Сергей Ф. Багненко, Аксель Р. Цандер, Герард Вагемакер, Рюдигер Хельманн, Борис Фезе, Александр Д. Кулагин, Людмила С. Зубаровская, Иван С. Моисеев, Инна В. Маркова, Алексей Б. Чухловин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "25943" ["VALUE"]=> array(2) { ["TEXT"]=> string(16260) "<p style="text-align: justify;">Санкт-Петербургский государственный медицинский университет им. И.П. Павлова потерял харизматического лидера, создавшего в Санкт-Петербурге признанный в мире ведущий центр по исследованию лейкозов и трансплантации стволовых гемопоэтических клеток.</p> <p style="text-align: justify;">С глубоким прискорбием извещаем наших читателей о безвременной кончине главного редактора журнала «Клеточная Терапия и Трансплантация». Профессор Борис Владимирович Афанасьев, директор НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой Первого Санкт-Петербургского государственного медицинского университета им. И.П. Павлова ушел из жизни 16 марта 2020 г. На протяжении последних трех десятилетий он был главным вдохновителем разработок по трансплантации стволовых кроветворных клеток в России, а также в области химиотерапии, иммунотерапии и таргетного лечения злокачественных и неопухолевых заболеваний системы крови, будучи широко известным как в России, так и в мире.</p> <p style="text-align: justify;">В 1971 г. Борис Владимирович Афанасьев с отличием окончил Первый Ленинградский медицинский институт. С 1973 г. он был ординатором в клинике факультетской терапии по специальности «Внутренние болезни». Будучи аспирантом (1974-1976), он был одним из первых в области фундаментальных исследований гемопоэтических стволовых клеток. Его кандидатская диссертация «Метод клонирования гемопоэтических стволовых клеток, изучение колониестимулирующей способности клеток костного мозга и крови гематологически здоровых лиц и больных с различными нейтропеническими состояниями» (1977) была посвящена регуляции роста нормальных и лейкозных клеток-предшественников гемопоэза, изученных в тест-системе «агаровой капли». В дальнейшем его наиболее выдающиеся исследования культур стволовых клеток включали описание терминальной дифференцировки лейкозных стволовых клеток.</p> <p style="text-align: justify;">Б.В. Афанасьев осуществил также ряд пионерских работ в области стромальной регуляции гемопоэза мезенхимными стволовыми клетками и показал, что они оказывают как стимулирующие, так и модулирующие эффекты на клетки-предшественники гранулоцитов, которые показаны им в разработанной им системе «агаровая капля в жидкой среде».</p> <p style="text-align: justify;">В 1976-1979 гг. Б.В. Афанасьев возглавлял отделение гематологии клиники факультетской терапии. В 1982 г., на основании данных о характере роста гемопоэтических клеток <i>in vitro</i>, он первым описал миелодиспдастический синдром у детей. Его докторская диссертация была посвящена теме «Грануломоноцитопоэз при остром лейкозе и бластном кризе» (1983). Он был одним из первых, кто описал клеточные механизмы миелоидного и лимфоидного типов бластного криза при хроническом миелоидном лейкозе. В 1985 г. он опубликовал широко известную монографию «Родоначальные кроветворные клетки человека» в соавторстве с профессором В.А. Алмазовым. В течение последующих 8 лет Б.В. Афанасьев был старшим научным сотрудником при кафедре факультетской терапии Первого Ленинградского медицинского института им. Павлова, где изучал биологические механизмы злокачественных клеток при острых и хронических лейкозах, тяжелых нейтропениях.</p> <p style="text-align: justify;">В 1986 г. Б.В. Афанасьев проходил клиническую стажировку в знаменитом онкологическом центре Фреда Хатчинсона в Сиэттле (США) под руководством Нобелевского лауреата, профессора Доннелла Томаса. В 1987 г. Б.В. Афанасьев организовал первое отделение трансплантации костного мозга (ТКМ) в Советском Союзе на базе НИИ онкологии им. Н.Н. Петрова. В 1991 г. он выполнил первую в России аллогенную ТКМ ребенку.</p> <p style="text-align: justify;">В 2000 он организовал первое университетское отделение ТКМ в России на базе Первого Санкт-Петербургского государственного медицинского университета им. И. П. Павлова (ПСПбГМУ). В 2003 г. он основал кафедру гематологии, трансфузиологии и трансплантологии последипломного образования в ПСПбГМУ.</p> <p style="text-align: justify;">В 2007 г., по инициативе Б.В. Афанасьева и ПСПбГМУ, при поддержке Фонда Горбачева и банка «Национальный Резерв», был построен НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой в составе ПСПбГМУ им. И.П. Павлова. С 2007 г. профессор Б.В. Афанасьев был директором этого института.</p> <p style="text-align: justify;">Интересы Б.В. Афанасьева на протяжении последнего десятилетия были сосредоточены на разработке протоколов лечения больных с рецидивирующими и рефрактерными формами злокачественных заболеваний. Под его руководством созданы новые протоколы промежуточной (bridging) и посттрансплантационной терапии таргетными лекарственными агентами и моноклональными антителами. Он также был сосредоточен на долгосрочных осложнениях ТКМ у детей и взрослых, и был одним из первых в применении режимов кондиционирования сниженной интенсивности у детей, что приводило к менее выраженным долговременным токсическим эффектам. Научное наследие профессора Б.В. Афанасьева включает более 300 научных статей и 6 монографий. Эти клинические исследования позволили значительно расширить трансплантационные программы, которые стали самыми большими в России и одной из самых больших в Европе.</p> <p style="text-align: justify;">С 1990 г. Б.В. Афанасьев прилагал усилия к развитию Регистра неродственных доноров костного мозга в России. Эта работа в 2013 г. привела к созданию кооперативной базы данных, которая сейчас способствует проведению примерно половины неродственных трансплантаций в России. На уровне правительства принят план расширения базы данных до Национальной программы неродственных доноров костного мозга, что также является наследием профессора Б.В. Афанасьева.</p> <p style="text-align: justify;">На протяжении всех тех лет, которые вели к успеху программы ТКМ, организованной Б.В. Афанасьевым, он был глубоко благодарен поддержке коллег и друзей за рубежом: Рольфа Нета, Клауса Винклера, Акселя Цандера, Николауса Крегера, Бориса Фезе (Гамбург Германия), Томаса Бюхнера (Мюнстер, Германия), Дитера Хельцера (Франкфурт, Германия), Ханса-Иохена Кольба (Мюнхен, Германия), Герарда Вагемакера (Роттердам, Нидерланды), Роберта Гэйла (Лондон, Великобритания), Андреа Бачигалупо (Рим, Италия), Магне Борсета (Трондхейм, Норвегия), Арнона Наглера (Тель-Авив, Израиль), Тапани Рууту (Хельсинки, Финляндия) и многих других, кто способствовал обучению более чем 70 российских гематологов в европейских центрах.</p> <p style="text-align: justify;">Начиная с 2007 г., ежегодные научные форумы памяти Р.М. Горбачевой в медицинском университете им. И. Павлова стали центральными событиями в России и международном сообществе врачей и исследователей лейкозов, в целях образования, сотрудничества, обмена мнениями, новыми научными данными и распространения передового опыта в области трансплантации стволовых клеток.</p> <p style="text-align: justify;">Профессор Борис В. Афанасьев был членом редакционных советов многих российских и международных журналов. В качестве главного редактора он играл ведущую роль в основании и развитии журнала «Клеточная Терапия и Трансплантация», где российские и зарубежные авторы могли представлять данные своих исследований по онкологии, гематологии и в смежных областях для международного сообщества. Нам, его коллегам и друзьям, а также всему сообществу гематологов и специалистов по трансплантации костного мозга, будет остро не хватать его огромного энтузиазма, творческого мышления и дружелюбия.</p> <p style="text-align: justify;">Борис В. Афанасьев всегда служил общей пользе и ушел из жизни так, как жил, завершив дело жизненной важности для Института, для Университета, для гематологии и для общества. Ему была присуждена награда за клинические достижения от Европейского Общества трансплантации костного мозга на конгрессе в Лиссабоне в 2018 г. У него остались жена Ольга, дочь Анастасия, внуки Эмиль-Борис и Антония.</p> <p><b>Сергей Ф. Багненко,</b><br> профессор, д.м.н., академик РАН,<br> ректор Первого Санкт-Петербургского государственного медицинского университета им. И. Павлова, Россия<br> <b>Аксель Р. Цандер,</b><br> профессор, почетный доктор, Университетский медицинский центр Гамбург-Эппендорф, Германия<br> <b>Герард Вагемакер,</b><br> профессор, почетный доктор, Университет Эразмус, Нидерланды<br> <b>Рюдигер Хельманн,</b><br> профессор, почетный доктор, Факультет Мангейм, Гейдельбергский университет, Германия<br> <b>Борис Фезе,</b><br> профессор, доктор, Университет Гамбурга, Германия<br> <b>Александр Д. Кулагин,</b> проф., д.м.н.,<br> <b>Людмила С. Зубаровская,</b> проф., д.м.н.,<br> <b> Иван С. Моисеев,</b> д.м.н.,<br> <b>Инна В. Маркова,</b> к.м.н.<br> и <b>Алексей Б. Чухловин,</b> проф. д.м.н.<br> НИИ детской онкологии, гематологии и трансплантологии <br>им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. Павлова, Россия<br> <br></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(15694) "Санкт-Петербургский государственный медицинский университет им. И.П. Павлова потерял харизматического лидера, создавшего в Санкт-Петербурге признанный в мире ведущий центр по исследованию лейкозов и трансплантации стволовых гемопоэтических клеток.
С глубоким прискорбием извещаем наших читателей о безвременной кончине главного редактора журнала «Клеточная Терапия и Трансплантация». Профессор Борис Владимирович Афанасьев, директор НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой Первого Санкт-Петербургского государственного медицинского университета им. И.П. Павлова ушел из жизни 16 марта 2020 г. На протяжении последних трех десятилетий он был главным вдохновителем разработок по трансплантации стволовых кроветворных клеток в России, а также в области химиотерапии, иммунотерапии и таргетного лечения злокачественных и неопухолевых заболеваний системы крови, будучи широко известным как в России, так и в мире.
В 1971 г. Борис Владимирович Афанасьев с отличием окончил Первый Ленинградский медицинский институт. С 1973 г. он был ординатором в клинике факультетской терапии по специальности «Внутренние болезни». Будучи аспирантом (1974-1976), он был одним из первых в области фундаментальных исследований гемопоэтических стволовых клеток. Его кандидатская диссертация «Метод клонирования гемопоэтических стволовых клеток, изучение колониестимулирующей способности клеток костного мозга и крови гематологически здоровых лиц и больных с различными нейтропеническими состояниями» (1977) была посвящена регуляции роста нормальных и лейкозных клеток-предшественников гемопоэза, изученных в тест-системе «агаровой капли». В дальнейшем его наиболее выдающиеся исследования культур стволовых клеток включали описание терминальной дифференцировки лейкозных стволовых клеток.
Б.В. Афанасьев осуществил также ряд пионерских работ в области стромальной регуляции гемопоэза мезенхимными стволовыми клетками и показал, что они оказывают как стимулирующие, так и модулирующие эффекты на клетки-предшественники гранулоцитов, которые показаны им в разработанной им системе «агаровая капля в жидкой среде».
В 1976-1979 гг. Б.В. Афанасьев возглавлял отделение гематологии клиники факультетской терапии. В 1982 г., на основании данных о характере роста гемопоэтических клеток in vitro, он первым описал миелодиспдастический синдром у детей. Его докторская диссертация была посвящена теме «Грануломоноцитопоэз при остром лейкозе и бластном кризе» (1983). Он был одним из первых, кто описал клеточные механизмы миелоидного и лимфоидного типов бластного криза при хроническом миелоидном лейкозе. В 1985 г. он опубликовал широко известную монографию «Родоначальные кроветворные клетки человека» в соавторстве с профессором В.А. Алмазовым. В течение последующих 8 лет Б.В. Афанасьев был старшим научным сотрудником при кафедре факультетской терапии Первого Ленинградского медицинского института им. Павлова, где изучал биологические механизмы злокачественных клеток при острых и хронических лейкозах, тяжелых нейтропениях.
В 1986 г. Б.В. Афанасьев проходил клиническую стажировку в знаменитом онкологическом центре Фреда Хатчинсона в Сиэттле (США) под руководством Нобелевского лауреата, профессора Доннелла Томаса. В 1987 г. Б.В. Афанасьев организовал первое отделение трансплантации костного мозга (ТКМ) в Советском Союзе на базе НИИ онкологии им. Н.Н. Петрова. В 1991 г. он выполнил первую в России аллогенную ТКМ ребенку.
В 2000 он организовал первое университетское отделение ТКМ в России на базе Первого Санкт-Петербургского государственного медицинского университета им. И. П. Павлова (ПСПбГМУ). В 2003 г. он основал кафедру гематологии, трансфузиологии и трансплантологии последипломного образования в ПСПбГМУ.
В 2007 г., по инициативе Б.В. Афанасьева и ПСПбГМУ, при поддержке Фонда Горбачева и банка «Национальный Резерв», был построен НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой в составе ПСПбГМУ им. И.П. Павлова. С 2007 г. профессор Б.В. Афанасьев был директором этого института.
Интересы Б.В. Афанасьева на протяжении последнего десятилетия были сосредоточены на разработке протоколов лечения больных с рецидивирующими и рефрактерными формами злокачественных заболеваний. Под его руководством созданы новые протоколы промежуточной (bridging) и посттрансплантационной терапии таргетными лекарственными агентами и моноклональными антителами. Он также был сосредоточен на долгосрочных осложнениях ТКМ у детей и взрослых, и был одним из первых в применении режимов кондиционирования сниженной интенсивности у детей, что приводило к менее выраженным долговременным токсическим эффектам. Научное наследие профессора Б.В. Афанасьева включает более 300 научных статей и 6 монографий. Эти клинические исследования позволили значительно расширить трансплантационные программы, которые стали самыми большими в России и одной из самых больших в Европе.
С 1990 г. Б.В. Афанасьев прилагал усилия к развитию Регистра неродственных доноров костного мозга в России. Эта работа в 2013 г. привела к созданию кооперативной базы данных, которая сейчас способствует проведению примерно половины неродственных трансплантаций в России. На уровне правительства принят план расширения базы данных до Национальной программы неродственных доноров костного мозга, что также является наследием профессора Б.В. Афанасьева.
На протяжении всех тех лет, которые вели к успеху программы ТКМ, организованной Б.В. Афанасьевым, он был глубоко благодарен поддержке коллег и друзей за рубежом: Рольфа Нета, Клауса Винклера, Акселя Цандера, Николауса Крегера, Бориса Фезе (Гамбург Германия), Томаса Бюхнера (Мюнстер, Германия), Дитера Хельцера (Франкфурт, Германия), Ханса-Иохена Кольба (Мюнхен, Германия), Герарда Вагемакера (Роттердам, Нидерланды), Роберта Гэйла (Лондон, Великобритания), Андреа Бачигалупо (Рим, Италия), Магне Борсета (Трондхейм, Норвегия), Арнона Наглера (Тель-Авив, Израиль), Тапани Рууту (Хельсинки, Финляндия) и многих других, кто способствовал обучению более чем 70 российских гематологов в европейских центрах.
Начиная с 2007 г., ежегодные научные форумы памяти Р.М. Горбачевой в медицинском университете им. И. Павлова стали центральными событиями в России и международном сообществе врачей и исследователей лейкозов, в целях образования, сотрудничества, обмена мнениями, новыми научными данными и распространения передового опыта в области трансплантации стволовых клеток.
Профессор Борис В. Афанасьев был членом редакционных советов многих российских и международных журналов. В качестве главного редактора он играл ведущую роль в основании и развитии журнала «Клеточная Терапия и Трансплантация», где российские и зарубежные авторы могли представлять данные своих исследований по онкологии, гематологии и в смежных областях для международного сообщества. Нам, его коллегам и друзьям, а также всему сообществу гематологов и специалистов по трансплантации костного мозга, будет остро не хватать его огромного энтузиазма, творческого мышления и дружелюбия.
Борис В. Афанасьев всегда служил общей пользе и ушел из жизни так, как жил, завершив дело жизненной важности для Института, для Университета, для гематологии и для общества. Ему была присуждена награда за клинические достижения от Европейского Общества трансплантации костного мозга на конгрессе в Лиссабоне в 2018 г. У него остались жена Ольга, дочь Анастасия, внуки Эмиль-Борис и Антония.
Сергей Ф. Багненко,
профессор, д.м.н., академик РАН,
ректор Первого Санкт-Петербургского государственного медицинского университета им. И. Павлова, Россия
Аксель Р. Цандер,
профессор, почетный доктор, Университетский медицинский центр Гамбург-Эппендорф, Германия
Герард Вагемакер,
профессор, почетный доктор, Университет Эразмус, Нидерланды
Рюдигер Хельманн,
профессор, почетный доктор, Факультет Мангейм, Гейдельбергский университет, Германия
Борис Фезе,
профессор, доктор, Университет Гамбурга, Германия
Александр Д. Кулагин, проф., д.м.н.,
Людмила С. Зубаровская, проф., д.м.н.,
Иван С. Моисеев, д.м.н.,
Инна В. Маркова, к.м.н.
и Алексей Б. Чухловин, проф. д.м.н.
НИИ детской онкологии, гематологии и трансплантологии
им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. Павлова, Россия
Sergey F. Bagnenko, Axel R. Zander, Gerard Wagemaker, Rudiger Hehlmann, Boris Fehse, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Ivan S. Moiseev, Inna V. Markova, Alexei B. Chukhlovin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "25997" ["VALUE"]=> array(2) { ["TEXT"]=> string(10061) "<p> </p> <img width="500" alt="Afanasyev_small.jpg" src="/upload/medialibrary/517/afanasyev_small.jpg" height="333" title="Afanasyev_small.jpg" align="middle"><br> <br> <p style="text-align: justify;"> Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide. </p> <p style="text-align: justify;"> Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: <i>Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions</i>. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system. </p> <p style="text-align: justify;"> From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the <i>in vitro</i> growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: <i>Granulomonocytopoiesis in acute leukemia and in blast crisis</i>. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph <i>Ancestral Human Hematopoietic Cells</i> in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions. </p> <p style="text-align: justify;"> In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia. </p> <p style="text-align: justify;"> <img width="500" alt="Gorbachev1_small.jpg" src="/upload/medialibrary/12f/gorbachev1_small.jpg" height="335" title="Gorbachev1_small.jpg"><br> </p> <p style="text-align: justify;"> In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University. </p> <p style="text-align: justify;"> By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute. </p> <p style="text-align: justify;"> Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe. </p> <p style="text-align: justify;"> Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev. </p> <p style="text-align: justify;"> During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers. </p> <p style="text-align: justify;"> Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation. </p> <p style="text-align: justify;"> <img width="500" alt="CTT_9-1-Afanasyev-Photo-Award_small.jpg" src="/upload/medialibrary/931/ctt_9_1_afanasyev_photo_award_small.jpg" height="333" title="CTT_9-1-Afanasyev-Photo-Award_small.jpg"><br> </p> <p style="text-align: justify;"> Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual <i>Cellular Therapy and Transplantation</i> journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness. </p> <p style="text-align: justify;"> Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.<br> </p> <p> <b>Sergey F. Bagnenko,</b><br> Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia<br> <b>Axel R. Zander,</b><br> Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany<br> <b>Gerard Wagemaker,</b><br> Prof. Dr. Dr.h.c. Erasmus University, The Netherlands<br> <b>Rudiger Hehlmann,</b><br> Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany<br> <b>Boris Fehse,</b><br> Prof. Dr., Hamburg University, Germany <br> <b> Alexander D. Kulagin,</b> Prof. Dr.,<br> <b>Ludmila S. Zubarovskaya,</b> Prof. Dr.,<br> <b>Ivan S. Moiseev,</b> PhD, MD<br> <b>Inna V. Markova,</b> PhD<br> and <b>Alexei B. Chukhlovin,</b> Prof. Dr.<br> Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia<br> <br> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(9229) "

Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide.
Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system.
From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the in vitro growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: Granulomonocytopoiesis in acute leukemia and in blast crisis. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph Ancestral Human Hematopoietic Cells in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions.
In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia.

In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University.
By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute.
Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe.
Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev.
During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers.
Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation.

Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual Cellular Therapy and Transplantation journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness.
Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.
Sergey F. Bagnenko,
Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia
Axel R. Zander,
Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany
Gerard Wagemaker,
Prof. Dr. Dr.h.c. Erasmus University, The Netherlands
Rudiger Hehlmann,
Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany
Boris Fehse,
Prof. Dr., Hamburg University, Germany
Alexander D. Kulagin, Prof. Dr.,
Ludmila S. Zubarovskaya, Prof. Dr.,
Ivan S. Moiseev, PhD, MD
Inna V. Markova, PhD
and Alexei B. Chukhlovin, Prof. Dr.
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia
Sergey F. Bagnenko, Axel R. Zander, Gerard Wagemaker, Rudiger Hehlmann, Boris Fehse, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Ivan S. Moiseev, Inna V. Markova, Alexei B. Chukhlovin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(193) "Sergey F. Bagnenko, Axel R. Zander, Gerard Wagemaker, Rudiger Hehlmann, Boris Fehse, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Ivan S. Moiseev, Inna V. Markova, Alexei B. Chukhlovin
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "25997" ["VALUE"]=> array(2) { ["TEXT"]=> string(10061) "<p> </p> <img width="500" alt="Afanasyev_small.jpg" src="/upload/medialibrary/517/afanasyev_small.jpg" height="333" title="Afanasyev_small.jpg" align="middle"><br> <br> <p style="text-align: justify;"> Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide. </p> <p style="text-align: justify;"> Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: <i>Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions</i>. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system. </p> <p style="text-align: justify;"> From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the <i>in vitro</i> growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: <i>Granulomonocytopoiesis in acute leukemia and in blast crisis</i>. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph <i>Ancestral Human Hematopoietic Cells</i> in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions. </p> <p style="text-align: justify;"> In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia. </p> <p style="text-align: justify;"> <img width="500" alt="Gorbachev1_small.jpg" src="/upload/medialibrary/12f/gorbachev1_small.jpg" height="335" title="Gorbachev1_small.jpg"><br> </p> <p style="text-align: justify;"> In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University. </p> <p style="text-align: justify;"> By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute. </p> <p style="text-align: justify;"> Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe. </p> <p style="text-align: justify;"> Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev. </p> <p style="text-align: justify;"> During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers. </p> <p style="text-align: justify;"> Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation. </p> <p style="text-align: justify;"> <img width="500" alt="CTT_9-1-Afanasyev-Photo-Award_small.jpg" src="/upload/medialibrary/931/ctt_9_1_afanasyev_photo_award_small.jpg" height="333" title="CTT_9-1-Afanasyev-Photo-Award_small.jpg"><br> </p> <p style="text-align: justify;"> Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual <i>Cellular Therapy and Transplantation</i> journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness. </p> <p style="text-align: justify;"> Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.<br> </p> <p> <b>Sergey F. Bagnenko,</b><br> Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia<br> <b>Axel R. Zander,</b><br> Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany<br> <b>Gerard Wagemaker,</b><br> Prof. Dr. Dr.h.c. Erasmus University, The Netherlands<br> <b>Rudiger Hehlmann,</b><br> Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany<br> <b>Boris Fehse,</b><br> Prof. Dr., Hamburg University, Germany <br> <b> Alexander D. Kulagin,</b> Prof. Dr.,<br> <b>Ludmila S. Zubarovskaya,</b> Prof. Dr.,<br> <b>Ivan S. Moiseev,</b> PhD, MD<br> <b>Inna V. Markova,</b> PhD<br> and <b>Alexei B. Chukhlovin,</b> Prof. Dr.<br> Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia<br> <br> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(9229) "

Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide.
Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system.
From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the in vitro growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: Granulomonocytopoiesis in acute leukemia and in blast crisis. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph Ancestral Human Hematopoietic Cells in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions.
In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia.

In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University.
By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute.
Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe.
Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev.
During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers.
Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation.

Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual Cellular Therapy and Transplantation journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness.
Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.
Sergey F. Bagnenko,
Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia
Axel R. Zander,
Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany
Gerard Wagemaker,
Prof. Dr. Dr.h.c. Erasmus University, The Netherlands
Rudiger Hehlmann,
Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany
Boris Fehse,
Prof. Dr., Hamburg University, Germany
Alexander D. Kulagin, Prof. Dr.,
Ludmila S. Zubarovskaya, Prof. Dr.,
Ivan S. Moiseev, PhD, MD
Inna V. Markova, PhD
and Alexei B. Chukhlovin, Prof. Dr.
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia

Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide.
Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system.
From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the in vitro growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: Granulomonocytopoiesis in acute leukemia and in blast crisis. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph Ancestral Human Hematopoietic Cells in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions.
In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia.

In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University.
By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute.
Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe.
Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev.
During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers.
Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation.

Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual Cellular Therapy and Transplantation journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness.
Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.
Sergey F. Bagnenko,
Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia
Axel R. Zander,
Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany
Gerard Wagemaker,
Prof. Dr. Dr.h.c. Erasmus University, The Netherlands
Rudiger Hehlmann,
Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany
Boris Fehse,
Prof. Dr., Hamburg University, Germany
Alexander D. Kulagin, Prof. Dr.,
Ludmila S. Zubarovskaya, Prof. Dr.,
Ivan S. Moiseev, PhD, MD
Inna V. Markova, PhD
and Alexei B. Chukhlovin, Prof. Dr.
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia
Сергей Ф. Багненко, Аксель Р. Цандер, Герард Вагемакер, Рюдигер Хельманн, Борис Фезе, Александр Д. Кулагин, Людмила С. Зубаровская, Иван С. Моисеев, Инна В. Маркова, Алексей Б. Чухловин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(335) "Сергей Ф. Багненко, Аксель Р. Цандер, Герард Вагемакер, Рюдигер Хельманн, Борис Фезе, Александр Д. Кулагин, Людмила С. Зубаровская, Иван С. Моисеев, Инна В. Маркова, Алексей Б. Чухловин
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "25931" ["VALUE"]=> string(22) "03/20/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/20/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/20/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "25932" ["VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/27/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "25943" ["VALUE"]=> array(2) { ["TEXT"]=> string(16260) "<p style="text-align: justify;">Санкт-Петербургский государственный медицинский университет им. И.П. Павлова потерял харизматического лидера, создавшего в Санкт-Петербурге признанный в мире ведущий центр по исследованию лейкозов и трансплантации стволовых гемопоэтических клеток.</p> <p style="text-align: justify;">С глубоким прискорбием извещаем наших читателей о безвременной кончине главного редактора журнала «Клеточная Терапия и Трансплантация». Профессор Борис Владимирович Афанасьев, директор НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой Первого Санкт-Петербургского государственного медицинского университета им. И.П. Павлова ушел из жизни 16 марта 2020 г. На протяжении последних трех десятилетий он был главным вдохновителем разработок по трансплантации стволовых кроветворных клеток в России, а также в области химиотерапии, иммунотерапии и таргетного лечения злокачественных и неопухолевых заболеваний системы крови, будучи широко известным как в России, так и в мире.</p> <p style="text-align: justify;">В 1971 г. Борис Владимирович Афанасьев с отличием окончил Первый Ленинградский медицинский институт. С 1973 г. он был ординатором в клинике факультетской терапии по специальности «Внутренние болезни». Будучи аспирантом (1974-1976), он был одним из первых в области фундаментальных исследований гемопоэтических стволовых клеток. Его кандидатская диссертация «Метод клонирования гемопоэтических стволовых клеток, изучение колониестимулирующей способности клеток костного мозга и крови гематологически здоровых лиц и больных с различными нейтропеническими состояниями» (1977) была посвящена регуляции роста нормальных и лейкозных клеток-предшественников гемопоэза, изученных в тест-системе «агаровой капли». В дальнейшем его наиболее выдающиеся исследования культур стволовых клеток включали описание терминальной дифференцировки лейкозных стволовых клеток.</p> <p style="text-align: justify;">Б.В. Афанасьев осуществил также ряд пионерских работ в области стромальной регуляции гемопоэза мезенхимными стволовыми клетками и показал, что они оказывают как стимулирующие, так и модулирующие эффекты на клетки-предшественники гранулоцитов, которые показаны им в разработанной им системе «агаровая капля в жидкой среде».</p> <p style="text-align: justify;">В 1976-1979 гг. Б.В. Афанасьев возглавлял отделение гематологии клиники факультетской терапии. В 1982 г., на основании данных о характере роста гемопоэтических клеток <i>in vitro</i>, он первым описал миелодиспдастический синдром у детей. Его докторская диссертация была посвящена теме «Грануломоноцитопоэз при остром лейкозе и бластном кризе» (1983). Он был одним из первых, кто описал клеточные механизмы миелоидного и лимфоидного типов бластного криза при хроническом миелоидном лейкозе. В 1985 г. он опубликовал широко известную монографию «Родоначальные кроветворные клетки человека» в соавторстве с профессором В.А. Алмазовым. В течение последующих 8 лет Б.В. Афанасьев был старшим научным сотрудником при кафедре факультетской терапии Первого Ленинградского медицинского института им. Павлова, где изучал биологические механизмы злокачественных клеток при острых и хронических лейкозах, тяжелых нейтропениях.</p> <p style="text-align: justify;">В 1986 г. Б.В. Афанасьев проходил клиническую стажировку в знаменитом онкологическом центре Фреда Хатчинсона в Сиэттле (США) под руководством Нобелевского лауреата, профессора Доннелла Томаса. В 1987 г. Б.В. Афанасьев организовал первое отделение трансплантации костного мозга (ТКМ) в Советском Союзе на базе НИИ онкологии им. Н.Н. Петрова. В 1991 г. он выполнил первую в России аллогенную ТКМ ребенку.</p> <p style="text-align: justify;">В 2000 он организовал первое университетское отделение ТКМ в России на базе Первого Санкт-Петербургского государственного медицинского университета им. И. П. Павлова (ПСПбГМУ). В 2003 г. он основал кафедру гематологии, трансфузиологии и трансплантологии последипломного образования в ПСПбГМУ.</p> <p style="text-align: justify;">В 2007 г., по инициативе Б.В. Афанасьева и ПСПбГМУ, при поддержке Фонда Горбачева и банка «Национальный Резерв», был построен НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой в составе ПСПбГМУ им. И.П. Павлова. С 2007 г. профессор Б.В. Афанасьев был директором этого института.</p> <p style="text-align: justify;">Интересы Б.В. Афанасьева на протяжении последнего десятилетия были сосредоточены на разработке протоколов лечения больных с рецидивирующими и рефрактерными формами злокачественных заболеваний. Под его руководством созданы новые протоколы промежуточной (bridging) и посттрансплантационной терапии таргетными лекарственными агентами и моноклональными антителами. Он также был сосредоточен на долгосрочных осложнениях ТКМ у детей и взрослых, и был одним из первых в применении режимов кондиционирования сниженной интенсивности у детей, что приводило к менее выраженным долговременным токсическим эффектам. Научное наследие профессора Б.В. Афанасьева включает более 300 научных статей и 6 монографий. Эти клинические исследования позволили значительно расширить трансплантационные программы, которые стали самыми большими в России и одной из самых больших в Европе.</p> <p style="text-align: justify;">С 1990 г. Б.В. Афанасьев прилагал усилия к развитию Регистра неродственных доноров костного мозга в России. Эта работа в 2013 г. привела к созданию кооперативной базы данных, которая сейчас способствует проведению примерно половины неродственных трансплантаций в России. На уровне правительства принят план расширения базы данных до Национальной программы неродственных доноров костного мозга, что также является наследием профессора Б.В. Афанасьева.</p> <p style="text-align: justify;">На протяжении всех тех лет, которые вели к успеху программы ТКМ, организованной Б.В. Афанасьевым, он был глубоко благодарен поддержке коллег и друзей за рубежом: Рольфа Нета, Клауса Винклера, Акселя Цандера, Николауса Крегера, Бориса Фезе (Гамбург Германия), Томаса Бюхнера (Мюнстер, Германия), Дитера Хельцера (Франкфурт, Германия), Ханса-Иохена Кольба (Мюнхен, Германия), Герарда Вагемакера (Роттердам, Нидерланды), Роберта Гэйла (Лондон, Великобритания), Андреа Бачигалупо (Рим, Италия), Магне Борсета (Трондхейм, Норвегия), Арнона Наглера (Тель-Авив, Израиль), Тапани Рууту (Хельсинки, Финляндия) и многих других, кто способствовал обучению более чем 70 российских гематологов в европейских центрах.</p> <p style="text-align: justify;">Начиная с 2007 г., ежегодные научные форумы памяти Р.М. Горбачевой в медицинском университете им. И. Павлова стали центральными событиями в России и международном сообществе врачей и исследователей лейкозов, в целях образования, сотрудничества, обмена мнениями, новыми научными данными и распространения передового опыта в области трансплантации стволовых клеток.</p> <p style="text-align: justify;">Профессор Борис В. Афанасьев был членом редакционных советов многих российских и международных журналов. В качестве главного редактора он играл ведущую роль в основании и развитии журнала «Клеточная Терапия и Трансплантация», где российские и зарубежные авторы могли представлять данные своих исследований по онкологии, гематологии и в смежных областях для международного сообщества. Нам, его коллегам и друзьям, а также всему сообществу гематологов и специалистов по трансплантации костного мозга, будет остро не хватать его огромного энтузиазма, творческого мышления и дружелюбия.</p> <p style="text-align: justify;">Борис В. Афанасьев всегда служил общей пользе и ушел из жизни так, как жил, завершив дело жизненной важности для Института, для Университета, для гематологии и для общества. Ему была присуждена награда за клинические достижения от Европейского Общества трансплантации костного мозга на конгрессе в Лиссабоне в 2018 г. У него остались жена Ольга, дочь Анастасия, внуки Эмиль-Борис и Антония.</p> <p><b>Сергей Ф. Багненко,</b><br> профессор, д.м.н., академик РАН,<br> ректор Первого Санкт-Петербургского государственного медицинского университета им. И. Павлова, Россия<br> <b>Аксель Р. Цандер,</b><br> профессор, почетный доктор, Университетский медицинский центр Гамбург-Эппендорф, Германия<br> <b>Герард Вагемакер,</b><br> профессор, почетный доктор, Университет Эразмус, Нидерланды<br> <b>Рюдигер Хельманн,</b><br> профессор, почетный доктор, Факультет Мангейм, Гейдельбергский университет, Германия<br> <b>Борис Фезе,</b><br> профессор, доктор, Университет Гамбурга, Германия<br> <b>Александр Д. Кулагин,</b> проф., д.м.н.,<br> <b>Людмила С. Зубаровская,</b> проф., д.м.н.,<br> <b> Иван С. Моисеев,</b> д.м.н.,<br> <b>Инна В. Маркова,</b> к.м.н.<br> и <b>Алексей Б. Чухловин,</b> проф. д.м.н.<br> НИИ детской онкологии, гематологии и трансплантологии <br>им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. Павлова, Россия<br> <br></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(15694) "Санкт-Петербургский государственный медицинский университет им. И.П. Павлова потерял харизматического лидера, создавшего в Санкт-Петербурге признанный в мире ведущий центр по исследованию лейкозов и трансплантации стволовых гемопоэтических клеток.
С глубоким прискорбием извещаем наших читателей о безвременной кончине главного редактора журнала «Клеточная Терапия и Трансплантация». Профессор Борис Владимирович Афанасьев, директор НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой Первого Санкт-Петербургского государственного медицинского университета им. И.П. Павлова ушел из жизни 16 марта 2020 г. На протяжении последних трех десятилетий он был главным вдохновителем разработок по трансплантации стволовых кроветворных клеток в России, а также в области химиотерапии, иммунотерапии и таргетного лечения злокачественных и неопухолевых заболеваний системы крови, будучи широко известным как в России, так и в мире.
В 1971 г. Борис Владимирович Афанасьев с отличием окончил Первый Ленинградский медицинский институт. С 1973 г. он был ординатором в клинике факультетской терапии по специальности «Внутренние болезни». Будучи аспирантом (1974-1976), он был одним из первых в области фундаментальных исследований гемопоэтических стволовых клеток. Его кандидатская диссертация «Метод клонирования гемопоэтических стволовых клеток, изучение колониестимулирующей способности клеток костного мозга и крови гематологически здоровых лиц и больных с различными нейтропеническими состояниями» (1977) была посвящена регуляции роста нормальных и лейкозных клеток-предшественников гемопоэза, изученных в тест-системе «агаровой капли». В дальнейшем его наиболее выдающиеся исследования культур стволовых клеток включали описание терминальной дифференцировки лейкозных стволовых клеток.
Б.В. Афанасьев осуществил также ряд пионерских работ в области стромальной регуляции гемопоэза мезенхимными стволовыми клетками и показал, что они оказывают как стимулирующие, так и модулирующие эффекты на клетки-предшественники гранулоцитов, которые показаны им в разработанной им системе «агаровая капля в жидкой среде».
В 1976-1979 гг. Б.В. Афанасьев возглавлял отделение гематологии клиники факультетской терапии. В 1982 г., на основании данных о характере роста гемопоэтических клеток in vitro, он первым описал миелодиспдастический синдром у детей. Его докторская диссертация была посвящена теме «Грануломоноцитопоэз при остром лейкозе и бластном кризе» (1983). Он был одним из первых, кто описал клеточные механизмы миелоидного и лимфоидного типов бластного криза при хроническом миелоидном лейкозе. В 1985 г. он опубликовал широко известную монографию «Родоначальные кроветворные клетки человека» в соавторстве с профессором В.А. Алмазовым. В течение последующих 8 лет Б.В. Афанасьев был старшим научным сотрудником при кафедре факультетской терапии Первого Ленинградского медицинского института им. Павлова, где изучал биологические механизмы злокачественных клеток при острых и хронических лейкозах, тяжелых нейтропениях.
В 1986 г. Б.В. Афанасьев проходил клиническую стажировку в знаменитом онкологическом центре Фреда Хатчинсона в Сиэттле (США) под руководством Нобелевского лауреата, профессора Доннелла Томаса. В 1987 г. Б.В. Афанасьев организовал первое отделение трансплантации костного мозга (ТКМ) в Советском Союзе на базе НИИ онкологии им. Н.Н. Петрова. В 1991 г. он выполнил первую в России аллогенную ТКМ ребенку.
В 2000 он организовал первое университетское отделение ТКМ в России на базе Первого Санкт-Петербургского государственного медицинского университета им. И. П. Павлова (ПСПбГМУ). В 2003 г. он основал кафедру гематологии, трансфузиологии и трансплантологии последипломного образования в ПСПбГМУ.
В 2007 г., по инициативе Б.В. Афанасьева и ПСПбГМУ, при поддержке Фонда Горбачева и банка «Национальный Резерв», был построен НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой в составе ПСПбГМУ им. И.П. Павлова. С 2007 г. профессор Б.В. Афанасьев был директором этого института.
Интересы Б.В. Афанасьева на протяжении последнего десятилетия были сосредоточены на разработке протоколов лечения больных с рецидивирующими и рефрактерными формами злокачественных заболеваний. Под его руководством созданы новые протоколы промежуточной (bridging) и посттрансплантационной терапии таргетными лекарственными агентами и моноклональными антителами. Он также был сосредоточен на долгосрочных осложнениях ТКМ у детей и взрослых, и был одним из первых в применении режимов кондиционирования сниженной интенсивности у детей, что приводило к менее выраженным долговременным токсическим эффектам. Научное наследие профессора Б.В. Афанасьева включает более 300 научных статей и 6 монографий. Эти клинические исследования позволили значительно расширить трансплантационные программы, которые стали самыми большими в России и одной из самых больших в Европе.
С 1990 г. Б.В. Афанасьев прилагал усилия к развитию Регистра неродственных доноров костного мозга в России. Эта работа в 2013 г. привела к созданию кооперативной базы данных, которая сейчас способствует проведению примерно половины неродственных трансплантаций в России. На уровне правительства принят план расширения базы данных до Национальной программы неродственных доноров костного мозга, что также является наследием профессора Б.В. Афанасьева.
На протяжении всех тех лет, которые вели к успеху программы ТКМ, организованной Б.В. Афанасьевым, он был глубоко благодарен поддержке коллег и друзей за рубежом: Рольфа Нета, Клауса Винклера, Акселя Цандера, Николауса Крегера, Бориса Фезе (Гамбург Германия), Томаса Бюхнера (Мюнстер, Германия), Дитера Хельцера (Франкфурт, Германия), Ханса-Иохена Кольба (Мюнхен, Германия), Герарда Вагемакера (Роттердам, Нидерланды), Роберта Гэйла (Лондон, Великобритания), Андреа Бачигалупо (Рим, Италия), Магне Борсета (Трондхейм, Норвегия), Арнона Наглера (Тель-Авив, Израиль), Тапани Рууту (Хельсинки, Финляндия) и многих других, кто способствовал обучению более чем 70 российских гематологов в европейских центрах.
Начиная с 2007 г., ежегодные научные форумы памяти Р.М. Горбачевой в медицинском университете им. И. Павлова стали центральными событиями в России и международном сообществе врачей и исследователей лейкозов, в целях образования, сотрудничества, обмена мнениями, новыми научными данными и распространения передового опыта в области трансплантации стволовых клеток.
Профессор Борис В. Афанасьев был членом редакционных советов многих российских и международных журналов. В качестве главного редактора он играл ведущую роль в основании и развитии журнала «Клеточная Терапия и Трансплантация», где российские и зарубежные авторы могли представлять данные своих исследований по онкологии, гематологии и в смежных областях для международного сообщества. Нам, его коллегам и друзьям, а также всему сообществу гематологов и специалистов по трансплантации костного мозга, будет остро не хватать его огромного энтузиазма, творческого мышления и дружелюбия.
Борис В. Афанасьев всегда служил общей пользе и ушел из жизни так, как жил, завершив дело жизненной важности для Института, для Университета, для гематологии и для общества. Ему была присуждена награда за клинические достижения от Европейского Общества трансплантации костного мозга на конгрессе в Лиссабоне в 2018 г. У него остались жена Ольга, дочь Анастасия, внуки Эмиль-Борис и Антония.
Сергей Ф. Багненко,
профессор, д.м.н., академик РАН,
ректор Первого Санкт-Петербургского государственного медицинского университета им. И. Павлова, Россия
Аксель Р. Цандер,
профессор, почетный доктор, Университетский медицинский центр Гамбург-Эппендорф, Германия
Герард Вагемакер,
профессор, почетный доктор, Университет Эразмус, Нидерланды
Рюдигер Хельманн,
профессор, почетный доктор, Факультет Мангейм, Гейдельбергский университет, Германия
Борис Фезе,
профессор, доктор, Университет Гамбурга, Германия
Александр Д. Кулагин, проф., д.м.н.,
Людмила С. Зубаровская, проф., д.м.н.,
Иван С. Моисеев, д.м.н.,
Инна В. Маркова, к.м.н.
и Алексей Б. Чухловин, проф. д.м.н.
НИИ детской онкологии, гематологии и трансплантологии
им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. Павлова, Россия
Санкт-Петербургский государственный медицинский университет им. И.П. Павлова потерял харизматического лидера, создавшего в Санкт-Петербурге признанный в мире ведущий центр по исследованию лейкозов и трансплантации стволовых гемопоэтических клеток.
С глубоким прискорбием извещаем наших читателей о безвременной кончине главного редактора журнала «Клеточная Терапия и Трансплантация». Профессор Борис Владимирович Афанасьев, директор НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой Первого Санкт-Петербургского государственного медицинского университета им. И.П. Павлова ушел из жизни 16 марта 2020 г. На протяжении последних трех десятилетий он был главным вдохновителем разработок по трансплантации стволовых кроветворных клеток в России, а также в области химиотерапии, иммунотерапии и таргетного лечения злокачественных и неопухолевых заболеваний системы крови, будучи широко известным как в России, так и в мире.
В 1971 г. Борис Владимирович Афанасьев с отличием окончил Первый Ленинградский медицинский институт. С 1973 г. он был ординатором в клинике факультетской терапии по специальности «Внутренние болезни». Будучи аспирантом (1974-1976), он был одним из первых в области фундаментальных исследований гемопоэтических стволовых клеток. Его кандидатская диссертация «Метод клонирования гемопоэтических стволовых клеток, изучение колониестимулирующей способности клеток костного мозга и крови гематологически здоровых лиц и больных с различными нейтропеническими состояниями» (1977) была посвящена регуляции роста нормальных и лейкозных клеток-предшественников гемопоэза, изученных в тест-системе «агаровой капли». В дальнейшем его наиболее выдающиеся исследования культур стволовых клеток включали описание терминальной дифференцировки лейкозных стволовых клеток.
Б.В. Афанасьев осуществил также ряд пионерских работ в области стромальной регуляции гемопоэза мезенхимными стволовыми клетками и показал, что они оказывают как стимулирующие, так и модулирующие эффекты на клетки-предшественники гранулоцитов, которые показаны им в разработанной им системе «агаровая капля в жидкой среде».
В 1976-1979 гг. Б.В. Афанасьев возглавлял отделение гематологии клиники факультетской терапии. В 1982 г., на основании данных о характере роста гемопоэтических клеток in vitro, он первым описал миелодиспдастический синдром у детей. Его докторская диссертация была посвящена теме «Грануломоноцитопоэз при остром лейкозе и бластном кризе» (1983). Он был одним из первых, кто описал клеточные механизмы миелоидного и лимфоидного типов бластного криза при хроническом миелоидном лейкозе. В 1985 г. он опубликовал широко известную монографию «Родоначальные кроветворные клетки человека» в соавторстве с профессором В.А. Алмазовым. В течение последующих 8 лет Б.В. Афанасьев был старшим научным сотрудником при кафедре факультетской терапии Первого Ленинградского медицинского института им. Павлова, где изучал биологические механизмы злокачественных клеток при острых и хронических лейкозах, тяжелых нейтропениях.
В 1986 г. Б.В. Афанасьев проходил клиническую стажировку в знаменитом онкологическом центре Фреда Хатчинсона в Сиэттле (США) под руководством Нобелевского лауреата, профессора Доннелла Томаса. В 1987 г. Б.В. Афанасьев организовал первое отделение трансплантации костного мозга (ТКМ) в Советском Союзе на базе НИИ онкологии им. Н.Н. Петрова. В 1991 г. он выполнил первую в России аллогенную ТКМ ребенку.
В 2000 он организовал первое университетское отделение ТКМ в России на базе Первого Санкт-Петербургского государственного медицинского университета им. И. П. Павлова (ПСПбГМУ). В 2003 г. он основал кафедру гематологии, трансфузиологии и трансплантологии последипломного образования в ПСПбГМУ.
В 2007 г., по инициативе Б.В. Афанасьева и ПСПбГМУ, при поддержке Фонда Горбачева и банка «Национальный Резерв», был построен НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой в составе ПСПбГМУ им. И.П. Павлова. С 2007 г. профессор Б.В. Афанасьев был директором этого института.
Интересы Б.В. Афанасьева на протяжении последнего десятилетия были сосредоточены на разработке протоколов лечения больных с рецидивирующими и рефрактерными формами злокачественных заболеваний. Под его руководством созданы новые протоколы промежуточной (bridging) и посттрансплантационной терапии таргетными лекарственными агентами и моноклональными антителами. Он также был сосредоточен на долгосрочных осложнениях ТКМ у детей и взрослых, и был одним из первых в применении режимов кондиционирования сниженной интенсивности у детей, что приводило к менее выраженным долговременным токсическим эффектам. Научное наследие профессора Б.В. Афанасьева включает более 300 научных статей и 6 монографий. Эти клинические исследования позволили значительно расширить трансплантационные программы, которые стали самыми большими в России и одной из самых больших в Европе.
С 1990 г. Б.В. Афанасьев прилагал усилия к развитию Регистра неродственных доноров костного мозга в России. Эта работа в 2013 г. привела к созданию кооперативной базы данных, которая сейчас способствует проведению примерно половины неродственных трансплантаций в России. На уровне правительства принят план расширения базы данных до Национальной программы неродственных доноров костного мозга, что также является наследием профессора Б.В. Афанасьева.
На протяжении всех тех лет, которые вели к успеху программы ТКМ, организованной Б.В. Афанасьевым, он был глубоко благодарен поддержке коллег и друзей за рубежом: Рольфа Нета, Клауса Винклера, Акселя Цандера, Николауса Крегера, Бориса Фезе (Гамбург Германия), Томаса Бюхнера (Мюнстер, Германия), Дитера Хельцера (Франкфурт, Германия), Ханса-Иохена Кольба (Мюнхен, Германия), Герарда Вагемакера (Роттердам, Нидерланды), Роберта Гэйла (Лондон, Великобритания), Андреа Бачигалупо (Рим, Италия), Магне Борсета (Трондхейм, Норвегия), Арнона Наглера (Тель-Авив, Израиль), Тапани Рууту (Хельсинки, Финляндия) и многих других, кто способствовал обучению более чем 70 российских гематологов в европейских центрах.
Начиная с 2007 г., ежегодные научные форумы памяти Р.М. Горбачевой в медицинском университете им. И. Павлова стали центральными событиями в России и международном сообществе врачей и исследователей лейкозов, в целях образования, сотрудничества, обмена мнениями, новыми научными данными и распространения передового опыта в области трансплантации стволовых клеток.
Профессор Борис В. Афанасьев был членом редакционных советов многих российских и международных журналов. В качестве главного редактора он играл ведущую роль в основании и развитии журнала «Клеточная Терапия и Трансплантация», где российские и зарубежные авторы могли представлять данные своих исследований по онкологии, гематологии и в смежных областях для международного сообщества. Нам, его коллегам и друзьям, а также всему сообществу гематологов и специалистов по трансплантации костного мозга, будет остро не хватать его огромного энтузиазма, творческого мышления и дружелюбия.
Борис В. Афанасьев всегда служил общей пользе и ушел из жизни так, как жил, завершив дело жизненной важности для Института, для Университета, для гематологии и для общества. Ему была присуждена награда за клинические достижения от Европейского Общества трансплантации костного мозга на конгрессе в Лиссабоне в 2018 г. У него остались жена Ольга, дочь Анастасия, внуки Эмиль-Борис и Антония.
Сергей Ф. Багненко,
профессор, д.м.н., академик РАН,
ректор Первого Санкт-Петербургского государственного медицинского университета им. И. Павлова, Россия
Аксель Р. Цандер,
профессор, почетный доктор, Университетский медицинский центр Гамбург-Эппендорф, Германия
Герард Вагемакер,
профессор, почетный доктор, Университет Эразмус, Нидерланды
Рюдигер Хельманн,
профессор, почетный доктор, Факультет Мангейм, Гейдельбергский университет, Германия
Борис Фезе,
профессор, доктор, Университет Гамбурга, Германия
Александр Д. Кулагин, проф., д.м.н.,
Людмила С. Зубаровская, проф., д.м.н.,
Иван С. Моисеев, д.м.н.,
Инна В. Маркова, к.м.н.
и Алексей Б. Чухловин, проф. д.м.н.
НИИ детской онкологии, гематологии и трансплантологии
им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. Павлова, Россия
Hematopoietic cell counts in peripheral blood
Some data exist concerning HPC contents in peripheral blood. In an early study, semisolid methyl cellulose-based clonogenic cultures were assayed in 43 healthy volunteers including 22 males and 21 females at the age of 21 to 39 years old [1]. Comparative studies have shown increased numbers of colony-forming cells (CFC), erythroblastic progenitors (BFU-E), and non-differentiated myeloid precursors (GM-CFU) in peripheral blood (PB) in male individuals. However, the numbers of pluripotent CFU-GEMM in peripheral blood did not differ between females and males. Probable effects of sex steroids on these cell populations still remain open.
A more recent study from USA was carried out in a large cohort of 1786 Framingham Heart Study participants who underwent counting of CD34(+) cells in peripheral blood [2]. Among 1595 persons without cardiovascular diseases, CD34(+) frequency tended to decrease with older age and in females, thus suggesting higher numbers of peripheral hematopoietic cells in males.
Sex differences in circulating HSC subpopulations were also found in a group of 642 persons (mean age, 48 years) either healthy, or with cardiovascular disorders [3]. CD34+ CD45med+ and some other HSC markers were determined by flow cytometry. After adjustment for age, cardiovascular risk, and body mass, the individual counts for CD34+, CD34+/CD133+, and CD34+/chemokine receptor 4+ counts, were found to be lower in women than in male persons.
Thus, one may consider higher background counts of hematopoietic stem cells in HSC grafts from males compared to female donors.
The potentially higher amounts of hematopoietic stem cells in males may be translated into higher incidence of hematological malignancies, i.e., CML (and, probably, of chronic myelomonocytic leukemia) in male persons rather than in females, as shown in a big cohort, e.g., Hiroshima survivors observed over decades [4]. The authors consider two hypotheses: increased actual risk for this disorder, or shorter latency time in males needed for development of the disorder.
Gender differences in pharmacokinetics
Cytostatic drugs, CYPs and pGp effects
Intensive conditioning treatment is a basic pre-requisite of hematopoietic stem cell transplantation procedure. In leukemia treatment, it is intended for eradication of malignant cells, as well as for engraftment and proliferation of donor hematopoietic cells. Therefore, sex-specific effects of cytostatic drugs should be realized when planning optimal conditioning protocols. Studies of these sex differences in response to the common cytostatic drugs, generally, show that female patients are, generally, more stable than males, and moreover, depend on the type and dose of cytostatic agent applied [5].
Influence of gender may be exerted at different steps of drug processing in the body, i.e., absorption, distribution, metabolism (mainly in gut and liver), and excretion, corresponding to the ADME concept. Among other cytostatics used during HSCT, a decreased clearance of MTX, etoposide, doxorubicine and melphalan was found in women. Prolonged half-life was reported for different therapeutic antibodies, e.g., widely used Rituximab [6]. E.g., intersexual differences in equivalent doses of steroid drugs are readily explained by their more rapid metabolization (mostly activation) in women caused by sex-dependent CYP3A4 expression in liver [7].
An early well-known study by Hunt et al. [8] showed a significant increase of CYP3A activity (assessed by erythromycin demethylation) in liver tissues from normal females compared to males. Meanwhile, Wolbold et al. [7] reported 2-fold increase in CYP3A levels in females accompanied by 50% increase in the CYP3A measured as verapamil conversion rates. As reviewed by Fujita [9], there are numerous CYP3A substrates among anticancer drugs used in oncohematology, e.g. cyclophosphamide, taxanes, tyrosine kinase inhibitors etc.
At the present time, most authors agree that CYP3A4 expression and function in the liver of females is higher than in male persons [10]. However, the CYP3A4 activity is suppressed in acute inflammation and subject to unpredictable changes in cases of multiple drugs used in HSCT practice. Therefore, its role in pharmacokinetics requires special evaluation in cases of acute tissue damage, such as intensive anticancer therapy [11].
P-glycoprotein, is another potent modifier of drug effects. It is an important tool for the drug efflux from target cells [12]. Worth of note, most drugs metabolized by CYP3A4 in liver, are also subjected to P-glycoprotein-mediated efflux from the cells, thus providing a dual pharmacodynamic effect. Interestingly, the drug efflux could be reduced in females due to lower expression of P-glycoprotein in liver cells [13].
In general, available data on drug metabolism in females suggest an evidence for decreased clearance, higher activation rates due to increased CYP3A enzyme activities, and decreased drug efflux from liver cells, thus suggesting higher accumulation of active cytostatic drugs in the body, thus being predisposed to more expressed cytotoxicity towards malignant cells and normal tissues. However, individual gender-based drug dosage is not possible, due to drug interactions, genetic polymorphisms (e.g., CYP3A4) and other numerous factors modifying the effects of metabolizing enzymes and p-glycoprotein.
Immune suppressor drugs
Immune suppressor drugs used in post-transplant period usually affect cellular immunity, mostly, T cell populations. Some data about sexual dimorphism of their effects are obtained from studies in organ transplantation. E.g., the impact of gender on survival of renal allografts was thoroughly discussed in a review by Momper et al. [14]. Some controversies on sex differences in renal transplant survival that could be partly explained by the patients’ age, due to differential hormonal patterns at pre-puberty and post-menopause periods of life.
Whatever the exact mechanisms, calcineurin inhibitors (cyclosporine and tacrolimus), generally, used in posttransplant patients exhibited higher clearance rates in women than in males [15, 16].
Similar increase of sirolimus clearance in females as compared with males was shown elsewhere [17]. This difference may be again coupled to the mentioned CYP3A and P-glycoprotein alterations in women.
By contrary, mycophenolic acid (MPA) is also commonly used in post-transplant patients. Appropriate studies in a group of 100 renal transplants have showed a significantly increased MPA kinetics in males against females, due to higher glucuronate conjugation of MPA in male patients [18]. Indeed, the in vitro studies have shown that the liver samples in men exhibited a 3 to 4-fold higher gene expression and microsomal activity levels for UGT2B17 conjugating enzyme compared to women [19]. A conclusion could be made that the observed sex-dependent differences in pharmacokinetics of immunosuppressor drugs occur due to main metabolic mechanisms, i.e., drug efflux or conjugation via common liver enzymes.
Gender differences for glucocorticoid kinetics were intensively studied over last 25 years. Female sex is, generally, associated with lower clearance and, therefore, increased prednisolone exposure, as reviewed by Momper et al. [14]. Indeed, total body weight-normalized free prednisolone oral clearance was shown to be higher in men vs women [20], regardless of race (by 22% in whites and 40% in blacks for oral clearance, p < 0.01).
Thus, clearance of different immune suppressors in women is higher for cyclosporin A, tacrolimus and sirolimus, whereas clearance of MPA and glucocorticoids is higher in males, probably, due to different conjugation mechanisms of specific drugs.
Infections and immune response
A number of works have demonstrated lesser risks for severe posttraumatic infections and multiple organ failure in females. Most of these studies were performed in experimental models [21].
In a seminal large multicentric study involving 20.000 post-traumatic patients, the significant survival benefit was shown for female patients younger than 50 years [22]. In later studies, significantly better outcome was revealed for women after traumatic injury, severe blood loss and sepsis. Better survival of females in these settings are thought to be mediated via sex hormone effects and in particular, estrogen binding to their specific cell receptors [23].
Most clinical and experimental studies suggest increased immunoreactivity, especially, humoral response in females compared to males [24].
At the average, innate and adaptive immune response in females is higher than in, thus resulting in lesser susceptibility to bacterial, fungal and viral infections, however more predisposed for autoimmune conditions [25]. Both genetic factors and hormonal molecules contribute independently to gender differences in immune response. Some immune-related genes are located on the X chromosome, thus determining lesser immune response in young males. At later ages, estrogen and testosterone become the major regulators of immune cell kinetics, their maturation and functions. Thus, sexual dimorphism may cause significant differences in occurrence of infectious or autoimmune diseases between females and males.
A number of studies have shown higher antibody responses and longer half-life of immunoglobulins in females (e.g. following vaccination) that are extensively discussed by Fischinger et al. [26]. Vice versa, innate immunity and NK activity seem to be increased in males.
Some experimental and clinical studies have shown that the ratio of different IgG subclass can vary between in sex-specific manner. E.g., Simon et al. [27] have revealed that females during cytomegalovirus (CMV) infection produce higher levels of IgG3, the most active antibody subclass. Moreover, as shown by oncologists, female patients respond more effectively to rituximab treatment, probably, due to slower clearance and longer half-life of the antibody-based drugs [28].
Aiming for space economy, we cannot extensively discuss clear sex differences in gut microbiome that was already discussed by Ruggieri et al. [29]. The numerous bacteria inhabiting normal microbiome may both metabolize sex hormones and produce antigenic landscape for optimal antibody response.
When studying intestinal biopsies in healthy persons, women had higher levels of immune activation and inflammation-related gene expression in gut mucosal samples [30], as well as significantly higher levels of immune activation-associated phenotype of CD4+ and CD8+ T cells from peripheral blood. Higher humoral immune response to viruses in female HSCT patients was confirmed in several works. E.g., an extensive HSCT population was studied by Ljungman and Brandan [31]. To study factors determining CMV status, 40.311 patients and 23.048 donors were identified in the EBMT registry. Female patients were more likely to be CMV-seropositive (p<0.001). Adjusted for patient serostatus, the risk of a donor being seropositive was higher in females (p<0.001) and in older donors (p<0.001).
Hence, a more effective immune response may be observed in females compared to males. This difference may be explained by dominant effects of female steroid hormones which seem to exert mostly proinflammatory effects, whereas androgens exerting immunomodulatory properties. Most sex-dependent immune differences observed in males and females, i.e, lower response to infection and vaccination in males, or the higher incidence of autoimmunity seen in females, are ascribed to female steroid hormones which seem to exert mostly proinflammatory effects [32].
There is controversial information on rates of viral infections. As expected, male sex was shown to be the risk factor for BK virus infection following organ transplantation [33]. On other hand, as shown by Freeman et al. [34], female sex was proven to be associated with CMV disease in solid organ transplants (OR, 2.19; 95% CI 1.21, 3.99) and CMV viremia (OR, 1.65; 95% CI 1.03, 2.65).
Therefore, active treatment with antiviral drugs is often required in cases of viral activation. Sex-dependence of antiviral drug kinetics is poorly studied. E.g., transport of these drugs to renal tubules is controlled, e.g., by organic anion 1 (OAT1), an efflux transporter at the membranes of renal proximal tubules [35]. A higher ganciclovir clearance (+24%) was previously shown for female patients.
GvHD incidence
Concerning frequency and severity of acute GvHD in male vs female recipients of allografts, most studies did not show any sex differences incidence or severity when comparing male and female patients. The only condition associated with more common aGvHD and inferior survival is transplantation of female HSCs transplanted to male recipients, e.g., see Inamoto et al. [36]. In this study, GvHD- and relapse-free survival was assessed at 1 year post-HSCT based on 23.000 cases of first transplants. It was associated with some factors, including, anti-thymocyte globulin prophylaxis (for standard-risk-disease), recent years of transplantation etc. Better survival was observed with sex combinations other than from a female donor to a male patient. Therefore, female-to-male grafting was found to be of maximal risk for the male patients.
Meanwhile, prevalence of chronic GvHD showed a more distinct dependence on the donor gender. In an early study by Locatelli et al. [37], a group of 145 patients with acute GCHD and 114 cases at risk for chronic GvHD. Of them, 107 (74%) presented with acute GvHD (of them, grade II-IV developed in 35%). I.e., the incidence of chronic GvHD was higher in female-to-male than in male-male donor-recipient sex pairs (33% vs 11%, p <0.05), with no difference between female-to-female and male-to-female pairs. In patients of >10 years old, a higher incidence of chronic GvHD was observed in both female donors and recipients compared with male donors and recipients (48% vs 20% and 47% vs 19%, respectively, p <0.05).
Possible role of female donorship for chronic GvHD was also suggested for patients reported by Gaziev et al. [38]. Several risk factors included grade I-IV acute GvHD, prior aGvHD (p=0.000), female donor sex (p=0.000), use of alloimmune female donors for male patients (0.009) that predicted chronic GvHD.
Reduced salivary function (dry mouth) is a common sign of chronic GvHD developing after allo-HSCT. A total of 74 adolescents received allo-HSCT and conditioning with busulfan or total-body irradiation [39]. In the multivariate model, only female sex was significantly correlated with low salivation rates several years later.
Therefore, donor/recipient sex differences, along with other biological factors, were still under discussion if compared to dominant influence of, e.g., HLA matching which may control the HCST outcomes, as suggested by Confer et al. [40]. In cases of several available donors, one may select them for age, sex, parity, cytomegalovirus (CMV) serostatus, ethnicity and ABO blood type.
Relapses and overall survival
Solh et al. [41] analyzed medical histories for 389 post allo-HSCT patients who survived and were disease-free for at least one year post-transplant, with a median follow-up of 48.2 months. The median 5-year OS overall survival and DFS at 5 years were 78 and 74%, respectively. The most common causes of late mortality were high-risk disease and relapses, chronic GvHD, infections, and male sex. The risk factors for late relapse included male sex and high/very high disease risk index.
Another Turkish study [42] was performed in 193 patients with CML subjected to allo-HSCTs between 1989 and 2012 (before and after TKI advent). Despite quite different clinical state at HSCT, the rates of hematologic remission at 3 months after allo-HSCT were similar between TKI and pre-TKI eras, the patients having remission had better disease-free survival. Interestingly, male allo-HSCT recipients had worse DFS and OS (RR: 1.7, p=0.007) than females.
Sex differences are also shown for late outcomes of auto- and allo-HSCTs in r/r Hodgkin’s lymphoma by Japanese workers [43]. They treated 298 and 122 patients, respectively. For both autologous and allogeneic HSCT, overall 3-year survival was dependent on sex, and performance status (PS) at HSCT. Following 1st allo-HSCT, female sex at HSCT was significantly associated with better progression-free survival [RR=0.55 (0.32-0.94)].
In a comprehensive study by Schmid et al. [44], clinical efficiency of donor lymphocyte infusions was evaluated in 399 AML cases after allo-HSCT (of them, 228 DLI-treated patients), with a median follow-up of 27-40 months, respectively. Estimated survival at 2 years (+/- standard deviation) was 21% +/- 3% for patients receiving DLI and 9% +/- 2% for patients not receiving DLI. Among DLI recipients, female sex (p=0.02), along with lower tumor burden, were predictive for survival in a multivariate analysis.
Early clinical works on sex differences in HSCT outcome male-female differences in HSCT were presented by Gratwohl et al. [45]. The authors analyzed clinical outcomes of HSCT from HLA-identical female donors for 782 patients (438 males and 344 females) with chronic myeloid leukemia (chronic phase). The risk of transplant-related mortality (TRM) and relapse incidence (RI) were compared for male and female recipients at early period (up to 3 months), and at later times (up to 2-5 years post-transplant). Non-relapse-related mortality risk over 5 years proved to be significantly increased in male patients than in females (42% vs 27%; p=0.02), however, without detectable summary effect on relapse incidence. There was no difference in mortality and relapse rates at early terms (0 to 3 mo) (zero to three months). Meanwhile, the risk of CML relapse was shown to be diminished in male patients; this difference became detectable only after two years of observation (p=0.01).
A summary of clinical studies showing different outcomes of HSCT in females vs male patients is presented in Table 1.
Hence, one may suggest that male patients with hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses and late mortality.
Table 1. Gender differences in survival and/or relapse rates for the patients subjected to allo- or allogeneic HSCT

Female-to-male grafting: Y-encoded antigens?
Consequences of sex-mismatched HSCT and role of male minor antigens in hematopoietic transplantation were discussed for decades as a factor of HSCT outcomes. E.g., sex chromosome-linked minor histocompatibility determinants have been shown to affect the incidence and severity of graft-versus-host disease (GvHD) in both humans and animals [46]. On the basis of earlier studies done in mice and humans, it has often been assumed that this effect is due to a simple response of female donor cells recognizing recipient male HY antigens as foreign and reacting against them.
Minor histocompatibility antigens (mHAs) recognized by donor T cells play a central role as immunologic targets of graft-versus-host disease (GvHD) and graft-versus-leukemia after allogeneic hematopoietic stem cell transplantation (HSCT). Men who have undergone sex-mismatched allogeneic HSCT are at high risk for GvHD because of immune responses directed against mHAs encoded by antigens encoded by some Y chromosome genes [47].
Higher GvL effects associated with female donors were suggested by Randolph et al. [48] based on analysis of 3238 patients who were subjected to HLA-identical HSCT from siblings. As shown in a large sample of HLA-identical HSCTs, the male patients transplanted from female donors showed higher chances for clinical GvHD, but significantly lower incidence of malignancy relapse. The "graft-versus-leukemia" effect in female-to-male HSCT seems to be independent from GvHD in the cases of acute leukemias and chronic myeloid leukemia. Thus, possible role of H-Y minor male antigens in GvHD could be suggested.
A clinical study by Alhashim et al. [49] was performed in a group of patients with AML in complete remission (215 cases) after allo-HCT from matched siblings, after myeloablative conditioning. Seventy-seven (35.8%) patients experienced disease relapse, 45 had BMR, and 32 had extramedullary relapses (EMR). Male sex was the only variable that was statistically associated with EMR post allo-HCT, with odds ratio of 3.2 (p=0.01).
Recent work by Ayuk et al. [50] was aimed for searching potential HLA-related and non-HLA factors impacting overall survival in general HSCT practice. The authors observed 3215 HSCTs performed between 2005 and 2013 in Germany for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Their donors were HLA-matched related (MRD; n=872) or unrelated (10/10 MUD, n=1553) or HLA-mismatched unrelated (<10/10 MMUD, n=790). Among non-HLA related factors, age, sex mismatching (male recipient-female donor), and cytomegalovirus (CMV) mismatching (positive recipient-negative donor) seem to influence overall survival. Their adverse effects may be comparable with a single HLA antigen mismatch.
Hence, an increased risk for acute GvHD is observed in cohorts with female-to male HSCT. Association with graft-versus-leukemia effect in these donor-recipient pairs is not proven.
Sex hormones as potential modifiers of HSCT-associated events
In this respect, experimental animal studies have demonstrated that a single, small-volume infusion of ethinyl estradiol-3-sulfate (EES) has beneficial effects following trauma-hemorrhage, even in the absence of fluid resuscitation [51].
It was demonstrated that estradiol treatment exerted these effects via estradiol receptors then being able to downregulate proinflammatory cytokine cascade and intracellular oxidative damage following experimental trauma/hemorrhage [52].
In fact, administration of extradiol in experimental trauma/hemorrhage was associated with reduced TNF-α and increased IL-10 in affected rats [53].
Hence, the survival rates in sepsis may be, in principle, improved by estrogen administration [54]. Vice versa, blockade of male sex hormone receptors by antagonist drugs is also suggested to boost immune responses in complicated sepsis.
Similarly, treatment with luteinizing hormone release hormone (LHRH) after allo-HSCT in murine experiments caused an enhanced thymic reconstitution and better T cell recovery, predominantly, among in naive T cell populations, with increased T cell functions in vivo and in vitro [55]. Since LHRH provides sex steroid ablation (a decrease in luteinizing hormone and follicle-stimulating hormone production, causing a decrease in gonadal sex steroid production) it may represent a promising therapy for enhancement of immunity in secondary immunosuppression. However, these experiments were performed with female mice only.
Conclusion and future prospects
1. Higher proliferative activity of hematopoietic cells of males could be demonstrated by several independent studies, causing increased background counts of hematopoietic stem cells in HSC grafts from males compared to female donors.
2. Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher activation rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic drugs in the body.
3. More effective and stable immune functions, especially, more active antibody response is shown in females compared to males. In allo-HSCT setting, this difference may be translated into higher risk of chronic GvHD in female patients after allo-HSCT.
4. One may suggest that male patients with hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses and late mortality.
5. A number of extensive studies have shown increased risk of acute GvHD in cases of female-to male HSCT. The issue of graft-versus-leukemia effect in this donor-recipient pairs still remains open.
6. Estrogen hormones seem to be to most probable cause of gender-dependent HSCT-associated events. However, their modifying role should vary, depending on the age of patients. Therefore, age dependence of sex differences in HSCT deserves further studies.
Conflicts of interest
Non declared.
References
- Hörner S, Pasternak G, Hehlmann R. A statistically significant sex difference in the number of colony-forming cells from human peripheral blood. Ann Hematol. 1997;74(6):259-263.
- Cohen KS, Cheng S, Larson MG, Cupples LA, McCabe EL, Wang YA, Ngwa JS, Martin RP, Klein RJ, Hashmi B, Ge Y, O'Donnell CJ, Vasan RS, Shaw SY, Wang TJ. Circulating CD34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121(8):e50-6. doi: 10.1182/blood-2012-05-424846.
- Topel ML, Hayek SS, Ko YA, Sandesara PB, Samman Tahhan A, Hesaroieh I, Mahar E, Martin GS, Waller EK, Quyyumi AA. Sex Differences in Circulating Progenitor Cells. J Am Heart Assoc. 2017; 6(10). pii: e006245. doi: 10.1161/JAHA.117.006245.
- Radivoyevitch T, Jankovic GM, Tiu RV, Saunthararajah Y, Jackson RC, Hlatky LR, Gale RP, Sachs RK. Sex differences in the incidence of chronic myeloid leukemia. Radiat Environ Biophys, 2014; 53 (1): 55-63.
- Schmetzer O, Floercken A, Sex differences in the drug therapy for oncologic diseases. Handb Exp Pharmacol. 2012; 214: 411-442.
- European Medicines Agency (EMA): http://www.ema. europa.eu.
- Wolbold R, Klein K, Burk O, et al. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003; 38(4):978-988.
- Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992; 44(2):275-283.
- Fujita KI. Cytochrome P450 and anticancer drugs. Curr Drug Metab. 2006; 7:23-37.
- Bebawy M, Chetty M. Gender differences in P-glycoprotein expression and function: effects on drug disposition and outcome. Curr Drug Metab. 2009; 10(4) 322-328.
- Kacevska M, Robertson GR, Clarke SJ, Liddle C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: impact and implications for chemotherapeutic drug dosing. Exp Opin Drug Metab Toxicol 2008; 4 (2): 137-149.
- Benet LZ, Cummins CL et al (2004) Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int J Pharm 277(1-2):3-9.
- Schuetz EG, Furuya KN, Schuetz JD. Interindividual variation in expression of P-glycoprotein in normal human liver and secondary hepatic neoplasms. J Pharmacol Exp Ther. 1995, 275(2): 1011-1018.
- Momper JD, Misel ML, McKay DB. Sex Differences in Transplantation. Transplant Rev. 2017; 31(3):145-150.
- Bleck JS, Thiesemann C, Kliem V, et al. Diltiazem increases blood concentrations of cyclized cyclosporine metabolites resulting in different cyclosporine metabolite patterns in stable male and female renal allograft recipients. Br J Clin Pharmacol. 1996; 41(6):551-556.
- Velickovic-Radovanovic R, Mikov M, Paunovic G, et al. Gender differences in pharmacokinetics of tacrolimus and their clinical significance in kidney transplant recipients. Gender Med. 2011; 8(1):23-31.
- Zimmerman JJ. Exposure-response relationships and drug interactions of sirolimus. The AAPS journal. 2004; 6(4):e28.
- Morissette P, Albert C, Busque S, St-Louis G, Vinet B. In vivo higher glucuronidation of mycophenolic acid in male than in female recipients of a cadaveric kidney allograft and under immunosuppressive therapy with mycophenolate mofetil. Ther Drug Monit. 2001; 23(5):520-525.
- Gallagher CJ, Balliet RM, Sun D, Chen G, Lazarus P. Sex differences in UDP-glucuronosyltransferase 2B17 expression and activity. Drug Metab Disposition. 2010; 38(12):2204-2209.
- Magee MH, Blum RA, Lates CD, Jusko WJ. Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J Clin Pharmacol. 2001; 41(11):1180-1194.
- Bösch F, Angele MK, Chaudry IH. Gender differences in trauma, shock and sepsis. Military Medical Research (2018) 5:35 doi: 10.1186/s40779-018-0182-5-PDF.
- Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181(4):297-300.
- Weniger M, Angele MK, Chaudry IH. The role and use of estrogens following trauma. Shock. 2016;46(1):4-11.
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature Rev Immunol. 2008; 8(9):737-744.
- Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol. 2019; 56:308-321.
- Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019; 41:239-249.
- Simon B, Kundi M, Puchhammer-Stockl E Association of HCMV specific IgG subclass antibody levels with gender and age. Exp Gerontol 2013; 48:472-475.
- Müller C et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012; 119:3276-3284.
- Ruggieri A, Anticoli S, D’Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016. 52:198-204.
- Sankaran-Walters S, Macal M, Grishina I, Nagy L, Goulart L, Coolidge K, Li J, Fenton A, Williams T, Miller MK, Flamm J, Prindiville T, George M, Dandekar S. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013; 4:10. doi: 10.1186/2042-6410-4-10.
- Ljungman P, Brandan R. Factors influencing cytomegalovirus seropositivity in stem cell transplant patients and donors. Haematologica. 2007 Aug;92(8):1139-42.
- Furman D. Sexual dimorphism in immunity: improving our understanding of vaccine immune responses in men. Expert Rev Vaccines. 2015 Mar;14(3):461-71.
- Hirsch HH, Randhawa P, Practice ASTIDCo. BK polyomavirus in solid organ transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013; 13(Suppl 4):179-188.
- Freeman RB, Paya C, Pescovitz MD, et al. Risk factors for cytomegalovirus viremia and disease developing after prophylaxis in high-risk solid-organ transplant recipients. Transplantation. 2004; 78(12):1765-1773.
- Perrottet N, Csajka C, Pascual M, et al. Population pharmacokinetics of ganciclovir in solid-organ transplant recipients receiving oral valganciclovir. Antimicrob Agents Chemother. 2009; 53(7):3017-3023.
- Inamoto Y, Kimura F, Kanda J, Sugita J, Ikegame K, Nakasone H, Nannya Y, Uchida N, Fukuda T, Yoshioka K, Ozawa Y, Kawano I, Atsuta Y, Kato K, Ichinohe T, Inoue M, Teshima T. Comparison of graft-versus-host disease-free, relapse-free survival according to a variety of graft sources: antithymocyte globulin and single cord blood provide favorable outcomes in some subgroups. Haematologica. 2016; 101(11):1592-1602.
- Locatelli F, Uderzo C, Dini G, Zecca M, Arcese W, Messina C, Andolina M, Miniero R, Porta F, Rovelli A, et al. Graft-versus-host disease in children: the AIEOP-BMT Group experience with Cyclosporin A. Bone Marrow Transplant 1993;12(6):627-633.
- Gaziev D, Polchi P, Galimberti M, Angelucci E, Giardini C, Baronciani D, Erer B, Lucarelli G. Graft-versus-host disease after bone marrow transplantation for thalassemia: an analysis of incidence and risk factors. Transplantation 1997, 63 (6), 854-60.
- Garming-Legert K, Remberger M, Ringdén O, Hassan M, Dahllöf G. Long-term salivary function after conditioning with busulfan, fractionated or single-dose TBI. Oral Dis. 2011; 17 (7): 670-676.
- Confer DL, Abress LK, Navarro W, Madrigal A. Selection of adult unrelated hematopoietic stem cell donors: beyond HLA. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S8-S11.
- Solh MM, Bashey A, Solomon SR, Morris LE, Zhang X, Brown S, Holland HK.. Long term survival among patients who are disease free at 1-year post allogeneic hematopoietic cell transplantation: a single center analysis of 389 consecutive patients. Bone Marrow Transplant. 2018; 53(5): 576-583.
- Özen M, Üstün C, Öztürk B, Topçuoğlu P, Arat M, Gündüz M, Atilla E, Bolat G, Arslan Ö, Demirer T, Akan H, İlhan O, Beksa M, Gürman G, Özcan M. Allogeneic transplantation in chronic myeloid leukemia and the effect of tyrosine kinase inhibitors on survival: a quasi-experimental study. Turk J Haematol. 2017, 34(1), 16-26.
- Kako S, Izutsu K, Kato K, Kim S-W, Mori T, Fukuda T, Kobayashi N, Taji H, Hashimoto H, Kondo T, Sakamaki H, Morishima Y, Kato K, Suzuki R, Suzumiya J, et al.. The role of hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma Am J Hematol ,2015, 90 (2), 132-138.
- Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, Volin L, Gürman G, Maertens J, Bordigoni P, Holler E, Ehninger G, Polge E, Gorin N-C, Kolb H-J, Rocha V, EBMT Acute Leukemia Working Party. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT acute leukemia Working Party. J Clin Oncol, 2007;25 (31), 4938-4945.
- Gratwohl A, Hermans J, Niederwieser D, van Biezen A, van Houwelingen HC, Apperley J; Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation EBMT. Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J. 2001;2(6):363-370.
- OKunewick JP, Kociban DL, Machen LL, Buffo MJ. Effect of donor and recipient gender disparities on fatal graft-vs.-host disease in a mouse model for major histocompatibility complex-matched unrelated-donor bone marrow transplantation. Exp Hematol 1993; 21(12): 1570-1576.
- Miklos DB, Miklos, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, Viatte S, Soiffer RJ, Antin JH, Ritz J. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004; 103(1):353-359.
- Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347-352.
- Alhashim N, Aljurf M , Hassanein M, Chaudhri N, Hashmi S, El-Gohary G, Alsharif F, Alsermani M, Alhumaid M, Al Beihany A, Shaheen M, Hanbali A, Alfraih F, Mohamed S, Alzahrani H, Elhassan T, Eldali A, Rasheed W, Ahmed S, Almohareb F, El Fakih R. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia: clinical characteristics, incidence, risk factors and outcomes. Bone Marrow Transplant, 2018; 53 (7), 838-843.
- Ayuk F, Beelen DW, Bornhäuser M, Stelljes M, Zabelina T, Finke J, Kobbe G, Wolff D, Wagner EM, Christopeit M, Schmid C, Ottinger H, Groth C, Faul C, Bertz H, Rachlis E, Wolschke C, Schetelig J, Horn PA, Mytilineos J, Guellstorf M, Kelsch R, Fleischhauer K, Kröger N, Bethge W. Relative impact of HLA matching and non-HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018;24(12):2558-2567.
- Hubbard W, Keith J, Berman J, Miller M, Scott C, Peck C, et al. 17alpha-ethynylestradiol-3-sulfate treatment of severe blood loss in rats. J Surg Res. 2015;193(1):355-360.
- Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA. Bland KI, et al. 17 beta-estradiol administration following trauma-hemorrhage prevents the increase in Kupffer cell cytokine production and MAPK activation predominately via estrogen receptor-alpha. Surgery. 2006;140(2):141-148.
- Wu CC, Chang CY, Chang ST, Chen SH. 17beta-estradiol accelerated renal tubule regeneration in male rats after ischemia/reperfusion-induced acute kidney injury. Shock. 2016;46(2):158-163.
- Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5(1):12-19.
- Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, Samstein RM, Dudakov JA, Chidgey AP, Chen-Kiang S, Boyd RL, van den Brink MR. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol, 2009,182 (9), 5846-5854. " ["~DETAIL_TEXT"]=> string(39746) "
- Hörner S, Pasternak G, Hehlmann R. A statistically significant sex difference in the number of colony-forming cells from human peripheral blood. Ann Hematol. 1997;74(6):259-263.
- Cohen KS, Cheng S, Larson MG, Cupples LA, McCabe EL, Wang YA, Ngwa JS, Martin RP, Klein RJ, Hashmi B, Ge Y, O'Donnell CJ, Vasan RS, Shaw SY, Wang TJ. Circulating CD34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121(8):e50-6. doi: 10.1182/blood-2012-05-424846.
- Topel ML, Hayek SS, Ko YA, Sandesara PB, Samman Tahhan A, Hesaroieh I, Mahar E, Martin GS, Waller EK, Quyyumi AA. Sex Differences in Circulating Progenitor Cells. J Am Heart Assoc. 2017; 6(10). pii: e006245. doi: 10.1161/JAHA.117.006245.
- Radivoyevitch T, Jankovic GM, Tiu RV, Saunthararajah Y, Jackson RC, Hlatky LR, Gale RP, Sachs RK. Sex differences in the incidence of chronic myeloid leukemia. Radiat Environ Biophys, 2014; 53 (1): 55-63.
- Schmetzer O, Floercken A, Sex differences in the drug therapy for oncologic diseases. Handb Exp Pharmacol. 2012; 214: 411-442.
- European Medicines Agency (EMA): http://www.ema. europa.eu.
- Wolbold R, Klein K, Burk O, et al. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003; 38(4):978-988.
- Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992; 44(2):275-283.
- Fujita KI. Cytochrome P450 and anticancer drugs. Curr Drug Metab. 2006; 7:23-37.
- Bebawy M, Chetty M. Gender differences in P-glycoprotein expression and function: effects on drug disposition and outcome. Curr Drug Metab. 2009; 10(4) 322-328.
- Kacevska M, Robertson GR, Clarke SJ, Liddle C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: impact and implications for chemotherapeutic drug dosing. Exp Opin Drug Metab Toxicol 2008; 4 (2): 137-149.
- Benet LZ, Cummins CL et al (2004) Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int J Pharm 277(1-2):3-9.
- Schuetz EG, Furuya KN, Schuetz JD. Interindividual variation in expression of P-glycoprotein in normal human liver and secondary hepatic neoplasms. J Pharmacol Exp Ther. 1995, 275(2): 1011-1018.
- Momper JD, Misel ML, McKay DB. Sex Differences in Transplantation. Transplant Rev. 2017; 31(3):145-150.
- Bleck JS, Thiesemann C, Kliem V, et al. Diltiazem increases blood concentrations of cyclized cyclosporine metabolites resulting in different cyclosporine metabolite patterns in stable male and female renal allograft recipients. Br J Clin Pharmacol. 1996; 41(6):551-556.
- Velickovic-Radovanovic R, Mikov M, Paunovic G, et al. Gender differences in pharmacokinetics of tacrolimus and their clinical significance in kidney transplant recipients. Gender Med. 2011; 8(1):23-31.
- Zimmerman JJ. Exposure-response relationships and drug interactions of sirolimus. The AAPS journal. 2004; 6(4):e28.
- Morissette P, Albert C, Busque S, St-Louis G, Vinet B. In vivo higher glucuronidation of mycophenolic acid in male than in female recipients of a cadaveric kidney allograft and under immunosuppressive therapy with mycophenolate mofetil. Ther Drug Monit. 2001; 23(5):520-525.
- Gallagher CJ, Balliet RM, Sun D, Chen G, Lazarus P. Sex differences in UDP-glucuronosyltransferase 2B17 expression and activity. Drug Metab Disposition. 2010; 38(12):2204-2209.
- Magee MH, Blum RA, Lates CD, Jusko WJ. Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J Clin Pharmacol. 2001; 41(11):1180-1194.
- Bösch F, Angele MK, Chaudry IH. Gender differences in trauma, shock and sepsis. Military Medical Research (2018) 5:35 doi: 10.1186/s40779-018-0182-5-PDF.
- Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, et al. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181(4):297-300.
- Weniger M, Angele MK, Chaudry IH. The role and use of estrogens following trauma. Shock. 2016;46(1):4-11.
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature Rev Immunol. 2008; 8(9):737-744.
- Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol. 2019; 56:308-321.
- Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019; 41:239-249.
- Simon B, Kundi M, Puchhammer-Stockl E Association of HCMV specific IgG subclass antibody levels with gender and age. Exp Gerontol 2013; 48:472-475.
- Müller C et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012; 119:3276-3284.
- Ruggieri A, Anticoli S, D’Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016. 52:198-204.
- Sankaran-Walters S, Macal M, Grishina I, Nagy L, Goulart L, Coolidge K, Li J, Fenton A, Williams T, Miller MK, Flamm J, Prindiville T, George M, Dandekar S. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013; 4:10. doi: 10.1186/2042-6410-4-10.
- Ljungman P, Brandan R. Factors influencing cytomegalovirus seropositivity in stem cell transplant patients and donors. Haematologica. 2007 Aug;92(8):1139-42.
- Furman D. Sexual dimorphism in immunity: improving our understanding of vaccine immune responses in men. Expert Rev Vaccines. 2015 Mar;14(3):461-71.
- Hirsch HH, Randhawa P, Practice ASTIDCo. BK polyomavirus in solid organ transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013; 13(Suppl 4):179-188.
- Freeman RB, Paya C, Pescovitz MD, et al. Risk factors for cytomegalovirus viremia and disease developing after prophylaxis in high-risk solid-organ transplant recipients. Transplantation. 2004; 78(12):1765-1773.
- Perrottet N, Csajka C, Pascual M, et al. Population pharmacokinetics of ganciclovir in solid-organ transplant recipients receiving oral valganciclovir. Antimicrob Agents Chemother. 2009; 53(7):3017-3023.
- Inamoto Y, Kimura F, Kanda J, Sugita J, Ikegame K, Nakasone H, Nannya Y, Uchida N, Fukuda T, Yoshioka K, Ozawa Y, Kawano I, Atsuta Y, Kato K, Ichinohe T, Inoue M, Teshima T. Comparison of graft-versus-host disease-free, relapse-free survival according to a variety of graft sources: antithymocyte globulin and single cord blood provide favorable outcomes in some subgroups. Haematologica. 2016; 101(11):1592-1602.
- Locatelli F, Uderzo C, Dini G, Zecca M, Arcese W, Messina C, Andolina M, Miniero R, Porta F, Rovelli A, et al. Graft-versus-host disease in children: the AIEOP-BMT Group experience with Cyclosporin A. Bone Marrow Transplant 1993;12(6):627-633.
- Gaziev D, Polchi P, Galimberti M, Angelucci E, Giardini C, Baronciani D, Erer B, Lucarelli G. Graft-versus-host disease after bone marrow transplantation for thalassemia: an analysis of incidence and risk factors. Transplantation 1997, 63 (6), 854-60.
- Garming-Legert K, Remberger M, Ringdén O, Hassan M, Dahllöf G. Long-term salivary function after conditioning with busulfan, fractionated or single-dose TBI. Oral Dis. 2011; 17 (7): 670-676.
- Confer DL, Abress LK, Navarro W, Madrigal A. Selection of adult unrelated hematopoietic stem cell donors: beyond HLA. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S8-S11.
- Solh MM, Bashey A, Solomon SR, Morris LE, Zhang X, Brown S, Holland HK.. Long term survival among patients who are disease free at 1-year post allogeneic hematopoietic cell transplantation: a single center analysis of 389 consecutive patients. Bone Marrow Transplant. 2018; 53(5): 576-583.
- Özen M, Üstün C, Öztürk B, Topçuoğlu P, Arat M, Gündüz M, Atilla E, Bolat G, Arslan Ö, Demirer T, Akan H, İlhan O, Beksa M, Gürman G, Özcan M. Allogeneic transplantation in chronic myeloid leukemia and the effect of tyrosine kinase inhibitors on survival: a quasi-experimental study. Turk J Haematol. 2017, 34(1), 16-26.
- Kako S, Izutsu K, Kato K, Kim S-W, Mori T, Fukuda T, Kobayashi N, Taji H, Hashimoto H, Kondo T, Sakamaki H, Morishima Y, Kato K, Suzuki R, Suzumiya J, et al.. The role of hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma Am J Hematol ,2015, 90 (2), 132-138.
- Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, Volin L, Gürman G, Maertens J, Bordigoni P, Holler E, Ehninger G, Polge E, Gorin N-C, Kolb H-J, Rocha V, EBMT Acute Leukemia Working Party. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT acute leukemia Working Party. J Clin Oncol, 2007;25 (31), 4938-4945.
- Gratwohl A, Hermans J, Niederwieser D, van Biezen A, van Houwelingen HC, Apperley J; Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation EBMT. Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J. 2001;2(6):363-370.
- OKunewick JP, Kociban DL, Machen LL, Buffo MJ. Effect of donor and recipient gender disparities on fatal graft-vs.-host disease in a mouse model for major histocompatibility complex-matched unrelated-donor bone marrow transplantation. Exp Hematol 1993; 21(12): 1570-1576.
- Miklos DB, Miklos, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, Viatte S, Soiffer RJ, Antin JH, Ritz J. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004; 103(1):353-359.
- Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103(1):347-352.
- Alhashim N, Aljurf M , Hassanein M, Chaudhri N, Hashmi S, El-Gohary G, Alsharif F, Alsermani M, Alhumaid M, Al Beihany A, Shaheen M, Hanbali A, Alfraih F, Mohamed S, Alzahrani H, Elhassan T, Eldali A, Rasheed W, Ahmed S, Almohareb F, El Fakih R. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia: clinical characteristics, incidence, risk factors and outcomes. Bone Marrow Transplant, 2018; 53 (7), 838-843.
- Ayuk F, Beelen DW, Bornhäuser M, Stelljes M, Zabelina T, Finke J, Kobbe G, Wolff D, Wagner EM, Christopeit M, Schmid C, Ottinger H, Groth C, Faul C, Bertz H, Rachlis E, Wolschke C, Schetelig J, Horn PA, Mytilineos J, Guellstorf M, Kelsch R, Fleischhauer K, Kröger N, Bethge W. Relative impact of HLA matching and non-HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018;24(12):2558-2567.
- Hubbard W, Keith J, Berman J, Miller M, Scott C, Peck C, et al. 17alpha-ethynylestradiol-3-sulfate treatment of severe blood loss in rats. J Surg Res. 2015;193(1):355-360.
- Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA. Bland KI, et al. 17 beta-estradiol administration following trauma-hemorrhage prevents the increase in Kupffer cell cytokine production and MAPK activation predominately via estrogen receptor-alpha. Surgery. 2006;140(2):141-148.
- Wu CC, Chang CY, Chang ST, Chen SH. 17beta-estradiol accelerated renal tubule regeneration in male rats after ischemia/reperfusion-induced acute kidney injury. Shock. 2016;46(2):158-163.
- Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5(1):12-19.
- Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, Samstein RM, Dudakov JA, Chidgey AP, Chen-Kiang S, Boyd RL, van den Brink MR. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol, 2009,182 (9), 5846-5854. " ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(3) "500" ["~SORT"]=> string(3) "500" ["CODE"]=> string(67) "faktor-pola-pri-transplantatsii-gemopoeticheskikh-stvolovykh-kletok" ["~CODE"]=> string(67) "faktor-pola-pri-transplantatsii-gemopoeticheskikh-stvolovykh-kletok" ["EXTERNAL_ID"]=> string(4) "1835" ["~EXTERNAL_ID"]=> string(4) "1835" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(176) "Фактор пола при трансплантации гемопоэтических стволовых клетокGender factor in hematopoietic stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4342) "<p style="text-align: justify;">Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_META_TITLE"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_META_KEYWORDS"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_META_DESCRIPTION"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_ALT"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_TITLE"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_NAME"]=> string(71) "faktor-pola-pri-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(120) "Фактор пола при трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(71) "faktor-pola-pri-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(71) "faktor-pola-pri-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(71) "faktor-pola-pri-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "143" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26057" ["VALUE"]=> string(22) "02/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26058" ["VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(1) { [0]=> string(5) "26076" } ["VALUE"]=> array(1) { [0]=> string(3) "119" } ["DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(1) { [0]=> string(3) "119" } ["~DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26060" ["VALUE"]=> array(2) { ["TEXT"]=> string(54) "<p>Алексей Б. Чухловин</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(42) "
- Ruutu T, Nihtinen A, Niittyvuopio R, Juvonen E, Volin L. A randomized study of cyclosporine and methotrexate with or without methylprednisolone for the prevention of graft-versus-host disease: Improved long-term survival with triple prophylaxis. Cancer. 2018; 124: 727-733.
- Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, Ciceri F, Cornelissen J, Malladi R, Duarte RF, Giebel S, Greinix H, Holler E, Lawitschka A, Mielke S, Mohty M, Arat M, Nagler A, Passweg J, Schoemans H, Socié G, Solano C, Vrhovac R, Zeiser R, Kröger N, Basak GW. Prophylaxis and management of graft-versus-host disease after stem cell transplantation for haematologic malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020; 7: e157-e167.
- Storb R, Pepe M, Anasetti C, Appelbaum FR, Beatty P, Doney K, Martin P, Stewart P, Sullivan KM, Witherspoon R, Bensinger W, Buckner CD, Clift R, Hansen J, Longton G, Loughran T, Petersen FB, Singer J, Sanders J, Thomas ED. What role for prednisone in prevention of acute graft-versus-host disease in patients undergoing marrow transplant? Blood. 1990; 76: 1037-1045.
- Atkinson K, Biggs J, Concannon A, Dodds A, Young S, Wilson F, Ashby M, Downs K. A prospective randomized trial of cyclosporine and methotrexate versus cyclosporine, methotrexate and prednisolone for prevention of graft-versus-host disease after HLA-identical sibling marrow transplantation for haematological malignancy. Aust NZ J Med. 1991; 21: 850-856.
- Hoyt R, Ritchie DS, Roberts AW, MacGregor L, Curtis DJ, Szer J, Grigg AP. Cyclosporin, methotrexate and prednisolone for graft-versus-host disease prophylaxis in allogeneic peripheral blood progenitor cell transplants. Bone Marrow Transplant. 2008; 41: 651-658.
- Ruutu T, Volin L, Parkkali T, Juvonen E, Elonen E. Cyclosporine, methotrexate, and methylprednisolone compared with cyclosporine and methotrexate for the prevention of graft-versus-host disease in bone marrow transplantation from HLA-identical sibling donor: a prospective randomized study. Blood. 2000; 96:2391-2398.
- Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014; 124: 374-384.
- Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, Travis LB, Travis WD, Flowers MED, Friedman DL, Horowitz MM, Wingard JR, Deeg HJ. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009; 113: 1175-1183.
- Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, Bolwell B, Wingard JR, Socie G. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011; 117: 316-322.
- Shimoni A, Shem-Tov N, Chetrit A, Volchek Y, Tallis E, Avigdor A, Sadetzki S, Yerushalmi R, Nagler A. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia. 2013; 27: 829-835.
- Chow EJ, Wong K, Lee SJ, Cushing-Haugen KL, Flowers MED, Friedman DL, Leisenring WL, Martin PJ, Mueller BA, Baker KS. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014; 20: 794-800.
- Deeg HJ, Flowers MED, Leisenring W, Appelbaum FR, Martin PJ, Storb RF. Cyclosporine (CSP) or CSP plus methylprednisolone for graft-versus-host-disease prophylaxis in patients with high-risk lymphohemopoietic malignancies: long-term follow-up of a randomized trial. Blood. 2000; 96:1194-1195.
- Deeg HJ, Lin D, Leisenring W, Boeckh R, Anasetti C, Appelbaum FR, Chauncey TR, Doney K, Flowers M, Martin P, Nash R, Schoch G, Sullivan KM, Witherspoon RP, Storb R. Cyclosporine or cyclosporine plus methylprednisolone for prophylaxis of graft-versus-host disease: a prospective randomized trial. Blood. 1997; 89: 3880-3887.
- Alyea EP. Graft-versus-host disease prevention: corticosteroids revisited. J Clin Oncol. 2016; 34: 1836-1837.
- Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Huang XJ. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J Clin Oncol. 2016; 34: 1855-1863.
- Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Huang XJ. Effect of low-dose glucocorticoid prophylaxis on chronic graft-versus-host disease and graft-versus-host disease-free, relapse-free survival after haploidentical transplantation: long-term follow-up of a controlled, randomized open-label trial. Biol Blood Marrow Transplant. 2019; 25: 529-537.
- Ruutu T, Nihtinen A, Niittyvuopio R, Juvonen E, Volin L. A randomized study of cyclosporine and methotrexate with or without methylprednisolone for the prevention of graft-versus-host disease: Improved long-term survival with triple prophylaxis. Cancer. 2018; 124: 727-733.
- Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, Ciceri F, Cornelissen J, Malladi R, Duarte RF, Giebel S, Greinix H, Holler E, Lawitschka A, Mielke S, Mohty M, Arat M, Nagler A, Passweg J, Schoemans H, Socié G, Solano C, Vrhovac R, Zeiser R, Kröger N, Basak GW. Prophylaxis and management of graft-versus-host disease after stem cell transplantation for haematologic malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020; 7: e157-e167.
- Storb R, Pepe M, Anasetti C, Appelbaum FR, Beatty P, Doney K, Martin P, Stewart P, Sullivan KM, Witherspoon R, Bensinger W, Buckner CD, Clift R, Hansen J, Longton G, Loughran T, Petersen FB, Singer J, Sanders J, Thomas ED. What role for prednisone in prevention of acute graft-versus-host disease in patients undergoing marrow transplant? Blood. 1990; 76: 1037-1045.
- Atkinson K, Biggs J, Concannon A, Dodds A, Young S, Wilson F, Ashby M, Downs K. A prospective randomized trial of cyclosporine and methotrexate versus cyclosporine, methotrexate and prednisolone for prevention of graft-versus-host disease after HLA-identical sibling marrow transplantation for haematological malignancy. Aust NZ J Med. 1991; 21: 850-856.
- Hoyt R, Ritchie DS, Roberts AW, MacGregor L, Curtis DJ, Szer J, Grigg AP. Cyclosporin, methotrexate and prednisolone for graft-versus-host disease prophylaxis in allogeneic peripheral blood progenitor cell transplants. Bone Marrow Transplant. 2008; 41: 651-658.
- Ruutu T, Volin L, Parkkali T, Juvonen E, Elonen E. Cyclosporine, methotrexate, and methylprednisolone compared with cyclosporine and methotrexate for the prevention of graft-versus-host disease in bone marrow transplantation from HLA-identical sibling donor: a prospective randomized study. Blood. 2000; 96:2391-2398.
- Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014; 124: 374-384.
- Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, Travis LB, Travis WD, Flowers MED, Friedman DL, Horowitz MM, Wingard JR, Deeg HJ. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009; 113: 1175-1183.
- Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, Horowitz MM, Bolwell B, Wingard JR, Socie G. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011; 117: 316-322.
- Shimoni A, Shem-Tov N, Chetrit A, Volchek Y, Tallis E, Avigdor A, Sadetzki S, Yerushalmi R, Nagler A. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia. 2013; 27: 829-835.
- Chow EJ, Wong K, Lee SJ, Cushing-Haugen KL, Flowers MED, Friedman DL, Leisenring WL, Martin PJ, Mueller BA, Baker KS. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014; 20: 794-800.
- Deeg HJ, Flowers MED, Leisenring W, Appelbaum FR, Martin PJ, Storb RF. Cyclosporine (CSP) or CSP plus methylprednisolone for graft-versus-host-disease prophylaxis in patients with high-risk lymphohemopoietic malignancies: long-term follow-up of a randomized trial. Blood. 2000; 96:1194-1195.
- Deeg HJ, Lin D, Leisenring W, Boeckh R, Anasetti C, Appelbaum FR, Chauncey TR, Doney K, Flowers M, Martin P, Nash R, Schoch G, Sullivan KM, Witherspoon RP, Storb R. Cyclosporine or cyclosporine plus methylprednisolone for prophylaxis of graft-versus-host disease: a prospective randomized trial. Blood. 1997; 89: 3880-3887.
- Alyea EP. Graft-versus-host disease prevention: corticosteroids revisited. J Clin Oncol. 2016; 34: 1836-1837.
- Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Huang XJ. Controlled, randomized, open-label trial of risk-stratified corticosteroid prevention of acute graft-versus-host disease after haploidentical transplantation. J Clin Oncol. 2016; 34: 1855-1863.
- Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, Wang FR, Han W, Sun YQ, Yan CH, Tang FF, Mo XD, Liu KY, Huang XJ. Effect of low-dose glucocorticoid prophylaxis on chronic graft-versus-host disease and graft-versus-host disease-free, relapse-free survival after haploidentical transplantation: long-term follow-up of a controlled, randomized open-label trial. Biol Blood Marrow Transplant. 2019; 25: 529-537.
- Swerdlow SH, Campo E, Pileri SA, Harris NL et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127: 2375-2390.
- Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood 2014; 123: 2636-2644.
- Wilcox RA. Optimising initial treatment for peripheral T-cell lymphoma: a tough nut to CHOP. Lancet Haematol. 2018; 5: e182-e183.
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 2004; 15: 1467-1475.
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2006; 24: 2472-2479.
- Schmitz N, Trumper L, Ziepert M, Nickelsen M et al. of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non Hodgkin Lymphoma Study Group. Blood 2010; 116: 3418-3425.
- Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T- cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 2014; 124: 1570-1577.
- Carson KR, Horwitz SM, Pinter-Brown LC, Rosen ST et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer 2017; 123: 1174-1183.
- Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M et al. ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma(ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019; 393:229-240.
- National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology for Non-Hodgkin’s Lymphomas. Version 2.2019.
- Schmitz N, de Leval L. How I manage peripheral T-cell lymphoma, not otherwise specified and angioimmunoblastic T-cell lymphoma: current practice and a glimpse into the future. Br J Haematol 2017; 176: 851-866.
- Zain JM. Aggressive T-cell lymphomas: 2019 updates on diagnosis, risk stratification, and management. Am J Hematol 2019; 94: 929-946.
- Schmitz N, Lenz G, Stelljes M. Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood 2018; 132: 245-253.
- Kharfan-Dabaja MA, Kumar A, Ayala E, Hamadani M, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T cell and NK/T cell lymphomas: an international collaborative effort on behalf of the guidelines committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017; 23(11):1826-1838.
- Rohlfing S, Dietrich S, Witzens-Harig M, Hegenbart U et al. The impact of stem cell transplantation on the natural course of peripheral T-cell lymphoma: a real-world experience. Ann Hematol 2018; 97: 1241-1250.
- Fossard G, Broussais F, Coelho I, Bailly S et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncology 2018; 29: 715-723.
- Epperla N, Ahn KW, Litovich C, Ahmed A et al. Allogeneic hematopoietic cell transplantation provides effective salvage despite refractory disease or failed prior autologous transplant in angioimmunoblastic T-cell lymphoma: a CIBMTR analysis. J Hematol Oncol 2019; 6: doi: 10.1186/s13045-018-0696-z.
- Duarte R, Labopin M, Bader P, Basak GW et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumors and immune disorders; current practice in Europe, 2019. Bone Marrow Transplant 2019; 54: 1525-15.
- Park SI, Horwitz SM, Foss FM, Pinter-Brown LC, Carson KR et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer 2019; 125:1507-1517.
- Swerdlow SH, Campo E, Pileri SA, Harris NL et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127: 2375-2390.
- Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood 2014; 123: 2636-2644.
- Wilcox RA. Optimising initial treatment for peripheral T-cell lymphoma: a tough nut to CHOP. Lancet Haematol. 2018; 5: e182-e183.
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 2004; 15: 1467-1475.
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2006; 24: 2472-2479.
- Schmitz N, Trumper L, Ziepert M, Nickelsen M et al. of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non Hodgkin Lymphoma Study Group. Blood 2010; 116: 3418-3425.
- Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T- cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 2014; 124: 1570-1577.
- Carson KR, Horwitz SM, Pinter-Brown LC, Rosen ST et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer 2017; 123: 1174-1183.
- Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M et al. ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma(ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019; 393:229-240.
- National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology for Non-Hodgkin’s Lymphomas. Version 2.2019.
- Schmitz N, de Leval L. How I manage peripheral T-cell lymphoma, not otherwise specified and angioimmunoblastic T-cell lymphoma: current practice and a glimpse into the future. Br J Haematol 2017; 176: 851-866.
- Zain JM. Aggressive T-cell lymphomas: 2019 updates on diagnosis, risk stratification, and management. Am J Hematol 2019; 94: 929-946.
- Schmitz N, Lenz G, Stelljes M. Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood 2018; 132: 245-253.
- Kharfan-Dabaja MA, Kumar A, Ayala E, Hamadani M, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T cell and NK/T cell lymphomas: an international collaborative effort on behalf of the guidelines committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017; 23(11):1826-1838.
- Rohlfing S, Dietrich S, Witzens-Harig M, Hegenbart U et al. The impact of stem cell transplantation on the natural course of peripheral T-cell lymphoma: a real-world experience. Ann Hematol 2018; 97: 1241-1250.
- Fossard G, Broussais F, Coelho I, Bailly S et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncology 2018; 29: 715-723.
- Epperla N, Ahn KW, Litovich C, Ahmed A et al. Allogeneic hematopoietic cell transplantation provides effective salvage despite refractory disease or failed prior autologous transplant in angioimmunoblastic T-cell lymphoma: a CIBMTR analysis. J Hematol Oncol 2019; 6: doi: 10.1186/s13045-018-0696-z.
- Duarte R, Labopin M, Bader P, Basak GW et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumors and immune disorders; current practice in Europe, 2019. Bone Marrow Transplant 2019; 54: 1525-15.
- Park SI, Horwitz SM, Foss FM, Pinter-Brown LC, Carson KR et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer 2019; 125:1507-1517.
- WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008.
- Mikhailova NB, Morozova EV, Leenman EE, Afanasyev BV. Subcutaneous panniculitis-like T-cell lymphoma. Case report and literature review. Clinical Oncohematology. 2008; 1(4):356-360 (In Russian).
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
- Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
- Bellei M, Foss FM, Shustov AR, Horwitz SM , Marcheselli L, Kim WS, Cabrera ME, Dlouhy I, Nagler A, Advani RH, Pesce EA, Ko Y-H, Martinez V, Montoto Silvia, Chiattone C, Moskowitz A, Spina M, Biasoli I, Manni M, Federico M, International T-cell Project Network. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica. 2018;103(7):1191-1197.
- Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S, Suzushima H, Tsukasaki K, Nosaka K, Fujiwara H, Ishitsuka K, Inagaki H, Ogura M, Akinaga S, Tomonaga M, Tobinai K, Ueda R. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837-842.
- Phillips AA, Fields PA, Hermine O, Ramos JC, Beltran BE, Pereira J, Wandroo F, Feldman T, Taylor GP, Sawas A, Humphrey J, Kurman M, Moriya J, Dwyer K, Leoni M, Conlon K, Cook L, Gonsky J, Horwitz SM, 0761-009 Study Group. Mogamulizumab versus investigator's choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 2019;104(5):993-1003.
- Duvic M, Pinter-Brown LC, Foss FM, Sokol L, Jorgensen JL, Challagundla P, Dwyer KM, Zhang X, Kurman MR, Ballerini R, Liu L, Kim YH. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883-1889.
- Ogura M, Ishida T, Hatake K, Taniwaki M, Ando K, Tobinai K, Fujimoto K, Yamamoto K, Miyamoto T, Uike N, Tanimoto M, Tsukasaki K, Ishizawa K, Suzumiya J, Inagaki H, Tamura K, Akinaga S, Tomonaga M, Ueda R. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157-1163.
- Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, Whittaker S, Tokura Y, Vermeer M, Zinzani PL, Sokol L, Morris S, Kim EJ, Ortiz-Romero PL, Eradat H, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204.
- Lundin J, Hagberg H, Repp R, Cavallin-Ståhl E, Fredén S, Juliusson G, Rosenblad E, Tjønnfjord G, Wiklund T, Osterborg A. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101(11):4267-4272.
- Enblad G, Hagberg H, Erlanson M, Lundin J, MacDonald AP, Repp R, Schetelig J, Seipelt G, Osterborg A. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103(8):2920-2924.
- de Masson A, Guitera P, Brice P, Moulonguet I, Mouly F, Bouaziz JD, Battistella M, Madelaine I, Roux J, Ram-Wolff C, Cayuela JM, Bachelez H, Bensussan A, Michel L, Bagot M. Long-term efficacy and safety of alemtuzumab in advanced primary cutaneous T-cell lymphomas. Br J Dermatol. 2014; 170(3):720-724.
- Zinzani PL, Alinari L, Tani M, Fina M, Pileri S, Baccarani M. Preliminary observations of a phase II study of reduced-dose alemtuzumab treatment in patients with pretreated T-cell lymphoma. Haematologica. 2005;90(5):702-703.
- Kim SJ, Kim K, Park Y, Kim BS, Huh J, Ko YH, Park K, Suh C, Kim WS. Dose modification of alemtuzumab in combination with dexamethasone, cytarabine, and cisplatin in patients with relapsed or refractory peripheral T-cell lymphoma: analysis of efficacy and toxicity. Invest New Drugs. 2012;30(1):368-375.
- Kim JG, Sohn SK, Chae YS, Cho YY, Yang DH, Lee JJ, Kim HJ, Shin HJ, Chung JS, Cho GJ, Lee WS, Joo YD, Sohn CH, Oh SJ. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother Pharmacol. 2007;60(1):129-134.
- Gallamini A, Zaja F, Patti C, Billio A, Specchia MR, Tucci A, Levis A, Manna A, Secondo V, Rigacci L, Pinto A, Iannitto E, Zoli V, Torchio P, Pileri S, Tarella C. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110(7):2316-2323.
- Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, van Putten WL, Luten M, Oudejans J, van Imhoff GW. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol. 2011;22(7):1595-600.
- Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ, Habermann TM, Kahl BS, Advani RH. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma. 2014;55(4):768-772.
- Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126(1):17-25.
- Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar M, Avigan D, Chapuy B, Ligon AH, Freeman GJ, Rodig SJ, Cattry D, Zhu L, Grosso JF, Bradley Garelik MB, Shipp MA, Borrello I, Timmerman J. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698-2704.
- Khodadoust M, Rook AH, Porcu P, Foss FM, Moskowitz AJ, Shustov AR, Shanbhag S, Sokol L, Shine R, Fling S, Li S, Rabhar Z, Kim J, Yang Y, Yearley J et al. Pembrolizumab for treatment of relapsed/refractory mycosis fungoides and Sezary syndrome: clinical efficacy in a Citn multicenter phase 2 study. Blood. 2016; 128 (22): 181.
- Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C, Tse E. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017 Apr 27;129(17):2437-2442.
- Barta SK, Zain J, MacFarlane AW 4th, Smith SM, Ruan J, Fung HC, Tan CR, Yang Y, Alpaugh RK, Dulaimi E, Ross EA, Campbell KS, Khan N, Siddharta R, Fowler NH, Fisher RI, Oki Y. Phase II study of the PD-1 inhibitor pembrolizumab for the treatment of relapsed or refractory mature T-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(6):356-364.e3.
- Rong Tao, Lei Fan, Yongping Song, Yu Hu, Wei Zhang, Yafei Wang, Linxinyu Xu, Hui Zhou, Jianyong Li. Sintilimab for relapsed/refractory (r/r) extranodal NK/T-cell lymphoma (ENKTL): A multicenter, single-arm, phase 2 trial (ORIENT-4). J Clin Oncol. 2019; 37(15 Suppl): 7504-7504.
- Ansell S, Gutierrez ME, Shipp MA, Gladstone DE, Moskowitz AJ, Borello I, Popa-Mckiver M, Farsaci B, Zhu L, Lesokhin AM, Armand P. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039). Blood (2016) 128 (22): 183. doi.org/10.1182/blood.V128.22.183.183.
- Querfeld C, Zain JM, Wakefield DL, Jovanovic-Talisman T, Kil SH, Estephan R, Sanchez J, Palmer J, Rosen ST. Phase 1/2 trial of durvalumab and lenalidomide in patients with cutaneous T cell lymphoma (CTCL): preliminary results of Phase I results and correlative studies. Blood 2018; 132 (Supplement 1): 2931. doi: https://doi.org/10.1182/blood-2018-99-114001.
- Eyal S, Yagen B, Shimshoni J, Bialer M. Histone deacetylases inhibition and tumor cells cytotoxicity by CNS-active VPA constitutional isomers and derivatives. Biochem Pharmacol. 2005;69(10):1501-1508.
- O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S, Bhat G, Choi MR, Walewski J, Savage K, Foss F, Allen LF, Shustov A. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33(23):2492-2499.
- Duvic M, Dummer R, Becker JC, Poulalhon N, Ortiz Romero P, Grazia Bernengo M, Lebbé C, Assaf C, Squier M, Williams D, Marshood M, Tai F, Prince HM. Panobinostat activity in both bexarotene-exposed and -naïve patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer. 2013:49(2):386-394.
- Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, Fan Y, Li W, Zhao X, Qi J, Huang H, Zhou D, Ning Z, Lu X. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26(8):1766-1771.
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Borchmann P, Morschhauser F, Wilhelm M, Pinter-Brown L, Padmanabhan S, Shustov A, Nichols J, Carroll S, Balser J, Balser B, Horwitz S. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631-636.
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109(1):31-39.
- Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS. Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol. 2016;27(3):508-513.
- Poggio T, Duyster J, Illert AL. Current immunotherapeutic approaches in T cell non-Hodgkin lymphomas. Cancers (Basel). 2018;10(9):339. 2018 Sep 18. doi:10.3390/cancers10090339.
- Scarfò I, Frigault MJ, Maus MV. CAR-based approaches to cutaneous T-cell lymphoma. Front Oncol. 2019;9:259. doi:10.3389/fonc.2019.00259.
- Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, Morales A, Abdulla F, Xing L, Navi D, Tibshirani RJ, Advani RH, Lingala B, Shah S, Hoppe RT, Levy R. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119(2):355-363.
- Abecasis M, Gomez C, Ferreira I, Gomes da Silva M, Miranda N, Teixeira G, Leal da Costa F, João Gutierrez M. Stem cell transplantation as consolidation in peripheral T-cell lymphomas. Cell Ther Transplant 2020; 9(1): 22-27.
- Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, Bartlett NL, Christensen JH, Morschhauser F, Domingo-Domenech E, Rossi G, Kim WS, Feldman T, Lennard A, Belada D, Illés A. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240.
- Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Fenton K, Huebner D, Pinelli JM, Kennedy DA, Shustov A. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709-2717.
- Kim YH, Tavallaee M, Sundram U, Salva KA, Wood GS, Li S, Rozati S, Nagpal S, Krathen M, Reddy S, Hoppe RT, Nguyen-Lin A, Weng WK, Armstrong R, Pulitzer M, Advani RH, Horwitz SM. Phase II investigator-initiated study of Brentuximab Vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: a multi-institution collaborative project. J Clin Oncol. 2015;33(32):3750-3758.
- Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O'Connor OA, Siddiqi T, Kennedy DA, OkI Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095-3100.
- Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, Corbacioglu S, Dreger P, Dufour C, Gennery AR, Kuball J, Lankester AC, Lanza F, Montoto S, Nagler A, Peffault de Latour R, Snowden JA, Styczynski J, Yakoub-Agha I, Kröger N, Mohty M; European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019;54(10):1525-1552.
- Trajkovska I, Georgievski B, Cevreska L, Gacovski A, Hasan T, Nedeska-Minova N. Early and late complications in patients with allogeneic transplantation of hematopoietic stem cell – case report. Open Access Maced J Med Sci. 2017;5(3):340-343. Publ. 2017 May 9. doi:10.3889/oamjms.2017.038
- Marchi E, O’Connor OA. The rapidly changing landscape in mature T‐cell lymphoma (MTCL) biology and management. CA A Cancer J Clin. 2020;70:47-70.
- WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008.
- Mikhailova NB, Morozova EV, Leenman EE, Afanasyev BV. Subcutaneous panniculitis-like T-cell lymphoma. Case report and literature review. Clinical Oncohematology. 2008; 1(4):356-360 (In Russian).
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
- Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
- Bellei M, Foss FM, Shustov AR, Horwitz SM , Marcheselli L, Kim WS, Cabrera ME, Dlouhy I, Nagler A, Advani RH, Pesce EA, Ko Y-H, Martinez V, Montoto Silvia, Chiattone C, Moskowitz A, Spina M, Biasoli I, Manni M, Federico M, International T-cell Project Network. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica. 2018;103(7):1191-1197.
- Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, Saburi Y, Miyamoto T, Takemoto S, Suzushima H, Tsukasaki K, Nosaka K, Fujiwara H, Ishitsuka K, Inagaki H, Ogura M, Akinaga S, Tomonaga M, Tobinai K, Ueda R. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837-842.
- Phillips AA, Fields PA, Hermine O, Ramos JC, Beltran BE, Pereira J, Wandroo F, Feldman T, Taylor GP, Sawas A, Humphrey J, Kurman M, Moriya J, Dwyer K, Leoni M, Conlon K, Cook L, Gonsky J, Horwitz SM, 0761-009 Study Group. Mogamulizumab versus investigator's choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 2019;104(5):993-1003.
- Duvic M, Pinter-Brown LC, Foss FM, Sokol L, Jorgensen JL, Challagundla P, Dwyer KM, Zhang X, Kurman MR, Ballerini R, Liu L, Kim YH. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883-1889.
- Ogura M, Ishida T, Hatake K, Taniwaki M, Ando K, Tobinai K, Fujimoto K, Yamamoto K, Miyamoto T, Uike N, Tanimoto M, Tsukasaki K, Ishizawa K, Suzumiya J, Inagaki H, Tamura K, Akinaga S, Tomonaga M, Ueda R. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157-1163.
- Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, Whittaker S, Tokura Y, Vermeer M, Zinzani PL, Sokol L, Morris S, Kim EJ, Ortiz-Romero PL, Eradat H, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204.
- Lundin J, Hagberg H, Repp R, Cavallin-Ståhl E, Fredén S, Juliusson G, Rosenblad E, Tjønnfjord G, Wiklund T, Osterborg A. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101(11):4267-4272.
- Enblad G, Hagberg H, Erlanson M, Lundin J, MacDonald AP, Repp R, Schetelig J, Seipelt G, Osterborg A. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103(8):2920-2924.
- de Masson A, Guitera P, Brice P, Moulonguet I, Mouly F, Bouaziz JD, Battistella M, Madelaine I, Roux J, Ram-Wolff C, Cayuela JM, Bachelez H, Bensussan A, Michel L, Bagot M. Long-term efficacy and safety of alemtuzumab in advanced primary cutaneous T-cell lymphomas. Br J Dermatol. 2014; 170(3):720-724.
- Zinzani PL, Alinari L, Tani M, Fina M, Pileri S, Baccarani M. Preliminary observations of a phase II study of reduced-dose alemtuzumab treatment in patients with pretreated T-cell lymphoma. Haematologica. 2005;90(5):702-703.
- Kim SJ, Kim K, Park Y, Kim BS, Huh J, Ko YH, Park K, Suh C, Kim WS. Dose modification of alemtuzumab in combination with dexamethasone, cytarabine, and cisplatin in patients with relapsed or refractory peripheral T-cell lymphoma: analysis of efficacy and toxicity. Invest New Drugs. 2012;30(1):368-375.
- Kim JG, Sohn SK, Chae YS, Cho YY, Yang DH, Lee JJ, Kim HJ, Shin HJ, Chung JS, Cho GJ, Lee WS, Joo YD, Sohn CH, Oh SJ. Alemtuzumab plus CHOP as front-line chemotherapy for patients with peripheral T-cell lymphomas: a phase II study. Cancer Chemother Pharmacol. 2007;60(1):129-134.
- Gallamini A, Zaja F, Patti C, Billio A, Specchia MR, Tucci A, Levis A, Manna A, Secondo V, Rigacci L, Pinto A, Iannitto E, Zoli V, Torchio P, Pileri S, Tarella C. Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) prospective multicenter trial. Blood. 2007;110(7):2316-2323.
- Kluin-Nelemans HC, van Marwijk Kooy M, Lugtenburg PJ, van Putten WL, Luten M, Oudejans J, van Imhoff GW. Intensified alemtuzumab-CHOP therapy for peripheral T-cell lymphoma. Ann Oncol. 2011;22(7):1595-600.
- Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ, Habermann TM, Kahl BS, Advani RH. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma. 2014;55(4):768-772.
- Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126(1):17-25.
- Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar M, Avigan D, Chapuy B, Ligon AH, Freeman GJ, Rodig SJ, Cattry D, Zhu L, Grosso JF, Bradley Garelik MB, Shipp MA, Borrello I, Timmerman J. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698-2704.
- Khodadoust M, Rook AH, Porcu P, Foss FM, Moskowitz AJ, Shustov AR, Shanbhag S, Sokol L, Shine R, Fling S, Li S, Rabhar Z, Kim J, Yang Y, Yearley J et al. Pembrolizumab for treatment of relapsed/refractory mycosis fungoides and Sezary syndrome: clinical efficacy in a Citn multicenter phase 2 study. Blood. 2016; 128 (22): 181.
- Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C, Tse E. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017 Apr 27;129(17):2437-2442.
- Barta SK, Zain J, MacFarlane AW 4th, Smith SM, Ruan J, Fung HC, Tan CR, Yang Y, Alpaugh RK, Dulaimi E, Ross EA, Campbell KS, Khan N, Siddharta R, Fowler NH, Fisher RI, Oki Y. Phase II study of the PD-1 inhibitor pembrolizumab for the treatment of relapsed or refractory mature T-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(6):356-364.e3.
- Rong Tao, Lei Fan, Yongping Song, Yu Hu, Wei Zhang, Yafei Wang, Linxinyu Xu, Hui Zhou, Jianyong Li. Sintilimab for relapsed/refractory (r/r) extranodal NK/T-cell lymphoma (ENKTL): A multicenter, single-arm, phase 2 trial (ORIENT-4). J Clin Oncol. 2019; 37(15 Suppl): 7504-7504.
- Ansell S, Gutierrez ME, Shipp MA, Gladstone DE, Moskowitz AJ, Borello I, Popa-Mckiver M, Farsaci B, Zhu L, Lesokhin AM, Armand P. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039). Blood (2016) 128 (22): 183. doi.org/10.1182/blood.V128.22.183.183.
- Querfeld C, Zain JM, Wakefield DL, Jovanovic-Talisman T, Kil SH, Estephan R, Sanchez J, Palmer J, Rosen ST. Phase 1/2 trial of durvalumab and lenalidomide in patients with cutaneous T cell lymphoma (CTCL): preliminary results of Phase I results and correlative studies. Blood 2018; 132 (Supplement 1): 2931. doi: https://doi.org/10.1182/blood-2018-99-114001.
- Eyal S, Yagen B, Shimshoni J, Bialer M. Histone deacetylases inhibition and tumor cells cytotoxicity by CNS-active VPA constitutional isomers and derivatives. Biochem Pharmacol. 2005;69(10):1501-1508.
- O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S, Bhat G, Choi MR, Walewski J, Savage K, Foss F, Allen LF, Shustov A. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33(23):2492-2499.
- Duvic M, Dummer R, Becker JC, Poulalhon N, Ortiz Romero P, Grazia Bernengo M, Lebbé C, Assaf C, Squier M, Williams D, Marshood M, Tai F, Prince HM. Panobinostat activity in both bexarotene-exposed and -naïve patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer. 2013:49(2):386-394.
- Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, Fan Y, Li W, Zhao X, Qi J, Huang H, Zhou D, Ning Z, Lu X. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26(8):1766-1771.
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, Caballero D, Borchmann P, Morschhauser F, Wilhelm M, Pinter-Brown L, Padmanabhan S, Shustov A, Nichols J, Carroll S, Balser J, Balser B, Horwitz S. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631-636.
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109(1):31-39.
- Kim SJ, Kim JH, Ki CS, Ko YH, Kim JS, Kim WS. Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol. 2016;27(3):508-513.
- Poggio T, Duyster J, Illert AL. Current immunotherapeutic approaches in T cell non-Hodgkin lymphomas. Cancers (Basel). 2018;10(9):339. 2018 Sep 18. doi:10.3390/cancers10090339.
- Scarfò I, Frigault MJ, Maus MV. CAR-based approaches to cutaneous T-cell lymphoma. Front Oncol. 2019;9:259. doi:10.3389/fonc.2019.00259.
- Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, Morales A, Abdulla F, Xing L, Navi D, Tibshirani RJ, Advani RH, Lingala B, Shah S, Hoppe RT, Levy R. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119(2):355-363.
- Abecasis M, Gomez C, Ferreira I, Gomes da Silva M, Miranda N, Teixeira G, Leal da Costa F, João Gutierrez M. Stem cell transplantation as consolidation in peripheral T-cell lymphomas. Cell Ther Transplant 2020; 9(1): 22-27.
- Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, Bartlett NL, Christensen JH, Morschhauser F, Domingo-Domenech E, Rossi G, Kim WS, Feldman T, Lennard A, Belada D, Illés A. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240.
- Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Fenton K, Huebner D, Pinelli JM, Kennedy DA, Shustov A. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709-2717.
- Kim YH, Tavallaee M, Sundram U, Salva KA, Wood GS, Li S, Rozati S, Nagpal S, Krathen M, Reddy S, Hoppe RT, Nguyen-Lin A, Weng WK, Armstrong R, Pulitzer M, Advani RH, Horwitz SM. Phase II investigator-initiated study of Brentuximab Vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: a multi-institution collaborative project. J Clin Oncol. 2015;33(32):3750-3758.
- Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O'Connor OA, Siddiqi T, Kennedy DA, OkI Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095-3100.
- Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, Corbacioglu S, Dreger P, Dufour C, Gennery AR, Kuball J, Lankester AC, Lanza F, Montoto S, Nagler A, Peffault de Latour R, Snowden JA, Styczynski J, Yakoub-Agha I, Kröger N, Mohty M; European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019;54(10):1525-1552.
- Trajkovska I, Georgievski B, Cevreska L, Gacovski A, Hasan T, Nedeska-Minova N. Early and late complications in patients with allogeneic transplantation of hematopoietic stem cell – case report. Open Access Maced J Med Sci. 2017;5(3):340-343. Publ. 2017 May 9. doi:10.3889/oamjms.2017.038
- Marchi E, O’Connor OA. The rapidly changing landscape in mature T‐cell lymphoma (MTCL) biology and management. CA A Cancer J Clin. 2020;70:47-70.
- Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985; 316:354-356.
- Baagen A, Van De Griend R, Clark M, Geerars A, Bast B, De Gast B. Killing of human leukaemia/lymphoma B cells by activated cytotoxic T lymphocytes in the presence of a bispecific monoclonal antibody (mCD3/mCD19). Clin Exp Immunol. 1992; 90: 368-375.
- Przepiorka D, Ko C-W, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu H-J, Gehrke BJ, Gomez-Broughton C, Kane RC, Kirshner S, Mehrotra N, Ricks TK, Schmiel D, Song P, Zhao P, Zhou Q, Farrell AT, Pazdur R. FDA Approval: Blinatumomab. Clin Cancer Res. 2015; 21(18):4035-4039.
- Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, Foà R, Litzow M, Ribera JM, Rambaldi A, Schiller G. Safety and activity of Blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm phase 2 study. Lancet Oncol. 2015;16:57-66.
- Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera J-M, Wei A, Dombret H, Foà R, Bassan R, Arslan Ö, Sanz MA, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Eng J Med. 2017;376:836-847.
- Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, Fielding AK, Rambaldi A, Ritchie EK, Papayannidis C, Sterling LR, Benjamin J, Stein A. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with Blinatumomab: Results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35:1795-1802.
- von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, Bader P, O'Brien MM, Brethon B, Bhojwani D, Schlegel PG, Borkhardt A, Rheingold SR, Cooper TM, Zwaan CM et al. Phase I/phase II study of Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
- Gokbuget N, Dombret H, Ribera JM, Fielding AK, Advani A, Bassan R, Chia V, Doubek M, Giebel S, Hoelzer D, Ifrah N, Katz A, Kelsh M, Martinelli G, Morgades M, O'Brien S et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016; 101:1524-1533.
- Sokolov A, Parovichnikova E, Troitskaya V, Galtseva I, Firsova M, Davidova J, Kapranov N, Savchenko V. Combined Blinatumomab + Dasatinib/Ibrutinib therapy of relapsed acute lymphoblastic leukemia patients – antileukemic effect on the T-helper and T-regulatory cells reduction background. Haematologica. 2016; 101: 354-355 (E867).
- Sokolov AN, Parovichnikova EN, Troitskaya VV, Kuzmina LA, Galtseva IV, Kulikov SM, Bondarenko SN, Davidova JO, Kapranov NM, Lukyanova IA, Lobanova TI, Usikova EI, Zarubina KI, Savchenko VG. Blinatumomab + tyrosine kinase inhibitors with no chemotherapy in BCR-ABL-positive or IKZF1-deleted or FLT3-ITD-positive relapsed/refractory acute lymphoblastic leukemia patients: high molecular remission rate and toxicity profile. Blood. 2017;130:3884.
- Assi R, Kantarjian H, Short NJ, Daver N, Takahashi K, Garcia-Manero G, DiNardo C, Burger J, Cortes J, Jain N, Wierda W, Chamoun S, Konopleva M, Jabbour E. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897-901.
- Churchman ML, Low J, Qu C, Paietta EM, Kasper LH, Chang Y, Payne-Turner D, Althoff MJ, Song G, Chen SC, Ma J, Rusch M, McGoldrick D, Edmonson M, Gupta P, Wang YD et al. Efficacy of retinoids in IKZF1-mutated BCR-ABL1 acute lymphoblastic leukemia. Cancer Cell. 2015;28(3):343-356.
- Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, Rasche L, Hartmann E, Dandekar T, Einsele H, Topp MS. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. (2017);31, 2181-2190.
- Zugmaier G, Gokbuget N, Klinger M, Viardot A, Stelljes M, Neumann S, Horst H-A, Marks R, Faul C, Diedrich H, Reichle A, Brüggemann M, Holland C, Schmidt M, Einsele H, Bargou RC, Topp MS. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood. 2015;126(24):2578-2584.
- Perez P, Hoffman RW, Shaw S, Bluestone JA, Segal DM. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985; 316:354-356.
- Baagen A, Van De Griend R, Clark M, Geerars A, Bast B, De Gast B. Killing of human leukaemia/lymphoma B cells by activated cytotoxic T lymphocytes in the presence of a bispecific monoclonal antibody (mCD3/mCD19). Clin Exp Immunol. 1992; 90: 368-375.
- Przepiorka D, Ko C-W, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu H-J, Gehrke BJ, Gomez-Broughton C, Kane RC, Kirshner S, Mehrotra N, Ricks TK, Schmiel D, Song P, Zhao P, Zhou Q, Farrell AT, Pazdur R. FDA Approval: Blinatumomab. Clin Cancer Res. 2015; 21(18):4035-4039.
- Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, Foà R, Litzow M, Ribera JM, Rambaldi A, Schiller G. Safety and activity of Blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm phase 2 study. Lancet Oncol. 2015;16:57-66.
- Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera J-M, Wei A, Dombret H, Foà R, Bassan R, Arslan Ö, Sanz MA, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Eng J Med. 2017;376:836-847.
- Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, Fielding AK, Rambaldi A, Ritchie EK, Papayannidis C, Sterling LR, Benjamin J, Stein A. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with Blinatumomab: Results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35:1795-1802.
- von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, Bader P, O'Brien MM, Brethon B, Bhojwani D, Schlegel PG, Borkhardt A, Rheingold SR, Cooper TM, Zwaan CM et al. Phase I/phase II study of Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389.
- Gokbuget N, Dombret H, Ribera JM, Fielding AK, Advani A, Bassan R, Chia V, Doubek M, Giebel S, Hoelzer D, Ifrah N, Katz A, Kelsh M, Martinelli G, Morgades M, O'Brien S et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016; 101:1524-1533.
- Sokolov A, Parovichnikova E, Troitskaya V, Galtseva I, Firsova M, Davidova J, Kapranov N, Savchenko V. Combined Blinatumomab + Dasatinib/Ibrutinib therapy of relapsed acute lymphoblastic leukemia patients – antileukemic effect on the T-helper and T-regulatory cells reduction background. Haematologica. 2016; 101: 354-355 (E867).
- Sokolov AN, Parovichnikova EN, Troitskaya VV, Kuzmina LA, Galtseva IV, Kulikov SM, Bondarenko SN, Davidova JO, Kapranov NM, Lukyanova IA, Lobanova TI, Usikova EI, Zarubina KI, Savchenko VG. Blinatumomab + tyrosine kinase inhibitors with no chemotherapy in BCR-ABL-positive or IKZF1-deleted or FLT3-ITD-positive relapsed/refractory acute lymphoblastic leukemia patients: high molecular remission rate and toxicity profile. Blood. 2017;130:3884.
- Assi R, Kantarjian H, Short NJ, Daver N, Takahashi K, Garcia-Manero G, DiNardo C, Burger J, Cortes J, Jain N, Wierda W, Chamoun S, Konopleva M, Jabbour E. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897-901.
- Churchman ML, Low J, Qu C, Paietta EM, Kasper LH, Chang Y, Payne-Turner D, Althoff MJ, Song G, Chen SC, Ma J, Rusch M, McGoldrick D, Edmonson M, Gupta P, Wang YD et al. Efficacy of retinoids in IKZF1-mutated BCR-ABL1 acute lymphoblastic leukemia. Cancer Cell. 2015;28(3):343-356.
- Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, Rasche L, Hartmann E, Dandekar T, Einsele H, Topp MS. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. (2017);31, 2181-2190.
- Zugmaier G, Gokbuget N, Klinger M, Viardot A, Stelljes M, Neumann S, Horst H-A, Marks R, Faul C, Diedrich H, Reichle A, Brüggemann M, Holland C, Schmidt M, Einsele H, Bargou RC, Topp MS. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood. 2015;126(24):2578-2584.
- Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, Slesarchuk OA, Bondarenko SN, Afanasyev BV. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus and mycophenalate mofetil. Biol Blood Marrow Transplant. 2016; 22(6):1037-1042.
- Shaheen M, Ivanova MO, Moiseev IS, Bondarchuk SV, Afanasyev BV. Impact of initial serum ferritin on early post-HSCT complications: a single center study. Cell Ther Transplant. 2016;5(2): 40-49.
- Morozova EV, Moiseev IS, Barabanshikova MV, Darskaya EI, Bondarenko SN, Zubarovskaya LS, Baykov VV, Alyanski AL, Barkhatov IM, Zander AR, Afanasyev BV. Graft-versus-host disease prophylaxis with posttransplantation cyclophosphamide and ruxolitinib in patients with myelofibrosis. Blood 2017; 130 (Supplement 1): 4492.
- Afanasyev BV, Zubarovskaya LS, Semenova EV, Ivanova NE, Alyanskyi AL, Morozova EV, Mikhailova N.B, Darskaya EI, Estrina MA, Golovacheva AA, Babenko EV, Bondarenko SN, Ganapiev AA, Bogomolny MP. Experience with unrelated allogeneic transplantation of hematopoietic stem cells in bone marrow transplantation clinic. Terap Arkhiv. 2007; 7: 35-42 (In Russian).
- Shcherbakov AA, Kucher AA, Shvetcov AN, Paina OV, Slesarchuk OA, Klementeva RV, Goloshchapov AV, Zubarovskaya LS, Afanasyev BV. Hemorrhagic cystitis after allogeneic transplantation of hematopoietic stem cells in children with hematological, oncological and hereditary diseases. Pediatriya – Zhurnal im G.N. Speranskogo 2018; 97(5): 41-46 (In Russian).
- Cesaro S, Dalianis T, Hanssen Rinaldo C, Koskenvuo M, Pegoraro A, Einsele H, Cordonnier C, Hirsch HH, Members of the ECIL-6 Group, ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2018; 73(1):12-21.
- Han SB, Cho B, Kang JH. BK virus-associated hemorrhagic cystitis after pediatric stem cell. Korean J Pediatr. 2014; 57(12):514-519.
- Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, Han XY, Tarrand J, Ribeiro R, Gulbis A, Shpall EJ, Jones R, Popat U, Walker JA, Petropoulos D, Chiattone A, Stewart J, El-Zimaity M, Anderlini P, Giralt S, Champlin RE, de Lima M. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95(7):1183-1190.
- Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, Mellstedt H, Remberger M, Ljungman P, Winiarski J, Dalianis T. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J Clin Microbiol. 2004;42(11):5394-5396.
- Mori T, Aisa Y, Shimizu T, Ikeda Y, Okamoto S, Okada K, Kazuyama Y. Hemorrhagic cystitis caused by adenovirus type 34 after allogeneic bone marrow transplantation. Transplantation. 2005;79(5):624.
- Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41(1):11-18.
- Chukhlovin AB, Eismont YuA, Vavilov VN, Zubarovskaya LS, Afanasyev BV. Time- and sample-dependent differences in polyomavirus incidence following hematopoietic stem cell transplantation. Cell Ther Transplant. 2016; 5(1):26-30.
- Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89(12):1462-1465.
- Sener A, House AA, Jevnikar AM, Boudville N, McAlister VC, Muirhead N, Rehman F, Luke PP. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81(1):117-120.
- Anyaegbu EI, Almond PS, Milligan T, Allen WR, Gharaybeh S, Al-Akash SI. Intravenous immunoglobulin therapy in the treatment of BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr Transplant. 2012 Feb; 16(1): E19-E24.
- Mert D, Batgi H, Merdin A, Çeken S, Dal MS, Tekgündüz E, Altuntaş F, Ertek M. BK Virus-associated hemorrhagic cystitis in patients with allogeneic hematopoietic cell transplantation: report of three cases. Hematol Rep. 2017;9(2):7205. 2017 Jun 26.
- World Health Organization. WHO model formulary 2008, Editors: Marc C. Stuart, Maria Kouimtzi, Suzanne R. Hill.
- Washburn N, Meccariello R, Hu S, Hains M, Bhatnagar N, Sarvaiya H, et al. High-resolution physicochemical characterization of different intravenous immunoglobulin products. PLoS ONE. 2017; 12(7): e0181251. https://doi.org/10.1371/journal.pone.0181251.
- Kopterides P, Theodorakopoulou M, Mentzelopoulos S, Armaganidis A. Cyclophosphamide-induced hemorrhagic cystitis successfully treated with conjugated estrogens. Am J Hematol. 2005;80(2):166-167.
- Walker RD. Cyclophosphamide-induced hemorrhagic cystitis. J Urol. 1999;161(6):1747.
- Haselberger MB, Schwinghammer TL. Efficacy of Mesna for prevention of hemorrhagic cystitis after high-dose cyclophosphamide therapy. Ann Pharmacother. 1995;29(9):918-921.
- Wang CC, Weng TI, Wu ET, Wu MH, Yang RS, Liu SH. Involvement of interleukin-6-regulated nitric oxide synthase in hemorrhagic cystitis and impaired bladder contractions in young rats induced by acrolein, a urinary metabolite of cyclophosphamide. Toxicol Sci. 2013;131(1):302-310.
- Gilis L, Morisset S, Billaud G, Ducastelle-Leprêtre S, Labussière-Wallet H, Nicolini F-E, Barraco F, Detrait M, Thomas X, Tedone N. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2014;49(5):664-670.
- Zubarovskaya LS. Efficiency evaluation of high-dose cytostatic therapy with hematopoietic stem cell transplantation in treatment of hematological and oncological disorders in children and adolescents. Doctoral Thesis. St.Petersburg, 2005:1-31 (In Russian).
- Vitrischak A, Semenova E, Ovsyannikova M, Pugachev A, Morozova E, Mikhailova N, Zubarovskaya L, Afanasyev B. Risk factors for acute GvHD after allogeneic stem cell transplantation in children. 30th Annual EBMT Meeting, Barcelona, 2004. Bone Marrow Transplant; 33 (Suppl 1 R1117): S312.
- Uhm J, Hamad N, Michelis FV, Shanavas M, Kuruvilla J, Gupta V, Lipton JH, Messner HA, Seftel M, Kim DD. The risk of polyomavirus BK-associated hemorrhagic cystitis after allogeneic hematopoietic SCT is associated with myeloablative conditioning, CMV viremia and severe acute GvHD. Bone Marrow Transplant. 2014; 49 (12): 1528-1534.
- Dalianis T, Ljungman P. Full myeloablative conditioning and an unrelated HLA-mismatched donor increase the risk for BK virus-positive hemorrhagic cystitis in allogeneic hematopoetic stem cell transplanted patients. Anticancer Res. 2011;31(3):939-944.
- Lunde LE, Dasaraju S, Cao Q, Cohn CS, Reding M, Bejanyan N, Trottier B, Rogosheske J, Brunstein C, Warlick E, Young JA, Weisdorf DJ, Ustun C. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant. 2015;50(11):1432-1437.
- Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, Slesarchuk OA, Bondarenko SN, Afanasyev BV. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus and mycophenalate mofetil. Biol Blood Marrow Transplant. 2016; 22(6):1037-1042.
- Shaheen M, Ivanova MO, Moiseev IS, Bondarchuk SV, Afanasyev BV. Impact of initial serum ferritin on early post-HSCT complications: a single center study. Cell Ther Transplant. 2016;5(2): 40-49.
- Morozova EV, Moiseev IS, Barabanshikova MV, Darskaya EI, Bondarenko SN, Zubarovskaya LS, Baykov VV, Alyanski AL, Barkhatov IM, Zander AR, Afanasyev BV. Graft-versus-host disease prophylaxis with posttransplantation cyclophosphamide and ruxolitinib in patients with myelofibrosis. Blood 2017; 130 (Supplement 1): 4492.
- Afanasyev BV, Zubarovskaya LS, Semenova EV, Ivanova NE, Alyanskyi AL, Morozova EV, Mikhailova N.B, Darskaya EI, Estrina MA, Golovacheva AA, Babenko EV, Bondarenko SN, Ganapiev AA, Bogomolny MP. Experience with unrelated allogeneic transplantation of hematopoietic stem cells in bone marrow transplantation clinic. Terap Arkhiv. 2007; 7: 35-42 (In Russian).
- Shcherbakov AA, Kucher AA, Shvetcov AN, Paina OV, Slesarchuk OA, Klementeva RV, Goloshchapov AV, Zubarovskaya LS, Afanasyev BV. Hemorrhagic cystitis after allogeneic transplantation of hematopoietic stem cells in children with hematological, oncological and hereditary diseases. Pediatriya – Zhurnal im G.N. Speranskogo 2018; 97(5): 41-46 (In Russian).
- Cesaro S, Dalianis T, Hanssen Rinaldo C, Koskenvuo M, Pegoraro A, Einsele H, Cordonnier C, Hirsch HH, Members of the ECIL-6 Group, ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2018; 73(1):12-21.
- Han SB, Cho B, Kang JH. BK virus-associated hemorrhagic cystitis after pediatric stem cell. Korean J Pediatr. 2014; 57(12):514-519.
- Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, Han XY, Tarrand J, Ribeiro R, Gulbis A, Shpall EJ, Jones R, Popat U, Walker JA, Petropoulos D, Chiattone A, Stewart J, El-Zimaity M, Anderlini P, Giralt S, Champlin RE, de Lima M. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95(7):1183-1190.
- Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, Mellstedt H, Remberger M, Ljungman P, Winiarski J, Dalianis T. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J Clin Microbiol. 2004;42(11):5394-5396.
- Mori T, Aisa Y, Shimizu T, Ikeda Y, Okamoto S, Okada K, Kazuyama Y. Hemorrhagic cystitis caused by adenovirus type 34 after allogeneic bone marrow transplantation. Transplantation. 2005;79(5):624.
- Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41(1):11-18.
- Chukhlovin AB, Eismont YuA, Vavilov VN, Zubarovskaya LS, Afanasyev BV. Time- and sample-dependent differences in polyomavirus incidence following hematopoietic stem cell transplantation. Cell Ther Transplant. 2016; 5(1):26-30.
- Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89(12):1462-1465.
- Sener A, House AA, Jevnikar AM, Boudville N, McAlister VC, Muirhead N, Rehman F, Luke PP. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81(1):117-120.
- Anyaegbu EI, Almond PS, Milligan T, Allen WR, Gharaybeh S, Al-Akash SI. Intravenous immunoglobulin therapy in the treatment of BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr Transplant. 2012 Feb; 16(1): E19-E24.
- Mert D, Batgi H, Merdin A, Çeken S, Dal MS, Tekgündüz E, Altuntaş F, Ertek M. BK Virus-associated hemorrhagic cystitis in patients with allogeneic hematopoietic cell transplantation: report of three cases. Hematol Rep. 2017;9(2):7205. 2017 Jun 26.
- World Health Organization. WHO model formulary 2008, Editors: Marc C. Stuart, Maria Kouimtzi, Suzanne R. Hill.
- Washburn N, Meccariello R, Hu S, Hains M, Bhatnagar N, Sarvaiya H, et al. High-resolution physicochemical characterization of different intravenous immunoglobulin products. PLoS ONE. 2017; 12(7): e0181251. https://doi.org/10.1371/journal.pone.0181251.
- Kopterides P, Theodorakopoulou M, Mentzelopoulos S, Armaganidis A. Cyclophosphamide-induced hemorrhagic cystitis successfully treated with conjugated estrogens. Am J Hematol. 2005;80(2):166-167.
- Walker RD. Cyclophosphamide-induced hemorrhagic cystitis. J Urol. 1999;161(6):1747.
- Haselberger MB, Schwinghammer TL. Efficacy of Mesna for prevention of hemorrhagic cystitis after high-dose cyclophosphamide therapy. Ann Pharmacother. 1995;29(9):918-921.
- Wang CC, Weng TI, Wu ET, Wu MH, Yang RS, Liu SH. Involvement of interleukin-6-regulated nitric oxide synthase in hemorrhagic cystitis and impaired bladder contractions in young rats induced by acrolein, a urinary metabolite of cyclophosphamide. Toxicol Sci. 2013;131(1):302-310.
- Gilis L, Morisset S, Billaud G, Ducastelle-Leprêtre S, Labussière-Wallet H, Nicolini F-E, Barraco F, Detrait M, Thomas X, Tedone N. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2014;49(5):664-670.
- Zubarovskaya LS. Efficiency evaluation of high-dose cytostatic therapy with hematopoietic stem cell transplantation in treatment of hematological and oncological disorders in children and adolescents. Doctoral Thesis. St.Petersburg, 2005:1-31 (In Russian).
- Vitrischak A, Semenova E, Ovsyannikova M, Pugachev A, Morozova E, Mikhailova N, Zubarovskaya L, Afanasyev B. Risk factors for acute GvHD after allogeneic stem cell transplantation in children. 30th Annual EBMT Meeting, Barcelona, 2004. Bone Marrow Transplant; 33 (Suppl 1 R1117): S312.
- Uhm J, Hamad N, Michelis FV, Shanavas M, Kuruvilla J, Gupta V, Lipton JH, Messner HA, Seftel M, Kim DD. The risk of polyomavirus BK-associated hemorrhagic cystitis after allogeneic hematopoietic SCT is associated with myeloablative conditioning, CMV viremia and severe acute GvHD. Bone Marrow Transplant. 2014; 49 (12): 1528-1534.
- Dalianis T, Ljungman P. Full myeloablative conditioning and an unrelated HLA-mismatched donor increase the risk for BK virus-positive hemorrhagic cystitis in allogeneic hematopoetic stem cell transplanted patients. Anticancer Res. 2011;31(3):939-944.
- Lunde LE, Dasaraju S, Cao Q, Cohn CS, Reding M, Bejanyan N, Trottier B, Rogosheske J, Brunstein C, Warlick E, Young JA, Weisdorf DJ, Ustun C. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant. 2015;50(11):1432-1437.
- Passweg JR, Baldomero H, Bader P, Basak GW, Bonini C, Duarte R et al. Is the use of unrelated donor transplantation leveling off in Europe? The 2016 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2018;53(9):1139-1148.
- Radujkovic A, Dietrich S, Blok HJ, Nagler A, Ayuk F, Finke J et al. Allogeneic Stem Cell Transplantation for Blast Crisis Chronic Myeloid Leukemia in the Era of Tyrosine Kinase Inhibitors: A Retrospective Study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2019;25(10):2008-2016.
- Carré M, Porcher R, Finke J, Ehninger G, Koster L, Beelen D et al. Role of age and hematopoietic cell transplantation-specific comorbidity index in myelodysplastic patients undergoing an allotransplant. A retrospective study from the CMWP (Chronic Malignancies Working Party) of the EBMT. Biol Blood Marrow Transplant. 2019 Oct 21. pii: S1083-8791(19)30672-X. doi: 10.1016/j.bbmt.2019.10.015.
- Onida F, de Wreede LC, van Biezen A, Eikema DJ, Byrne JL, Iori AP et al. Allogeneic stem cell transplantation in patients with atypical chronic myeloid leukaemia: a retrospective study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol. 2017;177(5):759-765.
- Sharma P, Shinde SS, Damlaj M, Hefazi Rorghabeh M, Hashmi SK, Litzow MR et al. Allogeneic hematopoietic stem cell transplant in adult patients with myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) overlap syndromes. Leuk Lymphoma. 2017;58(4):872-881.
- Olsson RF, Logan BR, Chaudhury S, Zhu X, Akpek G, Bolwell BJ et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015;29(8):1754-62.
- Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134(11):892-899.
- Piemontese S, Ciceri F, Labopin M, Bacigalupo A, Huang H, Santarone S et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(5):1069-75.
- Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104(3):524-532.
- Shah MV, Saliba RM, Rondon G, Chen J, Soebbing D, Rus I et al. Pilot study using post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate GvHD prophylaxis for older patients receiving 10/10 HLA-matched unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54(4):601-606.
- Ayuk F, Beelen DW, Bornhäuser M, Stelljes M, Zabelina T, Finke J et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2018;24(12):2558-2567.
- Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333-1338.
- Przepiorka D1, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GvHD Grading. Bone Marrow Transplant. 1995;15:825-828.
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945-956.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813-1821.
- Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives – a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50(6):781-789.
- Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144(6):407-414. Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683-693.
- Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6(3):e132-e143.
- Bryant A, Mallick R, Huebsch L, Allan D, Atkins H, Anstee G et al. Low-Dose Antithymocyte Globulin for Graft-versus-Host-Disease Prophylaxis in Matched Unrelated Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(12):2096-2101.
- Kennedy VE, Chen H, Savani BN, Greer J, Kassim AA, Engelhardt BG et al. Optimizing Antithymocyte Globulin Dosing for Unrelated Donor Allogeneic Hematopoietic Cell Transplantation Based on Recipient Absolute Lymphocyte Count. Biol Blood Marrow Transplant. 2018;24(1):150-155.
- Alessandrino EP, Della Porta MG, Bacigalupo A, Malcovati L, Angelucci E, Van Lint MT et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95(3):476-484.
- Passweg JR, Baldomero H, Bader P, Basak GW, Bonini C, Duarte R et al. Is the use of unrelated donor transplantation leveling off in Europe? The 2016 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2018;53(9):1139-1148.
- Radujkovic A, Dietrich S, Blok HJ, Nagler A, Ayuk F, Finke J et al. Allogeneic Stem Cell Transplantation for Blast Crisis Chronic Myeloid Leukemia in the Era of Tyrosine Kinase Inhibitors: A Retrospective Study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2019;25(10):2008-2016.
- Carré M, Porcher R, Finke J, Ehninger G, Koster L, Beelen D et al. Role of age and hematopoietic cell transplantation-specific comorbidity index in myelodysplastic patients undergoing an allotransplant. A retrospective study from the CMWP (Chronic Malignancies Working Party) of the EBMT. Biol Blood Marrow Transplant. 2019 Oct 21. pii: S1083-8791(19)30672-X. doi: 10.1016/j.bbmt.2019.10.015.
- Onida F, de Wreede LC, van Biezen A, Eikema DJ, Byrne JL, Iori AP et al. Allogeneic stem cell transplantation in patients with atypical chronic myeloid leukaemia: a retrospective study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Br J Haematol. 2017;177(5):759-765.
- Sharma P, Shinde SS, Damlaj M, Hefazi Rorghabeh M, Hashmi SK, Litzow MR et al. Allogeneic hematopoietic stem cell transplant in adult patients with myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) overlap syndromes. Leuk Lymphoma. 2017;58(4):872-881.
- Olsson RF, Logan BR, Chaudhury S, Zhu X, Akpek G, Bolwell BJ et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015;29(8):1754-62.
- Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134(11):892-899.
- Piemontese S, Ciceri F, Labopin M, Bacigalupo A, Huang H, Santarone S et al. A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(5):1069-75.
- Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104(3):524-532.
- Shah MV, Saliba RM, Rondon G, Chen J, Soebbing D, Rus I et al. Pilot study using post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate GvHD prophylaxis for older patients receiving 10/10 HLA-matched unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54(4):601-606.
- Ayuk F, Beelen DW, Bornhäuser M, Stelljes M, Zabelina T, Finke J et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2018;24(12):2558-2567.
- Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333-1338.
- Przepiorka D1, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GvHD Grading. Bone Marrow Transplant. 1995;15:825-828.
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945-956.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813-1821.
- Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives – a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50(6):781-789.
- Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144(6):407-414. Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683-693.
- Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6(3):e132-e143.
- Bryant A, Mallick R, Huebsch L, Allan D, Atkins H, Anstee G et al. Low-Dose Antithymocyte Globulin for Graft-versus-Host-Disease Prophylaxis in Matched Unrelated Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(12):2096-2101.
- Kennedy VE, Chen H, Savani BN, Greer J, Kassim AA, Engelhardt BG et al. Optimizing Antithymocyte Globulin Dosing for Unrelated Donor Allogeneic Hematopoietic Cell Transplantation Based on Recipient Absolute Lymphocyte Count. Biol Blood Marrow Transplant. 2018;24(1):150-155.
- Alessandrino EP, Della Porta MG, Bacigalupo A, Malcovati L, Angelucci E, Van Lint MT et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica. 2010;95(3):476-484.
- Pui CH, Mullighan Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood, 2012, 120(6): 1165-1174.
- Bussan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011; 29(5): 532-543.
- Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, Mann C, Hahlen K, Gobel U, Klingebiel T, Ludwig WD, Henze G. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Münster Group 87. J Clin Oncol. 2005; 23(31): 7942-7950.
- Fielding A, Richards S, Chopra R, Lazarus H, Litzow M, Buck G, Durrant J, Luger S, Marks D, Franklin I, McMillan A, Tallman M, Rowe J, Goldstone A. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 Study. Blood. 2007;109(3):944-950.
- O’Brien S, Thomas D, Ravandi F, Faderi S, Cortes J, Borthakur G, Pierse S, Garsia-Manero G, Kantarjian H. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113(11):3186-3191.
- Semenova EV, Stancheva NV, Bondarenko SN, Vavilov VN, Bagge DA, Paina OV, Razumova SV, Borovkova AS, Bykova TA, Rats AA, Zubarovskaya LS, Afanasyev BV. Treatment of refractory acute lymphoblastic leukemia in children and adolescents: remission reinduction followed by allogeneic hematopoietic stem cell transplantation. Klinicheskaya Onkogematologia. 2013; 6(1):53-58 (In Russian).
- Paina OV, Semenova EV, Markova IV, Zubarovskaya LS, Afanasyev BV. Modern opinions in therapy of acute leukemia in children under 1 year. Russian Journal of Pediatric Hematology and Oncology. 2019; 2:11-19 (In Russian).
- Bondarenko SN, Semenova EV, Stancheva NV, Vavilov VN, Gindina TL, Babenko EV, Alyanskyi АL, Zubarovskaya LS, Afanasyev BV. Efficiency of hematopoietic stem cell transplantation in acute lymphoblastic leukemia depending on the disease stage. Hematologia Transfusiologia. 2014; 59(1):14 (In Russian).
- Afanasyev B, Zubarovskaya L, Moiseev I. Allogeneic hematopoietic stem cell transplantation in children: now, problems and prospects. Russian Journal of Pediatric Hematology and Oncology. 2015;2(2):28-42 (In Russian).
- Kozhokar PV, Paina OV, Frolova AS, Rakhmanova ZZ, Borovkova AS, Semenova EV, Osipova AA, Ekushov KA, Ovechkina VN, Babenko EV, Vitrishchak AA, Smirnov BI, Zubarovskaya LS, Afanasyev BV. Efficiency of second allogeneic HSCT in the children with acute leukemias with relapses after first transplantation. Cell Ther Transplant. 2019; 8(4):33-40.
- Topp MC, Gokbuget N, Stein AS, Zugmaer G, O’Brien S, Bargou RS, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, Foa R, Lizow M, Ribera JM, Rambaldi A, Schiller G, Bruggemann M, Horst HA, Holland C, Jia C, Maniar T, Hubber B, Nagorsen D, Forman SJ, Kantarijan HM. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16 (1): 57-66.
- Stackelberg A, Locatelli F, Zugmaier G, Handgreteinger R, Trippett T, Rizzari C, Bader P, O’Brien MM, Brethon B, Bhojwani D, Schlegel PG, Borkhardt A, Reingold S, Cooper TM, Zwaan CM, Barnette P, Messina C, Michel G, DuBois SG, Hu K, Zhu M, Whitlock J, Gore L. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016: 34(936):4281-4389.
- De Angelo D, Stock W, Stein AS, Shustov A, Liedtke M, Schiffer CA, Vandendries E, Ananthakrishnan KLR, Boni J, Laird AD, Fostvedt L, Kantarjian H, Advani AS. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-posi-tive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 2017: 1(15):1167-1179.
- Bhoiwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, O’Brien MM, McNeer JL, Quereshi A, Cabannes A, Schlegel P, Rossig C, Dalla-Pozza L, August K, Alexander Sarah, Bourquin J-P, Zwaan M, Raetz EA, Loh ML, Rheingold SR. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia, 2019, 33, 884-892.
- Markova IV, Zubarovskaya LS, Paina OV, Bondarenko SN, Kozhokar PV, Frolova AS, Rahmanova Zh, Galas MA, Ekushov KA, Babenko EV Gindina TL, Barkhatov IM, Semenova EV, Moiseev IS, Afanasyev BV. Efficiency and safety evaluation of Blinatumomab in therapy of relapses and refractory forms of B-lineage acute lymphoblastic leukemia in children. Pediatriya. 2019; 19(4): 158-164.
- Bondarenko SN, Parovichnikova EN, Maschan AA, Baranova OY, Shelikhova TV, Doronin VA, Melnichenko VYa, Kaplanov KD, Uspenskaya OS, Sokolov AN, Myakova NV, Moiseev IS, Markova IV, Darskaya EV, Smirnova AG, Bykova TA, Ayubova BI, Samorodova IA, Babenko EV, Barkhatov IM, Gindina TL, Kulagin AD, Afanasyev BV. Blinatumomab in therapy of acute lymphoblastic leukemia: a Russian multicentric study. Klinicheskaya Onkogematologia. 2019; 12(2):145-153 (In Russian).
- Kantarjian H, De-Angelo DJ, Stelljes M, Liedtke M, Stock W, Gokbuget N, O’Brien S, Jabbour E, Wang T, White JL, Sleight B, Vandendries E, Advani AS. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019; 125(14):2474-2487.
- Kantarjian H, Stein A, Gokbuget N, Fielding AK , Schuh AC, Ribera J-M, Wei A, Dombret H, Foà R, Bassan R, Arslan O, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Eng J Med. 2017; 376:836-847.
- Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO State of the art update and next question: Philadelphia chromosome-positive acute lymphpblastic leukemia. Clinical lymphoma, myeloma and leukemia. 2018; 18 (7): 439-446.
- Liu YF, Wang BY, Zhang WN, Huang JY, Li BS, Zhang M, Jiang L, Li JF, Wang MJ, Dai YJ, Zhang ZG, Wang Q , Kong J, Chen B, Zhu YM, Weng XQ et al. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. EBioMedicine. 2016; 8: 173-183.
- Olin A, Henckel E, Chen Y. Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, Brodin P. Stereotypic immune system development in newborn children. 2018, Cell 174(5): 1277-1292.e14.
- Gokbuget N, Dombert H, Bonifacio M, Reichle A, Graux C, Faul C, Diedrich H, Topp M, Bruggemann M, Horst H-A, Havelange V, Steglmaier J, Wessels H, Haddad V, Benjamin JE, Zuggmaier G, Nagorsen D, Bargou RC. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018; 131(14):1522-1531.
- Pui CH, Mullighan Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood, 2012, 120(6): 1165-1174.
- Bussan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011; 29(5): 532-543.
- Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, Mann C, Hahlen K, Gobel U, Klingebiel T, Ludwig WD, Henze G. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Münster Group 87. J Clin Oncol. 2005; 23(31): 7942-7950.
- Fielding A, Richards S, Chopra R, Lazarus H, Litzow M, Buck G, Durrant J, Luger S, Marks D, Franklin I, McMillan A, Tallman M, Rowe J, Goldstone A. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 Study. Blood. 2007;109(3):944-950.
- O’Brien S, Thomas D, Ravandi F, Faderi S, Cortes J, Borthakur G, Pierse S, Garsia-Manero G, Kantarjian H. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008;113(11):3186-3191.
- Semenova EV, Stancheva NV, Bondarenko SN, Vavilov VN, Bagge DA, Paina OV, Razumova SV, Borovkova AS, Bykova TA, Rats AA, Zubarovskaya LS, Afanasyev BV. Treatment of refractory acute lymphoblastic leukemia in children and adolescents: remission reinduction followed by allogeneic hematopoietic stem cell transplantation. Klinicheskaya Onkogematologia. 2013; 6(1):53-58 (In Russian).
- Paina OV, Semenova EV, Markova IV, Zubarovskaya LS, Afanasyev BV. Modern opinions in therapy of acute leukemia in children under 1 year. Russian Journal of Pediatric Hematology and Oncology. 2019; 2:11-19 (In Russian).
- Bondarenko SN, Semenova EV, Stancheva NV, Vavilov VN, Gindina TL, Babenko EV, Alyanskyi АL, Zubarovskaya LS, Afanasyev BV. Efficiency of hematopoietic stem cell transplantation in acute lymphoblastic leukemia depending on the disease stage. Hematologia Transfusiologia. 2014; 59(1):14 (In Russian).
- Afanasyev B, Zubarovskaya L, Moiseev I. Allogeneic hematopoietic stem cell transplantation in children: now, problems and prospects. Russian Journal of Pediatric Hematology and Oncology. 2015;2(2):28-42 (In Russian).
- Kozhokar PV, Paina OV, Frolova AS, Rakhmanova ZZ, Borovkova AS, Semenova EV, Osipova AA, Ekushov KA, Ovechkina VN, Babenko EV, Vitrishchak AA, Smirnov BI, Zubarovskaya LS, Afanasyev BV. Efficiency of second allogeneic HSCT in the children with acute leukemias with relapses after first transplantation. Cell Ther Transplant. 2019; 8(4):33-40.
- Topp MC, Gokbuget N, Stein AS, Zugmaer G, O’Brien S, Bargou RS, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, Foa R, Lizow M, Ribera JM, Rambaldi A, Schiller G, Bruggemann M, Horst HA, Holland C, Jia C, Maniar T, Hubber B, Nagorsen D, Forman SJ, Kantarijan HM. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16 (1): 57-66.
- Stackelberg A, Locatelli F, Zugmaier G, Handgreteinger R, Trippett T, Rizzari C, Bader P, O’Brien MM, Brethon B, Bhojwani D, Schlegel PG, Borkhardt A, Reingold S, Cooper TM, Zwaan CM, Barnette P, Messina C, Michel G, DuBois SG, Hu K, Zhu M, Whitlock J, Gore L. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016: 34(936):4281-4389.
- De Angelo D, Stock W, Stein AS, Shustov A, Liedtke M, Schiffer CA, Vandendries E, Ananthakrishnan KLR, Boni J, Laird AD, Fostvedt L, Kantarjian H, Advani AS. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-posi-tive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 2017: 1(15):1167-1179.
- Bhoiwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, O’Brien MM, McNeer JL, Quereshi A, Cabannes A, Schlegel P, Rossig C, Dalla-Pozza L, August K, Alexander Sarah, Bourquin J-P, Zwaan M, Raetz EA, Loh ML, Rheingold SR. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia, 2019, 33, 884-892.
- Markova IV, Zubarovskaya LS, Paina OV, Bondarenko SN, Kozhokar PV, Frolova AS, Rahmanova Zh, Galas MA, Ekushov KA, Babenko EV Gindina TL, Barkhatov IM, Semenova EV, Moiseev IS, Afanasyev BV. Efficiency and safety evaluation of Blinatumomab in therapy of relapses and refractory forms of B-lineage acute lymphoblastic leukemia in children. Pediatriya. 2019; 19(4): 158-164.
- Bondarenko SN, Parovichnikova EN, Maschan AA, Baranova OY, Shelikhova TV, Doronin VA, Melnichenko VYa, Kaplanov KD, Uspenskaya OS, Sokolov AN, Myakova NV, Moiseev IS, Markova IV, Darskaya EV, Smirnova AG, Bykova TA, Ayubova BI, Samorodova IA, Babenko EV, Barkhatov IM, Gindina TL, Kulagin AD, Afanasyev BV. Blinatumomab in therapy of acute lymphoblastic leukemia: a Russian multicentric study. Klinicheskaya Onkogematologia. 2019; 12(2):145-153 (In Russian).
- Kantarjian H, De-Angelo DJ, Stelljes M, Liedtke M, Stock W, Gokbuget N, O’Brien S, Jabbour E, Wang T, White JL, Sleight B, Vandendries E, Advani AS. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019; 125(14):2474-2487.
- Kantarjian H, Stein A, Gokbuget N, Fielding AK , Schuh AC, Ribera J-M, Wei A, Dombret H, Foà R, Bassan R, Arslan O, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Eng J Med. 2017; 376:836-847.
- Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO State of the art update and next question: Philadelphia chromosome-positive acute lymphpblastic leukemia. Clinical lymphoma, myeloma and leukemia. 2018; 18 (7): 439-446.
- Liu YF, Wang BY, Zhang WN, Huang JY, Li BS, Zhang M, Jiang L, Li JF, Wang MJ, Dai YJ, Zhang ZG, Wang Q , Kong J, Chen B, Zhu YM, Weng XQ et al. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. EBioMedicine. 2016; 8: 173-183.
- Olin A, Henckel E, Chen Y. Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, Brodin P. Stereotypic immune system development in newborn children. 2018, Cell 174(5): 1277-1292.e14.
- Gokbuget N, Dombert H, Bonifacio M, Reichle A, Graux C, Faul C, Diedrich H, Topp M, Bruggemann M, Horst H-A, Havelange V, Steglmaier J, Wessels H, Haddad V, Benjamin JE, Zuggmaier G, Nagorsen D, Bargou RC. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018; 131(14):1522-1531.
- Gonçalves FDC, Luk F, Korevaar SS, Bouzid R, Paz AH, López-Iglesias C, Baan CC, Merino A, Hoogduijn MJ. Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro-inflammatory monocytes. Sci Rep. 2017;7(1):12100. doi: 10.1038/s41598-017-12121-z.
- Hong KY, Kim IK, Park SO, Jin US, Chang H. Systemic administration of adipose-derived stromal cells concurrent with fat grafting. Plast Reconstr Surg. 2019;143(5):973e-982e. doi: 10.1097/PRS.0000000000005513.
- Lei Y, Tang H, Yao L, Yu R, Feng M, Zou B. Applications of mesenchymal stem cells labeled with Tat peptide conjugated quantum dots to cell tracking in mouse body. Bioconjug Chem. 2008;19(2):421-427.
- Takasaki Y, Watanabe M, Yukawa H, Sabarudin A, Inagaki K, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Umemura T, Hayashi S, Baba Y, Haraguchi H. Estimation of the distribution of intravenously injected adipose tissue-derived stem cells labeled with quantum dots in mice organs through the determination of their metallic components by ICPMS. Anal Chem. 2011;83(21):8252-8258.
- Maiborodin IV, Morozov VV, Anikeev АА, Figurenko NF, Maslov RV, Chastikin GA, Matveeva VА, Maiborodina VI. Macrophage reaction to multipotent mesenchymal stromal cells introduction into surgical trauma site in rats. Novosti Khirurgii. 2017;25(3):233-241 (in Russian).
- Maiborodin IV, Maslov RV, Mikheeva TV, Elovskiy AA, Figurenko NF, Maiborodina VI, Shevela AI, Anishchenko VV. Macrophagal adsorption of multipotent mesenchymal stromal cells and their debris from vascular bed proves the migration of these cellular elements through the vessels after tissue injection. Molekulyarnaya Meditsina. 2018;16(3):56-61 (in Russian).
- Detante O, Moisan A, Dimastromatteo J, Richard MJ, Riou L, Grillon E, Barbier E, Desruet MD, De Fraipont F, Segebarth C, Jaillard A, Hommel M, Ghezzi C, Remy C. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant. 2009;18(12):1369-1379.
- Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R, Reisner Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865-2874. doi: 10.1002/stem.217.
- Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416). pii: eaam7828. doi: 10.1126/scitranslmed.aam7828.
- Khabbal J, Kerkelä E, Mitkari B, Raki M, Nystedt J, Mikkonen V, Bergström K, Laitinen S, Korhonen M, Jolkkonen J. Differential clearance of rat and human bone marrow-derived mesenchymal stem cells from the brain after intra-arterial infusion in rats. Cell Transplant. 2015;24(5):819-828.
- Bian SY, Cui H, Zhang XN, Qi LP, Li DY. Mesenchymal stem cells release membrane microparticles in the process of apoptosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20(2):453-457 (in Chinese).
- Detante O, Moisan A, Dimastromatteo J, Richard MJ, Riou L, Grillon E, Barbier E, Desruet MD, De Fraipont F, Segebarth C, Jaillard A, Hommel M, Ghezzi C, Remy C. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant. 2009;18(12):1369-1379.
- Shevela AA, Toder MS, Matveeva VA, Artemeva LV, Matveev AL, Meisner SN, Meisner LL, Shevela AI, Anikeev AA, Figurenko NF, Maslov RV, Bayborodin SI, Maiborodin IV. Chemically pure silicon and titanium coating is not toxic for mesenchymal stromal cells and improves cytological compatibility of electropolished TiNi alloy. Issues of Reconstructive and Plastic Surgery. 2017;(362):45–50 (in Russian). doi: 10.17223/1814147/62/06.
- Rodrigues M, Yates CC, Nuschke A, Griffith L, Wells A. The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Eng Part A. 2013;19(17-18):1972-1983.
- Yates CC, Nuschke A, Rodrigues M, Whaley D, Dechant JJ, Taylor DP, Wells A. Improved transplanted stem cell survival in a polymer gel supplemented with Tenascin C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017;26(1):103-113.
- Gonçalves FDC, Luk F, Korevaar SS, Bouzid R, Paz AH, López-Iglesias C, Baan CC, Merino A, Hoogduijn MJ. Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro-inflammatory monocytes. Sci Rep. 2017;7(1):12100. doi: 10.1038/s41598-017-12121-z.
- Hong KY, Kim IK, Park SO, Jin US, Chang H. Systemic administration of adipose-derived stromal cells concurrent with fat grafting. Plast Reconstr Surg. 2019;143(5):973e-982e. doi: 10.1097/PRS.0000000000005513.
- Lei Y, Tang H, Yao L, Yu R, Feng M, Zou B. Applications of mesenchymal stem cells labeled with Tat peptide conjugated quantum dots to cell tracking in mouse body. Bioconjug Chem. 2008;19(2):421-427.
- Takasaki Y, Watanabe M, Yukawa H, Sabarudin A, Inagaki K, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Umemura T, Hayashi S, Baba Y, Haraguchi H. Estimation of the distribution of intravenously injected adipose tissue-derived stem cells labeled with quantum dots in mice organs through the determination of their metallic components by ICPMS. Anal Chem. 2011;83(21):8252-8258.
- Maiborodin IV, Morozov VV, Anikeev АА, Figurenko NF, Maslov RV, Chastikin GA, Matveeva VА, Maiborodina VI. Macrophage reaction to multipotent mesenchymal stromal cells introduction into surgical trauma site in rats. Novosti Khirurgii. 2017;25(3):233-241 (in Russian).
- Maiborodin IV, Maslov RV, Mikheeva TV, Elovskiy AA, Figurenko NF, Maiborodina VI, Shevela AI, Anishchenko VV. Macrophagal adsorption of multipotent mesenchymal stromal cells and their debris from vascular bed proves the migration of these cellular elements through the vessels after tissue injection. Molekulyarnaya Meditsina. 2018;16(3):56-61 (in Russian).
- Detante O, Moisan A, Dimastromatteo J, Richard MJ, Riou L, Grillon E, Barbier E, Desruet MD, De Fraipont F, Segebarth C, Jaillard A, Hommel M, Ghezzi C, Remy C. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant. 2009;18(12):1369-1379.
- Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R, Reisner Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865-2874. doi: 10.1002/stem.217.
- Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416). pii: eaam7828. doi: 10.1126/scitranslmed.aam7828.
- Khabbal J, Kerkelä E, Mitkari B, Raki M, Nystedt J, Mikkonen V, Bergström K, Laitinen S, Korhonen M, Jolkkonen J. Differential clearance of rat and human bone marrow-derived mesenchymal stem cells from the brain after intra-arterial infusion in rats. Cell Transplant. 2015;24(5):819-828.
- Bian SY, Cui H, Zhang XN, Qi LP, Li DY. Mesenchymal stem cells release membrane microparticles in the process of apoptosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20(2):453-457 (in Chinese).
- Detante O, Moisan A, Dimastromatteo J, Richard MJ, Riou L, Grillon E, Barbier E, Desruet MD, De Fraipont F, Segebarth C, Jaillard A, Hommel M, Ghezzi C, Remy C. Intravenous administration of 99mTc-HMPAO-labeled human mesenchymal stem cells after stroke: in vivo imaging and biodistribution. Cell Transplant. 2009;18(12):1369-1379.
- Shevela AA, Toder MS, Matveeva VA, Artemeva LV, Matveev AL, Meisner SN, Meisner LL, Shevela AI, Anikeev AA, Figurenko NF, Maslov RV, Bayborodin SI, Maiborodin IV. Chemically pure silicon and titanium coating is not toxic for mesenchymal stromal cells and improves cytological compatibility of electropolished TiNi alloy. Issues of Reconstructive and Plastic Surgery. 2017;(362):45–50 (in Russian). doi: 10.17223/1814147/62/06.
- Rodrigues M, Yates CC, Nuschke A, Griffith L, Wells A. The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Eng Part A. 2013;19(17-18):1972-1983.
- Yates CC, Nuschke A, Rodrigues M, Whaley D, Dechant JJ, Taylor DP, Wells A. Improved transplanted stem cell survival in a polymer gel supplemented with Tenascin C accelerates healing and reduces scarring of murine skin wounds. Cell Transplant. 2017;26(1):103-113.
Hematopoietic cell counts in peripheral blood
Some data exist concerning HPC contents in peripheral blood. In an early study, semisolid methyl cellulose-based clonogenic cultures were assayed in 43 healthy volunteers including 22 males and 21 females at the age of 21 to 39 years old [1]. Comparative studies have shown increased numbers of colony-forming cells (CFC), erythroblastic progenitors (BFU-E), and non-differentiated myeloid precursors (GM-CFU) in peripheral blood (PB) in male individuals. However, the numbers of pluripotent CFU-GEMM in peripheral blood did not differ between females and males. Probable effects of sex steroids on these cell populations still remain open.
A more recent study from USA was carried out in a large cohort of 1786 Framingham Heart Study participants who underwent counting of CD34(+) cells in peripheral blood [2]. Among 1595 persons without cardiovascular diseases, CD34(+) frequency tended to decrease with older age and in females, thus suggesting higher numbers of peripheral hematopoietic cells in males.
Sex differences in circulating HSC subpopulations were also found in a group of 642 persons (mean age, 48 years) either healthy, or with cardiovascular disorders [3]. CD34+ CD45med+ and some other HSC markers were determined by flow cytometry. After adjustment for age, cardiovascular risk, and body mass, the individual counts for CD34+, CD34+/CD133+, and CD34+/chemokine receptor 4+ counts, were found to be lower in women than in male persons.
Thus, one may consider higher background counts of hematopoietic stem cells in HSC grafts from males compared to female donors.
The potentially higher amounts of hematopoietic stem cells in males may be translated into higher incidence of hematological malignancies, i.e., CML (and, probably, of chronic myelomonocytic leukemia) in male persons rather than in females, as shown in a big cohort, e.g., Hiroshima survivors observed over decades [4]. The authors consider two hypotheses: increased actual risk for this disorder, or shorter latency time in males needed for development of the disorder.
Gender differences in pharmacokinetics
Cytostatic drugs, CYPs and pGp effects
Intensive conditioning treatment is a basic pre-requisite of hematopoietic stem cell transplantation procedure. In leukemia treatment, it is intended for eradication of malignant cells, as well as for engraftment and proliferation of donor hematopoietic cells. Therefore, sex-specific effects of cytostatic drugs should be realized when planning optimal conditioning protocols. Studies of these sex differences in response to the common cytostatic drugs, generally, show that female patients are, generally, more stable than males, and moreover, depend on the type and dose of cytostatic agent applied [5].
Influence of gender may be exerted at different steps of drug processing in the body, i.e., absorption, distribution, metabolism (mainly in gut and liver), and excretion, corresponding to the ADME concept. Among other cytostatics used during HSCT, a decreased clearance of MTX, etoposide, doxorubicine and melphalan was found in women. Prolonged half-life was reported for different therapeutic antibodies, e.g., widely used Rituximab [6]. E.g., intersexual differences in equivalent doses of steroid drugs are readily explained by their more rapid metabolization (mostly activation) in women caused by sex-dependent CYP3A4 expression in liver [7].
An early well-known study by Hunt et al. [8] showed a significant increase of CYP3A activity (assessed by erythromycin demethylation) in liver tissues from normal females compared to males. Meanwhile, Wolbold et al. [7] reported 2-fold increase in CYP3A levels in females accompanied by 50% increase in the CYP3A measured as verapamil conversion rates. As reviewed by Fujita [9], there are numerous CYP3A substrates among anticancer drugs used in oncohematology, e.g. cyclophosphamide, taxanes, tyrosine kinase inhibitors etc.
At the present time, most authors agree that CYP3A4 expression and function in the liver of females is higher than in male persons [10]. However, the CYP3A4 activity is suppressed in acute inflammation and subject to unpredictable changes in cases of multiple drugs used in HSCT practice. Therefore, its role in pharmacokinetics requires special evaluation in cases of acute tissue damage, such as intensive anticancer therapy [11].
P-glycoprotein, is another potent modifier of drug effects. It is an important tool for the drug efflux from target cells [12]. Worth of note, most drugs metabolized by CYP3A4 in liver, are also subjected to P-glycoprotein-mediated efflux from the cells, thus providing a dual pharmacodynamic effect. Interestingly, the drug efflux could be reduced in females due to lower expression of P-glycoprotein in liver cells [13].
In general, available data on drug metabolism in females suggest an evidence for decreased clearance, higher activation rates due to increased CYP3A enzyme activities, and decreased drug efflux from liver cells, thus suggesting higher accumulation of active cytostatic drugs in the body, thus being predisposed to more expressed cytotoxicity towards malignant cells and normal tissues. However, individual gender-based drug dosage is not possible, due to drug interactions, genetic polymorphisms (e.g., CYP3A4) and other numerous factors modifying the effects of metabolizing enzymes and p-glycoprotein.
Immune suppressor drugs
Immune suppressor drugs used in post-transplant period usually affect cellular immunity, mostly, T cell populations. Some data about sexual dimorphism of their effects are obtained from studies in organ transplantation. E.g., the impact of gender on survival of renal allografts was thoroughly discussed in a review by Momper et al. [14]. Some controversies on sex differences in renal transplant survival that could be partly explained by the patients’ age, due to differential hormonal patterns at pre-puberty and post-menopause periods of life.
Whatever the exact mechanisms, calcineurin inhibitors (cyclosporine and tacrolimus), generally, used in posttransplant patients exhibited higher clearance rates in women than in males [15, 16].
Similar increase of sirolimus clearance in females as compared with males was shown elsewhere [17]. This difference may be again coupled to the mentioned CYP3A and P-glycoprotein alterations in women.
By contrary, mycophenolic acid (MPA) is also commonly used in post-transplant patients. Appropriate studies in a group of 100 renal transplants have showed a significantly increased MPA kinetics in males against females, due to higher glucuronate conjugation of MPA in male patients [18]. Indeed, the in vitro studies have shown that the liver samples in men exhibited a 3 to 4-fold higher gene expression and microsomal activity levels for UGT2B17 conjugating enzyme compared to women [19]. A conclusion could be made that the observed sex-dependent differences in pharmacokinetics of immunosuppressor drugs occur due to main metabolic mechanisms, i.e., drug efflux or conjugation via common liver enzymes.
Gender differences for glucocorticoid kinetics were intensively studied over last 25 years. Female sex is, generally, associated with lower clearance and, therefore, increased prednisolone exposure, as reviewed by Momper et al. [14]. Indeed, total body weight-normalized free prednisolone oral clearance was shown to be higher in men vs women [20], regardless of race (by 22% in whites and 40% in blacks for oral clearance, p < 0.01).
Thus, clearance of different immune suppressors in women is higher for cyclosporin A, tacrolimus and sirolimus, whereas clearance of MPA and glucocorticoids is higher in males, probably, due to different conjugation mechanisms of specific drugs.
Infections and immune response
A number of works have demonstrated lesser risks for severe posttraumatic infections and multiple organ failure in females. Most of these studies were performed in experimental models [21].
In a seminal large multicentric study involving 20.000 post-traumatic patients, the significant survival benefit was shown for female patients younger than 50 years [22]. In later studies, significantly better outcome was revealed for women after traumatic injury, severe blood loss and sepsis. Better survival of females in these settings are thought to be mediated via sex hormone effects and in particular, estrogen binding to their specific cell receptors [23].
Most clinical and experimental studies suggest increased immunoreactivity, especially, humoral response in females compared to males [24].
At the average, innate and adaptive immune response in females is higher than in, thus resulting in lesser susceptibility to bacterial, fungal and viral infections, however more predisposed for autoimmune conditions [25]. Both genetic factors and hormonal molecules contribute independently to gender differences in immune response. Some immune-related genes are located on the X chromosome, thus determining lesser immune response in young males. At later ages, estrogen and testosterone become the major regulators of immune cell kinetics, their maturation and functions. Thus, sexual dimorphism may cause significant differences in occurrence of infectious or autoimmune diseases between females and males.
A number of studies have shown higher antibody responses and longer half-life of immunoglobulins in females (e.g. following vaccination) that are extensively discussed by Fischinger et al. [26]. Vice versa, innate immunity and NK activity seem to be increased in males.
Some experimental and clinical studies have shown that the ratio of different IgG subclass can vary between in sex-specific manner. E.g., Simon et al. [27] have revealed that females during cytomegalovirus (CMV) infection produce higher levels of IgG3, the most active antibody subclass. Moreover, as shown by oncologists, female patients respond more effectively to rituximab treatment, probably, due to slower clearance and longer half-life of the antibody-based drugs [28].
Aiming for space economy, we cannot extensively discuss clear sex differences in gut microbiome that was already discussed by Ruggieri et al. [29]. The numerous bacteria inhabiting normal microbiome may both metabolize sex hormones and produce antigenic landscape for optimal antibody response.
When studying intestinal biopsies in healthy persons, women had higher levels of immune activation and inflammation-related gene expression in gut mucosal samples [30], as well as significantly higher levels of immune activation-associated phenotype of CD4+ and CD8+ T cells from peripheral blood. Higher humoral immune response to viruses in female HSCT patients was confirmed in several works. E.g., an extensive HSCT population was studied by Ljungman and Brandan [31]. To study factors determining CMV status, 40.311 patients and 23.048 donors were identified in the EBMT registry. Female patients were more likely to be CMV-seropositive (p<0.001). Adjusted for patient serostatus, the risk of a donor being seropositive was higher in females (p<0.001) and in older donors (p<0.001).
Hence, a more effective immune response may be observed in females compared to males. This difference may be explained by dominant effects of female steroid hormones which seem to exert mostly proinflammatory effects, whereas androgens exerting immunomodulatory properties. Most sex-dependent immune differences observed in males and females, i.e, lower response to infection and vaccination in males, or the higher incidence of autoimmunity seen in females, are ascribed to female steroid hormones which seem to exert mostly proinflammatory effects [32].
There is controversial information on rates of viral infections. As expected, male sex was shown to be the risk factor for BK virus infection following organ transplantation [33]. On other hand, as shown by Freeman et al. [34], female sex was proven to be associated with CMV disease in solid organ transplants (OR, 2.19; 95% CI 1.21, 3.99) and CMV viremia (OR, 1.65; 95% CI 1.03, 2.65).
Therefore, active treatment with antiviral drugs is often required in cases of viral activation. Sex-dependence of antiviral drug kinetics is poorly studied. E.g., transport of these drugs to renal tubules is controlled, e.g., by organic anion 1 (OAT1), an efflux transporter at the membranes of renal proximal tubules [35]. A higher ganciclovir clearance (+24%) was previously shown for female patients.
GvHD incidence
Concerning frequency and severity of acute GvHD in male vs female recipients of allografts, most studies did not show any sex differences incidence or severity when comparing male and female patients. The only condition associated with more common aGvHD and inferior survival is transplantation of female HSCs transplanted to male recipients, e.g., see Inamoto et al. [36]. In this study, GvHD- and relapse-free survival was assessed at 1 year post-HSCT based on 23.000 cases of first transplants. It was associated with some factors, including, anti-thymocyte globulin prophylaxis (for standard-risk-disease), recent years of transplantation etc. Better survival was observed with sex combinations other than from a female donor to a male patient. Therefore, female-to-male grafting was found to be of maximal risk for the male patients.
Meanwhile, prevalence of chronic GvHD showed a more distinct dependence on the donor gender. In an early study by Locatelli et al. [37], a group of 145 patients with acute GCHD and 114 cases at risk for chronic GvHD. Of them, 107 (74%) presented with acute GvHD (of them, grade II-IV developed in 35%). I.e., the incidence of chronic GvHD was higher in female-to-male than in male-male donor-recipient sex pairs (33% vs 11%, p <0.05), with no difference between female-to-female and male-to-female pairs. In patients of >10 years old, a higher incidence of chronic GvHD was observed in both female donors and recipients compared with male donors and recipients (48% vs 20% and 47% vs 19%, respectively, p <0.05).
Possible role of female donorship for chronic GvHD was also suggested for patients reported by Gaziev et al. [38]. Several risk factors included grade I-IV acute GvHD, prior aGvHD (p=0.000), female donor sex (p=0.000), use of alloimmune female donors for male patients (0.009) that predicted chronic GvHD.
Reduced salivary function (dry mouth) is a common sign of chronic GvHD developing after allo-HSCT. A total of 74 adolescents received allo-HSCT and conditioning with busulfan or total-body irradiation [39]. In the multivariate model, only female sex was significantly correlated with low salivation rates several years later.
Therefore, donor/recipient sex differences, along with other biological factors, were still under discussion if compared to dominant influence of, e.g., HLA matching which may control the HCST outcomes, as suggested by Confer et al. [40]. In cases of several available donors, one may select them for age, sex, parity, cytomegalovirus (CMV) serostatus, ethnicity and ABO blood type.
Relapses and overall survival
Solh et al. [41] analyzed medical histories for 389 post allo-HSCT patients who survived and were disease-free for at least one year post-transplant, with a median follow-up of 48.2 months. The median 5-year OS overall survival and DFS at 5 years were 78 and 74%, respectively. The most common causes of late mortality were high-risk disease and relapses, chronic GvHD, infections, and male sex. The risk factors for late relapse included male sex and high/very high disease risk index.
Another Turkish study [42] was performed in 193 patients with CML subjected to allo-HSCTs between 1989 and 2012 (before and after TKI advent). Despite quite different clinical state at HSCT, the rates of hematologic remission at 3 months after allo-HSCT were similar between TKI and pre-TKI eras, the patients having remission had better disease-free survival. Interestingly, male allo-HSCT recipients had worse DFS and OS (RR: 1.7, p=0.007) than females.
Sex differences are also shown for late outcomes of auto- and allo-HSCTs in r/r Hodgkin’s lymphoma by Japanese workers [43]. They treated 298 and 122 patients, respectively. For both autologous and allogeneic HSCT, overall 3-year survival was dependent on sex, and performance status (PS) at HSCT. Following 1st allo-HSCT, female sex at HSCT was significantly associated with better progression-free survival [RR=0.55 (0.32-0.94)].
In a comprehensive study by Schmid et al. [44], clinical efficiency of donor lymphocyte infusions was evaluated in 399 AML cases after allo-HSCT (of them, 228 DLI-treated patients), with a median follow-up of 27-40 months, respectively. Estimated survival at 2 years (+/- standard deviation) was 21% +/- 3% for patients receiving DLI and 9% +/- 2% for patients not receiving DLI. Among DLI recipients, female sex (p=0.02), along with lower tumor burden, were predictive for survival in a multivariate analysis.
Early clinical works on sex differences in HSCT outcome male-female differences in HSCT were presented by Gratwohl et al. [45]. The authors analyzed clinical outcomes of HSCT from HLA-identical female donors for 782 patients (438 males and 344 females) with chronic myeloid leukemia (chronic phase). The risk of transplant-related mortality (TRM) and relapse incidence (RI) were compared for male and female recipients at early period (up to 3 months), and at later times (up to 2-5 years post-transplant). Non-relapse-related mortality risk over 5 years proved to be significantly increased in male patients than in females (42% vs 27%; p=0.02), however, without detectable summary effect on relapse incidence. There was no difference in mortality and relapse rates at early terms (0 to 3 mo) (zero to three months). Meanwhile, the risk of CML relapse was shown to be diminished in male patients; this difference became detectable only after two years of observation (p=0.01).
A summary of clinical studies showing different outcomes of HSCT in females vs male patients is presented in Table 1.
Hence, one may suggest that male patients with hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses and late mortality.
Table 1. Gender differences in survival and/or relapse rates for the patients subjected to allo- or allogeneic HSCT

Female-to-male grafting: Y-encoded antigens?
Consequences of sex-mismatched HSCT and role of male minor antigens in hematopoietic transplantation were discussed for decades as a factor of HSCT outcomes. E.g., sex chromosome-linked minor histocompatibility determinants have been shown to affect the incidence and severity of graft-versus-host disease (GvHD) in both humans and animals [46]. On the basis of earlier studies done in mice and humans, it has often been assumed that this effect is due to a simple response of female donor cells recognizing recipient male HY antigens as foreign and reacting against them.
Minor histocompatibility antigens (mHAs) recognized by donor T cells play a central role as immunologic targets of graft-versus-host disease (GvHD) and graft-versus-leukemia after allogeneic hematopoietic stem cell transplantation (HSCT). Men who have undergone sex-mismatched allogeneic HSCT are at high risk for GvHD because of immune responses directed against mHAs encoded by antigens encoded by some Y chromosome genes [47].
Higher GvL effects associated with female donors were suggested by Randolph et al. [48] based on analysis of 3238 patients who were subjected to HLA-identical HSCT from siblings. As shown in a large sample of HLA-identical HSCTs, the male patients transplanted from female donors showed higher chances for clinical GvHD, but significantly lower incidence of malignancy relapse. The "graft-versus-leukemia" effect in female-to-male HSCT seems to be independent from GvHD in the cases of acute leukemias and chronic myeloid leukemia. Thus, possible role of H-Y minor male antigens in GvHD could be suggested.
A clinical study by Alhashim et al. [49] was performed in a group of patients with AML in complete remission (215 cases) after allo-HCT from matched siblings, after myeloablative conditioning. Seventy-seven (35.8%) patients experienced disease relapse, 45 had BMR, and 32 had extramedullary relapses (EMR). Male sex was the only variable that was statistically associated with EMR post allo-HCT, with odds ratio of 3.2 (p=0.01).
Recent work by Ayuk et al. [50] was aimed for searching potential HLA-related and non-HLA factors impacting overall survival in general HSCT practice. The authors observed 3215 HSCTs performed between 2005 and 2013 in Germany for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Their donors were HLA-matched related (MRD; n=872) or unrelated (10/10 MUD, n=1553) or HLA-mismatched unrelated (<10/10 MMUD, n=790). Among non-HLA related factors, age, sex mismatching (male recipient-female donor), and cytomegalovirus (CMV) mismatching (positive recipient-negative donor) seem to influence overall survival. Their adverse effects may be comparable with a single HLA antigen mismatch.
Hence, an increased risk for acute GvHD is observed in cohorts with female-to male HSCT. Association with graft-versus-leukemia effect in these donor-recipient pairs is not proven.
Sex hormones as potential modifiers of HSCT-associated events
In this respect, experimental animal studies have demonstrated that a single, small-volume infusion of ethinyl estradiol-3-sulfate (EES) has beneficial effects following trauma-hemorrhage, even in the absence of fluid resuscitation [51].
It was demonstrated that estradiol treatment exerted these effects via estradiol receptors then being able to downregulate proinflammatory cytokine cascade and intracellular oxidative damage following experimental trauma/hemorrhage [52].
In fact, administration of extradiol in experimental trauma/hemorrhage was associated with reduced TNF-α and increased IL-10 in affected rats [53].
Hence, the survival rates in sepsis may be, in principle, improved by estrogen administration [54]. Vice versa, blockade of male sex hormone receptors by antagonist drugs is also suggested to boost immune responses in complicated sepsis.
Similarly, treatment with luteinizing hormone release hormone (LHRH) after allo-HSCT in murine experiments caused an enhanced thymic reconstitution and better T cell recovery, predominantly, among in naive T cell populations, with increased T cell functions in vivo and in vitro [55]. Since LHRH provides sex steroid ablation (a decrease in luteinizing hormone and follicle-stimulating hormone production, causing a decrease in gonadal sex steroid production) it may represent a promising therapy for enhancement of immunity in secondary immunosuppression. However, these experiments were performed with female mice only.
Conclusion and future prospects
1. Higher proliferative activity of hematopoietic cells of males could be demonstrated by several independent studies, causing increased background counts of hematopoietic stem cells in HSC grafts from males compared to female donors.
2. Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher activation rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic drugs in the body.
3. More effective and stable immune functions, especially, more active antibody response is shown in females compared to males. In allo-HSCT setting, this difference may be translated into higher risk of chronic GvHD in female patients after allo-HSCT.
4. One may suggest that male patients with hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses and late mortality.
5. A number of extensive studies have shown increased risk of acute GvHD in cases of female-to male HSCT. The issue of graft-versus-leukemia effect in this donor-recipient pairs still remains open.
6. Estrogen hormones seem to be to most probable cause of gender-dependent HSCT-associated events. However, their modifying role should vary, depending on the age of patients. Therefore, age dependence of sex differences in HSCT deserves further studies.
Conflicts of interest
Non declared.
References
Алексей Б. Чухловин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26061" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26062" ["VALUE"]=> array(2) { ["TEXT"]=> string(4342) "<p style="text-align: justify;">Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4286) "Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26063" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-13-21" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-13-21" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26067" ["VALUE"]=> array(2) { ["TEXT"]=> string(39) "<p>Alexei B. Chukhlovin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(27) "Alexei B. Chukhlovin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26068" ["VALUE"]=> array(2) { ["TEXT"]=> string(512) "<p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p> <br> <p><b>Correspondence</b><br>Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 325 00 94<br> E-mail: alexei.chukh@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(452) "Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial
Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 325 00 94
E-mail: alexei.chukh@mail.ru
There is a huge number of studies concerning metabolic, immunological and other differences between males and females caused by their differential hormonal and physiological background. However, only few studies are dedicated to sex-dependent differences in amounts of donor hematopoietic cells used for stem cell transplantation (HSCT), kinetics of cytostatic drugs used for conditioning treatment and immunosuppressors for GvHD prophylaxis, as well as differences in common posttransplant complications. The following differences significant for evaluation of HSCT results may be derived from previous studies: (1) Higher counts of CD34+ cells in hematopoietic grafts from males compared to female donors; (2) Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher modification rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic metabolites in female patients; (3) More effective and stable humoral immune response in females compared to males could be translated into better anti-infectious response, along with higher risk of chronic GvHD in females after allo-HSCT; (4) Male patients with some hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses, however, conflicting data are reported; (5) Increased risk of acute GvHD in males exists in cases of allo-HSCT from female donors. The issue of graft-versus-leukemia effect in this setting still remains open. In sum, estrogen hormones seem to be to the most probable cause of gender differences in HSCT-associated risks. However, modifying role of sex steroids is not well studied, and it should vary, depending on the age of patients. Therefore, real significance of sex differences in HSCT deserves further extensive studies in large databases.
Keywords
Hematopoietic stem cell transplantation, sex differences, drug kinetics, immune response, GvHD, relapses, survival, estrogens, outcomes.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26064" ["VALUE"]=> string(56) "Gender factor in hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(56) "Gender factor in hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26065" ["VALUE"]=> string(4) "1941" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1941" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26069" ["VALUE"]=> string(4) "1942" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1942" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(11) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26067" ["VALUE"]=> array(2) { ["TEXT"]=> string(39) "<p>Alexei B. Chukhlovin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(27) "Alexei B. Chukhlovin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(27) "Alexei B. Chukhlovin
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26072" ["VALUE"]=> array(2) { ["TEXT"]=> string(2162) "<p style="text-align: justify;">There is a huge number of studies concerning metabolic, immunological and other differences between males and females caused by their differential hormonal and physiological background. However, only few studies are dedicated to sex-dependent differences in amounts of donor hematopoietic cells used for stem cell transplantation (HSCT), kinetics of cytostatic drugs used for conditioning treatment and immunosuppressors for GvHD prophylaxis, as well as differences in common posttransplant complications. The following differences significant for evaluation of HSCT results may be derived from previous studies: (1) Higher counts of CD34+ cells in hematopoietic grafts from males compared to female donors; (2) Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher modification rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic metabolites in female patients; (3) More effective and stable humoral immune response in females compared to males could be translated into better anti-infectious response, along with higher risk of chronic GvHD in females after allo-HSCT; (4) Male patients with some hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses, however, conflicting data are reported; (5) Increased risk of acute GvHD in males exists in cases of allo-HSCT from female donors. The issue of graft-versus-leukemia effect in this setting still remains open. In sum, estrogen hormones seem to be to the most probable cause of gender differences in HSCT-associated risks. However, modifying role of sex steroids is not well studied, and it should vary, depending on the age of patients. Therefore, real significance of sex differences in HSCT deserves further extensive studies in large databases.</p> <h2>Keywords</h2> <p style="text-align: justify;">Hematopoietic stem cell transplantation, sex differences, drug kinetics, immune response, GvHD, relapses, survival, estrogens, outcomes.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2106) "There is a huge number of studies concerning metabolic, immunological and other differences between males and females caused by their differential hormonal and physiological background. However, only few studies are dedicated to sex-dependent differences in amounts of donor hematopoietic cells used for stem cell transplantation (HSCT), kinetics of cytostatic drugs used for conditioning treatment and immunosuppressors for GvHD prophylaxis, as well as differences in common posttransplant complications. The following differences significant for evaluation of HSCT results may be derived from previous studies: (1) Higher counts of CD34+ cells in hematopoietic grafts from males compared to female donors; (2) Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher modification rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic metabolites in female patients; (3) More effective and stable humoral immune response in females compared to males could be translated into better anti-infectious response, along with higher risk of chronic GvHD in females after allo-HSCT; (4) Male patients with some hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses, however, conflicting data are reported; (5) Increased risk of acute GvHD in males exists in cases of allo-HSCT from female donors. The issue of graft-versus-leukemia effect in this setting still remains open. In sum, estrogen hormones seem to be to the most probable cause of gender differences in HSCT-associated risks. However, modifying role of sex steroids is not well studied, and it should vary, depending on the age of patients. Therefore, real significance of sex differences in HSCT deserves further extensive studies in large databases.
Keywords
Hematopoietic stem cell transplantation, sex differences, drug kinetics, immune response, GvHD, relapses, survival, estrogens, outcomes.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2106) "There is a huge number of studies concerning metabolic, immunological and other differences between males and females caused by their differential hormonal and physiological background. However, only few studies are dedicated to sex-dependent differences in amounts of donor hematopoietic cells used for stem cell transplantation (HSCT), kinetics of cytostatic drugs used for conditioning treatment and immunosuppressors for GvHD prophylaxis, as well as differences in common posttransplant complications. The following differences significant for evaluation of HSCT results may be derived from previous studies: (1) Higher counts of CD34+ cells in hematopoietic grafts from males compared to female donors; (2) Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher modification rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic metabolites in female patients; (3) More effective and stable humoral immune response in females compared to males could be translated into better anti-infectious response, along with higher risk of chronic GvHD in females after allo-HSCT; (4) Male patients with some hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses, however, conflicting data are reported; (5) Increased risk of acute GvHD in males exists in cases of allo-HSCT from female donors. The issue of graft-versus-leukemia effect in this setting still remains open. In sum, estrogen hormones seem to be to the most probable cause of gender differences in HSCT-associated risks. However, modifying role of sex steroids is not well studied, and it should vary, depending on the age of patients. Therefore, real significance of sex differences in HSCT deserves further extensive studies in large databases.
Keywords
Hematopoietic stem cell transplantation, sex differences, drug kinetics, immune response, GvHD, relapses, survival, estrogens, outcomes.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26063" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-13-21" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-13-21" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-13-21" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26064" ["VALUE"]=> string(56) "Gender factor in hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(56) "Gender factor in hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(56) "Gender factor in hematopoietic stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26068" ["VALUE"]=> array(2) { ["TEXT"]=> string(512) "<p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p> <br> <p><b>Correspondence</b><br>Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 325 00 94<br> E-mail: alexei.chukh@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(452) "Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial
Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 325 00 94
E-mail: alexei.chukh@mail.ru
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial
Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 325 00 94
E-mail: alexei.chukh@mail.ru
Алексей Б. Чухловин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(42) "Алексей Б. Чухловин
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26057" ["VALUE"]=> string(22) "02/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "02/27/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26058" ["VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/27/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26062" ["VALUE"]=> array(2) { ["TEXT"]=> string(4342) "<p style="text-align: justify;">Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4286) "Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4286) "Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26061" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [2]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "143" ["~IBLOCK_SECTION_ID"]=> string(3) "143" ["ID"]=> string(4) "1834" ["~ID"]=> string(4) "1834" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(95) "Играют ли роль кортикостероиды в профилактике РТПХ?" ["~NAME"]=> string(95) "Играют ли роль кортикостероиды в профилактике РТПХ?" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "04/24/2020 02:34:59 pm" ["~TIMESTAMP_X"]=> string(22) "04/24/2020 02:34:59 pm" ["DETAIL_PAGE_URL"]=> string(93) "/en/archive/tom-9-nomer-1/obzornye-stati/igrayut-li-rol-kortikosteroidy-v-profilaktike-rtpkh/" ["~DETAIL_PAGE_URL"]=> string(93) "/en/archive/tom-9-nomer-1/obzornye-stati/igrayut-li-rol-kortikosteroidy-v-profilaktike-rtpkh/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(15219) "Introduction
Corticosteroids have an established role in the treatment of graft-versus-host disease (GvHD). Practically all patients who develop clinically significant acute GvHD after hematopoietic stem cell transplantation (HSCT) are primarily treated with corticosteroids, and about half of them show a good response. However, the role of corticosteroids in the prophylaxis of GvHD is much less clear. They have been used in the prophylaxis in combination with a large number of other drugs including cyclosporine A (CsA), tacrolimus, methotrexate (Mtx), mycophenolate mofetil, cyclophosphamide, antilymphocyte globulin, and monoclonal ricin-combined or other antibodies [1]. The proportion of allogeneic HSCT patients given prophylactic corticosteroid has, however, been low. Among the patients reported to the EBMT registry, overall approximately 4 per cent had received corticosteroid prophylaxis, and this proportion has been declining, from about 10 per cent in the 1990s to approximately 2 per cent in the most recent years.
Studies on the addition of corticosteroid to CsA + Mtx
The combination of cyclosporine and a short course of methotrexate is the most widely used regimen for GvHD prophylaxis [2]. The addition of corticosteroid to this regimen has been studied in a few prospective randomized trials. Storb et al. [3] found that the addition of corticosteroid resulted in an increased incidence of acute GvHD, whereas Atkinson and coworkers [4] did not observe any significant effect. In the study of Hoyt et al. [5] a delayed onset of acute GvHD in the group given the triple prophylaxis was seen, but the incidence remained similar to the control group.
Table 1. Schedule of methylprednisolone administration [6]
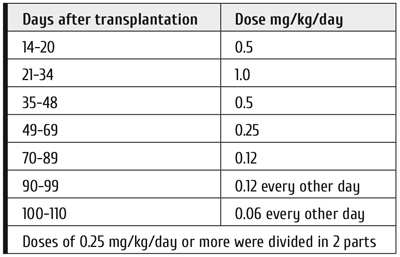
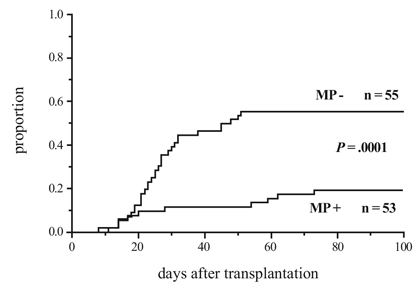
Figure 1. Cumulative incidence of grade I-IV acute GvHD in patients given or not given methylprednisolone (MP) [6]
We carried out a prospective randomized comparison of CsA + Mtx + methylprednisolone (MP) vs CsA + Mtx in the years 1989-1994 [6]. In this single-center study, 108 consecutive adult allogeneic transplant patients treated for a malignant blood disease were randomized to receive CsA + Mtx with (53 patients) or without MP (55 patients) for GvHD prophylaxis. They received myeloablative conditioning based on total body irradiation (68 patients) or busulfan (40 patients) and a non-manipulated bone marrow graft from an HLA-identical sibling donor. The schedule of MP administration is shown in Table 1.
We saw a markedly and significantly reduced overall incidence of acute GvHD in the MP+ arm (Fig. 1). Also the incidence of grade II-IV acute GvHD was significantly reduced (13% vs 36%). There was a non-significant trend towards a lower incidence of chronic GvHD in the MP+ arm. The relapse rates did not differ. There was no significant difference in the survival rates (at 6 years 60 per cent and 51 per cent in the MP + and MP- arms, respectively). In the MP+ group the neutrophil recovery was faster, there were fewer infections, and the hospitalization time was shorter. The total amount of MP given was similar in the two arms due to markedly higher incidence of acute GvHD in the MP- arm and aggressive GvHD treatment policy.
We performed a long-term follow-up of the patients in our study after a median follow-up of 24.5 (22.7-26.9) years in living patients [1]. The overall survival had remained similar in the study arms until 15 years post-transplantation (Fig. 2). Thereafter the curves deviated; by the end of the follow-up eleven patients had died in the MP- arm more than 15 years after the transplantation, but no patient in the MP+ arm. The mortality was due to non-relapse causes (Fig. 3), there was no difference in the relapse rate. At the end of the follow-up, 55% of the patients were alive in the MP+ arm, compared with 20% in the control arm.
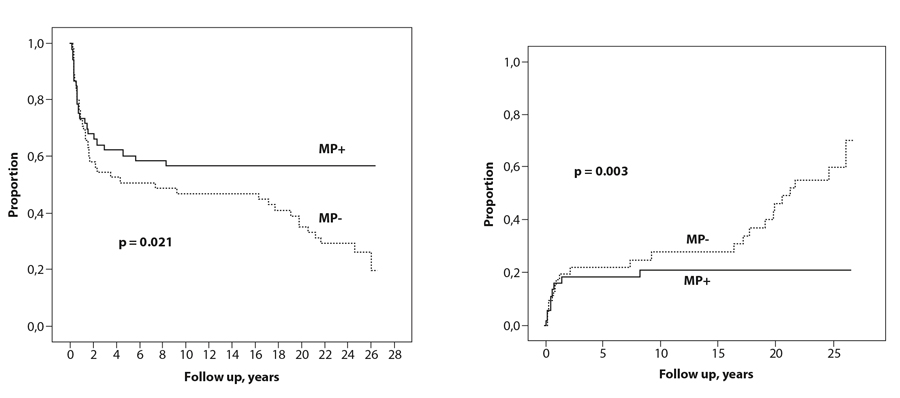
Figure 2. Overall survival of patients given or not given methylprednisolone (MP) [1]
Figure 3. Non-relapse mortality of patients given or not given methylprednisolone [1]
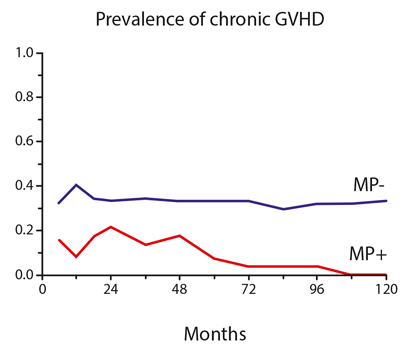
Figure 4. Prevalence of chronic GvHD in patients given or not given methylprednisolone (MP) [1]
In the patients who died in the MP- group more than 15 years post-transplantation, the causes of death were: bacterial infection in 3 patients, obstructive bronchiolitis in 1, confirmed or probable cardiovascular cause in 3, and second cancer in 4 patients.
We had detailed follow-up data of the patients for the first ten years after the transplantation, and during this period the prevalence of chronic GvHD was significantly lower in the MP+ arm (Fig. 4). At ten years, 28% of the patients in the MP- group but no one in the MP+ group had active chronic GvHD. Of the eleven patients who had a late death in the MP- arm, nine had had chronic GvHD. Of the remaining two patients, one died suddenly of an obviously cardiovascular cause, the other one of bacterial infection.
Discussion
The effects of the addition of corticosteroid to CsA + MP for GvHD prophylaxis have been conflicting in short-term reports [3, 4, 5, 6]. The reasons remain uncertain, but some possible factors can be identified. An important factor may have been the timing of the corticosteroid administration. In the two studies showing no useful effect of corticosteroid by Storb et al. [3] and Atkinson et al. [4], the administration was initiated at the time of the transplantation and given simultaneously with the other components of the regimen, whereas in the studies of Ruutu et al. [6] and Hoyt et al. [5], corticosteroid was initiated only after the short course of Mtx. It is possible that corticosteroid interfered with the effect of the other prophylactic drugs. In the Seattle study [3] the corticosteroid addition resulted in an increased incidence of acute GvHD, but this effect disappeared if the corticoid treatment was postponed to day 15 and started only after the methotrexate course. Another factor may be the duration of corticosteroid administration. In the studies by Storb et al. [3] and Atkinson et al. [4], corticosteroids were given for only 30-35 days, whereas in our study [6] this treatment was given until day 110 and in that of Hoyt et al. [5] until day 100. A third factor may be differences in the target CsA concentrations applied [6]. It looks likely that the conflicting results of the corticosteroid addition to CsA + Mtx are at least partly due to differences in the treatment schedule.
The cause of the difference between the study groups in non-relapse mortality due to high late mortality in the MP- group in our long-term follow-up study is not fully obvious, but chronic GvHD is a likely candidate. The prevalence of chronic GvHD was higher during the first ten years after the transplantation in the group of patients not given corticosteroid.
Nine of eleven patients who died more than 15 years after the transplantation had had chronic GvHD. The main causes of death were infection, cardiovascular event and second cancer. Immune deficiency associated with chronic GvHD is a major cause of morbidity and mortality from infections [7]. Chronic GvHD has been shown to be a risk factor for secondary malignancy [8, 9, 10], and active chronic GvHD is associated with an increased risk of cardiovascular morbidity and mortality [7, 11].
The findings of our long-term study would naturally need confirmation from other studies. However, to our knowledge no study on this subject with a follow-up time long enough to cover the time when the late complications took place in our study has been published. Deeg and coworkers [12] published a long-term follow-up of their randomized study where the addition of MP to CsA prophylaxis had been investigated. In the original publication [13] there was significantly less grade II-IV acute GvHD and more chronic GvHD in the MP+ arm, but no difference in the survival. In the long-term follow-up, the median follow-up time was only six years, and no effect of the corticoid addition on the survival was seen. This is in line with our study with the same follow-up.
The prophylactic use of corticosteroid for GvHD is infrequent at present. This reflects the sparsity of documentation to support such use and variable results in the literature. Our study of the addition of corticosteroid to the combination CsA + Mtx, demonstrating a marked decrease in the incidence of acute GvHD, also showed a beneficial effect on long-term survival, most likely by reducing chronic GvHD and its consequences. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated, as also suggested in another transplant setting, haploidentical transplantation [14, 15, 16].
Conflict of interest
No conflicts of interest reported.
References
Introduction
Corticosteroids have an established role in the treatment of graft-versus-host disease (GvHD). Practically all patients who develop clinically significant acute GvHD after hematopoietic stem cell transplantation (HSCT) are primarily treated with corticosteroids, and about half of them show a good response. However, the role of corticosteroids in the prophylaxis of GvHD is much less clear. They have been used in the prophylaxis in combination with a large number of other drugs including cyclosporine A (CsA), tacrolimus, methotrexate (Mtx), mycophenolate mofetil, cyclophosphamide, antilymphocyte globulin, and monoclonal ricin-combined or other antibodies [1]. The proportion of allogeneic HSCT patients given prophylactic corticosteroid has, however, been low. Among the patients reported to the EBMT registry, overall approximately 4 per cent had received corticosteroid prophylaxis, and this proportion has been declining, from about 10 per cent in the 1990s to approximately 2 per cent in the most recent years.
Studies on the addition of corticosteroid to CsA + Mtx
The combination of cyclosporine and a short course of methotrexate is the most widely used regimen for GvHD prophylaxis [2]. The addition of corticosteroid to this regimen has been studied in a few prospective randomized trials. Storb et al. [3] found that the addition of corticosteroid resulted in an increased incidence of acute GvHD, whereas Atkinson and coworkers [4] did not observe any significant effect. In the study of Hoyt et al. [5] a delayed onset of acute GvHD in the group given the triple prophylaxis was seen, but the incidence remained similar to the control group.
Table 1. Schedule of methylprednisolone administration [6]


Figure 1. Cumulative incidence of grade I-IV acute GvHD in patients given or not given methylprednisolone (MP) [6]
We carried out a prospective randomized comparison of CsA + Mtx + methylprednisolone (MP) vs CsA + Mtx in the years 1989-1994 [6]. In this single-center study, 108 consecutive adult allogeneic transplant patients treated for a malignant blood disease were randomized to receive CsA + Mtx with (53 patients) or without MP (55 patients) for GvHD prophylaxis. They received myeloablative conditioning based on total body irradiation (68 patients) or busulfan (40 patients) and a non-manipulated bone marrow graft from an HLA-identical sibling donor. The schedule of MP administration is shown in Table 1.
We saw a markedly and significantly reduced overall incidence of acute GvHD in the MP+ arm (Fig. 1). Also the incidence of grade II-IV acute GvHD was significantly reduced (13% vs 36%). There was a non-significant trend towards a lower incidence of chronic GvHD in the MP+ arm. The relapse rates did not differ. There was no significant difference in the survival rates (at 6 years 60 per cent and 51 per cent in the MP + and MP- arms, respectively). In the MP+ group the neutrophil recovery was faster, there were fewer infections, and the hospitalization time was shorter. The total amount of MP given was similar in the two arms due to markedly higher incidence of acute GvHD in the MP- arm and aggressive GvHD treatment policy.
We performed a long-term follow-up of the patients in our study after a median follow-up of 24.5 (22.7-26.9) years in living patients [1]. The overall survival had remained similar in the study arms until 15 years post-transplantation (Fig. 2). Thereafter the curves deviated; by the end of the follow-up eleven patients had died in the MP- arm more than 15 years after the transplantation, but no patient in the MP+ arm. The mortality was due to non-relapse causes (Fig. 3), there was no difference in the relapse rate. At the end of the follow-up, 55% of the patients were alive in the MP+ arm, compared with 20% in the control arm.

Figure 2. Overall survival of patients given or not given methylprednisolone (MP) [1]
Figure 3. Non-relapse mortality of patients given or not given methylprednisolone [1]

Figure 4. Prevalence of chronic GvHD in patients given or not given methylprednisolone (MP) [1]
In the patients who died in the MP- group more than 15 years post-transplantation, the causes of death were: bacterial infection in 3 patients, obstructive bronchiolitis in 1, confirmed or probable cardiovascular cause in 3, and second cancer in 4 patients.
We had detailed follow-up data of the patients for the first ten years after the transplantation, and during this period the prevalence of chronic GvHD was significantly lower in the MP+ arm (Fig. 4). At ten years, 28% of the patients in the MP- group but no one in the MP+ group had active chronic GvHD. Of the eleven patients who had a late death in the MP- arm, nine had had chronic GvHD. Of the remaining two patients, one died suddenly of an obviously cardiovascular cause, the other one of bacterial infection.
Discussion
The effects of the addition of corticosteroid to CsA + MP for GvHD prophylaxis have been conflicting in short-term reports [3, 4, 5, 6]. The reasons remain uncertain, but some possible factors can be identified. An important factor may have been the timing of the corticosteroid administration. In the two studies showing no useful effect of corticosteroid by Storb et al. [3] and Atkinson et al. [4], the administration was initiated at the time of the transplantation and given simultaneously with the other components of the regimen, whereas in the studies of Ruutu et al. [6] and Hoyt et al. [5], corticosteroid was initiated only after the short course of Mtx. It is possible that corticosteroid interfered with the effect of the other prophylactic drugs. In the Seattle study [3] the corticosteroid addition resulted in an increased incidence of acute GvHD, but this effect disappeared if the corticoid treatment was postponed to day 15 and started only after the methotrexate course. Another factor may be the duration of corticosteroid administration. In the studies by Storb et al. [3] and Atkinson et al. [4], corticosteroids were given for only 30-35 days, whereas in our study [6] this treatment was given until day 110 and in that of Hoyt et al. [5] until day 100. A third factor may be differences in the target CsA concentrations applied [6]. It looks likely that the conflicting results of the corticosteroid addition to CsA + Mtx are at least partly due to differences in the treatment schedule.
The cause of the difference between the study groups in non-relapse mortality due to high late mortality in the MP- group in our long-term follow-up study is not fully obvious, but chronic GvHD is a likely candidate. The prevalence of chronic GvHD was higher during the first ten years after the transplantation in the group of patients not given corticosteroid.
Nine of eleven patients who died more than 15 years after the transplantation had had chronic GvHD. The main causes of death were infection, cardiovascular event and second cancer. Immune deficiency associated with chronic GvHD is a major cause of morbidity and mortality from infections [7]. Chronic GvHD has been shown to be a risk factor for secondary malignancy [8, 9, 10], and active chronic GvHD is associated with an increased risk of cardiovascular morbidity and mortality [7, 11].
The findings of our long-term study would naturally need confirmation from other studies. However, to our knowledge no study on this subject with a follow-up time long enough to cover the time when the late complications took place in our study has been published. Deeg and coworkers [12] published a long-term follow-up of their randomized study where the addition of MP to CsA prophylaxis had been investigated. In the original publication [13] there was significantly less grade II-IV acute GvHD and more chronic GvHD in the MP+ arm, but no difference in the survival. In the long-term follow-up, the median follow-up time was only six years, and no effect of the corticoid addition on the survival was seen. This is in line with our study with the same follow-up.
The prophylactic use of corticosteroid for GvHD is infrequent at present. This reflects the sparsity of documentation to support such use and variable results in the literature. Our study of the addition of corticosteroid to the combination CsA + Mtx, demonstrating a marked decrease in the incidence of acute GvHD, also showed a beneficial effect on long-term survival, most likely by reducing chronic GvHD and its consequences. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated, as also suggested in another transplant setting, haploidentical transplantation [14, 15, 16].
Conflict of interest
No conflicts of interest reported.
References
Тапани Рууту
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26033" ["VALUE"]=> array(2) { ["TEXT"]=> string(173) "<p>Институт клинических исследований, Университетский госпиталь Хельсинки, Финляндия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(161) "Институт клинических исследований, Университетский госпиталь Хельсинки, Финляндия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26034" ["VALUE"]=> array(2) { ["TEXT"]=> string(2706) "<p style="text-align: justify;">Кортикостероиды играют определенную роль в качестве терапии первой линии реакции «трансплантат против хозяина» (РТПХ), но их значение в профилактике РТПХ менее ясно. В настоящее время кортикостероиды лишь в редких случаях включают в профилактические режимы. Исследования по добавлению кортикостероидов в наиболее широко применяемые режимы профилактики, с циклоспорином А и коротким курсом метотрексата приводили к противоречивым результатам, вероятно – из-за различий в схеме лечения. В нашем ранее опубликованном рандомизированном проспективном исследовании, добавление метилпреднизолона (МП) к циклоспорину и метотрексату вело к значительному снижению частоты острой РТПХ. Не отмечалось различий по выживаемости. При долгосрочном обследовании в этом исследовании, после 24,5 лет наблюдения у живущих пациентов мы отмечали существенную позднюю смертность, не связанную с рецидивами, среди пациентов, которые не получали профилактику МП, вероятно – из-за более высокой частоты хронической РТПХ в этой линии исследования. По окончании наблюдения, 55% пациентов, которым назначали МП для профилактики, были живы, по сравнению с 20% в контрольной группе. Эти находки предполагают, что роль кортикостероидов в профилактике РТПХT следует переоценить.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Реакция «трансплантат против хозяина», острая, хроническая, профилактика, кортикостероиды, глюкокортикоиды, благоприятный эффект.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2650) "Кортикостероиды играют определенную роль в качестве терапии первой линии реакции «трансплантат против хозяина» (РТПХ), но их значение в профилактике РТПХ менее ясно. В настоящее время кортикостероиды лишь в редких случаях включают в профилактические режимы. Исследования по добавлению кортикостероидов в наиболее широко применяемые режимы профилактики, с циклоспорином А и коротким курсом метотрексата приводили к противоречивым результатам, вероятно – из-за различий в схеме лечения. В нашем ранее опубликованном рандомизированном проспективном исследовании, добавление метилпреднизолона (МП) к циклоспорину и метотрексату вело к значительному снижению частоты острой РТПХ. Не отмечалось различий по выживаемости. При долгосрочном обследовании в этом исследовании, после 24,5 лет наблюдения у живущих пациентов мы отмечали существенную позднюю смертность, не связанную с рецидивами, среди пациентов, которые не получали профилактику МП, вероятно – из-за более высокой частоты хронической РТПХ в этой линии исследования. По окончании наблюдения, 55% пациентов, которым назначали МП для профилактики, были живы, по сравнению с 20% в контрольной группе. Эти находки предполагают, что роль кортикостероидов в профилактике РТПХT следует переоценить.
Ключевые слова
Реакция «трансплантат против хозяина», острая, хроническая, профилактика, кортикостероиды, глюкокортикоиды, благоприятный эффект.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26035" ["VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-1-8-12" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-1-8-12" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26039" ["VALUE"]=> array(2) { ["TEXT"]=> string(31) "<p>Tapani Ruutu</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(19) "Tapani Ruutu
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26040" ["VALUE"]=> array(2) { ["TEXT"]=> string(309) "<p>Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland</p><br> <p><b>Correspondence</b><br> Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland<br> E-mail: tapani.ruutu@hus.fi</p><br> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(249) "Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland
Correspondence
Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland
E-mail: tapani.ruutu@hus.fi
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26045" ["VALUE"]=> array(2) { ["TEXT"]=> string(1428) "<p style="text-align: justify;">Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.</p> <h2>Keywords</h2> <p style="text-align: justify;">Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1372) "
Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.
Keywords
Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26036" ["VALUE"]=> string(58) "Is there any role for corticosteroids in GvHD prophylaxis?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(58) "Is there any role for corticosteroids in GvHD prophylaxis?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26037" ["VALUE"]=> string(4) "1935" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1935" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26041" ["VALUE"]=> string(4) "1936" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1936" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(11) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26039" ["VALUE"]=> array(2) { ["TEXT"]=> string(31) "<p>Tapani Ruutu</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(19) "Tapani Ruutu
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(19) "Tapani Ruutu
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26045" ["VALUE"]=> array(2) { ["TEXT"]=> string(1428) "<p style="text-align: justify;">Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.</p> <h2>Keywords</h2> <p style="text-align: justify;">Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1372) "Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.
Keywords
Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1372) "Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.
Keywords
Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26035" ["VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-1-8-12" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-1-8-12" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-1-8-12" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26036" ["VALUE"]=> string(58) "Is there any role for corticosteroids in GvHD prophylaxis?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(58) "Is there any role for corticosteroids in GvHD prophylaxis?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(58) "Is there any role for corticosteroids in GvHD prophylaxis?" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26040" ["VALUE"]=> array(2) { ["TEXT"]=> string(309) "<p>Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland</p><br> <p><b>Correspondence</b><br> Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland<br> E-mail: tapani.ruutu@hus.fi</p><br> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(249) "Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland
Correspondence
Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland
E-mail: tapani.ruutu@hus.fi
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(249) "
Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland
Correspondence
Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland
E-mail: tapani.ruutu@hus.fi
" } ["AUTHORS"]=> array(38) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(1) { [0]=> string(5) "26056" } ["VALUE"]=> array(1) { [0]=> string(1) "7" } ["DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(1) { [0]=> string(1) "7" } ["~DESCRIPTION"]=> array(1) { [0]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(53) "Tapani Ruutu" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26032" ["VALUE"]=> array(2) { ["TEXT"]=> string(42) "<p>Тапани Рууту</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(30) "
Тапани Рууту
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(30) "Тапани Рууту
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26029" ["VALUE"]=> string(22) "12/15/2019 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/15/2019 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/15/2019 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26030" ["VALUE"]=> string(22) "01/24/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "01/24/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "01/24/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26034" ["VALUE"]=> array(2) { ["TEXT"]=> string(2706) "<p style="text-align: justify;">Кортикостероиды играют определенную роль в качестве терапии первой линии реакции «трансплантат против хозяина» (РТПХ), но их значение в профилактике РТПХ менее ясно. В настоящее время кортикостероиды лишь в редких случаях включают в профилактические режимы. Исследования по добавлению кортикостероидов в наиболее широко применяемые режимы профилактики, с циклоспорином А и коротким курсом метотрексата приводили к противоречивым результатам, вероятно – из-за различий в схеме лечения. В нашем ранее опубликованном рандомизированном проспективном исследовании, добавление метилпреднизолона (МП) к циклоспорину и метотрексату вело к значительному снижению частоты острой РТПХ. Не отмечалось различий по выживаемости. При долгосрочном обследовании в этом исследовании, после 24,5 лет наблюдения у живущих пациентов мы отмечали существенную позднюю смертность, не связанную с рецидивами, среди пациентов, которые не получали профилактику МП, вероятно – из-за более высокой частоты хронической РТПХ в этой линии исследования. По окончании наблюдения, 55% пациентов, которым назначали МП для профилактики, были живы, по сравнению с 20% в контрольной группе. Эти находки предполагают, что роль кортикостероидов в профилактике РТПХT следует переоценить.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Реакция «трансплантат против хозяина», острая, хроническая, профилактика, кортикостероиды, глюкокортикоиды, благоприятный эффект.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2650) "Кортикостероиды играют определенную роль в качестве терапии первой линии реакции «трансплантат против хозяина» (РТПХ), но их значение в профилактике РТПХ менее ясно. В настоящее время кортикостероиды лишь в редких случаях включают в профилактические режимы. Исследования по добавлению кортикостероидов в наиболее широко применяемые режимы профилактики, с циклоспорином А и коротким курсом метотрексата приводили к противоречивым результатам, вероятно – из-за различий в схеме лечения. В нашем ранее опубликованном рандомизированном проспективном исследовании, добавление метилпреднизолона (МП) к циклоспорину и метотрексату вело к значительному снижению частоты острой РТПХ. Не отмечалось различий по выживаемости. При долгосрочном обследовании в этом исследовании, после 24,5 лет наблюдения у живущих пациентов мы отмечали существенную позднюю смертность, не связанную с рецидивами, среди пациентов, которые не получали профилактику МП, вероятно – из-за более высокой частоты хронической РТПХ в этой линии исследования. По окончании наблюдения, 55% пациентов, которым назначали МП для профилактики, были живы, по сравнению с 20% в контрольной группе. Эти находки предполагают, что роль кортикостероидов в профилактике РТПХT следует переоценить.
Ключевые слова
Реакция «трансплантат против хозяина», острая, хроническая, профилактика, кортикостероиды, глюкокортикоиды, благоприятный эффект.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2650) "Кортикостероиды играют определенную роль в качестве терапии первой линии реакции «трансплантат против хозяина» (РТПХ), но их значение в профилактике РТПХ менее ясно. В настоящее время кортикостероиды лишь в редких случаях включают в профилактические режимы. Исследования по добавлению кортикостероидов в наиболее широко применяемые режимы профилактики, с циклоспорином А и коротким курсом метотрексата приводили к противоречивым результатам, вероятно – из-за различий в схеме лечения. В нашем ранее опубликованном рандомизированном проспективном исследовании, добавление метилпреднизолона (МП) к циклоспорину и метотрексату вело к значительному снижению частоты острой РТПХ. Не отмечалось различий по выживаемости. При долгосрочном обследовании в этом исследовании, после 24,5 лет наблюдения у живущих пациентов мы отмечали существенную позднюю смертность, не связанную с рецидивами, среди пациентов, которые не получали профилактику МП, вероятно – из-за более высокой частоты хронической РТПХ в этой линии исследования. По окончании наблюдения, 55% пациентов, которым назначали МП для профилактики, были живы, по сравнению с 20% в контрольной группе. Эти находки предполагают, что роль кортикостероидов в профилактике РТПХT следует переоценить.
Ключевые слова
Реакция «трансплантат против хозяина», острая, хроническая, профилактика, кортикостероиды, глюкокортикоиды, благоприятный эффект.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26033" ["VALUE"]=> array(2) { ["TEXT"]=> string(173) "<p>Институт клинических исследований, Университетский госпиталь Хельсинки, Финляндия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(161) "Институт клинических исследований, Университетский госпиталь Хельсинки, Финляндия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(161) "Институт клинических исследований, Университетский госпиталь Хельсинки, Финляндия
" } } } [3]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "144" ["~IBLOCK_SECTION_ID"]=> string(3) "144" ["ID"]=> string(4) "1844" ["~ID"]=> string(4) "1844" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(188) "Трансплантация стволовых клеток как консолидирующее лечение при периферических Т-клеточных лимфомах" ["~NAME"]=> string(188) "Трансплантация стволовых клеток как консолидирующее лечение при периферических Т-клеточных лимфомах" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "04/27/2020 12:31:28 pm" ["~TIMESTAMP_X"]=> string(22) "04/27/2020 12:31:28 pm" ["DETAIL_PAGE_URL"]=> string(154) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/transplantatsiya-stvolovykh-kletok-kak-konsolidiruyushchee-lechenie-pri-perifericheskikh-t-kletochny/" ["~DETAIL_PAGE_URL"]=> string(154) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/transplantatsiya-stvolovykh-kletok-kak-konsolidiruyushchee-lechenie-pri-perifericheskikh-t-kletochny/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(21016) "Introduction
Peripheral T-cell lymphomas (PTCLs) derived from post-thymic T cells or mature NK cells, are a rare and heterogeneous group of aggressive lymphomas comprising approximately 10% of all non-Hodgkin lymphomas diagnosed in the western world. In 2016, the WHO classification of lymphoid neoplasms recognized more than 20 different T-cell lymphoma entities that broadly segregate into lymphomas with predominant nodal involvement, extra-nodal involvement, leukemic, or cutaneous manifestations [1].
Nodal PTCLs are the most common of the PTCL subtypes and include peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), systemic anaplastic large cell lymphoma (sALCL) and angioimmunoblastic T-cell lymphoma (AITL). Together these 3 subtypes account for about 60% of all PTCL lymphomas. Given their heterogeneity, there is no consensus regarding the best first-line treatment, and the role of autologous/allogeneic (ASCT/alloSCT) stem cell transplantation as consolidation is controversial.
Table 1. Expected outcomes (5-year PFS) in PTCL subtypes treated with CHOP as first-line therapy [4, 5]

Conventional treatment with CHOP or CHOP-like regimen induces CR in about 50% of cases, with a 5-year survival of 30%-35% [2, 3]. Expected outcomes in terms of 5-year PFS/EFS in PTCL with initial CHOP treatment are based on 2 large retrospective series: the studies performed by the International T-Cell Project (ITCP) and the British Columbia Cancer Agency (BCCA) [4, 5] shown in Table 1.
Although CHOP is the most commonly used first-line regimen for patients with PTCL outcomes are disappointing with the exception of patients with ALK-positive sALCL. The most compelling evidence supporting the benefits of building on CHOP come from studies adding etoposide (CHOEP). It appears to offer an advantage over CHOP in younger patients (<60 years) based on a retrospective analysis of 7 prospective phase 2 or 3 German protocols including 343 patients [6]. CHOEP improved EFS from 51% to 71%. A registry study from Sweden found a superior PFS for CHOEP, also in patients <60 years [7]. This was seen particularly in those with ALCL and normal concentrations of lactate dehydrogenase. However, no improvement in OS was revealed, and greater toxicity was observed in older patients. This is also supported by the data from the COMPLETE study and suggests that any overall survival benefit associated with etoposide use in patients with PTCL is at best modest [8].
Although CHOP is the most commonly used first-line regimen for patients with PTCL outcomes are disappointing with the exception of patients with ALK-positive sALCL. The most compelling evidence supporting the benefits of building on CHOP come from studies adding etoposide (CHOEP). It appears to offer an advantage over CHOP in younger patients (<60 years) based on a retrospective analysis of 7 prospective phase 2 or 3 German protocols including 343 patients [6]. CHOEP improved EFS from 51% to 71%. A registry study from Sweden found a superior PFS for CHOEP, also in patients <60 years [7]. This was seen particularly in those with ALCL and normal concentrations of lactate dehydrogenase. However, no improvement in OS was revealed, and greater toxicity was observed in older patients. This is also supported by the data from the COMPLETE study and suggests that any overall survival benefit associated with etoposide use in patients with PTCL is at best modest [8].
CD30 is expressed in all sALCL and among other nodal variants of PTCL its expression is variable from 58-64% in PTCL NOS and 43-63% in AITL. Brentuximab vedotin (BV, Adcetris) is an antibody-drug conjugate active against CD30-positive lymphomas. The ECHELON-2 trial included a randomized, double-blind, phase 3 study of Brentuximab vedotin and CHP (A+CHP) versus CHOP in the frontline treatment of patients with CD30+ PTCLs [9].
The A+CHP schedule showed superior PFS and significantly longer OS than CHOP in patients with nodal CD30+ PTCLs. The trial also demonstrated improvement in the complete remission rate (68% vs 56%), and overall response rate (83% vs 72%) with A+CHP. Adverse events, including fatal AEs, were similar between groups. Of note, consolidative SCT was given to 22% patients in the A+CHP group and to 17% in the CHOP group. The treatment with ASCT did not affect PFS nor OS rates.
The NCCN guidelines suggest the following treatment regimens as first line therapy: BV+CHP regimen for sALCL cases; in other tumor histologies (PTCL NOS, AITL, nodal PTCL TFH), suggested regimens are as follows: BV+CHP for CD30+ histologies, and CHOP or CHOEP for other histologies, according to the patient’s age and performance status. As first-line consolidation high-dose therapy and stem cell rescue may be considered in appropriate patients [10].
High dose chemotherapy followed by autologous stem cell transplantation (ASCT) is a reasonable treatment option as front-line consolidation with resulting OS rates of 54% to 68% and low non-relapse mortality. A major problem is early relapse/progression in up to 40% of patients starting first-line therapy. In the relapse situation the overall prognosis is dismal, and the best treatment has not been defined yet [11, 12]. Chemorefractory patients should proceed to allogeneic SCT (allo-SCT) whenever possible, and the use of single agents as a bridge to transplant for these patients may be more appropriate because there is a need to sustain response until a compatible donor is identified and worked up [10-13].
Clinical practice recommendations on indication and timing of hematopoietic stem cell transplantation have recently been published by the American Society for Blood and Marrow Transplantation [14]. Registry studies on the role of autologous and allogeneic transplantation in PTCL have also been the subject of recent publications [15-17].
The current EBMT indications for hematopoietic transplantation in PTCL are illustrated in Table 2 [18].
Table 2. EBMT indications for HSCT in PTCL [18]

CO: clinical option; S: standard of care; GNR: generally not recommended; Grade I: at least 1 randomized trial; Grade II: at least 1 well-designed non-randomized CT; Grade III: expert opinion
To examine the real-world outcomes for patients with PTCL who underwent hematopoietic stem cell transplantation in our institution we conducted a retrospective review and report the clinical outcomes of 26 consecutive patients who were treated either as first-line consolidation or in the relapse setting between January 2000 and July 2018.
Patients and methods
Table 3. Clinical characteristics of the patients at diagnosis (n=26)

Abbreviations: AILT, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ALK, anaplastic lymphoma kinase; aaIPI, age-adjusted International Prognostic Index; BM, bone marrow; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified
Table 4. Treatment lines used in PTCL patients

Twenty-six patients were identified, with a median age of 46 years. Ninety-two percent of patients presented with advanced-stage at diagnosis (Ann Arbor stage III or IV) and 38% had B symptoms. The age-adjusted IPI (aaIPI) was low-intermediate in 15 patients and intermediate high/high in 11 patients (Table 3).
According to the 2016 revision of the WHO classification of lymphoid neoplasms, the most common PTCL subtypes within our cohort were AILT (46%), ALCL (26%) and PTCL NOS (20%) (1).
Most patients received CHOP chemotherapy as induction treatment regimen, with CHOEP being less frequently used (Table 4).
Twenty-seven transplants were performed in 26 patients (one patient had an ASCT followed by an allo-SCT after relapsing).
Nineteen patients had an ASCT with the BEAM conditioning regimen (Fig. 1). Sixteen patients were given an ASCT upfront as consolidation after induction treatment, with 14 being in CR and 2 in PR. Of these 16 patients, 5 had a relapse after ASCT and none survived, even though one was given an allo-SCT. Of the 3 patients given an ASCT as rescue treatment for relapse, 2 are alive in CR.
Seven patients underwent an allo-SCT, of whom 4 had it upfront and 3 after relapsing from a previous ASCT (Fig. 2). All patients received tacrolimus plus mycophenolate mofetil as GvHD prophylaxis and ATG was added to the conditioning regimen of the 2 patients transplanted with a mismatched unrelated donor. Five patients were conditioned with the Flu Mel protocol, 1 with Flu 2Gy TBI and 1 with the Flu BiCNU Mel protocol. All 4 patients treated upfront were given a transplant from unrelated donors of whom 1 was a 9/10 mismatch, none relapsed and all are alive. Of the 3 patients having a transplant after relapse, 2 are alive with no evidence of disease and one died of transplant related mortality.
Two patients deserve a special mention:
• Patient 19, a 58-y.o. male had a diagnosis of classic Hodgkin’s disease (cHD) in 2009, was treated with 8 cycles of ABVD and relapsed in 2011being treated with ICE followed by ASCT at another institution. In 2013, he presented with PTCL AITL subtype infiltrated with EBV+ B cells and was treated with R-ICE before being submitted to a matched unrelated allo-SCT in CR.
• Patient 21, a 42-y.o. male patient had a diagnosis of cHD in 1995, and achieved complete remission after 6 cycles of ABVD. He had a localized relapse of cHD in 1999, treated with local radiotherapy. He then presented in 2015 with a PTCL AITL subtype infiltrated with EBV+ B cells and CNS infiltration. He was treated with ESHAP followed by the LMB-96 protocol and was then submitted to a mismatched unrelated allo-SCT in CR.
Both patients are alive, one with moderate cGvHD.

Figure 1. Flow diagram of treatment and outcome of patients submitted to ASCT
Figure 2. Flow diagram of patients submitted to allo-SCT
Results
We evaluated the overall survival (OS) and progression-free survival (PFS) in our cohort of patients. The median follow-up time was 6.3 years (1-18.1 years). OS was calculated from the date of diagnosis until death due to any cause, and PFS was measured from transplant until relapse, progressive disease or last follow-up. Survival-based analysis were performed with the Kaplan-Meier methodology with censoring as appropriate and were evaluated with a log-rank test, with a 2-tailed P value ≤.05 used to reject the null hypothesis.
Nineteen patients underwent ASCT, of whom 16 (8 AITL, 4 PTCL NOS, 4 ALCL) as upfront consolidation treatment and 3 (2 ALCL, 1 AITL) for relapsed disease. Fifteen of the patients having an ASCT upfront were in CR1 and 4 relapsed, all within 6 months of transplant; the patient transplanted upfront in PR is alive in CR.
Three patients had an ASCT as rescue for recurrent disease, 1 died with relapse and 2 are alive in CR. The OS and RFS at 6-years for the 19 patients having an ASCT are 62% and 59%, respectively.
Seven patients had an allograft. Four (2 AITL, 1 ALCL, 1 NOS) as upfront consolidation, of whom 3 with a matched unrelated donor and 1 with a mismatched unrelated donor. They all are alive in CR.
Three patients (2 AITL, 1 PTCL NOS) had an alloSCT after relapsing. Two with an unrelated donor, after having failed an ASCT, and 1 with a matched sibling. Two are alive in CR and 1 died due to transplant-related complications.
Chronic GvHD was the most relevant complication observed in 50% of patients submitted to allo-SCT. It resolved in all except 2 patients who still have cGvHD needing immunosuppression.
The OS and RFS at 6-years for the 7 patients submitted to allo-SCT are 87% and 85%, respectively. Transplant-related mortality (TRM) was 3.7% for the entire population. Out of the 26 patients, seven patients died, 6 with progressive disease after auto HSCT and 1 with multiorgan failure after allo-HSCT. The 6-year OS and PFS for the entire population were 74% and 69% respectively (Fig. 3). Overall survival and PFS for patients submitted to ASCT and allo-SCT were similar, as shown in Fig. 4.

Figure 3. Kaplan-Meier estimates of 6-year OS (A) and PFS (B) for the entire patient population

Figure 4. Kaplan-Meier estimates of overall survival and relapse free survival of PTCL patient cohorts submitted to autologous and allogeneic transplantation
Conclusions
The results of this retrospective study, taking into account the adverse risk profile of the population, suggest that autologous/allogeneic stem cell transplantation is an effective and safe option for the consolidation of patients with PTCLs.
The recently published results of the COMPLETE consortium analyzed the impact of ASCT on the clinical outcomes of patients with newly diagnosed nodal PTCL in CR1 patients. They suggested that certain subgroups of patients with PTCL, i.e. those with AITL and/or high-risk features (advanced-stage disease or intermediate-to-high IPI scores) might benefit from consolidative ASCT in CR1 [19]. Still they concluded that the broader applicability of this strategy should be determined in prospective, randomized trials.
In our small study, some key questions remain on the real role of ASCT and allo-SCT in PTCL. Notwithstanding these limitations our study shows that HSCT is feasible and may benefit patients with high risk PTCL. The outcomes did not differ significantly between ASCT and allo-SCT approaches, but the latter is probably more effective in patients with refractory disease.
These results need to be validated in prospective studies, including a larger number of patients and may provide a platform for designing future and larger studies on the role of HSCT in PTCL.
Conflict of interest
M. Abecasis is in the speakers bureau of Takeda.
References
Introduction
Peripheral T-cell lymphomas (PTCLs) derived from post-thymic T cells or mature NK cells, are a rare and heterogeneous group of aggressive lymphomas comprising approximately 10% of all non-Hodgkin lymphomas diagnosed in the western world. In 2016, the WHO classification of lymphoid neoplasms recognized more than 20 different T-cell lymphoma entities that broadly segregate into lymphomas with predominant nodal involvement, extra-nodal involvement, leukemic, or cutaneous manifestations [1].
Nodal PTCLs are the most common of the PTCL subtypes and include peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), systemic anaplastic large cell lymphoma (sALCL) and angioimmunoblastic T-cell lymphoma (AITL). Together these 3 subtypes account for about 60% of all PTCL lymphomas. Given their heterogeneity, there is no consensus regarding the best first-line treatment, and the role of autologous/allogeneic (ASCT/alloSCT) stem cell transplantation as consolidation is controversial.
Table 1. Expected outcomes (5-year PFS) in PTCL subtypes treated with CHOP as first-line therapy [4, 5]

Conventional treatment with CHOP or CHOP-like regimen induces CR in about 50% of cases, with a 5-year survival of 30%-35% [2, 3]. Expected outcomes in terms of 5-year PFS/EFS in PTCL with initial CHOP treatment are based on 2 large retrospective series: the studies performed by the International T-Cell Project (ITCP) and the British Columbia Cancer Agency (BCCA) [4, 5] shown in Table 1.
Although CHOP is the most commonly used first-line regimen for patients with PTCL outcomes are disappointing with the exception of patients with ALK-positive sALCL. The most compelling evidence supporting the benefits of building on CHOP come from studies adding etoposide (CHOEP). It appears to offer an advantage over CHOP in younger patients (<60 years) based on a retrospective analysis of 7 prospective phase 2 or 3 German protocols including 343 patients [6]. CHOEP improved EFS from 51% to 71%. A registry study from Sweden found a superior PFS for CHOEP, also in patients <60 years [7]. This was seen particularly in those with ALCL and normal concentrations of lactate dehydrogenase. However, no improvement in OS was revealed, and greater toxicity was observed in older patients. This is also supported by the data from the COMPLETE study and suggests that any overall survival benefit associated with etoposide use in patients with PTCL is at best modest [8].
Although CHOP is the most commonly used first-line regimen for patients with PTCL outcomes are disappointing with the exception of patients with ALK-positive sALCL. The most compelling evidence supporting the benefits of building on CHOP come from studies adding etoposide (CHOEP). It appears to offer an advantage over CHOP in younger patients (<60 years) based on a retrospective analysis of 7 prospective phase 2 or 3 German protocols including 343 patients [6]. CHOEP improved EFS from 51% to 71%. A registry study from Sweden found a superior PFS for CHOEP, also in patients <60 years [7]. This was seen particularly in those with ALCL and normal concentrations of lactate dehydrogenase. However, no improvement in OS was revealed, and greater toxicity was observed in older patients. This is also supported by the data from the COMPLETE study and suggests that any overall survival benefit associated with etoposide use in patients with PTCL is at best modest [8].
CD30 is expressed in all sALCL and among other nodal variants of PTCL its expression is variable from 58-64% in PTCL NOS and 43-63% in AITL. Brentuximab vedotin (BV, Adcetris) is an antibody-drug conjugate active against CD30-positive lymphomas. The ECHELON-2 trial included a randomized, double-blind, phase 3 study of Brentuximab vedotin and CHP (A+CHP) versus CHOP in the frontline treatment of patients with CD30+ PTCLs [9].
The A+CHP schedule showed superior PFS and significantly longer OS than CHOP in patients with nodal CD30+ PTCLs. The trial also demonstrated improvement in the complete remission rate (68% vs 56%), and overall response rate (83% vs 72%) with A+CHP. Adverse events, including fatal AEs, were similar between groups. Of note, consolidative SCT was given to 22% patients in the A+CHP group and to 17% in the CHOP group. The treatment with ASCT did not affect PFS nor OS rates.
The NCCN guidelines suggest the following treatment regimens as first line therapy: BV+CHP regimen for sALCL cases; in other tumor histologies (PTCL NOS, AITL, nodal PTCL TFH), suggested regimens are as follows: BV+CHP for CD30+ histologies, and CHOP or CHOEP for other histologies, according to the patient’s age and performance status. As first-line consolidation high-dose therapy and stem cell rescue may be considered in appropriate patients [10].
High dose chemotherapy followed by autologous stem cell transplantation (ASCT) is a reasonable treatment option as front-line consolidation with resulting OS rates of 54% to 68% and low non-relapse mortality. A major problem is early relapse/progression in up to 40% of patients starting first-line therapy. In the relapse situation the overall prognosis is dismal, and the best treatment has not been defined yet [11, 12]. Chemorefractory patients should proceed to allogeneic SCT (allo-SCT) whenever possible, and the use of single agents as a bridge to transplant for these patients may be more appropriate because there is a need to sustain response until a compatible donor is identified and worked up [10-13].
Clinical practice recommendations on indication and timing of hematopoietic stem cell transplantation have recently been published by the American Society for Blood and Marrow Transplantation [14]. Registry studies on the role of autologous and allogeneic transplantation in PTCL have also been the subject of recent publications [15-17].
The current EBMT indications for hematopoietic transplantation in PTCL are illustrated in Table 2 [18].
Table 2. EBMT indications for HSCT in PTCL [18]

CO: clinical option; S: standard of care; GNR: generally not recommended; Grade I: at least 1 randomized trial; Grade II: at least 1 well-designed non-randomized CT; Grade III: expert opinion
To examine the real-world outcomes for patients with PTCL who underwent hematopoietic stem cell transplantation in our institution we conducted a retrospective review and report the clinical outcomes of 26 consecutive patients who were treated either as first-line consolidation or in the relapse setting between January 2000 and July 2018.
Patients and methods
Table 3. Clinical characteristics of the patients at diagnosis (n=26)

Abbreviations: AILT, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; ALK, anaplastic lymphoma kinase; aaIPI, age-adjusted International Prognostic Index; BM, bone marrow; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified
Table 4. Treatment lines used in PTCL patients

Twenty-six patients were identified, with a median age of 46 years. Ninety-two percent of patients presented with advanced-stage at diagnosis (Ann Arbor stage III or IV) and 38% had B symptoms. The age-adjusted IPI (aaIPI) was low-intermediate in 15 patients and intermediate high/high in 11 patients (Table 3).
According to the 2016 revision of the WHO classification of lymphoid neoplasms, the most common PTCL subtypes within our cohort were AILT (46%), ALCL (26%) and PTCL NOS (20%) (1).
Most patients received CHOP chemotherapy as induction treatment regimen, with CHOEP being less frequently used (Table 4).
Twenty-seven transplants were performed in 26 patients (one patient had an ASCT followed by an allo-SCT after relapsing).
Nineteen patients had an ASCT with the BEAM conditioning regimen (Fig. 1). Sixteen patients were given an ASCT upfront as consolidation after induction treatment, with 14 being in CR and 2 in PR. Of these 16 patients, 5 had a relapse after ASCT and none survived, even though one was given an allo-SCT. Of the 3 patients given an ASCT as rescue treatment for relapse, 2 are alive in CR.
Seven patients underwent an allo-SCT, of whom 4 had it upfront and 3 after relapsing from a previous ASCT (Fig. 2). All patients received tacrolimus plus mycophenolate mofetil as GvHD prophylaxis and ATG was added to the conditioning regimen of the 2 patients transplanted with a mismatched unrelated donor. Five patients were conditioned with the Flu Mel protocol, 1 with Flu 2Gy TBI and 1 with the Flu BiCNU Mel protocol. All 4 patients treated upfront were given a transplant from unrelated donors of whom 1 was a 9/10 mismatch, none relapsed and all are alive. Of the 3 patients having a transplant after relapse, 2 are alive with no evidence of disease and one died of transplant related mortality.
Two patients deserve a special mention:
• Patient 19, a 58-y.o. male had a diagnosis of classic Hodgkin’s disease (cHD) in 2009, was treated with 8 cycles of ABVD and relapsed in 2011being treated with ICE followed by ASCT at another institution. In 2013, he presented with PTCL AITL subtype infiltrated with EBV+ B cells and was treated with R-ICE before being submitted to a matched unrelated allo-SCT in CR.
• Patient 21, a 42-y.o. male patient had a diagnosis of cHD in 1995, and achieved complete remission after 6 cycles of ABVD. He had a localized relapse of cHD in 1999, treated with local radiotherapy. He then presented in 2015 with a PTCL AITL subtype infiltrated with EBV+ B cells and CNS infiltration. He was treated with ESHAP followed by the LMB-96 protocol and was then submitted to a mismatched unrelated allo-SCT in CR.
Both patients are alive, one with moderate cGvHD.

Figure 1. Flow diagram of treatment and outcome of patients submitted to ASCT
Figure 2. Flow diagram of patients submitted to allo-SCT
Results
We evaluated the overall survival (OS) and progression-free survival (PFS) in our cohort of patients. The median follow-up time was 6.3 years (1-18.1 years). OS was calculated from the date of diagnosis until death due to any cause, and PFS was measured from transplant until relapse, progressive disease or last follow-up. Survival-based analysis were performed with the Kaplan-Meier methodology with censoring as appropriate and were evaluated with a log-rank test, with a 2-tailed P value ≤.05 used to reject the null hypothesis.
Nineteen patients underwent ASCT, of whom 16 (8 AITL, 4 PTCL NOS, 4 ALCL) as upfront consolidation treatment and 3 (2 ALCL, 1 AITL) for relapsed disease. Fifteen of the patients having an ASCT upfront were in CR1 and 4 relapsed, all within 6 months of transplant; the patient transplanted upfront in PR is alive in CR.
Three patients had an ASCT as rescue for recurrent disease, 1 died with relapse and 2 are alive in CR. The OS and RFS at 6-years for the 19 patients having an ASCT are 62% and 59%, respectively.
Seven patients had an allograft. Four (2 AITL, 1 ALCL, 1 NOS) as upfront consolidation, of whom 3 with a matched unrelated donor and 1 with a mismatched unrelated donor. They all are alive in CR.
Three patients (2 AITL, 1 PTCL NOS) had an alloSCT after relapsing. Two with an unrelated donor, after having failed an ASCT, and 1 with a matched sibling. Two are alive in CR and 1 died due to transplant-related complications.
Chronic GvHD was the most relevant complication observed in 50% of patients submitted to allo-SCT. It resolved in all except 2 patients who still have cGvHD needing immunosuppression.
The OS and RFS at 6-years for the 7 patients submitted to allo-SCT are 87% and 85%, respectively. Transplant-related mortality (TRM) was 3.7% for the entire population. Out of the 26 patients, seven patients died, 6 with progressive disease after auto HSCT and 1 with multiorgan failure after allo-HSCT. The 6-year OS and PFS for the entire population were 74% and 69% respectively (Fig. 3). Overall survival and PFS for patients submitted to ASCT and allo-SCT were similar, as shown in Fig. 4.

Figure 3. Kaplan-Meier estimates of 6-year OS (A) and PFS (B) for the entire patient population

Figure 4. Kaplan-Meier estimates of overall survival and relapse free survival of PTCL patient cohorts submitted to autologous and allogeneic transplantation
Conclusions
The results of this retrospective study, taking into account the adverse risk profile of the population, suggest that autologous/allogeneic stem cell transplantation is an effective and safe option for the consolidation of patients with PTCLs.
The recently published results of the COMPLETE consortium analyzed the impact of ASCT on the clinical outcomes of patients with newly diagnosed nodal PTCL in CR1 patients. They suggested that certain subgroups of patients with PTCL, i.e. those with AITL and/or high-risk features (advanced-stage disease or intermediate-to-high IPI scores) might benefit from consolidative ASCT in CR1 [19]. Still they concluded that the broader applicability of this strategy should be determined in prospective, randomized trials.
In our small study, some key questions remain on the real role of ASCT and allo-SCT in PTCL. Notwithstanding these limitations our study shows that HSCT is feasible and may benefit patients with high risk PTCL. The outcomes did not differ significantly between ASCT and allo-SCT approaches, but the latter is probably more effective in patients with refractory disease.
These results need to be validated in prospective studies, including a larger number of patients and may provide a platform for designing future and larger studies on the role of HSCT in PTCL.
Conflict of interest
M. Abecasis is in the speakers bureau of Takeda.
References
Мануэль Абекасис, Каталина Гомез, Изабелина Феррейра, Мария Гомес да Силва, Нуньо Миранда, Гилда Тейксейра, Фернандо Леаль да Коста, Мария Жоао Гутьеррез
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26146" ["VALUE"]=> array(2) { ["TEXT"]=> string(155) "<p>Португальский институт онкологии Франциско Жентиль, Лиссабон, Португалия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(143) "Португальский институт онкологии Франциско Жентиль, Лиссабон, Португалия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26147" ["VALUE"]=> array(2) { ["TEXT"]=> string(3244) "<p style="text-align: justify;">Периферические Т-клеточные лимфомы (ПТКЛ) являются гетерогенной группой редких агрессивных лимфом, состоящей из более чем 20 различных клинических форм и представляющих около 10% всех неходжкинских лимфом, диагностированных в Западном мире. Учитывая их гетерогенность, отсутствует консенсус, касающийся наилучшего лечения первой линии, роль аутологичной или аллогенной трансплантации гемопоэтических клеток (ТГСК) в качестве консолидации пока противоречива. Чтобы изучить реальные исходы у пациентов с ПТКЛ, направленных для ТГСК в наше учреждение, мы ретроспективно изучили клинические исходы у 26 пациентов, которым выполняли трансплантацию либо в качестве консолидирующей терапии первой линии или при рецидиве заболевания в период с января 2000 по июль 2018 г. Медианный срок наблюдения составлял 6,3 года; 19 больных получили ауто-ТГСК, 16 – в качестве первой консолидации и 3 – по поводу рецидива болезни. Общая выживаемость (ОВ) и безрецидивная выживаемость (БРВ) были, соответственно, 62% и 59%. Семи больным было проведена алло-ТГСК, четыре – консолидация первой линии и трем – после рецидива. Шесть больных живы и один умер в связи с процедурой ТГСК. Этот вид летальности составил 3.7% для всей группы, а 6-летние показатели ОВ и БРВ были, соответственно, 74% и 69%. Наши результаты предполагают, что аутологичные и аллогенные ТГСК являются эффективным и безопасным вариантом в целях консолидации больных ПТКЛ с неблагоприятным профилем риска, но нужна их валидация в проспективных исследованиях, включающих большое число пациентов.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Периферические Т-клеточные лимфомы, терапия первой линии, CHOP, Брентуксимаб ведотин, трансплантация гемопоэтических клеток, аллогенная, аутологичная. </p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3188) "Периферические Т-клеточные лимфомы (ПТКЛ) являются гетерогенной группой редких агрессивных лимфом, состоящей из более чем 20 различных клинических форм и представляющих около 10% всех неходжкинских лимфом, диагностированных в Западном мире. Учитывая их гетерогенность, отсутствует консенсус, касающийся наилучшего лечения первой линии, роль аутологичной или аллогенной трансплантации гемопоэтических клеток (ТГСК) в качестве консолидации пока противоречива. Чтобы изучить реальные исходы у пациентов с ПТКЛ, направленных для ТГСК в наше учреждение, мы ретроспективно изучили клинические исходы у 26 пациентов, которым выполняли трансплантацию либо в качестве консолидирующей терапии первой линии или при рецидиве заболевания в период с января 2000 по июль 2018 г. Медианный срок наблюдения составлял 6,3 года; 19 больных получили ауто-ТГСК, 16 – в качестве первой консолидации и 3 – по поводу рецидива болезни. Общая выживаемость (ОВ) и безрецидивная выживаемость (БРВ) были, соответственно, 62% и 59%. Семи больным было проведена алло-ТГСК, четыре – консолидация первой линии и трем – после рецидива. Шесть больных живы и один умер в связи с процедурой ТГСК. Этот вид летальности составил 3.7% для всей группы, а 6-летние показатели ОВ и БРВ были, соответственно, 74% и 69%. Наши результаты предполагают, что аутологичные и аллогенные ТГСК являются эффективным и безопасным вариантом в целях консолидации больных ПТКЛ с неблагоприятным профилем риска, но нужна их валидация в проспективных исследованиях, включающих большое число пациентов.
Ключевые слова
Периферические Т-клеточные лимфомы, терапия первой линии, CHOP, Брентуксимаб ведотин, трансплантация гемопоэтических клеток, аллогенная, аутологичная.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26135" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-22-27" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-22-27" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26157" ["VALUE"]=> array(2) { ["TEXT"]=> string(169) "<p>Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(157) "Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26158" ["VALUE"]=> array(2) { ["TEXT"]=> string(324) "<p>Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal</p> <br> <p><b>Correspondence</b><br> Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia, <br>R. Professor Lima Basto 1099-093, Lisboa, Portugal</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(270) "Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal
Correspondence
Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia,
R. Professor Lima Basto 1099-093, Lisboa, Portugal
Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of aggressive lymphomas comprising more than 20 different entities and representing about 10% of all non-Hodgkin lymphomas diagnosed in the Western World. Given their heterogeneity, there is no consensus regarding the best first-line treatment and the role of autologous/allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation is controversial. To examine the real-world outcomes for patients with PTCL submitted to HSCT in our institution we retrospectively reviewed the clinical outcomes of 26 patients who were given a transplant either as first-line consolidation or in relapse between January 2000 and July 2018. The median follow-up is 6.3 years; 19 patients had an autologous HSCT, 16 as upfront consolidation and 3, for relapsed disease. Overall survival (OS) and relapse free survival (RFS) were, respectively, 62% and 59%. Seven patients had an allograft, 4 as upfront consolidation and 3 after relapse; 6 are alive and 1 died due to transplant-related mortality (TRM). TRM was 3.7% for the entire population and the 6-year OS and PFS were 74% and 69%, respectively. Our results suggest that autologous/allogeneic HSCT is an effective and safe option for the consolidation of adverse-risk profile patients with PTCL, but they need to be validated in prospective studies including a larger number of patients.
Keywords
Peripheral T-cell lymphomas, first-line therapy, CHOP, Brentuximab vedotin, hematopoietic stem cell transplantation, allogeneic, autologous.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26136" ["VALUE"]=> string(73) "Stem cell transplantation as consolidation in peripheral T-cell lymphomas" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(73) "Stem cell transplantation as consolidation in peripheral T-cell lymphomas" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26148" ["VALUE"]=> string(4) "1944" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1944" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26159" ["VALUE"]=> string(4) "1945" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1945" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(11) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26157" ["VALUE"]=> array(2) { ["TEXT"]=> string(169) "<p>Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(157) "Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(157) "Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26176" ["VALUE"]=> array(2) { ["TEXT"]=> string(1698) "<p style="text-align: justify;">Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of aggressive lymphomas comprising more than 20 different entities and representing about 10% of all non-Hodgkin lymphomas diagnosed in the Western World. Given their heterogeneity, there is no consensus regarding the best first-line treatment and the role of autologous/allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation is controversial. To examine the real-world outcomes for patients with PTCL submitted to HSCT in our institution we retrospectively reviewed the clinical outcomes of 26 patients who were given a transplant either as first-line consolidation or in relapse between January 2000 and July 2018. The median follow-up is 6.3 years; 19 patients had an autologous HSCT, 16 as upfront consolidation and 3, for relapsed disease. Overall survival (OS) and relapse free survival (RFS) were, respectively, 62% and 59%. Seven patients had an allograft, 4 as upfront consolidation and 3 after relapse; 6 are alive and 1 died due to transplant-related mortality (TRM). TRM was 3.7% for the entire population and the 6-year OS and PFS were 74% and 69%, respectively. Our results suggest that autologous/allogeneic HSCT is an effective and safe option for the consolidation of adverse-risk profile patients with PTCL, but they need to be validated in prospective studies including a larger number of patients.</p> <h2>Keywords</h2> <p style="text-align: justify;">Peripheral T-cell lymphomas, first-line therapy, CHOP, Brentuximab vedotin, hematopoietic stem cell transplantation, allogeneic, autologous. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1642) "Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of aggressive lymphomas comprising more than 20 different entities and representing about 10% of all non-Hodgkin lymphomas diagnosed in the Western World. Given their heterogeneity, there is no consensus regarding the best first-line treatment and the role of autologous/allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation is controversial. To examine the real-world outcomes for patients with PTCL submitted to HSCT in our institution we retrospectively reviewed the clinical outcomes of 26 patients who were given a transplant either as first-line consolidation or in relapse between January 2000 and July 2018. The median follow-up is 6.3 years; 19 patients had an autologous HSCT, 16 as upfront consolidation and 3, for relapsed disease. Overall survival (OS) and relapse free survival (RFS) were, respectively, 62% and 59%. Seven patients had an allograft, 4 as upfront consolidation and 3 after relapse; 6 are alive and 1 died due to transplant-related mortality (TRM). TRM was 3.7% for the entire population and the 6-year OS and PFS were 74% and 69%, respectively. Our results suggest that autologous/allogeneic HSCT is an effective and safe option for the consolidation of adverse-risk profile patients with PTCL, but they need to be validated in prospective studies including a larger number of patients.
Keywords
Peripheral T-cell lymphomas, first-line therapy, CHOP, Brentuximab vedotin, hematopoietic stem cell transplantation, allogeneic, autologous.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1642) "Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of aggressive lymphomas comprising more than 20 different entities and representing about 10% of all non-Hodgkin lymphomas diagnosed in the Western World. Given their heterogeneity, there is no consensus regarding the best first-line treatment and the role of autologous/allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation is controversial. To examine the real-world outcomes for patients with PTCL submitted to HSCT in our institution we retrospectively reviewed the clinical outcomes of 26 patients who were given a transplant either as first-line consolidation or in relapse between January 2000 and July 2018. The median follow-up is 6.3 years; 19 patients had an autologous HSCT, 16 as upfront consolidation and 3, for relapsed disease. Overall survival (OS) and relapse free survival (RFS) were, respectively, 62% and 59%. Seven patients had an allograft, 4 as upfront consolidation and 3 after relapse; 6 are alive and 1 died due to transplant-related mortality (TRM). TRM was 3.7% for the entire population and the 6-year OS and PFS were 74% and 69%, respectively. Our results suggest that autologous/allogeneic HSCT is an effective and safe option for the consolidation of adverse-risk profile patients with PTCL, but they need to be validated in prospective studies including a larger number of patients.
Keywords
Peripheral T-cell lymphomas, first-line therapy, CHOP, Brentuximab vedotin, hematopoietic stem cell transplantation, allogeneic, autologous.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26135" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-22-27" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-22-27" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-22-27" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26136" ["VALUE"]=> string(73) "Stem cell transplantation as consolidation in peripheral T-cell lymphomas" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(73) "Stem cell transplantation as consolidation in peripheral T-cell lymphomas" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(73) "Stem cell transplantation as consolidation in peripheral T-cell lymphomas" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26158" ["VALUE"]=> array(2) { ["TEXT"]=> string(324) "<p>Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal</p> <br> <p><b>Correspondence</b><br> Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia, <br>R. Professor Lima Basto 1099-093, Lisboa, Portugal</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(270) "Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal
Correspondence
Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia,
R. Professor Lima Basto 1099-093, Lisboa, Portugal
Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal
Correspondence
Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia,
R. Professor Lima Basto 1099-093, Lisboa, Portugal
Мануэль Абекасис, Каталина Гомез, Изабелина Феррейра, Мария Гомес да Силва, Нуньо Миранда, Гилда Тейксейра, Фернандо Леаль да Коста, Мария Жоао Гутьеррез
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(286) "Мануэль Абекасис, Каталина Гомез, Изабелина Феррейра, Мария Гомес да Силва, Нуньо Миранда, Гилда Тейксейра, Фернандо Леаль да Коста, Мария Жоао Гутьеррез
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26125" ["VALUE"]=> string(22) "10/10/2019 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/10/2019 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "10/10/2019 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26126" ["VALUE"]=> string(22) "01/24/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "01/24/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "01/24/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26147" ["VALUE"]=> array(2) { ["TEXT"]=> string(3244) "<p style="text-align: justify;">Периферические Т-клеточные лимфомы (ПТКЛ) являются гетерогенной группой редких агрессивных лимфом, состоящей из более чем 20 различных клинических форм и представляющих около 10% всех неходжкинских лимфом, диагностированных в Западном мире. Учитывая их гетерогенность, отсутствует консенсус, касающийся наилучшего лечения первой линии, роль аутологичной или аллогенной трансплантации гемопоэтических клеток (ТГСК) в качестве консолидации пока противоречива. Чтобы изучить реальные исходы у пациентов с ПТКЛ, направленных для ТГСК в наше учреждение, мы ретроспективно изучили клинические исходы у 26 пациентов, которым выполняли трансплантацию либо в качестве консолидирующей терапии первой линии или при рецидиве заболевания в период с января 2000 по июль 2018 г. Медианный срок наблюдения составлял 6,3 года; 19 больных получили ауто-ТГСК, 16 – в качестве первой консолидации и 3 – по поводу рецидива болезни. Общая выживаемость (ОВ) и безрецидивная выживаемость (БРВ) были, соответственно, 62% и 59%. Семи больным было проведена алло-ТГСК, четыре – консолидация первой линии и трем – после рецидива. Шесть больных живы и один умер в связи с процедурой ТГСК. Этот вид летальности составил 3.7% для всей группы, а 6-летние показатели ОВ и БРВ были, соответственно, 74% и 69%. Наши результаты предполагают, что аутологичные и аллогенные ТГСК являются эффективным и безопасным вариантом в целях консолидации больных ПТКЛ с неблагоприятным профилем риска, но нужна их валидация в проспективных исследованиях, включающих большое число пациентов.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Периферические Т-клеточные лимфомы, терапия первой линии, CHOP, Брентуксимаб ведотин, трансплантация гемопоэтических клеток, аллогенная, аутологичная. </p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3188) "Периферические Т-клеточные лимфомы (ПТКЛ) являются гетерогенной группой редких агрессивных лимфом, состоящей из более чем 20 различных клинических форм и представляющих около 10% всех неходжкинских лимфом, диагностированных в Западном мире. Учитывая их гетерогенность, отсутствует консенсус, касающийся наилучшего лечения первой линии, роль аутологичной или аллогенной трансплантации гемопоэтических клеток (ТГСК) в качестве консолидации пока противоречива. Чтобы изучить реальные исходы у пациентов с ПТКЛ, направленных для ТГСК в наше учреждение, мы ретроспективно изучили клинические исходы у 26 пациентов, которым выполняли трансплантацию либо в качестве консолидирующей терапии первой линии или при рецидиве заболевания в период с января 2000 по июль 2018 г. Медианный срок наблюдения составлял 6,3 года; 19 больных получили ауто-ТГСК, 16 – в качестве первой консолидации и 3 – по поводу рецидива болезни. Общая выживаемость (ОВ) и безрецидивная выживаемость (БРВ) были, соответственно, 62% и 59%. Семи больным было проведена алло-ТГСК, четыре – консолидация первой линии и трем – после рецидива. Шесть больных живы и один умер в связи с процедурой ТГСК. Этот вид летальности составил 3.7% для всей группы, а 6-летние показатели ОВ и БРВ были, соответственно, 74% и 69%. Наши результаты предполагают, что аутологичные и аллогенные ТГСК являются эффективным и безопасным вариантом в целях консолидации больных ПТКЛ с неблагоприятным профилем риска, но нужна их валидация в проспективных исследованиях, включающих большое число пациентов.
Ключевые слова
Периферические Т-клеточные лимфомы, терапия первой линии, CHOP, Брентуксимаб ведотин, трансплантация гемопоэтических клеток, аллогенная, аутологичная.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3188) "Периферические Т-клеточные лимфомы (ПТКЛ) являются гетерогенной группой редких агрессивных лимфом, состоящей из более чем 20 различных клинических форм и представляющих около 10% всех неходжкинских лимфом, диагностированных в Западном мире. Учитывая их гетерогенность, отсутствует консенсус, касающийся наилучшего лечения первой линии, роль аутологичной или аллогенной трансплантации гемопоэтических клеток (ТГСК) в качестве консолидации пока противоречива. Чтобы изучить реальные исходы у пациентов с ПТКЛ, направленных для ТГСК в наше учреждение, мы ретроспективно изучили клинические исходы у 26 пациентов, которым выполняли трансплантацию либо в качестве консолидирующей терапии первой линии или при рецидиве заболевания в период с января 2000 по июль 2018 г. Медианный срок наблюдения составлял 6,3 года; 19 больных получили ауто-ТГСК, 16 – в качестве первой консолидации и 3 – по поводу рецидива болезни. Общая выживаемость (ОВ) и безрецидивная выживаемость (БРВ) были, соответственно, 62% и 59%. Семи больным было проведена алло-ТГСК, четыре – консолидация первой линии и трем – после рецидива. Шесть больных живы и один умер в связи с процедурой ТГСК. Этот вид летальности составил 3.7% для всей группы, а 6-летние показатели ОВ и БРВ были, соответственно, 74% и 69%. Наши результаты предполагают, что аутологичные и аллогенные ТГСК являются эффективным и безопасным вариантом в целях консолидации больных ПТКЛ с неблагоприятным профилем риска, но нужна их валидация в проспективных исследованиях, включающих большое число пациентов.
Ключевые слова
Периферические Т-клеточные лимфомы, терапия первой линии, CHOP, Брентуксимаб ведотин, трансплантация гемопоэтических клеток, аллогенная, аутологичная.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26146" ["VALUE"]=> array(2) { ["TEXT"]=> string(155) "<p>Португальский институт онкологии Франциско Жентиль, Лиссабон, Португалия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(143) "Португальский институт онкологии Франциско Жентиль, Лиссабон, Португалия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(143) "Португальский институт онкологии Франциско Жентиль, Лиссабон, Португалия
" } } } [4]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "144" ["~IBLOCK_SECTION_ID"]=> string(3) "144" ["ID"]=> string(4) "1845" ["~ID"]=> string(4) "1845" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(134) "Варианты лечения Т-клеточных лимфом: данные одноцентрового исследования" ["~NAME"]=> string(134) "Варианты лечения Т-клеточных лимфом: данные одноцентрового исследования" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "04/27/2020 02:21:47 pm" ["~TIMESTAMP_X"]=> string(22) "04/27/2020 02:21:47 pm" ["DETAIL_PAGE_URL"]=> string(130) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/varianty-lecheniya-t-kletochnykh-limfom-dannye-odnotsentrovogo-issledovaniya/" ["~DETAIL_PAGE_URL"]=> string(130) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/varianty-lecheniya-t-kletochnykh-limfom-dannye-odnotsentrovogo-issledovaniya/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(28885) "Introduction
The T-cell/natural killer (NK) cell lymphomas are a heterogeneous group of generally aggressive neoplasms that constitute less than 10-15 percent of all non-Hodgkin lymphomas (NHLs) in adults [1, 2]. Modern classification of T/NK-cell lymphomas was developed on the basis of histological, immunohistochemical studies of tumor tissue and clinical presentation of lymphomas. In 2016, the World Health Organization classification revised the classification of both nodal and extranodal T/NK-cell lymphomas, which led to the introduction of new temporary structures [3]. Many of these changes were caused by the results of genomic studies using approaches to study gene expression profiling (GEP) and the genetic landscape of T/NK cell lymphomas. However, despite advances in molecular diagnostic methods, histological examination of tumor tissue and clinical manifestations remain the main diagnostic approach.
Another area of research concerns dismal outcomes in the patients with T/NK-cell lymphomas, except for anaplastic large-cell lymphoma (ALCL) positive for anaplastic lymphoma kinase (ALK) [4]. There are currently no successful treatment standards for the T/NK-cell lymphomas, both in the first-line therapy, and in refractory/relapsed (r/r) settings. Today, almost 70% of patients undergoing first-line therapy develop a relapse or refractory disease. A review of the scientific literature allowed us to identify a range of therapeutic options for patients with T/NK-cell lymphomas [5]. It includes the following approaches:
- Hematopoietic stem cells transplantation (HSCT);
- Therapy with monoclonal antibodies (Mabs), e.g., anti-CD30 antibody-drug conjugate (Brentuximab vedotin), anti-CC chemokine receptor 4 antibodies (Mogamulizumab), a monoclonal antibody that binds to CD52 (Alemtuzumab), the monoclonal anti-vascular endothelial growth factor (Bevacizumab) [6-19];
- ALK inhibitors (Crizotinib, Ceritinib) [20];
- Therapy with immune checkpoint inhibitors, e.g., Nivolumab, Pembrolizumab, Ipilimumab, Durvalumab [21-27];
- Histone deacetylase inhibitors (Belinostat, Panobinostat, Chidamide, Romidepsin, Vorinostat) [28-34];
- CAR-T/NK-cell therapy [35, 36];
- In situ vaccination [37].
There are a lot of studies and a large number of drugs being introduced into clinical studies, but at the present time, greatest evidence base should be recognized for brentuximab and bone marrow transplantation. E.g., patients with r/r T/NK-cell lymphomas who received hematopoietic stem cell transplantation (allogeneic and/or autologous) had a better outcome compared to the subset of non-transplanted patients (3-year survival rates of 48% and 18%, respectively) [5]. These conclusions also supported by data of Abekasis et al., demonstrating that autologous and/or allogeneic HSCT is an effective and safe option for the consolidation of patients with TCL [38].
Recently published results of a clinical study ECHELON-2 showed that front-line treatment with Adcetris (brentuximab vedotin) + CHP protocol is superior to CHOP for patients with CD30-positive peripheral T-cell lymphomas, thus suggesting that A+CHP is likely to be the standard first-line therapy [39].
Also, brentuximab vedotin showed good results in the treatment of r/r CD30 positive TCL [40]. Mostly, there were anaplastic large cell lymphoma (ALСL), angioimmunoblastic T-cell lymphoma (AITL), Sézary syndrome and Mycosis Fungoides (MF) [7]. The objective response was from 92% to 40%, with best results obtained in ALСL treatment [41, 42]. These methods are actively used at our center.
Hence, the aim of this study was to present our single-center experience in the treatment of patients with T/NK-cell lymphomas.
Patients and methods

Figure 1. Histological subtypes of patients with T-cell lymphomas treated at the R. Gorbacheva Memorial Institute
We analyzed data of 47 patients with TCL eligible for stem cells transplantation treated in the R. Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at the Pavlov University from 2005 to 2019 including 44 cases with r/r TCL and 3 patients being in complete response after first-line therapy. Among them, 10 patients were diagnosed with anaplastic large cell lymphoma (ALK+); 5 cases with anaplastic large cell lymphoma (ALK); 4 cases with angioimmunoblastic TCL (AITL); 4 patients with hepatosplenic T-cell lymphoma (HSTCL); 1 case of γδ T cell lymphoma (γδ TCL); 20 patients with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS); 1 patient with mucosis fungoides (MF); 1, with primary cutaneous CD4+ T-cell lymphoma (CD4+ PCSM-TCL), and one patient with subcutaneous panniculitis-like T-cell lymphoma (SPTCL), as seen from Fig. 1.
General characteristics of the patients are outlined in Table 1.
Table 1. Clinical characteristics of T-cell lymphoma patients

Table 2. Clinical state of the patients with T-cell lymphoma subjected to auto-HSCT

The treatment was tailored according to biological tumor markers revealed in the patients. In 10 patients with CD30+ PTCL (n=4 with PTCL-NOS; n=4 with ALK+; n=1 with ALK; n=1 with AITL), Brentuximab vedotin was used. One patient with ALK+ anaplastic lymphoma received ALK inhibitor crizotinib. Five patients with PD-L1 hyperexpression (n=4 with PTCL-NOS; n=1 with γδ TCL) were treated with nivolumab. Overall 24 patients underwent HSCT: high-dose chemotherapy with auto-HSCT was performed in 16 patients, 13 patients underwent allo-HSCT (among them 5 patients with relapses after auto-HSCT). Place of auto-HSCT in the treatment of TCL in our center could be seen from Table 2.
Main characteristics of patients who underwent allogeneic hematopoietic stem cells transplantation are shown in Table 3.
Table 3. Main clinical characteristics of 13 patients with r/r TCL subjected to allo-HSCT

Statistical analysis
Data analysis was performed using SPSS software. The descriptive statistics methods were applied when appropriate. Both OS and PFS were censored at the date of the last contact and were estimated using the Kaplan-Meier method. The difference in OS was tested with log-rank test.
Results

Figure 2. Histological subtypes of the TCL patients remain alive. For abbreviations, see Materials and Methods
At the time of analysis, 35 patients remained alive. These were mainly patients with peripheral T-cell lymphoma not otherwise specified (TCL-NOS), and anaplastic large cell lymphoma (LCL), ALK+ as the most common histological subtype and the histological subtype with the best prognosis, respectively (Fig. 2).
Results of treatment of patients of our center are presented in the Table 4.
The median follow-up of alive patients was 35 months (6-122 mo). The median overall survival was not reached, 5-year survival rate was 81% and 8-year survival rate was 78% (Fig. 3 and 4).

Figure 3, 4. 5-year (A) and 8-year (B) overall survival patients with T-cell lymphoma treated at R. Gorbacheva Memorial Institute
Table 4. Clinical state and survival rates of the TCL patients after HSCT

Complete remission (CR) state was maintained at the last follow up in 22 patients, partial remission (PR) was documented in 4 patients and PD, in 21 case. Among factors significantly associated with adverse prognosis were: lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06) (Fig. 5 and 6).

Figure 5, 6. 5-year survival rates depend on ECOG performance status (A), and presence of B-symptoms (B). The difference is significant by p=0.06

Figure 7. Disease status for patients remain alive
Patients that had undergo HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) in patients who did not undergo HSCT (Fig. 7). This can probably be attributed to the fact that patients had a good disease status before transplantation. I would also like to note that such a large number of patients who have not undergone HSCT is associated with age and severe comorbid status, the absence of a donor, unsuccessful apheresis of hematopoietic stem cell, or patient refusal from HSCT.
3-year progression-free survival in patients with TCL after auto-HSCT and allo-HSCT was 50%. And 61%, respectively (Fig. 8, 9).

Figure 8, 9. 5-year progression-free survival for patients with T-cell lymphoma after autoHSCT (A) and allogeneic HSCT (B)
5-year overall survival after auto-HSCT and allo-HSCT rate was 87% and 77% respectively (Fig. 10 and 11).

Figure 10, 11. 5-year overall survival for patients with T-cell lymphoma after auto-HSCT (A) and allo-HSCT (B)
Discussion
Today there is no generally adopted strategy for achievement of responses for r/r patients with PTCL and CTCL (cutaneous TCL), and significant improvements are needed in treatment methods for all subtypes of T/NK-cell lymphomas.
One such solution may be in the future, that we can expect combination therapies of new agents with cytotoxic chemotherapies, therapeutic combinations consisting of new agents and the use of these combinations in earlier in the course of treatment – addition to the first line of therapy. This statement is generally confirmed by the successful experience of our center, which consists in the use of some new drugs, taking into account biological tumor markers.
Our study also shows that HSCT improves the results, which is comparable with the data in world literature.
The consolidation auto-HSCT of achieved remission after first-line therapy can change overall survival for the better [5].
Allo-HSCT has the most solid evidence of the potential to significantly prolong survival or for the cure of disease [43]. But this method is not perfect due to a number of reasons: transplant-related mortality, absence of a donor for allo-HSCT, few appropriate candidates because of age, lack of adequate response to primary therapy, and/or absence of effective agents in the relapsed/refractory setting [44].
Nevertheless, over the past year, there has been a positive trend in allogeneic bone marrow transplantation, which is associated with the introduction of new conditioning regimens, prevention of graft-versus-host disease, new accompanying therapy.
These methods are used at our institute, but it should be noted that not only the improvement of approaches to the treatment of patients with TCL led to good results, but also the artificial selection of patients. Historically, we have accommodated patients who could theoretically become candidates for HSCT, which means patients with good comorbid status.
For the patients who are not candidates for hematopoietic stem cell transplantation the novel therapies (CAR-T/NK-cell therapy; monoclonal antibodies therapy, molecularly targeted therapy) may become the new successful standard of treatment [45].
Conclusion
Our results show that introduction of novel agents and consolidation with high dose chemotherapy and auto-HSCT or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab based treatment may be successfully used as a bridge therapy before HSCT. The treatment was tailored according to hyperexpression CD30 and PD-L1.
Conflict of interest
The authors report no conflicts of interest.
References
Introduction
The T-cell/natural killer (NK) cell lymphomas are a heterogeneous group of generally aggressive neoplasms that constitute less than 10-15 percent of all non-Hodgkin lymphomas (NHLs) in adults [1, 2]. Modern classification of T/NK-cell lymphomas was developed on the basis of histological, immunohistochemical studies of tumor tissue and clinical presentation of lymphomas. In 2016, the World Health Organization classification revised the classification of both nodal and extranodal T/NK-cell lymphomas, which led to the introduction of new temporary structures [3]. Many of these changes were caused by the results of genomic studies using approaches to study gene expression profiling (GEP) and the genetic landscape of T/NK cell lymphomas. However, despite advances in molecular diagnostic methods, histological examination of tumor tissue and clinical manifestations remain the main diagnostic approach.
Another area of research concerns dismal outcomes in the patients with T/NK-cell lymphomas, except for anaplastic large-cell lymphoma (ALCL) positive for anaplastic lymphoma kinase (ALK) [4]. There are currently no successful treatment standards for the T/NK-cell lymphomas, both in the first-line therapy, and in refractory/relapsed (r/r) settings. Today, almost 70% of patients undergoing first-line therapy develop a relapse or refractory disease. A review of the scientific literature allowed us to identify a range of therapeutic options for patients with T/NK-cell lymphomas [5]. It includes the following approaches:
- Hematopoietic stem cells transplantation (HSCT);
- Therapy with monoclonal antibodies (Mabs), e.g., anti-CD30 antibody-drug conjugate (Brentuximab vedotin), anti-CC chemokine receptor 4 antibodies (Mogamulizumab), a monoclonal antibody that binds to CD52 (Alemtuzumab), the monoclonal anti-vascular endothelial growth factor (Bevacizumab) [6-19];
- ALK inhibitors (Crizotinib, Ceritinib) [20];
- Therapy with immune checkpoint inhibitors, e.g., Nivolumab, Pembrolizumab, Ipilimumab, Durvalumab [21-27];
- Histone deacetylase inhibitors (Belinostat, Panobinostat, Chidamide, Romidepsin, Vorinostat) [28-34];
- CAR-T/NK-cell therapy [35, 36];
- In situ vaccination [37].
There are a lot of studies and a large number of drugs being introduced into clinical studies, but at the present time, greatest evidence base should be recognized for brentuximab and bone marrow transplantation. E.g., patients with r/r T/NK-cell lymphomas who received hematopoietic stem cell transplantation (allogeneic and/or autologous) had a better outcome compared to the subset of non-transplanted patients (3-year survival rates of 48% and 18%, respectively) [5]. These conclusions also supported by data of Abekasis et al., demonstrating that autologous and/or allogeneic HSCT is an effective and safe option for the consolidation of patients with TCL [38].
Recently published results of a clinical study ECHELON-2 showed that front-line treatment with Adcetris (brentuximab vedotin) + CHP protocol is superior to CHOP for patients with CD30-positive peripheral T-cell lymphomas, thus suggesting that A+CHP is likely to be the standard first-line therapy [39].
Also, brentuximab vedotin showed good results in the treatment of r/r CD30 positive TCL [40]. Mostly, there were anaplastic large cell lymphoma (ALСL), angioimmunoblastic T-cell lymphoma (AITL), Sézary syndrome and Mycosis Fungoides (MF) [7]. The objective response was from 92% to 40%, with best results obtained in ALСL treatment [41, 42]. These methods are actively used at our center.
Hence, the aim of this study was to present our single-center experience in the treatment of patients with T/NK-cell lymphomas.
Patients and methods

Figure 1. Histological subtypes of patients with T-cell lymphomas treated at the R. Gorbacheva Memorial Institute
We analyzed data of 47 patients with TCL eligible for stem cells transplantation treated in the R. Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at the Pavlov University from 2005 to 2019 including 44 cases with r/r TCL and 3 patients being in complete response after first-line therapy. Among them, 10 patients were diagnosed with anaplastic large cell lymphoma (ALK+); 5 cases with anaplastic large cell lymphoma (ALK); 4 cases with angioimmunoblastic TCL (AITL); 4 patients with hepatosplenic T-cell lymphoma (HSTCL); 1 case of γδ T cell lymphoma (γδ TCL); 20 patients with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS); 1 patient with mucosis fungoides (MF); 1, with primary cutaneous CD4+ T-cell lymphoma (CD4+ PCSM-TCL), and one patient with subcutaneous panniculitis-like T-cell lymphoma (SPTCL), as seen from Fig. 1.
General characteristics of the patients are outlined in Table 1.
Table 1. Clinical characteristics of T-cell lymphoma patients

Table 2. Clinical state of the patients with T-cell lymphoma subjected to auto-HSCT

The treatment was tailored according to biological tumor markers revealed in the patients. In 10 patients with CD30+ PTCL (n=4 with PTCL-NOS; n=4 with ALK+; n=1 with ALK; n=1 with AITL), Brentuximab vedotin was used. One patient with ALK+ anaplastic lymphoma received ALK inhibitor crizotinib. Five patients with PD-L1 hyperexpression (n=4 with PTCL-NOS; n=1 with γδ TCL) were treated with nivolumab. Overall 24 patients underwent HSCT: high-dose chemotherapy with auto-HSCT was performed in 16 patients, 13 patients underwent allo-HSCT (among them 5 patients with relapses after auto-HSCT). Place of auto-HSCT in the treatment of TCL in our center could be seen from Table 2.
Main characteristics of patients who underwent allogeneic hematopoietic stem cells transplantation are shown in Table 3.
Table 3. Main clinical characteristics of 13 patients with r/r TCL subjected to allo-HSCT

Statistical analysis
Data analysis was performed using SPSS software. The descriptive statistics methods were applied when appropriate. Both OS and PFS were censored at the date of the last contact and were estimated using the Kaplan-Meier method. The difference in OS was tested with log-rank test.
Results

Figure 2. Histological subtypes of the TCL patients remain alive. For abbreviations, see Materials and Methods
At the time of analysis, 35 patients remained alive. These were mainly patients with peripheral T-cell lymphoma not otherwise specified (TCL-NOS), and anaplastic large cell lymphoma (LCL), ALK+ as the most common histological subtype and the histological subtype with the best prognosis, respectively (Fig. 2).
Results of treatment of patients of our center are presented in the Table 4.
The median follow-up of alive patients was 35 months (6-122 mo). The median overall survival was not reached, 5-year survival rate was 81% and 8-year survival rate was 78% (Fig. 3 and 4).

Figure 3, 4. 5-year (A) and 8-year (B) overall survival patients with T-cell lymphoma treated at R. Gorbacheva Memorial Institute
Table 4. Clinical state and survival rates of the TCL patients after HSCT

Complete remission (CR) state was maintained at the last follow up in 22 patients, partial remission (PR) was documented in 4 patients and PD, in 21 case. Among factors significantly associated with adverse prognosis were: lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06) (Fig. 5 and 6).

Figure 5, 6. 5-year survival rates depend on ECOG performance status (A), and presence of B-symptoms (B). The difference is significant by p=0.06

Figure 7. Disease status for patients remain alive
Patients that had undergo HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) in patients who did not undergo HSCT (Fig. 7). This can probably be attributed to the fact that patients had a good disease status before transplantation. I would also like to note that such a large number of patients who have not undergone HSCT is associated with age and severe comorbid status, the absence of a donor, unsuccessful apheresis of hematopoietic stem cell, or patient refusal from HSCT.
3-year progression-free survival in patients with TCL after auto-HSCT and allo-HSCT was 50%. And 61%, respectively (Fig. 8, 9).

Figure 8, 9. 5-year progression-free survival for patients with T-cell lymphoma after autoHSCT (A) and allogeneic HSCT (B)
5-year overall survival after auto-HSCT and allo-HSCT rate was 87% and 77% respectively (Fig. 10 and 11).

Figure 10, 11. 5-year overall survival for patients with T-cell lymphoma after auto-HSCT (A) and allo-HSCT (B)
Discussion
Today there is no generally adopted strategy for achievement of responses for r/r patients with PTCL and CTCL (cutaneous TCL), and significant improvements are needed in treatment methods for all subtypes of T/NK-cell lymphomas.
One such solution may be in the future, that we can expect combination therapies of new agents with cytotoxic chemotherapies, therapeutic combinations consisting of new agents and the use of these combinations in earlier in the course of treatment – addition to the first line of therapy. This statement is generally confirmed by the successful experience of our center, which consists in the use of some new drugs, taking into account biological tumor markers.
Our study also shows that HSCT improves the results, which is comparable with the data in world literature.
The consolidation auto-HSCT of achieved remission after first-line therapy can change overall survival for the better [5].
Allo-HSCT has the most solid evidence of the potential to significantly prolong survival or for the cure of disease [43]. But this method is not perfect due to a number of reasons: transplant-related mortality, absence of a donor for allo-HSCT, few appropriate candidates because of age, lack of adequate response to primary therapy, and/or absence of effective agents in the relapsed/refractory setting [44].
Nevertheless, over the past year, there has been a positive trend in allogeneic bone marrow transplantation, which is associated with the introduction of new conditioning regimens, prevention of graft-versus-host disease, new accompanying therapy.
These methods are used at our institute, but it should be noted that not only the improvement of approaches to the treatment of patients with TCL led to good results, but also the artificial selection of patients. Historically, we have accommodated patients who could theoretically become candidates for HSCT, which means patients with good comorbid status.
For the patients who are not candidates for hematopoietic stem cell transplantation the novel therapies (CAR-T/NK-cell therapy; monoclonal antibodies therapy, molecularly targeted therapy) may become the new successful standard of treatment [45].
Conclusion
Our results show that introduction of novel agents and consolidation with high dose chemotherapy and auto-HSCT or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab based treatment may be successfully used as a bridge therapy before HSCT. The treatment was tailored according to hyperexpression CD30 and PD-L1.
Conflict of interest
The authors report no conflicts of interest.
References
Елена Е. Лепик1, Андрей В. Козлов1, Евгения С. Борзенкова1, Юрий Р. Залялов1, Кирилл В. Лепик1,
Елена В. Кондакова1, Вадим В. Байков1, Иван С. Моисеев1, Татьяна В. Шнайдер2, Наталья Б. Михайлова1,
Борис В. Афанасьев1
1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Ленинградская областная клиническая больница, Санкт-Петербург, Россия
Т-клеточные лимфомы (ТКЛ) представляют собой агрессивные неходжкинские лимфомы, которые не имеют успешных стандартов лечения. Почти у 70% пациентов после первой линии терапии развивается рецидив или рефрактерное течение (р/р). Ряд новых терапевтических подходов нацелены на улучшение результатов лечения у пациентов с р/р ТКЛ. В данной статье обобщен опыт Первого Санкт-Петербургского государственного медицинского университета им. Павлова в лечении пациентов с Т-клеточными лимфомами. Мы проанализировали данные 47 пациентов, которые проходила лечение с 2005 по 2019 год – на момент анализа 44 пациента имеют р/р течение заболевания и 3 пациента находятся в полном ответе после проведенной первой линии терапии. Средний возраст составил 45 лет (от 1 года до 72 лет). Это были преимущественно пациенты с периферическими Т-клеточными лимфомами, неуточненными (41%). Среди всех пациентов n26 (55%) имели первичное химиорезистентное течение, в то время как у остальных n18 (38%) был рецидив после первой линии терапии.
Опыт нашего центра включает в себя использование новых вариантов лечения р/р ТКЛ: антиCD30 моноклональное антитело – брентуксимаб, ингибитор ALK – кризотиниб, ингибитор контрольных точек – ниволумаб и иммунотерапию, включающую проведение трансплантации гемопоэтических стволовых клеток (ТГСК). В общей сложности 24 пациентам выполнена ТГСК: высокодозная химиотерапия с последующей аутологичной ТГСК (ауто-ТГСК) проведена 16 пациентам, 13 пациентам – аллогенная ТГСК (алло-ТГСК) (из них 5 пациентов с рецидивами после ауто-ТГСК). На момент анализа 35 пациентов были живы. Медиана наблюдения за живыми пациентами составила 35 месяцев (6-122 мес.). Медиана общей выживаемости не была достигнута, 5-летняя выживаемость составила 81%, 8-летняя общая выживаемость – 78%. Статус заболевания при последнем наблюдении был следующий: полный ответ у 22 пациентов, частичный ответ у 4 пациентов и прогрессия заболевания у 21. Среди факторов, значительно связанных с неблагоприятным прогнозом, были низкий статус ECOG и B-симптомы на момент постановки диагноза (p=0,06). Пациенты, которые перенесли ТГСК, имели лучший статус заболевания на момент последнего наблюдения: 17/19 (89%) были в полном ответе, по сравнению с 5/16 (31%) у пациентов, которым не проводилась ТГСК. 5-летняя общая выживаемость у пациентов с ТКЛ после ауто-ТГСК и алло-ТГСК составила 87% и 77% соответственно. Результаты анализа показывают, что введение новых агентов и консолидация с помощью высокодозной химиотерапии с последующей ауто-ТГСК или алло-ТГСК в отдельных случаях могут улучшить результаты у пациентов с рецидивирующей или рефрактерной Т-клеточной лимфомой. Схемы, основанные на применении брентуксимаба ведотина и ниволумаба, могут быть успешно использованы в качестве bridge-терапии перед алло-ТГСК.
Ключевые слова
T-клеточные лимфомы, аутологичная трансплантация гемопоэтических стволовых клеток, аллогенная трансплантация гемопоэтических стволовых клеток, брентуксимаб ведотин, ниволумаб.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26308" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-28-37" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-28-37" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26314" ["VALUE"]=> array(2) { ["TEXT"]=> string(622) "<p> Elena E. Lepik<sup>1</sup>, Andrey V. Kozlov<sup>1</sup>, Evgenia S. Borzenkova<sup>1</sup>,<br> Yury R. Zalyalov<sup>1</sup>, Kirill V. Lepik<sup>1</sup>, Elena V. Kondakova<sup>1</sup>, Vadim V. Baykov<sup>1</sup>, Ivan S. Moiseev<sup>1</sup>, Tatiana V. Schneider<sup>2</sup>, <br> Natalia B. Mikhaylova<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 1px 2px;">Boris V. Afanasyev<sup>1</sup></span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(444) "
Elena E. Lepik1, Andrey V. Kozlov1, Evgenia S. Borzenkova1,
Yury R. Zalyalov1, Kirill V. Lepik1, Elena V. Kondakova1, Vadim V. Baykov1, Ivan S. Moiseev1, Tatiana V. Schneider2,
Natalia B. Mikhaylova1, Boris V. Afanasyev1
1 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
2 Leningrad Regional Clinical Hospital, St. Petersburg, Russia
Correspondence
Dr. Elena E. Lepik, Clinical Hematologist, Oncology Department, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (905) 226 8922
E-mail: ee.dav@mail.ru
T-cell lymphomas (TCL) comprise a group of aggressive non-Hodgkin lymphomas, which do not have successful treatment standards. Almost 70% of patients undergoing first-line therapy develop a relapse or refractory (r/r) disease. A number of novel therapy approaches are aimed for improvement of outcomes in patients with r/r TCL. This report summarizes clinical experience of Pavlov Medical University in the treatment of T cell lymphomas. We analyzed data of 47 patients with TCL treated from 2005 to 2019. Of them, 44 had r/r TCL, and 3 patients were in complete response after first line therapy. The median age was 45 years (range 1-72 years). These were predominantly patients with peripheral T-cell lymphomas not otherwise specified (TCL-NOS, 41%). 26 patients (55% of total) had a primary chemoresistant disease, while the remaining 18 patients (38% of total) had a relapse after initial treatment.
Our center has implemented new treatment options for r/r TCL, i.e., anti CD30 monoclonal antibody brentuximab, ALK inhibitor crizotinib, immunotherapy with checkpoint inhibitors nivolumab, and hematopoietic stem cell transplantation (HSCT). A total of 24 patients underwent: high-dose chemotherapy with autologous HSCT (auto-HSCT) was performed in 16 cases, 13 patients were subjected to allogeneic HSCT (allo-HSCT), including 5 relapsed patients after auto-HSCT. At the time of analysis, 35 patients remained alive. The median follow-up of surviving patients was 35 months (6-122 mo). The median overall survival (OS) was not reached, 5-year survival rate was 81%, and 8-year survival rate was 78%. Complete remission (CR) at the last follow-up was diagnosed in 22 patients; partial response (PR), in 4 cases, and progression of the disease (PD) was revealed in 21 patients. Among factors significantly associated with adverse prognosis were lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06). The patients who underwent HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) among the patients not subjected to HSCT. 5-year overall survival rates after auto-HSCT and allo-HSCT were 87% and 77%, respectively. The results show that implementation of novel therapeutic agents, as well as consolidation with high-dose chemotherapy and auto- or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab-based regimens may be successfully used as a bridge therapy before HSCT.
Keywords
T-cell lymphoma, autologous hematopoietic stem cells transplantation, allogeneic hematopoietic stem cells transplantation, brentuximab vedotin, nivolumab.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26309" ["VALUE"]=> string(61) "Treatment options for T-cell lymphomas: a single-center study" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(61) "Treatment options for T-cell lymphomas: a single-center study" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26310" ["VALUE"]=> string(4) "1955" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1955" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26315" ["VALUE"]=> string(4) "1956" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1956" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26314" ["VALUE"]=> array(2) { ["TEXT"]=> string(622) "<p> Elena E. Lepik<sup>1</sup>, Andrey V. Kozlov<sup>1</sup>, Evgenia S. Borzenkova<sup>1</sup>,<br> Yury R. Zalyalov<sup>1</sup>, Kirill V. Lepik<sup>1</sup>, Elena V. Kondakova<sup>1</sup>, Vadim V. Baykov<sup>1</sup>, Ivan S. Moiseev<sup>1</sup>, Tatiana V. Schneider<sup>2</sup>, <br> Natalia B. Mikhaylova<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 1px 2px;">Boris V. Afanasyev<sup>1</sup></span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(444) "
Elena E. Lepik1, Andrey V. Kozlov1, Evgenia S. Borzenkova1,
Yury R. Zalyalov1, Kirill V. Lepik1, Elena V. Kondakova1, Vadim V. Baykov1, Ivan S. Moiseev1, Tatiana V. Schneider2,
Natalia B. Mikhaylova1, Boris V. Afanasyev1
Elena E. Lepik1, Andrey V. Kozlov1, Evgenia S. Borzenkova1,
Yury R. Zalyalov1, Kirill V. Lepik1, Elena V. Kondakova1, Vadim V. Baykov1, Ivan S. Moiseev1, Tatiana V. Schneider2,
Natalia B. Mikhaylova1, Boris V. Afanasyev1
T-cell lymphomas (TCL) comprise a group of aggressive non-Hodgkin lymphomas, which do not have successful treatment standards. Almost 70% of patients undergoing first-line therapy develop a relapse or refractory (r/r) disease. A number of novel therapy approaches are aimed for improvement of outcomes in patients with r/r TCL. This report summarizes clinical experience of Pavlov Medical University in the treatment of T cell lymphomas. We analyzed data of 47 patients with TCL treated from 2005 to 2019. Of them, 44 had r/r TCL, and 3 patients were in complete response after first line therapy. The median age was 45 years (range 1-72 years). These were predominantly patients with peripheral T-cell lymphomas not otherwise specified (TCL-NOS, 41%). 26 patients (55% of total) had a primary chemoresistant disease, while the remaining 18 patients (38% of total) had a relapse after initial treatment.
Our center has implemented new treatment options for r/r TCL, i.e., anti CD30 monoclonal antibody brentuximab, ALK inhibitor crizotinib, immunotherapy with checkpoint inhibitors nivolumab, and hematopoietic stem cell transplantation (HSCT). A total of 24 patients underwent: high-dose chemotherapy with autologous HSCT (auto-HSCT) was performed in 16 cases, 13 patients were subjected to allogeneic HSCT (allo-HSCT), including 5 relapsed patients after auto-HSCT. At the time of analysis, 35 patients remained alive. The median follow-up of surviving patients was 35 months (6-122 mo). The median overall survival (OS) was not reached, 5-year survival rate was 81%, and 8-year survival rate was 78%. Complete remission (CR) at the last follow-up was diagnosed in 22 patients; partial response (PR), in 4 cases, and progression of the disease (PD) was revealed in 21 patients. Among factors significantly associated with adverse prognosis were lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06). The patients who underwent HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) among the patients not subjected to HSCT. 5-year overall survival rates after auto-HSCT and allo-HSCT were 87% and 77%, respectively. The results show that implementation of novel therapeutic agents, as well as consolidation with high-dose chemotherapy and auto- or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab-based regimens may be successfully used as a bridge therapy before HSCT.
Keywords
T-cell lymphoma, autologous hematopoietic stem cells transplantation, allogeneic hematopoietic stem cells transplantation, brentuximab vedotin, nivolumab.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2814) "T-cell lymphomas (TCL) comprise a group of aggressive non-Hodgkin lymphomas, which do not have successful treatment standards. Almost 70% of patients undergoing first-line therapy develop a relapse or refractory (r/r) disease. A number of novel therapy approaches are aimed for improvement of outcomes in patients with r/r TCL. This report summarizes clinical experience of Pavlov Medical University in the treatment of T cell lymphomas. We analyzed data of 47 patients with TCL treated from 2005 to 2019. Of them, 44 had r/r TCL, and 3 patients were in complete response after first line therapy. The median age was 45 years (range 1-72 years). These were predominantly patients with peripheral T-cell lymphomas not otherwise specified (TCL-NOS, 41%). 26 patients (55% of total) had a primary chemoresistant disease, while the remaining 18 patients (38% of total) had a relapse after initial treatment.
Our center has implemented new treatment options for r/r TCL, i.e., anti CD30 monoclonal antibody brentuximab, ALK inhibitor crizotinib, immunotherapy with checkpoint inhibitors nivolumab, and hematopoietic stem cell transplantation (HSCT). A total of 24 patients underwent: high-dose chemotherapy with autologous HSCT (auto-HSCT) was performed in 16 cases, 13 patients were subjected to allogeneic HSCT (allo-HSCT), including 5 relapsed patients after auto-HSCT. At the time of analysis, 35 patients remained alive. The median follow-up of surviving patients was 35 months (6-122 mo). The median overall survival (OS) was not reached, 5-year survival rate was 81%, and 8-year survival rate was 78%. Complete remission (CR) at the last follow-up was diagnosed in 22 patients; partial response (PR), in 4 cases, and progression of the disease (PD) was revealed in 21 patients. Among factors significantly associated with adverse prognosis were lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06). The patients who underwent HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) among the patients not subjected to HSCT. 5-year overall survival rates after auto-HSCT and allo-HSCT were 87% and 77%, respectively. The results show that implementation of novel therapeutic agents, as well as consolidation with high-dose chemotherapy and auto- or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab-based regimens may be successfully used as a bridge therapy before HSCT.
Keywords
T-cell lymphoma, autologous hematopoietic stem cells transplantation, allogeneic hematopoietic stem cells transplantation, brentuximab vedotin, nivolumab.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26308" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-28-37" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-28-37" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-28-37" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26309" ["VALUE"]=> string(61) "Treatment options for T-cell lymphomas: a single-center study" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(61) "Treatment options for T-cell lymphomas: a single-center study" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(61) "Treatment options for T-cell lymphomas: a single-center study" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26316" ["VALUE"]=> array(2) { ["TEXT"]=> string(676) "<p><sup>1</sup> Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br> St. Petersburg, Russia<br> <sup>2</sup> Leningrad Regional Clinical Hospital, St. Petersburg, Russia </p> <br> <p><b>Correspondence</b><br> Dr. Elena E. Lepik, Clinical Hematologist, Oncology Department, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (905) 226 8922<br> E-mail: ee.dav@mail.ru</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(580) "1 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
2 Leningrad Regional Clinical Hospital, St. Petersburg, Russia
Correspondence
Dr. Elena E. Lepik, Clinical Hematologist, Oncology Department, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (905) 226 8922
E-mail: ee.dav@mail.ru
1 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
2 Leningrad Regional Clinical Hospital, St. Petersburg, Russia
Correspondence
Dr. Elena E. Lepik, Clinical Hematologist, Oncology Department, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (905) 226 8922
E-mail: ee.dav@mail.ru
Елена Е. Лепик1, Андрей В. Козлов1, Евгения С. Борзенкова1, Юрий Р. Залялов1, Кирилл В. Лепик1,
Елена В. Кондакова1, Вадим В. Байков1, Иван С. Моисеев1, Татьяна В. Шнайдер2, Наталья Б. Михайлова1,
Борис В. Афанасьев1
Елена Е. Лепик1, Андрей В. Козлов1, Евгения С. Борзенкова1, Юрий Р. Залялов1, Кирилл В. Лепик1,
Елена В. Кондакова1, Вадим В. Байков1, Иван С. Моисеев1, Татьяна В. Шнайдер2, Наталья Б. Михайлова1,
Борис В. Афанасьев1
Т-клеточные лимфомы (ТКЛ) представляют собой агрессивные неходжкинские лимфомы, которые не имеют успешных стандартов лечения. Почти у 70% пациентов после первой линии терапии развивается рецидив или рефрактерное течение (р/р). Ряд новых терапевтических подходов нацелены на улучшение результатов лечения у пациентов с р/р ТКЛ. В данной статье обобщен опыт Первого Санкт-Петербургского государственного медицинского университета им. Павлова в лечении пациентов с Т-клеточными лимфомами. Мы проанализировали данные 47 пациентов, которые проходила лечение с 2005 по 2019 год – на момент анализа 44 пациента имеют р/р течение заболевания и 3 пациента находятся в полном ответе после проведенной первой линии терапии. Средний возраст составил 45 лет (от 1 года до 72 лет). Это были преимущественно пациенты с периферическими Т-клеточными лимфомами, неуточненными (41%). Среди всех пациентов n26 (55%) имели первичное химиорезистентное течение, в то время как у остальных n18 (38%) был рецидив после первой линии терапии.
Опыт нашего центра включает в себя использование новых вариантов лечения р/р ТКЛ: антиCD30 моноклональное антитело – брентуксимаб, ингибитор ALK – кризотиниб, ингибитор контрольных точек – ниволумаб и иммунотерапию, включающую проведение трансплантации гемопоэтических стволовых клеток (ТГСК). В общей сложности 24 пациентам выполнена ТГСК: высокодозная химиотерапия с последующей аутологичной ТГСК (ауто-ТГСК) проведена 16 пациентам, 13 пациентам – аллогенная ТГСК (алло-ТГСК) (из них 5 пациентов с рецидивами после ауто-ТГСК). На момент анализа 35 пациентов были живы. Медиана наблюдения за живыми пациентами составила 35 месяцев (6-122 мес.). Медиана общей выживаемости не была достигнута, 5-летняя выживаемость составила 81%, 8-летняя общая выживаемость – 78%. Статус заболевания при последнем наблюдении был следующий: полный ответ у 22 пациентов, частичный ответ у 4 пациентов и прогрессия заболевания у 21. Среди факторов, значительно связанных с неблагоприятным прогнозом, были низкий статус ECOG и B-симптомы на момент постановки диагноза (p=0,06). Пациенты, которые перенесли ТГСК, имели лучший статус заболевания на момент последнего наблюдения: 17/19 (89%) были в полном ответе, по сравнению с 5/16 (31%) у пациентов, которым не проводилась ТГСК. 5-летняя общая выживаемость у пациентов с ТКЛ после ауто-ТГСК и алло-ТГСК составила 87% и 77% соответственно. Результаты анализа показывают, что введение новых агентов и консолидация с помощью высокодозной химиотерапии с последующей ауто-ТГСК или алло-ТГСК в отдельных случаях могут улучшить результаты у пациентов с рецидивирующей или рефрактерной Т-клеточной лимфомой. Схемы, основанные на применении брентуксимаба ведотина и ниволумаба, могут быть успешно использованы в качестве bridge-терапии перед алло-ТГСК.
Ключевые слова
T-клеточные лимфомы, аутологичная трансплантация гемопоэтических стволовых клеток, аллогенная трансплантация гемопоэтических стволовых клеток, брентуксимаб ведотин, ниволумаб.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(5496) "Т-клеточные лимфомы (ТКЛ) представляют собой агрессивные неходжкинские лимфомы, которые не имеют успешных стандартов лечения. Почти у 70% пациентов после первой линии терапии развивается рецидив или рефрактерное течение (р/р). Ряд новых терапевтических подходов нацелены на улучшение результатов лечения у пациентов с р/р ТКЛ. В данной статье обобщен опыт Первого Санкт-Петербургского государственного медицинского университета им. Павлова в лечении пациентов с Т-клеточными лимфомами. Мы проанализировали данные 47 пациентов, которые проходила лечение с 2005 по 2019 год – на момент анализа 44 пациента имеют р/р течение заболевания и 3 пациента находятся в полном ответе после проведенной первой линии терапии. Средний возраст составил 45 лет (от 1 года до 72 лет). Это были преимущественно пациенты с периферическими Т-клеточными лимфомами, неуточненными (41%). Среди всех пациентов n26 (55%) имели первичное химиорезистентное течение, в то время как у остальных n18 (38%) был рецидив после первой линии терапии.
Опыт нашего центра включает в себя использование новых вариантов лечения р/р ТКЛ: антиCD30 моноклональное антитело – брентуксимаб, ингибитор ALK – кризотиниб, ингибитор контрольных точек – ниволумаб и иммунотерапию, включающую проведение трансплантации гемопоэтических стволовых клеток (ТГСК). В общей сложности 24 пациентам выполнена ТГСК: высокодозная химиотерапия с последующей аутологичной ТГСК (ауто-ТГСК) проведена 16 пациентам, 13 пациентам – аллогенная ТГСК (алло-ТГСК) (из них 5 пациентов с рецидивами после ауто-ТГСК). На момент анализа 35 пациентов были живы. Медиана наблюдения за живыми пациентами составила 35 месяцев (6-122 мес.). Медиана общей выживаемости не была достигнута, 5-летняя выживаемость составила 81%, 8-летняя общая выживаемость – 78%. Статус заболевания при последнем наблюдении был следующий: полный ответ у 22 пациентов, частичный ответ у 4 пациентов и прогрессия заболевания у 21. Среди факторов, значительно связанных с неблагоприятным прогнозом, были низкий статус ECOG и B-симптомы на момент постановки диагноза (p=0,06). Пациенты, которые перенесли ТГСК, имели лучший статус заболевания на момент последнего наблюдения: 17/19 (89%) были в полном ответе, по сравнению с 5/16 (31%) у пациентов, которым не проводилась ТГСК. 5-летняя общая выживаемость у пациентов с ТКЛ после ауто-ТГСК и алло-ТГСК составила 87% и 77% соответственно. Результаты анализа показывают, что введение новых агентов и консолидация с помощью высокодозной химиотерапии с последующей ауто-ТГСК или алло-ТГСК в отдельных случаях могут улучшить результаты у пациентов с рецидивирующей или рефрактерной Т-клеточной лимфомой. Схемы, основанные на применении брентуксимаба ведотина и ниволумаба, могут быть успешно использованы в качестве bridge-терапии перед алло-ТГСК.
Ключевые слова
T-клеточные лимфомы, аутологичная трансплантация гемопоэтических стволовых клеток, аллогенная трансплантация гемопоэтических стволовых клеток, брентуксимаб ведотин, ниволумаб.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26312" ["VALUE"]=> array(2) { ["TEXT"]=> string(560) "<p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br> <sup>2</sup> Ленинградская областная клиническая больница, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(518) "1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Ленинградская областная клиническая больница, Санкт-Петербург, Россия
1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Ленинградская областная клиническая больница, Санкт-Петербург, Россия
Introduction
Bispecific monoclonal antibodies for targeting tumor cells have a long story from 1980's to produce approved drug in 2014 [1-3]. Several trials for relapsed/refractory acute lymphoblastic leukemia (R/R ALL) treatment including ALCANTARA for Philadelphia chromosome-positive (Ph-positive) R/R ALL were performed and showed clinical effectiveness and even an advantage over chemotherapy (TOWER Study) [4-8]. Recently published results on blinatumomab combined with TKI for therapy of Ph-positive R/R ALL characterized this approach to be safe and effective [11]. Combination of Blinatumomab and TKI could elicit lower toxicity and improve primary results in managing heavily pretreated R/R ALL patients. In preclinical studies, retinoids have shown antileukemic activity in IKZF-deleted ALL [12]. We previously reported about effective combination of blinatumomab with tyrosine kinase inhibitors (TKI) for the treatment of Ph-positive and FLT3-ITD R/R ALL and blinatumomab with TKI and all-trans retinoic acid in IKZF-deleted R/R ALL patients [9, 10]. The aim of our study was to present the data on 11 R/R ALL patients treated with Blinatumomab and TKI+/-ATRA, omitting simultaneous standard chemotherapy.
Patients and methods
Eleven patients (pts) with R/R ALL were treated with Blinatumomab + TKI/TKI+ATRA at the National Research Center for Hematology, Moscow, Russia, and in Raisa Gorbacheva Memorial Institute of Pediatric Oncology, Hematology and Transplantation, Saint Petersburg, Russia, from October 2015 to October 2018. The study was approved by the Institutional Review Board. The treatment was administered after the patient has signed informed consent. Blinatumomab was provided by Amgen as part of the expanded access program. The treatment consisted of 4-5 cycles of blinatumomab 28 mcg/day by continuous infusion for 4 weeks each cycle. Over 1st week of 1st cycle, Blinatumomab was administered at the dose of 9 mcg/day. The 2-week intervals followed between the rounds of blinatumomab treatment. All the patients were administered one of TKIs: 7 Ph-positive and 2 IKZF1-deleted ALL pts were initially treated with dasatinib (140 mg/day) per os, 1 FLT3-ITD ALL patient received sorafenib (800 mg/day) per os, and one Ph-positive ALL patient with a T315I mutation received ponatinib (45 mg/day). All the TKIs were administered continuously, from the 1st day of starting blinatumomab. All-trans-retinoic acid (ATRA) at a dose of 45 mg/m2/day was administered per os in the IKZF1-deleted pts during 4 weeks of the 1st blinatumomab cycle, and during first two weeks of subsequent blinatumomab cycles.
Complete remission (CR) was diagnosed if less than 5% of blasts were present in bone marrow. Cytogenetic remission (CyR) was diagnosed in the absence of BCR-ABL positive nuclei per 200 nuclei by fluorescence in situ hybridization (FISH). Molecular CR (MolCR) was diagnosed if BCR-ABL/ABL at a ratio of <0.01 was detected in bone marrow samples by RT-qPCR. The CD3+/CD4+/CD8- T-helper, CD3+/CD4-/CD8+ T-cytotoxic, CD3+/CD4-/CD8- T-Double-negative, CD3+/CD4+/CD25+ T-regulatory, CD3-/CD56+ NK subpopulations were measured in peripheral blood lymphocytes by flow cytometry weekly during blinatumomab treatment in all the pts (1 to 16 samples, 4 points per each blinatumomab cycle). Serum immunoglobulins (Ig) G, M and A were measured during each cycle of blinatumomab (a total of 4 sampling points).
The SAS software was used for statistical evaluation using regression analysis. The MIXED SAS procedure was used to perform repeated measures analysis and to estimate parameters of linear regression of average time-dependent trend.
Results
Median follow-up was 23 months (19 to 36 months). Median age is 32 years (24 to 49 years). Eight pts were females and 3, males. 7 pts received 4 cycles; 1 pt, 5 cycles; 2 pts underwent 2 cycles, and 1 pt was subjected to 1 cycle of blinatumomab treatment. Nine patients had febrile reactions during first two weeks at the 1st cycle of blinatumomab. No one cycle of blinatumomab therapy was not interrupted. Neurological toxicity (1-2 grades) was observed in 2 cases manifesting as headaches in 1 patient, and ulnar neuropathy in 1 case. One patient treated with sorafenib has hand-foot skin syndrome. The syndrome completely resolved after 2 weeks interruption of sorafenib treatment.
Pulmonary infiltrates and pleural effusion in one dasatinib-treated case were completely resolved after switching to nilotinib. Diarrhea associated with dasatinib therapy was observed in 3 patients and resolved after its replacement with bosutinib in 2 cases, and with nilotinib in 1 patient. CMV-associated colitis was diagnosed in 2 cases using virus-specific PCR in stool samples. Massive intestinal bleeding and multiple intestinal ulceration were observed in one patient with CMV colitis, as confirmed by colonoscopy. Facial edema and hyperemia were evident in 1 patient upon dasatinib treatment. These symptoms resolved after passage from dasatinib to nilotinib. Greyness of hair was detected in one patient treated with ponatinib. The main clinical characteristics of individual patients and the events are listed in Table 1.
Table 1. Basic clinical characteristics of blinatumomab + TKI-treated ALL patients, response to therapy, and adverse effects

The weekly performed counts of T-cytotoxic, NK, T-helper and T-regulatory cells in peripheral blood were decreased during the 1st blinatumomab cycle. T-cytotoxic and NK cells returned to normal ranges over the 2nd to 4th blinatumomab cycles. T-regulatory cell counts remained decreased or approached low-normal limits at all terms, except of 2nd blinatumomab cycle (Fig. 1).

Table 2. Weekly gaining effect in distinct T-cell subpopulations. Each sampling point was measured once a week (four points per cycle measured weekly) during 4 cycles of blinatumomab

Table 3. Weekly decrement in serum IgG, IgA and IgM upon blinatumomab therapy. Each measurement was performed at every treatment cycle (four points weekly over 4 cycles)

Regression analysis (the MIXED procedure in SAS system) was performed for each cell subpopulation, in order to detect significant changes of the T cell subpopulation kinetics (Table 2 and Fig. 2 a-e). As seen in table 2 in T-helper, T-cytotoxic and NK-subpopulations the weekly gaining effect was statistically significant.
Hypogammaglobulinemia during blinatumomab treatment was frequently observed (Fig. 3), and, in eight patients, treatment with intravenous human normal immunoglobulin was used. To check if the IgG, IgA and IgM kinetics were statistically significant, the regression analysis (the MIXED procedure in SAS system) was performed for each Ig (Table 3 and Fig. 4). As seen in Table 3, a gradual decrease in immunoglobulins with each cycle of blinatumomab was statistically significant for IgG, IgA and IgM. Fig. 4 shows the statistically significant effects of Blinatumomab treatment upon IgG (p=0.0446), IgA (p<.0001), and IgM (p=0.0186) (Table 3).
In 10 cases of 11, complete remission (CR) was achieved after 1st blimatumomab cycle. Progression of the disease was observed in 1 patient during 1st cycle of blinatumomab treatment, thus urging us to stop this therapy. Molecular CR (MolCR) was achieved in 9 cases, and cytogenetic CR was detected in 1 patient during consequent blinatumomab cycles. Allogeneic BMT was performed in 9 cases, and auto-BMT, in 1 patient with MolCR. Overt rapid hematological relapse was diagnosed in 1 patient under bosutinib maintenance therapy while waiting for alloBMT. Subsequent MolCR was achieved in the patient with bortezomib-based chemotherapy + dasatinib. One cytogenetic relapse was observed in 1 patient with complete cytogenetic remission before alloBMT. One patient treated with dasatinib as maintenance therapy after auto-BMT had molecular relapse, and second MolCR was achieved after Blinatumomab retreatment + dasatinib + ATRA. Allo-BMT from haploidentical donor was performed in this case. One patient treated with ponatinib and one patient receiving dasatinib maintenance after allo-BMT had CNS relapse and CNS lesions that regressed after intrathecal chemotherapy and cranial irradiation. One patient in MolCR died from septic shock 5 months after allo-BMT.



Discussion
Each patient in the study received combined treatment with blimatumomab immunotherapy and TKI target therapy. The treatment was well tolerated and complications were rare and curable. Hypogammaglobulinemia was common during blinatumomab treatment reflecting strong mature B-cell depletion on anti CD19 treatment. High rate of hypogammaglobulinemia lead to high rate of CMV infection, that was severe in 1 case. Rapid CR achievement and granulocyte recovery permits to treat almost all patients from 2nd to 4th cycles in outpatient settings. Nine molCR and one CyR with only one case of progressive disease permit to recognize this approach as highly effective, with low toxicity profile. Complementary treatment with ATRA in IKZF-deleted pts was also effective strategy though the number of such cases is too small. TKI treatment allows to perform prolonged maintenance, in order to control remaining leukemic populations in some cases. Though the rate of relapses is high, i.e., one half of the pts. However, a cohort of patients had extremely high risk of subsequent relapses and our approach did not exclude this risk entirely. In a single case, replacement of effective TKIs due to toxicity resulted in overt hematological relapse.
Prolonged neutropenia after Allo-BMT resulted in postponing of TKI maintenance and, in all these cases, the dose of eventually administered TKI was lowered by half. Heavily pre-treated patients have higher post-Allo-BMT toxicity, and the maintenance therapy after BMT was not proper. Auto-BMT in Ph-positive patient was not a curative strategy, though in IKZF-deleted patient, AutoBMT with lower toxicity regimen enables us to perform adequate maintenance with two agents, i.e., TKI and ATRA. Recently published results of combined treatment with blinatumomab and TKIs in Ph-positive relapsed ALL had also shown high rates of subsequent remissions and OS values (73% to 75%) in a small cohort of patients [11]. Statistical evaluation of changes observed for different lymphoid subpopulations revealed a strong evidence for T-cytotoxic and NK cells recovery in the course of effective combined treatment with blinatumomab and TKI. Duell et al. have demonstrated that lower frequency of T-regulatory cells correlates with higher response to blinatumomab in B-ALL patients [13]. T-regulatory and double-negative cells may potentially inhibit cytotoxic and other effector lymphocytes subpopulations and we observed their fluctuation within lower values of absolute peripheral blood counts in responders to the drug. Rapid and strong B-cell depletion as demonstrated by Zugmaier et al. [14], could explain the dropping immunoglobulin synthesis and prolonged hypogammaglobulinemia in most patients treated with blinatumomab.
Conclusion
Combined treatment with blinatumomab and TKI has acceptable and curable toxicity and demonstrates high rate of MolCR in high risk R/R ALL pts. The results are promising, with respect of using this treatment as induction and consolidation therapy without standard chemotherapy. IKZF deletions and FLT3-ITD are the new targets for chemo-free combined immunotherapy and TKI in ALL patients.
Acknowledgements
We thank Amgen for blinatumomab providing. We thank the Russian Acute Leukemia Study Group, National Hematology Society of Russia and also Margarita Anukhina for data management.
References
Introduction
Bispecific monoclonal antibodies for targeting tumor cells have a long story from 1980's to produce approved drug in 2014 [1-3]. Several trials for relapsed/refractory acute lymphoblastic leukemia (R/R ALL) treatment including ALCANTARA for Philadelphia chromosome-positive (Ph-positive) R/R ALL were performed and showed clinical effectiveness and even an advantage over chemotherapy (TOWER Study) [4-8]. Recently published results on blinatumomab combined with TKI for therapy of Ph-positive R/R ALL characterized this approach to be safe and effective [11]. Combination of Blinatumomab and TKI could elicit lower toxicity and improve primary results in managing heavily pretreated R/R ALL patients. In preclinical studies, retinoids have shown antileukemic activity in IKZF-deleted ALL [12]. We previously reported about effective combination of blinatumomab with tyrosine kinase inhibitors (TKI) for the treatment of Ph-positive and FLT3-ITD R/R ALL and blinatumomab with TKI and all-trans retinoic acid in IKZF-deleted R/R ALL patients [9, 10]. The aim of our study was to present the data on 11 R/R ALL patients treated with Blinatumomab and TKI+/-ATRA, omitting simultaneous standard chemotherapy.
Patients and methods
Eleven patients (pts) with R/R ALL were treated with Blinatumomab + TKI/TKI+ATRA at the National Research Center for Hematology, Moscow, Russia, and in Raisa Gorbacheva Memorial Institute of Pediatric Oncology, Hematology and Transplantation, Saint Petersburg, Russia, from October 2015 to October 2018. The study was approved by the Institutional Review Board. The treatment was administered after the patient has signed informed consent. Blinatumomab was provided by Amgen as part of the expanded access program. The treatment consisted of 4-5 cycles of blinatumomab 28 mcg/day by continuous infusion for 4 weeks each cycle. Over 1st week of 1st cycle, Blinatumomab was administered at the dose of 9 mcg/day. The 2-week intervals followed between the rounds of blinatumomab treatment. All the patients were administered one of TKIs: 7 Ph-positive and 2 IKZF1-deleted ALL pts were initially treated with dasatinib (140 mg/day) per os, 1 FLT3-ITD ALL patient received sorafenib (800 mg/day) per os, and one Ph-positive ALL patient with a T315I mutation received ponatinib (45 mg/day). All the TKIs were administered continuously, from the 1st day of starting blinatumomab. All-trans-retinoic acid (ATRA) at a dose of 45 mg/m2/day was administered per os in the IKZF1-deleted pts during 4 weeks of the 1st blinatumomab cycle, and during first two weeks of subsequent blinatumomab cycles.
Complete remission (CR) was diagnosed if less than 5% of blasts were present in bone marrow. Cytogenetic remission (CyR) was diagnosed in the absence of BCR-ABL positive nuclei per 200 nuclei by fluorescence in situ hybridization (FISH). Molecular CR (MolCR) was diagnosed if BCR-ABL/ABL at a ratio of <0.01 was detected in bone marrow samples by RT-qPCR. The CD3+/CD4+/CD8- T-helper, CD3+/CD4-/CD8+ T-cytotoxic, CD3+/CD4-/CD8- T-Double-negative, CD3+/CD4+/CD25+ T-regulatory, CD3-/CD56+ NK subpopulations were measured in peripheral blood lymphocytes by flow cytometry weekly during blinatumomab treatment in all the pts (1 to 16 samples, 4 points per each blinatumomab cycle). Serum immunoglobulins (Ig) G, M and A were measured during each cycle of blinatumomab (a total of 4 sampling points).
The SAS software was used for statistical evaluation using regression analysis. The MIXED SAS procedure was used to perform repeated measures analysis and to estimate parameters of linear regression of average time-dependent trend.
Results
Median follow-up was 23 months (19 to 36 months). Median age is 32 years (24 to 49 years). Eight pts were females and 3, males. 7 pts received 4 cycles; 1 pt, 5 cycles; 2 pts underwent 2 cycles, and 1 pt was subjected to 1 cycle of blinatumomab treatment. Nine patients had febrile reactions during first two weeks at the 1st cycle of blinatumomab. No one cycle of blinatumomab therapy was not interrupted. Neurological toxicity (1-2 grades) was observed in 2 cases manifesting as headaches in 1 patient, and ulnar neuropathy in 1 case. One patient treated with sorafenib has hand-foot skin syndrome. The syndrome completely resolved after 2 weeks interruption of sorafenib treatment.
Pulmonary infiltrates and pleural effusion in one dasatinib-treated case were completely resolved after switching to nilotinib. Diarrhea associated with dasatinib therapy was observed in 3 patients and resolved after its replacement with bosutinib in 2 cases, and with nilotinib in 1 patient. CMV-associated colitis was diagnosed in 2 cases using virus-specific PCR in stool samples. Massive intestinal bleeding and multiple intestinal ulceration were observed in one patient with CMV colitis, as confirmed by colonoscopy. Facial edema and hyperemia were evident in 1 patient upon dasatinib treatment. These symptoms resolved after passage from dasatinib to nilotinib. Greyness of hair was detected in one patient treated with ponatinib. The main clinical characteristics of individual patients and the events are listed in Table 1.
Table 1. Basic clinical characteristics of blinatumomab + TKI-treated ALL patients, response to therapy, and adverse effects

The weekly performed counts of T-cytotoxic, NK, T-helper and T-regulatory cells in peripheral blood were decreased during the 1st blinatumomab cycle. T-cytotoxic and NK cells returned to normal ranges over the 2nd to 4th blinatumomab cycles. T-regulatory cell counts remained decreased or approached low-normal limits at all terms, except of 2nd blinatumomab cycle (Fig. 1).

Table 2. Weekly gaining effect in distinct T-cell subpopulations. Each sampling point was measured once a week (four points per cycle measured weekly) during 4 cycles of blinatumomab

Table 3. Weekly decrement in serum IgG, IgA and IgM upon blinatumomab therapy. Each measurement was performed at every treatment cycle (four points weekly over 4 cycles)

Regression analysis (the MIXED procedure in SAS system) was performed for each cell subpopulation, in order to detect significant changes of the T cell subpopulation kinetics (Table 2 and Fig. 2 a-e). As seen in table 2 in T-helper, T-cytotoxic and NK-subpopulations the weekly gaining effect was statistically significant.
Hypogammaglobulinemia during blinatumomab treatment was frequently observed (Fig. 3), and, in eight patients, treatment with intravenous human normal immunoglobulin was used. To check if the IgG, IgA and IgM kinetics were statistically significant, the regression analysis (the MIXED procedure in SAS system) was performed for each Ig (Table 3 and Fig. 4). As seen in Table 3, a gradual decrease in immunoglobulins with each cycle of blinatumomab was statistically significant for IgG, IgA and IgM. Fig. 4 shows the statistically significant effects of Blinatumomab treatment upon IgG (p=0.0446), IgA (p<.0001), and IgM (p=0.0186) (Table 3).
In 10 cases of 11, complete remission (CR) was achieved after 1st blimatumomab cycle. Progression of the disease was observed in 1 patient during 1st cycle of blinatumomab treatment, thus urging us to stop this therapy. Molecular CR (MolCR) was achieved in 9 cases, and cytogenetic CR was detected in 1 patient during consequent blinatumomab cycles. Allogeneic BMT was performed in 9 cases, and auto-BMT, in 1 patient with MolCR. Overt rapid hematological relapse was diagnosed in 1 patient under bosutinib maintenance therapy while waiting for alloBMT. Subsequent MolCR was achieved in the patient with bortezomib-based chemotherapy + dasatinib. One cytogenetic relapse was observed in 1 patient with complete cytogenetic remission before alloBMT. One patient treated with dasatinib as maintenance therapy after auto-BMT had molecular relapse, and second MolCR was achieved after Blinatumomab retreatment + dasatinib + ATRA. Allo-BMT from haploidentical donor was performed in this case. One patient treated with ponatinib and one patient receiving dasatinib maintenance after allo-BMT had CNS relapse and CNS lesions that regressed after intrathecal chemotherapy and cranial irradiation. One patient in MolCR died from septic shock 5 months after allo-BMT.



Discussion
Each patient in the study received combined treatment with blimatumomab immunotherapy and TKI target therapy. The treatment was well tolerated and complications were rare and curable. Hypogammaglobulinemia was common during blinatumomab treatment reflecting strong mature B-cell depletion on anti CD19 treatment. High rate of hypogammaglobulinemia lead to high rate of CMV infection, that was severe in 1 case. Rapid CR achievement and granulocyte recovery permits to treat almost all patients from 2nd to 4th cycles in outpatient settings. Nine molCR and one CyR with only one case of progressive disease permit to recognize this approach as highly effective, with low toxicity profile. Complementary treatment with ATRA in IKZF-deleted pts was also effective strategy though the number of such cases is too small. TKI treatment allows to perform prolonged maintenance, in order to control remaining leukemic populations in some cases. Though the rate of relapses is high, i.e., one half of the pts. However, a cohort of patients had extremely high risk of subsequent relapses and our approach did not exclude this risk entirely. In a single case, replacement of effective TKIs due to toxicity resulted in overt hematological relapse.
Prolonged neutropenia after Allo-BMT resulted in postponing of TKI maintenance and, in all these cases, the dose of eventually administered TKI was lowered by half. Heavily pre-treated patients have higher post-Allo-BMT toxicity, and the maintenance therapy after BMT was not proper. Auto-BMT in Ph-positive patient was not a curative strategy, though in IKZF-deleted patient, AutoBMT with lower toxicity regimen enables us to perform adequate maintenance with two agents, i.e., TKI and ATRA. Recently published results of combined treatment with blinatumomab and TKIs in Ph-positive relapsed ALL had also shown high rates of subsequent remissions and OS values (73% to 75%) in a small cohort of patients [11]. Statistical evaluation of changes observed for different lymphoid subpopulations revealed a strong evidence for T-cytotoxic and NK cells recovery in the course of effective combined treatment with blinatumomab and TKI. Duell et al. have demonstrated that lower frequency of T-regulatory cells correlates with higher response to blinatumomab in B-ALL patients [13]. T-regulatory and double-negative cells may potentially inhibit cytotoxic and other effector lymphocytes subpopulations and we observed their fluctuation within lower values of absolute peripheral blood counts in responders to the drug. Rapid and strong B-cell depletion as demonstrated by Zugmaier et al. [14], could explain the dropping immunoglobulin synthesis and prolonged hypogammaglobulinemia in most patients treated with blinatumomab.
Conclusion
Combined treatment with blinatumomab and TKI has acceptable and curable toxicity and demonstrates high rate of MolCR in high risk R/R ALL pts. The results are promising, with respect of using this treatment as induction and consolidation therapy without standard chemotherapy. IKZF deletions and FLT3-ITD are the new targets for chemo-free combined immunotherapy and TKI in ALL patients.
Acknowledgements
We thank Amgen for blinatumomab providing. We thank the Russian Acute Leukemia Study Group, National Hematology Society of Russia and also Margarita Anukhina for data management.
References
Андрей Н. Соколов1, Елена Н. Паровичникова1, Вера В. Троицкая1, Лариса А. Кузьмина1, Ирина В. Гальцева1, Сергей М. Куликов1, Сергей Н. Бондаренко2, Ирина А. Лукьянова1, Татьяна И. Лобанова1, Екатерина И. Усикова1, Ксения И. Зарубина1, Ольга А. Гаврилина1, Юлия О. Давыдова1, Николай М. Капранов1, Валерий Г. Савченко1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26321" ["VALUE"]=> array(2) { ["TEXT"]=> string(679) "<p><sup>1</sup> Федеральное государственное бюджетное учреждение научный медицинский исследовательский центр гематологии Минздрава России, Москва, Россия<br> <sup>2</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(637) "1 Федеральное государственное бюджетное учреждение научный медицинский исследовательский центр гематологии Минздрава России, Москва, Россия
2 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
Биспецифическое моноклональное антитело блинатумомаб против антигена CD19 используется для лечения острого лимфобластного лейкоза (ОЛЛ). Несколько дополнительных молекулярных мишеней могут быть использованы для комбинированного лечения без обычной химиотерапии, а именно ингибиторы тирозинкиназ, в т.ч. BCR-ABL, FLT3 и делеции IKZF1. Целью данного исследования было определение токсичности и клинической эффективности комбинированного лечения блинатумомабом и несколькими ингибиторами тирозинкиназы.
Пациенты и методы
С октября 2015 по октябрь 2018 г. нами пролечены 11 пациентов с рецидивируюшим/рефрактерным течением ОЛЛ. Терапия блинатумомабом состояла из 4-5 циклов с 2-недельными интервалами (28 мкг/день посредством постоянной инфузии в течение 28 дней, при дозе 9 мкг/день в течении 1-й недели 1-го цикла). Семь BCR-ABL-позитивных больных и 2 пациента с делецией IKZF1 получали исходно дазатиниб (140 мг/день), один пациент с FLT3-ITD – сорафениб (800 мг/день). Один BCR-ABL-позитивный больной с мутацией T315I получал понатиниб (45 мг/день). Пациентам с делециями IKZF1 назначалась ATRA (45 мг/м2/день в течение 4 недель) на 1-м цикле блинатумомаба и в первые 2 недели последующих циклов лечения антителом.
Результаты
У пациентов, отвечающих на лечение блинатумомабом, отмечалось статистически достоверное повышение абсолютного количества Т-хелперных лимфоцитов (p=0.0034), T-цитотоксических клеток (p<0.0001) и субпопуляций естественных киллеров (p=0.0006) в периферической крови на протяжении всего периода лечения. T-регуляторные и дубль-негативные T-клетки – потенциальные ингибиторы Т-клеточного ответа на блинатумомаб оставались в пределах нижних значений нормы. Гипогаммаглобулинемия наблюдалась у 8 из 11 пациентов. Полная ремиссия (ПР) была получена у 10 больных после 1-го цикла блинатумомаба, прогрессия заболевания – в одном случае. Из 10 пациентов, в 9 случаях достигнута полная молекулярная ремиссия (ПМР) и в одном – полная цитогенетическая ремиссия. Выполнены девять аллогенных трансплантаций гемопоэтических стволовых клеток (ТГСК) и одна аутологичная ТГСК в 10 случаях ПР. Выявлены три рецидива в ЦНС после алло-ТГСК и один молекулярный рецидив после ауто-ТГСК. Один пациент скончался от септического шока после алло-ТГСК.
Выводы
Блинатомомаб в коимбинации с ингибиторами тирозинкиназ имеет высокий терапевтический потенциал для индукции ремиссии в определенных случаях ОЛЛ без применения обычной химиотерапии. Высокая частота потенциальных рецидивов в ЦНС может быть снижена за счет более интенсивной интратекальной профилактики.
Ключевые слова
Острый лимфобластный лейкоз, блинатумомаб, ингибиторы тирозинкиназ.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26323" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-38-46" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-38-46" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26326" ["VALUE"]=> array(2) { ["TEXT"]=> string(684) "<p>Andrey N. Sokolov<sup>1</sup>, Elena N. Parovichnikova<sup>1</sup>, Vera V. Troitskaya<sup>1</sup>, Larisa A. Kuzmina<sup>1</sup>, Irina V. Galtseva<sup>1</sup>, Sergei M. Kulikov<sup>1</sup>, Sergey N. Bondarenko<sup>2</sup>, Irina A. Lukyanova<sup>1</sup>, Tatiana I. Lobanova<sup>1</sup>, Ekaterina I. Usikova<sup>1</sup>, Ksenia I. Zarubina<sup>1</sup>, Olga A. Gavrilina<sup>1</sup>, Julia O. Davidova<sup>1</sup>, Nikolai M. Kapranov<sup>1</sup>, Valeriy G. Savchenko<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(492) "Andrey N. Sokolov1, Elena N. Parovichnikova1, Vera V. Troitskaya1, Larisa A. Kuzmina1, Irina V. Galtseva1, Sergei M. Kulikov1, Sergey N. Bondarenko2, Irina A. Lukyanova1, Tatiana I. Lobanova1, Ekaterina I. Usikova1, Ksenia I. Zarubina1, Olga A. Gavrilina1, Julia O. Davidova1, Nikolai M. Kapranov1, Valeriy G. Savchenko1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26327" ["VALUE"]=> array(2) { ["TEXT"]=> string(607) "<p><sup>1</sup> National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia<br> <sup>2</sup> Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br> St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia<br> Phone: +7 (495) 612 4592<br> E-mail: sokolov.a@blood.ru</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(511) "1 National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia
2 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia
Phone: +7 (495) 612 4592
E-mail: sokolov.a@blood.ru
Bispecific monoclonal antibody blinatumomab is targeting CD19, being applied for treatment of acute lymphoblastic leukemia (ALL). Several additional targets could be used for combined chemo-free treatment with thyrosine kinase inhibitors: BCR-ABL, FLT3 and IKZF1 deletions are amongst them. In the following study, we aimed to assess toxicity and clinical effectiveness of the combination of blinatumomab and several thyrosine kinase inhibitors.
Patients and methods
From October 2015 to October 2018, we treated 11 relapsed/refractory (R/R) ALL patients (pts). The blinatumomab treatment consisted of 4-5 cycles with 2-week intervals (28 mcg/day by continuous infusion during 28 days per cycle with 9 mcg/day during the first week of the first cycle). Seven BCR-ABL-positive and 2 IKZF1-deleted pts received initially dasatinib at 140 mg/day, one case, with FLT3-ITD received sorafenib (800 mg/day). One BCR-ABL-positive pt with T315I mutation was administered ponatinib (45 mg/day). Pts with IKZF1 deletions received ATRA (45 mg/m2/day for 4 weeks) of the 1st blinatumomab cycle and during first 2 weeks of subsequent blinatumomab cycles.
Results
In the patients responding to blinatumomab treatment, we observed a statistically significant increment of absolute values in T-helper (p=0.0034), T-cytotoxic (p<0.0001) and NK (p=0.0006) cell subpopulations in peripheral blood over the entire treatment period. T-regulatory and double-negative T-cells as potentially inhibitors of T cell response to blinatumomab remained within low values of normal ranges. Hypogammaglobulinemia was observed in 8 of 11 pts. CR was achieved in 10 pts after 1st cycle of blinatumomab, progressive disease was detected in 1 pt. Nine cases of complete molecular remissions (MolCR) and one cytogenetic complete remission were achieved in ten CR pts. Nine allo-BMT and one auto-BMT were performed in 10 CR pts. Three CNS relapses after allo-BMT and one molecular relapse after auto-BMT were diagnosed. One pt died from septic shock after allo-BMT.
Conclusion
Blinatumomab combined with TKI has high therapeutic potential as induction remission treatment in targeted ALL population without conventional chemotherapy. High rate of potential CNS relapses could be decreased with more intensive intrathecal prophylaxis.
Keywords
Acute lymphoblastic leukemia, blinatumomab, tyrosine kinase inhibitors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26324" ["VALUE"]=> string(185) "BCR/ABL, IKZF deletions and FLT3-ITD as the targets for relapsed/refractory B-cell acute lymphoblastic leukemia treatment: Blinatumomab combined with Tyrosine kinase inhibitors and ATRA" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(185) "BCR/ABL, IKZF deletions and FLT3-ITD as the targets for relapsed/refractory B-cell acute lymphoblastic leukemia treatment: Blinatumomab combined with Tyrosine kinase inhibitors and ATRA" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26325" ["VALUE"]=> string(4) "1968" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1968" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26329" ["VALUE"]=> string(4) "1969" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1969" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26326" ["VALUE"]=> array(2) { ["TEXT"]=> string(684) "<p>Andrey N. Sokolov<sup>1</sup>, Elena N. Parovichnikova<sup>1</sup>, Vera V. Troitskaya<sup>1</sup>, Larisa A. Kuzmina<sup>1</sup>, Irina V. Galtseva<sup>1</sup>, Sergei M. Kulikov<sup>1</sup>, Sergey N. Bondarenko<sup>2</sup>, Irina A. Lukyanova<sup>1</sup>, Tatiana I. Lobanova<sup>1</sup>, Ekaterina I. Usikova<sup>1</sup>, Ksenia I. Zarubina<sup>1</sup>, Olga A. Gavrilina<sup>1</sup>, Julia O. Davidova<sup>1</sup>, Nikolai M. Kapranov<sup>1</sup>, Valeriy G. Savchenko<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(492) "Andrey N. Sokolov1, Elena N. Parovichnikova1, Vera V. Troitskaya1, Larisa A. Kuzmina1, Irina V. Galtseva1, Sergei M. Kulikov1, Sergey N. Bondarenko2, Irina A. Lukyanova1, Tatiana I. Lobanova1, Ekaterina I. Usikova1, Ksenia I. Zarubina1, Olga A. Gavrilina1, Julia O. Davidova1, Nikolai M. Kapranov1, Valeriy G. Savchenko1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(492) "Andrey N. Sokolov1, Elena N. Parovichnikova1, Vera V. Troitskaya1, Larisa A. Kuzmina1, Irina V. Galtseva1, Sergei M. Kulikov1, Sergey N. Bondarenko2, Irina A. Lukyanova1, Tatiana I. Lobanova1, Ekaterina I. Usikova1, Ksenia I. Zarubina1, Olga A. Gavrilina1, Julia O. Davidova1, Nikolai M. Kapranov1, Valeriy G. Savchenko1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26328" ["VALUE"]=> array(2) { ["TEXT"]=> string(2821) "<p style="text-align: justify;">Bispecific monoclonal antibody blinatumomab is targeting CD19, being applied for treatment of acute lymphoblastic leukemia (ALL). Several additional targets could be used for combined chemo-free treatment with thyrosine kinase inhibitors: BCR-ABL, FLT3 and IKZF1 deletions are amongst them. In the following study, we aimed to assess toxicity and clinical effectiveness of the combination of blinatumomab and several thyrosine kinase inhibitors. </p> <h3>Patients and methods</h3> <p style="text-align: justify;"> From October 2015 to October 2018, we treated 11 relapsed/refractory (R/R) ALL patients (pts). The blinatumomab treatment consisted of 4-5 cycles with 2-week intervals (28 mcg/day by continuous infusion during 28 days per cycle with 9 mcg/day during the first week of the first cycle). Seven BCR-ABL-positive and 2 IKZF1-deleted pts received initially dasatinib at 140 mg/day, one case, with FLT3-ITD received sorafenib (800 mg/day). One BCR-ABL-positive pt with T315I mutation was administered ponatinib (45 mg/day). Pts with IKZF1 deletions received ATRA (45 mg/m<sup>2</sup>/day for 4 weeks) of the 1<sup>st</sup> blinatumomab cycle and during first 2 weeks of subsequent blinatumomab cycles. </p> <h3>Results</h3> <p style="text-align: justify;"> In the patients responding to blinatumomab treatment, we observed a statistically significant increment of absolute values in T-helper (p=0.0034), T-cytotoxic (p<0.0001) and NK (p=0.0006) cell subpopulations in peripheral blood over the entire treatment period. T-regulatory and double-negative T-cells as potentially inhibitors of T cell response to blinatumomab remained within low values of normal ranges. Hypogammaglobulinemia was observed in 8 of 11 pts. CR was achieved in 10 pts after 1st cycle of blinatumomab, progressive disease was detected in 1 pt. Nine cases of complete molecular remissions (MolCR) and one cytogenetic complete remission were achieved in ten CR pts. Nine allo-BMT and one auto-BMT were performed in 10 CR pts. Three CNS relapses after allo-BMT and one molecular relapse after auto-BMT were diagnosed. One pt died from septic shock after allo-BMT. </p> <h3>Conclusion</h3> <p style="text-align: justify;"> Blinatumomab combined with TKI has high therapeutic potential as induction remission treatment in targeted ALL population without conventional chemotherapy. High rate of potential CNS relapses could be decreased with more intensive intrathecal prophylaxis.</p> <h2>Keywords</h2> <p style="text-align: justify;"> Acute lymphoblastic leukemia, blinatumomab, tyrosine kinase inhibitors. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2636) "Bispecific monoclonal antibody blinatumomab is targeting CD19, being applied for treatment of acute lymphoblastic leukemia (ALL). Several additional targets could be used for combined chemo-free treatment with thyrosine kinase inhibitors: BCR-ABL, FLT3 and IKZF1 deletions are amongst them. In the following study, we aimed to assess toxicity and clinical effectiveness of the combination of blinatumomab and several thyrosine kinase inhibitors.
Patients and methods
From October 2015 to October 2018, we treated 11 relapsed/refractory (R/R) ALL patients (pts). The blinatumomab treatment consisted of 4-5 cycles with 2-week intervals (28 mcg/day by continuous infusion during 28 days per cycle with 9 mcg/day during the first week of the first cycle). Seven BCR-ABL-positive and 2 IKZF1-deleted pts received initially dasatinib at 140 mg/day, one case, with FLT3-ITD received sorafenib (800 mg/day). One BCR-ABL-positive pt with T315I mutation was administered ponatinib (45 mg/day). Pts with IKZF1 deletions received ATRA (45 mg/m2/day for 4 weeks) of the 1st blinatumomab cycle and during first 2 weeks of subsequent blinatumomab cycles.
Results
In the patients responding to blinatumomab treatment, we observed a statistically significant increment of absolute values in T-helper (p=0.0034), T-cytotoxic (p<0.0001) and NK (p=0.0006) cell subpopulations in peripheral blood over the entire treatment period. T-regulatory and double-negative T-cells as potentially inhibitors of T cell response to blinatumomab remained within low values of normal ranges. Hypogammaglobulinemia was observed in 8 of 11 pts. CR was achieved in 10 pts after 1st cycle of blinatumomab, progressive disease was detected in 1 pt. Nine cases of complete molecular remissions (MolCR) and one cytogenetic complete remission were achieved in ten CR pts. Nine allo-BMT and one auto-BMT were performed in 10 CR pts. Three CNS relapses after allo-BMT and one molecular relapse after auto-BMT were diagnosed. One pt died from septic shock after allo-BMT.
Conclusion
Blinatumomab combined with TKI has high therapeutic potential as induction remission treatment in targeted ALL population without conventional chemotherapy. High rate of potential CNS relapses could be decreased with more intensive intrathecal prophylaxis.
Keywords
Acute lymphoblastic leukemia, blinatumomab, tyrosine kinase inhibitors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2636) "Bispecific monoclonal antibody blinatumomab is targeting CD19, being applied for treatment of acute lymphoblastic leukemia (ALL). Several additional targets could be used for combined chemo-free treatment with thyrosine kinase inhibitors: BCR-ABL, FLT3 and IKZF1 deletions are amongst them. In the following study, we aimed to assess toxicity and clinical effectiveness of the combination of blinatumomab and several thyrosine kinase inhibitors.
Patients and methods
From October 2015 to October 2018, we treated 11 relapsed/refractory (R/R) ALL patients (pts). The blinatumomab treatment consisted of 4-5 cycles with 2-week intervals (28 mcg/day by continuous infusion during 28 days per cycle with 9 mcg/day during the first week of the first cycle). Seven BCR-ABL-positive and 2 IKZF1-deleted pts received initially dasatinib at 140 mg/day, one case, with FLT3-ITD received sorafenib (800 mg/day). One BCR-ABL-positive pt with T315I mutation was administered ponatinib (45 mg/day). Pts with IKZF1 deletions received ATRA (45 mg/m2/day for 4 weeks) of the 1st blinatumomab cycle and during first 2 weeks of subsequent blinatumomab cycles.
Results
In the patients responding to blinatumomab treatment, we observed a statistically significant increment of absolute values in T-helper (p=0.0034), T-cytotoxic (p<0.0001) and NK (p=0.0006) cell subpopulations in peripheral blood over the entire treatment period. T-regulatory and double-negative T-cells as potentially inhibitors of T cell response to blinatumomab remained within low values of normal ranges. Hypogammaglobulinemia was observed in 8 of 11 pts. CR was achieved in 10 pts after 1st cycle of blinatumomab, progressive disease was detected in 1 pt. Nine cases of complete molecular remissions (MolCR) and one cytogenetic complete remission were achieved in ten CR pts. Nine allo-BMT and one auto-BMT were performed in 10 CR pts. Three CNS relapses after allo-BMT and one molecular relapse after auto-BMT were diagnosed. One pt died from septic shock after allo-BMT.
Conclusion
Blinatumomab combined with TKI has high therapeutic potential as induction remission treatment in targeted ALL population without conventional chemotherapy. High rate of potential CNS relapses could be decreased with more intensive intrathecal prophylaxis.
Keywords
Acute lymphoblastic leukemia, blinatumomab, tyrosine kinase inhibitors.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26323" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-38-46" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-38-46" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-38-46" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26324" ["VALUE"]=> string(185) "BCR/ABL, IKZF deletions and FLT3-ITD as the targets for relapsed/refractory B-cell acute lymphoblastic leukemia treatment: Blinatumomab combined with Tyrosine kinase inhibitors and ATRA" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(185) "BCR/ABL, IKZF deletions and FLT3-ITD as the targets for relapsed/refractory B-cell acute lymphoblastic leukemia treatment: Blinatumomab combined with Tyrosine kinase inhibitors and ATRA" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(185) "BCR/ABL, IKZF deletions and FLT3-ITD as the targets for relapsed/refractory B-cell acute lymphoblastic leukemia treatment: Blinatumomab combined with Tyrosine kinase inhibitors and ATRA" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26327" ["VALUE"]=> array(2) { ["TEXT"]=> string(607) "<p><sup>1</sup> National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia<br> <sup>2</sup> Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br> St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia<br> Phone: +7 (495) 612 4592<br> E-mail: sokolov.a@blood.ru</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(511) "1 National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia
2 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia
Phone: +7 (495) 612 4592
E-mail: sokolov.a@blood.ru
1 National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia
2 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia
Phone: +7 (495) 612 4592
E-mail: sokolov.a@blood.ru
Андрей Н. Соколов1, Елена Н. Паровичникова1, Вера В. Троицкая1, Лариса А. Кузьмина1, Ирина В. Гальцева1, Сергей М. Куликов1, Сергей Н. Бондаренко2, Ирина А. Лукьянова1, Татьяна И. Лобанова1, Екатерина И. Усикова1, Ксения И. Зарубина1, Ольга А. Гаврилина1, Юлия О. Давыдова1, Николай М. Капранов1, Валерий Г. Савченко1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(718) "Андрей Н. Соколов1, Елена Н. Паровичникова1, Вера В. Троицкая1, Лариса А. Кузьмина1, Ирина В. Гальцева1, Сергей М. Куликов1, Сергей Н. Бондаренко2, Ирина А. Лукьянова1, Татьяна И. Лобанова1, Екатерина И. Усикова1, Ксения И. Зарубина1, Ольга А. Гаврилина1, Юлия О. Давыдова1, Николай М. Капранов1, Валерий Г. Савченко1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26318" ["VALUE"]=> string(22) "02/28/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/28/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "02/28/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26319" ["VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/27/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26322" ["VALUE"]=> array(2) { ["TEXT"]=> string(5159) "<p style="text-align: justify;">Биспецифическое моноклональное антитело блинатумомаб против антигена CD19 используется для лечения острого лимфобластного лейкоза (ОЛЛ). Несколько дополнительных молекулярных мишеней могут быть использованы для комбинированного лечения без обычной химиотерапии, а именно ингибиторы тирозинкиназ, в т.ч. BCR-ABL, FLT3 и делеции IKZF1. Целью данного исследования было определение токсичности и клинической эффективности комбинированного лечения блинатумомабом и несколькими ингибиторами тирозинкиназы. </p> <h3>Пациенты и методы</h3> <p style="text-align: justify;"> С октября 2015 по октябрь 2018 г. нами пролечены 11 пациентов с рецидивируюшим/рефрактерным течением ОЛЛ. Терапия блинатумомабом состояла из 4-5 циклов с 2-недельными интервалами (28 мкг/день посредством постоянной инфузии в течение 28 дней, при дозе 9 мкг/день в течении 1-й недели 1-го цикла). Семь BCR-ABL-позитивных больных и 2 пациента с делецией IKZF1 получали исходно дазатиниб (140 мг/день), один пациент с FLT3-ITD – сорафениб (800 мг/день). Один BCR-ABL-позитивный больной с мутацией T315I получал понатиниб (45 мг/день). Пациентам с делециями IKZF1 назначалась ATRA (45 мг/м<sup>2</sup>/день в течение 4 недель) на 1-м цикле блинатумомаба и в первые 2 недели последующих циклов лечения антителом.</p> <h3>Результаты</h3> <p style="text-align: justify;"> У пациентов, отвечающих на лечение блинатумомабом, отмечалось статистически достоверное повышение абсолютного количества Т-хелперных лимфоцитов (p=0.0034), T-цитотоксических клеток (p<0.0001) и субпопуляций естественных киллеров (p=0.0006) в периферической крови на протяжении всего периода лечения. T-регуляторные и дубль-негативные T-клетки – потенциальные ингибиторы Т-клеточного ответа на блинатумомаб оставались в пределах нижних значений нормы. Гипогаммаглобулинемия наблюдалась у 8 из 11 пациентов. Полная ремиссия (ПР) была получена у 10 больных после 1-го цикла блинатумомаба, прогрессия заболевания – в одном случае. Из 10 пациентов, в 9 случаях достигнута полная молекулярная ремиссия (ПМР) и в одном – полная цитогенетическая ремиссия. Выполнены девять аллогенных трансплантаций гемопоэтических стволовых клеток (ТГСК) и одна аутологичная ТГСК в 10 случаях ПР. Выявлены три рецидива в ЦНС после алло-ТГСК и один молекулярный рецидив после ауто-ТГСК. Один пациент скончался от септического шока после алло-ТГСК. </p> <h3>Выводы</h3> <p style="text-align: justify;"> Блинатомомаб в коимбинации с ингибиторами тирозинкиназ имеет высокий терапевтический потенциал для индукции ремиссии в определенных случаях ОЛЛ без применения обычной химиотерапии. Высокая частота потенциальных рецидивов в ЦНС может быть снижена за счет более интенсивной интратекальной профилактики. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Острый лимфобластный лейкоз, блинатумомаб, ингибиторы тирозинкиназ. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4986) "Биспецифическое моноклональное антитело блинатумомаб против антигена CD19 используется для лечения острого лимфобластного лейкоза (ОЛЛ). Несколько дополнительных молекулярных мишеней могут быть использованы для комбинированного лечения без обычной химиотерапии, а именно ингибиторы тирозинкиназ, в т.ч. BCR-ABL, FLT3 и делеции IKZF1. Целью данного исследования было определение токсичности и клинической эффективности комбинированного лечения блинатумомабом и несколькими ингибиторами тирозинкиназы.
Пациенты и методы
С октября 2015 по октябрь 2018 г. нами пролечены 11 пациентов с рецидивируюшим/рефрактерным течением ОЛЛ. Терапия блинатумомабом состояла из 4-5 циклов с 2-недельными интервалами (28 мкг/день посредством постоянной инфузии в течение 28 дней, при дозе 9 мкг/день в течении 1-й недели 1-го цикла). Семь BCR-ABL-позитивных больных и 2 пациента с делецией IKZF1 получали исходно дазатиниб (140 мг/день), один пациент с FLT3-ITD – сорафениб (800 мг/день). Один BCR-ABL-позитивный больной с мутацией T315I получал понатиниб (45 мг/день). Пациентам с делециями IKZF1 назначалась ATRA (45 мг/м2/день в течение 4 недель) на 1-м цикле блинатумомаба и в первые 2 недели последующих циклов лечения антителом.
Результаты
У пациентов, отвечающих на лечение блинатумомабом, отмечалось статистически достоверное повышение абсолютного количества Т-хелперных лимфоцитов (p=0.0034), T-цитотоксических клеток (p<0.0001) и субпопуляций естественных киллеров (p=0.0006) в периферической крови на протяжении всего периода лечения. T-регуляторные и дубль-негативные T-клетки – потенциальные ингибиторы Т-клеточного ответа на блинатумомаб оставались в пределах нижних значений нормы. Гипогаммаглобулинемия наблюдалась у 8 из 11 пациентов. Полная ремиссия (ПР) была получена у 10 больных после 1-го цикла блинатумомаба, прогрессия заболевания – в одном случае. Из 10 пациентов, в 9 случаях достигнута полная молекулярная ремиссия (ПМР) и в одном – полная цитогенетическая ремиссия. Выполнены девять аллогенных трансплантаций гемопоэтических стволовых клеток (ТГСК) и одна аутологичная ТГСК в 10 случаях ПР. Выявлены три рецидива в ЦНС после алло-ТГСК и один молекулярный рецидив после ауто-ТГСК. Один пациент скончался от септического шока после алло-ТГСК.
Выводы
Блинатомомаб в коимбинации с ингибиторами тирозинкиназ имеет высокий терапевтический потенциал для индукции ремиссии в определенных случаях ОЛЛ без применения обычной химиотерапии. Высокая частота потенциальных рецидивов в ЦНС может быть снижена за счет более интенсивной интратекальной профилактики.
Ключевые слова
Острый лимфобластный лейкоз, блинатумомаб, ингибиторы тирозинкиназ.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4986) "Биспецифическое моноклональное антитело блинатумомаб против антигена CD19 используется для лечения острого лимфобластного лейкоза (ОЛЛ). Несколько дополнительных молекулярных мишеней могут быть использованы для комбинированного лечения без обычной химиотерапии, а именно ингибиторы тирозинкиназ, в т.ч. BCR-ABL, FLT3 и делеции IKZF1. Целью данного исследования было определение токсичности и клинической эффективности комбинированного лечения блинатумомабом и несколькими ингибиторами тирозинкиназы.
Пациенты и методы
С октября 2015 по октябрь 2018 г. нами пролечены 11 пациентов с рецидивируюшим/рефрактерным течением ОЛЛ. Терапия блинатумомабом состояла из 4-5 циклов с 2-недельными интервалами (28 мкг/день посредством постоянной инфузии в течение 28 дней, при дозе 9 мкг/день в течении 1-й недели 1-го цикла). Семь BCR-ABL-позитивных больных и 2 пациента с делецией IKZF1 получали исходно дазатиниб (140 мг/день), один пациент с FLT3-ITD – сорафениб (800 мг/день). Один BCR-ABL-позитивный больной с мутацией T315I получал понатиниб (45 мг/день). Пациентам с делециями IKZF1 назначалась ATRA (45 мг/м2/день в течение 4 недель) на 1-м цикле блинатумомаба и в первые 2 недели последующих циклов лечения антителом.
Результаты
У пациентов, отвечающих на лечение блинатумомабом, отмечалось статистически достоверное повышение абсолютного количества Т-хелперных лимфоцитов (p=0.0034), T-цитотоксических клеток (p<0.0001) и субпопуляций естественных киллеров (p=0.0006) в периферической крови на протяжении всего периода лечения. T-регуляторные и дубль-негативные T-клетки – потенциальные ингибиторы Т-клеточного ответа на блинатумомаб оставались в пределах нижних значений нормы. Гипогаммаглобулинемия наблюдалась у 8 из 11 пациентов. Полная ремиссия (ПР) была получена у 10 больных после 1-го цикла блинатумомаба, прогрессия заболевания – в одном случае. Из 10 пациентов, в 9 случаях достигнута полная молекулярная ремиссия (ПМР) и в одном – полная цитогенетическая ремиссия. Выполнены девять аллогенных трансплантаций гемопоэтических стволовых клеток (ТГСК) и одна аутологичная ТГСК в 10 случаях ПР. Выявлены три рецидива в ЦНС после алло-ТГСК и один молекулярный рецидив после ауто-ТГСК. Один пациент скончался от септического шока после алло-ТГСК.
Выводы
Блинатомомаб в коимбинации с ингибиторами тирозинкиназ имеет высокий терапевтический потенциал для индукции ремиссии в определенных случаях ОЛЛ без применения обычной химиотерапии. Высокая частота потенциальных рецидивов в ЦНС может быть снижена за счет более интенсивной интратекальной профилактики.
Ключевые слова
Острый лимфобластный лейкоз, блинатумомаб, ингибиторы тирозинкиназ.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26321" ["VALUE"]=> array(2) { ["TEXT"]=> string(679) "<p><sup>1</sup> Федеральное государственное бюджетное учреждение научный медицинский исследовательский центр гематологии Минздрава России, Москва, Россия<br> <sup>2</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(637) "1 Федеральное государственное бюджетное учреждение научный медицинский исследовательский центр гематологии Минздрава России, Москва, Россия
2 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
1 Федеральное государственное бюджетное учреждение научный медицинский исследовательский центр гематологии Минздрава России, Москва, Россия
2 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
Introduction
Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo-HSCT) with incidence of 9% to 31% for different cohorts [1-7]. Early clinical form of HC usually develops within ten days post allo-HSCT. In this cases HC is considered a direct consequence of cytotoxic conditioning therapy and graft-versus-host disease (GvHD) prophylaxis [8]. Along with cytotoxic drugs, some viral agents, e.g., BK- and JC- polyomaviruses, adenovirus and cytomegalovirus may be also involved in HC pathogenesis. Among them, human BK-polyomavirus (BKPyV) is the most frequently activated virus in urological setting, and, therefore, its elimination is a primary aim in HC treatment [8-12].
Some preclinical evidence for intravenous immunoglobulin (IVIG) effectiveness was reported earlier. Parmjeet S Randhawa et al. have demonstrated an ability of commercially available IVIG to neutralize BKPyV in human and mice cell cultures [13]. E.g., a 5-gram IVIG volume (one standard bottle) was able to inactivate 1.9*106 BKPyV/mL of plasma in 5.000 ml of blood, thus roughly corresponding to mean circulating blood volume in adults. It was, therefore, supposed that these preparations contain sufficient amounts of BKPyV-specific antibodies to neutralize clinically significant viral loads.
There is also some clinical evidence of IVIG effectiveness in BK virus nephropathy following renal transplantation. The latter condition has much common in origin with HC post allo-HSCT since it also occurs in immunocompromised host with preexisting urinary system lesions. The current treatment approaches for BKPyV nephropathy are immunosuppression reduction and IVIG administration at a total dose of 2 g/kg over 2-5 consecutive days [14, 15].
No standard strategy is, however, developed for HC treatment after allo-HSCT, as all the published data on IVIG treatment in these settings are limited to several case reports [16]. Despite the lack of available data, IVIG may be regarded as a feasible option based on its favorable safety profile and potential clinical efficiency [6].
It is even more notable, since some other established treatment options, e.g. intravenous Cidofovir infusions (CII evidence level) are, besides being potentially nephrotoxic, not currently available in Russian Federation. Meanwhile, the IVIG efficiency in HC has not been previously evaluated, although it is routinely used in our practice.
The current study is, therefore, aimed at evaluating the efficiency of IVIG infusions in HC patients after allo-HSCT, taking into account such additional clinical variables as conditioning regimen intensity and GvHD prophylaxis used.
Materials and methods
A total of 1037 allo-HSCT recipients transplanted in R. M. Gor- bacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included to the retrospective open single-center cohort study. Hemorrhagic cystitis (HC) was registered in 118 cases (11.4%). According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included the patients with standard HC treatment with addition of IVIG; the control group (n=48) received standard HC therapy only. Patients developing allegedly cytotoxic HC before Day+7 post allo-HSCT and second allo-HSCT recipients were excluded from the analysis. Patients in the intervention group were also subdivided based on IVIG therapy timing, dose and duration. Only those patients were included who received IVIG for at least 3 days at the doses of >400 mg per kg, being administered not later than three days since HC onset.
HC diagnosis was based on the presence of hematuria and dysuria. The laboratory criteria included at least one episode of high-grade hematuria (>50 red blood cells per high-power field). The presence of GvHD I-IV was considered as an additional risk factor in statistical evaluation. The standard HC treatment, which all patients received, consisted of intensive iv fluids with forced diuresis, NSAIDs and antispasmodics. Elastic urinary catheters were used in cases of high-risk bladder tamponade, in order to provide bladder washing and evacuation of blood clots.
In the intervention group, we used a commercially available IVIG (Immunovenin® 5%, 50 mg/mL), manufactured by Microgen, Russia. The IVIG treatment began within the first three days since the HC onset, in most patients it was started within 24 hours. Estimation of treatment effectiveness was based on HC symptoms duration. These results were compared to the ones in the control group, which did not receive IVIG. Additional risk factors included conditioning regimen intensity, GvHD prophylaxis, and presence of GvHD.
Statistical evaluation
The data was analyzed with SPSS Statistics v.23 software. The groups were characterized via qualitative characteristics, which were grouped for comparison via chi-square test in conjugated tables. One sample Kolmogorov-Smirnov test used for testing if a variable follows a given distribution in a population. Mann-Whitney U test was used to compare outcomes between two independent groups. To compare HC duration depending on different factors (IVIG therapy, conditioning regimen intensity, and presence of GvHD) Kaplan-Meier curves were used. HC duration was a median (95% CI) according Kaplan-Meier test. The correlation strength was evaluated via log rank test. All values with significant correlation were combined as Cox regression model for multivariate analysis. The p values of ≤0.05 were considered statistically significant.
Results
The HC development rate in the total allo-HSCT recipient group (intervention and control) was 11.4% (n=118). Only 90 patients matched all the above clinical criteria and were included in the analysis, 42 of them (46.7%) fell into the intervention group (with IVIG), and 48 (53.3%), into control group. The median HC duration in common group was 21 days.
Both groups were balanced by gender, diagnosis (malignant and non-malignant conditions), donor type, hematopoietic stem cells (HSC) source, number of patients with clinical signs of GvHD at first signs of HC. The groups were different by two parameters: median age (17 in the intervention group, and 23 in control group, p=0.02), and underlying malignant conditions spectrum (more ALL patients among IVIG recipients, and more AML patients in control group).
The majority of patients received allo-HSCT from unrelated donor (70.8% in the intervention group, and 71% in control group, respectively). There was no significant difference in HSC source between the groups. Bone marrow (BM) and peripheral blood stem cells (PBSC) were used 43.7% and 56.3% in the intervention group, and 45.2% and 54.8% allo-HSCT cases in control group, respectively. The patients’ characteristics are presented in Table 1.
Table 1. Characteristics of patients with hemorrhagic cystitis in IVIG-treated and control groups

A total IVIG dose ranged from 1.2 g/kg to 4 g/kg was used (usually 400 mg/kg/day for 3 consecutive days, except one patient with 10-days IVIG treatment) with a median single dose of 1.2 g/kg and a median total dose of 35 g per patient. There was no statistically significant difference in HC duration between groups with the median of 24 days (16, 32; 95% CI) in the intervention group and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39; Fig. 1).
The main factors affecting HC duration were conditioning regimen intensity, presence of GvHD at the moment of the first HC signs. The median HC duration in MAC and RIC recipients were 35 days (18, 52; 95% CI) and 17 days (14, 20; 95% CI), respectively (p<0.01; Fig. 1).
The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with, and 18 days (15, 21; 95% CI) in patients without active GvHD (p=0.013).

Figure 1. Univariate analysis (Kaplan-Meier method) of HC cumulative incidence in the intervention group and control group, then based on conditioning regimen intensity (MAC vs RIC), and presence of GvHD I-IV at the time of HC manifestation
The multivariate analysis was performed by Cox proportional hazard regression model including the following factors: IVIG vs no IVIG in therapy regimen, conditioning regimen intensity (MAC vs RIC), and presence of HC at the time of HC symptoms onset. It has shown MAC and GvHD presence to be independent risk factors associated with longer HC duration (Table 2).
Table 2. Cox proportional hazard regression model data for the three studied clinical parameters in HSCT patients with hemorrhagic cystitis

Discussion
While there was no statistically significant difference between cohorts in our study, it had some significant limitations, which could compromise the results: the retrospective design of the study, single-center experience and unknown HC etiology; varied IVIG doses – the median single dose was 1.2 g/kg, which is significantly lower than dose recommended for patients with BKPyV-associated nephropathy (2 g/kg) [14, 15]. Therefore, the IVIG therapy at the dose recommended for BKPyV-associated nephropathy may still be effective.
According to World Health Organization Model Formulary 2008, Chapter – IVIG: "Formulations from different manufacturers vary and should not be regarded as equivalent" [17]. IVIG can have significant differences not only between manufacturers and commercial brands, but also between series of the same drug, which is well discussed by Nathaniel Washburn et al. It is a challenge to assess the influence of these data on clinical effectiveness, especially when IVIG mechanism of action in HC is partly unclear. Also there is lack of data in drug’s instructions for use, given by manufacturers about IVIG class and mechanism of action [18]. The prospective study design involving higher IVIG doses (at least 2 g/kg) and from different manufactures could be more exemplary.
According to ECIL (European Conference on Infections in Leukaemia) 2018 guidelines on post allo-HSCT BK-associated HC, IVIG therapy is not recommended as a routine treatment option and graded experimental among such methods as intravesical Cidofovir or Sodium Hyaluronate infusions, iv estrogens, mesenchymal cells etc. This is explained by current lack of evidence with most evident-based method (AIII level) being best supportive care consisting of hydratation, platelets transfusion and pain control [6]. Our data is also not yet sufficient to recommend IVIG as a standard treatment option.
Many researches have demonstrated the association between conditioning regimen intensity and HC incidence, some of them presumed cyclophosphamide toxicity, but this link has not always been confirmed [19-25]. The reasons for GvHD association with HC development are not yet completely clear. The direct immune damage hypothesis has not been proved [26, 27]. However, as GvHD treatment causes additional severe immunosuppression, viral reactivation and active replication may appear which may be a direct cause of HC [28]. We demonstrated the association between certain risk factors (condition regimen intensity and concurrent GvHD) and not only HC incidence, but also its duration, which may be due to stronger immunosuppression in these conditions.
Conclusions
Our data didn’t prove superior therapeutic effect of 1.2 g/kg IVIG (Immunovenin® 5% (50mg/ml) by Microgen) in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. A further prospective study is needed to make final conclusions on method’s effectiveness.
Conflict of interest
None declared.
References
Introduction
Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo-HSCT) with incidence of 9% to 31% for different cohorts [1-7]. Early clinical form of HC usually develops within ten days post allo-HSCT. In this cases HC is considered a direct consequence of cytotoxic conditioning therapy and graft-versus-host disease (GvHD) prophylaxis [8]. Along with cytotoxic drugs, some viral agents, e.g., BK- and JC- polyomaviruses, adenovirus and cytomegalovirus may be also involved in HC pathogenesis. Among them, human BK-polyomavirus (BKPyV) is the most frequently activated virus in urological setting, and, therefore, its elimination is a primary aim in HC treatment [8-12].
Some preclinical evidence for intravenous immunoglobulin (IVIG) effectiveness was reported earlier. Parmjeet S Randhawa et al. have demonstrated an ability of commercially available IVIG to neutralize BKPyV in human and mice cell cultures [13]. E.g., a 5-gram IVIG volume (one standard bottle) was able to inactivate 1.9*106 BKPyV/mL of plasma in 5.000 ml of blood, thus roughly corresponding to mean circulating blood volume in adults. It was, therefore, supposed that these preparations contain sufficient amounts of BKPyV-specific antibodies to neutralize clinically significant viral loads.
There is also some clinical evidence of IVIG effectiveness in BK virus nephropathy following renal transplantation. The latter condition has much common in origin with HC post allo-HSCT since it also occurs in immunocompromised host with preexisting urinary system lesions. The current treatment approaches for BKPyV nephropathy are immunosuppression reduction and IVIG administration at a total dose of 2 g/kg over 2-5 consecutive days [14, 15].
No standard strategy is, however, developed for HC treatment after allo-HSCT, as all the published data on IVIG treatment in these settings are limited to several case reports [16]. Despite the lack of available data, IVIG may be regarded as a feasible option based on its favorable safety profile and potential clinical efficiency [6].
It is even more notable, since some other established treatment options, e.g. intravenous Cidofovir infusions (CII evidence level) are, besides being potentially nephrotoxic, not currently available in Russian Federation. Meanwhile, the IVIG efficiency in HC has not been previously evaluated, although it is routinely used in our practice.
The current study is, therefore, aimed at evaluating the efficiency of IVIG infusions in HC patients after allo-HSCT, taking into account such additional clinical variables as conditioning regimen intensity and GvHD prophylaxis used.
Materials and methods
A total of 1037 allo-HSCT recipients transplanted in R. M. Gor- bacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included to the retrospective open single-center cohort study. Hemorrhagic cystitis (HC) was registered in 118 cases (11.4%). According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included the patients with standard HC treatment with addition of IVIG; the control group (n=48) received standard HC therapy only. Patients developing allegedly cytotoxic HC before Day+7 post allo-HSCT and second allo-HSCT recipients were excluded from the analysis. Patients in the intervention group were also subdivided based on IVIG therapy timing, dose and duration. Only those patients were included who received IVIG for at least 3 days at the doses of >400 mg per kg, being administered not later than three days since HC onset.
HC diagnosis was based on the presence of hematuria and dysuria. The laboratory criteria included at least one episode of high-grade hematuria (>50 red blood cells per high-power field). The presence of GvHD I-IV was considered as an additional risk factor in statistical evaluation. The standard HC treatment, which all patients received, consisted of intensive iv fluids with forced diuresis, NSAIDs and antispasmodics. Elastic urinary catheters were used in cases of high-risk bladder tamponade, in order to provide bladder washing and evacuation of blood clots.
In the intervention group, we used a commercially available IVIG (Immunovenin® 5%, 50 mg/mL), manufactured by Microgen, Russia. The IVIG treatment began within the first three days since the HC onset, in most patients it was started within 24 hours. Estimation of treatment effectiveness was based on HC symptoms duration. These results were compared to the ones in the control group, which did not receive IVIG. Additional risk factors included conditioning regimen intensity, GvHD prophylaxis, and presence of GvHD.
Statistical evaluation
The data was analyzed with SPSS Statistics v.23 software. The groups were characterized via qualitative characteristics, which were grouped for comparison via chi-square test in conjugated tables. One sample Kolmogorov-Smirnov test used for testing if a variable follows a given distribution in a population. Mann-Whitney U test was used to compare outcomes between two independent groups. To compare HC duration depending on different factors (IVIG therapy, conditioning regimen intensity, and presence of GvHD) Kaplan-Meier curves were used. HC duration was a median (95% CI) according Kaplan-Meier test. The correlation strength was evaluated via log rank test. All values with significant correlation were combined as Cox regression model for multivariate analysis. The p values of ≤0.05 were considered statistically significant.
Results
The HC development rate in the total allo-HSCT recipient group (intervention and control) was 11.4% (n=118). Only 90 patients matched all the above clinical criteria and were included in the analysis, 42 of them (46.7%) fell into the intervention group (with IVIG), and 48 (53.3%), into control group. The median HC duration in common group was 21 days.
Both groups were balanced by gender, diagnosis (malignant and non-malignant conditions), donor type, hematopoietic stem cells (HSC) source, number of patients with clinical signs of GvHD at first signs of HC. The groups were different by two parameters: median age (17 in the intervention group, and 23 in control group, p=0.02), and underlying malignant conditions spectrum (more ALL patients among IVIG recipients, and more AML patients in control group).
The majority of patients received allo-HSCT from unrelated donor (70.8% in the intervention group, and 71% in control group, respectively). There was no significant difference in HSC source between the groups. Bone marrow (BM) and peripheral blood stem cells (PBSC) were used 43.7% and 56.3% in the intervention group, and 45.2% and 54.8% allo-HSCT cases in control group, respectively. The patients’ characteristics are presented in Table 1.
Table 1. Characteristics of patients with hemorrhagic cystitis in IVIG-treated and control groups

A total IVIG dose ranged from 1.2 g/kg to 4 g/kg was used (usually 400 mg/kg/day for 3 consecutive days, except one patient with 10-days IVIG treatment) with a median single dose of 1.2 g/kg and a median total dose of 35 g per patient. There was no statistically significant difference in HC duration between groups with the median of 24 days (16, 32; 95% CI) in the intervention group and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39; Fig. 1).
The main factors affecting HC duration were conditioning regimen intensity, presence of GvHD at the moment of the first HC signs. The median HC duration in MAC and RIC recipients were 35 days (18, 52; 95% CI) and 17 days (14, 20; 95% CI), respectively (p<0.01; Fig. 1).
The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with, and 18 days (15, 21; 95% CI) in patients without active GvHD (p=0.013).

Figure 1. Univariate analysis (Kaplan-Meier method) of HC cumulative incidence in the intervention group and control group, then based on conditioning regimen intensity (MAC vs RIC), and presence of GvHD I-IV at the time of HC manifestation
The multivariate analysis was performed by Cox proportional hazard regression model including the following factors: IVIG vs no IVIG in therapy regimen, conditioning regimen intensity (MAC vs RIC), and presence of HC at the time of HC symptoms onset. It has shown MAC and GvHD presence to be independent risk factors associated with longer HC duration (Table 2).
Table 2. Cox proportional hazard regression model data for the three studied clinical parameters in HSCT patients with hemorrhagic cystitis

Discussion
While there was no statistically significant difference between cohorts in our study, it had some significant limitations, which could compromise the results: the retrospective design of the study, single-center experience and unknown HC etiology; varied IVIG doses – the median single dose was 1.2 g/kg, which is significantly lower than dose recommended for patients with BKPyV-associated nephropathy (2 g/kg) [14, 15]. Therefore, the IVIG therapy at the dose recommended for BKPyV-associated nephropathy may still be effective.
According to World Health Organization Model Formulary 2008, Chapter – IVIG: "Formulations from different manufacturers vary and should not be regarded as equivalent" [17]. IVIG can have significant differences not only between manufacturers and commercial brands, but also between series of the same drug, which is well discussed by Nathaniel Washburn et al. It is a challenge to assess the influence of these data on clinical effectiveness, especially when IVIG mechanism of action in HC is partly unclear. Also there is lack of data in drug’s instructions for use, given by manufacturers about IVIG class and mechanism of action [18]. The prospective study design involving higher IVIG doses (at least 2 g/kg) and from different manufactures could be more exemplary.
According to ECIL (European Conference on Infections in Leukaemia) 2018 guidelines on post allo-HSCT BK-associated HC, IVIG therapy is not recommended as a routine treatment option and graded experimental among such methods as intravesical Cidofovir or Sodium Hyaluronate infusions, iv estrogens, mesenchymal cells etc. This is explained by current lack of evidence with most evident-based method (AIII level) being best supportive care consisting of hydratation, platelets transfusion and pain control [6]. Our data is also not yet sufficient to recommend IVIG as a standard treatment option.
Many researches have demonstrated the association between conditioning regimen intensity and HC incidence, some of them presumed cyclophosphamide toxicity, but this link has not always been confirmed [19-25]. The reasons for GvHD association with HC development are not yet completely clear. The direct immune damage hypothesis has not been proved [26, 27]. However, as GvHD treatment causes additional severe immunosuppression, viral reactivation and active replication may appear which may be a direct cause of HC [28]. We demonstrated the association between certain risk factors (condition regimen intensity and concurrent GvHD) and not only HC incidence, but also its duration, which may be due to stronger immunosuppression in these conditions.
Conclusions
Our data didn’t prove superior therapeutic effect of 1.2 g/kg IVIG (Immunovenin® 5% (50mg/ml) by Microgen) in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. A further prospective study is needed to make final conclusions on method’s effectiveness.
Conflict of interest
None declared.
References
Александр А. Щербаков, Алена Н. Зайцева, Максим А. Кучер, Ирина С. Ярушкина, Ольга Н. Зацепина, Екатерина С. Кульнева, Олеся В. Паина, Руслана В. Клементьева, Олег В. Голощапов, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26399" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26400" ["VALUE"]=> array(2) { ["TEXT"]=> string(4154) "<p style="text-align: justify;"> Геморрагический цистит (ГЦ) – это частое осложнение при аллогенной трансплантации гемопоэтических стволовых клеток (алло-ТГСК). Персистирование BK полиомавируса (BKPyV) является одной из основных предполагаемых причин, приводящих к развитию позднего ГЦ после алло-ТГСК. Имеются доклинические данные об эффективной нейтрализации BKPyV внутривенным иммуноглобулином G (ВВИГ) в клеточных культурах, а также при BK вирусной нефропатии при трансплантации почки. </p> <h3>Пациенты и методы</h3> <p style="text-align: justify;"> Ретроспективно было изучено 1037 пациентов получивших алло-ТГСК в НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой с 2013 по 2018 год. ГЦ был зарегистрирован в 118 (11,4%) случаях, из них, согласно критериям включения, 90 пациентов включены в анализ: группа сравнения с ВВИГ (n=42) и контрольная группа (n=48) со стандартной терапией ГЦ. </p> <h3>Результаты</h3> <p style="text-align: justify;"> Продолжительность ГЦ в общей группе составила 21 день. Не было статистически значимой разницы в продолжительности ГЦ между группой сравнения и контрольной группой – медиана 24 дня (16, 32; 95% CI) и 24 дня (2, 46; 95% CI), соответственно (p=0,39). Продолжительность ГЦ при миелоаблативном режиме кондиционирования составила 35 дней (18, 52; 95% ДИ) и 17 дней (14, 20; 95% ДИ) при немиелоаблативном режиме кондиционирования, р <0,01. Одновременное наличие симптомов ГЦ и РТПХ I-IV степени, также характеризовалось положительной корреляцией с длительностью ГЦ – 36 дней (22, 50; 95% ДИ) у пациентов с РТПХ и 18 дней (15, 21; 95% ДИ) у пациентов без РТПХ, р=0,013. </p> <h3>Выводы</h3> <p style="text-align: justify;"> Настоящее исследование не подтвердило клиническую эффективность ВВИГ (Иммуновенин 5% (50мг/мл) Микроген) в дозе 1,2 гр/кг у пациентов с ГЦ после алло-ТГСК. Факторами риска более длительного течения ГЦ были интенсивность режима кондиционирования и наличие РТПХ I-IV степени. Требуется проведение проспективного исследования для определения эффективности ВВИГ в качестве терапии позднего ГЦ после алло-ТГСК. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Аллогенная трансплантация гемопоэтических стволовых клеток, геморрагический цистит, внутривенный иммуноглобулин, ВВИГ, факторы риска, реакция «трансплантат против хозяина» (РТПХ). </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3992) "Геморрагический цистит (ГЦ) – это частое осложнение при аллогенной трансплантации гемопоэтических стволовых клеток (алло-ТГСК). Персистирование BK полиомавируса (BKPyV) является одной из основных предполагаемых причин, приводящих к развитию позднего ГЦ после алло-ТГСК. Имеются доклинические данные об эффективной нейтрализации BKPyV внутривенным иммуноглобулином G (ВВИГ) в клеточных культурах, а также при BK вирусной нефропатии при трансплантации почки.
Пациенты и методы
Ретроспективно было изучено 1037 пациентов получивших алло-ТГСК в НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой с 2013 по 2018 год. ГЦ был зарегистрирован в 118 (11,4%) случаях, из них, согласно критериям включения, 90 пациентов включены в анализ: группа сравнения с ВВИГ (n=42) и контрольная группа (n=48) со стандартной терапией ГЦ.
Результаты
Продолжительность ГЦ в общей группе составила 21 день. Не было статистически значимой разницы в продолжительности ГЦ между группой сравнения и контрольной группой – медиана 24 дня (16, 32; 95% CI) и 24 дня (2, 46; 95% CI), соответственно (p=0,39). Продолжительность ГЦ при миелоаблативном режиме кондиционирования составила 35 дней (18, 52; 95% ДИ) и 17 дней (14, 20; 95% ДИ) при немиелоаблативном режиме кондиционирования, р <0,01. Одновременное наличие симптомов ГЦ и РТПХ I-IV степени, также характеризовалось положительной корреляцией с длительностью ГЦ – 36 дней (22, 50; 95% ДИ) у пациентов с РТПХ и 18 дней (15, 21; 95% ДИ) у пациентов без РТПХ, р=0,013.
Выводы
Настоящее исследование не подтвердило клиническую эффективность ВВИГ (Иммуновенин 5% (50мг/мл) Микроген) в дозе 1,2 гр/кг у пациентов с ГЦ после алло-ТГСК. Факторами риска более длительного течения ГЦ были интенсивность режима кондиционирования и наличие РТПХ I-IV степени. Требуется проведение проспективного исследования для определения эффективности ВВИГ в качестве терапии позднего ГЦ после алло-ТГСК.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, геморрагический цистит, внутривенный иммуноглобулин, ВВИГ, факторы риска, реакция «трансплантат против хозяина» (РТПХ).
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26401" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-60-66" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-60-66" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26404" ["VALUE"]=> array(2) { ["TEXT"]=> string(378) "<p>Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina, Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev, Ludmila S. Zubarovskaya,<br> <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(338) "Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina,
Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev,
Ludmila S. Zubarovskaya,
Boris V. Afanasyev
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Alexander A. Shcherbakov, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(904) 604 0884
E-mail: xihmrx@gmail.com
Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo- HSCT). Human BK-polyomavirus (BKPyV) may be one of the main agents found in late HC developing after allo-HSCT. In previous studies, intravenous immunoglobulin (IVIG) preparations were shown to neutralize BKPyV in mice cell cultures and to reduce BK virus nephropathy in kidney transplantation. The aim of current study was to evaluate efficiency of HC treatment with IVIG in allo-HSCT recipients, with respect to conditioning regimen intensity and presence of graft-versus-host disease (GvHD).
Patients and methods
A total of 1037 allo-HSCT recipients transplanted in R. M. Gorbacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included into retrospective open single-center cohort study. HC was registered in 118 (11.4%) cases. According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included patients with standard HC treatment with addition of IVIG; the control group (n=48) – with standard HC therapy only.
Results
The median HC duration in common group (IVIG and control) was 21 days. There was no statistically significant difference in HC duration between the two groups, with the median of 24 days (16, 32; 95% CI) in the intervention group (standard therapy + IVIG), and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39). The median HC duration in MAC and RIC was 35 days (18, 52; 95% CI) versus 17 days (14, 20; 95% CI), respectively (p<0.01). The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with GvHD and 18 days (15, 21; 95% CI) in patients without GvHD (p=0.013).
Conclusions
Our data didn’t show any clinical efficiency of 1.2 g/kg IVIG (Immunovenin® 5%, 50mg/mL by Microgen) effectiveness in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. The further prospective study is needed to make final conclusions on method’s effectiveness.
Keywords
Allogeneic hematopoietic stem cell transplantation, hemorrhagic cystitis, intravenous immunoglobulin, IVIG, risk factors, graft-versus-host disease (GvHD).
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26402" ["VALUE"]=> string(119) "Intravenous immunoglobulin G treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(119) "Intravenous immunoglobulin G treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26403" ["VALUE"]=> string(4) "1987" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1987" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26406" ["VALUE"]=> string(4) "1988" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1988" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26404" ["VALUE"]=> array(2) { ["TEXT"]=> string(378) "<p>Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina, Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev, Ludmila S. Zubarovskaya,<br> <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(338) "Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina,
Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev,
Ludmila S. Zubarovskaya,
Boris V. Afanasyev
Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina,
Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev,
Ludmila S. Zubarovskaya,
Boris V. Afanasyev
Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo- HSCT). Human BK-polyomavirus (BKPyV) may be one of the main agents found in late HC developing after allo-HSCT. In previous studies, intravenous immunoglobulin (IVIG) preparations were shown to neutralize BKPyV in mice cell cultures and to reduce BK virus nephropathy in kidney transplantation. The aim of current study was to evaluate efficiency of HC treatment with IVIG in allo-HSCT recipients, with respect to conditioning regimen intensity and presence of graft-versus-host disease (GvHD).
Patients and methods
A total of 1037 allo-HSCT recipients transplanted in R. M. Gorbacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included into retrospective open single-center cohort study. HC was registered in 118 (11.4%) cases. According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included patients with standard HC treatment with addition of IVIG; the control group (n=48) – with standard HC therapy only.
Results
The median HC duration in common group (IVIG and control) was 21 days. There was no statistically significant difference in HC duration between the two groups, with the median of 24 days (16, 32; 95% CI) in the intervention group (standard therapy + IVIG), and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39). The median HC duration in MAC and RIC was 35 days (18, 52; 95% CI) versus 17 days (14, 20; 95% CI), respectively (p<0.01). The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with GvHD and 18 days (15, 21; 95% CI) in patients without GvHD (p=0.013).
Conclusions
Our data didn’t show any clinical efficiency of 1.2 g/kg IVIG (Immunovenin® 5%, 50mg/mL by Microgen) effectiveness in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. The further prospective study is needed to make final conclusions on method’s effectiveness.
Keywords
Allogeneic hematopoietic stem cell transplantation, hemorrhagic cystitis, intravenous immunoglobulin, IVIG, risk factors, graft-versus-host disease (GvHD).
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2654) "Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo- HSCT). Human BK-polyomavirus (BKPyV) may be one of the main agents found in late HC developing after allo-HSCT. In previous studies, intravenous immunoglobulin (IVIG) preparations were shown to neutralize BKPyV in mice cell cultures and to reduce BK virus nephropathy in kidney transplantation. The aim of current study was to evaluate efficiency of HC treatment with IVIG in allo-HSCT recipients, with respect to conditioning regimen intensity and presence of graft-versus-host disease (GvHD).
Patients and methods
A total of 1037 allo-HSCT recipients transplanted in R. M. Gorbacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included into retrospective open single-center cohort study. HC was registered in 118 (11.4%) cases. According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included patients with standard HC treatment with addition of IVIG; the control group (n=48) – with standard HC therapy only.
Results
The median HC duration in common group (IVIG and control) was 21 days. There was no statistically significant difference in HC duration between the two groups, with the median of 24 days (16, 32; 95% CI) in the intervention group (standard therapy + IVIG), and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39). The median HC duration in MAC and RIC was 35 days (18, 52; 95% CI) versus 17 days (14, 20; 95% CI), respectively (p<0.01). The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with GvHD and 18 days (15, 21; 95% CI) in patients without GvHD (p=0.013).
Conclusions
Our data didn’t show any clinical efficiency of 1.2 g/kg IVIG (Immunovenin® 5%, 50mg/mL by Microgen) effectiveness in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. The further prospective study is needed to make final conclusions on method’s effectiveness.
Keywords
Allogeneic hematopoietic stem cell transplantation, hemorrhagic cystitis, intravenous immunoglobulin, IVIG, risk factors, graft-versus-host disease (GvHD).
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26401" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-60-66" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-60-66" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-60-66" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26402" ["VALUE"]=> string(119) "Intravenous immunoglobulin G treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(119) "Intravenous immunoglobulin G treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(119) "Intravenous immunoglobulin G treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26405" ["VALUE"]=> array(2) { ["TEXT"]=> string(521) "<p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br> St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Alexander A. Shcherbakov, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7(904) 604 0884<br> E-mail: xihmrx@gmail.com</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(455) "Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Alexander A. Shcherbakov, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(904) 604 0884
E-mail: xihmrx@gmail.com
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Alexander A. Shcherbakov, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(904) 604 0884
E-mail: xihmrx@gmail.com
Александр А. Щербаков, Алена Н. Зайцева, Максим А. Кучер, Ирина С. Ярушкина, Ольга Н. Зацепина, Екатерина С. Кульнева, Олеся В. Паина, Руслана В. Клементьева, Олег В. Голощапов, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(504) "Александр А. Щербаков, Алена Н. Зайцева, Максим А. Кучер, Ирина С. Ярушкина, Ольга Н. Зацепина, Екатерина С. Кульнева, Олеся В. Паина, Руслана В. Клементьева, Олег В. Голощапов, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26396" ["VALUE"]=> string(22) "02/19/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/19/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "02/19/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26397" ["VALUE"]=> string(22) "03/28/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/28/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/28/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26400" ["VALUE"]=> array(2) { ["TEXT"]=> string(4154) "<p style="text-align: justify;"> Геморрагический цистит (ГЦ) – это частое осложнение при аллогенной трансплантации гемопоэтических стволовых клеток (алло-ТГСК). Персистирование BK полиомавируса (BKPyV) является одной из основных предполагаемых причин, приводящих к развитию позднего ГЦ после алло-ТГСК. Имеются доклинические данные об эффективной нейтрализации BKPyV внутривенным иммуноглобулином G (ВВИГ) в клеточных культурах, а также при BK вирусной нефропатии при трансплантации почки. </p> <h3>Пациенты и методы</h3> <p style="text-align: justify;"> Ретроспективно было изучено 1037 пациентов получивших алло-ТГСК в НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой с 2013 по 2018 год. ГЦ был зарегистрирован в 118 (11,4%) случаях, из них, согласно критериям включения, 90 пациентов включены в анализ: группа сравнения с ВВИГ (n=42) и контрольная группа (n=48) со стандартной терапией ГЦ. </p> <h3>Результаты</h3> <p style="text-align: justify;"> Продолжительность ГЦ в общей группе составила 21 день. Не было статистически значимой разницы в продолжительности ГЦ между группой сравнения и контрольной группой – медиана 24 дня (16, 32; 95% CI) и 24 дня (2, 46; 95% CI), соответственно (p=0,39). Продолжительность ГЦ при миелоаблативном режиме кондиционирования составила 35 дней (18, 52; 95% ДИ) и 17 дней (14, 20; 95% ДИ) при немиелоаблативном режиме кондиционирования, р <0,01. Одновременное наличие симптомов ГЦ и РТПХ I-IV степени, также характеризовалось положительной корреляцией с длительностью ГЦ – 36 дней (22, 50; 95% ДИ) у пациентов с РТПХ и 18 дней (15, 21; 95% ДИ) у пациентов без РТПХ, р=0,013. </p> <h3>Выводы</h3> <p style="text-align: justify;"> Настоящее исследование не подтвердило клиническую эффективность ВВИГ (Иммуновенин 5% (50мг/мл) Микроген) в дозе 1,2 гр/кг у пациентов с ГЦ после алло-ТГСК. Факторами риска более длительного течения ГЦ были интенсивность режима кондиционирования и наличие РТПХ I-IV степени. Требуется проведение проспективного исследования для определения эффективности ВВИГ в качестве терапии позднего ГЦ после алло-ТГСК. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Аллогенная трансплантация гемопоэтических стволовых клеток, геморрагический цистит, внутривенный иммуноглобулин, ВВИГ, факторы риска, реакция «трансплантат против хозяина» (РТПХ). </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3992) "Геморрагический цистит (ГЦ) – это частое осложнение при аллогенной трансплантации гемопоэтических стволовых клеток (алло-ТГСК). Персистирование BK полиомавируса (BKPyV) является одной из основных предполагаемых причин, приводящих к развитию позднего ГЦ после алло-ТГСК. Имеются доклинические данные об эффективной нейтрализации BKPyV внутривенным иммуноглобулином G (ВВИГ) в клеточных культурах, а также при BK вирусной нефропатии при трансплантации почки.
Пациенты и методы
Ретроспективно было изучено 1037 пациентов получивших алло-ТГСК в НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой с 2013 по 2018 год. ГЦ был зарегистрирован в 118 (11,4%) случаях, из них, согласно критериям включения, 90 пациентов включены в анализ: группа сравнения с ВВИГ (n=42) и контрольная группа (n=48) со стандартной терапией ГЦ.
Результаты
Продолжительность ГЦ в общей группе составила 21 день. Не было статистически значимой разницы в продолжительности ГЦ между группой сравнения и контрольной группой – медиана 24 дня (16, 32; 95% CI) и 24 дня (2, 46; 95% CI), соответственно (p=0,39). Продолжительность ГЦ при миелоаблативном режиме кондиционирования составила 35 дней (18, 52; 95% ДИ) и 17 дней (14, 20; 95% ДИ) при немиелоаблативном режиме кондиционирования, р <0,01. Одновременное наличие симптомов ГЦ и РТПХ I-IV степени, также характеризовалось положительной корреляцией с длительностью ГЦ – 36 дней (22, 50; 95% ДИ) у пациентов с РТПХ и 18 дней (15, 21; 95% ДИ) у пациентов без РТПХ, р=0,013.
Выводы
Настоящее исследование не подтвердило клиническую эффективность ВВИГ (Иммуновенин 5% (50мг/мл) Микроген) в дозе 1,2 гр/кг у пациентов с ГЦ после алло-ТГСК. Факторами риска более длительного течения ГЦ были интенсивность режима кондиционирования и наличие РТПХ I-IV степени. Требуется проведение проспективного исследования для определения эффективности ВВИГ в качестве терапии позднего ГЦ после алло-ТГСК.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, геморрагический цистит, внутривенный иммуноглобулин, ВВИГ, факторы риска, реакция «трансплантат против хозяина» (РТПХ).
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3992) "Геморрагический цистит (ГЦ) – это частое осложнение при аллогенной трансплантации гемопоэтических стволовых клеток (алло-ТГСК). Персистирование BK полиомавируса (BKPyV) является одной из основных предполагаемых причин, приводящих к развитию позднего ГЦ после алло-ТГСК. Имеются доклинические данные об эффективной нейтрализации BKPyV внутривенным иммуноглобулином G (ВВИГ) в клеточных культурах, а также при BK вирусной нефропатии при трансплантации почки.
Пациенты и методы
Ретроспективно было изучено 1037 пациентов получивших алло-ТГСК в НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой с 2013 по 2018 год. ГЦ был зарегистрирован в 118 (11,4%) случаях, из них, согласно критериям включения, 90 пациентов включены в анализ: группа сравнения с ВВИГ (n=42) и контрольная группа (n=48) со стандартной терапией ГЦ.
Результаты
Продолжительность ГЦ в общей группе составила 21 день. Не было статистически значимой разницы в продолжительности ГЦ между группой сравнения и контрольной группой – медиана 24 дня (16, 32; 95% CI) и 24 дня (2, 46; 95% CI), соответственно (p=0,39). Продолжительность ГЦ при миелоаблативном режиме кондиционирования составила 35 дней (18, 52; 95% ДИ) и 17 дней (14, 20; 95% ДИ) при немиелоаблативном режиме кондиционирования, р <0,01. Одновременное наличие симптомов ГЦ и РТПХ I-IV степени, также характеризовалось положительной корреляцией с длительностью ГЦ – 36 дней (22, 50; 95% ДИ) у пациентов с РТПХ и 18 дней (15, 21; 95% ДИ) у пациентов без РТПХ, р=0,013.
Выводы
Настоящее исследование не подтвердило клиническую эффективность ВВИГ (Иммуновенин 5% (50мг/мл) Микроген) в дозе 1,2 гр/кг у пациентов с ГЦ после алло-ТГСК. Факторами риска более длительного течения ГЦ были интенсивность режима кондиционирования и наличие РТПХ I-IV степени. Требуется проведение проспективного исследования для определения эффективности ВВИГ в качестве терапии позднего ГЦ после алло-ТГСК.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, геморрагический цистит, внутривенный иммуноглобулин, ВВИГ, факторы риска, реакция «трансплантат против хозяина» (РТПХ).
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26399" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [7]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "144" ["~IBLOCK_SECTION_ID"]=> string(3) "144" ["ID"]=> string(4) "1848" ["~ID"]=> string(4) "1848" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(339) "Рандомизированное исследование тимоглобулина и посттрансплантационного циклофосфана при аллогенной неродственной трансплантации у взрослых с хроническими миелоидными неоплазиями" ["~NAME"]=> string(339) "Рандомизированное исследование тимоглобулина и посттрансплантационного циклофосфана при аллогенной неродственной трансплантации у взрослых с хроническими миелоидными неоплазиями" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "04/28/2020 11:01:35 am" ["~TIMESTAMP_X"]=> string(22) "04/28/2020 11:01:35 am" ["DETAIL_PAGE_URL"]=> string(154) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/randomizirovannoe-issledovanie-timoglobulina-i-posttransplantatsionnogo-tsiklofosfana-pri-allogennoy/" ["~DETAIL_PAGE_URL"]=> string(154) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/randomizirovannoe-issledovanie-timoglobulina-i-posttransplantatsionnogo-tsiklofosfana-pri-allogennoy/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(22436) "Introduction
Myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms (MPN) and chronic myeloid leukemia (CML) comprise about 15% of patients with indication for allogeneic stem cell transplantation (SCT) [1]. Nonetheless SCT in this group of patients is associated with significant non-relapse mortality reaching 26% in advanced chronic myeloid leukemia [2], 32% in myelodysplastic syndrome [3] and 25-36% in various types of MPN [4, 5]. The main reasons of mortality in these diseases are infectious complications, graft-versus-host disease and primary graft failure which is significantly more frequent than in other hematological malignancies [6].
Posttransplant cyclophosphamide (PTCY) is an approach which is gaining popularity for haploidentical (Haplo) and mismatched unrelated donor (MUD) transplantation. This approach in the large retrospective study demonstrated reduced incidence of GvHD, reduced non-relapse mortality and improved GvHD-relapse-free survival (GFRS) compared to in vivo T-cell depletion methods both in Haplo and MUD settings [7, 8]. But most of the supportive data describes acute leukemia patients [9] or MDS/ MPN patients are combined in the analysis with acute leukemia [10]. Thus there is limited data whether PTCY in the group of chronic myeloid neoplasms provide the same benefit as in acute leukemias. Also there was a concern that in the setting of MDS/CML/MPN PTCy might increase the primary graft failure incidence.
The majority of SCTs in MDS and CML patients are unrelated, because of older age and comorbidities of the related donors and better outcomes after transplantation from a young donor [11]. So we conducted a single-center prospective randomized study in unrelated SCT recipients between the standard approach of GvHD prophylaxis with thymoglobulin and PTCY. The other components of GvHD prophylaxis was the same as well as the conditioning and supportive care.
Patients and methods
General parameters of the patients and transplants
The prospective single-center randomized study was conducted in the First Pavlov Medical university. The study was approved by the local Ethical committee and was conducted according to the good clinical practices and Helsinki declaration. All patients signed informed consent to participate in the study and processing of the personal data. The study was registered at clinicaltrials.gov, NCT02627573. The conclusion criteria were the diagnosis of chronic myeloid leukemia or myelodysplastic Syndrome or myeloprolipherative neoplasm unclassified or atypical chronic myelogenous leukemia with indications for SCT and presence of 10/10 HLA-matched unrelated donor. The donor and recipient must be identical by the following genetic loci: HLA-A, HLA-B, HLA-Cw, HLA-DRB1, and HLA-DQB1. Mismatches in these loci were not allowed. Also only patients were included in whom the donor agreed to donate peripheral blood stem cells (PBSC). The exclusion criteria were the presence of severe concurrent illness, prior history of anaphylaxis to thymoglobulin, moderate or severe cardiac dysfunction, left ventricular ejection fraction <50%, moderate or severe decrease in pulmonary function, FEV1 <70% or DLCO <70% of predicted, respiratory distress at least grade I, severe organ dysfunction: AST or ALT >5 upper normal limits, bilirubin >1.5 upper normal limits, creatinine >2 upper normal limits, creatinine clearance <60 mL/min., uncontrolled bacterial or fungal infection at the time of enrollment, requirement for vasopressor support at the time of enrollment, Karnofsky index <30%, pregnancy, and somatic or psychiatric disorder making the patient unable to sign informed consent. The study during 2015-2019 has enrolled 33 patients: 16 in the thymoglobulin arm and 17 in the PTCY arm. The study was terminated prematurely due to poor recruitment. All recruited patients were included in the analysis. The study group was well balanced with no significant differences in characteristics of patients (Table 1).
Table 1. Characteristics of patients

CML = chronic myeloid leukemia, MDS = myelodysplastic syndrome, MPN = myeloproliferative neoplasm, CP = chronic phase, AP = acceleration phase, BC = blast crisis, AEB – access blasts, MLD – multileaneage dysplasia.
Study procedures
All patients received conditioning with fludarabine 180 mg/m2 over 6 days and oral busulfan 10 mg/kg over 3 days. Patients in the thymoglobulin group received GvHD prophylaxis with thymoglobulin 2.5 mg/kg on days -3 and -2, tacrolimus 0.03 mg/kg/day starting day -1 with target concentration 5-15 ng/ml and mycophenolate mofetil (MMF) 30 mg/kg/day starting. The MMF was continued until day +30. Tacrolimus was continued until day +100 and gradually tapered over 1 month in absence of GvHD. Steroids were used in this group as the pre-medication to thymoglobulin and graft infusion. In the PTCy group patients received cyclophosphamide 50 mg/kg on days +3 and +4. Mesna 50 mg/kg was administered as 24-hour infusion on the days of cyclophosphamide administration. MMF 30 mg/kg/day was started on day +5 and continued until day +35. Tacrolimus 0.03 mg/kg/day was started on day +5 with target concentration 5-15 ng/ml. Tacrolimus in this arm was in the same way continued until day +100 and gradually tapered over 1 month in absence of GvHD. Use of steroids was prohibited from day-5 to day+5. The rest of the supportive care was the same in both arms. The standard antifungal prophylaxis was fluconazole starting on day 0 and continued until engraftment.
Clinical outcomes
Time to disease relapse, acute GvHD (GvHD), moderate to severe chronic GvHD (cGvHD), non-relapse mortality (NRM), overall survival (OS), event-free survival (EFS), and GvHD-relapse free survival (GRFS) were defined as the time from transplantation to the event. Incidence of aGvHD was calculated at 125 days after HSCT, and the time frame for the other outcomes was free years. Events for EFS were relapse or death. Events for GRFS were either death, relapse, grades III-IV acute GvHD or systemic therapy-requiring chronic GvHD [12]. Patients were censored at the time of last contact or a second transplantation for all outcomes. Disease relapse was defined as morphologic or cytogenetic evidence of disease with pre-transplantation characteristics. Disease staging, including bone marrow biopsies, was routinely performed on days +30,+60,+100, +180, +365 post-transplant. The Consensus Conference criteria and National Institutes of Health criteria were used for aGvHD and cGvHD grading [13, 14]. Primary graft failure was defined as the complete absence of donor chimerism in bone marrow biopsy by day +40. Time to engraftment was calculated as time from HSCT to unsupported neutrophil count > 500/µl and white blood cell count >1000/µl for 3 consecutive days. Toxicity was assessed with CTCAE ver. 4.03. Sepsis and severe sepsis were diagnosed based on International Guidelines for Management of Severe Sepsis and Septic Shock [15]. Invasive mycosis was diagnosed in case of probable or proven infection according to EORTC/MSG guidelines [16]. The threshold for cytomegalovirus (CMV) reactivation was >500 copies/ml. Clinically significant CMV reactivation was defined as >10000 copies/mL. Veno-occlusive disease was diagnosed and graded based on EBMT criteria [17].
Statistical evaluation
The study primary endpoint was the incidence of primary graft failure. The study was planned to enroll 60 patients, 30 patients in each arm. With the study group size was calculated with 30% margin, 0.65 study power and 0.05 significance using Fisher’s criterion. Secondary endpoints were incidence of acute grade II-IV acute GvHD, incidence moderate and severe chronic GvHD, non-relapse mortality, OS, EFS, GFRS, relapse incidence, toxicity and infectious complications. The strata for randomization was a pretransplant assessment of mortality (PAM) score [18]. The stratification was performed using Mann-Whitney U-criterion to achieve the minimal differences in PAM between the study groups. The final median PAM values were 14.1 (range 7.8-25.2) and 14.1 (6.9-21.9) in the thymoglobulin and PTCY groups, respectively (p=0.98).
Comparison of patient characteristics was performed by Mann-Whitney test. The survival distributions for OS, EFS, GRFS were calculated using Kaplan-Meier methodology. The comparisons were made using the log-rank test. Cumulative incidence analysis with competing risks for aGvHD, cGvHD, relapse incidence and NRM was performed using Gray test. Relapse and NRM were accounted as competing risks. Incidence and severity of complications was compared using Chi-square and Mann-Whitney test. Analyses were conducted in SAS 9.3 (SAS Institute, Inc.).
Results
Median follow up in the study was 29 months (range 7-48). There was no difference in the incidence of primary graft failure: 12.50% (95%CI 2%-38%) in the thymoglobulin arm and 11.8% (95%CI 1%-36%) in the PTCY arm, p=0.95. Three out of four patients with primary graft failure died, one was salvaged with second transplantation. All patients without primary graft failure survived to the day of engraftment. Median time to neutrophil engraftment was longer in the PTCY group: 20 (16-33) vs 16 (11-30) days, p=0.0017. Time to white blood cell engraftment was also significantly longer: 21 (16-33) vs 15 (11-30) days, p=0.0016. No differences were observed in the time to platelet engraftment: median 12 (8-40) vs 15 (11-45) days, p=0.1482.
Acute GvHD grade II-IV was documented in 3 (23%) patients in the thymoglobulin group and 1 (6%) patients in the PTCY group (p=0.20). No cases of acute GvHD grade III-IV were documented. Moderate and severe chronic GvHD was diagnosed in 3 (23%) patient after thymoglobulin and 4 (25%) patients after PTCY (p=0.41). Median time to chronic GvHD was a little shorter after thymoglobulin (5.5 months) compared to PTCY (15 months), p=0.11. One patient in thymoglobulin and 2 patients in the PTCY group had mild chronic GvHD.
Patients after PTCY prophylaxis had significantly better outcomes of transplantation. 4-year OS was 82% (95%CI 55-94%) vs 30% (95%CI 6-59%), p=0.0126; EFS was 61% (95%CI 31-81%) vs 26% (95%CI 5-54%), p=0.0335, GFRS 61% 95%CI (31-81%) vs 16% (95%CI 1-46%), p=0.0072 (Figure 1). There was a comparable incidence of relapse between the groups (25% vs 29%, p=0.77), but significantly reduced non-relapse mortality in the PTCY group (6% vs 38%, p=0.0264), which was main cause of the differences in survival outcomes. Primary graft failure and infectious complications were the only causes of non-relapse mortality in the study groups.

Figure 1. Overall (A), event-free (B) and GvHD-relapse-free (C) survival. The percentages shown are 4-year Kaplan-Mayer estimates
No unexpected toxicity was observed during the study. There was no significant difference in the incidence of early complications (Table 2). No cases of neurotoxicity or veno-occlusive disease were documented during the study. The infusion reactions in the thymoglobulin group were allergic reactions and in the PTCY group – one case of cystalgia.
Table 2. Toxicity and complications observed in ATG and PTCY groups

Note: TA-TMA = transplant-associated microangiopathy. Infusion reaction is defined as any reaction during or immediately after infusion that required change in the systemic therapy
Discussion
The study was originally developed to demonstrate if there is a difference in primary graft failure after PTCY. Despite it was terminated prematurely due to poor recruitment, several approximations could be made. We have observed identical number of graft failures in the thymoglobulin and PTCY group. According to CIBMTR data the average number of primary graft failure is 5.5% and CML and MDS patients have the double risk. So the incidence in the current study was not only the same between groups, but almost identical to the one reported by large registry studies [6]. So it is likely that there is no impact of PTCY on the incidence of primary graft failure. On the other hand, we have not observed any positive impact of PTCY on engraftment in this patient population despite previous preclinical data [19]. Unlike primary graft failure engraftment of neutrophils is delayed by several days which in concordance with previously published data [7].
The study was not powered to capture the significance and survival and recruitment was not completed, but most striking results were obtained in terms of survival outcomes. PTCY prophylaxis was associated with significantly higher incidence of OS, EFS and GRFS. The differences were due to reduced non-relapse mortality, it was only 6% in the PTCY group. These extremely low incidence of NRM was recently confirmed in the retrospective [7] and prospective randomized [20] studies. Despite the situation in haploidentical transplantation where PTCY reduces mortality through more effective prevention of acute and chronic GvHD [8], here we observed identical incidence of both acute and chronic GvHD, but the difference was due to late severe bacterial infections. In this study 5 mg/kg dose of thymoglobulin was used which is reported to cause less profound immunosuppression and promote graft-versus-leukemia effect [21, 22]. However the population of MDS and MPN patients is different from acute leukemia. There is a significant iron overload, which may increase the risk of infections after SCT and thymoglobulin effects might overlap with these negative impacts of iron overload [23]. Otherwise this might be an indication of better immunological recovery after unrelated transplantation with PTCY. Additional studies are required to confirm these assumptions.
Conclusion
Despite incomplete recruitment, this study provides some evidence on the use of PTCY-based prophylaxis for unrelated transplantations in patients with CML, MDS and MPN. The study has demonstrated that there is likely no impact of PTCY on the incidence of primary graft failure. Also it creates the basis for future studies to evaluate survival and immunological recovery in this patient population.
Conflict of interest
No conflict of interest reported.
References
Introduction
Myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms (MPN) and chronic myeloid leukemia (CML) comprise about 15% of patients with indication for allogeneic stem cell transplantation (SCT) [1]. Nonetheless SCT in this group of patients is associated with significant non-relapse mortality reaching 26% in advanced chronic myeloid leukemia [2], 32% in myelodysplastic syndrome [3] and 25-36% in various types of MPN [4, 5]. The main reasons of mortality in these diseases are infectious complications, graft-versus-host disease and primary graft failure which is significantly more frequent than in other hematological malignancies [6].
Posttransplant cyclophosphamide (PTCY) is an approach which is gaining popularity for haploidentical (Haplo) and mismatched unrelated donor (MUD) transplantation. This approach in the large retrospective study demonstrated reduced incidence of GvHD, reduced non-relapse mortality and improved GvHD-relapse-free survival (GFRS) compared to in vivo T-cell depletion methods both in Haplo and MUD settings [7, 8]. But most of the supportive data describes acute leukemia patients [9] or MDS/ MPN patients are combined in the analysis with acute leukemia [10]. Thus there is limited data whether PTCY in the group of chronic myeloid neoplasms provide the same benefit as in acute leukemias. Also there was a concern that in the setting of MDS/CML/MPN PTCy might increase the primary graft failure incidence.
The majority of SCTs in MDS and CML patients are unrelated, because of older age and comorbidities of the related donors and better outcomes after transplantation from a young donor [11]. So we conducted a single-center prospective randomized study in unrelated SCT recipients between the standard approach of GvHD prophylaxis with thymoglobulin and PTCY. The other components of GvHD prophylaxis was the same as well as the conditioning and supportive care.
Patients and methods
General parameters of the patients and transplants
The prospective single-center randomized study was conducted in the First Pavlov Medical university. The study was approved by the local Ethical committee and was conducted according to the good clinical practices and Helsinki declaration. All patients signed informed consent to participate in the study and processing of the personal data. The study was registered at clinicaltrials.gov, NCT02627573. The conclusion criteria were the diagnosis of chronic myeloid leukemia or myelodysplastic Syndrome or myeloprolipherative neoplasm unclassified or atypical chronic myelogenous leukemia with indications for SCT and presence of 10/10 HLA-matched unrelated donor. The donor and recipient must be identical by the following genetic loci: HLA-A, HLA-B, HLA-Cw, HLA-DRB1, and HLA-DQB1. Mismatches in these loci were not allowed. Also only patients were included in whom the donor agreed to donate peripheral blood stem cells (PBSC). The exclusion criteria were the presence of severe concurrent illness, prior history of anaphylaxis to thymoglobulin, moderate or severe cardiac dysfunction, left ventricular ejection fraction <50%, moderate or severe decrease in pulmonary function, FEV1 <70% or DLCO <70% of predicted, respiratory distress at least grade I, severe organ dysfunction: AST or ALT >5 upper normal limits, bilirubin >1.5 upper normal limits, creatinine >2 upper normal limits, creatinine clearance <60 mL/min., uncontrolled bacterial or fungal infection at the time of enrollment, requirement for vasopressor support at the time of enrollment, Karnofsky index <30%, pregnancy, and somatic or psychiatric disorder making the patient unable to sign informed consent. The study during 2015-2019 has enrolled 33 patients: 16 in the thymoglobulin arm and 17 in the PTCY arm. The study was terminated prematurely due to poor recruitment. All recruited patients were included in the analysis. The study group was well balanced with no significant differences in characteristics of patients (Table 1).
Table 1. Characteristics of patients

CML = chronic myeloid leukemia, MDS = myelodysplastic syndrome, MPN = myeloproliferative neoplasm, CP = chronic phase, AP = acceleration phase, BC = blast crisis, AEB – access blasts, MLD – multileaneage dysplasia.
Study procedures
All patients received conditioning with fludarabine 180 mg/m2 over 6 days and oral busulfan 10 mg/kg over 3 days. Patients in the thymoglobulin group received GvHD prophylaxis with thymoglobulin 2.5 mg/kg on days -3 and -2, tacrolimus 0.03 mg/kg/day starting day -1 with target concentration 5-15 ng/ml and mycophenolate mofetil (MMF) 30 mg/kg/day starting. The MMF was continued until day +30. Tacrolimus was continued until day +100 and gradually tapered over 1 month in absence of GvHD. Steroids were used in this group as the pre-medication to thymoglobulin and graft infusion. In the PTCy group patients received cyclophosphamide 50 mg/kg on days +3 and +4. Mesna 50 mg/kg was administered as 24-hour infusion on the days of cyclophosphamide administration. MMF 30 mg/kg/day was started on day +5 and continued until day +35. Tacrolimus 0.03 mg/kg/day was started on day +5 with target concentration 5-15 ng/ml. Tacrolimus in this arm was in the same way continued until day +100 and gradually tapered over 1 month in absence of GvHD. Use of steroids was prohibited from day-5 to day+5. The rest of the supportive care was the same in both arms. The standard antifungal prophylaxis was fluconazole starting on day 0 and continued until engraftment.
Clinical outcomes
Time to disease relapse, acute GvHD (GvHD), moderate to severe chronic GvHD (cGvHD), non-relapse mortality (NRM), overall survival (OS), event-free survival (EFS), and GvHD-relapse free survival (GRFS) were defined as the time from transplantation to the event. Incidence of aGvHD was calculated at 125 days after HSCT, and the time frame for the other outcomes was free years. Events for EFS were relapse or death. Events for GRFS were either death, relapse, grades III-IV acute GvHD or systemic therapy-requiring chronic GvHD [12]. Patients were censored at the time of last contact or a second transplantation for all outcomes. Disease relapse was defined as morphologic or cytogenetic evidence of disease with pre-transplantation characteristics. Disease staging, including bone marrow biopsies, was routinely performed on days +30,+60,+100, +180, +365 post-transplant. The Consensus Conference criteria and National Institutes of Health criteria were used for aGvHD and cGvHD grading [13, 14]. Primary graft failure was defined as the complete absence of donor chimerism in bone marrow biopsy by day +40. Time to engraftment was calculated as time from HSCT to unsupported neutrophil count > 500/µl and white blood cell count >1000/µl for 3 consecutive days. Toxicity was assessed with CTCAE ver. 4.03. Sepsis and severe sepsis were diagnosed based on International Guidelines for Management of Severe Sepsis and Septic Shock [15]. Invasive mycosis was diagnosed in case of probable or proven infection according to EORTC/MSG guidelines [16]. The threshold for cytomegalovirus (CMV) reactivation was >500 copies/ml. Clinically significant CMV reactivation was defined as >10000 copies/mL. Veno-occlusive disease was diagnosed and graded based on EBMT criteria [17].
Statistical evaluation
The study primary endpoint was the incidence of primary graft failure. The study was planned to enroll 60 patients, 30 patients in each arm. With the study group size was calculated with 30% margin, 0.65 study power and 0.05 significance using Fisher’s criterion. Secondary endpoints were incidence of acute grade II-IV acute GvHD, incidence moderate and severe chronic GvHD, non-relapse mortality, OS, EFS, GFRS, relapse incidence, toxicity and infectious complications. The strata for randomization was a pretransplant assessment of mortality (PAM) score [18]. The stratification was performed using Mann-Whitney U-criterion to achieve the minimal differences in PAM between the study groups. The final median PAM values were 14.1 (range 7.8-25.2) and 14.1 (6.9-21.9) in the thymoglobulin and PTCY groups, respectively (p=0.98).
Comparison of patient characteristics was performed by Mann-Whitney test. The survival distributions for OS, EFS, GRFS were calculated using Kaplan-Meier methodology. The comparisons were made using the log-rank test. Cumulative incidence analysis with competing risks for aGvHD, cGvHD, relapse incidence and NRM was performed using Gray test. Relapse and NRM were accounted as competing risks. Incidence and severity of complications was compared using Chi-square and Mann-Whitney test. Analyses were conducted in SAS 9.3 (SAS Institute, Inc.).
Results
Median follow up in the study was 29 months (range 7-48). There was no difference in the incidence of primary graft failure: 12.50% (95%CI 2%-38%) in the thymoglobulin arm and 11.8% (95%CI 1%-36%) in the PTCY arm, p=0.95. Three out of four patients with primary graft failure died, one was salvaged with second transplantation. All patients without primary graft failure survived to the day of engraftment. Median time to neutrophil engraftment was longer in the PTCY group: 20 (16-33) vs 16 (11-30) days, p=0.0017. Time to white blood cell engraftment was also significantly longer: 21 (16-33) vs 15 (11-30) days, p=0.0016. No differences were observed in the time to platelet engraftment: median 12 (8-40) vs 15 (11-45) days, p=0.1482.
Acute GvHD grade II-IV was documented in 3 (23%) patients in the thymoglobulin group and 1 (6%) patients in the PTCY group (p=0.20). No cases of acute GvHD grade III-IV were documented. Moderate and severe chronic GvHD was diagnosed in 3 (23%) patient after thymoglobulin and 4 (25%) patients after PTCY (p=0.41). Median time to chronic GvHD was a little shorter after thymoglobulin (5.5 months) compared to PTCY (15 months), p=0.11. One patient in thymoglobulin and 2 patients in the PTCY group had mild chronic GvHD.
Patients after PTCY prophylaxis had significantly better outcomes of transplantation. 4-year OS was 82% (95%CI 55-94%) vs 30% (95%CI 6-59%), p=0.0126; EFS was 61% (95%CI 31-81%) vs 26% (95%CI 5-54%), p=0.0335, GFRS 61% 95%CI (31-81%) vs 16% (95%CI 1-46%), p=0.0072 (Figure 1). There was a comparable incidence of relapse between the groups (25% vs 29%, p=0.77), but significantly reduced non-relapse mortality in the PTCY group (6% vs 38%, p=0.0264), which was main cause of the differences in survival outcomes. Primary graft failure and infectious complications were the only causes of non-relapse mortality in the study groups.

Figure 1. Overall (A), event-free (B) and GvHD-relapse-free (C) survival. The percentages shown are 4-year Kaplan-Mayer estimates
No unexpected toxicity was observed during the study. There was no significant difference in the incidence of early complications (Table 2). No cases of neurotoxicity or veno-occlusive disease were documented during the study. The infusion reactions in the thymoglobulin group were allergic reactions and in the PTCY group – one case of cystalgia.
Table 2. Toxicity and complications observed in ATG and PTCY groups

Note: TA-TMA = transplant-associated microangiopathy. Infusion reaction is defined as any reaction during or immediately after infusion that required change in the systemic therapy
Discussion
The study was originally developed to demonstrate if there is a difference in primary graft failure after PTCY. Despite it was terminated prematurely due to poor recruitment, several approximations could be made. We have observed identical number of graft failures in the thymoglobulin and PTCY group. According to CIBMTR data the average number of primary graft failure is 5.5% and CML and MDS patients have the double risk. So the incidence in the current study was not only the same between groups, but almost identical to the one reported by large registry studies [6]. So it is likely that there is no impact of PTCY on the incidence of primary graft failure. On the other hand, we have not observed any positive impact of PTCY on engraftment in this patient population despite previous preclinical data [19]. Unlike primary graft failure engraftment of neutrophils is delayed by several days which in concordance with previously published data [7].
The study was not powered to capture the significance and survival and recruitment was not completed, but most striking results were obtained in terms of survival outcomes. PTCY prophylaxis was associated with significantly higher incidence of OS, EFS and GRFS. The differences were due to reduced non-relapse mortality, it was only 6% in the PTCY group. These extremely low incidence of NRM was recently confirmed in the retrospective [7] and prospective randomized [20] studies. Despite the situation in haploidentical transplantation where PTCY reduces mortality through more effective prevention of acute and chronic GvHD [8], here we observed identical incidence of both acute and chronic GvHD, but the difference was due to late severe bacterial infections. In this study 5 mg/kg dose of thymoglobulin was used which is reported to cause less profound immunosuppression and promote graft-versus-leukemia effect [21, 22]. However the population of MDS and MPN patients is different from acute leukemia. There is a significant iron overload, which may increase the risk of infections after SCT and thymoglobulin effects might overlap with these negative impacts of iron overload [23]. Otherwise this might be an indication of better immunological recovery after unrelated transplantation with PTCY. Additional studies are required to confirm these assumptions.
Conclusion
Despite incomplete recruitment, this study provides some evidence on the use of PTCY-based prophylaxis for unrelated transplantations in patients with CML, MDS and MPN. The study has demonstrated that there is likely no impact of PTCY on the incidence of primary graft failure. Also it creates the basis for future studies to evaluate survival and immunological recovery in this patient population.
Conflict of interest
No conflict of interest reported.
References
Елена В. Морозова, Иван С. Моисеев, Юлия Ю. Власова, Николай Ю. Цветков, Юлия В. Рудницкая, Мария В. Барабанщикова, Анна Г. Смирнова, Елена И. Дарская, Сергей Н. Бондаренко, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26387" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26388" ["VALUE"]=> array(2) { ["TEXT"]=> string(3984) "<p style="text-align: justify;">В настоящий момент отмечается рост числа публикаций по поводу использования посттрансплантационного циклофосфана (ПТЦФ) в профилактике реакции «трансплантат против хозяина» при неродственной трансплантации гемопоэтических стволовых клеток. Тем не менее, большинство этих публикаций включают только пациентов с острыми лейкозами. Данные о возможности применения ПТЦФ при хроническом миелолейкозе (ХМЛ), миелодиспластическом синдроме (МДС) и хронических миелопролиферативных заболеваниях (ХМПЗ) практически отсутствуют, поэтому в данной популяции пациентов было инициировано проспективное рандомизированное сравнение тимоглобулина и ПТЦФ в профилактики РТПХ при 10/10- HLA совместимой неродственной трансплантации (NCT02627573, clinicaltrials.gov). Стратой для рандомизации был индекс pretransplant assessment of mortality. Исследование было прекращено преждевременно в связи с медленным набором пациентов, тем не менее, за время исследования было включено 33 пациента, 16 пациентов в группу тимоглобулина и 17 пациентов в группу ПТЦФ. Медиана наблюдения составила 29 месяцев. Не было выявлено различий ни в частоте первичного неприживления трансплантата (12,50% против 11,8%, p=0,9), ни острой РТПХ II-IV степени (23% <i>vs</i> 6%, p=0,2), ни хронической РТПХ средней и тяжелой степени (25% <i>vs</i> 23%, p=0,4) в группах тимоглобулина и ПТЦФ, соответственно. Однако, было в группе ПТЦФ наблюдалась достоверно лучшая общая (82% <i>vs</i> 30%, p=0,0126) и бессобытийная (61% <i>vs</i> 26%, p=0,0335) выживаемость, а также выживаемость без рецидива и РТПХ (61% <i>vs</i> 16%, p=0,0072). Различия были связаны с поздней инфекционной летальностью. Никак достоверных различий в токсичности 2 режимов выявлено не было. Подводя итоги, данное исследование дает обоснование для применения ПТЦФ при неродственных трансплантациях у пациентов с ХМЛ и МДС. Требуются дальнейшие исследования для подтверждения преимущества ПТЦФ над классической профилактикой с тимоглобулином. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Посттрансплантационный циклофосфан, миелодиспластический синдром, хронический миелоидный лейкоз, хроническая миелопролиферативная неоплазия, совместимый неродственный донор. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3868) "В настоящий момент отмечается рост числа публикаций по поводу использования посттрансплантационного циклофосфана (ПТЦФ) в профилактике реакции «трансплантат против хозяина» при неродственной трансплантации гемопоэтических стволовых клеток. Тем не менее, большинство этих публикаций включают только пациентов с острыми лейкозами. Данные о возможности применения ПТЦФ при хроническом миелолейкозе (ХМЛ), миелодиспластическом синдроме (МДС) и хронических миелопролиферативных заболеваниях (ХМПЗ) практически отсутствуют, поэтому в данной популяции пациентов было инициировано проспективное рандомизированное сравнение тимоглобулина и ПТЦФ в профилактики РТПХ при 10/10- HLA совместимой неродственной трансплантации (NCT02627573, clinicaltrials.gov). Стратой для рандомизации был индекс pretransplant assessment of mortality. Исследование было прекращено преждевременно в связи с медленным набором пациентов, тем не менее, за время исследования было включено 33 пациента, 16 пациентов в группу тимоглобулина и 17 пациентов в группу ПТЦФ. Медиана наблюдения составила 29 месяцев. Не было выявлено различий ни в частоте первичного неприживления трансплантата (12,50% против 11,8%, p=0,9), ни острой РТПХ II-IV степени (23% vs 6%, p=0,2), ни хронической РТПХ средней и тяжелой степени (25% vs 23%, p=0,4) в группах тимоглобулина и ПТЦФ, соответственно. Однако, было в группе ПТЦФ наблюдалась достоверно лучшая общая (82% vs 30%, p=0,0126) и бессобытийная (61% vs 26%, p=0,0335) выживаемость, а также выживаемость без рецидива и РТПХ (61% vs 16%, p=0,0072). Различия были связаны с поздней инфекционной летальностью. Никак достоверных различий в токсичности 2 режимов выявлено не было. Подводя итоги, данное исследование дает обоснование для применения ПТЦФ при неродственных трансплантациях у пациентов с ХМЛ и МДС. Требуются дальнейшие исследования для подтверждения преимущества ПТЦФ над классической профилактикой с тимоглобулином.
Ключевые слова
Посттрансплантационный циклофосфан, миелодиспластический синдром, хронический миелоидный лейкоз, хроническая миелопролиферативная неоплазия, совместимый неродственный донор.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26389" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-53-59" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-53-59" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26392" ["VALUE"]=> array(2) { ["TEXT"]=> string(320) "<p>Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(286) "Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26394" ["VALUE"]=> array(2) { ["TEXT"]=> string(520) "<p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br> St. Petersburg, Russia </p><br> <p><b>Correspondence</b><br> Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (911) 927 8229<br> E-mail: dr_morozova@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(454) "Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 927 8229
E-mail: dr_morozova@mail.ru
Despite growing evidence on the use of posttransplantation cyclophosphamide (PTCY) in matched unrelated transplantation in acute leukemia patients, prospective evidence to support this type of prophylaxis in myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms and chronic myeloid leukemia (CML) patients is lacking. In this patient population we conducted a prospective randomized study of PTCY vs thymoglobulin for graft-versus-host disease (GvHD) prophylaxis in the setting of 10/10 HLA-matched unrelated transplantation (NCT02627573, clinicaltrials.gov). The strata for randomization was pretransplant assessment of mortality score. The study was terminated prematurely due to poor recruitment, but 33 patients were enrolled, 17 in the PTCY and 16 in the thymoglobulin group. Median follow up was 29 months. There was no difference between groups in the incidence primary graft failure (12.50% vs 11.8%, p=0.9), acute GvHD grade II-IV (23% vs 6%, p=0.2), moderate and severe chronic GvHD (25% vs 23%, p=0.4) in the thymoglobulin and PTCY arms, respectively. However there was a significantly higher overall survival (82% vs 30%, p=0.0126), event-free survival (61% vs 26%, p=0.0335), and GvHD-relapse free survival (61% vs 16%, p=0.0072) in the PTCY group due to reduced late infection-related mortality. No differences was observed in terms of toxicity. In conclusion, despite incomplete recruitment, the study created the basis for the use of PTCY in unrelated transplantations from fully matched unrelated donors in CML and MDS. Future studies are required to confirm if there is a benefit of PTCY-based prophylaxis over thymoglobulin.
Keywords
Posttransplant cyclophosphamide, myelodysplastic syndrome, chronic myeloid leukemia, chronic myeloproliferative neoplasm, matched unrelated donor.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26390" ["VALUE"]=> string(175) "Randomized study between thymoglobulin and posttransplant cyclophosphamide in patients with chronic myeloid neoplasms undergoing unrelated allogeneic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(175) "Randomized study between thymoglobulin and posttransplant cyclophosphamide in patients with chronic myeloid neoplasms undergoing unrelated allogeneic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26391" ["VALUE"]=> string(4) "1982" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1982" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26393" ["VALUE"]=> string(4) "1983" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1983" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26392" ["VALUE"]=> array(2) { ["TEXT"]=> string(320) "<p>Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(286) "Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(286) "Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, Boris V. Afanasyev
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26395" ["VALUE"]=> array(2) { ["TEXT"]=> string(2072) "<p style="text-align: justify;">Despite growing evidence on the use of posttransplantation cyclophosphamide (PTCY) in matched unrelated transplantation in acute leukemia patients, prospective evidence to support this type of prophylaxis in myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms and chronic myeloid leukemia (CML) patients is lacking. In this patient population we conducted a prospective randomized study of PTCY vs thymoglobulin for graft-versus-host disease (GvHD) prophylaxis in the setting of 10/10 HLA-matched unrelated transplantation (NCT02627573, clinicaltrials.gov). The strata for randomization was pretransplant assessment of mortality score. The study was terminated prematurely due to poor recruitment, but 33 patients were enrolled, 17 in the PTCY and 16 in the thymoglobulin group. Median follow up was 29 months. There was no difference between groups in the incidence primary graft failure (12.50% <i>vs</i> 11.8%, p=0.9), acute GvHD grade II-IV (23% <i>vs</i> 6%, p=0.2), moderate and severe chronic GvHD (25% <i>vs</i> 23%, p=0.4) in the thymoglobulin and PTCY arms, respectively. However there was a significantly higher overall survival (82% <i>vs</i> 30%, p=0.0126), event-free survival (61% <i>vs</i> 26%, p=0.0335), and GvHD-relapse free survival (61% <i>vs</i> 16%, p=0.0072) in the PTCY group due to reduced late infection-related mortality. No differences was observed in terms of toxicity. In conclusion, despite incomplete recruitment, the study created the basis for the use of PTCY in unrelated transplantations from fully matched unrelated donors in CML and MDS. Future studies are required to confirm if there is a benefit of PTCY-based prophylaxis over thymoglobulin. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Posttransplant cyclophosphamide, myelodysplastic syndrome, chronic myeloid leukemia, chronic myeloproliferative neoplasm, matched unrelated donor. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1944) "Despite growing evidence on the use of posttransplantation cyclophosphamide (PTCY) in matched unrelated transplantation in acute leukemia patients, prospective evidence to support this type of prophylaxis in myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms and chronic myeloid leukemia (CML) patients is lacking. In this patient population we conducted a prospective randomized study of PTCY vs thymoglobulin for graft-versus-host disease (GvHD) prophylaxis in the setting of 10/10 HLA-matched unrelated transplantation (NCT02627573, clinicaltrials.gov). The strata for randomization was pretransplant assessment of mortality score. The study was terminated prematurely due to poor recruitment, but 33 patients were enrolled, 17 in the PTCY and 16 in the thymoglobulin group. Median follow up was 29 months. There was no difference between groups in the incidence primary graft failure (12.50% vs 11.8%, p=0.9), acute GvHD grade II-IV (23% vs 6%, p=0.2), moderate and severe chronic GvHD (25% vs 23%, p=0.4) in the thymoglobulin and PTCY arms, respectively. However there was a significantly higher overall survival (82% vs 30%, p=0.0126), event-free survival (61% vs 26%, p=0.0335), and GvHD-relapse free survival (61% vs 16%, p=0.0072) in the PTCY group due to reduced late infection-related mortality. No differences was observed in terms of toxicity. In conclusion, despite incomplete recruitment, the study created the basis for the use of PTCY in unrelated transplantations from fully matched unrelated donors in CML and MDS. Future studies are required to confirm if there is a benefit of PTCY-based prophylaxis over thymoglobulin.
Keywords
Posttransplant cyclophosphamide, myelodysplastic syndrome, chronic myeloid leukemia, chronic myeloproliferative neoplasm, matched unrelated donor.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1944) "Despite growing evidence on the use of posttransplantation cyclophosphamide (PTCY) in matched unrelated transplantation in acute leukemia patients, prospective evidence to support this type of prophylaxis in myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms and chronic myeloid leukemia (CML) patients is lacking. In this patient population we conducted a prospective randomized study of PTCY vs thymoglobulin for graft-versus-host disease (GvHD) prophylaxis in the setting of 10/10 HLA-matched unrelated transplantation (NCT02627573, clinicaltrials.gov). The strata for randomization was pretransplant assessment of mortality score. The study was terminated prematurely due to poor recruitment, but 33 patients were enrolled, 17 in the PTCY and 16 in the thymoglobulin group. Median follow up was 29 months. There was no difference between groups in the incidence primary graft failure (12.50% vs 11.8%, p=0.9), acute GvHD grade II-IV (23% vs 6%, p=0.2), moderate and severe chronic GvHD (25% vs 23%, p=0.4) in the thymoglobulin and PTCY arms, respectively. However there was a significantly higher overall survival (82% vs 30%, p=0.0126), event-free survival (61% vs 26%, p=0.0335), and GvHD-relapse free survival (61% vs 16%, p=0.0072) in the PTCY group due to reduced late infection-related mortality. No differences was observed in terms of toxicity. In conclusion, despite incomplete recruitment, the study created the basis for the use of PTCY in unrelated transplantations from fully matched unrelated donors in CML and MDS. Future studies are required to confirm if there is a benefit of PTCY-based prophylaxis over thymoglobulin.
Keywords
Posttransplant cyclophosphamide, myelodysplastic syndrome, chronic myeloid leukemia, chronic myeloproliferative neoplasm, matched unrelated donor.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26389" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-53-59" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-53-59" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-53-59" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26390" ["VALUE"]=> string(175) "Randomized study between thymoglobulin and posttransplant cyclophosphamide in patients with chronic myeloid neoplasms undergoing unrelated allogeneic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(175) "Randomized study between thymoglobulin and posttransplant cyclophosphamide in patients with chronic myeloid neoplasms undergoing unrelated allogeneic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(175) "Randomized study between thymoglobulin and posttransplant cyclophosphamide in patients with chronic myeloid neoplasms undergoing unrelated allogeneic stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26394" ["VALUE"]=> array(2) { ["TEXT"]=> string(520) "<p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br> St. Petersburg, Russia </p><br> <p><b>Correspondence</b><br> Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (911) 927 8229<br> E-mail: dr_morozova@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(454) "Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 927 8229
E-mail: dr_morozova@mail.ru
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 927 8229
E-mail: dr_morozova@mail.ru
Елена В. Морозова, Иван С. Моисеев, Юлия Ю. Власова, Николай Ю. Цветков, Юлия В. Рудницкая, Мария В. Барабанщикова, Анна Г. Смирнова, Елена И. Дарская, Сергей Н. Бондаренко, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(418) "Елена В. Морозова, Иван С. Моисеев, Юлия Ю. Власова, Николай Ю. Цветков, Юлия В. Рудницкая, Мария В. Барабанщикова, Анна Г. Смирнова, Елена И. Дарская, Сергей Н. Бондаренко, Борис В. Афанасьев
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26384" ["VALUE"]=> string(22) "11/11/2019 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/11/2019 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/11/2019 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26385" ["VALUE"]=> string(22) "01/24/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "01/24/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "01/24/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26388" ["VALUE"]=> array(2) { ["TEXT"]=> string(3984) "<p style="text-align: justify;">В настоящий момент отмечается рост числа публикаций по поводу использования посттрансплантационного циклофосфана (ПТЦФ) в профилактике реакции «трансплантат против хозяина» при неродственной трансплантации гемопоэтических стволовых клеток. Тем не менее, большинство этих публикаций включают только пациентов с острыми лейкозами. Данные о возможности применения ПТЦФ при хроническом миелолейкозе (ХМЛ), миелодиспластическом синдроме (МДС) и хронических миелопролиферативных заболеваниях (ХМПЗ) практически отсутствуют, поэтому в данной популяции пациентов было инициировано проспективное рандомизированное сравнение тимоглобулина и ПТЦФ в профилактики РТПХ при 10/10- HLA совместимой неродственной трансплантации (NCT02627573, clinicaltrials.gov). Стратой для рандомизации был индекс pretransplant assessment of mortality. Исследование было прекращено преждевременно в связи с медленным набором пациентов, тем не менее, за время исследования было включено 33 пациента, 16 пациентов в группу тимоглобулина и 17 пациентов в группу ПТЦФ. Медиана наблюдения составила 29 месяцев. Не было выявлено различий ни в частоте первичного неприживления трансплантата (12,50% против 11,8%, p=0,9), ни острой РТПХ II-IV степени (23% <i>vs</i> 6%, p=0,2), ни хронической РТПХ средней и тяжелой степени (25% <i>vs</i> 23%, p=0,4) в группах тимоглобулина и ПТЦФ, соответственно. Однако, было в группе ПТЦФ наблюдалась достоверно лучшая общая (82% <i>vs</i> 30%, p=0,0126) и бессобытийная (61% <i>vs</i> 26%, p=0,0335) выживаемость, а также выживаемость без рецидива и РТПХ (61% <i>vs</i> 16%, p=0,0072). Различия были связаны с поздней инфекционной летальностью. Никак достоверных различий в токсичности 2 режимов выявлено не было. Подводя итоги, данное исследование дает обоснование для применения ПТЦФ при неродственных трансплантациях у пациентов с ХМЛ и МДС. Требуются дальнейшие исследования для подтверждения преимущества ПТЦФ над классической профилактикой с тимоглобулином. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Посттрансплантационный циклофосфан, миелодиспластический синдром, хронический миелоидный лейкоз, хроническая миелопролиферативная неоплазия, совместимый неродственный донор. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3868) "В настоящий момент отмечается рост числа публикаций по поводу использования посттрансплантационного циклофосфана (ПТЦФ) в профилактике реакции «трансплантат против хозяина» при неродственной трансплантации гемопоэтических стволовых клеток. Тем не менее, большинство этих публикаций включают только пациентов с острыми лейкозами. Данные о возможности применения ПТЦФ при хроническом миелолейкозе (ХМЛ), миелодиспластическом синдроме (МДС) и хронических миелопролиферативных заболеваниях (ХМПЗ) практически отсутствуют, поэтому в данной популяции пациентов было инициировано проспективное рандомизированное сравнение тимоглобулина и ПТЦФ в профилактики РТПХ при 10/10- HLA совместимой неродственной трансплантации (NCT02627573, clinicaltrials.gov). Стратой для рандомизации был индекс pretransplant assessment of mortality. Исследование было прекращено преждевременно в связи с медленным набором пациентов, тем не менее, за время исследования было включено 33 пациента, 16 пациентов в группу тимоглобулина и 17 пациентов в группу ПТЦФ. Медиана наблюдения составила 29 месяцев. Не было выявлено различий ни в частоте первичного неприживления трансплантата (12,50% против 11,8%, p=0,9), ни острой РТПХ II-IV степени (23% vs 6%, p=0,2), ни хронической РТПХ средней и тяжелой степени (25% vs 23%, p=0,4) в группах тимоглобулина и ПТЦФ, соответственно. Однако, было в группе ПТЦФ наблюдалась достоверно лучшая общая (82% vs 30%, p=0,0126) и бессобытийная (61% vs 26%, p=0,0335) выживаемость, а также выживаемость без рецидива и РТПХ (61% vs 16%, p=0,0072). Различия были связаны с поздней инфекционной летальностью. Никак достоверных различий в токсичности 2 режимов выявлено не было. Подводя итоги, данное исследование дает обоснование для применения ПТЦФ при неродственных трансплантациях у пациентов с ХМЛ и МДС. Требуются дальнейшие исследования для подтверждения преимущества ПТЦФ над классической профилактикой с тимоглобулином.
Ключевые слова
Посттрансплантационный циклофосфан, миелодиспластический синдром, хронический миелоидный лейкоз, хроническая миелопролиферативная неоплазия, совместимый неродственный донор.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3868) "В настоящий момент отмечается рост числа публикаций по поводу использования посттрансплантационного циклофосфана (ПТЦФ) в профилактике реакции «трансплантат против хозяина» при неродственной трансплантации гемопоэтических стволовых клеток. Тем не менее, большинство этих публикаций включают только пациентов с острыми лейкозами. Данные о возможности применения ПТЦФ при хроническом миелолейкозе (ХМЛ), миелодиспластическом синдроме (МДС) и хронических миелопролиферативных заболеваниях (ХМПЗ) практически отсутствуют, поэтому в данной популяции пациентов было инициировано проспективное рандомизированное сравнение тимоглобулина и ПТЦФ в профилактики РТПХ при 10/10- HLA совместимой неродственной трансплантации (NCT02627573, clinicaltrials.gov). Стратой для рандомизации был индекс pretransplant assessment of mortality. Исследование было прекращено преждевременно в связи с медленным набором пациентов, тем не менее, за время исследования было включено 33 пациента, 16 пациентов в группу тимоглобулина и 17 пациентов в группу ПТЦФ. Медиана наблюдения составила 29 месяцев. Не было выявлено различий ни в частоте первичного неприживления трансплантата (12,50% против 11,8%, p=0,9), ни острой РТПХ II-IV степени (23% vs 6%, p=0,2), ни хронической РТПХ средней и тяжелой степени (25% vs 23%, p=0,4) в группах тимоглобулина и ПТЦФ, соответственно. Однако, было в группе ПТЦФ наблюдалась достоверно лучшая общая (82% vs 30%, p=0,0126) и бессобытийная (61% vs 26%, p=0,0335) выживаемость, а также выживаемость без рецидива и РТПХ (61% vs 16%, p=0,0072). Различия были связаны с поздней инфекционной летальностью. Никак достоверных различий в токсичности 2 режимов выявлено не было. Подводя итоги, данное исследование дает обоснование для применения ПТЦФ при неродственных трансплантациях у пациентов с ХМЛ и МДС. Требуются дальнейшие исследования для подтверждения преимущества ПТЦФ над классической профилактикой с тимоглобулином.
Ключевые слова
Посттрансплантационный циклофосфан, миелодиспластический синдром, хронический миелоидный лейкоз, хроническая миелопролиферативная неоплазия, совместимый неродственный донор.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26387" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [8]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "144" ["~IBLOCK_SECTION_ID"]=> string(3) "144" ["ID"]=> string(4) "1847" ["~ID"]=> string(4) "1847" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(350) "Особенности ответа на терапию блинатумомабом и инотузумабом озогамицином у больных с рецидивирующим/рефрактерным B-клеточным острым лимфобластным лейкозом в реальной клинической практике" ["~NAME"]=> string(350) "Особенности ответа на терапию блинатумомабом и инотузумабом озогамицином у больных с рецидивирующим/рефрактерным B-клеточным острым лимфобластным лейкозом в реальной клинической практике" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "04/28/2020 09:23:01 am" ["~TIMESTAMP_X"]=> string(22) "04/28/2020 09:23:01 am" ["DETAIL_PAGE_URL"]=> string(154) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/osobennosti-otveta-na-terapiyu-blinatumomabom-i-inotuzumabom-ozogamitsinom-u-bolnykh-s-retsidiviruyu/" ["~DETAIL_PAGE_URL"]=> string(154) "/en/archive/tom-9-nomer-1/klinicheskie-issledovaniya/osobennosti-otveta-na-terapiyu-blinatumomabom-i-inotuzumabom-ozogamitsinom-u-bolnykh-s-retsidiviruyu/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(19640) "Introduction
In recent years, the outcome of patients with B-cell acute lymphoblastic leukemia (B-ALL) has improved dramatically due to the use of modern chemotherapy regimens. In children with B- ALL cure rate varies within 70%-80%, while in adults – within 15%-40% [1, 2] However, primary resistance and relapses that do occur in 15%-25% of children, and in 50%-70% of adults, and significantly impact survival [3, 4]. In relapsed patients, the prognosis is adverse and after each relapse, the outcome is deteriorating. In case of third and further relapses the probability to achieve remission by salvage high-dose chemotherapy is decreasing. In addition, when using intensive chemotherapy, commutative toxicity is accumulating which is an additional risk factor for increasing the incidence of non-relapse mortality during allogeneic hematopoietic stem cells transplantation (allo-HSCT) [5, 6, 7]. The depth of the response is another factor which impacts the allo-HSCT efficacy, and salvage chemotherapy rarely induces molecular remissions [7, 8, 9, 10].
The emergency of different monoclonal antibodies (BITE, conjugated) has significantly expanded our options for treatment of the patients with relapsed/refractory (r/r) B-cell ALL.
Currently blinatumomab, a bispecific T-cells engager monoclonal antibody (anti-CD19), and inotusumab ozogamicin, a conjugate monoclonal antibody (anti-CD22), are the most promising in treatment of r/r B-ALL in children and adults, which has been shown in a large number of studies [11, 12, 13, 14, 15, 16]. Their pharmacokinetics and pharmacodynamics have been well studied to date. But there are still many unresolved questions concerning use of these monoclonal antibodies in real clinical practice. Various pilot studies and randomized clinical trials have shown the advantages of blinatumomab and inotuzumab over standard and salvage chemotherapy [17, 18].
Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B-ALL.
Patients and methods
The study included patients, both children and adults, with different types of r/r B-ALL who were treated with monoclonal antibodies – blinatumomab and inotuzumab ozogamicin in real clinical setting. The patients were treated in Raisa Gorbacheva Memorial Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint-Petersburg from April 2015 to October 2019. All the patients gave informed consent for the treatment, each case was approved by the Russian Ministry of Health for individual use of investigational product.
The group of patients in our study was heterogenous for different parameters. The heterogeneity was due to genetic markers of r/r B-ALL patients, age differences, as well as different types of relapses, previous therapy and clinical state of the disease (hematological or molecular relapses) at the moment of starting therapy by blinatumomab or inotuzumab (Table 1). Hematological relapse (HR) was determined in cases with more than 5% blasts in bone marrow.
Table 1. Clinical characteristics of B-ALL patients included into the study

Molecular relapse (MR) was diagnosed if minimal reduced disease (MRD) was defined as presence of leukemia blasts not detectable by microscope, being, however, revealed by mutation-specific polymerase chain reaction (PCR) or flow cytometry, if the relevant result was >1 malignant cell per 10-4 normal counterparts.
The patients received blinatumomab by continuous intravenous infusions. One course included 4-week treatment followed by 2-weeks pause in treatment. The doses were as follows: in adult patients with hematologic relapse and children with hematologic and molecular relapses weighing >45 kg, at the dose of 9 mcg/d for the first week in cycle, and 28 mcg/d over the remaining 3 weeks. The patients with body mass of <45 kg received 5 mcg/m2/d during first week, and 15 mcg/m2/d over following 3 weeks. Adult patients with molecular relapses received 15 mcg/m2/d during 4 weeks. Inotuzumab ozogamycin was administered in adult and children groups at the same dosage: one cycle consisted of 3 dose regimens, i.e., 0.8 mg/m2 on week 1, and 0.5 mg/m2 on week 2 and week 3.
Patients with r/r Ph-positive B-ALL were treated with combination of blinatumomab or inotuzumab with different tyrosine kinase inhibitors at recommended doses administered during therapeutic cycles. Next types of response were registered: complete remission without MRD, i.e., molecular remission, and complete remission with MRD termed as hematological remission. Complete remission (CR) included variants with recovery and partial recovery of hemopoiesis. In several patients, complete remission was registered in bone marrow, but extramedullary lesions were found in them.
Statistical evaluation
Descriptive statistics were used for the patients’ characteristics and response to treatment using. Differences in response to treatment were evaluated with chi-square test. The variables with a significance levels of <=0.1 in the univariate analysis were selected for evaluation with logistic regression. The analyses were performed in SPSS ver. 17.0.
Results
The entire group of patients consisted of 182 children and adults at the age of 1 to 72 years old, including 78 children and adolescents up to 18 years, and 104 adults. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Baseline characteristics of the patients are shown in Table 1. The patients were classified by their mutational status at the time of onset of the disease, i.e., 161 (88%) cases of Ph-negative B-ALL and 21 (12%) patients with Ph-positive B-ALL. The mixed-lineage leukemia (MLL1) gene was detected in 17 (9%) patients: 9 with infant ALL, 2 in children over 1 year at the time of onset of disease and 6 in adults. Depending on occurrence of past relapses, the patients were divided into those who developed relapses after previous allo-HSCT 46 (25%) cases, or after chemotherapy (n=127, 70%) and primary resistance cases (n=9, i.e., 5%). Before blinatumomab treatment, 69 (54%) patients had molecular relapses, i.e., minimal residual disease (MRD), and 59 (46%) patients from this group. All the patients (n=54) who received inotuzumab had hematological relapses. Time of observation was from 23 to 730 days (median 244 days). In our study, most patients received 1-2 courses of blinatumomab and most patients received one cycle of inotuzumab ozogomicin. Patients with r/r Ph-positive B-ALL received different tyrosine kinase inhibitors (dasatinib 13, imatinib 6, nilotinib 2).
Clinical response in the whole group patients who received blinatumomab or inotuzumab was high both in adult and children groups, 83% and 69%, respectively, and most patients achieved complete remission (CR). In blinatumomab group, CR was registered in 57 adult patients (76%) and in 28 patients (52%) under 18 y.o. In inotuzumab group, overall response rate in adults was 82%, in children and adolescents, 81%; CR was achieved in 14 adult patients (50%), and in 14 children (58%) as seen from Fig. 1, A. The patients with cytogenetic or molecular genetics prognostic factors had high responses as well. Ph+ B-ALL in blinatumomab group had overall response in 84%, including 67% with CR. All Ph+ B-ALL patients in the inotuzumab group had hematological remission. The patients with MLL translocation had worst overall response compared to patients without MLL translocation, i.e., 41% and 65% of general group, respectively (Fig. 1, D). Overall responses in patients with relapses after previous HSCT and relapses after CT were comparable, 28 (63%) and 85 cases (63%) (Fig. 1, B). The patients who had molecular relapses before therapy with blinatumomab had higher response than patients with hematological relapses (90% vs 58%), as shown in Fig. 1, C. Multivariate analysis in our study showed that only age (p=0.001) and disease status (p=0.01) before blinatumomab or inotuzumab therapy showed statistical significance (Table 2). Previous exposition on blinatumab did not influence the response to inotuzumab ozogamicin, i.e., 89% vs 81% of responding patients (p=0.46) in blinatumomab-naïve versus blinatumomab-exposed cases.

Figure 1. Types of responses to antibody-based drug treatment in r/r B-ALL patients. A, Children vs adults; B, Patients with relapses following previous HSCT vs preceding CT; C, Patients with molecular relapses vs hematological relapses; D, Cases with MLL translocation vs patients without MLL translocation. The patients were classified by different types of minimal residual disease (bottom line of the pictures)
Table 2. Multivariate analysis of some factors predictive for treatment response

Discussion
We have assessed high efficacy of blinatumomab and inotuzumab ozogamicin in a heterogeneous cohort of r/r B-cell ALL of children and adults in real clinical practice. After these therapies, high rate of complete remission (CR) was observed in all subgroups, including patients with cytogenetics/ molecular genetic prognostic factors, e.g., in the cases with Ph-positive r/r B-ALL. If comparing our study with recent work that used combination of TKI and blinatumomab in patients with Ph-positive r/r B-ALL [19], our results seem to be something better. In our study, CR was achieved in 57% versus 36%, probably, due to high proportion of patients treated for MRD unlike in the previous study where majority of patients had hematological relapse. Patients with r/r B-ALL and MLL translocation achieved CR in 41% cases. These data demonstrate an opportunity of using this treatment in patients from high-risk group, but further studies are required to demonstrate whether the response rate is significantly lower than in general cohort.
Overall response in pediatric subgroup in our study differed significantly from adult patients because of probable differences in biology, e.g., genomic profiling in pediatric B-ALL more often reveals mutations in NRAS, KRAS genes, and MLL rearrangement. ETV6/RUNX1 (E/R) was the most common fusion gene in pediatric B-ALL as well [20]. Differences in immune system may also play a role in response rates [21] The observation that blinatumomab works better in MRD setting than in hematological remission confirms the previous report on clinical trials of Germany group, where 80% of molecular CR was documented [22]. Inotuzumab ozogamicin is effective, regardless the tumor burden: the overall response was 81%. The response rate was comparable to results of adult and pediatric registration study [11, 12, 13, 14]. Monoclonal antibodies present such opportunity, providing reliable approach to achieve CR in r/r B-ALL patients before sequential HSCT.
Conclusion
1. Both blinatumomab and inotuzumab ozogomicin are effective in patients with r/r B-ALL.
2. Blinatumomab is more effective in patients with molecular relapses.
3. Blinatumomab-refractory patients can be salvaged with inotuzumab ozogamicin.
4. Results in real-life clinical practice are comparable to extensive registration studies.
References
Introduction
In recent years, the outcome of patients with B-cell acute lymphoblastic leukemia (B-ALL) has improved dramatically due to the use of modern chemotherapy regimens. In children with B- ALL cure rate varies within 70%-80%, while in adults – within 15%-40% [1, 2] However, primary resistance and relapses that do occur in 15%-25% of children, and in 50%-70% of adults, and significantly impact survival [3, 4]. In relapsed patients, the prognosis is adverse and after each relapse, the outcome is deteriorating. In case of third and further relapses the probability to achieve remission by salvage high-dose chemotherapy is decreasing. In addition, when using intensive chemotherapy, commutative toxicity is accumulating which is an additional risk factor for increasing the incidence of non-relapse mortality during allogeneic hematopoietic stem cells transplantation (allo-HSCT) [5, 6, 7]. The depth of the response is another factor which impacts the allo-HSCT efficacy, and salvage chemotherapy rarely induces molecular remissions [7, 8, 9, 10].
The emergency of different monoclonal antibodies (BITE, conjugated) has significantly expanded our options for treatment of the patients with relapsed/refractory (r/r) B-cell ALL.
Currently blinatumomab, a bispecific T-cells engager monoclonal antibody (anti-CD19), and inotusumab ozogamicin, a conjugate monoclonal antibody (anti-CD22), are the most promising in treatment of r/r B-ALL in children and adults, which has been shown in a large number of studies [11, 12, 13, 14, 15, 16]. Their pharmacokinetics and pharmacodynamics have been well studied to date. But there are still many unresolved questions concerning use of these monoclonal antibodies in real clinical practice. Various pilot studies and randomized clinical trials have shown the advantages of blinatumomab and inotuzumab over standard and salvage chemotherapy [17, 18].
Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B-ALL.
Patients and methods
The study included patients, both children and adults, with different types of r/r B-ALL who were treated with monoclonal antibodies – blinatumomab and inotuzumab ozogamicin in real clinical setting. The patients were treated in Raisa Gorbacheva Memorial Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint-Petersburg from April 2015 to October 2019. All the patients gave informed consent for the treatment, each case was approved by the Russian Ministry of Health for individual use of investigational product.
The group of patients in our study was heterogenous for different parameters. The heterogeneity was due to genetic markers of r/r B-ALL patients, age differences, as well as different types of relapses, previous therapy and clinical state of the disease (hematological or molecular relapses) at the moment of starting therapy by blinatumomab or inotuzumab (Table 1). Hematological relapse (HR) was determined in cases with more than 5% blasts in bone marrow.
Table 1. Clinical characteristics of B-ALL patients included into the study

Molecular relapse (MR) was diagnosed if minimal reduced disease (MRD) was defined as presence of leukemia blasts not detectable by microscope, being, however, revealed by mutation-specific polymerase chain reaction (PCR) or flow cytometry, if the relevant result was >1 malignant cell per 10-4 normal counterparts.
The patients received blinatumomab by continuous intravenous infusions. One course included 4-week treatment followed by 2-weeks pause in treatment. The doses were as follows: in adult patients with hematologic relapse and children with hematologic and molecular relapses weighing >45 kg, at the dose of 9 mcg/d for the first week in cycle, and 28 mcg/d over the remaining 3 weeks. The patients with body mass of <45 kg received 5 mcg/m2/d during first week, and 15 mcg/m2/d over following 3 weeks. Adult patients with molecular relapses received 15 mcg/m2/d during 4 weeks. Inotuzumab ozogamycin was administered in adult and children groups at the same dosage: one cycle consisted of 3 dose regimens, i.e., 0.8 mg/m2 on week 1, and 0.5 mg/m2 on week 2 and week 3.
Patients with r/r Ph-positive B-ALL were treated with combination of blinatumomab or inotuzumab with different tyrosine kinase inhibitors at recommended doses administered during therapeutic cycles. Next types of response were registered: complete remission without MRD, i.e., molecular remission, and complete remission with MRD termed as hematological remission. Complete remission (CR) included variants with recovery and partial recovery of hemopoiesis. In several patients, complete remission was registered in bone marrow, but extramedullary lesions were found in them.
Statistical evaluation
Descriptive statistics were used for the patients’ characteristics and response to treatment using. Differences in response to treatment were evaluated with chi-square test. The variables with a significance levels of <=0.1 in the univariate analysis were selected for evaluation with logistic regression. The analyses were performed in SPSS ver. 17.0.
Results
The entire group of patients consisted of 182 children and adults at the age of 1 to 72 years old, including 78 children and adolescents up to 18 years, and 104 adults. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Baseline characteristics of the patients are shown in Table 1. The patients were classified by their mutational status at the time of onset of the disease, i.e., 161 (88%) cases of Ph-negative B-ALL and 21 (12%) patients with Ph-positive B-ALL. The mixed-lineage leukemia (MLL1) gene was detected in 17 (9%) patients: 9 with infant ALL, 2 in children over 1 year at the time of onset of disease and 6 in adults. Depending on occurrence of past relapses, the patients were divided into those who developed relapses after previous allo-HSCT 46 (25%) cases, or after chemotherapy (n=127, 70%) and primary resistance cases (n=9, i.e., 5%). Before blinatumomab treatment, 69 (54%) patients had molecular relapses, i.e., minimal residual disease (MRD), and 59 (46%) patients from this group. All the patients (n=54) who received inotuzumab had hematological relapses. Time of observation was from 23 to 730 days (median 244 days). In our study, most patients received 1-2 courses of blinatumomab and most patients received one cycle of inotuzumab ozogomicin. Patients with r/r Ph-positive B-ALL received different tyrosine kinase inhibitors (dasatinib 13, imatinib 6, nilotinib 2).
Clinical response in the whole group patients who received blinatumomab or inotuzumab was high both in adult and children groups, 83% and 69%, respectively, and most patients achieved complete remission (CR). In blinatumomab group, CR was registered in 57 adult patients (76%) and in 28 patients (52%) under 18 y.o. In inotuzumab group, overall response rate in adults was 82%, in children and adolescents, 81%; CR was achieved in 14 adult patients (50%), and in 14 children (58%) as seen from Fig. 1, A. The patients with cytogenetic or molecular genetics prognostic factors had high responses as well. Ph+ B-ALL in blinatumomab group had overall response in 84%, including 67% with CR. All Ph+ B-ALL patients in the inotuzumab group had hematological remission. The patients with MLL translocation had worst overall response compared to patients without MLL translocation, i.e., 41% and 65% of general group, respectively (Fig. 1, D). Overall responses in patients with relapses after previous HSCT and relapses after CT were comparable, 28 (63%) and 85 cases (63%) (Fig. 1, B). The patients who had molecular relapses before therapy with blinatumomab had higher response than patients with hematological relapses (90% vs 58%), as shown in Fig. 1, C. Multivariate analysis in our study showed that only age (p=0.001) and disease status (p=0.01) before blinatumomab or inotuzumab therapy showed statistical significance (Table 2). Previous exposition on blinatumab did not influence the response to inotuzumab ozogamicin, i.e., 89% vs 81% of responding patients (p=0.46) in blinatumomab-naïve versus blinatumomab-exposed cases.

Figure 1. Types of responses to antibody-based drug treatment in r/r B-ALL patients. A, Children vs adults; B, Patients with relapses following previous HSCT vs preceding CT; C, Patients with molecular relapses vs hematological relapses; D, Cases with MLL translocation vs patients without MLL translocation. The patients were classified by different types of minimal residual disease (bottom line of the pictures)
Table 2. Multivariate analysis of some factors predictive for treatment response

Discussion
We have assessed high efficacy of blinatumomab and inotuzumab ozogamicin in a heterogeneous cohort of r/r B-cell ALL of children and adults in real clinical practice. After these therapies, high rate of complete remission (CR) was observed in all subgroups, including patients with cytogenetics/ molecular genetic prognostic factors, e.g., in the cases with Ph-positive r/r B-ALL. If comparing our study with recent work that used combination of TKI and blinatumomab in patients with Ph-positive r/r B-ALL [19], our results seem to be something better. In our study, CR was achieved in 57% versus 36%, probably, due to high proportion of patients treated for MRD unlike in the previous study where majority of patients had hematological relapse. Patients with r/r B-ALL and MLL translocation achieved CR in 41% cases. These data demonstrate an opportunity of using this treatment in patients from high-risk group, but further studies are required to demonstrate whether the response rate is significantly lower than in general cohort.
Overall response in pediatric subgroup in our study differed significantly from adult patients because of probable differences in biology, e.g., genomic profiling in pediatric B-ALL more often reveals mutations in NRAS, KRAS genes, and MLL rearrangement. ETV6/RUNX1 (E/R) was the most common fusion gene in pediatric B-ALL as well [20]. Differences in immune system may also play a role in response rates [21] The observation that blinatumomab works better in MRD setting than in hematological remission confirms the previous report on clinical trials of Germany group, where 80% of molecular CR was documented [22]. Inotuzumab ozogamicin is effective, regardless the tumor burden: the overall response was 81%. The response rate was comparable to results of adult and pediatric registration study [11, 12, 13, 14]. Monoclonal antibodies present such opportunity, providing reliable approach to achieve CR in r/r B-ALL patients before sequential HSCT.
Conclusion
1. Both blinatumomab and inotuzumab ozogomicin are effective in patients with r/r B-ALL.
2. Blinatumomab is more effective in patients with molecular relapses.
3. Blinatumomab-refractory patients can be salvaged with inotuzumab ozogamicin.
4. Results in real-life clinical practice are comparable to extensive registration studies.
References
Инна В. Маркова, Сергей Н. Бондаренко, Олеся В. Паина, Белла И. Аюбова, Полина В. Кожокарь, Анастасия С. Фролова, Ильдар М. Бархатов, Елена В. Бабенко, Александр А. Алянский, Кирилл А. Екушов, Татьяна Л. Гиндина, Елена В. Семенова, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26375" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26376" ["VALUE"]=> array(2) { ["TEXT"]=> string(3129) "<p style="text-align: justify;">Аллогенная трансплантация гемопоэтических стволовых клеток остается наиболее эффективным методом излечения пациентов с рефрактерным течением/рецидивами В-клеточного острого лимфобластного лейкоза (р/р В-ОЛЛ). Полная ремиссия заболевания без признаков минимальной остаточной болезни является ключевым фактором успешного исхода. Моноклональные антитела (биспецифические и коньюгаты) представляют собой эффективные опции в достижении ремиссии у этих пациентов. Нашей целью было обобщение результатов одноцентрового нерандомизированного исследовапния для изучения результатов терапии блинатумамобом и инотузумаб-озогамицином у детей и взрослых в гетерогенной когорте больных р/р В-ОЛЛ. Результаты одноцентрового, нерандомизированного пилотного исследования в реальной клинической практике продемонстрировали высокий уровень ответа на данный вид терапии в гетерогенной когорте пациентов с р/р В-ОЛЛ. Исследуемая группа включала 182 пациента р/р В-ОЛЛ, возраст от одного года до 72 лет, 128 пациентов получали блинатумомаб, 54 пациента получали инотузумаб озогамицин. Уровень общего ответа был высоким в обеих группах – 96 (75%) и 44 случая (82%), соответственно. Главными предикторами ответа были взрослый возраст (OR=3,819, 95% CI=1,744-8,223, p=0,001) и показания к терапии – гематологический рецидив или персистенция минимальной остаточной болезни (OR=0,018, 95% CI=0,153-0,841, p=0,01). Другие клинические или патологические параметры не оказывали существенного влияния на ответы на терапию. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> В-клеточный острый лимфобластный лейкоз, рефрактерный и рецидивирующий, моноклональные антитела, блинатумомаб, инотузумаб озогамицин, общий ответ.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3073) "Аллогенная трансплантация гемопоэтических стволовых клеток остается наиболее эффективным методом излечения пациентов с рефрактерным течением/рецидивами В-клеточного острого лимфобластного лейкоза (р/р В-ОЛЛ). Полная ремиссия заболевания без признаков минимальной остаточной болезни является ключевым фактором успешного исхода. Моноклональные антитела (биспецифические и коньюгаты) представляют собой эффективные опции в достижении ремиссии у этих пациентов. Нашей целью было обобщение результатов одноцентрового нерандомизированного исследовапния для изучения результатов терапии блинатумамобом и инотузумаб-озогамицином у детей и взрослых в гетерогенной когорте больных р/р В-ОЛЛ. Результаты одноцентрового, нерандомизированного пилотного исследования в реальной клинической практике продемонстрировали высокий уровень ответа на данный вид терапии в гетерогенной когорте пациентов с р/р В-ОЛЛ. Исследуемая группа включала 182 пациента р/р В-ОЛЛ, возраст от одного года до 72 лет, 128 пациентов получали блинатумомаб, 54 пациента получали инотузумаб озогамицин. Уровень общего ответа был высоким в обеих группах – 96 (75%) и 44 случая (82%), соответственно. Главными предикторами ответа были взрослый возраст (OR=3,819, 95% CI=1,744-8,223, p=0,001) и показания к терапии – гематологический рецидив или персистенция минимальной остаточной болезни (OR=0,018, 95% CI=0,153-0,841, p=0,01). Другие клинические или патологические параметры не оказывали существенного влияния на ответы на терапию.
Ключевые слова
В-клеточный острый лимфобластный лейкоз, рефрактерный и рецидивирующий, моноклональные антитела, блинатумомаб, инотузумаб озогамицин, общий ответ.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26377" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-47-52" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-47-52" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26380" ["VALUE"]=> array(2) { ["TEXT"]=> string(421) "<p>Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova, <br>Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(381) "Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova,
Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Boris V. Afanasyev
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Inna V. Markova, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 964 6745
Allogeneic hematopoietic stem cell transplantation is an effective method to cure patients with relapse/refractory (r/r) B-cell acute lymphoblastic leukemia, and deep remission without minimal residual disease is the key factor for the favorable outcome. Monoclonal antibodies (bispecific T-cells engager and conjugates) are the promising option to achieve complete remission in these patients. Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B- ALL.
Results of the single-center non-randomized pilot study in real clinical practice showed high response rate in heterogeneous cohort of r/r B- ALL. The study group included 182 patients with r/r B-ALL, their age was from one to 72 years old. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Overall response was high in both groups, 96 (75%) and 44 (82%). The major predictors of response were adult age (OR= 3.819; 95% CI, 1.744-8.223; p=0.001) and clinical indications, i.e., active disease or measurable residual disease (OR= 0.018; 95% CI, 0.153-0.841; p=0.01). The other clinical or disease parameters had no significant impact on response.
Keywords
B-cell acute lymphoblastic leukemia, relapse/refractory, monoclonal antibodies, blinatumomab, inotuzumab ozogamicin, overall response.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26378" ["VALUE"]=> string(169) "Features of response to blinatumomab and inotuzumab ozogamicin therapy in patients with relapse/refractory B-cells acute lymphoblastic leukemia in real clinical practice" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(169) "Features of response to blinatumomab and inotuzumab ozogamicin therapy in patients with relapse/refractory B-cells acute lymphoblastic leukemia in real clinical practice" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26379" ["VALUE"]=> string(4) "1977" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1977" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26382" ["VALUE"]=> string(4) "1978" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1978" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26380" ["VALUE"]=> array(2) { ["TEXT"]=> string(421) "<p>Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova, <br>Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(381) "Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova,
Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Boris V. Afanasyev
Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova,
Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Boris V. Afanasyev
Allogeneic hematopoietic stem cell transplantation is an effective method to cure patients with relapse/refractory (r/r) B-cell acute lymphoblastic leukemia, and deep remission without minimal residual disease is the key factor for the favorable outcome. Monoclonal antibodies (bispecific T-cells engager and conjugates) are the promising option to achieve complete remission in these patients. Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B- ALL.
Results of the single-center non-randomized pilot study in real clinical practice showed high response rate in heterogeneous cohort of r/r B- ALL. The study group included 182 patients with r/r B-ALL, their age was from one to 72 years old. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Overall response was high in both groups, 96 (75%) and 44 (82%). The major predictors of response were adult age (OR= 3.819; 95% CI, 1.744-8.223; p=0.001) and clinical indications, i.e., active disease or measurable residual disease (OR= 0.018; 95% CI, 0.153-0.841; p=0.01). The other clinical or disease parameters had no significant impact on response.
Keywords
B-cell acute lymphoblastic leukemia, relapse/refractory, monoclonal antibodies, blinatumomab, inotuzumab ozogamicin, overall response.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1603) "Allogeneic hematopoietic stem cell transplantation is an effective method to cure patients with relapse/refractory (r/r) B-cell acute lymphoblastic leukemia, and deep remission without minimal residual disease is the key factor for the favorable outcome. Monoclonal antibodies (bispecific T-cells engager and conjugates) are the promising option to achieve complete remission in these patients. Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B- ALL.
Results of the single-center non-randomized pilot study in real clinical practice showed high response rate in heterogeneous cohort of r/r B- ALL. The study group included 182 patients with r/r B-ALL, their age was from one to 72 years old. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Overall response was high in both groups, 96 (75%) and 44 (82%). The major predictors of response were adult age (OR= 3.819; 95% CI, 1.744-8.223; p=0.001) and clinical indications, i.e., active disease or measurable residual disease (OR= 0.018; 95% CI, 0.153-0.841; p=0.01). The other clinical or disease parameters had no significant impact on response.
Keywords
B-cell acute lymphoblastic leukemia, relapse/refractory, monoclonal antibodies, blinatumomab, inotuzumab ozogamicin, overall response.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26377" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-47-52" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-47-52" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-47-52" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26378" ["VALUE"]=> string(169) "Features of response to blinatumomab and inotuzumab ozogamicin therapy in patients with relapse/refractory B-cells acute lymphoblastic leukemia in real clinical practice" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(169) "Features of response to blinatumomab and inotuzumab ozogamicin therapy in patients with relapse/refractory B-cells acute lymphoblastic leukemia in real clinical practice" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(169) "Features of response to blinatumomab and inotuzumab ozogamicin therapy in patients with relapse/refractory B-cells acute lymphoblastic leukemia in real clinical practice" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26381" ["VALUE"]=> array(2) { ["TEXT"]=> string(463) "<p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, <br>St. Petersburg, Russia</p><br> <p><b>Correspondence</b> Dr. Inna V. Markova, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (911) 964 6745</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(409) "Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Inna V. Markova, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 964 6745
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Inna V. Markova, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 964 6745
Инна В. Маркова, Сергей Н. Бондаренко, Олеся В. Паина, Белла И. Аюбова, Полина В. Кожокарь, Анастасия С. Фролова, Ильдар М. Бархатов, Елена В. Бабенко, Александр А. Алянский, Кирилл А. Екушов, Татьяна Л. Гиндина, Елена В. Семенова, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(591) "Инна В. Маркова, Сергей Н. Бондаренко, Олеся В. Паина, Белла И. Аюбова, Полина В. Кожокарь, Анастасия С. Фролова, Ильдар М. Бархатов, Елена В. Бабенко, Александр А. Алянский, Кирилл А. Екушов, Татьяна Л. Гиндина, Елена В. Семенова, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26372" ["VALUE"]=> string(22) "03/09/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/09/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/09/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26373" ["VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/27/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26376" ["VALUE"]=> array(2) { ["TEXT"]=> string(3129) "<p style="text-align: justify;">Аллогенная трансплантация гемопоэтических стволовых клеток остается наиболее эффективным методом излечения пациентов с рефрактерным течением/рецидивами В-клеточного острого лимфобластного лейкоза (р/р В-ОЛЛ). Полная ремиссия заболевания без признаков минимальной остаточной болезни является ключевым фактором успешного исхода. Моноклональные антитела (биспецифические и коньюгаты) представляют собой эффективные опции в достижении ремиссии у этих пациентов. Нашей целью было обобщение результатов одноцентрового нерандомизированного исследовапния для изучения результатов терапии блинатумамобом и инотузумаб-озогамицином у детей и взрослых в гетерогенной когорте больных р/р В-ОЛЛ. Результаты одноцентрового, нерандомизированного пилотного исследования в реальной клинической практике продемонстрировали высокий уровень ответа на данный вид терапии в гетерогенной когорте пациентов с р/р В-ОЛЛ. Исследуемая группа включала 182 пациента р/р В-ОЛЛ, возраст от одного года до 72 лет, 128 пациентов получали блинатумомаб, 54 пациента получали инотузумаб озогамицин. Уровень общего ответа был высоким в обеих группах – 96 (75%) и 44 случая (82%), соответственно. Главными предикторами ответа были взрослый возраст (OR=3,819, 95% CI=1,744-8,223, p=0,001) и показания к терапии – гематологический рецидив или персистенция минимальной остаточной болезни (OR=0,018, 95% CI=0,153-0,841, p=0,01). Другие клинические или патологические параметры не оказывали существенного влияния на ответы на терапию. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> В-клеточный острый лимфобластный лейкоз, рефрактерный и рецидивирующий, моноклональные антитела, блинатумомаб, инотузумаб озогамицин, общий ответ.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3073) "Аллогенная трансплантация гемопоэтических стволовых клеток остается наиболее эффективным методом излечения пациентов с рефрактерным течением/рецидивами В-клеточного острого лимфобластного лейкоза (р/р В-ОЛЛ). Полная ремиссия заболевания без признаков минимальной остаточной болезни является ключевым фактором успешного исхода. Моноклональные антитела (биспецифические и коньюгаты) представляют собой эффективные опции в достижении ремиссии у этих пациентов. Нашей целью было обобщение результатов одноцентрового нерандомизированного исследовапния для изучения результатов терапии блинатумамобом и инотузумаб-озогамицином у детей и взрослых в гетерогенной когорте больных р/р В-ОЛЛ. Результаты одноцентрового, нерандомизированного пилотного исследования в реальной клинической практике продемонстрировали высокий уровень ответа на данный вид терапии в гетерогенной когорте пациентов с р/р В-ОЛЛ. Исследуемая группа включала 182 пациента р/р В-ОЛЛ, возраст от одного года до 72 лет, 128 пациентов получали блинатумомаб, 54 пациента получали инотузумаб озогамицин. Уровень общего ответа был высоким в обеих группах – 96 (75%) и 44 случая (82%), соответственно. Главными предикторами ответа были взрослый возраст (OR=3,819, 95% CI=1,744-8,223, p=0,001) и показания к терапии – гематологический рецидив или персистенция минимальной остаточной болезни (OR=0,018, 95% CI=0,153-0,841, p=0,01). Другие клинические или патологические параметры не оказывали существенного влияния на ответы на терапию.
Ключевые слова
В-клеточный острый лимфобластный лейкоз, рефрактерный и рецидивирующий, моноклональные антитела, блинатумомаб, инотузумаб озогамицин, общий ответ.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3073) "Аллогенная трансплантация гемопоэтических стволовых клеток остается наиболее эффективным методом излечения пациентов с рефрактерным течением/рецидивами В-клеточного острого лимфобластного лейкоза (р/р В-ОЛЛ). Полная ремиссия заболевания без признаков минимальной остаточной болезни является ключевым фактором успешного исхода. Моноклональные антитела (биспецифические и коньюгаты) представляют собой эффективные опции в достижении ремиссии у этих пациентов. Нашей целью было обобщение результатов одноцентрового нерандомизированного исследовапния для изучения результатов терапии блинатумамобом и инотузумаб-озогамицином у детей и взрослых в гетерогенной когорте больных р/р В-ОЛЛ. Результаты одноцентрового, нерандомизированного пилотного исследования в реальной клинической практике продемонстрировали высокий уровень ответа на данный вид терапии в гетерогенной когорте пациентов с р/р В-ОЛЛ. Исследуемая группа включала 182 пациента р/р В-ОЛЛ, возраст от одного года до 72 лет, 128 пациентов получали блинатумомаб, 54 пациента получали инотузумаб озогамицин. Уровень общего ответа был высоким в обеих группах – 96 (75%) и 44 случая (82%), соответственно. Главными предикторами ответа были взрослый возраст (OR=3,819, 95% CI=1,744-8,223, p=0,001) и показания к терапии – гематологический рецидив или персистенция минимальной остаточной болезни (OR=0,018, 95% CI=0,153-0,841, p=0,01). Другие клинические или патологические параметры не оказывали существенного влияния на ответы на терапию.
Ключевые слова
В-клеточный острый лимфобластный лейкоз, рефрактерный и рецидивирующий, моноклональные антитела, блинатумомаб, инотузумаб озогамицин, общий ответ.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26375" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [9]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "145" ["~IBLOCK_SECTION_ID"]=> string(3) "145" ["ID"]=> string(4) "1850" ["~ID"]=> string(4) "1850" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(153) "Возможность элиминации введенных мультипотентных стромальных клеток через легкие" ["~NAME"]=> string(153) "Возможность элиминации введенных мультипотентных стромальных клеток через легкие" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "04/28/2020 11:00:18 am" ["~TIMESTAMP_X"]=> string(22) "04/28/2020 11:00:18 am" ["DETAIL_PAGE_URL"]=> string(141) "/en/archive/tom-9-nomer-1/eksperimentalnye-issledovaniya/vozmozhnost-eliminatsii-vvedennykh-multipotentnykh-stromalnykh-kletok-cherez-legkie/" ["~DETAIL_PAGE_URL"]=> string(141) "/en/archive/tom-9-nomer-1/eksperimentalnye-issledovaniya/vozmozhnost-eliminatsii-vvedennykh-multipotentnykh-stromalnykh-kletok-cherez-legkie/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(22884) "Introduction
The increasing implementation of cellular therapies into clinical practice and development of personalized medicine requires appropriate studies of differentiation, organ distribution, destruction and elimination of widely used multipotent stromal cells (MSC). According to the work of most researchers, the MSCs remain at the site of implantation after local administration, whereas intravenously infused MSCs first appear in lungs, being later on distributed to different tissues and organs [1, 2], however, at much fewer quantities. Moreover, there are reports on predominant migration of systemically administrated MSC into liver, and not into the lungs [3, 4].
The final fate of exogenously introduced MSC is still unclear. There are reports that MSCs are absorbed by immunocompetent cells of the recipient [5, 6], there is evidence of the production of antibodies against transplanted MSC with their rejection [7], the recipient can acquire immunity to MSC after repeated administration via increase in the percentage of T-cells [8]. Another possible way of eliminate MSC is that they can undergo apoptosis and disintegrate. The results of studies by A. Galleu et al. [9] are very interesting, they showed that apoptosis of MSC immediately after administration may be necessary for therapeutic efficacy. The destruction and elimination of introduced MSC, according to published data, occurs in the spleen [10], kidneys [10-12] and liver [10, 11]: the organs responsible for the destruction of other cells. However, Y. Takasaki et al. [4] found very few injected MSC in kidneys, and Y. Lei et al. [3] generally reject the possibility entering of such cells in the kidneys.
Due to the controversial data from literature about distribution and disappearance of MSC from the body, the aims of our study were as follows: to investigate final fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body via these organs.
Materials and methods
The experimental study was designed to perform morphological examination of lungs, liver and kidneys from male rats (Wag inbred strain) at 6 months of age (body mass 180-200 g).
It was performed at different terms after subcutaneous MMSC injections as made during cosmetic procedures. All manipulations with animals were carried out in aseptic operating room, in compliance with the "Rules for the Works Using Experimental Animals".
Isolation, characterization, preparation and introduction of MMSC in the experiment
MMSC were isolated from the bone marrow of syngeneic rats, characterized and cultured in accordance with our previous works [5, 6]. The second-passage MMSC were membrane-stained by Vybrant® CM-Dil solution (Thermo Fisher Scientific, USA), with maximal absorption wavelength of 553 nm, emission at 570 nm [5, 6]. The dye was added to MMSC suspension (5 µL per 106 cells in 1 mL serum-free culture medium), then gently and thoroughly mixed by pipetting, and left for 20 min in a CO2 incubator at 37°C, under 100% humidity. Then, MMSC were centrifuged for 5 min at 1500 rpm, the medium with the dye was discarded, and MMSC were resuspended in a new aliquote of warm medium. After 3-fold washing, MMSC were resuspended in warm medium and brought to a concentration of 106 cells/mL. Thereafter, following skin disinfection with ethyl alcohol, 100 μL of MMSC suspension in culture medium (1 mL contained 1x106 cells with a viability of >92%) was injected subcutaneously by insulin syringe into the region of upper third of the femur/inguinal ligament. The animals were sacrificed at 1; 2; 3; 4; and 5 weeks after MMSC injection. Intact rats were used as a control. In each group, 12 animals were used at any experimental point.
Sample processing for morphology studies
For morphological evaluation, the fragments of lungs, liver, and kidneys were isolated. The objects were fixed in a 4% paraformaldehyde in phosphate buffer solution (pH 7.4) for at least 24 hours, then dehydrated in a series of increasing ethanol concentrations. The samples were cleared with xylene, and embedded into Histoplast. The sections with a thickness of 5-7 μm were stained by hematoxylin and eosin, and examined with Axioimager M1 light microscope (Zeiss, Germany) at a magnification of ≤1200x.
In addition, unstained sections were examined in the luminescence mode of Axioimager M1 microscope using colour filters for Alexa Fluor 488 (excitation range 450-490 nm, registration range 515-∞ nm) and for Rhodamine (Rhod – excitation range 540-552 nm, registration range 575-640 nm). Tissue examination under UV light with Alexa Fluor 488 filter was aimed for detecting green background autofluorescence, which, firstly, provides good contrast for other luminescent objects, e.g., with Rhodamine filter (red color and its shades are clearly visible on a green background). Secondly, it is possible to see the structure of the studied tissues and better localize the objects with red luminescence in a distinct organ [5, 6].
Automatic exposure was used for obtaining microphotographs, when combining the images using Alexa Fluor 488 and rhodamine filters. Thus, one may obtain green and red (or orange and yellow) color, depending on prevalence of the glow intensity using distinct filters. Brighter fluorescence when using an Alexa Fluor 488 filter gives green luminescence, using a rhodamine filter produced red color. Yellow luminescence and its shades resulted from mixing green and red colors at different ratios [5, 6].
Results and discussion
The first luminescent objects of various shape and size, when applying rhodamine filter, were revealed in lungs by one week after MMSC injection (Fig. 1a). Numbers and fluorescence intensity of such luminous particles gradually increased over the observation period of 2-4 weeks (Fig. 1b, c, d). Only at 5th week, the number of luminescent structures decreased slightly, but remained detectable in significant number of cells. Most likely, the large fluorescent objects should represent macrophages, and smaller luminescent inclusions are their lysosomes. This conclusion was made on the basis of the following visual data [5, 6]: (1) Cell size: only few cells can exceed 20 µm in diameter, and macrophages are among such large cells; (2) Presence of many oval, clearly limited, fluorescent inclusions of different-size, which, most likely, are lysosomes. It should be noted that MMSC cytoplasm is rather evenly stained by Vybrant® CM-Dil [13]; (3) Irregular cell shape.

Figure 1. Rat lungs at different terms after inguinal subcutaneous MMSC injection. The combined images were obtained in luminescent mode of the microscope using Alexa 488 and rhodamine colour-filters
A, Numerous objects with predominant fluorescence obtained with Rhodamine filter are contained within alveolar wall one week after the MMSC injection.
B, Paravasal arrangement of structures with predominantly glowing inclusions with Rhodamine filter at 2 weeks after MMSC administration.
C, The objects with predominant fluorescence revealed with Rhodamine filter are observed in the wall of alveoli at 3 weeks after MMSC injection.
D, Four weeks after MMSC application: the particles with more intense fluoresce seen with Rhodamine filter are located in the alveolar wall.
Most likely, the Vybrant® CM-Dil-labeled MMSCs seem to penetrate into the bloodstream with time [6], migrate to the heart and arrive to lung, where are captured by numerous perivascular and alveolar macrophages, as it happens in other tissues [6]. Due to phagocytized material, the macrophage lysosomes become luminescent when exposed to UV light. When using Rhodamine colour filter [5, 6], one cannot also exclude staining of macrophage membranes by Vybrant® CM-Dil.
Due to short lifetime of injected MMSC in the tissues, an opportunity exists for migration of both cells and their fragments (debris) through blood flow into pulmonary circulation [5, 14, 15]. This MMSC debris is adsorbed by pulmonary macrophages causing staining of their structures. There is an opportunity of macrophage migration with other antigens, including those with Vybrant® CM-Dil-stained detritus, from the tissues via blood to the lungs. In any case, the lung tissues and structures do not contain any intact Vybrant® CM-Dil-labeled MMSC, but only their fragments, or debris-containing macrophages.
Hence, the described time-dependent dynamics of luminescent cell numbers in the lungs could be well explained by gradual replacement of injected MMSCs and relevant structures by cell populations differentiating from the own recipient cells. According to the literature data, this process is maximally expressed in rats within 2-3 weeks after the MMSC administration, when labeled cells disappear from the tissues, and the number of luminescent macrophages in regional lymph nodes becomes the largest [5].
Thus, one may suggest that, the detritus from injected MMSCs site early arrives to lungs, i.e., the cell fragments which were initially non-viable or died in situ soon after administration, due to drastic changes in their viability [5, 14, 15]. Then, starting from 2 weeks, when there is a massive elimination of labeled cells from tissues [5], the content of the dye-labeled objects in the lungs increases sharply, when using a Rhodamine colour-filter. It could be suggested that the stained MMSCs or their detritus do not enter lungs at later terms, and only macrophages that have previously absorbed a large volume of labeled objects will show fluorescence. Consequently, the luminescence intensity and the number of fluorescent cells will gradually decrease, due to the migration of such macrophages, their destruction and, possibly, due to the gradual lysis of stained structures, with a decrease in the Vybrant® CM-Dil concentration.
It should be especially noted that after 2 weeks, upon careful examination of the lungs, a red tint was found in the inclusions in the epithelial cells of some bronchioles (Fig. 2a), which contained a certain amount of cellular detritus, these was clearly visible when staining sections by hematoxylin and eosin (Fig. 2b). At week 3, the inclusions in epithelial cells of individual bronchioles showed a more distinct red staining when exposed to UV light via a Rhodamine filter. A careful study sometimes showed oval objects with weak red fluorescence, similar to cells with destruction phenomena: the empty cell membranes after loss of its content, organelles (Fig. 2c). Peribronchial tissues in such areas were abundantly infiltrated by macrophages (Fig. 2d).
It should be noted that sometimes, also at 2-3 weeks after MMSC injection, the Vybrant® CM-Dil-labeled objects were located on the very edge of alveolar lining, being even partially located in the alveolar lumen (Fig. 2e, g), where many macrophages with signs of destructive changes were detected in H&E-stained sections (Fig. 2f, h).


Fig. 2. Morphological data concerning possible elimination of MMSC detritus via the lungs in experiment
A, Small inclusions in the bronchiolar epithelium at 2 weeks after MMSC injection fluoresce red with Rhodamine filter. The combination of images obtained in the luminescent mode of the microscopy using Alexa 488- and rhodamine filters.
B, Cellular detritus in the bronchiolar lumen represented in the photo "a". (Hematoxylin/Eosin staining).
C, Cells with a significant predominance of fluorescence with Rhodamine filter are located next to bronchiole at 3 weeks after MMSC injection. An object similar to a cell with destruction phenomena, is weakly glowing when using Rhodamine filter, being located in the bronchiolar epithelium (arrow). Combined images obtained by luminescence microscopy using Alexa 488- and Rhodamine filters.
D, Tissues adjacent to the bronchiole represented in picture "c" are largely infiltrated by macrophages (Hematoxylin/Eosin staining).
E, Distinct objects located just along the edge of alveoli 2 weeks after the MMSC injection glow much brighter when using a Rhodamine filter: combined images obtained by luminescent microscopy with Alexa 488 and Rhodamine filters.
F, Oedematous effusion, macrophages and red blood cells are located in alveolar lumen, presented on the picture "e" (Hematoxylin/Eosin staining).
G, Objects of various sizes with intense fluorescence when using Rhodamine filter are located along the edge and in lumen of alveoli (arrow) 3 weeks after MMSC use. The combined images were obtained by luminescent microscopy with Alexa 488 and Rhodamine filters.
H, The limited area of alveolar lumen presented in picture "g" contains edematous effusion with fibrin, erythrocytes and macrophages, some phagocytes show destruction signs. (Hematoxylin/Eosin staining).
It is possible that such a pattern is associated with elimination of MMSC detritus (or macrophages with large amounts of such MMSC debris) from the body. It is likely that macrophages with large amounts of MMSC detritus from capillaries and larger vessels of the lungs, are also dying, and their fragments appear first at the edge, and then within alveolar lumens (or phagocytes migrate into the lumen of alveoli, where they die and undergo destruction) from where they are eliminated outwards through the bronchi and trachea. It is also possible that macrophages with MMSC detritus from other tissues can migrate to the lungs and undergo destruction. Labeled Vybrant® CM-Dil detritus of previous MMSCs, and now macrophages, appears in the alveolar lumens, from where it is eliminated to the ambient via bronchi of different sizes, staining some epithelial structures as well.
Thus, MMSC could get into the lungs from the inguinal subcutaneous tissues via blood vessels. The Vybrant® CM-Dil-labeled MMSC and their detritus are absorbed in lungs by numerous macrophages located near vessels and in alveolar septae. The macrophages may die, after accumulating a lot of antigenic particles, and these incompletely lysed substances, including Vybrant® CM-Dil-labeled MMSC detritus and, possibly, stained membranes of these phagocytes, first appear in alveolar walls, and, later, in their lumens. Consequently, these substances are eliminated through the bronchi outwards. This is evidenced by both stained inclusions in bronchiolar epithelium and Vybrant® CM-Dil-labeled fragments of cells, most likely macrophages, in alveoli and bronchi.
At all observation periods, the objects stained by Vybrant® CM-Dil were not found in liver and kidneys. Apparently, the subcutaneously injected MMSCs, if they enter blood flow, or these cells completely settle in the capillary network of the pulmonary circulation and do not reach the indicated organs. Another reason is that their macrophages do not adsorb antigens such as MMSC and their debris from the blood. This coincides with some results of Lei et al. [3] and Takasaki et al. [4], who detected no MSC or found very little this cells after their systemic administration in the kidneys, but contradicts other data from these and other researchers [3, 4, 10-12], especially reports of higher MSC numbers in liver than in lungs [3, 4], and on destruction and elimination of injected MSC by liver [10, 12] and/or kidneys [10-12].
Conclusion
Hence, one may conclude that MMSCs injected into the subcutaneous tissues and their detritus enter blood flow and end up in the lungs, where these cells are phagocytized by macrophages. The MMSC debris can disseminate throughout the organism only after filtration in lungs. In lungs, the elimination of detritus from injected MMSC could proceed via alveoli and further, through the bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys. Therefore, it contradicts some literature data on important role of these organs in destruction or removal of injected autologous or foreign MSCs.
Acknowledgements
This work was financially supported by the Fundamental Research Program of the State Academies of Sciences for 2017-2020 (VI.62.2.1, 0309-2016-0006) "Development of technologies for obtaining materials for regenerative medicine and development of methods for restoring reproductive health".
The authors declare no conflict of interest, they did not receive any financial support from the drug manufacturers.
References
Introduction
The increasing implementation of cellular therapies into clinical practice and development of personalized medicine requires appropriate studies of differentiation, organ distribution, destruction and elimination of widely used multipotent stromal cells (MSC). According to the work of most researchers, the MSCs remain at the site of implantation after local administration, whereas intravenously infused MSCs first appear in lungs, being later on distributed to different tissues and organs [1, 2], however, at much fewer quantities. Moreover, there are reports on predominant migration of systemically administrated MSC into liver, and not into the lungs [3, 4].
The final fate of exogenously introduced MSC is still unclear. There are reports that MSCs are absorbed by immunocompetent cells of the recipient [5, 6], there is evidence of the production of antibodies against transplanted MSC with their rejection [7], the recipient can acquire immunity to MSC after repeated administration via increase in the percentage of T-cells [8]. Another possible way of eliminate MSC is that they can undergo apoptosis and disintegrate. The results of studies by A. Galleu et al. [9] are very interesting, they showed that apoptosis of MSC immediately after administration may be necessary for therapeutic efficacy. The destruction and elimination of introduced MSC, according to published data, occurs in the spleen [10], kidneys [10-12] and liver [10, 11]: the organs responsible for the destruction of other cells. However, Y. Takasaki et al. [4] found very few injected MSC in kidneys, and Y. Lei et al. [3] generally reject the possibility entering of such cells in the kidneys.
Due to the controversial data from literature about distribution and disappearance of MSC from the body, the aims of our study were as follows: to investigate final fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body via these organs.
Materials and methods
The experimental study was designed to perform morphological examination of lungs, liver and kidneys from male rats (Wag inbred strain) at 6 months of age (body mass 180-200 g).
It was performed at different terms after subcutaneous MMSC injections as made during cosmetic procedures. All manipulations with animals were carried out in aseptic operating room, in compliance with the "Rules for the Works Using Experimental Animals".
Isolation, characterization, preparation and introduction of MMSC in the experiment
MMSC were isolated from the bone marrow of syngeneic rats, characterized and cultured in accordance with our previous works [5, 6]. The second-passage MMSC were membrane-stained by Vybrant® CM-Dil solution (Thermo Fisher Scientific, USA), with maximal absorption wavelength of 553 nm, emission at 570 nm [5, 6]. The dye was added to MMSC suspension (5 µL per 106 cells in 1 mL serum-free culture medium), then gently and thoroughly mixed by pipetting, and left for 20 min in a CO2 incubator at 37°C, under 100% humidity. Then, MMSC were centrifuged for 5 min at 1500 rpm, the medium with the dye was discarded, and MMSC were resuspended in a new aliquote of warm medium. After 3-fold washing, MMSC were resuspended in warm medium and brought to a concentration of 106 cells/mL. Thereafter, following skin disinfection with ethyl alcohol, 100 μL of MMSC suspension in culture medium (1 mL contained 1x106 cells with a viability of >92%) was injected subcutaneously by insulin syringe into the region of upper third of the femur/inguinal ligament. The animals were sacrificed at 1; 2; 3; 4; and 5 weeks after MMSC injection. Intact rats were used as a control. In each group, 12 animals were used at any experimental point.
Sample processing for morphology studies
For morphological evaluation, the fragments of lungs, liver, and kidneys were isolated. The objects were fixed in a 4% paraformaldehyde in phosphate buffer solution (pH 7.4) for at least 24 hours, then dehydrated in a series of increasing ethanol concentrations. The samples were cleared with xylene, and embedded into Histoplast. The sections with a thickness of 5-7 μm were stained by hematoxylin and eosin, and examined with Axioimager M1 light microscope (Zeiss, Germany) at a magnification of ≤1200x.
In addition, unstained sections were examined in the luminescence mode of Axioimager M1 microscope using colour filters for Alexa Fluor 488 (excitation range 450-490 nm, registration range 515-∞ nm) and for Rhodamine (Rhod – excitation range 540-552 nm, registration range 575-640 nm). Tissue examination under UV light with Alexa Fluor 488 filter was aimed for detecting green background autofluorescence, which, firstly, provides good contrast for other luminescent objects, e.g., with Rhodamine filter (red color and its shades are clearly visible on a green background). Secondly, it is possible to see the structure of the studied tissues and better localize the objects with red luminescence in a distinct organ [5, 6].
Automatic exposure was used for obtaining microphotographs, when combining the images using Alexa Fluor 488 and rhodamine filters. Thus, one may obtain green and red (or orange and yellow) color, depending on prevalence of the glow intensity using distinct filters. Brighter fluorescence when using an Alexa Fluor 488 filter gives green luminescence, using a rhodamine filter produced red color. Yellow luminescence and its shades resulted from mixing green and red colors at different ratios [5, 6].
Results and discussion
The first luminescent objects of various shape and size, when applying rhodamine filter, were revealed in lungs by one week after MMSC injection (Fig. 1a). Numbers and fluorescence intensity of such luminous particles gradually increased over the observation period of 2-4 weeks (Fig. 1b, c, d). Only at 5th week, the number of luminescent structures decreased slightly, but remained detectable in significant number of cells. Most likely, the large fluorescent objects should represent macrophages, and smaller luminescent inclusions are their lysosomes. This conclusion was made on the basis of the following visual data [5, 6]: (1) Cell size: only few cells can exceed 20 µm in diameter, and macrophages are among such large cells; (2) Presence of many oval, clearly limited, fluorescent inclusions of different-size, which, most likely, are lysosomes. It should be noted that MMSC cytoplasm is rather evenly stained by Vybrant® CM-Dil [13]; (3) Irregular cell shape.

Figure 1. Rat lungs at different terms after inguinal subcutaneous MMSC injection. The combined images were obtained in luminescent mode of the microscope using Alexa 488 and rhodamine colour-filters
A, Numerous objects with predominant fluorescence obtained with Rhodamine filter are contained within alveolar wall one week after the MMSC injection.
B, Paravasal arrangement of structures with predominantly glowing inclusions with Rhodamine filter at 2 weeks after MMSC administration.
C, The objects with predominant fluorescence revealed with Rhodamine filter are observed in the wall of alveoli at 3 weeks after MMSC injection.
D, Four weeks after MMSC application: the particles with more intense fluoresce seen with Rhodamine filter are located in the alveolar wall.
Most likely, the Vybrant® CM-Dil-labeled MMSCs seem to penetrate into the bloodstream with time [6], migrate to the heart and arrive to lung, where are captured by numerous perivascular and alveolar macrophages, as it happens in other tissues [6]. Due to phagocytized material, the macrophage lysosomes become luminescent when exposed to UV light. When using Rhodamine colour filter [5, 6], one cannot also exclude staining of macrophage membranes by Vybrant® CM-Dil.
Due to short lifetime of injected MMSC in the tissues, an opportunity exists for migration of both cells and their fragments (debris) through blood flow into pulmonary circulation [5, 14, 15]. This MMSC debris is adsorbed by pulmonary macrophages causing staining of their structures. There is an opportunity of macrophage migration with other antigens, including those with Vybrant® CM-Dil-stained detritus, from the tissues via blood to the lungs. In any case, the lung tissues and structures do not contain any intact Vybrant® CM-Dil-labeled MMSC, but only their fragments, or debris-containing macrophages.
Hence, the described time-dependent dynamics of luminescent cell numbers in the lungs could be well explained by gradual replacement of injected MMSCs and relevant structures by cell populations differentiating from the own recipient cells. According to the literature data, this process is maximally expressed in rats within 2-3 weeks after the MMSC administration, when labeled cells disappear from the tissues, and the number of luminescent macrophages in regional lymph nodes becomes the largest [5].
Thus, one may suggest that, the detritus from injected MMSCs site early arrives to lungs, i.e., the cell fragments which were initially non-viable or died in situ soon after administration, due to drastic changes in their viability [5, 14, 15]. Then, starting from 2 weeks, when there is a massive elimination of labeled cells from tissues [5], the content of the dye-labeled objects in the lungs increases sharply, when using a Rhodamine colour-filter. It could be suggested that the stained MMSCs or their detritus do not enter lungs at later terms, and only macrophages that have previously absorbed a large volume of labeled objects will show fluorescence. Consequently, the luminescence intensity and the number of fluorescent cells will gradually decrease, due to the migration of such macrophages, their destruction and, possibly, due to the gradual lysis of stained structures, with a decrease in the Vybrant® CM-Dil concentration.
It should be especially noted that after 2 weeks, upon careful examination of the lungs, a red tint was found in the inclusions in the epithelial cells of some bronchioles (Fig. 2a), which contained a certain amount of cellular detritus, these was clearly visible when staining sections by hematoxylin and eosin (Fig. 2b). At week 3, the inclusions in epithelial cells of individual bronchioles showed a more distinct red staining when exposed to UV light via a Rhodamine filter. A careful study sometimes showed oval objects with weak red fluorescence, similar to cells with destruction phenomena: the empty cell membranes after loss of its content, organelles (Fig. 2c). Peribronchial tissues in such areas were abundantly infiltrated by macrophages (Fig. 2d).
It should be noted that sometimes, also at 2-3 weeks after MMSC injection, the Vybrant® CM-Dil-labeled objects were located on the very edge of alveolar lining, being even partially located in the alveolar lumen (Fig. 2e, g), where many macrophages with signs of destructive changes were detected in H&E-stained sections (Fig. 2f, h).


Fig. 2. Morphological data concerning possible elimination of MMSC detritus via the lungs in experiment
A, Small inclusions in the bronchiolar epithelium at 2 weeks after MMSC injection fluoresce red with Rhodamine filter. The combination of images obtained in the luminescent mode of the microscopy using Alexa 488- and rhodamine filters.
B, Cellular detritus in the bronchiolar lumen represented in the photo "a". (Hematoxylin/Eosin staining).
C, Cells with a significant predominance of fluorescence with Rhodamine filter are located next to bronchiole at 3 weeks after MMSC injection. An object similar to a cell with destruction phenomena, is weakly glowing when using Rhodamine filter, being located in the bronchiolar epithelium (arrow). Combined images obtained by luminescence microscopy using Alexa 488- and Rhodamine filters.
D, Tissues adjacent to the bronchiole represented in picture "c" are largely infiltrated by macrophages (Hematoxylin/Eosin staining).
E, Distinct objects located just along the edge of alveoli 2 weeks after the MMSC injection glow much brighter when using a Rhodamine filter: combined images obtained by luminescent microscopy with Alexa 488 and Rhodamine filters.
F, Oedematous effusion, macrophages and red blood cells are located in alveolar lumen, presented on the picture "e" (Hematoxylin/Eosin staining).
G, Objects of various sizes with intense fluorescence when using Rhodamine filter are located along the edge and in lumen of alveoli (arrow) 3 weeks after MMSC use. The combined images were obtained by luminescent microscopy with Alexa 488 and Rhodamine filters.
H, The limited area of alveolar lumen presented in picture "g" contains edematous effusion with fibrin, erythrocytes and macrophages, some phagocytes show destruction signs. (Hematoxylin/Eosin staining).
It is possible that such a pattern is associated with elimination of MMSC detritus (or macrophages with large amounts of such MMSC debris) from the body. It is likely that macrophages with large amounts of MMSC detritus from capillaries and larger vessels of the lungs, are also dying, and their fragments appear first at the edge, and then within alveolar lumens (or phagocytes migrate into the lumen of alveoli, where they die and undergo destruction) from where they are eliminated outwards through the bronchi and trachea. It is also possible that macrophages with MMSC detritus from other tissues can migrate to the lungs and undergo destruction. Labeled Vybrant® CM-Dil detritus of previous MMSCs, and now macrophages, appears in the alveolar lumens, from where it is eliminated to the ambient via bronchi of different sizes, staining some epithelial structures as well.
Thus, MMSC could get into the lungs from the inguinal subcutaneous tissues via blood vessels. The Vybrant® CM-Dil-labeled MMSC and their detritus are absorbed in lungs by numerous macrophages located near vessels and in alveolar septae. The macrophages may die, after accumulating a lot of antigenic particles, and these incompletely lysed substances, including Vybrant® CM-Dil-labeled MMSC detritus and, possibly, stained membranes of these phagocytes, first appear in alveolar walls, and, later, in their lumens. Consequently, these substances are eliminated through the bronchi outwards. This is evidenced by both stained inclusions in bronchiolar epithelium and Vybrant® CM-Dil-labeled fragments of cells, most likely macrophages, in alveoli and bronchi.
At all observation periods, the objects stained by Vybrant® CM-Dil were not found in liver and kidneys. Apparently, the subcutaneously injected MMSCs, if they enter blood flow, or these cells completely settle in the capillary network of the pulmonary circulation and do not reach the indicated organs. Another reason is that their macrophages do not adsorb antigens such as MMSC and their debris from the blood. This coincides with some results of Lei et al. [3] and Takasaki et al. [4], who detected no MSC or found very little this cells after their systemic administration in the kidneys, but contradicts other data from these and other researchers [3, 4, 10-12], especially reports of higher MSC numbers in liver than in lungs [3, 4], and on destruction and elimination of injected MSC by liver [10, 12] and/or kidneys [10-12].
Conclusion
Hence, one may conclude that MMSCs injected into the subcutaneous tissues and their detritus enter blood flow and end up in the lungs, where these cells are phagocytized by macrophages. The MMSC debris can disseminate throughout the organism only after filtration in lungs. In lungs, the elimination of detritus from injected MMSC could proceed via alveoli and further, through the bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys. Therefore, it contradicts some literature data on important role of these organs in destruction or removal of injected autologous or foreign MSCs.
Acknowledgements
This work was financially supported by the Fundamental Research Program of the State Academies of Sciences for 2017-2020 (VI.62.2.1, 0309-2016-0006) "Development of technologies for obtaining materials for regenerative medicine and development of methods for restoring reproductive health".
The authors declare no conflict of interest, they did not receive any financial support from the drug manufacturers.
References
Игорь В. Майбородин1, Роман В. Маслов1, Татьяна В. Михеева1, Сергей В. Марчуков1, Виталина И. Майбородина2, Александр А. Шевела1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26411" ["VALUE"]=> array(2) { ["TEXT"]=> string(705) "<p><sup>1</sup> Центр новых медицинских технологий, Федеральное государственное бюджетное учреждение науки «Институт химической биологии и фундаментальной медицины СО РАН», Новосибирск, Россия<br> <sup>2</sup> Институт молекулярной патологии и патоморфологии, Федеральный научно-исследовательский центр фундаментальной и трансляционной медицины, Новосибирск, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(663) "1 Центр новых медицинских технологий, Федеральное государственное бюджетное учреждение науки «Институт химической биологии и фундаментальной медицины СО РАН», Новосибирск, Россия
2 Институт молекулярной патологии и патоморфологии, Федеральный научно-исследовательский центр фундаментальной и трансляционной медицины, Новосибирск, Россия
Согласно работам большинства исследователей, при местном применении мультипотентные стромальные клетки (МСК) остаются в месте имплантации, а введенные внутривенно МСК сначала оказываются в легких и только потом распределяются по всему организму, но уже в значительно меньшем количестве. Вместе с тем имеются сообщения о преимущественной миграции инфузированных системно МСК в печень, а не в легкие. Разрушение и элиминация введенных МСК, по данным литературы, происходит в селезенке, почках и печени. В связи с противоречивостью литературных данных о распределении и исчезновении МСК из организма была поставлена цель исследования – изучить возможность попадания в печень, почки и легкие мультипотентных мезенхимных стромальных клеток (ММСК) после их тканевой инъекции, а также получить новые доказательства их элиминации из организма указанными органами.
Материал и методика
Работа основана на результатах морфологического исследования легких, печени и почек крыс-самцов инбредной линии Wag в разные сроки после подкожной инъекции в паховую область ММСК, окрашенных мембрано-специфическим красителем Vybrant® CM-Dil. Методами световой и люминесцентной микроскопии регистрировали появление и распределение меченых объектов в указанных органах.
Результаты
Через 1 неделю после введения ММСК, в легких появились первые люминесцирующие макрофаги, выявляемые при посредстве светофильтра для родамина. В течение 2-4 недель количество таких светящихся клеток постепенно нарастает, также увеличивается интенсивность их флюоресценции. Спустя 2-3 недели был найден красный оттенок во включениях в эпителиальных клетках отдельных бронхиол. Иногда меченые Vybrant® CM-Dil объекты были расположены по самому краю альвеол и даже частично находились в просвете альвеол. Объекты, окрашенные Vybrant® CM-Dil, не были найдены в печени и почках во все сроки наблюдения.
Заключение
После инъекции в подкожно-жировую клетчатку ММСК и их фрагменты попадают в кровоток и оказываются в легких, где фагоцитируются макрофагами. Только после фильтрации в легких, дебрис ММСК может диссеминировать по всему организму. В легких возможна элиминация детрита инъецированных ММСК в альвеолы и далее, через систему бронхов, во внешнюю среду. В печени и почках меченые Vybrant® CM-Dil фрагменты ММСК найдены не были, что не соответствует данным литературы о важной роли этих органов в деструкции или удалении введенных извне МСК.
Ключевые слова
Мультипотентные стромальные клетки, элиминация, легкие, макрофаги, альвеолы, печень, почки.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26412" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-67-73" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-67-73" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26417" ["VALUE"]=> array(2) { ["TEXT"]=> string(288) "<p>Igor V. Maiborodin<sup>1</sup>, Roman V. Maslov<sup>1</sup>, Tatiana V. Mikheeva<sup>1</sup>, Sergey V. Marchukov<sup>1</sup>, Vitalina I. Maiborodina<sup>2</sup>, Aleksandr A. Shevela<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(204) "Igor V. Maiborodin1, Roman V. Maslov1, Tatiana V. Mikheeva1, Sergey V. Marchukov1, Vitalina I. Maiborodina2, Aleksandr A. Shevela1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26418" ["VALUE"]=> array(2) { ["TEXT"]=> string(789) "<p><sup>1</sup> Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia<br> <sup>2</sup> Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia</p> <br> <p><b>Correspondence</b><br> Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia<br> Phone: +7 (913) 753 0767 <br> Е-mail: imai@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(699) "1 Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia
2 Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia
Correspondence
Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia
Phone: +7 (913) 753 0767
Е-mail: imai@mail.ru
In compliance with most observations, the multipotent stromal cells (MSC) remain at the site of implantation after local application, whereas intravenously administrated MSC first appear in lungs followed by distribution to different tissues and organs, however, at much smaller quantities. Moreover, there are data on predominant migration of systemically infused MSC into liver, and not into the lungs. Destruction and elimination of introduced MSC, according to published data, occurs in the spleen, kidneys and liver. Due to controversial results on MSC distribution and elimination from the body, the aims of our study were as follows: to investigate the fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body via these organs.
Materials and methods
The work is based on morphological evaluation of lungs, liver and kidneys from 6-mo old male rats (Wag inbred strain) at different terms after subcutaneous injection of MMSC labeled with membrane-binding Vybrant® CM-Dil dye. Morphological patterns and distribution of labeled objects in these organs were investigated using visible and luminescent microscopy.
Results
The first luminescent macrophages observed with rhodamine colour-filter were initially revealed in the lungs one week after MMSC injections. The number of luminescent cells and intensity of their fluorescence increased gradually over successive 2-4 weeks. By 2-3 weeks, this red tint was revealed in the inclusions of epithelial cells in some bronchioles. The Vybrant® CM-Dil-labeled objects were sometimes detected at the very edge of alveoli, and even partially located in alveolar lumen. The objects stained by Vybrant® CM-Dil were not found in liver and kidneys at any observation terms.
Conclusion>/h3>
Following subcutaneous injection, MMSCs and their fragments seem to enter bloodstream and migrate to the lungs, where these cells are phagocytized by macrophages. Only after filtration in lungs, the MMSC debris can further disseminate throughout the body. Elimination of detritus from injected MMSC is possible in lung alveoli and further, via bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys, thus disagreeing the literature data on potential role of these organs in the destruction or removal of injected autologous or foreign MSC.
Keywords
Multipotent stromal cells, elimination, lungs, macrophages, alveoli, liver, kidneys.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26413" ["VALUE"]=> string(75) "Opportunity for elimination of injected multipotent stromal cells via lungs" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(75) "Opportunity for elimination of injected multipotent stromal cells via lungs" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26415" ["VALUE"]=> string(4) "1992" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1992" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26420" ["VALUE"]=> string(4) "1996" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "1996" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26417" ["VALUE"]=> array(2) { ["TEXT"]=> string(288) "<p>Igor V. Maiborodin<sup>1</sup>, Roman V. Maslov<sup>1</sup>, Tatiana V. Mikheeva<sup>1</sup>, Sergey V. Marchukov<sup>1</sup>, Vitalina I. Maiborodina<sup>2</sup>, Aleksandr A. Shevela<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(204) "Igor V. Maiborodin1, Roman V. Maslov1, Tatiana V. Mikheeva1, Sergey V. Marchukov1, Vitalina I. Maiborodina2, Aleksandr A. Shevela1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(204) "Igor V. Maiborodin1, Roman V. Maslov1, Tatiana V. Mikheeva1, Sergey V. Marchukov1, Vitalina I. Maiborodina2, Aleksandr A. Shevela1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26419" ["VALUE"]=> array(2) { ["TEXT"]=> string(2979) "<p style="text-align: justify;">In compliance with most observations, the multipotent stromal cells (MSC) remain at the site of implantation after local application, whereas intravenously administrated MSC first appear in lungs followed by distribution to different tissues and organs, however, at much smaller quantities. Moreover, there are data on predominant migration of systemically infused MSC into liver, and not into the lungs. Destruction and elimination of introduced MSC, according to published data, occurs in the spleen, kidneys and liver. Due to controversial results on MSC distribution and elimination from the body, the aims of our study were as follows: to investigate the fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body <i>via</i> these organs.</p> <h3>Materials and methods</h3> <p style="text-align: justify;"> The work is based on morphological evaluation of lungs, liver and kidneys from 6-mo old male rats (Wag inbred strain) at different terms after subcutaneous injection of MMSC labeled with membrane-binding Vybrant® CM-Dil dye. Morphological patterns and distribution of labeled objects in these organs were investigated using visible and luminescent microscopy.</p> <h3>Results</h3> <p style="text-align: justify;"> The first luminescent macrophages observed with rhodamine colour-filter were initially revealed in the lungs one week after MMSC injections. The number of luminescent cells and intensity of their fluorescence increased gradually over successive 2-4 weeks. By 2-3 weeks, this red tint was revealed in the inclusions of epithelial cells in some bronchioles. The Vybrant® CM-Dil-labeled objects were sometimes detected at the very edge of alveoli, and even partially located in alveolar lumen. The objects stained by Vybrant® CM-Dil were not found in liver and kidneys at any observation terms. </p> <h3>Conclusion>/h3> <p style="text-align: justify;"> Following subcutaneous injection, MMSCs and their fragments seem to enter bloodstream and migrate to the lungs, where these cells are phagocytized by macrophages. Only after filtration in lungs, the MMSC debris can further disseminate throughout the body. Elimination of detritus from injected MMSC is possible in lung alveoli and further, via bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys, thus disagreeing the literature data on potential role of these organs in the destruction or removal of injected autologous or foreign MSC. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Multipotent stromal cells, elimination, lungs, macrophages, alveoli, liver, kidneys.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2809) "In compliance with most observations, the multipotent stromal cells (MSC) remain at the site of implantation after local application, whereas intravenously administrated MSC first appear in lungs followed by distribution to different tissues and organs, however, at much smaller quantities. Moreover, there are data on predominant migration of systemically infused MSC into liver, and not into the lungs. Destruction and elimination of introduced MSC, according to published data, occurs in the spleen, kidneys and liver. Due to controversial results on MSC distribution and elimination from the body, the aims of our study were as follows: to investigate the fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body via these organs.
Materials and methods
The work is based on morphological evaluation of lungs, liver and kidneys from 6-mo old male rats (Wag inbred strain) at different terms after subcutaneous injection of MMSC labeled with membrane-binding Vybrant® CM-Dil dye. Morphological patterns and distribution of labeled objects in these organs were investigated using visible and luminescent microscopy.
Results
The first luminescent macrophages observed with rhodamine colour-filter were initially revealed in the lungs one week after MMSC injections. The number of luminescent cells and intensity of their fluorescence increased gradually over successive 2-4 weeks. By 2-3 weeks, this red tint was revealed in the inclusions of epithelial cells in some bronchioles. The Vybrant® CM-Dil-labeled objects were sometimes detected at the very edge of alveoli, and even partially located in alveolar lumen. The objects stained by Vybrant® CM-Dil were not found in liver and kidneys at any observation terms.
Conclusion>/h3>
Following subcutaneous injection, MMSCs and their fragments seem to enter bloodstream and migrate to the lungs, where these cells are phagocytized by macrophages. Only after filtration in lungs, the MMSC debris can further disseminate throughout the body. Elimination of detritus from injected MMSC is possible in lung alveoli and further, via bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys, thus disagreeing the literature data on potential role of these organs in the destruction or removal of injected autologous or foreign MSC.
Keywords
Multipotent stromal cells, elimination, lungs, macrophages, alveoli, liver, kidneys.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2809) "In compliance with most observations, the multipotent stromal cells (MSC) remain at the site of implantation after local application, whereas intravenously administrated MSC first appear in lungs followed by distribution to different tissues and organs, however, at much smaller quantities. Moreover, there are data on predominant migration of systemically infused MSC into liver, and not into the lungs. Destruction and elimination of introduced MSC, according to published data, occurs in the spleen, kidneys and liver. Due to controversial results on MSC distribution and elimination from the body, the aims of our study were as follows: to investigate the fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body via these organs.
Materials and methods
The work is based on morphological evaluation of lungs, liver and kidneys from 6-mo old male rats (Wag inbred strain) at different terms after subcutaneous injection of MMSC labeled with membrane-binding Vybrant® CM-Dil dye. Morphological patterns and distribution of labeled objects in these organs were investigated using visible and luminescent microscopy.
Results
The first luminescent macrophages observed with rhodamine colour-filter were initially revealed in the lungs one week after MMSC injections. The number of luminescent cells and intensity of their fluorescence increased gradually over successive 2-4 weeks. By 2-3 weeks, this red tint was revealed in the inclusions of epithelial cells in some bronchioles. The Vybrant® CM-Dil-labeled objects were sometimes detected at the very edge of alveoli, and even partially located in alveolar lumen. The objects stained by Vybrant® CM-Dil were not found in liver and kidneys at any observation terms.
Conclusion>/h3>
Following subcutaneous injection, MMSCs and their fragments seem to enter bloodstream and migrate to the lungs, where these cells are phagocytized by macrophages. Only after filtration in lungs, the MMSC debris can further disseminate throughout the body. Elimination of detritus from injected MMSC is possible in lung alveoli and further, via bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys, thus disagreeing the literature data on potential role of these organs in the destruction or removal of injected autologous or foreign MSC.
Keywords
Multipotent stromal cells, elimination, lungs, macrophages, alveoli, liver, kidneys.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26412" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-67-73" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-67-73" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-1-67-73" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26413" ["VALUE"]=> string(75) "Opportunity for elimination of injected multipotent stromal cells via lungs" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(75) "Opportunity for elimination of injected multipotent stromal cells via lungs" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(75) "Opportunity for elimination of injected multipotent stromal cells via lungs" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26418" ["VALUE"]=> array(2) { ["TEXT"]=> string(789) "<p><sup>1</sup> Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia<br> <sup>2</sup> Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia</p> <br> <p><b>Correspondence</b><br> Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia<br> Phone: +7 (913) 753 0767 <br> Е-mail: imai@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(699) "1 Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia
2 Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia
Correspondence
Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia
Phone: +7 (913) 753 0767
Е-mail: imai@mail.ru
1 Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia
2 Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia
Correspondence
Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia
Phone: +7 (913) 753 0767
Е-mail: imai@mail.ru
Игорь В. Майбородин1, Роман В. Маслов1, Татьяна В. Михеева1, Сергей В. Марчуков1, Виталина И. Майбородина2, Александр А. Шевела1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(297) "Игорь В. Майбородин1, Роман В. Маслов1, Татьяна В. Михеева1, Сергей В. Марчуков1, Виталина И. Майбородина2, Александр А. Шевела1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26408" ["VALUE"]=> string(22) "12/05/2019 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/05/2019 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/05/2019 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26409" ["VALUE"]=> string(22) "02/21/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/21/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "02/21/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26416" ["VALUE"]=> array(2) { ["TEXT"]=> string(4966) "<p style="text-align: justify;">Согласно работам большинства исследователей, при местном применении мультипотентные стромальные клетки (МСК) остаются в месте имплантации, а введенные внутривенно МСК сначала оказываются в легких и только потом распределяются по всему организму, но уже в значительно меньшем количестве. Вместе с тем имеются сообщения о преимущественной миграции инфузированных системно МСК в печень, а не в легкие. Разрушение и элиминация введенных МСК, по данным литературы, происходит в селезенке, почках и печени. В связи с противоречивостью литературных данных о распределении и исчезновении МСК из организма была поставлена цель исследования – изучить возможность попадания в печень, почки и легкие мультипотентных мезенхимных стромальных клеток (ММСК) после их тканевой инъекции, а также получить новые доказательства их элиминации из организма указанными органами.</p> <h3>Материал и методика</h3> <p style="text-align: justify;"> Работа основана на результатах морфологического исследования легких, печени и почек крыс-самцов инбредной линии Wag в разные сроки после подкожной инъекции в паховую область ММСК, окрашенных мембрано-специфическим красителем Vybrant® CM-Dil. Методами световой и люминесцентной микроскопии регистрировали появление и распределение меченых объектов в указанных органах. </p> <h3>Результаты</h3> <p style="text-align: justify;"> Через 1 неделю после введения ММСК, в легких появились первые люминесцирующие макрофаги, выявляемые при посредстве светофильтра для родамина. В течение 2-4 недель количество таких светящихся клеток постепенно нарастает, также увеличивается интенсивность их флюоресценции. Спустя 2-3 недели был найден красный оттенок во включениях в эпителиальных клетках отдельных бронхиол. Иногда меченые Vybrant® CM-Dil объекты были расположены по самому краю альвеол и даже частично находились в просвете альвеол. Объекты, окрашенные Vybrant® CM-Dil, не были найдены в печени и почках во все сроки наблюдения. </p> <h3>Заключение</h3> <p style="text-align: justify;"> После инъекции в подкожно-жировую клетчатку ММСК и их фрагменты попадают в кровоток и оказываются в легких, где фагоцитируются макрофагами. Только после фильтрации в легких, дебрис ММСК может диссеминировать по всему организму. В легких возможна элиминация детрита инъецированных ММСК в альвеолы и далее, через систему бронхов, во внешнюю среду. В печени и почках меченые Vybrant® CM-Dil фрагменты ММСК найдены не были, что не соответствует данным литературы о важной роли этих органов в деструкции или удалении введенных извне МСК. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Мультипотентные стромальные клетки, элиминация, легкие, макрофаги, альвеолы, печень, почки.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4808) "Согласно работам большинства исследователей, при местном применении мультипотентные стромальные клетки (МСК) остаются в месте имплантации, а введенные внутривенно МСК сначала оказываются в легких и только потом распределяются по всему организму, но уже в значительно меньшем количестве. Вместе с тем имеются сообщения о преимущественной миграции инфузированных системно МСК в печень, а не в легкие. Разрушение и элиминация введенных МСК, по данным литературы, происходит в селезенке, почках и печени. В связи с противоречивостью литературных данных о распределении и исчезновении МСК из организма была поставлена цель исследования – изучить возможность попадания в печень, почки и легкие мультипотентных мезенхимных стромальных клеток (ММСК) после их тканевой инъекции, а также получить новые доказательства их элиминации из организма указанными органами.
Материал и методика
Работа основана на результатах морфологического исследования легких, печени и почек крыс-самцов инбредной линии Wag в разные сроки после подкожной инъекции в паховую область ММСК, окрашенных мембрано-специфическим красителем Vybrant® CM-Dil. Методами световой и люминесцентной микроскопии регистрировали появление и распределение меченых объектов в указанных органах.
Результаты
Через 1 неделю после введения ММСК, в легких появились первые люминесцирующие макрофаги, выявляемые при посредстве светофильтра для родамина. В течение 2-4 недель количество таких светящихся клеток постепенно нарастает, также увеличивается интенсивность их флюоресценции. Спустя 2-3 недели был найден красный оттенок во включениях в эпителиальных клетках отдельных бронхиол. Иногда меченые Vybrant® CM-Dil объекты были расположены по самому краю альвеол и даже частично находились в просвете альвеол. Объекты, окрашенные Vybrant® CM-Dil, не были найдены в печени и почках во все сроки наблюдения.
Заключение
После инъекции в подкожно-жировую клетчатку ММСК и их фрагменты попадают в кровоток и оказываются в легких, где фагоцитируются макрофагами. Только после фильтрации в легких, дебрис ММСК может диссеминировать по всему организму. В легких возможна элиминация детрита инъецированных ММСК в альвеолы и далее, через систему бронхов, во внешнюю среду. В печени и почках меченые Vybrant® CM-Dil фрагменты ММСК найдены не были, что не соответствует данным литературы о важной роли этих органов в деструкции или удалении введенных извне МСК.
Ключевые слова
Мультипотентные стромальные клетки, элиминация, легкие, макрофаги, альвеолы, печень, почки.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4808) "Согласно работам большинства исследователей, при местном применении мультипотентные стромальные клетки (МСК) остаются в месте имплантации, а введенные внутривенно МСК сначала оказываются в легких и только потом распределяются по всему организму, но уже в значительно меньшем количестве. Вместе с тем имеются сообщения о преимущественной миграции инфузированных системно МСК в печень, а не в легкие. Разрушение и элиминация введенных МСК, по данным литературы, происходит в селезенке, почках и печени. В связи с противоречивостью литературных данных о распределении и исчезновении МСК из организма была поставлена цель исследования – изучить возможность попадания в печень, почки и легкие мультипотентных мезенхимных стромальных клеток (ММСК) после их тканевой инъекции, а также получить новые доказательства их элиминации из организма указанными органами.
Материал и методика
Работа основана на результатах морфологического исследования легких, печени и почек крыс-самцов инбредной линии Wag в разные сроки после подкожной инъекции в паховую область ММСК, окрашенных мембрано-специфическим красителем Vybrant® CM-Dil. Методами световой и люминесцентной микроскопии регистрировали появление и распределение меченых объектов в указанных органах.
Результаты
Через 1 неделю после введения ММСК, в легких появились первые люминесцирующие макрофаги, выявляемые при посредстве светофильтра для родамина. В течение 2-4 недель количество таких светящихся клеток постепенно нарастает, также увеличивается интенсивность их флюоресценции. Спустя 2-3 недели был найден красный оттенок во включениях в эпителиальных клетках отдельных бронхиол. Иногда меченые Vybrant® CM-Dil объекты были расположены по самому краю альвеол и даже частично находились в просвете альвеол. Объекты, окрашенные Vybrant® CM-Dil, не были найдены в печени и почках во все сроки наблюдения.
Заключение
После инъекции в подкожно-жировую клетчатку ММСК и их фрагменты попадают в кровоток и оказываются в легких, где фагоцитируются макрофагами. Только после фильтрации в легких, дебрис ММСК может диссеминировать по всему организму. В легких возможна элиминация детрита инъецированных ММСК в альвеолы и далее, через систему бронхов, во внешнюю среду. В печени и почках меченые Vybrant® CM-Dil фрагменты ММСК найдены не были, что не соответствует данным литературы о важной роли этих органов в деструкции или удалении введенных извне МСК.
Ключевые слова
Мультипотентные стромальные клетки, элиминация, легкие, макрофаги, альвеолы, печень, почки.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "26411" ["VALUE"]=> array(2) { ["TEXT"]=> string(705) "<p><sup>1</sup> Центр новых медицинских технологий, Федеральное государственное бюджетное учреждение науки «Институт химической биологии и фундаментальной медицины СО РАН», Новосибирск, Россия<br> <sup>2</sup> Институт молекулярной патологии и патоморфологии, Федеральный научно-исследовательский центр фундаментальной и трансляционной медицины, Новосибирск, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(663) "1 Центр новых медицинских технологий, Федеральное государственное бюджетное учреждение науки «Институт химической биологии и фундаментальной медицины СО РАН», Новосибирск, Россия
2 Институт молекулярной патологии и патоморфологии, Федеральный научно-исследовательский центр фундаментальной и трансляционной медицины, Новосибирск, Россия
1 Центр новых медицинских технологий, Федеральное государственное бюджетное учреждение науки «Институт химической биологии и фундаментальной медицины СО РАН», Новосибирск, Россия
2 Институт молекулярной патологии и патоморфологии, Федеральный научно-исследовательский центр фундаментальной и трансляционной медицины, Новосибирск, Россия
03/31/2020

Afanasyev B. V. (St. Petersburg, Russia)
Wagemaker G. (Rotterdam, Netherlands)
Zander A. R. (Hamburg, Germany)
Fehse B. (Hamburg, Germany)
Chukhlovin A. B. (St. Petersburg, Russia)
Aleynikova O. V. (Minsk, Republic of Belarus)
Borset M. (Trondheim, Norway)
Chechetkin A. V. (St. Petersburg, Russia)
Fibbe W. (Leiden, Netherlands)
Galibin O. V. (St. Petersburg, Russia)
Hölzer D. (Frankfurt a.M., Germany)
Klimko N. N. (St. Petersburg, Russia)
Kolb H.-J. (München, Germany)
Kröger N. (Hamburg, Germany)
Kulagin A. D. (St. Petersburg, Russia)
Lange C. (Hamburg, Germany)
Mamaev N. N. (St. Petersburg, Russia)
Mikhailova N. B. (St. Petersburg, Russia)
Moiseev I. S. (St. Petersburg, Russia)
Nagler A. (Tel-Aviv, Israel)
Nemkov A. S. (St. Petersburg, Russia)
Paramonov I. V. (Kirov, Russia)
Roumiantsev A. G. (Moscow, Russia)
Savchenko V. G. (Moscow, Russia)
Smirnov A. V. (St. Petersburg, Russia)
Uss A. L. (Minsk, Republic of Belarus)
Zubarovskaya L. S. (St. Petersburg, Russia)
Obituary
Sergey F. Bagnenko, Axel R. Zander, Gerard Wagemaker, Rudiger Hehlmann, Boris Fehse, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Ivan S. Moiseev, Inna V. Markova, Alexei B. Chukhlovin
Review articles
Alexei B. Chukhlovin
Clinical studies
Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez
Elena E. Lepik1, Andrey V. Kozlov1, Evgenia S. Borzenkova1,
Yury R. Zalyalov1, Kirill V. Lepik1, Elena V. Kondakova1, Vadim V. Baykov1, Ivan S. Moiseev1, Tatiana V. Schneider2,
Natalia B. Mikhaylova1, Boris V. Afanasyev1
Andrey N. Sokolov1, Elena N. Parovichnikova1, Vera V. Troitskaya1, Larisa A. Kuzmina1, Irina V. Galtseva1, Sergei M. Kulikov1, Sergey N. Bondarenko2, Irina A. Lukyanova1, Tatiana I. Lobanova1, Ekaterina I. Usikova1, Ksenia I. Zarubina1, Olga A. Gavrilina1, Julia O. Davidova1, Nikolai M. Kapranov1, Valeriy G. Savchenko1
Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina,
Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev,
Ludmila S. Zubarovskaya,
Boris V. Afanasyev
Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, Boris V. Afanasyev
Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova,
Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Boris V. Afanasyev
Experimental studies
Igor V. Maiborodin1, Roman V. Maslov1, Tatiana V. Mikheeva1, Sergey V. Marchukov1, Vitalina I. Maiborodina2, Aleksandr A. Shevela1
Obituary
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 25931
[VALUE] => 03/20/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/20/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 25932
[VALUE] => 03/27/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/27/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 26362
[1] => 26363
[2] => 26364
[3] => 26365
[4] => 26366
[5] => 26367
[6] => 26368
[7] => 26369
[8] => 26370
[9] => 26371
)
[VALUE] => Array
(
[0] => 1832
[1] => 11
[2] => 433
[3] => 87
[4] => 41
[5] => 503
[6] => 42
[7] => 60
[8] => 135
[9] => 119
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
[8] =>
[9] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 1832
[1] => 11
[2] => 433
[3] => 87
[4] => 41
[5] => 503
[6] => 42
[7] => 60
[8] => 135
[9] => 119
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
[8] =>
[9] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26340
[VALUE] => Array
(
[TEXT] => <p>Сергей Ф. Багненко, Аксель Р. Цандер, Герард Вагемакер, Рюдигер Хельманн, Борис Фезе, Александр Д. Кулагин, Людмила С. Зубаровская, Иван С. Моисеев, Инна В. Маркова, Алексей Б. Чухловин</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Сергей Ф. Багненко, Аксель Р. Цандер, Герард Вагемакер, Рюдигер Хельманн, Борис Фезе, Александр Д. Кулагин, Людмила С. Зубаровская, Иван С. Моисеев, Инна В. Маркова, Алексей Б. Чухловин
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 25943
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Санкт-Петербургский государственный медицинский университет им. И.П. Павлова потерял харизматического лидера, создавшего в Санкт-Петербурге признанный в мире ведущий центр по исследованию лейкозов и трансплантации стволовых гемопоэтических клеток.</p>
<p style="text-align: justify;">С глубоким прискорбием извещаем наших читателей о безвременной кончине главного редактора журнала «Клеточная Терапия и Трансплантация». Профессор Борис Владимирович Афанасьев, директор НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой Первого Санкт-Петербургского государственного медицинского университета им. И.П. Павлова ушел из жизни 16 марта 2020 г. На протяжении последних трех десятилетий он был главным вдохновителем разработок по трансплантации стволовых кроветворных клеток в России, а также в области химиотерапии, иммунотерапии и таргетного лечения злокачественных и неопухолевых заболеваний системы крови, будучи широко известным как в России, так и в мире.</p>
<p style="text-align: justify;">В 1971 г. Борис Владимирович Афанасьев с отличием окончил Первый Ленинградский медицинский институт. С 1973 г. он был ординатором в клинике факультетской терапии по специальности «Внутренние болезни». Будучи аспирантом (1974-1976), он был одним из первых в области фундаментальных исследований гемопоэтических стволовых клеток. Его кандидатская диссертация «Метод клонирования гемопоэтических стволовых клеток, изучение колониестимулирующей способности клеток костного мозга и крови гематологически здоровых лиц и больных с различными нейтропеническими состояниями» (1977) была посвящена регуляции роста нормальных и лейкозных клеток-предшественников гемопоэза, изученных в тест-системе «агаровой капли». В дальнейшем его наиболее выдающиеся исследования культур стволовых клеток включали описание терминальной дифференцировки лейкозных стволовых клеток.</p>
<p style="text-align: justify;">Б.В. Афанасьев осуществил также ряд пионерских работ в области стромальной регуляции гемопоэза мезенхимными стволовыми клетками и показал, что они оказывают как стимулирующие, так и модулирующие эффекты на клетки-предшественники гранулоцитов, которые показаны им в разработанной им системе «агаровая капля в жидкой среде».</p>
<p style="text-align: justify;">В 1976-1979 гг. Б.В. Афанасьев возглавлял отделение гематологии клиники факультетской терапии. В 1982 г., на основании данных о характере роста гемопоэтических клеток <i>in vitro</i>, он первым описал миелодиспдастический синдром у детей. Его докторская диссертация была посвящена теме «Грануломоноцитопоэз при остром лейкозе и бластном кризе» (1983). Он был одним из первых, кто описал клеточные механизмы миелоидного и лимфоидного типов бластного криза при хроническом миелоидном лейкозе. В 1985 г. он опубликовал широко известную монографию «Родоначальные кроветворные клетки человека» в соавторстве с профессором В.А. Алмазовым. В течение последующих 8 лет Б.В. Афанасьев был старшим научным сотрудником при кафедре факультетской терапии Первого Ленинградского медицинского института им. Павлова, где изучал биологические механизмы злокачественных клеток при острых и хронических лейкозах, тяжелых нейтропениях.</p>
<p style="text-align: justify;">В 1986 г. Б.В. Афанасьев проходил клиническую стажировку в знаменитом онкологическом центре Фреда Хатчинсона в Сиэттле (США) под руководством Нобелевского лауреата, профессора Доннелла Томаса. В 1987 г. Б.В. Афанасьев организовал первое отделение трансплантации костного мозга (ТКМ) в Советском Союзе на базе НИИ онкологии им. Н.Н. Петрова. В 1991 г. он выполнил первую в России аллогенную ТКМ ребенку.</p>
<p style="text-align: justify;">В 2000 он организовал первое университетское отделение ТКМ в России на базе Первого Санкт-Петербургского государственного медицинского университета им. И. П. Павлова (ПСПбГМУ). В 2003 г. он основал кафедру гематологии, трансфузиологии и трансплантологии последипломного образования в ПСПбГМУ.</p>
<p style="text-align: justify;">В 2007 г., по инициативе Б.В. Афанасьева и ПСПбГМУ, при поддержке Фонда Горбачева и банка «Национальный Резерв», был построен НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой в составе ПСПбГМУ им. И.П. Павлова. С 2007 г. профессор Б.В. Афанасьев был директором этого института.</p>
<p style="text-align: justify;">Интересы Б.В. Афанасьева на протяжении последнего десятилетия были сосредоточены на разработке протоколов лечения больных с рецидивирующими и рефрактерными формами злокачественных заболеваний. Под его руководством созданы новые протоколы промежуточной (bridging) и посттрансплантационной терапии таргетными лекарственными агентами и моноклональными антителами. Он также был сосредоточен на долгосрочных осложнениях ТКМ у детей и взрослых, и был одним из первых в применении режимов кондиционирования сниженной интенсивности у детей, что приводило к менее выраженным долговременным токсическим эффектам. Научное наследие профессора Б.В. Афанасьева включает более 300 научных статей и 6 монографий. Эти клинические исследования позволили значительно расширить трансплантационные программы, которые стали самыми большими в России и одной из самых больших в Европе.</p>
<p style="text-align: justify;">С 1990 г. Б.В. Афанасьев прилагал усилия к развитию Регистра неродственных доноров костного мозга в России. Эта работа в 2013 г. привела к созданию кооперативной базы данных, которая сейчас способствует проведению примерно половины неродственных трансплантаций в России. На уровне правительства принят план расширения базы данных до Национальной программы неродственных доноров костного мозга, что также является наследием профессора Б.В. Афанасьева.</p>
<p style="text-align: justify;">На протяжении всех тех лет, которые вели к успеху программы ТКМ, организованной Б.В. Афанасьевым, он был глубоко благодарен поддержке коллег и друзей за рубежом: Рольфа Нета, Клауса Винклера, Акселя Цандера, Николауса Крегера, Бориса Фезе (Гамбург Германия), Томаса Бюхнера (Мюнстер, Германия), Дитера Хельцера (Франкфурт, Германия), Ханса-Иохена Кольба (Мюнхен, Германия), Герарда Вагемакера (Роттердам, Нидерланды), Роберта Гэйла (Лондон, Великобритания), Андреа Бачигалупо (Рим, Италия), Магне Борсета (Трондхейм, Норвегия), Арнона Наглера (Тель-Авив, Израиль), Тапани Рууту (Хельсинки, Финляндия) и многих других, кто способствовал обучению более чем 70 российских гематологов в европейских центрах.</p>
<p style="text-align: justify;">Начиная с 2007 г., ежегодные научные форумы памяти Р.М. Горбачевой в медицинском университете им. И. Павлова стали центральными событиями в России и международном сообществе врачей и исследователей лейкозов, в целях образования, сотрудничества, обмена мнениями, новыми научными данными и распространения передового опыта в области трансплантации стволовых клеток.</p>
<p style="text-align: justify;">Профессор Борис В. Афанасьев был членом редакционных советов многих российских и международных журналов. В качестве главного редактора он играл ведущую роль в основании и развитии журнала «Клеточная Терапия и Трансплантация», где российские и зарубежные авторы могли представлять данные своих исследований по онкологии, гематологии и в смежных областях для международного сообщества. Нам, его коллегам и друзьям, а также всему сообществу гематологов и специалистов по трансплантации костного мозга, будет остро не хватать его огромного энтузиазма, творческого мышления и дружелюбия.</p>
<p style="text-align: justify;">Борис В. Афанасьев всегда служил общей пользе и ушел из жизни так, как жил, завершив дело жизненной важности для Института, для Университета, для гематологии и для общества. Ему была присуждена награда за клинические достижения от Европейского Общества трансплантации костного мозга на конгрессе в Лиссабоне в 2018 г. У него остались жена Ольга, дочь Анастасия, внуки Эмиль-Борис и Антония.</p>
<p><b>Сергей Ф. Багненко,</b><br>
профессор, д.м.н., академик РАН,<br> ректор Первого Санкт-Петербургского государственного медицинского университета им. И. Павлова, Россия<br>
<b>Аксель Р. Цандер,</b><br>
профессор, почетный доктор, Университетский медицинский центр Гамбург-Эппендорф, Германия<br>
<b>Герард Вагемакер,</b><br>
профессор, почетный доктор, Университет Эразмус, Нидерланды<br>
<b>Рюдигер Хельманн,</b><br>
профессор, почетный доктор, Факультет Мангейм, Гейдельбергский университет, Германия<br>
<b>Борис Фезе,</b><br>
профессор, доктор, Университет Гамбурга, Германия<br>
<b>Александр Д. Кулагин,</b> проф., д.м.н.,<br>
<b>Людмила С. Зубаровская,</b> проф., д.м.н.,<br>
<b> Иван С. Моисеев,</b> д.м.н.,<br>
<b>Инна В. Маркова,</b> к.м.н.<br>
и <b>Алексей Б. Чухловин,</b> проф. д.м.н.<br>
НИИ детской онкологии, гематологии и трансплантологии <br>им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. Павлова, Россия<br>
<br></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Санкт-Петербургский государственный медицинский университет им. И.П. Павлова потерял харизматического лидера, создавшего в Санкт-Петербурге признанный в мире ведущий центр по исследованию лейкозов и трансплантации стволовых гемопоэтических клеток.
С глубоким прискорбием извещаем наших читателей о безвременной кончине главного редактора журнала «Клеточная Терапия и Трансплантация». Профессор Борис Владимирович Афанасьев, директор НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой Первого Санкт-Петербургского государственного медицинского университета им. И.П. Павлова ушел из жизни 16 марта 2020 г. На протяжении последних трех десятилетий он был главным вдохновителем разработок по трансплантации стволовых кроветворных клеток в России, а также в области химиотерапии, иммунотерапии и таргетного лечения злокачественных и неопухолевых заболеваний системы крови, будучи широко известным как в России, так и в мире.
В 1971 г. Борис Владимирович Афанасьев с отличием окончил Первый Ленинградский медицинский институт. С 1973 г. он был ординатором в клинике факультетской терапии по специальности «Внутренние болезни». Будучи аспирантом (1974-1976), он был одним из первых в области фундаментальных исследований гемопоэтических стволовых клеток. Его кандидатская диссертация «Метод клонирования гемопоэтических стволовых клеток, изучение колониестимулирующей способности клеток костного мозга и крови гематологически здоровых лиц и больных с различными нейтропеническими состояниями» (1977) была посвящена регуляции роста нормальных и лейкозных клеток-предшественников гемопоэза, изученных в тест-системе «агаровой капли». В дальнейшем его наиболее выдающиеся исследования культур стволовых клеток включали описание терминальной дифференцировки лейкозных стволовых клеток.
Б.В. Афанасьев осуществил также ряд пионерских работ в области стромальной регуляции гемопоэза мезенхимными стволовыми клетками и показал, что они оказывают как стимулирующие, так и модулирующие эффекты на клетки-предшественники гранулоцитов, которые показаны им в разработанной им системе «агаровая капля в жидкой среде».
В 1976-1979 гг. Б.В. Афанасьев возглавлял отделение гематологии клиники факультетской терапии. В 1982 г., на основании данных о характере роста гемопоэтических клеток in vitro, он первым описал миелодиспдастический синдром у детей. Его докторская диссертация была посвящена теме «Грануломоноцитопоэз при остром лейкозе и бластном кризе» (1983). Он был одним из первых, кто описал клеточные механизмы миелоидного и лимфоидного типов бластного криза при хроническом миелоидном лейкозе. В 1985 г. он опубликовал широко известную монографию «Родоначальные кроветворные клетки человека» в соавторстве с профессором В.А. Алмазовым. В течение последующих 8 лет Б.В. Афанасьев был старшим научным сотрудником при кафедре факультетской терапии Первого Ленинградского медицинского института им. Павлова, где изучал биологические механизмы злокачественных клеток при острых и хронических лейкозах, тяжелых нейтропениях.
В 1986 г. Б.В. Афанасьев проходил клиническую стажировку в знаменитом онкологическом центре Фреда Хатчинсона в Сиэттле (США) под руководством Нобелевского лауреата, профессора Доннелла Томаса. В 1987 г. Б.В. Афанасьев организовал первое отделение трансплантации костного мозга (ТКМ) в Советском Союзе на базе НИИ онкологии им. Н.Н. Петрова. В 1991 г. он выполнил первую в России аллогенную ТКМ ребенку.
В 2000 он организовал первое университетское отделение ТКМ в России на базе Первого Санкт-Петербургского государственного медицинского университета им. И. П. Павлова (ПСПбГМУ). В 2003 г. он основал кафедру гематологии, трансфузиологии и трансплантологии последипломного образования в ПСПбГМУ.
В 2007 г., по инициативе Б.В. Афанасьева и ПСПбГМУ, при поддержке Фонда Горбачева и банка «Национальный Резерв», был построен НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой в составе ПСПбГМУ им. И.П. Павлова. С 2007 г. профессор Б.В. Афанасьев был директором этого института.
Интересы Б.В. Афанасьева на протяжении последнего десятилетия были сосредоточены на разработке протоколов лечения больных с рецидивирующими и рефрактерными формами злокачественных заболеваний. Под его руководством созданы новые протоколы промежуточной (bridging) и посттрансплантационной терапии таргетными лекарственными агентами и моноклональными антителами. Он также был сосредоточен на долгосрочных осложнениях ТКМ у детей и взрослых, и был одним из первых в применении режимов кондиционирования сниженной интенсивности у детей, что приводило к менее выраженным долговременным токсическим эффектам. Научное наследие профессора Б.В. Афанасьева включает более 300 научных статей и 6 монографий. Эти клинические исследования позволили значительно расширить трансплантационные программы, которые стали самыми большими в России и одной из самых больших в Европе.
С 1990 г. Б.В. Афанасьев прилагал усилия к развитию Регистра неродственных доноров костного мозга в России. Эта работа в 2013 г. привела к созданию кооперативной базы данных, которая сейчас способствует проведению примерно половины неродственных трансплантаций в России. На уровне правительства принят план расширения базы данных до Национальной программы неродственных доноров костного мозга, что также является наследием профессора Б.В. Афанасьева.
На протяжении всех тех лет, которые вели к успеху программы ТКМ, организованной Б.В. Афанасьевым, он был глубоко благодарен поддержке коллег и друзей за рубежом: Рольфа Нета, Клауса Винклера, Акселя Цандера, Николауса Крегера, Бориса Фезе (Гамбург Германия), Томаса Бюхнера (Мюнстер, Германия), Дитера Хельцера (Франкфурт, Германия), Ханса-Иохена Кольба (Мюнхен, Германия), Герарда Вагемакера (Роттердам, Нидерланды), Роберта Гэйла (Лондон, Великобритания), Андреа Бачигалупо (Рим, Италия), Магне Борсета (Трондхейм, Норвегия), Арнона Наглера (Тель-Авив, Израиль), Тапани Рууту (Хельсинки, Финляндия) и многих других, кто способствовал обучению более чем 70 российских гематологов в европейских центрах.
Начиная с 2007 г., ежегодные научные форумы памяти Р.М. Горбачевой в медицинском университете им. И. Павлова стали центральными событиями в России и международном сообществе врачей и исследователей лейкозов, в целях образования, сотрудничества, обмена мнениями, новыми научными данными и распространения передового опыта в области трансплантации стволовых клеток.
Профессор Борис В. Афанасьев был членом редакционных советов многих российских и международных журналов. В качестве главного редактора он играл ведущую роль в основании и развитии журнала «Клеточная Терапия и Трансплантация», где российские и зарубежные авторы могли представлять данные своих исследований по онкологии, гематологии и в смежных областях для международного сообщества. Нам, его коллегам и друзьям, а также всему сообществу гематологов и специалистов по трансплантации костного мозга, будет остро не хватать его огромного энтузиазма, творческого мышления и дружелюбия.
Борис В. Афанасьев всегда служил общей пользе и ушел из жизни так, как жил, завершив дело жизненной важности для Института, для Университета, для гематологии и для общества. Ему была присуждена награда за клинические достижения от Европейского Общества трансплантации костного мозга на конгрессе в Лиссабоне в 2018 г. У него остались жена Ольга, дочь Анастасия, внуки Эмиль-Борис и Антония.
Сергей Ф. Багненко,
профессор, д.м.н., академик РАН,
ректор Первого Санкт-Петербургского государственного медицинского университета им. И. Павлова, Россия
Аксель Р. Цандер,
профессор, почетный доктор, Университетский медицинский центр Гамбург-Эппендорф, Германия
Герард Вагемакер,
профессор, почетный доктор, Университет Эразмус, Нидерланды
Рюдигер Хельманн,
профессор, почетный доктор, Факультет Мангейм, Гейдельбергский университет, Германия
Борис Фезе,
профессор, доктор, Университет Гамбурга, Германия
Александр Д. Кулагин, проф., д.м.н.,
Людмила С. Зубаровская, проф., д.м.н.,
Иван С. Моисеев, д.м.н.,
Инна В. Маркова, к.м.н.
и Алексей Б. Чухловин, проф. д.м.н.
НИИ детской онкологии, гематологии и трансплантологии
им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. Павлова, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 25944
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-4-7
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-4-7
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26341
[VALUE] => Array
(
[TEXT] => <p>Sergey F. Bagnenko, Axel R. Zander, Gerard Wagemaker, Rudiger Hehlmann, Boris Fehse, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Ivan S. Moiseev, Inna V. Markova, Alexei B. Chukhlovin</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Sergey F. Bagnenko, Axel R. Zander, Gerard Wagemaker, Rudiger Hehlmann, Boris Fehse, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Ivan S. Moiseev, Inna V. Markova, Alexei B. Chukhlovin
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 25997
[VALUE] => Array
(
[TEXT] => <p>
</p>
<img width="500" alt="Afanasyev_small.jpg" src="/upload/medialibrary/517/afanasyev_small.jpg" height="333" title="Afanasyev_small.jpg" align="middle"><br>
<br>
<p style="text-align: justify;">
Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide.
</p>
<p style="text-align: justify;">
Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: <i>Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions</i>. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system.
</p>
<p style="text-align: justify;">
From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the <i>in vitro</i> growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: <i>Granulomonocytopoiesis in acute leukemia and in blast crisis</i>. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph <i>Ancestral Human Hematopoietic Cells</i> in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions.
</p>
<p style="text-align: justify;">
In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia.
</p>
<p style="text-align: justify;">
<img width="500" alt="Gorbachev1_small.jpg" src="/upload/medialibrary/12f/gorbachev1_small.jpg" height="335" title="Gorbachev1_small.jpg"><br>
</p>
<p style="text-align: justify;">
In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University.
</p>
<p style="text-align: justify;">
By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute.
</p>
<p style="text-align: justify;">
Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe.
</p>
<p style="text-align: justify;">
Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev.
</p>
<p style="text-align: justify;">
During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers.
</p>
<p style="text-align: justify;">
Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation.
</p>
<p style="text-align: justify;">
<img width="500" alt="CTT_9-1-Afanasyev-Photo-Award_small.jpg" src="/upload/medialibrary/931/ctt_9_1_afanasyev_photo_award_small.jpg" height="333" title="CTT_9-1-Afanasyev-Photo-Award_small.jpg"><br>
</p>
<p style="text-align: justify;">
Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual <i>Cellular Therapy and Transplantation</i> journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness.
</p>
<p style="text-align: justify;">
Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.<br>
</p>
<p>
<b>Sergey F. Bagnenko,</b><br>
Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia<br>
<b>Axel R. Zander,</b><br>
Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany<br>
<b>Gerard Wagemaker,</b><br>
Prof. Dr. Dr.h.c. Erasmus University, The Netherlands<br>
<b>Rudiger Hehlmann,</b><br>
Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany<br>
<b>Boris Fehse,</b><br>
Prof. Dr., Hamburg University, Germany <br>
<b> Alexander D. Kulagin,</b> Prof. Dr.,<br>
<b>Ludmila S. Zubarovskaya,</b> Prof. Dr.,<br>
<b>Ivan S. Moiseev,</b> PhD, MD<br>
<b>Inna V. Markova,</b> PhD<br>
and <b>Alexei B. Chukhlovin,</b> Prof. Dr.<br>
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia<br>
<br>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>

Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide.
Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system.
From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the in vitro growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: Granulomonocytopoiesis in acute leukemia and in blast crisis. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph Ancestral Human Hematopoietic Cells in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions.
In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia.

In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University.
By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute.
Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe.
Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev.
During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers.
Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation.

Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual Cellular Therapy and Transplantation journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness.
Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.
Sergey F. Bagnenko,
Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia
Axel R. Zander,
Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany
Gerard Wagemaker,
Prof. Dr. Dr.h.c. Erasmus University, The Netherlands
Rudiger Hehlmann,
Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany
Boris Fehse,
Prof. Dr., Hamburg University, Germany
Alexander D. Kulagin, Prof. Dr.,
Ludmila S. Zubarovskaya, Prof. Dr.,
Ivan S. Moiseev, PhD, MD
Inna V. Markova, PhD
and Alexei B. Chukhlovin, Prof. Dr.
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 25945
[VALUE] => In memory of Professor Boris Afanasyev (August 28, 1947 – March 16, 2020)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => In memory of Professor Boris Afanasyev (August 28, 1947 – March 16, 2020)
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 25946
[VALUE] => 1930
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1930
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 25998
[VALUE] => 1932
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1932
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Sergey F. Bagnenko, Axel R. Zander, Gerard Wagemaker, Rudiger Hehlmann, Boris Fehse, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Ivan S. Moiseev, Inna V. Markova, Alexei B. Chukhlovin

Pavlov University lost a charismatic leader who built in St. Petersburg an internationally visible center of excellence in leukemia research and hematopoietic stem cell transplantation. We sadly inform our readers of the untimely death of the Editor-in-Chief of CTT. Professor Boris V. Afanasyev, Director of Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation at Pavlov First Saint Petersburg State Medical University, passed away on Monday, March 16, 2020. Over the last three decades he was the major inspirator for the development of hematopoietic stem cell transplantation, as well as chemotherapy, immunotherapy and targeted therapy of malignant and non-malignant blood disorders in Russia. He was broadly known both in Russia and worldwide.
Boris V. Afanasyev graduated from Pavlov First Medical Institute in Leningrad with honors (1971). Till 1973, he was a resident at the Faculty Therapy (Internal Medicine). As a post-graduate student (1974-1976), he was one of the pioneers in basic hematopoietic stem cell research. His PhD Thesis (1977) was dedicated to growth regulation of normal and leukemic precursor cells observed in agar drop test system: Cloning of hematopoietic stem cells, studies of colony-forming ability of bone marrow and blood cells from healthy persons and patients with different neutropenic conditions. His most prominent studies of cultured stem cells included description of terminal differentiation of leukemic stem cells. Boris Afanasyev has also performed a number of pioneering works in stromal regulation of hematopoiesis by mesenchymal stem cells which proved to exert both stimulatory and modulatory effects upon granulocyte precursors that were performed by him in an original ‘agar drop-liquid culture’ system.
From 1976 to 1979, Prof. Boris V. Afanasyev headed the Department of Hematology at the Faculty of Internal Medicine. In 1982 based on the in vitro growth pattern of hematopoietic stem cells he was the first to describe myelodysplastic syndrome in children. His Habilitation Thesis (1983) was entitled: Granulomonocytopoiesis in acute leukemia and in blast crisis. He was one of the first to describe the cellular mechanisms of myeloid and lymphoid types of blast crisis in chronic myeloid leukemia. In 1985, he has published the well-known monograph Ancestral Human Hematopoietic Cells in co-authorship with Prof. V. A. Almazov. Over next 8 years, Boris Afanasyev was a Senior Research Associate at the Faculty of Internal Medicine in the First Leningrad Pavlov Medical Institute, studying biological mechanisms of malignant cells in acute and chronic leukemia, and in severe neutropenic conditions.
In 1986, Boris V. Afanasyev underwent clinical training at the famous Fred Hutchinson Cancer Center in Seattle under supervision of Nobel Laureate, Professor Donnall Thomas. In 1987, Boris V. Afanasyev organized the first Bone Marrow Transplantation (BMT) Department in the Soviet Union at the N. N. Petrov Research Institute of Oncology. In 1991 he performed the first pediatric allogeneic bone marrow transplantation in Russia.

In 2000 Prof. Boris V. Afanasyev organized the first university based BMT Department in Russia at the First St. Petersburg State I. Pavlov Medical University. In 2003, he established the Faculty of Hematology, Transfusiology and Transplantation for postgraduate education at the Pavlov University.
By 2007, based on the initiative of Prof. Boris V. Afanasyev and Pavlov University, with the support of Gorbachev Foundation and National Reserve Bank, the Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation was built and inaugurated in Pavlov University, which he since led as Founding Director of this Institute.
Over the last decade, his interests were focused on developing protocols for patients with relapsed and refractory malignant diseases. Under his supervision, novel protocols of bridging and posttransplant therapy with target agents and monoclonal antibodies were developed. He also was focused on long-term complications of BMT in children and adults and was one of the pioneers of reduced conditioning regimens in children that resulted in reduced long-term toxicity. The scientific heritage by Prof. Boris V. Afanasyev includes over 300 research papers and 6 monographs. This clinical research allowed to significantly expand the transplantation programs, which became the largest in Russia and are among the largest in Europe.
Since 1990, Prof. Afanasyev put effort into the development of unrelated bone marrow donor registry in Russia. These efforts in 2013 succeeded into the collaborative database which now helps recruiting about half of the unrelated donors in Russia. The governmental plan for expanding the database into a national unrelated bone marrow donor program has been approved, creating another legacy to Prof. Boris V. Afanasyev.
During the years that led to the success of BMT program organized by Prof. Afanasyev, he deeply acknowledged the support of his colleagues and friends from abroad: Rolf Neth, Klaus Winkler, Axel Zander, Nicolaus Kroeger, Boris Fehse (Hamburg, Germany), Thomas Buchner (Muenster, Germany), Dieter Hoelzer (Frankfurt, Germany), Hans-Jochem Kolb (Muenchen, Germany), Ruediger Hehlmann (Mannheim, Germany), Gerard Wagemaker (Rotterdam, Netherlands), Robert Gale (London, UK), Andrea Bacigalupo (Rome, Italy), Magne Børset (Trondheim, Norway), Arnon Nagler (Tel Aviv, Israel), Tapani Ruutu (Helsinki, Finland) and many others who facilitated the education of more than 70 Russian hematologists in the European centers.
Starting in 2007, the Annual Raisa Gorbacheva Memorial Meetings at Pavlov University has become the central event to russian and the international leukemia community focused on education, collaboration, exchanging opinions and sharing novel research data, and on spreading excellence in the field of stem cell transplantation.

Prof. Afanasyev was a Member of the Editorial Boards in many Russian and international journals. As Editor-in-Chief, he played a key role in starting and developing the bilingual Cellular Therapy and Transplantation journal, in which Russian and international authors in oncology, hematology and related fields could present their research to the international community. We, his colleagues and friends, but also the whole community of hematologists and stem cell transplanters, will strongly miss his huge enthusiasm, creativity and friendliness.
Boris Afanasyev served life-long the general interest and passed away as he lived, completing a task of vital importance to the Institute, to the University, to hematology, and to the society. He was awarded the Clinical Achievement Award from the EBMT at the Lisbon meeting in 2018. Boris Afanasiev was elected to be honored with an ELN Keynote Lecture this year on the topic: "The Raisa Gorbacheva Memorial Institute in St. Petersburg: Building a Center of Excellence in Russia" in recognition of his international visibility and esteem. He is survived by his wife Olga, daughter Anastasia and grandchildren Emil-Boris and Antonia.
Sergey F. Bagnenko,
Prof. Dr. Med., RAS Full Member, Rector, Pavlov University, Saint Petersburg, Russia
Axel R. Zander,
Prof. Dr. Dr. h.c., University Medical Center Hamburg-Eppendorf, Germany
Gerard Wagemaker,
Prof. Dr. Dr.h.c. Erasmus University, The Netherlands
Rudiger Hehlmann,
Prof. Dr., Dr.h.c. Med. Fakultät Mannheim, Heidelberg University, Germany
Boris Fehse,
Prof. Dr., Hamburg University, Germany
Alexander D. Kulagin, Prof. Dr.,
Ludmila S. Zubarovskaya, Prof. Dr.,
Ivan S. Moiseev, PhD, MD
Inna V. Markova, PhD
and Alexei B. Chukhlovin, Prof. Dr.
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Saint Petersburg, Russia
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26057
[VALUE] => 02/27/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/27/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26058
[VALUE] => 03/27/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/27/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 26076
)
[VALUE] => Array
(
[0] => 119
)
[DESCRIPTION] => Array
(
[0] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 119
)
[~DESCRIPTION] => Array
(
[0] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26060
[VALUE] => Array
(
[TEXT] => <p>Алексей Б. Чухловин</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Алексей Б. Чухловин
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26061
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26062
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Имеется огромное число исследований, касающихся метаболических, иммунологических и других различий между мужским и женским организмом, связанных с их различным гормональным и физиологическим фоном. Однако лишь немногие работы посвящены половым различиям в числе донорских гемопоэтических клеток для трансплантации гемопоэтических стволовых клеток (ТГСК), фармакокинетике цитостатических препаратов, используемых для кондиционирующей терапии и иммуносупрессоров для профилактики РТПХ, а также различий в плане частых посттрансплантационных осложнений. На основании предыдущих работ можно предположить о ряде различий, которые могут иметь значение для оценки результатов РТПХ: (1) Повышенные количества CD34+ клеток в трансплантатах от мужчин, по сравнению с донорами-женщинами; (2) Метаболизм цитостатических препаратов у женщин имеет тенденцию к сниженному клиренсу и повышенной скорости метаболической модификации в связи с повышенной активности цитохромов CYP3A, наряду со сниженным оттоком препаратов из клеток-мишеней, что предполагает более выраженное накопление активных цитостатических метаболитов в женском организме; (3) Более эффективный и стабильный гуморальный иммунный ответ у женщин по сравнению с мужчинами может выражаться, как в лучшем антиинфекционном ответе, так и повышенном риске хронической РТПХ у женщин после аллогенной ТГСК; (4) Пациенты мужского пола с некоторыми злокачественными заболеваниями системы крови после алло-ТГСК более склонны к посттрансплантационным рецидивам, хотя сообщают и иные результаты; (5) Повышенный риск острой РТПХ у мужчин имеется в случаях алло-ТГСК от доноров-женщин. Вопрос о наличии эффекта «трансплантат против лейкоза» в этой ситуации остается открытым. В целом, эстрогены, видимо, являются наиболее вероятной причиной половых различий при оценке ТГСК-ассоциированных рисков. Тем не менее, модифицирующая роль половых стероидных гормонов здесь изучена недостаточно, и она должна изменяться в зависимости от возраста пациентов. Поэтому реальная значимость половых различий при ТГСК заслуживает дальнейших углубленных исследований на больших базах данных.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, половые различия, фармакокинетика, иммунный ответ, РТПХ, рецидивы, выживаемость, эстрогены, исходы.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26063
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-13-21
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-13-21
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26067
[VALUE] => Array
(
[TEXT] => <p>Alexei B. Chukhlovin</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Alexei B. Chukhlovin
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26068
[VALUE] => Array
(
[TEXT] => <p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia</p>
<br>
<p><b>Correspondence</b><br>Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial
Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7 (921) 325 00 94<br>
E-mail: alexei.chukh@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial
Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 325 00 94
E-mail: alexei.chukh@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26072
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">There is a huge number of studies concerning metabolic, immunological and other differences between males and females caused by their differential hormonal and physiological background. However, only few studies are dedicated to sex-dependent differences in amounts of donor hematopoietic cells used for stem cell transplantation (HSCT), kinetics of cytostatic drugs used for conditioning treatment and immunosuppressors for GvHD prophylaxis, as well as differences in common posttransplant complications. The following differences significant for evaluation of HSCT results may be derived from previous studies: (1) Higher counts of CD34+ cells in hematopoietic grafts from males compared to female donors; (2) Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher modification rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic metabolites in female patients; (3) More effective and stable humoral immune response in females compared to males could be translated into better anti-infectious response, along with higher risk of chronic GvHD in females after allo-HSCT; (4) Male patients with some hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses, however, conflicting data are reported; (5) Increased risk of acute GvHD in males exists in cases of allo-HSCT from female donors. The issue of graft-versus-leukemia effect in this setting still remains open. In sum, estrogen hormones seem to be to the most probable cause of gender differences in HSCT-associated risks. However, modifying role of sex steroids is not well studied, and it should vary, depending on the age of patients. Therefore, real significance of sex differences in HSCT deserves further extensive studies in large databases.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Hematopoietic stem cell transplantation, sex differences, drug kinetics, immune response, GvHD, relapses, survival, estrogens, outcomes.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => There is a huge number of studies concerning metabolic, immunological and other differences between males and females caused by their differential hormonal and physiological background. However, only few studies are dedicated to sex-dependent differences in amounts of donor hematopoietic cells used for stem cell transplantation (HSCT), kinetics of cytostatic drugs used for conditioning treatment and immunosuppressors for GvHD prophylaxis, as well as differences in common posttransplant complications. The following differences significant for evaluation of HSCT results may be derived from previous studies: (1) Higher counts of CD34+ cells in hematopoietic grafts from males compared to female donors; (2) Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher modification rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic metabolites in female patients; (3) More effective and stable humoral immune response in females compared to males could be translated into better anti-infectious response, along with higher risk of chronic GvHD in females after allo-HSCT; (4) Male patients with some hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses, however, conflicting data are reported; (5) Increased risk of acute GvHD in males exists in cases of allo-HSCT from female donors. The issue of graft-versus-leukemia effect in this setting still remains open. In sum, estrogen hormones seem to be to the most probable cause of gender differences in HSCT-associated risks. However, modifying role of sex steroids is not well studied, and it should vary, depending on the age of patients. Therefore, real significance of sex differences in HSCT deserves further extensive studies in large databases.
Keywords
Hematopoietic stem cell transplantation, sex differences, drug kinetics, immune response, GvHD, relapses, survival, estrogens, outcomes.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26064
[VALUE] => Gender factor in hematopoietic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Gender factor in hematopoietic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26065
[VALUE] => 1941
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1941
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26069
[VALUE] => 1942
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1942
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Alexei B. Chukhlovin
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Prof. Alexei B. Chukhlovin, Raisa Gorbacheva Memorial
Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 325 00 94
E-mail: alexei.chukh@mail.ru
There is a huge number of studies concerning metabolic, immunological and other differences between males and females caused by their differential hormonal and physiological background. However, only few studies are dedicated to sex-dependent differences in amounts of donor hematopoietic cells used for stem cell transplantation (HSCT), kinetics of cytostatic drugs used for conditioning treatment and immunosuppressors for GvHD prophylaxis, as well as differences in common posttransplant complications. The following differences significant for evaluation of HSCT results may be derived from previous studies: (1) Higher counts of CD34+ cells in hematopoietic grafts from males compared to female donors; (2) Metabolism of cytostatic drug in females suggest a tendency for decreased clearance and higher modification rates due to increased CYP3A activities, along with decreased drug efflux from target cells, thus suggesting higher accumulation of active cytostatic metabolites in female patients; (3) More effective and stable humoral immune response in females compared to males could be translated into better anti-infectious response, along with higher risk of chronic GvHD in females after allo-HSCT; (4) Male patients with some hematological malignancies subjected to allo-HSCT are more prone to posttransplant relapses, however, conflicting data are reported; (5) Increased risk of acute GvHD in males exists in cases of allo-HSCT from female donors. The issue of graft-versus-leukemia effect in this setting still remains open. In sum, estrogen hormones seem to be to the most probable cause of gender differences in HSCT-associated risks. However, modifying role of sex steroids is not well studied, and it should vary, depending on the age of patients. Therefore, real significance of sex differences in HSCT deserves further extensive studies in large databases.
Keywords
Hematopoietic stem cell transplantation, sex differences, drug kinetics, immune response, GvHD, relapses, survival, estrogens, outcomes.
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26029
[VALUE] => 12/15/2019 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/15/2019 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26030
[VALUE] => 01/24/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 01/24/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 26056
)
[VALUE] => Array
(
[0] => 7
)
[DESCRIPTION] => Array
(
[0] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 7
)
[~DESCRIPTION] => Array
(
[0] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26032
[VALUE] => Array
(
[TEXT] => <p>Тапани Рууту</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Тапани Рууту
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26033
[VALUE] => Array
(
[TEXT] => <p>Институт клинических исследований, Университетский госпиталь Хельсинки, Финляндия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Институт клинических исследований, Университетский госпиталь Хельсинки, Финляндия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26034
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Кортикостероиды играют определенную роль в качестве терапии первой линии реакции «трансплантат против хозяина» (РТПХ), но их значение в профилактике РТПХ менее ясно. В настоящее время кортикостероиды лишь в редких случаях включают в профилактические режимы. Исследования по добавлению кортикостероидов в наиболее широко применяемые режимы профилактики, с циклоспорином А и коротким курсом метотрексата приводили к противоречивым результатам, вероятно – из-за различий в схеме лечения. В нашем ранее опубликованном рандомизированном проспективном исследовании, добавление метилпреднизолона (МП) к циклоспорину и метотрексату вело к значительному снижению частоты острой РТПХ. Не отмечалось различий по выживаемости. При долгосрочном обследовании в этом исследовании, после 24,5 лет наблюдения у живущих пациентов мы отмечали существенную позднюю смертность, не связанную с рецидивами, среди пациентов, которые не получали профилактику МП, вероятно – из-за более высокой частоты хронической РТПХ в этой линии исследования. По окончании наблюдения, 55% пациентов, которым назначали МП для профилактики, были живы, по сравнению с 20% в контрольной группе. Эти находки предполагают, что роль кортикостероидов в профилактике РТПХT следует переоценить.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Реакция «трансплантат против хозяина», острая, хроническая, профилактика, кортикостероиды, глюкокортикоиды, благоприятный эффект.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Кортикостероиды играют определенную роль в качестве терапии первой линии реакции «трансплантат против хозяина» (РТПХ), но их значение в профилактике РТПХ менее ясно. В настоящее время кортикостероиды лишь в редких случаях включают в профилактические режимы. Исследования по добавлению кортикостероидов в наиболее широко применяемые режимы профилактики, с циклоспорином А и коротким курсом метотрексата приводили к противоречивым результатам, вероятно – из-за различий в схеме лечения. В нашем ранее опубликованном рандомизированном проспективном исследовании, добавление метилпреднизолона (МП) к циклоспорину и метотрексату вело к значительному снижению частоты острой РТПХ. Не отмечалось различий по выживаемости. При долгосрочном обследовании в этом исследовании, после 24,5 лет наблюдения у живущих пациентов мы отмечали существенную позднюю смертность, не связанную с рецидивами, среди пациентов, которые не получали профилактику МП, вероятно – из-за более высокой частоты хронической РТПХ в этой линии исследования. По окончании наблюдения, 55% пациентов, которым назначали МП для профилактики, были живы, по сравнению с 20% в контрольной группе. Эти находки предполагают, что роль кортикостероидов в профилактике РТПХT следует переоценить.
Ключевые слова
Реакция «трансплантат против хозяина», острая, хроническая, профилактика, кортикостероиды, глюкокортикоиды, благоприятный эффект.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26035
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-8-12
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-8-12
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26039
[VALUE] => Array
(
[TEXT] => <p>Tapani Ruutu</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Tapani Ruutu
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26040
[VALUE] => Array
(
[TEXT] => <p>Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland</p><br>
<p><b>Correspondence</b><br>
Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland<br>
E-mail: tapani.ruutu@hus.fi</p><br>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland
Correspondence
Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland
E-mail: tapani.ruutu@hus.fi
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26045
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.
Keywords
Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26036
[VALUE] => Is there any role for corticosteroids in GvHD prophylaxis?
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Is there any role for corticosteroids in GvHD prophylaxis?
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26037
[VALUE] => 1935
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1935
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26041
[VALUE] => 1936
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1936
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Tapani Ruutu
Clinical Research Institute, Helsinki University Hospital, Helsinki, Finland
Correspondence
Professor Tapani Ruutu, Biomedicum 2 C, Tukholmankatu 8 C, 00029 HUS, Helsinki, Finland
E-mail: tapani.ruutu@hus.fi
Corticosteroids have an established role as the first-line treatment of graft-versus-host disease (GvHD), but their role in the prophylaxis of GvHD is less clear. At present, corticosteroids are included in the prophylaxis regimens only rarely. Studies of adding corticosteroid to the most widely used prophylactic regimen, cyclosporine A and a short course of methotrexate, have yielded conflicting results, possibly due to differences in the treatment schedule. In our earlier published randomized prospective study, the addition of methylprednisolone (MP) to cyclosporine and methotrexate resulted in a markedly reduced incidence of acute GvHD. No difference was seen in the survival. In long-term follow-up of this study, after a median follow-up of 24.5 years in living patients, we observed a marked late non-relapse mortality among the patients not given prophylactic MP, probably due to higher incidence of chronic GvHD in this study arm. At the end of the follow-up, 55% of the patients given MP in the prophylaxis were alive, compared with 20% in the control arm. These findings suggest that the role of corticosteroids in GvHD prophylaxis should be reevaluated.
Keywords
Graft-versus-host disease, acute, chronic, prophylaxis, corticosteroids, glucocorticoids, favorable effect.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26125
[VALUE] => 10/10/2019 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/10/2019 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26126
[VALUE] => 01/24/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 01/24/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 26297
[1] => 26298
[2] => 26299
[3] => 26300
[4] => 26301
[5] => 26302
[6] => 26303
[7] => 26304
)
[VALUE] => Array
(
[0] => 1836
[1] => 1837
[2] => 1838
[3] => 1839
[4] => 1840
[5] => 1841
[6] => 1842
[7] => 1843
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 1836
[1] => 1837
[2] => 1838
[3] => 1839
[4] => 1840
[5] => 1841
[6] => 1842
[7] => 1843
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26145
[VALUE] => Array
(
[TEXT] => <p>Мануэль Абекасис, Каталина Гомез, Изабелина Феррейра, Мария Гомес да Силва, Нуньо Миранда, Гилда Тейксейра, Фернандо Леаль да Коста, Мария Жоао Гутьеррез</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Мануэль Абекасис, Каталина Гомез, Изабелина Феррейра, Мария Гомес да Силва, Нуньо Миранда, Гилда Тейксейра, Фернандо Леаль да Коста, Мария Жоао Гутьеррез
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26146
[VALUE] => Array
(
[TEXT] => <p>Португальский институт онкологии Франциско Жентиль, Лиссабон, Португалия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Португальский институт онкологии Франциско Жентиль, Лиссабон, Португалия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26147
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Периферические Т-клеточные лимфомы (ПТКЛ) являются гетерогенной группой редких агрессивных лимфом, состоящей из более чем 20 различных клинических форм и представляющих около 10% всех неходжкинских лимфом, диагностированных в Западном мире. Учитывая их гетерогенность, отсутствует консенсус, касающийся наилучшего лечения первой линии, роль аутологичной или аллогенной трансплантации гемопоэтических клеток (ТГСК) в качестве консолидации пока противоречива. Чтобы изучить реальные исходы у пациентов с ПТКЛ, направленных для ТГСК в наше учреждение, мы ретроспективно изучили клинические исходы у 26 пациентов, которым выполняли трансплантацию либо в качестве консолидирующей терапии первой линии или при рецидиве заболевания в период с января 2000 по июль 2018 г. Медианный срок наблюдения составлял 6,3 года; 19 больных получили ауто-ТГСК, 16 – в качестве первой консолидации и 3 – по поводу рецидива болезни. Общая выживаемость (ОВ) и безрецидивная выживаемость (БРВ) были, соответственно, 62% и 59%. Семи больным было проведена алло-ТГСК, четыре – консолидация первой линии и трем – после рецидива. Шесть больных живы и один умер в связи с процедурой ТГСК. Этот вид летальности составил 3.7% для всей группы, а 6-летние показатели ОВ и БРВ были, соответственно, 74% и 69%. Наши результаты предполагают, что аутологичные и аллогенные ТГСК являются эффективным и безопасным вариантом в целях консолидации больных ПТКЛ с неблагоприятным профилем риска, но нужна их валидация в проспективных исследованиях, включающих большое число пациентов.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Периферические Т-клеточные лимфомы, терапия первой линии, CHOP, Брентуксимаб ведотин, трансплантация гемопоэтических клеток, аллогенная, аутологичная. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Периферические Т-клеточные лимфомы (ПТКЛ) являются гетерогенной группой редких агрессивных лимфом, состоящей из более чем 20 различных клинических форм и представляющих около 10% всех неходжкинских лимфом, диагностированных в Западном мире. Учитывая их гетерогенность, отсутствует консенсус, касающийся наилучшего лечения первой линии, роль аутологичной или аллогенной трансплантации гемопоэтических клеток (ТГСК) в качестве консолидации пока противоречива. Чтобы изучить реальные исходы у пациентов с ПТКЛ, направленных для ТГСК в наше учреждение, мы ретроспективно изучили клинические исходы у 26 пациентов, которым выполняли трансплантацию либо в качестве консолидирующей терапии первой линии или при рецидиве заболевания в период с января 2000 по июль 2018 г. Медианный срок наблюдения составлял 6,3 года; 19 больных получили ауто-ТГСК, 16 – в качестве первой консолидации и 3 – по поводу рецидива болезни. Общая выживаемость (ОВ) и безрецидивная выживаемость (БРВ) были, соответственно, 62% и 59%. Семи больным было проведена алло-ТГСК, четыре – консолидация первой линии и трем – после рецидива. Шесть больных живы и один умер в связи с процедурой ТГСК. Этот вид летальности составил 3.7% для всей группы, а 6-летние показатели ОВ и БРВ были, соответственно, 74% и 69%. Наши результаты предполагают, что аутологичные и аллогенные ТГСК являются эффективным и безопасным вариантом в целях консолидации больных ПТКЛ с неблагоприятным профилем риска, но нужна их валидация в проспективных исследованиях, включающих большое число пациентов.
Ключевые слова
Периферические Т-клеточные лимфомы, терапия первой линии, CHOP, Брентуксимаб ведотин, трансплантация гемопоэтических клеток, аллогенная, аутологичная.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26135
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-22-27
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-22-27
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26157
[VALUE] => Array
(
[TEXT] => <p>Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26158
[VALUE] => Array
(
[TEXT] => <p>Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal</p>
<br>
<p><b>Correspondence</b><br>
Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia, <br>R. Professor Lima Basto 1099-093, Lisboa, Portugal</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal
Correspondence
Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia,
R. Professor Lima Basto 1099-093, Lisboa, Portugal
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26176
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of aggressive lymphomas comprising more than 20 different entities and representing about 10% of all non-Hodgkin lymphomas diagnosed in the Western World. Given their heterogeneity, there is no consensus regarding the best first-line treatment and the role of autologous/allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation is controversial. To examine the real-world outcomes for patients with PTCL submitted to HSCT in our institution we retrospectively reviewed the clinical outcomes of 26 patients who were given a transplant either as first-line consolidation or in relapse between January 2000 and July 2018. The median follow-up is 6.3 years; 19 patients had an autologous HSCT, 16 as upfront consolidation and 3, for relapsed disease. Overall survival (OS) and relapse free survival (RFS) were, respectively, 62% and 59%. Seven patients had an allograft, 4 as upfront consolidation and 3 after relapse; 6 are alive and 1 died due to transplant-related mortality (TRM). TRM was 3.7% for the entire population and the 6-year OS and PFS were 74% and 69%, respectively. Our results suggest that autologous/allogeneic HSCT is an effective and safe option for the consolidation of adverse-risk profile patients with PTCL, but they need to be validated in prospective studies including a larger number of patients.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Peripheral T-cell lymphomas, first-line therapy, CHOP, Brentuximab vedotin, hematopoietic stem cell transplantation, allogeneic, autologous. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of aggressive lymphomas comprising more than 20 different entities and representing about 10% of all non-Hodgkin lymphomas diagnosed in the Western World. Given their heterogeneity, there is no consensus regarding the best first-line treatment and the role of autologous/allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation is controversial. To examine the real-world outcomes for patients with PTCL submitted to HSCT in our institution we retrospectively reviewed the clinical outcomes of 26 patients who were given a transplant either as first-line consolidation or in relapse between January 2000 and July 2018. The median follow-up is 6.3 years; 19 patients had an autologous HSCT, 16 as upfront consolidation and 3, for relapsed disease. Overall survival (OS) and relapse free survival (RFS) were, respectively, 62% and 59%. Seven patients had an allograft, 4 as upfront consolidation and 3 after relapse; 6 are alive and 1 died due to transplant-related mortality (TRM). TRM was 3.7% for the entire population and the 6-year OS and PFS were 74% and 69%, respectively. Our results suggest that autologous/allogeneic HSCT is an effective and safe option for the consolidation of adverse-risk profile patients with PTCL, but they need to be validated in prospective studies including a larger number of patients.
Keywords
Peripheral T-cell lymphomas, first-line therapy, CHOP, Brentuximab vedotin, hematopoietic stem cell transplantation, allogeneic, autologous.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26136
[VALUE] => Stem cell transplantation as consolidation in peripheral T-cell lymphomas
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Stem cell transplantation as consolidation in peripheral T-cell lymphomas
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26148
[VALUE] => 1944
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1944
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26159
[VALUE] => 1945
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1945
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Manuel Abecasis, Catalina Gomez, Isabelina Ferreira, Maria Gomes da Silva, Nuno Miranda, Gilda Teixeira, Fernando Leal da Costa, Maria João Gutierrez
Instituto Português Oncologia de Lisboa Francisco Gentil, Lisboa, Portugal
Correspondence
Prof. Dr. Manuel Abecasis, Director, Hematology Department, Instituto Português Oncologia,
R. Professor Lima Basto 1099-093, Lisboa, Portugal
Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of aggressive lymphomas comprising more than 20 different entities and representing about 10% of all non-Hodgkin lymphomas diagnosed in the Western World. Given their heterogeneity, there is no consensus regarding the best first-line treatment and the role of autologous/allogeneic hematopoietic stem cell transplantation (HSCT) as consolidation is controversial. To examine the real-world outcomes for patients with PTCL submitted to HSCT in our institution we retrospectively reviewed the clinical outcomes of 26 patients who were given a transplant either as first-line consolidation or in relapse between January 2000 and July 2018. The median follow-up is 6.3 years; 19 patients had an autologous HSCT, 16 as upfront consolidation and 3, for relapsed disease. Overall survival (OS) and relapse free survival (RFS) were, respectively, 62% and 59%. Seven patients had an allograft, 4 as upfront consolidation and 3 after relapse; 6 are alive and 1 died due to transplant-related mortality (TRM). TRM was 3.7% for the entire population and the 6-year OS and PFS were 74% and 69%, respectively. Our results suggest that autologous/allogeneic HSCT is an effective and safe option for the consolidation of adverse-risk profile patients with PTCL, but they need to be validated in prospective studies including a larger number of patients.
Keywords
Peripheral T-cell lymphomas, first-line therapy, CHOP, Brentuximab vedotin, hematopoietic stem cell transplantation, allogeneic, autologous.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26305
[VALUE] => 10/08/2019 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/08/2019 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26306
[VALUE] => 11/14/2019 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/14/2019 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26307
[VALUE] => Array
(
[TEXT] => <p>
Елена Е. Лепик<sup>1</sup>, Андрей В. Козлов<sup>1</sup>, Евгения С. Борзенкова<sup>1</sup>, Юрий Р. Залялов<sup>1</sup>, Кирилл В. Лепик<sup>1</sup>, <br>
Елена В. Кондакова<sup>1</sup>, Вадим В. Байков<sup>1</sup>, Иван С. Моисеев<sup>1</sup>, Татьяна В. Шнайдер<sup>2</sup>, Наталья Б. Михайлова<sup>1</sup>, <br>
<span style="border: 1px solid black; margin: 0; padding: 1px 2px;">Борис В. Афанасьев<sup>1</sup></span>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Елена Е. Лепик1, Андрей В. Козлов1, Евгения С. Борзенкова1, Юрий Р. Залялов1, Кирилл В. Лепик1,
Елена В. Кондакова1, Вадим В. Байков1, Иван С. Моисеев1, Татьяна В. Шнайдер2, Наталья Б. Михайлова1,
Борис В. Афанасьев1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26312
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br>
<sup>2</sup> Ленинградская областная клиническая больница, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Ленинградская областная клиническая больница, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26313
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Т-клеточные лимфомы (ТКЛ) представляют собой агрессивные неходжкинские лимфомы, которые не имеют успешных стандартов лечения. Почти у 70% пациентов после первой линии терапии развивается рецидив или рефрактерное течение (р/р). Ряд новых терапевтических подходов нацелены на улучшение результатов лечения у пациентов с р/р ТКЛ.
В данной статье обобщен опыт Первого Санкт-Петербургского государственного медицинского университета им. Павлова в лечении пациентов с Т-клеточными лимфомами. Мы проанализировали данные 47 пациентов, которые проходила лечение с 2005 по 2019 год – на момент анализа 44 пациента имеют р/р течение заболевания и 3 пациента находятся в полном ответе после проведенной первой линии терапии. Средний возраст составил 45 лет (от 1 года до 72 лет). Это были преимущественно пациенты с периферическими Т-клеточными лимфомами, неуточненными (41%). Среди всех пациентов n26 (55%) имели первичное химиорезистентное течение, в то время как у остальных n18 (38%) был рецидив после первой линии терапии.</p>
<p style="text-align: justify;">
Опыт нашего центра включает в себя использование новых вариантов лечения р/р ТКЛ: антиCD30 моноклональное антитело – брентуксимаб, ингибитор ALK – кризотиниб, ингибитор контрольных точек – ниволумаб и иммунотерапию, включающую проведение трансплантации гемопоэтических стволовых клеток (ТГСК). В общей сложности 24 пациентам выполнена ТГСК: высокодозная химиотерапия с последующей аутологичной ТГСК (ауто-ТГСК) проведена 16 пациентам, 13 пациентам – аллогенная ТГСК (алло-ТГСК) (из них 5 пациентов с рецидивами после ауто-ТГСК).
На момент анализа 35 пациентов были живы. Медиана наблюдения за живыми пациентами составила 35 месяцев (6-122 мес.). Медиана общей выживаемости не была достигнута, 5-летняя выживаемость составила 81%, 8-летняя общая выживаемость – 78%. Статус заболевания при последнем наблюдении был следующий: полный ответ у 22 пациентов, частичный ответ у 4 пациентов и прогрессия заболевания у 21. Среди факторов, значительно связанных с неблагоприятным прогнозом, были низкий статус ECOG и B-симптомы на момент постановки диагноза (p=0,06). Пациенты, которые перенесли ТГСК, имели лучший статус заболевания на момент последнего наблюдения: 17/19 (89%) были в полном ответе, по сравнению с 5/16 (31%) у пациентов, которым не проводилась ТГСК. 5-летняя общая выживаемость у пациентов с ТКЛ после ауто-ТГСК и алло-ТГСК составила 87% и 77% соответственно. Результаты анализа показывают, что введение новых агентов и консолидация с помощью высокодозной химиотерапии с последующей ауто-ТГСК или алло-ТГСК в отдельных случаях могут улучшить результаты у пациентов с рецидивирующей или рефрактерной Т-клеточной лимфомой. Схемы, основанные на применении брентуксимаба ведотина и ниволумаба, могут быть успешно использованы в качестве bridge-терапии перед алло-ТГСК.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
T-клеточные лимфомы, аутологичная трансплантация гемопоэтических стволовых клеток, аллогенная трансплантация гемопоэтических стволовых клеток, брентуксимаб ведотин, ниволумаб. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Т-клеточные лимфомы (ТКЛ) представляют собой агрессивные неходжкинские лимфомы, которые не имеют успешных стандартов лечения. Почти у 70% пациентов после первой линии терапии развивается рецидив или рефрактерное течение (р/р). Ряд новых терапевтических подходов нацелены на улучшение результатов лечения у пациентов с р/р ТКЛ.
В данной статье обобщен опыт Первого Санкт-Петербургского государственного медицинского университета им. Павлова в лечении пациентов с Т-клеточными лимфомами. Мы проанализировали данные 47 пациентов, которые проходила лечение с 2005 по 2019 год – на момент анализа 44 пациента имеют р/р течение заболевания и 3 пациента находятся в полном ответе после проведенной первой линии терапии. Средний возраст составил 45 лет (от 1 года до 72 лет). Это были преимущественно пациенты с периферическими Т-клеточными лимфомами, неуточненными (41%). Среди всех пациентов n26 (55%) имели первичное химиорезистентное течение, в то время как у остальных n18 (38%) был рецидив после первой линии терапии.
Опыт нашего центра включает в себя использование новых вариантов лечения р/р ТКЛ: антиCD30 моноклональное антитело – брентуксимаб, ингибитор ALK – кризотиниб, ингибитор контрольных точек – ниволумаб и иммунотерапию, включающую проведение трансплантации гемопоэтических стволовых клеток (ТГСК). В общей сложности 24 пациентам выполнена ТГСК: высокодозная химиотерапия с последующей аутологичной ТГСК (ауто-ТГСК) проведена 16 пациентам, 13 пациентам – аллогенная ТГСК (алло-ТГСК) (из них 5 пациентов с рецидивами после ауто-ТГСК).
На момент анализа 35 пациентов были живы. Медиана наблюдения за живыми пациентами составила 35 месяцев (6-122 мес.). Медиана общей выживаемости не была достигнута, 5-летняя выживаемость составила 81%, 8-летняя общая выживаемость – 78%. Статус заболевания при последнем наблюдении был следующий: полный ответ у 22 пациентов, частичный ответ у 4 пациентов и прогрессия заболевания у 21. Среди факторов, значительно связанных с неблагоприятным прогнозом, были низкий статус ECOG и B-симптомы на момент постановки диагноза (p=0,06). Пациенты, которые перенесли ТГСК, имели лучший статус заболевания на момент последнего наблюдения: 17/19 (89%) были в полном ответе, по сравнению с 5/16 (31%) у пациентов, которым не проводилась ТГСК. 5-летняя общая выживаемость у пациентов с ТКЛ после ауто-ТГСК и алло-ТГСК составила 87% и 77% соответственно. Результаты анализа показывают, что введение новых агентов и консолидация с помощью высокодозной химиотерапии с последующей ауто-ТГСК или алло-ТГСК в отдельных случаях могут улучшить результаты у пациентов с рецидивирующей или рефрактерной Т-клеточной лимфомой. Схемы, основанные на применении брентуксимаба ведотина и ниволумаба, могут быть успешно использованы в качестве bridge-терапии перед алло-ТГСК.
Ключевые слова
T-клеточные лимфомы, аутологичная трансплантация гемопоэтических стволовых клеток, аллогенная трансплантация гемопоэтических стволовых клеток, брентуксимаб ведотин, ниволумаб.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26308
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-28-37
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-28-37
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26314
[VALUE] => Array
(
[TEXT] => <p>
Elena E. Lepik<sup>1</sup>, Andrey V. Kozlov<sup>1</sup>, Evgenia S. Borzenkova<sup>1</sup>,<br>
Yury R. Zalyalov<sup>1</sup>, Kirill V. Lepik<sup>1</sup>, Elena V. Kondakova<sup>1</sup>, Vadim V. Baykov<sup>1</sup>, Ivan S. Moiseev<sup>1</sup>, Tatiana V. Schneider<sup>2</sup>, <br>
Natalia B. Mikhaylova<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 1px 2px;">Boris V. Afanasyev<sup>1</sup></span>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Elena E. Lepik1, Andrey V. Kozlov1, Evgenia S. Borzenkova1,
Yury R. Zalyalov1, Kirill V. Lepik1, Elena V. Kondakova1, Vadim V. Baykov1, Ivan S. Moiseev1, Tatiana V. Schneider2,
Natalia B. Mikhaylova1, Boris V. Afanasyev1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26316
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br> St. Petersburg, Russia<br>
<sup>2</sup> Leningrad Regional Clinical Hospital, St. Petersburg, Russia </p>
<br>
<p><b>Correspondence</b><br>
Dr. Elena E. Lepik, Clinical Hematologist, Oncology Department, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7 (905) 226 8922<br>
E-mail: ee.dav@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
2 Leningrad Regional Clinical Hospital, St. Petersburg, Russia
Correspondence
Dr. Elena E. Lepik, Clinical Hematologist, Oncology Department, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (905) 226 8922
E-mail: ee.dav@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26317
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">T-cell lymphomas (TCL) comprise a group of aggressive non-Hodgkin lymphomas, which do not have successful treatment standards. Almost 70% of patients undergoing first-line therapy develop a relapse or refractory (r/r) disease. A number of novel therapy approaches are aimed for improvement of outcomes in patients with r/r TCL. This report summarizes clinical experience of Pavlov Medical University in the treatment of T cell lymphomas. We analyzed data of 47 patients with TCL treated from 2005 to 2019. Of them, 44 had r/r TCL, and 3 patients were in complete response after first line therapy. The median age was 45 years (range 1-72 years). These were predominantly patients with peripheral T-cell lymphomas not otherwise specified (TCL-NOS, 41%).
26 patients (55% of total) had a primary chemoresistant disease, while the remaining 18 patients (38% of total) had a relapse after initial treatment. </p>
<p style="text-align: justify;">
Our center has implemented new treatment options for r/r TCL, i.e., anti CD30 monoclonal antibody brentuximab, ALK inhibitor crizotinib, immunotherapy with checkpoint inhibitors nivolumab, and hematopoietic stem cell transplantation (HSCT). A total of 24 patients underwent: high-dose chemotherapy with autologous HSCT (auto-HSCT) was performed in 16 cases, 13 patients were subjected to allogeneic HSCT (allo-HSCT), including 5 relapsed patients after auto-HSCT. At the time of analysis, 35 patients remained alive. The median follow-up of surviving patients was 35 months (6-122 mo). The median overall survival (OS) was not reached, 5-year survival rate was 81%, and 8-year survival rate was 78%. Complete remission (CR) at the last follow-up was diagnosed in 22 patients; partial response (PR), in 4 cases, and progression of the disease (PD) was revealed in 21 patients. Among factors significantly associated with adverse prognosis were lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06). The patients who underwent HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) among the patients not subjected to HSCT. 5-year overall survival rates after auto-HSCT and allo-HSCT were 87% and 77%, respectively. The results show that implementation of novel therapeutic agents, as well as consolidation with high-dose chemotherapy and auto- or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab-based regimens may be successfully used as a bridge therapy before HSCT. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">
T-cell lymphoma, autologous hematopoietic stem cells transplantation, allogeneic hematopoietic stem cells transplantation, brentuximab vedotin, nivolumab. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => T-cell lymphomas (TCL) comprise a group of aggressive non-Hodgkin lymphomas, which do not have successful treatment standards. Almost 70% of patients undergoing first-line therapy develop a relapse or refractory (r/r) disease. A number of novel therapy approaches are aimed for improvement of outcomes in patients with r/r TCL. This report summarizes clinical experience of Pavlov Medical University in the treatment of T cell lymphomas. We analyzed data of 47 patients with TCL treated from 2005 to 2019. Of them, 44 had r/r TCL, and 3 patients were in complete response after first line therapy. The median age was 45 years (range 1-72 years). These were predominantly patients with peripheral T-cell lymphomas not otherwise specified (TCL-NOS, 41%).
26 patients (55% of total) had a primary chemoresistant disease, while the remaining 18 patients (38% of total) had a relapse after initial treatment.
Our center has implemented new treatment options for r/r TCL, i.e., anti CD30 monoclonal antibody brentuximab, ALK inhibitor crizotinib, immunotherapy with checkpoint inhibitors nivolumab, and hematopoietic stem cell transplantation (HSCT). A total of 24 patients underwent: high-dose chemotherapy with autologous HSCT (auto-HSCT) was performed in 16 cases, 13 patients were subjected to allogeneic HSCT (allo-HSCT), including 5 relapsed patients after auto-HSCT. At the time of analysis, 35 patients remained alive. The median follow-up of surviving patients was 35 months (6-122 mo). The median overall survival (OS) was not reached, 5-year survival rate was 81%, and 8-year survival rate was 78%. Complete remission (CR) at the last follow-up was diagnosed in 22 patients; partial response (PR), in 4 cases, and progression of the disease (PD) was revealed in 21 patients. Among factors significantly associated with adverse prognosis were lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06). The patients who underwent HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) among the patients not subjected to HSCT. 5-year overall survival rates after auto-HSCT and allo-HSCT were 87% and 77%, respectively. The results show that implementation of novel therapeutic agents, as well as consolidation with high-dose chemotherapy and auto- or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab-based regimens may be successfully used as a bridge therapy before HSCT.
Keywords
T-cell lymphoma, autologous hematopoietic stem cells transplantation, allogeneic hematopoietic stem cells transplantation, brentuximab vedotin, nivolumab.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26309
[VALUE] => Treatment options for T-cell lymphomas: a single-center study
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Treatment options for T-cell lymphomas: a single-center study
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26310
[VALUE] => 1955
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1955
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26315
[VALUE] => 1956
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1956
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Elena E. Lepik1, Andrey V. Kozlov1, Evgenia S. Borzenkova1,
Yury R. Zalyalov1, Kirill V. Lepik1, Elena V. Kondakova1, Vadim V. Baykov1, Ivan S. Moiseev1, Tatiana V. Schneider2,
Natalia B. Mikhaylova1, Boris V. Afanasyev1
1 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
2 Leningrad Regional Clinical Hospital, St. Petersburg, Russia
Correspondence
Dr. Elena E. Lepik, Clinical Hematologist, Oncology Department, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (905) 226 8922
E-mail: ee.dav@mail.ru
T-cell lymphomas (TCL) comprise a group of aggressive non-Hodgkin lymphomas, which do not have successful treatment standards. Almost 70% of patients undergoing first-line therapy develop a relapse or refractory (r/r) disease. A number of novel therapy approaches are aimed for improvement of outcomes in patients with r/r TCL. This report summarizes clinical experience of Pavlov Medical University in the treatment of T cell lymphomas. We analyzed data of 47 patients with TCL treated from 2005 to 2019. Of them, 44 had r/r TCL, and 3 patients were in complete response after first line therapy. The median age was 45 years (range 1-72 years). These were predominantly patients with peripheral T-cell lymphomas not otherwise specified (TCL-NOS, 41%). 26 patients (55% of total) had a primary chemoresistant disease, while the remaining 18 patients (38% of total) had a relapse after initial treatment.
Our center has implemented new treatment options for r/r TCL, i.e., anti CD30 monoclonal antibody brentuximab, ALK inhibitor crizotinib, immunotherapy with checkpoint inhibitors nivolumab, and hematopoietic stem cell transplantation (HSCT). A total of 24 patients underwent: high-dose chemotherapy with autologous HSCT (auto-HSCT) was performed in 16 cases, 13 patients were subjected to allogeneic HSCT (allo-HSCT), including 5 relapsed patients after auto-HSCT. At the time of analysis, 35 patients remained alive. The median follow-up of surviving patients was 35 months (6-122 mo). The median overall survival (OS) was not reached, 5-year survival rate was 81%, and 8-year survival rate was 78%. Complete remission (CR) at the last follow-up was diagnosed in 22 patients; partial response (PR), in 4 cases, and progression of the disease (PD) was revealed in 21 patients. Among factors significantly associated with adverse prognosis were lower ECOG performance status and B-symptoms at the time of diagnosis (p=0.06). The patients who underwent HSCT showed significantly better disease status at the moment of last follow up: 17/19 (89%) were in CR, versus 5/16 (31%) among the patients not subjected to HSCT. 5-year overall survival rates after auto-HSCT and allo-HSCT were 87% and 77%, respectively. The results show that implementation of novel therapeutic agents, as well as consolidation with high-dose chemotherapy and auto- or allo-HSCT in selected cases improve outcomes in patients with r/r TCL. Brentuximab vedotin and nivolumab-based regimens may be successfully used as a bridge therapy before HSCT.
Keywords
T-cell lymphoma, autologous hematopoietic stem cells transplantation, allogeneic hematopoietic stem cells transplantation, brentuximab vedotin, nivolumab.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26318
[VALUE] => 02/28/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/28/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26319
[VALUE] => 03/27/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/27/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26320
[VALUE] => Array
(
[TEXT] => <p>Андрей Н. Соколов<sup>1</sup>, Елена Н. Паровичникова<sup>1</sup>, Вера В. Троицкая<sup>1</sup>, Лариса А. Кузьмина<sup>1</sup>, Ирина В. Гальцева<sup>1</sup>, Сергей М. Куликов<sup>1</sup>, Сергей Н. Бондаренко<sup>2</sup>, Ирина А. Лукьянова<sup>1</sup>, Татьяна И. Лобанова<sup>1</sup>, Екатерина И. Усикова<sup>1</sup>, Ксения И. Зарубина<sup>1</sup>, Ольга А. Гаврилина<sup>1</sup>, Юлия О. Давыдова<sup>1</sup>, Николай М. Капранов<sup>1</sup>, Валерий Г. Савченко<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Андрей Н. Соколов1, Елена Н. Паровичникова1, Вера В. Троицкая1, Лариса А. Кузьмина1, Ирина В. Гальцева1, Сергей М. Куликов1, Сергей Н. Бондаренко2, Ирина А. Лукьянова1, Татьяна И. Лобанова1, Екатерина И. Усикова1, Ксения И. Зарубина1, Ольга А. Гаврилина1, Юлия О. Давыдова1, Николай М. Капранов1, Валерий Г. Савченко1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26321
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Федеральное государственное бюджетное учреждение научный медицинский исследовательский центр гематологии Минздрава России, Москва, Россия<br>
<sup>2</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Федеральное государственное бюджетное учреждение научный медицинский исследовательский центр гематологии Минздрава России, Москва, Россия
2 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26322
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Биспецифическое моноклональное антитело блинатумомаб против антигена CD19 используется для лечения острого лимфобластного лейкоза (ОЛЛ). Несколько дополнительных молекулярных мишеней могут быть использованы для комбинированного лечения без обычной химиотерапии, а именно ингибиторы тирозинкиназ, в т.ч. BCR-ABL, FLT3 и делеции IKZF1. Целью данного исследования было определение токсичности и клинической эффективности комбинированного лечения блинатумомабом и несколькими ингибиторами тирозинкиназы. </p>
<h3>Пациенты и методы</h3>
<p style="text-align: justify;">
С октября 2015 по октябрь 2018 г. нами пролечены 11 пациентов с рецидивируюшим/рефрактерным течением ОЛЛ. Терапия блинатумомабом состояла из 4-5 циклов с 2-недельными интервалами (28 мкг/день посредством постоянной инфузии в течение 28 дней, при дозе 9 мкг/день в течении 1-й недели 1-го цикла). Семь BCR-ABL-позитивных больных и 2 пациента с делецией IKZF1 получали исходно дазатиниб (140 мг/день), один пациент с FLT3-ITD – сорафениб (800 мг/день). Один BCR-ABL-позитивный больной с мутацией T315I получал понатиниб (45 мг/день). Пациентам с делециями IKZF1 назначалась ATRA (45 мг/м<sup>2</sup>/день в течение 4 недель) на 1-м цикле блинатумомаба и в первые 2 недели последующих циклов лечения антителом.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">
У пациентов, отвечающих на лечение блинатумомабом, отмечалось статистически достоверное повышение абсолютного количества Т-хелперных лимфоцитов (p=0.0034), T-цитотоксических клеток (p<0.0001) и субпопуляций естественных киллеров (p=0.0006) в периферической крови на протяжении всего периода лечения. T-регуляторные и дубль-негативные T-клетки – потенциальные ингибиторы Т-клеточного ответа на блинатумомаб оставались в пределах нижних значений нормы. Гипогаммаглобулинемия наблюдалась у 8 из 11 пациентов. Полная ремиссия (ПР) была получена у 10 больных после 1-го цикла блинатумомаба, прогрессия заболевания – в одном случае. Из 10 пациентов, в 9 случаях достигнута полная молекулярная ремиссия (ПМР) и в одном – полная цитогенетическая ремиссия. Выполнены девять аллогенных трансплантаций гемопоэтических стволовых клеток (ТГСК) и одна аутологичная ТГСК в 10 случаях ПР. Выявлены три рецидива в ЦНС после алло-ТГСК и один молекулярный рецидив после ауто-ТГСК. Один пациент скончался от септического шока после алло-ТГСК. </p>
<h3>Выводы</h3>
<p style="text-align: justify;">
Блинатомомаб в коимбинации с ингибиторами тирозинкиназ имеет высокий терапевтический потенциал для индукции ремиссии в определенных случаях ОЛЛ без применения обычной химиотерапии. Высокая частота потенциальных рецидивов в ЦНС может быть снижена за счет более интенсивной интратекальной профилактики. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Острый лимфобластный лейкоз, блинатумомаб, ингибиторы тирозинкиназ. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Биспецифическое моноклональное антитело блинатумомаб против антигена CD19 используется для лечения острого лимфобластного лейкоза (ОЛЛ). Несколько дополнительных молекулярных мишеней могут быть использованы для комбинированного лечения без обычной химиотерапии, а именно ингибиторы тирозинкиназ, в т.ч. BCR-ABL, FLT3 и делеции IKZF1. Целью данного исследования было определение токсичности и клинической эффективности комбинированного лечения блинатумомабом и несколькими ингибиторами тирозинкиназы.
Пациенты и методы
С октября 2015 по октябрь 2018 г. нами пролечены 11 пациентов с рецидивируюшим/рефрактерным течением ОЛЛ. Терапия блинатумомабом состояла из 4-5 циклов с 2-недельными интервалами (28 мкг/день посредством постоянной инфузии в течение 28 дней, при дозе 9 мкг/день в течении 1-й недели 1-го цикла). Семь BCR-ABL-позитивных больных и 2 пациента с делецией IKZF1 получали исходно дазатиниб (140 мг/день), один пациент с FLT3-ITD – сорафениб (800 мг/день). Один BCR-ABL-позитивный больной с мутацией T315I получал понатиниб (45 мг/день). Пациентам с делециями IKZF1 назначалась ATRA (45 мг/м2/день в течение 4 недель) на 1-м цикле блинатумомаба и в первые 2 недели последующих циклов лечения антителом.
Результаты
У пациентов, отвечающих на лечение блинатумомабом, отмечалось статистически достоверное повышение абсолютного количества Т-хелперных лимфоцитов (p=0.0034), T-цитотоксических клеток (p<0.0001) и субпопуляций естественных киллеров (p=0.0006) в периферической крови на протяжении всего периода лечения. T-регуляторные и дубль-негативные T-клетки – потенциальные ингибиторы Т-клеточного ответа на блинатумомаб оставались в пределах нижних значений нормы. Гипогаммаглобулинемия наблюдалась у 8 из 11 пациентов. Полная ремиссия (ПР) была получена у 10 больных после 1-го цикла блинатумомаба, прогрессия заболевания – в одном случае. Из 10 пациентов, в 9 случаях достигнута полная молекулярная ремиссия (ПМР) и в одном – полная цитогенетическая ремиссия. Выполнены девять аллогенных трансплантаций гемопоэтических стволовых клеток (ТГСК) и одна аутологичная ТГСК в 10 случаях ПР. Выявлены три рецидива в ЦНС после алло-ТГСК и один молекулярный рецидив после ауто-ТГСК. Один пациент скончался от септического шока после алло-ТГСК.
Выводы
Блинатомомаб в коимбинации с ингибиторами тирозинкиназ имеет высокий терапевтический потенциал для индукции ремиссии в определенных случаях ОЛЛ без применения обычной химиотерапии. Высокая частота потенциальных рецидивов в ЦНС может быть снижена за счет более интенсивной интратекальной профилактики.
Ключевые слова
Острый лимфобластный лейкоз, блинатумомаб, ингибиторы тирозинкиназ.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26323
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-38-46
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-38-46
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26326
[VALUE] => Array
(
[TEXT] => <p>Andrey N. Sokolov<sup>1</sup>, Elena N. Parovichnikova<sup>1</sup>, Vera V. Troitskaya<sup>1</sup>, Larisa A. Kuzmina<sup>1</sup>, Irina V. Galtseva<sup>1</sup>, Sergei M. Kulikov<sup>1</sup>, Sergey N. Bondarenko<sup>2</sup>, Irina A. Lukyanova<sup>1</sup>, Tatiana I. Lobanova<sup>1</sup>, Ekaterina I. Usikova<sup>1</sup>, Ksenia I. Zarubina<sup>1</sup>, Olga A. Gavrilina<sup>1</sup>, Julia O. Davidova<sup>1</sup>, Nikolai M. Kapranov<sup>1</sup>, Valeriy G. Savchenko<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Andrey N. Sokolov1, Elena N. Parovichnikova1, Vera V. Troitskaya1, Larisa A. Kuzmina1, Irina V. Galtseva1, Sergei M. Kulikov1, Sergey N. Bondarenko2, Irina A. Lukyanova1, Tatiana I. Lobanova1, Ekaterina I. Usikova1, Ksenia I. Zarubina1, Olga A. Gavrilina1, Julia O. Davidova1, Nikolai M. Kapranov1, Valeriy G. Savchenko1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26327
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia<br>
<sup>2</sup> Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br>
St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia<br>
Phone: +7 (495) 612 4592<br>
E-mail: sokolov.a@blood.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia
2 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia
Phone: +7 (495) 612 4592
E-mail: sokolov.a@blood.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26328
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Bispecific monoclonal antibody blinatumomab is targeting CD19, being applied for treatment of acute lymphoblastic leukemia (ALL). Several additional targets could be used for combined chemo-free treatment with thyrosine kinase inhibitors: BCR-ABL, FLT3 and IKZF1 deletions are amongst them. In the following study, we aimed to assess toxicity and clinical effectiveness of the combination of blinatumomab and several thyrosine kinase inhibitors. </p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">
From October 2015 to October 2018, we treated 11 relapsed/refractory (R/R) ALL patients (pts). The blinatumomab treatment consisted of 4-5 cycles with 2-week intervals (28 mcg/day by continuous infusion during 28 days per cycle with 9 mcg/day during the first week of the first cycle). Seven BCR-ABL-positive and 2 IKZF1-deleted pts received initially dasatinib at 140 mg/day, one case, with FLT3-ITD received sorafenib (800 mg/day). One BCR-ABL-positive pt with T315I mutation was administered ponatinib (45 mg/day). Pts with IKZF1 deletions received ATRA (45 mg/m<sup>2</sup>/day for 4 weeks) of the 1<sup>st</sup> blinatumomab cycle and during first 2 weeks of subsequent blinatumomab cycles. </p>
<h3>Results</h3>
<p style="text-align: justify;">
In the patients responding to blinatumomab treatment, we observed a statistically significant increment of absolute values in T-helper (p=0.0034), T-cytotoxic (p<0.0001) and NK (p=0.0006) cell subpopulations in peripheral blood over the entire treatment period. T-regulatory and double-negative T-cells as potentially inhibitors of T cell response to blinatumomab remained within low values of normal ranges. Hypogammaglobulinemia was observed in 8 of 11 pts. CR was achieved in 10 pts after 1st cycle of blinatumomab, progressive disease was detected in 1 pt. Nine cases of complete molecular remissions (MolCR) and one cytogenetic complete remission were achieved in ten CR pts. Nine allo-BMT and one auto-BMT were performed in 10 CR pts. Three CNS relapses after allo-BMT and one molecular relapse after auto-BMT were diagnosed. One pt died from septic shock after allo-BMT. </p>
<h3>Conclusion</h3>
<p style="text-align: justify;">
Blinatumomab combined with TKI has high therapeutic potential as induction remission treatment in targeted ALL population without conventional chemotherapy. High rate of potential CNS relapses could be decreased with more intensive intrathecal prophylaxis.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Acute lymphoblastic leukemia, blinatumomab, tyrosine kinase inhibitors. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Bispecific monoclonal antibody blinatumomab is targeting CD19, being applied for treatment of acute lymphoblastic leukemia (ALL). Several additional targets could be used for combined chemo-free treatment with thyrosine kinase inhibitors: BCR-ABL, FLT3 and IKZF1 deletions are amongst them. In the following study, we aimed to assess toxicity and clinical effectiveness of the combination of blinatumomab and several thyrosine kinase inhibitors.
Patients and methods
From October 2015 to October 2018, we treated 11 relapsed/refractory (R/R) ALL patients (pts). The blinatumomab treatment consisted of 4-5 cycles with 2-week intervals (28 mcg/day by continuous infusion during 28 days per cycle with 9 mcg/day during the first week of the first cycle). Seven BCR-ABL-positive and 2 IKZF1-deleted pts received initially dasatinib at 140 mg/day, one case, with FLT3-ITD received sorafenib (800 mg/day). One BCR-ABL-positive pt with T315I mutation was administered ponatinib (45 mg/day). Pts with IKZF1 deletions received ATRA (45 mg/m2/day for 4 weeks) of the 1st blinatumomab cycle and during first 2 weeks of subsequent blinatumomab cycles.
Results
In the patients responding to blinatumomab treatment, we observed a statistically significant increment of absolute values in T-helper (p=0.0034), T-cytotoxic (p<0.0001) and NK (p=0.0006) cell subpopulations in peripheral blood over the entire treatment period. T-regulatory and double-negative T-cells as potentially inhibitors of T cell response to blinatumomab remained within low values of normal ranges. Hypogammaglobulinemia was observed in 8 of 11 pts. CR was achieved in 10 pts after 1st cycle of blinatumomab, progressive disease was detected in 1 pt. Nine cases of complete molecular remissions (MolCR) and one cytogenetic complete remission were achieved in ten CR pts. Nine allo-BMT and one auto-BMT were performed in 10 CR pts. Three CNS relapses after allo-BMT and one molecular relapse after auto-BMT were diagnosed. One pt died from septic shock after allo-BMT.
Conclusion
Blinatumomab combined with TKI has high therapeutic potential as induction remission treatment in targeted ALL population without conventional chemotherapy. High rate of potential CNS relapses could be decreased with more intensive intrathecal prophylaxis.
Keywords
Acute lymphoblastic leukemia, blinatumomab, tyrosine kinase inhibitors.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26324
[VALUE] => BCR/ABL, IKZF deletions and FLT3-ITD as the targets for relapsed/refractory B-cell acute lymphoblastic leukemia treatment: Blinatumomab combined with Tyrosine kinase inhibitors and ATRA
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => BCR/ABL, IKZF deletions and FLT3-ITD as the targets for relapsed/refractory B-cell acute lymphoblastic leukemia treatment: Blinatumomab combined with Tyrosine kinase inhibitors and ATRA
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26325
[VALUE] => 1968
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1968
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26329
[VALUE] => 1969
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1969
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Andrey N. Sokolov1, Elena N. Parovichnikova1, Vera V. Troitskaya1, Larisa A. Kuzmina1, Irina V. Galtseva1, Sergei M. Kulikov1, Sergey N. Bondarenko2, Irina A. Lukyanova1, Tatiana I. Lobanova1, Ekaterina I. Usikova1, Ksenia I. Zarubina1, Olga A. Gavrilina1, Julia O. Davidova1, Nikolai M. Kapranov1, Valeriy G. Savchenko1
1 National Research Center for Hematology of the Ministry of Healthcare of the Russian Federation, Moscow, Russia
2 Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Andrey N. Sokolov, National Research Center for Hematology, 4A Novyi Zykovskii Lane, 125167, Moscow, Russia
Phone: +7 (495) 612 4592
E-mail: sokolov.a@blood.ru
Bispecific monoclonal antibody blinatumomab is targeting CD19, being applied for treatment of acute lymphoblastic leukemia (ALL). Several additional targets could be used for combined chemo-free treatment with thyrosine kinase inhibitors: BCR-ABL, FLT3 and IKZF1 deletions are amongst them. In the following study, we aimed to assess toxicity and clinical effectiveness of the combination of blinatumomab and several thyrosine kinase inhibitors.
Patients and methods
From October 2015 to October 2018, we treated 11 relapsed/refractory (R/R) ALL patients (pts). The blinatumomab treatment consisted of 4-5 cycles with 2-week intervals (28 mcg/day by continuous infusion during 28 days per cycle with 9 mcg/day during the first week of the first cycle). Seven BCR-ABL-positive and 2 IKZF1-deleted pts received initially dasatinib at 140 mg/day, one case, with FLT3-ITD received sorafenib (800 mg/day). One BCR-ABL-positive pt with T315I mutation was administered ponatinib (45 mg/day). Pts with IKZF1 deletions received ATRA (45 mg/m2/day for 4 weeks) of the 1st blinatumomab cycle and during first 2 weeks of subsequent blinatumomab cycles.
Results
In the patients responding to blinatumomab treatment, we observed a statistically significant increment of absolute values in T-helper (p=0.0034), T-cytotoxic (p<0.0001) and NK (p=0.0006) cell subpopulations in peripheral blood over the entire treatment period. T-regulatory and double-negative T-cells as potentially inhibitors of T cell response to blinatumomab remained within low values of normal ranges. Hypogammaglobulinemia was observed in 8 of 11 pts. CR was achieved in 10 pts after 1st cycle of blinatumomab, progressive disease was detected in 1 pt. Nine cases of complete molecular remissions (MolCR) and one cytogenetic complete remission were achieved in ten CR pts. Nine allo-BMT and one auto-BMT were performed in 10 CR pts. Three CNS relapses after allo-BMT and one molecular relapse after auto-BMT were diagnosed. One pt died from septic shock after allo-BMT.
Conclusion
Blinatumomab combined with TKI has high therapeutic potential as induction remission treatment in targeted ALL population without conventional chemotherapy. High rate of potential CNS relapses could be decreased with more intensive intrathecal prophylaxis.
Keywords
Acute lymphoblastic leukemia, blinatumomab, tyrosine kinase inhibitors.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26396
[VALUE] => 02/19/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/19/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26397
[VALUE] => 03/28/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/28/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26398
[VALUE] => Array
(
[TEXT] => <p>
Александр А. Щербаков, Алена Н. Зайцева, Максим А. Кучер, Ирина С. Ярушкина, Ольга Н. Зацепина, Екатерина С. Кульнева, Олеся В. Паина, Руслана В. Клементьева, Олег В. Голощапов, Иван С. Моисеев, Людмила С. Зубаровская, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Александр А. Щербаков, Алена Н. Зайцева, Максим А. Кучер, Ирина С. Ярушкина, Ольга Н. Зацепина, Екатерина С. Кульнева, Олеся В. Паина, Руслана В. Клементьева, Олег В. Голощапов, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26399
[VALUE] => Array
(
[TEXT] => <p>
НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26400
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Геморрагический цистит (ГЦ) – это частое осложнение при аллогенной трансплантации гемопоэтических стволовых клеток (алло-ТГСК). Персистирование BK полиомавируса (BKPyV) является одной из основных предполагаемых причин, приводящих к развитию позднего ГЦ после алло-ТГСК. Имеются доклинические данные об эффективной нейтрализации BKPyV внутривенным иммуноглобулином G (ВВИГ) в клеточных культурах, а также при BK вирусной нефропатии при трансплантации почки.
</p>
<h3>Пациенты и методы</h3>
<p style="text-align: justify;">
Ретроспективно было изучено 1037 пациентов получивших алло-ТГСК в НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой с 2013 по 2018 год. ГЦ был зарегистрирован в 118 (11,4%) случаях, из них, согласно критериям включения, 90 пациентов включены в анализ: группа сравнения с ВВИГ (n=42) и контрольная группа (n=48) со стандартной терапией ГЦ.
</p>
<h3>Результаты</h3>
<p style="text-align: justify;">
Продолжительность ГЦ в общей группе составила 21 день. Не было статистически значимой разницы в продолжительности ГЦ между группой сравнения и контрольной группой – медиана 24 дня (16, 32; 95% CI) и 24 дня (2, 46; 95% CI), соответственно (p=0,39). Продолжительность ГЦ при миелоаблативном режиме кондиционирования составила 35 дней (18, 52; 95% ДИ) и 17 дней (14, 20; 95% ДИ) при немиелоаблативном режиме кондиционирования, р <0,01. Одновременное наличие симптомов ГЦ и РТПХ I-IV степени, также характеризовалось положительной корреляцией с длительностью ГЦ – 36 дней (22, 50; 95% ДИ) у пациентов с РТПХ и 18 дней (15, 21; 95% ДИ) у пациентов без РТПХ, р=0,013.
</p>
<h3>Выводы</h3>
<p style="text-align: justify;">
Настоящее исследование не подтвердило клиническую эффективность ВВИГ (Иммуновенин 5% (50мг/мл) Микроген) в дозе 1,2 гр/кг у пациентов с ГЦ после алло-ТГСК. Факторами риска более длительного течения ГЦ были интенсивность режима кондиционирования и наличие РТПХ I-IV степени. Требуется проведение проспективного исследования для определения эффективности ВВИГ в качестве терапии позднего ГЦ после алло-ТГСК.
</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Аллогенная трансплантация гемопоэтических стволовых клеток, геморрагический цистит, внутривенный иммуноглобулин, ВВИГ, факторы риска, реакция «трансплантат против хозяина» (РТПХ).
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Геморрагический цистит (ГЦ) – это частое осложнение при аллогенной трансплантации гемопоэтических стволовых клеток (алло-ТГСК). Персистирование BK полиомавируса (BKPyV) является одной из основных предполагаемых причин, приводящих к развитию позднего ГЦ после алло-ТГСК. Имеются доклинические данные об эффективной нейтрализации BKPyV внутривенным иммуноглобулином G (ВВИГ) в клеточных культурах, а также при BK вирусной нефропатии при трансплантации почки.
Пациенты и методы
Ретроспективно было изучено 1037 пациентов получивших алло-ТГСК в НИИ детской онкологии, гематологии и трансплантологии им. Р.М. Горбачевой с 2013 по 2018 год. ГЦ был зарегистрирован в 118 (11,4%) случаях, из них, согласно критериям включения, 90 пациентов включены в анализ: группа сравнения с ВВИГ (n=42) и контрольная группа (n=48) со стандартной терапией ГЦ.
Результаты
Продолжительность ГЦ в общей группе составила 21 день. Не было статистически значимой разницы в продолжительности ГЦ между группой сравнения и контрольной группой – медиана 24 дня (16, 32; 95% CI) и 24 дня (2, 46; 95% CI), соответственно (p=0,39). Продолжительность ГЦ при миелоаблативном режиме кондиционирования составила 35 дней (18, 52; 95% ДИ) и 17 дней (14, 20; 95% ДИ) при немиелоаблативном режиме кондиционирования, р <0,01. Одновременное наличие симптомов ГЦ и РТПХ I-IV степени, также характеризовалось положительной корреляцией с длительностью ГЦ – 36 дней (22, 50; 95% ДИ) у пациентов с РТПХ и 18 дней (15, 21; 95% ДИ) у пациентов без РТПХ, р=0,013.
Выводы
Настоящее исследование не подтвердило клиническую эффективность ВВИГ (Иммуновенин 5% (50мг/мл) Микроген) в дозе 1,2 гр/кг у пациентов с ГЦ после алло-ТГСК. Факторами риска более длительного течения ГЦ были интенсивность режима кондиционирования и наличие РТПХ I-IV степени. Требуется проведение проспективного исследования для определения эффективности ВВИГ в качестве терапии позднего ГЦ после алло-ТГСК.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, геморрагический цистит, внутривенный иммуноглобулин, ВВИГ, факторы риска, реакция «трансплантат против хозяина» (РТПХ).
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26401
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-60-66
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-60-66
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26404
[VALUE] => Array
(
[TEXT] => <p>Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina,
Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev,
Ludmila S. Zubarovskaya,<br> <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina,
Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev,
Ludmila S. Zubarovskaya,
Boris V. Afanasyev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26405
[VALUE] => Array
(
[TEXT] => <p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br>
St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Alexander A. Shcherbakov, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7(904) 604 0884<br>
E-mail: xihmrx@gmail.com</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Alexander A. Shcherbakov, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(904) 604 0884
E-mail: xihmrx@gmail.com
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26407
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo-
HSCT). Human BK-polyomavirus (BKPyV) may be one of the main agents found in late HC developing after allo-HSCT. In previous studies, intravenous immunoglobulin (IVIG) preparations were shown to neutralize BKPyV in mice cell cultures and to reduce BK virus nephropathy in kidney transplantation. The aim of current study was to evaluate efficiency of HC treatment with IVIG in allo-HSCT recipients, with respect to conditioning regimen intensity and presence of graft-versus-host disease (GvHD).</p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">A total of 1037 allo-HSCT recipients transplanted in R. M. Gorbacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included into retrospective open single-center cohort study. HC was registered in 118 (11.4%) cases. According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included patients with standard HC treatment with addition of IVIG; the control group (n=48) – with standard HC therapy only.</p>
<h3>Results</h3>
<p style="text-align: justify;">
The median HC duration in common group (IVIG and control) was 21 days. There was no statistically significant difference in HC duration between the two groups, with the median of 24 days (16, 32; 95% CI) in the intervention group (standard therapy + IVIG), and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39). The median HC duration in MAC and RIC was 35 days (18, 52; 95% CI) versus 17 days (14, 20; 95% CI), respectively (p<0.01). The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with GvHD and 18 days (15, 21; 95% CI) in patients without GvHD (p=0.013).</p>
<h3>Conclusions</h3>
<p style="text-align: justify;">Our data didn’t show any clinical efficiency of 1.2 g/kg IVIG (Immunovenin® 5%, 50mg/mL by Microgen) effectiveness in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. The further prospective study is needed to make final conclusions on method’s effectiveness.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Allogeneic hematopoietic stem cell transplantation, hemorrhagic cystitis, intravenous immunoglobulin, IVIG, risk factors, graft-versus-host disease (GvHD). </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo-
HSCT). Human BK-polyomavirus (BKPyV) may be one of the main agents found in late HC developing after allo-HSCT. In previous studies, intravenous immunoglobulin (IVIG) preparations were shown to neutralize BKPyV in mice cell cultures and to reduce BK virus nephropathy in kidney transplantation. The aim of current study was to evaluate efficiency of HC treatment with IVIG in allo-HSCT recipients, with respect to conditioning regimen intensity and presence of graft-versus-host disease (GvHD).
Patients and methods
A total of 1037 allo-HSCT recipients transplanted in R. M. Gorbacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included into retrospective open single-center cohort study. HC was registered in 118 (11.4%) cases. According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included patients with standard HC treatment with addition of IVIG; the control group (n=48) – with standard HC therapy only.
Results
The median HC duration in common group (IVIG and control) was 21 days. There was no statistically significant difference in HC duration between the two groups, with the median of 24 days (16, 32; 95% CI) in the intervention group (standard therapy + IVIG), and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39). The median HC duration in MAC and RIC was 35 days (18, 52; 95% CI) versus 17 days (14, 20; 95% CI), respectively (p<0.01). The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with GvHD and 18 days (15, 21; 95% CI) in patients without GvHD (p=0.013).
Conclusions
Our data didn’t show any clinical efficiency of 1.2 g/kg IVIG (Immunovenin® 5%, 50mg/mL by Microgen) effectiveness in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. The further prospective study is needed to make final conclusions on method’s effectiveness.
Keywords
Allogeneic hematopoietic stem cell transplantation, hemorrhagic cystitis, intravenous immunoglobulin, IVIG, risk factors, graft-versus-host disease (GvHD).
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26402
[VALUE] => Intravenous immunoglobulin G treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Intravenous immunoglobulin G treatment of hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26403
[VALUE] => 1987
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1987
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26406
[VALUE] => 1988
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1988
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Alexander A. Shcherbakov, Alena N. Zaytseva, Maksim A. Kucher, Irina S. Iarushkina, Olga N. Zatsepina,
Ekaterina S. Kulneva, Olesya V. Paina, Ruslana V. Klementeva, Оleg V. Goloshchapov, Ivan S. Moiseev,
Ludmila S. Zubarovskaya,
Boris V. Afanasyev
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Alexander A. Shcherbakov, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(904) 604 0884
E-mail: xihmrx@gmail.com
Hemorrhagic cystitis (HC) is a frequent complication in allogeneic hematopoietic stem cell transplantation (allo- HSCT). Human BK-polyomavirus (BKPyV) may be one of the main agents found in late HC developing after allo-HSCT. In previous studies, intravenous immunoglobulin (IVIG) preparations were shown to neutralize BKPyV in mice cell cultures and to reduce BK virus nephropathy in kidney transplantation. The aim of current study was to evaluate efficiency of HC treatment with IVIG in allo-HSCT recipients, with respect to conditioning regimen intensity and presence of graft-versus-host disease (GvHD).
Patients and methods
A total of 1037 allo-HSCT recipients transplanted in R. M. Gorbacheva Memorial institute for Pediatric Oncology, Hematology and Transplantation in 2013-2018 were included into retrospective open single-center cohort study. HC was registered in 118 (11.4%) cases. According to inclusion criteria, only 90 patients were enrolled to the final analysis. This cohort was divided into two groups based on HC therapy used: the intervention group (n=42) included patients with standard HC treatment with addition of IVIG; the control group (n=48) – with standard HC therapy only.
Results
The median HC duration in common group (IVIG and control) was 21 days. There was no statistically significant difference in HC duration between the two groups, with the median of 24 days (16, 32; 95% CI) in the intervention group (standard therapy + IVIG), and 24 days (2, 46; 95% CI) in control group, respectively (p=0.39). The median HC duration in MAC and RIC was 35 days (18, 52; 95% CI) versus 17 days (14, 20; 95% CI), respectively (p<0.01). The presence of GvHD I-IV at the time of HC symptoms manifestation was also characterized by positive correlation with HC duration. It was 36 days (22, 50; 95% CI) in patients with GvHD and 18 days (15, 21; 95% CI) in patients without GvHD (p=0.013).
Conclusions
Our data didn’t show any clinical efficiency of 1.2 g/kg IVIG (Immunovenin® 5%, 50mg/mL by Microgen) effectiveness in post allo-HSCT HC patients. Conditioning regimen intensity and GvHD I-IV are the risk factors for HC longer duration. The further prospective study is needed to make final conclusions on method’s effectiveness.
Keywords
Allogeneic hematopoietic stem cell transplantation, hemorrhagic cystitis, intravenous immunoglobulin, IVIG, risk factors, graft-versus-host disease (GvHD).
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26384
[VALUE] => 11/11/2019 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/11/2019 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26385
[VALUE] => 01/24/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 01/24/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26386
[VALUE] => Array
(
[TEXT] => <p>Елена В. Морозова, Иван С. Моисеев, Юлия Ю. Власова, Николай Ю. Цветков, Юлия В. Рудницкая, Мария В. Барабанщикова, Анна Г. Смирнова, Елена И. Дарская, Сергей Н. Бондаренко, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Елена В. Морозова, Иван С. Моисеев, Юлия Ю. Власова, Николай Ю. Цветков, Юлия В. Рудницкая, Мария В. Барабанщикова, Анна Г. Смирнова, Елена И. Дарская, Сергей Н. Бондаренко, Борис В. Афанасьев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26387
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26388
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">В настоящий момент отмечается рост числа публикаций по поводу использования посттрансплантационного циклофосфана (ПТЦФ) в профилактике реакции «трансплантат против хозяина» при неродственной трансплантации гемопоэтических стволовых клеток. Тем не менее, большинство этих публикаций включают только пациентов с острыми лейкозами. Данные о возможности применения ПТЦФ при хроническом миелолейкозе (ХМЛ), миелодиспластическом синдроме (МДС) и хронических миелопролиферативных заболеваниях (ХМПЗ) практически отсутствуют, поэтому в данной популяции пациентов было инициировано проспективное рандомизированное сравнение тимоглобулина и ПТЦФ в профилактики РТПХ при 10/10- HLA совместимой неродственной трансплантации (NCT02627573, clinicaltrials.gov). Стратой для рандомизации был индекс pretransplant assessment of mortality. Исследование было прекращено преждевременно в связи с медленным набором пациентов, тем не менее, за время исследования было включено 33 пациента, 16 пациентов в группу тимоглобулина и 17 пациентов в группу ПТЦФ. Медиана наблюдения составила 29 месяцев. Не было выявлено различий ни в частоте первичного неприживления трансплантата (12,50% против 11,8%, p=0,9), ни острой РТПХ II-IV степени (23% <i>vs</i> 6%, p=0,2), ни хронической РТПХ средней и тяжелой степени (25% <i>vs</i> 23%, p=0,4) в группах тимоглобулина и ПТЦФ, соответственно. Однако, было в группе ПТЦФ наблюдалась достоверно лучшая общая (82% <i>vs</i> 30%, p=0,0126) и бессобытийная (61% <i>vs</i> 26%, p=0,0335) выживаемость, а также выживаемость без рецидива и РТПХ (61% <i>vs</i> 16%, p=0,0072). Различия были связаны с поздней инфекционной летальностью. Никак достоверных различий в токсичности 2 режимов выявлено не было. Подводя итоги, данное исследование дает обоснование для применения ПТЦФ при неродственных трансплантациях у пациентов с ХМЛ и МДС. Требуются дальнейшие исследования для подтверждения преимущества ПТЦФ над классической профилактикой с тимоглобулином. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Посттрансплантационный циклофосфан, миелодиспластический синдром, хронический миелоидный лейкоз, хроническая миелопролиферативная неоплазия, совместимый неродственный донор. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => В настоящий момент отмечается рост числа публикаций по поводу использования посттрансплантационного циклофосфана (ПТЦФ) в профилактике реакции «трансплантат против хозяина» при неродственной трансплантации гемопоэтических стволовых клеток. Тем не менее, большинство этих публикаций включают только пациентов с острыми лейкозами. Данные о возможности применения ПТЦФ при хроническом миелолейкозе (ХМЛ), миелодиспластическом синдроме (МДС) и хронических миелопролиферативных заболеваниях (ХМПЗ) практически отсутствуют, поэтому в данной популяции пациентов было инициировано проспективное рандомизированное сравнение тимоглобулина и ПТЦФ в профилактики РТПХ при 10/10- HLA совместимой неродственной трансплантации (NCT02627573, clinicaltrials.gov). Стратой для рандомизации был индекс pretransplant assessment of mortality. Исследование было прекращено преждевременно в связи с медленным набором пациентов, тем не менее, за время исследования было включено 33 пациента, 16 пациентов в группу тимоглобулина и 17 пациентов в группу ПТЦФ. Медиана наблюдения составила 29 месяцев. Не было выявлено различий ни в частоте первичного неприживления трансплантата (12,50% против 11,8%, p=0,9), ни острой РТПХ II-IV степени (23% vs 6%, p=0,2), ни хронической РТПХ средней и тяжелой степени (25% vs 23%, p=0,4) в группах тимоглобулина и ПТЦФ, соответственно. Однако, было в группе ПТЦФ наблюдалась достоверно лучшая общая (82% vs 30%, p=0,0126) и бессобытийная (61% vs 26%, p=0,0335) выживаемость, а также выживаемость без рецидива и РТПХ (61% vs 16%, p=0,0072). Различия были связаны с поздней инфекционной летальностью. Никак достоверных различий в токсичности 2 режимов выявлено не было. Подводя итоги, данное исследование дает обоснование для применения ПТЦФ при неродственных трансплантациях у пациентов с ХМЛ и МДС. Требуются дальнейшие исследования для подтверждения преимущества ПТЦФ над классической профилактикой с тимоглобулином.
Ключевые слова
Посттрансплантационный циклофосфан, миелодиспластический синдром, хронический миелоидный лейкоз, хроническая миелопролиферативная неоплазия, совместимый неродственный донор.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26389
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-53-59
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-53-59
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26392
[VALUE] => Array
(
[TEXT] => <p>Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, Boris V. Afanasyev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26394
[VALUE] => Array
(
[TEXT] => <p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,<br>
St. Petersburg, Russia </p><br>
<p><b>Correspondence</b><br>
Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7 (911) 927 8229<br>
E-mail: dr_morozova@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 927 8229
E-mail: dr_morozova@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26395
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Despite growing evidence on the use of posttransplantation cyclophosphamide (PTCY) in matched unrelated transplantation in acute leukemia patients, prospective evidence to support this type of prophylaxis in myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms and chronic myeloid leukemia (CML) patients is lacking. In this patient population we conducted a prospective randomized study of PTCY vs thymoglobulin for graft-versus-host disease (GvHD) prophylaxis in the setting of 10/10 HLA-matched unrelated transplantation (NCT02627573, clinicaltrials.gov). The strata for randomization was pretransplant assessment of mortality score. The study was terminated prematurely due to poor recruitment, but 33 patients were enrolled, 17 in the PTCY and 16 in the thymoglobulin group. Median follow up was 29 months. There was no difference between groups in the incidence primary graft failure (12.50% <i>vs</i> 11.8%, p=0.9), acute GvHD grade II-IV (23% <i>vs</i> 6%, p=0.2), moderate and severe chronic GvHD (25% <i>vs</i> 23%, p=0.4) in the thymoglobulin and PTCY arms, respectively. However there was a significantly higher overall survival (82% <i>vs</i> 30%, p=0.0126), event-free survival (61% <i>vs</i> 26%, p=0.0335), and GvHD-relapse free survival (61% <i>vs</i> 16%, p=0.0072) in the PTCY group due to reduced late infection-related mortality. No differences was observed in terms of toxicity. In conclusion, despite incomplete recruitment, the study created the basis for the use of PTCY in unrelated transplantations from fully matched unrelated donors in CML and MDS. Future studies are required to confirm if there is a benefit of PTCY-based prophylaxis over thymoglobulin. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Posttransplant cyclophosphamide, myelodysplastic syndrome, chronic myeloid leukemia, chronic myeloproliferative neoplasm, matched unrelated donor. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Despite growing evidence on the use of posttransplantation cyclophosphamide (PTCY) in matched unrelated transplantation in acute leukemia patients, prospective evidence to support this type of prophylaxis in myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms and chronic myeloid leukemia (CML) patients is lacking. In this patient population we conducted a prospective randomized study of PTCY vs thymoglobulin for graft-versus-host disease (GvHD) prophylaxis in the setting of 10/10 HLA-matched unrelated transplantation (NCT02627573, clinicaltrials.gov). The strata for randomization was pretransplant assessment of mortality score. The study was terminated prematurely due to poor recruitment, but 33 patients were enrolled, 17 in the PTCY and 16 in the thymoglobulin group. Median follow up was 29 months. There was no difference between groups in the incidence primary graft failure (12.50% vs 11.8%, p=0.9), acute GvHD grade II-IV (23% vs 6%, p=0.2), moderate and severe chronic GvHD (25% vs 23%, p=0.4) in the thymoglobulin and PTCY arms, respectively. However there was a significantly higher overall survival (82% vs 30%, p=0.0126), event-free survival (61% vs 26%, p=0.0335), and GvHD-relapse free survival (61% vs 16%, p=0.0072) in the PTCY group due to reduced late infection-related mortality. No differences was observed in terms of toxicity. In conclusion, despite incomplete recruitment, the study created the basis for the use of PTCY in unrelated transplantations from fully matched unrelated donors in CML and MDS. Future studies are required to confirm if there is a benefit of PTCY-based prophylaxis over thymoglobulin.
Keywords
Posttransplant cyclophosphamide, myelodysplastic syndrome, chronic myeloid leukemia, chronic myeloproliferative neoplasm, matched unrelated donor.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26390
[VALUE] => Randomized study between thymoglobulin and posttransplant cyclophosphamide in patients with chronic myeloid neoplasms undergoing unrelated allogeneic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Randomized study between thymoglobulin and posttransplant cyclophosphamide in patients with chronic myeloid neoplasms undergoing unrelated allogeneic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26391
[VALUE] => 1982
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1982
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26393
[VALUE] => 1983
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1983
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Elena V. Morozova, Ivan S. Moiseev, Yulia Yu. Vlasova, Nikolay Yu. Tcvetkov, Yulia V. Rudnitskaya, Maria V. Barabanschikova, Anna G. Smirnova, Elena I. Darskaya, Sergey N. Bondarenko, Boris V. Afanasyev
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, PhD, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 927 8229
E-mail: dr_morozova@mail.ru
Despite growing evidence on the use of posttransplantation cyclophosphamide (PTCY) in matched unrelated transplantation in acute leukemia patients, prospective evidence to support this type of prophylaxis in myelodysplastic syndrome (MDS), chronic myeloproliferative neoplasms and chronic myeloid leukemia (CML) patients is lacking. In this patient population we conducted a prospective randomized study of PTCY vs thymoglobulin for graft-versus-host disease (GvHD) prophylaxis in the setting of 10/10 HLA-matched unrelated transplantation (NCT02627573, clinicaltrials.gov). The strata for randomization was pretransplant assessment of mortality score. The study was terminated prematurely due to poor recruitment, but 33 patients were enrolled, 17 in the PTCY and 16 in the thymoglobulin group. Median follow up was 29 months. There was no difference between groups in the incidence primary graft failure (12.50% vs 11.8%, p=0.9), acute GvHD grade II-IV (23% vs 6%, p=0.2), moderate and severe chronic GvHD (25% vs 23%, p=0.4) in the thymoglobulin and PTCY arms, respectively. However there was a significantly higher overall survival (82% vs 30%, p=0.0126), event-free survival (61% vs 26%, p=0.0335), and GvHD-relapse free survival (61% vs 16%, p=0.0072) in the PTCY group due to reduced late infection-related mortality. No differences was observed in terms of toxicity. In conclusion, despite incomplete recruitment, the study created the basis for the use of PTCY in unrelated transplantations from fully matched unrelated donors in CML and MDS. Future studies are required to confirm if there is a benefit of PTCY-based prophylaxis over thymoglobulin.
Keywords
Posttransplant cyclophosphamide, myelodysplastic syndrome, chronic myeloid leukemia, chronic myeloproliferative neoplasm, matched unrelated donor.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26372
[VALUE] => 03/09/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/09/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26373
[VALUE] => 03/27/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/27/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26374
[VALUE] => Array
(
[TEXT] => <p>Инна В. Маркова, Сергей Н. Бондаренко, Олеся В. Паина, Белла И. Аюбова, Полина В. Кожокарь, Анастасия С. Фролова, Ильдар М. Бархатов, Елена В. Бабенко, Александр А. Алянский, Кирилл А. Екушов, Татьяна Л. Гиндина, Елена В. Семенова, Иван С. Моисеев, Людмила С. Зубаровская, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Инна В. Маркова, Сергей Н. Бондаренко, Олеся В. Паина, Белла И. Аюбова, Полина В. Кожокарь, Анастасия С. Фролова, Ильдар М. Бархатов, Елена В. Бабенко, Александр А. Алянский, Кирилл А. Екушов, Татьяна Л. Гиндина, Елена В. Семенова, Иван С. Моисеев, Людмила С. Зубаровская, Борис В. Афанасьев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26375
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26376
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Аллогенная трансплантация гемопоэтических стволовых клеток остается наиболее эффективным методом излечения пациентов с рефрактерным течением/рецидивами В-клеточного острого лимфобластного лейкоза (р/р В-ОЛЛ). Полная ремиссия заболевания без признаков минимальной остаточной болезни является ключевым фактором успешного исхода. Моноклональные антитела (биспецифические и коньюгаты) представляют собой эффективные опции в достижении ремиссии у этих пациентов. Нашей целью было обобщение результатов одноцентрового нерандомизированного исследовапния для изучения результатов терапии блинатумамобом и инотузумаб-озогамицином у детей и взрослых в гетерогенной когорте больных р/р В-ОЛЛ. Результаты одноцентрового, нерандомизированного пилотного исследования в реальной клинической практике продемонстрировали высокий уровень ответа на данный вид терапии в гетерогенной когорте пациентов с р/р В-ОЛЛ. Исследуемая группа включала 182 пациента р/р В-ОЛЛ, возраст от одного года до 72 лет, 128 пациентов получали блинатумомаб, 54 пациента получали инотузумаб озогамицин. Уровень общего ответа был высоким в обеих группах – 96 (75%) и 44 случая (82%), соответственно. Главными предикторами ответа были взрослый возраст (OR=3,819, 95% CI=1,744-8,223, p=0,001) и показания к терапии – гематологический рецидив или персистенция минимальной остаточной болезни (OR=0,018, 95% CI=0,153-0,841, p=0,01). Другие клинические или патологические параметры не оказывали существенного влияния на ответы на терапию. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
В-клеточный острый лимфобластный лейкоз, рефрактерный и рецидивирующий, моноклональные антитела, блинатумомаб, инотузумаб озогамицин, общий ответ.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Аллогенная трансплантация гемопоэтических стволовых клеток остается наиболее эффективным методом излечения пациентов с рефрактерным течением/рецидивами В-клеточного острого лимфобластного лейкоза (р/р В-ОЛЛ). Полная ремиссия заболевания без признаков минимальной остаточной болезни является ключевым фактором успешного исхода. Моноклональные антитела (биспецифические и коньюгаты) представляют собой эффективные опции в достижении ремиссии у этих пациентов. Нашей целью было обобщение результатов одноцентрового нерандомизированного исследовапния для изучения результатов терапии блинатумамобом и инотузумаб-озогамицином у детей и взрослых в гетерогенной когорте больных р/р В-ОЛЛ. Результаты одноцентрового, нерандомизированного пилотного исследования в реальной клинической практике продемонстрировали высокий уровень ответа на данный вид терапии в гетерогенной когорте пациентов с р/р В-ОЛЛ. Исследуемая группа включала 182 пациента р/р В-ОЛЛ, возраст от одного года до 72 лет, 128 пациентов получали блинатумомаб, 54 пациента получали инотузумаб озогамицин. Уровень общего ответа был высоким в обеих группах – 96 (75%) и 44 случая (82%), соответственно. Главными предикторами ответа были взрослый возраст (OR=3,819, 95% CI=1,744-8,223, p=0,001) и показания к терапии – гематологический рецидив или персистенция минимальной остаточной болезни (OR=0,018, 95% CI=0,153-0,841, p=0,01). Другие клинические или патологические параметры не оказывали существенного влияния на ответы на терапию.
Ключевые слова
В-клеточный острый лимфобластный лейкоз, рефрактерный и рецидивирующий, моноклональные антитела, блинатумомаб, инотузумаб озогамицин, общий ответ.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26377
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-47-52
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-47-52
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26380
[VALUE] => Array
(
[TEXT] => <p>Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova, <br>Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova,
Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Boris V. Afanasyev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26381
[VALUE] => Array
(
[TEXT] => <p>Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
<br>St. Petersburg, Russia</p><br>
<p><b>Correspondence</b>
Dr. Inna V. Markova, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7 (911) 964 6745</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Inna V. Markova, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 964 6745
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26383
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Allogeneic hematopoietic stem cell transplantation is an effective method to cure patients with relapse/refractory (r/r) B-cell acute lymphoblastic leukemia, and deep remission without minimal residual disease is the key factor for the favorable outcome. Monoclonal antibodies (bispecific T-cells engager and conjugates) are the promising option to achieve complete remission in these patients. Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B- ALL.</p>
<p style="text-align: justify;">Results of the single-center non-randomized pilot study in real clinical practice showed high response rate in heterogeneous cohort of r/r B- ALL. The study group included 182 patients with r/r B-ALL, their age was from one to 72 years old. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Overall response was high in both groups, 96 (75%) and 44 (82%). The major predictors of response were adult age (OR= 3.819; 95% CI, 1.744-8.223; p=0.001) and clinical indications, i.e., active disease or measurable residual disease (OR= 0.018; 95% CI, 0.153-0.841; p=0.01). The other clinical or disease parameters had no significant impact on response.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
B-cell acute lymphoblastic leukemia, relapse/refractory, monoclonal antibodies, blinatumomab, inotuzumab ozogamicin, overall response.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Allogeneic hematopoietic stem cell transplantation is an effective method to cure patients with relapse/refractory (r/r) B-cell acute lymphoblastic leukemia, and deep remission without minimal residual disease is the key factor for the favorable outcome. Monoclonal antibodies (bispecific T-cells engager and conjugates) are the promising option to achieve complete remission in these patients. Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B- ALL.
Results of the single-center non-randomized pilot study in real clinical practice showed high response rate in heterogeneous cohort of r/r B- ALL. The study group included 182 patients with r/r B-ALL, their age was from one to 72 years old. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Overall response was high in both groups, 96 (75%) and 44 (82%). The major predictors of response were adult age (OR= 3.819; 95% CI, 1.744-8.223; p=0.001) and clinical indications, i.e., active disease or measurable residual disease (OR= 0.018; 95% CI, 0.153-0.841; p=0.01). The other clinical or disease parameters had no significant impact on response.
Keywords
B-cell acute lymphoblastic leukemia, relapse/refractory, monoclonal antibodies, blinatumomab, inotuzumab ozogamicin, overall response.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26378
[VALUE] => Features of response to blinatumomab and inotuzumab ozogamicin therapy in patients with relapse/refractory B-cells acute lymphoblastic leukemia in real clinical practice
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Features of response to blinatumomab and inotuzumab ozogamicin therapy in patients with relapse/refractory B-cells acute lymphoblastic leukemia in real clinical practice
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26379
[VALUE] => 1977
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1977
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26382
[VALUE] => 1978
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1978
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Inna V. Markova, Sergey N. Bondarenko, Olesya V. Paina, Bella I. Aubova, Polina V. Kozhokar, Anastasia S. Frolova,
Ildar M. Barkhatov, Elena V. Babenko, Alexander A. Alyanskiy, Kirill A. Ekushov, Tatyana L. Gindina, Elena V. Semenova, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Boris V. Afanasyev
Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University,
St. Petersburg, Russia
Correspondence
Dr. Inna V. Markova, Raisa Gorbacheva Memorial Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (911) 964 6745
Allogeneic hematopoietic stem cell transplantation is an effective method to cure patients with relapse/refractory (r/r) B-cell acute lymphoblastic leukemia, and deep remission without minimal residual disease is the key factor for the favorable outcome. Monoclonal antibodies (bispecific T-cells engager and conjugates) are the promising option to achieve complete remission in these patients. Our aim was to summarize the results of a single-center non-randomized study in order to investigate in real clinical practice the results of treatment with blinatumomab and inotuzumab ozogamicin in children and adults in heterogeneous cohort of r/r B- ALL.
Results of the single-center non-randomized pilot study in real clinical practice showed high response rate in heterogeneous cohort of r/r B- ALL. The study group included 182 patients with r/r B-ALL, their age was from one to 72 years old. 128 patients were treated with blinatumomab, 54 patients received inotuzumab ozogamicin. Overall response was high in both groups, 96 (75%) and 44 (82%). The major predictors of response were adult age (OR= 3.819; 95% CI, 1.744-8.223; p=0.001) and clinical indications, i.e., active disease or measurable residual disease (OR= 0.018; 95% CI, 0.153-0.841; p=0.01). The other clinical or disease parameters had no significant impact on response.
Keywords
B-cell acute lymphoblastic leukemia, relapse/refractory, monoclonal antibodies, blinatumomab, inotuzumab ozogamicin, overall response.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26408
[VALUE] => 12/05/2019 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/05/2019 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26409
[VALUE] => 02/21/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/21/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26410
[VALUE] => Array
(
[TEXT] => <p>Игорь В. Майбородин<sup>1</sup>, Роман В. Маслов<sup>1</sup>, Татьяна В. Михеева<sup>1</sup>, Сергей В. Марчуков<sup>1</sup>,
Виталина И. Майбородина<sup>2</sup>, Александр А. Шевела<sup>1</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Игорь В. Майбородин1, Роман В. Маслов1, Татьяна В. Михеева1, Сергей В. Марчуков1,
Виталина И. Майбородина2, Александр А. Шевела1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26411
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Центр новых медицинских технологий, Федеральное государственное бюджетное учреждение науки «Институт химической биологии и фундаментальной медицины СО РАН», Новосибирск, Россия<br>
<sup>2</sup> Институт молекулярной патологии и патоморфологии, Федеральный научно-исследовательский центр фундаментальной и трансляционной медицины, Новосибирск, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Центр новых медицинских технологий, Федеральное государственное бюджетное учреждение науки «Институт химической биологии и фундаментальной медицины СО РАН», Новосибирск, Россия
2 Институт молекулярной патологии и патоморфологии, Федеральный научно-исследовательский центр фундаментальной и трансляционной медицины, Новосибирск, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26416
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Согласно работам большинства исследователей, при местном применении мультипотентные стромальные клетки (МСК) остаются в месте имплантации, а введенные внутривенно МСК сначала оказываются в легких и только потом распределяются по всему организму, но уже в значительно меньшем количестве. Вместе с тем имеются сообщения о преимущественной миграции инфузированных системно МСК в печень, а не в легкие. Разрушение и элиминация введенных МСК, по данным литературы, происходит в селезенке, почках и печени.
В связи с противоречивостью литературных данных о распределении и исчезновении МСК из организма была поставлена цель исследования – изучить возможность попадания в печень, почки и легкие мультипотентных мезенхимных стромальных клеток (ММСК) после их тканевой инъекции, а также получить новые доказательства их элиминации из организма указанными органами.</p>
<h3>Материал и методика</h3>
<p style="text-align: justify;">
Работа основана на результатах морфологического исследования легких, печени и почек крыс-самцов инбредной линии Wag в разные сроки после подкожной инъекции в паховую область ММСК, окрашенных мембрано-специфическим красителем Vybrant® CM-Dil. Методами световой и люминесцентной микроскопии регистрировали появление и распределение меченых объектов в указанных
органах. </p>
<h3>Результаты</h3>
<p style="text-align: justify;">
Через 1 неделю после введения ММСК, в легких появились первые люминесцирующие макрофаги, выявляемые при посредстве светофильтра для родамина. В течение 2-4 недель количество таких светящихся клеток постепенно нарастает, также увеличивается интенсивность их флюоресценции. Спустя 2-3 недели был найден красный оттенок во включениях в эпителиальных клетках отдельных бронхиол. Иногда меченые Vybrant® CM-Dil объекты были расположены по самому краю альвеол и даже частично находились в просвете альвеол. Объекты, окрашенные Vybrant® CM-Dil, не были найдены в печени и почках во все сроки наблюдения. </p>
<h3>Заключение</h3>
<p style="text-align: justify;">
После инъекции в подкожно-жировую клетчатку ММСК и их фрагменты попадают в кровоток и оказываются в легких, где фагоцитируются макрофагами. Только после фильтрации в легких, дебрис ММСК может диссеминировать по всему организму. В легких возможна элиминация детрита инъецированных ММСК в альвеолы и далее, через систему бронхов, во внешнюю среду. В печени и почках меченые Vybrant® CM-Dil фрагменты ММСК найдены не были, что не соответствует данным литературы о важной роли этих органов в деструкции или удалении введенных извне МСК. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Мультипотентные стромальные клетки, элиминация, легкие, макрофаги, альвеолы, печень, почки.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Согласно работам большинства исследователей, при местном применении мультипотентные стромальные клетки (МСК) остаются в месте имплантации, а введенные внутривенно МСК сначала оказываются в легких и только потом распределяются по всему организму, но уже в значительно меньшем количестве. Вместе с тем имеются сообщения о преимущественной миграции инфузированных системно МСК в печень, а не в легкие. Разрушение и элиминация введенных МСК, по данным литературы, происходит в селезенке, почках и печени.
В связи с противоречивостью литературных данных о распределении и исчезновении МСК из организма была поставлена цель исследования – изучить возможность попадания в печень, почки и легкие мультипотентных мезенхимных стромальных клеток (ММСК) после их тканевой инъекции, а также получить новые доказательства их элиминации из организма указанными органами.
Материал и методика
Работа основана на результатах морфологического исследования легких, печени и почек крыс-самцов инбредной линии Wag в разные сроки после подкожной инъекции в паховую область ММСК, окрашенных мембрано-специфическим красителем Vybrant® CM-Dil. Методами световой и люминесцентной микроскопии регистрировали появление и распределение меченых объектов в указанных
органах.
Результаты
Через 1 неделю после введения ММСК, в легких появились первые люминесцирующие макрофаги, выявляемые при посредстве светофильтра для родамина. В течение 2-4 недель количество таких светящихся клеток постепенно нарастает, также увеличивается интенсивность их флюоресценции. Спустя 2-3 недели был найден красный оттенок во включениях в эпителиальных клетках отдельных бронхиол. Иногда меченые Vybrant® CM-Dil объекты были расположены по самому краю альвеол и даже частично находились в просвете альвеол. Объекты, окрашенные Vybrant® CM-Dil, не были найдены в печени и почках во все сроки наблюдения.
Заключение
После инъекции в подкожно-жировую клетчатку ММСК и их фрагменты попадают в кровоток и оказываются в легких, где фагоцитируются макрофагами. Только после фильтрации в легких, дебрис ММСК может диссеминировать по всему организму. В легких возможна элиминация детрита инъецированных ММСК в альвеолы и далее, через систему бронхов, во внешнюю среду. В печени и почках меченые Vybrant® CM-Dil фрагменты ММСК найдены не были, что не соответствует данным литературы о важной роли этих органов в деструкции или удалении введенных извне МСК.
Ключевые слова
Мультипотентные стромальные клетки, элиминация, легкие, макрофаги, альвеолы, печень, почки.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26412
[VALUE] => 10.18620/ctt-1866-8836-2020-9-1-67-73
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-1-67-73
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26417
[VALUE] => Array
(
[TEXT] => <p>Igor V. Maiborodin<sup>1</sup>, Roman V. Maslov<sup>1</sup>, Tatiana V. Mikheeva<sup>1</sup>, Sergey V. Marchukov<sup>1</sup>, Vitalina I. Maiborodina<sup>2</sup>, Aleksandr A. Shevela<sup>1</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Igor V. Maiborodin1, Roman V. Maslov1, Tatiana V. Mikheeva1, Sergey V. Marchukov1, Vitalina I. Maiborodina2, Aleksandr A. Shevela1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26418
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia<br>
<sup>2</sup> Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia</p>
<br>
<p><b>Correspondence</b><br>
Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia<br>
Phone: +7 (913) 753 0767 <br>
Е-mail: imai@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia
2 Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia
Correspondence
Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia
Phone: +7 (913) 753 0767
Е-mail: imai@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 26419
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">In compliance with most observations, the multipotent stromal cells (MSC) remain at the site of implantation after local application, whereas intravenously administrated MSC first appear in lungs followed by distribution to different tissues and organs, however, at much smaller quantities. Moreover, there are data on predominant migration of systemically infused MSC into liver, and not into the lungs. Destruction and elimination of introduced MSC, according to published data, occurs in the spleen, kidneys and liver. Due to controversial results on MSC distribution and elimination from the body, the aims of our study were as follows: to investigate the fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body <i>via</i> these organs.</p>
<h3>Materials and methods</h3>
<p style="text-align: justify;">
The work is based on morphological evaluation of lungs, liver and kidneys from 6-mo old male rats (Wag inbred strain) at different terms after subcutaneous injection of MMSC labeled with membrane-binding Vybrant® CM-Dil dye. Morphological patterns and distribution of labeled objects in these organs were investigated using visible and luminescent microscopy.</p>
<h3>Results</h3>
<p style="text-align: justify;">
The first luminescent macrophages observed with rhodamine colour-filter were initially revealed in the lungs one week after MMSC injections. The number of luminescent cells and intensity of their fluorescence increased gradually over successive 2-4 weeks. By 2-3 weeks, this red tint was revealed in the inclusions of epithelial cells in some bronchioles. The Vybrant® CM-Dil-labeled objects were sometimes detected at the very edge of alveoli, and even partially located in alveolar lumen. The objects stained by Vybrant® CM-Dil were not found in liver and kidneys at any observation terms. </p>
<h3>Conclusion>/h3>
<p style="text-align: justify;">
Following subcutaneous injection, MMSCs and their fragments seem to enter bloodstream and migrate to the lungs, where these cells are phagocytized by macrophages. Only after filtration in lungs, the MMSC debris can further disseminate throughout the body. Elimination of detritus from injected MMSC is possible in lung alveoli and further, via bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys, thus disagreeing the literature data on potential role of these organs in the destruction or removal of injected autologous or foreign MSC. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Multipotent stromal cells, elimination, lungs, macrophages, alveoli, liver, kidneys.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => In compliance with most observations, the multipotent stromal cells (MSC) remain at the site of implantation after local application, whereas intravenously administrated MSC first appear in lungs followed by distribution to different tissues and organs, however, at much smaller quantities. Moreover, there are data on predominant migration of systemically infused MSC into liver, and not into the lungs. Destruction and elimination of introduced MSC, according to published data, occurs in the spleen, kidneys and liver. Due to controversial results on MSC distribution and elimination from the body, the aims of our study were as follows: to investigate the fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body via these organs.
Materials and methods
The work is based on morphological evaluation of lungs, liver and kidneys from 6-mo old male rats (Wag inbred strain) at different terms after subcutaneous injection of MMSC labeled with membrane-binding Vybrant® CM-Dil dye. Morphological patterns and distribution of labeled objects in these organs were investigated using visible and luminescent microscopy.
Results
The first luminescent macrophages observed with rhodamine colour-filter were initially revealed in the lungs one week after MMSC injections. The number of luminescent cells and intensity of their fluorescence increased gradually over successive 2-4 weeks. By 2-3 weeks, this red tint was revealed in the inclusions of epithelial cells in some bronchioles. The Vybrant® CM-Dil-labeled objects were sometimes detected at the very edge of alveoli, and even partially located in alveolar lumen. The objects stained by Vybrant® CM-Dil were not found in liver and kidneys at any observation terms.
Conclusion>/h3>
Following subcutaneous injection, MMSCs and their fragments seem to enter bloodstream and migrate to the lungs, where these cells are phagocytized by macrophages. Only after filtration in lungs, the MMSC debris can further disseminate throughout the body. Elimination of detritus from injected MMSC is possible in lung alveoli and further, via bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys, thus disagreeing the literature data on potential role of these organs in the destruction or removal of injected autologous or foreign MSC.
Keywords
Multipotent stromal cells, elimination, lungs, macrophages, alveoli, liver, kidneys.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26413
[VALUE] => Opportunity for elimination of injected multipotent stromal cells via lungs
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Opportunity for elimination of injected multipotent stromal cells via lungs
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26415
[VALUE] => 1992
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1992
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 26420
[VALUE] => 1996
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 1996
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Igor V. Maiborodin1, Roman V. Maslov1, Tatiana V. Mikheeva1, Sergey V. Marchukov1, Vitalina I. Maiborodina2, Aleksandr A. Shevela1
1 Institute of Chemical Biology and Fundamental Medicine, Russian Academy of Sciences, Siberian Branch, Novosibirsk, Russia
2 Institute of Molecular Pathology and Pathomorphology, Federal Research Center of Fundamental and Translational Medicine, Novosibirsk, Russia
Correspondence
Professor Igor V. Maiborodin, Chief Research Associate, Laboratory of Health Management Technologies, The Center of New Medical Technologies, Institute of Chemical Biology and Fundamental Medicine, The Russian Academy of Sciences, Siberian Branch, Akad. Lavrenteva St. 8, Novosibirsk, 630090, Russia
Phone: +7 (913) 753 0767
Е-mail: imai@mail.ru
In compliance with most observations, the multipotent stromal cells (MSC) remain at the site of implantation after local application, whereas intravenously administrated MSC first appear in lungs followed by distribution to different tissues and organs, however, at much smaller quantities. Moreover, there are data on predominant migration of systemically infused MSC into liver, and not into the lungs. Destruction and elimination of introduced MSC, according to published data, occurs in the spleen, kidneys and liver. Due to controversial results on MSC distribution and elimination from the body, the aims of our study were as follows: to investigate the fate of tissue-injected autologous bone marrow-derived multipotent mesenchymal stromal cells (MMSC) entering liver, kidneys and lungs, as well as to obtain new evidence for their elimination from the body via these organs.
Materials and methods
The work is based on morphological evaluation of lungs, liver and kidneys from 6-mo old male rats (Wag inbred strain) at different terms after subcutaneous injection of MMSC labeled with membrane-binding Vybrant® CM-Dil dye. Morphological patterns and distribution of labeled objects in these organs were investigated using visible and luminescent microscopy.
Results
The first luminescent macrophages observed with rhodamine colour-filter were initially revealed in the lungs one week after MMSC injections. The number of luminescent cells and intensity of their fluorescence increased gradually over successive 2-4 weeks. By 2-3 weeks, this red tint was revealed in the inclusions of epithelial cells in some bronchioles. The Vybrant® CM-Dil-labeled objects were sometimes detected at the very edge of alveoli, and even partially located in alveolar lumen. The objects stained by Vybrant® CM-Dil were not found in liver and kidneys at any observation terms.
Conclusion>/h3>
Following subcutaneous injection, MMSCs and their fragments seem to enter bloodstream and migrate to the lungs, where these cells are phagocytized by macrophages. Only after filtration in lungs, the MMSC debris can further disseminate throughout the body. Elimination of detritus from injected MMSC is possible in lung alveoli and further, via bronchial system, to the ambient. Vybrant® CM-Dil-labeled MMSC fragments were not found in liver and kidneys, thus disagreeing the literature data on potential role of these organs in the destruction or removal of injected autologous or foreign MSC.
Keywords
Multipotent stromal cells, elimination, lungs, macrophages, alveoli, liver, kidneys.

