Composition and functional properties of monolayer cell culture from human umbilical cord blood
Barkhatov I. M.1, Roumiantsev S. A.2, Vladimirskaya E. B.2, Afanasyev B. V.1
1Saint-Petersburg Pavlov State Medical University, Russia;
2Russian Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia
Accepted 10 December 2008
Published 26 December 2008
Summary
Summary
Objectives
It’s known that during cultivation, adherent cells of umbilical cord blood (UCB) form a monolayer reminiscent, in its composition, of the stromal monolayer of bone marrow (BM) culture. However, the presence of mesenchymal stem cells (MSCs) in UCB still remains uncertain. This study was performed to investigate the composition and some functional characteristics of MSC-like cell populations revealed in the cord blood monolayer culture.
Materials and methods
Forty-three human UCB samples were under study. All the samples were obtained during full-term deliveries. To produce monolayer cultures, mononuclear cell fractions from UCB were cultivated in a culture medium containing DMEM with 20% FCS, supplied with 1% Pen/Strep. Phenotypic patterns of UCB culture were assessed with a panel of monoclonal antibodies specific for CD34; CD117; CD45; CD14; CD3; CD19; CD31; CD90; HLA DR; and HLA ABC. To determine the functional characteristics of MSCs derived from UCB culture, their differentiation ability and stimulation of hematopoietic colony formation activity were evaluated.
Results
In most cases, the cultures of plastic-adherent cells proved to be heterogeneous. Both spindle-shaped and polygonal cells were observed. In some samples, clonal growth could be detected. However, the number of fibroblastoid cells did not increase 100 cells per colony. Large colonies were registered in three UCB samples of the 43 under study. As evidenced by immune phenotyping, the monolayer UCB cultures were rather polymorphic and dissimilar in each sample. Most of the cells present in the cultures were macrophages (CD45+). However, we also found different amounts of presumably mesenchymal cells, including cells with an endothelial phenotype (CD34+CD31+).
Specific staining showed that the cells from a UCB monolayer culture have the capacity to differentiate into adipocytes and osteoblasts. In some cultures, however, induction of differentiation lead to the detachment of a major cell fraction. Hemostimulatory ability of UCB monolayer cultures depended on the phenotype composition of the monolayer culture. CD45+ and CD14+ cells, evidently, are stimulatory for granulocyte-macrophage colony formation. Moreover, levels of non-hematopoietic subpopulations (CD90+CD31-) in UCB cultures showed a direct correlation with the numbers of CFU-GM colonies produced.
Conclusion
UCB contains a subpopulation of non-hematopoietic cells possessing phenotypic and some functional characteristics of bone marrow derived mesenchymal stem cells. However, the low content and variable numbers of such cells provide some doubts on the viability of UCB as an alternative source for MSC.
Keywords
Mesenchymal stem cells, umbilical cord blood, immunophenotyping, cell culture
Introduction
Mesenchymal stem cells (MSC) constitute a rare population of multipotent progenitors capable of both supporting hematopoiesis and differentiating into at least osteogenic, adipogenic and chondrogenic lineages [1-3]. Moreover, they exhibit immunomodulatory activity, induce immunotolerance in case of allogenic transplantation [4], and posess the ability to expand in relatively simple in vitro systems [5-7]. These characteristics make MSCs very promising candidates in the development of new cell-based therapeutic strategies, such as the treatment of tissue injuries or the supportive application by hematopoietic stem cell transplantation. Taking into consideration that the number of MSCs and their differentiation capacity decline with age [8], most relevant is the search for alternative sources of these cells.
The advantages of umbilical cord blood (UCB), as a source of stem cells, include their accessibility, non-invasive sampling and, thus, their safety for potential donors [9-11]. Another reason for this study was due to some controversial data about amounts of mesenchymal precursors in UCB [12-17]. Mesenchymal precursors (CFU-F) are found in foetal blood in the early gestational period at concentrations equal to their amounts in the bone-marrow of newborns [18]. However, after the second trimester, the number of MSCs decreases considerably, and, at birth, their frequency in UCB is quite accidental [12,19]. The need for testing of some standards for culturing adhesive cell fractions from UCB, as well as assessment of their contents and functional characteristics provided other motivations for this study.
Materials and Methods
Collection of UCB
UCB samples (mean volume of 60 ml) from full-term deliveries were collected from the unborn placenta after obtaining the mothers' informed consent. A sterile bag system containing citrate phosphate dextrose (CPD) anticoagulant within the collection bag (manufactured by Terumo, Japan) was used. The units were stored at room temperature before processing (up to 33 hours).
Isolation and Culture of Adherent Cells from UCB
To isolate mononuclear cells (MNCs), each UCB unit was diluted 1:1 with phosphate-buffered saline (PBS)/2 mM EDTA (Biolot, Russia), and carefully loaded onto Ficoll-Hypaque solution (Lympho separation medium, ICN, USA, d=1.077). Following a density gradient centrifugation at 435 g for 30 minutes at room temperature, MNCs were removed from the interphase layer and washed two to three times with PBS/EDTA. UCB-derived MNCs were set at a density of 1 x 106/cm2 into six-well culture plates (Corning, USA) containing DMEM low-glucose medium (Gibco, USA) with 20% fetal calf serum (FCS) from selected lots, penicillin 100 UI/ml, and streptomycin 0.1 mg/ml (Gibco, USA).
After overnight incubation at 37°C in a humidified atmosphere containing 5% carbon dioxide, nonadherent cells were removed and fresh medium was added to the wells. Cell cultures were maintained, and remaining nonadherent cells were discarded via the complete exchange of culture medium every 7 days. Culture plates were screened continuously to detect developing colonies of adherent cells. The number of fibroblast colony forming units (CFU-F) was calculated by counting the number of colonies per 108 MNCs.
Fibroblast-like cells were detached between days 16 through 20 after initial plating using 0.04% Trypsin/0.03% EDTA (Gibco, USA). The recovered cells were replated at a density of 4,000 to 5,000 cells/cm2.
Collection and Isolation of Control MSCs from Bone Marrow
MSCs from bone marrow (BM) were obtained by bone marrow puncture. BM cells were aspirated into a 5-ml syringe containing CPD anticoagulant. A total of six samples were obtained, with the donor age ranging from 24 to 56 years. To isolate MSCs from BM, the aspirate was diluted 1:5 and processed as described above. In contrast to MNCs from UCB samples, BM-derived MNCs were cultured at a density of 1 x 106 cells/cm2 in T75 culture flasks (Corning, USA), and the first change of medium was performed 3 days after initial plating. Two weeks later, at reaching 80%–90% confluence, MSCs were detached using trypsin and replated as described for the UCB-derived adherent cells.
Primary Fibroblasts as Controls
Primary cultures of normal human dermal fibroblasts served as negative control in differentiation and comparative gene expression studies. Culture conditions were comparable to BM MSC’s expansion: DMEM low glucose medium (Gibco, USA) containing 20% fetal calf serum (FCS), penicillin 100 UI/ml, and streptomycin 0.1 mg/ml (Gibco, USA).
Immune Phenotypic Analysis
To analyze the cell-surface expression of typical marker proteins in UCB- and BM-derived adherent cells, each from primary culture and second passage, these were labeled with the following anti-human antibodies: CD34 PE; CD34 FITC, CD45 FITC; CD45 PE; CD14 FITC; CD31 PE; CD31 FITC; CD61 FITC; CD3 FITC; CD19 PE; CD117 PE; HLA ABC FITC; HLA DR,DP,DQ FITC; CD 90 PE (Becton Dickinson, USA). Murine isotype antibodies (Becton Dickinson, USA) served as respective controls. Ten thousand labeled cell aliquotes were analyzed using a FACScan flow cytometer running CellQuest software (Becton Dickinson, USA).
Aiming at a subset analysis of the population, we employed the following indices:
• hematopoietic cells index – CD45+ to CD45- cells
• myeloid cell maturation index – CD14+ to CD45+ cells
• hematopoietic progenitor cell index – CD34+CD45+ to CD45+ cells
• endothelial cell-precursors index – CD34+CD45- to CD45- cells
• mesenchymal precursors index – CD90+CD31- to CD45- cells
In vitro Osteogenic and Adipogenic Differentiation Studies
To induce osteogenic differentiation, the cells were seeded at a density of 3.1 x 103 cells/cm2 and cultured in six-well microplates (Costar, USA), until they reached approximately 80% confluence. Additional culture was performed in osteogenic differentiation medium supplemented with 0.1 µM dexamethasone, 10 mM ß-glycerophosphate, 0.05 mM ascorbate, and 10% FCS. The onset of osteoblast formation was evaluated after 3 weeks via calcium accumulation. Accumulation of mineralized calcium phosphate was assessed with von Kossa staining after the protocol from Cheng et al. [20], with a few modifications. The cells were fixed for 15 minutes in 10% formalin (Sigma-Aldrich), and, after washing, they were incubated with 5% silver nitrate (Sigma-Aldrich) for 15 to 30 minutes. Pyrogallol 1% (Merck, Canada) and sodium thiosulfate 5% (Sigma-Aldrich) were used to develop and register a resulting image. In addition, the mineralized matrix was also evaluated by Alizarin-red S staining using 4% formaldehyde for fixation and 1% aqueous Alizarin-Red S (Sigma-Aldrich) solution.
Adipogenic differentiation was induced according to the protocol of Pittenger et al. [2]. Special induction medium, containing DMEM (high glucose), 1 µM dexamethasone, 0.5 mM 3-isobutyl-1-methyl-xanthine, 10 µg/ml recombinant human (rh) insulin, 0.2 mM indomethacin, and 20% FCS, was added for 2 to 3 days to the culture microplates. It was then replaced by maintenance medium containing only rh-insulin and 20% FCS. Induction of adipogenic differentiation was apparent via the intracellular accumulation of lipid-rich vacuoles that stained with Oil Red O (Sigma-Aldrich). The cells were fixed with 10% formalin, washed, and stained with a working solution of 0.18% Oil Red O for 5 minutes.
In vitro Model for Studying Colony-stimulating Activity of UCB cells
An essential property of mesenchymal stem cells is their capacity to support hematopoiesis. In order to assess the hemostimulating capacity of the UCB monolayer culture, a modified technique proposed by Afanasyev [21,22] was used (Figure 1). This method determines the clonogenic capacity of granulocytic-macrophage colony forming units (CFU-GM) in “agar drop-liquid medium” culture.
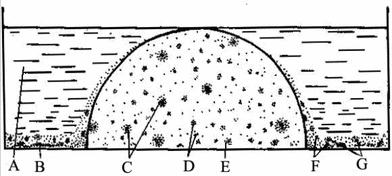
Figure 1. Agar drop-liquid culture system[22]
A-medium; B-feeder cells; C,D,E-colonies; F-clusters; G-agar
The monolayer culture of UCB-derived adherent cells (confluence rate: 70–80%) was used as a source of colony-stimulating activity of MSC from UCB. The cells (CFU-GM) from the UCB mononuclear fraction were targeted in this assay, providing clonal growth in agar cultures. Semisolid agar drops containing target cells were prepared on dry surfaces of sterile Petri dishes. The resulting semisolid drops were transferred to the dishes, and co-incubated for 7 days in the following four versions: 1) with complete culture medium only; 2) with medium containing standard-type leukocyte feeder; 3) with UCB confluent monolayer culture; 4) with both UCB confluent monolayer culture and standard-type leukocyte feeder. Each experiment was performed at least twice.
Colony-forming ability (CFA) and cluster-forming ability (ClFA) were classified according to the number of cells in the colonies (small colonies: containing 20–40 cells; medium-size colonies: 41–100 cells; and large colonies: more than 100 cells) and clusters (large clusters: 10–19 cells; small clusters: 5–9 cells). Depending on the total number of colonies and clusters, the cloning efficiency (CE) per 1х105 explanted mononuclear cells was assessed. A “colony-to-cluster” ratio (Co-Cl) and percentage of large colonies (LC) were supplemental parameters assessed in these cell cultures. Using such parameters, the proliferative potential of the target progenitor cells was tested.
Total RNA Isolation and RT-PCR
Total RNA was extracted from 3 to 30 x105 MSCs using Trizol Reagents (Invitrogen, USA), according to the manufacturer’s instructions. mRNA was subject to reverse transcription (RT) using Superscript II Kit (Invitrogen, USA), again using the manufacturer’s instructions. The resulting cDNA was amplified using an ABI GeneAmp PCR System 2400 (Perkin Elmer Applied Biosystems, Boston, MA) at 94°C for 40 seconds, 56°C for 50 seconds, and 72°C for 60 seconds for 35 cycles, after initial denaturation at 94°C for 5 minutes. Primers used for PCR are listed in Table 1. Fifteen microliters of PCR reaction were fractionated by agarose gel electrophoresis.
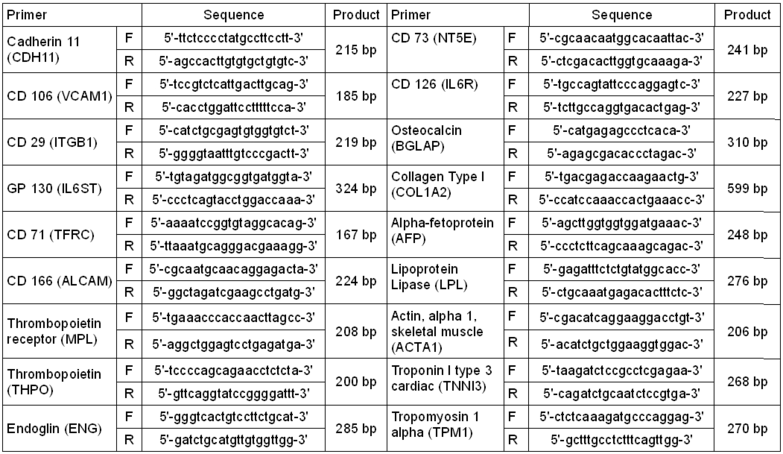
Table 1. Primers used for RT-PCR
Statistical Analysis
The statistical significance of the inter-group differences was evaluated with the Mann-Whitney test. The degrees of correlations between the parameters were evaluated by the Spearman test. The differences were considered significant by p values <0.05. The Statistica 6.0 software package was used for all statistical analyses.
Results and Discussion
In most cases, the cultures of plastic-adherent cells proved to be heterogenous. Two main morphological cell types were discernable: spindle-shaped cells that are presumably regarded as mesenchymal stem cells (MSC), and polygonal cells that are most likely of hematopoietic origin (Figure 2).
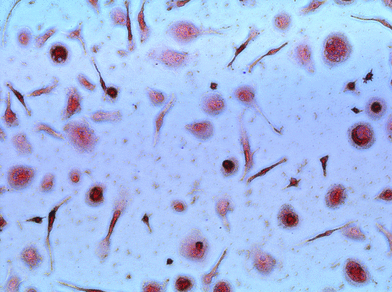
Figure 2. Heterogeneous UCB culture. No colony formation was observed. UCB samples produced a minimal, non-confluent adherent layer of heterogeneous cells.
In some UCB samples, clonal growth was observed, however the mean cell number per colony did not increase over 100 cells (Figure 3). Large colonies were detectable in 3 of the 40 UCB samples under study. These colonies (>1000 cells per colony) consisted of closely packed, spindle-shaped cells, typical of fibroblast morphology cells.
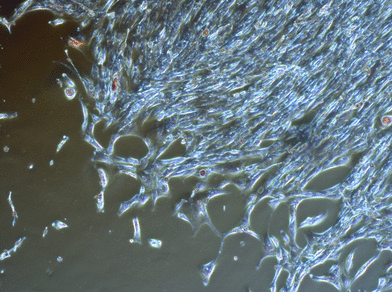
Figure 3. CFU-F in UCB culture. Adherently growing cells of fibroblastic morphology formed big colonies in 3 UCB samples.
The Phenotypic Composition of UCB Monolayer Cultures
Our efforts to define a distinct phenotype characteristic for MSC have been confounded by the fact that these cells can express a range of cell lineage-specific antigens [2,23].
During analysis of the predominant cellular types, it was discovered that the major fraction (median 60.17%) of plastic-adherent cells from UCB were of hematopoietic origin (CD45+) (Table 2). One-third of the CD45-positive cells belonged to the CD14-positive population fraction (median 14.81%). Cells in the monolayer with an osteoclast-like phenotype (CD45+CD61+) constituted approximately 1.5%. The cells phenotypically comparable with hematopoietic stem cells (HSC-like cells: CD34+CD45+, CD34+HLA DR-, CD117+) were present at a low concentration in the UCB monolayer culture - less than 1.5%.
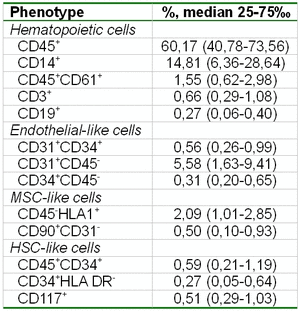
Table 2. The composition of the UCB monolayer culture.
The numbers of endothelial-like cells in the monolayer cultures were comparable to HSC-like cells. With this background, a heterogenous population of CD45-CD31+ phenotype was the most prominent one.
Despite a lack of specific markers that could characterize a population of MSC’s, we attempted to determine the quantity of these cells in the culture, based upon the fact that mesenchymal stem cells of the bone marrow do not express common leucocytic antigen CD45 and HLA class II antigens but do express class I HLA antigens and the CD90 marker. In this connection, we studied the following populations of phenotypically MSC-like cells: CD45-HLA ABC+ and CD90+CD31-.
Aiming for further validation of available cultural conditions for expansion of MSCs and endothelial precursors, we assessed the following factors influencing the terms of cultivation period in a primary culture under the conditions of initial culture and subsequent passages. During long term cultures (Table 3), the index of cells with hematopoietic markers decreased progressively, and the index of myeloid cell maturation decreased as well, providing evidence of the elimination of hematopoietic cells during the cultivation. We have revealed that the index of HSC-like cells (hematopoietic progenitor cell index) increases during prolonged cultivation, i.e. an index, reflecting a ratio of cells, phenotypically similar to hematopoietic stem cells, to the total number of СD45+-cells (CD34+CD45+, CD117+). This fact implies that this population is eliminated slower than other hematopoietic cells recovered in the UCB monolayer culture.
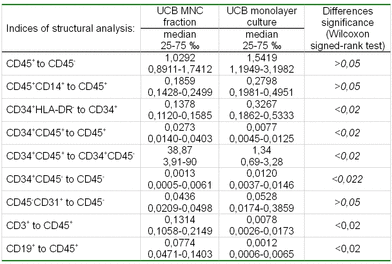
Table 3. Influence of cultivation period on composition of primary UCB culture
Moreover, the percentage of CD34+HLA DR- cells increases (37.33 to 81.98, p=0.001) in relation to general CD34-positive population, thus supposing that these cells undergo selective proliferation under the given cultural conditions. This result could indirectly confirm a theory that this population is presented via stromal component [24,25]. In the course of long-term cultivation, the index of endothelial cells is shown to be increased (0.013 to 0.025, p=0.02). This fact proves these cultural conditions are favorable for endothelial precursors' maintenance. When assessing the fractions that include mesenchymal precursors, we have found a tendency for an increase in the relative amounts of the CD45-HLA ABC+ subpopulation. However, when analyzing these populations with regard of all non-hematopoietic cells, we did not observe such a tendency. This fact may reflect the persistance of a steady phenotype of these cell populations upon durable cultivation.
During the process of culture passage, we could reveal a decrease of the hematopoietic cell index (3.19 to 1.6, p<0.016) (Table 4). This data may show the inability of the majority of hematopoietic cells for repeated adhesion, thus resulting in their elimination from the monolayer culture. At the same time, the cells phenotypically similar to HSC-like cells (CD34+CD45+) and lymphocytes (CD3+), were still able to adhere recurrently, when compared with other hematopoietic cells. Among cell populations containing mesenchymal precursors, it was noticed that the cells expressed class I HLA antigens, possessed a reduced adhesive ability, and were subject to elimination with sequential passing.
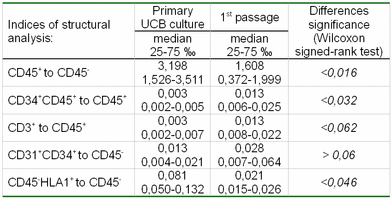
Table 4. Influence of culture passage on the structure of primary UCB culture
When comparing the phenotypical composition of mononuclear fraction and monolayer culture from UCB (Table 5) we discovered that the percentage of cells with CD34+CD45+ phenotype in the culture decreased considerably, along with an increased percentage of cells with СD34+HLA DR- phenotype. Therefore, we can suggest that these cultural conditions may be applied for isolation of the given cell type.
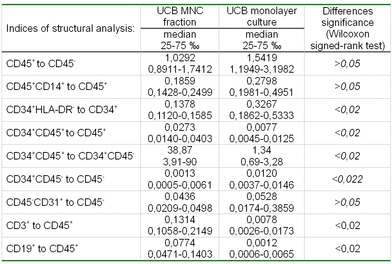
Table 5. The phenotypic composition of the mononuclear fraction and monolayer culture of UCB
This may be also proven by the data from semi-solid methylcellulose culture. The cells from monolayer cultures under study were incapable of forming hematopoietic colonies in the presence of standard hematopoietic growth factors (SCF, GM-CSF, IL-3, IL-6, G-CSF, EPO). This may result from a deficiency of clonogenic precursors in the monolayer, as well as an absence of clonogenic potential among hematopoietic stem-like cells.
The fraction of endothelial cells was higher in the monolayer culture. The percentage of hematopoietic-stem-like-cells and endothelial precursors decreased considerably during the initiation of the culture (38.87 to 1.34, p<0,02).
Analogous to Bieback's paper [12], when assessing the time parameters of sampling and storage of umbilical blood specimens we have revealed a tendency towards a decreased concentration of mesenchymal like stem-cells in the monolayer culture, when a prolonged time-period prior to processing umbilical blood sample with subsequent cultivation occurs (data not shown).
In vitro differentiation of UCB-derived MSC-like cells into adipocytes and osteocytes
Taking the lack of specific markers for identification of mesenchymal precursors into consideration, we tried to reveal the functional characteristics of the given types of cells and to assess the differential potentials in the framework of orthodox plasticity. It is shown that the cells of the UCB monolayer culture are capable of dividing and differentiating to adipocytes and osteoblasts, as was proven by their specific staining (Figure 4). In this figure (upper picture), red lipid inclusions are readily seen in differentiated adipocytes. In the lower picture, calcium insertions in the osteocytes are stainable red or black. In some cultures, however, induction of differentiation initiated detachment of most cells from plastic surface. As a result, the present conditions for differentiation of mesenchymal stem cells from BM are not quite appropriate for induced differentiation of UCB-derived mesenchymal precursors.
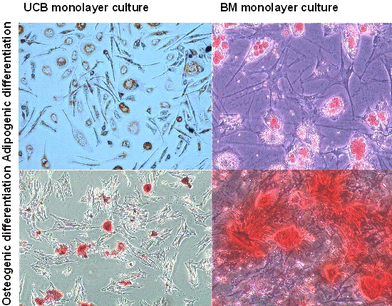
Figure 4. Differentiation assay of BM and UCB monolayer culture. Formation of mineralized matrix by Alizarin Red and von Kossa staining evidenced osteogenic differentiation. Adipogenic differentiation was evidenced by the formation of lipid vacuoles in phase-contrast photograph and by oil-red O staining.
Ability of UCB monolayer culture to support ex vivo expansion of CFU-GM
Concerning efficiency of CFU-GM cloning, the UCB monolayer culture does not differ from standard leucocytic feeder. However, when using umbilical cord blood culture as a feeder, UCB-generated growth-promoting capacity and percentage of large colonies were higher (data not shown). Additional analysis (Table 6) leads us to suggest that hematopoietic cells from a UCB monolayer culture have certain advantage over non-hematopoietic cells in terms of colony-stimulation activity (r=0.71, p=0.035), at least in this in vitro model. With respect to non-hematopoietic cells, growth of cellular elements bearing MSC-markers (CD90+CD31-) was accompanied by significant increase in CFU-GM proliferative activity (r=0.82, p=0.007). Higher percentages of monocytes/macrophages, as among hematopoietic elements, is accompanied by growing numbers of large colonies (r=0.67, p=0.045), without producing any significant impact upon cloning efficiency of the progenitors.
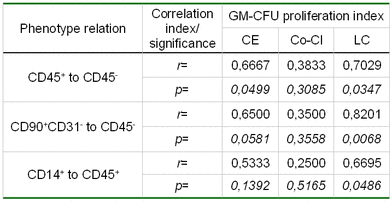
Table 6. Hemostimulating capacity of UCB-culture
Some references in the literature contain similar data obtained with another model, i.e., target cells were incubated with UCB cells in suspension cultures, followed by methyl cellulose cultures of non-attached cell populations in presence of standard growth factors, such as SCF, GM-CSF, G-CSF, IL3, IL6 and EPO [26,27]. Within our model, only umbilical cord blood cells of a monolayer culture were used as the colony stimulation source.
Comparative gene expression studies
When assessing the expression of some genes, we have found that the mRNA profile of bone marrow culture did not differ from the cells of UCB culture. Thrombopoietin was an exception, since specific mRNA was not detectable in the 1st passage culture of UCB cells (Table 7).
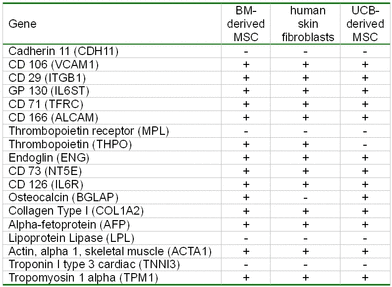
Table 7. mRNA profile of MSC-like cells from different sources
Conclusions
• Increased cultivation time of UCB mononuclear fraction (over 23 days) leads to a gradual elimination of hematopoietic cells from the culture and an increase in mesenchymal stem cells and endothelial progenitor cells.
• Cellular phenotype in the culture changes during passages, i.e., the quantity of hematopoietic cells is considerably decreased. This results in increased concentration of non-hematopoietic components the of umbilical blood monolayer culture during serial passaging.
• An increased time period of UCB sampling is associated with a decrease in the relative quantity of mesenchymal-like stem cells, along with an increase in the concentration of endothelial precursors in the culture.
• Mesenсhymal stem-like-cells of UCB have the capacity to differentiate into adipogenic and osteogenic lineages, thus suggesting their functional consistency.
• The adhesive fraction of the primary monolayer culture exerts a stimulatory effect upon the colony formation of GM precursors, being similar in type and degree of influence to a standard peripheral blood feeder. The primary effect upon their proliferative capacity may be produced by cellular elements with MSC (CD90+CD31-) markers.
• An increased time interval during sampling and storage of UCB leads to a decrease in the hemostimulating capacity.
• The variable contents of MSC-like cells and CFU-F in umbilical cord blood and/or their reduced repopulation ability may limit their application as an alternative source of MSCs.
References
1. Minguell JJ. Mesenchymal stem cells. Exp Biol Med. 2001;226:507-520.
2. Pittenger M, Mackay A, Beck S, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;84:143-147.
3. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74.
4. Le Blanc K, Ringden O. Mesenchymal stem cells, properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586-591.
5. Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the haematopoietic tissues: Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340.
6. Friedenstein AJ, Deriglasova UF, Kulagina, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974;2:83-92.
7. Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414-425.
8. Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. О Cell Biochem. 2001;82:583-590.
9. Vladimiskaya EB, Mayorova OA, Roumiantsev SA, Roumiantsev AG. Biological bases and therapy prospects with the stem cells. Moscow: Medpractica; 2005. 391 p.
10. Broxmeyer HE. Proliferation, self-renewal, and survival characteristics of cord blood hematopoietic stem and progenitor cells. In: Broxmeyer HE, ed. Cord Blood: Biology, Immunology, Banking, and Clinical Transplantation. Bethesda, MD: American Association of Blood Banking. 2004:1-21.
11. Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. 2005;7(3):209-218.
12. Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-634.
13. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242.
14. Koegler G, Sensken S, Airey J, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123-135.
15. Lee MW, Choi J, Yang MS, et al. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem Biophys Res Comm. 2004;320:273-278.
16. Mareschi K, Biasin E, Piacibello W, et al. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-1100.
17. Wexler S, Donaldson C, Denning-Kendall P, et al. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368-374.
18. Campagnoli C, Roberts I, Kumar S, Bennett P, Bellantuono I, Fisk N. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow Blood. 2001;98:2396-2402.
19. Goodwin HS, Bicknese AR, Chien SN, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat and neural markers. Biol Blood Marrow Transpl. 2001;7:581-588.
20. Cheng SL, Yang JW, Rifas L, et al. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277-286.
22. Afanasiev ВV, Almazov VA. Human hematopoietic progenitor cells. Leningrad: Nauka; 1985. 204 p.
23. Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69-80.
24. Huang S, Terstappen LW. Formation of haematopoietic microenvironment and haematopoietic stem cell from single human bone marrow stem cells. Nature. 1992;360:745-749.
25. Islam A. Hematopoietic stem cells: A new concept. Leuk Res. 1985;9:1415.
26. Ye ZQ, Burkholder JK, Qiu P, Schultz JC, Shahidi NT, Yang NS. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci USA. 1994;91:12140-12144.
27. Lu-Lu L, Liu Y-J, Yang S-G, Zhao Q-J, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017-1026.
Accepted 10 December 2008
Published 26 December 2008


