Efficacy of therapy with blinatumomab and autologous CD3 cell transfusions in relapsing acute lymphoblastic leukemia: A single center experience
Nara G. Stepanyan, Timur T. Valiev, Ramil R. Fatkhullin, Tatiana Y. Pavlova, Natalia S. Tsaplina, Kirill I. Kirgizov, Svetlana R. Varfolomeeva
N. N. Blokhin Russian Cancer Research Center, Moscow, Russia
Correspondence:
Dr. Nara G. Stepanyan, Bone Marrow Transplantation
Department, N. N. Blokhin Russian Cancer Research Center, 23B Kashirskaya St, 115522, Moscow, Russia
Phone: +7 (903) 247-30-82
E-mail: nara19922@yandex.ru
Citation: Stepanyan NG, Valiev TT, Fatkhullin RR, et al. Efficacy of therapy with blinatumomab and autologous CD3 cell transfusions in relapsing acute lymphoblastic leukemia: A single center experience. Cell Ther Transplant 2022; 11(3-4): 70-76.
Accepted 25 November 2022
Summary
About 85-90% of patients with acute lymphoblastic leukemia (ALL) recover after modern program therapy. However, the disease may relapse, or acquires a refractory course in 10-15% of the children. For this category of patients, second-line chemotherapy is standardly provided, followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, achievement of molecular/immunological remission is not always possible. In the era of targeted therapy, great hopes are placed on molecularly targeted drugs in order to reach complete molecular/immunological remission and obtain higher survival rates with allo-HSCT. Blinatumomab is among the targeted drugs used to achieve remission in ALL. Its effectiveness depends on a sufficient level of CD3-lymphocytes, CD19 expression, and the number of blast cells in peripheral blood and bone marrow. Our purpose was to present the experience of blinatumomab therapy with transfusions of autologous CD3 lymphocytes in children with recurrent ALL and to evaluate the efficiency of this technique in achieving remission.
Materials and methods
This article presents three clinical cases of patients with recurrent ALL treated at the Research Institute of Pediatric Oncology and Hematology at N. N. Blokhin National Cancer Research Center from July 2021 to February 2022.
Results
Two patients from our series were treated with blinatumomab and received 3 transfusions of CD3-lymphocytes followed by clinical and hematological remission with MRD-negative disease status. One patient, due to concomitant somatic pathology, received only 2 auto-transfusions, but ALL remission was not achieved.
Conclusion
Our experience of using autologous CD3+ lymphocyte transfusions in combination with blinatumomab showed that this technique is safe, reproducible, and may be effective in patients with ALL relapses. In 2 out of 3 patients with weekly infusion of autologous lymphocytes, a complete clinical, hematological and immunological remission of the disease was registered.
Keywords
Acute lymphoblastic leukemia, blinatumomab, lymphocytes, autologous, apheresis, children, MRD status.
Introduction
Acute lymphoblastic leukemia (ALL) is one of the most common malignant diseases in children. Despite the fact that overall survival rate in pediatric ALL reaches 90%, unfortunately, 10-15% of patients develop relapses after achieving first clinical and hematological remission [1]. Prior to the advent of immunotherapy, the only treatment for relapsing and refractory ALL was a more intensive second-line chemotherapy which usually included alternating blocks of high-dose chemotherapy, followed by allogeneic bone marrow or hematopoietic stem cell transplantation (allo-HSCT). However, this therapy increased the degree of toxicity and contributed to development of complications, with mortality of up to lethal in 10-15% of cases [2]. Moreover, it is necessary to achieve MRD-negative status prior to HSCT procedure. This task may represent a difficult challenge [3]. The success of allo-HSCT in pediatric ALL depends on the level of minimal residual disease (MRD) before transplantation. At the present time, all therapeutic options are aimed at achieving clinical and hematological remission and reducing the MRD level [4].
MRD-negative status in relapses and refractory forms of ALL may be achieved by usage of targeted drugs, e.g., bispecific antibodies BiTE (AT), which may activate effector T-cells providing antitumor effect [5]. Blinatumomab is a bispecific anti-СD19/CD3 antibody, consisting of 2 single-chain variable fragments (scFv), specific for to CD19 (B-lymphocyte marker) and epsilon chains of CD3+ T-lymphocytes [6]. The mechanism of the drug action is to "redirect" effector functions of the patient’s own T-lymphocytes to the target cells that carry CD19 including blast cells in B-ALL patients. Given the opportunity of such "redirection" at the time of blinatumomab administration, a mediated cytolytic effect is induced (with subsequent activation and proliferation of T cells) towards the CD19-expressing cells. Of note, the anti-CD19-scFv has a higher affinity than the anti-CD3 fragment, which may cast doubt on the drug efficiency at low levels of CD3 cells in the patient's peripheral blood [7], as seen in Fig. 1.
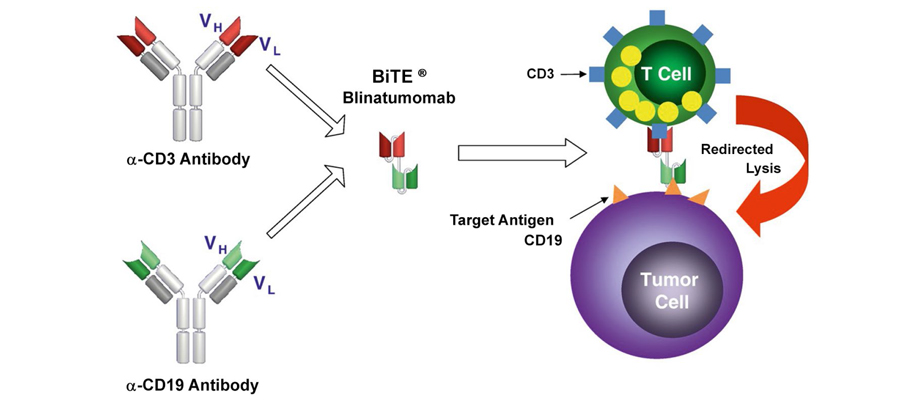
Figure 1. The mechanism of action of blinatumomab (bispecific BiTE molecule consisting of 2-single-chain variable fragments (scFv) specific for the CD19 and CD3 epsilon chain. The main mechanism involves recruitment of the own T-cells and redirecting their effector functions into target cells carrying the CD19 marker, which include B-lineage blast cells [8]
The advantage of blinatumomab is independence of this effect on the specificity of T-cell receptors (TCR), or presence of class 1 MHC molecules on the surface of antigen-presenting cells. T-cell activation increases the secretion of cytokines, especially IL-2, IFN-y, TNF-a, IL-4, IL-6, and IL-10. Moreover, at the moment of T-lymphocyte activation, granzyme B and perforin are released, which penetrate into CD19-positive tumor cells and activate apoptosis [9].
Taking into account the mechanism of action of the drug, one may assume that, during the period of immunotherapy, additional transfusions of autolymphocytes may increase the number of lymphocytes, thus promoting favorable effect of the drug and enhance its effectiveness. The transfusions should be performed at 7-day intervals, to maintain a stable concentration of CD3+cells in peripheral blood [10].
Despite the lack of convincing data on the correlation between the absolute levels of peripheral lymphocytes, especially, CD3+ cells, before starting the immunotherapy and transfusions of auto-lymphocytes may be reasonable, especially in severe aplasia of hematopoiesis [11].
Materials and methods
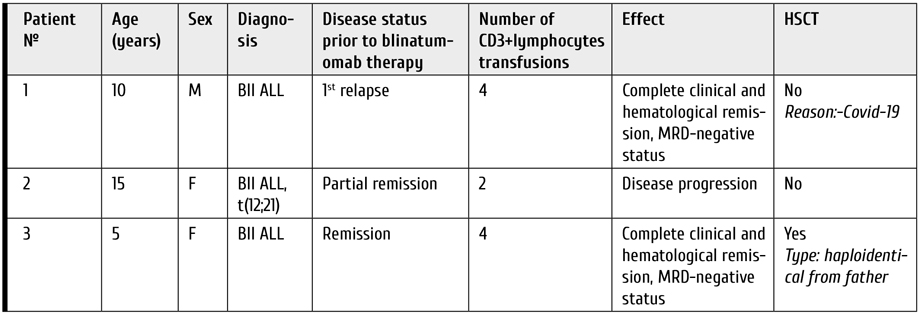
Transfusions of autologous CD3+ lymphocytes were performed in three patients with recurrent ALL who underwent immunotherapy with blinatumomab from July 2021 to February 2022 at the Research Institute of Pediatric Oncology and Hematology, N. N. Blokhin National Research Cancer Center. All patients were MRD-positive, and blinatumomab was administered as a bridge therapy to HSCT (Table 1).
Table 1. Characteristics of patients included in the study
Previously, the 3 patients at the N. N. Blokhin National Medical Research Center of Oncology underwent consolidation and anti-relapse therapy according to the ALL IC-BFM 2009 and ALL-REZ BFM 2002 protocols, which included distinct chemotherapy blocks with the following chemotherapy drugs:
• F1- dexamethasone, vincristine, PEG-asparaginase, methotrexate,
• F2- dexamethasone, cytarabine, PEG-asparaginase,
• R1- vincristine, methotrexate, cytarabine, dexamethasone, 6-mercaptopurine,
• R2- dexamethasone, 6-mercaptopurine, vincristine, methotrexate, ifosfamide, daunorubicin,
• FLAI- fludarabine, cytarabine, idarubicin.
Cell apheresis procedure
The lymphocyte collection was carried out at the confirmed levels of >107CD3+( cells per µL of peripheral blood, blood hemoglobin levels of >90 g/l; platelets, >50×109/L, without prior cell mobilization. To adjust correct anticoagulant ratio during cell collection, the blood clotting coagulation parameters were monitored a day before the procedure, due to possible hypocoagulation in excess of the ACD-A solution is possible with the introduction of anticoagulant [12].
Apheresis of autologous lymphocyte was performed at the Department of Bone Marrow Transplantation, using "Spectra Optia" cell separator (Terumo BCT Inc, USA). Detailed information on the technical features of collecting lymphocytes from patients of different groups and types of device software is described in the manufacturer’s instructions, as well as recommendations on the optimal procedure modes [13]. Central venous catheter (CVC) was pre-installed on the day of scheduled apheresis. Four units of the lymphocyte concentrates with predominant CD3+cells were prepared for each patient, at the median of 44.8% (32.8-55.7) of the nucleated cells in the mononuclear concentrat. Each patient received from 2 to 4 transfusions of autologous lymphocytes with the concentration of cells in the dose, respectively 25-36×106 cells in 35 mL. The number of transfusions depended on the total level of leukocytes, efficiency of lymphocyte harvesting, somatic and infectious status of the patient on the day of reinfusion.
An informed consent was obtained of the legal representative of the child before each session of lymphocyte apheresis.
Efficiency of the procedure was determined by achieving the target levels of CD3+ cells in apheresis product (>20 cells/µl). The average level of separated CD3+lymphocytes in the study group was 30.0 cells/µl, indicating a sufficient cell harvest. Lymphocyte concentration in the final product was assigned to the distinct patient. The lymphocyte-containing products underwent volume reduction up to 140 ml, and concentration 23-36×107 cells in 35 mL. The separated cells have been sent to the cryostorage being immersed in liquid nitrogen (-196°C).
The method for determining minimal residual disease(MRD) is represented by flow cytometry. The studies were carried out on a FACSCantoII cytometer (Becton Dickinson, USA). Flow cytometry data were analyzed with FACSDiva 6.1 software (Becton Dickinson)[14]. The result was given taking into account changes in the antigenic profile under the influence of therapy according to the ALL-REZ BFM 2002 protocol.
Therapy with blinatumomab
In all 3 patients, blinatumomab was administered by continuous intravenous infusion through a pre-installed central venous catheter (CVC). The initial dose was 5 µg/m2/day for 7 days followed by 15 µg/m2/day for 21 days. In one case, the patient's body weight exceeded 45 kg. Therefore, according to the official prescriptions, this patient received a dose of 9 μg/m2/day for 7 days then 15 μg/m2/day for 21 days. CD3+ lymphocytes have been infused to all three patients, at courses of every 7 days. Two patients from our group received the lymphocyte transfusions at +14, +21 and +28 days of therapy, one patient received only +7 and +14 days therapy, due to poor somatic condition. On the first day of therapy, all patients underwent spinal puncture with endolumbar chemoprophylaxis with methotrexate (at the age-matched doses) followed by bone marrow puncture, myelogram counts and MRD determination. When using blinatumomab in combination with lymphocyte transfusions, 2 out of 3 patients experienced skin allergic reactions [15].
Laboratory studies
Technical recommendations ISHAGE (International Society of Hematotherapy and Graft Engineering) were used to determine the numbers of CD3+ cells in peripheral blood before apheresis procedure. The results were presented both as a percentage and absolute values. The number of lymphocytes was determined by the expression of membrane CD3 and CD45 markers in the direct immunofluorescence reaction [14]. The result was evaluated by flow cytometry in the CD3+ PE-A population.
Case reports
Patient 1
The patient, a 10-year-old boy, was diagnosed with acute lymphoblastic leukemia LI-II according to the FAB classification, B-linear variant, BII immunosubvariant (pre-pre-B, common) with a chimeric gene TEL/AML 1, CNS 3, relapse I.
He received treatment according to the ALL-REZ BFM 2002, 1 course of induction anti-relapse blocks F1 and F2 were performed, given the lack of sanitation of cerebrospinal fluid after F1. After the end of therapy, control bone marrow punctures were performed. According to the myelograms, the presence of a blast population in the bone marrow remains 27% blast cells, as well as the persistence of minimal residual disease (MRD)-positive status. In the post-block period, complications were noted in the form of oropharyngeal mucositis and vincristine polyneuropathy (with following complete resolving). Next, the patient underwent an anti-relapse block R1 according to the ALL-REZ BFM 2002 protocol. The child tolerated the block of therapy satisfactorily, there were no complications. After the block of chemotherapy, repeated bone marrow punctures were performed which showed normal levels of blast cells. However, the MRD-positive status of the disease still persisted. Taking into account these results, the child has received the anti-relapse R2 block, according to the ALL-REZ BFM 2022 protocol. The block of therapy was tolerated satisfactorily, on control studies according to bone marrow punctures, the minimal residual disease positive status of the disease remains, which requires the start of an additional block of FLAI chemotherapy. After the chemotherapy, some complications were observed, i.e., febrile neutropenia, accompanied by multiple episodes of fever, requiring correction of antibacterial and supportive therapy. Upon restoration of hematopoiesis, control bone marrow punctures were performed which have shown MRD-positive status retained in the patient. Taking into account persistence of the MRD-positive status, the patient was administered immunotherapy with a bispecific T-cell activator drug – blinatumomab as part of the "bridge"-therapy with the reinfusion of CD3 autolymphocytes weekly. Control of MRD status on days 14 and 28 of immunotherapy. The patient received 4 transfusions of autologous CD3+lymphocytes, and achieved MRD-negative status, which further required HSCT, but during the pre-transplant examination he was infected with COVID-19. The persistence of the virus had been recorded for 2 months, on the 60th day of the disease, control punctures were carried out, which revealed a relapse of the underlying disease. The relapse occurred 55-60 days after the start of immunotherapy.
Patient 2
The patient, a 15-year-old girl, was diagnosed with acute lymphoblastic leukemia L-1 morphological variant, B-II immune subvariant. After therapy according to the ALL-MB-2015 protocol, her clinical condition corresponded to Relapse I. No specific gene aberration was found.
The patient maintained long-term clinical and hematological remission I, but 4 years later the disease relapsed, and she was administered the ALL-REZ BFM 2002 protocol, with second-line anti-relapse blocks been performed according to the F1, F2, R2, R1 blocks. After the end of anti-relapse therapy, control bone marrow punctures were performed. The myelograms revealed a remaining blast population in the bone marrow 38%, as well as the preservation of the MRD-positive status 0.8%. In the post-block period, the following complications were noted, i.e., grade 2 oropharyngeal mucositis. Further, according to the results of the myelogram, in view of the progression of the disease, a decision was made to intensify chemotherapy by the FLAI block regimen. The child tolerated the therapy block satisfactorily, there were no complications. After the end of this chemotherapy, the repeated bone marrow punctures were performed which have shown reduced level of blast cells (from 38 to 18%), but the bone marrow was not completely purged and the MRD-positive disease proved to persist (0.5%). Taking into account these results, the second course of the FLAI block was started for the child. The therapy block was satisfactorily tolerated, there were no pronounced infections. Examination of the bone marrow punctures still showed MRD-positive status, but the level of marrow blast cells was less than 5%. The following complications were associated with chemotherapy: febrile neutropenia, episodes of fever, requiring correction of antibacterial and supportive therapy. Due to persistence of MRD-positive status, the patient has received immunotherapy with blinatumomab, a bispecific T-cell activator drug as an anti-relapse treatment, with reinfusion of CD3+ autologous lymphocytes once every 7 days. Control of MRD status was performed on days 14 and 28 of immunotherapy. The patient received 2 transfusions of auto-lymphocytes, at +7 and +14 days from the start of Blinatumomab therapy. Then, according to the results of the bone marrow study on day +14 (during immunotherapy), an increased level of blast cells and MRD-positive status of the disease were noted, thus indicating the progression of the disease. The patient continued therapy with blinatumomab until the 21st day. Later on, the therapy was completed, due to worsening of the patient's somatic condition (development of pulmonary and renal failure).
Patient 3
The patient, a 5-year-old girl, was diagnosed with acute lymphoblastic leukemia B II immune subvariant, CNS 1. Relapse I. No specific gene aberration was found.
As part of intensive care, she received treatment according to the ALL-REZ BFM 2002 protocol, one course of induction anti-relapse blocks F1 and F2 were performed, due to the lack of CSF sanitation after F1. After the end of therapy, control bone marrow punctures were performed showing a detectable blast population in the bone marrow, as well as persistence of MRD-positive status. In the post-block period, some complications were noted, including oropharyngeal mucositis and vincristine polyneuropathy. Later on, the patient underwent an anti-relapse block R1 according to the ALL-REZ BFM 2002 protocol. The block of therapy was tolerated satisfactorily, without complications. At the end of chemotherapy block, a repeated bone marrow puncture has shown normal level of blast cells, however, with persistent MRD-positive status. In view of these findings, the child was subjected to the anti-relapse R2 block, according to the ALL-REZ BFM 2022 protocol. This block of therapy was tolerated satisfactorily, however, bone marrow examination revealed MRD-positive status of the disease, which required an additional block of FLAI chemotherapy. This therapy was followed by several complications including febrile neutropenia accompanied by multiple episodes of fever, requiring correction of antibacterial and accompanying therapy. Upon recovery of hematopoiesis, the control bone marrow punctures still confirmed the MRD-positive status. Taking into account the persistence of MRD-positive status, the patient was administered immunotherapy with blinatumomab in the frames of "bridge"-therapy along with weekly reinfusions of CD3+ autologous lymphocytes. MRD status was controlled on days +14 and +28 of immunotherapy. The patient received 4 auto-transfusions of CD3 lymphocytes, and achieved MRD-negative status, which allowed him to enter the next stage of treatment, i.e., HSCT. Two weeks later, after the pre-transplant examination, the patient underwent haploidentical transplantation from her father.
Discussion
The presented experience of using transfusions of CD3+ autolymphocytes in combination with blinatumomab showed that this technique is safe, reproducible, and can be effective in patients with relapses of ALL. In 2 out of 3 patients with the introduction of auto-lymphocytes, we have achieved a complete clinical, hematological and immunological remission of the disease.
Modern cellular and targeted technologies have improved the survival rate of patients with recurrent ALL. When conducting therapy with blinatumomab, it is necessary to take into account the level of CD3+ lymphocytes, which are an integral component of effect of blinatumomab against lymphoid malignancies. The drug is able to attract T-regulatory CD3+lymphocytes (Treg), which induce an immunosuppressive response, due to local increase in the IL-10 and TGF-b concentrations [16]. Thus, the transfusions of autologous lymphocytes with high contents of CD3+ cells is well justified, due to elevation of their contents in the patients’ blood and probable anti-leukemic effect of blinatumomab [11]. Infusions of donor lymphocytes to the patients after allogeneic HSCT may be also potentially effective, pursuing the goal of both replacing the pool of CD3 + cells (in case of lymphopenia), and contributing to the general phenomenon of "graft against leukemia" effect [17]. Both immunotherapeutic approaches require more detailed study.
Conclusion
Transfusion of CD3+ autologous lymphocytes in combination with blinatumomab therapy in pediatric patients with relapses and refractory forms of ALL is a quite safe therapeutic option being effective in achieving the disease remission, and as a tool of bridge therapy before allo-HSCT.
Conflict of interest
None declared.
References
- Jeha S, Pei D, Choi J, Cheng C, Sandlund JT, Coustan-Smith E, Campana D, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019; 37(35):3377-3391.
doi: 10.1200/JCO.19.01692 - Locatelli F, Moretta F, Rutella S. Management of relapsed acute lymphoblastic leukemia in childhood with conventional and innovative approaches. Curr Opin Oncol. 2013; 25:707-715. doi: 10.1097/CCO.0000000000000011
- Benjamin JE, Stein AS. The role of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Ther Adv Hematol. 2016;7(3);142-156. doi: 10.1177/2040620716640422
- Locatelly F, Whitlock J, Peters C, Chen-Santel C, Chia V, Dennis R, et al. Blinatumomab versus historical standard therapy in pediatric patients with relapsed/ refractory Ph-negative B-cell precursor acute lymphoblastic leukemia. Leukemia. 2020;34(9):2473-8.
doi: 10.1038/s41375-020-0770-8 - Le Jeune C, Thomas X. Potential for bispecific T-cell engagers: role of blinatumomab in acute lymphoblastic leukemia. Drug Des Devel Ther. 2016;10:757-765. doi: 10.2147/DDDT.S83848
- Achtari M, Kum-Ja L, Weissman A, Tulpule S, Aldoss I, Akhtari M. Clinical use of blinatumomab for B-cell acute lymphoblastic leukemia in adults. Ther Clin Risk Manag. 2016;12:1301-1310. doi: 10.2147/TCRM.S84261
- Zugmaier G, Klinger M, Schmidt M, Subklewe M. Clinical overview of anti- CD19 BiTE® and ex vivo data from anti- CD33 BiTE® as examples for retargeting T-cells in hematologic malignancies. Mol Immunol. 2015;67(2 Pt A):58-66. doi: 10.1016/j.molimm.2015.02.033
- Nagorsen D, Baeuerle P. Immunomodulatory therapy of cancer with T-cell-engaging BiTE antibody blinatumomab. Exp Cell Res. 2011;317(9):1255-1260. doi: 10.1016/j.yexcr.2011.03.010
- Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015 Sep 4;8:104. doi: 10.1186/s13045-015-0195-4
- Ueda M, de Lima M, Caimi P, Tomlinson B, Little J, Creger R, Lazarus H, Cooper B. Concurrent blinatumomab and donor lymphocyte infusions for treatment of relapsed pre-B-cell ALL after allogeneic hematopoietic cell transplant. Bone Marrow Transplant. 2016; 51(9):1253-1255. doi: 10.1038/bmt.2016.104
- Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 2017; 31(10):2181-2190. doi: 10.1038/leu.2017.41
- Barrett D, Fish JD, Grupp SA. Autologous and allogeneic cellular therapies for high-risk pediatric solid tumors. Pediatr Clin North Am. 2010; 57(1): 47-66. doi: 10.1016/j.pcl.2010.01.001
- Spectra Optia Apheresis System& Operator’s Manual. Part No. 777379-124. Reorder No. 703261-001 2018-04 Terumo BCT.
- Movchan LV. Leukemia-associated immunophenotype in children with B-precursor acute lymphoblastic leukemia. Onkogematologiya. 2012; 1:22-28. (In Russian).
- Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Ther. 2012; 136(3):334-342.
doi: 10.1016/j.pharmthera.2012.07.013 - Illarionova O, Gluhanyuk E, Kashpor S. Changes in leukemic blasts CD19 expression in children with relapsed/ refractory B-cell precursor ALL treated with blinatumomab. Blood. 2017;130(Suppl. 1):3991 (In Russian). doi: 10.3324/haematol.2019.2415966
- Chan WYK, Cheuk DKL, Lee PPW, Chan GCF, Leung W, Yeung EWM, et al. Blinatumomab with donor lymphocyte infusions post haploidentical hematopoietic stem cell transplantation as salvage therapy for relapsed refractory acute lymphoblastic leukemia post chimeric antigen receptor T-cell therapy. Pediat Blood Cancer 2022; Jun 21;e29852. doi: 10.1002/pbc.29852
Accepted 25 November 2022


