Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia
Elena V. Morozova, Tatiana A. Rudakova, Julia Ju. Vlasova, Maria V. Barabanshchikova, Tatiana L. Gindina, Alexander L. Alyanskiy, Maria D. Vladovskaya, Ivan S. Moiseev, Ludmila S. Zubarovskaya, Alexander D. Kulagin
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantology, Pavlov University, 6-8 L.Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (911) 927-82-29
E-mail: dr_morozova@mail.ru
Citation: Morozova EV, Rudakova TA, Vlasova JJ, et al. Pre- and post-transplantation factors associated with primary graft failure and severe poor graft function after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Cell Ther Transplant 2022; 11(1): 13-23.
Accepted 18 March 2022
Summary
Allogeneic stem cell transplantation (allo-HSCT) is used worldwide for long-term management and cure of hematological malignancies, still remaining a valuable option for treatment of chronic myeloid leukemia (CML) in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with tyrosine kinase inhibitors (TKIs), and in advanced-phase disease. Along with relapse risk, the unfavorable HSCT results may be associated with primary graft failure (PrGF), or poor graft function (PoGF). Hence, the aim of our study was to assess frequency and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Patients and methods
We performed a retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in the RM Gorbacheva Research Institute at the Pavlov University over 25 years. HSCT was indicated in cases of advanced-phase disease, or TKI resistance/intolerance of CML patients. BCR/ABL transcript levels and additional chromosomal abnormalities were used as laboratory markers of advanced disease. 80 patients (66%) were transplanted in chronic phase (CP); 41 patients (34%) were in acceleration phase (AP), or blast crisis (BC) at the time of HSCT. Matched unrelated donors were used in 65% of the cases; matched related donors, in 28%, and haploidentical donors, in 7% of cases.
Results
Engraftment was documented in 106 (88%) patients. Post-transplant relapses were registered in 31 patients within 15-333 days after HSCT. PrGF was documented in 8 cases (7%). Two patients developed secondary graft failure within two months after initial engraftment, with lethal infectious complications. Severe poor graft function (PoGF) was diagnosed in 11 cases (9%) at cumulative incidence of 10% within 1 year post-transplant. Among various pre-transplant characteristics, age factor, and, especially, presence of additional chromosomal abnormalities (ACA) were associated with cumulative incidence of PrGF and sPGF after HSCT. I.e., PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA (p=0.09). The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF.
Conclusions
Primary graft failure (PrGF) contributes to the non-relapse mortality during the first year after allo-HSCT in CML patients. Emergence of post-transplant relapses was not associated with PrGF and sPGF in CML. Further assessment of risk factors for the graft failure or poor graft function is required in order to improve the results of HSCT technologies.
Keywords
Chronic myeloid leukemia, hematopoietic stem cell transplantation, indications, graft failure, risk factors.
Introduction
Over the past decades, allogeneic stem cell transplantation (allo-HSCT) has been used worldwide as a technology aimed both the long-term management and cure of malignant hematological diseases [1]. Allo-HSCT remains a valuable option for treatment of chronic myeloid leukemia (CML) in era of tyrosine kinase inhibitors (TKI) [2-6]. The transplant-eligible population consists of the CML patients with predicted poor outcome if treated with TKIs alone. Despite the superiority of drug treatment, the development of transplant technology, i.e., usage of reduced intensity conditioning regimens, increased donor availability, led to improvement in the results of allo-HSCT in these patients [7,8]. Thus, transplantation is still a potentially curative therapeutic mode in all fit patients who are unable to achieve a durable complete cytogenetic response after treatment with 2 TKIs, and patients with advanced-phase CML.
Unfavorable results are mostly associated with impaired graft function, which is manifested in the lack of control over the underlying disease and subsequent relapse, as well as in primary graft failure (PrGF) and poor graft function (PoGF) [9-12]. Several risk factors of post-transplant graft failure were revealed, e.g., patient’s age, donor-recipient blood mismatch and CMV infection (13]. Treatment options for poor graft functioning are still limited. In addition to reinfusion of stem cell, some recent studies report, e.g., positive effects of Eltrombopag, a thrombopoietin mimetic [14].
The aim of the study was to assess the incidence and outcome of PrGF and severe poor graft function (sPGF) after allo-HSCT in CML patients.
Materials and methods
Patients and data collection
We carried out retrospective analysis of 121 consecutive patients with CML who received allo-HSCT in R. M. Gorbacheva Research Institute at the Pavlov University between 1995 and 2020. Information on the disease stage at diagnosis, time to allo-HSCT, transplantation procedure, relapse, and treatment following allo-HSCT was gathered via systematic reviews of the patient records. General approaches to evaluation of HSCT patients at our clinic were described elsewhere [15].
Definitions
CML was diagnosed on the basis of clinical and laboratory data, the detection of Philadelphia (Ph) chromosome and/or the chimeric BCR-ABL gene. Disease phase was defined according to the WHO classification [16]. The first chronic phase (CP1) was recognized in the absence of accelerated phase (AP) and/or blast crisis (BC) in the patient’s history, otherwise CP≥2 was registered. Hematological, cytogenetic and molecular response to the treatment prior to allo-HSCT was defined using ELN criteria [17].
Indications for HSCT
Indications for HSCT were as follows: 1) AP/BC at diagnosis or progression to AP/BC; 2) treatment failure in pre-TKI era; 3) treatment failure due to TKI resistance/intolerance; 4) T315I mutation. TKI resistance and TKI intolerance were defined according to ELN criteria [17].
Laboratory studies
For cytogenetic evaluation, conventional synchronized culture was performed for 48 hours with at least 20 metaphases analyzed per a sample (GTG method). Leukemia cell karyotype was evaluated according to International System for Human Cytogenetic Nomenclature (ISCN) [18]. In cases when the standard cytogenetic investigation was not available (i.e., insufficient material), the bone marrow was assessed with fluorescence in situ hybridization (FISH) probes aimed for detection of (9;22) variants (LSI BCR-ABL, Dual Color, Dual Fusion, "Vysis").
Additional chromosomal abnormalities (ACA) were defined as any structural and numerical chromosomal aberrations other than t(9;22)(q34;q11) (detected by cytogenetic or molecular assays for cryptic abnormalities).
Molecular response after allo-HSCT was evaluated according to the National Comprehensive Cancer Network (NCCN) criteria (2021). PCR monitoring of BCR/ABL was carried out according to NCCN Guidelines once in 3 months for 2 years, then once in every 3 to 6 months. The relative BCR-ABL1 expression level was evaluated according to method described by Gabert et al [19]. This technique includes the following stages: 1) total RNA extraction from peripheral blood of patients with CML, 2) reverse transcription with random hexameric primers, 3) real-time PCR with primers and probes specific to р210, р190 control ABL gene sequences.
Assessment of relative expression levels was based on evaluation of BCR-ABL1/ABL1 ratios in the studied cDNA samples. The ABL1 gene was used for normalization of the results. In order to determine copy numbers of the BCR-ABL1 and ABL1 transcripts, and to assess the reaction effectiveness, standard dilution curves were plotted using a plasmid with inserts of known target gene sequences (Invitrogen, USA), at a standard concentration ranges of 102-106 copies/mcl, according to 2020 European LeukemiaNet (ELN) Recommendations [17]. ABL kinase domain mutations were determined by Sanger direct sequencing.
Post-transplant monitoring
Post-transplant engraftment was defined as absolute neutrophil count (ANC) of >0.5×109/L without administration of colony-stimulating factor within 3 days. Primary graft failure (PrGF) was diagnosed in absence of donor cells in recipient’s bone marrow by the day +30. Donor chimerism was checked at the time of myelopoiesis recovery, i.e. ANC> 0.5×109/L, and by the days +30, 60, +100, +200, and in case of any cytopenia, or signs of relapse. Post-transplant relapse was diagnosed in cases of clinical progression to AP/BC, cytogenetic relapse, or molecular relapse defined as two consecutive positive PCR tests, or, at least, 1-log persistent increase of BCR/ABL transcript level.
The criteria for severe poor graft function (sPGF) were as follows: cytopenia in two or more hematopoietic lineages (platelets <20×109/l, ANC <0.5×109/l, hemoglobin <70 g/l) any time after documented engraftment in presence of full or stable mixed donor chimerism >90% without signs of relapse of underlying disease, rejection or acute graft-versus-host disease (GVHD) grade III-IV.
Secondary graft failure was defined as loss of donor hematopoiesis to <5% and/or ANC counts to <0.5×109/L after initial engraftment being not related to relapse, infection, or drug toxicity [20].
Statistical evaluation
Descriptive characteristics of the cohort included number of cases, proportions for discrete factors, medians and range for continuous values. Individual pre-transplant risk for the HSCT patients was evaluated according to Gratwohl [21]. Overall survival (OS) was assessed using the Kaplan-Meier method from the time of allo-HSCT to the date of last contact or the date of death. Death from any cause was considered as an event.
Survival analysis was performed using log-rank test. Relapse and non-relapse mortality (NRM) rates were summarized using cumulative incidence estimates, with NRM as competing risk for relapse, and relapse regarded as competing risk for NRM.
The event-free survival (EFS) was estimated as a period from allo-HSCT until last contact date, death, or any of the following events: any kind of post-transplant relapse, graft-versus-host disease (GVHD) grade III-IV, severe poor graft function, or secondary graft failure. PrGF and sPGF were estimated as a proportion of cases in the total cohort. Cumulative incidence of sPGF was calculated with respect to competing risks (death before day +30, any type of relapse, GVHD grade III-IV).
The differences between groups were assessed using Fisher's exact test, Pearson χ2 test, and Mann-Whitney U-test for categorical and quantitative characteristics respectively, and Gray’s test for cumulative incidences. All the tests were two-sided, and P-values <0.05 were assessed as indicating for significant associations. Statistical analysis was performed using SPSS, IBM Statistics and EZR free statistical environment, version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
General characteristics of the patients and HSCT procedure
A total of 121 patients diagnosed with CML had undergone allo-HSCT. The median patients’ age was 37 years (range: 18-66). Other baseline characteristics for these patients are presented in Table 1.
The median time between CML diagnosis and allo-HSCT was 31 months (4.5-260). A total of 80 (66%) patients were transplanted in chronic phase (CP), the remaining 41 (34%) patients were in the active phase (AP or BC) at the time of HSCT. The median follow-up from allo-HSCT to the time of the last contact was 15 months (0.5-294).
HLA-matched or partially mismatched unrelated donors were used in 78 cases (65%), while matched related donors were employed in 34 cases (28%), and 9 (7%) patients received haploidentical allo-HSCT. The proportion of bone marrow (BM) and peripheral blood stem cells (PBSC) as the graft sources was almost equal: 49% (n=59) versus 51% (n=62).
Conditioning regimen included oral busulfan 8-12 mg/kg or melphalan 140 mg/m2 in combination with fludarabine 180 mg/m2 or cyclophosphamide. GVHD prophylaxis included calcineurin inhibitor (tacrolimus or cyclosporine A) and mycophenolate mofetil (30 mg/kg), or short course of posttransplant metotrexate, with or without antithymocyte globulin (horse, 60 mg/kg, or rabbit preparations, 5 mg/kg on day -3, -2, or high-dose), post-transplant cyclophosphamide (50 mg/kg, day +3 and +4 after allo-HSCT)/Alemtuzumab was used in two cases (Table 2).


Figure 1. Cumulative incidence of poor graft function post-HSCT in CML patients
Survival and relapse rates
Engraftment was documented in 106 (88%) patients, with median time to neutrophil recovery of 22 (8-58) days. Early death with no signs of engraftment before day +30 occurred in two cases. Thirty-one patients developed post-transplant relapse of any type with median time after allo-HSCT of 106 days (range: 15-333), included early CML relapse/progression before day +30 in 5 cases.
PrGF was documented in 8 (7%) cases. Two patients developed secondary graft failure, both in about two months after initial engraftment with lethal outcome due to severe bacterial infection. Severe poor graft function was diagnosed in 11 (9% of engrafted patients) with cumulative incidence of 10% (95% CI, 5-19) within 1 year after allo-HSCT (Fig. 1). Median time from HSCT to sPGF diagnosis was 43 (18-114) days.
The 1-year cumulative incidence rates of relapse and NRM comprised 35% (95% CI, 26-46) and 26% (95% CI, 18-35), respectively (Fig. 2).
A total of 57 (47%) patients died, the 1-year OS was 60% (95% CI, 51-69). Median OS was not reached. One-year EFS was 41% (95% CI, 32-50), median EFS was 271 (95% CI, 96-365) days (Fig. 3).

Figure 2. One-year cumulative incidence of relapses and NRM after allo-HSCT in CML patients

Figure 3. One-year overall and event-free survival after allogeneic HSCT in CML patients
Factors associated with primary graft failure and severe poor graft function
Among various pre-transplant characteristics, the presence of additional chromosomal abnormalities (ACA) was associated with cumulative incidence of PrGF and sPGF after HSCT. Thus, PrGF was 14% in the group with detectable ACA versus 3% in the group without ACA, (p=0.02), whereas incidence of sPGF in patients with ACA was 2% versus 12% in those without ACA, p=0.09. HLA-matched allo-HSCTs were beneficial for engraftment: 96% for HLA-matched transplantations vs 89% for allo-HSCT from HLA-mismatched donors, and 78% for haploidentical donors (p=0.05). Other pre-transplant factors didn’t show any statistical correlation with graft failure syndromes after HSCT (Table 3).
Table 3. Potential risk factors for the graft failure after allo-HSCT in CML patients
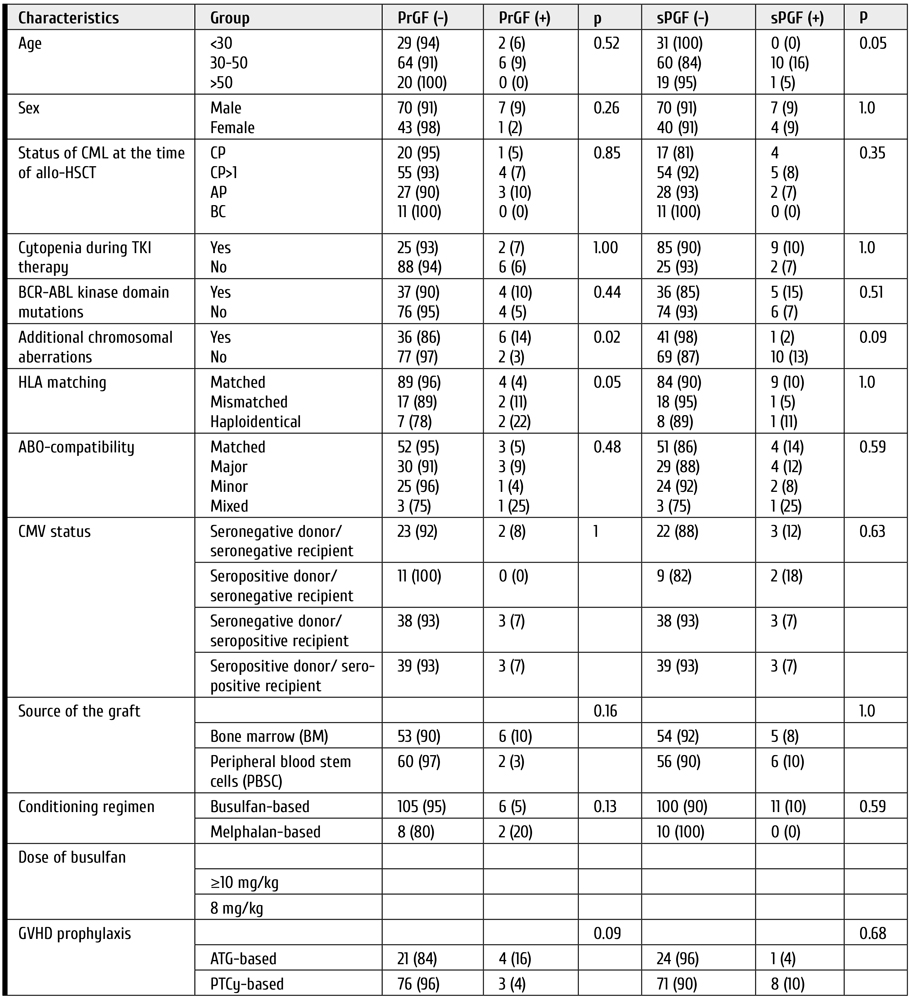
Clinical features and outcomes of graft failure and severe poor graft function
The median follow-up time after allo-HSCT was 68 days (range: 43-1792). All the patients with primary graft failure (PrGF) (n=8) were administered G-CSF, antimicrobial therapy and transfusion support. Two patients received donor lymphocyte infusions without any effect. The second allo-HSCT was performed in 4 cases. A total of 7 patients had lethal outcome (6, of infectious complications; 1, of relapse), whereas one patient is alive after the 2nd allo-HSCT (Fig. 4).

Figure 4. Treatment and outcomes of primary graft failure (PrGF) in CML patients after HSCT
Eleven patients exhibited severe poor graft function (sPGF) within median time of 21 (0-92) days after engraftment. Median length of sPGF was 52 days (range: 14-215). The median time of follow-up after allo-HSCT was 977 days (range: 45-2712).
Early sPGF with criterial cytopenia persisting after engraftment was diagnosed in 4 cases (36%), the remaining patients developed cytopenia after a period of normal graft function. A total of 3 cases of sPGF (27%) developed within 30 days after acute GVHD 2-3 grade (Fig. 5).

Figure 5. Timeline of severe poor graft function (SPGF) in a group of CML patients
All the patients with sPGF received antimicrobial therapy, transfusion support, and G-CSF in case of neutropenia. Other therapeutic options for sPGF therapy were: rituximab (n=4), the second allo-HSCT, or boost stem cell infusion (n=3); eltrombopag (n=1); supportive care (n=5), as seen from Fig. 4. Normal graft function was restored in 8 patients.
A total of 4 patients died. The causes of death were infectious complications (n=3) and late post-transplant relapse (n=1) (Fig. 6).
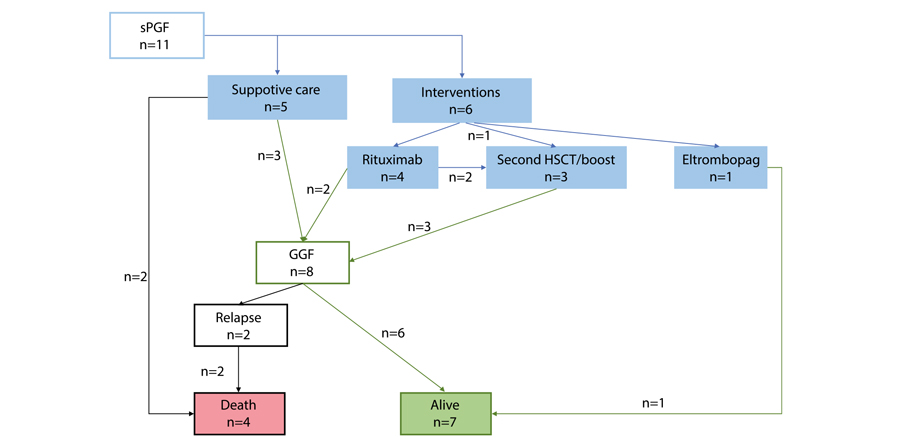
Figure 6. Treatment and outcomes of severe poor graft function (sPGF) in CML patients post-HSCT
Two cases of secondary graft failure occurred in about 3 months after allo-HSCT. Both patients died due to severe infection.
The incidence of post-transplant relapses did not differ in the patients with PrGF and sPGF as compared with those, who were free of these complications. Cumulative incidence of leukemia relapses was 31% (95% CI, 23-42), and 25% (95% CI, 4-87) in the patients with PrGF and engraftment (p=0.97), compared with 22% (95% CI, 3-54) and 32% (95% CI, 24-43) in the patients with sPGF and without sPGF (p=0.52), respectively.
Primary graft failure (PrGF) but not severe poor graft failure (sPGF) significantly increased non-relapse mortality during the first year after allo-HSCT. One-year NRM was 23% (95% CI, 15-32) in engrafted patients versus 71% (95% CI, 39-96) in the patients with PrGF (p<0.0001). Patients with and without sPGF had similar NRM: 20% (95% CI, 5-59) versus 26% (95% CI, 18-36) (p=0.74).
One-year OS was significantly lower in patients with PrGF: 13% (95% CI, 0.7-42) versus 64% (95% CI, 54-72) (p<0.0001) (Fig. 7). On the contrary, sPGF had no statistically significant influence on OS: 73% (95% CI, 37-90) versus 59% (95% CI, 49-69) (p=0.47).

Figure 7. Impact of primary graft failure (PrGF, A), and severe poor graft function (sPGF, B) on overall survival post-HSCT in CML patients
Discussion
In context of TKI therapy progress, the indications for allo-HSCT in CML are becoming more stringent, with respect both to selective TKI choice, relapse diagnostics, and improved transplant technologies [22]. In this regard, it becomes relevant to investigate the causes of allo-HSCT failure and to determine the risk factors for PrGF and sPGF in CML patients. While the factor of post-transplant relapse is discussed in most publications, the issues of PrGF and sPGF remain poorly reflected. Only few authors provided clear definitions and data on the incidence of these complications (mostly PrGF) in the patients with CML. At the same time, most studies of posttransplant graft failure syndromes show that the diagnosis of CML may be among risk factors of this complication. However, most previous studies concerned a heterogeneous range of diagnoses, e.g., acute leukemia, chronic myelo- and lymphoproliferative and non-malignant diseases. To our knowledge, the present work evaluates for the first time the incidence of both PrGF and PGF in a homogeneous cohort of CML patients.
According to our results, PrGF occurred in 7% of cases, thus being higher than in patients with acute leukemia as confirmed by other publications [10]. On the contrary, cumulative incidence of sPGF during the first year after allo-HSCT was 10%. This level is less than in general population of patients after allo-HSCT [15]. A prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT also showed increased rate of graft dysfunction in CML patients after allo-HSCT. Impact of pre-transplant treatment with tyrosine kinase inhibitors of second generation on the allograft function due to myelotoxicity is still under discussion [23]. Presumably, the similar factors may contribute to the development of both PrGF and sPoGF.
We analyzed the data associated with characteristics of patients, donors, and the HSCT procedure. Due to small number of cases in the target groups, only univariate analysis was performed. In contrast to many studies, conditioning regimen, the source and cellularity of the graft, CMV status of the donor and the patient, ABO incompatibility did not show any statistical significance of the disease status, although it is proved an important characteristic for the prognosis of primary graft failure and poor graft function [10, 11, 24, 25, 26, 27, 28, 29, 30].
Nevertheless, it was the presence of ACA that showed statistical significance for PrGF. This may suggest insufficient control of the underlying disease to be among the main causes of any type of graft dysfunction. However, no association between post-transplant relapse and sPGF was noted in our study. The disease recurrence after resolution of graft failure remains an important cause of treatment failure. Contribution of the underlying disease to development of PrGF and sPGF needs to be investigated in future.
HLA incompatibility was another factor for PrGF in univariate analysis. The importance of this characteristic for HSC engraftment is well known [13, 15, 30]. Haploidentical HSCTs in this analysis showed larger proportion of PrGF and sPGF, but this result needs further proofs, as our group was small and mostly retrospective.
The question still exists if the intensity of conditioning regimen may contribute to insufficient hematopoietic reconstitution after allo-HSCT. Impairment of bone marrow microenvironment exposed to high doses of alkylating agents may be one of the possible pathogenic pathways [13, 31]. Nevertheless, our study did not show significant influence of conditioning intensity upon the clinical outcomes.
Natural history of the patients who developed graft failure and poor graft function was of particular interest in this retrospective study. Despite various interventions, primary graft failure is still associated with poor outcomes and death, mostly, due to infectious complications.
Survival and NRM analysis showed that, despite the rare occurrence, PrGF and sPGF are life-threatening and resource-consuming problems. Both PrGF and sPGF need aggressive approach in order to improve outcomes of allo- HSCT. Intensive interventions might be a rescue for, at least, a part of the patients and lead to prolonged survival. Stimulation of residual HSCs by TPO agonists using in the setting of persistent cytopenia after HSCT by several groups might be a promising strategy, although influence of TPO agonists on the leukemic stem cells and risk of relapse is debated. Early employment of a CD34+-selected stem cell boost, or a second allogeneic HSCT to restore an effective haematopoiesis might also be a life-saving option. Identification of patients at high risk for these complications and development strategies for early intervention might be in scope of further investigation.
Conclusion
Both PrGF and sPGF are significant life-threatening problems in allo-HSCT. Specifically, PrGF but not severe poor graft failure (sPGF) significantly increased non-relapse mortality during the first year after allo-HSCT. Meanwhile, the incidence of post-transplant relapses did not differ in the CML patients exhibiting primary graft failure or severe poor graft function. Identification of risk factors for these complications can improve the results of this treatment, by planning HSCT technology, to minimize them and modify approaches to post-transplant therapy.
Conflict of interest
None declared.
References
- Afanasyev BV, Zubarovskaya LS. Role of hematopoietic stem cell transplantation in therapy of adult patients with acute leukemias. Oncohematology. 2006;1(1-2): 70-85. (In Russian).
- Lübking A, Dreimane A, Sandin F et al. Allogeneic stem cell transplantation for chronic myeloid leukemia in the TKI era: population-based data from the Swedish CML registry. Bone Marrow Transplant. 2019;54, 1764-1774. doi: 10.1038/s41409-019-0513-5
- Barrett AJ, Ito S. The role of stem cell transplantation for chronic myelogenous leukemia in the 21st century. Blood. 2015;125(21):3230-3235. doi: 10.1182/blood-2014-10-567784
- Lyubimova LS, Kuzmina LA, E.S.Urnova ES, Zhelnova ES, Anukhina MV, Mendeleyeva LP, et al. Early HLA-identical bone marrow transplantation during chronic myeloid leukemia chronic phase vs. long-term tyrosine kinase inhibitor therapy? Gematologyia i Transfusiologiya. 2012; 57 (3): 6-10. (In Russian).
- Morozova EV, Vlasova YI, Barabanshikova MV, Afanaseva KS, Iurovskaia KS, Gindina TL, Barchatov IM, Alyanskiy AL, Bakin EA, Bondarenko SN, Moiseev IS, Zubarovskaya LS, Afanasyev BV. Allogeneic haematopoietic stem cell transplantation with reduced-intensity conditioning in chronic myeloid leukaemia. Russian Journal of Hematology and Transfusiology. 2020; 65(4): 386-402. (In Russian.). doi: 10.35754/0234-5730-2020-65-4-386-402
- Boehm A, Walcherberger B, Sperr WR, Woehrer S, Dieckmann K, Rosenmayr A, et al. Improved outcome in patients with chronic myelogenous leukemia after allogeneic hematopoietic stem cell transplantation over the past 25 years: A single-center experience. Biol Blood Marrow Transplant. 2011; 17: 133-140. doi: 10.1016/j.bbmt.2010.06.019
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008; 112 (12): 4371-4383.
doi: 10.1182/blood-2008-03-077974 - Klyuchnikov E, Kröger N, Brummendorf TH et al. Current status and perspectives of tyrosine kinase inhibitor treatment in the posttransplant period in patients with chronic myelogenous leukemia (CML). Biol Blood Marrow Transplant. 2010; 16 (3): 301-310.
doi: 10.1016/j.bbmt.2009.08.019 - Bonifacio M, Stagno F, Scaffidi L, Krampera M, Raimondo FD. Management of chronic myeloid leukemia in advanced phase. Front Oncol. 2019; Oct 25; 9:1132. doi: 10.3389/fonc.2019.01132
- Olsson RF, Logan BR, Chaudhury S, Zhu X, Akpek G, Bolwell BJ, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015; 29(8), 1754-1762. doi: 10.1038/leu.2015.75
- Olsson RF, Berggren DM, Ringden O, Mattsson J, Remberger M. Graft failure in reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Blood. 2013; 122 (21): 4559. doi: 10.1182/blood.V122.21.4559.4559
- Locatelli F, Lucarelli B, Merli P. Current and future approaches to treat graft failure after allogeneic hematopoietic stem cell transplantation. Expert Opinion Pharmacother. 2014;15(1): 23-36. doi: 10.1517/14656566.2014.852537
- Xiao Y, Song J, Jiang Z, Li Y, Gao Y, Xu W, Lu Z, Wang Y, Xiao H. Risk-factor analysis of poor graft function after allogeneic hematopoietic stem cell transplantation. Int J Med Sci. 2014; 11(6):652-657. doi: 10.7150/ijms.6337
- Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, Trastulli F, Vitiello S, Cardano F, Pane F, Risitano AM. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019; 54(8):1346-1353. doi: 10.1038/s41409-019-0442-3
- Rudakova TA, Kulagin AD, Klimova OU, Golubovskaya IK, Darskaya EI, Bykova TA, Smirnova AG, Morozova EV, Bondarenko SN, Moiseev IS, et al. Severe poor graft function after allogeneic hematopoietic stem cell transplantation in adult patients: Incidence, risk factors, and outcomes. Clinical Oncohematology. 2019;12(3):309-318 (In Russ). doi: 10.21320/2500-2139-2019-12-3-309-318
- Turkina AG, Zaritsky AYu, Chelysheva EYu. et al. Clinical recommendations for the diagnosis and treatment of chronic myelogenous leukemia. Clinical Oncohematology. 2017;10(3):294-316. (In Russian). doi: 10.21320/2500-2139-2017-10-3-294-316
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
doi: 10.1182/blood-2013-05-501569 - McGowan-Jordan J, Simons A, Schmid M. International Standing Committee on Human Cytogenomic Nomenclature, 2016.
doi: 10.1159/isbn.978-3-318-06861-0 - Gabert J, Beillard E, van der Velden V., et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia- A Europe Against Cancer Program. Leukemia. 2003.17: 2318-2357. doi: 10.1038/sj.leu.2403135
- Valcárcel D, Sureda A. Graft Failure. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. 7th ed. Cham (CH): Springer; 2019. Chapter 41. PMID: 32091792. https://www.ncbi.nlm.nih.gov/books/NBK553942/. doi: 10.1007/978-3-030-02278-5
- Gratwohl A. The EBMT risk score. Bone Marrow Transplantation. 2012;47:749-56. doi: 10.1038/bmt.2011.110
- Craddock CF. We do still transplant CML, don’t we? Hematology Am Soc Hematol Educ Program. 2018 (1): 177-184.
https://doi.org/10.1182/asheducation-2018.1.177 - Masouridi-Levrat S, Olavarria E, Iacobelli S, Aljurf M, Morozova E, Niittyvuopio R, Sengeloev H, Reményi P, Helbig G, Browne P, et al. Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: a prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2022; 57(1):23-30. doi: 10.1038/s41409-021-01472-x
- Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, Ringden O. Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013 Apr; 48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. doi: 10.1038/bmt.2012.239
- Moiseev IS, Morozova EV, Vlasova YY, et al. Interim results of the randomized trial of post-transplanation cyclophosphamide versus rabbit ATG for graft-versus-host disease prophylaxis in chronic myeloproliferative neoplasms and myelodysplastic syndrome. Cell Ther Transplant. 2016; 5(3):62-63. doi: 10.18620/ctt-1866-8836-2016-5-3-49-50
- Hallemeier C. Letter to the editor. Re: High rate of graft failure in 25 patients with chronic myelogenous leukemia conditioned with a reduced-intensity regimen of 550 cGy total body irradiation and cyclophosphamide for unrelated donor transplantation. Biol Blood Marrow Transplant. 2004; 10:726-727. doi: 10.1016/j.bbmt.2004.07.001
- Khoury H, Adkins, Brown RH, Pence, Vij R, Goodnough LT, et al. Low incidence of transplantation-related acute complications in patients with chronic myeloid leukemia undergoing aiiogeneic stem cell transplantation with a low-dose (550 cGy) total body irradiation conditioning regimen. Biol Blood Marrow Transplant. 2001; 7(6):352-358. doi: 10.1016/S1083-8791(01)80006-9
- Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2012; 47, 810-816. doi: 10.1038/bmt.2011.194
- Radujkovic A, Dietrich S, Blok HJ, Nagler A, Ayuk F, Finke J, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: A retrospective study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2019; 25(10): 2008-2016. doi: 10.1016/j.bbmt.2019.06.028
- Kuzmich YV, Alyanskiy AI, Ivanova NY, Vitrischak AA, Vladovskaya MD, Morozova YV, Bondarenko SN, Semenova YV, Zubarovskaya LS, Afanasyev BV. Analysis of the results of allogeneic hematopoietic stem cell transplantation depending on HLA matching of the unrelated donor/recipient pair. Oncohematology. 2014; 9(3):25-31. (In Russian). doi: 10.17650/1818-8346-2014-9-3-25-31
- Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014; 20(9): 1440-1443. doi: 10.1016/j.bbmt.2014.05.016
Accepted 18 March 2022


