Introduction
There are many new therapies approved to treat acute myeloid leukaemia (AML) including new conventional and targeted drugs and immune therapy. A summary of new AML drugs is displayed in Figure 1 increasing from one approval every 6 years to one approval every 150 days, a 12-fold increase.
The question I consider is whether these new therapies will cure AML. My discussion is divided into 3 categories: (1) conventional drugs; (2) targeted therapies; and (3) immune therapy.

Figure 1. Drug approvals in AML 1973-2020
New drugs
I consider 4 new drugs: (a) venetoclax [1]; (b) CPX-351 [2]; (c) CC-486 [3]; and (d) glasdegib (± low-dose cytarabine) [1-4]. Results of these, quite recent trials demonstrate that, although each new drug, alone or combined with previously-approved drugs improved outcomes, there remains a high rate of failures by 2 years.
Targeted drugs
Four targeted drugs are approved in AML including: (1) midostaurin; (2) gilteritinib; (3) enasidenib, and (4) ivosidenib. Results of recent trials of these 3 drugs are published, and the survival curves can be compared [5-8]. Except for enasidenib, these drugs improve outcomes but 2-year failure rates are high. A US trial Beat AML in persons with newly-diagnosed AML assigned subjects with druggable mutations to targeted or conventional drugs. There was no important difference in outcomes [9]. Therefore, according to recent estimates, current targeted drugs are likely to help only a limited subgroup (ca. 10 percent) of patients with acute myeloid leukemia [10].
Immune therapy
Gemtuzumab, an anti-CD33 monoclonal toxin-linked antibody, is the only approved immune therapy of AML [11]. It modestly improves outcomes and is rarely used.
Table 1. Differential effects of azacitidine and venetoclax in sub-cohorts

Table 2. Impact of azacitidine and venetoclax on reversing loss in life-expectancy

Table 3. Relative per year costs of some new drugs

Issues in new drug approvals
Several important issues confound analyses of the appropriate use of new drugs in AML including: (1) who is unfit for intensive therapy? (2) no randomized trial proves less-intensive therapy is better than conventional intensive therapy amongst persons who could receive either; (3) what is the best endpoint for new drug approvals; (4) what is the appropriate comparator for a new drug approval; (5) several recent approvals are for unstudied populations; (6) recent approvals will decrease enrollment in clinical trials; and (7) most new drugs improve survival only slightly and long-term results remain unsatisfactory [12]. Table 1 displays data indicating not everyone benefits from a new drug such as venetoclax [1].
Table 2 shows although azacitidine and venetoclax improve survival of older persons with AML there remains a major loss of potential life-expectancy. Finally, Table 3 displays the cost of several new AML drugs compared with conventional drugs.
Conclusions
Based on the data I review above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new standard-of-care in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
Acknowledgement
Presented in part at the Raisa Gorbacheva Symposium in St. Petersburg on 17 September 2021. RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Conflict of interest
RPG is a consultant to: BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Pharmaceuticals Inc. and CStone Pharmaceuticals. Medical Director of FFF Enterprises Inc, on the Board of Directors: RakFond Foundation for Cancer Research Support. Scientific Advisory Board: Antegene Biotech LLC , StemRad Ltd. Author Contribution: I conceived, wrote and submitted the typescript for publication. Ethics Approval: None required.
References
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi: 10.1056/NEJMoa2012971
- Lancet JE, Uy GL, Newell LF, Lin TL, Ritchie EK, Stuart RK, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi: 10.1016/S2352-3026(21)00134-4. PMID: 34171279
- Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al.; QUAZAR AML-001 Trial Investigators. Oral Azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020; 383(26):2526-2537.
doi: 10.1056/NEJMoa2004444 - Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019; 33(2):379-389. doi: 10.1038/s41375-018-0312-9
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017; 377(5):454-464. doi: 10.1056/NEJMoa1614359
- Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019; 381(18):1728-1740. doi: 10.1056/NEJMoa1902688
- DiNardo CD, Schuh AC, Stein EM, Montesinos P, Wei AH, de Botton S. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021; 22(11):1597-1608. doi: 10.1016/S1470-2045(21)00494-0
- Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020 Feb 13;135(7):463-471. doi: 10.1182/blood.2019002140
- Burd A, Levine RL, Ruppert AS, Mims AS, Borate U, Stein EM, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020; 26(12):1852-1858. doi: 10.1038/s41591-020-1089-8
- Prasad V, Gale RP. Precision medicine in acute myeloid leukemia: Hope, hype or both? Leuk Res. 2016; 48:73-77.
doi: 10.1016/j.leukres.2016.07.011 - Lambert J, Pautas C, Terré C, Raffoux E, Turlure P, Caillot D, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019; 104(1):113-119.
doi: 10.3324/haematol.2018.188888 - Estey E, Karp JE, Emadi A, Othus M, Gale RP. Recent drug approvals for newly diagnosed acute myeloid leukemia: gifts or a Trojan horse? Leukemia. 2020;34(3):671-681. doi: 10.1038/s41375-019-0704-5
" ["~DETAIL_TEXT"]=> string(9815) "
Introduction
There are many new therapies approved to treat acute myeloid leukaemia (AML) including new conventional and targeted drugs and immune therapy. A summary of new AML drugs is displayed in Figure 1 increasing from one approval every 6 years to one approval every 150 days, a 12-fold increase.
The question I consider is whether these new therapies will cure AML. My discussion is divided into 3 categories: (1) conventional drugs; (2) targeted therapies; and (3) immune therapy.

Figure 1. Drug approvals in AML 1973-2020
New drugs
I consider 4 new drugs: (a) venetoclax [1]; (b) CPX-351 [2]; (c) CC-486 [3]; and (d) glasdegib (± low-dose cytarabine) [1-4]. Results of these, quite recent trials demonstrate that, although each new drug, alone or combined with previously-approved drugs improved outcomes, there remains a high rate of failures by 2 years.
Targeted drugs
Four targeted drugs are approved in AML including: (1) midostaurin; (2) gilteritinib; (3) enasidenib, and (4) ivosidenib. Results of recent trials of these 3 drugs are published, and the survival curves can be compared [5-8]. Except for enasidenib, these drugs improve outcomes but 2-year failure rates are high. A US trial Beat AML in persons with newly-diagnosed AML assigned subjects with druggable mutations to targeted or conventional drugs. There was no important difference in outcomes [9]. Therefore, according to recent estimates, current targeted drugs are likely to help only a limited subgroup (ca. 10 percent) of patients with acute myeloid leukemia [10].
Immune therapy
Gemtuzumab, an anti-CD33 monoclonal toxin-linked antibody, is the only approved immune therapy of AML [11]. It modestly improves outcomes and is rarely used.
Table 1. Differential effects of azacitidine and venetoclax in sub-cohorts

Table 2. Impact of azacitidine and venetoclax on reversing loss in life-expectancy

Table 3. Relative per year costs of some new drugs

Issues in new drug approvals
Several important issues confound analyses of the appropriate use of new drugs in AML including: (1) who is unfit for intensive therapy? (2) no randomized trial proves less-intensive therapy is better than conventional intensive therapy amongst persons who could receive either; (3) what is the best endpoint for new drug approvals; (4) what is the appropriate comparator for a new drug approval; (5) several recent approvals are for unstudied populations; (6) recent approvals will decrease enrollment in clinical trials; and (7) most new drugs improve survival only slightly and long-term results remain unsatisfactory [12]. Table 1 displays data indicating not everyone benefits from a new drug such as venetoclax [1].
Table 2 shows although azacitidine and venetoclax improve survival of older persons with AML there remains a major loss of potential life-expectancy. Finally, Table 3 displays the cost of several new AML drugs compared with conventional drugs.
Conclusions
Based on the data I review above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new standard-of-care in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
Acknowledgement
Presented in part at the Raisa Gorbacheva Symposium in St. Petersburg on 17 September 2021. RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Conflict of interest
RPG is a consultant to: BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Pharmaceuticals Inc. and CStone Pharmaceuticals. Medical Director of FFF Enterprises Inc, on the Board of Directors: RakFond Foundation for Cancer Research Support. Scientific Advisory Board: Antegene Biotech LLC , StemRad Ltd. Author Contribution: I conceived, wrote and submitted the typescript for publication. Ethics Approval: None required.
References
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi: 10.1056/NEJMoa2012971
- Lancet JE, Uy GL, Newell LF, Lin TL, Ritchie EK, Stuart RK, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-e491. doi: 10.1016/S2352-3026(21)00134-4. PMID: 34171279
- Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al.; QUAZAR AML-001 Trial Investigators. Oral Azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020; 383(26):2526-2537.
doi: 10.1056/NEJMoa2004444 - Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019; 33(2):379-389. doi: 10.1038/s41375-018-0312-9
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017; 377(5):454-464. doi: 10.1056/NEJMoa1614359
- Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019; 381(18):1728-1740. doi: 10.1056/NEJMoa1902688
- DiNardo CD, Schuh AC, Stein EM, Montesinos P, Wei AH, de Botton S. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021; 22(11):1597-1608. doi: 10.1016/S1470-2045(21)00494-0
- Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020 Feb 13;135(7):463-471. doi: 10.1182/blood.2019002140
- Burd A, Levine RL, Ruppert AS, Mims AS, Borate U, Stein EM, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020; 26(12):1852-1858. doi: 10.1038/s41591-020-1089-8
- Prasad V, Gale RP. Precision medicine in acute myeloid leukemia: Hope, hype or both? Leuk Res. 2016; 48:73-77.
doi: 10.1016/j.leukres.2016.07.011 - Lambert J, Pautas C, Terré C, Raffoux E, Turlure P, Caillot D, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019; 104(1):113-119.
doi: 10.3324/haematol.2018.188888 - Estey E, Karp JE, Emadi A, Othus M, Gale RP. Recent drug approvals for newly diagnosed acute myeloid leukemia: gifts or a Trojan horse? Leukemia. 2020;34(3):671-681. doi: 10.1038/s41375-019-0704-5
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(62) "budut-li-novyye-lekarstva-izlechivat-ostryy-miyeloidnyy-leykoz" ["~CODE"]=> string(62) "budut-li-novyye-lekarstva-izlechivat-ostryy-miyeloidnyy-leykoz" ["EXTERNAL_ID"]=> string(4) "2038" ["~EXTERNAL_ID"]=> string(4) "2038" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(158) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?Will new drugs cure acute myeloid leukaemia?" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2377) "<p style="text-align: justify;"> Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Острый миелоидный лейкоз, таргетная терапия, эффективность. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_META_TITLE"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_META_KEYWORDS"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_META_DESCRIPTION"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_PICTURE_FILE_ALT"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_PICTURE_FILE_TITLE"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_PICTURE_FILE_NAME"]=> string(64) "budut-li-novye-lekarstva-izlechivat-ostryy-mieloidnyy-leykoz-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(114) "Будут ли новые лекарства излечивать острый миелоидный лейкоз?" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(64) "budut-li-novye-lekarstva-izlechivat-ostryy-mieloidnyy-leykoz-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(64) "budut-li-novye-lekarstva-izlechivat-ostryy-mieloidnyy-leykoz-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(64) "budut-li-novye-lekarstva-izlechivat-ostryy-mieloidnyy-leykoz-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "199" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28385" ["VALUE"]=> array(2) { ["TEXT"]=> string(49) "<p> Роберт П. Гэйл </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(37) "
Роберт П. Гэйл
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28386" ["VALUE"]=> array(2) { ["TEXT"]=> string(228) "<p> Центр гематологии, Департамент иммунологии и воспаления, Лондонский Имперский Колледж, Лондон, Великобритания </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(216) "Центр гематологии, Департамент иммунологии и воспаления, Лондонский Имперский Колледж, Лондон, Великобритания
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28387" ["VALUE"]=> array(2) { ["TEXT"]=> string(2377) "<p style="text-align: justify;"> Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Острый миелоидный лейкоз, таргетная терапия, эффективность. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2321) "Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ.
Ключевые слова
Острый миелоидный лейкоз, таргетная терапия, эффективность.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28388" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-3-4-4-7" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-3-4-4-7" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28389" ["VALUE"]=> array(2) { ["TEXT"]=> string(44) "<p> Robert Peter Gale MD </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(32) "Robert Peter Gale MD
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28390" ["VALUE"]=> array(2) { ["TEXT"]=> string(735) "<p> Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK </p> <br> <p> <b>Correspondence:</b><br> Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ<br> Phone: +1 908 656 0484<br> Fax: +1 310 388 1320<br> E-Mail: <a href="mailto:robertpetergale@alumni.ucla.edu">robertpetergale@alumni.ucla.edu</a> </p> <p><b>Citation:</b> Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(623) "Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK
Correspondence:
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ
Phone: +1 908 656 0484
Fax: +1 310 388 1320
E-Mail: robertpetergale@alumni.ucla.edu
Citation: Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28391" ["VALUE"]=> array(2) { ["TEXT"]=> string(1132) "<p style="text-align: justify;"> There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new <i>standard-of-care</i> in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Acute myeloid leukemia, targeted therapy, efficiency. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1064) "There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new standard-of-care in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
Keywords
Acute myeloid leukemia, targeted therapy, efficiency.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28392" ["VALUE"]=> string(44) "Will new drugs cure acute myeloid leukaemia?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(44) "Will new drugs cure acute myeloid leukaemia?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28393" ["VALUE"]=> string(4) "2681" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2681" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28394" ["VALUE"]=> string(4) "2682" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2682" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28389" ["VALUE"]=> array(2) { ["TEXT"]=> string(44) "<p> Robert Peter Gale MD </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(32) "Robert Peter Gale MD
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(32) "Robert Peter Gale MD
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28391" ["VALUE"]=> array(2) { ["TEXT"]=> string(1132) "<p style="text-align: justify;"> There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new <i>standard-of-care</i> in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Acute myeloid leukemia, targeted therapy, efficiency. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1064) "There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new standard-of-care in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
Keywords
Acute myeloid leukemia, targeted therapy, efficiency.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1064) "There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new standard-of-care in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
Keywords
Acute myeloid leukemia, targeted therapy, efficiency.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28388" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-3-4-4-7" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-3-4-4-7" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-3-4-4-7" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28392" ["VALUE"]=> string(44) "Will new drugs cure acute myeloid leukaemia?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(44) "Will new drugs cure acute myeloid leukaemia?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(44) "Will new drugs cure acute myeloid leukaemia?" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28390" ["VALUE"]=> array(2) { ["TEXT"]=> string(735) "<p> Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK </p> <br> <p> <b>Correspondence:</b><br> Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ<br> Phone: +1 908 656 0484<br> Fax: +1 310 388 1320<br> E-Mail: <a href="mailto:robertpetergale@alumni.ucla.edu">robertpetergale@alumni.ucla.edu</a> </p> <p><b>Citation:</b> Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(623) "Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK
Correspondence:
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ
Phone: +1 908 656 0484
Fax: +1 310 388 1320
E-Mail: robertpetergale@alumni.ucla.edu
Citation: Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(623) "Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK
Correspondence:
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ
Phone: +1 908 656 0484
Fax: +1 310 388 1320
E-Mail: robertpetergale@alumni.ucla.edu
Citation: Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28385" ["VALUE"]=> array(2) { ["TEXT"]=> string(49) "<p> Роберт П. Гэйл </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(37) "Роберт П. Гэйл
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(37) "Роберт П. Гэйл
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28387" ["VALUE"]=> array(2) { ["TEXT"]=> string(2377) "<p style="text-align: justify;"> Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Острый миелоидный лейкоз, таргетная терапия, эффективность. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2321) "Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ.
Ключевые слова
Острый миелоидный лейкоз, таргетная терапия, эффективность.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2321) "Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ.
Ключевые слова
Острый миелоидный лейкоз, таргетная терапия, эффективность.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28386" ["VALUE"]=> array(2) { ["TEXT"]=> string(228) "<p> Центр гематологии, Департамент иммунологии и воспаления, Лондонский Имперский Колледж, Лондон, Великобритания </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(216) "Центр гематологии, Департамент иммунологии и воспаления, Лондонский Имперский Колледж, Лондон, Великобритания
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(216) "Центр гематологии, Департамент иммунологии и воспаления, Лондонский Имперский Колледж, Лондон, Великобритания
" } } } [1]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "200" ["~IBLOCK_SECTION_ID"]=> string(3) "200" ["ID"]=> string(4) "2041" ["~ID"]=> string(4) "2041" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["~NAME"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "01/25/2022 11:41:15 am" ["~TIMESTAMP_X"]=> string(22) "01/25/2022 11:41:15 am" ["DETAIL_PAGE_URL"]=> string(117) "/en/archive/tom-10-nomer-3-4/obzornye-stati/transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy/" ["~DETAIL_PAGE_URL"]=> string(117) "/en/archive/tom-10-nomer-3-4/obzornye-stati/transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(18776) "Introduction
Ornithine transcarbamylase deficiency (OTCD) is an X-linked genetic urea cycle disorder (UCD) caused by the mutation of the ornithine transcarbamylase (OTC, Xp2.1) gene. OTC is a mitochondrial enzyme synthesized in the cytoplasm. Following OTC transfer to the mitochondria, carbamoyl phosphate and ornithine are catalytically converted to citrulline. Then citrulline is transported to the cytoplasm to participate in the urea cycle reactions. The OTC gene mutations block normal urea metabolism. Therefore, increased blood ammonia, decreased blood citrulline and increased urine orotic acid are typical biochemical phenotypes of OTCD. High blood ammonia could cause the nervous system damage, epilepsy-like symptoms, disturbed consciousness, and cognitive impairment appear.
Early-onset OTCD mainly occurs in male heterozygous infants, usually with a rapid onset and a high mortality in the neonatal period [1]. The patient can be normal at birth. Then irritability, deteriorating feeding, drowsiness and tachypnea appear soon. It often develops into metabolic encephalopathy rapidly and leads to death if treatment is not applied. Severe intellectual impairment will be left in survivors due to the extensive damage to the brain caused by elevated blood ammonia [2]. Late-onset OTCD can occur in hemizygous males and heterozygous females. The clinical symptoms are variable and mild compared with early-onset OTCD.
The main principle of treatment is to control diet, reduce protein intake, avoid hyperammonemia, and use drugs to promote blood ammonia metabolism. However, excessive restriction of protein intake can lead to hypertrophy of endogenous protein catabolism, increase blood ammonia, and affect the patient's intelligence and physical development [3]. If the drug treatment is not effective, dialysis treatment should be considered as soon as possible.
Liver transplantation (LTx) is the most effective treatment of this disease, since OTC activity is mainly expressed in liver tissue. In these cases, the patients can stop anti-hyperammonemia drugs and return to normal diet after LTx. Hyperammonemia will not occur again, and the quality of life is significantly improved [4, 5]. Though LTx can correct the patient's urea cycle disorder and reduce blood ammonia substantially, it cannot reverse the nervous system damage that has occurred before LTx [5].
Indications for surgery
For the neonatal-onset patients, LTx should be performed as soon as possible if the patient’s condition is stable, independently on the blood ammonia levels. Considering the patient's tolerance for surgery and the risk of post-transplant hyperammonemia, the age of 3 months to 1 year, or body mass of >5 kg are appropriate pre-requisites for surgery [3]. It is usually done at six months of age. Early transplantation in the neonatal-onset patients may be associated with normal neurodevelopment compared with those without LTx.
For late-onset patients, it is now generally believed that, even with mild current manifestations, there is a risk of sudden, potentially life-threatening hyperammonemia at any age. Therefore, surgery should be considered for any OTCD patient. Final decision of LTx depends on the individual circumstances.
The peak of death with OTCD is noted at the age of 12-15 years in female patients, thus considering LTx before that time [6]. LTx in adolescents may also promote normal neurodevelopment. The patients should undergo LTx at peak blood ammonia levels of >300 μmol/L [7]. In cases of severe progressive liver disease, repeated metabolic abnormalities after standard treatment or poor compliance with current treatment, LTx can be also performed [3].
Analysis of data on the patients under 18 year subjected to LTx between February 2002 and September 2020, the waiting list time and male sex were associated with long-term risk for a cognitive delay. Minimizing the waiting time is quite important, in order to maintain the patient's cognition capacities at later terms and improve the quality of life [8].
All the patients with OTCD should be considered for LTx to prevent progressive neurological injury. But the decision is usually taken in cases of unstable condition and frequent episodes of hyperammonemia.
Donor selection
Liver transplants from either living or deceased donors are acceptable for the children of 1.5 to 3.0 years old. Three patients received cadaveric LTx at this age period. They developed well after this operation, and no recurrences were observed within follow-up for 13 years [9].
LTx from living donors is the most effective method in these cases. Living donors for the LTx should be in healthy condition, but sometimes there is no time to wait for another donor, except for subjects heterozygous for the mutated gene. A symptom-free carrier may be a donor for LTx, if OTC enzyme activity is high enough, and if no other options exist. The mutation carrier must undergo careful and comprehensive examination. OTC activity in liver biopsy samples must be tested to determine the suitability of heterozygote to be a donor [10]. According to Wakiya, T, the OTC activity of late-onset patients requiring LTx, ranges from 4.4% to 18.7%. Meanwhile, in those cases where LTx is not necessary, the residual enzyme activity ranges from 33% to 38% [11]. Rahayatri et al. [12] reported two 5-year-old girls who received liver transplants from heterozygous mutation carriers. The OTC activity in the first case and in her donor was 15% and 62%, respectively. She developed hyperammonemia within 2 months after the surgery. OTC activity in the second case and the donor was 9.7% and 42.6%, respectively. She developed hyperammonemia within 12 days after the surgery. Following continuous intravenous/venous hemodialysis, they were performing well without intensive care [12].
However, this method has potential risks. The enzyme activity in selected biopsy samples cannot represent its activity in other parts of the liver. Hence, one cannot accurately predict, whether the transplanted liver lobe exhibits sufficient activity, nor to predict whether total enzyme activity retained in the left liver is sufficient for heterozygous carrier donors.
Transplantation of hepatocytes may be another treatment option. Enosawa et al. reported an 11-day-old baby who underwent hepatocyte transplantation. The patient needed urgent LTx, but there was no source of liver, thus requiring hepatocyte transplantation. The patient was later in good condition and without recurrence within 3 months after the operation [13]. For the patients with poor overall clinical conditions, hepatocyte transplantation is less risky than liver grafting. Following hepatocyte transplantation, biochemical parameters of a 12-year-old patient with repeated metabolic decompensation showed decreased levels of plasma ammonia and increased urea production. However, the patient died because of a nosocomial fungal sepsis [14].
Surgical methods
Orthotopic LTx is still the best choice in OTCD. It has fewer complications than auxiliary LTx [5]. Over recent years, a domino cross-auxiliary LTx has been tried in the clinical setting. This method is based on exchanging part of liver tissue with patients suffering from other metabolic diseases aiming to achieve metabolic complementation. It does not require additional organ donation. Of the three OTCD patients in China, subjected to domino cross-auxiliary LTx, two cases recovered well after the operation, without any complications during the follow-up period. One patient experienced occult graft rejection resulting into graft dysfunction and eventual disease recurrence [15]. The domino cross-auxiliary LTx is a feasible method, without any problems caused by the operation itself.
Post-transplant management
Due to long-term therapy with immunosuppressive drugs and postoperative weakness, one should notice prevention of postsurgical infections, which may cause failure of this intervention and death of the patient.
Following transplantation, the liver function should be tested regularly, to discern graft injury. The graft-derived cell-free DNA in blood may be of similar discriminative value, it was also able to differentiate between the trend for graft injury and normal liver function. However, this technique is not as convenient as routine liver function tests [16]. The peak blood ammonia level of >356 μmol/L predicted poor neurodevelopmental outcomes in the patients undergoing LTx [17].
Clinical effect
According to the data from United Network for Organ Sharing (UNOS) database including 403 patients with urea cycle disorders (46.2% were OTCD) who underwent transplantation, the 1-, 3-, and 5-year graft survival rates were 90.4%, 86.3%, and 85.2%, respectively. Increased mass of the liver graft and male sex are related to decreased risk of graft loss [8]. In Japan, the 1-, 5-, 10-, and 15-year graft survival rates comprised 91.2%, 87.9%, 87.0%, and 79.3% among pediatric patients with metabolic disorders (OTCD, 20.6% of total) as shown by Kasahara et al. [18].
The 1-, 5-, and 10-year overall survival rates among 278 UCD patients who underwent LTx between 1987 and 2010 were 93%, 89%, and 87%, respectively, according to the UNOS database [19]. However, the article only stated that most UCD patients are OTCD, without any specific data on OTCD patients.
13 of 69 Chinese OTCD patients received LTx, at a median age of 3 years and one-year survival rate of 100% [20]. In Japan, the 1-, 5-, 10- and 15-year survival rates in 194 pediatric patients with metabolic disorders (OTCD=40) who underwent living donor LTx, were 91.2%, 87.9%, 86.1%, and 74.4% [18].
Hence, LTx can improve long-term survival rates of the patients, prevent recurrent hyperammonemia, and reduce the blood ammonia level. However, it did not improve neurodevelopmental outcomes in the patients with severe symptomatics, because hyperammonemia exerts early brain damage. Urgent LTx in another UCD, i.e., arginine succinate synthase deficiency, may improve the longitudinal cognitive and behavioural outcomes [17].
Conclusions
LTx can improve the long-term survival rate of patients with OTCD, but it cannot reverse the nervous system damage that occurred previously, and cannot improve cognitive impairment. However, neurodevelopment may normally proceed after LTx if it is performed early in childhood. The patients with late-onset disease should also be transplanted when required. Donorship of heterozygote carriers is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required post-transplant.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr. 2008; 97(10):1420-1425. doi: 10.1111/j.1651-2227.2008.00952.x
- Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984; 310(23):1500-1505. doi: 10.1056/NEJM198406073102304
- Haberle J, Burlina A, Chakrapani A, Dixon M, Karall D, Lindner M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis. 2019; 42(6): 1192-1230. doi: 10.1002/jimd.12100
- Kim IK, Niemi AK, Krueger C, Bonham CA, Concepcion W, Cowan TM, et al. Liver transplantation for urea cycle disorders in pediatric patients: a single-center experience. Pediatr Transplant. 2013; 17(2): 158-167. doi: 10.1111/petr.12041
- Morioka D, Kasahara M, Takada Y, Shirouzu Y, Taira K, Sakamoto S, et al. Current role of liver transplantation for the treatment of urea cycle disorders: a review of the worldwide English literature and 13 cases at Kyoto University. Liver Transpl. 2005; 11(11):1332-1342. doi: 10.1002/lt.20587
- Division of Genetics and Metabolism, Child Diseases and Health Care Branch, Chinese Association for Maternal and Child Health. Consensus on diagnosis and treatment of ornithine trans-carbamylase deficiency, J Zhejiang Univ (Med Sci), 2020; 49(5):539-547
(In Chinese). doi: 10.3785/j.issn.1008-9292.2020.04.11 - Kido J, Matsumoto S, Mitsubuchi H, Endo F, Nakamura K. Early liver transplantation in neonatal-onset and moderate urea cycle disorders may lead to normal neurodevelopment. Metab Brain Dis. 2018; 33(5): 1517-1523. doi: 10.1007/s11011-018-0259-6
- Ziogas IA, Wu WK, Matsuoka LK, Pai AK, Hafberg ET, Gillis LA, et al. Liver transplantation in children with urea cycle disorders: the importance of minimizing waiting time. Liver Transpl. 2021, May 31. doi: 10.1002/lt.26186
- Zhu Z, Sun L, Wei L, et al. Liver transplantation for the treatment of hyperammonemia due to urea cycle disorder: report of four cases. Zhonghua Er Ke Za Zhi (Chinese Journal of Pediatrics). 2015; 53(2): 136-139 (In Chinese). doi: 10.1159/000416533
- Wakiya T, Sanada Y, Urahashi T, Ihara Y, Yamada N, Okada N, et al. Living donor liver transplantation from an asymptomatic mother who was a carrier for ornithine transcarbamylase deficiency. Pediatr Transplant. 2012; 16(6):E196-200. doi: 10.1111/j.1399-3046.2012.01716.x
- Wakiya T, Sanada Y, Urahashi T, Ihara Y, Yamada N, Okada N, et al. Impact of enzyme activity assay on indication in liver transplantation for ornithine transcarbamylase deficiency. Mol Genet Metab. 2012; 105(3): 404-407. doi: 10.1016/j.ymgme.2011.12.019
- Rahayatri TH, Uchida H, Sasaki K, Shigeta T, Hirata Y, Kanazawa H, et al. Hyperammonemia in ornithine transcarbamylase-deficient recipients following living donor liver transplantation from heterozygous carrier donors. Pediatr Transplant. 2017; 21(1).
doi: 10.1111/petr.12848 - Enosawa S, Horikawa R, Yamamoto A, Sakamoto S, Shigeta T, Nosaka S, et al., Hepatocyte transplantation using a living donor reduced graft in a baby with ornithine transcarbamylase deficiency: a novel source of hepatocytes. Liver Transpl. 2014; 20(3): 391-393. doi: 10.1002/lt.23800
- Ribes-Koninckx C, Ibars ER, Calzado Agrasot MA, Bonora-Centelles A, Miquel BP, Vila Carbó JJ, et al. Clinical outcome of hepatocyte transplantation in four pediatric patients with inherited metabolic diseases. Cell Transplant. 2012; 21(10): 2267-2282. doi: 10.3727/096368912X637505
- Qu W, Wei L, Zhu ZJ, Sun LY, Liu Y, Zeng ZG. Considerations for use of domino cross-auxiliary liver transplantation in metabolic liver diseases: A review of case studies. Transplantation. 2019; 103(9):1916-1920. doi: 10.1097/TP.0000000000002602
- Ng HI, Sun LY, Zhu ZJ. Application of graft-derived cell-free DNA in ornithine transcarbamylase deficiency patient after living donor liver transplantation: Two case reports. Medicine (Baltimore). 2018; 97(51): e13843. doi: 10.1097/MD.0000000000013843
- Kido J, Matsumoto S, Haberle J, Inomata Y, Kasahara M, Sakamoto S, et al. Role of liver transplantation in urea cycle disorders: Report from a nationwide study in Japan. J Inherit Metab Dis. 2021. doi: 10.1002/jimd.12415
- Kasahara M, Sakamoto S, Horikawa R, Koji U, Mizuta K, Shinkai M, et al. Living donor liver transplantation for pediatric patients with metabolic disorders: the Japanese multicenter registry. Pediatr Transplant. 2014; 18(1): 6-15. doi: 10.1111/petr.12196
- Yu L, Rayhill SC, Hsu EK, Landis CS. Liver transplantation for urea cycle disorders: analysis of the united network for organ sharing database. Transplant Proc. 2015; 47(8): 2413-2418. doi: 10.1016/j.transproceed.2015.09.020
- Lu D, Han F, Qiu W, Zhang H, Ye J, Liang L, et al. Clinical and molecular characteristics of 69 Chinese patients with ornithine transcarbamylase deficiency. Orphanet J Rare Dis. 2020; 15(1): 340. doi: 10.1186/s13023-020-01606-2
" ["~DETAIL_TEXT"]=> string(18776) "
Introduction
Ornithine transcarbamylase deficiency (OTCD) is an X-linked genetic urea cycle disorder (UCD) caused by the mutation of the ornithine transcarbamylase (OTC, Xp2.1) gene. OTC is a mitochondrial enzyme synthesized in the cytoplasm. Following OTC transfer to the mitochondria, carbamoyl phosphate and ornithine are catalytically converted to citrulline. Then citrulline is transported to the cytoplasm to participate in the urea cycle reactions. The OTC gene mutations block normal urea metabolism. Therefore, increased blood ammonia, decreased blood citrulline and increased urine orotic acid are typical biochemical phenotypes of OTCD. High blood ammonia could cause the nervous system damage, epilepsy-like symptoms, disturbed consciousness, and cognitive impairment appear.
Early-onset OTCD mainly occurs in male heterozygous infants, usually with a rapid onset and a high mortality in the neonatal period [1]. The patient can be normal at birth. Then irritability, deteriorating feeding, drowsiness and tachypnea appear soon. It often develops into metabolic encephalopathy rapidly and leads to death if treatment is not applied. Severe intellectual impairment will be left in survivors due to the extensive damage to the brain caused by elevated blood ammonia [2]. Late-onset OTCD can occur in hemizygous males and heterozygous females. The clinical symptoms are variable and mild compared with early-onset OTCD.
The main principle of treatment is to control diet, reduce protein intake, avoid hyperammonemia, and use drugs to promote blood ammonia metabolism. However, excessive restriction of protein intake can lead to hypertrophy of endogenous protein catabolism, increase blood ammonia, and affect the patient's intelligence and physical development [3]. If the drug treatment is not effective, dialysis treatment should be considered as soon as possible.
Liver transplantation (LTx) is the most effective treatment of this disease, since OTC activity is mainly expressed in liver tissue. In these cases, the patients can stop anti-hyperammonemia drugs and return to normal diet after LTx. Hyperammonemia will not occur again, and the quality of life is significantly improved [4, 5]. Though LTx can correct the patient's urea cycle disorder and reduce blood ammonia substantially, it cannot reverse the nervous system damage that has occurred before LTx [5].
Indications for surgery
For the neonatal-onset patients, LTx should be performed as soon as possible if the patient’s condition is stable, independently on the blood ammonia levels. Considering the patient's tolerance for surgery and the risk of post-transplant hyperammonemia, the age of 3 months to 1 year, or body mass of >5 kg are appropriate pre-requisites for surgery [3]. It is usually done at six months of age. Early transplantation in the neonatal-onset patients may be associated with normal neurodevelopment compared with those without LTx.
For late-onset patients, it is now generally believed that, even with mild current manifestations, there is a risk of sudden, potentially life-threatening hyperammonemia at any age. Therefore, surgery should be considered for any OTCD patient. Final decision of LTx depends on the individual circumstances.
The peak of death with OTCD is noted at the age of 12-15 years in female patients, thus considering LTx before that time [6]. LTx in adolescents may also promote normal neurodevelopment. The patients should undergo LTx at peak blood ammonia levels of >300 μmol/L [7]. In cases of severe progressive liver disease, repeated metabolic abnormalities after standard treatment or poor compliance with current treatment, LTx can be also performed [3].
Analysis of data on the patients under 18 year subjected to LTx between February 2002 and September 2020, the waiting list time and male sex were associated with long-term risk for a cognitive delay. Minimizing the waiting time is quite important, in order to maintain the patient's cognition capacities at later terms and improve the quality of life [8].
All the patients with OTCD should be considered for LTx to prevent progressive neurological injury. But the decision is usually taken in cases of unstable condition and frequent episodes of hyperammonemia.
Donor selection
Liver transplants from either living or deceased donors are acceptable for the children of 1.5 to 3.0 years old. Three patients received cadaveric LTx at this age period. They developed well after this operation, and no recurrences were observed within follow-up for 13 years [9].
LTx from living donors is the most effective method in these cases. Living donors for the LTx should be in healthy condition, but sometimes there is no time to wait for another donor, except for subjects heterozygous for the mutated gene. A symptom-free carrier may be a donor for LTx, if OTC enzyme activity is high enough, and if no other options exist. The mutation carrier must undergo careful and comprehensive examination. OTC activity in liver biopsy samples must be tested to determine the suitability of heterozygote to be a donor [10]. According to Wakiya, T, the OTC activity of late-onset patients requiring LTx, ranges from 4.4% to 18.7%. Meanwhile, in those cases where LTx is not necessary, the residual enzyme activity ranges from 33% to 38% [11]. Rahayatri et al. [12] reported two 5-year-old girls who received liver transplants from heterozygous mutation carriers. The OTC activity in the first case and in her donor was 15% and 62%, respectively. She developed hyperammonemia within 2 months after the surgery. OTC activity in the second case and the donor was 9.7% and 42.6%, respectively. She developed hyperammonemia within 12 days after the surgery. Following continuous intravenous/venous hemodialysis, they were performing well without intensive care [12].
However, this method has potential risks. The enzyme activity in selected biopsy samples cannot represent its activity in other parts of the liver. Hence, one cannot accurately predict, whether the transplanted liver lobe exhibits sufficient activity, nor to predict whether total enzyme activity retained in the left liver is sufficient for heterozygous carrier donors.
Transplantation of hepatocytes may be another treatment option. Enosawa et al. reported an 11-day-old baby who underwent hepatocyte transplantation. The patient needed urgent LTx, but there was no source of liver, thus requiring hepatocyte transplantation. The patient was later in good condition and without recurrence within 3 months after the operation [13]. For the patients with poor overall clinical conditions, hepatocyte transplantation is less risky than liver grafting. Following hepatocyte transplantation, biochemical parameters of a 12-year-old patient with repeated metabolic decompensation showed decreased levels of plasma ammonia and increased urea production. However, the patient died because of a nosocomial fungal sepsis [14].
Surgical methods
Orthotopic LTx is still the best choice in OTCD. It has fewer complications than auxiliary LTx [5]. Over recent years, a domino cross-auxiliary LTx has been tried in the clinical setting. This method is based on exchanging part of liver tissue with patients suffering from other metabolic diseases aiming to achieve metabolic complementation. It does not require additional organ donation. Of the three OTCD patients in China, subjected to domino cross-auxiliary LTx, two cases recovered well after the operation, without any complications during the follow-up period. One patient experienced occult graft rejection resulting into graft dysfunction and eventual disease recurrence [15]. The domino cross-auxiliary LTx is a feasible method, without any problems caused by the operation itself.
Post-transplant management
Due to long-term therapy with immunosuppressive drugs and postoperative weakness, one should notice prevention of postsurgical infections, which may cause failure of this intervention and death of the patient.
Following transplantation, the liver function should be tested regularly, to discern graft injury. The graft-derived cell-free DNA in blood may be of similar discriminative value, it was also able to differentiate between the trend for graft injury and normal liver function. However, this technique is not as convenient as routine liver function tests [16]. The peak blood ammonia level of >356 μmol/L predicted poor neurodevelopmental outcomes in the patients undergoing LTx [17].
Clinical effect
According to the data from United Network for Organ Sharing (UNOS) database including 403 patients with urea cycle disorders (46.2% were OTCD) who underwent transplantation, the 1-, 3-, and 5-year graft survival rates were 90.4%, 86.3%, and 85.2%, respectively. Increased mass of the liver graft and male sex are related to decreased risk of graft loss [8]. In Japan, the 1-, 5-, 10-, and 15-year graft survival rates comprised 91.2%, 87.9%, 87.0%, and 79.3% among pediatric patients with metabolic disorders (OTCD, 20.6% of total) as shown by Kasahara et al. [18].
The 1-, 5-, and 10-year overall survival rates among 278 UCD patients who underwent LTx between 1987 and 2010 were 93%, 89%, and 87%, respectively, according to the UNOS database [19]. However, the article only stated that most UCD patients are OTCD, without any specific data on OTCD patients.
13 of 69 Chinese OTCD patients received LTx, at a median age of 3 years and one-year survival rate of 100% [20]. In Japan, the 1-, 5-, 10- and 15-year survival rates in 194 pediatric patients with metabolic disorders (OTCD=40) who underwent living donor LTx, were 91.2%, 87.9%, 86.1%, and 74.4% [18].
Hence, LTx can improve long-term survival rates of the patients, prevent recurrent hyperammonemia, and reduce the blood ammonia level. However, it did not improve neurodevelopmental outcomes in the patients with severe symptomatics, because hyperammonemia exerts early brain damage. Urgent LTx in another UCD, i.e., arginine succinate synthase deficiency, may improve the longitudinal cognitive and behavioural outcomes [17].
Conclusions
LTx can improve the long-term survival rate of patients with OTCD, but it cannot reverse the nervous system damage that occurred previously, and cannot improve cognitive impairment. However, neurodevelopment may normally proceed after LTx if it is performed early in childhood. The patients with late-onset disease should also be transplanted when required. Donorship of heterozygote carriers is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required post-transplant.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Summar ML, Dobbelaere D, Brusilow S, Lee B. Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatr. 2008; 97(10):1420-1425. doi: 10.1111/j.1651-2227.2008.00952.x
- Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984; 310(23):1500-1505. doi: 10.1056/NEJM198406073102304
- Haberle J, Burlina A, Chakrapani A, Dixon M, Karall D, Lindner M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis. 2019; 42(6): 1192-1230. doi: 10.1002/jimd.12100
- Kim IK, Niemi AK, Krueger C, Bonham CA, Concepcion W, Cowan TM, et al. Liver transplantation for urea cycle disorders in pediatric patients: a single-center experience. Pediatr Transplant. 2013; 17(2): 158-167. doi: 10.1111/petr.12041
- Morioka D, Kasahara M, Takada Y, Shirouzu Y, Taira K, Sakamoto S, et al. Current role of liver transplantation for the treatment of urea cycle disorders: a review of the worldwide English literature and 13 cases at Kyoto University. Liver Transpl. 2005; 11(11):1332-1342. doi: 10.1002/lt.20587
- Division of Genetics and Metabolism, Child Diseases and Health Care Branch, Chinese Association for Maternal and Child Health. Consensus on diagnosis and treatment of ornithine trans-carbamylase deficiency, J Zhejiang Univ (Med Sci), 2020; 49(5):539-547
(In Chinese). doi: 10.3785/j.issn.1008-9292.2020.04.11 - Kido J, Matsumoto S, Mitsubuchi H, Endo F, Nakamura K. Early liver transplantation in neonatal-onset and moderate urea cycle disorders may lead to normal neurodevelopment. Metab Brain Dis. 2018; 33(5): 1517-1523. doi: 10.1007/s11011-018-0259-6
- Ziogas IA, Wu WK, Matsuoka LK, Pai AK, Hafberg ET, Gillis LA, et al. Liver transplantation in children with urea cycle disorders: the importance of minimizing waiting time. Liver Transpl. 2021, May 31. doi: 10.1002/lt.26186
- Zhu Z, Sun L, Wei L, et al. Liver transplantation for the treatment of hyperammonemia due to urea cycle disorder: report of four cases. Zhonghua Er Ke Za Zhi (Chinese Journal of Pediatrics). 2015; 53(2): 136-139 (In Chinese). doi: 10.1159/000416533
- Wakiya T, Sanada Y, Urahashi T, Ihara Y, Yamada N, Okada N, et al. Living donor liver transplantation from an asymptomatic mother who was a carrier for ornithine transcarbamylase deficiency. Pediatr Transplant. 2012; 16(6):E196-200. doi: 10.1111/j.1399-3046.2012.01716.x
- Wakiya T, Sanada Y, Urahashi T, Ihara Y, Yamada N, Okada N, et al. Impact of enzyme activity assay on indication in liver transplantation for ornithine transcarbamylase deficiency. Mol Genet Metab. 2012; 105(3): 404-407. doi: 10.1016/j.ymgme.2011.12.019
- Rahayatri TH, Uchida H, Sasaki K, Shigeta T, Hirata Y, Kanazawa H, et al. Hyperammonemia in ornithine transcarbamylase-deficient recipients following living donor liver transplantation from heterozygous carrier donors. Pediatr Transplant. 2017; 21(1).
doi: 10.1111/petr.12848 - Enosawa S, Horikawa R, Yamamoto A, Sakamoto S, Shigeta T, Nosaka S, et al., Hepatocyte transplantation using a living donor reduced graft in a baby with ornithine transcarbamylase deficiency: a novel source of hepatocytes. Liver Transpl. 2014; 20(3): 391-393. doi: 10.1002/lt.23800
- Ribes-Koninckx C, Ibars ER, Calzado Agrasot MA, Bonora-Centelles A, Miquel BP, Vila Carbó JJ, et al. Clinical outcome of hepatocyte transplantation in four pediatric patients with inherited metabolic diseases. Cell Transplant. 2012; 21(10): 2267-2282. doi: 10.3727/096368912X637505
- Qu W, Wei L, Zhu ZJ, Sun LY, Liu Y, Zeng ZG. Considerations for use of domino cross-auxiliary liver transplantation in metabolic liver diseases: A review of case studies. Transplantation. 2019; 103(9):1916-1920. doi: 10.1097/TP.0000000000002602
- Ng HI, Sun LY, Zhu ZJ. Application of graft-derived cell-free DNA in ornithine transcarbamylase deficiency patient after living donor liver transplantation: Two case reports. Medicine (Baltimore). 2018; 97(51): e13843. doi: 10.1097/MD.0000000000013843
- Kido J, Matsumoto S, Haberle J, Inomata Y, Kasahara M, Sakamoto S, et al. Role of liver transplantation in urea cycle disorders: Report from a nationwide study in Japan. J Inherit Metab Dis. 2021. doi: 10.1002/jimd.12415
- Kasahara M, Sakamoto S, Horikawa R, Koji U, Mizuta K, Shinkai M, et al. Living donor liver transplantation for pediatric patients with metabolic disorders: the Japanese multicenter registry. Pediatr Transplant. 2014; 18(1): 6-15. doi: 10.1111/petr.12196
- Yu L, Rayhill SC, Hsu EK, Landis CS. Liver transplantation for urea cycle disorders: analysis of the united network for organ sharing database. Transplant Proc. 2015; 47(8): 2413-2418. doi: 10.1016/j.transproceed.2015.09.020
- Lu D, Han F, Qiu W, Zhang H, Ye J, Liang L, et al. Clinical and molecular characteristics of 69 Chinese patients with ornithine transcarbamylase deficiency. Orphanet J Rare Dis. 2020; 15(1): 340. doi: 10.1186/s13023-020-01606-2
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "30" ["~SORT"]=> string(2) "30" ["CODE"]=> string(72) "transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy" ["~CODE"]=> string(72) "transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy" ["EXTERNAL_ID"]=> string(4) "2041" ["~EXTERNAL_ID"]=> string(4) "2041" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(207) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазыLiver transplantation in the treatment of ornithine transcarbamylase deficiency" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2628) "<p style="text-align: justify;">Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_META_TITLE"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_META_KEYWORDS"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_META_DESCRIPTION"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_PICTURE_FILE_ALT"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_PICTURE_FILE_TITLE"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_PICTURE_FILE_NAME"]=> string(76) "transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(128) "Трансплантация печени при лечении дефицита орнитин-транскарбамилазы" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(76) "transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(76) "transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(76) "transplantatsiya-pecheni-pri-lechenii-defitsita-ornitin-transkarbamilazy-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "200" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28415" ["VALUE"]=> array(2) { ["TEXT"]=> string(53) "<p>Джингуа Вэй, Бо Хюи</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(41) "
Джингуа Вэй, Бо Хюи
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28416" ["VALUE"]=> array(2) { ["TEXT"]=> string(184) "<p>Департамент неврологии, Госпиталь Сиджин, 4-й Военно-Медицинский университет, Сиань, Китай</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(172) "Департамент неврологии, Госпиталь Сиджин, 4-й Военно-Медицинский университет, Сиань, Китай
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28417" ["VALUE"]=> array(2) { ["TEXT"]=> string(2628) "<p style="text-align: justify;">Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2572) "Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.
Ключевые слова
Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28418" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-26-29" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-26-29" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28419" ["VALUE"]=> array(2) { ["TEXT"]=> string(37) "<p>Jingya Wei, Bo Hui</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(25) "Jingya Wei, Bo Hui
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28420" ["VALUE"]=> array(2) { ["TEXT"]=> string(609) "<p>Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China </p><br> <p><b>Correspondence:</b><br> Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China<br> Phone: +86 29 8477 1055<br> E-mail: iamtrn@126.com</p><br> <p><b>Citation:</b> Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(519) "Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China
Correspondence:
Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China
Phone: +86 29 8477 1055
E-mail: iamtrn@126.com
Citation: Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28421" ["VALUE"]=> array(2) { ["TEXT"]=> string(1341) "<p style="text-align: justify;">Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.</p> <h2>Keywords</h2> <p style="text-align: justify;">Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1285) "Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.
Keywords
Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28422" ["VALUE"]=> string(79) "Liver transplantation in the treatment of ornithine transcarbamylase deficiency" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(79) "Liver transplantation in the treatment of ornithine transcarbamylase deficiency" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> &array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28423" ["VALUE"]=> string(4) "2698" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2698" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28424" ["VALUE"]=> string(4) "2699" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2699" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28419" ["VALUE"]=> array(2) { ["TEXT"]=> string(37) "<p>Jingya Wei, Bo Hui</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(25) "Jingya Wei, Bo Hui
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(25) "Jingya Wei, Bo Hui
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28421" ["VALUE"]=> array(2) { ["TEXT"]=> string(1341) "<p style="text-align: justify;">Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.</p> <h2>Keywords</h2> <p style="text-align: justify;">Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1285) "Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.
Keywords
Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1285) "Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.
Keywords
Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28418" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-26-29" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-26-29" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-26-29" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28422" ["VALUE"]=> string(79) "Liver transplantation in the treatment of ornithine transcarbamylase deficiency" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(79) "Liver transplantation in the treatment of ornithine transcarbamylase deficiency" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(79) "Liver transplantation in the treatment of ornithine transcarbamylase deficiency" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28420" ["VALUE"]=> array(2) { ["TEXT"]=> string(609) "<p>Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China </p><br> <p><b>Correspondence:</b><br> Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China<br> Phone: +86 29 8477 1055<br> E-mail: iamtrn@126.com</p><br> <p><b>Citation:</b> Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(519) "Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China
Correspondence:
Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China
Phone: +86 29 8477 1055
E-mail: iamtrn@126.com
Citation: Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(519) "Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China
Correspondence:
Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China
Phone: +86 29 8477 1055
E-mail: iamtrn@126.com
Citation: Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28415" ["VALUE"]=> array(2) { ["TEXT"]=> string(53) "<p>Джингуа Вэй, Бо Хюи</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(41) "Джингуа Вэй, Бо Хюи
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(41) "Джингуа Вэй, Бо Хюи
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28417" ["VALUE"]=> array(2) { ["TEXT"]=> string(2628) "<p style="text-align: justify;">Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2572) "Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.
Ключевые слова
Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2572) "Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.
Ключевые слова
Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28416" ["VALUE"]=> array(2) { ["TEXT"]=> string(184) "<p>Департамент неврологии, Госпиталь Сиджин, 4-й Военно-Медицинский университет, Сиань, Китай</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(172) "Департамент неврологии, Госпиталь Сиджин, 4-й Военно-Медицинский университет, Сиань, Китай
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(172) "Департамент неврологии, Госпиталь Сиджин, 4-й Военно-Медицинский университет, Сиань, Китай
" } } } [2]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "200" ["~IBLOCK_SECTION_ID"]=> string(3) "200" ["ID"]=> string(4) "2040" ["~ID"]=> string(4) "2040" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["~NAME"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "01/25/2022 11:17:58 am" ["~TIMESTAMP_X"]=> string(22) "01/25/2022 11:17:58 am" ["DETAIL_PAGE_URL"]=> string(145) "/en/archive/tom-10-nomer-3-4/obzornye-stati/potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov/" ["~DETAIL_PAGE_URL"]=> string(145) "/en/archive/tom-10-nomer-3-4/obzornye-stati/potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(29610) "Introduction
It is generally known that intestinal microbiota is a symbiotic community including many types of bacteria, fungi and viruses. It is a dynamic biological system with an average weight of 2-3 kg, producing many metabolites (metabolic substances, vitamins, neurotransmitters, etc.) necessary for existence and growth of the host organism. The gut microbiota interacts with intestinal epithelial cell layers which are exposed both to endogenous pathogenic (e.g., blood-borne) viruses, and to intestinal bacterial populations. In turn, the gut microbed strains are affected by various bacteriophage populations causing their lysis. The surviving bacteria may acquire mobile gene elements, including those providing resistance to antibiotics (Fig. 1). These polyresistant bacteria are an important cause of life-threatening infections in immunocompromised patients, e.g., after intensive cytostatic therapy and hematopoietic stem cell transplantation (HSCT).

Figure 1. Intestinal epithelium is exposed to endogenous host viruses (bottom), and interacts with gut bacteria (light green) which could be lysed by bacteriophages (top left). Some of them may harbor and transfer antibiotic resistance genes to the bacteria (top right)
Moreover, the host immune system develops in permanent contact with the surrounding microbial and viral antigens, especially with the endogenous intestinal microbiota, which includes all these components [1]. Microbial and viral antigens of the intestinal microbiota, passing through the epithelial barriers of the mucous membranes, penetrate into the regional blood vessels and lymph nodes, form an adaptive B- and T-cell immune response, being a key factor in the normal maturation and functioning of the immune system.
For decades, the main attention of bacteriologists was paid to cultivated, mainly aerobic microorganisms – Escherichia, Klebsiella, Enterococci that were studied in details for decades. Cultivable anaerobic gut bacteria were also exhaustively studied over last decades. However, with development of high-throughput DNA diagnostics, the spectrum of known microbial bacteria extended to sufficient degree. Currently, >1000 bacterial species are detectable in normal gut microbiota. Hence, over the past 10-20 years, the novel molecular biology approaches (multiple PCR, high-throughput DNA sequencing) enabled detailed classifying hundreds of species of anaerobic intestinal bacteria, which dominate the intestinal microbiota.
Human viral community (virome) is exhibiting even higher taxonomic and genetic complexity. Formerly, the main interest was drawn to the viruses of eukaryotic cells which could be pathogenic to humans. The modern NGS approach allows to detect both known viruses (herpes, anelloviruses, picobirnaviruses) and a number of previously unknown viral DNA sequences [2].
However, intestinal human virome is dominated by bacteriophages. These are largely represented by Caudiovirales. [3]. This order of phages includes 3 families (Myoviridae; Siphoviridae, and Podoviridae).
Along with double-stranded DNA phages, minor populations of single-stranded DNA and RNA phages are detectable in human gut virome which are also specific for distinct bacterial species as reviewed by Carding et al. [3].
The issues of viral sequences in bacterial and human cells (e.g., prophages, retroviral elements etc.) are also detectable by metagenomics but will not be covered here, because of undefined clinical significance and still undefined systemic approach. Hence, the question about functions and significance of virome abundance and diversity in healthy subjects and GIT disorders is still open.
Current methodology
Unlike the bacterial microbiota, viral intestinal population (gut virome) has been studied to a much lesser extent. Whereas bacterial species have the common target gene for sequencing, i.e., 16SrRNA (the main object for NGS analysis), the viruses lack such a common marker gene, thus hampering easy assessment of their diversity in biological samples.
Currently, there is a broad diagnostic choice in clinical virology, starting with cytopathogenic tests, large arrays of immune diagnostic sets, and modern whole-scale panels of PCR assays. To diagnose some pathogenic viruses (up to 10-15 species), multiplex PCR was developed, and it is commonly used in clinical laboratories. However, these commercial PCR panels cannot cover the entire spectrum of potential viral agents, usually targeting up to 10-12 pathogens.
Therefore, the most comprehensive approach to studying the entire viral community is whole-genome screening of viral sequences by means of NGS followed by extensive bioinformatics. E.g., a random metagenomic analysis may also allow detection of human pathogenic viruses. Methodology of metagenomic studies are well reviewed in [4].
Diversity of human viruses and bacteriophages
A number of endogenous viruses persisting lifelong may be found in stools under certain conditions, e.g., in immunocompromised patients or during immunosuppressive therapy.
Herperviruses in gut are often activated in the patients with inflammatory bowel diseases. E.g., the Japanese group, using high-capacity multiplex PCR with microchip electrophoresis, has detected as much as 191 different bacterial species and 206 viruses in 215 stool samples (71.7% positivity) from immunocompromised patients and subjects with ulcerative colitis [5]. Among viral pathogens, Epstein-Barr virus (EBV) was revealed in 90 samples (30.0%); HHV-6, in 53 cases (17.7%), and cytomegalovirus (CMV) in 37 specimens (12.3%).
Most often, however, human intestinal microbiome is extensively studied in search of the viruses known to be pathogenic (i.e., noroviruses, rotaviruses, astroviruses, adenoviruses etc.). Biological diversity of human pathogenic viruses was first studied in Asian countries, with high incidence of gastroenteritis in pediatric setting [6]. The authors examined stool samples from 7 children positive for picobirnavirus and identified, by means of metagenomics, enterovirus, gyrovirus, parechovirus in these samples, which are potentially pathogenic in gastroenteritis. In addition, a metagenomic study of stool microbiota allows detecting bacterial-viral associations (for example, C.difficile associated with a number of viruses) in intestinal syndromes as shown by a group from the USA [7]. Metagenomic analysis was also used for NGS analysis of stool samples in gastroenteritis, and, except of common noroviruses, additional pathogens were found from the genus Astroviridae and Caliciviridae [8].
Gut human virome after HSCT
A limited number of works concerned presence of intestinal viruses posttransplant. E.g., Legoff et al. have detected different viruses in stool and plasma following HSCT using electrospray ionization with mass-spectrometric [9]. The workers have revealed high adenovirus (AdV) viral load in stool samples. In an earlier study, AdV DNA was found in stools of 21 HSCT patients with AdV infection, from a total group of 182 patients. Of note, time dynamics of AdV viral loads in stool specimens was predictive for AdV viraemia, thus suggesting intestinal source to be the primary site of the viral infection [10].
Cytomegalovirus (CMV) viral load was assessed in stool of HSCT as possible marker of acute intestinal graft-versus-host disease posttransplant [11]. CMV DNA was found in 20 of 121 HSCT recipients. However, the authors did not find any correlation between CMV DNA loads and intestinal graft-versus-host disease (aGVHD), as well between viral contents in plasma and stools.
In view of scarce NGS-based virome studies in HSCT, it is very important to detect and realize significance of multiple viral species in progression of intestinal syndrome. Over the past 5 years, several papers have been published on the metagenomics of viruses after HSCT [12]. The authors traced the virome dynamics in 44 HSCT recipients using metagenomics techniques (NexTera, HiSeq platforms). Following transplantation (i.e., during transient immune deficiency), Anelloviridae, herpes viruses, papilloma and polyoma viruses, especially picobirnavirus were “flourishing” in intestinal GVHD. Moreover, decreased detection rates were noted for bacteriophages, except for Siphoviridae. A similar work was carried out by a Dutch group [13]. The authors used the original ViroCap system to study fecal samples, with a large panel of probes for 34 families of DNA and RNA viruses (a total of 337 species). After enrichment of viral DNA in the samples, the NGS procedures were performed. At the same time, it was possible to identify, along with known noro- and adenoviruses, also rhinovirus, herpesvirus-7, VK virus, astrovirus at higher sensitivity, than with conventional PCR tests. This approach revealed new associations between the findings of viral sequences and intestinal GVHD. Temporal changes of the patient's microbiota after GVHD and weekly fecal microbiota transplantation in a single patient was studied by Chinese workers [14]. Biodiversity of bacterial species gradually increased after successful TFM, whereas the spectrum of viruses, in general, expanded with time after FMT, e.g., Caudivirales content was increased.
Intestinal phageome
Current metagenomic approaches allowed to look for the whole spectrum of virome, including human viruses and bacteriophages in human microbiota which seems to become a feasible task of clinical significance [15].
For more than 100 years, the bacteriophages were extensively studied using bacteriolysis in classical cultures, morphological and, later molecular PCR methods. Therefore, current criteria for bacteriophage classification are based on their biological properties (lytic or temperate forms; bacterial targets), morphology, main location site etc. [16].
Some of the bacteriophages are widely used in clinical practice to combat antibiotic-resistant bacteria [17].
However, the full-scale analysis of multiple phage populations became available only few years ago, with an advent of high-throughput metagenomic sequencing performed at various NGS platforms [18].
This approach makes it possible to assess the entire community of pathogenic viruses, especially, different bacteriophage species in fecal samples. Different bacteriophages are, generally, specific for distinct bacterial species [19]. Therefore, spectrum and content of intestinal bacteriophages, by definition, depends on the dynamics of the bacterial hosts in the microbiota, and vice versa. Dietary habits, age and antibiotic therapy may influence composition and dynamics of both bacteria and their phages. E.g., starting from birth, the Caudovirales content and phage biodiversity change significantly with age [18].
Shotgun metagenomic methods allow evaluating the entire population of genes in a sample (though at different detection efficiencies). E.c., French authors have applied this technique to study the intestinal bacterial microbiota upon exposure to antimicrobial drug (cefprozil) Immediately after therapy, the total content of metagenomic sequences was expected to decrease. However, 3 months after the therapy, new previously absent gene sequences appeared in the intestinal microbiota and, what is important, the content of antibiotic resistance signatures increased, especially in individuals with initially low biodiversity of the intestinal microbiota. [20]. Different mechanisms of horizontal gene transfer via bacteriophages are shown in Fig. 2.

Figure 2. Horizontal gene transfer can commonly occur through conjugation and natural transformation. Additionally, it may occur through transduction, where resistance is transmitted via bacteriophage. (From [21])
Metagenomic studies of intestinal phageome and resistance genes
Previous knowledge of intestinal virome was, mostly, based on classical studies of bacteriophages detectable by their bacteriolytic effects. Tremendous information on intestinal phages accumulated over XX century. The data on eukaryotic cell viruses are also quite extensive, mostly concerning distinct pathogenic viral species.
Current technical advances, i.e., next-generation sequencing (NGS) enabled coverage of the whole intestinal virome. Metagenomics makes it possible to identify and evaluate both temporal dynamics of phage populations, and their relationship with antibiotic resistance genes that promoting colonization of intestines by pathogenic bacterial strains.
Over the past 3-5 years, the work was started with searching phage sequences and antibiotic resistance genes using new generation sequencing (NGS) methods. Thus, Fernández-Orth et al. investigated DNA from samples of intestinal microbiota in healthy individuals and after treatment with ciprofloxacin, using deep sequencing methods (MySec, Illumina). Assembly of the sequences obtained was evaluated using Kraken software. The samples were found to contain from 4 to 266 viruses. Caudovirales predominated among bacteriophages, and the phages of Siphoviridae and Myoviridae families were also identified. Antibiotic resistance genes were also found in bacterial DNA, and their content was significantly increased after treatment with ciprofloxacin [22].
In other study, bioinformatic analysis of multiple viral sequences from human intestinal microbiome was carried out 4 weeks after a course of antibiotics. The authors found a significant increase in the number of gene signatures (scaffolds) of antibiotic-resistant genes after antibiotic therapy [23].
However, current evidence show that the antibiotic resistance genes can be inserted into plasmids and other mobile genetic elements, which can be transmitted both vertically and by horizontal transfer within the bacterial population, which is essential for accumulation of resistant strains within the intestinal microbiota. Such evidence has been obtained recently. For example, gene sequences were screened for 254 strains of Klebsiella pneumoniae, which most often acquires the drug resistance genes [24]. They showed that most of the studied strains contained intact prophage sequences. An additional analysis of 42 K.pneumoniae strains showed that these phages belong to the families Myoviridae, Siphoviridae, and Podoviridae. Interestingly, no virulence genes were detected in these prophages; however, in 2% of cases, these prophages also encoded genes related to antibiotic resistance factors.
Probable bacteria-phage interactions in HSCT patients
However, most of these clinical studies dealt with the state and ways of restoring the bacterial component of the microbiota, while missing possible role of bacteriophages in severe intestinal dysbiosis. The latter direction can also be promising, as shown by the data of NGS studies on the stability of intestinal bacteriophage populations during fecal transplantation [25]. Meanwhile, there are still no proven positive results from phage therapy in severe intestinal dysbiosis. It is believed that in the future it will be possible to develop modified bacteriophages adapted to specific resistant bacteria [26]. So far, however, there is a process of accumulating information about the bacterial-viral complex of the intestinal microbiota in severe intestinal syndromes of infectious origin.
Posttransplant period is often associated with severe colitis often associated to persistent infection with K.pneumoniae, pathogenic E.coli, C.difficile as reviewed in [27]. Meanwhile, treatment of bacterial infections using species-specific bacteriophages, in particular – those against Staphylococci, E. coli, etc. has been successfully used for decades. The history of these developments is well described [17]. However, the desirable clinical effects were temporary, often due to the development of resistance to phage therapy [19]. British scientists have discovered that optimal combinations of several bacteriophages are most effective in suppressing and preventing phage resistance of C.difficile, as was previously shown experimentally [28]. In hamster experiments, treatment with optimized phage mixtures led to rapid decrease in the C.difficile colonization. Clinical transplantation of intestinal microbiota also suggests possible clinical effects of non-bacterial pathogens. E.g., Ott et al. [29] administered sterile filtrates of donor stool through a nasal cannule to the patients with Clostridium difficile infection. Usage of this bacteria-free drug in 5 patients led to long-term normalization of intestinal disorder, as well as appropriate changes in bacterial and intestinal microbiota. The authors suggest that bacteriophages from the stool filtrate may cause similar positive effects. Hence, these findings enable search and identification of intestinal bacteriophages associated with positive and persistent effects of colitis therapy, especially at the present time, when effective methods of metagenomic DNA sequencing are developed for such studies.
Conclusion
Gut microbiota is a complex symbiosis of bacteria, viruses and fungi. However, unlike the bacterial microbiota (bacteriome), the viral gut community (gut virome) is studied to much lesser degree, due to absence of common target gene for nucleic acid sequencing in viruses. Over last years, the whole-genome screening techniques using next-generation sequencing (NGS) promoted our knowledge in the field. This approach allowed to estimate the biodiversity of human viruses and bacteriophages in the individual samples of intestinal microbiota in health and disease.
Studies of the virome changes after HSCT are now launched, however, being at initial stage. Meanwhile, the changes in bacteriophage community (phageome) and its diversity post-transplant may explain both therapy-associated changes of bacteriome, and development of bacterial antibiotic resistance, due to phage-mediated transfer of antibiotic resistance genes.
Conflicts of interest
None conflicts of interest are declared.
Acknowledgements
The authors are much appreciated to the Pediatric Research and Clinical Center for Infectious Diseases, and dedicate the present article to the 95th anniversary of this institution.
References
- Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system Mil Med Res 2017; 4:14.
doi: 10.1186/s40779-017-0122-9 - Smits SL, Schapendonk CM, van Beek J, Vennema H, Schürch AC, Schipper D, et al. New viruses in idiopathic human diarrhea cases, the Netherlands. Emerg Infect Dis. 2014; 20(7):1218-22. doi: 10.3201/eid2007.140190
- Carding SR, Davis N, Hoyles L. Review article: the human intestinal virome in health and disease. Aliment Pharmacol Ther. 2017; 46(9):800-815. doi: 10.1111/apt.14280
- Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019; 14:319-338. doi: 10.1146/annurev-pathmechdis-012418-012751
- Nahar S, Iraha A, Hokama A, Uehara A, Parrott G, Ohira T, et al. Evaluation of a multiplex PCR assay for detection of cytomegalovirus in stool samples from patients with ulcerative colitis. World J Gastroenterol. 2015; 21(44):12667-75. doi: 10.3748/wjg.v21.i44.12667
- Sun G, Zang Q, Gu Y, Niu G, Ding C, Zhang P. Viral metagenomics analysis of picobirnavirus-positive feces from children with sporadic diarrhea in China. Arch Virol. 2016; 161 (4): 971-5. doi: 10.1007/s00705-015-2726-2
- Zhou Y, Wylie KM, El Feghaly RE, Mihindukulasuriya KA, Elward A, Haslam DB, Storch GA, Weinstock GM. Metagenomic Approach for Identification of the Pathogens Associated with Diarrhea in Stool Specimens. J Clin Microbiol. 2016; 54 (2): 368-75.
doi: 10.1128/JCM.01965-15 - Fernandez-Cassi X, Martínez-Puchol S, Silva-Sales M, Cornejo T, Bartolome R, Bofill-Mas S, Girones R. Unveiling Viruses Associated with Gastroenteritis Using a Metagenomics Approach. Viruses. 2020; 12 (12): 1432. doi: 10.3390/v12121432
- Legoff J, Feghoul L, Mercier-Delarue S, Dalle JH, Scieux C, Chérot J, et al. Broad-range PCR-electrospray ionization mass spectrometry for detection and typing of adenovirus and other opportunistic viruses in stem cell transplant patients. J Clin Microbiol. 2013; 51(12):4186-92. doi: 10.1128/JCM.01978-13
- Jeulin H, Salmon A, Bordigoni P, Venard V. Diagnostic value of quantitative PCR for adenovirus detection in stool samples as compared with antigen detection and cell culture in haematopoietic stem cell transplant recipients. Clin Microbiol Infect. 2011;17(11):1674-80. doi: 10.1111/j.1469-0691.2011.03488.x
- Bueno F, Albert E, Giménez E, Piñana JL, Pérez A, Gómez MD, et al., 2020. Cytomegalovirus DNA load monitoring in stool specimens for anticipating the occurrence of intestinal acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: Is it of any value? Transpl Infect Dis. 2020; 22(6):e13440. doi: 10.1111/tid.13440
- Legoff J, Resche-Rigon M, Bouquet J, Robin M, Naccache SN, Mercier-Delarue S, et al. The eukaryotic gut virome in hematopoietic stem cell transplantation: new clues in enteric graft-versus-host disease. Nat Med. 2017; 23 (9): 1080-1085. doi: 10.1038/nm.4380
- Jansen SA, Nijhuis W, Leavis HL, Riezebos-Brilman A, Lindemans CA, Schuurman R. Broad virus detection and variant discovery in fecal samples of hematopoietic transplant recipients using targeted sequence capture metagenomics. Front Microbiol. 2020; 11: 560179. doi: 10.3389/ fmicb.2020.560179
- Zhang F, Zuo T, Yeoh YK, Cheng FWT, Liu Q, Tang W, et al. Longitudinal dynamics of gut bacteriome, mycobiome and virome after fecal microbiota transplantation in graft-versus-host disease. Nat Commun. 2021; 12 (1): 65. doi: 10.1038/s41467-020-20240-x
- Wang W, Jovel J, Halloran B, Wine E, Patterson J, Ford G, et al. Metagenomic analysis of microbiome in colon tissue from subjects with inflammatory bowel diseases reveals interplay of viruses and bacteria. Inflamm Bowel Dis. 2015; 21 (6): 1419-27.
doi: 10.1097/MIB.0000000000000344 - Principi N, Silvestri E, Esposito S Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019;10:513. doi: 10.3389/fphar.2019.00513
- Brüssow H. Phage therapy for the treatment of human intestinal bacterial infections: soon to be a reality? Exp Rev Gastroenterol Hepatol. 2017. doi: 10.1080/17474124.2017.1342534
- Townsend EM, Kelly L, Muscatt G, Box JD, Hargraves N, Lilley D, Jameson E. The human gut phageome: Origins and roles in the human gut microbiome. Front Cell Infect Microbiol. 2021; 11: 643214. doi: 10.3389/fcimb.2021.643214
- Oechslin F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 2018, 10, 351.
doi: 10.3390/v10070351 - Raymond F, Déraspe M, Boissinot M, Bergeron MG, Corbeil J. Partial recovery of microbiomes after antibiotic treatment. Gut Microbes. 2016; 7 (5): 428-34. doi: 10.1080/ 19490976.2016.1216747
- Schroeder M, Brooks BD, Brooks AE. The Complex Relationship between Virulence and Antibiotic Resistance. Genes. 2017; 8(1):39. https://doi.org/10.3390/genes8010039
- Fernández-Orth D, Miró E, Brown-Jaque M, Rodríguez-Rubio L, Espinal P, Rodriguez-Navarro J, et al. Faecal phageome of healthy individuals: presence of antibiotic resistance genes and variations caused by ciprofloxacin treatment. J Antimicrob Chemother. 2019; 74 (4): 854-864. doi: 10.1093/jac/dky540
- Górska A, Peter S, Willmann M, Autenrieth I, Schlaberg R, Huson DH. Dynamics of the human gut phageome during antibiotic treatment Comput Biol Chem. 2018; 74: 420-427. doi: 10.1016/j.compbiolchem.2018.03.011
- Baliga P, Shekar M, Kallappa GS. Genome-wide identification and analysis of chromosomally integrated putative prophages associated with clinical Klebsiella pneumoniae strains. Curr Microbiol 2021; 78 (5): 2015-2024. doi: 10.1007/s00284-021-02472-2
- Broecker F, Russo G, Klumpp J, Moelling K. Stable core virome despite variable microbiome after fecal transfer. Gut Microbes. 2017; 8 (3): 214-220. doi: 10.1080/19490976.2016.1265196
- Paule A, Frezza D, Edeas M. Microbiota and phage therapy: Future challenges in medicine. Med Sci (Basel). 2018; 6 (4): 86.
doi: 10.3390/medsci6040086 - Goloshchapov OV, Kucher MA, Chukhlovin AB. Gut microbiome in hematopoietic stem cell transplantation: patient-and treatment-related factors. Cell Ther Transpant. 2018; 7(4): 16-28. doi: 10.18620/ctt-1866-8836-2018-7-4-16-28
- Nale JY, Spencer J, Hargreaves KR, Buckley AM, Trzepiński P, Douce GR, Clokie MR. Bacteriophage combinations significantly reduce Clostridium difficile growth in vitro and proliferation in vivo. Antimicrob Agents Chemother. 2015; 60 (2): 968-981.
doi: 10.1128/AAC.01774-15 - Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017; 152 (4): 799-811.e7. doi: 10.1053/ j.gastro.2016.11.010
" ["~DETAIL_TEXT"]=> string(29610) "
Introduction
It is generally known that intestinal microbiota is a symbiotic community including many types of bacteria, fungi and viruses. It is a dynamic biological system with an average weight of 2-3 kg, producing many metabolites (metabolic substances, vitamins, neurotransmitters, etc.) necessary for existence and growth of the host organism. The gut microbiota interacts with intestinal epithelial cell layers which are exposed both to endogenous pathogenic (e.g., blood-borne) viruses, and to intestinal bacterial populations. In turn, the gut microbed strains are affected by various bacteriophage populations causing their lysis. The surviving bacteria may acquire mobile gene elements, including those providing resistance to antibiotics (Fig. 1). These polyresistant bacteria are an important cause of life-threatening infections in immunocompromised patients, e.g., after intensive cytostatic therapy and hematopoietic stem cell transplantation (HSCT).

Figure 1. Intestinal epithelium is exposed to endogenous host viruses (bottom), and interacts with gut bacteria (light green) which could be lysed by bacteriophages (top left). Some of them may harbor and transfer antibiotic resistance genes to the bacteria (top right)
Moreover, the host immune system develops in permanent contact with the surrounding microbial and viral antigens, especially with the endogenous intestinal microbiota, which includes all these components [1]. Microbial and viral antigens of the intestinal microbiota, passing through the epithelial barriers of the mucous membranes, penetrate into the regional blood vessels and lymph nodes, form an adaptive B- and T-cell immune response, being a key factor in the normal maturation and functioning of the immune system.
For decades, the main attention of bacteriologists was paid to cultivated, mainly aerobic microorganisms – Escherichia, Klebsiella, Enterococci that were studied in details for decades. Cultivable anaerobic gut bacteria were also exhaustively studied over last decades. However, with development of high-throughput DNA diagnostics, the spectrum of known microbial bacteria extended to sufficient degree. Currently, >1000 bacterial species are detectable in normal gut microbiota. Hence, over the past 10-20 years, the novel molecular biology approaches (multiple PCR, high-throughput DNA sequencing) enabled detailed classifying hundreds of species of anaerobic intestinal bacteria, which dominate the intestinal microbiota.
Human viral community (virome) is exhibiting even higher taxonomic and genetic complexity. Formerly, the main interest was drawn to the viruses of eukaryotic cells which could be pathogenic to humans. The modern NGS approach allows to detect both known viruses (herpes, anelloviruses, picobirnaviruses) and a number of previously unknown viral DNA sequences [2].
However, intestinal human virome is dominated by bacteriophages. These are largely represented by Caudiovirales. [3]. This order of phages includes 3 families (Myoviridae; Siphoviridae, and Podoviridae).
Along with double-stranded DNA phages, minor populations of single-stranded DNA and RNA phages are detectable in human gut virome which are also specific for distinct bacterial species as reviewed by Carding et al. [3].
The issues of viral sequences in bacterial and human cells (e.g., prophages, retroviral elements etc.) are also detectable by metagenomics but will not be covered here, because of undefined clinical significance and still undefined systemic approach. Hence, the question about functions and significance of virome abundance and diversity in healthy subjects and GIT disorders is still open.
Current methodology
Unlike the bacterial microbiota, viral intestinal population (gut virome) has been studied to a much lesser extent. Whereas bacterial species have the common target gene for sequencing, i.e., 16SrRNA (the main object for NGS analysis), the viruses lack such a common marker gene, thus hampering easy assessment of their diversity in biological samples.
Currently, there is a broad diagnostic choice in clinical virology, starting with cytopathogenic tests, large arrays of immune diagnostic sets, and modern whole-scale panels of PCR assays. To diagnose some pathogenic viruses (up to 10-15 species), multiplex PCR was developed, and it is commonly used in clinical laboratories. However, these commercial PCR panels cannot cover the entire spectrum of potential viral agents, usually targeting up to 10-12 pathogens.
Therefore, the most comprehensive approach to studying the entire viral community is whole-genome screening of viral sequences by means of NGS followed by extensive bioinformatics. E.g., a random metagenomic analysis may also allow detection of human pathogenic viruses. Methodology of metagenomic studies are well reviewed in [4].
Diversity of human viruses and bacteriophages
A number of endogenous viruses persisting lifelong may be found in stools under certain conditions, e.g., in immunocompromised patients or during immunosuppressive therapy.
Herperviruses in gut are often activated in the patients with inflammatory bowel diseases. E.g., the Japanese group, using high-capacity multiplex PCR with microchip electrophoresis, has detected as much as 191 different bacterial species and 206 viruses in 215 stool samples (71.7% positivity) from immunocompromised patients and subjects with ulcerative colitis [5]. Among viral pathogens, Epstein-Barr virus (EBV) was revealed in 90 samples (30.0%); HHV-6, in 53 cases (17.7%), and cytomegalovirus (CMV) in 37 specimens (12.3%).
Most often, however, human intestinal microbiome is extensively studied in search of the viruses known to be pathogenic (i.e., noroviruses, rotaviruses, astroviruses, adenoviruses etc.). Biological diversity of human pathogenic viruses was first studied in Asian countries, with high incidence of gastroenteritis in pediatric setting [6]. The authors examined stool samples from 7 children positive for picobirnavirus and identified, by means of metagenomics, enterovirus, gyrovirus, parechovirus in these samples, which are potentially pathogenic in gastroenteritis. In addition, a metagenomic study of stool microbiota allows detecting bacterial-viral associations (for example, C.difficile associated with a number of viruses) in intestinal syndromes as shown by a group from the USA [7]. Metagenomic analysis was also used for NGS analysis of stool samples in gastroenteritis, and, except of common noroviruses, additional pathogens were found from the genus Astroviridae and Caliciviridae [8].
Gut human virome after HSCT
A limited number of works concerned presence of intestinal viruses posttransplant. E.g., Legoff et al. have detected different viruses in stool and plasma following HSCT using electrospray ionization with mass-spectrometric [9]. The workers have revealed high adenovirus (AdV) viral load in stool samples. In an earlier study, AdV DNA was found in stools of 21 HSCT patients with AdV infection, from a total group of 182 patients. Of note, time dynamics of AdV viral loads in stool specimens was predictive for AdV viraemia, thus suggesting intestinal source to be the primary site of the viral infection [10].
Cytomegalovirus (CMV) viral load was assessed in stool of HSCT as possible marker of acute intestinal graft-versus-host disease posttransplant [11]. CMV DNA was found in 20 of 121 HSCT recipients. However, the authors did not find any correlation between CMV DNA loads and intestinal graft-versus-host disease (aGVHD), as well between viral contents in plasma and stools.
In view of scarce NGS-based virome studies in HSCT, it is very important to detect and realize significance of multiple viral species in progression of intestinal syndrome. Over the past 5 years, several papers have been published on the metagenomics of viruses after HSCT [12]. The authors traced the virome dynamics in 44 HSCT recipients using metagenomics techniques (NexTera, HiSeq platforms). Following transplantation (i.e., during transient immune deficiency), Anelloviridae, herpes viruses, papilloma and polyoma viruses, especially picobirnavirus were “flourishing” in intestinal GVHD. Moreover, decreased detection rates were noted for bacteriophages, except for Siphoviridae. A similar work was carried out by a Dutch group [13]. The authors used the original ViroCap system to study fecal samples, with a large panel of probes for 34 families of DNA and RNA viruses (a total of 337 species). After enrichment of viral DNA in the samples, the NGS procedures were performed. At the same time, it was possible to identify, along with known noro- and adenoviruses, also rhinovirus, herpesvirus-7, VK virus, astrovirus at higher sensitivity, than with conventional PCR tests. This approach revealed new associations between the findings of viral sequences and intestinal GVHD. Temporal changes of the patient's microbiota after GVHD and weekly fecal microbiota transplantation in a single patient was studied by Chinese workers [14]. Biodiversity of bacterial species gradually increased after successful TFM, whereas the spectrum of viruses, in general, expanded with time after FMT, e.g., Caudivirales content was increased.
Intestinal phageome
Current metagenomic approaches allowed to look for the whole spectrum of virome, including human viruses and bacteriophages in human microbiota which seems to become a feasible task of clinical significance [15].
For more than 100 years, the bacteriophages were extensively studied using bacteriolysis in classical cultures, morphological and, later molecular PCR methods. Therefore, current criteria for bacteriophage classification are based on their biological properties (lytic or temperate forms; bacterial targets), morphology, main location site etc. [16].
Some of the bacteriophages are widely used in clinical practice to combat antibiotic-resistant bacteria [17].
However, the full-scale analysis of multiple phage populations became available only few years ago, with an advent of high-throughput metagenomic sequencing performed at various NGS platforms [18].
This approach makes it possible to assess the entire community of pathogenic viruses, especially, different bacteriophage species in fecal samples. Different bacteriophages are, generally, specific for distinct bacterial species [19]. Therefore, spectrum and content of intestinal bacteriophages, by definition, depends on the dynamics of the bacterial hosts in the microbiota, and vice versa. Dietary habits, age and antibiotic therapy may influence composition and dynamics of both bacteria and their phages. E.g., starting from birth, the Caudovirales content and phage biodiversity change significantly with age [18].
Shotgun metagenomic methods allow evaluating the entire population of genes in a sample (though at different detection efficiencies). E.c., French authors have applied this technique to study the intestinal bacterial microbiota upon exposure to antimicrobial drug (cefprozil) Immediately after therapy, the total content of metagenomic sequences was expected to decrease. However, 3 months after the therapy, new previously absent gene sequences appeared in the intestinal microbiota and, what is important, the content of antibiotic resistance signatures increased, especially in individuals with initially low biodiversity of the intestinal microbiota. [20]. Different mechanisms of horizontal gene transfer via bacteriophages are shown in Fig. 2.

Figure 2. Horizontal gene transfer can commonly occur through conjugation and natural transformation. Additionally, it may occur through transduction, where resistance is transmitted via bacteriophage. (From [21])
Metagenomic studies of intestinal phageome and resistance genes
Previous knowledge of intestinal virome was, mostly, based on classical studies of bacteriophages detectable by their bacteriolytic effects. Tremendous information on intestinal phages accumulated over XX century. The data on eukaryotic cell viruses are also quite extensive, mostly concerning distinct pathogenic viral species.
Current technical advances, i.e., next-generation sequencing (NGS) enabled coverage of the whole intestinal virome. Metagenomics makes it possible to identify and evaluate both temporal dynamics of phage populations, and their relationship with antibiotic resistance genes that promoting colonization of intestines by pathogenic bacterial strains.
Over the past 3-5 years, the work was started with searching phage sequences and antibiotic resistance genes using new generation sequencing (NGS) methods. Thus, Fernández-Orth et al. investigated DNA from samples of intestinal microbiota in healthy individuals and after treatment with ciprofloxacin, using deep sequencing methods (MySec, Illumina). Assembly of the sequences obtained was evaluated using Kraken software. The samples were found to contain from 4 to 266 viruses. Caudovirales predominated among bacteriophages, and the phages of Siphoviridae and Myoviridae families were also identified. Antibiotic resistance genes were also found in bacterial DNA, and their content was significantly increased after treatment with ciprofloxacin [22].
In other study, bioinformatic analysis of multiple viral sequences from human intestinal microbiome was carried out 4 weeks after a course of antibiotics. The authors found a significant increase in the number of gene signatures (scaffolds) of antibiotic-resistant genes after antibiotic therapy [23].
However, current evidence show that the antibiotic resistance genes can be inserted into plasmids and other mobile genetic elements, which can be transmitted both vertically and by horizontal transfer within the bacterial population, which is essential for accumulation of resistant strains within the intestinal microbiota. Such evidence has been obtained recently. For example, gene sequences were screened for 254 strains of Klebsiella pneumoniae, which most often acquires the drug resistance genes [24]. They showed that most of the studied strains contained intact prophage sequences. An additional analysis of 42 K.pneumoniae strains showed that these phages belong to the families Myoviridae, Siphoviridae, and Podoviridae. Interestingly, no virulence genes were detected in these prophages; however, in 2% of cases, these prophages also encoded genes related to antibiotic resistance factors.
Probable bacteria-phage interactions in HSCT patients
However, most of these clinical studies dealt with the state and ways of restoring the bacterial component of the microbiota, while missing possible role of bacteriophages in severe intestinal dysbiosis. The latter direction can also be promising, as shown by the data of NGS studies on the stability of intestinal bacteriophage populations during fecal transplantation [25]. Meanwhile, there are still no proven positive results from phage therapy in severe intestinal dysbiosis. It is believed that in the future it will be possible to develop modified bacteriophages adapted to specific resistant bacteria [26]. So far, however, there is a process of accumulating information about the bacterial-viral complex of the intestinal microbiota in severe intestinal syndromes of infectious origin.
Posttransplant period is often associated with severe colitis often associated to persistent infection with K.pneumoniae, pathogenic E.coli, C.difficile as reviewed in [27]. Meanwhile, treatment of bacterial infections using species-specific bacteriophages, in particular – those against Staphylococci, E. coli, etc. has been successfully used for decades. The history of these developments is well described [17]. However, the desirable clinical effects were temporary, often due to the development of resistance to phage therapy [19]. British scientists have discovered that optimal combinations of several bacteriophages are most effective in suppressing and preventing phage resistance of C.difficile, as was previously shown experimentally [28]. In hamster experiments, treatment with optimized phage mixtures led to rapid decrease in the C.difficile colonization. Clinical transplantation of intestinal microbiota also suggests possible clinical effects of non-bacterial pathogens. E.g., Ott et al. [29] administered sterile filtrates of donor stool through a nasal cannule to the patients with Clostridium difficile infection. Usage of this bacteria-free drug in 5 patients led to long-term normalization of intestinal disorder, as well as appropriate changes in bacterial and intestinal microbiota. The authors suggest that bacteriophages from the stool filtrate may cause similar positive effects. Hence, these findings enable search and identification of intestinal bacteriophages associated with positive and persistent effects of colitis therapy, especially at the present time, when effective methods of metagenomic DNA sequencing are developed for such studies.
Conclusion
Gut microbiota is a complex symbiosis of bacteria, viruses and fungi. However, unlike the bacterial microbiota (bacteriome), the viral gut community (gut virome) is studied to much lesser degree, due to absence of common target gene for nucleic acid sequencing in viruses. Over last years, the whole-genome screening techniques using next-generation sequencing (NGS) promoted our knowledge in the field. This approach allowed to estimate the biodiversity of human viruses and bacteriophages in the individual samples of intestinal microbiota in health and disease.
Studies of the virome changes after HSCT are now launched, however, being at initial stage. Meanwhile, the changes in bacteriophage community (phageome) and its diversity post-transplant may explain both therapy-associated changes of bacteriome, and development of bacterial antibiotic resistance, due to phage-mediated transfer of antibiotic resistance genes.
Conflicts of interest
None conflicts of interest are declared.
Acknowledgements
The authors are much appreciated to the Pediatric Research and Clinical Center for Infectious Diseases, and dedicate the present article to the 95th anniversary of this institution.
References
- Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system Mil Med Res 2017; 4:14.
doi: 10.1186/s40779-017-0122-9 - Smits SL, Schapendonk CM, van Beek J, Vennema H, Schürch AC, Schipper D, et al. New viruses in idiopathic human diarrhea cases, the Netherlands. Emerg Infect Dis. 2014; 20(7):1218-22. doi: 10.3201/eid2007.140190
- Carding SR, Davis N, Hoyles L. Review article: the human intestinal virome in health and disease. Aliment Pharmacol Ther. 2017; 46(9):800-815. doi: 10.1111/apt.14280
- Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019; 14:319-338. doi: 10.1146/annurev-pathmechdis-012418-012751
- Nahar S, Iraha A, Hokama A, Uehara A, Parrott G, Ohira T, et al. Evaluation of a multiplex PCR assay for detection of cytomegalovirus in stool samples from patients with ulcerative colitis. World J Gastroenterol. 2015; 21(44):12667-75. doi: 10.3748/wjg.v21.i44.12667
- Sun G, Zang Q, Gu Y, Niu G, Ding C, Zhang P. Viral metagenomics analysis of picobirnavirus-positive feces from children with sporadic diarrhea in China. Arch Virol. 2016; 161 (4): 971-5. doi: 10.1007/s00705-015-2726-2
- Zhou Y, Wylie KM, El Feghaly RE, Mihindukulasuriya KA, Elward A, Haslam DB, Storch GA, Weinstock GM. Metagenomic Approach for Identification of the Pathogens Associated with Diarrhea in Stool Specimens. J Clin Microbiol. 2016; 54 (2): 368-75.
doi: 10.1128/JCM.01965-15 - Fernandez-Cassi X, Martínez-Puchol S, Silva-Sales M, Cornejo T, Bartolome R, Bofill-Mas S, Girones R. Unveiling Viruses Associated with Gastroenteritis Using a Metagenomics Approach. Viruses. 2020; 12 (12): 1432. doi: 10.3390/v12121432
- Legoff J, Feghoul L, Mercier-Delarue S, Dalle JH, Scieux C, Chérot J, et al. Broad-range PCR-electrospray ionization mass spectrometry for detection and typing of adenovirus and other opportunistic viruses in stem cell transplant patients. J Clin Microbiol. 2013; 51(12):4186-92. doi: 10.1128/JCM.01978-13
- Jeulin H, Salmon A, Bordigoni P, Venard V. Diagnostic value of quantitative PCR for adenovirus detection in stool samples as compared with antigen detection and cell culture in haematopoietic stem cell transplant recipients. Clin Microbiol Infect. 2011;17(11):1674-80. doi: 10.1111/j.1469-0691.2011.03488.x
- Bueno F, Albert E, Giménez E, Piñana JL, Pérez A, Gómez MD, et al., 2020. Cytomegalovirus DNA load monitoring in stool specimens for anticipating the occurrence of intestinal acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: Is it of any value? Transpl Infect Dis. 2020; 22(6):e13440. doi: 10.1111/tid.13440
- Legoff J, Resche-Rigon M, Bouquet J, Robin M, Naccache SN, Mercier-Delarue S, et al. The eukaryotic gut virome in hematopoietic stem cell transplantation: new clues in enteric graft-versus-host disease. Nat Med. 2017; 23 (9): 1080-1085. doi: 10.1038/nm.4380
- Jansen SA, Nijhuis W, Leavis HL, Riezebos-Brilman A, Lindemans CA, Schuurman R. Broad virus detection and variant discovery in fecal samples of hematopoietic transplant recipients using targeted sequence capture metagenomics. Front Microbiol. 2020; 11: 560179. doi: 10.3389/ fmicb.2020.560179
- Zhang F, Zuo T, Yeoh YK, Cheng FWT, Liu Q, Tang W, et al. Longitudinal dynamics of gut bacteriome, mycobiome and virome after fecal microbiota transplantation in graft-versus-host disease. Nat Commun. 2021; 12 (1): 65. doi: 10.1038/s41467-020-20240-x
- Wang W, Jovel J, Halloran B, Wine E, Patterson J, Ford G, et al. Metagenomic analysis of microbiome in colon tissue from subjects with inflammatory bowel diseases reveals interplay of viruses and bacteria. Inflamm Bowel Dis. 2015; 21 (6): 1419-27.
doi: 10.1097/MIB.0000000000000344 - Principi N, Silvestri E, Esposito S Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019;10:513. doi: 10.3389/fphar.2019.00513
- Brüssow H. Phage therapy for the treatment of human intestinal bacterial infections: soon to be a reality? Exp Rev Gastroenterol Hepatol. 2017. doi: 10.1080/17474124.2017.1342534
- Townsend EM, Kelly L, Muscatt G, Box JD, Hargraves N, Lilley D, Jameson E. The human gut phageome: Origins and roles in the human gut microbiome. Front Cell Infect Microbiol. 2021; 11: 643214. doi: 10.3389/fcimb.2021.643214
- Oechslin F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 2018, 10, 351.
doi: 10.3390/v10070351 - Raymond F, Déraspe M, Boissinot M, Bergeron MG, Corbeil J. Partial recovery of microbiomes after antibiotic treatment. Gut Microbes. 2016; 7 (5): 428-34. doi: 10.1080/ 19490976.2016.1216747
- Schroeder M, Brooks BD, Brooks AE. The Complex Relationship between Virulence and Antibiotic Resistance. Genes. 2017; 8(1):39. https://doi.org/10.3390/genes8010039
- Fernández-Orth D, Miró E, Brown-Jaque M, Rodríguez-Rubio L, Espinal P, Rodriguez-Navarro J, et al. Faecal phageome of healthy individuals: presence of antibiotic resistance genes and variations caused by ciprofloxacin treatment. J Antimicrob Chemother. 2019; 74 (4): 854-864. doi: 10.1093/jac/dky540
- Górska A, Peter S, Willmann M, Autenrieth I, Schlaberg R, Huson DH. Dynamics of the human gut phageome during antibiotic treatment Comput Biol Chem. 2018; 74: 420-427. doi: 10.1016/j.compbiolchem.2018.03.011
- Baliga P, Shekar M, Kallappa GS. Genome-wide identification and analysis of chromosomally integrated putative prophages associated with clinical Klebsiella pneumoniae strains. Curr Microbiol 2021; 78 (5): 2015-2024. doi: 10.1007/s00284-021-02472-2
- Broecker F, Russo G, Klumpp J, Moelling K. Stable core virome despite variable microbiome after fecal transfer. Gut Microbes. 2017; 8 (3): 214-220. doi: 10.1080/19490976.2016.1265196
- Paule A, Frezza D, Edeas M. Microbiota and phage therapy: Future challenges in medicine. Med Sci (Basel). 2018; 6 (4): 86.
doi: 10.3390/medsci6040086 - Goloshchapov OV, Kucher MA, Chukhlovin AB. Gut microbiome in hematopoietic stem cell transplantation: patient-and treatment-related factors. Cell Ther Transpant. 2018; 7(4): 16-28. doi: 10.18620/ctt-1866-8836-2018-7-4-16-28
- Nale JY, Spencer J, Hargreaves KR, Buckley AM, Trzepiński P, Douce GR, Clokie MR. Bacteriophage combinations significantly reduce Clostridium difficile growth in vitro and proliferation in vivo. Antimicrob Agents Chemother. 2015; 60 (2): 968-981.
doi: 10.1128/AAC.01774-15 - Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology. 2017; 152 (4): 799-811.e7. doi: 10.1053/ j.gastro.2016.11.010
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(100) "potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov" ["~CODE"]=> string(100) "potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov" ["EXTERNAL_ID"]=> string(4) "2040" ["~EXTERNAL_ID"]=> string(4) "2040" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(346) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзорPossible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4017) "<p style="text-align: justify;"> Микробиота кишечника (сложный симбиоз бактерий, грибов и вирусов) – это динамическая биологическая система, необходимая для существования и роста человеческого организма. Состав и соотношение бактериальных популяций серьезно нарушаются при тяжелых колитах и болезни «трансплантат против хозяина» (GVHD), возникающих после трансплантации гемопоэтических стволовых клеток (HSCT), особенно при развитии устойчивых к антибиотикам бактериальных штаммов. В отличие от хорошо известной бактериальной микробиоты, изученной с помощью классической бактериологии и секвенирования гена 16S рРНК, вирусные популяции кишечной микробиоты (например, бактериофагов) в этих клинических условиях изучены недостаточно из-за отсутствия общего вирусного гена, пригодного для сравнительного молекулярно-генетического анализа. Оценка соотношений вирусной и бактериальной кишечной микробиоты возможна с помощью метагеномных методов анализа множества видов ДНК в образцах биоматериала. Объектом клинического исследования являются пациенты с инфекционными осложнениями, вызванными массивным антибактериальным и цитостатическим лечением. </p> <p style="text-align: justify;"> Особое внимание следует обратить на тяжелый колит с инфекцией <i>C.difficile</i> и устойчивой к антибиотикам <i>K. pneumoniae</i>, а также другими патогенами, как при трансплантации фекальной микробиоты (FMT), так и без нее. Обычная оценка кишечной микробиоты будет осуществляться путем секвенирования следующего поколения (NGS) на основе генного разнообразия 16S рДНК для бактериальных генов и метагеномного анализа NGS, чтобы оценить соотношение различных вирусов эукариотических клеток и, в частности, бактериофагов в случае дисбактериоза кишечника. Следует установить типичные нарушения кишечного вирома, а также их роль в колонизации кишечными бактериями, устойчивыми к антибиотикам, после интенсивной антибиотикотерапии и химиотерапии. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Кишечные инфекции, трансплантация, иммунологические осложнения, кишечная микробиота, вирусы, бактериофаги, антибиотикорезистентность, секвенирование нового поколения (NGS), ген 16S rRNA, метагеномика. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_META_TITLE"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_META_KEYWORDS"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_META_DESCRIPTION"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_PICTURE_FILE_ALT"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_PICTURE_FILE_TITLE"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(221) "Потенциальная роль вирусов человека и бактериофагов после трансплантации гемопоэтических стволовых клеток: мини-обзор" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "potentsialnaya-rol-virusov-cheloveka-i-bakteriofagov-posle-transplantatsii-gemopoeticheskikh-stvolov" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "200" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28405" ["VALUE"]=> array(2) { ["TEXT"]=> string(188) "<p>Олег В. Голощапов<sup>1</sup>, Алексей Б. Чухловин<sup>1,2</sup>, Олег С. Глотов<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(140) "
Олег В. Голощапов1, Алексей Б. Чухловин1,2, Олег С. Глотов2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28406" ["VALUE"]=> array(2) { ["TEXT"]=> string(594) "<p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> Детский научно-практический центр инфекционных болезней ФМБА, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(552) "1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Детский научно-практический центр инфекционных болезней ФМБА, Санкт-Петербург, Россия
Микробиота кишечника (сложный симбиоз бактерий, грибов и вирусов) – это динамическая биологическая система, необходимая для существования и роста человеческого организма. Состав и соотношение бактериальных популяций серьезно нарушаются при тяжелых колитах и болезни «трансплантат против хозяина» (GVHD), возникающих после трансплантации гемопоэтических стволовых клеток (HSCT), особенно при развитии устойчивых к антибиотикам бактериальных штаммов. В отличие от хорошо известной бактериальной микробиоты, изученной с помощью классической бактериологии и секвенирования гена 16S рРНК, вирусные популяции кишечной микробиоты (например, бактериофагов) в этих клинических условиях изучены недостаточно из-за отсутствия общего вирусного гена, пригодного для сравнительного молекулярно-генетического анализа. Оценка соотношений вирусной и бактериальной кишечной микробиоты возможна с помощью метагеномных методов анализа множества видов ДНК в образцах биоматериала. Объектом клинического исследования являются пациенты с инфекционными осложнениями, вызванными массивным антибактериальным и цитостатическим лечением.
Особое внимание следует обратить на тяжелый колит с инфекцией C.difficile и устойчивой к антибиотикам K. pneumoniae, а также другими патогенами, как при трансплантации фекальной микробиоты (FMT), так и без нее. Обычная оценка кишечной микробиоты будет осуществляться путем секвенирования следующего поколения (NGS) на основе генного разнообразия 16S рДНК для бактериальных генов и метагеномного анализа NGS, чтобы оценить соотношение различных вирусов эукариотических клеток и, в частности, бактериофагов в случае дисбактериоза кишечника. Следует установить типичные нарушения кишечного вирома, а также их роль в колонизации кишечными бактериями, устойчивыми к антибиотикам, после интенсивной антибиотикотерапии и химиотерапии.
Ключевые слова
Кишечные инфекции, трансплантация, иммунологические осложнения, кишечная микробиота, вирусы, бактериофаги, антибиотикорезистентность, секвенирование нового поколения (NGS), ген 16S rRNA, метагеномика.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28408" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-19-25" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-19-25" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28409" ["VALUE"]=> array(2) { ["TEXT"]=> string(152) "<p>Oleg V. Goloshchapov<sup>1</sup>, Alexei B. Chukhlovin<sup>1,2</sup>, Oleg S. Glotov<sup>2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(104) "Oleg V. Goloshchapov1, Alexei B. Chukhlovin1,2, Oleg S. Glotov2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28410" ["VALUE"]=> array(2) { ["TEXT"]=> string(956) "<p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Alexei B. Chukhlovin, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 325-00-94<br> E-mail: alexei.chukh@mail.ru</p><br> <p><b>Citation:</b> Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(836) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia
Correspondence:
Dr. Alexei B. Chukhlovin, RM Gorbacheva Research
Institute of Pediatric Oncology, Hematology and
Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 325-00-94
E-mail: alexei.chukh@mail.ru
Citation: Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28414" ["VALUE"]=> array(2) { ["TEXT"]=> string(2124) "<p style="text-align: justify;"> Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with <i>C.difficile</i> infection and antibiotic-resistant <i>K.pneumonia</i> and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2044) "Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with C.difficile infection and antibiotic-resistant K.pneumonia and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy.
Keywords
Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28411" ["VALUE"]=> string(125) "Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(125) "Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28412" ["VALUE"]=> string(4) "2694" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2694" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28413" ["VALUE"]=> string(4) "2695" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2695" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28409" ["VALUE"]=> array(2) { ["TEXT"]=> string(152) "<p>Oleg V. Goloshchapov<sup>1</sup>, Alexei B. Chukhlovin<sup>1,2</sup>, Oleg S. Glotov<sup>2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(104) "Oleg V. Goloshchapov1, Alexei B. Chukhlovin1,2, Oleg S. Glotov2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(104) "Oleg V. Goloshchapov1, Alexei B. Chukhlovin1,2, Oleg S. Glotov2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28414" ["VALUE"]=> array(2) { ["TEXT"]=> string(2124) "<p style="text-align: justify;"> Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with <i>C.difficile</i> infection and antibiotic-resistant <i>K.pneumonia</i> and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2044) "Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with C.difficile infection and antibiotic-resistant K.pneumonia and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy.
Keywords
Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2044) "Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with C.difficile infection and antibiotic-resistant K.pneumonia and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy.
Keywords
Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28408" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-19-25" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-19-25" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-19-25" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28411" ["VALUE"]=> string(125) "Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(125) "Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(125) "Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28410" ["VALUE"]=> array(2) { ["TEXT"]=> string(956) "<p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Alexei B. Chukhlovin, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 325-00-94<br> E-mail: alexei.chukh@mail.ru</p><br> <p><b>Citation:</b> Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(836) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia
Correspondence:
Dr. Alexei B. Chukhlovin, RM Gorbacheva Research
Institute of Pediatric Oncology, Hematology and
Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 325-00-94
E-mail: alexei.chukh@mail.ru
Citation: Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(836) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia
Correspondence:
Dr. Alexei B. Chukhlovin, RM Gorbacheva Research
Institute of Pediatric Oncology, Hematology and
Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 325-00-94
E-mail: alexei.chukh@mail.ru
Citation: Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28405" ["VALUE"]=> array(2) { ["TEXT"]=> string(188) "<p>Олег В. Голощапов<sup>1</sup>, Алексей Б. Чухловин<sup>1,2</sup>, Олег С. Глотов<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(140) "Олег В. Голощапов1, Алексей Б. Чухловин1,2, Олег С. Глотов2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(140) "Олег В. Голощапов1, Алексей Б. Чухловин1,2, Олег С. Глотов2
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28407" ["VALUE"]=> array(2) { ["TEXT"]=> string(4017) "<p style="text-align: justify;"> Микробиота кишечника (сложный симбиоз бактерий, грибов и вирусов) – это динамическая биологическая система, необходимая для существования и роста человеческого организма. Состав и соотношение бактериальных популяций серьезно нарушаются при тяжелых колитах и болезни «трансплантат против хозяина» (GVHD), возникающих после трансплантации гемопоэтических стволовых клеток (HSCT), особенно при развитии устойчивых к антибиотикам бактериальных штаммов. В отличие от хорошо известной бактериальной микробиоты, изученной с помощью классической бактериологии и секвенирования гена 16S рРНК, вирусные популяции кишечной микробиоты (например, бактериофагов) в этих клинических условиях изучены недостаточно из-за отсутствия общего вирусного гена, пригодного для сравнительного молекулярно-генетического анализа. Оценка соотношений вирусной и бактериальной кишечной микробиоты возможна с помощью метагеномных методов анализа множества видов ДНК в образцах биоматериала. Объектом клинического исследования являются пациенты с инфекционными осложнениями, вызванными массивным антибактериальным и цитостатическим лечением. </p> <p style="text-align: justify;"> Особое внимание следует обратить на тяжелый колит с инфекцией <i>C.difficile</i> и устойчивой к антибиотикам <i>K. pneumoniae</i>, а также другими патогенами, как при трансплантации фекальной микробиоты (FMT), так и без нее. Обычная оценка кишечной микробиоты будет осуществляться путем секвенирования следующего поколения (NGS) на основе генного разнообразия 16S рДНК для бактериальных генов и метагеномного анализа NGS, чтобы оценить соотношение различных вирусов эукариотических клеток и, в частности, бактериофагов в случае дисбактериоза кишечника. Следует установить типичные нарушения кишечного вирома, а также их роль в колонизации кишечными бактериями, устойчивыми к антибиотикам, после интенсивной антибиотикотерапии и химиотерапии. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Кишечные инфекции, трансплантация, иммунологические осложнения, кишечная микробиота, вирусы, бактериофаги, антибиотикорезистентность, секвенирование нового поколения (NGS), ген 16S rRNA, метагеномика. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3915) "Микробиота кишечника (сложный симбиоз бактерий, грибов и вирусов) – это динамическая биологическая система, необходимая для существования и роста человеческого организма. Состав и соотношение бактериальных популяций серьезно нарушаются при тяжелых колитах и болезни «трансплантат против хозяина» (GVHD), возникающих после трансплантации гемопоэтических стволовых клеток (HSCT), особенно при развитии устойчивых к антибиотикам бактериальных штаммов. В отличие от хорошо известной бактериальной микробиоты, изученной с помощью классической бактериологии и секвенирования гена 16S рРНК, вирусные популяции кишечной микробиоты (например, бактериофагов) в этих клинических условиях изучены недостаточно из-за отсутствия общего вирусного гена, пригодного для сравнительного молекулярно-генетического анализа. Оценка соотношений вирусной и бактериальной кишечной микробиоты возможна с помощью метагеномных методов анализа множества видов ДНК в образцах биоматериала. Объектом клинического исследования являются пациенты с инфекционными осложнениями, вызванными массивным антибактериальным и цитостатическим лечением.
Особое внимание следует обратить на тяжелый колит с инфекцией C.difficile и устойчивой к антибиотикам K. pneumoniae, а также другими патогенами, как при трансплантации фекальной микробиоты (FMT), так и без нее. Обычная оценка кишечной микробиоты будет осуществляться путем секвенирования следующего поколения (NGS) на основе генного разнообразия 16S рДНК для бактериальных генов и метагеномного анализа NGS, чтобы оценить соотношение различных вирусов эукариотических клеток и, в частности, бактериофагов в случае дисбактериоза кишечника. Следует установить типичные нарушения кишечного вирома, а также их роль в колонизации кишечными бактериями, устойчивыми к антибиотикам, после интенсивной антибиотикотерапии и химиотерапии.
Ключевые слова
Кишечные инфекции, трансплантация, иммунологические осложнения, кишечная микробиота, вирусы, бактериофаги, антибиотикорезистентность, секвенирование нового поколения (NGS), ген 16S rRNA, метагеномика.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3915) "Микробиота кишечника (сложный симбиоз бактерий, грибов и вирусов) – это динамическая биологическая система, необходимая для существования и роста человеческого организма. Состав и соотношение бактериальных популяций серьезно нарушаются при тяжелых колитах и болезни «трансплантат против хозяина» (GVHD), возникающих после трансплантации гемопоэтических стволовых клеток (HSCT), особенно при развитии устойчивых к антибиотикам бактериальных штаммов. В отличие от хорошо известной бактериальной микробиоты, изученной с помощью классической бактериологии и секвенирования гена 16S рРНК, вирусные популяции кишечной микробиоты (например, бактериофагов) в этих клинических условиях изучены недостаточно из-за отсутствия общего вирусного гена, пригодного для сравнительного молекулярно-генетического анализа. Оценка соотношений вирусной и бактериальной кишечной микробиоты возможна с помощью метагеномных методов анализа множества видов ДНК в образцах биоматериала. Объектом клинического исследования являются пациенты с инфекционными осложнениями, вызванными массивным антибактериальным и цитостатическим лечением.
Особое внимание следует обратить на тяжелый колит с инфекцией C.difficile и устойчивой к антибиотикам K. pneumoniae, а также другими патогенами, как при трансплантации фекальной микробиоты (FMT), так и без нее. Обычная оценка кишечной микробиоты будет осуществляться путем секвенирования следующего поколения (NGS) на основе генного разнообразия 16S рДНК для бактериальных генов и метагеномного анализа NGS, чтобы оценить соотношение различных вирусов эукариотических клеток и, в частности, бактериофагов в случае дисбактериоза кишечника. Следует установить типичные нарушения кишечного вирома, а также их роль в колонизации кишечными бактериями, устойчивыми к антибиотикам, после интенсивной антибиотикотерапии и химиотерапии.
Ключевые слова
Кишечные инфекции, трансплантация, иммунологические осложнения, кишечная микробиота, вирусы, бактериофаги, антибиотикорезистентность, секвенирование нового поколения (NGS), ген 16S rRNA, метагеномика.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28406" ["VALUE"]=> array(2) { ["TEXT"]=> string(594) "<p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> Детский научно-практический центр инфекционных болезней ФМБА, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(552) "1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Детский научно-практический центр инфекционных болезней ФМБА, Санкт-Петербург, Россия
1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Детский научно-практический центр инфекционных болезней ФМБА, Санкт-Петербург, Россия
Introduction
During the development of malignant neoplasia, a specific cellular environment is formed in the chronic inflammation site termed "inflammatory microenvironment of the tumour" (TME). This cell community consists of tumour-associated macrophages (MF), dendritic cells (DC), myeloid suppressor cells (MSC), neutrophils (NF), mast cells, natural killer cells (NK), T- and B-lymphocytes, cancer-associated fibroblasts (CAF) and endothelial cells. The interaction between tumour cells, myeloid cells and lymphocytes is a dynamic, bidirectional process and includes intercellular contacts, constant exchange of secreted soluble molecules, factors, vesicles, due to which an autonomous system is established that regulates tumour growth [1-5].
Neoplastic progression is associated with lack of oxygen, deficiency of nutrients causing hypoxia and development of metabolic acidosis in the tumour microenvironment. These factors promote selection of tumour cells with the gene mutations that allow them to survive under more severe microenvironmental conditions. Such adaptation of tumour cells is accompanied by increased production of various growth factors, cytokines, chemokines, which together present a triggering factor for enhancement of angiogenesis, metastases, and inhibition of local immune response. In turn, the normal TME cells also begin to secrete factors promoting tumour progression. As a result, a closed-circuit regulatory system is formed [6]. E.g., the content of IL-4 increases in TME, thus inducing differentiation of macrophages to the second-type (M2) resident cells. The M2 subpopulation may account for up to 50 % of the tumour mass and contribute to activation of pro-tumourigenic processes accompanied by the synthesis of IL-1, IL-1RA, IL-4, IL-6, IL-10, IL-12, L-arginine, prostaglandin E2, TNF-α, TGF-β, VEGF-A, and a variety of chemokines and their receptors CCL1, CCL5, CCL17, CCL22, CCL24, CCR2, CXCL10, CXCL16 [7-9]. These mediators are involved in angiogenesis, immunosuppression, and metastasis.
In tumour cells, increased production of some amino acid metabolism enzymes is revealed, e.g., of indolamine 2,3-dioxygenase, arginase-1. Activation of iNOS, as well as STAT3 transcription factor is noted, thereby initiating the differentiation of dendritic cells into tolerogenic tumour-associated dendritic cells (TADC) [10]. These cells produce TGF-β which promotes immunosuppression by stimulating Th2, Th17 and T regulatory cells [11].
Of interest, differentiation of neutrophils in the TME structures depends on the stage of the disease. Thus, the normally pro-inflammatory neutrophils differentiate at later phase to an immunosuppressive phenotype under the influence of TGF-β and angiotensin II [12]. The tumour-associated neutrophils synthesise collagenase IV, heparanase, elastase and matrix metalloproteinases (MMPs) which contribute to extracellular matrix degradation, tumour cell invasion and metastasis. The secreted proteinases destroy extracellular matrix, and degrade the pro-inflammatory cytokines, thus causing anti-inflammatory effects [13]. Neutrophils also produce oncostatin M, which enhances angiogenesis, as well as CXCL1, CXCL8, CCL-3, CXCL6, TGF-β, and prostaglandin E2 synthesis, thus supporting the neoplastic progression [14].
The CSF-1, HIF-1α, CCL2, CCL7, CXCL1 peptide factors synthesised by the TME cell populations are able to alter the metabolism of myeloid cells, leading to transition to MSC [15]. MSC enhance the synthesis of reactive oxygen species, arginase-1, prostaglandin E2, IL-4, IL-6, inhibit the function of T-lymphocytes [16], support the stemness of tumour cells [17], increase angiogenesis and metastasis [18]. It should be noted that MSC create background for spreading the tumour not only locally, but also to the target organ, inducing expression of adhesion molecules on the surface of endotheliocytes, e.g., E-selectin, intercellular adhesion molecules 1 (ICAM-1), and vascular cell adhesion molecules 1 (VCAM-1), promoting residence of tumour cells in the target organ [19].
M2 macrophages and MSCs are the main producers of IL-1β, which initiates a whole spectrum of procarcinogenic effects [20–23]. IL-1β provides both direct and indirect effects upon angiogenesis, by inducing the synthesis of various cytokines and angiogenesis factors [24]. Direct effects of IL-1β include activation of FGF-β expression in endothelial cells [24], VEGF-A and its receptors [25], regulation of endothelial progenitor cells, thus contributing to neovascularisation [25]. IL-1β also affects Bv8, CCL2, and CCL3 secretion, leading to (i) enhanced synthesis of VEGF-A, PLGF, bFGF by endothelial cells, (ii) VEGF-A secretion by myeloid cells (CD34+ or Flk-1+) [26], (iii) FGF1 secretion by mononuclear cells [27], (iv) IL-8 secretion by macrophages [28]. In general, IL-1β may affect cell differentiation, causing synthesis of either pro-inflammatory, or angiogenetic factors [28]. Immunosuppressive effect of IL-1β proceeds via stimulated synthesis of anti-inflammatory factors (IL-10, TGF-β, arginase-1) by tumour-associated macrophages and MSCs. This effect results into suppression of T cell and M1 activities [28], and the non-canonical signalling pathway of the NF-κB transcription factor is triggered, thus, in turn, suppressing antitumour immunity of T-regulatory cells [29]. The effect of IL-1β was shown to be associated with PD-L1 overexpression, an additional factor of immunity inhibition [29].
The role of immune cells in TME may be either to suppress tumour growth (antitumour TME), or to promote tumour growth (immunosuppressive TME). Thus, depending on the TME factors and type of malignancy, the immune cells may exert pro- or antitumour effects (Fig. 1) [30].

Figure 1. Influence of various immune cell populations upon TME
Abbreviations: NK, natural killers; Neu, neutrophils; MI, type I macrophages; DC, dendritic cells.
Cancer-associated fibroblasts (CAF) synthesise factor(s) inducing differentiation of macrophages to M2 and depletion of CD8+ T cells, which also stimulates proliferation, invasion and metastasis of tumour cells [31]. Along with cytokine production, tumour cells may activate pro-tumourigenic processes, due to metabolic acidosis in the extracellular matrix. E.g., acidification of extracellular matrix triggers p53-dependent apoptosis of surrounding cells and degradation of the basement membrane [32], along with increased secretion of cathepsin B, metalloproteinases, urokinase plasminogen activator, hydrolysing components of the extracellular matrix [33]. Also, acidification of extracellular matrix alters the lysosomal distribution, thus further enhancing secretion of proteinases causing destruction of extracellular matrix [34] and disruption of intercellular adhesion contacts by degradation of E-cadherin [35].
Several studies suggest acidosis to be a factor of immune therapy efficiency, as shown in murine models of melanoma and pancreatic adenocarcinoma, where the increased TME acidity correlated with inhibited antitumour T cell-mediated immunity, associated with lower efficacy of immune drugs [36, 37].
Increased acidity of TME leads to decreased efficiency of anticancer drugs, due to decreased transfer of drugs into the cells. For example, anthracyclines (doxorubicin), anthraquinones and alkaloids are weak bases and require optimal pH range (7.5-9.5) for their transmembrane transport [38-40]. Under these conditions, activity of a well-known glycoprotein transporter (pGP) is enhanced, thus promoting efflux of anticancer drugs from malignant cells. This effect was observed in tumour cells adapted for low extracellular pH, with acquired TP53 gene mutations [41–43]. These adaptive processes cause multidrug resistance and limit the choice of therapeutic options.
Hence, malignant cells may stimulate differentiation of tumour-associated immune cells to immunosuppressive phenotypes by synthesising a wide range of signalling molecules. In turn, these immune cells produce anti-inflammatory factors, growth factors, proteinases, enhance expression of adhesion molecules, causing invasion and metastasis of tumours, activation of angiogenesis. TME acidification is not only a factor leading to selection of the most resistant and aggressive tumour phenotypes, but it also induces destruction of intercellular contacts and extracellular matrix, which ultimately triggers the processes of invasion and metastasis.
Mutual influence of tumour cells and normal TME populations promotes development of cell associations, and their extracellular milieu protects from external impacts, e.g., anticancer therapy, immune surveillance. Even a small number of tumour cells forms a microenvironment resistant to antitumour therapy, especially if they are represented by stem or ‘dormant’ cells. Hence, a prevalence of the distinct component of the cellular microenvironment presumes differentiated approach to treatment. The strategic purpose in oncology is to transform cancer into a long-term chronic disease which is well controlled by the low-toxicity approaches. To implement this task, three areas of research could be highlighted: (i) a search for specific key target molecules, in order to create targeted drugs; (ii) personalisation of treatment programs based on molecular and cellular characteristics of the tumour, and individual clinical prognosis; (iii) use of nanomaterials when creating novel drugs, thus providing higher efficiency of treatment. The latter approach may increase concentrations of active substances in the tumour foci and reduce the drug toxicity. Long-term studies provide convincing evidence that carcinogenesis is a multistage multicomponent process, including disturbances of apoptosis, proliferation, angiogenesis and cell metabolism leading to the formation of altered pathological microenvironment.
Multimodality of carcinogenesis requires usage of combined treatment methods aimed at different molecular targets. In the past ten years, a series of nanomaterials has been developed in various laboratories around the world that have great potential for therapeutic use in oncology, e.g., liposomes, polymer carriers, carbon nanoparticles, iron oxide and gold nanoparticles. Currently, there is a technical opportunity of creating structures that would deliver active substances to the target cells and undergo biodegradation. However, the mentioned nanocarriers have a number of disadvantages. Liposomes, polymers, dendrimers can provide a significant decrease in overall toxicity of cytotoxic drugs, but they have a low potential in terms of targeted delivery. Carbon nanomaterials make it possible to create multicomponent and multitarget therapeutic constructs. Graphene and its derivatives are considered the most promising carbon nanomaterials for these purposes.
Current experience with graphene carriers
Initial experiments with unmodified graphene demonstrated its systemic toxicity [44,45] associated with accumulation in lungs [46], reticuloendothelial system including liver and spleen [47, 48] and provoking an inflammatory response [49]. Recent studies have shown that modified graphene oxide is promising for manipulating the tumour microenvironment; however, an analysis of the literature suggests that research in this area has just begun. For example, the use of graphene oxide functionalised with polyethylene glycol (GO-PEG) in photodynamic therapy led to a decrease in the interleukin-4-dependent polarisation of M2 in macrophages of the tumour microenvironment. The antitumour action of GO-PEG was to reduce the migration and invasion of subcutaneous osteosarcoma cells in mice [50]. In [51], the combined effect of low-frequency ultrasound therapy and graphene oxide-doxorubicin (GO-DOX) conjugate on local damage to endothelial cells lining the neovascular network was shown. This effect increased the penetration of the GO-DOX conjugate into the interstitial space of mouse liver carcinoma through damaged capillaries. Over past few years, a series of works was published at the Department of General and Bioorganic Chemistry (Pavlov University, St. Petersburg) which demonstrated an opportunity of reducing toxicity to acceptable level, due to functionalisation of the graphene carrier [52-54]. Moreover, the graphene-based nanomaterials (GBN) have been shown to have a number of advantages: stimulation of immune response, inhibition of tumour stem cells, regulation of angiogenesis and hypoxia [55]. In particular, it should be noted that the GBNs exhibit photodynamic and photothermal activity, and can be effectively used as nanoplatforms for targeted delivery of cytostatic drugs.

Figure 2. General structure of graphene oxide
Graphene is known to consist of sp2-hybridised carbon atoms forming two-dimensional nanolayers, while graphene oxide (GO) contains various oxygen-containing oxygen functional groups: (i) carboxyl, carbonyl, and lactol, located at the edges of GO layers; (ii) epoxy and hydroxyl groups distributed on the surface of the GO plane [56-61] (Fig. 2). Reduced GO (rGO) is a variant of GO in which most of the oxygen-containing functional groups are reduced by means of hydrazine hydrate or biomolecules [54, 62].
A graphene monolayer was obtained in 2004 by A. Geim and K. Novoselov [63], while GO was first synthesised in 1859 by B. Brodie by oxidising graphite using a mixture of oxidising agents (potassium chlorate and fuming nitric acid) [64]. However, the most effective method was developed by W. Hammers and R. Offeman in 1957, with a mixture of sulphuric acid, sodium nitrate and potassium permanganate [65]. GO functionalisation can be carried out using various reactions (Fig. 3): amidation, esterification, 1,3-dipolar cycloaddition, halogenation, as well as through non-covalent functionalisation through the formation of hydrogen bonds, π-π stacking and hydrophobic interactions. These reactions make it possible to obtain unique nanomaterials having a medical potential in cancer treatment [66], delivery of drugs and biomolecules [67, 68], development of biosensors [69], as well as substances with antiviral [70], antibacterial [71] and antifungal activity [72]. Among GBN, GO has the greatest potential for use in medicine for the following reasons: (i) GO contains various functional groups that allow further surface functionalisation; (ii) functionalisation of GO increases its biocompatibility; (iii) the presence of oxygen-containing functional groups ensures stability of aqueous GO dispersions.

Figure 3. Basic ways to functionalise graphene oxide
Analysis of the literature revealed a number of research works devoted to synthesis and biological activity of GBN-based conjugates. Zhang et al. [73] reported that covalent GO functionalisation with sulphonic acid and folic acid (GO-SO3H-FA) groups increased the specific cytotoxicity for MCF-7 cells (breast cancer-derived strain). Conjugation of doxorubicin (DOX) and camptothecin (CPT) with GO through its non-covalent functionalisation (due to π-π stacking and hydrophobic interactions) significantly increases therapeutic efficacy as compared to individual drugs. The CPT and DOX loading in the mixed GO-SO3H-FA-CPT-DOX conjugate was 4.5% and 400%, respectively.
Wang et al. [67] demonstrated that covalent functionalisation of GO with chlorotoxin (CTX) increases the efficiency of drug delivery to C6 glioma cells. At the same time, non-covalent DOX attachment with a loading of 570 mg DOX per gram of CTX-GO significantly increases efficiency of the conjugate (the release of the cytostatic drug was pH-dependent). Fan et al. [74] synthesised a covalent GO-based conjugate with adipic acid dihydrazide and sodium alginate (SA). Then, DOX.HCl was attached non-covalently to GO-SA. The maximum DOX loading was 1.8 mg per 1 mg GO-SA. The highest drug release rate was observed at pH 5.0. Cytotoxicity testing with HeLa (cervical carcinoma) cell line showed that the GO-SA conjugate is not cytotoxic, while GO-SA/DOX exhibits cytotoxicity due to the specific effect on CD44 receptors.
Qin et al. [75] synthesised GO non-covalently conjugated with polyvinylpyrrolidone (PVP, M=30 kDa), and then folic acid (FA) was covalently attached through the formation of an amide bond (carboxyl groups of GO and amino groups of FA). Next, the authors performed non-covalent DOX loading (due to π-π stacking and hydrophobic interactions).
The calculated value of the DOX loading on the FA-GO-PVP was 107.5 wt. %. The resulting conjugate demonstrated high antitumour efficacy against HeLa cells. Huang et al. [76] described the ability of FA-functionalised GO to efficiently bind the chlorine e6 photosensitiser for photodynamic therapy. Tiwari et al. [77] used the GO-PVP noncovalent conjugate for double noncovalent addition of quercetin (QS) and gefitinib (GF) and compared it with the GO-PVP-QS and GO-PVP-GF conjugates. The authors found that the combined loading of the drugs showed higher cytotoxicity against PA-1 (ovarian cancer) cell line compared to individual drugs. The amount of QS and GF in GO-PVP-QS-GF was 20% and 46%, respectively.
Deb et al. [78] functionalised GO with polyethylene glycol (PEG), FA, and CPT via non-covalent π-π stacking interactions (CPT loading was 45%). The resulting conjugate (C=100 μg∙ml−1) caused the death of 76% of cells compared with the control when using the MCF-7 cell line [78]. The same group functionalised GO with the natural polymer chitosan (CS) and FA, to deliver CPT and 3,3’-diindolylme- thane (DIM). The resulting conjugate (GO-CS-FA-CPT-DIM) demonstrated high cytotoxicity against the MCF-7 cell line (95.7% decrease in cell viability), which was significantly higher compared to individual DIM preparations (42.4%) and CPT (52.6%) [79]. Pei et al. [80] showed that simultaneous functionalisation of the GO surface with PEG (pGO) (pGO-CP-DOX, mass ratio: 1: 0.376: 0.376) with cisplatin (CP) and DOX leads to increased cytotoxicity towards Cal-27 (human squamous carcinoma) and MCF-7 cell lines. The authors observed higher inhibition of cell proliferation for the pGO-CP-DOX conjugate compared to individual preparations: IC50 (MCF-7)=14.5 μg∙ml−1 for pGO-CP-DOX, 22.5 μg∙ml−1 for pGO-DOX, and 22 μg∙ml−1 for pGO-CP [80]. Bullo et al. [81] demonstrated the ability of GO functionalisation using PEG, FA, and anticancer drugs: protocatechuic acid (23.5% PCA) and chlorogenic acid (18.3% CA). The authors investigated the effect of the GO-PEG-FA-PCA-CA conjugate on the HT29 (colon cancer) and HepG2 (liver cancer) cell lines. Cytotoxicity trials showed the following results: IC50 (HT29)=50.7 μg∙ml−1, IC50 (HepG2)=40.4 μg∙ml−1 [81]. Gong et al. [82] demonstrated that fluorinated graphene (FG) can be used to load a mixture of DOX and CPT after CS covalent functionalisation; DOX and CPT loading were 110% and 25%, respectively. The resulting FG-CS-DOX-CPT conjugate demonstrated a 60% and 75% decrease in the viability of HeLa cells with simultaneous laser irradiation (wavelength 808 nm) [82]. Gong et al. [83] evaluated the ability of non-covalent FG conjugation with a cytostatic DOX loading as high as 200%. The FG-DOX conjugate at 30 μg∙ml−1 drug content reduced HeLa cell line viability to 94% after 48 h of incubation [83].
In an in vivo study, Shim et al. [84] showed that rGO functionalised with low-molecular weight heparin (LHT7) acts as a targeted drug for the delivery of DOX. The rGO-LHT7-DOX conjugate with rGO: DOX mass ratios of 2, 1, 0.5, 0.1 demonstrated a high antitumour effect at the KB human carcinoma cell line (cell viability was decreased by 61.1%), along with significant decrease in tumour size by (92.5±3.1)% [84]. Table 1 shows the results of studying cytotoxic conjugates based on GBN and cytostatic drugs.
Table 1. Cytotoxicity of GBN and non-covalently linked cytostatic drugs


Conclusion
Current data provide sufficient results concerning molecular and cellular events providing mutual influence of tumour cells and TME cells, as well as factors of cancer progression. It has been shown that the tumour cells per se and their cellular TME create an integrated system that promotes tumour progression and development of multiple drug resistance.
Thus, the following requirements must be met for modern therapeutic agents: targeted action, polyfunctionality with respect to ability of loading various molecules on the GBN surface, low toxicity, opportunity of selective inactivation of immunosuppressive components in the TME. The last issue deserves special attention. The chance to resolve this complex problem is shown by the example of GBN usage.
List of abbreviations
IL – interleukin
TNF-α – tumour necrosis factor alpha
TGF-β1 – transforming growth factor receptor-β1
VEGF-A – vascular endothelial growth factor A
CAF – cancer-associated fibroblasts
CCL – C-C motif ligand
CCR2 – C-C chemokine receptor type 2
CPT – camptothecin
CSF-1 – the colony stimulating factor 1
CTX – chlorotoxin
CXCL – the chemokine (C-X-C motif) ligand
DOX – doxorubicin
FA – folic acid
GBN – graphene-based nanomaterials
GO – graphene oxide
iNOS – Inducible nitric oxide synthase
bFGF – basic fibroblast growth factor
FGF1 – fibroblast growth factor 1
HIF-1α – hypoxia inducible factor 1 subunit alpha
PD-L1 – ligand of programmed death-1 receptor
PLGF – placental growth factor
STAT3 – signal transducer and activator of transcription 3
Th – T helper cells
TME – tumour microenvironment
Acknowledgement
This work was financially supported by the Ministry of Health of the Russian Federation (state assignment Э.03-2021; 121040200136-0).
Conflict of interests
The authors declared no potential conflict of interest.
References
- Voronov E, Dotan S, Krelin Y, Song X, Elkabets M, Carmi Y, et al. Unique versus redundant functions of IL-1α and IL-1β in the tumor microenvironment. Front Immunol. 2013; 4:177. doi: 10.3389/fimmu.2013.00177
- Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006; 42:751-759. doi: 10.1016/j.ejca.2006.01.010
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006; 25:387-408. doi: 10.1007/s10555-006-9004-4
- Farc O, Cristea V. An overview of the tumor microenvironment, from cells to complex networks (Review). Exp Ther Med. 2021; 21:1-1. doi: 10.3892/ETM.2020.9528
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015; 348:74-80.
doi: 10.1126/SCIENCE.AAA6204 - Hinshaw DC, Shevde LA The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019; 79:4557-4567.
doi: 10.1158/0008-5472.CAN-18-3962 - Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010 1110. 2010; 11:889-896. doi: 10.1038/ni.1937
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010; 141:39-51.
doi: 10.1016/J.CELL.2010.03.014 - Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004; 25:677-686. doi: 10.1016/J.IT.2004.09.015
- Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012; 189:3848-3858. doi: 10.4049/jimmunol.1200819
- Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018; 154:3-20. doi: 10.1111/imm.12888
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: Friend or foe? Carcinogenesis. 2012; 33:949-955. doi: 10.1093/carcin/bgs123
- Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014; 20:511-517. doi: 10.1038/nm.3547
- Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016; 5. doi: 10.1080/2162402X.2015.1134073
- Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015; 3:1236-1247. doi: 10.1158/2326-6066.CIR-15-0036
- Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016; 37:208-220. doi: 10.1016/j.it.2016.01.004
- Guan J, Chen J. Mesenchymal stem cells in the tumor microenvironment (Review). Biomed Rep. 2013; 1:517-521.
doi: 10.3892/BR.2013.103 - Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015; 66:97-110. doi: 10.1146/annurev-med-051013-052304
- Shi H, Zhang J, Han X, Li H, Xie M, Sun Y, et al. Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1β-mediated increase in E-selectin expression. Int J Cancer. 2017; 140:1370-1383.
doi: 10.1002/ijc.30538 - Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, et al. CD11b + /Gr-1 + immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1β-secreting cells. J Immunol. 2005; 175:8200-8208. doi: 10.4049/jimmunol.175.12.8200
- Elkabets M, Ribeiro VSG, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, et al. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010; 40:3347-3357. doi: 10.1002/eji.201041037
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006; 176:284-290. doi: 10.4049/jimmunol.176.1.284
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253-268. doi: 10.1038/nri3175
- Hye WK, Torres D, Wald L, Weissleder R, Bogdanov AA. Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006; 86:599-609. doi: 10.1038/labinvest.3700421
- Nasu K, Itoh H, Yuge A, Kawano Y, Yoshimatsu J, Narahara H, et al. Interleukin-1β regulates vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 secretion by human oviductal epithelial cells and stromal fibroblasts. Gynecol Endocrinol. 2006; 22:495-500. doi: 10.1080/08916930600929487
- Qin SL, Li TS, Takahashi M, Hamano K. In vitro assessment of the effect of interleukin-1β on angiogenic potential of bone marrow cells. Circ J. 2006. doi: 10.1253/circj.70.1195
- Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, et al. The non-classical export routes: FGF1 and IL-1α point the way. J. Cell Sci. 2003; doi: 10.1242/jcs.00872
- Tannahill GM, Curtis AM, Adamik J, Palsson-Mcdermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013; 496:238-242. doi: 10.1038/nature11986
- Oh H, Grinberg-Bleyer Y, Liao W, Maloney D, Wang P, Wu Z, et al. An NF-κB transcription-factor-dependent lineage-specific transcriptional program promotes regulatory T cell identity and function. Immunity. 2017; 47:450-465.e5.
doi: 10.1016/j.immuni.2017.08.010 - Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020; 30:R921-R925. doi: 10.1016/J.CUB.2020.06.081
- Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T Cells to protect tumour cells. Nat Commun. 2018; 9. doi: 10.1038/s41467-018-03347-0
- Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: A multidisciplinary study. Cancer Res. 2006; 66:5216-5223. doi: 10.1158/0008-5472.CAN-05-4193
- Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, et al. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis. 2008; 25:411-425. doi: 10.1007/s10585-008-9145-7
- Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003; 5:533-545. doi: 10.1016/s1476-5586(03)80037-4
- Švastová E, Žilka N, Zat’ovičová M, Gibadulinová A, Čiampor F, Pastorek J, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with β-catenin. Exp Cell Res. 2003; 290:332-345. doi: 10.1016/S0014-4827(03)00351-3
- Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016; 76:1381-1390. doi: 10.1158/0008-5472.CAN-15-1743
- Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012; 72:2746-2756. doi: 10.1158/0008-5472.CAN-11-1272
- Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. 2003; 66:1219-1229. doi: 10.1016/S0006-2952(03)00468-4
- Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003; 66:1207-1218. doi: 10.1016/S0006-2952(03)00467-2
- Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006; 5:1275-1279. doi: 10.1158/1535-7163.MCT-06-0024
- Salerno M, Przewloka T, Fokt I, Priebe W, Garnier-Suillerot A Preferential efflux by P-glycoprotein, but not MRP1, of compounds containing a free electron donor amine. Biochem Pharmacol. 2002; 63:1471-1479. doi: 10.1016/S0006-2952(02)00895-X
- Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006; 8:143-152. doi: 10.1593/neo.05697
- Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O. Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007; 17:239-244. doi: 10.3892/or.17.1.239
- Seabra AB, Paula AJ, De Lima R, Alves OL, Durán N. Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol. 2014; 27:159-168. doi: 10.1021/TX400385X
- Lalwani G, D’Agati M, Khan AM, Sitharaman B. Toxicology of graphene-based nanomaterials. Adv Drug Deliv Rev. 2016; 105:109-144. doi: 10.1016/J.ADDR.2016.04.028
- Li B, Yang J, Huang Q, Zhang Y, Peng C, Zhang Y, et al. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice. NPG Asia Mater. 2013 54. 2013; 5:e44-e44. doi: 10.1038/am.2013.7
- Liu JH, Yang ST, Wang H, Chang Y, Cao A, Liu Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine (Lond). 2012;7(12):1801-1812. doi: 10.2217/Nnm.12.60
- Yang K, Gong H, Shi X, Wan J, Zhang Y, Liu Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials. 2013; 34:2787-2795. doi: 10.1016/J.BIOMATERIALS.2013.01.001
- Yue H, Wei W, Yue Z, Wang B, Luo N, Gao Y, et al. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012; 33:4013-4021. doi: 10.1016/J.BIOMATERIALS.2012.02.021
- Deng X, Liang H, Yang W, Shao Z. Polarization and function of tumor-associated macrophages mediate graphene oxide-induced photothermal cancer therapy. J Photochem Photobiol. B Biol. 2020; 208:111913. doi: 10.1016/J.JPHOTOBIOL.2020.111913
- Shen ZY, Shen BQ, Shen AJ, Zhu XH. Cavitation-enhanced delivery of the nanomaterial graphene oxide-doxorubicin to hepatic tumors in nude mice using 20 kHz low-frequency ultrasound and microbubbles. J Nanomater. 2020:. doi: 10.1155/2020/3136078
- Abdelhalim AOE, Sharoyko VV, Ageev SV, Farafonov VS, Nerukh DA, Postnov VN, et al. Graphene oxide of extra high oxidation: A wafer for loading guest molecules. J Phys Chem Lett. 2021;10015-10024. doi: 10.1021/ACS.JPCLETT.1C02766
- Abdelhalim AOE, Sharoyko VV, Meshcheriakov AA, Luttsev MD, Potanin AA, Iamalova NR, et al. Synthesis, characterisation and biocompatibility of graphene-L-methionine nanomaterial. J Mol Liq. 2020; 314:113605. doi: 10.1016/j.molliq.2020.113605
- Abdelhalim AOE, Sharoyko VV, Meshcheriakov AA, Martynova SD, Ageev SV, Iurev GO, et al., Reduction and functionalization of graphene oxide with L-cysteine: Synthesis, characterization and biocompatibility. Nanomedicine Nanotechnology, Biol Med. 2020; 29:102284. doi: 10.1016/j.nano.2020.102284
- Li Q, Shi Z, Zhang F, Zeng W, Zhu D, Mei L, Symphony of nanomaterials and immunotherapy based on the cancer-immunity cycle. Acta Pharm Sin B. 2021; doi: 10.1016/J.APSB.2021.05.031
- Kaplan A, Yuan Z, Benck JD, Govind Rajan A, Chu XS, Wang QH, et al. Current and future directions in electron transfer chemistry of graphene. Chem Soc Rev. 2017; 46:4530-4571. doi: 10.1039/c7cs00181a
- Sun Z, James DK, Tour JM. Graphene chemistry: synthesis and manipulation. J Phys Chem Lett. 2011; 2:2425-2432.
doi: 10.1021/jz201000a - Loh KP, Bao Q, Ang PK, Yang J. The chemistry of graphene. J Mater Chem. 2010; 20:2277-2289. doi: 10.1039/b920539j
- Sturala J, Luxa J, Pumera M, Sofer Z. Frontispiece: Chemistry of graphene derivatives: Synthesis, applications, and perspectives. Chem A Eur J. 2018; 24:. doi: 10.1002/chem.201882361
- Gao W. The chemistry of graphene oxide, in: Graphene Oxide Reduct. Recipes, Spectrosc. Appl., Springer Int Publishing, 2015: pp. 61-95. doi: 10.1007/978-3-319-15500-5_3
- Eigler S, Hirsch A. Chemistry with graphene and graphene oxide – Challenges for synthetic chemists. Angew Chemie-Int Ed. 2014; 53:7720-7738. doi: 10.1002/anie.201402780
- Lavin-Lopez MP, Paton-Carrero A, Sanchez-Silva L, Valverde JL, Romero A. Influence of the reduction strategy in the synthesis of reduced graphene oxide. Adv Powder Technol. 2017; 28:3195-3203. doi: 10.1016/j.apt.2017.09.032
- Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007; 6:183-191. doi: 10.1038/nmat1849
- [Brodie BC, XIII. On the atomic weight of graphite. Philos Trans R Soc London. 1859; 149:249-259.
- Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958; 80:1339. doi: 10.1021/ja01539a017
- Eskiizmir G, Baskin Y, Yapici K. Graphene-based nanomaterials in cancer treatment and diagnosis, in: Fullerenes, Graphenes Nanotub. A Pharm. Approach, Elsevier, 2018: pp. 331-374. doi: 10.1016/B978-0-12-813691-1.00009-9
- Wang H, Gu W, Xiao N, Ye L, Xu Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int J Nanomedicine. 2014; 9:1433-1442. doi: 10.2147/IJN.S58783
- Kim H, Namgung R, Singha K, Oh IK, Kim WJ. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug Chem. 2011; 22:2558-2567. doi: 10.1021/bc200397j
- Lee J, Kim J, Kim S, Min DH. Biosensors based on graphene oxide and its biomedical application. Adv Drug Deliv Rev. 2016; 105:275-287. doi: 10.1016/j.addr.2016.06.001
- Innocenzi P, Stagi L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020; 11:6606-6622. doi: 10.1039/d0sc02658a
- Szunerits S, Boukherroub R. Antibacterial activity of graphene-based materials. J Mater Chem. B. 2016; 4:6892-6912.
doi: 10.1039/c6tb01647b - Li C, Wang X, Chen F, Zhang C, Zhi X, Wang K, et al. The antifungal activity of graphene oxide-silver nanocomposites. Biomaterials. 2013; 34:3882-3890. doi: 10.1016/j.biomaterials.2013.02.001
- Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010; 6:537-544. doi: 10.1002/smll.200901680
- Fan L, Ge H, Zou S, Xiao Y, Wen H, Li Y, et al. Sodium alginate conjugated graphene oxide as a new carrier for drug delivery system. Int J Biol Macromol. 2016; 93:582-590. doi: 10.1016/j.ijbiomac.2016.09.026
- Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, Yang BW. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J Photochem Photobiol B Biol. 2013; 120:156-162. doi: 10.1016/j.jphotobiol.2012.12.005
- Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, et al. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2012; 1:240-250. doi: 10.7150/thno/v01p0240
- Tiwari H, Karki N, Pal M, Basak S, Verma RK, Bal R, et al. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surfaces B Biointerfaces. 2019; 178:452-459. doi: 10.1016/j.colsurfb.2019.03.037
- Deb A, Vimala R. Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J Drug Deliv Sci Technol. 2018; 43:333-342. doi: 10.1016/j.jddst.2017.10.025
- Deb A, Andrews NG, Raghavan V. Natural polymer functionalized graphene oxide for co-delivery of anticancer drugs: In-vitro and in-vivo. Int J Biol Macromol. 2018; 113:515-525. doi: 10.1016/j.ijbiomac.2018.02.153
- Pei X, Zhu Z, Gan Z, Chen J, Zhang X, Cheng X, et al. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci Rep. 2020; 10:1-15. doi: 10.1038/s41598-020-59624-w
- Bullo S, Buskaran K, Baby R, Dorniani D, Fakurazi S, Hussein MZ. Dual drugs anticancer nanoformulation using graphene oxide-PEG as nanocarrier for protocatechuic acid and chlorogenic acid. Pharm Res. 2019; 36:91. doi: 10.1007/s11095-019-2621-8
- Gong P, Zhao Q, Dai D, Zhang S, Tian Z, Sun L, et al. Functionalized ultrasmall fluorinated graphene with high NIR absorbance for controlled delivery of mixed anticancer drugs. Chem- A Eur J. 2017; 23:17531-17541. doi: 10.1002/chem.201702917
- Gong P, Du J, Wang D, Cao B, Tian M, Wang Y, et al. Fluorinated graphene as an anticancer nanocarrier: An experimental and DFT study. J Mater Chem B. 2018; 6:2769-2777. doi: 10.1039/c8tb00102b
- Shim G, Kim JY, Han J, Chung SW, Lee S, Byun Y, et al. Reduced graphene oxide nanosheets coated with an anti-angiogenic anticancer low-molecular-weight heparin derivative for delivery of anticancer drugs. J Control Release. 2014; 189:80-89.
doi: 10.1016/j.jconrel.2014.06.026 - Rosli NF, Fojtů M, Fisher AC, Pumera M. Graphene oxide nanoplatelets potentiate anticancer effect of cisplatin in human lung cancer cells. Langmuir. 2019; 35:3176-3182. doi: 10.1021/acs.langmuir.8b03086
- Pooresmaeil M, Namazi H. β-Cyclodextrin grafted magnetic graphene oxide applicable as cancer drug delivery agent: Synthesis and characterization. Mater Chem Phys. 2018; 218:62-69. doi: 10.1016/j.matchemphys.2018.07.022
- Ma N, Liu J, He W, Li Z, Luan Y, Song Y, et al. Folic acid-grafted bovine serum albumin decorated graphene oxide: An efficient drug carrier for targeted cancer therapy. J Colloid Interface Sci. 2017; 490:598-607. doi: 10.1016/j.jcis.2016.11.097
- Tian J, Luo Y, Huang L, Feng Y, Ju H, Yu BY. Pegylated folate and peptide-decorated graphene oxide nanovehicle for in vivo targeted delivery of anticancer drugs and therapeutic self-monitoring. Biosens Bioelectron. 2016; 80:519-524. doi: 10.1016/j.bios.2016.02.018
- Javanbakht S, Namazi H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater Sci Eng C. 2018; 87:50-59. doi: 10.1016/j.msec.2018.02.010
- Karki N, Tiwari H, Pal M, Chaurasia A, Bal R, Joshi P, et al. Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: A comparative study. Colloids Surfaces B Biointerfaces. 2018; 169:265-272.
doi: 10.1016/j.colsurfb.2018.05.022
" ["~DETAIL_TEXT"]=> string(51376) "
Introduction
During the development of malignant neoplasia, a specific cellular environment is formed in the chronic inflammation site termed "inflammatory microenvironment of the tumour" (TME). This cell community consists of tumour-associated macrophages (MF), dendritic cells (DC), myeloid suppressor cells (MSC), neutrophils (NF), mast cells, natural killer cells (NK), T- and B-lymphocytes, cancer-associated fibroblasts (CAF) and endothelial cells. The interaction between tumour cells, myeloid cells and lymphocytes is a dynamic, bidirectional process and includes intercellular contacts, constant exchange of secreted soluble molecules, factors, vesicles, due to which an autonomous system is established that regulates tumour growth [1-5].
Neoplastic progression is associated with lack of oxygen, deficiency of nutrients causing hypoxia and development of metabolic acidosis in the tumour microenvironment. These factors promote selection of tumour cells with the gene mutations that allow them to survive under more severe microenvironmental conditions. Such adaptation of tumour cells is accompanied by increased production of various growth factors, cytokines, chemokines, which together present a triggering factor for enhancement of angiogenesis, metastases, and inhibition of local immune response. In turn, the normal TME cells also begin to secrete factors promoting tumour progression. As a result, a closed-circuit regulatory system is formed [6]. E.g., the content of IL-4 increases in TME, thus inducing differentiation of macrophages to the second-type (M2) resident cells. The M2 subpopulation may account for up to 50 % of the tumour mass and contribute to activation of pro-tumourigenic processes accompanied by the synthesis of IL-1, IL-1RA, IL-4, IL-6, IL-10, IL-12, L-arginine, prostaglandin E2, TNF-α, TGF-β, VEGF-A, and a variety of chemokines and their receptors CCL1, CCL5, CCL17, CCL22, CCL24, CCR2, CXCL10, CXCL16 [7-9]. These mediators are involved in angiogenesis, immunosuppression, and metastasis.
In tumour cells, increased production of some amino acid metabolism enzymes is revealed, e.g., of indolamine 2,3-dioxygenase, arginase-1. Activation of iNOS, as well as STAT3 transcription factor is noted, thereby initiating the differentiation of dendritic cells into tolerogenic tumour-associated dendritic cells (TADC) [10]. These cells produce TGF-β which promotes immunosuppression by stimulating Th2, Th17 and T regulatory cells [11].
Of interest, differentiation of neutrophils in the TME structures depends on the stage of the disease. Thus, the normally pro-inflammatory neutrophils differentiate at later phase to an immunosuppressive phenotype under the influence of TGF-β and angiotensin II [12]. The tumour-associated neutrophils synthesise collagenase IV, heparanase, elastase and matrix metalloproteinases (MMPs) which contribute to extracellular matrix degradation, tumour cell invasion and metastasis. The secreted proteinases destroy extracellular matrix, and degrade the pro-inflammatory cytokines, thus causing anti-inflammatory effects [13]. Neutrophils also produce oncostatin M, which enhances angiogenesis, as well as CXCL1, CXCL8, CCL-3, CXCL6, TGF-β, and prostaglandin E2 synthesis, thus supporting the neoplastic progression [14].
The CSF-1, HIF-1α, CCL2, CCL7, CXCL1 peptide factors synthesised by the TME cell populations are able to alter the metabolism of myeloid cells, leading to transition to MSC [15]. MSC enhance the synthesis of reactive oxygen species, arginase-1, prostaglandin E2, IL-4, IL-6, inhibit the function of T-lymphocytes [16], support the stemness of tumour cells [17], increase angiogenesis and metastasis [18]. It should be noted that MSC create background for spreading the tumour not only locally, but also to the target organ, inducing expression of adhesion molecules on the surface of endotheliocytes, e.g., E-selectin, intercellular adhesion molecules 1 (ICAM-1), and vascular cell adhesion molecules 1 (VCAM-1), promoting residence of tumour cells in the target organ [19].
M2 macrophages and MSCs are the main producers of IL-1β, which initiates a whole spectrum of procarcinogenic effects [20–23]. IL-1β provides both direct and indirect effects upon angiogenesis, by inducing the synthesis of various cytokines and angiogenesis factors [24]. Direct effects of IL-1β include activation of FGF-β expression in endothelial cells [24], VEGF-A and its receptors [25], regulation of endothelial progenitor cells, thus contributing to neovascularisation [25]. IL-1β also affects Bv8, CCL2, and CCL3 secretion, leading to (i) enhanced synthesis of VEGF-A, PLGF, bFGF by endothelial cells, (ii) VEGF-A secretion by myeloid cells (CD34+ or Flk-1+) [26], (iii) FGF1 secretion by mononuclear cells [27], (iv) IL-8 secretion by macrophages [28]. In general, IL-1β may affect cell differentiation, causing synthesis of either pro-inflammatory, or angiogenetic factors [28]. Immunosuppressive effect of IL-1β proceeds via stimulated synthesis of anti-inflammatory factors (IL-10, TGF-β, arginase-1) by tumour-associated macrophages and MSCs. This effect results into suppression of T cell and M1 activities [28], and the non-canonical signalling pathway of the NF-κB transcription factor is triggered, thus, in turn, suppressing antitumour immunity of T-regulatory cells [29]. The effect of IL-1β was shown to be associated with PD-L1 overexpression, an additional factor of immunity inhibition [29].
The role of immune cells in TME may be either to suppress tumour growth (antitumour TME), or to promote tumour growth (immunosuppressive TME). Thus, depending on the TME factors and type of malignancy, the immune cells may exert pro- or antitumour effects (Fig. 1) [30].

Figure 1. Influence of various immune cell populations upon TME
Abbreviations: NK, natural killers; Neu, neutrophils; MI, type I macrophages; DC, dendritic cells.
Cancer-associated fibroblasts (CAF) synthesise factor(s) inducing differentiation of macrophages to M2 and depletion of CD8+ T cells, which also stimulates proliferation, invasion and metastasis of tumour cells [31]. Along with cytokine production, tumour cells may activate pro-tumourigenic processes, due to metabolic acidosis in the extracellular matrix. E.g., acidification of extracellular matrix triggers p53-dependent apoptosis of surrounding cells and degradation of the basement membrane [32], along with increased secretion of cathepsin B, metalloproteinases, urokinase plasminogen activator, hydrolysing components of the extracellular matrix [33]. Also, acidification of extracellular matrix alters the lysosomal distribution, thus further enhancing secretion of proteinases causing destruction of extracellular matrix [34] and disruption of intercellular adhesion contacts by degradation of E-cadherin [35].
Several studies suggest acidosis to be a factor of immune therapy efficiency, as shown in murine models of melanoma and pancreatic adenocarcinoma, where the increased TME acidity correlated with inhibited antitumour T cell-mediated immunity, associated with lower efficacy of immune drugs [36, 37].
Increased acidity of TME leads to decreased efficiency of anticancer drugs, due to decreased transfer of drugs into the cells. For example, anthracyclines (doxorubicin), anthraquinones and alkaloids are weak bases and require optimal pH range (7.5-9.5) for their transmembrane transport [38-40]. Under these conditions, activity of a well-known glycoprotein transporter (pGP) is enhanced, thus promoting efflux of anticancer drugs from malignant cells. This effect was observed in tumour cells adapted for low extracellular pH, with acquired TP53 gene mutations [41–43]. These adaptive processes cause multidrug resistance and limit the choice of therapeutic options.
Hence, malignant cells may stimulate differentiation of tumour-associated immune cells to immunosuppressive phenotypes by synthesising a wide range of signalling molecules. In turn, these immune cells produce anti-inflammatory factors, growth factors, proteinases, enhance expression of adhesion molecules, causing invasion and metastasis of tumours, activation of angiogenesis. TME acidification is not only a factor leading to selection of the most resistant and aggressive tumour phenotypes, but it also induces destruction of intercellular contacts and extracellular matrix, which ultimately triggers the processes of invasion and metastasis.
Mutual influence of tumour cells and normal TME populations promotes development of cell associations, and their extracellular milieu protects from external impacts, e.g., anticancer therapy, immune surveillance. Even a small number of tumour cells forms a microenvironment resistant to antitumour therapy, especially if they are represented by stem or ‘dormant’ cells. Hence, a prevalence of the distinct component of the cellular microenvironment presumes differentiated approach to treatment. The strategic purpose in oncology is to transform cancer into a long-term chronic disease which is well controlled by the low-toxicity approaches. To implement this task, three areas of research could be highlighted: (i) a search for specific key target molecules, in order to create targeted drugs; (ii) personalisation of treatment programs based on molecular and cellular characteristics of the tumour, and individual clinical prognosis; (iii) use of nanomaterials when creating novel drugs, thus providing higher efficiency of treatment. The latter approach may increase concentrations of active substances in the tumour foci and reduce the drug toxicity. Long-term studies provide convincing evidence that carcinogenesis is a multistage multicomponent process, including disturbances of apoptosis, proliferation, angiogenesis and cell metabolism leading to the formation of altered pathological microenvironment.
Multimodality of carcinogenesis requires usage of combined treatment methods aimed at different molecular targets. In the past ten years, a series of nanomaterials has been developed in various laboratories around the world that have great potential for therapeutic use in oncology, e.g., liposomes, polymer carriers, carbon nanoparticles, iron oxide and gold nanoparticles. Currently, there is a technical opportunity of creating structures that would deliver active substances to the target cells and undergo biodegradation. However, the mentioned nanocarriers have a number of disadvantages. Liposomes, polymers, dendrimers can provide a significant decrease in overall toxicity of cytotoxic drugs, but they have a low potential in terms of targeted delivery. Carbon nanomaterials make it possible to create multicomponent and multitarget therapeutic constructs. Graphene and its derivatives are considered the most promising carbon nanomaterials for these purposes.
Current experience with graphene carriers
Initial experiments with unmodified graphene demonstrated its systemic toxicity [44,45] associated with accumulation in lungs [46], reticuloendothelial system including liver and spleen [47, 48] and provoking an inflammatory response [49]. Recent studies have shown that modified graphene oxide is promising for manipulating the tumour microenvironment; however, an analysis of the literature suggests that research in this area has just begun. For example, the use of graphene oxide functionalised with polyethylene glycol (GO-PEG) in photodynamic therapy led to a decrease in the interleukin-4-dependent polarisation of M2 in macrophages of the tumour microenvironment. The antitumour action of GO-PEG was to reduce the migration and invasion of subcutaneous osteosarcoma cells in mice [50]. In [51], the combined effect of low-frequency ultrasound therapy and graphene oxide-doxorubicin (GO-DOX) conjugate on local damage to endothelial cells lining the neovascular network was shown. This effect increased the penetration of the GO-DOX conjugate into the interstitial space of mouse liver carcinoma through damaged capillaries. Over past few years, a series of works was published at the Department of General and Bioorganic Chemistry (Pavlov University, St. Petersburg) which demonstrated an opportunity of reducing toxicity to acceptable level, due to functionalisation of the graphene carrier [52-54]. Moreover, the graphene-based nanomaterials (GBN) have been shown to have a number of advantages: stimulation of immune response, inhibition of tumour stem cells, regulation of angiogenesis and hypoxia [55]. In particular, it should be noted that the GBNs exhibit photodynamic and photothermal activity, and can be effectively used as nanoplatforms for targeted delivery of cytostatic drugs.

Figure 2. General structure of graphene oxide
Graphene is known to consist of sp2-hybridised carbon atoms forming two-dimensional nanolayers, while graphene oxide (GO) contains various oxygen-containing oxygen functional groups: (i) carboxyl, carbonyl, and lactol, located at the edges of GO layers; (ii) epoxy and hydroxyl groups distributed on the surface of the GO plane [56-61] (Fig. 2). Reduced GO (rGO) is a variant of GO in which most of the oxygen-containing functional groups are reduced by means of hydrazine hydrate or biomolecules [54, 62].
A graphene monolayer was obtained in 2004 by A. Geim and K. Novoselov [63], while GO was first synthesised in 1859 by B. Brodie by oxidising graphite using a mixture of oxidising agents (potassium chlorate and fuming nitric acid) [64]. However, the most effective method was developed by W. Hammers and R. Offeman in 1957, with a mixture of sulphuric acid, sodium nitrate and potassium permanganate [65]. GO functionalisation can be carried out using various reactions (Fig. 3): amidation, esterification, 1,3-dipolar cycloaddition, halogenation, as well as through non-covalent functionalisation through the formation of hydrogen bonds, π-π stacking and hydrophobic interactions. These reactions make it possible to obtain unique nanomaterials having a medical potential in cancer treatment [66], delivery of drugs and biomolecules [67, 68], development of biosensors [69], as well as substances with antiviral [70], antibacterial [71] and antifungal activity [72]. Among GBN, GO has the greatest potential for use in medicine for the following reasons: (i) GO contains various functional groups that allow further surface functionalisation; (ii) functionalisation of GO increases its biocompatibility; (iii) the presence of oxygen-containing functional groups ensures stability of aqueous GO dispersions.

Figure 3. Basic ways to functionalise graphene oxide
Analysis of the literature revealed a number of research works devoted to synthesis and biological activity of GBN-based conjugates. Zhang et al. [73] reported that covalent GO functionalisation with sulphonic acid and folic acid (GO-SO3H-FA) groups increased the specific cytotoxicity for MCF-7 cells (breast cancer-derived strain). Conjugation of doxorubicin (DOX) and camptothecin (CPT) with GO through its non-covalent functionalisation (due to π-π stacking and hydrophobic interactions) significantly increases therapeutic efficacy as compared to individual drugs. The CPT and DOX loading in the mixed GO-SO3H-FA-CPT-DOX conjugate was 4.5% and 400%, respectively.
Wang et al. [67] demonstrated that covalent functionalisation of GO with chlorotoxin (CTX) increases the efficiency of drug delivery to C6 glioma cells. At the same time, non-covalent DOX attachment with a loading of 570 mg DOX per gram of CTX-GO significantly increases efficiency of the conjugate (the release of the cytostatic drug was pH-dependent). Fan et al. [74] synthesised a covalent GO-based conjugate with adipic acid dihydrazide and sodium alginate (SA). Then, DOX.HCl was attached non-covalently to GO-SA. The maximum DOX loading was 1.8 mg per 1 mg GO-SA. The highest drug release rate was observed at pH 5.0. Cytotoxicity testing with HeLa (cervical carcinoma) cell line showed that the GO-SA conjugate is not cytotoxic, while GO-SA/DOX exhibits cytotoxicity due to the specific effect on CD44 receptors.
Qin et al. [75] synthesised GO non-covalently conjugated with polyvinylpyrrolidone (PVP, M=30 kDa), and then folic acid (FA) was covalently attached through the formation of an amide bond (carboxyl groups of GO and amino groups of FA). Next, the authors performed non-covalent DOX loading (due to π-π stacking and hydrophobic interactions).
The calculated value of the DOX loading on the FA-GO-PVP was 107.5 wt. %. The resulting conjugate demonstrated high antitumour efficacy against HeLa cells. Huang et al. [76] described the ability of FA-functionalised GO to efficiently bind the chlorine e6 photosensitiser for photodynamic therapy. Tiwari et al. [77] used the GO-PVP noncovalent conjugate for double noncovalent addition of quercetin (QS) and gefitinib (GF) and compared it with the GO-PVP-QS and GO-PVP-GF conjugates. The authors found that the combined loading of the drugs showed higher cytotoxicity against PA-1 (ovarian cancer) cell line compared to individual drugs. The amount of QS and GF in GO-PVP-QS-GF was 20% and 46%, respectively.
Deb et al. [78] functionalised GO with polyethylene glycol (PEG), FA, and CPT via non-covalent π-π stacking interactions (CPT loading was 45%). The resulting conjugate (C=100 μg∙ml−1) caused the death of 76% of cells compared with the control when using the MCF-7 cell line [78]. The same group functionalised GO with the natural polymer chitosan (CS) and FA, to deliver CPT and 3,3’-diindolylme- thane (DIM). The resulting conjugate (GO-CS-FA-CPT-DIM) demonstrated high cytotoxicity against the MCF-7 cell line (95.7% decrease in cell viability), which was significantly higher compared to individual DIM preparations (42.4%) and CPT (52.6%) [79]. Pei et al. [80] showed that simultaneous functionalisation of the GO surface with PEG (pGO) (pGO-CP-DOX, mass ratio: 1: 0.376: 0.376) with cisplatin (CP) and DOX leads to increased cytotoxicity towards Cal-27 (human squamous carcinoma) and MCF-7 cell lines. The authors observed higher inhibition of cell proliferation for the pGO-CP-DOX conjugate compared to individual preparations: IC50 (MCF-7)=14.5 μg∙ml−1 for pGO-CP-DOX, 22.5 μg∙ml−1 for pGO-DOX, and 22 μg∙ml−1 for pGO-CP [80]. Bullo et al. [81] demonstrated the ability of GO functionalisation using PEG, FA, and anticancer drugs: protocatechuic acid (23.5% PCA) and chlorogenic acid (18.3% CA). The authors investigated the effect of the GO-PEG-FA-PCA-CA conjugate on the HT29 (colon cancer) and HepG2 (liver cancer) cell lines. Cytotoxicity trials showed the following results: IC50 (HT29)=50.7 μg∙ml−1, IC50 (HepG2)=40.4 μg∙ml−1 [81]. Gong et al. [82] demonstrated that fluorinated graphene (FG) can be used to load a mixture of DOX and CPT after CS covalent functionalisation; DOX and CPT loading were 110% and 25%, respectively. The resulting FG-CS-DOX-CPT conjugate demonstrated a 60% and 75% decrease in the viability of HeLa cells with simultaneous laser irradiation (wavelength 808 nm) [82]. Gong et al. [83] evaluated the ability of non-covalent FG conjugation with a cytostatic DOX loading as high as 200%. The FG-DOX conjugate at 30 μg∙ml−1 drug content reduced HeLa cell line viability to 94% after 48 h of incubation [83].
In an in vivo study, Shim et al. [84] showed that rGO functionalised with low-molecular weight heparin (LHT7) acts as a targeted drug for the delivery of DOX. The rGO-LHT7-DOX conjugate with rGO: DOX mass ratios of 2, 1, 0.5, 0.1 demonstrated a high antitumour effect at the KB human carcinoma cell line (cell viability was decreased by 61.1%), along with significant decrease in tumour size by (92.5±3.1)% [84]. Table 1 shows the results of studying cytotoxic conjugates based on GBN and cytostatic drugs.
Table 1. Cytotoxicity of GBN and non-covalently linked cytostatic drugs


Conclusion
Current data provide sufficient results concerning molecular and cellular events providing mutual influence of tumour cells and TME cells, as well as factors of cancer progression. It has been shown that the tumour cells per se and their cellular TME create an integrated system that promotes tumour progression and development of multiple drug resistance.
Thus, the following requirements must be met for modern therapeutic agents: targeted action, polyfunctionality with respect to ability of loading various molecules on the GBN surface, low toxicity, opportunity of selective inactivation of immunosuppressive components in the TME. The last issue deserves special attention. The chance to resolve this complex problem is shown by the example of GBN usage.
List of abbreviations
IL – interleukin
TNF-α – tumour necrosis factor alpha
TGF-β1 – transforming growth factor receptor-β1
VEGF-A – vascular endothelial growth factor A
CAF – cancer-associated fibroblasts
CCL – C-C motif ligand
CCR2 – C-C chemokine receptor type 2
CPT – camptothecin
CSF-1 – the colony stimulating factor 1
CTX – chlorotoxin
CXCL – the chemokine (C-X-C motif) ligand
DOX – doxorubicin
FA – folic acid
GBN – graphene-based nanomaterials
GO – graphene oxide
iNOS – Inducible nitric oxide synthase
bFGF – basic fibroblast growth factor
FGF1 – fibroblast growth factor 1
HIF-1α – hypoxia inducible factor 1 subunit alpha
PD-L1 – ligand of programmed death-1 receptor
PLGF – placental growth factor
STAT3 – signal transducer and activator of transcription 3
Th – T helper cells
TME – tumour microenvironment
Acknowledgement
This work was financially supported by the Ministry of Health of the Russian Federation (state assignment Э.03-2021; 121040200136-0).
Conflict of interests
The authors declared no potential conflict of interest.
References
- Voronov E, Dotan S, Krelin Y, Song X, Elkabets M, Carmi Y, et al. Unique versus redundant functions of IL-1α and IL-1β in the tumor microenvironment. Front Immunol. 2013; 4:177. doi: 10.3389/fimmu.2013.00177
- Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006; 42:751-759. doi: 10.1016/j.ejca.2006.01.010
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006; 25:387-408. doi: 10.1007/s10555-006-9004-4
- Farc O, Cristea V. An overview of the tumor microenvironment, from cells to complex networks (Review). Exp Ther Med. 2021; 21:1-1. doi: 10.3892/ETM.2020.9528
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015; 348:74-80.
doi: 10.1126/SCIENCE.AAA6204 - Hinshaw DC, Shevde LA The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019; 79:4557-4567.
doi: 10.1158/0008-5472.CAN-18-3962 - Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010 1110. 2010; 11:889-896. doi: 10.1038/ni.1937
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010; 141:39-51.
doi: 10.1016/J.CELL.2010.03.014 - Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004; 25:677-686. doi: 10.1016/J.IT.2004.09.015
- Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012; 189:3848-3858. doi: 10.4049/jimmunol.1200819
- Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018; 154:3-20. doi: 10.1111/imm.12888
- Fridlender ZG, Albelda SM. Tumor-associated neutrophils: Friend or foe? Carcinogenesis. 2012; 33:949-955. doi: 10.1093/carcin/bgs123
- Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014; 20:511-517. doi: 10.1038/nm.3547
- Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016; 5. doi: 10.1080/2162402X.2015.1134073
- Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015; 3:1236-1247. doi: 10.1158/2326-6066.CIR-15-0036
- Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016; 37:208-220. doi: 10.1016/j.it.2016.01.004
- Guan J, Chen J. Mesenchymal stem cells in the tumor microenvironment (Review). Biomed Rep. 2013; 1:517-521.
doi: 10.3892/BR.2013.103 - Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015; 66:97-110. doi: 10.1146/annurev-med-051013-052304
- Shi H, Zhang J, Han X, Li H, Xie M, Sun Y, et al. Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1β-mediated increase in E-selectin expression. Int J Cancer. 2017; 140:1370-1383.
doi: 10.1002/ijc.30538 - Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, et al. CD11b + /Gr-1 + immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1β-secreting cells. J Immunol. 2005; 175:8200-8208. doi: 10.4049/jimmunol.175.12.8200
- Elkabets M, Ribeiro VSG, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, et al. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010; 40:3347-3357. doi: 10.1002/eji.201041037
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006; 176:284-290. doi: 10.4049/jimmunol.176.1.284
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12:253-268. doi: 10.1038/nri3175
- Hye WK, Torres D, Wald L, Weissleder R, Bogdanov AA. Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006; 86:599-609. doi: 10.1038/labinvest.3700421
- Nasu K, Itoh H, Yuge A, Kawano Y, Yoshimatsu J, Narahara H, et al. Interleukin-1β regulates vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 secretion by human oviductal epithelial cells and stromal fibroblasts. Gynecol Endocrinol. 2006; 22:495-500. doi: 10.1080/08916930600929487
- Qin SL, Li TS, Takahashi M, Hamano K. In vitro assessment of the effect of interleukin-1β on angiogenic potential of bone marrow cells. Circ J. 2006. doi: 10.1253/circj.70.1195
- Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, et al. The non-classical export routes: FGF1 and IL-1α point the way. J. Cell Sci. 2003; doi: 10.1242/jcs.00872
- Tannahill GM, Curtis AM, Adamik J, Palsson-Mcdermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013; 496:238-242. doi: 10.1038/nature11986
- Oh H, Grinberg-Bleyer Y, Liao W, Maloney D, Wang P, Wu Z, et al. An NF-κB transcription-factor-dependent lineage-specific transcriptional program promotes regulatory T cell identity and function. Immunity. 2017; 47:450-465.e5.
doi: 10.1016/j.immuni.2017.08.010 - Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020; 30:R921-R925. doi: 10.1016/J.CUB.2020.06.081
- Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T Cells to protect tumour cells. Nat Commun. 2018; 9. doi: 10.1038/s41467-018-03347-0
- Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: A multidisciplinary study. Cancer Res. 2006; 66:5216-5223. doi: 10.1158/0008-5472.CAN-05-4193
- Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, et al. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis. 2008; 25:411-425. doi: 10.1007/s10585-008-9145-7
- Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003; 5:533-545. doi: 10.1016/s1476-5586(03)80037-4
- Švastová E, Žilka N, Zat’ovičová M, Gibadulinová A, Čiampor F, Pastorek J, et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with β-catenin. Exp Cell Res. 2003; 290:332-345. doi: 10.1016/S0014-4827(03)00351-3
- Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016; 76:1381-1390. doi: 10.1158/0008-5472.CAN-15-1743
- Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012; 72:2746-2756. doi: 10.1158/0008-5472.CAN-11-1272
- Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. 2003; 66:1219-1229. doi: 10.1016/S0006-2952(03)00468-4
- Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003; 66:1207-1218. doi: 10.1016/S0006-2952(03)00467-2
- Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006; 5:1275-1279. doi: 10.1158/1535-7163.MCT-06-0024
- Salerno M, Przewloka T, Fokt I, Priebe W, Garnier-Suillerot A Preferential efflux by P-glycoprotein, but not MRP1, of compounds containing a free electron donor amine. Biochem Pharmacol. 2002; 63:1471-1479. doi: 10.1016/S0006-2952(02)00895-X
- Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006; 8:143-152. doi: 10.1593/neo.05697
- Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O. Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007; 17:239-244. doi: 10.3892/or.17.1.239
- Seabra AB, Paula AJ, De Lima R, Alves OL, Durán N. Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol. 2014; 27:159-168. doi: 10.1021/TX400385X
- Lalwani G, D’Agati M, Khan AM, Sitharaman B. Toxicology of graphene-based nanomaterials. Adv Drug Deliv Rev. 2016; 105:109-144. doi: 10.1016/J.ADDR.2016.04.028
- Li B, Yang J, Huang Q, Zhang Y, Peng C, Zhang Y, et al. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice. NPG Asia Mater. 2013 54. 2013; 5:e44-e44. doi: 10.1038/am.2013.7
- Liu JH, Yang ST, Wang H, Chang Y, Cao A, Liu Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine (Lond). 2012;7(12):1801-1812. doi: 10.2217/Nnm.12.60
- Yang K, Gong H, Shi X, Wan J, Zhang Y, Liu Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials. 2013; 34:2787-2795. doi: 10.1016/J.BIOMATERIALS.2013.01.001
- Yue H, Wei W, Yue Z, Wang B, Luo N, Gao Y, et al. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012; 33:4013-4021. doi: 10.1016/J.BIOMATERIALS.2012.02.021
- Deng X, Liang H, Yang W, Shao Z. Polarization and function of tumor-associated macrophages mediate graphene oxide-induced photothermal cancer therapy. J Photochem Photobiol. B Biol. 2020; 208:111913. doi: 10.1016/J.JPHOTOBIOL.2020.111913
- Shen ZY, Shen BQ, Shen AJ, Zhu XH. Cavitation-enhanced delivery of the nanomaterial graphene oxide-doxorubicin to hepatic tumors in nude mice using 20 kHz low-frequency ultrasound and microbubbles. J Nanomater. 2020:. doi: 10.1155/2020/3136078
- Abdelhalim AOE, Sharoyko VV, Ageev SV, Farafonov VS, Nerukh DA, Postnov VN, et al. Graphene oxide of extra high oxidation: A wafer for loading guest molecules. J Phys Chem Lett. 2021;10015-10024. doi: 10.1021/ACS.JPCLETT.1C02766
- Abdelhalim AOE, Sharoyko VV, Meshcheriakov AA, Luttsev MD, Potanin AA, Iamalova NR, et al. Synthesis, characterisation and biocompatibility of graphene-L-methionine nanomaterial. J Mol Liq. 2020; 314:113605. doi: 10.1016/j.molliq.2020.113605
- Abdelhalim AOE, Sharoyko VV, Meshcheriakov AA, Martynova SD, Ageev SV, Iurev GO, et al., Reduction and functionalization of graphene oxide with L-cysteine: Synthesis, characterization and biocompatibility. Nanomedicine Nanotechnology, Biol Med. 2020; 29:102284. doi: 10.1016/j.nano.2020.102284
- Li Q, Shi Z, Zhang F, Zeng W, Zhu D, Mei L, Symphony of nanomaterials and immunotherapy based on the cancer-immunity cycle. Acta Pharm Sin B. 2021; doi: 10.1016/J.APSB.2021.05.031
- Kaplan A, Yuan Z, Benck JD, Govind Rajan A, Chu XS, Wang QH, et al. Current and future directions in electron transfer chemistry of graphene. Chem Soc Rev. 2017; 46:4530-4571. doi: 10.1039/c7cs00181a
- Sun Z, James DK, Tour JM. Graphene chemistry: synthesis and manipulation. J Phys Chem Lett. 2011; 2:2425-2432.
doi: 10.1021/jz201000a - Loh KP, Bao Q, Ang PK, Yang J. The chemistry of graphene. J Mater Chem. 2010; 20:2277-2289. doi: 10.1039/b920539j
- Sturala J, Luxa J, Pumera M, Sofer Z. Frontispiece: Chemistry of graphene derivatives: Synthesis, applications, and perspectives. Chem A Eur J. 2018; 24:. doi: 10.1002/chem.201882361
- Gao W. The chemistry of graphene oxide, in: Graphene Oxide Reduct. Recipes, Spectrosc. Appl., Springer Int Publishing, 2015: pp. 61-95. doi: 10.1007/978-3-319-15500-5_3
- Eigler S, Hirsch A. Chemistry with graphene and graphene oxide – Challenges for synthetic chemists. Angew Chemie-Int Ed. 2014; 53:7720-7738. doi: 10.1002/anie.201402780
- Lavin-Lopez MP, Paton-Carrero A, Sanchez-Silva L, Valverde JL, Romero A. Influence of the reduction strategy in the synthesis of reduced graphene oxide. Adv Powder Technol. 2017; 28:3195-3203. doi: 10.1016/j.apt.2017.09.032
- Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007; 6:183-191. doi: 10.1038/nmat1849
- [Brodie BC, XIII. On the atomic weight of graphite. Philos Trans R Soc London. 1859; 149:249-259.
- Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958; 80:1339. doi: 10.1021/ja01539a017
- Eskiizmir G, Baskin Y, Yapici K. Graphene-based nanomaterials in cancer treatment and diagnosis, in: Fullerenes, Graphenes Nanotub. A Pharm. Approach, Elsevier, 2018: pp. 331-374. doi: 10.1016/B978-0-12-813691-1.00009-9
- Wang H, Gu W, Xiao N, Ye L, Xu Q. Chlorotoxin-conjugated graphene oxide for targeted delivery of an anticancer drug. Int J Nanomedicine. 2014; 9:1433-1442. doi: 10.2147/IJN.S58783
- Kim H, Namgung R, Singha K, Oh IK, Kim WJ. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug Chem. 2011; 22:2558-2567. doi: 10.1021/bc200397j
- Lee J, Kim J, Kim S, Min DH. Biosensors based on graphene oxide and its biomedical application. Adv Drug Deliv Rev. 2016; 105:275-287. doi: 10.1016/j.addr.2016.06.001
- Innocenzi P, Stagi L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020; 11:6606-6622. doi: 10.1039/d0sc02658a
- Szunerits S, Boukherroub R. Antibacterial activity of graphene-based materials. J Mater Chem. B. 2016; 4:6892-6912.
doi: 10.1039/c6tb01647b - Li C, Wang X, Chen F, Zhang C, Zhi X, Wang K, et al. The antifungal activity of graphene oxide-silver nanocomposites. Biomaterials. 2013; 34:3882-3890. doi: 10.1016/j.biomaterials.2013.02.001
- Zhang L, Xia J, Zhao Q, Liu L, Zhang Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small. 2010; 6:537-544. doi: 10.1002/smll.200901680
- Fan L, Ge H, Zou S, Xiao Y, Wen H, Li Y, et al. Sodium alginate conjugated graphene oxide as a new carrier for drug delivery system. Int J Biol Macromol. 2016; 93:582-590. doi: 10.1016/j.ijbiomac.2016.09.026
- Qin XC, Guo ZY, Liu ZM, Zhang W, Wan MM, Yang BW. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J Photochem Photobiol B Biol. 2013; 120:156-162. doi: 10.1016/j.jphotobiol.2012.12.005
- Huang P, Xu C, Lin J, Wang C, Wang X, Zhang C, et al. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2012; 1:240-250. doi: 10.7150/thno/v01p0240
- Tiwari H, Karki N, Pal M, Basak S, Verma RK, Bal R, et al. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surfaces B Biointerfaces. 2019; 178:452-459. doi: 10.1016/j.colsurfb.2019.03.037
- Deb A, Vimala R. Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J Drug Deliv Sci Technol. 2018; 43:333-342. doi: 10.1016/j.jddst.2017.10.025
- Deb A, Andrews NG, Raghavan V. Natural polymer functionalized graphene oxide for co-delivery of anticancer drugs: In-vitro and in-vivo. Int J Biol Macromol. 2018; 113:515-525. doi: 10.1016/j.ijbiomac.2018.02.153
- Pei X, Zhu Z, Gan Z, Chen J, Zhang X, Cheng X, et al. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci Rep. 2020; 10:1-15. doi: 10.1038/s41598-020-59624-w
- Bullo S, Buskaran K, Baby R, Dorniani D, Fakurazi S, Hussein MZ. Dual drugs anticancer nanoformulation using graphene oxide-PEG as nanocarrier for protocatechuic acid and chlorogenic acid. Pharm Res. 2019; 36:91. doi: 10.1007/s11095-019-2621-8
- Gong P, Zhao Q, Dai D, Zhang S, Tian Z, Sun L, et al. Functionalized ultrasmall fluorinated graphene with high NIR absorbance for controlled delivery of mixed anticancer drugs. Chem- A Eur J. 2017; 23:17531-17541. doi: 10.1002/chem.201702917
- Gong P, Du J, Wang D, Cao B, Tian M, Wang Y, et al. Fluorinated graphene as an anticancer nanocarrier: An experimental and DFT study. J Mater Chem B. 2018; 6:2769-2777. doi: 10.1039/c8tb00102b
- Shim G, Kim JY, Han J, Chung SW, Lee S, Byun Y, et al. Reduced graphene oxide nanosheets coated with an anti-angiogenic anticancer low-molecular-weight heparin derivative for delivery of anticancer drugs. J Control Release. 2014; 189:80-89.
doi: 10.1016/j.jconrel.2014.06.026 - Rosli NF, Fojtů M, Fisher AC, Pumera M. Graphene oxide nanoplatelets potentiate anticancer effect of cisplatin in human lung cancer cells. Langmuir. 2019; 35:3176-3182. doi: 10.1021/acs.langmuir.8b03086
- Pooresmaeil M, Namazi H. β-Cyclodextrin grafted magnetic graphene oxide applicable as cancer drug delivery agent: Synthesis and characterization. Mater Chem Phys. 2018; 218:62-69. doi: 10.1016/j.matchemphys.2018.07.022
- Ma N, Liu J, He W, Li Z, Luan Y, Song Y, et al. Folic acid-grafted bovine serum albumin decorated graphene oxide: An efficient drug carrier for targeted cancer therapy. J Colloid Interface Sci. 2017; 490:598-607. doi: 10.1016/j.jcis.2016.11.097
- Tian J, Luo Y, Huang L, Feng Y, Ju H, Yu BY. Pegylated folate and peptide-decorated graphene oxide nanovehicle for in vivo targeted delivery of anticancer drugs and therapeutic self-monitoring. Biosens Bioelectron. 2016; 80:519-524. doi: 10.1016/j.bios.2016.02.018
- Javanbakht S, Namazi H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater Sci Eng C. 2018; 87:50-59. doi: 10.1016/j.msec.2018.02.010
- Karki N, Tiwari H, Pal M, Chaurasia A, Bal R, Joshi P, et al. Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: A comparative study. Colloids Surfaces B Biointerfaces. 2018; 169:265-272.
doi: 10.1016/j.colsurfb.2018.05.022
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(74) "vzaimnoye-vliyaniye-opukholevykh-kletok-i-kletok-mikrookruzheniya-opukholi" ["~CODE"]=> string(74) "vzaimnoye-vliyaniye-opukholevykh-kletok-i-kletok-mikrookruzheniya-opukholi" ["EXTERNAL_ID"]=> string(4) "2039" ["~EXTERNAL_ID"]=> string(4) "2039" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(415) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериаловMutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(3236) "<p style="text-align: justify;">Развитие и прогрессирование неоплазий происходит одновременно с изменениями окружающей стромы. Раковые клетки могут функционально формировать свое микроокружение за счет секреции различных цитокинов, хемокинов и формирования кислой среды. Данные факторы способствуют дифференциации иммунных клеток по иммуносупрессивному фенотипу, стимулируют синтез ряда ферментов обмена аминокислот, факторов роста, молекул адгезии, что промотирует инвазию, ангиогенез и метастазирование, а также снижает эффективность действия противоопухолевых препаратов и лучевой терапии. Для повышения эффективности химиотерапии возможно использование мультитаргетных углеродных наноматериалов. В частности, наноматериалы на основе модифицированного графена позволяют создавать многокомпонентные терапевтические конструкции, включающие макромолекулы, полимеры и эффекторные агенты. Первоначальные эксперименты с немодифицированными графенами продемонстрировали их токсичность, связанную с их накоплением в паренхиматозных органах и инициированием воспалительных процессов. В последние несколько лет вышла серия работ, в которых продемонстрирована возможность снижения токсичности оксида графена за счет функционализации. В данном обзоре обобщены экспериментальные данные по созданию ковалентных и нековалентных конъюгатов на основе оксида графена и показана их эффективность <i>in vitro</i> на различных опухолевых клеточных линиях. Отдельно представлены немногочисленные данные по влиянию наноматериалов на основе оксида графена на опухолевое микроокружение.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Опухоль, микроокружение, прогрессирование, цитокины, ацидоз, иммунная система, углеродные наноматериалы.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_META_TITLE"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_META_KEYWORDS"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_META_DESCRIPTION"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_PICTURE_FILE_ALT"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_PICTURE_FILE_TITLE"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_PICTURE_FILE_NAME"]=> string(101) "vzaimnoe-vliyanie-opukholevykh-kletok-i-kletok-mikrookruzheniya-opukholi-perspektivy-manipulirovaniya" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(279) "Взаимное влияние опухолевых клеток и клеток микроокружения опухоли. Перспективы манипулирования опухолевым микроокружением с помощью наноматериалов" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(101) "vzaimnoe-vliyanie-opukholevykh-kletok-i-kletok-mikrookruzheniya-opukholi-perspektivy-manipulirovaniya" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(101) "vzaimnoe-vliyanie-opukholevykh-kletok-i-kletok-mikrookruzheniya-opukholi-perspektivy-manipulirovaniya" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(101) "vzaimnoe-vliyanie-opukholevykh-kletok-i-kletok-mikrookruzheniya-opukholi-perspektivy-manipulirovaniya" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "200" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28395" ["VALUE"]=> array(2) { ["TEXT"]=> string(460) "<p>Анна М. Малкова<sup>1,2</sup>, Сергей В. Агеев<sup>1,2</sup>, Абделсаттар О. Е. Абделхалим<sup>2,3</sup>, Олег Е. Молчанов<sup>4</sup>, Дмитрий Н. Майстренко<sup>4</sup>, Константин Н. Семенов<sup>1,2,4</sup>, Владимир В. Шаройко<sup>1,2,4</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(364) "
Анна М. Малкова1,2, Сергей В. Агеев1,2, Абделсаттар О. Е. Абделхалим2,3, Олег Е. Молчанов4, Дмитрий Н. Майстренко4, Константин Н. Семенов1,2,4, Владимир В. Шаройко1,2,4
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28396" ["VALUE"]=> array(2) { ["TEXT"]=> string(976) "<p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> Институт химии, Санкт-Петербургский государственный университет, Санкт-Петербург, Россия<br> <sup>3</sup> Отдел исследования окружающей среды, Национальный центр социальных и криминологических исследований, Гиза, Арабская Республика Египет<br> <sup>4</sup> Российский научный центр радиологии и хирургических технологий имени A. M. Гранова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(898) "1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Институт химии, Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 Отдел исследования окружающей среды, Национальный центр социальных и криминологических исследований, Гиза, Арабская Республика Египет
4 Российский научный центр радиологии и хирургических технологий имени A. M. Гранова, Санкт-Петербург, Россия
Развитие и прогрессирование неоплазий происходит одновременно с изменениями окружающей стромы. Раковые клетки могут функционально формировать свое микроокружение за счет секреции различных цитокинов, хемокинов и формирования кислой среды. Данные факторы способствуют дифференциации иммунных клеток по иммуносупрессивному фенотипу, стимулируют синтез ряда ферментов обмена аминокислот, факторов роста, молекул адгезии, что промотирует инвазию, ангиогенез и метастазирование, а также снижает эффективность действия противоопухолевых препаратов и лучевой терапии. Для повышения эффективности химиотерапии возможно использование мультитаргетных углеродных наноматериалов. В частности, наноматериалы на основе модифицированного графена позволяют создавать многокомпонентные терапевтические конструкции, включающие макромолекулы, полимеры и эффекторные агенты. Первоначальные эксперименты с немодифицированными графенами продемонстрировали их токсичность, связанную с их накоплением в паренхиматозных органах и инициированием воспалительных процессов. В последние несколько лет вышла серия работ, в которых продемонстрирована возможность снижения токсичности оксида графена за счет функционализации. В данном обзоре обобщены экспериментальные данные по созданию ковалентных и нековалентных конъюгатов на основе оксида графена и показана их эффективность in vitro на различных опухолевых клеточных линиях. Отдельно представлены немногочисленные данные по влиянию наноматериалов на основе оксида графена на опухолевое микроокружение.
Ключевые слова
Опухоль, микроокружение, прогрессирование, цитокины, ацидоз, иммунная система, углеродные наноматериалы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28398" ["VALUE"]=> string(39) "10.18620/ctt-1866-8836-2021-10-3-4-8-18" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(39) "10.18620/ctt-1866-8836-2021-10-3-4-8-18" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28399" ["VALUE"]=> array(2) { ["TEXT"]=> string(328) "<p>Anna M. Malkova<sup>1,2</sup>, Sergei V. Ageev<sup>1,2</sup>, Abdelsattar O. E. Abdelhalim<sup>2,3</sup>, Oleg E. Molchanov<sup>4</sup>, Dmitrii N. Maistrenko 4, Konstantin N. Semenov<sup>1,2,4</sup>, Vladimir V. Sharoyko<sup>1,2,4</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(244) "Anna M. Malkova1,2, Sergei V. Ageev1,2, Abdelsattar O. E. Abdelhalim2,3, Oleg E. Molchanov4, Dmitrii N. Maistrenko 4, Konstantin N. Semenov1,2,4, Vladimir V. Sharoyko1,2,4
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28400" ["VALUE"]=> array(2) { ["TEXT"]=> string(992) "<p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia<br> <sup>3</sup> National Center for Social and Criminological Research, Giza, Arab Republic of Egypt<br> <sup>4</sup> A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Vladimir V. Sharoyko, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia<br> Phone: +7 (981) 936-41-51<br> E-mail: sharoyko@gmail.com</p><br> <p><b>Citation:</b> Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(836) "1 Pavlov University, St. Petersburg, Russia
2 Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia
3 National Center for Social and Criminological Research, Giza, Arab Republic of Egypt
4 A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Vladimir V. Sharoyko, Pavlov University,
6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
Phone: +7 (981) 936-41-51
E-mail: sharoyko@gmail.com
Citation: Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28401" ["VALUE"]=> array(2) { ["TEXT"]=> string(1764) "<p style="text-align: justify;">Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their <i>in vitro</i> efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.</p> <h2>Keywords</h2> <p style="text-align: justify;">Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1696) "Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their in vitro efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.
Keywords
Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28402" ["VALUE"]=> string(136) "Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(136) "Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28403" ["VALUE"]=> string(4) "2692" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2692" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28404" ["VALUE"]=> string(4) "2693" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2693" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28399" ["VALUE"]=> array(2) { ["TEXT"]=> string(328) "<p>Anna M. Malkova<sup>1,2</sup>, Sergei V. Ageev<sup>1,2</sup>, Abdelsattar O. E. Abdelhalim<sup>2,3</sup>, Oleg E. Molchanov<sup>4</sup>, Dmitrii N. Maistrenko 4, Konstantin N. Semenov<sup>1,2,4</sup>, Vladimir V. Sharoyko<sup>1,2,4</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(244) "Anna M. Malkova1,2, Sergei V. Ageev1,2, Abdelsattar O. E. Abdelhalim2,3, Oleg E. Molchanov4, Dmitrii N. Maistrenko 4, Konstantin N. Semenov1,2,4, Vladimir V. Sharoyko1,2,4
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(244) "Anna M. Malkova1,2, Sergei V. Ageev1,2, Abdelsattar O. E. Abdelhalim2,3, Oleg E. Molchanov4, Dmitrii N. Maistrenko 4, Konstantin N. Semenov1,2,4, Vladimir V. Sharoyko1,2,4
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28401" ["VALUE"]=> array(2) { ["TEXT"]=> string(1764) "<p style="text-align: justify;">Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their <i>in vitro</i> efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.</p> <h2>Keywords</h2> <p style="text-align: justify;">Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1696) "Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their in vitro efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.
Keywords
Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1696) "Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their in vitro efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.
Keywords
Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28398" ["VALUE"]=> string(39) "10.18620/ctt-1866-8836-2021-10-3-4-8-18" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(39) "10.18620/ctt-1866-8836-2021-10-3-4-8-18" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(39) "10.18620/ctt-1866-8836-2021-10-3-4-8-18" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28402" ["VALUE"]=> string(136) "Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(136) "Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(136) "Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28400" ["VALUE"]=> array(2) { ["TEXT"]=> string(992) "<p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia<br> <sup>3</sup> National Center for Social and Criminological Research, Giza, Arab Republic of Egypt<br> <sup>4</sup> A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Vladimir V. Sharoyko, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia<br> Phone: +7 (981) 936-41-51<br> E-mail: sharoyko@gmail.com</p><br> <p><b>Citation:</b> Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(836) "1 Pavlov University, St. Petersburg, Russia
2 Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia
3 National Center for Social and Criminological Research, Giza, Arab Republic of Egypt
4 A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Vladimir V. Sharoyko, Pavlov University,
6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
Phone: +7 (981) 936-41-51
E-mail: sharoyko@gmail.com
Citation: Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(836) "1 Pavlov University, St. Petersburg, Russia
2 Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia
3 National Center for Social and Criminological Research, Giza, Arab Republic of Egypt
4 A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Vladimir V. Sharoyko, Pavlov University,
6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
Phone: +7 (981) 936-41-51
E-mail: sharoyko@gmail.com
Citation: Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28395" ["VALUE"]=> array(2) { ["TEXT"]=> string(460) "<p>Анна М. Малкова<sup>1,2</sup>, Сергей В. Агеев<sup>1,2</sup>, Абделсаттар О. Е. Абделхалим<sup>2,3</sup>, Олег Е. Молчанов<sup>4</sup>, Дмитрий Н. Майстренко<sup>4</sup>, Константин Н. Семенов<sup>1,2,4</sup>, Владимир В. Шаройко<sup>1,2,4</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(364) "Анна М. Малкова1,2, Сергей В. Агеев1,2, Абделсаттар О. Е. Абделхалим2,3, Олег Е. Молчанов4, Дмитрий Н. Майстренко4, Константин Н. Семенов1,2,4, Владимир В. Шаройко1,2,4
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(364) "Анна М. Малкова1,2, Сергей В. Агеев1,2, Абделсаттар О. Е. Абделхалим2,3, Олег Е. Молчанов4, Дмитрий Н. Майстренко4, Константин Н. Семенов1,2,4, Владимир В. Шаройко1,2,4
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28397" ["VALUE"]=> array(2) { ["TEXT"]=> string(3236) "<p style="text-align: justify;">Развитие и прогрессирование неоплазий происходит одновременно с изменениями окружающей стромы. Раковые клетки могут функционально формировать свое микроокружение за счет секреции различных цитокинов, хемокинов и формирования кислой среды. Данные факторы способствуют дифференциации иммунных клеток по иммуносупрессивному фенотипу, стимулируют синтез ряда ферментов обмена аминокислот, факторов роста, молекул адгезии, что промотирует инвазию, ангиогенез и метастазирование, а также снижает эффективность действия противоопухолевых препаратов и лучевой терапии. Для повышения эффективности химиотерапии возможно использование мультитаргетных углеродных наноматериалов. В частности, наноматериалы на основе модифицированного графена позволяют создавать многокомпонентные терапевтические конструкции, включающие макромолекулы, полимеры и эффекторные агенты. Первоначальные эксперименты с немодифицированными графенами продемонстрировали их токсичность, связанную с их накоплением в паренхиматозных органах и инициированием воспалительных процессов. В последние несколько лет вышла серия работ, в которых продемонстрирована возможность снижения токсичности оксида графена за счет функционализации. В данном обзоре обобщены экспериментальные данные по созданию ковалентных и нековалентных конъюгатов на основе оксида графена и показана их эффективность <i>in vitro</i> на различных опухолевых клеточных линиях. Отдельно представлены немногочисленные данные по влиянию наноматериалов на основе оксида графена на опухолевое микроокружение.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Опухоль, микроокружение, прогрессирование, цитокины, ацидоз, иммунная система, углеродные наноматериалы.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3168) "Развитие и прогрессирование неоплазий происходит одновременно с изменениями окружающей стромы. Раковые клетки могут функционально формировать свое микроокружение за счет секреции различных цитокинов, хемокинов и формирования кислой среды. Данные факторы способствуют дифференциации иммунных клеток по иммуносупрессивному фенотипу, стимулируют синтез ряда ферментов обмена аминокислот, факторов роста, молекул адгезии, что промотирует инвазию, ангиогенез и метастазирование, а также снижает эффективность действия противоопухолевых препаратов и лучевой терапии. Для повышения эффективности химиотерапии возможно использование мультитаргетных углеродных наноматериалов. В частности, наноматериалы на основе модифицированного графена позволяют создавать многокомпонентные терапевтические конструкции, включающие макромолекулы, полимеры и эффекторные агенты. Первоначальные эксперименты с немодифицированными графенами продемонстрировали их токсичность, связанную с их накоплением в паренхиматозных органах и инициированием воспалительных процессов. В последние несколько лет вышла серия работ, в которых продемонстрирована возможность снижения токсичности оксида графена за счет функционализации. В данном обзоре обобщены экспериментальные данные по созданию ковалентных и нековалентных конъюгатов на основе оксида графена и показана их эффективность in vitro на различных опухолевых клеточных линиях. Отдельно представлены немногочисленные данные по влиянию наноматериалов на основе оксида графена на опухолевое микроокружение.
Ключевые слова
Опухоль, микроокружение, прогрессирование, цитокины, ацидоз, иммунная система, углеродные наноматериалы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3168) "Развитие и прогрессирование неоплазий происходит одновременно с изменениями окружающей стромы. Раковые клетки могут функционально формировать свое микроокружение за счет секреции различных цитокинов, хемокинов и формирования кислой среды. Данные факторы способствуют дифференциации иммунных клеток по иммуносупрессивному фенотипу, стимулируют синтез ряда ферментов обмена аминокислот, факторов роста, молекул адгезии, что промотирует инвазию, ангиогенез и метастазирование, а также снижает эффективность действия противоопухолевых препаратов и лучевой терапии. Для повышения эффективности химиотерапии возможно использование мультитаргетных углеродных наноматериалов. В частности, наноматериалы на основе модифицированного графена позволяют создавать многокомпонентные терапевтические конструкции, включающие макромолекулы, полимеры и эффекторные агенты. Первоначальные эксперименты с немодифицированными графенами продемонстрировали их токсичность, связанную с их накоплением в паренхиматозных органах и инициированием воспалительных процессов. В последние несколько лет вышла серия работ, в которых продемонстрирована возможность снижения токсичности оксида графена за счет функционализации. В данном обзоре обобщены экспериментальные данные по созданию ковалентных и нековалентных конъюгатов на основе оксида графена и показана их эффективность in vitro на различных опухолевых клеточных линиях. Отдельно представлены немногочисленные данные по влиянию наноматериалов на основе оксида графена на опухолевое микроокружение.
Ключевые слова
Опухоль, микроокружение, прогрессирование, цитокины, ацидоз, иммунная система, углеродные наноматериалы.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28396" ["VALUE"]=> array(2) { ["TEXT"]=> string(976) "<p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> Институт химии, Санкт-Петербургский государственный университет, Санкт-Петербург, Россия<br> <sup>3</sup> Отдел исследования окружающей среды, Национальный центр социальных и криминологических исследований, Гиза, Арабская Республика Египет<br> <sup>4</sup> Российский научный центр радиологии и хирургических технологий имени A. M. Гранова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(898) "1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Институт химии, Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 Отдел исследования окружающей среды, Национальный центр социальных и криминологических исследований, Гиза, Арабская Республика Египет
4 Российский научный центр радиологии и хирургических технологий имени A. M. Гранова, Санкт-Петербург, Россия
1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Институт химии, Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 Отдел исследования окружающей среды, Национальный центр социальных и криминологических исследований, Гиза, Арабская Республика Египет
4 Российский научный центр радиологии и хирургических технологий имени A. M. Гранова, Санкт-Петербург, Россия
Introduction
Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells (PS) or lymphocytes and their deposition in organs and tissues in the form of an insoluble fibrillar protein-amyloid. Cardiac involvement is the main predictor of patient outcome. The goal of therapy is to rapidly and profoundly suppress production of amyloidogenic light chains [1-2].
Autologous hematopoietic stem cell transplantation (ASCT) has been used in the treatment of systemic AL amyloidosis since 1994. Long-term follow-up demonstrate 15-year overall survival in 50% of patients who achieved complete hematological response (CR) after ASCT [3-4]. However, 80% of patients with newly diagnosed systemic AL amyloidosis are ineligible for ASCT.
For a long time, oral melphalan and dexamethasone (MDex) were considered a standard of care in AL amyloidosis, but the addition of proteasome inhibitor- bortezomib, significantly increased the efficacy of treatment [5]. However, bortezomib, cyclophosphamide and dexamethasone (CyBorD) regime currently is the standard of care for newly diagnosed patients. According to a large European retrospective study, the frequency of hematological response (HR) on CyBorD regime is 65%, cardiac response – 33%, renal response –15%, median overall survival (OS) was 72 months [6-8].
Despite higher frequency of clinical responses, the addition of bortezomib did not improve prognosis in the patients with IIIb heart stage, according to the Mayo Clinic classification (NT-proBNP >8500 ng/l), and 40% of these patients still die within 6 months after the diagnosis. Therefore, the question of the optimal regimens for these patients is still open.
Patients and methods
We analyzed the response to induction therapy in 105 newly diagnosed patients with systemic AL amyloidosis, treated at RM Gorbacheva Research Institute within a period from 2004 to 2021 [6]. All the patients signed informed consent for the use of personal data for research purposes. Their median age was 63 years (31-81). The percentage of men and women was 53% and 47%, respectively. Isolated AL amyloidosis was documented in 72% (n=76) of cases; in combination with multiple myeloma, in 27% (n=29). At the time of diagnosis, 62% of patients had three or more organs involved. The incidence of organ involvement was as follows: kidneys, 88% (n=93); heart, 87% (n=92); liver, 40% (n=43); nervous system, 48% (n=51); gastrointestinal tract, (GIT) 27% (n=29); lungs, 11% (n=12), and other organs (thyroid gland, lymph nodes, adrenal glands, etc.), 20% (n=21). In the patients with established Mayo stage, the following distribution was observed: stage I, 22% (n=20); stage II, 52% (n=47); stage III, 26% (n=24). The median NT-proBNP level was 1892 ng/l (35 to 34772), 15 patients had IIIb stage (NT-proBNP >8500 ng/l). Renal involvement was documented in 94 patients at the following severity distribution: stage I, 23% (n=22); stage II, 48% (n=45); stage III, 29% (n=27).
In our study, we assessed the efficacy of CyBorD compared with other treatment regimens. All consecutive patients were divided into 3 groups: group 1 was treated with CyBorD (26%, n=28); group 2 received other bortezomib-based regimens (bortezomib/dexamethasone (VD), bortezomib/melphalan/dexamethasone (BMDex)), 59% (n=62); group 3 included bortezomib-free regimens (melphalan/dexamethasone (MDex), cyclophosphamide/prednisolone, corticosteroids), 14% (n=15). Patients with autologous ASCT were excluded. The median number of therapeutic rounds in all groups was 4. The main patients’ characteristics are presented in Table 1.
Table 1. Patients characteristics

Clinical definitions
Hematological response (HR) evaluation was based on serum and urine immunofixation and determination of the level of free light chains (FLC) by nephelometry. Complete remission (CR) definition included negative serum and urine immunofixation and normal FLC ratio, very good partial response (VGPR) was based on difference between involved and uninvolved concentrations of free light chains (dFLС) <40 mg/L. Partial response (PR) was defined as a decrease of dFLC by >50%. Organ response was evaluated by changes in biomarkers: NT-proBNP for the heart, proteinuria for the kidney and alkaline phosphatase for the liver. Relapse or progression was defined as loss of previously achieved response, or organ progression, or increase in dFLС by 50% from the best response.
Statistical analysis
Descriptive statistics was used for the patient data evaluation. Chi-square test was used to compare response between the groups. Kaplan-Meier method and log-rank test was used to compare survival estimates. Cumulative incidence estimates were used to evaluate hematologic and organ responses. Gray’s test was applied to compare cumulative incidences between the groups. All the survival and cumulative incidence parameters were calculated from the date of systemic therapy initiation. For progression-free survival, the outcome measures were death or hematological relapse/progressive disease. Relapse/progressive disease were considered a competing risk for organ response. Multivariate analysis was done with proportional hazard analysis for survival estimates and Fyne-Gray regression for cumulative incidences. Variables with significance of <0.015 were selected for multivariate analysis.
Results
The 3-year OS was 70.3% (95% СI 61-80), the median follow-up was 27.8 months (22 days to 11 years). In the univariate analysis, unfavorable factors for OS were as follows: age over 70 years (p=0.007); male gender (p=0.015, 64% versus 86%); Mayo stage IIIb [OS was 48% (95% CI 21-75) vs 60% (95% CI 25-95) vs 72% (95% CI 59-85), and 79% (95% CI 58-100) in comparison with Mayo stages IIIа, II and I, respectively (p=0.07)]; renal injury stage III [65% (95% CI 46-84) vs 73% (95% CI 54-92) vs 79% (95% CI 68-90) compared with stages I and II, respectively, p<0.0001]. Improved 3-year OS was documented in the presence of hematological response [91% (95% CI 83-99) vs 40% (95% CI 25-54), p<0.001] (Figure 1B) and organ responses [93% (95% CI 86-100) vs 55% (95% CI 38-72), p=0.0003] in patients with cardiac response vs non-responders, [92% (95% CI 85-99) vs 77% (95% CI 60-94), p<0.0001, and 100% vs 33% (95% CI 4-62), p=0.0002 in the patients with renal and liver responses, respectively. Assessment of overall survival risk factors is presented in Table 2.
Table 2. Risk factors assessment for 3-year overall survival

Notes: NS, nervous system; GIT, gastrointestinal tract

Figure 1. Kinetics of hematologic and organ responses in the study group (A). Impact of hematologic response on overall survival (B)
The 3-year OS in CyBorD group was 95% (95% CI 85-99) versus 85% (95% CI 73-97) in other bortezomib-containing regimens, and 63% (95% CI 44-82) in non-bortezomib regimens (p=0.0337). In the multivariate analysis, when corrected for other confounding factors, any treatment except CyBorD was associated with worse 3-year OS (HR 4.9, 95% CI 1.4-17.2, p=0.012). Other factors which impacted the survival were: bone marrow plasma cell (BMPC) counts >2.5% (HR 0.2, 95% CI 0.1-0.5, p=0.0002), and NtproBNP levels >2500 ng/l OS (HR 4.6, 95% CI 1.0-20.6, p=0.04, Figure 2A).
The 3-year progression-free survival (PFS) in CyBorD group was 79% (95%CI 63-95), versus 48% (95%CI 35-61) in group 2, and 55% (95%CI 30-80) in group 3 (p=0.28).
Overall response rate (ORR) was 70% (n=74), and median time to HR was 10.3 (9-14.6) months.

Figure 2. Forest plot of multivariate analyses of 3-year overall survival (A), and hematological response (B)
The percentage of patients who had HR was significantly higher in the CyBoRD group, 94% (95% CI 84-99) vs 84% in group 2 (95% CI 73-95), and 63% in group 3 (95% CI 26-100), p=0.033. The HR was achieved earlier at the bortezomib-containing regimens, with median time to response of 9.6 (5.3-15), and 10 (7.7-12.4) months in groups 1 and 2 vs 32.7 (6.4-32.7) months in group 3.

Figure 3. Summary of the best hematologic responses according to the study groups
Group 1: CyBorD; group 2: other bortezomib-containing regimens; group 3: non-bortezomib treatments.
Abbreviations: Partial response (PR); Very good partial response (VGPR); Complete response (CR)
The frequency of CR was comparable between the groups: 52% (11/21), 76% (30/39) and 60% (3/5). VGPR was achieved in 19% (4/21), 8% (3/39), 20% (1/5) and PR 28% (6/21), 15% (6/39), 20% (1/5), in groups 1, 2 and 3 respectively (Fig. 3, p>0.05).
In the univariate analysis, gender, age, number of affected organs, presence of multiple myeloma did not affect frequency of hematological remission. Surprisingly, in the multivariate analysis, the cumulative HR incidence was significantly lower in patients with the presence of PC >2.5% (HR 0.67, 95%CI 0.54-0.83, p=0.0002) and it was the only significant factor (Fig. 2B).
The organ response (OR) was assessed over a 3-year period from the start of therapy (Fig. 1A). Assessment of heart response by cardiac biomarkers was possible in 77 patients (23, 45 and 9 patients in groups 1, 2, and 3, respectively). Cardiac responses were observed in 38 (49%) cases. The median time to response was 19.3 (12.8-70) months.
The percentage of cardiac responses was higher in CyBorD group, i.e., 78% (95% CI 59-97) vs 55% (95% CI 36-74) and 16 % (95% CI 16-44), in groups 2 and 3, respectively (p=0.050). In CyBorD group, the time to cardiac response was also shorter: 13 months versus 36 months versus not reached (p=0.050).
Renal responses were observed in 46 (63%) of 73 patients with median time to response 12 (9.5-18.3) months. The frequency of responses in groups 1, 2 and 3 was the following: 90% (95% СI 73-99), 92% (95% СI 81-99, and 57% (95% СI 22-92), p=0.01. The shortest median time to response was also in CyBorD group, i.e., 7 (2.7-11.9) vs 16.5 (10-29) months in group 2. For the group 3, median response was not reached at 3-year interval. Liver responses were documented in 11 (45%) of 24 patients, median time to response was 25.5 (13.3-70.7) months. The frequency of response in groups 1, 2 and 3 was 40% (95% CI 40-81), 77% (95% CI 42-99) and 100%, p=0.084. The median time to liver response was comparable among groups.
Discussion
According to the previous studies, overall response rate (ORR) to bortezomib-based treatment varies from 60% to 94%, and CR rates vary from 23% to 71%. Multicenter retrospective European study included 230 AL amyloidosis patients, and concluded that upfront CyBorD was unable to overcome poor outcomes in Mayo stage III patients, with median OS of 4.6 months. Also, long-term organ responses are rarely reported for bortezomib-based treatments [7].
A recent prospective observational study data, with 915 systemic AL amyloidosis patients, showed that upfront bortezomib confers durable hematologic responses [9]. The main goal of this study was to assess the impact of deep HR upon the patients’ outcomes. A dFLC <10 mg/L was evaluated as а new predictor of response depth ("stringent dFLC response"). All the patients were treated with bortezomib, and it was CyBorD regimen in 94.9% of cases. The median age, gender, number of therapy courses was comparable to our group of patients. The only difference was higher percentage of patients with stage III heart disease (50%), while percentage of patients with stage IIIb was comparable to our study (13%). In this retrospective study, median OS was 72 months. Overall response rate was 65%, with 49% of deep responses (CR, VGPR, stringent dFLC responses). Median time to next treatment (TNT) was not reached, and 55% had not proceeded to further treatment at 7 years [9]. Patients with stringent dFLC responses had significantly better OS and TNT and impressive organ responses than did those with lesser responses. The incidence of cardiac response was 61% compared to 45% with lesser responses (p=0.005).
Therefore, achievement of a stringent dFLC response may be a potential new therapy goal in AL. Our results are comparable to published data, and show a highly frequent HR, organ responses and improvement in OS with bortezomib-based treatments. Of note, like in the other studies [6, 10], we demonstrated that CyBorD was superior to other bortezomib-containing regimens not only in terms of time to organ response and probability of such response, but also for OS. Nonetheless, patients with IIIb stage still have a significantly lower OS.
The main limitation of our study were the absence of FLC analysis and cardiac biomarkers in some patients at the time of diagnosis and at the moment of data evaluation thus complicating the Mayo stage assessment, depth of hematological response and heart response, and could explain statistical insignificance for some comparisons.
However, absence of effective second-line treatments for a long time limited practical applications of the response criteria. Recent advances with introduction of daratumumab make the evaluation of response crucial for the prognosis and planning of therapies. According to the ANDROMEDA study, addition of daratumumab to the CyBorD regimen increased the rate of HR and organ response. Cardiac and renal response was achieved in the first 6 months of therapy with daratumumab in 41.5% versus 22.2% and 53.0% versus 23.9%, respectively [11].
Another unusual finding of this study is a correlation between the BMPC infiltration and outcomes in patients with AL amyloidosis. Upon analysis of our subgroups, higher percentage of BMPC was a significant factor of improved OS but negative factor for HR (p=0.0002). The significance of increased BMPCs in biology of AL amyloidosis is not clear. According to the literature, the patients with more than 10% BMPC had more frequent cardiac involvement (86% vs 63%), a significantly shorter PFS time (18 vs 48 months), and reduced OS (33 months vs not reached) [13]. However, in our study the high BMPC infiltration in patients with multiple myeloma and AL amyloidosis had an opposite effect on OS survival and HR rate, probably, due to low frequency of multiple myeloma cases in the study group. The issue of amyloidosis biology with high BMPC, but without multiple myeloma, should be yet to be understood.
Conclusions
CyBorD is an effective upfront option for the patients with systemic AL amyloidosis. However, the presence of a progressive heart damage remains a predictor of early mortality in these patients. Currently, only early diagnosis can improve the outcomes of these patients, so we need a good collaboration between hematologists, general physician, cardiologists and nephrologists, to recognize the disease before advanced stages. Risk stratification and response monitoring should be based on measurements of cardiac markers and markers of hematologic response.
Appropriate treatment should begin as soon as possible with rapidly acting regimens.
Acknowledgments
The authors acknowledge the laboratories of Histopathology, Immunology, specialists in echocardiography for their help with assessment of diagnosis as well as Charity foundation "AdVita" for help in purchasing reagents for the "Freelite" testing.
The authors declare no conflicts of interest.
References
- Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood. 2020; 136(23): 2620-2627. doi: 10.1182/blood.2020006913
- Morie A. Gertz. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol, 2020 Jul;95(7):848-860. doi: 10.1002/ajh.25819
- Sanchorawala V, Sun F, Quillen K, Sloan JM, Berk JL, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015; 126(20):2345-2347.
doi: 10.1182/blood-2015-08-662726 - Sidiqi M, Aljama M, Buadi F, Warsame R, Lacy M, Dispenzieri, et al A.Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018; 36(13):1323-1329. doi: 10.1200/JCO.2017.76.9554
- Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira M, Kwok F, et al. Bortezomib, melphalan and dexamethasone for light chain amyloidosis. J Clin Oncol. 2020; 38(28): 3252-3260. doi: 10.1200/JCO.20.01285
- Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: targeted therapy? Haematologica. 2007; 92(10):1302-1307. doi: 10.3324/haematol.12136
- Palladini G, Sachchithanantham S, Milani O, Gillmore J, Foli A, Lachmannet H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015; 126 (5): 612-615. doi: 10.1182/blood-2015-01-620302
- Landau H, Lahoud O, Devlin S, Lendvai N, Chung D, Dogan A, et al. Pilot study of bortezomib and dexamethasone preand post-risk-adapted autologous stem cell transplantation in AL amyloidosis. Biol Blood Marrow Transplant 2020; 26(1):204-208.
doi: 10.1016/j.bbmt.2019.08.016 - Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019; 134(25):2271-2280. doi: 10.1182/blood.2019000834
- Smirnova AG, Bondarenko SN, Kisina AA, Smirnov AV, Zander A, Afanasyev BV. Current therapies for AL amyloidosis: literature review and our data. Clinical Oncohematology. 2013; 6(3):303-311 (In Russian).
- Palladini G, Kastritis E, Maurer MS, Zonder J, Minnema M, Wechalekar A, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136(1): 71-80. doi: 10.1182/blood.2019004460
- Tovar N, Lobato LG, Cibeira MT, Magnano L, Isola I, Rosiñol L, et al. Bone marrow plasma cell infiltration in light chain amyloidosis: impact on organ involvement and outcome. Amyloid 2018; 25(2):79-85. doi: 10.1080/13506129.2018.1443439
" ["~DETAIL_TEXT"]=> string(21393) "
Introduction
Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells (PS) or lymphocytes and their deposition in organs and tissues in the form of an insoluble fibrillar protein-amyloid. Cardiac involvement is the main predictor of patient outcome. The goal of therapy is to rapidly and profoundly suppress production of amyloidogenic light chains [1-2].
Autologous hematopoietic stem cell transplantation (ASCT) has been used in the treatment of systemic AL amyloidosis since 1994. Long-term follow-up demonstrate 15-year overall survival in 50% of patients who achieved complete hematological response (CR) after ASCT [3-4]. However, 80% of patients with newly diagnosed systemic AL amyloidosis are ineligible for ASCT.
For a long time, oral melphalan and dexamethasone (MDex) were considered a standard of care in AL amyloidosis, but the addition of proteasome inhibitor- bortezomib, significantly increased the efficacy of treatment [5]. However, bortezomib, cyclophosphamide and dexamethasone (CyBorD) regime currently is the standard of care for newly diagnosed patients. According to a large European retrospective study, the frequency of hematological response (HR) on CyBorD regime is 65%, cardiac response – 33%, renal response –15%, median overall survival (OS) was 72 months [6-8].
Despite higher frequency of clinical responses, the addition of bortezomib did not improve prognosis in the patients with IIIb heart stage, according to the Mayo Clinic classification (NT-proBNP >8500 ng/l), and 40% of these patients still die within 6 months after the diagnosis. Therefore, the question of the optimal regimens for these patients is still open.
Patients and methods
We analyzed the response to induction therapy in 105 newly diagnosed patients with systemic AL amyloidosis, treated at RM Gorbacheva Research Institute within a period from 2004 to 2021 [6]. All the patients signed informed consent for the use of personal data for research purposes. Their median age was 63 years (31-81). The percentage of men and women was 53% and 47%, respectively. Isolated AL amyloidosis was documented in 72% (n=76) of cases; in combination with multiple myeloma, in 27% (n=29). At the time of diagnosis, 62% of patients had three or more organs involved. The incidence of organ involvement was as follows: kidneys, 88% (n=93); heart, 87% (n=92); liver, 40% (n=43); nervous system, 48% (n=51); gastrointestinal tract, (GIT) 27% (n=29); lungs, 11% (n=12), and other organs (thyroid gland, lymph nodes, adrenal glands, etc.), 20% (n=21). In the patients with established Mayo stage, the following distribution was observed: stage I, 22% (n=20); stage II, 52% (n=47); stage III, 26% (n=24). The median NT-proBNP level was 1892 ng/l (35 to 34772), 15 patients had IIIb stage (NT-proBNP >8500 ng/l). Renal involvement was documented in 94 patients at the following severity distribution: stage I, 23% (n=22); stage II, 48% (n=45); stage III, 29% (n=27).
In our study, we assessed the efficacy of CyBorD compared with other treatment regimens. All consecutive patients were divided into 3 groups: group 1 was treated with CyBorD (26%, n=28); group 2 received other bortezomib-based regimens (bortezomib/dexamethasone (VD), bortezomib/melphalan/dexamethasone (BMDex)), 59% (n=62); group 3 included bortezomib-free regimens (melphalan/dexamethasone (MDex), cyclophosphamide/prednisolone, corticosteroids), 14% (n=15). Patients with autologous ASCT were excluded. The median number of therapeutic rounds in all groups was 4. The main patients’ characteristics are presented in Table 1.
Table 1. Patients characteristics

Clinical definitions
Hematological response (HR) evaluation was based on serum and urine immunofixation and determination of the level of free light chains (FLC) by nephelometry. Complete remission (CR) definition included negative serum and urine immunofixation and normal FLC ratio, very good partial response (VGPR) was based on difference between involved and uninvolved concentrations of free light chains (dFLС) <40 mg/L. Partial response (PR) was defined as a decrease of dFLC by >50%. Organ response was evaluated by changes in biomarkers: NT-proBNP for the heart, proteinuria for the kidney and alkaline phosphatase for the liver. Relapse or progression was defined as loss of previously achieved response, or organ progression, or increase in dFLС by 50% from the best response.
Statistical analysis
Descriptive statistics was used for the patient data evaluation. Chi-square test was used to compare response between the groups. Kaplan-Meier method and log-rank test was used to compare survival estimates. Cumulative incidence estimates were used to evaluate hematologic and organ responses. Gray’s test was applied to compare cumulative incidences between the groups. All the survival and cumulative incidence parameters were calculated from the date of systemic therapy initiation. For progression-free survival, the outcome measures were death or hematological relapse/progressive disease. Relapse/progressive disease were considered a competing risk for organ response. Multivariate analysis was done with proportional hazard analysis for survival estimates and Fyne-Gray regression for cumulative incidences. Variables with significance of <0.015 were selected for multivariate analysis.
Results
The 3-year OS was 70.3% (95% СI 61-80), the median follow-up was 27.8 months (22 days to 11 years). In the univariate analysis, unfavorable factors for OS were as follows: age over 70 years (p=0.007); male gender (p=0.015, 64% versus 86%); Mayo stage IIIb [OS was 48% (95% CI 21-75) vs 60% (95% CI 25-95) vs 72% (95% CI 59-85), and 79% (95% CI 58-100) in comparison with Mayo stages IIIа, II and I, respectively (p=0.07)]; renal injury stage III [65% (95% CI 46-84) vs 73% (95% CI 54-92) vs 79% (95% CI 68-90) compared with stages I and II, respectively, p<0.0001]. Improved 3-year OS was documented in the presence of hematological response [91% (95% CI 83-99) vs 40% (95% CI 25-54), p<0.001] (Figure 1B) and organ responses [93% (95% CI 86-100) vs 55% (95% CI 38-72), p=0.0003] in patients with cardiac response vs non-responders, [92% (95% CI 85-99) vs 77% (95% CI 60-94), p<0.0001, and 100% vs 33% (95% CI 4-62), p=0.0002 in the patients with renal and liver responses, respectively. Assessment of overall survival risk factors is presented in Table 2.
Table 2. Risk factors assessment for 3-year overall survival

Notes: NS, nervous system; GIT, gastrointestinal tract

Figure 1. Kinetics of hematologic and organ responses in the study group (A). Impact of hematologic response on overall survival (B)
The 3-year OS in CyBorD group was 95% (95% CI 85-99) versus 85% (95% CI 73-97) in other bortezomib-containing regimens, and 63% (95% CI 44-82) in non-bortezomib regimens (p=0.0337). In the multivariate analysis, when corrected for other confounding factors, any treatment except CyBorD was associated with worse 3-year OS (HR 4.9, 95% CI 1.4-17.2, p=0.012). Other factors which impacted the survival were: bone marrow plasma cell (BMPC) counts >2.5% (HR 0.2, 95% CI 0.1-0.5, p=0.0002), and NtproBNP levels >2500 ng/l OS (HR 4.6, 95% CI 1.0-20.6, p=0.04, Figure 2A).
The 3-year progression-free survival (PFS) in CyBorD group was 79% (95%CI 63-95), versus 48% (95%CI 35-61) in group 2, and 55% (95%CI 30-80) in group 3 (p=0.28).
Overall response rate (ORR) was 70% (n=74), and median time to HR was 10.3 (9-14.6) months.

Figure 2. Forest plot of multivariate analyses of 3-year overall survival (A), and hematological response (B)
The percentage of patients who had HR was significantly higher in the CyBoRD group, 94% (95% CI 84-99) vs 84% in group 2 (95% CI 73-95), and 63% in group 3 (95% CI 26-100), p=0.033. The HR was achieved earlier at the bortezomib-containing regimens, with median time to response of 9.6 (5.3-15), and 10 (7.7-12.4) months in groups 1 and 2 vs 32.7 (6.4-32.7) months in group 3.

Figure 3. Summary of the best hematologic responses according to the study groups
Group 1: CyBorD; group 2: other bortezomib-containing regimens; group 3: non-bortezomib treatments.
Abbreviations: Partial response (PR); Very good partial response (VGPR); Complete response (CR)
The frequency of CR was comparable between the groups: 52% (11/21), 76% (30/39) and 60% (3/5). VGPR was achieved in 19% (4/21), 8% (3/39), 20% (1/5) and PR 28% (6/21), 15% (6/39), 20% (1/5), in groups 1, 2 and 3 respectively (Fig. 3, p>0.05).
In the univariate analysis, gender, age, number of affected organs, presence of multiple myeloma did not affect frequency of hematological remission. Surprisingly, in the multivariate analysis, the cumulative HR incidence was significantly lower in patients with the presence of PC >2.5% (HR 0.67, 95%CI 0.54-0.83, p=0.0002) and it was the only significant factor (Fig. 2B).
The organ response (OR) was assessed over a 3-year period from the start of therapy (Fig. 1A). Assessment of heart response by cardiac biomarkers was possible in 77 patients (23, 45 and 9 patients in groups 1, 2, and 3, respectively). Cardiac responses were observed in 38 (49%) cases. The median time to response was 19.3 (12.8-70) months.
The percentage of cardiac responses was higher in CyBorD group, i.e., 78% (95% CI 59-97) vs 55% (95% CI 36-74) and 16 % (95% CI 16-44), in groups 2 and 3, respectively (p=0.050). In CyBorD group, the time to cardiac response was also shorter: 13 months versus 36 months versus not reached (p=0.050).
Renal responses were observed in 46 (63%) of 73 patients with median time to response 12 (9.5-18.3) months. The frequency of responses in groups 1, 2 and 3 was the following: 90% (95% СI 73-99), 92% (95% СI 81-99, and 57% (95% СI 22-92), p=0.01. The shortest median time to response was also in CyBorD group, i.e., 7 (2.7-11.9) vs 16.5 (10-29) months in group 2. For the group 3, median response was not reached at 3-year interval. Liver responses were documented in 11 (45%) of 24 patients, median time to response was 25.5 (13.3-70.7) months. The frequency of response in groups 1, 2 and 3 was 40% (95% CI 40-81), 77% (95% CI 42-99) and 100%, p=0.084. The median time to liver response was comparable among groups.
Discussion
According to the previous studies, overall response rate (ORR) to bortezomib-based treatment varies from 60% to 94%, and CR rates vary from 23% to 71%. Multicenter retrospective European study included 230 AL amyloidosis patients, and concluded that upfront CyBorD was unable to overcome poor outcomes in Mayo stage III patients, with median OS of 4.6 months. Also, long-term organ responses are rarely reported for bortezomib-based treatments [7].
A recent prospective observational study data, with 915 systemic AL amyloidosis patients, showed that upfront bortezomib confers durable hematologic responses [9]. The main goal of this study was to assess the impact of deep HR upon the patients’ outcomes. A dFLC <10 mg/L was evaluated as а new predictor of response depth ("stringent dFLC response"). All the patients were treated with bortezomib, and it was CyBorD regimen in 94.9% of cases. The median age, gender, number of therapy courses was comparable to our group of patients. The only difference was higher percentage of patients with stage III heart disease (50%), while percentage of patients with stage IIIb was comparable to our study (13%). In this retrospective study, median OS was 72 months. Overall response rate was 65%, with 49% of deep responses (CR, VGPR, stringent dFLC responses). Median time to next treatment (TNT) was not reached, and 55% had not proceeded to further treatment at 7 years [9]. Patients with stringent dFLC responses had significantly better OS and TNT and impressive organ responses than did those with lesser responses. The incidence of cardiac response was 61% compared to 45% with lesser responses (p=0.005).
Therefore, achievement of a stringent dFLC response may be a potential new therapy goal in AL. Our results are comparable to published data, and show a highly frequent HR, organ responses and improvement in OS with bortezomib-based treatments. Of note, like in the other studies [6, 10], we demonstrated that CyBorD was superior to other bortezomib-containing regimens not only in terms of time to organ response and probability of such response, but also for OS. Nonetheless, patients with IIIb stage still have a significantly lower OS.
The main limitation of our study were the absence of FLC analysis and cardiac biomarkers in some patients at the time of diagnosis and at the moment of data evaluation thus complicating the Mayo stage assessment, depth of hematological response and heart response, and could explain statistical insignificance for some comparisons.
However, absence of effective second-line treatments for a long time limited practical applications of the response criteria. Recent advances with introduction of daratumumab make the evaluation of response crucial for the prognosis and planning of therapies. According to the ANDROMEDA study, addition of daratumumab to the CyBorD regimen increased the rate of HR and organ response. Cardiac and renal response was achieved in the first 6 months of therapy with daratumumab in 41.5% versus 22.2% and 53.0% versus 23.9%, respectively [11].
Another unusual finding of this study is a correlation between the BMPC infiltration and outcomes in patients with AL amyloidosis. Upon analysis of our subgroups, higher percentage of BMPC was a significant factor of improved OS but negative factor for HR (p=0.0002). The significance of increased BMPCs in biology of AL amyloidosis is not clear. According to the literature, the patients with more than 10% BMPC had more frequent cardiac involvement (86% vs 63%), a significantly shorter PFS time (18 vs 48 months), and reduced OS (33 months vs not reached) [13]. However, in our study the high BMPC infiltration in patients with multiple myeloma and AL amyloidosis had an opposite effect on OS survival and HR rate, probably, due to low frequency of multiple myeloma cases in the study group. The issue of amyloidosis biology with high BMPC, but without multiple myeloma, should be yet to be understood.
Conclusions
CyBorD is an effective upfront option for the patients with systemic AL amyloidosis. However, the presence of a progressive heart damage remains a predictor of early mortality in these patients. Currently, only early diagnosis can improve the outcomes of these patients, so we need a good collaboration between hematologists, general physician, cardiologists and nephrologists, to recognize the disease before advanced stages. Risk stratification and response monitoring should be based on measurements of cardiac markers and markers of hematologic response.
Appropriate treatment should begin as soon as possible with rapidly acting regimens.
Acknowledgments
The authors acknowledge the laboratories of Histopathology, Immunology, specialists in echocardiography for their help with assessment of diagnosis as well as Charity foundation "AdVita" for help in purchasing reagents for the "Freelite" testing.
The authors declare no conflicts of interest.
References
- Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood. 2020; 136(23): 2620-2627. doi: 10.1182/blood.2020006913
- Morie A. Gertz. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol, 2020 Jul;95(7):848-860. doi: 10.1002/ajh.25819
- Sanchorawala V, Sun F, Quillen K, Sloan JM, Berk JL, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015; 126(20):2345-2347.
doi: 10.1182/blood-2015-08-662726 - Sidiqi M, Aljama M, Buadi F, Warsame R, Lacy M, Dispenzieri, et al A.Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018; 36(13):1323-1329. doi: 10.1200/JCO.2017.76.9554
- Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira M, Kwok F, et al. Bortezomib, melphalan and dexamethasone for light chain amyloidosis. J Clin Oncol. 2020; 38(28): 3252-3260. doi: 10.1200/JCO.20.01285
- Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: targeted therapy? Haematologica. 2007; 92(10):1302-1307. doi: 10.3324/haematol.12136
- Palladini G, Sachchithanantham S, Milani O, Gillmore J, Foli A, Lachmannet H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015; 126 (5): 612-615. doi: 10.1182/blood-2015-01-620302
- Landau H, Lahoud O, Devlin S, Lendvai N, Chung D, Dogan A, et al. Pilot study of bortezomib and dexamethasone preand post-risk-adapted autologous stem cell transplantation in AL amyloidosis. Biol Blood Marrow Transplant 2020; 26(1):204-208.
doi: 10.1016/j.bbmt.2019.08.016 - Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019; 134(25):2271-2280. doi: 10.1182/blood.2019000834
- Smirnova AG, Bondarenko SN, Kisina AA, Smirnov AV, Zander A, Afanasyev BV. Current therapies for AL amyloidosis: literature review and our data. Clinical Oncohematology. 2013; 6(3):303-311 (In Russian).
- Palladini G, Kastritis E, Maurer MS, Zonder J, Minnema M, Wechalekar A, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136(1): 71-80. doi: 10.1182/blood.2019004460
- Tovar N, Lobato LG, Cibeira MT, Magnano L, Isola I, Rosiñol L, et al. Bone marrow plasma cell infiltration in light chain amyloidosis: impact on organ involvement and outcome. Amyloid 2018; 25(2):79-85. doi: 10.1080/13506129.2018.1443439
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(101) "sravnenie-bortezomib-soderzhashchikh-rezhimov-s-drugimi-variantami-terapii-dlya-lecheniya-pervichnykh" ["~CODE"]=> string(101) "sravnenie-bortezomib-soderzhashchikh-rezhimov-s-drugimi-variantami-terapii-dlya-lecheniya-pervichnykh" ["EXTERNAL_ID"]=> string(4) "2043" ["~EXTERNAL_ID"]=> string(4) "2043" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(566) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозгаComparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(5045) "<p style="text-align: justify;">Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.</p> <p style="text-align: justify;">В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.</p> <p style="text-align: justify;">Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Системный AL амилоидоз, CyBoRD, бортезомиб.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_META_TITLE"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_META_KEYWORDS"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_META_DESCRIPTION"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_PICTURE_FILE_ALT"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_PICTURE_FILE_TITLE"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_PICTURE_FILE_NAME"]=> string(101) "sravnenie-bortezomib-soderzhashchikh-rezhimov-s-drugimi-variantami-terapii-dlya-lecheniya-pervichnykh" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(367) "Сравнение бортезомиб-содержащих режимов с другими вариантами терапии для лечения первичных пациентов с системным амилоидозом легких цепей, не подлежащих аутологичной трансплантации костного мозга" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(101) "sravnenie-bortezomib-soderzhashchikh-rezhimov-s-drugimi-variantami-terapii-dlya-lecheniya-pervichnykh" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(101) "sravnenie-bortezomib-soderzhashchikh-rezhimov-s-drugimi-variantami-terapii-dlya-lecheniya-pervichnykh" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(101) "sravnenie-bortezomib-soderzhashchikh-rezhimov-s-drugimi-variantami-terapii-dlya-lecheniya-pervichnykh" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "201" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28435" ["VALUE"]=> array(2) { ["TEXT"]=> string(263) "<p>Ольга В. Кудяшева, Ольга В. Пирогова, Валентина В. Порунова, Светлана В. Толстова, Анна Г. Смирнова, Иван С. Моисеев, Александр Д. Кулагин</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(251) "
Ольга В. Кудяшева, Ольга В. Пирогова, Валентина В. Порунова, Светлана В. Толстова, Анна Г. Смирнова, Иван С. Моисеев, Александр Д. Кулагин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28436" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28437" ["VALUE"]=> array(2) { ["TEXT"]=> string(5045) "<p style="text-align: justify;">Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.</p> <p style="text-align: justify;">В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.</p> <p style="text-align: justify;">Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Системный AL амилоидоз, CyBoRD, бортезомиб.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4942) "Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.
В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.
Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические.
Ключевые слова
Системный AL амилоидоз, CyBoRD, бортезомиб.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28438" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-38-45" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-38-45" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28439" ["VALUE"]=> array(2) { ["TEXT"]=> string(157) "<p>Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(145) "Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28440" ["VALUE"]=> array(2) { ["TEXT"]=> string(790) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia<br> E-mail: bmt.myeloma@gmail.com</p><br> <p><b>Citation:</b> Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(706) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: bmt.myeloma@gmail.com
Citation: Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28441" ["VALUE"]=> array(2) { ["TEXT"]=> string(3153) "<p style="text-align: justify;"> Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses. </p> <p style="text-align: justify;"> In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens. </p> <p style="text-align: justify;"> The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), <i>vs </i>48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% <i>vs </i>84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% <i>vs </i>55% <i>vs </i>16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% <i>vs </i>92% <i>vs </i>57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months). </p> <p style="text-align: justify;"> In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Systemic AL amyloidosis, CyBoRD, bortezomib. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2951) "Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses.
In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens.
The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), vs 48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% vs 84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% vs 55% vs 16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% vs 92% vs 57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months).
In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response.
Keywords
Systemic AL amyloidosis, CyBoRD, bortezomib.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28442" ["VALUE"]=> string(199) "Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(199) "Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28443" ["VALUE"]=> string(4) "2716" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2716" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28444" ["VALUE"]=> string(4) "2717" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2717" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28439" ["VALUE"]=> array(2) { ["TEXT"]=> string(157) "<p>Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(145) "Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(145) "Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28441" ["VALUE"]=> array(2) { ["TEXT"]=> string(3153) "<p style="text-align: justify;"> Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses. </p> <p style="text-align: justify;"> In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens. </p> <p style="text-align: justify;"> The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), <i>vs </i>48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% <i>vs </i>84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% <i>vs </i>55% <i>vs </i>16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% <i>vs </i>92% <i>vs </i>57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months). </p> <p style="text-align: justify;"> In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Systemic AL amyloidosis, CyBoRD, bortezomib. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2951) "Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses.
In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens.
The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), vs 48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% vs 84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% vs 55% vs 16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% vs 92% vs 57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months).
In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response.
Keywords
Systemic AL amyloidosis, CyBoRD, bortezomib.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2951) "Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses.
In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens.
The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), vs 48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% vs 84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% vs 55% vs 16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% vs 92% vs 57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months).
In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response.
Keywords
Systemic AL amyloidosis, CyBoRD, bortezomib.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28438" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-38-45" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-38-45" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-38-45" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28442" ["VALUE"]=> string(199) "Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(199) "Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(199) "Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28440" ["VALUE"]=> array(2) { ["TEXT"]=> string(790) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia<br> E-mail: bmt.myeloma@gmail.com</p><br> <p><b>Citation:</b> Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(706) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: bmt.myeloma@gmail.com
Citation: Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(706) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: bmt.myeloma@gmail.com
Citation: Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28435" ["VALUE"]=> array(2) { ["TEXT"]=> string(263) "<p>Ольга В. Кудяшева, Ольга В. Пирогова, Валентина В. Порунова, Светлана В. Толстова, Анна Г. Смирнова, Иван С. Моисеев, Александр Д. Кулагин</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(251) "Ольга В. Кудяшева, Ольга В. Пирогова, Валентина В. Порунова, Светлана В. Толстова, Анна Г. Смирнова, Иван С. Моисеев, Александр Д. Кулагин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(251) "Ольга В. Кудяшева, Ольга В. Пирогова, Валентина В. Порунова, Светлана В. Толстова, Анна Г. Смирнова, Иван С. Моисеев, Александр Д. Кулагин
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28437" ["VALUE"]=> array(2) { ["TEXT"]=> string(5045) "<p style="text-align: justify;">Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.</p> <p style="text-align: justify;">В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.</p> <p style="text-align: justify;">Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Системный AL амилоидоз, CyBoRD, бортезомиб.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4942) "Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.
В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.
Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические.
Ключевые слова
Системный AL амилоидоз, CyBoRD, бортезомиб.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4942) "Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.
В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.
Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические.
Ключевые слова
Системный AL амилоидоз, CyBoRD, бортезомиб.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28436" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [5]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "201" ["~IBLOCK_SECTION_ID"]=> string(3) "201" ["ID"]=> string(4) "2042" ["~ID"]=> string(4) "2042" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["~NAME"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "01/25/2022 12:20:15 pm" ["~TIMESTAMP_X"]=> string(22) "01/25/2022 12:20:15 pm" ["DETAIL_PAGE_URL"]=> string(150) "/en/archive/tom-10-nomer-3-4/klinicheskie-raboty/cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto/" ["~DETAIL_PAGE_URL"]=> string(150) "/en/archive/tom-10-nomer-3-4/klinicheskie-raboty/cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(20675) "Introduction
In 2020, the COVID-19 pandemic has affected virtually all activities, both in Russia and globally [1-5]. The most significant damage occurred to the healthcare system. The first case of COVID-19 in St. Petersburg was registered on March 8, 2020. The nation-wide lockdown had been imposed in Russian Federation since March 30, 2020. Therefore, COVID-19 has become a real challenge for oncology and hematology services in Russia. The main task was to preserve the availability and quality of highly specialized medical care. Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for hematological patients. Despite the global trend towards a decrease in transplantation activity due to the pandemic, we tried to retain its capacity by introducing preventive measures and optimizing anti-infectious control in our Center. In the present study, we aim to present the workflow at the largest Russian transplant center in the era of COVID-19 pandemic.
Patients and methods
This work represents an observational study. The main methodology was to collect the data on clinical capacities of our transplant center from April 2020 to July 2021. Since the beginning of the COVID-19 pandemic, a series of measures have been taken at our Center to prevent spreading of the SARS-CoV-2 virus. Appropriate measures at the inpatient units included were as follows: a part of staff members was transferred to remote work; weekly PCR screening of the personnel and patients was arranged; the staff movement in the clinic was minimized, along with mandatory usage of personal protection equipment (PPE). Protection measures for outpatients included mandatory PCR screening of the patients before hospital admission, remote supervision of patients in permissible situations.
The main objectives included studying the influence of COVID-19 pandemic on the workflow of our HSCT Center, and assessing efficiency of the anti-epidemic measures taken. The main tasks were to estimate the rates of transplant-related mortality, incidence of COVID-19 infection in HSCT recipients and the characteristics of this group, to assess possibility of maintaining HSCT performance during the pandemic. The Kaplan-Meier method was used to estimate overall survival (OS) and transplant-related mortality (TRM). The log-rank test used for comparison of cumulative TRM incidence. All the calculations were performed using R version 3.3.2.
Results
Medical staff/Healthcare professionals
The medical staff of the center consists of 494 employees. The median age is 35 years (range 19 to 77). The key aspect was to preserve safety and efficiency of healthcare professionals. None of the employees were directly involved in COVID hospitals/red zones. Some of the staff switched to remote work, psychologists for example, who continued to work with the patients online. A total of 27 employees over 65 years old, including 10 medical doctors and 7 nurses, were transferred to self-isolation. In April 2020, due to limited diagnostic facilities, we were able to perform PCR testing for SARS-CoV-2 among employees only when they manifested with fever or respiratory symptoms. But already in May 2020, we started with weekly screening of clinical staff. During the COVID-19 pandemic, we performed a total of 21,702 PCR tests. Due to optimization of laboratory work, the PCR data became available within 24 hours. The symptom-free employees with positive PCR test results or those with mild clinical signs were sent to self-isolation. Employees with signs of respiratory failure were admitted to the specialized hospital for therapy. The movements of personnel within the clinic were minimized as much as possible. Regular use of personal protective equipment was mandatory.
From April 2020 to July 2021, a total of 252 (54.9%) of healthcare professionals suffered and recovered from COVID-19, including 83 physicians (51.2%) and 120 nurses (57.7%) who developed antiviral IgG antibodies in 44.4% and 48.1% of the cases, respectively. Other medical and non-medical staff members had documented disease in 55% (n=49) with antibody response in 37% of the cases (Fig. 1). The median time between the first positive and the first negative PCR test was 16.5 days (range 2 to 65). There were no COVID-19-related deaths among healthcare professionals in our Center.

Figure 1. Number of healthcare workers infected with SARS-CoV-2 from the start of pandemic
The largest number of COVID-19 cases among our staff was observed during the second wave of COVID-19 (autumn 2020), which correlated with general course of the pandemic in the Russian Federation (Fig. 2).

Figure 2. Time dynamics of confirmed cases in RM Gorbacheva Memorial Institute compared to general trends for the Russian Federation
Red curve, dynamics of confirmed cases in Russian Federation; blue curve, time course of confirmed cases in RM Gorbacheva Memorial Institute (St. Petersburg)
By July 1, 2021, 119 employees at our Center (25.9%) have been vaccinated: physicians, 43.2% (n=70); nurses, 15.8% (n=33); other medical and non-medical staff, 18% (n=16). Thus, 69.7% of employees (n=320) were immunized against COVID-19, including physicians (82.1%, n=133), nurses (66.8%, n=139), other medical and non-medical staff (54%, n=48) as shown in Fig. 3.

Figure 3. Dynamic of immune response in health-care workers in RM Gorbacheva Memorial Institute through each COVID-19 wave

Figure 4. Transplant activity in 2019 and 2020

Figure 5. Comparison of HSC donors in 2019 and 2020
Transplant activity in 2020
A total of 419 HSCTs performed in 2020, included 136 autologous and 283 allogeneic transplants. By comparison, 415 transplantations were performed in 2019, of which 144 and 271 were autologous and allogeneic, respectively (Fig. 4).
Due to closed access to international bone marrow donor registries, the HSCT structure was redistributed in favor of unrelated donors from the Russian registry and haploidentical donors (Fig. 5). During the pandemic from April 2020 to July 2021, 27 allogeneic grafts were cryopreserved and 24 (88.9%) of them were transfused to HSCT recipients. In 3 cases, graft transfusion was not performed due to recipient death: one patient deceased with COVID-19, two patients, due to progression of the underlying disease. However, we tried to use non-cryopreserved allogeneic grafts whenever possible, and succeeded in 90% of allografts. The reason for this practice is provided by the data from our center, published in 2018 showing higher frequency of primary graft failure with a cryopreserved allogeneic graft [6]. COVID-19-positive cases were recorded in eight donors, of which 6 were scheduled for adult patients and 2, for children. Of these cases, 4 donors were from the Russian unrelated donor registry; 3 donors were haploidentical, and a matched related donor was used in one case. In 4 patients, HSCT was postponed for a 1 to 8 weeks. During this period, no progression of the underlying disease was observed; in these patients, HSCT procedure was performed without complications. In 3 cases, the donor was changed to a haploidentical. In 1 case, a positive PCR test was received after bone marrow harvest, which did not affect the stem cell harvesting or HSCT procedure in any way. Upon receiving a PCR-positive test, donors were sent to self-isolation, and transplantation was postponed due to the absence of alternative donors.
HSCT recipients
Almost half of the HSCTs performed in 2020 were patients with acute leukemias, i.e., AML, 20.8% (n=87) and ALL, 26% (n=109). The detailed diagnosis distribution of HSCT recipients is presented in Table 1.
Table 1. Comparison of diagnosis structure in 2019 and 2020


Figure 6. Structure of initial diagnoses in the study group
From April 2020 to July 2021, COVID-19 was diagnosed in 39 HSCT recipients with ALL 11 (28%), AML 10 (25.5%), aplastic anemia 5 (13%), Hodgkin's lymphoma 3 (8%), non-Hodgkin lymphomas 2 (5%), multiple myeloma 3 (8%), multiple sclerosis 2 (5%) and 1 (2.5%) each with diagnoses of mucopolysaccharidosis type 1, chronic myeloid leukemia and CNS embryonal tumors (Fig. 6). During the pandemic, COVID-19 hospital has been deployed at the University, thus enabling continued specific therapies using distant consultation approach for HSCT patients who developed SARS-CoV-2 infection. It should be noted that immunosuppressive therapy with calcineurin and mTOR inhibitors as part of the GVHD prophylaxis was continued during the COVID-19 infection.
The overall group characteristics
The median age of infected patients was 27 years (4-66). There were 25 males (64.1%) and 14 females (35.9%). The majority of patients (n=29, 74.3%) had 0 points of the HCT CI comorbidity index. Allogeneic and autologous HSCT was performed in 31 (79.5%) and 8 (20.5%) patients, respectively. The HSC source were both PBSC (n=22, 56.4%), and bone marrow (n=17, 43.6%). Reduced-intensity conditioning (RIC) was used in 27 (69.3%) patients; MAC, in 12 cases (30.7%). Thirty patients (76.9%) were in complete remission of the underlying disease at the time of HSCT.
he median time between HSCT and COVID-19 infection was 68 days (-1 to +2093). At the time of COVID-19 onset, 33 patients (84.6%) were in remission of underlying disease. Most patients had ECOG status of 0-1 point at the onset of infection [11/18, (28.2%/46.1%)]. The median cycle threshold value (Ct) for the PCR of the first positive test was 31.8 (14.2-39.2). Two (5.1%) patients had an active acute graft-versus-host disease (GVHD) and 8 (20.5%) patients had chronic GVHD. The median duration of immunosuppressive therapy at the time of COVID-19 development was 36 days (-1 to +340).
Coronavirus infection was asymptomatic in 23 (59%) patients. Febrile fever was recorded in 16 (27%) patients; cough, in 8 (14%); respiratory failure, in 4 (7%); diarrhea, in 3 (5%); emetic syndrome, in 1 (2%); hypotension in 1 (2 %); rhinopharyngitis, in 1 (2%); anorexia, in 1 (2%). The median level of leukocytes was 3.6×109/L (0-14.7), neutrophils – 1.6×109/L (0-11.9), lymphocytes – 0.9×109/L (0-3.2), platelets – 115×109/L (6-298), hemoglobin – 98 g/L (58-155).
Seventeen (43.6%) patients were admitted to the hospital, and 22 (56.4%) were observed as outpatients. The median hospital stay was 15.5 days (1-47). Eight (20.5%) patients died. The median duration of COVID-19 before death was 20 days (1 to 47). After the infection, 21 (53.8%) patients were in remission of the underlying disease, 9 (23.1%) were in progression. In 6 (15.4%) patients, the disease staging was not performed due to a lethal outcome, in 2 (5.1%) patients with diagnoses of MPS type 1 and MS, the staging was not applicable. The disease status at the follow-up point was unknown in 1 patient (2.6%). The median period from the diagnosis of coronavirus infection to the follow-up term was 105 days (1-337).
The incidence of COVID-19 among HSCT recipients from April 2020 to July 2021 was 7.3% (n=39), in allo-HSCT, 8.6% (n=31); in auto-HSCT, 4.5% (n=8). A total of 536 HSCTs were performed during this period. The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609-0.884), as shown in Fig. 7A. The mortality rate was 20.5% (n=8). Among 8 patients, 50% (n=4) died in the early post-transplant period up to +100 days, and 37.5% (n=3) of them had pancytopenia at the time of the COVID-19 onset. The causes of death were COVID-19 infection (n=4, 50%), secondary infectious complications (n=2, 25%), relapse of the underlying disease (n=1, 12.5%), hemorrhagic complications (n=1, 12.5%).
The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95%CI 0.9-0.95) and 8.7% (95%CI 0.88-0.93) in 2019 and 2020, respectively (p=0.35) (Fig. 7B). The 100-day cumulative incidence of TRM among auto-HSCT recipients was 1.4% and 3.8% (95%CI 0.9-0.98) in 2019 and 2020, respectively (p=0.33) (Fig. 8B). The 100-day cumulative incidence of TRM among allo-HSCT recipients was 13.9% and 14.9% (95% CI 0.8-0.89) in 2019 and 2020 respectively (p=0.79) as seen from Fig. 8А.

Figure 7. Overall 100-day survival rate since the COVID-19 diagnosis (A), and 100-day cumulative incidence of transplant-related mortality (B) among all HSCT recipients

Figure 8. Comparison of 100-day transplant related mortality among allo-HSCT (A) and auto-HSCT (B) recipients in 2019 and 2020
Discussion
A number of published works to the similar topic point to decreased transplant activity in HSCT centers around the world during the COVID-19 pandemic [7-12]. We tried, however, to keep the same transplant activity. Despite the mortality rate of 20.5% in the group of HSCT recipients, diagnosed with COVID-19, the one-year TRM did not change significantly compared to 2019. In our opinion, the reason for these results is, firstly, low incidence of COVID-19 infection among HSCT recipients in our Center, and, secondly, maintenance of HSCT activity during the pandemic. This became possible due to the measures taken to limit spreading of SARS-CoV-2, as well as redistribution of the donor structure in favor of unrelated donors from the Russian registry, as well as participation of haploidentical donors.
Conclusions
Our experience confirms that with appropriate patient care resources, thorough screening and selection of donors, regular screening of patients and staff, vaccination, proper use of personal protective equipment, it is possible to maintain the same transplant activity during a pandemic without significantly increased TRM.
Financial support
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement № 075-15-2021-1086, contract № RF----193021X0015). No conflicts of interest are declared.
Acknowledgement
Special thanks to the nurses, patients and their relatives.
References
- Varma A, Kosuri S, Ustun C, Ibrahim U, Moreira J, Bishop M, et al. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia 34, 2809-2812 (2020). doi: 10.1038/s41375-020-01019-x
- Sultan AM, Mahmoud, HK, Fathy GM, Abdelfattah N. The outcome of hematopoietic stem cell transplantation patients with COVID-19 infection. Bone Marrow Transplant. 2021; 56, 971-973. doi: 10.1038/s41409-020-01094-9
- Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020; published online Aug 13. doi: 10.1016/S2352-3026(20)30251-9
- Orchard K, Dignan FL, Lee J, Pearce R, Desai M, McFarlane E, et al. The NICE COVID-19 rapid guideline on haematopoietic stem cell transplantation: development, implementation and impact. Br J Haematol. 2021; 192:467-473. https://doi.org/10.1111/bjh.17280
- Ljungman P, Mikulska M, de la Camara R, Basak G, Chabannon C, Corbacioglu S, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020; 55, 2071–2076. https://doi.org/10.1038/s41409-020-0919-0
- Babenko EV, Moiseev IS, Kanunnikov MM, Alyanskiy AL, Pevcov DE, Frolova AV, et al. Pair-matched study of cryopreserved versus native graft in adult and pediatric recipients of allogeneic hematopoietic stem cell transplantation. Cell Ther Transplant. 2018 7(1), 45–53. doi: 10.18620/ctt-1866-8836-2018-7-2-45-53
- Xu Z-L, Huang X-J. COVID-19 & allogeneic transplant: Activity and preventive measures for best outcomes in China. Adv Cell Gene Ther. 2020;00:e94. doi: 10.1002/acg2.94
- Ueda Oshima M, Sandmaier BM, Petersdorf E, Flowers M, Hill G, Lee S, et al. Blood and marrow transplantation during the emerging COVID-19 pandemic: the Seattle approach. Bone Marrow Transplant. 2021; 56, 305-313. doi: 10.1038/s41409-020-01068-x
- Maurer K, Saucier A, Kim HT, Acharya U, Mo CC, Porter J, et al. COVID-19 and hematopoietic stem cell transplantation and immune effector cell therapy: a US cancer center experience. Blood Adv. 2021; 5 (3): 861-871. doi: 10.1182/bloodadvances.2020003883
- Kanellopoulos A, Ahmed MZ, Kishore B, Lovell R, Horgan C, Paneesha S, et al. COVID-19 in bone marrow transplant recipients: reflecting on a single centre experience. Br J Haematol. 2020; l, 190: e67-e70. https://doi.org/10.1111/bjh.16856
- del Campo PL, López AR, de la Cruz Benito B, de Paz Arias R, de Soto Álvarez T, Sánchez Vadillo I, et al. Hematopoietic cell transplantation during COVID-19 pandemic: experience from a tertiary hospital in Madrid. Exp Rev Hematol. (2021); 14:1, 1-5.
doi: 10.1080/17474086.2021.1858789 - Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, Martínez-Fernández JR, Crespo M, Gayoso J, et al. COVID-19 in transplant recipients: The Spanish experience. Am J Transplant. 2021, 21: 1825-1837. https://doi.org/10.1111/ajt.16369
" ["~DETAIL_TEXT"]=> string(20675) "
Introduction
In 2020, the COVID-19 pandemic has affected virtually all activities, both in Russia and globally [1-5]. The most significant damage occurred to the healthcare system. The first case of COVID-19 in St. Petersburg was registered on March 8, 2020. The nation-wide lockdown had been imposed in Russian Federation since March 30, 2020. Therefore, COVID-19 has become a real challenge for oncology and hematology services in Russia. The main task was to preserve the availability and quality of highly specialized medical care. Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for hematological patients. Despite the global trend towards a decrease in transplantation activity due to the pandemic, we tried to retain its capacity by introducing preventive measures and optimizing anti-infectious control in our Center. In the present study, we aim to present the workflow at the largest Russian transplant center in the era of COVID-19 pandemic.
Patients and methods
This work represents an observational study. The main methodology was to collect the data on clinical capacities of our transplant center from April 2020 to July 2021. Since the beginning of the COVID-19 pandemic, a series of measures have been taken at our Center to prevent spreading of the SARS-CoV-2 virus. Appropriate measures at the inpatient units included were as follows: a part of staff members was transferred to remote work; weekly PCR screening of the personnel and patients was arranged; the staff movement in the clinic was minimized, along with mandatory usage of personal protection equipment (PPE). Protection measures for outpatients included mandatory PCR screening of the patients before hospital admission, remote supervision of patients in permissible situations.
The main objectives included studying the influence of COVID-19 pandemic on the workflow of our HSCT Center, and assessing efficiency of the anti-epidemic measures taken. The main tasks were to estimate the rates of transplant-related mortality, incidence of COVID-19 infection in HSCT recipients and the characteristics of this group, to assess possibility of maintaining HSCT performance during the pandemic. The Kaplan-Meier method was used to estimate overall survival (OS) and transplant-related mortality (TRM). The log-rank test used for comparison of cumulative TRM incidence. All the calculations were performed using R version 3.3.2.
Results
Medical staff/Healthcare professionals
The medical staff of the center consists of 494 employees. The median age is 35 years (range 19 to 77). The key aspect was to preserve safety and efficiency of healthcare professionals. None of the employees were directly involved in COVID hospitals/red zones. Some of the staff switched to remote work, psychologists for example, who continued to work with the patients online. A total of 27 employees over 65 years old, including 10 medical doctors and 7 nurses, were transferred to self-isolation. In April 2020, due to limited diagnostic facilities, we were able to perform PCR testing for SARS-CoV-2 among employees only when they manifested with fever or respiratory symptoms. But already in May 2020, we started with weekly screening of clinical staff. During the COVID-19 pandemic, we performed a total of 21,702 PCR tests. Due to optimization of laboratory work, the PCR data became available within 24 hours. The symptom-free employees with positive PCR test results or those with mild clinical signs were sent to self-isolation. Employees with signs of respiratory failure were admitted to the specialized hospital for therapy. The movements of personnel within the clinic were minimized as much as possible. Regular use of personal protective equipment was mandatory.
From April 2020 to July 2021, a total of 252 (54.9%) of healthcare professionals suffered and recovered from COVID-19, including 83 physicians (51.2%) and 120 nurses (57.7%) who developed antiviral IgG antibodies in 44.4% and 48.1% of the cases, respectively. Other medical and non-medical staff members had documented disease in 55% (n=49) with antibody response in 37% of the cases (Fig. 1). The median time between the first positive and the first negative PCR test was 16.5 days (range 2 to 65). There were no COVID-19-related deaths among healthcare professionals in our Center.

Figure 1. Number of healthcare workers infected with SARS-CoV-2 from the start of pandemic
The largest number of COVID-19 cases among our staff was observed during the second wave of COVID-19 (autumn 2020), which correlated with general course of the pandemic in the Russian Federation (Fig. 2).

Figure 2. Time dynamics of confirmed cases in RM Gorbacheva Memorial Institute compared to general trends for the Russian Federation
Red curve, dynamics of confirmed cases in Russian Federation; blue curve, time course of confirmed cases in RM Gorbacheva Memorial Institute (St. Petersburg)
By July 1, 2021, 119 employees at our Center (25.9%) have been vaccinated: physicians, 43.2% (n=70); nurses, 15.8% (n=33); other medical and non-medical staff, 18% (n=16). Thus, 69.7% of employees (n=320) were immunized against COVID-19, including physicians (82.1%, n=133), nurses (66.8%, n=139), other medical and non-medical staff (54%, n=48) as shown in Fig. 3.

Figure 3. Dynamic of immune response in health-care workers in RM Gorbacheva Memorial Institute through each COVID-19 wave

Figure 4. Transplant activity in 2019 and 2020

Figure 5. Comparison of HSC donors in 2019 and 2020
Transplant activity in 2020
A total of 419 HSCTs performed in 2020, included 136 autologous and 283 allogeneic transplants. By comparison, 415 transplantations were performed in 2019, of which 144 and 271 were autologous and allogeneic, respectively (Fig. 4).
Due to closed access to international bone marrow donor registries, the HSCT structure was redistributed in favor of unrelated donors from the Russian registry and haploidentical donors (Fig. 5). During the pandemic from April 2020 to July 2021, 27 allogeneic grafts were cryopreserved and 24 (88.9%) of them were transfused to HSCT recipients. In 3 cases, graft transfusion was not performed due to recipient death: one patient deceased with COVID-19, two patients, due to progression of the underlying disease. However, we tried to use non-cryopreserved allogeneic grafts whenever possible, and succeeded in 90% of allografts. The reason for this practice is provided by the data from our center, published in 2018 showing higher frequency of primary graft failure with a cryopreserved allogeneic graft [6]. COVID-19-positive cases were recorded in eight donors, of which 6 were scheduled for adult patients and 2, for children. Of these cases, 4 donors were from the Russian unrelated donor registry; 3 donors were haploidentical, and a matched related donor was used in one case. In 4 patients, HSCT was postponed for a 1 to 8 weeks. During this period, no progression of the underlying disease was observed; in these patients, HSCT procedure was performed without complications. In 3 cases, the donor was changed to a haploidentical. In 1 case, a positive PCR test was received after bone marrow harvest, which did not affect the stem cell harvesting or HSCT procedure in any way. Upon receiving a PCR-positive test, donors were sent to self-isolation, and transplantation was postponed due to the absence of alternative donors.
HSCT recipients
Almost half of the HSCTs performed in 2020 were patients with acute leukemias, i.e., AML, 20.8% (n=87) and ALL, 26% (n=109). The detailed diagnosis distribution of HSCT recipients is presented in Table 1.
Table 1. Comparison of diagnosis structure in 2019 and 2020


Figure 6. Structure of initial diagnoses in the study group
From April 2020 to July 2021, COVID-19 was diagnosed in 39 HSCT recipients with ALL 11 (28%), AML 10 (25.5%), aplastic anemia 5 (13%), Hodgkin's lymphoma 3 (8%), non-Hodgkin lymphomas 2 (5%), multiple myeloma 3 (8%), multiple sclerosis 2 (5%) and 1 (2.5%) each with diagnoses of mucopolysaccharidosis type 1, chronic myeloid leukemia and CNS embryonal tumors (Fig. 6). During the pandemic, COVID-19 hospital has been deployed at the University, thus enabling continued specific therapies using distant consultation approach for HSCT patients who developed SARS-CoV-2 infection. It should be noted that immunosuppressive therapy with calcineurin and mTOR inhibitors as part of the GVHD prophylaxis was continued during the COVID-19 infection.
The overall group characteristics
The median age of infected patients was 27 years (4-66). There were 25 males (64.1%) and 14 females (35.9%). The majority of patients (n=29, 74.3%) had 0 points of the HCT CI comorbidity index. Allogeneic and autologous HSCT was performed in 31 (79.5%) and 8 (20.5%) patients, respectively. The HSC source were both PBSC (n=22, 56.4%), and bone marrow (n=17, 43.6%). Reduced-intensity conditioning (RIC) was used in 27 (69.3%) patients; MAC, in 12 cases (30.7%). Thirty patients (76.9%) were in complete remission of the underlying disease at the time of HSCT.
he median time between HSCT and COVID-19 infection was 68 days (-1 to +2093). At the time of COVID-19 onset, 33 patients (84.6%) were in remission of underlying disease. Most patients had ECOG status of 0-1 point at the onset of infection [11/18, (28.2%/46.1%)]. The median cycle threshold value (Ct) for the PCR of the first positive test was 31.8 (14.2-39.2). Two (5.1%) patients had an active acute graft-versus-host disease (GVHD) and 8 (20.5%) patients had chronic GVHD. The median duration of immunosuppressive therapy at the time of COVID-19 development was 36 days (-1 to +340).
Coronavirus infection was asymptomatic in 23 (59%) patients. Febrile fever was recorded in 16 (27%) patients; cough, in 8 (14%); respiratory failure, in 4 (7%); diarrhea, in 3 (5%); emetic syndrome, in 1 (2%); hypotension in 1 (2 %); rhinopharyngitis, in 1 (2%); anorexia, in 1 (2%). The median level of leukocytes was 3.6×109/L (0-14.7), neutrophils – 1.6×109/L (0-11.9), lymphocytes – 0.9×109/L (0-3.2), platelets – 115×109/L (6-298), hemoglobin – 98 g/L (58-155).
Seventeen (43.6%) patients were admitted to the hospital, and 22 (56.4%) were observed as outpatients. The median hospital stay was 15.5 days (1-47). Eight (20.5%) patients died. The median duration of COVID-19 before death was 20 days (1 to 47). After the infection, 21 (53.8%) patients were in remission of the underlying disease, 9 (23.1%) were in progression. In 6 (15.4%) patients, the disease staging was not performed due to a lethal outcome, in 2 (5.1%) patients with diagnoses of MPS type 1 and MS, the staging was not applicable. The disease status at the follow-up point was unknown in 1 patient (2.6%). The median period from the diagnosis of coronavirus infection to the follow-up term was 105 days (1-337).
The incidence of COVID-19 among HSCT recipients from April 2020 to July 2021 was 7.3% (n=39), in allo-HSCT, 8.6% (n=31); in auto-HSCT, 4.5% (n=8). A total of 536 HSCTs were performed during this period. The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609-0.884), as shown in Fig. 7A. The mortality rate was 20.5% (n=8). Among 8 patients, 50% (n=4) died in the early post-transplant period up to +100 days, and 37.5% (n=3) of them had pancytopenia at the time of the COVID-19 onset. The causes of death were COVID-19 infection (n=4, 50%), secondary infectious complications (n=2, 25%), relapse of the underlying disease (n=1, 12.5%), hemorrhagic complications (n=1, 12.5%).
The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95%CI 0.9-0.95) and 8.7% (95%CI 0.88-0.93) in 2019 and 2020, respectively (p=0.35) (Fig. 7B). The 100-day cumulative incidence of TRM among auto-HSCT recipients was 1.4% and 3.8% (95%CI 0.9-0.98) in 2019 and 2020, respectively (p=0.33) (Fig. 8B). The 100-day cumulative incidence of TRM among allo-HSCT recipients was 13.9% and 14.9% (95% CI 0.8-0.89) in 2019 and 2020 respectively (p=0.79) as seen from Fig. 8А.

Figure 7. Overall 100-day survival rate since the COVID-19 diagnosis (A), and 100-day cumulative incidence of transplant-related mortality (B) among all HSCT recipients

Figure 8. Comparison of 100-day transplant related mortality among allo-HSCT (A) and auto-HSCT (B) recipients in 2019 and 2020
Discussion
A number of published works to the similar topic point to decreased transplant activity in HSCT centers around the world during the COVID-19 pandemic [7-12]. We tried, however, to keep the same transplant activity. Despite the mortality rate of 20.5% in the group of HSCT recipients, diagnosed with COVID-19, the one-year TRM did not change significantly compared to 2019. In our opinion, the reason for these results is, firstly, low incidence of COVID-19 infection among HSCT recipients in our Center, and, secondly, maintenance of HSCT activity during the pandemic. This became possible due to the measures taken to limit spreading of SARS-CoV-2, as well as redistribution of the donor structure in favor of unrelated donors from the Russian registry, as well as participation of haploidentical donors.
Conclusions
Our experience confirms that with appropriate patient care resources, thorough screening and selection of donors, regular screening of patients and staff, vaccination, proper use of personal protective equipment, it is possible to maintain the same transplant activity during a pandemic without significantly increased TRM.
Financial support
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement № 075-15-2021-1086, contract № RF----193021X0015). No conflicts of interest are declared.
Acknowledgement
Special thanks to the nurses, patients and their relatives.
References
- Varma A, Kosuri S, Ustun C, Ibrahim U, Moreira J, Bishop M, et al. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia 34, 2809-2812 (2020). doi: 10.1038/s41375-020-01019-x
- Sultan AM, Mahmoud, HK, Fathy GM, Abdelfattah N. The outcome of hematopoietic stem cell transplantation patients with COVID-19 infection. Bone Marrow Transplant. 2021; 56, 971-973. doi: 10.1038/s41409-020-01094-9
- Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020; published online Aug 13. doi: 10.1016/S2352-3026(20)30251-9
- Orchard K, Dignan FL, Lee J, Pearce R, Desai M, McFarlane E, et al. The NICE COVID-19 rapid guideline on haematopoietic stem cell transplantation: development, implementation and impact. Br J Haematol. 2021; 192:467-473. https://doi.org/10.1111/bjh.17280
- Ljungman P, Mikulska M, de la Camara R, Basak G, Chabannon C, Corbacioglu S, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020; 55, 2071–2076. https://doi.org/10.1038/s41409-020-0919-0
- Babenko EV, Moiseev IS, Kanunnikov MM, Alyanskiy AL, Pevcov DE, Frolova AV, et al. Pair-matched study of cryopreserved versus native graft in adult and pediatric recipients of allogeneic hematopoietic stem cell transplantation. Cell Ther Transplant. 2018 7(1), 45–53. doi: 10.18620/ctt-1866-8836-2018-7-2-45-53
- Xu Z-L, Huang X-J. COVID-19 & allogeneic transplant: Activity and preventive measures for best outcomes in China. Adv Cell Gene Ther. 2020;00:e94. doi: 10.1002/acg2.94
- Ueda Oshima M, Sandmaier BM, Petersdorf E, Flowers M, Hill G, Lee S, et al. Blood and marrow transplantation during the emerging COVID-19 pandemic: the Seattle approach. Bone Marrow Transplant. 2021; 56, 305-313. doi: 10.1038/s41409-020-01068-x
- Maurer K, Saucier A, Kim HT, Acharya U, Mo CC, Porter J, et al. COVID-19 and hematopoietic stem cell transplantation and immune effector cell therapy: a US cancer center experience. Blood Adv. 2021; 5 (3): 861-871. doi: 10.1182/bloodadvances.2020003883
- Kanellopoulos A, Ahmed MZ, Kishore B, Lovell R, Horgan C, Paneesha S, et al. COVID-19 in bone marrow transplant recipients: reflecting on a single centre experience. Br J Haematol. 2020; l, 190: e67-e70. https://doi.org/10.1111/bjh.16856
- del Campo PL, López AR, de la Cruz Benito B, de Paz Arias R, de Soto Álvarez T, Sánchez Vadillo I, et al. Hematopoietic cell transplantation during COVID-19 pandemic: experience from a tertiary hospital in Madrid. Exp Rev Hematol. (2021); 14:1, 1-5.
doi: 10.1080/17474086.2021.1858789 - Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, Martínez-Fernández JR, Crespo M, Gayoso J, et al. COVID-19 in transplant recipients: The Spanish experience. Am J Transplant. 2021, 21: 1825-1837. https://doi.org/10.1111/ajt.16369
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(100) "cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto" ["~CODE"]=> string(100) "cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto" ["EXTERNAL_ID"]=> string(4) "2042" ["~EXTERNAL_ID"]=> string(4) "2042" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(278) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клетокJourney of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(6316) "<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.</p> <h3>Материалы и методы</h3> <p style="text-align: justify;">Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.</p> <h3>Результаты</h3> <p style="text-align: justify;">Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы. </p> <p style="text-align: justify;">В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно. В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров. <p style="text-align: justify;"> Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66). Медиана времени развития COVID-19 после ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения – 12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).</p> <h3>Выводы</h3> <p style="text-align: justify;">Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_META_TITLE"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_META_KEYWORDS"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_META_DESCRIPTION"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_ALT"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_TITLE"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(172) "Через пандемию COVID-19: однолетний опыт центра трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "cherez-pandemiyu-covid-19-odnoletniy-opyt-tsentra-transplantatsii-gemopoeticheskikh-stvolovykh-kleto" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "201" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28425" ["VALUE"]=> array(2) { ["TEXT"]=> string(1339) "<p>Александр А. Синяев, Марина О. Попова, Юлия А. Рогачева, Анна А. Спиридонова, Мария Ю. Аверьянова, Александр Л. Алянский, Белла И. Аюбова, Елена В. Бабенко, Евгений А. Бакин, Ильдар М. Бархатов, Максим П. Богомольный, Татьяна А. Быкова, Алина А. Витрищак, Мария Д. Владовская, Юлия Ю. Власова, Алиса Г. Волкова, Асмик Г. Геворгян, Татьяна Л. Гиндина, Олег В. Голощапов, Кирилл А. Екушов, Мария А. Эстрина, Наталья Е. Иванова, Максим А. Кучер, Алексей Б. Чухловин, Кирилл В. Лепик, Инна В. Маркова, Наталья Б. Михайлова, Елена В. Морозова, Анна А. Осипова, Олеся В. Паина, Дмитрий Э. Певцов, Анна Г. Смирнова, Александр Н. Швецов, Лилия В. Стельмах, Галина Н. Столбенко, Людмила С. Зубаровская, Сергей Н. Бондаренко, Иван С. Моисеев, Александр Д. Кулагин</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1327) "
Александр А. Синяев, Марина О. Попова, Юлия А. Рогачева, Анна А. Спиридонова, Мария Ю. Аверьянова, Александр Л. Алянский, Белла И. Аюбова, Елена В. Бабенко, Евгений А. Бакин, Ильдар М. Бархатов, Максим П. Богомольный, Татьяна А. Быкова, Алина А. Витрищак, Мария Д. Владовская, Юлия Ю. Власова, Алиса Г. Волкова, Асмик Г. Геворгян, Татьяна Л. Гиндина, Олег В. Голощапов, Кирилл А. Екушов, Мария А. Эстрина, Наталья Е. Иванова, Максим А. Кучер, Алексей Б. Чухловин, Кирилл В. Лепик, Инна В. Маркова, Наталья Б. Михайлова, Елена В. Морозова, Анна А. Осипова, Олеся В. Паина, Дмитрий Э. Певцов, Анна Г. Смирнова, Александр Н. Швецов, Лилия В. Стельмах, Галина Н. Столбенко, Людмила С. Зубаровская, Сергей Н. Бондаренко, Иван С. Моисеев, Александр Д. Кулагин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28426" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28427" ["VALUE"]=> array(2) { ["TEXT"]=> string(6316) "<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.</p> <h3>Материалы и методы</h3> <p style="text-align: justify;">Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.</p> <h3>Результаты</h3> <p style="text-align: justify;">Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы. </p> <p style="text-align: justify;">В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно. В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров. <p style="text-align: justify;"> Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66). Медиана времени развития COVID-19 после ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения – 12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).</p> <h3>Выводы</h3> <p style="text-align: justify;">Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(6120) "Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.
Материалы и методы
Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.
Результаты
Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы.
В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно. В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров.
Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66). Медиана времени развития COVID-19 после ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения – 12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).
Выводы
Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.
Ключевые слова
Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28428" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-30-37" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-30-37" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28429" ["VALUE"]=> array(2) { ["TEXT"]=> string(795) "<p>Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova, Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny, atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian, Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher, Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova, Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko, Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(783) "Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova, Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny, atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian, Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher, Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova, Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko, Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28430" ["VALUE"]=> array(2) { ["TEXT"]=> string(701) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia<br> E-mail: drsiniaev@yandex.ru</p><br> <p><b> Citation:</b> Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(617) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
E-mail: drsiniaev@yandex.ru
Citation: Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28431" ["VALUE"]=> array(2) { ["TEXT"]=> string(3620) "<p style="text-align: justify;">Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.</p> <h3>Patients and methods</h3> <p style="text-align: justify;">This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity. </p> <h3>Results</h3> <p style="text-align: justify;">The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 – 50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 – 0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).</p> <h3>Conclusions</h3> <p style="text-align: justify;">Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center. </p> <h2>Keywords</h2> <p style="text-align: justify;">Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3462) "Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.
Patients and methods
This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity.
Results
The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 – 50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 – 0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).
Conclusions
Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center.
Keywords
Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28432" ["VALUE"]=> string(106) "Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(106) "Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28433" ["VALUE"]=> string(4) "2709" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2709" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28434" ["VALUE"]=> string(4) "2710" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2710" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28429" ["VALUE"]=> array(2) { ["TEXT"]=> string(795) "<p>Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova, Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny, atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian, Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher, Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova, Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko, Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(783) "Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova, Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny, atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian, Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher, Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova, Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko, Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(783) "Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova, Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny, atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian, Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher, Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova, Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko, Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28431" ["VALUE"]=> array(2) { ["TEXT"]=> string(3620) "<p style="text-align: justify;">Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.</p> <h3>Patients and methods</h3> <p style="text-align: justify;">This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity. </p> <h3>Results</h3> <p style="text-align: justify;">The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 – 50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 – 0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).</p> <h3>Conclusions</h3> <p style="text-align: justify;">Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center. </p> <h2>Keywords</h2> <p style="text-align: justify;">Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3462) "Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.
Patients and methods
This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity.
Results
The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 – 50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 – 0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).
Conclusions
Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center.
Keywords
Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3462) "Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.
Patients and methods
This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity.
Results
The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 – 50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 – 0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).
Conclusions
Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center.
Keywords
Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28428" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-30-37" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-30-37" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-30-37" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28432" ["VALUE"]=> string(106) "Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(106) "Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(106) "Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28430" ["VALUE"]=> array(2) { ["TEXT"]=> string(701) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia<br> E-mail: drsiniaev@yandex.ru</p><br> <p><b> Citation:</b> Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(617) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
E-mail: drsiniaev@yandex.ru
Citation: Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(617) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
E-mail: drsiniaev@yandex.ru
Citation: Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28425" ["VALUE"]=> array(2) { ["TEXT"]=> string(1339) "<p>Александр А. Синяев, Марина О. Попова, Юлия А. Рогачева, Анна А. Спиридонова, Мария Ю. Аверьянова, Александр Л. Алянский, Белла И. Аюбова, Елена В. Бабенко, Евгений А. Бакин, Ильдар М. Бархатов, Максим П. Богомольный, Татьяна А. Быкова, Алина А. Витрищак, Мария Д. Владовская, Юлия Ю. Власова, Алиса Г. Волкова, Асмик Г. Геворгян, Татьяна Л. Гиндина, Олег В. Голощапов, Кирилл А. Екушов, Мария А. Эстрина, Наталья Е. Иванова, Максим А. Кучер, Алексей Б. Чухловин, Кирилл В. Лепик, Инна В. Маркова, Наталья Б. Михайлова, Елена В. Морозова, Анна А. Осипова, Олеся В. Паина, Дмитрий Э. Певцов, Анна Г. Смирнова, Александр Н. Швецов, Лилия В. Стельмах, Галина Н. Столбенко, Людмила С. Зубаровская, Сергей Н. Бондаренко, Иван С. Моисеев, Александр Д. Кулагин</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1327) "Александр А. Синяев, Марина О. Попова, Юлия А. Рогачева, Анна А. Спиридонова, Мария Ю. Аверьянова, Александр Л. Алянский, Белла И. Аюбова, Елена В. Бабенко, Евгений А. Бакин, Ильдар М. Бархатов, Максим П. Богомольный, Татьяна А. Быкова, Алина А. Витрищак, Мария Д. Владовская, Юлия Ю. Власова, Алиса Г. Волкова, Асмик Г. Геворгян, Татьяна Л. Гиндина, Олег В. Голощапов, Кирилл А. Екушов, Мария А. Эстрина, Наталья Е. Иванова, Максим А. Кучер, Алексей Б. Чухловин, Кирилл В. Лепик, Инна В. Маркова, Наталья Б. Михайлова, Елена В. Морозова, Анна А. Осипова, Олеся В. Паина, Дмитрий Э. Певцов, Анна Г. Смирнова, Александр Н. Швецов, Лилия В. Стельмах, Галина Н. Столбенко, Людмила С. Зубаровская, Сергей Н. Бондаренко, Иван С. Моисеев, Александр Д. Кулагин
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1327) "Александр А. Синяев, Марина О. Попова, Юлия А. Рогачева, Анна А. Спиридонова, Мария Ю. Аверьянова, Александр Л. Алянский, Белла И. Аюбова, Елена В. Бабенко, Евгений А. Бакин, Ильдар М. Бархатов, Максим П. Богомольный, Татьяна А. Быкова, Алина А. Витрищак, Мария Д. Владовская, Юлия Ю. Власова, Алиса Г. Волкова, Асмик Г. Геворгян, Татьяна Л. Гиндина, Олег В. Голощапов, Кирилл А. Екушов, Мария А. Эстрина, Наталья Е. Иванова, Максим А. Кучер, Алексей Б. Чухловин, Кирилл В. Лепик, Инна В. Маркова, Наталья Б. Михайлова, Елена В. Морозова, Анна А. Осипова, Олеся В. Паина, Дмитрий Э. Певцов, Анна Г. Смирнова, Александр Н. Швецов, Лилия В. Стельмах, Галина Н. Столбенко, Людмила С. Зубаровская, Сергей Н. Бондаренко, Иван С. Моисеев, Александр Д. Кулагин
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28427" ["VALUE"]=> array(2) { ["TEXT"]=> string(6316) "<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.</p> <h3>Материалы и методы</h3> <p style="text-align: justify;">Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.</p> <h3>Результаты</h3> <p style="text-align: justify;">Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы. </p> <p style="text-align: justify;">В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно. В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров. <p style="text-align: justify;"> Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66). Медиана времени развития COVID-19 после ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения – 12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).</p> <h3>Выводы</h3> <p style="text-align: justify;">Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(6120) "Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.
Материалы и методы
Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.
Результаты
Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы.
В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно. В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров.
Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66). Медиана времени развития COVID-19 после ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения – 12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).
Выводы
Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.
Ключевые слова
Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(6120) "Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.
Материалы и методы
Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.
Результаты
Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы.
В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно. В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров.
Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66). Медиана времени развития COVID-19 после ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения – 12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).
Выводы
Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.
Ключевые слова
Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28426" ["VALUE"]=> array(2) { ["TEXT"]=> string(373) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(361) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [6]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "201" ["~IBLOCK_SECTION_ID"]=> string(3) "201" ["ID"]=> string(4) "2044" ["~ID"]=> string(4) "2044" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["~NAME"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "01/25/2022 02:06:49 pm" ["~TIMESTAMP_X"]=> string(22) "01/25/2022 02:06:49 pm" ["DETAIL_PAGE_URL"]=> string(150) "/en/archive/tom-10-nomer-3-4/klinicheskie-raboty/effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech/" ["~DETAIL_PAGE_URL"]=> string(150) "/en/archive/tom-10-nomer-3-4/klinicheskie-raboty/effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(29227) "Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the most common primary liver malignancy worldwide [1, 2]. The incidence of HCC has tripled in the United States during the past three decades, with an annual increase of 4.5% [3]. Additionally, the mortality rates associated with this disease have continued to rise [3, 4]. In Egypt, malignant liver tumors represent 1.7% of all malignancies and HCC constitutes more than 70% of these malignancies [5]. Hospital-based studies have reported an overall increase in relative frequency of all liver cancers in Egypt (mainly, HCC), from approximately 4% in 1993 to 7.3% in 2003 [6].
Currently, since introduction of Milan criteria (MC) of liver transplantation (LTx), this treatment has proven to be an excellent therapeutic option for HCC [7]. Due to the organ shortage, not all transplant-eligible HCC patients undergo LT at the optimal timing. Scarcity of liver grafts and, therefore, longer waiting time result into the disease progression. The predicted probability of dropping from waiting list is estimated as 12% for the patients with 6-month delay of tumor treatment [8, 9].
In view of sufficient organ shortage, the need for a model to select, prioritize and identify HCC patients with fruitful outcome became crucial. The established transplant criteria, like Milan and UCSF criteria have been well validated and were used as the guideline to select the patients for LTx, especially deceased donor LT [10-12]. Lymphovascular invasion and histological grade of tumor were found to be the most relevant tumor variables to the outcome of HCC patients. Despite that, LT for HCC currently relies on morphological criteria, i.e., number of tumor nodes?, size, and total tumor volume to select HCC patients for LT [6, 13-16].
Unfortunately, most HCC patients are beyond the MC at the time of presentation. Therefore, downstaging to the values fitting for MC is attempted in selected patients. Downstaging is usually performed by a variety of techniques called collectively locoregional therapies (LRT). LRT include radiofrequency ablation (RFA), transarterial chemoembolization (TACE), transarterial radioembolization (TARE), alone or a combination. Downstaging offers many advantages, including decreasing tumor burden and causing less aggressive tumor biology [17]. In 2001, Yao et al. reported the first successful LTx following the HCC downstaging. They showed that successful downstaging of HCC is feasible with a good post LTx outcome [18]. Subsequently, several groups obtained similar outcomes by using different protocols [18-23]. The shortage of the organ grafts requires proper evaluation of the downstaging LRT upon outcomes of HCC treated by LTx, thus determining the aim of our study.
Materials and methods
A cohort of 115 adult patients underwent LT for the presence of hepatocellular carcinoma (HCC) at our institution between August 2006 and December 2019. Approval from our institutional Research Ethical Committee was obtained before conduction of this study. Cases of pediatric LT or liver retransplantation were excluded from this study.
LTx was performed using both cadaveric and living donor liver transplantation (LDLT). Cases from LDLT were first- and second-degree relatives of their respective patients. HCC was diagnosed by contrast-enhanced computed tomography (CT) and/or abdominal magnetic resonance imaging (MRI). The disease staging was done by chest CT, cranial CT, and technetium-99m bone scintigraphy, to exclude extra-hepatic disease. HCC size, number, tumor grade, and lymph vascular invasion were diagnosed by an experienced pathologist.
For histopathological examination, the 7-point sampling procedure was employed as follows: (1) At least four tissue samples were biopsied from the junction of HCC with nearby hepatic tissue in a 1:1 proportion at 12, 3, 6, 9 o’clock locations, (2) at least one sample should be taken from intratumoral region to allow for molecular subtyping, (3) Aiming to exclude the presence of microvascular invasion, satellite, or dysplastic nodules, samples were also obtained from hepatic tissue ≤ 1 cm and >1 cm from HCC margin (adjacent and distant peritumoral locations respectively). (4) The size of tissue blocks was confirmed to be approximately 1.5 cm – 2.0 cm × 1.0 cm × 0.3 cm, (5) All the specimens were tagged according to the sampling locations.
Liver explant is defined as the native liver of the recipient which was removed in toto by the surgical team as a preparatory step for implanting a new whole liver, or a liver lobe during LTx procedure. Total tumor volume (TTV) was defined as the sum of individual tumor lesion volume; the tumor volume was estimated using the formula (T= {(4/3) π, r3} where r equals the maximum diameter of the lesion measured in cm). Presence of mixed HCC and cholangiocarcinoma was a criterion for excluding the case from our study.
Based on the usage of pre-transplant downstaging LRT, patients were divided into the following groups:
• Group A: Patients within Milan criteria (MC) who did not receive pretransplant downstaging LRT.
• Group B: Patients beyond MC who, therefore, received downstaging pretransplant LRT.
Our center currently adopts MC for HCC as the standard criteria for LTx. Any patient who is beyond MC is usually considered for a downstaging protocol by means of one or more locoregional therapies to downstage the tumor to the values fitting within MC. LTx for the downstaged patients is considered after subsequent confirmation of the absence of extrahepatic disease. Pretransplant locoregional therapies included RFA, TACE and TARE.
Evaluation of the response to locoregional therapy was done according to the mRECIST criteria [24]. Accordingly, complete response (CR) was defined as disappearance of any arterial enhancement in all target lesions. Meanwhile, partial response (PR) was defined by at least a 30% reduction in the sum of diameters of enhancing lesions. Progressive disease (PD) was identified as an increase for, at least, 20% in the sum of diameters of enhancing lesions. Finally, stable disease (SD) was defined as cases that do not qualify for either (PR) or (PD) [24].
A triple immunosuppression protocol was used for the LT recipients, including calcineurin inhibitor (CNI), corticosteroids and mycophenolate mofetil. Significance of differences and correlations between distinct tumor variables, effects of LRT downstaging, and posttransplant outcomes were assessed by t-test, Chi-square test, one-way ANOVA test, and Pearson’s correlation criterion. P-value of <0.05 was considered statistically significant. Kaplan-Meier curves were used to express patient survival, graft survival, and tumor-free survival and its significance was determined by log-rank test.
Results
A cohort of 115 patients underwent LTx between August 2006 and December 2019 at our center. Presence of HCC was the primary indication for LTx. Table 1 is demonstrating pretransplant, transplant, and explant variables for the studied HCC lesions.
Table 1. Pretransplant, transplant and explant characteristics of the studied HCC patients

Note: *, percentage of non necrotic HCC cases
The degree of tumor differentiation and vascular invasion could not be assessed in 15 cases (13% of lesions) due to complete necrosis of the lesion caused by LRT prior to LTx. Follow-up period ranged from 24.3-149.9 months, with a mean of 45.98±33.3 months. The overall 5-year patient and tumor-free survival, and graft survival rate were 79.7%, 90.4% and 88.2%, respectively (Fig. 1).

Figure 1. Overall and tumor-free survival, graft survival following LTx for HCC patients
Initially, 136 patients presented with HCC during the study period. Fig. 2 illustrates their distribution, according to their initial HCC burden, LRT and LTx.

Figure 2. Distribution of HCC patients by their clinical condition during the study period
Note: * Tumor response was assessed according to mRECIST Criteria
Twenty-one patients dropped out while awaiting LT, thus representing 15.4% dropout for the total HCC group. Therefore, thirteen patients from group A were not transplanted, i.e., 8 patients were delisted due to worsening of their clinical state, and 5 patients, due to progression of HCC beyond the MC (9.3% and 5.8% of HCC patients within MC, respectively).
On the other hand, fifty-five patients were subjected to downstaging LRT, and only 42 of them were eligible for transplant since they showed, at least, partial response. The remaining thirteen patients were excluded from the waiting list, due to stable or progressive disease following the use of LRT. Seventy-three patients had HCC within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). Only number of HCC nodules and TTV exhibited significant difference between both groups (Table 2).
Table 2. Comparison of different pretransplant variables between both groups

Note: *, percentage within the corresponding group; **, after exclusion of 11 cases with total tumor necrosis

Figure 3. Patient survival curves for group A and B patients following LTx

Figure 4. Tumor-free survival curves for group A and B patients following LTx

Figure 5. Graft survival curves for group A and B patients following LTx
Group A slightly differed from group B as regards the long-term outcomes, but this marginal difference did not reach the level of statistical significance. Patient survival was slightly better in Group A (78.6% vs 74.4% at 5 years), with p value of 0.57 (log-rank test). Similarly, tumor-free survival was slightly better in group A (83.6% vs 74.2%) at the 5-year interval (p=0.35). On the other hand, graft survival was almost the same 90.1% vs 90.5% at the 5 years interval in group A and B respectively (p=0.83). Kaplan-Meier graphs for patient, tumor free and graft survival are illustrated in Fig. 3, 4 and 5, respectively.
Twenty-five recipients (21.7%) died during the follow-up period. 15 were in group A, and 10, in group B. The causes of death among those recipients are listed in Table 3. The total number of mortalities was statistically insignificant between the groups A and B at p=0.82.
HCC recurrence was reported in a total of 11 patients (9.6% of transplanted HCC patients). Six cases were reported in group A and five, in group B. Recurrence rates were 8.2% and 11.9 % in group A and B, respectively (p=0.53).
Five patients diagnosed with HCC recurrence showed initially lymphovascular invasion in their liver explants, poor tumor differentiation was found in four cases, five patients had moderate tumor differentiation, and 7 patients showed total tumor volume (TTV) of >115 cm3. HCC recurrence was significantly related to the presence of vascular invasion and poor degree of differentiation. Moreover, both features were significantly related to patient survival. Vice versa, transplant criteria and tumor volume >115 cm3 showed no significant relation to the patients’ survival or tumor recurrence (Table 4).
Seven patients presented solely with distant metastases in lungs (n=6), bones (n=1), and the remaining four patients developed intrahepatic tumor recurrence. Treatment for these cases was scheduled as supportive therapy, along with palliative use of sorafenib if possible.

Discussion
Currently, LTx offers the best curative chance for patients with HCC on the top of liver cirrhosis, when a liver graft is available [7-9]. Our results correspond with such an opinion, i.e., the overall 5-year patient survival, graft survival, and tumor-free survival were 79.7%, 90.4%, and 88.2%, respectively. We observed LTx dropout rate of 15.1% for the patients with tumors that corresponded to Milan criteria. The institutional data showed dropout of 11.0%, and 57.4% at 6 and 12 months for those patients who initially were within MC [8].
Lack of organs for transplants led to development of prioritizing models to use the limited graft pool very efficiently and successfully. In this context, the accumulated evidence of excellent outcomes in the patients transplanted for HCC who fits the Milan criteria lead to adoption of the 22-point bonus system by UNOS [7-9]. There are endless debates on whether a transplant center should embark on adopting MC or more liberal criteria [10-12]. This might indicate that none of the single morphology-based transplant criteria is sufficient when predicting best transplant outcomes in HCC patients, thus raising a need for inclusion of some biological tumor factors into the predictive models [6, 13-16].
There have been numerous reports on the tumor downstaging approaches. However, these data are limited by the small number of patients and/or the use of varying downstaging protocols [17-23]. HCC progression in the patients being on waiting list for LTx was found to be an important prognostic factor. It was observed that tumor recurrence after LTx increased from 12% in the patients remaining within MC (either spontaneously or following bridging therapy), to 45% for those with tumor progression beyond the MC [24, 25]. Different criteria were developed to assess the response of HCC to LRT, with mRECIST assessment criteria being the commonly adopted [23, 26-29].
Morphological HCC criteria including TTV >115 cm3, or being within MC parameters were not shown to significantly influence post-LTx outcome in our HCC patients. Meanwhile, poor tumor differentiation and presence of lymphatic microvascular invasion proved to exert statistically significant influence upon patient survival and HCC recurrence. This finding highlights the importance of biological factors versus morphological variables in determining long-term outcomes following LTx.
Multiplicity of criteria for LTx might partially demonstrate insufficiency of tumor morphologic criteria alone to precise prediction of post-LTx outcomes. The current staging systems for HCC consider only gross tumor morphology. Tumor size and volume appear to reflect the more sufficient biological features of malignancies. Jonas et al. found that tumor diameter and number of HCC lesions in association with pathologic tumor grade predicted the presence of vascular invasion only in HCC lesions >5 cm [13, 15]. Moreover, molecular subtyping appears to be a useful approach to more individualized management of malignant tumors in general, this is also true in the context of HCC. Various studies concentrate now on genetic profiling of HCC lesions. E.g., Marsh et al. investigated DNA mutations in HCC lesions and found the fractional allelic loss (FAI, an index of mutation accumulation), and vascular invasion were the strongest predictors of tumor-free survival [14, 15].
Downstaging LRT was used to reverse HCC patients to the clinical stage fitting Milan criteria. This strategy proved to be successful in more than 75% of cases; 23.6% were not considered a successful downstaging, due to persistence of stable or progressing disease, according to mRECIST criteria.
The patients successfully downstaged to the condition within MC showed the rates of overall and recurrence-free survival comparable to the patients who corresponded to Milan criteria, being transplanted without initial LRT. In the light of organ shortage, LRT offers an opportunity to downstage HCC to clinical stage within MC, along with selection of more biologically favorable tumors.
Retrospective pattern and relatively small number are among the limitations of our study, thus requiring further studies to confirm such results and to define the role of LRT as a bridge for LTx, aiming for prevention of HCC progression while awaiting LTx.
The era of molecular HCC typing will soon have its influence on HCC management, and introduction of one or more biological markers to the HCC transplant criteria is one of the ways to improve its future therapy.
Conclusion
The overall results of LTx for HCC at our institution showed an excellent outcome. Presence of lymphatic vascular invasion and poor tumor differentiation are the main factors affecting the long-term post-LTx outcomes. This finding again highlights the importance of biological tumor criteria along with commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the clinical state which fits MC, along with choosing more biologically favorable tumors. The successfully downstaged HCC patients who fitted MC, showed overall and recurrence-free survival rates comparable to those who were initially transplanted within MC and without LRT.
Conflict of interest
Authors have neither conflict of interest, nor financial issues to be disclosed.
References
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi: 10.1016/S0140-6736(03)14964-1
- Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1(1):2-14. doi: 10.1159/000339016
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi: 10.1200/JCO.2008.20.7753
- Patel SS, Arrington AK, McKenzie S, Mailey B, Ding M, Lee W, et al. Milan criteria and UCSF criteria: A preliminary comparative study of liver transplantation outcomes in the United States. Int J Hepatol. 2012;2012:253517. doi: 10.1155/2012/253517
- Mokhtar N, Gouda I, Adel I. Malignant digestive system tumors. In: Mokhtar N, Gouda I, Adel I, Eds. Cancer pathology registry, 2003-2004 and time trend analysis. Cairo, Egypt: Elsheraa Press; 2007. pp. 55-67.
- el-Zayadi AR, Badran HM, Barakat EM, Attia Mel D, Shawky S, Mohamed MK, et al. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol. 2005;11(33):5193-5198. doi: 10.3748/wjg.v11.i33.5193
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699. doi: 10.1056/NEJM199603143341104
- Martin AP, Bartels M, Hauss J, Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc. 2007;39(10):3169-3174. doi: 10.1016/j.transproceed.2007.04.025
- United Network for Organ Sharing, "Data reports", https://optn.transplant.hrsa.gov/data/view-data-reports/national-data. Accessed December 17, 2019.
- Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15(4):1001-1007. doi: 10.1245/s10434-007-9559-5
- Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587-2596. doi: 10.1111/j.1600-6143.2007.01965.x
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43.
doi: 10.1016/S1470-2045(08)70284-5 - Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33(5):1080-1086.
doi: 10.1053/jhep.2001.23561 - Marsh JW, Finkelstein SD, Demetris AJ, Swalsky PA, Sasatomi E, Bandos A, et al. Genotyping of hepatocellular carcinoma in liver transplant recipients adds predictive power for determining recurrence-free survival. Liver Transpl. 2003;9(7):664-671.
doi: 10.1053/jlts.2003.50144 - Abdelfattah MR, Elsiesy H, Al-Manea H, Broering DC. Liver transplantation for hepatocellular carcinoma within the Milan criteria versus the University of California San Francisco criteria: a comparative study. Eur J Gastroenterol Hepatol. 2018;30(4):398-403.
doi: 10.1097/MEG.0000000000001044 - Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8(9):765-774.
doi: 10.1053/jlts.2002.34892 - Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015;21(9):1142-1152. doi: 10.1002/lt.24169
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-1403. doi: 10.1053/jhep.2001.24563
- Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248(4):617-625.
doi: 10.1097/SLA.0b013e31818a07d4 - Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12(8):1260-1267. doi: 10.1002/lt.20837
- Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8(12):2547-2557.
doi: 10.1111/j.1600-6143.2008.02409.x - Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968-1977. doi: 10.1002/hep.27752
- Tohme S, Sukato D, Chen HW, Amesur N, Zajko AB, Humar A, et al. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol. 2013;24(11):1632-1638. doi: 10.1016/j.jvir.2013.07.026
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60.
doi: 10.1055/s-0030-1247132 - Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9(7):684-692. doi: 10.1053/jlts.2003.50147
- Yamashiki N, Gaynor JJ, Kato T, Reddy KR, Sobhonslidsuk A, Levi D, et al. Competing risks analysis of predictors of delisting owing to tumor progression in liver transplant candidates with hepatocellular carcinoma. Am J Transplant. 2004;4(5):774-781.
doi: 10.1111/j.1600-6143.2004.00412.x - Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421-430. doi: 10.1016/s0168-8278(01)00130-1
- Bruix J, Sherman M, Practice Guidelines Committee AASLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208-1236. doi: 10.1002/hep.20933
- Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115(3):616-623.
doi: 10.1002/cncr.24050
" ["~DETAIL_TEXT"]=> string(29227) "
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the most common primary liver malignancy worldwide [1, 2]. The incidence of HCC has tripled in the United States during the past three decades, with an annual increase of 4.5% [3]. Additionally, the mortality rates associated with this disease have continued to rise [3, 4]. In Egypt, malignant liver tumors represent 1.7% of all malignancies and HCC constitutes more than 70% of these malignancies [5]. Hospital-based studies have reported an overall increase in relative frequency of all liver cancers in Egypt (mainly, HCC), from approximately 4% in 1993 to 7.3% in 2003 [6].
Currently, since introduction of Milan criteria (MC) of liver transplantation (LTx), this treatment has proven to be an excellent therapeutic option for HCC [7]. Due to the organ shortage, not all transplant-eligible HCC patients undergo LT at the optimal timing. Scarcity of liver grafts and, therefore, longer waiting time result into the disease progression. The predicted probability of dropping from waiting list is estimated as 12% for the patients with 6-month delay of tumor treatment [8, 9].
In view of sufficient organ shortage, the need for a model to select, prioritize and identify HCC patients with fruitful outcome became crucial. The established transplant criteria, like Milan and UCSF criteria have been well validated and were used as the guideline to select the patients for LTx, especially deceased donor LT [10-12]. Lymphovascular invasion and histological grade of tumor were found to be the most relevant tumor variables to the outcome of HCC patients. Despite that, LT for HCC currently relies on morphological criteria, i.e., number of tumor nodes?, size, and total tumor volume to select HCC patients for LT [6, 13-16].
Unfortunately, most HCC patients are beyond the MC at the time of presentation. Therefore, downstaging to the values fitting for MC is attempted in selected patients. Downstaging is usually performed by a variety of techniques called collectively locoregional therapies (LRT). LRT include radiofrequency ablation (RFA), transarterial chemoembolization (TACE), transarterial radioembolization (TARE), alone or a combination. Downstaging offers many advantages, including decreasing tumor burden and causing less aggressive tumor biology [17]. In 2001, Yao et al. reported the first successful LTx following the HCC downstaging. They showed that successful downstaging of HCC is feasible with a good post LTx outcome [18]. Subsequently, several groups obtained similar outcomes by using different protocols [18-23]. The shortage of the organ grafts requires proper evaluation of the downstaging LRT upon outcomes of HCC treated by LTx, thus determining the aim of our study.
Materials and methods
A cohort of 115 adult patients underwent LT for the presence of hepatocellular carcinoma (HCC) at our institution between August 2006 and December 2019. Approval from our institutional Research Ethical Committee was obtained before conduction of this study. Cases of pediatric LT or liver retransplantation were excluded from this study.
LTx was performed using both cadaveric and living donor liver transplantation (LDLT). Cases from LDLT were first- and second-degree relatives of their respective patients. HCC was diagnosed by contrast-enhanced computed tomography (CT) and/or abdominal magnetic resonance imaging (MRI). The disease staging was done by chest CT, cranial CT, and technetium-99m bone scintigraphy, to exclude extra-hepatic disease. HCC size, number, tumor grade, and lymph vascular invasion were diagnosed by an experienced pathologist.
For histopathological examination, the 7-point sampling procedure was employed as follows: (1) At least four tissue samples were biopsied from the junction of HCC with nearby hepatic tissue in a 1:1 proportion at 12, 3, 6, 9 o’clock locations, (2) at least one sample should be taken from intratumoral region to allow for molecular subtyping, (3) Aiming to exclude the presence of microvascular invasion, satellite, or dysplastic nodules, samples were also obtained from hepatic tissue ≤ 1 cm and >1 cm from HCC margin (adjacent and distant peritumoral locations respectively). (4) The size of tissue blocks was confirmed to be approximately 1.5 cm – 2.0 cm × 1.0 cm × 0.3 cm, (5) All the specimens were tagged according to the sampling locations.
Liver explant is defined as the native liver of the recipient which was removed in toto by the surgical team as a preparatory step for implanting a new whole liver, or a liver lobe during LTx procedure. Total tumor volume (TTV) was defined as the sum of individual tumor lesion volume; the tumor volume was estimated using the formula (T= {(4/3) π, r3} where r equals the maximum diameter of the lesion measured in cm). Presence of mixed HCC and cholangiocarcinoma was a criterion for excluding the case from our study.
Based on the usage of pre-transplant downstaging LRT, patients were divided into the following groups:
• Group A: Patients within Milan criteria (MC) who did not receive pretransplant downstaging LRT.
• Group B: Patients beyond MC who, therefore, received downstaging pretransplant LRT.
Our center currently adopts MC for HCC as the standard criteria for LTx. Any patient who is beyond MC is usually considered for a downstaging protocol by means of one or more locoregional therapies to downstage the tumor to the values fitting within MC. LTx for the downstaged patients is considered after subsequent confirmation of the absence of extrahepatic disease. Pretransplant locoregional therapies included RFA, TACE and TARE.
Evaluation of the response to locoregional therapy was done according to the mRECIST criteria [24]. Accordingly, complete response (CR) was defined as disappearance of any arterial enhancement in all target lesions. Meanwhile, partial response (PR) was defined by at least a 30% reduction in the sum of diameters of enhancing lesions. Progressive disease (PD) was identified as an increase for, at least, 20% in the sum of diameters of enhancing lesions. Finally, stable disease (SD) was defined as cases that do not qualify for either (PR) or (PD) [24].
A triple immunosuppression protocol was used for the LT recipients, including calcineurin inhibitor (CNI), corticosteroids and mycophenolate mofetil. Significance of differences and correlations between distinct tumor variables, effects of LRT downstaging, and posttransplant outcomes were assessed by t-test, Chi-square test, one-way ANOVA test, and Pearson’s correlation criterion. P-value of <0.05 was considered statistically significant. Kaplan-Meier curves were used to express patient survival, graft survival, and tumor-free survival and its significance was determined by log-rank test.
Results
A cohort of 115 patients underwent LTx between August 2006 and December 2019 at our center. Presence of HCC was the primary indication for LTx. Table 1 is demonstrating pretransplant, transplant, and explant variables for the studied HCC lesions.
Table 1. Pretransplant, transplant and explant characteristics of the studied HCC patients

Note: *, percentage of non necrotic HCC cases
The degree of tumor differentiation and vascular invasion could not be assessed in 15 cases (13% of lesions) due to complete necrosis of the lesion caused by LRT prior to LTx. Follow-up period ranged from 24.3-149.9 months, with a mean of 45.98±33.3 months. The overall 5-year patient and tumor-free survival, and graft survival rate were 79.7%, 90.4% and 88.2%, respectively (Fig. 1).

Figure 1. Overall and tumor-free survival, graft survival following LTx for HCC patients
Initially, 136 patients presented with HCC during the study period. Fig. 2 illustrates their distribution, according to their initial HCC burden, LRT and LTx.

Figure 2. Distribution of HCC patients by their clinical condition during the study period
Note: * Tumor response was assessed according to mRECIST Criteria
Twenty-one patients dropped out while awaiting LT, thus representing 15.4% dropout for the total HCC group. Therefore, thirteen patients from group A were not transplanted, i.e., 8 patients were delisted due to worsening of their clinical state, and 5 patients, due to progression of HCC beyond the MC (9.3% and 5.8% of HCC patients within MC, respectively).
On the other hand, fifty-five patients were subjected to downstaging LRT, and only 42 of them were eligible for transplant since they showed, at least, partial response. The remaining thirteen patients were excluded from the waiting list, due to stable or progressive disease following the use of LRT. Seventy-three patients had HCC within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). Only number of HCC nodules and TTV exhibited significant difference between both groups (Table 2).
Table 2. Comparison of different pretransplant variables between both groups

Note: *, percentage within the corresponding group; **, after exclusion of 11 cases with total tumor necrosis

Figure 3. Patient survival curves for group A and B patients following LTx

Figure 4. Tumor-free survival curves for group A and B patients following LTx

Figure 5. Graft survival curves for group A and B patients following LTx
Group A slightly differed from group B as regards the long-term outcomes, but this marginal difference did not reach the level of statistical significance. Patient survival was slightly better in Group A (78.6% vs 74.4% at 5 years), with p value of 0.57 (log-rank test). Similarly, tumor-free survival was slightly better in group A (83.6% vs 74.2%) at the 5-year interval (p=0.35). On the other hand, graft survival was almost the same 90.1% vs 90.5% at the 5 years interval in group A and B respectively (p=0.83). Kaplan-Meier graphs for patient, tumor free and graft survival are illustrated in Fig. 3, 4 and 5, respectively.
Twenty-five recipients (21.7%) died during the follow-up period. 15 were in group A, and 10, in group B. The causes of death among those recipients are listed in Table 3. The total number of mortalities was statistically insignificant between the groups A and B at p=0.82.
HCC recurrence was reported in a total of 11 patients (9.6% of transplanted HCC patients). Six cases were reported in group A and five, in group B. Recurrence rates were 8.2% and 11.9 % in group A and B, respectively (p=0.53).
Five patients diagnosed with HCC recurrence showed initially lymphovascular invasion in their liver explants, poor tumor differentiation was found in four cases, five patients had moderate tumor differentiation, and 7 patients showed total tumor volume (TTV) of >115 cm3. HCC recurrence was significantly related to the presence of vascular invasion and poor degree of differentiation. Moreover, both features were significantly related to patient survival. Vice versa, transplant criteria and tumor volume >115 cm3 showed no significant relation to the patients’ survival or tumor recurrence (Table 4).
Seven patients presented solely with distant metastases in lungs (n=6), bones (n=1), and the remaining four patients developed intrahepatic tumor recurrence. Treatment for these cases was scheduled as supportive therapy, along with palliative use of sorafenib if possible.

Discussion
Currently, LTx offers the best curative chance for patients with HCC on the top of liver cirrhosis, when a liver graft is available [7-9]. Our results correspond with such an opinion, i.e., the overall 5-year patient survival, graft survival, and tumor-free survival were 79.7%, 90.4%, and 88.2%, respectively. We observed LTx dropout rate of 15.1% for the patients with tumors that corresponded to Milan criteria. The institutional data showed dropout of 11.0%, and 57.4% at 6 and 12 months for those patients who initially were within MC [8].
Lack of organs for transplants led to development of prioritizing models to use the limited graft pool very efficiently and successfully. In this context, the accumulated evidence of excellent outcomes in the patients transplanted for HCC who fits the Milan criteria lead to adoption of the 22-point bonus system by UNOS [7-9]. There are endless debates on whether a transplant center should embark on adopting MC or more liberal criteria [10-12]. This might indicate that none of the single morphology-based transplant criteria is sufficient when predicting best transplant outcomes in HCC patients, thus raising a need for inclusion of some biological tumor factors into the predictive models [6, 13-16].
There have been numerous reports on the tumor downstaging approaches. However, these data are limited by the small number of patients and/or the use of varying downstaging protocols [17-23]. HCC progression in the patients being on waiting list for LTx was found to be an important prognostic factor. It was observed that tumor recurrence after LTx increased from 12% in the patients remaining within MC (either spontaneously or following bridging therapy), to 45% for those with tumor progression beyond the MC [24, 25]. Different criteria were developed to assess the response of HCC to LRT, with mRECIST assessment criteria being the commonly adopted [23, 26-29].
Morphological HCC criteria including TTV >115 cm3, or being within MC parameters were not shown to significantly influence post-LTx outcome in our HCC patients. Meanwhile, poor tumor differentiation and presence of lymphatic microvascular invasion proved to exert statistically significant influence upon patient survival and HCC recurrence. This finding highlights the importance of biological factors versus morphological variables in determining long-term outcomes following LTx.
Multiplicity of criteria for LTx might partially demonstrate insufficiency of tumor morphologic criteria alone to precise prediction of post-LTx outcomes. The current staging systems for HCC consider only gross tumor morphology. Tumor size and volume appear to reflect the more sufficient biological features of malignancies. Jonas et al. found that tumor diameter and number of HCC lesions in association with pathologic tumor grade predicted the presence of vascular invasion only in HCC lesions >5 cm [13, 15]. Moreover, molecular subtyping appears to be a useful approach to more individualized management of malignant tumors in general, this is also true in the context of HCC. Various studies concentrate now on genetic profiling of HCC lesions. E.g., Marsh et al. investigated DNA mutations in HCC lesions and found the fractional allelic loss (FAI, an index of mutation accumulation), and vascular invasion were the strongest predictors of tumor-free survival [14, 15].
Downstaging LRT was used to reverse HCC patients to the clinical stage fitting Milan criteria. This strategy proved to be successful in more than 75% of cases; 23.6% were not considered a successful downstaging, due to persistence of stable or progressing disease, according to mRECIST criteria.
The patients successfully downstaged to the condition within MC showed the rates of overall and recurrence-free survival comparable to the patients who corresponded to Milan criteria, being transplanted without initial LRT. In the light of organ shortage, LRT offers an opportunity to downstage HCC to clinical stage within MC, along with selection of more biologically favorable tumors.
Retrospective pattern and relatively small number are among the limitations of our study, thus requiring further studies to confirm such results and to define the role of LRT as a bridge for LTx, aiming for prevention of HCC progression while awaiting LTx.
The era of molecular HCC typing will soon have its influence on HCC management, and introduction of one or more biological markers to the HCC transplant criteria is one of the ways to improve its future therapy.
Conclusion
The overall results of LTx for HCC at our institution showed an excellent outcome. Presence of lymphatic vascular invasion and poor tumor differentiation are the main factors affecting the long-term post-LTx outcomes. This finding again highlights the importance of biological tumor criteria along with commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the clinical state which fits MC, along with choosing more biologically favorable tumors. The successfully downstaged HCC patients who fitted MC, showed overall and recurrence-free survival rates comparable to those who were initially transplanted within MC and without LRT.
Conflict of interest
Authors have neither conflict of interest, nor financial issues to be disclosed.
References
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907-1917. doi: 10.1016/S0140-6736(03)14964-1
- Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1(1):2-14. doi: 10.1159/000339016
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485-1491. doi: 10.1200/JCO.2008.20.7753
- Patel SS, Arrington AK, McKenzie S, Mailey B, Ding M, Lee W, et al. Milan criteria and UCSF criteria: A preliminary comparative study of liver transplantation outcomes in the United States. Int J Hepatol. 2012;2012:253517. doi: 10.1155/2012/253517
- Mokhtar N, Gouda I, Adel I. Malignant digestive system tumors. In: Mokhtar N, Gouda I, Adel I, Eds. Cancer pathology registry, 2003-2004 and time trend analysis. Cairo, Egypt: Elsheraa Press; 2007. pp. 55-67.
- el-Zayadi AR, Badran HM, Barakat EM, Attia Mel D, Shawky S, Mohamed MK, et al. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol. 2005;11(33):5193-5198. doi: 10.3748/wjg.v11.i33.5193
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699. doi: 10.1056/NEJM199603143341104
- Martin AP, Bartels M, Hauss J, Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc. 2007;39(10):3169-3174. doi: 10.1016/j.transproceed.2007.04.025
- United Network for Organ Sharing, "Data reports", https://optn.transplant.hrsa.gov/data/view-data-reports/national-data. Accessed December 17, 2019.
- Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15(4):1001-1007. doi: 10.1245/s10434-007-9559-5
- Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587-2596. doi: 10.1111/j.1600-6143.2007.01965.x
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43.
doi: 10.1016/S1470-2045(08)70284-5 - Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33(5):1080-1086.
doi: 10.1053/jhep.2001.23561 - Marsh JW, Finkelstein SD, Demetris AJ, Swalsky PA, Sasatomi E, Bandos A, et al. Genotyping of hepatocellular carcinoma in liver transplant recipients adds predictive power for determining recurrence-free survival. Liver Transpl. 2003;9(7):664-671.
doi: 10.1053/jlts.2003.50144 - Abdelfattah MR, Elsiesy H, Al-Manea H, Broering DC. Liver transplantation for hepatocellular carcinoma within the Milan criteria versus the University of California San Francisco criteria: a comparative study. Eur J Gastroenterol Hepatol. 2018;30(4):398-403.
doi: 10.1097/MEG.0000000000001044 - Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8(9):765-774.
doi: 10.1053/jlts.2002.34892 - Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015;21(9):1142-1152. doi: 10.1002/lt.24169
- Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-1403. doi: 10.1053/jhep.2001.24563
- Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248(4):617-625.
doi: 10.1097/SLA.0b013e31818a07d4 - Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12(8):1260-1267. doi: 10.1002/lt.20837
- Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8(12):2547-2557.
doi: 10.1111/j.1600-6143.2008.02409.x - Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968-1977. doi: 10.1002/hep.27752
- Tohme S, Sukato D, Chen HW, Amesur N, Zajko AB, Humar A, et al. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol. 2013;24(11):1632-1638. doi: 10.1016/j.jvir.2013.07.026
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60.
doi: 10.1055/s-0030-1247132 - Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9(7):684-692. doi: 10.1053/jlts.2003.50147
- Yamashiki N, Gaynor JJ, Kato T, Reddy KR, Sobhonslidsuk A, Levi D, et al. Competing risks analysis of predictors of delisting owing to tumor progression in liver transplant candidates with hepatocellular carcinoma. Am J Transplant. 2004;4(5):774-781.
doi: 10.1111/j.1600-6143.2004.00412.x - Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35(3):421-430. doi: 10.1016/s0168-8278(01)00130-1
- Bruix J, Sherman M, Practice Guidelines Committee AASLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208-1236. doi: 10.1002/hep.20933
- Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115(3):616-623.
doi: 10.1002/cncr.24050
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "30" ["~SORT"]=> string(2) "30" ["CODE"]=> string(100) "effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech" ["~CODE"]=> string(100) "effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech" ["EXTERNAL_ID"]=> string(4) "2044" ["~EXTERNAL_ID"]=> string(4) "2044" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(375) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномойThe effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4244) "<p style="text-align: justify;">Наша цель состояла в том, чтобы оценить влияние циторедуктивной локорегиональной терапии (ЛРТ) на исход гепатоцеллюлярной карциномы (ГЦК), леченной посредством трансплантации печени (LTx), а также оценить отдаленные результаты LTx при ГЦК и факторы, влияющие на них.</p> <h3>Материалы и методы</h3> <p style="text-align: justify;">Наблюдали 115 взрослых пациентов, которым проведена LTx в качестве лечения ГЦК в период с августа 2006 г. по декабрь 2019 г. В зависимости от предтрансплантационной циторедуктивной ЛРТ, пациенты были разделены на две группы следующим образом: группа A – пациенты, соответствующие миланским критериям (MК) для LTx, которые не получали циторедуктивной ЛРТ перед LTx; группа В – пациенты с параметрами опухоли, выходящими за рамки МК, которым была проведена предварительная циторедуктивная ЛРТ.</p> <h3>Результаты</h3> <p style="text-align: justify;">При анализе по всей выборке общая выживаемость пациентов, трансплантата и безрецидивная выживаемость составили, соответственно, 79,7%, 90,4% и 88,2%. Параметры опухоли у 73 пациентов соответствовали миланским критериям (63,5% пациентов с трансплантированным ГЦК), в то время как у остальных 36,5% ГЦК превышала критерии МК (42 пациента). У пациентов после успешной циторедукции ГЦК до значений, соответствующих критериям MК, общая и безрецидивная выживаемость были сравнимы с пациентами, которым была проведена трансплантация LRT при соответствии миланским критериям. Рецидив ГЦК достоверно коррелировал с выявленной инвазией в лимфатические сосуды и низкой степенью дифференцировки опухоли. Более того, обе характеристики были существенно связаны с выживаемостью пациентов, и, наоборот, критерии трансплантации и объем опухоли >115 см3 не показали значимой связи с выживаемостью пациента или рецидивом опухоли.</p> <h3>Заключение</h3> <p style="text-align: justify;">Наши результаты подтверждают большую важность биологических критериев опухоли, чем общепринятые морфологические критерии. ЛРТ обеспечивает возможность снизить стадию ГЦК до значений, соответствующих миланским критериям, при одновременном выборе более биологически благоприятных вариантов опухолей.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация печени, гепатоцеллюлярная карцинома, локорегионарная терапия, Миланские критерии.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_META_TITLE"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_META_KEYWORDS"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_META_DESCRIPTION"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_PICTURE_FILE_ALT"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_PICTURE_FILE_TITLE"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(264) "Эффект от применения претрансплантационной локорегионарной терапии на исходы трансплантации печени у больных с гепатоцеллюлярной карциномой" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "effekt-ot-primeneniya-pretransplantatsionnoy-lokoregionarnoy-terapii-na-iskhody-transplantatsii-pech" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "201" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28445" ["VALUE"]=> array(2) { ["TEXT"]=> string(261) "<p>Мохамед Рабеи Абдельфаттах<sup>1</sup>, Мохамед А. Шараан<sup>1</sup>, Mохамед С. Камель<sup>1</sup>, Хусейн Эльсиеси<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(201) "
Мохамед Рабеи Абдельфаттах1, Мохамед А. Шараан1, Mохамед С. Камель1, Хусейн Эльсиеси2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28446" ["VALUE"]=> array(2) { ["TEXT"]=> string(486) "<p><sup>1</sup> Департамент хирургии, Университет Александрии, Факультет медицины, Александрия, Египет<br> <sup>2</sup> Центр трансплантации органов, Специализированный научный госпитальный Центр короля Фейсала, Эр-Рияд, Королевство Саудовская Аравия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(444) "1 Департамент хирургии, Университет Александрии, Факультет медицины, Александрия, Египет
2 Центр трансплантации органов, Специализированный научный госпитальный Центр короля Фейсала, Эр-Рияд, Королевство Саудовская Аравия
Наша цель состояла в том, чтобы оценить влияние циторедуктивной локорегиональной терапии (ЛРТ) на исход гепатоцеллюлярной карциномы (ГЦК), леченной посредством трансплантации печени (LTx), а также оценить отдаленные результаты LTx при ГЦК и факторы, влияющие на них.
Материалы и методы
Наблюдали 115 взрослых пациентов, которым проведена LTx в качестве лечения ГЦК в период с августа 2006 г. по декабрь 2019 г. В зависимости от предтрансплантационной циторедуктивной ЛРТ, пациенты были разделены на две группы следующим образом: группа A – пациенты, соответствующие миланским критериям (MК) для LTx, которые не получали циторедуктивной ЛРТ перед LTx; группа В – пациенты с параметрами опухоли, выходящими за рамки МК, которым была проведена предварительная циторедуктивная ЛРТ.
Результаты
При анализе по всей выборке общая выживаемость пациентов, трансплантата и безрецидивная выживаемость составили, соответственно, 79,7%, 90,4% и 88,2%. Параметры опухоли у 73 пациентов соответствовали миланским критериям (63,5% пациентов с трансплантированным ГЦК), в то время как у остальных 36,5% ГЦК превышала критерии МК (42 пациента). У пациентов после успешной циторедукции ГЦК до значений, соответствующих критериям MК, общая и безрецидивная выживаемость были сравнимы с пациентами, которым была проведена трансплантация LRT при соответствии миланским критериям. Рецидив ГЦК достоверно коррелировал с выявленной инвазией в лимфатические сосуды и низкой степенью дифференцировки опухоли. Более того, обе характеристики были существенно связаны с выживаемостью пациентов, и, наоборот, критерии трансплантации и объем опухоли >115 см3 не показали значимой связи с выживаемостью пациента или рецидивом опухоли.
Заключение
Наши результаты подтверждают большую важность биологических критериев опухоли, чем общепринятые морфологические критерии. ЛРТ обеспечивает возможность снизить стадию ГЦК до значений, соответствующих миланским критериям, при одновременном выборе более биологически благоприятных вариантов опухолей.
Ключевые слова
Трансплантация печени, гепатоцеллюлярная карцинома, локорегионарная терапия, Миланские критерии.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28448" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-46-53" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-46-53" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28449" ["VALUE"]=> array(2) { ["TEXT"]=> string(195) "<p>Mohamed Rabei Abdelfattah<sup>1</sup>, Mohamed A. Sharaan<sup>1</sup>, Mohamed S. Kamel<sup>1</sup>, Hussein Elsiesy<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(135) "Mohamed Rabei Abdelfattah1, Mohamed A. Sharaan1, Mohamed S. Kamel1, Hussein Elsiesy2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28450" ["VALUE"]=> array(2) { ["TEXT"]=> string(942) "<p><sup>1</sup> Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt<br> <sup>2</sup> Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia</p><br> <p><b>Correspondence:</b><br> Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544<br> Phone: 00201023061111 (mob.)<br> E-mail: Mohamad.rabie@gmail.com</p><br> <p><b>Citation:</b> Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(822) "1 Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt
2 Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544
Phone: 00201023061111 (mob.)
E-mail: Mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28451" ["VALUE"]=> array(2) { ["TEXT"]=> string(2223) "<p style="text-align: justify;">Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.</p> <h3>Materials and methods</h3> <p style="text-align: justify;">115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.</p> <h3>Results</h3> <p style="text-align: justify;">Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm<sup>3</sup> did not show a significant relation to patient survival or tumor recurrence.</p> <h3>Conclusion</h3> <p style="text-align: justify;">Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors. </p> <h2>Keywords</h2> <p style="text-align: justify;">Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2050) "Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.
Materials and methods
115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.
Results
Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm3 did not show a significant relation to patient survival or tumor recurrence.
Conclusion
Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors.
Keywords
Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28452" ["VALUE"]=> string(111) "The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(111) "The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28453" ["VALUE"]=> string(4) "2725" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2725" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28454" ["VALUE"]=> string(4) "2726" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2726" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28449" ["VALUE"]=> array(2) { ["TEXT"]=> string(195) "<p>Mohamed Rabei Abdelfattah<sup>1</sup>, Mohamed A. Sharaan<sup>1</sup>, Mohamed S. Kamel<sup>1</sup>, Hussein Elsiesy<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(135) "Mohamed Rabei Abdelfattah1, Mohamed A. Sharaan1, Mohamed S. Kamel1, Hussein Elsiesy2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(135) "Mohamed Rabei Abdelfattah1, Mohamed A. Sharaan1, Mohamed S. Kamel1, Hussein Elsiesy2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28451" ["VALUE"]=> array(2) { ["TEXT"]=> string(2223) "<p style="text-align: justify;">Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.</p> <h3>Materials and methods</h3> <p style="text-align: justify;">115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.</p> <h3>Results</h3> <p style="text-align: justify;">Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm<sup>3</sup> did not show a significant relation to patient survival or tumor recurrence.</p> <h3>Conclusion</h3> <p style="text-align: justify;">Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors. </p> <h2>Keywords</h2> <p style="text-align: justify;">Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2050) "Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.
Materials and methods
115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.
Results
Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm3 did not show a significant relation to patient survival or tumor recurrence.
Conclusion
Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors.
Keywords
Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2050) "Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.
Materials and methods
115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.
Results
Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm3 did not show a significant relation to patient survival or tumor recurrence.
Conclusion
Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors.
Keywords
Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28448" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-46-53" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-46-53" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-46-53" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28452" ["VALUE"]=> string(111) "The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(111) "The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(111) "The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28450" ["VALUE"]=> array(2) { ["TEXT"]=> string(942) "<p><sup>1</sup> Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt<br> <sup>2</sup> Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia</p><br> <p><b>Correspondence:</b><br> Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544<br> Phone: 00201023061111 (mob.)<br> E-mail: Mohamad.rabie@gmail.com</p><br> <p><b>Citation:</b> Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(822) "1 Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt
2 Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544
Phone: 00201023061111 (mob.)
E-mail: Mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(822) "1 Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt
2 Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544
Phone: 00201023061111 (mob.)
E-mail: Mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28445" ["VALUE"]=> array(2) { ["TEXT"]=> string(261) "<p>Мохамед Рабеи Абдельфаттах<sup>1</sup>, Мохамед А. Шараан<sup>1</sup>, Mохамед С. Камель<sup>1</sup>, Хусейн Эльсиеси<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(201) "Мохамед Рабеи Абдельфаттах1, Мохамед А. Шараан1, Mохамед С. Камель1, Хусейн Эльсиеси2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(201) "Мохамед Рабеи Абдельфаттах1, Мохамед А. Шараан1, Mохамед С. Камель1, Хусейн Эльсиеси2
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28447" ["VALUE"]=> array(2) { ["TEXT"]=> string(4244) "<p style="text-align: justify;">Наша цель состояла в том, чтобы оценить влияние циторедуктивной локорегиональной терапии (ЛРТ) на исход гепатоцеллюлярной карциномы (ГЦК), леченной посредством трансплантации печени (LTx), а также оценить отдаленные результаты LTx при ГЦК и факторы, влияющие на них.</p> <h3>Материалы и методы</h3> <p style="text-align: justify;">Наблюдали 115 взрослых пациентов, которым проведена LTx в качестве лечения ГЦК в период с августа 2006 г. по декабрь 2019 г. В зависимости от предтрансплантационной циторедуктивной ЛРТ, пациенты были разделены на две группы следующим образом: группа A – пациенты, соответствующие миланским критериям (MК) для LTx, которые не получали циторедуктивной ЛРТ перед LTx; группа В – пациенты с параметрами опухоли, выходящими за рамки МК, которым была проведена предварительная циторедуктивная ЛРТ.</p> <h3>Результаты</h3> <p style="text-align: justify;">При анализе по всей выборке общая выживаемость пациентов, трансплантата и безрецидивная выживаемость составили, соответственно, 79,7%, 90,4% и 88,2%. Параметры опухоли у 73 пациентов соответствовали миланским критериям (63,5% пациентов с трансплантированным ГЦК), в то время как у остальных 36,5% ГЦК превышала критерии МК (42 пациента). У пациентов после успешной циторедукции ГЦК до значений, соответствующих критериям MК, общая и безрецидивная выживаемость были сравнимы с пациентами, которым была проведена трансплантация LRT при соответствии миланским критериям. Рецидив ГЦК достоверно коррелировал с выявленной инвазией в лимфатические сосуды и низкой степенью дифференцировки опухоли. Более того, обе характеристики были существенно связаны с выживаемостью пациентов, и, наоборот, критерии трансплантации и объем опухоли >115 см3 не показали значимой связи с выживаемостью пациента или рецидивом опухоли.</p> <h3>Заключение</h3> <p style="text-align: justify;">Наши результаты подтверждают большую важность биологических критериев опухоли, чем общепринятые морфологические критерии. ЛРТ обеспечивает возможность снизить стадию ГЦК до значений, соответствующих миланским критериям, при одновременном выборе более биологически благоприятных вариантов опухолей.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация печени, гепатоцеллюлярная карцинома, локорегионарная терапия, Миланские критерии.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4083) "Наша цель состояла в том, чтобы оценить влияние циторедуктивной локорегиональной терапии (ЛРТ) на исход гепатоцеллюлярной карциномы (ГЦК), леченной посредством трансплантации печени (LTx), а также оценить отдаленные результаты LTx при ГЦК и факторы, влияющие на них.
Материалы и методы
Наблюдали 115 взрослых пациентов, которым проведена LTx в качестве лечения ГЦК в период с августа 2006 г. по декабрь 2019 г. В зависимости от предтрансплантационной циторедуктивной ЛРТ, пациенты были разделены на две группы следующим образом: группа A – пациенты, соответствующие миланским критериям (MК) для LTx, которые не получали циторедуктивной ЛРТ перед LTx; группа В – пациенты с параметрами опухоли, выходящими за рамки МК, которым была проведена предварительная циторедуктивная ЛРТ.
Результаты
При анализе по всей выборке общая выживаемость пациентов, трансплантата и безрецидивная выживаемость составили, соответственно, 79,7%, 90,4% и 88,2%. Параметры опухоли у 73 пациентов соответствовали миланским критериям (63,5% пациентов с трансплантированным ГЦК), в то время как у остальных 36,5% ГЦК превышала критерии МК (42 пациента). У пациентов после успешной циторедукции ГЦК до значений, соответствующих критериям MК, общая и безрецидивная выживаемость были сравнимы с пациентами, которым была проведена трансплантация LRT при соответствии миланским критериям. Рецидив ГЦК достоверно коррелировал с выявленной инвазией в лимфатические сосуды и низкой степенью дифференцировки опухоли. Более того, обе характеристики были существенно связаны с выживаемостью пациентов, и, наоборот, критерии трансплантации и объем опухоли >115 см3 не показали значимой связи с выживаемостью пациента или рецидивом опухоли.
Заключение
Наши результаты подтверждают большую важность биологических критериев опухоли, чем общепринятые морфологические критерии. ЛРТ обеспечивает возможность снизить стадию ГЦК до значений, соответствующих миланским критериям, при одновременном выборе более биологически благоприятных вариантов опухолей.
Ключевые слова
Трансплантация печени, гепатоцеллюлярная карцинома, локорегионарная терапия, Миланские критерии.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4083) "Наша цель состояла в том, чтобы оценить влияние циторедуктивной локорегиональной терапии (ЛРТ) на исход гепатоцеллюлярной карциномы (ГЦК), леченной посредством трансплантации печени (LTx), а также оценить отдаленные результаты LTx при ГЦК и факторы, влияющие на них.
Материалы и методы
Наблюдали 115 взрослых пациентов, которым проведена LTx в качестве лечения ГЦК в период с августа 2006 г. по декабрь 2019 г. В зависимости от предтрансплантационной циторедуктивной ЛРТ, пациенты были разделены на две группы следующим образом: группа A – пациенты, соответствующие миланским критериям (MК) для LTx, которые не получали циторедуктивной ЛРТ перед LTx; группа В – пациенты с параметрами опухоли, выходящими за рамки МК, которым была проведена предварительная циторедуктивная ЛРТ.
Результаты
При анализе по всей выборке общая выживаемость пациентов, трансплантата и безрецидивная выживаемость составили, соответственно, 79,7%, 90,4% и 88,2%. Параметры опухоли у 73 пациентов соответствовали миланским критериям (63,5% пациентов с трансплантированным ГЦК), в то время как у остальных 36,5% ГЦК превышала критерии МК (42 пациента). У пациентов после успешной циторедукции ГЦК до значений, соответствующих критериям MК, общая и безрецидивная выживаемость были сравнимы с пациентами, которым была проведена трансплантация LRT при соответствии миланским критериям. Рецидив ГЦК достоверно коррелировал с выявленной инвазией в лимфатические сосуды и низкой степенью дифференцировки опухоли. Более того, обе характеристики были существенно связаны с выживаемостью пациентов, и, наоборот, критерии трансплантации и объем опухоли >115 см3 не показали значимой связи с выживаемостью пациента или рецидивом опухоли.
Заключение
Наши результаты подтверждают большую важность биологических критериев опухоли, чем общепринятые морфологические критерии. ЛРТ обеспечивает возможность снизить стадию ГЦК до значений, соответствующих миланским критериям, при одновременном выборе более биологически благоприятных вариантов опухолей.
Ключевые слова
Трансплантация печени, гепатоцеллюлярная карцинома, локорегионарная терапия, Миланские критерии.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28446" ["VALUE"]=> array(2) { ["TEXT"]=> string(486) "<p><sup>1</sup> Департамент хирургии, Университет Александрии, Факультет медицины, Александрия, Египет<br> <sup>2</sup> Центр трансплантации органов, Специализированный научный госпитальный Центр короля Фейсала, Эр-Рияд, Королевство Саудовская Аравия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(444) "1 Департамент хирургии, Университет Александрии, Факультет медицины, Александрия, Египет
2 Центр трансплантации органов, Специализированный научный госпитальный Центр короля Фейсала, Эр-Рияд, Королевство Саудовская Аравия
1 Департамент хирургии, Университет Александрии, Факультет медицины, Александрия, Египет
2 Центр трансплантации органов, Специализированный научный госпитальный Центр короля Фейсала, Эр-Рияд, Королевство Саудовская Аравия
Introduction
PTCL is a rare neoplasm in children and there are no standards and decisively effective approaches in the therapy. A number of subtypes with specific morphologic and clinical features comprise the diversity of this entity. Survival in children with PTCL is lower compared to other types of pediatric non-Hodgkin lymphoma (NHL) and is approximately 60%. Patients with non-anaplastic PTCL-not otherwise specified have higher chance of cure compared to other subtypes. According to World Health Organization, more than 20 subtypes of PTCL are recognized [1, 2]. PTCL is a diagnostic challenge with common mistakes occurring at initial diagnosis. Among 69 patients in Berlin-Frankfurt-Munster (BFM) database, the diagnosis was confirmed after reference only in 38 children (55%) [3]. Recent classification is based on adult data, and there are some concerns if it can be completely extrapolated in pediatric cohort. Distribution of PTCL subtypes differs with age, and there are often pre-existing conditions in 25% of pediatric patients, e.g., primary immunodeficiency, prior chemotherapy, etc. [4].
Natural killer (NK) cell lymphoblastic lymphoma/leukemia is uncommon pathology with only case reports published [5]. Its rarity prevents investigation of tumor biology and optimal treatment approaches. To establish this diagnosis, a combination of CD56 and immature T cell-associated markers should be present on the lymphoblasts. Misdiagnosis is a common situation with common discrepancies among pathologists on exact final diagnosis. Prognosis is dismal in these patients. Prior to WHO 2008 classification, the NK cell lymphoblastic lymphoma/leukemia was attributed to PTCL [6]. Nowadays, the disease is related to ambiguous leukemia [7].
The present case report illustrates 7-year-old child with unclassified NK/T cell lymphoma with blast morphology. The closest clinical entity in WHO classification is NK/T cell lymphoma, and it should be attributed to PTCL [8]. At the same time, blast morphology and clinical presentation share features of lymphoblastic lymphoma.

Figure 1. Histology of testicular bioptate (H&E staining)
Case description
Six-year-old otherwise healthy boy presented with febrile temperature, generalized macular/papular rash, hepatosplenomegaly and facial swelling. The onset of disease was sudden (15.01.2017). Initially, the patient was diagnosed with hemophagocytic lymphohistiocytosis, due to fever, progressive hepatosplenomegaly and increased ferritin level (535 ng/ml). The disease rapidly progressed, and pneumonia with pleuritis developed in several days, thus requiring admission to intensive care unit. Specific therapy with steroids, etoposide, intravenous immunoglobulins and cyclosporine A was started with transient response and further progression with enlargement of left testis, which was biopsied on March 29, 2017. Initial histological analysis in local lab demonstrated extranodal NK/T cell lymphoma, nasal type. Later on, the histology was revised in RM Gorbacheva Research Institute, Pavlov University (St. Petersburg), and the diagnosis was switched to NK/T lymphoma with blast morphology. The revised histology revealed overgrowth of lymphoid small- and middle-sized cells with narrow cytoplasmic area. The cells contained irregular and roundish nuclei with finely dispersed chromatin. Some nuclei contain solitary tiny nucleoli (Fig. 1).
Immunohistochemical study showed intensive expression of CD2, CD3, CD5, CD7, CD56, granzyme B, and co-expression of CD4 and CD8. Most cells were slightly CD33-positive. There was no detectable expression of CD30, CD57, CD99, TdT, CD1a, CD10. In situ hybridization failed to demonstrate presence of Epstein-Barr virus. Index of proliferative activity (Ki-67) was elevated (70-80%). PDL1 was not expressed on tumor cells but was clearly detectable on tumor microenvironment cells (macrophages) (Fig. 2 A-I). On the basis of these pathological findings, the pathologist concluded that the tumors with such immunophenotype were not previously described under known classifications. Extranodal NK/T cell lymphoma, nasal type, and blastic plasmacytoid dendritic cell neoplasm were excluded. This is an unclassified lymphoma with the features of peripheral T cell and lymphoblastic lymphoma (Fig. 2). It is typical for NK-cells to express CD2, CD16 and CD56. Presence of these molecules on tumor cells together with the absence of clonal receptor gene rearrangements are characteristic for NK-cell lymphomas [8].

Figure 2. Immunohistochemistry pattern of the unclassified NK/T cell lymphoma (testicular biopsy). Staining with antibodies for the following antigens: Ki-67 (A), CD3 (B); CD4 (C); CD5 (D); CD8 (E); CD33 (F); CD56 (G); Granzyme B(H), PDL1 (I)
Molecular biological analysis revealed mutation in enhancer of Zeste homolog 2 (EZH2) gene (p.Arg288Gln) in 5% of tumor cells.
Polychemotherapy according to SMILE protocol was started on 13 April 2017. Treatment schedule consisted of dexamethasone 20 mg/m2 №4, methotrexate 2000 mg/m2 №1, ifosfamide 1500 mg №3, L-asparaginase 6000 mg/m2 №7, and etoposide 100 mg/m2 №3. Due to presence of effusions and risk of unacceptable toxicity, methotrexate was skipped in the first cycle. Other drugs, doses and timing were applied according to standard protocol [9]. In general, the patient received 4 courses of SMILE (13.04.17-2.08.17). Aggressive progression after the fourth cycle of SMILE with involvement of liver, left testis and CNS was registered (15 August 2017). Active disease was confirmed by presence of tumor cells in the cytology specimens of left testis and cerebrospinal fluid. Bone marrow was not affected. Multiple rash elements that were present upon progression did not exhibit tumor biology, and were associated with paraneoplastic process, as revealed by biopsy.

Figure 3. Immunophenotyping of cerebrospinal fluid (CD2, CD3, CD4, CD8, CD45, CD56)

Figure 4. Acute graft-host-disease after allo-HSCT
The second-line induction therapy consisted of FLAG (fludarabine 30 mg/m2 №5, cytarabine 4000 mg/m2 №5, granulocyte colony-stimulating factor) and ICI (nivolumab 40 mg on Days 1, 14, 28). This strategy resulted into the first durable complete remission. Irradiation of testis, or orchidectomy was not performed due to refusal of parents. Further treatment consisted of nivolumab (3 mg/kg №9 biweekly, weekly gemcitabine 1000 mg/m2 №6, and intrathecal therapy (cytarabine 30 mg, metotrexate 12 mg, prednisolone 10 mg) №6. Autologous HSCT was performed on 15 February 2018 with BeEAM (bendamustine 160 mg/m2 on Days -7 and -6, etoposide 200 mg/m2 and cytarabine 400 mg/m2 on Days -5 to -2 and melphalan 140 mg/m2 on Day -1) as a conditioning regimen administered for remission consolidation. Afterwards, the therapy was stopped, hoping for curation. Unfortunately, 2 months after autologous HSCT, the patient developed facial asymmetry, and cerebrospinal fluid aspirate showed presence of tumor cells upon immunophenotyping (Fig. 3).
There were no structural abnormalities on magnetic resonance imaging. Third-line therapy consisted of single dose intravenous cytarabine 1000 mg/m2, biweekly intrathecal therapy (cytarabine 30 mg, metotrexate 12 mg, prednisolone 10 mg) №8 and biweekly nivolumab 40 mg №9. Second complete remission was achieved during the first week of treatment. To consolidate second remission, first haploidentical HSCT (from father) was performed (25 September 2018). Non-myeloablative conditioning (busulfan 8 mg/kg and fludarabine 150 mg/m2) was used prior to transplantation. Graft-versus-host disease (GVHD) prophylaxis was based on posttransplant cyclophosphamide (100 mg/kg), sirolimus and tacrolimus. Bone marrow was used as a transplant source, with total number of 8×106 CD34+ cells/kg. There were no signs of peripheral blood recovery, and primary non-engraftment occurred (Day+30). The patient received second haploidentical HSCT on 14 October 2018 with switching to other donor and transplant source (unmanipulated peripheral blood stem cells from mother). A total of 10×106 CD34+ cells/kg were infused. Reduced-intensity conditioning regimen consisted of fludarabine 150 mg/m2 and treosulfan 36 mg/m2.
Posttransplant cyclophosphamide (100 mg/m2) and sirolimus were used for GVHD prophylaxis. Engraftment was diagnosed on Day +19. Transplantation was complicated by stage IV steroid-refractory acute GVHD of skin (Day+25) that responded to ruxolitinib (Fig. 4).
The patient received monthly intrathecal therapy №6 after transplantation for the prophylaxis of CNS relapse. At the present time, he remains in complete remission without signs of GVHD and treatment-free for 3 years. There is a slight decrease in intellectual abilities that necessitated individualized learning program at school. This complication is probably associated with previous CNS affection by lymphoma and related treatment [10].
Discussion
Data on NK cell lymphoblastic leukemia/lymphoma is limited to case reports. PTCL is more common nosology, but non-anaplastic PTCL is also a rare entity in children [2, 11]. This case report represents rare unclassified NK/T lymphoma in a child. According to opinion of pathologists, this type of lymphoma was not previously described in WHO classification, sharing features of both PTCL and lymphoblastic lymphoma. This fact underlines the existence of lymphoma subtypes that do not match established classification variants. Regular revision of lymphoma classifications is partially explained by the discovery of new features or detailing of established lymphoma subtypes. An EZH2 gene mutation typical to PTCL was found in the minority of tumor cells (5%) of our patient [12]. It could be attributed to tumor heterogeneity and presence of subclone harboring the EZH2 mutation. Epstein-Barr virus (EBV) was not present in the tumor specimen. However, EBV plays an important role in the pathogenesis of many PTCL subtypes. Most extranodal NK/T lymphomas carry type A EBV. Despite certain role of EBV in NK/T cell lymphomas, exact mechanism of viral cancerogenesis is unclear in this setting [12]. Prevalence of NK/T cell lymphomas in distinct geographical regions despite worldwide EBV distribution suggests importance of ethnic genetic profile for NK/T lymphoma pathogenesis [8]. Initially, our patient was diagnosed with hemophagocytic lymphohistiocytosis (HLH) and started appropriate therapy. The diagnosis was not confirmed later since the patient did not fit all necessary HLH criteria (only 3 criteria of 5 required) [13]. Anyway, to our opinion, the initial therapy played an important role in patient rescue in the circumstances of limited time and progressive deterioration, because treatment with steroids and etoposide transiently stabilized clinical situation and gained time for correct diagnosis. Biopsy of enlarged testis became a game changer that enabled true PTCL diagnostics and corrected the subsequent therapy. Natural killer cells emerge from bone marrow and further migrate to nodal and extranodal sites. This fact can partially explain common extranodal localization of NK cell lymphomas [14]. According to Murphy classification, stage IV was diagnosed in our patient due to CNS involvement. Staging of non-Hodgkin lymphoma in adults is carried out according to Ann Arbor classification, which was primary used for Hodgkin lymphoma. In children, the modified Murphy classification is used, which was initially developed for the Burkitt and lymphoblastic lymphomas. Unlike Ann Arbor system, the Murphy classification assigns all intrathoracic and most intra-abdominal tumors to stage III, whereas only bone marrow and central nervous system lesions are classified as stage IV [15]. According to previously published data, most children with PTCL demonstrate advanced disease at the onset expect extranodal NK/T cell lymphoma [4].
Patient failed first-line therapy of lymphoma. He could be primary refractory to the therapy, or previous treatment of HLH interfered in natural course. Another reason of chemoresistance could be application of the SMILE chemotherapy protocol designed specifically for extranodal NK/T lymphoma, since this type of PTCL was initially diagnosed. There is no established treatment for non-anaplastic PTCL in children. Various treatment protocols, such as anaplastic large-cell lymphoma therapy or acute leukemia protocols are used with comparable efficiency [3, 16]. The treatment results vary between PTCL subgroups, indicating that a subtype-specific approach should be pursued. Except for subcutaneous panniculitis-like T-cell lymphoma, survival rates are poor and the optimal treatment strategy has not been defined yet. International registry with treatment recommendations is needed to improve outcomes for this rare and heterogeneous group of diseases. Results in adult PTCL after CHOP or other treatment approaches are disappointing [17, 18]. Difficulties in PTCL management are associated with unique tumor biology and rarity of the disease that prevents multicentre randomized clinical trials, especially in pediatric population. Prognosis in R-R non-anaplastic PTCL is dismal, with median OS of only 5.8 months. Our patient demonstrated progression of the disease after first-line therapy, and the panel of doctors decided to start FLAG protocol combined with nivolumab. This was a non-standard recommendation, since the second-line therapies in PTCL usually include platinum and/or gemcitabine [19]. Our choice was based on leukemia-like clinical features (involvement of CNS and testis), blast morphology of tumor cells and presence of PDL1 expression on tumor microenvironment. There are no data on combination of nivolumab with FLAG, but combination of nivolumab with cytarabine or fludarabine is feasible according to recently published data [20, 21]. Extranodal NK-cell lymphomas are more common in patients with definite HLA haplotypes while rarely occur in patients with other haplotypes suggesting that immune surveillance is important for this tumor. Thus, one may hypothesize on immunotherapy as a potentially effective approach in this clinical setting [22]. Informed consent was obtained from relatives of the patient prior to starting the therapy. This treatment plan resulted in first continuous complete remission. Further treatment was based on systemic immunochemotherapy (nivolumab and gemcitabine) combined with local tumor control (intrathecal therapy). The rationale for this approach was presence of PDL1 on tumor microenvironment, and published data on possible effectiveness of ICI in pediatric PTCL [24]. First remission was consolidated with autologous HSCT according to established recommendations for refractory NHL in children [25]. Allogeneic HSCT was reserved for further treatment if required. Previous therapy with nivolumab and, therefore, increased risk of severe GVHD was an important counter-argument for the ongoing allogeneic HSCT [26]. The role of allogeneic transplant in the management of pediatric R-R PTCL patients remains unclear. According to results obtained in adults, allo-HSCT may be beneficial in PTCL, due to graft-versus-tumor effect [27]. Allo-HSCT may be similarly effective as consolidation after first or second remission, being not a good option at progression [28]. Data in children with PTCL and HSCT are limited to sporadic reports and are not decisive [4]. Due to early relapse of lymphoma after autologous HSCT in our patient, the re-treatment with nivolumab and intrathecal therapy was initiated which resulted into second complete remission. There were no doubts that this was the optimal point for consolidation of second remission with allo-HSCT. Moreover, due to aggressive and unpredictable disease course there was no benefit for searching a matched unrelated donor. For the first haploidentical HSCT, a non-myeloablative conditioning regimen was chosen based on previous autologous HSCT. Unfortunately, this approach led to primary non-engraftment, probably, due to residual recipient cells that promoted the graft rejection. Risks of non-engraftment with standard transplant approaches with haploidentical stem cell source are well known and can be ameliorated with higher number of infused stem cells, more intense conditioning regimens and additional techniques of transplant processing [29, 30]. To enable engraftment, the second haploidentical HSCT was performed with more intense conditioning regimen and peripheral blood stem cells as transplant source. This procedure resulted into engraftment, but early posttransplant period was complicated with severe steroid-refractory cutaneous acute GVHD that responded to ruxolitinib. Further on, the patient underwent prophylactic intrathecal therapy due to high risk of CNS relapse and low effectiveness of graft-versus-tumor effect in the controlling barrier areas [31].
The limitation of this case report is absence of survey for underlying primary immunodeficiency syndrome, because many children with PTCL have some pre-existing disorders affecting immune system.
The present report demonstrates a case of rare unclassified NK/T cell lymphoma that was successfully treated with chemoimmunotherapy and repeated transplantations. No doubt, this tumor was sensitive to immunotherapy. Hence, the immune checkpoint inhibitors followed by allogeneic transplantation may be effective in therapy of pediatric PTCL.
Conflict of interest
None declared.
References
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H , Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. doi: 10.1182/blood-2016-01-643569
- Maciejka-Kemblowska L, Chaber R, Wrobel G, Maldyk J, Kozlowska M, Kulej D, et al. Clinical features and treatment outcomes of peripheral T-cell lymphoma in children. A current data report from Polish Pediatric Leukemia/Lymphoma Study Group (PPLLSG). Adv Med Sci. 2016; 61(2):311-316. doi: 10.1016/j.advms.2016.03.002
- Kontny U, Oschlies I, Woessmann W, Burkhardt B, Lisfeld J, Salzburg J et al. Non-anaplastic peripheral T-cell lymphoma in children and adolescents – a retrospective analysis of the NHL-BFM study group. Br J Haematol. 2015; 168(6):835-844. doi: 10.1111/bjh.13216
- Mellgren, K, Attarbaschi A, Abla, O, Alexander S, Bomken S, Bubanska E, et al. Non-anaplastic peripheral T cell lymphoma in children and adolescents – an international review of 143 cases. Ann Hematol. 2016; 95:1295-1305. doi: 10.1007/s00277-016-2722-y
- Jain S, Kumar R, Purohit A, Pati HP. Precursor NK cell lymphoblastic leukemia/lymphoma – report of a case with literature review. Ind J Hematol Blood Transfus. 2014; 30 (Suppl 1):283-285. doi: 10.1007/s12288-014-0360-x
- Frenkel’ MA, Baranova O Yu, Antipova AS, Kupryshina NA, Tupitsyn NN. NK-cell lymphoblastic leukemia/lymphoma (literature review and authors’ experience). Clinical Oncohematology. 2016;9(2):208-217 (In Russian). doi: 10.21320/2500-2139-2016-9-2-208-217
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi: 10.1182/blood-2016-03-643544
- Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol 10, 85 (2017).
doi: 10.1186/s13045-017-0452-9 - Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study.
J ClinOncol. 2011; 29(33):4410-4416. doi: 10.1200/JCO.2011.35.6287 - Turhan AB, Tülin Fidan S, Yarar C, NazlıSakallı E, Özdemir ZC, Bör Ö. Neurocognitive consequences of childhood leukemia and its treatment. Ind J Hematol Blood Transfus. 2018;34(1):62-69. doi: 10.1007/s12288-017-0846-4
- Al Mahmoud R, Weitzman S, Schechter T, Ngan B, Abdelhaleem M, Alexander S. Peripheral T-cell lymphoma in children and adolescents: a single-institution experience. J Pediatr Hematol Oncol. 2012; 34(8):611-616. doi: 10.1097/MPH.0b013e3182707592
- Zhang H, Lv H, Jia X, Hu G, Kong L, Zhang T, et al. Clinical significance of enhancer of zeste homolog 2 and histone deacetylases 1 and 2 expression in peripheral T-cell lymphoma. Oncol Lett. 2019;18(2):1415-1423. doi: 10.3892/ol.2019.10410
- Grzybowski B, Vishwanath VA. Hemophagocytic lymphohistiocytosis: A diagnostic conundrum. J Pediatr Neurosci. 2017;12(1):55-60. doi: 10.4103/jpn.JPN_140_16
- Bekiaris V, Lane PJL. The localization and migration of natural killer cells in health and disease. Natural Killer Cells. 2010; 137-153.
doi: 10.1016/B978-0-12-370454-2.00010-7 - Rosolen A, Perkins SL, Pinkerton CR, Guillerman GR, Sandlund JT, Reiter A, et al. Revised international pediatric non-Hodgkin lymphoma staging system. J Clin Oncol. 2015; 33 (18):2112-2118. doi: 10.1200/JCO.2014.59.7203
- Windsor R, Stiller C, Webb D. Peripheral T-cell lymphoma in childhood: population-based experience in the United Kingdom over 20 years. Pediatr Blood Cancer. 2008; 50(4): 784-787. doi: 10.1002/pbc.21293. PMID: 18022899
- Ma H, Bin Cheng, O'Connor OA. Survival outcomes of patients with peripheral T-cell lymphomas (PTCL) treated with chemotherapy and/or novel agents: The Columbia University experience. J Clin Oncol. 2019; 37:15 (Suppl), e19049-e19049.
doi: 10.1200/JCO.2019.37.15_suppl.e19049 - Lepik EE, Kozlov AV, Borzenkova ES, Zalyalov YR , Lepik KV, Kondakova EV, et al. Treatment options for T-cell lymphomas: a single-center study. Cell Ther Transplant. 2020; 9(1): 28-37. doi: 10.18620/ctt-1866-8836-2020-9-1-28-37
- Foster C, Kuruvilla J. Treatment approaches in relapsed or refractory peripheral T-cell lymphomas. F1000Res. 2020;9:F1000 Faculty Rev-1091. Published 2020 Sep 4. doi: 10.12688/f1000research.22257.1
- Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study. Lancet Haematol. 2019; 6(9):e480-e488. doi: 10.1016/S2352-3026(19)30114-0
- Creelan, BC, Wang, C, Teer, JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021; 27: 1410-1418. doi: 10.1038/s41591-021-01462-y
- Kanno H, Kojya S, Li T, Ohsawa M, Nakatsuka S, Miyaguchi M, et al. Low frequency of HLA-A*0201 allele in patients with Epstein-Barr virus-positive nasal lymphomas with polymorphic reticulosis morphology. Int J Cancer. 2000; 87(2):195-199.
doi: 10.1002/1097-0215(20000715)87:2<195::AID-IJC6>3.0.CO;2-0 - Sedick Q, Alotaibi S, Alshieban S, Naheet KB, Elyamany G. Natural killer cell lymphoblastic leukaemia/lymphoma: case report and review of the recent literature. Case Rep Oncol. 2017; 10(2): 588-595. doi: 10.1159/000477843
- Kozlov AV, Kazantsev IV, Yukhta TV, Tolkunova PS, Gevorgyan AG, Lepik KV, et al. The use of checkpoint inhibitors in children with non-Hodgkin lymphomas. Pediatric Hematology/Oncology and Immunopathology. 2020; 19(2):112-120. (In Russian).
doi: 10.24287/1726-1708-2020-19-2-112-120 - Afanasyev BV, Afanasyeva KS, Barabanshchikova MV, Bondarenko SN, Bykova TA, Vlasova JYu, Gevorgian AG, et al. Indications for hematopoietic stem cell transplantation. December 2019. Cell Ther Transplant. 2019; 8(4):101-145 (In Russian).
doi: 10.18620/ctt-1866-8836-2019-8-4-101-145 - Ijaz A, Khan AY, Malik SU, Faridi W, Fraz MA, Usman M, et al. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transplant. 2019; 25(1): 94-99.
doi: 10.1016/j.bbmt.2018.08.028 - Castagna L, Pagliardini T, Bramanti S, Schiano JM, de Oca CM, Bouabdallah R, et al. Allogeneic stem cell transplantation in poor prognosis peripheral T-cell lymphoma: the impact of different donor type on outcome. Bone Marrow Transplant. 2021; 56: 883-889. doi: 10.1038/s41409-020-01133-5
- Mamez AC, Dupont A, Blaise D, Chevallier P, Forcade E, Ceballos P, et al. Allogeneic stem cell transplantation for peripheral T cell lymphomas: a retrospective study in 285 patients from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). J Hematol Oncol. 2020; 13(1):56. Published 2020 May 19. doi: 10.1186/s13045-020-00892-4
- Motorin DV, Badaev RS, Babenetskaya DV, Ilyina NA, Silina TO, Baratashvili GG, et al. Haploidentical Hematopoietic Stem Cell Transplantation (Haplo-SCT) with Post-Transplant Cyclophosphamide for haematological malignancies: a single centre experience. Cell Ther Transplant. 2016; 5(3):51-53. doi: 10.18620/ctt-1866-8836-2016-5-3-51-53
- Maschan MA. Depletion of alpha/beta-T-cells is a robust platform for haploidentical hematopoietic stem cell transplantation results improvement. Russian Journal of Pediatric Hematology and Oncology. 2015; 2(3):34-38. (In Russian).
doi: 10.17650/2311-1267-2015-2-3-34-38 - Ostronoff F, Ostronoff M, Fernandes HS, Buessio R, Soriano S, Souto-Maior AS, et al. Evidence for a graft-versus-leukemia effect in the central nervous system. Leukemia & Lymphoma. 2008; 49(2): 365-369. doi: 10.1080/10428190701784417
" ["~DETAIL_TEXT"]=> string(30020) "
Introduction
PTCL is a rare neoplasm in children and there are no standards and decisively effective approaches in the therapy. A number of subtypes with specific morphologic and clinical features comprise the diversity of this entity. Survival in children with PTCL is lower compared to other types of pediatric non-Hodgkin lymphoma (NHL) and is approximately 60%. Patients with non-anaplastic PTCL-not otherwise specified have higher chance of cure compared to other subtypes. According to World Health Organization, more than 20 subtypes of PTCL are recognized [1, 2]. PTCL is a diagnostic challenge with common mistakes occurring at initial diagnosis. Among 69 patients in Berlin-Frankfurt-Munster (BFM) database, the diagnosis was confirmed after reference only in 38 children (55%) [3]. Recent classification is based on adult data, and there are some concerns if it can be completely extrapolated in pediatric cohort. Distribution of PTCL subtypes differs with age, and there are often pre-existing conditions in 25% of pediatric patients, e.g., primary immunodeficiency, prior chemotherapy, etc. [4].
Natural killer (NK) cell lymphoblastic lymphoma/leukemia is uncommon pathology with only case reports published [5]. Its rarity prevents investigation of tumor biology and optimal treatment approaches. To establish this diagnosis, a combination of CD56 and immature T cell-associated markers should be present on the lymphoblasts. Misdiagnosis is a common situation with common discrepancies among pathologists on exact final diagnosis. Prognosis is dismal in these patients. Prior to WHO 2008 classification, the NK cell lymphoblastic lymphoma/leukemia was attributed to PTCL [6]. Nowadays, the disease is related to ambiguous leukemia [7].
The present case report illustrates 7-year-old child with unclassified NK/T cell lymphoma with blast morphology. The closest clinical entity in WHO classification is NK/T cell lymphoma, and it should be attributed to PTCL [8]. At the same time, blast morphology and clinical presentation share features of lymphoblastic lymphoma.

Figure 1. Histology of testicular bioptate (H&E staining)
Case description
Six-year-old otherwise healthy boy presented with febrile temperature, generalized macular/papular rash, hepatosplenomegaly and facial swelling. The onset of disease was sudden (15.01.2017). Initially, the patient was diagnosed with hemophagocytic lymphohistiocytosis, due to fever, progressive hepatosplenomegaly and increased ferritin level (535 ng/ml). The disease rapidly progressed, and pneumonia with pleuritis developed in several days, thus requiring admission to intensive care unit. Specific therapy with steroids, etoposide, intravenous immunoglobulins and cyclosporine A was started with transient response and further progression with enlargement of left testis, which was biopsied on March 29, 2017. Initial histological analysis in local lab demonstrated extranodal NK/T cell lymphoma, nasal type. Later on, the histology was revised in RM Gorbacheva Research Institute, Pavlov University (St. Petersburg), and the diagnosis was switched to NK/T lymphoma with blast morphology. The revised histology revealed overgrowth of lymphoid small- and middle-sized cells with narrow cytoplasmic area. The cells contained irregular and roundish nuclei with finely dispersed chromatin. Some nuclei contain solitary tiny nucleoli (Fig. 1).
Immunohistochemical study showed intensive expression of CD2, CD3, CD5, CD7, CD56, granzyme B, and co-expression of CD4 and CD8. Most cells were slightly CD33-positive. There was no detectable expression of CD30, CD57, CD99, TdT, CD1a, CD10. In situ hybridization failed to demonstrate presence of Epstein-Barr virus. Index of proliferative activity (Ki-67) was elevated (70-80%). PDL1 was not expressed on tumor cells but was clearly detectable on tumor microenvironment cells (macrophages) (Fig. 2 A-I). On the basis of these pathological findings, the pathologist concluded that the tumors with such immunophenotype were not previously described under known classifications. Extranodal NK/T cell lymphoma, nasal type, and blastic plasmacytoid dendritic cell neoplasm were excluded. This is an unclassified lymphoma with the features of peripheral T cell and lymphoblastic lymphoma (Fig. 2). It is typical for NK-cells to express CD2, CD16 and CD56. Presence of these molecules on tumor cells together with the absence of clonal receptor gene rearrangements are characteristic for NK-cell lymphomas [8].

Figure 2. Immunohistochemistry pattern of the unclassified NK/T cell lymphoma (testicular biopsy). Staining with antibodies for the following antigens: Ki-67 (A), CD3 (B); CD4 (C); CD5 (D); CD8 (E); CD33 (F); CD56 (G); Granzyme B(H), PDL1 (I)
Molecular biological analysis revealed mutation in enhancer of Zeste homolog 2 (EZH2) gene (p.Arg288Gln) in 5% of tumor cells.
Polychemotherapy according to SMILE protocol was started on 13 April 2017. Treatment schedule consisted of dexamethasone 20 mg/m2 №4, methotrexate 2000 mg/m2 №1, ifosfamide 1500 mg №3, L-asparaginase 6000 mg/m2 №7, and etoposide 100 mg/m2 №3. Due to presence of effusions and risk of unacceptable toxicity, methotrexate was skipped in the first cycle. Other drugs, doses and timing were applied according to standard protocol [9]. In general, the patient received 4 courses of SMILE (13.04.17-2.08.17). Aggressive progression after the fourth cycle of SMILE with involvement of liver, left testis and CNS was registered (15 August 2017). Active disease was confirmed by presence of tumor cells in the cytology specimens of left testis and cerebrospinal fluid. Bone marrow was not affected. Multiple rash elements that were present upon progression did not exhibit tumor biology, and were associated with paraneoplastic process, as revealed by biopsy.

Figure 3. Immunophenotyping of cerebrospinal fluid (CD2, CD3, CD4, CD8, CD45, CD56)

Figure 4. Acute graft-host-disease after allo-HSCT
The second-line induction therapy consisted of FLAG (fludarabine 30 mg/m2 №5, cytarabine 4000 mg/m2 №5, granulocyte colony-stimulating factor) and ICI (nivolumab 40 mg on Days 1, 14, 28). This strategy resulted into the first durable complete remission. Irradiation of testis, or orchidectomy was not performed due to refusal of parents. Further treatment consisted of nivolumab (3 mg/kg №9 biweekly, weekly gemcitabine 1000 mg/m2 №6, and intrathecal therapy (cytarabine 30 mg, metotrexate 12 mg, prednisolone 10 mg) №6. Autologous HSCT was performed on 15 February 2018 with BeEAM (bendamustine 160 mg/m2 on Days -7 and -6, etoposide 200 mg/m2 and cytarabine 400 mg/m2 on Days -5 to -2 and melphalan 140 mg/m2 on Day -1) as a conditioning regimen administered for remission consolidation. Afterwards, the therapy was stopped, hoping for curation. Unfortunately, 2 months after autologous HSCT, the patient developed facial asymmetry, and cerebrospinal fluid aspirate showed presence of tumor cells upon immunophenotyping (Fig. 3).
There were no structural abnormalities on magnetic resonance imaging. Third-line therapy consisted of single dose intravenous cytarabine 1000 mg/m2, biweekly intrathecal therapy (cytarabine 30 mg, metotrexate 12 mg, prednisolone 10 mg) №8 and biweekly nivolumab 40 mg №9. Second complete remission was achieved during the first week of treatment. To consolidate second remission, first haploidentical HSCT (from father) was performed (25 September 2018). Non-myeloablative conditioning (busulfan 8 mg/kg and fludarabine 150 mg/m2) was used prior to transplantation. Graft-versus-host disease (GVHD) prophylaxis was based on posttransplant cyclophosphamide (100 mg/kg), sirolimus and tacrolimus. Bone marrow was used as a transplant source, with total number of 8×106 CD34+ cells/kg. There were no signs of peripheral blood recovery, and primary non-engraftment occurred (Day+30). The patient received second haploidentical HSCT on 14 October 2018 with switching to other donor and transplant source (unmanipulated peripheral blood stem cells from mother). A total of 10×106 CD34+ cells/kg were infused. Reduced-intensity conditioning regimen consisted of fludarabine 150 mg/m2 and treosulfan 36 mg/m2.
Posttransplant cyclophosphamide (100 mg/m2) and sirolimus were used for GVHD prophylaxis. Engraftment was diagnosed on Day +19. Transplantation was complicated by stage IV steroid-refractory acute GVHD of skin (Day+25) that responded to ruxolitinib (Fig. 4).
The patient received monthly intrathecal therapy №6 after transplantation for the prophylaxis of CNS relapse. At the present time, he remains in complete remission without signs of GVHD and treatment-free for 3 years. There is a slight decrease in intellectual abilities that necessitated individualized learning program at school. This complication is probably associated with previous CNS affection by lymphoma and related treatment [10].
Discussion
Data on NK cell lymphoblastic leukemia/lymphoma is limited to case reports. PTCL is more common nosology, but non-anaplastic PTCL is also a rare entity in children [2, 11]. This case report represents rare unclassified NK/T lymphoma in a child. According to opinion of pathologists, this type of lymphoma was not previously described in WHO classification, sharing features of both PTCL and lymphoblastic lymphoma. This fact underlines the existence of lymphoma subtypes that do not match established classification variants. Regular revision of lymphoma classifications is partially explained by the discovery of new features or detailing of established lymphoma subtypes. An EZH2 gene mutation typical to PTCL was found in the minority of tumor cells (5%) of our patient [12]. It could be attributed to tumor heterogeneity and presence of subclone harboring the EZH2 mutation. Epstein-Barr virus (EBV) was not present in the tumor specimen. However, EBV plays an important role in the pathogenesis of many PTCL subtypes. Most extranodal NK/T lymphomas carry type A EBV. Despite certain role of EBV in NK/T cell lymphomas, exact mechanism of viral cancerogenesis is unclear in this setting [12]. Prevalence of NK/T cell lymphomas in distinct geographical regions despite worldwide EBV distribution suggests importance of ethnic genetic profile for NK/T lymphoma pathogenesis [8]. Initially, our patient was diagnosed with hemophagocytic lymphohistiocytosis (HLH) and started appropriate therapy. The diagnosis was not confirmed later since the patient did not fit all necessary HLH criteria (only 3 criteria of 5 required) [13]. Anyway, to our opinion, the initial therapy played an important role in patient rescue in the circumstances of limited time and progressive deterioration, because treatment with steroids and etoposide transiently stabilized clinical situation and gained time for correct diagnosis. Biopsy of enlarged testis became a game changer that enabled true PTCL diagnostics and corrected the subsequent therapy. Natural killer cells emerge from bone marrow and further migrate to nodal and extranodal sites. This fact can partially explain common extranodal localization of NK cell lymphomas [14]. According to Murphy classification, stage IV was diagnosed in our patient due to CNS involvement. Staging of non-Hodgkin lymphoma in adults is carried out according to Ann Arbor classification, which was primary used for Hodgkin lymphoma. In children, the modified Murphy classification is used, which was initially developed for the Burkitt and lymphoblastic lymphomas. Unlike Ann Arbor system, the Murphy classification assigns all intrathoracic and most intra-abdominal tumors to stage III, whereas only bone marrow and central nervous system lesions are classified as stage IV [15]. According to previously published data, most children with PTCL demonstrate advanced disease at the onset expect extranodal NK/T cell lymphoma [4].
Patient failed first-line therapy of lymphoma. He could be primary refractory to the therapy, or previous treatment of HLH interfered in natural course. Another reason of chemoresistance could be application of the SMILE chemotherapy protocol designed specifically for extranodal NK/T lymphoma, since this type of PTCL was initially diagnosed. There is no established treatment for non-anaplastic PTCL in children. Various treatment protocols, such as anaplastic large-cell lymphoma therapy or acute leukemia protocols are used with comparable efficiency [3, 16]. The treatment results vary between PTCL subgroups, indicating that a subtype-specific approach should be pursued. Except for subcutaneous panniculitis-like T-cell lymphoma, survival rates are poor and the optimal treatment strategy has not been defined yet. International registry with treatment recommendations is needed to improve outcomes for this rare and heterogeneous group of diseases. Results in adult PTCL after CHOP or other treatment approaches are disappointing [17, 18]. Difficulties in PTCL management are associated with unique tumor biology and rarity of the disease that prevents multicentre randomized clinical trials, especially in pediatric population. Prognosis in R-R non-anaplastic PTCL is dismal, with median OS of only 5.8 months. Our patient demonstrated progression of the disease after first-line therapy, and the panel of doctors decided to start FLAG protocol combined with nivolumab. This was a non-standard recommendation, since the second-line therapies in PTCL usually include platinum and/or gemcitabine [19]. Our choice was based on leukemia-like clinical features (involvement of CNS and testis), blast morphology of tumor cells and presence of PDL1 expression on tumor microenvironment. There are no data on combination of nivolumab with FLAG, but combination of nivolumab with cytarabine or fludarabine is feasible according to recently published data [20, 21]. Extranodal NK-cell lymphomas are more common in patients with definite HLA haplotypes while rarely occur in patients with other haplotypes suggesting that immune surveillance is important for this tumor. Thus, one may hypothesize on immunotherapy as a potentially effective approach in this clinical setting [22]. Informed consent was obtained from relatives of the patient prior to starting the therapy. This treatment plan resulted in first continuous complete remission. Further treatment was based on systemic immunochemotherapy (nivolumab and gemcitabine) combined with local tumor control (intrathecal therapy). The rationale for this approach was presence of PDL1 on tumor microenvironment, and published data on possible effectiveness of ICI in pediatric PTCL [24]. First remission was consolidated with autologous HSCT according to established recommendations for refractory NHL in children [25]. Allogeneic HSCT was reserved for further treatment if required. Previous therapy with nivolumab and, therefore, increased risk of severe GVHD was an important counter-argument for the ongoing allogeneic HSCT [26]. The role of allogeneic transplant in the management of pediatric R-R PTCL patients remains unclear. According to results obtained in adults, allo-HSCT may be beneficial in PTCL, due to graft-versus-tumor effect [27]. Allo-HSCT may be similarly effective as consolidation after first or second remission, being not a good option at progression [28]. Data in children with PTCL and HSCT are limited to sporadic reports and are not decisive [4]. Due to early relapse of lymphoma after autologous HSCT in our patient, the re-treatment with nivolumab and intrathecal therapy was initiated which resulted into second complete remission. There were no doubts that this was the optimal point for consolidation of second remission with allo-HSCT. Moreover, due to aggressive and unpredictable disease course there was no benefit for searching a matched unrelated donor. For the first haploidentical HSCT, a non-myeloablative conditioning regimen was chosen based on previous autologous HSCT. Unfortunately, this approach led to primary non-engraftment, probably, due to residual recipient cells that promoted the graft rejection. Risks of non-engraftment with standard transplant approaches with haploidentical stem cell source are well known and can be ameliorated with higher number of infused stem cells, more intense conditioning regimens and additional techniques of transplant processing [29, 30]. To enable engraftment, the second haploidentical HSCT was performed with more intense conditioning regimen and peripheral blood stem cells as transplant source. This procedure resulted into engraftment, but early posttransplant period was complicated with severe steroid-refractory cutaneous acute GVHD that responded to ruxolitinib. Further on, the patient underwent prophylactic intrathecal therapy due to high risk of CNS relapse and low effectiveness of graft-versus-tumor effect in the controlling barrier areas [31].
The limitation of this case report is absence of survey for underlying primary immunodeficiency syndrome, because many children with PTCL have some pre-existing disorders affecting immune system.
The present report demonstrates a case of rare unclassified NK/T cell lymphoma that was successfully treated with chemoimmunotherapy and repeated transplantations. No doubt, this tumor was sensitive to immunotherapy. Hence, the immune checkpoint inhibitors followed by allogeneic transplantation may be effective in therapy of pediatric PTCL.
Conflict of interest
None declared.
References
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H , Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. doi: 10.1182/blood-2016-01-643569
- Maciejka-Kemblowska L, Chaber R, Wrobel G, Maldyk J, Kozlowska M, Kulej D, et al. Clinical features and treatment outcomes of peripheral T-cell lymphoma in children. A current data report from Polish Pediatric Leukemia/Lymphoma Study Group (PPLLSG). Adv Med Sci. 2016; 61(2):311-316. doi: 10.1016/j.advms.2016.03.002
- Kontny U, Oschlies I, Woessmann W, Burkhardt B, Lisfeld J, Salzburg J et al. Non-anaplastic peripheral T-cell lymphoma in children and adolescents – a retrospective analysis of the NHL-BFM study group. Br J Haematol. 2015; 168(6):835-844. doi: 10.1111/bjh.13216
- Mellgren, K, Attarbaschi A, Abla, O, Alexander S, Bomken S, Bubanska E, et al. Non-anaplastic peripheral T cell lymphoma in children and adolescents – an international review of 143 cases. Ann Hematol. 2016; 95:1295-1305. doi: 10.1007/s00277-016-2722-y
- Jain S, Kumar R, Purohit A, Pati HP. Precursor NK cell lymphoblastic leukemia/lymphoma – report of a case with literature review. Ind J Hematol Blood Transfus. 2014; 30 (Suppl 1):283-285. doi: 10.1007/s12288-014-0360-x
- Frenkel’ MA, Baranova O Yu, Antipova AS, Kupryshina NA, Tupitsyn NN. NK-cell lymphoblastic leukemia/lymphoma (literature review and authors’ experience). Clinical Oncohematology. 2016;9(2):208-217 (In Russian). doi: 10.21320/2500-2139-2016-9-2-208-217
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. doi: 10.1182/blood-2016-03-643544
- Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol 10, 85 (2017).
doi: 10.1186/s13045-017-0452-9 - Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study.
J ClinOncol. 2011; 29(33):4410-4416. doi: 10.1200/JCO.2011.35.6287 - Turhan AB, Tülin Fidan S, Yarar C, NazlıSakallı E, Özdemir ZC, Bör Ö. Neurocognitive consequences of childhood leukemia and its treatment. Ind J Hematol Blood Transfus. 2018;34(1):62-69. doi: 10.1007/s12288-017-0846-4
- Al Mahmoud R, Weitzman S, Schechter T, Ngan B, Abdelhaleem M, Alexander S. Peripheral T-cell lymphoma in children and adolescents: a single-institution experience. J Pediatr Hematol Oncol. 2012; 34(8):611-616. doi: 10.1097/MPH.0b013e3182707592
- Zhang H, Lv H, Jia X, Hu G, Kong L, Zhang T, et al. Clinical significance of enhancer of zeste homolog 2 and histone deacetylases 1 and 2 expression in peripheral T-cell lymphoma. Oncol Lett. 2019;18(2):1415-1423. doi: 10.3892/ol.2019.10410
- Grzybowski B, Vishwanath VA. Hemophagocytic lymphohistiocytosis: A diagnostic conundrum. J Pediatr Neurosci. 2017;12(1):55-60. doi: 10.4103/jpn.JPN_140_16
- Bekiaris V, Lane PJL. The localization and migration of natural killer cells in health and disease. Natural Killer Cells. 2010; 137-153.
doi: 10.1016/B978-0-12-370454-2.00010-7 - Rosolen A, Perkins SL, Pinkerton CR, Guillerman GR, Sandlund JT, Reiter A, et al. Revised international pediatric non-Hodgkin lymphoma staging system. J Clin Oncol. 2015; 33 (18):2112-2118. doi: 10.1200/JCO.2014.59.7203
- Windsor R, Stiller C, Webb D. Peripheral T-cell lymphoma in childhood: population-based experience in the United Kingdom over 20 years. Pediatr Blood Cancer. 2008; 50(4): 784-787. doi: 10.1002/pbc.21293. PMID: 18022899
- Ma H, Bin Cheng, O'Connor OA. Survival outcomes of patients with peripheral T-cell lymphomas (PTCL) treated with chemotherapy and/or novel agents: The Columbia University experience. J Clin Oncol. 2019; 37:15 (Suppl), e19049-e19049.
doi: 10.1200/JCO.2019.37.15_suppl.e19049 - Lepik EE, Kozlov AV, Borzenkova ES, Zalyalov YR , Lepik KV, Kondakova EV, et al. Treatment options for T-cell lymphomas: a single-center study. Cell Ther Transplant. 2020; 9(1): 28-37. doi: 10.18620/ctt-1866-8836-2020-9-1-28-37
- Foster C, Kuruvilla J. Treatment approaches in relapsed or refractory peripheral T-cell lymphomas. F1000Res. 2020;9:F1000 Faculty Rev-1091. Published 2020 Sep 4. doi: 10.12688/f1000research.22257.1
- Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study. Lancet Haematol. 2019; 6(9):e480-e488. doi: 10.1016/S2352-3026(19)30114-0
- Creelan, BC, Wang, C, Teer, JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021; 27: 1410-1418. doi: 10.1038/s41591-021-01462-y
- Kanno H, Kojya S, Li T, Ohsawa M, Nakatsuka S, Miyaguchi M, et al. Low frequency of HLA-A*0201 allele in patients with Epstein-Barr virus-positive nasal lymphomas with polymorphic reticulosis morphology. Int J Cancer. 2000; 87(2):195-199.
doi: 10.1002/1097-0215(20000715)87:2<195::AID-IJC6>3.0.CO;2-0 - Sedick Q, Alotaibi S, Alshieban S, Naheet KB, Elyamany G. Natural killer cell lymphoblastic leukaemia/lymphoma: case report and review of the recent literature. Case Rep Oncol. 2017; 10(2): 588-595. doi: 10.1159/000477843
- Kozlov AV, Kazantsev IV, Yukhta TV, Tolkunova PS, Gevorgyan AG, Lepik KV, et al. The use of checkpoint inhibitors in children with non-Hodgkin lymphomas. Pediatric Hematology/Oncology and Immunopathology. 2020; 19(2):112-120. (In Russian).
doi: 10.24287/1726-1708-2020-19-2-112-120 - Afanasyev BV, Afanasyeva KS, Barabanshchikova MV, Bondarenko SN, Bykova TA, Vlasova JYu, Gevorgian AG, et al. Indications for hematopoietic stem cell transplantation. December 2019. Cell Ther Transplant. 2019; 8(4):101-145 (In Russian).
doi: 10.18620/ctt-1866-8836-2019-8-4-101-145 - Ijaz A, Khan AY, Malik SU, Faridi W, Fraz MA, Usman M, et al. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transplant. 2019; 25(1): 94-99.
doi: 10.1016/j.bbmt.2018.08.028 - Castagna L, Pagliardini T, Bramanti S, Schiano JM, de Oca CM, Bouabdallah R, et al. Allogeneic stem cell transplantation in poor prognosis peripheral T-cell lymphoma: the impact of different donor type on outcome. Bone Marrow Transplant. 2021; 56: 883-889. doi: 10.1038/s41409-020-01133-5
- Mamez AC, Dupont A, Blaise D, Chevallier P, Forcade E, Ceballos P, et al. Allogeneic stem cell transplantation for peripheral T cell lymphomas: a retrospective study in 285 patients from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). J Hematol Oncol. 2020; 13(1):56. Published 2020 May 19. doi: 10.1186/s13045-020-00892-4
- Motorin DV, Badaev RS, Babenetskaya DV, Ilyina NA, Silina TO, Baratashvili GG, et al. Haploidentical Hematopoietic Stem Cell Transplantation (Haplo-SCT) with Post-Transplant Cyclophosphamide for haematological malignancies: a single centre experience. Cell Ther Transplant. 2016; 5(3):51-53. doi: 10.18620/ctt-1866-8836-2016-5-3-51-53
- Maschan MA. Depletion of alpha/beta-T-cells is a robust platform for haploidentical hematopoietic stem cell transplantation results improvement. Russian Journal of Pediatric Hematology and Oncology. 2015; 2(3):34-38. (In Russian).
doi: 10.17650/2311-1267-2015-2-3-34-38 - Ostronoff F, Ostronoff M, Fernandes HS, Buessio R, Soriano S, Souto-Maior AS, et al. Evidence for a graft-versus-leukemia effect in the central nervous system. Leukemia & Lymphoma. 2008; 49(2): 365-369. doi: 10.1080/10428190701784417
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(63) "redkaya-neklassifitsiruemaya-nk-t-kletochnaya-limfoma-u-rebenka" ["~CODE"]=> string(63) "redkaya-neklassifitsiruemaya-nk-t-kletochnaya-limfoma-u-rebenka" ["EXTERNAL_ID"]=> string(4) "2045" ["~EXTERNAL_ID"]=> string(4) "2045" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(165) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенкаA case of rare pediatric unclassified NK/T cell lymphoma" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2339) "<p style="text-align: justify;">Статья представляет собой демонстрацию клинического случая редкой неклассифицируемой периферической Т-клеточной лимфомы у ребенка. Заболевание характеризовалось рефрактерным течением. Ремиссия была получена после добавления ингибиторов иммунных контрольных точек (ИКТ) к химиотерапии. Основанием для начала иммунотерапии было присутствие PDL1 (лиганд рецептора программируемой клеточной смерти 1) на клетках микроокружения опухоли. Для консолидации первой ремиссии была проведена аутологичная ТГСК и для консолидации второй ремиссии – аллогенная. Течение посттрансплантационного периода осложнилось тяжелой реакцией «трансплантат против хозяина» (РТПХ) с поражением кожи, которая была вылечена с помощью руксолитиниба.</p> <p style="text-align: justify;">Для лечения и профилактики лимфомы ЦНС применялись интратекальные введения цитостатиков как до, так и после ТГСК. Пациент находится в полной ремиссии и без признаков РТПХ в течение 3-х лет после окончания лечения. В целом, качество жизни у ребенка хорошее, за исключением легких нарушений в когнитивной сфере.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Периферическая Т-клеточная лимфома, дети, химиоиммунотерапия, трансплантация гемопоэтических стволовых клеток.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_META_TITLE"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_META_KEYWORDS"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_META_DESCRIPTION"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_PICTURE_FILE_ALT"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_PICTURE_FILE_TITLE"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_PICTURE_FILE_NAME"]=> string(67) "redkaya-neklassifitsiruemaya-nk-t-kletochnaya-limfoma-u-rebenka-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(109) "Редкая неклассифицируемая НК/Т-клеточная лимфома у ребенка" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(67) "redkaya-neklassifitsiruemaya-nk-t-kletochnaya-limfoma-u-rebenka-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(67) "redkaya-neklassifitsiruemaya-nk-t-kletochnaya-limfoma-u-rebenka-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(67) "redkaya-neklassifitsiruemaya-nk-t-kletochnaya-limfoma-u-rebenka-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "202" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28455" ["VALUE"]=> array(2) { ["TEXT"]=> string(532) "<p>Андрей В. Козлов<sup>1</sup>, Ольга И. Богданова<sup>1</sup>, Асмик Г. Геворгян<sup>1</sup>, Егор В. Волчков<sup>2</sup>, Анна В. Ботина<sup>1</sup>, Вадим В. Байков<sup>1</sup>, Елена В. Морозова<sup>1</sup>, Наталия Б. Михайлова<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(412) "
Андрей В. Козлов1, Ольга И. Богданова1, Асмик Г. Геворгян1, Егор В. Волчков2, Анна В. Ботина1, Вадим В. Байков1, Елена В. Морозова1, Наталия Б. Михайлова1, Людмила С. Зубаровская1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28456" ["VALUE"]=> array(2) { ["TEXT"]=> string(518) "<p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> НМИЦ ДГОИ им. Дмитрия Рогачева, Москва, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(476) "1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 НМИЦ ДГОИ им. Дмитрия Рогачева, Москва, Россия
Статья представляет собой демонстрацию клинического случая редкой неклассифицируемой периферической Т-клеточной лимфомы у ребенка. Заболевание характеризовалось рефрактерным течением. Ремиссия была получена после добавления ингибиторов иммунных контрольных точек (ИКТ) к химиотерапии. Основанием для начала иммунотерапии было присутствие PDL1 (лиганд рецептора программируемой клеточной смерти 1) на клетках микроокружения опухоли. Для консолидации первой ремиссии была проведена аутологичная ТГСК и для консолидации второй ремиссии – аллогенная. Течение посттрансплантационного периода осложнилось тяжелой реакцией «трансплантат против хозяина» (РТПХ) с поражением кожи, которая была вылечена с помощью руксолитиниба.
Для лечения и профилактики лимфомы ЦНС применялись интратекальные введения цитостатиков как до, так и после ТГСК. Пациент находится в полной ремиссии и без признаков РТПХ в течение 3-х лет после окончания лечения. В целом, качество жизни у ребенка хорошее, за исключением легких нарушений в когнитивной сфере.
Ключевые слова
Периферическая Т-клеточная лимфома, дети, химиоиммунотерапия, трансплантация гемопоэтических стволовых клеток.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28458" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-54-60" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-54-60" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28459" ["VALUE"]=> array(2) { ["TEXT"]=> string(408) "<p>Andrey V. Kozlov<sup>1</sup>, Olga I. Bogdanova<sup>1</sup>, Asmik G. Gevorgian<sup>1</sup>, Egor V. Volchkov<sup>2</sup>, Anna V. Botina<sup>1</sup>, Vadim V. Baykov<sup>1</sup>, Elena V. Morozova<sup>1</sup>, Natalya B. Mikhailova<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(288) "Andrey V. Kozlov1, Olga I. Bogdanova1, Asmik G. Gevorgian1, Egor V. Volchkov2, Anna V. Botina1, Vadim V. Baykov1, Elena V. Morozova1, Natalya B. Mikhailova1, Ludmila S. Zubarovskaya1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28460" ["VALUE"]=> array(2) { ["TEXT"]=> string(769) "<p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Dmitry Rogachev National Research Center, Moscow, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia<br> E-mail: kozlovandrew1983@yandex.ru</p><br> <p><b>Citation:</b> Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(655) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Dmitry Rogachev National Research Center, Moscow, Russia
Correspondence:
Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute
of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: kozlovandrew1983@yandex.ru
Citation: Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28461" ["VALUE"]=> array(2) { ["TEXT"]=> string(1171) "<p style="text-align: justify;">The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.</p> <h2>Keywords</h2> <p style="text-align: justify;">Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1115) "The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.
Keywords
Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28462" ["VALUE"]=> string(56) "A case of rare pediatric unclassified NK/T cell lymphoma" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(56) "A case of rare pediatric unclassified NK/T cell lymphoma" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28463" ["VALUE"]=> string(4) "2732" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2732" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28464" ["VALUE"]=> string(4) "2733" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2733" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28459" ["VALUE"]=> array(2) { ["TEXT"]=> string(408) "<p>Andrey V. Kozlov<sup>1</sup>, Olga I. Bogdanova<sup>1</sup>, Asmik G. Gevorgian<sup>1</sup>, Egor V. Volchkov<sup>2</sup>, Anna V. Botina<sup>1</sup>, Vadim V. Baykov<sup>1</sup>, Elena V. Morozova<sup>1</sup>, Natalya B. Mikhailova<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(288) "Andrey V. Kozlov1, Olga I. Bogdanova1, Asmik G. Gevorgian1, Egor V. Volchkov2, Anna V. Botina1, Vadim V. Baykov1, Elena V. Morozova1, Natalya B. Mikhailova1, Ludmila S. Zubarovskaya1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(288) "Andrey V. Kozlov1, Olga I. Bogdanova1, Asmik G. Gevorgian1, Egor V. Volchkov2, Anna V. Botina1, Vadim V. Baykov1, Elena V. Morozova1, Natalya B. Mikhailova1, Ludmila S. Zubarovskaya1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28461" ["VALUE"]=> array(2) { ["TEXT"]=> string(1171) "<p style="text-align: justify;">The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.</p> <h2>Keywords</h2> <p style="text-align: justify;">Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1115) "The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.
Keywords
Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1115) "The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.
Keywords
Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28458" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-54-60" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-54-60" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-54-60" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28462" ["VALUE"]=> string(56) "A case of rare pediatric unclassified NK/T cell lymphoma" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(56) "A case of rare pediatric unclassified NK/T cell lymphoma" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(56) "A case of rare pediatric unclassified NK/T cell lymphoma" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28460" ["VALUE"]=> array(2) { ["TEXT"]=> string(769) "<p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Dmitry Rogachev National Research Center, Moscow, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia<br> E-mail: kozlovandrew1983@yandex.ru</p><br> <p><b>Citation:</b> Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(655) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Dmitry Rogachev National Research Center, Moscow, Russia
Correspondence:
Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute
of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: kozlovandrew1983@yandex.ru
Citation: Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(655) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Dmitry Rogachev National Research Center, Moscow, Russia
Correspondence:
Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute
of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: kozlovandrew1983@yandex.ru
Citation: Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28455" ["VALUE"]=> array(2) { ["TEXT"]=> string(532) "<p>Андрей В. Козлов<sup>1</sup>, Ольга И. Богданова<sup>1</sup>, Асмик Г. Геворгян<sup>1</sup>, Егор В. Волчков<sup>2</sup>, Анна В. Ботина<sup>1</sup>, Вадим В. Байков<sup>1</sup>, Елена В. Морозова<sup>1</sup>, Наталия Б. Михайлова<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(412) "Андрей В. Козлов1, Ольга И. Богданова1, Асмик Г. Геворгян1, Егор В. Волчков2, Анна В. Ботина1, Вадим В. Байков1, Елена В. Морозова1, Наталия Б. Михайлова1, Людмила С. Зубаровская1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(412) "Андрей В. Козлов1, Ольга И. Богданова1, Асмик Г. Геворгян1, Егор В. Волчков2, Анна В. Ботина1, Вадим В. Байков1, Елена В. Морозова1, Наталия Б. Михайлова1, Людмила С. Зубаровская1
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28457" ["VALUE"]=> array(2) { ["TEXT"]=> string(2339) "<p style="text-align: justify;">Статья представляет собой демонстрацию клинического случая редкой неклассифицируемой периферической Т-клеточной лимфомы у ребенка. Заболевание характеризовалось рефрактерным течением. Ремиссия была получена после добавления ингибиторов иммунных контрольных точек (ИКТ) к химиотерапии. Основанием для начала иммунотерапии было присутствие PDL1 (лиганд рецептора программируемой клеточной смерти 1) на клетках микроокружения опухоли. Для консолидации первой ремиссии была проведена аутологичная ТГСК и для консолидации второй ремиссии – аллогенная. Течение посттрансплантационного периода осложнилось тяжелой реакцией «трансплантат против хозяина» (РТПХ) с поражением кожи, которая была вылечена с помощью руксолитиниба.</p> <p style="text-align: justify;">Для лечения и профилактики лимфомы ЦНС применялись интратекальные введения цитостатиков как до, так и после ТГСК. Пациент находится в полной ремиссии и без признаков РТПХ в течение 3-х лет после окончания лечения. В целом, качество жизни у ребенка хорошее, за исключением легких нарушений в когнитивной сфере.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Периферическая Т-клеточная лимфома, дети, химиоиммунотерапия, трансплантация гемопоэтических стволовых клеток.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2261) "Статья представляет собой демонстрацию клинического случая редкой неклассифицируемой периферической Т-клеточной лимфомы у ребенка. Заболевание характеризовалось рефрактерным течением. Ремиссия была получена после добавления ингибиторов иммунных контрольных точек (ИКТ) к химиотерапии. Основанием для начала иммунотерапии было присутствие PDL1 (лиганд рецептора программируемой клеточной смерти 1) на клетках микроокружения опухоли. Для консолидации первой ремиссии была проведена аутологичная ТГСК и для консолидации второй ремиссии – аллогенная. Течение посттрансплантационного периода осложнилось тяжелой реакцией «трансплантат против хозяина» (РТПХ) с поражением кожи, которая была вылечена с помощью руксолитиниба.
Для лечения и профилактики лимфомы ЦНС применялись интратекальные введения цитостатиков как до, так и после ТГСК. Пациент находится в полной ремиссии и без признаков РТПХ в течение 3-х лет после окончания лечения. В целом, качество жизни у ребенка хорошее, за исключением легких нарушений в когнитивной сфере.
Ключевые слова
Периферическая Т-клеточная лимфома, дети, химиоиммунотерапия, трансплантация гемопоэтических стволовых клеток.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2261) "Статья представляет собой демонстрацию клинического случая редкой неклассифицируемой периферической Т-клеточной лимфомы у ребенка. Заболевание характеризовалось рефрактерным течением. Ремиссия была получена после добавления ингибиторов иммунных контрольных точек (ИКТ) к химиотерапии. Основанием для начала иммунотерапии было присутствие PDL1 (лиганд рецептора программируемой клеточной смерти 1) на клетках микроокружения опухоли. Для консолидации первой ремиссии была проведена аутологичная ТГСК и для консолидации второй ремиссии – аллогенная. Течение посттрансплантационного периода осложнилось тяжелой реакцией «трансплантат против хозяина» (РТПХ) с поражением кожи, которая была вылечена с помощью руксолитиниба.
Для лечения и профилактики лимфомы ЦНС применялись интратекальные введения цитостатиков как до, так и после ТГСК. Пациент находится в полной ремиссии и без признаков РТПХ в течение 3-х лет после окончания лечения. В целом, качество жизни у ребенка хорошее, за исключением легких нарушений в когнитивной сфере.
Ключевые слова
Периферическая Т-клеточная лимфома, дети, химиоиммунотерапия, трансплантация гемопоэтических стволовых клеток.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28456" ["VALUE"]=> array(2) { ["TEXT"]=> string(518) "<p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>2</sup> НМИЦ ДГОИ им. Дмитрия Рогачева, Москва, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(476) "1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 НМИЦ ДГОИ им. Дмитрия Рогачева, Москва, Россия
1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 НМИЦ ДГОИ им. Дмитрия Рогачева, Москва, Россия
Introduction
Doxorubicin is a very potent anticancer antibiotic that, unfortunately, demonstrates considerable cardiotoxicity, accumulation in liver and rapid clearance [1]. Use of various delivery systems for DOX in chemotherapy makes it possible to decrease negative influence of the drug and provides its prolonged release. In our previous work [2], it has been shown that porous carbonate vaterites modified with various polyanions can be successfully applied for encapsulation of DOX and facilitate prolonged in vitro release of the substance into blood plasma. Besides, it has been proven that these delivery systems (DS) are bio-resorbable and safe to use [3, 4]. The advantages of these DS in chemotherapy are related to some peculiar features of defense mechanisms of tumor cells against chemotherapeutic compounds. Drug efflux is among these mechanisms which does not affect DS (unlike free DOX). Phagocytosis of drugs by a cell is preceded by opsonization. Mechanism of opsonization (sorption of various plasma components on surface of delivery system) is impaired when a DOX-containing DS is coated with polyelectrolytes. As a result, opsonization is reduced, and the time of passive DS circulation is prolonged in the area of high capillary permeability and disturbed lymphatic drainage (typical of tumor tissues [5]); i.e., the drug is retained in the tumor tissue.
Among other delivery systems for DOX, polymer-drug conjugates (PDC) have been described [6]. In our earlier work [2], the profiles of DOX release into blood plasma have been compared for PDC (DexS+DOX) versus DexS coated СаСО3 cores.
Ascitic tumors (particularly, Seidel hepatoma, SH) are widely used as models for in vivo studies of regional intraperitoneal chemotherapy. These tumors are included into the list recommended by the Russian Ministry of Health and the State Pharmacological Committee for studies of antitumor mechanisms [7].
Revtovich et al. [8] compared antitumor activity of cisplatin and its prolonged pharmaceutical form based on dextran phosphate hydrogel in the rats inoculated with Seidel hepatoma. They have demonstrated higher efficiency of the modified drug.
When free DOX or DOX-containing delivery systems are used in chemotherapy, it is important to control concentration of the substance in blood. Administration of free DOX results in its distribution through all the organs and tissues. When delivery systems are used, distribution of the drug in organism largely depends on DS structure (i.e., drug release rate) and its administration route (intravenous, intraperitoneal, subcutaneous, etc. [9]). The amount of introduced drug and physiology of an organism are also of importance.
Comparing the literature data on DOX concentration in blood plasma after intravenous administration to different tumor-bearing mice and women, we can see that the amount of introduced DOX exerts relatively low influence on its content in plasma, whereas tumor type is a considerable factor [10-13].
The aims of the present work included (i) studies of the opportunity to use the proposed delivery model of antitumor preparation in laboratory animals; (ii) time course evaluation of DOX release into blood following intraperitoneal administration, using drug delivery systems based on CaCO3 cores coated with DexS, or DexS-DOX conjugates (HPLC studies of DOX release); (iii) comparison between the profiles of DOX release into rat blood (both in healthy rats and animals with Seidel hepatoma), and general condition of animals, thus discerning effects of the released DOX amounts upon malignant cells in tumor-bearing animals and upon intact rats.
Materials and methods
Reagents
Doxorubicin hydrochloride purchased as the “Sindroxocin” preparation, containing 17% of doxorubicin (DOX) and 83% of lactose, was from Actavis (Hafnarfjordur, Iceland). For experiments, doxorubicin salt with protonated amino group (–NH3+) was used. Salts (CaCl2 × 2H2O, Na2CO3), acetone, and dextran sulfate (Mw = 9-20 kDa) were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis of carbonate cores
Preparation techniques for porous carbonate vaterites, methods for coating vaterites with DexS polyanion and introducing DOX into these carriers are described in [14]. Briefly, porous vaterites (СаСО3 cores) were prepared by co-precipitation. Equal volumes of 1 M aqueous solutions of CaCl2 × 2H2O and Na2CO3 were rapidly mixed at stirring (1000 rpm) with an RW 20 anchor-type mechanical stirrer (Kika-Werk, Switzerland). The mixture was stirred for 30 s. The suspension was then filtered through Schott filter glass (#16), washed thrice with distilled water and with acetone/water mixtures with increasing acetone concentrations (33%, 50%, and 100%). The precipitate was dried in thermostat at 40-50°C until a constant weight was achieved.
Doping of carbonate cores with DexS and DOX loading into delivery systems
The cores were coated with a polyanion (sodium salt of dextran sulfate, DexS). СаСО3 cores (50 mg) were added to 1 mg/mL aqueous solution of DexS (10 mL). The suspension was stirred using a Multi Bio RS-24 rotor (Biosan, Latvia) for 1 h; the solid fraction was filtered off using a Schott glass filter (#16), washed thrice with distilled water and dried at 30°C.
DOX was loaded into doped CaCO3 under continuous stirring of the mixture of CaCO3 suspension and DOX solution (C=2 mg/mL) for 24 hours. The DOX/(CaCO3+DexS) ratio was 0.4. After mixing, the suspension was centrifuged at 8000 rpm for 3 min, and the DOX amount in supernatant was determined.
DOX load (L) was calculated using the following equation: L = (mi − ms)/mP, where mi is the initial amount of DOX (mg), ms is the amount of non-encapsulated DOX in supernatant solution (mg), mP is the amount of particles (mg).
DOX concentrations were determined using the calibration curves obtained from optical density measurements in the corresponding solvents at λ=480 nm. The measurements were carried out using a SF-2000 spectrophotometer (LOMO, St. Petersburg, Russia).
Polymer-drug conjugates and their loading
Polymer-drug conjugates (DexS+DOX) were prepared according to the technique described in [2]. The DOX solution (2 mg/mL) and solution of DexS polyanion (1 mg/mL) were mixed in volumes that provided an equimolar ratio of functional groups. Then the mixture was subjected to ultrasound treatment for 2 min and centrifuged at 8000 rpm for 10 min. The supernatant was removed, the residue was freeze-dried. The DOX concentration in supernatant was determined spectrophotometrically. The PDC load was determined by subtracting the amount of DOX in supernatant from total amount of introduced DOX followed by dividing this quantity by the weight of the residue. All the measurements were carried out thrice.
In vivo experiments with rats
Two groups of rats were used in the experiments with intraperitoneal administration of various DOX delivery systems. The first group (experimental) included the rats with inoculated Seidel hepatoma; DOX encapsulated into calcium carbonate cores doped with DexS polyanion was administrated intraperitoneally (i.p.). The second group consisted of healthy rats; they were treated i.p. with DOX incorporated into different delivery systems: (i) submicron-sized calcium carbonate cores coated with DexS polyanion (CaCO3+DexS); (ii) nanosized polymer-drug conjugates (DexS+DOX). Two reference groups were also included, i.e., the first reference group consisted of non-treated animals with inoculated Seidel hepatoma (no DOX included in the СаСО3+DexS delivery system was introduced). The second reference group consisted of healthy rats that were treated with free DOX (without delivery systems).
Thirty-six male and female outbred rats with body weight ranging from 256 to 312 g were used in the experiments ("Rappolovo" nursery for laboratory animals). All manipulations with animals were performed under general anesthesia: Sol. Zoletil 50 (0.05 mL per 0.1 kg of body mass), Sol. Rometаrum 20 mg/mL (0.0125 mL per 0.1 kg of body mass, intramuscularly). The animals were caged (2-5 individuals in a cage), had free access to water and food (4R F18 prolonged keeping formula for rodents, Macedonia, Italy). The animals were fed the standard diet for laboratory rats used in the vivarium of A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia.
The animals of experimental and reference groups were examined daily; consumption of water and food was registered, body temperature and weight were measured. Behavior of animals and life expectancy were estimated. Immediately after death, ascitic fluid was collected, and its volume was determined. All the manipulations with animals were performed in accordance with State Standard 33216-2014 "Regulations for work with laboratory rodents and rabbits".
Transplantation of ascitic Seidel hepatoma (SH)
Resuspended cells of ascitic Seidel hepatoma (freshly defrosted and washed from dimethyl sulfoxide) were injected into abdominal cavity of rats from the 1st group. Ascitic fluid containing Seidel hepatoma cells (1 mL) was introduced intraperitoneally using a needle (21 G). The dosage was calculated using the Freireich quotient [15].
Introduction of DOX into rats
Doxorubicin (both free and encapsulated in delivery systems) was administered to anaesthesized animals using Zoletil 50, 0.05 mL per 0.1 kg of body mass i.p.). The drug was injected in 1.5 mL of 5% glucose solution, containing DOX in CаСО3+DexS cores, or DOX in polymer drug conjugates (DexS+DOX), or free DOX (2 or 4 mg of DOX per 1 animal of the 1st and 2nd groups, respectively). The drug preparations were injected by means of 21-gauge needles. For the animals of the 1st experimental group, the tumor cells and DOX-containing delivery systems were applied simultaneously. Examination of animals from the 1st group was described elsewhere ("In vivo experiments with rats").
Along with visual inspection, peripheral blood (1.0 mL) was taken from tail vein of rats of the 1st group 24 h, at 4, 7, 14, 17 and 21 days after drug injection. Before blood sampling, the rat was examined, weighed, its body temperature was measured, then it was anesthetized and fixed in a holder for immobilizing rodents. Plasma was obtained from the blood specimens 10 min after blood sampling by centrifugation for 15 min at 1500 rpm. The supernatantses were frozen and stored in closed vessels at -40°C for further analysis.
Determination of DOX content in plasma of rats
Content of doxorubicin (DOX) in rat blood plasma was determined by high-performance liquid chromatography (HPLC) using Prominence-I LC 2030C 3D Plus instrument (Shimadzu) equipped with an RF-20A fluorimetric detector and a 5 µm Luna C18 column (Phenomenex). The excitation wavelength was 475 nm, emission wavelength was 555 nm. Analysis was performed in the gradient elution regime (with acetonitrile) in 0.01 N Na-formiate buffer (рН 3.68). Duration of experiment: was 20 min, at the detection limit of 1 ng/mL. All the measurements were carried out thrice.
Results and discussion
First group of rats
The influence of DOX delivery systems based on porous calcium carbonate cores coated with DexS (CaCO3+DexS) on development of Seidel hepatoma was studied in laboratory rats.
The first experimental group included 4 male and 4 female rats with body mass varying from 256 to 306 g. The first reference group consisted of 5 male and 3 female animals (266 to 312 g). The animals were followed up as described under Materials and Methods.
Six animals from the first experimental group died within a period from 10 to 14 days after the procedure (median, 14 days). All animals of the reference group died within a period from 8 to 13 days after the beginning of the experiment; the lifetime median was 8 days. Physical examination revealed ascites in animals of both groups starting from the 4th day after starting the experiment. The volume of ascites determined during autopsy in the reference group varied from 31 to 122 mL (median, 66 mL), compared with volume of 28 to 34 mL (median: 31 mL) in the 1st experimental group. The difference in ascites volumes between the two groups was statistically significant (р <0.05, according to the Mann-Whitney criterion). Two animals from the first experimental group survived for more than two weeks and were withdrawn from the experiment on day 207.
Changes in appearance and behavior of animals of the reference group were seen at 8 days and were observed for 2-3 days until death of animals. Their fur became lackluster and scraggly. The animals were depressed, lacking curiosity and reaction to other animals, low motor activity, with absence of vertical postures. The amount of consumed food decreased, whereas the amount of consumed water remained the same. If an animal died within 8 days after injecting hepatoma cells, the above changes were not observed.
In the animals treated with doxorubicin (the 1st experimental group), exterior and behavior started to change later (in 10 days). The changes in fur appearance were similar to those in reference group. The animals were depressed, moved slowly (crawled); reddish eyelids and developed yellowish crusts (simple blepharitis) were observed. Of note, no eye pathology was seen in the animals of the reference group. Two animals of experimental group that died within 10 days after treatment did not show these changes.
In two surviving animals from the 1st experimental group (that survived for 207 days after implantation of hepatoma cells and injection of DOX in CaCO3+DexS delivery systems), ascites was revealed in 4 days (physical examination). From 10 to 14 days of the experiment, changed appearance (dull and scraggly fur), behavior and motion activity (depression, absence of vertical postures) were observed. Slight reddening of eyelids, decrease in food consumption and weight loss also occurred. Then the above sickness symptoms disappeared, the animals started to feed normally and gained weight. No signs of Seidel hepatoma and ascites were revealed during autopsy.
The observations described above allowed us to make the following conclusions: DOX applied intraperitoneally in the DS based on calcium carbonate cores doped with DexS affects development of the tumor, manifesting as decreased volume of ascitic fluid. Low percentage of survival (only two animals) may be explained by low dose of antitumor preparation (2 mg per animal). In this series of experiments, concentrations of doxorubicin in blood were not determined.
Since the animals tolerated treatment with DOX in (CaCO3+DexS) delivery systems relatively well, and the applied dose (2 mg of doxorubicin per animal) inhibited development of Seidel hepatoma, one may assume that the amount of injected preparation in DS can be increased. Therefore, the next experiment with laboratory animals was designed.
Second group of rats
Time dynamics of DOX release in blood of laboratory animals after i.p. administration of two types of delivery systems were studied. The two kinds of delivery systems were used: porous calcium carbonate cores doped with DexS (CaCO3+DexS) (DS1), and DexS-DOX conjugates (DS2).
The 2nd experimental group of rats included 17 healthy animals (body weight 270-310 g). Six rats (2 females, 4 males) were treated with DOX in DS1 (4 mg per animal); the remaining eleven animals (6 females, 5 males) were treated with DOX in DS2 (4 mg of DOX per animal). The 2nd reference group for treatment with free DOX consisted of 3 rats (2 females, 1 male) weighing from 260 to 280 g.
Upon i.p. administration of CаСО3+DexS delivery systems containing DOX, the animals were active, no inflammation symptoms were observed in the injection area. During the experiment, no changes in the state of fur, eyes, neither abnormal behavior nor reactions were registered. The body temperature was typical of healthy rats. Starting from the 2nd day, consumption of food and water was common for the animals kept in the vivarium (from 3 to 8 mL daily per 100 g of body mass, and from 4.4 to 4.7 g of food daily per 100 g of body mass). One of 11 rats that were given DOX in DS2 (DexS-DOX conjugates) died at 12 days after drug administration. Autopsy of the dead rat revealed neither macroscopic changes in internal organs, nor defects of DOX administration.
Concentration of DOX in rat blood plasma after administration of various delivery systems

Figure 1. DOX concentrations in plasma after intraperitoneal administration to the rats: CaCO3+DexS+DOX (1); free DOX (2); DexS+DOX (3)
In what follows, we describe the results of experiments involving healthy rats; free DOX and two types of DOX-containing delivery systems were injected intraperitoneally. Like as in the first experimental series, DOX was encapsulated in calcium carbonate cores doped with DexS and included in conjugates with this polyanion. During i.p. administration, the delivery systems enter intercellular fluid which is nearly similar to blood plasma. In our earlier works [14, 16], it has been demonstrated that vaterites were gradually destroyed in plasma, resulting into increased release rate of encapsulated compounds from porous carrier. Moreover, treatment of vaterites with DexS made it possible to reach prolonged release of DOX into blood plasma. Scanning electron microscopy demonstrated that structural changes in hybrid delivery systems occurring in plasma correlated with DOX release profiles [14]. After introduction of various delivery systems, DOX concentration in rat blood plasma was determined and compared with the concentration reached after administration of free DOX. Time profiles of DOX concentrations are seen in Fig. 1.
Before analysis of the obtained results, let us compare the literature data on DOX concentrations in blood of various tumor-bearing animals. It should be noted that the results obtained in experiments with rats cannot be directly used in pharmacology. The authors of [17] emphasize that it is necessary to estimate interspecies differences in distribution and elimination of drugs.
Free DOX is rapidly assimilated by organisms of DBA2 mice. After intravenous introduction of 7 mg/kg of the drug, its concentration in plasma decreases by a factor of 100 within an hour (down to 0.2 nM/mL=116 ng/mL [10]). A 10-fold decrease in plasma DOX concentration (down to 1 µg/mL) was observed within 4 h, when almost similar amount of free DOX (5 mg/kg) was administered intravenously (i.v.) to tumor HeLa-bearing mice, [11]. Upon i.v. injection of DOX (10 mg/kg) to tumor MCF-7 bearing mice, the drug disappeared during 4 days. At 12 hrs, 70 ng/mL of DOX was found in plasma. Signs of cardiotoxicity (release of characteristic enzymes) were observed in 2 weeks after DOX administration [12]. The results of studies involving breast cancer patients treated with standard amounts of DOX showed that the concentrations of DOX in plasma ranged between 12 and 620 ng/mL. The risk of cardiomyopathy in such patients was under 4% [13]. Hence, DOX concentration in plasma after treatment may vary from 10 to 600 ng/mL. The total amount of drug in blood is directly proportional to animal size and, therefore, to blood volume.
One may assume that determination of DOX concentrations in rat blood plasma within the mentioned range, together with physical examination of animals, allows comparing effects of various DOX delivery systems after i.p. administration. Moreover, we should consider the fact that DOX is not only present in blood, but distributed in all organs of an animal.
Comparison of time profiles of DOX release from DexS-coated CaCO3 cores and from DexS+DОХ conjugates into human blood plasma is discussed in [2]. In the case of conjugates, the DOX release profile (unlike that from doped carbonate cores) demonstrated burst release of the drug for the first 24 h. Later on, gradual increase in the released amount of drug (up to 70%) was observed for 2 weeks. As shown in Fig. 1, (curve 3) injection of the fast-releasing carriers is associated with higher DOX levels in the bloodstream.
The authors of [11] compared time profiles of DOX concentration in blood plasma of HeLa-bearing mice after i.v. administration of free DOX versus DOX incorporated into nanoparticles of various compositions. Similar results were obtained in the present work. High amounts of DOX were released from DS2 nanocarriers into plasma during the first hour after administration, unlike the case of free DOX injection (Fig. 1, curve 2). Increased plasma DOX concentration early after injection may be caused by release of DOX molecules that were weakly bound to the carriers. Similar situation is possible in the case of polymer-drug conjugates formation; despite equimolar ratio of components, complete attachment of DOX to DexS did not occur, due to considerable difference in sizes of molecules.
Calcium carbonate cores doped with DexS provided prolonged release of DOX with gradually increased concentration (Fig.1, curve 1). Note that DOX concentrations in plasma lie in the nanogram range typical of humans and animals treated with DOX.
Besides, the size range of the DOX delivery systems should be taken into account; they determine ability of the drug to penetrate into organs and tissues. Of course, nanosized conjugates have advantages over submicron-sized doped carbonate cores. Probably, this factor is responsible for increased concentration of released DOX by the end of second week after injection of the drug within polymer-drug conjugates.
Conclusions
Intraperitoneal administration of 2 mg of DOX in DS based on calcium carbonate cores doped with DexS in rats inoculated with Seidel hepatoma resulted in increase of life expectancy by 1.75 times and in decrease in ascites volume in laboratory animals. It is expected that increase in the dosage up to 4 mg per animal will lead to more efficient inhibition of tumor growth. This dose was used in the studies of dynamics of DOX release into blood plasma after i.p. administration of the drug to intact rats using delivery systems of various structures. In 2 days after introduction of free DOX to rats, the drug disappeared from blood plasma. Application of the delivery systems made it possible to prolong presence of the drug in blood. When similar amounts of DOX (4 mg per animal) were introduced by means of various delivery systems, DOX was present in blood plasma at different amounts, depending on the structure of a delivery system. Porous calcium carbonate cores doped with DexS allowed for release of DOX within 2 weeks at the rates under cardiotoxic concentrations. The release profile of DOX in blood plasma after injection of polymer-drug conjugate DexS-DOX had a complex pattern, due to the carrier structure. These DS release significantly higher amounts of DOX into plasma by 14th day after beginning the experiment. Despite the difference in DOX release profiles, neither calcium carbonate, nor conjugate DS caused negative reactions in rats, as confirmed by observations of behavior and physical state of the animals, and autopsy results. Therefore, both studied drug delivery systems could be used for prolonged regional administration of antitumor DOX preparation.
Financial support
The study was performed within the framework of budget-supported research project №АААА-А20-120022090044-2, Institute of Macromolecular Compounds, RAS.
Conflict of interests
None declared.
References
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185-229. doi: 10.1124/pr.56.2.6
- Sudareva N, Suvorova O, Saprykina N, Vlasova E, Vilesov A. DOX delivery systems based on doped CaCO3 cores and polyanion-drug conjugates. J Microencapsul. 2021;38:164-176. doi: 10.1080/02652048.2021.1872724
- Popryadukhin P, Sudareva N, Suvorova O, Yukina G, Sukhorukova E, Saprykina N, et al. Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue. Cell Ther Transplant. 2020;9:78-84.
doi: 10.18620/ctt-1866-8836-2020-9-4-78-84 - Sudareva N, Suvorova O, Saprykina N, Smirnova N, Bel'tiukov P, Petunov S, et al. Two-level delivery systems based on CaCO3 cores for oral administration of therapeutic peptides. J Microencapsul. 2018; 35:619-634. doi: 10.1080/02652048.2018.1559247
- Youn Y, Bae H. Perspectives on the past, present, and future of cancer nanomedicine. Adv Drug Delivery Rev. 2018;130:3-11.
doi: 10.1016/j.addr.2018.05.008 - Alven S, Nqoro X, Buyana B, Aderibigbe B. Polymer-drug conjugate, a potential therapeutic to combat breast and lung cancer. Pharmaceutics, 2020;12:406. doi: 10.3390/pharmaceutics12050406
- Guidelines for the experimental (preclinical) study of new pharmacological substances. (Ed. Khabriev R). Medicine Publishers. 2005, 832 p (In Russian).
- Revtovich M, Istomin U, Krasko O, Treshchalina E, Bichkovsky P, Yurkschtovich T, et al. Antitumor activity of the hydrogel form of cisplatin on Seidel ascitic hepatoma. Russ Biother J. 2016;15:96-101. (In Russian). doi: 10.17650/1726-9784-2016-15-4-96-101
- Adiseshaiah P, Hall J, McNeil S. Nanomaterial standards for efficacy and toxicity assessment. WIREs Nanomed Nanobiotechnol. 2009;2:99,112. doi: 10.1002/wnan.66
- Baurain R, Deprez-De Campeneere D, Trouet A. Determination of Daunorubicin, Doxorubicin and their fluorescent metabolites by high-pressure liquid chromatography: plasma levels in DBA2mice. Cancer Chemother Pharmacol. 1979;2:11-14.
- Zhang C, Wu Y, Dong Y, Xu H, Zhao I. Quantification of DOX bioavailability in biological samples of mice by sensitive and precise HPLC assay. Pharm Biol. 2016; 54:55-61. doi: 10.3109/13880209.2015.1014918
- Mondal L, Mukherjee B, Das K, Bhattacharya S, Dutta D, Chakraborty S, et al. CD-340 functionalized doxorubicin-loaded nanoparticle induces apoptosis and reduces tumor volume along with drug-related cardiotoxicity in mice. Int J Nanomed. 2019;14:8073-8094.
doi: 10.2147/IJN.S220740 - Harahap Y, Ardiningsih P, Winarti A, Purwanto D. Analysis of the Doxorubicin and Doxorubicinol in the plasma of breast cancer patients for monitoring the toxicity of Doxorubicin. Drug Design Devel Ther. 2020;14:3469-3475. doi: 10.2147/DDDT.S251144
- Sudareva N, Suvorova O, Saprykina N, Tomson V, Suslov D, Galibin O, Vilesov A. Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma. Cell Ther Transplant. 2021; 10:79-85.
doi: 10.18620/ctt-1866-8836-2021-10-1-79-85 - Freireich E, Gehan E, Rall D, Schmidt L, Skipper H. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother Res. 1966;50:219-224.
- Sudareva N, Popryadukhin P, Saprykina N, Suvorova O, Yukina G, Galibin O, Vilesov A. CaCO3 vaterite as components of target drug delivery systems. Cell Ther Transplant. 2020;9:13-19. doi: 10.18620/ctt-1866-8836-2020-9-2-13-19
- Li Y, Zhang C, Zhou S, He M, Zhang H, et al. Species difference in paclitaxel disposition correlated with poor pharmacological efficacy translation from mice to humans. Clin Pharm Adv Appl. 2018;10:165-174. doi: 10.2147/CPAA.S185449
" ["~DETAIL_TEXT"]=> string(31571) "
Introduction
Doxorubicin is a very potent anticancer antibiotic that, unfortunately, demonstrates considerable cardiotoxicity, accumulation in liver and rapid clearance [1]. Use of various delivery systems for DOX in chemotherapy makes it possible to decrease negative influence of the drug and provides its prolonged release. In our previous work [2], it has been shown that porous carbonate vaterites modified with various polyanions can be successfully applied for encapsulation of DOX and facilitate prolonged in vitro release of the substance into blood plasma. Besides, it has been proven that these delivery systems (DS) are bio-resorbable and safe to use [3, 4]. The advantages of these DS in chemotherapy are related to some peculiar features of defense mechanisms of tumor cells against chemotherapeutic compounds. Drug efflux is among these mechanisms which does not affect DS (unlike free DOX). Phagocytosis of drugs by a cell is preceded by opsonization. Mechanism of opsonization (sorption of various plasma components on surface of delivery system) is impaired when a DOX-containing DS is coated with polyelectrolytes. As a result, opsonization is reduced, and the time of passive DS circulation is prolonged in the area of high capillary permeability and disturbed lymphatic drainage (typical of tumor tissues [5]); i.e., the drug is retained in the tumor tissue.
Among other delivery systems for DOX, polymer-drug conjugates (PDC) have been described [6]. In our earlier work [2], the profiles of DOX release into blood plasma have been compared for PDC (DexS+DOX) versus DexS coated СаСО3 cores.
Ascitic tumors (particularly, Seidel hepatoma, SH) are widely used as models for in vivo studies of regional intraperitoneal chemotherapy. These tumors are included into the list recommended by the Russian Ministry of Health and the State Pharmacological Committee for studies of antitumor mechanisms [7].
Revtovich et al. [8] compared antitumor activity of cisplatin and its prolonged pharmaceutical form based on dextran phosphate hydrogel in the rats inoculated with Seidel hepatoma. They have demonstrated higher efficiency of the modified drug.
When free DOX or DOX-containing delivery systems are used in chemotherapy, it is important to control concentration of the substance in blood. Administration of free DOX results in its distribution through all the organs and tissues. When delivery systems are used, distribution of the drug in organism largely depends on DS structure (i.e., drug release rate) and its administration route (intravenous, intraperitoneal, subcutaneous, etc. [9]). The amount of introduced drug and physiology of an organism are also of importance.
Comparing the literature data on DOX concentration in blood plasma after intravenous administration to different tumor-bearing mice and women, we can see that the amount of introduced DOX exerts relatively low influence on its content in plasma, whereas tumor type is a considerable factor [10-13].
The aims of the present work included (i) studies of the opportunity to use the proposed delivery model of antitumor preparation in laboratory animals; (ii) time course evaluation of DOX release into blood following intraperitoneal administration, using drug delivery systems based on CaCO3 cores coated with DexS, or DexS-DOX conjugates (HPLC studies of DOX release); (iii) comparison between the profiles of DOX release into rat blood (both in healthy rats and animals with Seidel hepatoma), and general condition of animals, thus discerning effects of the released DOX amounts upon malignant cells in tumor-bearing animals and upon intact rats.
Materials and methods
Reagents
Doxorubicin hydrochloride purchased as the “Sindroxocin” preparation, containing 17% of doxorubicin (DOX) and 83% of lactose, was from Actavis (Hafnarfjordur, Iceland). For experiments, doxorubicin salt with protonated amino group (–NH3+) was used. Salts (CaCl2 × 2H2O, Na2CO3), acetone, and dextran sulfate (Mw = 9-20 kDa) were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis of carbonate cores
Preparation techniques for porous carbonate vaterites, methods for coating vaterites with DexS polyanion and introducing DOX into these carriers are described in [14]. Briefly, porous vaterites (СаСО3 cores) were prepared by co-precipitation. Equal volumes of 1 M aqueous solutions of CaCl2 × 2H2O and Na2CO3 were rapidly mixed at stirring (1000 rpm) with an RW 20 anchor-type mechanical stirrer (Kika-Werk, Switzerland). The mixture was stirred for 30 s. The suspension was then filtered through Schott filter glass (#16), washed thrice with distilled water and with acetone/water mixtures with increasing acetone concentrations (33%, 50%, and 100%). The precipitate was dried in thermostat at 40-50°C until a constant weight was achieved.
Doping of carbonate cores with DexS and DOX loading into delivery systems
The cores were coated with a polyanion (sodium salt of dextran sulfate, DexS). СаСО3 cores (50 mg) were added to 1 mg/mL aqueous solution of DexS (10 mL). The suspension was stirred using a Multi Bio RS-24 rotor (Biosan, Latvia) for 1 h; the solid fraction was filtered off using a Schott glass filter (#16), washed thrice with distilled water and dried at 30°C.
DOX was loaded into doped CaCO3 under continuous stirring of the mixture of CaCO3 suspension and DOX solution (C=2 mg/mL) for 24 hours. The DOX/(CaCO3+DexS) ratio was 0.4. After mixing, the suspension was centrifuged at 8000 rpm for 3 min, and the DOX amount in supernatant was determined.
DOX load (L) was calculated using the following equation: L = (mi − ms)/mP, where mi is the initial amount of DOX (mg), ms is the amount of non-encapsulated DOX in supernatant solution (mg), mP is the amount of particles (mg).
DOX concentrations were determined using the calibration curves obtained from optical density measurements in the corresponding solvents at λ=480 nm. The measurements were carried out using a SF-2000 spectrophotometer (LOMO, St. Petersburg, Russia).
Polymer-drug conjugates and their loading
Polymer-drug conjugates (DexS+DOX) were prepared according to the technique described in [2]. The DOX solution (2 mg/mL) and solution of DexS polyanion (1 mg/mL) were mixed in volumes that provided an equimolar ratio of functional groups. Then the mixture was subjected to ultrasound treatment for 2 min and centrifuged at 8000 rpm for 10 min. The supernatant was removed, the residue was freeze-dried. The DOX concentration in supernatant was determined spectrophotometrically. The PDC load was determined by subtracting the amount of DOX in supernatant from total amount of introduced DOX followed by dividing this quantity by the weight of the residue. All the measurements were carried out thrice.
In vivo experiments with rats
Two groups of rats were used in the experiments with intraperitoneal administration of various DOX delivery systems. The first group (experimental) included the rats with inoculated Seidel hepatoma; DOX encapsulated into calcium carbonate cores doped with DexS polyanion was administrated intraperitoneally (i.p.). The second group consisted of healthy rats; they were treated i.p. with DOX incorporated into different delivery systems: (i) submicron-sized calcium carbonate cores coated with DexS polyanion (CaCO3+DexS); (ii) nanosized polymer-drug conjugates (DexS+DOX). Two reference groups were also included, i.e., the first reference group consisted of non-treated animals with inoculated Seidel hepatoma (no DOX included in the СаСО3+DexS delivery system was introduced). The second reference group consisted of healthy rats that were treated with free DOX (without delivery systems).
Thirty-six male and female outbred rats with body weight ranging from 256 to 312 g were used in the experiments ("Rappolovo" nursery for laboratory animals). All manipulations with animals were performed under general anesthesia: Sol. Zoletil 50 (0.05 mL per 0.1 kg of body mass), Sol. Rometаrum 20 mg/mL (0.0125 mL per 0.1 kg of body mass, intramuscularly). The animals were caged (2-5 individuals in a cage), had free access to water and food (4R F18 prolonged keeping formula for rodents, Macedonia, Italy). The animals were fed the standard diet for laboratory rats used in the vivarium of A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia.
The animals of experimental and reference groups were examined daily; consumption of water and food was registered, body temperature and weight were measured. Behavior of animals and life expectancy were estimated. Immediately after death, ascitic fluid was collected, and its volume was determined. All the manipulations with animals were performed in accordance with State Standard 33216-2014 "Regulations for work with laboratory rodents and rabbits".
Transplantation of ascitic Seidel hepatoma (SH)
Resuspended cells of ascitic Seidel hepatoma (freshly defrosted and washed from dimethyl sulfoxide) were injected into abdominal cavity of rats from the 1st group. Ascitic fluid containing Seidel hepatoma cells (1 mL) was introduced intraperitoneally using a needle (21 G). The dosage was calculated using the Freireich quotient [15].
Introduction of DOX into rats
Doxorubicin (both free and encapsulated in delivery systems) was administered to anaesthesized animals using Zoletil 50, 0.05 mL per 0.1 kg of body mass i.p.). The drug was injected in 1.5 mL of 5% glucose solution, containing DOX in CаСО3+DexS cores, or DOX in polymer drug conjugates (DexS+DOX), or free DOX (2 or 4 mg of DOX per 1 animal of the 1st and 2nd groups, respectively). The drug preparations were injected by means of 21-gauge needles. For the animals of the 1st experimental group, the tumor cells and DOX-containing delivery systems were applied simultaneously. Examination of animals from the 1st group was described elsewhere ("In vivo experiments with rats").
Along with visual inspection, peripheral blood (1.0 mL) was taken from tail vein of rats of the 1st group 24 h, at 4, 7, 14, 17 and 21 days after drug injection. Before blood sampling, the rat was examined, weighed, its body temperature was measured, then it was anesthetized and fixed in a holder for immobilizing rodents. Plasma was obtained from the blood specimens 10 min after blood sampling by centrifugation for 15 min at 1500 rpm. The supernatantses were frozen and stored in closed vessels at -40°C for further analysis.
Determination of DOX content in plasma of rats
Content of doxorubicin (DOX) in rat blood plasma was determined by high-performance liquid chromatography (HPLC) using Prominence-I LC 2030C 3D Plus instrument (Shimadzu) equipped with an RF-20A fluorimetric detector and a 5 µm Luna C18 column (Phenomenex). The excitation wavelength was 475 nm, emission wavelength was 555 nm. Analysis was performed in the gradient elution regime (with acetonitrile) in 0.01 N Na-formiate buffer (рН 3.68). Duration of experiment: was 20 min, at the detection limit of 1 ng/mL. All the measurements were carried out thrice.
Results and discussion
First group of rats
The influence of DOX delivery systems based on porous calcium carbonate cores coated with DexS (CaCO3+DexS) on development of Seidel hepatoma was studied in laboratory rats.
The first experimental group included 4 male and 4 female rats with body mass varying from 256 to 306 g. The first reference group consisted of 5 male and 3 female animals (266 to 312 g). The animals were followed up as described under Materials and Methods.
Six animals from the first experimental group died within a period from 10 to 14 days after the procedure (median, 14 days). All animals of the reference group died within a period from 8 to 13 days after the beginning of the experiment; the lifetime median was 8 days. Physical examination revealed ascites in animals of both groups starting from the 4th day after starting the experiment. The volume of ascites determined during autopsy in the reference group varied from 31 to 122 mL (median, 66 mL), compared with volume of 28 to 34 mL (median: 31 mL) in the 1st experimental group. The difference in ascites volumes between the two groups was statistically significant (р <0.05, according to the Mann-Whitney criterion). Two animals from the first experimental group survived for more than two weeks and were withdrawn from the experiment on day 207.
Changes in appearance and behavior of animals of the reference group were seen at 8 days and were observed for 2-3 days until death of animals. Their fur became lackluster and scraggly. The animals were depressed, lacking curiosity and reaction to other animals, low motor activity, with absence of vertical postures. The amount of consumed food decreased, whereas the amount of consumed water remained the same. If an animal died within 8 days after injecting hepatoma cells, the above changes were not observed.
In the animals treated with doxorubicin (the 1st experimental group), exterior and behavior started to change later (in 10 days). The changes in fur appearance were similar to those in reference group. The animals were depressed, moved slowly (crawled); reddish eyelids and developed yellowish crusts (simple blepharitis) were observed. Of note, no eye pathology was seen in the animals of the reference group. Two animals of experimental group that died within 10 days after treatment did not show these changes.
In two surviving animals from the 1st experimental group (that survived for 207 days after implantation of hepatoma cells and injection of DOX in CaCO3+DexS delivery systems), ascites was revealed in 4 days (physical examination). From 10 to 14 days of the experiment, changed appearance (dull and scraggly fur), behavior and motion activity (depression, absence of vertical postures) were observed. Slight reddening of eyelids, decrease in food consumption and weight loss also occurred. Then the above sickness symptoms disappeared, the animals started to feed normally and gained weight. No signs of Seidel hepatoma and ascites were revealed during autopsy.
The observations described above allowed us to make the following conclusions: DOX applied intraperitoneally in the DS based on calcium carbonate cores doped with DexS affects development of the tumor, manifesting as decreased volume of ascitic fluid. Low percentage of survival (only two animals) may be explained by low dose of antitumor preparation (2 mg per animal). In this series of experiments, concentrations of doxorubicin in blood were not determined.
Since the animals tolerated treatment with DOX in (CaCO3+DexS) delivery systems relatively well, and the applied dose (2 mg of doxorubicin per animal) inhibited development of Seidel hepatoma, one may assume that the amount of injected preparation in DS can be increased. Therefore, the next experiment with laboratory animals was designed.
Second group of rats
Time dynamics of DOX release in blood of laboratory animals after i.p. administration of two types of delivery systems were studied. The two kinds of delivery systems were used: porous calcium carbonate cores doped with DexS (CaCO3+DexS) (DS1), and DexS-DOX conjugates (DS2).
The 2nd experimental group of rats included 17 healthy animals (body weight 270-310 g). Six rats (2 females, 4 males) were treated with DOX in DS1 (4 mg per animal); the remaining eleven animals (6 females, 5 males) were treated with DOX in DS2 (4 mg of DOX per animal). The 2nd reference group for treatment with free DOX consisted of 3 rats (2 females, 1 male) weighing from 260 to 280 g.
Upon i.p. administration of CаСО3+DexS delivery systems containing DOX, the animals were active, no inflammation symptoms were observed in the injection area. During the experiment, no changes in the state of fur, eyes, neither abnormal behavior nor reactions were registered. The body temperature was typical of healthy rats. Starting from the 2nd day, consumption of food and water was common for the animals kept in the vivarium (from 3 to 8 mL daily per 100 g of body mass, and from 4.4 to 4.7 g of food daily per 100 g of body mass). One of 11 rats that were given DOX in DS2 (DexS-DOX conjugates) died at 12 days after drug administration. Autopsy of the dead rat revealed neither macroscopic changes in internal organs, nor defects of DOX administration.
Concentration of DOX in rat blood plasma after administration of various delivery systems

Figure 1. DOX concentrations in plasma after intraperitoneal administration to the rats: CaCO3+DexS+DOX (1); free DOX (2); DexS+DOX (3)
In what follows, we describe the results of experiments involving healthy rats; free DOX and two types of DOX-containing delivery systems were injected intraperitoneally. Like as in the first experimental series, DOX was encapsulated in calcium carbonate cores doped with DexS and included in conjugates with this polyanion. During i.p. administration, the delivery systems enter intercellular fluid which is nearly similar to blood plasma. In our earlier works [14, 16], it has been demonstrated that vaterites were gradually destroyed in plasma, resulting into increased release rate of encapsulated compounds from porous carrier. Moreover, treatment of vaterites with DexS made it possible to reach prolonged release of DOX into blood plasma. Scanning electron microscopy demonstrated that structural changes in hybrid delivery systems occurring in plasma correlated with DOX release profiles [14]. After introduction of various delivery systems, DOX concentration in rat blood plasma was determined and compared with the concentration reached after administration of free DOX. Time profiles of DOX concentrations are seen in Fig. 1.
Before analysis of the obtained results, let us compare the literature data on DOX concentrations in blood of various tumor-bearing animals. It should be noted that the results obtained in experiments with rats cannot be directly used in pharmacology. The authors of [17] emphasize that it is necessary to estimate interspecies differences in distribution and elimination of drugs.
Free DOX is rapidly assimilated by organisms of DBA2 mice. After intravenous introduction of 7 mg/kg of the drug, its concentration in plasma decreases by a factor of 100 within an hour (down to 0.2 nM/mL=116 ng/mL [10]). A 10-fold decrease in plasma DOX concentration (down to 1 µg/mL) was observed within 4 h, when almost similar amount of free DOX (5 mg/kg) was administered intravenously (i.v.) to tumor HeLa-bearing mice, [11]. Upon i.v. injection of DOX (10 mg/kg) to tumor MCF-7 bearing mice, the drug disappeared during 4 days. At 12 hrs, 70 ng/mL of DOX was found in plasma. Signs of cardiotoxicity (release of characteristic enzymes) were observed in 2 weeks after DOX administration [12]. The results of studies involving breast cancer patients treated with standard amounts of DOX showed that the concentrations of DOX in plasma ranged between 12 and 620 ng/mL. The risk of cardiomyopathy in such patients was under 4% [13]. Hence, DOX concentration in plasma after treatment may vary from 10 to 600 ng/mL. The total amount of drug in blood is directly proportional to animal size and, therefore, to blood volume.
One may assume that determination of DOX concentrations in rat blood plasma within the mentioned range, together with physical examination of animals, allows comparing effects of various DOX delivery systems after i.p. administration. Moreover, we should consider the fact that DOX is not only present in blood, but distributed in all organs of an animal.
Comparison of time profiles of DOX release from DexS-coated CaCO3 cores and from DexS+DОХ conjugates into human blood plasma is discussed in [2]. In the case of conjugates, the DOX release profile (unlike that from doped carbonate cores) demonstrated burst release of the drug for the first 24 h. Later on, gradual increase in the released amount of drug (up to 70%) was observed for 2 weeks. As shown in Fig. 1, (curve 3) injection of the fast-releasing carriers is associated with higher DOX levels in the bloodstream.
The authors of [11] compared time profiles of DOX concentration in blood plasma of HeLa-bearing mice after i.v. administration of free DOX versus DOX incorporated into nanoparticles of various compositions. Similar results were obtained in the present work. High amounts of DOX were released from DS2 nanocarriers into plasma during the first hour after administration, unlike the case of free DOX injection (Fig. 1, curve 2). Increased plasma DOX concentration early after injection may be caused by release of DOX molecules that were weakly bound to the carriers. Similar situation is possible in the case of polymer-drug conjugates formation; despite equimolar ratio of components, complete attachment of DOX to DexS did not occur, due to considerable difference in sizes of molecules.
Calcium carbonate cores doped with DexS provided prolonged release of DOX with gradually increased concentration (Fig.1, curve 1). Note that DOX concentrations in plasma lie in the nanogram range typical of humans and animals treated with DOX.
Besides, the size range of the DOX delivery systems should be taken into account; they determine ability of the drug to penetrate into organs and tissues. Of course, nanosized conjugates have advantages over submicron-sized doped carbonate cores. Probably, this factor is responsible for increased concentration of released DOX by the end of second week after injection of the drug within polymer-drug conjugates.
Conclusions
Intraperitoneal administration of 2 mg of DOX in DS based on calcium carbonate cores doped with DexS in rats inoculated with Seidel hepatoma resulted in increase of life expectancy by 1.75 times and in decrease in ascites volume in laboratory animals. It is expected that increase in the dosage up to 4 mg per animal will lead to more efficient inhibition of tumor growth. This dose was used in the studies of dynamics of DOX release into blood plasma after i.p. administration of the drug to intact rats using delivery systems of various structures. In 2 days after introduction of free DOX to rats, the drug disappeared from blood plasma. Application of the delivery systems made it possible to prolong presence of the drug in blood. When similar amounts of DOX (4 mg per animal) were introduced by means of various delivery systems, DOX was present in blood plasma at different amounts, depending on the structure of a delivery system. Porous calcium carbonate cores doped with DexS allowed for release of DOX within 2 weeks at the rates under cardiotoxic concentrations. The release profile of DOX in blood plasma after injection of polymer-drug conjugate DexS-DOX had a complex pattern, due to the carrier structure. These DS release significantly higher amounts of DOX into plasma by 14th day after beginning the experiment. Despite the difference in DOX release profiles, neither calcium carbonate, nor conjugate DS caused negative reactions in rats, as confirmed by observations of behavior and physical state of the animals, and autopsy results. Therefore, both studied drug delivery systems could be used for prolonged regional administration of antitumor DOX preparation.
Financial support
The study was performed within the framework of budget-supported research project №АААА-А20-120022090044-2, Institute of Macromolecular Compounds, RAS.
Conflict of interests
None declared.
References
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185-229. doi: 10.1124/pr.56.2.6
- Sudareva N, Suvorova O, Saprykina N, Vlasova E, Vilesov A. DOX delivery systems based on doped CaCO3 cores and polyanion-drug conjugates. J Microencapsul. 2021;38:164-176. doi: 10.1080/02652048.2021.1872724
- Popryadukhin P, Sudareva N, Suvorova O, Yukina G, Sukhorukova E, Saprykina N, et al. Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue. Cell Ther Transplant. 2020;9:78-84.
doi: 10.18620/ctt-1866-8836-2020-9-4-78-84 - Sudareva N, Suvorova O, Saprykina N, Smirnova N, Bel'tiukov P, Petunov S, et al. Two-level delivery systems based on CaCO3 cores for oral administration of therapeutic peptides. J Microencapsul. 2018; 35:619-634. doi: 10.1080/02652048.2018.1559247
- Youn Y, Bae H. Perspectives on the past, present, and future of cancer nanomedicine. Adv Drug Delivery Rev. 2018;130:3-11.
doi: 10.1016/j.addr.2018.05.008 - Alven S, Nqoro X, Buyana B, Aderibigbe B. Polymer-drug conjugate, a potential therapeutic to combat breast and lung cancer. Pharmaceutics, 2020;12:406. doi: 10.3390/pharmaceutics12050406
- Guidelines for the experimental (preclinical) study of new pharmacological substances. (Ed. Khabriev R). Medicine Publishers. 2005, 832 p (In Russian).
- Revtovich M, Istomin U, Krasko O, Treshchalina E, Bichkovsky P, Yurkschtovich T, et al. Antitumor activity of the hydrogel form of cisplatin on Seidel ascitic hepatoma. Russ Biother J. 2016;15:96-101. (In Russian). doi: 10.17650/1726-9784-2016-15-4-96-101
- Adiseshaiah P, Hall J, McNeil S. Nanomaterial standards for efficacy and toxicity assessment. WIREs Nanomed Nanobiotechnol. 2009;2:99,112. doi: 10.1002/wnan.66
- Baurain R, Deprez-De Campeneere D, Trouet A. Determination of Daunorubicin, Doxorubicin and their fluorescent metabolites by high-pressure liquid chromatography: plasma levels in DBA2mice. Cancer Chemother Pharmacol. 1979;2:11-14.
- Zhang C, Wu Y, Dong Y, Xu H, Zhao I. Quantification of DOX bioavailability in biological samples of mice by sensitive and precise HPLC assay. Pharm Biol. 2016; 54:55-61. doi: 10.3109/13880209.2015.1014918
- Mondal L, Mukherjee B, Das K, Bhattacharya S, Dutta D, Chakraborty S, et al. CD-340 functionalized doxorubicin-loaded nanoparticle induces apoptosis and reduces tumor volume along with drug-related cardiotoxicity in mice. Int J Nanomed. 2019;14:8073-8094.
doi: 10.2147/IJN.S220740 - Harahap Y, Ardiningsih P, Winarti A, Purwanto D. Analysis of the Doxorubicin and Doxorubicinol in the plasma of breast cancer patients for monitoring the toxicity of Doxorubicin. Drug Design Devel Ther. 2020;14:3469-3475. doi: 10.2147/DDDT.S251144
- Sudareva N, Suvorova O, Saprykina N, Tomson V, Suslov D, Galibin O, Vilesov A. Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma. Cell Ther Transplant. 2021; 10:79-85.
doi: 10.18620/ctt-1866-8836-2021-10-1-79-85 - Freireich E, Gehan E, Rall D, Schmidt L, Skipper H. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother Res. 1966;50:219-224.
- Sudareva N, Popryadukhin P, Saprykina N, Suvorova O, Yukina G, Galibin O, Vilesov A. CaCO3 vaterite as components of target drug delivery systems. Cell Ther Transplant. 2020;9:13-19. doi: 10.18620/ctt-1866-8836-2020-9-2-13-19
- Li Y, Zhang C, Zhou S, He M, Zhang H, et al. Species difference in paclitaxel disposition correlated with poor pharmacological efficacy translation from mice to humans. Clin Pharm Adv Appl. 2018;10:165-174. doi: 10.2147/CPAA.S185449
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(100) "saso3-vaterity-pokrytye-dekstransulfatom-kak-sistemy-dlya-regionarnogo-vvedeniya-doksorubitsina-krys" ["~CODE"]=> string(100) "saso3-vaterity-pokrytye-dekstransulfatom-kak-sistemy-dlya-regionarnogo-vvedeniya-doksorubitsina-krys" ["EXTERNAL_ID"]=> string(4) "2047" ["~EXTERNAL_ID"]=> string(4) "2047" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(295) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысамDextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(3344) "<p style="text-align: justify;">Доксорубицин (ДОХ) – водорастворимый антрациклиновый антибиотик, обладающий высокой противораковой эффективностью. Можно добиться уменьшения концентрации ДОХ в крови ниже кардиотоксического уровня в процессе терапии, формируя депо, содержащее системы доставки ДОХ с пролонгированным высвобождением лекарства. Для этих целей использовали кальций карбонатные пористые ватериты, допированные полианионом декстран сульфатом. Субмикронные размеры носителей не позволяют свободно включаться им в кровеносное русло. Распространяется токсическое лекарство по организму только после попадания в кровь в результате высвобождения из систем доставки. Внутрибрюшинное введение крысам с перевитой гепатомой Зайделя ДОХ-содержащих систем доставки позволило оценить эффективную концентрацию ДОХ, тормозящую рост опухоли и уменьшающую объем асцитной жидкости. Динамику поступления ДОХ в кровь здоровых крыс после внутрибрюшинного введения 4 мг ДОХ в системах доставки различной природы определяли методом ВЭЖХ. Введенное при помощи допированных декстрансульфатом субмикронных карбонатных ядер лекарство высвобождается в кровь крыс в течение двух недель в концентрациях, меньших токсичных значений. При использовании в качестве системы доставки наноразмерного конъюгата декстрансульфат+ДОХ в крови крыс обнаруживается лекарство в значительно больших концентрациях. Независимо от концентрации ДОХ в плазме результаты физикального осмотра, а также аутопсии крыс в течение 21 дня после внутрибрюшинного введения ДОХ в разных системах доставки, свидетельствуют об отсутствии негативных реакций у животных.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Доксорубицин, система доставки лекарства, СаСО<sub>3</sub>, декстрансульфат, конъюгат полимер-лекарство, плазма крови.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_META_TITLE"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_META_KEYWORDS"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_META_DESCRIPTION"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_PICTURE_FILE_ALT"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_PICTURE_FILE_TITLE"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "saso3-vaterity-pokrytye-dekstransulfatom-kak-sistemy-dlya-regionarnogo-vvedeniya-doksorubitsina-krys" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(191) "СаСО3 ватериты, покрытые декстрансульфатом, как системы для регионарного введения доксорубицина крысам" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "saso3-vaterity-pokrytye-dekstransulfatom-kak-sistemy-dlya-regionarnogo-vvedeniya-doksorubitsina-krys" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "saso3-vaterity-pokrytye-dekstransulfatom-kak-sistemy-dlya-regionarnogo-vvedeniya-doksorubitsina-krys" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "saso3-vaterity-pokrytye-dekstransulfatom-kak-sistemy-dlya-regionarnogo-vvedeniya-doksorubitsina-krys" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "203" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28475" ["VALUE"]=> array(2) { ["TEXT"]=> string(313) "<p>Наталья Н. Сударева<sup>1,2</sup>, Ольга М. Суворова<sup>1</sup>, Дмитрий Н. Суслов<sup>3</sup>, Олег В. Галибин<sup>2</sup>, Александр Д. Вилесов<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(241) "
Наталья Н. Сударева1,2, Ольга М. Суворова1, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28476" ["VALUE"]=> array(2) { ["TEXT"]=> string(660) "<p><sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия<br> <sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Российский научный центр радиологии и хирургических технологий им. акад. А.М. Гранова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(600) "1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. акад. А.М. Гранова, Санкт-Петербург, Россия
Доксорубицин (ДОХ) – водорастворимый антрациклиновый антибиотик, обладающий высокой противораковой эффективностью. Можно добиться уменьшения концентрации ДОХ в крови ниже кардиотоксического уровня в процессе терапии, формируя депо, содержащее системы доставки ДОХ с пролонгированным высвобождением лекарства. Для этих целей использовали кальций карбонатные пористые ватериты, допированные полианионом декстран сульфатом. Субмикронные размеры носителей не позволяют свободно включаться им в кровеносное русло. Распространяется токсическое лекарство по организму только после попадания в кровь в результате высвобождения из систем доставки. Внутрибрюшинное введение крысам с перевитой гепатомой Зайделя ДОХ-содержащих систем доставки позволило оценить эффективную концентрацию ДОХ, тормозящую рост опухоли и уменьшающую объем асцитной жидкости. Динамику поступления ДОХ в кровь здоровых крыс после внутрибрюшинного введения 4 мг ДОХ в системах доставки различной природы определяли методом ВЭЖХ. Введенное при помощи допированных декстрансульфатом субмикронных карбонатных ядер лекарство высвобождается в кровь крыс в течение двух недель в концентрациях, меньших токсичных значений. При использовании в качестве системы доставки наноразмерного конъюгата декстрансульфат+ДОХ в крови крыс обнаруживается лекарство в значительно больших концентрациях. Независимо от концентрации ДОХ в плазме результаты физикального осмотра, а также аутопсии крыс в течение 21 дня после внутрибрюшинного введения ДОХ в разных системах доставки, свидетельствуют об отсутствии негативных реакций у животных.
Ключевые слова
Доксорубицин, система доставки лекарства, СаСО3, декстрансульфат, конъюгат полимер-лекарство, плазма крови.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28478" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-71-77" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-71-77" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28479" ["VALUE"]=> array(2) { ["TEXT"]=> string(243) "<p> Natalia N. Sudareva<sup>1,2</sup>, Olga М. Suvorova<sup>1</sup>, Dmitry N. Suslov<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(171) "Natalia N. Sudareva1,2, Olga М. Suvorova1, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28480" ["VALUE"]=> array(2) { ["TEXT"]=> string(793) "<p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> <sup>2</sup> Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Natalia N. Sudareva, Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> E-mail: nnsas@mail.ru</p><br> <p><b>Citation:</b> Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO<sub>3</sub> vaterites as the systems for regional administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(649) "1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Natalia N. Sudareva, Institute of Macromolecular
Compounds RAS, St. Petersburg, Russia
E-mail: nnsas@mail.ru
Citation: Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28481" ["VALUE"]=> array(2) { ["TEXT"]=> string(1842) "<p style="text-align: justify;">Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.</p> <h2>Keywords</h2> <p style="text-align: justify;">Doxorubicin, drug delivery system, CaCO<sub>3</sub>, dextran sulfate, polymer-drug conjugate, blood plasma.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1774) "Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.
Keywords
Doxorubicin, drug delivery system, CaCO3, dextran sulfate, polymer-drug conjugate, blood plasma.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28482" ["VALUE"]=> string(104) "Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(104) "Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28483" ["VALUE"]=> string(4) "2745" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2745" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28484" ["VALUE"]=> string(4) "2746" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2746" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28479" ["VALUE"]=> array(2) { ["TEXT"]=> string(243) "<p> Natalia N. Sudareva<sup>1,2</sup>, Olga М. Suvorova<sup>1</sup>, Dmitry N. Suslov<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(171) "Natalia N. Sudareva1,2, Olga М. Suvorova1, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(171) "Natalia N. Sudareva1,2, Olga М. Suvorova1, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28481" ["VALUE"]=> array(2) { ["TEXT"]=> string(1842) "<p style="text-align: justify;">Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.</p> <h2>Keywords</h2> <p style="text-align: justify;">Doxorubicin, drug delivery system, CaCO<sub>3</sub>, dextran sulfate, polymer-drug conjugate, blood plasma.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1774) "Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.
Keywords
Doxorubicin, drug delivery system, CaCO3, dextran sulfate, polymer-drug conjugate, blood plasma.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1774) "Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.
Keywords
Doxorubicin, drug delivery system, CaCO3, dextran sulfate, polymer-drug conjugate, blood plasma.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28478" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-71-77" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-71-77" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-71-77" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28482" ["VALUE"]=> string(104) "Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(104) "Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(104) "Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28480" ["VALUE"]=> array(2) { ["TEXT"]=> string(793) "<p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> <sup>2</sup> Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Natalia N. Sudareva, Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> E-mail: nnsas@mail.ru</p><br> <p><b>Citation:</b> Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO<sub>3</sub> vaterites as the systems for regional administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(649) "1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Natalia N. Sudareva, Institute of Macromolecular
Compounds RAS, St. Petersburg, Russia
E-mail: nnsas@mail.ru
Citation: Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(649) "1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Natalia N. Sudareva, Institute of Macromolecular
Compounds RAS, St. Petersburg, Russia
E-mail: nnsas@mail.ru
Citation: Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28475" ["VALUE"]=> array(2) { ["TEXT"]=> string(313) "<p>Наталья Н. Сударева<sup>1,2</sup>, Ольга М. Суворова<sup>1</sup>, Дмитрий Н. Суслов<sup>3</sup>, Олег В. Галибин<sup>2</sup>, Александр Д. Вилесов<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(241) "Наталья Н. Сударева1,2, Ольга М. Суворова1, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(241) "Наталья Н. Сударева1,2, Ольга М. Суворова1, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28477" ["VALUE"]=> array(2) { ["TEXT"]=> string(3344) "<p style="text-align: justify;">Доксорубицин (ДОХ) – водорастворимый антрациклиновый антибиотик, обладающий высокой противораковой эффективностью. Можно добиться уменьшения концентрации ДОХ в крови ниже кардиотоксического уровня в процессе терапии, формируя депо, содержащее системы доставки ДОХ с пролонгированным высвобождением лекарства. Для этих целей использовали кальций карбонатные пористые ватериты, допированные полианионом декстран сульфатом. Субмикронные размеры носителей не позволяют свободно включаться им в кровеносное русло. Распространяется токсическое лекарство по организму только после попадания в кровь в результате высвобождения из систем доставки. Внутрибрюшинное введение крысам с перевитой гепатомой Зайделя ДОХ-содержащих систем доставки позволило оценить эффективную концентрацию ДОХ, тормозящую рост опухоли и уменьшающую объем асцитной жидкости. Динамику поступления ДОХ в кровь здоровых крыс после внутрибрюшинного введения 4 мг ДОХ в системах доставки различной природы определяли методом ВЭЖХ. Введенное при помощи допированных декстрансульфатом субмикронных карбонатных ядер лекарство высвобождается в кровь крыс в течение двух недель в концентрациях, меньших токсичных значений. При использовании в качестве системы доставки наноразмерного конъюгата декстрансульфат+ДОХ в крови крыс обнаруживается лекарство в значительно больших концентрациях. Независимо от концентрации ДОХ в плазме результаты физикального осмотра, а также аутопсии крыс в течение 21 дня после внутрибрюшинного введения ДОХ в разных системах доставки, свидетельствуют об отсутствии негативных реакций у животных.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Доксорубицин, система доставки лекарства, СаСО<sub>3</sub>, декстрансульфат, конъюгат полимер-лекарство, плазма крови.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3276) "Доксорубицин (ДОХ) – водорастворимый антрациклиновый антибиотик, обладающий высокой противораковой эффективностью. Можно добиться уменьшения концентрации ДОХ в крови ниже кардиотоксического уровня в процессе терапии, формируя депо, содержащее системы доставки ДОХ с пролонгированным высвобождением лекарства. Для этих целей использовали кальций карбонатные пористые ватериты, допированные полианионом декстран сульфатом. Субмикронные размеры носителей не позволяют свободно включаться им в кровеносное русло. Распространяется токсическое лекарство по организму только после попадания в кровь в результате высвобождения из систем доставки. Внутрибрюшинное введение крысам с перевитой гепатомой Зайделя ДОХ-содержащих систем доставки позволило оценить эффективную концентрацию ДОХ, тормозящую рост опухоли и уменьшающую объем асцитной жидкости. Динамику поступления ДОХ в кровь здоровых крыс после внутрибрюшинного введения 4 мг ДОХ в системах доставки различной природы определяли методом ВЭЖХ. Введенное при помощи допированных декстрансульфатом субмикронных карбонатных ядер лекарство высвобождается в кровь крыс в течение двух недель в концентрациях, меньших токсичных значений. При использовании в качестве системы доставки наноразмерного конъюгата декстрансульфат+ДОХ в крови крыс обнаруживается лекарство в значительно больших концентрациях. Независимо от концентрации ДОХ в плазме результаты физикального осмотра, а также аутопсии крыс в течение 21 дня после внутрибрюшинного введения ДОХ в разных системах доставки, свидетельствуют об отсутствии негативных реакций у животных.
Ключевые слова
Доксорубицин, система доставки лекарства, СаСО3, декстрансульфат, конъюгат полимер-лекарство, плазма крови.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3276) "Доксорубицин (ДОХ) – водорастворимый антрациклиновый антибиотик, обладающий высокой противораковой эффективностью. Можно добиться уменьшения концентрации ДОХ в крови ниже кардиотоксического уровня в процессе терапии, формируя депо, содержащее системы доставки ДОХ с пролонгированным высвобождением лекарства. Для этих целей использовали кальций карбонатные пористые ватериты, допированные полианионом декстран сульфатом. Субмикронные размеры носителей не позволяют свободно включаться им в кровеносное русло. Распространяется токсическое лекарство по организму только после попадания в кровь в результате высвобождения из систем доставки. Внутрибрюшинное введение крысам с перевитой гепатомой Зайделя ДОХ-содержащих систем доставки позволило оценить эффективную концентрацию ДОХ, тормозящую рост опухоли и уменьшающую объем асцитной жидкости. Динамику поступления ДОХ в кровь здоровых крыс после внутрибрюшинного введения 4 мг ДОХ в системах доставки различной природы определяли методом ВЭЖХ. Введенное при помощи допированных декстрансульфатом субмикронных карбонатных ядер лекарство высвобождается в кровь крыс в течение двух недель в концентрациях, меньших токсичных значений. При использовании в качестве системы доставки наноразмерного конъюгата декстрансульфат+ДОХ в крови крыс обнаруживается лекарство в значительно больших концентрациях. Независимо от концентрации ДОХ в плазме результаты физикального осмотра, а также аутопсии крыс в течение 21 дня после внутрибрюшинного введения ДОХ в разных системах доставки, свидетельствуют об отсутствии негативных реакций у животных.
Ключевые слова
Доксорубицин, система доставки лекарства, СаСО3, декстрансульфат, конъюгат полимер-лекарство, плазма крови.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28476" ["VALUE"]=> array(2) { ["TEXT"]=> string(660) "<p><sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия<br> <sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Российский научный центр радиологии и хирургических технологий им. акад. А.М. Гранова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(600) "1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. акад. А.М. Гранова, Санкт-Петербург, Россия
1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. акад. А.М. Гранова, Санкт-Петербург, Россия
Introduction
Bone marrow (BM) stroma is a complex tissue containing several cell types which provide a microenvironment for hematopoiesis and contribute to the maintenance and regeneration of skeletal tissues [1]. Two major stem cell populations with distinct progenies are found within adult BM, i.e., hematopoietic stem cells and mesenchymal stem cells (MSCs) [2]. MSCs were first described by Friedenstein et al. Who found that MSCs can be isolated by physical adhere to culture plates, able to form colonies with cells have fibroblast shape in vitro [3]. MSCs have also been defined as colony-forming fibroblastic cells, marrow stromal stem cells, mesenchymal progenitor cells [4], and, even, medicinal signaling cells [5].
MSCs are of interest in clinical applications for their ability to modulate the immune system, as well as their potential to regenerate tissues [6]. Medical applications of MSC require in vitro expansion in order to obtain sufficient cell numbers to achieve therapeutic results. Therefore, determination of optimal culture conditions is a prerequisite for usage of MSC in clinical setting [7].
However, prior to clinical implementation, the animal models are needed to confirm their efficacy and safety [8]. MSCs from mouse bone marrow (BM) provide valuable information on cell biology, potential function [9] and biochemical characteristics of MSC populations [8]. Human and rat bone marrow mesenchymal stem cells have been the most extensively characterized, due to relatively easy isolation procedure, their adherence to plastic dishes, and extensive in vitro expansion [10].
Isolation and purification of BM-MSCs from mouse are more difficult than from other species [9]. This observation is due to low frequency of BM-MSCs [11] and high proportion of hematopoietic stem cells (HSCs) in bone marrow [9]. The stromal cells exist near the surface of the bone, thus making it difficult to obtain enough MSCs, even after flushing BM by the cell preparation [8]. In addition, lack of specific mouse BM-MSC markers increases the difficulties [9].
To date, several suggestions were presented to improve the method of mouse BM-MSCs isolation, propagation, and culture, but none of them are widely acceptable [12]. We have reviewed most of the available protocols published so far, and attempted to develop an optimized, efficient, uncomplicated protocol suitable for production of a large number of MSC(M) in less than a week, thus enabling their use in regenerative medicine.
In this paper, we describe our MSC(M) isolation protocol in details, with appropriate illustrations, and index of some technical problems that occur when isolating and culturing MSCs, as well as expected causes of culture failures, and appropriate troubleshooting measures.
Materials and methods
1. Experimental Animals
Healthy mice (Balb/c, 4-6 weeks) were used in this study. Mice were housed in clean cages containing woodchip bedding, under a controlled temperature (24±2) and light (12h light/ dark cycle) conditions, with free access to food and water. All procedures were performed according to the Guide for the Care and Use of Laboratory Animals and the ethical standards of our institution [13].
2. Reagents and materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), L-glutamine, penicillin/streptomycin, amphotericin B, trypsin-EDTA (Euroclone, Italy), TRIzol Reagent (Sigma-Aldrich, USA), reverse transcriptase kit (Promega, USA), ladder (GeneDireX, Taiwan), Ethidium Bromide (Carl Roth, Germany).
3. Isolation and culture of mouse MSC(M)
1. To isolate marrow, the mouse is sacrificed by cervical dislocation. Then, the mouse is brought to a laminar hood.
2. Place the animal in dorsal position on the dissecting board and thoroughly spray the animal skin with 70% ethanol to prevent contamination from the skin.
3. Incise the skin around the perimeter of the hindlimbs, pull the skin down toward the foot, and cut at the anklebone along with the peeled skin. Then, disconnect the hind limbs from the trunk (Fig. 1).
4. Remove the muscles, ligaments, and tendons attached to the bones carefully using sterile surgical scissors, forceps and scalpel.
5. Separate tibias and femurs by bending them in opposite direction and cutting through the knee joint, ensuring that the epiphysis remains intact.
6. Transfer the bones to pre-warmed DMEM medium (37°C) in a sterile Petri dish to preserve viability.
7. Cut the epiphyses of the tibia and femur carefully just below the end of the marrow cavity using sterile, sharp scissors.
8. Insert a 27-gauge needle attached to a 1-ml insulin syringe filled with complete DMEM medium heated to 37°C, supplemented with 1% L-glutamine, 1% penicillin-streptomycin (P/S), and 1.5 µg/ml amphotericin B into the bone cavity and flush the marrow out of the cut end of the bone into a 15 ml Falcon tube. In order to obtain enough marrow cells, repeat this process from both ends of the bone (use 1.4 ml of DMEM for each bone). Add 10% FBS to the bone marrow suspension and gently mix it with a pipette. Transfer the suspension to a cell culture flask. Incubate this flask in an incubator at 37°C in a humidified atmosphere containing 5% CO2.
9. After flushing out the marrow, transfer the bones to a mortar containing 5 ml of complete DMEM medium, and crush bones gently with pestle using only enough force to crack open the bones and extract the remainder of the bone marrow. Mix gently and transfer the cell suspension to a plastic culture flask. Incubate the flask in an incubator at 37°C and 5% CO2.
10. Determine yield and viability of the isolated cells using a hemocytometer/automated cell counter and Trypan Blue staining.
11. After 24 h at 37°C, replace the culture medium to remove non-adherent cells and tissue debris with 5 mL of complete DMEM medium (20% FBS). Later on, replace the culture medium daily.
12. On the 5th day, remove the culture medium and wash the cells with phosphate buffer saline (PBS 1X), then detach the adherent cells by adding 1-1.5 ml of 0.25% trypsin into the flask for 2 min at 37°C. Neutralize trypsin by adding 3 ml of DMEM medium (20% FBS). Transfer the cell suspension into a 15 ml Falcon tube, and centrifuge for 3-5 min at 1000 rpm. Passage the resulting cells at a split ratio of 1:2 or 1:3.

Figure 1. Stepwise procedure of mesenchymal stromal cells isolation from the bone marrow of Balb/c mice
The mouse was sacrificed by a cervical dislocation, and the animal skin is sprayed with 70% ethanol (A). Skin was incised around the perimeter of the hind limbs (B). The foot with peeled skin was cut at the anklebone (C). Hind limbs were disconnected from the trunk, and transferred to a sterile Petri dish (D, E). Muscles, ligaments, and tendons were removed from the bones (F). Bones cleared from the rest of muscles (G). Tibias and femurs were separated by cutting through the knee joint (H), and transferred to pre-warmed DMEM medium in a sterile Petri dish (I). Epiphyses were cut using sterile, sharp scissors (J). Bone marrow was flushed out of the bone (K, L). Bones were crushed gently to extract the remainder of the bone marrow (M). Yield and viability of cells was determined using automated cell counter (N).
4. Gene expression assay for molecular phenotyping
Total RNA was isolated from MSC(M) using TRIzol Reagent. The quantity and quality of the isolated RNA were evaluated by Thermo Scientific NanoDrop 2000. RNA was reversely transcribed into complementary DNA (cDNA) from 1 μg of total RNA in a 20 μl reaction mixture by means of reverse transcriptase kit (Promega, USA), according to the manufacturer’s instruction. PCR of the cDNA samples was applied to detect expression of CD73, CD44, CD105, CD11b, CD34, with GAPDH gene used as a reference. To check expression of each gene, 4 μl of cDNA was added to the gene-specific primers in 25 μl total reaction mixture. Primer sequences for GAPDH and CD34 genes were obtained from the published reports [14, 15], whereas primers for CD11b, CD73, CD44, CD105 genes were designed using NCBI database. Table 1 summarizes gene names, forward and reverse primer sequences, annealing temperatures and expected amplicon length. The PCR conditions were as follows: (1) 10 minutes at 95°C, (2) 35 cycles of 30 seconds at 95°C, 45 seconds at primer annealing temperature, and 45 seconds at 72°C, (3) 10 minutes at 72°C. Following PCR, the amplicons were detected on 2% agarose gel using a 50 bp ladder (GeneDireX, Taiwan) and Ethidium Bromide to visualize the amplicons.
Table 1. Genes assayed, NCBI accession number, forward and reverse primer sequences, annealing temperatures and amplicon sizes for PCR analysis

5. Adipogenic Differentiation
Adipogenic differentiation ability was evaluated in vitro, according to [16], with slight modifications. The cells were plated in 96-well plate in complete DMEM medium (10% FBS). After 24 hours, the medium was replaced by adipogenic induction medium (complete DMEM medium supplemented with 10% FBS, 2 μM dexamethasone, 0.1 mM indomethacin, and 5 μg/ml insulin). Cells cultured in a complete DMEM medium (10% FBS) were used as a negative control. After one week, the cells were fixed with 10% Paraformaldehyde (PFA) for 20 min and stained with Sudan III (1%) to detect the formation of lipid droplets, then washed with distilled water. Lipid droplets were detected by inverted microscope (Olympus IX53, Japan).
6. Osteogenic differentiation
Osteogenic differentiation ability was evaluated according to [17], with slight modifications. Cells were plated in 96 well plate in complete DMEM medium (10% FBS). After 24 hours, the medium was replaced with osteogenic induction medium (complete DMEM medium supplemented with 10% FBS, 10 μM dexamethasone, 10mM β-glycerophosphate, and 50 μM ascorbic acid). Cells cultured in a complete DMEM medium (10% FBS) were used as a negative control. After 1 week, the cells were fixed with 10% Paraformaldehyde (PFA) for 20 min and stained with Alizarin Red (2%) to detect the presence of calcium deposits, then washed with distilled water. Calcium deposits were detected by inverted microscope.
Results
1. Morphology of isolated MSC(M) in culture
MSC(M) were isolated from the femurs and tibias of Balb/c mouse according to their adherence to a culture flask. Using our protocol, we obtained ~ 75-90*106 of BM mononuclear cells (BMMNCs) with 94% viability using flushing process, and ~ 10-20*106 of BMMNCs with 84% viability by crushing process. Isolated MSC(M) settled down within 10 minutes of seeding and showed round-shaped morphology with various sizes. After 24 hours, the MSC(M) attached to the flask surface and non-adherent cells were carefully removed by medium changing. After 24 hours of cultivation, some cells became spindle. On Day 3, the number of spindle-shaped cells increased dramatically. On Day 4, the spindle-shaped cells reached about 60-70% confluence. On Day 5, the cells grew and form 80-90% confluent monolayer (Fig. 2), and cells were passaged on this day.

Figure 2. Morphological features of cultured mouse MSC(M) at passage 0
(A) Rounded-shape cells settled down within 10 minutes of seeding. (B) Following 24 hours of cultivation, some spindle-shaped cells appeared. (C) The ratio of spindle-shaped cells increased on Day 3. (D) On Day 4, the spindle-shaped cells reached about 60-70% confluence. (E) On Day 5 of culture, the cells reached 80-90% confluence, being passaged at this term.

Figure 3. Expression of MSC gene markers detected by RT-PCR and agarose gel electrophoresis (lanes 1 to 6) for the following PCR products: (1) GAPDH (123bp) used as control; (2) CD73 (115 bp); (3) CD44 (136 bp); (4) CD11b (192 bp); (5) CD105 (344 bp); (6) CD34 (215 bp)
2. Characterization of cell surface markers
In this study, MSC(M) isolated from Balb/c mouse were characterized to investigate expression of surface antigens, and the results indicated that the cells were positive for CD73, and CD44, since the bands appeared at the expected distances when PCR products were subjected to electrophoresis in 2% agarose gel. Moreover, there was no detectable CD11b expression, along with only weak expression of CD34 and CD105 (Fig. 3).
3. Adipogenic and osteogenic differentiation
Adipogenic and osteogenic differentiation potential of isolated MSC(M) was tested. Following 7 days of culture under adipogenic induction conditions, MSC(M) showed marked morphological changes compared to the undifferentiated cells (control). They also showed accumulation of several cytoplasmic lipid droplets. After staining with lipophilic Sudan III, the control cells did not show any cytoplasmic changes and were negative for Sudan III staining, while distinct cytoplasmic lipid droplets were observed in MSC(M) grown in adipogenic medium (Fig. 4).

Figure 4. Mesodermal differentiation of MSC(M) at the passage 0
A: Adipogenic differentiation of MSCs: (a) BMSCs after induction with adipogenic medium, showing formation of intracytoplasmic lipid droplets; (b, c). Positive staining of lipid droplets with Sudan III. (B): Osteogenic differentiation: (a). BMSCs after induction with osteogenic medium showed cuboidal morphology (b). Alizarin Red staining indicated dark red precipitation of calcium deposits. (c) Control BMSCs cultured in DMEM medium (10%FBS).
Following 7 days of culture in osteogenic induction medium, MSC(M) showed morphological changes and acquired cubical shape, whereas control cells did not show any morphological changes. After staining with Alizarin Red, the control cells were dye-negative, whereas differentiated cells were positively stained, indicating the presence of calcium deposits (Fig. 4).
Discussion
Cell isolation procedure
Mesenchymal stromal cells (MSCs) are used in many research fields and have sparked great interest in cell therapies due to their ability to differentiate into various cell types [18]. MSC culture was first established by Friedenstein and his colleagues by virtue of the physical propensity of MSCs to adhere to plastic flasks, and the original method has been modified by others [19]. Several methods have been used for isolating MSC(M) including whole marrow direct adherence, density gradient centrifugation, flow cytometry and immunobead methods. However, the latter two methods are considered high in cost and technically difficult [20]. The direct culture method is best to culture high number of MSC(M) within very short time and at high viability rate [21]. It is clearly easier and reduce loss of MSCs compared to density gradient separation approach. However, the cells collected by this method represent a heterogeneous mixture of cells, including hematopoietic cells, endothelial cells and endothelial progenitor cells [11]. This problem was solved using frequent medium change that may prevent adherence of many of the non-MSCs and hematopoietic cells to the culture dishes [2].
Usage of adult MSCs in human therapeutic applications depends on the establishing of preclinical studies with other mammals such as mouse [22]. In order for such therapies to be reproducibly performed in preclinical animal models, a simple, high-yield method for obtaining MSCs is required [19]. In this study, MSC(M) were isolated from the mice aged 4-6 weeks. We obtained high cellular yield, and the isolated cells were able to grow and proliferate within a short period of time. Previous study indicated that age of the animals used as MSCs source directly influences their differentiation, proliferative and metabolism profiles [23]. According to [19], younger mice (sucklings of <3 weeks) produce more proliferative cell cultures. Long bones of newborn mice (up to one week of age) are quite fragile, thus being inconvenient for researchers who have no relevant experience handling them.
- We found that a delay in isolation process from sacrificing the mouse to BM collection and culture, greatly affects the yield and viability of the isolated cells. This step is very critical and requires experience and accuracy during work.
- In this study, MSC(M) were isolated using an adherence method, the culture medium was replaced daily during the first four days of culture, in order to provide the cells with the nutrients required for their growth and proliferation. At the same time, the daily change of the culture media may prevent adherence of many non-MSCs, e.g., hematopoietic populations, to the culture dishes. According to our experience, we do not recommend replacement of the culture medium two or three hours after primary cell culture because we found that this process results in loss of some MSCs and thus obtaining a lower cellular yield.
- Two types of culture media (DMEM or RPMI 1640) supplemented with 20% FBS, 1% L-glutamine, 1% penicillin/streptomycin and 1.5 µg/ml amphotericin B were used in our study in order to choose the optimal medium, and the results demonstrated that the both media are suitable for MSC(M) culture and proliferation, since no differences in cell viability, growth or proliferation were observed.
- Fetal bovine serum (FBS) seems to be the most popular choice of supplements in the culture media. It may play a key role in promoting cell attachment and proliferation [24]. In the current protocol, FBS concentration was increased to 20%, and we found that this concentration was suitable for obtaining sufficient numbers of MSCs within a short period of time.
- After separating bones from the muscles, they were directly transferred to a Petri dish containing 3-4 ml of pre-warmed DMEM medium (37°C), without additives of serum or antibiotics, to preserve cell viability.
- To reduce the possibility of contamination, the bones should not be transferred to the culture media before cleaning it from the muscle.
- To reduce the possibility of contamination, a fresh media should be used to flush the marrow out of the bone rather than using the media in which the bones were placed after separating from the muscle.
- In this study, the marrow was flushed out from both ends of the bone to obtain a high cellular yield, as proposed by [25].
- Only 1.4 ml of culture medium is sufficient for flushing the marrow out of the bone, then the bones could be transferred to a sterile mortar containing 5 ml of culture medium, and gently crushed to extract the remaining bone marrow. We found that this step is very useful to obtain a high MSC(M) yield, because the stromal cells locate near the surface of the bone, which makes it difficult to obtain enough MSCs, even after flushing BM during cell preparation [8].
- In our study, after collecting the BM suspension, it was immediately transferred to the culture flask, without centrifugation or filtration. MSC(M) were cultured into their initial niche composing of stromal cells, extracellular matrix elements, and secreting factors with minimal disturbance, to allow the initial adjusting time for the MSCs in culture, according to [26]. We found this method to be more efficient. However, in our protocol, the culture medium was replaced daily, contrary to what was suggested by [26] who kept the cells in their niche for 4 days without replacing the media.
- Using our modified protocol, we were able to isolate ~ 25% more BMMNCs than the commonly used protocol.
- On the fifth day of culture, MSC(M) reached about 80- 90% confluence. Hence, they were passaged to prevent overgrowth and cell detachment. MSC(M) are strongly attached to the flask and can't be easily detached from the flask using trypsin solution at lower concentration (0.05%). Therefore, a more concentrated trypsin solution (0.25%) was used to detach the cells.
- The trypsinization conditions should be carefully controlled. It is necessary to adhere to the concentration of the trypsin solution used to separate the cells, as well as incubation time. Insufficient trypsin treatment will reduce the yield of cells [11]. On the other hand, bone marrow-derived adherent cells were found to contain different cell types including fibroblasts, hematopoietic progenitor cells, macrophages, endothelial cells and adipocytes [4]. Long-term incubation with trypsin resulting in collection of other unwanted trypsin-resistant cells, e.g., macrophages [19], which firmly adhere to cell culture dishes, whereas MSCs were shown to be more responsive to trypsin [4]. Excessive trypsinisation may also damage cells by striping cell surface proteins. The most prominent problems that researchers may face in the mouse MSC(M) isolation and culture and possible solutions for them are listed in Table 2.
Table 2. Some problems and suggested solutions for mouse MSC(M) isolation and culture

Cell Surface Markers
Mammalian bone marrow contains a myriad of stem cells with distinctive morphological and functional features such as mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), multipotent adult progenitor cells (MAPCs) and very small embryonic-like stem cells (VSELs) [12]. Due to growing interest in using MSCs for cell-based therapy, the need to identify MSCs in a definitive way is not only of research interest but also derives from clinical requirements [27].
The isolation of distinct cell types from bone marrow stroma is hindered by the lack of known specific surface markers for those cells. To date, only a set of markers can identify stem/stromal cells from bone marrow, and none of them is unique [1].
In this study, MSC(M) isolated from Balb/c mice were characterized to investigate gene expression of the surface markers, and the results indicated that the cells were positive for (CD73 and CD44), negative for (CD11b), while they showed weak expression of CD34 and CD105 (Fig. 3). These results demonstrated that the isolated cells were MSC-like in morphology, adhesion to plastic surfaces, expression of surface markers, and in adipogenic/osteogenetic differentiation potential. Although the isolated MSC(M) showed characteristics of mesenchymal stromal cells, we suggest that there is a low proportion of hematopoietic stem cells, since the cells were characterized at the passage (P0), and it has been reported that hematopoietic stem cells still exist in the culture even after nine in vitro passages [19]. Increased number of cell passages could result in a significant reduction of hematopoietic stem cells.
Mouse MSCs are generally characterized by positive expression of CD44, CD73, CD105, CD29, CD106, Sca-1, and negative expression of hematopoietic and endothelial markers CD45, CD11b, Ter-119, and CD31 [6]. Our results showed that the surface marker CD profiles for the MSC(M) were compatible with those previously reported.
It has been demonstrated that CD34 expression is not specific for murine hematopoietic cells, as it was shown for human cells. Previous study reported that the expression of CD34 was strain-dependent in mice. Bl/6 MSCs expressed high levels of CD34, with moderate expression by FVB/N MSCs. Both Balb/c and DBA1 MSCs expressed low levels, while MSCs from the 4 standard inbred strains were negative for CD11b [10].
Adipogenic and osteogenic potentials
MSCs are defined by their ability to differentiate and generate cells of mesodermal origin like adipocytes, osteocytes and chondrocytes in culture [6]. The results of mesodermal differentiation assays showed that isolated MSC(M) were able to differentiate into adipocytes and osteocytes in vitro. MSC(M) cultured in adipogenic medium showed morphological changes and displayed accumulation of lipid vacuoles, which stained positively with Sudan III. Lipid droplets in differentiated cells became red due to their affinity for lipophilic Sudan III dye. Similarly, MSC(M) cultured in osteogenic medium showed morphological changes and stained positively with Alizarin Red, indicating presence of calcium deposits. Our results are consistent with previous reports, and demonstrate the opportunity of using these cells in both clinical and research applications.
In the current study, MSC(M) at passage 0 were able to differentiate into adipocyte and osteoblasts like cells within only seven days of incubating with differentiation medium. Meanwhile, many previous studies indicated that adipogenic and osteogenic differentiation takes about three weeks. This slow dynamic may be due to the fact that cell differentiation capability and function decline with repeated passaging [28].
Previous research demonstrated that BMSCs from passage 3 were inferior to bone marrow mononuclear cells (BMMNCs) not only in their chondrogenic differentiation capability but also as candidates for long-term repair of cartilage defects [28]. Previous study reported that MSCs from passage 1 have stronger osteogenic potential in vitro than those of passages 2 and 3, and might be suitable for clinical application to bone tissue engineering [29].
Differentiation of MSCs into various lineages is strictly regulated at multiple sequential steps, being represented by distinct morphological and molecular characteristics [12]. Until now, the mechanism of MSCs trans-differentiation is unclear. There are many internal and external factors that trigger MSCs differentiation.
Conclusion
Increased demand for MSCs in research and medical studies has led scientists to give priority to the development of standardized MSCs isolation and culture methods. In the present study, we have successfully isolated MSC(M) from Balb/c mouse under optimized isolation and culture conditions. We have also listed the most prominent problems that researchers may face and suggested the possible solutions for them. We hope that this simple and practical method will facilitate the study of MSCs, both for examining their biological properties, as well as their therapeutic potential in various murine disease models.
Acknowledgments
The authors are grateful to the Leishmania Center of Epidemiological and Biological Studies, National Commission for Biotechnology (Department of Pharmaceutical Biotechnology). We would like to thank Dr. Ruba Joujeh, Dr. Hassan Alkhoury, Dr. Majd Aljamali, Dr. Fateh Khatib, Ms. Reham Antaki, for their help and support. This research was funded by the University of Aleppo.
References
- Rostovskaya M, Anastassiadis K. Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PLoS One. 2012; 7(12): 1-12. doi: 10.1371/journal.pone.0051221
- Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nature Protocols. 2009; 4(1): 102-106. doi: 10.1038/nprot.2008.221
- Al-Qaisy B, Yaseen N, Alwachi S, AL-Shammari A. Comparison between three different protocols for isolation and culture of mouse bone marrow derived mesenchymal stem cells. Iraqi J Cancer Med Genet. 2014; 7(1): 26-35.
- Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int. J. Dev. Biol. 2007; 51: 723-729. doi: 10.1387/ijdb.072352ns
- Caplan A. Mesenchymal stem cells: Time to change the name. Stem Cells Transl Med. 2017; 1-7. https://doi.org/10.1002/sctm.17-0051
- Schachtele S, Clouser C, Aho J. Markers and methods to verify mesenchymal stem cell identity, potency, and quality. https://www.cellandgene.com/doc/markers-and-methods-to-verify-mesenchymal-stem-cell-identity-potency-and-quality-0001
- Oikonomopoulos A, Deen W, Manansala A, Lacey P, Tomakili T, Ziman A, Hommes D. Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media. Sci Rep. 2015; 5: 1-11. doi: 10.1038/srep16570
- Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ. Isolation and characterization of mouse mesenchymal stem cells. Transpl Proc. 2008; 40: 2649-2654. doi: 10.1016/j.transproceed.2008.08.009
- Hu Y, Lou B, Wu X, Wu R, Wang H, Gao L, Pi J, Xu Y. Comparative study on in vitro culture of mouse bone marrow mesenchymal stem cells. Stem Cells Intern. 2018; 1-14. doi: 10.1155/2018/6704583
- Peister A, Mellad J, Larson B, Hall B, Gibson L, Prockop D. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004; 103(5): 1662-1668.
doi: 10.1182/blood-2003-09-3070 - Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. BioMed Research International. 2014; 1-11. doi: 10.1155/2014/951512
- Chaudhary JK, Rath PC. A simple method for isolation, propagation, characterization, and differentiation of adult mouse bone marrow-derived multipotent mesenchymal stem cells. J Cell Sci Ther. 2017; 8(1): 1-10. doi: 10.4172/2157-7013.1000261
- Guide for the care and use of laboratory animals, Eighth Edition, 2011, National Academy of Sciences, 246 pages.
- Roderfeld M, Rath T, Voswinckel R, Dierkes C, Dietrich H, Zahner D, Graf J, Roeb E. Bone marrow transplantation demonstrates medullar origin of CD34+ fibrocytes and ameliorates hepatic fibrosis in Abcb4_/_ mice. Hepatology. 2010; 51:267-276.
doi: 10.1002/hep.23274 - Tohidi F, Toosi M, Azimian H, Khademi S, Fardid R, Sarab GH. The gene expression level of p53 and p21 in mouse brain exposed to radiofrequency field. Int J Radiat Res. 2015; 13(4): 337-343. doi: 10.7508/ijrr.2015.04.007
- Sibov Y, Severino P, Marti LC, Pavon LF, Oliveira DM, Tobo PR, Campos AH, Paes AT, Amaro E, Gamarra LF, Moreira-Filho CA. Mesenchymal stem cells from umbilical cord blood: parameters for isolation, characterization and adipogenic differentiation. Cytotechnology. 2012; 64: 511-521. doi: 10.1007/s10616-012-9428-3
- Castro-Silva I, Castro L, Machado J, Nicola M, Granjeiro J. Isolation of human umbilical cord blood-derived osteoprogenitor cells: a promising candidate for cell-based therapy for bone repair. Einstein. 2011; 9: 449-455. doi: 10.1590/s1679-45082011ao2196
- Baustian C, Hanley S, Ceredig R. Isolation, selection and culture methods to enhance clonogenicity of mouse bone marrow derived mesenchymal stromal cell precursors. Stem Cell Res Ther. 2015; 6(151): 1-13. doi: 10.1186/s13287-015-0139-5
- Zhu H, Guo Z, Jiang X, Li H, Wang X, Yao H, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nature Protoc. 2010; 5(3): 550-560. doi: 10.1038/nprot.2009.238
- Hasoon MF, Nader B, Mohammed MH. A study of the bone marrow derived mesenchymal stromal cells in rats - proliferation and immunophynotypic markers. Malaysian J Vet Res. 2018; 9(1): 73-80.
- Abdullah RH, Yaseen NY, Saleh SM, Mohamed MH, Al-Shammari A. Direct and simple method for mesenchymal stem cells isolation, culturing and detection. Int J Stem Cell Res. 2018; 5(2): 1-5. doi: 10.23937/2469-570X/1410054
- Tropel Ph, Noel D, Platet N, Legrand P, Benabid A, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004; 295: 395-406. doi: 10.1016/j.yexcr.2003.12.030
- Fafián-Labora J, Fernández-Pernas P, Fuentes I, De Toro J, Oreiro N, Sangiao-Alvarellos S, Mateos J, Arufe MC. Influence of age on rat bone marrow mesenchymal stem cells potential. Sci Rep. 2015; 5: 1-20. doi: 10.1038/srep16765
- Wang X, Yang J, Chen X, Pan X. Establishment and characterization of a fibroblast-like cell line from Anabarilius grahami (Cypriniformes: Cyprinidae). Dongwuxue Yanjiu. 2012; 33(E5-6): E89-E97. doi: 10.3724/SP.J.1141.2012.E05-06E89
- Madaan A, Verma R, Singh AT, Jain SK, Jaggi M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. Journal of Biological Methods. 2014; 1(e1): 1-6. doi: 10.14440/jbm.2014.12
- Huang S, Xu L, Sun Y, Wu T, Wang K, Li G. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J Orthop Transl. 2015; 3(1): 26-33. doi: 10.1016/j.jot.2014.07.005
- Augello A, Kurth TB, Bari CD. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010; 20: 121-133. doi:10.22203/ecm.v020a11
- Jiang T, Xu G, Wang Q, Yang L, Zheng L, Zhao J, Zhang X. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017; 8:1-12. doi: 10.1038/cddis.2017.215
- Chen J, Sotome S, Wang J, Orii H, Uemura T, Shinomiya K. Correlation of in vivo bone formation capability and in vitro differentiation of human bone marrow stromal cells. J Med Dent Sci. 2005; 52(1): 27-34. PMID: 15868738
" ["~DETAIL_TEXT"]=> string(38539) "
Introduction
Bone marrow (BM) stroma is a complex tissue containing several cell types which provide a microenvironment for hematopoiesis and contribute to the maintenance and regeneration of skeletal tissues [1]. Two major stem cell populations with distinct progenies are found within adult BM, i.e., hematopoietic stem cells and mesenchymal stem cells (MSCs) [2]. MSCs were first described by Friedenstein et al. Who found that MSCs can be isolated by physical adhere to culture plates, able to form colonies with cells have fibroblast shape in vitro [3]. MSCs have also been defined as colony-forming fibroblastic cells, marrow stromal stem cells, mesenchymal progenitor cells [4], and, even, medicinal signaling cells [5].
MSCs are of interest in clinical applications for their ability to modulate the immune system, as well as their potential to regenerate tissues [6]. Medical applications of MSC require in vitro expansion in order to obtain sufficient cell numbers to achieve therapeutic results. Therefore, determination of optimal culture conditions is a prerequisite for usage of MSC in clinical setting [7].
However, prior to clinical implementation, the animal models are needed to confirm their efficacy and safety [8]. MSCs from mouse bone marrow (BM) provide valuable information on cell biology, potential function [9] and biochemical characteristics of MSC populations [8]. Human and rat bone marrow mesenchymal stem cells have been the most extensively characterized, due to relatively easy isolation procedure, their adherence to plastic dishes, and extensive in vitro expansion [10].
Isolation and purification of BM-MSCs from mouse are more difficult than from other species [9]. This observation is due to low frequency of BM-MSCs [11] and high proportion of hematopoietic stem cells (HSCs) in bone marrow [9]. The stromal cells exist near the surface of the bone, thus making it difficult to obtain enough MSCs, even after flushing BM by the cell preparation [8]. In addition, lack of specific mouse BM-MSC markers increases the difficulties [9].
To date, several suggestions were presented to improve the method of mouse BM-MSCs isolation, propagation, and culture, but none of them are widely acceptable [12]. We have reviewed most of the available protocols published so far, and attempted to develop an optimized, efficient, uncomplicated protocol suitable for production of a large number of MSC(M) in less than a week, thus enabling their use in regenerative medicine.
In this paper, we describe our MSC(M) isolation protocol in details, with appropriate illustrations, and index of some technical problems that occur when isolating and culturing MSCs, as well as expected causes of culture failures, and appropriate troubleshooting measures.
Materials and methods
1. Experimental Animals
Healthy mice (Balb/c, 4-6 weeks) were used in this study. Mice were housed in clean cages containing woodchip bedding, under a controlled temperature (24±2) and light (12h light/ dark cycle) conditions, with free access to food and water. All procedures were performed according to the Guide for the Care and Use of Laboratory Animals and the ethical standards of our institution [13].
2. Reagents and materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), L-glutamine, penicillin/streptomycin, amphotericin B, trypsin-EDTA (Euroclone, Italy), TRIzol Reagent (Sigma-Aldrich, USA), reverse transcriptase kit (Promega, USA), ladder (GeneDireX, Taiwan), Ethidium Bromide (Carl Roth, Germany).
3. Isolation and culture of mouse MSC(M)
1. To isolate marrow, the mouse is sacrificed by cervical dislocation. Then, the mouse is brought to a laminar hood.
2. Place the animal in dorsal position on the dissecting board and thoroughly spray the animal skin with 70% ethanol to prevent contamination from the skin.
3. Incise the skin around the perimeter of the hindlimbs, pull the skin down toward the foot, and cut at the anklebone along with the peeled skin. Then, disconnect the hind limbs from the trunk (Fig. 1).
4. Remove the muscles, ligaments, and tendons attached to the bones carefully using sterile surgical scissors, forceps and scalpel.
5. Separate tibias and femurs by bending them in opposite direction and cutting through the knee joint, ensuring that the epiphysis remains intact.
6. Transfer the bones to pre-warmed DMEM medium (37°C) in a sterile Petri dish to preserve viability.
7. Cut the epiphyses of the tibia and femur carefully just below the end of the marrow cavity using sterile, sharp scissors.
8. Insert a 27-gauge needle attached to a 1-ml insulin syringe filled with complete DMEM medium heated to 37°C, supplemented with 1% L-glutamine, 1% penicillin-streptomycin (P/S), and 1.5 µg/ml amphotericin B into the bone cavity and flush the marrow out of the cut end of the bone into a 15 ml Falcon tube. In order to obtain enough marrow cells, repeat this process from both ends of the bone (use 1.4 ml of DMEM for each bone). Add 10% FBS to the bone marrow suspension and gently mix it with a pipette. Transfer the suspension to a cell culture flask. Incubate this flask in an incubator at 37°C in a humidified atmosphere containing 5% CO2.
9. After flushing out the marrow, transfer the bones to a mortar containing 5 ml of complete DMEM medium, and crush bones gently with pestle using only enough force to crack open the bones and extract the remainder of the bone marrow. Mix gently and transfer the cell suspension to a plastic culture flask. Incubate the flask in an incubator at 37°C and 5% CO2.
10. Determine yield and viability of the isolated cells using a hemocytometer/automated cell counter and Trypan Blue staining.
11. After 24 h at 37°C, replace the culture medium to remove non-adherent cells and tissue debris with 5 mL of complete DMEM medium (20% FBS). Later on, replace the culture medium daily.
12. On the 5th day, remove the culture medium and wash the cells with phosphate buffer saline (PBS 1X), then detach the adherent cells by adding 1-1.5 ml of 0.25% trypsin into the flask for 2 min at 37°C. Neutralize trypsin by adding 3 ml of DMEM medium (20% FBS). Transfer the cell suspension into a 15 ml Falcon tube, and centrifuge for 3-5 min at 1000 rpm. Passage the resulting cells at a split ratio of 1:2 or 1:3.

Figure 1. Stepwise procedure of mesenchymal stromal cells isolation from the bone marrow of Balb/c mice
The mouse was sacrificed by a cervical dislocation, and the animal skin is sprayed with 70% ethanol (A). Skin was incised around the perimeter of the hind limbs (B). The foot with peeled skin was cut at the anklebone (C). Hind limbs were disconnected from the trunk, and transferred to a sterile Petri dish (D, E). Muscles, ligaments, and tendons were removed from the bones (F). Bones cleared from the rest of muscles (G). Tibias and femurs were separated by cutting through the knee joint (H), and transferred to pre-warmed DMEM medium in a sterile Petri dish (I). Epiphyses were cut using sterile, sharp scissors (J). Bone marrow was flushed out of the bone (K, L). Bones were crushed gently to extract the remainder of the bone marrow (M). Yield and viability of cells was determined using automated cell counter (N).
4. Gene expression assay for molecular phenotyping
Total RNA was isolated from MSC(M) using TRIzol Reagent. The quantity and quality of the isolated RNA were evaluated by Thermo Scientific NanoDrop 2000. RNA was reversely transcribed into complementary DNA (cDNA) from 1 μg of total RNA in a 20 μl reaction mixture by means of reverse transcriptase kit (Promega, USA), according to the manufacturer’s instruction. PCR of the cDNA samples was applied to detect expression of CD73, CD44, CD105, CD11b, CD34, with GAPDH gene used as a reference. To check expression of each gene, 4 μl of cDNA was added to the gene-specific primers in 25 μl total reaction mixture. Primer sequences for GAPDH and CD34 genes were obtained from the published reports [14, 15], whereas primers for CD11b, CD73, CD44, CD105 genes were designed using NCBI database. Table 1 summarizes gene names, forward and reverse primer sequences, annealing temperatures and expected amplicon length. The PCR conditions were as follows: (1) 10 minutes at 95°C, (2) 35 cycles of 30 seconds at 95°C, 45 seconds at primer annealing temperature, and 45 seconds at 72°C, (3) 10 minutes at 72°C. Following PCR, the amplicons were detected on 2% agarose gel using a 50 bp ladder (GeneDireX, Taiwan) and Ethidium Bromide to visualize the amplicons.
Table 1. Genes assayed, NCBI accession number, forward and reverse primer sequences, annealing temperatures and amplicon sizes for PCR analysis

5. Adipogenic Differentiation
Adipogenic differentiation ability was evaluated in vitro, according to [16], with slight modifications. The cells were plated in 96-well plate in complete DMEM medium (10% FBS). After 24 hours, the medium was replaced by adipogenic induction medium (complete DMEM medium supplemented with 10% FBS, 2 μM dexamethasone, 0.1 mM indomethacin, and 5 μg/ml insulin). Cells cultured in a complete DMEM medium (10% FBS) were used as a negative control. After one week, the cells were fixed with 10% Paraformaldehyde (PFA) for 20 min and stained with Sudan III (1%) to detect the formation of lipid droplets, then washed with distilled water. Lipid droplets were detected by inverted microscope (Olympus IX53, Japan).
6. Osteogenic differentiation
Osteogenic differentiation ability was evaluated according to [17], with slight modifications. Cells were plated in 96 well plate in complete DMEM medium (10% FBS). After 24 hours, the medium was replaced with osteogenic induction medium (complete DMEM medium supplemented with 10% FBS, 10 μM dexamethasone, 10mM β-glycerophosphate, and 50 μM ascorbic acid). Cells cultured in a complete DMEM medium (10% FBS) were used as a negative control. After 1 week, the cells were fixed with 10% Paraformaldehyde (PFA) for 20 min and stained with Alizarin Red (2%) to detect the presence of calcium deposits, then washed with distilled water. Calcium deposits were detected by inverted microscope.
Results
1. Morphology of isolated MSC(M) in culture
MSC(M) were isolated from the femurs and tibias of Balb/c mouse according to their adherence to a culture flask. Using our protocol, we obtained ~ 75-90*106 of BM mononuclear cells (BMMNCs) with 94% viability using flushing process, and ~ 10-20*106 of BMMNCs with 84% viability by crushing process. Isolated MSC(M) settled down within 10 minutes of seeding and showed round-shaped morphology with various sizes. After 24 hours, the MSC(M) attached to the flask surface and non-adherent cells were carefully removed by medium changing. After 24 hours of cultivation, some cells became spindle. On Day 3, the number of spindle-shaped cells increased dramatically. On Day 4, the spindle-shaped cells reached about 60-70% confluence. On Day 5, the cells grew and form 80-90% confluent monolayer (Fig. 2), and cells were passaged on this day.

Figure 2. Morphological features of cultured mouse MSC(M) at passage 0
(A) Rounded-shape cells settled down within 10 minutes of seeding. (B) Following 24 hours of cultivation, some spindle-shaped cells appeared. (C) The ratio of spindle-shaped cells increased on Day 3. (D) On Day 4, the spindle-shaped cells reached about 60-70% confluence. (E) On Day 5 of culture, the cells reached 80-90% confluence, being passaged at this term.

Figure 3. Expression of MSC gene markers detected by RT-PCR and agarose gel electrophoresis (lanes 1 to 6) for the following PCR products: (1) GAPDH (123bp) used as control; (2) CD73 (115 bp); (3) CD44 (136 bp); (4) CD11b (192 bp); (5) CD105 (344 bp); (6) CD34 (215 bp)
2. Characterization of cell surface markers
In this study, MSC(M) isolated from Balb/c mouse were characterized to investigate expression of surface antigens, and the results indicated that the cells were positive for CD73, and CD44, since the bands appeared at the expected distances when PCR products were subjected to electrophoresis in 2% agarose gel. Moreover, there was no detectable CD11b expression, along with only weak expression of CD34 and CD105 (Fig. 3).
3. Adipogenic and osteogenic differentiation
Adipogenic and osteogenic differentiation potential of isolated MSC(M) was tested. Following 7 days of culture under adipogenic induction conditions, MSC(M) showed marked morphological changes compared to the undifferentiated cells (control). They also showed accumulation of several cytoplasmic lipid droplets. After staining with lipophilic Sudan III, the control cells did not show any cytoplasmic changes and were negative for Sudan III staining, while distinct cytoplasmic lipid droplets were observed in MSC(M) grown in adipogenic medium (Fig. 4).

Figure 4. Mesodermal differentiation of MSC(M) at the passage 0
A: Adipogenic differentiation of MSCs: (a) BMSCs after induction with adipogenic medium, showing formation of intracytoplasmic lipid droplets; (b, c). Positive staining of lipid droplets with Sudan III. (B): Osteogenic differentiation: (a). BMSCs after induction with osteogenic medium showed cuboidal morphology (b). Alizarin Red staining indicated dark red precipitation of calcium deposits. (c) Control BMSCs cultured in DMEM medium (10%FBS).
Following 7 days of culture in osteogenic induction medium, MSC(M) showed morphological changes and acquired cubical shape, whereas control cells did not show any morphological changes. After staining with Alizarin Red, the control cells were dye-negative, whereas differentiated cells were positively stained, indicating the presence of calcium deposits (Fig. 4).
Discussion
Cell isolation procedure
Mesenchymal stromal cells (MSCs) are used in many research fields and have sparked great interest in cell therapies due to their ability to differentiate into various cell types [18]. MSC culture was first established by Friedenstein and his colleagues by virtue of the physical propensity of MSCs to adhere to plastic flasks, and the original method has been modified by others [19]. Several methods have been used for isolating MSC(M) including whole marrow direct adherence, density gradient centrifugation, flow cytometry and immunobead methods. However, the latter two methods are considered high in cost and technically difficult [20]. The direct culture method is best to culture high number of MSC(M) within very short time and at high viability rate [21]. It is clearly easier and reduce loss of MSCs compared to density gradient separation approach. However, the cells collected by this method represent a heterogeneous mixture of cells, including hematopoietic cells, endothelial cells and endothelial progenitor cells [11]. This problem was solved using frequent medium change that may prevent adherence of many of the non-MSCs and hematopoietic cells to the culture dishes [2].
Usage of adult MSCs in human therapeutic applications depends on the establishing of preclinical studies with other mammals such as mouse [22]. In order for such therapies to be reproducibly performed in preclinical animal models, a simple, high-yield method for obtaining MSCs is required [19]. In this study, MSC(M) were isolated from the mice aged 4-6 weeks. We obtained high cellular yield, and the isolated cells were able to grow and proliferate within a short period of time. Previous study indicated that age of the animals used as MSCs source directly influences their differentiation, proliferative and metabolism profiles [23]. According to [19], younger mice (sucklings of <3 weeks) produce more proliferative cell cultures. Long bones of newborn mice (up to one week of age) are quite fragile, thus being inconvenient for researchers who have no relevant experience handling them.
- We found that a delay in isolation process from sacrificing the mouse to BM collection and culture, greatly affects the yield and viability of the isolated cells. This step is very critical and requires experience and accuracy during work.
- In this study, MSC(M) were isolated using an adherence method, the culture medium was replaced daily during the first four days of culture, in order to provide the cells with the nutrients required for their growth and proliferation. At the same time, the daily change of the culture media may prevent adherence of many non-MSCs, e.g., hematopoietic populations, to the culture dishes. According to our experience, we do not recommend replacement of the culture medium two or three hours after primary cell culture because we found that this process results in loss of some MSCs and thus obtaining a lower cellular yield.
- Two types of culture media (DMEM or RPMI 1640) supplemented with 20% FBS, 1% L-glutamine, 1% penicillin/streptomycin and 1.5 µg/ml amphotericin B were used in our study in order to choose the optimal medium, and the results demonstrated that the both media are suitable for MSC(M) culture and proliferation, since no differences in cell viability, growth or proliferation were observed.
- Fetal bovine serum (FBS) seems to be the most popular choice of supplements in the culture media. It may play a key role in promoting cell attachment and proliferation [24]. In the current protocol, FBS concentration was increased to 20%, and we found that this concentration was suitable for obtaining sufficient numbers of MSCs within a short period of time.
- After separating bones from the muscles, they were directly transferred to a Petri dish containing 3-4 ml of pre-warmed DMEM medium (37°C), without additives of serum or antibiotics, to preserve cell viability.
- To reduce the possibility of contamination, the bones should not be transferred to the culture media before cleaning it from the muscle.
- To reduce the possibility of contamination, a fresh media should be used to flush the marrow out of the bone rather than using the media in which the bones were placed after separating from the muscle.
- In this study, the marrow was flushed out from both ends of the bone to obtain a high cellular yield, as proposed by [25].
- Only 1.4 ml of culture medium is sufficient for flushing the marrow out of the bone, then the bones could be transferred to a sterile mortar containing 5 ml of culture medium, and gently crushed to extract the remaining bone marrow. We found that this step is very useful to obtain a high MSC(M) yield, because the stromal cells locate near the surface of the bone, which makes it difficult to obtain enough MSCs, even after flushing BM during cell preparation [8].
- In our study, after collecting the BM suspension, it was immediately transferred to the culture flask, without centrifugation or filtration. MSC(M) were cultured into their initial niche composing of stromal cells, extracellular matrix elements, and secreting factors with minimal disturbance, to allow the initial adjusting time for the MSCs in culture, according to [26]. We found this method to be more efficient. However, in our protocol, the culture medium was replaced daily, contrary to what was suggested by [26] who kept the cells in their niche for 4 days without replacing the media.
- Using our modified protocol, we were able to isolate ~ 25% more BMMNCs than the commonly used protocol.
- On the fifth day of culture, MSC(M) reached about 80- 90% confluence. Hence, they were passaged to prevent overgrowth and cell detachment. MSC(M) are strongly attached to the flask and can't be easily detached from the flask using trypsin solution at lower concentration (0.05%). Therefore, a more concentrated trypsin solution (0.25%) was used to detach the cells.
- The trypsinization conditions should be carefully controlled. It is necessary to adhere to the concentration of the trypsin solution used to separate the cells, as well as incubation time. Insufficient trypsin treatment will reduce the yield of cells [11]. On the other hand, bone marrow-derived adherent cells were found to contain different cell types including fibroblasts, hematopoietic progenitor cells, macrophages, endothelial cells and adipocytes [4]. Long-term incubation with trypsin resulting in collection of other unwanted trypsin-resistant cells, e.g., macrophages [19], which firmly adhere to cell culture dishes, whereas MSCs were shown to be more responsive to trypsin [4]. Excessive trypsinisation may also damage cells by striping cell surface proteins. The most prominent problems that researchers may face in the mouse MSC(M) isolation and culture and possible solutions for them are listed in Table 2.
Table 2. Some problems and suggested solutions for mouse MSC(M) isolation and culture

Cell Surface Markers
Mammalian bone marrow contains a myriad of stem cells with distinctive morphological and functional features such as mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), multipotent adult progenitor cells (MAPCs) and very small embryonic-like stem cells (VSELs) [12]. Due to growing interest in using MSCs for cell-based therapy, the need to identify MSCs in a definitive way is not only of research interest but also derives from clinical requirements [27].
The isolation of distinct cell types from bone marrow stroma is hindered by the lack of known specific surface markers for those cells. To date, only a set of markers can identify stem/stromal cells from bone marrow, and none of them is unique [1].
In this study, MSC(M) isolated from Balb/c mice were characterized to investigate gene expression of the surface markers, and the results indicated that the cells were positive for (CD73 and CD44), negative for (CD11b), while they showed weak expression of CD34 and CD105 (Fig. 3). These results demonstrated that the isolated cells were MSC-like in morphology, adhesion to plastic surfaces, expression of surface markers, and in adipogenic/osteogenetic differentiation potential. Although the isolated MSC(M) showed characteristics of mesenchymal stromal cells, we suggest that there is a low proportion of hematopoietic stem cells, since the cells were characterized at the passage (P0), and it has been reported that hematopoietic stem cells still exist in the culture even after nine in vitro passages [19]. Increased number of cell passages could result in a significant reduction of hematopoietic stem cells.
Mouse MSCs are generally characterized by positive expression of CD44, CD73, CD105, CD29, CD106, Sca-1, and negative expression of hematopoietic and endothelial markers CD45, CD11b, Ter-119, and CD31 [6]. Our results showed that the surface marker CD profiles for the MSC(M) were compatible with those previously reported.
It has been demonstrated that CD34 expression is not specific for murine hematopoietic cells, as it was shown for human cells. Previous study reported that the expression of CD34 was strain-dependent in mice. Bl/6 MSCs expressed high levels of CD34, with moderate expression by FVB/N MSCs. Both Balb/c and DBA1 MSCs expressed low levels, while MSCs from the 4 standard inbred strains were negative for CD11b [10].
Adipogenic and osteogenic potentials
MSCs are defined by their ability to differentiate and generate cells of mesodermal origin like adipocytes, osteocytes and chondrocytes in culture [6]. The results of mesodermal differentiation assays showed that isolated MSC(M) were able to differentiate into adipocytes and osteocytes in vitro. MSC(M) cultured in adipogenic medium showed morphological changes and displayed accumulation of lipid vacuoles, which stained positively with Sudan III. Lipid droplets in differentiated cells became red due to their affinity for lipophilic Sudan III dye. Similarly, MSC(M) cultured in osteogenic medium showed morphological changes and stained positively with Alizarin Red, indicating presence of calcium deposits. Our results are consistent with previous reports, and demonstrate the opportunity of using these cells in both clinical and research applications.
In the current study, MSC(M) at passage 0 were able to differentiate into adipocyte and osteoblasts like cells within only seven days of incubating with differentiation medium. Meanwhile, many previous studies indicated that adipogenic and osteogenic differentiation takes about three weeks. This slow dynamic may be due to the fact that cell differentiation capability and function decline with repeated passaging [28].
Previous research demonstrated that BMSCs from passage 3 were inferior to bone marrow mononuclear cells (BMMNCs) not only in their chondrogenic differentiation capability but also as candidates for long-term repair of cartilage defects [28]. Previous study reported that MSCs from passage 1 have stronger osteogenic potential in vitro than those of passages 2 and 3, and might be suitable for clinical application to bone tissue engineering [29].
Differentiation of MSCs into various lineages is strictly regulated at multiple sequential steps, being represented by distinct morphological and molecular characteristics [12]. Until now, the mechanism of MSCs trans-differentiation is unclear. There are many internal and external factors that trigger MSCs differentiation.
Conclusion
Increased demand for MSCs in research and medical studies has led scientists to give priority to the development of standardized MSCs isolation and culture methods. In the present study, we have successfully isolated MSC(M) from Balb/c mouse under optimized isolation and culture conditions. We have also listed the most prominent problems that researchers may face and suggested the possible solutions for them. We hope that this simple and practical method will facilitate the study of MSCs, both for examining their biological properties, as well as their therapeutic potential in various murine disease models.
Acknowledgments
The authors are grateful to the Leishmania Center of Epidemiological and Biological Studies, National Commission for Biotechnology (Department of Pharmaceutical Biotechnology). We would like to thank Dr. Ruba Joujeh, Dr. Hassan Alkhoury, Dr. Majd Aljamali, Dr. Fateh Khatib, Ms. Reham Antaki, for their help and support. This research was funded by the University of Aleppo.
References
- Rostovskaya M, Anastassiadis K. Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PLoS One. 2012; 7(12): 1-12. doi: 10.1371/journal.pone.0051221
- Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nature Protocols. 2009; 4(1): 102-106. doi: 10.1038/nprot.2008.221
- Al-Qaisy B, Yaseen N, Alwachi S, AL-Shammari A. Comparison between three different protocols for isolation and culture of mouse bone marrow derived mesenchymal stem cells. Iraqi J Cancer Med Genet. 2014; 7(1): 26-35.
- Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int. J. Dev. Biol. 2007; 51: 723-729. doi: 10.1387/ijdb.072352ns
- Caplan A. Mesenchymal stem cells: Time to change the name. Stem Cells Transl Med. 2017; 1-7. https://doi.org/10.1002/sctm.17-0051
- Schachtele S, Clouser C, Aho J. Markers and methods to verify mesenchymal stem cell identity, potency, and quality. https://www.cellandgene.com/doc/markers-and-methods-to-verify-mesenchymal-stem-cell-identity-potency-and-quality-0001
- Oikonomopoulos A, Deen W, Manansala A, Lacey P, Tomakili T, Ziman A, Hommes D. Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media. Sci Rep. 2015; 5: 1-11. doi: 10.1038/srep16570
- Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ. Isolation and characterization of mouse mesenchymal stem cells. Transpl Proc. 2008; 40: 2649-2654. doi: 10.1016/j.transproceed.2008.08.009
- Hu Y, Lou B, Wu X, Wu R, Wang H, Gao L, Pi J, Xu Y. Comparative study on in vitro culture of mouse bone marrow mesenchymal stem cells. Stem Cells Intern. 2018; 1-14. doi: 10.1155/2018/6704583
- Peister A, Mellad J, Larson B, Hall B, Gibson L, Prockop D. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004; 103(5): 1662-1668.
doi: 10.1182/blood-2003-09-3070 - Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. BioMed Research International. 2014; 1-11. doi: 10.1155/2014/951512
- Chaudhary JK, Rath PC. A simple method for isolation, propagation, characterization, and differentiation of adult mouse bone marrow-derived multipotent mesenchymal stem cells. J Cell Sci Ther. 2017; 8(1): 1-10. doi: 10.4172/2157-7013.1000261
- Guide for the care and use of laboratory animals, Eighth Edition, 2011, National Academy of Sciences, 246 pages.
- Roderfeld M, Rath T, Voswinckel R, Dierkes C, Dietrich H, Zahner D, Graf J, Roeb E. Bone marrow transplantation demonstrates medullar origin of CD34+ fibrocytes and ameliorates hepatic fibrosis in Abcb4_/_ mice. Hepatology. 2010; 51:267-276.
doi: 10.1002/hep.23274 - Tohidi F, Toosi M, Azimian H, Khademi S, Fardid R, Sarab GH. The gene expression level of p53 and p21 in mouse brain exposed to radiofrequency field. Int J Radiat Res. 2015; 13(4): 337-343. doi: 10.7508/ijrr.2015.04.007
- Sibov Y, Severino P, Marti LC, Pavon LF, Oliveira DM, Tobo PR, Campos AH, Paes AT, Amaro E, Gamarra LF, Moreira-Filho CA. Mesenchymal stem cells from umbilical cord blood: parameters for isolation, characterization and adipogenic differentiation. Cytotechnology. 2012; 64: 511-521. doi: 10.1007/s10616-012-9428-3
- Castro-Silva I, Castro L, Machado J, Nicola M, Granjeiro J. Isolation of human umbilical cord blood-derived osteoprogenitor cells: a promising candidate for cell-based therapy for bone repair. Einstein. 2011; 9: 449-455. doi: 10.1590/s1679-45082011ao2196
- Baustian C, Hanley S, Ceredig R. Isolation, selection and culture methods to enhance clonogenicity of mouse bone marrow derived mesenchymal stromal cell precursors. Stem Cell Res Ther. 2015; 6(151): 1-13. doi: 10.1186/s13287-015-0139-5
- Zhu H, Guo Z, Jiang X, Li H, Wang X, Yao H, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nature Protoc. 2010; 5(3): 550-560. doi: 10.1038/nprot.2009.238
- Hasoon MF, Nader B, Mohammed MH. A study of the bone marrow derived mesenchymal stromal cells in rats - proliferation and immunophynotypic markers. Malaysian J Vet Res. 2018; 9(1): 73-80.
- Abdullah RH, Yaseen NY, Saleh SM, Mohamed MH, Al-Shammari A. Direct and simple method for mesenchymal stem cells isolation, culturing and detection. Int J Stem Cell Res. 2018; 5(2): 1-5. doi: 10.23937/2469-570X/1410054
- Tropel Ph, Noel D, Platet N, Legrand P, Benabid A, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004; 295: 395-406. doi: 10.1016/j.yexcr.2003.12.030
- Fafián-Labora J, Fernández-Pernas P, Fuentes I, De Toro J, Oreiro N, Sangiao-Alvarellos S, Mateos J, Arufe MC. Influence of age on rat bone marrow mesenchymal stem cells potential. Sci Rep. 2015; 5: 1-20. doi: 10.1038/srep16765
- Wang X, Yang J, Chen X, Pan X. Establishment and characterization of a fibroblast-like cell line from Anabarilius grahami (Cypriniformes: Cyprinidae). Dongwuxue Yanjiu. 2012; 33(E5-6): E89-E97. doi: 10.3724/SP.J.1141.2012.E05-06E89
- Madaan A, Verma R, Singh AT, Jain SK, Jaggi M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. Journal of Biological Methods. 2014; 1(e1): 1-6. doi: 10.14440/jbm.2014.12
- Huang S, Xu L, Sun Y, Wu T, Wang K, Li G. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J Orthop Transl. 2015; 3(1): 26-33. doi: 10.1016/j.jot.2014.07.005
- Augello A, Kurth TB, Bari CD. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010; 20: 121-133. doi:10.22203/ecm.v020a11
- Jiang T, Xu G, Wang Q, Yang L, Zheng L, Zhao J, Zhang X. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017; 8:1-12. doi: 10.1038/cddis.2017.215
- Chen J, Sotome S, Wang J, Orii H, Uemura T, Shinomiya K. Correlation of in vivo bone formation capability and in vitro differentiation of human bone marrow stromal cells. J Med Dent Sci. 2005; 52(1): 27-34. PMID: 15868738
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(99) "optimizirovannyy-protokol-vydeleniya-i-kultivirovaniya-myshinykh-mezenkhimalnykh-stromalnykh-kletok" ["~CODE"]=> string(99) "optimizirovannyy-protokol-vydeleniya-i-kultivirovaniya-myshinykh-mezenkhimalnykh-stromalnykh-kletok" ["EXTERNAL_ID"]=> string(4) "2046" ["~EXTERNAL_ID"]=> string(4) "2046" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(273) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клетокAn optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2317) "<p style="text-align: justify;">Мезенхимные стволовые клетки (МСК) вызвали большой интерес в научном сообществе. МСК мышей служат идеальной моделью для изучения клеточной биологии и терапевтического потенциала этой популяции клеток. Поэтому необходимо создание оптимального стандартизованного протокола для выделения и культивирования мышиных МСК. Нашей целью было разработать и описать эффективный, надежный и простой в выполнении протокол для выделения и культивирования мезенхимальных стромальных клеток костного мозга мышей (МСКМ). Наш протокол основан на сочетании метода промывки и механического дробления костей. МСКМ, выделенные с использованием нашего протокола, имеют веретенообразную форму, проявляется экспрессия маркеров CD73 и CD44, слабая экспрессия CD34 и CD105 и отсутствие CD11b. Эти клетки также способны дифференцироваться в другие ростки мезодермы, такие как клоны адипоцитов и остеоцитов. Мы надеемся, что данные, представленные в этой статье, имеют практическое значение и могут быть использованы в клинических и исследовательских приложениях, а также при заготовке клеток для банкирования. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Мыши Balb/c, костный мозг, изоляция клеток, мезенхимные стволовые клетки, первичная культура.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_META_TITLE"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_META_KEYWORDS"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_META_DESCRIPTION"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_PICTURE_FILE_ALT"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_PICTURE_FILE_TITLE"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "optimizirovannyy-protokol-vydeleniya-i-kultivirovaniya-myshinykh-mezenkhimalnykh-stromalnykh-kletok-" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(182) "Оптимизированный протокол выделения и культивирования мышиных мезенхимальных стромальных клеток" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "optimizirovannyy-protokol-vydeleniya-i-kultivirovaniya-myshinykh-mezenkhimalnykh-stromalnykh-kletok-" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "optimizirovannyy-protokol-vydeleniya-i-kultivirovaniya-myshinykh-mezenkhimalnykh-stromalnykh-kletok-" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "optimizirovannyy-protokol-vydeleniya-i-kultivirovaniya-myshinykh-mezenkhimalnykh-stromalnykh-kletok-" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "203" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28465" ["VALUE"]=> array(2) { ["TEXT"]=> string(299) "<p>Дима Джуджех<sup>1</sup>, Абдулджалил Гревати<sup>1</sup>, Чади Суккариех<sup>2</sup>, Аднан Альмаррави<sup>1</sup>, Джамал А.Н. Дарвича<sup>3</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(227) "
Дима Джуджех1, Абдулджалил Гревати1, Чади Суккариех2, Аднан Альмаррави1, Джамал А.Н. Дарвича3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28466" ["VALUE"]=> array(2) { ["TEXT"]=> string(635) "<p><sup>1</sup> Департамент биотехнологической инженерии, Факультет технической инженерии, Университет Алеппо, Сирия<br> <sup>2</sup> Департамент биологии животных, Факультет наук, Университет Дамаска, Сирия<br> <sup>3</sup> Департамент фармакологии и токсикологии, Факультет фармации, Арабский международный университет, Сирия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(575) "1 Департамент биотехнологической инженерии, Факультет технической инженерии, Университет Алеппо, Сирия
2 Департамент биологии животных, Факультет наук, Университет Дамаска, Сирия
3 Департамент фармакологии и токсикологии, Факультет фармации, Арабский международный университет, Сирия
Мезенхимные стволовые клетки (МСК) вызвали большой интерес в научном сообществе. МСК мышей служат идеальной моделью для изучения клеточной биологии и терапевтического потенциала этой популяции клеток. Поэтому необходимо создание оптимального стандартизованного протокола для выделения и культивирования мышиных МСК. Нашей целью было разработать и описать эффективный, надежный и простой в выполнении протокол для выделения и культивирования мезенхимальных стромальных клеток костного мозга мышей (МСКМ). Наш протокол основан на сочетании метода промывки и механического дробления костей. МСКМ, выделенные с использованием нашего протокола, имеют веретенообразную форму, проявляется экспрессия маркеров CD73 и CD44, слабая экспрессия CD34 и CD105 и отсутствие CD11b. Эти клетки также способны дифференцироваться в другие ростки мезодермы, такие как клоны адипоцитов и остеоцитов. Мы надеемся, что данные, представленные в этой статье, имеют практическое значение и могут быть использованы в клинических и исследовательских приложениях, а также при заготовке клеток для банкирования.
Ключевые слова
Мыши Balb/c, костный мозг, изоляция клеток, мезенхимные стволовые клетки, первичная культура.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28468" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-61-70" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-61-70" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28469" ["VALUE"]=> array(2) { ["TEXT"]=> string(235) "<p>Dima Joujeh<sup>1</sup>, Abduljalil Ghrewaty<sup>1</sup>, Chadi Soukkarieh<sup>2</sup>, Adnan Almarrawi<sup>1</sup>, Jamal Abdul Naser Darwicha<sup>3</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(163) "Dima Joujeh1, Abduljalil Ghrewaty1, Chadi Soukkarieh2, Adnan Almarrawi1, Jamal Abdul Naser Darwicha3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28470" ["VALUE"]=> array(2) { ["TEXT"]=> string(880) "<p> <sup>1</sup> Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria<br> <sup>2</sup> Department of Animal Biology, Faculty of Sciences, Damascus University, Syria<br> <sup>3</sup> Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria </p> <br> <p><b>Correspondence:</b><br> Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria<br> E-mail: dimajoujeh@gmail.com</p><br> <p><b>Citation:</b> Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(748) "
1 Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
2 Department of Animal Biology, Faculty of Sciences, Damascus University, Syria
3 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria
Correspondence:
Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
E-mail: dimajoujeh@gmail.com
Citation: Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28471" ["VALUE"]=> array(2) { ["TEXT"]=> string(1223) "<p style="text-align: justify;">Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking. </p> <h2>Keywords</h2> <p style="text-align: justify;">Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1167) "Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking.
Keywords
Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28472" ["VALUE"]=> string(91) "An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(91) "An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28473" ["VALUE"]=> string(4) "2742" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2742" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28474" ["VALUE"]=> string(4) "2743" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2743" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28469" ["VALUE"]=> array(2) { ["TEXT"]=> string(235) "<p>Dima Joujeh<sup>1</sup>, Abduljalil Ghrewaty<sup>1</sup>, Chadi Soukkarieh<sup>2</sup>, Adnan Almarrawi<sup>1</sup>, Jamal Abdul Naser Darwicha<sup>3</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(163) "Dima Joujeh1, Abduljalil Ghrewaty1, Chadi Soukkarieh2, Adnan Almarrawi1, Jamal Abdul Naser Darwicha3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(163) "Dima Joujeh1, Abduljalil Ghrewaty1, Chadi Soukkarieh2, Adnan Almarrawi1, Jamal Abdul Naser Darwicha3
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28471" ["VALUE"]=> array(2) { ["TEXT"]=> string(1223) "<p style="text-align: justify;">Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking. </p> <h2>Keywords</h2> <p style="text-align: justify;">Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1167) "Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking.
Keywords
Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1167) "Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking.
Keywords
Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28468" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-61-70" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-61-70" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-61-70" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28472" ["VALUE"]=> string(91) "An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(91) "An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(91) "An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28470" ["VALUE"]=> array(2) { ["TEXT"]=> string(880) "<p> <sup>1</sup> Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria<br> <sup>2</sup> Department of Animal Biology, Faculty of Sciences, Damascus University, Syria<br> <sup>3</sup> Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria </p> <br> <p><b>Correspondence:</b><br> Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria<br> E-mail: dimajoujeh@gmail.com</p><br> <p><b>Citation:</b> Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(748) "
1 Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
2 Department of Animal Biology, Faculty of Sciences, Damascus University, Syria
3 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria
Correspondence:
Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
E-mail: dimajoujeh@gmail.com
Citation: Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(748) "
1 Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
2 Department of Animal Biology, Faculty of Sciences, Damascus University, Syria
3 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria
Correspondence:
Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
E-mail: dimajoujeh@gmail.com
Citation: Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28465" ["VALUE"]=> array(2) { ["TEXT"]=> string(299) "<p>Дима Джуджех<sup>1</sup>, Абдулджалил Гревати<sup>1</sup>, Чади Суккариех<sup>2</sup>, Аднан Альмаррави<sup>1</sup>, Джамал А.Н. Дарвича<sup>3</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(227) "Дима Джуджех1, Абдулджалил Гревати1, Чади Суккариех2, Аднан Альмаррави1, Джамал А.Н. Дарвича3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(227) "Дима Джуджех1, Абдулджалил Гревати1, Чади Суккариех2, Аднан Альмаррави1, Джамал А.Н. Дарвича3
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28467" ["VALUE"]=> array(2) { ["TEXT"]=> string(2317) "<p style="text-align: justify;">Мезенхимные стволовые клетки (МСК) вызвали большой интерес в научном сообществе. МСК мышей служат идеальной моделью для изучения клеточной биологии и терапевтического потенциала этой популяции клеток. Поэтому необходимо создание оптимального стандартизованного протокола для выделения и культивирования мышиных МСК. Нашей целью было разработать и описать эффективный, надежный и простой в выполнении протокол для выделения и культивирования мезенхимальных стромальных клеток костного мозга мышей (МСКМ). Наш протокол основан на сочетании метода промывки и механического дробления костей. МСКМ, выделенные с использованием нашего протокола, имеют веретенообразную форму, проявляется экспрессия маркеров CD73 и CD44, слабая экспрессия CD34 и CD105 и отсутствие CD11b. Эти клетки также способны дифференцироваться в другие ростки мезодермы, такие как клоны адипоцитов и остеоцитов. Мы надеемся, что данные, представленные в этой статье, имеют практическое значение и могут быть использованы в клинических и исследовательских приложениях, а также при заготовке клеток для банкирования. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Мыши Balb/c, костный мозг, изоляция клеток, мезенхимные стволовые клетки, первичная культура.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2261) "Мезенхимные стволовые клетки (МСК) вызвали большой интерес в научном сообществе. МСК мышей служат идеальной моделью для изучения клеточной биологии и терапевтического потенциала этой популяции клеток. Поэтому необходимо создание оптимального стандартизованного протокола для выделения и культивирования мышиных МСК. Нашей целью было разработать и описать эффективный, надежный и простой в выполнении протокол для выделения и культивирования мезенхимальных стромальных клеток костного мозга мышей (МСКМ). Наш протокол основан на сочетании метода промывки и механического дробления костей. МСКМ, выделенные с использованием нашего протокола, имеют веретенообразную форму, проявляется экспрессия маркеров CD73 и CD44, слабая экспрессия CD34 и CD105 и отсутствие CD11b. Эти клетки также способны дифференцироваться в другие ростки мезодермы, такие как клоны адипоцитов и остеоцитов. Мы надеемся, что данные, представленные в этой статье, имеют практическое значение и могут быть использованы в клинических и исследовательских приложениях, а также при заготовке клеток для банкирования.
Ключевые слова
Мыши Balb/c, костный мозг, изоляция клеток, мезенхимные стволовые клетки, первичная культура.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2261) "Мезенхимные стволовые клетки (МСК) вызвали большой интерес в научном сообществе. МСК мышей служат идеальной моделью для изучения клеточной биологии и терапевтического потенциала этой популяции клеток. Поэтому необходимо создание оптимального стандартизованного протокола для выделения и культивирования мышиных МСК. Нашей целью было разработать и описать эффективный, надежный и простой в выполнении протокол для выделения и культивирования мезенхимальных стромальных клеток костного мозга мышей (МСКМ). Наш протокол основан на сочетании метода промывки и механического дробления костей. МСКМ, выделенные с использованием нашего протокола, имеют веретенообразную форму, проявляется экспрессия маркеров CD73 и CD44, слабая экспрессия CD34 и CD105 и отсутствие CD11b. Эти клетки также способны дифференцироваться в другие ростки мезодермы, такие как клоны адипоцитов и остеоцитов. Мы надеемся, что данные, представленные в этой статье, имеют практическое значение и могут быть использованы в клинических и исследовательских приложениях, а также при заготовке клеток для банкирования.
Ключевые слова
Мыши Balb/c, костный мозг, изоляция клеток, мезенхимные стволовые клетки, первичная культура.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28466" ["VALUE"]=> array(2) { ["TEXT"]=> string(635) "<p><sup>1</sup> Департамент биотехнологической инженерии, Факультет технической инженерии, Университет Алеппо, Сирия<br> <sup>2</sup> Департамент биологии животных, Факультет наук, Университет Дамаска, Сирия<br> <sup>3</sup> Департамент фармакологии и токсикологии, Факультет фармации, Арабский международный университет, Сирия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(575) "1 Департамент биотехнологической инженерии, Факультет технической инженерии, Университет Алеппо, Сирия
2 Департамент биологии животных, Факультет наук, Университет Дамаска, Сирия
3 Департамент фармакологии и токсикологии, Факультет фармации, Арабский международный университет, Сирия
1 Департамент биотехнологической инженерии, Факультет технической инженерии, Университет Алеппо, Сирия
2 Департамент биологии животных, Факультет наук, Университет Дамаска, Сирия
3 Департамент фармакологии и токсикологии, Факультет фармации, Арабский международный университет, Сирия
Introduction
Medical imaging is one of the main diagnostic methods that defines the treatment strategy. The main advantage of X-ray data examination is its ability for digitilization. The data obtained by the digitization provide multidimensional data which, due to appropriate standardization and analysis, enable additional information about specific features of the diseases [1].
Radiomics is a quantitative method for the analysis of digitized X-ray images, which is based on mathematical analysis [2]. Radiomics allows quantifying texture information by mathematically extracting the spatial distribution of signal intensities and pixel relationships [2, 3]. The concept of radiomics is based on the search for imaging biomarkers that are specific to certain pathological processes undetectable by standard visual inspection of the generated images. Visually noticeable differences in the intensity, shape and texture of the image can be quantified using radiomics, which allows the more objective process of interpreting X-ray images [3]. The key purpose of radiomics applied for medical image analysis is to reveal objective non-invasive prognostic biomarkers of the disease, presuming its further transition to personalized medicine [4].
Four main steps for extraction of radiomic data from medical images are as follows:
1. Acquisition of images using methods of radiological diagnostics;
2. Segmentation of the region of interest (ROI or volume of interest – VOI);
3. Extraction of radiomic features;
4. Obtaining biomarkers of imaging [5].
However, in order to improve the accuracy of diagnostic procedures, such as biopsy of the neoplasm, the obtained data require integration into the clinical picture. This can be done by directly overlaying the region of the location of the imaging biomarker on the region of neoplasm through augmented reality.
Augmented reality is the projection of any digital information (images, video, text, graphics, etc.) over a real image [6]. As a result, the real world is supplemented with artificial elements and new information. This technology is becoming increasingly popular in various fields of medicine, including maxillofacial surgery [6]. The following is required with respect to ensurance of the technology functioning:
1. Acquisition of images using methods of conventional radiology diagnostics (obtaining data on a three-dimensional object);
2. Segmentation of the region of interest (e.g., neoplasms);
3. Loading the region of interest into augmented reality glasses;
4. Conducting surgery in augmented reality, focusing on the location of the region of interest.
As seen from the below algorithms, working in radiomics and augmented reality has several identical stages. Moreover, integration of the latter technique contributes to creation of a radio-oriented navigation system (Fig. 1).
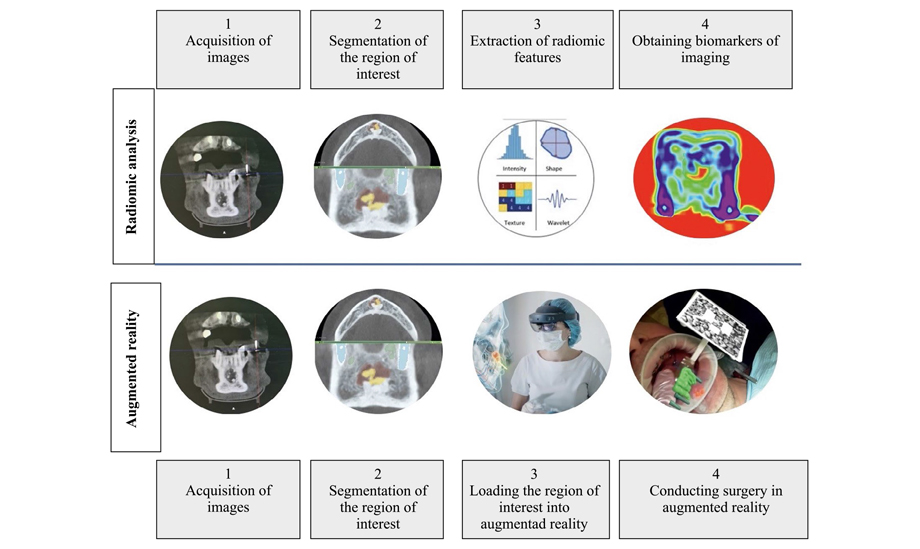
Figure 1. Algorithm for radiome analysis of Cone Beam Computed Tomography images and the algorithm for the operation of augmented reality technology (Original picture)
The purpose hereof is to present an algorithm for radiomic image analysis using the example of a neoplasm of the lower jaw and to demonstrate practical applicability of this technology, i.e., to conduct a radiomically targeted biopsy of a neoplasm directed by the augmented reality technology.
Materials and methods
The authors used a comprehensive open source platform PyRadiomics, built into 3D Slicer, the interface of the software was designed for analyzing and working with medical images, to handle the radiological data. The software is intended for processing and extraction of radiomic characteristics from medical images using a large panel of hard-coded function algorithms (Fig. 2).
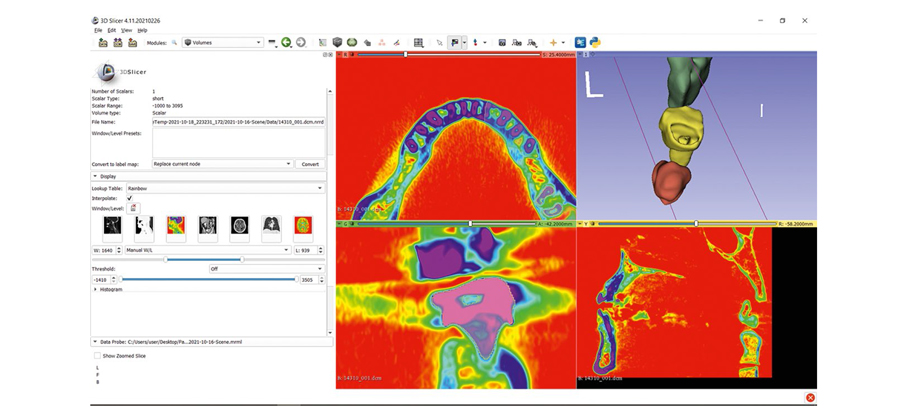
Figure 2. Appearance of the software 3D Slicer
Stage 1. Acquisition of images using methods of radiology diagnostics
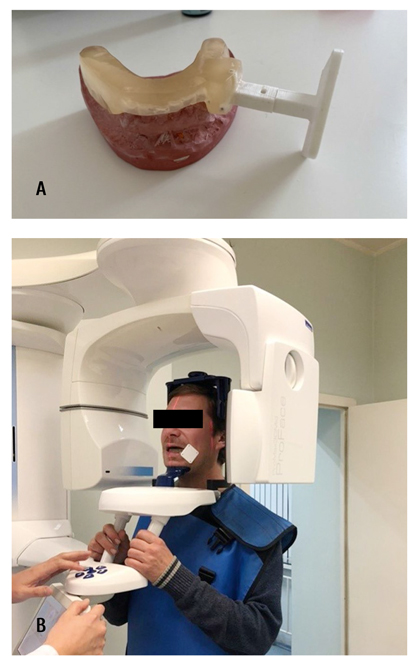
Figure 3. Appearance of the occlusal splint with X-ray contrast marks (A); Patient positioning at the cone-beam computed tomography with occlusal splint (B)
The first stage is to identify the clinical problem and obtain a digital image, excluding low quality studies. When planning to perform an intervention in augmented reality, it is necessary to perform an X-ray examination using X-ray contrast markers, according to which the augmented reality marker will be localized and calibrated for the prospective surgery.
Taking into account the mobility of lower jaw, an individual occlusal splint with X-ray contrast marks and an integrated holder for the augmented reality marker was pre-fabricated. Cone beam computed tomography (CBCT) was performed with a mouthguard containing X-ray contrast marks fixed in the patient's mouth (Fig. 3).
Stage 2. Segmentation of the region of interest
Upon receipt of the radiological data in DICOM format, one should define the region of interest (ROI) in two-dimensional projection (2D), or volume of interest (VOI) in three-dimensional (3D) projection begins. ROI/VOI determine the region in which the radiomic features are calculated. The image segmentation may be performed manually, semi-automatically or fully automatically (Fig. 4).
Stage 3. Extraction of radiomic features
After image segmentation and processing, the extraction of radiomic features can be performed, which is performed automatically after activation of the PyRadiomics module. The features extracted from images could be divided into morphological parameters (volume and shape), histogram features (description of gray tone intensities, texture analysis) (Fig. 5).
Stage 4. Obtaining imaging biomarkers
After receiving the radiomic data, selection or reduction of specific features is carried out. After that, the multivariate data analysis is started: a connection is established between the texture features of the gray level coincidence matrix and the morphological type of neoplasms. Isolated radiomic features that closely correlate with clinical findings may be assigned to the imaging biomarkers (Fig. 6).
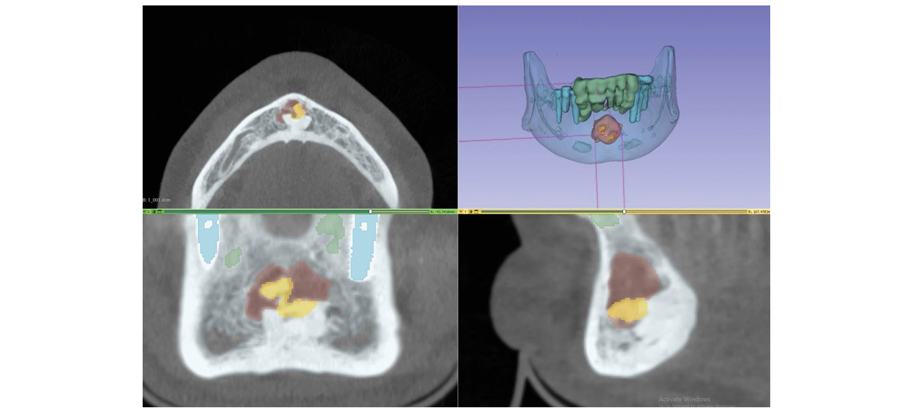
Figure 4. Segmentation of the area of interest in 3D Slicer
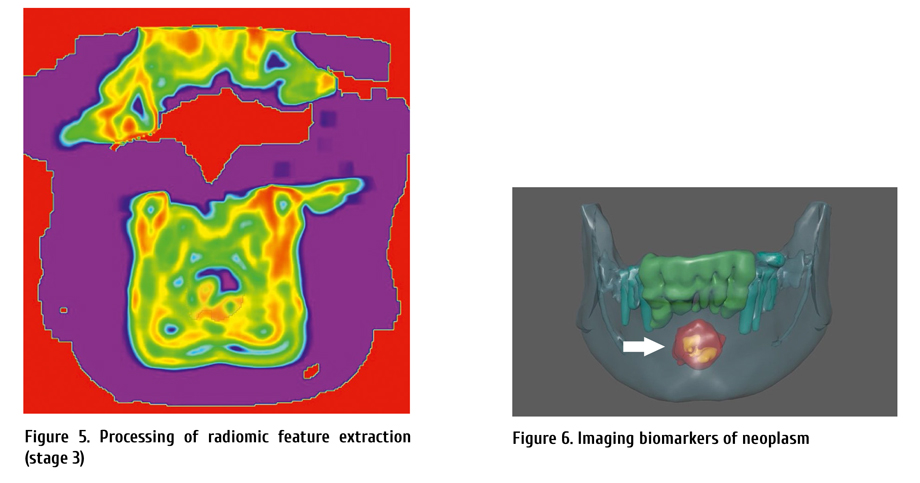
Stage 5. Creation of a radiomally oriented augmented reality navigation system
To perform the surgery, two augmented reality markers were used. Marker 1 was attached to a holder embedded into the occlusal splint (as in cone-beam computer tomography?? CTCBCT). Marker 1 was associated with an image of a segmented neoplasm of the lower jaw with a highlighted visualization biomarker. Marker 2 was attached to the handle of the dental handpiece. Projection of the bone trephine was attached to marker 2 (Fig. 7 a, b).
Figure 7. Marker 1 fixed in the oral cavity (A); marker 2, at the dental handpiece (B)
Stage 6. Radiomic-guided biopsy of mandibular neoplasm in augmented reality
During the surgical intervention after fixation of the augmented reality markers, we checked calibration of the marker positions, projection of the lower jaw neoplasm, and the tip of the biopsy instrument. The surgery was performed under local anesthesia. Skeletonization of the outer plate of the lower jaw in the area 3.3-4.3 was performed. Bone tissue in the region under inspection had no visible abnormal changes (Fig. 8).
Figure 8. The body of the lower jaw in the projection of the neoplasm. Pathological changes are not visualized
The neoplasm imaging was made in augmented reality. Using a bone trephine, a fragment of the formation was sampled in projection of the imaging biomarker (Fig. 9).
Figure 9. Location of the neoplasm in virtual imaging (A), and actual location of the object (B)
The material was sent for histological examination. The postoperative period was uneventful.
Histological report was as follows: an area of fibrous dysplasia of the lower jaw.
Discussion
In the presented clinical case, according to the results of the radiomic analysis of the segmented neoplasm of the lower jaw, its distinctive radiomic features were revealed - imaging biomarkers, that were transferred to the surgical field using augmented reality technology.
The object of a lower jaw neoplasm obtained as a result of segmentation with a highlighted area of region biomarkers is combined with real anatomical structures in the oral cavity at the time of surgery by means of augmented reality technology. The combined model of this neoplasm demonstrates its anatomical and topographic position and structure, thus making it possible to determine the optimal operative access and most informative biopsy area for histological examination. Usage of this approach to tracking the instrument tip (bone trephine) in mixed reality enables control of its position, immersion depth, and reduced risk of traumas to adjacent anatomical structures. Real volume and topography of the object and adjacent anatomical structures fully corresponded to the data obtained in the course of virtual planning.
Conclusion
Analysis of medical images allows non-invasive assessment of the characteristics of human tissues. However, the interpretation of research results is often quite subjective. Recent advances in acquisition and analysis of medical imaging allow high-precision digital data extraction to quantify the difference between healthy and diseased tissue.
Radiomics applies mathematical analysis and advanced computational methodologies to medical imaging data to provide quantitative descriptors of pathological tissues. This is especially true for oncology.
Radiomics analysis of X-ray images has the potential to promote development of personalized medicine.
Clinical application of radiomic data is of the greatest interest for targeted biopsy of neoplasms in maxillofacial area. In order to improve accuracy of the diagnostic procedure, we recommend usage of augmented reality technology, which makes it possible to accurately visualize the most informative biopsy area for histological examination.
On the basis of advances in modern digital technologies such as radiomics and augmented reality, one can personalize and adapt established methods of treating diseases in the maxillofacial region for each patient, to improve the results of diagnosis and treatment, as well as reducing the number of complications.
References
- Van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V. Computational radiomics system to decode the radiographic phenotype. Cancer Research 2017; 77(21) :104-107. doi: 10.1158/0008-5472.CAN-17-0339
- Aerts HJWL, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature Сommun. 2014; 5(1): 1-9. doi: 10.1038/ncomms5006
- Caudell JJ, Torres-Roca JF, Gillies RJ, Enderling H, Kim S, Rishi A, et al. The future of personalised radiotherapy for head and neck cancer. The Lancet Oncology 2017; 18( 5):266-273. doi: 10.1016/S1470-2045(17)30252-8
- van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging – "How-to" guide and critical reflection. Insights Imag. 2020; 11(1): 1-16. doi: 10.1186/s13244-020-00887-2
- Yaremenko АI, Lysenko AV, Ivanova EA, Galibin OV. Augmented reality technology for auricular reconstruction in the treatment of microtia. Cell Ther Transplant. 2020; 9(2): 78-82. doi: 10.18620/ctt-1866-8836-2020-9-2-78-82
- Lysenko AV, Razumova AYa, Yaremenko AI, Mirzakhmedov RR. Augmented reality in the treatment of sialolithiasis. Stomatology. 2020; 99(4): 64-66. doi: 10.17116/stomat20209904164
" ["~DETAIL_TEXT"]=> string(14372) "
Introduction
Medical imaging is one of the main diagnostic methods that defines the treatment strategy. The main advantage of X-ray data examination is its ability for digitilization. The data obtained by the digitization provide multidimensional data which, due to appropriate standardization and analysis, enable additional information about specific features of the diseases [1].
Radiomics is a quantitative method for the analysis of digitized X-ray images, which is based on mathematical analysis [2]. Radiomics allows quantifying texture information by mathematically extracting the spatial distribution of signal intensities and pixel relationships [2, 3]. The concept of radiomics is based on the search for imaging biomarkers that are specific to certain pathological processes undetectable by standard visual inspection of the generated images. Visually noticeable differences in the intensity, shape and texture of the image can be quantified using radiomics, which allows the more objective process of interpreting X-ray images [3]. The key purpose of radiomics applied for medical image analysis is to reveal objective non-invasive prognostic biomarkers of the disease, presuming its further transition to personalized medicine [4].
Four main steps for extraction of radiomic data from medical images are as follows:
1. Acquisition of images using methods of radiological diagnostics;
2. Segmentation of the region of interest (ROI or volume of interest – VOI);
3. Extraction of radiomic features;
4. Obtaining biomarkers of imaging [5].
However, in order to improve the accuracy of diagnostic procedures, such as biopsy of the neoplasm, the obtained data require integration into the clinical picture. This can be done by directly overlaying the region of the location of the imaging biomarker on the region of neoplasm through augmented reality.
Augmented reality is the projection of any digital information (images, video, text, graphics, etc.) over a real image [6]. As a result, the real world is supplemented with artificial elements and new information. This technology is becoming increasingly popular in various fields of medicine, including maxillofacial surgery [6]. The following is required with respect to ensurance of the technology functioning:
1. Acquisition of images using methods of conventional radiology diagnostics (obtaining data on a three-dimensional object);
2. Segmentation of the region of interest (e.g., neoplasms);
3. Loading the region of interest into augmented reality glasses;
4. Conducting surgery in augmented reality, focusing on the location of the region of interest.
As seen from the below algorithms, working in radiomics and augmented reality has several identical stages. Moreover, integration of the latter technique contributes to creation of a radio-oriented navigation system (Fig. 1).

Figure 1. Algorithm for radiome analysis of Cone Beam Computed Tomography images and the algorithm for the operation of augmented reality technology (Original picture)
The purpose hereof is to present an algorithm for radiomic image analysis using the example of a neoplasm of the lower jaw and to demonstrate practical applicability of this technology, i.e., to conduct a radiomically targeted biopsy of a neoplasm directed by the augmented reality technology.
Materials and methods
The authors used a comprehensive open source platform PyRadiomics, built into 3D Slicer, the interface of the software was designed for analyzing and working with medical images, to handle the radiological data. The software is intended for processing and extraction of radiomic characteristics from medical images using a large panel of hard-coded function algorithms (Fig. 2).

Figure 2. Appearance of the software 3D Slicer
Stage 1. Acquisition of images using methods of radiology diagnostics

Figure 3. Appearance of the occlusal splint with X-ray contrast marks (A); Patient positioning at the cone-beam computed tomography with occlusal splint (B)
The first stage is to identify the clinical problem and obtain a digital image, excluding low quality studies. When planning to perform an intervention in augmented reality, it is necessary to perform an X-ray examination using X-ray contrast markers, according to which the augmented reality marker will be localized and calibrated for the prospective surgery.
Taking into account the mobility of lower jaw, an individual occlusal splint with X-ray contrast marks and an integrated holder for the augmented reality marker was pre-fabricated. Cone beam computed tomography (CBCT) was performed with a mouthguard containing X-ray contrast marks fixed in the patient's mouth (Fig. 3).
Stage 2. Segmentation of the region of interest
Upon receipt of the radiological data in DICOM format, one should define the region of interest (ROI) in two-dimensional projection (2D), or volume of interest (VOI) in three-dimensional (3D) projection begins. ROI/VOI determine the region in which the radiomic features are calculated. The image segmentation may be performed manually, semi-automatically or fully automatically (Fig. 4).
Stage 3. Extraction of radiomic features
After image segmentation and processing, the extraction of radiomic features can be performed, which is performed automatically after activation of the PyRadiomics module. The features extracted from images could be divided into morphological parameters (volume and shape), histogram features (description of gray tone intensities, texture analysis) (Fig. 5).
Stage 4. Obtaining imaging biomarkers
After receiving the radiomic data, selection or reduction of specific features is carried out. After that, the multivariate data analysis is started: a connection is established between the texture features of the gray level coincidence matrix and the morphological type of neoplasms. Isolated radiomic features that closely correlate with clinical findings may be assigned to the imaging biomarkers (Fig. 6).

Figure 4. Segmentation of the area of interest in 3D Slicer

Stage 5. Creation of a radiomally oriented augmented reality navigation system
To perform the surgery, two augmented reality markers were used. Marker 1 was attached to a holder embedded into the occlusal splint (as in cone-beam computer tomography?? CTCBCT). Marker 1 was associated with an image of a segmented neoplasm of the lower jaw with a highlighted visualization biomarker. Marker 2 was attached to the handle of the dental handpiece. Projection of the bone trephine was attached to marker 2 (Fig. 7 a, b).

Figure 7. Marker 1 fixed in the oral cavity (A); marker 2, at the dental handpiece (B)
Stage 6. Radiomic-guided biopsy of mandibular neoplasm in augmented reality
During the surgical intervention after fixation of the augmented reality markers, we checked calibration of the marker positions, projection of the lower jaw neoplasm, and the tip of the biopsy instrument. The surgery was performed under local anesthesia. Skeletonization of the outer plate of the lower jaw in the area 3.3-4.3 was performed. Bone tissue in the region under inspection had no visible abnormal changes (Fig. 8).

Figure 8. The body of the lower jaw in the projection of the neoplasm. Pathological changes are not visualized
The neoplasm imaging was made in augmented reality. Using a bone trephine, a fragment of the formation was sampled in projection of the imaging biomarker (Fig. 9).

Figure 9. Location of the neoplasm in virtual imaging (A), and actual location of the object (B)
The material was sent for histological examination. The postoperative period was uneventful.
Histological report was as follows: an area of fibrous dysplasia of the lower jaw.
Discussion
In the presented clinical case, according to the results of the radiomic analysis of the segmented neoplasm of the lower jaw, its distinctive radiomic features were revealed - imaging biomarkers, that were transferred to the surgical field using augmented reality technology.
The object of a lower jaw neoplasm obtained as a result of segmentation with a highlighted area of region biomarkers is combined with real anatomical structures in the oral cavity at the time of surgery by means of augmented reality technology. The combined model of this neoplasm demonstrates its anatomical and topographic position and structure, thus making it possible to determine the optimal operative access and most informative biopsy area for histological examination. Usage of this approach to tracking the instrument tip (bone trephine) in mixed reality enables control of its position, immersion depth, and reduced risk of traumas to adjacent anatomical structures. Real volume and topography of the object and adjacent anatomical structures fully corresponded to the data obtained in the course of virtual planning.
Conclusion
Analysis of medical images allows non-invasive assessment of the characteristics of human tissues. However, the interpretation of research results is often quite subjective. Recent advances in acquisition and analysis of medical imaging allow high-precision digital data extraction to quantify the difference between healthy and diseased tissue.
Radiomics applies mathematical analysis and advanced computational methodologies to medical imaging data to provide quantitative descriptors of pathological tissues. This is especially true for oncology.
Radiomics analysis of X-ray images has the potential to promote development of personalized medicine.
Clinical application of radiomic data is of the greatest interest for targeted biopsy of neoplasms in maxillofacial area. In order to improve accuracy of the diagnostic procedure, we recommend usage of augmented reality technology, which makes it possible to accurately visualize the most informative biopsy area for histological examination.
On the basis of advances in modern digital technologies such as radiomics and augmented reality, one can personalize and adapt established methods of treating diseases in the maxillofacial region for each patient, to improve the results of diagnosis and treatment, as well as reducing the number of complications.
References
- Van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V. Computational radiomics system to decode the radiographic phenotype. Cancer Research 2017; 77(21) :104-107. doi: 10.1158/0008-5472.CAN-17-0339
- Aerts HJWL, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature Сommun. 2014; 5(1): 1-9. doi: 10.1038/ncomms5006
- Caudell JJ, Torres-Roca JF, Gillies RJ, Enderling H, Kim S, Rishi A, et al. The future of personalised radiotherapy for head and neck cancer. The Lancet Oncology 2017; 18( 5):266-273. doi: 10.1016/S1470-2045(17)30252-8
- van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging – "How-to" guide and critical reflection. Insights Imag. 2020; 11(1): 1-16. doi: 10.1186/s13244-020-00887-2
- Yaremenko АI, Lysenko AV, Ivanova EA, Galibin OV. Augmented reality technology for auricular reconstruction in the treatment of microtia. Cell Ther Transplant. 2020; 9(2): 78-82. doi: 10.18620/ctt-1866-8836-2020-9-2-78-82
- Lysenko AV, Razumova AYa, Yaremenko AI, Mirzakhmedov RR. Augmented reality in the treatment of sialolithiasis. Stomatology. 2020; 99(4): 64-66. doi: 10.17116/stomat20209904164
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "30" ["~SORT"]=> string(2) "30" ["CODE"]=> string(100) "opyt-primeneniya-radiomicheski-orientirovannoy-navigatsionnoy-sistemy-dopolnennoy-realnosti-pri-biop" ["~CODE"]=> string(100) "opyt-primeneniya-radiomicheski-orientirovannoy-navigatsionnoy-sistemy-dopolnennoy-realnosti-pri-biop" ["EXTERNAL_ID"]=> string(4) "2048" ["~EXTERNAL_ID"]=> string(4) "2048" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(371) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюстиExperience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2286) "<p style="text-align: justify;">Радиомика – это количественный подход к медицинской визуализации, который направлен на улучшение существующих данных, доступных клиницистам, с помощью передового математического анализа. Посредством математического извлечения пространственного распределения интенсивностей сигналов и взаимосвязей пикселей, радиомика количественно определяет текстурную информацию, используя методы анализа из области искусственного интеллекта. Данные, извлеченные из рентгенологических изображений, при их сопоставлении с клиническими данными, потенциально могут предоставить дополнительную информацию для поддержки принятия решений в клинической медицине.</p> <p style="text-align: justify;">В данном исследовании выполнен предварительный радиомический анализ новообразования нижней челюсти. На основании полученных данных произведен выбор оптимального участка для биопсии. Во время проведения диагностического вмешательства использовалась навигационная система дополненной реальности, которая учитывала результаты данного математического анализа.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Радиомика, дополненная реальность, динамические навигационные системы, новообразования челюстей.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_META_TITLE"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_META_KEYWORDS"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_META_DESCRIPTION"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_PICTURE_FILE_ALT"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_PICTURE_FILE_TITLE"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "opyt-primeneniya-radiomicheski-orientirovannoy-navigatsionnoy-sistemy-dopolnennoy-realnosti-pri-biop" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(252) "Опыт применения радиомически-ориентированной навигационной системы дополненной реальности при биопсии новообразования нижней челюсти" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "opyt-primeneniya-radiomicheski-orientirovannoy-navigatsionnoy-sistemy-dopolnennoy-realnosti-pri-biop" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "opyt-primeneniya-radiomicheski-orientirovannoy-navigatsionnoy-sistemy-dopolnennoy-realnosti-pri-biop" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "opyt-primeneniya-radiomicheski-orientirovannoy-navigatsionnoy-sistemy-dopolnennoy-realnosti-pri-biop" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "203" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28485" ["VALUE"]=> array(2) { ["TEXT"]=> string(310) "<p>Анна В. Лысенко<sup>1</sup>, Андрей И. Яременко<sup>2</sup>, Владимир М. Иванов<sup>3</sup>, Сергей В. Стрелков<sup>3</sup>, Елизавета А. Иванова<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(238) "
Анна В. Лысенко1, Андрей И. Яременко2, Владимир М. Иванов3, Сергей В. Стрелков3, Елизавета А. Иванова2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28486" ["VALUE"]=> array(2) { ["TEXT"]=> string(751) "<p><sup>1</sup> Отдел челюстно-лицевой хирургии НИИ стоматологии и челюстно-лицевой хирургии, Санкт-Петербург, Россия<br> <sup>2</sup> Кафедра челюстно-лицевой хирургии, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Санкт-Петербургский политехнический университет Петра Великого, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(691) "1 Отдел челюстно-лицевой хирургии НИИ стоматологии и челюстно-лицевой хирургии, Санкт-Петербург, Россия
2 Кафедра челюстно-лицевой хирургии, Первый Санкт-Петербургский государственный медицинский университет
им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Санкт-Петербургский политехнический университет Петра Великого, Санкт-Петербург, Россия
Радиомика – это количественный подход к медицинской визуализации, который направлен на улучшение существующих данных, доступных клиницистам, с помощью передового математического анализа. Посредством математического извлечения пространственного распределения интенсивностей сигналов и взаимосвязей пикселей, радиомика количественно определяет текстурную информацию, используя методы анализа из области искусственного интеллекта. Данные, извлеченные из рентгенологических изображений, при их сопоставлении с клиническими данными, потенциально могут предоставить дополнительную информацию для поддержки принятия решений в клинической медицине.
В данном исследовании выполнен предварительный радиомический анализ новообразования нижней челюсти. На основании полученных данных произведен выбор оптимального участка для биопсии. Во время проведения диагностического вмешательства использовалась навигационная система дополненной реальности, которая учитывала результаты данного математического анализа.
Ключевые слова
Радиомика, дополненная реальность, динамические навигационные системы, новообразования челюстей.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28488" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-78-83" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-78-83" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28489" ["VALUE"]=> array(2) { ["TEXT"]=> string(238) "<p>Anna V. Lysenko<sup>1</sup>, Andrey I. Yaremenko<sup>2</sup>, Vladimir M. Ivanov<sup>3</sup>, Sergey V. Strelkov<sup>3</sup>, Elizaveta A. Ivanova<sup>2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(166) "Anna V. Lysenko1, Andrey I. Yaremenko2, Vladimir M. Ivanov3, Sergey V. Strelkov3, Elizaveta A. Ivanova2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28490" ["VALUE"]=> array(2) { ["TEXT"]=> string(1002) "<p><sup>1</sup> Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia<br> <sup>2</sup> Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101, St. Petersburg, Russia<br> Phone: +7 (812) 429-03-33<br> E-mail: lysenko.anna@mail.ru</p><br> <p><b>Citation:</b> Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(864) "1 Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia
2 Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia
3 Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia
Correspondence:
Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101,
St. Petersburg, Russia
Phone: +7 (812) 429-03-33
E-mail: lysenko.anna@mail.ru
Citation: Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28491" ["VALUE"]=> array(2) { ["TEXT"]=> string(1153) "<p style="text-align: justify;">Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.</p> <h2>Keywords</h2> <p style="text-align: justify;">Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1097) "Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.
Keywords
Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28492" ["VALUE"]=> string(119) "Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(119) "Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28493" ["VALUE"]=> string(4) "2750" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2750" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28494" ["VALUE"]=> string(4) "2751" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2751" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(8) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28489" ["VALUE"]=> array(2) { ["TEXT"]=> string(238) "<p>Anna V. Lysenko<sup>1</sup>, Andrey I. Yaremenko<sup>2</sup>, Vladimir M. Ivanov<sup>3</sup>, Sergey V. Strelkov<sup>3</sup>, Elizaveta A. Ivanova<sup>2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(166) "Anna V. Lysenko1, Andrey I. Yaremenko2, Vladimir M. Ivanov3, Sergey V. Strelkov3, Elizaveta A. Ivanova2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(166) "Anna V. Lysenko1, Andrey I. Yaremenko2, Vladimir M. Ivanov3, Sergey V. Strelkov3, Elizaveta A. Ivanova2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28491" ["VALUE"]=> array(2) { ["TEXT"]=> string(1153) "<p style="text-align: justify;">Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.</p> <h2>Keywords</h2> <p style="text-align: justify;">Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1097) "Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.
Keywords
Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1097) "Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.
Keywords
Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28488" ["VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-78-83" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-78-83" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(40) "10.18620/ctt-1866-8836-2021-10-3-4-78-83" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28492" ["VALUE"]=> string(119) "Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(119) "Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(119) "Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28490" ["VALUE"]=> array(2) { ["TEXT"]=> string(1002) "<p><sup>1</sup> Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia<br> <sup>2</sup> Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia</p><br> <p><b>Correspondence:</b><br> Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101, St. Petersburg, Russia<br> Phone: +7 (812) 429-03-33<br> E-mail: lysenko.anna@mail.ru</p><br> <p><b>Citation:</b> Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(864) "1 Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia
2 Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia
3 Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia
Correspondence:
Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101,
St. Petersburg, Russia
Phone: +7 (812) 429-03-33
E-mail: lysenko.anna@mail.ru
Citation: Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(864) "1 Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia
2 Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia
3 Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia
Correspondence:
Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101,
St. Petersburg, Russia
Phone: +7 (812) 429-03-33
E-mail: lysenko.anna@mail.ru
Citation: Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.
" } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28485" ["VALUE"]=> array(2) { ["TEXT"]=> string(310) "<p>Анна В. Лысенко<sup>1</sup>, Андрей И. Яременко<sup>2</sup>, Владимир М. Иванов<sup>3</sup>, Сергей В. Стрелков<sup>3</sup>, Елизавета А. Иванова<sup>2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(238) "Анна В. Лысенко1, Андрей И. Яременко2, Владимир М. Иванов3, Сергей В. Стрелков3, Елизавета А. Иванова2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(238) "Анна В. Лысенко1, Андрей И. Яременко2, Владимир М. Иванов3, Сергей В. Стрелков3, Елизавета А. Иванова2
" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28487" ["VALUE"]=> array(2) { ["TEXT"]=> string(2286) "<p style="text-align: justify;">Радиомика – это количественный подход к медицинской визуализации, который направлен на улучшение существующих данных, доступных клиницистам, с помощью передового математического анализа. Посредством математического извлечения пространственного распределения интенсивностей сигналов и взаимосвязей пикселей, радиомика количественно определяет текстурную информацию, используя методы анализа из области искусственного интеллекта. Данные, извлеченные из рентгенологических изображений, при их сопоставлении с клиническими данными, потенциально могут предоставить дополнительную информацию для поддержки принятия решений в клинической медицине.</p> <p style="text-align: justify;">В данном исследовании выполнен предварительный радиомический анализ новообразования нижней челюсти. На основании полученных данных произведен выбор оптимального участка для биопсии. Во время проведения диагностического вмешательства использовалась навигационная система дополненной реальности, которая учитывала результаты данного математического анализа.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Радиомика, дополненная реальность, динамические навигационные системы, новообразования челюстей.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2208) "Радиомика – это количественный подход к медицинской визуализации, который направлен на улучшение существующих данных, доступных клиницистам, с помощью передового математического анализа. Посредством математического извлечения пространственного распределения интенсивностей сигналов и взаимосвязей пикселей, радиомика количественно определяет текстурную информацию, используя методы анализа из области искусственного интеллекта. Данные, извлеченные из рентгенологических изображений, при их сопоставлении с клиническими данными, потенциально могут предоставить дополнительную информацию для поддержки принятия решений в клинической медицине.
В данном исследовании выполнен предварительный радиомический анализ новообразования нижней челюсти. На основании полученных данных произведен выбор оптимального участка для биопсии. Во время проведения диагностического вмешательства использовалась навигационная система дополненной реальности, которая учитывала результаты данного математического анализа.
Ключевые слова
Радиомика, дополненная реальность, динамические навигационные системы, новообразования челюстей.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2208) "Радиомика – это количественный подход к медицинской визуализации, который направлен на улучшение существующих данных, доступных клиницистам, с помощью передового математического анализа. Посредством математического извлечения пространственного распределения интенсивностей сигналов и взаимосвязей пикселей, радиомика количественно определяет текстурную информацию, используя методы анализа из области искусственного интеллекта. Данные, извлеченные из рентгенологических изображений, при их сопоставлении с клиническими данными, потенциально могут предоставить дополнительную информацию для поддержки принятия решений в клинической медицине.
В данном исследовании выполнен предварительный радиомический анализ новообразования нижней челюсти. На основании полученных данных произведен выбор оптимального участка для биопсии. Во время проведения диагностического вмешательства использовалась навигационная система дополненной реальности, которая учитывала результаты данного математического анализа.
Ключевые слова
Радиомика, дополненная реальность, динамические навигационные системы, новообразования челюстей.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "28486" ["VALUE"]=> array(2) { ["TEXT"]=> string(751) "<p><sup>1</sup> Отдел челюстно-лицевой хирургии НИИ стоматологии и челюстно-лицевой хирургии, Санкт-Петербург, Россия<br> <sup>2</sup> Кафедра челюстно-лицевой хирургии, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Санкт-Петербургский политехнический университет Петра Великого, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(691) "1 Отдел челюстно-лицевой хирургии НИИ стоматологии и челюстно-лицевой хирургии, Санкт-Петербург, Россия
2 Кафедра челюстно-лицевой хирургии, Первый Санкт-Петербургский государственный медицинский университет
им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Санкт-Петербургский политехнический университет Петра Великого, Санкт-Петербург, Россия
1 Отдел челюстно-лицевой хирургии НИИ стоматологии и челюстно-лицевой хирургии, Санкт-Петербург, Россия
2 Кафедра челюстно-лицевой хирургии, Первый Санкт-Петербургский государственный медицинский университет
им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Санкт-Петербургский политехнический университет Петра Великого, Санкт-Петербург, Россия
12/01/2021

Kulagin A. D. (St. Petersburg, Russia)
Wagemaker G. (Rotterdam, Netherlands)
Zander A. R. (Hamburg, Germany)
Fehse B. (Hamburg, Germany)
Chukhlovin A. B. (St. Petersburg, Russia)
Aleynikova O. V. (Minsk, Belarus)
Borset M. (Trondheim, Norway)
Chechetkin A. V. (St. Petersburg, Russia)
Fibbe W. (Leiden, Netherlands)
Gale R. P. (Los Angeles, USA)
Galibin O. V. (St. Petersburg, Russia)
Hehlmann R. (Mannheim, Germany)
Hölzer D. (Frankfurt a.M., Germany)
Klimko N. N. (St. Petersburg, Russia)
Kolb H.-J. (München, Germany)
Kröger N. (Hamburg, Germany)
Lange C. (Hamburg, Germany)
Mamaev N. N. (St. Petersburg, Russia)
Mikhailova N. B. (St. Petersburg, Russia)
Moiseev I. S. (St. Petersburg, Russia)
Nagler A. (Tel-Aviv, Israel)
Nemkov A. S. (St. Petersburg, Russia)
Paramonov I. V. (Kirov, Russia)
Roumiantsev A. G. (Moscow, Russia)
Savchenko V. G. (Moscow, Russia)
Smirnov A. V. (St. Petersburg, Russia)
Uss A. L. (Minsk, Belarus)
Zubarovskaya L. S. (St. Petersburg, Russia)
Expert opinion
Robert Peter Gale MD
Review articles
Oleg V. Goloshchapov1, Alexei B. Chukhlovin1,2, Oleg S. Glotov2
Anna M. Malkova1,2, Sergei V. Ageev1,2, Abdelsattar O. E. Abdelhalim2,3, Oleg E. Molchanov4, Dmitrii N. Maistrenko 4, Konstantin N. Semenov1,2,4, Vladimir V. Sharoyko1,2,4
Clinical studies
Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin
Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova, Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny, atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian, Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher, Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova, Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko, Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin
Mohamed Rabei Abdelfattah1, Mohamed A. Sharaan1, Mohamed S. Kamel1, Hussein Elsiesy2
Clinical case
Andrey V. Kozlov1, Olga I. Bogdanova1, Asmik G. Gevorgian1, Egor V. Volchkov2, Anna V. Botina1, Vadim V. Baykov1, Elena V. Morozova1, Natalya B. Mikhailova1, Ludmila S. Zubarovskaya1
Experimental studies
Natalia N. Sudareva1,2, Olga М. Suvorova1, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
Dima Joujeh1, Abduljalil Ghrewaty1, Chadi Soukkarieh2, Adnan Almarrawi1, Jamal Abdul Naser Darwicha3
Anna V. Lysenko1, Andrey I. Yaremenko2, Vladimir M. Ivanov3, Sergey V. Strelkov3, Elizaveta A. Ivanova2
Expert opinion
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28385
[VALUE] => Array
(
[TEXT] => <p>
Роберт П. Гэйл
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Роберт П. Гэйл
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28386
[VALUE] => Array
(
[TEXT] => <p>
Центр гематологии, Департамент иммунологии и воспаления, Лондонский Имперский Колледж, Лондон, Великобритания
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Центр гематологии, Департамент иммунологии и воспаления, Лондонский Имперский Колледж, Лондон, Великобритания
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28387
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ.
</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Острый миелоидный лейкоз, таргетная терапия, эффективность.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Существует много новых методов лечения, одобренных для лечения острого миелоидного лейкоза (ОМЛ), включая традиционные и таргетные препараты, а также иммунотерапию. Большинство из них улучшают различные исходы, включая бессобытийную и безрецидивную выживаемость. Однако в большинстве случаев выраженность эффекта невелика, и высока частота неуспешной терапии при 2-летнем наблюдении. Основываясь на данных, рассмотренных выше, сделаны выводы о том, что: (1) многие новые методы лечения ОМЛ направлены на терапию определенных подтипов ОМЛ; (2) ни один из них не оказался лучше, чем интенсивная химиолучевая терапия пациентов, которые могли бы получать любой из этих видов лечения; (3) существуют разногласия по поводу того, кто может или не может получать интенсивную терапию; (4) существуют серьезные проблемы с одобрением нескольких новых лекарственных препаратов; (5) азацитидин и венетоклакс могут быть новым стандартом лечения пожилых людей с ОМЛ, признанных неспособными получать интенсивную терапию; и (6) новые препараты должны рассматриваться, но пока не оказали большого влияния на долгосрочное выживание большинства пациентов с ОМЛ.
Ключевые слова
Острый миелоидный лейкоз, таргетная терапия, эффективность.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28388
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-4-7
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-4-7
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28389
[VALUE] => Array
(
[TEXT] => <p>
Robert Peter Gale MD
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Robert Peter Gale MD
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28390
[VALUE] => Array
(
[TEXT] => <p>
Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK
</p>
<br>
<p>
<b>Correspondence:</b><br>
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ<br>
Phone: +1 908 656 0484<br>
Fax: +1 310 388 1320<br>
E-Mail: <a href="mailto:robertpetergale@alumni.ucla.edu">robertpetergale@alumni.ucla.edu</a>
</p>
<p><b>Citation:</b> Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK
Correspondence:
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ
Phone: +1 908 656 0484
Fax: +1 310 388 1320
E-Mail: robertpetergale@alumni.ucla.edu
Citation: Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28391
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new <i>standard-of-care</i> in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Acute myeloid leukemia, targeted therapy, efficiency.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new standard-of-care in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
Keywords
Acute myeloid leukemia, targeted therapy, efficiency.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28392
[VALUE] => Will new drugs cure acute myeloid leukaemia?
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Will new drugs cure acute myeloid leukaemia?
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28393
[VALUE] => 2681
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2681
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28394
[VALUE] => 2682
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2682
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Robert Peter Gale MD
Centre for Haematology, Department of Immunology and Inflammation, Imperial College London, London, UK
Correspondence:
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Centre for Haematology Research, Department of Immunology and Inflammation, Imperial College London, London, UK SW7 2AZ
Phone: +1 908 656 0484
Fax: +1 310 388 1320
E-Mail: robertpetergale@alumni.ucla.edu
Citation: Gale RP. Will new drugs cure acute myeloid leukaemia? Cell Ther Transplant 2021; 10(3-4): 4-7.
There are many new therapies approved to treat acute myeloid leukaemia (AML) including conventional and targeted drugs, and immune therapy. Most improve diverse outcomes including event- and relapse-free survivals and survival. However, most effect sizes are small and failure rates by 2 years are high. Based on the data reviewed above I conclude: (1) many new AML therapies target specific AML sub-types; (2) none are proved better than intensive radiochemotherapy in persons who could receive either therapy; (3) there is disagreement defining who can or cannot receive intensive therapy; (4) there are important problems with several new drug approvals; (5) azacitidine and venetoclax may be the new standard-of-care in elderly persons with AML judged unable to receive intensive therapy; and (6) new drugs are welcome but have not had a big impact on long-term survival of most people with AML.
Keywords
Acute myeloid leukemia, targeted therapy, efficiency.
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28415
[VALUE] => Array
(
[TEXT] => <p>Джингуа Вэй, Бо Хюи</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Джингуа Вэй, Бо Хюи
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28416
[VALUE] => Array
(
[TEXT] => <p>Департамент неврологии, Госпиталь Сиджин, 4-й Военно-Медицинский университет, Сиань, Китай</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Департамент неврологии, Госпиталь Сиджин, 4-й Военно-Медицинский университет, Сиань, Китай
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28417
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Дефицит орнитин-транскарбамилазы (ДОТК) представляет собой наследственное заболевание с нарушением цикла обмена мочевины, характеризующееся высокой летальностью. Это генетическое нарушение обмена веществ проявляется гипераммониемией. Лекарства и гемодиализ могут снизить уровень аммиака в крови у пациентов. Трансплантация печени может улучшить долгосрочную выживаемость пациентов, но не может излечить необратимые повреждения нервной системы, возникшие ранее, и не может улучшить когнитивные функции. Если трансплантацию печени проводят в раннем детстве, впоследствии нервное развитие может быть нормальным. При необходимости пациентам с поздним дебютом также следует проводить трансплантацию. Гетерозиготность по ДОТК у донора все же представляет существенный риск, и ее следует использовать только тогда, когда нет других вариантов. При необходимости можно попытаться сделать трансплантацию гепатоцитов. После трансплантации необходима профилактика инфекции, длительный контроль функции печени и содержания аммиака в крови. Трансплантация печени должна рассматриваться для всех пациентов с генетическим ДОТК. Окончательное решение о том, следует ли и как использовать этот режим лечения, зависит от индивидуальной клинической ситуации.
Ключевые слова
Дефицит орнитин-транскарбамилазы, нарушение цикла мочевины, трансплантация печени.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28418
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-26-29
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-26-29
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28419
[VALUE] => Array
(
[TEXT] => <p>Jingya Wei, Bo Hui</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Jingya Wei, Bo Hui
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28420
[VALUE] => Array
(
[TEXT] => <p>Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China </p><br>
<p><b>Correspondence:</b><br>
Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China<br>
Phone: +86 29 8477 1055<br>
E-mail: iamtrn@126.com</p><br>
<p><b>Citation:</b> Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China
Correspondence:
Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China
Phone: +86 29 8477 1055
E-mail: iamtrn@126.com
Citation: Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28421
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.
Keywords
Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28422
[VALUE] => Liver transplantation in the treatment of ornithine transcarbamylase deficiency
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Liver transplantation in the treatment of ornithine transcarbamylase deficiency
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28423
[VALUE] => 2698
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2698
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28424
[VALUE] => 2699
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2699
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Jingya Wei, Bo Hui
Department of Neurology, Xijing Hospital, Fourth Military Medical University, Xi’an, China
Correspondence:
Dr. Jingya Wei, Department of Neurology, Xijing Hospital, Fourth Military Medical University, 15 Changle-xi Road, Xi’an 710032, Shaanxi province, China
Phone: +86 29 8477 1055
E-mail: iamtrn@126.com
Citation: Wei J, Hui B. Liver transplantation in the treatment of ornithine transcarbamylase deficiency. Cell Ther Transplant 2021; 10(3-4): 26-29.
Ornithine transcarbamylase deficiency (OTCD) is a genetic disorder causing disturbed urea metabolic cycle with a high mortality rates. It’s a genetic metabolic disease manifesting as hyperammonemia. Drugs and hemodialysis may reduce blood ammonia levels in the patients. Liver transplantation may improve the long-term survival rate of patients, but it cannot reverse the nervous system damage that has occurred before, and cannot improve cognition. If the liver transplant is performed early in childhood, neurodevelopment may be normal at later terms. Late-onset patients should also be transplanted when required. Heterozygosity for OTCD in the donor is still risky and should only be used when there are no other options. Hepatocyte transplantation can be tried if necessary. Prevention of infection, long-term monitoring of liver function and blood ammonia are required posttransplant. Liver transplantation should be considered for all patients with genetic OTCD. The final decision of whether and how to use this treatment mode depends on individual clinical circumstances.
Keywords
Ornithine transcarbamylase deficiency, urea cycle disorder, liver transplantation, hepatocyte transplantation.
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28405
[VALUE] => Array
(
[TEXT] => <p>Олег В. Голощапов<sup>1</sup>, Алексей Б. Чухловин<sup>1,2</sup>, Олег С. Глотов<sup>2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Олег В. Голощапов1, Алексей Б. Чухловин1,2, Олег С. Глотов2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28406
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>2</sup> Детский научно-практический центр инфекционных болезней ФМБА, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Детский научно-практический центр инфекционных болезней ФМБА, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28407
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Микробиота кишечника (сложный симбиоз бактерий, грибов и вирусов) – это динамическая биологическая система, необходимая для существования и роста человеческого организма. Состав и соотношение бактериальных популяций серьезно нарушаются при тяжелых колитах и болезни «трансплантат против хозяина» (GVHD), возникающих после трансплантации гемопоэтических стволовых клеток (HSCT), особенно при развитии устойчивых к антибиотикам бактериальных штаммов. В отличие от хорошо известной бактериальной микробиоты, изученной с помощью классической бактериологии и секвенирования гена 16S рРНК, вирусные популяции кишечной микробиоты (например, бактериофагов) в этих клинических условиях изучены недостаточно из-за отсутствия общего вирусного гена, пригодного для сравнительного молекулярно-генетического анализа. Оценка соотношений вирусной и бактериальной кишечной микробиоты возможна с помощью метагеномных методов анализа множества видов ДНК в образцах биоматериала. Объектом клинического исследования являются пациенты с инфекционными осложнениями, вызванными массивным антибактериальным и цитостатическим лечением.
</p>
<p style="text-align: justify;">
Особое внимание следует обратить на тяжелый колит с инфекцией <i>C.difficile</i> и устойчивой к антибиотикам <i>K. pneumoniae</i>, а также другими патогенами, как при трансплантации фекальной микробиоты (FMT), так и без нее. Обычная оценка кишечной микробиоты будет осуществляться путем секвенирования следующего поколения (NGS) на основе генного разнообразия 16S рДНК для бактериальных генов и метагеномного анализа NGS, чтобы оценить соотношение различных вирусов эукариотических клеток и, в частности, бактериофагов в случае дисбактериоза кишечника. Следует установить типичные нарушения кишечного вирома, а также их роль в колонизации кишечными бактериями, устойчивыми к антибиотикам, после интенсивной антибиотикотерапии и химиотерапии.
</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Кишечные инфекции, трансплантация, иммунологические осложнения, кишечная микробиота, вирусы, бактериофаги, антибиотикорезистентность, секвенирование нового поколения (NGS), ген 16S rRNA, метагеномика.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Микробиота кишечника (сложный симбиоз бактерий, грибов и вирусов) – это динамическая биологическая система, необходимая для существования и роста человеческого организма. Состав и соотношение бактериальных популяций серьезно нарушаются при тяжелых колитах и болезни «трансплантат против хозяина» (GVHD), возникающих после трансплантации гемопоэтических стволовых клеток (HSCT), особенно при развитии устойчивых к антибиотикам бактериальных штаммов. В отличие от хорошо известной бактериальной микробиоты, изученной с помощью классической бактериологии и секвенирования гена 16S рРНК, вирусные популяции кишечной микробиоты (например, бактериофагов) в этих клинических условиях изучены недостаточно из-за отсутствия общего вирусного гена, пригодного для сравнительного молекулярно-генетического анализа. Оценка соотношений вирусной и бактериальной кишечной микробиоты возможна с помощью метагеномных методов анализа множества видов ДНК в образцах биоматериала. Объектом клинического исследования являются пациенты с инфекционными осложнениями, вызванными массивным антибактериальным и цитостатическим лечением.
Особое внимание следует обратить на тяжелый колит с инфекцией C.difficile и устойчивой к антибиотикам K. pneumoniae, а также другими патогенами, как при трансплантации фекальной микробиоты (FMT), так и без нее. Обычная оценка кишечной микробиоты будет осуществляться путем секвенирования следующего поколения (NGS) на основе генного разнообразия 16S рДНК для бактериальных генов и метагеномного анализа NGS, чтобы оценить соотношение различных вирусов эукариотических клеток и, в частности, бактериофагов в случае дисбактериоза кишечника. Следует установить типичные нарушения кишечного вирома, а также их роль в колонизации кишечными бактериями, устойчивыми к антибиотикам, после интенсивной антибиотикотерапии и химиотерапии.
Ключевые слова
Кишечные инфекции, трансплантация, иммунологические осложнения, кишечная микробиота, вирусы, бактериофаги, антибиотикорезистентность, секвенирование нового поколения (NGS), ген 16S rRNA, метагеномика.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28408
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-19-25
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-19-25
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28409
[VALUE] => Array
(
[TEXT] => <p>Oleg V. Goloshchapov<sup>1</sup>, Alexei B. Chukhlovin<sup>1,2</sup>, Oleg S. Glotov<sup>2</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Oleg V. Goloshchapov1, Alexei B. Chukhlovin1,2, Oleg S. Glotov2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28410
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia<br>
<sup>2</sup> Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Alexei B. Chukhlovin, RM Gorbacheva Research
Institute of Pediatric Oncology, Hematology and
Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia<br>
Phone: +7 (921) 325-00-94<br>
E-mail: alexei.chukh@mail.ru</p><br>
<p><b>Citation:</b> Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia
Correspondence:
Dr. Alexei B. Chukhlovin, RM Gorbacheva Research
Institute of Pediatric Oncology, Hematology and
Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 325-00-94
E-mail: alexei.chukh@mail.ru
Citation: Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28414
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with <i>C.difficile</i> infection and antibiotic-resistant <i>K.pneumonia</i> and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with C.difficile infection and antibiotic-resistant K.pneumonia and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy.
Keywords
Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28411
[VALUE] => Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28412
[VALUE] => 2694
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2694
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28413
[VALUE] => 2695
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2695
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Oleg V. Goloshchapov1, Alexei B. Chukhlovin1,2, Oleg S. Glotov2
1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Children's Scientific and Clinical Center for Infectious Diseases of the Federal Medical and Biological Agency, St. Petersburg, Russia
Correspondence:
Dr. Alexei B. Chukhlovin, RM Gorbacheva Research
Institute of Pediatric Oncology, Hematology and
Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
Phone: +7 (921) 325-00-94
E-mail: alexei.chukh@mail.ru
Citation: Goloshchapov OV, Chukhlovin AB, Glotov OS. Possible role of intestinal human viruses and bacteriophages following hematopoietic stem cell transplantation: a mini-review. Cell Ther Transplant 2021; 10(3-4): 19-25.
Gut microbiota (a complex community of bacteria, fungi and viruses) is a dynamic biological system adapted for co-existence and symbiosis with host organism. Composition and ratio of bacterial populations is severely impaired in severe colitis and graft-versus-host disease (GVHD) occurring after hematopoietic stem cell transplantation (HSCT), especially, upon development of antibiotic-resistant bacterial strains. In contrast to the well-known bacterial microbiota studied by classic bacteriology and 16S rRNA gene sequencing, the viral populations of intestinal microbiota (e.g., bacteriophages) in are poorly studied in these clinical conditions, due to absence of a common viral gene suitable for comparative molecular genetic analysis. Assessing the ratios for viral and bacterial intestinal microbiota is feasible by means of metagenomic methods assaying multiple DNA species in the samples of biomaterial. As an object of clinical research, the patients with infectious complications caused by massive antibacterial and cytostatic treatment. Special attention should be drawn to severe colitis with C.difficile infection and antibiotic-resistant K.pneumonia and other pathogens with/without fecal microbiota transplantation (FMT). Conventional assessment of intestinal microbiota will be accomplished by next-generation sequencing (NGS) based on 16S rDNA gene diversity for bacterial genes, and metagenomic NGS analysis, in order to assess the ratio of various viruses of eukaryotic cells and, in particular, bacteriophages in cases of gut dysbiosis. Typical disturbances of the gut virome should be established, as well as role of bacteriophages in emergence of antibiotic-resistant intestinal bacteria after intensive antibiotic and chemotherapy.
Keywords
Intestinal microbiota, gut microbiota, viruses, bacteriophages, transplantation, immune complications, antibiotic resistance, NGS sequencing, 16S rRNA gene, metagenomics.
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28395
[VALUE] => Array
(
[TEXT] => <p>Анна М. Малкова<sup>1,2</sup>, Сергей В. Агеев<sup>1,2</sup>, Абделсаттар О. Е. Абделхалим<sup>2,3</sup>, Олег Е. Молчанов<sup>4</sup>, Дмитрий Н. Майстренко<sup>4</sup>, Константин Н. Семенов<sup>1,2,4</sup>, Владимир В. Шаройко<sup>1,2,4</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Анна М. Малкова1,2, Сергей В. Агеев1,2, Абделсаттар О. Е. Абделхалим2,3, Олег Е. Молчанов4, Дмитрий Н. Майстренко4, Константин Н. Семенов1,2,4, Владимир В. Шаройко1,2,4
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28396
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>2</sup> Институт химии, Санкт-Петербургский государственный университет, Санкт-Петербург, Россия<br>
<sup>3</sup> Отдел исследования окружающей среды, Национальный центр социальных и криминологических исследований, Гиза, Арабская Республика Египет<br>
<sup>4</sup> Российский научный центр радиологии и хирургических технологий имени A. M. Гранова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Институт химии, Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 Отдел исследования окружающей среды, Национальный центр социальных и криминологических исследований, Гиза, Арабская Республика Египет
4 Российский научный центр радиологии и хирургических технологий имени A. M. Гранова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28397
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Развитие и прогрессирование неоплазий происходит одновременно с изменениями окружающей стромы. Раковые клетки могут функционально формировать свое микроокружение за счет секреции различных цитокинов, хемокинов и формирования кислой среды. Данные факторы способствуют дифференциации иммунных клеток по иммуносупрессивному фенотипу, стимулируют синтез ряда ферментов обмена аминокислот, факторов роста, молекул адгезии, что промотирует инвазию, ангиогенез и метастазирование, а также снижает эффективность действия противоопухолевых препаратов и лучевой терапии. Для повышения эффективности химиотерапии возможно использование мультитаргетных углеродных наноматериалов. В частности, наноматериалы на основе модифицированного графена позволяют создавать многокомпонентные терапевтические конструкции, включающие макромолекулы, полимеры и эффекторные агенты. Первоначальные эксперименты с немодифицированными графенами продемонстрировали их токсичность, связанную с их накоплением в паренхиматозных органах и инициированием воспалительных процессов. В последние несколько лет вышла серия работ, в которых продемонстрирована возможность снижения токсичности оксида графена за счет функционализации. В данном обзоре обобщены экспериментальные данные по созданию ковалентных и нековалентных конъюгатов на основе оксида графена и показана их эффективность <i>in vitro</i> на различных опухолевых клеточных линиях. Отдельно представлены немногочисленные данные по влиянию наноматериалов на основе оксида графена на опухолевое микроокружение.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Опухоль, микроокружение, прогрессирование, цитокины, ацидоз, иммунная система, углеродные наноматериалы.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Развитие и прогрессирование неоплазий происходит одновременно с изменениями окружающей стромы. Раковые клетки могут функционально формировать свое микроокружение за счет секреции различных цитокинов, хемокинов и формирования кислой среды. Данные факторы способствуют дифференциации иммунных клеток по иммуносупрессивному фенотипу, стимулируют синтез ряда ферментов обмена аминокислот, факторов роста, молекул адгезии, что промотирует инвазию, ангиогенез и метастазирование, а также снижает эффективность действия противоопухолевых препаратов и лучевой терапии. Для повышения эффективности химиотерапии возможно использование мультитаргетных углеродных наноматериалов. В частности, наноматериалы на основе модифицированного графена позволяют создавать многокомпонентные терапевтические конструкции, включающие макромолекулы, полимеры и эффекторные агенты. Первоначальные эксперименты с немодифицированными графенами продемонстрировали их токсичность, связанную с их накоплением в паренхиматозных органах и инициированием воспалительных процессов. В последние несколько лет вышла серия работ, в которых продемонстрирована возможность снижения токсичности оксида графена за счет функционализации. В данном обзоре обобщены экспериментальные данные по созданию ковалентных и нековалентных конъюгатов на основе оксида графена и показана их эффективность in vitro на различных опухолевых клеточных линиях. Отдельно представлены немногочисленные данные по влиянию наноматериалов на основе оксида графена на опухолевое микроокружение.
Ключевые слова
Опухоль, микроокружение, прогрессирование, цитокины, ацидоз, иммунная система, углеродные наноматериалы.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28398
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-8-18
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-8-18
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28399
[VALUE] => Array
(
[TEXT] => <p>Anna M. Malkova<sup>1,2</sup>, Sergei V. Ageev<sup>1,2</sup>, Abdelsattar O. E. Abdelhalim<sup>2,3</sup>, Oleg E. Molchanov<sup>4</sup>, Dmitrii N. Maistrenko 4, Konstantin N. Semenov<sup>1,2,4</sup>, Vladimir V. Sharoyko<sup>1,2,4</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Anna M. Malkova1,2, Sergei V. Ageev1,2, Abdelsattar O. E. Abdelhalim2,3, Oleg E. Molchanov4, Dmitrii N. Maistrenko 4, Konstantin N. Semenov1,2,4, Vladimir V. Sharoyko1,2,4
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28400
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br>
<sup>2</sup> Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia<br>
<sup>3</sup> National Center for Social and Criminological Research, Giza, Arab Republic of Egypt<br>
<sup>4</sup> A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Vladimir V. Sharoyko, Pavlov University,
6-8 L. Tolstoy St., 197022, St. Petersburg, Russia<br>
Phone: +7 (981) 936-41-51<br>
E-mail: sharoyko@gmail.com</p><br>
<p><b>Citation:</b> Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Pavlov University, St. Petersburg, Russia
2 Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia
3 National Center for Social and Criminological Research, Giza, Arab Republic of Egypt
4 A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Vladimir V. Sharoyko, Pavlov University,
6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
Phone: +7 (981) 936-41-51
E-mail: sharoyko@gmail.com
Citation: Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28401
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their <i>in vitro</i> efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their in vitro efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.
Keywords
Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28402
[VALUE] => Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28403
[VALUE] => 2692
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2692
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28404
[VALUE] => 2693
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2693
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Anna M. Malkova1,2, Sergei V. Ageev1,2, Abdelsattar O. E. Abdelhalim2,3, Oleg E. Molchanov4, Dmitrii N. Maistrenko 4, Konstantin N. Semenov1,2,4, Vladimir V. Sharoyko1,2,4
1 Pavlov University, St. Petersburg, Russia
2 Institute of Chemistry, St. Petersburg State University, Saint Petersburg, Russia
3 National Center for Social and Criminological Research, Giza, Arab Republic of Egypt
4 A. M. Granov Russian Research Centre for Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Vladimir V. Sharoyko, Pavlov University,
6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
Phone: +7 (981) 936-41-51
E-mail: sharoyko@gmail.com
Citation: Malkova AM, Ageev SV, Abdelhalim AOE et al. Mutual influence of malignant cells and cellular microenvironment: prospects for manipulating tumour microenvironment with nanomaterials. Cell Ther Transplant 2021; 10(3-4): 8-18.
Development and progression of neoplasia occurs in parallel with changes in the surrounding stroma. Cancer cells may functionally reshape their microenvironment by secreting various cytokines, chemokines and generation of acidic medium. These factors contribute to differentiation of immune cells into immunosuppressive phenotype, stimulate the synthesis of a number of amino acid metabolism enzymes, growth factors, adhesion molecules, which promote invasion, angiogenesis and metastasis, and also reduce efficiency of anticancer drugs and radiation therapy. To increase effectiveness of the chemotherapy, multitargeted carbon nanomaterials may be applied. In particular, nanomaterials based on modified graphene make it possible to create multicomponent therapeutic constructs, including macromolecules, polymers, and effector agents. Initial experiments with unmodified graphenes demonstrated their toxicity associated with their accumulation in parenchymal organs and initiation of inflammatory processes. In the past few years, a series of works has been published in which the possibility of reducing the toxicity of graphene oxide through functionalisation has been demonstrated. This review summarises the experimental data on the creation of covalent and non-covalent conjugates based on graphene oxide and demonstrates their in vitro efficacy on various tumour cell lines. Separately, there are few data on the effect of nanomaterials based on graphene oxide on the tumour microenvironment.
Keywords
Tumour, microenvironment, progression, cytokines, acidosis, immune system, carbon nanomaterials.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28435
[VALUE] => Array
(
[TEXT] => <p>Ольга В. Кудяшева, Ольга В. Пирогова, Валентина В. Порунова, Светлана В. Толстова, Анна Г. Смирнова,
Иван С. Моисеев, Александр Д. Кулагин</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Ольга В. Кудяшева, Ольга В. Пирогова, Валентина В. Порунова, Светлана В. Толстова, Анна Г. Смирнова,
Иван С. Моисеев, Александр Д. Кулагин
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28436
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28437
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.</p>
<p style="text-align: justify;">В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.</p>
<p style="text-align: justify;">Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Системный AL амилоидоз, CyBoRD, бортезомиб.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Системный амилоидоз легких цепей (AL) – одна из форм плазмоклеточных дискразий, характеризующаяся гиперпродукцией свободных легких цепей иммуноглобулинов клональными плазматическими клетками и их отложением в органах и тканях в виде нерастворимого фибриллярного белка-амилоида. Основная цель терапии – быстрое и глубокое подавление продукции амилоидогенных легких цепей, а поражение сердца при этом заболевании является основным предиктором выживаемости пациентов. Терапия на основе бортезомиба, циклофосфамида и дексаметазона (CyBorD) в последнее время считается стандартом лечения впервые выявленных пациентов. Однако, количество исследований режима CyBorD с оценкой долгосрочного гематологического и органного ответа ограничено.
В нашем исследовании мы проанализировали ответ на индукционную терапию у 105 пациентов с впервые выявленным системным AL амилоидозом, не являющихся кандидатами на проведение аутологичной трансплантации гемопоэтических стволовых клеток (АТГСК). Вся терапия была разделена на 3 группы: 1 – CyBorD, 2 – другие схемы на основе бортезомиба и 3 группа – схемы без бортезомиба.
Общая 3-х летняя выживаемость составила 70,3% с медианой наблюдения 27,8 месяцев (22 дня-11 лет). Неблагоприятными факторами в отношении прогноза при однофакторном анализе были возраст старше 70 лет (p=0,007), мужской пол (p=0,015), стадия Mayo IIIb (p=0,07) и III (p<0,0001) стадия поражения почек. В многофакторном анализе применение любых других режимов, помимо CyBorD, негативно сказывалось на показателях 3-х летней выживаемости (ОР 4,9, 95% CI 1,4-17,2, p=0,012). 3-летняя беспрогрессивная выживаемость при использовании схемы CyBorD составила 79% против 48% в группе 2, и 55% в группе 3 (p=0,28). Общая частота ответов составила 70% (n=74). Процент пациентов с гематологическим ответом был достоверно выше в группе CyBoRD, 94% против 84% и 63% в группах 2 и 3, соответственно (p=0,033), как и медиана времени до ответа (9,6, 95% ДИ 5,3-15 месяцев). Органные ответы были оценены за 3-х летний период от начала терапии. Процент кардиальных и почечных ответов так же был выше в группе CyBorD: для сердца он составил 78% против 55% и 16% (p=0,050) в 1, 2, 3 группах соответственно, а для почек 90% против 92% против 57% (p=0,01). В общей группе, гематологический (медиана 10 месяцев) и почечный ответ (медиана 12 месяцев) наблюдались раньше, чем кардиальный и печеночный ответы (медиана 26 месяцев). Наши результаты сопоставимы с ранее опубликованными данными европейских исследований и показывают высокую частоту ГО и органных ответов, улучшение общей выживаемости при использовании схемы CyBorD. Органные ответы при этом наблюдались существенно позже, чем гематологические.
Ключевые слова
Системный AL амилоидоз, CyBoRD, бортезомиб.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28438
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-38-45
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-38-45
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28439
[VALUE] => Array
(
[TEXT] => <p>Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28440
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia<br>
E-mail: bmt.myeloma@gmail.com</p><br>
<p><b>Citation:</b> Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: bmt.myeloma@gmail.com
Citation: Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28441
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses.
</p>
<p style="text-align: justify;">
In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens.
</p>
<p style="text-align: justify;">
The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), <i>vs </i>48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% <i>vs </i>84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% <i>vs </i>55% <i>vs </i>16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% <i>vs </i>92% <i>vs </i>57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months).
</p>
<p style="text-align: justify;">
In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Systemic AL amyloidosis, CyBoRD, bortezomib.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses.
In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens.
The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), vs 48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% vs 84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% vs 55% vs 16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% vs 92% vs 57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months).
In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response.
Keywords
Systemic AL amyloidosis, CyBoRD, bortezomib.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28442
[VALUE] => Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28443
[VALUE] => 2716
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2716
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28444
[VALUE] => 2717
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2717
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Olga V. Kudyasheva, Olga V. Pirogova, Valentina V. Porunova, Svetlana V. Tolstova, Anna G. Smirnova, Ivan S. Moiseev, Alexander D. Kulagin
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Olga V. Kudyasheva, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, 6-8 L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: bmt.myeloma@gmail.com
Citation: Kudyasheva OV, Pirogova OV, Porunova VV. Comparison of bortesomib-based induction regimens with other treatment modalities in patients with newly diagnosed systemic light chain amyloidosis ineligible for autologous stem cell transplantation. Cell Ther Transplant 2021; 10(3-4): 38-45.
Systemic light chain (AL) amyloidosis is a form of plasma cell disorders, characterized by overproduction of immunoglobulin light chains by clonal plasma cells and their deposition in organs and tissues as an insoluble fibrillar protein-amyloid. Suppression of amyloid production is the main goal of therapy, whereas cardiac involvement is the main predictor of survival. Therapeutic regimen containing bortezomib, cyclophosphamide and dexamethasone (CyBorD) was recently introduced as the standard of care for newly diagnosed patients. However, there are only few longitudinal comparative studies of this regimen with evaluation of organ responses.
In our study we analyzed the response to induction therapy in 105 patients with newly diagnosed patients with systemic AL amyloidosis ineligible for autologous stem cell transplantation (ASCT). All the patients were divided into three groups: group 1 received CyBorD; group 2 was treated with other bortezomib-based regimens, and group 3 received bortezomib-free regimens.
The 3-year OS was 70.3% (95% СI 61-80) with the median follow-up of 27.8 months (22 days to 11 years). Unfavorable factors for OS were as follows: age >70 years (p=0.007), male gender (p=0.015), Mayo stage IIIb (p=0.07) and renal damage stage III (p<0.0001). In the multivariate analysis, all other treatments than CyBorD were associated with decreased 3-year OS values (HR 4.9, 95% CI 1.4-17.2, p=0.012). 3-year progression-free survival (PFS) in CyBorD group was 79% (95% CI 63-95), vs 48% (95% CI 35-61) in group 2, and 55% (95% CI 30-80) in group 3 (p=0.28). Overall response rate (ORR) was 70% (n=74). The percentage of patients who showed hematological response was significantly higher in the CyBoRD group, 94% vs 84% in group 2 and 63% in group 3 (p=0.033), and median time to response in this group was 9.6 (5.3-15) months. The organ response (OR) was assessed over a 3-year period. The percentage of heart and renal responses was higher in CyBorD group. For cardiac responses, the rate was 78% vs 55% vs 16% (p=0.05) for groups 1, 2 and 3 respectively. Renal responses were observed in 90% vs 92% vs 57% of the patients (p=0.01). Overall median time of hematologic response (median, 10 months) and renal response (median, 12 months) occurred earlier than cardiac and hepatic responses (median, 26 months).
In summary, our results are comparable with previously published studies, demonstrating faster hematological response and organ responses after CyBorD treatment, which is translated into improved overall survival. Organ responses were observed significantly later than hematologic response.
Keywords
Systemic AL amyloidosis, CyBoRD, bortezomib.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28425
[VALUE] => Array
(
[TEXT] => <p>Александр А. Синяев, Марина О. Попова, Юлия А. Рогачева, Анна А. Спиридонова, Мария Ю. Аверьянова,
Александр Л. Алянский, Белла И. Аюбова, Елена В. Бабенко, Евгений А. Бакин, Ильдар М. Бархатов,
Максим П. Богомольный, Татьяна А. Быкова, Алина А. Витрищак, Мария Д. Владовская, Юлия Ю. Власова,
Алиса Г. Волкова, Асмик Г. Геворгян, Татьяна Л. Гиндина, Олег В. Голощапов, Кирилл А. Екушов,
Мария А. Эстрина, Наталья Е. Иванова, Максим А. Кучер, Алексей Б. Чухловин, Кирилл В. Лепик,
Инна В. Маркова, Наталья Б. Михайлова, Елена В. Морозова, Анна А. Осипова, Олеся В. Паина,
Дмитрий Э. Певцов, Анна Г. Смирнова, Александр Н. Швецов, Лилия В. Стельмах, Галина Н. Столбенко,
Людмила С. Зубаровская, Сергей Н. Бондаренко, Иван С. Моисеев, Александр Д. Кулагин</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Александр А. Синяев, Марина О. Попова, Юлия А. Рогачева, Анна А. Спиридонова, Мария Ю. Аверьянова,
Александр Л. Алянский, Белла И. Аюбова, Елена В. Бабенко, Евгений А. Бакин, Ильдар М. Бархатов,
Максим П. Богомольный, Татьяна А. Быкова, Алина А. Витрищак, Мария Д. Владовская, Юлия Ю. Власова,
Алиса Г. Волкова, Асмик Г. Геворгян, Татьяна Л. Гиндина, Олег В. Голощапов, Кирилл А. Екушов,
Мария А. Эстрина, Наталья Е. Иванова, Максим А. Кучер, Алексей Б. Чухловин, Кирилл В. Лепик,
Инна В. Маркова, Наталья Б. Михайлова, Елена В. Морозова, Анна А. Осипова, Олеся В. Паина,
Дмитрий Э. Певцов, Анна Г. Смирнова, Александр Н. Швецов, Лилия В. Стельмах, Галина Н. Столбенко,
Людмила С. Зубаровская, Сергей Н. Бондаренко, Иван С. Моисеев, Александр Д. Кулагин
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28426
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28427
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.</p>
<h3>Материалы и методы</h3>
<p style="text-align: justify;">Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы. </p>
<p style="text-align: justify;">В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно.
В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров.
<p style="text-align: justify;">
Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66).
Медиана времени развития COVID-19 после
ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения –
12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).</p>
<h3>Выводы</h3>
<p style="text-align: justify;">Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Трансплантация гемопоэтических стволовых клеток (ТГСК) – это жизнеспасающая процедура при онкологических, гематологических и доброкачественных заболеваниях. Несмотря на глобальную тенденцию к снижению трансплантационной активности на фоне пандемии COVID-19, мы старались ее сохранить, внедряя профилактические меры и оптимизируя инфекционный контроль в нашем центре.
Материалы и методы
Это наблюдательное исследование. Мы собрали данные о работе нашего трансплантационного центра с апреля 2020 года по июль 2021 года в течение двух волн пандемии. Основной задачей было изучение влияния пандемии COVID-19 на работу центра ТГСК, включая заболеваемость сотрудников и реципиентов ТГСК, а также трансплантационную активность.
Результаты
Первый случай заражения COVID-19 в Санкт-Петербурге был зарегистрирован 8 марта 2020 года. 30 марта 2020 года в Российской Федерации был объявлен всеобщий режим самоизоляции. Вторая волна COVID-19 началась в октябре 2020 года. Основным инструментом инфекционного контроля, помимо требований государственных органов, был еженедельный скрининг персонала и пациентов. Всего за период с мая 2020 по июль 2021 было проведено 21702 ПЦР исследований. На 1 июля 2021 года 69,7% сотрудников были иммунизированы в результате перенесенной инфекции или вакцинированы.
В 2020 году, на который пришлась первая волна пандемии, нам удалось провести 419 ТГСК: 136 аутологичных и 283 аллогенных. Для сравнения: в 2019 году выполнено 415 трансплантаций, из них 144 аутологичных и 271 аллогенных соответственно.
В 2020 году структура доноров для ТГСК была перераспределена в пользу неродственных из Российского реестра и гаплоидентичных доноров.
Заболеваемость COVID-19 среди реципиентов ТГСК в период с апреля 2020 года по июль 2021 года составила 7,3% (n=39), после алло-ТГСК – 8,6% (n=31), после ауто-ТГСК – 4,5% (n=8). Медиана возраста пациентов с COVID-19 составила 27 лет (4-66).
Медиана времени развития COVID-19 после
ТГСК составила 68 дней (-1-2093). У большинства пациентов – 29 (74,3%) индекс коморбидности HCT CI на момент трансплантации был равен 0. Источником стволовых клеток были как ПСКК – 22 (56,4%), так и КМ – 17 (43,6%). У большинства пациентов на момент проведения ТГСК была полная ремиссия основного заболевания (n=30, 76,9%). Общая 100-дневная выживаемость среди реципиентов ТГСК с момента постановки диагноза COVID-19 составила 79,5% (95% ДИ 0,609-0,884). Летальность составила 20,5% (n=8). Причины смерти: инфекция COVID-19 – 50% (n=4), вторичные инфекционные осложнения – 25% (n=2), рецидив основного заболевания – 12,5% (n=1), геморрагические осложнения –
12, 5% (n=1). 100-дневная кумулятивная частота трансплантационной летальности среди всех реципиентов ТГСК составила 7% (95% ДИ 0,9-0,95) и 8,7% (95% ДИ 0,88-0,93) в 2019 и 2020 годах соответственно (p=0,35).
Выводы
Благодаря профилактическим мерам, регулярному скринингу ПЦР, а также использованию доноров, как из Российского регистра, так и гаплоидентичных, нам удалось сохранить трансплантационную активность на прежнем уровне. Заболеваемость COVID-19 среди реципиентов ТГСК составила 7,3%. Несмотря на то, что летальность от COVID-19 среди реципиентов ТГСК составила 20,5%, пандемия не повлияла на трансплантационную летальность среди всех реципиентов ТГСК в нашем центре.
Ключевые слова
Пандемия, COVID-19, SARS-CoV-2, трансплантация гемопоэтических стволовых клеток.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28428
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-30-37
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-30-37
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28429
[VALUE] => Array
(
[TEXT] => <p>Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova,
Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny,
atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian,
Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher,
Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova,
Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko,
Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova,
Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny,
atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian,
Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher,
Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova,
Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko,
Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28430
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia<br>
E-mail: drsiniaev@yandex.ru</p><br>
<p><b>
Citation:</b> Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
E-mail: drsiniaev@yandex.ru
Citation: Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28431
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.</p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity. </p>
<h3>Results</h3>
<p style="text-align: justify;">The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 –
50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 –
0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).</p>
<h3>Conclusions</h3>
<p style="text-align: justify;">Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.
Patients and methods
This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity.
Results
The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 –
50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 –
0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).
Conclusions
Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center.
Keywords
Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28432
[VALUE] => Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28433
[VALUE] => 2709
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2709
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28434
[VALUE] => 2710
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2710
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Aleksandr A. Siniaev, Marina O. Popova, Yulia A. Rogacheva, Anna A. Spiridonova, Maria Y. Averyanova, Alexander L. Alyanskiy, Bella I. Ayubova, Elena V. Babenko, Evgenii A. Bakin, Ildar M. Barkhatov, Maxim P. Bogomolny, atiana A. Bykova, Alina A. Vitrischak, Maria D. Vladovskaya, Yulia Y. Vlasova, Alisa G. Volkova, Asmik G. Gevorgian, Tatiana L. Gindina, Oleg V. Goloshchapov, Kirill A. Ekushov, Maria A. Estrina, Natalia E. Ivanova, Maxim A. Kucher, Alexei B. Chukhlovin, Kirill V. Lepik, Inna V. Markova, Natalia B. Mikhailova, Elena V. Morozova, Anna A. Osipova, Olesya V. Paina, Dmitrii E. Pevtsov, Anna G. Smirnova, Alexandr N. Shvetsov, Lilia V. Stelmakh, Galina N. Stolbenko, Ludmila S. Zubarovskaya, Sergey N. Bondarenko, Ivan S. Moiseev, Alexander D. Kulagin
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
Correspondence:
Dr. Aleksandr A. Siniaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transpantation, Pavlov University, 6-8 L. Tolstoy St., 197022, St. Petersburg, Russia
E-mail: drsiniaev@yandex.ru
Citation: Siniaev AA, Popova MO, Rogacheva YA et al. Journey of a hematopoietic stem cell transplantation center through COVID-19 pandemic: one-year experience. Cell Ther Transplant 2021; 10(3-4): 30-37.
Hematopoietic stem cell transplantation (HSCT) is a life-saving procedure for oncological, hematological and non-malignant disorders. Despite global trend for a decrease of transplantation activity in view of the COVID-19 pandemic, we tried to maintain it by taking preventive measures and optimizing infection control in our center.
Patients and methods
This is an observational study. We collected the performance data of our transplant center from April 2020 to July 2021, i.e., during two waves of the pandemic. The main objectives were to study the influence of COVID-19 pandemic on the workflow of the HSCT center, including morbidity among employees and HSCT recipients, as well as on the transplant activity.
Results
The first case of COVID-19 infection in St. Petersburg was recorded on March 8, 2020. On March 30, 2020, a national lockdown had been imposed in the Russian Federation. The second wave of COVID-19 started in October 2020. Weekly screening of staff and patients was the main diagnostic tool, in addition to the governmental requirements. In sum, a total of 21702 PCR tests for SARS-CoV-2 were performed over the study period. As for July 1, 2021, 69.7% of employees became immune to the virus, due to previous COVID-19 disease, or by vaccination. In 2020, we managed to perform 419 HSCT, including 136 autologous and 283 allogeneic transplants. For comparison, 415 HSCTs were carried out in 2019, with 144 autologous and 271 allogeneic transplants. In 2020, the HSC donorship was shifted towards unrelated donors from the Russian Registry and haploidentical donors. Incidence of COVID-19 among HSCT recipients between April 2020 and July 2021 was 7.3% (n=39), being 8.6% (n=31) after allogeneic HSCT, and 4.5% (n=8) following auto-HSCT. The median age of patients with COVID-19 was 27 years (4-66). The median term for the COVID-19 onset was 68 days post-transplant (-1 to +2093). In most patients – 29 (74.3%) the HCT CI comorbidity index at the time of transplantation was 0. The stem cell source were either peripheral blood stem cells (n=22, 56.4%), or bone marrow (n=17, 43.6%). Most of the patients achieved complete remission of the underlying disease at the time of HSCT (n=30, 76.9%). The overall 100-day survival rate among HSCT recipients since the diagnosis of the COVID-19 was 79.5% (95% CI 0.609 – 0.884). The mortality rate was 20.5% (n=8). The causes of death were as follows: COVID-19 – 50% (n=4); secondary infectious complications, 25% (n=2); relapse of the underlying disease, 12.5% (n=1); hemorrhagic complications, 12.5% (n=1). The 100-day cumulative incidence of transplant-related mortality (TRM) among all HSCT recipients was 7% (95% CI 0.9 – 0.95) and 8.7% (95% CI 0.88 – 0.93) in 2019 and 2020, respectively (p=0.35).
Conclusions
Due to preventive measures, regular PCR screening, as well as the use of donors from the Russian Registry or haploidentical donors, we managed to maintain HSCT activity at the same level. The COVID-19 morbidity of HSCT recipients was 7.3%, their mortality rate – 20.5%. In summary, the pandemic did not affect transplant-related mortality among the HSCT recipients in our center.
Keywords
Pandemic, COVID-19, SARS-CoV-2, HSC transplantation.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28445
[VALUE] => Array
(
[TEXT] => <p>Мохамед Рабеи Абдельфаттах<sup>1</sup>, Мохамед А. Шараан<sup>1</sup>, Mохамед С. Камель<sup>1</sup>, Хусейн Эльсиеси<sup>2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Мохамед Рабеи Абдельфаттах1, Мохамед А. Шараан1, Mохамед С. Камель1, Хусейн Эльсиеси2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28446
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Департамент хирургии, Университет Александрии, Факультет медицины, Александрия, Египет<br>
<sup>2</sup> Центр трансплантации органов, Специализированный научный госпитальный Центр короля Фейсала, Эр-Рияд, Королевство Саудовская Аравия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Департамент хирургии, Университет Александрии, Факультет медицины, Александрия, Египет
2 Центр трансплантации органов, Специализированный научный госпитальный Центр короля Фейсала, Эр-Рияд, Королевство Саудовская Аравия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28447
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Наша цель состояла в том, чтобы оценить влияние циторедуктивной локорегиональной терапии (ЛРТ) на исход гепатоцеллюлярной карциномы (ГЦК), леченной посредством трансплантации печени (LTx), а также оценить отдаленные результаты LTx при ГЦК и факторы, влияющие на них.</p>
<h3>Материалы и методы</h3>
<p style="text-align: justify;">Наблюдали 115 взрослых пациентов, которым проведена LTx в качестве лечения ГЦК в период с августа 2006 г. по декабрь 2019 г. В зависимости от предтрансплантационной циторедуктивной ЛРТ, пациенты были разделены на две группы следующим образом: группа A – пациенты, соответствующие миланским критериям (MК) для LTx, которые не получали циторедуктивной ЛРТ перед LTx; группа В – пациенты с параметрами опухоли, выходящими за рамки МК, которым была проведена предварительная циторедуктивная ЛРТ.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">При анализе по всей выборке общая выживаемость пациентов, трансплантата и безрецидивная выживаемость составили, соответственно, 79,7%, 90,4% и 88,2%. Параметры опухоли у 73 пациентов соответствовали миланским критериям (63,5% пациентов с трансплантированным ГЦК), в то время как у остальных 36,5% ГЦК превышала критерии МК (42 пациента). У пациентов после успешной циторедукции ГЦК до значений, соответствующих критериям MК, общая и безрецидивная выживаемость были сравнимы с пациентами, которым была проведена трансплантация LRT при соответствии миланским критериям. Рецидив ГЦК достоверно коррелировал с выявленной инвазией в лимфатические сосуды и низкой степенью дифференцировки опухоли. Более того, обе характеристики были существенно связаны с выживаемостью пациентов, и, наоборот, критерии трансплантации и объем опухоли >115 см3 не показали значимой связи с выживаемостью пациента или рецидивом опухоли.</p>
<h3>Заключение</h3>
<p style="text-align: justify;">Наши результаты подтверждают большую важность биологических критериев опухоли, чем общепринятые морфологические критерии. ЛРТ обеспечивает возможность снизить стадию ГЦК до значений, соответствующих миланским критериям, при одновременном выборе более биологически благоприятных вариантов опухолей.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Трансплантация печени, гепатоцеллюлярная карцинома, локорегионарная терапия, Миланские критерии.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Наша цель состояла в том, чтобы оценить влияние циторедуктивной локорегиональной терапии (ЛРТ) на исход гепатоцеллюлярной карциномы (ГЦК), леченной посредством трансплантации печени (LTx), а также оценить отдаленные результаты LTx при ГЦК и факторы, влияющие на них.
Материалы и методы
Наблюдали 115 взрослых пациентов, которым проведена LTx в качестве лечения ГЦК в период с августа 2006 г. по декабрь 2019 г. В зависимости от предтрансплантационной циторедуктивной ЛРТ, пациенты были разделены на две группы следующим образом: группа A – пациенты, соответствующие миланским критериям (MК) для LTx, которые не получали циторедуктивной ЛРТ перед LTx; группа В – пациенты с параметрами опухоли, выходящими за рамки МК, которым была проведена предварительная циторедуктивная ЛРТ.
Результаты
При анализе по всей выборке общая выживаемость пациентов, трансплантата и безрецидивная выживаемость составили, соответственно, 79,7%, 90,4% и 88,2%. Параметры опухоли у 73 пациентов соответствовали миланским критериям (63,5% пациентов с трансплантированным ГЦК), в то время как у остальных 36,5% ГЦК превышала критерии МК (42 пациента). У пациентов после успешной циторедукции ГЦК до значений, соответствующих критериям MК, общая и безрецидивная выживаемость были сравнимы с пациентами, которым была проведена трансплантация LRT при соответствии миланским критериям. Рецидив ГЦК достоверно коррелировал с выявленной инвазией в лимфатические сосуды и низкой степенью дифференцировки опухоли. Более того, обе характеристики были существенно связаны с выживаемостью пациентов, и, наоборот, критерии трансплантации и объем опухоли >115 см3 не показали значимой связи с выживаемостью пациента или рецидивом опухоли.
Заключение
Наши результаты подтверждают большую важность биологических критериев опухоли, чем общепринятые морфологические критерии. ЛРТ обеспечивает возможность снизить стадию ГЦК до значений, соответствующих миланским критериям, при одновременном выборе более биологически благоприятных вариантов опухолей.
Ключевые слова
Трансплантация печени, гепатоцеллюлярная карцинома, локорегионарная терапия, Миланские критерии.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28448
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-46-53
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-46-53
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28449
[VALUE] => Array
(
[TEXT] => <p>Mohamed Rabei Abdelfattah<sup>1</sup>, Mohamed A. Sharaan<sup>1</sup>, Mohamed S. Kamel<sup>1</sup>, Hussein Elsiesy<sup>2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Mohamed Rabei Abdelfattah1, Mohamed A. Sharaan1, Mohamed S. Kamel1, Hussein Elsiesy2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28450
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt<br>
<sup>2</sup> Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia</p><br>
<p><b>Correspondence:</b><br>
Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544<br>
Phone: 00201023061111 (mob.)<br>
E-mail: Mohamad.rabie@gmail.com</p><br>
<p><b>Citation:</b> Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt
2 Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544
Phone: 00201023061111 (mob.)
E-mail: Mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28451
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.</p>
<h3>Materials and methods</h3>
<p style="text-align: justify;">115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.</p>
<h3>Results</h3>
<p style="text-align: justify;">Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm<sup>3</sup> did not show a significant relation to patient survival or tumor recurrence.</p>
<h3>Conclusion</h3>
<p style="text-align: justify;">Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.
Materials and methods
115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.
Results
Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm3 did not show a significant relation to patient survival or tumor recurrence.
Conclusion
Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors.
Keywords
Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28452
[VALUE] => The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28453
[VALUE] => 2725
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2725
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28454
[VALUE] => 2726
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2726
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Mohamed Rabei Abdelfattah1, Mohamed A. Sharaan1, Mohamed S. Kamel1, Hussein Elsiesy2
1 Department of Surgery, University of Alexandria, Faculty of Medicine, Alexandria, Arab Republic of Egypt
2 Organ Transplant Center, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia
Correspondence:
Prof. Dr. Mohamed Rabei Abdelfattah. MD, Associate Professor of Surgery, Department of Surgery, Faculty of Medicine, University of Alexandria, AL Khartoum Square, Azzaritta, Alexandria, Arab Republic of Egypt, PO BOX 21544
Phone: 00201023061111 (mob.)
E-mail: Mohamad.rabie@gmail.com
Citation: Abdelfattah MR, Sharaan MA, Kamel MS, Elsiesi H. The effect of using pretransplant locoregional therapy on the outcome of liver transplantation for HCC patients. Cell Ther Transplant 2021; 10(3-4): 46-53.
Our objective was to assess impact of downstaging locoregional therapy (LRT) on outcome of HCC treated by liver transplantation (LTx), and to assess long-term outcomes of LTx for hepatocellular carcinoma (HCC) and factors affecting them.
Materials and methods
115 Adult patients underwent LTx as a treatment of HCC between August 2006 and December 2019. As dependent on pre-transplant downstaging LRT, the patients were divided in two groups as follows: group A, patients corresponding to Milan criteria for LTx (MC) who did not receive downstaging LRT prior to LTx; group B included patients beyond Milan criteria who received downstaging LRT pretransplant.
Results
Among the entire LTx group, the patient, graft, and tumor-free survival rates were 79.7%, 90.4% and 88.2% respectively. 73 patients had HCC classified within MC (63.5% of transplanted HCC patients), while the remaining 36.5% were beyond MC (42 patients). The HCC patients successfully downstaged to the values corresponding to MC criteria showed overall survival and recurrence-free survival comparable to those who were transplanted within MC without LRT. HCC recurrence significantly correlated with detectable vascular invasion and poor degree of tumor differentiation. Moreover, the both features were significantly related to patient survival. Conversely, the transplant criteria and tumor volume >115 cm3 did not show a significant relation to patient survival or tumor recurrence.
Conclusion
Our results confirm the importance of biological tumor criteria over the commonly adopted morphological criteria. LRT offers an opportunity to downstage HCC to the values which fit the Milan criteria, while selecting more biologically favorable tumors.
Keywords
Liver transplantation, hepatocellular carcinoma, locoregional therapy, Milan criteria.
Clinical case
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28455
[VALUE] => Array
(
[TEXT] => <p>Андрей В. Козлов<sup>1</sup>, Ольга И. Богданова<sup>1</sup>, Асмик Г. Геворгян<sup>1</sup>, Егор В. Волчков<sup>2</sup>, Анна В. Ботина<sup>1</sup>, Вадим В. Байков<sup>1</sup>, Елена В. Морозова<sup>1</sup>, Наталия Б. Михайлова<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Андрей В. Козлов1, Ольга И. Богданова1, Асмик Г. Геворгян1, Егор В. Волчков2, Анна В. Ботина1, Вадим В. Байков1, Елена В. Морозова1, Наталия Б. Михайлова1, Людмила С. Зубаровская1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28456
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>2</sup> НМИЦ ДГОИ им. Дмитрия Рогачева, Москва, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 НМИЦ ДГОИ им. Дмитрия Рогачева, Москва, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28457
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Статья представляет собой демонстрацию клинического случая редкой неклассифицируемой периферической Т-клеточной лимфомы у ребенка. Заболевание характеризовалось рефрактерным течением. Ремиссия была получена после добавления ингибиторов иммунных контрольных точек (ИКТ) к химиотерапии. Основанием для начала иммунотерапии было присутствие PDL1 (лиганд рецептора программируемой клеточной смерти 1) на клетках микроокружения опухоли. Для консолидации первой ремиссии была проведена аутологичная ТГСК и для консолидации второй ремиссии – аллогенная. Течение посттрансплантационного периода осложнилось тяжелой реакцией «трансплантат против хозяина» (РТПХ) с поражением кожи, которая была вылечена с помощью руксолитиниба.</p>
<p style="text-align: justify;">Для лечения и профилактики лимфомы ЦНС применялись интратекальные введения цитостатиков как до, так и после ТГСК. Пациент находится в полной ремиссии и без признаков РТПХ в течение 3-х лет после окончания лечения. В целом, качество жизни у ребенка хорошее, за исключением легких нарушений в когнитивной сфере.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Периферическая Т-клеточная лимфома, дети, химиоиммунотерапия, трансплантация гемопоэтических стволовых клеток.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Статья представляет собой демонстрацию клинического случая редкой неклассифицируемой периферической Т-клеточной лимфомы у ребенка. Заболевание характеризовалось рефрактерным течением. Ремиссия была получена после добавления ингибиторов иммунных контрольных точек (ИКТ) к химиотерапии. Основанием для начала иммунотерапии было присутствие PDL1 (лиганд рецептора программируемой клеточной смерти 1) на клетках микроокружения опухоли. Для консолидации первой ремиссии была проведена аутологичная ТГСК и для консолидации второй ремиссии – аллогенная. Течение посттрансплантационного периода осложнилось тяжелой реакцией «трансплантат против хозяина» (РТПХ) с поражением кожи, которая была вылечена с помощью руксолитиниба.
Для лечения и профилактики лимфомы ЦНС применялись интратекальные введения цитостатиков как до, так и после ТГСК. Пациент находится в полной ремиссии и без признаков РТПХ в течение 3-х лет после окончания лечения. В целом, качество жизни у ребенка хорошее, за исключением легких нарушений в когнитивной сфере.
Ключевые слова
Периферическая Т-клеточная лимфома, дети, химиоиммунотерапия, трансплантация гемопоэтических стволовых клеток.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28458
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-54-60
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-54-60
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28459
[VALUE] => Array
(
[TEXT] => <p>Andrey V. Kozlov<sup>1</sup>, Olga I. Bogdanova<sup>1</sup>, Asmik G. Gevorgian<sup>1</sup>, Egor V. Volchkov<sup>2</sup>, Anna V. Botina<sup>1</sup>, Vadim V. Baykov<sup>1</sup>, Elena V. Morozova<sup>1</sup>, Natalya B. Mikhailova<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Andrey V. Kozlov1, Olga I. Bogdanova1, Asmik G. Gevorgian1, Egor V. Volchkov2, Anna V. Botina1, Vadim V. Baykov1, Elena V. Morozova1, Natalya B. Mikhailova1, Ludmila S. Zubarovskaya1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28460
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia<br>
<sup>2</sup> Dmitry Rogachev National Research Center, Moscow, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute
of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia<br>
E-mail: kozlovandrew1983@yandex.ru</p><br>
<p><b>Citation:</b> Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Dmitry Rogachev National Research Center, Moscow, Russia
Correspondence:
Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute
of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: kozlovandrew1983@yandex.ru
Citation: Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28461
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.
Keywords
Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28462
[VALUE] => A case of rare pediatric unclassified NK/T cell lymphoma
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => A case of rare pediatric unclassified NK/T cell lymphoma
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28463
[VALUE] => 2732
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2732
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28464
[VALUE] => 2733
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2733
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Andrey V. Kozlov1, Olga I. Bogdanova1, Asmik G. Gevorgian1, Egor V. Volchkov2, Anna V. Botina1, Vadim V. Baykov1, Elena V. Morozova1, Natalya B. Mikhailova1, Ludmila S. Zubarovskaya1
1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantology, Pavlov University, St. Petersburg, Russia
2 Dmitry Rogachev National Research Center, Moscow, Russia
Correspondence:
Dr. Andrey V. Kozlov, RM Gorbacheva Research Institute
of Pediatric Oncology, Hematology and Transplantology, Pavlov University, L. Tolstoy St, 197022, St. Petersburg, Russia
E-mail: kozlovandrew1983@yandex.ru
Citation: Kozlov AV, Bogdanova OI, Gevorgian AG et al. A case of rare pediatric unclassified NK/T cell lymphoma. Cell Ther Transplant 2021; 10(3-4): 54-60.
The article presents a case report of rare unclassified peripheral T-cell lymphoma (PTCL) in a child. The disease was characterized by refractory course and addition of immune checkpoint inhibitors (ICI) to chemotherapy resulted in remission induction. The rationale for immunotherapy was presence of programmed cell death ligand 1 (PDL1) on the cells of tumor microenvironment. First remission was consolidated with autologous hematopoietic stem cell transplantation (HSCT), and second remission was followed by allogeneic HSCT. Post-transplant period was complicated by severe acute graft-versus-host disease (GVHD) successfully managed with ruxolitinib. To perform local control, intrathecal cytostatic therapy was used prior to HSCT and post-transplant. Three years after last transplantation, the patient is disease-free and GVHD-free, with overall good quality of life and only mild impairment of cognitive functions.
Keywords
Peripheral T-cell lymphoma, children, chemoimmunotherapy, hematopoietic stem cell transplantation.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28475
[VALUE] => Array
(
[TEXT] => <p>Наталья Н. Сударева<sup>1,2</sup>, Ольга М. Суворова<sup>1</sup>, Дмитрий Н. Суслов<sup>3</sup>, Олег В. Галибин<sup>2</sup>, Александр Д. Вилесов<sup>1,2</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Наталья Н. Сударева1,2, Ольга М. Суворова1, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28476
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия<br>
<sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>3</sup> Российский научный центр радиологии и хирургических технологий им. акад. А.М. Гранова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. акад. А.М. Гранова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28477
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Доксорубицин (ДОХ) – водорастворимый антрациклиновый антибиотик, обладающий высокой противораковой эффективностью. Можно добиться уменьшения концентрации ДОХ в крови ниже кардиотоксического уровня в процессе терапии, формируя депо, содержащее системы доставки ДОХ с пролонгированным высвобождением лекарства. Для этих целей использовали кальций карбонатные пористые ватериты, допированные полианионом декстран сульфатом. Субмикронные размеры носителей не позволяют свободно включаться им в кровеносное русло. Распространяется токсическое лекарство по организму только после попадания в кровь в результате высвобождения из систем доставки. Внутрибрюшинное введение крысам с перевитой гепатомой Зайделя ДОХ-содержащих систем доставки позволило оценить эффективную концентрацию ДОХ, тормозящую рост опухоли и уменьшающую объем асцитной жидкости. Динамику поступления ДОХ в кровь здоровых крыс после внутрибрюшинного введения 4 мг ДОХ в системах доставки различной природы определяли методом ВЭЖХ.
Введенное при помощи допированных декстрансульфатом субмикронных карбонатных ядер лекарство высвобождается в кровь крыс в течение двух недель в концентрациях, меньших токсичных значений. При использовании в качестве системы доставки наноразмерного конъюгата декстрансульфат+ДОХ в крови крыс обнаруживается лекарство в значительно больших концентрациях. Независимо от концентрации ДОХ в плазме результаты физикального осмотра, а также аутопсии крыс в течение 21 дня после внутрибрюшинного введения ДОХ в разных системах доставки, свидетельствуют об отсутствии негативных реакций у животных.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Доксорубицин, система доставки лекарства, СаСО<sub>3</sub>, декстрансульфат, конъюгат полимер-лекарство, плазма крови.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Доксорубицин (ДОХ) – водорастворимый антрациклиновый антибиотик, обладающий высокой противораковой эффективностью. Можно добиться уменьшения концентрации ДОХ в крови ниже кардиотоксического уровня в процессе терапии, формируя депо, содержащее системы доставки ДОХ с пролонгированным высвобождением лекарства. Для этих целей использовали кальций карбонатные пористые ватериты, допированные полианионом декстран сульфатом. Субмикронные размеры носителей не позволяют свободно включаться им в кровеносное русло. Распространяется токсическое лекарство по организму только после попадания в кровь в результате высвобождения из систем доставки. Внутрибрюшинное введение крысам с перевитой гепатомой Зайделя ДОХ-содержащих систем доставки позволило оценить эффективную концентрацию ДОХ, тормозящую рост опухоли и уменьшающую объем асцитной жидкости. Динамику поступления ДОХ в кровь здоровых крыс после внутрибрюшинного введения 4 мг ДОХ в системах доставки различной природы определяли методом ВЭЖХ.
Введенное при помощи допированных декстрансульфатом субмикронных карбонатных ядер лекарство высвобождается в кровь крыс в течение двух недель в концентрациях, меньших токсичных значений. При использовании в качестве системы доставки наноразмерного конъюгата декстрансульфат+ДОХ в крови крыс обнаруживается лекарство в значительно больших концентрациях. Независимо от концентрации ДОХ в плазме результаты физикального осмотра, а также аутопсии крыс в течение 21 дня после внутрибрюшинного введения ДОХ в разных системах доставки, свидетельствуют об отсутствии негативных реакций у животных.
Ключевые слова
Доксорубицин, система доставки лекарства, СаСО3, декстрансульфат, конъюгат полимер-лекарство, плазма крови.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28478
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-71-77
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-71-77
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28479
[VALUE] => Array
(
[TEXT] => <p>
Natalia N. Sudareva<sup>1,2</sup>, Olga М. Suvorova<sup>1</sup>, Dmitry N. Suslov<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Natalia N. Sudareva1,2, Olga М. Suvorova1, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28480
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br>
<sup>2</sup> Pavlov University, St. Petersburg, Russia<br>
<sup>3</sup> Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Natalia N. Sudareva, Institute of Macromolecular
Compounds RAS, St. Petersburg, Russia<br>
E-mail: nnsas@mail.ru</p><br>
<p><b>Citation:</b> Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO<sub>3</sub> vaterites as the systems for regional
administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Natalia N. Sudareva, Institute of Macromolecular
Compounds RAS, St. Petersburg, Russia
E-mail: nnsas@mail.ru
Citation: Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO3 vaterites as the systems for regional
administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28481
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Doxorubicin, drug delivery system, CaCO<sub>3</sub>, dextran sulfate, polymer-drug conjugate, blood plasma.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.
Keywords
Doxorubicin, drug delivery system, CaCO3, dextran sulfate, polymer-drug conjugate, blood plasma.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28482
[VALUE] => Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28483
[VALUE] => 2745
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2745
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28484
[VALUE] => 2746
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2746
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Natalia N. Sudareva1,2, Olga М. Suvorova1, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence:
Dr. Natalia N. Sudareva, Institute of Macromolecular
Compounds RAS, St. Petersburg, Russia
E-mail: nnsas@mail.ru
Citation: Sudareva NN, Suvorova OM, Suslov DN et al. Dextran sulfate coated CaCO3 vaterites as the systems for regional administration of doxorubicin to rats. Cell Ther Transplant 2021; 10(3-4): 71-77.
Doxorubicin (DOX) is a water-soluble anthracycline antibiotic possessing high anti-cancer activity. It is possible to achieve decrease in DOX concentration in blood (below the cardiotoxic level) during therapy by forming a depot containing DOX delivery systems that provide prolonged release of the drug. To this purpose, porous calcium carbonate particles (vaterites) coated with polyanion (dextran sulfate) were used. Due to submicron sizes of carriers, they do not freely enter bloodstream. The toxic drug is distributed in the organism only after entering the blood due to release from the delivery systems. Upon intraperitoneal administration of the DOX-containing delivery systems to rats inoculated with Seidel hepatoma, an efficient DOX concentration has been achieved which inhibited tumor growth and reduced the amount of ascitic fluid. Time profiles of DOX release into bloodstream of healthy rats were studied by HPLC after intraperitoneal administration of 4 mg of DOX, using various delivery systems. The drug injected in the form of dextran sulfate coated submicron carbonate cores was released within two weeks, and its concentrations were under the toxicity levels. When the nano-sized DexS+DOX conjugate was used for the drug delivery, DOX was found in rat blood at significantly higher concentrations. Irrespective of drug concentration in plasma, the results of physical examination and autopsy of rats performed on day 21 after intraperitoneal administration of DOX by various delivery systems indicated the absence of any negative reactions in animals.
Keywords
Doxorubicin, drug delivery system, CaCO3, dextran sulfate, polymer-drug conjugate, blood plasma.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28465
[VALUE] => Array
(
[TEXT] => <p>Дима Джуджех<sup>1</sup>, Абдулджалил Гревати<sup>1</sup>, Чади Суккариех<sup>2</sup>, Аднан Альмаррави<sup>1</sup>, Джамал А.Н. Дарвича<sup>3</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Дима Джуджех1, Абдулджалил Гревати1, Чади Суккариех2, Аднан Альмаррави1, Джамал А.Н. Дарвича3
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28466
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Департамент биотехнологической инженерии, Факультет технической инженерии, Университет Алеппо, Сирия<br>
<sup>2</sup> Департамент биологии животных, Факультет наук, Университет Дамаска, Сирия<br>
<sup>3</sup> Департамент фармакологии и токсикологии, Факультет фармации, Арабский международный университет, Сирия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Департамент биотехнологической инженерии, Факультет технической инженерии, Университет Алеппо, Сирия
2 Департамент биологии животных, Факультет наук, Университет Дамаска, Сирия
3 Департамент фармакологии и токсикологии, Факультет фармации, Арабский международный университет, Сирия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28467
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Мезенхимные стволовые клетки (МСК) вызвали большой интерес в научном сообществе. МСК мышей служат идеальной моделью для изучения клеточной биологии и терапевтического потенциала этой популяции клеток. Поэтому необходимо создание оптимального стандартизованного протокола для выделения и культивирования мышиных МСК. Нашей целью было разработать и описать эффективный, надежный и простой в выполнении протокол для выделения и культивирования мезенхимальных стромальных клеток костного мозга мышей (МСКМ). Наш протокол основан на сочетании метода промывки и механического дробления костей. МСКМ, выделенные с использованием нашего протокола, имеют веретенообразную форму, проявляется экспрессия маркеров CD73 и CD44, слабая экспрессия CD34 и CD105 и отсутствие CD11b.
Эти клетки также способны дифференцироваться в другие ростки мезодермы, такие как клоны адипоцитов и остеоцитов. Мы надеемся, что данные, представленные в этой статье, имеют практическое значение и могут быть использованы в клинических и исследовательских приложениях, а также при заготовке клеток для банкирования. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Мыши Balb/c, костный мозг, изоляция клеток, мезенхимные стволовые клетки, первичная культура.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Мезенхимные стволовые клетки (МСК) вызвали большой интерес в научном сообществе. МСК мышей служат идеальной моделью для изучения клеточной биологии и терапевтического потенциала этой популяции клеток. Поэтому необходимо создание оптимального стандартизованного протокола для выделения и культивирования мышиных МСК. Нашей целью было разработать и описать эффективный, надежный и простой в выполнении протокол для выделения и культивирования мезенхимальных стромальных клеток костного мозга мышей (МСКМ). Наш протокол основан на сочетании метода промывки и механического дробления костей. МСКМ, выделенные с использованием нашего протокола, имеют веретенообразную форму, проявляется экспрессия маркеров CD73 и CD44, слабая экспрессия CD34 и CD105 и отсутствие CD11b.
Эти клетки также способны дифференцироваться в другие ростки мезодермы, такие как клоны адипоцитов и остеоцитов. Мы надеемся, что данные, представленные в этой статье, имеют практическое значение и могут быть использованы в клинических и исследовательских приложениях, а также при заготовке клеток для банкирования.
Ключевые слова
Мыши Balb/c, костный мозг, изоляция клеток, мезенхимные стволовые клетки, первичная культура.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28468
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-61-70
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-61-70
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28469
[VALUE] => Array
(
[TEXT] => <p>Dima Joujeh<sup>1</sup>, Abduljalil Ghrewaty<sup>1</sup>, Chadi Soukkarieh<sup>2</sup>, Adnan Almarrawi<sup>1</sup>, Jamal Abdul Naser Darwicha<sup>3</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Dima Joujeh1, Abduljalil Ghrewaty1, Chadi Soukkarieh2, Adnan Almarrawi1, Jamal Abdul Naser Darwicha3
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28470
[VALUE] => Array
(
[TEXT] => <p>
<sup>1</sup> Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria<br>
<sup>2</sup> Department of Animal Biology, Faculty of Sciences, Damascus University, Syria<br>
<sup>3</sup> Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria
</p>
<br>
<p><b>Correspondence:</b><br>
Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria<br>
E-mail: dimajoujeh@gmail.com</p><br>
<p><b>Citation:</b> Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
1 Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
2 Department of Animal Biology, Faculty of Sciences, Damascus University, Syria
3 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria
Correspondence:
Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
E-mail: dimajoujeh@gmail.com
Citation: Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28471
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking.
Keywords
Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28472
[VALUE] => An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28473
[VALUE] => 2742
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2742
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28474
[VALUE] => 2743
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2743
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Dima Joujeh1, Abduljalil Ghrewaty1, Chadi Soukkarieh2, Adnan Almarrawi1, Jamal Abdul Naser Darwicha3
1 Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
2 Department of Animal Biology, Faculty of Sciences, Damascus University, Syria
3 Department of Pharmacology and Toxicology, Faculty of Pharmacy, Arab International University, Syria
Correspondence:
Dr. Dima Joujeh, Department of Biotechnology Engineering, Faculty of Technical Engineering, University of Aleppo, Syria
E-mail: dimajoujeh@gmail.com
Citation: Joujeh D, Ghrewaty A, Soukkarieh C, et al. An optimized protocol for mouse bone marrow mesenchymal stromal cells isolation and culture. Cell Ther Transplant 2021; 10(3-4): 61-70.
Mesenchymal stromal cells (MSCs) have stimulated much interest in the scientific community. Mouse MSCs serve as an ideal tool to explore cell biology and therapeutic potential of MSCs. Therefore, establishment of optimal, standardized protocol for mouse MSCs isolation and culture is required. Our aim was to develop and describe an efficient, reliable, and easy-to-perform protocol for isolation and culture of mouse bone marrow mesenchymal stromal cells MSC(M). Our protocol is based on a combination of flushing method and mechanical crushing of the bones. MSC(M) isolated using our protocol showed spindle-shaped appearance, positive expression of CD73 and CD44 markers, weak expression of CD34 and CD105, and negative expression for CD11b. They were also able to differentiate into mesodermal lineages such as adipocytes, and osteocytes. We hope that the data presented in this paper are of practical importance and can be used in clinical and research applications, and cell banking.
Keywords
Balb/c mice, bone marrow cells, isolation, mesenchymal stem cells, primary culture.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28485
[VALUE] => Array
(
[TEXT] => <p>Анна В. Лысенко<sup>1</sup>, Андрей И. Яременко<sup>2</sup>, Владимир М. Иванов<sup>3</sup>, Сергей В. Стрелков<sup>3</sup>, Елизавета А. Иванова<sup>2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Анна В. Лысенко1, Андрей И. Яременко2, Владимир М. Иванов3, Сергей В. Стрелков3, Елизавета А. Иванова2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28486
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Отдел челюстно-лицевой хирургии НИИ стоматологии и челюстно-лицевой хирургии, Санкт-Петербург, Россия<br>
<sup>2</sup> Кафедра челюстно-лицевой хирургии, Первый Санкт-Петербургский государственный медицинский университет
им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>3</sup> Санкт-Петербургский политехнический университет Петра Великого, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Отдел челюстно-лицевой хирургии НИИ стоматологии и челюстно-лицевой хирургии, Санкт-Петербург, Россия
2 Кафедра челюстно-лицевой хирургии, Первый Санкт-Петербургский государственный медицинский университет
им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Санкт-Петербургский политехнический университет Петра Великого, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28487
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Радиомика – это количественный подход к медицинской визуализации, который направлен на улучшение существующих данных, доступных клиницистам, с помощью передового математического анализа. Посредством математического извлечения пространственного распределения интенсивностей сигналов и взаимосвязей пикселей, радиомика количественно определяет текстурную информацию, используя методы анализа из области искусственного интеллекта. Данные, извлеченные из рентгенологических изображений, при их сопоставлении с клиническими данными, потенциально могут предоставить дополнительную информацию для поддержки принятия решений в клинической медицине.</p>
<p style="text-align: justify;">В данном исследовании выполнен предварительный радиомический анализ новообразования нижней челюсти. На основании полученных данных произведен выбор оптимального участка для биопсии. Во время проведения диагностического вмешательства использовалась навигационная система дополненной реальности, которая учитывала результаты данного математического анализа.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Радиомика, дополненная реальность, динамические навигационные системы, новообразования челюстей.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Радиомика – это количественный подход к медицинской визуализации, который направлен на улучшение существующих данных, доступных клиницистам, с помощью передового математического анализа. Посредством математического извлечения пространственного распределения интенсивностей сигналов и взаимосвязей пикселей, радиомика количественно определяет текстурную информацию, используя методы анализа из области искусственного интеллекта. Данные, извлеченные из рентгенологических изображений, при их сопоставлении с клиническими данными, потенциально могут предоставить дополнительную информацию для поддержки принятия решений в клинической медицине.
В данном исследовании выполнен предварительный радиомический анализ новообразования нижней челюсти. На основании полученных данных произведен выбор оптимального участка для биопсии. Во время проведения диагностического вмешательства использовалась навигационная система дополненной реальности, которая учитывала результаты данного математического анализа.
Ключевые слова
Радиомика, дополненная реальность, динамические навигационные системы, новообразования челюстей.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28488
[VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-78-83
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-3-4-78-83
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28489
[VALUE] => Array
(
[TEXT] => <p>Anna V. Lysenko<sup>1</sup>, Andrey I. Yaremenko<sup>2</sup>, Vladimir M. Ivanov<sup>3</sup>, Sergey V. Strelkov<sup>3</sup>, Elizaveta A. Ivanova<sup>2</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Anna V. Lysenko1, Andrey I. Yaremenko2, Vladimir M. Ivanov3, Sergey V. Strelkov3, Elizaveta A. Ivanova2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28490
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia<br>
<sup>2</sup> Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia<br>
<sup>3</sup> Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia</p><br>
<p><b>Correspondence:</b><br>
Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101,
St. Petersburg, Russia<br>
Phone: +7 (812) 429-03-33<br>
E-mail: lysenko.anna@mail.ru</p><br>
<p><b>Citation:</b> Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia
2 Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia
3 Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia
Correspondence:
Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101,
St. Petersburg, Russia
Phone: +7 (812) 429-03-33
E-mail: lysenko.anna@mail.ru
Citation: Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 28491
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.
Keywords
Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28492
[VALUE] => Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28493
[VALUE] => 2750
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2750
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 28494
[VALUE] => 2751
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2751
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Anna V. Lysenko1, Andrey I. Yaremenko2, Vladimir M. Ivanov3, Sergey V. Strelkov3, Elizaveta A. Ivanova2
1 Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, St. Petersburg, Russia
2 Department of Maxillofacial Surgery, Pavlov University, St. Petersburg, Russia
3 Peter the Great St. Petersburg Polytechnic University, St. Petersburg, Russia
Correspondence:
Dr. Anna V. Lysenko, Department of Maxillofacial Surgery, Research Institute of Dentistry and Maxillofacial Surgery, Pavlov University, 44 Petrogradskaya Emb., 197101,
St. Petersburg, Russia
Phone: +7 (812) 429-03-33
E-mail: lysenko.anna@mail.ru
Citation: Lysenko AV, Yaremenko AI, Ivanov VM et al. Experience in the use of a radio-oriented augmented reality navigation system for biopsy of a neoplasm of the lower jaw. Cell Ther Transplant 2021; 10(3-4): 78-83.
Radiomics is a quantitative approach to medical imaging that applies advanced mathematical analysis in order to improve the existing data available to clinicians. Radiomics quantifies texture information by mathematical extraction of spatial distribution of the signal intensities and pixel relationships. Quantitative evaluation of the texture 2-D information employs analytic techniques from the field of artificial intelligence. The data derived from radiographic images, when compared with clinical data, may potentially provide additional information aiming for support of decision-making in clinical medicine. In this study, a preliminary radiomic analysis of a lower jaw neoplasm was performed. Based on the data obtained, the optimal site for tissue biopsy was chosen. During diagnostic intervention, an augmented reality navigation system was used which took into account the results of the mentioned mathematical analysis.
Keywords
Radiomics, augmented reality, dynamic navigation systems, jaw neoplasms.

