Detection of minimal residual disease in patients with acute myeloid leukemia with multicolor flow cytometry
Konstantin U. Slobodnyuk
Center for laboratory diagnostics, Pavlov State Medical University, St. Petersburg, Russia
Correspondence
Konstantin U. Slobodnyuk, Center for laboratory diagnostics, Pavlov State Medical University, 6/8 Lev Tolstoy Str., St. Petersburg, 199022, Russia
E-mail: k.slobodnyuk@gmail.com
Accepted 10 December 2011
Published 10 January 2012
Summary
Minimal residual disease (MRD) in patients diagnosed with acute myeloid leukemia (AML) can be detected by different methods. Knowing the MRD value is highly important for (i) stratification of patients in to risk groups and (ii) monitoring treatment's efficiency. Flow cytometry procedures have been developed to identify surface and intracellular antigens in cells. It has become an extremely useful tool for detecting the leukemia-associated immunophenotype of leukemia cells in bone marrow and it can be provided to virtually all patients with acute myeloid leukemia. This short report focuses on the assessment of AML MRD with multicolor flow cytometry.
Keywords
acute myeloid leukemia, multicolor flow cytometry, leukemia-associated immunophenotype
Introduction
Acute myeloid leukemia (AML) is a disease of malignant hematopoietic cells, which initially affect bone marrow with further suppression of its normal counterparts and infiltrate solid organs and tissues. The aims of AML patients’ treatment are the elimination of malignant cells for the further achievement of remission (complete, morphological or molecular) and the recovery of patient's bone marrow hematopoiesis. The best expected result of such treatment is the achievement of complete remission, i.e., molecular complete remission as the result of eradication of AML symptoms. The possibility of AML relapse depends upon the quality of the remission [6]. Herein, the measurement of minimal residual disease (MRD) is required for (i) the stratification of a patient’s risk and (ii) monitoring the efficiency of treatment [13].
Modern treatment regimens allow the achievement of complete morphological remission for most patients. Unfortunately, the minimal quantity of initial leukemic cells (less than 5% of nucleated cells of the bone marrow) are able to develop the relapse of AML and the further fatal outcome.
In the primary diagnosis of AML, the quantity of leukemic cells reaches up to 1010–1012 cells. After the first course of induction therapy the number of malignant cells is reduced almost 3 times, however there are millions of leukemic cells still alive. Herein the minimal residual disease is a small population of leukemic cells, which can be detected in the bone marrow provided that there are normal peripheral blood characteristics and not any extramedullar lesion focuses in patients. Thereby the MRD of AML is a persistent number of leukemic cells in bone marrow during complete remission.
Flow cytometry versus Polymerase Chain Reaction
There are two fundamental methods for the submorphological evaluation of MRD with a high sensitivity in clinical diagnostics – Polymerase Chain Reaction (PCR) and Flow Cytometry (FC). Both the PCR and FC were invented in 1980s and these methods are the gold standard of up-to-date routine diagnostics. Flow cytometry is based on the aberrant immunophenotype surface and/or intracellular characteristics of leukemic cells, whereas PCR is based on molecular or genetic aberrations of malignant cells such as (i) gene mutations, (ii) the over-expression of mRNA, (iii) fusion transcripts and so on.
The PCR method is considered to be a highly specific tool with the sensitivity of one leukemic cell in 104–106 of nucleated bone marrow cells, provided that the necessary technical processes are followed [8]. The main disadvantage is a limitation in using PCR in a number of patients who do not have specific genetic aberrations. According to a number of authors, half of all patients do not have molecular – specific aberrations, so this method is difficult to use for monitoring the level of MRD in such cases [11].
Multicolor flow cytometry permits the detection of one leukemic cell in 103–104 of normal nucleated bone marrow cells. The main benefit of this method is that it can be used for virtually all patients with AML (up to 95% of patients) for the monitoring of MRD in the dynamics of treatment [5], except in cases of promyelocytic leukemia where WHO strongly recommend using PCR to detect PML-RARa transcript or t(15;17).
MRD is difficult to evaluate because the development of myeloid cells occurs entirely in the bone marrow; therefore evaluation should be based on the detection of immunophenotypic characteristics, which will differ between normal and malignant bone marrow cells [12, 15]. Unfortunately, leukemia cells do not express any specific antigens that clearly distinguish them from healthy regenerating bone marrow cells. However, some authors report that malignant cells have a fixed aberrant expression of myeloid inhibitory c-type lectin (hMICL) and CD123 and this could be used as the immunophenotypic marker for an assessment of MRD [14].
Leukemia-associated immunophenotype of AML
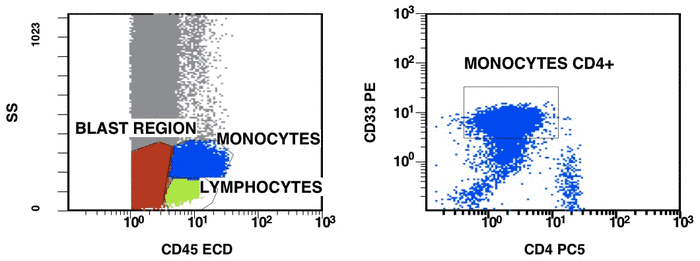
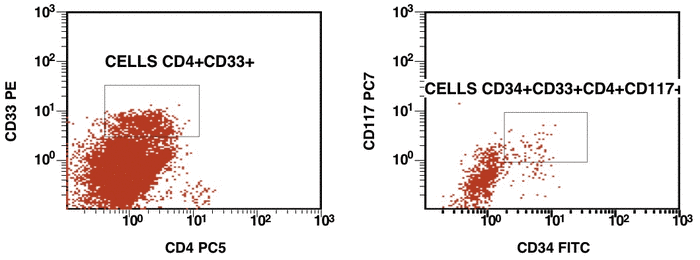
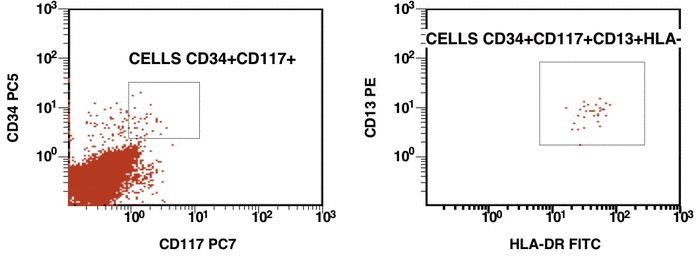
There are some recommendations for the initial diagnosis of AML [4]. In the primary diagnosis of immunophenotype leukemic cells, the application of combination multicolor monoclonal antibodies can be used later in the detection of aberrations in MRD [17, 16]. The leukemia-associated immunophenotype (LAIP) of the malignant cells is detected individually for a patient. The aberration detection of surface immunophenotype can be performed as (i) an asynchronous expression in one or more antigens, (ii) a cross-lineage expression in one or more antigens, (ii) a lack of expression in one or more antigens, and (iv) an over-expression in one or more markers. The precise evaluation of MRD in patients with AML with multicolor FC depends upon many different factors including the strategy of gating (using the CD45/SS or FS/SS gating strategies) [9], a broad panel of monoclonal antibodies, instrument settings (a compensation), and the operator’s qualification (Fig. 1, Fig. 2).
Some complications arise at the diagnosis of AML during the detection of LAIP. AML in comparison with ALL is rather heterogeneous disease, and very often it produces two or more subpopulations. Some authors very actively propose using a special strategy for the measurement of LAIP. On the other hand, there are some questions such as whether we should evaluate the subpopulation of “leukemic stem” cells CD34+CD38- at diagnosis even if it appears at very low frequency (for instance, in the case of promyelocytic leukemia) and should we take into account the changes of granularity according to SS parameter in CD45/SS gating strategy at the diagnosis and during the follow-up of patients.
Another issue is stability of the LAIPs during treatment and at the relapse of AML. This question merits additional discussion. Briefly, in concordance with a number of authors the best way to reveal alterations in LAIPs is to use a broad panel of monoclonal antibody at the primary diagnosis of AML. It increases the probability of detecting LAIPs during follow-up.
Figure 1. Detection of a LAIP at the diagnosis of AML using multicolor flow cytometry and CD45-SS gating strategies – CD45dimCD4+CD13+CD33+CD34+CD38+CD117+ with the co-expression of CD7 and CD64. The type of aberrant expression is asynchronous expression of CD4 and co-expression CD64 with early myeloid antigens
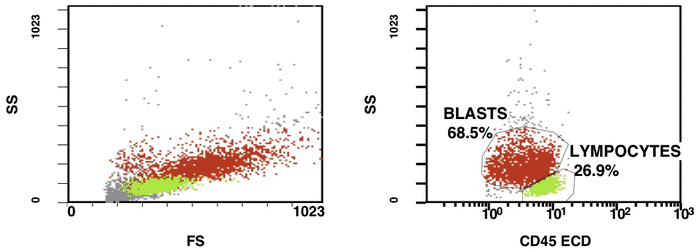
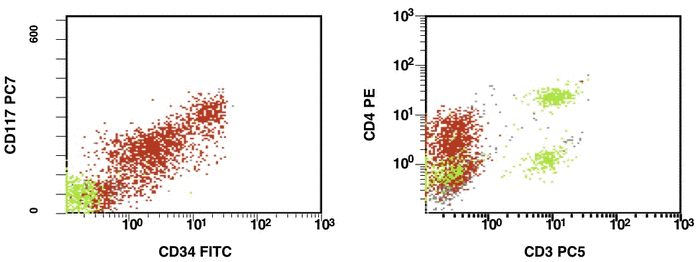
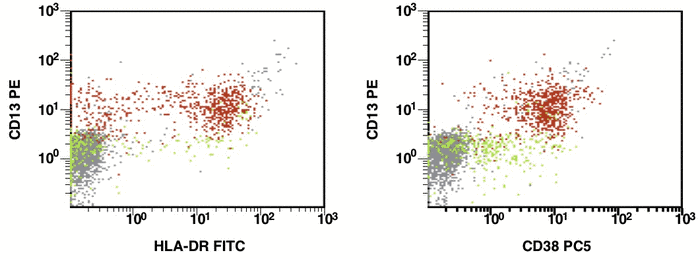
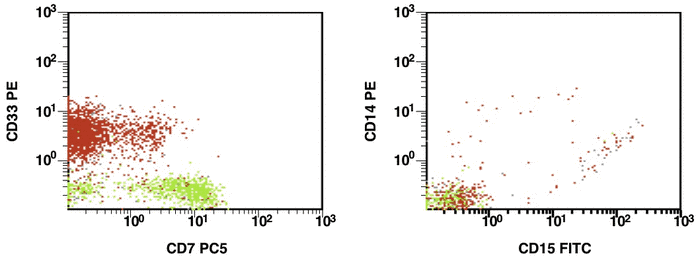
Figure 2. After induction chemotherapy and leukocyte recovery, persistent cells with LAIP are detectable at a very low frequency
Conclusion
There are different options to detect MRD for patients with AML. In patients with detectable molecular markers, both PCR and multicolor FC might be used for receiving more sensitive and specific information about MRD. The leukemic cells are detectable by FC with sensitivity lower than molecular aberrations by PCR, however some patients do not have such mutations. One of the main problems is standardizing procedures in diagnostic AML by both PCR and FC. There is no official protocol with comparisons between PCR and FC during a follow-up patient with AML.
The identification of LAIPs in patients with AML depends upon (i) how broad the panel of reagents is used at diagnosis and during an evaluation of MRD, (ii) how appropriate settings are used in flow cytometer (compensation, laser stability, voltage) and (iii) the operator’s qualifications [1].
The search for new markers to distinguish normal from malignant cells after chemotherapy in bone marrow by multicolor FC is a future direction of development in clinical laboratory diagnostics, and the possibility to identify new target treatment with AML patients [7].
Acknowledgements
The author wish to thank Yekaterina Ye. Zueva (Center for laboratory diagnostics, Pavlov Medical University of St. Petersburg, Russia) for providing the supervision.
References
4. Campana D. Progress of minimal residual disease studies in childhood acute leukemia. Curr Hematol Malig Rep. 2010 Jul;5(3):169-7. doi: 10.1007/s11899-010-0056-8.
5. Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of Clinical Oncology. 2003;21(24):4642-9.
6. Chung NG, Buxhofer-Ausch V, Radich JP. The detection and significance of minimal residual disease in acute and chronic leukemia. Tissue Antigens. 2006 Nov;68(5):371-85. doi: 10.1111/j.1399-0039.2006.00714.x.
9. Kern W, Voskova D, Schnittger S, Schoch C, Hiddemann W, Haferlach T. Four-fold staining including CD45 gating improves the sensitivity of multiparameter flow cytometric assessment of minimal residual disease in patients with acute myeloid leukemia. Hematol J. 2004;5(5):410-8.
11. Kern W, Bacher U, Haferlach C, Schnittger S, Haferlach T. The role of multiparameter flow cytometry for disease monitoring in AML. Best Pract Res Clin Haematol. 2010 Sep;23(3):379-90. doi: 10.1016/j.beha.2010.06.007.
12. Kern W, Schoch C, Haferlach T, Schnittger S. Monitoring of minimal residual disease in acute myeloid leukemia. Crit Rev Oncol Hematol. 2005 Nov;56(2):283-309. doi: 10.1016/j.critrevonc.2004.06.004.
14. Sperr WR, Valent P. Novel developments in AML – ASH 2010. ASH report. Memo 2011;4:106-9.
15. Voskova D, Schnittger S, Schoch C, Haferlach T, Kern W. Use of five-color staining improves the sensitivity of multiparameter flow cytomeric assessment of minimal residual disease in patients with acute myeloid leukemia. Leuk Lymphoma 2007;48(1):80-8. doi: 10.1080/10428190600886164.
16. Inoue D, Maruoka H, Takahashi T. Clinical analysis and optimization of postremission therapy for acute myeloid leukemia patients with minimal residual disease as determined by flow cytometry. Mediterr J Hematol Infect Dis. 2010; 2(2):e2010020. doi: 10.4084/MJHID.2010.020.
17. Peters JM, Ansari MQ. Multiparameter flow cytometry in the diagnosis and management of acute leukemia. Arch Pathol Lab Med. 2011;135(1):44-54. Review.
Accepted 10 December 2011
Published 10 January 2012


