Subcutaneous administration of doxorubicin delivery systems based on CaCO3 vaterites coated with dextran sulfate
Natalia N. Sudareva1,2, Olga М. Suvorova1, Konstantin A. Kolbe1, Dmitry N. Suslov3, Oleg V. Galibin2, Alexander D. Vilesov1, Galina Y. Yukina2, Elena G. Sukhorukova2
1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 Granov Russian Research Center for Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Natalia N. Sudareva, Institute of Macromolecular
Compounds RAS, St. Petersburg, Russia
E-mail: nnsas@mail.ru
Citation: Sudareva NN, Suvorova OM, Kolbe KA et al. Subcutaneous administration of doxorubicin delivery systems based on CaCO3 vaterites coated with dextran sulfate. Cell Ther Transplant 2022; 11(3-4): 87-92.
Accepted 28 October 2022
Summary
Subcutaneous administration of drug delivery systems containing antitumor drug doxorubicin (DOX) was studied in laboratory rats. The drug delivery systems consisted of СаСО3 vaterites coated with the dextran sulfate polyanion; these particles have been used earlier for intraperitoneal administration of DOX. Time profile of DOX release into blood plasma of rats after subcutaneous administration of the delivery systems loaded with 4 mg of DOX was studied by high-performance liquid chromatography (HPLC). The working concentration of DOX in blood was maintained for 10 days after subcutaneous administration, which is comparable with the results obtained after intraperitoneal introduction of similar DOX carriers. To estimate toxicity of the used DOX delivery systems, histological studies of different rat organs (liver, intestines, lungs) were performed at 17 and 23 days after beginning of the experiment. Histological analysis of the material revealed morphological changes in rat liver and lungs, while the morphological pattern of intestines remained unchanged. No changes in animal behavior (including their feeding behavior) were observed.
Keywords
Doxorubicin, drug delivery system, calcium carbonate, dextran sulfate, blood plasma, subcutaneous administration.
Introduction
Doxorubicin (DOX) is a common potent antibiotic and anticancer agent. Virtually all highly efficient antibiotics exhibit some degree of toxicity; particularly, DOX demonstrates the dose-dependent toxicity [1]. To reduce toxicity and provide prolonged release of the preparation into blood, delivery systems of various structures are used [2]. Porous СаСО3 vaterites were proposed as delivery systems for biologically active compounds as early as 2004 [3]. The vaterite carriers have been modified with polymers (alginate and sodium dextran sulfate, DexS [4, 5]) with the objective of stabilizing vaterites in active media of an organism and reducing their size [6, 7]. This modification made it possible to increase bioavailability of carbonate cores and to use these carriers for delivery of various biologically active compounds [8-10].
Distribution of a drug preparation within organs and systems depends not only on its structure, but also on the used administration method [11, 12]. Recently, the trends are developed towards use of less invasive routes of drug administration, which reduce the possibility of entry of non-sterile components into blood. In our study, along with in vivo intraperitoneal administration [13], attention is given to subcutaneous introduction of the DOX delivery systems. Creation of a depot that will provide prolonged release of certain doses of the medicinal compound and maintain relatively low concentration of the drug in blood (thus preventing toxic effects) can increase DOX therapeutic efficiency. The micron-sized drug carriers are more suitable for this purpose; unlike nano-sized carriers, they do not enter bloodstream and only provide prolonged drug release. The duration of drug release also depends on the carrier. For instance, release of DOX from the carriers 3 µm in size (СаСО3 doped with carboxymethyl chitosan) proceeds longer than from the nano-sized carriers (650 nm) of the same composition. In the first case (micron-sized particles), 60% of the administered DOX was released from the carriers in 10 days, whereas, in the second case (nano-sized particles), 90% of the drug was released in 6 days [14]. In the present work, the micron-sized СаСО3 vaterites were used to create a depot.
The aim of this work was to investigate behavior of DOX administered subcutaneously (4 mg) to a healthy animal; the drug was loaded into the delivery systems based on СаСО3 vaterites modified with DexS. The experimental tasks included establishing time profiles of DOX concentration in rat blood; revealing toxic action of the preparation on particular organs by morphological analysis; confirmation of the absence of cytotoxicity of the studied delivery systems (without DOX) as tested with MCF7 tumor cells.
Materials and methods
Reagents
Doxorubicin hydrochloride in the form of pharmaceutical preparation "Sindroxocin", which contains 17% of doxorubicin (DOX) and 83% of lactose, was purchased from Actavis (Hafnarfjordur, Iceland). In the experiments, doxorubicin salt with protonated amino group (–NH3+) was used. Salts (CaCl2 × 2H2O, Na2CO3), acetone, and dextran sulfate (Mw = 9-20 kDa) were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis of carbonate cores
The synthesis of porous СаСО3 vaterites was performed according to the modified technique [3] described in [13]. Co-precipitation of 1 M solutions of CaCl2 × 2H2O and Na2CO3 was carried out at vigorous stirring for 30 s; then the suspension was centrifuged, the precipitate was rinsed and dried.
Doping of carbonate cores with DexS and loading DOX into delivery systems
Coating of carbonate cores with dextran sulfate polyanions was performed as follows: the suspension of СаСО3 vaterites (С=10 mg/mL) in aqueous solution of the polymer (С=5 mg/mL) was stirred for 1 h, then centrifuged; the precipitate was washed and dried [15].
Loading of DOX into the doped CaCO3 cores proceeded in the mixture of CaCO3 suspension and DOX solution (C=2 mg/mL) at continuous stirring for 24 hours. The DOX/(CaCO3+DexS) ratio was 0.4. After mixing, the suspension was centrifuged at 8000 rpm for 3 min, and the DOX amount in supernatant was determined. The DOX load (L) was calculated using the following equation:
L = (mi − ms)/mP
where mi is the initial weight of DOX (mg), ms is the weight of non-encapsulated DOX in supernatant solution (mg), mP is the weight of particles (mg). DOX concentrations were determined using the calibration curves obtained from optical density measurements in the corresponding solvents at λ = 480 nm. The DOX load (L) varied from 230 to 250 µg/mg.
Animal experiments
Three healthy female outbred rats with body weight ranging from 256 to 300 g ("Rappolovo" nursery for laboratory animals) were used in the experiments with subcutaneous (s.c.) administration of DOX delivery systems. All manipulations with animals were performed under general anesthesia: Sol. Zoletil 50 (0.05 mL per 0.1 kg of body mass), Sol. Rometаrum 20 mg/mL (0.0125 mL per 0.1 kg of body mass) intramuscularly. The animals were caged and given free access to water and food. They were fed the standard diet for laboratory rats used in the vivarium of Granov Russian Research Center for Radiology and Surgical Technologies, St. Petersburg, Russia (4R F18 prolonged keeping formula for rodents, Macedonia, Italy).
The animals were examined daily; consumption of water and food was registered, body temperature and weight were measured. Behavior of animals and life span were estimated. All manipulations with animals were performed in accordance with State Standard 33216-2014 "Regulations for work with laboratory rodents and rabbits".
Administration of encapsulated DOX into rats
DOX encapsulated into calcium carbonate cores doped with the dextran sulfate polyanion was administered subcutaneously (s.c.) to the rat's nape. The procedure was carried out under anesthesia. The drug (DOX in CаСО3+DexS cores, 4 mg of DOX per 1 animal) was injected in 1.5 mL of 5% glucose solution. The preparations were injected with the use of 21-gauge needles.
Along with visual inspection, peripheral blood samples (1.0 mL) were taken from the rat tail vein on the 1st, 4th, 7th, 14th, 17th and 21st days after s.c. drug injection.
Before blood sampling, the animal was examined and weighed; its body temperature was measured, then it was anesthetized and fixed in a holder for immobilizing rodents. Plasma was obtained from the blood specimens 10 min after blood sampling by centrifugation for 15 min at 1500 rpm. The supernatants were frozen and stored in closed vessels at -40°C for further analysis.
Determination of DOX content in rat blood plasma
Content of doxorubicin (DOX) in rat blood plasma was determined by high performance liquid chromatography (HPLC) with the aid of a Prominence-I LC 2030C 3D Plus instrument (Shimadzu) equipped with an RF-20A fluorimetric detector and a 5 µm Luna C18 column (Phenomenex). The excitation wavelength was 475 nm, the emission wavelength was 555 nm. The analysis was performed in the gradient elution regime (with acetonitrile) in 0.01 N sodium formiate buffer (рН 3.68). The experiment duration was 20 min; the detection limit was 1 ng/mL. All the measurements were carried out thrice.
Morphological studies
Prior to histological studies, the material was fixated in 10% neutral formalin in phosphate buffer (рН = 7.4) for not less than 24 hrs, dehydrated using a series of ethanol solutions with increasing concentrations, and enclosed in paraffin blocks according to the standard histological technique. To obtain comparable results, the samples were treated simultaneously under similar conditions. The paraffin cuts (5 μm thick) were prepared with the use of an Accu-Cut SRT 200 microtome (Sakura, Japan) and stained with Mayer hematoxylin and eosin (BioVitrum, Russia). Microscopic analysis was performed using a Nikon Eclipse E200 light microscope (Nikon, Japan) with a 10× ocular and 4, 10, 20, and 40× objectives. Digital images were recorded with a Nikon DS-Fi3 camera (Nikon, Japan).
Cytological experiments
Before contact with cells, the delivery systems were sterilized for 40 min at 120°С. Proliferation dynamics of MCF7 cells (breast adenocarcinoma) was studied with the use of an RTCA iCELLIgence System cell analyzer (ACEA Biosciences, USA) that enables to determine the real-time state of the cell cultures without additional coloring. Cell growth was monitored by changing the impedance created by cells in the wells equipped with gold electrodes upon contact of the cells with different types of DS. The adhesion and proliferation of cells on the surface of the electrodes increase the medium impedance and are recorded as a cellular index (CI): the ratio of the impedance at a particular time to the initial value of the impedance. When there are no cells, the value of CI is equal to the background value (about zero). The CI values increase as the cells attach to the electrodes.
The cells were incubated in DMEM nutrient medium (Pane-co, Russia). Cultivation was carried out in an incubator (Thermo Fisher Scientific, USA) at 37°С and at increased humidity; the CO2 concentration was 5%. 35 000 MCF7 cells were placed in each well containing the nutrient medium (0.2 mL). In 24 h, DexS, empty DS (CaCO3+DexS) and the DS loaded with DOX were added to the wells. As a result, the time dependencies of the CI of the control and cells with the addition of DS averaged over three wells. The graphs were used to evaluate the dynamics of adhesion, cell proliferation, and the beginning of the stationary phase of cell growth.
Statistical evaluations of CI were carried out by the iCELLIgence System cell analyzer program.
Results and discussion
To form the depot providing prolonged DOX release, the micron-sized СаСО3+DexS vaterites were used. The core size varied from 1 to 3 µm. Subcutaneous administration of the calcium carbonate DOX delivery systems to healthy female rats was performed as follows. DOX (4 mg) in СаСО3+DexS carriers was introduced into three rats. One rat (№1) died in 17 days, rats №2 and №3 were sacrificed on the 23rd day.
Macroscopic description
After administering vaterites to laboratory rats, no changes in behavior, consumption of food and water, state of hair coat were observed. No signs of inflammation at the injection site were revealed.
Autopsy of the rat that died on the 17th day showed sanguine organs. The large intestine (colon, sigmoid colon, upper rectum) was enlarged; the content was semi-liquid. The intestine wall was moderately edematous. Histological analysis of rat liver, one lung, a fragment of large intestine, and a fragment of adipose tissue taken near the injection site of DOX delivery systems was performed.
The large intestine (colon, sigmoid colon, upper rectum) of sacrificed rats (№2 and №3) was not enlarged and contained small amounts of feces. The intestinal wall was edematous. The liver was uniformly colored, lungs were pink. The changes were less pronounced than those in rat №1. Histological analysis of the liver, a lung, and a fragment of large intestine was performed.
Tissue morphology studies
Microscopic study of adipose tissue of rat № 1 (that died on the 17th day) taken from the injection site of delivery systems (СаСО3+DexS) containing DOX revealed bright red calcium carbonate cores surrounded with loose fibrous connective tissue with high amount of adipose cells. The vessels were thick, varicose and plethoric; small extravasates were observed. Endothelium of vessels was swollen; sludges of erythrocytes were visible. Epithelium was absent in an extended segment of intestinal mucosa. The villous stroma (a connective tissue layer) on this part of mucous membrane was strongly infiltrated with macrophages and lymphocytes. Epithelium remained in small parts of intestinal mucosa, mainly in intestinal crypts.
Histological analysis of the material taken from rats №2 and №3 revealed morphological changes in liver and lungs, while the morphology picture of the large intestine remained unchanged. The change in cytoarchitectonics of hepatic lobules (manifested as a disorder in the hepatocyte plates) was observed. Granular dystrophy was observed in the cytoplasm of most hepatocytes; some hepatocytes showed signs of hydropic degeneration. All vessels in the lung were broad, varicose and plethoric which could be, in part, ascribed to potential toxic action of the antitumor preparation. These changes seemed, however, mostly physiologically tolerated, thus, probably, being reversible.
In our previous works, it has been shown [17] that morphological changes in liver occurring upon intramuscular administration of DOX-containing delivery systems were reversible. The experiment that involved observation of morphological changes in the tissues at the implantation site lasted from 3 days to 3 months. At the early stages of experiment, we revealed doxorubicin toxicity toward the surrounding muscle tissue and liver. In the course of time, manifestations of DOX toxicity became less pronounced, and complete bioresorption of the introduced sample occurred within 3 months after beginning of the experiment.
It should be noted that the procedure of implantation of delivery systems causes a muscle tissue trauma, no matter how carefully it is performed. This is why its recovery takes sufficient time. In the case of subcutaneous administration, there is no extensive trauma, which is an additional reason for using this administration route.
DOX concentrations in rat blood plasma after subcutaneous administration of delivery systems
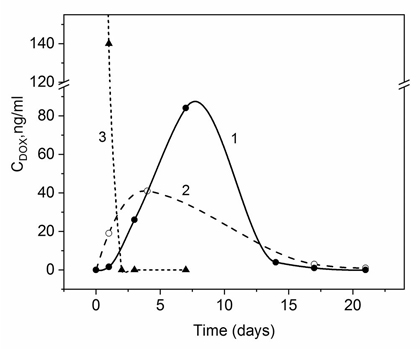
Figure 1. Release profile of DOX into rat blood plasma after intraperitoneal (1, 3) and subcutaneous administration (2) of 4 mg DOX using delivery systems based on CaCO3 vaterites doped by DexS (1, 2) and free DOX (3)
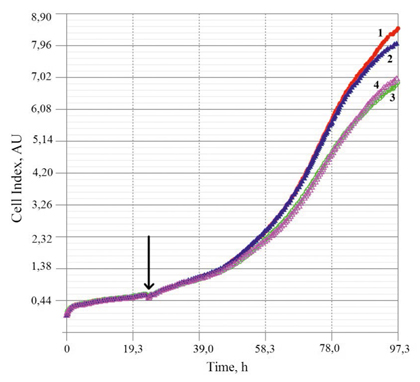
Figure 2. Increase in the cell index value in the course of three days during interaction between MCF7 cells and DS (СаСО3+DexS), their components, and the DS containing DOX (0.05 mg/mL). 1 – control sample; 2 – DS; 3 – DS+DOX; 4 – DexS. The arrow shows the moment when DS and their components were added to cultivated cells (in 24 h after beginning of growth)
Time profiles of DOX concentration in rat blood plasma after subcutaneous administration of the preparation in СаСО3 + DexS delivery systems (Fig. 1, curve 2) were compared with the previously observed concentration profiles [13] during the same period of time after intraperitoneal administration of DOX with the same delivery system DS (curve 1). The amounts of injected DOX were similar in both cases (4 mg per animal). As is seen in Fig. 1, DOX is present in circulating blood after subcutaneous and intraperitoneal administration for at least two weeks, while after i.p. administration of free DOX, the preparation is cleared already on the 3rd day of the experiment (curve 3). The concentration of drug in blood after subcutaneous administration of free DOX was not determined, since such route of DOX injection may cause a severe local inflammation and necrosis of muscle tissue within one week after injection [18]. This fact further confirms efficiency of using these delivery systems for subcutaneous administration.
Dextran sulfate effects on the rat organism and tumor cells
The question now arises of whether DexS included in the delivery systems could cause acute colitis observed in one of the rats (№1). Indeed, DexS is used for inducing colitis in rats and mice, for which purpose DexS is administered orally (510 mg and 150 mg, respectively) daily for a week [16]. In our experiments, the DS containing 4 mg DOX were introduced into rats. When the DOX load was 230-250 µg per 1 mg of DS, which, in turn, contained 20% of DexS (determined by the combustion method from the sulfur content in DS), one dose of DexS introduced into rat organism was equal to 3.3 mg. This is much lower than the amount of the polymer inducing appearance of ulcerative colitis.
The influence of DexS and the DS based on CaCO3+DexS on proliferation of tumor cells was also studied.
Our further studies will involve administration of DOX in the studied DS into rats with breast adenocarcinoma MCF7. These cells were selected for estimation of toxicity of the delivery systems and their components. Fig. 2 presents cell indices of growing MCF7 cells obtained in real time in the presence of delivery systems (drug-free calcium carbonate cores and the carriers containing encapsulated DOX). In addition, profiles of cell proliferation in the presence of DexS (a component of delivery systems) are given (see Fig. 2).
The presented results indicate insignificant toxicity of the DexS polyanion. One may suggest the influence of negatively charged polymer molecules on cell proliferative activity and viability. However, similar amount of DexS included in the СаСО3-based delivery system did not cause toxic effects. Apparently, the excess negative charge (δ-) present on the surface of DexS molecules may be compensated after interaction with calcium carbonate (which carries the excess positive charge (δ+) at рН< 8.5 [3]).
Conclusion
Aiming for design of a depot tool containing anti-cancer drug doxorubicin, we studied the consequences of subcutaneous administration of DOX delivery systems based on porous calcium carbonate vaterites doped with dextran sulfate polyanion. It was demonstrated that this method of DOX administration facilitated prolonged (up to two weeks) presence of the preparation in blood of laboratory rats. The time-concentration profile of DOX in blood after less invasive subcutaneous administration was comparable to that obtained after intraperitoneal injection of the delivery systems containing similar amount of DOX. Subcutaneous administration of DOX delivery systems did not cause local inflammation (unlike introduction of free DOX which resulted in acute inflammation reaction). Histological analysis of rat organs revealed morphological changes in liver and lungs; no changes in the state of intestines were observed. Since the revealed internal changes were tolerated by the rats without noticeable clinical manifestations, there is a good chance for their reversibility.
Hence, subcutaneous administration of the DOX delivery systems based on СаСО3+ DexS can be considered efficient method for formation of minimally invasive depot with prolonged release of this antitumor drug.
Financial support
The study was performed within the framework of budget-supported research project №АААА-А20-120022090044-2, Institute of Macromolecular Compounds, RAS.
Conflict of interests
None declared.
References
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185-229. doi: 10.1124/pr.56.2.6
- Matyszewska D. Drug delivery systems in the transport of doxorubicin. Inst Civil Eng Surface Innovat. 2014;2(4):201-210.
doi: 10.1680/si.13.00040 - Volodkin D, Petrov A, Prevot M, Sukhorukov G. Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir. 2004;20: 3398-3406. doi: 10.1021/la036177z
- Zhao D, Zhuo R, Cheng S. Alginate modified nanostructured calcium carbonate with enhanced delivery efficiency for gene and drug delivery. Mol. BioSystems. 2012; 8: 753-759. doi: 10.1039/C1MB05337J
- Sudareva N, Suvorova O, Tarasenko I, Saprykina N, Smirnova N, Petunov S, et al. Hybrid systems for oral delivery of a therapeutic neuropeptide. Mendeleev Commun. 2020;30: 25-27. doi: 10.1016/j.mencom.2020.01.008
- Svenskaya Y, Parakhonskiy B, Haase A, Atkin V, Lukyanets E, Gorin D et al. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys Chem. 2013;182:11-15. doi: 10.1016/j.bpc.2013.07.006
- Feoktistova N. Vaterite microspheres as a basis for the preparation of multifunctional carriers of biologically active substances. PhD in Chemistry, Moscow State University M. V. Lomonosov, Moscow, 2019 (In Russian).
- Sudareva N, Suvorova O, Saprykina N, Vilesov A, Bel'tiukov P, Petunov S. Alginate-containing systems for oral delivery of superoxide dismutase. Comparison of various configurations and their properties. J Microencapsul. 2016; 33(5): 487-496. doi: 10.1080/02652048.2016.1206146
- Mydin R, Zahidi I, Ishak N, Ghazali N, Moshawih S, Siddiquee S. Potential of calcium carbonate nanoparticles for therapeutic applications. Mal J Med Health Sci. 2018;201-206.
- Sudareva N, Suvorova O, Saprykina N, Smirnova N, Bel'tiukov P, Petunov S et al. Two-level delivery systems based on CaCO3 cores for oral administration of therapeutic peptides. J Microencapsul. 2018;35: 619-634. doi: 10.1080/02652048.2018.1559247
- Adiseshaiah P, Hall J, McNeil S. Nanomaterial standards for efficacy and toxicity assessment. WIREs Nanomed Nanobiotechnol. 2009;2: 99-112. doi: 10.1002/wnan.66
- Ansar F, Latifah S, Kamal W, Khong K, Ng Y, Foong J, et al. Pharmacokinetics and biodistribution of thymoquinone-loaded nanostructured lipid carrier after oral and intravenous administration into rats. Int J Nanomed. 2020;15:7703-7717.
doi: 10.2147/IJN.S262395 - Sudareva N, Suvorova O, Suslov D, Galibin O, Vilesov A. Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats. Cell Ther Transplant. 2021;10(3/4):71-77. doi: 10.18620/ctt-1866-8836-2021-10-3-4-71-77
- Wang J, Chen J, Zong J, Zhao D, Li F, Zhuo R, et al. Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. J Phys Chem C. 2010; 114:18940-18945. doi: 10.1021/jp105906p
- Sudareva N, Suvorova O, Saprykina N, Tomson V, Suslov D, Galibin O, et al. Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma. Cell Ther Transplant. 2021;10(1):79-85.
doi: 10.18620/ctt-1866-8836-2021-10-1-79-85 - Wang J, Zhang C, Guo C, Li X. Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int J Mol Sci. 2019;20:5751. doi: 10.3390/ijms20225751
- Sudareva N, Popryadukhin P, Suvorova O, Yukina G, Sukhorukova E. Morphology of rat muscle tissue after implantation of delivery systems consisting of porous CaCO3 vaterites doped with dextran sulfate and containing doxorubicin. Cell Tissue Biol 2022; 16(4): 392-399. doi: 10.1134/S1990519X22040083
- Oussoren C, Eling W, Crommelin D, Storm G, Zuidema J. The influence of the route of administration and liposome composition on the potential of liposomes to protect tissue against local toxicity of two antitumor drugs. Biochim Biophys Acta. 1998:1369;159-172.
Accepted 28 October 2022


