Experience of clinical mesenchymal stem cells (MSCs) usage for prophylaxis and GVHD treatment in patients undergoing allo-HSCT
Stankevich Y.1, Golovacheva A.1, Babenko E.1, Alyansky A.1, Paina O.1, Zubarovskaya L.1, Semenova E.1, Polintsev D.2,
Kruglyakov P.2, Afanasyev B.1
1Pavlov State Medical University, St. Petersburg, Russia;
2"TransTechnology" LtD, St. Petersburg, Russia
Accepted 09 December 2008
Published 23 December 2008
Summary
Summary
Within bone marrow stroma, there exist subsets of nonhematopoietic cells referred to as mesenchymal stem cells (MSCs), or mesenchymal stromal cells [1]. These cells may not only improve HSC engraftment and regeneration of damaged tissues after allogeneic transplantation [7], but also modulate immune responses in vitro and in vivo [8]. Hence, co-transplantation of allogeneic HSC together with allogeneic MSC hypothetically could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution [4], GVHD suppression, and it may be used for GVHD prophylaxis, like as for treatment of severe acute or chronic GVHD. This study shows that more than a half of the patients with steroid-refractory acute GVHD responded to treatment with MSCs. However, further randomized clinical trials are necessary for estimation of therapeutic effect of MSCs in allo-HSCT patients and definition of important and significant factors influenced upon MSCs infusion.
Keywords
Hematopoietic stem cell transplantation, mesenchymal stem cells, acute gvhd, chronic gvhd
Introduction
Graft-versus-host disease (GVHD) remains a major obstacle to successful allogeneic hematopoietic stem cell transplantation (HSCT), causing significant morbidity and mortality, especially in a case of allogeneic unrelated and haploidentical settings. The ability to prevent and treat GVHD is a key to success. A calcineurin inhibitor in combination with methotrexate is still the basic regimen for prophylaxis of both acute GVHD (aGVHD) and chronic GVHD (chGVHD). Steroid therapy still represents the first-line treatment for established GVHD, with a response rate of 30 to 50 %. However, the outcome for patients with severe, steroid-resistant, acute GVHD is poor, and overall survival is low, despite of steady increasing repertoire of available drugs. Improved knowledge of GVHD pathophysiology has led to rational approaches to both prophylaxis and therapy.
Within bone marrow (BM) stroma, there exist subsets of non-hematopoietic cells referred to as mesenchymal stem cells, or mesenchymal stromal cells [1]. MSCs comprise a population of nonhematopoietic bone marrow cells that possess an extensive proliferative potential and ability to differentiate into various cell types [2]. Therefore, it may be used to improve rate and quality of haematopoietic engraftment by regenerating the marrow microenvironment [1,3,5].
MSCs play a significant role in bone marrow microenvironment. The major function of these cells is to provide mechanical support to hematopoietic cells. MCSs express a large number of adhesion molecules, extracellular matrix proteins, cytokines and growth factor receptors, associated with their function and cell interactions within bone marrow stroma [2]. Moreover, MSCs are known to produce a variety of cytokines that are involved in homing (stromal derived factor-1, SDF-1), or proliferation and differentiation of hematopoietic cells (GM-CSF, SCF, IL-6). E.g., the engrafted MSCs may support human hematopoiesis via secreted factors and by physical interactions with hematopoietic cells [7,13].
Moreover, MSCs are able of modifying cellular immune response by multiple mechanisms, suppressing various T cell, B cell and NK cell functions [4,6,8,9], thus suggesting their possible use for treatment of immune-mediated disorders, like as GVHD [11,12]. Thus, MSC are currently under investigation for their potential reparative and immunosuppressive effects.
An opportunity of tolerance induction to allogeneic or xenogeneic grafts following incompatible bone marrow stem cell transplantation into a mismatched recipient was proposed since 1984 [14]. However, only in 2002 it has been clearly demonstrated that human MSCs may inhibit proliferation of T cells [6,8,9]. MSCs are generally considered to be poorly immunogenic cells, since they do not express neither HLA MHC class II antigens, FAS ligand, nor costimulatory molecules, such as В7-1, В7-2, CD40, CD40L on their surface [10]. In addition, MSCs are able to suppress a variety of T-, B-, and NK cell functions, and may affect also dendritic cell activities [9]. However, little is known about probable molecular mechanism(s) responsible for these effects.
Hence, potential applications of MSCs for prophylaxis and treatment of both acute and chronic severe GVHD seem to be quite reasonable [15,16,17]. Co-transplantation of allogeneic MSC and allogeneic HSCs could provide some beneficial effects, such as enhanced engraftment, acceleration of immune reconstitution and suppression of GVHD in HSCT.
The aim of our present study was to test a hypothesis that co-transplantations of MSCs could be used either for GVHD prophylaxis, or treatment of severe acute or chronic GVHD following allogeneic HSCT.
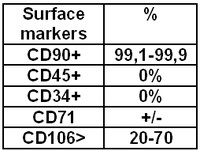
Table 1. MSCs phenotype
Patients and methods
Eligible for current study were children and adults (their age ranged from 6 to 53) with different hematological malignancies, such as acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia (AML), non-Hodgkin lymphoma (NHL), myelodisplastic syndrome (MDS), chronic myeloid leukemia (CML).
From October 2005 to May 2008, eight patients received co-transplantation of HSC and MSCs for speeding up engraftment and prophylaxis of GVHD.
Sixteen pts received isolated infusions of MSCs for treatment of steroid-resistant GVHD. Patients or/and their caregivers were fully informed about all aspects of their participation in the study. A signed informed consent form was obtained in all cases.
When performing MSC co-transplantation, related allo-HSCTs were performed in five patients, unrelated allo-HSCTs, in two cases, and haploidentical HCST in one patient. The source of HSC was BM (six cases), peripheral blood stem cells (PBSC) in one patient, and a combination of BM and PBSC in one case.
Seven patients received nonmyeloablative conditioning regimen (fludarabine+melphalan in five pts, fludarabine+busulfan in two pts), and one patient was subject to a myeloablative treatment (busulfane+cyclophosphamide).
Acute GVHD prophylaxis was cyclosporineA (CsA) and methotrexate (Mtx) (short course) in seven patients and CsA and mycophenotate mofetil (MMF) in one case. Patients and transplant characteristics are presented in Table 2 and 3.
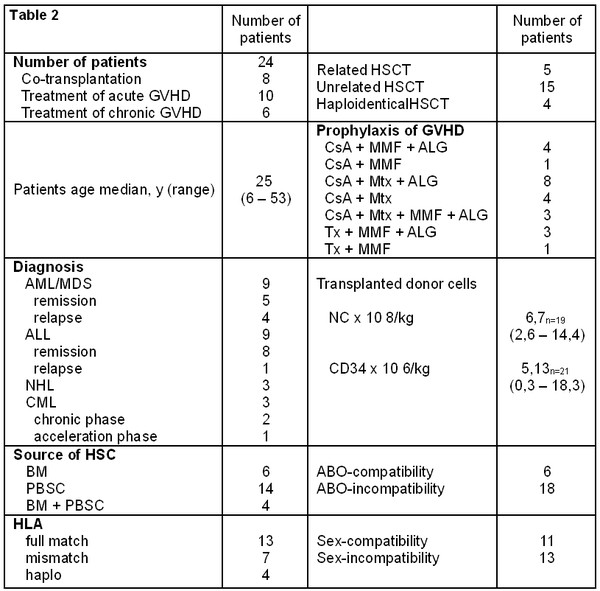
Table 2. Patient’s and transplant’s characteristics
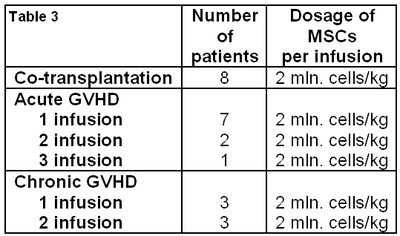
Table 3. Number of MSC’s infusion and dosage of MSCs
MSCs were harvested from the BM of HLA-identical sibling in cases of related allo-HSCT (n=5), or from the BM of haploidentical donors in cases of unrelated allo-HSCT (n=3).
Before starting the treatment, BM was aspirated from MSC donor, MSCs been collected and selected. Bone marrow-derived MSCs for transplantation were produced by “Trans-Technology” Ltd Company (license № 99-01-002224 dd. 14.07.2005). Generally, in vitro MSC processing included their specific selection and expansion in culture during 21-28 days, until achieving sufficient therapeutical dose MSC for co-transplantation into HSC recipient (2.0x10^6 cells/kg body weight). (Figure 1 and Table 3). This study used PCR-based testing of common infectious pathogens in bone marrow.
The patients were given in vitro expanded MSCs intravenously 24 hours before HSC infusion. Design of use MSCs shown in Fig.1.
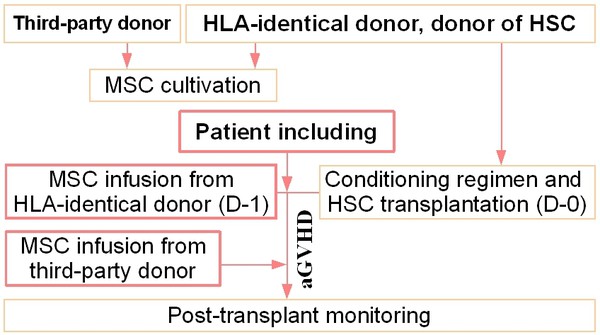
Figure 1. Design of usage of MSC for allogeneic hematopoietic stem cell transplantation
Isolated infusions of MSCs was performed in cases of steroid-resistant acute or chronic GVHD in patients after unrelated HSCT. Two patients had a mismatch in C locus, one, in DRB1 locus, one in B and C locus, one in DRB1 and C locus and three patients were haploidentical to their donors.
Ten patients received single MSC doses, five recipients - 2 doses, and one patient received three MSC doses (Table 2).
For thirteen patients, PBSCs were used as an HSC source. In cases of haplo-HSCT, HSCs represented a combination of G-CSF-primed marrow cells and T-cell depleted PBSCs (CliniMacs technique, Miltenyi Biotec).
MSCs were harvested from the BM of third-party donors in all cases before transplantation, and were cryopreserved until their use.
Results and discussion
A total of thirty-one infusions of mesenchymal stem cells were performed. Eight patients received co-transplantation of HSC and MSCs aiming to improve engraftment, and for GVHD prophylaxis. Sixteen patients received isolated infusions of MSCs for treatment of acute or chronic steroid-resistant GVHD.
Among them, ten patients received single MSC doses, five patients were treated with double MSC infusions, and one patient has got three doses (Table 3). MSC infusion was well tolerated, safe, without immediate infusion-related or late MSC-associated toxicities. Due to rather different indications for MSC infusions in cases of MSC co-transplantation versus isolated infusions, their results will be reviewed and discussed separately.
Results of MSCs use for speeding up engraftment and prevention of GVHD (co-transplantation of MSC and HSC)
According to peripheral leukocyte recovery, HSC engraftment was observed in seven pts (D+16 to +38), whereas platelet reconstitution proceeded by D+14 to +45 post-transplant. Hence, infusion of MSCs before HSCs did not improve engraftment rates as compared to HSCT without co-infusion of MSCs during conditioning (Table 4). After co-transplantation, six patients remained alive between 3 and 25 months. Severe aGVHD (grade III to IV) was not observed in MSC group. Six patients had aGVHD stage 0-I, and one patient exhibited stage II aGVHD. Chronic GVHD was not registered.
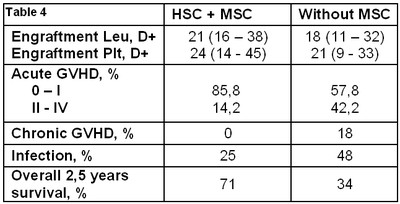
Table 4. Result of usage of MSC after co-transplantation
Additional infusions of MSCs for treatment of GVHD were not required due to the absence of severe GVHD. Two patients of this group died. In first case, graft failure was observed by D+16, complicated with disseminated intravenous coagulation and cerebral stroke. The second patient had disease progression and died at D+186. No treatment-related toxicities could be immediately ascribed to infusions of MSCs.
Overall 2.5 years relapse-free survival was 71%. No clinical complications were detected that could be attributed to MSC treatment.
Since non-myelоablative conditioning was used for 88% of co-transplanted patients, we have also compared the outcomes in these cases with general group after HSCT with reduced conditioning regimen.
Overall survival in MSC-treated group proved to be significantly higher, i.e., 71% after co-transplantation versus 34% after HSCT without MSCs (P=0,05). However, these data are rather preliminary and need further confirmation in larger series.
Mean incidence of infections in co-transplanted group was lower (25% against 48% in HSCT group). Two of eight patients developed respiratory, severe CMV and Aspergillus infection.
Result of MSC use for treatment of GVHD
Acute GVHD with involvement of skin was diagnosed in all patients (n=16), isolated involvement of skin (stage II-III) was detectable in eight patients. Combined aGVHD grade II with involvement of skin and liver was registered in one case, liver and gut GVHD, grade II-III was evident in four patients, and gut GVHD grade II-IV was found in three cases. One, two, or three MSC doses were administered, respectively, to ten, five, and one patient. Therefore, a total of twenty-three MSC infusions were performed. In ten patients, MSCs were used for treatment of acute GVHD, and in six cases they were applied for therapy of chronic GVHD.
After isolated infusions of MSCs in steroid-resistant aGVHD, a partial response (PR) was observed in five cases, complete response in two cases, without improvement in three cases. (Tab.5). Hence, after infusion of MSCs in the patients with aGVHD, a detectable response was observed in seven pts of ten (overall response rate 70%). Positive results of MSC administration for treatment of chGVHD were observed in 67%.
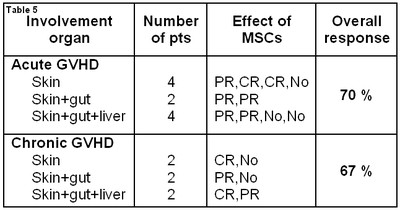
Table 5. Result of use MSC for treatment of GVHD
The median MSC dose did not differ for those patients who responded to the therapy, as compared with nonresponder group (2.0 x 10^6/kg b.w.of recipient). Six patients who responded to the first infusion were given a second infusion, to prevent GVHD recurrence upon reduction of immunosuppressive drug treatment. Two patients had complete response but received several (two or three) doses of MSCs because of GVHD recurrence. Four patients had partial responses and were given multiple (two or three) doses. Six of ten patients with involvement of one or two organs in aGVHD did respond to the therapy, as compared to four (of ten) patients with involvement of three organs.
Median time interval from onset of aGVHD to the start of treatment with MSC was 36 days (range 3-116).
Five patients were alive at the time of data analysis (May of 2008), with a median follow-up of 6,3 months (3-14,5 months) after infusion of MSCs.
Three patients had recurrences of their basic diseases, one with NHL, one with acute lymphoblastic leukaemia, and one with acute myeloid leukaemia. Fatal outcome was registered in all these cases. Acute GVHD was the most common cause of death in other cases (six patients of ten), with or without concomitant infection. One patient died with multiorgan failure. Infections in patients who died with acute or chronic GVHD included cytomegalovirus, aspergillosis and an unidentified pathogen.
In more than a half of patients with both acute and chGVHD, a single MSC dose produced a response, whereas in a few patients with partial response or with recurrence of acute or chronic GVHD, several doses were needed to induce a lasting response.
We have analyzed dependence of the response to MSCs infusion of various factors, such as conditioning regimen, GVHD prophylaxis, transplant type, donor and recipient characteristics. However, no significant differences were found, probably because of small and variable group of patients. There was relation between the treatment given before infusion of MSCs and response. In case of MSC infusion in the patients (n=7) after myeloablative HSCT, immunosupression and result of treatment of acute GVHD were better than after non-myeloablative regimen (Figure 2). Usage of ALG (Atgam by Pfizer) in GVHD prophylaxis did worsen the response to GVHD treatment with MSCs (Figure 3). In cases of HLA mismatch, HSCT response to MSCs infusion was more significant than after full-match HSCs. Patients transplanted from donor of opposite sex exhibited a more pronounced response to MSCs. ABO-incompatibility between donor and recipient did not influence response to MSCs. There are no differences in reactions to MSCs after GVHD prophylaxis with cyclosporine A versus tacrolimus.
Efficiency of MSCs therapy of GVHD with depending on:
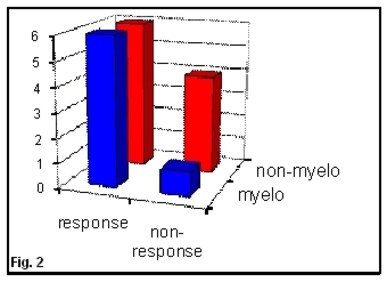
Figure 2. Conditioning regimen
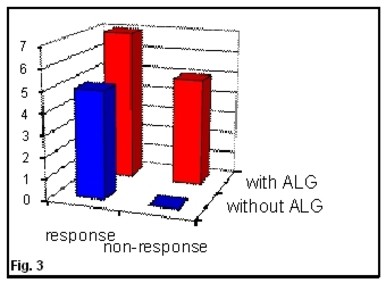
Figure 3. Usage of ALG
Conclusions
1. MSCs infusion in patients, who underwent allo-HSCT, is well-tolerated, safe, without immediate infusion-related or late MSC-associated toxicities.
2. Infusion of MSCs before HSCs transplantation did not influence duration of engraftment.
3. Infusion of MSCs during conditioning therapy before HSCT may prevent severe acute and chronic GVHD.
4. Infusion of MSCs for treatment-resistant both acute and chronic GVHD lead to reduction of GVHD grade in some patients.
5. Usage MSCs before HSC transplantation did not increase frequency of malignancy relapses.
6. Usage of MSCs seems to be more effective in patients after HSCT with myeloablative regimen and GVHD prophylaxis without ATG.
7. Large randomized clinical trials are necessary for evaluation of therapeutic effect of MSCs in allo-HSCT patients.
References
1. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow: analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-47.
2. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81-88.
3. Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307-16.
4. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11-20.
5. Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34 (+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870-78.
6. Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48.
7. Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733-38.
8. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-22.
9. Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, and Koc ON. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplantation. 2004;33:597-604.
10. Eliopoulos N, Stagg J, Lejeune L, Pommey S, and Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005 Dec 15;106(13).
11. Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557-64.
12. Fibbe WE and Noort WA. Mesenchymal stem cell and hematopoietic stem cell transplantation. Ann N Y Acad Sci. 2003;996:235-244.
13. Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764-67.
14. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263-72.
15. Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390-97.
16. Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-41.
17. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo M, Remberger M, Dini G, Egeler R, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008 May 10;371.
Accepted 09 December 2008
Published 23 December 2008


