Gilteritinib as a bridge to allogeneic hematopoietic stem cell transplantation in adult patients with refractory/relapsed acute myeloid leukemia with FLT3 mutations
Bella I. Ayubova1, Sergey N. Bondarenko1, Anna G. Smirnova1, Yulia Yu. Vlasova1, Nikolay Yu. Tsvetkov1, Michail M. Kanunnikov1, Dmitry K. Zhogolev1, Yuliya D. Oleynikova1, Elena V. Karyagina2, Ridvan K. Ilyasov3, Natalya A. Zorina4, Svetlana S. Belyaeva5, Yulia S. Neredko6, Irina A. Samorodova7, Yulia B. Chernih8, Mikhail Yu. Lazarev9, Anna P. Kochergina10, Anna A. Nasredinova1, Ildar M. Barkhatov1, Tatyana L. Gindina1, Ivan S. Moiseev1, Alexander D. Kulagin1
1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 City Hospital No. 15 of St. Petersburg, Russia
3 Crimean V. М. Efetov Republican Oncological Clinical Dispensary, Simferopol, Russia
4 Kirov Research Institute of Hematology and Blood Transfusion, Kirov, Russia
5 Belgorod Regional Clinical Hospital of St. Joasaph, Belgorod, Russia
6 Stavropol Regional Clinical Oncological Dispensary, Stavropol, Russia
7 City Hospital No. 31 of St. Petersburg, Russia
8 Moscow M. F. Vladimirsky Regional Research Clinical Institute, Moscow, Russia
9 City Clinical Hospital No. 40, Moscow, Russia
10 Regional Clinical Hospital, Barnaul, Russia
Correspondence:
Dr. Bella I. Ayubova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia
E-mail: bella_ayubova@mail.ru
Citation: Ayubova BI, Bondarenko SN, Smirnova AG et al. Gilteritinib as a bridge to allogeneic hematopoietic stem cell transplantation in adult patients with refractory/relapsed acute myeloid leukemia with FLT3 mutations. Cell Ther Transplant 2022; 11(3-4): 60-69.
Accepted 28 October 2022
Summary
Acute myeloid leukemia (AML) with FLT3 mutations, being a clinical subtype of AML, is associated with higher relapse rate, shorter remission period, decreased survival, especially without NPM1 co-mutation, and at high FLT3-ITD/wild-type allelic ratios. FLT3 mutations can be considered as a promising molecular target for the treatment of patients with FLT3-mutated AML, particularly in refractory or relapsed (R/R) AML cases. Allogeneic hematopoietic stem cell transplantation (allo- HSCT) gives chance for a potential cure in patients with R/R AML. The present study included 48 patients with R/R AML with FLT3-mutation, at a median age of 53 (18-79) years. Primarily refractory patients (p/r AML) made 31.2% of them; among recurrent AML, 54.2% had a first relapse, and 14.6% had 2 or more relapses (relAML). The ITD mutation was detected in 89.6% and TKD-mutation in 10.4% of cases. The patients received gilteritinib 120 mg once daily, administered as one (in most cases), or >2 28-day cycles of therapy (56.2% and 27.1%, respectively). Overall response (OR) rate was 77.1% (CI 95% 65.7-88.3): complete remission (CR) was documented in 15 (31.2%) patients, remission with incomplete recovery (CRi/r) and morphological leukemia-free state (MLFS) were observed in 11 patients (22.9%) each. Allo-HSCT was subsequently performed in 29.2% (CI 95% 18.2-43.2) of all treated patients. The median age of this group of patients was 43.1 (18-68) years. The median time from overall response achieved after gilteritinib to allo-HSCT was 45 (24-156) days. One-year overall survival was 39.9%, with median survival terms of 6.3 months (95% CI: 4.7-12.0). Disease-free survival in the group of patients who achieved remission (37 patients) was 40.5%, and the median was 7.7 months (4.4-11.0 months). In the multivariate analysis, event-free survival was significantly associated with successful bridging to allo-HSCT (HR=0.16; CI 95%: 0.04-0.62; p<0.01) and in patients who achieved OR (HR=0.11; CI 95%: 0.04-0.33; p<0.01). In contrast, the patients with late relapse showed an increase in the risk of the events (HR=3.42; CI 95%: 1.2-9.64; p=0.02). No unexpected toxicity was observed after the therapy. Gilteritinib in patients with R/R FLT3-mutated AML demonstrated favorable outcomes with a satisfactory tolerance to therapy. Thus, gilteritinib may be used for a bridge therapy to allo-HSCT in adult patients with R/R FLT3-mutated AML.
Keywords
Acute myeloid leukemia, target therapy, gilteritinib.
Introduction
Acute myeloid leukemia (AML) with a mutation in tyrosine kinase 3 (FLT3) gene represents one-third of the de novo AML cases [1, 2, 3, 4]. Mutations in the FLT3 gene (in particular, internal tandem duplication (ITD)) tend to cause poor outcomes, with an increased risk of relapse and shorter overall survival (OS) compared to patients without the mutation [4, 5, 6]. Thus, according to the NCCN Clinical Practice Guidelines in Oncology, AML cases with FLT3-ITD mutation in presence of normal karyotype should be interpreted as patients with poor prognosis [5, 7]. Based on European LeukemiaNet risk stratification by genetics (ELN risk), the prognostic risk in AML with FLT3-mutation also depends on FLT3-ITD allelic ratio, presence of an additional NPM1-mutation and accessory karyotype abnormalities [3, 5, 8].
The rate of remission after standard chemotherapy (ChT) varies from 60 to 70% for patients with de novo AML younger than 50 years [10]. The addition of midostaurin to frontline ChT improved OS (by 7.1% after 4 years to 51.4%). Also event-free survival (EFS) and disease-free survival (DFS) showed better result in the midostaurin group (8.2 months vs. 3.0 months and 26.7 months vs. 15.5 months, respectively) [9]. Nevertheless, challenges of the treatment of patients with refractory/relapsed (R/R) AML remain, where the chances for achieving remission do not exceed 30% [12]. As a result, patients with R/R AML have poor outcomes with 3-year overall survival (OS) around 10% [11].
A retrospective analysis of the 2-year overall survival (OS2) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) in a second complete remission demonstrated promising 50% OS, compared to allo-HSCT performed in active disease (first relapsed or refractory AML) with OS2 of ca. 20% [13, 14]. Thus, the main goal of therapy in patients with R/R AML is to achieve remission then followed by allogeneic HSCT.
A combination of high-dose cytarabine and purine analogues (fludarabine, cladribine) demonstrated a higher response rate in patients with R/R AML being, however, associated with considerable toxicity [16, 17, 18]. For patients with FLT3-mutated AML, therapy with FLT3 inhibitors showed promising results even in the case of R/R AML [19].
The current study aims to evaluate the response rate, overall survival (OS), relapse-free survival (RFS) and toxicity of gilteritinib in adult patients with R/R AML with FLT3-mutation.
Patients and methods
Between 2019 and 2021, 48 patients with R/R AML FLT3-mutated with a median age of 53 (18-79) years were included in the study. Most of the patients were with de novo AML (79.2%). Patients with secondary AML (20.8%) included transition form myelodysplastic syndrome (MDS), chronic myelomonocytic leukemia (CMML) and chronic myeloproliferative neoplasm (CMN). Primary-refractory patients (p/r AML) comprised 31.2%; among relapsed AML, 54.2% had a first relapse and 14.6% had 2 or more relapses (relAML). The ITD mutation was detected in 89.6% and TKD –mutation in 10.4% of cases.
Despite the median age of patients included in the study, allo-HSCT after gilteritinib was performed in 29% of cases. The type of donors was as follows: unrelated, in 9 cases; haploidentical, in 5 patients. The median time between treatment by gilteritinib and allo-HSCT was 12 days (range 7-54 days). In 3 patients, the relapses after first allo-HSCT were treated with ginteritinib. Characteristics of the patients are presented in Table 1.
Table 1. Characteristics of the patients
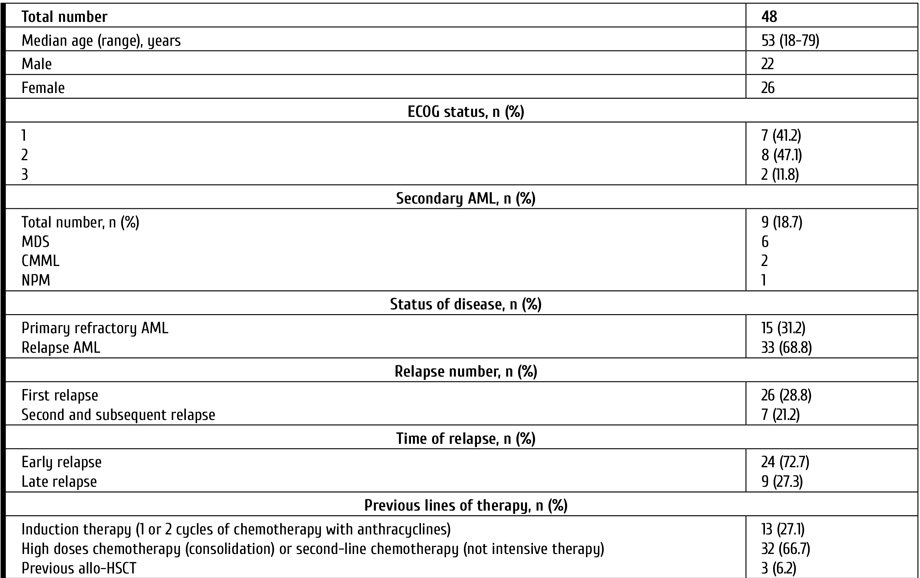
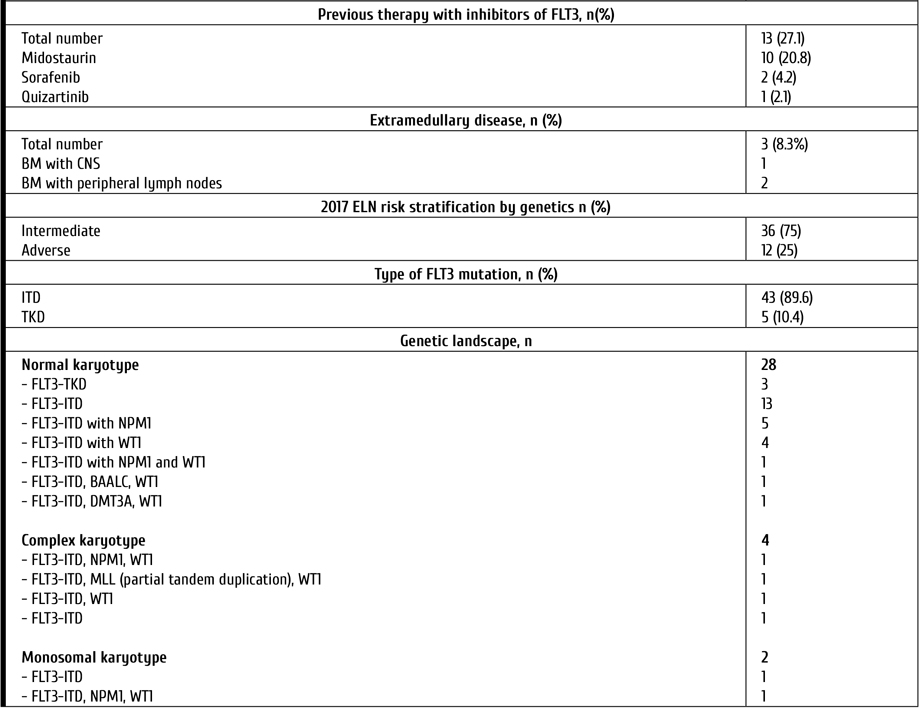
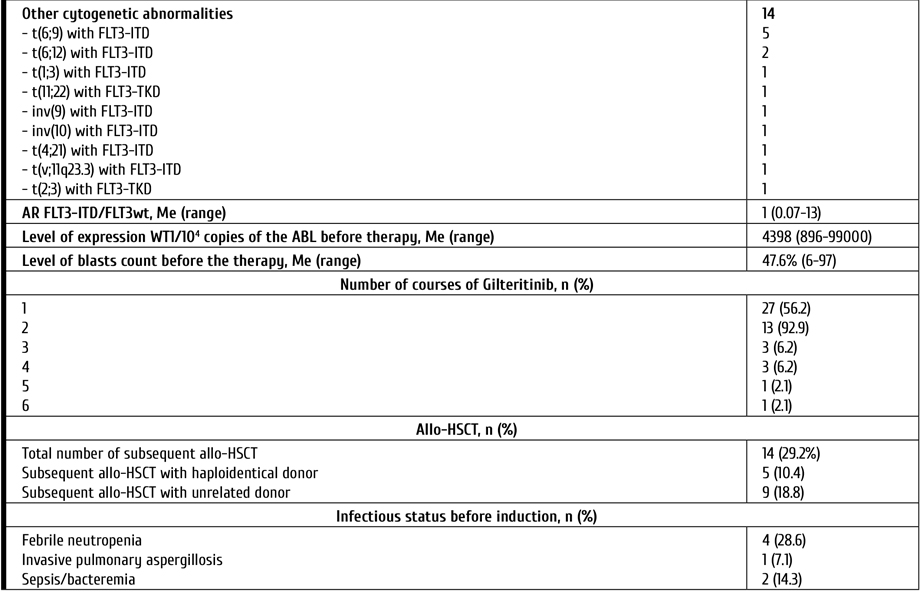
Notes: AML, acute myeloid leukemia; MDS, Myelodysplastic syndrome; CMML, Chronic myelomonocytic leukemia; CMN, Chronic myeloproliferative neoplasm; ECOG, Eastern Cooperative Oncology Group (ECOG) Performance Status; FLT3, FMS-like tyrosin kinase 3; ITD, internal tandem mutation; TKD, mutation surrounding D835 in TK domain; BM, bone marrow; CNS, central nervous system; allo-HSCT, allogeneic hematopoietic stem cell transplantation.
Morphological evaluation of bone marrow (BM) aspirate, immunophenotyping of bone marrow cells, standard cytogenetic and molecular assays were performed in all patients before therapy. The median level of blasts in the BM was 47.6% (6-97). Cytogenetic analysis was performed on R-banded metaphases after 24-h culture using standard procedures. The following targets were evaluated by real-time polymerase chain reaction (PCR): CBFB-MYH11, BCR/ABL p210 and BCR/ABL p190, PML/RARa, RUNX1-RUNX1T1, EVI1, FLT3-ITD, FLT3-TKD (D835), DMT3A, MLL partial tandem duplication, WT1, BAALC gene expression. Assessment of minimal residual disease (MRD) status included both flow cytometric assays (FCI) and PCR.
This study included patients with Eastern Cooperative Oncology Group (ECOG) performance status ≤3 as well as without moderate/severe hepatic dysfunction or heart disease. P/r AML was defined as more than 5% of clonal blast cells in bone marrow after one (or more) induction chemotherapy with anthracyclines (with or without FLT3-inhibitors). Response criteria after treatment were based on The European Leukemia Net (ELN) 2017 Recommendations [8]. Response was assessed on day 28 of 1st course (or 2nd in cases without response after first course). Toxicity was determined by the National Cancer Institute Grading Scale (NCI Grading Scale) version 5.0.
All patients recieved gilteritinib 120 mg once daily for at least 28 days (single-course therapy) [19]. Patients, who achieved response after 1st course, continued the therapy up to allo-HSCT. Those who achieved reduced blasts count in the bone marrow were able to receive 2nd course. Antiviral prophylaxis (acyclovir) and Pneumocystis prophylaxis (trimethoprim-sulfamethoxazole) were given to all patients. Primary antifungal prophylaxis for patients was performed with voriconazole. All patients received prophylactic treatment for infections during the period when the absolute neutrophil count was less than 0.5×109/l.
Statistical analysis
Overall survival (OS) and disease-free survival (DFS), event-free survival (EFS) were evaluated using Kaplan-Meier method, the influence of factors on OS and RFS were evaluated by the Mantel-Cox log-rank criterion. Multivariate analysis was performed with Cox model after the proportional hazards assumption was checked. The OS was calculated from the starting date of the treatment to the date of the last contact. RFS was defined from the date of overall response (complete remission, remission with incomplete recovery) to the date of relapse, death or last contact. EFS was defined as the time from the start date of treatment to the time when refractory disease was confirmed (the date of failure to achieve a response), relapse, or death from any cause (censored at last contact). Overall response (OR) assessment included patients with complete remission (CR), remission with incomplete recovery (CRi/r), morphological leukaemia-free state (MLFS). Therapy-related mortality was defined as death within 30 days of Giteritinib initiation. Pearson's chi-square test was used for binary variables with the number of expected observations in any of the fields in a four-field table less than 5, Fisher's exact test was used to assess the level of significance of the differences. Multivariate analysis was done using proportional hazard regression. Differences at a p-value less than 0.05 were considered statistically significant. Statistical analyses were performed with the standard statistical software package StatTech 2.8.4.
Results
Clinical response to gilteritinib
More than a half of the patients received the 28-day gilteritinib therapy administered in 1 or >2 cycles (56.2% and 27.1%, respectively), as seen from Table 1. Overall response (OR) rate was 77.1% (CI 95% 65.7-88.3): complete remission (CR) was documented in 15 (31.2%) patients, remission with incomplete recovery (CRi/r) and morphological leukemia-free state (MLFS) were registered in 11 patients (22.9%) each. Among the responders, CR/CRi/r/MLFS were achieved in 83.8% (31 patients) after 1st cycle and in 16.2% after 2nd treatment cycle. Among 15 patients with CR, MRD was evaluated in 6 patients on day 28 after starting the therapy), being positive in 5 cases.
In 14 of 48 patients, allo-HSCT was subsequently performed, thus making up 29.2% (CI 95% 18.2-43.2) of all treated patients. The median age of this group was 43.1 (18-68) years. The median time from OR after gilteritinib to allo-HSCT was 45 (24-156) days. The main reasons for failure to undergo allo-HSCT was elderly age (18 patients over 65), and sufficient co-morbidities; relapse of AML during donor search revealed in 6 cases (at <60 days); early death (<60 days of therapy), in 8 patients. Poor performance status was the reason for postponing allograft in 2 patients.
In the univariate and multivariate analysis, there was no correlations between the response rates and clinical factors, e.g., patient’s age, ELN risk, WT1 levels before therapy, presence ITD or TKD mutation in the FLT3 gene, previous therapy, relAML or p/r AML, early versus late relapse, blast cell counts.
Analysis of survival outcomes after gilteritinib therapy
The median follow-up period was 16.5 (CI 95% 11.4-21.6) months. One-year OS (OS1) after gilteritinib was 39.9%, with a median survival of 6.3 months (95% CI: 4.7-12.0 months). DFS (DFS1) in the group of patients who achieved remission (37 patients) was 40.5%. The median DFS1 was 7.7 months (4.4-11.0 months) in this group of patients.
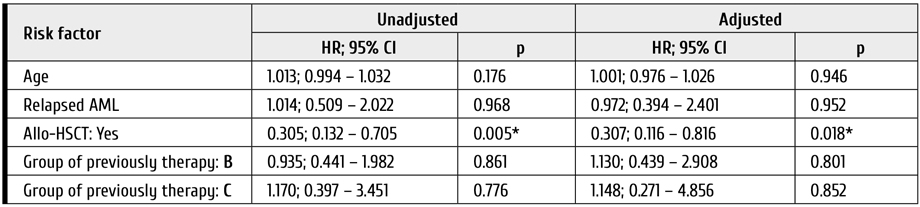
In the multivariate analysis, statistically significant factors influencing OS were as follows: achievement of response (HR=0.16; CI 95%: 0.05-0.47; p<0.01) (Table 2; Fig. 1) and allo-HSCT (HR=0.06; CI 95%: 0.01-0.44; p<0.01) (Table 2; Fig. 2). DFS was also significantly better in group of patients with subsequent allo-HSCT (HR=0.18; CI 95%: 0.04-0.8; p=0.02) (Fig. 2).
Table 2. Analysis of potential OS predictors in the patients treated with gilteritinib
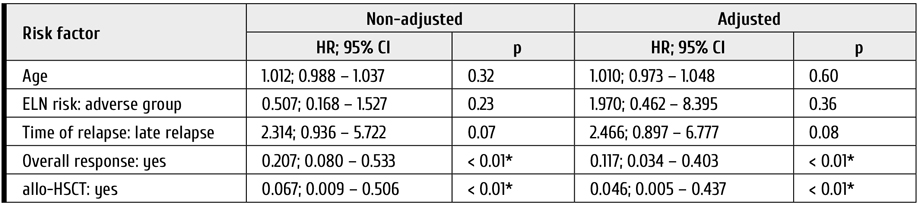
* – association of the outcome value with the predictor value is statistically significant (p < 0.05)
Notes: allo-HSCT, allogeneic hematopoietic stem cell transplantation; ELN risk, genetic risk stratification scale by ELN criteria (2017)
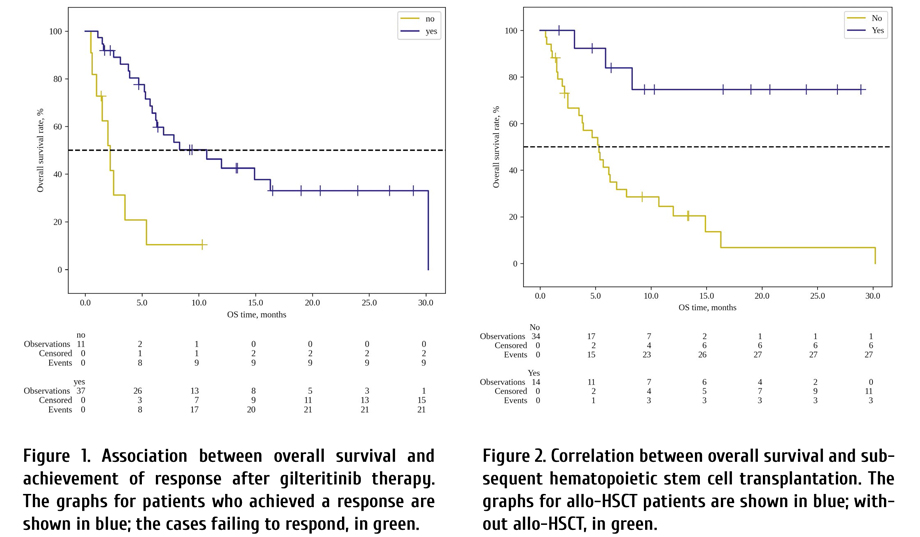
Median survival in patient without OR was 2.2 months (95% CI: 0.6-3.5 months), median survival in patients with OR was 10.7 months (95% CI: 5.9-30.2 months).
Median survival in patients without allo-HSCT was 5.3 months (95% CI: 2.5-6.9 months), whereas median survival in patients with subsequent allo-HSCT cannot be estimated.
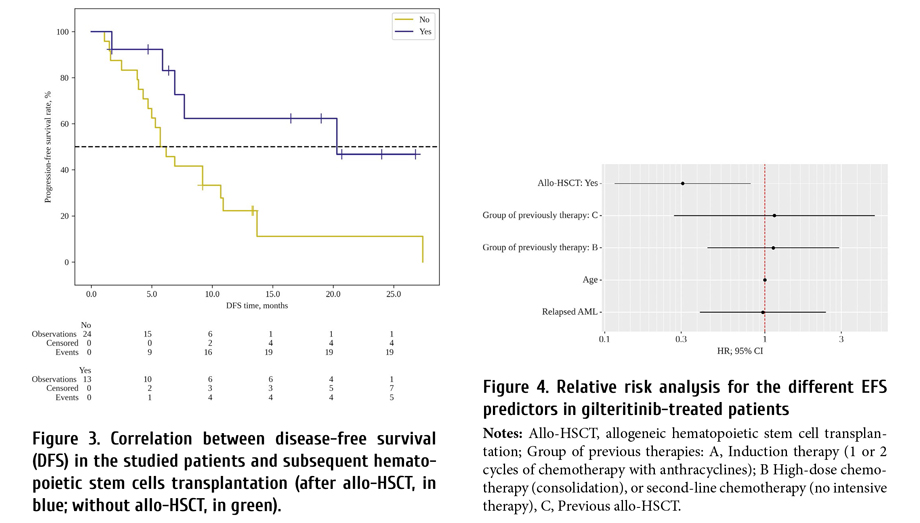
Median survival in cases without allo-HSCT was 5.7 months (95% CI: 4.3-10.7 months), median survival in patients with subsequent allo-HSCT was 20.3 months as seen from Fig. 3 (95% CI: 5.9 > months).
In the multivariate analysis, EFS was significantly associated with successful bridging to allo-HSCT (HR=0.31; CI 95%: 0.12-0.82; p<0.018). Age, status of AML before gilteritinib therapy, previously therapy showed no correlation with EFS, as seen from Fig. 4 and Table 3.
Table 3. Relative risk analysis for the different EFS predictors in the gilteritinib-treated patients
* – association of the outcome value with the predictor value is statistically significant (p < 0.05)
Notes: Allo-HSCT, allogeneic hematopoietic stem cell transplantation; Previous therapies: A, Induction therapy (1 or 2 cycles of chemotherapy with anthracyclines); B, High-dose chemotherapy (consolidation) or second-line chemotherapy (no intensive therapy); C, Previous allo-HSCT.
Toxicity of gilteritinib therapy
Hematological toxicity was the most common complication of the therapy. Among 39 patients, neutropenia (grade 3-4) was observed in 97.4% of cases (38/39); grade 3-4 thrombocytopenia, in 69.3% (27/39), and grade 3-4 anemia was registered in 37.2% (18/35). The median duration of grade 4 neutropenia and thrombocytopenia was 36 (4-425) days and 55 (4-325) days, respectively. Severe hemorrhagic complications occurred in 1 of 48 patients (cerebral hemorrhage, 2.1%). In 29.4% (10/34), we have diagnosed infectious complications. Sepsis/bacteremia developed in 7 of 34 patients, 20.6% (CI 95% 10.4-36.8). Bacterial pneumonia was observed in 3 cases (8.8%, CI 95% 3.1-23).
Non-haematological toxicity of any grade included myalgia or arthralgia (15.2%), dry skin (12.1%), dyspnoea (6.1%), nausea (6.1%), headache (3%), arrhythmia (3%), increased transaminases (3%), and high blood pressure (3%). A prolonged QT syndrome has been reported in one patient. The observed complications after gilteritinib therapy are summarized in Fig. 5.
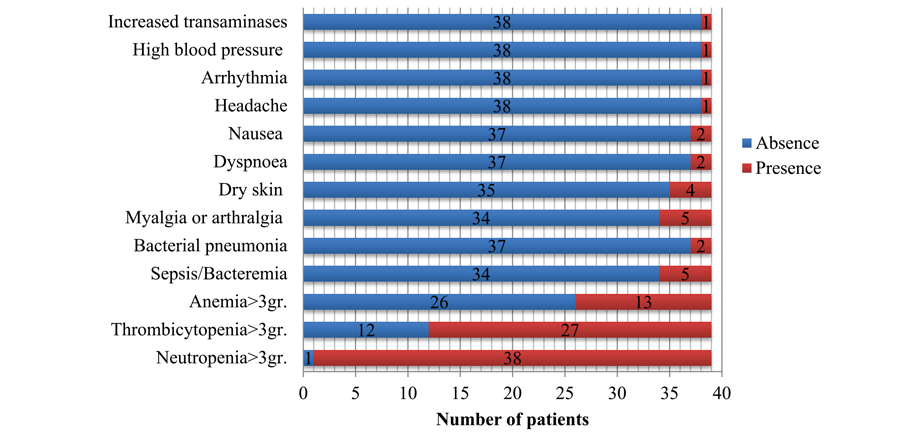
Figure 5. Frequencies of complications observed after gilteritinib treatment
Mortality during treatment (up to 30 days) was 6.3% (CI95% 3.3-19.6), i.e. 3 of 48 patients. The causes of death in 2 patients were infectious complications (including 1 case of COVID-19), and 1 patient died after cerebral hemorrhage.
Discussion
AML with FLT3 mutations is of particular interest thus being quite often considered a separate group for several reasons, e.g.: epidemiological, prognostic, and special therapeutic features options. Mutations in FMS-like tyrosin kinase 3 proteins occur in approximately 30% of patients with AML. ITD is the most common mutation which is found in up to 25% (and in >30% of patients older than 55 years). Approximately 10% of mutations in the FLT3 gene involve point mutations, deletions, and insertions in codons surrounding D835 position within the FLT3 TK domain (FLT3-TKD) [1-8].
AML with FLT3 mutations (primary FLT3-ITD) is associated as subtype of AML with higher relapse rate, shorter remission period, decreased survival, especially when NPM1 is not co-mutated and the allelic FLT3-ITD/wild-type ratio is high [6, 7, 20]. According to some recent studies by Kiyoi et al., Sakaguchi M. et al, which concerned Japanese AML patients, the long-term prognosis in FLT3-ITDlow AML was similar to FLT3-ITDhigh patient group [21, 22].
In a view of the above, mutations in FLT3 can be considered as a promising molecular target for the treatment of patients with FLT3-mutated AML. The first-generation FLT3 inhibitors (sunitinib, tandutinib, and lestaurtinib) applied as monotherapy for AML patients with FLT3 mutation did not demonstrate efficacy and have shown clinically relevant adverse events [6, 21]. However, according to the CALGB 10603 (RATIFY) trial, the addition of midostaurin to induction ChT improved OS by 7.1% after 4 years to 51.4%, with satisfactory toxicity profile of the therapy [9]. Based on the results of this study, midostaurin was approved as a combination agent with standard ("7+3" ChT) induction therapy for newly diagnosed AML by the US Food and Drug Administration (FDA) in 2017. In the RATIFY study, allo-HSCT in first remission was performed in 28.1% (n=101) of patients, and the transplants did not show therapeutic benefit (p=0.85). Observations are still ongoing, but the currently available data show up to 8-11% of relapses even after allo- HSCT in the first remission on the background of FLT3 inhibitor therapy [6]. Of note, the patients in the midostaurin group underwent allo-HSCT earlier than those in the control group. Hence, the timing of allo-HSCT in the first remission may influence the positive outcomes [23].
The outcome in the patients with primary refractory or relapsed AML remains dismal. Retrospective analysis found OS rates from 4% to 38%, being dependent on time of relapse and previous allo-HSCT [3, 24]. Allo-HSCT remains the only option to achieve a long-term favorable outcomes in R/R AML [3, 15, 25]. A retrospective analysis of the 2-year overall survival (OS2) after allo-HSCT in the 2nd complete remission demonstrated promising 50% OS, compared to allo-HSCT in active disease (first relapsed or RefAML) where OS2 was around 20% [13, 14]. Multivariate analysis in these studies identified allo-HSCT in a first CR as the single favorable prognostic factor (RFS, p=0.001; OS, p=0.001). Sakaguchi M. et al. also found that prognosis was unfavorable in NPM1mut AML with FLT3-ITDlow in patients, who have not received allo-HSCT in first CR [22].
Intensive ChT may improve outcomes for R/R AML by increasing remission rates to 40-60%, but it is associated with considerable toxicity [16, 17, 18]. FLT3 inhibitors demonstrate encouraging results in AML patients with FLT3 mutations [3, 19, 24]. Based on the results of the ADMIRAL study, the FDA approved gilteritinib for R/R FLT3-mutated AML therapy in adult patients in 2018 [7]. This study demonstrated a favorable response rate (ORR was achieved in 67.2%) and improved OS compared with ChT (median OS 9.3 vs 5.6 months) [4].
Treatment-related adverse events of grade ≥ 3 were reported in 60.2% of patients. The most common were, e.g., anemia (40.7%), febrile neutropenia (45.9%) and thrombocytopenia (22.8%). Increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were observed in 13.8% and 14.6% of patients, respectively. Dose reduction or interruption of the therapy due to a toxicity were required in 6% and 29%, respectively [4].
Despite favorable response rate and lower toxicity of therapy compared to salvage ChT, only 25.5% (63 of 247 patients) of gilteritinib-treated patients underwent allo-HSCT in the ADMIRAL study [7]. In our study, this rate was slightly higher and reached 29.2%. The main reason for refusal of allo-HSCT was older age of patients (in the study ADMIRAL the median age of patients ware 62 years, in our study, 53 years with 18 patients over 65 years). Considering the importance of early allo-HSCT option, finding a relative donor for patients over the age of 65 is a challenging task. In our study, allo-HSCT was performed from haploidentical donors in 5 cases and from unrelated donors, in 9 cases, with median time of 45 days from OR after gilteritinib to HSCT.
According to the data of ADMIRAL trial, the median event-free survival was 2.8 months in the gilteritinib group. In our study, subsequent transplantation was an independent factor improving the outcome of leukemia. An extremely short window for transplantation after remission might be the key issue for favorable outcome.
Conclusion
Gilteritinib in patients with R/R FLT3-mutated AML demonstrated favorable outcomes with a satisfactory tolerance to the therapy. Patients who subsequently underwent allo-HSCT had statistically significantly better OS, DFS and EFS. Thus, gilteritinib can be used as a bridge to allo-HSCT in adult patients with R/R FLT3-mutated AML.
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
All human studies have been approved by the appropriate University Ethics Committee and have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Savchenko VG. ed. Programmed treatment of diseases of the blood system. Moscow.: Praktika; 2012: 155-245 (In Russian).
- Savchenko VG, Parovichnikova EN, Afanasiev BV, Gritsayev SV, Semochkin SV, Bondarenko SN, et al. National clinical guidelines for diagnosis and treatment of adult acute myeloid leukemia. Hematol and Transfusiol. 2014; 59(1-S2):2-29. (In Russian).
- Heuser M, Ofran Y, Boissel N, Brunet Mauri S, Craddock C, Janssen J, Wierzbowska A, Buske C; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020 Jun;31(6):697-712. doi: 10.1016/j.annonc.2020.02.018
- Kang C, Blair HA. Gilteritinib: A Review in Relapsed or Refractory FLT3-Mutated Acute Myeloid Leukaemia. Target Oncol. 2020;15(5):681-689. doi: 10.1007/s11523-020-00749-3
- Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019; 33(2):299-312. doi: 10.1038/s41375-018-0357-9
- Medeiros BC, Chan SM, Daver NG, Jonas BA, Pollyea DA. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia. Am J Hematol. 2019; 94(7):803-811. doi: 10.1002/ajh.25484
- National Comprehensive Cancer Network. Bone Cancer (Version 2.2022).
https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf - Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T. et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129(4):424-447. doi: 10.1182/blood-2016-08-733196
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017; 377(5):454-464. doi: 10.1056/NEJMoa1614359
- Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31(27):3360-3368. doi: 10.1200/JCO.2012.47.4874
- Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol. 2017; 18(3):17.
doi: 10.1007/s11864-017-0456-2 - Ferguson P, Craddock C. Allogeneic transplantation in primary refractory AML. Bone Marrow Transplant. 2017; 52(7):950-951.
doi: 10.1038/bmt.2017.61 - Weisdorf DJ, Millard HR, Horowitz MM, Hyare PS, Champlin R, Ho V, Mielcarek M, et al. Allogeneic transplantation for advanced acute myeloid leukemia: The value of complete remission. Cancer. 2017; 123(11):2025-2034. doi: 10.1002/cncr.30536
- Loke J, Buka R, Craddock C. Allogeneic stem cell transplantation for acute myeloid leukemia: who, when, and how? Front Immunol. 2021; 12:659595. doi: 10.3389/fimmu.2021.659595
- Schlenk RF, Döhner K, Mack S, Stoppel M, Király F, Götze K, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010; 28(30):4642-4648. doi: 10.1200/JCO.2010.28.6856
- Westhus J, Noppeney R, Dührsen U, Hanoun M. FLAG salvage therapy combined with idarubicin in relapsed/refractory acute myeloid leukemia. Leuk Lymphoma. 2019; 60(4):1014-1022. doi: 10.1080/10428194.2018.1508670
- Tenold ME, Moskoff BN, Krishnan R, Rosenberg AS, Hoeg RT, Abedi M, et al. Retrospective analysis of adult patients with relapsed/refractory acute myeloid leukemia treated with FLAG at a comprehensive cancer center. Clin Lymphoma Myeloma Leuk. 2021; 21(7):e611-e618. doi: 10.1016/j.clml.2021.02.007
- Montillo M, Mirto S, Petti MC, Latagliata R, Magrin S, Pinto A, et al. Fludarabine, cytarabine, and G-CSF (FLAG) for the treatment of poor risk acute myeloid leukemia. Am J Hematol. 1998; 58(2):105-109. doi: 10.1002/(sici)1096-8652(199806)58:2<105::aid-ajh3>3.0.co;2-w
- Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019; 381(18):1728-1740. doi: 10.1056/NEJMoa1902688
- Bertoli S, Dumas PY, Bérard E, Largeaud L, Bidet A, Delabesse E, et al. Outcome of relapsed or refractory FLT3-mutated acute myeloid leukemia before second-generation FLT3 tyrosine kinase inhibitors: A Toulouse-Bordeaux DATAML Registry Study. Cancers (Basel). 2020; 25;12(4):773. doi: 10.3390/cancers12040773
- Kiyoi H, Kawashima N, Ishikawa Y. FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci. 2020; 111(2):312-322. doi: 10.1111/cas.14274
- Sakaguchi M, Yamaguchi H, Najima Y, Usuki K, Ueki T, Oh I, Mori S, et al. Prognostic impact of low allelic ratio FLT3-ITD and NPM1 mutation in acute myeloid leukemia. Blood Adv. 2018; 2(20): 2744-2754. doi: 10.1182/bloodadvances.2018020305
- Keiffer G, Aderhold KL, Palmisiano ND. Upfront treatment of FLT3-mutated AML: A look back at the RATIFY trial and beyond. Front Oncol. 2020; 10:562219. doi: 10.3389/fonc.2020.562219
- Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019; 20(7):984-997. doi: 10.1016/S1470-2045(19)30150-0
- Heinicke T, Labopin M, Schmid C, Polge E, Socié G, Blaise D, et al. Reduced relapse incidence with FLAMSA-RIC compared with busulfan/fludarabine for acute myelogenous leukemia patients in first or second complete remission: A sudy from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018; 24(11):2224-2232. doi: 10.1016/j.bbmt.2018.07.007
Accepted 28 October 2022


