Introduction
Clinical gene therapy is a very ambitious intent. The general principle sounds as easy as it is ingenious. Instead of treating the symptoms of severe genetic diseases such as immunodeficiencies or metabolic disorders, gene therapy intends to cure the underlying genetic defect by introducing a corrected copy of the mutated gene, or even by correcting the affected gene itself. In the context of disorders affecting the hematopoietic system, patient-derived cells can be treated ex vivo with engineered gene vectors designed to deliver the therapeutic gene. Except in those diseases associated with a strong selective advantage of the gene-modified cells, a preparatory “conditioning” by cytoreductive treatment may be required to promote engraftment.
Stable gene transfer, and therefore long-term genetic modification, is achieved by gene vectors, which integrate their genetic material and the therapeutic gene respectively into the chromatin of the target cells. Unlike classical pharmaceutical drug treatment, gene therapy is an approach highly specific to the patient and the disease. Parameters like cell source and origin, vector type, therapeutic dose, route of administration and, in particular, the transgene itself have to be adapted to each specific approach and medicinal purpose. For example, between 1989 and 2009 there were 1537 clinical gene therapy trials approved employing 35 different vector types (predominantly derived from Adenovirus, Retrovirus or naked DNA) in more than 8 different fields of medicine (predominantly cancer) (http://www.wiley.co.uk/genetherapy/clinical/). The consequence of the diversity of the products is a challenge for legal regulation, which should normally be universally valid while giving specific guidance to certain therapies in order to guarantee the safety and efficacy of the individual products.
While a gene therapy approach allows for the desired efficient long-term correction of a genetic defect on the one hand, it also brings up the concern of side effects on the other. The most noted example has been the clinical gene therapy trial for treatment of the rare genetic disorder X-linked severe combined immunodeficiency (X-SCID). While the majority of treated patients benefited from a life-saving and long-term immune reconstitution, the occurrence of lymphoproliferative disease due to insertional mutagenesis in 5 patients to date has gained notoriety [1, 2]. Integration site analysis revealed vector integrations close to cellular proto-oncogenes such as the LMO2 gene, known to be activated by chromosomal translocations in T-lymphoblastic leukemia [3, 4]. These severe adverse events made the theoretical concerns a reality. Additional concerns related to immunogenicity, spread of genetic sequences or toxic and infectious byproducts of vector preparations cause many gene therapy products to be classified as high-risk products that need to be strongly regulated by the authorities. This poses a tremendous challenge, since regulation can only be defined in general terms and an all-in-one document suitable for every purpose and individual need of a product cannot be established. To overcome this dilemma, case-by-case considerations are indicated.
Since researchers who invent the individual product initiate many clinical gene therapy trials, this review targets those investigators planning to enter the clinical development phase and initiating a clinical trial. It will clarify the complex situation and will give an overview of the current regulatory status as well as important points to consider before applying for a clinical trial authorization. This review involves preclinical and clinical issues as well as references to the necessary documents to be prepared.
Viral gene transfer and its associated risks
The regulatory framework should define uniform requirements in order to ensure compliance with quality standards and therefore guarantee the safety and well being of trial participants. In consideration of the major risks accompanied by viral gene transfer, it becomes clear that a thorough and complex regulatory framework is needed to control these biological products. As indicated above, the complexity of the individual product itself accounts for the necessity for evaluation of the risks and benefits of a certain gene therapy application on a case-by-case basis.
Integrating replication-defective vectors based on gamma-retroviruses have been frequently used in initial gene therapy protocols, and their risks will therefore be discussed as an example. Target cells are transduced in most cases ex vivo with the viral vector preparation. Shortly after entry of the retroviral particles, the viral RNA carrying the transgene of choice is reverse-transcribed into dsDNA, and a preintegration complex (PIC) is formed in which the DNA is associated with retroviral and cellular proteins (Figure 1). After translocation to the nucleus, the retroviral DNA including the transgene is stably integrated into the chromosomal DNA by the viral protein integrase.
Figure 1. The retroviral life cycle (reproduced with permission from the Journal Molecular Therapy (Nature Publishing Group); adapted from [5])
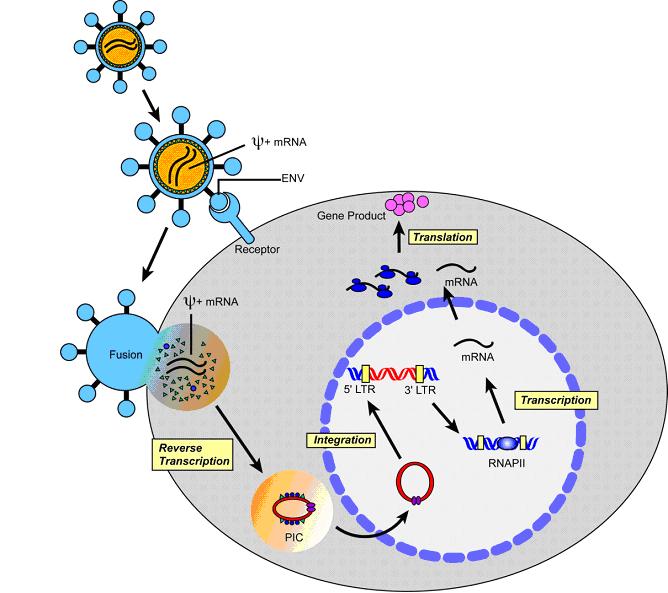
The integration of the transgene makes the desired correction of the genetic defect possible in the first place, not only in the transduced cell but also in its progeny. However, at the same time it forms the basis of one of the major safety concerns of integrating retroviral vectors: insertional mutagenesis. The process of integration is highly efficient but occurs in a semi-random manner with respect to the targeted genetic loci. Although gamma-retroviral integration tends to occur preferentially in open chromatin regions, which is associated with actively transcribed genes [6], it does not favor a specific target sequence. Therefore, insertion of the transgene in a non-predictable unspecific way may lead to activation, inactivation or truncation of cellular genes adjacent to the site of integration. Cellular proto-oncogenes might be placed under control of the viral enhancer/promoter elements resulting in a non-physiological expression of the respective gene with loss of cellular regulation. The tumorigenic risk associated with such an event appears to be highly context dependent. Available evidence suggests that additional mutations are required before cells transform to overt malignancy [7].
The concern of genotoxicity especially affects the first generation of retroviral-based gene transfer vectors in which the transgene is driven by the strong long terminal repeat (LTR) enhancer/promoter elements of the virus. New strategies in vector design with reduced potential for enhancer-mediated interaction with adjacent cellular genes may decrease the risk of genotoxicity, e.g., the use of self-inactivating (SIN) vectors [8], the incorporation of cellular promoters [9], the use of chromatin insulators [10], or the use of lentivirus-based vectors, which have a lower likelihood of integration in promoter-proximal or other regulatory gene regions [11]. In all circumstances, the genotoxic potential of a given vector designed for clinical use needs to be preclinically assessed and evaluated.
Another concern regarding the risk of oncogenesis is the formation of replication-competent retrovirus (RCR). Although retroviral vectors are replication-deficient due to the lack of the sequences encoding for the structural and enzymatic viral proteins, there is still the possibility of formation of RCR by recombination of the vector sequence with the so-called “helper” plasmids or with other, endogenous retroviral genetic information present in the producer or target cells [12]. As a consequence, unintended infection of target or even off-target cells might occur. This might introduce harmful viral pathogens into treated patients.
In order to assess the risk of RCR formation, investigators should evaluate the presence of homologous sequences suitable for recombination in the vector constructs, as well as the presence of endogenous viruses in the packaging cells. If possible, such sequences should be avoided or at least limited. Nevertheless, strict testing for the presence of RCR in retroviral vector batches intended for clinical application is required.
Another major concern of viral gene transfer is vertical germ line transmission. In general, gene therapy trials with the aim of direct germ line manipulation are prohibited [28]. Nevertheless, also in somatic gene therapy the concern of inadvertent germ line integration exists and gained new attention when semen of clinical trial participants for treatment of hemophilia tested positive for vector sequences [13, 14]. Although this does not necessarily imply that vector sequences were present in germ cells, this observation underlines the need for diligent biodistribution studies. The risk of germline transmission is in particular dependent on the biodistribution pattern of a given vector. In this regard, the route of administration, vector type and dose, and the innate and adapted immunity of the patient play pivotal roles. A replication deficient integrating vector used for ex vivo transduction of the target cell might have a much lower risk than the same vector applied systemically in an in vivo application. At the same time, a gamma-retroviral vector which can only transduce dividing cells might have a lower risk in transducing mature sperm cells than a lentiviral vector, which has the ability to also infect non-dividing cells. Still, spermatogonial stem cells that have a high proliferation activity might be accessible for gamma-retroviral vectors. Therefore, regulatory agencies have developed guidelines describing how to address the risk of inadvertent germ line transmission in preclinical studies (see below and Table 1).
Table 1. Applicable guidelines addressing GTMPs

Regulatory framework for Gene Therapy Medicinal Products
Currently, the regulatory situation for Gene Therapy Medicinal Products (GTMPs) is evolving rapidly on the European level. The new core regulation (EC) No 1394/2007 on Advanced Therapy Medicinal Products (ATMPs), which became effective in December 2008 (from here on referred to as ‘ATMP regulation’), lays the foundation for a harmonized regulatory situation applicable for all member states in the European Communion. According to Article 2 of this regulation, GTMPs together with Somatic Cell Therapy Medicinal Products and Tissue Engineered Products are classified as ATMPs (Figure 2). Until incorporation of this regulation, these products fell in a regulatory gap somewhere in between legislation 93/42/EEC on Medical Devices and Directive 2001/83/EC on Medicinal Products. The new ‘ATMP regulation’ fills this gap and "lays down specific rules concerning the authorization, supervision and pharmacovigilance of Advanced Therapy Medicinal Products” [50, Article 1].
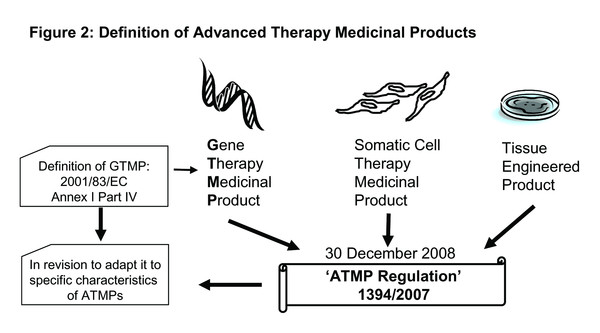
For the definition of a GTMP itself, the regulation refers to Annex I Part IV of Directive 2001/83/EC, as amended by Directive 2003/63/EC, which states: “For the purposes of this Annex, Gene Therapy Medicinal Product shall mean a product obtained through a set of manufacturing processes aimed at the transfer, to be performed either in vivo or ex vivo, of a prophylactic, diagnostic or therapeutic gene (i.e. a piece of nucleic acid), to human/animal cells and its subsequent expression in vivo.” Within the implementation of the ‘ATMP regulation’, this annex is currently under revision in order to adjust it to the specific characteristics of ATMPs (Figure 2). There is no draft version currently available, but the outcome of the public consultation paper as well as single contributions can be found on the webpage of the European Commission.
On the national German level, GTMPs are similarly defined as Medicinal Products in the Medicinal Products Act (“AMG”), Section 4 No. 9, [48]. Within the 15th amendment of the Medicinal Products Act, the new ATMP regulation will be implemented into national German law. Though a regulation, the translation into national law is necessary because the ‘ATMP regulation’ amends Directive 2001/83/EC.
GTMPs are often very complex products that may contain other components that are regulated by additional legislation. The GTMP may for instance contain human cells or tissues. Regarding the quality and safety for the donation, procurement and testing of these cells or tissues, Directive 2004/23/EC as implemented by Directive 2006/17/EC applies (Figure 3A). Directive 2004/23/EC is already transposed into national law by the German Tissue Act (“Gewebegesetz”). The processing, preservation, storage and distribution of these cells and tissues, however, fall under Section 14 of the ‘ATMP regulation’. When the GTMP also contains human blood or blood components, Directive 2002/98/EC as implemented by Directives 2004/33/EC, 2005/61/EC and 2005/62/EC applies (Figure 3B). On the German level, these aspects are addressed in the Medicinal Products Act and Transfusion Law.
In addition, the GTMP may be a combination product consistent of a Medicinal Product and Medical Device. This would, for instance, be the case if the gene-modified cells are applied to the patient via a specific biodelivery implant. Whether the regulatory rules and standards of Medicinal Products or of Medical Devices apply to combination products generally depends on their mode of action. Nevertheless, if ATMPs (and therefore GTMPs) are incorporated into a combination product, the ‘ATMP regulation’ applies regardless of the function of the Medical Device [50, Section 4]. However, the latter have to furthermore fulfill the quality and safety requirements of Directive 93/42/EEC (in case of a Medical Device) and accordingly Directive 90/385/EEC (in case of an active implantable Medical Device) as amended by Directive 2007/47/EC (Figure 3C). On the German level, both directives are implemented by the German Act on Medical Devices (“MPG”).
For clinical trials involving GTMPs, the overall requirements and ethical standards of the Clinical Trial Directive 2001/20/EC apply as well as for all other medicinal products (Figure 3D). In Germany, this directive is translated into national law by the GCP Ordinance (“GCP-Verordnung”). Furthermore, the German Medicinal Products Act applies, in particular concerning clinical trials in Chapter 6, together with the general considerations for clinical trials laid down in ICH E8 Step 5 [27]. Within the scope of the implementation of the ‘ATMP regulation’, the standards of good clinical practices shall be expanded to the specific needs of ATMPs [50, Article 4]. The adaption process is currently proceeding. A public consultation paper is already published on the European Commission webpage. The public consultation process has been closed so that a draft document on good clinical practice specific for ATMPs is expected to be published soon.
In Germany, the competent authority concerning clinical trial authorization related to GTMPs is the Paul-Ehrlich-Institute (PEI). General principles for requesting the authorization of a clinical trial in Germany are laid down in the third Notification of the joint announcement from PEI and the Federal Institute for Drugs and Medical Devices (BfArM) (third Notification).
In Germany there are some particularities for GTMPs compared to conventional medicinal products. While for example the ethics committee has to give an opinion within 60 days after a clinical trial application (multicentric trial) for conventional medicinal products, the time period extends up to 180 days if GTMPs are concerned [44, Section 8(4)]. Furthermore, the competent authority has to provide a written approval (explicit authorization) for clinical trials concerning GTMPs within 90 days after complete clinical trial application. This period may be extended to 180 days if the authority consults experts or professional opinions for decision-making [44, Section 9(4)].
The manufacture of GTMPs needs to be consistent with the requirements of Directive 2003/94/EC on Good Manufacturing Practice (“GMP“) (Figure 3E). Due to the complexity of ATMPs (and GTMPs) and the extensive manufacturing processes, the ‘ATMP regulation’ implicates an adaption of the guidelines for GMP to the specific situation of ATMPs [50, Article 5].
Figure 3. Regulatory framework for Gene Therapy Medicinal Products

Currently, a draft version of the adapted Eudralex Volume 4 Annex 2 on the manufacture of biological medicinal products is published. Furthermore, the European Pharmacopoeia serves as a legally binding framework for quality standards of medicinal products in Europe [43]. The General Chapter 5.14, which deals with GTMPs, gives instructions for the testing of batches of recombinant vectors and gene-modified cells. In Germany, good manufacturing practice is regulated in the ordinance for the manufacture of medicinal products and active pharmaceutical ingredients (AMWHV).
There are in addition various guidelines published addressing manufacturing and quality aspects during the development of ATMPs (Table 1). One is the multidisciplinary “Guideline on human Cell-Based Medicinal Products” of the European Medicines Agency (EMEA) [35], which gives advice for the “development, manufacturing and quality control as well as non-clinical and clinical development of Cell-Based Medicinal Products” which also include GTMPs. The guideline is aimed at products already in the phase of marketing authorization but the general considerations also apply for clinical trials. The EMEA “Note for guidance on the quality, preclinical and clinical aspects of Gene Transfer Medicinal Products” [25] gives recommendations for producing data aiming at an application for marketing authorization. Affiliated is the “Concept paper on the development of a guideline on the quality, preclinical and clinical aspects of medicinal products containing genetically modified cells” by EMEA [40]. If the GTMP was generated with the help of lentiviral vectors, the EMEA “Guideline on development and manufacture of lentiviral vectors” gives advice regarding quality and safety of the vectors [24].
Importantly, the specific safety aspects related to GTMPs have to be considered and analyzed before the products can be used in the clinic. The EMEA “Guideline on the non-clinical studies required before first clinical use of Gene Therapy Medicinal Products” [38] specifies which studies are essential prior to the first application to humans. This includes but is not limited to non-clinical proof of concept studies, biodistribution studies, as well as studies on dose finding, germ line transmission, immunotoxicity and studies addressing the environmental risks/shedding. Specific recommendations for the later are defined in the EMEA “Guideline on scientific requirements for the environmental risk assessment of Gene Therapy Medicinal Products” [39]. This guideline states: "Generally the purpose of clinical trials are to study the adsorption, distribution, metabolism and excretion of one or more Investigational Medicinal Products with the object of ascertaining its (their) safety and/or efficacy ([28]; definition of a clinical trial). As such the evaluation of vector shedding is a requirement for a phase I study.” Data of clinical trials regarding environmental risks need to be collected and integrated in a full environmental risk assessment (ERA) needed for a marketing authorization procedure. Other guidelines dealing with environmental risk assessments of genetically modified organisms are EMEA/BWP/473191/06-corr and EMEA/CHMP/BWP/135148/04.
Furthermore, in terms of studies addressing the risk and ethical concerns of inadvertent germline transmission, separate guidelines are available. The EMEA “Guideline on non-clinical testing for inadvertent germline transmission of gene transfer vectors” [34] recommends adjusted study designs depending on the type of vector used, as well as providing a decision tree regarding the necessary questions to be addressed by biodistribution and germ line transmission studies. Also a considerations paper of the International Conference On Harmonization Of Technical Requirements For Registration Of Pharmaceuticals For Human Use (ICH) is available on this topic and can be consulted in this regard (ICH considerations, general principles to address the risk of inadvertent germline integration of gene therapy vectors). The ICH S2B document “Note for guidance on genotoxicity” [26], however, gives recommendations for studies to address the risk of genotoxicity and can give advice in these questions.
If patients have been treated with a gene therapy approach in a clinical trial, clinical monitoring as well as intensive follow-up care should be performed in order to detect adverse events at an early stage to avoid clinical implications and to collect safety data. The “Guideline on follow-up of patients administered with Gene Therapy Medicinal Products” [41] gives recommendations in this regard.
Regulation (EC) No 726/2004 already regulates the marketing authorization procedure of biotechnology medicinal products on the European level. In this context, the products pass through a centralized authorization procedure at the EMEA in order to assess their quality, safety and efficacy. The technical requirements needed to prove the latter are defined in Annex I to Directive 2001/83/EC.
Nevertheless, the complexity and variety of ATMPs may result in a situation of very specific and individual questions to be addressed. Within the scope of the ‘ATMP regulation’, the Committee for Advanced Therapies (CAT) will be established within the EMEA in order to provide strong expertise in this field and exhibit draft opinions on ATMP applications presented to EMEA [50, Chapter 7]. Therefore, Regulation (EC) No 726/2004 needs to be amended. Currently, the CAT is in the process of establishment within the EMEA. Updated details can be found on the respective webpage of EMEA. Within the context of a marketing authorization, the standards for pharmacovigilance described in Regulation 726/2004 apply. Since clinical trials are typically powered for efficacy and furthermore the duration of the respective trials might not allow the detection of long term adverse events, the application has to provide a plan to ensure the follow-up of adverse reactions after marketing authorization. A specific EMEA guideline giving further recommendations on “Safety and Efficacy Follow-Up – Risk management of Advanced Therapy Medicinal Products” is already published [33]. Another measure of safety is the full traceability of the product (including the starting materials) and the treated patient. According to the ‘ATMP regulation’, the marketing authorization holder for an ATMP is responsible for setting up a traceability system [50, Article 15]. If human tissues or cells are involved, the traceability standards defined in Directive 2004/23/EC also apply, as do the requirements of Directive 2002/98/EC concerning human blood and blood components, respectively.
Finally, it should be noted that both the European agency EMEA and the German agency PEI (in charge of ATMPs) provide scientific advice. Investigators are welcome to consult the agencies in all phases of project development in order to obtain important project-specific advice, e.g., before applying for a clinical trial or marketing authorization. Details can be found on the webpages of EMEA or PEI, respectively.
Outlook
Application of new technologies of gene therapy to the treatment of diseases will yield the greatest benefits if approached on a sound scientific and medical basis. Regulatory guidelines are the basis of (i) current scientific knowledge, (ii) scientific expertise in a given research area, and (iii) the result of a continuous interaction between researchers and regulatory experts. Gene therapy has entered the stage of clinical evaluation. As always at the frontier of bench to bedside research, patient safety must be the first and foremost consideration in human gene therapy before one enters the stage of extensive clinical trials to assess efficacy.
References
Index of scientific references
1. Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132-3142.
2. Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143-50.
3. Boehm T, Foroni L, Kaneko Y, et al. The rhombotin family of cysteine-rich LIM-domain oncogenes: Distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc. Natl. Acad. Sci. 1991;88:4367–4371.
4. Royer-Pokora B, Loos U, and Ludwig WD. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukemia with the t(11;14)(p13;q11). Oncogene. 1991;6:1887–1893.
5. Baum C, Schambach A, Bohne J, and Galla M. Retrovirus Vectors: Toward the Plentivirus? Mol Ther. 2006;13:1050-1063.
6. Wu X, Li Y, Crise B, and Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300(5626):1749-1751.
7. Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758-764.
8. Modlich U, Bohne J, Schmidt M, et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545-2553.
9. Zychlinski D, Schambach A, Modlich U, et al. Physiological Promoters Reduce the Genotoxic Risk of Integrating Gene Vectors. Mol Ther. 2008;16(4):718-725.
10. Emery DW, Yannaki E, Tubb J, et al. Development of virus vectors for gene therapy of beta chain hemoglobinopathies: flanking with a chromatin insulator reduces gamma-globin gene silencing in vivo. Blood. 2002;100:2012-2019.
11. De Palma M, Montini E, Santoni de Sio FR, et al. Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells. Blood.2005;105(6):2307-15.
12. Chong H, Starkey W, and Vile RG. A replication-competent retrovirus arising from a split-function packaging cell line was generated by recombination events between the vector, one of the packaging constructs, and endogenous retroviral sequences. J Virol.1998;72:2663–2670.
13. Marshall E. Gene therapy. Viral vectors still pack surprises. Science. 2001;294(5547):1640.
14. Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342-7.
Index of regulatory references
15. 3. Notification (“3. Bekanntmachung”) of the Federal Institute for Drugs and Medical Devices and the Paul Ehrlich Institute of 10 August 2006 on the clinical trial of medicinal products for human use.
16. Commission Directive 2003/63/EC of 25 June 2003 amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use. OJ L 159, 27.06.2006, p.46.
17. Commission Directive 2003/94/EC of 8 October 2003 laying down the principles and guidelines of good manufacturing practice in respect of medicinal products for human use and investigational medicinal products for human use.OJ L 262, 14.10.2003, p.22.
18. Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards certain technical requirements for blood and blood components. OJ L 91, 30.03.2004, p.25.
19. Commission Directive 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards traceability requirements and notification of serious adverse reactions and events. OJ L 256, 01.10.2005, p.32.
20. Commission Directive 2005/62/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards Community standards and specifications relating to a quality system for blood establishments. OJ L 256, 01.10.2005, p.41.
21. Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. OJ L 38, 09.02.2006, p.40.
22. Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices. OJ L 189, 20.07.1990, p.17
23. Council Directive 93/42/EEC of 14 June 1993 concerning medical devices. OJ L 169, 12.07.1993, p.1.
24. CHMP/BWP/2458/03 of the Committee for Medicinal Products for Human Use (CHMP) of 26 May 2005. Guideline on development and manufacture of lentiviral vectors.
25. CPMP/BWP/3088/99 of the Committee for Proprietary Medicinal Products (CPMP) of 24 April 2001. Note for Guidance on the quality, preclinical and clinical aspects of gene transfer medicinal products.
26. CPMP/ICH/174/95 ICH Topic S 2 B of March 1998. Genotoxicity: A standard battery for genotoxicity testing of pharmaceuticals, Step 5. Note for guidance on genotoxicity: A standard battery for genotoxicity testing of pharmaceuticals.
27. CPMP/ICH/291/95 ICH Topic E 8 of March 1998. General considerations for clinical trials, step 5. Note for guidance on general considerations for clinical trials.
28. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. OJ L 121, 1.5.2001, p.34.
29. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. OJ L 311, 28.11.2001, p.67.
30. Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood componentsand amending Directive 2001/83/EC. OJ L 33, 08.02.2003, p.30.
31. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. OJ L 102, 07.04.2004, p.48.
32. Directive 2007/47/EC of the European Parliament and of the Council of 5 September 2007 amending Council Directive 90/385/EEC on the approximation of the laws of the Member States relating to active implantable medical devices, Council Directive 93/42/EEC concerning medical devices and Directive 98/8/EC concerning the placing of biocidal products on the market.
33. EMEA/149995/08 of the Committee for Medicinal Products for Human Use (CHMP) of 20 November 2008. Guideline on safety and efficacy follow-up – risk management of advanced therapy medicinal products.
34. EMEA/273974/05 of the Committee for Medicinal Products for Human Use (CHMP) of 16 November 2006. Guideline on non-clinical testing for inadvertent germline transmission of gene transfer vectors.
35. EMEA/CHMP/410869/06 of the Committee for Medicinal Products for Human Use (CHMP) of 21 May 2008. Guideline on human cell-based medicinal products.
36. EMEA/CHMP/BWP/135148/04 of the Committee for Medicinal Products for Human Use (CHMP) of 20 January 2005. Environmental risk assessments for medicinal products containing, or consisting of, genetically modified organisms (GMOs) (Module 1.6.2).
37. EMEA/CHMP/BWP/473191/06 - Corr of the Committee for Medicinal Products for Human Use (CHMP) of 11 December 2006. Guideline on environmental risk assessments for medicinal products consisting of, or containing, genetically modified organisms (GMOs).
38. EMEA/CHMP/GTWP/125459/06 of the Committee for Medicinal Products for Human Use (CHMP) of 30 May 2008. Guideline on the non-clinical studies required before first clinical use of gene therapy medicinal products.
39. EMEA/CHMP/GTWP/125491/06 of the Committee for Medicinal Products for Human Use (CHMP) of 30 May 2008. Guideline on scientific requirements for the environmental risk assessment of gene therapy medicinal products.
40. EMEA/CHMP/GTWP/405681/06 of the Committee for Medicinal Products for Human Use (CHMP) of 24 May 2007. Concept paper on the development of a guideline on the quality, preclinical and clinical aspects of medicinal products containing genetically modified cells.
41. EMEA/CHMP/GTWP/60436/07 of the Committee for Medicinal Products for Human Use (CHMP) of 30 May 2008. Draft guideline on follow-up of patients administered with gene therapy medicinal products.
42. Eudralex Volume 4. Good manufacturing practice guidelines. Volume 4 of “The rules governing medicinal products in the European Union”. http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol4_en.htm
43. European Pharacopoeia. http://www.pheur.org
44. GCP Ordinance (“GCP-Verordnung“). Of the German Federal Ministry for Health and Social Security of 09 August 2004 for good clinical practice in the conduct of clinical trials on medicinal products for human use. Federal Law Gazette I, 12.08.2004, p.2081.
45. German Act on Medical Devices (“Medizinproduktegesetz”) of the Federal Republic of Germany of 02.08.1994 in the version of the notification of the Law of 07.08. 2002. Federal Law Gazette I p.3146.
46. German Tissue Act (“Gewebegesetz“) of the German Federal Parliament of 20 July 2007 on the quality and safety of human tissues and cells. Federal Law Gazette I, 27.07.2007, p.1574.
47. ICH Considerations of the International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use of 25 October 2006. General principles to address the risk of inadvertent germline integration of gene therapy vectors.
48. Medicinal Products Act (“Arzneimittelgesetz”) of the Federal Republic of Germany of 24.08.1976 in the version of the notification of the Law of 12th December 2005. Federal Law Gazette I p.3394.
49. Ordinance for the manufacture of medicinal products and active pharmaceutical ingredients (“AMWHV”) of the German Federal Ministries for Health and Food, Agriculture and Consumer Protection of 03 November 2006. Federal Law Gazette I, 09.11.2006, p.2523.
50. Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. OJ L 324, 10.12.2007, p.121.
51. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. OJ L 136, 30.04.2004, p.1.
52. Transfusion Law (“Transfusionsgesetz”) of the Federal Republic of Germany of 01.07.1998 in the version of the notification of the Law of 28.08.2007. Federal Law Gazette I p.2169.
Introduction
Clinical gene therapy is a very ambitious intent. The general principle sounds as easy as it is ingenious. Instead of treating the symptoms of severe genetic diseases such as immunodeficiencies or metabolic disorders, gene therapy intends to cure the underlying genetic defect by introducing a corrected copy of the mutated gene, or even by correcting the affected gene itself. In the context of disorders affecting the hematopoietic system, patient-derived cells can be treated ex vivo with engineered gene vectors designed to deliver the therapeutic gene. Except in those diseases associated with a strong selective advantage of the gene-modified cells, a preparatory “conditioning” by cytoreductive treatment may be required to promote engraftment.
Stable gene transfer, and therefore long-term genetic modification, is achieved by gene vectors, which integrate their genetic material and the therapeutic gene respectively into the chromatin of the target cells. Unlike classical pharmaceutical drug treatment, gene therapy is an approach highly specific to the patient and the disease. Parameters like cell source and origin, vector type, therapeutic dose, route of administration and, in particular, the transgene itself have to be adapted to each specific approach and medicinal purpose. For example, between 1989 and 2009 there were 1537 clinical gene therapy trials approved employing 35 different vector types (predominantly derived from Adenovirus, Retrovirus or naked DNA) in more than 8 different fields of medicine (predominantly cancer) (http://www.wiley.co.uk/genetherapy/clinical/). The consequence of the diversity of the products is a challenge for legal regulation, which should normally be universally valid while giving specific guidance to certain therapies in order to guarantee the safety and efficacy of the individual products.
While a gene therapy approach allows for the desired efficient long-term correction of a genetic defect on the one hand, it also brings up the concern of side effects on the other. The most noted example has been the clinical gene therapy trial for treatment of the rare genetic disorder X-linked severe combined immunodeficiency (X-SCID). While the majority of treated patients benefited from a life-saving and long-term immune reconstitution, the occurrence of lymphoproliferative disease due to insertional mutagenesis in 5 patients to date has gained notoriety [1, 2]. Integration site analysis revealed vector integrations close to cellular proto-oncogenes such as the LMO2 gene, known to be activated by chromosomal translocations in T-lymphoblastic leukemia [3, 4]. These severe adverse events made the theoretical concerns a reality. Additional concerns related to immunogenicity, spread of genetic sequences or toxic and infectious byproducts of vector preparations cause many gene therapy products to be classified as high-risk products that need to be strongly regulated by the authorities. This poses a tremendous challenge, since regulation can only be defined in general terms and an all-in-one document suitable for every purpose and individual need of a product cannot be established. To overcome this dilemma, case-by-case considerations are indicated.
Since researchers who invent the individual product initiate many clinical gene therapy trials, this review targets those investigators planning to enter the clinical development phase and initiating a clinical trial. It will clarify the complex situation and will give an overview of the current regulatory status as well as important points to consider before applying for a clinical trial authorization. This review involves preclinical and clinical issues as well as references to the necessary documents to be prepared.
Viral gene transfer and its associated risks
The regulatory framework should define uniform requirements in order to ensure compliance with quality standards and therefore guarantee the safety and well being of trial participants. In consideration of the major risks accompanied by viral gene transfer, it becomes clear that a thorough and complex regulatory framework is needed to control these biological products. As indicated above, the complexity of the individual product itself accounts for the necessity for evaluation of the risks and benefits of a certain gene therapy application on a case-by-case basis.
Integrating replication-defective vectors based on gamma-retroviruses have been frequently used in initial gene therapy protocols, and their risks will therefore be discussed as an example. Target cells are transduced in most cases ex vivo with the viral vector preparation. Shortly after entry of the retroviral particles, the viral RNA carrying the transgene of choice is reverse-transcribed into dsDNA, and a preintegration complex (PIC) is formed in which the DNA is associated with retroviral and cellular proteins (Figure 1). After translocation to the nucleus, the retroviral DNA including the transgene is stably integrated into the chromosomal DNA by the viral protein integrase.
Figure 1. The retroviral life cycle (reproduced with permission from the Journal Molecular Therapy (Nature Publishing Group); adapted from [5])

The integration of the transgene makes the desired correction of the genetic defect possible in the first place, not only in the transduced cell but also in its progeny. However, at the same time it forms the basis of one of the major safety concerns of integrating retroviral vectors: insertional mutagenesis. The process of integration is highly efficient but occurs in a semi-random manner with respect to the targeted genetic loci. Although gamma-retroviral integration tends to occur preferentially in open chromatin regions, which is associated with actively transcribed genes [6], it does not favor a specific target sequence. Therefore, insertion of the transgene in a non-predictable unspecific way may lead to activation, inactivation or truncation of cellular genes adjacent to the site of integration. Cellular proto-oncogenes might be placed under control of the viral enhancer/promoter elements resulting in a non-physiological expression of the respective gene with loss of cellular regulation. The tumorigenic risk associated with such an event appears to be highly context dependent. Available evidence suggests that additional mutations are required before cells transform to overt malignancy [7].
The concern of genotoxicity especially affects the first generation of retroviral-based gene transfer vectors in which the transgene is driven by the strong long terminal repeat (LTR) enhancer/promoter elements of the virus. New strategies in vector design with reduced potential for enhancer-mediated interaction with adjacent cellular genes may decrease the risk of genotoxicity, e.g., the use of self-inactivating (SIN) vectors [8], the incorporation of cellular promoters [9], the use of chromatin insulators [10], or the use of lentivirus-based vectors, which have a lower likelihood of integration in promoter-proximal or other regulatory gene regions [11]. In all circumstances, the genotoxic potential of a given vector designed for clinical use needs to be preclinically assessed and evaluated.
Another concern regarding the risk of oncogenesis is the formation of replication-competent retrovirus (RCR). Although retroviral vectors are replication-deficient due to the lack of the sequences encoding for the structural and enzymatic viral proteins, there is still the possibility of formation of RCR by recombination of the vector sequence with the so-called “helper” plasmids or with other, endogenous retroviral genetic information present in the producer or target cells [12]. As a consequence, unintended infection of target or even off-target cells might occur. This might introduce harmful viral pathogens into treated patients.
In order to assess the risk of RCR formation, investigators should evaluate the presence of homologous sequences suitable for recombination in the vector constructs, as well as the presence of endogenous viruses in the packaging cells. If possible, such sequences should be avoided or at least limited. Nevertheless, strict testing for the presence of RCR in retroviral vector batches intended for clinical application is required.
Another major concern of viral gene transfer is vertical germ line transmission. In general, gene therapy trials with the aim of direct germ line manipulation are prohibited [28]. Nevertheless, also in somatic gene therapy the concern of inadvertent germ line integration exists and gained new attention when semen of clinical trial participants for treatment of hemophilia tested positive for vector sequences [13, 14]. Although this does not necessarily imply that vector sequences were present in germ cells, this observation underlines the need for diligent biodistribution studies. The risk of germline transmission is in particular dependent on the biodistribution pattern of a given vector. In this regard, the route of administration, vector type and dose, and the innate and adapted immunity of the patient play pivotal roles. A replication deficient integrating vector used for ex vivo transduction of the target cell might have a much lower risk than the same vector applied systemically in an in vivo application. At the same time, a gamma-retroviral vector which can only transduce dividing cells might have a lower risk in transducing mature sperm cells than a lentiviral vector, which has the ability to also infect non-dividing cells. Still, spermatogonial stem cells that have a high proliferation activity might be accessible for gamma-retroviral vectors. Therefore, regulatory agencies have developed guidelines describing how to address the risk of inadvertent germ line transmission in preclinical studies (see below and Table 1).
Table 1. Applicable guidelines addressing GTMPs

Regulatory framework for Gene Therapy Medicinal Products
Currently, the regulatory situation for Gene Therapy Medicinal Products (GTMPs) is evolving rapidly on the European level. The new core regulation (EC) No 1394/2007 on Advanced Therapy Medicinal Products (ATMPs), which became effective in December 2008 (from here on referred to as ‘ATMP regulation’), lays the foundation for a harmonized regulatory situation applicable for all member states in the European Communion. According to Article 2 of this regulation, GTMPs together with Somatic Cell Therapy Medicinal Products and Tissue Engineered Products are classified as ATMPs (Figure 2). Until incorporation of this regulation, these products fell in a regulatory gap somewhere in between legislation 93/42/EEC on Medical Devices and Directive 2001/83/EC on Medicinal Products. The new ‘ATMP regulation’ fills this gap and "lays down specific rules concerning the authorization, supervision and pharmacovigilance of Advanced Therapy Medicinal Products” [50, Article 1].

For the definition of a GTMP itself, the regulation refers to Annex I Part IV of Directive 2001/83/EC, as amended by Directive 2003/63/EC, which states: “For the purposes of this Annex, Gene Therapy Medicinal Product shall mean a product obtained through a set of manufacturing processes aimed at the transfer, to be performed either in vivo or ex vivo, of a prophylactic, diagnostic or therapeutic gene (i.e. a piece of nucleic acid), to human/animal cells and its subsequent expression in vivo.” Within the implementation of the ‘ATMP regulation’, this annex is currently under revision in order to adjust it to the specific characteristics of ATMPs (Figure 2). There is no draft version currently available, but the outcome of the public consultation paper as well as single contributions can be found on the webpage of the European Commission.
On the national German level, GTMPs are similarly defined as Medicinal Products in the Medicinal Products Act (“AMG”), Section 4 No. 9, [48]. Within the 15th amendment of the Medicinal Products Act, the new ATMP regulation will be implemented into national German law. Though a regulation, the translation into national law is necessary because the ‘ATMP regulation’ amends Directive 2001/83/EC.
GTMPs are often very complex products that may contain other components that are regulated by additional legislation. The GTMP may for instance contain human cells or tissues. Regarding the quality and safety for the donation, procurement and testing of these cells or tissues, Directive 2004/23/EC as implemented by Directive 2006/17/EC applies (Figure 3A). Directive 2004/23/EC is already transposed into national law by the German Tissue Act (“Gewebegesetz”). The processing, preservation, storage and distribution of these cells and tissues, however, fall under Section 14 of the ‘ATMP regulation’. When the GTMP also contains human blood or blood components, Directive 2002/98/EC as implemented by Directives 2004/33/EC, 2005/61/EC and 2005/62/EC applies (Figure 3B). On the German level, these aspects are addressed in the Medicinal Products Act and Transfusion Law.
In addition, the GTMP may be a combination product consistent of a Medicinal Product and Medical Device. This would, for instance, be the case if the gene-modified cells are applied to the patient via a specific biodelivery implant. Whether the regulatory rules and standards of Medicinal Products or of Medical Devices apply to combination products generally depends on their mode of action. Nevertheless, if ATMPs (and therefore GTMPs) are incorporated into a combination product, the ‘ATMP regulation’ applies regardless of the function of the Medical Device [50, Section 4]. However, the latter have to furthermore fulfill the quality and safety requirements of Directive 93/42/EEC (in case of a Medical Device) and accordingly Directive 90/385/EEC (in case of an active implantable Medical Device) as amended by Directive 2007/47/EC (Figure 3C). On the German level, both directives are implemented by the German Act on Medical Devices (“MPG”).
For clinical trials involving GTMPs, the overall requirements and ethical standards of the Clinical Trial Directive 2001/20/EC apply as well as for all other medicinal products (Figure 3D). In Germany, this directive is translated into national law by the GCP Ordinance (“GCP-Verordnung”). Furthermore, the German Medicinal Products Act applies, in particular concerning clinical trials in Chapter 6, together with the general considerations for clinical trials laid down in ICH E8 Step 5 [27]. Within the scope of the implementation of the ‘ATMP regulation’, the standards of good clinical practices shall be expanded to the specific needs of ATMPs [50, Article 4]. The adaption process is currently proceeding. A public consultation paper is already published on the European Commission webpage. The public consultation process has been closed so that a draft document on good clinical practice specific for ATMPs is expected to be published soon.
In Germany, the competent authority concerning clinical trial authorization related to GTMPs is the Paul-Ehrlich-Institute (PEI). General principles for requesting the authorization of a clinical trial in Germany are laid down in the third Notification of the joint announcement from PEI and the Federal Institute for Drugs and Medical Devices (BfArM) (third Notification).
In Germany there are some particularities for GTMPs compared to conventional medicinal products. While for example the ethics committee has to give an opinion within 60 days after a clinical trial application (multicentric trial) for conventional medicinal products, the time period extends up to 180 days if GTMPs are concerned [44, Section 8(4)]. Furthermore, the competent authority has to provide a written approval (explicit authorization) for clinical trials concerning GTMPs within 90 days after complete clinical trial application. This period may be extended to 180 days if the authority consults experts or professional opinions for decision-making [44, Section 9(4)].
The manufacture of GTMPs needs to be consistent with the requirements of Directive 2003/94/EC on Good Manufacturing Practice (“GMP“) (Figure 3E). Due to the complexity of ATMPs (and GTMPs) and the extensive manufacturing processes, the ‘ATMP regulation’ implicates an adaption of the guidelines for GMP to the specific situation of ATMPs [50, Article 5].
Figure 3. Regulatory framework for Gene Therapy Medicinal Products

Currently, a draft version of the adapted Eudralex Volume 4 Annex 2 on the manufacture of biological medicinal products is published. Furthermore, the European Pharmacopoeia serves as a legally binding framework for quality standards of medicinal products in Europe [43]. The General Chapter 5.14, which deals with GTMPs, gives instructions for the testing of batches of recombinant vectors and gene-modified cells. In Germany, good manufacturing practice is regulated in the ordinance for the manufacture of medicinal products and active pharmaceutical ingredients (AMWHV).
There are in addition various guidelines published addressing manufacturing and quality aspects during the development of ATMPs (Table 1). One is the multidisciplinary “Guideline on human Cell-Based Medicinal Products” of the European Medicines Agency (EMEA) [35], which gives advice for the “development, manufacturing and quality control as well as non-clinical and clinical development of Cell-Based Medicinal Products” which also include GTMPs. The guideline is aimed at products already in the phase of marketing authorization but the general considerations also apply for clinical trials. The EMEA “Note for guidance on the quality, preclinical and clinical aspects of Gene Transfer Medicinal Products” [25] gives recommendations for producing data aiming at an application for marketing authorization. Affiliated is the “Concept paper on the development of a guideline on the quality, preclinical and clinical aspects of medicinal products containing genetically modified cells” by EMEA [40]. If the GTMP was generated with the help of lentiviral vectors, the EMEA “Guideline on development and manufacture of lentiviral vectors” gives advice regarding quality and safety of the vectors [24].
Importantly, the specific safety aspects related to GTMPs have to be considered and analyzed before the products can be used in the clinic. The EMEA “Guideline on the non-clinical studies required before first clinical use of Gene Therapy Medicinal Products” [38] specifies which studies are essential prior to the first application to humans. This includes but is not limited to non-clinical proof of concept studies, biodistribution studies, as well as studies on dose finding, germ line transmission, immunotoxicity and studies addressing the environmental risks/shedding. Specific recommendations for the later are defined in the EMEA “Guideline on scientific requirements for the environmental risk assessment of Gene Therapy Medicinal Products” [39]. This guideline states: "Generally the purpose of clinical trials are to study the adsorption, distribution, metabolism and excretion of one or more Investigational Medicinal Products with the object of ascertaining its (their) safety and/or efficacy ([28]; definition of a clinical trial). As such the evaluation of vector shedding is a requirement for a phase I study.” Data of clinical trials regarding environmental risks need to be collected and integrated in a full environmental risk assessment (ERA) needed for a marketing authorization procedure. Other guidelines dealing with environmental risk assessments of genetically modified organisms are EMEA/BWP/473191/06-corr and EMEA/CHMP/BWP/135148/04.
Furthermore, in terms of studies addressing the risk and ethical concerns of inadvertent germline transmission, separate guidelines are available. The EMEA “Guideline on non-clinical testing for inadvertent germline transmission of gene transfer vectors” [34] recommends adjusted study designs depending on the type of vector used, as well as providing a decision tree regarding the necessary questions to be addressed by biodistribution and germ line transmission studies. Also a considerations paper of the International Conference On Harmonization Of Technical Requirements For Registration Of Pharmaceuticals For Human Use (ICH) is available on this topic and can be consulted in this regard (ICH considerations, general principles to address the risk of inadvertent germline integration of gene therapy vectors). The ICH S2B document “Note for guidance on genotoxicity” [26], however, gives recommendations for studies to address the risk of genotoxicity and can give advice in these questions.
If patients have been treated with a gene therapy approach in a clinical trial, clinical monitoring as well as intensive follow-up care should be performed in order to detect adverse events at an early stage to avoid clinical implications and to collect safety data. The “Guideline on follow-up of patients administered with Gene Therapy Medicinal Products” [41] gives recommendations in this regard.
Regulation (EC) No 726/2004 already regulates the marketing authorization procedure of biotechnology medicinal products on the European level. In this context, the products pass through a centralized authorization procedure at the EMEA in order to assess their quality, safety and efficacy. The technical requirements needed to prove the latter are defined in Annex I to Directive 2001/83/EC.
Nevertheless, the complexity and variety of ATMPs may result in a situation of very specific and individual questions to be addressed. Within the scope of the ‘ATMP regulation’, the Committee for Advanced Therapies (CAT) will be established within the EMEA in order to provide strong expertise in this field and exhibit draft opinions on ATMP applications presented to EMEA [50, Chapter 7]. Therefore, Regulation (EC) No 726/2004 needs to be amended. Currently, the CAT is in the process of establishment within the EMEA. Updated details can be found on the respective webpage of EMEA. Within the context of a marketing authorization, the standards for pharmacovigilance described in Regulation 726/2004 apply. Since clinical trials are typically powered for efficacy and furthermore the duration of the respective trials might not allow the detection of long term adverse events, the application has to provide a plan to ensure the follow-up of adverse reactions after marketing authorization. A specific EMEA guideline giving further recommendations on “Safety and Efficacy Follow-Up – Risk management of Advanced Therapy Medicinal Products” is already published [33]. Another measure of safety is the full traceability of the product (including the starting materials) and the treated patient. According to the ‘ATMP regulation’, the marketing authorization holder for an ATMP is responsible for setting up a traceability system [50, Article 15]. If human tissues or cells are involved, the traceability standards defined in Directive 2004/23/EC also apply, as do the requirements of Directive 2002/98/EC concerning human blood and blood components, respectively.
Finally, it should be noted that both the European agency EMEA and the German agency PEI (in charge of ATMPs) provide scientific advice. Investigators are welcome to consult the agencies in all phases of project development in order to obtain important project-specific advice, e.g., before applying for a clinical trial or marketing authorization. Details can be found on the webpages of EMEA or PEI, respectively.
Outlook
Application of new technologies of gene therapy to the treatment of diseases will yield the greatest benefits if approached on a sound scientific and medical basis. Regulatory guidelines are the basis of (i) current scientific knowledge, (ii) scientific expertise in a given research area, and (iii) the result of a continuous interaction between researchers and regulatory experts. Gene therapy has entered the stage of clinical evaluation. As always at the frontier of bench to bedside research, patient safety must be the first and foremost consideration in human gene therapy before one enters the stage of extensive clinical trials to assess efficacy.
References
Index of scientific references
1. Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132-3142.
2. Howe SJ, Mansour MR, Schwarzwaelder K, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143-50.
3. Boehm T, Foroni L, Kaneko Y, et al. The rhombotin family of cysteine-rich LIM-domain oncogenes: Distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc. Natl. Acad. Sci. 1991;88:4367–4371.
4. Royer-Pokora B, Loos U, and Ludwig WD. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukemia with the t(11;14)(p13;q11). Oncogene. 1991;6:1887–1893.
5. Baum C, Schambach A, Bohne J, and Galla M. Retrovirus Vectors: Toward the Plentivirus? Mol Ther. 2006;13:1050-1063.
6. Wu X, Li Y, Crise B, and Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300(5626):1749-1751.
7. Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758-764.
8. Modlich U, Bohne J, Schmidt M, et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545-2553.
9. Zychlinski D, Schambach A, Modlich U, et al. Physiological Promoters Reduce the Genotoxic Risk of Integrating Gene Vectors. Mol Ther. 2008;16(4):718-725.
10. Emery DW, Yannaki E, Tubb J, et al. Development of virus vectors for gene therapy of beta chain hemoglobinopathies: flanking with a chromatin insulator reduces gamma-globin gene silencing in vivo. Blood. 2002;100:2012-2019.
11. De Palma M, Montini E, Santoni de Sio FR, et al. Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells. Blood.2005;105(6):2307-15.
12. Chong H, Starkey W, and Vile RG. A replication-competent retrovirus arising from a split-function packaging cell line was generated by recombination events between the vector, one of the packaging constructs, and endogenous retroviral sequences. J Virol.1998;72:2663–2670.
13. Marshall E. Gene therapy. Viral vectors still pack surprises. Science. 2001;294(5547):1640.
14. Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342-7.
Index of regulatory references
15. 3. Notification (“3. Bekanntmachung”) of the Federal Institute for Drugs and Medical Devices and the Paul Ehrlich Institute of 10 August 2006 on the clinical trial of medicinal products for human use.
16. Commission Directive 2003/63/EC of 25 June 2003 amending Directive 2001/83/EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use. OJ L 159, 27.06.2006, p.46.
17. Commission Directive 2003/94/EC of 8 October 2003 laying down the principles and guidelines of good manufacturing practice in respect of medicinal products for human use and investigational medicinal products for human use.OJ L 262, 14.10.2003, p.22.
18. Commission Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards certain technical requirements for blood and blood components. OJ L 91, 30.03.2004, p.25.
19. Commission Directive 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards traceability requirements and notification of serious adverse reactions and events. OJ L 256, 01.10.2005, p.32.
20. Commission Directive 2005/62/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards Community standards and specifications relating to a quality system for blood establishments. OJ L 256, 01.10.2005, p.41.
21. Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. OJ L 38, 09.02.2006, p.40.
22. Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices. OJ L 189, 20.07.1990, p.17
23. Council Directive 93/42/EEC of 14 June 1993 concerning medical devices. OJ L 169, 12.07.1993, p.1.
24. CHMP/BWP/2458/03 of the Committee for Medicinal Products for Human Use (CHMP) of 26 May 2005. Guideline on development and manufacture of lentiviral vectors.
25. CPMP/BWP/3088/99 of the Committee for Proprietary Medicinal Products (CPMP) of 24 April 2001. Note for Guidance on the quality, preclinical and clinical aspects of gene transfer medicinal products.
26. CPMP/ICH/174/95 ICH Topic S 2 B of March 1998. Genotoxicity: A standard battery for genotoxicity testing of pharmaceuticals, Step 5. Note for guidance on genotoxicity: A standard battery for genotoxicity testing of pharmaceuticals.
27. CPMP/ICH/291/95 ICH Topic E 8 of March 1998. General considerations for clinical trials, step 5. Note for guidance on general considerations for clinical trials.
28. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. OJ L 121, 1.5.2001, p.34.
29. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. OJ L 311, 28.11.2001, p.67.
30. Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood componentsand amending Directive 2001/83/EC. OJ L 33, 08.02.2003, p.30.
31. Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. OJ L 102, 07.04.2004, p.48.
32. Directive 2007/47/EC of the European Parliament and of the Council of 5 September 2007 amending Council Directive 90/385/EEC on the approximation of the laws of the Member States relating to active implantable medical devices, Council Directive 93/42/EEC concerning medical devices and Directive 98/8/EC concerning the placing of biocidal products on the market.
33. EMEA/149995/08 of the Committee for Medicinal Products for Human Use (CHMP) of 20 November 2008. Guideline on safety and efficacy follow-up – risk management of advanced therapy medicinal products.
34. EMEA/273974/05 of the Committee for Medicinal Products for Human Use (CHMP) of 16 November 2006. Guideline on non-clinical testing for inadvertent germline transmission of gene transfer vectors.
35. EMEA/CHMP/410869/06 of the Committee for Medicinal Products for Human Use (CHMP) of 21 May 2008. Guideline on human cell-based medicinal products.
36. EMEA/CHMP/BWP/135148/04 of the Committee for Medicinal Products for Human Use (CHMP) of 20 January 2005. Environmental risk assessments for medicinal products containing, or consisting of, genetically modified organisms (GMOs) (Module 1.6.2).
37. EMEA/CHMP/BWP/473191/06 - Corr of the Committee for Medicinal Products for Human Use (CHMP) of 11 December 2006. Guideline on environmental risk assessments for medicinal products consisting of, or containing, genetically modified organisms (GMOs).
38. EMEA/CHMP/GTWP/125459/06 of the Committee for Medicinal Products for Human Use (CHMP) of 30 May 2008. Guideline on the non-clinical studies required before first clinical use of gene therapy medicinal products.
39. EMEA/CHMP/GTWP/125491/06 of the Committee for Medicinal Products for Human Use (CHMP) of 30 May 2008. Guideline on scientific requirements for the environmental risk assessment of gene therapy medicinal products.
40. EMEA/CHMP/GTWP/405681/06 of the Committee for Medicinal Products for Human Use (CHMP) of 24 May 2007. Concept paper on the development of a guideline on the quality, preclinical and clinical aspects of medicinal products containing genetically modified cells.
41. EMEA/CHMP/GTWP/60436/07 of the Committee for Medicinal Products for Human Use (CHMP) of 30 May 2008. Draft guideline on follow-up of patients administered with gene therapy medicinal products.
42. Eudralex Volume 4. Good manufacturing practice guidelines. Volume 4 of “The rules governing medicinal products in the European Union”. http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol4_en.htm
43. European Pharacopoeia. http://www.pheur.org
44. GCP Ordinance (“GCP-Verordnung“). Of the German Federal Ministry for Health and Social Security of 09 August 2004 for good clinical practice in the conduct of clinical trials on medicinal products for human use. Federal Law Gazette I, 12.08.2004, p.2081.
45. German Act on Medical Devices (“Medizinproduktegesetz”) of the Federal Republic of Germany of 02.08.1994 in the version of the notification of the Law of 07.08. 2002. Federal Law Gazette I p.3146.
46. German Tissue Act (“Gewebegesetz“) of the German Federal Parliament of 20 July 2007 on the quality and safety of human tissues and cells. Federal Law Gazette I, 27.07.2007, p.1574.
47. ICH Considerations of the International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use of 25 October 2006. General principles to address the risk of inadvertent germline integration of gene therapy vectors.
48. Medicinal Products Act (“Arzneimittelgesetz”) of the Federal Republic of Germany of 24.08.1976 in the version of the notification of the Law of 12th December 2005. Federal Law Gazette I p.3394.
49. Ordinance for the manufacture of medicinal products and active pharmaceutical ingredients (“AMWHV”) of the German Federal Ministries for Health and Food, Agriculture and Consumer Protection of 03 November 2006. Federal Law Gazette I, 09.11.2006, p.2523.
50. Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. OJ L 324, 10.12.2007, p.121.
51. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. OJ L 136, 30.04.2004, p.1.
52. Transfusion Law (“Transfusionsgesetz”) of the Federal Republic of Germany of 01.07.1998 in the version of the notification of the Law of 28.08.2007. Federal Law Gazette I p.2169.
Велькель К., Люрманн А., Баум К., фон дер Ляйен Х.Э.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13490" ["VALUE"]=> array(2) { ["TEXT"]=> string(6323) "<p class="bodytext">Генная терапия предназначена для лечения генетических дефектов путем введения корригированной копии мутантного гена. Однако, в отличие от классического лекарственного лечения, генная терапия является подходом, высокоспецифичным в отношении больного и заболевания. Трансген как таковой должен быть адаптирован к каждому специфическому подходу и медицинской цели. С 1989 по 2009 гг. были одобрены 1537 клинических испытаний в области генной терапии с применением 35 различных типов векторов. Кроме того, с одной стороны, генно-терапевтический подход позволяет достичь эффективной долгосрочной коррекции генетического дефекта, а с другой стороны – несет опасность побочных эффектов. Ряд работ по лечению тяжелого комбинированного иммунодефицита, сцепленного с Х-хромосомой, показал развитие у нескольких больных лимфопролиферативных заболеваний в связи с инсерционным мутагенезом. Такие побочные эффекты, наряду с иммуногенностью, передачей генетических последовательностей, а также токсичных и инфекционных побочных продуктов, связанных с приготовлением вектора, делает необходимой строгое регулирование их применения со стороны властей. Соответствующие правила могут быть определены лишь в общих понятиях, и нельзя создать единый документ, пригодный для конкретной цели и индивидуального использования каждого продукта. <br /><br />Встраивающиеся векторы, дефектные по репликационным свойствам, основанные на гамма-ретровирусах, часто применялись в исходных протоколах генной терапии, и их опасность может обсуждаться в качестве примера, в частности, инсерционный мутагенез, который возникает во многом из-за случайного характера встраивания вируса по отношению к целевым генным локусам. Кроме того, могут быть вызваны и генотоксические эффекты, в основном связанные с дизрегуляцией активности нормальных генов при введении регуляторных элементов в составе вектора. Другой крупной проблемой вирусного переноса генов является их «вертикальный» перенос в зародышевых клеточных линиях, в частности, через пролиферирующие сперматогониальные клетки, которые могут быть мишенью для гамма-ретровирусных векторов. <br /><br />Для того, чтобы упредить эти опасности, регулирующая система должна уточнить единообразные требования для того, чтобы обеспечить соответствия между стандартами качества и, тем самым, гарантировать безопасность участников испытания. Данная статья суммирует наиболее важные регулирующие документы, которые следует учитывать до вхождения в фазу клинической разработки – не только для Германии, но и в европейской перспективе. Приводятся ссылки на применимые для этого руководящие указания в отношении генно-терапевтических медицинских продуктов (ГТМП), с соответствующими определениями для таких продуктов. <br /><br />Производство ГТМП должно согласовываться с требованиями Директивы Европейского Союза 2003/94/EC о качественной практике производства (GMP). В Германии эта практика регулируется Предписаниями по производству медицинских продуктов и активных фармацевтических ингредиентов (AMWHV). Важно, чтобы особые аспекты безопасности в отношении ГТМП учитывались до использования этих продуктов в клинике. В любом случае, регулирующие указания имеют в своей основе: (1) имеющиеся научные знания, (2) научный опыт в данной области исследований, и они возникают в результате постоянного взаимодействия между исследователями и экспертами в области регулирования. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(6265) "Генная терапия предназначена для лечения генетических дефектов путем введения корригированной копии мутантного гена. Однако, в отличие от классического лекарственного лечения, генная терапия является подходом, высокоспецифичным в отношении больного и заболевания. Трансген как таковой должен быть адаптирован к каждому специфическому подходу и медицинской цели. С 1989 по 2009 гг. были одобрены 1537 клинических испытаний в области генной терапии с применением 35 различных типов векторов. Кроме того, с одной стороны, генно-терапевтический подход позволяет достичь эффективной долгосрочной коррекции генетического дефекта, а с другой стороны – несет опасность побочных эффектов. Ряд работ по лечению тяжелого комбинированного иммунодефицита, сцепленного с Х-хромосомой, показал развитие у нескольких больных лимфопролиферативных заболеваний в связи с инсерционным мутагенезом. Такие побочные эффекты, наряду с иммуногенностью, передачей генетических последовательностей, а также токсичных и инфекционных побочных продуктов, связанных с приготовлением вектора, делает необходимой строгое регулирование их применения со стороны властей. Соответствующие правила могут быть определены лишь в общих понятиях, и нельзя создать единый документ, пригодный для конкретной цели и индивидуального использования каждого продукта.
Встраивающиеся векторы, дефектные по репликационным свойствам, основанные на гамма-ретровирусах, часто применялись в исходных протоколах генной терапии, и их опасность может обсуждаться в качестве примера, в частности, инсерционный мутагенез, который возникает во многом из-за случайного характера встраивания вируса по отношению к целевым генным локусам. Кроме того, могут быть вызваны и генотоксические эффекты, в основном связанные с дизрегуляцией активности нормальных генов при введении регуляторных элементов в составе вектора. Другой крупной проблемой вирусного переноса генов является их «вертикальный» перенос в зародышевых клеточных линиях, в частности, через пролиферирующие сперматогониальные клетки, которые могут быть мишенью для гамма-ретровирусных векторов.
Для того, чтобы упредить эти опасности, регулирующая система должна уточнить единообразные требования для того, чтобы обеспечить соответствия между стандартами качества и, тем самым, гарантировать безопасность участников испытания. Данная статья суммирует наиболее важные регулирующие документы, которые следует учитывать до вхождения в фазу клинической разработки – не только для Германии, но и в европейской перспективе. Приводятся ссылки на применимые для этого руководящие указания в отношении генно-терапевтических медицинских продуктов (ГТМП), с соответствующими определениями для таких продуктов.
Производство ГТМП должно согласовываться с требованиями Директивы Европейского Союза 2003/94/EC о качественной практике производства (GMP). В Германии эта практика регулируется Предписаниями по производству медицинских продуктов и активных фармацевтических ингредиентов (AMWHV). Важно, чтобы особые аспекты безопасности в отношении ГТМП учитывались до использования этих продуктов в клинике. В любом случае, регулирующие указания имеют в своей основе: (1) имеющиеся научные знания, (2) научный опыт в данной области исследований, и они возникают в результате постоянного взаимодействия между исследователями и экспертами в области регулирования.
Christine Voelkel1,2, Anke Lührmann2, Christopher Baum1,3, and Heiko E. von der Leyen2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13503" ["VALUE"]=> array(2) { ["TEXT"]=> string(878) "<p class="bodytext"><sup>1</sup>Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;<br><sup> 2</sup>Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany; <br><sup>3</sup>Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA </p> <br> <p> <b>Correspondence </b><br>Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany, <br>Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.zhpicirDgpmrmgep1xvmep1girxiv2hi');">vdleyen@<span style="display:none;">spam is bad</span>clinical-trial-center.de</a> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(722) "1Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;
2Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany;
3Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA
Correspondence
Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany,
Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: vdleyen@clinical-trial-center.de
Retrovirus mediated gene therapy has already proven to be more than just a theoretical option to treat patients with severe genetic defects. Clinical gene therapy trials of X-linked severe combined immunodeficiency or adenosine deaminase deficiency have demonstrated the success and potential benefit of the therapy. Nevertheless, the complexity of the therapeutic products and their biological origin, as well as virus-related safety concerns require the need of a strict regulatory framework in order to guarantee the quality of the individual products and safety of the patients. The aim of this review is to give an overview of the rapidly evolving regulatory framework of Advanced Therapy Medicinal Products in Europe. We will summarize the most important regulatory documents to be considered before entering the clinical development phase – not only from a German but also from a European perspective.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13453" ["VALUE"]=> string(128) "Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(128) "Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> &array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13491" ["VALUE"]=> string(3) "647" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "647" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13492" ["VALUE"]=> string(3) "648" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "648" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(13) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13502" ["VALUE"]=> array(2) { ["TEXT"]=> string(203) "<p> Christine Voelkel<sup>1,2</sup>, Anke Lührmann<sup>2</sup>, Christopher Baum<sup>1,3</sup>, and Heiko E. von der Leyen<sup>2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(143) "
Christine Voelkel1,2, Anke Lührmann2, Christopher Baum1,3, and Heiko E. von der Leyen2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(143) "Christine Voelkel1,2, Anke Lührmann2, Christopher Baum1,3, and Heiko E. von der Leyen2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13504" ["VALUE"]=> array(2) { ["TEXT"]=> string(955) "<p class="bodytext">Retrovirus mediated gene therapy has already proven to be more than just a theoretical option to treat patients with severe genetic defects. Clinical gene therapy trials of X-linked severe combined immunodeficiency or adenosine deaminase deficiency have demonstrated the success and potential benefit of the therapy. Nevertheless, the complexity of the therapeutic products and their biological origin, as well as virus-related safety concerns require the need of a strict regulatory framework in order to guarantee the quality of the individual products and safety of the patients. The aim of this review is to give an overview of the rapidly evolving regulatory framework of Advanced Therapy Medicinal Products in Europe. We will summarize the most important regulatory documents to be considered before entering the clinical development phase – not only from a German but also from a European perspective.<p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(933) "Retrovirus mediated gene therapy has already proven to be more than just a theoretical option to treat patients with severe genetic defects. Clinical gene therapy trials of X-linked severe combined immunodeficiency or adenosine deaminase deficiency have demonstrated the success and potential benefit of the therapy. Nevertheless, the complexity of the therapeutic products and their biological origin, as well as virus-related safety concerns require the need of a strict regulatory framework in order to guarantee the quality of the individual products and safety of the patients. The aim of this review is to give an overview of the rapidly evolving regulatory framework of Advanced Therapy Medicinal Products in Europe. We will summarize the most important regulatory documents to be considered before entering the clinical development phase – not only from a German but also from a European perspective.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(933) "
Retrovirus mediated gene therapy has already proven to be more than just a theoretical option to treat patients with severe genetic defects. Clinical gene therapy trials of X-linked severe combined immunodeficiency or adenosine deaminase deficiency have demonstrated the success and potential benefit of the therapy. Nevertheless, the complexity of the therapeutic products and their biological origin, as well as virus-related safety concerns require the need of a strict regulatory framework in order to guarantee the quality of the individual products and safety of the patients. The aim of this review is to give an overview of the rapidly evolving regulatory framework of Advanced Therapy Medicinal Products in Europe. We will summarize the most important regulatory documents to be considered before entering the clinical development phase – not only from a German but also from a European perspective.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13452" ["VALUE"]=> string(29) "10.3205/ctt-2009-en-000044.01" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(29) "10.3205/ctt-2009-en-000044.01" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(29) "10.3205/ctt-2009-en-000044.01" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13453" ["VALUE"]=> string(128) "Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(128) "Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(128) "Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13503" ["VALUE"]=> array(2) { ["TEXT"]=> string(878) "<p class="bodytext"><sup>1</sup>Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;<br><sup> 2</sup>Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany; <br><sup>3</sup>Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA </p> <br> <p> <b>Correspondence </b><br>Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany, <br>Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.zhpicirDgpmrmgep1xvmep1girxiv2hi');">vdleyen@<span style="display:none;">spam is bad</span>clinical-trial-center.de</a> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(722) "
1Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;
2Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany;
3Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA
Correspondence
Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany,
Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: vdleyen@clinical-trial-center.de
1Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;
2Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany;
3Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA
Correspondence
Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany,
Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: vdleyen@clinical-trial-center.de
Велькель К., Люрманн А., Баум К., фон дер Ляйен Х.Э.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(95) "Велькель К., Люрманн А., Баум К., фон дер Ляйен Х.Э.
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13473" ["VALUE"]=> string(22) "03/13/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/13/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/13/2009 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13474" ["VALUE"]=> string(22) "03/30/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "03/30/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "03/30/2009 12:00:00 am" } ["PUBLISHED"]=> array(37) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13475" ["VALUE"]=> string(22) "05/14/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "05/14/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "05/14/2009 12:00:00 am" } ["KEYWORDS"]=> array(38) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(5) { [0]=> string(5) "13541" [1]=> string(5) "13542" [2]=> string(5) "13543" [3]=> string(5) "13544" [4]=> string(5) "13545" } ["VALUE"]=> array(5) { [0]=> string(3) "688" [1]=> string(3) "955" [2]=> string(3) "956" [3]=> string(3) "804" [4]=> string(3) "957" } ["DESCRIPTION"]=> array(5) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(5) { [0]=> string(3) "688" [1]=> string(3) "955" [2]=> string(3) "956" [3]=> string(3) "804" [4]=> string(3) "957" } ["~DESCRIPTION"]=> array(5) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(5) { [0]=> string(71) "генная терапия" [1]=> string(75) "побочные эффекты" [2]=> string(83) "регулирующая система" [3]=> string(85) "клинические испытания" [4]=> string(64) "ретровирус" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["CONTACT"]=> array(38) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13448" ["VALUE"]=> string(3) "952" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "952" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(59) "Christopher Baum" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13490" ["VALUE"]=> array(2) { ["TEXT"]=> string(6323) "<p class="bodytext">Генная терапия предназначена для лечения генетических дефектов путем введения корригированной копии мутантного гена. Однако, в отличие от классического лекарственного лечения, генная терапия является подходом, высокоспецифичным в отношении больного и заболевания. Трансген как таковой должен быть адаптирован к каждому специфическому подходу и медицинской цели. С 1989 по 2009 гг. были одобрены 1537 клинических испытаний в области генной терапии с применением 35 различных типов векторов. Кроме того, с одной стороны, генно-терапевтический подход позволяет достичь эффективной долгосрочной коррекции генетического дефекта, а с другой стороны – несет опасность побочных эффектов. Ряд работ по лечению тяжелого комбинированного иммунодефицита, сцепленного с Х-хромосомой, показал развитие у нескольких больных лимфопролиферативных заболеваний в связи с инсерционным мутагенезом. Такие побочные эффекты, наряду с иммуногенностью, передачей генетических последовательностей, а также токсичных и инфекционных побочных продуктов, связанных с приготовлением вектора, делает необходимой строгое регулирование их применения со стороны властей. Соответствующие правила могут быть определены лишь в общих понятиях, и нельзя создать единый документ, пригодный для конкретной цели и индивидуального использования каждого продукта. <br /><br />Встраивающиеся векторы, дефектные по репликационным свойствам, основанные на гамма-ретровирусах, часто применялись в исходных протоколах генной терапии, и их опасность может обсуждаться в качестве примера, в частности, инсерционный мутагенез, который возникает во многом из-за случайного характера встраивания вируса по отношению к целевым генным локусам. Кроме того, могут быть вызваны и генотоксические эффекты, в основном связанные с дизрегуляцией активности нормальных генов при введении регуляторных элементов в составе вектора. Другой крупной проблемой вирусного переноса генов является их «вертикальный» перенос в зародышевых клеточных линиях, в частности, через пролиферирующие сперматогониальные клетки, которые могут быть мишенью для гамма-ретровирусных векторов. <br /><br />Для того, чтобы упредить эти опасности, регулирующая система должна уточнить единообразные требования для того, чтобы обеспечить соответствия между стандартами качества и, тем самым, гарантировать безопасность участников испытания. Данная статья суммирует наиболее важные регулирующие документы, которые следует учитывать до вхождения в фазу клинической разработки – не только для Германии, но и в европейской перспективе. Приводятся ссылки на применимые для этого руководящие указания в отношении генно-терапевтических медицинских продуктов (ГТМП), с соответствующими определениями для таких продуктов. <br /><br />Производство ГТМП должно согласовываться с требованиями Директивы Европейского Союза 2003/94/EC о качественной практике производства (GMP). В Германии эта практика регулируется Предписаниями по производству медицинских продуктов и активных фармацевтических ингредиентов (AMWHV). Важно, чтобы особые аспекты безопасности в отношении ГТМП учитывались до использования этих продуктов в клинике. В любом случае, регулирующие указания имеют в своей основе: (1) имеющиеся научные знания, (2) научный опыт в данной области исследований, и они возникают в результате постоянного взаимодействия между исследователями и экспертами в области регулирования. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(6265) "Генная терапия предназначена для лечения генетических дефектов путем введения корригированной копии мутантного гена. Однако, в отличие от классического лекарственного лечения, генная терапия является подходом, высокоспецифичным в отношении больного и заболевания. Трансген как таковой должен быть адаптирован к каждому специфическому подходу и медицинской цели. С 1989 по 2009 гг. были одобрены 1537 клинических испытаний в области генной терапии с применением 35 различных типов векторов. Кроме того, с одной стороны, генно-терапевтический подход позволяет достичь эффективной долгосрочной коррекции генетического дефекта, а с другой стороны – несет опасность побочных эффектов. Ряд работ по лечению тяжелого комбинированного иммунодефицита, сцепленного с Х-хромосомой, показал развитие у нескольких больных лимфопролиферативных заболеваний в связи с инсерционным мутагенезом. Такие побочные эффекты, наряду с иммуногенностью, передачей генетических последовательностей, а также токсичных и инфекционных побочных продуктов, связанных с приготовлением вектора, делает необходимой строгое регулирование их применения со стороны властей. Соответствующие правила могут быть определены лишь в общих понятиях, и нельзя создать единый документ, пригодный для конкретной цели и индивидуального использования каждого продукта.
Встраивающиеся векторы, дефектные по репликационным свойствам, основанные на гамма-ретровирусах, часто применялись в исходных протоколах генной терапии, и их опасность может обсуждаться в качестве примера, в частности, инсерционный мутагенез, который возникает во многом из-за случайного характера встраивания вируса по отношению к целевым генным локусам. Кроме того, могут быть вызваны и генотоксические эффекты, в основном связанные с дизрегуляцией активности нормальных генов при введении регуляторных элементов в составе вектора. Другой крупной проблемой вирусного переноса генов является их «вертикальный» перенос в зародышевых клеточных линиях, в частности, через пролиферирующие сперматогониальные клетки, которые могут быть мишенью для гамма-ретровирусных векторов.
Для того, чтобы упредить эти опасности, регулирующая система должна уточнить единообразные требования для того, чтобы обеспечить соответствия между стандартами качества и, тем самым, гарантировать безопасность участников испытания. Данная статья суммирует наиболее важные регулирующие документы, которые следует учитывать до вхождения в фазу клинической разработки – не только для Германии, но и в европейской перспективе. Приводятся ссылки на применимые для этого руководящие указания в отношении генно-терапевтических медицинских продуктов (ГТМП), с соответствующими определениями для таких продуктов.
Производство ГТМП должно согласовываться с требованиями Директивы Европейского Союза 2003/94/EC о качественной практике производства (GMP). В Германии эта практика регулируется Предписаниями по производству медицинских продуктов и активных фармацевтических ингредиентов (AMWHV). Важно, чтобы особые аспекты безопасности в отношении ГТМП учитывались до использования этих продуктов в клинике. В любом случае, регулирующие указания имеют в своей основе: (1) имеющиеся научные знания, (2) научный опыт в данной области исследований, и они возникают в результате постоянного взаимодействия между исследователями и экспертами в области регулирования.
Генная терапия предназначена для лечения генетических дефектов путем введения корригированной копии мутантного гена. Однако, в отличие от классического лекарственного лечения, генная терапия является подходом, высокоспецифичным в отношении больного и заболевания. Трансген как таковой должен быть адаптирован к каждому специфическому подходу и медицинской цели. С 1989 по 2009 гг. были одобрены 1537 клинических испытаний в области генной терапии с применением 35 различных типов векторов. Кроме того, с одной стороны, генно-терапевтический подход позволяет достичь эффективной долгосрочной коррекции генетического дефекта, а с другой стороны – несет опасность побочных эффектов. Ряд работ по лечению тяжелого комбинированного иммунодефицита, сцепленного с Х-хромосомой, показал развитие у нескольких больных лимфопролиферативных заболеваний в связи с инсерционным мутагенезом. Такие побочные эффекты, наряду с иммуногенностью, передачей генетических последовательностей, а также токсичных и инфекционных побочных продуктов, связанных с приготовлением вектора, делает необходимой строгое регулирование их применения со стороны властей. Соответствующие правила могут быть определены лишь в общих понятиях, и нельзя создать единый документ, пригодный для конкретной цели и индивидуального использования каждого продукта.
Встраивающиеся векторы, дефектные по репликационным свойствам, основанные на гамма-ретровирусах, часто применялись в исходных протоколах генной терапии, и их опасность может обсуждаться в качестве примера, в частности, инсерционный мутагенез, который возникает во многом из-за случайного характера встраивания вируса по отношению к целевым генным локусам. Кроме того, могут быть вызваны и генотоксические эффекты, в основном связанные с дизрегуляцией активности нормальных генов при введении регуляторных элементов в составе вектора. Другой крупной проблемой вирусного переноса генов является их «вертикальный» перенос в зародышевых клеточных линиях, в частности, через пролиферирующие сперматогониальные клетки, которые могут быть мишенью для гамма-ретровирусных векторов.
Для того, чтобы упредить эти опасности, регулирующая система должна уточнить единообразные требования для того, чтобы обеспечить соответствия между стандартами качества и, тем самым, гарантировать безопасность участников испытания. Данная статья суммирует наиболее важные регулирующие документы, которые следует учитывать до вхождения в фазу клинической разработки – не только для Германии, но и в европейской перспективе. Приводятся ссылки на применимые для этого руководящие указания в отношении генно-терапевтических медицинских продуктов (ГТМП), с соответствующими определениями для таких продуктов.
Производство ГТМП должно согласовываться с требованиями Директивы Европейского Союза 2003/94/EC о качественной практике производства (GMP). В Германии эта практика регулируется Предписаниями по производству медицинских продуктов и активных фармацевтических ингредиентов (AMWHV). Важно, чтобы особые аспекты безопасности в отношении ГТМП учитывались до использования этих продуктов в клинике. В любом случае, регулирующие указания имеют в своей основе: (1) имеющиеся научные знания, (2) научный опыт в данной области исследований, и они возникают в результате постоянного взаимодействия между исследователями и экспертами в области регулирования.
Introduction
The young field of stem cell research leads to a change of paradigm in the modern medicinal world from a symptomatic to a causal treatment of previously untreatable diseases such as Parkinson’s disease, Diabetes mellitus, and heart failure. In 1998, the first human embryonic stem cell (hESC) lines were established in the USA [1]. It was in 2002 that the combination of somatic cell nuclear transfer (SCNT), ES cell derivation, and gene therapy in a mouse model provided indications for autologous treatment of immune deficiencies in the near future [2]. The reprogramming of adult somatic cells into pluripotent cells was achieved by 2006 [3], marking a milestone with fundamental implications on developmental dynamics, which also challenged the categorization of cells by potency. The first human-induced pluripotent stem cells (hiPSC) via transference of pluripotence-associated genes were produced in Japan [4]. Although hiPSC have already had a great impact on the development of therapeutic strategies, hESC remain the benchmark for characterization of pluripotency. A unifying problem for the research on hESC or hiPSC is the lack of standardized methods and criteria to characterize pluripotency, which are essential for comparing scientific results and controlling the risks in future clinical and diagnostic applications. A harmonized regulatory landscape of stem cell research would promote an environment for the efficient development of internationally accepted standards for pluripotent cells characterization and their donation, handling, and application.
In order to reach this goal it is necessary to make regulations and their current changes known and transparent to the stem cell research community, regulators, and stakeholders. hESCreg provides an efficient tool and central platform for the registration and documentation of human embryonic stem cell (hESC) lines being derived and available to the European Union and beyond [5, 6, 7, 8]. Currently, the registry holds information on more than 700 hESC-lines and several hiPSC-lines (see figure 1). hESCreg is coordinated by the Berlin-Brandenburg Center for Regenerative Therapies (BCRT) and the Center for Regenerative Medicine Barcelona, with the UK Stem Cell Bank as a lead partner. Community representatives from more than 15 countries act as National Contacts for hESCreg. These are Australia, Belgium, the Czech Republic, China, Denmark, Finland, France, India, Israel, the Netherlands, Spain, Sweden, Switzerland, Turkey, the United Kingdom, and the USA.
Table 1.
Regulatory Variations for Embryonic Stem Cell Research in National Contact countries of hESCreg. Whilst certain countries enable specific legislation on hESC research there may be an effective ban on such research in others. The three different colored sections in the table resemble the three different positions on hESC research in Europe and beyond. For a geographical overview please see Figure 1.
1creation of interspecies embryos allowed,
2law open to interpretation as regards SCNT,
3compensation for embryo or cell donation,
4not with federal funding as outlined in Dickey-Wicker.

Relevant legislation and its practical interpretation in selected countries
The following section describes the legal situation in countries where a) hESCreg has a National Contact representative, and b) which have been selected due to recent or upcoming changes in their hESC-specific legislation: Australia, China, India, Finland, the Netherlands, Norway, Turkey, UK, and the USA (see also Figure 1 and Table 1).
Figure 1.
The Status of hESC Research Legislation in National Contact countries of hESCreg. The three different colors resemble the three different positions on hESC research: For a detailed overview please refer to table 1. Dark green: hESC research and derivation of hESC lines from supernumerary IVF embryos permitted, SCNT permitted, Light green: hESC research and derivation of hESC lines from supernumerary IVF embryos permitted, SCNT prohibited, Red: hESC research permitted only with imported hESC lines, hESC derivation and SCNT prohibited. Number of hESC provided = number of hESC lines derived in the respective country.

Information on hESC legislation in other National Contact countries of hESCreg, such as Belgium, the Czech Republic, Denmark, France, Germany, Hungary, Israel, Italy, Portugal, Spain, Sweden, and Switzerland can be found in another of our recent publications [6]. In both articles we focus on regulatory issues such as pre-implantation genetic diagnosis (PGD), procurement of embryos for research, research on and derivation of hESC lines from a) supernumerary embryos coming from in-vitro fertilization (IVF) programs and that are no longer intended for clinical use, and b) embryos created for research, i.e. human embryos created by IVF with donated gametes and not intended to induce pregnancy, creation of embryos by SCNT, and the creation of interspecies embryos including cytoplasmic hybrid embryos and hiPSC.
The legislative bases for human embryo and hESC research in Australia are the Act on Prohibition of Human Cloning for Reproduction (2002), and the Act on Research Involving Human Embryos (2002), both being amended in December 2006 and effective from June 2007 on. They allow the creation of IVF and SCNT embryos as well as the derivation of hESC lines from these sources as well as research on hESC lines. PGD is allowed. Further regulatory changes are already expected in 2010.
In China the guidelines on human embryonic stem cell research from 2003, which were amended in March 2009, now allow the creation of supernumerary IVF and SCNT embryos for research. Moreover, PGD is permitted and the use of surplus PGD embryos and interspecies embryos are allowed.
Finland experienced the last amendment of the Medical Research Act 488 from 1999 in November 2009. The production of embryos for research purposes is prohibited. The legislative text is open to interpretation whether the creation of SCNT embryos is permitted or not, since the definition of an embryo refers to a “living group of cells resulting from fertilization”. The creation of an IVF embryo is not regulated in the law but the research on surplus IVF embryos is allowed. Regulation of the research on PGD embryos as well as the derivation of hESC lines from supernumerary PGD or IVF embryos is open to interpretation of the legislative text.
In India the DBT-ICMR guidelines regulate issues on stem cell research but have not become law yet. The creation of embryos by SCNT is allowed, this issue falls under “restricted areas of research” of the DBT-ICMR guidelines. The derivation of hESC lines from supernumerary IVF embryos is allowed, as is the research on embryos and hESC lines.
The Netherlands’ Embryo Act from 2002 was amended in 2007. The creation of embryos solely for research purposes is not allowed. The creation of IVF embryos for research purposes is not allowed. The derivation of hESC lines from supernumerary IVF and SCNT embryos for research is allowed if no other source than embryonic stem cells can be used as an alternative. PGD is allowed. Both, hESC line derivation and PGD must be applied at and approved by the CCMO (Central Committee for Research Involving Human Subjects). There are no further changes expected before 2012.
The Norwegian Biotechnology Act from 2007 was revised in 2008. It does not allow the creation of IVF embryos for research purposes. Research on supernumerary IVF embryos and PGD embryos is allowed. The derivation of hESC from supernumerary IVF embryos and SCNT for medical and biological research is allowed.
In Turkey the act “By-law on centers for in vitro fertilization and embryo transfer” concerning research on human embryos from 1987 was amended in 2005. IVF and PDG are legal in the country. hESC research is not regulated by a specific law. However, the Ministry of Health announced a 2005 memorandum stating that until specific guidelines are established, hESC derivation and research should not be performed in the country. Research with imported hESC lines may be performed. A new act is expected to be announced in 2010. For that, it is expected that hESC derivation for research purposes and hESC research will be regulated and allowed in certain qualified institutions and under certain conditions (such as lines with genetic diseases).
In the United Kingdom the Human Fertilisation and Embryology Act, which became law in 1990, was amended at the end of 2008 and legalizes the creation of embryos (including artificial techniques as SCNT) and the creation of interspecies embryos for research. The derivation of hESC lines from supernumerary IVF, SCNT and interspecies embryos are allowed. The definition of an embryo now includes embryos created by cloning and other processes.
In the USA there are different legal situations with respect to embryo and hESC research. The federal law concerning human embryo and embryonic stem cell (hESC) research is the Dickey-Wicker Amendment of 1995, which was again amended in 2009. The research on hESC lines and the derivation of hESC lines from IVF embryos, from embryos created by SCNT and from human admixed embryos is allowed but not for those projects with federal funding as outlined in the Dickey-Wicker Amendment. NIH-funded research is allowed with NIH-approved cell lines only. The NIH reviews information on informed consent and derivation for each line it registers. Non-federally funded research is reviewed by institutional review boards (IRB/SCRO). Creation of embryos for research must follow an IRB-approved protocol (45 CFR 46.107). In addition, IRB/SCRO committees tend to follow national guidelines such as those established by the National Academies of Science and the ISSCR.
Conclusion
Novel legislation and legislative changes need to take into account scientific progress and must be flexible enough to accommodate novel research results and the subsequent changes in the foundations of ethical views. It is unlikely that there will be a single universally accepted view on the ethical, legal, and moral status of the embryo as such, nor on the use of hESC lines derived thereof [5, 6]. But the recent advances in reprogramming show an enormous dynamic and developmental potential of almost any cell and thus question the basis for defining the embryo only as inherently totipotent. Totipotency is probably not inherent to cells derived from embryos and may become inducible by technical means from any differentiated adult cell, for example through the generation of sperm and oocytes from hiPSC. These questions are not yet or only partially reflected in legislative texts. Japan, for example, has prohibited the use of hiPSC for the creation of human gametes, embryos, and their implantation into animal or human wombs [9, 10]. It is therefore expected that the legislative and regulatory landscape will continue to change and that the pressure for international harmonization and transparency will increase.
An essential requirement is therefore the transparent information of what can be done with hiPSC and hESC. One of the tasks of the hESCreg is the reflection of international pluralism by providing information on each registered cell including its source and characteristics, as well as the ethical provenance [6, 7, 8]. This will be one aspect for safeguarding high standards in relation to ethical concerns and transparency in research. Consequently, the European Commission might use hESCreg as a central reference for all funding decisions on proposed hESC research projects within the Seventh EU Framework Programme.
Furthermore, hESCreg is also involved in providing information to the public about this highly sensitive field of research and its legal framework. By providing transparency an increased acceptance of this work is expected. Future tasks of the registry will include the establishment of a pluripotent stem cell registry at the international level, the inclusion of all types of pluripotent stem cells (hiPSC and others), the integration of a knowledge-service tool, the inclusion of banking service tools, the provision of an open and standardized common forum for exchange, and last but not least the development of guidelines for standards, governance, and ethical procurement of pluripotent stem cells.
References
4. Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081-9.
5. Cervera RP, Stojkovic M. Developments and Challenges in Human Embryonic Stem Cell Research in Spain. Stem Cell Rev Rep. 2009 Oct 3. [Epub ahead of print]
6. Elstner A, Damaschun A, Kurtz A, Stacey G, Arán B, Veiga A, Borstlap J. The changing landscape of European and international regulation on embryonic stem cell research. Stem Cell Res. 2009 Mar;2(2):101-7. Epub 2008 Nov 17.
7. Borstlap J, Stacey G, Kurtz A, Elstner A, Damaschun A, Arán B, Veiga A. First evaluation of the European hESCreg. Nat Biotechnol. 2008 Aug; 26(8):859-60.
8. Sipp, D., 2008. Q & A: Anna Veiga. Nat Med. 2008 Mar;14(3):234
10. Cyranoski D. Stem cells: 5 things to know before jumping on the iPS bandwagon. Nature 452: 406-408; 2008.
Acknowledgements
The authors gratefully acknowledge the representatives of the National Contacts of the Human European Stem Cell Registry — hESCreg — for their contributions, input and helpful discussions. Especially we would like to thank Claus Yding Andersen, Necati Findikli, Derek Hei, Maneesha Inamdar, Anna Michalska, Christine Mummery, Timo Otonkoski, Glyn Stacey Benjamin Reubinoff, Arne Sunde and Fanyi Zeng. Many thanks to Luis Huegel for his assistance in layout work.
" ["~DETAIL_TEXT"]=> string(16664) "Introduction
The young field of stem cell research leads to a change of paradigm in the modern medicinal world from a symptomatic to a causal treatment of previously untreatable diseases such as Parkinson’s disease, Diabetes mellitus, and heart failure. In 1998, the first human embryonic stem cell (hESC) lines were established in the USA [1]. It was in 2002 that the combination of somatic cell nuclear transfer (SCNT), ES cell derivation, and gene therapy in a mouse model provided indications for autologous treatment of immune deficiencies in the near future [2]. The reprogramming of adult somatic cells into pluripotent cells was achieved by 2006 [3], marking a milestone with fundamental implications on developmental dynamics, which also challenged the categorization of cells by potency. The first human-induced pluripotent stem cells (hiPSC) via transference of pluripotence-associated genes were produced in Japan [4]. Although hiPSC have already had a great impact on the development of therapeutic strategies, hESC remain the benchmark for characterization of pluripotency. A unifying problem for the research on hESC or hiPSC is the lack of standardized methods and criteria to characterize pluripotency, which are essential for comparing scientific results and controlling the risks in future clinical and diagnostic applications. A harmonized regulatory landscape of stem cell research would promote an environment for the efficient development of internationally accepted standards for pluripotent cells characterization and their donation, handling, and application.
In order to reach this goal it is necessary to make regulations and their current changes known and transparent to the stem cell research community, regulators, and stakeholders. hESCreg provides an efficient tool and central platform for the registration and documentation of human embryonic stem cell (hESC) lines being derived and available to the European Union and beyond [5, 6, 7, 8]. Currently, the registry holds information on more than 700 hESC-lines and several hiPSC-lines (see figure 1). hESCreg is coordinated by the Berlin-Brandenburg Center for Regenerative Therapies (BCRT) and the Center for Regenerative Medicine Barcelona, with the UK Stem Cell Bank as a lead partner. Community representatives from more than 15 countries act as National Contacts for hESCreg. These are Australia, Belgium, the Czech Republic, China, Denmark, Finland, France, India, Israel, the Netherlands, Spain, Sweden, Switzerland, Turkey, the United Kingdom, and the USA.
Table 1.
Regulatory Variations for Embryonic Stem Cell Research in National Contact countries of hESCreg. Whilst certain countries enable specific legislation on hESC research there may be an effective ban on such research in others. The three different colored sections in the table resemble the three different positions on hESC research in Europe and beyond. For a geographical overview please see Figure 1.
1creation of interspecies embryos allowed,
2law open to interpretation as regards SCNT,
3compensation for embryo or cell donation,
4not with federal funding as outlined in Dickey-Wicker.

Relevant legislation and its practical interpretation in selected countries
The following section describes the legal situation in countries where a) hESCreg has a National Contact representative, and b) which have been selected due to recent or upcoming changes in their hESC-specific legislation: Australia, China, India, Finland, the Netherlands, Norway, Turkey, UK, and the USA (see also Figure 1 and Table 1).
Figure 1.
The Status of hESC Research Legislation in National Contact countries of hESCreg. The three different colors resemble the three different positions on hESC research: For a detailed overview please refer to table 1. Dark green: hESC research and derivation of hESC lines from supernumerary IVF embryos permitted, SCNT permitted, Light green: hESC research and derivation of hESC lines from supernumerary IVF embryos permitted, SCNT prohibited, Red: hESC research permitted only with imported hESC lines, hESC derivation and SCNT prohibited. Number of hESC provided = number of hESC lines derived in the respective country.

Information on hESC legislation in other National Contact countries of hESCreg, such as Belgium, the Czech Republic, Denmark, France, Germany, Hungary, Israel, Italy, Portugal, Spain, Sweden, and Switzerland can be found in another of our recent publications [6]. In both articles we focus on regulatory issues such as pre-implantation genetic diagnosis (PGD), procurement of embryos for research, research on and derivation of hESC lines from a) supernumerary embryos coming from in-vitro fertilization (IVF) programs and that are no longer intended for clinical use, and b) embryos created for research, i.e. human embryos created by IVF with donated gametes and not intended to induce pregnancy, creation of embryos by SCNT, and the creation of interspecies embryos including cytoplasmic hybrid embryos and hiPSC.
The legislative bases for human embryo and hESC research in Australia are the Act on Prohibition of Human Cloning for Reproduction (2002), and the Act on Research Involving Human Embryos (2002), both being amended in December 2006 and effective from June 2007 on. They allow the creation of IVF and SCNT embryos as well as the derivation of hESC lines from these sources as well as research on hESC lines. PGD is allowed. Further regulatory changes are already expected in 2010.
In China the guidelines on human embryonic stem cell research from 2003, which were amended in March 2009, now allow the creation of supernumerary IVF and SCNT embryos for research. Moreover, PGD is permitted and the use of surplus PGD embryos and interspecies embryos are allowed.
Finland experienced the last amendment of the Medical Research Act 488 from 1999 in November 2009. The production of embryos for research purposes is prohibited. The legislative text is open to interpretation whether the creation of SCNT embryos is permitted or not, since the definition of an embryo refers to a “living group of cells resulting from fertilization”. The creation of an IVF embryo is not regulated in the law but the research on surplus IVF embryos is allowed. Regulation of the research on PGD embryos as well as the derivation of hESC lines from supernumerary PGD or IVF embryos is open to interpretation of the legislative text.
In India the DBT-ICMR guidelines regulate issues on stem cell research but have not become law yet. The creation of embryos by SCNT is allowed, this issue falls under “restricted areas of research” of the DBT-ICMR guidelines. The derivation of hESC lines from supernumerary IVF embryos is allowed, as is the research on embryos and hESC lines.
The Netherlands’ Embryo Act from 2002 was amended in 2007. The creation of embryos solely for research purposes is not allowed. The creation of IVF embryos for research purposes is not allowed. The derivation of hESC lines from supernumerary IVF and SCNT embryos for research is allowed if no other source than embryonic stem cells can be used as an alternative. PGD is allowed. Both, hESC line derivation and PGD must be applied at and approved by the CCMO (Central Committee for Research Involving Human Subjects). There are no further changes expected before 2012.
The Norwegian Biotechnology Act from 2007 was revised in 2008. It does not allow the creation of IVF embryos for research purposes. Research on supernumerary IVF embryos and PGD embryos is allowed. The derivation of hESC from supernumerary IVF embryos and SCNT for medical and biological research is allowed.
In Turkey the act “By-law on centers for in vitro fertilization and embryo transfer” concerning research on human embryos from 1987 was amended in 2005. IVF and PDG are legal in the country. hESC research is not regulated by a specific law. However, the Ministry of Health announced a 2005 memorandum stating that until specific guidelines are established, hESC derivation and research should not be performed in the country. Research with imported hESC lines may be performed. A new act is expected to be announced in 2010. For that, it is expected that hESC derivation for research purposes and hESC research will be regulated and allowed in certain qualified institutions and under certain conditions (such as lines with genetic diseases).
In the United Kingdom the Human Fertilisation and Embryology Act, which became law in 1990, was amended at the end of 2008 and legalizes the creation of embryos (including artificial techniques as SCNT) and the creation of interspecies embryos for research. The derivation of hESC lines from supernumerary IVF, SCNT and interspecies embryos are allowed. The definition of an embryo now includes embryos created by cloning and other processes.
In the USA there are different legal situations with respect to embryo and hESC research. The federal law concerning human embryo and embryonic stem cell (hESC) research is the Dickey-Wicker Amendment of 1995, which was again amended in 2009. The research on hESC lines and the derivation of hESC lines from IVF embryos, from embryos created by SCNT and from human admixed embryos is allowed but not for those projects with federal funding as outlined in the Dickey-Wicker Amendment. NIH-funded research is allowed with NIH-approved cell lines only. The NIH reviews information on informed consent and derivation for each line it registers. Non-federally funded research is reviewed by institutional review boards (IRB/SCRO). Creation of embryos for research must follow an IRB-approved protocol (45 CFR 46.107). In addition, IRB/SCRO committees tend to follow national guidelines such as those established by the National Academies of Science and the ISSCR.
Conclusion
Novel legislation and legislative changes need to take into account scientific progress and must be flexible enough to accommodate novel research results and the subsequent changes in the foundations of ethical views. It is unlikely that there will be a single universally accepted view on the ethical, legal, and moral status of the embryo as such, nor on the use of hESC lines derived thereof [5, 6]. But the recent advances in reprogramming show an enormous dynamic and developmental potential of almost any cell and thus question the basis for defining the embryo only as inherently totipotent. Totipotency is probably not inherent to cells derived from embryos and may become inducible by technical means from any differentiated adult cell, for example through the generation of sperm and oocytes from hiPSC. These questions are not yet or only partially reflected in legislative texts. Japan, for example, has prohibited the use of hiPSC for the creation of human gametes, embryos, and their implantation into animal or human wombs [9, 10]. It is therefore expected that the legislative and regulatory landscape will continue to change and that the pressure for international harmonization and transparency will increase.
An essential requirement is therefore the transparent information of what can be done with hiPSC and hESC. One of the tasks of the hESCreg is the reflection of international pluralism by providing information on each registered cell including its source and characteristics, as well as the ethical provenance [6, 7, 8]. This will be one aspect for safeguarding high standards in relation to ethical concerns and transparency in research. Consequently, the European Commission might use hESCreg as a central reference for all funding decisions on proposed hESC research projects within the Seventh EU Framework Programme.
Furthermore, hESCreg is also involved in providing information to the public about this highly sensitive field of research and its legal framework. By providing transparency an increased acceptance of this work is expected. Future tasks of the registry will include the establishment of a pluripotent stem cell registry at the international level, the inclusion of all types of pluripotent stem cells (hiPSC and others), the integration of a knowledge-service tool, the inclusion of banking service tools, the provision of an open and standardized common forum for exchange, and last but not least the development of guidelines for standards, governance, and ethical procurement of pluripotent stem cells.
References
4. Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081-9.
5. Cervera RP, Stojkovic M. Developments and Challenges in Human Embryonic Stem Cell Research in Spain. Stem Cell Rev Rep. 2009 Oct 3. [Epub ahead of print]
6. Elstner A, Damaschun A, Kurtz A, Stacey G, Arán B, Veiga A, Borstlap J. The changing landscape of European and international regulation on embryonic stem cell research. Stem Cell Res. 2009 Mar;2(2):101-7. Epub 2008 Nov 17.
7. Borstlap J, Stacey G, Kurtz A, Elstner A, Damaschun A, Arán B, Veiga A. First evaluation of the European hESCreg. Nat Biotechnol. 2008 Aug; 26(8):859-60.
8. Sipp, D., 2008. Q & A: Anna Veiga. Nat Med. 2008 Mar;14(3):234
10. Cyranoski D. Stem cells: 5 things to know before jumping on the iPS bandwagon. Nature 452: 406-408; 2008.
Acknowledgements
The authors gratefully acknowledge the representatives of the National Contacts of the Human European Stem Cell Registry — hESCreg — for their contributions, input and helpful discussions. Especially we would like to thank Claus Yding Andersen, Necati Findikli, Derek Hei, Maneesha Inamdar, Anna Michalska, Christine Mummery, Timo Otonkoski, Glyn Stacey Benjamin Reubinoff, Arne Sunde and Fanyi Zeng. Many thanks to Luis Huegel for his assistance in layout work.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(3) "500" ["~SORT"]=> string(3) "500" ["CODE"]=> string(100) "tekushchaya-dinamika-pravovogo-regulirovaniya-issledovaniy-embrionalnykh-stvolovykh-kletok-cheloveka" ["~CODE"]=> string(100) "tekushchaya-dinamika-pravovogo-regulirovaniya-issledovaniy-embrionalnykh-stvolovykh-kletok-cheloveka" ["EXTERNAL_ID"]=> string(3) "961" ["~EXTERNAL_ID"]=> string(3) "961" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["ELEMENT_META_KEYWORDS"]=> string(144) "эмбриональные стволовые клетки человек законодательство Европейский регистр" ["ELEMENT_META_DESCRIPTION"]=> string(260) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человекаOngoing dynamics in the regulatory landscape of human embryonic stem cell research" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2890) "<p class="bodytext">Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, <a href="http://www.hescreg.eu/" target="_blank">www.hescreg.eu</a>), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_META_TITLE"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_META_KEYWORDS"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_META_DESCRIPTION"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_PICTURE_FILE_ALT"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_PICTURE_FILE_TITLE"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "tekushchaya-dinamika-pravovogo-regulirovaniya-issledovaniy-embrionalnykh-stvolovykh-kletok-cheloveka" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(178) "Текущая динамика правового регулирования исследований эмбриональных стволовых клеток человека" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "tekushchaya-dinamika-pravovogo-regulirovaniya-issledovaniy-embrionalnykh-stvolovykh-kletok-cheloveka" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "tekushchaya-dinamika-pravovogo-regulirovaniya-issledovaniy-embrionalnykh-stvolovykh-kletok-cheloveka" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "tekushchaya-dinamika-pravovogo-regulirovaniya-issledovaniy-embrionalnykh-stvolovykh-kletok-cheloveka" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(2) "44" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(4) { [0]=> string(5) "13670" [1]=> string(5) "13671" [2]=> string(5) "13672" [3]=> string(5) "13673" } ["VALUE"]=> array(4) { [0]=> string(3) "968" [1]=> string(3) "969" [2]=> string(3) "970" [3]=> string(3) "971" } ["DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(4) { [0]=> string(3) "968" [1]=> string(3) "969" [2]=> string(3) "970" [3]=> string(3) "971" } ["~DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13611" ["VALUE"]=> string(22) "12/02/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/02/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13612" ["VALUE"]=> string(22) "12/07/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/07/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13613" ["VALUE"]=> string(22) "12/30/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/30/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13569" ["VALUE"]=> string(3) "958" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "958" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(9) { [0]=> string(5) "13674" [1]=> string(5) "13675" [2]=> string(5) "13676" [3]=> string(5) "13677" [4]=> string(5) "13678" [5]=> string(5) "13679" [6]=> string(5) "13680" [7]=> string(5) "13681" [8]=> string(5) "13682" } ["VALUE"]=> array(9) { [0]=> string(3) "958" [1]=> string(3) "959" [2]=> string(3) "960" [3]=> string(3) "962" [4]=> string(3) "963" [5]=> string(3) "964" [6]=> string(3) "965" [7]=> string(3) "966" [8]=> string(3) "967" } ["DESCRIPTION"]=> array(9) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" [6]=> string(0) "" [7]=> string(0) "" [8]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(9) { [0]=> string(3) "958" [1]=> string(3) "959" [2]=> string(3) "960" [3]=> string(3) "962" [4]=> string(3) "963" [5]=> string(3) "964" [6]=> string(3) "965" [7]=> string(3) "966" [8]=> string(3) "967" } ["~DESCRIPTION"]=> array(9) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" [6]=> string(0) "" [7]=> string(0) "" [8]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13650" ["VALUE"]=> array(2) { ["TEXT"]=> string(340) "<p>Аня Эльстнер*, Аннетт Ноак*, Александр Дамашун, Глин Стэйси, Бегонья Аран, Илона Гавронска, Анна Вейга, <br>Йори Борстлап, Андреас Куртц</p><p>* Вклад обоих авторов одинаков</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(310) "Аня Эльстнер*, Аннетт Ноак*, Александр Дамашун, Глин Стэйси, Бегонья Аран, Илона Гавронска, Анна Вейга,
Йори Борстлап, Андреас Куртц
* Вклад обоих авторов одинаков
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13651" ["VALUE"]=> array(2) { ["TEXT"]=> string(2890) "<p class="bodytext">Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, <a href="http://www.hescreg.eu/" target="_blank">www.hescreg.eu</a>), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2836) "Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, www.hescreg.eu), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13573" ["VALUE"]=> string(29) "10.3205/ctt-2009-en-000052.01" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(29) "10.3205/ctt-2009-en-000052.01" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13665" ["VALUE"]=> array(2) { ["TEXT"]=> string(470) "<p>Anja Elstner*<sup>1</sup>, Annette Noack*<sup>1</sup>, Alexander Damaschun<sup>1</sup>, Glyn Stacey<sup>2</sup>, Begoňa Arán<sup>3</sup>, Ilona Gawronska<sup>1</sup>, Anna Veiga<sup>3,4</sup>, <br>Joeri Borstlap<sup>1</sup> and Andreas Kurtz<sup>1</sup></p> <p class="bodytext">* Both authors contributed equally</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(322) "Anja Elstner*1, Annette Noack*1, Alexander Damaschun1, Glyn Stacey2, Begoňa Arán3, Ilona Gawronska1, Anna Veiga3,4,
Joeri Borstlap1 and Andreas Kurtz1
* Both authors contributed equally
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13666" ["VALUE"]=> array(2) { ["TEXT"]=> string(464) "<p><sup>1</sup>Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; <sup>2</sup>The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; <sup>3</sup>Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); <sup>4</sup>Institut Universitari Dexeus, Barcelona, Spain </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(404) "1Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; 2The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; 3Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); 4Institut Universitari Dexeus, Barcelona, Spain
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_EN"]=> array(36) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13667" ["VALUE"]=> array(2) { ["TEXT"]=> string(1400) "<p class="bodytext">The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1378) "The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13574" ["VALUE"]=> string(82) "Ongoing dynamics in the regulatory landscape of human embryonic stem cell research" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(82) "Ongoing dynamics in the regulatory landscape of human embryonic stem cell research" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13668" ["VALUE"]=> string(3) "656" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "656" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13669" ["VALUE"]=> string(3) "657" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "657" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(13) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13665" ["VALUE"]=> array(2) { ["TEXT"]=> string(470) "<p>Anja Elstner*<sup>1</sup>, Annette Noack*<sup>1</sup>, Alexander Damaschun<sup>1</sup>, Glyn Stacey<sup>2</sup>, Begoňa Arán<sup>3</sup>, Ilona Gawronska<sup>1</sup>, Anna Veiga<sup>3,4</sup>, <br>Joeri Borstlap<sup>1</sup> and Andreas Kurtz<sup>1</sup></p> <p class="bodytext">* Both authors contributed equally</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(322) "Anja Elstner*1, Annette Noack*1, Alexander Damaschun1, Glyn Stacey2, Begoňa Arán3, Ilona Gawronska1, Anna Veiga3,4,
Joeri Borstlap1 and Andreas Kurtz1
* Both authors contributed equally
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(322) "Anja Elstner*1, Annette Noack*1, Alexander Damaschun1, Glyn Stacey2, Begoňa Arán3, Ilona Gawronska1, Anna Veiga3,4,
Joeri Borstlap1 and Andreas Kurtz1
* Both authors contributed equally
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13667" ["VALUE"]=> array(2) { ["TEXT"]=> string(1400) "<p class="bodytext">The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1378) "The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1378) "The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13573" ["VALUE"]=> string(29) "10.3205/ctt-2009-en-000052.01" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(29) "10.3205/ctt-2009-en-000052.01" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(29) "10.3205/ctt-2009-en-000052.01" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13574" ["VALUE"]=> string(82) "Ongoing dynamics in the regulatory landscape of human embryonic stem cell research" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(82) "Ongoing dynamics in the regulatory landscape of human embryonic stem cell research" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(82) "Ongoing dynamics in the regulatory landscape of human embryonic stem cell research" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13666" ["VALUE"]=> array(2) { ["TEXT"]=> string(464) "<p><sup>1</sup>Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; <sup>2</sup>The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; <sup>3</sup>Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); <sup>4</sup>Institut Universitari Dexeus, Barcelona, Spain </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(404) "1Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; 2The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; 3Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); 4Institut Universitari Dexeus, Barcelona, Spain
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Organization" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(404) "1Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; 2The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; 3Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); 4Institut Universitari Dexeus, Barcelona, Spain
" } ["AUTHORS"]=> array(38) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(9) { [0]=> string(5) "13674" [1]=> string(5) "13675" [2]=> string(5) "13676" [3]=> string(5) "13677" [4]=> string(5) "13678" [5]=> string(5) "13679" [6]=> string(5) "13680" [7]=> string(5) "13681" [8]=> string(5) "13682" } ["VALUE"]=> array(9) { [0]=> string(3) "958" [1]=> string(3) "959" [2]=> string(3) "960" [3]=> string(3) "962" [4]=> string(3) "963" [5]=> string(3) "964" [6]=> string(3) "965" [7]=> string(3) "966" [8]=> string(3) "967" } ["DESCRIPTION"]=> array(9) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" [6]=> string(0) "" [7]=> string(0) "" [8]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(9) { [0]=> string(3) "958" [1]=> string(3) "959" [2]=> string(3) "960" [3]=> string(3) "962" [4]=> string(3) "963" [5]=> string(3) "964" [6]=> string(3) "965" [7]=> string(3) "966" [8]=> string(3) "967" } ["~DESCRIPTION"]=> array(9) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" [6]=> string(0) "" [7]=> string(0) "" [8]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(9) { [0]=> string(55) "Anja Elstner" [1]=> string(56) "Annette Noack" [2]=> string(62) "Alexander Damaschun" [3]=> string(54) "Glyn Stacey" [4]=> string(56) "Begoňa Arán" [5]=> string(58) "Ilona Gawronska" [6]=> string(53) "Anna Veiga" [7]=> string(57) "Joeri Borstlap" [8]=> string(56) "Andreas Kurtz" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["AUTHOR_RU"]=> array(37) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13650" ["VALUE"]=> array(2) { ["TEXT"]=> string(340) "<p>Аня Эльстнер*, Аннетт Ноак*, Александр Дамашун, Глин Стэйси, Бегонья Аран, Илона Гавронска, Анна Вейга, <br>Йори Борстлап, Андреас Куртц</p><p>* Вклад обоих авторов одинаков</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(310) "Аня Эльстнер*, Аннетт Ноак*, Александр Дамашун, Глин Стэйси, Бегонья Аран, Илона Гавронска, Анна Вейга,
Йори Борстлап, Андреас Куртц
* Вклад обоих авторов одинаков
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(310) "Аня Эльстнер*, Аннетт Ноак*, Александр Дамашун, Глин Стэйси, Бегонья Аран, Илона Гавронска, Анна Вейга,
Йори Борстлап, Андреас Куртц
* Вклад обоих авторов одинаков
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13611" ["VALUE"]=> string(22) "12/02/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/02/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/02/2009 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13612" ["VALUE"]=> string(22) "12/07/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/07/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/07/2009 12:00:00 am" } ["PUBLISHED"]=> array(37) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13613" ["VALUE"]=> string(22) "12/30/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/30/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/30/2009 12:00:00 am" } ["KEYWORDS"]=> array(38) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(4) { [0]=> string(5) "13670" [1]=> string(5) "13671" [2]=> string(5) "13672" [3]=> string(5) "13673" } ["VALUE"]=> array(4) { [0]=> string(3) "968" [1]=> string(3) "969" [2]=> string(3) "970" [3]=> string(3) "971" } ["DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(4) { [0]=> string(3) "968" [1]=> string(3) "969" [2]=> string(3) "970" [3]=> string(3) "971" } ["~DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(4) { [0]=> string(102) "эмбриональные стволовые клетки" [1]=> string(58) "человек" [2]=> string(76) "законодательство" [3]=> string(81) "Европейский регистр" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["CONTACT"]=> array(38) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13569" ["VALUE"]=> string(3) "958" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "958" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(55) "Anja Elstner" ["LINK_ELEMENT_VALUE"]=> bool(false) } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13651" ["VALUE"]=> array(2) { ["TEXT"]=> string(2890) "<p class="bodytext">Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, <a href="http://www.hescreg.eu/" target="_blank">www.hescreg.eu</a>), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2836) "Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, www.hescreg.eu), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2836) "Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, www.hescreg.eu), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.
" } } } [2]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(2) "44" ["~IBLOCK_SECTION_ID"]=> string(2) "44" ["ID"]=> string(3) "974" ["~ID"]=> string(3) "974" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["~NAME"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "06/23/2017 03:59:48 pm" ["~TIMESTAMP_X"]=> string(22) "06/23/2017 03:59:48 pm" ["DETAIL_PAGE_URL"]=> string(133) "/en/archive/tom-1-nomer-4/stati/autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss/" ["~DETAIL_PAGE_URL"]=> string(133) "/en/archive/tom-1-nomer-4/stati/autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(45767) "Introduction
Multiple studies have been performed investigating the kinetics, safety and efficiency of mesenchymal cell isolation, characterization, culture-expanding, and clinical approaches [26, 10, 1, 31, 14, 18, 21, 27, 28]. In recent years the biological activity of MSC has been very actively discussed for their potential use for suppression of autoimmune and transplant-related reactions, but also with the aim of speeding up of hematopoiesis recovery after bone marrow transplantation [15, 16, 29, 24]. Many existing protocols suppose the use of autologous MSCs [23, 9]. However, the use of autologous MSCs may be limited in a clinical setting, due to imperfect and often controversial knowledge of the functional competence and integrity of immunosuppressive and hematopoietic-supporting potentials of these cells [19, 5, 8, 4]. As shown earlier, bone marrow-derived mesenchymal stromal stem cells provide support for hematopoiesis in vitro and in experimental animal models [3, 15, 16, 24]. In this study we compared the morphology, immunophenotype, and immunoregulatory activities of MSCs derived from the bone marrow of healthy donors and patients with hematological malignancies. Our results demonstrated that MSCs derived from bone marrow suffering from hemoblastoses can be successfully expanded in culture and infused along with PBSCs after high-dose chemotherapy without any toxicity. Moreover, one of the promising results is the decrease in the period of critical neutropenia and thrombocytopenia in the patient after high-doses chemotherapy, when PBSCs and MSCs are co-transplanted. Our results show that co-transplantation of MSCs is associated with rapid hematopoietic recovery when PBSC counts are suboptimal.
Patients and methods
From April 2005 through June 2007, after obtaining written informed consent, 39 patients who were eligible for high-dose chemotherapy and PBSC transplantation were enrolled into single center investigation of the feasibility, safety, and hematopoietic effects of autologous culture-expanded MSCs (Table 1). The clinical trial protocol and the consent form were approved by the Local Ethics committee of the Institute of Clinical Immunology SB RAMS.
Table 1. Characteristics of patients who were treated with (n=39) and without (n=42) mesenchymal stem cells
|
Control |
MSC supported |
|
|---|---|---|
|
Median age, years (range) |
32 (17–57) |
31 (7–55) |
|
Gender (male/female) |
16/23 |
22/21 |
|
Diagnoses |
||
|
HD |
CR 4 |
CR 0 |
|
NHL |
CR 4 |
CR 4 |
|
MM |
CR 2 |
CR 0 |
|
AML (1st remission) |
2 |
11 |
|
ALL (2nd remission) |
4 |
4 |
|
SLE (SLEDAI >16) |
3 |
2 |
|
Conditioning regimens |
BEAM for HD and NHL; |
|
|
Mean dose of CD34+/CD45+ cells |
5.57/kgx106(1.81–9.5x106/kg) |
5.64/kgx106(2.5–8.7x106/kg) |
|
Mean dose of MSCs |
-- |
6.64x106 (0.8–23.0x106) |
Patients with lymphomas and leukemia were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1 and were required to have adequate visceral organ function, including a left ventricular ejection fraction of at least 50%, forced expiratory volume in 1 second, serum direct bilirubin less than 2.0 mg/dl, and an actual or calculated creatinine clearance greater than 60 ml/min. Also all patients with hematological diseases underwent restaging evaluation with blood count, biochemical activity, trepanobiopsy, and computed tomography.
At the start of therapy, a neutrophil count greater than 1.2x106/ml and a platelet count greater than 100x106/ml were required. Patients were excluded if they had bone marrow involvement or active infection. Patients were excluded for evidence of tumor on routine histological staining of bilateral paraffin-embedded posterior iliac crest bone marrow biopsy specimens. In the “refractory group”, the patient was required to have no effects after standard chemotherapy.
SLE patient eligibility depended on a refractory to standard immunosuppression therapies and either organ- or life-threatening visceral involvement. The inclusion criteria were: not controlled with pulse therapy Cy glomerulonephritis (World Health Organization (WHO) class III–IV), central nervous system (CNS) lupus, vasculitis involving the lung or heart, or transfusion-dependent autoimmune cytopenias. Evaluation of eligible patients by two independent rheumatologists and transplant physicians, informed consent, and approval by the ethical committee were part of the protocol of Tyndall et al. [29]
High-Dose Chemotherapy and PBSC Infusion
The PBSC mobilization regimen consisted of Cyclophosphamide 4.0 g/m2 IV infusion over 6 hours on day 1, along with Mesna (first 3.0 g/m2 IV, then 500 mg every 3 hours orally/IV for eight doses). On day 7 after completion of the Cyclophosphamide, patients began subcutaneous injections of recombinant human G-CSF (Neupogen, Granocyte, Leukostim) 10 mg/kg/d. On recovery of neutrophils to a level greater than 1.2x109/l (usually 12 to 15 days after Cyclophosphamide treatment), patients underwent a leukapheresis procedure using AS TEC 204 (Fresenius, Germany) or Spectra LRS 07 (COBE, Lakewood, CO) apheresis equipment. Cells were cryopreserved using a controlled-rate liquid nitrogen freezer using previously published methods [10]. After PBSC procurement, high-dose chemotherapy with BEAM (BCNU 300 mg/m2 on day -7, Etoposid 800 mg/m2 and Ara-C 800 mg/m2 on days -6, -5, -4, -3, Melphalan 120 mg/m2 on day -2) for NHL and HD patients, Alceran (200 mg/m2 on day -2) for patients with AL, Cy (200-140 mg/kg in divided doses for 4 days -6, -5, -4, -3 with/or not ATG (total doses 60 mg/kg) for patients who suffered from SLE were administered. PBSCs were thawed and infused 24 hours after the completion of high-dose chemotherapy.
Phenotypic analysis of mobilized cells (expression of CD 34 and CD45) was performed using multicolor flow cytometry (FACSCalibur, Becton Dickinson). Moreover, cancer cell contamination of every PBSC specimen was excluded by the use of cytological analysis.
Supportive Care
Before, during, and for 24 hours after treatment with high dose Cyclophosphamide, patients received hyperhydration with forced diuresis and Urometexan (Mesna) infusion for the prevention of hemorrhagic cystitis. Patients were treated in a single room without air filtration. All patients followed a standardized supportive care protocol including antiemetic therapy, analgesia for mucositis, transfusion support, and venoocclusive disease prophylaxis. A low microbial diet, oral daily Fluoroquinolone (1 g/d) changed to intravenous Cefepime on neutropenic fever, Fluconazole (400 mg/d) and Acyclovir (10 mg/kg/d) and aerosolized Amphotericin B (10 mg twice daily) were started upon admission and discontinued when the ANC rebounded to 0.5x109/l. Platelets irradiated with 25 Gy and red blood cells were given to maintain a platelet count greater than 20 000/μl and a hemoglobin level greater than 8.0 g/dl. For the first 6 months after transplantation, patients were treated orally with daily oral Fluconazole and Trimethoprim/Sulfamethoxazole three times a week.
Harvesting and Ex Vivo MSC Culture
A median of 50 ml of bone marrow aspirate was obtained under sterile conditions by puncture of posterior iliac crests of patients or donors (n=9) under local anesthesia during standard pre-transplant staging approximately 2 days before high-dose Cyclophosphamide mobilization and 30 days before scheduled PBSC infusion. Washed heparinized bone marrow cells were re-suspended in phosphate-buffered solution (PBS, Sigma-Aldrich, Germany) and overlaid on Ficoll density gradient (1.078 g/l), then centrifuged for 20 min at 1000 g. A median of 320x106 (range 250–600 x106) mononuclear cells (MNC) were collected from the interface, washed three times in PBS, and re-suspended in α-modified minimum essential medium (αMEM, Sigma-Aldrich, Germany) containing 100 μg/ml gentamycin and 5% human platelet lysate (PL). After the cell number was counted, 30 ml of cell suspension was plated in a 175 cm2 plastic culture flask (Nunc, Denmark).
It is important to note that for clinical use with a therapeutic intent we used 5% platelet lysate to replace fetal calf serum (FCS) in the MSC culture. Platelet lysate was obtained from several allogeneic platelet units prepared by machine trombocytaphereis using AS TEC 204 (Fresenius, Germany). The platelet units were frozen at -40 C, thawed, mixed, aliquoted and lysate frozen at -20 C until use.
MSCs were cultured in humidified incubators with 5% CO2 and initially allowed to adhere for 72 hours, followed by a media change every 3 days. When cultures reached more than 90% confluence, adherent cells were detached with 0.05% trypsin-EDTA (Sigma-Aldrich, Germany) and replated at a density of 0.8x106 per 175 cm2 flask until processing for cryopreservation. Harvested MSCs from 1-2 passages were cryopreserved with a rate-controlled freezer (Planer Kryo 560-16) in 10% human Albumin solution (Microgen, Russia) and in a final concentration of 10% Dimethylsulfoxide (Sigma-Aldrich, Germany) and 10% Hydroxyethylstarch in freezing bags.
Cell cultures were tested for sterility weekly and before cryopreservation (Municipal Hospitals N1, Microbiology Laboratory, Novosibirsk) for the presence of bacterial/fungal contamination via microbiological cultural tests. All cell manipulations were performed in a sterile class II biological safety cabinet.
The number of MSCs was estimated by the quantity of colony forming unit-fibroblasts (CFU-F). For that, 106 of bone marrow MNCs were cultivated in Petri dishes for 14 days and stained using Giemsa protocol, then the number of spindle-shaped cell colonies consisting of more than 50 cells were counted.
Flow Cytometry
Phenotypic analysis of MSCs (expression of CD3, CD14, CD16, CD20, CD34, CD73, CD90, CD105 and HLA-DR molecules) was performed using multicolor flow cytometry (FACSCalibur, Becton Dickinson). Cells were detached with 0.05% trypsin-EDTA (Sigma, USA), washed with PBS plus 2% bovine albumin, fixed in 1% paraformaldehyde, blocked with 10% normal goat serum, and incubated separately with appropriate primary antibodies (BD Bioscience, USA). Non-treated MSCs were used as negative control.
The immunosuppressive properties of MSCs
To study MSC effects on immune cell function, MSCs were first cultivated for 24 h in flat-bottomed 96-well or 24-well plates in α-MEM/20% fetal calf serum (FCS, Gibco, USA). After this, peripheral blood MNCs (PBMNCs) from donors were added to MSC monolayer and stimulated with mitogens or alloantigens (mixed lymphocyte culture, MLC). For MLC, 0.1x106 PBMNCs from two donors were cultivated for 5 days in RPMI-1640 medium supplemented with 0.3 mg/ml L-glutamine, 100 μg/ml Gentamycine and 10% of heat-inactivated donor AB (IV) serum. Mitogen-induced proliferative response was studied in 3-day cultures of donor PBMNCs, which were activated by Concanavalin A (ConA, 15 μg/ml, Sigma-Aldrich, Germany) or 1 μg/ml monoclonal antibodies against CD3 (anti-CD3, Becton Dickinson, USA). Cell proliferation was measured by incorporation of 3H-thymidine that was added at 1 μCi/well during the last 18 h of cultivation. Proliferative response of MNCs cultivated without MSCs was used as control.
Support for hematopoiesis by MSC (CFU Assay)
To estimate MSCs' effect on colony-forming activity of hematopoietic progenitor cells, bone marrow MNC were grown in “complete” methylcellulose medium (Methocult H4344, Stem Cell Technologies, Vancouver, BC), containing hSCF, hGM-CSF, hIL-3, and hEPO at a density of 25x105/ml. Autologous MSC were added to MNCs in MNC: MSC ratios of 1:1 and 10:1 in triplicates. Cultures were grown at 37°C, 5% CO2 for 14 days, then the numbers of erythroid- and granulocyte-macrophage colony-forming units (CFU-E and CFU-GM, respectively) were calculated. Colonies were considered if consisted of at least 50 cells.
MSC Infusion
On the day of infusion, cryopreserved units were thawed ex tempore in a 37°C water bath, washed twice with PBS, transferred into 60-ml syringes with 10% human Albumin and infused into patients one hour after the PBSC infusion through a side port of a running 0.9% saline IV infusion into a central catheter over 10 to 20 minutes. Patients were premedicated with Acetaminophen (650 mg) and Diphenhydramine. Vital and clinical signs and symptoms were monitored at the time of infusion and every 15 minutes thereafter for 3 hours, followed by every 2 hours for 6 hours and every 8 hours for 3 days. There was no immediate or delayed toxicity related to MSC infusion. None of the patients experienced allergic reactions or respiratory symptoms.
Statistical analysis
Statistica 6.0 software for Windows, StatSoft Inc. USA was used for basic descriptive, and correlation analysis of data. The statistical significance was assessed with the Mann-Whitney U-test. The T-test was used to analyze the difference in means when comparing two groups. In assessing correlation, we used 2D scatterplots estimated correlation coefficient r and P-value for Rank Order Correlation Spearmen test. A p-value of less than 0.05 was considered statistically significant.
Results
MSC characteristics
At the first stage we compared the quantity of MSCs in bone marrow of patients with hemoblastosis and healthy donors. Since MSCs were first described as fibroblast-like cells capable of forming colonies 13], the initial content of MSC precursors among bone marrow MNCs was measured as a colony-forming unit fibroblast (CFU-F) number. The mean number of CFU-F in the control group was 36±3 colonies per 106 bone marrow-derived MNCs (min-max range 16–70). The CFU-F number in patients with hemoblastosis was 21±3 (p<0.05). The range of values in the patient group widely varied (from 1 to 67 CFU-F), and 70% of samples showed CFU-F numbers that were below the lower quartile value of control group (<25 CFU-F). The most significant decrease of CFU-F number was revealed in patients with NHL (16±5, p<0.01). In patients with MM and HL number of MSC precursors was also decreased (19±10 and 26±7, respectively; p>0.05). Decreasing CFU-F numbers mean that MSC progenitors in bone marrow of investigated patients are present at a lower amount.
Taking into consideration the MSC deficiency in patients with hemoblastosis, it seemed important to evaluate their ability for expansion in vitro. With that aim we analyzed the growth rate of MSC by measuring cultivation time until confluence. In healthy volunteers, confluent growth in MSC cultures was reached by day 15±0.5 (range 11–22 days). Meanwhile, in the patient group 80–90% confluence took on average 26±2 days (range 10–50); 23±3 days in patients with HL (p<0.01); 29±3.5 days in patients with NHL (p< 0.01), and 30±4 days in patients with MM (p<0.01). It seems to be important that despite a low CFU-F number in patient cultures it was possible to isolate and in vitro expand MSCs from patients with hematological malignancies.
MSC immunophenotype
Further, we characterized the immunophenotype of MSCs. Classically, MSCs are defined as cells expressing STRO-1, CD105 (SH2), CD71, CD73 (SH3, SH4), CD90 (Thy1) surface markers and not expressing lineage (CD3, CD14, CD20, CD16) and hematopoietic (CD34, CD45) markers along with HLA-DR antigen [11, 30]. We observed that on the first passage the MSCs of healthy volunteers expressed CD73 (88±3.7%), CD90 (83±2.6%) and CD105 (90±6.3%), while only some few MSCs expressed CD34 (0.7±0.3%), CD3 (3.9±1.5%), CD20 (5.3±2.3%), CD16 (5.6±1.7%), CD14 (5.6±2.3%), and HLA-DR (11.1±1.6%).
The MSCs of patients with ALL, HL, NHL and MM were found to express a similar immunophenotype. However, several differences were revealed as well. Firstly, CD73, CD90 and CD105-positive cells were lowered (78±6.8, 71±8.7 and 82±10% correspondingly; p>0.05). Secondly, in contrast to healthy volunteers, MSCs of patients with hemoblastosis showed a two-fold increased number of HLA-DR-positive cells (23±4.8 vs 11.1±1.6%, p<0.05), which is in agreement with published data [6].
Immunosuppressive properties of MSCs
Evaluation of the immunosuppressive activity of MSCs showed that donor MSCs rendered a dose-dependent suppressive effect on T-cell proliferation in mixed lymphocyte cultures (Fig. 1) as well as in cultures stimulated with ConA or monoclonal anti-CD3 antibodies (data not shown). The most pronounced effect was observed at the MSC:MNC ratio of 1:1. However, even at lower concentrations of MSCs (MSC:MNC ratio of 1:2 and 1:4) their suppressive activity remained high, and averaged 44–47%. In patients with hemoblastosis a significant suppressive effect of MSCs was registered only at the highest concentration of MSCs in culture (MSC:MNC ratio of 1:1). Nevertheless, even in this case the level of immunosuppressive activity of patient MSCs was lower compared to the level in control group (52±7 and 72±18%, correspondingly; p>0.05), varying from 18 to 64%. Reduction of MSC dose in mixed lymphocyte cultures strongly decreased ability of MSCs to block T-cell proliferative response.

Figure 1. Effects of donor and patient MSCs on proliferative response of T-cells in MLC.
MNCs from two donors (both 0.1x106 per well) have been cultivated for 5 days in the absence (control) or presence of MSCs of healthy donors (n=7) or hematologic patients (n=10). Cellular uptake of 3H-thymidine added at 18 hours before the end of cultivation was used to measure cell proliferation rate. Results are expressed as a suppression rate in percents calculated using the following formula: ![]() , where cpmMNC+MSC is proliferation rate of MNCs in presence of MSCs, and cpmMNC is proliferation rate of MNCs in absence of MSCs (control).
, where cpmMNC+MSC is proliferation rate of MNCs in presence of MSCs, and cpmMNC is proliferation rate of MNCs in absence of MSCs (control).
Support of hematopoiesis by MSC
One of the promising fields of therapeutic application of MSCs is their use for the rapid recovery of hematopoiesis after bone marrow transplantations. This statement is based on the ability of mesenchymal cells to support the hematopoietic progenitors [9, 15]. However, the question about the integrity of hematopoiesis-supporting potential of MSCs in case of hemoblastosis still remains open. Assessment of the colony-forming potential of patient bone marrow MNCs under co-incubation with autologous MSCs showed that the presence of MSCs (in ratio of 1:1) increased the number of CFU-E and CFU-GM approximately five times (Fig. 2). It should be noted that even in the presence of lower MSC concentration (in ratio of 1:10) a two-fold increase of hematopoietic colonies was also registered. The ability of MSCs to support hematopoietic progenitors in vitro was observed in all investigated cases (ALL, n=3; HL, n=3; NHL, n=3).

Figures 2a/b. Effects of MSCs on colony-forming activity of bone marrow MNCs.
Bone marrow MNCs of hematologic patients (n=9) were cultivated for 14 days in absence (control) or presence of autologous MSC at various concentrations (1:1 and 1:10) in semi-liquid methylcellulose medium. At the end of cultivation the amounts of erythroid (A) and granulocyte-macrophage (B) colonies per 105 MNCs were calculated. * - pU < 0,05 and ** - pU < 0,01 – versus control; Mann-Whitney U-test.

Hematopoietic engraftment and clinical outcome
Patients of both groups received a PBSC infusion containing equal doses of CD34+cells: the mean number of CD34+ mononuclear cells was 5.57х106/kg in the control group and 5.64х106/kg in the group with MSC co-transplantation. The dose of mesenchymal cells infused varied from 0.8x106 to 23x106 (mean 6.64x106).

Figure 3. Effects of MSCs infusions on neutrophil recovery. The t-test was used to evaluate a difference in period of neutrophil recovery.

Figure 4. Effects of MSCs infusions on neutrophil recovery. The t-test was used to evaluate a difference in period of platelets recovery.
There was no significant correlation between the number of MSCs and days of neutropenia or thrombocytopenia. However, we found a significant invert correlation between the number of MSCs and days of neutropenia in selected group, when infused PBSC were <3.0x106/kg.
No differences in analysis of severity and rate of complication, transfusion dependence, total or disease-free survival between the groups compared were revealed. Patients underwent standard restaging evaluation with computed tomography 42 days after transplantation and every 3 months thereafter. All patients evaluated on Day +60 were free of symptoms and clinical findings. Median follow-up of the patients is 9 months (range 4 to 22 months). Four patients died as a result of early disease progression (NHL, n= 2; HD, n=1; MM, n=1). All of them had refractory disease before PBSC transplantation. Of the 34 remaining patients, 33 are without evidence of disease; one has a stable disease (plato-phase in MM).
Transplant-related mortality / Toxicity
Two patients died on Day +7 and +21 due to transplant-related complications. There was one patient who died before engraftment. The cause of death in patient 1 on Day +7 was pneumonia and Candida sepsis as background. Patient 2 recovered hematopoiesis on Day +11, but died from pneumonia on Day +21. Progression of HD: lymphoadenopathy in the mediastinum was also revealed on autopsy of patient 2. Similar toxicity and causes of death were found in the control group: 3 fatal outcomes due to infection among 42 transplanted patients.
Discussion
The work presented here originated from the question of whether MSCs isolated from bone marrow of patients with hematologic malignancies possess intact functional properties including immunosuppressive activity and hematopoiesis-supporting ability. The transplantation of autologous bone marrow is widely used in clinical practice, but very little data exists on the characteristics of MSCs from the bone marrow of these patients, and the reported results often differ from one other.
Some authors have shown a statistically significant decrease of CFU-F numbers in cases of acute myeloid leukemia either in primary patients and patients after PCT courses [5, 6, 8] or for patients suffering from lymphomas, acute lymphoid leukemia and multiple myeloma [6, 23]. Notable is that the CFU-F number in patients varies from total absence to donor values. Moreover, neither sex nor age or involvement of bone marrow or time of the last PCT course affects the number of CFU-F [23]. In this study we also found a decrease in the CFU-F numbers for bone marrow samples obtained from patients with hemoblastosis. However, despite a low CFU-F number in patients our data display the principal possibility of isolation and in vitro expansion of MSC from patients with hematological malignancies.
Moreover, our results demonstrated a decrease in the proliferative capacity of in vitro expanded patient MSCs. For achieving confluence growth of patients’ MSCs, cultivation time needed to be twice as long. On the one hand, this may be caused by inhibition of proliferative potential in the patients’ MSCs, and on the other hand it may be caused by initially lowered content of MSC in bone marrow MNCs under hematological malignancies.
Our data shows that the MSCs of patients with ALL, HL, NHL, and MM do not express linear and hematopoietic antigens but are positive for some “stromal” markers. Along with that we managed to elicit some other special features. Namely, in the MSC population there was a lower number of CD73, CD90, and CD105-positive cells. Moreover, patient MSCs exhibited a two-fold increased number of HLA-DR-positive cells. Our data corresponds with Campioni D. et al [6], who used 4-color flow cytometry to show significant decreases in CD73, CD90, and CD105 expression on MSCs of 43 hematological patients. We have found this fact just as a tendency but this probably was caused by small array of patients (n=16) or by using 1-2-color flow cytometry.
We further found that in contrast to donor cells, patient MSCs in some cases did not suppress but even increased a proliferative response in MLC (data not shown), which corresponds with published data [17]. The low number of such observations did not allow us to connect this feature either with a distinct nosologic form of hemoblastosis, nor with particular phenotype or morphology of MSCs. One of the possible reasons for this phenomenon could be the ability of MSCs to secrete IL-7, which induces and supports lymphocyte proliferation [9].
Finally, in all investigated cultures (ALL, HL, NHL) we observed the ability of MSCs to support growth of hematopoietic progenitors in vitro. Published data concerning this issue can not be called univocal. For example, it was demonstated that the stromal cells of patients with acute myeloid leukemia possessed a low potential to stimulate growth of allogeneic hematopoietic precursors [8]. Meanwhile Mayani et al. [19] have shown that stromal cells of patients with AML display intact hematopoiesis-supporting activity, which strongly depends on the presence of CFU-F.
Summarizing the given experimental data we can conclude that MSCs of patients with hemoblastosis used in our study correspond to the Minimal criteria for defining multipotent mesenchymal stromal cells settled in the International Society for Cellular Therapy position statement [11]. These cells are characterized by adhesiveness, show fibroblast-like morphology, and a specific phenotype. Moreover, patients' MSCs display immunosuppressive and hematopoiesis-supporting activity. However, they have a number of distinct features. For instance, patient stromal cells in comparison with healthy donor cells contain lower numbers of CD73, CD90, and CD105-positive cells and a two-fold increased number of HLA-DR-positive cells. In addition, MSCs in hemoblastosis display lower levels of suppressive activity that is only become apparent at high concentrations of MSCs. It is important to note that described features of MSCs in hemoblastosis are not crucial for their hematopoiesis-supporting activity. This fact in combination with the ability of MSCs to expand ex vivo represents the basis for the clinical application of hematopoietic stem cell co-transplantation with mesenchymal stromal cells in oncohematology for the acceleration of hematopoiesis recovery.
Based on our clinical findings, we propose that culture-expanded autologous MSCs can be used to improve the rate and quality of hematopoietic engraftment, particularly in patients who previously received stroma-damaging therapy. Indeed, our report demonstrates that autologous MSCs can be successfully culture-expanded in FCS-free conditioning media, and infused IV for therapeutic intent with efficacy and without toxicity into different nosologic groups of patients at the time of PBSC transplantation. We have optimized MSC culture expansion methods to generate optimal numbers of autologous MSCs in a relatively short period of time for clinical use with a therapeutic intent without xenogenic components (e.g., FCS) [7].
Patients treated with high-dose chemotherapy generally experience complete and rapid neutrophil and platelet engraftment when supported with mobilized PBSCs containing >2–3 106 CD34+ cells. On the other hand, patients receiving lower doses of CD34+ cells are at increased risk for delayed platelet engraftment [3, 2].
Unsuccessful mobilization of CD34+ cells is commonly associated with extensive previous therapy, which is also associated with microenvironment damage. Coupled with low doses of CD34+ cells, an abnormal marrow microenvironment increases the risk of delayed engraftment. Sequential use of high-dose chemotherapy is also toxic to the marrow microenvironment, as evidenced by the delayed hematopoietic recovery after transplantation despite infusion of equal numbers of stem cells [12, 20, 22, 25]. Therefore, attempts to protect and improve the bone marrow microenvironment ex vivo are likely to better hematopoiesis engraftment after suboptimal СD34+ transplantation.
In conclusion our results indicate that the proposed cellular therapy protocol is feasible and may have a number of beneficial clinical effects in the setting of hematopoietic stem-cell transplantation. We therefore suppose studying it in more depths in randomized trials.
References
2. Akard L. Optimum methods to mobilize stem cells. J Clin Oncol 2000; 18: 3063.
3. Appelbaum F. The current status of hematopoietic cell transplantation. Annu Rev Med 2003; 54: 491.
5. Campioni D, Lanza F, Moretti S, Dominici M, Punturieri M, Pauli1 S et al. Functional and immunophenotypic characteristics of isolated CD105(+) and fibroblast(+) stromal cells from AML: implications for their plasticity along endothelial lineage. Cytotherapy 2003; 5: 66-79.
11. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315-317.
13. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974; 17:331-340.
16. Le Blanc K , Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363:1439-1441.
18. Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 2003; 10:228-241.
19. Mayani H., Guilbert LJ, Janowska-Wieczorek A. Functional characterization of fibroblastic cells in long-term marrow cultures from patients with acute myelogenous leukemia. Leukemia 1993; 7:1564-1569.
25. O’Flaherty E, Sparrow R, Szer J: Bone marrow stromal function from patients after bone marrow transplantation. Bone Marrow Transplant 1995; 15:207-212.
26. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143-147.
29. Tyndall A, Fassas A, Passweg J, Ruiz de Elvira C, Attal M, et al.: Autologous haematopoietic stem cell transplants for autoimmune disease — feasibility and transplant-related mortality. Autoimmune Disease and Lymphoma Working Parties of the European Group for Blood and Marrow Transplantation, the European League Against Rheumatism and theInternational Stem Cell Project for Autoimmune Disease. Bone Marrow Transplant 1999; 24:729-734.
31. Zhang W, Ge W, Li C, You S, Liao L, Han Q et al. Effects of mesenchymal stem cells on differentiation, maturation and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004; 13:263-71.
" ["~DETAIL_TEXT"]=> string(45767) "Introduction
Multiple studies have been performed investigating the kinetics, safety and efficiency of mesenchymal cell isolation, characterization, culture-expanding, and clinical approaches [26, 10, 1, 31, 14, 18, 21, 27, 28]. In recent years the biological activity of MSC has been very actively discussed for their potential use for suppression of autoimmune and transplant-related reactions, but also with the aim of speeding up of hematopoiesis recovery after bone marrow transplantation [15, 16, 29, 24]. Many existing protocols suppose the use of autologous MSCs [23, 9]. However, the use of autologous MSCs may be limited in a clinical setting, due to imperfect and often controversial knowledge of the functional competence and integrity of immunosuppressive and hematopoietic-supporting potentials of these cells [19, 5, 8, 4]. As shown earlier, bone marrow-derived mesenchymal stromal stem cells provide support for hematopoiesis in vitro and in experimental animal models [3, 15, 16, 24]. In this study we compared the morphology, immunophenotype, and immunoregulatory activities of MSCs derived from the bone marrow of healthy donors and patients with hematological malignancies. Our results demonstrated that MSCs derived from bone marrow suffering from hemoblastoses can be successfully expanded in culture and infused along with PBSCs after high-dose chemotherapy without any toxicity. Moreover, one of the promising results is the decrease in the period of critical neutropenia and thrombocytopenia in the patient after high-doses chemotherapy, when PBSCs and MSCs are co-transplanted. Our results show that co-transplantation of MSCs is associated with rapid hematopoietic recovery when PBSC counts are suboptimal.
Patients and methods
From April 2005 through June 2007, after obtaining written informed consent, 39 patients who were eligible for high-dose chemotherapy and PBSC transplantation were enrolled into single center investigation of the feasibility, safety, and hematopoietic effects of autologous culture-expanded MSCs (Table 1). The clinical trial protocol and the consent form were approved by the Local Ethics committee of the Institute of Clinical Immunology SB RAMS.
Table 1. Characteristics of patients who were treated with (n=39) and without (n=42) mesenchymal stem cells
|
Control |
MSC supported |
|
|---|---|---|
|
Median age, years (range) |
32 (17–57) |
31 (7–55) |
|
Gender (male/female) |
16/23 |
22/21 |
|
Diagnoses |
||
|
HD |
CR 4 |
CR 0 |
|
NHL |
CR 4 |
CR 4 |
|
MM |
CR 2 |
CR 0 |
|
AML (1st remission) |
2 |
11 |
|
ALL (2nd remission) |
4 |
4 |
|
SLE (SLEDAI >16) |
3 |
2 |
|
Conditioning regimens |
BEAM for HD and NHL; |
|
|
Mean dose of CD34+/CD45+ cells |
5.57/kgx106(1.81–9.5x106/kg) |
5.64/kgx106(2.5–8.7x106/kg) |
|
Mean dose of MSCs |
-- |
6.64x106 (0.8–23.0x106) |
Patients with lymphomas and leukemia were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1 and were required to have adequate visceral organ function, including a left ventricular ejection fraction of at least 50%, forced expiratory volume in 1 second, serum direct bilirubin less than 2.0 mg/dl, and an actual or calculated creatinine clearance greater than 60 ml/min. Also all patients with hematological diseases underwent restaging evaluation with blood count, biochemical activity, trepanobiopsy, and computed tomography.
At the start of therapy, a neutrophil count greater than 1.2x106/ml and a platelet count greater than 100x106/ml were required. Patients were excluded if they had bone marrow involvement or active infection. Patients were excluded for evidence of tumor on routine histological staining of bilateral paraffin-embedded posterior iliac crest bone marrow biopsy specimens. In the “refractory group”, the patient was required to have no effects after standard chemotherapy.
SLE patient eligibility depended on a refractory to standard immunosuppression therapies and either organ- or life-threatening visceral involvement. The inclusion criteria were: not controlled with pulse therapy Cy glomerulonephritis (World Health Organization (WHO) class III–IV), central nervous system (CNS) lupus, vasculitis involving the lung or heart, or transfusion-dependent autoimmune cytopenias. Evaluation of eligible patients by two independent rheumatologists and transplant physicians, informed consent, and approval by the ethical committee were part of the protocol of Tyndall et al. [29]
High-Dose Chemotherapy and PBSC Infusion
The PBSC mobilization regimen consisted of Cyclophosphamide 4.0 g/m2 IV infusion over 6 hours on day 1, along with Mesna (first 3.0 g/m2 IV, then 500 mg every 3 hours orally/IV for eight doses). On day 7 after completion of the Cyclophosphamide, patients began subcutaneous injections of recombinant human G-CSF (Neupogen, Granocyte, Leukostim) 10 mg/kg/d. On recovery of neutrophils to a level greater than 1.2x109/l (usually 12 to 15 days after Cyclophosphamide treatment), patients underwent a leukapheresis procedure using AS TEC 204 (Fresenius, Germany) or Spectra LRS 07 (COBE, Lakewood, CO) apheresis equipment. Cells were cryopreserved using a controlled-rate liquid nitrogen freezer using previously published methods [10]. After PBSC procurement, high-dose chemotherapy with BEAM (BCNU 300 mg/m2 on day -7, Etoposid 800 mg/m2 and Ara-C 800 mg/m2 on days -6, -5, -4, -3, Melphalan 120 mg/m2 on day -2) for NHL and HD patients, Alceran (200 mg/m2 on day -2) for patients with AL, Cy (200-140 mg/kg in divided doses for 4 days -6, -5, -4, -3 with/or not ATG (total doses 60 mg/kg) for patients who suffered from SLE were administered. PBSCs were thawed and infused 24 hours after the completion of high-dose chemotherapy.
Phenotypic analysis of mobilized cells (expression of CD 34 and CD45) was performed using multicolor flow cytometry (FACSCalibur, Becton Dickinson). Moreover, cancer cell contamination of every PBSC specimen was excluded by the use of cytological analysis.
Supportive Care
Before, during, and for 24 hours after treatment with high dose Cyclophosphamide, patients received hyperhydration with forced diuresis and Urometexan (Mesna) infusion for the prevention of hemorrhagic cystitis. Patients were treated in a single room without air filtration. All patients followed a standardized supportive care protocol including antiemetic therapy, analgesia for mucositis, transfusion support, and venoocclusive disease prophylaxis. A low microbial diet, oral daily Fluoroquinolone (1 g/d) changed to intravenous Cefepime on neutropenic fever, Fluconazole (400 mg/d) and Acyclovir (10 mg/kg/d) and aerosolized Amphotericin B (10 mg twice daily) were started upon admission and discontinued when the ANC rebounded to 0.5x109/l. Platelets irradiated with 25 Gy and red blood cells were given to maintain a platelet count greater than 20 000/μl and a hemoglobin level greater than 8.0 g/dl. For the first 6 months after transplantation, patients were treated orally with daily oral Fluconazole and Trimethoprim/Sulfamethoxazole three times a week.
Harvesting and Ex Vivo MSC Culture
A median of 50 ml of bone marrow aspirate was obtained under sterile conditions by puncture of posterior iliac crests of patients or donors (n=9) under local anesthesia during standard pre-transplant staging approximately 2 days before high-dose Cyclophosphamide mobilization and 30 days before scheduled PBSC infusion. Washed heparinized bone marrow cells were re-suspended in phosphate-buffered solution (PBS, Sigma-Aldrich, Germany) and overlaid on Ficoll density gradient (1.078 g/l), then centrifuged for 20 min at 1000 g. A median of 320x106 (range 250–600 x106) mononuclear cells (MNC) were collected from the interface, washed three times in PBS, and re-suspended in α-modified minimum essential medium (αMEM, Sigma-Aldrich, Germany) containing 100 μg/ml gentamycin and 5% human platelet lysate (PL). After the cell number was counted, 30 ml of cell suspension was plated in a 175 cm2 plastic culture flask (Nunc, Denmark).
It is important to note that for clinical use with a therapeutic intent we used 5% platelet lysate to replace fetal calf serum (FCS) in the MSC culture. Platelet lysate was obtained from several allogeneic platelet units prepared by machine trombocytaphereis using AS TEC 204 (Fresenius, Germany). The platelet units were frozen at -40 C, thawed, mixed, aliquoted and lysate frozen at -20 C until use.
MSCs were cultured in humidified incubators with 5% CO2 and initially allowed to adhere for 72 hours, followed by a media change every 3 days. When cultures reached more than 90% confluence, adherent cells were detached with 0.05% trypsin-EDTA (Sigma-Aldrich, Germany) and replated at a density of 0.8x106 per 175 cm2 flask until processing for cryopreservation. Harvested MSCs from 1-2 passages were cryopreserved with a rate-controlled freezer (Planer Kryo 560-16) in 10% human Albumin solution (Microgen, Russia) and in a final concentration of 10% Dimethylsulfoxide (Sigma-Aldrich, Germany) and 10% Hydroxyethylstarch in freezing bags.
Cell cultures were tested for sterility weekly and before cryopreservation (Municipal Hospitals N1, Microbiology Laboratory, Novosibirsk) for the presence of bacterial/fungal contamination via microbiological cultural tests. All cell manipulations were performed in a sterile class II biological safety cabinet.
The number of MSCs was estimated by the quantity of colony forming unit-fibroblasts (CFU-F). For that, 106 of bone marrow MNCs were cultivated in Petri dishes for 14 days and stained using Giemsa protocol, then the number of spindle-shaped cell colonies consisting of more than 50 cells were counted.
Flow Cytometry
Phenotypic analysis of MSCs (expression of CD3, CD14, CD16, CD20, CD34, CD73, CD90, CD105 and HLA-DR molecules) was performed using multicolor flow cytometry (FACSCalibur, Becton Dickinson). Cells were detached with 0.05% trypsin-EDTA (Sigma, USA), washed with PBS plus 2% bovine albumin, fixed in 1% paraformaldehyde, blocked with 10% normal goat serum, and incubated separately with appropriate primary antibodies (BD Bioscience, USA). Non-treated MSCs were used as negative control.
The immunosuppressive properties of MSCs
To study MSC effects on immune cell function, MSCs were first cultivated for 24 h in flat-bottomed 96-well or 24-well plates in α-MEM/20% fetal calf serum (FCS, Gibco, USA). After this, peripheral blood MNCs (PBMNCs) from donors were added to MSC monolayer and stimulated with mitogens or alloantigens (mixed lymphocyte culture, MLC). For MLC, 0.1x106 PBMNCs from two donors were cultivated for 5 days in RPMI-1640 medium supplemented with 0.3 mg/ml L-glutamine, 100 μg/ml Gentamycine and 10% of heat-inactivated donor AB (IV) serum. Mitogen-induced proliferative response was studied in 3-day cultures of donor PBMNCs, which were activated by Concanavalin A (ConA, 15 μg/ml, Sigma-Aldrich, Germany) or 1 μg/ml monoclonal antibodies against CD3 (anti-CD3, Becton Dickinson, USA). Cell proliferation was measured by incorporation of 3H-thymidine that was added at 1 μCi/well during the last 18 h of cultivation. Proliferative response of MNCs cultivated without MSCs was used as control.
Support for hematopoiesis by MSC (CFU Assay)
To estimate MSCs' effect on colony-forming activity of hematopoietic progenitor cells, bone marrow MNC were grown in “complete” methylcellulose medium (Methocult H4344, Stem Cell Technologies, Vancouver, BC), containing hSCF, hGM-CSF, hIL-3, and hEPO at a density of 25x105/ml. Autologous MSC were added to MNCs in MNC: MSC ratios of 1:1 and 10:1 in triplicates. Cultures were grown at 37°C, 5% CO2 for 14 days, then the numbers of erythroid- and granulocyte-macrophage colony-forming units (CFU-E and CFU-GM, respectively) were calculated. Colonies were considered if consisted of at least 50 cells.
MSC Infusion
On the day of infusion, cryopreserved units were thawed ex tempore in a 37°C water bath, washed twice with PBS, transferred into 60-ml syringes with 10% human Albumin and infused into patients one hour after the PBSC infusion through a side port of a running 0.9% saline IV infusion into a central catheter over 10 to 20 minutes. Patients were premedicated with Acetaminophen (650 mg) and Diphenhydramine. Vital and clinical signs and symptoms were monitored at the time of infusion and every 15 minutes thereafter for 3 hours, followed by every 2 hours for 6 hours and every 8 hours for 3 days. There was no immediate or delayed toxicity related to MSC infusion. None of the patients experienced allergic reactions or respiratory symptoms.
Statistical analysis
Statistica 6.0 software for Windows, StatSoft Inc. USA was used for basic descriptive, and correlation analysis of data. The statistical significance was assessed with the Mann-Whitney U-test. The T-test was used to analyze the difference in means when comparing two groups. In assessing correlation, we used 2D scatterplots estimated correlation coefficient r and P-value for Rank Order Correlation Spearmen test. A p-value of less than 0.05 was considered statistically significant.
Results
MSC characteristics
At the first stage we compared the quantity of MSCs in bone marrow of patients with hemoblastosis and healthy donors. Since MSCs were first described as fibroblast-like cells capable of forming colonies 13], the initial content of MSC precursors among bone marrow MNCs was measured as a colony-forming unit fibroblast (CFU-F) number. The mean number of CFU-F in the control group was 36±3 colonies per 106 bone marrow-derived MNCs (min-max range 16–70). The CFU-F number in patients with hemoblastosis was 21±3 (p<0.05). The range of values in the patient group widely varied (from 1 to 67 CFU-F), and 70% of samples showed CFU-F numbers that were below the lower quartile value of control group (<25 CFU-F). The most significant decrease of CFU-F number was revealed in patients with NHL (16±5, p<0.01). In patients with MM and HL number of MSC precursors was also decreased (19±10 and 26±7, respectively; p>0.05). Decreasing CFU-F numbers mean that MSC progenitors in bone marrow of investigated patients are present at a lower amount.
Taking into consideration the MSC deficiency in patients with hemoblastosis, it seemed important to evaluate their ability for expansion in vitro. With that aim we analyzed the growth rate of MSC by measuring cultivation time until confluence. In healthy volunteers, confluent growth in MSC cultures was reached by day 15±0.5 (range 11–22 days). Meanwhile, in the patient group 80–90% confluence took on average 26±2 days (range 10–50); 23±3 days in patients with HL (p<0.01); 29±3.5 days in patients with NHL (p< 0.01), and 30±4 days in patients with MM (p<0.01). It seems to be important that despite a low CFU-F number in patient cultures it was possible to isolate and in vitro expand MSCs from patients with hematological malignancies.
MSC immunophenotype
Further, we characterized the immunophenotype of MSCs. Classically, MSCs are defined as cells expressing STRO-1, CD105 (SH2), CD71, CD73 (SH3, SH4), CD90 (Thy1) surface markers and not expressing lineage (CD3, CD14, CD20, CD16) and hematopoietic (CD34, CD45) markers along with HLA-DR antigen [11, 30]. We observed that on the first passage the MSCs of healthy volunteers expressed CD73 (88±3.7%), CD90 (83±2.6%) and CD105 (90±6.3%), while only some few MSCs expressed CD34 (0.7±0.3%), CD3 (3.9±1.5%), CD20 (5.3±2.3%), CD16 (5.6±1.7%), CD14 (5.6±2.3%), and HLA-DR (11.1±1.6%).
The MSCs of patients with ALL, HL, NHL and MM were found to express a similar immunophenotype. However, several differences were revealed as well. Firstly, CD73, CD90 and CD105-positive cells were lowered (78±6.8, 71±8.7 and 82±10% correspondingly; p>0.05). Secondly, in contrast to healthy volunteers, MSCs of patients with hemoblastosis showed a two-fold increased number of HLA-DR-positive cells (23±4.8 vs 11.1±1.6%, p<0.05), which is in agreement with published data [6].
Immunosuppressive properties of MSCs
Evaluation of the immunosuppressive activity of MSCs showed that donor MSCs rendered a dose-dependent suppressive effect on T-cell proliferation in mixed lymphocyte cultures (Fig. 1) as well as in cultures stimulated with ConA or monoclonal anti-CD3 antibodies (data not shown). The most pronounced effect was observed at the MSC:MNC ratio of 1:1. However, even at lower concentrations of MSCs (MSC:MNC ratio of 1:2 and 1:4) their suppressive activity remained high, and averaged 44–47%. In patients with hemoblastosis a significant suppressive effect of MSCs was registered only at the highest concentration of MSCs in culture (MSC:MNC ratio of 1:1). Nevertheless, even in this case the level of immunosuppressive activity of patient MSCs was lower compared to the level in control group (52±7 and 72±18%, correspondingly; p>0.05), varying from 18 to 64%. Reduction of MSC dose in mixed lymphocyte cultures strongly decreased ability of MSCs to block T-cell proliferative response.

Figure 1. Effects of donor and patient MSCs on proliferative response of T-cells in MLC.
MNCs from two donors (both 0.1x106 per well) have been cultivated for 5 days in the absence (control) or presence of MSCs of healthy donors (n=7) or hematologic patients (n=10). Cellular uptake of 3H-thymidine added at 18 hours before the end of cultivation was used to measure cell proliferation rate. Results are expressed as a suppression rate in percents calculated using the following formula: ![]() , where cpmMNC+MSC is proliferation rate of MNCs in presence of MSCs, and cpmMNC is proliferation rate of MNCs in absence of MSCs (control).
, where cpmMNC+MSC is proliferation rate of MNCs in presence of MSCs, and cpmMNC is proliferation rate of MNCs in absence of MSCs (control).
Support of hematopoiesis by MSC
One of the promising fields of therapeutic application of MSCs is their use for the rapid recovery of hematopoiesis after bone marrow transplantations. This statement is based on the ability of mesenchymal cells to support the hematopoietic progenitors [9, 15]. However, the question about the integrity of hematopoiesis-supporting potential of MSCs in case of hemoblastosis still remains open. Assessment of the colony-forming potential of patient bone marrow MNCs under co-incubation with autologous MSCs showed that the presence of MSCs (in ratio of 1:1) increased the number of CFU-E and CFU-GM approximately five times (Fig. 2). It should be noted that even in the presence of lower MSC concentration (in ratio of 1:10) a two-fold increase of hematopoietic colonies was also registered. The ability of MSCs to support hematopoietic progenitors in vitro was observed in all investigated cases (ALL, n=3; HL, n=3; NHL, n=3).

Figures 2a/b. Effects of MSCs on colony-forming activity of bone marrow MNCs.
Bone marrow MNCs of hematologic patients (n=9) were cultivated for 14 days in absence (control) or presence of autologous MSC at various concentrations (1:1 and 1:10) in semi-liquid methylcellulose medium. At the end of cultivation the amounts of erythroid (A) and granulocyte-macrophage (B) colonies per 105 MNCs were calculated. * - pU < 0,05 and ** - pU < 0,01 – versus control; Mann-Whitney U-test.

Hematopoietic engraftment and clinical outcome
Patients of both groups received a PBSC infusion containing equal doses of CD34+cells: the mean number of CD34+ mononuclear cells was 5.57х106/kg in the control group and 5.64х106/kg in the group with MSC co-transplantation. The dose of mesenchymal cells infused varied from 0.8x106 to 23x106 (mean 6.64x106).

Figure 3. Effects of MSCs infusions on neutrophil recovery. The t-test was used to evaluate a difference in period of neutrophil recovery.

Figure 4. Effects of MSCs infusions on neutrophil recovery. The t-test was used to evaluate a difference in period of platelets recovery.
There was no significant correlation between the number of MSCs and days of neutropenia or thrombocytopenia. However, we found a significant invert correlation between the number of MSCs and days of neutropenia in selected group, when infused PBSC were <3.0x106/kg.
No differences in analysis of severity and rate of complication, transfusion dependence, total or disease-free survival between the groups compared were revealed. Patients underwent standard restaging evaluation with computed tomography 42 days after transplantation and every 3 months thereafter. All patients evaluated on Day +60 were free of symptoms and clinical findings. Median follow-up of the patients is 9 months (range 4 to 22 months). Four patients died as a result of early disease progression (NHL, n= 2; HD, n=1; MM, n=1). All of them had refractory disease before PBSC transplantation. Of the 34 remaining patients, 33 are without evidence of disease; one has a stable disease (plato-phase in MM).
Transplant-related mortality / Toxicity
Two patients died on Day +7 and +21 due to transplant-related complications. There was one patient who died before engraftment. The cause of death in patient 1 on Day +7 was pneumonia and Candida sepsis as background. Patient 2 recovered hematopoiesis on Day +11, but died from pneumonia on Day +21. Progression of HD: lymphoadenopathy in the mediastinum was also revealed on autopsy of patient 2. Similar toxicity and causes of death were found in the control group: 3 fatal outcomes due to infection among 42 transplanted patients.
Discussion
The work presented here originated from the question of whether MSCs isolated from bone marrow of patients with hematologic malignancies possess intact functional properties including immunosuppressive activity and hematopoiesis-supporting ability. The transplantation of autologous bone marrow is widely used in clinical practice, but very little data exists on the characteristics of MSCs from the bone marrow of these patients, and the reported results often differ from one other.
Some authors have shown a statistically significant decrease of CFU-F numbers in cases of acute myeloid leukemia either in primary patients and patients after PCT courses [5, 6, 8] or for patients suffering from lymphomas, acute lymphoid leukemia and multiple myeloma [6, 23]. Notable is that the CFU-F number in patients varies from total absence to donor values. Moreover, neither sex nor age or involvement of bone marrow or time of the last PCT course affects the number of CFU-F [23]. In this study we also found a decrease in the CFU-F numbers for bone marrow samples obtained from patients with hemoblastosis. However, despite a low CFU-F number in patients our data display the principal possibility of isolation and in vitro expansion of MSC from patients with hematological malignancies.
Moreover, our results demonstrated a decrease in the proliferative capacity of in vitro expanded patient MSCs. For achieving confluence growth of patients’ MSCs, cultivation time needed to be twice as long. On the one hand, this may be caused by inhibition of proliferative potential in the patients’ MSCs, and on the other hand it may be caused by initially lowered content of MSC in bone marrow MNCs under hematological malignancies.
Our data shows that the MSCs of patients with ALL, HL, NHL, and MM do not express linear and hematopoietic antigens but are positive for some “stromal” markers. Along with that we managed to elicit some other special features. Namely, in the MSC population there was a lower number of CD73, CD90, and CD105-positive cells. Moreover, patient MSCs exhibited a two-fold increased number of HLA-DR-positive cells. Our data corresponds with Campioni D. et al [6], who used 4-color flow cytometry to show significant decreases in CD73, CD90, and CD105 expression on MSCs of 43 hematological patients. We have found this fact just as a tendency but this probably was caused by small array of patients (n=16) or by using 1-2-color flow cytometry.
We further found that in contrast to donor cells, patient MSCs in some cases did not suppress but even increased a proliferative response in MLC (data not shown), which corresponds with published data [17]. The low number of such observations did not allow us to connect this feature either with a distinct nosologic form of hemoblastosis, nor with particular phenotype or morphology of MSCs. One of the possible reasons for this phenomenon could be the ability of MSCs to secrete IL-7, which induces and supports lymphocyte proliferation [9].
Finally, in all investigated cultures (ALL, HL, NHL) we observed the ability of MSCs to support growth of hematopoietic progenitors in vitro. Published data concerning this issue can not be called univocal. For example, it was demonstated that the stromal cells of patients with acute myeloid leukemia possessed a low potential to stimulate growth of allogeneic hematopoietic precursors [8]. Meanwhile Mayani et al. [19] have shown that stromal cells of patients with AML display intact hematopoiesis-supporting activity, which strongly depends on the presence of CFU-F.
Summarizing the given experimental data we can conclude that MSCs of patients with hemoblastosis used in our study correspond to the Minimal criteria for defining multipotent mesenchymal stromal cells settled in the International Society for Cellular Therapy position statement [11]. These cells are characterized by adhesiveness, show fibroblast-like morphology, and a specific phenotype. Moreover, patients' MSCs display immunosuppressive and hematopoiesis-supporting activity. However, they have a number of distinct features. For instance, patient stromal cells in comparison with healthy donor cells contain lower numbers of CD73, CD90, and CD105-positive cells and a two-fold increased number of HLA-DR-positive cells. In addition, MSCs in hemoblastosis display lower levels of suppressive activity that is only become apparent at high concentrations of MSCs. It is important to note that described features of MSCs in hemoblastosis are not crucial for their hematopoiesis-supporting activity. This fact in combination with the ability of MSCs to expand ex vivo represents the basis for the clinical application of hematopoietic stem cell co-transplantation with mesenchymal stromal cells in oncohematology for the acceleration of hematopoiesis recovery.
Based on our clinical findings, we propose that culture-expanded autologous MSCs can be used to improve the rate and quality of hematopoietic engraftment, particularly in patients who previously received stroma-damaging therapy. Indeed, our report demonstrates that autologous MSCs can be successfully culture-expanded in FCS-free conditioning media, and infused IV for therapeutic intent with efficacy and without toxicity into different nosologic groups of patients at the time of PBSC transplantation. We have optimized MSC culture expansion methods to generate optimal numbers of autologous MSCs in a relatively short period of time for clinical use with a therapeutic intent without xenogenic components (e.g., FCS) [7].
Patients treated with high-dose chemotherapy generally experience complete and rapid neutrophil and platelet engraftment when supported with mobilized PBSCs containing >2–3 106 CD34+ cells. On the other hand, patients receiving lower doses of CD34+ cells are at increased risk for delayed platelet engraftment [3, 2].
Unsuccessful mobilization of CD34+ cells is commonly associated with extensive previous therapy, which is also associated with microenvironment damage. Coupled with low doses of CD34+ cells, an abnormal marrow microenvironment increases the risk of delayed engraftment. Sequential use of high-dose chemotherapy is also toxic to the marrow microenvironment, as evidenced by the delayed hematopoietic recovery after transplantation despite infusion of equal numbers of stem cells [12, 20, 22, 25]. Therefore, attempts to protect and improve the bone marrow microenvironment ex vivo are likely to better hematopoiesis engraftment after suboptimal СD34+ transplantation.
In conclusion our results indicate that the proposed cellular therapy protocol is feasible and may have a number of beneficial clinical effects in the setting of hematopoietic stem-cell transplantation. We therefore suppose studying it in more depths in randomized trials.
References
2. Akard L. Optimum methods to mobilize stem cells. J Clin Oncol 2000; 18: 3063.
3. Appelbaum F. The current status of hematopoietic cell transplantation. Annu Rev Med 2003; 54: 491.
5. Campioni D, Lanza F, Moretti S, Dominici M, Punturieri M, Pauli1 S et al. Functional and immunophenotypic characteristics of isolated CD105(+) and fibroblast(+) stromal cells from AML: implications for their plasticity along endothelial lineage. Cytotherapy 2003; 5: 66-79.
11. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315-317.
13. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974; 17:331-340.
16. Le Blanc K , Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363:1439-1441.
18. Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 2003; 10:228-241.
19. Mayani H., Guilbert LJ, Janowska-Wieczorek A. Functional characterization of fibroblastic cells in long-term marrow cultures from patients with acute myelogenous leukemia. Leukemia 1993; 7:1564-1569.
25. O’Flaherty E, Sparrow R, Szer J: Bone marrow stromal function from patients after bone marrow transplantation. Bone Marrow Transplant 1995; 15:207-212.
26. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143-147.
29. Tyndall A, Fassas A, Passweg J, Ruiz de Elvira C, Attal M, et al.: Autologous haematopoietic stem cell transplants for autoimmune disease — feasibility and transplant-related mortality. Autoimmune Disease and Lymphoma Working Parties of the European Group for Blood and Marrow Transplantation, the European League Against Rheumatism and theInternational Stem Cell Project for Autoimmune Disease. Bone Marrow Transplant 1999; 24:729-734.
31. Zhang W, Ge W, Li C, You S, Liao L, Han Q et al. Effects of mesenchymal stem cells on differentiation, maturation and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004; 13:263-71.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(3) "500" ["~SORT"]=> string(3) "500" ["CODE"]=> string(100) "autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss" ["~CODE"]=> string(100) "autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss" ["EXTERNAL_ID"]=> string(3) "974" ["~EXTERNAL_ID"]=> string(3) "974" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["ELEMENT_META_KEYWORDS"]=> string(174) "гемобластозы костный мозг мезенхимальные стромальные клетки трансплантация стволовых клеток" ["ELEMENT_META_DESCRIPTION"]=> string(469) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клетокAutologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(2757) "<p class="bodytext">Мезенхимальные стромальные клетки (МСК), выделенные из костного мозга, обладают иммунорегуляторной активностью и способны поддерживать гемопоэз. К сожалению, данные, характеризующие биологические свойства МСК при различных патологических состояниях, очень немногочисленны и зачастую противоречивы. В настоящей работе мы показали, что МСК, полученные из костного мозга больных гемобластозами, имеют морфологию фибробластоподобных клеток и характерный фенотип. Более того, МСК больных обладают хорошо выраженной способностью к стимуляции гемопоэза, в сочетании со сниженным иммуносупрессорным потенциалом. Эти свойства МСК послужили основанием для клинического применения котрансплантации аутологичных гемопоэтических стволовых клеток и МСК в онкогематологии с целью ускорения восстановления гемопоэза. Нами были исследованы безопасность и гемопоэтические эффекты мезенхимальных стромальных клеток у пациентов с гемобластозами, которым выполнялась трансплантация периферических стволовых кроветворных клеток (ПСКК). Использование ex vivo культивированных МСК, котрансплантированных с аутологичными ПСКК, позволило нам выявить снижение периодов критической нейтропении и тромбоцитопении у больных гемобластозами после высокодозной химиотерапии. Полученные результаты демонстрируют возможность и безопасность совместных трансплантаций МСК и ПСКК. Сокращение периода восстановления кроветворения свидетельствует о позитивном влиянии МСК на гемопоэз.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_META_TITLE"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_META_KEYWORDS"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_META_DESCRIPTION"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_PICTURE_FILE_ALT"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_PICTURE_FILE_TITLE"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(332) "Аутологичные мезенхимальные стромальные клетки больных гемобластозами эффективно поддерживают восстановление кроветворения после трансплантации стволовых кроветворных клеток" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "autologichnye-mezenkhimalnye-stromalnye-kletki-bolnykh-gemoblastozami-effektivno-podderzhivayut-voss" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(2) "44" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(4) { [0]=> string(5) "13812" [1]=> string(5) "13813" [2]=> string(5) "13814" [3]=> string(5) "13815" } ["VALUE"]=> array(4) { [0]=> string(3) "977" [1]=> string(2) "81" [2]=> string(3) "978" [3]=> string(2) "58" } ["DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(4) { [0]=> string(3) "977" [1]=> string(2) "81" [2]=> string(3) "978" [3]=> string(2) "58" } ["~DESCRIPTION"]=> array(4) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13734" ["VALUE"]=> string(22) "08/25/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "08/25/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13735" ["VALUE"]=> string(22) "01/20/2010 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "01/20/2010 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13736" ["VALUE"]=> string(22) "02/16/2010 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/16/2010 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13737" ["VALUE"]=> string(3) "450" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "450" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(10) { [0]=> string(5) "13816" [1]=> string(5) "13817" [2]=> string(5) "13818" [3]=> string(5) "13819" [4]=> string(5) "13820" [5]=> string(5) "13821" [6]=> string(5) "13822" [7]=> string(5) "13823" [8]=> string(5) "13824" [9]=> string(5) "13825" } ["VALUE"]=> array(10) { [0]=> string(3) "551" [1]=> string(3) "972" [2]=> string(3) "552" [3]=> string(3) "503" [4]=> string(3) "973" [5]=> string(3) "555" [6]=> string(3) "450" [7]=> string(3) "975" [8]=> string(3) "976" [9]=> string(3) "556" } ["DESCRIPTION"]=> array(10) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" [6]=> string(0) "" [7]=> string(0) "" [8]=> string(0) "" [9]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(10) { [0]=> string(3) "551" [1]=> string(3) "972" [2]=> string(3) "552" [3]=> string(3) "503" [4]=> string(3) "973" [5]=> string(3) "555" [6]=> string(3) "450" [7]=> string(3) "975" [8]=> string(3) "976" [9]=> string(3) "556" } ["~DESCRIPTION"]=> array(10) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" [5]=> string(0) "" [6]=> string(0) "" [7]=> string(0) "" [8]=> string(0) "" [9]=> string(0) "" } ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13762" ["VALUE"]=> array(2) { ["TEXT"]=> string(515) "<p>Сергеевичева В. В.<sup>1</sup>, Шевела Е. Я.<sup>1</sup>, Сизикова С. А.<sup>1</sup>, Кулагин А. Д.<sup>2</sup>, Крючкова И. В.<sup>1</sup>, Гилевич А. В.<sup>1</sup>, Лисуков И. А.<sup>2</sup>, <br>Козлов В. А.<sup>1</sup>, Останин А. А.<sup>1</sup>, Черных Е .Р.<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(377) "Сергеевичева В. В.1, Шевела Е. Я.1, Сизикова С. А.1, Кулагин А. Д.2, Крючкова И. В.1, Гилевич А. В.1, Лисуков И. А.2,
Козлов В. А.1, Останин А. А.1, Черных Е .Р.1
1Институт клинической иммунологии СО РАМН, Новосибирск, Россия
2Новосибирский государственный медицинский университет, Новосибирск, Россия
Мезенхимальные стромальные клетки (МСК), выделенные из костного мозга, обладают иммунорегуляторной активностью и способны поддерживать гемопоэз. К сожалению, данные, характеризующие биологические свойства МСК при различных патологических состояниях, очень немногочисленны и зачастую противоречивы. В настоящей работе мы показали, что МСК, полученные из костного мозга больных гемобластозами, имеют морфологию фибробластоподобных клеток и характерный фенотип. Более того, МСК больных обладают хорошо выраженной способностью к стимуляции гемопоэза, в сочетании со сниженным иммуносупрессорным потенциалом. Эти свойства МСК послужили основанием для клинического применения котрансплантации аутологичных гемопоэтических стволовых клеток и МСК в онкогематологии с целью ускорения восстановления гемопоэза. Нами были исследованы безопасность и гемопоэтические эффекты мезенхимальных стромальных клеток у пациентов с гемобластозами, которым выполнялась трансплантация периферических стволовых кроветворных клеток (ПСКК). Использование ex vivo культивированных МСК, котрансплантированных с аутологичными ПСКК, позволило нам выявить снижение периодов критической нейтропении и тромбоцитопении у больных гемобластозами после высокодозной химиотерапии. Полученные результаты демонстрируют возможность и безопасность совместных трансплантаций МСК и ПСКК. Сокращение периода восстановления кроветворения свидетельствует о позитивном влиянии МСК на гемопоэз.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13705" ["VALUE"]=> string(29) "10.3205/ctt-2010-en-000055.01" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(29) "10.3205/ctt-2010-en-000055.01" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13781" ["VALUE"]=> array(2) { ["TEXT"]=> string(474) "<p>Vera V. Sergeevicheva<sup>1</sup>, Ekaterina Y. Shevela<sup>1</sup>, Svetlana A. Sizikova<sup>1</sup>, Alexander D. Kulagin<sup>2</sup>, Irina V. Kruchkova<sup>1</sup>, <br>Andrey V. Gilevich<sup>1</sup>, Igor A. Lisukov<sup>2</sup>, Vladimir A. Kozlov<sup>1</sup>, Alexander A. Ostanin<sup>1</sup>, Elena R. Chernykh<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(336) "Vera V. Sergeevicheva1, Ekaterina Y. Shevela1, Svetlana A. Sizikova1, Alexander D. Kulagin2, Irina V. Kruchkova1,
Andrey V. Gilevich1, Igor A. Lisukov2, Vladimir A. Kozlov1, Alexander A. Ostanin1, Elena R. Chernykh1
1Institute of Clinical Immunology SB RAMS, Novosibirsk, Russia;
2Novosibirsk State Medical University, Novosibirsk, Russia
Mesenchymal stromal cells (MSCs) derived from bone marrow possess immunoregulatory activity and are able to support hematopoiesis. Unfortunately, data concerning the biological properties of MSCs in various pathologies is poor and often discrepant. In this study, we demonstrated that MSCs derived from bone marrow of patients with hemoblastoses have fibroblast-like morphology and a typical phenotype. Moreover, the patients' MSCs possess well-defined hematopoietic-supporting activity coupled with decreased immunosuppressive potential. These properties prove the clinical application of co-transplantation of autologous hematopoietic stem cells and MSCs in oncohematology to achieve a rapid hematopoietic recovery. Therefore we investigated the safety and hematopoietic effects of MSCs in patients with hematological malignancies receiving peripheral blood hematopoietic stem cell (PBSC) transplantation. We revealed the decreasing of the period of neutropenia and thrombocytopenia in the patients with hematological tumors after high-dose chemotherapy, when autologous PBSC were co-transplanted with ex vivo expanded autologous MSCs. Our results show that co-transplantation of autologous MSCs with PBSC is feasible and safe. The shortening of hematopoietic recovery time suggests that MSC may have a positive impact on hematopoiesis.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13706" ["VALUE"]=> string(137) "Autologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(137) "Autologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13765" ["VALUE"]=> string(3) "658" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "658" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13766" ["VALUE"]=> string(3) "659" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(3) "659" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(14) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13781" ["VALUE"]=> array(2) { ["TEXT"]=> string(474) "<p>Vera V. Sergeevicheva<sup>1</sup>, Ekaterina Y. Shevela<sup>1</sup>, Svetlana A. Sizikova<sup>1</sup>, Alexander D. Kulagin<sup>2</sup>, Irina V. Kruchkova<sup>1</sup>, <br>Andrey V. Gilevich<sup>1</sup>, Igor A. Lisukov<sup>2</sup>, Vladimir A. Kozlov<sup>1</sup>, Alexander A. Ostanin<sup>1</sup>, Elena R. Chernykh<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(336) "Vera V. Sergeevicheva1, Ekaterina Y. Shevela1, Svetlana A. Sizikova1, Alexander D. Kulagin2, Irina V. Kruchkova1,
Andrey V. Gilevich1, Igor A. Lisukov2, Vladimir A. Kozlov1, Alexander A. Ostanin1, Elena R. Chernykh1
Vera V. Sergeevicheva1, Ekaterina Y. Shevela1, Svetlana A. Sizikova1, Alexander D. Kulagin2, Irina V. Kruchkova1,
Andrey V. Gilevich1, Igor A. Lisukov2, Vladimir A. Kozlov1, Alexander A. Ostanin1, Elena R. Chernykh1
Mesenchymal stromal cells (MSCs) derived from bone marrow possess immunoregulatory activity and are able to support hematopoiesis. Unfortunately, data concerning the biological properties of MSCs in various pathologies is poor and often discrepant. In this study, we demonstrated that MSCs derived from bone marrow of patients with hemoblastoses have fibroblast-like morphology and a typical phenotype. Moreover, the patients' MSCs possess well-defined hematopoietic-supporting activity coupled with decreased immunosuppressive potential. These properties prove the clinical application of co-transplantation of autologous hematopoietic stem cells and MSCs in oncohematology to achieve a rapid hematopoietic recovery. Therefore we investigated the safety and hematopoietic effects of MSCs in patients with hematological malignancies receiving peripheral blood hematopoietic stem cell (PBSC) transplantation. We revealed the decreasing of the period of neutropenia and thrombocytopenia in the patients with hematological tumors after high-dose chemotherapy, when autologous PBSC were co-transplanted with ex vivo expanded autologous MSCs. Our results show that co-transplantation of autologous MSCs with PBSC is feasible and safe. The shortening of hematopoietic recovery time suggests that MSC may have a positive impact on hematopoiesis.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1362) "Mesenchymal stromal cells (MSCs) derived from bone marrow possess immunoregulatory activity and are able to support hematopoiesis. Unfortunately, data concerning the biological properties of MSCs in various pathologies is poor and often discrepant. In this study, we demonstrated that MSCs derived from bone marrow of patients with hemoblastoses have fibroblast-like morphology and a typical phenotype. Moreover, the patients' MSCs possess well-defined hematopoietic-supporting activity coupled with decreased immunosuppressive potential. These properties prove the clinical application of co-transplantation of autologous hematopoietic stem cells and MSCs in oncohematology to achieve a rapid hematopoietic recovery. Therefore we investigated the safety and hematopoietic effects of MSCs in patients with hematological malignancies receiving peripheral blood hematopoietic stem cell (PBSC) transplantation. We revealed the decreasing of the period of neutropenia and thrombocytopenia in the patients with hematological tumors after high-dose chemotherapy, when autologous PBSC were co-transplanted with ex vivo expanded autologous MSCs. Our results show that co-transplantation of autologous MSCs with PBSC is feasible and safe. The shortening of hematopoietic recovery time suggests that MSC may have a positive impact on hematopoiesis.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13705" ["VALUE"]=> string(29) "10.3205/ctt-2010-en-000055.01" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(29) "10.3205/ctt-2010-en-000055.01" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(29) "10.3205/ctt-2010-en-000055.01" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13706" ["VALUE"]=> string(137) "Autologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(137) "Autologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(137) "Autologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13782" ["VALUE"]=> array(2) { ["TEXT"]=> string(223) "<p class="bodytext"><sup>1</sup>Institute of Clinical Immunology SB RAMS, Novosibirsk, Russia;<br><sup>2</sup>Novosibirsk State Medical University, Novosibirsk, Russia</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(171) "1Institute of Clinical Immunology SB RAMS, Novosibirsk, Russia;
2Novosibirsk State Medical University, Novosibirsk, Russia
1Institute of Clinical Immunology SB RAMS, Novosibirsk, Russia;
2Novosibirsk State Medical University, Novosibirsk, Russia
Сергеевичева В. В.1, Шевела Е. Я.1, Сизикова С. А.1, Кулагин А. Д.2, Крючкова И. В.1, Гилевич А. В.1, Лисуков И. А.2,
Козлов В. А.1, Останин А. А.1, Черных Е .Р.1
Сергеевичева В. В.1, Шевела Е. Я.1, Сизикова С. А.1, Кулагин А. Д.2, Крючкова И. В.1, Гилевич А. В.1, Лисуков И. А.2,
Козлов В. А.1, Останин А. А.1, Черных Е .Р.1
Мезенхимальные стромальные клетки (МСК), выделенные из костного мозга, обладают иммунорегуляторной активностью и способны поддерживать гемопоэз. К сожалению, данные, характеризующие биологические свойства МСК при различных патологических состояниях, очень немногочисленны и зачастую противоречивы. В настоящей работе мы показали, что МСК, полученные из костного мозга больных гемобластозами, имеют морфологию фибробластоподобных клеток и характерный фенотип. Более того, МСК больных обладают хорошо выраженной способностью к стимуляции гемопоэза, в сочетании со сниженным иммуносупрессорным потенциалом. Эти свойства МСК послужили основанием для клинического применения котрансплантации аутологичных гемопоэтических стволовых клеток и МСК в онкогематологии с целью ускорения восстановления гемопоэза. Нами были исследованы безопасность и гемопоэтические эффекты мезенхимальных стромальных клеток у пациентов с гемобластозами, которым выполнялась трансплантация периферических стволовых кроветворных клеток (ПСКК). Использование ex vivo культивированных МСК, котрансплантированных с аутологичными ПСКК, позволило нам выявить снижение периодов критической нейтропении и тромбоцитопении у больных гемобластозами после высокодозной химиотерапии. Полученные результаты демонстрируют возможность и безопасность совместных трансплантаций МСК и ПСКК. Сокращение периода восстановления кроветворения свидетельствует о позитивном влиянии МСК на гемопоэз.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2735) "Мезенхимальные стромальные клетки (МСК), выделенные из костного мозга, обладают иммунорегуляторной активностью и способны поддерживать гемопоэз. К сожалению, данные, характеризующие биологические свойства МСК при различных патологических состояниях, очень немногочисленны и зачастую противоречивы. В настоящей работе мы показали, что МСК, полученные из костного мозга больных гемобластозами, имеют морфологию фибробластоподобных клеток и характерный фенотип. Более того, МСК больных обладают хорошо выраженной способностью к стимуляции гемопоэза, в сочетании со сниженным иммуносупрессорным потенциалом. Эти свойства МСК послужили основанием для клинического применения котрансплантации аутологичных гемопоэтических стволовых клеток и МСК в онкогематологии с целью ускорения восстановления гемопоэза. Нами были исследованы безопасность и гемопоэтические эффекты мезенхимальных стромальных клеток у пациентов с гемобластозами, которым выполнялась трансплантация периферических стволовых кроветворных клеток (ПСКК). Использование ex vivo культивированных МСК, котрансплантированных с аутологичными ПСКК, позволило нам выявить снижение периодов критической нейтропении и тромбоцитопении у больных гемобластозами после высокодозной химиотерапии. Полученные результаты демонстрируют возможность и безопасность совместных трансплантаций МСК и ПСКК. Сокращение периода восстановления кроветворения свидетельствует о позитивном влиянии МСК на гемопоэз.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13763" ["VALUE"]=> array(2) { ["TEXT"]=> string(366) "<p class="bodytext"><sup>1</sup>Институт клинической иммунологии СО РАМН, Новосибирск, Россия <br /><sup>2</sup>Новосибирский государственный медицинский университет, Новосибирск, Россия </p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(314) "1Институт клинической иммунологии СО РАМН, Новосибирск, Россия
2Новосибирский государственный медицинский университет, Новосибирск, Россия
1Институт клинической иммунологии СО РАМН, Новосибирск, Россия
2Новосибирский государственный медицинский университет, Новосибирск, Россия
Video description
Human mesenchymal stromal cells (hMSCs) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. Although a unique marker for prospective isolation of hMSC is still lacking, they can be easily isolated by plastic adherence from bone marrow and other tissues. Furthermore, hMSCs are capable of expanding in vitro as undifferentiated cells [1]. In vitro, human MSCs are heterogeneous in that they contain morphologically distinct kinds of cells: spindle-shaped and large cuboidal or flattened cells. Colter et al. described an additional population of small and rounded cells with rapidly self-renewing capacity [2].
Some therapeutic approaches have demonstrated the ability of MSCs to regenerate injured tissues [3, 4]. Transplantation experiments in mice and primates have shown that intravenously administered MSCs distribute to several tissues. This implies that MSCs are not only able to migrate but also that the direction of migration is controlled. This migration ability is highly dependent on the flexibility of the cell. For example, basic fibroblast growth factor (bFGF) applied in vitro has been shown to influence not only an attraction but also routing of MSCs by manipulating the orientation of the cytoskeleton. Basic FGF attracted the MSCs by directly influencing the migratory ability through a parallel-orientation of the cells' actin filament [5]. In contrast, the inhibition of the FGF pathway indirectly might cause differentiation [6].
Recently, several factors have been identified which mediate homing and migration of MSCs to injured tissues. For example, ischemia-reperfusion-induced acute kidney injury leads to an increase of the stroma-derived factor 1 (SDF-1) expression in the kidney. At the same time, SDF-1 expression decreases in the bone marrow, thereby reversing the normal gradient between bone marrow and the periphery, and thus mediating homing to the injured tissue and migration of MSCs expressing SDF-1 receptor CXCR4 [7]. Additionally, MSCs were strongly attracted by hepatocyte growth factor (HGF) gradients as well, and proved their chemo-invasiveness across a reconstituted basement membrane in vitro [8]. These results suggest that the SDF-1-CXCR4 and HGF-c-met axes may be involved in recruitment of expanded MSCs for damaged tissues. Finally, the investigation of the traversal mechanism by which MSCs migrate into injured tissues revealed that both P-selectin and VCAM-1 are equally required for this process. Here, MSCs were able to roll and adhere to post-capillary venules in vivo showing similar migratory behaviour to leukocytes [9].
The presented videos show the proliferation of hMSC kept in DMEM-low glucose with fetal calf serum as described (1). Pictures were taken in the phase-contrast mode every 15 min for a time period of 2 days from cultures incubated on an Olympus IX81 microscope equipped with incubation chamber and SIS software. In video 1, on the right picture's edge, a large flattened cell remains almost stationary for the complete recording time. It communicates, “pulsing” with neighbouring cells by extending protrusions forming filopodiae/lobopodiae/lamellopodiae, coming close together or only contacting slightly at the surface. In this cell, the floating changes of the cell body are visible, combined with changes of the nucleus location. The cytoplasmic changes suggest an active orientation of actin-fibers, visible as dark lines directed to the outer borders of the cell. The high count of actin-fibers is particularly characteristic for cells that are not undergoing any more cell division but possibly serving as “nursery” cells. The actin fibers can regularly be observed in old and stressed cultures of hMSC as well (Fig.1).

Figure 1. hMSC culture after prolonged propagation. Ageing cultures show a majority of flattened cells with actin fibres along the cell body. No small and round cells are visible, suggesting a stationary phase of this culture.
Departing cells might leave behind molecular signatures in form of extra-cellular matrices seen as dark-gray lines without any cell body. These traces often are “visited” by trampers, sometimes taking up particles of the traces, sometimes "sniffing" only and continuing on their way. The vagabonds easily might travel more than 500 µm, themselves being only a few µm in size. The length of other cells might reach 300‒600 µm (see upper quarter, video 1), with a width of only ca. 5 µm. During the travel, cells might condense, forming small and round, only loosely adherent cells (see lower quarter, Video 1).
Video 1.
This is the moment when cells prepare for division. The cell divisions itself takes roughly 15‒20 min, and afterwards the new 2 daughter cells either continue to travel as long stretched or short cells or stay in place and provide contact and information to other arriving cells. It is known that MSCs itself express SDF-1 and HGF. Although we do not show stainings for these factors, they might be one part of the interaction of MSCs seen in vitro.
In video 2, the initial cell number was higher, the pictures taken every 10 min for a time period of 2 days, and the cell body shown in semi-3D-dimensionality. Here, the video shows the active interaction of MSCs.
Video 2.
The observer has the impression of witnessing a highly communicative community where the members have a lively exchange experience. The movements of MSCs are accompanied by 1. change of cell shape, 2. polymerisation of cellular actin, 3. formation of lamellopodiae at the leading edge, 4. concentration of the main cell body on the leading edge, and 5. movement of the whole cell body via 6. formation of uropodiae at the lateral edge. The whole process of the MSCs' proliferation and movement observed in vitro is similar to that described for leukocytes during the process of homing to inflamed tissues. Thus, our results support the notion of similar behaviour of MSCs and leukocytes.
However, the high motility of MSCs in vitro also suggests that cloning with cloning rings even at low density of 1‒5 cells/cm2 might not lead to the pure clonal populations clearly derived from 1 cell only. Single travellers easily might contaminate colonies. Therefore, we suggest a more rigid cloning technique either by seeding a limited number of mononuclear cells (MNCs), supposed that the MSC frequency in MNCs is 1/104‒105 or by rigid cloning of 0.3 cells/well and regular observation of the forming clones.
Acknowledgements
This work was supported by the Federal Ministry of Education and Research Germany; Contract grant number: 13N8904 (HyCelex). We thank B. Brunswig-Spickenheier for critical reading of the manuscript.
References
1. Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213(1):18-26.
6. Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bossé M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448(7157):1015-21.
Video description
Human mesenchymal stromal cells (hMSCs) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. Although a unique marker for prospective isolation of hMSC is still lacking, they can be easily isolated by plastic adherence from bone marrow and other tissues. Furthermore, hMSCs are capable of expanding in vitro as undifferentiated cells [1]. In vitro, human MSCs are heterogeneous in that they contain morphologically distinct kinds of cells: spindle-shaped and large cuboidal or flattened cells. Colter et al. described an additional population of small and rounded cells with rapidly self-renewing capacity [2].
Some therapeutic approaches have demonstrated the ability of MSCs to regenerate injured tissues [3, 4]. Transplantation experiments in mice and primates have shown that intravenously administered MSCs distribute to several tissues. This implies that MSCs are not only able to migrate but also that the direction of migration is controlled. This migration ability is highly dependent on the flexibility of the cell. For example, basic fibroblast growth factor (bFGF) applied in vitro has been shown to influence not only an attraction but also routing of MSCs by manipulating the orientation of the cytoskeleton. Basic FGF attracted the MSCs by directly influencing the migratory ability through a parallel-orientation of the cells' actin filament [5]. In contrast, the inhibition of the FGF pathway indirectly might cause differentiation [6].
Recently, several factors have been identified which mediate homing and migration of MSCs to injured tissues. For example, ischemia-reperfusion-induced acute kidney injury leads to an increase of the stroma-derived factor 1 (SDF-1) expression in the kidney. At the same time, SDF-1 expression decreases in the bone marrow, thereby reversing the normal gradient between bone marrow and the periphery, and thus mediating homing to the injured tissue and migration of MSCs expressing SDF-1 receptor CXCR4 [7]. Additionally, MSCs were strongly attracted by hepatocyte growth factor (HGF) gradients as well, and proved their chemo-invasiveness across a reconstituted basement membrane in vitro [8]. These results suggest that the SDF-1-CXCR4 and HGF-c-met axes may be involved in recruitment of expanded MSCs for damaged tissues. Finally, the investigation of the traversal mechanism by which MSCs migrate into injured tissues revealed that both P-selectin and VCAM-1 are equally required for this process. Here, MSCs were able to roll and adhere to post-capillary venules in vivo showing similar migratory behaviour to leukocytes [9].
The presented videos show the proliferation of hMSC kept in DMEM-low glucose with fetal calf serum as described (1). Pictures were taken in the phase-contrast mode every 15 min for a time period of 2 days from cultures incubated on an Olympus IX81 microscope equipped with incubation chamber and SIS software. In video 1, on the right picture's edge, a large flattened cell remains almost stationary for the complete recording time. It communicates, “pulsing” with neighbouring cells by extending protrusions forming filopodiae/lobopodiae/lamellopodiae, coming close together or only contacting slightly at the surface. In this cell, the floating changes of the cell body are visible, combined with changes of the nucleus location. The cytoplasmic changes suggest an active orientation of actin-fibers, visible as dark lines directed to the outer borders of the cell. The high count of actin-fibers is particularly characteristic for cells that are not undergoing any more cell division but possibly serving as “nursery” cells. The actin fibers can regularly be observed in old and stressed cultures of hMSC as well (Fig.1).

Figure 1. hMSC culture after prolonged propagation. Ageing cultures show a majority of flattened cells with actin fibres along the cell body. No small and round cells are visible, suggesting a stationary phase of this culture.
Departing cells might leave behind molecular signatures in form of extra-cellular matrices seen as dark-gray lines without any cell body. These traces often are “visited” by trampers, sometimes taking up particles of the traces, sometimes "sniffing" only and continuing on their way. The vagabonds easily might travel more than 500 µm, themselves being only a few µm in size. The length of other cells might reach 300‒600 µm (see upper quarter, video 1), with a width of only ca. 5 µm. During the travel, cells might condense, forming small and round, only loosely adherent cells (see lower quarter, Video 1).
Video 1.
This is the moment when cells prepare for division. The cell divisions itself takes roughly 15‒20 min, and afterwards the new 2 daughter cells either continue to travel as long stretched or short cells or stay in place and provide contact and information to other arriving cells. It is known that MSCs itself express SDF-1 and HGF. Although we do not show stainings for these factors, they might be one part of the interaction of MSCs seen in vitro.
In video 2, the initial cell number was higher, the pictures taken every 10 min for a time period of 2 days, and the cell body shown in semi-3D-dimensionality. Here, the video shows the active interaction of MSCs.
Video 2.
The observer has the impression of witnessing a highly communicative community where the members have a lively exchange experience. The movements of MSCs are accompanied by 1. change of cell shape, 2. polymerisation of cellular actin, 3. formation of lamellopodiae at the leading edge, 4. concentration of the main cell body on the leading edge, and 5. movement of the whole cell body via 6. formation of uropodiae at the lateral edge. The whole process of the MSCs' proliferation and movement observed in vitro is similar to that described for leukocytes during the process of homing to inflamed tissues. Thus, our results support the notion of similar behaviour of MSCs and leukocytes.
However, the high motility of MSCs in vitro also suggests that cloning with cloning rings even at low density of 1‒5 cells/cm2 might not lead to the pure clonal populations clearly derived from 1 cell only. Single travellers easily might contaminate colonies. Therefore, we suggest a more rigid cloning technique either by seeding a limited number of mononuclear cells (MNCs), supposed that the MSC frequency in MNCs is 1/104‒105 or by rigid cloning of 0.3 cells/well and regular observation of the forming clones.
Acknowledgements
This work was supported by the Federal Ministry of Education and Research Germany; Contract grant number: 13N8904 (HyCelex). We thank B. Brunswig-Spickenheier for critical reading of the manuscript.
References
1. Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213(1):18-26.
6. Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bossé M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448(7157):1015-21.
Саша Ланге, Аксель Цандер, Клаудиа Ланге
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13848" ["VALUE"]=> array(2) { ["TEXT"]=> string(1078) "<p class="bodytext">Мезенхимные стволовые клетки человека (МСКЧ) в настоящее время интенсивно изучаются на предмет их потенциального применения в качестве средства клеточной терапии в регенеративной медицине. Их можно легко выделять, например, из костного мозга посредством залипания на пластике, и размножать in vitro. Представленные видеокадры показывают пролиферацию МСКЧ, их взаимодействия между собой и процесс их деления. Высокая степень подвижности МСКЧ может быть причиной возможных нарушений формирования «чистых» клонов даже при низкой плотности посева клеток.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1056) "Мезенхимные стволовые клетки человека (МСКЧ) в настоящее время интенсивно изучаются на предмет их потенциального применения в качестве средства клеточной терапии в регенеративной медицине. Их можно легко выделять, например, из костного мозга посредством залипания на пластике, и размножать in vitro. Представленные видеокадры показывают пролиферацию МСКЧ, их взаимодействия между собой и процесс их деления. Высокая степень подвижности МСКЧ может быть причиной возможных нарушений формирования «чистых» клонов даже при низкой плотности посева клеток.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13849" ["VALUE"]=> string(29) "10.3205/ctt-2010-en-000051.01" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(29) "10.3205/ctt-2010-en-000051.01" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13859" ["VALUE"]=> array(2) { ["TEXT"]=> string(67) "<p> Sascha Lange, Axel R. Zander, Claudia Lange </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(55) "Sascha Lange, Axel R. Zander, Claudia Lange
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13860" ["VALUE"]=> array(2) { ["TEXT"]=> string(563) "<p class="bodytext"> Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany<br> <br> <b>Correspondence: </b><br> Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.gpperkiDyoi2yrm1leqfyvk2hi');">cllange@<span style="display:none;">spam is bad</span>uke.uni-hamburg.de</a> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(467) "
Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany
Correspondence:
Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: cllange@uke.uni-hamburg.de
Human mesenchymal stromal cells (hMSC) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. They can be easily isolated, e.g., from bone marrow by plastic adherence, and expanded in vitro. These videos show the proliferation of hMSC, and how they interact with each other and divide. The large distances hMSC can conquer call into question the cloning carried out by low-density seeding.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13850" ["VALUE"]=> string(42) "The MSCs' in vitro community: where to go?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(42) "The MSCs' in vitro community: where to go?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(12) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13859" ["VALUE"]=> array(2) { ["TEXT"]=> string(67) "<p> Sascha Lange, Axel R. Zander, Claudia Lange </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(55) "Sascha Lange, Axel R. Zander, Claudia Lange
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(55) "Sascha Lange, Axel R. Zander, Claudia Lange
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13861" ["VALUE"]=> array(2) { ["TEXT"]=> string(505) "<p class="bodytext"> Human mesenchymal stromal cells (hMSC) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. They can be easily isolated, e.g., from bone marrow by plastic adherence, and expanded in vitro. These videos show the proliferation of hMSC, and how they interact with each other and divide. The large distances hMSC can conquer call into question the cloning carried out by low-density seeding. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(483) "Human mesenchymal stromal cells (hMSC) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. They can be easily isolated, e.g., from bone marrow by plastic adherence, and expanded in vitro. These videos show the proliferation of hMSC, and how they interact with each other and divide. The large distances hMSC can conquer call into question the cloning carried out by low-density seeding.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(483) "Human mesenchymal stromal cells (hMSC) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. They can be easily isolated, e.g., from bone marrow by plastic adherence, and expanded in vitro. These videos show the proliferation of hMSC, and how they interact with each other and divide. The large distances hMSC can conquer call into question the cloning carried out by low-density seeding.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13849" ["VALUE"]=> string(29) "10.3205/ctt-2010-en-000051.01" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(29) "10.3205/ctt-2010-en-000051.01" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(29) "10.3205/ctt-2010-en-000051.01" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13850" ["VALUE"]=> string(42) "The MSCs' in vitro community: where to go?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(42) "The MSCs' in vitro community: where to go?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(42) "The MSCs' in vitro community: where to go?" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13860" ["VALUE"]=> array(2) { ["TEXT"]=> string(563) "<p class="bodytext"> Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany<br> <br> <b>Correspondence: </b><br> Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.gpperkiDyoi2yrm1leqfyvk2hi');">cllange@<span style="display:none;">spam is bad</span>uke.uni-hamburg.de</a> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(467) "
Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany
Correspondence:
Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: cllange@uke.uni-hamburg.de
Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany
Correspondence:
Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: cllange@uke.uni-hamburg.de
Саша Ланге, Аксель Цандер, Клаудиа Ланге
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(80) "Саша Ланге, Аксель Цандер, Клаудиа Ланге
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13840" ["VALUE"]=> string(22) "10/29/2009 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/29/2009 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "10/29/2009 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13841" ["VALUE"]=> string(22) "02/12/2010 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/12/2010 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "02/12/2010 12:00:00 am" } ["PUBLISHED"]=> array(37) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13842" ["VALUE"]=> string(22) "02/12/2010 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "02/12/2010 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "02/12/2010 12:00:00 am" } ["KEYWORDS"]=> array(38) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> array(5) { [0]=> string(5) "13910" [1]=> string(5) "13911" [2]=> string(5) "13912" [3]=> string(5) "13913" [4]=> string(5) "13914" } ["VALUE"]=> array(5) { [0]=> string(2) "83" [1]=> string(3) "980" [2]=> string(3) "981" [3]=> string(3) "982" [4]=> string(3) "983" } ["DESCRIPTION"]=> array(5) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" } ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(5) { [0]=> string(2) "83" [1]=> string(3) "980" [2]=> string(3) "981" [3]=> string(3) "982" [4]=> string(3) "983" } ["~DESCRIPTION"]=> array(5) { [0]=> string(0) "" [1]=> string(0) "" [2]=> string(0) "" [3]=> string(0) "" [4]=> string(0) "" } ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> array(5) { [0]=> string(97) "мезенхимные стволовые клетки" [1]=> string(52) "МСКЧ" [2]=> string(68) "пролиферация" [3]=> string(67) "деление in vitro" [4]=> string(87) "замедленная фотосъемка" } ["LINK_ELEMENT_VALUE"]=> bool(false) } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "13848" ["VALUE"]=> array(2) { ["TEXT"]=> string(1078) "<p class="bodytext">Мезенхимные стволовые клетки человека (МСКЧ) в настоящее время интенсивно изучаются на предмет их потенциального применения в качестве средства клеточной терапии в регенеративной медицине. Их можно легко выделять, например, из костного мозга посредством залипания на пластике, и размножать in vitro. Представленные видеокадры показывают пролиферацию МСКЧ, их взаимодействия между собой и процесс их деления. Высокая степень подвижности МСКЧ может быть причиной возможных нарушений формирования «чистых» клонов даже при низкой плотности посева клеток.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1056) "Мезенхимные стволовые клетки человека (МСКЧ) в настоящее время интенсивно изучаются на предмет их потенциального применения в качестве средства клеточной терапии в регенеративной медицине. Их можно легко выделять, например, из костного мозга посредством залипания на пластике, и размножать in vitro. Представленные видеокадры показывают пролиферацию МСКЧ, их взаимодействия между собой и процесс их деления. Высокая степень подвижности МСКЧ может быть причиной возможных нарушений формирования «чистых» клонов даже при низкой плотности посева клеток.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1056) "Мезенхимные стволовые клетки человека (МСКЧ) в настоящее время интенсивно изучаются на предмет их потенциального применения в качестве средства клеточной терапии в регенеративной медицине. Их можно легко выделять, например, из костного мозга посредством залипания на пластике, и размножать in vitro. Представленные видеокадры показывают пролиферацию МСКЧ, их взаимодействия между собой и процесс их деления. Высокая степень подвижности МСКЧ может быть причиной возможных нарушений формирования «чистых» клонов даже при низкой плотности посева клеток.
" } } } }Articles
Christine Voelkel1,2, Anke Lührmann2, Christopher Baum1,3, and Heiko E. von der Leyen2
Anja Elstner*1, Annette Noack*1, Alexander Damaschun1, Glyn Stacey2, Begoňa Arán3, Ilona Gawronska1, Anna Veiga3,4,
Joeri Borstlap1 and Andreas Kurtz1
* Both authors contributed equally
Vera V. Sergeevicheva1, Ekaterina Y. Shevela1, Svetlana A. Sizikova1, Alexander D. Kulagin2, Irina V. Kruchkova1,
Andrey V. Gilevich1, Igor A. Lisukov2, Vladimir A. Kozlov1, Alexander A. Ostanin1, Elena R. Chernykh1
Sascha Lange, Axel R. Zander, Claudia Lange
Articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13541
[1] => 13542
[2] => 13543
[3] => 13544
[4] => 13545
)
[VALUE] => Array
(
[0] => 688
[1] => 955
[2] => 956
[3] => 804
[4] => 957
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 688
[1] => 955
[2] => 956
[3] => 804
[4] => 957
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
)
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13473
[VALUE] => 03/13/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/13/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13474
[VALUE] => 03/30/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 03/30/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13475
[VALUE] => 05/14/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 05/14/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13448
[VALUE] => 952
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 952
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13546
[1] => 13547
[2] => 13548
[3] => 13549
)
[VALUE] => Array
(
[0] => 950
[1] => 951
[2] => 952
[3] => 954
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 950
[1] => 951
[2] => 952
[3] => 954
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13489
[VALUE] => Array
(
[TEXT] => <p>Велькель К., Люрманн А., Баум К., фон дер Ляйен Х.Э. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Велькель К., Люрманн А., Баум К., фон дер Ляйен Х.Э.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13490
[VALUE] => Array
(
[TEXT] => <p class="bodytext">Генная терапия предназначена для лечения генетических дефектов путем введения корригированной копии мутантного гена. Однако, в отличие от классического лекарственного лечения, генная терапия является подходом, высокоспецифичным в отношении больного и заболевания. Трансген как таковой должен быть адаптирован к каждому специфическому подходу и медицинской цели. С 1989 по 2009 гг. были одобрены 1537 клинических испытаний в области генной терапии с применением 35 различных типов векторов. Кроме того, с одной стороны, генно-терапевтический подход позволяет достичь эффективной долгосрочной коррекции генетического дефекта, а с другой стороны – несет опасность побочных эффектов. Ряд работ по лечению тяжелого комбинированного иммунодефицита, сцепленного с Х-хромосомой, показал развитие у нескольких больных лимфопролиферативных заболеваний в связи с инсерционным мутагенезом. Такие побочные эффекты, наряду с иммуногенностью, передачей генетических последовательностей, а также токсичных и инфекционных побочных продуктов, связанных с приготовлением вектора, делает необходимой строгое регулирование их применения со стороны властей. Соответствующие правила могут быть определены лишь в общих понятиях, и нельзя создать единый документ, пригодный для конкретной цели и индивидуального использования каждого продукта. <br /><br />Встраивающиеся векторы, дефектные по репликационным свойствам, основанные на гамма-ретровирусах, часто применялись в исходных протоколах генной терапии, и их опасность может обсуждаться в качестве примера, в частности, инсерционный мутагенез, который возникает во многом из-за случайного характера встраивания вируса по отношению к целевым генным локусам. Кроме того, могут быть вызваны и генотоксические эффекты, в основном связанные с дизрегуляцией активности нормальных генов при введении регуляторных элементов в составе вектора. Другой крупной проблемой вирусного переноса генов является их «вертикальный» перенос в зародышевых клеточных линиях, в частности, через пролиферирующие сперматогониальные клетки, которые могут быть мишенью для гамма-ретровирусных векторов. <br /><br />Для того, чтобы упредить эти опасности, регулирующая система должна уточнить единообразные требования для того, чтобы обеспечить соответствия между стандартами качества и, тем самым, гарантировать безопасность участников испытания. Данная статья суммирует наиболее важные регулирующие документы, которые следует учитывать до вхождения в фазу клинической разработки – не только для Германии, но и в европейской перспективе. Приводятся ссылки на применимые для этого руководящие указания в отношении генно-терапевтических медицинских продуктов (ГТМП), с соответствующими определениями для таких продуктов. <br /><br />Производство ГТМП должно согласовываться с требованиями Директивы Европейского Союза 2003/94/EC о качественной практике производства (GMP). В Германии эта практика регулируется Предписаниями по производству медицинских продуктов и активных фармацевтических ингредиентов (AMWHV). Важно, чтобы особые аспекты безопасности в отношении ГТМП учитывались до использования этих продуктов в клинике. В любом случае, регулирующие указания имеют в своей основе: (1) имеющиеся научные знания, (2) научный опыт в данной области исследований, и они возникают в результате постоянного взаимодействия между исследователями и экспертами в области регулирования.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Генная терапия предназначена для лечения генетических дефектов путем введения корригированной копии мутантного гена. Однако, в отличие от классического лекарственного лечения, генная терапия является подходом, высокоспецифичным в отношении больного и заболевания. Трансген как таковой должен быть адаптирован к каждому специфическому подходу и медицинской цели. С 1989 по 2009 гг. были одобрены 1537 клинических испытаний в области генной терапии с применением 35 различных типов векторов. Кроме того, с одной стороны, генно-терапевтический подход позволяет достичь эффективной долгосрочной коррекции генетического дефекта, а с другой стороны – несет опасность побочных эффектов. Ряд работ по лечению тяжелого комбинированного иммунодефицита, сцепленного с Х-хромосомой, показал развитие у нескольких больных лимфопролиферативных заболеваний в связи с инсерционным мутагенезом. Такие побочные эффекты, наряду с иммуногенностью, передачей генетических последовательностей, а также токсичных и инфекционных побочных продуктов, связанных с приготовлением вектора, делает необходимой строгое регулирование их применения со стороны властей. Соответствующие правила могут быть определены лишь в общих понятиях, и нельзя создать единый документ, пригодный для конкретной цели и индивидуального использования каждого продукта.
Встраивающиеся векторы, дефектные по репликационным свойствам, основанные на гамма-ретровирусах, часто применялись в исходных протоколах генной терапии, и их опасность может обсуждаться в качестве примера, в частности, инсерционный мутагенез, который возникает во многом из-за случайного характера встраивания вируса по отношению к целевым генным локусам. Кроме того, могут быть вызваны и генотоксические эффекты, в основном связанные с дизрегуляцией активности нормальных генов при введении регуляторных элементов в составе вектора. Другой крупной проблемой вирусного переноса генов является их «вертикальный» перенос в зародышевых клеточных линиях, в частности, через пролиферирующие сперматогониальные клетки, которые могут быть мишенью для гамма-ретровирусных векторов.
Для того, чтобы упредить эти опасности, регулирующая система должна уточнить единообразные требования для того, чтобы обеспечить соответствия между стандартами качества и, тем самым, гарантировать безопасность участников испытания. Данная статья суммирует наиболее важные регулирующие документы, которые следует учитывать до вхождения в фазу клинической разработки – не только для Германии, но и в европейской перспективе. Приводятся ссылки на применимые для этого руководящие указания в отношении генно-терапевтических медицинских продуктов (ГТМП), с соответствующими определениями для таких продуктов.
Производство ГТМП должно согласовываться с требованиями Директивы Европейского Союза 2003/94/EC о качественной практике производства (GMP). В Германии эта практика регулируется Предписаниями по производству медицинских продуктов и активных фармацевтических ингредиентов (AMWHV). Важно, чтобы особые аспекты безопасности в отношении ГТМП учитывались до использования этих продуктов в клинике. В любом случае, регулирующие указания имеют в своей основе: (1) имеющиеся научные знания, (2) научный опыт в данной области исследований, и они возникают в результате постоянного взаимодействия между исследователями и экспертами в области регулирования.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13452
[VALUE] => 10.3205/ctt-2009-en-000044.01
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.3205/ctt-2009-en-000044.01
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13502
[VALUE] => Array
(
[TEXT] => <p>
Christine Voelkel<sup>1,2</sup>, Anke Lührmann<sup>2</sup>, Christopher Baum<sup>1,3</sup>, and Heiko E. von der Leyen<sup>2</sup>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Christine Voelkel1,2, Anke Lührmann2, Christopher Baum1,3, and Heiko E. von der Leyen2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13503
[VALUE] => Array
(
[TEXT] => <p class="bodytext"><sup>1</sup>Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;<br><sup> 2</sup>Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany; <br><sup>3</sup>Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA
</p>
<br>
<p>
<b>Correspondence </b><br>Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany, <br>Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.zhpicirDgpmrmgep1xvmep1girxiv2hi');">vdleyen@<span style="display:none;">spam is bad</span>clinical-trial-center.de</a>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;
2Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany;
3Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA
Correspondence
Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany,
Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: vdleyen@clinical-trial-center.de
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13504
[VALUE] => Array
(
[TEXT] => <p class="bodytext">Retrovirus mediated gene therapy has already proven to be more than just a theoretical option to treat patients with severe genetic defects. Clinical gene therapy trials of X-linked severe combined immunodeficiency or adenosine deaminase deficiency have demonstrated the success and potential benefit of the therapy. Nevertheless, the complexity of the therapeutic products and their biological origin, as well as virus-related safety concerns require the need of a strict regulatory framework in order to guarantee the quality of the individual products and safety of the patients. The aim of this review is to give an overview of the rapidly evolving regulatory framework of Advanced Therapy Medicinal Products in Europe. We will summarize the most important regulatory documents to be considered before entering the clinical development phase – not only from a German but also from a European perspective.<p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Retrovirus mediated gene therapy has already proven to be more than just a theoretical option to treat patients with severe genetic defects. Clinical gene therapy trials of X-linked severe combined immunodeficiency or adenosine deaminase deficiency have demonstrated the success and potential benefit of the therapy. Nevertheless, the complexity of the therapeutic products and their biological origin, as well as virus-related safety concerns require the need of a strict regulatory framework in order to guarantee the quality of the individual products and safety of the patients. The aim of this review is to give an overview of the rapidly evolving regulatory framework of Advanced Therapy Medicinal Products in Europe. We will summarize the most important regulatory documents to be considered before entering the clinical development phase – not only from a German but also from a European perspective.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13453
[VALUE] => Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Retrovirus mediated hematopoietic gene therapy: A European regulatory perspective with special focus on the situation in Germany
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13491
[VALUE] => 647
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 647
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13492
[VALUE] => 648
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 648
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Christine Voelkel1,2, Anke Lührmann2, Christopher Baum1,3, and Heiko E. von der Leyen2
1Department of Experimental Hematology, Hannover Medical School, D-30625 Hannover, Germany;
2Hannover Clinical Trial Center GmbH, D-30625 Hannover, Germany;
3Division of Experimental Hematology, Cincinnati Children’s Research Foundation, Cincinnati, Ohio, USA
Correspondence
Prof. Dr. Heiko E. von der Leyen, Hannover Clinical Trial Center GmbH, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany,
Tel.: +49 511 533 333 0, Fax: +49 511 533 333 99, E-mail: vdleyen@clinical-trial-center.de
Retrovirus mediated gene therapy has already proven to be more than just a theoretical option to treat patients with severe genetic defects. Clinical gene therapy trials of X-linked severe combined immunodeficiency or adenosine deaminase deficiency have demonstrated the success and potential benefit of the therapy. Nevertheless, the complexity of the therapeutic products and their biological origin, as well as virus-related safety concerns require the need of a strict regulatory framework in order to guarantee the quality of the individual products and safety of the patients. The aim of this review is to give an overview of the rapidly evolving regulatory framework of Advanced Therapy Medicinal Products in Europe. We will summarize the most important regulatory documents to be considered before entering the clinical development phase – not only from a German but also from a European perspective.
Articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13670
[1] => 13671
[2] => 13672
[3] => 13673
)
[VALUE] => Array
(
[0] => 968
[1] => 969
[2] => 970
[3] => 971
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 968
[1] => 969
[2] => 970
[3] => 971
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13611
[VALUE] => 12/02/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/02/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13612
[VALUE] => 12/07/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/07/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13613
[VALUE] => 12/30/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/30/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13569
[VALUE] => 958
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 958
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13674
[1] => 13675
[2] => 13676
[3] => 13677
[4] => 13678
[5] => 13679
[6] => 13680
[7] => 13681
[8] => 13682
)
[VALUE] => Array
(
[0] => 958
[1] => 959
[2] => 960
[3] => 962
[4] => 963
[5] => 964
[6] => 965
[7] => 966
[8] => 967
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
[8] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 958
[1] => 959
[2] => 960
[3] => 962
[4] => 963
[5] => 964
[6] => 965
[7] => 966
[8] => 967
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
[8] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13650
[VALUE] => Array
(
[TEXT] => <p>Аня Эльстнер*, Аннетт Ноак*, Александр Дамашун, Глин Стэйси, Бегонья Аран, Илона Гавронска, Анна Вейга, <br>Йори Борстлап, Андреас Куртц</p><p>* Вклад обоих авторов одинаков</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Аня Эльстнер*, Аннетт Ноак*, Александр Дамашун, Глин Стэйси, Бегонья Аран, Илона Гавронска, Анна Вейга,
Йори Борстлап, Андреас Куртц
* Вклад обоих авторов одинаков
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13651
[VALUE] => Array
(
[TEXT] => <p class="bodytext">Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, <a href="http://www.hescreg.eu/" target="_blank">www.hescreg.eu</a>), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Специфика межнациональных особенностей основ правового регулирования в области научных исследований эмбриональных стволовых клеток человека (ЭСКЧ) все еще остается весьма сложным вопросом из-за множества исторических, культурных и этических мнений, которые преобладают в тех или иных странах. Путем учреждения Европейского Регистра стволовых клеток человека (ЕРСК, hESCreg, www.hescreg.eu), Европейский Союз в 2007 г. инициировал первую концепцию, касающуюся сравнений и научно обоснованной гармонизации законодательства в области ЭСКЧ. Консорциум ЕРСК в настоящее время включает в себя представителей 15 стран (в том числе европейских и неевропейских государств), которые действуют в качестве национальных контактных организаций, и регулярно обновляют Регистр информацией о стволовых клетках, а также о законодательных и этических дискуссиях в области исследований стволовых клеток. Некоторые из этих стран недавно испытали серьезные изменения законодательства, которые вступили в силу в Китае, Финляндии, Норвегии, Великобритании и США. В других странах ожидаются изменения правил в близком будущем, как, например, в Австралии, Индии и Турции. В то время как многие страны ввели законоположения, направленные на либерализацию исследований ЭСК, некоторые государства придерживаются более строгих правил, касающихся ЭСК (например, Турция, Германия. Венгрия и Италия). В данной статье обобщена и собрана информация о нынешнем состоянии международных правил ЕРСК, которая представлена нами в настоящем докладе.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13573
[VALUE] => 10.3205/ctt-2009-en-000052.01
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.3205/ctt-2009-en-000052.01
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13665
[VALUE] => Array
(
[TEXT] => <p>Anja Elstner*<sup>1</sup>, Annette Noack*<sup>1</sup>, Alexander Damaschun<sup>1</sup>, Glyn Stacey<sup>2</sup>, Begoňa Arán<sup>3</sup>, Ilona Gawronska<sup>1</sup>, Anna Veiga<sup>3,4</sup>, <br>Joeri Borstlap<sup>1</sup> and Andreas Kurtz<sup>1</sup></p>
<p class="bodytext">* Both authors contributed equally</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Anja Elstner*1, Annette Noack*1, Alexander Damaschun1, Glyn Stacey2, Begoňa Arán3, Ilona Gawronska1, Anna Veiga3,4,
Joeri Borstlap1 and Andreas Kurtz1
* Both authors contributed equally
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13666
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup>Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; <sup>2</sup>The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; <sup>3</sup>Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); <sup>4</sup>Institut Universitari Dexeus, Barcelona, Spain
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; 2The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; 3Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); 4Institut Universitari Dexeus, Barcelona, Spain
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13667
[VALUE] => Array
(
[TEXT] => <p class="bodytext">The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13574
[VALUE] => Ongoing dynamics in the regulatory landscape of human embryonic stem cell research
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Ongoing dynamics in the regulatory landscape of human embryonic stem cell research
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13668
[VALUE] => 656
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 656
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13669
[VALUE] => 657
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 657
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Anja Elstner*1, Annette Noack*1, Alexander Damaschun1, Glyn Stacey2, Begoňa Arán3, Ilona Gawronska1, Anna Veiga3,4,
Joeri Borstlap1 and Andreas Kurtz1
* Both authors contributed equally
1Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, Germany; 2The UK Stem Cell Bank, National Institute for Biological Standards and Control, South Mimms, UK; 3Banc de Linies Cel.lulars, Center of Regenerative Medicine Barcelona (CMRB); 4Institut Universitari Dexeus, Barcelona, Spain
The international situation regarding the specific nature of regulation on human embryonic stem cell research is still quite complex due to pluralistic historical, cultural and ethical opinions that dominate in respective countries. By establishing the Human European Stem Cell Registry (hESCreg, www.hescreg.eu) in 2007, the EU initiated the first steps towards comparison and science-driven harmonization of hESC legislation. The hESCreg consortium currently includes representatives from 15 countries (including European and non-European countries), who act as National Contacts for hESCreg and regularly update the registry with information on stem cells as well as legislative and ethical discussions in the field of stem cell research. Several of these countries have experienced recent legislative changes; these were implemented in China, Finland, the Netherlands, Norway, the United Kingdom, and the USA. Others expect regulatory changes in the near future, such as in Australia, India, and Turkey. Whilst many countries have introduced legislation to liberalize embryonic stem cell research, others hold to stricter regulations on embryo-derived stem cells (e.g. Turkey, Germany, Hungary, and Italy). In this article we summarize and complete the information on the current status of international hESC regulation provided in our recent report.
Articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13812
[1] => 13813
[2] => 13814
[3] => 13815
)
[VALUE] => Array
(
[0] => 977
[1] => 81
[2] => 978
[3] => 58
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 977
[1] => 81
[2] => 978
[3] => 58
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
)
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13734
[VALUE] => 08/25/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 08/25/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13735
[VALUE] => 01/20/2010 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 01/20/2010 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13736
[VALUE] => 02/16/2010 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/16/2010 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13737
[VALUE] => 450
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 450
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13816
[1] => 13817
[2] => 13818
[3] => 13819
[4] => 13820
[5] => 13821
[6] => 13822
[7] => 13823
[8] => 13824
[9] => 13825
)
[VALUE] => Array
(
[0] => 551
[1] => 972
[2] => 552
[3] => 503
[4] => 973
[5] => 555
[6] => 450
[7] => 975
[8] => 976
[9] => 556
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
[8] =>
[9] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 551
[1] => 972
[2] => 552
[3] => 503
[4] => 973
[5] => 555
[6] => 450
[7] => 975
[8] => 976
[9] => 556
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
[5] =>
[6] =>
[7] =>
[8] =>
[9] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13762
[VALUE] => Array
(
[TEXT] => <p>Сергеевичева В. В.<sup>1</sup>, Шевела Е. Я.<sup>1</sup>, Сизикова С. А.<sup>1</sup>, Кулагин А. Д.<sup>2</sup>, Крючкова И. В.<sup>1</sup>, Гилевич А. В.<sup>1</sup>, Лисуков И. А.<sup>2</sup>, <br>Козлов В. А.<sup>1</sup>, Останин А. А.<sup>1</sup>, Черных Е .Р.<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Сергеевичева В. В.1, Шевела Е. Я.1, Сизикова С. А.1, Кулагин А. Д.2, Крючкова И. В.1, Гилевич А. В.1, Лисуков И. А.2,
Козлов В. А.1, Останин А. А.1, Черных Е .Р.1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13763
[VALUE] => Array
(
[TEXT] => <p class="bodytext"><sup>1</sup>Институт клинической иммунологии СО РАМН, Новосибирск, Россия <br /><sup>2</sup>Новосибирский государственный медицинский университет, Новосибирск, Россия
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1Институт клинической иммунологии СО РАМН, Новосибирск, Россия
2Новосибирский государственный медицинский университет, Новосибирск, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13764
[VALUE] => Array
(
[TEXT] => <p class="bodytext">Мезенхимальные стромальные клетки (МСК), выделенные из костного мозга, обладают иммунорегуляторной активностью и способны поддерживать гемопоэз. К сожалению, данные, характеризующие биологические свойства МСК при различных патологических состояниях, очень немногочисленны и зачастую противоречивы. В настоящей работе мы показали, что МСК, полученные из костного мозга больных гемобластозами, имеют морфологию фибробластоподобных клеток и характерный фенотип. Более того, МСК больных обладают хорошо выраженной способностью к стимуляции гемопоэза, в сочетании со сниженным иммуносупрессорным потенциалом. Эти свойства МСК послужили основанием для клинического применения котрансплантации аутологичных гемопоэтических стволовых клеток и МСК в онкогематологии с целью ускорения восстановления гемопоэза. Нами были исследованы безопасность и гемопоэтические эффекты мезенхимальных стромальных клеток у пациентов с гемобластозами, которым выполнялась трансплантация периферических стволовых кроветворных клеток (ПСКК). Использование ex vivo культивированных МСК, котрансплантированных с аутологичными ПСКК, позволило нам выявить снижение периодов критической нейтропении и тромбоцитопении у больных гемобластозами после высокодозной химиотерапии. Полученные результаты демонстрируют возможность и безопасность совместных трансплантаций МСК и ПСКК. Сокращение периода восстановления кроветворения свидетельствует о позитивном влиянии МСК на гемопоэз.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Мезенхимальные стромальные клетки (МСК), выделенные из костного мозга, обладают иммунорегуляторной активностью и способны поддерживать гемопоэз. К сожалению, данные, характеризующие биологические свойства МСК при различных патологических состояниях, очень немногочисленны и зачастую противоречивы. В настоящей работе мы показали, что МСК, полученные из костного мозга больных гемобластозами, имеют морфологию фибробластоподобных клеток и характерный фенотип. Более того, МСК больных обладают хорошо выраженной способностью к стимуляции гемопоэза, в сочетании со сниженным иммуносупрессорным потенциалом. Эти свойства МСК послужили основанием для клинического применения котрансплантации аутологичных гемопоэтических стволовых клеток и МСК в онкогематологии с целью ускорения восстановления гемопоэза. Нами были исследованы безопасность и гемопоэтические эффекты мезенхимальных стромальных клеток у пациентов с гемобластозами, которым выполнялась трансплантация периферических стволовых кроветворных клеток (ПСКК). Использование ex vivo культивированных МСК, котрансплантированных с аутологичными ПСКК, позволило нам выявить снижение периодов критической нейтропении и тромбоцитопении у больных гемобластозами после высокодозной химиотерапии. Полученные результаты демонстрируют возможность и безопасность совместных трансплантаций МСК и ПСКК. Сокращение периода восстановления кроветворения свидетельствует о позитивном влиянии МСК на гемопоэз.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13705
[VALUE] => 10.3205/ctt-2010-en-000055.01
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.3205/ctt-2010-en-000055.01
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13781
[VALUE] => Array
(
[TEXT] => <p>Vera V. Sergeevicheva<sup>1</sup>, Ekaterina Y. Shevela<sup>1</sup>, Svetlana A. Sizikova<sup>1</sup>, Alexander D. Kulagin<sup>2</sup>, Irina V. Kruchkova<sup>1</sup>, <br>Andrey V. Gilevich<sup>1</sup>, Igor A. Lisukov<sup>2</sup>, Vladimir A. Kozlov<sup>1</sup>, Alexander A. Ostanin<sup>1</sup>, Elena R. Chernykh<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Vera V. Sergeevicheva1, Ekaterina Y. Shevela1, Svetlana A. Sizikova1, Alexander D. Kulagin2, Irina V. Kruchkova1,
Andrey V. Gilevich1, Igor A. Lisukov2, Vladimir A. Kozlov1, Alexander A. Ostanin1, Elena R. Chernykh1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13782
[VALUE] => Array
(
[TEXT] => <p class="bodytext"><sup>1</sup>Institute of Clinical Immunology SB RAMS, Novosibirsk, Russia;<br><sup>2</sup>Novosibirsk State Medical University, Novosibirsk, Russia</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1Institute of Clinical Immunology SB RAMS, Novosibirsk, Russia;
2Novosibirsk State Medical University, Novosibirsk, Russia
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13783
[VALUE] => Array
(
[TEXT] => <p class="bodytext">Mesenchymal stromal cells (MSCs) derived from bone marrow possess immunoregulatory activity and are able to support hematopoiesis. Unfortunately, data concerning the biological properties of MSCs in various pathologies is poor and often discrepant. In this study, we demonstrated that MSCs derived from bone marrow of patients with hemoblastoses have fibroblast-like morphology and a typical phenotype. Moreover, the patients' MSCs possess well-defined hematopoietic-supporting activity coupled with decreased immunosuppressive potential. These properties prove the clinical application of co-transplantation of autologous hematopoietic stem cells and MSCs in oncohematology to achieve a rapid hematopoietic recovery. Therefore we investigated the safety and hematopoietic effects of MSCs in patients with hematological malignancies receiving peripheral blood hematopoietic stem cell (PBSC) transplantation. We revealed the decreasing of the period of neutropenia and thrombocytopenia in the patients with hematological tumors after high-dose chemotherapy, when autologous PBSC were co-transplanted with ex vivo expanded autologous MSCs. Our results show that co-transplantation of autologous MSCs with PBSC is feasible and safe. The shortening of hematopoietic recovery time suggests that MSC may have a positive impact on hematopoiesis.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Mesenchymal stromal cells (MSCs) derived from bone marrow possess immunoregulatory activity and are able to support hematopoiesis. Unfortunately, data concerning the biological properties of MSCs in various pathologies is poor and often discrepant. In this study, we demonstrated that MSCs derived from bone marrow of patients with hemoblastoses have fibroblast-like morphology and a typical phenotype. Moreover, the patients' MSCs possess well-defined hematopoietic-supporting activity coupled with decreased immunosuppressive potential. These properties prove the clinical application of co-transplantation of autologous hematopoietic stem cells and MSCs in oncohematology to achieve a rapid hematopoietic recovery. Therefore we investigated the safety and hematopoietic effects of MSCs in patients with hematological malignancies receiving peripheral blood hematopoietic stem cell (PBSC) transplantation. We revealed the decreasing of the period of neutropenia and thrombocytopenia in the patients with hematological tumors after high-dose chemotherapy, when autologous PBSC were co-transplanted with ex vivo expanded autologous MSCs. Our results show that co-transplantation of autologous MSCs with PBSC is feasible and safe. The shortening of hematopoietic recovery time suggests that MSC may have a positive impact on hematopoiesis.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13706
[VALUE] => Autologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Autologous mesenchymal stromal cells of hemoblastosis patients efficiently support hematopoietic recovery after stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13765
[VALUE] => 658
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 658
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13766
[VALUE] => 659
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 659
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Vera V. Sergeevicheva1, Ekaterina Y. Shevela1, Svetlana A. Sizikova1, Alexander D. Kulagin2, Irina V. Kruchkova1,
Andrey V. Gilevich1, Igor A. Lisukov2, Vladimir A. Kozlov1, Alexander A. Ostanin1, Elena R. Chernykh1
1Institute of Clinical Immunology SB RAMS, Novosibirsk, Russia;
2Novosibirsk State Medical University, Novosibirsk, Russia
Mesenchymal stromal cells (MSCs) derived from bone marrow possess immunoregulatory activity and are able to support hematopoiesis. Unfortunately, data concerning the biological properties of MSCs in various pathologies is poor and often discrepant. In this study, we demonstrated that MSCs derived from bone marrow of patients with hemoblastoses have fibroblast-like morphology and a typical phenotype. Moreover, the patients' MSCs possess well-defined hematopoietic-supporting activity coupled with decreased immunosuppressive potential. These properties prove the clinical application of co-transplantation of autologous hematopoietic stem cells and MSCs in oncohematology to achieve a rapid hematopoietic recovery. Therefore we investigated the safety and hematopoietic effects of MSCs in patients with hematological malignancies receiving peripheral blood hematopoietic stem cell (PBSC) transplantation. We revealed the decreasing of the period of neutropenia and thrombocytopenia in the patients with hematological tumors after high-dose chemotherapy, when autologous PBSC were co-transplanted with ex vivo expanded autologous MSCs. Our results show that co-transplantation of autologous MSCs with PBSC is feasible and safe. The shortening of hematopoietic recovery time suggests that MSC may have a positive impact on hematopoiesis.
Articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13910
[1] => 13911
[2] => 13912
[3] => 13913
[4] => 13914
)
[VALUE] => Array
(
[0] => 83
[1] => 980
[2] => 981
[3] => 982
[4] => 983
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 83
[1] => 980
[2] => 981
[3] => 982
[4] => 983
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
[3] =>
[4] =>
)
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13840
[VALUE] => 10/29/2009 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/29/2009 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13841
[VALUE] => 02/12/2010 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/12/2010 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13842
[VALUE] => 02/12/2010 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 02/12/2010 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] => Array
(
[0] => 13915
[1] => 13916
[2] => 13917
)
[VALUE] => Array
(
[0] => 979
[1] => 11
[2] => 515
)
[DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
)
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[0] => 979
[1] => 11
[2] => 515
)
[~DESCRIPTION] => Array
(
[0] =>
[1] =>
[2] =>
)
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13847
[VALUE] => Array
(
[TEXT] => <p>Саша Ланге, Аксель Цандер, Клаудиа Ланге</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Саша Ланге, Аксель Цандер, Клаудиа Ланге
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13848
[VALUE] => Array
(
[TEXT] => <p class="bodytext">Мезенхимные стволовые клетки человека (МСКЧ) в настоящее время интенсивно изучаются на предмет их потенциального применения в качестве средства клеточной терапии в регенеративной медицине. Их можно легко выделять, например, из костного мозга посредством залипания на пластике, и размножать in vitro. Представленные видеокадры показывают пролиферацию МСКЧ, их взаимодействия между собой и процесс их деления. Высокая степень подвижности МСКЧ может быть причиной возможных нарушений формирования «чистых» клонов даже при низкой плотности посева клеток.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Мезенхимные стволовые клетки человека (МСКЧ) в настоящее время интенсивно изучаются на предмет их потенциального применения в качестве средства клеточной терапии в регенеративной медицине. Их можно легко выделять, например, из костного мозга посредством залипания на пластике, и размножать in vitro. Представленные видеокадры показывают пролиферацию МСКЧ, их взаимодействия между собой и процесс их деления. Высокая степень подвижности МСКЧ может быть причиной возможных нарушений формирования «чистых» клонов даже при низкой плотности посева клеток.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13849
[VALUE] => 10.3205/ctt-2010-en-000051.01
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.3205/ctt-2010-en-000051.01
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13859
[VALUE] => Array
(
[TEXT] => <p>
Sascha Lange, Axel R. Zander, Claudia Lange
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Sascha Lange, Axel R. Zander, Claudia Lange
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13860
[VALUE] => Array
(
[TEXT] => <p class="bodytext">
Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany<br>
<br>
<b>Correspondence: </b><br>
Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: <a href="javascript:linkTo_UnCryptMailto('qempxs.gpperkiDyoi2yrm1leqfyvk2hi');">cllange@<span style="display:none;">spam is bad</span>uke.uni-hamburg.de</a>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany
Correspondence:
Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: cllange@uke.uni-hamburg.de
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 13861
[VALUE] => Array
(
[TEXT] => <p class="bodytext">
Human mesenchymal stromal cells (hMSC) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. They can be easily isolated, e.g., from bone marrow by plastic adherence, and expanded in vitro. These videos show the proliferation of hMSC, and how they interact with each other and divide. The large distances hMSC can conquer call into question the cloning carried out by low-density seeding.
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Human mesenchymal stromal cells (hMSC) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. They can be easily isolated, e.g., from bone marrow by plastic adherence, and expanded in vitro. These videos show the proliferation of hMSC, and how they interact with each other and divide. The large distances hMSC can conquer call into question the cloning carried out by low-density seeding.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 13850
[VALUE] => The MSCs' in vitro community: where to go?
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => The MSCs' in vitro community: where to go?
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Sascha Lange, Axel R. Zander, Claudia Lange
Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Germany
Correspondence:
Claudia Lange, Clinic for Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20246 Hamburg, Germany, E-mail: cllange@uke.uni-hamburg.de
Human mesenchymal stromal cells (hMSC) are presently being investigated extensively for their potential use as cellular therapeutics in regenerative medicine. They can be easily isolated, e.g., from bone marrow by plastic adherence, and expanded in vitro. These videos show the proliferation of hMSC, and how they interact with each other and divide. The large distances hMSC can conquer call into question the cloning carried out by low-density seeding.

