Results of allogeneic hemopoietic stem cell transplantation in patients with acute leukemia for different age groups
Elena V. Semenova1, Olesya V. Paina1, Polina V. Kozhokar1, Olga A. Slesarchuk1, Anastasia S. Borovkova1, Anastasia S. Frolova1, Zhemal Z. Rakhmanova1, Anna A. Osipova1, Liubov A. Tsvetkova1, Svetlana V. Razumova1, Tatyana A. Bykova1, Sergey N. Bondarenko1, Maria V. Latypova1, Tatyana L. Gindina1, Elena V. Babenko1, Alexander L. Alyanskiy1, Ildar M. Barkhatov1, Boris I. Smirnov2, Ludmila S. Zubarovskaya1
1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 St. Petersburg State Electrotechnical University "LETI", St. Petersburg, Russia
Correspondence:
Prof. Elena V. Semenova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 420-46-22
E-mail: alena-semenova@yandex.ru
Citation: Semenova EV, Paina OV, Kozokhar PV, et al. Results of allogeneic hemopoietic stem cell transplantation in patients with acute leukemia for different age groups. Cell Ther Transplant 2022; 11(3-4): 36-44.
Accepted 25 November 2022
Summary
To analyze the effect of age on the overall survival (OS) of patients with acute leukemia (AL) after allogeneic hematopoietic cell transplantation (allo-HSCT).
Materials
The data of 712 patients with AL (from 0.5 to 29 years old) who underwent allo-HSCT at the R. M. Gorbacheva Research Institute from 2000 to 2019 y.
Results
The OS of children with ALL under one year and from 1 to 10 years was 79% and 65%, these indicators were higher in comparison with the OS of adolescents (11-20 years) – 46% and young adults (21-29 years) – 47% (p=0.039). OS of infants with AML – 78%, children – 59%, adolescents – 59%, young adults – 70% (p=0.564).
Conclusion
Age has a significant effect on OS after allo-HSCT in patients with ALL and to a lesser extent in patients with AML.
Keywords
Acute leukemia, allogeneic hematopoietic stem cell transplantation, age groups.
Introduction
Allogeneic hemopoietic stem cell transplantation (allo-HSCT) is still the only potentially curative treatment option for most children with high-risk acute leukemia [1, 2, 3] as standard chemotherapy is drastically ineffective in these cases. However, in spite of allo-HSCT being actively used up to this moment, its indications and place in treatment strategy are constantly subject to changes as new targeted drugs and immunotherapy methods enter into clinical practice. The undisputable indications are presence of unfavorable risk factors, relapse development (especially an early one), therapy resistance, and, what’s important, an ability to obtain second or subsequent remission. Patient’s age at diagnosis is still an important factor when actual treatment decision is made. We, therefore, find it important to evaluate its contribution into long-term survival of children and young adults with acute leukemia undergoing allo-HSCT.
Materials and methods
A total of 712 patients with acute leukemia (AL) of different age (median age of 14 years, range 6 months to 29 years) undergoing allo-HSCT in R. M. Gorbacheva research institute clinic in 2000-2019 were included in our retrospective study cohort. The median follow-up is 5-5.4 years. Based on the age at diagnosis all patients were divided into the following four age groups:
• Group 1 (infants): children below the age of 1 year (n=57);
• Group 2 (children): 1 to 10 years (n=248);
• Group 3 (adolescents): 11 to 20 years (n=227);
• Group 4 (young adults): 21 to 29 years (n=180).
Most patients (n=390, 55%) received an allo-HSCT from matched unrelated donor, in 139 (20%) cases a matched related, and in 183 (25%) – a mismatched related (haploidentical) donor was used. The hemopoietic stem cells (HSC) sources were bone marrow (BM) in 399 (56%), and peripheral blood stem cells (PBSC) in 313 (44%) cases. A total of 375 patients were initially diagnosed acute lymphoblastic leukemia (ALL), and 337 – acute myeloblastic leukemia (AML). The age distribution and disease status at allo-HSCT are summarized in Table 1.
Table 1. Diagnoses, cytogenetic aberrations and disease status distribution at the moment of allo-HSCT
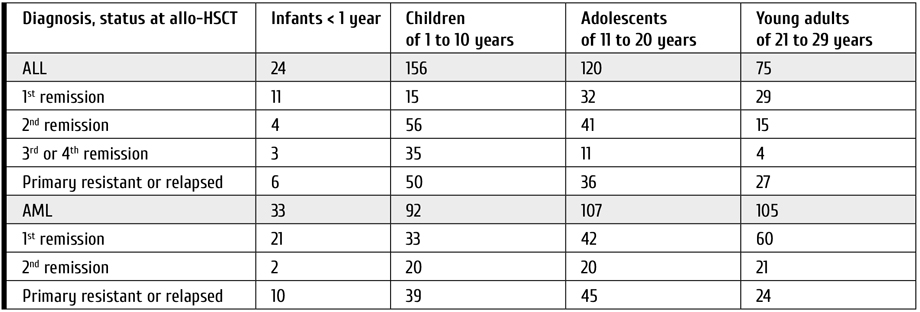
The indications for allo-HSCT in infants with ALL in 1st remission were induction failure (n=5), unfavorable biological factors (KMT2A (11q23)-r), early disease manifestation (in children younger than 6 months) or high initial white blood cells counts (n=6). For children of 1 to 20 years the following: induction failure, minimal residual disease-positive (MRD+) status (n=28), and presence of the following unfavorable cytogenetic or molecular aberrations: KMT2A (11q23)-r (n=15), and Ph+ ALL (valid till 2010; n=4). In young adults the criteria were induction failure or MRD+ status (n=13), KMT2A (11q23)-r (n=7), Ph+ ALL (n=4), or hypodiploidy (n=3).
In patients with AML the indications were induction failure or MRD+ status (n=26), as well as presence of monosomy 7 (n=12), KMT2A (11q23)-r (n=23), t(6;9) (p23;q34,1) (n=4), FLT3 ITD (n=12), inv3/t(3;3) (q21;q26) MECOM(EVI1) (n=7), and М5 (n=30), М6 (n=7), or М7 (n=11) variants according to FAB classification. AML, myelodysplasia-related (AML-MR) (n=18) and secondary AML (n=6) were also viewed as unfavorable factors and indications for allo-HSCT.
Remission was defined as lack of blasts in peripheral blood and <5% blasts in bone marrow as well as absence of extramedullary disease and with recovery of peripheral counts.
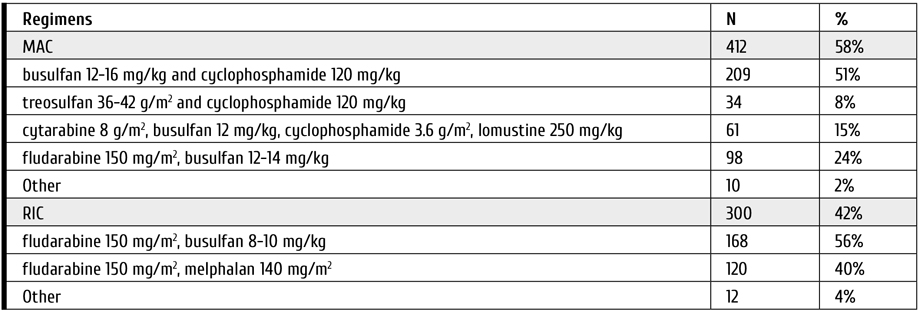
Conditioning regimens used were based on different combinations of cytostatics (see Tab. 2). Myeloablative conditioning (MAC) regimens were used in 412 (58%), and reduced intensity conditioning (RIC) in 300 (42%) cases.
Table 2. Conditioning regimens in allo-HSCT
In 2003-2013 the following indications for RIC regimens were employed:
• "Pretreatment" status seriously affecting patient’s performance score (Karnofsky/Lansky score value of <80%),
• History of severe complications after preceding chemotherapy courses (serious adverse events, grade 3-4 toxicity, infectious complications),
• Verified ongoing infection at the time of allo-HSCT,
• History of autologous HSCT.
Since 2014 the indications for RIC regimens were reviewed and limited to:
• History of severe complications after preceding chemotherapy courses (serious adverse events, grade 3-4 toxicity, infectious complications),
• Verified ongoing infection at the time of allo-HSCT.
Patient cohorts receiving MAC and RIC regimens were comparable by the following characteristics: age (р=0.087), diagnosis (р=0.567), status at allo-HSCT (р=0.721), HSC donor type (р=0.878). The only factor different was performance status evaluated via Karnofsky/Lansky score value. There were 198 (66%) patients with <80% values in RIC cohort, while in MAC recipients these values were only registered in 39 (9%) cases (р=0.021).
Since 2013 post-transplant cyclophosphamide given at 50 mg/kg on days +3 and +4 was used as a backbone for acute graft-versus-host disease (aGVHD) prophylaxis. As a whole, these regimens were used in 447 (63%) patients. Also in 282 case the GVHD prophylaxis was based on pre-transplant antithymocyte/antilymphocyte globulins, and in 3 cases transplant modification by TCR α/β immunomagnetic depletion was used. The basic immunosupression by calceneurin inhibitors was used in most cases with cyclosporine-A based regimen in 242 and tacrolimus-based one in 398 patients.
Statistical analysis was performed with SPSS statistics 20.0 software. Patients alive at the time of analysis were censored at October 1st 2020. Survival data was evaluated by Kaplan-Meier method. Survival comparison was performed using log-rаnk test. The difference was deemed statistically significant in cases when р value was <0.05. (CES B. I. Smirnov).
Results
Cytogenetic markers: age dependence
The cytogenetic aberrations found in ALL and AML patients differed for various age groups (Fig. 1).
Based in prognostic significance of genetic aberrations all patients were divided into 3 groups (see Tab. 3 and Tab. 4).
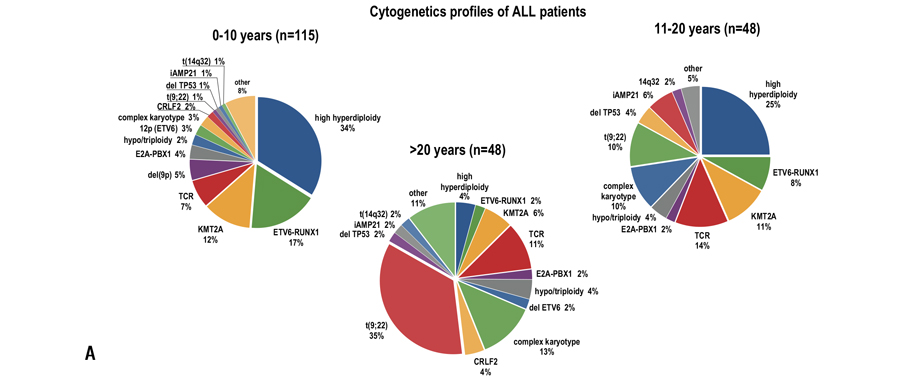
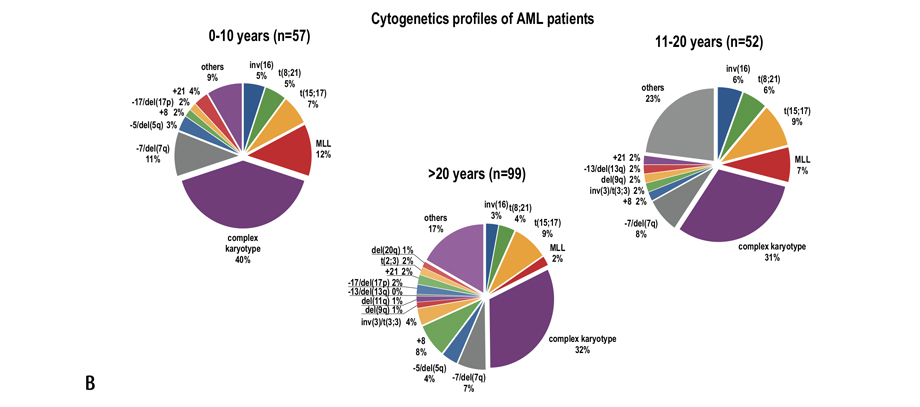
Figure 1. Cytogenetic aberrations rate found in patients with ALL (A) and AML (B) of different age groups. The data was provided by R. M. Gorbacheva research institute cytegenetics lab (headed by T. L. Gindina)
Table 3. Frequency of different cytogenetic and molecular aberrations in different age groups based on their prognostic significance for children with ALL
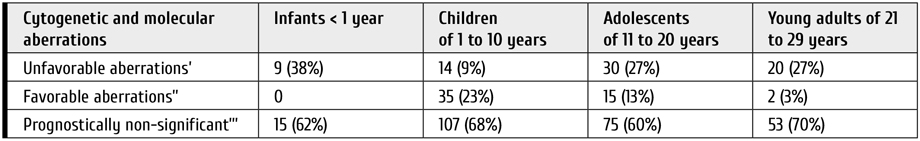
Notes: ’– t(9;22) (q34; q11), KMT2A-r, hypodiploidy, iAMP21, t(17;19)(q22;p13) TCF3-HLF, CRLF2-r; ’’ – t(12;21) (p13; q21) ETV6RUNX1, hyperdiploidy; ’’’ – all other aberrations and normal karyotype.
Table 4. Frequency of different cytogenetic and molecular aberrations in different age groups based on their prognostic significance for children with AML
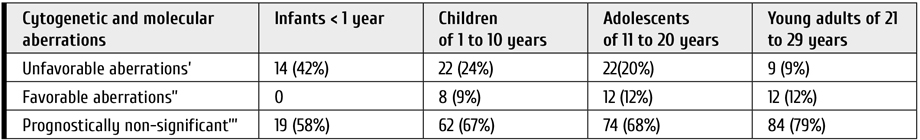
Notes: ’ – KMT2A-r (except t(9;11) ad t(1;11)), FLT3ITD, t (6;9)(p23;q34), inv3/t(3;3) (q21;q26) MECOM(EVI1), chromosome 7 involvement; ’’ – t(8;21) (q22; q22) RUNX1-RUNX1T1, inv 16(p13;q22) and t(16;16) (p13;q22) CBFB-MYH11; ’’’ – all other aberrations and normal karyotype.
Acute lymphoblastic leukemia
As the disease status at allo-HSCT (presence or absence of remission) is indisputably a major influence at allo-HSCT outcome, all patients were divided into 2 groups: ones receiving allo-HSCT in 1st or 2nd remission (n=203), and patients, in whom allo-HSCT was performed in 3rd to 4th remission or in presence of active disease (n=172).
While analyzing the influence of conditioning regimen (RIC vs. MAC) on outcome of allo-HSCT performed in infants, children or young adults with ALL achieving 1st or 2nd remission prior to allo-HSCT, there was no significant difference in overall survival (OS). The values for RIC and MAC recipients were 100% vs. 75% (р=0.511) in infants, 55% vs. 69% (р=0.263) in children, 47% vs. 46% (р=0.865) in adolescents, and 52% vs. 44% (р=0.547) in young adults, accordingly.
The OS of patients in 1st or 2nd remission was significantly higher in children younger than 1 year (79%) and 1 to 10 years (65%) compared to values of 46% achieved in adolescents (11-20 years) and 47% achieved in adults (21-29 years) (р=0.043 and р=0.050, accordingly).
In patients receiving allo-HSCT outside of 1st or 2nd remission the age also influenced the outcome with OS being 22% in infants (<1 year) and 30% in children (1-10 years), which was significantly better compared to results achieved in adolescents (11-20 years) and young adults (21-29 years), in whom the OS did not exceed 10% and 6%, accordingly (р=0.001) (Fig. 2).
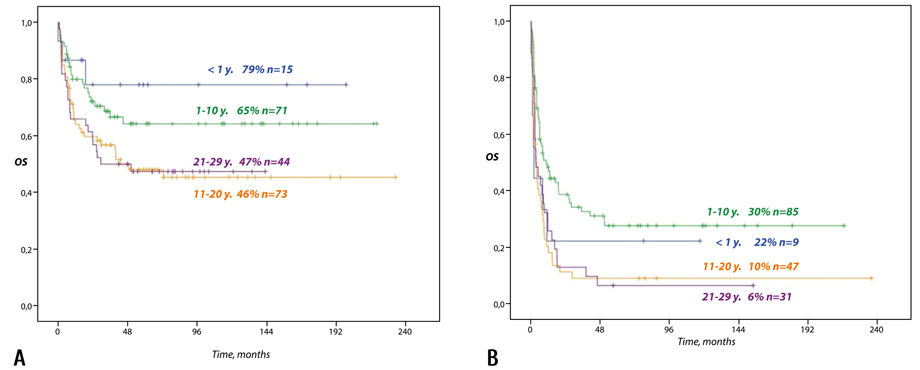
Figure 2. The overall survival of patients (0.5-29 years) with high-risk ALL based on their age and disease status at allo-HSCT (20 years of follow-up). А: patients in 1st or 2nd remission. B: patients outside of 1st or 2nd remission.
The cytogenetic and molecular factors assessment in different age groups has shown that older age is associated in ALL patients with prevalence of unfavorable and lack of favorable aberrations (р=0.034) (Table 3).
The overall survival of patients with "favorable" genetic aberrations was significantly higher and reached 82% in patients achieving remission at the time of allo-HSCT. On the contrary, the corresponding OS values for patients with "unfavorable" and "neutral" aberrations were only 44% and 45%, accordingly (р=0.002).
The positive influence of "favorable" aberrations presence on OS is evident for each age group with OS being 85% in children aged 1 to 10 years and 73% in adolescents. The rate of these genetic changes is also higher in children aged 1 to 10 years compared to other age cohorts. Unfavorable aberrations show universally bad influence for all age groups with OS being 54% in children 1 to 10 years, 42% in adolescents, and 23% in young adults, accordingly. While neutral aberrations influence on outcomes seemed to be age-dependent (OS of 60% in infants, 49% in children, 35% in adolescents, and 48% in young adults, accordingly), this difference was not statistically significant (р>0.05).
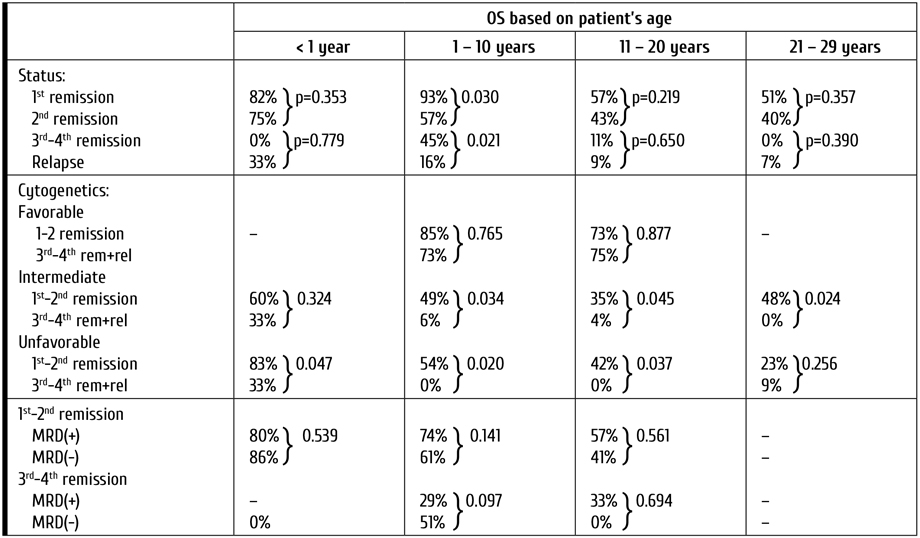
The influence of the disease status on outcome was performed separately for each age group. The overall survival in children younger than 1 year achieving 1st hematological remission prior to allo-HSCT was 82%. For children in 2nd remission, 3rd to 4th remission and relapse it was 75%, 0%, and 33%, accordingly. For children aged 1 to 10 years, adolescents and young adults these values amounted to 93%, 57%, 45% and 16%; 56%, 43%, 11%, and 9%; and 51%, 40%, 0%, and 7%, accordingly.
In patients with ALL aged 1 to 10 years at diagnosis there was a statistically significant difference in OS between groups with different disease status (р<0.001). Also, if allo-HSCT was performed in 1st remission the overall survival was significantly better compared to all other groups including the one consisting of children transplanted in 2nd remission (р=0.030).
In infants (<1 year), adolescents (11-20 years) and young adults (21-29 years) groups the OS also consistently decreased in more advanced disease stages, although there was no significant difference in OS between patients receiving allo-HSCT in 1st and 2nd remission (р=0.353, р=0.219, р=0.357) or between ones transplanted in 3rd to 4th remission or relapse (р=0.779, р=0.650, р=0.390). Therefore, for these age groups allo-HSCT was equally effective when performed during 1st as well 2nd remission being also much better compared to results seen in patients with 3rd to 4th remission or relapse (р<0.001).
This correlation between long-term OS and disease status at HSCT is highly expected for patients with ALL. This factor could, however, be omitted due to significant body of data confirming the negative influence of ALL MRD-positive status on allo-HSCT results [13]. The OS of children in 1st to 2nd remission with present of absent MRD at allo-HSCT was 80% vs. 86% for infants (р=0.539), 74% vs. 61% for children aged 1 to 10 years (р=0.141), and 57% vs. 41% for adolescents (р=0.561), accordingly.
The influence of studied parameters on overall survival is summarized in Table 5.
Table 5. Overall survival based on patient’s age, disease status, cytogenetic prognostic group and MRD presence for children with ALL
The post allo-HSCT ALL relapse rate in infants, children aged 1 to 10 years and adolescents was very similar in case the 1st or 2nd CR achievement (27%, 35% and 34%, accordingly). In patients transplanted outside of 1st or 2nd remission it was as high as78%, 58%, and 57%, accordingly. The relapse rate as also significantly higher for young adults with any disease status being 55% for patients with and 97% for patients without remission at allo-HSCT (р=0.001). All patients developing a post-transplant relapse received some kind of therapy of varying intensity. Its effectiveness differed based on age group. With a median follow-up of 46 (1-125) months a total of 36% of infants, 24% of children aged 1 to 10 years, 12% of adolescents, and 4% of young adults are alive (р=0.005).
Acute myeloid leukemia
All patients with AML were divided into two groups based on their disease status at allo-HSCT. Group 1 (n=219) consisted of ones receiving allo-HSCT in 1st or 2nd remission, while Group 2 (n=118) included patients with primary resistant disease or resistant relapse.
We evaluated the conditioning regimen intensity (MAC vs. RIC) influence on OS in each AML patient age group, but the analysis performed yielded no significant difference between MAC and RIC recipients. The overall survival of patients with high-risk AML in remission (MAC vs. RIC) was 80% vs. 70% for infants (р=0.737), 60% vs. 52% for children aged 1 to 10 years (p=0.731), 50% vs. 67% for adolescents (р=0.413), and 73% vs. 67% for young adults (р=0.523), accordingly.
The overall survival after allo-HSCT performed in 1st or 2nd remission was 78% for infants, 59% for children aged 1 to 10 years, 59% for adolescents, and 70% for young adults, while in patients transplanted outside of remission these values were as low as 30%, 7%, 11%, and 17%, accordingly.
In spite of OS advantage observed in younger patients with AML, this different was not statistically significant for either Group 1 (р=0.564) or Group 2 (р=0.604) (Fig. 3).
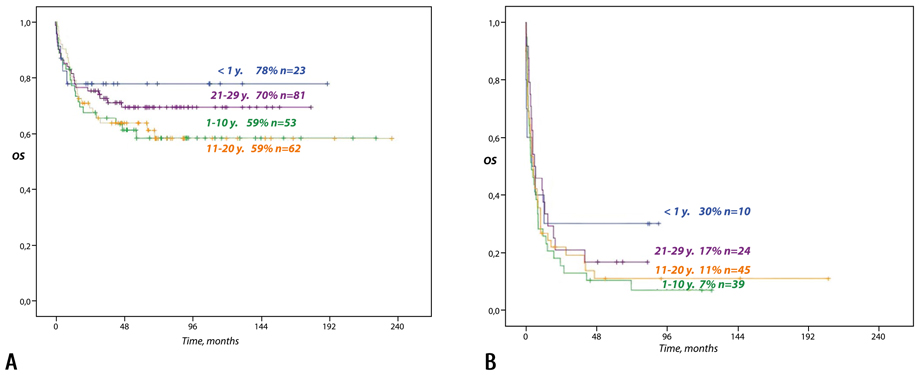
Figure 3. The overall survival of patients (6 months to 29 years) with high-risk AML based on their age at diagnosis and disease status at allo-HSCT (20 years of follow-up). А: patients in 1st or 2nd remission. B: patients outside of 1st or 2nd remission.
We analyzed the influence of genetic changes on overall survival of patients with AML belonging to different age groups. Unlike ALL cohort, the age distribution of different prognostically significant aberrations in AML patients was relatively even (р=0.546), while young adult group being also characterized by lower "unfavorable" aberrations rate compared to other age cohorts (р=0.002) (Table 4).
The overall survival of patients with "favorable" aberrations was slightly better (86%) compared to ones harboring "unfavorable" (71%) or "neutral" genetic defects (59%), with difference tending to be statistically significant in responders (р=0.060) as well as in ones with resistant disease at the moment of allo-HSCT (р=0.062).
The further statistical analysis has shown disease status to have more important influence on prognosis than genetic changes for all AML patients’ age groups.
The OS of patients in 1st remission, 2nd remission or resistant disease was 80%, 50% and 30% for infants vs. 59%, 52%, and 7% for children aged 1 to 10 years. The OS was not significantly different for children receiving allo-HSCT in 1st or 2nd remission for both these age groups (р=0.231 and р=0.577, accordingly), although the OS of patients transplanted outside of remission was significantly worse (р=0.003 and р<0.001, accordingly). This correlation was also seen in young adults, in whom the OS was 69% if transplanted in 1st and 71% in transplanted in 2nd remission (р=0.888) with corresponding value in patients with resistant disease being as low as 16% (р<0.001). There was a significant difference in OS between all disease stages in adolescents: 74% when transplanted in 1st, 30% in 2nd remission, and 10% in patients with resistant disease (р=0.009 and р<0.001).
The post-transplant relapse rate in patients with AML depended on disease status prior to allo-HSCT and did not differ between age groups. For infants in 1st or 2nd remission relapses were seen in 22% and 50% (р=0.067), in children ages 1 to 10 years in 36% and 62% (р=0.013), in adolescents in 15% and 67% (р=0.001), and in young adults in 11% and 92% of cases (р=0.001), accordingly.
Infant leukemia
We performed a separate outcomes analysis for patients with infant leukemia (n=57) combining both patients with AML (n=33) and ALL (n=24) younger than 1 year at the time of diagnosis. The overall survival of infants with AML or ALL was 64% and 59%, accordingly (р=0.762). It did not differ between children with AML and ALL achieving 1st or 2nd remission prior to allo-HSCT (79% and 80%, accordingly; р=0.924). There was also no difference between survival of MAC and RIC regimens recipients with OS being 79% in prior and 80% in the latter group (р=0.897).
Discussion
For several last decades the survival registered in children with acute leukemia was better compared to one observed in adolescents and young adults. There is a number of researchers advocating for age-related biological factors being the reason [4, 5] as there is an evident tendency to higher risk factors and somatic mutations rate (manifesting as molecular and cytogenetic aberrations) in young adults accompanied by age-related changes in immune response, which may as a whole change disease course to the worse [6, 7, 8]. Some also point at very young (infant) age as a potent adverse prognostic factor [6, 9].
We analyzed in our cohort the influence of age on results of allo-HSCT, which is an important stage for high-risk leukemia patients’ treatment. All patients were diagnosed high-risk leukemia and were divided into infants (<1 year), children (1-10 years), adolescents (11-20 years), and young adults (21-29 years) subcohorts. The data obtained suggests an increase in "unfavorable" genetic changes rate and decrease in one for "favorable" aberrations for overall ALL group, which may explain worse long-term results in older patients.
There is an evidence for more important role of older age in allo-HSCT recipients with ALL compared to ones diagnosed AML, although this relation is not universal and is more pronounced in certain subgroups. Thus, in Group 1 consisting of patients achieving remission prior to allo-HSCT the OS was 79% in infants and 65% in children aged 1 to 10 years, which was significantly better compared to results obtained in adolescents (46%; p=0.043) or young adults (47%; р=0.050).
The overall survival of infants with ALL in 1st remission was 82%, in group of children of 1 to 10 years it reached 93%. There was no difference in OS in children with 1st and 2nd ALL remission prior to allo-HSCT for all age groups except the group of 1 to 10 years, where OS was 93% and 57% (р=0.030), accordingly. The long-term OS in children with infant leukemia was higher compared to historic cohorts receiving no allo-HSCT [10, 11]. Thus, international high-risk infant ALL trial Interfant-06 noted unsatisfactory results in children not receiving allo-HSCT with 6-year OS being only 20.9% [9]. The allo-HSCT may be the factor helping overcome the negative effect of KMT2A rearrangements in this age group.
The analysis of our data was not able to pinpoint a negative influence of MRD positivity for any pediatric subcohort with difference being insignificant in infants (р=0.539), children aged 1 to 10 years (р=0.141), and adolescents (р=0.561). Although this data contradicts the results of some well-organized studies [12], it may be explained by retrospective nature of studied cohort, in which most patients were treated before modern targeted and immunotherapeutic interventions were introduced. Also, constant MRD status monitoring after allo-HSCT allows counterbalancing this factor by timely interventions via prophylactic or preventive immunotherapy (donor lymphocyte infusions, monoclonal antibodies) or targeted therapy in cases with persistent MRD.
Well-timed allo-HSCT scheduling is extremely important for children (younger than 10 years) with adverse prognostic factors. It should, therefore, be provided for as soon as high-risk leukemia is diagnosed. On the other hand, our data suggests equal allo-HSCT effectiveness in adolescents and young adults with ALL when performed in 1st and 2nd remission (р=0.231 and р=0.339, accordingly), while the results in 3rd or 4th remission are as poor as ones obtained in patients with resistant disease (р=0.697 and р=0.390, accordingly).
The overall survival analysis in high-risk AML patients of different age groups uncovered a very different trend as long-term results depended much more on disease status at allo-HSCT, than patient’s age (р=0.564 and р=0.604). This correlation was, however, a bit different for various age groups. The OS achieved in children aged 1 to 10 years and young adults transplanted in 1st remission was not significantly different from patients of the same age cohorts receiving transplant in 2nd remission (р=0.218 and р=0.888, accordingly). On the contrary, in adolescent cohort the transplantation in 1st remission provided much better results, even compared to transplants performed in 2nd remission (р<0.001). Thereof, the patient’s age should be taken into account when allo-HSCT is planned.
Our clinic is actively implementing RIC regimens in patients with high-risk leukemia from all age groups in order to alleviate the cytostatic burden and mitigate long-term negative effects of anticancer treatment. We have been monitoring and comparing the effectiveness of different intensity conditioning regimens for more than 20 years. The first results of allo-HSCT with RIC regimens in children with ALL were published in 2010 [13], but up to this moment there is very few data on RIC regimens effectiveness in adolescent and young adult acute leukemia cohorts [14]. IN this retrospective study we have compared the OS obtained in children of different age groups receiving allo-HSCT with RIC. It is important that there was no statistically significant difference in OS for any of age groups in RIC vs. MAC comparison with 100% vs. 75% (р=0.511) in infants, 55% vs. 69% (р=0.263) in children aged 1 to 10 years, 47% vs. 46% (р=0.865) in adolescents, and 52% vs. 44% (р=0.547) in young adults, accordingly.
The long-term OS in patients receiving allo-HSCT outside of 1st or 2nd remission was 22% for infants and 30% for children aged 1 to 10 years, which was significantly higher than 10% and 6% OS obtained in adolescents and young adults, accordingly (р=0.001). The relapse treatment effectiveness was also different depending on patient’s age with 36% of infants, 24% of children aged 1 to 10 years, 12% of adolescent and only 4% of young adults being able to achieve subsequent remission (р=0.005). In order to further consolidate the remission (based on relapse risk factors present) some of the patients needed preventive/prophylactic interventions. The factors taken into account here may include patient’s age, genetic aberrations, disease and MRD status at the moment of allo-HSCT.
Conclusions
The allo-HSCT improved the survival in patients with high-risk acute leukemia of different age allowing to reach long-term survival values comparable to those seen in standard and intermediate-risk cohorts, in which treatment intensification is not necessary. Allo-HSCT improves the OS in infants with ALL, when additional adverse risk factors are present. It also provides sizable advantage in children with high-risk ALL aged 1 to 10 years when performed in 1st remission. The overall survival of children younger than 10 years with high-risk ALL is better compared to older cohorts, which is probably explained by presence of additional unfavorable genetic aberrations in latter cases. Although MRD status at time of allo-HSCT is an important factor for patients with ALL, it may be mitigated by timely use of post-transplant targeted and immunotherapy. The long-term results in cohorts receiving allo-HSCT with RIC regimens suggest this tactic to be non-inferior compared to more traditional MAC-based approach.
Authors contribution: all authors contributed equally to this manuscript, revised its final version and agreed for the publication.
Funding: all authors received no financial support for this manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- Afanasyev B, Afanasyeva KS, Barabanshchikova MV, Bondarenko SN, Bykova TA, Vlasova JY, et al. Indications for hematopoietic stem cell transplantation. Cell Ther Transplant. 2019; 8(4):101-145. doi: 10.18620/ctt-1866-8836-2019-8-4-101-145
- Afanasyev BV, Zubarovskaya LS, Moiseev IS. Allogeneic hematopoietic stem cell transplantation in children: present, future, prospective. Russian J Pediatric Hematology/Oncology. 2015; 2(2): 28-42. (In Russian). doi: 10.17650/2311-1267-2015-2-2-28-42
- Bondarenko SN, Moiseev IS, Slesarchuk OA, Darskaya EI, Ekushev KA, Smirnova AG, et al. Allogeneic hematopoietic stem cell transplantation in children and adults with acute lymphoblastic leukemia. Cell Ther Transplant. 2016; 5(2): 12-20.
doi: 10.18620/1866-8836-2016-5-2-12-20 - Gu Z, Churchman ML, Roberts KG, Moore I, Zhou X, Nakitandwe J, et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat Genet. 2019;51(2):296-307. doi: 10.1038/s41588-018-0315-5
- Paina OV, Semenova EV, Markova IV, Zubarovskaya LS, Afanasyev BV. Modern views on the treatment of acute leukemia in children under 1 year. Russ J Pediatric Hematology/ Oncology. 2019; 6(2):11-19. (In Russian). doi: 10.21682/2311-1267-2019-6-2-11-19
- Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020; 1; 105(11):2524-2539. doi: 10.3324/haematol.2020.247031
- Roberts KG. Genetics and prognosis of ALL in children vs adults. Hematology Am Soc Hematol Educ Program. 2018; 30;2018(1):137-145. doi: 10.1182/asheducation-2018.1.137
- Conneely SE, Rau RE. The genomics of acute myeloid leukemia in children. Cancer Metastasis Rev. 2020;39(1):189-209.
doi: 10.1007/s10555-020-09846-1 - Pieters R, De Lorenzo P, Ancliffe P, Aversa LA, Brethon B, Biondi A, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: Results from an international Phase III randomized study. J Clin Oncol. 2019;37(25):2246-2256. doi: 10.1200/JCO.19.00261
- Clesham K, Rao V, Bartram J, Ancliff P, Ghorashian S, O'Connor D , et al. Blinatumomab for infant acute lymphoblastic leukemia. Blood. 2020 23;135(17):1501-1504. doi: 10.1182/blood.2019004008
- Brown P, Pieters R, Biondi A. How I treat infant leukemia. Blood. 2019 17;133(3):205-214. doi: 10.1182/blood-2018-04-785980
- Health Quality Ontario. Minimal Residual Disease Evaluation in Childhood Acute Lymphoblastic Leukemia: A Clinical Evidence Review. Ont Health Technol Assess Ser. 2016; 16(7):1-52. PMID: 27099643; eCollection 2016.
- Verneris MR, Eapen M, Duerst R, Carpenter PA, Burke MJ, Afanasyev BV, et al. Reduced-intensity conditioning regimens for allogeneic transplantation in children with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2010;16(9):1237-1244.
doi: 10.1016/j.bbmt.2010.03.009 - Tracey J, Zhang MJ, Thiel E, Sobocinski KA, Eapen M. Transplantation conditioning regimens and outcomes after allogeneic hematopoietic cell transplantation in children and adolescents with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2013; 19(2):255-259. doi: 10.1016/j.bbmt.2012.09.019
Accepted 25 November 2022


