A unique population of mobile very small embryonic/epiblast like (VSEL) stem cells resides in adult tissues: physiological and pathological consequences
Mariusz Z. Ratajczak1,2, Magda Kucia1, Dong-Myung Shin1, Liu Rui1, Justyna Drukala1, Wojtek Marlicz2, Janina Ratajczak1, Ewa K. Zuba-Surma1
1Stem Cell Institute at James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202, USA;
2Department of Physiopathology, Pomeranian Medical University, Szczecin, Poland
Accepted 21 November 2008
Published 27 November 2008
Summary
Accumulating evidence demonstrates that adult tissue contains a population of very primitive pluripotent stem cells (PSCs). Recently, our group identified a population of very small SCs in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that these cells, which are deposited during early gastrulation in developing tissues/organs, play an important role in the turnover of tissue-specific/committed SCs. Based on this, we envision that germ line is not only the origin but also a “basis/skeleton” for the SC compartment in adult life forms. We noticed that VSELs could be mobilized into peripheral blood (PB) and the number of these cells circulating in PB increases during stress and tissue/organ injuries (e.g., heart infarct, stroke). Furthermore, our data indicates that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique pattern of methylation of selected somatic imprinted genes. Finally, we envision that in pathological situations, VSELs could be involved in the development of some malignancies (e.g., teratomas, germinal tumors, pediatric sarcomas).
Keywords
Vsels, pluripotency, imprinting, mobilization, cxcr4, oct-4, nanog, ssea
Introduction
An adult organism develops from the most primitive stem cell (SC) called a zygote, which is an oocyte that is fertilized by a sperm cell. This totipotent zygote, the “mother of all stem cells” in the developing body, first gives rise to pluripotent (P)SCs that form morula and, subsequently, to the SCs committed to trophoblasts that will give rise to the placenta and the pluripotent SC population that forms the inner cell mass of the blastocyst. The cells from the inner cell mass of the blastocyst will give rise to the epiblast, a part of the developing embryo, which is the origin of SCs committed to all the three germ layers (meso-, ecto-, and endoderm) [1-5].
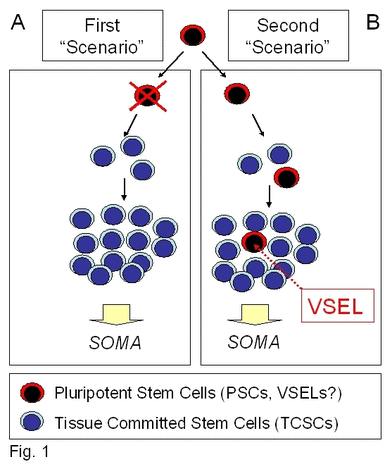
Thus, the epiblast could be considered the origin for the SCs committed for all the organs and tissues in developing the embryo proper. PSCs in the epiblast undergo a sequence of specification events, first into multipotent and subsequently into versatile tissue-committed SCs, which play a role in the formation and rejuvenation of various organs [5-7]. The most important questions emerge of whether some of these primitive epiblast-forming PSCs can “escape” specification into more differentiated populations of SCs and retain their pluripotential character, thus surviving among differentiated daughter tissue-committed SCs. Conversely, would all of them undergo tissue/organ specific differentiation and then “disappear” after embryogenesis, and not be found in the adult body (Figure 1)?
Figure 1. Potential VSELs contribution to tissue rejuvenation.
Panel A: VSELs deposited in adult tissues during embryogenesis/gastrulation may become eliminated after giving rise to TCSCs. Panel B: Conversely, they may survive among TCSCs and serve as a potential back-up/reserve source of TCSCs.
Recently, our group obtained several pieces of evidence that may lend some support for the first possibility. Accordingly, we have identified a population of very primitive SCs in adult tissues that express many markers characteristic for epiblast Scs [8]. Based on this we named these rare cells “very small embryonic like stem cells (VSELs).” We hypothesized that they are deposited during early gastrulation in developing tissues/organs, survive into adulthood, and play an important role as a back-up population of PSCs in the turnover of tissue-specific/committed SCs (TCSCs) [5, 7, 8].
The presence of pluripotent VSELs in adult tissues may reconcile all previously published data stating that adult tissues may contain a population of PSCs [9, 10]. The existence of such cells had been postulated by several investigators [11, 12]. However, such cells were never purified and identified at the single cell level. Their presence was accepted mainly based on experiments showing that some populations of cells were enriched with adherent cell populations isolated from the bone marrow (BM) or that certain solid organs contain some primitive cells that may differentiate into various tissues.
Accordingly, several populations of non-hematopoietic primitive SCs have been described in the BM and other adult tissues, including: i) mesenchymal (M)SCs [13]; ii) multipotent adult progenitor cells (MAPCs) [14]; iii) marrow-isolated adult multilineage inducible (MIAMI) cells [15]; iv) multipotent adult (MA)SCs [16]; and v) Omnicytes [17, 18]. It is conceivable that all these cells are closely related, overlapping populations of SCs described by different investigators and given various names according to circumstance. Furthermore, the potential relationship between these cells and VSELs is not clear. Since MSCs, MAPCs, MIAMIs, and MASCs are largely derived from the adherent fraction of BM- or adult organ-derived cells, these cells could potentially contain some VSELs attached to or associated with them due to emperipolesis. This requires further investigation.
“Of germ line and soma”: germ line as origin and skeleton of the SC system in the adult body
From a developmental point of view, cells that are “immortal” in mammals are those that belong to the germ line. Accordingly, the germ line passes genomic and mitochondrial DNA to the next generations and creates “mortal soma”, which helps the germ line to fulfill this reproductive mission [19-21]. The most primitive cell in the germ line is the above-mentioned zygote, which is a result of fusion of two gametes (germ cells), i.e., the oocyte and sperm, during the process of fertilization. Germ line potential is subsequently maintained in blastomers of morula and in the cells of the inner cell mass of the blastocyst. At the level of the blastocyst, however, a part of the cells that surrounds the blastula “buds out” from the germ line lineage and differentiates toward throphoblasts, which give rise to the placenta. After implantation of the blastocyst in the uterus, a germ line potential is maintained in the epiblast [19-21].
In mice, at 7.25 days post-conception (dpc), a part of epiblast PSCs is specified into a population of primordial germ cells (PGCs) that will migrate to the genital ridgesahre where they subsequently differentiate into oocytes or sperm during gametogenesis [6, 22]. Shortly after PGC specification, the remaining epiblast PSCs, which we envision to be related to the germ line lineage, become specified to multipotent/unipotent SCs for developing tissues and organs [20]. These primitive epiblast/germ line-derived PCSs, as we hypothesize, are not completely eliminated from the developing organism by the differentiation process. We believe that some of them survive (e.g., VSELs?) among tissue-committed SCs [20].
PGCs are the most important population of SCs. As precursors of germ cells/gametes, they are directly responsible for passing genetic information on to the next generation. However, these developmentally early cells, if isolated from the developing embryo after 11 dpc (at the time while they migrate to genital ridges) and cultured ex vivo, surprisingly will undergo rapid terminal differentiation or apoptosis [23]. Interestingly, they also do not complement blastocyst development, are not able to provide fully functional nuclei during nuclear transfer in the process of clonote formation, and do not grow teratomas [20, 24-26]. Therefore, these cells lack the currently approved criteria of pluripotentiality. This also indicates that PGCs have to be somehow protected from uncontrolled expansion by certain important regulatory mechanisms.
One explanation for this obvious lack of pluripotentiality is that PGCs undergo epigenetic modification and erasure of the somatic imprint on differently methylated regions (DMRs) of somatic imprinted genes [27, 28]. Somatic imprinted genes show a different methylation pattern in some of the genes located either on maternal or paternal chromosomes (e.g., H19, Igf-2, Igf-2R, Snrpn). As a result of the imprint, for example, Igf-2 is expressed from the paternal and H19 is expressed from the maternal chromosome [20, 27, 28].
The process of erasure of the somatic imprint occurs very early during gastrulation when the PGCs begin to migrate to the genital ridges [27, 28]. It is one of the basic mechanisms that prevents their uncontrolled proliferation, parthenogenesis, and prevents potential teratoma formation by these cells. Thus, the proper somatic imprint seems to be required for PSCs to be able to complement blastocyst development, provide nuclei for clonote formation after nuclear transfer, and to grow teratomas in immunodeficient mice. Because migrating PGCs erase the imprint, they are not able to display these “golden standard” pluripotency criteria in appropriate experimental models [26-28].
However, if plated over murine fetal fibroblasts in the presence of selected growth factors, i.e., leukemia inhibitory factor (LIF), basic fibroblast growth factor (bFGF), and kit ligand, PGCs may undergo epigenetic changes forced by in vitro culture conditions that regain the somatic imprint, which endows them with “immortality” [29, 30]. Thus, this change of “PGC fate” is connected with a proper re-methylation of somatic imprinted genes. This immortalized population of PGCs known as embryonic germ cells (EGCs) is in many aspects the equivalent to embryonic (E)SCs [31]. For example, similarly to ESCs, EGCs contribute to all three germ layers including the germ cell lineage after injection into a blastocyst (blastocyst complementation assay), provide functional nuclei for clonote after nuclear transfer, and, after injection into living mice, these cells form teratomas [21, 31]. Thus, it is evident that a proper somatic imprint is vital for cells from the germ line to retain full pluripotentiality. Based on this, we postulate that erasure of the somatic imprint of some crucial developmentally genes is involved in controlling the quiescent status of VSELs [20, 32]. However, as in the case of PGCs, this process should be potentially reversible. As such, we will have to focus on reestablishing the proper somatic imprint in these cells. We envision that such reverted VSELs could potentially become equivalent to cells isolated from the embryos, e.g., after nuclear transfer or even to inducible (i)PSCs [33, 34].
The hunt for PSCs in the adult body
The presence of PSCs in adult tissues was postulated in the past by several investigators, but such cells were never purified at the single cell level. A few years ago, our team began to search for such cells in murine BM. Based on our preliminary experimental data, we assumed that the cells we were looking for would be CD133+ CXCR4+ in mice and humans as well as Sca-1+ in mice. We also assumed that they would be negative in both species for the pan-hematopoietic marker, which is the CD45 antigen (CD45-) [8, 35, 36]. In addition, our preliminary chemotactic experiments revealed that BM contains a very rare population of small cells (3-5 µm in diameter) that robustly respond by chemotaxis to stromal-derived factor-1 (SDF-1), which is a ligand for the CXCR4 receptor [37]. These small CXCR4+ cells expressed some early developmental markers characteristic for very primitive cells [8, 37].
Based on this information, we decided to sort a population of small (<6 µm) Sca-1+lin-CD45- cells from the murine BM and other tissues. By employing a fluorescence activated cell sorter (FACS), we isolated a population of rare Sca-1+Lin-CD45- cells from several adult tissues including BM, brain, liver, pancreas, kidney, muscles, heart, testes, and thymus [9]. As determined by real time RT-PCR (RQ-PCR) by employing sequence specific primers and by immunohistochemistry, these cells express several markers of PSCs such as SSEA-1, Oct-4, Nanog, and Rex-1 as well as Rif-1 telomerase protein [8, 9]. Based on the expression of these early developmental markers and morphology, we named these cells “very small embryonic-like stem cells (VSELs)” [8].
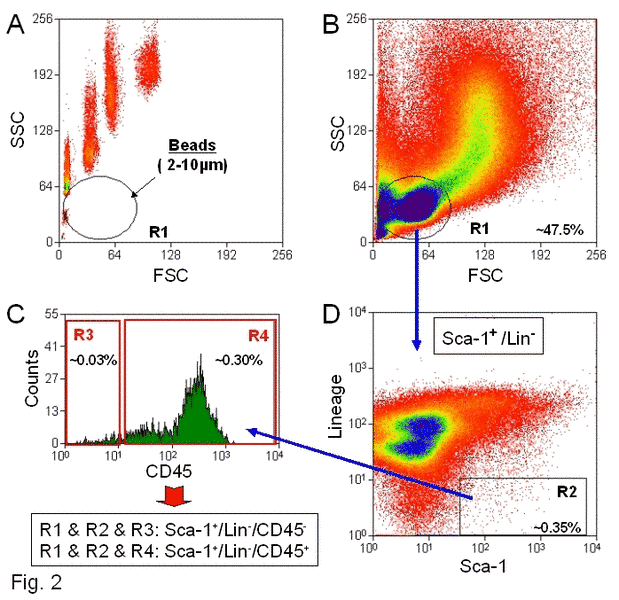
To isolate VSELs from the BM by FACS, we employed a novel size-based approach controlled by size bead markers (Figure 2). The overall sorting strategy was to gate in regions containing small events (2–10 µm), which is shown as Region R1 on the dot plot (Figure 2: Panel A). This region mostly contains cell debris, but also includes some rare nucleated cell events. Because it is well known that most of the sorting protocols exclude events smaller than 6 µm in diameter that contain cell debris, erythrocytes, and platelets, small VSELs are usually excluded from the sorted cell populations. Thus, in our sorting strategy to isolate VSELs and define a region of cells from which we could sort these cells, the size of the sorted cells was controlled by beads with predefined sizes (1, 2, 4, 6, 10, and 15 µm in diameter) (Figure 2: Panel A).
Figure 2. Sorting strategy for isolation of murine BM-derived VSELs by FACS.
BM-derived VSELs were sorted by MoFlo cell sorter (Dako, Glostrup, DEN) following immunofluorescence staining for Sca-1, CD45, and hematopoietic Lin. Panel A: Distribution of six predefined, sized beads populations according to their FSCs vs. SSCs (forward and side scatter characteristics, respectively). Gate R1 includes objects between 2 to 10µm in size after comparison to bead particles with standard sizes of 1, 2, 4, 6, 10, and 15µm (Flow Cytometry Size beads, Invitrogen; Molecular Probes, Carlsbad, CA, USA). Panel B: BMNCs visualized on dot plots showing their FSC and SSC signals related to the size and granularity/complexity of the cell, respectively. Small, agranular cells included in Region R1 are further visualized based on the expression of Sca-1 and Lin markers (Panel D). Region R2 includes only Sca-1+/Lin-, which are subsequently sorted based on CD45 marker expression into CD45- and CD45+ subpopulations visualized on histogram (Panel C). Sca-1+/Lin-/CD45- cells (VSELs) are sorted as events enclosed in a logical gate including Regions R1, R2, and R3, while Sca-1+/Lin-/CD45+ cells (HSCs) from gate include Regions R1, R2, and R4. Approximate percent contents of each cellular subpopulation are indicated on the plots.
The events enclosed in region R1 (Figure 2: Panel B), which include an average of ~50% of total collected events, are further analyzed for the expression of Sca-1 and lineage markers (Lin). The Sca-1+Lin- events shown in region R2 (Figure 2: Panel D) consist of 0.38 ± 0.05% of total analyzed BM nucleated cells (BMNCs) on average. Cells from region R2 are subsequently sorted according to the expression of CD45- as Sca-1+Lin-CD45- (region R3) and Sca-1+Lin-CD45+ (region R4) subpopulations (Figure 2: Panel C) that contain VSELs and HSCs, respectively. We found that VSELs comprise ~0.03% while HSCs are ~0.35% of total BMNCs (Figure 2: Panel C). We found that 95% of Sca-1+Lin-CD45- (VSELs) are located within the 2–6 µm size range, while 86% of Sca-1+Lin-CD45+ (HSCs) are found in the 6–10 µm size range [36]. Thus, by employing flow cytometry and the size marker beads, we confirmed our previous transmission electron microscopy (TEM) data showing that the majority of Sca-1+Lin-CD45- cells isolated from adult BM are unusually small (<6 µm) [8]. In conclusion, VSELs are larger than PB platelets and smaller than erythrocytes. Direct TEM analysis revealed that these cells display several features typical for ESCs such as small size, a large nucleus surrounded by a narrow rim of cytoplasm, and open-type chromatin (euchromatin).
ImageStream system (ISS) analysis was also employed to further evaluate the morphological features of VSELs [36]. The ISS-based analysis is a new flow cytometry-based analytical strategy that employs flow cytometry combined with microscopy. This allows for statistical analyses of various cellular parameters as well as direct visualization of cells acquired by FACS in suspension during flow analysis via high-resolution brightfield, darkfield, and fluorescence images [36]. The high resolution of ISS imaging enables the identification of objects as small as 1 µm in diameter [38-40].
In employing ISS analysis, we confirmed with greater precision that VSELs are ~3.6 μm in diameter, while Sca-1+Lin-CD45+ HSCs are larger at ~6.5 μm in diameter. We also noticed that VSELs have a significantly higher (P < 0.05) nuclear/cytoplasmic ratio as compared with HSCs (1.47 ± 0.17 and 0.82 ± 0.03, respectively). Furthermore, VSELs had significantly lower (P < 0.05) cytoplasmic area as compared with HSCs (5.41 ± 0.58 and 33.78 ± 1.68, respectively) [36]. Despite their small size, VSELs possess diploid DNA. They do not express MHC-1 and HLA-DR antigens and are CD90- CD105- CD29- [41].
Interestingly, if plated over a C2C12 murine sarcoma cell feeder layer, ~5–10% of purified VSELs are able to form spheres that resemble embryoid bodies. Cells from these VSEL-derived spheres (VSEL-DSs) are composed of immature cells with large nuclei containing euchromatin and are CXCR4+SSEA-1+Oct-4+, just like purified VSELs [8>]. Similar spheres were also formed by VSELs isolated from murine fetal liver, spleen, thymus, and kidney. Interestingly, formation of VSEL-DSs was associated with a young age in mice with no VSEL-DSs observed in cells isolated from older mice (> 2 years) [10]. This age-dependent content of VSELs in BM may somehow explain why the regeneration processes is more efficient in younger individuals. There are also differences in the content of these cells among BM mononuclear cells (MNCs) between long- and short-lived mouse strains. The concentration of these cells is much higher in the BM of long-lived (e.g., C57Bl6) as compared to short-lived (DBA/2J) mice [8]. In the future, it would be interesting to identify the genes responsible for tissue distribution and expansion of these cells, as they could be involved in controlling the life span of mammals.
Furthermore, since VSELs express several markers of PGCs (fetal-type alkaline phosphatase, Oct-4, SSEA-1, CXCR4, Mvh, Stella, Fragilis, Nobox, Hdac6), they could be closely related to a population of epiblast-derived PGCs. VSELs are also highly mobile and respond robustly to an SDF-1 gradient, adhere to fibronectin and fibrinogen, and may interact with BM-derived stromal fibroblasts [8]. Confocal microscopy and time-lapse studies revealed that these cells attach rapidly to, migrate beneath, and undergo emperipolesis in marrow-derived fibroblasts. This is explainable by fibroblasts secreting SDF-1 and other chemoattractants, which may create a homing environment for small CXCR4+ VSELs [8]. This robust interaction of VSELs with BM-derived fibroblasts has an important implication. It is possible that isolated BM and other tissues’ fibroblastic cells (e.g., MSCs, USSCs, MACSs, MAPCs, or MIAMI cells) may be to some degree contaminated by these tiny cells from the beginning. This observation may explain the unexpected plasticity of marrow-derived fibroblastic cells, e.g., MSCs.
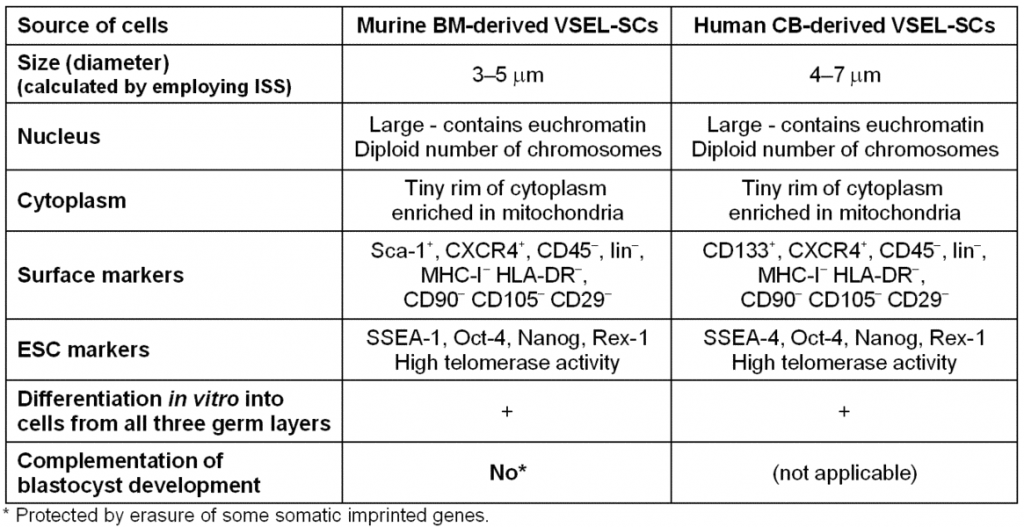
Recently, evidence has also mounted to suggest that similar cells corresponding to those found in murine tissues are also present particularly in the human BM, umbilical cord blood (UCB), and mobilized (m)PB (Table I). Overall, it is anticipated that VSELs could become an important source of PSCs for regeneration. Thus, researchers working with animal models must determine whether these cells could be efficiently employed in the clinic or whether they are merely developmental remnants found in the BM that cannot be harnessed effectively for regeneration. Our initial collaborative studies indicate an efficacy of these cells in improving heart function in an animal model of acute myocardiac infarction in mice [42, 43]. We anticipate seeing similar phenomena in humans.
Table I. Morphological and phenotypic comparison of murine and human VSELs.
VSELs as circulating “paramedics” in the body
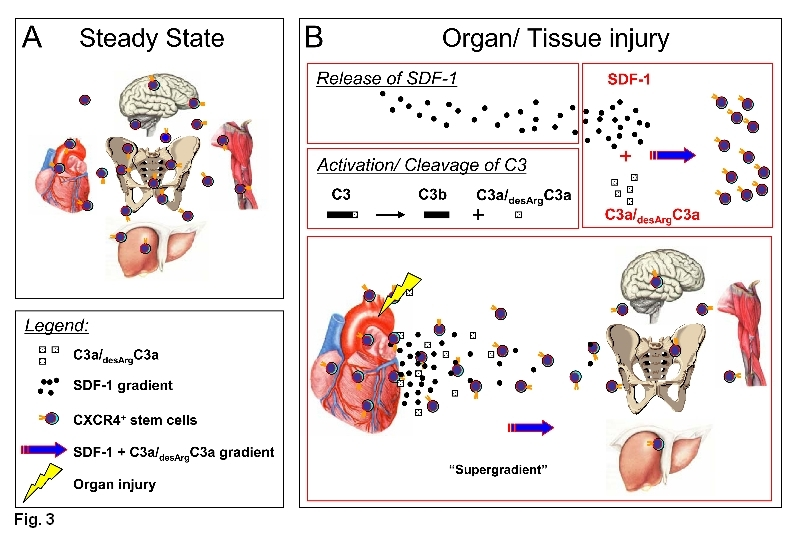
Our data also indicates that VSELs may be released during stress situations or tissue/organ injury from their tissue niches and circulate in the PB both in humans (e.g., after heart infarct or stroke) and in mice (e.g., after granulocyte colony growth factor [G-CSF]-induced mobilization, experimental heart infarct and stroke, as well as liver and skeletal muscle injury) [44-46]. The trafficking of VSELs is orchestrated by several chemotactic factors that are upregulated in damaged tissues during tissue organ injury such as α-chemokine SDF-1, hepatocyte growth factor/scatter factor (HGF/SF), LIF, and vascular endothelial growth factor (VEGF) [47-50]. Complement cascade cleavage fragments also play an important role in this process, such as C3a anaplylatoxin for example, which enhances responsiveness of VSELs to SDF-1 gradient (Figure 3). Thus, a concept emerged where chemotactic factors that are upregulated in damaged tissues may orchestrate the release of non-hematopoietic SCs from BM into mPB.
For instance, in a murine model of G-CSF-induced mobilization, we noticed that VSELs are detectable at a very low level in steady state conditions in murine PB (~160 cells/ml) and that their number increases ~6 times during G-CSF-induced mobilization events [44]. Increases in the number of these cells circulating in PB are further supported by an increase in expression of mRNA for early developmental markers expressed in VSELs, such as the embryonic transcription factors Oct-4, Nanog, and Rex-1 as well as the expression of Rif1 and Dppa3 [44]. Furthermore, at the same time, MNCs mobilized into PB are highly enriched for mRNA for several early developmental tissue-specific markers, a phenomenon that could be explained as mentioned above by the open-type status of chromatin in these cells. Finally, we sorted these rare cells from murine PB by FACS for immunofluorescence staining and provided evidence that they express SSEA-1 antigen on the surface and Oct-4 in the nucleus.
To provide evidence that mobilized VSELs not only express PSC markers but also are able to differentiate into cells from all three germ layers, we performed differentiation studies in vitro. To provide such proof, VSELs were cultured in appropriate differentiation media on the layer of BM-derived stromal support. We found that mobilized VSELs are able to differentiate into cardiomyocytes, neurons, and pancreatic cell-like clusters [44]. The analysis of DNA content in GFP+ cells isolated from the co-cultures excluded the contribution of cell fusion to this effect. Thus, these experiments revealed the in vitro pluripotency of VSELs mobilized by G-CSF and circulating in mPB by demonstrating their ability to differentiate into cells from all three germ layers. We envision that VSELs mobilized into PB in humans, such as after G-CSF administration, could be harvested by leucopheresis as a potential source of SCs for regenerative medicine.
Figure 3. VSELs are mobilized into PB.
Panel A: Under normal steady state conditions, VSELs may circulate in PB to keep a pool of SCs in balance in distant niches of the same tissue.
Panel B: The number of these cells increases during stress related to organ/tissue damage. During organ damage (e.g., heart infarct), the level of SDF-1 is upregulated in the affected tissues and C3 becomes activated leading to the accumulation of C3 cleavage fragments (C3a and desArgC3a). C3 cleavage fragments enhance/prime the responsiveness of circulating CXCR4+ SCs to an SDF-1 gradient. This leads to more efficient chemoattraction of SCs for potential regeneration of the damaged tissue by creating “a super gradient,” as shown in Panel B for infracted myocardium, for example. In addition to SDF-1, other chemoattractants also play important roles here (e.g., HGF/SF, LIF, and VEGF).
Do Oct-4+ VSELs initiate tumor development?
Several investigators have proposed theories regarding cancer formation in the germ cell compartment. Accordingly, Recamier (1829), Remak (1854), and Virchow (1958) proposed that cancer arises from embryo-like cells. Subsequently, Durante and J. Cohnheim in 1874 and 1875, respectively, suggested adult tissues contain embryonic remnants that normally lie dormant, but can be activated to become cancerous. In 1910, Wright proposed the germinal cell origin of Willm’s tumor (nephroblastoma) and in 1911, J. Beard postulated that tumors arise from displaced trophoblast or activated germ cells. We envision the Oct-4+ VSEL recently identified in adult tissues could unify and fully support all these theories. First, we envision that if the genomic imprint in VSELs is not erased, they may retain post-developmental in vivo pluripotency and grow teratomas and teratocarcinomas [5, 20]. Second, if they are closely related to migratory PGCs, which go astray from the major migratory route to the genital ridges, they may ultimately give rise to germinomas and seminomas, for example. Third, if these cells acquire critical mutations, they may develop into the several types of pediatric sarcomas (e.g., rhabdomyosarcoma, neuroblastoma, Ewing-sarcoma, or Willm's tumor). In support of this, there is a strong correlation between the number of these Oct-4+ cells that persist in postnatal tissues and the coincidence with these types of tumors in pediatric patients. Finally, it is possible that these cells, if mobilized at the wrong time into the PB and deposited in areas of chronic inflammation, may not play a role in regeneration but may contribute to the development of other malignancies (e.g., stomach cancer or lung cancer). To support this further, several tumor types may express embryonic markers including Oct-4 and, as reported, BM-derived SCs that may develop in the presence of carcinogens to some sarcomas or teratomas. Furthermore, we hypothesize that VSELs hiding among BM-derived fibroblasts could also be responsible for sarcoma formation by MSC cells. Circulating VSELs also could be also chemoattracted by the hypoxic/chemoattractant-rich environment of a growing tumor and provide stroma and vessels for expanding that tumor. Finally, it is also possible that circulating VSELs or cells very closely related to this population may also act in progressive fibrosis of some organs such as the lung.
Closing remarks
Several attempts have been made in the past few years to purify a population of PSCs from adult tissues including BM, UCB, and mPB that could give rise in vitro to cells from all three germ layers (meso-, ecto-, and endoderm) [8, 16, 44] and in vivo as well as in mice after injection into the developing blastocyst that would contribute to the development of multiple organs and tissues. In contrast to positive data in vitro, this latter criterion for pluripotentiality in vivo for several potential candidates for PSCs has not yet been demonstrated in a reproducible manner with any SC type isolated from the adult tissues. This is also true for VSELs. The reason for this could be that PSCs deposited in adult tissues erase the imprint on some crucial maternal or paternal imprinted genes. This phenomenon keeps these cells under control from unleashed proliferation and not only prevents the possibility of teratoma formation in vivo by these cells, but also simultaneously will affect their ability to complete blastocyst development after injection into developing blastocyst.
VSELs isolated from adult tissues are an alternative and not ethically controversial source of SCs for regenerative medicine. However, there are several missing answers to this timely issue, especially in view of the current and widely performed clinical trials with BM-derived SCs in cardiology and neurology, before VSELs can find their potential application in regenerative medicine.
First, there is the obvious problem of isolating a sufficient number of VSELs from the BM, UCB, or mPB. The number of these cells among BM MNCs is very low. For example, VSELs represent ~1 cell in 105 of BM MNCs [8, 35, 36]. Furthermore, our data shows that these cells are enriched in the BM of young mammals and their number decreases with age. It is also likely that if VSELs are released from the BM, even if they are able to home to the areas of tissue/organ injury, they may function only in the regeneration of minor tissue injuries. Heart infarct or stroke, on the other hand, may involve severe tissue damage beyond the effective repair capacity of these rare cells. Second, the allocation of these cells to the damaged areas depends on homing signals that may be inefficient in the presence of proteolytic enzymes released from leukocytes and macrophages associated with damaged tissue. For example, matrix metalloproteinases (MMPs) released from inflammatory cells may degrade SDF-1 locally and perturb homing of CXCR4+ SCs [51]. Thus, VSEL-SCs may potentially circulate as a homeless population of SCs in PB and return to the BM or home to other organs. Third, to reveal their full regenerative potential, these cells have to be fully functional. We cannot exclude the possibility that VSEL-SCs, while residing or being trapped in the BM, not only erase appropriate methylation on differently methylated regions of some important somatic imprinted genes but also are not fully functional and remain locked in a dormant state. They require the appropriate activation signals by unidentified factors. Finally, we have to develop efficient ex vivo culture conditions that will allow for efficient expansion of VSEL-SCs without supportive feeder layer cells (e.g., C2C12, BM-derived fibroblasts).
Nevertheless, our data strongly indicates that VSEL-SCs could potentially provide a therapeutic alternative to the controversial use of human ESCs and strategies based on therapeutic cloning. Hence, while the ethical debate on the application of ESCs in therapy continues, the potential of VSELs is ripe for exploration. The current work in our laboratory indicates that VSELs could be efficiently employed in the realm of regenerative medicine and that they are physiologically more important than merely being potential developmental remnants. Finally, we believe that the controlled modulation of somatic imprint status in VSELs such as we hypothesized, a proper de novo methylation of somatic imprinted genes on maternal and paternal chromosomes, could increase a regenerative power of these cells. The coming years will bring important answers to these questions.
References
1. Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872-884.
2. Darr H, Benvenisty N. Human embryonic stem cells: the battle between self-renewal and differentiation. Regen Med. 2006;1:317-325.
3. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634-7638.
4. O'Farrell PH, Stumpff J, Su TT. Embryonic cleavage cycles: how is a mouse like a fly? Curr Biol. 2004;14:R35-45.
5. Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W, Kucia M: Hunt for pluripotent stem cell - Regenerative medicine search for almighty cell. J Autoimmun. 2008;30:151-162.
6. McLaren A, Lawson KA. How is the mouse germ-cell lineage established? Differentiation. 2005;73:435-437.
7. Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J, Kucia M. Very Small Embryonic-Like (VSEL) Stem Cells: Purification from Adult Organs, Characterization, and Biological Significance. Stem Cell Rev. 2008;4:89-99.
8. Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857-869.
9. Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr., Ratajczak J, Ratajczak MZ. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A. 2008 Oct;24.
10. Zuba-Surma EK, Wu W, Ratajczak J, Kucia M, Ratajczak MZ. Very small embryonic-like stem cells in adult tissues-Potential implications for aging. Mech Ageing Dev. 2008 Feb;14.
11. Jones RJ, Wagner JE, Celano P, Zicha MS, Sharkis SJ. Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature. 1990;347:188-189.
12. Vacanti MP, Roy A, Cortiella J, Bonassar L, Vacanti CA. Identification and initial characterization of spore-like cells in adult mammals. J Cell Biochem. 2001;80:455-460.
13. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74.
14. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49.
15. D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971-2981.
16. Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow). Blood. 2007;110:3438-3446.
17. Gordon MY. Stem cells for regenerative medicine--biological attributes and clinical application. Exp Hematol. 2008;36:726-732.
18. Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M'Hamdi H, Thalji T, Welsh JP, Marley SB, Davies J, Dazzi F, Marelli-Berg F, Tait P, Playford R, Jiao L, Jensen S, Nicholls JP, Ayav A, Nohandani M, Farzaneh F, Gaken J, Dodge R, Alison M, Apperley JF, Lechler R, Habib NA. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822-1830.
19. Donovan PJ. The germ cell - the mother of all stem cells. Int J Dev Biol. 1998;42:1043-1050.
20. Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4+ stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860-867.
21. Zwaka TP, Thomson JA. A germ cell origin of embryonic stem cells? Development. 2005;132:227-233.
22. McLaren A. Primordial germ cells in the mouse. Dev Biol. 2003;262:1-15.
23. De Felici M, McLaren A. In vitro culture of mouse primordial germ cells. Exp Cell Res. 1983;144:417-427.
24. Macchiarini P, Ostertag H. Uncommon primary mediastinal tumours. Lancet Oncol. 2004;5:107-118.
25. Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210-222.
26. Yamazaki Y, Mann MR, Lee SS, Marh J, McCarrey JR, Yanagimachi R, Bartolomei MS. Reprogramming of primordial germ cells begins before migration into the genital ridge, making these cells inadequate donors for reproductive cloning. Proc Natl Acad Sci U S A. 2003;100:12207-12212.
27. Lee J, Inoue K, Ono R, Ogonuki N, Kohda T, Kaneko-Ishino T, Ogura A, Ishino F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development. 2002;129:1807-1817.
28. Mann JR. Imprinting in the germ line. Stem Cells. 2001;19:287-294.
29. Donovan PJ. Growth factor regulation of mouse primordial germ cell development. Curr Top Dev Biol. 1994;29.
30. Resnick JL, Ortiz M, Keller JR, Donovan PJ. Role of fibroblast growth factors and their receptors in mouse primordial germ cell growth. Biol Reprod. 1998;59:1224-1229.
31. Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841-847.
32. Kucia M, Wu W, Ratajczak MZ. Bone marrow-derived very small embryonic-like stem cells: Their developmental origin and biological significance. Dev Dyn. 2007;12:3309-20.
33. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
34. Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-324.
35. Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4(+) SSEA-4(+) Oct-4(+) very small embryonic-like cells purified from human cord blood - preliminary report. Leukemia. 2007;21:297-303.
36. Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawnn B, Hall B, Singh R, Lillard JW, Ratajczak MZ. Morphological characterization of Very Small Embryonic-Like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292-303.
37. Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52-57.
38. Basiji DA, Ortyn WE, Liang L, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med. 2007;27:653-670.
39. Zuba-Surma EK, Kucia M, Abdel-Latif A, Lillard JJ, Ratajczak MZ. The ImageStream System: a key step to a new era in imaging. Folia Histochem Cytobiol. 2007;45:279-290.
40. Zuba-Surma EK, Kucia M, Ratajczak MZ. “Decoding of Dot”: The ImageStream System (ISS) as a Supportive Tool for Flow Cytometric Analysis. Cent Eur J Biol. 2008;3:1-10.
41. Kucia M, Wysoczynski M, Ratajczak J, Ratajczak MZ. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331:125-134.
42. Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646-1655.
43. Zuba-Surma EK, Taher H, Kucia M, Guo Y, SanganalMath SK, Hunt G, Vincent RJ, Abdel-Latif A, Dawn B, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived Very Small Embryonic-Like stem cells (VSELs) improves left ventricular function and remodeling after myocardial infarction. Circulation. 2007:204.
44. Kucia M, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that Very Small Embryonic Like (VSEL) Stem Cells are Mobilized into Peripheral Blood. Stem Cells. 2008;26:2083-2092.
45. Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865-873.
46. Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213-3220.
47. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858-864.
48. Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A, Fujii N, Nagler A, Lahav M, Szyper-Kravitz M, Zipori D, Lapidot T. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331-1339.
49. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434-438.
50. Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772-1784.
51. McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503-43508.
Accepted 21 November 2008
Published 27 November 2008