In vitro models for stem cell transplantation
Sergey A. Sergeev, Yulia V. Khramova
Lomonosov MSU, Moscow, Russia
Correspondence
Lomonosov MSU, Moscow, Russia, Leninskie gory 1/12, 119234, Moscow, Russia; Phone: +79035187482
E-mail: embryossa@gmail.com
Accepted 10 December 2011
Published 14 December 2011
Summary
Transplanted NSPC and MMSC quickly migrated in laser damaged retina explants (significantly differs (p<0.05) from the undamaged explants) during the first 24 hours, formed neuritis and connections with the retina cells, and were detected up to 50 days later in damaged recipient tissue. Individual transplanted cells were associated with each other and migrated chaotically with glial differentiation. Seven days after transplantation EGFP+ cells were observed in different distances (600 mkm, 1000 mkm, 3000 mkm) populating damaged zones in 90%, 56%, and 27% respectively. NSPC migration and differentiation in cell layers of neuroretina were higher (p<0.05) than for NSPC transplanted to the explant's surface, and no significant data was obtained for MMSC.
Keywords
Stem cells, in vitro transplantation, retina explant culture
Introduction
Stem cell (SC) transplantation is very topical in the fields of developmental biology and regenerative surgery. A variety of types of SC obtained from different sources are successfully used for reparation of tissues defects. The functional rehabilitation of damaged retinas in different cases of pathology with SC transplantation is a current theme in ophthalmology [1] and can be used as a model for studying cell engrafting in brain tissue [2]. Currently we have plenty of experience in SC transplantation procedures and a huge number of published works; however it is difficult to predict the effects of such transplantation in vivo due to scarce information about SC behavior in recipient tissue. In vivo studies can’t show us all changes in SC and recipient interactions after transplantation, and it is impossible to obtain enough information about transplanted cells behavior experimentally. However, many questions about the communication of transplanted cells with recipient tissue and the processes of functional contact development and mechanisms of the new microenvironment on transplanted cells still remain open. To answer these questions new adequate model systems with the ability to investigate the destiny of transplanted cells are required. That’s why it is necessary to design ex vivo models that register individual cell behavior after transplantation and clear visualization of these changes [3]. In vitro injection of xenogeny cells into 3D rat retina culture seems to be an attractive method in this case. Additionally, organotyping explant culture damaged by laser emission is used in this work.
Materials and methods
Seven-day-old rats’ retina explant culture (DMEM/F12 with 20 ng/ml FGF and EGF, 7% FCS, В12 and N2 supplements) damaged with laser Zilos-tk (300mW, 1000 mc) was used. Neuronal stem/progenitor cells (NSPC), bone marrow stromal cells (MMSC) [4], and pigment epithelium cells (PE) of С57BL/6-Tg(ACTB-EGFP)/Osb/J GFP+ mice had been transplanted in them (300-300000 cells in 0.2mkl) in vitro. AFM data were obtained from an Atomic force microscope Solver BIO Olympus with the scanning field 100х100х7mkm3. Data analysis was done with the programs Nova (НТ-МDТ) and STATISTICA 8.0. The MMSC reaction to external irritation was estimated by fluorescent dye RH 795 after electro stimulation on an ASL-1 device.
Results
Injected SC stayed alive in culture (negative stain for propidium iodide) for more than 2 months and rapidly migrated (for longer than 3000mkm in distance) from the injection point during only the first 48 hours to the laser-damaged area. Thus injected SC had good live potency, migrated for long distances and morphologically differentiated according to a new microenvironment in all our culture systems, and these culture methods were adequate and easy-to-use 3D model systems for stem cells research.
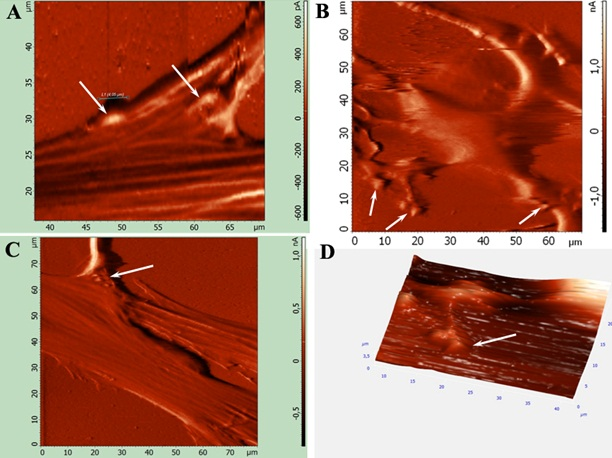
After MMSC transplantation two populations of small (≈15mkm) rapidly migrating cells and almost motionless big (≈30mkm) cells were notable among injected MMSC. On the third day after injection into intact retinas, MMSC changed their morphology by spreading long branched outgrowths but were still negative for neural and glial cell markers (β-III-tubulin and GFAP). Analogous morphology changes in transplanted MMSC in damaged retinas were observed after 24 hours (Fig. 1). MMSC injection had a noticeable neuroprotective effect on the retina. ASM assay showed significant difference (p<0.01) between glial thickness and endothelial retina cells processes and neurites and neuro-like transplanted MMSC processes. Also it was shown that MMSC form synapses up to 2.5 ±0.06mkm in diameter on the 4th day after transplantation (Fig. 2). After electrostimulation (20V, 0.5Hz, 200ms), clear depolarization of retina neurons and their processes was detected. It was shown that some GFP+ MMSC which had changed their morphology to neuro-like after transplantation into the retina explants were able to depolarize after exogenic stimulation. This means that the MMSC population is highly heterogenic. Those cells that quickly migrated and formed neurit-like processes had an ability to generate a response to stimulation. Big (>30mkm) passive migrating cells that had taken a fibroblast-like morphology did not answer to stimulation. But it was shown that many transplanted MMSC had changed their morphology but did not respond to stimulation. Because of the absence of transplanted GFP+ MMSC and retina neuron fusion detected by fluorescent DiI staining, it is reasonable to suppose that there is a small MMSC subpopulation that has the ability for neuronal transdifferentiation.
Figure 1. MMSC morphology changes at 7 days after transplantation in retina explants. Cells positive for GFP with bipolar neuromorphology were presented (E-F). Many neurite-like processes were sprouted from MMSC during their migration and differentiation (A-D)
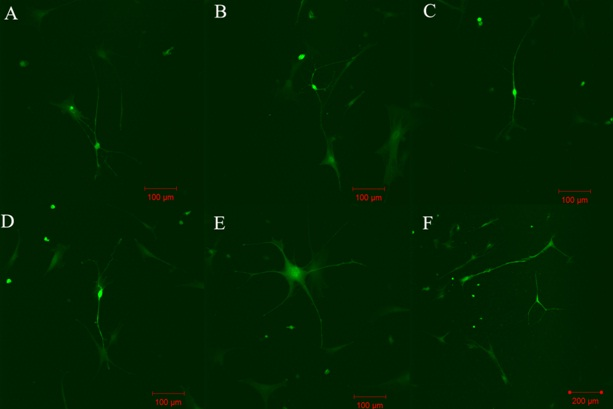
Figure 2. AFM analysis injected MMSC surface in full contact and semi contact mode. A, C: Processes connections between transplanted MMSC and retina cells (arrows). B: MMSC surface reconstruction after morphology changes. D: 3D neurite-like processes surface reconstruction with synaptic connections
NSPC quickly migrated (significantly differs (p<0.05) from the NSPC in undamaged explants) during the first 24 hours for a distance more than 3 mm from the damaged zone in the case when aggregates (1–5 thousands cells) were transplanted, formed neuritis and connections with the retina cells, and were detected up to 50 days in damaged recipient tissue. Individual transplanted cells were associated with each other and migrated chaotically with glial differentiation. Seven days after transplantation GFP+ cells were observed at different distances (600mkm, 1000mkm, 3000mkm) populating damaged zones at a rate of 90%, 56%, and 27% respectively. NSPC migration and differentiation in cell layers of neuroretina were higher (p<0.05) than for NSPC transplanted to the explant surface. In comparison, PE cells were available for migration and proliferation activity only after photoreceptor surface transplantation. The same data were obtained after NSPC and PE cell in vivo injection in Campbell rat eyes (intravitreal, retrobulbar and suprachoroidal transplantation of 50–100 thousands cells in 2µl).
Conclusions
Therefore the obtained data indicate: an effective in vitro model of explantation culture for detecting cell migration, differentiation and communication processes in recipient tissue directly after transplantation was developed and successfully applied for NSPC and MMSC in vitro transplantation. Using this technique it is possible to observe the reparation time for damaged tissue after SC transplantation; minimal cell-migration time to the damaged area; optimal transplantation time after damage; minimal number of transplanted SC required to start the reparation process; necessity of tissue micro surrounding for SC migration and choosing the best strategy for cell injection. Using this method it is possible to optimize clinical transplantation protocols and make them more effective.
Acknowledgements
This work is a realization of the Federal program for the years 2009–2013 entitled “Scientific and science-pedagogic staff of innovation Russia”.
References
1. Bull ND, Martin KR. Using stem cells to mend the retina in ocular disease. Regen Med. 2009 Nov;4(6):855-64. doi: 10.2217/rme.09.59.
2. Johansson K, Ehinger B. Structural changes in the developing retina maintained in vitro. Vision Res. 2005 Nov;45(25-26):3235-43. doi: 10.1016/j.visres.2005.05.022.
3. Kretz A, Hermening SH, Isenmann S. A novel primary culture technique for adult retina allows for evaluation of CNS axon regeneration in rodents. J Neurosci Methods. 2004 Jul 30;136(2):207-19. doi: 10.1016/j.jneumeth.2004.01.012.
4. Schrepfer S, Deuse T, Lange C, Katzenberg R, Reichenspurner H, Robbins RC, Pelletier MP. Simplified protocol to isolate, purify, and culture expand mesenchymal stem cells. Stem Cells Dev. 2007 Feb;16(1):105-7. doi: 10.1089/scd.2006.0041.
Accepted 10 December 2011
Published 14 December 2011


