Detecting the V617F mutation of the Jak2 gene in patients with myeloproliferative disorders
Irina Y. Saburova, Yana S. Onikiychuk, Irina I. Zotova, Galina N. Sologub, Mikhail I. Zarayskiy
Saint-Petersburg Pavlov State Medical University, Russia
Accepted 11 December 2008
Published 17 December 2008
Summary
Myeloproliferative disorders (MPD) are a heterogeneous group of hematopoietic diseases accompanied by multiple hyperplasia of bone marrow cells. They are rather difficult for diagnostics and often only revealed by excluding other conditions. One of the most valuable diagnostic criteria for MPD is the V617F mutation of the JAK2 gene. The main subject of this study was to develop a routine detection technique for the JAK2V617F mutation that will be useful for primary diagnostics. To do so, we developed two pairs of primers specific for mutated and wild-type JAK2. To ensure high sensitivity and specificity in JAK2V617F detection we first adjusted the novel PCR technique on the UKE1 cell line previously shown to be homozygous for the JAK2V617F mutation. Next we isolated genomic DNA from 58 MPD patients with different diagnoses using standard techniques. The overall mutation rate in this group was found to be 29.3%. The frequency of the JAK2V617F mutation in newly diagnosed patients with non-verified MPD was 25.7%. We conclude that the detection technique for the JAK2V617F mutation developed in our laboratory represents a useful tool as a diagnostic screening method in patients with myeloproliferative disorders.
Keywords
Myeloproliferative disorders, v617f mutation of jak2 gene, polymerase chain reaction (pcr)
Introduction
Myeloproliferative disorders (MPDs) comprise a group of hematopoietic malignancies that are characterized by enhanced proliferation and survival of one or more myeloid line cells [1]. According to the World Health Organization classification, MPDs include polycythemia vera (PV), essential thrombocythemia (ET), idiopathic myelofibrosis (IMF) and chronic myeloid leukemia (CML), plus rarer subtypes, such as chronic neutrophilic leukemia, hypereosinophilic syndrome and chronic eosinophilic leukemia [8]. The clinical picture of these disorders has many features: all malignant cells originate from a single, multipotent hematopoietic stem cell that predominates over nontransformed progenitors; hypercellularity of the bone marrow, with apparently unstimulated overproduction of one or more of the blood corpuscles; and increased risk of thrombosis and bleeding, spontaneous transformation into acute leukemia and marrow fibrosis [3]. Until very recently MPDs continued to be separated and diagnosed on the basis of their clinical and laboratory findings [4]. The identification of new genetic markers represents a major advance in the understanding of the molecular pathogenesis of MPDs, which will likely result in new classifications and the development of novel therapeutic strategies for these diseases.
The most extensively studied mutation is BCR/ABL, the pathogenetic mutation in CML [5]. CML was the first leukemia to be described and associated with a consistent cytogenetic abnormality, the Philadelphia chromosome (Ph1). Since then new approaches, based on detection of different mutations, have been effectively developed.
The discovery of the JAK2V617F mutation has already greatly influenced the diagnostic approach for MPDs, as well as research strategies in terms of both molecular pathogenesis and drug development [6].
JAK2V617F, a somatic gain-of-function mutation involving the JAK2 tyrosine kinase gene, could be found in nearly all patients with polycythemia vera (PV), in approximately 50% in both essential thrombocythemia (ET) and myelofibrosis (MF) patients, up to 20% of the time in certain subcategories of atypical MPD, in less than 3% in de novo MDS or acute myeloid leukemia patients, and 0% of cases of CML [7].
The Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway plays a central role in initiating signal transduction from hematopoietic growth factor receptors. Non-receptor tyrosine kinases JAK2 are normally responsible for signaling from various growth factor receptors, including those for erythropoietin and thrombopoietin. Each JAK protein has two active tyrosine kinase domains and a catalytically inactive pseudokinase domain. Under normal physiological circumstances, the pseudokinase domain prevents the closure of the two tyrosine kinase domains and auto-activation. When a ligand (for example erythropoietin) binds with a receptor, a conformational change occurs. The JAK2 protein then contacts the cytoplasmic domain of the receptor, where it catalyses tyrosine phosphorylation. This primarily leads to the recruitment of STAT (signal transducer and activator of transcription) molecules, which are then phosphorylated, homodimerize and translocate to the nucleus, where they act as transcription factors [8]. These processes are key events in the modification of regulatory pathways for cell proliferation and survival.
The specific genetic mutation G1849T observed in exon 14 results in the substitution of phenylalanine by valine, both hydrophobic nonpolar amino acids, at position 617 of the JAK2 protein within the JH2 pseudokinase domain [9]. Loss of JAK2 auto-inhibition results in constitutive activation of the kinase. It results in deregulation of intracellular signaling and disturbance of cell proliferation, which becomes independent of normal growth factor control. Since the mutation has a high specificity for clonal myeloid diseases, the presence of JAK2 V617F can definitively confirm an MPD diagnosis [10].
The main aim of this study was to develop a routine detection technique for the V617F mutation of the JAK2 gene that will be useful both for primary diagnostics and for semiquantative estimation during treatment.
Patients, materials, and methods
Patient characteristics
Fifty-eight patients from hematological clinics of the Saint Petersburg Pavlov State Medical University were included in the study: 8 patients with PV, 7 with ET, 2 with MF and 35 with primary diagnosed MPD. The median age was 55 years (range 20–86 years). All patients did not receive any specific therapy. The control group included 20 standard blood donors.
Cell line
As a positive control we used cell line UKE1 [11], donated by Professor B. Fehse (Germany). The UKE1 cell line is homozygous for the V617F mutation in the Jak2 gene (G1849T substitution in exon 14) [12].
DNA samples
DNA was isolated from 200 µl of bone marrow or 1 ml whole blood using sorbate methods (DNA Technology, Russia). This procedure regularly results in 3 µg to 8 µg DNA in a final volume of 100 µl. After isolation from blood samples, DNA was stored at -20 °C until analysis.
PCR analysis
PCR was performed on an amplificator “Terzik” (DNA Technology, Russia) with standard PCR mix. The program for PCR includes initial denaturation (3 minutes at 95°C) and 40 cycles at 94°C for 20 seconds; 61°C for 30 seconds and 72°C for 60 seconds. The following primers were used: Jak2-F (forward): 5'-GGGTTTCCTCAGAACGTTGA-3'; Jak2-RW (reverse wild type): 5'-TTTACTTACTCTCGTCTCCACATAC-3'; Jak2-RM (reverse mutated): 5'-TTTACTTACTCTCGTCTCCACATAA-3'. After amplification PCR products were visualized in 2% agarose gel and photographed by means of a "Gel Imager, 08-111" (DNA Technology, Russia).
Real-time PCR was performed on a DT-96 amplificator (DNA Technology, Russia), with standard PCR real time mix SYBR GREEN and the same primers. After initial denaturation (3 minutes at 95°C), PCR was carried out for 40 cycles at standard conditions (94°C for 15 seconds; 61°C for 40 seconds). The estimation of "threshold" was performed automatically; "melting curve" analysis was used for discrimination of the nonspecific results.
Results assessment
To assess the specificity of the method we used the UKE1 cell line and 20 donor samples as negative control.
A) Quality assessment
Each sample was assessed on two lines: line 1 – PCR specific for the wild- type of the Jak2 gene, and line 2 – specific for the mutated gene. If the mutation-specific signal in line 2 was detected, such samples were considered positive for the V617F mutation in the Jak2 gene (see Figure 1).
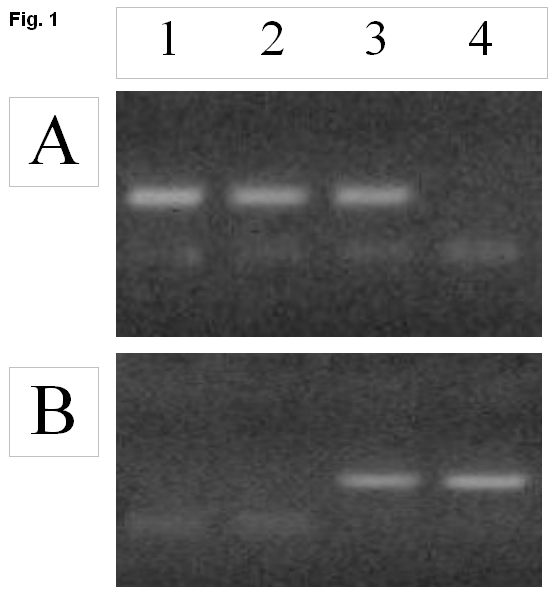
Panel А: Jak2 "wild" type; panel B: Jak2 mutated type.
Samples: 1 – donor, 2 and 3 – patients, 4 – cell line (UKE1). Samples 1 and 2 are homozygous for Jak2 wild-type, sample 3 is heterozygous, and sample 4 is homozygous for the Jak2 mutant type.
B) Semi quantitative assessment
A semi-quantitative assessment was performed by means of the GelPro Analyzer 3.1 computer program. This program allows the quantitative valuation of luminescence levels and assesses those in Relative Units of Luminescence (RU). The sum of RU from two lines (wild and mutated type of gene) was assessed as 100%. Relative intensity of mutant type gene signals thus indicates the number of mutated cells.
Results and discussion
Screening for the JAK2V617F mutation in MPD diagnostics is a predictive and specific approach [13]. In most cases the JAK2 V617F mutation was examined using polymerase chain reaction (PCR)-amplified genomic DNA with two primers (for mutant and wild type gene) [12]. Concerning the results of this study such a method is appropriate for screening the mutation.
As a positive control we used cell line UKE1, which is homozygous for the V617F JAK2 mutation. For negative control we used samples from 20 healthy donors who do not carry this mutation after informed consent. The correlation analysis showed high-level convergence between real time and standard PCR dates. “Melting curve” analysis showed high level specificity and sensitivity.
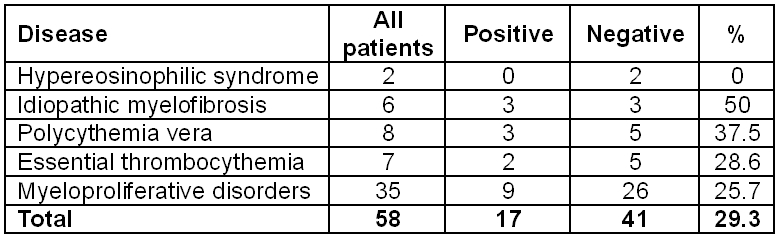
Prospective evaluation of the V617F JAK2 mutation was implemented at Saint Petersburg Pavlov State Medical University. Fifty-eight patients with a preliminary diagnosis of MPD were examined. The overall mutation frequency was 29.3%. The incidence of different diseases is shown in Table 1.
Table 1. Overall frequency of the V617F JAK2 mutation in 58 patients with different myeloproliferative disorders at the Saint Petersburg Pavlov State Medical University.
Detection of V617F mutation of JAK2 gene in patients with chronic myeloproliferative disorders is a common criterion for diagnosis. For primary diagnostics, screening methods are widely used. Such methods should be quick in performance, rather cheap and reproducible in laboratories with technical equipment of middle level.
For screening methods, qualitative detection of the JAK2 gene mutation without its quantitative assessment is sufficient. Detection of this mutation helps not only in diagnostics, but also in determining the treatment strategy for a concrete patient.
After specific therapy (standard chemotherapy and particularly hematopoietic stem cells transplantation) it is rather important to assess dynamics of reduction of the tumor clone that bears the mutation in the JAK2 gene. Another substantial goal is to follow minimal amounts of tumor cells (“minimal residual disease”) to predict potential recurrence of the disease. For this purpose both semiquantitative and quantitative methods are appropriate. Quantitative methods need verified controls (e.g. cell line or plasmid dilutions) for establishing a calibration curve.
Concerning the abovementioned protocol we have developed a screening method for the detection of the V617F mutation in the JAK2 gene. This method is based on common allele-specific amplification with further detection in agarose gel. Specificity and sensitivity of this method were tested on the cell line UКE1 (data are not presented). Using this protocol we retrospectively investigated 23 patients with clinically verified diagnoses (see Table 1), and performed prospective trial on 35 outpatients with suspected myeloproliferative disorders.
Comparing our results with data from literature we indicated similarity of incidence rates for V617F mutation in JAK2 gene in patients with idiopathic myelofibrosis and essential thrombocythemia. Discrepancy between incidence rates in patients with polycythemia vera and unspecified myeloproliferative disorders and data from literature could be referred to small sample and/or and shortage of clinical criteria for diagnosis verification. Besides specificity we assessed reproducibility of this method on different types of amplificators – «GeneAmp»-9600 (USA), DT-96 (Russia), «Tercic» (Russia). We showed identity of data obtained on different amplificators, which confirms high reproducibility of the proposed method. All samples positive for V617F mutation in JAK2 gene were analyzed with computer program “GelPrо” to determine percentage ratio of tumor and normal cells.
At the second phase of our study we developed a uniform qualitative method for detection of the JAK2V617F mutation using real-time PCR. Therefore we used the same samples as for the standard PCR analysis. Preliminary data of qPCR analysis were in full agreement with those of standard PCR (not shown). Further refinement may be carried out in order to develop a simple quantitative method to assess minimal residual disease after standard treatment and transplantation.
In conclusion the proposed PCR method for the detection of the JAK2 mutation seems to be suitable for the initial evaluation of patients with myeloproliferative disorders.
References
1. Steensma DP. JAK2 V617F in Myeloid Disorders: Molecular Diagnostic Techniques and Their Clinical Utility. JMD. 2006;8(4):397-410.
2. Tefferi А, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14-22.
3. Yamaoka K, Saharinen P, Pesu M, Holt VE 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks). Genome Biology. 2004;5:253.
4. Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372-375.
5. Wilson-Rawls J, Xie S, Liu J, Laneuville P, Arlinghaus RB. P210 Bcr-Abl interacts with the interleukin 3 receptor beta(c) subunit and constitutively induces its tyrosine phosphorylation. Cancer Res. 1996;56:3426-3430.
6. Spivak JL, Barosi G, Tognoni G, Barbui T, Finazzi G, Marchioli R, Marchetti M. Chronic myeloproliferative disorders. Hematology: American Society of Hematology Education Program Book. 2003:200-224.
7. McLornan D, Percy M, McMullin MF. JAK2 V617F: A Single Mutation in the Myeloproliferative Group of Disorders. Ulster Med J. 2006;75(2):112-119.
8. Tefferi А. Classification, Diagnosis and Management of Myeloproliferative Disorders in the JAK2V617F Era. Hematology. 2006;240-245.
9. Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ, et al. JAK2V617F mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005;131(2):208-13.
10. Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824-830.
11. Fiedler W, Henke RP, Ergün S, Schumacher U, Gehling UM, Vohwinkel G, Kilic N, Hossfeld DK. Derivation of a new hematopoietic cell line with endothelial features from a patient with transformed myeloproliferative syndrome: a case report. Cancer. 2000 Jan 15;88(2):344-51.
12. Lippert E, Girodon F, Hammond E, et al. Concordance of assays designed for the quantification of JAK2V617F: a multicenter study. Haematologica. 2008 Nov 10. Epub ahead of print.
13. Levine RL, Wernig G. Role of JAK-STAT Signaling in the Pathogenesis of Myeloproliferative Disorders. Hematology. 2006:233-239.
Accepted 11 December 2008
Published 17 December 2008


