Introduction
HSCT is a potentially life-saving procedure, often the only curative option for a variety of diseases. This procedure is, however, associated with both immediate and long-term complications requiring increased vigilance and monitoring. Some HSCT complications can result in decreased quality of life and shortened life expectancy [1].
Early HSCT complications generally occur within 100 days post HSCT. They include: oral complications/mucositis and sepsis, neutropenic fever, haemorrhagic cystitis, endothelial damage, engraftment syndrome, idiopathic pneumonia syndrome, diffuse alveolar haemorrhage, transplant-associated microangiopathy (TAM) and sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD), infection due to compromised immune function, organ toxicity, secondary malignancy, cancer relapse, graft failure and graft rejection.
Late post-transplant complications occur ≥1 year after treatment with a wide spectrum in terms of latency, intensity, reversibility, and lethality. The most frequent causes of delayed mortality are cardiac/vascular, subsequent neoplasms, infections, and pulmonary disorders. It is noteworthy that the incidence for cardiac/vascular and subsequent neoplasms continues to increase with time after HCT and does not peak before the completion of the second decade of survivorship. Timely diagnostics of hematopoiesis reconstitution is the management cornerstone in these patients.
Immature subsets of RBC and platelets as markers of blood recovery
At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients.
• Reticulocyte count
• IRF (immature reticulocytes fraction): Fig. 1A
• IPF (immature platelets fraction)/reticulated platelets: Fig. 1B
• RBC fragments.

Figure 1. Immature reticulocytes (A) and one reticulated platelets (B) in blood smears
Reticulocytes represent the terminal stage of RBC maturation in bone marrow. Reticulocytosis rates are changed in a variety of blood disorders [2]. Immature reticulocyte fraction (IRF) arises at the late stage of erythrocyte differentiation, recognizable by trace amounts of nucleic acids seen as visible reticular substance which normally fades away within 3 days of terminal maturation. Similar reticular substance is seen in immature platelet fraction (IPF) budding from megakaryocytes in bone marrow. Following HSCT, both erythropoiesis and platelet generation proceed at much faster rates, thus exhibiting higher levels of IRF and IPF in peripheral blood.
State of the art in reticulocyte count
Reticulocyte count in the last-generation hematological analyzers correlate with the reference methods, using different staining and registration approaches:
Fluorochromes:
- ReticFit, ReticCount (thiazol orange);
- Sysmex R-1000/3000, SE 9500 (auramine O);
- Sysmex XE-2100;
- Abbott Cell Dyn 4000 (CD4K530);
- ABX Pentra (thiazol orange);
- Mindray BC‐6800 (asymmetric cyanine).
Stains and light scatter/absorption:
- Bayer H*3/Advia (oxazine 750);
- Abbott Cell Dyn 3500-3700 (NMB);
- Coulter STKS/MAXM/GEN-S (NMB);
- Microscope image analysis (Micro 21).
Constant monitoring of reticulocytes count is critical in the post-transplant period, in order to monitor engraftment and hematopoietic recovery as well as to intercept in real time changes in the erythropoiesis. Parvovirus B19 (PVB19) infection may represent such clinical situation. PVB19 is a single-stranded DNA virus of the family Parvoviridae with tropism to erythroid progenitor cells. In healthy immunocompetent individuals it causes rash of erythema infectiosum (fifth disease) in children and acute symmetric polyarthropathia in adults. Transient aplastic crisis may be induced in individuals with congenital hemolytic disorders. Persistent B19 infection in immunocompromised patients is manifested as pure red cell aplasia and chronic anemia. In peripheral blood of these patients, one may observe marked reticulocytopenia; in bone marrow, marked erythroid hypoplasia, absence of maturing precursors and presence of giant pro-erythroblasts.
Studies of immature reticulocytes (IRF) may be performed by manual or automated methods, as follows:
Morphology:
- polychromatophylic red cells;
- reticulocyte differential counts.
Flow cytometry:
- RNA content (fluorochromes, dyes);
- CD71, CD36;
- fetal hemoglobin or antigens;
- RMI = mean fluorescence-intensity (i.e., mean channel);
- Fraction(s) of RNA-rich reticulocytes:
- single thresholds (IRF);
- two thresholds (H, M, L).
Worth of note, reference intervals and diagnostic cutoff values are dependent on the methodology used.

Figure 2. Dynamics of immature (higly-fluorescent) reticulocytes following PBSCT, adapted from [4]

Figure 3. General view on thrombocyte production by megakaryocytes (MK), and their maturation [9]

Figure 4. Immature platelets in blood film
In previous studies, a very early increase of highly fluorescent reticulocytes was shown in peripheral blood after stem cell mobilization for stem cell harvests [3]. Similar peak in immature reticulocytes is observed at the very onset of hematopoiesis recovery post-HSCT [4], as seen in Fig. 2.
Several studies have confirmed the role of the IRF as early markers in predicting hematopoietic engraftment [5-8].
Thrombocytopoiesis
The most relevant facts were summarized by Wang, Zheng [9]. A common scheme of thrombopoirsis is shown in Fig. 3.
• A healthy human produces approximately 1-2 million platelets per second.
• In response to thrombocytopenia, platelet production can be accelerated by up to a 10-fold increase.
• MGKs may form platelet ribbons into blood vessels. The ribbons are formed via pseudopodia and they are able to continuously emit platelets into circulation.
• Platelets are released from the megakaryocyte cytoplasm, they still contain a small amount of RNA.
• They represent the youngest platelets in the circulation and are named reticulated or immature platelets that are not necessarily large platelets.
• When correlated with megakaryocyte, their concentration in BM is 2-3 times higher than in PB.
• Platelets persist in the circulation for 7-10 days, immature PLT have a much shorter lifespan ( < 1 day).
RetPlt/IPF Reference ranges
It should be noted that flow cytometric assay of immature RBC and platelet forms yields sufficient scatter of reference values reported to range between 1% and 15%, due to lack of standardization. IPF ranges in normal state differ among published studies, due to differences in counting methods (Fig. 4). Normal reference ranges are reported to range between 1% and 15%. Our personal published data showed a range of 2.39% (0.8-5.1%). Several papers within the next 10 years have shown associations between RetPlt/IPF and such disordrs as thrombocytopenia, thrombocytosis, hereditary platelet diseases, after HSCT, thrombo-embolic disorders, kidney disease, preeclampsia, hyperthyroidism, infections and in healthy and thrombocytopenic neonates. An overall conclusion may be drawn as follows: retPLT/IPF in blood (Fig. 4) represent a useful non-invasive marker of megakaryopoietic activity in the bone marrow.
In the literature, IPF was confirmed as one of the earliest predictors of hematopoietic recovery following peripheral blood HPC transplantation [10]. Six patients with primary diagnosis of AML or NHL were subjected to HSCT. Increased percentage of immature platelets in peripheral blood was revealed early post-transplant. According to later studies by several groups, a general consensus with rare contrary opinions exists on the role of the IPF as a useful non-invasive marker predicting hematopoietic engraftment [11-14]. An interesting association was also found between IPF ratios and bacterial nosocomial infections [15].
Erythrocyte schistocytosis
Schistocytosis in peripheral blood is another important marker revealed in the posttransplant period. Schistocytes are fragmented red blood cells (FRBC) formed in peripheral blood (PB) as a consequence of a mechanical damage. These abnormal red blood cells assume a triangular, helmet-shaped form, with two or three pointed extremities. The most frequent cause of erythrocyte fragmentation is the formation of thrombi in the microvasculature as in thrombotic microangiopathies (TMA), with consumption of fibrin and platelets. RBC fragmentation occurs after the passage of erythrocytes through the fibrin cords of microthrombi. For decades, this RBC anomaly is considered a good TMA marker [16].
The present consensus on schistocyte morphology is as follows (Fig. 5):
Helmet shape: decreased size (surface/area), irregular shape, with a defective amputated zone highlighted by a rectilinear border ending with usually 2 (1 or 3 possible) angulated spicules. Pale central zone is not observed.
Triangular shape: reduced size, irregular shape with a defective amputated zone highlighted by a rectilinear sometimes spiculated border, ending with 2 angulated spicules. Pale central zone is not observed.
Bitten erythrocyte: Normal size, shape irregular with a defective amputated zone.
Fragments: Small/very small size, highly irregular shape.
Microspherocytes: Small size, round shape, hyperdensity (increased staining).
Irregularly contracted cells: Small size, hyperdensity, irregular outline sometimes with small protrusions and Heinz bodies.

Figure 5. Morphological anomalies associated with schistocytosis: Helmet shape (A); triangle cells (B); bitten RBC (C); microspherocyte (D)
The following literature reference values for schistocytosis (blood smears, pro mille counts) are:
• for adults: ≤ 0.1%;
• for newborns: 0.3-1.9%;
• for preterm infants: ≤ 5.5%.
Appropriate guidelines for identification, counting and clinical interpretation of schistocytes were published by Zini et al. [17]. This recommendation was cited in 93 papers. Most of them did confirm the utility of the Recommendation. Papers questionnaire-based highlight its role in reducing the intra- and inter-laboratories variability of schistocyte identification and enumeration at microscope. Several published papers are focused on transplanted patients. Some studies are focused on the correlation with instrumental flags, count and/or digital RBC morphology. The latest study concerned inter-laboratory reproducibility of schistocytosis counts [18]. In conclusion, the survey identified the need for the standardization of TA-TMA morphological diagnosis, in order to optimize general diagnostic criteria (Table 1).
Table 1. Comparison of diagnostic criteria for TMA from different sources

To confirm the morphologic rules in schistocyte identification.
• To provide common rules for laboratory staff to check at microscope the samples with flags for “fragments” and/or images from digitized systems.
• To mention new published data not only in the clinical setting of TTP.
• To implement it providing reference values in post BMT patients in collaboration with Prof. G.W. Basak, Chairman of the EBMT TCWP.
There are different methods for automated red cell fragments counts proposed by several manufacturers. E.g., Vcoulter approach based in classical flow cytometry. ADVIA 2120 method offers integrated analysis of RBC and platelet count: events with an object volume smaller than 30 fL and with a refractive index greater than 1.4 frequence of events above the threshold of 10.000/μL. Mindray BC 6800 method considers microcytic/fragmented RBCs or large platelets. Abbott Sapphire method detects RBC- specific (CD235a) and platelet-specific (CD61) surface antigensantigens to identify abnormal cell populations.
Schistocytes, RBC fragments and Laws of thought: three Aristotle’s axioms
1. Contradiction/self-evident proposition: Schistocytes are red blood cell fragments (RBCFr).
2. Exclusion middle/third: Schistocytes are RBCFr identified by a peculiar shape.
3. Identity (non-identity): Not all red blood cell fragments are schistocytes.
There is a number of studies which show some differences in FRBC numbers upon manual vs automatical counting. E.g., Banno et al. [23] have shown that the RBC fragments determined visually was higher than that determined by XE-2100 hematology analyzer, suggesting over-estimation of the FRBC counts by visual microscopy. Similar comparative aspects were considered in the study by Lesesve et al. [24]. In summary, automated FRC count vs schistocytes visual count lead to the conclusion that not all automated red cell fragments are schistocytes.
Conflict of interest
No conflicts of interest reported.
References
- Styczynski J, Tridello G., Koster L, Iacobelli S, van Biezen A, van der Werf S, Mikulska M, Gil L, Cordonnier C, Ljungman P, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020; 55:126-136. doi: 10.1038/s41409-019-0624-z.
- d’Onofrio G, Kuse R, Foures C, Jou JM, Pradella M, Zini G. Reticulocytes in haematological disorders. Clin Lab Haematol. 1996; 18(Suppl 1):29-34. PMID: 9054716.
- Remacha AF, Martino R, Sureda A, Sarda M, Solá C, Tugues D, Amill B, García J, Oliver A. Changes in the reticulocyte fraction during peripheral stem cell harvesting: role in monitoring stem cell collection. Bone Marrow Transplant 1996;17(2):163–168. PMID: 8640161.
- Sica S, Salutari P, Laurenti L, Ortu La Barbera E, Zini G, d’Onofrio G, Serafin R, Leone G. Highly fluorescent reticulocytes after CD34+ peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1998; 21:361-364. doi: 10.1038/sj.bmt.1701098.
- George P, Wyre RM, Bruty SJ, Sweetenham JW, Duncombe AS. Automated immature reticulocyte counts are early markers of engraftment following autologous PBSC transplantation in patients with lymphoma. J Hematother Stem Cell Res. 2000; 9(2): 219-223. doi: 10.1089/152581600319432.
- Lesesve JF, Lenormand B, Lacombe F. The role of high fluorescent reticulocytes in monitoring Platelet generation in vivo and in vitro the aplasia outcome and optimizing the timing of peripheral blood stem cell harvesting. J Hematother Stem Cell Res. 2002; 11(6):987-989. doi: 10.1089/152581602321080664.
- Grazziuti ML, Dong L, Miceli MH, Cottler-Fox M, Krishna SG, Fassas A, van Rhee F, Barlogie BM, Anaissie EJ. Recovery from neutropenia can be predicted by he immature reticulocyte fraction several days before neutrophil recovery in autologous stem cell transplant recipients. Bone Marrow Transplant. 2006; 37: 403-409. doi: 10.1038/sj.bmt.1705251.
- Molina JR, Sanchez-Garcia J, Torres A, Alvarez MA, Serrano J, Casano J, Gomez P, Martinez F, Rodriguez A, Martin C. Reticulocyte maturation parameters are reliable early predictors of hematopoietic engraftment after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007; 13: 172-182. doi:10.1016/j.bbmt.2006.09.007.
- Wang B, Zheng J. Platelet generation in vivo and in vitro. SpringerPlus. 2016. 5:787. doi: 10.1186/s40064-016-2384-1.
- Zucker ML, Murphy CA, Rachel JM, Martinez GA, Abhyankar S, McGuirk JP, Reid KJ, Plapp FV. Immature platelet fraction as a predictor of platelet recovery following hematopoietic progenitor cell transplantation. Lab Hematol 2006;12(3):125–130. doi: 10.1532/LH96.06012.
- Briggs C, Hart D, Kunka S, Oguni S, Machin SJ. Immature platelet fraction measurement: a future guide to platelet transfusion requirement after haematopoietic stem cell transplantation. Transfusion Medicine. 2006; 16(2):101-109. doi: 10.1111/j.1365-3148.2006.00654.x.
- Goncalo AP, Barbosa IL, Campiho F, Campos A, Mendes C. Predictive value of immature reticulocyte and platelet fractions in hematopoietic recovery of allograft patients. Transplant Proc. 2011; 43(1):241-243. doi: 10.1016/j.transproceed.2010.12.030.
- Morkis IVC, Farias MG, Rigoni LDC, Scotti L, Gregianin LJ, Daudt LE, LM Da R Silla LM, Paz AA. Assessment of immature platelet fraction and immature reticulocyte fraction as predictors of engraftment after hematopoietic stem cell transplantation. Int J Lab Hem. 2015; 37: 259-264. doi: 10.1111/ijlh.12278.
- Meintker L, Fritsch JD, Ringwald J, Krause SW. Immature platelets do reliably predict platelet recovery in patients with intensive chemotherapy or stem cell transplantation. Vox Sang. 2017; 112(2):132-139. doi: 10.1111/vox.12483.
- Di Mario A, Garzia M, Leone F, Arcangeli A, Pagano L, Zini G. Immature platelet fraction (IPF) in hospitalized patients with neutrophilia and suspected bacterial infection. J Infect. 2009; 59(3):201-216. doi: 10.1016/j.jinf.2009.07.007.
- Bessis M. Blood Smears Reinterpreted. Springer-Verlag, 1977.
- Zini G, d’Onofrio G, Briggs C, Erber W, Jou JM, Lee SH, McFadden S, Vives-Corrons JL, Yutaka N, Lesesve JF. ICSH recommendations for identification, diagnostic value and quantitation of schistocytes. Int J Lab Hematol. 2011; 34(2):107-116. DOI: 10.1111/j.1751-553X.2011.01380.x.
- Moiseev IS, Tsvetkova T, Aljurf M, Alnounou RM, Bogardt J, Chalandon, Y, Drokov M Yu, Dvirnyk V, Faraci M, Friis LS, et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019; 54:1022-1028. doi: 10.1038/s41409-018-0374-3.
- Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, Ferrara J, Soiffer R, Giralt S. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571-575. doi: 10.1016/j.bbmt.2005.06.001.
- Ruutu T, Barosi G, Benjamin R, Clark R, George J, Gratwohl A, Holler E, Iacobelli M, Kentouche K, Lämmle B et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: Results of a consensus process by an International Working Group. 2007. Haematologica; 92(1):95-100. doi: 10.3324/haematol.10699.
- Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010; 90(8):918-926. doi: 10.1097/TP.0b013e3181f24e8d.
- Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, Goebel J, Dixon BP. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev 2015;29(3):191‒204. doi:10.1016/j.blre.2014.11.001.
- Banno S, Ito Y, Tanaka C, Hori T, Fujimoto K, Suzuki T, Hashimoto T, Ueda R, Mizokami M. Quantification of red blood cell fragmentation by the automated hematology analyzer XE-2100 in patients with living donor liver transplantation. Clin Lab Haematol. 2005; 27(5):292-296. doi: 10.1111/j.1365-2257.2005.00704.x.
- Lesesve JF, Asnafi V, Braun F, Zini G. Fragmented red blood cells automated measurement is a useful parameter to exclude shisticytes on the blood film. Int J Lab Hematol. 2012; 34(6):566-576. doi: 10.1111/j.1751-553X.2012.01434.x.
" ["~DETAIL_TEXT"]=> string(24276) "
Introduction
HSCT is a potentially life-saving procedure, often the only curative option for a variety of diseases. This procedure is, however, associated with both immediate and long-term complications requiring increased vigilance and monitoring. Some HSCT complications can result in decreased quality of life and shortened life expectancy [1].
Early HSCT complications generally occur within 100 days post HSCT. They include: oral complications/mucositis and sepsis, neutropenic fever, haemorrhagic cystitis, endothelial damage, engraftment syndrome, idiopathic pneumonia syndrome, diffuse alveolar haemorrhage, transplant-associated microangiopathy (TAM) and sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD), infection due to compromised immune function, organ toxicity, secondary malignancy, cancer relapse, graft failure and graft rejection.
Late post-transplant complications occur ≥1 year after treatment with a wide spectrum in terms of latency, intensity, reversibility, and lethality. The most frequent causes of delayed mortality are cardiac/vascular, subsequent neoplasms, infections, and pulmonary disorders. It is noteworthy that the incidence for cardiac/vascular and subsequent neoplasms continues to increase with time after HCT and does not peak before the completion of the second decade of survivorship. Timely diagnostics of hematopoiesis reconstitution is the management cornerstone in these patients.
Immature subsets of RBC and platelets as markers of blood recovery
At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients.
• Reticulocyte count
• IRF (immature reticulocytes fraction): Fig. 1A
• IPF (immature platelets fraction)/reticulated platelets: Fig. 1B
• RBC fragments.

Figure 1. Immature reticulocytes (A) and one reticulated platelets (B) in blood smears
Reticulocytes represent the terminal stage of RBC maturation in bone marrow. Reticulocytosis rates are changed in a variety of blood disorders [2]. Immature reticulocyte fraction (IRF) arises at the late stage of erythrocyte differentiation, recognizable by trace amounts of nucleic acids seen as visible reticular substance which normally fades away within 3 days of terminal maturation. Similar reticular substance is seen in immature platelet fraction (IPF) budding from megakaryocytes in bone marrow. Following HSCT, both erythropoiesis and platelet generation proceed at much faster rates, thus exhibiting higher levels of IRF and IPF in peripheral blood.
State of the art in reticulocyte count
Reticulocyte count in the last-generation hematological analyzers correlate with the reference methods, using different staining and registration approaches:
Fluorochromes:
- ReticFit, ReticCount (thiazol orange);
- Sysmex R-1000/3000, SE 9500 (auramine O);
- Sysmex XE-2100;
- Abbott Cell Dyn 4000 (CD4K530);
- ABX Pentra (thiazol orange);
- Mindray BC‐6800 (asymmetric cyanine).
Stains and light scatter/absorption:
- Bayer H*3/Advia (oxazine 750);
- Abbott Cell Dyn 3500-3700 (NMB);
- Coulter STKS/MAXM/GEN-S (NMB);
- Microscope image analysis (Micro 21).
Constant monitoring of reticulocytes count is critical in the post-transplant period, in order to monitor engraftment and hematopoietic recovery as well as to intercept in real time changes in the erythropoiesis. Parvovirus B19 (PVB19) infection may represent such clinical situation. PVB19 is a single-stranded DNA virus of the family Parvoviridae with tropism to erythroid progenitor cells. In healthy immunocompetent individuals it causes rash of erythema infectiosum (fifth disease) in children and acute symmetric polyarthropathia in adults. Transient aplastic crisis may be induced in individuals with congenital hemolytic disorders. Persistent B19 infection in immunocompromised patients is manifested as pure red cell aplasia and chronic anemia. In peripheral blood of these patients, one may observe marked reticulocytopenia; in bone marrow, marked erythroid hypoplasia, absence of maturing precursors and presence of giant pro-erythroblasts.
Studies of immature reticulocytes (IRF) may be performed by manual or automated methods, as follows:
Morphology:
- polychromatophylic red cells;
- reticulocyte differential counts.
Flow cytometry:
- RNA content (fluorochromes, dyes);
- CD71, CD36;
- fetal hemoglobin or antigens;
- RMI = mean fluorescence-intensity (i.e., mean channel);
- Fraction(s) of RNA-rich reticulocytes:
- single thresholds (IRF);
- two thresholds (H, M, L).
Worth of note, reference intervals and diagnostic cutoff values are dependent on the methodology used.

Figure 2. Dynamics of immature (higly-fluorescent) reticulocytes following PBSCT, adapted from [4]

Figure 3. General view on thrombocyte production by megakaryocytes (MK), and their maturation [9]

Figure 4. Immature platelets in blood film
In previous studies, a very early increase of highly fluorescent reticulocytes was shown in peripheral blood after stem cell mobilization for stem cell harvests [3]. Similar peak in immature reticulocytes is observed at the very onset of hematopoiesis recovery post-HSCT [4], as seen in Fig. 2.
Several studies have confirmed the role of the IRF as early markers in predicting hematopoietic engraftment [5-8].
Thrombocytopoiesis
The most relevant facts were summarized by Wang, Zheng [9]. A common scheme of thrombopoirsis is shown in Fig. 3.
• A healthy human produces approximately 1-2 million platelets per second.
• In response to thrombocytopenia, platelet production can be accelerated by up to a 10-fold increase.
• MGKs may form platelet ribbons into blood vessels. The ribbons are formed via pseudopodia and they are able to continuously emit platelets into circulation.
• Platelets are released from the megakaryocyte cytoplasm, they still contain a small amount of RNA.
• They represent the youngest platelets in the circulation and are named reticulated or immature platelets that are not necessarily large platelets.
• When correlated with megakaryocyte, their concentration in BM is 2-3 times higher than in PB.
• Platelets persist in the circulation for 7-10 days, immature PLT have a much shorter lifespan ( < 1 day).
RetPlt/IPF Reference ranges
It should be noted that flow cytometric assay of immature RBC and platelet forms yields sufficient scatter of reference values reported to range between 1% and 15%, due to lack of standardization. IPF ranges in normal state differ among published studies, due to differences in counting methods (Fig. 4). Normal reference ranges are reported to range between 1% and 15%. Our personal published data showed a range of 2.39% (0.8-5.1%). Several papers within the next 10 years have shown associations between RetPlt/IPF and such disordrs as thrombocytopenia, thrombocytosis, hereditary platelet diseases, after HSCT, thrombo-embolic disorders, kidney disease, preeclampsia, hyperthyroidism, infections and in healthy and thrombocytopenic neonates. An overall conclusion may be drawn as follows: retPLT/IPF in blood (Fig. 4) represent a useful non-invasive marker of megakaryopoietic activity in the bone marrow.
In the literature, IPF was confirmed as one of the earliest predictors of hematopoietic recovery following peripheral blood HPC transplantation [10]. Six patients with primary diagnosis of AML or NHL were subjected to HSCT. Increased percentage of immature platelets in peripheral blood was revealed early post-transplant. According to later studies by several groups, a general consensus with rare contrary opinions exists on the role of the IPF as a useful non-invasive marker predicting hematopoietic engraftment [11-14]. An interesting association was also found between IPF ratios and bacterial nosocomial infections [15].
Erythrocyte schistocytosis
Schistocytosis in peripheral blood is another important marker revealed in the posttransplant period. Schistocytes are fragmented red blood cells (FRBC) formed in peripheral blood (PB) as a consequence of a mechanical damage. These abnormal red blood cells assume a triangular, helmet-shaped form, with two or three pointed extremities. The most frequent cause of erythrocyte fragmentation is the formation of thrombi in the microvasculature as in thrombotic microangiopathies (TMA), with consumption of fibrin and platelets. RBC fragmentation occurs after the passage of erythrocytes through the fibrin cords of microthrombi. For decades, this RBC anomaly is considered a good TMA marker [16].
The present consensus on schistocyte morphology is as follows (Fig. 5):
Helmet shape: decreased size (surface/area), irregular shape, with a defective amputated zone highlighted by a rectilinear border ending with usually 2 (1 or 3 possible) angulated spicules. Pale central zone is not observed.
Triangular shape: reduced size, irregular shape with a defective amputated zone highlighted by a rectilinear sometimes spiculated border, ending with 2 angulated spicules. Pale central zone is not observed.
Bitten erythrocyte: Normal size, shape irregular with a defective amputated zone.
Fragments: Small/very small size, highly irregular shape.
Microspherocytes: Small size, round shape, hyperdensity (increased staining).
Irregularly contracted cells: Small size, hyperdensity, irregular outline sometimes with small protrusions and Heinz bodies.

Figure 5. Morphological anomalies associated with schistocytosis: Helmet shape (A); triangle cells (B); bitten RBC (C); microspherocyte (D)
The following literature reference values for schistocytosis (blood smears, pro mille counts) are:
• for adults: ≤ 0.1%;
• for newborns: 0.3-1.9%;
• for preterm infants: ≤ 5.5%.
Appropriate guidelines for identification, counting and clinical interpretation of schistocytes were published by Zini et al. [17]. This recommendation was cited in 93 papers. Most of them did confirm the utility of the Recommendation. Papers questionnaire-based highlight its role in reducing the intra- and inter-laboratories variability of schistocyte identification and enumeration at microscope. Several published papers are focused on transplanted patients. Some studies are focused on the correlation with instrumental flags, count and/or digital RBC morphology. The latest study concerned inter-laboratory reproducibility of schistocytosis counts [18]. In conclusion, the survey identified the need for the standardization of TA-TMA morphological diagnosis, in order to optimize general diagnostic criteria (Table 1).
Table 1. Comparison of diagnostic criteria for TMA from different sources

To confirm the morphologic rules in schistocyte identification.
• To provide common rules for laboratory staff to check at microscope the samples with flags for “fragments” and/or images from digitized systems.
• To mention new published data not only in the clinical setting of TTP.
• To implement it providing reference values in post BMT patients in collaboration with Prof. G.W. Basak, Chairman of the EBMT TCWP.
There are different methods for automated red cell fragments counts proposed by several manufacturers. E.g., Vcoulter approach based in classical flow cytometry. ADVIA 2120 method offers integrated analysis of RBC and platelet count: events with an object volume smaller than 30 fL and with a refractive index greater than 1.4 frequence of events above the threshold of 10.000/μL. Mindray BC 6800 method considers microcytic/fragmented RBCs or large platelets. Abbott Sapphire method detects RBC- specific (CD235a) and platelet-specific (CD61) surface antigensantigens to identify abnormal cell populations.
Schistocytes, RBC fragments and Laws of thought: three Aristotle’s axioms
1. Contradiction/self-evident proposition: Schistocytes are red blood cell fragments (RBCFr).
2. Exclusion middle/third: Schistocytes are RBCFr identified by a peculiar shape.
3. Identity (non-identity): Not all red blood cell fragments are schistocytes.
There is a number of studies which show some differences in FRBC numbers upon manual vs automatical counting. E.g., Banno et al. [23] have shown that the RBC fragments determined visually was higher than that determined by XE-2100 hematology analyzer, suggesting over-estimation of the FRBC counts by visual microscopy. Similar comparative aspects were considered in the study by Lesesve et al. [24]. In summary, automated FRC count vs schistocytes visual count lead to the conclusion that not all automated red cell fragments are schistocytes.
Conflict of interest
No conflicts of interest reported.
References
- Styczynski J, Tridello G., Koster L, Iacobelli S, van Biezen A, van der Werf S, Mikulska M, Gil L, Cordonnier C, Ljungman P, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020; 55:126-136. doi: 10.1038/s41409-019-0624-z.
- d’Onofrio G, Kuse R, Foures C, Jou JM, Pradella M, Zini G. Reticulocytes in haematological disorders. Clin Lab Haematol. 1996; 18(Suppl 1):29-34. PMID: 9054716.
- Remacha AF, Martino R, Sureda A, Sarda M, Solá C, Tugues D, Amill B, García J, Oliver A. Changes in the reticulocyte fraction during peripheral stem cell harvesting: role in monitoring stem cell collection. Bone Marrow Transplant 1996;17(2):163–168. PMID: 8640161.
- Sica S, Salutari P, Laurenti L, Ortu La Barbera E, Zini G, d’Onofrio G, Serafin R, Leone G. Highly fluorescent reticulocytes after CD34+ peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1998; 21:361-364. doi: 10.1038/sj.bmt.1701098.
- George P, Wyre RM, Bruty SJ, Sweetenham JW, Duncombe AS. Automated immature reticulocyte counts are early markers of engraftment following autologous PBSC transplantation in patients with lymphoma. J Hematother Stem Cell Res. 2000; 9(2): 219-223. doi: 10.1089/152581600319432.
- Lesesve JF, Lenormand B, Lacombe F. The role of high fluorescent reticulocytes in monitoring Platelet generation in vivo and in vitro the aplasia outcome and optimizing the timing of peripheral blood stem cell harvesting. J Hematother Stem Cell Res. 2002; 11(6):987-989. doi: 10.1089/152581602321080664.
- Grazziuti ML, Dong L, Miceli MH, Cottler-Fox M, Krishna SG, Fassas A, van Rhee F, Barlogie BM, Anaissie EJ. Recovery from neutropenia can be predicted by he immature reticulocyte fraction several days before neutrophil recovery in autologous stem cell transplant recipients. Bone Marrow Transplant. 2006; 37: 403-409. doi: 10.1038/sj.bmt.1705251.
- Molina JR, Sanchez-Garcia J, Torres A, Alvarez MA, Serrano J, Casano J, Gomez P, Martinez F, Rodriguez A, Martin C. Reticulocyte maturation parameters are reliable early predictors of hematopoietic engraftment after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007; 13: 172-182. doi:10.1016/j.bbmt.2006.09.007.
- Wang B, Zheng J. Platelet generation in vivo and in vitro. SpringerPlus. 2016. 5:787. doi: 10.1186/s40064-016-2384-1.
- Zucker ML, Murphy CA, Rachel JM, Martinez GA, Abhyankar S, McGuirk JP, Reid KJ, Plapp FV. Immature platelet fraction as a predictor of platelet recovery following hematopoietic progenitor cell transplantation. Lab Hematol 2006;12(3):125–130. doi: 10.1532/LH96.06012.
- Briggs C, Hart D, Kunka S, Oguni S, Machin SJ. Immature platelet fraction measurement: a future guide to platelet transfusion requirement after haematopoietic stem cell transplantation. Transfusion Medicine. 2006; 16(2):101-109. doi: 10.1111/j.1365-3148.2006.00654.x.
- Goncalo AP, Barbosa IL, Campiho F, Campos A, Mendes C. Predictive value of immature reticulocyte and platelet fractions in hematopoietic recovery of allograft patients. Transplant Proc. 2011; 43(1):241-243. doi: 10.1016/j.transproceed.2010.12.030.
- Morkis IVC, Farias MG, Rigoni LDC, Scotti L, Gregianin LJ, Daudt LE, LM Da R Silla LM, Paz AA. Assessment of immature platelet fraction and immature reticulocyte fraction as predictors of engraftment after hematopoietic stem cell transplantation. Int J Lab Hem. 2015; 37: 259-264. doi: 10.1111/ijlh.12278.
- Meintker L, Fritsch JD, Ringwald J, Krause SW. Immature platelets do reliably predict platelet recovery in patients with intensive chemotherapy or stem cell transplantation. Vox Sang. 2017; 112(2):132-139. doi: 10.1111/vox.12483.
- Di Mario A, Garzia M, Leone F, Arcangeli A, Pagano L, Zini G. Immature platelet fraction (IPF) in hospitalized patients with neutrophilia and suspected bacterial infection. J Infect. 2009; 59(3):201-216. doi: 10.1016/j.jinf.2009.07.007.
- Bessis M. Blood Smears Reinterpreted. Springer-Verlag, 1977.
- Zini G, d’Onofrio G, Briggs C, Erber W, Jou JM, Lee SH, McFadden S, Vives-Corrons JL, Yutaka N, Lesesve JF. ICSH recommendations for identification, diagnostic value and quantitation of schistocytes. Int J Lab Hematol. 2011; 34(2):107-116. DOI: 10.1111/j.1751-553X.2011.01380.x.
- Moiseev IS, Tsvetkova T, Aljurf M, Alnounou RM, Bogardt J, Chalandon, Y, Drokov M Yu, Dvirnyk V, Faraci M, Friis LS, et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019; 54:1022-1028. doi: 10.1038/s41409-018-0374-3.
- Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, Ferrara J, Soiffer R, Giralt S. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571-575. doi: 10.1016/j.bbmt.2005.06.001.
- Ruutu T, Barosi G, Benjamin R, Clark R, George J, Gratwohl A, Holler E, Iacobelli M, Kentouche K, Lämmle B et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: Results of a consensus process by an International Working Group. 2007. Haematologica; 92(1):95-100. doi: 10.3324/haematol.10699.
- Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010; 90(8):918-926. doi: 10.1097/TP.0b013e3181f24e8d.
- Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, Goebel J, Dixon BP. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev 2015;29(3):191‒204. doi:10.1016/j.blre.2014.11.001.
- Banno S, Ito Y, Tanaka C, Hori T, Fujimoto K, Suzuki T, Hashimoto T, Ueda R, Mizokami M. Quantification of red blood cell fragmentation by the automated hematology analyzer XE-2100 in patients with living donor liver transplantation. Clin Lab Haematol. 2005; 27(5):292-296. doi: 10.1111/j.1365-2257.2005.00704.x.
- Lesesve JF, Asnafi V, Braun F, Zini G. Fragmented red blood cells automated measurement is a useful parameter to exclude shisticytes on the blood film. Int J Lab Hematol. 2012; 34(6):566-576. doi: 10.1111/j.1751-553X.2012.01434.x.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(100) "laboratornaya-diagnostika-oslozhneniy-transplantatsii-gemopoeticheskikh-kletok-nezrelye-trombotsity-" ["~CODE"]=> string(100) "laboratornaya-diagnostika-oslozhneniy-transplantatsii-gemopoeticheskikh-kletok-nezrelye-trombotsity-" ["EXTERNAL_ID"]=> string(4) "1947" ["~EXTERNAL_ID"]=> string(4) "1947" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(277) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроцитыLaboratory diagnostics of HSCT complications: immature platelets and RBCs" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2981) "<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.</p> <p style="text-align: justify;">В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.</p> <p style="text-align: justify;">Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_META_TITLE"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_META_KEYWORDS"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_META_DESCRIPTION"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_PICTURE_FILE_ALT"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_PICTURE_FILE_TITLE"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "laboratornaya-diagnostika-oslozhneniy-transplantatsii-gemopoeticheskikh-kletok-nezrelye-trombotsity-" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(204) "Лабораторная диагностика осложнений трансплантации гемопоэтических клеток: незрелые тромбоциты и эритроциты" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "laboratornaya-diagnostika-oslozhneniy-transplantatsii-gemopoeticheskikh-kletok-nezrelye-trombotsity-" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "laboratornaya-diagnostika-oslozhneniy-transplantatsii-gemopoeticheskikh-kletok-nezrelye-trombotsity-" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "laboratornaya-diagnostika-oslozhneniy-transplantatsii-gemopoeticheskikh-kletok-nezrelye-trombotsity-" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "174" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27432" ["VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27433" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27434" ["VALUE"]=> array(2) { ["TEXT"]=> string(37) "<p>Джина Зини</>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(25) "
Джина Зини" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27435" ["VALUE"]=> array(2) { ["TEXT"]=> string(322) "<p>Отдел визуальной диагностики, онкологической радиотерапии и гематологии, Университетская поликлиника «Гемелли», Католический Университет Сакро Куоре, Рим, Италия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(310) "
Отдел визуальной диагностики, онкологической радиотерапии и гематологии, Университетская поликлиника «Гемелли», Католический Университет Сакро Куоре, Рим, Италия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27436" ["VALUE"]=> array(2) { ["TEXT"]=> string(2981) "<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.</p> <p style="text-align: justify;">В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.</p> <p style="text-align: justify;">Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2881) "Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.
В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.
Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.
Ключевые слова
Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27437" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2021-10-1-6-12" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2021-10-1-6-12" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27439" ["VALUE"]=> array(2) { ["TEXT"]=> string(28) "<p>Gina Zini</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(16) "Gina Zini
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27440" ["VALUE"]=> array(2) { ["TEXT"]=> string(510) "<p>Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy</p><br> <p><b>Correspondence</b><br> <p style="text-align: justify;">Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario <br>A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore <br> E-mail: gina.zini@unicatt.it</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(434) "Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy
Correspondence
Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario
A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore
E-mail: gina.zini@unicatt.it
HSCT is a potentially life-saving procedure, associated with complications requiring increased vigilance and monitoring. In the immediate follow-up of patients, the blood count provides precise quantitative indications upon the post-transplant course, allowing to follow haematopoietic recovery, evaluating hemoglobin values, and the numbers of platelets and white blood cells. At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients, that are the immature reticulocyte fraction (IRF), immature platelet fraction/reticulated platelets (IPF) and red blood cell (RBC) fragments. In the presence of post-transplant recovery of erythropoiesis and thrombocytopoiesis, IRF and IPF increase in the peripheral blood correlates in real time with haematopoietic recovery. Another useful parameter in the follow-up of these patients is the number of red blood cell fragments (FRC) which can correlate with presence of shistocytes. Availability of this parameter allows to intercept in routine practice the samples that could contain schistocytes: immediate evaluation of the peripheral blood smear will confirm or not the diagnostic suspicion.
Keywords
Automated blood analysis, immature reticulocytes fraction, immature platelets fraction, red blood cell fragments, hematopoietic cell transplantation, complications.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27438" ["VALUE"]=> string(73) "Laboratory diagnostics of HSCT complications: immature platelets and RBCs" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(73) "Laboratory diagnostics of HSCT complications: immature platelets and RBCs" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27522" ["VALUE"]=> string(4) "2410" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2410" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27523" ["VALUE"]=> string(4) "2411" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2411" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27439" ["VALUE"]=> array(2) { ["TEXT"]=> string(28) "<p>Gina Zini</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(16) "Gina Zini
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(16) "Gina Zini
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27441" ["VALUE"]=> array(2) { ["TEXT"]=> string(1553) "<p style="text-align: justify;">HSCT is a potentially life-saving procedure, associated with complications requiring increased vigilance and monitoring. In the immediate follow-up of patients, the blood count provides precise quantitative indications upon the post-transplant course, allowing to follow haematopoietic recovery, evaluating hemoglobin values, and the numbers of platelets and white blood cells. At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients, that are the immature reticulocyte fraction (IRF), immature platelet fraction/reticulated platelets (IPF) and red blood cell (RBC) fragments. In the presence of post-transplant recovery of erythropoiesis and thrombocytopoiesis, IRF and IPF increase in the peripheral blood correlates in real time with haematopoietic recovery. Another useful parameter in the follow-up of these patients is the number of red blood cell fragments (FRC) which can correlate with presence of shistocytes. Availability of this parameter allows to intercept in routine practice the samples that could contain schistocytes: immediate evaluation of the peripheral blood smear will confirm or not the diagnostic suspicion.</p> <h2>Keywords</h2> <p style="text-align: justify;">Automated blood analysis, immature reticulocytes fraction, immature platelets fraction, red blood cell fragments, hematopoietic cell transplantation, complications.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1497) "HSCT is a potentially life-saving procedure, associated with complications requiring increased vigilance and monitoring. In the immediate follow-up of patients, the blood count provides precise quantitative indications upon the post-transplant course, allowing to follow haematopoietic recovery, evaluating hemoglobin values, and the numbers of platelets and white blood cells. At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients, that are the immature reticulocyte fraction (IRF), immature platelet fraction/reticulated platelets (IPF) and red blood cell (RBC) fragments. In the presence of post-transplant recovery of erythropoiesis and thrombocytopoiesis, IRF and IPF increase in the peripheral blood correlates in real time with haematopoietic recovery. Another useful parameter in the follow-up of these patients is the number of red blood cell fragments (FRC) which can correlate with presence of shistocytes. Availability of this parameter allows to intercept in routine practice the samples that could contain schistocytes: immediate evaluation of the peripheral blood smear will confirm or not the diagnostic suspicion.
Keywords
Automated blood analysis, immature reticulocytes fraction, immature platelets fraction, red blood cell fragments, hematopoietic cell transplantation, complications.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1497) "HSCT is a potentially life-saving procedure, associated with complications requiring increased vigilance and monitoring. In the immediate follow-up of patients, the blood count provides precise quantitative indications upon the post-transplant course, allowing to follow haematopoietic recovery, evaluating hemoglobin values, and the numbers of platelets and white blood cells. At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients, that are the immature reticulocyte fraction (IRF), immature platelet fraction/reticulated platelets (IPF) and red blood cell (RBC) fragments. In the presence of post-transplant recovery of erythropoiesis and thrombocytopoiesis, IRF and IPF increase in the peripheral blood correlates in real time with haematopoietic recovery. Another useful parameter in the follow-up of these patients is the number of red blood cell fragments (FRC) which can correlate with presence of shistocytes. Availability of this parameter allows to intercept in routine practice the samples that could contain schistocytes: immediate evaluation of the peripheral blood smear will confirm or not the diagnostic suspicion.
Keywords
Automated blood analysis, immature reticulocytes fraction, immature platelets fraction, red blood cell fragments, hematopoietic cell transplantation, complications.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27437" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2021-10-1-6-12" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2021-10-1-6-12" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2021-10-1-6-12" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27438" ["VALUE"]=> string(73) "Laboratory diagnostics of HSCT complications: immature platelets and RBCs" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(73) "Laboratory diagnostics of HSCT complications: immature platelets and RBCs" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(73) "Laboratory diagnostics of HSCT complications: immature platelets and RBCs" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27440" ["VALUE"]=> array(2) { ["TEXT"]=> string(510) "<p>Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy</p><br> <p><b>Correspondence</b><br> <p style="text-align: justify;">Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario <br>A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore <br> E-mail: gina.zini@unicatt.it</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(434) "Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy
Correspondence
Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario
A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore
E-mail: gina.zini@unicatt.it
Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy
Correspondence
Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario
A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore
E-mail: gina.zini@unicatt.it
Джина Зини" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(25) "
Джина Зини" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27432" ["VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/08/2021 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27433" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "04/09/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27436" ["VALUE"]=> array(2) { ["TEXT"]=> string(2981) "<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.</p> <p style="text-align: justify;">В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.</p> <p style="text-align: justify;">Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2881) "
Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.
В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.
Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.
Ключевые слова
Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2881) "Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.
В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.
Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.
Ключевые слова
Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27435" ["VALUE"]=> array(2) { ["TEXT"]=> string(322) "<p>Отдел визуальной диагностики, онкологической радиотерапии и гематологии, Университетская поликлиника «Гемелли», Католический Университет Сакро Куоре, Рим, Италия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(310) "Отдел визуальной диагностики, онкологической радиотерапии и гематологии, Университетская поликлиника «Гемелли», Католический Университет Сакро Куоре, Рим, Италия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(310) "Отдел визуальной диагностики, онкологической радиотерапии и гематологии, Университетская поликлиника «Гемелли», Католический Университет Сакро Куоре, Рим, Италия
" } } } [1]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "174" ["~IBLOCK_SECTION_ID"]=> string(3) "174" ["ID"]=> string(4) "1948" ["~ID"]=> string(4) "1948" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["~NAME"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "05/31/2021 11:48:45 am" ["~TIMESTAMP_X"]=> string(22) "05/31/2021 11:48:45 am" ["DETAIL_PAGE_URL"]=> string(114) "/en/archive/tom-10-nomer-1/obzornye-stati/immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla/" ["~DETAIL_PAGE_URL"]=> string(114) "/en/archive/tom-10-nomer-1/obzornye-stati/immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(53987) "Introduction
Human leukocyte antigens (HLA) on the cell surface play a pivotal role in recognizing inter-and extracellular proteins as "self" or "non-self". A unique set of HLA alleles keeps our immunological identity. The immune response of the host organism is based on the detection of "non-self" protein epitopes. E.g., in viral infections, Class I HLA molecules recognize viral peptides produced inside the infected cells, causing the recruitment of CD8+ cytotoxic populations and destruction of the target cells.
Class II antigens on the antigen-presenting immune cells mostly determine the risk of graft rejection after allogeneic transplantation of organs or bone marrow, thus requiring maximal HLA-similarity between recipient and donor. Since the 1970’s, the high-throughput and precise techniques of HLA-typing were introduced for allogeneic transplantation of hematopoietic cells (HSCT). More than 30.000 HLA variations of HLA proteins are registered and DNA-sequenced by the classical Sanger technique or NGS approach which is implemented for HLA typing worldwide [1]. The resulting databases contain quite extensive data on HLA polymorphic regions and provide excellent opportunities for in silico modeling of best matches between HLA and any foreign proteins, including fragments of viral antigens.
Our review aimed to discuss common bioinformatic approaches to the search of target HLA epitopes and immunity-related proteins when designing novel antiviral vaccines, in particular, against SARS-CoV-2. This new beta-coronavirus of the Coronaviridae family, Betacoronavirus genus was identified as a pathogen causing severe acute respiratory infection. On February 11, 2020, The World Health Organization has assigned the official name COVID-19 ("CОronaVIrus Disease 2019"), whereas an International Committee for Taxonomy of Viruses, ICTV) Committee for Taxonomy of Viruses, ICTV) designates the causative agent of this infection as SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2). At sooner time, WHO has claimed SARS-CoV-2 pandemia.
Clinical course in SARS-CoV-2 infection is quite variable. i.e., from mild or symptom-free clinical forms to severe or extremely severe COVID-19 with high mortality levels. Acute respiratory distress syndrome was registered in some patients, and 11% of the patients deceased with multi-organ failure in a short time [2]. Most patients with COVID-19 manifest with fever (90%); cough (80%); apnea (55%); loss of smell and taste (50%); myalgia and fatigability (44%); chest pressure and/or pain (20%), as well as headache (8%), hemoptysis (5%), diarrhea, nausea (3%) [3]. SARS-CoV-2 exhibits pronounced lung tropism causing severe respiratory failure in some patients with COVID-19-associated pneumonia thus requiring invasive mechanical ventilation with a mortality risk of up to 60% [4]. The specific clinical course of COVID-19 infection suggests a unique immune dysfunction with pronounced lymphopenia, excessive IL-6 production, and enhanced blood clotting [5].
The results of computer modeling (in silico experiments) show that there are genetic differences, especially in the human immune system, which may explain the different ability to respond to SARS-CoV-2 infection, differences in symptoms, and severity of COVID-19.
Following infection of human cells by a coronavirus, the organism responds by antiviral signaling. These alarms identify the virus and mobilize the immune system for sending cytotoxic T cells to destroy the infected cells. To find out if different alleles of signaling system may explain the diversity of immune responses to SARS-CoV-2, numerous research teams use computer algorithms to analyze all coronavirus proteins to predict how different versions of antiviral signaling system recognize the coronavirus proteins, in particular, to get data on the role of HLA haplotype predisposing for the clinical course of the infection, its diagnostics and therapy [6].
HLA alleles predispose for differential susceptibility to viral disease and its clinical course. Genetic variability of the main HLA genes may influence the severity of COVID-19 severity. In particular, understanding these variabilities may be helpful for detection of the persons with a high risk of the disease. The combination of HLA typing with SARS-CoV-2 testing will improve risk assessment in the general population. Following the development of the anti-SARS-CoV-2 vaccine, the persons with high-risk HLA alleles should have priority for vaccination.
When binding the virus or its fragment, HLA antigen exposes it on the cell surface, thus tagging the cell as an infected one, promoting its killing by specific immune cells. Generally, the more viral peptides could be recognized by the HLA system, the more pronounced is the immune response. The results of computer modeling predict that some HLA alleles are binding numerous peptides of SARS-CoV-2 proteins whereas others are binding with only a few of them, thus determining the extent and efficiency of an immune response. Therefore, a consensus was reached on the biological significance of HLA differences, i.e., these variations may partially explain the wide variability of differences for the COVID-19 infection severity. The differences for HLA loci seem to be not the only genetic factor influencing COVID-19 severity. However, understanding their actual effects upon the clinical course of COVID-19 may help to reveal the persons with a higher risk for the disease and to develop vaccines against SARS-CoV-2.
Biology and genetic properties of SARS-CoV-2
SARS-CoV-2 belongs to the single-stranded coronavirus family. Its genome encodes a series of structural and non-structural genes. It consists of a single-stranded positive-sense RNA 30 kb long and contains two flanking untranslated regions, and one long open reading frame (ORF) encoding a polyprotein including replicase complex (ORF1ab), and the genes of four structural proteins: spike glycoprotein (S), membrane glycoprotein (M), an envelope protein (E), and nucleocapsid phosphoprotein (N). The ORF1ab sequence encodes 16 non-structural proteins [7]. Two SARS-CoV-2 proteins, ORF-3a and ORF-7a, are the suggested determinants for T cell recognition. Both proteins are important for viral replication and may influence the pathogenesis and dissemination of the disease [8].
Clinical features of COVID-19
COVID-19 in SARS-CoV-2-positive patients is classified as mild, severe, and critically severe disease. The absolute and relative number of CD4+, CD8+ T cells, and B cells is reduced with increased severity of the disease. The levels of pro-inflammatory IL-2, TNF-α, IL-6 cytokines, as well as CRP in blood plasma are increased, whereas activation of dendritic cell and B cells are decreased in severe clinical cases [9].
Lymphopenia is among the typical characteristics of SARS-CoV-2 infection. Lymphocyte migration from blood to lungs may be a reason for lymphocyte deficiency in peripheral blood, due to antigenic stimulation. If CD8+ T cells are unable to eliminate the virus, CD4 T cells will be activated to further enhancement of immune response. Continuous and excessive inflammatory reactions finally cause apoptosis and anergy of the lymphocytes. Hence, the lymphocyte function can be completely different in different stages of infection [9].
Severe complications develop in 20% of the COVID-19 patients, being connected with an uncontrolled systemic hyperinflammatory immune response to SARS-CoV-2 infection, the so-called "cytokine storm". Excessive IL-6 production is a trigger of this life-threatening condition. Suppression of this inflammatory immune response could be considered a target for anti-inflammatory and immune-modulating therapy in severe COVID-19 [10].
The results of computer modeling (in silico experiments) show that there are genetic differences, especially in the human immune system, which may explain the different ability to respond to SARS-CoV-2 infection, differences in symptoms, and severity of COVID-19.
Following infection of human cells by a coronavirus, the organism responds by antiviral signaling. These alarm signals identify the virus and mobilize immune cells, especially, cytotoxic T cells, to destroy the infected cells. To search for distinct alleles of signaling system predisposing for diverse immune responses to SARS-CoV-2, numerous research teams use computer algorithm to analyze all coronavirus proteins, to predict how different versions of antiviral signaling system recognize the coronavirus proteins, in particular, to get data on the role of HLA haplotype predisposing for the clinical course of the infection, its diagnostics and therapy [6].
Biology of an anti-COVID immune response
HLA alleles predispose for differential susceptibility to viral disease and its clinical course. Genetic variability of the main HLA genes may influence COVID-19 severity. In particular, understanding this variability may be helpful for detection of the persons with a high risk of the disease. The combination of HLA typing with SARS-CoV-2 testing will improve risk assessment in the general population. Following the development of an anti-SARS-CoV-2 vaccine, the persons with high-risk HLA alleles should have priority for vaccination.
When binding the virus or its fragment, HLA antigen exposes it on the cell surface, thus tagging the cell as an infected one, promoting its killing by specific immune cells. Generally, the more viral peptides could be recognized by the HLA system, the more pronounced is the immune response. The results of computer modeling predict that some HLA alleles are binding numerous peptides of SARS-CoV-2 proteins whereas others are binding with only a few of them, thus determining the extent and efficiency of an immune response. Therefore, a consensus was reached on the biological significance of HLA differences, i.e., these variations may partially explain the wide variability of differences for the COVID-19 infection severity. The differences for HLA loci seem to be not the only genetic factor influencing COVID-19 severity. However, understanding their actual effects upon the clinical course of COVID-19 may help to reveal the persons with a higher risk for the disease and to develop vaccines against SARS-CoV-2.
Distinct HLA haplotypes are associated with different susceptibility for infections, mainly, due to T cell receptors which recognize the conformational structure of the HLA antigen-binding domain in a complex with corresponding antigenic peptides. Hence, the advantage in the immune response is to express HLA molecules with increased specificity of binding to SARS-CoV-2 viral peptides on the cell surface of antigen-presenting cells (APCs). Identification of class I and II HLA alleles associated with the immune response against SARS-CoV-2 is, therefore, important for the development of diagnostic test-systems and evaluation of the vaccine efficiency [6, 11].
Following viral penetration to the target cell, its antigens presented by HLA molecules are recognized by virus-specific cytotoxic T lymphocytes (CTL). Numerous HLA alleles, e.g., HLA-B*46:01, HLA-B*07:03, HLA-DRB1*12:02, and HLA-C*08:01, (most frequently can be found in Chinese and Indonesian population) are associated with susceptibility to SARS-CoV, whereas HLA-DR*03:01, HLA-C*15:02, and HLA-A*02:01 correlate with protection against SARS-CoV infection and European Population. HLA-II molecules, such as HLA-DRB1*11:01 and HLA-DQB1*02:02, are associated with susceptibility to MERS-CoV infection. These data provide valuable clues for studying the pathogenetic mechanisms of COVID-19. The antigen presentation stimulates humoral and cellular immune response mediated by virus-specific B and T cells, respectively. The number of SARS-CoV-2-infected CD4+ and CD8+ T cells in peripheral blood of patients was sufficiently decreased, while their immune status is excessive activation, as evidenced by high proportions of HLA-DR-positive CD4+Т cells and CD38+ CD8+ T cells [12]. The SARS-CoV-2 epitope screening identified 2013 and 1399 peptide epitopes with high-affinity for HLA class I and class II molecules, respectively. These epitopes are distributed across structural proteins (S, M, E, and N), and non-structural proteins, being able to induce the CD8+ and CD4+ T cell responses. Several regions are enriched in high-affinity epitopes. These data are important for the development of vaccines against SARS-CoV-2 and T cell response monitoring [13].
The signal pathway of HLA-G and its receptor, expressed on the surface of immune cells, participates in viral infection by downregulation of T-, B-, and natural killer (NK) cells. Increased HLA-G expression is a strategy of viral escape from an immune response. The main evasion mechanism is that HLA-G binds to the immuno-inhibitory CTL receptors. Comparison of HLA-G expression and its receptors on peripheral blood lymphocytes between the day of an initial positive result for SARS-CoV-2 and the day of negative result has shown a positive relation between HLA-G expression on В cells and IFN-γ levels, while HLA-G expression in monocytes was negatively related to IL-2 levels. The percentage of HLA-G+ Т cells and expression of immune-inhibiting ILT4 receptor on B cells showed a negative correlation with TNF-α levels, the expression on the monocytes exhibited a positive correlation with IL-6, IL-10, and IFN-γ contents. The HLA-G expression pattern on peripheral immune cells may reflect three phases of the disease, i.e., primary infection, replication, and clearance of SARS-CoV-2. The time course of HLA-G expression on the peripheral immune cells termed as high/low/high dynamics from SARS-CoV-2 positive state to the SARS-CoV-2 negative condition suggests that the SARS-CoV-2 infection state is connected with cytokine regulation of HLA-G expression. Given that HLA-G is an antigen-presenting molecule, suppression of HLA-G expression by the SARS-CoV-2 virus can disrupt virus recognition by CD8 + T cells and maintain immunity evasion [14].
Genotyping of the COVID-19 patients by HLA loci has shown that the frequencies of HLA-C*07:29, C*08:01G, B*15:27, B*40:06, DRB1*04:06 and DPB1*36:01 (all of these alleles can be found mostly in the Asian population) alleles are higher, whereas frequencies of DRB1*12:02 and DPB1*04:01 alleles are lower in COVID-19 patients than in control population. Only the HLA-C*07:29 and B*15:27 frequencies were statistically different, with regard to corrected statistical significance. These data demonstrate some HLA alleles to be associated with COVID-19 [15].
The unique pattern of altered immunity regulation in SARS-CoV-2 patients is characterized, firstly, by increased circulating pro-inflammatory cytokine levels (especially, IL-6), secondly, by the functional lymphoid defect connected with IL-6-mediated decrease in HLA-DR expression. As mentioned above, the HLA class II, especially, HLA-DR, are constitutively expressed, mainly, by antigen-presenting cells (APC), В cells, and T cell subpopulations. IFN-γ is able to induce HLA-DR gene, as well as proinflammatory TNF-α, IL-1β и IL-6 cytokine expression. HLA class II activation enhances the HLA-restricted antigen presentation and adaptive immune response [5].
All the patients with SARS-CoV-2-associated pneumonia who develop severe respiratory failure, exhibit excessive inflammatory responses with immune dysregulation caused by IL-6, or macrophage activation syndrome (macrophage activation syndrome, MAS) caused by IL-1β, or very low HLA-DR expression, accompanied by a sharp decrease in CD4+T lymphocytes and NK. Blood plasma from the SARS-CoV-2 patients is shown to inhibit HLA-DR expression which could be partially restored by tocilizumab, an IL-6 blocking antibody. Tocilizumab treatment is accompanied by an increase in circulating lymphocyte numbers. Hence, the unique pattern of immune dysregulation in severe COVID-19 is characterized by the IL-6-mediated suppression of HLA-DR expression and lymphopenia due to permanently enhanced cytokine production and excessive inflammation [5].
In COVID-19, the patients show a decreased HLA-DR expression on the CD14+ monocytes, whereas blood ferritin levels sufficiently exceed normal values, a pattern revealed only in SARS-CoV-2 patients, with increased haemophagocytosis being of high diagnostic value. In the patients with bacterial pneumonia, HLA-DR on the CD14+ monocytes is below normal values, being, however, close to normal levels in SARS-CoV-2-associated pneumonia. In cases of its sudden drop, severe respiratory failure develops. The circulating IFN-γ concentrations were very low in all patients with SARS-CoV-2 infection. On contrary, the IL-6 and CRP levels were sufficiently higher in the patients with disturbed immunity than in cases with intermediate immune activation. IL-6 inhibits HLA-DR expression in COVID-19 patients. Accordingly, a negative correlation was found between serum level of IL-6 and absolute counts of HLA-DR on the CD14+ monocytes, as well as between absolute lymphocyte counts and HLA-DR contents on CD14+ monocytes in COVID-19 patients [5]. The role of IL-6 as a factor of HLA-DR decrease on the CD14+ monocytes is confirmed by an increase in HLA-DR+ circulating cells upon recovery from COVID-19 [16]. Tocilizumab can partially restore HLA-DR expression on the monocytes, thus increasing the number of circulating lymphocytes [17].
Development of vaccines against SARS-CoV-2
Aiming for the development of epitope-based subunit vaccines against SARS-CoV-2, one perform studies using reverse vaccinology and immune informatics. The tools of bioinformatics are used in reverse vaccinology to analyze the genome and proteome of the pathogen, identification, and analysis of neoantigens (Fig. 1). This approach allows choosing the viral antigenic segments that should be addressed when developing vaccines, being a more efficient, simple, time- and cost-effective method for the vaccine design.

Figure 1. Step-by-step strategies of reverse vaccinology approach of vaccine development [23]
During viral infection, the viral proteins are processed into small peptides within the proteasomes of infected cells. As mentioned above, the viral peptides are presented by HLA molecules at the surface of infected cells and recognized by T cells. The epitopes potentially recognizable by the T cells could belong to any viral structural or non-structural proteins.
Prediction of SARS-CoV-2 epitopes and appropriate immune responses is quite important to design and evaluate the immunogenicity of a vaccine against SARS-CoV-2. Prediction of antigenicity for the candidate vaccines presumes determination of numeric index for the ability of the vaccine to bind the В- and Т cell receptors and augment the immune response. To construct a vaccine, only highly antigenic sequences are chosen. At present, however, only scarce information exists on what SARS-CoV-2 sequences may induce a strong immune response.
The virus antigen-activated CD4+ helper T cells boost B cells to produce a lot of specific antibodies. Macrophages and CD8+ CTLs are also activated by the T-helpers, thus causing the final destruction of the target antigen. T cell epitopes interact with HLA class I and II alleles. This approach is used in the modern vaccine design since it provides a benefit, concerning time and cost expenditures over classical "test and error" strategy of "wet" labs (unlike dry labs, i.e., in silico experiments using mathematic or computer analysis). T cell epitopes were identified which can elicit a stable immune response against SARS-CoV-2 in the general human population. However, the ability of these epitopes to serve as vaccine candidates should be analyzed and validated at the molecular biology laboratories. Prediction of B cell epitopes seems less reliable than T cell epitopes since the B epitopes do not elicit a strong humoral response. For this reason, only T cell epitopes able to generate longitudinal CD4+ and CD8+T cell responses were analyzed in most in silico studies to choose epitopes for subsequent testing traditionally ("wet" laboratory) [8].
К. Kiyotani et al. [13] have performed immunoinformatic epitope prediction of S, E, N, M, and ORFs proteins from SARS-CoV-2 reference sample. They selected The HLA-A, HLA-B и HLA-C alleles presented in more than 5% frequencies in a Japanese population. To predict HLA class II epitopes, the HLA-DPA1-DPB1, HLA-DRB1 alleles, and HLA-DQA1-DQB1 haplotypes were used which encounter in Japan at frequencies of 5 to 38%. The work involved a total of 6421 sequences of SARS-CoV-2 isolated in various regions including 587 sequences from Asia, 1918 from North America, 3190 from Europe, and 726 from the Pacific Region deposited in the Global Initiative on Sharing Avian Influenza Database.
The next step included a screening of high-affinity peptide epitopes from the SARS-CoV-2 proteins which could be presented by molecules HLA class I and II. The two T cell epitopes in ORF1ab protein, ORF1ab2168-2176, and ORF1ab4089-4098, that were predicted to have a strong affinity to HLA-A*24:02, HLA-A*02:01 and HLA-A*02:06, have shown the broadest coverage of the Japanese population (83.8%). ORF1ab2168-2176, a predicted epitope binding HLA-C molecules (C*01:02, C*08:01, C*12: 02, and C*14:02), is present in 76.5%; the S268-277 and S448-457 epitopes in S protein were detected in 70% of Japanee individuals. The complexes of HLA with these peptides are useful for monitoring CD8+Т cell responses in patients and symptom-free infected individuals. No mutations were found in the sequences of the above epitopes. Therefore, the authors believe that these potential candidate epitopes can contribute to the development of rationally designed peptide vaccines based on epitopes against SARS-CoV-2 [13].
A complex analysis using in silico computer modeling of binding affinity between the peptides-HLA class I for 145 HLA-A, -B, and -C genotypes and whole proteome of the SARS-CoV-2, as well as cross-protective immunity resulting from preliminary action of the 4 widespread human coronaviruses, has shown that the HLA-B*46:01 antigen binds a minimal number of predicted SARS-CoV-2 peptides. This fact allows suggesting that the individuals carrying this allele could be especially susceptible for COVID-19, as it was previously shown with SARS-CoV, thus corresponding to clinical data which associate this allele with severe disease. And, vice versa, HLA-B*15:03 allele has demonstrated maximal ability to present highly conserved SARS-CoV-2 peptide sequences [18]. Hence, distinct HLA genotypes may differentially induce T cell antiviral response, thus influencing clinical course of infection and its transmission.
For 32257 unique 8-12-mer SARS-CoV-2 peptides, which are predicted to pass through the proteasomal processing pathway, a SARS-CoV-2-specific distribution was shown for presentation by the HLA class I molecules. The presumed ability of SARS-CoV-2 peptides for antigenic presentation is unrelated with the population frequency of HLA alleles. When reporting the global frequency cards for the 145 studied HLA alleles, the authors highlight the global distribution of the three best (A*02:02, B*15:03, C*12:03), and three worst (A*25:01, B*46:01, C*01:02) HLA-presenting alleles, by their ability to generate the SARS-CoV-2 epitope repertoire, in order to support T cell immune response. These differences remain significant at the haplotype level. SARS-CoV-2 evolution in the population may modify the repertoire of presented viral epitopes or otherwise modulate HLA-independent epitopes. However, the authors recommend integrating HLA-typing into clinical trials, and combining it with COVID-19 testing to apply it as a potential predictor(s) of the disease severity among the population, and, probably, for adaptation of future vaccination strategies for genotypic risk groups. This approach could be used to control a broad spectrum of other viruses [18].
Aiming for epitope-based development of a vaccine against SARS-CoV-2 employing analysis of viral proteome using immunoinformatic tools, А. Joshi et al. have chosen antigenic non-toxic non-allergenic peptides from non-allergenic proteins based on their interactions with HLA allelic sets. Among the identified T cell epitopes, the ITLCFTLKR epitope is a candidate for the anti-SARS-CoV-2 vaccine, exhibiting better binding indexes in the epitope-HLA complexes, as well as acceptable stability, toxicity, and population coverage. This epitope should now undergo laboratory verification [8].
The spike protein (S protein) is most commonly analyzed to detect immunogenic epitopes which are highly affine for the cellular ACE2 receptor when penetrating human cells. On this basis, S protein is considered a potential target for the coronavirus vaccine. Using immunoinformatics and computer modeling tools, М. Bhattacharya et al. [19] have revealed 34 linear B cell epitopes for S protein of the SARS-CoV-2 and identified among them 13 HLA I-binding epitopes and 3 HLA II-binding epitopes with antigenic features of SARS-CoV-2 S protein, which are recognized by Т cell receptor (TLR-5, Toll-like receptor-5). The antigenic epitopes are converted into the single vaccine component using a linker peptide that promoted stability and assembly of the modeled and validated vaccine component. Molecular docking between the vaccine component and TLR5 caused spontaneous reactivity in the receptor-ligand complex which activates immune cascades for the destruction of viral antigens. Hence, the selected antigenic SARS-CoV-2 epitopes are good candidates for the design of the immunogenic multi-epitope peptide vaccine against SARS-CoV-2.
A similar immunoinformatic approach was used by V.Baruah and S.Bose aiming to reveal significant epitopes in the S protein of SARS-CoV-2. The authors identified five CTL-specific epitopes (YLQPRTFLL, GVYFASTEK, EPVLKGVKL, VVNQNAQAL, WTAGAAAYY), that were highly affine for appropriate class I HLA alleles (A*02:01, A*03:01, B*07:02, B*07:02, HLA‐B*15:01). Upon modeling of molecular dynamics, it was shown that these epitopes bind the peptide-binding groove of the HLA-I molecule used for antigen presentation through multiple contacts, showing their potential for immune response generation. Some of these epitopes are candidates for the design of anti-SARS-CoV-2 vaccines. In addition to activating CTLs, the successful immunogens should generate a persistent humoral immunity. This study has revealed three such B-cell epitopes unique to SARS-CoV-2 [20].
D. Santoni et al. [21] used a bioinformatic methodology based on the selection of viral peptides which are at a distance of more than 3 mutational steps from humans, presuming that the probability of binding to HLA antigens increases with longer evolutionary distance from humans. In other words, the researchers seek those viral peptides which are absent in humans (nullomeres). Identification of the most human-distant peptides is quite important to minimize the autoimmunity risks. Of the 27 nullomeres, 25 were common to all the known SARS-CoV-2 strains. These peptides were called the third-order nullomeres that belong to the most distant class from humans were subjected to three additional selection stages, to choose those with the highest probability of exposure on the cell surface, i.e., (1) being the product of proteasomal cleavage, 2) being transferred to the cell surface, and (3) strongly binds to HLA molecules. A set of nine peptides was identified for subsequent experimental studies. The in silico selected SARS-CoV-2 peptides represent potential targets for the immune system, that should be then tested experimentally to confirm their immunogenicity. According to in silico prediction, the YVMHANYIF peptide is strongly binding to 27 various HLA antigens, the FLCWHTNCY and YIKWPWYIW peptides may bind 11 and 10 different HLA molecules, respectively, YYHKNNKSW peptide may interact with 8 HLA allelic variants.
The concept of multi-epitopic vaccine addresses identification and assembly of В- and Т cell-specific epitopes into an integral immunogen able to induce effective response at both immunity arms. The peptides and epitopes proved to be preferred candidates for vaccine development, due to simpler technology production, chemical stability, and absence of infectious potential. The multi-epitopic vaccines may contain epitopes for B cells, CD8+ CTLs, and CD4+ helper T cells.
Using immunobioinformatic approach, the five domains rich in common T- and B-cell epitopes were selected and connected by linker chains, in order to generate a more diverse and stable immune response. The resulting multi-epitope candidate vaccine NOM (nucleocapsid, ORF3a, membrane protein) represents itself a recombinant multi-epitope protein constructed and validated utilizing bioinformatics tools which include, as the name suggests, antigenic immune epitopes from three SARS-CoV-2 viral proteins. The optimized NOM protein structure is adapted to interactions with specific immune receptors. Upon modeling the molecular dynamics, maximal stability was shown for the NOM binding with TLR4 and HLA-A*11:01 (i.e., NOM–TLR4 and NOM–HLA-A*11:01 models). The in silico testing has shown that interaction of the chimeric protein with TLR4 and HLA-A*11:01 receptors induces both humoral and cellular immune responses, due to the composite nature of the construct including В- and Т cell epitopes [22].
С.Н. Lee et al. have reported on in silico identification of a comprehensive list of SARS-CoV-2 immunogenic peptides which could be used as target molecules for vaccine development. Among them 48 viral peptides showing a high degree of similarity with immunogenic peptides deposited in the Immune Epitope Database (IEDB), and 63 more new peptides with high immunogenic potential, which could be recognized by T-cell receptors. Searching for the immunogenic SARS-CoV-2 peptides, the authors focused on the haplotypes common in Europe and China. The 28 most promising SARS-CoV-2 peptides are shown to bind different HLA class I and HLA class II alleles (HLA-A*01:01, DRB1*07:01, DRB1*04:01), and could serve as a target for, respectively, CD8+ and CD4+ T cells. Based on HLА presentation and immunogenicity prediction, the five peptides were selected for subsequent experimental validation (VQMAPISAM, AMYTPHTVL, TLDSKTQSL, KVDGVVQQL, KVDGVDVEL), which potentially would specifically bind four different HLA variants (A*01:01, HLA-B*07:02, HLA-B*40:01, and, with the highest affinity, HLA-A*02:01 [23].
To perform prediction and cluster analysis of probable HLA alleles, which could interact with SARS-CoV-2 epitopes, В. Sarkar et al. [24] have used the HLAcluster 2.0 online tool (http://www.cbs.dtu.dk/services/HLAcluster/). After predicting the three-dimensional structure of highly antigenic, non-allergenic, and nontoxic Т- and B-cell epitopes, they compared the ability of these epitopes to bind HLA molecules. The HLA-A*11:01 allele was used as a receptor for docking with HLA-I epitopes, whereas HLA-DRB1*04:01 was tested with HLA-II epitopes. The best results were obtained for viral N-protein, the QLESKMSGK peptide for docking with HLA-I and LIRQGTDYKHWP peptide with HLA-II epitopes. The GVLTESNKK peptide from S-protein was the best for HLA-I epitopes, whereas the TSNFRVQPTESI peptide of the viral surface glycoprotein showed the best binding properties for HLA class II.
As based on successful modeling of peptide/protein docking, three anti-SARS-CoV-2 vaccine variants were proposed, using the selected epitopes, CV-1, CV-2 и CV-3. The CV-1 proved to be the best, by the molecular docking criteria. CV-2 showed maximal binding efficiency with HLA-DRB3*02:02 and HLA-DRB1*03:01. The CV-3 vaccine has better binding energy parameters with most HLA alleles (DRB5*01:01, DRB1*01:01, and DRB3*01:01). Since CV-1 showed the best results for protein/protein docking, it was recognized as the best construct of those three. Modeling of molecular dynamics and in vitro adaptation studies have been performed with only this vaccinal variant. The proposed vaccine constructs could be used for SARS-CoV-2 vaccination if the validation trials will yield satisfactory results [24].

Figure 2. Scheme of design of a multi-epitope subunit candidate vaccine using epitopes of B-cells, CTLs, and T-helper cells [25]
The unique construct of a recombinant multi-peptide subunit vaccine against COVID-19 contains 18 epitopes for CTLs, 6 epitopes for T-helper cells, and 9 epitopes for В cells from three SARS-CoV-2 proteins which participate in recognition of cellular receptors and viral penetration into the target cells (Fig. 2). To enhance immunogenicity, the construct is supplied by a sequence from human β-defensin (TLR3 agonist) which serves as a binding adjuvant with epitopes and linkers. Computational studies suggest that the vaccine is not allergenic, stable, and can elicit humoral and cellular immune responses. The synthesis and experimental evaluation of this vaccine are pending to determine its immunogenic activity. The authors hope that this vaccine will be synthesized and used in public healthcare [25].
SARS-CoV-2 possesses self-amplifying RNA in the cytosol, which allows the development of an RNA-based vaccine. These vaccines use the mRNA sequence of recombinant target protein, rather than the sequence of the target antibody. The vaccinal mRNA is then transferred by lipid nanoparticles to the cytoplasm where the immunogenic protein is translated. After release from the cell, this protein is quickly captured and processed by APCs, followed by HLA-mediated presentation on the surface of the antigen-presenting cell, followed by the activation of B and T cells and, accordingly, the antigen-specific humoral and cytotoxic responses.
The vaccines based on the cytoplasmic expression of chimeric viral mRNA are potentially superior to protein-based vaccines: they are more safe, efficient, easier to produce, and they can block chromosomal integration of the virus. The manufacturing of RNA-based vaccines is the most promising trend in vaccinology, due to capacity of wide-scale production, thus spating time during pandemics. Following injection, the vaccine RNA could be processed by immune cells and produce specific protein directly through translation followed by activation of other immune cells and antibody synthesis (Fig. 3) [7].

Figure 3. Schematic diagram of the mRNA-based vaccine targeted to the S protein of SARS-CoV-2
The mRNA-based vaccine targeted to the S protein of SARS-CoV-2 works by active immunization. This technique uses mRNA of the S protein, coated with lipid nanoparticles for effective delivery. Once injected into the muscle, the myocytes take up the lipid nanoparticle and then release the mRNAs into the cytoplasm for translation into the S proteins. These endogenously synthesized S proteins will be secreted to activate both humoral and cellular immune responses. S protein – spike protein; IM – intramuscular, LNP – lipid nanoparticle; DC – dendritic cell; MHC – major histocompatibility complex; Ag – antigen [7].
The lipid nanoparticle (LNP)-encapsulated mRNA-1273 based vaccine, produced by Moderna Inc. (USA) encodes SARS-CoV-2 S-protein. Upon the formation of sufficient antibody titers to S-protein, a double therapeutic effect may occur, i.e., the host immune system could eliminate the antigen-antibody complex, thus promoting virus clearance and alleviating its contagiosity [7]. Preliminary results of clinical trials have shown that the mRNA-1273 vaccine has induced high levels of both virus-binding and neutralizing antibodies, as well as strong cytokine response with participation of CD4 helper T cells, type 1 [26] and that the mRNA-1273 immunogenicity retained for, at least, 3 months [27].
Prefusion-stabilized protein immunogens that preserve neutralization-sensitive epitopes are an effective vaccine strategy for enveloped viruses. Structural studies have led to the search for mutations that stabilize Betacoronavirus spike proteins in the prefusion state, improving their expression and increasing immunogenicity. This principle has been applied to design mRNA-1273, an mRNA vaccine that encodes a SARS-CoV-2 spike protein that is stabilized in the prefusion conformation. Here we show that mRNA-1273 induces potent neutralizing antibody responses to both wild-type (D614) and D614G mutant2 SARS-CoV-2 as well as CD8+ T cell responses, and protects against SARS-CoV-2 infection in the lungs and noses of mice without evidence of immunopathology. mRNA-1273 is currently in a phase III trial to evaluate its efficacy.
K.S. Corbett et al. [28] identified 2 proline substitutions (2P) at positions 986 and 987 of S protein that effectively stabilized S proteins in the pre-fusion conformation. The authors performed structural analysis and developed serological tests in silico, without additional experimental verification. Similar to other prefusion-stabilized fusion proteins, S (2P) protein SARS-CoV-2 was more immunogenic at lower doses than wild-type S protein [28]. The 2P mutation has similar effects on the stability of S proteins and from other beta coronaviruses, suggesting a generalizable approach for designing S protein antigens for vaccination. Such generalizability is fundamental to pandemic preparedness [29].
The production of mRNA encoding the S-protein (2P) SARS-CoV-2 (mRNA-1273) was started in parallel with the preclinical evaluation. This led to the first phase I human clinical trial, which began on March 16, 2020. Thus, concepts based on new technologies, such as synthetic vaccinology, can facilitate and accelerate a vaccine development program based on pathogen sequences.
Protein glycosylation is a very common biological process, a form of posttransplant protein modification and regulation of protein location and functioning. Glycosylation of viral structural proteins is closely related to their replication and cell invasion, thus helping to escape the host immune response. Unusually high glycosylation degree of SARS-CoV-2 presumes its high mutation rates, thus sufficiently complicating the vaccine development. However, the mRNA-based vaccine technologies and targeting for S-protein may result in the production of antibodies to S-protein only, irrespectively of its glycosylation status. Taking into account useful features of mRNA-based vaccines, such as lack of integration into the genome, lack of induction of autoantibodies, the feasibility to produce mRNA vaccines in large quantities, and their high purity, mRNA-based vaccines are a promising choice for combating COVID-19 [7].
Conclusion
The development of vaccines is a long-term and costly process with high failure rates which requires several years for the production of a commercial product. Optimization of the manufacturing process allows sooner testing of the subunit vaccines and their release to the market. Protein-based vaccines include only antigenic parts of the pathogen causing an immediate immune response. Moreover, the vaccine does not contain a live pathogen. Hence, it could be used in immunocompromised patients. Computer-assisted studies show that the multi-epitope subunit vaccine is safe and effective against SARS-CoV-2 infection.
Common vaccines, attenuated, or inactivated, are not always able to provide immunity for the target antigen. Besides, the generally accepted approach to vaccine development causes multiple concerns for safety during pre-clinical and clinical trials. The subunit vaccines designed using in silico computer modeling may overcome these difficulties. Numerous study groups perform studies and publish their results in this field. The independent works carried out with different computational techniques provide higher reliability and reproducibility level of the results.
Individual genetic variations of HLA allelic variants may explain diverse immune responses to the virus infection and variable clinical outcomes in the population. Moreover, a detailed analysis of the common HLA alleles will help to develop more effective anti-COVID-19 vaccines, since searching for optimal vaccine constructs strongly depends on the detection of significant epitopes in spike glycoprotein, and other SARS-CoV-2 proteins recognized by the antigen-presenting cells, cytotoxic T-cells, and B cells using bioinformatics methodologies. This review presents a profile of in silico predicted SARS-CoV-2 immunogenic peptides for functional validation and vaccine development. Computer-assisted prediction plays an important role in rapid and cost-effective decision making for the prevention of infection spreading, and, finally, overcoming pandemics. Most attempts in the field of SARS-CoV-2 prevention and treatment are focused on the viral S protein, the main inducer of virus-neutralizing antibodies. The article concerns current achievements in the development of modern SARS-CoV-2 vaccines which, in sum, may stop this new viral infection.
Conflict of Interest
None declared.
References
- Glotov OS, Romanova OV, Eismont YA, Sarana AM, Scherbak SG, Kuzmich EV, Alyansky AL, Ivanova NE, Teplyashina VV, Serov YA, Zubarovskaya LS, Afanasyev BV. Comparative analysis of HGS and Sanger sequencing methods for HLA typing at a Russian university clinic. Cell Ther Transplant. 2018; 7(2): 72-82. doi: 10.18620/ctt-1866-8836-2018-7-4-72-82.
- Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microb Infect. 2020;9(1):382-385. doi: 10.1080/22221751.2020.1729069.
- Pathological anatomy of lungs in COVID-19. Histology atlas. Ryasan, 2020. 52 p (In Russian).
- Arabi YM, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020;46(5):833-836. doi: 10.1007/s00134-020-05955-1.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020; 27(6):992-1000.e3. doi: 10.1016/j.chom.2020.04.009.
- Forouzesh M, Rahimi A, Valizadeh R, Dadashzadeh N, Mirzazadeh A. Clinical display, diagnostics and genetic implication of novel Coronavirus (COVID-19) epidemic. Eur Rev Med Pharmacol Sci. 2020;24(8):4607-4615. doi: 10.26355/eurrev_202004_21047.
- Wang F, Kream RM, Stefano GB. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med Sci Monit. 2020 May 5;26:e924700. doi: 10.12659/MSM.924700.
- Joshi A, Joshi BC, Mannan MA, Kaushik V. Epitope based vaccine prediction for SARS-COV-2 by deploying immuno-informatics approach. Inform Med Unlocked. 2020 Apr 29:100338. doi: 10.1016/j.imu.2020.100338.
- Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, Liu W, Zhu Y, Lin Q, Mao L et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020 May 21;5(10):e137799. doi: 10.1172/jci.insight.137799.
- Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, Mekinian A, Anunciacion-Llunell A, Miró-Mur F. et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev. 2020 Jul;19(7):102569. doi: 10.1016/j.autrev.2020.102569.
- Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451-1454. doi: 10.1038/s41418-020-0530-3.
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020; 10(2):102-108. doi: 10.1016/j.jpha.2020.03.001.
- Kiyotani K, Toyoshima Y, Nemoto K, Nakamura Y. Bioinformatic prediction of potential T cell epitopes for SARS-Cov-2. J Hum Genet. 2020 Jul;65(7):569-575. doi: 10.1038/s10038-020-0771-5.
- Zhang S, Gan J, Chen BG, Zheng D, Zhang JG, Lin RH, Zhou YP, Yang WY, Lin A, Yan WH. Dynamics of peripheral immune cells and their HLA-G and receptor expressions in a patient suffering from critical COVID-19 pneumonia to convalescence. Clin Transl Immunology. 2020 May 10;9(5):e1128. doi: 10.1002/cti2.1128.
- Wang W, Zhang W, Zhang J, He J, Zhu F. Distribution of HLA allele frequencies in 82 chinese individuals with Coronavirus disease-2019. HLA. 2020; 96(2):194-196. doi: 10.1111/tan.13941.
- Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, Jia X, Nicholson S, Catton M, Cowie B et al. Breadth of concomitant immune responses prior to patient recovery: a case-report of non-severe COVID-10. Nat. Med. 2020; 26(4):453-455. doi: 10.1038/s41591-020-0819-2.
- Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, Walzer T, François B, Sève P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020 Jul;19(7):102567. doi: 10.1016/j.autrev.2020.102567.
- Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, Nellore A, Thompson RF. Human leukocyte antigen susceptibility map for SARS-CoV-2. J Virol. 2020 Jun 16;94(13):e00510-e00520. doi: 10.1128/JVI.00510-20.
- Bhattacharya M, Sharma AR, Patra P, Ghosh P, Sharma G, Patra BC, Lee SS, Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J Med Virol. 2020;92(6):618-631. doi: 10.1002/jmv.25736.
- Baruah V, Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of SARS-CoV-2. J Med Virol. 2020;92(5):495-500. doi: 10.1002/jmv.25698.
- Santoni D, Vergni D. In the search of potential epitopes for Wuhan seafood market pneumonia virus using high order nullomers. J Immunol Methods. 2020;481-482:112787. doi: 10.1016/j.jim.2020.112787.
- Enayatkhani M, Hasaniazad M, Faezi S, Guklani H, Davoodian P, Ahmadi N, Einakian MA, Karmostaji A, Ahmadi K. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: an in silico study. J Biomol Struct Dyn. 2020 May 2:1-16. doi: 10.1080/07391102.2020.1756411.
- Lee CH, Pinho MP, Buckley PR, Woodhouse IB, Ogg G, Simmons A, Napolitani G, Koohy H. Potential CD8+ T Cell Cross-Reactivity Against SARS-CoV-2 Conferred by Other Coronavirus Strains. Front Immunol. 2020 Nov 5;11:579480. doi: 10.3389/fimmu.2020.579480.
- Sarkar B, Ullah A, Johora FT, Taniya MA, Araf Y. Immunoinformatics-guided designing of epitope-based subunit vaccine against the SARS Coronavirus-2 (SARS-CoV-2). Immunobiology. 2020 May 11;225(3):151955. doi: 10.1016/j.imbio.2020.151955.
- Kalita P, Padhi AK, Zhang KYJ, Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020 May 4;145:104236. doi: 10.1016/j.micpath.2020.104236.
- Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020; 383(25):2427-2438. doi: 10.1056/NEJMoa2028436.
- Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021; 384(1):80-82. doi: 10.1056/NEJMc2032195.
- Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567-571. doi: 10.1038/s41586-020-2622-0.
- Graham BS, Corbett KS. Prototype pathogen approach for pandemic preparedness: world on fire. J. Clin. Invest. J Clin Invest. 2020;130(7):3348-3349. doi: 10.1172/JCI139601.
" ["~DETAIL_TEXT"]=> string(53987) "
Introduction
Human leukocyte antigens (HLA) on the cell surface play a pivotal role in recognizing inter-and extracellular proteins as "self" or "non-self". A unique set of HLA alleles keeps our immunological identity. The immune response of the host organism is based on the detection of "non-self" protein epitopes. E.g., in viral infections, Class I HLA molecules recognize viral peptides produced inside the infected cells, causing the recruitment of CD8+ cytotoxic populations and destruction of the target cells.
Class II antigens on the antigen-presenting immune cells mostly determine the risk of graft rejection after allogeneic transplantation of organs or bone marrow, thus requiring maximal HLA-similarity between recipient and donor. Since the 1970’s, the high-throughput and precise techniques of HLA-typing were introduced for allogeneic transplantation of hematopoietic cells (HSCT). More than 30.000 HLA variations of HLA proteins are registered and DNA-sequenced by the classical Sanger technique or NGS approach which is implemented for HLA typing worldwide [1]. The resulting databases contain quite extensive data on HLA polymorphic regions and provide excellent opportunities for in silico modeling of best matches between HLA and any foreign proteins, including fragments of viral antigens.
Our review aimed to discuss common bioinformatic approaches to the search of target HLA epitopes and immunity-related proteins when designing novel antiviral vaccines, in particular, against SARS-CoV-2. This new beta-coronavirus of the Coronaviridae family, Betacoronavirus genus was identified as a pathogen causing severe acute respiratory infection. On February 11, 2020, The World Health Organization has assigned the official name COVID-19 ("CОronaVIrus Disease 2019"), whereas an International Committee for Taxonomy of Viruses, ICTV) Committee for Taxonomy of Viruses, ICTV) designates the causative agent of this infection as SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2). At sooner time, WHO has claimed SARS-CoV-2 pandemia.
Clinical course in SARS-CoV-2 infection is quite variable. i.e., from mild or symptom-free clinical forms to severe or extremely severe COVID-19 with high mortality levels. Acute respiratory distress syndrome was registered in some patients, and 11% of the patients deceased with multi-organ failure in a short time [2]. Most patients with COVID-19 manifest with fever (90%); cough (80%); apnea (55%); loss of smell and taste (50%); myalgia and fatigability (44%); chest pressure and/or pain (20%), as well as headache (8%), hemoptysis (5%), diarrhea, nausea (3%) [3]. SARS-CoV-2 exhibits pronounced lung tropism causing severe respiratory failure in some patients with COVID-19-associated pneumonia thus requiring invasive mechanical ventilation with a mortality risk of up to 60% [4]. The specific clinical course of COVID-19 infection suggests a unique immune dysfunction with pronounced lymphopenia, excessive IL-6 production, and enhanced blood clotting [5].
The results of computer modeling (in silico experiments) show that there are genetic differences, especially in the human immune system, which may explain the different ability to respond to SARS-CoV-2 infection, differences in symptoms, and severity of COVID-19.
Following infection of human cells by a coronavirus, the organism responds by antiviral signaling. These alarms identify the virus and mobilize the immune system for sending cytotoxic T cells to destroy the infected cells. To find out if different alleles of signaling system may explain the diversity of immune responses to SARS-CoV-2, numerous research teams use computer algorithms to analyze all coronavirus proteins to predict how different versions of antiviral signaling system recognize the coronavirus proteins, in particular, to get data on the role of HLA haplotype predisposing for the clinical course of the infection, its diagnostics and therapy [6].
HLA alleles predispose for differential susceptibility to viral disease and its clinical course. Genetic variability of the main HLA genes may influence the severity of COVID-19 severity. In particular, understanding these variabilities may be helpful for detection of the persons with a high risk of the disease. The combination of HLA typing with SARS-CoV-2 testing will improve risk assessment in the general population. Following the development of the anti-SARS-CoV-2 vaccine, the persons with high-risk HLA alleles should have priority for vaccination.
When binding the virus or its fragment, HLA antigen exposes it on the cell surface, thus tagging the cell as an infected one, promoting its killing by specific immune cells. Generally, the more viral peptides could be recognized by the HLA system, the more pronounced is the immune response. The results of computer modeling predict that some HLA alleles are binding numerous peptides of SARS-CoV-2 proteins whereas others are binding with only a few of them, thus determining the extent and efficiency of an immune response. Therefore, a consensus was reached on the biological significance of HLA differences, i.e., these variations may partially explain the wide variability of differences for the COVID-19 infection severity. The differences for HLA loci seem to be not the only genetic factor influencing COVID-19 severity. However, understanding their actual effects upon the clinical course of COVID-19 may help to reveal the persons with a higher risk for the disease and to develop vaccines against SARS-CoV-2.
Biology and genetic properties of SARS-CoV-2
SARS-CoV-2 belongs to the single-stranded coronavirus family. Its genome encodes a series of structural and non-structural genes. It consists of a single-stranded positive-sense RNA 30 kb long and contains two flanking untranslated regions, and one long open reading frame (ORF) encoding a polyprotein including replicase complex (ORF1ab), and the genes of four structural proteins: spike glycoprotein (S), membrane glycoprotein (M), an envelope protein (E), and nucleocapsid phosphoprotein (N). The ORF1ab sequence encodes 16 non-structural proteins [7]. Two SARS-CoV-2 proteins, ORF-3a and ORF-7a, are the suggested determinants for T cell recognition. Both proteins are important for viral replication and may influence the pathogenesis and dissemination of the disease [8].
Clinical features of COVID-19
COVID-19 in SARS-CoV-2-positive patients is classified as mild, severe, and critically severe disease. The absolute and relative number of CD4+, CD8+ T cells, and B cells is reduced with increased severity of the disease. The levels of pro-inflammatory IL-2, TNF-α, IL-6 cytokines, as well as CRP in blood plasma are increased, whereas activation of dendritic cell and B cells are decreased in severe clinical cases [9].
Lymphopenia is among the typical characteristics of SARS-CoV-2 infection. Lymphocyte migration from blood to lungs may be a reason for lymphocyte deficiency in peripheral blood, due to antigenic stimulation. If CD8+ T cells are unable to eliminate the virus, CD4 T cells will be activated to further enhancement of immune response. Continuous and excessive inflammatory reactions finally cause apoptosis and anergy of the lymphocytes. Hence, the lymphocyte function can be completely different in different stages of infection [9].
Severe complications develop in 20% of the COVID-19 patients, being connected with an uncontrolled systemic hyperinflammatory immune response to SARS-CoV-2 infection, the so-called "cytokine storm". Excessive IL-6 production is a trigger of this life-threatening condition. Suppression of this inflammatory immune response could be considered a target for anti-inflammatory and immune-modulating therapy in severe COVID-19 [10].
The results of computer modeling (in silico experiments) show that there are genetic differences, especially in the human immune system, which may explain the different ability to respond to SARS-CoV-2 infection, differences in symptoms, and severity of COVID-19.
Following infection of human cells by a coronavirus, the organism responds by antiviral signaling. These alarm signals identify the virus and mobilize immune cells, especially, cytotoxic T cells, to destroy the infected cells. To search for distinct alleles of signaling system predisposing for diverse immune responses to SARS-CoV-2, numerous research teams use computer algorithm to analyze all coronavirus proteins, to predict how different versions of antiviral signaling system recognize the coronavirus proteins, in particular, to get data on the role of HLA haplotype predisposing for the clinical course of the infection, its diagnostics and therapy [6].
Biology of an anti-COVID immune response
HLA alleles predispose for differential susceptibility to viral disease and its clinical course. Genetic variability of the main HLA genes may influence COVID-19 severity. In particular, understanding this variability may be helpful for detection of the persons with a high risk of the disease. The combination of HLA typing with SARS-CoV-2 testing will improve risk assessment in the general population. Following the development of an anti-SARS-CoV-2 vaccine, the persons with high-risk HLA alleles should have priority for vaccination.
When binding the virus or its fragment, HLA antigen exposes it on the cell surface, thus tagging the cell as an infected one, promoting its killing by specific immune cells. Generally, the more viral peptides could be recognized by the HLA system, the more pronounced is the immune response. The results of computer modeling predict that some HLA alleles are binding numerous peptides of SARS-CoV-2 proteins whereas others are binding with only a few of them, thus determining the extent and efficiency of an immune response. Therefore, a consensus was reached on the biological significance of HLA differences, i.e., these variations may partially explain the wide variability of differences for the COVID-19 infection severity. The differences for HLA loci seem to be not the only genetic factor influencing COVID-19 severity. However, understanding their actual effects upon the clinical course of COVID-19 may help to reveal the persons with a higher risk for the disease and to develop vaccines against SARS-CoV-2.
Distinct HLA haplotypes are associated with different susceptibility for infections, mainly, due to T cell receptors which recognize the conformational structure of the HLA antigen-binding domain in a complex with corresponding antigenic peptides. Hence, the advantage in the immune response is to express HLA molecules with increased specificity of binding to SARS-CoV-2 viral peptides on the cell surface of antigen-presenting cells (APCs). Identification of class I and II HLA alleles associated with the immune response against SARS-CoV-2 is, therefore, important for the development of diagnostic test-systems and evaluation of the vaccine efficiency [6, 11].
Following viral penetration to the target cell, its antigens presented by HLA molecules are recognized by virus-specific cytotoxic T lymphocytes (CTL). Numerous HLA alleles, e.g., HLA-B*46:01, HLA-B*07:03, HLA-DRB1*12:02, and HLA-C*08:01, (most frequently can be found in Chinese and Indonesian population) are associated with susceptibility to SARS-CoV, whereas HLA-DR*03:01, HLA-C*15:02, and HLA-A*02:01 correlate with protection against SARS-CoV infection and European Population. HLA-II molecules, such as HLA-DRB1*11:01 and HLA-DQB1*02:02, are associated with susceptibility to MERS-CoV infection. These data provide valuable clues for studying the pathogenetic mechanisms of COVID-19. The antigen presentation stimulates humoral and cellular immune response mediated by virus-specific B and T cells, respectively. The number of SARS-CoV-2-infected CD4+ and CD8+ T cells in peripheral blood of patients was sufficiently decreased, while their immune status is excessive activation, as evidenced by high proportions of HLA-DR-positive CD4+Т cells and CD38+ CD8+ T cells [12]. The SARS-CoV-2 epitope screening identified 2013 and 1399 peptide epitopes with high-affinity for HLA class I and class II molecules, respectively. These epitopes are distributed across structural proteins (S, M, E, and N), and non-structural proteins, being able to induce the CD8+ and CD4+ T cell responses. Several regions are enriched in high-affinity epitopes. These data are important for the development of vaccines against SARS-CoV-2 and T cell response monitoring [13].
The signal pathway of HLA-G and its receptor, expressed on the surface of immune cells, participates in viral infection by downregulation of T-, B-, and natural killer (NK) cells. Increased HLA-G expression is a strategy of viral escape from an immune response. The main evasion mechanism is that HLA-G binds to the immuno-inhibitory CTL receptors. Comparison of HLA-G expression and its receptors on peripheral blood lymphocytes between the day of an initial positive result for SARS-CoV-2 and the day of negative result has shown a positive relation between HLA-G expression on В cells and IFN-γ levels, while HLA-G expression in monocytes was negatively related to IL-2 levels. The percentage of HLA-G+ Т cells and expression of immune-inhibiting ILT4 receptor on B cells showed a negative correlation with TNF-α levels, the expression on the monocytes exhibited a positive correlation with IL-6, IL-10, and IFN-γ contents. The HLA-G expression pattern on peripheral immune cells may reflect three phases of the disease, i.e., primary infection, replication, and clearance of SARS-CoV-2. The time course of HLA-G expression on the peripheral immune cells termed as high/low/high dynamics from SARS-CoV-2 positive state to the SARS-CoV-2 negative condition suggests that the SARS-CoV-2 infection state is connected with cytokine regulation of HLA-G expression. Given that HLA-G is an antigen-presenting molecule, suppression of HLA-G expression by the SARS-CoV-2 virus can disrupt virus recognition by CD8 + T cells and maintain immunity evasion [14].
Genotyping of the COVID-19 patients by HLA loci has shown that the frequencies of HLA-C*07:29, C*08:01G, B*15:27, B*40:06, DRB1*04:06 and DPB1*36:01 (all of these alleles can be found mostly in the Asian population) alleles are higher, whereas frequencies of DRB1*12:02 and DPB1*04:01 alleles are lower in COVID-19 patients than in control population. Only the HLA-C*07:29 and B*15:27 frequencies were statistically different, with regard to corrected statistical significance. These data demonstrate some HLA alleles to be associated with COVID-19 [15].
The unique pattern of altered immunity regulation in SARS-CoV-2 patients is characterized, firstly, by increased circulating pro-inflammatory cytokine levels (especially, IL-6), secondly, by the functional lymphoid defect connected with IL-6-mediated decrease in HLA-DR expression. As mentioned above, the HLA class II, especially, HLA-DR, are constitutively expressed, mainly, by antigen-presenting cells (APC), В cells, and T cell subpopulations. IFN-γ is able to induce HLA-DR gene, as well as proinflammatory TNF-α, IL-1β и IL-6 cytokine expression. HLA class II activation enhances the HLA-restricted antigen presentation and adaptive immune response [5].
All the patients with SARS-CoV-2-associated pneumonia who develop severe respiratory failure, exhibit excessive inflammatory responses with immune dysregulation caused by IL-6, or macrophage activation syndrome (macrophage activation syndrome, MAS) caused by IL-1β, or very low HLA-DR expression, accompanied by a sharp decrease in CD4+T lymphocytes and NK. Blood plasma from the SARS-CoV-2 patients is shown to inhibit HLA-DR expression which could be partially restored by tocilizumab, an IL-6 blocking antibody. Tocilizumab treatment is accompanied by an increase in circulating lymphocyte numbers. Hence, the unique pattern of immune dysregulation in severe COVID-19 is characterized by the IL-6-mediated suppression of HLA-DR expression and lymphopenia due to permanently enhanced cytokine production and excessive inflammation [5].
In COVID-19, the patients show a decreased HLA-DR expression on the CD14+ monocytes, whereas blood ferritin levels sufficiently exceed normal values, a pattern revealed only in SARS-CoV-2 patients, with increased haemophagocytosis being of high diagnostic value. In the patients with bacterial pneumonia, HLA-DR on the CD14+ monocytes is below normal values, being, however, close to normal levels in SARS-CoV-2-associated pneumonia. In cases of its sudden drop, severe respiratory failure develops. The circulating IFN-γ concentrations were very low in all patients with SARS-CoV-2 infection. On contrary, the IL-6 and CRP levels were sufficiently higher in the patients with disturbed immunity than in cases with intermediate immune activation. IL-6 inhibits HLA-DR expression in COVID-19 patients. Accordingly, a negative correlation was found between serum level of IL-6 and absolute counts of HLA-DR on the CD14+ monocytes, as well as between absolute lymphocyte counts and HLA-DR contents on CD14+ monocytes in COVID-19 patients [5]. The role of IL-6 as a factor of HLA-DR decrease on the CD14+ monocytes is confirmed by an increase in HLA-DR+ circulating cells upon recovery from COVID-19 [16]. Tocilizumab can partially restore HLA-DR expression on the monocytes, thus increasing the number of circulating lymphocytes [17].
Development of vaccines against SARS-CoV-2
Aiming for the development of epitope-based subunit vaccines against SARS-CoV-2, one perform studies using reverse vaccinology and immune informatics. The tools of bioinformatics are used in reverse vaccinology to analyze the genome and proteome of the pathogen, identification, and analysis of neoantigens (Fig. 1). This approach allows choosing the viral antigenic segments that should be addressed when developing vaccines, being a more efficient, simple, time- and cost-effective method for the vaccine design.

Figure 1. Step-by-step strategies of reverse vaccinology approach of vaccine development [23]
During viral infection, the viral proteins are processed into small peptides within the proteasomes of infected cells. As mentioned above, the viral peptides are presented by HLA molecules at the surface of infected cells and recognized by T cells. The epitopes potentially recognizable by the T cells could belong to any viral structural or non-structural proteins.
Prediction of SARS-CoV-2 epitopes and appropriate immune responses is quite important to design and evaluate the immunogenicity of a vaccine against SARS-CoV-2. Prediction of antigenicity for the candidate vaccines presumes determination of numeric index for the ability of the vaccine to bind the В- and Т cell receptors and augment the immune response. To construct a vaccine, only highly antigenic sequences are chosen. At present, however, only scarce information exists on what SARS-CoV-2 sequences may induce a strong immune response.
The virus antigen-activated CD4+ helper T cells boost B cells to produce a lot of specific antibodies. Macrophages and CD8+ CTLs are also activated by the T-helpers, thus causing the final destruction of the target antigen. T cell epitopes interact with HLA class I and II alleles. This approach is used in the modern vaccine design since it provides a benefit, concerning time and cost expenditures over classical "test and error" strategy of "wet" labs (unlike dry labs, i.e., in silico experiments using mathematic or computer analysis). T cell epitopes were identified which can elicit a stable immune response against SARS-CoV-2 in the general human population. However, the ability of these epitopes to serve as vaccine candidates should be analyzed and validated at the molecular biology laboratories. Prediction of B cell epitopes seems less reliable than T cell epitopes since the B epitopes do not elicit a strong humoral response. For this reason, only T cell epitopes able to generate longitudinal CD4+ and CD8+T cell responses were analyzed in most in silico studies to choose epitopes for subsequent testing traditionally ("wet" laboratory) [8].
К. Kiyotani et al. [13] have performed immunoinformatic epitope prediction of S, E, N, M, and ORFs proteins from SARS-CoV-2 reference sample. They selected The HLA-A, HLA-B и HLA-C alleles presented in more than 5% frequencies in a Japanese population. To predict HLA class II epitopes, the HLA-DPA1-DPB1, HLA-DRB1 alleles, and HLA-DQA1-DQB1 haplotypes were used which encounter in Japan at frequencies of 5 to 38%. The work involved a total of 6421 sequences of SARS-CoV-2 isolated in various regions including 587 sequences from Asia, 1918 from North America, 3190 from Europe, and 726 from the Pacific Region deposited in the Global Initiative on Sharing Avian Influenza Database.
The next step included a screening of high-affinity peptide epitopes from the SARS-CoV-2 proteins which could be presented by molecules HLA class I and II. The two T cell epitopes in ORF1ab protein, ORF1ab2168-2176, and ORF1ab4089-4098, that were predicted to have a strong affinity to HLA-A*24:02, HLA-A*02:01 and HLA-A*02:06, have shown the broadest coverage of the Japanese population (83.8%). ORF1ab2168-2176, a predicted epitope binding HLA-C molecules (C*01:02, C*08:01, C*12: 02, and C*14:02), is present in 76.5%; the S268-277 and S448-457 epitopes in S protein were detected in 70% of Japanee individuals. The complexes of HLA with these peptides are useful for monitoring CD8+Т cell responses in patients and symptom-free infected individuals. No mutations were found in the sequences of the above epitopes. Therefore, the authors believe that these potential candidate epitopes can contribute to the development of rationally designed peptide vaccines based on epitopes against SARS-CoV-2 [13].
A complex analysis using in silico computer modeling of binding affinity between the peptides-HLA class I for 145 HLA-A, -B, and -C genotypes and whole proteome of the SARS-CoV-2, as well as cross-protective immunity resulting from preliminary action of the 4 widespread human coronaviruses, has shown that the HLA-B*46:01 antigen binds a minimal number of predicted SARS-CoV-2 peptides. This fact allows suggesting that the individuals carrying this allele could be especially susceptible for COVID-19, as it was previously shown with SARS-CoV, thus corresponding to clinical data which associate this allele with severe disease. And, vice versa, HLA-B*15:03 allele has demonstrated maximal ability to present highly conserved SARS-CoV-2 peptide sequences [18]. Hence, distinct HLA genotypes may differentially induce T cell antiviral response, thus influencing clinical course of infection and its transmission.
For 32257 unique 8-12-mer SARS-CoV-2 peptides, which are predicted to pass through the proteasomal processing pathway, a SARS-CoV-2-specific distribution was shown for presentation by the HLA class I molecules. The presumed ability of SARS-CoV-2 peptides for antigenic presentation is unrelated with the population frequency of HLA alleles. When reporting the global frequency cards for the 145 studied HLA alleles, the authors highlight the global distribution of the three best (A*02:02, B*15:03, C*12:03), and three worst (A*25:01, B*46:01, C*01:02) HLA-presenting alleles, by their ability to generate the SARS-CoV-2 epitope repertoire, in order to support T cell immune response. These differences remain significant at the haplotype level. SARS-CoV-2 evolution in the population may modify the repertoire of presented viral epitopes or otherwise modulate HLA-independent epitopes. However, the authors recommend integrating HLA-typing into clinical trials, and combining it with COVID-19 testing to apply it as a potential predictor(s) of the disease severity among the population, and, probably, for adaptation of future vaccination strategies for genotypic risk groups. This approach could be used to control a broad spectrum of other viruses [18].
Aiming for epitope-based development of a vaccine against SARS-CoV-2 employing analysis of viral proteome using immunoinformatic tools, А. Joshi et al. have chosen antigenic non-toxic non-allergenic peptides from non-allergenic proteins based on their interactions with HLA allelic sets. Among the identified T cell epitopes, the ITLCFTLKR epitope is a candidate for the anti-SARS-CoV-2 vaccine, exhibiting better binding indexes in the epitope-HLA complexes, as well as acceptable stability, toxicity, and population coverage. This epitope should now undergo laboratory verification [8].
The spike protein (S protein) is most commonly analyzed to detect immunogenic epitopes which are highly affine for the cellular ACE2 receptor when penetrating human cells. On this basis, S protein is considered a potential target for the coronavirus vaccine. Using immunoinformatics and computer modeling tools, М. Bhattacharya et al. [19] have revealed 34 linear B cell epitopes for S protein of the SARS-CoV-2 and identified among them 13 HLA I-binding epitopes and 3 HLA II-binding epitopes with antigenic features of SARS-CoV-2 S protein, which are recognized by Т cell receptor (TLR-5, Toll-like receptor-5). The antigenic epitopes are converted into the single vaccine component using a linker peptide that promoted stability and assembly of the modeled and validated vaccine component. Molecular docking between the vaccine component and TLR5 caused spontaneous reactivity in the receptor-ligand complex which activates immune cascades for the destruction of viral antigens. Hence, the selected antigenic SARS-CoV-2 epitopes are good candidates for the design of the immunogenic multi-epitope peptide vaccine against SARS-CoV-2.
A similar immunoinformatic approach was used by V.Baruah and S.Bose aiming to reveal significant epitopes in the S protein of SARS-CoV-2. The authors identified five CTL-specific epitopes (YLQPRTFLL, GVYFASTEK, EPVLKGVKL, VVNQNAQAL, WTAGAAAYY), that were highly affine for appropriate class I HLA alleles (A*02:01, A*03:01, B*07:02, B*07:02, HLA‐B*15:01). Upon modeling of molecular dynamics, it was shown that these epitopes bind the peptide-binding groove of the HLA-I molecule used for antigen presentation through multiple contacts, showing their potential for immune response generation. Some of these epitopes are candidates for the design of anti-SARS-CoV-2 vaccines. In addition to activating CTLs, the successful immunogens should generate a persistent humoral immunity. This study has revealed three such B-cell epitopes unique to SARS-CoV-2 [20].
D. Santoni et al. [21] used a bioinformatic methodology based on the selection of viral peptides which are at a distance of more than 3 mutational steps from humans, presuming that the probability of binding to HLA antigens increases with longer evolutionary distance from humans. In other words, the researchers seek those viral peptides which are absent in humans (nullomeres). Identification of the most human-distant peptides is quite important to minimize the autoimmunity risks. Of the 27 nullomeres, 25 were common to all the known SARS-CoV-2 strains. These peptides were called the third-order nullomeres that belong to the most distant class from humans were subjected to three additional selection stages, to choose those with the highest probability of exposure on the cell surface, i.e., (1) being the product of proteasomal cleavage, 2) being transferred to the cell surface, and (3) strongly binds to HLA molecules. A set of nine peptides was identified for subsequent experimental studies. The in silico selected SARS-CoV-2 peptides represent potential targets for the immune system, that should be then tested experimentally to confirm their immunogenicity. According to in silico prediction, the YVMHANYIF peptide is strongly binding to 27 various HLA antigens, the FLCWHTNCY and YIKWPWYIW peptides may bind 11 and 10 different HLA molecules, respectively, YYHKNNKSW peptide may interact with 8 HLA allelic variants.
The concept of multi-epitopic vaccine addresses identification and assembly of В- and Т cell-specific epitopes into an integral immunogen able to induce effective response at both immunity arms. The peptides and epitopes proved to be preferred candidates for vaccine development, due to simpler technology production, chemical stability, and absence of infectious potential. The multi-epitopic vaccines may contain epitopes for B cells, CD8+ CTLs, and CD4+ helper T cells.
Using immunobioinformatic approach, the five domains rich in common T- and B-cell epitopes were selected and connected by linker chains, in order to generate a more diverse and stable immune response. The resulting multi-epitope candidate vaccine NOM (nucleocapsid, ORF3a, membrane protein) represents itself a recombinant multi-epitope protein constructed and validated utilizing bioinformatics tools which include, as the name suggests, antigenic immune epitopes from three SARS-CoV-2 viral proteins. The optimized NOM protein structure is adapted to interactions with specific immune receptors. Upon modeling the molecular dynamics, maximal stability was shown for the NOM binding with TLR4 and HLA-A*11:01 (i.e., NOM–TLR4 and NOM–HLA-A*11:01 models). The in silico testing has shown that interaction of the chimeric protein with TLR4 and HLA-A*11:01 receptors induces both humoral and cellular immune responses, due to the composite nature of the construct including В- and Т cell epitopes [22].
С.Н. Lee et al. have reported on in silico identification of a comprehensive list of SARS-CoV-2 immunogenic peptides which could be used as target molecules for vaccine development. Among them 48 viral peptides showing a high degree of similarity with immunogenic peptides deposited in the Immune Epitope Database (IEDB), and 63 more new peptides with high immunogenic potential, which could be recognized by T-cell receptors. Searching for the immunogenic SARS-CoV-2 peptides, the authors focused on the haplotypes common in Europe and China. The 28 most promising SARS-CoV-2 peptides are shown to bind different HLA class I and HLA class II alleles (HLA-A*01:01, DRB1*07:01, DRB1*04:01), and could serve as a target for, respectively, CD8+ and CD4+ T cells. Based on HLА presentation and immunogenicity prediction, the five peptides were selected for subsequent experimental validation (VQMAPISAM, AMYTPHTVL, TLDSKTQSL, KVDGVVQQL, KVDGVDVEL), which potentially would specifically bind four different HLA variants (A*01:01, HLA-B*07:02, HLA-B*40:01, and, with the highest affinity, HLA-A*02:01 [23].
To perform prediction and cluster analysis of probable HLA alleles, which could interact with SARS-CoV-2 epitopes, В. Sarkar et al. [24] have used the HLAcluster 2.0 online tool (http://www.cbs.dtu.dk/services/HLAcluster/). After predicting the three-dimensional structure of highly antigenic, non-allergenic, and nontoxic Т- and B-cell epitopes, they compared the ability of these epitopes to bind HLA molecules. The HLA-A*11:01 allele was used as a receptor for docking with HLA-I epitopes, whereas HLA-DRB1*04:01 was tested with HLA-II epitopes. The best results were obtained for viral N-protein, the QLESKMSGK peptide for docking with HLA-I and LIRQGTDYKHWP peptide with HLA-II epitopes. The GVLTESNKK peptide from S-protein was the best for HLA-I epitopes, whereas the TSNFRVQPTESI peptide of the viral surface glycoprotein showed the best binding properties for HLA class II.
As based on successful modeling of peptide/protein docking, three anti-SARS-CoV-2 vaccine variants were proposed, using the selected epitopes, CV-1, CV-2 и CV-3. The CV-1 proved to be the best, by the molecular docking criteria. CV-2 showed maximal binding efficiency with HLA-DRB3*02:02 and HLA-DRB1*03:01. The CV-3 vaccine has better binding energy parameters with most HLA alleles (DRB5*01:01, DRB1*01:01, and DRB3*01:01). Since CV-1 showed the best results for protein/protein docking, it was recognized as the best construct of those three. Modeling of molecular dynamics and in vitro adaptation studies have been performed with only this vaccinal variant. The proposed vaccine constructs could be used for SARS-CoV-2 vaccination if the validation trials will yield satisfactory results [24].

Figure 2. Scheme of design of a multi-epitope subunit candidate vaccine using epitopes of B-cells, CTLs, and T-helper cells [25]
The unique construct of a recombinant multi-peptide subunit vaccine against COVID-19 contains 18 epitopes for CTLs, 6 epitopes for T-helper cells, and 9 epitopes for В cells from three SARS-CoV-2 proteins which participate in recognition of cellular receptors and viral penetration into the target cells (Fig. 2). To enhance immunogenicity, the construct is supplied by a sequence from human β-defensin (TLR3 agonist) which serves as a binding adjuvant with epitopes and linkers. Computational studies suggest that the vaccine is not allergenic, stable, and can elicit humoral and cellular immune responses. The synthesis and experimental evaluation of this vaccine are pending to determine its immunogenic activity. The authors hope that this vaccine will be synthesized and used in public healthcare [25].
SARS-CoV-2 possesses self-amplifying RNA in the cytosol, which allows the development of an RNA-based vaccine. These vaccines use the mRNA sequence of recombinant target protein, rather than the sequence of the target antibody. The vaccinal mRNA is then transferred by lipid nanoparticles to the cytoplasm where the immunogenic protein is translated. After release from the cell, this protein is quickly captured and processed by APCs, followed by HLA-mediated presentation on the surface of the antigen-presenting cell, followed by the activation of B and T cells and, accordingly, the antigen-specific humoral and cytotoxic responses.
The vaccines based on the cytoplasmic expression of chimeric viral mRNA are potentially superior to protein-based vaccines: they are more safe, efficient, easier to produce, and they can block chromosomal integration of the virus. The manufacturing of RNA-based vaccines is the most promising trend in vaccinology, due to capacity of wide-scale production, thus spating time during pandemics. Following injection, the vaccine RNA could be processed by immune cells and produce specific protein directly through translation followed by activation of other immune cells and antibody synthesis (Fig. 3) [7].

Figure 3. Schematic diagram of the mRNA-based vaccine targeted to the S protein of SARS-CoV-2
The mRNA-based vaccine targeted to the S protein of SARS-CoV-2 works by active immunization. This technique uses mRNA of the S protein, coated with lipid nanoparticles for effective delivery. Once injected into the muscle, the myocytes take up the lipid nanoparticle and then release the mRNAs into the cytoplasm for translation into the S proteins. These endogenously synthesized S proteins will be secreted to activate both humoral and cellular immune responses. S protein – spike protein; IM – intramuscular, LNP – lipid nanoparticle; DC – dendritic cell; MHC – major histocompatibility complex; Ag – antigen [7].
The lipid nanoparticle (LNP)-encapsulated mRNA-1273 based vaccine, produced by Moderna Inc. (USA) encodes SARS-CoV-2 S-protein. Upon the formation of sufficient antibody titers to S-protein, a double therapeutic effect may occur, i.e., the host immune system could eliminate the antigen-antibody complex, thus promoting virus clearance and alleviating its contagiosity [7]. Preliminary results of clinical trials have shown that the mRNA-1273 vaccine has induced high levels of both virus-binding and neutralizing antibodies, as well as strong cytokine response with participation of CD4 helper T cells, type 1 [26] and that the mRNA-1273 immunogenicity retained for, at least, 3 months [27].
Prefusion-stabilized protein immunogens that preserve neutralization-sensitive epitopes are an effective vaccine strategy for enveloped viruses. Structural studies have led to the search for mutations that stabilize Betacoronavirus spike proteins in the prefusion state, improving their expression and increasing immunogenicity. This principle has been applied to design mRNA-1273, an mRNA vaccine that encodes a SARS-CoV-2 spike protein that is stabilized in the prefusion conformation. Here we show that mRNA-1273 induces potent neutralizing antibody responses to both wild-type (D614) and D614G mutant2 SARS-CoV-2 as well as CD8+ T cell responses, and protects against SARS-CoV-2 infection in the lungs and noses of mice without evidence of immunopathology. mRNA-1273 is currently in a phase III trial to evaluate its efficacy.
K.S. Corbett et al. [28] identified 2 proline substitutions (2P) at positions 986 and 987 of S protein that effectively stabilized S proteins in the pre-fusion conformation. The authors performed structural analysis and developed serological tests in silico, without additional experimental verification. Similar to other prefusion-stabilized fusion proteins, S (2P) protein SARS-CoV-2 was more immunogenic at lower doses than wild-type S protein [28]. The 2P mutation has similar effects on the stability of S proteins and from other beta coronaviruses, suggesting a generalizable approach for designing S protein antigens for vaccination. Such generalizability is fundamental to pandemic preparedness [29].
The production of mRNA encoding the S-protein (2P) SARS-CoV-2 (mRNA-1273) was started in parallel with the preclinical evaluation. This led to the first phase I human clinical trial, which began on March 16, 2020. Thus, concepts based on new technologies, such as synthetic vaccinology, can facilitate and accelerate a vaccine development program based on pathogen sequences.
Protein glycosylation is a very common biological process, a form of posttransplant protein modification and regulation of protein location and functioning. Glycosylation of viral structural proteins is closely related to their replication and cell invasion, thus helping to escape the host immune response. Unusually high glycosylation degree of SARS-CoV-2 presumes its high mutation rates, thus sufficiently complicating the vaccine development. However, the mRNA-based vaccine technologies and targeting for S-protein may result in the production of antibodies to S-protein only, irrespectively of its glycosylation status. Taking into account useful features of mRNA-based vaccines, such as lack of integration into the genome, lack of induction of autoantibodies, the feasibility to produce mRNA vaccines in large quantities, and their high purity, mRNA-based vaccines are a promising choice for combating COVID-19 [7].
Conclusion
The development of vaccines is a long-term and costly process with high failure rates which requires several years for the production of a commercial product. Optimization of the manufacturing process allows sooner testing of the subunit vaccines and their release to the market. Protein-based vaccines include only antigenic parts of the pathogen causing an immediate immune response. Moreover, the vaccine does not contain a live pathogen. Hence, it could be used in immunocompromised patients. Computer-assisted studies show that the multi-epitope subunit vaccine is safe and effective against SARS-CoV-2 infection.
Common vaccines, attenuated, or inactivated, are not always able to provide immunity for the target antigen. Besides, the generally accepted approach to vaccine development causes multiple concerns for safety during pre-clinical and clinical trials. The subunit vaccines designed using in silico computer modeling may overcome these difficulties. Numerous study groups perform studies and publish their results in this field. The independent works carried out with different computational techniques provide higher reliability and reproducibility level of the results.
Individual genetic variations of HLA allelic variants may explain diverse immune responses to the virus infection and variable clinical outcomes in the population. Moreover, a detailed analysis of the common HLA alleles will help to develop more effective anti-COVID-19 vaccines, since searching for optimal vaccine constructs strongly depends on the detection of significant epitopes in spike glycoprotein, and other SARS-CoV-2 proteins recognized by the antigen-presenting cells, cytotoxic T-cells, and B cells using bioinformatics methodologies. This review presents a profile of in silico predicted SARS-CoV-2 immunogenic peptides for functional validation and vaccine development. Computer-assisted prediction plays an important role in rapid and cost-effective decision making for the prevention of infection spreading, and, finally, overcoming pandemics. Most attempts in the field of SARS-CoV-2 prevention and treatment are focused on the viral S protein, the main inducer of virus-neutralizing antibodies. The article concerns current achievements in the development of modern SARS-CoV-2 vaccines which, in sum, may stop this new viral infection.
Conflict of Interest
None declared.
References
- Glotov OS, Romanova OV, Eismont YA, Sarana AM, Scherbak SG, Kuzmich EV, Alyansky AL, Ivanova NE, Teplyashina VV, Serov YA, Zubarovskaya LS, Afanasyev BV. Comparative analysis of HGS and Sanger sequencing methods for HLA typing at a Russian university clinic. Cell Ther Transplant. 2018; 7(2): 72-82. doi: 10.18620/ctt-1866-8836-2018-7-4-72-82.
- Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microb Infect. 2020;9(1):382-385. doi: 10.1080/22221751.2020.1729069.
- Pathological anatomy of lungs in COVID-19. Histology atlas. Ryasan, 2020. 52 p (In Russian).
- Arabi YM, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020;46(5):833-836. doi: 10.1007/s00134-020-05955-1.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020; 27(6):992-1000.e3. doi: 10.1016/j.chom.2020.04.009.
- Forouzesh M, Rahimi A, Valizadeh R, Dadashzadeh N, Mirzazadeh A. Clinical display, diagnostics and genetic implication of novel Coronavirus (COVID-19) epidemic. Eur Rev Med Pharmacol Sci. 2020;24(8):4607-4615. doi: 10.26355/eurrev_202004_21047.
- Wang F, Kream RM, Stefano GB. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med Sci Monit. 2020 May 5;26:e924700. doi: 10.12659/MSM.924700.
- Joshi A, Joshi BC, Mannan MA, Kaushik V. Epitope based vaccine prediction for SARS-COV-2 by deploying immuno-informatics approach. Inform Med Unlocked. 2020 Apr 29:100338. doi: 10.1016/j.imu.2020.100338.
- Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, Liu W, Zhu Y, Lin Q, Mao L et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020 May 21;5(10):e137799. doi: 10.1172/jci.insight.137799.
- Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, Mekinian A, Anunciacion-Llunell A, Miró-Mur F. et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev. 2020 Jul;19(7):102569. doi: 10.1016/j.autrev.2020.102569.
- Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451-1454. doi: 10.1038/s41418-020-0530-3.
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020; 10(2):102-108. doi: 10.1016/j.jpha.2020.03.001.
- Kiyotani K, Toyoshima Y, Nemoto K, Nakamura Y. Bioinformatic prediction of potential T cell epitopes for SARS-Cov-2. J Hum Genet. 2020 Jul;65(7):569-575. doi: 10.1038/s10038-020-0771-5.
- Zhang S, Gan J, Chen BG, Zheng D, Zhang JG, Lin RH, Zhou YP, Yang WY, Lin A, Yan WH. Dynamics of peripheral immune cells and their HLA-G and receptor expressions in a patient suffering from critical COVID-19 pneumonia to convalescence. Clin Transl Immunology. 2020 May 10;9(5):e1128. doi: 10.1002/cti2.1128.
- Wang W, Zhang W, Zhang J, He J, Zhu F. Distribution of HLA allele frequencies in 82 chinese individuals with Coronavirus disease-2019. HLA. 2020; 96(2):194-196. doi: 10.1111/tan.13941.
- Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, Jia X, Nicholson S, Catton M, Cowie B et al. Breadth of concomitant immune responses prior to patient recovery: a case-report of non-severe COVID-10. Nat. Med. 2020; 26(4):453-455. doi: 10.1038/s41591-020-0819-2.
- Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, Walzer T, François B, Sève P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020 Jul;19(7):102567. doi: 10.1016/j.autrev.2020.102567.
- Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, Nellore A, Thompson RF. Human leukocyte antigen susceptibility map for SARS-CoV-2. J Virol. 2020 Jun 16;94(13):e00510-e00520. doi: 10.1128/JVI.00510-20.
- Bhattacharya M, Sharma AR, Patra P, Ghosh P, Sharma G, Patra BC, Lee SS, Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J Med Virol. 2020;92(6):618-631. doi: 10.1002/jmv.25736.
- Baruah V, Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of SARS-CoV-2. J Med Virol. 2020;92(5):495-500. doi: 10.1002/jmv.25698.
- Santoni D, Vergni D. In the search of potential epitopes for Wuhan seafood market pneumonia virus using high order nullomers. J Immunol Methods. 2020;481-482:112787. doi: 10.1016/j.jim.2020.112787.
- Enayatkhani M, Hasaniazad M, Faezi S, Guklani H, Davoodian P, Ahmadi N, Einakian MA, Karmostaji A, Ahmadi K. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: an in silico study. J Biomol Struct Dyn. 2020 May 2:1-16. doi: 10.1080/07391102.2020.1756411.
- Lee CH, Pinho MP, Buckley PR, Woodhouse IB, Ogg G, Simmons A, Napolitani G, Koohy H. Potential CD8+ T Cell Cross-Reactivity Against SARS-CoV-2 Conferred by Other Coronavirus Strains. Front Immunol. 2020 Nov 5;11:579480. doi: 10.3389/fimmu.2020.579480.
- Sarkar B, Ullah A, Johora FT, Taniya MA, Araf Y. Immunoinformatics-guided designing of epitope-based subunit vaccine against the SARS Coronavirus-2 (SARS-CoV-2). Immunobiology. 2020 May 11;225(3):151955. doi: 10.1016/j.imbio.2020.151955.
- Kalita P, Padhi AK, Zhang KYJ, Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020 May 4;145:104236. doi: 10.1016/j.micpath.2020.104236.
- Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020; 383(25):2427-2438. doi: 10.1056/NEJMoa2028436.
- Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021; 384(1):80-82. doi: 10.1056/NEJMc2032195.
- Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, Himansu S, Schäfer A, Ziwawo CT, DiPiazza AT et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567-571. doi: 10.1038/s41586-020-2622-0.
- Graham BS, Corbett KS. Prototype pathogen approach for pandemic preparedness: world on fire. J. Clin. Invest. J Clin Invest. 2020;130(7):3348-3349. doi: 10.1172/JCI139601.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(71) "immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla" ["~CODE"]=> string(71) "immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla" ["EXTERNAL_ID"]=> string(4) "1948" ["~EXTERNAL_ID"]=> string(4) "1948" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(197) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLAImmunoinformatics in COVID-19 Vaccine Development: The Role of HLA System" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2762) "<p style="text-align: justify;"> Индивидуальные генетические вариации могут объяснить различные иммунные реакции на инфекцию коронавируса SARS-CoV-2 в популяции. Методология компьютерного моделирования <i>in silico</i> предоставляет экспериментальному сообществу список иммуногенных пептидов SARS-CoV-2, презентируемых антигенами HLA-системы. В этом обзоре представлен профиль предсказанных <i>in silico</i> иммуногенных пептидов вируса SARS-CoV-2 для функциональной валидации и разработки вакцин. Исследования, проводимые независимо друг от друга, дают высокую степень уверенности в воспроизводимости результатов. Вычислительное прогнозирование является инструментом быстрого и экономичного решения для предотвращения распространения и, в конечном итоге, устранения инфекции. Большая часть усилий по разработке вакцин и лекарств против SARS-CoV-2 направлена на гликопротеин шипа (S-белок), главный индуктор нейтрализующих антител. Несколько вакцин-кандидатов продемонстрировали эффективность в исследованиях <i>in vitro</i> и перешли в рандомизированные испытания на животных или людях для противодействия инфекции COVID-19. В этой статье освещаются текущие достижения в разработке субъединичных вакцин для борьбы с COVID-19, которые сокращают время и затраты при разработке вакцин. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Коронавирус, SARS-CoV-2, COVID-19, HLA, иммуногенный пептид, антиген, эпитоп, вакцина, компьютерное прогнозирование, компьютерное моделирование <i>in silico</i>, иммуноинформатика. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_META_TITLE"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_META_KEYWORDS"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_META_DESCRIPTION"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_PICTURE_FILE_ALT"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_PICTURE_FILE_TITLE"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_PICTURE_FILE_NAME"]=> string(75) "immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(124) "Иммуноинформатика в разработке вакцины против COVID-19: роль системы HLA" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(75) "immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(75) "immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(75) "immunoinformatika-v-razrabotke-vaktsiny-protiv-covid-19-rol-sistemy-hla-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "174" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27442" ["VALUE"]=> string(22) "12/25/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/25/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27443" ["VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27444" ["VALUE"]=> array(2) { ["TEXT"]=> string(860) "<p>Анна Ю. Анисенкова<sup>1</sup>, Александр С. Голота<sup>1</sup>, Дмитрий А. Вологжанин<sup>1</sup>, Татьяна А. Камилова<sup>1</sup>, Станислав В. Макаренко<sup>1,2</sup>, Ольга В. Шнейдер<sup>1</sup>, Олег С. Глотов<sup>1,3</sup>, Юрий А. Серов<sup>4</sup>, Сергей В. Мосенко<sup>1</sup>, Сергей В. Азаренко<sup>1</sup>, Константин В. Сманцерев<sup>1</sup>, Дмитрий Н. Хоботников<sup>1</sup>, Татьяна В. Гладышева<sup>1</sup>, Сергей Г. Щербак<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(680) "
Анна Ю. Анисенкова1, Александр С. Голота1, Дмитрий А. Вологжанин1, Татьяна А. Камилова1, Станислав В. Макаренко1,2, Ольга В. Шнейдер1, Олег С. Глотов1,3, Юрий А. Серов4, Сергей В. Мосенко1, Сергей В. Азаренко1, Константин В. Сманцерев1, Дмитрий Н. Хоботников1, Татьяна В. Гладышева1, Сергей Г. Щербак1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27445" ["VALUE"]=> array(2) { ["TEXT"]=> string(787) "<p><sup>1</sup> СПб ГБУЗ «Городская больница №40 Курортного района», Сестрорецк, Россия <br> <sup>2</sup> Санкт-Петербургский государственный университет, Санкт-Петербург, Россия<br> <sup>3</sup> НИИ акушерства, гинекологии и репродуктологии им. Д. О. Отта, Санкт-Петербург, Россия<br> <sup>4</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(709) "1 СПб ГБУЗ «Городская больница №40 Курортного района», Сестрорецк, Россия
2 Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 НИИ акушерства, гинекологии и репродуктологии им. Д. О. Отта, Санкт-Петербург, Россия
4 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
Индивидуальные генетические вариации могут объяснить различные иммунные реакции на инфекцию коронавируса SARS-CoV-2 в популяции. Методология компьютерного моделирования in silico предоставляет экспериментальному сообществу список иммуногенных пептидов SARS-CoV-2, презентируемых антигенами HLA-системы. В этом обзоре представлен профиль предсказанных in silico иммуногенных пептидов вируса SARS-CoV-2 для функциональной валидации и разработки вакцин. Исследования, проводимые независимо друг от друга, дают высокую степень уверенности в воспроизводимости результатов. Вычислительное прогнозирование является инструментом быстрого и экономичного решения для предотвращения распространения и, в конечном итоге, устранения инфекции. Большая часть усилий по разработке вакцин и лекарств против SARS-CoV-2 направлена на гликопротеин шипа (S-белок), главный индуктор нейтрализующих антител. Несколько вакцин-кандидатов продемонстрировали эффективность в исследованиях in vitro и перешли в рандомизированные испытания на животных или людях для противодействия инфекции COVID-19. В этой статье освещаются текущие достижения в разработке субъединичных вакцин для борьбы с COVID-19, которые сокращают время и затраты при разработке вакцин.
Ключевые слова
Коронавирус, SARS-CoV-2, COVID-19, HLA, иммуногенный пептид, антиген, эпитоп, вакцина, компьютерное прогнозирование, компьютерное моделирование in silico, иммуноинформатика.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27447" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-13-23" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-13-23" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27449" ["VALUE"]=> array(2) { ["TEXT"]=> string(650) "<p>Anna Yu. Anisenkova<sup>1</sup>, Aleksander S. Golota<sup>1</sup>, Dmitry A. Vologzhanin<sup>1</sup>, Tatyana A. Kamilova<sup>1</sup>, Stanislav V. Makarenko<sup>1,2</sup>, Olga V. Shneider<sup>1</sup>, Oleg S. Glotov<sup>1,3</sup>, Yury A. Serov<sup>4</sup>, Sergey V. Mosenko<sup>1</sup>, Sergey V. Azarenko<sup>1</sup>, Konstantin V. Smanzerev<sup>1</sup>, Dmitry N. Khobotnikov<sup>1</sup>, Tatyana V. Gladisheva<sup>1</sup>, Sergey G. Shcherbak<sup>1,2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(470) "Anna Yu. Anisenkova1, Aleksander S. Golota1, Dmitry A. Vologzhanin1, Tatyana A. Kamilova1, Stanislav V. Makarenko1,2, Olga V. Shneider1, Oleg S. Glotov1,3, Yury A. Serov4, Sergey V. Mosenko1, Sergey V. Azarenko1, Konstantin V. Smanzerev1, Dmitry N. Khobotnikov1, Tatyana V. Gladisheva1, Sergey G. Shcherbak1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27450" ["VALUE"]=> array(2) { ["TEXT"]=> string(700) "<p><sup>1</sup> St. Petersburg City Hospital №40, St. Petersburg, Russia <br> <sup>2</sup> St. Petersburg State University School of Medicine, St. Petersburg, Russia<br> <sup>3</sup> D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia<br> <sup>4</sup> Pavlov University, St.Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia<br> E-mail: golotaa@yahoo.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(580) "1 St. Petersburg City Hospital №40, St. Petersburg, Russia
2 St. Petersburg State University School of Medicine, St. Petersburg, Russia
3 D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia
4 Pavlov University, St.Petersburg, Russia
Correspondence
Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia
E-mail: golotaa@yahoo.com
Individual genetic variation may help to explain different immune responses to a coronavirus SARS-CoV-2 across a population. The in silico computer simulation methodology provides the experimental community with a more complete list of SARS-CoV-2 immunogenic peptides presented by the antigens of the HLA system. This review considers an array of computationally predicted immunogenic peptides from SARS-CoV-2 for in vitro functional validation and potential vaccine developments. Several independent studies conducted with different approaches showed a high degree of confidence and reproducibility of the results. Computer-assisted prediction is instrumental for a quick and cost-effective solution to prevent the spread and ultimately eliminate the infection.
Most efforts to develop vaccines and drugs against SARS-CoV-2 target the spike glycoprotein (protein S), the major inducer of neutralizing antibodies. Several candidates have been shown to be effective in in vitro studies and have progressed to randomized trials in animals or humans against COVID-19 infection. This article highlights current advances in the development of subunit vaccines to combat COVID-19 that are reducing the time and costs of vaccine development.
Keywords
Coronavirus, SARS-CoV-2, COVID-19, immunogenic peptides, antigen, HLA, vaccine, epitope, computational prediction, computer simulation in silico, immunoinformatics.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27448" ["VALUE"]=> string(73) "Immunoinformatics in COVID-19 Vaccine Development: The Role of HLA System" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(73) "Immunoinformatics in COVID-19 Vaccine Development: The Role of HLA System" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27524" ["VALUE"]=> string(4) "2414" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2414" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27525" ["VALUE"]=> string(4) "2415" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2415" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27449" ["VALUE"]=> array(2) { ["TEXT"]=> string(650) "<p>Anna Yu. Anisenkova<sup>1</sup>, Aleksander S. Golota<sup>1</sup>, Dmitry A. Vologzhanin<sup>1</sup>, Tatyana A. Kamilova<sup>1</sup>, Stanislav V. Makarenko<sup>1,2</sup>, Olga V. Shneider<sup>1</sup>, Oleg S. Glotov<sup>1,3</sup>, Yury A. Serov<sup>4</sup>, Sergey V. Mosenko<sup>1</sup>, Sergey V. Azarenko<sup>1</sup>, Konstantin V. Smanzerev<sup>1</sup>, Dmitry N. Khobotnikov<sup>1</sup>, Tatyana V. Gladisheva<sup>1</sup>, Sergey G. Shcherbak<sup>1,2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(470) "Anna Yu. Anisenkova1, Aleksander S. Golota1, Dmitry A. Vologzhanin1, Tatyana A. Kamilova1, Stanislav V. Makarenko1,2, Olga V. Shneider1, Oleg S. Glotov1,3, Yury A. Serov4, Sergey V. Mosenko1, Sergey V. Azarenko1, Konstantin V. Smanzerev1, Dmitry N. Khobotnikov1, Tatyana V. Gladisheva1, Sergey G. Shcherbak1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(470) "Anna Yu. Anisenkova1, Aleksander S. Golota1, Dmitry A. Vologzhanin1, Tatyana A. Kamilova1, Stanislav V. Makarenko1,2, Olga V. Shneider1, Oleg S. Glotov1,3, Yury A. Serov4, Sergey V. Mosenko1, Sergey V. Azarenko1, Konstantin V. Smanzerev1, Dmitry N. Khobotnikov1, Tatyana V. Gladisheva1, Sergey G. Shcherbak1,2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27451" ["VALUE"]=> array(2) { ["TEXT"]=> string(1697) "<p style="text-align: justify;"> Individual genetic variation may help to explain different immune responses to a coronavirus SARS-CoV-2 across a population. The <i>in silico</i> computer simulation methodology provides the experimental community with a more complete list of SARS-CoV-2 immunogenic peptides presented by the antigens of the HLA system. This review considers an array of computationally predicted immunogenic peptides from SARS-CoV-2 for <i>in vitro</i> functional validation and potential vaccine developments. Several independent studies conducted with different approaches showed a high degree of confidence and reproducibility of the results. Computer-assisted prediction is instrumental for a quick and cost-effective solution to prevent the spread and ultimately eliminate the infection. </p> <p style="text-align: justify;"> Most efforts to develop vaccines and drugs against SARS-CoV-2 target the spike glycoprotein (protein S), the major inducer of neutralizing antibodies. Several candidates have been shown to be effective in <i>in vitro</i> studies and have progressed to randomized trials in animals or humans against COVID-19 infection. This article highlights current advances in the development of subunit vaccines to combat COVID-19 that are reducing the time and costs of vaccine development. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Coronavirus, SARS-CoV-2, COVID-19, immunogenic peptides, antigen, HLA, vaccine, epitope, computational prediction, computer simulation<i> in silico</i>, immunoinformatics. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1571) "Individual genetic variation may help to explain different immune responses to a coronavirus SARS-CoV-2 across a population. The in silico computer simulation methodology provides the experimental community with a more complete list of SARS-CoV-2 immunogenic peptides presented by the antigens of the HLA system. This review considers an array of computationally predicted immunogenic peptides from SARS-CoV-2 for in vitro functional validation and potential vaccine developments. Several independent studies conducted with different approaches showed a high degree of confidence and reproducibility of the results. Computer-assisted prediction is instrumental for a quick and cost-effective solution to prevent the spread and ultimately eliminate the infection.
Most efforts to develop vaccines and drugs against SARS-CoV-2 target the spike glycoprotein (protein S), the major inducer of neutralizing antibodies. Several candidates have been shown to be effective in in vitro studies and have progressed to randomized trials in animals or humans against COVID-19 infection. This article highlights current advances in the development of subunit vaccines to combat COVID-19 that are reducing the time and costs of vaccine development.
Keywords
Coronavirus, SARS-CoV-2, COVID-19, immunogenic peptides, antigen, HLA, vaccine, epitope, computational prediction, computer simulation in silico, immunoinformatics.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1571) "Individual genetic variation may help to explain different immune responses to a coronavirus SARS-CoV-2 across a population. The in silico computer simulation methodology provides the experimental community with a more complete list of SARS-CoV-2 immunogenic peptides presented by the antigens of the HLA system. This review considers an array of computationally predicted immunogenic peptides from SARS-CoV-2 for in vitro functional validation and potential vaccine developments. Several independent studies conducted with different approaches showed a high degree of confidence and reproducibility of the results. Computer-assisted prediction is instrumental for a quick and cost-effective solution to prevent the spread and ultimately eliminate the infection.
Most efforts to develop vaccines and drugs against SARS-CoV-2 target the spike glycoprotein (protein S), the major inducer of neutralizing antibodies. Several candidates have been shown to be effective in in vitro studies and have progressed to randomized trials in animals or humans against COVID-19 infection. This article highlights current advances in the development of subunit vaccines to combat COVID-19 that are reducing the time and costs of vaccine development.
Keywords
Coronavirus, SARS-CoV-2, COVID-19, immunogenic peptides, antigen, HLA, vaccine, epitope, computational prediction, computer simulation in silico, immunoinformatics.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27447" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-13-23" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-13-23" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-13-23" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27448" ["VALUE"]=> string(73) "Immunoinformatics in COVID-19 Vaccine Development: The Role of HLA System" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(73) "Immunoinformatics in COVID-19 Vaccine Development: The Role of HLA System" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(73) "Immunoinformatics in COVID-19 Vaccine Development: The Role of HLA System" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27450" ["VALUE"]=> array(2) { ["TEXT"]=> string(700) "<p><sup>1</sup> St. Petersburg City Hospital №40, St. Petersburg, Russia <br> <sup>2</sup> St. Petersburg State University School of Medicine, St. Petersburg, Russia<br> <sup>3</sup> D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia<br> <sup>4</sup> Pavlov University, St.Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia<br> E-mail: golotaa@yahoo.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(580) "1 St. Petersburg City Hospital №40, St. Petersburg, Russia
2 St. Petersburg State University School of Medicine, St. Petersburg, Russia
3 D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia
4 Pavlov University, St.Petersburg, Russia
Correspondence
Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia
E-mail: golotaa@yahoo.com
1 St. Petersburg City Hospital №40, St. Petersburg, Russia
2 St. Petersburg State University School of Medicine, St. Petersburg, Russia
3 D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia
4 Pavlov University, St.Petersburg, Russia
Correspondence
Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia
E-mail: golotaa@yahoo.com
Анна Ю. Анисенкова1, Александр С. Голота1, Дмитрий А. Вологжанин1, Татьяна А. Камилова1, Станислав В. Макаренко1,2, Ольга В. Шнейдер1, Олег С. Глотов1,3, Юрий А. Серов4, Сергей В. Мосенко1, Сергей В. Азаренко1, Константин В. Сманцерев1, Дмитрий Н. Хоботников1, Татьяна В. Гладышева1, Сергей Г. Щербак1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(680) "Анна Ю. Анисенкова1, Александр С. Голота1, Дмитрий А. Вологжанин1, Татьяна А. Камилова1, Станислав В. Макаренко1,2, Ольга В. Шнейдер1, Олег С. Глотов1,3, Юрий А. Серов4, Сергей В. Мосенко1, Сергей В. Азаренко1, Константин В. Сманцерев1, Дмитрий Н. Хоботников1, Татьяна В. Гладышева1, Сергей Г. Щербак1,2
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27442" ["VALUE"]=> string(22) "12/25/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/25/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/25/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27443" ["VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/12/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27446" ["VALUE"]=> array(2) { ["TEXT"]=> string(2762) "<p style="text-align: justify;"> Индивидуальные генетические вариации могут объяснить различные иммунные реакции на инфекцию коронавируса SARS-CoV-2 в популяции. Методология компьютерного моделирования <i>in silico</i> предоставляет экспериментальному сообществу список иммуногенных пептидов SARS-CoV-2, презентируемых антигенами HLA-системы. В этом обзоре представлен профиль предсказанных <i>in silico</i> иммуногенных пептидов вируса SARS-CoV-2 для функциональной валидации и разработки вакцин. Исследования, проводимые независимо друг от друга, дают высокую степень уверенности в воспроизводимости результатов. Вычислительное прогнозирование является инструментом быстрого и экономичного решения для предотвращения распространения и, в конечном итоге, устранения инфекции. Большая часть усилий по разработке вакцин и лекарств против SARS-CoV-2 направлена на гликопротеин шипа (S-белок), главный индуктор нейтрализующих антител. Несколько вакцин-кандидатов продемонстрировали эффективность в исследованиях <i>in vitro</i> и перешли в рандомизированные испытания на животных или людях для противодействия инфекции COVID-19. В этой статье освещаются текущие достижения в разработке субъединичных вакцин для борьбы с COVID-19, которые сокращают время и затраты при разработке вакцин. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Коронавирус, SARS-CoV-2, COVID-19, HLA, иммуногенный пептид, антиген, эпитоп, вакцина, компьютерное прогнозирование, компьютерное моделирование <i>in silico</i>, иммуноинформатика. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2658) "Индивидуальные генетические вариации могут объяснить различные иммунные реакции на инфекцию коронавируса SARS-CoV-2 в популяции. Методология компьютерного моделирования in silico предоставляет экспериментальному сообществу список иммуногенных пептидов SARS-CoV-2, презентируемых антигенами HLA-системы. В этом обзоре представлен профиль предсказанных in silico иммуногенных пептидов вируса SARS-CoV-2 для функциональной валидации и разработки вакцин. Исследования, проводимые независимо друг от друга, дают высокую степень уверенности в воспроизводимости результатов. Вычислительное прогнозирование является инструментом быстрого и экономичного решения для предотвращения распространения и, в конечном итоге, устранения инфекции. Большая часть усилий по разработке вакцин и лекарств против SARS-CoV-2 направлена на гликопротеин шипа (S-белок), главный индуктор нейтрализующих антител. Несколько вакцин-кандидатов продемонстрировали эффективность в исследованиях in vitro и перешли в рандомизированные испытания на животных или людях для противодействия инфекции COVID-19. В этой статье освещаются текущие достижения в разработке субъединичных вакцин для борьбы с COVID-19, которые сокращают время и затраты при разработке вакцин.
Ключевые слова
Коронавирус, SARS-CoV-2, COVID-19, HLA, иммуногенный пептид, антиген, эпитоп, вакцина, компьютерное прогнозирование, компьютерное моделирование in silico, иммуноинформатика.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2658) "Индивидуальные генетические вариации могут объяснить различные иммунные реакции на инфекцию коронавируса SARS-CoV-2 в популяции. Методология компьютерного моделирования in silico предоставляет экспериментальному сообществу список иммуногенных пептидов SARS-CoV-2, презентируемых антигенами HLA-системы. В этом обзоре представлен профиль предсказанных in silico иммуногенных пептидов вируса SARS-CoV-2 для функциональной валидации и разработки вакцин. Исследования, проводимые независимо друг от друга, дают высокую степень уверенности в воспроизводимости результатов. Вычислительное прогнозирование является инструментом быстрого и экономичного решения для предотвращения распространения и, в конечном итоге, устранения инфекции. Большая часть усилий по разработке вакцин и лекарств против SARS-CoV-2 направлена на гликопротеин шипа (S-белок), главный индуктор нейтрализующих антител. Несколько вакцин-кандидатов продемонстрировали эффективность в исследованиях in vitro и перешли в рандомизированные испытания на животных или людях для противодействия инфекции COVID-19. В этой статье освещаются текущие достижения в разработке субъединичных вакцин для борьбы с COVID-19, которые сокращают время и затраты при разработке вакцин.
Ключевые слова
Коронавирус, SARS-CoV-2, COVID-19, HLA, иммуногенный пептид, антиген, эпитоп, вакцина, компьютерное прогнозирование, компьютерное моделирование in silico, иммуноинформатика.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27445" ["VALUE"]=> array(2) { ["TEXT"]=> string(787) "<p><sup>1</sup> СПб ГБУЗ «Городская больница №40 Курортного района», Сестрорецк, Россия <br> <sup>2</sup> Санкт-Петербургский государственный университет, Санкт-Петербург, Россия<br> <sup>3</sup> НИИ акушерства, гинекологии и репродуктологии им. Д. О. Отта, Санкт-Петербург, Россия<br> <sup>4</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(709) "1 СПб ГБУЗ «Городская больница №40 Курортного района», Сестрорецк, Россия
2 Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 НИИ акушерства, гинекологии и репродуктологии им. Д. О. Отта, Санкт-Петербург, Россия
4 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
1 СПб ГБУЗ «Городская больница №40 Курортного района», Сестрорецк, Россия
2 Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 НИИ акушерства, гинекологии и репродуктологии им. Д. О. Отта, Санкт-Петербург, Россия
4 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
Introduction
Over recent decades, significant advances were made in treatment of hematological malignancies, which were primarily associated with intensive antitumor therapy and hematopoietic stem cell transplantation (HSCT). Probability of 10-year survival among hematological cancer patients subjected to HSCT is 85%, as shown by the large study performed by Wingard et al. [1].
Multiple myeloma accounts for approximately 10% of oncohematological disorders, and 1% of total cancer incidence. The concept of high-dose chemotherapy followed by autologous HSCT was dated back to early 980s and remains the ‘golden standard’ for treating newly diagnosed MM in young and some older patients. The advent of novel agents, such as immunomodulatory drugs, proteasome inhibitors and monoclonal antibodies does not compete with ASCT. Instead, the novel approaches supported its central role as the standard of care [2].
However, implementation of modern therapy programs in MM may increase the risk of infectious complications that require urgent treatment [3]. In turn, early administration of antibiotic therapy requires rapid identification of infectious agents, in particular, over recent years, due to widespread prevalence of nosocomial multidrug-resistant (MDR) microorganisms [4]. Septic complications in patients with hemoblastoses after HSCT develop, mostly, due to bacterial translocation from intestines to the bloodstream, though other ways of pathogen penetration are possible [5]. Suppression of immune system during chemotherapy of oncohematological diseases is an additional prerequisite for severe infectious complications, thus leading to impairment of complex anti-infectious defense systems.
Hence, the aim of our study was to determine the frequency of infectious complications in patients with multiple myeloma in the post-transplant period and their influence on the results of autologous hematopoietic stem cell transplantation.
Patients and methods
The study included 38 adult patients with MM who were treated at the Russian Research Institute of Hematology and Transfusiology, from January 2018 to February 2020. All the patients received a conditioning regimen with melphalan: monotherapy at a dose of 200 mg/m2 (29 patients), and 140 mg/m2 (4 cases), or combined therapy with melphalan and carfilzomib (5 patients).
Infectious complications were diagnosed, as based on routine clinical examination and laboratory tests, including bacterial cultures and tests for antibiotic resistance. Herpesviruses in peripheral blood were detected by means of PCR techniques.
Antibacterial therapy was started immediately upon diagnosis of infection. First-line anti-infectious therapy was usually performed at empirical basis, in accordance with conventional regimens, at average doses of antibiotic drugs [3]. The antimicrobial therapy could be modified upon changing data on antibiotics resistance, carbapenems were added in the most severe cases. When detecting CMV or EBV, the therapy was accomplished by antiviral drugs, i.e., Acyclovir, Ganciclovir.
The Sepsis-3 Criteria were used to characterize bacterial infections. In accordance with the definition adopted by the International Consensus Commission on Sepsis and Septic Shock [6], sepsis was defined as a condition characterized by the presence of an infectious locus and multiple organ failure. For the diagnosis of sepsis (SEPSIS 3), the Quick SOFA scale (rapid scale for assessing the severity of organ dysfunction) was used, which included only 3 criteria: (1) respiratory rate of >22/min or higher; (2) changing mental status, and (3) systolic blood pressure of <100 mm Hg. The patients with proven or suspected bacterial infection, organ dysfunction and more than 2 points on the Quick SOFA scale were defined as patients with sepsis. Clinical verification of septic shock was based on the need for vasopressor support to the mean arterial pressure values of >65 mm Hg, along with serum lactate over 2 mmol/l (18 mg/dl), in the absence of hypovolemia [6].
Before starting antibacterial therapy, and later, during febrile episodes, 8 mL of peripheral blood were taken regularly, both from peripheral vein and central venous catheter, with standard aseptic precautions, and incubated in aerobic/anaerobic bioMerieux BacT/ALERT culture media using BacT/ALERT 3D automated microbial detection system until positive results, or till day 7. Bacteriological analyses and identification of micromycetes were performed by routine technique over the entire study period, according to current guidelines [7]. We have also used a molecular biology method for rapid identification of microorganisms in blood cultures by means of real-time multiplex PCR technique [8]. Upon the RT-PCR-based detection of Gram-negative microorganisms in the cultures, we tested them for acquired carbapenemase genes, i.e., metallo-β-lactamases (VIM, IMP, NDM groups), KPC, and OXA-48-like groups were. To this purpose, we used PCR kits manufactured by InterLabService (AmpliSens®, Moscow, Russia).
RT-PCR was also used to detect respiratory viruses in nucleic acids extracted from the throat swabs. The diagnostic panel included PCR kits from InterLabService (AmpliSens®, Moscow, Russia) for the following pathogens: respiratory syncytial virus (RSV), 4 types of parainfluenza virus (PIV), rhinoviruses, coronaviruses (OS43, 229E, NL63, HKU1), metapneumovirus, adenovirus and human bocavirus, as well as Clamydophila pneumoniae and Mycoplasma pneumoniae. To detect the genomes of herpesviruses in blood, we used RT-PCR. The herpesvirus panel included herpes simplex viruses type 1 and 2 (HSV 1,2); cytomegalovirus (CMV); Epstein-Barr virus (EBV), and human herpesvirus type 6 (HHV6). PCR techniques were performed according to the manufacturer instructions (AmpliSens®, Moscow, Russia). The declared analytical sensitivity for the test systems was 500…1000 copies/mL for HSV1/2, and 5×105 per 105 leukocytes for EBV, CMV, and HHV type 6.
Statistical evaluation
The analysis of the data obtained was performed with IBM SPSS Statistics 22 software. To evaluate the results, mean values, medians, and ratios were presented as percentage values. The life expectancy curves according to Kaplan-Meier were calculated from the day of ASCT (D0) to the time of death, or date of last contact with the patient. Competing conditions (death from progression, or alternative reasons) have not been revealed in this group. Statistically the difference was considered significant at p <0.05. ROC-analysis was used, in order to analyze probability of CMV and EBV reactivation and development of bacterial complications in the patients at different ages.
Table 1. Demographic and clinical baseline characteristics of patients, included in the study

Table 2. Antitumor response to CVD therapy of MM achieved before ASCT (n=38) 

Figure 1. Overall survival rate after ASCT during the 826-day follow-up (Kaplan-Meier method)

Figure 2. ROC curve that shows dependence of the probability between the "CMV and EBV reactivation" index and the “Age” index

Figure 3. ROC curve that shows dependence of the probability between the "Bloodstream infection" index and the “Age” index
Results
Clinical response and survival
We studied 38 patients (18 women and 20 men) aged 39-70 years treated at the Russian Research Institute of Hematology and Transfusiology, during the period of January 2018 to February 2020. All the patients were diagnosed with multiple myeloma. Baseline demographic and clinical characteristics of the patients included into the study are shown in Table 1.
The median age of patients at the time of ASCT was 57 years. Most patients were at the III, Durie-Salmon stage of MM. Renal impairment was found in 15.8% of the cases. The patients were treated with bortezomib-based combination (CVD), followed by ASCT (29 single and 9 tandem). Clinical response rates to CVD therapy are shown in Table 2.
In 28.9% of the patients, complete response (CR) to therapy was shown, very good partial response (VGPR) was observed in 21.1%, and partial response (PR), in 50.0%. The median follow-up was 370 days (range, 9 to 826 days). During first 60 days after ASCT, one patient (2.6%) died due to acute heart failure. Among the remaining 37 patients, there were no deaths during the follow-up period (Fig. 1).
Infectious complications after ASCT
During the first 60 days after the ASCT, 12 episodes of infectious complications were recorded: CMV reactivation occurred in 5 cases, EBV was detected in 3 patients. In one case, a mixed-pathogen pneumonia was diagnosed, as based on conventional symptoms of chest infection and radiographic findings. In this patient, respiratory syncytial virus (RSV) genome was detected in throat swab, along with S.aureus in sputum. EBV was detected in blood at the same terms. Bacterial infections of the bloodstream were diagnosed in 3 patients (7.1%).
We have also asked a question of chances to develop infections for the patients of different ages. ROC-analysis was used to assess the probability of reactivation of CMV and EBV herpes viruses in patients after ASCT (Fig. 2).
The threshold value of the "age" index at the cut-off point of 57 years was determined by the ROC-analysis, i.e., if the value of the index "age" is higher or equal to 57 years, the probability of reactivation of CMV and EBV infections is predictable. The resulting model was statistically significant (p=0.036). The sensitivity and specificity of the method were 69% and 66.7%, respectively.
Bacterial bloodstream infections
According to the Sepsis-3 criteria [6], bacterial infections of different severity were diagnosed in three patients, as follows: bloodstream infection, sepsis, and septic shock. In the first patient, the infection occurred on the 6th day after transplantation in presence of agranulocytosis and grade 4 thrombocytopenia, with fever up to 38.3°C, and a decrease in blood pressure to 80/40 mm Hg, heart rate – 114/min, and respiratory rate 20/min. The condition was regarded as septic shock. The second patient had an episode of E. coli infection found in peripheral blood by the day +40 after ASCT, regarded as septic state (2 points on the Quick SOFA scale). In both cases, the infection was caused by multidrug-resistant E. coli. In the third patient, on the 6th day after ASCT, in presence of fever (up to 38°C), a Gram-negative microorganism of the Enterobacteriaceae family was detected. No symptoms compliant with Sepsis-3 criteria were registered, and this infectious episode was considered as a bloodstream infection. Despite severity of clinical manifestations, these infectious complications did not have a negative prognostic effect on the 100-day overall survival.
ROC-analysis was used to assess the probability of developing bloodstream infections in patients from different age groups in the period after ASCT (Fig. 3).
The threshold value of the "age" index was established at the cutoff point of 53. If the value of the age index is higher or equal to 53 years, the probability of developing bloodstream infections is well predicted. The resulting model was statistically significant (p<0.001). The sensitivity and specificity of the method were 80% and 66.7%, respectively.
Discussion
Multiple myeloma, a bone marrow-resident plasma cell malignancy, still remains largely incurable, despite dramatic improvements in the patient outcomes with advent of myeloma-targeted and immunomodulatory agents. ASCT remains an important consolidation treatment in the patients with MM. It has recently become clear that T cells from the MM patients are able to recognize and eliminate myeloma [9]. These data provide new insights into mechanisms of action of ASCT and provide rational approaches to improving clinical outcomes.
Infectious complications in MM, especially in elderly patients, remain an important issue. They restrict further development and improvement of therapies in hemato-oncology, in particular, after HSCT. The difficulty of treating and preventing infections in HSCT is associated with a variety of potential infectious pathogens, a need for their rapid detection, and early causal treatment.
The role of CMV infection in patients with hemoblastoses is well known [10]. CMV reactivation is often diagnosed in allogeneic HSCT recipients and therefore could lead to CMV-related disease, involving many organs in these immunocompromised patients. We have also previously found mixed bacterial/viral infections [11]. In contrast, few studies concerned CMV reactivation in ASCT since these patients are considered at low risk for both CMV reactivation and disease [12]. Our study demonstrated that CMV activation is quite often observed in the patients during early post-transplant period, e.g., after ASCT.
As to the EBV, in most cases its reactivation proceeds subclinically, and does not require special therapy. However, in some HSCT patients, EBV may cause life-threatening complications: post-transplant lymphoproliferative disorder (PTLD), or end-organ diseases such as encephalitis/myelitis, pneumonia, or hepatitis. EBV might be transmitted with the graft, since EBV-PTLD after HSCT is usually of donor origin. The risk of EBV-PTLD is higher, when the donor is seropositive [12]. However, there is a report on PTLD cases in MM patients after ASCT [13].
In the present work, we revealed that, at the age of more than 57 years, reactivation of CMV and EBV infection is highly probable. On the other hand, no clinical manifestations of CMV or EBM infection were documented. We can speculate that this can be explained by the increased probability of developing systemic bacterial infections, due to immunosuppression under the influence of CMV and EBV activation, especially in elderly patients. Previously, we performed a comparative study of herpesvirus frequency in cases of proven bacterial infections in bloodstream. We have revealed a statistically significant (p<0.05) increase of CMV and EBV incidence in blood of the patients who developed bacteremia, as compared with bacteremia-free cases [11].
In one case, pneumonia was diagnosed on day +16 after ASCT. Pneumonia diagnosis was based on traditional signs and symptoms of chest infection and chest radiography, as well as RSV and EBV positivity. We have previously shown the importance of respiratory viruses and, especially, RSV and their associations with CMV in the development of bacterial complications in MM following allogeneic bone marrow transplantation [14]. The present results suggest that respiratory viral infections should be carefully controlled also in ASCT patients.
Development of bacterial infections of the bloodstream and sepsis is a serious issue in HSCT setting. Among the causative agents of sepsis in hematological cancer patients, Gram-negative microbes are more common and dangerous, the mortality rate with these infections can reach 57% [11].
In our study, bloodstream infections were revealed in three patients. In all cases, they were caused by gram-negative microorganisms. In addition, multidrug-resistant (MDR) bacteria were identified by detecting carbapenemase genes, and faster diagnostic methods by PCR-RT were applied, thus reducing analysis time by 24-48 hours [8].
Despite severe clinical manifestations of bloodstream infections caused by gram-negative microbes, their presence did not adversely affect the overall survival rate. In our opinion, this is to a certain extent related to the use of infection control methods in clinical practice: detection of MDR strains, the use of accelerated methods for identifying bacteria and timely determination of antimicrobial therapy strategy, taking into account the results obtained.
Conclusion
Over decades, ASCT remains the standard of care for young patients with newly diagnosed multiple myeloma. Autologous stem cell transplant is increasingly used also in older patients with MM. Meanwhile, such care causes negative effects, associated with significant risks of infectious complications, such as bacterial sepsis and activation of viruses, especially herpes viruses (CMV and EBV).
Cytomegalovirus reactivation is often diagnosed in allogeneic hematopoietic cell transplant recipients and, therefore, could lead to CMV disease in immunocompromised patients. In contrast, few studies investigated CMV reactivation in the patients undergoing ASCT, since they are considered at low risk for both reactivation and disease. In present work we revealed a significant role of CMV also in ASCT setting.
The present work has supported our previous results on the role of respiratory viruses and especially RSV and their associations with CMV in development of bacterial infectious complications, i.e., pneumonia in MM following allogeneic bone marrow transplantation [12]. Hence, the respiratory viral infections should be thoroughly controlled also in ASCT patients.
Development of bloodstream bacterial infections and sepsis remain a serious issue. The Sepsis 3 Criteria can be successfully used in patients with ASCT to discern the groups with dismal prognosis (progression to severe sepsis and septic shock).
In our study, bloodstream infections were diagnosed in three patients. In all cases, they were caused by Gram-negative microorganisms. In addition, the multidrug-resistant bacteria were monitored, and the methods of rapid identification of bacteria by RT-PCR were applied, thus sufficiently shortening the terms of analysis. More fast and precise methods of revealing the infectious pathogens in clinical practice (identification of multidrug-resistant strains and usage of antimicrobial therapy control strategies) will improve efficiency of treatment in MM patients following ASCT.
Conflict of interest
None declared.
References
- Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, Sorror ML, Horowitz MM,Bolwell B, Rizzo JD, Socié G. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Clin Oncol. 2011; 29(16):2230-2239. doi: 10.1200/JCO.2010.33.7212.
- Hamed A, Bazarbachi A, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019 Apr 8;9(4):44. doi: 10.1038/s41408-019-0205-9.
- Bessmeltsev SS, Abdulkadyrov KM. Multiple myeloma: The Physicians’ Guide. Moscow: MK Publishers. 2016, 326 p. (In Russian).
- Abraham E. New definitions for sepsis and septic shock: continuing evolution but with much still to be done. JAMA. 2016;315(8):757-759. doi: 10.1001/jama.2016.0290.
- Chukhlovin AB, Pankratova OS. Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency. Cell Ther Transplant. 2017; 6(4): 28-41.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016: 315(8):801-810. doi: 10.1001/jama.2016.0287.
- Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (eds). Manual of Clinical Microbiology, 10th Edition, Vol. 1, 2011, ASM Press.
- Chebotkevich VN, Martens JA, Sidorenko SV, Kiseleva EE. Accelerated method of identification of bacteria and micromycetes in hemocultures in children using multiplex PCR in real time. Zhurnal infektologii. 2019;11(4): 107-112 (in Russian).
- Minnie SA, Geoffrey RH. Immunotherapy of multiple myeloma. J Clin Invest. 2020;130(4):1565-1575. doi: 10.1172/JCI129205.
- Moiseev SI, Nuĭia ML, Chebotkevich VN, Gonchar VA, Abdulkadyrov KM. Cytomegalovirus infection in bone marrow transplantation. Ter Arkh. 2002;74(7):44-48. PMID: 12181834 (In Russian).
- Chebotkevich VN, Bessmeltsev SS, Kiseleva EE, Stizhak NP, Kaytandzhan EI, Burylev VV. Cellular bloodstream infections and herpesvirus activation following intensive chemotherapy of adult oncohematological patients. Cell Ther Transplant. 2016;5(4):21-31. doi: 10.18620/ctt-1866-8836-2016-5-4-21-31.
- Ljungman P, Styczynski J, Einsele H. The EBMT Handbook Hematopoietic Stem Cell Transplantation and Cellular Therapies, 2019, Chapter 39: Viral Infections, p.281-290.
- Ishikawa T, Shimizu H, Takei T, Koya H, Iriuchishima H, Hosiho T, Hirato J, Kojima M, Handa H, Nojima Y et al. Monomorphic post-transplant T-lymphoproliferative disorder after autologous stem cell transplantation for multiple myeloma. Jap J Clin Hematol. 2016; 57(1):36-40. doi: 10.11406/rinketsu.57.36
- Tchebotkevitch V, Roomel N, Bessmeltsev S, Abdoulkadirov K. Respiratory syncytial virus in oncohematologic patients. Supportive Care in Cancer. 2000; 8(3):247.
" ["~DETAIL_TEXT"]=> string(24328) "
Introduction
Over recent decades, significant advances were made in treatment of hematological malignancies, which were primarily associated with intensive antitumor therapy and hematopoietic stem cell transplantation (HSCT). Probability of 10-year survival among hematological cancer patients subjected to HSCT is 85%, as shown by the large study performed by Wingard et al. [1].
Multiple myeloma accounts for approximately 10% of oncohematological disorders, and 1% of total cancer incidence. The concept of high-dose chemotherapy followed by autologous HSCT was dated back to early 980s and remains the ‘golden standard’ for treating newly diagnosed MM in young and some older patients. The advent of novel agents, such as immunomodulatory drugs, proteasome inhibitors and monoclonal antibodies does not compete with ASCT. Instead, the novel approaches supported its central role as the standard of care [2].
However, implementation of modern therapy programs in MM may increase the risk of infectious complications that require urgent treatment [3]. In turn, early administration of antibiotic therapy requires rapid identification of infectious agents, in particular, over recent years, due to widespread prevalence of nosocomial multidrug-resistant (MDR) microorganisms [4]. Septic complications in patients with hemoblastoses after HSCT develop, mostly, due to bacterial translocation from intestines to the bloodstream, though other ways of pathogen penetration are possible [5]. Suppression of immune system during chemotherapy of oncohematological diseases is an additional prerequisite for severe infectious complications, thus leading to impairment of complex anti-infectious defense systems.
Hence, the aim of our study was to determine the frequency of infectious complications in patients with multiple myeloma in the post-transplant period and their influence on the results of autologous hematopoietic stem cell transplantation.
Patients and methods
The study included 38 adult patients with MM who were treated at the Russian Research Institute of Hematology and Transfusiology, from January 2018 to February 2020. All the patients received a conditioning regimen with melphalan: monotherapy at a dose of 200 mg/m2 (29 patients), and 140 mg/m2 (4 cases), or combined therapy with melphalan and carfilzomib (5 patients).
Infectious complications were diagnosed, as based on routine clinical examination and laboratory tests, including bacterial cultures and tests for antibiotic resistance. Herpesviruses in peripheral blood were detected by means of PCR techniques.
Antibacterial therapy was started immediately upon diagnosis of infection. First-line anti-infectious therapy was usually performed at empirical basis, in accordance with conventional regimens, at average doses of antibiotic drugs [3]. The antimicrobial therapy could be modified upon changing data on antibiotics resistance, carbapenems were added in the most severe cases. When detecting CMV or EBV, the therapy was accomplished by antiviral drugs, i.e., Acyclovir, Ganciclovir.
The Sepsis-3 Criteria were used to characterize bacterial infections. In accordance with the definition adopted by the International Consensus Commission on Sepsis and Septic Shock [6], sepsis was defined as a condition characterized by the presence of an infectious locus and multiple organ failure. For the diagnosis of sepsis (SEPSIS 3), the Quick SOFA scale (rapid scale for assessing the severity of organ dysfunction) was used, which included only 3 criteria: (1) respiratory rate of >22/min or higher; (2) changing mental status, and (3) systolic blood pressure of <100 mm Hg. The patients with proven or suspected bacterial infection, organ dysfunction and more than 2 points on the Quick SOFA scale were defined as patients with sepsis. Clinical verification of septic shock was based on the need for vasopressor support to the mean arterial pressure values of >65 mm Hg, along with serum lactate over 2 mmol/l (18 mg/dl), in the absence of hypovolemia [6].
Before starting antibacterial therapy, and later, during febrile episodes, 8 mL of peripheral blood were taken regularly, both from peripheral vein and central venous catheter, with standard aseptic precautions, and incubated in aerobic/anaerobic bioMerieux BacT/ALERT culture media using BacT/ALERT 3D automated microbial detection system until positive results, or till day 7. Bacteriological analyses and identification of micromycetes were performed by routine technique over the entire study period, according to current guidelines [7]. We have also used a molecular biology method for rapid identification of microorganisms in blood cultures by means of real-time multiplex PCR technique [8]. Upon the RT-PCR-based detection of Gram-negative microorganisms in the cultures, we tested them for acquired carbapenemase genes, i.e., metallo-β-lactamases (VIM, IMP, NDM groups), KPC, and OXA-48-like groups were. To this purpose, we used PCR kits manufactured by InterLabService (AmpliSens®, Moscow, Russia).
RT-PCR was also used to detect respiratory viruses in nucleic acids extracted from the throat swabs. The diagnostic panel included PCR kits from InterLabService (AmpliSens®, Moscow, Russia) for the following pathogens: respiratory syncytial virus (RSV), 4 types of parainfluenza virus (PIV), rhinoviruses, coronaviruses (OS43, 229E, NL63, HKU1), metapneumovirus, adenovirus and human bocavirus, as well as Clamydophila pneumoniae and Mycoplasma pneumoniae. To detect the genomes of herpesviruses in blood, we used RT-PCR. The herpesvirus panel included herpes simplex viruses type 1 and 2 (HSV 1,2); cytomegalovirus (CMV); Epstein-Barr virus (EBV), and human herpesvirus type 6 (HHV6). PCR techniques were performed according to the manufacturer instructions (AmpliSens®, Moscow, Russia). The declared analytical sensitivity for the test systems was 500…1000 copies/mL for HSV1/2, and 5×105 per 105 leukocytes for EBV, CMV, and HHV type 6.
Statistical evaluation
The analysis of the data obtained was performed with IBM SPSS Statistics 22 software. To evaluate the results, mean values, medians, and ratios were presented as percentage values. The life expectancy curves according to Kaplan-Meier were calculated from the day of ASCT (D0) to the time of death, or date of last contact with the patient. Competing conditions (death from progression, or alternative reasons) have not been revealed in this group. Statistically the difference was considered significant at p <0.05. ROC-analysis was used, in order to analyze probability of CMV and EBV reactivation and development of bacterial complications in the patients at different ages.
Table 1. Demographic and clinical baseline characteristics of patients, included in the study

Table 2. Antitumor response to CVD therapy of MM achieved before ASCT (n=38) 

Figure 1. Overall survival rate after ASCT during the 826-day follow-up (Kaplan-Meier method)

Figure 2. ROC curve that shows dependence of the probability between the "CMV and EBV reactivation" index and the “Age” index

Figure 3. ROC curve that shows dependence of the probability between the "Bloodstream infection" index and the “Age” index
Results
Clinical response and survival
We studied 38 patients (18 women and 20 men) aged 39-70 years treated at the Russian Research Institute of Hematology and Transfusiology, during the period of January 2018 to February 2020. All the patients were diagnosed with multiple myeloma. Baseline demographic and clinical characteristics of the patients included into the study are shown in Table 1.
The median age of patients at the time of ASCT was 57 years. Most patients were at the III, Durie-Salmon stage of MM. Renal impairment was found in 15.8% of the cases. The patients were treated with bortezomib-based combination (CVD), followed by ASCT (29 single and 9 tandem). Clinical response rates to CVD therapy are shown in Table 2.
In 28.9% of the patients, complete response (CR) to therapy was shown, very good partial response (VGPR) was observed in 21.1%, and partial response (PR), in 50.0%. The median follow-up was 370 days (range, 9 to 826 days). During first 60 days after ASCT, one patient (2.6%) died due to acute heart failure. Among the remaining 37 patients, there were no deaths during the follow-up period (Fig. 1).
Infectious complications after ASCT
During the first 60 days after the ASCT, 12 episodes of infectious complications were recorded: CMV reactivation occurred in 5 cases, EBV was detected in 3 patients. In one case, a mixed-pathogen pneumonia was diagnosed, as based on conventional symptoms of chest infection and radiographic findings. In this patient, respiratory syncytial virus (RSV) genome was detected in throat swab, along with S.aureus in sputum. EBV was detected in blood at the same terms. Bacterial infections of the bloodstream were diagnosed in 3 patients (7.1%).
We have also asked a question of chances to develop infections for the patients of different ages. ROC-analysis was used to assess the probability of reactivation of CMV and EBV herpes viruses in patients after ASCT (Fig. 2).
The threshold value of the "age" index at the cut-off point of 57 years was determined by the ROC-analysis, i.e., if the value of the index "age" is higher or equal to 57 years, the probability of reactivation of CMV and EBV infections is predictable. The resulting model was statistically significant (p=0.036). The sensitivity and specificity of the method were 69% and 66.7%, respectively.
Bacterial bloodstream infections
According to the Sepsis-3 criteria [6], bacterial infections of different severity were diagnosed in three patients, as follows: bloodstream infection, sepsis, and septic shock. In the first patient, the infection occurred on the 6th day after transplantation in presence of agranulocytosis and grade 4 thrombocytopenia, with fever up to 38.3°C, and a decrease in blood pressure to 80/40 mm Hg, heart rate – 114/min, and respiratory rate 20/min. The condition was regarded as septic shock. The second patient had an episode of E. coli infection found in peripheral blood by the day +40 after ASCT, regarded as septic state (2 points on the Quick SOFA scale). In both cases, the infection was caused by multidrug-resistant E. coli. In the third patient, on the 6th day after ASCT, in presence of fever (up to 38°C), a Gram-negative microorganism of the Enterobacteriaceae family was detected. No symptoms compliant with Sepsis-3 criteria were registered, and this infectious episode was considered as a bloodstream infection. Despite severity of clinical manifestations, these infectious complications did not have a negative prognostic effect on the 100-day overall survival.
ROC-analysis was used to assess the probability of developing bloodstream infections in patients from different age groups in the period after ASCT (Fig. 3).
The threshold value of the "age" index was established at the cutoff point of 53. If the value of the age index is higher or equal to 53 years, the probability of developing bloodstream infections is well predicted. The resulting model was statistically significant (p<0.001). The sensitivity and specificity of the method were 80% and 66.7%, respectively.
Discussion
Multiple myeloma, a bone marrow-resident plasma cell malignancy, still remains largely incurable, despite dramatic improvements in the patient outcomes with advent of myeloma-targeted and immunomodulatory agents. ASCT remains an important consolidation treatment in the patients with MM. It has recently become clear that T cells from the MM patients are able to recognize and eliminate myeloma [9]. These data provide new insights into mechanisms of action of ASCT and provide rational approaches to improving clinical outcomes.
Infectious complications in MM, especially in elderly patients, remain an important issue. They restrict further development and improvement of therapies in hemato-oncology, in particular, after HSCT. The difficulty of treating and preventing infections in HSCT is associated with a variety of potential infectious pathogens, a need for their rapid detection, and early causal treatment.
The role of CMV infection in patients with hemoblastoses is well known [10]. CMV reactivation is often diagnosed in allogeneic HSCT recipients and therefore could lead to CMV-related disease, involving many organs in these immunocompromised patients. We have also previously found mixed bacterial/viral infections [11]. In contrast, few studies concerned CMV reactivation in ASCT since these patients are considered at low risk for both CMV reactivation and disease [12]. Our study demonstrated that CMV activation is quite often observed in the patients during early post-transplant period, e.g., after ASCT.
As to the EBV, in most cases its reactivation proceeds subclinically, and does not require special therapy. However, in some HSCT patients, EBV may cause life-threatening complications: post-transplant lymphoproliferative disorder (PTLD), or end-organ diseases such as encephalitis/myelitis, pneumonia, or hepatitis. EBV might be transmitted with the graft, since EBV-PTLD after HSCT is usually of donor origin. The risk of EBV-PTLD is higher, when the donor is seropositive [12]. However, there is a report on PTLD cases in MM patients after ASCT [13].
In the present work, we revealed that, at the age of more than 57 years, reactivation of CMV and EBV infection is highly probable. On the other hand, no clinical manifestations of CMV or EBM infection were documented. We can speculate that this can be explained by the increased probability of developing systemic bacterial infections, due to immunosuppression under the influence of CMV and EBV activation, especially in elderly patients. Previously, we performed a comparative study of herpesvirus frequency in cases of proven bacterial infections in bloodstream. We have revealed a statistically significant (p<0.05) increase of CMV and EBV incidence in blood of the patients who developed bacteremia, as compared with bacteremia-free cases [11].
In one case, pneumonia was diagnosed on day +16 after ASCT. Pneumonia diagnosis was based on traditional signs and symptoms of chest infection and chest radiography, as well as RSV and EBV positivity. We have previously shown the importance of respiratory viruses and, especially, RSV and their associations with CMV in the development of bacterial complications in MM following allogeneic bone marrow transplantation [14]. The present results suggest that respiratory viral infections should be carefully controlled also in ASCT patients.
Development of bacterial infections of the bloodstream and sepsis is a serious issue in HSCT setting. Among the causative agents of sepsis in hematological cancer patients, Gram-negative microbes are more common and dangerous, the mortality rate with these infections can reach 57% [11].
In our study, bloodstream infections were revealed in three patients. In all cases, they were caused by gram-negative microorganisms. In addition, multidrug-resistant (MDR) bacteria were identified by detecting carbapenemase genes, and faster diagnostic methods by PCR-RT were applied, thus reducing analysis time by 24-48 hours [8].
Despite severe clinical manifestations of bloodstream infections caused by gram-negative microbes, their presence did not adversely affect the overall survival rate. In our opinion, this is to a certain extent related to the use of infection control methods in clinical practice: detection of MDR strains, the use of accelerated methods for identifying bacteria and timely determination of antimicrobial therapy strategy, taking into account the results obtained.
Conclusion
Over decades, ASCT remains the standard of care for young patients with newly diagnosed multiple myeloma. Autologous stem cell transplant is increasingly used also in older patients with MM. Meanwhile, such care causes negative effects, associated with significant risks of infectious complications, such as bacterial sepsis and activation of viruses, especially herpes viruses (CMV and EBV).
Cytomegalovirus reactivation is often diagnosed in allogeneic hematopoietic cell transplant recipients and, therefore, could lead to CMV disease in immunocompromised patients. In contrast, few studies investigated CMV reactivation in the patients undergoing ASCT, since they are considered at low risk for both reactivation and disease. In present work we revealed a significant role of CMV also in ASCT setting.
The present work has supported our previous results on the role of respiratory viruses and especially RSV and their associations with CMV in development of bacterial infectious complications, i.e., pneumonia in MM following allogeneic bone marrow transplantation [12]. Hence, the respiratory viral infections should be thoroughly controlled also in ASCT patients.
Development of bloodstream bacterial infections and sepsis remain a serious issue. The Sepsis 3 Criteria can be successfully used in patients with ASCT to discern the groups with dismal prognosis (progression to severe sepsis and septic shock).
In our study, bloodstream infections were diagnosed in three patients. In all cases, they were caused by Gram-negative microorganisms. In addition, the multidrug-resistant bacteria were monitored, and the methods of rapid identification of bacteria by RT-PCR were applied, thus sufficiently shortening the terms of analysis. More fast and precise methods of revealing the infectious pathogens in clinical practice (identification of multidrug-resistant strains and usage of antimicrobial therapy control strategies) will improve efficiency of treatment in MM patients following ASCT.
Conflict of interest
None declared.
References
- Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, Sorror ML, Horowitz MM,Bolwell B, Rizzo JD, Socié G. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Clin Oncol. 2011; 29(16):2230-2239. doi: 10.1200/JCO.2010.33.7212.
- Hamed A, Bazarbachi A, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019 Apr 8;9(4):44. doi: 10.1038/s41408-019-0205-9.
- Bessmeltsev SS, Abdulkadyrov KM. Multiple myeloma: The Physicians’ Guide. Moscow: MK Publishers. 2016, 326 p. (In Russian).
- Abraham E. New definitions for sepsis and septic shock: continuing evolution but with much still to be done. JAMA. 2016;315(8):757-759. doi: 10.1001/jama.2016.0290.
- Chukhlovin AB, Pankratova OS. Opportunistic microflora at unusual sites: marker pathogens in severe posttransplant immune deficiency. Cell Ther Transplant. 2017; 6(4): 28-41.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016: 315(8):801-810. doi: 10.1001/jama.2016.0287.
- Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (eds). Manual of Clinical Microbiology, 10th Edition, Vol. 1, 2011, ASM Press.
- Chebotkevich VN, Martens JA, Sidorenko SV, Kiseleva EE. Accelerated method of identification of bacteria and micromycetes in hemocultures in children using multiplex PCR in real time. Zhurnal infektologii. 2019;11(4): 107-112 (in Russian).
- Minnie SA, Geoffrey RH. Immunotherapy of multiple myeloma. J Clin Invest. 2020;130(4):1565-1575. doi: 10.1172/JCI129205.
- Moiseev SI, Nuĭia ML, Chebotkevich VN, Gonchar VA, Abdulkadyrov KM. Cytomegalovirus infection in bone marrow transplantation. Ter Arkh. 2002;74(7):44-48. PMID: 12181834 (In Russian).
- Chebotkevich VN, Bessmeltsev SS, Kiseleva EE, Stizhak NP, Kaytandzhan EI, Burylev VV. Cellular bloodstream infections and herpesvirus activation following intensive chemotherapy of adult oncohematological patients. Cell Ther Transplant. 2016;5(4):21-31. doi: 10.18620/ctt-1866-8836-2016-5-4-21-31.
- Ljungman P, Styczynski J, Einsele H. The EBMT Handbook Hematopoietic Stem Cell Transplantation and Cellular Therapies, 2019, Chapter 39: Viral Infections, p.281-290.
- Ishikawa T, Shimizu H, Takei T, Koya H, Iriuchishima H, Hosiho T, Hirato J, Kojima M, Handa H, Nojima Y et al. Monomorphic post-transplant T-lymphoproliferative disorder after autologous stem cell transplantation for multiple myeloma. Jap J Clin Hematol. 2016; 57(1):36-40. doi: 10.11406/rinketsu.57.36
- Tchebotkevitch V, Roomel N, Bessmeltsev S, Abdoulkadirov K. Respiratory syncytial virus in oncohematologic patients. Supportive Care in Cancer. 2000; 8(3):247.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "50" ["~SORT"]=> string(2) "50" ["CODE"]=> string(100) "infektsionnye-oslozhneniya-u-patsientov-s-mnozhestvennoy-mielomoy-perenesshikh-autologichnuyu-transp" ["~CODE"]=> string(100) "infektsionnye-oslozhneniya-u-patsientov-s-mnozhestvennoy-mielomoy-perenesshikh-autologichnuyu-transp" ["EXTERNAL_ID"]=> string(4) "1953" ["~EXTERNAL_ID"]=> string(4) "1953" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(380) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической кровиInfectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4584) "<p style="text-align: justify;">Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.</p> <p style="text-align: justify;">Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_META_TITLE"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_META_KEYWORDS"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_META_DESCRIPTION"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_PICTURE_FILE_ALT"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_PICTURE_FILE_TITLE"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "infektsionnye-oslozhneniya-u-patsientov-s-mnozhestvennoy-mielomoy-perenesshikh-autologichnuyu-transp" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(262) "Инфекционные осложнения у пациентов с множественной миеломой, перенесших аутологичную трансплантацию стволовых клеток периферической крови" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "infektsionnye-oslozhneniya-u-patsientov-s-mnozhestvennoy-mielomoy-perenesshikh-autologichnuyu-transp" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "infektsionnye-oslozhneniya-u-patsientov-s-mnozhestvennoy-mielomoy-perenesshikh-autologichnuyu-transp" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "infektsionnye-oslozhneniya-u-patsientov-s-mnozhestvennoy-mielomoy-perenesshikh-autologichnuyu-transp" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "175" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27492" ["VALUE"]=> string(22) "12/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27493" ["VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27494" ["VALUE"]=> array(2) { ["TEXT"]=> string(401) "<p>Виталий Н. Чеботкевич, Алена В. Кулешова, Анастасия А. Жернякова, Иван И. Кострома, Екатерина Е. Киселева, Елена И. Кайтанджан, Наталья Ю. Семенова, Станислав С. Бессмельцев, Александр В. Чечеткин, Сергей В. Грицаев</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(389) "
Виталий Н. Чеботкевич, Алена В. Кулешова, Анастасия А. Жернякова, Иван И. Кострома, Екатерина Е. Киселева, Елена И. Кайтанджан, Наталья Ю. Семенова, Станислав С. Бессмельцев, Александр В. Чечеткин, Сергей В. Грицаев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27495" ["VALUE"]=> array(2) { ["TEXT"]=> string(206) "<p>Российский научно-исследовательский институт гематологии и трансфузиологии, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(194) "Российский научно-исследовательский институт гематологии и трансфузиологии, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27496" ["VALUE"]=> array(2) { ["TEXT"]=> string(4584) "<p style="text-align: justify;">Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.</p> <p style="text-align: justify;">Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4506) "Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.
Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.
Ключевые слова
Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27497" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-63-68" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-63-68" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27499" ["VALUE"]=> array(2) { ["TEXT"]=> string(243) "<p>Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(231) "Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27500" ["VALUE"]=> array(2) { ["TEXT"]=> string(397) "<p>Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia </p><br> <p><b>Correspondence</b><br> Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia<br> Phone: +7 (906) 267 0266<br> E-mail: vitnikcheb@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(337) "Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia
Phone: +7 (906) 267 0266
E-mail: vitnikcheb@mail.ru
Multiple myeloma (MM) accounts for approximately 10% of blood malignancies and 1% of all cancers in general. The concept of high-dose chemotherapy followed by transplantation of autologous hematopoietic stem cells (ASCT) remains the standard for treating newly diagnosed multiple myeloma in young and in selected, fit, elderly patients. Infectious complications represent important cause of morbidity and mortality in MM patients. Bloodstream infections remain the most severe bacterial complication in recipients of hematopoietic stem cell transplantation, whereas herpesviruses, especially, cytomegalovirus (CMV), dominate among viral complications. We analyzed data on 38 patients with MM who underwent ASCT from January 2018 to February 2020. Reactivation of cytomegalovirus (CMV) was revealed in 5 cases (13.2%), and Epstein-Barr virus (EBV), in 3 patients (7.9%). Pneumonia was diagnosed in one case (2.6%). Bacterial bloodstream infections were detected in 3 patients (7.9%). The bloodstream infections were stratified in accordance with the Sepsis-3 criteria, thus enabling us to identify patients with unfavorable prognosis who developed sepsis and/or septic shock. Infectious complications were observed over the period of 60 days after ASCT. Meanwhile, CMV and EBV reactivation and bloodstream bacterial infections did not affect overall survival rate.
Conclusion
Our results demonstrate that bacterial complications and viral (CMV and EBV) reactivation aggravate the course of primary disease in MM patients over the post-transplant period. The methods of infection control in clinical practice (genotyping of multidrug-resistant strains, and antimicrobial control protocols) should improve the treatment strategies in patients with MM following ASCT. Keywords Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
Keywords
Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27498" ["VALUE"]=> string(118) "Infectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(118) "Infectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27534" ["VALUE"]=> string(4) "2424" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2424" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27535" ["VALUE"]=> string(4) "2425" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2425" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27499" ["VALUE"]=> array(2) { ["TEXT"]=> string(243) "<p>Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(231) "Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(231) "Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27501" ["VALUE"]=> array(2) { ["TEXT"]=> string(2257) "<p style="text-align: justify;"> Multiple myeloma (MM) accounts for approximately 10% of blood malignancies and 1% of all cancers in general. The concept of high-dose chemotherapy followed by transplantation of autologous hematopoietic stem cells (ASCT) remains the standard for treating newly diagnosed multiple myeloma in young and in selected, fit, elderly patients. Infectious complications represent important cause of morbidity and mortality in MM patients. Bloodstream infections remain the most severe bacterial complication in recipients of hematopoietic stem cell transplantation, whereas herpesviruses, especially, cytomegalovirus (CMV), dominate among viral complications. We analyzed data on 38 patients with MM who underwent ASCT from January 2018 to February 2020. Reactivation of cytomegalovirus (CMV) was revealed in 5 cases (13.2%), and Epstein-Barr virus (EBV), in 3 patients (7.9%). Pneumonia was diagnosed in one case (2.6%). Bacterial bloodstream infections were detected in 3 patients (7.9%). The bloodstream infections were stratified in accordance with the Sepsis-3 criteria, thus enabling us to identify patients with unfavorable prognosis who developed sepsis and/or septic shock. Infectious complications were observed over the period of 60 days after ASCT. Meanwhile, CMV and EBV reactivation and bloodstream bacterial infections did not affect overall survival rate. </p> <h3> Conclusion</h3> <p style="text-align: justify;"> Our results demonstrate that bacterial complications and viral (CMV and EBV) reactivation aggravate the course of primary disease in MM patients over the post-transplant period. The methods of infection control in clinical practice (genotyping of multidrug-resistant strains, and antimicrobial control protocols) should improve the treatment strategies in patients with MM following ASCT. Keywords Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2167) "Multiple myeloma (MM) accounts for approximately 10% of blood malignancies and 1% of all cancers in general. The concept of high-dose chemotherapy followed by transplantation of autologous hematopoietic stem cells (ASCT) remains the standard for treating newly diagnosed multiple myeloma in young and in selected, fit, elderly patients. Infectious complications represent important cause of morbidity and mortality in MM patients. Bloodstream infections remain the most severe bacterial complication in recipients of hematopoietic stem cell transplantation, whereas herpesviruses, especially, cytomegalovirus (CMV), dominate among viral complications. We analyzed data on 38 patients with MM who underwent ASCT from January 2018 to February 2020. Reactivation of cytomegalovirus (CMV) was revealed in 5 cases (13.2%), and Epstein-Barr virus (EBV), in 3 patients (7.9%). Pneumonia was diagnosed in one case (2.6%). Bacterial bloodstream infections were detected in 3 patients (7.9%). The bloodstream infections were stratified in accordance with the Sepsis-3 criteria, thus enabling us to identify patients with unfavorable prognosis who developed sepsis and/or septic shock. Infectious complications were observed over the period of 60 days after ASCT. Meanwhile, CMV and EBV reactivation and bloodstream bacterial infections did not affect overall survival rate.
Conclusion
Our results demonstrate that bacterial complications and viral (CMV and EBV) reactivation aggravate the course of primary disease in MM patients over the post-transplant period. The methods of infection control in clinical practice (genotyping of multidrug-resistant strains, and antimicrobial control protocols) should improve the treatment strategies in patients with MM following ASCT. Keywords Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
Keywords
Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2167) "Multiple myeloma (MM) accounts for approximately 10% of blood malignancies and 1% of all cancers in general. The concept of high-dose chemotherapy followed by transplantation of autologous hematopoietic stem cells (ASCT) remains the standard for treating newly diagnosed multiple myeloma in young and in selected, fit, elderly patients. Infectious complications represent important cause of morbidity and mortality in MM patients. Bloodstream infections remain the most severe bacterial complication in recipients of hematopoietic stem cell transplantation, whereas herpesviruses, especially, cytomegalovirus (CMV), dominate among viral complications. We analyzed data on 38 patients with MM who underwent ASCT from January 2018 to February 2020. Reactivation of cytomegalovirus (CMV) was revealed in 5 cases (13.2%), and Epstein-Barr virus (EBV), in 3 patients (7.9%). Pneumonia was diagnosed in one case (2.6%). Bacterial bloodstream infections were detected in 3 patients (7.9%). The bloodstream infections were stratified in accordance with the Sepsis-3 criteria, thus enabling us to identify patients with unfavorable prognosis who developed sepsis and/or septic shock. Infectious complications were observed over the period of 60 days after ASCT. Meanwhile, CMV and EBV reactivation and bloodstream bacterial infections did not affect overall survival rate.
Conclusion
Our results demonstrate that bacterial complications and viral (CMV and EBV) reactivation aggravate the course of primary disease in MM patients over the post-transplant period. The methods of infection control in clinical practice (genotyping of multidrug-resistant strains, and antimicrobial control protocols) should improve the treatment strategies in patients with MM following ASCT. Keywords Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
Keywords
Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27497" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-63-68" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-63-68" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-63-68" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27498" ["VALUE"]=> string(118) "Infectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(118) "Infectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(118) "Infectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27500" ["VALUE"]=> array(2) { ["TEXT"]=> string(397) "<p>Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia </p><br> <p><b>Correspondence</b><br> Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia<br> Phone: +7 (906) 267 0266<br> E-mail: vitnikcheb@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(337) "Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia
Phone: +7 (906) 267 0266
E-mail: vitnikcheb@mail.ru
Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia
Phone: +7 (906) 267 0266
E-mail: vitnikcheb@mail.ru
Виталий Н. Чеботкевич, Алена В. Кулешова, Анастасия А. Жернякова, Иван И. Кострома, Екатерина Е. Киселева, Елена И. Кайтанджан, Наталья Ю. Семенова, Станислав С. Бессмельцев, Александр В. Чечеткин, Сергей В. Грицаев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(389) "Виталий Н. Чеботкевич, Алена В. Кулешова, Анастасия А. Жернякова, Иван И. Кострома, Екатерина Е. Киселева, Елена И. Кайтанджан, Наталья Ю. Семенова, Станислав С. Бессмельцев, Александр В. Чечеткин, Сергей В. Грицаев
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27492" ["VALUE"]=> string(22) "12/27/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/27/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/27/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27493" ["VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/12/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27496" ["VALUE"]=> array(2) { ["TEXT"]=> string(4584) "<p style="text-align: justify;">Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.</p> <p style="text-align: justify;">Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4506) "Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.
Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.
Ключевые слова
Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4506) "Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.
Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.
Ключевые слова
Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27495" ["VALUE"]=> array(2) { ["TEXT"]=> string(206) "<p>Российский научно-исследовательский институт гематологии и трансфузиологии, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(194) "Российский научно-исследовательский институт гематологии и трансфузиологии, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(194) "Российский научно-исследовательский институт гематологии и трансфузиологии, Санкт-Петербург, Россия
" } } } [3]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "175" ["~IBLOCK_SECTION_ID"]=> string(3) "175" ["ID"]=> string(4) "1954" ["~ID"]=> string(4) "1954" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["~NAME"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "06/02/2021 03:27:37 pm" ["~TIMESTAMP_X"]=> string(22) "06/02/2021 03:27:37 pm" ["DETAIL_PAGE_URL"]=> string(148) "/en/archive/tom-10-nomer-1/klinicheskie-raboty/kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra/" ["~DETAIL_PAGE_URL"]=> string(148) "/en/archive/tom-10-nomer-1/klinicheskie-raboty/kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(48488) "Introduction
Our knowledge on gut microbiota significantly extended over last decades, due to improved cultivation of fastidious bacteria and DNA-based classification. As a result, big classes of anaerobic microbes were revealed, thus allowing renewing phylogenetic tree of gut microflora. Over 80% of gut bacterial species do not grow on available bacteriological media [1]. Therefore, molecular biology methods (PCR and DNA sequencing) proved to be the most effective methods, both in experimental and clinical studies [1, 2]. Sequencing of 16S rRNA gene became the standard approach to phylogenetic attribution of numerous bacterial types and families inhabiting gut microbiota [3]. Implementation of these echniques allowed us both to assess species composition of gut microbiota (GM), and to revise the role of some bacterial groups in pathogenesis of oncological, infectious and autoimmune diseases. At the present time, two main types of bacteria dominate in human intestines, i.e, Firmicutes and Bacteroidetes [4]. Their ratio and species composition are changing from the first days of life and depends on the mode of birth. Early predomination of Bacteroidetes over Firmicutes is observed in the children born by physiological way. Such altered ratio may influence gut immunity, cause metabolic syndrome, as well as affect maturation of nervous system and immune response [5-9].
Impact of intestinal microbiota upon carcinogenesis and clinical course of malignant diseases is studied to much lesser degree. E.g., well-known antitumor immune surveillance depends on proper maturation of CD4+ T cells as well as cytotoxic NK and NK-T cells [10, 11]. Gut microbiota is known to be a potent educating factor of adaptive immunity. Hence, it may modulate growth of malignant cells, both in children and adults.
It is known, however, that anticancer therapy of leukemias and solid tumors in children is often associated with severe and durable disruption of intestinal microbiota. These changes are caused by damage of intestinal wall, inhibition of local anti-infectious immunity, usage of systemic broad-spectrum antibiotics. Such negative background results into decrease of the bacterial species with favorable immunological effects, e.g., Bacteroidetes, Ruminococcaceae, Prevotella, Blautia, and expansion of Enterococci, Staphylococci and Gram-negative microbiota which may cause septic complications [12-14].
Recent studies of gut microbiota with deep sequencing technologies have shown that qualitative and quantitative composition of this bacterial community determine the balance between antitumor immune response and immune-mediated complications. This effect is implemented via immune system, lymphocyte subpopulations and cytokine profile. Cancer patients with severe bacterial complications are virtually lacking both anti- as well a proinflammatory populations. Worth of note, neither significant anaerobic group was considered a potentially therapeutic tool. Most of existing bacterial probiotics did not influence prognosis, as shown in oncological patients using sequencing of target bacterial DNA’s. Transplantation of fecal microbiota remains the only currently available technique aimed for correction of the required bacterial populations. Of course, FMT is primarily used for treatment of disorders that do not respond to conventional therapies, e.g., resistant pseudomembranous colitis, or intestinal graft-versus-host disease (GVHD) refractory to immunosuppressive treatment.
The aim of current work is to assess effects of gut microbiota transplantation upon the course of severe inflammatory disorder, immune system changes, and gut microbiota correction in cases of life-threatening conditions in pediatric patients.
Patients and methods
Clinical characteristics and procedures
Over a period of 2015 to 2020, seven children (4 females and 3 males, 3 to 10 years old) were subjected to FMT. These patients were initially treated for acute lymphoblastic leukemia (n=4), hereditary disorders (n=3), or myelodysplastic syndrome (n=1). The reduced-intensity conditioning regimen included fludarabine with busulfan (n=3), melphalan (n=3), or cyclophosphamide (Cy) (n=1). Hematopoietic stem cell transplantation (HSCT) was performed from haploidentical donors (n=5), or matched related donors (n=2). Posttransplant prophylaxis of acute GVHD was carried out with Cy in 5 patients, combined with tacrolimus (n=5), mycophenolate mofetil (n=3), antilymphocyte globulin was used in three cases. In one patient, GVHD prophylaxis was performed with TCR alpha/beta depletion, rituximab, tocilizumab and abatacept. Despite the preventive therapy, severe intestinal GVHD with intestinal bleeding has been developed in all the described patients. In 2 patients, acute/chronic GVHD (overlap syndrome) was documented. Median term from beginning of acute intestinal GVHD or overlap-syndrome to FMT was 56 (8 to 889) days. Severity grade of acute GVHD was scored as grade IV in four cases, grade 3 in 2 children, and grade II, in one patient. In all the cases, treatment of acute intestinal GVHD was started with steroids at the dose of 2 mg/kg/day. In cases of failure, the therapy was escalated with ruxolitinib (0.3 mg/kg/day), within clinical testing program performed by Russian Ministry of Healthcare (No.2016-29-1). Etanercept was added in 3 patients later in cases of poor effect (25 mg twice a week). Other therapies were applied, i.e., extracorporeal photopheresis (n=3), injections of mesenchymal stem cells (n=2), sirolimus (n=1) or tacrolimus (n=1).
General state of the patients at FMT was as follows: satisfactory in one case; moderate severity, in 2 patients; and four children were in severe condition. Clinical severity was determined, mainly, by assessment of pain and dyspeptic syndrome pronounced in all the patients. Intestinal bleeding with severe anemia was diagnosed in four patients, bilirubinemia, in 3 cases, pronounced encephalopathy was revealed in 2 children. Due to absence of sufficient clinical effect, FMT was administered, as adopted by the Pavlov University Ethical Board of 30.01.2017, №192). Both examination and treatment was performed under the conditions of Helsinki Declaration. The parents or caretakers signed appropriate informed consent. Inclusion criteria were as follows: acute GVHD grade II-IV, or overlap syndrome with intestinal disorder, steroid-refractory form of GVHD and failure of preceding treatment with etanercept or ruxolitinib. Exclusion criteria were not applied, due to severity of the disorder. All the children were in remission of primary disease.
Evaluation of acute intestinal GVHD severity, clinical response in the patients, characteristics of stool, undesirable adverse effects (AE) and pain syndrome was performed according to standard scales [15-19]. Histological examination of colon biopsies was carried out to confirm the intestinal GVHD diagnosis.
The FMT procedure in 5 patients was performed at the intensive care unit. In two cases, FMT was made in the outpatient setting. In four patients (57%), FT from third-party donors was used, and relatives served as donors in 3 cases (mother, in 1 case, and father, in 2 patients). In all these cases, the FT donors were also donors of hematopoietic stem cells for the same patients. Median age of the donors was 33 (19 to 38) years (4 females and 3 males). All the donors kept Mediterranean diet.
The routes of fecal transplant administration were as follows: EGD, 2 patients; EGD+ nasointestinal tube, 2 patients. Three children received fecal transplant (FT) per os, in gelatin capsules with frozen fecal microbiota. This route of administration was used for FMT in the patients and control persons [20]. Minimal age of the child treated with capsules was 4 years old.
Upon endoscopic FT administration, fresh native substance was used in two patients, and frozen native material was applied in other 2 cases. The frozen material was stored for a median time of 25 (2-104) days at -80°C. FT was delivered to upper intestine by means of nasointestinal tube, in 3.25 (2-5) séances; to lower intestine, using colonoscopy, 3 times. The gelatin capsules with frozen FT were administered daily 8 (5 to 10) times, at a dose of 3 to 6 capsules. Single FT dose applied via gastroscopy/nasointestinal tube was 1.7 (0.8-4.8) ml/kg; by colonoscopy, 9 and 6 ml/kg. Total dose of FT administered by the encapsulation method was 1.1 (0.6-1.7) g/kg.
Antibacterial prophylaxis was cancelled before FMT period in 3 patients; in one case, the therapy was resumed. Four other patients continued therapy with antibiotics. Due to proven viral gut affection (HHV 6 or EBV virus), four patients received gancyclovir therapy at a dose of 10 mg/kg daily.
Six patients recovered from GVHD and were followed after FMT at long terms (320-1964 days). Only one patient of the seven died on day +34 with pan-resistant Klebsiella sepsis.
Special laboratory tests
All the patients underwent clinical and laboratory examinations at the following terms: before FMT, on days +3, +16, +30, +60, and +120 after the procedure. The last day of FTM was considered Day 0.
Study protocols for FT donors, preparation of frozen encapsulated microbiota, native and frozen material, procedures for injection by intestinal catheters, storage and transportation of capsules with FT were described elsewhere in details [21].
Time dynamics of fecal microbiota before and after FMT was assessed by gene-specific PCR detection of DNA samples extracted from fecal samples using commercial Colonoflor test kit (Explana, St. Petersburg, Russia). This test system allowed quantitative detection of common gut bacteria, as based on multiplex real-time PCR (up to 20 specific bacterial genes and total molecular mass are targeted), being recalculated for CFU numbers as proposed by the manufacturer.
Immunophenotyping of lymphocyte subpopulations was performed by flow cytometry (Cytomics FC500, Beckman Coulter, USA), with CXP Analysis software (Beckman Coulter) using fluorochrome-labeled antibodies (CD45 FITC/CD4 PE/CD8 ECD/CD3 PC5, CD19PC7, CD3 FITC/CD(16+56) PE, CD45 PC5, CD5 FITC/CD23 PE/CD19 ECD, CD27 PC7, Beckman Coulter, USA), Versalyse protocol (Beckman Coulter, USA).
The patients were observed for a median of 585 (34-1948) days after FMT. Clinical response was evaluated according to common scales, assessing intestinal GVHD severity, and Bristol scale of stool quality.
Statistical evaluation
Due to small sample size, only methods of descriptive non-parametric statistics were used with Statistica software, to evaluate levels of significance for the differences between pre- and post-FMT indices.
Results
Clinical effects of FMT using clinical response scales
According to the results of clinical assessment by the mentioned scales, complete or partial response (resp., CR and PR) to the intestinal GVHD therapy was achieved in 6 patients out of 7 by the D+120 post-FMT, with a median time for CR and PR, of, respectively, 9 (2-45) and 34 (4-50) days.
Upon evaluation of the patients’ stool by the Bristol scale, partial response (>4 points) was achieved in 6 patients (86%) after 120 days, complete response (<4 points) was registered in 5 cases (71%). Median time for PR and CR following FMT comprised 23 (8-45) and 50 (10-90) days, respectively.
The patients reported positive dynamics after FMT, i.e., reduction of gut-related GVHD symptoms (stool volume, blood admixtures, abdominal pain), mitigated dyspeptic syndrome (vomiting, nausea, anorexia), as shown in Table 1.
Table 1. Time course of intestinal symptoms at different terms after fecal microbiota transplantation in children with acute GVHD (7 cases)

Composition and changes of fecal microbiota in FMT donors and recipients
According to the results obtained by multiplex PCR (Colonoflor system), total intestinal bacterial mass (TBM) in the FMT donors was as follows: 3×1012 (9×1011-8×1012) CFU/g, Lactobacillus spp., 3×106 (1×106-2×108) CFU/g; Bifidobacterium spp, 2×109 (9×107-3×109) CFU/g; E.coli, 3×108 (1×106-3×109) CFU/g; B.fragilis group, 2×1012 (2×1010-5×1012) CFU/g; F.prausnitzii, 1×1010 (4×108-2×1011) CFU/g.

Figure 1. Time-dependent changes of the gut microbiota in pediatric patients (n=7) before and at different terms after fecal microbiota transplantation. Colored bars show median amounts of specific bacterial species and total bacterial mass (TBM)
Abscissa, days posttransplant. Ordinate, log10 of CFU numbers.
In the patients, when comparing median values for microbial contents post-FMT, some sufficient dynamics was revealed against initial values (Fig. 1). E.g., a significant increase of total bacterial mass was registered on D+120 (р<0.02). Since D+3, increased values against initial levels were found for B.fragilis group, F.prausnitzii and E.coli (respectively, р<0.048, р<0.001 and р<0.048), as seen from Fig. 1.
E.coli strains found in donor microbiota were tested by Colonoflor system (multiple PCR panel) and no enteropathogenic E.coli genes were revealed.
By the day +120, no significant differences were noted for Bifidobacterium spp and Lactobacillus spp, compared with their initial levels.
Individual changes of gut bacteria amounts are shown in Fig. 2. In cases of early death (n=1) and of the malignancy relapse (n=1), a decrease of B.fragilis and, to lesser degree, Faecalibacterium prausnitzii was noted by the end of observation periods (resp., on D+15 and D+120). Hence, recovery of B.fragilis may be an informative marker of gut microbiota recovery which should be tested in more representative groups of patients.

Figure 2. Time-dependent changes of the gut microbiota species and total bacterial mass in pediatric patients before and after fecal microbiota transplantation (FMT). Time dynamics of dead, recovered and recurred (relapsed) patients is marked, respectively, in red, green and blue
Abscissa, terms after FMT, days. Ordinate, bacterial amounts per g of stool derived from multiplex PCR results, recalculated for CFU contents per sample.
Immunological effects of FMT
Upon increase in major commensal microorganisms (Bacteroides fragilis, Faecalibacterium prausnitzii) shown by multiple PCR following FMT in patients with acute intestinal GVHD, the number of major lymphocyte subpopulations among surviving children was stable, or became substantially increased for NK cells (p<0.05), as seen from Table 2. On the contrary, in a patient with relapse of primary malignancy, and in the patient who died due to subsequent sepsis, the absolute numbers of major lymphocyte subpopulations were either decreased, or remained at subnormal levels with time, compared to the surviving patients (data not shown).
Table 2. Time-dependent changes of main lymphocyte populations in peripheral blood of five patients with acute GVHD following fecal microbiota transplantation

Adverse effects of FMT
In 6 patients (86%), we have observed undesirable events, probably connected with FMT procedure. All these symptoms manifested within 7 days after FMT. However, these side effects were not referred to serious events. Nausea, abdominal pains and stomach rumbling were the most common events (43%) followed by subfebrile rise of body temperature (29%). In a single case, vomiting, pronounced abdominal flatulence and intestinal paresis were documented. One patient died on D+34 after FMT with K.pneumonia sepsis diagnosed on D+5, accomplished by fast progression of intestinal GVHD. The patient was previously colonized with pan-resistant K.pneumoniae strain, thus excluding potential negative consequence of FMT procedure.
Discussion
FMT is presently introduced as experimental therapeutic option for treatment of clinical syndromes with pronounced gut dysbiosis. E.g., incidence of C.difficile infection (CDI) in children and adults is sufficiently increased since 2000 [22-24]. Recurrent CDI develop in ca. 15-30% of pediatric patients with this infection [25, 26]. C.difficile-associated syndrome may cause different clinical pattern: from gut colonization to severe fulminant colitis. Many effective antibiotics and immunological therapies are adopted for C.difficile management in adults. However, therapeutic options for children are currently limited [27]. A recent large multicenter study of more than 300 patients aged from 11 to 23 years with C.difficile-associated infection has shown that FMT was clinically successful in 81% and 90% of the cases after 1st and 2nd FMT, respectively [28]. FMT becomes a part of therapeutic protocols in pediatrics, when treating recurrent C.difficile infection, being introduced into appropriate guidelines for pediatric patients [29].
There are also some studies of FMT in children with ulcerative colitis [30]. FMT was performed in 21 patients at a mean age of 12 years, in whom clinical response was observed in 57% and 28%, respectively, at 1 and 6 months after the procedure [31].
Of interest, the prospective study of FMT effects in 20 patients colonized with multiple drug-resistant (MDR) bacteria. Full decolonization of MDR microorganisms was registered in 15 patients [32]. In other study, decolonization of carbapenemase-producing and vancomycin-resistant bacteria was achieved following FMT in 7 of 10 patients [33]. To our knowledge, usage of FMT for decolonization of pan-resistant Gram-negative microbes was not yet reported in pediatric practice. However, FMT in childhood is used rather rarely, due to potential hazards and non-validated treatment techniques. First pediatric FMT was performed 10 years ago in a child 2 years old with recurrent Clostridium difficile infection resistant to standard therapy [34].
Acute GVHD after HSCT, with mortality rates up to 90%, is another complication treated by FMT [35]. Gut dysbiosis in children with acute GVHD was recently demonstrated, with sufficient decrease in anaerobic Firmicutes associated with "anti-inflammatory" effects (Clostridiaceae, Erysipelotrichaceae, Eubacteriaceae, Lachnospiraceae and Ruminococcaceae), compared to GVHD-free patients [36].
Interestingly, the children free of intestinal GHVD exhibited high intestinal levels of obligate anaerobes (Ruminococcaceae) accompanied by fast NK- and B cell reconstitution, and decreased mortality. Vice versa, severe GVHD was related to higher contents of Lactobacillaceae in gut microbiota [37].
The ratio of Enterococcus in adult patients without acute intestinal GVHD was 21% compared to 46% incidence in those who subsequently developed this complication, being increased to 74% in presence of active intestinal GVHD [38]. Meanwhile, Enterococcus spp. was shown to affect epithelial barrier and promote TNF production by the macrophages [39].
Potential efficiency of FMT in acute intestinal GVHD was initially shown in [40]. Complete remission of steroid-resistant intestinal GVHD was reported in 3 of 4 patients following FMT, thus considering this option a quite promising approach for this indication. Current data on FMT usage in acute intestinal GVHD in pediatric patients are quite limited [41].
While being an experimental therapeutic option, pediatric FMT may be applied only at large medical centers, due to lacking treatment standards and absence of appropriate clinical and laboratory staff. However, distinct clinical situations may require urgent FMT intervention, e.g., recurrent fulminant colitis associated with C.difficile infection, or acute severe intestinal GVHD refractory to any immunosuppressive therapy, thus presuming optional off-label FMT procedure. To perform the off-label FMT treatment, this approach meets the following criteria: (1) Severe disorder which is life-threatening, or causing a long-term impaired quality of life; (2) Absence of specific treatment methods; (3) Analysis of research data allows to suggest that the given preparation may achieve clinical effect in the given patients. Moreover, FDA (USA) interprets FMT usage in the following way: if the state of patient without any treatment may move from less severe to more serious, and if a potentially better therapy is available, compared to existing treatment methods. Indications for FMT procedure in acute intestinal GVHD seem to be consistent with these requirements [42].
Safety issues are of most importance in pediatrics, especially, for immunocompromised children following allogeneic HSCT. A sufficient retrospective safety study was performed in 49 children who received 114 FMT procedures [43]. Incidence of short-term undesirable events (UDE) within 48 hours was 26% (30/114). Two severe UDE were observed, and one patient was lost due to bloodstream infection and hepatic failure at 4th week following FMT, with total mortality of 2.04%. Notably, immune state was an independent factor which sufficiently influenced clinical outcomes (р=0.002). Risk quotient in the patients with immune deficiency proved to be 3.1. Hence, we must be cautious when performing FMT in children with immune deficiencies.
In the present study, we observed potentially FMT-related adverse effects in 86% of the cases. However, the undesirable events were not classified as serious, requiring only symptomatic therapy. Longitudinal changes in gut microbiota, metabolome and immune system were not assessed. The observation terms after our 1st pediatric FMT exceed 5 years, without detectable anomalies in growth and developmental, or behavioral deviations. One should observe other patients from our FMT series to these purposes.
To perform FMT in children with intestinal GVHD, encapsulated form of frozen microbiota was used. The youngest patient who received capsules with fecal microbiota was 4 years old. Some FMT studies with capsule administration suggest lower age limits (over 7 years old) for the patients with C.difficile-associated infection [44]. We guess, however, that the main factor is the patient’s ability to take encapsulated preparation, not age. According to a survey among 58 gastroenterologists, administration of oral capsules with microbiota to the patients was preferred by 34% of clinicians [45].
Usage of encapsulated form in FMT allows to avoid more invasive procedures, thus being more safe for the children, decreasing both complication rates and treatment costs. In our study, we, generally, followed the FMT protocol proposed by Shouval et al. [46].
There are still insufficient data for a full objective assessment of FMT efficiency in pediatric patients with acute intestinal GVHD, e.g., due to lack of appropriate comparison group. However, our results provide evidence of complete clinical response in 6 of 7 cases within 34 (4 to 50) days after FMT. We guess that, in intestinal GVHD, clinical evaluation by means of validated Bristol scale is quite indicative to assess stool normalization and recovery of intestinal functions. By these criteria, complete response (<4 points, solid faeces) was achieved in 5 children (70%) with steroid-resistant gut GVHD within 10-90 days (median of 50 days), thus being an impressive result. In our previous study which included a mixed-age group treated by FMT (13 adults and 6 children), and 8 control cases (4 adults and 4 children), we have obtained similar results, i.e., a distinct trend for a faster clinical response was observed in FMT group [47]. The median terms for development of complete response were 34 (3–90) versus 75 (6–91) days in controls. Complete clinical response (CCR) in the FMT group was documented in majority of cases by the day +60. Clinical improvement in the patients with intestinal GVHD was accompanied by restoration of intestinal B.fragilis, F.prausnitzii and E.coli contents. In contrast, placebo-treated patients did not exhibit any CCR by the day +30, and only one patient achieved complete response by the day +60.
Immunotropic effects of commensal gut bacteria, especially, upon major lymphocyte subpopulations may be of great importance for successful therapy of acute intestinal GVHD and survival of children following HSCT. Different lymphocyte dynamics in surviving and deceased patients is in accordance with current views that microbiota may influence human immunity. In our previous study, distinct changes in immune state were shown after FMT in healthy volunteers [20]. An increase in absolute and relative counts of T-helper cells (CD3+ CD4+ CD19+ CD23+) was found by D+9, along with decline in the numbers of cytotoxic Т cells (CD3+ CD8+ ) and NK-cells (CD3-CD16+ 56+). A reversal to normal values was revealed by the D+30.
Other indications for FMT in hematology malignancy are widely discussed recently. Most effects of FMT are yet poorly explored. However, one may formulate several clinical applications for FMT: decolonization of the microorganisms with multiple drug resistance; treatment of C.difficile-associated infection; therapy of autoimmune colitis developing after treatment with PD-1 inhibitors (Nivolumab), or anti-CD20 antibodies (Rituximab) therapy; gut dysbiosis following massive antibacterial therapy; changing sensitivity of malignancies for targeted drugs.
Ethical aspects should be definitely observed, i.e., FMT perception by the children with acute GVHD and their patients. When treating pediatric patients with ulcerative colitis, the authors report tolerance for FMT, and readiness for continuing the therapy [48]. In our opinion, education of parents about the main purposes, mechanisms of FMT action, and its safety should be of utmost importance.
Conclusion
FMT might be effective in children with severe intestinal GVHD following HSCT. FMT is a relatively safe and feasible procedure in immunocompromised patients upon careful screening of the third-party donors. However, our study has some limitations, i.e., small number of the group and absence of randomized controls. Therefore, larger prospective studies should be performed to better assess safety of FMT in pediatric patients with severe GVHD. Moreover, additional longitudinal studies are required, especially in pediatric patients, in order to assess potential changes in microbiota, metabolome, and immune system. Effective usage of FMT is a pre-requisite for implementation of novel anaerobic bacterial preparations which seem to be used in future, not only for treatment of antibiotic-resistant infections, but also to prevent tumor progression and autoimmune diseases. High-throughput DNA sequencing may provide additional evidence for application of microbiota-based biotherapy in pediatrics.
Acknowledgement
The study was partially supported by the AdVita Charity Foundation.
Conflicts of interest
None reported.
References
- Rheims H, Rainey FA, Stackebrandt E. A molecular approach to search for diversity among bacteria in the environment. J Ind Microbiol. 1996; 17: 159-169. doi: 10.1007/BF01574689.
- Hugenholtz P, Tyson GW, Webb RI, Wagner AM, Blackall LL. Investigation of candidate division TM7, a recently recognized major lineage of the domain Bacteria with no known pure-culture representatives. Appl Environ Microbiol. 2001; 67(1):411-419. doi: 10.1128/AEM.67.1.411-419.2001.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012; 488(7410):178-184. doi: 10.1038/nature11319.
- Tanaka М, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 2017; 66: 515-522. doi: 10.1016/j.alit.2017.07.010.
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-20. doi: 10.1126/science.1104816.
- Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut. 2011;60:288-289. doi: 10.1136/gut.2010.226779.
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187-192. doi: 10.1111/j.1365-2982.2010.01664.x.
- Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs. 2016;30:1019-1041. doi: 10.1007/s40263-016-0370-3.
- Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595:489-503. doi: 10.1113/JP273106.
- Torow N, Yu K, Hassani K, et al. Active suppression of intestinal CD4(+)TCRαβ(+) T-lymphocyte maturation during the postnatal period. Nat Commun. 2015; 21(6):7725. doi:10.1038/ncomms8725.
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489-493. doi: 10.1126/science.1219328.
- Hakim H, Dallas R, Wolf J, Tang L, Schultz-Cherry S, Darling V, Johnson C, Karlsson EA, Chang TC, Jeha S, Pui CH, Sun Y, Pounds S, Hayden RT, Tuomanen E, Rosch JW. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin Infect Dis. 2018 Aug 1;67(4):541-548. doi: 10.1093/cid/ciy153.
- Taur Y, Jenq RR, Ubeda C, van den Brink M, Pamer EG. Role of intestinal microbiota in transplantation outcomes. Best Pract Res Clin Haematol. 2015;28(2-3):155-161. doi: 10.1016/j.beha.2015.10.013
- Galloway-Peña JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG, Chemaly RF, Marsh L, Ghantoji SS, Pemmaraju N et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer. 2016;122(14):2186-2196. doi:10.1002/cncr.30039.
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18(4):295-304.
- Martin PJ, Bachier CR, Klingemann HG, McCarthy PL, Szabolcs P, Uberti JP, Schuster MW, Weisdorf D, Chao NJ, Kebriaei P, Shpall EJ, Macmillan ML, Soiffer RJ. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a consensus document. Biol Blood Marrow Transplant. 2009; 15(7):777-784. doi: 10.1016/j.bbmt.2009.03.012.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920-924. doi: 10.3109/00365529709011203.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.....
- WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva: World Health Organization; 2018. https://www.who.int/ncds/management/palliative-care/cancer-pain-guidelines/en/
- Goloshchapov, OV, Olekhnovich EI, Sidorenko SV, Moiseev IS, Kucher MA, Fedorov DE, Pavlenko AV, Manolov AI, Gostev VV, Veselovsky VA et al. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol. 2019; 19(1), 312. doi: 10.1186/s12866-019-1689-y.
- Goloshchapov OV, Churakina DV, Kucher MA, Klement’eva RV, Sidorenko SV, Gostev VV, Karev VE, Suvorova MA, Shlyk IV, Zubarovskaya LS, Afanasyev BV. Fecal microbiota transplantation in critically ill patients in oncohematological practice. Vestnik Anaesthesiol Reanimatol. 2019; 16(3):63-73 (In Russian). doi: 10.21292/2078-5658-2019-16-3-63-73.
- Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol 2007; 28:1233–1235. doi: 10.1086/520732.
- Khanna S, Baddour LM, Huskins WC et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 2013; 56:1401-1406. doi: 10.1093/cid/cit075.
- Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001-2006. Pediatrics 2008; 122:1266-70. doi: 10.1542/peds.2008-0469.
- Nicholson MR, Thomsen IP, Slaughter JC, Creech CB, Edwards KM. Novel risk factors for recurrent Clostridium difficult infection in children. J Pediatr Gastroenterol Nutr. 2015; 60(1):18-22. doi: 10.1097/MPG.0000000000000553.
- Kociolek LK, Palac HL, Patel SJ, Shulman ST, Gerding DN. Risk factors for recurrent Clostridium difficile infection in children: A Nested Case-Control Study. J Pediatr. 2015 Aug; 167(2):384-389. doi: 10.1016/j.jpeds.2015.04.052.
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll K, Coffin S, Dubberke E, Garey K, Gould C, Kelly C et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. doi:10.1093/cid/cix1085.
- Nicholson MR, Mitchell PD, Alexander E, Ballal S, Bartlett M, Becker P, Davidovics Z, Docktor M, Dole M, Felix G, et al. Efficacy of fecal microbiota transplantation for Clostridium difficile infection in children. Clin Gastroenterol Hepatol. 2020 Mar;18(3):612-619.e1. doi: 10.1016/j.cgh.2019.04.037.
- Davidovics ZH, Michail S, Nicholson MR, Kociolek L, Pai N, Hansen R, Schwerd T, Maspons A, Shamir R, Szajewska H et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection and other conditions in children: A joint position paper from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2019;68(1):130-143. doi:10.1097/MPG.0000000000002205.
- Quagliariello A, Del Chierico F, Reddel S, Russo A, Muda AO, D’Argenio P, Angelino G, Romeo EF, Dall’Oglio L, De Angelis P et al. Fecal Microbiota Transplant in Two Ulcerative Colitis Pediatric Cases: Gut Microbiota and Clinical Course Correlations. Microorganisms. 2020;8(10):1486. doi:10.3390/microorganisms810148631.
- Goyal A, Yeh A, Bush BR, Firek BA, Siebold LM, Rogers MB, Kufen AD, Morowitz MJ. Safety, Clinical Response, and Microbiome Findings Following Fecal Microbiota Transplant in Children With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24(2):410-421. doi: 10.1093/ibd/izx035.
- Bilinski J, Grzesiowski P, Sorensen N, Madry K, Muszynski J, Robak K, Wroblewska M, Dzieciatkowski T, Dulny G, Dwilewicz-Trojaczek J et al. Fecal Microbiota Transplantation in Patients With Blood Disorders Inhibits Gut Colonization With Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin Infect Dis. 2017;65(3):364-370. doi: 10.1093/cid/cix252.
- Battipaglia G, Malard F, Rubio MT, Ruggeri A, Mamez AC, Brissot E, Giannotti F, Dulery R, Joly AC, Baylatry MT et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica. 2019;104(8):1682-1628. doi: 10.3324/haematol.2018.198549.
- Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126 (1): e239‒е242. doi: 10.1542/peds.2009-3363.
- Steinbach G, Hockenbery DM, Huls G, Furlong T, Myerson D, Loeb KR, Fann JR, Castilla-Llorente C, McDonald GB, Martin PJ. Pilot study of lithium to restore intestinal barrier function in severe graft-versus-host disease. PLoS One. 2017;12(8):e0183284. doi: 10.1371/journal.pone.0183284.
- Simms-Waldrip TR, Sunkersett G, Coughlin LA, Savani MR, Arana C, Kim J, Kim M, Zhan X, Greenberg DE, Xie Y, Davies SM, Koh AY. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol Blood Marrow Transplant. 2017; 23(5):820-829. doi: 10.1016/j.bbmt.2017.02.004.
- Ingham AC, Kielsen K, Cilieborg MS, Lund O, Holmes S, Aarestrup FM, Müller KG, Pamp SJ. Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome. 2019; 7(1):131. doi: 10.1186/s40168-019-0745-z.
- Taur Y. Intestinal microbiome changes and stem cell transplantation: Lessons learned. Virulence 2016;7(8):930-938. doi: 10.1080/21505594.2016.1250982.
- Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, Mair K, Krueger D, Pruteanu M, Shanahan F et al Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology.2011; 141 (3): 959–971. doi: 10.1053/j.gastro.2011.05.035.
- Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, Mimura I, Morita H, Sugiyama D, Nishikawa H, Hattori M, Hino Y, Ikegawa S, Yamamoto K, Toya T, Doki N, Koizumi K, Honda K, Ohashi K. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016; 128(16):2083-2088. doi: 10.1182/blood-2016-05-717652.
- Zhong S, Zeng J, Deng Z, Jiang L, Zhang B, Yang K, Wang W, Zhang T. Fecal microbiota transplantation for refractory diarrhea in immunocompromised diseases: a pediatric case report. Ital J Pediatr. 2019;45(1):116. doi:10.1186/s13052-019-0708-9.
- Use of fecal microbiota for transplantation to treat Clostridium difficile. Federal Register 2019; 84 (176): 471911. www.federalregister.gov/documents/2019/09/11/2019-19643.
- Zhang XY, Wang YZ, Li XL, Hu H, Liu HF, Li D, Xiao YM, Zhang T. Safety of fecal microbiota transplantation in Chinese children: A single-center retrospective study. World J Clin Cases. 2018;6(16):1121-1127. doi: 10.12998/wjcc.v6.i16.1121.
- Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, Kaplan JL, Hohmann EL. . Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14(1):134. doi:10.1186/s12916-016-0680-9.
- McIlroy JR, Nalagatla N, Hansen R, Hart A, Hold GL. Faecal microbiota transplantation as a treatment for inflammatory bowel disease: a national survey of adult and paediatric gastroenterologists in the UK. Frontline Gastroenterol. 2018;9(4):250-255. doi: 10.1136/flgastro-2017-100936.
- Shouval R, Geva M, Nagler A, Youngster I. Fecal microbiota transplantation for treatment of acute graft-versus-host disease. Clin Hematol Internat. 2019; 1(1):28-35. doi: 10.2991/chi.d.190316.002.
- Goloshchapov OV, Bakin EA, Kucher MA, Stanevich OV, Suvorova MA, Gostev VV, Glotov OS, Eismont YA, Polev DE, Lobenskaya A. Bacteroides fragilis is a potential marker of effective microbiota recolonization in acute graft-versus-host disease treatment. Cell Ther Transplant. 2020; 9(2): 47-59.doi:10.18620/ctt-1866-8836-2020-9-2-47-59.
- Popov J, Hartung E, Hill L, Chauhan U, Pai N. Pediatric Patient and Parent Perceptions of Fecal Microbiota Transplantation for the Treatment of Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2020. doi: 10.1097/MPG.0000000000002995. Epub ahead of print. PMID: 33230077.
" ["~DETAIL_TEXT"]=> string(48488) "
Introduction
Our knowledge on gut microbiota significantly extended over last decades, due to improved cultivation of fastidious bacteria and DNA-based classification. As a result, big classes of anaerobic microbes were revealed, thus allowing renewing phylogenetic tree of gut microflora. Over 80% of gut bacterial species do not grow on available bacteriological media [1]. Therefore, molecular biology methods (PCR and DNA sequencing) proved to be the most effective methods, both in experimental and clinical studies [1, 2]. Sequencing of 16S rRNA gene became the standard approach to phylogenetic attribution of numerous bacterial types and families inhabiting gut microbiota [3]. Implementation of these echniques allowed us both to assess species composition of gut microbiota (GM), and to revise the role of some bacterial groups in pathogenesis of oncological, infectious and autoimmune diseases. At the present time, two main types of bacteria dominate in human intestines, i.e, Firmicutes and Bacteroidetes [4]. Their ratio and species composition are changing from the first days of life and depends on the mode of birth. Early predomination of Bacteroidetes over Firmicutes is observed in the children born by physiological way. Such altered ratio may influence gut immunity, cause metabolic syndrome, as well as affect maturation of nervous system and immune response [5-9].
Impact of intestinal microbiota upon carcinogenesis and clinical course of malignant diseases is studied to much lesser degree. E.g., well-known antitumor immune surveillance depends on proper maturation of CD4+ T cells as well as cytotoxic NK and NK-T cells [10, 11]. Gut microbiota is known to be a potent educating factor of adaptive immunity. Hence, it may modulate growth of malignant cells, both in children and adults.
It is known, however, that anticancer therapy of leukemias and solid tumors in children is often associated with severe and durable disruption of intestinal microbiota. These changes are caused by damage of intestinal wall, inhibition of local anti-infectious immunity, usage of systemic broad-spectrum antibiotics. Such negative background results into decrease of the bacterial species with favorable immunological effects, e.g., Bacteroidetes, Ruminococcaceae, Prevotella, Blautia, and expansion of Enterococci, Staphylococci and Gram-negative microbiota which may cause septic complications [12-14].
Recent studies of gut microbiota with deep sequencing technologies have shown that qualitative and quantitative composition of this bacterial community determine the balance between antitumor immune response and immune-mediated complications. This effect is implemented via immune system, lymphocyte subpopulations and cytokine profile. Cancer patients with severe bacterial complications are virtually lacking both anti- as well a proinflammatory populations. Worth of note, neither significant anaerobic group was considered a potentially therapeutic tool. Most of existing bacterial probiotics did not influence prognosis, as shown in oncological patients using sequencing of target bacterial DNA’s. Transplantation of fecal microbiota remains the only currently available technique aimed for correction of the required bacterial populations. Of course, FMT is primarily used for treatment of disorders that do not respond to conventional therapies, e.g., resistant pseudomembranous colitis, or intestinal graft-versus-host disease (GVHD) refractory to immunosuppressive treatment.
The aim of current work is to assess effects of gut microbiota transplantation upon the course of severe inflammatory disorder, immune system changes, and gut microbiota correction in cases of life-threatening conditions in pediatric patients.
Patients and methods
Clinical characteristics and procedures
Over a period of 2015 to 2020, seven children (4 females and 3 males, 3 to 10 years old) were subjected to FMT. These patients were initially treated for acute lymphoblastic leukemia (n=4), hereditary disorders (n=3), or myelodysplastic syndrome (n=1). The reduced-intensity conditioning regimen included fludarabine with busulfan (n=3), melphalan (n=3), or cyclophosphamide (Cy) (n=1). Hematopoietic stem cell transplantation (HSCT) was performed from haploidentical donors (n=5), or matched related donors (n=2). Posttransplant prophylaxis of acute GVHD was carried out with Cy in 5 patients, combined with tacrolimus (n=5), mycophenolate mofetil (n=3), antilymphocyte globulin was used in three cases. In one patient, GVHD prophylaxis was performed with TCR alpha/beta depletion, rituximab, tocilizumab and abatacept. Despite the preventive therapy, severe intestinal GVHD with intestinal bleeding has been developed in all the described patients. In 2 patients, acute/chronic GVHD (overlap syndrome) was documented. Median term from beginning of acute intestinal GVHD or overlap-syndrome to FMT was 56 (8 to 889) days. Severity grade of acute GVHD was scored as grade IV in four cases, grade 3 in 2 children, and grade II, in one patient. In all the cases, treatment of acute intestinal GVHD was started with steroids at the dose of 2 mg/kg/day. In cases of failure, the therapy was escalated with ruxolitinib (0.3 mg/kg/day), within clinical testing program performed by Russian Ministry of Healthcare (No.2016-29-1). Etanercept was added in 3 patients later in cases of poor effect (25 mg twice a week). Other therapies were applied, i.e., extracorporeal photopheresis (n=3), injections of mesenchymal stem cells (n=2), sirolimus (n=1) or tacrolimus (n=1).
General state of the patients at FMT was as follows: satisfactory in one case; moderate severity, in 2 patients; and four children were in severe condition. Clinical severity was determined, mainly, by assessment of pain and dyspeptic syndrome pronounced in all the patients. Intestinal bleeding with severe anemia was diagnosed in four patients, bilirubinemia, in 3 cases, pronounced encephalopathy was revealed in 2 children. Due to absence of sufficient clinical effect, FMT was administered, as adopted by the Pavlov University Ethical Board of 30.01.2017, №192). Both examination and treatment was performed under the conditions of Helsinki Declaration. The parents or caretakers signed appropriate informed consent. Inclusion criteria were as follows: acute GVHD grade II-IV, or overlap syndrome with intestinal disorder, steroid-refractory form of GVHD and failure of preceding treatment with etanercept or ruxolitinib. Exclusion criteria were not applied, due to severity of the disorder. All the children were in remission of primary disease.
Evaluation of acute intestinal GVHD severity, clinical response in the patients, characteristics of stool, undesirable adverse effects (AE) and pain syndrome was performed according to standard scales [15-19]. Histological examination of colon biopsies was carried out to confirm the intestinal GVHD diagnosis.
The FMT procedure in 5 patients was performed at the intensive care unit. In two cases, FMT was made in the outpatient setting. In four patients (57%), FT from third-party donors was used, and relatives served as donors in 3 cases (mother, in 1 case, and father, in 2 patients). In all these cases, the FT donors were also donors of hematopoietic stem cells for the same patients. Median age of the donors was 33 (19 to 38) years (4 females and 3 males). All the donors kept Mediterranean diet.
The routes of fecal transplant administration were as follows: EGD, 2 patients; EGD+ nasointestinal tube, 2 patients. Three children received fecal transplant (FT) per os, in gelatin capsules with frozen fecal microbiota. This route of administration was used for FMT in the patients and control persons [20]. Minimal age of the child treated with capsules was 4 years old.
Upon endoscopic FT administration, fresh native substance was used in two patients, and frozen native material was applied in other 2 cases. The frozen material was stored for a median time of 25 (2-104) days at -80°C. FT was delivered to upper intestine by means of nasointestinal tube, in 3.25 (2-5) séances; to lower intestine, using colonoscopy, 3 times. The gelatin capsules with frozen FT were administered daily 8 (5 to 10) times, at a dose of 3 to 6 capsules. Single FT dose applied via gastroscopy/nasointestinal tube was 1.7 (0.8-4.8) ml/kg; by colonoscopy, 9 and 6 ml/kg. Total dose of FT administered by the encapsulation method was 1.1 (0.6-1.7) g/kg.
Antibacterial prophylaxis was cancelled before FMT period in 3 patients; in one case, the therapy was resumed. Four other patients continued therapy with antibiotics. Due to proven viral gut affection (HHV 6 or EBV virus), four patients received gancyclovir therapy at a dose of 10 mg/kg daily.
Six patients recovered from GVHD and were followed after FMT at long terms (320-1964 days). Only one patient of the seven died on day +34 with pan-resistant Klebsiella sepsis.
Special laboratory tests
All the patients underwent clinical and laboratory examinations at the following terms: before FMT, on days +3, +16, +30, +60, and +120 after the procedure. The last day of FTM was considered Day 0.
Study protocols for FT donors, preparation of frozen encapsulated microbiota, native and frozen material, procedures for injection by intestinal catheters, storage and transportation of capsules with FT were described elsewhere in details [21].
Time dynamics of fecal microbiota before and after FMT was assessed by gene-specific PCR detection of DNA samples extracted from fecal samples using commercial Colonoflor test kit (Explana, St. Petersburg, Russia). This test system allowed quantitative detection of common gut bacteria, as based on multiplex real-time PCR (up to 20 specific bacterial genes and total molecular mass are targeted), being recalculated for CFU numbers as proposed by the manufacturer.
Immunophenotyping of lymphocyte subpopulations was performed by flow cytometry (Cytomics FC500, Beckman Coulter, USA), with CXP Analysis software (Beckman Coulter) using fluorochrome-labeled antibodies (CD45 FITC/CD4 PE/CD8 ECD/CD3 PC5, CD19PC7, CD3 FITC/CD(16+56) PE, CD45 PC5, CD5 FITC/CD23 PE/CD19 ECD, CD27 PC7, Beckman Coulter, USA), Versalyse protocol (Beckman Coulter, USA).
The patients were observed for a median of 585 (34-1948) days after FMT. Clinical response was evaluated according to common scales, assessing intestinal GVHD severity, and Bristol scale of stool quality.
Statistical evaluation
Due to small sample size, only methods of descriptive non-parametric statistics were used with Statistica software, to evaluate levels of significance for the differences between pre- and post-FMT indices.
Results
Clinical effects of FMT using clinical response scales
According to the results of clinical assessment by the mentioned scales, complete or partial response (resp., CR and PR) to the intestinal GVHD therapy was achieved in 6 patients out of 7 by the D+120 post-FMT, with a median time for CR and PR, of, respectively, 9 (2-45) and 34 (4-50) days.
Upon evaluation of the patients’ stool by the Bristol scale, partial response (>4 points) was achieved in 6 patients (86%) after 120 days, complete response (<4 points) was registered in 5 cases (71%). Median time for PR and CR following FMT comprised 23 (8-45) and 50 (10-90) days, respectively.
The patients reported positive dynamics after FMT, i.e., reduction of gut-related GVHD symptoms (stool volume, blood admixtures, abdominal pain), mitigated dyspeptic syndrome (vomiting, nausea, anorexia), as shown in Table 1.
Table 1. Time course of intestinal symptoms at different terms after fecal microbiota transplantation in children with acute GVHD (7 cases)

Composition and changes of fecal microbiota in FMT donors and recipients
According to the results obtained by multiplex PCR (Colonoflor system), total intestinal bacterial mass (TBM) in the FMT donors was as follows: 3×1012 (9×1011-8×1012) CFU/g, Lactobacillus spp., 3×106 (1×106-2×108) CFU/g; Bifidobacterium spp, 2×109 (9×107-3×109) CFU/g; E.coli, 3×108 (1×106-3×109) CFU/g; B.fragilis group, 2×1012 (2×1010-5×1012) CFU/g; F.prausnitzii, 1×1010 (4×108-2×1011) CFU/g.

Figure 1. Time-dependent changes of the gut microbiota in pediatric patients (n=7) before and at different terms after fecal microbiota transplantation. Colored bars show median amounts of specific bacterial species and total bacterial mass (TBM)
Abscissa, days posttransplant. Ordinate, log10 of CFU numbers.
In the patients, when comparing median values for microbial contents post-FMT, some sufficient dynamics was revealed against initial values (Fig. 1). E.g., a significant increase of total bacterial mass was registered on D+120 (р<0.02). Since D+3, increased values against initial levels were found for B.fragilis group, F.prausnitzii and E.coli (respectively, р<0.048, р<0.001 and р<0.048), as seen from Fig. 1.
E.coli strains found in donor microbiota were tested by Colonoflor system (multiple PCR panel) and no enteropathogenic E.coli genes were revealed.
By the day +120, no significant differences were noted for Bifidobacterium spp and Lactobacillus spp, compared with their initial levels.
Individual changes of gut bacteria amounts are shown in Fig. 2. In cases of early death (n=1) and of the malignancy relapse (n=1), a decrease of B.fragilis and, to lesser degree, Faecalibacterium prausnitzii was noted by the end of observation periods (resp., on D+15 and D+120). Hence, recovery of B.fragilis may be an informative marker of gut microbiota recovery which should be tested in more representative groups of patients.

Figure 2. Time-dependent changes of the gut microbiota species and total bacterial mass in pediatric patients before and after fecal microbiota transplantation (FMT). Time dynamics of dead, recovered and recurred (relapsed) patients is marked, respectively, in red, green and blue
Abscissa, terms after FMT, days. Ordinate, bacterial amounts per g of stool derived from multiplex PCR results, recalculated for CFU contents per sample.
Immunological effects of FMT
Upon increase in major commensal microorganisms (Bacteroides fragilis, Faecalibacterium prausnitzii) shown by multiple PCR following FMT in patients with acute intestinal GVHD, the number of major lymphocyte subpopulations among surviving children was stable, or became substantially increased for NK cells (p<0.05), as seen from Table 2. On the contrary, in a patient with relapse of primary malignancy, and in the patient who died due to subsequent sepsis, the absolute numbers of major lymphocyte subpopulations were either decreased, or remained at subnormal levels with time, compared to the surviving patients (data not shown).
Table 2. Time-dependent changes of main lymphocyte populations in peripheral blood of five patients with acute GVHD following fecal microbiota transplantation

Adverse effects of FMT
In 6 patients (86%), we have observed undesirable events, probably connected with FMT procedure. All these symptoms manifested within 7 days after FMT. However, these side effects were not referred to serious events. Nausea, abdominal pains and stomach rumbling were the most common events (43%) followed by subfebrile rise of body temperature (29%). In a single case, vomiting, pronounced abdominal flatulence and intestinal paresis were documented. One patient died on D+34 after FMT with K.pneumonia sepsis diagnosed on D+5, accomplished by fast progression of intestinal GVHD. The patient was previously colonized with pan-resistant K.pneumoniae strain, thus excluding potential negative consequence of FMT procedure.
Discussion
FMT is presently introduced as experimental therapeutic option for treatment of clinical syndromes with pronounced gut dysbiosis. E.g., incidence of C.difficile infection (CDI) in children and adults is sufficiently increased since 2000 [22-24]. Recurrent CDI develop in ca. 15-30% of pediatric patients with this infection [25, 26]. C.difficile-associated syndrome may cause different clinical pattern: from gut colonization to severe fulminant colitis. Many effective antibiotics and immunological therapies are adopted for C.difficile management in adults. However, therapeutic options for children are currently limited [27]. A recent large multicenter study of more than 300 patients aged from 11 to 23 years with C.difficile-associated infection has shown that FMT was clinically successful in 81% and 90% of the cases after 1st and 2nd FMT, respectively [28]. FMT becomes a part of therapeutic protocols in pediatrics, when treating recurrent C.difficile infection, being introduced into appropriate guidelines for pediatric patients [29].
There are also some studies of FMT in children with ulcerative colitis [30]. FMT was performed in 21 patients at a mean age of 12 years, in whom clinical response was observed in 57% and 28%, respectively, at 1 and 6 months after the procedure [31].
Of interest, the prospective study of FMT effects in 20 patients colonized with multiple drug-resistant (MDR) bacteria. Full decolonization of MDR microorganisms was registered in 15 patients [32]. In other study, decolonization of carbapenemase-producing and vancomycin-resistant bacteria was achieved following FMT in 7 of 10 patients [33]. To our knowledge, usage of FMT for decolonization of pan-resistant Gram-negative microbes was not yet reported in pediatric practice. However, FMT in childhood is used rather rarely, due to potential hazards and non-validated treatment techniques. First pediatric FMT was performed 10 years ago in a child 2 years old with recurrent Clostridium difficile infection resistant to standard therapy [34].
Acute GVHD after HSCT, with mortality rates up to 90%, is another complication treated by FMT [35]. Gut dysbiosis in children with acute GVHD was recently demonstrated, with sufficient decrease in anaerobic Firmicutes associated with "anti-inflammatory" effects (Clostridiaceae, Erysipelotrichaceae, Eubacteriaceae, Lachnospiraceae and Ruminococcaceae), compared to GVHD-free patients [36].
Interestingly, the children free of intestinal GHVD exhibited high intestinal levels of obligate anaerobes (Ruminococcaceae) accompanied by fast NK- and B cell reconstitution, and decreased mortality. Vice versa, severe GVHD was related to higher contents of Lactobacillaceae in gut microbiota [37].
The ratio of Enterococcus in adult patients without acute intestinal GVHD was 21% compared to 46% incidence in those who subsequently developed this complication, being increased to 74% in presence of active intestinal GVHD [38]. Meanwhile, Enterococcus spp. was shown to affect epithelial barrier and promote TNF production by the macrophages [39].
Potential efficiency of FMT in acute intestinal GVHD was initially shown in [40]. Complete remission of steroid-resistant intestinal GVHD was reported in 3 of 4 patients following FMT, thus considering this option a quite promising approach for this indication. Current data on FMT usage in acute intestinal GVHD in pediatric patients are quite limited [41].
While being an experimental therapeutic option, pediatric FMT may be applied only at large medical centers, due to lacking treatment standards and absence of appropriate clinical and laboratory staff. However, distinct clinical situations may require urgent FMT intervention, e.g., recurrent fulminant colitis associated with C.difficile infection, or acute severe intestinal GVHD refractory to any immunosuppressive therapy, thus presuming optional off-label FMT procedure. To perform the off-label FMT treatment, this approach meets the following criteria: (1) Severe disorder which is life-threatening, or causing a long-term impaired quality of life; (2) Absence of specific treatment methods; (3) Analysis of research data allows to suggest that the given preparation may achieve clinical effect in the given patients. Moreover, FDA (USA) interprets FMT usage in the following way: if the state of patient without any treatment may move from less severe to more serious, and if a potentially better therapy is available, compared to existing treatment methods. Indications for FMT procedure in acute intestinal GVHD seem to be consistent with these requirements [42].
Safety issues are of most importance in pediatrics, especially, for immunocompromised children following allogeneic HSCT. A sufficient retrospective safety study was performed in 49 children who received 114 FMT procedures [43]. Incidence of short-term undesirable events (UDE) within 48 hours was 26% (30/114). Two severe UDE were observed, and one patient was lost due to bloodstream infection and hepatic failure at 4th week following FMT, with total mortality of 2.04%. Notably, immune state was an independent factor which sufficiently influenced clinical outcomes (р=0.002). Risk quotient in the patients with immune deficiency proved to be 3.1. Hence, we must be cautious when performing FMT in children with immune deficiencies.
In the present study, we observed potentially FMT-related adverse effects in 86% of the cases. However, the undesirable events were not classified as serious, requiring only symptomatic therapy. Longitudinal changes in gut microbiota, metabolome and immune system were not assessed. The observation terms after our 1st pediatric FMT exceed 5 years, without detectable anomalies in growth and developmental, or behavioral deviations. One should observe other patients from our FMT series to these purposes.
To perform FMT in children with intestinal GVHD, encapsulated form of frozen microbiota was used. The youngest patient who received capsules with fecal microbiota was 4 years old. Some FMT studies with capsule administration suggest lower age limits (over 7 years old) for the patients with C.difficile-associated infection [44]. We guess, however, that the main factor is the patient’s ability to take encapsulated preparation, not age. According to a survey among 58 gastroenterologists, administration of oral capsules with microbiota to the patients was preferred by 34% of clinicians [45].
Usage of encapsulated form in FMT allows to avoid more invasive procedures, thus being more safe for the children, decreasing both complication rates and treatment costs. In our study, we, generally, followed the FMT protocol proposed by Shouval et al. [46].
There are still insufficient data for a full objective assessment of FMT efficiency in pediatric patients with acute intestinal GVHD, e.g., due to lack of appropriate comparison group. However, our results provide evidence of complete clinical response in 6 of 7 cases within 34 (4 to 50) days after FMT. We guess that, in intestinal GVHD, clinical evaluation by means of validated Bristol scale is quite indicative to assess stool normalization and recovery of intestinal functions. By these criteria, complete response (<4 points, solid faeces) was achieved in 5 children (70%) with steroid-resistant gut GVHD within 10-90 days (median of 50 days), thus being an impressive result. In our previous study which included a mixed-age group treated by FMT (13 adults and 6 children), and 8 control cases (4 adults and 4 children), we have obtained similar results, i.e., a distinct trend for a faster clinical response was observed in FMT group [47]. The median terms for development of complete response were 34 (3–90) versus 75 (6–91) days in controls. Complete clinical response (CCR) in the FMT group was documented in majority of cases by the day +60. Clinical improvement in the patients with intestinal GVHD was accompanied by restoration of intestinal B.fragilis, F.prausnitzii and E.coli contents. In contrast, placebo-treated patients did not exhibit any CCR by the day +30, and only one patient achieved complete response by the day +60.
Immunotropic effects of commensal gut bacteria, especially, upon major lymphocyte subpopulations may be of great importance for successful therapy of acute intestinal GVHD and survival of children following HSCT. Different lymphocyte dynamics in surviving and deceased patients is in accordance with current views that microbiota may influence human immunity. In our previous study, distinct changes in immune state were shown after FMT in healthy volunteers [20]. An increase in absolute and relative counts of T-helper cells (CD3+ CD4+ CD19+ CD23+) was found by D+9, along with decline in the numbers of cytotoxic Т cells (CD3+ CD8+ ) and NK-cells (CD3-CD16+ 56+). A reversal to normal values was revealed by the D+30.
Other indications for FMT in hematology malignancy are widely discussed recently. Most effects of FMT are yet poorly explored. However, one may formulate several clinical applications for FMT: decolonization of the microorganisms with multiple drug resistance; treatment of C.difficile-associated infection; therapy of autoimmune colitis developing after treatment with PD-1 inhibitors (Nivolumab), or anti-CD20 antibodies (Rituximab) therapy; gut dysbiosis following massive antibacterial therapy; changing sensitivity of malignancies for targeted drugs.
Ethical aspects should be definitely observed, i.e., FMT perception by the children with acute GVHD and their patients. When treating pediatric patients with ulcerative colitis, the authors report tolerance for FMT, and readiness for continuing the therapy [48]. In our opinion, education of parents about the main purposes, mechanisms of FMT action, and its safety should be of utmost importance.
Conclusion
FMT might be effective in children with severe intestinal GVHD following HSCT. FMT is a relatively safe and feasible procedure in immunocompromised patients upon careful screening of the third-party donors. However, our study has some limitations, i.e., small number of the group and absence of randomized controls. Therefore, larger prospective studies should be performed to better assess safety of FMT in pediatric patients with severe GVHD. Moreover, additional longitudinal studies are required, especially in pediatric patients, in order to assess potential changes in microbiota, metabolome, and immune system. Effective usage of FMT is a pre-requisite for implementation of novel anaerobic bacterial preparations which seem to be used in future, not only for treatment of antibiotic-resistant infections, but also to prevent tumor progression and autoimmune diseases. High-throughput DNA sequencing may provide additional evidence for application of microbiota-based biotherapy in pediatrics.
Acknowledgement
The study was partially supported by the AdVita Charity Foundation.
Conflicts of interest
None reported.
References
- Rheims H, Rainey FA, Stackebrandt E. A molecular approach to search for diversity among bacteria in the environment. J Ind Microbiol. 1996; 17: 159-169. doi: 10.1007/BF01574689.
- Hugenholtz P, Tyson GW, Webb RI, Wagner AM, Blackall LL. Investigation of candidate division TM7, a recently recognized major lineage of the domain Bacteria with no known pure-culture representatives. Appl Environ Microbiol. 2001; 67(1):411-419. doi: 10.1128/AEM.67.1.411-419.2001.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012; 488(7410):178-184. doi: 10.1038/nature11319.
- Tanaka М, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 2017; 66: 515-522. doi: 10.1016/j.alit.2017.07.010.
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-20. doi: 10.1126/science.1104816.
- Bercik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut. 2011;60:288-289. doi: 10.1136/gut.2010.226779.
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187-192. doi: 10.1111/j.1365-2982.2010.01664.x.
- Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs. 2016;30:1019-1041. doi: 10.1007/s40263-016-0370-3.
- Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595:489-503. doi: 10.1113/JP273106.
- Torow N, Yu K, Hassani K, et al. Active suppression of intestinal CD4(+)TCRαβ(+) T-lymphocyte maturation during the postnatal period. Nat Commun. 2015; 21(6):7725. doi:10.1038/ncomms8725.
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489-493. doi: 10.1126/science.1219328.
- Hakim H, Dallas R, Wolf J, Tang L, Schultz-Cherry S, Darling V, Johnson C, Karlsson EA, Chang TC, Jeha S, Pui CH, Sun Y, Pounds S, Hayden RT, Tuomanen E, Rosch JW. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin Infect Dis. 2018 Aug 1;67(4):541-548. doi: 10.1093/cid/ciy153.
- Taur Y, Jenq RR, Ubeda C, van den Brink M, Pamer EG. Role of intestinal microbiota in transplantation outcomes. Best Pract Res Clin Haematol. 2015;28(2-3):155-161. doi: 10.1016/j.beha.2015.10.013
- Galloway-Peña JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG, Chemaly RF, Marsh L, Ghantoji SS, Pemmaraju N et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer. 2016;122(14):2186-2196. doi:10.1002/cncr.30039.
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18(4):295-304.
- Martin PJ, Bachier CR, Klingemann HG, McCarthy PL, Szabolcs P, Uberti JP, Schuster MW, Weisdorf D, Chao NJ, Kebriaei P, Shpall EJ, Macmillan ML, Soiffer RJ. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a consensus document. Biol Blood Marrow Transplant. 2009; 15(7):777-784. doi: 10.1016/j.bbmt.2009.03.012.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920-924. doi: 10.3109/00365529709011203.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.....
- WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva: World Health Organization; 2018. https://www.who.int/ncds/management/palliative-care/cancer-pain-guidelines/en/
- Goloshchapov, OV, Olekhnovich EI, Sidorenko SV, Moiseev IS, Kucher MA, Fedorov DE, Pavlenko AV, Manolov AI, Gostev VV, Veselovsky VA et al. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol. 2019; 19(1), 312. doi: 10.1186/s12866-019-1689-y.
- Goloshchapov OV, Churakina DV, Kucher MA, Klement’eva RV, Sidorenko SV, Gostev VV, Karev VE, Suvorova MA, Shlyk IV, Zubarovskaya LS, Afanasyev BV. Fecal microbiota transplantation in critically ill patients in oncohematological practice. Vestnik Anaesthesiol Reanimatol. 2019; 16(3):63-73 (In Russian). doi: 10.21292/2078-5658-2019-16-3-63-73.
- Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol 2007; 28:1233–1235. doi: 10.1086/520732.
- Khanna S, Baddour LM, Huskins WC et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 2013; 56:1401-1406. doi: 10.1093/cid/cit075.
- Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001-2006. Pediatrics 2008; 122:1266-70. doi: 10.1542/peds.2008-0469.
- Nicholson MR, Thomsen IP, Slaughter JC, Creech CB, Edwards KM. Novel risk factors for recurrent Clostridium difficult infection in children. J Pediatr Gastroenterol Nutr. 2015; 60(1):18-22. doi: 10.1097/MPG.0000000000000553.
- Kociolek LK, Palac HL, Patel SJ, Shulman ST, Gerding DN. Risk factors for recurrent Clostridium difficile infection in children: A Nested Case-Control Study. J Pediatr. 2015 Aug; 167(2):384-389. doi: 10.1016/j.jpeds.2015.04.052.
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll K, Coffin S, Dubberke E, Garey K, Gould C, Kelly C et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. doi:10.1093/cid/cix1085.
- Nicholson MR, Mitchell PD, Alexander E, Ballal S, Bartlett M, Becker P, Davidovics Z, Docktor M, Dole M, Felix G, et al. Efficacy of fecal microbiota transplantation for Clostridium difficile infection in children. Clin Gastroenterol Hepatol. 2020 Mar;18(3):612-619.e1. doi: 10.1016/j.cgh.2019.04.037.
- Davidovics ZH, Michail S, Nicholson MR, Kociolek L, Pai N, Hansen R, Schwerd T, Maspons A, Shamir R, Szajewska H et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection and other conditions in children: A joint position paper from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2019;68(1):130-143. doi:10.1097/MPG.0000000000002205.
- Quagliariello A, Del Chierico F, Reddel S, Russo A, Muda AO, D’Argenio P, Angelino G, Romeo EF, Dall’Oglio L, De Angelis P et al. Fecal Microbiota Transplant in Two Ulcerative Colitis Pediatric Cases: Gut Microbiota and Clinical Course Correlations. Microorganisms. 2020;8(10):1486. doi:10.3390/microorganisms810148631.
- Goyal A, Yeh A, Bush BR, Firek BA, Siebold LM, Rogers MB, Kufen AD, Morowitz MJ. Safety, Clinical Response, and Microbiome Findings Following Fecal Microbiota Transplant in Children With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24(2):410-421. doi: 10.1093/ibd/izx035.
- Bilinski J, Grzesiowski P, Sorensen N, Madry K, Muszynski J, Robak K, Wroblewska M, Dzieciatkowski T, Dulny G, Dwilewicz-Trojaczek J et al. Fecal Microbiota Transplantation in Patients With Blood Disorders Inhibits Gut Colonization With Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin Infect Dis. 2017;65(3):364-370. doi: 10.1093/cid/cix252.
- Battipaglia G, Malard F, Rubio MT, Ruggeri A, Mamez AC, Brissot E, Giannotti F, Dulery R, Joly AC, Baylatry MT et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematologic malignancies carrying multidrug-resistance bacteria. Haematologica. 2019;104(8):1682-1628. doi: 10.3324/haematol.2018.198549.
- Russell G, Kaplan J, Ferraro M, Michelow IC. Fecal bacteriotherapy for relapsing Clostridium difficile infection in a child: a proposed treatment protocol. Pediatrics. 2010;126 (1): e239‒е242. doi: 10.1542/peds.2009-3363.
- Steinbach G, Hockenbery DM, Huls G, Furlong T, Myerson D, Loeb KR, Fann JR, Castilla-Llorente C, McDonald GB, Martin PJ. Pilot study of lithium to restore intestinal barrier function in severe graft-versus-host disease. PLoS One. 2017;12(8):e0183284. doi: 10.1371/journal.pone.0183284.
- Simms-Waldrip TR, Sunkersett G, Coughlin LA, Savani MR, Arana C, Kim J, Kim M, Zhan X, Greenberg DE, Xie Y, Davies SM, Koh AY. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol Blood Marrow Transplant. 2017; 23(5):820-829. doi: 10.1016/j.bbmt.2017.02.004.
- Ingham AC, Kielsen K, Cilieborg MS, Lund O, Holmes S, Aarestrup FM, Müller KG, Pamp SJ. Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome. 2019; 7(1):131. doi: 10.1186/s40168-019-0745-z.
- Taur Y. Intestinal microbiome changes and stem cell transplantation: Lessons learned. Virulence 2016;7(8):930-938. doi: 10.1080/21505594.2016.1250982.
- Steck N, Hoffmann M, Sava IG, Kim SC, Hahne H, Tonkonogy SL, Mair K, Krueger D, Pruteanu M, Shanahan F et al Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology.2011; 141 (3): 959–971. doi: 10.1053/j.gastro.2011.05.035.
- Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, Mimura I, Morita H, Sugiyama D, Nishikawa H, Hattori M, Hino Y, Ikegawa S, Yamamoto K, Toya T, Doki N, Koizumi K, Honda K, Ohashi K. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016; 128(16):2083-2088. doi: 10.1182/blood-2016-05-717652.
- Zhong S, Zeng J, Deng Z, Jiang L, Zhang B, Yang K, Wang W, Zhang T. Fecal microbiota transplantation for refractory diarrhea in immunocompromised diseases: a pediatric case report. Ital J Pediatr. 2019;45(1):116. doi:10.1186/s13052-019-0708-9.
- Use of fecal microbiota for transplantation to treat Clostridium difficile. Federal Register 2019; 84 (176): 471911. www.federalregister.gov/documents/2019/09/11/2019-19643.
- Zhang XY, Wang YZ, Li XL, Hu H, Liu HF, Li D, Xiao YM, Zhang T. Safety of fecal microbiota transplantation in Chinese children: A single-center retrospective study. World J Clin Cases. 2018;6(16):1121-1127. doi: 10.12998/wjcc.v6.i16.1121.
- Youngster I, Mahabamunuge J, Systrom HK, Sauk J, Khalili H, Levin J, Kaplan JL, Hohmann EL. . Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016;14(1):134. doi:10.1186/s12916-016-0680-9.
- McIlroy JR, Nalagatla N, Hansen R, Hart A, Hold GL. Faecal microbiota transplantation as a treatment for inflammatory bowel disease: a national survey of adult and paediatric gastroenterologists in the UK. Frontline Gastroenterol. 2018;9(4):250-255. doi: 10.1136/flgastro-2017-100936.
- Shouval R, Geva M, Nagler A, Youngster I. Fecal microbiota transplantation for treatment of acute graft-versus-host disease. Clin Hematol Internat. 2019; 1(1):28-35. doi: 10.2991/chi.d.190316.002.
- Goloshchapov OV, Bakin EA, Kucher MA, Stanevich OV, Suvorova MA, Gostev VV, Glotov OS, Eismont YA, Polev DE, Lobenskaya A. Bacteroides fragilis is a potential marker of effective microbiota recolonization in acute graft-versus-host disease treatment. Cell Ther Transplant. 2020; 9(2): 47-59.doi:10.18620/ctt-1866-8836-2020-9-2-47-59.
- Popov J, Hartung E, Hill L, Chauhan U, Pai N. Pediatric Patient and Parent Perceptions of Fecal Microbiota Transplantation for the Treatment of Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2020. doi: 10.1097/MPG.0000000000002995. Epub ahead of print. PMID: 33230077.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "60" ["~SORT"]=> string(2) "60" ["CODE"]=> string(100) "kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra" ["~CODE"]=> string(100) "kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra" ["EXTERNAL_ID"]=> string(4) "1954" ["~EXTERNAL_ID"]=> string(4) "1954" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(343) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяинаClinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4520) "<p style="text-align: justify;"> На протяжении последних лет установлена важная роль нарушений кишечной микробиоты и ее потенуиальной коррекции при злокачественных новообразованиях и аутоиммунных заболеваниях у детей. Данные последнего десятилетия выявили существенное влияние основных классов кишечных бактерий (<i>Firmicutes</i> и <i>Bacteroides</i>) на развитие иммунного ответа при онкологических заболеваниях и аутоагрессивных состояниях, а также роль их дисбаланса и коррекции посредством трансплантации фекальной микробиоты (ТФМ). Наши предыдущие исследования показали выраженный клинический эффект ТФМ, в основном, у взрослых пациентов с тяжелой острой реакцией «трансплантат против хозяина» (РТПХ). Целью настоящей работы было представить наш опыт ТФМ у детей с кишечной формой острой РТПХ, резистентной к обычному лечению. </p> <h3>Материалы и методы</h3> <p style="text-align: justify;"> Проспективное одноцентровое исследование включало 7 пациентов в возрасте от 3 до 10 лет с тяжелой кишечной РТПХ после аллогенной трансплантации гемопоэтических стволовых клеток. Клинические эффекты ТФМ определяли с применением стандартных шкал на протяжении 120 сут. после данной процедуры. Временную динамику состава фекальной микробиоты оценивали, главным образом, посредством мультиплексной ПЦР тест-системы. Результаты Мы представили собственный опыт ТФМ у 7 детей с кишечной формой острой РТПХ и антибиотикорезистентным колитом. Полный или частичный ответ на этот вид лечения РТПХ был достигнут в 6 случаях (86%) в течение 120 сут. в отсутствии серьезных нежелательных эффектов после ТФМ. С 8-го дня после ТФМ было отмечено нарастание содержания <i>B. fragilis gr., Faecalibacterium prausnitzii</i> и <i>E. coli</i> в фекальной микробиоте (р< 0,048, р< 0,001, и р<0,048, соответственно), при отсутствии различий по <i>Bifidobacterium spp.</i> и <i>Lactobacillus spp</i>.</p> <h3>Выводы</h3> <p style="text-align: justify;"> Комбинированная терапия иммуносупрессивными препаратами и ФМТ у пациентов с кишечной формой РТПХ, резистентной к стандартной терапии, сопровождается выраженным клиническим ответом, коррелирующим с определенными изменениями кишечной микробиоты и приемлемыми показателями безопасности процедуры.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантации фекальной микробиоты, трансплантация гемопоэтических клеток, реакция «трансплантат против хозяина», фекальная микробиота, трансплантация, клиническая эффективность, побочные эффекты. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_META_TITLE"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_META_KEYWORDS"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_META_DESCRIPTION"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_PICTURE_FILE_ALT"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_PICTURE_FILE_TITLE"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(231) "Клинико-иммунологические эффекты трансплантации фекальной микробиоты у детей с острой реакцией трансплантат против хозяина" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "kliniko-immunologicheskie-effekty-transplantatsii-fekalnoy-mikrobioty-u-detey-s-ostroy-reaktsiey-tra" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "175" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27502" ["VALUE"]=> string(22) "02/05/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/05/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27503" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27504" ["VALUE"]=> array(2) { ["TEXT"]=> string(969) "<p>Олег В. Голощапов<sup>1</sup>, Евгений А. Бакин<sup>1</sup>, Оксана В. Станевич<sup>1</sup>, Руслана В. Клементьева<sup>1</sup>, Александр А. Щербаков<sup>1</sup>, Александр Н. Швецов<sup>1</sup>, Олеся В. Паина<sup>1</sup>, Полина В. Кожокарь<sup>1</sup>, Маргарита В. Горчакова<sup>1</sup>, Елена В. Бабенко<sup>1</sup>, Мария А. Суворова<sup>2</sup>, Сергей Н. Бондаренко<sup>1</sup>, Максим А. Кучер<sup>1</sup>, Александр Д. Кулагин<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup>, Иван С. Моисеев<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(765) "
Олег В. Голощапов1, Евгений А. Бакин1, Оксана В. Станевич1, Руслана В. Клементьева1, Александр А. Щербаков1, Александр Н. Швецов1, Олеся В. Паина1, Полина В. Кожокарь1, Маргарита В. Горчакова1, Елена В. Бабенко1, Мария А. Суворова2, Сергей Н. Бондаренко1, Максим А. Кучер1, Александр Д. Кулагин1, Людмила С. Зубаровская1, Иван С. Моисеев1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27505" ["VALUE"]=> array(2) { ["TEXT"]=> string(365) "<p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br> <sup>2</sup> ООО «Эксплана», Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(323) "1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ООО «Эксплана», Санкт-Петербург, Россия
На протяжении последних лет установлена важная роль нарушений кишечной микробиоты и ее потенуиальной коррекции при злокачественных новообразованиях и аутоиммунных заболеваниях у детей. Данные последнего десятилетия выявили существенное влияние основных классов кишечных бактерий (Firmicutes и Bacteroides) на развитие иммунного ответа при онкологических заболеваниях и аутоагрессивных состояниях, а также роль их дисбаланса и коррекции посредством трансплантации фекальной микробиоты (ТФМ). Наши предыдущие исследования показали выраженный клинический эффект ТФМ, в основном, у взрослых пациентов с тяжелой острой реакцией «трансплантат против хозяина» (РТПХ). Целью настоящей работы было представить наш опыт ТФМ у детей с кишечной формой острой РТПХ, резистентной к обычному лечению.
Материалы и методы
Проспективное одноцентровое исследование включало 7 пациентов в возрасте от 3 до 10 лет с тяжелой кишечной РТПХ после аллогенной трансплантации гемопоэтических стволовых клеток. Клинические эффекты ТФМ определяли с применением стандартных шкал на протяжении 120 сут. после данной процедуры. Временную динамику состава фекальной микробиоты оценивали, главным образом, посредством мультиплексной ПЦР тест-системы. Результаты Мы представили собственный опыт ТФМ у 7 детей с кишечной формой острой РТПХ и антибиотикорезистентным колитом. Полный или частичный ответ на этот вид лечения РТПХ был достигнут в 6 случаях (86%) в течение 120 сут. в отсутствии серьезных нежелательных эффектов после ТФМ. С 8-го дня после ТФМ было отмечено нарастание содержания B. fragilis gr., Faecalibacterium prausnitzii и E. coli в фекальной микробиоте (р< 0,048, р< 0,001, и р<0,048, соответственно), при отсутствии различий по Bifidobacterium spp. и Lactobacillus spp.
Выводы
Комбинированная терапия иммуносупрессивными препаратами и ФМТ у пациентов с кишечной формой РТПХ, резистентной к стандартной терапии, сопровождается выраженным клиническим ответом, коррелирующим с определенными изменениями кишечной микробиоты и приемлемыми показателями безопасности процедуры.
Ключевые слова
Трансплантации фекальной микробиоты, трансплантация гемопоэтических клеток, реакция «трансплантат против хозяина», фекальная микробиота, трансплантация, клиническая эффективность, побочные эффекты.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27507" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-69-78" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-69-78" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27509" ["VALUE"]=> array(2) { ["TEXT"]=> string(735) "<p>Oleg V. Goloshchapov<sup>1</sup>, Evgeny A. Bakin<sup>1</sup>, Oksana V. Stanevich<sup>1</sup>, Ruslana V. Klementeva<sup>1</sup>, Alexander A. Shcherbakov<sup>1</sup>, Alexander N. Shvetsov<sup>1</sup>, Olesya V. Paina<sup>1</sup>, Polina V. Kozhokar<sup>1</sup>, Margarita V. Gorchakova<sup>1</sup>, Elena V. Babenko<sup>1</sup>, Maria A. Suvorova<sup>2</sup>, Sergey N. Bondarenko<sup>1</sup>, Maxim A. Kucher<sup>1</sup>, Alexander D. Kulagin<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Ivan S. Moiseev<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(531) "Oleg V. Goloshchapov1, Evgeny A. Bakin1, Oksana V. Stanevich1, Ruslana V. Klementeva1, Alexander A. Shcherbakov1, Alexander N. Shvetsov1, Olesya V. Paina1, Polina V. Kozhokar1, Margarita V. Gorchakova1, Elena V. Babenko1, Maria A. Suvorova2, Sergey N. Bondarenko1, Maxim A. Kucher1, Alexander D. Kulagin1, Ludmila S. Zubarovskaya1, Ivan S. Moiseev1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27510" ["VALUE"]=> array(2) { ["TEXT"]=> string(488) "<p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Explana Research Laboratory, St. Petersbug, Russia</p><br> <p><b>Correspondence</b><br> Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br> Phone: +7 (921) 979 2913<br> E-mail: golocht@yandex.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(398) "1 Pavlov University, St. Petersburg, Russia
2 Explana Research Laboratory, St. Petersbug, Russia
Correspondence
Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Phone: +7 (921) 979 2913
E-mail: golocht@yandex.ru
Over last years, an important role of altered gut microbiota and its potential correction was suggested for pediatric cancer and autoimmune disorders. The data from last decade highlight sufficient influence of the main classes of gut bacteria (Firmicutes and Bacteroides) upon development of immune response in oncological disorders and autoagressive conditions, as well as role of their imbalance and its correction using fecal microbiota transplantation (FMT) approach. Our previous studies have shown a pronounced clinical effect of FMT, mostly, in adult patients with severe acute graft-versus-host disease (GVHD). The aim of this article was to present our own experience of FMT in children with intestinal form of GVHD resistant to conventional treatment.
Materials and methods
A prospective single-center study included 7 patients aged from 3 to 10 years with severe intestinal GVHD developed after allogeneic hematopoietic stem cell transplantation. Clinical effects of FMT were evaluated by conventional scales during 120 days after the procedure. Time-dependent changes of fecal microbiota were assayed, mainly, by the multiplex polymerase chain reaction (PCR) test-system.
Results
We present our own experience of FMT in 7 children with intestinal GVHD and antibiotic-resistant colitis. Complete or partial response to the GVHD treatment was achieved in 6 cases (86%) by 120 days, in absence of serious adverse events following FMT. Since day +8 after TFM, increased amounts of B. fragilis gr., Faecalibacterium prausnitzii and E. coli were registered in fecal microbiota (р< 0.048, р< 0.001, and р<0.048, respectively), in absence of differences for Bifidobacterium spp and Lactobacillus spp.
Conclusion
Combined therapy with immunosuppressive agents and FMT procedure in the patients with intestinal GVHD resistant to standard therapy is associated with pronounced clinical responses correlating with distinct changes of intestinal microbiota, with acceptable safety profile.
Keywords
Hematopoietic stem cell transplantation, graft-versus-host disease, fecal microbiota transplantation, clinical efficiency.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27508" ["VALUE"]=> string(112) "Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(112) "Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> &array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27536" ["VALUE"]=> string(4) "2428" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2428" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27537" ["VALUE"]=> string(4) "2429" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2429" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27509" ["VALUE"]=> array(2) { ["TEXT"]=> string(735) "<p>Oleg V. Goloshchapov<sup>1</sup>, Evgeny A. Bakin<sup>1</sup>, Oksana V. Stanevich<sup>1</sup>, Ruslana V. Klementeva<sup>1</sup>, Alexander A. Shcherbakov<sup>1</sup>, Alexander N. Shvetsov<sup>1</sup>, Olesya V. Paina<sup>1</sup>, Polina V. Kozhokar<sup>1</sup>, Margarita V. Gorchakova<sup>1</sup>, Elena V. Babenko<sup>1</sup>, Maria A. Suvorova<sup>2</sup>, Sergey N. Bondarenko<sup>1</sup>, Maxim A. Kucher<sup>1</sup>, Alexander D. Kulagin<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Ivan S. Moiseev<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(531) "Oleg V. Goloshchapov1, Evgeny A. Bakin1, Oksana V. Stanevich1, Ruslana V. Klementeva1, Alexander A. Shcherbakov1, Alexander N. Shvetsov1, Olesya V. Paina1, Polina V. Kozhokar1, Margarita V. Gorchakova1, Elena V. Babenko1, Maria A. Suvorova2, Sergey N. Bondarenko1, Maxim A. Kucher1, Alexander D. Kulagin1, Ludmila S. Zubarovskaya1, Ivan S. Moiseev1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(531) "Oleg V. Goloshchapov1, Evgeny A. Bakin1, Oksana V. Stanevich1, Ruslana V. Klementeva1, Alexander A. Shcherbakov1, Alexander N. Shvetsov1, Olesya V. Paina1, Polina V. Kozhokar1, Margarita V. Gorchakova1, Elena V. Babenko1, Maria A. Suvorova2, Sergey N. Bondarenko1, Maxim A. Kucher1, Alexander D. Kulagin1, Ludmila S. Zubarovskaya1, Ivan S. Moiseev1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27511" ["VALUE"]=> array(2) { ["TEXT"]=> string(2672) "<p style="text-align: justify;"> Over last years, an important role of altered gut microbiota and its potential correction was suggested for pediatric cancer and autoimmune disorders. The data from last decade highlight sufficient influence of the main classes of gut bacteria (<i>Firmicutes</i> and <i>Bacteroides</i>) upon development of immune response in oncological disorders and autoagressive conditions, as well as role of their imbalance and its correction using fecal microbiota transplantation (FMT) approach. Our previous studies have shown a pronounced clinical effect of FMT, mostly, in adult patients with severe acute graft-versus-host disease (GVHD). The aim of this article was to present our own experience of FMT in children with intestinal form of GVHD resistant to conventional treatment. </p> <h3>Materials and methods</h3> <p style="text-align: justify;"> A prospective single-center study included 7 patients aged from 3 to 10 years with severe intestinal GVHD developed after allogeneic hematopoietic stem cell transplantation. Clinical effects of FMT were evaluated by conventional scales during 120 days after the procedure. Time-dependent changes of fecal microbiota were assayed, mainly, by the multiplex polymerase chain reaction (PCR) test-system. </p> <h3> Results</h3> <p style="text-align: justify;"> We present our own experience of FMT in 7 children with intestinal GVHD and antibiotic-resistant colitis. Complete or partial response to the GVHD treatment was achieved in 6 cases (86%) by 120 days, in absence of serious adverse events following FMT. Since day +8 after TFM, increased amounts of <i>B. fragilis gr., Faecalibacterium prausnitzii</i> and <i>E. coli </i>were registered in fecal microbiota (р< 0.048, р< 0.001, and р<0.048, respectively), in absence of differences for <i>Bifidobacterium spp </i>and <i>Lactobacillus spp</i>. </p> <h3>Conclusion</h3> <p style="text-align: justify;"> Combined therapy with immunosuppressive agents and FMT procedure in the patients with intestinal GVHD resistant to standard therapy is associated with pronounced clinical responses correlating with distinct changes of intestinal microbiota, with acceptable safety profile. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Hematopoietic stem cell transplantation, graft-versus-host disease, fecal microbiota transplantation, clinical efficiency. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2430) "Over last years, an important role of altered gut microbiota and its potential correction was suggested for pediatric cancer and autoimmune disorders. The data from last decade highlight sufficient influence of the main classes of gut bacteria (Firmicutes and Bacteroides) upon development of immune response in oncological disorders and autoagressive conditions, as well as role of their imbalance and its correction using fecal microbiota transplantation (FMT) approach. Our previous studies have shown a pronounced clinical effect of FMT, mostly, in adult patients with severe acute graft-versus-host disease (GVHD). The aim of this article was to present our own experience of FMT in children with intestinal form of GVHD resistant to conventional treatment.
Materials and methods
A prospective single-center study included 7 patients aged from 3 to 10 years with severe intestinal GVHD developed after allogeneic hematopoietic stem cell transplantation. Clinical effects of FMT were evaluated by conventional scales during 120 days after the procedure. Time-dependent changes of fecal microbiota were assayed, mainly, by the multiplex polymerase chain reaction (PCR) test-system.
Results
We present our own experience of FMT in 7 children with intestinal GVHD and antibiotic-resistant colitis. Complete or partial response to the GVHD treatment was achieved in 6 cases (86%) by 120 days, in absence of serious adverse events following FMT. Since day +8 after TFM, increased amounts of B. fragilis gr., Faecalibacterium prausnitzii and E. coli were registered in fecal microbiota (р< 0.048, р< 0.001, and р<0.048, respectively), in absence of differences for Bifidobacterium spp and Lactobacillus spp.
Conclusion
Combined therapy with immunosuppressive agents and FMT procedure in the patients with intestinal GVHD resistant to standard therapy is associated with pronounced clinical responses correlating with distinct changes of intestinal microbiota, with acceptable safety profile.
Keywords
Hematopoietic stem cell transplantation, graft-versus-host disease, fecal microbiota transplantation, clinical efficiency.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2430) "Over last years, an important role of altered gut microbiota and its potential correction was suggested for pediatric cancer and autoimmune disorders. The data from last decade highlight sufficient influence of the main classes of gut bacteria (Firmicutes and Bacteroides) upon development of immune response in oncological disorders and autoagressive conditions, as well as role of their imbalance and its correction using fecal microbiota transplantation (FMT) approach. Our previous studies have shown a pronounced clinical effect of FMT, mostly, in adult patients with severe acute graft-versus-host disease (GVHD). The aim of this article was to present our own experience of FMT in children with intestinal form of GVHD resistant to conventional treatment.
Materials and methods
A prospective single-center study included 7 patients aged from 3 to 10 years with severe intestinal GVHD developed after allogeneic hematopoietic stem cell transplantation. Clinical effects of FMT were evaluated by conventional scales during 120 days after the procedure. Time-dependent changes of fecal microbiota were assayed, mainly, by the multiplex polymerase chain reaction (PCR) test-system.
Results
We present our own experience of FMT in 7 children with intestinal GVHD and antibiotic-resistant colitis. Complete or partial response to the GVHD treatment was achieved in 6 cases (86%) by 120 days, in absence of serious adverse events following FMT. Since day +8 after TFM, increased amounts of B. fragilis gr., Faecalibacterium prausnitzii and E. coli were registered in fecal microbiota (р< 0.048, р< 0.001, and р<0.048, respectively), in absence of differences for Bifidobacterium spp and Lactobacillus spp.
Conclusion
Combined therapy with immunosuppressive agents and FMT procedure in the patients with intestinal GVHD resistant to standard therapy is associated with pronounced clinical responses correlating with distinct changes of intestinal microbiota, with acceptable safety profile.
Keywords
Hematopoietic stem cell transplantation, graft-versus-host disease, fecal microbiota transplantation, clinical efficiency.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27507" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-69-78" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-69-78" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-69-78" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27508" ["VALUE"]=> string(112) "Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(112) "Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(112) "Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27510" ["VALUE"]=> array(2) { ["TEXT"]=> string(488) "<p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Explana Research Laboratory, St. Petersbug, Russia</p><br> <p><b>Correspondence</b><br> Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br> Phone: +7 (921) 979 2913<br> E-mail: golocht@yandex.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(398) "1 Pavlov University, St. Petersburg, Russia
2 Explana Research Laboratory, St. Petersbug, Russia
Correspondence
Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Phone: +7 (921) 979 2913
E-mail: golocht@yandex.ru
1 Pavlov University, St. Petersburg, Russia
2 Explana Research Laboratory, St. Petersbug, Russia
Correspondence
Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Phone: +7 (921) 979 2913
E-mail: golocht@yandex.ru
Олег В. Голощапов1, Евгений А. Бакин1, Оксана В. Станевич1, Руслана В. Клементьева1, Александр А. Щербаков1, Александр Н. Швецов1, Олеся В. Паина1, Полина В. Кожокарь1, Маргарита В. Горчакова1, Елена В. Бабенко1, Мария А. Суворова2, Сергей Н. Бондаренко1, Максим А. Кучер1, Александр Д. Кулагин1, Людмила С. Зубаровская1, Иван С. Моисеев1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(765) "Олег В. Голощапов1, Евгений А. Бакин1, Оксана В. Станевич1, Руслана В. Клементьева1, Александр А. Щербаков1, Александр Н. Швецов1, Олеся В. Паина1, Полина В. Кожокарь1, Маргарита В. Горчакова1, Елена В. Бабенко1, Мария А. Суворова2, Сергей Н. Бондаренко1, Максим А. Кучер1, Александр Д. Кулагин1, Людмила С. Зубаровская1, Иван С. Моисеев1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27502" ["VALUE"]=> string(22) "02/05/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/05/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "02/05/2021 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27503" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "04/09/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27506" ["VALUE"]=> array(2) { ["TEXT"]=> string(4520) "<p style="text-align: justify;"> На протяжении последних лет установлена важная роль нарушений кишечной микробиоты и ее потенуиальной коррекции при злокачественных новообразованиях и аутоиммунных заболеваниях у детей. Данные последнего десятилетия выявили существенное влияние основных классов кишечных бактерий (<i>Firmicutes</i> и <i>Bacteroides</i>) на развитие иммунного ответа при онкологических заболеваниях и аутоагрессивных состояниях, а также роль их дисбаланса и коррекции посредством трансплантации фекальной микробиоты (ТФМ). Наши предыдущие исследования показали выраженный клинический эффект ТФМ, в основном, у взрослых пациентов с тяжелой острой реакцией «трансплантат против хозяина» (РТПХ). Целью настоящей работы было представить наш опыт ТФМ у детей с кишечной формой острой РТПХ, резистентной к обычному лечению. </p> <h3>Материалы и методы</h3> <p style="text-align: justify;"> Проспективное одноцентровое исследование включало 7 пациентов в возрасте от 3 до 10 лет с тяжелой кишечной РТПХ после аллогенной трансплантации гемопоэтических стволовых клеток. Клинические эффекты ТФМ определяли с применением стандартных шкал на протяжении 120 сут. после данной процедуры. Временную динамику состава фекальной микробиоты оценивали, главным образом, посредством мультиплексной ПЦР тест-системы. Результаты Мы представили собственный опыт ТФМ у 7 детей с кишечной формой острой РТПХ и антибиотикорезистентным колитом. Полный или частичный ответ на этот вид лечения РТПХ был достигнут в 6 случаях (86%) в течение 120 сут. в отсутствии серьезных нежелательных эффектов после ТФМ. С 8-го дня после ТФМ было отмечено нарастание содержания <i>B. fragilis gr., Faecalibacterium prausnitzii</i> и <i>E. coli</i> в фекальной микробиоте (р< 0,048, р< 0,001, и р<0,048, соответственно), при отсутствии различий по <i>Bifidobacterium spp.</i> и <i>Lactobacillus spp</i>.</p> <h3>Выводы</h3> <p style="text-align: justify;"> Комбинированная терапия иммуносупрессивными препаратами и ФМТ у пациентов с кишечной формой РТПХ, резистентной к стандартной терапии, сопровождается выраженным клиническим ответом, коррелирующим с определенными изменениями кишечной микробиоты и приемлемыми показателями безопасности процедуры.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантации фекальной микробиоты, трансплантация гемопоэтических клеток, реакция «трансплантат против хозяина», фекальная микробиота, трансплантация, клиническая эффективность, побочные эффекты. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4312) "На протяжении последних лет установлена важная роль нарушений кишечной микробиоты и ее потенуиальной коррекции при злокачественных новообразованиях и аутоиммунных заболеваниях у детей. Данные последнего десятилетия выявили существенное влияние основных классов кишечных бактерий (Firmicutes и Bacteroides) на развитие иммунного ответа при онкологических заболеваниях и аутоагрессивных состояниях, а также роль их дисбаланса и коррекции посредством трансплантации фекальной микробиоты (ТФМ). Наши предыдущие исследования показали выраженный клинический эффект ТФМ, в основном, у взрослых пациентов с тяжелой острой реакцией «трансплантат против хозяина» (РТПХ). Целью настоящей работы было представить наш опыт ТФМ у детей с кишечной формой острой РТПХ, резистентной к обычному лечению.
Материалы и методы
Проспективное одноцентровое исследование включало 7 пациентов в возрасте от 3 до 10 лет с тяжелой кишечной РТПХ после аллогенной трансплантации гемопоэтических стволовых клеток. Клинические эффекты ТФМ определяли с применением стандартных шкал на протяжении 120 сут. после данной процедуры. Временную динамику состава фекальной микробиоты оценивали, главным образом, посредством мультиплексной ПЦР тест-системы. Результаты Мы представили собственный опыт ТФМ у 7 детей с кишечной формой острой РТПХ и антибиотикорезистентным колитом. Полный или частичный ответ на этот вид лечения РТПХ был достигнут в 6 случаях (86%) в течение 120 сут. в отсутствии серьезных нежелательных эффектов после ТФМ. С 8-го дня после ТФМ было отмечено нарастание содержания B. fragilis gr., Faecalibacterium prausnitzii и E. coli в фекальной микробиоте (р< 0,048, р< 0,001, и р<0,048, соответственно), при отсутствии различий по Bifidobacterium spp. и Lactobacillus spp.
Выводы
Комбинированная терапия иммуносупрессивными препаратами и ФМТ у пациентов с кишечной формой РТПХ, резистентной к стандартной терапии, сопровождается выраженным клиническим ответом, коррелирующим с определенными изменениями кишечной микробиоты и приемлемыми показателями безопасности процедуры.
Ключевые слова
Трансплантации фекальной микробиоты, трансплантация гемопоэтических клеток, реакция «трансплантат против хозяина», фекальная микробиота, трансплантация, клиническая эффективность, побочные эффекты.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4312) "На протяжении последних лет установлена важная роль нарушений кишечной микробиоты и ее потенуиальной коррекции при злокачественных новообразованиях и аутоиммунных заболеваниях у детей. Данные последнего десятилетия выявили существенное влияние основных классов кишечных бактерий (Firmicutes и Bacteroides) на развитие иммунного ответа при онкологических заболеваниях и аутоагрессивных состояниях, а также роль их дисбаланса и коррекции посредством трансплантации фекальной микробиоты (ТФМ). Наши предыдущие исследования показали выраженный клинический эффект ТФМ, в основном, у взрослых пациентов с тяжелой острой реакцией «трансплантат против хозяина» (РТПХ). Целью настоящей работы было представить наш опыт ТФМ у детей с кишечной формой острой РТПХ, резистентной к обычному лечению.
Материалы и методы
Проспективное одноцентровое исследование включало 7 пациентов в возрасте от 3 до 10 лет с тяжелой кишечной РТПХ после аллогенной трансплантации гемопоэтических стволовых клеток. Клинические эффекты ТФМ определяли с применением стандартных шкал на протяжении 120 сут. после данной процедуры. Временную динамику состава фекальной микробиоты оценивали, главным образом, посредством мультиплексной ПЦР тест-системы. Результаты Мы представили собственный опыт ТФМ у 7 детей с кишечной формой острой РТПХ и антибиотикорезистентным колитом. Полный или частичный ответ на этот вид лечения РТПХ был достигнут в 6 случаях (86%) в течение 120 сут. в отсутствии серьезных нежелательных эффектов после ТФМ. С 8-го дня после ТФМ было отмечено нарастание содержания B. fragilis gr., Faecalibacterium prausnitzii и E. coli в фекальной микробиоте (р< 0,048, р< 0,001, и р<0,048, соответственно), при отсутствии различий по Bifidobacterium spp. и Lactobacillus spp.
Выводы
Комбинированная терапия иммуносупрессивными препаратами и ФМТ у пациентов с кишечной формой РТПХ, резистентной к стандартной терапии, сопровождается выраженным клиническим ответом, коррелирующим с определенными изменениями кишечной микробиоты и приемлемыми показателями безопасности процедуры.
Ключевые слова
Трансплантации фекальной микробиоты, трансплантация гемопоэтических клеток, реакция «трансплантат против хозяина», фекальная микробиота, трансплантация, клиническая эффективность, побочные эффекты.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27505" ["VALUE"]=> array(2) { ["TEXT"]=> string(365) "<p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br> <sup>2</sup> ООО «Эксплана», Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(323) "1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ООО «Эксплана», Санкт-Петербург, Россия
1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ООО «Эксплана», Санкт-Петербург, Россия
Introduction
Over last decade, several experimental studies showed a heterogeneity of myeloid leukemia precursor cells in human acute myeloid leukemia (AML)[1-4]. This population includes, at least, immature leukemia-initiating cells (LIC) with CD34+CD38– – immune phenotype, being able for selective BAALC gene expression [5], whereas more mature LIC were attributed to acute promyelocytic leukemia (APL) origin. Biology of the latter cell population has been yet poorly studied. From some indirect data, one may suggest that these precursors, along to blast cells [6-11], may participate in WT1 gene expression [11, 12]. One line of evidence is based on some cases of acute promyelocytic leukemia (APL), where higher level of gene WT1 expression was coupled to lower blast cell numbers in bone marrow [12]. Moreover, this conclusion is also supported by similar discrepancy between higher WT1 gene expression and lower numbers of bone marrow blasts in 30% to 40% AML patients, both before and after HSCT [6, 9, 10, 13, 14]. In our opinion, this phenomenon may be also explained by presence of active expression of WT1 gene by more mature precursors [12, 15]. Confirmation of this hypothesis should extend our opportunities for evaluation of leukemic hematopoiesis on the level of leukemic precursors, using quantitative qRT-PCR [12, 14, 15]. To test this hypothesis, we performed parallel measurements of BAALC and WT1 expression levels in 14 AML patients with different cytological, cytogenetic and molecular variants of AML treated with combination of high-dose chemotherapy (ChT) and Mylotarg. Moreover, allogeneic hematopoietic stem cell transplantation (HSCT) was performed in 11 cases. It should be noticed here that our choice of Mylotarg was determined by some biological effects of this drug. Firstly, we have recently revealed unexpected response of leukemic cells to this drug, accompanied by elevation of WT1 gene expression [13]. Secondly, some data suggest a direct action of Mylotarg upon immature precursor cells with CD34+CD38– – phenotype [16]. Worth of note, more pronounced response to Mylotarg was revealed in AML with normal karyotypes and, especially, in those with FLT3 gene mutations [17]. This effect may be explained by higher expression of CD33 antigens on the surface of leukemic cells in AML with mutated FLT3 [18, 19]. On the contrary, overall survival in AML patients with WT1 gene overexpression became shorter, being in good accordance with our present findings.
Hence, the aim of our work was to study feasibility of BAALC/WT1 molecular panel for evaluation of hematopoiesis on the level of variously differentiated leukemic progenitors in the group of treated with Mylotarg patents with various cytogenetic and molecular AML variants, using standardized qRT-PCR approach.
Patients and methods
Clinical evaluation
Our study included fourteen patients (4 pediatric, 10 adults) at the age of 3 to 67 years (median of 25.7 years) with various morphological, cytogenetic and molecular variants of AML (Table 1). All the patients were resistant to standard chemotherapy, thus using Gemtuzumab ozogamicin (GO, Mylotarg) in subsequent treatment. One of these patients and his WT1 changes was earlier reported as a case No.22 [13]. These data were supplemented by new results concerning BAALC gene expression. The basic part of present work concerns clinical and molecular biology data obtained in 13 newly studied AML patients treated at our Center by combination of high-doses ChT, GO treatment and HSCT (n=11).
Table 1. Clinical and laboratory parameters of AML patients subjected to combined treatment with high-dose ChT, GO, and allo-HSCT performed in 11 cases

Laboratory studies
Routine laboratory counts of blood and bone marrow cells were performed, along with regular testing of blood chemistry. Cytogenetic studies were carried out using standard criteria to detect chromosome aberrations. Simultaneous serial measurements of BAALC and WT1 gene expressions levels were performed with quantitative real-time polymerase chain reaction (qRT-PCR), according to earlier described technique [20]. In brief, RNA was isolated from the fresh bone marrow samples by guanidine-phenol-chloroform extraction ("Ribozol-DF" kit reagent, InterLabService, Russia), according to the manufacturer’s instructions. Aliquots of extracted RNA (11 mcL) were used for reverse transcription with cDNA Synthesis Kit (LifeTechnologics, USA). The multiplex PCR of BAALC, WT1 and ABL genes was performed for each cDNA sample. Reaction conditions were as follows; 10 μcL of PCR reaction mixture ("Syntol", Russia), containing dNTP mix of 2.5 mM each, 10×PCR buffer, 5 Units of Taq-DNA polymerase and 2.5 μcL of 25 mM MgCl2, 7 pmol of each gene-specific primers, 5 pmol of Taqman probes for both BAALC, WT1 and ABL genes. Cutoff value of 31% was accepted for BAALC expression. The threshold level for WT1 gene was 250 copies/104 copies of BCR/ABL1 gene.
Along with individual assessment of clinical response, the patients were classified in 2 groups: (1) with FLT3 mutations and normal/near-normal karyotypes, and (2) more complex chromosome aberrations and EVI1 gene overexpression.
The prognostic significance of GO treatment in common group with normal, near-normal karyotypes and FLT3-mutated AML were compared to those with complex chromosome aberrations and EVI1 gene overexpression estimated by plotting of overall survival (OS) and relapse risk curves, according to Kaplan-Meier, SPSS software version 22.0 (IBM corporation, Armonk, NY, USA) were used for statistical analysis.

Figure 1. 3-year overall survival in AML patents with normal, near-normal karyotypes and FLT3-mutated variants (group 1) and those with more complex karyotypes and EVI1-positive leukemia (group 2)

Figure 2. Relative BAALC- and WT1-expression by the bone marrow precursors in a 67-year old patient with M0 AML showing opposite responses to Mylotarg therapy without significant effect upon bone marrow blast counts
Ordinate: percentage of blast cells (left); relative BAALC- and WT1 gene expression, % and copies, respectively.
Results
Basic clinical and laboratory findings in 13 newly studied AML patients treated with GO (Mylotarg) were presented in Table 1. In general, the results obtained in Mylotarg-treated group have shown that AML patients with normal or near-normal karyotypes, and mutated FLT3 variants (subgroup 1) exhibited higher 3-year OS rates, as compared to AML cases with complex karyotypes and EVI1-positivity (Fig. 1). The difference in 3-year overall survival (OS) proved to be statistically significant (85.7% vs 16.7%; P<0.032).
To interpret the differences observed, we would like to report in details some indicative cases with significant changes of BAALC expression. E.g., we observed a 67-year-old man with AML (M0 FAB variant) diagnosed in 2016. Interestingly, the cytogenetic analysis revealed karyotype with trisomy of chromosome 8, where the BAALC gene is mapped. As shown in Fig. 2, the BAALC expression was sufficiently increased (833%) which cannot be explained by trisomy 8 only. Moreover, the BAALC overexpression was accomplished by similar increase of WT1 gene up to 681 copies which could correlate with higher number of blasts in the tested bone marrow (59.8%).
This ratio was sufficiently changed after Mylotarg treatment, i.e., BAALC gene expression associated with contents of common BAALC-expressing precursors decreased to the threshold level (37%). In contrast, 13-fold elevation of WT1 expression was revealed, whereas percentage of marrow blasts was only slightly increased. Hence, we may assume that, at least in this case, higher WT1 expression might be provided by some distinct precursor subpopulation, along with blast cells.
Apart from this patient, the highest levels of BAALC gene expression (>200%) before Mylotarg therapy were observed in patients #2, 3 and 6 who had normal or nearly-normal karyotypes. Intermediate levels of this gene (from 100 to 199%) were found in patients #1 and #7 with normal or moderately changed karyotypes. Lower levels of BAALC expression were characteristic for all other patients including three cases (#4, 11 and 13) with sub-threshold levels of this gene expression.
As mentioned above, clinical response to GO therapy, in terms of leukemic cell counts, was not uniform. In most cases (9/11), the level of BAALC expression was decreased, thus presuming a direct inhibitory effect of GO upon immature leukemic precursors. Some of GO-treated patients (#1-3) showed a simultaneous decrease of both WT1 gene and blast cell counts in the tested bone marrows.
However, such response was not revealed in AML case #12, as shown in Table 2.
Table 2. Parallel serial measurements of BAALC, WT1, EVI1 gene expression and bone marrow blasts in the patient with M5 FAB-variant of AML (№12) treated by Mylotarg and HSCT

As shown above (Fig. 1), this positive response of Mylotarg was more pronounced in patients with normal or close to normal karyotypes, as well as in 6/8 (75%) cases with tandem duplication of FLT3 gene, except of a patient #8 with М1 FAB-variant, in whom, along with FLT3 duplication, multiple chromosome aberrations and NPMI mutation were found (Table 3).
At diagnosis, this patient had WBC counts of 75.2×109/L and high blast numbers (88%) in bone marrow and peripheral blood. Initial therapy included hydroxyurea (3 g), followed by standard 7+3 protocol. Clinical and hematological remission with 3.8% blasts in bone marrow was established on 5.09.17. However, the blast cell number had already increased to 70% a month later. As seen from data at Table 3, these leukemic cells contained complex karyotype with 3 various translocations, 2 deletions, inv(3), internal duplication of FLT3 receptor and NPM1 gene mutation. Initially, BAALC and EVI1 expression levels were 54 and 0.1%, respectively. At the same time, the level of WT1 (2849 copies) and the numbers of blast cells in bone marrow sample (92%) were higher. Meanwhile, the initial effects of 7+3 and FIAMx2 protocols were insufficient, since the WT1 gene expression decreased to 1471 copies only.
Table 3. Simultaneous serial measurements of BAALC, WT1 and EVI1 expression levels, and blast percentage in bone marrow aspirates of a female patient with M2 FAB variant of AML (#8) treated by GO only

In addition, the number of blasts in bone marrow samples increased initially to 83.4%, but soon reached 92%. Under these conditions, HSCT procedure became impossible, despite presence of available HLA-matched related donor. Death was registered on 24.12.17., 145 days after GO therapy. As example of positive therapeutic effect of combined therapy with Mylotarg and HSCT, we present here detailed clinical and laboratory data from 2 of 13 patients (# 4 and #9).
Table 4. Simultaneous serial measurements of BAALC, WT1 and EVI1 expression levels, and blast percentage in bone marrow aspirates of a female patient with M2 FAB variant of AML (№4) in whom initial remission was achieved by high- dosage ChT (protocol FLAG) combined with double HSCT, followed by GO and Gilteritinib

On the other hand, the expected negative reactions of leukemic hematopoiesis to GO therapy were noticed in 3 of 4 patients with EVI1-positive variants of AML (#10-12). The only exclusion was a young patient (#9) with more prognostically favorable for this group translocation t(9;11)(p22;q23). At diagnosis, he had no increased numbers of blast cells in peripheral blood, whereas it reached 77.5% in bone marrow.
Cytogenetic study revealed a standard translocation t(9;11)(p22;q23), which involves KMT2A gene and is, generally, associated with intermediate risk (Table 5). After a course of standard chemotherapy (7+3 protocol), her WBC decreased to 3×109/L with 43% of blast cells. Further treatment included two more intensive chemotherapy courses (FLAM protocol). Consequently, clinical, cytogenetic and molecular remissions were established. At this time (14.03.15), HSCT was performed, which was soon complicated by unexpected elevation of WT1 and MLL/AF9 expression, although essential increase of bone marrow blasts number (to 81.4%) appeared much later (31.08.17). Therefore, GO was administered for further treatment. The patient is alive now, and her survival following GO and haplo-HSCT has reached 1200 days.
Table 5. Simultaneous serial measurements of BAALC, WT1 and EVI1 expression levels, and blast percentage in bone marrow aspirates of a female patient with M2 FAB variant of AML (№9) treated by Mylotarg and HSCT

Discussion
Our preliminary study contains data which support earlier data on better response to Mylotarg therapy patients with normal and favorable cytogenetic variants, as well as higher sensitivity to Mylotarg treatment in AML with FLT3 mutations [17]. Vice versa, the results of this therapy are significantly worse in AML with complex karyotypes and EVI1 gene overexpression which may be mutually combined. A significant decrease of BAALC gene expression in most GO-treated AML patients is in accordance with earlier conclusions about inhibitory effect of Mylotarg upon immature BAALC-expressing precursors [16].
On the other hand, the data presented here suggest a possible dichotomy for BAALC and WT1 gene expression, due to their divergent changes, thus allowing an existence of hypothetical regulatory switch in leukemic hematopoiesis from earlier BAALC-expressing precursors to their more mature WT1-expressing progeny.
In general, the molecular diagnostic panel suggested by us, based on simultaneous quantitative measurements of BAALC- and WT1-expressing mRNA using standard qRT-PCR, allows to obtain useful fundamental and applied data which might be available for further search in subtle molecular mechanisms of AML resistance to therapy, as well as emerging relapses at the precursor cell level in different cytogenetic and molecular AML variants.
Conclusion
The study of leukemic hematopoiesis in AML patients with different cytogenetic and molecular variants treated with GO in combination with high doses ChT and HSCT, using simultaneous quantitative monitoring of BAALC- and WT1-expression by leukemic precursors, provides novel tools for deeper studying the precise mechanisms of AML resistance to therapy and relapse emergence at the level of earlier precursors, thus being important in fundamental and applied aspects.
Acknowledgements
The authors would like to acknowledge the assistance of Professor A. B. Chukhlovin in the preparation of this paper.
Conflict of interest
No conflict of interest are reported.
References
- Lapidot T, Sirard C, Vorm J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367(6464): 645-648. doi: 10.1038/367645a0.
- Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, Lillington D, Oakervee H, Cavenagh J, Agrawal SG et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34- fraction. Blood 2010; 115 (10): 1976-1984. doi: 10.1182/blood-2009-02-206565.
- Patel S, Zhang Y, Cassinat B, Zassadowski F, Ferré N, Cuccuini W, Cayuela JM, Fenaux P, Bonnet D, Chomienne C, Louache F. Successful xenografts of AML3 samples in immunodeficient NOD/shi-SCID IL2Rγ–/– mice. Leukemia 2012; 26(11):2432-2435. doi: 10.1038/leu.2012.154.
- Walter R, Appelbaum F, Estey E, Bernstein I. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 2012; 119: 6198-6208. doi: 10.1182/blood-2011-11-325050.
- Morita K, Masamoto Y, Kataoka K, Koya J, Kagoya Y, Yashiroda H, Sato T, Murata S, Kurokawa M. BAALC potentiates oncogenic ERK pathway through interactions with MEKK1 and KLF4. Leukemia 2015; 29(11): 2248-2256. doi: 10.1038/leu.2015.137.
- Cilloni D, Gottardi A, De Micheli D, Serra A, Volpe G, Messa F, Rege-Cambrin G, Guerrasio A, Divona M, Coco FL, Saglio G. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia 2002; 16: 2115-2121. doi: 10.1038/sj.leu.2402675.
- Cilloni D, Saglio G.WT1 as a universal marker for minimal residual disease detection and quantification in myeloid leukemias and in myelodysplastic syndrome. Acta Hematol 2004; 112: 79-84. doi: 10.1159/000077562.
- Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, Gottardi E, Fava M, Schnittger S, Weiss T et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol 2009; 27 (31): 5195-5201. doi: 10.1200/JCO.2009.22.4865.
- Candoni A, Toffoletti E, Gallina R, Simeone E, Chiozzotto M, Volpetti S, Fanin R. Monitoring of minimal residual disease by quantitative WT1 gene expression following reduced intensity conditioning allogeneic stem cell transplantation in acute myeloid leukemia. Clin Transplant 2011; 25(2): 308-316. doi: 10.1111/j.1399-0012.2010.01251.x.
- Gudozhnikova YaV, Mamaev NN, Barkhatov IM, Katerina VA, Gindina TL, Shakirova AI, Bondarenko SN, Slesarchuk OA, Darskaya EI, Paina OV, Zubarovskaya LS, Afanasyev BV. Results of molecular monitoring in posttransplant period by means of series investigation of WT1 gene expression in patients with acute myeloid leukemia. Clinical Oncohematology 2018; 11(3): 241-251. doi: 10.21320/2500-2139-2018-11-3-241-251 (In Russian).
- Goel H, Rahul E, Gupta AK, Meena JP, Chopra A, Ranjan A, Hussain S, Rath GK, Tanwar P. Molecular update on biology of Wilms Tumor 1 gene and its applications in acute myeloid leukemia. Am J Blood Res 2020; 10(5): 151-160. PMCID: PMC7675129. PMID: 33224559.
- Mamaev NN, Shakirova AI, Barkhatov IM, et al. New opportunities for assay of leukemia initiating cells (LICs) participating in post-transplant relapse development in the patients with acute myeloid leukemia. 3rd Annual IACH Meeting, 1-3 October, 2020, Paris, report #12.
- Mamaev NN, Gudozhnikova YaV, Gindina TL, Barkhatov IM, Shakirova AI, Katerina VA, Gubina MV, Nikolaeva ES, Semenova EV, Paina OV et al. Efficacy of Chemotherapy in Acute Leukemia Patients Resistant to Previous Standard Treatment According to the Series Measurement of WT1 Gene Expression. Clinical Oncohematology 2018; 11 (1): 78-88. doi: 10.21320/2500-2139-2018-11-1-78-88 (In Russian).
- Mamaev NN, Shakirova AI, Gudozhnikova YaV, Barkhatov IM, Gindina TL, Paina OV, Zubarovskaya LS, Afanasyev BV. Crucial role of BAALC-expressing progenitor cells in emergence and development of post-transplantation relapses in patients with acute myeloid leukemia. Clinical Oncohematology 2020; 13(1):75-88. doi: 10.21320/2500-2139-2020-13-1-75-88 (In Russian).
- Mamaev NN, Shakirova AI, Barkhatov IM, Gudozhnikova YV, Gindina TL, Kanunnikov MM, Kravtsova VM, Rakhmanova ZZ, Paina OV, Zubarovskaya LS. Crucial role of BAALC-expressing leukemic precursors in origin and development of posttransplant relapses in patients with acute myeloid leukemias. Int J Hematol 2020; 8(6): 127-131. doi: 10.15406/htij.2020.08.00240.
- Jawad M, Yu N, Seedhouse C, Tandon K, Russell NH, Pallis M. Targeting of CD34+CD38– cells using Gemtuzumab ozogamicin (Mylotarg) in combination with tipifarnib (Zarnestra) in acute Myeloid Leukaemia. BMC Cancer 2012; 12(1): 431. doi:10.1186/1471-2407-12-431.
- Jawad M, Seedhouse C, Mony U. Grundy M, Russell NH, Pallis M. Analysis of factors that affect in vitro chemosensitivity of leukemic stem and progenitor cells to gemtuzumab ozogomicin (Mylotarg) in acute myeloid leukemia. Leukemia 2010; 24(1):74-80. doi: 10.1038/leu.2009.199.
- Ehninger A, Kramer M, Röllig C, Thiede C, Bornhäuser M, von Bonin M, Wermke M, Feldmann A, Bachmann M, Ehninger G, Oelschlägel U. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J 2014; 4, e218. doi: 10.1038/bcj. 2014.39.
- Olombel G, Guérin E, Guy J, Perrot JY, Dumezy F, de Labarthe A, Bastie JN, Legrand O, Raffoux E, Plesa A et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin patients with acute myeloid leukemia. Blood 2016; 127(17): 2157-2160. doi: 10.1182/blood-2016-01-689976.
- Shakirova A, Barkhatov I, Churkina A, Moiseev IS, Gindina TL, Bondarenko SN, Afanasyev BV. Prognostic significance of BAALC overexpression in patients with AML during the posttransplant period. Cell Ther Transplant 2018; 7(2):54-63. doi: 10.18620/ctt-1866-8836-2018-7-2-64-69.
" ["~DETAIL_TEXT"]=> string(26327) "
Introduction
Over last decade, several experimental studies showed a heterogeneity of myeloid leukemia precursor cells in human acute myeloid leukemia (AML)[1-4]. This population includes, at least, immature leukemia-initiating cells (LIC) with CD34+CD38– – immune phenotype, being able for selective BAALC gene expression [5], whereas more mature LIC were attributed to acute promyelocytic leukemia (APL) origin. Biology of the latter cell population has been yet poorly studied. From some indirect data, one may suggest that these precursors, along to blast cells [6-11], may participate in WT1 gene expression [11, 12]. One line of evidence is based on some cases of acute promyelocytic leukemia (APL), where higher level of gene WT1 expression was coupled to lower blast cell numbers in bone marrow [12]. Moreover, this conclusion is also supported by similar discrepancy between higher WT1 gene expression and lower numbers of bone marrow blasts in 30% to 40% AML patients, both before and after HSCT [6, 9, 10, 13, 14]. In our opinion, this phenomenon may be also explained by presence of active expression of WT1 gene by more mature precursors [12, 15]. Confirmation of this hypothesis should extend our opportunities for evaluation of leukemic hematopoiesis on the level of leukemic precursors, using quantitative qRT-PCR [12, 14, 15]. To test this hypothesis, we performed parallel measurements of BAALC and WT1 expression levels in 14 AML patients with different cytological, cytogenetic and molecular variants of AML treated with combination of high-dose chemotherapy (ChT) and Mylotarg. Moreover, allogeneic hematopoietic stem cell transplantation (HSCT) was performed in 11 cases. It should be noticed here that our choice of Mylotarg was determined by some biological effects of this drug. Firstly, we have recently revealed unexpected response of leukemic cells to this drug, accompanied by elevation of WT1 gene expression [13]. Secondly, some data suggest a direct action of Mylotarg upon immature precursor cells with CD34+CD38– – phenotype [16]. Worth of note, more pronounced response to Mylotarg was revealed in AML with normal karyotypes and, especially, in those with FLT3 gene mutations [17]. This effect may be explained by higher expression of CD33 antigens on the surface of leukemic cells in AML with mutated FLT3 [18, 19]. On the contrary, overall survival in AML patients with WT1 gene overexpression became shorter, being in good accordance with our present findings.
Hence, the aim of our work was to study feasibility of BAALC/WT1 molecular panel for evaluation of hematopoiesis on the level of variously differentiated leukemic progenitors in the group of treated with Mylotarg patents with various cytogenetic and molecular AML variants, using standardized qRT-PCR approach.
Patients and methods
Clinical evaluation
Our study included fourteen patients (4 pediatric, 10 adults) at the age of 3 to 67 years (median of 25.7 years) with various morphological, cytogenetic and molecular variants of AML (Table 1). All the patients were resistant to standard chemotherapy, thus using Gemtuzumab ozogamicin (GO, Mylotarg) in subsequent treatment. One of these patients and his WT1 changes was earlier reported as a case No.22 [13]. These data were supplemented by new results concerning BAALC gene expression. The basic part of present work concerns clinical and molecular biology data obtained in 13 newly studied AML patients treated at our Center by combination of high-doses ChT, GO treatment and HSCT (n=11).
Table 1. Clinical and laboratory parameters of AML patients subjected to combined treatment with high-dose ChT, GO, and allo-HSCT performed in 11 cases

Laboratory studies
Routine laboratory counts of blood and bone marrow cells were performed, along with regular testing of blood chemistry. Cytogenetic studies were carried out using standard criteria to detect chromosome aberrations. Simultaneous serial measurements of BAALC and WT1 gene expressions levels were performed with quantitative real-time polymerase chain reaction (qRT-PCR), according to earlier described technique [20]. In brief, RNA was isolated from the fresh bone marrow samples by guanidine-phenol-chloroform extraction ("Ribozol-DF" kit reagent, InterLabService, Russia), according to the manufacturer’s instructions. Aliquots of extracted RNA (11 mcL) were used for reverse transcription with cDNA Synthesis Kit (LifeTechnologics, USA). The multiplex PCR of BAALC, WT1 and ABL genes was performed for each cDNA sample. Reaction conditions were as follows; 10 μcL of PCR reaction mixture ("Syntol", Russia), containing dNTP mix of 2.5 mM each, 10×PCR buffer, 5 Units of Taq-DNA polymerase and 2.5 μcL of 25 mM MgCl2, 7 pmol of each gene-specific primers, 5 pmol of Taqman probes for both BAALC, WT1 and ABL genes. Cutoff value of 31% was accepted for BAALC expression. The threshold level for WT1 gene was 250 copies/104 copies of BCR/ABL1 gene.
Along with individual assessment of clinical response, the patients were classified in 2 groups: (1) with FLT3 mutations and normal/near-normal karyotypes, and (2) more complex chromosome aberrations and EVI1 gene overexpression.
The prognostic significance of GO treatment in common group with normal, near-normal karyotypes and FLT3-mutated AML were compared to those with complex chromosome aberrations and EVI1 gene overexpression estimated by plotting of overall survival (OS) and relapse risk curves, according to Kaplan-Meier, SPSS software version 22.0 (IBM corporation, Armonk, NY, USA) were used for statistical analysis.

Figure 1. 3-year overall survival in AML patents with normal, near-normal karyotypes and FLT3-mutated variants (group 1) and those with more complex karyotypes and EVI1-positive leukemia (group 2)

Figure 2. Relative BAALC- and WT1-expression by the bone marrow precursors in a 67-year old patient with M0 AML showing opposite responses to Mylotarg therapy without significant effect upon bone marrow blast counts
Ordinate: percentage of blast cells (left); relative BAALC- and WT1 gene expression, % and copies, respectively.
Results
Basic clinical and laboratory findings in 13 newly studied AML patients treated with GO (Mylotarg) were presented in Table 1. In general, the results obtained in Mylotarg-treated group have shown that AML patients with normal or near-normal karyotypes, and mutated FLT3 variants (subgroup 1) exhibited higher 3-year OS rates, as compared to AML cases with complex karyotypes and EVI1-positivity (Fig. 1). The difference in 3-year overall survival (OS) proved to be statistically significant (85.7% vs 16.7%; P<0.032).
To interpret the differences observed, we would like to report in details some indicative cases with significant changes of BAALC expression. E.g., we observed a 67-year-old man with AML (M0 FAB variant) diagnosed in 2016. Interestingly, the cytogenetic analysis revealed karyotype with trisomy of chromosome 8, where the BAALC gene is mapped. As shown in Fig. 2, the BAALC expression was sufficiently increased (833%) which cannot be explained by trisomy 8 only. Moreover, the BAALC overexpression was accomplished by similar increase of WT1 gene up to 681 copies which could correlate with higher number of blasts in the tested bone marrow (59.8%).
This ratio was sufficiently changed after Mylotarg treatment, i.e., BAALC gene expression associated with contents of common BAALC-expressing precursors decreased to the threshold level (37%). In contrast, 13-fold elevation of WT1 expression was revealed, whereas percentage of marrow blasts was only slightly increased. Hence, we may assume that, at least in this case, higher WT1 expression might be provided by some distinct precursor subpopulation, along with blast cells.
Apart from this patient, the highest levels of BAALC gene expression (>200%) before Mylotarg therapy were observed in patients #2, 3 and 6 who had normal or nearly-normal karyotypes. Intermediate levels of this gene (from 100 to 199%) were found in patients #1 and #7 with normal or moderately changed karyotypes. Lower levels of BAALC expression were characteristic for all other patients including three cases (#4, 11 and 13) with sub-threshold levels of this gene expression.
As mentioned above, clinical response to GO therapy, in terms of leukemic cell counts, was not uniform. In most cases (9/11), the level of BAALC expression was decreased, thus presuming a direct inhibitory effect of GO upon immature leukemic precursors. Some of GO-treated patients (#1-3) showed a simultaneous decrease of both WT1 gene and blast cell counts in the tested bone marrows.
However, such response was not revealed in AML case #12, as shown in Table 2.
Table 2. Parallel serial measurements of BAALC, WT1, EVI1 gene expression and bone marrow blasts in the patient with M5 FAB-variant of AML (№12) treated by Mylotarg and HSCT

As shown above (Fig. 1), this positive response of Mylotarg was more pronounced in patients with normal or close to normal karyotypes, as well as in 6/8 (75%) cases with tandem duplication of FLT3 gene, except of a patient #8 with М1 FAB-variant, in whom, along with FLT3 duplication, multiple chromosome aberrations and NPMI mutation were found (Table 3).
At diagnosis, this patient had WBC counts of 75.2×109/L and high blast numbers (88%) in bone marrow and peripheral blood. Initial therapy included hydroxyurea (3 g), followed by standard 7+3 protocol. Clinical and hematological remission with 3.8% blasts in bone marrow was established on 5.09.17. However, the blast cell number had already increased to 70% a month later. As seen from data at Table 3, these leukemic cells contained complex karyotype with 3 various translocations, 2 deletions, inv(3), internal duplication of FLT3 receptor and NPM1 gene mutation. Initially, BAALC and EVI1 expression levels were 54 and 0.1%, respectively. At the same time, the level of WT1 (2849 copies) and the numbers of blast cells in bone marrow sample (92%) were higher. Meanwhile, the initial effects of 7+3 and FIAMx2 protocols were insufficient, since the WT1 gene expression decreased to 1471 copies only.
Table 3. Simultaneous serial measurements of BAALC, WT1 and EVI1 expression levels, and blast percentage in bone marrow aspirates of a female patient with M2 FAB variant of AML (#8) treated by GO only

In addition, the number of blasts in bone marrow samples increased initially to 83.4%, but soon reached 92%. Under these conditions, HSCT procedure became impossible, despite presence of available HLA-matched related donor. Death was registered on 24.12.17., 145 days after GO therapy. As example of positive therapeutic effect of combined therapy with Mylotarg and HSCT, we present here detailed clinical and laboratory data from 2 of 13 patients (# 4 and #9).
Table 4. Simultaneous serial measurements of BAALC, WT1 and EVI1 expression levels, and blast percentage in bone marrow aspirates of a female patient with M2 FAB variant of AML (№4) in whom initial remission was achieved by high- dosage ChT (protocol FLAG) combined with double HSCT, followed by GO and Gilteritinib

On the other hand, the expected negative reactions of leukemic hematopoiesis to GO therapy were noticed in 3 of 4 patients with EVI1-positive variants of AML (#10-12). The only exclusion was a young patient (#9) with more prognostically favorable for this group translocation t(9;11)(p22;q23). At diagnosis, he had no increased numbers of blast cells in peripheral blood, whereas it reached 77.5% in bone marrow.
Cytogenetic study revealed a standard translocation t(9;11)(p22;q23), which involves KMT2A gene and is, generally, associated with intermediate risk (Table 5). After a course of standard chemotherapy (7+3 protocol), her WBC decreased to 3×109/L with 43% of blast cells. Further treatment included two more intensive chemotherapy courses (FLAM protocol). Consequently, clinical, cytogenetic and molecular remissions were established. At this time (14.03.15), HSCT was performed, which was soon complicated by unexpected elevation of WT1 and MLL/AF9 expression, although essential increase of bone marrow blasts number (to 81.4%) appeared much later (31.08.17). Therefore, GO was administered for further treatment. The patient is alive now, and her survival following GO and haplo-HSCT has reached 1200 days.
Table 5. Simultaneous serial measurements of BAALC, WT1 and EVI1 expression levels, and blast percentage in bone marrow aspirates of a female patient with M2 FAB variant of AML (№9) treated by Mylotarg and HSCT

Discussion
Our preliminary study contains data which support earlier data on better response to Mylotarg therapy patients with normal and favorable cytogenetic variants, as well as higher sensitivity to Mylotarg treatment in AML with FLT3 mutations [17]. Vice versa, the results of this therapy are significantly worse in AML with complex karyotypes and EVI1 gene overexpression which may be mutually combined. A significant decrease of BAALC gene expression in most GO-treated AML patients is in accordance with earlier conclusions about inhibitory effect of Mylotarg upon immature BAALC-expressing precursors [16].
On the other hand, the data presented here suggest a possible dichotomy for BAALC and WT1 gene expression, due to their divergent changes, thus allowing an existence of hypothetical regulatory switch in leukemic hematopoiesis from earlier BAALC-expressing precursors to their more mature WT1-expressing progeny.
In general, the molecular diagnostic panel suggested by us, based on simultaneous quantitative measurements of BAALC- and WT1-expressing mRNA using standard qRT-PCR, allows to obtain useful fundamental and applied data which might be available for further search in subtle molecular mechanisms of AML resistance to therapy, as well as emerging relapses at the precursor cell level in different cytogenetic and molecular AML variants.
Conclusion
The study of leukemic hematopoiesis in AML patients with different cytogenetic and molecular variants treated with GO in combination with high doses ChT and HSCT, using simultaneous quantitative monitoring of BAALC- and WT1-expression by leukemic precursors, provides novel tools for deeper studying the precise mechanisms of AML resistance to therapy and relapse emergence at the level of earlier precursors, thus being important in fundamental and applied aspects.
Acknowledgements
The authors would like to acknowledge the assistance of Professor A. B. Chukhlovin in the preparation of this paper.
Conflict of interest
No conflict of interest are reported.
References
- Lapidot T, Sirard C, Vorm J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367(6464): 645-648. doi: 10.1038/367645a0.
- Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, Lillington D, Oakervee H, Cavenagh J, Agrawal SG et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34- fraction. Blood 2010; 115 (10): 1976-1984. doi: 10.1182/blood-2009-02-206565.
- Patel S, Zhang Y, Cassinat B, Zassadowski F, Ferré N, Cuccuini W, Cayuela JM, Fenaux P, Bonnet D, Chomienne C, Louache F. Successful xenografts of AML3 samples in immunodeficient NOD/shi-SCID IL2Rγ–/– mice. Leukemia 2012; 26(11):2432-2435. doi: 10.1038/leu.2012.154.
- Walter R, Appelbaum F, Estey E, Bernstein I. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 2012; 119: 6198-6208. doi: 10.1182/blood-2011-11-325050.
- Morita K, Masamoto Y, Kataoka K, Koya J, Kagoya Y, Yashiroda H, Sato T, Murata S, Kurokawa M. BAALC potentiates oncogenic ERK pathway through interactions with MEKK1 and KLF4. Leukemia 2015; 29(11): 2248-2256. doi: 10.1038/leu.2015.137.
- Cilloni D, Gottardi A, De Micheli D, Serra A, Volpe G, Messa F, Rege-Cambrin G, Guerrasio A, Divona M, Coco FL, Saglio G. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia 2002; 16: 2115-2121. doi: 10.1038/sj.leu.2402675.
- Cilloni D, Saglio G.WT1 as a universal marker for minimal residual disease detection and quantification in myeloid leukemias and in myelodysplastic syndrome. Acta Hematol 2004; 112: 79-84. doi: 10.1159/000077562.
- Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV, Gottardi E, Fava M, Schnittger S, Weiss T et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol 2009; 27 (31): 5195-5201. doi: 10.1200/JCO.2009.22.4865.
- Candoni A, Toffoletti E, Gallina R, Simeone E, Chiozzotto M, Volpetti S, Fanin R. Monitoring of minimal residual disease by quantitative WT1 gene expression following reduced intensity conditioning allogeneic stem cell transplantation in acute myeloid leukemia. Clin Transplant 2011; 25(2): 308-316. doi: 10.1111/j.1399-0012.2010.01251.x.
- Gudozhnikova YaV, Mamaev NN, Barkhatov IM, Katerina VA, Gindina TL, Shakirova AI, Bondarenko SN, Slesarchuk OA, Darskaya EI, Paina OV, Zubarovskaya LS, Afanasyev BV. Results of molecular monitoring in posttransplant period by means of series investigation of WT1 gene expression in patients with acute myeloid leukemia. Clinical Oncohematology 2018; 11(3): 241-251. doi: 10.21320/2500-2139-2018-11-3-241-251 (In Russian).
- Goel H, Rahul E, Gupta AK, Meena JP, Chopra A, Ranjan A, Hussain S, Rath GK, Tanwar P. Molecular update on biology of Wilms Tumor 1 gene and its applications in acute myeloid leukemia. Am J Blood Res 2020; 10(5): 151-160. PMCID: PMC7675129. PMID: 33224559.
- Mamaev NN, Shakirova AI, Barkhatov IM, et al. New opportunities for assay of leukemia initiating cells (LICs) participating in post-transplant relapse development in the patients with acute myeloid leukemia. 3rd Annual IACH Meeting, 1-3 October, 2020, Paris, report #12.
- Mamaev NN, Gudozhnikova YaV, Gindina TL, Barkhatov IM, Shakirova AI, Katerina VA, Gubina MV, Nikolaeva ES, Semenova EV, Paina OV et al. Efficacy of Chemotherapy in Acute Leukemia Patients Resistant to Previous Standard Treatment According to the Series Measurement of WT1 Gene Expression. Clinical Oncohematology 2018; 11 (1): 78-88. doi: 10.21320/2500-2139-2018-11-1-78-88 (In Russian).
- Mamaev NN, Shakirova AI, Gudozhnikova YaV, Barkhatov IM, Gindina TL, Paina OV, Zubarovskaya LS, Afanasyev BV. Crucial role of BAALC-expressing progenitor cells in emergence and development of post-transplantation relapses in patients with acute myeloid leukemia. Clinical Oncohematology 2020; 13(1):75-88. doi: 10.21320/2500-2139-2020-13-1-75-88 (In Russian).
- Mamaev NN, Shakirova AI, Barkhatov IM, Gudozhnikova YV, Gindina TL, Kanunnikov MM, Kravtsova VM, Rakhmanova ZZ, Paina OV, Zubarovskaya LS. Crucial role of BAALC-expressing leukemic precursors in origin and development of posttransplant relapses in patients with acute myeloid leukemias. Int J Hematol 2020; 8(6): 127-131. doi: 10.15406/htij.2020.08.00240.
- Jawad M, Yu N, Seedhouse C, Tandon K, Russell NH, Pallis M. Targeting of CD34+CD38– cells using Gemtuzumab ozogamicin (Mylotarg) in combination with tipifarnib (Zarnestra) in acute Myeloid Leukaemia. BMC Cancer 2012; 12(1): 431. doi:10.1186/1471-2407-12-431.
- Jawad M, Seedhouse C, Mony U. Grundy M, Russell NH, Pallis M. Analysis of factors that affect in vitro chemosensitivity of leukemic stem and progenitor cells to gemtuzumab ozogomicin (Mylotarg) in acute myeloid leukemia. Leukemia 2010; 24(1):74-80. doi: 10.1038/leu.2009.199.
- Ehninger A, Kramer M, Röllig C, Thiede C, Bornhäuser M, von Bonin M, Wermke M, Feldmann A, Bachmann M, Ehninger G, Oelschlägel U. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J 2014; 4, e218. doi: 10.1038/bcj. 2014.39.
- Olombel G, Guérin E, Guy J, Perrot JY, Dumezy F, de Labarthe A, Bastie JN, Legrand O, Raffoux E, Plesa A et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin patients with acute myeloid leukemia. Blood 2016; 127(17): 2157-2160. doi: 10.1182/blood-2016-01-689976.
- Shakirova A, Barkhatov I, Churkina A, Moiseev IS, Gindina TL, Bondarenko SN, Afanasyev BV. Prognostic significance of BAALC overexpression in patients with AML during the posttransplant period. Cell Ther Transplant 2018; 7(2):54-63. doi: 10.18620/ctt-1866-8836-2018-7-2-64-69.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "40" ["~SORT"]=> string(2) "40" ["CODE"]=> string(100) "kolichestvennoe-izuchenie-baalc-i-wt1-ekspressiruyushchikh-kletok-predshestvennits-u-bolnykh-c-razli" ["~CODE"]=> string(100) "kolichestvennoe-izuchenie-baalc-i-wt1-ekspressiruyushchikh-kletok-predshestvennits-u-bolnykh-c-razli" ["EXTERNAL_ID"]=> string(4) "1952" ["~EXTERNAL_ID"]=> string(4) "1952" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(639) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клетокQuantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4834) "<p style="text-align: justify;"> Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34<sup>+</sup>CD38<sup>–</sup>, которые, в свою очередь, способны к избирательной экспрессии гена <i>BAALC</i>. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена <i>WT1</i>. Поскольку можно количественно оценить содержание мРНК <i>BAALC</i> и <i>WT1</i> посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов <i>BAALC </i>и <i>WT1</i>, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена <i>EVI1</i> (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых <i>BAALC</i>-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена <i>WT1</i>, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых <i>BAALC</i>-экспрессирующих предшественников на более зрелые <i>WT1</i>-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии<i> BAALC</i>, <i>WT1</i> и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия <i>BAALC</i>, экспрессия <i>WT1</i>, прекурсоры лейкозных клеток, РТ-ПЦР. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_META_TITLE"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_META_KEYWORDS"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_META_DESCRIPTION"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_ALT"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_TITLE"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "kolichestvennoe-izuchenie-baalc-i-wt1-ekspressiruyushchikh-kletok-predshestvennits-u-bolnykh-c-razli" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(427) "Количественное изучение BAALC- и WT1-экспрессирующих клеток-предшественниц у больных c различными цитогенетическими и молекулярными вариантами ОМЛ, леченных гемтузумаб озогамицином и трансплантацией гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "kolichestvennoe-izuchenie-baalc-i-wt1-ekspressiruyushchikh-kletok-predshestvennits-u-bolnykh-c-razli" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "kolichestvennoe-izuchenie-baalc-i-wt1-ekspressiruyushchikh-kletok-predshestvennits-u-bolnykh-c-razli" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "kolichestvennoe-izuchenie-baalc-i-wt1-ekspressiruyushchikh-kletok-predshestvennits-u-bolnykh-c-razli" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "175" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27482" ["VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27483" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27484" ["VALUE"]=> array(2) { ["TEXT"]=> string(476) "<p>Николай Н. Мамаев, Алена И. Шакирова, Татьяна Л. Гиндина, Сергей Н. Бондаренко, Белла И. Аюбова, Ильдар М. Бархатов, Яна В. Гудожникова, Валентина М. Кравцова, Михаил М. Канунников, Олеся В. Паина, Жемал Ж. Рахманова, Татьяна Ю. Грачева, Людмила С. Зубаровская</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(464) "
Николай Н. Мамаев, Алена И. Шакирова, Татьяна Л. Гиндина, Сергей Н. Бондаренко, Белла И. Аюбова, Ильдар М. Бархатов, Яна В. Гудожникова, Валентина М. Кравцова, Михаил М. Канунников, Олеся В. Паина, Жемал Ж. Рахманова, Татьяна Ю. Грачева, Людмила С. Зубаровская
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27485" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27486" ["VALUE"]=> array(2) { ["TEXT"]=> string(4834) "<p style="text-align: justify;"> Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34<sup>+</sup>CD38<sup>–</sup>, которые, в свою очередь, способны к избирательной экспрессии гена <i>BAALC</i>. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена <i>WT1</i>. Поскольку можно количественно оценить содержание мРНК <i>BAALC</i> и <i>WT1</i> посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов <i>BAALC </i>и <i>WT1</i>, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена <i>EVI1</i> (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых <i>BAALC</i>-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена <i>WT1</i>, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых <i>BAALC</i>-экспрессирующих предшественников на более зрелые <i>WT1</i>-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии<i> BAALC</i>, <i>WT1</i> и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия <i>BAALC</i>, экспрессия <i>WT1</i>, прекурсоры лейкозных клеток, РТ-ПЦР. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4574) "Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34+CD38–, которые, в свою очередь, способны к избирательной экспрессии гена BAALC. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена WT1. Поскольку можно количественно оценить содержание мРНК BAALC и WT1 посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов BAALC и WT1, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена EVI1 (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых BAALC-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена WT1, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых BAALC-экспрессирующих предшественников на более зрелые WT1-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии BAALC, WT1 и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов.
Ключевые слова
Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия BAALC, экспрессия WT1, прекурсоры лейкозных клеток, РТ-ПЦР.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27487" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-55-62" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-55-62" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27489" ["VALUE"]=> array(2) { ["TEXT"]=> string(292) "<p>Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(280) "Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27490" ["VALUE"]=> array(2) { ["TEXT"]=> string(484) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022, St. Petersburg, Russia<br> Phone: +7 (911) 760 5086<br> E-mail: nikmamev524@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(424) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022,
St. Petersburg, Russia
Phone: +7 (911) 760 5086
E-mail: nikmamev524@gmail.com
There is evidence that relapses of acute myeloid leukemia (AML) are closely related to heterogeneous population of leukemic precursors. At least, two classes of the leukemia-initiating cells (LIC) may be discerned, according to recent experimental studies with hematopoietic cell transplants to immunodeficient mice. The main class of LICs is presented by immature precursors with CD34+CD38– immunophenotype which, in turn, are capable of selective expression of BAALC gene. The second class of LICs is presented by relatively mature precursors with more differentiated immunophenotypes. According to indirect findings, they are able of WT1 gene expression, along with blast cells. Since both BAALC and WT1 mRNAs may be quantitatively evaluated by means of standardized quantitative polymerase reaction in real time (qRT-PCR), this approach may be effective for specifying the mechanisms of relapses and resistance to therapy in AML patients. The aim of this work was to perform simultaneous dynamic evaluation of BAALC and WT1 genes expressions along with determination of blast numbers in the tested bone marrow samples in 14 AML patients treated at our Center with Gemtuzumab ozogamicin (GO, Mylotarg), which was combined with high-dose chemotherapy (ChT), followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Our preliminary results are as follows: a) superior 3-year overall survival (OS) in general group of patients with normal or nearly-normal karyotypes, and FLT3-mutated AML variants as compared to those with more complex karyotypes and EVI1 gene overexpression (85.7% vs 16.7%; p=0.032); b) highly sensitive response of immature BAALC-expressing precursors to combined ChT and GO treatment; c) hypothetical participation of some mature precursors, along with blast cells, in WT1 gene expression; d) real evidence for switching hematopoietic regulation from immature BAALC-expressing precursors to more mature WT1-expressing progeny. These results suggest diagnostic utility of combined BAALC/WT1/blast counts panel for quantitative studies and assessment of distinct precursors in AML progression and emergence of relapses.
Keywords
Acute myeloid leukemia, resistance to therapy, relapses, pathogenesis, Gemtuzumab ozogamycin, BAALC expression, WT1 expression, leukemic cell precursors, qRT-PCR.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27488" ["VALUE"]=> string(212) "Quantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(212) "Quantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27532" ["VALUE"]=> string(4) "2422" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2422" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27533" ["VALUE"]=> string(4) "2423" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2423" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27489" ["VALUE"]=> array(2) { ["TEXT"]=> string(292) "<p>Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(280) "Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(280) "Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27491" ["VALUE"]=> array(2) { ["TEXT"]=> string(2831) "<p style="text-align: justify;"> There is evidence that relapses of acute myeloid leukemia (AML) are closely related to heterogeneous population of leukemic precursors. At least, two classes of the leukemia-initiating cells (LIC) may be discerned, according to recent experimental studies with hematopoietic cell transplants to immunodeficient mice. The main class of LICs is presented by immature precursors with CD34<sup>+</sup>CD38<sup>–</sup> immunophenotype which, in turn, are capable of selective expression of <i>BAALC</i> gene. The second class of LICs is presented by relatively mature precursors with more differentiated immunophenotypes. According to indirect findings, they are able of <i>WT1</i> gene expression, along with blast cells. Since both <i>BAALC</i> and <i>WT1</i> mRNAs may be quantitatively evaluated by means of standardized quantitative polymerase reaction in real time (qRT-PCR), this approach may be effective for specifying the mechanisms of relapses and resistance to therapy in AML patients. The aim of this work was to perform simultaneous dynamic evaluation of <i>BAALC</i> and <i>WT1</i> genes expressions along with determination of blast numbers in the tested bone marrow samples in 14 AML patients treated at our Center with Gemtuzumab ozogamicin (GO, Mylotarg), which was combined with high-dose chemotherapy (ChT), followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Our preliminary results are as follows: a) superior 3-year overall survival (OS) in general group of patients with normal or nearly-normal karyotypes, and FLT3-mutated AML variants as compared to those with more complex karyotypes and <i>EVI1</i> gene overexpression (85.7% <i>vs</i> 16.7%; p=0.032); b) highly sensitive response of immature <i>BAALC</i>-expressing precursors to combined ChT and GO treatment; c) hypothetical participation of some mature precursors, along with blast cells, in <i>WT1</i> gene expression; d) real evidence for switching hematopoietic regulation from immature <i>BAALC</i>-expressing precursors to more mature <i>WT1</i>-expressing progeny. These results suggest diagnostic utility of combined <i>BAALC</i>/<i>WT1</i>/blast counts panel for quantitative studies and assessment of distinct precursors in AML progression and emergence of relapses. </p> <h2>Keywords</h2> <p style="text-align: justify;"> Acute myeloid leukemia, resistance to therapy, relapses, pathogenesis, Gemtuzumab ozogamycin, <i>BAALC</i> expression, <i>WT1</i> expression, leukemic cell precursors, qRT-PCR. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2559) "There is evidence that relapses of acute myeloid leukemia (AML) are closely related to heterogeneous population of leukemic precursors. At least, two classes of the leukemia-initiating cells (LIC) may be discerned, according to recent experimental studies with hematopoietic cell transplants to immunodeficient mice. The main class of LICs is presented by immature precursors with CD34+CD38– immunophenotype which, in turn, are capable of selective expression of BAALC gene. The second class of LICs is presented by relatively mature precursors with more differentiated immunophenotypes. According to indirect findings, they are able of WT1 gene expression, along with blast cells. Since both BAALC and WT1 mRNAs may be quantitatively evaluated by means of standardized quantitative polymerase reaction in real time (qRT-PCR), this approach may be effective for specifying the mechanisms of relapses and resistance to therapy in AML patients. The aim of this work was to perform simultaneous dynamic evaluation of BAALC and WT1 genes expressions along with determination of blast numbers in the tested bone marrow samples in 14 AML patients treated at our Center with Gemtuzumab ozogamicin (GO, Mylotarg), which was combined with high-dose chemotherapy (ChT), followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Our preliminary results are as follows: a) superior 3-year overall survival (OS) in general group of patients with normal or nearly-normal karyotypes, and FLT3-mutated AML variants as compared to those with more complex karyotypes and EVI1 gene overexpression (85.7% vs 16.7%; p=0.032); b) highly sensitive response of immature BAALC-expressing precursors to combined ChT and GO treatment; c) hypothetical participation of some mature precursors, along with blast cells, in WT1 gene expression; d) real evidence for switching hematopoietic regulation from immature BAALC-expressing precursors to more mature WT1-expressing progeny. These results suggest diagnostic utility of combined BAALC/WT1/blast counts panel for quantitative studies and assessment of distinct precursors in AML progression and emergence of relapses.
Keywords
Acute myeloid leukemia, resistance to therapy, relapses, pathogenesis, Gemtuzumab ozogamycin, BAALC expression, WT1 expression, leukemic cell precursors, qRT-PCR.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2559) "There is evidence that relapses of acute myeloid leukemia (AML) are closely related to heterogeneous population of leukemic precursors. At least, two classes of the leukemia-initiating cells (LIC) may be discerned, according to recent experimental studies with hematopoietic cell transplants to immunodeficient mice. The main class of LICs is presented by immature precursors with CD34+CD38– immunophenotype which, in turn, are capable of selective expression of BAALC gene. The second class of LICs is presented by relatively mature precursors with more differentiated immunophenotypes. According to indirect findings, they are able of WT1 gene expression, along with blast cells. Since both BAALC and WT1 mRNAs may be quantitatively evaluated by means of standardized quantitative polymerase reaction in real time (qRT-PCR), this approach may be effective for specifying the mechanisms of relapses and resistance to therapy in AML patients. The aim of this work was to perform simultaneous dynamic evaluation of BAALC and WT1 genes expressions along with determination of blast numbers in the tested bone marrow samples in 14 AML patients treated at our Center with Gemtuzumab ozogamicin (GO, Mylotarg), which was combined with high-dose chemotherapy (ChT), followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Our preliminary results are as follows: a) superior 3-year overall survival (OS) in general group of patients with normal or nearly-normal karyotypes, and FLT3-mutated AML variants as compared to those with more complex karyotypes and EVI1 gene overexpression (85.7% vs 16.7%; p=0.032); b) highly sensitive response of immature BAALC-expressing precursors to combined ChT and GO treatment; c) hypothetical participation of some mature precursors, along with blast cells, in WT1 gene expression; d) real evidence for switching hematopoietic regulation from immature BAALC-expressing precursors to more mature WT1-expressing progeny. These results suggest diagnostic utility of combined BAALC/WT1/blast counts panel for quantitative studies and assessment of distinct precursors in AML progression and emergence of relapses.
Keywords
Acute myeloid leukemia, resistance to therapy, relapses, pathogenesis, Gemtuzumab ozogamycin, BAALC expression, WT1 expression, leukemic cell precursors, qRT-PCR.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27487" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-55-62" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-55-62" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-55-62" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27488" ["VALUE"]=> string(212) "Quantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(212) "Quantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(212) "Quantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27490" ["VALUE"]=> array(2) { ["TEXT"]=> string(484) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022, St. Petersburg, Russia<br> Phone: +7 (911) 760 5086<br> E-mail: nikmamev524@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(424) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022,
St. Petersburg, Russia
Phone: +7 (911) 760 5086
E-mail: nikmamev524@gmail.com
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022,
St. Petersburg, Russia
Phone: +7 (911) 760 5086
E-mail: nikmamev524@gmail.com
Николай Н. Мамаев, Алена И. Шакирова, Татьяна Л. Гиндина, Сергей Н. Бондаренко, Белла И. Аюбова, Ильдар М. Бархатов, Яна В. Гудожникова, Валентина М. Кравцова, Михаил М. Канунников, Олеся В. Паина, Жемал Ж. Рахманова, Татьяна Ю. Грачева, Людмила С. Зубаровская
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(464) "Николай Н. Мамаев, Алена И. Шакирова, Татьяна Л. Гиндина, Сергей Н. Бондаренко, Белла И. Аюбова, Ильдар М. Бархатов, Яна В. Гудожникова, Валентина М. Кравцова, Михаил М. Канунников, Олеся В. Паина, Жемал Ж. Рахманова, Татьяна Ю. Грачева, Людмила С. Зубаровская
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27482" ["VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/12/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/12/2021 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27483" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "04/09/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27486" ["VALUE"]=> array(2) { ["TEXT"]=> string(4834) "<p style="text-align: justify;"> Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34<sup>+</sup>CD38<sup>–</sup>, которые, в свою очередь, способны к избирательной экспрессии гена <i>BAALC</i>. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена <i>WT1</i>. Поскольку можно количественно оценить содержание мРНК <i>BAALC</i> и <i>WT1</i> посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов <i>BAALC </i>и <i>WT1</i>, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена <i>EVI1</i> (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых <i>BAALC</i>-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена <i>WT1</i>, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых <i>BAALC</i>-экспрессирующих предшественников на более зрелые <i>WT1</i>-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии<i> BAALC</i>, <i>WT1</i> и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;"> Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия <i>BAALC</i>, экспрессия <i>WT1</i>, прекурсоры лейкозных клеток, РТ-ПЦР. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4574) "Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34+CD38–, которые, в свою очередь, способны к избирательной экспрессии гена BAALC. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена WT1. Поскольку можно количественно оценить содержание мРНК BAALC и WT1 посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов BAALC и WT1, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена EVI1 (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых BAALC-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена WT1, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых BAALC-экспрессирующих предшественников на более зрелые WT1-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии BAALC, WT1 и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов.
Ключевые слова
Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия BAALC, экспрессия WT1, прекурсоры лейкозных клеток, РТ-ПЦР.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4574) "Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34+CD38–, которые, в свою очередь, способны к избирательной экспрессии гена BAALC. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена WT1. Поскольку можно количественно оценить содержание мРНК BAALC и WT1 посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов BAALC и WT1, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена EVI1 (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых BAALC-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена WT1, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых BAALC-экспрессирующих предшественников на более зрелые WT1-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии BAALC, WT1 и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов.
Ключевые слова
Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия BAALC, экспрессия WT1, прекурсоры лейкозных клеток, РТ-ПЦР.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27485" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [5]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "175" ["~IBLOCK_SECTION_ID"]=> string(3) "175" ["ID"]=> string(4) "1951" ["~ID"]=> string(4) "1951" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["~NAME"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "06/01/2021 03:42:16 pm" ["~TIMESTAMP_X"]=> string(22) "06/01/2021 03:42:16 pm" ["DETAIL_PAGE_URL"]=> string(148) "/en/archive/tom-10-nomer-1/klinicheskie-raboty/vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto/" ["~DETAIL_PAGE_URL"]=> string(148) "/en/archive/tom-10-nomer-1/klinicheskie-raboty/vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(29744) "Introduction
Central nervous system (CNS) atypical teratoid rhabdoid tumor (ATRT) is a rare aggressive malignancy, which comprises 1-2% of brain and spinal neoplasms [1, 2]. Median age at diagnosis is 1 year with male predominance 1.5:1.3 [3, 4, 5, 6]. Nowadays, there are no established standards in the treatment of CNS ATRT. Various management approaches exist for different countries and institutions. Despite this uncertainty, surgery followed by adjuvant chemotherapy and radiotherapy (in older children) is, without doubt, the cornerstone of their treatment [7, 8]. HDCT/auto-HSCT is used in children under 3 years, intending for canceling or postponing radiation therapy (RT) and reduce the risk of long-term neuro-cognitive disorders and improve outcome [9, 10, 11]. Despite intensive multimodal therapy, the majority of patients with ATRT develop relapses [10, 11, 12]. Prognosis for CNS ATRT remains dismal, especially in children with residual tumor and metastatic disease.
The aim of present study was to assess effectiveness and define prognostic factors in CNS ATRT patients after HDCT/auto-HSCT.
Patients and methods
Trial design
This retrospective multicentric continuous cooperative study was performed between 2008 and 2020 in V. A. Almazov NMRC, RM Gorbacheva Research Institute, Pavlov University, and Pirogov Russian National Research Medical University. Eligibility criteria: the study included 30 patients aged ≤18 with histologically verified CNS ATRT and complex treatment performed according to different protocols, including HDCT/auto-HSCT.
The surviving patients were censored by 1 February 2021. Overall survival was calculated from the date of surgery up to death or up to the last follow up. Event-free survival was calculated from the date of surgery up to the date of unfavorable event (death, relapse, progression), or up to last follow up.
Clinical characteristics of patients
A total of 30 patients ≤ 18 years old with median age of 19.5 months were enrolled in the study from different regions of Russian Federation. Initial characteristics of patients are presented in Table 1. There were 11 (36.6%) infants, and 19 (63.4%) children aged over 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial – in 13 (43.3%). Molecular biologic subgroups were identified only in 7 patients: ATRT-SHH (n=4), ATRT-MYC (n=1) and ATRT TYR (n=2). All children initially received surgery with total tumor resection (n=8, 26.7%), subtotal resection (n=9, 30.0%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and minor subgroup had M-0 stage (n=12, 40%), with non-classified stage (Mx) in 2 cases (6.7%). After surgery, everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). There was no radiotherapy (RT) prior to HDCT/auto-HSCT in children with ATRT. RT after transplantation was performed in 24 children (80%): local RT (n=16, 60%), cranio-spinal irradiation (n=6, 20%), and no RT (n=6, 20%). The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. Disease status was assessed in all 12 cases (40%) prior to HDCT/auto-HSCT with complete response (CR); partial response (PR), in 8 (26.7%), and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in majority of patients (n=21, 70%), and tandem transplant, only in 9 cases (30%). Total number of transplants was 39. Prior to auto-HSCT peripheral blood hematopoietic stem cells (PBSC) or bone marrow (BM) were harvested. PBSC were the transplant source in 27 children (90%), and combination of PBSC and BM was used in 3 cases (10%).
Table 1. Characteristics of CNS ATRT patients and HDCT/auto-HSCT

Conditioning regimen for single auto-HSCT in the majority of cases (n=13, 43.3%) consisted of thiotepa 300 mg/m2, carboplatin 500 mg/m2 and etoposide 250 mg/m2 on Days -6, -5, -4 (Table 2). Carboplatin 500 mg/m2 and thiotepa 300 mg/m2 on Days -6, -5, -4 were used in 8 patients (26.7%). First conditioning regimen consisted of carboplatin 500 mg/m2 and etoposide 250 mg/m2 on Days -8, -7, -6, -5 and second conditioning regimen consisted of thiotepa 300 mg/m2 and cyclophosphamide 1500 mg/m2 on Days -4,-3,-2 in 7 children (23.3%) with tandem transplantation. Carboplatin 510 mg/m2 and thiotepa 300 mg/m2 on Days -4, -3 for both transplantations were used in 2 patients (6.7%). Time interval between first and second HDCT was 4-6 weeks. Chemotherapy was calculated according to pre-transplant levels of glomerular filtration rate, cardiac output and audiometry. Mean number of infused CD34+ cells was 4.98×106/kg (1.9-9.2).
Table 2. Conditioning regimens for HDCT/auto-HSCT in CNS ATRT patients

Statistical evaluation
Data collection and clarification, systematization of initial information and visualization in digital tables were performed by means of Microsoft Office Excel (2016). Python was used for statistical analysis (Python 3.8.). Calculations were based on built-in function modules (Scipy and Lifelines).
The Shapiro-Wilk test demonstrated absence of normal distribution of data in the study. It required usage of non-parametric statistics for further analysis.
The surviving patients were censored by 1 February 2021. Overall survival was calculated from the date of surgery up to death or last follow-up. Event-free survival was calculated from the date of intervention up to the date of unfavorable event (death, relapse, progression), or to the last follow-up. Median values describe the central values of distribution. Quantiles (Me [Q1; Q3]) and range of variation were used for assessment of variables. Mann-Whitney U-test compared independent samples in the absence of normal distribution. Nominal data obtained for independent research groups were compared using Pearson's Chi-squared test. Survival and cumulative incidence of events were calculated according to Kaplan-Meier method. Survival curves were compared using the log-rank test. Clinical outcomes were analyzed by multifactorial analysis (Cox regression). Stepwise regression was chosen for regression assessment, starting with maximal number of predictors. At each next step, the model excludes less valuable predictors. The procedure was stopped when the only independent variables remained, that were statistically significant. Statistical significance was assumed at p-value of ≤ 0.05.
Results
At the time of analysis, 18 children (60%) were alive and 12 (40%) died, among them 11 (91.6%) succumbed to ATRT progression and 1 (8.4%) to infectious complications in early post-trasnplant period. There were no cases of secondary tumors. Among children of ATRT-SHH molecular subgroup 2 died of progression and 2 were alive and in remission. Patient with ATRT-MYC molecular subgroup died of progression and 2 children with ATRT TYR stayed alive and in remission at the last follow up.
According to Kaplan-Meier statistical method EFS of the whole cohort (n=30) was 0.87 [0.68; 0.95] at 1 year, 0.49 [0.3; 0.67] at 2 years and 0.44 [0.24; 0.62] at 5 years (Fig. 1). Median EFS was 23 months [16.0; 102]. Worth of note, the majority of relapses in children with CNS ATRT occured during first 24 months after diagnosis.
OS was 0.97 [0.79; 1.0] at 1 year, 0.7 [0.49; 0.84] at 2 years, 0.44 [0.22; 0.64] at 5 years (Fig. 2). Median OS was 44 months [22.0; 102].

Analysis of prognostic factors in CNS ATRT patients after HDCT/auto-HSCT was performed. The results of univariate analysis are presented in Table 3.
Table 3. Prognosis of CNS ATRT patients after HDCT/auto-HSCT according to different factors

In univariate analysis extent of resection, radiotherapy, intraventricular/intrathecal chemotherapy and disease status prior to auto-HSCT demonstrated statistically significant impact on EFS. Survival curves are presented on Figures 3a-3d.
Upon univariate analysis, EFS in patients with CNS ATRT after HDCT/auto-HSCT was statistically significantly higher after total resection compared to subtotal resection, partial resection or biopsy: 1.0 [1.0; 1.0]; 0.37 [0.07; 0.69]; 0.15 [0.01; 0.46]; 0,00 [0.00; 0.00], respectively (p<0.001) (Fig. 3A); in the patients with RT versus children without RT: 0.56 [0.31; 0.75] and 0.00 [0.00; 0.00], respectively (р<0.001) (Fig. 3B); in the patients with intraventricular/intrathecal chemotherapy than in children without this local approach: 0.55 [0.29; 0.75] and 0.13 [0.01; 0.42], respectively (p=0.0005) (Fig. 3C); and in complete responders prior to auto-HSCT compared to PR and stable disease: 0.88 [0.39; 0.98]; 0.0 [0.0; 0.0] and 0.47 [0.12; 0.76], respectively (р<0.001), as seen from Fig. 3D.

Figure 3. Event-free survival in CNS ATRT patients after HDCT/auto-HSCT according to different factors: a – extent of resection; b – radiotherapy, c – intraventricular/intrathecal chemotherapy; d – disease status prior to auto-HSCT
Such factors as extent of tumor resection, radiotherapy, intraventricular/intrathecal chemotherapy, disease status prior to HDCT demonstrated statistically significant impact on OS (the survival curves are shown on Figures 4A to 4D).
Upon univariate analysis, OS in CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total resection compared to subtotal resection, partial resection or biopsy: 1.0 [1.0; 1.0]; 0.62 [0.23; 0.86]; 0.0 [0.0; 0.0]; 0,00 [0.00; 0.00] 0,00 [0.00; 0.00], respectively (p<0.001), as senn in Fig. 4A; in the patients with RT compared to children without RT: 0.57 [0.29; 0.78] and 0,00 [0.00; 0.00], respectively (р<0.001) depicted in Fig. 4B; in the patients with intraventricular/intrathecal chemotherapy than in children without this local approach: 0.56 [0.25; 0.78] and 0.13 [0.01; 0.42], respectively (p=0.0001, Fig. 4C); and in complete responders prior to auto-HSCT compared to PR and stable disease: 1.0 [1.0; 1.0]; 0.0 [0.0; 0.0] and 0.0 [0.0; 0.0], respectively (р<0.001), as shown in Fig. 4D.

Figure 4. Overall survival of CNS ATRT patients after HDCT/auto-HSCT according to different factors: a, extent of tumor resection; b, radiotherapy; c, intraventricular/intrathecal chemotherapy; d, disease status prior to auto-HSCT
Figure 5A demonstrates that final model of EFS includes such variables as RT, disease stage, intraventricular/intrathecal chemotherapy, disease status prior to auto-HSCT, number of transplants.
Figure 5B demonstrates that final model of OS includes age, tumor localization, extent of resection, radiotherapy, intraventricular/intrathecal chemotherapy and disease status prior to auto-HSCT.

Figure 5. Multivariate analysis of EFS (a) and OS (b) by Cox Proportional Hazard Model

Figure 6. Toxicity distribution according to CTCAE in CNS ATRT patients after HDCT/auto-HSCT
Duration of cytopenia varied from 6 to 24 days with median of 11 days after hematopoietic stem cell reinfusion. Toxicity of HDCT/auto-HSCT was assessed according to international criteria (СТСAE v.4.0), with the following items: ototoxicity, neurotoxicity, infections, nephrotoxicity, nausea/vomiting, mucositis, skin toxicity, hepatotoxicity, lung toxicity, cardiotoxicity.
During the study period, 21 single and 9 tandem transplants were performed in 30 patients. Total number of transplants reached 39, and they were separately analyzed according to 5-scale CTCAE recommendations. Distribution according to toxicity was as follows: Grade I – 32 %( n=58), Grade II – 46% (n=83), Grade III – 15% (n=27) and Grade IV – 7% (n=13) as shown in Fig. 6.
Fig. 7 demonstrates that that the majority of Stage IV complications were mucositis and infections (sepsis).
Distribution of Grade II-IV toxicity, of the most commonly affected organs and systems was as follows: gastrointestinal mucositis (90%, n=35), infectious complications (85%, n=33), nausea/vomiting (49%, n=19), hepatotoxicity (49%, n=19) as shown in Fig. 8.
There were no statistically significant differences in organ and system toxicity after single versus tandem transplants (Fig. 9).

Figure 7. Toxicity distribution according to grade, organs and systems in CNS ATRT patients after HDCT/auto-HSCT
Note: Grade 0, marked grey; Grade I, blue; Grade II, green; Grade III, yellow, and Grade IV, in red colour

Figure 8. Distribution of Grade II-I toxicity according to organs and systems in CNS ATRT patients after HDCT/auto-HSCT

Figure 9. Comparative toxicity characteristics after single (blue bars) vs tandem (red bars) HDCT/auto-HSCT in CNS ATRT patients
Discussion
CNS ATRT is a rare malignancy with poor prognosis that is predominantly diagnosed in young children [3, 4]. Nowadays there are no established standards for the treatment of CNS ATRT and prognosis remains dismal. It is generally accepted that in ATRT surgery should be followed by adjuvant chemotherapy with probable inclusion of RT depending on age [7, 8]. HDCT/auto-HSCT may be used for the intensification of first line therapy with the intention to postpone RT in young children. Nowadays there is no consensus concerning the role of HDCT/auto-HSCT in the treatment of ATRT due to limited patient number, differences in conditioning regimens and RT.
Initially HDCT/auto-HSCT was used by Hilden and coauthors in 2004 as part of therapy for CNS ATRT in 13 patients. Among 9 patients that received auto-HSCT 46% remained alive and disease-free with total tumor resection in half and RT in only third [1]. According to data of Tekautz and coauthors [2] that were published in 2005 nine patients older than 3 years that were treated with craniospinal irradiation and HDCT demonstrated 2-year OS of 89±11%. In the Head Start II study 1-3 cycles of HDCT with carboplatin, thiotepa and etoposide were performed after induction therapy with high dose metotrexate (HD-MTX). It is worth mentioning that patients after HS I scheme (without HD-MTX) demonstrated inferior outcome compared to children with HDCT HS II (all 6 patients died of progression as opposed to 3 out of 7 alive and disease free patients). There was no RT in long term survivors [13]. Headstart III trial (n=19) that included surgical resection of the tumor, 5 cycles of induction chemotherapy with HD-MTX and myeloablative HDCT/auto-HSCT demonstrated 3-year OS and EFS of 26% and 21%, respectively [14]. It is important to mention that 5 cases of treatment related mortality (TRM) on induction therapy were registered.
Lafay-Cousin and coauthors observed higher OS rates in the patients after HDCT/auto-HSCT compared to standard chemotherapy alone (2-year OS 47.9±12.1% and 27.3±9.5%, respectively) [15]. At the same time, it should be emphasized that among 9 survived patients after HDCT total tumor resection was performed in 55% and 67% had localized disease at diagnosis. In the EU-RHAB study (n=19) various conditioning regimens for tandem and single auto-HSCT were used. OS and EFS at 2 years were 50% and 29%, respectively. There was no TRM [16]. In our study 12 patients treated according to EU-RHAВ demonstrated OS and EFS of 40% and 32%, respectively.
Fossey and coauthors showed improved 5-year OS in ATRT patients under 1 year of age after HDCT/auto-HSCT compared to infants without HDCT (52.0% vs 10.7% respectively, p <0.001). Patients with CR prior to HDCT had significantly higher OS [17]. It underlines the importance of disease status prior to HDCT for prognosis and it is necessary to thoroughly select patients for HDCT.
It is worth of discussing the results obtained by Medical University of Vienna in CNS ATRT patients with М0-М3 stage (MUV). Treatment in post-surgery period included 3 blocks of 9-week chemotherapy with anthracyclines, alkylating agents, HD-MTX with addition of intrathecal chemotherapy (etoposide and cytarabine) and subsequent HDCT/auto-HSCT. Local RT was postponed until the end of chemotherapy. This cohort of patients demonstrated 100% OS and 88.9±10.58% EFS at 5 years, thus being significantly higher as for the control group (OS = 56.3±11.3%; EFS = 52.9±11%) treated with various other approaches. According to data of these workers, chemotherapy was well tolerated, timing delays and dose reduction due to toxicity were minimal [18]. In our study, 11 patients treated according to MUV protocol showed 3-year OS and EFS of 70% and 50%, respectively. These results are higher than in patients treated by other protocols, however, without significant difference.
In a recent study by Yamasaky et al. [19], in a group of 34 CNS ATRT patients, 19 received HDCT/auto-HSCT with tandem (n=13) transplantations in the majority of cases. Conditioning regimen consisted of thiotepa and melphalan. Two patients succumbed to sepsis in early post-transplant period. The study demonstrated better OS (p=0.025) in CNS ATRT patients after HDCT/auto-HSCT compared to patients without HDCT. Protocol ACNS 0333 for the treatment of ATRT has been recently developed by the Children's Oncology Group (COG). It consists of induction chemotherapy combined with 3 cycles of HDCT/auto-HSCT and RT. The abovementioned study (n=65) demonstrated 4-year EFS and OS of 48% and 57%, respectively [9].
Conclusion
HDCT/auto-HSCT is an important treatment option for children with chemosensitive CNS ATRT. In our study, 5-year EFS and OS rates after transplantation were 44%. The majority of relapses occurred during 24 months after diagnosis. These results are comparable to the majority of international studies. Survival of CNS ATRT patients after HDCT/auto-HSCT was significantly higher after total tumor resection, radiotherapy, intraventricular/intrathecal chemotherapy and complete response prior to auto-HSCT. Thus HDCT/auto-HSCT can postpone RT in younger children with CNS ATRT, but cannot substitute it. There was no statistical significant difference in survival between the patients following single and tandem transplantations. According to our data, HDCT/auto-HSCT demonstrated acceptable toxicity. Low incidence of CNS ATRT in children requires a large-scale multicentre randomized trials aiming for stratifying the patients into risk groups on the basis of clinical data, and clear indications for HDCT/auto-HSCT are crucial.
Conflict of interest
None reported.
References
- Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, Walter AW, Rorke LB, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004; 22(14):2877-2884. doi: 10.1200/ JCO.2004.07.073.
- Tekautz T, Fuller C, Blaney S, Foulad M, Fouladi M, Broniscer A, Merchant TE, Krasin M, Dalton J, Hale G, Kun LE, et al. Atypical teratoid/rhabdoid tumors (ATRT): Improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol.2005;23(7):1491-1499. doi: 10.1200/JCO.2005.05.187.
- Ostrom QT, Chen Y, de Blank P, Ondracek A, Farah P, Gittleman H, Wolinsky Y, Kruchko C, Cohen ML, Brat DJ, Barnholtz-Sloan JS. The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001-2010. Neuro-Oncology. 2014; 16 (10): 1392-1399. doi: 10.1093/neuonc/nou090.
- Lau CS, Mahendraraj K, Chamberlain RS. Atypical teratoid rhabdoid tumors: a population-based clinical outcomes study involving 174 patients from the Surveillance, Epidemiology, and End Results database (1973-2010). Cancer Manag Res. 2015; 7: 301-309. doi: 10.2147/CMAR.S88561.
- Zheludkova OG, Korshunov AG, Gorbatykh SV, Livshitz MI, Popov VE, Tarasova IS, Gorelyshex SK, Ozerova VI, Shcherbenko OI, Litvinov DV, Polushkina OB, Belogurova MB, Rusanova MG. Malignant theratoid-rhabdoid tumors of central nervous system in children. Voprosy Gematologii/oncologii i Immunopathologii v Pediatrii. 2003; 2(3): 32-39 (In Russian).
- Olkhova LV, Zheludkova OG, Kushel YuV, Melikyan AG, Gorelyshev SK, Ryzhova MV, Korshunov AG, Gorbatykh SV, Popov VE, Privalova LP, et al. Results of multidisciplinary study of atypical teratoid-rabdhoid CNS tumor treatment in children. Pediatriya. 2020; 99(4): 18-26 (In Russian). doi: 10.24110/0031-403X-2020-99-4-18-27.
- Richardson EA, Ho B, Huang A. Atypical Teratoid Rhabdoid tumour: from tumours to therapies. J Korean Neurosurg Soc. 2018; 61(3): 302-311. doi: 10.3340/jkns.2018.0061.
- Biswas A, Kashyap L, Kakkar A, Sarkar C, Julka PK. Аtypical teratoid/rhabdoid tumors: challenges and search for solutions. Cancer Manag Res. 2016; 8:115-125. doi: 10.2147/CMAR.S83472.
- Reddy AT, Strother DR, Judkins AR, Burger PC, Pollack IF, Krailo MD, Buxton AB, Williams-Hughes C, Fouladi M, Mahajan A, et al. Efficacy of high-dose chemotherapy and three-dimensional conformal radiation for atypical teratoid/rhabdoid tumor: a report from the Children's Oncology Group trial ACNS0333. J Clin Oncol. 2020; 38(11):1175-1185. doi: 10.1200/JCO.19.01776.
- Park M, Han JW, Hahn SM, Lee JA, Kim JY, Shin SH, Kim DS, Yoon HI, Hong KT, Choi JY et al. Atypical teratoid/rhabdoid tumor of the central nervous system in children under the age of 3 years. Cancer Res Treat. 2020. Oct 28. doi: 10.4143/crt.2020.756. PMID: 33138347.
- Yang WC, Yen HJ, Liang ML, Chen HH, Lee YY, Chang FC, Lin SC, Wong TT, Hu YW, Chen YW. Effect of early radiotherapy initiation and high-dose chemotherapy on the prognosis of pediatric atypical teratoid rhabdoid tumors in different age groups. J Neurooncol. 2020; 147 (3): 619-631. doi: 10.1007/s11060-020-03456-1.
- Bartelheim K, Nemes K, Seeringer A, Kerl K, Buechner J, Boos J, Graf N, Dürken M, Gerss J, Hasselblatt M, et al. Improved 6-year overall survival in AT/RT – results of the registry study Rhabdoid 2007. Cancer Med. 2016; 5(8):1765-1775. doi: 10.1002/cam4.741.
- Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008; 51(2):235-240. doi: 10.1002/pbc.21578.
- Zaky W, Dhall G, Ji L, Haley K, Allen J, Atlas M, Bertolone S, Cornelius A, Gardner S, Patel R et al. Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer. 2014; 61(1):95-101. doi: 10.1002/pbc.24648.
- Lafay-Cousin L, Hawkins C, Carret AS, Johnston D, Zelcer S, Wilson B, Jabado N, Scheinemann K, Eisenstat D, Fryer C, et al. Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012; 48(3):353-359. doi: 10.1016/j.ejca.2011.09.005.
- Benesch M, Bartelheim K, Fleischhack G, Gruhn B, Schlegel PG, Witt O, Stachel KD, Hauch H, Urban C, Quehenberger F, et al. High-dose chemotherapy (HDCT) with auto-SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU-RHAB). Bone Marrow Transplant. 2014; 49(3):370-375. doi: 10.1038/bmt.2013.208.
- Fossey M, Li H, Afzal S, Carret AS, Eisenstat DD, Fleming A, Hukin J, Hawkins C, Jabado N, Johnston D, et al. Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. J Neurooncol. 2017; 132(1):155-162. doi: 10.1007/s11060-016-2353-0.
- Slavc I, Chocholous M, Leiss U, Haberler C, Peyrl A, Azizi AA, Dieckmann K, Woehrer A, Peters C, Widhalm G, Dorfer C, Czech T. Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992-2012. Cancer Med. 2014 Feb;3(1):91-100. doi: 10.1002/cam4.161. Epub 2013 Dec 11. PMID: 24402832; PMCID: PMC3930393.
- Yamasaki K, Kiyotani C, Terashima K, Watanabe Y, Kanamori M, Koga Y, Hata N, Iwasaki F, Goto H, Koh K, et al. Clinical characteristics, treatment, and survival outcome in pediatric patients with atypical teratoid/rhabdoid tumors: a retrospective study by the Japan Children's Cancer Group. J Neurosurg Pediatr. 2019 :1-10. doi: 10.3171/ 2019.9.PEDS19367.
" ["~DETAIL_TEXT"]=> string(29744) "
Introduction
Central nervous system (CNS) atypical teratoid rhabdoid tumor (ATRT) is a rare aggressive malignancy, which comprises 1-2% of brain and spinal neoplasms [1, 2]. Median age at diagnosis is 1 year with male predominance 1.5:1.3 [3, 4, 5, 6]. Nowadays, there are no established standards in the treatment of CNS ATRT. Various management approaches exist for different countries and institutions. Despite this uncertainty, surgery followed by adjuvant chemotherapy and radiotherapy (in older children) is, without doubt, the cornerstone of their treatment [7, 8]. HDCT/auto-HSCT is used in children under 3 years, intending for canceling or postponing radiation therapy (RT) and reduce the risk of long-term neuro-cognitive disorders and improve outcome [9, 10, 11]. Despite intensive multimodal therapy, the majority of patients with ATRT develop relapses [10, 11, 12]. Prognosis for CNS ATRT remains dismal, especially in children with residual tumor and metastatic disease.
The aim of present study was to assess effectiveness and define prognostic factors in CNS ATRT patients after HDCT/auto-HSCT.
Patients and methods
Trial design
This retrospective multicentric continuous cooperative study was performed between 2008 and 2020 in V. A. Almazov NMRC, RM Gorbacheva Research Institute, Pavlov University, and Pirogov Russian National Research Medical University. Eligibility criteria: the study included 30 patients aged ≤18 with histologically verified CNS ATRT and complex treatment performed according to different protocols, including HDCT/auto-HSCT.
The surviving patients were censored by 1 February 2021. Overall survival was calculated from the date of surgery up to death or up to the last follow up. Event-free survival was calculated from the date of surgery up to the date of unfavorable event (death, relapse, progression), or up to last follow up.
Clinical characteristics of patients
A total of 30 patients ≤ 18 years old with median age of 19.5 months were enrolled in the study from different regions of Russian Federation. Initial characteristics of patients are presented in Table 1. There were 11 (36.6%) infants, and 19 (63.4%) children aged over 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial – in 13 (43.3%). Molecular biologic subgroups were identified only in 7 patients: ATRT-SHH (n=4), ATRT-MYC (n=1) and ATRT TYR (n=2). All children initially received surgery with total tumor resection (n=8, 26.7%), subtotal resection (n=9, 30.0%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and minor subgroup had M-0 stage (n=12, 40%), with non-classified stage (Mx) in 2 cases (6.7%). After surgery, everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). There was no radiotherapy (RT) prior to HDCT/auto-HSCT in children with ATRT. RT after transplantation was performed in 24 children (80%): local RT (n=16, 60%), cranio-spinal irradiation (n=6, 20%), and no RT (n=6, 20%). The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. Disease status was assessed in all 12 cases (40%) prior to HDCT/auto-HSCT with complete response (CR); partial response (PR), in 8 (26.7%), and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in majority of patients (n=21, 70%), and tandem transplant, only in 9 cases (30%). Total number of transplants was 39. Prior to auto-HSCT peripheral blood hematopoietic stem cells (PBSC) or bone marrow (BM) were harvested. PBSC were the transplant source in 27 children (90%), and combination of PBSC and BM was used in 3 cases (10%).
Table 1. Characteristics of CNS ATRT patients and HDCT/auto-HSCT

Conditioning regimen for single auto-HSCT in the majority of cases (n=13, 43.3%) consisted of thiotepa 300 mg/m2, carboplatin 500 mg/m2 and etoposide 250 mg/m2 on Days -6, -5, -4 (Table 2). Carboplatin 500 mg/m2 and thiotepa 300 mg/m2 on Days -6, -5, -4 were used in 8 patients (26.7%). First conditioning regimen consisted of carboplatin 500 mg/m2 and etoposide 250 mg/m2 on Days -8, -7, -6, -5 and second conditioning regimen consisted of thiotepa 300 mg/m2 and cyclophosphamide 1500 mg/m2 on Days -4,-3,-2 in 7 children (23.3%) with tandem transplantation. Carboplatin 510 mg/m2 and thiotepa 300 mg/m2 on Days -4, -3 for both transplantations were used in 2 patients (6.7%). Time interval between first and second HDCT was 4-6 weeks. Chemotherapy was calculated according to pre-transplant levels of glomerular filtration rate, cardiac output and audiometry. Mean number of infused CD34+ cells was 4.98×106/kg (1.9-9.2).
Table 2. Conditioning regimens for HDCT/auto-HSCT in CNS ATRT patients

Statistical evaluation
Data collection and clarification, systematization of initial information and visualization in digital tables were performed by means of Microsoft Office Excel (2016). Python was used for statistical analysis (Python 3.8.). Calculations were based on built-in function modules (Scipy and Lifelines).
The Shapiro-Wilk test demonstrated absence of normal distribution of data in the study. It required usage of non-parametric statistics for further analysis.
The surviving patients were censored by 1 February 2021. Overall survival was calculated from the date of surgery up to death or last follow-up. Event-free survival was calculated from the date of intervention up to the date of unfavorable event (death, relapse, progression), or to the last follow-up. Median values describe the central values of distribution. Quantiles (Me [Q1; Q3]) and range of variation were used for assessment of variables. Mann-Whitney U-test compared independent samples in the absence of normal distribution. Nominal data obtained for independent research groups were compared using Pearson's Chi-squared test. Survival and cumulative incidence of events were calculated according to Kaplan-Meier method. Survival curves were compared using the log-rank test. Clinical outcomes were analyzed by multifactorial analysis (Cox regression). Stepwise regression was chosen for regression assessment, starting with maximal number of predictors. At each next step, the model excludes less valuable predictors. The procedure was stopped when the only independent variables remained, that were statistically significant. Statistical significance was assumed at p-value of ≤ 0.05.
Results
At the time of analysis, 18 children (60%) were alive and 12 (40%) died, among them 11 (91.6%) succumbed to ATRT progression and 1 (8.4%) to infectious complications in early post-trasnplant period. There were no cases of secondary tumors. Among children of ATRT-SHH molecular subgroup 2 died of progression and 2 were alive and in remission. Patient with ATRT-MYC molecular subgroup died of progression and 2 children with ATRT TYR stayed alive and in remission at the last follow up.
According to Kaplan-Meier statistical method EFS of the whole cohort (n=30) was 0.87 [0.68; 0.95] at 1 year, 0.49 [0.3; 0.67] at 2 years and 0.44 [0.24; 0.62] at 5 years (Fig. 1). Median EFS was 23 months [16.0; 102]. Worth of note, the majority of relapses in children with CNS ATRT occured during first 24 months after diagnosis.
OS was 0.97 [0.79; 1.0] at 1 year, 0.7 [0.49; 0.84] at 2 years, 0.44 [0.22; 0.64] at 5 years (Fig. 2). Median OS was 44 months [22.0; 102].

Analysis of prognostic factors in CNS ATRT patients after HDCT/auto-HSCT was performed. The results of univariate analysis are presented in Table 3.
Table 3. Prognosis of CNS ATRT patients after HDCT/auto-HSCT according to different factors

In univariate analysis extent of resection, radiotherapy, intraventricular/intrathecal chemotherapy and disease status prior to auto-HSCT demonstrated statistically significant impact on EFS. Survival curves are presented on Figures 3a-3d.
Upon univariate analysis, EFS in patients with CNS ATRT after HDCT/auto-HSCT was statistically significantly higher after total resection compared to subtotal resection, partial resection or biopsy: 1.0 [1.0; 1.0]; 0.37 [0.07; 0.69]; 0.15 [0.01; 0.46]; 0,00 [0.00; 0.00], respectively (p<0.001) (Fig. 3A); in the patients with RT versus children without RT: 0.56 [0.31; 0.75] and 0.00 [0.00; 0.00], respectively (р<0.001) (Fig. 3B); in the patients with intraventricular/intrathecal chemotherapy than in children without this local approach: 0.55 [0.29; 0.75] and 0.13 [0.01; 0.42], respectively (p=0.0005) (Fig. 3C); and in complete responders prior to auto-HSCT compared to PR and stable disease: 0.88 [0.39; 0.98]; 0.0 [0.0; 0.0] and 0.47 [0.12; 0.76], respectively (р<0.001), as seen from Fig. 3D.

Figure 3. Event-free survival in CNS ATRT patients after HDCT/auto-HSCT according to different factors: a – extent of resection; b – radiotherapy, c – intraventricular/intrathecal chemotherapy; d – disease status prior to auto-HSCT
Such factors as extent of tumor resection, radiotherapy, intraventricular/intrathecal chemotherapy, disease status prior to HDCT demonstrated statistically significant impact on OS (the survival curves are shown on Figures 4A to 4D).
Upon univariate analysis, OS in CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total resection compared to subtotal resection, partial resection or biopsy: 1.0 [1.0; 1.0]; 0.62 [0.23; 0.86]; 0.0 [0.0; 0.0]; 0,00 [0.00; 0.00] 0,00 [0.00; 0.00], respectively (p<0.001), as senn in Fig. 4A; in the patients with RT compared to children without RT: 0.57 [0.29; 0.78] and 0,00 [0.00; 0.00], respectively (р<0.001) depicted in Fig. 4B; in the patients with intraventricular/intrathecal chemotherapy than in children without this local approach: 0.56 [0.25; 0.78] and 0.13 [0.01; 0.42], respectively (p=0.0001, Fig. 4C); and in complete responders prior to auto-HSCT compared to PR and stable disease: 1.0 [1.0; 1.0]; 0.0 [0.0; 0.0] and 0.0 [0.0; 0.0], respectively (р<0.001), as shown in Fig. 4D.

Figure 4. Overall survival of CNS ATRT patients after HDCT/auto-HSCT according to different factors: a, extent of tumor resection; b, radiotherapy; c, intraventricular/intrathecal chemotherapy; d, disease status prior to auto-HSCT
Figure 5A demonstrates that final model of EFS includes such variables as RT, disease stage, intraventricular/intrathecal chemotherapy, disease status prior to auto-HSCT, number of transplants.
Figure 5B demonstrates that final model of OS includes age, tumor localization, extent of resection, radiotherapy, intraventricular/intrathecal chemotherapy and disease status prior to auto-HSCT.

Figure 5. Multivariate analysis of EFS (a) and OS (b) by Cox Proportional Hazard Model

Figure 6. Toxicity distribution according to CTCAE in CNS ATRT patients after HDCT/auto-HSCT
Duration of cytopenia varied from 6 to 24 days with median of 11 days after hematopoietic stem cell reinfusion. Toxicity of HDCT/auto-HSCT was assessed according to international criteria (СТСAE v.4.0), with the following items: ototoxicity, neurotoxicity, infections, nephrotoxicity, nausea/vomiting, mucositis, skin toxicity, hepatotoxicity, lung toxicity, cardiotoxicity.
During the study period, 21 single and 9 tandem transplants were performed in 30 patients. Total number of transplants reached 39, and they were separately analyzed according to 5-scale CTCAE recommendations. Distribution according to toxicity was as follows: Grade I – 32 %( n=58), Grade II – 46% (n=83), Grade III – 15% (n=27) and Grade IV – 7% (n=13) as shown in Fig. 6.
Fig. 7 demonstrates that that the majority of Stage IV complications were mucositis and infections (sepsis).
Distribution of Grade II-IV toxicity, of the most commonly affected organs and systems was as follows: gastrointestinal mucositis (90%, n=35), infectious complications (85%, n=33), nausea/vomiting (49%, n=19), hepatotoxicity (49%, n=19) as shown in Fig. 8.
There were no statistically significant differences in organ and system toxicity after single versus tandem transplants (Fig. 9).

Figure 7. Toxicity distribution according to grade, organs and systems in CNS ATRT patients after HDCT/auto-HSCT
Note: Grade 0, marked grey; Grade I, blue; Grade II, green; Grade III, yellow, and Grade IV, in red colour

Figure 8. Distribution of Grade II-I toxicity according to organs and systems in CNS ATRT patients after HDCT/auto-HSCT

Figure 9. Comparative toxicity characteristics after single (blue bars) vs tandem (red bars) HDCT/auto-HSCT in CNS ATRT patients
Discussion
CNS ATRT is a rare malignancy with poor prognosis that is predominantly diagnosed in young children [3, 4]. Nowadays there are no established standards for the treatment of CNS ATRT and prognosis remains dismal. It is generally accepted that in ATRT surgery should be followed by adjuvant chemotherapy with probable inclusion of RT depending on age [7, 8]. HDCT/auto-HSCT may be used for the intensification of first line therapy with the intention to postpone RT in young children. Nowadays there is no consensus concerning the role of HDCT/auto-HSCT in the treatment of ATRT due to limited patient number, differences in conditioning regimens and RT.
Initially HDCT/auto-HSCT was used by Hilden and coauthors in 2004 as part of therapy for CNS ATRT in 13 patients. Among 9 patients that received auto-HSCT 46% remained alive and disease-free with total tumor resection in half and RT in only third [1]. According to data of Tekautz and coauthors [2] that were published in 2005 nine patients older than 3 years that were treated with craniospinal irradiation and HDCT demonstrated 2-year OS of 89±11%. In the Head Start II study 1-3 cycles of HDCT with carboplatin, thiotepa and etoposide were performed after induction therapy with high dose metotrexate (HD-MTX). It is worth mentioning that patients after HS I scheme (without HD-MTX) demonstrated inferior outcome compared to children with HDCT HS II (all 6 patients died of progression as opposed to 3 out of 7 alive and disease free patients). There was no RT in long term survivors [13]. Headstart III trial (n=19) that included surgical resection of the tumor, 5 cycles of induction chemotherapy with HD-MTX and myeloablative HDCT/auto-HSCT demonstrated 3-year OS and EFS of 26% and 21%, respectively [14]. It is important to mention that 5 cases of treatment related mortality (TRM) on induction therapy were registered.
Lafay-Cousin and coauthors observed higher OS rates in the patients after HDCT/auto-HSCT compared to standard chemotherapy alone (2-year OS 47.9±12.1% and 27.3±9.5%, respectively) [15]. At the same time, it should be emphasized that among 9 survived patients after HDCT total tumor resection was performed in 55% and 67% had localized disease at diagnosis. In the EU-RHAB study (n=19) various conditioning regimens for tandem and single auto-HSCT were used. OS and EFS at 2 years were 50% and 29%, respectively. There was no TRM [16]. In our study 12 patients treated according to EU-RHAВ demonstrated OS and EFS of 40% and 32%, respectively.
Fossey and coauthors showed improved 5-year OS in ATRT patients under 1 year of age after HDCT/auto-HSCT compared to infants without HDCT (52.0% vs 10.7% respectively, p <0.001). Patients with CR prior to HDCT had significantly higher OS [17]. It underlines the importance of disease status prior to HDCT for prognosis and it is necessary to thoroughly select patients for HDCT.
It is worth of discussing the results obtained by Medical University of Vienna in CNS ATRT patients with М0-М3 stage (MUV). Treatment in post-surgery period included 3 blocks of 9-week chemotherapy with anthracyclines, alkylating agents, HD-MTX with addition of intrathecal chemotherapy (etoposide and cytarabine) and subsequent HDCT/auto-HSCT. Local RT was postponed until the end of chemotherapy. This cohort of patients demonstrated 100% OS and 88.9±10.58% EFS at 5 years, thus being significantly higher as for the control group (OS = 56.3±11.3%; EFS = 52.9±11%) treated with various other approaches. According to data of these workers, chemotherapy was well tolerated, timing delays and dose reduction due to toxicity were minimal [18]. In our study, 11 patients treated according to MUV protocol showed 3-year OS and EFS of 70% and 50%, respectively. These results are higher than in patients treated by other protocols, however, without significant difference.
In a recent study by Yamasaky et al. [19], in a group of 34 CNS ATRT patients, 19 received HDCT/auto-HSCT with tandem (n=13) transplantations in the majority of cases. Conditioning regimen consisted of thiotepa and melphalan. Two patients succumbed to sepsis in early post-transplant period. The study demonstrated better OS (p=0.025) in CNS ATRT patients after HDCT/auto-HSCT compared to patients without HDCT. Protocol ACNS 0333 for the treatment of ATRT has been recently developed by the Children's Oncology Group (COG). It consists of induction chemotherapy combined with 3 cycles of HDCT/auto-HSCT and RT. The abovementioned study (n=65) demonstrated 4-year EFS and OS of 48% and 57%, respectively [9].
Conclusion
HDCT/auto-HSCT is an important treatment option for children with chemosensitive CNS ATRT. In our study, 5-year EFS and OS rates after transplantation were 44%. The majority of relapses occurred during 24 months after diagnosis. These results are comparable to the majority of international studies. Survival of CNS ATRT patients after HDCT/auto-HSCT was significantly higher after total tumor resection, radiotherapy, intraventricular/intrathecal chemotherapy and complete response prior to auto-HSCT. Thus HDCT/auto-HSCT can postpone RT in younger children with CNS ATRT, but cannot substitute it. There was no statistical significant difference in survival between the patients following single and tandem transplantations. According to our data, HDCT/auto-HSCT demonstrated acceptable toxicity. Low incidence of CNS ATRT in children requires a large-scale multicentre randomized trials aiming for stratifying the patients into risk groups on the basis of clinical data, and clear indications for HDCT/auto-HSCT are crucial.
Conflict of interest
None reported.
References
- Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, Walter AW, Rorke LB, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004; 22(14):2877-2884. doi: 10.1200/ JCO.2004.07.073.
- Tekautz T, Fuller C, Blaney S, Foulad M, Fouladi M, Broniscer A, Merchant TE, Krasin M, Dalton J, Hale G, Kun LE, et al. Atypical teratoid/rhabdoid tumors (ATRT): Improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol.2005;23(7):1491-1499. doi: 10.1200/JCO.2005.05.187.
- Ostrom QT, Chen Y, de Blank P, Ondracek A, Farah P, Gittleman H, Wolinsky Y, Kruchko C, Cohen ML, Brat DJ, Barnholtz-Sloan JS. The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001-2010. Neuro-Oncology. 2014; 16 (10): 1392-1399. doi: 10.1093/neuonc/nou090.
- Lau CS, Mahendraraj K, Chamberlain RS. Atypical teratoid rhabdoid tumors: a population-based clinical outcomes study involving 174 patients from the Surveillance, Epidemiology, and End Results database (1973-2010). Cancer Manag Res. 2015; 7: 301-309. doi: 10.2147/CMAR.S88561.
- Zheludkova OG, Korshunov AG, Gorbatykh SV, Livshitz MI, Popov VE, Tarasova IS, Gorelyshex SK, Ozerova VI, Shcherbenko OI, Litvinov DV, Polushkina OB, Belogurova MB, Rusanova MG. Malignant theratoid-rhabdoid tumors of central nervous system in children. Voprosy Gematologii/oncologii i Immunopathologii v Pediatrii. 2003; 2(3): 32-39 (In Russian).
- Olkhova LV, Zheludkova OG, Kushel YuV, Melikyan AG, Gorelyshev SK, Ryzhova MV, Korshunov AG, Gorbatykh SV, Popov VE, Privalova LP, et al. Results of multidisciplinary study of atypical teratoid-rabdhoid CNS tumor treatment in children. Pediatriya. 2020; 99(4): 18-26 (In Russian). doi: 10.24110/0031-403X-2020-99-4-18-27.
- Richardson EA, Ho B, Huang A. Atypical Teratoid Rhabdoid tumour: from tumours to therapies. J Korean Neurosurg Soc. 2018; 61(3): 302-311. doi: 10.3340/jkns.2018.0061.
- Biswas A, Kashyap L, Kakkar A, Sarkar C, Julka PK. Аtypical teratoid/rhabdoid tumors: challenges and search for solutions. Cancer Manag Res. 2016; 8:115-125. doi: 10.2147/CMAR.S83472.
- Reddy AT, Strother DR, Judkins AR, Burger PC, Pollack IF, Krailo MD, Buxton AB, Williams-Hughes C, Fouladi M, Mahajan A, et al. Efficacy of high-dose chemotherapy and three-dimensional conformal radiation for atypical teratoid/rhabdoid tumor: a report from the Children's Oncology Group trial ACNS0333. J Clin Oncol. 2020; 38(11):1175-1185. doi: 10.1200/JCO.19.01776.
- Park M, Han JW, Hahn SM, Lee JA, Kim JY, Shin SH, Kim DS, Yoon HI, Hong KT, Choi JY et al. Atypical teratoid/rhabdoid tumor of the central nervous system in children under the age of 3 years. Cancer Res Treat. 2020. Oct 28. doi: 10.4143/crt.2020.756. PMID: 33138347.
- Yang WC, Yen HJ, Liang ML, Chen HH, Lee YY, Chang FC, Lin SC, Wong TT, Hu YW, Chen YW. Effect of early radiotherapy initiation and high-dose chemotherapy on the prognosis of pediatric atypical teratoid rhabdoid tumors in different age groups. J Neurooncol. 2020; 147 (3): 619-631. doi: 10.1007/s11060-020-03456-1.
- Bartelheim K, Nemes K, Seeringer A, Kerl K, Buechner J, Boos J, Graf N, Dürken M, Gerss J, Hasselblatt M, et al. Improved 6-year overall survival in AT/RT – results of the registry study Rhabdoid 2007. Cancer Med. 2016; 5(8):1765-1775. doi: 10.1002/cam4.741.
- Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008; 51(2):235-240. doi: 10.1002/pbc.21578.
- Zaky W, Dhall G, Ji L, Haley K, Allen J, Atlas M, Bertolone S, Cornelius A, Gardner S, Patel R et al. Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer. 2014; 61(1):95-101. doi: 10.1002/pbc.24648.
- Lafay-Cousin L, Hawkins C, Carret AS, Johnston D, Zelcer S, Wilson B, Jabado N, Scheinemann K, Eisenstat D, Fryer C, et al. Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012; 48(3):353-359. doi: 10.1016/j.ejca.2011.09.005.
- Benesch M, Bartelheim K, Fleischhack G, Gruhn B, Schlegel PG, Witt O, Stachel KD, Hauch H, Urban C, Quehenberger F, et al. High-dose chemotherapy (HDCT) with auto-SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU-RHAB). Bone Marrow Transplant. 2014; 49(3):370-375. doi: 10.1038/bmt.2013.208.
- Fossey M, Li H, Afzal S, Carret AS, Eisenstat DD, Fleming A, Hukin J, Hawkins C, Jabado N, Johnston D, et al. Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. J Neurooncol. 2017; 132(1):155-162. doi: 10.1007/s11060-016-2353-0.
- Slavc I, Chocholous M, Leiss U, Haberler C, Peyrl A, Azizi AA, Dieckmann K, Woehrer A, Peters C, Widhalm G, Dorfer C, Czech T. Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992-2012. Cancer Med. 2014 Feb;3(1):91-100. doi: 10.1002/cam4.161. Epub 2013 Dec 11. PMID: 24402832; PMCID: PMC3930393.
- Yamasaki K, Kiyotani C, Terashima K, Watanabe Y, Kanamori M, Koga Y, Hata N, Iwasaki F, Goto H, Koh K, et al. Clinical characteristics, treatment, and survival outcome in pediatric patients with atypical teratoid/rhabdoid tumors: a retrospective study by the Japan Children's Cancer Group. J Neurosurg Pediatr. 2019 :1-10. doi: 10.3171/ 2019.9.PEDS19367.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "30" ["~SORT"]=> string(2) "30" ["CODE"]=> string(100) "vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto" ["~CODE"]=> string(100) "vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto" ["EXTERNAL_ID"]=> string(4) "1951" ["~EXTERNAL_ID"]=> string(4) "1951" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(456) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системыHigh-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(5390) "<p style="text-align: justify;">Атипичная тератоид-рабдоидная опухоль (АТРО) центральной нервной системы (ЦНС) – это агрессивная злокачественная опухоль, встречается преимущественно у детей младшего возраста и характеризуется плохим прогнозом. В настоящее время нет единых стандартов лечения АТРО ЦНС. Данное ретроспективное исследование выполнено с целью оценки результатов высокодозной полихимиотерапии (ВДХТ) с последующей аутологичной трансплантацией гемопоэтических стволовых клеток (ауто-ТГСК) у детей с АТРО ЦНС и определения влияния различных факторов прогноза на выживаемость. В исследование включены 30 больных с АТРО ЦНС, которые в протоколе терапии получали ВДХТ с ауто-ТГСК. Медиана возраста пациентов составила 19,5 месяцев [9; 27]. Распределение пациентов в зависимости от возраста было следующим: младше 12 месяцев – 11 (36,6%), старше 12 месяцев – 19 (63,4%); по полу: мальчиков – 21 (70%) и девочек – 9 (30%). Опухоль у 17 пациентов (56,7%) локализовалась инфратенториально, у 13 (43,3%) – супратенториально. Всем пациентам инициально выполнено хирургическое лечение в различных объемах: тотальное удаление опухоли – у 8 (26,7%) пациентов, субтотальное – у 9 (30,0%), частичное удаление – у 11 (36,6%), биопсия – у 2 (6,7%). В анализируемой группе преобладали больные с М+ стадией заболевания – 16 пациентов (53,3%), у 12 (40,0%) метастазирование и опухолевые клетки отсутствовали, установлена М0-стадия, у 2 (6,7%) – стадия заболевания не уточнена (Мх). Все пациенты после удаления опухоли получали лечение по различным протоколам терапии: 12 пациентов (40,0%) – по протоколу EU-RHAB, 11 (36,7%) – по протоколу MUV-ATRT, у 7 (23,3%) больных выполняли индивидуальные схемы. ЛТ проведена 24 больным (80%) после ВДХТ с ауто-ТГСК. Интратекальное/интравентрикулярное введение химиопрепаратов получили большинство пациентов (n=22, 73,3%). Статус заболевания оценивался у всех пациентов до выполнения ВДХТ с ауто-ТГСК: полный ответ (ПО) был зарегистрирован у 12 пациентов (40%), стабилизация болезни (СБ) – у 10 (33,3%), частичный ответ (ЧО) – у 8 (26,7%). Большинству пациентов выполнена однократная ауто-ТГСК – 21 (70%), тандемная ауто-ТГСК – у 9 (30%) пациентов. Общее количество выполненных ауто-ТГСК у пациентов с АТРО ЦНС составило 39. В качестве источника трансплантата в 90% (n=27) были использованы СКПК, у 10% (n=3) больных использовали комбинацию СКПК+КМ. Результаты: 5-летняя БСВ и ОВ составили 44%, большинство рецидивов диагностировано в течение 24 месяцев после постановки диагноза. Эти результаты сопоставимы с большинством международных данных. Выживаемость пациентов с АТРО ЦНС, получивших ВДХТ с ауто-ТГСК, статистически достоверно выше была при выполнении тотальной резекции опухоли, проведении ЛТ и регионарной химиотерапии и достижении ПО к моменту проведения ТГСК. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Дети, младший возраст, атипичная тератоид-рабдоидная опухоль, центральная нервная система, химиотерапия, высокодозная, лучевая терапия, результаты лечения, выживаемость, прогностические факторы.</p> " ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_META_TITLE"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_META_KEYWORDS"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_META_DESCRIPTION"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_PICTURE_FILE_ALT"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_PICTURE_FILE_TITLE"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(323) "Высокодозная полихимиотерапия с аутологичной трансплантацией гемопоэтических стволовых клеток у детей с атипичной тератоид-рабдоидной опухолью центральной нервной системы" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "vysokodoznaya-polikhimioterapiya-s-autologichnoy-transplantatsiey-gemopoeticheskikh-stvolovykh-kleto" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "175" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27472" ["VALUE"]=> string(22) "03/11/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/11/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27473" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27474" ["VALUE"]=> array(2) { ["TEXT"]=> string(495) "<p>Людмила В. Ольхова<sup>1</sup>, Ольга Г. Желудкова<sup>2</sup>, Людмила С. Зубаровская<sup>3</sup>, Анна Ю. Смирнова<sup>4</sup>, Юлия В. Диникина<sup>4,5</sup>, Асмик Г. Геворгян<sup>3</sup>, Андрей С. Левашов<sup>6</sup>, Елена В. Скоробогатова<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(387) "
Людмила В. Ольхова1, Ольга Г. Желудкова2, Людмила С. Зубаровская3, Анна Ю. Смирнова4, Юлия В. Диникина4,5, Асмик Г. Геворгян3, Андрей С. Левашов6, Елена В. Скоробогатова1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27475" ["VALUE"]=> array(2) { ["TEXT"]=> string(1612) "<p><sup>1</sup> Обособленное структурное подразделение «Российская детская клиническая больница», Российский национальный медицинский университет им. Н. И. Пирогова, Москва, Россия<br> <sup>2</sup> Научно-практический центр специализированной медицинской помощи им. В. Ф. Войно-Ясенецкого, Москва, Россия<br> <sup>3</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>4</sup> Национальный медицинский исследовательский центр им. В. А. Алмазова, Санкт-Петербург, Россия<br> <sup>5</sup> Санкт-Петербургский государственный педиатрический медицинский университет, Санкт-Петербург, Россия<br> <sup>6</sup> Национальный медицинский исследовательский центр онкологии им. Н. Н. Блохина, Москва, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1498) "1 Обособленное структурное подразделение «Российская детская клиническая больница», Российский национальный медицинский университет им. Н. И. Пирогова, Москва, Россия
2 Научно-практический центр специализированной медицинской помощи им. В. Ф. Войно-Ясенецкого, Москва, Россия
3 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
4 Национальный медицинский исследовательский центр им. В. А. Алмазова, Санкт-Петербург, Россия
5 Санкт-Петербургский государственный педиатрический медицинский университет, Санкт-Петербург, Россия
6 Национальный медицинский исследовательский центр онкологии им. Н. Н. Блохина, Москва, Россия
Атипичная тератоид-рабдоидная опухоль (АТРО) центральной нервной системы (ЦНС) – это агрессивная злокачественная опухоль, встречается преимущественно у детей младшего возраста и характеризуется плохим прогнозом. В настоящее время нет единых стандартов лечения АТРО ЦНС. Данное ретроспективное исследование выполнено с целью оценки результатов высокодозной полихимиотерапии (ВДХТ) с последующей аутологичной трансплантацией гемопоэтических стволовых клеток (ауто-ТГСК) у детей с АТРО ЦНС и определения влияния различных факторов прогноза на выживаемость. В исследование включены 30 больных с АТРО ЦНС, которые в протоколе терапии получали ВДХТ с ауто-ТГСК. Медиана возраста пациентов составила 19,5 месяцев [9; 27]. Распределение пациентов в зависимости от возраста было следующим: младше 12 месяцев – 11 (36,6%), старше 12 месяцев – 19 (63,4%); по полу: мальчиков – 21 (70%) и девочек – 9 (30%). Опухоль у 17 пациентов (56,7%) локализовалась инфратенториально, у 13 (43,3%) – супратенториально. Всем пациентам инициально выполнено хирургическое лечение в различных объемах: тотальное удаление опухоли – у 8 (26,7%) пациентов, субтотальное – у 9 (30,0%), частичное удаление – у 11 (36,6%), биопсия – у 2 (6,7%). В анализируемой группе преобладали больные с М+ стадией заболевания – 16 пациентов (53,3%), у 12 (40,0%) метастазирование и опухолевые клетки отсутствовали, установлена М0-стадия, у 2 (6,7%) – стадия заболевания не уточнена (Мх). Все пациенты после удаления опухоли получали лечение по различным протоколам терапии: 12 пациентов (40,0%) – по протоколу EU-RHAB, 11 (36,7%) – по протоколу MUV-ATRT, у 7 (23,3%) больных выполняли индивидуальные схемы. ЛТ проведена 24 больным (80%) после ВДХТ с ауто-ТГСК. Интратекальное/интравентрикулярное введение химиопрепаратов получили большинство пациентов (n=22, 73,3%). Статус заболевания оценивался у всех пациентов до выполнения ВДХТ с ауто-ТГСК: полный ответ (ПО) был зарегистрирован у 12 пациентов (40%), стабилизация болезни (СБ) – у 10 (33,3%), частичный ответ (ЧО) – у 8 (26,7%). Большинству пациентов выполнена однократная ауто-ТГСК – 21 (70%), тандемная ауто-ТГСК – у 9 (30%) пациентов. Общее количество выполненных ауто-ТГСК у пациентов с АТРО ЦНС составило 39. В качестве источника трансплантата в 90% (n=27) были использованы СКПК, у 10% (n=3) больных использовали комбинацию СКПК+КМ. Результаты: 5-летняя БСВ и ОВ составили 44%, большинство рецидивов диагностировано в течение 24 месяцев после постановки диагноза. Эти результаты сопоставимы с большинством международных данных. Выживаемость пациентов с АТРО ЦНС, получивших ВДХТ с ауто-ТГСК, статистически достоверно выше была при выполнении тотальной резекции опухоли, проведении ЛТ и регионарной химиотерапии и достижении ПО к моменту проведения ТГСК.
Ключевые слова
Дети, младший возраст, атипичная тератоид-рабдоидная опухоль, центральная нервная система, химиотерапия, высокодозная, лучевая терапия, результаты лечения, выживаемость, прогностические факторы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27477" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-44-54" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-44-54" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27479" ["VALUE"]=> array(2) { ["TEXT"]=> string(380) "<p>Liudmila V. Olkhova<sup>1</sup>, Olga G. Zheludkova<sup>2</sup>, Ludmila S. Zubarovskaya<sup>3</sup>, Anna Yu. Smirnova<sup>4</sup>, Yulia V. Dinikina<sup>4,5</sup>, Asmik G. Gevorgyan<sup>3</sup>, Andrey S. Levashov<sup>6</sup>, Elena V. Skorobogatova<sup>1</sup></p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(272) "Liudmila V. Olkhova1, Olga G. Zheludkova2, Ludmila S. Zubarovskaya3, Anna Yu. Smirnova4, Yulia V. Dinikina4,5, Asmik G. Gevorgyan3, Andrey S. Levashov6, Elena V. Skorobogatova1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27480" ["VALUE"]=> array(2) { ["TEXT"]=> string(1114) "<p><sup>1</sup> Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia<br> <sup>2</sup> St. Luke Clinical Research Center for Children, Moscow, Russia<br> <sup>3</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br> <sup>4</sup> V. A. Almazov National Medical Research Center, St. Petersburg, Russia<br> <sup>5</sup> St. Petersburg State Pediatric Medical University, St. Petersburg, Russia<br> <sup>6</sup> N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia</p><br> <p><b>Correspondence</b><br> Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia<br> Phone: +7 (995) 707 5140<br> E-mail: rylkova87@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(952) "1 Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia
2 St. Luke Clinical Research Center for Children, Moscow, Russia
3 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
4 V. A. Almazov National Medical Research Center, St. Petersburg, Russia
5 St. Petersburg State Pediatric Medical University, St. Petersburg, Russia
6 N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia
Correspondence
Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia
Phone: +7 (995) 707 5140
E-mail: rylkova87@mail.ru
Atypical teratoid rhabdoid tumor (ATRT) of central nervous system (CNS) is an aggressive malignancy with poor prognosis, predominantly observed in young children. There are no established approaches to CNS ATRT management nowadays. This retrospective study aimed to analyze the effectiveness and prognostic factors of high dose chemotherapy with autologous hematopoietic stem cell transplantation HDCT/auto-HSCT in pediatric CNS ATRT. Thirty CNS ATRT patients treated with HDCT/auto-HSCT were enrolled in the analysis. Median age was 19.5 months. There were 11 (36.6%) infants and 19 (63.4%) children older than 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial in 13 (43.3%). All children initially received surgery with total resection (n=8, 26.7%), subtotal resection (n=9, 30%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and the minority had M-0 stage (n=12, 40%), while stage wasn't clarified (Mx) in 2 (6.7%) cases. After surgery everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). Radiotherapy (RT) was performed in 24 children (80%) after HDCT/auto-HSCT. The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. The disease status was assessed in all cases prior to HDCT/auto-HSCT with complete response (CR) in 12 (40%), partial response (PR) in 8 (26.7%) and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in the majority of patients (n=21, 70%) and tandem transplants were carried out in 9 cases only (30%). In total, 39 transplants were performed. Peripheral blood hematopoietic stem cells (PBSC) were the transplant source in 27 children (90%), and combination of PBSC and bone marrow (BM), in 3 (10%). Five-year event-free survival (EFS) and overall survival (OS) were 44%. The majority of relapses were diagnosed during first 24 months after disease onset. These data are comparable to the most international results. Survival of CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total tumor resection, RT, intraventricular/intrathecal chemotherapy, and CR prior to transplantation.
Keywords
Сhildren, young age, atypical teratoid rhabdoid tumor, central nervous system, chemotherapy, high-dose, radiation therapy, clinical outcomes, survival, prognostic factors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27478" ["VALUE"]=> string(133) "High-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(133) "High-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27530" ["VALUE"]=> string(4) "2420" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2420" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27531" ["VALUE"]=> string(4) "2421" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2421" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27479" ["VALUE"]=> array(2) { ["TEXT"]=> string(380) "<p>Liudmila V. Olkhova<sup>1</sup>, Olga G. Zheludkova<sup>2</sup>, Ludmila S. Zubarovskaya<sup>3</sup>, Anna Yu. Smirnova<sup>4</sup>, Yulia V. Dinikina<sup>4,5</sup>, Asmik G. Gevorgyan<sup>3</sup>, Andrey S. Levashov<sup>6</sup>, Elena V. Skorobogatova<sup>1</sup></p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(272) "Liudmila V. Olkhova1, Olga G. Zheludkova2, Ludmila S. Zubarovskaya3, Anna Yu. Smirnova4, Yulia V. Dinikina4,5, Asmik G. Gevorgyan3, Andrey S. Levashov6, Elena V. Skorobogatova1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(272) "Liudmila V. Olkhova1, Olga G. Zheludkova2, Ludmila S. Zubarovskaya3, Anna Yu. Smirnova4, Yulia V. Dinikina4,5, Asmik G. Gevorgyan3, Andrey S. Levashov6, Elena V. Skorobogatova1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27481" ["VALUE"]=> array(2) { ["TEXT"]=> string(2639) "<p style="text-align: justify;">Atypical teratoid rhabdoid tumor (ATRT) of central nervous system (CNS) is an aggressive malignancy with poor prognosis, predominantly observed in young children. There are no established approaches to CNS ATRT management nowadays. This retrospective study aimed to analyze the effectiveness and prognostic factors of high dose chemotherapy with autologous hematopoietic stem cell transplantation HDCT/auto-HSCT in pediatric CNS ATRT. Thirty CNS ATRT patients treated with HDCT/auto-HSCT were enrolled in the analysis. Median age was 19.5 months. There were 11 (36.6%) infants and 19 (63.4%) children older than 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial in 13 (43.3%). All children initially received surgery with total resection (n=8, 26.7%), subtotal resection (n=9, 30%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and the minority had M-0 stage (n=12, 40%), while stage wasn't clarified (Mx) in 2 (6.7%) cases. After surgery everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). Radiotherapy (RT) was performed in 24 children (80%) after HDCT/auto-HSCT. The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. The disease status was assessed in all cases prior to HDCT/auto-HSCT with complete response (CR) in 12 (40%), partial response (PR) in 8 (26.7%) and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in the majority of patients (n=21, 70%) and tandem transplants were carried out in 9 cases only (30%). In total, 39 transplants were performed. Peripheral blood hematopoietic stem cells (PBSC) were the transplant source in 27 children (90%), and combination of PBSC and bone marrow (BM), in 3 (10%). Five-year event-free survival (EFS) and overall survival (OS) were 44%. The majority of relapses were diagnosed during first 24 months after disease onset. These data are comparable to the most international results. Survival of CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total tumor resection, RT, intraventricular/intrathecal chemotherapy, and CR prior to transplantation. </p> <h2>Keywords</h2> <p style="text-align: justify;">Сhildren, young age, atypical teratoid rhabdoid tumor, central nervous system, chemotherapy, high-dose, radiation therapy, clinical outcomes, survival, prognostic factors.</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2583) "Atypical teratoid rhabdoid tumor (ATRT) of central nervous system (CNS) is an aggressive malignancy with poor prognosis, predominantly observed in young children. There are no established approaches to CNS ATRT management nowadays. This retrospective study aimed to analyze the effectiveness and prognostic factors of high dose chemotherapy with autologous hematopoietic stem cell transplantation HDCT/auto-HSCT in pediatric CNS ATRT. Thirty CNS ATRT patients treated with HDCT/auto-HSCT were enrolled in the analysis. Median age was 19.5 months. There were 11 (36.6%) infants and 19 (63.4%) children older than 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial in 13 (43.3%). All children initially received surgery with total resection (n=8, 26.7%), subtotal resection (n=9, 30%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and the minority had M-0 stage (n=12, 40%), while stage wasn't clarified (Mx) in 2 (6.7%) cases. After surgery everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). Radiotherapy (RT) was performed in 24 children (80%) after HDCT/auto-HSCT. The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. The disease status was assessed in all cases prior to HDCT/auto-HSCT with complete response (CR) in 12 (40%), partial response (PR) in 8 (26.7%) and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in the majority of patients (n=21, 70%) and tandem transplants were carried out in 9 cases only (30%). In total, 39 transplants were performed. Peripheral blood hematopoietic stem cells (PBSC) were the transplant source in 27 children (90%), and combination of PBSC and bone marrow (BM), in 3 (10%). Five-year event-free survival (EFS) and overall survival (OS) were 44%. The majority of relapses were diagnosed during first 24 months after disease onset. These data are comparable to the most international results. Survival of CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total tumor resection, RT, intraventricular/intrathecal chemotherapy, and CR prior to transplantation.
Keywords
Сhildren, young age, atypical teratoid rhabdoid tumor, central nervous system, chemotherapy, high-dose, radiation therapy, clinical outcomes, survival, prognostic factors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2583) "Atypical teratoid rhabdoid tumor (ATRT) of central nervous system (CNS) is an aggressive malignancy with poor prognosis, predominantly observed in young children. There are no established approaches to CNS ATRT management nowadays. This retrospective study aimed to analyze the effectiveness and prognostic factors of high dose chemotherapy with autologous hematopoietic stem cell transplantation HDCT/auto-HSCT in pediatric CNS ATRT. Thirty CNS ATRT patients treated with HDCT/auto-HSCT were enrolled in the analysis. Median age was 19.5 months. There were 11 (36.6%) infants and 19 (63.4%) children older than 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial in 13 (43.3%). All children initially received surgery with total resection (n=8, 26.7%), subtotal resection (n=9, 30%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and the minority had M-0 stage (n=12, 40%), while stage wasn't clarified (Mx) in 2 (6.7%) cases. After surgery everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). Radiotherapy (RT) was performed in 24 children (80%) after HDCT/auto-HSCT. The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. The disease status was assessed in all cases prior to HDCT/auto-HSCT with complete response (CR) in 12 (40%), partial response (PR) in 8 (26.7%) and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in the majority of patients (n=21, 70%) and tandem transplants were carried out in 9 cases only (30%). In total, 39 transplants were performed. Peripheral blood hematopoietic stem cells (PBSC) were the transplant source in 27 children (90%), and combination of PBSC and bone marrow (BM), in 3 (10%). Five-year event-free survival (EFS) and overall survival (OS) were 44%. The majority of relapses were diagnosed during first 24 months after disease onset. These data are comparable to the most international results. Survival of CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total tumor resection, RT, intraventricular/intrathecal chemotherapy, and CR prior to transplantation.
Keywords
Сhildren, young age, atypical teratoid rhabdoid tumor, central nervous system, chemotherapy, high-dose, radiation therapy, clinical outcomes, survival, prognostic factors.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27477" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-44-54" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-44-54" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-44-54" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27478" ["VALUE"]=> string(133) "High-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(133) "High-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(133) "High-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27480" ["VALUE"]=> array(2) { ["TEXT"]=> string(1114) "<p><sup>1</sup> Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia<br> <sup>2</sup> St. Luke Clinical Research Center for Children, Moscow, Russia<br> <sup>3</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br> <sup>4</sup> V. A. Almazov National Medical Research Center, St. Petersburg, Russia<br> <sup>5</sup> St. Petersburg State Pediatric Medical University, St. Petersburg, Russia<br> <sup>6</sup> N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia</p><br> <p><b>Correspondence</b><br> Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia<br> Phone: +7 (995) 707 5140<br> E-mail: rylkova87@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(952) "1 Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia
2 St. Luke Clinical Research Center for Children, Moscow, Russia
3 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
4 V. A. Almazov National Medical Research Center, St. Petersburg, Russia
5 St. Petersburg State Pediatric Medical University, St. Petersburg, Russia
6 N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia
Correspondence
Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia
Phone: +7 (995) 707 5140
E-mail: rylkova87@mail.ru
1 Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia
2 St. Luke Clinical Research Center for Children, Moscow, Russia
3 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
4 V. A. Almazov National Medical Research Center, St. Petersburg, Russia
5 St. Petersburg State Pediatric Medical University, St. Petersburg, Russia
6 N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia
Correspondence
Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia
Phone: +7 (995) 707 5140
E-mail: rylkova87@mail.ru
Людмила В. Ольхова1, Ольга Г. Желудкова2, Людмила С. Зубаровская3, Анна Ю. Смирнова4, Юлия В. Диникина4,5, Асмик Г. Геворгян3, Андрей С. Левашов6, Елена В. Скоробогатова1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(387) "Людмила В. Ольхова1, Ольга Г. Желудкова2, Людмила С. Зубаровская3, Анна Ю. Смирнова4, Юлия В. Диникина4,5, Асмик Г. Геворгян3, Андрей С. Левашов6, Елена В. Скоробогатова1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27472" ["VALUE"]=> string(22) "03/11/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/11/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/11/2021 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27473" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "04/09/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27476" ["VALUE"]=> array(2) { ["TEXT"]=> string(5390) "<p style="text-align: justify;">Атипичная тератоид-рабдоидная опухоль (АТРО) центральной нервной системы (ЦНС) – это агрессивная злокачественная опухоль, встречается преимущественно у детей младшего возраста и характеризуется плохим прогнозом. В настоящее время нет единых стандартов лечения АТРО ЦНС. Данное ретроспективное исследование выполнено с целью оценки результатов высокодозной полихимиотерапии (ВДХТ) с последующей аутологичной трансплантацией гемопоэтических стволовых клеток (ауто-ТГСК) у детей с АТРО ЦНС и определения влияния различных факторов прогноза на выживаемость. В исследование включены 30 больных с АТРО ЦНС, которые в протоколе терапии получали ВДХТ с ауто-ТГСК. Медиана возраста пациентов составила 19,5 месяцев [9; 27]. Распределение пациентов в зависимости от возраста было следующим: младше 12 месяцев – 11 (36,6%), старше 12 месяцев – 19 (63,4%); по полу: мальчиков – 21 (70%) и девочек – 9 (30%). Опухоль у 17 пациентов (56,7%) локализовалась инфратенториально, у 13 (43,3%) – супратенториально. Всем пациентам инициально выполнено хирургическое лечение в различных объемах: тотальное удаление опухоли – у 8 (26,7%) пациентов, субтотальное – у 9 (30,0%), частичное удаление – у 11 (36,6%), биопсия – у 2 (6,7%). В анализируемой группе преобладали больные с М+ стадией заболевания – 16 пациентов (53,3%), у 12 (40,0%) метастазирование и опухолевые клетки отсутствовали, установлена М0-стадия, у 2 (6,7%) – стадия заболевания не уточнена (Мх). Все пациенты после удаления опухоли получали лечение по различным протоколам терапии: 12 пациентов (40,0%) – по протоколу EU-RHAB, 11 (36,7%) – по протоколу MUV-ATRT, у 7 (23,3%) больных выполняли индивидуальные схемы. ЛТ проведена 24 больным (80%) после ВДХТ с ауто-ТГСК. Интратекальное/интравентрикулярное введение химиопрепаратов получили большинство пациентов (n=22, 73,3%). Статус заболевания оценивался у всех пациентов до выполнения ВДХТ с ауто-ТГСК: полный ответ (ПО) был зарегистрирован у 12 пациентов (40%), стабилизация болезни (СБ) – у 10 (33,3%), частичный ответ (ЧО) – у 8 (26,7%). Большинству пациентов выполнена однократная ауто-ТГСК – 21 (70%), тандемная ауто-ТГСК – у 9 (30%) пациентов. Общее количество выполненных ауто-ТГСК у пациентов с АТРО ЦНС составило 39. В качестве источника трансплантата в 90% (n=27) были использованы СКПК, у 10% (n=3) больных использовали комбинацию СКПК+КМ. Результаты: 5-летняя БСВ и ОВ составили 44%, большинство рецидивов диагностировано в течение 24 месяцев после постановки диагноза. Эти результаты сопоставимы с большинством международных данных. Выживаемость пациентов с АТРО ЦНС, получивших ВДХТ с ауто-ТГСК, статистически достоверно выше была при выполнении тотальной резекции опухоли, проведении ЛТ и регионарной химиотерапии и достижении ПО к моменту проведения ТГСК. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Дети, младший возраст, атипичная тератоид-рабдоидная опухоль, центральная нервная система, химиотерапия, высокодозная, лучевая терапия, результаты лечения, выживаемость, прогностические факторы.</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(5334) "Атипичная тератоид-рабдоидная опухоль (АТРО) центральной нервной системы (ЦНС) – это агрессивная злокачественная опухоль, встречается преимущественно у детей младшего возраста и характеризуется плохим прогнозом. В настоящее время нет единых стандартов лечения АТРО ЦНС. Данное ретроспективное исследование выполнено с целью оценки результатов высокодозной полихимиотерапии (ВДХТ) с последующей аутологичной трансплантацией гемопоэтических стволовых клеток (ауто-ТГСК) у детей с АТРО ЦНС и определения влияния различных факторов прогноза на выживаемость. В исследование включены 30 больных с АТРО ЦНС, которые в протоколе терапии получали ВДХТ с ауто-ТГСК. Медиана возраста пациентов составила 19,5 месяцев [9; 27]. Распределение пациентов в зависимости от возраста было следующим: младше 12 месяцев – 11 (36,6%), старше 12 месяцев – 19 (63,4%); по полу: мальчиков – 21 (70%) и девочек – 9 (30%). Опухоль у 17 пациентов (56,7%) локализовалась инфратенториально, у 13 (43,3%) – супратенториально. Всем пациентам инициально выполнено хирургическое лечение в различных объемах: тотальное удаление опухоли – у 8 (26,7%) пациентов, субтотальное – у 9 (30,0%), частичное удаление – у 11 (36,6%), биопсия – у 2 (6,7%). В анализируемой группе преобладали больные с М+ стадией заболевания – 16 пациентов (53,3%), у 12 (40,0%) метастазирование и опухолевые клетки отсутствовали, установлена М0-стадия, у 2 (6,7%) – стадия заболевания не уточнена (Мх). Все пациенты после удаления опухоли получали лечение по различным протоколам терапии: 12 пациентов (40,0%) – по протоколу EU-RHAB, 11 (36,7%) – по протоколу MUV-ATRT, у 7 (23,3%) больных выполняли индивидуальные схемы. ЛТ проведена 24 больным (80%) после ВДХТ с ауто-ТГСК. Интратекальное/интравентрикулярное введение химиопрепаратов получили большинство пациентов (n=22, 73,3%). Статус заболевания оценивался у всех пациентов до выполнения ВДХТ с ауто-ТГСК: полный ответ (ПО) был зарегистрирован у 12 пациентов (40%), стабилизация болезни (СБ) – у 10 (33,3%), частичный ответ (ЧО) – у 8 (26,7%). Большинству пациентов выполнена однократная ауто-ТГСК – 21 (70%), тандемная ауто-ТГСК – у 9 (30%) пациентов. Общее количество выполненных ауто-ТГСК у пациентов с АТРО ЦНС составило 39. В качестве источника трансплантата в 90% (n=27) были использованы СКПК, у 10% (n=3) больных использовали комбинацию СКПК+КМ. Результаты: 5-летняя БСВ и ОВ составили 44%, большинство рецидивов диагностировано в течение 24 месяцев после постановки диагноза. Эти результаты сопоставимы с большинством международных данных. Выживаемость пациентов с АТРО ЦНС, получивших ВДХТ с ауто-ТГСК, статистически достоверно выше была при выполнении тотальной резекции опухоли, проведении ЛТ и регионарной химиотерапии и достижении ПО к моменту проведения ТГСК.
Ключевые слова
Дети, младший возраст, атипичная тератоид-рабдоидная опухоль, центральная нервная система, химиотерапия, высокодозная, лучевая терапия, результаты лечения, выживаемость, прогностические факторы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(5334) "Атипичная тератоид-рабдоидная опухоль (АТРО) центральной нервной системы (ЦНС) – это агрессивная злокачественная опухоль, встречается преимущественно у детей младшего возраста и характеризуется плохим прогнозом. В настоящее время нет единых стандартов лечения АТРО ЦНС. Данное ретроспективное исследование выполнено с целью оценки результатов высокодозной полихимиотерапии (ВДХТ) с последующей аутологичной трансплантацией гемопоэтических стволовых клеток (ауто-ТГСК) у детей с АТРО ЦНС и определения влияния различных факторов прогноза на выживаемость. В исследование включены 30 больных с АТРО ЦНС, которые в протоколе терапии получали ВДХТ с ауто-ТГСК. Медиана возраста пациентов составила 19,5 месяцев [9; 27]. Распределение пациентов в зависимости от возраста было следующим: младше 12 месяцев – 11 (36,6%), старше 12 месяцев – 19 (63,4%); по полу: мальчиков – 21 (70%) и девочек – 9 (30%). Опухоль у 17 пациентов (56,7%) локализовалась инфратенториально, у 13 (43,3%) – супратенториально. Всем пациентам инициально выполнено хирургическое лечение в различных объемах: тотальное удаление опухоли – у 8 (26,7%) пациентов, субтотальное – у 9 (30,0%), частичное удаление – у 11 (36,6%), биопсия – у 2 (6,7%). В анализируемой группе преобладали больные с М+ стадией заболевания – 16 пациентов (53,3%), у 12 (40,0%) метастазирование и опухолевые клетки отсутствовали, установлена М0-стадия, у 2 (6,7%) – стадия заболевания не уточнена (Мх). Все пациенты после удаления опухоли получали лечение по различным протоколам терапии: 12 пациентов (40,0%) – по протоколу EU-RHAB, 11 (36,7%) – по протоколу MUV-ATRT, у 7 (23,3%) больных выполняли индивидуальные схемы. ЛТ проведена 24 больным (80%) после ВДХТ с ауто-ТГСК. Интратекальное/интравентрикулярное введение химиопрепаратов получили большинство пациентов (n=22, 73,3%). Статус заболевания оценивался у всех пациентов до выполнения ВДХТ с ауто-ТГСК: полный ответ (ПО) был зарегистрирован у 12 пациентов (40%), стабилизация болезни (СБ) – у 10 (33,3%), частичный ответ (ЧО) – у 8 (26,7%). Большинству пациентов выполнена однократная ауто-ТГСК – 21 (70%), тандемная ауто-ТГСК – у 9 (30%) пациентов. Общее количество выполненных ауто-ТГСК у пациентов с АТРО ЦНС составило 39. В качестве источника трансплантата в 90% (n=27) были использованы СКПК, у 10% (n=3) больных использовали комбинацию СКПК+КМ. Результаты: 5-летняя БСВ и ОВ составили 44%, большинство рецидивов диагностировано в течение 24 месяцев после постановки диагноза. Эти результаты сопоставимы с большинством международных данных. Выживаемость пациентов с АТРО ЦНС, получивших ВДХТ с ауто-ТГСК, статистически достоверно выше была при выполнении тотальной резекции опухоли, проведении ЛТ и регионарной химиотерапии и достижении ПО к моменту проведения ТГСК.
Ключевые слова
Дети, младший возраст, атипичная тератоид-рабдоидная опухоль, центральная нервная система, химиотерапия, высокодозная, лучевая терапия, результаты лечения, выживаемость, прогностические факторы.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27475" ["VALUE"]=> array(2) { ["TEXT"]=> string(1612) "<p><sup>1</sup> Обособленное структурное подразделение «Российская детская клиническая больница», Российский национальный медицинский университет им. Н. И. Пирогова, Москва, Россия<br> <sup>2</sup> Научно-практический центр специализированной медицинской помощи им. В. Ф. Войно-Ясенецкого, Москва, Россия<br> <sup>3</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>4</sup> Национальный медицинский исследовательский центр им. В. А. Алмазова, Санкт-Петербург, Россия<br> <sup>5</sup> Санкт-Петербургский государственный педиатрический медицинский университет, Санкт-Петербург, Россия<br> <sup>6</sup> Национальный медицинский исследовательский центр онкологии им. Н. Н. Блохина, Москва, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1498) "1 Обособленное структурное подразделение «Российская детская клиническая больница», Российский национальный медицинский университет им. Н. И. Пирогова, Москва, Россия
2 Научно-практический центр специализированной медицинской помощи им. В. Ф. Войно-Ясенецкого, Москва, Россия
3 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
4 Национальный медицинский исследовательский центр им. В. А. Алмазова, Санкт-Петербург, Россия
5 Санкт-Петербургский государственный педиатрический медицинский университет, Санкт-Петербург, Россия
6 Национальный медицинский исследовательский центр онкологии им. Н. Н. Блохина, Москва, Россия
1 Обособленное структурное подразделение «Российская детская клиническая больница», Российский национальный медицинский университет им. Н. И. Пирогова, Москва, Россия
2 Научно-практический центр специализированной медицинской помощи им. В. Ф. Войно-Ясенецкого, Москва, Россия
3 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
4 Национальный медицинский исследовательский центр им. В. А. Алмазова, Санкт-Петербург, Россия
5 Санкт-Петербургский государственный педиатрический медицинский университет, Санкт-Петербург, Россия
6 Национальный медицинский исследовательский центр онкологии им. Н. Н. Блохина, Москва, Россия
Introduction
Myelodysplastic syndrome (MDS) represents a heterogenous group of clonal diseases caused by alterations in hematopoietic stem cells due to hereditary predisposal, along with variable somatic gene mutations, and/or abnormal epigenetic regulation, including those induced by altered microenvironment and disrupted immune surveillance [1].
Pre-leukemic features of MDS were explored since early 80’s based on atypical in vitro growth of hematopoietic precursor cells from MDS patients [2-4]. Further on, some newer laboratory techniques, like flow cytometry and next-generation sequencing (NGS), allowed better insight into the distinct pathological events underlying MDS development. Over last decades, with increased lifespan, a number of peripheral blood cytopenias were found to precede clinical MDS, especially, in the patients >65 years old, i.e., ICUS (idiopathic cytopenia of undetermined significance); CHIP (Clonal hematopoiesis of indeterminate potential); CCUS (clonal cytopenia of undetermined significance). Such conditions are determined by the age-dependent accumulation of somatic mutations which may play a role in subsequent MDS deve- lopment.
These disorders may be transformed to hematological malignancy at a frequency of ca. 0.5-1% per year [5-7]. The majority of associated gene mutations (e.g., DNMT3A, TET2, ASXL1, TP53, and JAK2) affect RNA splicing or epigenetic regulation. However, patients with long-term cytopenias and somatic mutations do not always exhibit morphological changes of blood cells corresponding to MDS criteria. Moreover, only distinct cytogenetic aberrations (del5q) and point mutations (SF3B1) are considered specific for MDS, whereas other mutations are relatively non-specific and could be revealed in other pathologies, e.g., in the myeloproliferative disorders [8] or aplastic anemia (Fig. 1). In the latter case, MDS frequency was demonstrated to be sufficiently higher than in general population which is determined by the mutational profile [9].
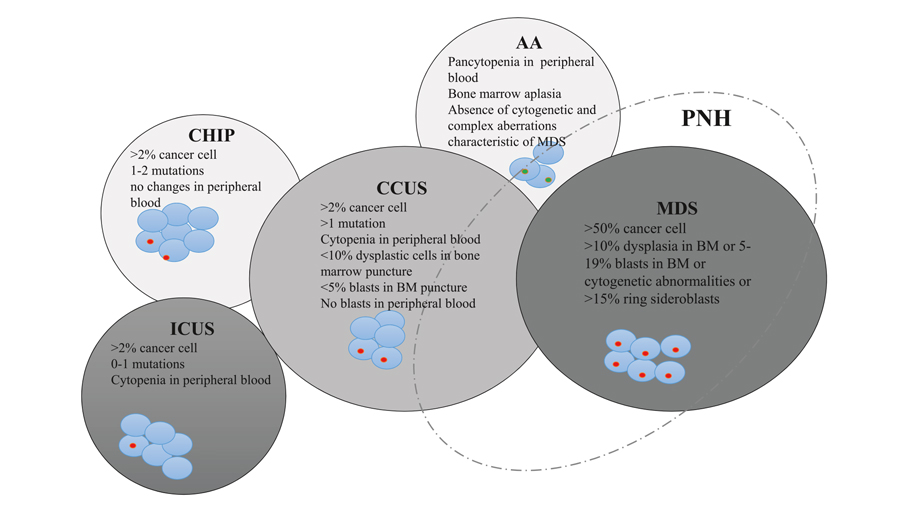
Figure 1. Interactions and characteristic features of clonal hematopoiesis of indetermined potential (CHIP), idiopathic cytopenia of undetermined significance (ICUS), clonal cytopenia of undetermined significance (CCUS), aplastic anemia (АА), and myelodysplastic syndrome (MDS). Adapted from Young N., 2002 [13]
Occurrence of additional potentially pathogenic mutations is associated with increased MDS risk [10]. At diagnosis, two or more marker somatic mutations can be determined in most MDS patients [11]. In parallel to additional mutagenesis and clonal evolution of hematopoietic cells, the clinical manifestations undergo several stages, from a cytopenia of undetermined significance to MDS, and, in some cases, to leukemic transformation into acute myeloid leukemia (AML) [12]. Due to this complex pathogenesis effective target therapy is still not available for this group of diseases despite better awareness of MDS development mechanisms.
Hematopoietic cell transplantation and current therapeutic options
At the present time, allogeneic HSCT is the only curative treatment method for MDS. However, patients with MDS still remain the most complicated candidates for allo-HSCT, due to a number of additional unfavorable factors, including advanced age of the patients. MDS is more common in the patients over 65 years old, which determines a sufficient number of co-morbidities. In addition, preceding prolonged therapy is associated with significant number of blood transfusions with high frequency of clinical and laboratory signs of iron overload, as well as immunization due to these transfusions. In clinical practice, reduced-intensity conditioning regimens are often applied, taking into account somatic state of patients [14]. However, hematological remission is not achieved at the time of allo-HSCT in the majority of patients.
Along with gene mutations in stem cells, a special feature of MDS pathogenesis are certain defects of hematopoietic microenvironment, which alter functioning of hematopoietic niches [15]. These alterations contribute to higher incidence of graft failure, including primary graft failure and severe poor graft function after engraftment, as well as increased probability of early relapse. Long cytopenias after allo-HSCT is associated with higher risk of infectious complications, which are the main cause of posttransplant mortality in MDS patients. All issues described above determine higher post-transplant mortality in MDS compared to the other groups of HSCT recipients [16, 17]. Moreover, a significant proportion of patients is lacking HLA-compatible related or unrelated donor, and efficiency of haploidentical HSCT in MDS is still not supported by large studies [18]. According to the current European Blood and Marrow Transplantation (EBMT) guidelines, allo-HSCT from haploidentical donor is a clinical option and could be considered only after thorough evaluation of potential risks and benefits associated with this procedure.
Nonetheless, allo-HSCT facilitates cure in 30-40% of MDS patients. Several studies are ongoing aiming to improve allo-HSCT outcomes with posttransplant relapse preventive strategies [19, 20].
Due to clinical heterogeneity of MDS and risks associated with HSCT procedure, the strategy of treatment is largely personalized, dependent on the prognostic group, according to national and international recommendations [21, 22].
In low- and very low-risk patients with predominant clinical signs of anemia, the main therapeutic goal is to reduce transfusion dependence and to prevent organ damage caused by iron overload. Erythropoiesis-stimulating agents (ESA) could be administered, which, however, are clinically effective in about 1/3 of the patients, according to the multicenter studies [23]. The responders are predominantly those with initially low erythropoietin levels (<200 ng/ml) and the median duration of clinical response is about 1 year. Some studies suggest the possibility of decreased transfusion requirements with chelator therapy [24]. However, a randomized study did not show any improvement of erythropoietic response compared to ESA monotherapy [25].
In low-risk MDS cases with 5q- chromosomal aberration, immunomodulating therapy may result into cytogenetic remission in about 50% of the patients, with 2/3 of cases becoming transfusion-independent [26, 27]. In other low-risk MDS variants, there are only several single-center studies, where transfusion independence was achieved after short courses of hypomethylating agents (HMA) [28], and immunosuppressive treatment (IST), including cyclosporine A and anti-thymocyte globulin [29]. The median duration of clinical response using these therapies was, respectively, 16 and 18 months. Only small proportion of MDS patients has long-term remissions, either with HMA or IST protocols. However Kokhno A. et al. have shown that IST proved to be more effective in the patients with hypocellular bone marrow, or non-uniform cellularity with hypoplastic/aplastic areas, in absence of poor and very poor karyotype abnormalities according to IPSS-R scale, or without 7q-, i(17q) aberration [30].
In high-risk MDS, low-dose cytosine arabinoside (LDAC) treatment was a long-standing approach to therapy in myeloid dysplasia since mid-80’s [31]. Median survival time in clinical studies was ca. 8 months for these cohorts, with maximal lifespan of 2-3 years and frequency of clinical responses of 15 to 30%. However, some studies did not show any differences in survival rates between LDAC protocol and best supporting therapy [32-34]. At the present time, HMA became another standard of therapy in high-risk MDS. In registration randomized studies, decitabine and 5-azacitidine were associated with prolonged median survival by 2.7 and 10 months, respectively, compared with best available treatment [35, 36]. Nevertheless, large clinical studies, e.g., Surveillance, Epidemiology, and End Result‐Medicare database embracing 532 MDS high-risk patients, have shown somewhat higher results, i.e., median overall survival (OS) of, respectively, 11 and 12 months for decitabine and 5-azacitidine [37]. Despite MDS complete remissions are rare in HMA-treated MDS patients, these drugs enable temporary control of the disease with good quality of life and low hematological toxicity. Moreover, in the context of allo-HSCT, HMA may be effective in the pre-transplant period, by improving the patient’s state during search and activation of potential donors without increase of hematological toxicity [38].
There also are some studies on improvement of platelet counts with supplementary treatment with Eltrombopag, however, without evidence of higher response rate [39, 40].
Despite improvements with HMA introduction, recent studies in fundamental biology and pathogenesis of MDS revealed potential opportunities for studying novel treatments able to modify specific signaling and metabolic pathways, as well as hematopoietic and immune microenvironment.
Transforming growth factor-beta (TGFβ) antagonists
TGFβ antagonists, e.g. luspatercept, proved to be potentially effective in low-risk MDS with transfusion dependence, given the key role of TGFβ ligands in hematopoiesis. This drug represents a recombinant protein able to bind the TGFβ superfamily ligands, thus blocking SMAD2 and SMAD3 signaling pathways, regulating differentiation and maturation of erythroid precursors [41, 42]. These pathways play an important role in MDS pathogenesis by inhibition of SMAD7 and SKI expression [43, 44]. Luspatercept binds TGFβ ligands, that abrogating negative erythropoiesis regulation, accelerating events during late RBC maturation, unlike erythropoietin which regulates early stages of erythropoiesis [45], as shown in Fig. 2.
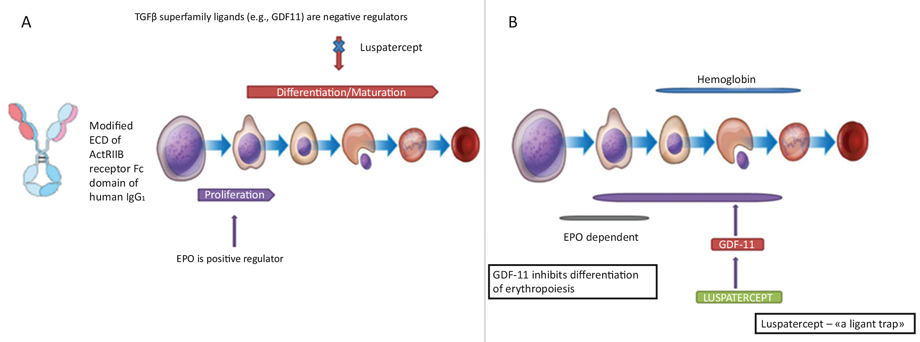
Figure 2. Hematopoietic effects of luspatercept (A); Molecular mechanisms of luspatercept action (B)
A general reduction in transfusion dependence was demonstrated in phase II clinical trial PACE-MDS with 29% of cases being with high transfusion requirements. Independence of blood transfusions was achieved in 36% of the cases. In particular, clinical responses were more often observed among patients with marrow ring sideroblasts and SF3B1 mutation [46].
In the phase 3 MEDALIST study, 37.9% of low-risk MDS patients became transfusion-independent in the treatment with luspatercept compared to 13.2% in the placebo group. According to the results of longitudinal observation (MEDALIST study), independence on red blood cell (RBC) transfusions maintained for at least 8 weeks at any period of treatment, and it was more frequent among patients treated with luspatercept (47.7%) than in placebo group (15.8%). Approximately 1 year after initiation of luspatercept, 31.4% did not require RBC transfusions, against 0% in placebo group. Among luspatercept-treated patients, the overall transfusion-free period and clinical improvement was observed, independent on previous transfusion burden. Transfusion independence persisted in 12 cases (7.8%) out of 153 patients who received luspatercept after 48 weeks of follow up. Some adverse effects associated with luspatercept administration rarely caused early discontinuation of therapy, and their occurrence decreased over time. Frequency of progression to AML (5%) was similar for luspatercept-treated patients and placebo group [47].
Another randomized phase III study COMMANDS is ongoing, where a comparison is made between luspatercept and erythropoietin α, the current standard of therapy [48]. The results of this study may influence the subsequent treatment standards in low-risk MDS patients. Similarly, some preclinical and clinical studies of kinase inhibitors involved into the TGFβ signal pathway are currently underway.
Galunisertib, an inhibitor of ALK5 kinase related to the signal transduction for TGFβ receptor activation, was studied in phase 2 clinical trials in low- and intermediate risk MDS, according to the IWG 2000 criteria. In 43.9% of patients, erythroid response was observed, hematological improvement was registered in 24.4% of cases, along with reduction of weakness in 44% of the patients. The results may justify its application in transfusion-dependent MDS patients who are non-responding to ESA [49]. Thus, a new class of TGFβ-directed drugs can be registered in the near future. The ongoing studies will show if these drugs are applicable only in MDS with ring sideroblasts, or they could be extended to other subgroups of low-risk MDS patients.
Another agent for low-risk MDS in late clinical trials is Roxadustat. It is a protein factor regulating HIF-1α (hypoxia-inducible factor-α) [50]. The drug influences erythropoietin production and iron uptake from macrophages, enhances iron metabolism, stabilizes blood HIF levels and prevents its degradation, thus promoting erythropoiesis [51]. Interim results of a multicenter study involved 24 patients with low-risk MDS and demonstrated a decrease of transfusion dependence by 50% [52]. Roxadustat inhibits HIF-α decay followed by its dimerization with HIF-β and nuclear translocation to cellular nucleus where the response to hypoxia is mediated at the transcriptional level (Fig. 3).
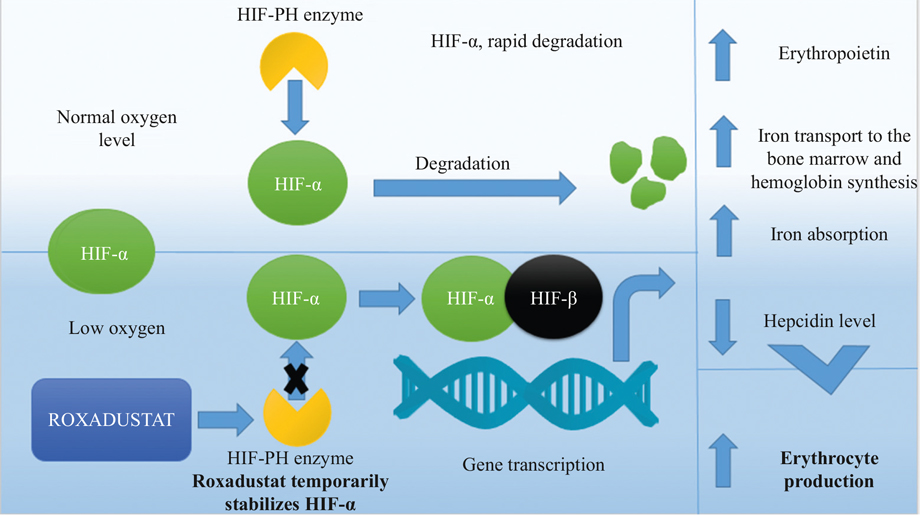
Figure 3. Main effects of roxadustat upon different organs and cells
Immune checkpoint inhibitors
The inhibitors of immune checkpoints (ICP) are highly effective in a treatment of some solid tumors. Their versatile effects are based on reactivation of exhausted immune cells via blocking appropriate inhibitory signal, leading to a recovery of antitumor immunity. Nevertheless, the characteristics and expression of different ligands of ICP strongly depends on the type of malignancy. Pronounced clinical response to ICP is prominent in tumors with high neo-antigen contents, or amplification of genes encoding ICP ligands. Highest sensitivity to ICP treatment was demonstrated for Hodgkin’s lymphoma, melanoma, cancers of urinary tract, lung cancer, head and neck malignancies, solid cancers with microsatellite instability [53]. There are usually only a small number of somatic mutations in MDS patients despite variable mutation profile [11], however hematopoietic cells in MDS exhibit high expression of the ICP ligands which may be increased during HMA therapy [54, 55].
Nonetheless, the ICP monotherapy was not associated with significant response in clinical trials [56]. The failure of nivolumab or ipilimumab monotherapy may be due to simultaneous expression of several inhibitors of immune response as confirmed by experimental data, including our own results [57]. Garcia-Manero G. et al. have shown the overall response rate (69%) to 5-azacitidine+nivolumab treatment in MDS, including complete remission in 2 patients. Higher response rate in this combined treatment schedule correlates with induction of neoantigen expression in tumor cells under HMA, thus enhancing the T cell immune response.
Failure of ICP therapy in MDS could be also determined by immunosuppressive effects in the hematopoietic niches. As possible mechanisms, one may consider high indoleamine-pyrrole 2,3-dioxygenase (IDO) expression in cellular microenvironment, T cell differentiation towards regulatory T cells, low interferon-α expression by T cells, induction of T cell apoptosis due to activation of CD33-S100A9 signaling, increased levels of myeloid suppressor cells [58-68]. In future, ICP-based treatment seems to enter the standards of MDS therapy, however, not as monotherapy, but as combined therapeutic schedules, e.g., with PD-1, CTLA4 and TIM3 inhibitors [69]. Clinical trials with anti-TIM3 monoclonal antibodies are underway now. A multicenter study has shown that combined treatment with MBG453В, an anti-TIM3 antibody, and decitabine resulted into hematological or molecular remission in 50% of high-risk MDS patients [70].
Macrophage ICPs represent a fundamentally new class of such regulatory molecules. CD47, being expressed on macrophages, is a key molecule able to inhibit their response, and its interaction with SIRPα ligand protein causes suppression of phagocytic function. This effect is called a blocking "don't eat me" signal of CD47-SIRPα. This signal pathway is active during interactions between hematopoietic cells and macrophages, also serving as a tolerance mechanism in hematopoietic malignancies [71]. Magrolimab, or 5F9 antibody, is a humanized monoclonal antibody (MAb) which blocks CD47 and activates SIRPα pathway, promotes phagocytosis of tumor cells. Combined application of this drug with 5-azacitidine in preclinical model of acute myeloid leukemia (AML) has shown high survival rates in laboratory animals [72]. During the Phase 1 clinical trial, 5-azacitidine and Magrolimab was administered to 35 high-risk MDS patients. The response was evaluated in 24 patients, with hematological response in 92% and complete remission in 50% of the cases. Further observations are required to assess duration of the responses [73].
Distinct effects of the CD47-blocking antibodies are considered, as follows: (a) under normal conditions, both healthy and malignant cells are avoiding phagocytosis by CD47 expression. CD47 is overexpressed by cancer cells for protection from eat me/prophagocyte signals. (b) After the Mab-induced CD47 blockage, the malignant cells are phagocytized, thus causing exposure of the eat me signal. By contrast, normal cells remain intact due to absent expression of prophagocytic signals.
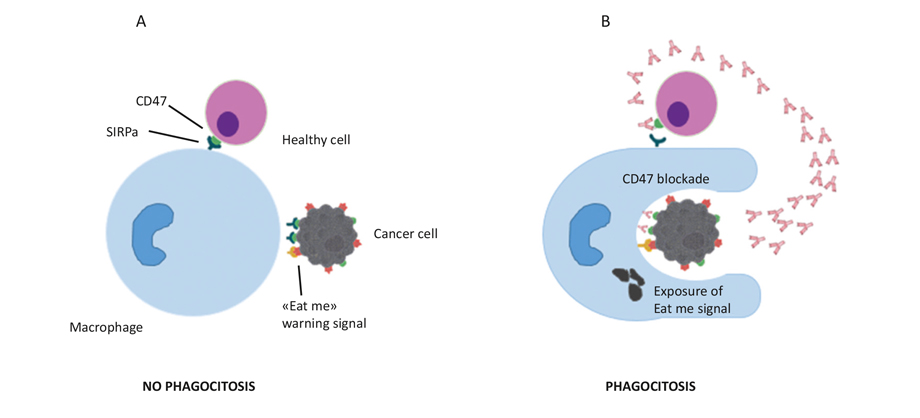
Figure 4. Possible effects of CD47-blocking antibodies upon immune cells
Inhibitors of bcl2, hedgehog, IDH and other molecular targets
Over last years, a significant role of bcl2 in AML progression and drug resistance was elucidated. Venetoclax, a specific bcl2 inhibitor, was shown to increase the rates of complete remission in combination with LDAC or HMA [74, 75]. However, the results observed in AML cannot be blindly extended to MDS. BCL2 inhibition in an MDS models leads to suppression of apoptosis in hematopoietic cells and transition to the resting phase of a cell cycle. As a result a reduced DNA damage of erythropoietic precursors was observed. However, some doubts exist since apoptosis blockage may promote faster transition to AML [76].
Overall response to venetoclax was 21% in a group of chemotherapy-refractory AML and MDS patients [77]. Clinical studies are ongoing with early venetoclax and HMA administration in MDS. So far only the results of non-randomized studies are published demonstrating promising overall and complete remission rates (59% and 14%, respectively) even in HMA-exposed patients [78].
Glasdegib, another inhibitor of signaling pathways, was registered in 2019 for therapy of AML and high-risk MDS in combination with chemotherapy. The drug inhibits Hedgehog (Hh) pathway previously described as an embryogenesis regulator, since Hh proteins are involved in cell and tissue differentiation. Like other intracellular signaling systems, the Hh pathway plays an important role in cell cycle regulation of malignant cells and is involved into the mechanisms of chemotherapy resistance [79].
In a Phase I study, 31% of AML and high-risk MDS patients have achieved complete remission during the glasdegib therapy combined with LDAC and decitabine [80]. Complete remission was achieved in 46% of the patients with similar disorders in another study using glasdegib combined with systemic 7+3 chemotherapy [81]. In a randomized study comparing glasdegib plus LDAC against LDAC a 3-month increase in OS was demonstrated, along with long-term stabilization of the disease in some patients. The rates of response to glasdegib monotherapy in refractory MDS comprise only 6% [82]. Chaudhry et al. noted that therapeutic activity of glasdegib requires expression of GLI3 suppressor gene which may be hypermethylated in MDS and AML. Determination of GLI3 expression may serve as predictor of response to glasdegib treatment [83]. Despite relatively low efficiency, glasdegib is well tolerated, thus allowing to suggest it as a component of combined therapy in MDS.
About 5% of MDS cases are associated with IDH1 and IDH2 gene mutations. The mutated IDH variants are associated with excessive production of R2-hydroxyglutarate which causes functional insufficiency of TET2 gene [84, 85]. Presently, two IDH inhibitors for oral administration are under clinical trials, enasidenib, and ivosidenib (respectively for IDH2 and IDH1 inhibition). The Phase II study in AML and high-risk MDS patients, enasidenib therapy was associated with response in 53% of the patients including complete remission in 7% [86]. There are no preliminary results on ivosidenib in MDS at the present moment. However, in elderly AML ivosidenib induced complete remission, including one with partial hematologic recovery, in 42.4% of patients and median duration of remission was not reached with 2-year follow up [87].
Rigosertib is another clinically tested inhibitor of signal pathways which is able to suppress several kinases, e.g., Akt, PI3K. A clinical study in MDS patients has demonstrated reduction of blastosis [88]. However, Phase III study in HMA-resistant patients did not show any differences in survival between Rigosertib treatment and best available therapy [89]. At present, Rigosertib is tested in combination with 5-azacitidine [90].
A small group of MDS patients exhibits FLT3 mutation [11]. These patients are prone to rapid transformation to AML, thus precluding data accumulation on clinical efficiency of FLT3 inhibitors in this MDS variant. Nevertheless, the MDS experience shows that addition of Midostaurin to chemotherapy is associated with 8% increase in relapse-free survival [91]. The response rate in combined therapy with 5-azacitidine was 26% [92]. Meanwhile, the second-generation FLT3 inhibitors (Gilteritinib and Quizartinib) demonstrate more optimistic results [93, 94], thus creating the basis for addition of these agents to standard therapy in MDS patients with FLT3 mutations.
Summarizing the overview of developing targeted therapies, it is important to mention that few of them induce high complete remission rate and these complete remissions are not durable in the high proportion of the patients. When keeping in mind the complex pathogenesis of this disease, it is clear that complex approaches to therapy are required. The successful examples of other hematological diseases give us hope for long-term improvement of survival in MDS (Fig. 5).
Figure 5. Potential drug combinations in personalized therapy of high-risk MDS
Allogeneic HSCT as a platform for immune therapy in MDS patients
Along with development of novel therapeutic molecules, some feasible options of cellular therapy are under investigation in MDS [95]. At the moment, allo-HSCT is the only cellular therapy in MDS which is widely used in clinical practice. However, allo-HSCT is a high-risk procedure with potentially severe complications that may cause sufficiently decreased quality of life and shorter survival of the patients. This issue is especially important due to advanced age of most MDS patients, thus increasing risk for dismal outcome.
To evaluate the impact of allo-HSCT on survival in MDS we compared the survival of two contemporary cohorts of patients treated at RM Gorbacheva Research Institute. The first cohort agreed to allograft procedure while the second refused to undergo allo-HSCT and was treated with available therapies. Although the groups were not well matched, however represent the real life clinical practice at large HSCT center. The comparison of these two groups revealed that two-year OS in MDS patients without allo-HSCT was 36.7% (n=68), while it was 69.1% (n=83) among the patients after HSCT (p<0.05) (Fig. 6A). In allo-HSCT group, the 2-year OS was comparable in IPPS-R intermediate-2 versus high-risk groups (n=62), and intermediate-1 versus low-risk groups (n=21, 68.2% vs 71.4%, respectively). Two-year overall survival was lower in similar patient groups without allo-HSCT: 31.2% (n=43) and 47% (n=25, p<0.05), respectively (Fig. 6B). The survival rates following HSCT depended on the following items: disease status, graft composition, infectious complications, acute GvHD grade I-II [16]. Interestingly, the evaluation of all existing MDS prognostic scales in our MDS allo-HSCT group did not exert any significant effects on survival after allo-HSCT. This observation highlights a necessity to validate developing scales in the center- or country-stratified manner. Hence, it is clear that the risk-adapted strategies in MDS patients are of extreme importance.
Figure 6. Two-year overall survival of MDS patients depending on allo-HSCT performed (A). The two-year overall survival of MDS patients depending on allo-HSCT performed and IPSS risk group (B)
So far, it is unclear how the role of allo-HSCT in MDS will change. It can follow the track of chronic lymphocytic leukemia [96] and Hodgkin’s lymphoma [97] where effective bridging before allo-HSCT brought the results of allo-HSCT in refractory disease close to the results of primary treatment in these malignancies. In this situation, allo-HSCT will be more broadly used in MDS. On the other hand, combination therapies can bring durable remissions to this difficult population of patients. In this case allo-HSCT will be brought from first line to second or subsequent lines of therapy like it happened with chronic myeloid leukemia [98]. Given the preliminary favorable results of allo-HSCT in MDS after therapy with ICPs [99], it is likely that at least the first scenario with effective bridging therapies will be implemented in the near future.
Conclusion
With respect to recent findings and genetic studies made by means of NGS techniques, some radical changes are expected in MDS therapies. The drug selection will be based on evaluation of mutational profile, expression of checkpoint molecules and methylation profile. The results of these studies will determine a combination of target agents, ICP inhibitors and hypomethylating agents. It is also clear that allo-HSCT will remain in MDS clinical practice, however its place in a sequence of therapies will be rapidly changing.
Acknowledgements
The work was supported by Russian Science Foundation grant 17-75-20145-П.
Conflict of interest
The authors declare no conflicts of interest.
References
- Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat Rev Cancer. 2017;17(1):5-19. doi:10.1038/nrc.2016.112.
- Tiranova SA, Alekseev NA, Petrova EM, et al. On the question of the existence of hematopoietic dysplasia (preleukemia) in children. Terapevticheskii arkhiv. 1982;54(8):1-16 (In Russian).
- Afanasyev BV, Tiranova SA, Kulibaba TG, et al. Cloning of human hematopoietic cells in agar drop-liquid medium system. Terapevticheskii arkhiv. 1983;54(8):114-22 (In Russian).
- Kleihauer E. The preleukemic syndromes (hematopoietic dysplasia) in childhood. Eur J Pediatr. 1980;133(1):5-10.
- Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44(11):1179-1181. doi:10.1038/ng.2413.
- Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477-2487. doi:10.1056/NEJMoa1409405.
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498. doi:10.1056/NEJMoa1408617.
- Malcovati L, Cazzola M. The shadowlands of MDS: idiopathic cytopenias of undetermined significance (ICUS) and clonal hematopoiesis of indeterminate potential (CHIP). Hematology Am Soc Hematol Educ Program. 2015;2015:299-307. doi:10.1182/asheducation-2015.1.299.
- Afanasyev BV, Zubarovskaya L. Pediatric myelodysplastic syndrome. Russian Journal of Pediatric Hematology and Oncology. 2018;5(3):23-35. (In Russian). doi: 10.17650/2311-1267-2018-5-3-23-35.
- Malcovati L, Gallì A, Travaglino E, Ambaglio I, Rizzo E, Molteni E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129(25):3371-3378. doi:10.1182/blood-2017-01-763425.
- Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616-3699. doi:10.1182/blood-2013-08-518886.
- Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019;54(10):1525-1552. doi:10.1038/s41409-019-0516-2.
- Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136(7):534-546. doi:10.7326/0003-4819-136-7-200204020-00011.
- Kröger N, Iacobelli S, Franke GN, Platzbecker U, Uddin R, Hübel K, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: A prospective randomized Phase III Study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35(19):2157-2164. doi:10.1200/JCO.2016.70.7349.
- Li AJ, Calvi LM. The microenvironment in myelodysplastic syndromes: Niche-mediated disease initiation and progression. Exp Hematol. 2017;55:3-18. doi:10.1016/j.exphem.2017.08.003.
- Morozova EV, Barabanshikova MV, Tcvetkov NY, Melsitova KV, Rudnitskaya JV, Darskaya EI, et al. Application of standard and novel prognostic systems in patients with myelodysplastic syndrome undergoing allogeneic hematopoietic stem cell transplantation. Cell Ther Transplant 2019; 8(1): 36-45. doi: 10.18620/ctt-1866-8836-2019-8-1-36-45.
- Kröger N, Zabelina T, de Wreede L, Berger J, Alchalby H, van Biezen A, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia. 2013;27(3):604-609. doi:10.1038/leu.2012.210.
- Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30(10):2055-2063. doi:10.1038/leu.2016.110.
- El-Cheikh J, Massoud R, Fares E, Kreidieh N, Mahfouz R, Charafeddine M, et al. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT. Bone Marrow Transplant. 2017;52(6):918-921. doi:10.1038/bmt.2017.31.
- Ovechkina VN, Bondarenko SN, Morozova EV, et al. The role of hypomethylating agents prior to allogeneic hematopoietic stem cells transplantation in acute myeloid leukemia and myelodysplastic syndrome. Clinical Oncohematology. 2017;10(3):351–357 (In Russian). doi: 10.21320/2500-2139-2017-10-3-351-7.
- Savchenko VG, Parovichnikova EN, Kokhno AV, et al. National clinical guidelines for the diagnosis and treatment of myelodysplastic syndromes adults. Hematology and Transfusiology (Gematologiya i transfusiologiya). 2016; 61 (1-32). doi: 10.18821/0234-5730-2016-61-1 (In Russian).
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. doi:10.1182/blood-2012-03-420489.
- Fenaux P, Santini V, Spiriti MAA, Giagounidis A, Schlag R, Radinoff A, et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-α in anemic patients with low-risk MDS. Leukemia. 2018;32(12):2648-2658. doi:10.1038/s41375-018-0118-9.
- Latagliata R, Montagna C, Porrini R, Di Veroli A, Leonetti SC, Niscola P, et al. Chelation efficacy and erythroid response during deferasirox treatment in patients with myeloproliferative neoplasms in fibrotic phase. Eur J Haematol. 2016;96(6):643-649. doi:10.1111/ejh.12674.
- Gattermann N, Coll R, Jacobasch L, Allameddine A, Azmon A, DeBonnett L, et al. Effect of deferasirox + erythropoietin vs erythropoietin on erythroid response in Low/Int-1-risk MDS patients: Results of the phase II KALLISTO trial [published online ahead of print, 2018 May 19]. Eur J Haematol. 2018;10.1111/ejh.13096. doi:10.1111/ejh.13096.
- Garcia-Manero G, Almeida A, Fenaux P, Gattermann N, Giagounidis A, Goldberg SL, et al. Clinical benefit-risk profile of lenalidomide in patients with lower-risk myelodysplastic syndromes without del(5q): Results of a Phase III Trial. Clin Lymphoma Myeloma Leuk. 2019;19(4):213-219.e4. doi:10.1016/j.clml.2018.12.012.
- Lee JH, List A, Sallman DA. Molecular pathogenesis of myelodysplastic syndromes with deletion 5q. Eur J Haematol. 2019;102(3):203-209. doi:10.1111/ejh.13207.
- Jabbour E, Short NJ, Montalban-Bravo G, Huang X, Bueso-Ramos C, Qiao W, et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood. 2017;130(13):1514-1522. doi:10.1182/blood-2017-06-788497.
- Passweg JR, Giagounidis AA, Simcock M, Aul C, Dobbelstein C, Stadler M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol. 2011;29(3):303-309. doi:10.1200/JCO.2010.31.2686.
- Kokhno AV, Parovichnikova EN, Mikhailova EA, et al. Efficiency of cyclosporin A therapy in patients with myelodysplastic syndrome. Terapevticheskii Arkhiv. 2010; 82(8): 48-53 (In Russian).
- Patterson D, Kravtsova VM, Petrova EM, et al. Therapy of patients with myeloid leukemia with low doses of cytosine arabinoside. Terapevticheskii Arkhiv. 1987; 12: 81-86 (In Russian).
- Gerhartz HH, Marcus R, Delmer A, Zwierzina H, de Witte T, Jacobs A, et al. Treatment of myelodysplastic syndromes (MDS) and high leukaemic risk with low-dose cytosine arabinoside (LD-AraC) plus granulocyte-macrophage colony-stimulating factor (rh GM-CSF). The EORTC Leukaemia Group. Infection, 1992; 20 (Suppl 2):S116-S123. doi:10.1007/BF01705030.
- Zwierzina H, Suciu S, Loeffler-Ragg J, Neuwirtova R, Fenaux P, Beksac M, et al. Low-dose cytosine arabinoside (LD-AraC) vs LD-AraC plus granulocyte/macrophage colony stimulating factor vs LD-AraC plus Interleukin-3 for myelodysplastic syndrome patients with a high risk of developing acute leukemia: final results of a randomized phase III study (06903) of the EORTC Leukemia Cooperative Group. Leukemia. 2005;19(11):1929-1933. doi:10.1038/sj.leu.2403934.
- Kokhno AV, Parovichnikova EN, Mikhailova EA, et al. Myelodysplastic syndromes and aplastic anemia. In the manual "Programmed treatment of diseases of the blood system: Collection of diagnostic algorithms and treatment protocols for diseases of the blood system" (ed. by VG Savchenko). State Research Center of the Russian Healthcare Ministry; Moscow: Practice (2012): 83-150 (In Russian).
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. doi:10.1200/JCO.2011.38.9429.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009; 10(3):223-232. doi:10.1016/S1470-2045(09)70003-8.
- Zeidan AM, Davidoff AJ, Long JB, Hu X, Wang R, Ma X, et al. Comparative clinical effectiveness of azacitidine versus decitabine in older patients with myelodysplastic syndromes. Br J Haematol. 2016;175(5):829-840. doi:10.1111/bjh.14305.
- Field T, Perkins J, Huang Y, Kharfan-Dabaja MA, Alsina M, Ayala E, et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010;45(2):255-260. doi:10.1038/bmt.2009.134.
- Mittelman M, Platzbecker U, Afanasyev B, et al. Eltrombopag for advanced myelodysplastic syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. Lancet Haematol. 2018;5(1):e34-e43. doi:10.1016/S2352-3026(17)30228-4.
- Dickinson M, Cherif H, Fenaux P, Mittelman M, Verma A, Portella MSO, et al. Azacitidine with or without eltrombopag for first-line treatment of intermediate- or high-risk MDS with thrombocytopenia. Blood. 2018; 132(25):2629-2638. doi:10.1182/blood-2018-06-855221.
- Muench DE, Ferchen K, Velu CS, Pradhan K, Chetal K, Chen X, et al. SKI controls MDS-associated chronic TGF-β signaling, aberrant splicing, and stem cell fitness. Blood. 2018;132(21):e24-e34. doi:10.1182/blood-2018-06-860890.
- Zermati Y, Fichelson S, Valensi F, Freyssinier JM, Rouyer-Fessard P, Cramer E, et al. Transforming growth factor inhibits erythropoiesis by blocking proliferation and accelerating differentiation of erythroid progenitors. Exp Hematol. 2000;28(8):885-894. doi:10.1016/s0301-472x(00)00488-4.
- Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, Fazzari M, et al. Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71(3):955-963. doi:10.1158/0008-5472.CAN-10-2933.
- Bachegowda L, Gligich O, Mantzaris I, Schinke C, Wyville D, Carrillo T, et al. Signal transduction inhibitors in treatment of myelodysplastic syndromes. J Hematol Oncol. 2013;6:50. Published 2013 Jul 10. doi:10.1186/1756-8722-6-50.
- Hanke T, Wong JF, Berger BT, Abdi I, Berger LM, Tesch R, et al. A highly selective chemical probe for activin receptor-like kinases ALK4 and ALK5. ACS Chem Biol. 2020;15(4):862-870. doi:10.1021/acschembio.0c00076.
- Platzbecker U, Germing U, Götze KS, Kiewe P, Mayer K, Chromik J, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017;18(10):1338-1347. doi:10.1016/S1470-2045(17)30615-0.
- Fenaux P, Platzbecker U, Mufti GJ, Garcia-Manero G, Buckstein R, Santini V, et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N Engl J Med. 2020;382(2):140-151. doi:10.1056/NEJMoa1908892.
- https://clinicaltrials.gov/ct2/show/NCT03682536, as of 12/2019.
- Santini V, Valcárcel D, Platzbecker U, Komrokji RS, Cleverly AL, Lahn MM, et al. Phase II study of the ALK5 inhibitor galunisertib in very low-, low-, and intermediate-risk myelodysplastic syndromes. Clin Cancer Res. 2019; 25(23):6976-6985. doi:10.1158/1078-0432.CCR-19-1338.
- https://clinicaltrials.gov/ct2/show/NCT03263091, as of 12/2019.
- Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32(8):1373-1386. doi:10.1093/ndt/gfx011.
- Henry DH, Glaspy J, Harrup RA, Mittelman M, Zhou A, Bradley C, et al. Roxadustat (FG4592; ASP1517; AZD9941) in the treatment of anemia in patients with lower risk myelodysplastic syndrome (LR-MDS) and low red blood cell (RBC) transfusion burden (LTB). Blood 2019; 134 (Supplement_1): 843. doi: 10.1182/blood-2019-128714.
- Allison JP. Checkpoints. Cell. 2015;162(6):1202-1205. doi:10.1016/j.cell.2015.08.047.
- Chokr N, Patel R, Wattamwar K, Chokr S. The rising era of immune checkpoint inhibitors in myelodysplastic syndromes. Adv Hematol. 2018;2018:2458679. doi:10.1155/2018/2458679.
- Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280-1288. doi:10.1038/leu.2013.355.
- Garcia-Manero G, Daver NG, Montalban-Bravo G, Jabbour EJ, DiNardo CD, Kornblau SM, et al. A Phase II Study evaluating the combination of Nivolumab (Nivo) or Ipilimumab (Ipi) with Azacitidine in patients with previously treated or untreated myelodysplastic syndromes (MDS). Blood 2016; 128 (22): 344. doi: 10.1182/blood.V128.22.344.344.
- Tcvetkov N, Gusak A, Morozova E, et al. Immune checkpoints bone marrow expression as the predictor of clinical outcome in myelodysplastic syndrome. Leuk Res Rep. 2020;14:100215. Published 2020 Jun 28. doi:10.1016/j.lrr.2020.100215.
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619-4621. doi:10.1182/blood-2003-11-3909.
- Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, et al. Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One. 2011;6(2):e14698. Published 2011 Feb 16. doi:10.1371/journal.pone.0014698.
- Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients' sera by HPLC and is inducible by IFN-gamma. Leuk Res. 2009;33(3):490-494. doi:10.1016/j.leukres.2008.06.014.
- Yuan Y, Lu X, Tao CL, Chen X, Shao HW, Huang SL. Forced expression of indoleamine-2,3-dioxygenase in human umbilical cord-derived mesenchymal stem cells abolishes their anti-apoptotic effect on leukemia cell lines in vitro. In Vitro Cell Dev Biol Anim. 2013;49(10):752-758. doi:10.1007/s11626-013-9667-4.
- Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109(7):2871-2877. doi:10.1182/blood-2006-07-036863.
- Schnorfeil FM, Lichtenegger FS, Emmerig K, Schlueter M, Neitz JS, Draenert R, et al. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J Hematol Oncol. 2015;8:93. Published 2015 Jul 30. doi:10.1186/s13045-015-0189-2.
- Wendelbo Ø, Nesthus I, Sjo M, Paulsen K, Ernst P, Bruserud Ø. Functional characterization of T lymphocytes derived from patients with acute myelogenous leukemia and chemotherapy-induced leukopenia. Cancer Immunol Immunother. 2004;53(8):740-747. doi:10.1007/s00262-004-0505-0.
- Lamble A, Kosaka Y, Huang F, Sasser AK, Adams H, Tognon CE, et al. Mass cytometry as a modality to identify candidates for immune checkpoint inhibitor therapy within acute myeloid leukemia. Blood 2016;128:2829. doi: https://doi.org/10.1182/blood.V128.22.2829.2829.
- Legat A, Speiser DE, Pircher H, Zehn D, Marraco SAF. Inhibitory receptor expression depends more dominantly on differentiation and activation than "exhaustion" of human CD8 T cells. Front Immunol. 2013;4:455. Published 2013 Dec 19. doi:10.3389/fimmu.2013.00455.
- Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824-837. doi:10.1016/j.stem.2014.02.014.
- Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest. 2013;123(11):4595-4611. doi:10.1172/JCI67580.
- Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. 2018;32(5):1094-1105. doi:10.1038/s41375-018-0070-8.
- Borate U, Esteve J, Porkka K, Knapper S, Vey N, Scholl S, et al. Phase Ib study of the anti-TIM-3 antibody MBG453 in combination with Decitabine in patients with high-risk myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). ASH 2019, O570. doi: https://doi.org/10.1182/blood-2019-128178.
- Russ A, Hua AB, Montfort WR, Rahman B, Riaz IB, Khalid MU, et al. Blocking "don't eat me" signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018;32(6):480-489. doi:10.1016/j.blre.2018.04.005.
- Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015;10(9):e0137345. Published 2015 Sep 21. doi:10.1371/journal.pone.0137345.
- Sallman DA, Asch AS, Al Malki MM, Lee DJ, Donnellan WB, Marcucci G, et al. The first-in-class anti-CD47 antibody Magrolimab (5F9) in combination with Azacitidine is effective in MDS and AML patients: Ongoing Phase 1b results. Blood 2019; 134 (Supplement_1): 569. doi: https://doi.org/10.1182/blood-2019-126271.
- Konopleva M, Letai A. BCL-2 inhibition in AML: an unexpected bonus?. Blood. 2018;132(10):1007-1012. doi:10.1182/blood-2018-03-828269.
- DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216-228. doi:10.1016/S1470-2045(18)30010-X.
- Slape CI, Saw J, Jowett JB, Aplan PD, Strasser A, Jane SM, et al. Inhibition of apoptosis by BCL2 prevents leukemic transformation of a murine myelodysplastic syndrome. Blood. 2012;120(12):2475-2483. doi:10.1182/blood-2012-05-430736.
- DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401-407. doi:10.1002/ajh.25000.
- Ball BJ, Famulare CA, Stein EM, Tallman MS, Derkach A, Roshal M, et al. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020;4(13):2866-2870. doi:10.1182/bloodadvances.2020001482.
- Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12(6):393-406. doi:10.1038/nrg2984.
- Savona MR, Pollyea DA, Stock W, Oehler VG, Schroeder MA, Lancet J, et al. Phase Ib study of Glasdegib, a hedgehog pathway inhibitor, in combination with standard chemotherapy in patients with AML or high-risk MDS. Clin Cancer Res. 2018;24(10):2294-2303. doi:10.1158/1078-0432.CCR-17-2824.
- Cortes JE, Douglas Smith B, Wang ES, Merchant A, Oehler VG, Arellano M, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: Phase 2 study results. Am J Hematol. 2018;93(11):1301-1310. doi:10.1002/ajh.25238.
- Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9.
- Chaudhry P, Singh M, Triche TJ, Guzman M, Merchant AA. GLI3 repressor determines Hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood. 2017;129(26):3465-3475. doi:10.1182/blood-2016-05-718585.
- Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12(5):463-469. doi:10.1038/embor.2011.43.
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17-30. doi:10.1016/j.ccr.2010.12.014.
- Stein EM, Fathi AT, DiNardo CD, Pollyea DA, Swords RT, Roboz GJ, et al. Enasidenib (AG-221), a potent oral inhibitor of mutant isocitrate dehydrogenase 2(IDH2) enzyme, induces hematologic responses in patients with myelodysplastic syndromes (MDS). Blood. 2016;128(22). Abstract 343. doi: 10.1182/blood.V128.22.343.343.
- Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 2020; 135 (7): 463-471. doi: 10.1182/blood.2019002140.
- Chun AW, Cosenza SC, Taft DR, Maniar M. Preclinical pharmacokinetics and in vitro activity of ON 01910.Na, a novel anti-cancer agent. Cancer Chemother Pharmacol. 2009;65(1):177-186. doi:10.1007/s00280-009-1022-9.
- Garcia-Manero G, Fenaux P, Al-Kali A, Baer MR, Sekeres MA, Roboz GJ, et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(4):496-508. doi:10.1016/S1470-2045(16)00009-7.
- Navada SC, Gacia-Manero G, Atallah EL, Rajeh MN, Shammo JM, Griffith EA, et al. Phase 2 expansion study of oral rigosertib combined with azacitidine (AZA) in patients (Pts) with higher-risk (HR) myelodysplastic syndromes (MDS): efficacy and safety results in HMA treatment naïve & relapsed (Rel)/refractory (Ref) patients [abstract]. Blood. 2018;132. Abstract 230. doi: 10.1182/blood-2018-99-119259.
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
- Strati P, Kantarjian H, Ravandi F, Nazha A, Borthakur G, Daver N, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90(4):276-281. doi:10.1002/ajh.23924.
- Pratz KW, Cherry M, Altman JK, Cooper B, Cruz JC, Jurcic JG, et al. Updated results from a Phase 1 Study of Gilteritinib in combination with induction and consolidation chemotherapy in subjects with newly diagnosed acute myeloid leukemia (AML). Blood 2018; 132 (Supplement 1): 564. doi: 10.1182/blood-2018-99-110975.
- Altman JK, Foran JM, Pratz KW, Trone D, Cortes JE, Tallman MS. Phase 1 study of quizartinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed acute myeloid leukemia. Am J Hematol. 2018;93(2):213-221. doi:10.1002/ajh.24974.
- Stevens BM, Zhang W, Pollyea DA, Winters A, Gutman J, Smith C, et al. CD123 CAR T cells for the treatment of myelodysplastic syndrome. Exp Hematol. 2019;74:52-63.e3. doi:10.1016/j.exphem.2019.05.002.
- Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020;4(16):3977-3989. doi:10.1182/bloodadvances.2020001956.
- Paul S, Zahurak M, Luznik L, Ambinder RF, Fuchs EJ, Bolaños-Meade J, et al. Non-myeloablative allogeneic transplantation with post-transplant cyclophosphamide after immune checkpoint inhibition for classic Hodgkin lymphoma: A retrospective cohort study. Biol Blood Marrow Transplant. 2020;26(9):1679-1688. doi:10.1016/j.bbmt.2020.06.012.
- Morozova EV, Vlasova YuYu, Barabanshchikova MV, et al. Chronic myeloid leukemia: role of allogeneic hematopoietic stem cell transplantation in the era of tyrosine kinase inhibitors. Clinical Oncohematology. 2020;13(2):193-198 (In Russian). doi: 10.21320/2500-2139-2020-13-2-193-198.
- Oran B, Garcia-Manero G, Saliba RM, Alatrash G, Jabbour EJ, Popat U, et al. Post Allogeneic Stem Cell Transplant (SCT) Cyclophosphamide improves progression free survival (PFS) in pts with AML/MDS treated with CTLA-4 or PD-1 blockade prior to SCT. Blood 2018; 132 (Supplement 1): 483. https://doi.org/10.1182/blood-2018-99-115382.
" ["~DETAIL_TEXT"]=> string(65964) "
Introduction
Myelodysplastic syndrome (MDS) represents a heterogenous group of clonal diseases caused by alterations in hematopoietic stem cells due to hereditary predisposal, along with variable somatic gene mutations, and/or abnormal epigenetic regulation, including those induced by altered microenvironment and disrupted immune surveillance [1].
Pre-leukemic features of MDS were explored since early 80’s based on atypical in vitro growth of hematopoietic precursor cells from MDS patients [2-4]. Further on, some newer laboratory techniques, like flow cytometry and next-generation sequencing (NGS), allowed better insight into the distinct pathological events underlying MDS development. Over last decades, with increased lifespan, a number of peripheral blood cytopenias were found to precede clinical MDS, especially, in the patients >65 years old, i.e., ICUS (idiopathic cytopenia of undetermined significance); CHIP (Clonal hematopoiesis of indeterminate potential); CCUS (clonal cytopenia of undetermined significance). Such conditions are determined by the age-dependent accumulation of somatic mutations which may play a role in subsequent MDS deve- lopment.
These disorders may be transformed to hematological malignancy at a frequency of ca. 0.5-1% per year [5-7]. The majority of associated gene mutations (e.g., DNMT3A, TET2, ASXL1, TP53, and JAK2) affect RNA splicing or epigenetic regulation. However, patients with long-term cytopenias and somatic mutations do not always exhibit morphological changes of blood cells corresponding to MDS criteria. Moreover, only distinct cytogenetic aberrations (del5q) and point mutations (SF3B1) are considered specific for MDS, whereas other mutations are relatively non-specific and could be revealed in other pathologies, e.g., in the myeloproliferative disorders [8] or aplastic anemia (Fig. 1). In the latter case, MDS frequency was demonstrated to be sufficiently higher than in general population which is determined by the mutational profile [9].

Figure 1. Interactions and characteristic features of clonal hematopoiesis of indetermined potential (CHIP), idiopathic cytopenia of undetermined significance (ICUS), clonal cytopenia of undetermined significance (CCUS), aplastic anemia (АА), and myelodysplastic syndrome (MDS). Adapted from Young N., 2002 [13]
Occurrence of additional potentially pathogenic mutations is associated with increased MDS risk [10]. At diagnosis, two or more marker somatic mutations can be determined in most MDS patients [11]. In parallel to additional mutagenesis and clonal evolution of hematopoietic cells, the clinical manifestations undergo several stages, from a cytopenia of undetermined significance to MDS, and, in some cases, to leukemic transformation into acute myeloid leukemia (AML) [12]. Due to this complex pathogenesis effective target therapy is still not available for this group of diseases despite better awareness of MDS development mechanisms.
Hematopoietic cell transplantation and current therapeutic options
At the present time, allogeneic HSCT is the only curative treatment method for MDS. However, patients with MDS still remain the most complicated candidates for allo-HSCT, due to a number of additional unfavorable factors, including advanced age of the patients. MDS is more common in the patients over 65 years old, which determines a sufficient number of co-morbidities. In addition, preceding prolonged therapy is associated with significant number of blood transfusions with high frequency of clinical and laboratory signs of iron overload, as well as immunization due to these transfusions. In clinical practice, reduced-intensity conditioning regimens are often applied, taking into account somatic state of patients [14]. However, hematological remission is not achieved at the time of allo-HSCT in the majority of patients.
Along with gene mutations in stem cells, a special feature of MDS pathogenesis are certain defects of hematopoietic microenvironment, which alter functioning of hematopoietic niches [15]. These alterations contribute to higher incidence of graft failure, including primary graft failure and severe poor graft function after engraftment, as well as increased probability of early relapse. Long cytopenias after allo-HSCT is associated with higher risk of infectious complications, which are the main cause of posttransplant mortality in MDS patients. All issues described above determine higher post-transplant mortality in MDS compared to the other groups of HSCT recipients [16, 17]. Moreover, a significant proportion of patients is lacking HLA-compatible related or unrelated donor, and efficiency of haploidentical HSCT in MDS is still not supported by large studies [18]. According to the current European Blood and Marrow Transplantation (EBMT) guidelines, allo-HSCT from haploidentical donor is a clinical option and could be considered only after thorough evaluation of potential risks and benefits associated with this procedure.
Nonetheless, allo-HSCT facilitates cure in 30-40% of MDS patients. Several studies are ongoing aiming to improve allo-HSCT outcomes with posttransplant relapse preventive strategies [19, 20].
Due to clinical heterogeneity of MDS and risks associated with HSCT procedure, the strategy of treatment is largely personalized, dependent on the prognostic group, according to national and international recommendations [21, 22].
In low- and very low-risk patients with predominant clinical signs of anemia, the main therapeutic goal is to reduce transfusion dependence and to prevent organ damage caused by iron overload. Erythropoiesis-stimulating agents (ESA) could be administered, which, however, are clinically effective in about 1/3 of the patients, according to the multicenter studies [23]. The responders are predominantly those with initially low erythropoietin levels (<200 ng/ml) and the median duration of clinical response is about 1 year. Some studies suggest the possibility of decreased transfusion requirements with chelator therapy [24]. However, a randomized study did not show any improvement of erythropoietic response compared to ESA monotherapy [25].
In low-risk MDS cases with 5q- chromosomal aberration, immunomodulating therapy may result into cytogenetic remission in about 50% of the patients, with 2/3 of cases becoming transfusion-independent [26, 27]. In other low-risk MDS variants, there are only several single-center studies, where transfusion independence was achieved after short courses of hypomethylating agents (HMA) [28], and immunosuppressive treatment (IST), including cyclosporine A and anti-thymocyte globulin [29]. The median duration of clinical response using these therapies was, respectively, 16 and 18 months. Only small proportion of MDS patients has long-term remissions, either with HMA or IST protocols. However Kokhno A. et al. have shown that IST proved to be more effective in the patients with hypocellular bone marrow, or non-uniform cellularity with hypoplastic/aplastic areas, in absence of poor and very poor karyotype abnormalities according to IPSS-R scale, or without 7q-, i(17q) aberration [30].
In high-risk MDS, low-dose cytosine arabinoside (LDAC) treatment was a long-standing approach to therapy in myeloid dysplasia since mid-80’s [31]. Median survival time in clinical studies was ca. 8 months for these cohorts, with maximal lifespan of 2-3 years and frequency of clinical responses of 15 to 30%. However, some studies did not show any differences in survival rates between LDAC protocol and best supporting therapy [32-34]. At the present time, HMA became another standard of therapy in high-risk MDS. In registration randomized studies, decitabine and 5-azacitidine were associated with prolonged median survival by 2.7 and 10 months, respectively, compared with best available treatment [35, 36]. Nevertheless, large clinical studies, e.g., Surveillance, Epidemiology, and End Result‐Medicare database embracing 532 MDS high-risk patients, have shown somewhat higher results, i.e., median overall survival (OS) of, respectively, 11 and 12 months for decitabine and 5-azacitidine [37]. Despite MDS complete remissions are rare in HMA-treated MDS patients, these drugs enable temporary control of the disease with good quality of life and low hematological toxicity. Moreover, in the context of allo-HSCT, HMA may be effective in the pre-transplant period, by improving the patient’s state during search and activation of potential donors without increase of hematological toxicity [38].
There also are some studies on improvement of platelet counts with supplementary treatment with Eltrombopag, however, without evidence of higher response rate [39, 40].
Despite improvements with HMA introduction, recent studies in fundamental biology and pathogenesis of MDS revealed potential opportunities for studying novel treatments able to modify specific signaling and metabolic pathways, as well as hematopoietic and immune microenvironment.
Transforming growth factor-beta (TGFβ) antagonists
TGFβ antagonists, e.g. luspatercept, proved to be potentially effective in low-risk MDS with transfusion dependence, given the key role of TGFβ ligands in hematopoiesis. This drug represents a recombinant protein able to bind the TGFβ superfamily ligands, thus blocking SMAD2 and SMAD3 signaling pathways, regulating differentiation and maturation of erythroid precursors [41, 42]. These pathways play an important role in MDS pathogenesis by inhibition of SMAD7 and SKI expression [43, 44]. Luspatercept binds TGFβ ligands, that abrogating negative erythropoiesis regulation, accelerating events during late RBC maturation, unlike erythropoietin which regulates early stages of erythropoiesis [45], as shown in Fig. 2.

Figure 2. Hematopoietic effects of luspatercept (A); Molecular mechanisms of luspatercept action (B)
A general reduction in transfusion dependence was demonstrated in phase II clinical trial PACE-MDS with 29% of cases being with high transfusion requirements. Independence of blood transfusions was achieved in 36% of the cases. In particular, clinical responses were more often observed among patients with marrow ring sideroblasts and SF3B1 mutation [46].
In the phase 3 MEDALIST study, 37.9% of low-risk MDS patients became transfusion-independent in the treatment with luspatercept compared to 13.2% in the placebo group. According to the results of longitudinal observation (MEDALIST study), independence on red blood cell (RBC) transfusions maintained for at least 8 weeks at any period of treatment, and it was more frequent among patients treated with luspatercept (47.7%) than in placebo group (15.8%). Approximately 1 year after initiation of luspatercept, 31.4% did not require RBC transfusions, against 0% in placebo group. Among luspatercept-treated patients, the overall transfusion-free period and clinical improvement was observed, independent on previous transfusion burden. Transfusion independence persisted in 12 cases (7.8%) out of 153 patients who received luspatercept after 48 weeks of follow up. Some adverse effects associated with luspatercept administration rarely caused early discontinuation of therapy, and their occurrence decreased over time. Frequency of progression to AML (5%) was similar for luspatercept-treated patients and placebo group [47].
Another randomized phase III study COMMANDS is ongoing, where a comparison is made between luspatercept and erythropoietin α, the current standard of therapy [48]. The results of this study may influence the subsequent treatment standards in low-risk MDS patients. Similarly, some preclinical and clinical studies of kinase inhibitors involved into the TGFβ signal pathway are currently underway.
Galunisertib, an inhibitor of ALK5 kinase related to the signal transduction for TGFβ receptor activation, was studied in phase 2 clinical trials in low- and intermediate risk MDS, according to the IWG 2000 criteria. In 43.9% of patients, erythroid response was observed, hematological improvement was registered in 24.4% of cases, along with reduction of weakness in 44% of the patients. The results may justify its application in transfusion-dependent MDS patients who are non-responding to ESA [49]. Thus, a new class of TGFβ-directed drugs can be registered in the near future. The ongoing studies will show if these drugs are applicable only in MDS with ring sideroblasts, or they could be extended to other subgroups of low-risk MDS patients.
Another agent for low-risk MDS in late clinical trials is Roxadustat. It is a protein factor regulating HIF-1α (hypoxia-inducible factor-α) [50]. The drug influences erythropoietin production and iron uptake from macrophages, enhances iron metabolism, stabilizes blood HIF levels and prevents its degradation, thus promoting erythropoiesis [51]. Interim results of a multicenter study involved 24 patients with low-risk MDS and demonstrated a decrease of transfusion dependence by 50% [52]. Roxadustat inhibits HIF-α decay followed by its dimerization with HIF-β and nuclear translocation to cellular nucleus where the response to hypoxia is mediated at the transcriptional level (Fig. 3).

Figure 3. Main effects of roxadustat upon different organs and cells
Immune checkpoint inhibitors
The inhibitors of immune checkpoints (ICP) are highly effective in a treatment of some solid tumors. Their versatile effects are based on reactivation of exhausted immune cells via blocking appropriate inhibitory signal, leading to a recovery of antitumor immunity. Nevertheless, the characteristics and expression of different ligands of ICP strongly depends on the type of malignancy. Pronounced clinical response to ICP is prominent in tumors with high neo-antigen contents, or amplification of genes encoding ICP ligands. Highest sensitivity to ICP treatment was demonstrated for Hodgkin’s lymphoma, melanoma, cancers of urinary tract, lung cancer, head and neck malignancies, solid cancers with microsatellite instability [53]. There are usually only a small number of somatic mutations in MDS patients despite variable mutation profile [11], however hematopoietic cells in MDS exhibit high expression of the ICP ligands which may be increased during HMA therapy [54, 55].
Nonetheless, the ICP monotherapy was not associated with significant response in clinical trials [56]. The failure of nivolumab or ipilimumab monotherapy may be due to simultaneous expression of several inhibitors of immune response as confirmed by experimental data, including our own results [57]. Garcia-Manero G. et al. have shown the overall response rate (69%) to 5-azacitidine+nivolumab treatment in MDS, including complete remission in 2 patients. Higher response rate in this combined treatment schedule correlates with induction of neoantigen expression in tumor cells under HMA, thus enhancing the T cell immune response.
Failure of ICP therapy in MDS could be also determined by immunosuppressive effects in the hematopoietic niches. As possible mechanisms, one may consider high indoleamine-pyrrole 2,3-dioxygenase (IDO) expression in cellular microenvironment, T cell differentiation towards regulatory T cells, low interferon-α expression by T cells, induction of T cell apoptosis due to activation of CD33-S100A9 signaling, increased levels of myeloid suppressor cells [58-68]. In future, ICP-based treatment seems to enter the standards of MDS therapy, however, not as monotherapy, but as combined therapeutic schedules, e.g., with PD-1, CTLA4 and TIM3 inhibitors [69]. Clinical trials with anti-TIM3 monoclonal antibodies are underway now. A multicenter study has shown that combined treatment with MBG453В, an anti-TIM3 antibody, and decitabine resulted into hematological or molecular remission in 50% of high-risk MDS patients [70].
Macrophage ICPs represent a fundamentally new class of such regulatory molecules. CD47, being expressed on macrophages, is a key molecule able to inhibit their response, and its interaction with SIRPα ligand protein causes suppression of phagocytic function. This effect is called a blocking "don't eat me" signal of CD47-SIRPα. This signal pathway is active during interactions between hematopoietic cells and macrophages, also serving as a tolerance mechanism in hematopoietic malignancies [71]. Magrolimab, or 5F9 antibody, is a humanized monoclonal antibody (MAb) which blocks CD47 and activates SIRPα pathway, promotes phagocytosis of tumor cells. Combined application of this drug with 5-azacitidine in preclinical model of acute myeloid leukemia (AML) has shown high survival rates in laboratory animals [72]. During the Phase 1 clinical trial, 5-azacitidine and Magrolimab was administered to 35 high-risk MDS patients. The response was evaluated in 24 patients, with hematological response in 92% and complete remission in 50% of the cases. Further observations are required to assess duration of the responses [73].
Distinct effects of the CD47-blocking antibodies are considered, as follows: (a) under normal conditions, both healthy and malignant cells are avoiding phagocytosis by CD47 expression. CD47 is overexpressed by cancer cells for protection from eat me/prophagocyte signals. (b) After the Mab-induced CD47 blockage, the malignant cells are phagocytized, thus causing exposure of the eat me signal. By contrast, normal cells remain intact due to absent expression of prophagocytic signals.

Figure 4. Possible effects of CD47-blocking antibodies upon immune cells
Inhibitors of bcl2, hedgehog, IDH and other molecular targets
Over last years, a significant role of bcl2 in AML progression and drug resistance was elucidated. Venetoclax, a specific bcl2 inhibitor, was shown to increase the rates of complete remission in combination with LDAC or HMA [74, 75]. However, the results observed in AML cannot be blindly extended to MDS. BCL2 inhibition in an MDS models leads to suppression of apoptosis in hematopoietic cells and transition to the resting phase of a cell cycle. As a result a reduced DNA damage of erythropoietic precursors was observed. However, some doubts exist since apoptosis blockage may promote faster transition to AML [76].
Overall response to venetoclax was 21% in a group of chemotherapy-refractory AML and MDS patients [77]. Clinical studies are ongoing with early venetoclax and HMA administration in MDS. So far only the results of non-randomized studies are published demonstrating promising overall and complete remission rates (59% and 14%, respectively) even in HMA-exposed patients [78].
Glasdegib, another inhibitor of signaling pathways, was registered in 2019 for therapy of AML and high-risk MDS in combination with chemotherapy. The drug inhibits Hedgehog (Hh) pathway previously described as an embryogenesis regulator, since Hh proteins are involved in cell and tissue differentiation. Like other intracellular signaling systems, the Hh pathway plays an important role in cell cycle regulation of malignant cells and is involved into the mechanisms of chemotherapy resistance [79].
In a Phase I study, 31% of AML and high-risk MDS patients have achieved complete remission during the glasdegib therapy combined with LDAC and decitabine [80]. Complete remission was achieved in 46% of the patients with similar disorders in another study using glasdegib combined with systemic 7+3 chemotherapy [81]. In a randomized study comparing glasdegib plus LDAC against LDAC a 3-month increase in OS was demonstrated, along with long-term stabilization of the disease in some patients. The rates of response to glasdegib monotherapy in refractory MDS comprise only 6% [82]. Chaudhry et al. noted that therapeutic activity of glasdegib requires expression of GLI3 suppressor gene which may be hypermethylated in MDS and AML. Determination of GLI3 expression may serve as predictor of response to glasdegib treatment [83]. Despite relatively low efficiency, glasdegib is well tolerated, thus allowing to suggest it as a component of combined therapy in MDS.
About 5% of MDS cases are associated with IDH1 and IDH2 gene mutations. The mutated IDH variants are associated with excessive production of R2-hydroxyglutarate which causes functional insufficiency of TET2 gene [84, 85]. Presently, two IDH inhibitors for oral administration are under clinical trials, enasidenib, and ivosidenib (respectively for IDH2 and IDH1 inhibition). The Phase II study in AML and high-risk MDS patients, enasidenib therapy was associated with response in 53% of the patients including complete remission in 7% [86]. There are no preliminary results on ivosidenib in MDS at the present moment. However, in elderly AML ivosidenib induced complete remission, including one with partial hematologic recovery, in 42.4% of patients and median duration of remission was not reached with 2-year follow up [87].
Rigosertib is another clinically tested inhibitor of signal pathways which is able to suppress several kinases, e.g., Akt, PI3K. A clinical study in MDS patients has demonstrated reduction of blastosis [88]. However, Phase III study in HMA-resistant patients did not show any differences in survival between Rigosertib treatment and best available therapy [89]. At present, Rigosertib is tested in combination with 5-azacitidine [90].
A small group of MDS patients exhibits FLT3 mutation [11]. These patients are prone to rapid transformation to AML, thus precluding data accumulation on clinical efficiency of FLT3 inhibitors in this MDS variant. Nevertheless, the MDS experience shows that addition of Midostaurin to chemotherapy is associated with 8% increase in relapse-free survival [91]. The response rate in combined therapy with 5-azacitidine was 26% [92]. Meanwhile, the second-generation FLT3 inhibitors (Gilteritinib and Quizartinib) demonstrate more optimistic results [93, 94], thus creating the basis for addition of these agents to standard therapy in MDS patients with FLT3 mutations.
Summarizing the overview of developing targeted therapies, it is important to mention that few of them induce high complete remission rate and these complete remissions are not durable in the high proportion of the patients. When keeping in mind the complex pathogenesis of this disease, it is clear that complex approaches to therapy are required. The successful examples of other hematological diseases give us hope for long-term improvement of survival in MDS (Fig. 5).

Figure 5. Potential drug combinations in personalized therapy of high-risk MDS
Allogeneic HSCT as a platform for immune therapy in MDS patients
Along with development of novel therapeutic molecules, some feasible options of cellular therapy are under investigation in MDS [95]. At the moment, allo-HSCT is the only cellular therapy in MDS which is widely used in clinical practice. However, allo-HSCT is a high-risk procedure with potentially severe complications that may cause sufficiently decreased quality of life and shorter survival of the patients. This issue is especially important due to advanced age of most MDS patients, thus increasing risk for dismal outcome.
To evaluate the impact of allo-HSCT on survival in MDS we compared the survival of two contemporary cohorts of patients treated at RM Gorbacheva Research Institute. The first cohort agreed to allograft procedure while the second refused to undergo allo-HSCT and was treated with available therapies. Although the groups were not well matched, however represent the real life clinical practice at large HSCT center. The comparison of these two groups revealed that two-year OS in MDS patients without allo-HSCT was 36.7% (n=68), while it was 69.1% (n=83) among the patients after HSCT (p<0.05) (Fig. 6A). In allo-HSCT group, the 2-year OS was comparable in IPPS-R intermediate-2 versus high-risk groups (n=62), and intermediate-1 versus low-risk groups (n=21, 68.2% vs 71.4%, respectively). Two-year overall survival was lower in similar patient groups without allo-HSCT: 31.2% (n=43) and 47% (n=25, p<0.05), respectively (Fig. 6B). The survival rates following HSCT depended on the following items: disease status, graft composition, infectious complications, acute GvHD grade I-II [16]. Interestingly, the evaluation of all existing MDS prognostic scales in our MDS allo-HSCT group did not exert any significant effects on survival after allo-HSCT. This observation highlights a necessity to validate developing scales in the center- or country-stratified manner. Hence, it is clear that the risk-adapted strategies in MDS patients are of extreme importance.

Figure 6. Two-year overall survival of MDS patients depending on allo-HSCT performed (A). The two-year overall survival of MDS patients depending on allo-HSCT performed and IPSS risk group (B)
So far, it is unclear how the role of allo-HSCT in MDS will change. It can follow the track of chronic lymphocytic leukemia [96] and Hodgkin’s lymphoma [97] where effective bridging before allo-HSCT brought the results of allo-HSCT in refractory disease close to the results of primary treatment in these malignancies. In this situation, allo-HSCT will be more broadly used in MDS. On the other hand, combination therapies can bring durable remissions to this difficult population of patients. In this case allo-HSCT will be brought from first line to second or subsequent lines of therapy like it happened with chronic myeloid leukemia [98]. Given the preliminary favorable results of allo-HSCT in MDS after therapy with ICPs [99], it is likely that at least the first scenario with effective bridging therapies will be implemented in the near future.
Conclusion
With respect to recent findings and genetic studies made by means of NGS techniques, some radical changes are expected in MDS therapies. The drug selection will be based on evaluation of mutational profile, expression of checkpoint molecules and methylation profile. The results of these studies will determine a combination of target agents, ICP inhibitors and hypomethylating agents. It is also clear that allo-HSCT will remain in MDS clinical practice, however its place in a sequence of therapies will be rapidly changing.
Acknowledgements
The work was supported by Russian Science Foundation grant 17-75-20145-П.
Conflict of interest
The authors declare no conflicts of interest.
References
- Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat Rev Cancer. 2017;17(1):5-19. doi:10.1038/nrc.2016.112.
- Tiranova SA, Alekseev NA, Petrova EM, et al. On the question of the existence of hematopoietic dysplasia (preleukemia) in children. Terapevticheskii arkhiv. 1982;54(8):1-16 (In Russian).
- Afanasyev BV, Tiranova SA, Kulibaba TG, et al. Cloning of human hematopoietic cells in agar drop-liquid medium system. Terapevticheskii arkhiv. 1983;54(8):114-22 (In Russian).
- Kleihauer E. The preleukemic syndromes (hematopoietic dysplasia) in childhood. Eur J Pediatr. 1980;133(1):5-10.
- Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44(11):1179-1181. doi:10.1038/ng.2413.
- Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477-2487. doi:10.1056/NEJMoa1409405.
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498. doi:10.1056/NEJMoa1408617.
- Malcovati L, Cazzola M. The shadowlands of MDS: idiopathic cytopenias of undetermined significance (ICUS) and clonal hematopoiesis of indeterminate potential (CHIP). Hematology Am Soc Hematol Educ Program. 2015;2015:299-307. doi:10.1182/asheducation-2015.1.299.
- Afanasyev BV, Zubarovskaya L. Pediatric myelodysplastic syndrome. Russian Journal of Pediatric Hematology and Oncology. 2018;5(3):23-35. (In Russian). doi: 10.17650/2311-1267-2018-5-3-23-35.
- Malcovati L, Gallì A, Travaglino E, Ambaglio I, Rizzo E, Molteni E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129(25):3371-3378. doi:10.1182/blood-2017-01-763425.
- Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616-3699. doi:10.1182/blood-2013-08-518886.
- Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019;54(10):1525-1552. doi:10.1038/s41409-019-0516-2.
- Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136(7):534-546. doi:10.7326/0003-4819-136-7-200204020-00011.
- Kröger N, Iacobelli S, Franke GN, Platzbecker U, Uddin R, Hübel K, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: A prospective randomized Phase III Study of the EBMT (RICMAC Trial). J Clin Oncol. 2017;35(19):2157-2164. doi:10.1200/JCO.2016.70.7349.
- Li AJ, Calvi LM. The microenvironment in myelodysplastic syndromes: Niche-mediated disease initiation and progression. Exp Hematol. 2017;55:3-18. doi:10.1016/j.exphem.2017.08.003.
- Morozova EV, Barabanshikova MV, Tcvetkov NY, Melsitova KV, Rudnitskaya JV, Darskaya EI, et al. Application of standard and novel prognostic systems in patients with myelodysplastic syndrome undergoing allogeneic hematopoietic stem cell transplantation. Cell Ther Transplant 2019; 8(1): 36-45. doi: 10.18620/ctt-1866-8836-2019-8-1-36-45.
- Kröger N, Zabelina T, de Wreede L, Berger J, Alchalby H, van Biezen A, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia. 2013;27(3):604-609. doi:10.1038/leu.2012.210.
- Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30(10):2055-2063. doi:10.1038/leu.2016.110.
- El-Cheikh J, Massoud R, Fares E, Kreidieh N, Mahfouz R, Charafeddine M, et al. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT. Bone Marrow Transplant. 2017;52(6):918-921. doi:10.1038/bmt.2017.31.
- Ovechkina VN, Bondarenko SN, Morozova EV, et al. The role of hypomethylating agents prior to allogeneic hematopoietic stem cells transplantation in acute myeloid leukemia and myelodysplastic syndrome. Clinical Oncohematology. 2017;10(3):351–357 (In Russian). doi: 10.21320/2500-2139-2017-10-3-351-7.
- Savchenko VG, Parovichnikova EN, Kokhno AV, et al. National clinical guidelines for the diagnosis and treatment of myelodysplastic syndromes adults. Hematology and Transfusiology (Gematologiya i transfusiologiya). 2016; 61 (1-32). doi: 10.18821/0234-5730-2016-61-1 (In Russian).
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. doi:10.1182/blood-2012-03-420489.
- Fenaux P, Santini V, Spiriti MAA, Giagounidis A, Schlag R, Radinoff A, et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-α in anemic patients with low-risk MDS. Leukemia. 2018;32(12):2648-2658. doi:10.1038/s41375-018-0118-9.
- Latagliata R, Montagna C, Porrini R, Di Veroli A, Leonetti SC, Niscola P, et al. Chelation efficacy and erythroid response during deferasirox treatment in patients with myeloproliferative neoplasms in fibrotic phase. Eur J Haematol. 2016;96(6):643-649. doi:10.1111/ejh.12674.
- Gattermann N, Coll R, Jacobasch L, Allameddine A, Azmon A, DeBonnett L, et al. Effect of deferasirox + erythropoietin vs erythropoietin on erythroid response in Low/Int-1-risk MDS patients: Results of the phase II KALLISTO trial [published online ahead of print, 2018 May 19]. Eur J Haematol. 2018;10.1111/ejh.13096. doi:10.1111/ejh.13096.
- Garcia-Manero G, Almeida A, Fenaux P, Gattermann N, Giagounidis A, Goldberg SL, et al. Clinical benefit-risk profile of lenalidomide in patients with lower-risk myelodysplastic syndromes without del(5q): Results of a Phase III Trial. Clin Lymphoma Myeloma Leuk. 2019;19(4):213-219.e4. doi:10.1016/j.clml.2018.12.012.
- Lee JH, List A, Sallman DA. Molecular pathogenesis of myelodysplastic syndromes with deletion 5q. Eur J Haematol. 2019;102(3):203-209. doi:10.1111/ejh.13207.
- Jabbour E, Short NJ, Montalban-Bravo G, Huang X, Bueso-Ramos C, Qiao W, et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood. 2017;130(13):1514-1522. doi:10.1182/blood-2017-06-788497.
- Passweg JR, Giagounidis AA, Simcock M, Aul C, Dobbelstein C, Stadler M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol. 2011;29(3):303-309. doi:10.1200/JCO.2010.31.2686.
- Kokhno AV, Parovichnikova EN, Mikhailova EA, et al. Efficiency of cyclosporin A therapy in patients with myelodysplastic syndrome. Terapevticheskii Arkhiv. 2010; 82(8): 48-53 (In Russian).
- Patterson D, Kravtsova VM, Petrova EM, et al. Therapy of patients with myeloid leukemia with low doses of cytosine arabinoside. Terapevticheskii Arkhiv. 1987; 12: 81-86 (In Russian).
- Gerhartz HH, Marcus R, Delmer A, Zwierzina H, de Witte T, Jacobs A, et al. Treatment of myelodysplastic syndromes (MDS) and high leukaemic risk with low-dose cytosine arabinoside (LD-AraC) plus granulocyte-macrophage colony-stimulating factor (rh GM-CSF). The EORTC Leukaemia Group. Infection, 1992; 20 (Suppl 2):S116-S123. doi:10.1007/BF01705030.
- Zwierzina H, Suciu S, Loeffler-Ragg J, Neuwirtova R, Fenaux P, Beksac M, et al. Low-dose cytosine arabinoside (LD-AraC) vs LD-AraC plus granulocyte/macrophage colony stimulating factor vs LD-AraC plus Interleukin-3 for myelodysplastic syndrome patients with a high risk of developing acute leukemia: final results of a randomized phase III study (06903) of the EORTC Leukemia Cooperative Group. Leukemia. 2005;19(11):1929-1933. doi:10.1038/sj.leu.2403934.
- Kokhno AV, Parovichnikova EN, Mikhailova EA, et al. Myelodysplastic syndromes and aplastic anemia. In the manual "Programmed treatment of diseases of the blood system: Collection of diagnostic algorithms and treatment protocols for diseases of the blood system" (ed. by VG Savchenko). State Research Center of the Russian Healthcare Ministry; Moscow: Practice (2012): 83-150 (In Russian).
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. doi:10.1200/JCO.2011.38.9429.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009; 10(3):223-232. doi:10.1016/S1470-2045(09)70003-8.
- Zeidan AM, Davidoff AJ, Long JB, Hu X, Wang R, Ma X, et al. Comparative clinical effectiveness of azacitidine versus decitabine in older patients with myelodysplastic syndromes. Br J Haematol. 2016;175(5):829-840. doi:10.1111/bjh.14305.
- Field T, Perkins J, Huang Y, Kharfan-Dabaja MA, Alsina M, Ayala E, et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010;45(2):255-260. doi:10.1038/bmt.2009.134.
- Mittelman M, Platzbecker U, Afanasyev B, et al. Eltrombopag for advanced myelodysplastic syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. Lancet Haematol. 2018;5(1):e34-e43. doi:10.1016/S2352-3026(17)30228-4.
- Dickinson M, Cherif H, Fenaux P, Mittelman M, Verma A, Portella MSO, et al. Azacitidine with or without eltrombopag for first-line treatment of intermediate- or high-risk MDS with thrombocytopenia. Blood. 2018; 132(25):2629-2638. doi:10.1182/blood-2018-06-855221.
- Muench DE, Ferchen K, Velu CS, Pradhan K, Chetal K, Chen X, et al. SKI controls MDS-associated chronic TGF-β signaling, aberrant splicing, and stem cell fitness. Blood. 2018;132(21):e24-e34. doi:10.1182/blood-2018-06-860890.
- Zermati Y, Fichelson S, Valensi F, Freyssinier JM, Rouyer-Fessard P, Cramer E, et al. Transforming growth factor inhibits erythropoiesis by blocking proliferation and accelerating differentiation of erythroid progenitors. Exp Hematol. 2000;28(8):885-894. doi:10.1016/s0301-472x(00)00488-4.
- Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, Fazzari M, et al. Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71(3):955-963. doi:10.1158/0008-5472.CAN-10-2933.
- Bachegowda L, Gligich O, Mantzaris I, Schinke C, Wyville D, Carrillo T, et al. Signal transduction inhibitors in treatment of myelodysplastic syndromes. J Hematol Oncol. 2013;6:50. Published 2013 Jul 10. doi:10.1186/1756-8722-6-50.
- Hanke T, Wong JF, Berger BT, Abdi I, Berger LM, Tesch R, et al. A highly selective chemical probe for activin receptor-like kinases ALK4 and ALK5. ACS Chem Biol. 2020;15(4):862-870. doi:10.1021/acschembio.0c00076.
- Platzbecker U, Germing U, Götze KS, Kiewe P, Mayer K, Chromik J, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017;18(10):1338-1347. doi:10.1016/S1470-2045(17)30615-0.
- Fenaux P, Platzbecker U, Mufti GJ, Garcia-Manero G, Buckstein R, Santini V, et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N Engl J Med. 2020;382(2):140-151. doi:10.1056/NEJMoa1908892.
- https://clinicaltrials.gov/ct2/show/NCT03682536, as of 12/2019.
- Santini V, Valcárcel D, Platzbecker U, Komrokji RS, Cleverly AL, Lahn MM, et al. Phase II study of the ALK5 inhibitor galunisertib in very low-, low-, and intermediate-risk myelodysplastic syndromes. Clin Cancer Res. 2019; 25(23):6976-6985. doi:10.1158/1078-0432.CCR-19-1338.
- https://clinicaltrials.gov/ct2/show/NCT03263091, as of 12/2019.
- Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32(8):1373-1386. doi:10.1093/ndt/gfx011.
- Henry DH, Glaspy J, Harrup RA, Mittelman M, Zhou A, Bradley C, et al. Roxadustat (FG4592; ASP1517; AZD9941) in the treatment of anemia in patients with lower risk myelodysplastic syndrome (LR-MDS) and low red blood cell (RBC) transfusion burden (LTB). Blood 2019; 134 (Supplement_1): 843. doi: 10.1182/blood-2019-128714.
- Allison JP. Checkpoints. Cell. 2015;162(6):1202-1205. doi:10.1016/j.cell.2015.08.047.
- Chokr N, Patel R, Wattamwar K, Chokr S. The rising era of immune checkpoint inhibitors in myelodysplastic syndromes. Adv Hematol. 2018;2018:2458679. doi:10.1155/2018/2458679.
- Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280-1288. doi:10.1038/leu.2013.355.
- Garcia-Manero G, Daver NG, Montalban-Bravo G, Jabbour EJ, DiNardo CD, Kornblau SM, et al. A Phase II Study evaluating the combination of Nivolumab (Nivo) or Ipilimumab (Ipi) with Azacitidine in patients with previously treated or untreated myelodysplastic syndromes (MDS). Blood 2016; 128 (22): 344. doi: 10.1182/blood.V128.22.344.344.
- Tcvetkov N, Gusak A, Morozova E, et al. Immune checkpoints bone marrow expression as the predictor of clinical outcome in myelodysplastic syndrome. Leuk Res Rep. 2020;14:100215. Published 2020 Jun 28. doi:10.1016/j.lrr.2020.100215.
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619-4621. doi:10.1182/blood-2003-11-3909.
- Croitoru-Lamoury J, Lamoury FM, Caristo M, Suzuki K, Walker D, Takikawa O, et al. Interferon-γ regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One. 2011;6(2):e14698. Published 2011 Feb 16. doi:10.1371/journal.pone.0014698.
- Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients' sera by HPLC and is inducible by IFN-gamma. Leuk Res. 2009;33(3):490-494. doi:10.1016/j.leukres.2008.06.014.
- Yuan Y, Lu X, Tao CL, Chen X, Shao HW, Huang SL. Forced expression of indoleamine-2,3-dioxygenase in human umbilical cord-derived mesenchymal stem cells abolishes their anti-apoptotic effect on leukemia cell lines in vitro. In Vitro Cell Dev Biol Anim. 2013;49(10):752-758. doi:10.1007/s11626-013-9667-4.
- Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109(7):2871-2877. doi:10.1182/blood-2006-07-036863.
- Schnorfeil FM, Lichtenegger FS, Emmerig K, Schlueter M, Neitz JS, Draenert R, et al. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J Hematol Oncol. 2015;8:93. Published 2015 Jul 30. doi:10.1186/s13045-015-0189-2.
- Wendelbo Ø, Nesthus I, Sjo M, Paulsen K, Ernst P, Bruserud Ø. Functional characterization of T lymphocytes derived from patients with acute myelogenous leukemia and chemotherapy-induced leukopenia. Cancer Immunol Immunother. 2004;53(8):740-747. doi:10.1007/s00262-004-0505-0.
- Lamble A, Kosaka Y, Huang F, Sasser AK, Adams H, Tognon CE, et al. Mass cytometry as a modality to identify candidates for immune checkpoint inhibitor therapy within acute myeloid leukemia. Blood 2016;128:2829. doi: https://doi.org/10.1182/blood.V128.22.2829.2829.
- Legat A, Speiser DE, Pircher H, Zehn D, Marraco SAF. Inhibitory receptor expression depends more dominantly on differentiation and activation than "exhaustion" of human CD8 T cells. Front Immunol. 2013;4:455. Published 2013 Dec 19. doi:10.3389/fimmu.2013.00455.
- Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824-837. doi:10.1016/j.stem.2014.02.014.
- Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest. 2013;123(11):4595-4611. doi:10.1172/JCI67580.
- Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. 2018;32(5):1094-1105. doi:10.1038/s41375-018-0070-8.
- Borate U, Esteve J, Porkka K, Knapper S, Vey N, Scholl S, et al. Phase Ib study of the anti-TIM-3 antibody MBG453 in combination with Decitabine in patients with high-risk myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). ASH 2019, O570. doi: https://doi.org/10.1182/blood-2019-128178.
- Russ A, Hua AB, Montfort WR, Rahman B, Riaz IB, Khalid MU, et al. Blocking "don't eat me" signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018;32(6):480-489. doi:10.1016/j.blre.2018.04.005.
- Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015;10(9):e0137345. Published 2015 Sep 21. doi:10.1371/journal.pone.0137345.
- Sallman DA, Asch AS, Al Malki MM, Lee DJ, Donnellan WB, Marcucci G, et al. The first-in-class anti-CD47 antibody Magrolimab (5F9) in combination with Azacitidine is effective in MDS and AML patients: Ongoing Phase 1b results. Blood 2019; 134 (Supplement_1): 569. doi: https://doi.org/10.1182/blood-2019-126271.
- Konopleva M, Letai A. BCL-2 inhibition in AML: an unexpected bonus?. Blood. 2018;132(10):1007-1012. doi:10.1182/blood-2018-03-828269.
- DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216-228. doi:10.1016/S1470-2045(18)30010-X.
- Slape CI, Saw J, Jowett JB, Aplan PD, Strasser A, Jane SM, et al. Inhibition of apoptosis by BCL2 prevents leukemic transformation of a murine myelodysplastic syndrome. Blood. 2012;120(12):2475-2483. doi:10.1182/blood-2012-05-430736.
- DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401-407. doi:10.1002/ajh.25000.
- Ball BJ, Famulare CA, Stein EM, Tallman MS, Derkach A, Roshal M, et al. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020;4(13):2866-2870. doi:10.1182/bloodadvances.2020001482.
- Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12(6):393-406. doi:10.1038/nrg2984.
- Savona MR, Pollyea DA, Stock W, Oehler VG, Schroeder MA, Lancet J, et al. Phase Ib study of Glasdegib, a hedgehog pathway inhibitor, in combination with standard chemotherapy in patients with AML or high-risk MDS. Clin Cancer Res. 2018;24(10):2294-2303. doi:10.1158/1078-0432.CCR-17-2824.
- Cortes JE, Douglas Smith B, Wang ES, Merchant A, Oehler VG, Arellano M, et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: Phase 2 study results. Am J Hematol. 2018;93(11):1301-1310. doi:10.1002/ajh.25238.
- Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379-389. doi:10.1038/s41375-018-0312-9.
- Chaudhry P, Singh M, Triche TJ, Guzman M, Merchant AA. GLI3 repressor determines Hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood. 2017;129(26):3465-3475. doi:10.1182/blood-2016-05-718585.
- Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12(5):463-469. doi:10.1038/embor.2011.43.
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17-30. doi:10.1016/j.ccr.2010.12.014.
- Stein EM, Fathi AT, DiNardo CD, Pollyea DA, Swords RT, Roboz GJ, et al. Enasidenib (AG-221), a potent oral inhibitor of mutant isocitrate dehydrogenase 2(IDH2) enzyme, induces hematologic responses in patients with myelodysplastic syndromes (MDS). Blood. 2016;128(22). Abstract 343. doi: 10.1182/blood.V128.22.343.343.
- Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 2020; 135 (7): 463-471. doi: 10.1182/blood.2019002140.
- Chun AW, Cosenza SC, Taft DR, Maniar M. Preclinical pharmacokinetics and in vitro activity of ON 01910.Na, a novel anti-cancer agent. Cancer Chemother Pharmacol. 2009;65(1):177-186. doi:10.1007/s00280-009-1022-9.
- Garcia-Manero G, Fenaux P, Al-Kali A, Baer MR, Sekeres MA, Roboz GJ, et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(4):496-508. doi:10.1016/S1470-2045(16)00009-7.
- Navada SC, Gacia-Manero G, Atallah EL, Rajeh MN, Shammo JM, Griffith EA, et al. Phase 2 expansion study of oral rigosertib combined with azacitidine (AZA) in patients (Pts) with higher-risk (HR) myelodysplastic syndromes (MDS): efficacy and safety results in HMA treatment naïve & relapsed (Rel)/refractory (Ref) patients [abstract]. Blood. 2018;132. Abstract 230. doi: 10.1182/blood-2018-99-119259.
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
- Strati P, Kantarjian H, Ravandi F, Nazha A, Borthakur G, Daver N, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90(4):276-281. doi:10.1002/ajh.23924.
- Pratz KW, Cherry M, Altman JK, Cooper B, Cruz JC, Jurcic JG, et al. Updated results from a Phase 1 Study of Gilteritinib in combination with induction and consolidation chemotherapy in subjects with newly diagnosed acute myeloid leukemia (AML). Blood 2018; 132 (Supplement 1): 564. doi: 10.1182/blood-2018-99-110975.
- Altman JK, Foran JM, Pratz KW, Trone D, Cortes JE, Tallman MS. Phase 1 study of quizartinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed acute myeloid leukemia. Am J Hematol. 2018;93(2):213-221. doi:10.1002/ajh.24974.
- Stevens BM, Zhang W, Pollyea DA, Winters A, Gutman J, Smith C, et al. CD123 CAR T cells for the treatment of myelodysplastic syndrome. Exp Hematol. 2019;74:52-63.e3. doi:10.1016/j.exphem.2019.05.002.
- Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020;4(16):3977-3989. doi:10.1182/bloodadvances.2020001956.
- Paul S, Zahurak M, Luznik L, Ambinder RF, Fuchs EJ, Bolaños-Meade J, et al. Non-myeloablative allogeneic transplantation with post-transplant cyclophosphamide after immune checkpoint inhibition for classic Hodgkin lymphoma: A retrospective cohort study. Biol Blood Marrow Transplant. 2020;26(9):1679-1688. doi:10.1016/j.bbmt.2020.06.012.
- Morozova EV, Vlasova YuYu, Barabanshchikova MV, et al. Chronic myeloid leukemia: role of allogeneic hematopoietic stem cell transplantation in the era of tyrosine kinase inhibitors. Clinical Oncohematology. 2020;13(2):193-198 (In Russian). doi: 10.21320/2500-2139-2020-13-2-193-198.
- Oran B, Garcia-Manero G, Saliba RM, Alatrash G, Jabbour EJ, Popat U, et al. Post Allogeneic Stem Cell Transplant (SCT) Cyclophosphamide improves progression free survival (PFS) in pts with AML/MDS treated with CTLA-4 or PD-1 blockade prior to SCT. Blood 2018; 132 (Supplement 1): 483. https://doi.org/10.1182/blood-2018-99-115382.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(100) "novye-optsii-lecheniya-patsientov-s-mielodisplasticheskim-sindromom-obzor-literatury-i-rezultaty-odn" ["~CODE"]=> string(100) "novye-optsii-lecheniya-patsientov-s-mielodisplasticheskim-sindromom-obzor-literatury-i-rezultaty-odn" ["EXTERNAL_ID"]=> string(4) "1949" ["~EXTERNAL_ID"]=> string(4) "1949" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(335) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследованияNew therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(1906) "<p style="text-align: justify;">Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.</p> <p style="text-align: justify;">В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_META_TITLE"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_META_KEYWORDS"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_META_DESCRIPTION"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_PICTURE_FILE_ALT"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_PICTURE_FILE_TITLE"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "novye-optsii-lecheniya-patsientov-s-mielodisplasticheskim-sindromom-obzor-literatury-i-rezultaty-odn" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(229) "Новые опции лечения пациентов с миелодиспластическим синдромом: обзор литературы и результаты одноцентрового исследования" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "novye-optsii-lecheniya-patsientov-s-mielodisplasticheskim-sindromom-obzor-literatury-i-rezultaty-odn" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "novye-optsii-lecheniya-patsientov-s-mielodisplasticheskim-sindromom-obzor-literatury-i-rezultaty-odn" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "novye-optsii-lecheniya-patsientov-s-mielodisplasticheskim-sindromom-obzor-literatury-i-rezultaty-odn" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "175" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27452" ["VALUE"]=> string(22) "01/14/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "01/14/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27453" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27454" ["VALUE"]=> array(2) { ["TEXT"]=> string(149) "<p>Елена В. Морозова, Николай Ю. Цветков, Ирина О. Туртанова, Иван С. Моисеев</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(137) "
Елена В. Морозова, Николай Ю. Цветков, Ирина О. Туртанова, Иван С. Моисеев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27455" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27456" ["VALUE"]=> array(2) { ["TEXT"]=> string(1906) "<p style="text-align: justify;">Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.</p> <p style="text-align: justify;">В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1828) "Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.
В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.
Ключевые слова
Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27457" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-24-36" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-24-36" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27459" ["VALUE"]=> array(2) { ["TEXT"]=> string(95) "<p>Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(83) "Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27460" ["VALUE"]=> array(2) { ["TEXT"]=> string(481) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> <p>Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 796 1951<br> E-mail: moisiv@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(415) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 796 1951
E-mail: moisiv@mail.ru
Myelodysplastic syndromes (MDS) comprise a group (continuum) of clonal hematopoietic diseases associated with high risk of transformation into acute myeloid leukemia (AML) and unfavorable prognosis. Compared to the other hematologic malignant diseases, there was only a modest improvement in survival of MDS patients over the last years. Allogeneic stem cell transplantation remains the only curative option for these patients, however, most of them are not candidates for transplantation. This review focuses on the long-term outcomes of existing therapies and novel agents that are currently tested at different stages of clinical trials. These include inhibitors of TGFβ, various kinase inhibitors, and immune checkpoint inhibitors. Administration of new therapies in the patients with different pathogenetic MDS variants is discussed.
Keywords
Myelodysplastic syndrome, treatment, checkpoint inhibitors, luspatercept, glasdegib, venetoclax, IDH inhibitors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27458" ["VALUE"]=> string(106) "New therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(106) "New therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27526" ["VALUE"]=> string(4) "2416" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2416" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27527" ["VALUE"]=> string(4) "2417" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2417" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27459" ["VALUE"]=> array(2) { ["TEXT"]=> string(95) "<p>Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(83) "Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(83) "Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27461" ["VALUE"]=> array(2) { ["TEXT"]=> string(1100) "<p style="text-align: justify;">Myelodysplastic syndromes (MDS) comprise a group (continuum) of clonal hematopoietic diseases associated with high risk of transformation into acute myeloid leukemia (AML) and unfavorable prognosis. Compared to the other hematologic malignant diseases, there was only a modest improvement in survival of MDS patients over the last years. Allogeneic stem cell transplantation remains the only curative option for these patients, however, most of them are not candidates for transplantation. This review focuses on the long-term outcomes of existing therapies and novel agents that are currently tested at different stages of clinical trials. These include inhibitors of TGFβ, various kinase inhibitors, and immune checkpoint inhibitors. Administration of new therapies in the patients with different pathogenetic MDS variants is discussed.</p> <h2>Keywords</h2> <p style="text-align: justify;">Myelodysplastic syndrome, treatment, checkpoint inhibitors, luspatercept, glasdegib, venetoclax, IDH inhibitors.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1044) "Myelodysplastic syndromes (MDS) comprise a group (continuum) of clonal hematopoietic diseases associated with high risk of transformation into acute myeloid leukemia (AML) and unfavorable prognosis. Compared to the other hematologic malignant diseases, there was only a modest improvement in survival of MDS patients over the last years. Allogeneic stem cell transplantation remains the only curative option for these patients, however, most of them are not candidates for transplantation. This review focuses on the long-term outcomes of existing therapies and novel agents that are currently tested at different stages of clinical trials. These include inhibitors of TGFβ, various kinase inhibitors, and immune checkpoint inhibitors. Administration of new therapies in the patients with different pathogenetic MDS variants is discussed.
Keywords
Myelodysplastic syndrome, treatment, checkpoint inhibitors, luspatercept, glasdegib, venetoclax, IDH inhibitors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1044) "Myelodysplastic syndromes (MDS) comprise a group (continuum) of clonal hematopoietic diseases associated with high risk of transformation into acute myeloid leukemia (AML) and unfavorable prognosis. Compared to the other hematologic malignant diseases, there was only a modest improvement in survival of MDS patients over the last years. Allogeneic stem cell transplantation remains the only curative option for these patients, however, most of them are not candidates for transplantation. This review focuses on the long-term outcomes of existing therapies and novel agents that are currently tested at different stages of clinical trials. These include inhibitors of TGFβ, various kinase inhibitors, and immune checkpoint inhibitors. Administration of new therapies in the patients with different pathogenetic MDS variants is discussed.
Keywords
Myelodysplastic syndrome, treatment, checkpoint inhibitors, luspatercept, glasdegib, venetoclax, IDH inhibitors.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27457" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-24-36" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-24-36" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-24-36" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27458" ["VALUE"]=> string(106) "New therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(106) "New therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(106) "New therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27460" ["VALUE"]=> array(2) { ["TEXT"]=> string(481) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> <p>Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7 (921) 796 1951<br> E-mail: moisiv@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(415) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 796 1951
E-mail: moisiv@mail.ru
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 796 1951
E-mail: moisiv@mail.ru
Елена В. Морозова, Николай Ю. Цветков, Ирина О. Туртанова, Иван С. Моисеев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(137) "Елена В. Морозова, Николай Ю. Цветков, Ирина О. Туртанова, Иван С. Моисеев
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27452" ["VALUE"]=> string(22) "01/14/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "01/14/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "01/14/2021 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27453" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "04/09/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27456" ["VALUE"]=> array(2) { ["TEXT"]=> string(1906) "<p style="text-align: justify;">Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.</p> <p style="text-align: justify;">В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1828) "Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.
В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.
Ключевые слова
Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1828) "Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.
В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.
Ключевые слова
Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27455" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [7]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "175" ["~IBLOCK_SECTION_ID"]=> string(3) "175" ["ID"]=> string(4) "1950" ["~ID"]=> string(4) "1950" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["~NAME"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "06/01/2021 02:56:17 pm" ["~TIMESTAMP_X"]=> string(22) "06/01/2021 02:56:17 pm" ["DETAIL_PAGE_URL"]=> string(148) "/en/archive/tom-10-nomer-1/klinicheskie-raboty/immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas/" ["~DETAIL_PAGE_URL"]=> string(148) "/en/archive/tom-10-nomer-1/klinicheskie-raboty/immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(20823) "Introduction
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and classical Hodgkin lymphoma (cHL) was initially described in 1998 [1] and first recognized in the World Health Organization classification as a distinct entity in 2008 [2]. It is a rare and aggressive lymphoma, also known as gray zone lymphoma (GZL), with morphologic and immunophenotypic features overlap with both of primary mediastinal large B-cell lymphoma (PMBL) and cHL, nodular sclerosis [3]. Initially, GZL with mediastinum involvement, i.e., mediastinal GZL (MGZL), was described, whereas data on non-mediastinal GZL (NMGZL) were obtained later. MGZL typically occurs in young adults. In contrast, patients with NMGZL are usually older, with advanced-stage disease and extranodal involvement. However, a large retrospective study in the North American population with 112 cases of GZL demonstrated that the patients with MGZL and NMGZL have similar outcomes [4].
GZL shows a wide variety of morphological features and can be represented by tumor cells cytologically related to cHL (cHL-like GZL), but with large B-cell lymphoma (LBCL) immunophenotype (CD20, CD79a, PAX5, and OCT2), while another type of GZL can demonstrate large B-cell lymphoma morphology (LBCL-like GZL) with cHL immunophenotype (CD15 and CD30). Similar to cHL and PMBL, genetic alterations involving CIITA (16p13.13) and CD274 (PD-L1)/PDCD1LG2 (PD-L2) (9p24.1) have been described in GZL [5]. Epigenetic profile of GZL demonstrated close relationship of GZL with cHL/PMBL and the difference between GZL and DLBCL [6].
Due to relative rarity, difficult diagnosis and lack of randomized trials, clinical management of GZL is challenging. There is no standard for a frontline therapy for GZL, but regimens for aggressive large B-cell lymphoma (CHOP +/-R, EPOCH, DA-EPOCH +/-R, etc.) are preferable with consideration for consolidative radiotherapy for bulk disease [4, 7]. GZL has a worse prognosis than either cHL, or PMBL. Compared to PMBL treated with R-DA-EPOCH, the GZL outcomes were significantly lower with 5-year overall survival (OS) 74% vs 97% and 5- year event-free survival (EFS) of 62% vs 93%, respectively [8, 9]. About 59% of patients achieved a complete response, but 33% had primary refractory disease and 58% relapsed with a median time to relapse of 7 months [4]. The optimal treatment strategy for relapsed/refractory (r/r) GZL is not yet established. Most of the patients received standard salvage chemotherapy and, in case of chemosensitivity, autologous hematopoietic stem cell transplant (HSCT) can be performed. Two-year OS for patients with r/r GZL who underwent HSCT was 88% versus 67% for the patients who did not undergo HSCT [4]. However, some patients cannot be candidates for high-dose chemotherapy with HSCT due to age, comorbidity, or chemoresistant disease.
As based on biological characteristics of GZL, search for novel target and immunotherapy is necessary. Most cases of GZL show expression of CD30 [8]. Brentuximab vedotin (BV) is an anti-CD30 antibody-drug conjugate that is effective in HL and some T-cell lymphomas. Due to the expression of CD30 in GZL, BV is an attractive treatment strategy in patients with GZL and some data on BV therapy in patients with GZL have been published [10].
Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion [11]. Only case series of patients with r/r GZL with complete and durable responses after checkpoint inhibitor treatment were published [12, 13]. In this view, immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL.
Patients and methods
The single-center study included nine patients (6 men and 3 women) with histologically confirmed GZL, relapsed or refractory to at least 2 lines of previous therapy, and treated in RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University. All the patients had active disease following the previous therapy. Patients received nivolumab in dose of 0.5-3 mg/kg every 2 weeks as monotherapy or in combination with lenalidomide or chemotherapy. Total-body PET/CT scans were performed before treatment initiation, and clinical response was assessed by investigators using LYmphoma Response to Immunomodulatory therapy Criteria (LYRIC) every 3 months. Treatment toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v 4.03.
The primary efficacy endpoint was the overall response rate during nivolumab therapy, defined as the proportion of patients with complete response (CR) or partial response (PR) in measurable lesions by LYRIC criteria within a timeframe of 24 months. The efficacy and safety evaluable population included those patients who received at least 1 cycle of therapy. To evaluate the best response, all assessments during therapy were analyzed up to the treatment discontinuation or initiation of other therapy. Secondary endpoints included frequency of grade 3 or higher treatment-related adverse events by NCI CTCAE 4.03, duration of response (DOR), progression-free survival (PFS) and overall survival (OS). Duration of response was defined as the time from initial objective response to documented disease progression or death; PFS was defined as the time from the first nivolumab infusion to disease progression, relapse or death; overall survival (OS) defined as the time from the first nivolumab infusion to death from any reason. In each survival outcome, data were censored at the date of last contact for patients who have not experienced the events of interest during their follow-up. Both OS and PFS were censored at the date of last contact and were estimated using Kaplan-Meier method with 95% CI estimates. All analyses were performed using SPSS system v 17.0 and R 3.6.1 software.
Results
Therapeutic effects of nivolumab-containing treatment schedules
All the patients (n=9) were included into safety and efficacy analysis. The median age was 35 (23–87) years. Six patients (67%) were initially diagnosed as cHL, one patient (11%), as PMBL, and two patients, as GZL (22%). Thus, 6 patients at the first-line therapy received regimens for cHL (ABVD, BEACOPP), and 3 patients were treated with regimens for aggressive large B-cell lymphoma (R-CHOP, R-DA-EPOCH). Most patients (n=7, 78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment, the disease stage III-IV was assessed in 6 cases (67%), and bulky disease was registred in 3 patients (33%).
All nine patients showed high levels of PD-L1 expression (80%-100%) on their tumor cells. In this group, 4 patients (44%) received nivolumab as monotherapy, 3 patients (33%) received nivolumab in combination with chemotherapy, one patient (11%) was treated with nivolumab in combination with BV, and nivolumab in combination with lenalidomide was used in one case (11%). Demographic characteristics and clinical data are summarized in Table 1.
Table 1. Demographic and clinical characteristics of the studied GZL patients
Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Objective response rate among all the treated patients was 89%, with complete response (CR) in 6 cases (67%), and partial response (PR) in 2 cases (22%). One patient (11%) had shown stabilization of the disease as best response. Median duration of response (DOR) was 14 months (range 5-26). After immunotherapy, one patient in CR underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) from haploidentical donor, and 3 patients in CR underwent autologous hematopoietic stem cell transplantation (auto-HSCT) as the consolidation treatment. One patient after allo-HSCT and two patients after auto-HSCT are alive in CR. Another patient after auto-HSCT relapsed and was re-treated with nivolumab combined with brentuximab vedotin, followed by CR achievement. Overall, 4 patients received additional therapy after nivolumab-based treatment, due to disease progression. At the time of analysis, 6 patients are alive and in CR, one patient is alive in PR (proceeding with nivolumab therapy), one patient is alive in progression (receiving another therapy), and one patient died, due to the disease progression. Overall survival and progression-free survival rates were 83% and 48%, respectively. Clinical outcomes are presented in Table 2 and Fig. 1, 2.
Table 2. Clinical complications and outcomes in the studied lymphoma patients

Figure 1. Characteristics of clinical response achieved during observation period
Numbers of the left ordinate axis represent the patient number (see Table 1 and Table 2), OS, overall survival; PFS, progression-free survival; CR, complete remission achieved; PR, partial remission achieved; PD/Relapse, time point of documented progression or relapse; Retreatment, time point of starting repeated nivolumab treatment.
Figure 2. Two-year overall survival (A) and progression-free survival (B) for the patients with r/r GZL after nivolumab-based treatment
Nivolumab toxicity
During nivolumab treatment, 3 (33%) patients experienced grade 3-4 adverse events (AEs), which included two cases of neutropenia (these two patients received nivolumab in combination with chemotherapy) and one case of autoimmune pneumonitis. Due to severe adverse events, nivolumab treatment was discontinued, and the patient received glucocorticoids (1 mg/kg methylprednisolone) with complete resolution of pneumonitis.
Discussion
The diagnosis of GZL is difficult, due to clinical, morphological and immunophenotypic features [14,15]. In our study, only 2 of 9 patients were initially diagnosed with GZL, while 6 patients were diagnosed as classical Hodgkin’s lymphoma (cHL). This group of patients received cHL-like regimens (ABVD or BEACOPP), as frontline therapy. However, dose-intensive chemotherapy might be more effective in GZL, and 5 of 6 patients who received cHL-like regimen showed primary chemoresistance. Retrospective study of the 112 patients with GZL who received ABVD, R-CHOP or R-DA-EPOCH, as frontline therapy, demonstrated significantly inferior PFS rate for the patients treated with ABVD versus DLBCL-based regimen with two-year rates of 23% versus 52%, respectively. In patients with сhemosensitive r/r GZL, high-dose chemotherapy with auto-HSCT may be beneficial [4]. However, in patients with chemoresistant disease, the optimal treatment strategy is not well established, and there are only scarce clinical data on usage of biological agents for r/r GZL.
GZL share a common genetic signature and high rates of 9p24.1 gain, similar to cHL. Blockade of PD-1/PD-L1 pathway demonstrated beneficial therapeutic effect in cHL patients [16,17], but there is limited data on effects of checkpoint inhibitors in GZL. In our study, all 9 patients initially demonstrated high-level (80%-100%) PD-L1 expression. Depending on the stage of the disease, patients received nivolumab at different regimens, i.e., nivolumab as monotherapy, or in combination with other drugs (lenalidomide, BV, chemotherapy), dose of nivolumab was 0.5-3 mg/kg. Decrease of tumor mass was noted in all patients during nivolumab-based therapy, with objective response in 8 of 9 patients, regardless of nivolumab regimen and dose. However, an elderly patient (87 y.o.) who achieved only stabilization of the disease, well tolerated the nivolumab therapy, showing suppression of tumor growth and good quality of life.
BV may augment the effect of PD-1 blockade, and combination of nivolumab with BV demonstrated efficacy in r/r cHL [18]. In this study, 2 patients received nivolumab with BV, one of them relapsed after earlier nivolumab treatment. Both patients achieved CR after this treatment. Thus, nivolumab with BV may be perspective treatment option for the patients with r/r GZL. Moreover, among 8 patients with objective response to nivolumab-based treatment, four patients received hematopoietic stem cell transplantation as the consolidation of response. Thus, nivolumab can be used as a "bridge" to HSCT.
Conclusion
Diagnosis and treatment of GZL still represent a challenge to clinicians. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL. Larger studies of nivolumab therapy and development of optimal combinations of target and immunotherapy for GZL are required in future.
Acknowledgments
The authors thank the patients as well as research and medical staff for making this study possible.
Financial disclosure
The authors have nothing to disclose.
Conflict of interest statement
There are no conflicts of interest to report.
References
- Rüdiger T, Jaffe ES, Delsol G, deWolf-Peeters C, Gascoyne RD, Georgii A, et al. Workshop report on Hodgkin's disease and related diseases ('grey zone' lymphoma). Ann Oncol. 1998;9 Suppl 5:S31-8. doi: 10.1093/annonc/9.suppl_5.s31.
- Swerdlow S, Campo E, Harris N, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edition. Lyon (France): International Agency for Research on Cancer; 2008.
- Pilichowska M, Pittaluga S, Ferry JA, Hemminger J, Chang H, Kanakry JA, et al. Clinicopathologic consensus study of gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv. 2017;1(26):2600-2609. doi: 10.1182/bloodadvances.2017009472.
- Evens AM, Kanakry JA, Sehn LH, Kritharis A, Feldman T, Kroll A, et al. Gray zone lymphoma with features intermediate between classical Hodgkin lymphoma and diffuse large B-cell lymphoma: characteristics, outcomes, and prognostication among a large multicenter cohort. Am J Hematol. 2015; 90(9):778-783. doi: 10.1002/ajh.24082.
- Sarkozy C, Copie-Bergman C, Damotte D, Ben-Neriah S, Burroni B, Cornillon J, et al. Gray-zone Lymphoma Between cHL and Large B-Cell Lymphoma: A Histopathologic Series From the LYSA. Am J Surg Pathol. 2019; 43(3):341-351. doi: 10.1097/PAS.0000000000001198.
- Eberle FC, Rodriguez-Canales J, Wei L, Hanson JC, Killian JK, Sun HW, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin's lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. 2011; 96(4):558-566. doi: 10.3324/haematol.2010.033167.
- Chihara D, Westin JR, Miranda RN, Cheah CY, Oki Y, Turturro F, et al. Dose adjusted-EPOCH-R and mediastinal disease may improve outcomes for patients with gray-zone lymphoma. Br J Haematol. 2017;179(3):503-506. doi: 10.1111/bjh.14226.
- Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood. 2015; 125(1):33-9. doi: 10.1182/blood-2014-05-575092.
- Wilson WH, Pittaluga S, Nicolae A, Camphausen K, Shovlin M, Steinberg SM, Roschewski M, et al. A prospective study of mediastinal gray-zone lymphoma. Blood. 2014; 124(10):1563-1569. doi: 10.1182/blood-2014-03-564906.
- Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394-1402. doi: 10.1182/blood-2014-09-598763.
- Eberle FC, Salaverria I, Steidl C, Summers TA Jr, Pittaluga S, Neriah SB, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol. 2011; 24(12):1586-1597. doi: 10.1038/modpathol.2011.116.
- Melani C, Major A, Schowinsky J, Roschewski M, Pittaluga S, Jaffe ES, et al. PD-1 Blockade in Mediastinal Gray-Zone Lymphoma. N Engl J Med. 2017; 377(1):89-91. doi: 10.1056/NEJMc1704767.
- Rosales YMZ, Mesquita JL, Garcia YDO, Paz FRF, Campos NCB, de Vasconcelos Leitão JP, et al. Use of checkpoint inhibitors in gray zone lymphoma. Hematol Oncol Stem Cell Ther. 2020:S1658-3876(20)30112-6. doi: 10.1016/j.hemonc.2020.06.001.
- Misyurina AE, Kravchenko SK, Mangasarova YK, et al. Gray-zone lymphoma. Examples of rare clinical manifestation. Therapeutic Archive. 2019; 91 (4): 107-113. doi: 10.26442/00403660.2019.04.000071.
- Kovrigina AM, Probatova NA. Hodgkin’s lymphoma and large cell lymphomas. Moscow: MIA Publ.; 2007 (In Russian).
- Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018; 36(14):1428-1439. doi: 10.1200/JCO.2017.76.0793.
- Lepik KV, Mikhailova NB, Moiseev IS, Kondakova EV, Tsvetkova LA, Zalyalov YR, et al. Nivolumab for the treatment of relapsed and refractory classical Hodgkin lymphoma after ASCT and in ASCT-naïve patients. Leuk Lymphoma. 2019; 60(9):2316-2319. doi: 10.1080/10428194.2019.1573368.
- Herrera A, Moskowitz A, Bartlett N, Vose JM, Ramchandren R, Feldman TA, LaCasce AS, Ansell SM, Moskowitz CH, Fenton K, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018;131:1183-1194. doi: 10.1182/blood-2017-10-811224.
" ["~DETAIL_TEXT"]=> string(20823) "
Introduction
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and classical Hodgkin lymphoma (cHL) was initially described in 1998 [1] and first recognized in the World Health Organization classification as a distinct entity in 2008 [2]. It is a rare and aggressive lymphoma, also known as gray zone lymphoma (GZL), with morphologic and immunophenotypic features overlap with both of primary mediastinal large B-cell lymphoma (PMBL) and cHL, nodular sclerosis [3]. Initially, GZL with mediastinum involvement, i.e., mediastinal GZL (MGZL), was described, whereas data on non-mediastinal GZL (NMGZL) were obtained later. MGZL typically occurs in young adults. In contrast, patients with NMGZL are usually older, with advanced-stage disease and extranodal involvement. However, a large retrospective study in the North American population with 112 cases of GZL demonstrated that the patients with MGZL and NMGZL have similar outcomes [4].
GZL shows a wide variety of morphological features and can be represented by tumor cells cytologically related to cHL (cHL-like GZL), but with large B-cell lymphoma (LBCL) immunophenotype (CD20, CD79a, PAX5, and OCT2), while another type of GZL can demonstrate large B-cell lymphoma morphology (LBCL-like GZL) with cHL immunophenotype (CD15 and CD30). Similar to cHL and PMBL, genetic alterations involving CIITA (16p13.13) and CD274 (PD-L1)/PDCD1LG2 (PD-L2) (9p24.1) have been described in GZL [5]. Epigenetic profile of GZL demonstrated close relationship of GZL with cHL/PMBL and the difference between GZL and DLBCL [6].
Due to relative rarity, difficult diagnosis and lack of randomized trials, clinical management of GZL is challenging. There is no standard for a frontline therapy for GZL, but regimens for aggressive large B-cell lymphoma (CHOP +/-R, EPOCH, DA-EPOCH +/-R, etc.) are preferable with consideration for consolidative radiotherapy for bulk disease [4, 7]. GZL has a worse prognosis than either cHL, or PMBL. Compared to PMBL treated with R-DA-EPOCH, the GZL outcomes were significantly lower with 5-year overall survival (OS) 74% vs 97% and 5- year event-free survival (EFS) of 62% vs 93%, respectively [8, 9]. About 59% of patients achieved a complete response, but 33% had primary refractory disease and 58% relapsed with a median time to relapse of 7 months [4]. The optimal treatment strategy for relapsed/refractory (r/r) GZL is not yet established. Most of the patients received standard salvage chemotherapy and, in case of chemosensitivity, autologous hematopoietic stem cell transplant (HSCT) can be performed. Two-year OS for patients with r/r GZL who underwent HSCT was 88% versus 67% for the patients who did not undergo HSCT [4]. However, some patients cannot be candidates for high-dose chemotherapy with HSCT due to age, comorbidity, or chemoresistant disease.
As based on biological characteristics of GZL, search for novel target and immunotherapy is necessary. Most cases of GZL show expression of CD30 [8]. Brentuximab vedotin (BV) is an anti-CD30 antibody-drug conjugate that is effective in HL and some T-cell lymphomas. Due to the expression of CD30 in GZL, BV is an attractive treatment strategy in patients with GZL and some data on BV therapy in patients with GZL have been published [10].
Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion [11]. Only case series of patients with r/r GZL with complete and durable responses after checkpoint inhibitor treatment were published [12, 13]. In this view, immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL.
Patients and methods
The single-center study included nine patients (6 men and 3 women) with histologically confirmed GZL, relapsed or refractory to at least 2 lines of previous therapy, and treated in RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University. All the patients had active disease following the previous therapy. Patients received nivolumab in dose of 0.5-3 mg/kg every 2 weeks as monotherapy or in combination with lenalidomide or chemotherapy. Total-body PET/CT scans were performed before treatment initiation, and clinical response was assessed by investigators using LYmphoma Response to Immunomodulatory therapy Criteria (LYRIC) every 3 months. Treatment toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v 4.03.
The primary efficacy endpoint was the overall response rate during nivolumab therapy, defined as the proportion of patients with complete response (CR) or partial response (PR) in measurable lesions by LYRIC criteria within a timeframe of 24 months. The efficacy and safety evaluable population included those patients who received at least 1 cycle of therapy. To evaluate the best response, all assessments during therapy were analyzed up to the treatment discontinuation or initiation of other therapy. Secondary endpoints included frequency of grade 3 or higher treatment-related adverse events by NCI CTCAE 4.03, duration of response (DOR), progression-free survival (PFS) and overall survival (OS). Duration of response was defined as the time from initial objective response to documented disease progression or death; PFS was defined as the time from the first nivolumab infusion to disease progression, relapse or death; overall survival (OS) defined as the time from the first nivolumab infusion to death from any reason. In each survival outcome, data were censored at the date of last contact for patients who have not experienced the events of interest during their follow-up. Both OS and PFS were censored at the date of last contact and were estimated using Kaplan-Meier method with 95% CI estimates. All analyses were performed using SPSS system v 17.0 and R 3.6.1 software.
Results
Therapeutic effects of nivolumab-containing treatment schedules
All the patients (n=9) were included into safety and efficacy analysis. The median age was 35 (23–87) years. Six patients (67%) were initially diagnosed as cHL, one patient (11%), as PMBL, and two patients, as GZL (22%). Thus, 6 patients at the first-line therapy received regimens for cHL (ABVD, BEACOPP), and 3 patients were treated with regimens for aggressive large B-cell lymphoma (R-CHOP, R-DA-EPOCH). Most patients (n=7, 78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment, the disease stage III-IV was assessed in 6 cases (67%), and bulky disease was registred in 3 patients (33%).
All nine patients showed high levels of PD-L1 expression (80%-100%) on their tumor cells. In this group, 4 patients (44%) received nivolumab as monotherapy, 3 patients (33%) received nivolumab in combination with chemotherapy, one patient (11%) was treated with nivolumab in combination with BV, and nivolumab in combination with lenalidomide was used in one case (11%). Demographic characteristics and clinical data are summarized in Table 1.
Table 1. Demographic and clinical characteristics of the studied GZL patients

Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Objective response rate among all the treated patients was 89%, with complete response (CR) in 6 cases (67%), and partial response (PR) in 2 cases (22%). One patient (11%) had shown stabilization of the disease as best response. Median duration of response (DOR) was 14 months (range 5-26). After immunotherapy, one patient in CR underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) from haploidentical donor, and 3 patients in CR underwent autologous hematopoietic stem cell transplantation (auto-HSCT) as the consolidation treatment. One patient after allo-HSCT and two patients after auto-HSCT are alive in CR. Another patient after auto-HSCT relapsed and was re-treated with nivolumab combined with brentuximab vedotin, followed by CR achievement. Overall, 4 patients received additional therapy after nivolumab-based treatment, due to disease progression. At the time of analysis, 6 patients are alive and in CR, one patient is alive in PR (proceeding with nivolumab therapy), one patient is alive in progression (receiving another therapy), and one patient died, due to the disease progression. Overall survival and progression-free survival rates were 83% and 48%, respectively. Clinical outcomes are presented in Table 2 and Fig. 1, 2.
Table 2. Clinical complications and outcomes in the studied lymphoma patients


Figure 1. Characteristics of clinical response achieved during observation period
Numbers of the left ordinate axis represent the patient number (see Table 1 and Table 2), OS, overall survival; PFS, progression-free survival; CR, complete remission achieved; PR, partial remission achieved; PD/Relapse, time point of documented progression or relapse; Retreatment, time point of starting repeated nivolumab treatment.

Figure 2. Two-year overall survival (A) and progression-free survival (B) for the patients with r/r GZL after nivolumab-based treatment
Nivolumab toxicity
During nivolumab treatment, 3 (33%) patients experienced grade 3-4 adverse events (AEs), which included two cases of neutropenia (these two patients received nivolumab in combination with chemotherapy) and one case of autoimmune pneumonitis. Due to severe adverse events, nivolumab treatment was discontinued, and the patient received glucocorticoids (1 mg/kg methylprednisolone) with complete resolution of pneumonitis.
Discussion
The diagnosis of GZL is difficult, due to clinical, morphological and immunophenotypic features [14,15]. In our study, only 2 of 9 patients were initially diagnosed with GZL, while 6 patients were diagnosed as classical Hodgkin’s lymphoma (cHL). This group of patients received cHL-like regimens (ABVD or BEACOPP), as frontline therapy. However, dose-intensive chemotherapy might be more effective in GZL, and 5 of 6 patients who received cHL-like regimen showed primary chemoresistance. Retrospective study of the 112 patients with GZL who received ABVD, R-CHOP or R-DA-EPOCH, as frontline therapy, demonstrated significantly inferior PFS rate for the patients treated with ABVD versus DLBCL-based regimen with two-year rates of 23% versus 52%, respectively. In patients with сhemosensitive r/r GZL, high-dose chemotherapy with auto-HSCT may be beneficial [4]. However, in patients with chemoresistant disease, the optimal treatment strategy is not well established, and there are only scarce clinical data on usage of biological agents for r/r GZL.
GZL share a common genetic signature and high rates of 9p24.1 gain, similar to cHL. Blockade of PD-1/PD-L1 pathway demonstrated beneficial therapeutic effect in cHL patients [16,17], but there is limited data on effects of checkpoint inhibitors in GZL. In our study, all 9 patients initially demonstrated high-level (80%-100%) PD-L1 expression. Depending on the stage of the disease, patients received nivolumab at different regimens, i.e., nivolumab as monotherapy, or in combination with other drugs (lenalidomide, BV, chemotherapy), dose of nivolumab was 0.5-3 mg/kg. Decrease of tumor mass was noted in all patients during nivolumab-based therapy, with objective response in 8 of 9 patients, regardless of nivolumab regimen and dose. However, an elderly patient (87 y.o.) who achieved only stabilization of the disease, well tolerated the nivolumab therapy, showing suppression of tumor growth and good quality of life.
BV may augment the effect of PD-1 blockade, and combination of nivolumab with BV demonstrated efficacy in r/r cHL [18]. In this study, 2 patients received nivolumab with BV, one of them relapsed after earlier nivolumab treatment. Both patients achieved CR after this treatment. Thus, nivolumab with BV may be perspective treatment option for the patients with r/r GZL. Moreover, among 8 patients with objective response to nivolumab-based treatment, four patients received hematopoietic stem cell transplantation as the consolidation of response. Thus, nivolumab can be used as a "bridge" to HSCT.
Conclusion
Diagnosis and treatment of GZL still represent a challenge to clinicians. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL. Larger studies of nivolumab therapy and development of optimal combinations of target and immunotherapy for GZL are required in future.
Acknowledgments
The authors thank the patients as well as research and medical staff for making this study possible.
Financial disclosure
The authors have nothing to disclose.
Conflict of interest statement
There are no conflicts of interest to report.
References
- Rüdiger T, Jaffe ES, Delsol G, deWolf-Peeters C, Gascoyne RD, Georgii A, et al. Workshop report on Hodgkin's disease and related diseases ('grey zone' lymphoma). Ann Oncol. 1998;9 Suppl 5:S31-8. doi: 10.1093/annonc/9.suppl_5.s31.
- Swerdlow S, Campo E, Harris N, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edition. Lyon (France): International Agency for Research on Cancer; 2008.
- Pilichowska M, Pittaluga S, Ferry JA, Hemminger J, Chang H, Kanakry JA, et al. Clinicopathologic consensus study of gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv. 2017;1(26):2600-2609. doi: 10.1182/bloodadvances.2017009472.
- Evens AM, Kanakry JA, Sehn LH, Kritharis A, Feldman T, Kroll A, et al. Gray zone lymphoma with features intermediate between classical Hodgkin lymphoma and diffuse large B-cell lymphoma: characteristics, outcomes, and prognostication among a large multicenter cohort. Am J Hematol. 2015; 90(9):778-783. doi: 10.1002/ajh.24082.
- Sarkozy C, Copie-Bergman C, Damotte D, Ben-Neriah S, Burroni B, Cornillon J, et al. Gray-zone Lymphoma Between cHL and Large B-Cell Lymphoma: A Histopathologic Series From the LYSA. Am J Surg Pathol. 2019; 43(3):341-351. doi: 10.1097/PAS.0000000000001198.
- Eberle FC, Rodriguez-Canales J, Wei L, Hanson JC, Killian JK, Sun HW, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin's lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. 2011; 96(4):558-566. doi: 10.3324/haematol.2010.033167.
- Chihara D, Westin JR, Miranda RN, Cheah CY, Oki Y, Turturro F, et al. Dose adjusted-EPOCH-R and mediastinal disease may improve outcomes for patients with gray-zone lymphoma. Br J Haematol. 2017;179(3):503-506. doi: 10.1111/bjh.14226.
- Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood. 2015; 125(1):33-9. doi: 10.1182/blood-2014-05-575092.
- Wilson WH, Pittaluga S, Nicolae A, Camphausen K, Shovlin M, Steinberg SM, Roschewski M, et al. A prospective study of mediastinal gray-zone lymphoma. Blood. 2014; 124(10):1563-1569. doi: 10.1182/blood-2014-03-564906.
- Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394-1402. doi: 10.1182/blood-2014-09-598763.
- Eberle FC, Salaverria I, Steidl C, Summers TA Jr, Pittaluga S, Neriah SB, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol. 2011; 24(12):1586-1597. doi: 10.1038/modpathol.2011.116.
- Melani C, Major A, Schowinsky J, Roschewski M, Pittaluga S, Jaffe ES, et al. PD-1 Blockade in Mediastinal Gray-Zone Lymphoma. N Engl J Med. 2017; 377(1):89-91. doi: 10.1056/NEJMc1704767.
- Rosales YMZ, Mesquita JL, Garcia YDO, Paz FRF, Campos NCB, de Vasconcelos Leitão JP, et al. Use of checkpoint inhibitors in gray zone lymphoma. Hematol Oncol Stem Cell Ther. 2020:S1658-3876(20)30112-6. doi: 10.1016/j.hemonc.2020.06.001.
- Misyurina AE, Kravchenko SK, Mangasarova YK, et al. Gray-zone lymphoma. Examples of rare clinical manifestation. Therapeutic Archive. 2019; 91 (4): 107-113. doi: 10.26442/00403660.2019.04.000071.
- Kovrigina AM, Probatova NA. Hodgkin’s lymphoma and large cell lymphomas. Moscow: MIA Publ.; 2007 (In Russian).
- Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018; 36(14):1428-1439. doi: 10.1200/JCO.2017.76.0793.
- Lepik KV, Mikhailova NB, Moiseev IS, Kondakova EV, Tsvetkova LA, Zalyalov YR, et al. Nivolumab for the treatment of relapsed and refractory classical Hodgkin lymphoma after ASCT and in ASCT-naïve patients. Leuk Lymphoma. 2019; 60(9):2316-2319. doi: 10.1080/10428194.2019.1573368.
- Herrera A, Moskowitz A, Bartlett N, Vose JM, Ramchandren R, Feldman TA, LaCasce AS, Ansell SM, Moskowitz CH, Fenton K, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018;131:1183-1194. doi: 10.1182/blood-2017-10-811224.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(100) "immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas" ["~CODE"]=> string(100) "immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas" ["EXTERNAL_ID"]=> string(4) "1950" ["~EXTERNAL_ID"]=> string(4) "1950" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(581) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой ХоджкинаNivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(3650) "<p style="text-align: justify;">Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_META_TITLE"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_META_KEYWORDS"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_META_DESCRIPTION"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_PICTURE_FILE_ALT"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_PICTURE_FILE_TITLE"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(400) "Иммунотерапия на основе ниволумаба при рецидивирующей/рефрактерной В-клеточной лимфоме, неклассифициируемой, с промежуточными признаками между диффузной крупноклеточной В-лимфомой и классической лимфомой Ходжкина" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "immunoterapiya-na-osnove-nivolumaba-pri-retsidiviruyushchey-refrakternoy-v-kletochnoy-limfome-neklas" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "175" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27462" ["VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27463" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27464" ["VALUE"]=> array(2) { ["TEXT"]=> string(511) "<p>Олеся Г. Смыкова, Кирилл В. Лепик, Наталья Б. Михайлова, Елена В. Кондакова, Евгения С. Борзенкова, Елена Е. Лепик, Юрий Р. Залялов, Лилия В. Стельмах, Вадим В. Байков, Иван С. Моисеев, Александр Д. Кулагин, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(477) "
Олеся Г. Смыкова, Кирилл В. Лепик, Наталья Б. Михайлова, Елена В. Кондакова, Евгения С. Борзенкова, Елена Е. Лепик, Юрий Р. Залялов, Лилия В. Стельмах, Вадим В. Байков, Иван С. Моисеев, Александр Д. Кулагин, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27465" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27466" ["VALUE"]=> array(2) { ["TEXT"]=> string(3650) "<p style="text-align: justify;">Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3594) "Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.
Ключевые слова
Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27467" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-37-43" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-37-43" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27469" ["VALUE"]=> array(2) { ["TEXT"]=> string(345) "<p>Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(311) "Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27470" ["VALUE"]=> array(2) { ["TEXT"]=> string(441) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia<br> E-mail: olesya.gen@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(387) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia
E-mail: olesya.gen@gmail.com
The treatment of relapsed/refractory gray zone lymphoma (r/r GZL) remains challenging. Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion. Immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL. We have retrospectively assessed efficacy and toxicity of nivolumab-based regimens in nine patients with r/r GZL. Most of the patients n=7 (78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment disease stage III-IV was in n=6 (67%) patients and bulky disease was in n=3 (33%) patients. All nine patients had high-level of PD-L1 expression (80%-100%) on tumor cells. In this group n=4 (44%) patients received nivolumab as monotherapy, n=3 (33%) received nivolumab in combination with chemotherapy, n=1 (11%) received nivolumab in combination with BV and n=1 (11%) received nivolumab in combination with lenalidomide. The objective response rate among all treated patients was 89% with 6 cases (67%) of complete response and 2 (22%), with partial response. One patient (11%) had stabilization of the disease as best response. Median duration of response was 14 (range 5-26) months. Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Overall survival and progression free survival rates were 83% and 38%, respectively. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL.
Keywords
Gray-zone lymphoma, relapse, immune checkpoints inhibitors, nivolumab, hematopoietic stem cell transplantation, allogeneic, autologous.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27468" ["VALUE"]=> string(181) "Nivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(181) "Nivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27528" ["VALUE"]=> string(4) "2418" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2418" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27529" ["VALUE"]=> string(4) "2419" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2419" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27469" ["VALUE"]=> array(2) { ["TEXT"]=> string(345) "<p>Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(311) "Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(311) "Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, Boris V. Afanasyev
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27471" ["VALUE"]=> array(2) { ["TEXT"]=> string(1906) "<p style="text-align: justify;">The treatment of relapsed/refractory gray zone lymphoma (r/r GZL) remains challenging. Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion. Immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL. We have retrospectively assessed efficacy and toxicity of nivolumab-based regimens in nine patients with r/r GZL. Most of the patients n=7 (78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment disease stage III-IV was in n=6 (67%) patients and bulky disease was in n=3 (33%) patients. All nine patients had high-level of PD-L1 expression (80%-100%) on tumor cells. In this group n=4 (44%) patients received nivolumab as monotherapy, n=3 (33%) received nivolumab in combination with chemotherapy, n=1 (11%) received nivolumab in combination with BV and n=1 (11%) received nivolumab in combination with lenalidomide. The objective response rate among all treated patients was 89% with 6 cases (67%) of complete response and 2 (22%), with partial response. One patient (11%) had stabilization of the disease as best response. Median duration of response was 14 (range 5-26) months. Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Overall survival and progression free survival rates were 83% and 38%, respectively. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL. </p> <h2>Keywords</h2> <p style="text-align: justify;">Gray-zone lymphoma, relapse, immune checkpoints inhibitors, nivolumab, hematopoietic stem cell transplantation, allogeneic, autologous.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1850) "The treatment of relapsed/refractory gray zone lymphoma (r/r GZL) remains challenging. Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion. Immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL. We have retrospectively assessed efficacy and toxicity of nivolumab-based regimens in nine patients with r/r GZL. Most of the patients n=7 (78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment disease stage III-IV was in n=6 (67%) patients and bulky disease was in n=3 (33%) patients. All nine patients had high-level of PD-L1 expression (80%-100%) on tumor cells. In this group n=4 (44%) patients received nivolumab as monotherapy, n=3 (33%) received nivolumab in combination with chemotherapy, n=1 (11%) received nivolumab in combination with BV and n=1 (11%) received nivolumab in combination with lenalidomide. The objective response rate among all treated patients was 89% with 6 cases (67%) of complete response and 2 (22%), with partial response. One patient (11%) had stabilization of the disease as best response. Median duration of response was 14 (range 5-26) months. Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Overall survival and progression free survival rates were 83% and 38%, respectively. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL.
Keywords
Gray-zone lymphoma, relapse, immune checkpoints inhibitors, nivolumab, hematopoietic stem cell transplantation, allogeneic, autologous.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1850) "The treatment of relapsed/refractory gray zone lymphoma (r/r GZL) remains challenging. Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion. Immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL. We have retrospectively assessed efficacy and toxicity of nivolumab-based regimens in nine patients with r/r GZL. Most of the patients n=7 (78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment disease stage III-IV was in n=6 (67%) patients and bulky disease was in n=3 (33%) patients. All nine patients had high-level of PD-L1 expression (80%-100%) on tumor cells. In this group n=4 (44%) patients received nivolumab as monotherapy, n=3 (33%) received nivolumab in combination with chemotherapy, n=1 (11%) received nivolumab in combination with BV and n=1 (11%) received nivolumab in combination with lenalidomide. The objective response rate among all treated patients was 89% with 6 cases (67%) of complete response and 2 (22%), with partial response. One patient (11%) had stabilization of the disease as best response. Median duration of response was 14 (range 5-26) months. Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Overall survival and progression free survival rates were 83% and 38%, respectively. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL.
Keywords
Gray-zone lymphoma, relapse, immune checkpoints inhibitors, nivolumab, hematopoietic stem cell transplantation, allogeneic, autologous.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27467" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-37-43" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-37-43" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-37-43" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27468" ["VALUE"]=> string(181) "Nivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(181) "Nivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(181) "Nivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27470" ["VALUE"]=> array(2) { ["TEXT"]=> string(441) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia<br> E-mail: olesya.gen@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(387) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia
E-mail: olesya.gen@gmail.com
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia
E-mail: olesya.gen@gmail.com
Олеся Г. Смыкова, Кирилл В. Лепик, Наталья Б. Михайлова, Елена В. Кондакова, Евгения С. Борзенкова, Елена Е. Лепик, Юрий Р. Залялов, Лилия В. Стельмах, Вадим В. Байков, Иван С. Моисеев, Александр Д. Кулагин, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(477) "Олеся Г. Смыкова, Кирилл В. Лепик, Наталья Б. Михайлова, Елена В. Кондакова, Евгения С. Борзенкова, Елена Е. Лепик, Юрий Р. Залялов, Лилия В. Стельмах, Вадим В. Байков, Иван С. Моисеев, Александр Д. Кулагин, Борис В. Афанасьев
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27462" ["VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/08/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/08/2021 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27463" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "04/09/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27466" ["VALUE"]=> array(2) { ["TEXT"]=> string(3650) "<p style="text-align: justify;">Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3594) "Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.
Ключевые слова
Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3594) "Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.
Ключевые слова
Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27465" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [8]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "176" ["~IBLOCK_SECTION_ID"]=> string(3) "176" ["ID"]=> string(4) "1955" ["~ID"]=> string(4) "1955" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["~NAME"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "06/03/2021 10:06:33 am" ["~TIMESTAMP_X"]=> string(22) "06/03/2021 10:06:33 am" ["DETAIL_PAGE_URL"]=> string(159) "/en/archive/tom-10-nomer-1/eksperimentalnye-issledovaniya/morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl/" ["~DETAIL_PAGE_URL"]=> string(159) "/en/archive/tom-10-nomer-1/eksperimentalnye-issledovaniya/morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(27779) "Introduction
Oncological diseases are among the main causes of death worldwide: cancer mortality reaches 20%. The World Health Organization predicts that in 20 years this value will double. In addition to existing methods of cancer treatment (surgery, radiotherapy, chemotherapy), a new method (targeted drug therapy) has been developed over last decade.
In many countries, doxorubicin monotherapy is considered to be the standard chemotherapy procedure [1]. DОХ efficiently inhibits tumor growth by intercalation into DNA of cancer cells, which prevents replication process, thus leading to cell death. However, its clinical applications are limited by the dose-dependent cardiac toxicity. Moreover, this substance shows some disadvantages, i.e., short lifetime, accumulation in liver, and multidrug resistance of malignant cells [2]. Different delivery systems (DS) may be used in order to reduce these side effects. Prolongation of DOX release from DS can reduce probability of overdosage.
In the recent years, a large number of nano- and submicron-sized delivery systems for anti-cancer drugs have been developed [3]. The basic structure of carriers may vary; bio-organic objects are commonly used as carriers: exosomes [4], liposomes [5], dendrimers, macrophages [6], micelles [7], as well as inorganic substances, e.g., carbon nanotubes [8], calcium phosphates [9, 10], SiO2 [11], and CaCO3 [12]. It should be noted that manufacturing of most DS is rather laborious; in a number of cases, it is necessary to synthesize complex polymers. Meanwhile, preparation of submicron-sized porous СаСО3 vaterites is very simple, i.e., co-precipitation of СаСl2 and Na2CO3 solutions [13]. Nanomicron-sized particles can be prepared by increasing viscosity of solutions [14] or changing ratio between concentrations of salts [15]. Of the three CaCO3 polymorphs, porous vaterite is the least stable. Other forms (calcite and needle-shaped aragonite) are not porous. The CaCO3 vaterites are biocompatible and biodegradable [16]. The particles have anti-cancer activity, even without encapsulated drugs, since CaCO3 vaterites are shown to modulate local pH and inhibit tumor growth in vivo [17].
There are few studies on usage of different drug delivery systems based on CaCO3 for tumor suppression, e.g., the pH dependence of CaCO3 may control release of photosensitizer leading to cancer cell apoptosis [14]. The review [18] gives several examples of applications for CaCO3 nanoparticles as carriers for various anti-cancer drugs, including DOX.
Various methods have been proposed to increase efficiency of delivery systems. Modification of polymer particles with polyelectrolyte coating leads to prolonged drug circulation time [19]. Similar effect can be expected upon treatment of CaCO3 carriers with polymers. Special study concerned behavior and fate of hybrid phospholipid-treated CaCO3 nanoparticles without drugs in vitro and in tumor-bearing mice [20]. Their uptake, penetration and distribution in tissues were investigated. It was demonstrated that the hybrid nanoparticles showed almost no cytotoxicity in vitro or in vivo and had high biocompatibility. The lipid-coated CaCO3 cores saturated with DOX help to overcome chemoresistance in hepatocellular carcinoma [21]. In our earlier works, it has been demonstrated [12] that the properties of CaCO3-based delivery systems for DOX may be improved. In particular, the drug load can be increased, the carrier morphology can be stabilized, and drug release can be prolonged by covering the cores with polyelectrolytes (dextran sulfate, polyacrylic acid, and polyvinyl sulfonate). In our recent works [22, 23], behavior of the DS based on submicron-sized CaCO3 particles in mouse muscle tissue was studied for the first time. We have revealed that the DS based on parent porous vaterites and the vaterites coated by DexS undergo complete bioresorption in mice within, respectively, 2 and 3 weeks after administration. This process involves the stage of transition to needle-shaped aragonite structures. No pathological effects of the carriers on the surrounding tissues have been observed.
Anti-cancer therapy involves different ways of drug administration, including local introduction of DS containing active drugs [22]. For example, during treatment of hepatocellular cancer, the drug is introduced intraperitoneally. In this case, the DS will be exposed to interstitial fluids, which differ from blood plasma, mainly, in lesser protein contents.
Thus, the goals of the present work were: (i) to study in vitro structural changes in hybrid DOX delivery systems (based on porous CaCO3 vaterites modified with DexS) in human blood plasma; (ii) to assess correlation between DOX release profiles from these DS into human plasma, and concomitant structural changes of the carrier substance.
Materials and methods
Reagents
Doxorubicin hydrochloride under the brand name of Sindroxocin, which contains 17% of doxorubicin (DOX) and 83% of lactose, was purchased from Actavis (Hafnarfjordur, Iceland). In experimental studies, doxorubicin salt with protonated amino group (–NH3+) was used. Inorganic salts (CaCl2 × 2H2O, Na2CO3), acetone, and dextran sulfate, Mw=9-20 kDa were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis of carbonate cores
Porous vaterites (СаСО3 cores) were prepared by co-precipitation according to the technique described in [13], with some modifications. Equal volumes of 1 M aqueous solutions of CaCl2×2H2O and Na2CO3 were rapidly mixed with stirring (1000 rpm) for 30 s, using RW 20 anchor-type mechanical stirrer (Kika-Werk, Switzerland). The resulting suspension was then filtered through Schott glass filter (#16), washed thrice with distilled water, then with acetone/water mixtures at increasing acetone concentrations (33%, 50%, and 100%). The precipitate was dried in thermostat at 40-50°C, until achieving constant weight.
Modification of carbonate cores with DexS and preparation of hybrid DS
The carbonate cores were coated with polyanions (sodium salt of dextran sulfate). СаСО3 cores (50 mg) were added to 1 mg/mL aqueous solution of DexS (10 mL). The suspension was stirred using a Multi Bio RS-24 rotor (Biosan, Latvia) for 1 h. Solid fraction was filtered off using a Schott glass filter (#16), washed thrice with distilled water and dried at 30°C.
DOX loading and release
Loading of hybrid DS with DOX was performed by continuous stirring of the mixture of DSs suspension and DOX solution (C=2 mg/mL) for 24 hours. The DOX/(СаСО3+DexS) ratio was 0.4. After mixing, the suspension was centrifuged at 8000 rpm for 3 min, and DOX amounts were determined in supernatants.
DOX loading (L) was calculated by the following equation: L = (mi − ms)/mp, where mi is initial amount of DOX (mg); ms, the amount of non-encapsulated DOX in supernatant solution (mg); mp, amount of particles (mg). DOX concentrations were determined using the calibration curves obtained from optical density values, using appropriate solvents (λ = 480 nm). The measurements were carried out using SF-2000 spectrophotometer (LOMO, St. Petersburg, Russia).
In vitro release of DOX from hybrid DSs was analyzed in human blood plasma obtained from the Department of Blood Transfusion, Pavlov University. Incubation of DS in plasma was carried out at 20°C, with constant mixing. Aliquots were collected from incubation mixtures every 1-2 hours after centrifugation of core suspension at 3000 rpm for 3 min. The DOX contents in aliquots were determined by spectrophotometry. The supernatants obtained for similarly incubated DOX-free hybrid CaCO3 cores were used as a reference solution. The cumulative release was determined as percentage of DOX amounts loaded into the initial cores.
All the measurements were carried out in triplicate.
Interaction between DexS-modified CaCO3 and human blood plasma
The interaction between hybrid carbonate cores and human blood plasma occurred in suspension at continuous stirring for different periods of time. Upon completion of the reaction, the cores were centrifuged (5 min at 3000 rpm), the supernatant was discarded and substituted by blood plasma. The procedure was performed twice. The cores were dried at 40°C until constant weight was achieved.
Scanning electron microscopy (SEM)
SEM micrographs of DexS-coated CaCO3 cores were obtained by means Supra 55VP scanning electron microscope (Carl Zeiss, Germany) using secondary electron imaging. Before imaging procedure, the samples were coated with a thin platinum layer.
Results and discussion
Efficiency of the therapy with encapsulated drugs and various methods of administration (intravenous, intramuscular, etc.) depends on interaction between delivery systems and media of an organism, thus resulting into the drug release. In the case of local administration of chemotherapeutic preparations into tumor area, the drug and a delivery system are exposed to the interstitial fluid, which, unlike blood plasma, contain lesser protein amounts. In the present work, we studied structural and functional characteristics of hybrid delivery systems for DOX based on CaCO3 vaterites modified with DexS polyanions. Porous vaterite is the least stable of three CaCO3 polymorphs, whereas calcite and needle-shaped aragonite are not porous. In our previous publications [12, 22], it has been shown that the exposure of vaterites to blood plasma led to gradual destruction of calcium carbonate cores. This destruction may be caused, e.g., by interaction between vaterites and phosphate ions present in blood plasma, thus causing gradual development of macroporous СаНРО4 structures [24].
Structural changes of CaCO3 vaterites coated with DexS upon exposure to blood plasma for various periods of time (1, 4 and 7 days) are presented in Fig. 1. SEM micrographs of non-coated vaterites and calcites in plasma are also provided [12].
SEM micrographs demonstrate that delivery systems of various structures have different resistance upon incubation with blood plasma. DexS coating protects CaCO3 vaterites from aggressive medium. Comparison of SEM micrographs of hybrid CaCO3 and non-coated vaterites following exposure to blood plasma for 4 days (Figures 1a3 and 1b3) shows a more pronounced destruction of non-coated vaterites.
After 1 week of exposure to blood plasma, the hybrid delivery systems were also destroyed to a large degree (Fig. 1, a4). The DexS coating was partially separated from CaCO3 cores, and the exposed vaterites are strongly damaged. Non-porous calcites do not undergo any significant structural changes under the contact with blood plasma. Thus, they are not suitable for use as delivery systems.

Figure 1. SEM micrographs of CaCO3 vaterites coated with DexS (a), non-coated vaterites (b), and calcites (c). Images of the initial cores (1) were made upon interaction with human blood plasma for various periods of time: 1 day (2); 4 days (3); 7 days (4). Magnification: 20x. Marker size: 2 µm.

Figure 2. Release of DOX into blood plasma from CaCO3 cores of different morphologies: calcites (1); vaterites (2); vaterites coated with DexS (3). Abscissa, incubation terms, days; ordinate, DOX release, %
An important question arises concerning relations between structural characteristics of DOX delivery systems and their functional characteristics, i.e., DOX loading and time-dependent release profile of the drug? The process of DOX loading into various CaCO3-based DS involves sorption, diffusion, and ionic interactions. Calcites interact with DOX mainly via sorption mechanism. The vaterite loading proceeds via sorption and diffusion interactions between DOX and porous core matter. When hybrid delivery systems (CaCO3 vaterites coated with DexS polyanions) are used, additional ionic interaction takes place between DOX cations and excess negative charges present on the cores covered with polyanions (non-compensated charges). Thus, one may expect that, all other factors being equal, loading of DOX into hybrid DSs will be the highest of these three systems. Loading values (L) of non-coated and hybrid CaCO3 cores were 370 and 400 µg/mg, respectively. Loading of calcites was about 100 µg/mg. Fig. 2 presents the DOX release profiles from CaCO3-based DS of various structures. For comparison experiments, DS of various structures, however, with similar DOX loading values were selected.
Release of DOX from hybrid cores was delayed, but more intensive as compared to those for the non-coated cores (curves 3 and 2). Therefore, one may expect that the amount of drug entering the bloodstream will be higher in the first case. The cumulative release of DOX from CaCO3 coated with DexS into plasma was 55% in 2 weeks. For comparison, we now give the data on DOX release into model media from the delivery systems prepared with layer-by-layer coating of CaCO3 vaterites with polyelectrolytes, followed by dissolution of carbonate cores [25]. During first 24 h of the experiment, 60% of DOX was released from these DS, and no increase in cumulative drug release was observed in the next 10 days. These results indicate more efficient prolongation of drug release from the DS based on hybrid CaCO3 vaterites.
We may correlate the DOX release profiles (Fig. 2) with SEM micrographs of hybrid DS (Fig. 1a) and non-coated CaCO3 vaterites (Fig. 1b). Exposure to blood plasma causes similar structural changes in hybrid and non-coated vaterites (Fig. 1, a2 and Fig.1, b2). Release profiles of DOX from hybrid and non-coated vaterites (Fig. 2, curves 3 and 2) are also similar for both DS exposed to blood plasma for 1 day. Similar results were obtained in experiments with CaCO3 calcites. The structure of non-porous calcites virtually does not change even upon prolonged exposure to blood plasma (Fig.1, c1 to c3). The DOX release profile from calcites increases up to 11% in the first 2 days, then the release is ceased (Fig. 2, curve 1). Hence, we have shown that DOX release profiles correlate with structural changes of the tested delivery systems.
Conclusion
This work is a continuation of the research concerning the in vitro and in vivo behavior of targeted drug delivery systems based on CaCO3 vaterites and carrying anti-cancer drug doxorubicin as described in our earlier publications [12, 22, 23]. Our previous results suggest that porous CaCO3 vaterites coated with DexS may be safe for medicinal use and undergo bioresorption in a living organism. In order to model behavior of the developed DOX-containing delivery systems in the interstitial fluid after intraperitoneal administration, we assessed structural and functional changes in these DS occurring during interaction with human blood plasma. Ionic composition of a medium is an important characteristic when degradation processes of different DS based on CaCO3 cores are concerned. Treatment of CaCO3 vaterites with DexS polyanion allowed us to obtain the delivery systems that provide a prolonged release of 55% of encapsulated anti-cancer drug DOX, with increased terms of drug release by more than 2 weeks. Using DS of various structures as examples, a correlation between functional characteristics of delivery systems (DOX release profiles into human blood plasma) and their structural changes was demonstrated. DOX is excreted from bloodstream very rapidly upon intravenous administration. According to chromatography data [26], DOX concentration in plasma becomes 100 times lower in 40-60 min after a single drug injection into mice (7 mg/kg). Since achievement of prolonged drug release is one of the main goals in DS development, our further studies will be focused on determination of DOX concentrations released into blood from DS of various structures.
Acknowledgement
The study was performed within the framework of budget-supported research project №АААА-А20-120022090044-2, Institute of Macromolecular Compounds, RAS.
Conflict of interest
The authors declare no conflict of interest.
References
- Basin IS. Hepatocellular cancer – modern status. Problems of Practical Oncology. 2008;9(4):216-228 (In Russian).
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185-229. doi: 10.1124/pr.56.2.6.
- Ruman U, Fakurazi S, Masarudin M, Hussein M. Nanocarrier-based therapeutics and theranostics drug delivery systems for next generation of liver cancer nanodrug modalities. Int J Nanomed. 2020;15:1437-1456. doi: 10.2147/IJN.S236927.
- Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomed. 2020;15:9355-9371. doi: 10.2147/IJN.S281890.
- Lian B, Wei H, Pan R, Sun J, Zhang B, Wu J, et al. Galactose-modified liposomes for effective co-delivery of doxorubicin and combretastatin A4. Int J Nanomed. 2021;16:457-467. doi: 10.2147/IJN.S283793.
- Lu Y, Jun Y, Tang Z, Li X, Tao M, Zhang Z, et al. Enhanced antitumor efficacy of macrophage-mediated egg yolk lipid-derived delivery system against breast cancer. Int J Nanomed. 2020;15:10075-10084. doi: 10.2147/IJN.S271310.
- Li Z, Tian G, Jiang H , Pan R, Lian B, Wang M, et al. Liver-targeting and pH-sensitive sulfated hyaluronic acid mixed micelles for hepatoma therapy. Int J Nanomed. 2019;14:9437-9452. doi: 10.2147/IJN.S214528.
- Yan H, Xue Z, Xie J, Dong Y, Ma Z, Sun X, et al. Toxicity of carbon nanotubes as anti-cancer drug carriers. Int J Nanomed. 2019;14:10179-10194. doi: 10.2147/IJN.S220087.
- Zhang J, Zhang H, Jiang J, Cui N, Xue X, Wang T, et al. Doxorubicin-loaded carbon dots lipid-coated calcium phosphate nanoparticles for visual targeted delivery and therapy of tumor. Int J Nanomed. 2020;15:433-444. doi: 10.2147/IJN.S229154.
- Zhang N, Yu R, Xu M, Cheng X, Chen C, Xu X, et al. Visual targeted therapy of hepatic cancer using homing peptide modified calcium phosphate nanoparticles loading doxorubicin guided by T1 weighted MRI. Nanomedicine. 2018;14:2167-2178. doi: 10.1016/j.nano.2018.06.014.
- Shahein S, Aboul-Enein A, Higazy I, Abou-Elella F, Lojkowski W, Ahmed E, et al. Targeted anticancer potential against glioma cells of thymoquinone delivered by mesoporous silica core-shell nanoformulations with pH-dependent release. Int J Nanomed. 2019;14:5503-5526. doi: 10.2147/IJN.S206899.
- Sudareva N, Suvorova O, Saprykina N, Vlasova H, Vilesov A. Doxorubicin delivery systems based on doped CaCO3 cores and polyanion drug conjugates, J Microencapsul. 2021; 38:3,164-176. doi: 10.1080/02652048.2021.1872724.
- Volodkin DV, Petrov AI, Prevot M, Sukhorukov GB. Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir. 2004;20:3398-3406. doi: 10.1021/LA036177Z.
- Svenskaya Y, Parakhonskiy B, Haase A, Atkin V, Lukyanets E, Gorin D et al. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys Chem. 2013;182:11-15. doi: 10.1016/j.bpc.2013.07.006.
- Chen S, Zhao D, Li F, Zhuo R, Cheng S. Co-delivery of genes and drugs with nanostructured calcium carbonate for cancer therapy. RSC Adv. 2012;2:1820-1826. doi: 10.1039/C1RA00527H.
- Binevski P, Balabushevich N, Uvarova V, Vikulina A, Volodkin D. Bio-friendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology. Colloids Surf B Biointerfaces. 2019;181:437-449. doi: 10.1016/j.colsurfb.2019.05.077.
- Som A, Raliya R, Tian L, Akers W, Ippolito J, Singamaneni S, et al. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale. 2016;8:12639-12647. doi: 10.1039/C5NR06162H.
- Dizaj M, Sharifi S, Ahmadian E, Eftekhari A, Adibkia K, Lotfipour F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin Drug Deliv. 2019;16:331-345. doi: 10.1080/17425247.2019.1587408.
- Karabasz A, Bzowska M, Szczepanowicz K. Biomedical applications of multifunctional polymeric nanocarriers: A review of current literature. Int J Nanomed. 2020;15:8673-8696. doi: 10.2147/IJN.S231477.
- Wang C, Chen S, Bao L, Liu X, Hu F, Yuan H. Size-controlled preparation and behavior study of phospholipid-calcium carbonate hybrid nanoparticles. Int J Nanomed. 2020;15:4049-4062. doi: 10.2147/IJN.S237156.
- Zhao P, Wu S, Cheng Y, You J, Chen Y, Li M, et al. MiR-375 delivered by lipid-coated doxorubicin calcium carbonate nanoparticles overcomes chemoresistance in hepatocellular carcinoma. Nanomedicine. 2017;13(8):2507-2516. doi: 10.1016/j.nano.2017.05.010.
- Sudareva NN, Popryadukhin PV, Saprykina NN, Suvorova OM, Yukina GY, Galibin OV, Vilesov AD. CaCO3 vaterites as components of target drug delivery systems. Cell Ther Transplant. 2020;9(2):13-19. doi: 10.18620/ctt-1866-8836-2020-9-2-13-19.
- Popryadukhin PV, Sudareva NN, Suvorova OM, Yukina GY, Sukhorukova EG, Saprykina NN, Galibin OV, Vilesov AD. Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue. Cell Ther Transplant. 2020; 9(4):78-84. doi: 10.18620/ctt-1866-8836-2020-9-4-78-84.
- Sudareva NN, Saprykina NN, Popova EV, Vilesov AD. Porous calcium carbonate cores as templates for preparation of peroral proteins delivery systems. The influence of composition of simulated gastrointestinal fluids on the structure and morphology of carbonate cores. Chapter 4. In: "Calcium Carbonate: Occurrence, Characterization and Applications". (Ed. A.Cohen), Nova Science Publishers, Inc (NOVA). 2015, pp.73-95.
- Zhao Q, Han B, Wang Z, Gao C, Shenc J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: doxorubicin loading and in vitro and in vivo studies. Nanomedicine. 2007; 3:63-74. doi: 10.1016/j.nano. 2006.11.007.
- Baurain R, Deprez-De Campeneere D, Trouet A. Determination of daunorubicin, doxorubicin and their fluorescent metabolites by high-pressure liquid chromatography: Plasma levels in DBA2 mice. Cancer Chemother, Pharmacol. 1979;2:11-14.
" ["~DETAIL_TEXT"]=> string(27779) "
Introduction
Oncological diseases are among the main causes of death worldwide: cancer mortality reaches 20%. The World Health Organization predicts that in 20 years this value will double. In addition to existing methods of cancer treatment (surgery, radiotherapy, chemotherapy), a new method (targeted drug therapy) has been developed over last decade.
In many countries, doxorubicin monotherapy is considered to be the standard chemotherapy procedure [1]. DОХ efficiently inhibits tumor growth by intercalation into DNA of cancer cells, which prevents replication process, thus leading to cell death. However, its clinical applications are limited by the dose-dependent cardiac toxicity. Moreover, this substance shows some disadvantages, i.e., short lifetime, accumulation in liver, and multidrug resistance of malignant cells [2]. Different delivery systems (DS) may be used in order to reduce these side effects. Prolongation of DOX release from DS can reduce probability of overdosage.
In the recent years, a large number of nano- and submicron-sized delivery systems for anti-cancer drugs have been developed [3]. The basic structure of carriers may vary; bio-organic objects are commonly used as carriers: exosomes [4], liposomes [5], dendrimers, macrophages [6], micelles [7], as well as inorganic substances, e.g., carbon nanotubes [8], calcium phosphates [9, 10], SiO2 [11], and CaCO3 [12]. It should be noted that manufacturing of most DS is rather laborious; in a number of cases, it is necessary to synthesize complex polymers. Meanwhile, preparation of submicron-sized porous СаСО3 vaterites is very simple, i.e., co-precipitation of СаСl2 and Na2CO3 solutions [13]. Nanomicron-sized particles can be prepared by increasing viscosity of solutions [14] or changing ratio between concentrations of salts [15]. Of the three CaCO3 polymorphs, porous vaterite is the least stable. Other forms (calcite and needle-shaped aragonite) are not porous. The CaCO3 vaterites are biocompatible and biodegradable [16]. The particles have anti-cancer activity, even without encapsulated drugs, since CaCO3 vaterites are shown to modulate local pH and inhibit tumor growth in vivo [17].
There are few studies on usage of different drug delivery systems based on CaCO3 for tumor suppression, e.g., the pH dependence of CaCO3 may control release of photosensitizer leading to cancer cell apoptosis [14]. The review [18] gives several examples of applications for CaCO3 nanoparticles as carriers for various anti-cancer drugs, including DOX.
Various methods have been proposed to increase efficiency of delivery systems. Modification of polymer particles with polyelectrolyte coating leads to prolonged drug circulation time [19]. Similar effect can be expected upon treatment of CaCO3 carriers with polymers. Special study concerned behavior and fate of hybrid phospholipid-treated CaCO3 nanoparticles without drugs in vitro and in tumor-bearing mice [20]. Their uptake, penetration and distribution in tissues were investigated. It was demonstrated that the hybrid nanoparticles showed almost no cytotoxicity in vitro or in vivo and had high biocompatibility. The lipid-coated CaCO3 cores saturated with DOX help to overcome chemoresistance in hepatocellular carcinoma [21]. In our earlier works, it has been demonstrated [12] that the properties of CaCO3-based delivery systems for DOX may be improved. In particular, the drug load can be increased, the carrier morphology can be stabilized, and drug release can be prolonged by covering the cores with polyelectrolytes (dextran sulfate, polyacrylic acid, and polyvinyl sulfonate). In our recent works [22, 23], behavior of the DS based on submicron-sized CaCO3 particles in mouse muscle tissue was studied for the first time. We have revealed that the DS based on parent porous vaterites and the vaterites coated by DexS undergo complete bioresorption in mice within, respectively, 2 and 3 weeks after administration. This process involves the stage of transition to needle-shaped aragonite structures. No pathological effects of the carriers on the surrounding tissues have been observed.
Anti-cancer therapy involves different ways of drug administration, including local introduction of DS containing active drugs [22]. For example, during treatment of hepatocellular cancer, the drug is introduced intraperitoneally. In this case, the DS will be exposed to interstitial fluids, which differ from blood plasma, mainly, in lesser protein contents.
Thus, the goals of the present work were: (i) to study in vitro structural changes in hybrid DOX delivery systems (based on porous CaCO3 vaterites modified with DexS) in human blood plasma; (ii) to assess correlation between DOX release profiles from these DS into human plasma, and concomitant structural changes of the carrier substance.
Materials and methods
Reagents
Doxorubicin hydrochloride under the brand name of Sindroxocin, which contains 17% of doxorubicin (DOX) and 83% of lactose, was purchased from Actavis (Hafnarfjordur, Iceland). In experimental studies, doxorubicin salt with protonated amino group (–NH3+) was used. Inorganic salts (CaCl2 × 2H2O, Na2CO3), acetone, and dextran sulfate, Mw=9-20 kDa were purchased from Sigma-Aldrich (St. Louis, MO).
Synthesis of carbonate cores
Porous vaterites (СаСО3 cores) were prepared by co-precipitation according to the technique described in [13], with some modifications. Equal volumes of 1 M aqueous solutions of CaCl2×2H2O and Na2CO3 were rapidly mixed with stirring (1000 rpm) for 30 s, using RW 20 anchor-type mechanical stirrer (Kika-Werk, Switzerland). The resulting suspension was then filtered through Schott glass filter (#16), washed thrice with distilled water, then with acetone/water mixtures at increasing acetone concentrations (33%, 50%, and 100%). The precipitate was dried in thermostat at 40-50°C, until achieving constant weight.
Modification of carbonate cores with DexS and preparation of hybrid DS
The carbonate cores were coated with polyanions (sodium salt of dextran sulfate). СаСО3 cores (50 mg) were added to 1 mg/mL aqueous solution of DexS (10 mL). The suspension was stirred using a Multi Bio RS-24 rotor (Biosan, Latvia) for 1 h. Solid fraction was filtered off using a Schott glass filter (#16), washed thrice with distilled water and dried at 30°C.
DOX loading and release
Loading of hybrid DS with DOX was performed by continuous stirring of the mixture of DSs suspension and DOX solution (C=2 mg/mL) for 24 hours. The DOX/(СаСО3+DexS) ratio was 0.4. After mixing, the suspension was centrifuged at 8000 rpm for 3 min, and DOX amounts were determined in supernatants.
DOX loading (L) was calculated by the following equation: L = (mi − ms)/mp, where mi is initial amount of DOX (mg); ms, the amount of non-encapsulated DOX in supernatant solution (mg); mp, amount of particles (mg). DOX concentrations were determined using the calibration curves obtained from optical density values, using appropriate solvents (λ = 480 nm). The measurements were carried out using SF-2000 spectrophotometer (LOMO, St. Petersburg, Russia).
In vitro release of DOX from hybrid DSs was analyzed in human blood plasma obtained from the Department of Blood Transfusion, Pavlov University. Incubation of DS in plasma was carried out at 20°C, with constant mixing. Aliquots were collected from incubation mixtures every 1-2 hours after centrifugation of core suspension at 3000 rpm for 3 min. The DOX contents in aliquots were determined by spectrophotometry. The supernatants obtained for similarly incubated DOX-free hybrid CaCO3 cores were used as a reference solution. The cumulative release was determined as percentage of DOX amounts loaded into the initial cores.
All the measurements were carried out in triplicate.
Interaction between DexS-modified CaCO3 and human blood plasma
The interaction between hybrid carbonate cores and human blood plasma occurred in suspension at continuous stirring for different periods of time. Upon completion of the reaction, the cores were centrifuged (5 min at 3000 rpm), the supernatant was discarded and substituted by blood plasma. The procedure was performed twice. The cores were dried at 40°C until constant weight was achieved.
Scanning electron microscopy (SEM)
SEM micrographs of DexS-coated CaCO3 cores were obtained by means Supra 55VP scanning electron microscope (Carl Zeiss, Germany) using secondary electron imaging. Before imaging procedure, the samples were coated with a thin platinum layer.
Results and discussion
Efficiency of the therapy with encapsulated drugs and various methods of administration (intravenous, intramuscular, etc.) depends on interaction between delivery systems and media of an organism, thus resulting into the drug release. In the case of local administration of chemotherapeutic preparations into tumor area, the drug and a delivery system are exposed to the interstitial fluid, which, unlike blood plasma, contain lesser protein amounts. In the present work, we studied structural and functional characteristics of hybrid delivery systems for DOX based on CaCO3 vaterites modified with DexS polyanions. Porous vaterite is the least stable of three CaCO3 polymorphs, whereas calcite and needle-shaped aragonite are not porous. In our previous publications [12, 22], it has been shown that the exposure of vaterites to blood plasma led to gradual destruction of calcium carbonate cores. This destruction may be caused, e.g., by interaction between vaterites and phosphate ions present in blood plasma, thus causing gradual development of macroporous СаНРО4 structures [24].
Structural changes of CaCO3 vaterites coated with DexS upon exposure to blood plasma for various periods of time (1, 4 and 7 days) are presented in Fig. 1. SEM micrographs of non-coated vaterites and calcites in plasma are also provided [12].
SEM micrographs demonstrate that delivery systems of various structures have different resistance upon incubation with blood plasma. DexS coating protects CaCO3 vaterites from aggressive medium. Comparison of SEM micrographs of hybrid CaCO3 and non-coated vaterites following exposure to blood plasma for 4 days (Figures 1a3 and 1b3) shows a more pronounced destruction of non-coated vaterites.
After 1 week of exposure to blood plasma, the hybrid delivery systems were also destroyed to a large degree (Fig. 1, a4). The DexS coating was partially separated from CaCO3 cores, and the exposed vaterites are strongly damaged. Non-porous calcites do not undergo any significant structural changes under the contact with blood plasma. Thus, they are not suitable for use as delivery systems.

Figure 1. SEM micrographs of CaCO3 vaterites coated with DexS (a), non-coated vaterites (b), and calcites (c). Images of the initial cores (1) were made upon interaction with human blood plasma for various periods of time: 1 day (2); 4 days (3); 7 days (4). Magnification: 20x. Marker size: 2 µm.

Figure 2. Release of DOX into blood plasma from CaCO3 cores of different morphologies: calcites (1); vaterites (2); vaterites coated with DexS (3). Abscissa, incubation terms, days; ordinate, DOX release, %
An important question arises concerning relations between structural characteristics of DOX delivery systems and their functional characteristics, i.e., DOX loading and time-dependent release profile of the drug? The process of DOX loading into various CaCO3-based DS involves sorption, diffusion, and ionic interactions. Calcites interact with DOX mainly via sorption mechanism. The vaterite loading proceeds via sorption and diffusion interactions between DOX and porous core matter. When hybrid delivery systems (CaCO3 vaterites coated with DexS polyanions) are used, additional ionic interaction takes place between DOX cations and excess negative charges present on the cores covered with polyanions (non-compensated charges). Thus, one may expect that, all other factors being equal, loading of DOX into hybrid DSs will be the highest of these three systems. Loading values (L) of non-coated and hybrid CaCO3 cores were 370 and 400 µg/mg, respectively. Loading of calcites was about 100 µg/mg. Fig. 2 presents the DOX release profiles from CaCO3-based DS of various structures. For comparison experiments, DS of various structures, however, with similar DOX loading values were selected.
Release of DOX from hybrid cores was delayed, but more intensive as compared to those for the non-coated cores (curves 3 and 2). Therefore, one may expect that the amount of drug entering the bloodstream will be higher in the first case. The cumulative release of DOX from CaCO3 coated with DexS into plasma was 55% in 2 weeks. For comparison, we now give the data on DOX release into model media from the delivery systems prepared with layer-by-layer coating of CaCO3 vaterites with polyelectrolytes, followed by dissolution of carbonate cores [25]. During first 24 h of the experiment, 60% of DOX was released from these DS, and no increase in cumulative drug release was observed in the next 10 days. These results indicate more efficient prolongation of drug release from the DS based on hybrid CaCO3 vaterites.
We may correlate the DOX release profiles (Fig. 2) with SEM micrographs of hybrid DS (Fig. 1a) and non-coated CaCO3 vaterites (Fig. 1b). Exposure to blood plasma causes similar structural changes in hybrid and non-coated vaterites (Fig. 1, a2 and Fig.1, b2). Release profiles of DOX from hybrid and non-coated vaterites (Fig. 2, curves 3 and 2) are also similar for both DS exposed to blood plasma for 1 day. Similar results were obtained in experiments with CaCO3 calcites. The structure of non-porous calcites virtually does not change even upon prolonged exposure to blood plasma (Fig.1, c1 to c3). The DOX release profile from calcites increases up to 11% in the first 2 days, then the release is ceased (Fig. 2, curve 1). Hence, we have shown that DOX release profiles correlate with structural changes of the tested delivery systems.
Conclusion
This work is a continuation of the research concerning the in vitro and in vivo behavior of targeted drug delivery systems based on CaCO3 vaterites and carrying anti-cancer drug doxorubicin as described in our earlier publications [12, 22, 23]. Our previous results suggest that porous CaCO3 vaterites coated with DexS may be safe for medicinal use and undergo bioresorption in a living organism. In order to model behavior of the developed DOX-containing delivery systems in the interstitial fluid after intraperitoneal administration, we assessed structural and functional changes in these DS occurring during interaction with human blood plasma. Ionic composition of a medium is an important characteristic when degradation processes of different DS based on CaCO3 cores are concerned. Treatment of CaCO3 vaterites with DexS polyanion allowed us to obtain the delivery systems that provide a prolonged release of 55% of encapsulated anti-cancer drug DOX, with increased terms of drug release by more than 2 weeks. Using DS of various structures as examples, a correlation between functional characteristics of delivery systems (DOX release profiles into human blood plasma) and their structural changes was demonstrated. DOX is excreted from bloodstream very rapidly upon intravenous administration. According to chromatography data [26], DOX concentration in plasma becomes 100 times lower in 40-60 min after a single drug injection into mice (7 mg/kg). Since achievement of prolonged drug release is one of the main goals in DS development, our further studies will be focused on determination of DOX concentrations released into blood from DS of various structures.
Acknowledgement
The study was performed within the framework of budget-supported research project №АААА-А20-120022090044-2, Institute of Macromolecular Compounds, RAS.
Conflict of interest
The authors declare no conflict of interest.
References
- Basin IS. Hepatocellular cancer – modern status. Problems of Practical Oncology. 2008;9(4):216-228 (In Russian).
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185-229. doi: 10.1124/pr.56.2.6.
- Ruman U, Fakurazi S, Masarudin M, Hussein M. Nanocarrier-based therapeutics and theranostics drug delivery systems for next generation of liver cancer nanodrug modalities. Int J Nanomed. 2020;15:1437-1456. doi: 10.2147/IJN.S236927.
- Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomed. 2020;15:9355-9371. doi: 10.2147/IJN.S281890.
- Lian B, Wei H, Pan R, Sun J, Zhang B, Wu J, et al. Galactose-modified liposomes for effective co-delivery of doxorubicin and combretastatin A4. Int J Nanomed. 2021;16:457-467. doi: 10.2147/IJN.S283793.
- Lu Y, Jun Y, Tang Z, Li X, Tao M, Zhang Z, et al. Enhanced antitumor efficacy of macrophage-mediated egg yolk lipid-derived delivery system against breast cancer. Int J Nanomed. 2020;15:10075-10084. doi: 10.2147/IJN.S271310.
- Li Z, Tian G, Jiang H , Pan R, Lian B, Wang M, et al. Liver-targeting and pH-sensitive sulfated hyaluronic acid mixed micelles for hepatoma therapy. Int J Nanomed. 2019;14:9437-9452. doi: 10.2147/IJN.S214528.
- Yan H, Xue Z, Xie J, Dong Y, Ma Z, Sun X, et al. Toxicity of carbon nanotubes as anti-cancer drug carriers. Int J Nanomed. 2019;14:10179-10194. doi: 10.2147/IJN.S220087.
- Zhang J, Zhang H, Jiang J, Cui N, Xue X, Wang T, et al. Doxorubicin-loaded carbon dots lipid-coated calcium phosphate nanoparticles for visual targeted delivery and therapy of tumor. Int J Nanomed. 2020;15:433-444. doi: 10.2147/IJN.S229154.
- Zhang N, Yu R, Xu M, Cheng X, Chen C, Xu X, et al. Visual targeted therapy of hepatic cancer using homing peptide modified calcium phosphate nanoparticles loading doxorubicin guided by T1 weighted MRI. Nanomedicine. 2018;14:2167-2178. doi: 10.1016/j.nano.2018.06.014.
- Shahein S, Aboul-Enein A, Higazy I, Abou-Elella F, Lojkowski W, Ahmed E, et al. Targeted anticancer potential against glioma cells of thymoquinone delivered by mesoporous silica core-shell nanoformulations with pH-dependent release. Int J Nanomed. 2019;14:5503-5526. doi: 10.2147/IJN.S206899.
- Sudareva N, Suvorova O, Saprykina N, Vlasova H, Vilesov A. Doxorubicin delivery systems based on doped CaCO3 cores and polyanion drug conjugates, J Microencapsul. 2021; 38:3,164-176. doi: 10.1080/02652048.2021.1872724.
- Volodkin DV, Petrov AI, Prevot M, Sukhorukov GB. Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir. 2004;20:3398-3406. doi: 10.1021/LA036177Z.
- Svenskaya Y, Parakhonskiy B, Haase A, Atkin V, Lukyanets E, Gorin D et al. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys Chem. 2013;182:11-15. doi: 10.1016/j.bpc.2013.07.006.
- Chen S, Zhao D, Li F, Zhuo R, Cheng S. Co-delivery of genes and drugs with nanostructured calcium carbonate for cancer therapy. RSC Adv. 2012;2:1820-1826. doi: 10.1039/C1RA00527H.
- Binevski P, Balabushevich N, Uvarova V, Vikulina A, Volodkin D. Bio-friendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology. Colloids Surf B Biointerfaces. 2019;181:437-449. doi: 10.1016/j.colsurfb.2019.05.077.
- Som A, Raliya R, Tian L, Akers W, Ippolito J, Singamaneni S, et al. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale. 2016;8:12639-12647. doi: 10.1039/C5NR06162H.
- Dizaj M, Sharifi S, Ahmadian E, Eftekhari A, Adibkia K, Lotfipour F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin Drug Deliv. 2019;16:331-345. doi: 10.1080/17425247.2019.1587408.
- Karabasz A, Bzowska M, Szczepanowicz K. Biomedical applications of multifunctional polymeric nanocarriers: A review of current literature. Int J Nanomed. 2020;15:8673-8696. doi: 10.2147/IJN.S231477.
- Wang C, Chen S, Bao L, Liu X, Hu F, Yuan H. Size-controlled preparation and behavior study of phospholipid-calcium carbonate hybrid nanoparticles. Int J Nanomed. 2020;15:4049-4062. doi: 10.2147/IJN.S237156.
- Zhao P, Wu S, Cheng Y, You J, Chen Y, Li M, et al. MiR-375 delivered by lipid-coated doxorubicin calcium carbonate nanoparticles overcomes chemoresistance in hepatocellular carcinoma. Nanomedicine. 2017;13(8):2507-2516. doi: 10.1016/j.nano.2017.05.010.
- Sudareva NN, Popryadukhin PV, Saprykina NN, Suvorova OM, Yukina GY, Galibin OV, Vilesov AD. CaCO3 vaterites as components of target drug delivery systems. Cell Ther Transplant. 2020;9(2):13-19. doi: 10.18620/ctt-1866-8836-2020-9-2-13-19.
- Popryadukhin PV, Sudareva NN, Suvorova OM, Yukina GY, Sukhorukova EG, Saprykina NN, Galibin OV, Vilesov AD. Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue. Cell Ther Transplant. 2020; 9(4):78-84. doi: 10.18620/ctt-1866-8836-2020-9-4-78-84.
- Sudareva NN, Saprykina NN, Popova EV, Vilesov AD. Porous calcium carbonate cores as templates for preparation of peroral proteins delivery systems. The influence of composition of simulated gastrointestinal fluids on the structure and morphology of carbonate cores. Chapter 4. In: "Calcium Carbonate: Occurrence, Characterization and Applications". (Ed. A.Cohen), Nova Science Publishers, Inc (NOVA). 2015, pp.73-95.
- Zhao Q, Han B, Wang Z, Gao C, Shenc J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: doxorubicin loading and in vitro and in vivo studies. Nanomedicine. 2007; 3:63-74. doi: 10.1016/j.nano. 2006.11.007.
- Baurain R, Deprez-De Campeneere D, Trouet A. Determination of daunorubicin, doxorubicin and their fluorescent metabolites by high-pressure liquid chromatography: Plasma levels in DBA2 mice. Cancer Chemother, Pharmacol. 1979;2:11-14.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(100) "morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl" ["~CODE"]=> string(100) "morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl" ["EXTERNAL_ID"]=> string(4) "1955" ["~EXTERNAL_ID"]=> string(4) "1955" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(331) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человекаMorphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2161) "<p style="text-align: justify;">Регионарное введение химиотерапевтических препаратов в область опухоли может во многих случаях повысить эффективность лечения злокачественных опухолей. После введения в организм, лечебный препарат или система доставки препарата могут подвергаться воздействию внутритканевых жидкостей, которые отличаются от плазмы крови тем, что они содержат меньше белков. В настоящей работе мы изучали влияние плазмы крови человека на структуру и свойства гибридной системы доставки, основанной на кальций-карбонатных ватеритах (СаСО<sub>3</sub>), покрытых полиэлектролитом декстран-сульфатом (DexS). Эти системы доставки содержали противораковый препарат доксорубицин.</p> <p style="text-align: justify;">Было показано, что ватериты, модифицированные DexS, обеспечивали пролонгированный выход доксорубицина в плазму крови. Сканирующие микрофотографии выявили структурные изменения гибридных систем доставки, возникающие при контакте с плазмой и коррелирующие с профилем высвобождения доксорубицина. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Доксорубицин, система доставки препаратов, СаСО<sub>3</sub>, декстран сульфат, плазма крови.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_META_TITLE"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_META_KEYWORDS"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_META_DESCRIPTION"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_PICTURE_FILE_ALT"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_PICTURE_FILE_TITLE"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(219) "Морфология гибридных систем доставки доксорубицина (ватериты CaCO3, покрытые декстран-сульфатом) в плазме крови человека" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "morfologiya-gibridnykh-sistem-dostavki-doksorubitsina-vaterity-caco3-pokrytye-dekstran-sulfatom-v-pl" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "176" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27512" ["VALUE"]=> string(22) "03/10/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/10/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27513" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27514" ["VALUE"]=> array(2) { ["TEXT"]=> string(434) "<p>Наталья Н. Сударева<sup>1,2</sup>, Ольга М. Суворова<sup>1</sup>, Наталья Н. Сапрыкина<sup>1</sup>, Владимир В. Томсон<sup>2</sup>, Дмитрий Н. Суслов<sup>3</sup>, Олег В. Галибин<sup>2</sup>, Александр Д. Вилесов<sup>1,2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(338) "
Наталья Н. Сударева1,2, Ольга М. Суворова1, Наталья Н. Сапрыкина1, Владимир В. Томсон2, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27515" ["VALUE"]=> array(2) { ["TEXT"]=> string(650) "<p><sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт- Петербург, Россия<br> <sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Российский научный центр радиологии и хирургических технологий им. А. М. Гранова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(590) "1 Институт высокомолекулярных соединений РАН, Санкт- Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. А. М. Гранова, Санкт-Петербург, Россия
Регионарное введение химиотерапевтических препаратов в область опухоли может во многих случаях повысить эффективность лечения злокачественных опухолей. После введения в организм, лечебный препарат или система доставки препарата могут подвергаться воздействию внутритканевых жидкостей, которые отличаются от плазмы крови тем, что они содержат меньше белков. В настоящей работе мы изучали влияние плазмы крови человека на структуру и свойства гибридной системы доставки, основанной на кальций-карбонатных ватеритах (СаСО3), покрытых полиэлектролитом декстран-сульфатом (DexS). Эти системы доставки содержали противораковый препарат доксорубицин.
Было показано, что ватериты, модифицированные DexS, обеспечивали пролонгированный выход доксорубицина в плазму крови. Сканирующие микрофотографии выявили структурные изменения гибридных систем доставки, возникающие при контакте с плазмой и коррелирующие с профилем высвобождения доксорубицина.
Ключевые слова
Доксорубицин, система доставки препаратов, СаСО3, декстран сульфат, плазма крови.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27517" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-79-85" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-79-85" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27519" ["VALUE"]=> array(2) { ["TEXT"]=> string(328) "<p>Natalia N. Sudareva<sup>1,2</sup>, Olga М. Suvorova<sup>1</sup>, Natalia N. Saprykina<sup>1</sup>, Vladimir V. Tomson<sup>2</sup>, Dmitry N. Suslov<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(232) "Natalia N. Sudareva1,2, Olga М. Suvorova1, Natalia N. Saprykina1, Vladimir V. Tomson2, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27520" ["VALUE"]=> array(2) { ["TEXT"]=> string(565) "<p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> <sup>2</sup> Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Natalya N. Sudareva, Institute of Macromolecular Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004, St. Petersburg, Russia<br> E-mail: nnsas@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(463) "1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence
Dr. Natalya N. Sudareva, Institute of Macromolecular
Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004,
St. Petersburg, Russia
E-mail: nnsas@mail.ru
In a number of cases, efficiency of cancer therapy may be enhanced by local administration of chemotherapeutic drugs into the tumor area. After regional injection, a drug, or appropriate delivery system is exposed to the interstitial fluid, which differs from blood plasma, mainly, in lesser protein amounts. In the present work, we studied the influence of human blood plasma upon structure and properties of hybrid drug delivery systems based on calcium carbonate (СаСО3) vaterites coated with polyelectrolyte dextran sulfate (DexS). These delivery systems included anti-cancer drug doxorubicin (DOX).
It has been shown that DexS-modified vaterites provided the prolonged release of DOX to blood plasma. SEM microphotographs revealed structural changes in hybrid delivery systems occurring in plasma and correlating with the DOX release profiles.
Keywords
Doxorubicin, drug delivery system, СаСО3, dextran sulfate, blood plasma.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27518" ["VALUE"]=> string(112) "Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(112) "Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27538" ["VALUE"]=> string(4) "2430" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2430" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27539" ["VALUE"]=> string(4) "2431" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2431" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27519" ["VALUE"]=> array(2) { ["TEXT"]=> string(328) "<p>Natalia N. Sudareva<sup>1,2</sup>, Olga М. Suvorova<sup>1</sup>, Natalia N. Saprykina<sup>1</sup>, Vladimir V. Tomson<sup>2</sup>, Dmitry N. Suslov<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(232) "Natalia N. Sudareva1,2, Olga М. Suvorova1, Natalia N. Saprykina1, Vladimir V. Tomson2, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(232) "Natalia N. Sudareva1,2, Olga М. Suvorova1, Natalia N. Saprykina1, Vladimir V. Tomson2, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27521" ["VALUE"]=> array(2) { ["TEXT"]=> string(1183) "<p style="text-align: justify;">In a number of cases, efficiency of cancer therapy may be enhanced by local administration of chemotherapeutic drugs into the tumor area. After regional injection, a drug, or appropriate delivery system is exposed to the interstitial fluid, which differs from blood plasma, mainly, in lesser protein amounts. In the present work, we studied the influence of human blood plasma upon structure and properties of hybrid drug delivery systems based on calcium carbonate (СаСО<sub>3</sub>) vaterites coated with polyelectrolyte dextran sulfate (DexS). These delivery systems included anti-cancer drug doxorubicin (DOX).</p> <p style="text-align: justify;">It has been shown that DexS-modified vaterites provided the prolonged release of DOX to blood plasma. SEM microphotographs revealed structural changes in hybrid delivery systems occurring in plasma and correlating with the DOX release profiles. </p> <h2>Keywords</h2> <p style="text-align: justify;">Doxorubicin, drug delivery system, СаСО<sub>3</sub>, dextran sulfate, blood plasma.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1081) "In a number of cases, efficiency of cancer therapy may be enhanced by local administration of chemotherapeutic drugs into the tumor area. After regional injection, a drug, or appropriate delivery system is exposed to the interstitial fluid, which differs from blood plasma, mainly, in lesser protein amounts. In the present work, we studied the influence of human blood plasma upon structure and properties of hybrid drug delivery systems based on calcium carbonate (СаСО3) vaterites coated with polyelectrolyte dextran sulfate (DexS). These delivery systems included anti-cancer drug doxorubicin (DOX).
It has been shown that DexS-modified vaterites provided the prolonged release of DOX to blood plasma. SEM microphotographs revealed structural changes in hybrid delivery systems occurring in plasma and correlating with the DOX release profiles.
Keywords
Doxorubicin, drug delivery system, СаСО3, dextran sulfate, blood plasma.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1081) "In a number of cases, efficiency of cancer therapy may be enhanced by local administration of chemotherapeutic drugs into the tumor area. After regional injection, a drug, or appropriate delivery system is exposed to the interstitial fluid, which differs from blood plasma, mainly, in lesser protein amounts. In the present work, we studied the influence of human blood plasma upon structure and properties of hybrid drug delivery systems based on calcium carbonate (СаСО3) vaterites coated with polyelectrolyte dextran sulfate (DexS). These delivery systems included anti-cancer drug doxorubicin (DOX).
It has been shown that DexS-modified vaterites provided the prolonged release of DOX to blood plasma. SEM microphotographs revealed structural changes in hybrid delivery systems occurring in plasma and correlating with the DOX release profiles.
Keywords
Doxorubicin, drug delivery system, СаСО3, dextran sulfate, blood plasma.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27517" ["VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-79-85" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-79-85" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(38) "10.18620/ctt-1866-8836-2021-10-1-79-85" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27518" ["VALUE"]=> string(112) "Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(112) "Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(112) "Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27520" ["VALUE"]=> array(2) { ["TEXT"]=> string(565) "<p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> <sup>2</sup> Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Natalya N. Sudareva, Institute of Macromolecular Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004, St. Petersburg, Russia<br> E-mail: nnsas@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(463) "1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence
Dr. Natalya N. Sudareva, Institute of Macromolecular
Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004,
St. Petersburg, Russia
E-mail: nnsas@mail.ru
1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence
Dr. Natalya N. Sudareva, Institute of Macromolecular
Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004,
St. Petersburg, Russia
E-mail: nnsas@mail.ru
Наталья Н. Сударева1,2, Ольга М. Суворова1, Наталья Н. Сапрыкина1, Владимир В. Томсон2, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(338) "Наталья Н. Сударева1,2, Ольга М. Суворова1, Наталья Н. Сапрыкина1, Владимир В. Томсон2, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27512" ["VALUE"]=> string(22) "03/10/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/10/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/10/2021 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27513" ["VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "04/09/2021 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "04/09/2021 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27516" ["VALUE"]=> array(2) { ["TEXT"]=> string(2161) "<p style="text-align: justify;">Регионарное введение химиотерапевтических препаратов в область опухоли может во многих случаях повысить эффективность лечения злокачественных опухолей. После введения в организм, лечебный препарат или система доставки препарата могут подвергаться воздействию внутритканевых жидкостей, которые отличаются от плазмы крови тем, что они содержат меньше белков. В настоящей работе мы изучали влияние плазмы крови человека на структуру и свойства гибридной системы доставки, основанной на кальций-карбонатных ватеритах (СаСО<sub>3</sub>), покрытых полиэлектролитом декстран-сульфатом (DexS). Эти системы доставки содержали противораковый препарат доксорубицин.</p> <p style="text-align: justify;">Было показано, что ватериты, модифицированные DexS, обеспечивали пролонгированный выход доксорубицина в плазму крови. Сканирующие микрофотографии выявили структурные изменения гибридных систем доставки, возникающие при контакте с плазмой и коррелирующие с профилем высвобождения доксорубицина. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Доксорубицин, система доставки препаратов, СаСО<sub>3</sub>, декстран сульфат, плазма крови.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2059) "Регионарное введение химиотерапевтических препаратов в область опухоли может во многих случаях повысить эффективность лечения злокачественных опухолей. После введения в организм, лечебный препарат или система доставки препарата могут подвергаться воздействию внутритканевых жидкостей, которые отличаются от плазмы крови тем, что они содержат меньше белков. В настоящей работе мы изучали влияние плазмы крови человека на структуру и свойства гибридной системы доставки, основанной на кальций-карбонатных ватеритах (СаСО3), покрытых полиэлектролитом декстран-сульфатом (DexS). Эти системы доставки содержали противораковый препарат доксорубицин.
Было показано, что ватериты, модифицированные DexS, обеспечивали пролонгированный выход доксорубицина в плазму крови. Сканирующие микрофотографии выявили структурные изменения гибридных систем доставки, возникающие при контакте с плазмой и коррелирующие с профилем высвобождения доксорубицина.
Ключевые слова
Доксорубицин, система доставки препаратов, СаСО3, декстран сульфат, плазма крови.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2059) "Регионарное введение химиотерапевтических препаратов в область опухоли может во многих случаях повысить эффективность лечения злокачественных опухолей. После введения в организм, лечебный препарат или система доставки препарата могут подвергаться воздействию внутритканевых жидкостей, которые отличаются от плазмы крови тем, что они содержат меньше белков. В настоящей работе мы изучали влияние плазмы крови человека на структуру и свойства гибридной системы доставки, основанной на кальций-карбонатных ватеритах (СаСО3), покрытых полиэлектролитом декстран-сульфатом (DexS). Эти системы доставки содержали противораковый препарат доксорубицин.
Было показано, что ватериты, модифицированные DexS, обеспечивали пролонгированный выход доксорубицина в плазму крови. Сканирующие микрофотографии выявили структурные изменения гибридных систем доставки, возникающие при контакте с плазмой и коррелирующие с профилем высвобождения доксорубицина.
Ключевые слова
Доксорубицин, система доставки препаратов, СаСО3, декстран сульфат, плазма крови.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27515" ["VALUE"]=> array(2) { ["TEXT"]=> string(650) "<p><sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт- Петербург, Россия<br> <sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Российский научный центр радиологии и хирургических технологий им. А. М. Гранова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(590) "1 Институт высокомолекулярных соединений РАН, Санкт- Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. А. М. Гранова, Санкт-Петербург, Россия
1 Институт высокомолекулярных соединений РАН, Санкт- Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. А. М. Гранова, Санкт-Петербург, Россия
04/30/2021

Kulagin A. D. (St. Petersburg, Russia)
Wagemaker G. (Rotterdam, Netherlands)
Zander A. R. (Hamburg, Germany)
Fehse B. (Hamburg, Germany)
Chukhlovin A. B. (St. Petersburg, Russia)
Aleynikova O. V. (Minsk, Belarus)
Borset M. (Trondheim, Norway)
Chechetkin A. V. (St. Petersburg, Russia)
Fibbe W. (Leiden, Netherlands)
Gale R. P. (Los Angeles, USA)
Galibin O. V. (St. Petersburg, Russia)
Hehlmann R. (Mannheim, Germany)
Hölzer D. (Frankfurt a.M., Germany)
Klimko N. N. (St. Petersburg, Russia)
Kolb H.-J. (München, Germany)
Kröger N. (Hamburg, Germany)
Lange C. (Hamburg, Germany)
Mamaev N. N. (St. Petersburg, Russia)
Mikhailova N. B. (St. Petersburg, Russia)
Moiseev I. S. (St. Petersburg, Russia)
Nagler A. (Tel-Aviv, Israel)
Nemkov A. S. (St. Petersburg, Russia)
Paramonov I. V. (Kirov, Russia)
Roumiantsev A. G. (Moscow, Russia)
Savchenko V. G. (Moscow, Russia)
Smirnov A. V. (St. Petersburg, Russia)
Uss A. L. (Minsk, Belarus)
Zubarovskaya L. S. (St. Petersburg, Russia)
Dear CTT authors and readers,
The continuing COVID-19 pandemia presents great challenges to hemato-oncological and BMT clinics worldwide.
Like our colleagues in Western and Eastern World, we have encountered the new coronavirus infection in March 2020, thus requiring a variety of urgent epidemiological measures and early diagnostics of SARS-CoV-2-infected persons, involving our clinical staff and the patients admitted or those, who were on the waiting list. To this purpose, a countrywide training of medical staff was urgently performed, according to the temporary Guidelines from Russian Health Ministry (with later amendments), thus providing a uniform strategy to combat the epidemics in St. Petersburg and in dozens hematological clinics all over the country, and creating a basis for training of our doctors, nurses and relatives of the patients in this items.
Huge problems initially arised for our epidemiologists, clinical infectologists and laboratory staff who resolved appropriate organizational and educational tasks. Within several weeks, first native PCR kits for SARS-CoV2 were approved by the central surveillance authorities, their number then extended to >30 PCR test systems widely used by the network of clinical laboratories throughout Russia. About 2 months were sufficient to develop tests for detection of IgM and IgG antibodies against SARS-CoV-2 in recovered patients.
Hence, within several weeks, the infrastructure of oncohematological clinics and related clinical departments was adapted for diagnostics, isolation and observation of SARS-CoV-2-infected persons, as well as special prophylactic measures to prevent further spread of infection. In our specific setting, we were obliged to arrange ‘red’ (wards) for patients with COVID-19, with special regimen of their handling and care.
Аnother crucial problem was to adapt intensive care units for management of severe patients following chemotherapy and HSCT, and to expand diagnostic facilities at the University Department of Microbiology which worked at their extreme limits with PCR testing of the virus and antibody diagnostics over last year.
Isolation of immunocompromised patients at our clinics became extremely strict. The infected patients were displaced to the COVID hospital at our University, thus presenting a favorable opportunity to our transplantation clinics.
To minimize epidemiological risks, we were obliged to delay intensive treatment for infected patients with leukemias and lymphomas undergoing HSCT. At longer periods, the scheduled follow-up of the patients was required, however, distant counseling was arranged, if possible.
In the frames of post-COVID and post-transplant rehabilitation, clinical psychologists should support both patients, their relatives and clinicians employed in the “red” areas.
COVID vaccination is another unresolved issue, which should be considered. The existing vaccines are not yet studied for appropriate safety and specific protective ability in cancer patients, especially, in pediatric immunocompromised cohorts subjected to cytostatic and immunosuppressive therapies.
While making our efforts, we are much appreciated to international authorities, and leading medical journals, which shared their indispensable experience in this field, hoping for further multicentric studies on this issues. Of course, it is too soon to make some definite conclusions on COVID-19 impact on clinical course and outcomes in our patients. The effects of past COVID-19 upon overall and event-free survival require long-term follow-up, and, at least, several years are required to summarize these data with our colleages worldwide.
In any case, we invite our present and future authors to submit to Cellular Therapy and Transplantation their studies about specific aspects of COVID-19 infection in oncohematology and stem cell transplantation clinics.
Professor Alexander D. Kulagin, Editor-in-Chief, Cellular Therapy and Transplantation Journal
Review articles
Anna Yu. Anisenkova1, Aleksander S. Golota1, Dmitry A. Vologzhanin1, Tatyana A. Kamilova1, Stanislav V. Makarenko1,2, Olga V. Shneider1, Oleg S. Glotov1,3, Yury A. Serov4, Sergey V. Mosenko1, Sergey V. Azarenko1, Konstantin V. Smanzerev1, Dmitry N. Khobotnikov1, Tatyana V. Gladisheva1, Sergey G. Shcherbak1,2
Clinical studies
Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev
Oleg V. Goloshchapov1, Evgeny A. Bakin1, Oksana V. Stanevich1, Ruslana V. Klementeva1, Alexander A. Shcherbakov1, Alexander N. Shvetsov1, Olesya V. Paina1, Polina V. Kozhokar1, Margarita V. Gorchakova1, Elena V. Babenko1, Maria A. Suvorova2, Sergey N. Bondarenko1, Maxim A. Kucher1, Alexander D. Kulagin1, Ludmila S. Zubarovskaya1, Ivan S. Moiseev1
Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya
Liudmila V. Olkhova1, Olga G. Zheludkova2, Ludmila S. Zubarovskaya3, Anna Yu. Smirnova4, Yulia V. Dinikina4,5, Asmik G. Gevorgyan3, Andrey S. Levashov6, Elena V. Skorobogatova1
Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev
Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, Boris V. Afanasyev
Experimental studies
Natalia N. Sudareva1,2, Olga М. Suvorova1, Natalia N. Saprykina1, Vladimir V. Tomson2, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27432
[VALUE] => 03/08/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/08/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27433
[VALUE] => 04/09/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 04/09/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27434
[VALUE] => Array
(
[TEXT] => <p>Джина Зини</>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Джина Зини
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27435
[VALUE] => Array
(
[TEXT] => <p>Отдел визуальной диагностики, онкологической радиотерапии и гематологии, Университетская поликлиника «Гемелли», Католический Университет Сакро Куоре, Рим, Италия </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Отдел визуальной диагностики, онкологической радиотерапии и гематологии, Университетская поликлиника «Гемелли», Католический Университет Сакро Куоре, Рим, Италия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27436
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.</p>
<p style="text-align: justify;">В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.</p>
<p style="text-align: justify;">Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Трансплантация гемопоэтических стволовых клеток (ТГСК) является потенциально жизнеспасающей процедурой, ассоциированной с осложнениями, которые требуют повышенного внимания и мониторинга. При непосредственном динамическом наблюдении за пациентом, подсчет клеток крови обеспечивает точные количественные показания о течении посттрансплантационного периода, что позволяет отслеживать восстановление гемопоэза, уровни гемоглобина, число тромбоцитов и лейкоцитов. В настоящее время доступны полезные и дешевые автоматизированные гематологические методы для того, чтобы улучшить ведение и наблюдение пациентов после ТГСК. Это – незрелая фракция ретикулоцитов (IRF), незрелые тромбоциты/ретикулярные тромбоциты (IPF) и фрагменты эритроцитов.
В процессе посттрансплантационного восстановления эритро- и тромбоцитопоэза, отмечается повышение IRF и IPF в периферической крови, что коррелирует во времени реконституцией гемопоэза.
Другим полезным параметром при ведении этих больных является число фрагментов эритроцитов, которые могут коррелировать с присутствием шистоцитов. Доступность этого параметра позволяет выявлять в рутинной практике те образцы, которые могут содержать шистоциты: немедленное исследование мазка периферической крови подтверждает (или нет) диагностическую гипотезу.
Ключевые слова
Автоматический анализ крови, фракция незрелых ретикулоцитов, фракция незрелых тромбоцитов, эритроцитарные фрагменты, трансплантация гемопоэтических клеток, осложнения.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27437
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-6-12
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-6-12
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27439
[VALUE] => Array
(
[TEXT] => <p>Gina Zini</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Gina Zini
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27440
[VALUE] => Array
(
[TEXT] => <p>Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy</p><br>
<p><b>Correspondence</b><br>
<p style="text-align: justify;">Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario <br>A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore <br>
E-mail: gina.zini@unicatt.it</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy
Correspondence
Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario
A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore
E-mail: gina.zini@unicatt.it
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27441
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">HSCT is a potentially life-saving procedure, associated with complications requiring increased vigilance and monitoring. In the immediate follow-up of patients, the blood count provides precise quantitative indications upon the post-transplant course, allowing to follow haematopoietic recovery, evaluating hemoglobin values, and the numbers of platelets and white blood cells. At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients, that are the immature reticulocyte fraction (IRF), immature platelet fraction/reticulated platelets (IPF) and red blood cell (RBC) fragments. In the presence of post-transplant recovery of erythropoiesis and thrombocytopoiesis, IRF and IPF increase in the peripheral blood correlates in real time with haematopoietic recovery. Another useful parameter in the follow-up of these patients is the number of red blood cell fragments (FRC) which can correlate with presence of shistocytes. Availability of this parameter allows to intercept in routine practice the samples that could contain schistocytes: immediate evaluation of the peripheral blood smear will confirm or not the diagnostic suspicion.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Automated blood analysis, immature reticulocytes fraction, immature platelets fraction, red blood cell fragments, hematopoietic cell transplantation, complications.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => HSCT is a potentially life-saving procedure, associated with complications requiring increased vigilance and monitoring. In the immediate follow-up of patients, the blood count provides precise quantitative indications upon the post-transplant course, allowing to follow haematopoietic recovery, evaluating hemoglobin values, and the numbers of platelets and white blood cells. At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients, that are the immature reticulocyte fraction (IRF), immature platelet fraction/reticulated platelets (IPF) and red blood cell (RBC) fragments. In the presence of post-transplant recovery of erythropoiesis and thrombocytopoiesis, IRF and IPF increase in the peripheral blood correlates in real time with haematopoietic recovery. Another useful parameter in the follow-up of these patients is the number of red blood cell fragments (FRC) which can correlate with presence of shistocytes. Availability of this parameter allows to intercept in routine practice the samples that could contain schistocytes: immediate evaluation of the peripheral blood smear will confirm or not the diagnostic suspicion.
Keywords
Automated blood analysis, immature reticulocytes fraction, immature platelets fraction, red blood cell fragments, hematopoietic cell transplantation, complications.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27438
[VALUE] => Laboratory diagnostics of HSCT complications: immature platelets and RBCs
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Laboratory diagnostics of HSCT complications: immature platelets and RBCs
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27522
[VALUE] => 2410
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2410
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27523
[VALUE] => 2411
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2411
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Gina Zini
Fondazione Policlinico Universitario A, Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore, Roma, Italy
Correspondence
Professor Gina Zini, MD, PhD, Dpt. Diagnostica per immagini, Radioterapia oncologica e Ematologia, Fondazione Policlinico Universitario
A. Gemelli IRCCS – Roma, Università Cattolica del Sacro Cuore
E-mail: gina.zini@unicatt.it
HSCT is a potentially life-saving procedure, associated with complications requiring increased vigilance and monitoring. In the immediate follow-up of patients, the blood count provides precise quantitative indications upon the post-transplant course, allowing to follow haematopoietic recovery, evaluating hemoglobin values, and the numbers of platelets and white blood cells. At the present time, useful and cheap automated hematology parameters are available, in order to improve both management and follow-up of HSC transplanted patients, that are the immature reticulocyte fraction (IRF), immature platelet fraction/reticulated platelets (IPF) and red blood cell (RBC) fragments. In the presence of post-transplant recovery of erythropoiesis and thrombocytopoiesis, IRF and IPF increase in the peripheral blood correlates in real time with haematopoietic recovery. Another useful parameter in the follow-up of these patients is the number of red blood cell fragments (FRC) which can correlate with presence of shistocytes. Availability of this parameter allows to intercept in routine practice the samples that could contain schistocytes: immediate evaluation of the peripheral blood smear will confirm or not the diagnostic suspicion.
Keywords
Automated blood analysis, immature reticulocytes fraction, immature platelets fraction, red blood cell fragments, hematopoietic cell transplantation, complications.
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27442
[VALUE] => 12/25/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/25/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27443
[VALUE] => 03/12/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/12/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27444
[VALUE] => Array
(
[TEXT] => <p>Анна Ю. Анисенкова<sup>1</sup>, Александр С. Голота<sup>1</sup>, Дмитрий А. Вологжанин<sup>1</sup>, Татьяна А. Камилова<sup>1</sup>, Станислав В. Макаренко<sup>1,2</sup>, Ольга В. Шнейдер<sup>1</sup>, Олег С. Глотов<sup>1,3</sup>, Юрий А. Серов<sup>4</sup>, Сергей В. Мосенко<sup>1</sup>, Сергей В. Азаренко<sup>1</sup>, Константин В. Сманцерев<sup>1</sup>, Дмитрий Н. Хоботников<sup>1</sup>, Татьяна В. Гладышева<sup>1</sup>, Сергей Г. Щербак<sup>1,2</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Анна Ю. Анисенкова1, Александр С. Голота1, Дмитрий А. Вологжанин1, Татьяна А. Камилова1, Станислав В. Макаренко1,2, Ольга В. Шнейдер1, Олег С. Глотов1,3, Юрий А. Серов4, Сергей В. Мосенко1, Сергей В. Азаренко1, Константин В. Сманцерев1, Дмитрий Н. Хоботников1, Татьяна В. Гладышева1, Сергей Г. Щербак1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27445
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> СПб ГБУЗ «Городская больница №40 Курортного района», Сестрорецк, Россия <br>
<sup>2</sup> Санкт-Петербургский государственный университет, Санкт-Петербург, Россия<br>
<sup>3</sup> НИИ акушерства, гинекологии и репродуктологии им. Д. О. Отта, Санкт-Петербург, Россия<br>
<sup>4</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 СПб ГБУЗ «Городская больница №40 Курортного района», Сестрорецк, Россия
2 Санкт-Петербургский государственный университет, Санкт-Петербург, Россия
3 НИИ акушерства, гинекологии и репродуктологии им. Д. О. Отта, Санкт-Петербург, Россия
4 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27446
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Индивидуальные генетические вариации могут объяснить различные иммунные реакции на инфекцию коронавируса SARS-CoV-2 в популяции. Методология компьютерного моделирования <i>in silico</i> предоставляет экспериментальному сообществу список иммуногенных пептидов SARS-CoV-2, презентируемых антигенами HLA-системы. В этом обзоре представлен профиль предсказанных <i>in silico</i> иммуногенных пептидов вируса SARS-CoV-2 для функциональной валидации и разработки вакцин. Исследования, проводимые независимо друг от друга, дают высокую степень уверенности в воспроизводимости результатов. Вычислительное прогнозирование является инструментом быстрого и экономичного решения для предотвращения распространения и, в конечном итоге, устранения инфекции. Большая часть усилий по разработке вакцин и лекарств против SARS-CoV-2 направлена на гликопротеин шипа (S-белок), главный индуктор нейтрализующих антител. Несколько вакцин-кандидатов продемонстрировали эффективность в исследованиях <i>in vitro</i> и перешли в рандомизированные испытания на животных или людях для противодействия инфекции COVID-19. В этой статье освещаются текущие достижения в разработке субъединичных вакцин для борьбы с COVID-19, которые сокращают время и затраты при разработке вакцин.
</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Коронавирус, SARS-CoV-2, COVID-19, HLA, иммуногенный пептид, антиген, эпитоп, вакцина, компьютерное прогнозирование, компьютерное моделирование <i>in silico</i>, иммуноинформатика.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Индивидуальные генетические вариации могут объяснить различные иммунные реакции на инфекцию коронавируса SARS-CoV-2 в популяции. Методология компьютерного моделирования in silico предоставляет экспериментальному сообществу список иммуногенных пептидов SARS-CoV-2, презентируемых антигенами HLA-системы. В этом обзоре представлен профиль предсказанных in silico иммуногенных пептидов вируса SARS-CoV-2 для функциональной валидации и разработки вакцин. Исследования, проводимые независимо друг от друга, дают высокую степень уверенности в воспроизводимости результатов. Вычислительное прогнозирование является инструментом быстрого и экономичного решения для предотвращения распространения и, в конечном итоге, устранения инфекции. Большая часть усилий по разработке вакцин и лекарств против SARS-CoV-2 направлена на гликопротеин шипа (S-белок), главный индуктор нейтрализующих антител. Несколько вакцин-кандидатов продемонстрировали эффективность в исследованиях in vitro и перешли в рандомизированные испытания на животных или людях для противодействия инфекции COVID-19. В этой статье освещаются текущие достижения в разработке субъединичных вакцин для борьбы с COVID-19, которые сокращают время и затраты при разработке вакцин.
Ключевые слова
Коронавирус, SARS-CoV-2, COVID-19, HLA, иммуногенный пептид, антиген, эпитоп, вакцина, компьютерное прогнозирование, компьютерное моделирование in silico, иммуноинформатика.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27447
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-13-23
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-13-23
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27449
[VALUE] => Array
(
[TEXT] => <p>Anna Yu. Anisenkova<sup>1</sup>, Aleksander S. Golota<sup>1</sup>, Dmitry A. Vologzhanin<sup>1</sup>, Tatyana A. Kamilova<sup>1</sup>, Stanislav V. Makarenko<sup>1,2</sup>, Olga V. Shneider<sup>1</sup>, Oleg S. Glotov<sup>1,3</sup>, Yury A. Serov<sup>4</sup>, Sergey V. Mosenko<sup>1</sup>, Sergey V. Azarenko<sup>1</sup>, Konstantin V. Smanzerev<sup>1</sup>, Dmitry N. Khobotnikov<sup>1</sup>, Tatyana V. Gladisheva<sup>1</sup>, Sergey G. Shcherbak<sup>1,2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Anna Yu. Anisenkova1, Aleksander S. Golota1, Dmitry A. Vologzhanin1, Tatyana A. Kamilova1, Stanislav V. Makarenko1,2, Olga V. Shneider1, Oleg S. Glotov1,3, Yury A. Serov4, Sergey V. Mosenko1, Sergey V. Azarenko1, Konstantin V. Smanzerev1, Dmitry N. Khobotnikov1, Tatyana V. Gladisheva1, Sergey G. Shcherbak1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27450
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> St. Petersburg City Hospital №40, St. Petersburg, Russia <br>
<sup>2</sup> St. Petersburg State University School of Medicine, St. Petersburg, Russia<br>
<sup>3</sup> D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia<br>
<sup>4</sup> Pavlov University, St.Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia<br>
E-mail: golotaa@yahoo.com</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 St. Petersburg City Hospital №40, St. Petersburg, Russia
2 St. Petersburg State University School of Medicine, St. Petersburg, Russia
3 D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia
4 Pavlov University, St.Petersburg, Russia
Correspondence
Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia
E-mail: golotaa@yahoo.com
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27451
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Individual genetic variation may help to explain different immune responses to a coronavirus SARS-CoV-2 across a population. The <i>in silico</i> computer simulation methodology provides the experimental community with a more complete list of SARS-CoV-2 immunogenic peptides presented by the antigens of the HLA system. This review considers an array of computationally predicted immunogenic peptides from SARS-CoV-2 for <i>in vitro</i> functional validation and potential vaccine developments. Several independent studies conducted with different approaches showed a high degree of confidence and reproducibility of the results. Computer-assisted prediction is instrumental for a quick and cost-effective solution to prevent the spread and ultimately eliminate the infection.
</p>
<p style="text-align: justify;">
Most efforts to develop vaccines and drugs against SARS-CoV-2 target the spike glycoprotein (protein S), the major inducer of neutralizing antibodies. Several candidates have been shown to be effective in <i>in vitro</i> studies and have progressed to randomized trials in animals or humans against COVID-19 infection. This article highlights current advances in the development of subunit vaccines to combat COVID-19 that are reducing the time and costs of vaccine development.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Coronavirus, SARS-CoV-2, COVID-19, immunogenic peptides, antigen, HLA, vaccine, epitope, computational prediction, computer simulation<i> in silico</i>, immunoinformatics.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Individual genetic variation may help to explain different immune responses to a coronavirus SARS-CoV-2 across a population. The in silico computer simulation methodology provides the experimental community with a more complete list of SARS-CoV-2 immunogenic peptides presented by the antigens of the HLA system. This review considers an array of computationally predicted immunogenic peptides from SARS-CoV-2 for in vitro functional validation and potential vaccine developments. Several independent studies conducted with different approaches showed a high degree of confidence and reproducibility of the results. Computer-assisted prediction is instrumental for a quick and cost-effective solution to prevent the spread and ultimately eliminate the infection.
Most efforts to develop vaccines and drugs against SARS-CoV-2 target the spike glycoprotein (protein S), the major inducer of neutralizing antibodies. Several candidates have been shown to be effective in in vitro studies and have progressed to randomized trials in animals or humans against COVID-19 infection. This article highlights current advances in the development of subunit vaccines to combat COVID-19 that are reducing the time and costs of vaccine development.
Keywords
Coronavirus, SARS-CoV-2, COVID-19, immunogenic peptides, antigen, HLA, vaccine, epitope, computational prediction, computer simulation in silico, immunoinformatics.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27448
[VALUE] => Immunoinformatics in COVID-19 Vaccine Development: The Role of HLA System
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Immunoinformatics in COVID-19 Vaccine Development: The Role of HLA System
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27524
[VALUE] => 2414
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2414
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27525
[VALUE] => 2415
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2415
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Anna Yu. Anisenkova1, Aleksander S. Golota1, Dmitry A. Vologzhanin1, Tatyana A. Kamilova1, Stanislav V. Makarenko1,2, Olga V. Shneider1, Oleg S. Glotov1,3, Yury A. Serov4, Sergey V. Mosenko1, Sergey V. Azarenko1, Konstantin V. Smanzerev1, Dmitry N. Khobotnikov1, Tatyana V. Gladisheva1, Sergey G. Shcherbak1,2
1 St. Petersburg City Hospital №40, St. Petersburg, Russia
2 St. Petersburg State University School of Medicine, St. Petersburg, Russia
3 D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg, Russia
4 Pavlov University, St.Petersburg, Russia
Correspondence
Dr. Aleksander S. Golota, Ph.D., Head, Clinical Research Branch for Medical Rehabilitation, City Hospital №40, Borisova St. 9B, Sestrorezk, St.Petersburg, Russia
E-mail: golotaa@yahoo.com
Individual genetic variation may help to explain different immune responses to a coronavirus SARS-CoV-2 across a population. The in silico computer simulation methodology provides the experimental community with a more complete list of SARS-CoV-2 immunogenic peptides presented by the antigens of the HLA system. This review considers an array of computationally predicted immunogenic peptides from SARS-CoV-2 for in vitro functional validation and potential vaccine developments. Several independent studies conducted with different approaches showed a high degree of confidence and reproducibility of the results. Computer-assisted prediction is instrumental for a quick and cost-effective solution to prevent the spread and ultimately eliminate the infection.
Most efforts to develop vaccines and drugs against SARS-CoV-2 target the spike glycoprotein (protein S), the major inducer of neutralizing antibodies. Several candidates have been shown to be effective in in vitro studies and have progressed to randomized trials in animals or humans against COVID-19 infection. This article highlights current advances in the development of subunit vaccines to combat COVID-19 that are reducing the time and costs of vaccine development.
Keywords
Coronavirus, SARS-CoV-2, COVID-19, immunogenic peptides, antigen, HLA, vaccine, epitope, computational prediction, computer simulation in silico, immunoinformatics.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27492
[VALUE] => 12/27/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/27/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27493
[VALUE] => 03/12/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/12/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27494
[VALUE] => Array
(
[TEXT] => <p>Виталий Н. Чеботкевич, Алена В. Кулешова, Анастасия А. Жернякова, Иван И. Кострома, Екатерина Е. Киселева, Елена И. Кайтанджан, Наталья Ю. Семенова, Станислав С. Бессмельцев, Александр В. Чечеткин, Сергей В. Грицаев</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Виталий Н. Чеботкевич, Алена В. Кулешова, Анастасия А. Жернякова, Иван И. Кострома, Екатерина Е. Киселева, Елена И. Кайтанджан, Наталья Ю. Семенова, Станислав С. Бессмельцев, Александр В. Чечеткин, Сергей В. Грицаев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27495
[VALUE] => Array
(
[TEXT] => <p>Российский научно-исследовательский институт гематологии и трансфузиологии, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Российский научно-исследовательский институт гематологии и трансфузиологии, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27496
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.</p>
<p style="text-align: justify;">Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Множественная миелома (MM) составляет примерно 10% гемобластозов и 1% всех онкологических заболеваний в целом. Концепция высокодозной химиотерапии с последующей трансплантацией аутологичных гемопоэтических стволовых клеток (AТСК) была разработана еще в 1980-х годах и остается стандартом лечения впервые выявленной множественной миеломы у молодых и избранных пожилых пациентов. Появление новых агентов, таких как иммуномодулирующие препараты, ингибиторы протеасом и моноклональные антитела, не заменили AТСК, а лишь укрепили его центральную роль в качестве стандарта лечения. Таким образом, в эпоху появления новых агентов трансплантация подвергается дальнейшему изучению. Важной причиной заболеваемости и смертности больных ММ являются инфекционные осложнения. Инфекции кровотока остаются наиболее серьезным бактериальным осложнением у реципиентов трансплантации гемопоэтических стволовых клеток, тогда как вирусы герпеса, особенно цитомегаловирус (ЦМВ), преобладают среди вирусных осложнений. Мы проанализировали данные 38 пациентов с ММ, которым была проведена AТСК в период с января 2018 г. по февраль 2020 г. Частота реактивации цитомегаловируса (ЦМВ) выявлена у 5 (13,2%), и вируса Эпштейна-Барра (ВЭБ) у 3-х (7,9%) пациентов. Пневмония диагностирована в 1 (2,6%) случае. Бактериальные инфекции кровотока выявлены у 3 (7,9%) пациентов. Инфекции кровотока были стратифицированы в соответствии с критериями сепсиса-3. Это позволило выявить пациентов с неблагоприятным прогнозом (развитие сепсиса и септического шока). Инфекционные осложнения наблюдались в период до 60 дней после AТСК.
Однако влияния реактивации ЦМВ и ВЭБ и инфекций кровотока на общую выживаемость (ОВ) не отмечено. Результат показывает, что бактериальные и вирусные (реактивация ЦМВ и ВЭБ) инфекционные осложнения усугубляют течение заболевания у гематологических больных. Критерии «Сепсис-3» позволяют своевременно выделить группы пациентов с неблагоприятным прогнозом (возможность развития сепсиса и септического шока). Использование методов инфекционного контроля в клинической практике (выявление штаммов с множественной лекарственной устойчивостью и использование стратегий контроля антимикробной терапии) улучшает тактику лечения пациентов с ММ в период после AТСК.
Ключевые слова
Трансплантация аутологичных гемопоэтических стволовых клеток, бактериальные и вирусные инфекционные осложнения, сепсис, септический шок.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27497
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-63-68
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-63-68
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27499
[VALUE] => Array
(
[TEXT] => <p>Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27500
[VALUE] => Array
(
[TEXT] => <p>Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia </p><br>
<p><b>Correspondence</b><br>
Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia<br>
Phone: +7 (906) 267 0266<br>
E-mail: vitnikcheb@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia
Phone: +7 (906) 267 0266
E-mail: vitnikcheb@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27501
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Multiple myeloma (MM) accounts for approximately 10% of blood malignancies and 1% of all cancers in general. The concept of high-dose chemotherapy followed by transplantation of autologous hematopoietic stem cells (ASCT) remains the standard for treating newly diagnosed multiple myeloma in young and in selected, fit, elderly patients. Infectious complications represent important cause of morbidity and mortality in MM patients. Bloodstream infections remain the most severe bacterial complication in recipients of hematopoietic stem cell transplantation, whereas herpesviruses, especially, cytomegalovirus (CMV), dominate among viral complications. We analyzed data on 38 patients with MM who underwent ASCT from January 2018 to February 2020. Reactivation of cytomegalovirus (CMV) was revealed in 5 cases (13.2%), and Epstein-Barr virus (EBV), in 3 patients (7.9%). Pneumonia was diagnosed in one case (2.6%). Bacterial bloodstream infections were detected in 3 patients (7.9%). The bloodstream infections were stratified in accordance with the Sepsis-3 criteria, thus enabling us to identify patients with unfavorable prognosis who developed sepsis and/or septic shock. Infectious complications were observed over the period of 60 days after ASCT. Meanwhile, CMV and EBV reactivation and bloodstream bacterial infections did not affect overall survival rate.
</p>
<h3>
Conclusion</h3>
<p style="text-align: justify;">
Our results demonstrate that bacterial complications and viral (CMV and EBV) reactivation aggravate the course of primary disease in MM patients over the post-transplant period. The methods of infection control in clinical practice (genotyping of multidrug-resistant strains, and antimicrobial control protocols) should improve the treatment strategies in patients with MM following ASCT. Keywords Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Multiple myeloma (MM) accounts for approximately 10% of blood malignancies and 1% of all cancers in general. The concept of high-dose chemotherapy followed by transplantation of autologous hematopoietic stem cells (ASCT) remains the standard for treating newly diagnosed multiple myeloma in young and in selected, fit, elderly patients. Infectious complications represent important cause of morbidity and mortality in MM patients. Bloodstream infections remain the most severe bacterial complication in recipients of hematopoietic stem cell transplantation, whereas herpesviruses, especially, cytomegalovirus (CMV), dominate among viral complications. We analyzed data on 38 patients with MM who underwent ASCT from January 2018 to February 2020. Reactivation of cytomegalovirus (CMV) was revealed in 5 cases (13.2%), and Epstein-Barr virus (EBV), in 3 patients (7.9%). Pneumonia was diagnosed in one case (2.6%). Bacterial bloodstream infections were detected in 3 patients (7.9%). The bloodstream infections were stratified in accordance with the Sepsis-3 criteria, thus enabling us to identify patients with unfavorable prognosis who developed sepsis and/or septic shock. Infectious complications were observed over the period of 60 days after ASCT. Meanwhile, CMV and EBV reactivation and bloodstream bacterial infections did not affect overall survival rate.
Conclusion
Our results demonstrate that bacterial complications and viral (CMV and EBV) reactivation aggravate the course of primary disease in MM patients over the post-transplant period. The methods of infection control in clinical practice (genotyping of multidrug-resistant strains, and antimicrobial control protocols) should improve the treatment strategies in patients with MM following ASCT. Keywords Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
Keywords
Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27498
[VALUE] => Infectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Infectious complications in multiple myeloma patients undergoing autologous peripheral blood stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27534
[VALUE] => 2424
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2424
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27535
[VALUE] => 2425
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2425
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Vitaly N. Chebotkevich, Alena V. Kuleshova, Anastasia A. Zhernyakova, Ivan I. Kostroma, Ekaterina E. Kiseleva, Elena I. Kaytandzhan, Natalia Yu. Semenova, Stanislav S. Bessmeltsev, Alexander V. Chechetkin, Sergei V. Gritsaev
Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Prof. Vitaly N. Chebotkevich, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St. 16, 191024, St. Petersburg, Russia
Phone: +7 (906) 267 0266
E-mail: vitnikcheb@mail.ru
Multiple myeloma (MM) accounts for approximately 10% of blood malignancies and 1% of all cancers in general. The concept of high-dose chemotherapy followed by transplantation of autologous hematopoietic stem cells (ASCT) remains the standard for treating newly diagnosed multiple myeloma in young and in selected, fit, elderly patients. Infectious complications represent important cause of morbidity and mortality in MM patients. Bloodstream infections remain the most severe bacterial complication in recipients of hematopoietic stem cell transplantation, whereas herpesviruses, especially, cytomegalovirus (CMV), dominate among viral complications. We analyzed data on 38 patients with MM who underwent ASCT from January 2018 to February 2020. Reactivation of cytomegalovirus (CMV) was revealed in 5 cases (13.2%), and Epstein-Barr virus (EBV), in 3 patients (7.9%). Pneumonia was diagnosed in one case (2.6%). Bacterial bloodstream infections were detected in 3 patients (7.9%). The bloodstream infections were stratified in accordance with the Sepsis-3 criteria, thus enabling us to identify patients with unfavorable prognosis who developed sepsis and/or septic shock. Infectious complications were observed over the period of 60 days after ASCT. Meanwhile, CMV and EBV reactivation and bloodstream bacterial infections did not affect overall survival rate.
Conclusion
Our results demonstrate that bacterial complications and viral (CMV and EBV) reactivation aggravate the course of primary disease in MM patients over the post-transplant period. The methods of infection control in clinical practice (genotyping of multidrug-resistant strains, and antimicrobial control protocols) should improve the treatment strategies in patients with MM following ASCT. Keywords Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
Keywords
Autologous hematopoietic stem cells transplantation, bacterial and viral infectious complications, sepsis, septic shock.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27502
[VALUE] => 02/05/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/05/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27503
[VALUE] => 04/09/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 04/09/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27504
[VALUE] => Array
(
[TEXT] => <p>Олег В. Голощапов<sup>1</sup>, Евгений А. Бакин<sup>1</sup>, Оксана В. Станевич<sup>1</sup>, Руслана В. Клементьева<sup>1</sup>, Александр А. Щербаков<sup>1</sup>, Александр Н. Швецов<sup>1</sup>, Олеся В. Паина<sup>1</sup>, Полина В. Кожокарь<sup>1</sup>, Маргарита В. Горчакова<sup>1</sup>, Елена В. Бабенко<sup>1</sup>, Мария А. Суворова<sup>2</sup>, Сергей Н. Бондаренко<sup>1</sup>, Максим А. Кучер<sup>1</sup>, Александр Д. Кулагин<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup>, Иван С. Моисеев<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Олег В. Голощапов1, Евгений А. Бакин1, Оксана В. Станевич1, Руслана В. Клементьева1, Александр А. Щербаков1, Александр Н. Швецов1, Олеся В. Паина1, Полина В. Кожокарь1, Маргарита В. Горчакова1, Елена В. Бабенко1, Мария А. Суворова2, Сергей Н. Бондаренко1, Максим А. Кучер1, Александр Д. Кулагин1, Людмила С. Зубаровская1, Иван С. Моисеев1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27505
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br>
<sup>2</sup> ООО «Эксплана», Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 ООО «Эксплана», Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27506
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
На протяжении последних лет установлена важная роль нарушений кишечной микробиоты и ее потенуиальной коррекции при злокачественных новообразованиях и аутоиммунных заболеваниях у детей. Данные последнего десятилетия выявили существенное влияние основных классов кишечных бактерий (<i>Firmicutes</i> и <i>Bacteroides</i>) на развитие иммунного ответа при онкологических заболеваниях и аутоагрессивных состояниях, а также роль их дисбаланса и коррекции посредством трансплантации фекальной микробиоты (ТФМ). Наши предыдущие исследования показали выраженный клинический эффект ТФМ, в основном, у взрослых пациентов с тяжелой острой реакцией «трансплантат против хозяина» (РТПХ). Целью настоящей работы было представить наш опыт ТФМ у детей с кишечной формой острой РТПХ, резистентной к обычному лечению.
</p>
<h3>Материалы и методы</h3>
<p style="text-align: justify;">
Проспективное одноцентровое исследование включало 7 пациентов в возрасте от 3 до 10 лет с тяжелой кишечной РТПХ после аллогенной трансплантации гемопоэтических стволовых клеток. Клинические эффекты ТФМ определяли с применением стандартных шкал на протяжении 120 сут. после данной процедуры. Временную динамику состава фекальной микробиоты оценивали, главным образом, посредством мультиплексной ПЦР тест-системы. Результаты Мы представили собственный опыт ТФМ у 7 детей с кишечной формой острой РТПХ и антибиотикорезистентным колитом. Полный или частичный ответ на этот вид лечения РТПХ был достигнут в 6 случаях (86%) в течение 120 сут. в отсутствии серьезных нежелательных эффектов после ТФМ. С 8-го дня после ТФМ было отмечено нарастание содержания <i>B. fragilis gr., Faecalibacterium prausnitzii</i> и <i>E. coli</i> в фекальной микробиоте (р< 0,048, р< 0,001, и р<0,048, соответственно), при отсутствии различий по <i>Bifidobacterium spp.</i> и <i>Lactobacillus spp</i>.</p>
<h3>Выводы</h3>
<p style="text-align: justify;"> Комбинированная терапия иммуносупрессивными препаратами и ФМТ у пациентов с кишечной формой РТПХ, резистентной к стандартной терапии, сопровождается выраженным клиническим ответом, коррелирующим с определенными изменениями кишечной микробиоты и приемлемыми показателями безопасности процедуры.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Трансплантации фекальной микробиоты, трансплантация гемопоэтических клеток, реакция «трансплантат против хозяина», фекальная микробиота, трансплантация, клиническая эффективность, побочные эффекты.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
На протяжении последних лет установлена важная роль нарушений кишечной микробиоты и ее потенуиальной коррекции при злокачественных новообразованиях и аутоиммунных заболеваниях у детей. Данные последнего десятилетия выявили существенное влияние основных классов кишечных бактерий (Firmicutes и Bacteroides) на развитие иммунного ответа при онкологических заболеваниях и аутоагрессивных состояниях, а также роль их дисбаланса и коррекции посредством трансплантации фекальной микробиоты (ТФМ). Наши предыдущие исследования показали выраженный клинический эффект ТФМ, в основном, у взрослых пациентов с тяжелой острой реакцией «трансплантат против хозяина» (РТПХ). Целью настоящей работы было представить наш опыт ТФМ у детей с кишечной формой острой РТПХ, резистентной к обычному лечению.
Материалы и методы
Проспективное одноцентровое исследование включало 7 пациентов в возрасте от 3 до 10 лет с тяжелой кишечной РТПХ после аллогенной трансплантации гемопоэтических стволовых клеток. Клинические эффекты ТФМ определяли с применением стандартных шкал на протяжении 120 сут. после данной процедуры. Временную динамику состава фекальной микробиоты оценивали, главным образом, посредством мультиплексной ПЦР тест-системы. Результаты Мы представили собственный опыт ТФМ у 7 детей с кишечной формой острой РТПХ и антибиотикорезистентным колитом. Полный или частичный ответ на этот вид лечения РТПХ был достигнут в 6 случаях (86%) в течение 120 сут. в отсутствии серьезных нежелательных эффектов после ТФМ. С 8-го дня после ТФМ было отмечено нарастание содержания B. fragilis gr., Faecalibacterium prausnitzii и E. coli в фекальной микробиоте (р< 0,048, р< 0,001, и р<0,048, соответственно), при отсутствии различий по Bifidobacterium spp. и Lactobacillus spp.
Выводы
Комбинированная терапия иммуносупрессивными препаратами и ФМТ у пациентов с кишечной формой РТПХ, резистентной к стандартной терапии, сопровождается выраженным клиническим ответом, коррелирующим с определенными изменениями кишечной микробиоты и приемлемыми показателями безопасности процедуры.
Ключевые слова
Трансплантации фекальной микробиоты, трансплантация гемопоэтических клеток, реакция «трансплантат против хозяина», фекальная микробиота, трансплантация, клиническая эффективность, побочные эффекты.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27507
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-69-78
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-69-78
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27509
[VALUE] => Array
(
[TEXT] => <p>Oleg V. Goloshchapov<sup>1</sup>, Evgeny A. Bakin<sup>1</sup>, Oksana V. Stanevich<sup>1</sup>, Ruslana V. Klementeva<sup>1</sup>, Alexander A. Shcherbakov<sup>1</sup>, Alexander N. Shvetsov<sup>1</sup>, Olesya V. Paina<sup>1</sup>, Polina V. Kozhokar<sup>1</sup>, Margarita V. Gorchakova<sup>1</sup>, Elena V. Babenko<sup>1</sup>, Maria A. Suvorova<sup>2</sup>, Sergey N. Bondarenko<sup>1</sup>, Maxim A. Kucher<sup>1</sup>, Alexander D. Kulagin<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Ivan S. Moiseev<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Oleg V. Goloshchapov1, Evgeny A. Bakin1, Oksana V. Stanevich1, Ruslana V. Klementeva1, Alexander A. Shcherbakov1, Alexander N. Shvetsov1, Olesya V. Paina1, Polina V. Kozhokar1, Margarita V. Gorchakova1, Elena V. Babenko1, Maria A. Suvorova2, Sergey N. Bondarenko1, Maxim A. Kucher1, Alexander D. Kulagin1, Ludmila S. Zubarovskaya1, Ivan S. Moiseev1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27510
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Pavlov University, St. Petersburg, Russia<br>
<sup>2</sup> Explana Research Laboratory, St. Petersbug, Russia</p><br>
<p><b>Correspondence</b><br>
Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br>
Phone: +7 (921) 979 2913<br>
E-mail: golocht@yandex.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Pavlov University, St. Petersburg, Russia
2 Explana Research Laboratory, St. Petersbug, Russia
Correspondence
Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Phone: +7 (921) 979 2913
E-mail: golocht@yandex.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27511
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Over last years, an important role of altered gut microbiota and its potential correction was suggested for pediatric cancer and autoimmune disorders. The data from last decade highlight sufficient influence of the main classes of gut bacteria (<i>Firmicutes</i> and <i>Bacteroides</i>) upon development of immune response in oncological disorders and autoagressive conditions, as well as role of their imbalance and its correction using fecal microbiota transplantation (FMT) approach. Our previous studies have shown a pronounced clinical effect of FMT, mostly, in adult patients with severe acute graft-versus-host disease (GVHD). The aim of this article was to present our own experience of FMT in children with intestinal form of GVHD resistant to conventional treatment.
</p>
<h3>Materials and methods</h3>
<p style="text-align: justify;">
A prospective single-center study included 7 patients aged from 3 to 10 years with severe intestinal GVHD developed after allogeneic hematopoietic stem cell transplantation. Clinical effects of FMT were evaluated by conventional scales during 120 days after the procedure. Time-dependent changes of fecal microbiota were assayed, mainly, by the multiplex polymerase chain reaction (PCR) test-system.
</p>
<h3> Results</h3>
<p style="text-align: justify;">
We present our own experience of FMT in 7 children with intestinal GVHD and antibiotic-resistant colitis. Complete or partial response to the GVHD treatment was achieved in 6 cases (86%) by 120 days, in absence of serious adverse events following FMT. Since day +8 after TFM, increased amounts of <i>B. fragilis gr., Faecalibacterium prausnitzii</i> and <i>E. coli </i>were registered in fecal microbiota (р< 0.048, р< 0.001, and р<0.048, respectively), in absence of differences for <i>Bifidobacterium spp </i>and <i>Lactobacillus spp</i>.
</p>
<h3>Conclusion</h3>
<p style="text-align: justify;">
Combined therapy with immunosuppressive agents and FMT procedure in the patients with intestinal GVHD resistant to standard therapy is associated with pronounced clinical responses correlating with distinct changes of intestinal microbiota, with acceptable safety profile.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Hematopoietic stem cell transplantation, graft-versus-host disease, fecal microbiota transplantation, clinical efficiency.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Over last years, an important role of altered gut microbiota and its potential correction was suggested for pediatric cancer and autoimmune disorders. The data from last decade highlight sufficient influence of the main classes of gut bacteria (Firmicutes and Bacteroides) upon development of immune response in oncological disorders and autoagressive conditions, as well as role of their imbalance and its correction using fecal microbiota transplantation (FMT) approach. Our previous studies have shown a pronounced clinical effect of FMT, mostly, in adult patients with severe acute graft-versus-host disease (GVHD). The aim of this article was to present our own experience of FMT in children with intestinal form of GVHD resistant to conventional treatment.
Materials and methods
A prospective single-center study included 7 patients aged from 3 to 10 years with severe intestinal GVHD developed after allogeneic hematopoietic stem cell transplantation. Clinical effects of FMT were evaluated by conventional scales during 120 days after the procedure. Time-dependent changes of fecal microbiota were assayed, mainly, by the multiplex polymerase chain reaction (PCR) test-system.
Results
We present our own experience of FMT in 7 children with intestinal GVHD and antibiotic-resistant colitis. Complete or partial response to the GVHD treatment was achieved in 6 cases (86%) by 120 days, in absence of serious adverse events following FMT. Since day +8 after TFM, increased amounts of B. fragilis gr., Faecalibacterium prausnitzii and E. coli were registered in fecal microbiota (р< 0.048, р< 0.001, and р<0.048, respectively), in absence of differences for Bifidobacterium spp and Lactobacillus spp.
Conclusion
Combined therapy with immunosuppressive agents and FMT procedure in the patients with intestinal GVHD resistant to standard therapy is associated with pronounced clinical responses correlating with distinct changes of intestinal microbiota, with acceptable safety profile.
Keywords
Hematopoietic stem cell transplantation, graft-versus-host disease, fecal microbiota transplantation, clinical efficiency.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27508
[VALUE] => Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Clinical and immune effects of fecal microbiota transplantation in children with acute graft-versus-host disease
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27536
[VALUE] => 2428
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2428
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27537
[VALUE] => 2429
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2429
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Oleg V. Goloshchapov1, Evgeny A. Bakin1, Oksana V. Stanevich1, Ruslana V. Klementeva1, Alexander A. Shcherbakov1, Alexander N. Shvetsov1, Olesya V. Paina1, Polina V. Kozhokar1, Margarita V. Gorchakova1, Elena V. Babenko1, Maria A. Suvorova2, Sergey N. Bondarenko1, Maxim A. Kucher1, Alexander D. Kulagin1, Ludmila S. Zubarovskaya1, Ivan S. Moiseev1
1 Pavlov University, St. Petersburg, Russia
2 Explana Research Laboratory, St. Petersbug, Russia
Correspondence
Oleg V. Goloshchapov, ICU Department, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Phone: +7 (921) 979 2913
E-mail: golocht@yandex.ru
Over last years, an important role of altered gut microbiota and its potential correction was suggested for pediatric cancer and autoimmune disorders. The data from last decade highlight sufficient influence of the main classes of gut bacteria (Firmicutes and Bacteroides) upon development of immune response in oncological disorders and autoagressive conditions, as well as role of their imbalance and its correction using fecal microbiota transplantation (FMT) approach. Our previous studies have shown a pronounced clinical effect of FMT, mostly, in adult patients with severe acute graft-versus-host disease (GVHD). The aim of this article was to present our own experience of FMT in children with intestinal form of GVHD resistant to conventional treatment.
Materials and methods
A prospective single-center study included 7 patients aged from 3 to 10 years with severe intestinal GVHD developed after allogeneic hematopoietic stem cell transplantation. Clinical effects of FMT were evaluated by conventional scales during 120 days after the procedure. Time-dependent changes of fecal microbiota were assayed, mainly, by the multiplex polymerase chain reaction (PCR) test-system.
Results
We present our own experience of FMT in 7 children with intestinal GVHD and antibiotic-resistant colitis. Complete or partial response to the GVHD treatment was achieved in 6 cases (86%) by 120 days, in absence of serious adverse events following FMT. Since day +8 after TFM, increased amounts of B. fragilis gr., Faecalibacterium prausnitzii and E. coli were registered in fecal microbiota (р< 0.048, р< 0.001, and р<0.048, respectively), in absence of differences for Bifidobacterium spp and Lactobacillus spp.
Conclusion
Combined therapy with immunosuppressive agents and FMT procedure in the patients with intestinal GVHD resistant to standard therapy is associated with pronounced clinical responses correlating with distinct changes of intestinal microbiota, with acceptable safety profile.
Keywords
Hematopoietic stem cell transplantation, graft-versus-host disease, fecal microbiota transplantation, clinical efficiency.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27482
[VALUE] => 03/12/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/12/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27483
[VALUE] => 04/09/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 04/09/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27484
[VALUE] => Array
(
[TEXT] => <p>Николай Н. Мамаев, Алена И. Шакирова, Татьяна Л. Гиндина, Сергей Н. Бондаренко, Белла И. Аюбова, Ильдар М. Бархатов, Яна В. Гудожникова, Валентина М. Кравцова, Михаил М. Канунников, Олеся В. Паина, Жемал Ж. Рахманова, Татьяна Ю. Грачева, Людмила С. Зубаровская</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Николай Н. Мамаев, Алена И. Шакирова, Татьяна Л. Гиндина, Сергей Н. Бондаренко, Белла И. Аюбова, Ильдар М. Бархатов, Яна В. Гудожникова, Валентина М. Кравцова, Михаил М. Канунников, Олеся В. Паина, Жемал Ж. Рахманова, Татьяна Ю. Грачева, Людмила С. Зубаровская
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27485
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27486
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34<sup>+</sup>CD38<sup>–</sup>, которые, в свою очередь, способны к избирательной экспрессии гена <i>BAALC</i>. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена <i>WT1</i>. Поскольку можно количественно оценить содержание мРНК <i>BAALC</i> и <i>WT1</i> посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов <i>BAALC </i>и <i>WT1</i>, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена <i>EVI1</i> (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых <i>BAALC</i>-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена <i>WT1</i>, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых <i>BAALC</i>-экспрессирующих предшественников на более зрелые <i>WT1</i>-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии<i> BAALC</i>, <i>WT1</i> и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов.
</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">
Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия <i>BAALC</i>, экспрессия <i>WT1</i>, прекурсоры лейкозных клеток, РТ-ПЦР.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Имеются данные о том, что рецидивы острого миелобластного лейкоза (ОМЛ) тесно связаны с гетерогенностью популяции лейкозных предшественников. Можно выделить, по меньшей мере, два класса лейкоз-инициирующих клеток (ЛИК), исходя из недавних экспериментальных работ по трансплантации гемопоэтических стволовых клеток (ТГСК) иммунодефицитным мышам. Основные классы ЛИК представлены незрелыми предшественниками с иммунным фенотипом CD34+CD38–, которые, в свою очередь, способны к избирательной экспрессии гена BAALC. Другой класс ЛИК состоит из относительно зрелых предшественников с более дифференцированными фенотипами. Согласно косвенным результатам, они, наряду с бластными формами, способны к экспрессии гена WT1. Поскольку можно количественно оценить содержание мРНК BAALC и WT1 посредством стандартизированной real-time-ПЦР, то этот подход может быть эффективным для уточнения механизмов рецидивов и резистентности к терапии ОМЛ. Целью данной работы была одновременная динамическая оценка экспрессии генов BAALC и WT1, наряду с определением числа бластных форм в исследуемых образцах костного мозга у 14 пациентов с ОМЛ, леченных в нашем центре Гемтузумаб-озогамицином (ГО, Милотарг), который комбинировали с высокодозной терапией (ВДХТ) и аллогенной ТГСК. Наши предварительные результаты показали следующее: a) более высокую общую 3-летнюю выживаемость в группе пациентов ОМЛ с нормальным кариотипом или малыми нарушениями кариотипа и мутациями гена FLT3, по сравнению с больными с более сложными кариотипами и гиперэкспрессией гена EVI1 (85,7% против 16,7%; p=0,032); б) выраженный клинический ответ незрелых BAALC-экспрессирующих предшественников на комбинированную ВДХТ и терапию ГО; в) возможное участие некоторых более зрелых предшественников в экспрессии гена WT1, наряду с бластными формами; г) реальное доказательство переключения гемопоэтической регуляции с незрелых BAALC-экспрессирующих предшественников на более зрелые WT1-экспрессирующие клетки. Эти результаты указывают на диагностическую ценность комбинированной панели из экспрессии BAALC, WT1 и содержания бластных форм для количественных исследований и определения различных классов клеток-предшественников в прогрессии ОМЛ и возникновении рецидивов.
Ключевые слова
Острый миелобластный лейкоз, резистентность к терапии, рецидивы, патогенез, Гемтузумаб озогамицин, экспрессия BAALC, экспрессия WT1, прекурсоры лейкозных клеток, РТ-ПЦР.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27487
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-55-62
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-55-62
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27489
[VALUE] => Array
(
[TEXT] => <p>Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27490
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022,
St. Petersburg, Russia<br>
Phone: +7 (911) 760 5086<br>
E-mail: nikmamev524@gmail.com</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022,
St. Petersburg, Russia
Phone: +7 (911) 760 5086
E-mail: nikmamev524@gmail.com
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27491
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">
There is evidence that relapses of acute myeloid leukemia (AML) are closely related to heterogeneous population of leukemic precursors. At least, two classes of the leukemia-initiating cells (LIC) may be discerned, according to recent experimental studies with hematopoietic cell transplants to immunodeficient mice. The main class of LICs is presented by immature precursors with CD34<sup>+</sup>CD38<sup>–</sup> immunophenotype which, in turn, are capable of selective expression of <i>BAALC</i> gene. The second class of LICs is presented by relatively mature precursors with more differentiated immunophenotypes. According to indirect findings, they are able of <i>WT1</i> gene expression, along with blast cells. Since both <i>BAALC</i> and <i>WT1</i> mRNAs may be quantitatively evaluated by means of standardized quantitative polymerase reaction in real time (qRT-PCR), this approach may be effective for specifying the mechanisms of relapses and resistance to therapy in AML patients. The aim of this work was to perform simultaneous dynamic evaluation of <i>BAALC</i> and <i>WT1</i> genes expressions along with determination of blast numbers in the tested bone marrow samples in 14 AML patients treated at our Center with Gemtuzumab ozogamicin (GO, Mylotarg), which was combined with high-dose chemotherapy (ChT), followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Our preliminary results are as follows: a) superior 3-year overall survival (OS) in general group of patients with normal or nearly-normal karyotypes, and FLT3-mutated AML variants as compared to those with more complex karyotypes and <i>EVI1</i> gene overexpression (85.7% <i>vs</i> 16.7%; p=0.032); b) highly sensitive response of immature <i>BAALC</i>-expressing precursors to combined ChT and GO treatment; c) hypothetical participation of some mature precursors, along with blast cells, in <i>WT1</i> gene expression; d) real evidence for switching hematopoietic regulation from immature <i>BAALC</i>-expressing precursors to more mature <i>WT1</i>-expressing progeny. These results suggest diagnostic utility of combined <i>BAALC</i>/<i>WT1</i>/blast counts panel for quantitative studies and assessment of distinct precursors in AML progression and emergence of relapses.
</p>
<h2>Keywords</h2>
<p style="text-align: justify;">
Acute myeloid leukemia, resistance to therapy, relapses, pathogenesis, Gemtuzumab ozogamycin, <i>BAALC</i> expression, <i>WT1</i> expression, leukemic cell precursors, qRT-PCR.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
There is evidence that relapses of acute myeloid leukemia (AML) are closely related to heterogeneous population of leukemic precursors. At least, two classes of the leukemia-initiating cells (LIC) may be discerned, according to recent experimental studies with hematopoietic cell transplants to immunodeficient mice. The main class of LICs is presented by immature precursors with CD34+CD38– immunophenotype which, in turn, are capable of selective expression of BAALC gene. The second class of LICs is presented by relatively mature precursors with more differentiated immunophenotypes. According to indirect findings, they are able of WT1 gene expression, along with blast cells. Since both BAALC and WT1 mRNAs may be quantitatively evaluated by means of standardized quantitative polymerase reaction in real time (qRT-PCR), this approach may be effective for specifying the mechanisms of relapses and resistance to therapy in AML patients. The aim of this work was to perform simultaneous dynamic evaluation of BAALC and WT1 genes expressions along with determination of blast numbers in the tested bone marrow samples in 14 AML patients treated at our Center with Gemtuzumab ozogamicin (GO, Mylotarg), which was combined with high-dose chemotherapy (ChT), followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Our preliminary results are as follows: a) superior 3-year overall survival (OS) in general group of patients with normal or nearly-normal karyotypes, and FLT3-mutated AML variants as compared to those with more complex karyotypes and EVI1 gene overexpression (85.7% vs 16.7%; p=0.032); b) highly sensitive response of immature BAALC-expressing precursors to combined ChT and GO treatment; c) hypothetical participation of some mature precursors, along with blast cells, in WT1 gene expression; d) real evidence for switching hematopoietic regulation from immature BAALC-expressing precursors to more mature WT1-expressing progeny. These results suggest diagnostic utility of combined BAALC/WT1/blast counts panel for quantitative studies and assessment of distinct precursors in AML progression and emergence of relapses.
Keywords
Acute myeloid leukemia, resistance to therapy, relapses, pathogenesis, Gemtuzumab ozogamycin, BAALC expression, WT1 expression, leukemic cell precursors, qRT-PCR.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27488
[VALUE] => Quantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Quantitative study of BAALC- and WT1-expressing cell precursors in the patients with different cytogenetic and molecular AML variants treated with Gemtuzumab ozogamicin and hematopoietic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27532
[VALUE] => 2422
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2422
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27533
[VALUE] => 2423
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2423
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Nikolay N. Mamaev, Alyona I. Shakirova, Tatiana L. Gindina, Sergey N. Bondarenko, Bella I. Ayubova, Ildar M. Barkhatov, Yana V. Gudozhnikova, Valentina M. Kravtsova, Mikhail M. Kanunnikov, Olesya V. Paina, Zhemal Z. Rakhmanova, Tatiana Yu. Gracheva, Ludmila S. Zubarovskaya
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Professor Nikolay N. Mamaev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, 12 Roentgen St, 197022,
St. Petersburg, Russia
Phone: +7 (911) 760 5086
E-mail: nikmamev524@gmail.com
There is evidence that relapses of acute myeloid leukemia (AML) are closely related to heterogeneous population of leukemic precursors. At least, two classes of the leukemia-initiating cells (LIC) may be discerned, according to recent experimental studies with hematopoietic cell transplants to immunodeficient mice. The main class of LICs is presented by immature precursors with CD34+CD38– immunophenotype which, in turn, are capable of selective expression of BAALC gene. The second class of LICs is presented by relatively mature precursors with more differentiated immunophenotypes. According to indirect findings, they are able of WT1 gene expression, along with blast cells. Since both BAALC and WT1 mRNAs may be quantitatively evaluated by means of standardized quantitative polymerase reaction in real time (qRT-PCR), this approach may be effective for specifying the mechanisms of relapses and resistance to therapy in AML patients. The aim of this work was to perform simultaneous dynamic evaluation of BAALC and WT1 genes expressions along with determination of blast numbers in the tested bone marrow samples in 14 AML patients treated at our Center with Gemtuzumab ozogamicin (GO, Mylotarg), which was combined with high-dose chemotherapy (ChT), followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Our preliminary results are as follows: a) superior 3-year overall survival (OS) in general group of patients with normal or nearly-normal karyotypes, and FLT3-mutated AML variants as compared to those with more complex karyotypes and EVI1 gene overexpression (85.7% vs 16.7%; p=0.032); b) highly sensitive response of immature BAALC-expressing precursors to combined ChT and GO treatment; c) hypothetical participation of some mature precursors, along with blast cells, in WT1 gene expression; d) real evidence for switching hematopoietic regulation from immature BAALC-expressing precursors to more mature WT1-expressing progeny. These results suggest diagnostic utility of combined BAALC/WT1/blast counts panel for quantitative studies and assessment of distinct precursors in AML progression and emergence of relapses.
Keywords
Acute myeloid leukemia, resistance to therapy, relapses, pathogenesis, Gemtuzumab ozogamycin, BAALC expression, WT1 expression, leukemic cell precursors, qRT-PCR.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27472
[VALUE] => 03/11/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/11/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27473
[VALUE] => 04/09/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 04/09/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27474
[VALUE] => Array
(
[TEXT] => <p>Людмила В. Ольхова<sup>1</sup>, Ольга Г. Желудкова<sup>2</sup>, Людмила С. Зубаровская<sup>3</sup>, Анна Ю. Смирнова<sup>4</sup>, Юлия В. Диникина<sup>4,5</sup>, Асмик Г. Геворгян<sup>3</sup>, Андрей С. Левашов<sup>6</sup>, Елена В. Скоробогатова<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Людмила В. Ольхова1, Ольга Г. Желудкова2, Людмила С. Зубаровская3, Анна Ю. Смирнова4, Юлия В. Диникина4,5, Асмик Г. Геворгян3, Андрей С. Левашов6, Елена В. Скоробогатова1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27475
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Обособленное структурное подразделение «Российская детская клиническая больница», Российский национальный медицинский университет им. Н. И. Пирогова, Москва, Россия<br>
<sup>2</sup> Научно-практический центр специализированной медицинской помощи им. В. Ф. Войно-Ясенецкого, Москва, Россия<br>
<sup>3</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>4</sup> Национальный медицинский исследовательский центр им. В. А. Алмазова, Санкт-Петербург, Россия<br>
<sup>5</sup> Санкт-Петербургский государственный педиатрический медицинский университет, Санкт-Петербург, Россия<br>
<sup>6</sup> Национальный медицинский исследовательский центр онкологии им. Н. Н. Блохина, Москва, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Обособленное структурное подразделение «Российская детская клиническая больница», Российский национальный медицинский университет им. Н. И. Пирогова, Москва, Россия
2 Научно-практический центр специализированной медицинской помощи им. В. Ф. Войно-Ясенецкого, Москва, Россия
3 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
4 Национальный медицинский исследовательский центр им. В. А. Алмазова, Санкт-Петербург, Россия
5 Санкт-Петербургский государственный педиатрический медицинский университет, Санкт-Петербург, Россия
6 Национальный медицинский исследовательский центр онкологии им. Н. Н. Блохина, Москва, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27476
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Атипичная тератоид-рабдоидная опухоль (АТРО) центральной нервной системы (ЦНС) – это агрессивная злокачественная опухоль, встречается преимущественно у детей младшего возраста и характеризуется плохим прогнозом. В настоящее время нет единых стандартов лечения АТРО ЦНС. Данное ретроспективное исследование выполнено с целью оценки результатов высокодозной полихимиотерапии (ВДХТ) с последующей аутологичной трансплантацией гемопоэтических стволовых клеток (ауто-ТГСК) у детей с АТРО ЦНС и определения влияния различных факторов прогноза на выживаемость. В исследование включены 30 больных с АТРО ЦНС, которые в протоколе терапии получали ВДХТ с ауто-ТГСК. Медиана возраста пациентов составила 19,5 месяцев [9; 27]. Распределение пациентов в зависимости от возраста было следующим: младше 12 месяцев – 11 (36,6%), старше 12 месяцев –
19 (63,4%); по полу: мальчиков – 21 (70%) и девочек –
9 (30%). Опухоль у 17 пациентов (56,7%) локализовалась инфратенториально, у 13 (43,3%) – супратенториально. Всем пациентам инициально выполнено хирургическое лечение в различных объемах: тотальное удаление опухоли – у 8 (26,7%) пациентов, субтотальное – у 9 (30,0%), частичное удаление –
у 11 (36,6%), биопсия – у 2 (6,7%). В анализируемой группе преобладали больные с М+ стадией заболевания – 16 пациентов (53,3%), у 12 (40,0%) метастазирование и опухолевые клетки отсутствовали, установлена М0-стадия, у 2 (6,7%) – стадия заболевания не уточнена (Мх). Все пациенты после удаления опухоли получали лечение по различным протоколам терапии: 12 пациентов (40,0%) – по протоколу EU-RHAB, 11 (36,7%) – по протоколу MUV-ATRT, у 7 (23,3%) больных выполняли индивидуальные схемы. ЛТ проведена 24 больным (80%) после ВДХТ с ауто-ТГСК. Интратекальное/интравентрикулярное введение химиопрепаратов получили большинство пациентов (n=22, 73,3%). Статус заболевания оценивался у всех пациентов до выполнения ВДХТ с ауто-ТГСК: полный ответ (ПО) был зарегистрирован у 12 пациентов (40%), стабилизация болезни (СБ) – у 10 (33,3%), частичный ответ (ЧО) – у 8 (26,7%). Большинству пациентов выполнена однократная ауто-ТГСК – 21 (70%), тандемная ауто-ТГСК – у 9 (30%) пациентов. Общее количество выполненных ауто-ТГСК у пациентов с АТРО ЦНС составило 39. В качестве источника трансплантата в 90% (n=27) были использованы СКПК, у 10% (n=3) больных использовали комбинацию СКПК+КМ. Результаты: 5-летняя БСВ и ОВ составили 44%, большинство рецидивов диагностировано в течение 24 месяцев после постановки диагноза. Эти результаты сопоставимы с большинством международных данных. Выживаемость пациентов с АТРО ЦНС, получивших ВДХТ с ауто-ТГСК, статистически достоверно выше была при выполнении тотальной резекции опухоли, проведении ЛТ и регионарной химиотерапии и достижении ПО к моменту проведения ТГСК. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Дети, младший возраст, атипичная тератоид-рабдоидная опухоль, центральная нервная система, химиотерапия, высокодозная, лучевая терапия, результаты лечения, выживаемость, прогностические факторы.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Атипичная тератоид-рабдоидная опухоль (АТРО) центральной нервной системы (ЦНС) – это агрессивная злокачественная опухоль, встречается преимущественно у детей младшего возраста и характеризуется плохим прогнозом. В настоящее время нет единых стандартов лечения АТРО ЦНС. Данное ретроспективное исследование выполнено с целью оценки результатов высокодозной полихимиотерапии (ВДХТ) с последующей аутологичной трансплантацией гемопоэтических стволовых клеток (ауто-ТГСК) у детей с АТРО ЦНС и определения влияния различных факторов прогноза на выживаемость. В исследование включены 30 больных с АТРО ЦНС, которые в протоколе терапии получали ВДХТ с ауто-ТГСК. Медиана возраста пациентов составила 19,5 месяцев [9; 27]. Распределение пациентов в зависимости от возраста было следующим: младше 12 месяцев – 11 (36,6%), старше 12 месяцев –
19 (63,4%); по полу: мальчиков – 21 (70%) и девочек –
9 (30%). Опухоль у 17 пациентов (56,7%) локализовалась инфратенториально, у 13 (43,3%) – супратенториально. Всем пациентам инициально выполнено хирургическое лечение в различных объемах: тотальное удаление опухоли – у 8 (26,7%) пациентов, субтотальное – у 9 (30,0%), частичное удаление –
у 11 (36,6%), биопсия – у 2 (6,7%). В анализируемой группе преобладали больные с М+ стадией заболевания – 16 пациентов (53,3%), у 12 (40,0%) метастазирование и опухолевые клетки отсутствовали, установлена М0-стадия, у 2 (6,7%) – стадия заболевания не уточнена (Мх). Все пациенты после удаления опухоли получали лечение по различным протоколам терапии: 12 пациентов (40,0%) – по протоколу EU-RHAB, 11 (36,7%) – по протоколу MUV-ATRT, у 7 (23,3%) больных выполняли индивидуальные схемы. ЛТ проведена 24 больным (80%) после ВДХТ с ауто-ТГСК. Интратекальное/интравентрикулярное введение химиопрепаратов получили большинство пациентов (n=22, 73,3%). Статус заболевания оценивался у всех пациентов до выполнения ВДХТ с ауто-ТГСК: полный ответ (ПО) был зарегистрирован у 12 пациентов (40%), стабилизация болезни (СБ) – у 10 (33,3%), частичный ответ (ЧО) – у 8 (26,7%). Большинству пациентов выполнена однократная ауто-ТГСК – 21 (70%), тандемная ауто-ТГСК – у 9 (30%) пациентов. Общее количество выполненных ауто-ТГСК у пациентов с АТРО ЦНС составило 39. В качестве источника трансплантата в 90% (n=27) были использованы СКПК, у 10% (n=3) больных использовали комбинацию СКПК+КМ. Результаты: 5-летняя БСВ и ОВ составили 44%, большинство рецидивов диагностировано в течение 24 месяцев после постановки диагноза. Эти результаты сопоставимы с большинством международных данных. Выживаемость пациентов с АТРО ЦНС, получивших ВДХТ с ауто-ТГСК, статистически достоверно выше была при выполнении тотальной резекции опухоли, проведении ЛТ и регионарной химиотерапии и достижении ПО к моменту проведения ТГСК.
Ключевые слова
Дети, младший возраст, атипичная тератоид-рабдоидная опухоль, центральная нервная система, химиотерапия, высокодозная, лучевая терапия, результаты лечения, выживаемость, прогностические факторы.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27477
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-44-54
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-44-54
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27479
[VALUE] => Array
(
[TEXT] => <p>Liudmila V. Olkhova<sup>1</sup>, Olga G. Zheludkova<sup>2</sup>, Ludmila S. Zubarovskaya<sup>3</sup>, Anna Yu. Smirnova<sup>4</sup>, Yulia V. Dinikina<sup>4,5</sup>, Asmik G. Gevorgyan<sup>3</sup>, Andrey S. Levashov<sup>6</sup>, Elena V. Skorobogatova<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Liudmila V. Olkhova1, Olga G. Zheludkova2, Ludmila S. Zubarovskaya3, Anna Yu. Smirnova4, Yulia V. Dinikina4,5, Asmik G. Gevorgyan3, Andrey S. Levashov6, Elena V. Skorobogatova1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27480
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia<br>
<sup>2</sup> St. Luke Clinical Research Center for Children, Moscow, Russia<br>
<sup>3</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br>
<sup>4</sup> V. A. Almazov National Medical Research Center, St. Petersburg, Russia<br>
<sup>5</sup> St. Petersburg State Pediatric Medical University, St. Petersburg, Russia<br>
<sup>6</sup> N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia<br>
Phone: +7 (995) 707 5140<br>
E-mail: rylkova87@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia
2 St. Luke Clinical Research Center for Children, Moscow, Russia
3 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
4 V. A. Almazov National Medical Research Center, St. Petersburg, Russia
5 St. Petersburg State Pediatric Medical University, St. Petersburg, Russia
6 N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia
Correspondence
Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia
Phone: +7 (995) 707 5140
E-mail: rylkova87@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27481
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Atypical teratoid rhabdoid tumor (ATRT) of central nervous system (CNS) is an aggressive malignancy with poor prognosis, predominantly observed in young children. There are no established approaches to CNS ATRT management nowadays. This retrospective study aimed to analyze the effectiveness and prognostic factors of high dose chemotherapy with autologous hematopoietic stem cell transplantation HDCT/auto-HSCT in pediatric CNS ATRT. Thirty CNS ATRT patients treated with HDCT/auto-HSCT were enrolled in the analysis. Median age was 19.5 months. There were 11 (36.6%) infants and 19 (63.4%) children older than 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial in 13 (43.3%). All children initially received surgery with total resection (n=8, 26.7%), subtotal resection (n=9, 30%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and the minority had M-0 stage (n=12, 40%), while stage wasn't clarified (Mx) in 2 (6.7%) cases. After surgery everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). Radiotherapy (RT) was performed in 24 children (80%) after HDCT/auto-HSCT. The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. The disease status was assessed in all cases prior to HDCT/auto-HSCT with complete response (CR) in 12 (40%), partial response (PR) in 8 (26.7%) and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in the majority of patients (n=21, 70%) and tandem transplants were carried out in 9 cases only (30%). In total, 39 transplants were performed. Peripheral blood hematopoietic stem cells (PBSC) were the transplant source in 27 children (90%), and combination of PBSC and bone marrow (BM), in 3 (10%). Five-year event-free survival (EFS) and overall survival (OS) were 44%. The majority of relapses were diagnosed during first 24 months after disease onset. These data are comparable to the most international results. Survival of CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total tumor resection, RT, intraventricular/intrathecal chemotherapy, and CR prior to transplantation. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Сhildren, young age, atypical teratoid rhabdoid tumor, central nervous system, chemotherapy, high-dose, radiation therapy, clinical outcomes, survival, prognostic factors.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Atypical teratoid rhabdoid tumor (ATRT) of central nervous system (CNS) is an aggressive malignancy with poor prognosis, predominantly observed in young children. There are no established approaches to CNS ATRT management nowadays. This retrospective study aimed to analyze the effectiveness and prognostic factors of high dose chemotherapy with autologous hematopoietic stem cell transplantation HDCT/auto-HSCT in pediatric CNS ATRT. Thirty CNS ATRT patients treated with HDCT/auto-HSCT were enrolled in the analysis. Median age was 19.5 months. There were 11 (36.6%) infants and 19 (63.4%) children older than 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial in 13 (43.3%). All children initially received surgery with total resection (n=8, 26.7%), subtotal resection (n=9, 30%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and the minority had M-0 stage (n=12, 40%), while stage wasn't clarified (Mx) in 2 (6.7%) cases. After surgery everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). Radiotherapy (RT) was performed in 24 children (80%) after HDCT/auto-HSCT. The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. The disease status was assessed in all cases prior to HDCT/auto-HSCT with complete response (CR) in 12 (40%), partial response (PR) in 8 (26.7%) and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in the majority of patients (n=21, 70%) and tandem transplants were carried out in 9 cases only (30%). In total, 39 transplants were performed. Peripheral blood hematopoietic stem cells (PBSC) were the transplant source in 27 children (90%), and combination of PBSC and bone marrow (BM), in 3 (10%). Five-year event-free survival (EFS) and overall survival (OS) were 44%. The majority of relapses were diagnosed during first 24 months after disease onset. These data are comparable to the most international results. Survival of CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total tumor resection, RT, intraventricular/intrathecal chemotherapy, and CR prior to transplantation.
Keywords
Сhildren, young age, atypical teratoid rhabdoid tumor, central nervous system, chemotherapy, high-dose, radiation therapy, clinical outcomes, survival, prognostic factors.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27478
[VALUE] => High-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => High-dose chemotherapy with autologous hematopoietic stem cell transplantation in children with atypical teratoid/rhabdoid CNS tumors
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27530
[VALUE] => 2420
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2420
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27531
[VALUE] => 2421
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2421
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Liudmila V. Olkhova1, Olga G. Zheludkova2, Ludmila S. Zubarovskaya3, Anna Yu. Smirnova4, Yulia V. Dinikina4,5, Asmik G. Gevorgyan3, Andrey S. Levashov6, Elena V. Skorobogatova1
1 Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Moscow, Russia
2 St. Luke Clinical Research Center for Children, Moscow, Russia
3 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
4 V. A. Almazov National Medical Research Center, St. Petersburg, Russia
5 St. Petersburg State Pediatric Medical University, St. Petersburg, Russia
6 N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia
Correspondence
Dr. Liudmila V. Olkhova, Pediatric Oncologist, Department of Bone Marrow Transplantation, Russian Pediatric Clinical Hospital, N. N. Pirogov Russian National Medical University, Leninsky Ave 117, 119571, Moscow, Russia
Phone: +7 (995) 707 5140
E-mail: rylkova87@mail.ru
Atypical teratoid rhabdoid tumor (ATRT) of central nervous system (CNS) is an aggressive malignancy with poor prognosis, predominantly observed in young children. There are no established approaches to CNS ATRT management nowadays. This retrospective study aimed to analyze the effectiveness and prognostic factors of high dose chemotherapy with autologous hematopoietic stem cell transplantation HDCT/auto-HSCT in pediatric CNS ATRT. Thirty CNS ATRT patients treated with HDCT/auto-HSCT were enrolled in the analysis. Median age was 19.5 months. There were 11 (36.6%) infants and 19 (63.4%) children older than 12 months, among them 21 (70%) boys and 9 (30%) girls. Infratentorial tumor was diagnosed in 7 patients (56.7%) and supratentorial in 13 (43.3%). All children initially received surgery with total resection (n=8, 26.7%), subtotal resection (n=9, 30%), partial resection (n=11, 36.6%) and biopsy (n=2, 6.7%). The majority of patients had M+ stage (n=16, 53.3%) and the minority had M-0 stage (n=12, 40%), while stage wasn't clarified (Mx) in 2 (6.7%) cases. After surgery everyone received treatment according to various protocols: EU-RHAB (n=12, 40%), MUV-ATRT (n=11, 36.7%), individual therapy (n=7, 23.3%). Radiotherapy (RT) was performed in 24 children (80%) after HDCT/auto-HSCT. The majority of patients (n=22, 73.3%) received intraventricular/intrathecal chemotherapy. The disease status was assessed in all cases prior to HDCT/auto-HSCT with complete response (CR) in 12 (40%), partial response (PR) in 8 (26.7%) and stabilization (S) in 10 (33.3%). Single auto-HSCT was performed in the majority of patients (n=21, 70%) and tandem transplants were carried out in 9 cases only (30%). In total, 39 transplants were performed. Peripheral blood hematopoietic stem cells (PBSC) were the transplant source in 27 children (90%), and combination of PBSC and bone marrow (BM), in 3 (10%). Five-year event-free survival (EFS) and overall survival (OS) were 44%. The majority of relapses were diagnosed during first 24 months after disease onset. These data are comparable to the most international results. Survival of CNS ATRT patients after HDCT/auto-HSCT was statistically significantly higher after total tumor resection, RT, intraventricular/intrathecal chemotherapy, and CR prior to transplantation.
Keywords
Сhildren, young age, atypical teratoid rhabdoid tumor, central nervous system, chemotherapy, high-dose, radiation therapy, clinical outcomes, survival, prognostic factors.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27452
[VALUE] => 01/14/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 01/14/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27453
[VALUE] => 04/09/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 04/09/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27454
[VALUE] => Array
(
[TEXT] => <p>Елена В. Морозова, Николай Ю. Цветков, Ирина О. Туртанова, Иван С. Моисеев</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Елена В. Морозова, Николай Ю. Цветков, Ирина О. Туртанова, Иван С. Моисеев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27455
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27456
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.</p>
<p style="text-align: justify;">В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Миелодиспластический синдром (МДС) – это гетерогенная группа клональных заболеваний, в основе которой находится поражение гемопоэтической стволовой клетки, как следствие наследственной предрасположенности, а также соматических мутаций различных генов и/или эпигенетической регуляции, в том числе индуцированных нарушением микроокружения и нарушениями в иммунной системе противоопухолевого надзора.
В обзоре освещаются долгосрочные результаты существующих методов лечения МДС, а также эффективность новых препаратов, находящихся на различных стадиях клинических испытаний, включая ингибиторы сигнальных путей, ингибиторы контрольных точек, антагонисты трансформирующего ростового фактора бета. Характеризуется взаимосвязь новых методов терапии с патогенетическими основами МДС.
Ключевые слова
Миелодиспластический синдром, терапия, ингибиторы контрольных точек, луспатерцепт, гласдегиб, венетоклакс, IDH ингибиторы.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27457
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-24-36
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-24-36
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27459
[VALUE] => Array
(
[TEXT] => <p>Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27460
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
<p>Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7 (921) 796 1951<br>
E-mail: moisiv@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 796 1951
E-mail: moisiv@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27461
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Myelodysplastic syndromes (MDS) comprise a group (continuum) of clonal hematopoietic diseases associated with high risk of transformation into acute myeloid leukemia (AML) and unfavorable prognosis. Compared to the other hematologic malignant diseases, there was only a modest improvement in survival of MDS patients over the last years. Allogeneic stem cell transplantation remains the only curative option for these patients, however, most of them are not candidates for transplantation. This review focuses on the long-term outcomes of existing therapies and novel agents that are currently tested at different stages of clinical trials. These include inhibitors of TGFβ, various kinase inhibitors, and immune checkpoint inhibitors. Administration of new therapies in the patients with different pathogenetic MDS variants is discussed.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Myelodysplastic syndrome, treatment, checkpoint inhibitors, luspatercept, glasdegib, venetoclax, IDH inhibitors.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Myelodysplastic syndromes (MDS) comprise a group (continuum) of clonal hematopoietic diseases associated with high risk of transformation into acute myeloid leukemia (AML) and unfavorable prognosis. Compared to the other hematologic malignant diseases, there was only a modest improvement in survival of MDS patients over the last years. Allogeneic stem cell transplantation remains the only curative option for these patients, however, most of them are not candidates for transplantation. This review focuses on the long-term outcomes of existing therapies and novel agents that are currently tested at different stages of clinical trials. These include inhibitors of TGFβ, various kinase inhibitors, and immune checkpoint inhibitors. Administration of new therapies in the patients with different pathogenetic MDS variants is discussed.
Keywords
Myelodysplastic syndrome, treatment, checkpoint inhibitors, luspatercept, glasdegib, venetoclax, IDH inhibitors.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27458
[VALUE] => New therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => New therapeutic options in myelodysplastic syndrome: literature review and single-center treatment results
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27526
[VALUE] => 2416
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2416
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27527
[VALUE] => 2417
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2417
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Elena V. Morozova, Nikolai Yu. Tsvetkov, Irina O. Turtanova, Ivan S. Moiseev
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Ivan S. Moiseev, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L. Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7 (921) 796 1951
E-mail: moisiv@mail.ru
Myelodysplastic syndromes (MDS) comprise a group (continuum) of clonal hematopoietic diseases associated with high risk of transformation into acute myeloid leukemia (AML) and unfavorable prognosis. Compared to the other hematologic malignant diseases, there was only a modest improvement in survival of MDS patients over the last years. Allogeneic stem cell transplantation remains the only curative option for these patients, however, most of them are not candidates for transplantation. This review focuses on the long-term outcomes of existing therapies and novel agents that are currently tested at different stages of clinical trials. These include inhibitors of TGFβ, various kinase inhibitors, and immune checkpoint inhibitors. Administration of new therapies in the patients with different pathogenetic MDS variants is discussed.
Keywords
Myelodysplastic syndrome, treatment, checkpoint inhibitors, luspatercept, glasdegib, venetoclax, IDH inhibitors.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27462
[VALUE] => 03/08/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/08/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27463
[VALUE] => 04/09/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 04/09/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27464
[VALUE] => Array
(
[TEXT] => <p>Олеся Г. Смыкова, Кирилл В. Лепик, Наталья Б. Михайлова, Елена В. Кондакова, Евгения С. Борзенкова,
Елена Е. Лепик, Юрий Р. Залялов, Лилия В. Стельмах, Вадим В. Байков, Иван С. Моисеев, Александр Д. Кулагин, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Олеся Г. Смыкова, Кирилл В. Лепик, Наталья Б. Михайлова, Елена В. Кондакова, Евгения С. Борзенкова,
Елена Е. Лепик, Юрий Р. Залялов, Лилия В. Стельмах, Вадим В. Байков, Иван С. Моисеев, Александр Д. Кулагин, Борис В. Афанасьев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27465
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27466
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Терапия рецидивирующей/рефрактерной лимфомы серой зоны (р/р ЛСЗ) остается сложной задачей. Генетические аберрации с участием 9p24.1 и связанные с активацией лиганда запрограммированной клеточной гибели (PD-L1/L2) важны в патогенезе ЛСЗ и уклонении опухолевых клеток от иммунитета. Применение ингибитора иммунных контрольных точек – ниволумаба (антитело, блокирующее PD-1) может быть привлекательной стратегией в терапии ЛСЗ. Мы ретроспективно оценили эффективность и токсичность схем на основе ниволумаба у девяти пациентов с р/р ЛСЗ. Большинство пациентов n=7 (78%) имели первичную химиорезистентность, медиана предшествующих линий терапии составила 3 линии (диапазон от 2 до 5 линий). На момент начала терапии ниволумабом III-IV стадия заболевания была у n=6 (67%) пациентов, а объемное образование у n=3 (33%) пациентов. У всех девяти пациентов был высокий уровень экспрессии PD-L1 (80%-100%) на опухолевых клетках. В этой группе n=4 (44%) пациента получили ниволумаб в монотерапии, n=3 (33%) – ниволумаб в комбинации с химиотерапией, n=1 (11%) – ниволумаб в комбинации с брентуксимабом ведотином и n=1 (11%) – ниволумаб в сочетании с леналидомидом. Частота объективного ответа среди всех пациентов составила 89%, у 6 (67%) пациентов был достигнут полный ответ и 2-х (22%) пациентов – частичный ответ. У одного пациента (11%) стабилизация заболевания была лучшим ответом. Медиана продолжительности достигнутого ответа составила 14 месяцев (диапазон 5-26 месяцев). Медиана времени наблюдения составила 25 месяцев (от 6 до 30 месяцев) от начала терапии ниволумабом. Общая выживаемость и выживаемость без прогрессирования заболевания составили 83% и 38%, соответственно. Эта серия клинических случаев продемонстрировала, что схемы на основе ниволумаба могут быть эффективным вариантом терапии пациентов с р/р ЛСЗ.
Ключевые слова
Лимфома «серой зоны», рецидив, ингибиторы иммунных точек, ниволумаб, трансплантация гемопоэтических стволовых клеток, аллогенная, аутологичная.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27467
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-37-43
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-37-43
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27469
[VALUE] => Array
(
[TEXT] => <p>Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, Boris V. Afanasyev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27470
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia<br>
E-mail: olesya.gen@gmail.com</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia
E-mail: olesya.gen@gmail.com
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27471
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">The treatment of relapsed/refractory gray zone lymphoma (r/r GZL) remains challenging. Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion. Immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL. We have retrospectively assessed efficacy and toxicity of nivolumab-based regimens in nine patients with r/r GZL. Most of the patients n=7 (78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment disease stage III-IV was in n=6 (67%) patients and bulky disease was in n=3 (33%) patients. All nine patients had high-level of PD-L1 expression (80%-100%) on tumor cells. In this group n=4 (44%) patients received nivolumab as monotherapy, n=3 (33%) received nivolumab in combination with chemotherapy, n=1 (11%) received nivolumab in combination with BV and n=1 (11%) received nivolumab in combination with lenalidomide. The objective response rate among all treated patients was 89% with 6 cases (67%) of complete response and 2 (22%), with partial response. One patient (11%) had stabilization of the disease as best response. Median duration of response was 14 (range 5-26) months. Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Overall survival and progression free survival rates were 83% and 38%, respectively. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Gray-zone lymphoma, relapse, immune checkpoints inhibitors, nivolumab, hematopoietic stem cell transplantation, allogeneic, autologous.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => The treatment of relapsed/refractory gray zone lymphoma (r/r GZL) remains challenging. Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion. Immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL. We have retrospectively assessed efficacy and toxicity of nivolumab-based regimens in nine patients with r/r GZL. Most of the patients n=7 (78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment disease stage III-IV was in n=6 (67%) patients and bulky disease was in n=3 (33%) patients. All nine patients had high-level of PD-L1 expression (80%-100%) on tumor cells. In this group n=4 (44%) patients received nivolumab as monotherapy, n=3 (33%) received nivolumab in combination with chemotherapy, n=1 (11%) received nivolumab in combination with BV and n=1 (11%) received nivolumab in combination with lenalidomide. The objective response rate among all treated patients was 89% with 6 cases (67%) of complete response and 2 (22%), with partial response. One patient (11%) had stabilization of the disease as best response. Median duration of response was 14 (range 5-26) months. Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Overall survival and progression free survival rates were 83% and 38%, respectively. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL.
Keywords
Gray-zone lymphoma, relapse, immune checkpoints inhibitors, nivolumab, hematopoietic stem cell transplantation, allogeneic, autologous.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27468
[VALUE] => Nivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Nivolumab-based immunotherapy in relapsed/refractory B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27528
[VALUE] => 2418
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2418
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27529
[VALUE] => 2419
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2419
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Olesya G. Smykova, Kirill V. Lepik, Natalia B. Mikhailova, Elena V. Kondakova, Evgenia S. Borzenkova, Elena E. Lepik, Yuri R. Zalyalov, Lilia V. Stelmakh, Vadim V. Baykov, Ivan S. Moiseev, Alexander D. Kulagin, Boris V. Afanasyev
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Olesya G. Smykova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, Roentgen St. 12, 197022, St. Petersburg, Russia
E-mail: olesya.gen@gmail.com
The treatment of relapsed/refractory gray zone lymphoma (r/r GZL) remains challenging. Genetic aberrations involving 9p24.1 and associated with programmed death ligand (PD-L1/L2) upregulation are important in GZL pathogenesis and immune evasion. Immune checkpoint inhibitor nivolumab (PD-1-blocking antibody) may be an attractive treatment strategy in GZL. We have retrospectively assessed efficacy and toxicity of nivolumab-based regimens in nine patients with r/r GZL. Most of the patients n=7 (78%) had primary chemoresistance and the median number of prior therapy lines was 3 (range, 2-5). At the start of nivolumab treatment disease stage III-IV was in n=6 (67%) patients and bulky disease was in n=3 (33%) patients. All nine patients had high-level of PD-L1 expression (80%-100%) on tumor cells. In this group n=4 (44%) patients received nivolumab as monotherapy, n=3 (33%) received nivolumab in combination with chemotherapy, n=1 (11%) received nivolumab in combination with BV and n=1 (11%) received nivolumab in combination with lenalidomide. The objective response rate among all treated patients was 89% with 6 cases (67%) of complete response and 2 (22%), with partial response. One patient (11%) had stabilization of the disease as best response. Median duration of response was 14 (range 5-26) months. Median follow‐up time was 25 months (range, 6-30) from the start of nivolumab-based treatment. Overall survival and progression free survival rates were 83% and 38%, respectively. This case series demonstrated that nivolumab-based regimen may be an effective treatment option for patients with r/r GZL.
Keywords
Gray-zone lymphoma, relapse, immune checkpoints inhibitors, nivolumab, hematopoietic stem cell transplantation, allogeneic, autologous.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27512
[VALUE] => 03/10/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/10/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27513
[VALUE] => 04/09/2021 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 04/09/2021 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27514
[VALUE] => Array
(
[TEXT] => <p>Наталья Н. Сударева<sup>1,2</sup>, Ольга М. Суворова<sup>1</sup>, Наталья Н. Сапрыкина<sup>1</sup>, Владимир В. Томсон<sup>2</sup>, Дмитрий Н. Суслов<sup>3</sup>, Олег В. Галибин<sup>2</sup>, Александр Д. Вилесов<sup>1,2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Наталья Н. Сударева1,2, Ольга М. Суворова1, Наталья Н. Сапрыкина1, Владимир В. Томсон2, Дмитрий Н. Суслов3, Олег В. Галибин2, Александр Д. Вилесов1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27515
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт- Петербург, Россия<br>
<sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>3</sup> Российский научный центр радиологии и хирургических технологий им. А. М. Гранова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Институт высокомолекулярных соединений РАН, Санкт- Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Российский научный центр радиологии и хирургических технологий им. А. М. Гранова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27516
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Регионарное введение химиотерапевтических препаратов в область опухоли может во многих случаях повысить эффективность лечения злокачественных опухолей. После введения в организм, лечебный препарат или система доставки препарата могут подвергаться воздействию внутритканевых жидкостей, которые отличаются от плазмы крови тем, что они содержат меньше белков. В настоящей работе мы изучали влияние плазмы крови человека на структуру и свойства гибридной системы доставки, основанной на кальций-карбонатных ватеритах (СаСО<sub>3</sub>), покрытых полиэлектролитом декстран-сульфатом (DexS). Эти системы доставки содержали противораковый препарат доксорубицин.</p>
<p style="text-align: justify;">Было показано, что ватериты, модифицированные DexS, обеспечивали пролонгированный выход доксорубицина в плазму крови. Сканирующие микрофотографии выявили структурные изменения гибридных систем доставки, возникающие при контакте с плазмой и коррелирующие с профилем высвобождения доксорубицина. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Доксорубицин, система доставки препаратов, СаСО<sub>3</sub>, декстран сульфат, плазма крови.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Регионарное введение химиотерапевтических препаратов в область опухоли может во многих случаях повысить эффективность лечения злокачественных опухолей. После введения в организм, лечебный препарат или система доставки препарата могут подвергаться воздействию внутритканевых жидкостей, которые отличаются от плазмы крови тем, что они содержат меньше белков. В настоящей работе мы изучали влияние плазмы крови человека на структуру и свойства гибридной системы доставки, основанной на кальций-карбонатных ватеритах (СаСО3), покрытых полиэлектролитом декстран-сульфатом (DexS). Эти системы доставки содержали противораковый препарат доксорубицин.
Было показано, что ватериты, модифицированные DexS, обеспечивали пролонгированный выход доксорубицина в плазму крови. Сканирующие микрофотографии выявили структурные изменения гибридных систем доставки, возникающие при контакте с плазмой и коррелирующие с профилем высвобождения доксорубицина.
Ключевые слова
Доксорубицин, система доставки препаратов, СаСО3, декстран сульфат, плазма крови.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27517
[VALUE] => 10.18620/ctt-1866-8836-2021-10-1-79-85
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2021-10-1-79-85
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27519
[VALUE] => Array
(
[TEXT] => <p>Natalia N. Sudareva<sup>1,2</sup>, Olga М. Suvorova<sup>1</sup>, Natalia N. Saprykina<sup>1</sup>, Vladimir V. Tomson<sup>2</sup>, Dmitry N. Suslov<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Natalia N. Sudareva1,2, Olga М. Suvorova1, Natalia N. Saprykina1, Vladimir V. Tomson2, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27520
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br>
<sup>2</sup> Pavlov University, St. Petersburg, Russia<br>
<sup>3</sup> A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Natalya N. Sudareva, Institute of Macromolecular
Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004,
St. Petersburg, Russia<br>
E-mail: nnsas@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence
Dr. Natalya N. Sudareva, Institute of Macromolecular
Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004,
St. Petersburg, Russia
E-mail: nnsas@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27521
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">In a number of cases, efficiency of cancer therapy may be enhanced by local administration of chemotherapeutic drugs into the tumor area. After regional injection, a drug, or appropriate delivery system is exposed to the interstitial fluid, which differs from blood plasma, mainly, in lesser protein amounts. In the present work, we studied the influence of human blood plasma upon structure and properties of hybrid drug delivery systems based on calcium carbonate (СаСО<sub>3</sub>) vaterites coated with polyelectrolyte dextran sulfate (DexS). These delivery systems included anti-cancer drug doxorubicin (DOX).</p>
<p style="text-align: justify;">It has been shown that DexS-modified vaterites provided the prolonged release of DOX to blood plasma. SEM microphotographs revealed structural changes in hybrid delivery systems occurring in plasma and correlating with the DOX release profiles. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Doxorubicin, drug delivery system, СаСО<sub>3</sub>, dextran sulfate, blood plasma.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => In a number of cases, efficiency of cancer therapy may be enhanced by local administration of chemotherapeutic drugs into the tumor area. After regional injection, a drug, or appropriate delivery system is exposed to the interstitial fluid, which differs from blood plasma, mainly, in lesser protein amounts. In the present work, we studied the influence of human blood plasma upon structure and properties of hybrid drug delivery systems based on calcium carbonate (СаСО3) vaterites coated with polyelectrolyte dextran sulfate (DexS). These delivery systems included anti-cancer drug doxorubicin (DOX).
It has been shown that DexS-modified vaterites provided the prolonged release of DOX to blood plasma. SEM microphotographs revealed structural changes in hybrid delivery systems occurring in plasma and correlating with the DOX release profiles.
Keywords
Doxorubicin, drug delivery system, СаСО3, dextran sulfate, blood plasma.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27518
[VALUE] => Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Morphology of hybrid doxorubicin delivery systems (dextran sulfate-coated CaCO3 vaterites) in human blood plasma
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27538
[VALUE] => 2430
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2430
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27539
[VALUE] => 2431
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2431
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Natalia N. Sudareva1,2, Olga М. Suvorova1, Natalia N. Saprykina1, Vladimir V. Tomson2, Dmitry N. Suslov3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 A. M. Granov Russian Research Center of Radiology and Surgical Technologies, St. Petersburg, Russia
Correspondence
Dr. Natalya N. Sudareva, Institute of Macromolecular
Compounds, Bolshoy Ave 31, Vassilievsky Island, 199004,
St. Petersburg, Russia
E-mail: nnsas@mail.ru
In a number of cases, efficiency of cancer therapy may be enhanced by local administration of chemotherapeutic drugs into the tumor area. After regional injection, a drug, or appropriate delivery system is exposed to the interstitial fluid, which differs from blood plasma, mainly, in lesser protein amounts. In the present work, we studied the influence of human blood plasma upon structure and properties of hybrid drug delivery systems based on calcium carbonate (СаСО3) vaterites coated with polyelectrolyte dextran sulfate (DexS). These delivery systems included anti-cancer drug doxorubicin (DOX).
It has been shown that DexS-modified vaterites provided the prolonged release of DOX to blood plasma. SEM microphotographs revealed structural changes in hybrid delivery systems occurring in plasma and correlating with the DOX release profiles.
Keywords
Doxorubicin, drug delivery system, СаСО3, dextran sulfate, blood plasma.

