germ cells), i.e., the oocyte and sperm, during the process of fertilization. Germ line potential is subsequently maintained in blastomers of morula and in the cells of the inner cell mass of the blastocyst. At the level of the blastocyst, however, a part of the cells that surrounds the blastula “buds out” from the germ line lineage and differentiates toward throphoblasts, which give rise to the placenta. After implantation of the blastocyst in the uterus, a germ line potential is maintained in the epiblast [19-21].
In mice, at 7.25 days post-conception (dpc), a part of epiblast PSCs is specified into a population of primordial germ cells (PGCs) that will migrate to the genital ridgesahre where they subsequently differentiate into oocytes or sperm during gametogenesis [6, 22]. Shortly after PGC specification, the remaining epiblast PSCs, which we envision to be related to the germ line lineage, become specified to multipotent/unipotent SCs for developing tissues and organs [20]. These primitive epiblast/germ line-derived PCSs, as we hypothesize, are not completely eliminated from the developing organism by the differentiation process. We believe that some of them survive (e.g., VSELs?) among tissue-committed SCs [20].
PGCs are the most important population of SCs. As precursors of germ cells/gametes, they are directly responsible for passing genetic information on to the next generation. However, these developmentally early cells, if isolated from the developing embryo after 11 dpc (at the time while they migrate to genital ridges) and cultured ex vivo, surprisingly will undergo rapid terminal differentiation or apoptosis [23]. Interestingly, they also do not complement blastocyst development, are not able to provide fully functional nuclei during nuclear transfer in the process of clonote formation, and do not grow teratomas [20, 24-26]. Therefore, these cells lack the currently approved criteria of pluripotentiality. This also indicates that PGCs have to be somehow protected from uncontrolled expansion by certain important regulatory mechanisms.
One explanation for this obvious lack of pluripotentiality is that PGCs undergo epigenetic modification and erasure of the somatic imprint on differently methylated regions (DMRs) of somatic imprinted genes [27, 28]. Somatic imprinted genes show a different methylation pattern in some of the genes located either on maternal or paternal chromosomes (e.g., H19, Igf-2, Igf-2R, Snrpn). As a result of the imprint, for example, Igf-2 is expressed from the paternal and H19 is expressed from the maternal chromosome [20, 27, 28].
The process of erasure of the somatic imprint occurs very early during gastrulation when the PGCs begin to migrate to the genital ridges [27, 28]. It is one of the basic mechanisms that prevents their uncontrolled proliferation, parthenogenesis, and prevents potential teratoma formation by these cells. Thus, the proper somatic imprint seems to be required for PSCs to be able to complement blastocyst development, provide nuclei for clonote formation after nuclear transfer, and to grow teratomas in immunodeficient mice. Because migrating PGCs erase the imprint, they are not able to display these “golden standard” pluripotency criteria in appropriate experimental models [26-28].
However, if plated over murine fetal fibroblasts in the presence of selected growth factors, i.e., leukemia inhibitory factor (LIF), basic fibroblast growth factor (bFGF), and kit ligand, PGCs may undergo epigenetic changes forced by in vitro culture conditions that regain the somatic imprint, which endows them with “immortality” [29, 30]. Thus, this change of “PGC fate” is connected with a proper re-methylation of somatic imprinted genes. This immortalized population of PGCs known as embryonic germ cells (EGCs) is in many aspects the equivalent to embryonic (E)SCs [31]. For example, similarly to ESCs, EGCs contribute to all three germ layers including the germ cell lineage after injection into a blastocyst (blastocyst complementation assay), provide functional nuclei for clonote after nuclear transfer, and, after injection into living mice, these cells form teratomas [21, 31]. Thus, it is evident that a proper somatic imprint is vital for cells from the germ line to retain full pluripotentiality. Based on this, we postulate that erasure of the somatic imprint of some crucial developmentally genes is involved in controlling the quiescent status of VSELs [20, 32]. However, as in the case of PGCs, this process should be potentially reversible. As such, we will have to focus on reestablishing the proper somatic imprint in these cells. We envision that such reverted VSELs could potentially become equivalent to cells isolated from the embryos, e.g., after nuclear transfer or even to inducible (i)PSCs [33, 34].
The hunt for PSCs in the adult body
The presence of PSCs in adult tissues was postulated in the past by several investigators, but such cells were never purified at the single cell level. A few years ago, our team began to search for such cells in murine BM. Based on our preliminary experimental data, we assumed that the cells we were looking for would be CD133+ CXCR4+ in mice and humans as well as Sca-1+ in mice. We also assumed that they would be negative in both species for the pan-hematopoietic marker, which is the CD45 antigen (CD45-) [8, 35, 36]. In addition, our preliminary chemotactic experiments revealed that BM contains a very rare population of small cells (3-5 µm in diameter) that robustly respond by chemotaxis to stromal-derived factor-1 (SDF-1), which is a ligand for the CXCR4 receptor [37]. These small CXCR4+ cells expressed some early developmental markers characteristic for very primitive cells [8, 37].
Based on this information, we decided to sort a population of small (<6 µm) Sca-1+lin-CD45- cells from the murine BM and other tissues. By employing a fluorescence activated cell sorter (FACS), we isolated a population of rare Sca-1+Lin-CD45- cells from several adult tissues including BM, brain, liver, pancreas, kidney, muscles, heart, testes, and thymus [9]. As determined by real time RT-PCR (RQ-PCR) by employing sequence specific primers and by immunohistochemistry, these cells express several markers of PSCs such as SSEA-1, Oct-4, Nanog, and Rex-1 as well as Rif-1 telomerase protein [8, 9]. Based on the expression of these early developmental markers and morphology, we named these cells “very small embryonic-like stem cells (VSELs)” [8].
To isolate VSELs from the BM by FACS, we employed a novel size-based approach controlled by size bead markers (Figure 2). The overall sorting strategy was to gate in regions containing small events (2–10 µm), which is shown as Region R1 on the dot plot (Figure 2: Panel A). This region mostly contains cell debris, but also includes some rare nucleated cell events. Because it is well known that most of the sorting protocols exclude events smaller than 6 µm in diameter that contain cell debris, erythrocytes, and platelets, small VSELs are usually excluded from the sorted cell populations. Thus, in our sorting strategy to isolate VSELs and define a region of cells from which we could sort these cells, the size of the sorted cells was controlled by beads with predefined sizes (1, 2, 4, 6, 10, and 15 µm in diameter) (Figure 2: Panel A).
-
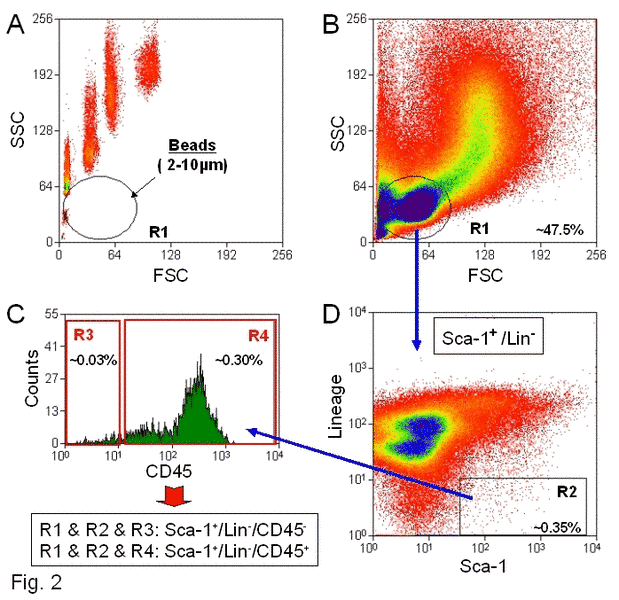
Figure 2. Sorting strategy for isolation of murine BM-derived VSELs by FACS.
BM-derived VSELs were sorted by MoFlo cell sorter (Dako, Glostrup, DEN) following immunofluorescence staining for Sca-1, CD45, and hematopoietic Lin. Panel A: Distribution of six predefined, sized beads populations according to their FSCs vs. SSCs (forward and side scatter characteristics, respectively). Gate R1 includes objects between 2 to 10µm in size after comparison to bead particles with standard sizes of 1, 2, 4, 6, 10, and 15µm (Flow Cytometry Size beads, Invitrogen; Molecular Probes, Carlsbad, CA, USA). Panel B: BMNCs visualized on dot plots showing their FSC and SSC signals related to the size and granularity/complexity of the cell, respectively. Small, agranular cells included in Region R1 are further visualized based on the expression of Sca-1 and Lin markers (Panel D). Region R2 includes only Sca-1+/Lin-, which are subsequently sorted based on CD45 marker expression into CD45- and CD45+ subpopulations visualized on histogram (Panel C). Sca-1+/Lin-/CD45- cells (VSELs) are sorted as events enclosed in a logical gate including Regions R1, R2, and R3, while Sca-1+/Lin-/CD45+ cells (HSCs) from gate include Regions R1, R2, and R4. Approximate percent contents of each cellular subpopulation are indicated on the plots.
The events enclosed in region R1 (Figure 2: Panel B), which include an average of ~50% of total collected events, are further analyzed for the expression of Sca-1 and lineage markers (Lin). The Sca-1+Lin- events shown in region R2 (Figure 2: Panel D) consist of 0.38 ± 0.05% of total analyzed BM nucleated cells (BMNCs) on average. Cells from region R2 are subsequently sorted according to the expression of CD45- as Sca-1+Lin-CD45- (region R3) and Sca-1+Lin-CD45+ (region R4) subpopulations (Figure 2: Panel C) that contain VSELs and HSCs, respectively. We found that VSELs comprise ~0.03% while HSCs are ~0.35% of total BMNCs (Figure 2: Panel C). We found that 95% of Sca-1+Lin-CD45- (VSELs) are located within the 2–6 µm size range, while 86% of Sca-1+Lin-CD45+ (HSCs) are found in the 6–10 µm size range [36]. Thus, by employing flow cytometry and the size marker beads, we confirmed our previous transmission electron microscopy (TEM) data showing that the majority of Sca-1+Lin-CD45- cells isolated from adult BM are unusually small (<6 µm) [8]. In conclusion, VSELs are larger than PB platelets and smaller than erythrocytes. Direct TEM analysis revealed that these cells display several features typical for ESCs such as small size, a large nucleus surrounded by a narrow rim of cytoplasm, and open-type chromatin (euchromatin).
ImageStream system (ISS) analysis was also employed to further evaluate the morphological features of VSELs [36]. The ISS-based analysis is a new flow cytometry-based analytical strategy that employs flow cytometry combined with microscopy. This allows for statistical analyses of various cellular parameters as well as direct visualization of cells acquired by FACS in suspension during flow analysis via high-resolution brightfield, darkfield, and fluorescence images [36]. The high resolution of ISS imaging enables the identification of objects as small as 1 µm in diameter [38-40].
In employing ISS analysis, we confirmed with greater precision that VSELs are ~3.6 μm in diameter, while Sca-1+Lin-CD45+ HSCs are larger at ~6.5 μm in diameter. We also noticed that VSELs have a significantly higher (P < 0.05) nuclear/cytoplasmic ratio as compared with HSCs (1.47 ± 0.17 and 0.82 ± 0.03, respectively). Furthermore, VSELs had significantly lower (P < 0.05) cytoplasmic area as compared with HSCs (5.41 ± 0.58 and 33.78 ± 1.68, respectively) [36]. Despite their small size, VSELs possess diploid DNA. They do not express MHC-1 and HLA-DR antigens and are CD90- CD105- CD29- [41].
Interestingly, if plated over a C2C12 murine sarcoma cell feeder layer, ~5–10% of purified VSELs are able to form spheres that resemble embryoid bodies. Cells from these VSEL-derived spheres (VSEL-DSs) are composed of immature cells with large nuclei containing euchromatin and are CXCR4+SSEA-1+Oct-4+, just like purified VSELs [8>]. Similar spheres were also formed by VSELs isolated from murine fetal liver, spleen, thymus, and kidney. Interestingly, formation of VSEL-DSs was associated with a young age in mice with no VSEL-DSs observed in cells isolated from older mice (> 2 years) [10]. This age-dependent content of VSELs in BM may somehow explain why the regeneration processes is more efficient in younger individuals. There are also differences in the content of these cells among BM mononuclear cells (MNCs) between long- and short-lived mouse strains. The concentration of these cells is much higher in the BM of long-lived (e.g., C57Bl6) as compared to short-lived (DBA/2J) mice [8]. In the future, it would be interesting to identify the genes responsible for tissue distribution and expansion of these cells, as they could be involved in controlling the life span of mammals.
Furthermore, since VSELs express several markers of PGCs (fetal-type alkaline phosphatase, Oct-4, SSEA-1, CXCR4, Mvh, Stella, Fragilis, Nobox, Hdac6), they could be closely related to a population of epiblast-derived PGCs. VSELs are also highly mobile and respond robustly to an SDF-1 gradient, adhere to fibronectin and fibrinogen, and may interact with BM-derived stromal fibroblasts [8]. Confocal microscopy and time-lapse studies revealed that these cells attach rapidly to, migrate beneath, and undergo emperipolesis in marrow-derived fibroblasts. This is explainable by fibroblasts secreting SDF-1 and other chemoattractants, which may create a homing environment for small CXCR4+ VSELs [8]. This robust interaction of VSELs with BM-derived fibroblasts has an important implication. It is possible that isolated BM and other tissues’ fibroblastic cells (e.g., MSCs, USSCs, MACSs, MAPCs, or MIAMI cells) may be to some degree contaminated by these tiny cells from the beginning. This observation may explain the unexpected plasticity of marrow-derived fibroblastic cells, e.g., MSCs.
Recently, evidence has also mounted to suggest that similar cells corresponding to those found in murine tissues are also present particularly in the human BM, umbilical cord blood (UCB), and mobilized (m)PB (Table I). Overall, it is anticipated that VSELs could become an important source of PSCs for regeneration. Thus, researchers working with animal models must determine whether these cells could be efficiently employed in the clinic or whether they are merely developmental remnants found in the BM that cannot be harnessed effectively for regeneration. Our initial collaborative studies indicate an efficacy of these cells in improving heart function in an animal model of acute myocardiac infarction in mice [42, 43]. We anticipate seeing similar phenomena in humans.
Table I. Morphological and phenotypic comparison of murine and human VSELs.
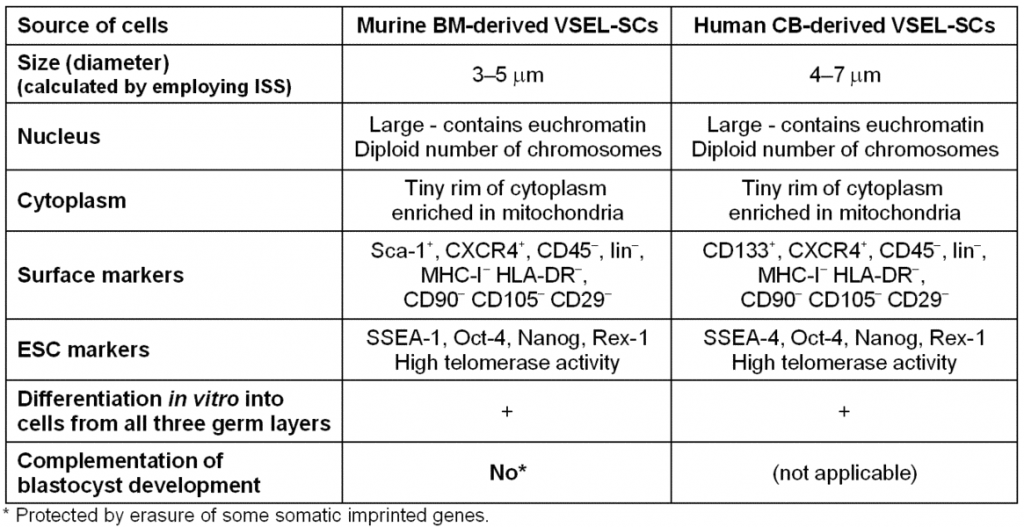
VSELs as circulating “paramedics” in the body
Our data also indicates that VSELs may be released during stress situations or tissue/organ injury from their tissue niches and circulate in the PB both in humans (e.g., after heart infarct or stroke) and in mice (e.g., after granulocyte colony growth factor [G-CSF]-induced mobilization, experimental heart infarct and stroke, as well as liver and skeletal muscle injury) [44-46]. The trafficking of VSELs is orchestrated by several chemotactic factors that are upregulated in damaged tissues during tissue organ injury such as α-chemokine SDF-1, hepatocyte growth factor/scatter factor (HGF/SF), LIF, and vascular endothelial growth factor (VEGF) [47-50]. Complement cascade cleavage fragments also play an important role in this process, such as C3a anaplylatoxin for example, which enhances responsiveness of VSELs to SDF-1 gradient (Figure 3). Thus, a concept emerged where chemotactic factors that are upregulated in damaged tissues may orchestrate the release of non-hematopoietic SCs from BM into mPB.
For instance, in a murine model of G-CSF-induced mobilization, we noticed that VSELs are detectable at a very low level in steady state conditions in murine PB (~160 cells/ml) and that their number increases ~6 times during G-CSF-induced mobilization events [44]. Increases in the number of these cells circulating in PB are further supported by an increase in expression of mRNA for early developmental markers expressed in VSELs, such as the embryonic transcription factors Oct-4, Nanog, and Rex-1 as well as the expression of Rif1 and Dppa3 [44]. Furthermore, at the same time, MNCs mobilized into PB are highly enriched for mRNA for several early developmental tissue-specific markers, a phenomenon that could be explained as mentioned above by the open-type status of chromatin in these cells. Finally, we sorted these rare cells from murine PB by FACS for immunofluorescence staining and provided evidence that they express SSEA-1 antigen on the surface and Oct-4 in the nucleus.
To provide evidence that mobilized VSELs not only express PSC markers but also are able to differentiate into cells from all three germ layers, we performed differentiation studies in vitro. To provide such proof, VSELs were cultured in appropriate differentiation media on the layer of BM-derived stromal support. We found that mobilized VSELs are able to differentiate into cardiomyocytes, neurons, and pancreatic cell-like clusters [44]. The analysis of DNA content in GFP+ cells isolated from the co-cultures excluded the contribution of cell fusion to this effect. Thus, these experiments revealed the in vitro pluripotency of VSELs mobilized by G-CSF and circulating in mPB by demonstrating their ability to differentiate into cells from all three germ layers. We envision that VSELs mobilized into PB in humans, such as after G-CSF administration, could be harvested by leucopheresis as a potential source of SCs for regenerative medicine.
-
Figure 3. VSELs are mobilized into PB.
Panel A: Under normal steady state conditions, VSELs may circulate in PB to keep a pool of SCs in balance in distant niches of the same tissue.
Panel B: The number of these cells increases during stress related to organ/tissue damage. During organ damage (e.g., heart infarct), the level of SDF-1 is upregulated in the affected tissues and C3 becomes activated leading to the accumulation of C3 cleavage fragments (C3a and desArgC3a). C3 cleavage fragments enhance/prime the responsiveness of circulating CXCR4+ SCs to an SDF-1 gradient. This leads to more efficient chemoattraction of SCs for potential regeneration of the damaged tissue by creating “a super gradient,” as shown in Panel B for infracted myocardium, for example. In addition to SDF-1, other chemoattractants also play important roles here (e.g., HGF/SF, LIF, and VEGF).
Do Oct-4+ VSELs initiate tumor development?
Several investigators have proposed theories regarding cancer formation in the germ cell compartment. Accordingly, Recamier (1829), Remak (1854), and Virchow (1958) proposed that cancer arises from embryo-like cells. Subsequently, Durante and J. Cohnheim in 1874 and 1875, respectively, suggested adult tissues contain embryonic remnants that normally lie dormant, but can be activated to become cancerous. In 1910, Wright proposed the germinal cell origin of Willm’s tumor (nephroblastoma) and in 1911, J. Beard postulated that tumors arise from displaced trophoblast or activated germ cells. We envision the Oct-4+ VSEL recently identified in adult tissues could unify and fully support all these theories. First, we envision that if the genomic imprint in VSELs is not erased, they may retain post-developmental in vivo pluripotency and grow teratomas and teratocarcinomas [5, 20]. Second, if they are closely related to migratory PGCs, which go astray from the major migratory route to the genital ridges, they may ultimately give rise to germinomas and seminomas, for example. Third, if these cells acquire critical mutations, they may develop into the several types of pediatric sarcomas (e.g., rhabdomyosarcoma, neuroblastoma, Ewing-sarcoma, or Willm's tumor). In support of this, there is a strong correlation between the number of these Oct-4+ cells that persist in postnatal tissues and the coincidence with these types of tumors in pediatric patients. Finally, it is possible that these cells, if mobilized at the wrong time into the PB and deposited in areas of chronic inflammation, may not play a role in regeneration but may contribute to the development of other malignancies (e.g., stomach cancer or lung cancer). To support this further, several tumor types may express embryonic markers including Oct-4 and, as reported, BM-derived SCs that may develop in the presence of carcinogens to some sarcomas or teratomas. Furthermore, we hypothesize that VSELs hiding among BM-derived fibroblasts could also be responsible for sarcoma formation by MSC cells. Circulating VSELs also could be also chemoattracted by the hypoxic/chemoattractant-rich environment of a growing tumor and provide stroma and vessels for expanding that tumor. Finally, it is also possible that circulating VSELs or cells very closely related to this population may also act in progressive fibrosis of some organs such as the lung.
Closing remarks
Several attempts have been made in the past few years to purify a population of PSCs from adult tissues including BM, UCB, and mPB that could give rise in vitro to cells from all three germ layers (meso-, ecto-, and endoderm) [8, 16, 44] and in vivo as well as in mice after injection into the developing blastocyst that would contribute to the development of multiple organs and tissues. In contrast to positive data in vitro, this latter criterion for pluripotentiality in vivo for several potential candidates for PSCs has not yet been demonstrated in a reproducible manner with any SC type isolated from the adult tissues. This is also true for VSELs. The reason for this could be that PSCs deposited in adult tissues erase the imprint on some crucial maternal or paternal imprinted genes. This phenomenon keeps these cells under control from unleashed proliferation and not only prevents the possibility of teratoma formation in vivo by these cells, but also simultaneously will affect their ability to complete blastocyst development after injection into developing blastocyst.
VSELs isolated from adult tissues are an alternative and not ethically controversial source of SCs for regenerative medicine. However, there are several missing answers to this timely issue, especially in view of the current and widely performed clinical trials with BM-derived SCs in cardiology and neurology, before VSELs can find their potential application in regenerative medicine.
First, there is the obvious problem of isolating a sufficient number of VSELs from the BM, UCB, or mPB. The number of these cells among BM MNCs is very low. For example, VSELs represent ~1 cell in 105 of BM MNCs [8, 35, 36]. Furthermore, our data shows that these cells are enriched in the BM of young mammals and their number decreases with age. It is also likely that if VSELs are released from the BM, even if they are able to home to the areas of tissue/organ injury, they may function only in the regeneration of minor tissue injuries. Heart infarct or stroke, on the other hand, may involve severe tissue damage beyond the effective repair capacity of these rare cells. Second, the allocation of these cells to the damaged areas depends on homing signals that may be inefficient in the presence of proteolytic enzymes released from leukocytes and macrophages associated with damaged tissue. For example, matrix metalloproteinases (MMPs) released from inflammatory cells may degrade SDF-1 locally and perturb homing of CXCR4+ SCs [51]. Thus, VSEL-SCs may potentially circulate as a homeless population of SCs in PB and return to the BM or home to other organs. Third, to reveal their full regenerative potential, these cells have to be fully functional. We cannot exclude the possibility that VSEL-SCs, while residing or being trapped in the BM, not only erase appropriate methylation on differently methylated regions of some important somatic imprinted genes but also are not fully functional and remain locked in a dormant state. They require the appropriate activation signals by unidentified factors. Finally, we have to develop efficient ex vivo culture conditions that will allow for efficient expansion of VSEL-SCs without supportive feeder layer cells (e.g., C2C12, BM-derived fibroblasts).
Nevertheless, our data strongly indicates that VSEL-SCs could potentially provide a therapeutic alternative to the controversial use of human ESCs and strategies based on therapeutic cloning. Hence, while the ethical debate on the application of ESCs in therapy continues, the potential of VSELs is ripe for exploration. The current work in our laboratory indicates that VSELs could be efficiently employed in the realm of regenerative medicine and that they are physiologically more important than merely being potential developmental remnants. Finally, we believe that the controlled modulation of somatic imprint status in VSELs such as we hypothesized, a proper de novo methylation of somatic imprinted genes on maternal and paternal chromosomes, could increase a regenerative power of these cells. The coming years will bring important answers to these questions.
References
1. Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872-884.
2. Darr H, Benvenisty N. Human embryonic stem cells: the battle between self-renewal and differentiation. Regen Med. 2006;1:317-325.
3. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634-7638.
4. O'Farrell PH, Stumpff J, Su TT. Embryonic cleavage cycles: how is a mouse like a fly? Curr Biol. 2004;14:R35-45.
5. Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W, Kucia M: Hunt for pluripotent stem cell - Regenerative medicine search for almighty cell. J Autoimmun. 2008;30:151-162.
6. McLaren A, Lawson KA. How is the mouse germ-cell lineage established? Differentiation. 2005;73:435-437.
7. Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J, Kucia M. Very Small Embryonic-Like (VSEL) Stem Cells: Purification from Adult Organs, Characterization, and Biological Significance. Stem Cell Rev. 2008;4:89-99.
8. Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857-869.
9. Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr., Ratajczak J, Ratajczak MZ. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A. 2008 Oct;24.
10. Zuba-Surma EK, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Very small embryonic-like stem cells in adult tissues-Potential implications for aging. Mech Ageing Dev. 2008 Feb;14.
11. Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188-189.
12. Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti CA. Identification and initial characterization of spore-like cells in adult mammals. J Cell Biochem. 2001;80:455-460.
13. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74.
14. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49.
15. D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971-2981.
16. Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow). Blood. 2007;110:3438-3446.
17. Gordon MY. Stem cells for regenerative medicine--biological attributes and clinical application. Exp Hematol. 2008;36:726-732.
18. Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M'Hamdi H, Thalji T, Welsh JP, Marley SB, Davies J, Dazzi F, Marelli-Berg F, Tait P, Playford R, Jiao L, Jensen S, Nicholls JP, Ayav A, Nohandani M, Farzaneh F, Gaken J, Dodge R, Alison M, Apperley JF, Lechler R, Habib NA. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830.
19. Donovan PJ. The germ cell - the mother of all stem cells. Int J Dev Biol. 1998;42:1043-1050.
20. Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4+ stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860-867.
21. Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227-233.
22. McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1-15.
23. De Felici M, McLaren A. In vitro culture of mouse primordial germ cells. Exp Cell Res. 1983;144:417-427.
24. Macchiarini P, Ostertag H. Uncommon primary mediastinal tumours. Lancet Oncol. 2004;5:107-118.
25. Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210-222.
26. Yamazaki Y, Mann MR, Lee SS, Marh J, McCarrey JR, Yanagimachi R, Bartolomei MS. Reprogramming of primordial germ cells begins before migration into the genital ridge, making these cells inadequate donors for reproductive cloning. Proc Natl Acad Sci U S A. 2003;100:12207-12212.
27. Lee J, Inoue K, Ono R, Ogonuki N, Kohda T, Kaneko-Ishino T, Ogura A, Ishino F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development. 2002;129:1807-1817.
28. Mann JR. Imprinting in the germ line. Stem Cells. 2001;19:287-294.
29. Donovan PJ. Growth factor regulation of mouse primordial germ cell development. Curr Top Dev Biol. 1994;29.
30. Resnick JL, Ortiz M, Keller JR, Donovan PJ. Role of fibroblast growth factors and their receptors in mouse primordial germ cell growth. Biol Reprod. 1998;59:1224-1229.
31. Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841-847.
32. Kucia M, Wu W, Ratajczak MZ. Bone marrow-derived very small embryonic-like stem cells: Their developmental origin and biological significance. Dev Dyn. 2007;12:3309-20.
33. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
34. Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-324.
35. Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4(+) SSEA-4(+) Oct-4(+) very small embryonic-like cells purified from human cord blood - preliminary report. Leukemia. 2007;21:297-303.
36. Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawnn B, Hall B, Singh R, Lillard JW, Ratajczak MZ. Morphological characterization of Very Small Embryonic-Like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292-303.
37. Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52-57.
38. Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med. 2007;27:653-670.
39. Zuba-Surma EK, Kucia M, Abdel-Latif A, Lillard JJ, Ratajczak MZ. The ImageStream System: a key step to a new era in imaging. Folia Histochem Cytobiol. 2007;45:279-290.
40. Zuba-Surma EK, Kucia M, Ratajczak MZ. “Decoding of Dot”: The ImageStream System (ISS) as a Supportive Tool for Flow Cytometric Analysis. Cent Eur J Biol. 2008;3:1-10.
41. Kucia M, Wysoczynski M, Ratajczak J, Ratajczak MZ. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331:125-134.
42. Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646-1655.
43. Zuba-Surma EK, Taher H, Kucia M, Guo Y, SanganalMath SK, Hunt G, Vincent RJ, Abdel-Latif A, Dawn B, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived Very Small Embryonic-Like stem cells (VSELs) improves left ventricular function and remodeling after myocardial infarction. Circulation. 2007:204.
44. Kucia M, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that Very Small Embryonic Like (VSEL) Stem Cells are Mobilized into Peripheral Blood. Stem Cells. 2008;26:2083-2092.
45. Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865-873.
46. Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213-3220.
47. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858-864.
48. Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331-1339.
49. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434-438.
50. Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772-1784.
51. McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503-43508.
"
["~DETAIL_TEXT"]=>
string(45132) "
Introduction
An adult organism develops from the most primitive stem cell (SC) called a zygote, which is an oocyte that is fertilized by a sperm cell. This totipotent zygote, the “mother of all stem cells” in the developing body, first gives rise to pluripotent (P)SCs that form morula and, subsequently, to the SCs committed to trophoblasts that will give rise to the placenta and the pluripotent SC population that forms the inner cell mass of the blastocyst. The cells from the inner cell mass of the blastocyst will give rise to the epiblast, a part of the developing embryo, which is the origin of SCs committed to all the three germ layers (meso-, ecto-, and endoderm) [1-5].
Thus, the epiblast could be considered the origin for the SCs committed for all the organs and tissues in developing the embryo proper. PSCs in the epiblast undergo a sequence of specification events, first into multipotent and subsequently into versatile tissue-committed SCs, which play a role in the formation and rejuvenation of various organs [5-7]. The most important questions emerge of whether some of these primitive epiblast-forming PSCs can “escape” specification into more differentiated populations of SCs and retain their pluripotential character, thus surviving among differentiated daughter tissue-committed SCs. Conversely, would all of them undergo tissue/organ specific differentiation and then “disappear” after embryogenesis, and not be found in the adult body (Figure 1)?
-

Figure 1. Potential VSELs contribution to tissue rejuvenation.
Panel A: VSELs deposited in adult tissues during embryogenesis/gastrulation may become eliminated after giving rise to TCSCs. Panel B: Conversely, they may survive among TCSCs and serve as a potential back-up/reserve source of TCSCs.
Recently, our group obtained several pieces of evidence that may lend some support for the first possibility. Accordingly, we have identified a population of very primitive SCs in adult tissues that express many markers characteristic for epiblast Scs [8]. Based on this we named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that they are deposited during early gastrulation in developing tissues/organs, survive into adulthood, and play an important role as a back-up population of PSCs in the turnover of tissue-specific/committed SCs (TCSCs) [5, 7, 8].
The presence of pluripotent VSELs in adult tissues may reconcile all previously published data stating that adult tissues may contain a population of PSCs [9, 10]. The existence of such cells had been postulated by several investigators [11, 12]. However, such cells were never purified and identified at the single cell level. Their presence was accepted mainly based on experiments showing that some populations of cells were enriched with adherent cell populations isolated from the bone marrow (BM) or that certain solid organs contain some primitive cells that may differentiate into various tissues.
Accordingly, several populations of non-hematopoietic primitive SCs have been described in the BM and other adult tissues, including: i) mesenchymal (M)SCs [13]; ii) multipotent adult progenitor cells (MAPCs) [14]; iii) marrow-isolated adult multilineage inducible (MIAMI) cells [15]; iv) multipotent adult (MA)SCs [16]; and v) Omnicytes [17, 18]. It is conceivable that all these cells are closely related, overlapping populations of SCs described by different investigators and given various names according to circumstance. Furthermore, the potential relationship between these cells and VSELs is not clear. Since MSCs, MAPCs, MIAMIs, and MASCs are largely derived from the adherent fraction of BM- or adult organ-derived cells, these cells could potentially contain some VSELs attached to or associated with them due to emperipolesis. This requires further investigation.
“Of germ line and soma”: germ line as origin and skeleton of the SC system in the adult body
From a developmental point of view, cells that are “immortal” in mammals are those that belong to the germ line. Accordingly, the germ line passes genomic and mitochondrial DNA to the next generations and creates “mortal soma”, which helps the germ line to fulfill this reproductive mission [19-21]. The most primitive cell in the germ line is the above-mentioned zygote, which is a result of fusion of two gametes (germ cells), i.e., the oocyte and sperm, during the process of fertilization. Germ line potential is subsequently maintained in blastomers of morula and in the cells of the inner cell mass of the blastocyst. At the level of the blastocyst, however, a part of the cells that surrounds the blastula “buds out” from the germ line lineage and differentiates toward throphoblasts, which give rise to the placenta. After implantation of the blastocyst in the uterus, a germ line potential is maintained in the epiblast [19-21].
In mice, at 7.25 days post-conception (dpc), a part of epiblast PSCs is specified into a population of primordial germ cells (PGCs) that will migrate to the genital ridgesahre where they subsequently differentiate into oocytes or sperm during gametogenesis [6, 22]. Shortly after PGC specification, the remaining epiblast PSCs, which we envision to be related to the germ line lineage, become specified to multipotent/unipotent SCs for developing tissues and organs [20]. These primitive epiblast/germ line-derived PCSs, as we hypothesize, are not completely eliminated from the developing organism by the differentiation process. We believe that some of them survive (e.g., VSELs?) among tissue-committed SCs [20].
PGCs are the most important population of SCs. As precursors of germ cells/gametes, they are directly responsible for passing genetic information on to the next generation. However, these developmentally early cells, if isolated from the developing embryo after 11 dpc (at the time while they migrate to genital ridges) and cultured ex vivo, surprisingly will undergo rapid terminal differentiation or apoptosis [23]. Interestingly, they also do not complement blastocyst development, are not able to provide fully functional nuclei during nuclear transfer in the process of clonote formation, and do not grow teratomas [20, 24-26]. Therefore, these cells lack the currently approved criteria of pluripotentiality. This also indicates that PGCs have to be somehow protected from uncontrolled expansion by certain important regulatory mechanisms.
One explanation for this obvious lack of pluripotentiality is that PGCs undergo epigenetic modification and erasure of the somatic imprint on differently methylated regions (DMRs) of somatic imprinted genes [27, 28]. Somatic imprinted genes show a different methylation pattern in some of the genes located either on maternal or paternal chromosomes (e.g., H19, Igf-2, Igf-2R, Snrpn). As a result of the imprint, for example, Igf-2 is expressed from the paternal and H19 is expressed from the maternal chromosome [20, 27, 28].
The process of erasure of the somatic imprint occurs very early during gastrulation when the PGCs begin to migrate to the genital ridges [27, 28]. It is one of the basic mechanisms that prevents their uncontrolled proliferation, parthenogenesis, and prevents potential teratoma formation by these cells. Thus, the proper somatic imprint seems to be required for PSCs to be able to complement blastocyst development, provide nuclei for clonote formation after nuclear transfer, and to grow teratomas in immunodeficient mice. Because migrating PGCs erase the imprint, they are not able to display these “golden standard” pluripotency criteria in appropriate experimental models [26-28].
However, if plated over murine fetal fibroblasts in the presence of selected growth factors, i.e., leukemia inhibitory factor (LIF), basic fibroblast growth factor (bFGF), and kit ligand, PGCs may undergo epigenetic changes forced by in vitro culture conditions that regain the somatic imprint, which endows them with “immortality” [29, 30]. Thus, this change of “PGC fate” is connected with a proper re-methylation of somatic imprinted genes. This immortalized population of PGCs known as embryonic germ cells (EGCs) is in many aspects the equivalent to embryonic (E)SCs [31]. For example, similarly to ESCs, EGCs contribute to all three germ layers including the germ cell lineage after injection into a blastocyst (blastocyst complementation assay), provide functional nuclei for clonote after nuclear transfer, and, after injection into living mice, these cells form teratomas [21, 31]. Thus, it is evident that a proper somatic imprint is vital for cells from the germ line to retain full pluripotentiality. Based on this, we postulate that erasure of the somatic imprint of some crucial developmentally genes is involved in controlling the quiescent status of VSELs [20, 32]. However, as in the case of PGCs, this process should be potentially reversible. As such, we will have to focus on reestablishing the proper somatic imprint in these cells. We envision that such reverted VSELs could potentially become equivalent to cells isolated from the embryos, e.g., after nuclear transfer or even to inducible (i)PSCs [33, 34].
The hunt for PSCs in the adult body
The presence of PSCs in adult tissues was postulated in the past by several investigators, but such cells were never purified at the single cell level. A few years ago, our team began to search for such cells in murine BM. Based on our preliminary experimental data, we assumed that the cells we were looking for would be CD133+ CXCR4+ in mice and humans as well as Sca-1+ in mice. We also assumed that they would be negative in both species for the pan-hematopoietic marker, which is the CD45 antigen (CD45-) [8, 35, 36]. In addition, our preliminary chemotactic experiments revealed that BM contains a very rare population of small cells (3-5 µm in diameter) that robustly respond by chemotaxis to stromal-derived factor-1 (SDF-1), which is a ligand for the CXCR4 receptor [37]. These small CXCR4+ cells expressed some early developmental markers characteristic for very primitive cells [8, 37].
Based on this information, we decided to sort a population of small (<6 µm) Sca-1+lin-CD45- cells from the murine BM and other tissues. By employing a fluorescence activated cell sorter (FACS), we isolated a population of rare Sca-1+Lin-CD45- cells from several adult tissues including BM, brain, liver, pancreas, kidney, muscles, heart, testes, and thymus [9]. As determined by real time RT-PCR (RQ-PCR) by employing sequence specific primers and by immunohistochemistry, these cells express several markers of PSCs such as SSEA-1, Oct-4, Nanog, and Rex-1 as well as Rif-1 telomerase protein [8, 9]. Based on the expression of these early developmental markers and morphology, we named these cells “very small embryonic-like stem cells (VSELs)” [8].
To isolate VSELs from the BM by FACS, we employed a novel size-based approach controlled by size bead markers (Figure 2). The overall sorting strategy was to gate in regions containing small events (2–10 µm), which is shown as Region R1 on the dot plot (Figure 2: Panel A). This region mostly contains cell debris, but also includes some rare nucleated cell events. Because it is well known that most of the sorting protocols exclude events smaller than 6 µm in diameter that contain cell debris, erythrocytes, and platelets, small VSELs are usually excluded from the sorted cell populations. Thus, in our sorting strategy to isolate VSELs and define a region of cells from which we could sort these cells, the size of the sorted cells was controlled by beads with predefined sizes (1, 2, 4, 6, 10, and 15 µm in diameter) (Figure 2: Panel A).
-

Figure 2. Sorting strategy for isolation of murine BM-derived VSELs by FACS.
BM-derived VSELs were sorted by MoFlo cell sorter (Dako, Glostrup, DEN) following immunofluorescence staining for Sca-1, CD45, and hematopoietic Lin. Panel A: Distribution of six predefined, sized beads populations according to their FSCs vs. SSCs (forward and side scatter characteristics, respectively). Gate R1 includes objects between 2 to 10µm in size after comparison to bead particles with standard sizes of 1, 2, 4, 6, 10, and 15µm (Flow Cytometry Size beads, Invitrogen; Molecular Probes, Carlsbad, CA, USA). Panel B: BMNCs visualized on dot plots showing their FSC and SSC signals related to the size and granularity/complexity of the cell, respectively. Small, agranular cells included in Region R1 are further visualized based on the expression of Sca-1 and Lin markers (Panel D). Region R2 includes only Sca-1+/Lin-, which are subsequently sorted based on CD45 marker expression into CD45- and CD45+ subpopulations visualized on histogram (Panel C). Sca-1+/Lin-/CD45- cells (VSELs) are sorted as events enclosed in a logical gate including Regions R1, R2, and R3, while Sca-1+/Lin-/CD45+ cells (HSCs) from gate include Regions R1, R2, and R4. Approximate percent contents of each cellular subpopulation are indicated on the plots.
The events enclosed in region R1 (Figure 2: Panel B), which include an average of ~50% of total collected events, are further analyzed for the expression of Sca-1 and lineage markers (Lin). The Sca-1+Lin- events shown in region R2 (Figure 2: Panel D) consist of 0.38 ± 0.05% of total analyzed BM nucleated cells (BMNCs) on average. Cells from region R2 are subsequently sorted according to the expression of CD45- as Sca-1+Lin-CD45- (region R3) and Sca-1+Lin-CD45+ (region R4) subpopulations (Figure 2: Panel C) that contain VSELs and HSCs, respectively. We found that VSELs comprise ~0.03% while HSCs are ~0.35% of total BMNCs (Figure 2: Panel C). We found that 95% of Sca-1+Lin-CD45- (VSELs) are located within the 2–6 µm size range, while 86% of Sca-1+Lin-CD45+ (HSCs) are found in the 6–10 µm size range [36]. Thus, by employing flow cytometry and the size marker beads, we confirmed our previous transmission electron microscopy (TEM) data showing that the majority of Sca-1+Lin-CD45- cells isolated from adult BM are unusually small (<6 µm) [8]. In conclusion, VSELs are larger than PB platelets and smaller than erythrocytes. Direct TEM analysis revealed that these cells display several features typical for ESCs such as small size, a large nucleus surrounded by a narrow rim of cytoplasm, and open-type chromatin (euchromatin).
ImageStream system (ISS) analysis was also employed to further evaluate the morphological features of VSELs [36]. The ISS-based analysis is a new flow cytometry-based analytical strategy that employs flow cytometry combined with microscopy. This allows for statistical analyses of various cellular parameters as well as direct visualization of cells acquired by FACS in suspension during flow analysis via high-resolution brightfield, darkfield, and fluorescence images [36]. The high resolution of ISS imaging enables the identification of objects as small as 1 µm in diameter [38-40].
In employing ISS analysis, we confirmed with greater precision that VSELs are ~3.6 μm in diameter, while Sca-1+Lin-CD45+ HSCs are larger at ~6.5 μm in diameter. We also noticed that VSELs have a significantly higher (P < 0.05) nuclear/cytoplasmic ratio as compared with HSCs (1.47 ± 0.17 and 0.82 ± 0.03, respectively). Furthermore, VSELs had significantly lower (P < 0.05) cytoplasmic area as compared with HSCs (5.41 ± 0.58 and 33.78 ± 1.68, respectively) [36]. Despite their small size, VSELs possess diploid DNA. They do not express MHC-1 and HLA-DR antigens and are CD90- CD105- CD29- [41].
Interestingly, if plated over a C2C12 murine sarcoma cell feeder layer, ~5–10% of purified VSELs are able to form spheres that resemble embryoid bodies. Cells from these VSEL-derived spheres (VSEL-DSs) are composed of immature cells with large nuclei containing euchromatin and are CXCR4+SSEA-1+Oct-4+, just like purified VSELs [8>]. Similar spheres were also formed by VSELs isolated from murine fetal liver, spleen, thymus, and kidney. Interestingly, formation of VSEL-DSs was associated with a young age in mice with no VSEL-DSs observed in cells isolated from older mice (> 2 years) [10]. This age-dependent content of VSELs in BM may somehow explain why the regeneration processes is more efficient in younger individuals. There are also differences in the content of these cells among BM mononuclear cells (MNCs) between long- and short-lived mouse strains. The concentration of these cells is much higher in the BM of long-lived (e.g., C57Bl6) as compared to short-lived (DBA/2J) mice [8]. In the future, it would be interesting to identify the genes responsible for tissue distribution and expansion of these cells, as they could be involved in controlling the life span of mammals.
Furthermore, since VSELs express several markers of PGCs (fetal-type alkaline phosphatase, Oct-4, SSEA-1, CXCR4, Mvh, Stella, Fragilis, Nobox, Hdac6), they could be closely related to a population of epiblast-derived PGCs. VSELs are also highly mobile and respond robustly to an SDF-1 gradient, adhere to fibronectin and fibrinogen, and may interact with BM-derived stromal fibroblasts [8]. Confocal microscopy and time-lapse studies revealed that these cells attach rapidly to, migrate beneath, and undergo emperipolesis in marrow-derived fibroblasts. This is explainable by fibroblasts secreting SDF-1 and other chemoattractants, which may create a homing environment for small CXCR4+ VSELs [8]. This robust interaction of VSELs with BM-derived fibroblasts has an important implication. It is possible that isolated BM and other tissues’ fibroblastic cells (e.g., MSCs, USSCs, MACSs, MAPCs, or MIAMI cells) may be to some degree contaminated by these tiny cells from the beginning. This observation may explain the unexpected plasticity of marrow-derived fibroblastic cells, e.g., MSCs.
Recently, evidence has also mounted to suggest that similar cells corresponding to those found in murine tissues are also present particularly in the human BM, umbilical cord blood (UCB), and mobilized (m)PB (Table I). Overall, it is anticipated that VSELs could become an important source of PSCs for regeneration. Thus, researchers working with animal models must determine whether these cells could be efficiently employed in the clinic or whether they are merely developmental remnants found in the BM that cannot be harnessed effectively for regeneration. Our initial collaborative studies indicate an efficacy of these cells in improving heart function in an animal model of acute myocardiac infarction in mice [42, 43]. We anticipate seeing similar phenomena in humans.
Table I. Morphological and phenotypic comparison of murine and human VSELs.

VSELs as circulating “paramedics” in the body
Our data also indicates that VSELs may be released during stress situations or tissue/organ injury from their tissue niches and circulate in the PB both in humans (e.g., after heart infarct or stroke) and in mice (e.g., after granulocyte colony growth factor [G-CSF]-induced mobilization, experimental heart infarct and stroke, as well as liver and skeletal muscle injury) [44-46]. The trafficking of VSELs is orchestrated by several chemotactic factors that are upregulated in damaged tissues during tissue organ injury such as α-chemokine SDF-1, hepatocyte growth factor/scatter factor (HGF/SF), LIF, and vascular endothelial growth factor (VEGF) [47-50]. Complement cascade cleavage fragments also play an important role in this process, such as C3a anaplylatoxin for example, which enhances responsiveness of VSELs to SDF-1 gradient (Figure 3). Thus, a concept emerged where chemotactic factors that are upregulated in damaged tissues may orchestrate the release of non-hematopoietic SCs from BM into mPB.
For instance, in a murine model of G-CSF-induced mobilization, we noticed that VSELs are detectable at a very low level in steady state conditions in murine PB (~160 cells/ml) and that their number increases ~6 times during G-CSF-induced mobilization events [44]. Increases in the number of these cells circulating in PB are further supported by an increase in expression of mRNA for early developmental markers expressed in VSELs, such as the embryonic transcription factors Oct-4, Nanog, and Rex-1 as well as the expression of Rif1 and Dppa3 [44]. Furthermore, at the same time, MNCs mobilized into PB are highly enriched for mRNA for several early developmental tissue-specific markers, a phenomenon that could be explained as mentioned above by the open-type status of chromatin in these cells. Finally, we sorted these rare cells from murine PB by FACS for immunofluorescence staining and provided evidence that they express SSEA-1 antigen on the surface and Oct-4 in the nucleus.
To provide evidence that mobilized VSELs not only express PSC markers but also are able to differentiate into cells from all three germ layers, we performed differentiation studies in vitro. To provide such proof, VSELs were cultured in appropriate differentiation media on the layer of BM-derived stromal support. We found that mobilized VSELs are able to differentiate into cardiomyocytes, neurons, and pancreatic cell-like clusters [44]. The analysis of DNA content in GFP+ cells isolated from the co-cultures excluded the contribution of cell fusion to this effect. Thus, these experiments revealed the in vitro pluripotency of VSELs mobilized by G-CSF and circulating in mPB by demonstrating their ability to differentiate into cells from all three germ layers. We envision that VSELs mobilized into PB in humans, such as after G-CSF administration, could be harvested by leucopheresis as a potential source of SCs for regenerative medicine.
-

Figure 3. VSELs are mobilized into PB.
Panel A: Under normal steady state conditions, VSELs may circulate in PB to keep a pool of SCs in balance in distant niches of the same tissue.
Panel B: The number of these cells increases during stress related to organ/tissue damage. During organ damage (e.g., heart infarct), the level of SDF-1 is upregulated in the affected tissues and C3 becomes activated leading to the accumulation of C3 cleavage fragments (C3a and desArgC3a). C3 cleavage fragments enhance/prime the responsiveness of circulating CXCR4+ SCs to an SDF-1 gradient. This leads to more efficient chemoattraction of SCs for potential regeneration of the damaged tissue by creating “a super gradient,” as shown in Panel B for infracted myocardium, for example. In addition to SDF-1, other chemoattractants also play important roles here (e.g., HGF/SF, LIF, and VEGF).
Do Oct-4+ VSELs initiate tumor development?
Several investigators have proposed theories regarding cancer formation in the germ cell compartment. Accordingly, Recamier (1829), Remak (1854), and Virchow (1958) proposed that cancer arises from embryo-like cells. Subsequently, Durante and J. Cohnheim in 1874 and 1875, respectively, suggested adult tissues contain embryonic remnants that normally lie dormant, but can be activated to become cancerous. In 1910, Wright proposed the germinal cell origin of Willm’s tumor (nephroblastoma) and in 1911, J. Beard postulated that tumors arise from displaced trophoblast or activated germ cells. We envision the Oct-4+ VSEL recently identified in adult tissues could unify and fully support all these theories. First, we envision that if the genomic imprint in VSELs is not erased, they may retain post-developmental in vivo pluripotency and grow teratomas and teratocarcinomas [5, 20]. Second, if they are closely related to migratory PGCs, which go astray from the major migratory route to the genital ridges, they may ultimately give rise to germinomas and seminomas, for example. Third, if these cells acquire critical mutations, they may develop into the several types of pediatric sarcomas (e.g., rhabdomyosarcoma, neuroblastoma, Ewing-sarcoma, or Willm's tumor). In support of this, there is a strong correlation between the number of these Oct-4+ cells that persist in postnatal tissues and the coincidence with these types of tumors in pediatric patients. Finally, it is possible that these cells, if mobilized at the wrong time into the PB and deposited in areas of chronic inflammation, may not play a role in regeneration but may contribute to the development of other malignancies (e.g., stomach cancer or lung cancer). To support this further, several tumor types may express embryonic markers including Oct-4 and, as reported, BM-derived SCs that may develop in the presence of carcinogens to some sarcomas or teratomas. Furthermore, we hypothesize that VSELs hiding among BM-derived fibroblasts could also be responsible for sarcoma formation by MSC cells. Circulating VSELs also could be also chemoattracted by the hypoxic/chemoattractant-rich environment of a growing tumor and provide stroma and vessels for expanding that tumor. Finally, it is also possible that circulating VSELs or cells very closely related to this population may also act in progressive fibrosis of some organs such as the lung.
Closing remarks
Several attempts have been made in the past few years to purify a population of PSCs from adult tissues including BM, UCB, and mPB that could give rise in vitro to cells from all three germ layers (meso-, ecto-, and endoderm) [8, 16, 44] and in vivo as well as in mice after injection into the developing blastocyst that would contribute to the development of multiple organs and tissues. In contrast to positive data in vitro, this latter criterion for pluripotentiality in vivo for several potential candidates for PSCs has not yet been demonstrated in a reproducible manner with any SC type isolated from the adult tissues. This is also true for VSELs. The reason for this could be that PSCs deposited in adult tissues erase the imprint on some crucial maternal or paternal imprinted genes. This phenomenon keeps these cells under control from unleashed proliferation and not only prevents the possibility of teratoma formation in vivo by these cells, but also simultaneously will affect their ability to complete blastocyst development after injection into developing blastocyst.
VSELs isolated from adult tissues are an alternative and not ethically controversial source of SCs for regenerative medicine. However, there are several missing answers to this timely issue, especially in view of the current and widely performed clinical trials with BM-derived SCs in cardiology and neurology, before VSELs can find their potential application in regenerative medicine.
First, there is the obvious problem of isolating a sufficient number of VSELs from the BM, UCB, or mPB. The number of these cells among BM MNCs is very low. For example, VSELs represent ~1 cell in 105 of BM MNCs [8, 35, 36]. Furthermore, our data shows that these cells are enriched in the BM of young mammals and their number decreases with age. It is also likely that if VSELs are released from the BM, even if they are able to home to the areas of tissue/organ injury, they may function only in the regeneration of minor tissue injuries. Heart infarct or stroke, on the other hand, may involve severe tissue damage beyond the effective repair capacity of these rare cells. Second, the allocation of these cells to the damaged areas depends on homing signals that may be inefficient in the presence of proteolytic enzymes released from leukocytes and macrophages associated with damaged tissue. For example, matrix metalloproteinases (MMPs) released from inflammatory cells may degrade SDF-1 locally and perturb homing of CXCR4+ SCs [51]. Thus, VSEL-SCs may potentially circulate as a homeless population of SCs in PB and return to the BM or home to other organs. Third, to reveal their full regenerative potential, these cells have to be fully functional. We cannot exclude the possibility that VSEL-SCs, while residing or being trapped in the BM, not only erase appropriate methylation on differently methylated regions of some important somatic imprinted genes but also are not fully functional and remain locked in a dormant state. They require the appropriate activation signals by unidentified factors. Finally, we have to develop efficient ex vivo culture conditions that will allow for efficient expansion of VSEL-SCs without supportive feeder layer cells (e.g., C2C12, BM-derived fibroblasts).
Nevertheless, our data strongly indicates that VSEL-SCs could potentially provide a therapeutic alternative to the controversial use of human ESCs and strategies based on therapeutic cloning. Hence, while the ethical debate on the application of ESCs in therapy continues, the potential of VSELs is ripe for exploration. The current work in our laboratory indicates that VSELs could be efficiently employed in the realm of regenerative medicine and that they are physiologically more important than merely being potential developmental remnants. Finally, we believe that the controlled modulation of somatic imprint status in VSELs such as we hypothesized, a proper de novo methylation of somatic imprinted genes on maternal and paternal chromosomes, could increase a regenerative power of these cells. The coming years will bring important answers to these questions.
References
1. Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872-884.
2. Darr H, Benvenisty N. Human embryonic stem cells: the battle between self-renewal and differentiation. Regen Med. 2006;1:317-325.
3. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634-7638.
4. O'Farrell PH, Stumpff J, Su TT. Embryonic cleavage cycles: how is a mouse like a fly? Curr Biol. 2004;14:R35-45.
5. Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W, Kucia M: Hunt for pluripotent stem cell - Regenerative medicine search for almighty cell. J Autoimmun. 2008;30:151-162.
6. McLaren A, Lawson KA. How is the mouse germ-cell lineage established? Differentiation. 2005;73:435-437.
7. Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J, Kucia M. Very Small Embryonic-Like (VSEL) Stem Cells: Purification from Adult Organs, Characterization, and Biological Significance. Stem Cell Rev. 2008;4:89-99.
8. Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857-869.
9. Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr., Ratajczak J, Ratajczak MZ. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A. 2008 Oct;24.
10. Zuba-Surma EK, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Very small embryonic-like stem cells in adult tissues-Potential implications for aging. Mech Ageing Dev. 2008 Feb;14.
11. Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188-189.
12. Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti CA. Identification and initial characterization of spore-like cells in adult mammals. J Cell Biochem. 2001;80:455-460.
13. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74.
14. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49.
15. D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971-2981.
16. Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow). Blood. 2007;110:3438-3446.
17. Gordon MY. Stem cells for regenerative medicine--biological attributes and clinical application. Exp Hematol. 2008;36:726-732.
18. Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M'Hamdi H, Thalji T, Welsh JP, Marley SB, Davies J, Dazzi F, Marelli-Berg F, Tait P, Playford R, Jiao L, Jensen S, Nicholls JP, Ayav A, Nohandani M, Farzaneh F, Gaken J, Dodge R, Alison M, Apperley JF, Lechler R, Habib NA. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830.
19. Donovan PJ. The germ cell - the mother of all stem cells. Int J Dev Biol. 1998;42:1043-1050.
20. Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4+ stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860-867.
21. Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227-233.
22. McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1-15.
23. De Felici M, McLaren A. In vitro culture of mouse primordial germ cells. Exp Cell Res. 1983;144:417-427.
24. Macchiarini P, Ostertag H. Uncommon primary mediastinal tumours. Lancet Oncol. 2004;5:107-118.
25. Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210-222.
26. Yamazaki Y, Mann MR, Lee SS, Marh J, McCarrey JR, Yanagimachi R, Bartolomei MS. Reprogramming of primordial germ cells begins before migration into the genital ridge, making these cells inadequate donors for reproductive cloning. Proc Natl Acad Sci U S A. 2003;100:12207-12212.
27. Lee J, Inoue K, Ono R, Ogonuki N, Kohda T, Kaneko-Ishino T, Ogura A, Ishino F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development. 2002;129:1807-1817.
28. Mann JR. Imprinting in the germ line. Stem Cells. 2001;19:287-294.
29. Donovan PJ. Growth factor regulation of mouse primordial germ cell development. Curr Top Dev Biol. 1994;29.
30. Resnick JL, Ortiz M, Keller JR, Donovan PJ. Role of fibroblast growth factors and their receptors in mouse primordial germ cell growth. Biol Reprod. 1998;59:1224-1229.
31. Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841-847.
32. Kucia M, Wu W, Ratajczak MZ. Bone marrow-derived very small embryonic-like stem cells: Their developmental origin and biological significance. Dev Dyn. 2007;12:3309-20.
33. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
34. Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-324.
35. Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4(+) SSEA-4(+) Oct-4(+) very small embryonic-like cells purified from human cord blood - preliminary report. Leukemia. 2007;21:297-303.
36. Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawnn B, Hall B, Singh R, Lillard JW, Ratajczak MZ. Morphological characterization of Very Small Embryonic-Like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292-303.
37. Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52-57.
38. Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med. 2007;27:653-670.
39. Zuba-Surma EK, Kucia M, Abdel-Latif A, Lillard JJ, Ratajczak MZ. The ImageStream System: a key step to a new era in imaging. Folia Histochem Cytobiol. 2007;45:279-290.
40. Zuba-Surma EK, Kucia M, Ratajczak MZ. “Decoding of Dot”: The ImageStream System (ISS) as a Supportive Tool for Flow Cytometric Analysis. Cent Eur J Biol. 2008;3:1-10.
41. Kucia M, Wysoczynski M, Ratajczak J, Ratajczak MZ. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331:125-134.
42. Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646-1655.
43. Zuba-Surma EK, Taher H, Kucia M, Guo Y, SanganalMath SK, Hunt G, Vincent RJ, Abdel-Latif A, Dawn B, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived Very Small Embryonic-Like stem cells (VSELs) improves left ventricular function and remodeling after myocardial infarction. Circulation. 2007:204.
44. Kucia M, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that Very Small Embryonic Like (VSEL) Stem Cells are Mobilized into Peripheral Blood. Stem Cells. 2008;26:2083-2092.
45. Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865-873.
46. Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213-3220.
47. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858-864.
48. Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331-1339.
49. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434-438.
50. Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772-1784.
51. McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503-43508.
"
["DETAIL_TEXT_TYPE"]=>
string(4) "html"
["~DETAIL_TEXT_TYPE"]=>
string(4) "html"
["PREVIEW_TEXT"]=>
string(0) ""
["~PREVIEW_TEXT"]=>
string(0) ""
["PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["~PREVIEW_TEXT_TYPE"]=>
string(4) "text"
["PREVIEW_PICTURE"]=>
NULL
["~PREVIEW_PICTURE"]=>
NULL
["LANG_DIR"]=>
string(4) "/ru/"
["~LANG_DIR"]=>
string(4) "/ru/"
["SORT"]=>
string(3) "500"
["~SORT"]=>
string(3) "500"
["CODE"]=>
string(100) "unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya"
["~CODE"]=>
string(100) "unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya"
["EXTERNAL_ID"]=>
string(3) "825"
["~EXTERNAL_ID"]=>
string(3) "825"
["IBLOCK_TYPE_ID"]=>
string(7) "journal"
["~IBLOCK_TYPE_ID"]=>
string(7) "journal"
["IBLOCK_CODE"]=>
string(7) "volumes"
["~IBLOCK_CODE"]=>
string(7) "volumes"
["IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["~IBLOCK_EXTERNAL_ID"]=>
string(1) "2"
["LID"]=>
string(2) "s2"
["~LID"]=>
string(2) "s2"
["EDIT_LINK"]=>
NULL
["DELETE_LINK"]=>
NULL
["DISPLAY_ACTIVE_FROM"]=>
string(0) ""
["IPROPERTY_VALUES"]=>
array(18) {
["ELEMENT_META_TITLE"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["ELEMENT_META_KEYWORDS"]=>
string(175) "эмбрионоподобные стволовые клетки (МСКЭ) плюрипотентность импринтинг мобилизация CXCR4 Oct-4 Nanog SSEA"
["ELEMENT_META_DESCRIPTION"]=>
string(469) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствияA unique population of mobile very small embryonic/epiblast like (VSEL) stem cells resides in adult tissues: physiological and pathological consequences"
["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=>
string(2847) "<p class="bodytext">Накапливаются сведения о том, что ткани взрослого организма содержат популяцию весьма примитивных плюрипотентных стволовых клеток (СК). В недавних исследованиях наша группа провела идентификацию небольших по размеру стволовых клеток в костном мозге мыши и других органах взрослыго организма. Эти клетки экспрессируют маркеры, характерные для стволовых клеток, происходящих эпибласта/зародышевых клеток. Мы назвали эти клетки «очень маленькими стволовыми клетками, схожими с эмбриональным» (МСКЭ). Мы предположили, что эти клетки, которые накапливаются в период ранней гаструляции в развивающихся тканях/органах, играют важную роль в обороте тканеспецифических/коммитированных популяций СК. На основании этого, мы допускаем, что зародышевая линия клеток является не только источником, но и «основой или костяком» для фракции стволовых клеток во взрослом организме. Мы показали, что МСКЭ могут быть мобилизованы в периферическую кровь, и число этих циркулирующих клеток повышается в период стресса и повреждений тканей/органов (например, при инфаркте миокарда, инсульте). Кроме того, наши данные указывают на то, что МСКЭ защищены от неконтролируемой пролиферации и образования тератом вследствие уникального типа метилирования отдельных генов, который реализуется по механизму соматического геномного импринтинга. Наконец, мы предполагаем, что МСКЭ в патологических ситуациях могут быть вовлечены в развитие некоторых злокачественных заболеваний (например, таратом, герминальных опухолей, сарком в детском возрасте).</p>"
["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_META_TITLE"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_META_KEYWORDS"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_META_DESCRIPTION"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_PICTURE_FILE_ALT"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_PICTURE_FILE_TITLE"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_PICTURE_FILE_NAME"]=>
string(100) "unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya"
["SECTION_DETAIL_PICTURE_FILE_ALT"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_DETAIL_PICTURE_FILE_TITLE"]=>
string(317) "Уникальная популяция мобильных небольших эмбрионоподобных стволовых клеток (МСКЭ) сохраняется в тканях взрослого организма: физиологические и патологические последствия"
["SECTION_DETAIL_PICTURE_FILE_NAME"]=>
string(100) "unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya"
["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=>
string(100) "unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya"
["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=>
string(100) "unikalnaya-populyatsiya-mobilnykh-nebolshikh-embrionopodobnykh-stvolovykh-kletok-mske-sokhranyaetsya"
}
["FIELDS"]=>
array(1) {
["IBLOCK_SECTION_ID"]=>
string(2) "10"
}
["PROPERTIES"]=>
array(18) {
["KEYWORDS"]=>
array(36) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(8) {
[0]=>
string(5) "11348"
[1]=>
string(5) "11349"
[2]=>
string(5) "11350"
[3]=>
string(5) "11351"
[4]=>
string(5) "11352"
[5]=>
string(5) "11353"
[6]=>
string(5) "11354"
[7]=>
string(5) "11355"
}
["VALUE"]=>
array(8) {
[0]=>
string(3) "817"
[1]=>
string(3) "818"
[2]=>
string(3) "819"
[3]=>
string(3) "820"
[4]=>
string(3) "821"
[5]=>
string(3) "822"
[6]=>
string(3) "823"
[7]=>
string(3) "824"
}
["DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(8) {
[0]=>
string(3) "817"
[1]=>
string(3) "818"
[2]=>
string(3) "819"
[3]=>
string(3) "820"
[4]=>
string(3) "821"
[5]=>
string(3) "822"
[6]=>
string(3) "823"
[7]=>
string(3) "824"
}
["~DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["SUBMITTED"]=>
array(36) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11084"
["VALUE"]=>
string(10) "30.10.2088"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "30.10.2088"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
}
["ACCEPTED"]=>
array(36) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11085"
["VALUE"]=>
string(10) "21.11.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "21.11.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
}
["PUBLISHED"]=>
array(36) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11086"
["VALUE"]=>
string(10) "27.11.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "27.11.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
}
["CONTACT"]=>
array(36) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11087"
["VALUE"]=>
string(3) "808"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "808"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHORS"]=>
array(36) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(8) {
[0]=>
string(5) "11356"
[1]=>
string(5) "11357"
[2]=>
string(5) "11358"
[3]=>
string(5) "11359"
[4]=>
string(5) "11360"
[5]=>
string(5) "11361"
[6]=>
string(5) "11362"
[7]=>
string(5) "11363"
}
["VALUE"]=>
array(8) {
[0]=>
string(3) "808"
[1]=>
string(3) "809"
[2]=>
string(3) "810"
[3]=>
string(3) "811"
[4]=>
string(3) "812"
[5]=>
string(3) "813"
[6]=>
string(3) "814"
[7]=>
string(3) "815"
}
["DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(8) {
[0]=>
string(3) "808"
[1]=>
string(3) "809"
[2]=>
string(3) "810"
[3]=>
string(3) "811"
[4]=>
string(3) "812"
[5]=>
string(3) "813"
[6]=>
string(3) "814"
[7]=>
string(3) "815"
}
["~DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_RU"]=>
array(36) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11114"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(197) "<p class="Autor">Ратайчак М.З., Кучал М., Шин Д.М., Руи Л., Друкала Ю., Марлиш В., Ратайчак Я., Зуба-Сурма Э.К.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(175) "
Ратайчак М.З., Кучал М., Шин Д.М., Руи Л., Друкала Ю., Марлиш В., Ратайчак Я., Зуба-Сурма Э.К.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_RU"]=>
array(36) {
["ID"]=>
string(2) "26"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(22) "Организации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "26"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(22) "Организации"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_RU"]=>
array(36) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11115"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(2847) "<p class="bodytext">Накапливаются сведения о том, что ткани взрослого организма содержат популяцию весьма примитивных плюрипотентных стволовых клеток (СК). В недавних исследованиях наша группа провела идентификацию небольших по размеру стволовых клеток в костном мозге мыши и других органах взрослыго организма. Эти клетки экспрессируют маркеры, характерные для стволовых клеток, происходящих эпибласта/зародышевых клеток. Мы назвали эти клетки «очень маленькими стволовыми клетками, схожими с эмбриональным» (МСКЭ). Мы предположили, что эти клетки, которые накапливаются в период ранней гаструляции в развивающихся тканях/органах, играют важную роль в обороте тканеспецифических/коммитированных популяций СК. На основании этого, мы допускаем, что зародышевая линия клеток является не только источником, но и «основой или костяком» для фракции стволовых клеток во взрослом организме. Мы показали, что МСКЭ могут быть мобилизованы в периферическую кровь, и число этих циркулирующих клеток повышается в период стресса и повреждений тканей/органов (например, при инфаркте миокарда, инсульте). Кроме того, наши данные указывают на то, что МСКЭ защищены от неконтролируемой пролиферации и образования тератом вследствие уникального типа метилирования отдельных генов, который реализуется по механизму соматического геномного импринтинга. Наконец, мы предполагаем, что МСКЭ в патологических ситуациях могут быть вовлечены в развитие некоторых злокачественных заболеваний (например, таратом, герминальных опухолей, сарком в детском возрасте).</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(2825) "
Накапливаются сведения о том, что ткани взрослого организма содержат популяцию весьма примитивных плюрипотентных стволовых клеток (СК). В недавних исследованиях наша группа провела идентификацию небольших по размеру стволовых клеток в костном мозге мыши и других органах взрослыго организма. Эти клетки экспрессируют маркеры, характерные для стволовых клеток, происходящих эпибласта/зародышевых клеток. Мы назвали эти клетки «очень маленькими стволовыми клетками, схожими с эмбриональным» (МСКЭ). Мы предположили, что эти клетки, которые накапливаются в период ранней гаструляции в развивающихся тканях/органах, играют важную роль в обороте тканеспецифических/коммитированных популяций СК. На основании этого, мы допускаем, что зародышевая линия клеток является не только источником, но и «основой или костяком» для фракции стволовых клеток во взрослом организме. Мы показали, что МСКЭ могут быть мобилизованы в периферическую кровь, и число этих циркулирующих клеток повышается в период стресса и повреждений тканей/органов (например, при инфаркте миокарда, инсульте). Кроме того, наши данные указывают на то, что МСКЭ защищены от неконтролируемой пролиферации и образования тератом вследствие уникального типа метилирования отдельных генов, который реализуется по механизму соматического геномного импринтинга. Наконец, мы предполагаем, что МСКЭ в патологических ситуациях могут быть вовлечены в развитие некоторых злокачественных заболеваний (например, таратом, герминальных опухолей, сарком в детском возрасте).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["DOI"]=>
array(36) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11096"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000023.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000023.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["AUTHOR_EN"]=>
array(36) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11142"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(374) "<p class="Autor">Mariusz Z. Ratajczak<sup>1,2</sup>, Magda Kucia<sup>1</sup>, Dong-Myung Shin<sup>1</sup>, Liu Rui<sup>1</sup>, Justyna Drukala<sup>1</sup>, Wojtek Marlicz<sup>2</sup>, Janina Ratajczak<sup>1</sup>, Ewa K. Zuba-Surma<sup>1</sup></p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(256) "
Mariusz Z. Ratajczak1,2, Magda Kucia1, Dong-Myung Shin1, Liu Rui1, Justyna Drukala1, Wojtek Marlicz2, Janina Ratajczak1, Ewa K. Zuba-Surma1
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["ORGANIZATION_EN"]=>
array(36) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11143"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(299) "<p class="bodytext"><sup>1</sup>Stem Cell Institute at James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202, USA; <br>
<sup>2</sup>Department of Physiopathology, Pomeranian Medical University, Szczecin, Poland</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(247) "
1Stem Cell Institute at James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202, USA;
2Department of Physiopathology, Pomeranian Medical University, Szczecin, Poland
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["SUMMARY_EN"]=>
array(36) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11144"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1318) "<p class="bodytext">Accumulating evidence demonstrates that adult tissue contains a population of very primitive pluripotent stem cells (PSCs). Recently, our group identified a population of very small SCs in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that these cells, which are deposited during early gastrulation in developing tissues/organs, play an important role in the turnover of tissue-specific/committed SCs. Based on this, we envision that germ line is not only the origin but also a “basis/skeleton” for the SC compartment in adult life forms. We noticed that VSELs could be mobilized into peripheral blood (PB) and the number of these cells circulating in PB increases during stress and tissue/organ injuries (e.g., heart infarct, stroke). Furthermore, our data indicates that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique pattern of methylation of selected somatic imprinted genes. Finally, we envision that in pathological situations, VSELs could be involved in the development of some malignancies (e.g., teratomas, germinal tumors, pediatric sarcomas).</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1296) "
Accumulating evidence demonstrates that adult tissue contains a population of very primitive pluripotent stem cells (PSCs). Recently, our group identified a population of very small SCs in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that these cells, which are deposited during early gastrulation in developing tissues/organs, play an important role in the turnover of tissue-specific/committed SCs. Based on this, we envision that germ line is not only the origin but also a “basis/skeleton” for the SC compartment in adult life forms. We noticed that VSELs could be mobilized into peripheral blood (PB) and the number of these cells circulating in PB increases during stress and tissue/organ injuries (e.g., heart infarct, stroke). Furthermore, our data indicates that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique pattern of methylation of selected somatic imprinted genes. Finally, we envision that in pathological situations, VSELs could be involved in the development of some malignancies (e.g., teratomas, germinal tumors, pediatric sarcomas).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["NAME_EN"]=>
array(36) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11097"
["VALUE"]=>
string(152) "A unique population of mobile very small embryonic/epiblast like (VSEL) stem cells resides in adult tissues: physiological and pathological consequences"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(152) "A unique population of mobile very small embryonic/epiblast like (VSEL) stem cells resides in adult tissues: physiological and pathological consequences"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["FULL_TEXT_RU"]=>
array(36) {
["ID"]=>
string(2) "42"
["TIMESTAMP_X"]=>
string(19) "2015-09-07 20:29:18"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(23) "Полный текст"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(12) "FULL_TEXT_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "42"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(23) "Полный текст"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
}
["PDF_RU"]=>
array(36) {
["ID"]=>
string(2) "43"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF RUS"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_RU"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "43"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11116"
["VALUE"]=>
string(3) "464"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "464"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF RUS"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["PDF_EN"]=>
array(36) {
["ID"]=>
string(2) "44"
["TIMESTAMP_X"]=>
string(19) "2015-09-09 16:05:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(7) "PDF ENG"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(6) "PDF_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "F"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "44"
["FILE_TYPE"]=>
string(18) "doc, txt, rtf, pdf"
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11117"
["VALUE"]=>
string(3) "465"
["DESCRIPTION"]=>
NULL
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "465"
["~DESCRIPTION"]=>
NULL
["~NAME"]=>
string(7) "PDF ENG"
["~DEFAULT_VALUE"]=>
string(0) ""
}
["NAME_LONG"]=>
array(36) {
["ID"]=>
string(2) "45"
["TIMESTAMP_X"]=>
string(19) "2023-04-13 00:55:00"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "NAME_LONG"
["DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "45"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(80)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
NULL
["VALUE"]=>
string(0) ""
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(0) ""
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(72) "Название (для очень длинных заголовков)"
["~DEFAULT_VALUE"]=>
array(2) {
["TYPE"]=>
string(4) "HTML"
["TEXT"]=>
string(0) ""
}
}
}
["DISPLAY_PROPERTIES"]=>
array(13) {
["AUTHOR_EN"]=>
array(37) {
["ID"]=>
string(2) "37"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(6) "Author"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "37"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11142"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(374) "<p class="Autor">Mariusz Z. Ratajczak<sup>1,2</sup>, Magda Kucia<sup>1</sup>, Dong-Myung Shin<sup>1</sup>, Liu Rui<sup>1</sup>, Justyna Drukala<sup>1</sup>, Wojtek Marlicz<sup>2</sup>, Janina Ratajczak<sup>1</sup>, Ewa K. Zuba-Surma<sup>1</sup></p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(256) "
Mariusz Z. Ratajczak1,2, Magda Kucia1, Dong-Myung Shin1, Liu Rui1, Justyna Drukala1, Wojtek Marlicz2, Janina Ratajczak1, Ewa K. Zuba-Surma1
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(6) "Author"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(256) "
Mariusz Z. Ratajczak1,2, Magda Kucia1, Dong-Myung Shin1, Liu Rui1, Justyna Drukala1, Wojtek Marlicz2, Janina Ratajczak1, Ewa K. Zuba-Surma1
"
}
["SUMMARY_EN"]=>
array(37) {
["ID"]=>
string(2) "39"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Description / Summary"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "39"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11144"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(1318) "<p class="bodytext">Accumulating evidence demonstrates that adult tissue contains a population of very primitive pluripotent stem cells (PSCs). Recently, our group identified a population of very small SCs in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that these cells, which are deposited during early gastrulation in developing tissues/organs, play an important role in the turnover of tissue-specific/committed SCs. Based on this, we envision that germ line is not only the origin but also a “basis/skeleton” for the SC compartment in adult life forms. We noticed that VSELs could be mobilized into peripheral blood (PB) and the number of these cells circulating in PB increases during stress and tissue/organ injuries (e.g., heart infarct, stroke). Furthermore, our data indicates that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique pattern of methylation of selected somatic imprinted genes. Finally, we envision that in pathological situations, VSELs could be involved in the development of some malignancies (e.g., teratomas, germinal tumors, pediatric sarcomas).</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(1296) "
Accumulating evidence demonstrates that adult tissue contains a population of very primitive pluripotent stem cells (PSCs). Recently, our group identified a population of very small SCs in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that these cells, which are deposited during early gastrulation in developing tissues/organs, play an important role in the turnover of tissue-specific/committed SCs. Based on this, we envision that germ line is not only the origin but also a “basis/skeleton” for the SC compartment in adult life forms. We noticed that VSELs could be mobilized into peripheral blood (PB) and the number of these cells circulating in PB increases during stress and tissue/organ injuries (e.g., heart infarct, stroke). Furthermore, our data indicates that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique pattern of methylation of selected somatic imprinted genes. Finally, we envision that in pathological situations, VSELs could be involved in the development of some malignancies (e.g., teratomas, germinal tumors, pediatric sarcomas).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Description / Summary"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(1296) "
Accumulating evidence demonstrates that adult tissue contains a population of very primitive pluripotent stem cells (PSCs). Recently, our group identified a population of very small SCs in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that these cells, which are deposited during early gastrulation in developing tissues/organs, play an important role in the turnover of tissue-specific/committed SCs. Based on this, we envision that germ line is not only the origin but also a “basis/skeleton” for the SC compartment in adult life forms. We noticed that VSELs could be mobilized into peripheral blood (PB) and the number of these cells circulating in PB increases during stress and tissue/organ injuries (e.g., heart infarct, stroke). Furthermore, our data indicates that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique pattern of methylation of selected somatic imprinted genes. Finally, we envision that in pathological situations, VSELs could be involved in the development of some malignancies (e.g., teratomas, germinal tumors, pediatric sarcomas).
"
}
["DOI"]=>
array(37) {
["ID"]=>
string(2) "28"
["TIMESTAMP_X"]=>
string(19) "2016-04-06 14:11:12"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(3) "DOI"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(3) "DOI"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "28"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11096"
["VALUE"]=>
string(29) "10.3205/ctt-2008-en-000023.01"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(29) "10.3205/ctt-2008-en-000023.01"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(3) "DOI"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(29) "10.3205/ctt-2008-en-000023.01"
}
["NAME_EN"]=>
array(37) {
["ID"]=>
string(2) "40"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:49:47"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(4) "Name"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "NAME_EN"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "80"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "40"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
NULL
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11097"
["VALUE"]=>
string(152) "A unique population of mobile very small embryonic/epiblast like (VSEL) stem cells resides in adult tissues: physiological and pathological consequences"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(152) "A unique population of mobile very small embryonic/epiblast like (VSEL) stem cells resides in adult tissues: physiological and pathological consequences"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(4) "Name"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(152) "A unique population of mobile very small embryonic/epiblast like (VSEL) stem cells resides in adult tissues: physiological and pathological consequences"
}
["ORGANIZATION_EN"]=>
array(37) {
["ID"]=>
string(2) "38"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:02:59"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Organization"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(15) "ORGANIZATION_EN"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "38"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11143"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(299) "<p class="bodytext"><sup>1</sup>Stem Cell Institute at James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202, USA; <br>
<sup>2</sup>Department of Physiopathology, Pomeranian Medical University, Szczecin, Poland</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(247) "
1Stem Cell Institute at James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202, USA;
2Department of Physiopathology, Pomeranian Medical University, Szczecin, Poland
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Organization"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(247) "
1Stem Cell Institute at James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202, USA;
2Department of Physiopathology, Pomeranian Medical University, Szczecin, Poland
"
}
["AUTHORS"]=>
array(38) {
["ID"]=>
string(2) "24"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:45:07"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "AUTHORS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "24"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(8) {
[0]=>
string(5) "11356"
[1]=>
string(5) "11357"
[2]=>
string(5) "11358"
[3]=>
string(5) "11359"
[4]=>
string(5) "11360"
[5]=>
string(5) "11361"
[6]=>
string(5) "11362"
[7]=>
string(5) "11363"
}
["VALUE"]=>
array(8) {
[0]=>
string(3) "808"
[1]=>
string(3) "809"
[2]=>
string(3) "810"
[3]=>
string(3) "811"
[4]=>
string(3) "812"
[5]=>
string(3) "813"
[6]=>
string(3) "814"
[7]=>
string(3) "815"
}
["DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(8) {
[0]=>
string(3) "808"
[1]=>
string(3) "809"
[2]=>
string(3) "810"
[3]=>
string(3) "811"
[4]=>
string(3) "812"
[5]=>
string(3) "813"
[6]=>
string(3) "814"
[7]=>
string(3) "815"
}
["~DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(8) {
[0]=>
string(63) "
Mariusz Z. Ratajczak"
[1]=>
string(54) "
Magda Kucia"
[2]=>
string(58) "
Dong-Myung Shin"
[3]=>
string(50) "
Liu Rui"
[4]=>
string(58) "
Justyna Drukala"
[5]=>
string(57) "
Wojtek Marlicz"
[6]=>
string(59) "
Janina Ratajczak"
[7]=>
string(60) "
Ewa K. Zuba-Surma"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["AUTHOR_RU"]=>
array(37) {
["ID"]=>
string(2) "25"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(12) "Авторы"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "AUTHOR_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "25"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11114"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(197) "<p class="Autor">Ратайчак М.З., Кучал М., Шин Д.М., Руи Л., Друкала Ю., Марлиш В., Ратайчак Я., Зуба-Сурма Э.К.</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(175) "
Ратайчак М.З., Кучал М., Шин Д.М., Руи Л., Друкала Ю., Марлиш В., Ратайчак Я., Зуба-Сурма Э.К.
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(12) "Авторы"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(175) "
Ратайчак М.З., Кучал М., Шин Д.М., Руи Л., Друкала Ю., Марлиш В., Ратайчак Я., Зуба-Сурма Э.К.
"
}
["SUBMITTED"]=>
array(37) {
["ID"]=>
string(2) "20"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(21) "Дата подачи"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "SUBMITTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "20"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11084"
["VALUE"]=>
string(10) "30.10.2088"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "30.10.2088"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(21) "Дата подачи"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(10) "30.10.2088"
}
["ACCEPTED"]=>
array(37) {
["ID"]=>
string(2) "21"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(25) "Дата принятия"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "ACCEPTED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "21"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11085"
["VALUE"]=>
string(10) "21.11.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "21.11.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(25) "Дата принятия"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(10) "21.11.2008"
}
["PUBLISHED"]=>
array(37) {
["ID"]=>
string(2) "22"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 17:21:42"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Дата публикации"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(9) "PUBLISHED"
["DEFAULT_VALUE"]=>
NULL
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "22"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(8) "DateTime"
["USER_TYPE_SETTINGS"]=>
NULL
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11086"
["VALUE"]=>
string(10) "27.11.2008"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(10) "27.11.2008"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Дата публикации"
["~DEFAULT_VALUE"]=>
NULL
["DISPLAY_VALUE"]=>
string(10) "27.11.2008"
}
["KEYWORDS"]=>
array(38) {
["ID"]=>
string(2) "19"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 10:46:01"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(27) "Ключевые слова"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(8) "KEYWORDS"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "Y"
["XML_ID"]=>
string(2) "19"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "4"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "Y"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "Y"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
array(8) {
[0]=>
string(5) "11348"
[1]=>
string(5) "11349"
[2]=>
string(5) "11350"
[3]=>
string(5) "11351"
[4]=>
string(5) "11352"
[5]=>
string(5) "11353"
[6]=>
string(5) "11354"
[7]=>
string(5) "11355"
}
["VALUE"]=>
array(8) {
[0]=>
string(3) "817"
[1]=>
string(3) "818"
[2]=>
string(3) "819"
[3]=>
string(3) "820"
[4]=>
string(3) "821"
[5]=>
string(3) "822"
[6]=>
string(3) "823"
[7]=>
string(3) "824"
}
["DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(8) {
[0]=>
string(3) "817"
[1]=>
string(3) "818"
[2]=>
string(3) "819"
[3]=>
string(3) "820"
[4]=>
string(3) "821"
[5]=>
string(3) "822"
[6]=>
string(3) "823"
[7]=>
string(3) "824"
}
["~DESCRIPTION"]=>
array(8) {
[0]=>
string(0) ""
[1]=>
string(0) ""
[2]=>
string(0) ""
[3]=>
string(0) ""
[4]=>
string(0) ""
[5]=>
string(0) ""
[6]=>
string(0) ""
[7]=>
string(0) ""
}
["~NAME"]=>
string(27) "Ключевые слова"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
array(8) {
[0]=>
string(119) "
эмбрионоподобные стволовые клетки (МСКЭ)"
[1]=>
string(76) "
плюрипотентность"
[2]=>
string(64) "
импринтинг"
[3]=>
string(66) "
мобилизация"
[4]=>
string(49) "
CXCR4"
[5]=>
string(49) "
Oct-4"
[6]=>
string(49) "
Nanog"
[7]=>
string(48) "
SSEA"
}
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["CONTACT"]=>
array(38) {
["ID"]=>
string(2) "23"
["TIMESTAMP_X"]=>
string(19) "2015-09-03 14:43:05"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(14) "Контакт"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(7) "CONTACT"
["DEFAULT_VALUE"]=>
string(0) ""
["PROPERTY_TYPE"]=>
string(1) "E"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "23"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "3"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "Y"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(13) "EAutocomplete"
["USER_TYPE_SETTINGS"]=>
array(9) {
["VIEW"]=>
string(1) "E"
["SHOW_ADD"]=>
string(1) "Y"
["MAX_WIDTH"]=>
int(0)
["MIN_HEIGHT"]=>
int(24)
["MAX_HEIGHT"]=>
int(1000)
["BAN_SYM"]=>
string(2) ",;"
["REP_SYM"]=>
string(1) " "
["OTHER_REP_SYM"]=>
string(0) ""
["IBLOCK_MESS"]=>
string(1) "N"
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11087"
["VALUE"]=>
string(3) "808"
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
string(3) "808"
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(14) "Контакт"
["~DEFAULT_VALUE"]=>
string(0) ""
["DISPLAY_VALUE"]=>
string(63) "
Mariusz Z. Ratajczak"
["LINK_ELEMENT_VALUE"]=>
bool(false)
}
["SUMMARY_RU"]=>
array(37) {
["ID"]=>
string(2) "27"
["TIMESTAMP_X"]=>
string(19) "2015-09-02 18:01:20"
["IBLOCK_ID"]=>
string(1) "2"
["NAME"]=>
string(29) "Описание/Резюме"
["ACTIVE"]=>
string(1) "Y"
["SORT"]=>
string(3) "500"
["CODE"]=>
string(10) "SUMMARY_RU"
["DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["PROPERTY_TYPE"]=>
string(1) "S"
["ROW_COUNT"]=>
string(1) "1"
["COL_COUNT"]=>
string(2) "30"
["LIST_TYPE"]=>
string(1) "L"
["MULTIPLE"]=>
string(1) "N"
["XML_ID"]=>
string(2) "27"
["FILE_TYPE"]=>
string(0) ""
["MULTIPLE_CNT"]=>
string(1) "5"
["TMP_ID"]=>
NULL
["LINK_IBLOCK_ID"]=>
string(1) "0"
["WITH_DESCRIPTION"]=>
string(1) "N"
["SEARCHABLE"]=>
string(1) "N"
["FILTRABLE"]=>
string(1) "N"
["IS_REQUIRED"]=>
string(1) "N"
["VERSION"]=>
string(1) "1"
["USER_TYPE"]=>
string(4) "HTML"
["USER_TYPE_SETTINGS"]=>
array(1) {
["height"]=>
int(200)
}
["HINT"]=>
string(0) ""
["PROPERTY_VALUE_ID"]=>
string(5) "11115"
["VALUE"]=>
array(2) {
["TEXT"]=>
string(2847) "<p class="bodytext">Накапливаются сведения о том, что ткани взрослого организма содержат популяцию весьма примитивных плюрипотентных стволовых клеток (СК). В недавних исследованиях наша группа провела идентификацию небольших по размеру стволовых клеток в костном мозге мыши и других органах взрослыго организма. Эти клетки экспрессируют маркеры, характерные для стволовых клеток, происходящих эпибласта/зародышевых клеток. Мы назвали эти клетки «очень маленькими стволовыми клетками, схожими с эмбриональным» (МСКЭ). Мы предположили, что эти клетки, которые накапливаются в период ранней гаструляции в развивающихся тканях/органах, играют важную роль в обороте тканеспецифических/коммитированных популяций СК. На основании этого, мы допускаем, что зародышевая линия клеток является не только источником, но и «основой или костяком» для фракции стволовых клеток во взрослом организме. Мы показали, что МСКЭ могут быть мобилизованы в периферическую кровь, и число этих циркулирующих клеток повышается в период стресса и повреждений тканей/органов (например, при инфаркте миокарда, инсульте). Кроме того, наши данные указывают на то, что МСКЭ защищены от неконтролируемой пролиферации и образования тератом вследствие уникального типа метилирования отдельных генов, который реализуется по механизму соматического геномного импринтинга. Наконец, мы предполагаем, что МСКЭ в патологических ситуациях могут быть вовлечены в развитие некоторых злокачественных заболеваний (например, таратом, герминальных опухолей, сарком в детском возрасте).</p>"
["TYPE"]=>
string(4) "HTML"
}
["DESCRIPTION"]=>
string(0) ""
["VALUE_ENUM"]=>
NULL
["VALUE_XML_ID"]=>
NULL
["VALUE_SORT"]=>
NULL
["~VALUE"]=>
array(2) {
["TEXT"]=>
string(2825) "
Накапливаются сведения о том, что ткани взрослого организма содержат популяцию весьма примитивных плюрипотентных стволовых клеток (СК). В недавних исследованиях наша группа провела идентификацию небольших по размеру стволовых клеток в костном мозге мыши и других органах взрослыго организма. Эти клетки экспрессируют маркеры, характерные для стволовых клеток, происходящих эпибласта/зародышевых клеток. Мы назвали эти клетки «очень маленькими стволовыми клетками, схожими с эмбриональным» (МСКЭ). Мы предположили, что эти клетки, которые накапливаются в период ранней гаструляции в развивающихся тканях/органах, играют важную роль в обороте тканеспецифических/коммитированных популяций СК. На основании этого, мы допускаем, что зародышевая линия клеток является не только источником, но и «основой или костяком» для фракции стволовых клеток во взрослом организме. Мы показали, что МСКЭ могут быть мобилизованы в периферическую кровь, и число этих циркулирующих клеток повышается в период стресса и повреждений тканей/органов (например, при инфаркте миокарда, инсульте). Кроме того, наши данные указывают на то, что МСКЭ защищены от неконтролируемой пролиферации и образования тератом вследствие уникального типа метилирования отдельных генов, который реализуется по механизму соматического геномного импринтинга. Наконец, мы предполагаем, что МСКЭ в патологических ситуациях могут быть вовлечены в развитие некоторых злокачественных заболеваний (например, таратом, герминальных опухолей, сарком в детском возрасте).
"
["TYPE"]=>
string(4) "HTML"
}
["~DESCRIPTION"]=>
string(0) ""
["~NAME"]=>
string(29) "Описание/Резюме"
["~DEFAULT_VALUE"]=>
array(2) {
["TEXT"]=>
string(0) ""
["TYPE"]=>
string(4) "HTML"
}
["DISPLAY_VALUE"]=>
string(2825) "
Накапливаются сведения о том, что ткани взрослого организма содержат популяцию весьма примитивных плюрипотентных стволовых клеток (СК). В недавних исследованиях наша группа провела идентификацию небольших по размеру стволовых клеток в костном мозге мыши и других органах взрослыго организма. Эти клетки экспрессируют маркеры, характерные для стволовых клеток, происходящих эпибласта/зародышевых клеток. Мы назвали эти клетки «очень маленькими стволовыми клетками, схожими с эмбриональным» (МСКЭ). Мы предположили, что эти клетки, которые накапливаются в период ранней гаструляции в развивающихся тканях/органах, играют важную роль в обороте тканеспецифических/коммитированных популяций СК. На основании этого, мы допускаем, что зародышевая линия клеток является не только источником, но и «основой или костяком» для фракции стволовых клеток во взрослом организме. Мы показали, что МСКЭ могут быть мобилизованы в периферическую кровь, и число этих циркулирующих клеток повышается в период стресса и повреждений тканей/органов (например, при инфаркте миокарда, инсульте). Кроме того, наши данные указывают на то, что МСКЭ защищены от неконтролируемой пролиферации и образования тератом вследствие уникального типа метилирования отдельных генов, который реализуется по механизму соматического геномного импринтинга. Наконец, мы предполагаем, что МСКЭ в патологических ситуациях могут быть вовлечены в развитие некоторых злокачественных заболеваний (например, таратом, герминальных опухолей, сарком в детском возрасте).
"
}
}
}
}
Статьи о терапии мезенхимными клетками
Трансплантация кроветворных стволовых клеток при рассеянном склерозе
Ю. Л. Шевченко, А. А. Новик, А. Н. Кузнецов, Б. В. Афанасьев, И. А. Лисуков, O. А. Рукавицын, А. А. Мясников,
В. Я. Мельниченко, Д. А. Федоренко, T. И. Ионова, Р. А. Иванов, Г. Городокин
Предварительные данные клинического использования мезенхимных стволовых клеток для профилактики и лечения РТПХ у пациентов после аллогенной ТГСК
Станкевич Ю. А., Головачева А. А., Бабенко Е. В., Алянский А. Л., Паина О. В., Зубаровская Л. С., Семенова E. В., Полынцев Д. Г., Кругляков П. В., Афанасьев Б. В.
Первичный отчет о фазе I клинических испытаний: профилактика и лечение острого послеоперационного повреждения почек аллогенными мезенхимными стволовыми клетками у кардиохирургических больных при операциях на открытом сердце
Гуч А., Доти Дж., Флорес Дж., Свенсон Л., Тегель Ф., Райсс Р. Г., Ланге К., Цандер А. Р., Ху Дж., Пул С., Жанг П., Вестенвельдер К.