This paper is based on the lecture held at the R. Gorbacheva Memorial Meeting in Hematopoietic Stem Cell Transplantation and Gene Therapy (Sept 19, 2020, St. Petersburg, Russia)
Background
Table 1. Indications for haematopoietic cell transplants in the US 2018 (https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx)

Table 2. Some new drugs for blood and bone marrow cancers


Figure 1. Results of a randomized trial of azacitidine with or without venetoclax in persons with newly diagnosed AML judged inappropriate to receive intensive chemotherapy [1]
Haematopoietic cell transplants are increasingly done to treat blood and bone marrow cancers, bone marrow failure and genetic disorders. In the US there were about 14,000 autotransplants and 9,000 allotransplants in 2018. Global autotransplants in 2019 were about 28,000 and allotransplants, about 17,000 with a total of about 45,000. These rates are expected to increase. In the US most autotransplants are for plasma cell myeloma (PCM) and non-Hodgkin lymphoma (NHL) whereas most allotransplants are for acute myeloid leukaemia (AML) and myelodysplastic syndromes (MDS). Relatively few allotransplants were done for acute lymphoblastic leukaemia (ALL) or myelo-proliferative neoplasms (MPNs; Table 1). This distribution is important as most immune therapies are for cancers not currently treated with haematopoietic cell transplants as I discuss below.
Discussion
Recent interventions which might compete with haematopoietic cell transplants include new anti-cancer chemotherapy and targeted and immune therapies. Several new drugs used to treat blood and bone marrow cancers are listed in Table 2.
Although progress in developing new drugs is encouraging the magnitude of benefit with several of these drugs is modest. An example of the impact of azacitidine and venetoclax in newly diagnosed persons with AML judged inappropriate to receive intensive chemotherapy is shown in Figure 1. Although adding venetoclax to azacitidine significantly improved survival, 65% of subjects died by 2.5 years.
Table 3 shows the 5-year survivals of persons with AML and PCM treated in 1999-2003, and treated 2010-2016. These data indicate only a modest improvement with about 70 percent of persons with AML and 45 percent of those with PCM dying before 5 years.
Examples of targeted therapies for myeloid cancers, especially AML are shown in Table 4 along with the target gene.
An example of the impact of targeted FLT3 mutation therapy in AML is shown in Fig. 2 [2]. Although adding midostaurin to cytarabine and daunorubicin improves survival, 55 percent of subjects died by 5 years and many survivors also received an allotransplant in 1st remission. These data indicate a modest impact of targeted therapy in AML.
Fig. 3 displays the estimated impact of targeted therapies on survival of persons with acute myeloblastic leukemia (AML) [3]. It seems only about 10 percent of people will benefit.

Table 5. Some examples of immune therapies in blood and bone marrow cancers

Immune therapy is another new therapy of blood and bone marrow cancers. Some examples are displayed in Table 5.
However, these therapies are effective only in B-cell cancers and are lineage- but not cancer-specific (Fig. 4). Other than gemtuzumab ozogamicin, an anti-CD33 monoclonal antibody, there is no proved safe and effective therapy of myeloid cancers.
CAR-T-cells are an important advance in therapy of lymphoid cancers with high response rates. However, often responses are not sustained unless a subsequent allogeneic haematopoietic cell transplant is done. An example of event-free survival (EFS) in children and young adults with advanced ALL is shown in Figure 5 with a failure rate of 40 percent at 1.5 years in complete responders [4].

Similarly, there are high rates of therapy-failure after successful CAR-T-cell therapy of diffuse large B-cell (DLBCL; Figure 6), follicular (FL) and marginal zone lymphomas (MZL; Fig. 7). [5] (https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.8008).

The argument CAR-T-cell therapy serves predominately as a bridge to an allotransplant is unconvincing as there are no strong data survival of persons with ALL transplanted in 2nd remission are better than those transplanted in relapse when all subjects are accounted for. Consequently, the best approach to someone responding to CAR-T-cell therapy is controversial. It may be reasonable to consider an allotransplant in persons with ALL and in those with MZL after responding to CAR-T-cell therapy, but wait for therapy failure in those with DLBCL and FL (Fig. 8).
It is also important to recall much of the efficacy of allotransplants results from an allogeneic anti-cancer effect so far difficult to distinguish from graft-versus-host disease (GvHD) [6]. These data are displayed in Fig. 9. For example, whether there is a specific anti-leukaemia effect is controversial, as reviewed in [7].
Some data suggest the potential use of donor-derived CAR-T-cells in persons relapsing after an allotransplant for B-cell cancers [8].

Conclusion
Advances in treating ALL, AML, lymphomas and PCM are important and exciting but the magnitude of benefit is modest and few people are cured. Many persons with immune therapy successes go on to receive an allotransplant. Most cancers where immune therapy is effective are B-cell cancers whereas most allotransplants are done for myeloid cancers. Moreover, these immune therapies are lineage-, not cancer-specific and lack the allogeneic anti-cancer effect associated with allotransplants. In young persons with PCM, autotransplants are better than new drugs in randomized clinical trials. CAR-T-cell therapy of B-cell cancers is effective but mostly not a cure. Donor CAR-T-cells are a promising in persons relapsing after an allogeneic haematopoietic cell transplant. Transplants will remain an important therapy in the immediate future. Put in terms of food, although there is nouvelle cuisine Boef Stroganoff remains a classic and my 1st dinner choice at Chekhov restaurant in Petersburg.
Acknowledgement
Supported by the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. Prof. Elena Parovichnikova and Drs. Kristina Zakurdaeva and Saida Kadyrova provided expert advice on Boef Stroganoff and Dr. Ivan Moiseev and the late Prof. Boris Afanasyev introduced me to Chekhov restaurant. My preference is for Boef Stroganoff with kasha. Bon Appetit.
Conflict of interest
RPG is a consultant to BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals; advisor to Antegene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd.
References
- DiNarco CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617-629.
- Stone RM, Mandrekar SJ, Sanford BL, Lauman K, Geyer S, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with FLT3 mutation. N Engl J Med. 2017;377:454-464.
- Prasad V, Gale RP. Precision medicine in acute myeloid leukemia: Hope, hype or both? Leuk Res. 2016;48:73-77.
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Enl J Med. 2018;378:439-448.
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019; 20: 31-42.
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555-562.
- Gale RP, Fuchs EJ. Is there really a specific graft-versus-leukaemia effect? Bone Marrow Transplant. 2016;51:1413-1415.
- Zhang C, Wang XQ, Zhang RL, Liu F, Wang Y, Yan ZL, et al. Donor-derived CD19 CAR-T-cell therapy of relapse of CD 19-positive B-ALL posttransplant. Leukemia.2020; In Press.
" ["~DETAIL_TEXT"]=> string(10791) "
This paper is based on the lecture held at the R. Gorbacheva Memorial Meeting in Hematopoietic Stem Cell Transplantation and Gene Therapy (Sept 19, 2020, St. Petersburg, Russia)
Background
Table 1. Indications for haematopoietic cell transplants in the US 2018 (https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx)

Table 2. Some new drugs for blood and bone marrow cancers


Figure 1. Results of a randomized trial of azacitidine with or without venetoclax in persons with newly diagnosed AML judged inappropriate to receive intensive chemotherapy [1]
Haematopoietic cell transplants are increasingly done to treat blood and bone marrow cancers, bone marrow failure and genetic disorders. In the US there were about 14,000 autotransplants and 9,000 allotransplants in 2018. Global autotransplants in 2019 were about 28,000 and allotransplants, about 17,000 with a total of about 45,000. These rates are expected to increase. In the US most autotransplants are for plasma cell myeloma (PCM) and non-Hodgkin lymphoma (NHL) whereas most allotransplants are for acute myeloid leukaemia (AML) and myelodysplastic syndromes (MDS). Relatively few allotransplants were done for acute lymphoblastic leukaemia (ALL) or myelo-proliferative neoplasms (MPNs; Table 1). This distribution is important as most immune therapies are for cancers not currently treated with haematopoietic cell transplants as I discuss below.
Discussion
Recent interventions which might compete with haematopoietic cell transplants include new anti-cancer chemotherapy and targeted and immune therapies. Several new drugs used to treat blood and bone marrow cancers are listed in Table 2.
Although progress in developing new drugs is encouraging the magnitude of benefit with several of these drugs is modest. An example of the impact of azacitidine and venetoclax in newly diagnosed persons with AML judged inappropriate to receive intensive chemotherapy is shown in Figure 1. Although adding venetoclax to azacitidine significantly improved survival, 65% of subjects died by 2.5 years.
Table 3 shows the 5-year survivals of persons with AML and PCM treated in 1999-2003, and treated 2010-2016. These data indicate only a modest improvement with about 70 percent of persons with AML and 45 percent of those with PCM dying before 5 years.
Examples of targeted therapies for myeloid cancers, especially AML are shown in Table 4 along with the target gene.
An example of the impact of targeted FLT3 mutation therapy in AML is shown in Fig. 2 [2]. Although adding midostaurin to cytarabine and daunorubicin improves survival, 55 percent of subjects died by 5 years and many survivors also received an allotransplant in 1st remission. These data indicate a modest impact of targeted therapy in AML.
Fig. 3 displays the estimated impact of targeted therapies on survival of persons with acute myeloblastic leukemia (AML) [3]. It seems only about 10 percent of people will benefit.

Table 5. Some examples of immune therapies in blood and bone marrow cancers

Immune therapy is another new therapy of blood and bone marrow cancers. Some examples are displayed in Table 5.
However, these therapies are effective only in B-cell cancers and are lineage- but not cancer-specific (Fig. 4). Other than gemtuzumab ozogamicin, an anti-CD33 monoclonal antibody, there is no proved safe and effective therapy of myeloid cancers.
CAR-T-cells are an important advance in therapy of lymphoid cancers with high response rates. However, often responses are not sustained unless a subsequent allogeneic haematopoietic cell transplant is done. An example of event-free survival (EFS) in children and young adults with advanced ALL is shown in Figure 5 with a failure rate of 40 percent at 1.5 years in complete responders [4].

Similarly, there are high rates of therapy-failure after successful CAR-T-cell therapy of diffuse large B-cell (DLBCL; Figure 6), follicular (FL) and marginal zone lymphomas (MZL; Fig. 7). [5] (https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.8008).

The argument CAR-T-cell therapy serves predominately as a bridge to an allotransplant is unconvincing as there are no strong data survival of persons with ALL transplanted in 2nd remission are better than those transplanted in relapse when all subjects are accounted for. Consequently, the best approach to someone responding to CAR-T-cell therapy is controversial. It may be reasonable to consider an allotransplant in persons with ALL and in those with MZL after responding to CAR-T-cell therapy, but wait for therapy failure in those with DLBCL and FL (Fig. 8).
It is also important to recall much of the efficacy of allotransplants results from an allogeneic anti-cancer effect so far difficult to distinguish from graft-versus-host disease (GvHD) [6]. These data are displayed in Fig. 9. For example, whether there is a specific anti-leukaemia effect is controversial, as reviewed in [7].
Some data suggest the potential use of donor-derived CAR-T-cells in persons relapsing after an allotransplant for B-cell cancers [8].

Conclusion
Advances in treating ALL, AML, lymphomas and PCM are important and exciting but the magnitude of benefit is modest and few people are cured. Many persons with immune therapy successes go on to receive an allotransplant. Most cancers where immune therapy is effective are B-cell cancers whereas most allotransplants are done for myeloid cancers. Moreover, these immune therapies are lineage-, not cancer-specific and lack the allogeneic anti-cancer effect associated with allotransplants. In young persons with PCM, autotransplants are better than new drugs in randomized clinical trials. CAR-T-cell therapy of B-cell cancers is effective but mostly not a cure. Donor CAR-T-cells are a promising in persons relapsing after an allogeneic haematopoietic cell transplant. Transplants will remain an important therapy in the immediate future. Put in terms of food, although there is nouvelle cuisine Boef Stroganoff remains a classic and my 1st dinner choice at Chekhov restaurant in Petersburg.
Acknowledgement
Supported by the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme. Prof. Elena Parovichnikova and Drs. Kristina Zakurdaeva and Saida Kadyrova provided expert advice on Boef Stroganoff and Dr. Ivan Moiseev and the late Prof. Boris Afanasyev introduced me to Chekhov restaurant. My preference is for Boef Stroganoff with kasha. Bon Appetit.
Conflict of interest
RPG is a consultant to BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals; advisor to Antegene Biotech LLC, Medical Director, FFF Enterprises Inc.; partner, AZAC Inc.; Board of Directors, Russian Foundation for Cancer Research Support; and Scientific Advisory Board: StemRad Ltd.
References
- DiNarco CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617-629.
- Stone RM, Mandrekar SJ, Sanford BL, Lauman K, Geyer S, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with FLT3 mutation. N Engl J Med. 2017;377:454-464.
- Prasad V, Gale RP. Precision medicine in acute myeloid leukemia: Hope, hype or both? Leuk Res. 2016;48:73-77.
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N Enl J Med. 2018;378:439-448.
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019; 20: 31-42.
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555-562.
- Gale RP, Fuchs EJ. Is there really a specific graft-versus-leukaemia effect? Bone Marrow Transplant. 2016;51:1413-1415.
- Zhang C, Wang XQ, Zhang RL, Liu F, Wang Y, Yan ZL, et al. Donor-derived CD19 CAR-T-cell therapy of relapse of CD 19-positive B-ALL posttransplant. Leukemia.2020; In Press.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(1) "-" ["~CODE"]=> string(1) "-" ["EXTERNAL_ID"]=> string(4) "1936" ["~EXTERNAL_ID"]=> string(4) "1936" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(159) "Есть ли будущее у трансплантации гемопоэтических клеток?Is there a Future for Haematopoietic Cell Transplants?" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(3055) "<p style="text-align: justify;">Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен</p> <p style="text-align: justify;">Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ). </p> <p style="text-align: justify;">Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.</p> <p style="text-align: justify;">Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.</p> <p style="text-align: justify;">Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_META_TITLE"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_META_KEYWORDS"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_META_DESCRIPTION"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_PICTURE_FILE_ALT"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_PICTURE_FILE_TITLE"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_PICTURE_FILE_NAME"]=> string(64) "est-li-budushchee-u-transplantatsii-gemopoeticheskikh-kletok-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(105) "Есть ли будущее у трансплантации гемопоэтических клеток?" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(64) "est-li-budushchee-u-transplantatsii-gemopoeticheskikh-kletok-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(64) "est-li-budushchee-u-transplantatsii-gemopoeticheskikh-kletok-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(64) "est-li-budushchee-u-transplantatsii-gemopoeticheskikh-kletok-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "168" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27299" ["VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27300" ["VALUE"]=> string(22) "10/23/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/23/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27301" ["VALUE"]=> array(2) { ["TEXT"]=> string(188) "<p>Роберт П. Гэйл </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(176) "
Роберт П. Гэйл
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27302" ["VALUE"]=> array(2) { ["TEXT"]=> string(199) "<p>Центр гематологии, Департамент иммунологии и воспаления, Имперский коледж Лондон, Великобритания</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(187) "Центр гематологии, Департамент иммунологии и воспаления, Имперский коледж Лондон, Великобритания
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27303" ["VALUE"]=> array(2) { ["TEXT"]=> string(3055) "<p style="text-align: justify;">Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен</p> <p style="text-align: justify;">Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ). </p> <p style="text-align: justify;">Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.</p> <p style="text-align: justify;">Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.</p> <p style="text-align: justify;">Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2911) "Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен
Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ).
Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.
Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.
Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27304" ["VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-4-6-10" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-4-6-10" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27307" ["VALUE"]=> array(2) { ["TEXT"]=> string(105) "<p>Robert P. Gale </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(93) "Robert P. Gale
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27308" ["VALUE"]=> array(2) { ["TEXT"]=> string(564) "<p style="text-align: justify;">Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK</p><br> <p><b>Correspondence</b><br> <p style="text-align: justify;"> Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK<br> Phone: +1-908-656-0484<br> Fax: +1-310-388-1320<br> E-mail: robertpetergale@alumni.ucla.edu</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(472) "Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Correspondence
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Phone: +1-908-656-0484
Fax: +1-310-388-1320
E-mail: robertpetergale@alumni.ucla.edu
Recent advances in anti-cancer chemotherapy and in targeted and immune therapies raise the question whether there is a future for haematopoietic cell transplants. I discuss their importance but in the end the magnitude of these improvements is modest. I point out the efficacy of immune therapy is predominately restricted to B-cell cancers and that many if not most successful immune therapy recipients eventually receive an allogeneic haematopoietic cell transplant, especially those with acute lymphoblastic leukaemia (ALL).
I also discuss most allotransplants are done for cancers not treated with current immune therapy. Randomized trials show an autotransplant is better than new drugs in young persons with plasma cell myeloma. Considerable data indicate much of the efficacy of allotransplants results from a non-cancer-specific allogeneic effect not expected to operate with current immune therapies. Lastly, I discuss a role for donor-derived chimeric antigen receptor (CAR)-T-cells in persons relapsing after an allotransplant for B-cell cancers. The sum of these considerations suggest an ongoing role for haematopoietic cell transplants in diverse settings.
Keywords
Hematopoietic stem cell transplantation, targeted drugs, immune therapy, (CAR)-T cells, efficiency.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27305" ["VALUE"]=> string(54) "Is there a Future for Haematopoietic Cell Transplants?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(54) "Is there a Future for Haematopoietic Cell Transplants?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27306" ["VALUE"]=> string(4) "2285" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2285" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27310" ["VALUE"]=> string(4) "2286" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2286" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27307" ["VALUE"]=> array(2) { ["TEXT"]=> string(105) "<p>Robert P. Gale </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(93) "Robert P. Gale
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(93) "Robert P. Gale
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27309" ["VALUE"]=> array(2) { ["TEXT"]=> string(1477) "<p style="text-align: justify;">Recent advances in anti-cancer chemotherapy and in targeted and immune therapies raise the question whether there is a future for haematopoietic cell transplants. I discuss their importance but in the end the magnitude of these improvements is modest. I point out the efficacy of immune therapy is predominately restricted to B-cell cancers and that many if not most successful immune therapy recipients eventually receive an allogeneic haematopoietic cell transplant, especially those with acute lymphoblastic leukaemia (ALL).</p> <p style="text-align: justify;">I also discuss most allotransplants are done for cancers not treated with current immune therapy. Randomized trials show an autotransplant is better than new drugs in young persons with plasma cell myeloma. Considerable data indicate much of the efficacy of allotransplants results from a non-cancer-specific allogeneic effect not expected to operate with current immune therapies. Lastly, I discuss a role for donor-derived chimeric antigen receptor (CAR)-T-cells in persons relapsing after an allotransplant for B-cell cancers. The sum of these considerations suggest an ongoing role for haematopoietic cell transplants in diverse settings.</p> <h2>Keywords</h2> <p style="text-align: justify;">Hematopoietic stem cell transplantation, targeted drugs, immune therapy, (CAR)-T cells, efficiency.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1399) "Recent advances in anti-cancer chemotherapy and in targeted and immune therapies raise the question whether there is a future for haematopoietic cell transplants. I discuss their importance but in the end the magnitude of these improvements is modest. I point out the efficacy of immune therapy is predominately restricted to B-cell cancers and that many if not most successful immune therapy recipients eventually receive an allogeneic haematopoietic cell transplant, especially those with acute lymphoblastic leukaemia (ALL).
I also discuss most allotransplants are done for cancers not treated with current immune therapy. Randomized trials show an autotransplant is better than new drugs in young persons with plasma cell myeloma. Considerable data indicate much of the efficacy of allotransplants results from a non-cancer-specific allogeneic effect not expected to operate with current immune therapies. Lastly, I discuss a role for donor-derived chimeric antigen receptor (CAR)-T-cells in persons relapsing after an allotransplant for B-cell cancers. The sum of these considerations suggest an ongoing role for haematopoietic cell transplants in diverse settings.
Keywords
Hematopoietic stem cell transplantation, targeted drugs, immune therapy, (CAR)-T cells, efficiency.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1399) "Recent advances in anti-cancer chemotherapy and in targeted and immune therapies raise the question whether there is a future for haematopoietic cell transplants. I discuss their importance but in the end the magnitude of these improvements is modest. I point out the efficacy of immune therapy is predominately restricted to B-cell cancers and that many if not most successful immune therapy recipients eventually receive an allogeneic haematopoietic cell transplant, especially those with acute lymphoblastic leukaemia (ALL).
I also discuss most allotransplants are done for cancers not treated with current immune therapy. Randomized trials show an autotransplant is better than new drugs in young persons with plasma cell myeloma. Considerable data indicate much of the efficacy of allotransplants results from a non-cancer-specific allogeneic effect not expected to operate with current immune therapies. Lastly, I discuss a role for donor-derived chimeric antigen receptor (CAR)-T-cells in persons relapsing after an allotransplant for B-cell cancers. The sum of these considerations suggest an ongoing role for haematopoietic cell transplants in diverse settings.
Keywords
Hematopoietic stem cell transplantation, targeted drugs, immune therapy, (CAR)-T cells, efficiency.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27304" ["VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-4-6-10" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-4-6-10" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(36) "10.18620/ctt-1866-8836-2020-9-4-6-10" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27305" ["VALUE"]=> string(54) "Is there a Future for Haematopoietic Cell Transplants?" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(54) "Is there a Future for Haematopoietic Cell Transplants?" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(54) "Is there a Future for Haematopoietic Cell Transplants?" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27308" ["VALUE"]=> array(2) { ["TEXT"]=> string(564) "<p style="text-align: justify;">Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK</p><br> <p><b>Correspondence</b><br> <p style="text-align: justify;"> Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK<br> Phone: +1-908-656-0484<br> Fax: +1-310-388-1320<br> E-mail: robertpetergale@alumni.ucla.edu</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(472) "Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Correspondence
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Phone: +1-908-656-0484
Fax: +1-310-388-1320
E-mail: robertpetergale@alumni.ucla.edu
Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Correspondence
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Phone: +1-908-656-0484
Fax: +1-310-388-1320
E-mail: robertpetergale@alumni.ucla.edu
Роберт П. Гэйл
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(176) "Роберт П. Гэйл
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27299" ["VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "10/02/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27300" ["VALUE"]=> string(22) "10/23/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/23/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "10/23/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27303" ["VALUE"]=> array(2) { ["TEXT"]=> string(3055) "<p style="text-align: justify;">Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен</p> <p style="text-align: justify;">Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ). </p> <p style="text-align: justify;">Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.</p> <p style="text-align: justify;">Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.</p> <p style="text-align: justify;">Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2911) "Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен
Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ).
Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.
Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.
Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2911) "Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен
Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ).
Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.
Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.
Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27302" ["VALUE"]=> array(2) { ["TEXT"]=> string(199) "<p>Центр гематологии, Департамент иммунологии и воспаления, Имперский коледж Лондон, Великобритания</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(187) "Центр гематологии, Департамент иммунологии и воспаления, Имперский коледж Лондон, Великобритания
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(187) "Центр гематологии, Департамент иммунологии и воспаления, Имперский коледж Лондон, Великобритания
" } } } [1]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "169" ["~IBLOCK_SECTION_ID"]=> string(3) "169" ["ID"]=> string(4) "1937" ["~ID"]=> string(4) "1937" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["~NAME"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "02/03/2021 04:48:07 pm" ["~TIMESTAMP_X"]=> string(22) "02/03/2021 04:48:07 pm" ["DETAIL_PAGE_URL"]=> string(25) "/en/archive/-9-4/-/-eln-/" ["~DETAIL_PAGE_URL"]=> string(25) "/en/archive/-9-4/-/-eln-/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(29015) "Introduction
Since the first attempts at treating CML with arsenic in 1865, treatment has been mostly palliative. Some modest prolongation of survival was reported with hydroxyurea and interferon alpha, for review see Hehlmann (2020) [1]. The only curative approach was allogeneic transplantation which, however, was available only to those few patients who had a donor and could tolerate the procedure. The advent of tyrosine kinase inhibitors has profoundly changed CML management as normal survival has been achieved for most patients as seen from Table 1 [2-11]. The new goal for treating CML is now survival at good quality of life without life-long treatment: treatment discontinuation in sustained deep molecular remission (DMR) and treatment-free remission (TFR). The European LeukemiaNet (ELN) has accounted for this development with an update of its recommendations [12]. This review summarizes the most important new developments and recommendations for treating CML including early transplantation of patients with high-risk additional chromosomal abnormalities (ACA) in early CML end-phase [13].
Table 1. Survival of CML patients in clinical trials: update 2020

Note: IM = imatinib, Nilo = nilotinib, Dasa = dasatinib, BOS = bosutinib, NA = not assessed
Diagnosis
At diagnosis, ELN recommends a complete blood count with microscopic differential and a physical examination with special reference to spleen and liver size. Marrow cytology, cytogenetics for securing the Philadelphia (Ph) chromosome and a qualitative polymerase chain reaction (PCR) for BCR-ABL1 transcripts detection and typing are also recommended as well as an EKG, standard clinical chemistry and a hepatitis serology [12].
Risk score
The preferred risk score is the new EUTOS score for long-term survival (ELTS), since it predicts death by CML better than all other scores [14, 15]. ELTS uses the same variables as the Sokal score, but with different weights. Age is much less important in the TKI era, since TKI treatment is virtually equally successful in older patients. The variables of the ELTS score and the calculation of relative risk are shown in Table 2.
Table 2. Risk assessment by ELTS14

To calculate the ELTS scores go to: http://www.leukemia-net.org/content/leukemia/cml/elts score/index_eng.html
Molecular monitoring, response milestones and deep molecular response
Molecular monitoring has replaced cytogenetics in clinical routine and is considered mainstay of treatment monitoring. Cytogenetics is still needed in the case of atypical translocations or atypical transcripts that cannot be measured by standard PCR, and in the case of failure/resistance or progression for detecting additional chromosomal abnormalities (ACA).
Quantitative real-time PCR (RT-PCR) should be performed on blood cells by standard methodology and reported as % BCR-ABL1 transcripts on the international scale (IS) [16,17]. BCR-ABL1 in %IS underlies the response milestones guiding treatment (Table 3).
Table 3. Response milestones expressed as % BCR-ABL1IS

*Loss of MMR indicates failure after treatment-free remission (TFR)

Figure 1. Benchmark times for molecular responses with imatinib (updated from Kalmanti et al.) [19]
Deep molecular responses (DMR; MR4 or deeper) indicate a state of disease with a very low probability of progress [18]. They are observed in the majority of TKI treated patients.
Benchmark times for what can be expected have been determined in imatinib treated patients and are depicted in Fig. 1. Most molecular responses are stable. After 10 years, 92% of patients in MMR reached MR4.5, 88% in MR4 reached MR5. Only one of 1326 patients in MR4 progressed during a median of 3.8 years, and none of 1302 patients in MR4.5 during a median of 3 years [18].
Failure or intolerance (not for not-reaching MMR) in imatinib treated patients with treatment change to 2G-TKI were observed in 26.5% over 9.5 years after a median of 34 months [2]. Changing treatment identified patients who did worse than the rest of the cohort, thus representing a poorer risk group. Most imatinib-treated patients, however, are candidates for treatment discontinuation.
First-line treatment
At present, 4 drugs are approved for 1st line therapy in CML by EMA and FDA:
• imatinib;
• dasatinib;
• nilotinib;
• bosutinib.
Approved in Korea only:
• radotinib.
Generic imatinib, now available worldwide, is the cost-effective initial therapy in chronic phase (CP) CML. Dosing of generics should be the same as brand dosing. Patients should continue the same generic brand in order to avoid potential side-effects due to changes in drug structure, bioavailability and drug preparation [12].
Second- and higher-line treatment
Second and higher lines of treatment after intolerance or resistance to the first-line TKI usually also consist of a TKI, but may include allogeneic transplantation of hematopoietic cells (allo-HCT), see below.
Table 4. TKI drugs recommended in case of BCR-ABL1 resistance mutations

In the instance of treatment failure/resistance or progression to accelerated phase or blast crisis a mutational analysis should be initiated (Table 4) and the treatment changed. If available, next-generation sequencing (NGS) should be used for mutational analysis [12, 20]. Imatinib resistance mutations are relatively rare in CP2, but are more frequent in advanced phases.
If 2G-TKI are applied, the following comorbidities and contraindications have to be considered:
• Dasatinib:
- Previous pleuro-pulmonary diseases are strong contraindications (cave pleural effusion).
- Uncontrolled hypertension, pulmonary arterial hypertension (PAH) and bleeding due to impaired platelet function (cave anticoagulation) are relative contraindications.
• Nilotinib:
- Coronary heart disease, cerebrovascular accidents and peripheral arterial occlusive disease represent strong contraindications.
- Also, hypertension, diabetes mellitus, hypercholesterolemia and a history of pancreatitis may represent contraindications.
• Bosutinib:
- No relevant comorbidities have been determined yet. Frequent and annoying, but mostly self-limited diarrhea occurs. Loperamide may be indicated.
• Ponatinib:
- Ponatinib is a third generation (3G-)TKI and the only TKI with activity against the T315I mutation.
- Because of its cardiotoxicity dosing is critical. An initial dose of 45 mg/day should be reduced to a lower dose (15 mg/day) as soon as a response has been achieved [21].
Allogeneic transplantation
Although drug therapy is clearly superior to transplantation in CP [22], transplantation still plays an important role in CML treatment. Indications have moved from CP to more advanced phases, accelerated phase (AP) and blast crisis (BC), but transplantation in CP has to be considered in high-risk patients. Transplantation in CP is still indicated in:
• TKI resistant disease
• Rare patients who are intolerant to all currently available TKI
• Resistance to initial 2G-TKI
• Resistance to 3G-TKI indicating high risk of progression
• End-phase CML with high-risk ACA.

Figure 2. Clinical strategies in evolving acceleration phase and blast crisis of CML
Fig. 2 illustrates the management of progression and emerging AP and BC1. Outcome of transplantation in AP and BC is worse than in CP, but transplantation provides probably the best outcome in BC. In an analysis of 786 BC patients managed by the German CML Study Group, 29 of the 40 long-term survivors (72.5%) had received a transplant [23].
Since earlier transplantations have better outcomes [23], the strategy is to recognize emerging progression to BC earlier. High-risk ACA indicate emerging progression. High-risk ACA are observed with increasing frequency in the later course of CML and have a negative impact on survival (Fig. 3). High-risk ACA are as follows [13, 24-26]:
• +8
• +Ph
• i(17q)
• +19
• +21
• +17
• -7/7q-
• 3q26.2
• 11q23
• complex karyotypes (3 or more aberrations).

High-risk ACA are used to define CML end-phase. CML end-phase comprises early progression with emerging high-risk ACA and late progression with failing hematopoiesis and blast proliferation (Fig. 4).

Figure 4. High-risk ACA and progression to blast crisis [13, 27, 28]

Figure 5. Early versus late transplantation in CML patients with high-risk ACA [13]
Table 5. Benchmark times for DMR (MR4, MR4.5)

Notes: *imatinib (n=1442), **nilotinib 300 mg twice daily (n=282), imatinib 400 mg daily (n=283), ***dasatinib 100 mg once daily (n=259), imatinib 400 mg daily (n=260), ****bosutinib 400 mg once daily (n=268), imatinib 400 mg daily (n=268), NA = not available
DMR rates of these trials cannot be directly compared owing to different methods of trial evaluation.
A total of 42 patients with high-risk ACA were transplanted in CML Study IV. Transplantation in early CML end-phase with emerging high-risk ACA, but without progression to AP or BC has shown superior survival (Fig. 5), although the survival difference, due to the small numbers (n=13 without progression; n=26 with progression to AP or BC; n=3 phase unknown), has not reached statistical significance at p=0.09 [13].
High-risk ACA at low blast counts herald death by CML [13]. The hazard to die with high-risk ACA compared with no ACA is increased:
• Up to 3.9-fold at blood blast levels of 1-5%;
• Up to 6.5-fold at marrow blast levels of 1-15%.
The lower the blast count, the higher is the predictive power of high-risk ACA. Low-risk ACA are associated with lesser or non-increased hazard.
Treatment discontinuation and TFR
Achievement of TFR after treatment discontinuation in sustained DMR is a new goal in the management of CML[12]. The majority of imatinib-treated patients in CP have reached DMR (MR4 or deeper) after 3 years as seen from Fig. 1 [18, 19]. Benchmark times for DMR have been determined in long-term clinical trials for imatinib [2], dasatinib [7], nilotinib [6], and bosutinib [8] and are shown in Table 5.
After the first pioneering studies have been published by the French CML group [29,30] many more studies have followed. Table 6 shows a selection of 21 studies totaling close to 3000 patients. Rates of relapse-free remissions at 2 years range around 50% (33% to 72% at 0.5-10 years). The largest of the studies, the EURO-SKI study (n=755), reports a TFR rate of 49% at 2 years [31].
Duration of TFR and of TKI treatment appear to be the most important predictors of successful TFR [31]. Loss of MMR indicates failure after TFR [32]. After resumption of treatment, 95% of patients will regain pre-discontinuation response levels.
The ELN considers the following requirements mandatory for TKI discontinuation [12]:
• CML in first CP only (data are lacking outside this setting);
• motivated patient with structured communication;
• accessibility to high quality quantitative PCR using the International Scale (IS) with rapid turn-around of PCR test results;
• patient agreement to more frequent monitoring after stopping treatment meaning;
• monthly for the first 6 months, every 2 months for months 6-12, and every 3 months thereafter.
Table 6. Selected TKI-discontinuation studies, update 2020


Notes: Updated from [1]. ND = not defined; UMRD = undetectable minimal residual disease; IM = Imatinib; Nilo = Nilotinib; Dasa = Dasatinib; MR = molecular response; RFS = relapse free survival.
Conclusion
By 2020, survival of patients with CML has approached that of the general population. ELTS score is the preferred risk score in the TKI era. Molecular monitoring of minimal residual disease has replaced cytogenetics in routine monitoring. The four TKIs available for first-line therapy show different adverse effects profiles, but no differences in survival. Generic imatinib is the cost-effective initial treatment in chronic phase CML. Usage of second and higher-line TKI therapy is specified by mutational analysis and comorbidities. Early allogeneic hematopoietic cell transplantation is indicated in high-risk patients, e.g. with high-risk ACA. The new treatment goal is TFR. TKI discontinuation is feasible and safe. The rate of successful TFR ranges around 50% at 2 years.
Conflict of interest
None declared.
References
- Hehlmann, R. Chronic Myeloid Leukemia in 2020. HemaSphere. 2020; 4(5): e468.
- Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017; 31(11):2398-2406.
- Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. New Engl J Med. 2017; 376:917-927.
- Sasaki K, Strom SS, O’Brien S, Jabbour E, Ravandi F, Konopleva M et al. Relative survival in patients with chronic-phase chronic myeloid leukemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015; 2:e186-e193.
- ESH Update 2019 to: Preudhomme C, Guilhot J, Nicolini F et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010; 363:2511-2521.
- Hughes TP, Saglio G, Larson RA, Kantarjian HM, Kim D‐W, Issaragrisil S, et al. Long-term outcomes in patients with chronic myeloid leukemia in chronic phase receiving frontline nilotinib versus imatinib: Enestnd 10-year analysis. Blood. 2019; 134:2924-2924.
- Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patient trial. J Clin Oncol. 2016; 34:2333-2340.
- Brümmendorf TH, Cortes JE, Milojkovic D, Gambacorti-Passerini C, Clark RE, Le Coutre PD, et al. Bosutinib (BOS) versus imatinib (IM) for newly diagnosed chronic myeloid leukemia (CML): Final 5-year results from the BFORE trial. Blood. 2020; 136 (Supplement 1): 41-42.
- Thielen N, Visser O, Ossenkoppele G, Janssen J. Chronic myeloid leukemia in the Netherlands: a population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur J Haematol. 2016; 97(2): 145-154.
- Bower H, Bjorkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016; 34(24): 2851-2857.
- Welch HG, Kramer BS, Black WC. Epidemiologic signatures in cancer. N Engl J Med. 2019; 381(14): 1378-1386.
- Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley J F, Cervantes F et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020; 34(4):966-984.
- Hehlmann R, Voskanyan A, Lauseker M, Pfirrmann M, Kalmanti L, Rinaldetti S et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. 2020; 34: 2074-2086.
- Pfirrmann M, Baccarani M, Saußele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016; 30(1), 48-56.
- Pfirrmann M, Clark RE, Prejzner W, Lauseker M, Baccarani M, Saussele S, et al. The EUTOS long-term survival (ELTS) score is superior to the Sokal score for predicting survival in chronic myeloid leukemia. Leukemia. 2020; 34(8), 2138-2149.
- Cross NCP, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012; 26:2172-2175.
- Cross NCP, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015; 29:999-1003.
- Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014; 32:415-423.
- Kalmanti L, Saußele S, Lauseker M, Müller MC, Dietz CT, Heinrich L et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015; 29:1123-1132.
- Soverini S, Bavaro L, De Benedittis C, Martelli M, Iurlo A, Orofino N, et al. Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: the NEXT-in-CML study. Blood. 2020; 135:534-541.
- Cortes J, Lomaia E, Turkina A, Moiraghi B, Undurraga Sutton M, et al. Interim analysis from the OPTIC trial – a dose-ranging study of 3 starting doses of ponatinib. Clin Lymph Myel & Leuk. 2020; 20: S234-S234.
- Hehlmann R, Berger U, Pfirrmann M, Heimpel H, Hochhaus A, Hasford J, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007; 109(11): 4686-4692.
- Gratwohl A, Pfirrmann M, Zander A, Kröger N, Beelen D, Novotny J, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016; 30(3): 562-569.
- Fabarius A, Kalmanti L, Dietz CT, Lauseker M, Rinaldetti S, Haferlach C, et al. SAKK and the German CML Study Group. Impact of unbalanced minor route versus major route karyotypes at diagnosis on prognosis of CML. Ann Hematol. 2015;94(12):2015-2024.
- Wang W, Cortes JE, Tang G. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016; 127(22):2742-2750.
- Gong Z, Medeiros LJ, Cortes JE, et al. Cytogenetics-based risk prediction of blastic transformation of chronic myeloid leukemia in the era of TKI therapy. Blood Adv. 2017; 1:2541-2552.
- Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006; 108(1): 319-327.
- Ko TK, Javed A, Lee KL, Pathiraja TN, Liu X, Malik S, et al. An integrative model of pathway convergence in genetically heterogeneous blast crisis chronic myeloid leukemia. Blood. 2020;135(26):2337-2353.
- Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007; 109(1): 58-60.
- Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicenter Stop Imatinib (STIM) trial. Lancet Oncol. 2010; 11(11), 1029-1035.
- Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia (EURO-SKI): a prespecified interim analysis of a prospective, multicenter, non-randomized, trial. Lancet Oncol. 2018;19(6): 747-757.
- Rousselot P, Charbonnier A, Cony-Makhoul et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5): 424-430.
- Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017; 35(3): 298-305.
- Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT et al (2013). Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013; 122(4): 515-522.
- Lee SE, Choi SY, Song HY, Kim SH, Choi MY, Park JS et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016; 101(6): 717-723.
- Zang DY, Lee WS, Mun YC, et al. Long-term follow-up after treatment discontinuation in patients with chronic myeloid leukemia: the Korean Imatinib Discontinuation (KID) study. Blood. 2018; 132(Supplement 1): 4252-4252.
- Nicolini FE, Dulucq S, Guilhot J, Etienne G, Mahon FX. The evaluation of residual disease by digital PCR and TKI duration are critical predictive factors for molecular recurrence after stopping imatinib first-line in chronic phase CML patients: Results of the STIM2 Study. Blood. 2018; 132; ASH abstract 462.
- Mori S, Vagge E, Le Coutre P et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am J Hematol. 2015; 90(10): 910-914.
- Mori S, le Coutre P, Abruzzese E, et al. Imatinib Suspension and Validation (ISAV) study: final results at 79 months. Blood. 2018; 132(Supplement 1): 461-461.
- Rea D, Nicolini FE, Tulliez M et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017; 29(7): 846-854.
- Imagawa J, Tanaka H, Okada M et al. Discontinuation of dasatinib in patients with chronic myeloid leukemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicenter phase 2 trial. Lancet Haematol. 2015; 2(12): e528-e535.
- Kadowaki N, Kawaguchi T, Kuroda J et al. Discontinuation of nilotinib in patients with chronic myeloid leukemia who have maintained deep molecular responses for at least 2 years: a multicenter phase 2 stop nilotinib (Nilst) trial. Blood 2016; 128 (ASH Abstracts): 790-790.
- Kim DDH, Bence-Bruckler I, Forrest DL et al. Treatment-free remission accomplished by dasatinib (TRAD): Preliminary results of the Pan-Canadian tyrosine kinase inhibitor discontinuation trial. Blood. 2016; 128 (ASH Abstracts): 1922-1922.
- Shah N, García-Gutiérrez JV, Jiménez-Velasco A et al. Dasfree 2-year update: dasatinib discontinuation in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) and deep molecular response (DMR): PF408. HemaSphere. 2019; 3: 156.
- Hughes TP, Boquimpani CM, Takahashi N et al. Treatment-free remission in patients with chronic myeloid leukemia in chronic phase according to reasons for switching from imatinib to nilotinib: subgroup analysis from ENESTop. Blood. 2016; 128 (ASH Abstracts): 792-792.
- Takahashi N, Nishiwaki K, Nakaseko C et al. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica. 2018; 103(11): 1835-1842.
- Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7): 1525-1531.
- Kumagai T, Nakaseko C, Nishiwaki K et al. Discontinuation of dasatinib after deep molecular response for over 2 years in patients with chronic myelogenous leukemia and the unique profiles of lymphocyte subsets for successful discontinuation: a prospective, multicenter Japanese trial (D-STOP Trial). Blood. 2016; 128 (ASH Abstracts): 791-791.
- Hernández-Boluda JC, Pereira A, Pastor-Galán I, Alvarez-Larran A, Savchuk A, Puerta Puerta JM et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer Journal. 2018; 8(10):1-8.
- Clark RE, Polydoros F, Apperley J F, Milojkovic D, Rothwell K, Pocock C. Initial reduction of therapy prior to complete treatment discontinuation in chronic myeloid leukaemia: final results of the British DESTINY Study. Lancet Haematol. 2019;6:e375-e383.
- Rousselot P, Loiseau C, Delord M, Cayuela JM, Spentchian M. Late molecular recurrences in patients with chronic myeloid leukemia experiencing treatment-free remission. Blood Adv. 2020; 4(13): 3034-3040.
- Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017; 123(22), 4403-4410.
" ["~DETAIL_TEXT"]=> string(29015) "
Introduction
Since the first attempts at treating CML with arsenic in 1865, treatment has been mostly palliative. Some modest prolongation of survival was reported with hydroxyurea and interferon alpha, for review see Hehlmann (2020) [1]. The only curative approach was allogeneic transplantation which, however, was available only to those few patients who had a donor and could tolerate the procedure. The advent of tyrosine kinase inhibitors has profoundly changed CML management as normal survival has been achieved for most patients as seen from Table 1 [2-11]. The new goal for treating CML is now survival at good quality of life without life-long treatment: treatment discontinuation in sustained deep molecular remission (DMR) and treatment-free remission (TFR). The European LeukemiaNet (ELN) has accounted for this development with an update of its recommendations [12]. This review summarizes the most important new developments and recommendations for treating CML including early transplantation of patients with high-risk additional chromosomal abnormalities (ACA) in early CML end-phase [13].
Table 1. Survival of CML patients in clinical trials: update 2020

Note: IM = imatinib, Nilo = nilotinib, Dasa = dasatinib, BOS = bosutinib, NA = not assessed
Diagnosis
At diagnosis, ELN recommends a complete blood count with microscopic differential and a physical examination with special reference to spleen and liver size. Marrow cytology, cytogenetics for securing the Philadelphia (Ph) chromosome and a qualitative polymerase chain reaction (PCR) for BCR-ABL1 transcripts detection and typing are also recommended as well as an EKG, standard clinical chemistry and a hepatitis serology [12].
Risk score
The preferred risk score is the new EUTOS score for long-term survival (ELTS), since it predicts death by CML better than all other scores [14, 15]. ELTS uses the same variables as the Sokal score, but with different weights. Age is much less important in the TKI era, since TKI treatment is virtually equally successful in older patients. The variables of the ELTS score and the calculation of relative risk are shown in Table 2.
Table 2. Risk assessment by ELTS14

To calculate the ELTS scores go to: http://www.leukemia-net.org/content/leukemia/cml/elts score/index_eng.html
Molecular monitoring, response milestones and deep molecular response
Molecular monitoring has replaced cytogenetics in clinical routine and is considered mainstay of treatment monitoring. Cytogenetics is still needed in the case of atypical translocations or atypical transcripts that cannot be measured by standard PCR, and in the case of failure/resistance or progression for detecting additional chromosomal abnormalities (ACA).
Quantitative real-time PCR (RT-PCR) should be performed on blood cells by standard methodology and reported as % BCR-ABL1 transcripts on the international scale (IS) [16,17]. BCR-ABL1 in %IS underlies the response milestones guiding treatment (Table 3).
Table 3. Response milestones expressed as % BCR-ABL1IS

*Loss of MMR indicates failure after treatment-free remission (TFR)

Figure 1. Benchmark times for molecular responses with imatinib (updated from Kalmanti et al.) [19]
Deep molecular responses (DMR; MR4 or deeper) indicate a state of disease with a very low probability of progress [18]. They are observed in the majority of TKI treated patients.
Benchmark times for what can be expected have been determined in imatinib treated patients and are depicted in Fig. 1. Most molecular responses are stable. After 10 years, 92% of patients in MMR reached MR4.5, 88% in MR4 reached MR5. Only one of 1326 patients in MR4 progressed during a median of 3.8 years, and none of 1302 patients in MR4.5 during a median of 3 years [18].
Failure or intolerance (not for not-reaching MMR) in imatinib treated patients with treatment change to 2G-TKI were observed in 26.5% over 9.5 years after a median of 34 months [2]. Changing treatment identified patients who did worse than the rest of the cohort, thus representing a poorer risk group. Most imatinib-treated patients, however, are candidates for treatment discontinuation.
First-line treatment
At present, 4 drugs are approved for 1st line therapy in CML by EMA and FDA:
• imatinib;
• dasatinib;
• nilotinib;
• bosutinib.
Approved in Korea only:
• radotinib.
Generic imatinib, now available worldwide, is the cost-effective initial therapy in chronic phase (CP) CML. Dosing of generics should be the same as brand dosing. Patients should continue the same generic brand in order to avoid potential side-effects due to changes in drug structure, bioavailability and drug preparation [12].
Second- and higher-line treatment
Second and higher lines of treatment after intolerance or resistance to the first-line TKI usually also consist of a TKI, but may include allogeneic transplantation of hematopoietic cells (allo-HCT), see below.
Table 4. TKI drugs recommended in case of BCR-ABL1 resistance mutations

In the instance of treatment failure/resistance or progression to accelerated phase or blast crisis a mutational analysis should be initiated (Table 4) and the treatment changed. If available, next-generation sequencing (NGS) should be used for mutational analysis [12, 20]. Imatinib resistance mutations are relatively rare in CP2, but are more frequent in advanced phases.
If 2G-TKI are applied, the following comorbidities and contraindications have to be considered:
• Dasatinib:
- Previous pleuro-pulmonary diseases are strong contraindications (cave pleural effusion).
- Uncontrolled hypertension, pulmonary arterial hypertension (PAH) and bleeding due to impaired platelet function (cave anticoagulation) are relative contraindications.
• Nilotinib:
- Coronary heart disease, cerebrovascular accidents and peripheral arterial occlusive disease represent strong contraindications.
- Also, hypertension, diabetes mellitus, hypercholesterolemia and a history of pancreatitis may represent contraindications.
• Bosutinib:
- No relevant comorbidities have been determined yet. Frequent and annoying, but mostly self-limited diarrhea occurs. Loperamide may be indicated.
• Ponatinib:
- Ponatinib is a third generation (3G-)TKI and the only TKI with activity against the T315I mutation.
- Because of its cardiotoxicity dosing is critical. An initial dose of 45 mg/day should be reduced to a lower dose (15 mg/day) as soon as a response has been achieved [21].
Allogeneic transplantation
Although drug therapy is clearly superior to transplantation in CP [22], transplantation still plays an important role in CML treatment. Indications have moved from CP to more advanced phases, accelerated phase (AP) and blast crisis (BC), but transplantation in CP has to be considered in high-risk patients. Transplantation in CP is still indicated in:
• TKI resistant disease
• Rare patients who are intolerant to all currently available TKI
• Resistance to initial 2G-TKI
• Resistance to 3G-TKI indicating high risk of progression
• End-phase CML with high-risk ACA.

Figure 2. Clinical strategies in evolving acceleration phase and blast crisis of CML
Fig. 2 illustrates the management of progression and emerging AP and BC1. Outcome of transplantation in AP and BC is worse than in CP, but transplantation provides probably the best outcome in BC. In an analysis of 786 BC patients managed by the German CML Study Group, 29 of the 40 long-term survivors (72.5%) had received a transplant [23].
Since earlier transplantations have better outcomes [23], the strategy is to recognize emerging progression to BC earlier. High-risk ACA indicate emerging progression. High-risk ACA are observed with increasing frequency in the later course of CML and have a negative impact on survival (Fig. 3). High-risk ACA are as follows [13, 24-26]:
• +8
• +Ph
• i(17q)
• +19
• +21
• +17
• -7/7q-
• 3q26.2
• 11q23
• complex karyotypes (3 or more aberrations).

High-risk ACA are used to define CML end-phase. CML end-phase comprises early progression with emerging high-risk ACA and late progression with failing hematopoiesis and blast proliferation (Fig. 4).

Figure 4. High-risk ACA and progression to blast crisis [13, 27, 28]

Figure 5. Early versus late transplantation in CML patients with high-risk ACA [13]
Table 5. Benchmark times for DMR (MR4, MR4.5)

Notes: *imatinib (n=1442), **nilotinib 300 mg twice daily (n=282), imatinib 400 mg daily (n=283), ***dasatinib 100 mg once daily (n=259), imatinib 400 mg daily (n=260), ****bosutinib 400 mg once daily (n=268), imatinib 400 mg daily (n=268), NA = not available
DMR rates of these trials cannot be directly compared owing to different methods of trial evaluation.
A total of 42 patients with high-risk ACA were transplanted in CML Study IV. Transplantation in early CML end-phase with emerging high-risk ACA, but without progression to AP or BC has shown superior survival (Fig. 5), although the survival difference, due to the small numbers (n=13 without progression; n=26 with progression to AP or BC; n=3 phase unknown), has not reached statistical significance at p=0.09 [13].
High-risk ACA at low blast counts herald death by CML [13]. The hazard to die with high-risk ACA compared with no ACA is increased:
• Up to 3.9-fold at blood blast levels of 1-5%;
• Up to 6.5-fold at marrow blast levels of 1-15%.
The lower the blast count, the higher is the predictive power of high-risk ACA. Low-risk ACA are associated with lesser or non-increased hazard.
Treatment discontinuation and TFR
Achievement of TFR after treatment discontinuation in sustained DMR is a new goal in the management of CML[12]. The majority of imatinib-treated patients in CP have reached DMR (MR4 or deeper) after 3 years as seen from Fig. 1 [18, 19]. Benchmark times for DMR have been determined in long-term clinical trials for imatinib [2], dasatinib [7], nilotinib [6], and bosutinib [8] and are shown in Table 5.
After the first pioneering studies have been published by the French CML group [29,30] many more studies have followed. Table 6 shows a selection of 21 studies totaling close to 3000 patients. Rates of relapse-free remissions at 2 years range around 50% (33% to 72% at 0.5-10 years). The largest of the studies, the EURO-SKI study (n=755), reports a TFR rate of 49% at 2 years [31].
Duration of TFR and of TKI treatment appear to be the most important predictors of successful TFR [31]. Loss of MMR indicates failure after TFR [32]. After resumption of treatment, 95% of patients will regain pre-discontinuation response levels.
The ELN considers the following requirements mandatory for TKI discontinuation [12]:
• CML in first CP only (data are lacking outside this setting);
• motivated patient with structured communication;
• accessibility to high quality quantitative PCR using the International Scale (IS) with rapid turn-around of PCR test results;
• patient agreement to more frequent monitoring after stopping treatment meaning;
• monthly for the first 6 months, every 2 months for months 6-12, and every 3 months thereafter.
Table 6. Selected TKI-discontinuation studies, update 2020


Notes: Updated from [1]. ND = not defined; UMRD = undetectable minimal residual disease; IM = Imatinib; Nilo = Nilotinib; Dasa = Dasatinib; MR = molecular response; RFS = relapse free survival.
Conclusion
By 2020, survival of patients with CML has approached that of the general population. ELTS score is the preferred risk score in the TKI era. Molecular monitoring of minimal residual disease has replaced cytogenetics in routine monitoring. The four TKIs available for first-line therapy show different adverse effects profiles, but no differences in survival. Generic imatinib is the cost-effective initial treatment in chronic phase CML. Usage of second and higher-line TKI therapy is specified by mutational analysis and comorbidities. Early allogeneic hematopoietic cell transplantation is indicated in high-risk patients, e.g. with high-risk ACA. The new treatment goal is TFR. TKI discontinuation is feasible and safe. The rate of successful TFR ranges around 50% at 2 years.
Conflict of interest
None declared.
References
- Hehlmann, R. Chronic Myeloid Leukemia in 2020. HemaSphere. 2020; 4(5): e468.
- Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017; 31(11):2398-2406.
- Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. New Engl J Med. 2017; 376:917-927.
- Sasaki K, Strom SS, O’Brien S, Jabbour E, Ravandi F, Konopleva M et al. Relative survival in patients with chronic-phase chronic myeloid leukemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol. 2015; 2:e186-e193.
- ESH Update 2019 to: Preudhomme C, Guilhot J, Nicolini F et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010; 363:2511-2521.
- Hughes TP, Saglio G, Larson RA, Kantarjian HM, Kim D‐W, Issaragrisil S, et al. Long-term outcomes in patients with chronic myeloid leukemia in chronic phase receiving frontline nilotinib versus imatinib: Enestnd 10-year analysis. Blood. 2019; 134:2924-2924.
- Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patient trial. J Clin Oncol. 2016; 34:2333-2340.
- Brümmendorf TH, Cortes JE, Milojkovic D, Gambacorti-Passerini C, Clark RE, Le Coutre PD, et al. Bosutinib (BOS) versus imatinib (IM) for newly diagnosed chronic myeloid leukemia (CML): Final 5-year results from the BFORE trial. Blood. 2020; 136 (Supplement 1): 41-42.
- Thielen N, Visser O, Ossenkoppele G, Janssen J. Chronic myeloid leukemia in the Netherlands: a population-based study on incidence, treatment, and survival in 3585 patients from 1989 to 2012. Eur J Haematol. 2016; 97(2): 145-154.
- Bower H, Bjorkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016; 34(24): 2851-2857.
- Welch HG, Kramer BS, Black WC. Epidemiologic signatures in cancer. N Engl J Med. 2019; 381(14): 1378-1386.
- Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley J F, Cervantes F et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020; 34(4):966-984.
- Hehlmann R, Voskanyan A, Lauseker M, Pfirrmann M, Kalmanti L, Rinaldetti S et al. High-risk additional chromosomal abnormalities at low blast counts herald death by CML. Leukemia. 2020; 34: 2074-2086.
- Pfirrmann M, Baccarani M, Saußele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016; 30(1), 48-56.
- Pfirrmann M, Clark RE, Prejzner W, Lauseker M, Baccarani M, Saussele S, et al. The EUTOS long-term survival (ELTS) score is superior to the Sokal score for predicting survival in chronic myeloid leukemia. Leukemia. 2020; 34(8), 2138-2149.
- Cross NCP, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012; 26:2172-2175.
- Cross NCP, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015; 29:999-1003.
- Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014; 32:415-423.
- Kalmanti L, Saußele S, Lauseker M, Müller MC, Dietz CT, Heinrich L et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015; 29:1123-1132.
- Soverini S, Bavaro L, De Benedittis C, Martelli M, Iurlo A, Orofino N, et al. Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: the NEXT-in-CML study. Blood. 2020; 135:534-541.
- Cortes J, Lomaia E, Turkina A, Moiraghi B, Undurraga Sutton M, et al. Interim analysis from the OPTIC trial – a dose-ranging study of 3 starting doses of ponatinib. Clin Lymph Myel & Leuk. 2020; 20: S234-S234.
- Hehlmann R, Berger U, Pfirrmann M, Heimpel H, Hochhaus A, Hasford J, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007; 109(11): 4686-4692.
- Gratwohl A, Pfirrmann M, Zander A, Kröger N, Beelen D, Novotny J, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2016; 30(3): 562-569.
- Fabarius A, Kalmanti L, Dietz CT, Lauseker M, Rinaldetti S, Haferlach C, et al. SAKK and the German CML Study Group. Impact of unbalanced minor route versus major route karyotypes at diagnosis on prognosis of CML. Ann Hematol. 2015;94(12):2015-2024.
- Wang W, Cortes JE, Tang G. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016; 127(22):2742-2750.
- Gong Z, Medeiros LJ, Cortes JE, et al. Cytogenetics-based risk prediction of blastic transformation of chronic myeloid leukemia in the era of TKI therapy. Blood Adv. 2017; 1:2541-2552.
- Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006; 108(1): 319-327.
- Ko TK, Javed A, Lee KL, Pathiraja TN, Liu X, Malik S, et al. An integrative model of pathway convergence in genetically heterogeneous blast crisis chronic myeloid leukemia. Blood. 2020;135(26):2337-2353.
- Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007; 109(1): 58-60.
- Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicenter Stop Imatinib (STIM) trial. Lancet Oncol. 2010; 11(11), 1029-1035.
- Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia (EURO-SKI): a prespecified interim analysis of a prospective, multicenter, non-randomized, trial. Lancet Oncol. 2018;19(6): 747-757.
- Rousselot P, Charbonnier A, Cony-Makhoul et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5): 424-430.
- Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017; 35(3): 298-305.
- Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT et al (2013). Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013; 122(4): 515-522.
- Lee SE, Choi SY, Song HY, Kim SH, Choi MY, Park JS et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016; 101(6): 717-723.
- Zang DY, Lee WS, Mun YC, et al. Long-term follow-up after treatment discontinuation in patients with chronic myeloid leukemia: the Korean Imatinib Discontinuation (KID) study. Blood. 2018; 132(Supplement 1): 4252-4252.
- Nicolini FE, Dulucq S, Guilhot J, Etienne G, Mahon FX. The evaluation of residual disease by digital PCR and TKI duration are critical predictive factors for molecular recurrence after stopping imatinib first-line in chronic phase CML patients: Results of the STIM2 Study. Blood. 2018; 132; ASH abstract 462.
- Mori S, Vagge E, Le Coutre P et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am J Hematol. 2015; 90(10): 910-914.
- Mori S, le Coutre P, Abruzzese E, et al. Imatinib Suspension and Validation (ISAV) study: final results at 79 months. Blood. 2018; 132(Supplement 1): 461-461.
- Rea D, Nicolini FE, Tulliez M et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017; 29(7): 846-854.
- Imagawa J, Tanaka H, Okada M et al. Discontinuation of dasatinib in patients with chronic myeloid leukemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicenter phase 2 trial. Lancet Haematol. 2015; 2(12): e528-e535.
- Kadowaki N, Kawaguchi T, Kuroda J et al. Discontinuation of nilotinib in patients with chronic myeloid leukemia who have maintained deep molecular responses for at least 2 years: a multicenter phase 2 stop nilotinib (Nilst) trial. Blood 2016; 128 (ASH Abstracts): 790-790.
- Kim DDH, Bence-Bruckler I, Forrest DL et al. Treatment-free remission accomplished by dasatinib (TRAD): Preliminary results of the Pan-Canadian tyrosine kinase inhibitor discontinuation trial. Blood. 2016; 128 (ASH Abstracts): 1922-1922.
- Shah N, García-Gutiérrez JV, Jiménez-Velasco A et al. Dasfree 2-year update: dasatinib discontinuation in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) and deep molecular response (DMR): PF408. HemaSphere. 2019; 3: 156.
- Hughes TP, Boquimpani CM, Takahashi N et al. Treatment-free remission in patients with chronic myeloid leukemia in chronic phase according to reasons for switching from imatinib to nilotinib: subgroup analysis from ENESTop. Blood. 2016; 128 (ASH Abstracts): 792-792.
- Takahashi N, Nishiwaki K, Nakaseko C et al. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica. 2018; 103(11): 1835-1842.
- Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7): 1525-1531.
- Kumagai T, Nakaseko C, Nishiwaki K et al. Discontinuation of dasatinib after deep molecular response for over 2 years in patients with chronic myelogenous leukemia and the unique profiles of lymphocyte subsets for successful discontinuation: a prospective, multicenter Japanese trial (D-STOP Trial). Blood. 2016; 128 (ASH Abstracts): 791-791.
- Hernández-Boluda JC, Pereira A, Pastor-Galán I, Alvarez-Larran A, Savchuk A, Puerta Puerta JM et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer Journal. 2018; 8(10):1-8.
- Clark RE, Polydoros F, Apperley J F, Milojkovic D, Rothwell K, Pocock C. Initial reduction of therapy prior to complete treatment discontinuation in chronic myeloid leukaemia: final results of the British DESTINY Study. Lancet Haematol. 2019;6:e375-e383.
- Rousselot P, Loiseau C, Delord M, Cayuela JM, Spentchian M. Late molecular recurrences in patients with chronic myeloid leukemia experiencing treatment-free remission. Blood Adv. 2020; 4(13): 3034-3040.
- Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017; 123(22), 4403-4410.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(5) "-eln-" ["~CODE"]=> string(5) "-eln-" ["EXTERNAL_ID"]=> string(4) "1937" ["~EXTERNAL_ID"]=> string(4) "1937" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(395) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого рискаThe new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(1785) "<p style="text-align: justify;">Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_META_TITLE"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_META_KEYWORDS"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_META_DESCRIPTION"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_PICTURE_FILE_ALT"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_PICTURE_FILE_TITLE"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "novye-rekomendatsii-eln-po-lecheniyu-khronicheskogo-mieloidnogo-leykoza-rannyaya-transplantatsiya-u-" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(297) "Новые рекомендации ELN по лечению хронического миелоидного лейкоза. Ранняя трансплантация у пациентов с дополнительными хромосомными аберрациями высокого риска" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "novye-rekomendatsii-eln-po-lecheniyu-khronicheskogo-mieloidnogo-leykoza-rannyaya-transplantatsiya-u-" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "novye-rekomendatsii-eln-po-lecheniyu-khronicheskogo-mieloidnogo-leykoza-rannyaya-transplantatsiya-u-" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "novye-rekomendatsii-eln-po-lecheniyu-khronicheskogo-mieloidnogo-leykoza-rannyaya-transplantatsiya-u-" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "169" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27311" ["VALUE"]=> string(22) "09/28/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "09/28/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27312" ["VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27313" ["VALUE"]=> array(2) { ["TEXT"]=> string(194) "<p>Рюдигер Хельманн </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(182) "
Рюдигер Хельманн
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27314" ["VALUE"]=> array(2) { ["TEXT"]=> string(184) "<p>Медицинский факультет Маннгейма, Гейдельбергский университет; Фонд ELN, Вайнхайм, Германия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(172) "Медицинский факультет Маннгейма, Гейдельбергский университет; Фонд ELN, Вайнхайм, Германия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27315" ["VALUE"]=> array(2) { ["TEXT"]=> string(1785) "<p style="text-align: justify;">Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1729) "Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.
Ключевые слова
Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27316" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-11-19" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-11-19" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27319" ["VALUE"]=> array(2) { ["TEXT"]=> string(180) "<p>Rüdiger Hehlmann </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(168) "Rüdiger Hehlmann
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27320" ["VALUE"]=> array(2) { ["TEXT"]=> string(328) "<p>Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany</p><br> <p><b>Correspondence</b><br> Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany<br> E-mail: Hehlmann.eln@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(274) "Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
Correspondence
Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
E-mail: Hehlmann.eln@gmail.com
After 150 years of mostly palliative CML therapy, treatment advances with BCR-ABL1 tyrosine kinase inhibitors (TKI) have resulted in normal survival for most patients with CML. The new treatment goal is treatment-free remission (TFR) with survival at good quality of life without life-long treatment. The European LeukemiaNet (ELN) has accounted for this development with its most recent recommendations. Hematopoietic stem cell transplantation has retained an important role in patients who have become resistant or intolerant to all TKI or progress to advanced phases. This review focuses on the ELN 2020 recommendations for treating CML and on early transplantation in high-risk patients.
Keywords
Chronic myeloid leukemia, high-risk group, tyrosine kinase inhibitors, hematopoietic stem cell transplantation, ELN recommendations.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27317" ["VALUE"]=> string(98) "The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(98) "The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27318" ["VALUE"]=> string(4) "2297" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2297" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27322" ["VALUE"]=> string(4) "2298" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2298" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27319" ["VALUE"]=> array(2) { ["TEXT"]=> string(180) "<p>Rüdiger Hehlmann </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(168) "Rüdiger Hehlmann
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(168) "Rüdiger Hehlmann
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27321" ["VALUE"]=> array(2) { ["TEXT"]=> string(972) "<p style="text-align: justify;">After 150 years of mostly palliative CML therapy, treatment advances with BCR-ABL1 tyrosine kinase inhibitors (TKI) have resulted in normal survival for most patients with CML. The new treatment goal is treatment-free remission (TFR) with survival at good quality of life without life-long treatment. The European LeukemiaNet (ELN) has accounted for this development with its most recent recommendations. Hematopoietic stem cell transplantation has retained an important role in patients who have become resistant or intolerant to all TKI or progress to advanced phases. This review focuses on the ELN 2020 recommendations for treating CML and on early transplantation in high-risk patients.</p> <h2>Keywords</h2> <p style="text-align: justify;">Chronic myeloid leukemia, high-risk group, tyrosine kinase inhibitors, hematopoietic stem cell transplantation, ELN recommendations.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(916) "After 150 years of mostly palliative CML therapy, treatment advances with BCR-ABL1 tyrosine kinase inhibitors (TKI) have resulted in normal survival for most patients with CML. The new treatment goal is treatment-free remission (TFR) with survival at good quality of life without life-long treatment. The European LeukemiaNet (ELN) has accounted for this development with its most recent recommendations. Hematopoietic stem cell transplantation has retained an important role in patients who have become resistant or intolerant to all TKI or progress to advanced phases. This review focuses on the ELN 2020 recommendations for treating CML and on early transplantation in high-risk patients.
Keywords
Chronic myeloid leukemia, high-risk group, tyrosine kinase inhibitors, hematopoietic stem cell transplantation, ELN recommendations.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(916) "After 150 years of mostly palliative CML therapy, treatment advances with BCR-ABL1 tyrosine kinase inhibitors (TKI) have resulted in normal survival for most patients with CML. The new treatment goal is treatment-free remission (TFR) with survival at good quality of life without life-long treatment. The European LeukemiaNet (ELN) has accounted for this development with its most recent recommendations. Hematopoietic stem cell transplantation has retained an important role in patients who have become resistant or intolerant to all TKI or progress to advanced phases. This review focuses on the ELN 2020 recommendations for treating CML and on early transplantation in high-risk patients.
Keywords
Chronic myeloid leukemia, high-risk group, tyrosine kinase inhibitors, hematopoietic stem cell transplantation, ELN recommendations.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27316" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-11-19" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-11-19" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-11-19" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27317" ["VALUE"]=> string(98) "The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(98) "The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(98) "The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27320" ["VALUE"]=> array(2) { ["TEXT"]=> string(328) "<p>Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany</p><br> <p><b>Correspondence</b><br> Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany<br> E-mail: Hehlmann.eln@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(274) "Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
Correspondence
Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
E-mail: Hehlmann.eln@gmail.com
Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
Correspondence
Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
E-mail: Hehlmann.eln@gmail.com
Рюдигер Хельманн
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(182) "Рюдигер Хельманн
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27311" ["VALUE"]=> string(22) "09/28/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "09/28/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "09/28/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27312" ["VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/13/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27315" ["VALUE"]=> array(2) { ["TEXT"]=> string(1785) "<p style="text-align: justify;">Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1729) "Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.
Ключевые слова
Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1729) "Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.
Ключевые слова
Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27314" ["VALUE"]=> array(2) { ["TEXT"]=> string(184) "<p>Медицинский факультет Маннгейма, Гейдельбергский университет; Фонд ELN, Вайнхайм, Германия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(172) "Медицинский факультет Маннгейма, Гейдельбергский университет; Фонд ELN, Вайнхайм, Германия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(172) "Медицинский факультет Маннгейма, Гейдельбергский университет; Фонд ELN, Вайнхайм, Германия
" } } } [2]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "170" ["~IBLOCK_SECTION_ID"]=> string(3) "170" ["ID"]=> string(4) "1939" ["~ID"]=> string(4) "1939" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["~NAME"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "02/05/2021 11:57:40 am" ["~TIMESTAMP_X"]=> string(22) "02/05/2021 11:57:40 am" ["DETAIL_PAGE_URL"]=> string(138) "/en/archive/-9-4/klinicheskie-raboty/dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so/" ["~DETAIL_PAGE_URL"]=> string(138) "/en/archive/-9-4/klinicheskie-raboty/dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(25361) "Introduction
Chronic graft-versus-host disease (cGvHD) is a complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT), which is associated both with long-term mortality and significant disability in long-term survivors. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and several other risk factors [1-4]. Although cGvHD is associated with reduced risk of relapse and improved survival in the majority of malignant diseases [5], the persistence of clinical signs is associated with long-term mortality due to cardiovascular disease, infections and secondary malignancies [6]. Also cGvHD is the major cause of decline in the quality of life (QoL), social and professional disability. Gastrointestinal, joint and kidney problems are the main drivers of QoL decline [7-9].
The early studies of cGvHD treatment demonstrated a superiority of steroids over other agents in the treatment of cGvHD in terms of survival [10, 11]. However all subsequent attempts to improve response rate with augmented immunosuppression were not successful. Addition of thalidomide and mycofenolate mofetil resulted in higher frequency of adverse events and infection-related mortality [12, 13]. The only combination with some benefit in terms of steroid sparing was the combination of steroids and cyclosporine A (CsA), which demonstrated comparable response rate and duration of immunosuppression, however the cumulative dose of steroids was less in the combination arm, which resulted in the reduced frequency of femur aseptic necrosis [14]. The failure of these clinical trials to demonstrate improved response rate lead to the shift in the concept of cGvHD treatment. Currenly it is considered that immunosuppressive therapy (IST) does not induce tolerance, but rather alleviates target organ damage before the tolerance between donor and recipient cells occur. This understanding creates a dissonance between endpoints from the clinical studies and the real clinical practice where the formal response criteria, like decrease in the severity score or improved 2-minute walk test results, does not necessarily correlate with long-term prognosis.
While several studies focus on the clinical features of cGvHD that are associated with adverse prognosis [15, 16], few focus on the prognosis according to the response to treatment. Now we have novel effective treatments for steroid-refractory disease, which could be steroid-sparing and facilitate better clinical responses [17, 18]. Thus it is important to define the goals of therapy for cGvHD. In this single center study we did not evaluate the outcomes of certain treatment modalities for chronic GvHD but rather focused on IST discontinuation, complete response of cGvHD and survival. For this purpose we included only patients who have long-term follow up after onset of cGvHD. As the first line of therapy 62% of patients received prednisone 1 mg/kg daily in combination with calcineurin inhibitor (CNI), 22% received CNI as the monotherapy, 16% received monotherapy with a second line treatments.
Patients and methods
Table 1.

Patients and transplantation procedures
Two hundred and nine patients transplanted in 2006-2017 in Pavlov First Saint Petersburg State Medical University were included in the study. The inclusion criteria were moderate or severe disease according to National Institute of Health (NIH) 2015 criteria [19], administration of systemic treatment for cGvHD, transplantation from 9-10/10 HLA-matched related or unrelated donor. All patients signed informed consent for the use of their medical data in research purposes. Two thirds of patients had either acute myeloblastic leukemia or acute lymphoblastic leukemia, 49% had severe cGvHD, 51% – moderate. Median time from HSCT to cGvHD onset was 166 days. Twenty three percent received GvHD prophylaxis with post-ransplantation cyclophosphamide (PTCY) and the rest – conventional prophylaxis with calcineurin inhibitor and antimetabolite. Median follow up time after the onset of cGvHD was 52 months. More than 56% had three or more organ involvement (Table 1).
Clinical definitions
Time to disease relapse incidence (RI), complete response (CR), non-relapse mortality (NRM), overall survival (OS) and event-free survival (EFS), were defined as the time from cGvHD onset to the event. RI and NRM were considered a competing risk events. RI and CR were also considered competing risks. cGvHD severity was evaluated using NIH 2015 criteria [19], while response using 2006 NIH criteria [20]. Complete response was defined as absence of cGvHD clinical signs with IST discontinued. Partial response (PR) was defined as decrease in the total NIH score without increase in each individual organ score.
Statistical analysis
Non-parametric analysis included Chi-square test, Mann-Whitney test according to the type of data. The survival distributions for OS, EFS, were calculated using Kaplan-Meier methodology. The comparisons were made using the log-rank test. Cumulative incidence analysis with competing risks RI, NRM, CR was performed using Gray test. Relapse and NRM were accounted as competing risks as well as RI and CR. Fine and Grey regression was used for the multivariate analysis of cumulative incidences. Factors used for multivariate correction had at least p=0.10 significance in the univariate analysis.
Results
As the first line of therapy 62% of patients received prednisone 1 mg/kg daily in combination with calcineurin inhibitor (CNI), 22% received CNI as the monotherapy, 16% received monotherapy with a second line treatment (pharmacological or extracorporeal photopheresis without steroids. Beyond the first line 39.56% of patients required additional treatment. The most frequent options were ECP, IL-2, JAK inhibitors, BTK inhibitors, tyrosine-kinase inhibitors (TKI).
At five years, the cumulative incidence of CR was 16.9% (95% CI 10.5-24.7%). The proportion of patients with CR was 18.68%. However the cumulative incidence of IST discontinuation without GVHD flare was higher – 51.2% (95% CI 40.0-61.2%), and close to the proportion of patients with CR and mild chronic GvHD manifestations after treatment (44.5%). The competing risk of relapse was 25.4% (95% CI 18.6-32.8%) (Fig. 1).

Figure 1. (A) Initial severity of cGvHD before treatment and at last follow up. (B) Cumulative incidence of complete remission and immunosuppression (IST) discontinuation. Relapse was accounted as competing risk
In the multivariate analysis there was only one significant predictor of CR – severe form of chronic GvHD comparing to the moderate disease (HR 0.26, 95%CI 0.08-0.728, p=0.0194). The other factors significant in the univariate analysis like type of initial treatment (HR 0.84, 95%CI 0.44-1.46, p=0.5154), type of GvHD prophylaxis (HR 0.95, 95%CI 0.34-2.48, p=0.9122), previous acute GvHD grade 3-4 (HR 0.82, 95%CI 0.28-2.40, p=0.82) and number of organs involved (HR 0.77, 95%CI 0.52-1.08, p=0.1751) had no impact on CR cumulative incidence.
In the multivariate analysis of IST discontinuation, the statistical significance was observed for overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The other factors like type of the donor (HR 0.70, 95%CI 0.37-1.38, p=0.2909), previous severe acute GvHD (HR 0.96, 95%CI 0.49-1.82, p=0.9379), type of initial GvHD treatment (HR 0.93, 95%CI 0.63-1.33, p=0.7021), GI involvement (HR 0.76, 95%CI 0.53-1.04, p=0.1223), or lung involvement (HR 0.78, 95%CI 0.47-1.17, p=0.2288) were not statistically significant (Figure 2A). However it is worth mentioning that 55% of patients without GI cGvHD discontinued IST, while 28% achieved this goal with mild GI GvHD, 26% with moderate and 8% with severe. The same pattern was observed for lung GvHD: 47% discontinued systemic IST without lung involvement and 25% with mild bronchiolitis obliterans (BO), 29% with moderate and only 10% with severe. Absence of significance in the multivariate analysis may be partially explained by certain overlap of these variables with overall severity of cGvHD. Among patients with moderate disease 56% discontinued IST, but with severe disease – only 25%. At the end of the follow up patients with CR discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases.
The analysis of NRM demonstrated that the major factors with impact on 5-year NRM were severe form of cGvHD (32% vs 13%, p=0.0050), discontinuation of systemic IST (2% vs 42%, p<0.0001) and surprisingly steroid-free first-line therapy (8% vs 32%, p=0.0006). Although administration of second-line regimens were not statistically significant in this data set (NRM 20% vs 27%, p= 0.7092) (Fig. 3), it was forced in the subsequent multivariate analysis due to significant literature data on increased mortality in steroid- refractory GvHD.
In the multivariate analysis it was demonstrated that the initial severity of cGvHD did not influenced the NRM (HR 1.70, 95%CI 0.80-3.97, p=0.1959), while early discontinuation of IST (HR 0.03, 95%CI 0.01-0.15, p=0.0005), steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322) were protective against NRM (Fig. 2B). Since it was a non-randomized study patients with steroid-free starting therapy more often had moderate disease compared to patients in the steroids group (41% vs 66%, p= 0.0011). The same is true for additional cGvHD therapy: 54% received it in the severe group, while only 20% received it in the mode-rate cGvHD group.

Figure 2. (A) Multivariate analysis of predictors for successful IST discontinuation. (B) Multivariate analysis of predictors for non-relapse mortality
MUD=matched unrelated donor, MRD=matched related donor, GI=gastrointestinal. IST= immunosuppressive treatment. Factors with significance <0.1 in the univariate analysis were included.

Figure 3. Major predictors of non-relapse mortality
Discussion
This retrospective analysis of the large single center-cohort is not in line with several previous studies. The initial studies of cGvHD treatment identified prednisone as an optimal therapy among the existing at that time immunosuppressive agents [10, 11]. Many clinics even do not use CNIs in combination with steroids for the treatment, given the comparable response rate [14]. All the subsequent studies demonstrated that addition of thalidomide [13], or MMF [21], or ECP [22] in the first line of therapy did not improve response or survival. In our single-center study of patients with cGvHD many did not receive first line steroids. Partly, this was due to the single-agent PTCY prophylaxis protocol involving first line CsA for both acute and chronic GvHD, but also due national peculiarities of healthcare when a patient cannot easily travel to the transplant center and CNIs had to be introduced during distant consultations, while treatment with steroids were saved only for patients who could be admitted to the outpatient care. Secondly, there was an internal policy of faster steroid tapper after introduction of second line treatment than in the majority of centers [23]. Hence, if the patient did not show the signs of the flair he usually completely discontinued steroids within a month and continued only second line treatment, while the standard policy is to continue steroids until response. These differences in the internal policies led to several interesting discoveries.
First, patients initially treated without steroids had significantly reduced NRM. Although it is not well documented in the literature, but the majority of early deaths in chronic GvHD patients occur not due to cGvHD clinical manifestations, but due to recurrent infections [24]. Hence, the modality of immunosuppressive therapy should focus on minimal increase in the rate of infectious complications while providing at least minimal continuous GvHD improvement. This goes in line with recent single cell sequencing studies demonstrating that variation in cGvHD manifestation is due to the mixture of alloreactive graft-derived cells and de novo T-cells generated in thymus. Exhaustion of these clones is associated with cGvHD amelioration or resolution [25, 26]. Now there is not enough data to support that exhaustion and elimination of GvHD-related T-cells is a consequence of IST. This might be as well the result of restored process of negative T-cell cell selection in the thymus [27]. This study proposes the idea that minimally effective immunosuppression should be used.
At the time R. Storb et al. compared the efficacy of various IST with prednisone the choice of agents was limited to azathioprine, methotrexate and cyclophosphamide. Now we have several effective therapy options for cGVHD, including ECP [28], JAK inhibitors [18], BTK inhibitors [17]. All of them were used either as early therapeutic intervention in the first or second lines of therapy in this study in a small proportion of patients. None of these agents were previously randomized against steroids but rather randomized on top of steroids. Second line therapy with kinase inhibitors demonstrated excellent survival, so moving this agents in the first line might reduce infection-related NRM [17, 19, 29]. Despite this was not a randomized study and steroid-free first line therapy group had less patients with severe cGvHD, at least these results warrant randomized studies of novel therapies against steroids, but not with steroids.
Although it was demonstrated previously that patients with improvement in cGvHD manifestations have better survival compared to patients without improvement [30], this study demonstrated how long the IST should continue and when it should be stopped. The ideal situation is reaching CR or mild manifestations of cGvHD when systemic IST could be stopped and GvHD controlled by topical therapy. A quarter of patients with formal moderate disease can also stop systemic IST without a flare. Usually, these are lung GvHD patients who may never restore the lung capacity to normal, or patients with eye involvement in whom it will be controlled with topical therapy. Still there is a problem with patients who still have severe disease after several years of therapy. Despite they will have higher mortality than patients with GvHD resolution, in this study we demonstrated that they may benefit in terms of NRM from early intervention with second-line therapies or using them in the first line. Also prospective trials are required to confirm these observations. The long-term results of this approach is unknown, however we know that prolonged use of steroids is associated with dismal prognosis [6].
Financial Disclosure Statement
The authors have nothing to disclaim.
Acknowledgements
The authors declare no conflicts of interest.
References
- Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, Berenson R, Buckner CD, Clift R, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73(6):1729-1734. PMID: 2653461.
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, Oneto R, Bruno B, Barbanti M, Sacchi N, Van Lint MT, Bosi A. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98(10):2942-2947.
- Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D, Persiantseva M, Skvortsova Y, Laberko A, Muzalevskii Y, et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplant. 2016;51(5):668-674.
- Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, Wang SA, Konopleva M, Fernandez-Vina M, Montes N, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835-1844.
- Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, de Witte TM, Kröger N, Ruutu T. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28(11):2235-2240.
- Ruutu T, Nihtinen A, Niittyvuopio R, Juvonen E, Volin L. A randomized study of cyclosporine and methotrexate with or without methylprednisolone for the prevention of graft-versus-host disease: Improved long-term survival with triple prophylaxis. Cancer. 2018;124(4):727-733.
- Inamoto Y, Pidala J, Chai X, Kurland BF, Weisdorf D, Flowers ME, Palmer J, Arai S, Jacobsohn D, Cutler C, Jagasia M, Goldberg JD, Martin PJ, Pavletic SZ, Vogelsang GB, Lee SJ, Carpenter PA; Chronic GVHD Consortium. Assessment of joint and fascia manifestations in chronic graft-versus-host disease. Arthritis Rheumatol. 2014;66(4):1044-1052.
- Glezerman IG, Jhaveri KD, Watson TH, Edwards AM, Papadopoulos EB, Young JW, Flombaum CD, Jakubowski AA. Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(7):976-984.
- Worel N, Biener D, Kalhs P, Mitterbauer M, Keil F, Schulenburg A, Höcker P, Dieckmann K, Fischer G, Rosenmayr A, et al. Long-term outcome and quality of life of patients who are alive and in complete remission more than two years after allogeneic and syngeneic stem cell transplantation. Bone Marrow Transplant. 2002;30(9):619-626.
- Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, Schubert MM, Atkinson K, Thomas ED. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267-276. PMID: 7004534.
- Sullivan KM, Witherspoon RP, Storb R, Deeg HJ, Dahlberg S, Sanders JE, Appelbaum FR, Doney KC, Weiden P, Anasetti C, et al. Alternating-day cyclosporine and prednisone for treatment of high-risk chronic graft-v-host disease. Blood. 1988;72(2):555-561.
- Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, Wingard JR, Shaughnessy PJ, DeVetten MP, Jagasia M, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113(21):5074-5082.
- Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, Sanders JE, Witherspoon RP, Appelbaum FR, Storb R, Martin PJ. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96(12):3995-3996.
- Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, Sanders JE, Witherspoon RP, Storb R, Appelbaum FR, Martin PJ. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48-51.
- Ayuk F, Veit R, Zabelina T, Bussmann L, Christopeit M, Alchalby H, Wolschke C, Lellek H, Bacher U, Zander AR, Kröger N. Prognostic factors for survival of patients with newly diagnosed chronic GvHD according to NIH criteria. Ann Hematol. 2015;94(10):1727-1732.
- Pavletic SZ, Smith LM, Bishop MR, Lynch JC, Tarantolo SR, Vose JM, Bierman PJ, Hadi A, Armitage JO, Kessinger A. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78(4):265-274.
- Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, Flowers ME, Logan AC, Nakamura R, Blazar BR, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243-2250. doi: 10.1182/blood-2017-07-793786.
- Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, Ditschkowski M, Ecsedi M, Sockel K, Ayuk F, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062-2068.
- Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21(3):389-401.e1.
- Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006; 12: 252-266.
- Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, Wingard JR, Shaughnessy PJ, DeVetten MP, Jagasia M, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009; 113(21):5074-5082.
- Jagasia M, Scheid C, Socié G, Ayuk FA, Tischer J, Donato ML, Bátai Á, Chen H, Chen SC, Chin T, et al. Randomized controlled study of ECP with methoxsalen as first-line treatment of patients with moderate to severe cGVHD. Blood Adv. 2019;3(14):2218-2229.
- Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133(11):1191-1200.
- Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, Sorror ML, Horowitz MM, Bolwell B, Rizzo JD, Socié G. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230-2239.
- Balakrishnan A, Gloude N, Sasik R, Ball ED, Morris GP. Proinflammatory dual receptor T cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23(11):1852-1860.
- Kosugi-Kanaya M, Ueha S, Abe J, et al. Long-lasting graft-derived donor T cells contribute to the pathogenesis of chronic graft-versus-host disease in mice. Front Immunol. 2017;8:1842. doi:10.3389/fimmu.2017.01842.
- Klein L, Robey EA, Hsieh CS. Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat Rev Immunol. 2019;19(1):7-18.
- Greinix HT, Worel N, Just U, Knobler R. Extracorporeal photopheresis in acute and chronic graft-versus-host disease. Transfus Apher Sci. 2014;50(3):349-357.
- Escamilla Gómez V, García-Gutiérrez V, López Corral L, García Cadenas I, Pérez Martínez A, Márquez Malaver FJ, Caballero-Velázquez T, González Sierra PA, Viguria Alegría MC, Parra Salinas IM, et al.; Grupo Español de Trasplante Hematopoyético (GETH). Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transplant. 2020;55(3):641-648.
- Murata M, Nakasone H, Kanda J, Nakane T, Furukawa T, Fukuda T, Mori T, Taniguchi S, Eto T, Ohashi K, et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2013;19(8):1183-1189.
" ["~DETAIL_TEXT"]=> string(25361) "
Introduction
Chronic graft-versus-host disease (cGvHD) is a complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT), which is associated both with long-term mortality and significant disability in long-term survivors. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and several other risk factors [1-4]. Although cGvHD is associated with reduced risk of relapse and improved survival in the majority of malignant diseases [5], the persistence of clinical signs is associated with long-term mortality due to cardiovascular disease, infections and secondary malignancies [6]. Also cGvHD is the major cause of decline in the quality of life (QoL), social and professional disability. Gastrointestinal, joint and kidney problems are the main drivers of QoL decline [7-9].
The early studies of cGvHD treatment demonstrated a superiority of steroids over other agents in the treatment of cGvHD in terms of survival [10, 11]. However all subsequent attempts to improve response rate with augmented immunosuppression were not successful. Addition of thalidomide and mycofenolate mofetil resulted in higher frequency of adverse events and infection-related mortality [12, 13]. The only combination with some benefit in terms of steroid sparing was the combination of steroids and cyclosporine A (CsA), which demonstrated comparable response rate and duration of immunosuppression, however the cumulative dose of steroids was less in the combination arm, which resulted in the reduced frequency of femur aseptic necrosis [14]. The failure of these clinical trials to demonstrate improved response rate lead to the shift in the concept of cGvHD treatment. Currenly it is considered that immunosuppressive therapy (IST) does not induce tolerance, but rather alleviates target organ damage before the tolerance between donor and recipient cells occur. This understanding creates a dissonance between endpoints from the clinical studies and the real clinical practice where the formal response criteria, like decrease in the severity score or improved 2-minute walk test results, does not necessarily correlate with long-term prognosis.
While several studies focus on the clinical features of cGvHD that are associated with adverse prognosis [15, 16], few focus on the prognosis according to the response to treatment. Now we have novel effective treatments for steroid-refractory disease, which could be steroid-sparing and facilitate better clinical responses [17, 18]. Thus it is important to define the goals of therapy for cGvHD. In this single center study we did not evaluate the outcomes of certain treatment modalities for chronic GvHD but rather focused on IST discontinuation, complete response of cGvHD and survival. For this purpose we included only patients who have long-term follow up after onset of cGvHD. As the first line of therapy 62% of patients received prednisone 1 mg/kg daily in combination with calcineurin inhibitor (CNI), 22% received CNI as the monotherapy, 16% received monotherapy with a second line treatments.
Patients and methods
Table 1.

Patients and transplantation procedures
Two hundred and nine patients transplanted in 2006-2017 in Pavlov First Saint Petersburg State Medical University were included in the study. The inclusion criteria were moderate or severe disease according to National Institute of Health (NIH) 2015 criteria [19], administration of systemic treatment for cGvHD, transplantation from 9-10/10 HLA-matched related or unrelated donor. All patients signed informed consent for the use of their medical data in research purposes. Two thirds of patients had either acute myeloblastic leukemia or acute lymphoblastic leukemia, 49% had severe cGvHD, 51% – moderate. Median time from HSCT to cGvHD onset was 166 days. Twenty three percent received GvHD prophylaxis with post-ransplantation cyclophosphamide (PTCY) and the rest – conventional prophylaxis with calcineurin inhibitor and antimetabolite. Median follow up time after the onset of cGvHD was 52 months. More than 56% had three or more organ involvement (Table 1).
Clinical definitions
Time to disease relapse incidence (RI), complete response (CR), non-relapse mortality (NRM), overall survival (OS) and event-free survival (EFS), were defined as the time from cGvHD onset to the event. RI and NRM were considered a competing risk events. RI and CR were also considered competing risks. cGvHD severity was evaluated using NIH 2015 criteria [19], while response using 2006 NIH criteria [20]. Complete response was defined as absence of cGvHD clinical signs with IST discontinued. Partial response (PR) was defined as decrease in the total NIH score without increase in each individual organ score.
Statistical analysis
Non-parametric analysis included Chi-square test, Mann-Whitney test according to the type of data. The survival distributions for OS, EFS, were calculated using Kaplan-Meier methodology. The comparisons were made using the log-rank test. Cumulative incidence analysis with competing risks RI, NRM, CR was performed using Gray test. Relapse and NRM were accounted as competing risks as well as RI and CR. Fine and Grey regression was used for the multivariate analysis of cumulative incidences. Factors used for multivariate correction had at least p=0.10 significance in the univariate analysis.
Results
As the first line of therapy 62% of patients received prednisone 1 mg/kg daily in combination with calcineurin inhibitor (CNI), 22% received CNI as the monotherapy, 16% received monotherapy with a second line treatment (pharmacological or extracorporeal photopheresis without steroids. Beyond the first line 39.56% of patients required additional treatment. The most frequent options were ECP, IL-2, JAK inhibitors, BTK inhibitors, tyrosine-kinase inhibitors (TKI).
At five years, the cumulative incidence of CR was 16.9% (95% CI 10.5-24.7%). The proportion of patients with CR was 18.68%. However the cumulative incidence of IST discontinuation without GVHD flare was higher – 51.2% (95% CI 40.0-61.2%), and close to the proportion of patients with CR and mild chronic GvHD manifestations after treatment (44.5%). The competing risk of relapse was 25.4% (95% CI 18.6-32.8%) (Fig. 1).

Figure 1. (A) Initial severity of cGvHD before treatment and at last follow up. (B) Cumulative incidence of complete remission and immunosuppression (IST) discontinuation. Relapse was accounted as competing risk
In the multivariate analysis there was only one significant predictor of CR – severe form of chronic GvHD comparing to the moderate disease (HR 0.26, 95%CI 0.08-0.728, p=0.0194). The other factors significant in the univariate analysis like type of initial treatment (HR 0.84, 95%CI 0.44-1.46, p=0.5154), type of GvHD prophylaxis (HR 0.95, 95%CI 0.34-2.48, p=0.9122), previous acute GvHD grade 3-4 (HR 0.82, 95%CI 0.28-2.40, p=0.82) and number of organs involved (HR 0.77, 95%CI 0.52-1.08, p=0.1751) had no impact on CR cumulative incidence.
In the multivariate analysis of IST discontinuation, the statistical significance was observed for overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The other factors like type of the donor (HR 0.70, 95%CI 0.37-1.38, p=0.2909), previous severe acute GvHD (HR 0.96, 95%CI 0.49-1.82, p=0.9379), type of initial GvHD treatment (HR 0.93, 95%CI 0.63-1.33, p=0.7021), GI involvement (HR 0.76, 95%CI 0.53-1.04, p=0.1223), or lung involvement (HR 0.78, 95%CI 0.47-1.17, p=0.2288) were not statistically significant (Figure 2A). However it is worth mentioning that 55% of patients without GI cGvHD discontinued IST, while 28% achieved this goal with mild GI GvHD, 26% with moderate and 8% with severe. The same pattern was observed for lung GvHD: 47% discontinued systemic IST without lung involvement and 25% with mild bronchiolitis obliterans (BO), 29% with moderate and only 10% with severe. Absence of significance in the multivariate analysis may be partially explained by certain overlap of these variables with overall severity of cGvHD. Among patients with moderate disease 56% discontinued IST, but with severe disease – only 25%. At the end of the follow up patients with CR discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases.
The analysis of NRM demonstrated that the major factors with impact on 5-year NRM were severe form of cGvHD (32% vs 13%, p=0.0050), discontinuation of systemic IST (2% vs 42%, p<0.0001) and surprisingly steroid-free first-line therapy (8% vs 32%, p=0.0006). Although administration of second-line regimens were not statistically significant in this data set (NRM 20% vs 27%, p= 0.7092) (Fig. 3), it was forced in the subsequent multivariate analysis due to significant literature data on increased mortality in steroid- refractory GvHD.
In the multivariate analysis it was demonstrated that the initial severity of cGvHD did not influenced the NRM (HR 1.70, 95%CI 0.80-3.97, p=0.1959), while early discontinuation of IST (HR 0.03, 95%CI 0.01-0.15, p=0.0005), steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322) were protective against NRM (Fig. 2B). Since it was a non-randomized study patients with steroid-free starting therapy more often had moderate disease compared to patients in the steroids group (41% vs 66%, p= 0.0011). The same is true for additional cGvHD therapy: 54% received it in the severe group, while only 20% received it in the mode-rate cGvHD group.

Figure 2. (A) Multivariate analysis of predictors for successful IST discontinuation. (B) Multivariate analysis of predictors for non-relapse mortality
MUD=matched unrelated donor, MRD=matched related donor, GI=gastrointestinal. IST= immunosuppressive treatment. Factors with significance <0.1 in the univariate analysis were included.

Figure 3. Major predictors of non-relapse mortality
Discussion
This retrospective analysis of the large single center-cohort is not in line with several previous studies. The initial studies of cGvHD treatment identified prednisone as an optimal therapy among the existing at that time immunosuppressive agents [10, 11]. Many clinics even do not use CNIs in combination with steroids for the treatment, given the comparable response rate [14]. All the subsequent studies demonstrated that addition of thalidomide [13], or MMF [21], or ECP [22] in the first line of therapy did not improve response or survival. In our single-center study of patients with cGvHD many did not receive first line steroids. Partly, this was due to the single-agent PTCY prophylaxis protocol involving first line CsA for both acute and chronic GvHD, but also due national peculiarities of healthcare when a patient cannot easily travel to the transplant center and CNIs had to be introduced during distant consultations, while treatment with steroids were saved only for patients who could be admitted to the outpatient care. Secondly, there was an internal policy of faster steroid tapper after introduction of second line treatment than in the majority of centers [23]. Hence, if the patient did not show the signs of the flair he usually completely discontinued steroids within a month and continued only second line treatment, while the standard policy is to continue steroids until response. These differences in the internal policies led to several interesting discoveries.
First, patients initially treated without steroids had significantly reduced NRM. Although it is not well documented in the literature, but the majority of early deaths in chronic GvHD patients occur not due to cGvHD clinical manifestations, but due to recurrent infections [24]. Hence, the modality of immunosuppressive therapy should focus on minimal increase in the rate of infectious complications while providing at least minimal continuous GvHD improvement. This goes in line with recent single cell sequencing studies demonstrating that variation in cGvHD manifestation is due to the mixture of alloreactive graft-derived cells and de novo T-cells generated in thymus. Exhaustion of these clones is associated with cGvHD amelioration or resolution [25, 26]. Now there is not enough data to support that exhaustion and elimination of GvHD-related T-cells is a consequence of IST. This might be as well the result of restored process of negative T-cell cell selection in the thymus [27]. This study proposes the idea that minimally effective immunosuppression should be used.
At the time R. Storb et al. compared the efficacy of various IST with prednisone the choice of agents was limited to azathioprine, methotrexate and cyclophosphamide. Now we have several effective therapy options for cGVHD, including ECP [28], JAK inhibitors [18], BTK inhibitors [17]. All of them were used either as early therapeutic intervention in the first or second lines of therapy in this study in a small proportion of patients. None of these agents were previously randomized against steroids but rather randomized on top of steroids. Second line therapy with kinase inhibitors demonstrated excellent survival, so moving this agents in the first line might reduce infection-related NRM [17, 19, 29]. Despite this was not a randomized study and steroid-free first line therapy group had less patients with severe cGvHD, at least these results warrant randomized studies of novel therapies against steroids, but not with steroids.
Although it was demonstrated previously that patients with improvement in cGvHD manifestations have better survival compared to patients without improvement [30], this study demonstrated how long the IST should continue and when it should be stopped. The ideal situation is reaching CR or mild manifestations of cGvHD when systemic IST could be stopped and GvHD controlled by topical therapy. A quarter of patients with formal moderate disease can also stop systemic IST without a flare. Usually, these are lung GvHD patients who may never restore the lung capacity to normal, or patients with eye involvement in whom it will be controlled with topical therapy. Still there is a problem with patients who still have severe disease after several years of therapy. Despite they will have higher mortality than patients with GvHD resolution, in this study we demonstrated that they may benefit in terms of NRM from early intervention with second-line therapies or using them in the first line. Also prospective trials are required to confirm these observations. The long-term results of this approach is unknown, however we know that prolonged use of steroids is associated with dismal prognosis [6].
Financial Disclosure Statement
The authors have nothing to disclaim.
Acknowledgements
The authors declare no conflicts of interest.
References
- Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, Berenson R, Buckner CD, Clift R, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73(6):1729-1734. PMID: 2653461.
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, Oneto R, Bruno B, Barbanti M, Sacchi N, Van Lint MT, Bosi A. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98(10):2942-2947.
- Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D, Persiantseva M, Skvortsova Y, Laberko A, Muzalevskii Y, et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplant. 2016;51(5):668-674.
- Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, Wang SA, Konopleva M, Fernandez-Vina M, Montes N, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835-1844.
- Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, de Witte TM, Kröger N, Ruutu T. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28(11):2235-2240.
- Ruutu T, Nihtinen A, Niittyvuopio R, Juvonen E, Volin L. A randomized study of cyclosporine and methotrexate with or without methylprednisolone for the prevention of graft-versus-host disease: Improved long-term survival with triple prophylaxis. Cancer. 2018;124(4):727-733.
- Inamoto Y, Pidala J, Chai X, Kurland BF, Weisdorf D, Flowers ME, Palmer J, Arai S, Jacobsohn D, Cutler C, Jagasia M, Goldberg JD, Martin PJ, Pavletic SZ, Vogelsang GB, Lee SJ, Carpenter PA; Chronic GVHD Consortium. Assessment of joint and fascia manifestations in chronic graft-versus-host disease. Arthritis Rheumatol. 2014;66(4):1044-1052.
- Glezerman IG, Jhaveri KD, Watson TH, Edwards AM, Papadopoulos EB, Young JW, Flombaum CD, Jakubowski AA. Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(7):976-984.
- Worel N, Biener D, Kalhs P, Mitterbauer M, Keil F, Schulenburg A, Höcker P, Dieckmann K, Fischer G, Rosenmayr A, et al. Long-term outcome and quality of life of patients who are alive and in complete remission more than two years after allogeneic and syngeneic stem cell transplantation. Bone Marrow Transplant. 2002;30(9):619-626.
- Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, Schubert MM, Atkinson K, Thomas ED. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267-276. PMID: 7004534.
- Sullivan KM, Witherspoon RP, Storb R, Deeg HJ, Dahlberg S, Sanders JE, Appelbaum FR, Doney KC, Weiden P, Anasetti C, et al. Alternating-day cyclosporine and prednisone for treatment of high-risk chronic graft-v-host disease. Blood. 1988;72(2):555-561.
- Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, Wingard JR, Shaughnessy PJ, DeVetten MP, Jagasia M, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113(21):5074-5082.
- Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, Sanders JE, Witherspoon RP, Appelbaum FR, Storb R, Martin PJ. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96(12):3995-3996.
- Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, Sanders JE, Witherspoon RP, Storb R, Appelbaum FR, Martin PJ. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48-51.
- Ayuk F, Veit R, Zabelina T, Bussmann L, Christopeit M, Alchalby H, Wolschke C, Lellek H, Bacher U, Zander AR, Kröger N. Prognostic factors for survival of patients with newly diagnosed chronic GvHD according to NIH criteria. Ann Hematol. 2015;94(10):1727-1732.
- Pavletic SZ, Smith LM, Bishop MR, Lynch JC, Tarantolo SR, Vose JM, Bierman PJ, Hadi A, Armitage JO, Kessinger A. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78(4):265-274.
- Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, Flowers ME, Logan AC, Nakamura R, Blazar BR, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243-2250. doi: 10.1182/blood-2017-07-793786.
- Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, Ditschkowski M, Ecsedi M, Sockel K, Ayuk F, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062-2068.
- Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21(3):389-401.e1.
- Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006; 12: 252-266.
- Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, Wingard JR, Shaughnessy PJ, DeVetten MP, Jagasia M, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009; 113(21):5074-5082.
- Jagasia M, Scheid C, Socié G, Ayuk FA, Tischer J, Donato ML, Bátai Á, Chen H, Chen SC, Chin T, et al. Randomized controlled study of ECP with methoxsalen as first-line treatment of patients with moderate to severe cGVHD. Blood Adv. 2019;3(14):2218-2229.
- Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133(11):1191-1200.
- Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, Sorror ML, Horowitz MM, Bolwell B, Rizzo JD, Socié G. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230-2239.
- Balakrishnan A, Gloude N, Sasik R, Ball ED, Morris GP. Proinflammatory dual receptor T cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23(11):1852-1860.
- Kosugi-Kanaya M, Ueha S, Abe J, et al. Long-lasting graft-derived donor T cells contribute to the pathogenesis of chronic graft-versus-host disease in mice. Front Immunol. 2017;8:1842. doi:10.3389/fimmu.2017.01842.
- Klein L, Robey EA, Hsieh CS. Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat Rev Immunol. 2019;19(1):7-18.
- Greinix HT, Worel N, Just U, Knobler R. Extracorporeal photopheresis in acute and chronic graft-versus-host disease. Transfus Apher Sci. 2014;50(3):349-357.
- Escamilla Gómez V, García-Gutiérrez V, López Corral L, García Cadenas I, Pérez Martínez A, Márquez Malaver FJ, Caballero-Velázquez T, González Sierra PA, Viguria Alegría MC, Parra Salinas IM, et al.; Grupo Español de Trasplante Hematopoyético (GETH). Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transplant. 2020;55(3):641-648.
- Murata M, Nakasone H, Kanda J, Nakane T, Furukawa T, Fukuda T, Mori T, Taniguchi S, Eto T, Ohashi K, et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2013;19(8):1183-1189.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(100) "dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so" ["~CODE"]=> string(100) "dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so" ["EXTERNAL_ID"]=> string(4) "1939" ["~EXTERNAL_ID"]=> string(4) "1939" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(424) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клетокLong-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(5075) "<p style="text-align: justify;">Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.</p> <h3>Результаты</h3> <p style="text-align: justify;">Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).</p> <h3>Выводы</h3> <p style="text-align: justify;">Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_META_TITLE"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_META_KEYWORDS"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_META_DESCRIPTION"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_ALT"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_TITLE"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(290) "Долгосрочные цели терапии хронической реакции «трансплантат против хозяина» после аллогенной совместимой трансплантации гемопоэтических стволовых клеток" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "dolgosrochnye-tseli-terapii-khronicheskoy-reaktsii-transplantat-protiv-khozyaina-posle-allogennoy-so" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "170" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27333" ["VALUE"]=> string(22) "10/22/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/22/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27334" ["VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27339" ["VALUE"]=> array(2) { ["TEXT"]=> string(339) "<p>Иван С. Моисеев, Анна А. Доценко, Анна Г. Смирнова, Юлия Ю. Власова, Елена В. Морозова, Сергей Н. Бондаренко, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(305) "
Иван С. Моисеев, Анна А. Доценко, Анна Г. Смирнова, Юлия Ю. Власова, Елена В. Морозова, Сергей Н. Бондаренко, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27340" ["VALUE"]=> array(2) { ["TEXT"]=> string(368) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(356) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27341" ["VALUE"]=> array(2) { ["TEXT"]=> string(5075) "<p style="text-align: justify;">Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.</p> <h3>Результаты</h3> <p style="text-align: justify;">Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).</p> <h3>Выводы</h3> <p style="text-align: justify;">Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4917) "Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.
Пациенты и методы
В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.
Результаты
Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).
Выводы
Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.
Ключевые слова
Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27335" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-29-36" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-29-36" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27343" ["VALUE"]=> array(2) { ["TEXT"]=> string(247) "<p>Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(213) "Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27344" ["VALUE"]=> array(2) { ["TEXT"]=> string(527) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7(812) 338 6259, +7 (921) 796 1951<br> Fax: +7(812) 338 6263<br> E-mail: moisiv@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(461) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(812) 338 6259, +7 (921) 796 1951
Fax: +7(812) 338 6263
E-mail: moisiv@mail.ru
Chronic graft-versus-host disease (cGvHD) is a common complication of allogeneic hematopoietic stem cell transplantation. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and other risk factors. Although cGvHD is associated with reduced risk of relapse, the persistence of clinical signs is associated with long-term mortality, morbidity and disability. Despite there are clear endpoints for the clinical trials of novel agents, the choices in clinical practice should involve long-term goals like in all autoimmune disease. So far, there is no consensus on these goals. Analyzing the results of cGvHD therapy in the large single-center cohort of patients we tried to focus on predictors of long-term prognosis and their association with therapy.
Patients and methods
The study included 182 patients with moderate and severe cGvHD. The majority of patients were allografted for malignant diseases and 49% had severe cGvHD, 51% – moderate disease. Median follow up time was 52 months. Beyond the first line 39.56% of patients required additional treatment.
Results
At five years the cumulative incidence of complete responses was 16.9% (95% CI 10.5-24.7%) and immunosuppressive therapy (IST) discontinuation without GvHD flare was 51.2% (95% CI 40.0-61.2%). The major predictors of IST discontinuation were overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The analysis of non-relapse mortality (NRM) demonstrated that discontinuation of IST was the major predictor (2% vs 42%, HR 0.03, 95%CI 0.01-0.15, p=0.0005). At the end of the follow up patients with complete response discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases. The other significant factors for NRM were steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and early use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322). In conclusion, the study demonstrated that discontinuation of systemic IST therapy without the flare of cGvHD should be the goal of therapy. Also the study creates a rationale for randomized studies of novel second-line options not with but against steroids in the first line.
Keywords
Chronic graft-versus-host disease, therapy, long-term outcomes, goals of therapy.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27336" ["VALUE"]=> string(134) "Long-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(134) "Long-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27342" ["VALUE"]=> string(4) "2320" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2320" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27346" ["VALUE"]=> string(4) "2321" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2321" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27343" ["VALUE"]=> array(2) { ["TEXT"]=> string(247) "<p>Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(213) "Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(213) "Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, Boris V. Afanasyev
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27345" ["VALUE"]=> array(2) { ["TEXT"]=> string(2710) "<p style="text-align: justify;">Chronic graft-versus-host disease (cGvHD) is a common complication of allogeneic hematopoietic stem cell transplantation. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and other risk factors. Although cGvHD is associated with reduced risk of relapse, the persistence of clinical signs is associated with long-term mortality, morbidity and disability. Despite there are clear endpoints for the clinical trials of novel agents, the choices in clinical practice should involve long-term goals like in all autoimmune disease. So far, there is no consensus on these goals. Analyzing the results of cGvHD therapy in the large single-center cohort of patients we tried to focus on predictors of long-term prognosis and their association with therapy.</p> <h3>Patients and methods</h3> <p style="text-align: justify;">The study included 182 patients with moderate and severe cGvHD. The majority of patients were allografted for malignant diseases and 49% had severe cGvHD, 51% – moderate disease. Median follow up time was 52 months. Beyond the first line 39.56% of patients required additional treatment.</p> <h3>Results</h3> <p style="text-align: justify;">At five years the cumulative incidence of complete responses was 16.9% (95% CI 10.5-24.7%) and immunosuppressive therapy (IST) discontinuation without GvHD flare was 51.2% (95% CI 40.0-61.2%). The major predictors of IST discontinuation were overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The analysis of non-relapse mortality (NRM) demonstrated that discontinuation of IST was the major predictor (2% <i>vs</i> 42%, HR 0.03, 95%CI 0.01-0.15, p=0.0005). At the end of the follow up patients with complete response discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases. The other significant factors for NRM were steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and early use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322). In conclusion, the study demonstrated that discontinuation of systemic IST therapy without the flare of cGvHD should be the goal of therapy. Also the study creates a rationale for randomized studies of novel second-line options not with but against steroids in the first line. </p> <h2>Keywords</h2> <p style="text-align: justify;">Chronic graft-versus-host disease, therapy, long-term outcomes, goals of therapy.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2574) "Chronic graft-versus-host disease (cGvHD) is a common complication of allogeneic hematopoietic stem cell transplantation. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and other risk factors. Although cGvHD is associated with reduced risk of relapse, the persistence of clinical signs is associated with long-term mortality, morbidity and disability. Despite there are clear endpoints for the clinical trials of novel agents, the choices in clinical practice should involve long-term goals like in all autoimmune disease. So far, there is no consensus on these goals. Analyzing the results of cGvHD therapy in the large single-center cohort of patients we tried to focus on predictors of long-term prognosis and their association with therapy.
Patients and methods
The study included 182 patients with moderate and severe cGvHD. The majority of patients were allografted for malignant diseases and 49% had severe cGvHD, 51% – moderate disease. Median follow up time was 52 months. Beyond the first line 39.56% of patients required additional treatment.
Results
At five years the cumulative incidence of complete responses was 16.9% (95% CI 10.5-24.7%) and immunosuppressive therapy (IST) discontinuation without GvHD flare was 51.2% (95% CI 40.0-61.2%). The major predictors of IST discontinuation were overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The analysis of non-relapse mortality (NRM) demonstrated that discontinuation of IST was the major predictor (2% vs 42%, HR 0.03, 95%CI 0.01-0.15, p=0.0005). At the end of the follow up patients with complete response discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases. The other significant factors for NRM were steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and early use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322). In conclusion, the study demonstrated that discontinuation of systemic IST therapy without the flare of cGvHD should be the goal of therapy. Also the study creates a rationale for randomized studies of novel second-line options not with but against steroids in the first line.
Keywords
Chronic graft-versus-host disease, therapy, long-term outcomes, goals of therapy.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2574) "Chronic graft-versus-host disease (cGvHD) is a common complication of allogeneic hematopoietic stem cell transplantation. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and other risk factors. Although cGvHD is associated with reduced risk of relapse, the persistence of clinical signs is associated with long-term mortality, morbidity and disability. Despite there are clear endpoints for the clinical trials of novel agents, the choices in clinical practice should involve long-term goals like in all autoimmune disease. So far, there is no consensus on these goals. Analyzing the results of cGvHD therapy in the large single-center cohort of patients we tried to focus on predictors of long-term prognosis and their association with therapy.
Patients and methods
The study included 182 patients with moderate and severe cGvHD. The majority of patients were allografted for malignant diseases and 49% had severe cGvHD, 51% – moderate disease. Median follow up time was 52 months. Beyond the first line 39.56% of patients required additional treatment.
Results
At five years the cumulative incidence of complete responses was 16.9% (95% CI 10.5-24.7%) and immunosuppressive therapy (IST) discontinuation without GvHD flare was 51.2% (95% CI 40.0-61.2%). The major predictors of IST discontinuation were overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The analysis of non-relapse mortality (NRM) demonstrated that discontinuation of IST was the major predictor (2% vs 42%, HR 0.03, 95%CI 0.01-0.15, p=0.0005). At the end of the follow up patients with complete response discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases. The other significant factors for NRM were steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and early use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322). In conclusion, the study demonstrated that discontinuation of systemic IST therapy without the flare of cGvHD should be the goal of therapy. Also the study creates a rationale for randomized studies of novel second-line options not with but against steroids in the first line.
Keywords
Chronic graft-versus-host disease, therapy, long-term outcomes, goals of therapy.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27335" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-29-36" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-29-36" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-29-36" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27336" ["VALUE"]=> string(134) "Long-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(134) "Long-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(134) "Long-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27344" ["VALUE"]=> array(2) { ["TEXT"]=> string(527) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7(812) 338 6259, +7 (921) 796 1951<br> Fax: +7(812) 338 6263<br> E-mail: moisiv@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(461) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(812) 338 6259, +7 (921) 796 1951
Fax: +7(812) 338 6263
E-mail: moisiv@mail.ru
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(812) 338 6259, +7 (921) 796 1951
Fax: +7(812) 338 6263
E-mail: moisiv@mail.ru
Иван С. Моисеев, Анна А. Доценко, Анна Г. Смирнова, Юлия Ю. Власова, Елена В. Морозова, Сергей Н. Бондаренко, Борис В. Афанасьев
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(305) "Иван С. Моисеев, Анна А. Доценко, Анна Г. Смирнова, Юлия Ю. Власова, Елена В. Морозова, Сергей Н. Бондаренко, Борис В. Афанасьев
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27333" ["VALUE"]=> string(22) "10/22/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/22/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "10/22/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27334" ["VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/13/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27341" ["VALUE"]=> array(2) { ["TEXT"]=> string(5075) "<p style="text-align: justify;">Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.</p> <h3>Результаты</h3> <p style="text-align: justify;">Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).</p> <h3>Выводы</h3> <p style="text-align: justify;">Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4917) "Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.
Пациенты и методы
В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.
Результаты
Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).
Выводы
Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.
Ключевые слова
Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4917) "Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.
Пациенты и методы
В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.
Результаты
Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).
Выводы
Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.
Ключевые слова
Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27340" ["VALUE"]=> array(2) { ["TEXT"]=> string(368) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(356) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(356) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [3]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "170" ["~IBLOCK_SECTION_ID"]=> string(3) "170" ["ID"]=> string(4) "1941" ["~ID"]=> string(4) "1941" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["~NAME"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "02/05/2021 02:52:04 pm" ["~TIMESTAMP_X"]=> string(22) "02/05/2021 02:52:04 pm" ["DETAIL_PAGE_URL"]=> string(138) "/en/archive/-9-4/klinicheskie-raboty/anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli/" ["~DETAIL_PAGE_URL"]=> string(138) "/en/archive/-9-4/klinicheskie-raboty/anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(13933) "Introduction
Fanconi anemia (FA) is a rare inherited condition characterized by heterogeneity of clinical signs and underling genetic mutations, which significantly increase the risk of bone marrow failure or malignancy (leukemia, solid tumors) development. Fanconi anemia is characterized by the presence of congenital anomalies occurring in approximately 75-80% of affected individuals, which include one or more of the following features: small stature, abnormal skin pigmentation, upper or lower limb skeletal malformation, microcephaly, visual and urogenital anomalies [1, 2]. The cumulative risk of developing bone marrow failure is 50%. Progressive bone marrow failure typically occurs in the first decade of life often starting with thrombocytopenia or leukopenia. Leukemia and solid tumors may occur as early as in 16 years. The diagnosis of FA is based on increased chromosome fragility detection in a test with or without an agent for DNA cross-link sensitivity testing, diepoxybutane (DEB) or mitomycin C (MMC). If hematopoietic sell still possess an intact DNA repair mechanism due to mosaicism these tests may be negative in blood cells cultures. Although these situations are rare, they may complicate diagnosis and warrant for test performed in non-hematopoietic cells culture, usually fibroblasts [3]. The final diagnosis nowadays is often confirmed by detection of a mutation in one of Fanconi complex genes [4].
Gene mutations in Fanconi anemia
• Bialellic pathogenic variant of one of 19 genes causing autosomal recessive form of FA.
• Heterozygous pathogenic variant in gene RAD51 (de novo; FANCO) causing autosomal dominant form of FA.
• Hemizygous pathogenic variant in gene FANCB causing X-linked form of FA.
There is a distinct flowchart for stepwise post-diagnostic monitoring and decision making in therapy of the Fanconi anemia patients, as described, e.g., by Dufour [5].
FA epidemiology in Czech Republic
The Czech population in 2020 is 10.7 millions (11th among EU countries) including 2.0 million children of 0 to 17.99 years with a birth rate of 115,000 per year. A total of 35 probands with Fanconi anemia were born in 1985-2019 in the Czech Republic with diagnosis confirmed in 1986-2020, it correlates with probable incidence of ca.1 patient/year. Diagnosis of FA was confirmed at the median age of 6 (0-24.3) years, 34/35 of patients were children at the moment of diagnosis. In all cases the diagnostic procedure included spontaneous and induced chromosomal breakage evaluation, then the diagnosis was prospectively or retrospectively confirmed by specific mutation detection in 33/35 patients.
The initial symptoms registered in FA patients included congenital effects in 5, thrombocytopenia in 9, bicytopenia or pancytopenia in 13 cases, accordingly. Also, 8 patients had characteristic family history (BMF, malignancies).
The main physical abnormalities and laboratory signs incidence in our FA group (n=35) at the time of diagnoses are summarized in Table 1. Table 2 and Fig.1 contain the list and relative frequency of mutations revealed in available patients (33 of 35 cases).

Bone marrow failure (BMF) and malignancies, outcome
During the follow-up 17/31 (55%) children developed BMF at the median age of 8 (4.0-17.1) years. Five patients (3 boys and 2 girls) had prior history of anabolics treatment with some effect in two girls [6]. Among these, 15 patients consequently underwent allogeneic HSCT (2004-2020) at the median age of 9.3 (4.6-24.3) years.
Also, 7/35 (20%) patients developed the following malignancies during follow-up with a median age of 9.8 (1.0 - 32.3) at cancer diagnosis:
- Spinocellular carcinoma of GI (32 years); the patient died due to cancer progression,
- Gastrointestinal adenocarcinoma (at 15 years), later a squamous cell carcinoma of tongue (at 18 years); this patient had a history of HSCT at 5 years, then multiple surgical interventions were performed in order to eradicate cancers, is currently alive,
- Acute myeloid leukemia (at 6 years); this child died due to rapid leukemia progression.
Children with FANCD1 mutation (homozygous BRCA2 mutations) do not have a risk of bone marrow failure, but still the malignancies incidence is very high [7]. Among our cohort all 4 children with this mutation developed cancer early in their life. The following malignancies were registered:
- Acute lymphoblastic leukemia (at 1 year); the child responded to chemotherapy, but then a secondary acute myeloid lekemia developed and death of disease progression followed,
- Meduloblastoma (at 4.6 years); the patients died of relapse,
- Nephroblastoma (at 3.7 years); the child is alive and in complete remission (these three patients are siblings),
- Medulloblastoma (1.4 years); the patient has recently finished treatment and is currently alive.
At last visit with a median follow-up of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died after HSCT and one due to severe congenital defects.
Results of HSCT
A total of 15 patients with bone marrow failure due to FA underwent allogeneic hematopoietic stem cell transplantation (HSCT) at a median age of 9.3 (4.6-24.3) years from a matched sibling donor (MSD, n=3) or matched unrelated donor (MUD, n=12). The bone marrow was used in as graft source in 6, peripheral blood stem cells (PBSC) in 7, and umbilical cord blood in 2 cases, accordingly. In al cases the pre-transplant conditioning regimen was irradiation-free. The following regimens were used: FluCy ATG in 10 cases, FluCy MbC in 1 patient, and FluCy/Bu MbC(3)/ATG(1) in 4 patients. The regimen toxicity was acceptable and no early mortality (till D+100) was observed. All transplanted patients achieved stable hematopoietic engraftment after HSCT. Late mortality (after D+100) was registered in 2/15 patients (13%), both suffered from chronic graft- versus-host disease (GvHD), with extensive form in one case. These patients developed fatal infections due to inadequate immune reconstitution, dying of CMV pneumonia and invasive aspergillosis 12 and 14 months after HSCT, accordingly. Also, three patients require long-term immunosuppressive therapy due to chronic GvHD (limited in two cases, and extensive in one case). The patient who consequently developed two different malignancies did not suffer from chronic GvHD.
The overall survival (OS) and event-free survival (EFS) in total FA group are shown in Fig. 2.
Overall and event-free survival among the Fanconi anemia patients subjected to HSCT are shown in Fig. 3.

Long-term monitoring
A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease [8, 9, 10].
The outpatient care includes long-term follow-up by hematologist/oncologist or bone marrow transplant specialist.
The following surveillance program should be scheduled for all the FA patients, whether they underwent allogeneic HSCT or not:
Evaluation by oncologists in order to check for signs of head and neck tumors, oral cancer, and gynecologic cancers (including breast carcinoma) once in every 6 months.
Hematological evaluation (every 6 months) due to the risk of bone marrow failure, myelodysplastic syndrome or acute leukemia. One should remember that 1/3 of FA patients may maintain mild/moderate cytopenia, while 2/3 cases will later develop progression.
Examination by specialists in endocrinology. Endocrinopathies, including thyroid dysfunction, growth hormone deficiency, and glucose intolerance, are common in patients with FA, even in ones without history of HSCT.
- Examination by specialists in dermatology, hearing, cardiology, pulmonology (every 12 months).
- Recommendations: no smoking, no alcohol use, correct oral hygiene, limited radiation exposure, limited sun and ultraviolet exposure.
- Human papilloma virus vaccination should be initiated at nine years in order to reduce the risk of gynecologic cancer in females and possibly reduce the risk of oral cancer in all individuals.
Conclusion
While HSCT is a demanding medical procedure in patients with FA due to disease biology leading to higher transplant-associated risk, it allows achieving very good results when performed in centers with adequate expertise using appropriate conditioning regimens. It reverses bone marrow failure and prevents further development of hematological malignancies. However, the indications have to be considered very carefully. Clinically significant chemotherapy and radiation toxicity due to impaired DNA damage repair mechanisms have historically made allogeneic HSCT for patients with FA extremely challenging. Chronic graft-versus-host disease of mouth and/or genitourinary tract has been associated with higher baseline risk of spinocellular carcinoma. HSCT may also increase the risk of other secondary solid tumors and therapy-related MDS or leukemia as it does the non-FA population, but to greater extent. Renal failure is rare in patients with FA despite the fact that about one-quarter of them have structural abnormalities involving kidneys and urinary tract. Renal function may be compromised during and after HSCT by chemotherapy and calcineurin inhibitors toxicity. HSCT can restore long-term hematopoiesis and cure the hematologic complications of FA; however, when compared with age-matched controls, these patients do not achieve complete health or normal life expectancy. The risk of long-term disease- or transplantation-related complications remain and patients with FA are still at risk of conditions caused by congenital anomalies, endocrinopathy, and cancer. Still, HSCT significantly increases a life span if indications were chosen carefully. However, a lifelong multidisciplinary follow-up of all patients with FA is essential for early detection of bone marrow failure or any malignant disease. Preventive measures include minimizing radiation exposure and contact with harmful substances (including smoking). Vaccination against human papillomavirus is recommended to reduce the risk of gynecological cancer in women and oral cancer in all subjects.
Conflict of interest
None declared.
References
- Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018; 103(1): 30-39.
- Fiesco-Roa MO, Giri N, McReynolds LJ, Best AF, Alter BP. Genotype-phenotype associations in Fanconi anemia: A literature review. Blood Rev. 2019; 37: 100589.
- Soulier J, Leblanc T, Larghero J, Dastot H, Shimamura A, Guardiola P, et al. Detection of somatic mosaicism and classification of Fanconi anemia patients by analysis of the FA/BRCA pathway. Blood. 2005; 105(3): 1329-1336.
- Bogliolo M, Pujol R, Aza-Carmona M, Munoz-Subirana N, Rodriguez-Santiago B, Casado JA, et al. Optimised molecular genetic diagnostics of Fanconi anaemia by whole exome sequencing and functional studies. J Med Genet. 2020; 57(4): 258-268.
- Dufour C. How I manage patients with Fanconi anaemia. Br J Haematol. 2017;178(1):32-47
- Paustian L, Chao MM, Hanenberg H, Schindler D, Neitzel H, Kratz CP, et al. Androgen therapy in Fanconi anemia: A retrospective analysis of 30 years in Germany. Pediatr Hematol Oncol. 2016; 33(1): 5-12.
- Alter BP, Rosenberg PS, Brody LC. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J Med Genet. 2007; 44(1): 1-9.
- Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003; 129(1): 106-112.
- Kelaidi C, Makis A, Petrikkos L, Antoniadi K, Selenti N, Tzotzola V, et al. Bone marrow failure in Fanconi anemia: clinical and genetic spectrum in a cohort of 20 pediatric patients. J Pediatr Hematol Oncol. 2019; 41(8): 612-617.
- Dietz AC, Savage SA, Vlachos A, Mehta PA, Bresters D, Tolar J, et al. Late effects screening guidelines after hematopoietic cell transplantation for inherited bone marrow failure syndromes: consensus statement from the second Pediatric Blood and Marrow Transplant Consortium International Conference on late effects after pediatric HCT. Biol Blood Marrow Transplant. 2017; 23(9):1422-1428.
" ["~DETAIL_TEXT"]=> string(13933) "
Introduction
Fanconi anemia (FA) is a rare inherited condition characterized by heterogeneity of clinical signs and underling genetic mutations, which significantly increase the risk of bone marrow failure or malignancy (leukemia, solid tumors) development. Fanconi anemia is characterized by the presence of congenital anomalies occurring in approximately 75-80% of affected individuals, which include one or more of the following features: small stature, abnormal skin pigmentation, upper or lower limb skeletal malformation, microcephaly, visual and urogenital anomalies [1, 2]. The cumulative risk of developing bone marrow failure is 50%. Progressive bone marrow failure typically occurs in the first decade of life often starting with thrombocytopenia or leukopenia. Leukemia and solid tumors may occur as early as in 16 years. The diagnosis of FA is based on increased chromosome fragility detection in a test with or without an agent for DNA cross-link sensitivity testing, diepoxybutane (DEB) or mitomycin C (MMC). If hematopoietic sell still possess an intact DNA repair mechanism due to mosaicism these tests may be negative in blood cells cultures. Although these situations are rare, they may complicate diagnosis and warrant for test performed in non-hematopoietic cells culture, usually fibroblasts [3]. The final diagnosis nowadays is often confirmed by detection of a mutation in one of Fanconi complex genes [4].
Gene mutations in Fanconi anemia
• Bialellic pathogenic variant of one of 19 genes causing autosomal recessive form of FA.
• Heterozygous pathogenic variant in gene RAD51 (de novo; FANCO) causing autosomal dominant form of FA.
• Hemizygous pathogenic variant in gene FANCB causing X-linked form of FA.
There is a distinct flowchart for stepwise post-diagnostic monitoring and decision making in therapy of the Fanconi anemia patients, as described, e.g., by Dufour [5].
FA epidemiology in Czech Republic
The Czech population in 2020 is 10.7 millions (11th among EU countries) including 2.0 million children of 0 to 17.99 years with a birth rate of 115,000 per year. A total of 35 probands with Fanconi anemia were born in 1985-2019 in the Czech Republic with diagnosis confirmed in 1986-2020, it correlates with probable incidence of ca.1 patient/year. Diagnosis of FA was confirmed at the median age of 6 (0-24.3) years, 34/35 of patients were children at the moment of diagnosis. In all cases the diagnostic procedure included spontaneous and induced chromosomal breakage evaluation, then the diagnosis was prospectively or retrospectively confirmed by specific mutation detection in 33/35 patients.
The initial symptoms registered in FA patients included congenital effects in 5, thrombocytopenia in 9, bicytopenia or pancytopenia in 13 cases, accordingly. Also, 8 patients had characteristic family history (BMF, malignancies).
The main physical abnormalities and laboratory signs incidence in our FA group (n=35) at the time of diagnoses are summarized in Table 1. Table 2 and Fig.1 contain the list and relative frequency of mutations revealed in available patients (33 of 35 cases).

Bone marrow failure (BMF) and malignancies, outcome
During the follow-up 17/31 (55%) children developed BMF at the median age of 8 (4.0-17.1) years. Five patients (3 boys and 2 girls) had prior history of anabolics treatment with some effect in two girls [6]. Among these, 15 patients consequently underwent allogeneic HSCT (2004-2020) at the median age of 9.3 (4.6-24.3) years.
Also, 7/35 (20%) patients developed the following malignancies during follow-up with a median age of 9.8 (1.0 - 32.3) at cancer diagnosis:
- Spinocellular carcinoma of GI (32 years); the patient died due to cancer progression,
- Gastrointestinal adenocarcinoma (at 15 years), later a squamous cell carcinoma of tongue (at 18 years); this patient had a history of HSCT at 5 years, then multiple surgical interventions were performed in order to eradicate cancers, is currently alive,
- Acute myeloid leukemia (at 6 years); this child died due to rapid leukemia progression.
Children with FANCD1 mutation (homozygous BRCA2 mutations) do not have a risk of bone marrow failure, but still the malignancies incidence is very high [7]. Among our cohort all 4 children with this mutation developed cancer early in their life. The following malignancies were registered:
- Acute lymphoblastic leukemia (at 1 year); the child responded to chemotherapy, but then a secondary acute myeloid lekemia developed and death of disease progression followed,
- Meduloblastoma (at 4.6 years); the patients died of relapse,
- Nephroblastoma (at 3.7 years); the child is alive and in complete remission (these three patients are siblings),
- Medulloblastoma (1.4 years); the patient has recently finished treatment and is currently alive.
At last visit with a median follow-up of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died after HSCT and one due to severe congenital defects.
Results of HSCT
A total of 15 patients with bone marrow failure due to FA underwent allogeneic hematopoietic stem cell transplantation (HSCT) at a median age of 9.3 (4.6-24.3) years from a matched sibling donor (MSD, n=3) or matched unrelated donor (MUD, n=12). The bone marrow was used in as graft source in 6, peripheral blood stem cells (PBSC) in 7, and umbilical cord blood in 2 cases, accordingly. In al cases the pre-transplant conditioning regimen was irradiation-free. The following regimens were used: FluCy ATG in 10 cases, FluCy MbC in 1 patient, and FluCy/Bu MbC(3)/ATG(1) in 4 patients. The regimen toxicity was acceptable and no early mortality (till D+100) was observed. All transplanted patients achieved stable hematopoietic engraftment after HSCT. Late mortality (after D+100) was registered in 2/15 patients (13%), both suffered from chronic graft- versus-host disease (GvHD), with extensive form in one case. These patients developed fatal infections due to inadequate immune reconstitution, dying of CMV pneumonia and invasive aspergillosis 12 and 14 months after HSCT, accordingly. Also, three patients require long-term immunosuppressive therapy due to chronic GvHD (limited in two cases, and extensive in one case). The patient who consequently developed two different malignancies did not suffer from chronic GvHD.
The overall survival (OS) and event-free survival (EFS) in total FA group are shown in Fig. 2.
Overall and event-free survival among the Fanconi anemia patients subjected to HSCT are shown in Fig. 3.

Long-term monitoring
A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease [8, 9, 10].
The outpatient care includes long-term follow-up by hematologist/oncologist or bone marrow transplant specialist.
The following surveillance program should be scheduled for all the FA patients, whether they underwent allogeneic HSCT or not:
Evaluation by oncologists in order to check for signs of head and neck tumors, oral cancer, and gynecologic cancers (including breast carcinoma) once in every 6 months.
Hematological evaluation (every 6 months) due to the risk of bone marrow failure, myelodysplastic syndrome or acute leukemia. One should remember that 1/3 of FA patients may maintain mild/moderate cytopenia, while 2/3 cases will later develop progression.
Examination by specialists in endocrinology. Endocrinopathies, including thyroid dysfunction, growth hormone deficiency, and glucose intolerance, are common in patients with FA, even in ones without history of HSCT.
- Examination by specialists in dermatology, hearing, cardiology, pulmonology (every 12 months).
- Recommendations: no smoking, no alcohol use, correct oral hygiene, limited radiation exposure, limited sun and ultraviolet exposure.
- Human papilloma virus vaccination should be initiated at nine years in order to reduce the risk of gynecologic cancer in females and possibly reduce the risk of oral cancer in all individuals.
Conclusion
While HSCT is a demanding medical procedure in patients with FA due to disease biology leading to higher transplant-associated risk, it allows achieving very good results when performed in centers with adequate expertise using appropriate conditioning regimens. It reverses bone marrow failure and prevents further development of hematological malignancies. However, the indications have to be considered very carefully. Clinically significant chemotherapy and radiation toxicity due to impaired DNA damage repair mechanisms have historically made allogeneic HSCT for patients with FA extremely challenging. Chronic graft-versus-host disease of mouth and/or genitourinary tract has been associated with higher baseline risk of spinocellular carcinoma. HSCT may also increase the risk of other secondary solid tumors and therapy-related MDS or leukemia as it does the non-FA population, but to greater extent. Renal failure is rare in patients with FA despite the fact that about one-quarter of them have structural abnormalities involving kidneys and urinary tract. Renal function may be compromised during and after HSCT by chemotherapy and calcineurin inhibitors toxicity. HSCT can restore long-term hematopoiesis and cure the hematologic complications of FA; however, when compared with age-matched controls, these patients do not achieve complete health or normal life expectancy. The risk of long-term disease- or transplantation-related complications remain and patients with FA are still at risk of conditions caused by congenital anomalies, endocrinopathy, and cancer. Still, HSCT significantly increases a life span if indications were chosen carefully. However, a lifelong multidisciplinary follow-up of all patients with FA is essential for early detection of bone marrow failure or any malignant disease. Preventive measures include minimizing radiation exposure and contact with harmful substances (including smoking). Vaccination against human papillomavirus is recommended to reduce the risk of gynecological cancer in women and oral cancer in all subjects.
Conflict of interest
None declared.
References
- Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow-up. Haematologica. 2018; 103(1): 30-39.
- Fiesco-Roa MO, Giri N, McReynolds LJ, Best AF, Alter BP. Genotype-phenotype associations in Fanconi anemia: A literature review. Blood Rev. 2019; 37: 100589.
- Soulier J, Leblanc T, Larghero J, Dastot H, Shimamura A, Guardiola P, et al. Detection of somatic mosaicism and classification of Fanconi anemia patients by analysis of the FA/BRCA pathway. Blood. 2005; 105(3): 1329-1336.
- Bogliolo M, Pujol R, Aza-Carmona M, Munoz-Subirana N, Rodriguez-Santiago B, Casado JA, et al. Optimised molecular genetic diagnostics of Fanconi anaemia by whole exome sequencing and functional studies. J Med Genet. 2020; 57(4): 258-268.
- Dufour C. How I manage patients with Fanconi anaemia. Br J Haematol. 2017;178(1):32-47
- Paustian L, Chao MM, Hanenberg H, Schindler D, Neitzel H, Kratz CP, et al. Androgen therapy in Fanconi anemia: A retrospective analysis of 30 years in Germany. Pediatr Hematol Oncol. 2016; 33(1): 5-12.
- Alter BP, Rosenberg PS, Brody LC. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J Med Genet. 2007; 44(1): 1-9.
- Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003; 129(1): 106-112.
- Kelaidi C, Makis A, Petrikkos L, Antoniadi K, Selenti N, Tzotzola V, et al. Bone marrow failure in Fanconi anemia: clinical and genetic spectrum in a cohort of 20 pediatric patients. J Pediatr Hematol Oncol. 2019; 41(8): 612-617.
- Dietz AC, Savage SA, Vlachos A, Mehta PA, Bresters D, Tolar J, et al. Late effects screening guidelines after hematopoietic cell transplantation for inherited bone marrow failure syndromes: consensus statement from the second Pediatric Blood and Marrow Transplant Consortium International Conference on late effects after pediatric HCT. Biol Blood Marrow Transplant. 2017; 23(9):1422-1428.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "40" ["~SORT"]=> string(2) "40" ["CODE"]=> string(100) "anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli" ["~CODE"]=> string(100) "anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli" ["EXTERNAL_ID"]=> string(4) "1941" ["~EXTERNAL_ID"]=> string(4) "1941" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(289) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюденияFanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4777) "<p style="text-align: justify;">Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.</p> <h3>Методы</h3> <p style="text-align: justify;">Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB – у двух сиблингов.</p> <h3>Результаты</h3> <p style="text-align: justify;">По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками. </p> <h3>Выводы</h3> <p style="text-align: justify;">ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_META_TITLE"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_META_KEYWORDS"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_META_DESCRIPTION"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_PICTURE_FILE_ALT"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_PICTURE_FILE_TITLE"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(215) "Анемия Фанкони в Чешской республике: роль трансплантации гемопоэтических стволовых клеток и длительного наблюдения" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "anemiya-fankoni-v-cheshskoy-respublike-rol-transplantatsii-gemopoeticheskikh-stvolovykh-kletok-i-dli" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "170" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27359" ["VALUE"]=> string(22) "11/09/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/09/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27360" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27361" ["VALUE"]=> array(2) { ["TEXT"]=> string(235) "<p>Петр Седлачек, Петра Кеслова, Петр Смишек, Мартина Сукова, Марцела Маликова, Спирос Тавандзис, Ярослав Чермак, Ян Стари</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(223) "
Петр Седлачек, Петра Кеслова, Петр Смишек, Мартина Сукова, Марцела Маликова, Спирос Тавандзис, Ярослав Чермак, Ян Стари
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27362" ["VALUE"]=> array(2) { ["TEXT"]=> string(292) "<p>Департамент детской гематологии и онкологии, 2-я медицинская школа Карлова Университета, университетский госпиталь Мотол, Прага, Чешская республика</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(280) "Департамент детской гематологии и онкологии, 2-я медицинская школа Карлова Университета, университетский госпиталь Мотол, Прага, Чешская республика
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27363" ["VALUE"]=> array(2) { ["TEXT"]=> string(4777) "<p style="text-align: justify;">Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.</p> <h3>Методы</h3> <p style="text-align: justify;">Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB – у двух сиблингов.</p> <h3>Результаты</h3> <p style="text-align: justify;">По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками. </p> <h3>Выводы</h3> <p style="text-align: justify;">ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4619) "Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.
Методы
Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB – у двух сиблингов.
Результаты
По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками.
Выводы
ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.
Ключевые слова
Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27364" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-48-52" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-48-52" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27367" ["VALUE"]=> array(2) { ["TEXT"]=> string(141) "<p>Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(129) "Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27368" ["VALUE"]=> array(2) { ["TEXT"]=> string(557) "<p>Department of Pediatric Hematology and Oncology, 2<sup>nd</sup> Medical School at Charles University, University Hospital Motol, Prague, Czech Republic</p><br> <p><b>Correspondence</b><br> Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic<br> Phone: +420 22443 6552<br> Fax: +420 22443 6519<br> E-mail: petr.sedlacek@lfmotol.cuni.cz</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(479) "Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, Prague, Czech Republic
Correspondence
Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic
Phone: +420 22443 6552
Fax: +420 22443 6519
E-mail: petr.sedlacek@lfmotol.cuni.cz
Fanconi anemia (FA) is a rare and heterogeneous syndrome associated with bone marrow failure and increased risk of cancer. While FA is often characterized by the presence of congenital malformations, in some patients cytopenia may be the only sign. In our cohort diagnosis was based on evidence of increased chromosome fragility with subsequent confirmation by gene mutation detection.
Methods
Our cohort includes 35 probands diagnosed with FA aged 0-24.3 (median 6) years between January 1986 and August 2020. Congenital anomalies at diagnosis were seen in 5 and cytopenia in 22 patients, 8 patients had family history of FA. Genetic test confirmed FANCA gene mutations in 24, FANCG in 3, FANCD1 in 4, and FANCB in 2 siblings.
Results
During follow-up 7 patients developed malignancy (among them all 4 patients with FANCD1 mutation). Seventeen patients developed marrow failure, for which 15 patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the median age of 9.3 (4.6-24.3) years. All transplanted patients achieved stable hematopoietic engraftment. However, in 2 patients due to inadequate immune reconstitution developed fatal CMV pneumonia and invasive aspergillosis 12 and 14 months post HSCT, accordingly. In one patient we have diagnosed adenocarcinoma of the gut 10 years and squamous cell carcinoma of tongue 13 years after HSCT. Thirteen patients are alive with a median follow-up of 10.6 (0.3 – 15.1 years) years after HSCT. With a median follow-up till the last visit of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died due to HSCT-related complications, and one due to severe congenital somatic defects.
Conclusions
HSCT is effective in FA patients with bone marrow failure and prevents further development of hematological malignancies. A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease.
Keywords
Fanconi anemia, somatic anomalies, gene mutations, bone marrow failure, solid tumors, hematopoietic stem cell transplantation, long-term outpatient observation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27365" ["VALUE"]=> string(74) "Fanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(74) "Fanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27366" ["VALUE"]=> string(4) "2335" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2335" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27370" ["VALUE"]=> string(4) "2336" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2336" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27367" ["VALUE"]=> array(2) { ["TEXT"]=> string(141) "<p>Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(129) "Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(129) "Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27369" ["VALUE"]=> array(2) { ["TEXT"]=> string(2524) "<p style="text-align: justify;">Fanconi anemia (FA) is a rare and heterogeneous syndrome associated with bone marrow failure and increased risk of cancer. While FA is often characterized by the presence of congenital malformations, in some patients cytopenia may be the only sign. In our cohort diagnosis was based on evidence of increased chromosome fragility with subsequent confirmation by gene mutation detection. </p> <h3>Methods</h3> <p style="text-align: justify;">Our cohort includes 35 probands diagnosed with FA aged 0-24.3 (median 6) years between January 1986 and August 2020. Congenital anomalies at diagnosis were seen in 5 and cytopenia in 22 patients, 8 patients had family history of FA. Genetic test confirmed FANCA gene mutations in 24, FANCG in 3, FANCD1 in 4, and FANCB in 2 siblings.</p> <h3>Results</h3> <p style="text-align: justify;">During follow-up 7 patients developed malignancy (among them all 4 patients with FANCD1 mutation). Seventeen patients developed marrow failure, for which 15 patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the median age of 9.3 (4.6-24.3) years. All transplanted patients achieved stable hematopoietic engraftment. However, in 2 patients due to inadequate immune reconstitution developed fatal CMV pneumonia and invasive aspergillosis 12 and 14 months post HSCT, accordingly. In one patient we have diagnosed adenocarcinoma of the gut 10 years and squamous cell carcinoma of tongue 13 years after HSCT. Thirteen patients are alive with a median follow-up of 10.6 (0.3 – 15.1 years) years after HSCT. With a median follow-up till the last visit of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died due to HSCT-related complications, and one due to severe congenital somatic defects.</p> <h3>Conclusions</h3> <p style="text-align: justify;">HSCT is effective in FA patients with bone marrow failure and prevents further development of hematological malignancies. A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease. </p> <h2>Keywords</h2> <p style="text-align: justify;">Fanconi anemia, somatic anomalies, gene mutations, bone marrow failure, solid tumors, hematopoietic stem cell transplantation, long-term outpatient observation.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2366) "Fanconi anemia (FA) is a rare and heterogeneous syndrome associated with bone marrow failure and increased risk of cancer. While FA is often characterized by the presence of congenital malformations, in some patients cytopenia may be the only sign. In our cohort diagnosis was based on evidence of increased chromosome fragility with subsequent confirmation by gene mutation detection.
Methods
Our cohort includes 35 probands diagnosed with FA aged 0-24.3 (median 6) years between January 1986 and August 2020. Congenital anomalies at diagnosis were seen in 5 and cytopenia in 22 patients, 8 patients had family history of FA. Genetic test confirmed FANCA gene mutations in 24, FANCG in 3, FANCD1 in 4, and FANCB in 2 siblings.
Results
During follow-up 7 patients developed malignancy (among them all 4 patients with FANCD1 mutation). Seventeen patients developed marrow failure, for which 15 patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the median age of 9.3 (4.6-24.3) years. All transplanted patients achieved stable hematopoietic engraftment. However, in 2 patients due to inadequate immune reconstitution developed fatal CMV pneumonia and invasive aspergillosis 12 and 14 months post HSCT, accordingly. In one patient we have diagnosed adenocarcinoma of the gut 10 years and squamous cell carcinoma of tongue 13 years after HSCT. Thirteen patients are alive with a median follow-up of 10.6 (0.3 – 15.1 years) years after HSCT. With a median follow-up till the last visit of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died due to HSCT-related complications, and one due to severe congenital somatic defects.
Conclusions
HSCT is effective in FA patients with bone marrow failure and prevents further development of hematological malignancies. A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease.
Keywords
Fanconi anemia, somatic anomalies, gene mutations, bone marrow failure, solid tumors, hematopoietic stem cell transplantation, long-term outpatient observation.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2366) "Fanconi anemia (FA) is a rare and heterogeneous syndrome associated with bone marrow failure and increased risk of cancer. While FA is often characterized by the presence of congenital malformations, in some patients cytopenia may be the only sign. In our cohort diagnosis was based on evidence of increased chromosome fragility with subsequent confirmation by gene mutation detection.
Methods
Our cohort includes 35 probands diagnosed with FA aged 0-24.3 (median 6) years between January 1986 and August 2020. Congenital anomalies at diagnosis were seen in 5 and cytopenia in 22 patients, 8 patients had family history of FA. Genetic test confirmed FANCA gene mutations in 24, FANCG in 3, FANCD1 in 4, and FANCB in 2 siblings.
Results
During follow-up 7 patients developed malignancy (among them all 4 patients with FANCD1 mutation). Seventeen patients developed marrow failure, for which 15 patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the median age of 9.3 (4.6-24.3) years. All transplanted patients achieved stable hematopoietic engraftment. However, in 2 patients due to inadequate immune reconstitution developed fatal CMV pneumonia and invasive aspergillosis 12 and 14 months post HSCT, accordingly. In one patient we have diagnosed adenocarcinoma of the gut 10 years and squamous cell carcinoma of tongue 13 years after HSCT. Thirteen patients are alive with a median follow-up of 10.6 (0.3 – 15.1 years) years after HSCT. With a median follow-up till the last visit of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died due to HSCT-related complications, and one due to severe congenital somatic defects.
Conclusions
HSCT is effective in FA patients with bone marrow failure and prevents further development of hematological malignancies. A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease.
Keywords
Fanconi anemia, somatic anomalies, gene mutations, bone marrow failure, solid tumors, hematopoietic stem cell transplantation, long-term outpatient observation.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27364" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-48-52" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-48-52" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-48-52" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27365" ["VALUE"]=> string(74) "Fanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(74) "Fanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(74) "Fanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27368" ["VALUE"]=> array(2) { ["TEXT"]=> string(557) "<p>Department of Pediatric Hematology and Oncology, 2<sup>nd</sup> Medical School at Charles University, University Hospital Motol, Prague, Czech Republic</p><br> <p><b>Correspondence</b><br> Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic<br> Phone: +420 22443 6552<br> Fax: +420 22443 6519<br> E-mail: petr.sedlacek@lfmotol.cuni.cz</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(479) "Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, Prague, Czech Republic
Correspondence
Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic
Phone: +420 22443 6552
Fax: +420 22443 6519
E-mail: petr.sedlacek@lfmotol.cuni.cz
Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, Prague, Czech Republic
Correspondence
Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic
Phone: +420 22443 6552
Fax: +420 22443 6519
E-mail: petr.sedlacek@lfmotol.cuni.cz
Петр Седлачек, Петра Кеслова, Петр Смишек, Мартина Сукова, Марцела Маликова, Спирос Тавандзис, Ярослав Чермак, Ян Стари
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(223) "Петр Седлачек, Петра Кеслова, Петр Смишек, Мартина Сукова, Марцела Маликова, Спирос Тавандзис, Ярослав Чермак, Ян Стари
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27359" ["VALUE"]=> string(22) "11/09/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/09/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/09/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27360" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/04/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27363" ["VALUE"]=> array(2) { ["TEXT"]=> string(4777) "<p style="text-align: justify;">Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.</p> <h3>Методы</h3> <p style="text-align: justify;">Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB – у двух сиблингов.</p> <h3>Результаты</h3> <p style="text-align: justify;">По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками. </p> <h3>Выводы</h3> <p style="text-align: justify;">ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4619) "Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.
Методы
Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB – у двух сиблингов.
Результаты
По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками.
Выводы
ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.
Ключевые слова
Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4619) "Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.
Методы
Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB – у двух сиблингов.
Результаты
По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками.
Выводы
ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.
Ключевые слова
Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27362" ["VALUE"]=> array(2) { ["TEXT"]=> string(292) "<p>Департамент детской гематологии и онкологии, 2-я медицинская школа Карлова Университета, университетский госпиталь Мотол, Прага, Чешская республика</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(280) "Департамент детской гематологии и онкологии, 2-я медицинская школа Карлова Университета, университетский госпиталь Мотол, Прага, Чешская республика
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(280) "Департамент детской гематологии и онкологии, 2-я медицинская школа Карлова Университета, университетский госпиталь Мотол, Прага, Чешская республика
" } } } [4]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "170" ["~IBLOCK_SECTION_ID"]=> string(3) "170" ["ID"]=> string(4) "1942" ["~ID"]=> string(4) "1942" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["~NAME"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "02/05/2021 03:36:20 pm" ["~TIMESTAMP_X"]=> string(22) "02/05/2021 03:36:20 pm" ["DETAIL_PAGE_URL"]=> string(138) "/en/archive/-9-4/klinicheskie-raboty/vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto/" ["~DETAIL_PAGE_URL"]=> string(138) "/en/archive/-9-4/klinicheskie-raboty/vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(20509) "Introduction
Hematopoietic stem cell transplantation (HSCT) from haploidentical donors (haplo-HSCT) increasingly becomes the method of choice in the treatment of patients with hemoblastoses and hematopoietic depressions. HLA-matched related donors are often not available, and there is no sufficient time for searching unrelated donors, or matched unrelated donors cannot be found neither in the domestic nor in foreign bone marrow registers. New conditioning regimens implementation, improvement of graft processing technologies, as well as the use of effective methods of graft-versus-host disease (GvHD) prevention significantly reduced the risk of severe complications and allowed to expand the indications for such a kind of transplantations [1, 2]. Thus, indication of cyclophosphamide following haplo-HSCT for the GvHD-prevention and selective αβ-depletion of T-cells from a graft significantly increased patient survival rates [2, 3, 4, 5].
HLA-haploidentical donor may be available for the most of patients: first- and second-line relatives who inherit the same HLA-haplotype as the patient may be related HLA-haplocompatible, or even HLA-haploidentical donors. According to Fuchs [6] the related HLA-haploidentical donor in pediatric practice can be found for more than 95% of patients, and the average number of haploidentical donors for one patient can be two or more. Such donors are usually available and motivated to donate. But haplocompatible/haploidentical donors are just partially compatible with the patient's HLA-antigens. If the patient develops antibodies against HLA-antigens of its donor's cells, i.e., the donor-specific antibodies (DSA), these DSA may potentially cause the immunomediated haplo-HSCT complications. Primary graft failure is the most dangerous hazard. Ciurea et al. have found DSA in 18% (22 out of 122) of patients before haplo-HSCT. In the group of patients with DSA, the frequency of primary graft failure was significantly higher (32%) than in patients without DSA (4%) (p<0.001) [7]. Chang et al. found DSA in 11.3% of patients (39 out of 345) for whom then haplo-HSCT has been performed [8]. With DSA levels of more than 2000 units, the incidence of primary graft failure was significantly higher (23.7%) than at their absence, and DSA levels of less than 2000 units (1.9%) (p=0.003). Due to primary graft failure, the survival rate is significantly lower in patients with DSA after haplo-HSCT [9].
The aim of the current study was to determine frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay (ELISA) and to prove an association between the DSA presence and risk of primary graft failure and (or) graft hypofunction in children after haplo-HSCT.
Materials and methods
Table 1. Demographic and clinical characteristics of the patients
Note: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; AA, aplastic anemia; JMML, juvenile myelomonocytic leukemia.
Current publication presents the results of a pilot retrospective single-center study. Forty pediatric patients who received haplo-HSCT with unmanipulated graft have been included into the study. The study proceeded in the clinic of R. M. Gorbacheva Memorial Research Institute for Pediatric Oncology, Hematology and Transplantation over the period from July 2019 to March 2020. HLA-typing of recipients and their donors was performed with molecular-genetic methods. If based on the results of HLA-typing (HLA-A, B, C, DRB1, DQB1) in the patient and first-line relatives at low-resolution level, it was possible to establish four family haplotypes, then the recipient and the donor, matched by one of these haplotypes were considered as haploidentical. If the family study did not allow to determine four haplotypes, then the high-resolution HLA-typing has been performed by Sanger sequencing. The recipient/donor concordance for, at least, 5 alleles out of 10 was considered haplo-compatible. However, in vast majority of patients (95%), haploidentity could not be determined, so we considered haplo-compatible all the performed HSCTs. Demographics and clinical characteristics of the patients are presented in Table 1.
The median age of patients at the time of HSCT was 8 (1-17) years. The cohort was dominated by patients with ALL (67.5%) and AML (20%). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×108/kg (2.3-17.9×108/kg) and 7.6×106/kg (2.3-8.2×106/kg), respectively. Stimulated, unmanipulated bone marrow was a source of CD34+ cells in all cases. The donor's hematopoesis was stimulated by G-CSF at the dose of 5 mcg/kg/day during 3 days, and the bone marrow myeloexfusion has been performed on the 4th day. 26 recipients (65%) received myeloablative conditioning (MAC) based on busulfan at a total dose of 12 mg/kg and fludarabine (150 mg/m2). The reduced-intensity conditioning (RIC) has been received by 14 (35%) of patients, i.e.,: melphalan at the total dose of 140 mg/m2 and fludarabine (150 mg/m2). Post-transplant cyclophosphamide at the days +3 and +4 post-HSCT was administered at the dose of 50 mg/kg per day, calcineurin inhibitor tacrolimus (0.03 mg/kg/day with a target concentration of 3-5 ng/ml), and mTOR inhibitor – sirolimus (1 mg/m2/day, with a target concentration of 3-5 ng/ml) were used to prevent acute GvHD. The distribution of HLA-haploidentical donors was as follows: fathers, 24 cases; mothers, 14 cases; siblings, in 2 cases.
The primary graft failure criterion was considered as failure to reach the level of neutrophils of more than 0.5×109/l within three consequent days +35 to 42 posttransplant. The hypofunction of the transplant was diagnosed as two- or three-lineage cytopenia (Hb< 100 g/l, neutrophils <0.5×109/l, platelets <109/l), regardless of the time post-transplant, requirements for blood transfusion, and hypocellular bone marrow in presence of complete donor chimerism and absence of acute GvHD, or a disease relapse.
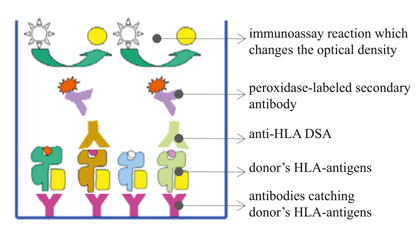
Figure 1. Enzyme Immunoassay in the presence of DSA to HLA-antigens of the donor
All the patients were examined for the presence of anti-HLA DSA before haplo-HSCT. The laboratory method selected for DSA detection was an enzyme immunoassay analysis (XMatch®, PROTRANS, Germany). From the donor blood samples lymphocytes were isolated and then lysed. The cell lysates containing donor HLA-molecules were then incubated with capture antibodies precipitated at the bottom of ELISA-wells separately for HLA class I and II (Fig. 1).
The donor's native HLA-antigens were bound to the capturing antibodies fixed to the bottom of the wells. Then the patient's blood serum samples were incubated at this carrier. In the case of DSA presence, these antibodies were attached to the donor's HLA-antigens. After series of washings from unbound antibodies, secondary antibodies labeled with horseradish peroxidase were added. These antibodies could only bind to DSA previously fixed to the donor's HLA-antigens. To visualize the results, a peroxidase-oxidized substrate was added to change the color of the solution. Optical density of the reaction products was measured by using an enzyme immunoassay analyzer (Immunochem-2100, High Technology Inc, USA) at wavelengths of 450 and 620 nm. The result was accepted as valid if the optical density in positive control was ≥0.350 OD and in negative control ≤0.150 OD. The result was considered positive, if the optical density was at least twice higher than negative control.
We have used ELISA-based crossmatch to detect DSA de facto opposite to virtual crossmatching based on the analysis of Luminex results. The idea was to prove the direct interaction between HLA-antigens of the certain donor with antibodies of the patient. ELISA was also the method of choice since it allowed to avoid false-positive results that occur in CDC or FC-XM due to therapiutic monoclonal antibodies received by the patients before haplo-HSCT. Thus, the chosen method could provide the direct interaction evidence, and could be used qualitatively.
The differences in the frequency of primary graft failure and graft hypofunction between the analyzed groups were evaluated by means of the two-tailed Fisher's exact test. For statistical data processing, STATISTICA 8 software was used (StatSoft Inc., USA).
Results
Among all the examined children, three patients (7.5%) were found to be DSA-positive, while the vast majority (n=37; 92.5%) had no DSA. It was also found that, in all cases, DSA were directed against HLA class II antigens.
Brief description of patients with DSA is as follows:
1) Patient K.P., 10 years old; acquired idiopathic aplastic anemia, severe form; duration of the disease before haplo-HSCT was 5 years; previous history of multiple hemotransfusions.
2) Patient I.V., 15 years old; acute myeloblastic leukemia with interstitial 7p and 9q deletions; high risk group; allogeneic unrelated bone marrow transplantation (28.04.2018); engraftment by the day +20; donor lymphocyte infusion (DLI, n=5); isolated bone marrow relapse (of 11.02.2019); transplant rejection (25.03.2019); a history of multiple hemotransfusions; duration of the disease prior to haplo-HSCT was 1.5 years.
3) Patient K.P., 14 years old; acute myeloblastic leukemia (FAB M4) with co-expression of CD7, CD79A; NUP98 gene rearrangement; therapy according to the AML-BFM 2004 Protocol; the first early isolated bone marrow relapse documented at 07.03.2019 with resistance to therapy; duration of the disease from diagnosis to haplo-HSCT was 9 months.
When analyzing efficiency of performed haplo-HSCTs, we have found that nine patients (22.5%) had either primary graft failure (n=6; 15%) or graft hypofunction (n=3; 7.5%).
Primary graft failure and allograft hypofunction, being a sign of poor engraftment in these patients, required then a repeated allo-HSCT from other donor. Of note, the improved analytic technologies for evaluating donor chimerism have shown that some patients can develop graft hypofunction even when the complete donor chimerism is observed.
Comparative analysis of the results shows that patients with DSA prior to haplo-HSCT are more likely to develop primary graft failure, or hypofunction compared to the group of recipients without DSA. All three patients with detected antibodies were diagnosed with either primary graft failure (n=1; 33%) or graft hypofunction (n=2; 67%). In the control group of patients without DSA, primary graft failure or graft hypofunction were observed in 5 (13.5 %) and 1 (2.7 %) cases respectively, with a total of 6 cases (16.2%) (Table 2).
Table 2. Frequency of primary graft failure and graft hypofunction depending on the presence of DSA (n=40)
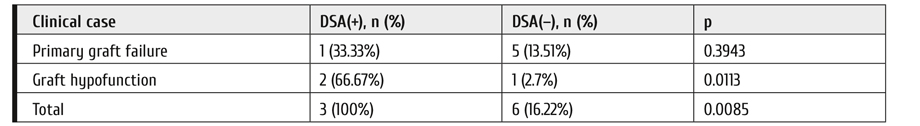
Discussion
The role of DSA in hematopoietic cell transplantation remained unclear for a long time. For allogeneic HSCT, clinicians tried to search for either HLA-identical related, or HLA-matched unrelated donors, in these cases DSA to HLA-antigens would not develop in principle. Therefore, when studying graft failure mechanisms, the cellular immunity has drawn much attention. Nowadays, its generally accepted that, following organ and tissue transplantation, the immune system reacts to the HLA-antigens differing from recipient HLA-antigens by the mechanisms of both cellular and humoral immune response. At the same time, it would be noted that the exact mechanism by which DSA causes graft failure in haplo-HSCT, remains undetermined.
The impact of DSA to the outcome of organ transplantation became the point for some studies on their role in haplo-HSCT. The results of these works represented the basis of clinical recommendations aimed for identification and elimination of DSA in patients before haplo-HSCT, published by the European Society for Blood and Marrow Transplantation (EBMT) in 2018 [10]. In adult patients with hemoblastosis requiring allo-HSCT, the detection frequency of anti-HLA antibodies may reach 40%, [11, 12, 13]. However, not all of these anti-HLA antibodies are directed against donor HLA-antigens. Using highly sensitive methods of solid-phase analysis, it was detected that anti-HLA antibodies were donor-specific in 24% of haplo-HSCT recipients [14]. According to the most researchers, the prevalence of DSA varies from 10% to 21% [9]. In the present study, we were able to identify DSA only in 7.5% of examined patients. Probably, the lower rate in children is observed due to anti-HLA antibodies arising after hemotransfusions and, in some cases, following previous HSCT from a partially matched donor. Among adults, HLA-allosensitization is most often detected in women (up to 86%, against 5% in men), with pregnancy being the most common reason.
Our data on the high frequency of primary graft failure and/or graft hypofunction in the recipient group with the presence of DSA (100%), compared to the group, in which DSA is not detectable (16.22%) agreed with previous data [9] about the influence of DSA upon the outcome of haplo-HSCT. However, due to minority of patients with identified DSA, one should continue this study in order to confirm these results in a more representative sample.
It is recommended to determine DSAs in all patients 1 month before haplo-HSCT. When DSA are detected, a search for alternative haplo-compatible donor should be considered. If such donors are not available, it is necessary to use the treatment reducing the DSA-levels and to prevent the development of new anti-HLA DSA. Laboratory monitoring of DSA would be used as a way to assess effectiveness of treatment, and should be performed just before transplantation and every week after it [10].
Conclusion
The method we used to determine DSA is sufficiently informative, has a number of advantages over the serological method using a lymphocytotoxic test, flow cytometry, as well as multiplex analysis methods, and it does not require intact donor lymphocytes incubated immediately with DSA. The test does not give false positive results in the presence of any non-anti-HLA IgG’s in the patient's serum that can contact the membrane and activate the complement. HLA-molecules of a real donor as an antigen material are used for the test. Results are recorded automatically. It is necessary to perform a comparative study of the results of DSA testing using enzyme immunoassay with a multiplex analysis which is more common method in routine practice, using the Luminex platform. Implementation of DSA-testing for the routine practice in recipients prior to haplo-HSCT will optimize the choice of a donor, as well as to select a group of potential recipients who requires desensitization treatment over the pre-transplant period.
Conflict of interest
None declared.
References
- Afanasyev BV, Zubarovskaya LS, Alyanskiy AL, et al. The donor choice for allogeneic stem cell transplantation. Russian Journal of Children Hematology and Oncology. 2016; 3(3): 30-36 (In Russian).
- Maschan MA. Т-lymphocytes alpha/beta depletion – reliable platform for the development of stem cell transplantaion from haploidentical donors. Russian Journal of Children Hematology and Oncology. 2015; 2 (3):34-38. (In Russian).
- Paina OV, Stancheva NV, Semenova EV, et al. Haploidentical transplantation of hematopoetic stem cells in treatment of children and adolescents with resistant forms of acute leucosis. Russian Journal of Children Hematology and Oncology. 2015; 2(3):39-45 (In Russian).
- Paina OV, Kojokar' PV, Borovkova AS, et al. Results of alogeneic stem cell transplantation from haploidentical donor with unmanipulated bone marrowtransplant in children and adolescents with acute leucosis from high risk group: 10 years experience. Issues in Hematology/Oncology and Immunology in Pediatrics. 2018; 17(2): 21-27 (In Russian).
- Lee CJ, Savani BN, Mohty M, Labopin M, Ruggeri A et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017; 102(11);1810-1822.
- Fuchs EJ. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematol. Am.Soc.Hematol.Educ.Program. 2012; 2012: 230-236.
- Ciurea SO, Mulanovich V, Jiang Y, et al. Lymphocyte recovery predicts outcomes in cord blood and T cell-depleted haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1169-1175.
- Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015; 8:84.
- Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1392-1398.
- The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the detection and treatment of donor specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplantation. 2018; 53: 521-534.
- Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012; 47:508-515.
- Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015; 8:84.
- Ruggeri A, Rocha V, Masson E, Labopin M, Cunha R, Absi L, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Societe Francophone d’Histocompatibilite et d’Immunogenetique (SFHI) and Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) analysis. Haematologica. 2013; 98:1154-1160.
- Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19:647-652.
" ["~DETAIL_TEXT"]=> string(20509) "
Introduction
Hematopoietic stem cell transplantation (HSCT) from haploidentical donors (haplo-HSCT) increasingly becomes the method of choice in the treatment of patients with hemoblastoses and hematopoietic depressions. HLA-matched related donors are often not available, and there is no sufficient time for searching unrelated donors, or matched unrelated donors cannot be found neither in the domestic nor in foreign bone marrow registers. New conditioning regimens implementation, improvement of graft processing technologies, as well as the use of effective methods of graft-versus-host disease (GvHD) prevention significantly reduced the risk of severe complications and allowed to expand the indications for such a kind of transplantations [1, 2]. Thus, indication of cyclophosphamide following haplo-HSCT for the GvHD-prevention and selective αβ-depletion of T-cells from a graft significantly increased patient survival rates [2, 3, 4, 5].
HLA-haploidentical donor may be available for the most of patients: first- and second-line relatives who inherit the same HLA-haplotype as the patient may be related HLA-haplocompatible, or even HLA-haploidentical donors. According to Fuchs [6] the related HLA-haploidentical donor in pediatric practice can be found for more than 95% of patients, and the average number of haploidentical donors for one patient can be two or more. Such donors are usually available and motivated to donate. But haplocompatible/haploidentical donors are just partially compatible with the patient's HLA-antigens. If the patient develops antibodies against HLA-antigens of its donor's cells, i.e., the donor-specific antibodies (DSA), these DSA may potentially cause the immunomediated haplo-HSCT complications. Primary graft failure is the most dangerous hazard. Ciurea et al. have found DSA in 18% (22 out of 122) of patients before haplo-HSCT. In the group of patients with DSA, the frequency of primary graft failure was significantly higher (32%) than in patients without DSA (4%) (p<0.001) [7]. Chang et al. found DSA in 11.3% of patients (39 out of 345) for whom then haplo-HSCT has been performed [8]. With DSA levels of more than 2000 units, the incidence of primary graft failure was significantly higher (23.7%) than at their absence, and DSA levels of less than 2000 units (1.9%) (p=0.003). Due to primary graft failure, the survival rate is significantly lower in patients with DSA after haplo-HSCT [9].
The aim of the current study was to determine frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay (ELISA) and to prove an association between the DSA presence and risk of primary graft failure and (or) graft hypofunction in children after haplo-HSCT.
Materials and methods
Table 1. Demographic and clinical characteristics of the patients

Note: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; AA, aplastic anemia; JMML, juvenile myelomonocytic leukemia.
Current publication presents the results of a pilot retrospective single-center study. Forty pediatric patients who received haplo-HSCT with unmanipulated graft have been included into the study. The study proceeded in the clinic of R. M. Gorbacheva Memorial Research Institute for Pediatric Oncology, Hematology and Transplantation over the period from July 2019 to March 2020. HLA-typing of recipients and their donors was performed with molecular-genetic methods. If based on the results of HLA-typing (HLA-A, B, C, DRB1, DQB1) in the patient and first-line relatives at low-resolution level, it was possible to establish four family haplotypes, then the recipient and the donor, matched by one of these haplotypes were considered as haploidentical. If the family study did not allow to determine four haplotypes, then the high-resolution HLA-typing has been performed by Sanger sequencing. The recipient/donor concordance for, at least, 5 alleles out of 10 was considered haplo-compatible. However, in vast majority of patients (95%), haploidentity could not be determined, so we considered haplo-compatible all the performed HSCTs. Demographics and clinical characteristics of the patients are presented in Table 1.
The median age of patients at the time of HSCT was 8 (1-17) years. The cohort was dominated by patients with ALL (67.5%) and AML (20%). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×108/kg (2.3-17.9×108/kg) and 7.6×106/kg (2.3-8.2×106/kg), respectively. Stimulated, unmanipulated bone marrow was a source of CD34+ cells in all cases. The donor's hematopoesis was stimulated by G-CSF at the dose of 5 mcg/kg/day during 3 days, and the bone marrow myeloexfusion has been performed on the 4th day. 26 recipients (65%) received myeloablative conditioning (MAC) based on busulfan at a total dose of 12 mg/kg and fludarabine (150 mg/m2). The reduced-intensity conditioning (RIC) has been received by 14 (35%) of patients, i.e.,: melphalan at the total dose of 140 mg/m2 and fludarabine (150 mg/m2). Post-transplant cyclophosphamide at the days +3 and +4 post-HSCT was administered at the dose of 50 mg/kg per day, calcineurin inhibitor tacrolimus (0.03 mg/kg/day with a target concentration of 3-5 ng/ml), and mTOR inhibitor – sirolimus (1 mg/m2/day, with a target concentration of 3-5 ng/ml) were used to prevent acute GvHD. The distribution of HLA-haploidentical donors was as follows: fathers, 24 cases; mothers, 14 cases; siblings, in 2 cases.
The primary graft failure criterion was considered as failure to reach the level of neutrophils of more than 0.5×109/l within three consequent days +35 to 42 posttransplant. The hypofunction of the transplant was diagnosed as two- or three-lineage cytopenia (Hb< 100 g/l, neutrophils <0.5×109/l, platelets <109/l), regardless of the time post-transplant, requirements for blood transfusion, and hypocellular bone marrow in presence of complete donor chimerism and absence of acute GvHD, or a disease relapse.

Figure 1. Enzyme Immunoassay in the presence of DSA to HLA-antigens of the donor
All the patients were examined for the presence of anti-HLA DSA before haplo-HSCT. The laboratory method selected for DSA detection was an enzyme immunoassay analysis (XMatch®, PROTRANS, Germany). From the donor blood samples lymphocytes were isolated and then lysed. The cell lysates containing donor HLA-molecules were then incubated with capture antibodies precipitated at the bottom of ELISA-wells separately for HLA class I and II (Fig. 1).
The donor's native HLA-antigens were bound to the capturing antibodies fixed to the bottom of the wells. Then the patient's blood serum samples were incubated at this carrier. In the case of DSA presence, these antibodies were attached to the donor's HLA-antigens. After series of washings from unbound antibodies, secondary antibodies labeled with horseradish peroxidase were added. These antibodies could only bind to DSA previously fixed to the donor's HLA-antigens. To visualize the results, a peroxidase-oxidized substrate was added to change the color of the solution. Optical density of the reaction products was measured by using an enzyme immunoassay analyzer (Immunochem-2100, High Technology Inc, USA) at wavelengths of 450 and 620 nm. The result was accepted as valid if the optical density in positive control was ≥0.350 OD and in negative control ≤0.150 OD. The result was considered positive, if the optical density was at least twice higher than negative control.
We have used ELISA-based crossmatch to detect DSA de facto opposite to virtual crossmatching based on the analysis of Luminex results. The idea was to prove the direct interaction between HLA-antigens of the certain donor with antibodies of the patient. ELISA was also the method of choice since it allowed to avoid false-positive results that occur in CDC or FC-XM due to therapiutic monoclonal antibodies received by the patients before haplo-HSCT. Thus, the chosen method could provide the direct interaction evidence, and could be used qualitatively.
The differences in the frequency of primary graft failure and graft hypofunction between the analyzed groups were evaluated by means of the two-tailed Fisher's exact test. For statistical data processing, STATISTICA 8 software was used (StatSoft Inc., USA).
Results
Among all the examined children, three patients (7.5%) were found to be DSA-positive, while the vast majority (n=37; 92.5%) had no DSA. It was also found that, in all cases, DSA were directed against HLA class II antigens.
Brief description of patients with DSA is as follows:
1) Patient K.P., 10 years old; acquired idiopathic aplastic anemia, severe form; duration of the disease before haplo-HSCT was 5 years; previous history of multiple hemotransfusions.
2) Patient I.V., 15 years old; acute myeloblastic leukemia with interstitial 7p and 9q deletions; high risk group; allogeneic unrelated bone marrow transplantation (28.04.2018); engraftment by the day +20; donor lymphocyte infusion (DLI, n=5); isolated bone marrow relapse (of 11.02.2019); transplant rejection (25.03.2019); a history of multiple hemotransfusions; duration of the disease prior to haplo-HSCT was 1.5 years.
3) Patient K.P., 14 years old; acute myeloblastic leukemia (FAB M4) with co-expression of CD7, CD79A; NUP98 gene rearrangement; therapy according to the AML-BFM 2004 Protocol; the first early isolated bone marrow relapse documented at 07.03.2019 with resistance to therapy; duration of the disease from diagnosis to haplo-HSCT was 9 months.
When analyzing efficiency of performed haplo-HSCTs, we have found that nine patients (22.5%) had either primary graft failure (n=6; 15%) or graft hypofunction (n=3; 7.5%).
Primary graft failure and allograft hypofunction, being a sign of poor engraftment in these patients, required then a repeated allo-HSCT from other donor. Of note, the improved analytic technologies for evaluating donor chimerism have shown that some patients can develop graft hypofunction even when the complete donor chimerism is observed.
Comparative analysis of the results shows that patients with DSA prior to haplo-HSCT are more likely to develop primary graft failure, or hypofunction compared to the group of recipients without DSA. All three patients with detected antibodies were diagnosed with either primary graft failure (n=1; 33%) or graft hypofunction (n=2; 67%). In the control group of patients without DSA, primary graft failure or graft hypofunction were observed in 5 (13.5 %) and 1 (2.7 %) cases respectively, with a total of 6 cases (16.2%) (Table 2).
Table 2. Frequency of primary graft failure and graft hypofunction depending on the presence of DSA (n=40)

Discussion
The role of DSA in hematopoietic cell transplantation remained unclear for a long time. For allogeneic HSCT, clinicians tried to search for either HLA-identical related, or HLA-matched unrelated donors, in these cases DSA to HLA-antigens would not develop in principle. Therefore, when studying graft failure mechanisms, the cellular immunity has drawn much attention. Nowadays, its generally accepted that, following organ and tissue transplantation, the immune system reacts to the HLA-antigens differing from recipient HLA-antigens by the mechanisms of both cellular and humoral immune response. At the same time, it would be noted that the exact mechanism by which DSA causes graft failure in haplo-HSCT, remains undetermined.
The impact of DSA to the outcome of organ transplantation became the point for some studies on their role in haplo-HSCT. The results of these works represented the basis of clinical recommendations aimed for identification and elimination of DSA in patients before haplo-HSCT, published by the European Society for Blood and Marrow Transplantation (EBMT) in 2018 [10]. In adult patients with hemoblastosis requiring allo-HSCT, the detection frequency of anti-HLA antibodies may reach 40%, [11, 12, 13]. However, not all of these anti-HLA antibodies are directed against donor HLA-antigens. Using highly sensitive methods of solid-phase analysis, it was detected that anti-HLA antibodies were donor-specific in 24% of haplo-HSCT recipients [14]. According to the most researchers, the prevalence of DSA varies from 10% to 21% [9]. In the present study, we were able to identify DSA only in 7.5% of examined patients. Probably, the lower rate in children is observed due to anti-HLA antibodies arising after hemotransfusions and, in some cases, following previous HSCT from a partially matched donor. Among adults, HLA-allosensitization is most often detected in women (up to 86%, against 5% in men), with pregnancy being the most common reason.
Our data on the high frequency of primary graft failure and/or graft hypofunction in the recipient group with the presence of DSA (100%), compared to the group, in which DSA is not detectable (16.22%) agreed with previous data [9] about the influence of DSA upon the outcome of haplo-HSCT. However, due to minority of patients with identified DSA, one should continue this study in order to confirm these results in a more representative sample.
It is recommended to determine DSAs in all patients 1 month before haplo-HSCT. When DSA are detected, a search for alternative haplo-compatible donor should be considered. If such donors are not available, it is necessary to use the treatment reducing the DSA-levels and to prevent the development of new anti-HLA DSA. Laboratory monitoring of DSA would be used as a way to assess effectiveness of treatment, and should be performed just before transplantation and every week after it [10].
Conclusion
The method we used to determine DSA is sufficiently informative, has a number of advantages over the serological method using a lymphocytotoxic test, flow cytometry, as well as multiplex analysis methods, and it does not require intact donor lymphocytes incubated immediately with DSA. The test does not give false positive results in the presence of any non-anti-HLA IgG’s in the patient's serum that can contact the membrane and activate the complement. HLA-molecules of a real donor as an antigen material are used for the test. Results are recorded automatically. It is necessary to perform a comparative study of the results of DSA testing using enzyme immunoassay with a multiplex analysis which is more common method in routine practice, using the Luminex platform. Implementation of DSA-testing for the routine practice in recipients prior to haplo-HSCT will optimize the choice of a donor, as well as to select a group of potential recipients who requires desensitization treatment over the pre-transplant period.
Conflict of interest
None declared.
References
- Afanasyev BV, Zubarovskaya LS, Alyanskiy AL, et al. The donor choice for allogeneic stem cell transplantation. Russian Journal of Children Hematology and Oncology. 2016; 3(3): 30-36 (In Russian).
- Maschan MA. Т-lymphocytes alpha/beta depletion – reliable platform for the development of stem cell transplantaion from haploidentical donors. Russian Journal of Children Hematology and Oncology. 2015; 2 (3):34-38. (In Russian).
- Paina OV, Stancheva NV, Semenova EV, et al. Haploidentical transplantation of hematopoetic stem cells in treatment of children and adolescents with resistant forms of acute leucosis. Russian Journal of Children Hematology and Oncology. 2015; 2(3):39-45 (In Russian).
- Paina OV, Kojokar' PV, Borovkova AS, et al. Results of alogeneic stem cell transplantation from haploidentical donor with unmanipulated bone marrowtransplant in children and adolescents with acute leucosis from high risk group: 10 years experience. Issues in Hematology/Oncology and Immunology in Pediatrics. 2018; 17(2): 21-27 (In Russian).
- Lee CJ, Savani BN, Mohty M, Labopin M, Ruggeri A et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017; 102(11);1810-1822.
- Fuchs EJ. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematol. Am.Soc.Hematol.Educ.Program. 2012; 2012: 230-236.
- Ciurea SO, Mulanovich V, Jiang Y, et al. Lymphocyte recovery predicts outcomes in cord blood and T cell-depleted haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1169-1175.
- Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015; 8:84.
- Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1392-1398.
- The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the detection and treatment of donor specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplantation. 2018; 53: 521-534.
- Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012; 47:508-515.
- Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015; 8:84.
- Ruggeri A, Rocha V, Masson E, Labopin M, Cunha R, Absi L, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Societe Francophone d’Histocompatibilite et d’Immunogenetique (SFHI) and Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) analysis. Haematologica. 2013; 98:1154-1160.
- Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19:647-652.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "50" ["~SORT"]=> string(2) "50" ["CODE"]=> string(100) "vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto" ["~CODE"]=> string(100) "vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto" ["EXTERNAL_ID"]=> string(4) "1942" ["~EXTERNAL_ID"]=> string(4) "1942" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(552) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донораDonor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(5034) "<p style="text-align: justify;">Донор-специфические анти-HLA антитела (ДСА) могут являться важным фактором риска недостаточного приживления HLA-гаплосовместимых донорских гемопоэтических клеток (ГСК). Цель настоящего исследования – определить частоту выявления донор-специфических анти-HLA антител у реципиентов перед проведением гаплосовместимой трансплантации ГСК (ТГСК) с помощью метода иммуноферментного анализа и установить взаимосвязь наличия ДСА с первичным неприживлением трансплантата и (или) гипофункцией трансплантата у детей, перенесших ГТГСК.</p> <p style="text-align: justify;">В работе представлены результаты пилотного ретроспективного одноцентрового исследования. Обследованы 40 пациентов детского возраста, медиана возраста составила 8,5 лет (1 год – 17 лет), которым были выполнены гаплосовместимые трансплантации неманипулированных ГСК в клинике НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой (22-M, 18-ж): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. Средний возраст пациентов составил 8,5 лет (1-17 лет). Медианы общего числа трансплантированных ядросодержащих и CD34+ клеток составляли 8,5×10<sup>8</sup>/кг (2,3-17,9×10<sup>8</sup>/кг) и 7,6×10<sup>6</sup>/кг (2,3-8,2×10<sup>6</sup>/кг) соответственно. Во всех случаях источником CD34+ клеток был стимулированный неманипулированный костный мозг. Определение ДСА в сыворотке крови реципиентов выполнено с использованием коммерческого набора (XMatch®, Protrans), который позволяет определять антитела против донорских антигенов HLA- класса I и класса II отдельно в перекрестном сопоставлении ELISA de facto. Среди всех обследованных детей у 3-х пациентов (7,5%) выявлены ДСА, в то время как у подавляющего большинства (n=37; 92,5%) ДСА не было, во всех случаях ДСА были направлены против антигенов HLA класса II. Сравнительный анализ результатов показывает, что у пациентов с наличием ДСА до гаплосовместимой ТГСК более вероятно развитие первичной недостаточности трансплантата или гипофункции по сравнению с группой реципиентов без DSA. У всех 3 пациентов (100%) с обнаруженными антителами была диагностирована либо первичная недостаточность трансплантата (n=1, 33%), либо гипофункция трансплантата (n=2, 67%). В группе пациентов без ДСА первичная недостаточность трансплантата или гипофункция трансплантата наблюдались в 5 (13,5%) и 1 (2,7%) случаях соответственно, всего в 6 случаях (16,2%). Внедрение DSA-тестирования в рутинную практику у реципиентов перед гапло-ТГСК оптимизирует выбор донора, а также выберет группу потенциальных реципиентов, которым необходимо пройти курс лечения в предтрансплантационный период для десенсибилизации.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Донор-специфические антитела, трансплантация гемопоэтических стволовых клеток, HLA-гаплосовместимые доноры, HLA-гаплоидентичные доноры.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_META_TITLE"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_META_KEYWORDS"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_META_DESCRIPTION"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_PICTURE_FILE_ALT"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_PICTURE_FILE_TITLE"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(393) "Выявление донор-специфических анти-HLA-антител методом индивидуальной перекрестной пробы де-факто у педиатрических реципиентов перед трансплантацией гемопоэтических стволовых клеток от гаплосовместимого донора" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "vyyavlenie-donor-spetsificheskikh-anti-hla-antitel-metodom-individualnoy-perekrestnoy-proby-de-fakto" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "170" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27371" ["VALUE"]=> string(22) "09/02/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "09/02/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27372" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27373" ["VALUE"]=> array(2) { ["TEXT"]=> string(593) "<p>Олеся В. Паина<sup>1</sup>, Ирина Е. Павлова<sup>1,2</sup>, Наталья Е. Иванова<sup>1</sup>, Александр Л. Алянский<sup>1</sup>, Татьяна А. Быкова<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup>, Александр Д. Кулагин<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев<sup>1</sup></span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(463) "
Олеся В. Паина1, Ирина Е. Павлова1,2, Наталья Е. Иванова1, Александр Л. Алянский1, Татьяна А. Быкова1, Людмила С. Зубаровская1, Александр Д. Кулагин1, Борис В. Афанасьев1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27374" ["VALUE"]=> array(2) { ["TEXT"]=> string(703) "<p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br> <sup>2</sup> Российский научно-исследовательский институт гематологии и трансфузиологии Федерального медико-биологического агентства, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(661) "1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Российский научно-исследовательский институт гематологии и трансфузиологии Федерального медико-биологического агентства, Санкт-Петербург, Россия
Донор-специфические анти-HLA антитела (ДСА) могут являться важным фактором риска недостаточного приживления HLA-гаплосовместимых донорских гемопоэтических клеток (ГСК). Цель настоящего исследования – определить частоту выявления донор-специфических анти-HLA антител у реципиентов перед проведением гаплосовместимой трансплантации ГСК (ТГСК) с помощью метода иммуноферментного анализа и установить взаимосвязь наличия ДСА с первичным неприживлением трансплантата и (или) гипофункцией трансплантата у детей, перенесших ГТГСК.
В работе представлены результаты пилотного ретроспективного одноцентрового исследования. Обследованы 40 пациентов детского возраста, медиана возраста составила 8,5 лет (1 год – 17 лет), которым были выполнены гаплосовместимые трансплантации неманипулированных ГСК в клинике НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой (22-M, 18-ж): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. Средний возраст пациентов составил 8,5 лет (1-17 лет). Медианы общего числа трансплантированных ядросодержащих и CD34+ клеток составляли 8,5×108/кг (2,3-17,9×108/кг) и 7,6×106/кг (2,3-8,2×106/кг) соответственно. Во всех случаях источником CD34+ клеток был стимулированный неманипулированный костный мозг. Определение ДСА в сыворотке крови реципиентов выполнено с использованием коммерческого набора (XMatch®, Protrans), который позволяет определять антитела против донорских антигенов HLA- класса I и класса II отдельно в перекрестном сопоставлении ELISA de facto. Среди всех обследованных детей у 3-х пациентов (7,5%) выявлены ДСА, в то время как у подавляющего большинства (n=37; 92,5%) ДСА не было, во всех случаях ДСА были направлены против антигенов HLA класса II. Сравнительный анализ результатов показывает, что у пациентов с наличием ДСА до гаплосовместимой ТГСК более вероятно развитие первичной недостаточности трансплантата или гипофункции по сравнению с группой реципиентов без DSA. У всех 3 пациентов (100%) с обнаруженными антителами была диагностирована либо первичная недостаточность трансплантата (n=1, 33%), либо гипофункция трансплантата (n=2, 67%). В группе пациентов без ДСА первичная недостаточность трансплантата или гипофункция трансплантата наблюдались в 5 (13,5%) и 1 (2,7%) случаях соответственно, всего в 6 случаях (16,2%). Внедрение DSA-тестирования в рутинную практику у реципиентов перед гапло-ТГСК оптимизирует выбор донора, а также выберет группу потенциальных реципиентов, которым необходимо пройти курс лечения в предтрансплантационный период для десенсибилизации.
Ключевые слова
Донор-специфические антитела, трансплантация гемопоэтических стволовых клеток, HLA-гаплосовместимые доноры, HLA-гаплоидентичные доноры.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27376" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-53-58" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-53-58" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27379" ["VALUE"]=> array(2) { ["TEXT"]=> string(475) "<p>Olesya V. Paina<sup>1</sup>, Irina E. Pavlova<sup>1,2</sup>, Natalia E. Ivanova<sup>1</sup>, Alexander L. Alyanskiy<sup>1</sup>, Tatiana A. Bykova<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Alexander D. Kulagin<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev<sup>1</sup></span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(345) "Olesya V. Paina1, Irina E. Pavlova1,2, Natalia E. Ivanova1, Alexander L. Alyanskiy1, Tatiana A. Bykova1, Ludmila S. Zubarovskaya1, Alexander D. Kulagin1, Boris V. Afanasyev1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27380" ["VALUE"]=> array(2) { ["TEXT"]=> string(607) "<p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2<sup>nd</sup> Sovetskaya St 16, 191024, St. Petersburg, Russia<br> Phone: +7 (921) 983 6664<br> E-mail: dr_pavlova_irina@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(505) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
2 Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St 16, 191024, St. Petersburg, Russia
Phone: +7 (921) 983 6664
E-mail: dr_pavlova_irina@mail.ru
Donor-specific antibodies (DSA) have been recently recognized as an important risk factor for the failing engraftment of HLA-haploidentical donor cells. In this study, we aimed to determine the frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay and establishing an association between the DSA presence and primary graft failure and/or graft hypofunction in children after haplo-HSCT. 40 patients have been tested (22-M, 18-F): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. The median age of the patients was 8.5 years (1-17 years). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×108/kg (2.3-17.9×108/kg) and 7.6×106/kg (2.3-8.2×106/kg) respectively. The donor bone marrow was the source of stem cells in all cases. Detection of DSA has been performed by using the commercial kit (XMatch®, Protrans) that allows determining antibodies against donor's HLA class I and class II separately in the ELISA de facto crossmatching. Among all the examined children, 3 patients (7.5%) were found to be positive for DSA, while the vast majority (n=37; 92.5%) had no detectable DSA. It was also found that in all cases DSA were directed against HLA class II antigens.
Comparative analysis of the results shows that patients with DSA before haplo-HSCT are more likely to develop primary graft failure or hypofunction compared to the group of recipients without DSA. All 3 patients with detected antibodies were diagnosed with either primary graft failure (n=1, 33%) or graft hypofunction (n=2, 67%). In the control group of patients without DSA primary graft failure or graft hypofunction were observed in 5 (13.5%) and 1 (2.7%) cases respectively, with a total of 6 cases (16.2%). Implementation of DSA-testing into routine practice in recipients before haplo-HSCT will optimize the choice of a donor, as well as select a group of potential recipients who need to be treated in the pre-transplant period for desensitization.
Keywords
Donor-specific antibodies, hematopoietic stem cell transplantation, HLA-haplocompatible donors, HLA-haploidentical donors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27377" ["VALUE"]=> string(159) "Donor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(159) "Donor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> &array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27378" ["VALUE"]=> string(4) "2339" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2339" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27382" ["VALUE"]=> string(4) "2340" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2340" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27379" ["VALUE"]=> array(2) { ["TEXT"]=> string(475) "<p>Olesya V. Paina<sup>1</sup>, Irina E. Pavlova<sup>1,2</sup>, Natalia E. Ivanova<sup>1</sup>, Alexander L. Alyanskiy<sup>1</sup>, Tatiana A. Bykova<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Alexander D. Kulagin<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev<sup>1</sup></span> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(345) "Olesya V. Paina1, Irina E. Pavlova1,2, Natalia E. Ivanova1, Alexander L. Alyanskiy1, Tatiana A. Bykova1, Ludmila S. Zubarovskaya1, Alexander D. Kulagin1, Boris V. Afanasyev1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(345) "Olesya V. Paina1, Irina E. Pavlova1,2, Natalia E. Ivanova1, Alexander L. Alyanskiy1, Tatiana A. Bykova1, Ludmila S. Zubarovskaya1, Alexander D. Kulagin1, Boris V. Afanasyev1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27381" ["VALUE"]=> array(2) { ["TEXT"]=> string(2415) "<p style="text-align: justify;">Donor-specific antibodies (DSA) have been recently recognized as an important risk factor for the failing engraftment of HLA-haploidentical donor cells. In this study, we aimed to determine the frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay and establishing an association between the DSA presence and primary graft failure and/or graft hypofunction in children after haplo-HSCT. 40 patients have been tested (22-M, 18-F): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. The median age of the patients was 8.5 years (1-17 years). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×10<sup>8</sup>/kg (2.3-17.9×10<sup>8</sup>/kg) and 7.6×10<sup>6</sup>/kg (2.3-8.2×10<sup>6</sup>/kg) respectively. The donor bone marrow was the source of stem cells in all cases. Detection of DSA has been performed by using the commercial kit (XMatch®, Protrans) that allows determining antibodies against donor's HLA class I and class II separately in the ELISA de facto crossmatching. Among all the examined children, 3 patients (7.5%) were found to be positive for DSA, while the vast majority (n=37; 92.5%) had no detectable DSA. It was also found that in all cases DSA were directed against HLA class II antigens.</p> <p style="text-align: justify;">Comparative analysis of the results shows that patients with DSA before haplo-HSCT are more likely to develop primary graft failure or hypofunction compared to the group of recipients without DSA. All 3 patients with detected antibodies were diagnosed with either primary graft failure (n=1, 33%) or graft hypofunction (n=2, 67%). In the control group of patients without DSA primary graft failure or graft hypofunction were observed in 5 (13.5%) and 1 (2.7%) cases respectively, with a total of 6 cases (16.2%). Implementation of DSA-testing into routine practice in recipients before haplo-HSCT will optimize the choice of a donor, as well as select a group of potential recipients who need to be treated in the pre-transplant period for desensitization.</p> <h2>Keywords</h2> <p style="text-align: justify;">Donor-specific antibodies, hematopoietic stem cell transplantation, HLA-haplocompatible donors, HLA-haploidentical donors.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2289) "Donor-specific antibodies (DSA) have been recently recognized as an important risk factor for the failing engraftment of HLA-haploidentical donor cells. In this study, we aimed to determine the frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay and establishing an association between the DSA presence and primary graft failure and/or graft hypofunction in children after haplo-HSCT. 40 patients have been tested (22-M, 18-F): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. The median age of the patients was 8.5 years (1-17 years). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×108/kg (2.3-17.9×108/kg) and 7.6×106/kg (2.3-8.2×106/kg) respectively. The donor bone marrow was the source of stem cells in all cases. Detection of DSA has been performed by using the commercial kit (XMatch®, Protrans) that allows determining antibodies against donor's HLA class I and class II separately in the ELISA de facto crossmatching. Among all the examined children, 3 patients (7.5%) were found to be positive for DSA, while the vast majority (n=37; 92.5%) had no detectable DSA. It was also found that in all cases DSA were directed against HLA class II antigens.
Comparative analysis of the results shows that patients with DSA before haplo-HSCT are more likely to develop primary graft failure or hypofunction compared to the group of recipients without DSA. All 3 patients with detected antibodies were diagnosed with either primary graft failure (n=1, 33%) or graft hypofunction (n=2, 67%). In the control group of patients without DSA primary graft failure or graft hypofunction were observed in 5 (13.5%) and 1 (2.7%) cases respectively, with a total of 6 cases (16.2%). Implementation of DSA-testing into routine practice in recipients before haplo-HSCT will optimize the choice of a donor, as well as select a group of potential recipients who need to be treated in the pre-transplant period for desensitization.
Keywords
Donor-specific antibodies, hematopoietic stem cell transplantation, HLA-haplocompatible donors, HLA-haploidentical donors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2289) "Donor-specific antibodies (DSA) have been recently recognized as an important risk factor for the failing engraftment of HLA-haploidentical donor cells. In this study, we aimed to determine the frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay and establishing an association between the DSA presence and primary graft failure and/or graft hypofunction in children after haplo-HSCT. 40 patients have been tested (22-M, 18-F): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. The median age of the patients was 8.5 years (1-17 years). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×108/kg (2.3-17.9×108/kg) and 7.6×106/kg (2.3-8.2×106/kg) respectively. The donor bone marrow was the source of stem cells in all cases. Detection of DSA has been performed by using the commercial kit (XMatch®, Protrans) that allows determining antibodies against donor's HLA class I and class II separately in the ELISA de facto crossmatching. Among all the examined children, 3 patients (7.5%) were found to be positive for DSA, while the vast majority (n=37; 92.5%) had no detectable DSA. It was also found that in all cases DSA were directed against HLA class II antigens.
Comparative analysis of the results shows that patients with DSA before haplo-HSCT are more likely to develop primary graft failure or hypofunction compared to the group of recipients without DSA. All 3 patients with detected antibodies were diagnosed with either primary graft failure (n=1, 33%) or graft hypofunction (n=2, 67%). In the control group of patients without DSA primary graft failure or graft hypofunction were observed in 5 (13.5%) and 1 (2.7%) cases respectively, with a total of 6 cases (16.2%). Implementation of DSA-testing into routine practice in recipients before haplo-HSCT will optimize the choice of a donor, as well as select a group of potential recipients who need to be treated in the pre-transplant period for desensitization.
Keywords
Donor-specific antibodies, hematopoietic stem cell transplantation, HLA-haplocompatible donors, HLA-haploidentical donors.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27376" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-53-58" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-53-58" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-53-58" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27377" ["VALUE"]=> string(159) "Donor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(159) "Donor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(159) "Donor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27380" ["VALUE"]=> array(2) { ["TEXT"]=> string(607) "<p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br> <sup>2</sup> Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2<sup>nd</sup> Sovetskaya St 16, 191024, St. Petersburg, Russia<br> Phone: +7 (921) 983 6664<br> E-mail: dr_pavlova_irina@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(505) "1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
2 Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St 16, 191024, St. Petersburg, Russia
Phone: +7 (921) 983 6664
E-mail: dr_pavlova_irina@mail.ru
1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
2 Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St 16, 191024, St. Petersburg, Russia
Phone: +7 (921) 983 6664
E-mail: dr_pavlova_irina@mail.ru
Олеся В. Паина1, Ирина Е. Павлова1,2, Наталья Е. Иванова1, Александр Л. Алянский1, Татьяна А. Быкова1, Людмила С. Зубаровская1, Александр Д. Кулагин1, Борис В. Афанасьев1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(463) "Олеся В. Паина1, Ирина Е. Павлова1,2, Наталья Е. Иванова1, Александр Л. Алянский1, Татьяна А. Быкова1, Людмила С. Зубаровская1, Александр Д. Кулагин1, Борис В. Афанасьев1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27371" ["VALUE"]=> string(22) "09/02/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "09/02/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "09/02/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27372" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/04/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27375" ["VALUE"]=> array(2) { ["TEXT"]=> string(5034) "<p style="text-align: justify;">Донор-специфические анти-HLA антитела (ДСА) могут являться важным фактором риска недостаточного приживления HLA-гаплосовместимых донорских гемопоэтических клеток (ГСК). Цель настоящего исследования – определить частоту выявления донор-специфических анти-HLA антител у реципиентов перед проведением гаплосовместимой трансплантации ГСК (ТГСК) с помощью метода иммуноферментного анализа и установить взаимосвязь наличия ДСА с первичным неприживлением трансплантата и (или) гипофункцией трансплантата у детей, перенесших ГТГСК.</p> <p style="text-align: justify;">В работе представлены результаты пилотного ретроспективного одноцентрового исследования. Обследованы 40 пациентов детского возраста, медиана возраста составила 8,5 лет (1 год – 17 лет), которым были выполнены гаплосовместимые трансплантации неманипулированных ГСК в клинике НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой (22-M, 18-ж): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. Средний возраст пациентов составил 8,5 лет (1-17 лет). Медианы общего числа трансплантированных ядросодержащих и CD34+ клеток составляли 8,5×10<sup>8</sup>/кг (2,3-17,9×10<sup>8</sup>/кг) и 7,6×10<sup>6</sup>/кг (2,3-8,2×10<sup>6</sup>/кг) соответственно. Во всех случаях источником CD34+ клеток был стимулированный неманипулированный костный мозг. Определение ДСА в сыворотке крови реципиентов выполнено с использованием коммерческого набора (XMatch®, Protrans), который позволяет определять антитела против донорских антигенов HLA- класса I и класса II отдельно в перекрестном сопоставлении ELISA de facto. Среди всех обследованных детей у 3-х пациентов (7,5%) выявлены ДСА, в то время как у подавляющего большинства (n=37; 92,5%) ДСА не было, во всех случаях ДСА были направлены против антигенов HLA класса II. Сравнительный анализ результатов показывает, что у пациентов с наличием ДСА до гаплосовместимой ТГСК более вероятно развитие первичной недостаточности трансплантата или гипофункции по сравнению с группой реципиентов без DSA. У всех 3 пациентов (100%) с обнаруженными антителами была диагностирована либо первичная недостаточность трансплантата (n=1, 33%), либо гипофункция трансплантата (n=2, 67%). В группе пациентов без ДСА первичная недостаточность трансплантата или гипофункция трансплантата наблюдались в 5 (13,5%) и 1 (2,7%) случаях соответственно, всего в 6 случаях (16,2%). Внедрение DSA-тестирования в рутинную практику у реципиентов перед гапло-ТГСК оптимизирует выбор донора, а также выберет группу потенциальных реципиентов, которым необходимо пройти курс лечения в предтрансплантационный период для десенсибилизации.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Донор-специфические антитела, трансплантация гемопоэтических стволовых клеток, HLA-гаплосовместимые доноры, HLA-гаплоидентичные доноры.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4908) "Донор-специфические анти-HLA антитела (ДСА) могут являться важным фактором риска недостаточного приживления HLA-гаплосовместимых донорских гемопоэтических клеток (ГСК). Цель настоящего исследования – определить частоту выявления донор-специфических анти-HLA антител у реципиентов перед проведением гаплосовместимой трансплантации ГСК (ТГСК) с помощью метода иммуноферментного анализа и установить взаимосвязь наличия ДСА с первичным неприживлением трансплантата и (или) гипофункцией трансплантата у детей, перенесших ГТГСК.
В работе представлены результаты пилотного ретроспективного одноцентрового исследования. Обследованы 40 пациентов детского возраста, медиана возраста составила 8,5 лет (1 год – 17 лет), которым были выполнены гаплосовместимые трансплантации неманипулированных ГСК в клинике НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой (22-M, 18-ж): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. Средний возраст пациентов составил 8,5 лет (1-17 лет). Медианы общего числа трансплантированных ядросодержащих и CD34+ клеток составляли 8,5×108/кг (2,3-17,9×108/кг) и 7,6×106/кг (2,3-8,2×106/кг) соответственно. Во всех случаях источником CD34+ клеток был стимулированный неманипулированный костный мозг. Определение ДСА в сыворотке крови реципиентов выполнено с использованием коммерческого набора (XMatch®, Protrans), который позволяет определять антитела против донорских антигенов HLA- класса I и класса II отдельно в перекрестном сопоставлении ELISA de facto. Среди всех обследованных детей у 3-х пациентов (7,5%) выявлены ДСА, в то время как у подавляющего большинства (n=37; 92,5%) ДСА не было, во всех случаях ДСА были направлены против антигенов HLA класса II. Сравнительный анализ результатов показывает, что у пациентов с наличием ДСА до гаплосовместимой ТГСК более вероятно развитие первичной недостаточности трансплантата или гипофункции по сравнению с группой реципиентов без DSA. У всех 3 пациентов (100%) с обнаруженными антителами была диагностирована либо первичная недостаточность трансплантата (n=1, 33%), либо гипофункция трансплантата (n=2, 67%). В группе пациентов без ДСА первичная недостаточность трансплантата или гипофункция трансплантата наблюдались в 5 (13,5%) и 1 (2,7%) случаях соответственно, всего в 6 случаях (16,2%). Внедрение DSA-тестирования в рутинную практику у реципиентов перед гапло-ТГСК оптимизирует выбор донора, а также выберет группу потенциальных реципиентов, которым необходимо пройти курс лечения в предтрансплантационный период для десенсибилизации.
Ключевые слова
Донор-специфические антитела, трансплантация гемопоэтических стволовых клеток, HLA-гаплосовместимые доноры, HLA-гаплоидентичные доноры.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4908) "Донор-специфические анти-HLA антитела (ДСА) могут являться важным фактором риска недостаточного приживления HLA-гаплосовместимых донорских гемопоэтических клеток (ГСК). Цель настоящего исследования – определить частоту выявления донор-специфических анти-HLA антител у реципиентов перед проведением гаплосовместимой трансплантации ГСК (ТГСК) с помощью метода иммуноферментного анализа и установить взаимосвязь наличия ДСА с первичным неприживлением трансплантата и (или) гипофункцией трансплантата у детей, перенесших ГТГСК.
В работе представлены результаты пилотного ретроспективного одноцентрового исследования. Обследованы 40 пациентов детского возраста, медиана возраста составила 8,5 лет (1 год – 17 лет), которым были выполнены гаплосовместимые трансплантации неманипулированных ГСК в клинике НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой (22-M, 18-ж): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. Средний возраст пациентов составил 8,5 лет (1-17 лет). Медианы общего числа трансплантированных ядросодержащих и CD34+ клеток составляли 8,5×108/кг (2,3-17,9×108/кг) и 7,6×106/кг (2,3-8,2×106/кг) соответственно. Во всех случаях источником CD34+ клеток был стимулированный неманипулированный костный мозг. Определение ДСА в сыворотке крови реципиентов выполнено с использованием коммерческого набора (XMatch®, Protrans), который позволяет определять антитела против донорских антигенов HLA- класса I и класса II отдельно в перекрестном сопоставлении ELISA de facto. Среди всех обследованных детей у 3-х пациентов (7,5%) выявлены ДСА, в то время как у подавляющего большинства (n=37; 92,5%) ДСА не было, во всех случаях ДСА были направлены против антигенов HLA класса II. Сравнительный анализ результатов показывает, что у пациентов с наличием ДСА до гаплосовместимой ТГСК более вероятно развитие первичной недостаточности трансплантата или гипофункции по сравнению с группой реципиентов без DSA. У всех 3 пациентов (100%) с обнаруженными антителами была диагностирована либо первичная недостаточность трансплантата (n=1, 33%), либо гипофункция трансплантата (n=2, 67%). В группе пациентов без ДСА первичная недостаточность трансплантата или гипофункция трансплантата наблюдались в 5 (13,5%) и 1 (2,7%) случаях соответственно, всего в 6 случаях (16,2%). Внедрение DSA-тестирования в рутинную практику у реципиентов перед гапло-ТГСК оптимизирует выбор донора, а также выберет группу потенциальных реципиентов, которым необходимо пройти курс лечения в предтрансплантационный период для десенсибилизации.
Ключевые слова
Донор-специфические антитела, трансплантация гемопоэтических стволовых клеток, HLA-гаплосовместимые доноры, HLA-гаплоидентичные доноры.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27374" ["VALUE"]=> array(2) { ["TEXT"]=> string(703) "<p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br> <sup>2</sup> Российский научно-исследовательский институт гематологии и трансфузиологии Федерального медико-биологического агентства, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(661) "1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Российский научно-исследовательский институт гематологии и трансфузиологии Федерального медико-биологического агентства, Санкт-Петербург, Россия
1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Российский научно-исследовательский институт гематологии и трансфузиологии Федерального медико-биологического агентства, Санкт-Петербург, Россия
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a potentially curative therapy for hematological diseases. Sustained engraftment of the donor stem cells is essential for transplant success and overall favourable outcomes. Graft failure (GF) is an uncommon (incidence 5-6%) complication post-allo-HCT and is associated with a poor prognosis, especially in allo-HCT for malignant disorders [1]. There are differences in the definition of primary and secondary graft failure [1-10]. Numerous risk factors have been previously associated with GF such as myelofibrosis (MF), aplastic anemia (AA), bone marrow as a graft source or donor HLA mismatch, while other factors such as ABO mismatch, graft-versus-host disease prophylaxis or infections, particularly viral reactivation, remain a matter of debate [11]. The incidence of GF may increase to 20-25% with the use of alternative modalities of HCT, including non-myeloablative conditioning, intensive T-cell depletion of the graft, human leukocyte antigen (HLA) disparity between donor and recipient or cord blood as the progenitor source [1, 11]. The use of filgrastim-mobilized peripheral blood (PB) stem cells instead of bone marrow (BM) decreases the risk of GF, especially in unrelated allo-HCT [8]. Many factors have been proposed to be involved in the etiology of GF, including defects in the BM microenvironment, immune-mediated rejection, drug toxicity or viral infections [12, 13].
The management of GF includes the administration of growth factor, additional hematopoietic progenitor boost or a second allo-HCT with conditioning therapy [11, 13]. Data available on patient outcomes after the development of GF are limited and heterogeneous. Strategies for reversing GF depend on the options available in each situation, and there is no clear recommendation for the best approach to this complication. Some reports have suggested that a second transplant could benefit patients who develop GF [14]. However, transplant-related mortality compromises the overall survival (OS) of these patients. In the present study, we assessed the incidence and the risk factors for GF in a single-center population and the impact on the patients’ outcome.
Patients and methods
Between January 1, 2015, and December 31, 2018, 557 patients underwent allo-HCT at the Princess Margaret Cancer Center. Data was collected retrospectively and updated in June 2019. Cases were included regardless of the underlying diagnosis, disease status prior to transplant, preparative regimen, or stem cell source. Acute (aGvHD) and chronic GvHD (cGvHD) were diagnosed and graded using the aGvHD consensus conference criteria and the NIH consensus criteria for cGvHD, respectively [15, 16]. Conditioning therapy was considered as nonmyeloablative when patients received busulfan < 9 mg/kg, and total body irradiation ≤500 cGy as a single fraction or ≤800 cGy if fractionated. All patients received granulocyte colony-stimulating factor from day +6 following transplant until neutrophil engraftment.
Primary graft failure was defined as the failure to achieve an absolute neutrophil count (ANC) of 0.5×109/L by 28 days after BM or PB [17]. Secondary graft failure was defined as sustained fall in ANC <0.5×109/L after initial engraftment, and one of the following: (a) donor chimerism of less than 5%, or (b) intervention such as use of DLI or second transplant for falling blood counts, or (c) patient death due to cytopenia, with falling donor chimerism, but level of donor chimerism >5% but <95% [1, 17]. Decrease in peripheral blood counts due to relapse of disease was excluded as a cause of GF. Outcomes examined included overall survival (OS), the cumulative incidence of GF and non-relapse mortality (NRM) as well as the cause of death.
Patients were managed clinically according to Princess Margaret Cancer Center guidelines. BM aspirates were monitored for disease status and donor chimerism was assessed in sex-mismatched donor–recipient pairs by metaphase karyotype analyses; restriction fragment length DNA polymorphisms were compared in sex-matched pairs; whole blood chimerism was assay for the duration of this study.
This study was approved by the institutional research ethics board of our center, and consent had been obtained from all the patients for transplant procedures and sharing data following local policies.
Overall survival (OS) was calculated using the Kaplan-Meier method and compared with the log-rank test. Survival time was calculated from the day of first transplantation until death or last follow-up. Incidences of graft failure (GF) were obtained using an estimator of cumulative incidence curves. Patients were censored at the time of death or last follow-up. Competing events for GF were death or relapse without GF. For SGF, PGF was also considered as a competing event.
Uni- and multivariate predictive analyses for GF were performed with the proportional sub-distribution hazard regression model of Fine and Gray [18]. Factors with a p-value <0.10 in the univariate analysis were included in the backwards elimination multivariate analysis.
Analyses were performed using the EZR freely available software and Statistica 13 (TIBCO, Palo Alto, CA, USA) software [19].
Results
Baseline characteristics are summarized in Table 1. Pretransplant therapies where indicated for hematological malignancies consisted of current North American induction protocols: for ALL, Dana Farber Cancer Institute regimen for ALL (1 patient received blinatumomab); for AML either daunorubicin and cytarabine (‘3+7’) or FLAG-Ida; for MDS/MPN supportive care, azacytidine, hydroxyurea or ‘3+7’; ruxolitinib for MF, ibrutinib for CLL, and standard salvage regimens (Bendamustine/rituximab or ‘ICE’) for non-Hodgkin lymphoma.
Table 1. Patients’ characteristics and main factors contributing to GF

Abbreviations: MDS, myelodysplasia; MF, myelofibrosis; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MUD, matched unrelated donor; MRD, matched related donor; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; PTCY, post-transplant cyclophosphamide; ATG, anti-thymocyte globulin; MMF, myecophenolate; CSA, cyclosporine; MTX, methotrexate; CMV, cytomegalovirus; BM, bone marrow; PBSC, peripheral blood stem cells; BSI, bloodstream infections; HCT-CI, hematopoietic stem cell transplant co-morbidity index; KPS, Karnofsky performance score
The cumulative incidence of relapse (CIR) for the entire cohort (n=557) was 7.9% (CI95%; 5.9-10.3) at 100 days and 24.3% (CI95%; 20.6-28.1) at 2 years. In particular, the CIR for patients transplanted for AML (n=284) was 7.7% (CI95%; 5.0-11.2) at 100 days and 21.4% (CI95%; 16.7-26.6) at 2 years, whereas it was 14.6% (CI95%; 5.8-27.2) at 100 days and 45.0% (CI95%; 27.9-60.6%) at 2 years in patients transplanted for ALL (n=41). For MF (n=47), CIR was 2.1% (CI95%; 0.2-9.9) at 100 days and 10.2% (CI95%; 2.9-22.8) at 2 years, CIR for MDS/MPN (n=102) was 11.8% (CI95%; 6.4-18.9) at 100 days and 30.4% CI95%; (21.5-39.8) 2 years. Finally, CIR of patients transplanted for Lymphoma (n=31) was 6.5% (CI95%; 1.1-18.9) at 100 days, and 36.6% (CI95%; 19.6-53.8) at 2 years.
Median survival following PGF was 41 days, while in SGF it was 144 days (Figure 1b). The one hundred days OS in PGF was 22%, whereas it was 64% for SGF. One-year and two-year OS for SGF were 33% and 28%, respectively, Figure 1b. Survival of GF patients was calculated from the date of GF.
Percentage donor chimerism (Figure 1c), was as follows: for primary graft failure, at day 30 post-transplant, n=9, median 19.9% (0.8-75.7%), at day 60 post-transplant, n=5, median 0% (0-27%), at day 90 post-transplant, n=3, median 1% (0-1%). For secondary graft failure, at day 30 post-transplant, n=33, median 96.85% (70.4-100%) (missing data for one patient), at day 60 post-transplant, n=32, median 87.65% (0-99.3%), at day 90 post-transplant, n=24, median 74.15% (3.2-98.2%).

Figure 1. A, cumulative incidence to primary and secondary graft failure (GF). Numbers represent median number of days to graft failure diagnosis with range. B, median survival from primary and secondary graft failure. C, percentage donor chimerism in patients with primary and secondary graft failure. Bars represent the inter-quartile range, and round circles, the median. The percentage donor chimerism at day 30 (D30), day 60 (D60) and day 90 (D90) post-transplant are represented. D, overall survival from allogeneic stem cell transplant for the whole cohort, and patients with GF. Survival of GF patients was calculated from date of GF.
Percentage donor chimerism (Figure 1c), was as follows: for primary graft failure, at day 30 post-transplant, n=9, median 19.9% (0.8-75.7%), at day 60 post-transplant, n=5, median 0% (0-27%), at day 90 post-transplant, n=3, median 1% (0-1%). For secondary graft failure, at day 30 post-transplant, n=33, median 96.85% (70.4-100%) (missing data for one patient), at day 60 post-transplant, n=32, median 87.65% (0-99.3%), at day 90 post-transplant, n=24, median 74.15% (3.2-98.2%).
Table 2. Results of univariate analysis


Abbreviations: MAC, myeloablative conditioning; RIC, reduced intensity conditioning; PTCY, post-transplant cyclophosphamide; ATG, anti-thymocyteglobulin; BM, bone marrow; PBSC, peripheral blood stem cells; BSI, bloodstream infection; HCT-CI, hematopoietic stem cell transplant co-morbidity index; KPS, Karnofsky performance score; SCT, stem cell transplant; CR, complete remission
DLI was administered to nine patients with SGF. Five of these experienced grade I-II acute GvHD, and two, grade III-IV acute GvHD. Two subsequently experienced moderately severe GvHD. Of these nine patients, one experienced relapse and died, another demised with post-transplant lymphoproliferative disorder, two with GvHD, and ultimately 5 were alive at last follow-up.
Second allo-HCT was performed in 5 patients (56%) with PGF and 15 patients (44%) with SGF (see Table 4). Another 8 patients with SGF received donor lymphocyte infusions (DLI). The primary complication following second allo-HCT was infection. Median time from GF diagnosis to second allo-HCT was 35 days (20-172), whilst median time from GF diagnosis to DLI was 90 days (7-288). Six out of 15 patients who received a second transplant for SGF developed Grade 3/4 acute GvHD and 4 out of these patients developed moderately severe chronic GvHD.
Median survival (Figure 1d) post second transplant was 109 days (9-1014); survival was calculated from second HCT. Median survival post DLI was 293 days (22-868).
Overall, all the patients with PGF died because of GF or complications related to it. In SGF 22 of 43 patients (51%) died, the most common causes of death were infections (30%), GvHD (18%) and one patient died related to veno-occlusive disease. The median neutrophil count for the SGF patients that died in GF was 0 ×109/L (0-0.5×109/L) and median platelet count was 6 ×109/L (3-41×109/L).
Table 3. Results of multivariate analysis for factors associated with GF

Table 4. Characteristics of patients who underwent a second transplant for GF

Abbreviations: GF, graft failure; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MF, myelofibrosis; MDS, myelodysplasia; MPN, myeloproliferative neoplasm; MUD, matched unrelated donor; GvHD, graft versus host disease
Factors associated with graft failure on univariate analysis are shown in Table 2. Diagnoses associated with GF were MDS (p=0.04), MF (p=0.005), lymphoma (p=0.002) and non-malignant conditions (aplastic anemia, adrenoleukodystrophy, mitochondrial neurogastrointestinal encephalopathy syndrome) (p<0.001). Other significant factors were incomplete remissions prior to allo-HCT (p=0.01), mismatched unrelated donors (p=0.002) or haploidentical donors (p=0.004), use of BM as graft source (p=0.008). CMV mismatch, CD34 stem cell dose, and freezing of the stem cell product were not significant factors.
Multivariate analysis was undertaken with the following variables: diagnosis, age, donor type, sex, disease stage at allo-HCT, donor age, stem cell source, CD34 dose, stem cells frozen or fresh, myeloablative or reduced-intensity conditioning, use of post-transplant cyclophosphamide, use of anti-thymocyte globulin as GvHD prophylaxis, presence of CMV serological mismatch, presence of bloodstream infection before D+20 post-allo-HCT, primary disease, disease risk index, Karnofsky performance score (KPS) at allo-HCT, HCT-CI (hematopoietic cell transplant co-morbidity index20) score, presence of donor and recipient of blood group mismatch, and time from diagnosis to transplant.
Multivariate analysis of all GF demonstrated that transplant indication (MDS, MF, lymphoma or non-malignant diseases) and donor type (HLA-mismatched unrelated or haploidentical) were the significant factors associated with increased GF (Table 3). For PGF significant factors were: non-malignant disease (HR 114.3 95% confidence interval [CI] [4.53-2881], p<0.004), MF (HR 27.6 [2.84-268], p=0.004), MDS (HR 18.2 [1.99-166], p=0.01) and graft from haploidentical donor (HR 12.5 [3-51.6], p<0.001), Figure 2c. For SGF significant factors were: non-malignant disease (HR 4.31 [1.06-17.5], p=0.04), and lymphoma (HR 4.19 [1.71-10.3], p=0.002), Figure 2d. Taken together, non-leukemia diagnosis and mismatched unrelated or haploidentical donors were significantly associated with graft failure (see Figure 2e).
The effect of more than one of the previously described risk factors (non-leukemia diagnosis and mismatched unrelated or haploidentical donors) on the occurrence of graft failure is shown in Figure 2e. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5%.

Figure 2. A, cumulative incidence of GF according to transplant indication/disease. B, cumulative incidence of GF according to donor type. C, cumulative incidence of primary GF according to the presence of risk factors from multivariable analysis. D, cumulative incidence of secondary GF according to the presence of risk factors from multivariable analysis. E, cumulative incidence of overall GF from multivariable analysis according to the presence of no risk factors (3.6%), 1 risk factor (9.9%) and 2 risk factors (24.5%).
Abbreviations: Haplo, haploidentical; MUD, matched unrelated donor; MMURD, mismatched unrelated donor; MRD, matched related donor; Other Mal, other malignancies; RF, risk factor
Discussion
GF remains a significant clinical problem post allo-HCT. It is important to identify patients who are at risk of GF to potentially limit the number of risk factors for prevention of this high-risk complication. Our study showed an increased risk for GF following the use of mismatched unrelated or haploidentical donors for diseases such as lymphoma, myelofibrosis, myelodysplastic syndrome and non-malignant diseases.
A number of factors found significant in other studies were not significant on multivariate analysis in the present study. Cryopreservation was a significant factor in a large retrospective study on PGF from CIBMTR data, but this was not significant in the present cohort (p=0.79) [6]. This may be due to the more rigorous standardization of cryopreservation and thawing techniques at our center, compared to the multicenter data in the CIBMTR study. Other stated significant factors from the CIBMTR study, including age, bone marrow source, ABO incompatibility and sex-mismatched transplants (male recipients of female grafts) were similarly not significant on multivariate analysis in the present study. This may be related to our limited numbers compared to the 23,272 transplants examined in the CIBMTR study. Both the present study and the CIBMTR study are in concordance that HLA-mismatch and non-leukemic myeloid malignancies are significant risk factors for GF. Possible explanations for the differences in other results include the use of myeloablative conditioning as an inclusion criterion in the CIBMTR study, the use of haploidentical donors at our center, and the exclusive examination of PGF in the CIBMTR study. In support of our findings, another report has similarly identified mismatched donors and non-malignant conditions as significantly associated with GF on multivariate analysis [1]. Other factors also identified in this single-center report which identified GF in 54 (5.6%) patients of 967 transplants undertaken from 1995 to 2010, were non-myeloablative conditioning, total nucleated cell dose <2.4×108/kg, HLA-mismatch and ex vivo T-cell depletion. We analyzed separately effects of CD34 dose above and below the median, as well as stem cell source, but there were no significant associations in all GF or in PGF-only patients.
Treatment strategies for GF vary, while second transplant remains a common option, particularly in our center, as CD34-selected stem cell boost is not available. Using data reported to the National Marrow Donor Program (NMDP) of 14,564 transplants, of which 981 experienced PGF, Schriber, et al. (2010) described 122 patients who received a subsequent second unrelated transplant [21]. One-year OS after the second HCT was 11%, with only 10 patients alive at the last follow-up. The cumulative probability of NRM was high at 39% and 75% at 30 and 100 days, respectively. In this study, engraftment data from 79 patients were included, and the cumulative incidence of neutrophil engraftment at 28 days was 66%. Survival was poor (10%) for patients who received a second allo-HCT; however, the mortality rate was 99% at 1 year in patients not undergoing a second HCT. Only 162 patients out of 981 reported with GF had received a second HCT, of which 122 were from unrelated donors. This could be because the first HCT was from an unrelated donor, making the attainment of a second donation more complicated than from a related donor. However, where donors were available for the second SCT, no differences were observed between patients who received grafts from the same (80%) or a different donor, or if the stem cells had been previously cryopreserved.
In contrast to our findings and that of the CIBMTR study, the MD Anderson group7 reported 68 patients out of 1726 who experienced GF, showing that a diagnosis of acute leukemia was found to be the only prognostic marker for survival on multivariate analysis [7]. A patient was considered to have GF if any of these 3 conditions were met: (1) PGF: failure to achieve ANC of <0.5×109/L by 28 days after BM or PB progenitor cell transplantation or 42 days after cord blood (CB) transplantation, (2) SGF: loss of neutrophil engraftment as determined by an ANC of <0.5×109/L for 3 consecutive days after having achieved neutrophil engraftment with documented donor cell chimerism and no evidence of disease progression in the marrow, or (3) PGF with autologous reconstitution defined as achievement of an ANC of at least 0.5×109/L but without evidence of at least 5% or more donor cell chimerism as defined by cytogenetics or molecular techniques [7]. They reported a 1-, 2- and 5-year OS of 31%, 24% and 15%, respectively. The most common causes of death were original malignancy (41%), infection (27%), and GF (18%). Twenty-nine patients had PGF, with a median survival of 2.9 months, and 7 were re-transplanted, with only 2 surviving over 5 months. 9 patients had GF with autologous reconstitution, and their median survival was 13.7 months. Thirty patients had SGF; 3 patients survived over 4 years. Five of the 30 received a second transplant, but none survived longer than a year. Overall, patients with autologous reconstitution survived longer, but long-term outcomes were similar.
Using different methodology, a Spanish group reported the outcome of 89 patients with GF, irrespective of donor chimerism [2]. This group used the standard criteria for PGF and SGF was defined as a recurrent ANC <0.5×109/L for at least 7 days. They did not state chimerism as a requirement for SGF. Of these, 80 patients received a second allo-HCT, with a 5-year survival probability of 31% (95% confidence interval [CI]: 18-44%), NRM 47% (95% CI 36-58%), and relapse 21% (95% CI 4-28%).
A review of various studies highlights the significant discordance in definitions of SGF: some authors include the presence of donor chimerism in the definition, whilst others do not [1, 2, 4, 6, 7, 8, 9, 10]. Including those cases with low blood counts which may be secondary to causes such as drugs, infection and GvHD may lead to over-diagnosis and possibly inappropriate treatment, given that a proportion of these will be transient and demonstrate recovery of blood counts. This lack of uniformity may hamper common definitions of what constitutes GF [3]. A definition of SGF which requires donor chimerism <5% will exclude patients who had falling chimerism (between 5% and 95%) but insufficient time for this to fall below 5%, or those who died due to cytopenia without documenting <5% donor chimerism. We, therefore, included patients with cytopenia, who had falling donor chimerism (Figure 1c) and died due to complications or received salvage DLI or second transplant. Importantly, we excluded those patients whose cytopenia was due to relapse. We were assiduous in ensuring that the presenting cytopenia were not due to relapse of the underlying disease. This serves to reduce ambiguity and focus resources on those patients in urgent need of salvage from GF. Often, relapsed myelodysplasia or acute leukemia may present with progressive single- or multi-lineage cytopenia, and it is crucial to make every attempt to exclude relapse, either by repeated bone marrow examination or the use of modern techniques for minimal or measurable residual disease by flow cytometry and/or quantitative polymerase chain reaction, as appropriate.
Interestingly, the significant factors for primary and SGF in our cohort were slightly different, which may represent that these types of GF represent different phenomena, with differing mechanisms. This is evidenced by the fact that PGF risks included myelofibrosis, myelodysplasia, myeloproliferative neoplasm, while these were absent from those factors found significant for SGF (lymphoma/non-malignant diagnosis, Figures 2c and 2d).
There are limitations to our analysis. Numbers of patients with primary or SGF are few and therefore the analysis was improved by evaluating all GF together. Nevertheless, analysis within primary and SGF cohorts gave broadly similar results. The inclusion of patients who died with cytopenia in the SGF may not be conventional, but this allowed study of relevant groups which would have otherwise have been excluded.
Conclusion
According to the results from the present study, the prognosis of GF remains poor even after successful treatment of GF, with a few long-term survivors. Those patients with mismatched donors and certain hematological conditions as previously mentioned, should be counselled carefully that the risk of GF may be near 25%, with a mortality rate of approximately 73-89% should it occur. Whereas risk factors such as primary diagnosis are unavoidable, it may be possible to reduce the other risk factors, including careful donor selection. However, most patients with MMUD or haploidentical donors will not have a readily available alternative donor. Measures that could be taken to reduce risk of GF include choice of haploidentical donor, utilizing a more immunosuppressive conditioning regimen or increasing the immunosuppressive component of the GvHD prophylaxis. If appropriate, a myeloablative regimen is more likely to ensure full donor engraftment. Furthermore, careful attention could be paid to the presence of donor-specific antibodies (DSA) and their titers when using MMURD and haploidentical donors. Post-transplant monitoring modification could be implemented, such as lineage-specific chimerism, which our center has recently introduced, and more frequent monitoring of chimerism, e.g. every 30 days with priority for rapid results. More careful use of myelo-suppressive medication such as valganciclovir and co-trimoxazole in these patients is another suggested strategy, with early adoption of alternative therapies in the event of cytopenias. These special precautions could be taken in those cases with 1 or more risk factors as identified in this study. Further studies are required to establish whether close monitoring and early intervention improve outcomes. The optimal treatment strategies for graft failure remain to be established.
Conflicts of interests
None declared.
References
- Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48(4):537-543.
- Ferra C, Sanz J, Diaz-Perez MA, et al. Outcome of graft failure after allogeneic stem cell transplant: study of 89 patients. Leuk Lymphoma. 2015;56(3):656-662.
- Gale RP. Early and late graft-failure after transplants. Bone Marrow Transplant. 2016;51(2):182-183.
- Satwani P, Jin Z, Duffy D, et al. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013;19(4):552-561.
- Ozdemir ZN, Civriz Bozdag S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2018;57(2):163-167.
- Olsson RF, Logan BR, Chaudhury S, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015;29(8):1754-1762.
- Rondon G, Saliba RM, Khouri I, et al. Long-term follow-up of patients who experienced graft failure postallogeneic progenitor cell transplantation. Results of a single institution analysis. Biol Blood Marrow Transplant. 2008;14(8):859-866.
- Passweg JR, Zhang MJ, Rocha V, et al. Donor characteristics affecting graft failure, graft-versus-host disease, and survival after unrelated donor transplantation with reduced-intensity conditioning for hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(12):1869-1873.
- Hutt D. Engraftment, Graft Failure, and Rejection. In: Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses: Under the Auspices of EBMT. Cham (CH)2018:259-270.
- Lund TC, Liegel J, Bejanyan N, et al. Second allogeneic hematopoietic cell transplantation for graft failure: poor outcomes for neutropenic graft failure. Am J Hematol. 2015;90(10):892-896.
- Mattsson J, Ringden O, Storb R. Graft failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(1 Suppl 1):165-170.
- Masouridi-Levrat S, Simonetta F, Chalandon Y. Immunological Basis of Bone Marrow Failure after Allogeneic Hematopoietic Stem Cell Transplantation. Frontiers in immunology. 2016;7:362.
- Wolff SN. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant. 2002;29(7):545-552.
- Stucki A, Leisenring W, Sandmaier BM, Sanders J, Anasetti C, Storb R. Decreased Rejection and Improved Survival of First and Second Marrow Transplants for Severe Aplastic Anemia (A 26-Year Retrospective Analysis). Blood. 1998;92(8):2742-2749.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828.
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401 e381.
- Ferrà C, Sanz J, Díaz-Pérez M-A, et al. Outcome of graft failure after allogeneic stem cell transplant: study of 89 patients. Leukemia & Lymphoma. 2015;56(3):656-662.
- Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496-509.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific co-morbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919.
- Schriber J, Agovi MA, Ho V, et al. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol Blood Marrow Transplant. 2010;16(8):1099-1106.
- Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905-913.
" ["~DETAIL_TEXT"]=> string(32360) "
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a potentially curative therapy for hematological diseases. Sustained engraftment of the donor stem cells is essential for transplant success and overall favourable outcomes. Graft failure (GF) is an uncommon (incidence 5-6%) complication post-allo-HCT and is associated with a poor prognosis, especially in allo-HCT for malignant disorders [1]. There are differences in the definition of primary and secondary graft failure [1-10]. Numerous risk factors have been previously associated with GF such as myelofibrosis (MF), aplastic anemia (AA), bone marrow as a graft source or donor HLA mismatch, while other factors such as ABO mismatch, graft-versus-host disease prophylaxis or infections, particularly viral reactivation, remain a matter of debate [11]. The incidence of GF may increase to 20-25% with the use of alternative modalities of HCT, including non-myeloablative conditioning, intensive T-cell depletion of the graft, human leukocyte antigen (HLA) disparity between donor and recipient or cord blood as the progenitor source [1, 11]. The use of filgrastim-mobilized peripheral blood (PB) stem cells instead of bone marrow (BM) decreases the risk of GF, especially in unrelated allo-HCT [8]. Many factors have been proposed to be involved in the etiology of GF, including defects in the BM microenvironment, immune-mediated rejection, drug toxicity or viral infections [12, 13].
The management of GF includes the administration of growth factor, additional hematopoietic progenitor boost or a second allo-HCT with conditioning therapy [11, 13]. Data available on patient outcomes after the development of GF are limited and heterogeneous. Strategies for reversing GF depend on the options available in each situation, and there is no clear recommendation for the best approach to this complication. Some reports have suggested that a second transplant could benefit patients who develop GF [14]. However, transplant-related mortality compromises the overall survival (OS) of these patients. In the present study, we assessed the incidence and the risk factors for GF in a single-center population and the impact on the patients’ outcome.
Patients and methods
Between January 1, 2015, and December 31, 2018, 557 patients underwent allo-HCT at the Princess Margaret Cancer Center. Data was collected retrospectively and updated in June 2019. Cases were included regardless of the underlying diagnosis, disease status prior to transplant, preparative regimen, or stem cell source. Acute (aGvHD) and chronic GvHD (cGvHD) were diagnosed and graded using the aGvHD consensus conference criteria and the NIH consensus criteria for cGvHD, respectively [15, 16]. Conditioning therapy was considered as nonmyeloablative when patients received busulfan < 9 mg/kg, and total body irradiation ≤500 cGy as a single fraction or ≤800 cGy if fractionated. All patients received granulocyte colony-stimulating factor from day +6 following transplant until neutrophil engraftment.
Primary graft failure was defined as the failure to achieve an absolute neutrophil count (ANC) of 0.5×109/L by 28 days after BM or PB [17]. Secondary graft failure was defined as sustained fall in ANC <0.5×109/L after initial engraftment, and one of the following: (a) donor chimerism of less than 5%, or (b) intervention such as use of DLI or second transplant for falling blood counts, or (c) patient death due to cytopenia, with falling donor chimerism, but level of donor chimerism >5% but <95% [1, 17]. Decrease in peripheral blood counts due to relapse of disease was excluded as a cause of GF. Outcomes examined included overall survival (OS), the cumulative incidence of GF and non-relapse mortality (NRM) as well as the cause of death.
Patients were managed clinically according to Princess Margaret Cancer Center guidelines. BM aspirates were monitored for disease status and donor chimerism was assessed in sex-mismatched donor–recipient pairs by metaphase karyotype analyses; restriction fragment length DNA polymorphisms were compared in sex-matched pairs; whole blood chimerism was assay for the duration of this study.
This study was approved by the institutional research ethics board of our center, and consent had been obtained from all the patients for transplant procedures and sharing data following local policies.
Overall survival (OS) was calculated using the Kaplan-Meier method and compared with the log-rank test. Survival time was calculated from the day of first transplantation until death or last follow-up. Incidences of graft failure (GF) were obtained using an estimator of cumulative incidence curves. Patients were censored at the time of death or last follow-up. Competing events for GF were death or relapse without GF. For SGF, PGF was also considered as a competing event.
Uni- and multivariate predictive analyses for GF were performed with the proportional sub-distribution hazard regression model of Fine and Gray [18]. Factors with a p-value <0.10 in the univariate analysis were included in the backwards elimination multivariate analysis.
Analyses were performed using the EZR freely available software and Statistica 13 (TIBCO, Palo Alto, CA, USA) software [19].
Results
Baseline characteristics are summarized in Table 1. Pretransplant therapies where indicated for hematological malignancies consisted of current North American induction protocols: for ALL, Dana Farber Cancer Institute regimen for ALL (1 patient received blinatumomab); for AML either daunorubicin and cytarabine (‘3+7’) or FLAG-Ida; for MDS/MPN supportive care, azacytidine, hydroxyurea or ‘3+7’; ruxolitinib for MF, ibrutinib for CLL, and standard salvage regimens (Bendamustine/rituximab or ‘ICE’) for non-Hodgkin lymphoma.
Table 1. Patients’ characteristics and main factors contributing to GF

Abbreviations: MDS, myelodysplasia; MF, myelofibrosis; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MUD, matched unrelated donor; MRD, matched related donor; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning; PTCY, post-transplant cyclophosphamide; ATG, anti-thymocyte globulin; MMF, myecophenolate; CSA, cyclosporine; MTX, methotrexate; CMV, cytomegalovirus; BM, bone marrow; PBSC, peripheral blood stem cells; BSI, bloodstream infections; HCT-CI, hematopoietic stem cell transplant co-morbidity index; KPS, Karnofsky performance score
The cumulative incidence of relapse (CIR) for the entire cohort (n=557) was 7.9% (CI95%; 5.9-10.3) at 100 days and 24.3% (CI95%; 20.6-28.1) at 2 years. In particular, the CIR for patients transplanted for AML (n=284) was 7.7% (CI95%; 5.0-11.2) at 100 days and 21.4% (CI95%; 16.7-26.6) at 2 years, whereas it was 14.6% (CI95%; 5.8-27.2) at 100 days and 45.0% (CI95%; 27.9-60.6%) at 2 years in patients transplanted for ALL (n=41). For MF (n=47), CIR was 2.1% (CI95%; 0.2-9.9) at 100 days and 10.2% (CI95%; 2.9-22.8) at 2 years, CIR for MDS/MPN (n=102) was 11.8% (CI95%; 6.4-18.9) at 100 days and 30.4% CI95%; (21.5-39.8) 2 years. Finally, CIR of patients transplanted for Lymphoma (n=31) was 6.5% (CI95%; 1.1-18.9) at 100 days, and 36.6% (CI95%; 19.6-53.8) at 2 years.
Median survival following PGF was 41 days, while in SGF it was 144 days (Figure 1b). The one hundred days OS in PGF was 22%, whereas it was 64% for SGF. One-year and two-year OS for SGF were 33% and 28%, respectively, Figure 1b. Survival of GF patients was calculated from the date of GF.
Percentage donor chimerism (Figure 1c), was as follows: for primary graft failure, at day 30 post-transplant, n=9, median 19.9% (0.8-75.7%), at day 60 post-transplant, n=5, median 0% (0-27%), at day 90 post-transplant, n=3, median 1% (0-1%). For secondary graft failure, at day 30 post-transplant, n=33, median 96.85% (70.4-100%) (missing data for one patient), at day 60 post-transplant, n=32, median 87.65% (0-99.3%), at day 90 post-transplant, n=24, median 74.15% (3.2-98.2%).

Figure 1. A, cumulative incidence to primary and secondary graft failure (GF). Numbers represent median number of days to graft failure diagnosis with range. B, median survival from primary and secondary graft failure. C, percentage donor chimerism in patients with primary and secondary graft failure. Bars represent the inter-quartile range, and round circles, the median. The percentage donor chimerism at day 30 (D30), day 60 (D60) and day 90 (D90) post-transplant are represented. D, overall survival from allogeneic stem cell transplant for the whole cohort, and patients with GF. Survival of GF patients was calculated from date of GF.
Percentage donor chimerism (Figure 1c), was as follows: for primary graft failure, at day 30 post-transplant, n=9, median 19.9% (0.8-75.7%), at day 60 post-transplant, n=5, median 0% (0-27%), at day 90 post-transplant, n=3, median 1% (0-1%). For secondary graft failure, at day 30 post-transplant, n=33, median 96.85% (70.4-100%) (missing data for one patient), at day 60 post-transplant, n=32, median 87.65% (0-99.3%), at day 90 post-transplant, n=24, median 74.15% (3.2-98.2%).
Table 2. Results of univariate analysis


Abbreviations: MAC, myeloablative conditioning; RIC, reduced intensity conditioning; PTCY, post-transplant cyclophosphamide; ATG, anti-thymocyteglobulin; BM, bone marrow; PBSC, peripheral blood stem cells; BSI, bloodstream infection; HCT-CI, hematopoietic stem cell transplant co-morbidity index; KPS, Karnofsky performance score; SCT, stem cell transplant; CR, complete remission
DLI was administered to nine patients with SGF. Five of these experienced grade I-II acute GvHD, and two, grade III-IV acute GvHD. Two subsequently experienced moderately severe GvHD. Of these nine patients, one experienced relapse and died, another demised with post-transplant lymphoproliferative disorder, two with GvHD, and ultimately 5 were alive at last follow-up.
Second allo-HCT was performed in 5 patients (56%) with PGF and 15 patients (44%) with SGF (see Table 4). Another 8 patients with SGF received donor lymphocyte infusions (DLI). The primary complication following second allo-HCT was infection. Median time from GF diagnosis to second allo-HCT was 35 days (20-172), whilst median time from GF diagnosis to DLI was 90 days (7-288). Six out of 15 patients who received a second transplant for SGF developed Grade 3/4 acute GvHD and 4 out of these patients developed moderately severe chronic GvHD.
Median survival (Figure 1d) post second transplant was 109 days (9-1014); survival was calculated from second HCT. Median survival post DLI was 293 days (22-868).
Overall, all the patients with PGF died because of GF or complications related to it. In SGF 22 of 43 patients (51%) died, the most common causes of death were infections (30%), GvHD (18%) and one patient died related to veno-occlusive disease. The median neutrophil count for the SGF patients that died in GF was 0 ×109/L (0-0.5×109/L) and median platelet count was 6 ×109/L (3-41×109/L).
Table 3. Results of multivariate analysis for factors associated with GF

Table 4. Characteristics of patients who underwent a second transplant for GF

Abbreviations: GF, graft failure; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MF, myelofibrosis; MDS, myelodysplasia; MPN, myeloproliferative neoplasm; MUD, matched unrelated donor; GvHD, graft versus host disease
Factors associated with graft failure on univariate analysis are shown in Table 2. Diagnoses associated with GF were MDS (p=0.04), MF (p=0.005), lymphoma (p=0.002) and non-malignant conditions (aplastic anemia, adrenoleukodystrophy, mitochondrial neurogastrointestinal encephalopathy syndrome) (p<0.001). Other significant factors were incomplete remissions prior to allo-HCT (p=0.01), mismatched unrelated donors (p=0.002) or haploidentical donors (p=0.004), use of BM as graft source (p=0.008). CMV mismatch, CD34 stem cell dose, and freezing of the stem cell product were not significant factors.
Multivariate analysis was undertaken with the following variables: diagnosis, age, donor type, sex, disease stage at allo-HCT, donor age, stem cell source, CD34 dose, stem cells frozen or fresh, myeloablative or reduced-intensity conditioning, use of post-transplant cyclophosphamide, use of anti-thymocyte globulin as GvHD prophylaxis, presence of CMV serological mismatch, presence of bloodstream infection before D+20 post-allo-HCT, primary disease, disease risk index, Karnofsky performance score (KPS) at allo-HCT, HCT-CI (hematopoietic cell transplant co-morbidity index20) score, presence of donor and recipient of blood group mismatch, and time from diagnosis to transplant.
Multivariate analysis of all GF demonstrated that transplant indication (MDS, MF, lymphoma or non-malignant diseases) and donor type (HLA-mismatched unrelated or haploidentical) were the significant factors associated with increased GF (Table 3). For PGF significant factors were: non-malignant disease (HR 114.3 95% confidence interval [CI] [4.53-2881], p<0.004), MF (HR 27.6 [2.84-268], p=0.004), MDS (HR 18.2 [1.99-166], p=0.01) and graft from haploidentical donor (HR 12.5 [3-51.6], p<0.001), Figure 2c. For SGF significant factors were: non-malignant disease (HR 4.31 [1.06-17.5], p=0.04), and lymphoma (HR 4.19 [1.71-10.3], p=0.002), Figure 2d. Taken together, non-leukemia diagnosis and mismatched unrelated or haploidentical donors were significantly associated with graft failure (see Figure 2e).
The effect of more than one of the previously described risk factors (non-leukemia diagnosis and mismatched unrelated or haploidentical donors) on the occurrence of graft failure is shown in Figure 2e. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5%.

Figure 2. A, cumulative incidence of GF according to transplant indication/disease. B, cumulative incidence of GF according to donor type. C, cumulative incidence of primary GF according to the presence of risk factors from multivariable analysis. D, cumulative incidence of secondary GF according to the presence of risk factors from multivariable analysis. E, cumulative incidence of overall GF from multivariable analysis according to the presence of no risk factors (3.6%), 1 risk factor (9.9%) and 2 risk factors (24.5%).
Abbreviations: Haplo, haploidentical; MUD, matched unrelated donor; MMURD, mismatched unrelated donor; MRD, matched related donor; Other Mal, other malignancies; RF, risk factor
Discussion
GF remains a significant clinical problem post allo-HCT. It is important to identify patients who are at risk of GF to potentially limit the number of risk factors for prevention of this high-risk complication. Our study showed an increased risk for GF following the use of mismatched unrelated or haploidentical donors for diseases such as lymphoma, myelofibrosis, myelodysplastic syndrome and non-malignant diseases.
A number of factors found significant in other studies were not significant on multivariate analysis in the present study. Cryopreservation was a significant factor in a large retrospective study on PGF from CIBMTR data, but this was not significant in the present cohort (p=0.79) [6]. This may be due to the more rigorous standardization of cryopreservation and thawing techniques at our center, compared to the multicenter data in the CIBMTR study. Other stated significant factors from the CIBMTR study, including age, bone marrow source, ABO incompatibility and sex-mismatched transplants (male recipients of female grafts) were similarly not significant on multivariate analysis in the present study. This may be related to our limited numbers compared to the 23,272 transplants examined in the CIBMTR study. Both the present study and the CIBMTR study are in concordance that HLA-mismatch and non-leukemic myeloid malignancies are significant risk factors for GF. Possible explanations for the differences in other results include the use of myeloablative conditioning as an inclusion criterion in the CIBMTR study, the use of haploidentical donors at our center, and the exclusive examination of PGF in the CIBMTR study. In support of our findings, another report has similarly identified mismatched donors and non-malignant conditions as significantly associated with GF on multivariate analysis [1]. Other factors also identified in this single-center report which identified GF in 54 (5.6%) patients of 967 transplants undertaken from 1995 to 2010, were non-myeloablative conditioning, total nucleated cell dose <2.4×108/kg, HLA-mismatch and ex vivo T-cell depletion. We analyzed separately effects of CD34 dose above and below the median, as well as stem cell source, but there were no significant associations in all GF or in PGF-only patients.
Treatment strategies for GF vary, while second transplant remains a common option, particularly in our center, as CD34-selected stem cell boost is not available. Using data reported to the National Marrow Donor Program (NMDP) of 14,564 transplants, of which 981 experienced PGF, Schriber, et al. (2010) described 122 patients who received a subsequent second unrelated transplant [21]. One-year OS after the second HCT was 11%, with only 10 patients alive at the last follow-up. The cumulative probability of NRM was high at 39% and 75% at 30 and 100 days, respectively. In this study, engraftment data from 79 patients were included, and the cumulative incidence of neutrophil engraftment at 28 days was 66%. Survival was poor (10%) for patients who received a second allo-HCT; however, the mortality rate was 99% at 1 year in patients not undergoing a second HCT. Only 162 patients out of 981 reported with GF had received a second HCT, of which 122 were from unrelated donors. This could be because the first HCT was from an unrelated donor, making the attainment of a second donation more complicated than from a related donor. However, where donors were available for the second SCT, no differences were observed between patients who received grafts from the same (80%) or a different donor, or if the stem cells had been previously cryopreserved.
In contrast to our findings and that of the CIBMTR study, the MD Anderson group7 reported 68 patients out of 1726 who experienced GF, showing that a diagnosis of acute leukemia was found to be the only prognostic marker for survival on multivariate analysis [7]. A patient was considered to have GF if any of these 3 conditions were met: (1) PGF: failure to achieve ANC of <0.5×109/L by 28 days after BM or PB progenitor cell transplantation or 42 days after cord blood (CB) transplantation, (2) SGF: loss of neutrophil engraftment as determined by an ANC of <0.5×109/L for 3 consecutive days after having achieved neutrophil engraftment with documented donor cell chimerism and no evidence of disease progression in the marrow, or (3) PGF with autologous reconstitution defined as achievement of an ANC of at least 0.5×109/L but without evidence of at least 5% or more donor cell chimerism as defined by cytogenetics or molecular techniques [7]. They reported a 1-, 2- and 5-year OS of 31%, 24% and 15%, respectively. The most common causes of death were original malignancy (41%), infection (27%), and GF (18%). Twenty-nine patients had PGF, with a median survival of 2.9 months, and 7 were re-transplanted, with only 2 surviving over 5 months. 9 patients had GF with autologous reconstitution, and their median survival was 13.7 months. Thirty patients had SGF; 3 patients survived over 4 years. Five of the 30 received a second transplant, but none survived longer than a year. Overall, patients with autologous reconstitution survived longer, but long-term outcomes were similar.
Using different methodology, a Spanish group reported the outcome of 89 patients with GF, irrespective of donor chimerism [2]. This group used the standard criteria for PGF and SGF was defined as a recurrent ANC <0.5×109/L for at least 7 days. They did not state chimerism as a requirement for SGF. Of these, 80 patients received a second allo-HCT, with a 5-year survival probability of 31% (95% confidence interval [CI]: 18-44%), NRM 47% (95% CI 36-58%), and relapse 21% (95% CI 4-28%).
A review of various studies highlights the significant discordance in definitions of SGF: some authors include the presence of donor chimerism in the definition, whilst others do not [1, 2, 4, 6, 7, 8, 9, 10]. Including those cases with low blood counts which may be secondary to causes such as drugs, infection and GvHD may lead to over-diagnosis and possibly inappropriate treatment, given that a proportion of these will be transient and demonstrate recovery of blood counts. This lack of uniformity may hamper common definitions of what constitutes GF [3]. A definition of SGF which requires donor chimerism <5% will exclude patients who had falling chimerism (between 5% and 95%) but insufficient time for this to fall below 5%, or those who died due to cytopenia without documenting <5% donor chimerism. We, therefore, included patients with cytopenia, who had falling donor chimerism (Figure 1c) and died due to complications or received salvage DLI or second transplant. Importantly, we excluded those patients whose cytopenia was due to relapse. We were assiduous in ensuring that the presenting cytopenia were not due to relapse of the underlying disease. This serves to reduce ambiguity and focus resources on those patients in urgent need of salvage from GF. Often, relapsed myelodysplasia or acute leukemia may present with progressive single- or multi-lineage cytopenia, and it is crucial to make every attempt to exclude relapse, either by repeated bone marrow examination or the use of modern techniques for minimal or measurable residual disease by flow cytometry and/or quantitative polymerase chain reaction, as appropriate.
Interestingly, the significant factors for primary and SGF in our cohort were slightly different, which may represent that these types of GF represent different phenomena, with differing mechanisms. This is evidenced by the fact that PGF risks included myelofibrosis, myelodysplasia, myeloproliferative neoplasm, while these were absent from those factors found significant for SGF (lymphoma/non-malignant diagnosis, Figures 2c and 2d).
There are limitations to our analysis. Numbers of patients with primary or SGF are few and therefore the analysis was improved by evaluating all GF together. Nevertheless, analysis within primary and SGF cohorts gave broadly similar results. The inclusion of patients who died with cytopenia in the SGF may not be conventional, but this allowed study of relevant groups which would have otherwise have been excluded.
Conclusion
According to the results from the present study, the prognosis of GF remains poor even after successful treatment of GF, with a few long-term survivors. Those patients with mismatched donors and certain hematological conditions as previously mentioned, should be counselled carefully that the risk of GF may be near 25%, with a mortality rate of approximately 73-89% should it occur. Whereas risk factors such as primary diagnosis are unavoidable, it may be possible to reduce the other risk factors, including careful donor selection. However, most patients with MMUD or haploidentical donors will not have a readily available alternative donor. Measures that could be taken to reduce risk of GF include choice of haploidentical donor, utilizing a more immunosuppressive conditioning regimen or increasing the immunosuppressive component of the GvHD prophylaxis. If appropriate, a myeloablative regimen is more likely to ensure full donor engraftment. Furthermore, careful attention could be paid to the presence of donor-specific antibodies (DSA) and their titers when using MMURD and haploidentical donors. Post-transplant monitoring modification could be implemented, such as lineage-specific chimerism, which our center has recently introduced, and more frequent monitoring of chimerism, e.g. every 30 days with priority for rapid results. More careful use of myelo-suppressive medication such as valganciclovir and co-trimoxazole in these patients is another suggested strategy, with early adoption of alternative therapies in the event of cytopenias. These special precautions could be taken in those cases with 1 or more risk factors as identified in this study. Further studies are required to establish whether close monitoring and early intervention improve outcomes. The optimal treatment strategies for graft failure remain to be established.
Conflicts of interests
None declared.
References
- Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48(4):537-543.
- Ferra C, Sanz J, Diaz-Perez MA, et al. Outcome of graft failure after allogeneic stem cell transplant: study of 89 patients. Leuk Lymphoma. 2015;56(3):656-662.
- Gale RP. Early and late graft-failure after transplants. Bone Marrow Transplant. 2016;51(2):182-183.
- Satwani P, Jin Z, Duffy D, et al. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013;19(4):552-561.
- Ozdemir ZN, Civriz Bozdag S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2018;57(2):163-167.
- Olsson RF, Logan BR, Chaudhury S, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015;29(8):1754-1762.
- Rondon G, Saliba RM, Khouri I, et al. Long-term follow-up of patients who experienced graft failure postallogeneic progenitor cell transplantation. Results of a single institution analysis. Biol Blood Marrow Transplant. 2008;14(8):859-866.
- Passweg JR, Zhang MJ, Rocha V, et al. Donor characteristics affecting graft failure, graft-versus-host disease, and survival after unrelated donor transplantation with reduced-intensity conditioning for hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(12):1869-1873.
- Hutt D. Engraftment, Graft Failure, and Rejection. In: Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses: Under the Auspices of EBMT. Cham (CH)2018:259-270.
- Lund TC, Liegel J, Bejanyan N, et al. Second allogeneic hematopoietic cell transplantation for graft failure: poor outcomes for neutropenic graft failure. Am J Hematol. 2015;90(10):892-896.
- Mattsson J, Ringden O, Storb R. Graft failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(1 Suppl 1):165-170.
- Masouridi-Levrat S, Simonetta F, Chalandon Y. Immunological Basis of Bone Marrow Failure after Allogeneic Hematopoietic Stem Cell Transplantation. Frontiers in immunology. 2016;7:362.
- Wolff SN. Second hematopoietic stem cell transplantation for the treatment of graft failure, graft rejection or relapse after allogeneic transplantation. Bone Marrow Transplant. 2002;29(7):545-552.
- Stucki A, Leisenring W, Sandmaier BM, Sanders J, Anasetti C, Storb R. Decreased Rejection and Improved Survival of First and Second Marrow Transplants for Severe Aplastic Anemia (A 26-Year Retrospective Analysis). Blood. 1998;92(8):2742-2749.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828.
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401 e381.
- Ferrà C, Sanz J, Díaz-Pérez M-A, et al. Outcome of graft failure after allogeneic stem cell transplant: study of 89 patients. Leukemia & Lymphoma. 2015;56(3):656-662.
- Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496-509.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific co-morbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919.
- Schriber J, Agovi MA, Ho V, et al. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol Blood Marrow Transplant. 2010;16(8):1099-1106.
- Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905-913.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "30" ["~SORT"]=> string(2) "30" ["CODE"]=> string(100) "faktory-riska-nedostatochnosti-transplantata-pri-allogennoy-transplantatsii-gemopoeticheskikh-stvolo" ["~CODE"]=> string(100) "faktory-riska-nedostatochnosti-transplantata-pri-allogennoy-transplantatsii-gemopoeticheskikh-stvolo" ["EXTERNAL_ID"]=> string(4) "1940" ["~EXTERNAL_ID"]=> string(4) "1940" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(363) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследованиеRisk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4106) "<h3>Цель работы</h3> <p style="text-align: justify;">Недостаточность трансплантата (НТ) после аллогенной трансплантации гемопоэтических клеток (алло-ТГСК) имеет плохой прогноз. Целью данного исследования было установление частоты возникновения, факторов риска (Фри исходов НТ в контингенте из одного центра.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">В период с 2015 по 2018 гг., алло-ТГСК была выполнена у 557 больных. Первичную НТ (ПНТ) определяли как отсутствие роста абсолютного количества нейтрофилов (АКН) до >0.5×10<sup>9</sup>/л к 28 сут. после алло-ТГСК. Вторичную НТ (ВНТ) характеризовало снижение числа донорских клеток после начального приживления с возвратом КН к уровням <0.5×10<sup>9</sup>/л без рецидива или иных причин цитопении. Конечными результатами исследования была кумулятивная встречаемость НТ и общая выживаемость (ОВ); факторы риска НТ определяли путем многофакторного анализа.</p> <h3>Результаты</h3> <p style="text-align: justify;">В 9 случаях выявлена ПНТ, и у 34 больных была ВНТ. Кумулятивная встречаемость НТ была, соответственно, 1,6% ко дню +100 (CI95%; 0,8- 3,0%), и 6,5% – (CI95%; 4,5-8,8%) ко дню +800. Многофакторный анализ показал, что лиагноз (миелодиспластический синдром, миелофиброз, лимфома или неопухолевые заболевания), а также тип донора (HLA-несовместимый неродственный или гаплоидентичный) были достоверно ассоциированы с НТ. Частота НТ составила 3,6% при отсутствии указанных факторов риска, 9.9% при наличии одного ФР и 24.5% при двух ФР. Медиана выживаемости пациентов после ПНТ составила 41 сут., после ВНТ – 144 сут. Общая выживаемость на день +100 при ПНТ была 22%, при ВНТ – 64%. Двухлетняя общая выживаемость в случаях ВНТ была 28%.</p> <h3>Выводы</h3> <p style="text-align: justify;">Данное исследование показало наличие повышенного риска недостаточности трансплантата после алло-ТГСК от несовместимого/неродственного или гаплоидентичного донора, или при нелейкемическом диагнозе. Для таких случаев мы предлагаем тщательный мониторинг, ранние диагностические и терапевтические мероприятия и для улучшения клинических исходов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Аллогенная трансплантация гемопоэтических стволовых клеток, недостаточность трансплантата, встречаемость, факторы риска. </p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_META_TITLE"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_META_KEYWORDS"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_META_DESCRIPTION"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_PICTURE_FILE_ALT"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_PICTURE_FILE_TITLE"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "faktory-riska-nedostatochnosti-transplantata-pri-allogennoy-transplantatsii-gemopoeticheskikh-stvolo" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(256) "Факторы риска недостаточности трансплантата при аллогенной трансплантации гемопоэтических стволовых клеток: одноцентровое исследование" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "faktory-riska-nedostatochnosti-transplantata-pri-allogennoy-transplantatsii-gemopoeticheskikh-stvolo" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "faktory-riska-nedostatochnosti-transplantata-pri-allogennoy-transplantatsii-gemopoeticheskikh-stvolo" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "faktory-riska-nedostatochnosti-transplantata-pri-allogennoy-transplantatsii-gemopoeticheskikh-stvolo" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "170" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27347" ["VALUE"]=> string(22) "09/22/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "09/22/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27348" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27349" ["VALUE"]=> array(2) { ["TEXT"]=> string(803) "<p>Игорь Новицки-Бассо<sup>1</sup>, Эшрак Аль-Шайбани<sup>1</sup>, Мата Рембергер<sup>2</sup>, Кэрол Чен<sup>1</sup>, Уилсон Лэм<sup>1</sup>, Арджун Д. Лоу<sup>1</sup>, Айвэн Пазич<sup>1</sup>, Фотиос В. Микелис<sup>1</sup>, Ауро Висвабандья<sup>1</sup>, Деннис Д. Ким<sup>1</sup>, Джеффри Х. Липтон<sup>1</sup>, Армин Гербитц<sup>1</sup>, Ионас Маттсон<sup>1</sup>, Раджат Кумар<sup>1</sup>, Зейяд Аль-Шайбани<sup>1</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(611) "
Игорь Новицки-Бассо1, Эшрак Аль-Шайбани1, Мата Рембергер2, Кэрол Чен1, Уилсон Лэм1, Арджун Д. Лоу1, Айвэн Пазич1, Фотиос В. Микелис1, Ауро Висвабандья1, Деннис Д. Ким1, Джеффри Х. Липтон1, Армин Гербитц1, Ионас Маттсон1, Раджат Кумар1, Зейяд Аль-Шайбани1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27350" ["VALUE"]=> array(2) { ["TEXT"]=> string(585) "<p><sup>1</sup> Программа аллогенных трансплантаций Ханса Месснера, Онкологический Центр принцессы Маргарет, университетская сеть здравоохранения, Университет Торонто, Торонто, Канада<br> <sup>2</sup> Департамент медицинских наук, университет Уппсала, университетский госпиталь Уппсала, Уппсала, Швеция</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(543) "1 Программа аллогенных трансплантаций Ханса Месснера, Онкологический Центр принцессы Маргарет, университетская сеть здравоохранения, Университет Торонто, Торонто, Канада
2 Департамент медицинских наук, университет Уппсала, университетский госпиталь Уппсала, Уппсала, Швеция
Цель работы
Недостаточность трансплантата (НТ) после аллогенной трансплантации гемопоэтических клеток (алло-ТГСК) имеет плохой прогноз. Целью данного исследования было установление частоты возникновения, факторов риска (Фри исходов НТ в контингенте из одного центра.
Пациенты и методы
В период с 2015 по 2018 гг., алло-ТГСК была выполнена у 557 больных. Первичную НТ (ПНТ) определяли как отсутствие роста абсолютного количества нейтрофилов (АКН) до >0.5×109/л к 28 сут. после алло-ТГСК. Вторичную НТ (ВНТ) характеризовало снижение числа донорских клеток после начального приживления с возвратом КН к уровням <0.5×109/л без рецидива или иных причин цитопении. Конечными результатами исследования была кумулятивная встречаемость НТ и общая выживаемость (ОВ); факторы риска НТ определяли путем многофакторного анализа.
Результаты
В 9 случаях выявлена ПНТ, и у 34 больных была ВНТ. Кумулятивная встречаемость НТ была, соответственно, 1,6% ко дню +100 (CI95%; 0,8- 3,0%), и 6,5% – (CI95%; 4,5-8,8%) ко дню +800. Многофакторный анализ показал, что лиагноз (миелодиспластический синдром, миелофиброз, лимфома или неопухолевые заболевания), а также тип донора (HLA-несовместимый неродственный или гаплоидентичный) были достоверно ассоциированы с НТ. Частота НТ составила 3,6% при отсутствии указанных факторов риска, 9.9% при наличии одного ФР и 24.5% при двух ФР. Медиана выживаемости пациентов после ПНТ составила 41 сут., после ВНТ – 144 сут. Общая выживаемость на день +100 при ПНТ была 22%, при ВНТ – 64%. Двухлетняя общая выживаемость в случаях ВНТ была 28%.
Выводы
Данное исследование показало наличие повышенного риска недостаточности трансплантата после алло-ТГСК от несовместимого/неродственного или гаплоидентичного донора, или при нелейкемическом диагнозе. Для таких случаев мы предлагаем тщательный мониторинг, ранние диагностические и терапевтические мероприятия и для улучшения клинических исходов.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, недостаточность трансплантата, встречаемость, факторы риска.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27352" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-37-47" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-37-47" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27355" ["VALUE"]=> array(2) { ["TEXT"]=> string(620) "<p>Igor Novitzky-Basso<sup>1</sup>, Eshrak Al-Shaibani<sup>1</sup>, Mats Remberger<sup>2</sup>, Carol Chen<sup>1</sup>, Wilson Lam<sup>1</sup>, Arjun D. Law<sup>1</sup>, Ivan Pasic<sup>1</sup>, Fotios V. Michelis<sup>1</sup>, Auro Viswabandya<sup>1</sup>, Dennis D. Kim<sup>1</sup>, Jeffrey H. Lipton<sup>1</sup>, Armin Gerbitz<sup>1</sup>, Jonas Mattsson<sup>1</sup>, Rajat Kumar<sup>1</sup>, Zeyad Al-Shaibani<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(428) "Igor Novitzky-Basso1, Eshrak Al-Shaibani1, Mats Remberger2, Carol Chen1, Wilson Lam1, Arjun D. Law1, Ivan Pasic1, Fotios V. Michelis1, Auro Viswabandya1, Dennis D. Kim1, Jeffrey H. Lipton1, Armin Gerbitz1, Jonas Mattsson1, Rajat Kumar1, Zeyad Al-Shaibani1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27356" ["VALUE"]=> array(2) { ["TEXT"]=> string(721) "<p><sup>1</sup> Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada<br> <sup>2</sup> Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden</p><br> <p><b>Correspondence</b><br> Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada<br> Phone: +1 647 927 8700<br> Fax: +1 416 946 4407<br> E-mail: igor.novitzkybasso@uhn.ca</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(625) "1 Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada
2 Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden
Correspondence
Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada
Phone: +1 647 927 8700
Fax: +1 416 946 4407
E-mail: igor.novitzkybasso@uhn.ca
Objectives
Graft failure (GF) following allogeneic hematopoietic stem cell transplantation (allo-HCT) has a dismal prognosis. This study assessed incidence, risk factors (RF) and outcome of GF in a single-center population.
Patients and methods
Between 2015-2018, 557 patients underwent allo-HCT. Primary GF (PGF) was defined as failure to achieve absolute neutrophil count (ANC) of >0.5×109/L by 28 days after allo-HCT. Secondary GF (SGF) was characterized by loss of donor cells after initial engraftment with recurrence of ANC <0.5×109/L without relapse or other causes of cytopenia. Endpoints of the study were cumulative incidence of GF and overall survival (OS). Risk factors for GF were assessed in multivariate analysis.
Results
Nine patients had PGF, and 34 had SGF. The cumulative incidence of GF overall (primary and secondary) is 1.6% (CI95%; 0.8-3.0%) at day 100 and 6.5% (CI95%; 4.5-8.8%) at day 800 respectively. Multivariate analysis showed diagnosis (myelodysplastic syndrome [MDS], myelofibrosis [MF], lymphoma or non-malignant diseases) and donor type (HLA-mismatched-unrelated or haploidentical) were significantly associated with GF. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5% (p=0.002). Median OS of patients following PGF was 41 days, SGF – 144 days. The D100 OS in PGF was 22%, SGF – 64%; 2-year overall survival for SGF was 28%.
Conclusions
This study showed increased risk of GF following mismatched-unrelated or haploidentical donor allo-HCT or for non-leukemia diagnosis, for which we suggest close monitoring for early diagnostic and therapeutic interventions, in order to improve clinical outcomes.
Keywords
Allogeneic hematopoietic stem cell transplantation, graft failure, incidence, risk factors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27353" ["VALUE"]=> string(107) "Risk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(107) "Risk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27354" ["VALUE"]=> string(4) "2326" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2326" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27357" ["VALUE"]=> string(4) "2327" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2327" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27355" ["VALUE"]=> array(2) { ["TEXT"]=> string(620) "<p>Igor Novitzky-Basso<sup>1</sup>, Eshrak Al-Shaibani<sup>1</sup>, Mats Remberger<sup>2</sup>, Carol Chen<sup>1</sup>, Wilson Lam<sup>1</sup>, Arjun D. Law<sup>1</sup>, Ivan Pasic<sup>1</sup>, Fotios V. Michelis<sup>1</sup>, Auro Viswabandya<sup>1</sup>, Dennis D. Kim<sup>1</sup>, Jeffrey H. Lipton<sup>1</sup>, Armin Gerbitz<sup>1</sup>, Jonas Mattsson<sup>1</sup>, Rajat Kumar<sup>1</sup>, Zeyad Al-Shaibani<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(428) "Igor Novitzky-Basso1, Eshrak Al-Shaibani1, Mats Remberger2, Carol Chen1, Wilson Lam1, Arjun D. Law1, Ivan Pasic1, Fotios V. Michelis1, Auro Viswabandya1, Dennis D. Kim1, Jeffrey H. Lipton1, Armin Gerbitz1, Jonas Mattsson1, Rajat Kumar1, Zeyad Al-Shaibani1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(428) "Igor Novitzky-Basso1, Eshrak Al-Shaibani1, Mats Remberger2, Carol Chen1, Wilson Lam1, Arjun D. Law1, Ivan Pasic1, Fotios V. Michelis1, Auro Viswabandya1, Dennis D. Kim1, Jeffrey H. Lipton1, Armin Gerbitz1, Jonas Mattsson1, Rajat Kumar1, Zeyad Al-Shaibani1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27358" ["VALUE"]=> array(2) { ["TEXT"]=> string(2384) "<h3>Objectives</h3> <p style="text-align: justify;">Graft failure (GF) following allogeneic hematopoietic stem cell transplantation (allo-HCT) has a dismal prognosis. This study assessed incidence, risk factors (RF) and outcome of GF in a single-center population.</p> <h3>Patients and methods</h3> <p style="text-align: justify;">Between 2015-2018, 557 patients underwent allo-HCT. Primary GF (PGF) was defined as failure to achieve absolute neutrophil count (ANC) of >0.5×10<sup>9</sup>/L by 28 days after allo-HCT. Secondary GF (SGF) was characterized by loss of donor cells after initial engraftment with recurrence of ANC <0.5×10<sup>9</sup>/L without relapse or other causes of cytopenia. Endpoints of the study were cumulative incidence of GF and overall survival (OS). Risk factors for GF were assessed in multivariate analysis.</p> <h3>Results</h3> <p style="text-align: justify;">Nine patients had PGF, and 34 had SGF. The cumulative incidence of GF overall (primary and secondary) is 1.6% (CI95%; 0.8-3.0%) at day 100 and 6.5% (CI95%; 4.5-8.8%) at day 800 respectively. Multivariate analysis showed diagnosis (myelodysplastic syndrome [MDS], myelofibrosis [MF], lymphoma or non-malignant diseases) and donor type (HLA-mismatched-unrelated or haploidentical) were significantly associated with GF. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5% (p=0.002). Median OS of patients following PGF was 41 days, SGF – 144 days. The D100 OS in PGF was 22%, SGF – 64%; 2-year overall survival for SGF was 28%.</p> <h3>Conclusions</h3> <p style="text-align: justify;">This study showed increased risk of GF following mismatched-unrelated or haploidentical donor allo-HCT or for non-leukemia diagnosis, for which we suggest close monitoring for early diagnostic and therapeutic interventions, in order to improve clinical outcomes. </p> <h2>Keywords</h2> <p style="text-align: justify;">Allogeneic hematopoietic stem cell transplantation, graft failure, incidence, risk factors. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2184) "Objectives
Graft failure (GF) following allogeneic hematopoietic stem cell transplantation (allo-HCT) has a dismal prognosis. This study assessed incidence, risk factors (RF) and outcome of GF in a single-center population.
Patients and methods
Between 2015-2018, 557 patients underwent allo-HCT. Primary GF (PGF) was defined as failure to achieve absolute neutrophil count (ANC) of >0.5×109/L by 28 days after allo-HCT. Secondary GF (SGF) was characterized by loss of donor cells after initial engraftment with recurrence of ANC <0.5×109/L without relapse or other causes of cytopenia. Endpoints of the study were cumulative incidence of GF and overall survival (OS). Risk factors for GF were assessed in multivariate analysis.
Results
Nine patients had PGF, and 34 had SGF. The cumulative incidence of GF overall (primary and secondary) is 1.6% (CI95%; 0.8-3.0%) at day 100 and 6.5% (CI95%; 4.5-8.8%) at day 800 respectively. Multivariate analysis showed diagnosis (myelodysplastic syndrome [MDS], myelofibrosis [MF], lymphoma or non-malignant diseases) and donor type (HLA-mismatched-unrelated or haploidentical) were significantly associated with GF. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5% (p=0.002). Median OS of patients following PGF was 41 days, SGF – 144 days. The D100 OS in PGF was 22%, SGF – 64%; 2-year overall survival for SGF was 28%.
Conclusions
This study showed increased risk of GF following mismatched-unrelated or haploidentical donor allo-HCT or for non-leukemia diagnosis, for which we suggest close monitoring for early diagnostic and therapeutic interventions, in order to improve clinical outcomes.
Keywords
Allogeneic hematopoietic stem cell transplantation, graft failure, incidence, risk factors.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2184) "Objectives
Graft failure (GF) following allogeneic hematopoietic stem cell transplantation (allo-HCT) has a dismal prognosis. This study assessed incidence, risk factors (RF) and outcome of GF in a single-center population.
Patients and methods
Between 2015-2018, 557 patients underwent allo-HCT. Primary GF (PGF) was defined as failure to achieve absolute neutrophil count (ANC) of >0.5×109/L by 28 days after allo-HCT. Secondary GF (SGF) was characterized by loss of donor cells after initial engraftment with recurrence of ANC <0.5×109/L without relapse or other causes of cytopenia. Endpoints of the study were cumulative incidence of GF and overall survival (OS). Risk factors for GF were assessed in multivariate analysis.
Results
Nine patients had PGF, and 34 had SGF. The cumulative incidence of GF overall (primary and secondary) is 1.6% (CI95%; 0.8-3.0%) at day 100 and 6.5% (CI95%; 4.5-8.8%) at day 800 respectively. Multivariate analysis showed diagnosis (myelodysplastic syndrome [MDS], myelofibrosis [MF], lymphoma or non-malignant diseases) and donor type (HLA-mismatched-unrelated or haploidentical) were significantly associated with GF. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5% (p=0.002). Median OS of patients following PGF was 41 days, SGF – 144 days. The D100 OS in PGF was 22%, SGF – 64%; 2-year overall survival for SGF was 28%.
Conclusions
This study showed increased risk of GF following mismatched-unrelated or haploidentical donor allo-HCT or for non-leukemia diagnosis, for which we suggest close monitoring for early diagnostic and therapeutic interventions, in order to improve clinical outcomes.
Keywords
Allogeneic hematopoietic stem cell transplantation, graft failure, incidence, risk factors.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27352" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-37-47" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-37-47" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-37-47" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27353" ["VALUE"]=> string(107) "Risk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(107) "Risk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(107) "Risk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27356" ["VALUE"]=> array(2) { ["TEXT"]=> string(721) "<p><sup>1</sup> Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada<br> <sup>2</sup> Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden</p><br> <p><b>Correspondence</b><br> Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada<br> Phone: +1 647 927 8700<br> Fax: +1 416 946 4407<br> E-mail: igor.novitzkybasso@uhn.ca</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(625) "1 Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada
2 Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden
Correspondence
Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada
Phone: +1 647 927 8700
Fax: +1 416 946 4407
E-mail: igor.novitzkybasso@uhn.ca
1 Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada
2 Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden
Correspondence
Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada
Phone: +1 647 927 8700
Fax: +1 416 946 4407
E-mail: igor.novitzkybasso@uhn.ca
Игорь Новицки-Бассо1, Эшрак Аль-Шайбани1, Мата Рембергер2, Кэрол Чен1, Уилсон Лэм1, Арджун Д. Лоу1, Айвэн Пазич1, Фотиос В. Микелис1, Ауро Висвабандья1, Деннис Д. Ким1, Джеффри Х. Липтон1, Армин Гербитц1, Ионас Маттсон1, Раджат Кумар1, Зейяд Аль-Шайбани1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(611) "Игорь Новицки-Бассо1, Эшрак Аль-Шайбани1, Мата Рембергер2, Кэрол Чен1, Уилсон Лэм1, Арджун Д. Лоу1, Айвэн Пазич1, Фотиос В. Микелис1, Ауро Висвабандья1, Деннис Д. Ким1, Джеффри Х. Липтон1, Армин Гербитц1, Ионас Маттсон1, Раджат Кумар1, Зейяд Аль-Шайбани1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27347" ["VALUE"]=> string(22) "09/22/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "09/22/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "09/22/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27348" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/04/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27351" ["VALUE"]=> array(2) { ["TEXT"]=> string(4106) "<h3>Цель работы</h3> <p style="text-align: justify;">Недостаточность трансплантата (НТ) после аллогенной трансплантации гемопоэтических клеток (алло-ТГСК) имеет плохой прогноз. Целью данного исследования было установление частоты возникновения, факторов риска (Фри исходов НТ в контингенте из одного центра.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">В период с 2015 по 2018 гг., алло-ТГСК была выполнена у 557 больных. Первичную НТ (ПНТ) определяли как отсутствие роста абсолютного количества нейтрофилов (АКН) до >0.5×10<sup>9</sup>/л к 28 сут. после алло-ТГСК. Вторичную НТ (ВНТ) характеризовало снижение числа донорских клеток после начального приживления с возвратом КН к уровням <0.5×10<sup>9</sup>/л без рецидива или иных причин цитопении. Конечными результатами исследования была кумулятивная встречаемость НТ и общая выживаемость (ОВ); факторы риска НТ определяли путем многофакторного анализа.</p> <h3>Результаты</h3> <p style="text-align: justify;">В 9 случаях выявлена ПНТ, и у 34 больных была ВНТ. Кумулятивная встречаемость НТ была, соответственно, 1,6% ко дню +100 (CI95%; 0,8- 3,0%), и 6,5% – (CI95%; 4,5-8,8%) ко дню +800. Многофакторный анализ показал, что лиагноз (миелодиспластический синдром, миелофиброз, лимфома или неопухолевые заболевания), а также тип донора (HLA-несовместимый неродственный или гаплоидентичный) были достоверно ассоциированы с НТ. Частота НТ составила 3,6% при отсутствии указанных факторов риска, 9.9% при наличии одного ФР и 24.5% при двух ФР. Медиана выживаемости пациентов после ПНТ составила 41 сут., после ВНТ – 144 сут. Общая выживаемость на день +100 при ПНТ была 22%, при ВНТ – 64%. Двухлетняя общая выживаемость в случаях ВНТ была 28%.</p> <h3>Выводы</h3> <p style="text-align: justify;">Данное исследование показало наличие повышенного риска недостаточности трансплантата после алло-ТГСК от несовместимого/неродственного или гаплоидентичного донора, или при нелейкемическом диагнозе. Для таких случаев мы предлагаем тщательный мониторинг, ранние диагностические и терапевтические мероприятия и для улучшения клинических исходов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Аллогенная трансплантация гемопоэтических стволовых клеток, недостаточность трансплантата, встречаемость, факторы риска. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3906) "Цель работы
Недостаточность трансплантата (НТ) после аллогенной трансплантации гемопоэтических клеток (алло-ТГСК) имеет плохой прогноз. Целью данного исследования было установление частоты возникновения, факторов риска (Фри исходов НТ в контингенте из одного центра.
Пациенты и методы
В период с 2015 по 2018 гг., алло-ТГСК была выполнена у 557 больных. Первичную НТ (ПНТ) определяли как отсутствие роста абсолютного количества нейтрофилов (АКН) до >0.5×109/л к 28 сут. после алло-ТГСК. Вторичную НТ (ВНТ) характеризовало снижение числа донорских клеток после начального приживления с возвратом КН к уровням <0.5×109/л без рецидива или иных причин цитопении. Конечными результатами исследования была кумулятивная встречаемость НТ и общая выживаемость (ОВ); факторы риска НТ определяли путем многофакторного анализа.
Результаты
В 9 случаях выявлена ПНТ, и у 34 больных была ВНТ. Кумулятивная встречаемость НТ была, соответственно, 1,6% ко дню +100 (CI95%; 0,8- 3,0%), и 6,5% – (CI95%; 4,5-8,8%) ко дню +800. Многофакторный анализ показал, что лиагноз (миелодиспластический синдром, миелофиброз, лимфома или неопухолевые заболевания), а также тип донора (HLA-несовместимый неродственный или гаплоидентичный) были достоверно ассоциированы с НТ. Частота НТ составила 3,6% при отсутствии указанных факторов риска, 9.9% при наличии одного ФР и 24.5% при двух ФР. Медиана выживаемости пациентов после ПНТ составила 41 сут., после ВНТ – 144 сут. Общая выживаемость на день +100 при ПНТ была 22%, при ВНТ – 64%. Двухлетняя общая выживаемость в случаях ВНТ была 28%.
Выводы
Данное исследование показало наличие повышенного риска недостаточности трансплантата после алло-ТГСК от несовместимого/неродственного или гаплоидентичного донора, или при нелейкемическом диагнозе. Для таких случаев мы предлагаем тщательный мониторинг, ранние диагностические и терапевтические мероприятия и для улучшения клинических исходов.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, недостаточность трансплантата, встречаемость, факторы риска.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3906) "Цель работы
Недостаточность трансплантата (НТ) после аллогенной трансплантации гемопоэтических клеток (алло-ТГСК) имеет плохой прогноз. Целью данного исследования было установление частоты возникновения, факторов риска (Фри исходов НТ в контингенте из одного центра.
Пациенты и методы
В период с 2015 по 2018 гг., алло-ТГСК была выполнена у 557 больных. Первичную НТ (ПНТ) определяли как отсутствие роста абсолютного количества нейтрофилов (АКН) до >0.5×109/л к 28 сут. после алло-ТГСК. Вторичную НТ (ВНТ) характеризовало снижение числа донорских клеток после начального приживления с возвратом КН к уровням <0.5×109/л без рецидива или иных причин цитопении. Конечными результатами исследования была кумулятивная встречаемость НТ и общая выживаемость (ОВ); факторы риска НТ определяли путем многофакторного анализа.
Результаты
В 9 случаях выявлена ПНТ, и у 34 больных была ВНТ. Кумулятивная встречаемость НТ была, соответственно, 1,6% ко дню +100 (CI95%; 0,8- 3,0%), и 6,5% – (CI95%; 4,5-8,8%) ко дню +800. Многофакторный анализ показал, что лиагноз (миелодиспластический синдром, миелофиброз, лимфома или неопухолевые заболевания), а также тип донора (HLA-несовместимый неродственный или гаплоидентичный) были достоверно ассоциированы с НТ. Частота НТ составила 3,6% при отсутствии указанных факторов риска, 9.9% при наличии одного ФР и 24.5% при двух ФР. Медиана выживаемости пациентов после ПНТ составила 41 сут., после ВНТ – 144 сут. Общая выживаемость на день +100 при ПНТ была 22%, при ВНТ – 64%. Двухлетняя общая выживаемость в случаях ВНТ была 28%.
Выводы
Данное исследование показало наличие повышенного риска недостаточности трансплантата после алло-ТГСК от несовместимого/неродственного или гаплоидентичного донора, или при нелейкемическом диагнозе. Для таких случаев мы предлагаем тщательный мониторинг, ранние диагностические и терапевтические мероприятия и для улучшения клинических исходов.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, недостаточность трансплантата, встречаемость, факторы риска.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27350" ["VALUE"]=> array(2) { ["TEXT"]=> string(585) "<p><sup>1</sup> Программа аллогенных трансплантаций Ханса Месснера, Онкологический Центр принцессы Маргарет, университетская сеть здравоохранения, Университет Торонто, Торонто, Канада<br> <sup>2</sup> Департамент медицинских наук, университет Уппсала, университетский госпиталь Уппсала, Уппсала, Швеция</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(543) "1 Программа аллогенных трансплантаций Ханса Месснера, Онкологический Центр принцессы Маргарет, университетская сеть здравоохранения, Университет Торонто, Торонто, Канада
2 Департамент медицинских наук, университет Уппсала, университетский госпиталь Уппсала, Уппсала, Швеция
1 Программа аллогенных трансплантаций Ханса Месснера, Онкологический Центр принцессы Маргарет, университетская сеть здравоохранения, Университет Торонто, Торонто, Канада
2 Департамент медицинских наук, университет Уппсала, университетский госпиталь Уппсала, Уппсала, Швеция
Introduction
The cases of chronic myeloid leukemia (CML) in acceleration phase (AP) and blast crisis (BC) are still associated with very unfavorable prognosis. Introduction of low-molecular BCR/ABL1 inhibitors into clinical practice has caused a decrease in BC incidence from 1.5-3.7% to 0.3-2.2% per year [1].
The median overall survival (OS) in untreated patients with BC CML does not exceed 3-6 months. The results of conservative treatment approach with chemotherapy and tyrosine kinase inhibitors (TKIs) are also unsatisfactory. The median OS in patients with BC is about 12 months [2]. According to ELN and NCCN guidelines, the evolving AP or BCs upon TKIs therapy are indications for allogeneic hemopoietic stem cell transplantation (allo-HSCT). The latter represents the only curative option for some CML patients. Starting from 2004, the cohort of allo-HSCT recipients shifted significantly from the 1st chronic phase to TKIs non-responders and initially advanced-stage CML [3-5].
The CML evolution to BC is characterized by rather distinct biological features, which make it quite different from chronic phase [6]. These differences are evident not only in such characteristics as cellular proliferation, differentiation and apoptosis, but also in clinical course and therapy response rate which is associated with clonal evolution [6].
However, according to EBMT data, the long-term prognosis for patients transplanted in BC is still unfavorable [7]. The status at the time of allo-HSCT is still one of the most important prognostic factors along with donor HLA-compatibility, disease duration, and recipient’s age [7].
According to the data by Khoury et al. the 3-year event-free survival (EFS) for patients transplanted in BC and AP is 8-11% and 26-27%, accordingly [8]. Also, in 40% of cases patients died of disease progression.
The aim of our retrospective study was to compare two patient’s cohorts receiving allo-HSCT or conservative TKI therpy in order to evaluate the therapeutic approaches that provide a survival advantage.
Patients and methods
Clinical characteristics
A total of 162 patients with CML, who had AP or BC were included in this retrospective study. All the patients included into the study were under the age of 62 years without severe cardiac, pulmonary, renal, and other comorbidities. A cohort of 82 patients received TKIs and allo-HSCT (allo-HSCT+TKI) in RM Gorbacheva Research Institute, Pavlov University since 2002 to 2019. Moreover, eighty patients received only TKIs or their combination with chemotherapy according to acute leukemia protocol, as reported elsewhere [9]. TKIs were given according to ELN recommendations [9]. The patients in TKI group did not proceed to allo-HSCT due to lack of potential stem cell donor, due to refusal for personal reasons, or delay in referral to transplant center.
The CML diagnosis was based on clinical criteria and presence of Philadelphia (Ph) chromosome and/or chimeric BCR-ABL gene [15]. The disease stage was established according to the WHO criteria [9]. Hematological, cytogenetic and molecular responses were evaluated in compliance with ELN criteria [9]. Molecular response after allo-HSCT was evaluated according to the NCCN criteria. PCR monitoring of BCR/ABL was carried out according to NCCN recommendations once in 3 months for 2 years, then once in every 3 to 6 months. Cytogenetic investigation of bone marrow was performed according to a standard procedure with at least 20 metaphases analyzed per a sample (GTG method). The karyotype was evaluated according to International System for Human Cytogenetic Nomenclature (ISCN) [10]. In cases when the standard cytogenetic investigation was not available (insufficient material), the bone marrow was assessed by fluorescence in situ hybridization (FISH) probes aiming for detection of (9;22) variants (LSI BCR-ABL , Dual Color, Dual Fusion, "Vysis").
The relative BCR-ABL1 expression level was evaluated according to method described by Gabert et al [11]. The approach consists of the following stages: 1) total RNA extraction from peripheral blood of patients with CML, 2) reverse transcription with random hexameric primers, 3) real-time PCR with primers and probes specific to р210, р190 control ABL gene sequences. Assessment of relative expression levels is based on evaluation of BCR-ABL1/ABL1 ratios in the studied cDNA samples. The ABL1 gene was used for normalization of the results. In order to determine copy numbers of the BCR-ABL1 and ABL1 transcripts, and to assess the reaction effectiveness, standard dilution curves were plotted using a plasmid with inserts of known target gene sequences (Invitrogen, USA), at a standard concentration ranges of 102-106 copies/mcl, according to 2020 European LeukemiaNet (ELN) Recommendations [9] [12].
Treatment options
The conditioning regimen included fludarabine (180 mg/m2) and busulfan (8-12 mg/kg), or 140 mg/m2 of melphalan. Fifty-four patients (66%) received post-transplant cyclophosphamide (PTCy)-based graft-versus-host disease (GvHD) prophylaxis. The PTCy was given at 50 mg/kg on D +3 and +4 after allo-HSCT 5 mg/kg of rabbit antithymocyte globulin (n=3) in combination with tacrolimus (target concentration of 5-10 ng/ml) from D+5 to D+120, and 30 mg/kg of mycophenolate mofetil (MMF) from D+5 to D+30, or 60 mg/kg of horse (n=12), or 5 mg/kg of rabbit antithymocyte globulin (n=3) in combination with tacrolimus (target concentration, 5-10 ng/ml) from D-1 to D+120, and 30 mg/kg of MMF from D-1 to D+30. If allo-HSCT was performed from matched related donor, the GvHD prophylaxis consisted of tacrolimus (target concentration of 5-10 ng/ml) from D-1 to D+120, 30 mg/kg of MMF rom D-1 to D+30, 15 mg/m2 of methotrexate on D+1, and 10 mg/m2 of methotrexate on D+3 and D+6.
The acute and chronic GvHD stage was assessed according to the common Glucksberg criteria [13], and NIH criteria [14]. The engraftment was confirmed in patients with WBC count >1 × 109/l, neutrophils of > 0.5 × 109/l without granulocyte colony stimulating factor support for 3 days, platelets count of > 20 × 109/l for 3 days. The primary non-engraftment was diagnosed, if no complete donor chimerism was observed on D+40. The comorbidity index was determined according to the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) [15]. The allo-HSCT associated risk was evaluated in accordance with the Group for Blood and Marrow Transplantation Scale [7]. The immunosuppression was ceased, If there was a disease relapse, followed by donor lymphocyte infusions and/or TKIs were given as described before [16].
The TKIs were given in the post-transplant period to prevent relapses, or in cases of persistent BCR-ABL transcription as shown by PCR. TKIs were given if the neutrophil counts exceeded 0.5×109/l, or at platelet counts of > 50×109/l on D+60. The TKI choice was based on resistance pattern or history of intolerability. In 86% of cases, the patients were given 2nd generation TKI dasatinib, since it proved to be more effective in BC, and is able to penetrate the blood-brain barrier [17]. All the patients signed an informed consent for processing of personal data; the trial was approved by the Pavlov University Local Ethical Committee.
Evaluation of results in TKI and TKI+ allo-HSCT groups
The overall survival (OS) was estimated as a period from treatment initiation of allo-HSCT until death by any cause, or till last contact date. The event-free survival (EFS) was estimated as a period from treatment initiation of allo-HSCT until death, last contact date, or any of the following events: failure to obtain hematological response within 3 months, loss of previously achieved complete molecular response (CMR), complete cytogenetic response (CCR), or complete hematological response (CHR), post-transplant molecular relapse. Post-transplant relapse was diagnosed in case of two consecutive positive PCR assays or at least 1-log persistent increase of BCR/ABL transcript level. If patient received a second allo-HSCT due to primary non-engraftment or a relapse, the survival terms were dated back from second allo-HSCT.
Statistical analysis
The standard SPSS, IBM Statistics and R 1.41 software was used for statistical evaluation. The quantitative attributes of groups were compared using Mann-Whitney U-test. The qualitative attributes were compared by Chi-square test, Fisher’s exact test. Survival charts were plotted using Kaplan-Meier method. The statistical significance of differences evaluated via Kaplan-Meier test was checked by Log-rank test, the differences at р <0.05 were considered statistically significant. The cumulative risk of non relapse-related mortality was evaluated as competing risk.
Results
A total of 162 patients with advanced CML were included into the study. In 82 cases, the allo-HSCT was performed. The remaining 80 patients received only TKIs or TKIs in combination with chemotherapy. The median follow-up was 44 (1 to 344) months. There were no significant differences in gender, age, somatic status, disease phase or presence of additional chromosomal aberrations (ACAs) between allo-HSCT and TKI groups (see Tab. 1). The number of patient with BC was higher in allo-HSCT group compared to TKI group, i.e., 28% versus 12%. At the same time, non-transplanted patients were more likely to receive 3rd line of TKIs than allo-HSCT group (37% versus 18%) as seen from Table 1.
Table 1. Characteristics of patient groups receiving allo-HSCT or TKIs



Therapy and clinical response prior to allo-HSCT
A total of 82 patients received allo-HSCT. In 48% of cases, the disease was initially in CP, and it progressed later to more advanced stage (AC or BC). 52% of the cases had an advanced-stage disease at diagnosis. The median time span from diagnosis to allo-HSCT was 2.2 (0.3-21.4) years. Most patients received chemotherapy in combination with TKIs, 37% of patients was administered only TKIs. All but two patients, who received allo-HSCT in 2002, had previous history of TKI treatment. Most patients (61%) had 2 or 3 lines of TKIs, with imatinib, dasatinib or nilotinib used. Only seven patients received bosutinib and two patients received ponatinib.
Patients in CP at diagnosis
A total of 29 (74%) of patients who were initially in CP, later developed BC. In 17 cases, a 2nd CP was achieved prior to allo-HSCT. Six patients were subjected to allo-HSCT in AP, six patients did not respond to therapy and underwent allo-HSCT in BC. In ten patients, the AP developed, and it persisted until allo-HSCT.
Patients in AP at diagnosis
A total of 20 patients had AP at diagnosis, ten of them subsequently developed blast crisis (BC). In six cases, the CP was achieved after TKI + chemotherapy (n=4) or TKI treatment (n=2); two patients were in AP after TKI + chemotherapy, and two patients still had BC after TKI + chemotherapy (n=1) or TKI only (n=1) at the moment of allo-HSCT. The remaining 10 patients had no history of BC. Six of them subsequently achieved CP, four patients were still in AP at the moment of allo-HSCT.
Patients with BC at diagnosis
A total of 23 patients had BC at the time of diagnosis. In 20 cases, a subsequent chronic phase was achieved after TKI + chemotherapy (n=17) or TKI therapy (n=3), in one case, AP was documented after TKI therapy, and two patients were still in BC after TKI + chemotherapy (n=2) at the moment of allo-HSCT.

Figure 1. Cumulative 2-year relapse rate and 1-year non-relapse mortality after allo-HSCT.
Abbreviations: NRM, non-relapse mortality
Engraftment, causes of death and non-relapse mortality
Post-transplant engraftment was achieved in 71 (86%) patients. The median time to WBC engraftment was 22 (8 to 39) days, median time to the neutrophil engraftment was 22 (8 to 35) days. Median time to the platelet engraftment was 19 (6 to 57) days. In 9 cases of primary non-engraftment, the 2nd allo-HSCT was performed. The median follow-up was 35 (1 to 161) months.
Thirty-two patients died after allo-HSCT. The most common causes of death were relapse (n=16, 50%); GvHD (n=8, 25%); infectious complications (n=5, 16%); heart failure (n=2, 6%); hepatic veno-occlusive disease (n=1, 3%). One-year NRM was 18% (95% CI 10-28%), 100-day NRM 10% (95% CI 5-18%), as shown in Fig. 1.
Also, 48 (58%) patients received TKIs after allo-HSCT, 45 (88%) of them as relapse prophylaxis. 28 patients did not develop subsequent relapses. Two patients received bosutinib, 36 dasatinib, 8 nilotinib, and 2 ponatinib.
In 31 cases (38%), a relapse was developed, 16 of patients who relapsed received TKI prophylaxis. The cumulative relapse incidence was 39% (95% CI 28-51%). In 11 patients (34%), molecular relapse was shown; in one case, cytogenetic (3%), and in 19 cases (63%), hematological relapses were documented. Six patients received donor lymphocyte infusions (DLIs) for relapse treatment, five subjects received only TKIs; TKIs, chemotherapy and DLI were used in four cases; TKIs and DLIs were applied in 15 patients, and a combination of DLI and chemotherapy was used in one case. In 29% of the cases (n=9), the patients had durable molecular response, in 61% (n=19), the disease progression. Evaluation of therapeutic response was impossible for 10% of the patients. DLI was performed in the remaining six 6 cases, due to primary non-engraftment or poor graft function post-transplant. In three cases, DLIs were carried out due to persisting BCR/ABL transcript.
The incidence of grade 2-4 acute GvHD was 29% (n=21), grade 3-4 acute GvHD was registered in 20% (n=14). Chronic GvHD (cGvHD) incidence was 27% (n=18), having been mild in 6 cases (9%). Moderate cGvHD was observed in 8 cases (12%), and four patients (6%) developed severe cGvHD.
Patients who received only TKIs
A cohort of 80 patients received only TKIs, or their combination with chemotherapy. The median follow-up was 93 (13-344) months. The data on outcomes was available for 71 patients. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among 10 patients without history of BC, one patient did not respond, 5 achieved CHR; 2 patients developed CCR, and CMR was registered in 2 cases. Sixty-nine patients died, more than half of them deceased due to disease progression/relapse.

Figure 2. Four-year OS for allo-HSCT+TKIs and TKIs groups (А), and four-year EFS for allo-HSCT+TKIs and TKIs groups (B)
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; TKI, tyrosine kinase inhibitor

Figure 3. Four-year OS for patients treated in BC who received allo-HSCT+TKIs in CP≥2/AP+TKI, TKIs, and allo- HSCT in BC
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; CP≥2, chronic phase; AP, acceleration phase; TKI, therapy with tyrosine kinase inhibitors; allo-HSCT in BC +TKI, allogeneic hematopoietic stem cell transplantation in blast crisis
Comparisons between allo-HSCT+TKIs and TKIs groups
The CML patients who received allo-HSCT exhibited significantly better 4-year OS compared to the TKI-treated group, i.e., 58% (95% CI 44%-69%) versus 33% (95% CI 23-44%), accordingly (р=0.032), as shown in Fig. 2А). At the same time, no statistically significant differences were found between the 4-year EFS, which was 35% (95% CI 24%-47%) in allo-HSCT, and 17% (95% CI 10%-26%) in TKIs group (р=0.5) (Fig. 2B). Also, there was no statistically significant difference between the 4-year OS receiving allo-HSCT in BC CML. This value was 33% for TKIs group (95% CI 23%-44%), and 23% for allo-HSCT patients (95% CI 3-52%) (р=0.217). However, the 4-year OS reached 63% (95% CI 48%-74%) for the patients transplanted in AP or CP after TKIs therapy, which is significantly better compared to other groups (р=0.005) (Fig. 3).
Discussion
In our study, we compared therapy results for advanced-phase patients with CML who received TKIs with or without allo- HSCT. In allo-HSCT group, the 4-year OS was significantly longer if compared to patients, who were not transplanted (58% versus 33%, accordingly). The 4-year EFS was also longer in allo-HSCT group (35% versus 17%, accordingly), although without statistical significance in this case.
One should note that the relapse rate in allotransplanted patients is still as high as 39%. However, CML is one of the most immunotherapy-responsive malignancies. Kolb et al. have first shown clinical success of DLIs in allo-HSCT recipients with CML [18]. Moreover, development of acute or chronic GvHD was more important for CML patients as a factor able to decrease relapse rate compared to patients with AML, MDS or plasma cell disorders [19, 20].
This may be a reason for higher OS in allo-HSCT group, while EFS rates have not differed significantly. As many relapses were sensitive to immune therapy, they were not invariably fatal, and many patients responded to post-transplant therapy. In our study, 29% of the patients achieved durable CMR upon DLI-containing treatment. However, usage of this method may be limited due to the risk of GvHD, since grade 2-4 acute GVHD develops in 15%, and chronic GvHD occurs in 22% of patients [21]. Donor lymphocyte infusions should not be performed in patients with a history of chronic GvHD. Moreover, donor cells are not available in some cases.
Efficiency of donor lymphocyte infusions depends on many factors. The study by Basak et al. in patients with CML has shown that PBSCs as graft source worsen the long-term OS among DLI recipients. The authors note that there were more patients with advanced stages in PBSC group, and suppose a connection between worse DLI effect and immune response attenuation due to lower immunogenicity of malignant cells in advanced stage patients [22]. Early CML relapse is another potentially negative factor making DLIs less effective [22].
The response to DLI may depend on the type of relapse. In this study we performed DLI for 26 patients who usually presented with hematological relapses. In a study by Radujkovic et al., this method was more effective in treatment of patients with molecular or cytogenetic, but not hematologic relapses. Five-year relapse and GvHD-free survival in patients with cytogenetic and hematological relapses was 40% and 20%, accordingly [22]. In our study, more than a half of relapsed patients developed hematological relapse, which may be a reason for lower response rate observed.
Prophylactic use of TKIs may exert important influence on the allo-HSCT outcomes. The relapses developed only in 38% of HSCT recipients after TKI-based prophylaxis. However, the actual role of prophylactic interventions is yet not quite clear. The largest patient cohort described by DeFilipp et al. included 89 patients [23]. The authors have not found significant differences in OS, which was 61% in recipients with post-transplant TKI prophylaxis versus 57% in the patients without such prophylaxis, and EFS was 42% versus 44%, accordingly (all differences not statistically significant). Hence, the results of our retrospective study may suggest whether prophylactic TKIs improve allo-HSCT results.
Some studies have compared the results of allo-HSCT and TKIs with or without chemotherapy in advanced-stage CML patients. Worth of note, the 1st line therapy was performed in most of these reports. In our study, however, nearly half of patients in each group received 2nd line TKIs; 38% in TKIs and 20% in allo-HSCT+TKIs group received 3 or more lines of TKIs. Allo-HSCT was not performed, due to the lack of potential donor or failure to sign an informed consent. Therefore, the non-transplant group was more likely to receive 3rd line TKIs than the allo-HSCT group. In study by Jiang et al., the results of TKIs with or without allo-HSCT were evaluated in 83 patients with CML BC. The allo-HSCT proved to be advantageous in 4-year OS compared to other treatment modalities, with OS of 46.7% versus 9.7%, and EFS of 47.1% versus 6.7%, accordingly [24]. Jiang et al. analyzed imatinib therapy results compared with allo-HSCT in 132 AP CML cases and found survival advantage for allo-HSCT compared to TKI group [25], with 5-year OS of 100% versus 18% and EFS 67% versus 9%, accordingly. Jain et al. had analyzed survival rates from the moment of BC diagnosis in a group including 104 allo-HSCT recipients and demonstrated that allo-HSCT decreases risk of death [2].
Allo-HSCT in patients with BC lead to the same results as other treatment options, with 4-year OS of 33% and 23% in TKIs and allo-HSCT groups, respectively. It corresponds to the published data from some other studies [24, 26, 27, 28], which makes us to recommend preferential usage of this approach in the patients with therapy-resistant disease.
Of course, the retrospective nature of our study urges us to be cautious for interpretation of its results. Meanwhile, we compared here extensive groups without showing statistically significant differences for the main clinical characteristics that could influence the CML outcomes.
Conclusions
This study has shown allo-HSCT still to be a curative method in many patients with AP and BC of CML in the presence of new-generation TKIs. However, the relapses are probable even after allo-HSCT. Due to good response to immune therapy in CML patients, a long-term remission and even curation may be achieved even after relapse of malignancy following allo-HSCT.
Tyrosine kinase inhibitors may be used as additional method for relapse therapy and prophylaxis. Performing allo-HSCT in patients with BC without achievement of hematological response does not lead to significantly better outcome. The indications for allo-HSCT should be discussed individually for each patient.
Ackhowledgements
No conflicts of interest reported.
References
- Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017;31:2398-2406.
- Jain P, Kantarjian HM, Ghorab A, Sasaki K, Jabbour EJ, Nogueras Gonzalez G, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: Cohort study of 477 patients. Cancer 2017;123:4391-4402.
- Bacher U, Klyuchnikov E, Zabelina T, Ottinger H, Beelen DW, Schrezenmeier H, et al. The changing scene of allogeneic stem cell transplantation for chronic myeloid leukemia – A report from the German Registry covering the period from 1998 to 2004. Ann Hematol 2009;88:1237-1247.
- Morozova E, Barabanshchikova M, Mamaev N, Barkhatov I, Alyanskii A, Darskaya E, et al. Chronic myeloid leukemia: Role of allogeneic hematopoietic stem cell transplantation in the era of tyrosine kinase inhibitors. Clin Oncohematology 2020;13:193-198 In Russian).
- Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: Evaluation of its impact within a subgroup of the randomized German CML study IV. Blood 2010;115:1880-1885.
- Calabretta B, Perrotti D. Review in translational hematology The biology of CML blast crisis. Blood 2004;103:4010-4022.
- Gratwohl A. The EBMT risk score. Bone Marrow Transplant 2012;47:749-756.
- Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: A CIBMTR analysis. Bone Marrow Transplant 2012;47:810-816.
- Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020;34:966-984.
- Simons A, Shaffer LG, Hastings RJ. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. Cytogenet Genome Res 2013;141:1-6.
- Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of "real time" quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – A Europe Against Cancer Program. Leukemia 2003;17:2318-2357.
- Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Recommendations from an expert panel on behalf of European LeukemiaNet. Blood 2011;118:1208-1215.
- Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Jean Henslee‐Downey P, et al. IBMTR Severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855-864.
- Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant 2015;21:389-401.e1.
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912-2919.
- Radujkovic A, Guglielmi C, Bergantini S, Iacobelli S, van Biezen A, Milojkovic D, et al. Donor lymphocyte infusions for chronic myeloid leukemia relapsing after allogeneic stem cell transplantation: May we predict graft-versus-leukemia without graft-versus-host disease? Biol Blood Marrow Transplant 2015;21:1230-1236.
- Porkka K, Koskenvesa P, Lundán T, Rimpiläinen J, Mustjoki S, Smykla R, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system philadelphia chromosome positive leukemia. Blood 2008;112:1005-1012.
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990;76:2462-2465.
- Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Impact of graft-versus-host disease on relapse after allogeneic hematopoietic stem cell transplantation, an EBMT Megafile Study. Blood 2012;120:469-469.
- Oyekunle AA, Kröger N, Zabelina T, Ayuk F, Schieder H, Renges H, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: A long-term follow-up. Bone Marrow Transplant 2006;37:45-50.
- Chalandon Y, Passweg JR, Guglielmi C, Iacobelli S, Apperley J, Schaap NPM, et al. Early administration of donor lymphocyte infusions upon molecular relapse after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia: A study by the chronic malignancies working party of the EBMT. Haematologica 2014;99:1492-1498.
- Basak GW, De Wreede LC, Van Biezen A, Wiktor-Jedrzejczak W, Halaburda K, Schmid C, et al. Donor lymphocyte infusions for the treatment of chronic myeloid leukemia relapse following peripheral blood or bone marrow stem cell transplantation. Bone Marrow Transplant 2013;48:837-842.
- DeFilipp Z, Ancheta R, Liu Y, Hu ZH, Gale RP, Snyder D, et al. Maintenance tyrosine kinase inhibitors following allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia: A center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 2020;26:472-479.
- Jiang H, Xu LP, Liu DH, Liu KY, Chen SS, Jiang B, et al. Allogeneic hematopoietic SCT in combination with tyrosine kinase inhibitor treatment compared with TKI treatment alone in CML blast crisis. Bone Marrow Transplant 2014;49:1146-1154.
- Jiang Q, Xu LP, Liu DH, Liu KY, Chen SS, Jiang B, et al. Imatinib mesylate versus allogeneic hematopoietic stem cell transplantation for patients with chronic myelogenous leukemia in the accelerated phase. Blood 2011;117:3032-3040.
- Gratwohl A, Pfirrmann M, Zander A, Kröger N, Beelen D, Novotny J, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: A randomized comparison of stem cell transplantation with drug treatment. Leukemia 2016;30:562-569.
- Radujkovic A, Dietrich S, Blok H-J, Nagler A, Ayuk F, Finke J, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: A retrospective study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant 2019;25:2008-2016.
- Hehlmann R. The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA. Cell Ther Transplant. 2020; 9(4): 11-19.
" ["~DETAIL_TEXT"]=> string(32457) "
Introduction
The cases of chronic myeloid leukemia (CML) in acceleration phase (AP) and blast crisis (BC) are still associated with very unfavorable prognosis. Introduction of low-molecular BCR/ABL1 inhibitors into clinical practice has caused a decrease in BC incidence from 1.5-3.7% to 0.3-2.2% per year [1].
The median overall survival (OS) in untreated patients with BC CML does not exceed 3-6 months. The results of conservative treatment approach with chemotherapy and tyrosine kinase inhibitors (TKIs) are also unsatisfactory. The median OS in patients with BC is about 12 months [2]. According to ELN and NCCN guidelines, the evolving AP or BCs upon TKIs therapy are indications for allogeneic hemopoietic stem cell transplantation (allo-HSCT). The latter represents the only curative option for some CML patients. Starting from 2004, the cohort of allo-HSCT recipients shifted significantly from the 1st chronic phase to TKIs non-responders and initially advanced-stage CML [3-5].
The CML evolution to BC is characterized by rather distinct biological features, which make it quite different from chronic phase [6]. These differences are evident not only in such characteristics as cellular proliferation, differentiation and apoptosis, but also in clinical course and therapy response rate which is associated with clonal evolution [6].
However, according to EBMT data, the long-term prognosis for patients transplanted in BC is still unfavorable [7]. The status at the time of allo-HSCT is still one of the most important prognostic factors along with donor HLA-compatibility, disease duration, and recipient’s age [7].
According to the data by Khoury et al. the 3-year event-free survival (EFS) for patients transplanted in BC and AP is 8-11% and 26-27%, accordingly [8]. Also, in 40% of cases patients died of disease progression.
The aim of our retrospective study was to compare two patient’s cohorts receiving allo-HSCT or conservative TKI therpy in order to evaluate the therapeutic approaches that provide a survival advantage.
Patients and methods
Clinical characteristics
A total of 162 patients with CML, who had AP or BC were included in this retrospective study. All the patients included into the study were under the age of 62 years without severe cardiac, pulmonary, renal, and other comorbidities. A cohort of 82 patients received TKIs and allo-HSCT (allo-HSCT+TKI) in RM Gorbacheva Research Institute, Pavlov University since 2002 to 2019. Moreover, eighty patients received only TKIs or their combination with chemotherapy according to acute leukemia protocol, as reported elsewhere [9]. TKIs were given according to ELN recommendations [9]. The patients in TKI group did not proceed to allo-HSCT due to lack of potential stem cell donor, due to refusal for personal reasons, or delay in referral to transplant center.
The CML diagnosis was based on clinical criteria and presence of Philadelphia (Ph) chromosome and/or chimeric BCR-ABL gene [15]. The disease stage was established according to the WHO criteria [9]. Hematological, cytogenetic and molecular responses were evaluated in compliance with ELN criteria [9]. Molecular response after allo-HSCT was evaluated according to the NCCN criteria. PCR monitoring of BCR/ABL was carried out according to NCCN recommendations once in 3 months for 2 years, then once in every 3 to 6 months. Cytogenetic investigation of bone marrow was performed according to a standard procedure with at least 20 metaphases analyzed per a sample (GTG method). The karyotype was evaluated according to International System for Human Cytogenetic Nomenclature (ISCN) [10]. In cases when the standard cytogenetic investigation was not available (insufficient material), the bone marrow was assessed by fluorescence in situ hybridization (FISH) probes aiming for detection of (9;22) variants (LSI BCR-ABL , Dual Color, Dual Fusion, "Vysis").
The relative BCR-ABL1 expression level was evaluated according to method described by Gabert et al [11]. The approach consists of the following stages: 1) total RNA extraction from peripheral blood of patients with CML, 2) reverse transcription with random hexameric primers, 3) real-time PCR with primers and probes specific to р210, р190 control ABL gene sequences. Assessment of relative expression levels is based on evaluation of BCR-ABL1/ABL1 ratios in the studied cDNA samples. The ABL1 gene was used for normalization of the results. In order to determine copy numbers of the BCR-ABL1 and ABL1 transcripts, and to assess the reaction effectiveness, standard dilution curves were plotted using a plasmid with inserts of known target gene sequences (Invitrogen, USA), at a standard concentration ranges of 102-106 copies/mcl, according to 2020 European LeukemiaNet (ELN) Recommendations [9] [12].
Treatment options
The conditioning regimen included fludarabine (180 mg/m2) and busulfan (8-12 mg/kg), or 140 mg/m2 of melphalan. Fifty-four patients (66%) received post-transplant cyclophosphamide (PTCy)-based graft-versus-host disease (GvHD) prophylaxis. The PTCy was given at 50 mg/kg on D +3 and +4 after allo-HSCT 5 mg/kg of rabbit antithymocyte globulin (n=3) in combination with tacrolimus (target concentration of 5-10 ng/ml) from D+5 to D+120, and 30 mg/kg of mycophenolate mofetil (MMF) from D+5 to D+30, or 60 mg/kg of horse (n=12), or 5 mg/kg of rabbit antithymocyte globulin (n=3) in combination with tacrolimus (target concentration, 5-10 ng/ml) from D-1 to D+120, and 30 mg/kg of MMF from D-1 to D+30. If allo-HSCT was performed from matched related donor, the GvHD prophylaxis consisted of tacrolimus (target concentration of 5-10 ng/ml) from D-1 to D+120, 30 mg/kg of MMF rom D-1 to D+30, 15 mg/m2 of methotrexate on D+1, and 10 mg/m2 of methotrexate on D+3 and D+6.
The acute and chronic GvHD stage was assessed according to the common Glucksberg criteria [13], and NIH criteria [14]. The engraftment was confirmed in patients with WBC count >1 × 109/l, neutrophils of > 0.5 × 109/l without granulocyte colony stimulating factor support for 3 days, platelets count of > 20 × 109/l for 3 days. The primary non-engraftment was diagnosed, if no complete donor chimerism was observed on D+40. The comorbidity index was determined according to the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI) [15]. The allo-HSCT associated risk was evaluated in accordance with the Group for Blood and Marrow Transplantation Scale [7]. The immunosuppression was ceased, If there was a disease relapse, followed by donor lymphocyte infusions and/or TKIs were given as described before [16].
The TKIs were given in the post-transplant period to prevent relapses, or in cases of persistent BCR-ABL transcription as shown by PCR. TKIs were given if the neutrophil counts exceeded 0.5×109/l, or at platelet counts of > 50×109/l on D+60. The TKI choice was based on resistance pattern or history of intolerability. In 86% of cases, the patients were given 2nd generation TKI dasatinib, since it proved to be more effective in BC, and is able to penetrate the blood-brain barrier [17]. All the patients signed an informed consent for processing of personal data; the trial was approved by the Pavlov University Local Ethical Committee.
Evaluation of results in TKI and TKI+ allo-HSCT groups
The overall survival (OS) was estimated as a period from treatment initiation of allo-HSCT until death by any cause, or till last contact date. The event-free survival (EFS) was estimated as a period from treatment initiation of allo-HSCT until death, last contact date, or any of the following events: failure to obtain hematological response within 3 months, loss of previously achieved complete molecular response (CMR), complete cytogenetic response (CCR), or complete hematological response (CHR), post-transplant molecular relapse. Post-transplant relapse was diagnosed in case of two consecutive positive PCR assays or at least 1-log persistent increase of BCR/ABL transcript level. If patient received a second allo-HSCT due to primary non-engraftment or a relapse, the survival terms were dated back from second allo-HSCT.
Statistical analysis
The standard SPSS, IBM Statistics and R 1.41 software was used for statistical evaluation. The quantitative attributes of groups were compared using Mann-Whitney U-test. The qualitative attributes were compared by Chi-square test, Fisher’s exact test. Survival charts were plotted using Kaplan-Meier method. The statistical significance of differences evaluated via Kaplan-Meier test was checked by Log-rank test, the differences at р <0.05 were considered statistically significant. The cumulative risk of non relapse-related mortality was evaluated as competing risk.
Results
A total of 162 patients with advanced CML were included into the study. In 82 cases, the allo-HSCT was performed. The remaining 80 patients received only TKIs or TKIs in combination with chemotherapy. The median follow-up was 44 (1 to 344) months. There were no significant differences in gender, age, somatic status, disease phase or presence of additional chromosomal aberrations (ACAs) between allo-HSCT and TKI groups (see Tab. 1). The number of patient with BC was higher in allo-HSCT group compared to TKI group, i.e., 28% versus 12%. At the same time, non-transplanted patients were more likely to receive 3rd line of TKIs than allo-HSCT group (37% versus 18%) as seen from Table 1.
Table 1. Characteristics of patient groups receiving allo-HSCT or TKIs



Therapy and clinical response prior to allo-HSCT
A total of 82 patients received allo-HSCT. In 48% of cases, the disease was initially in CP, and it progressed later to more advanced stage (AC or BC). 52% of the cases had an advanced-stage disease at diagnosis. The median time span from diagnosis to allo-HSCT was 2.2 (0.3-21.4) years. Most patients received chemotherapy in combination with TKIs, 37% of patients was administered only TKIs. All but two patients, who received allo-HSCT in 2002, had previous history of TKI treatment. Most patients (61%) had 2 or 3 lines of TKIs, with imatinib, dasatinib or nilotinib used. Only seven patients received bosutinib and two patients received ponatinib.
Patients in CP at diagnosis
A total of 29 (74%) of patients who were initially in CP, later developed BC. In 17 cases, a 2nd CP was achieved prior to allo-HSCT. Six patients were subjected to allo-HSCT in AP, six patients did not respond to therapy and underwent allo-HSCT in BC. In ten patients, the AP developed, and it persisted until allo-HSCT.
Patients in AP at diagnosis
A total of 20 patients had AP at diagnosis, ten of them subsequently developed blast crisis (BC). In six cases, the CP was achieved after TKI + chemotherapy (n=4) or TKI treatment (n=2); two patients were in AP after TKI + chemotherapy, and two patients still had BC after TKI + chemotherapy (n=1) or TKI only (n=1) at the moment of allo-HSCT. The remaining 10 patients had no history of BC. Six of them subsequently achieved CP, four patients were still in AP at the moment of allo-HSCT.
Patients with BC at diagnosis
A total of 23 patients had BC at the time of diagnosis. In 20 cases, a subsequent chronic phase was achieved after TKI + chemotherapy (n=17) or TKI therapy (n=3), in one case, AP was documented after TKI therapy, and two patients were still in BC after TKI + chemotherapy (n=2) at the moment of allo-HSCT.

Figure 1. Cumulative 2-year relapse rate and 1-year non-relapse mortality after allo-HSCT.
Abbreviations: NRM, non-relapse mortality
Engraftment, causes of death and non-relapse mortality
Post-transplant engraftment was achieved in 71 (86%) patients. The median time to WBC engraftment was 22 (8 to 39) days, median time to the neutrophil engraftment was 22 (8 to 35) days. Median time to the platelet engraftment was 19 (6 to 57) days. In 9 cases of primary non-engraftment, the 2nd allo-HSCT was performed. The median follow-up was 35 (1 to 161) months.
Thirty-two patients died after allo-HSCT. The most common causes of death were relapse (n=16, 50%); GvHD (n=8, 25%); infectious complications (n=5, 16%); heart failure (n=2, 6%); hepatic veno-occlusive disease (n=1, 3%). One-year NRM was 18% (95% CI 10-28%), 100-day NRM 10% (95% CI 5-18%), as shown in Fig. 1.
Also, 48 (58%) patients received TKIs after allo-HSCT, 45 (88%) of them as relapse prophylaxis. 28 patients did not develop subsequent relapses. Two patients received bosutinib, 36 dasatinib, 8 nilotinib, and 2 ponatinib.
In 31 cases (38%), a relapse was developed, 16 of patients who relapsed received TKI prophylaxis. The cumulative relapse incidence was 39% (95% CI 28-51%). In 11 patients (34%), molecular relapse was shown; in one case, cytogenetic (3%), and in 19 cases (63%), hematological relapses were documented. Six patients received donor lymphocyte infusions (DLIs) for relapse treatment, five subjects received only TKIs; TKIs, chemotherapy and DLI were used in four cases; TKIs and DLIs were applied in 15 patients, and a combination of DLI and chemotherapy was used in one case. In 29% of the cases (n=9), the patients had durable molecular response, in 61% (n=19), the disease progression. Evaluation of therapeutic response was impossible for 10% of the patients. DLI was performed in the remaining six 6 cases, due to primary non-engraftment or poor graft function post-transplant. In three cases, DLIs were carried out due to persisting BCR/ABL transcript.
The incidence of grade 2-4 acute GvHD was 29% (n=21), grade 3-4 acute GvHD was registered in 20% (n=14). Chronic GvHD (cGvHD) incidence was 27% (n=18), having been mild in 6 cases (9%). Moderate cGvHD was observed in 8 cases (12%), and four patients (6%) developed severe cGvHD.
Patients who received only TKIs
A cohort of 80 patients received only TKIs, or their combination with chemotherapy. The median follow-up was 93 (13-344) months. The data on outcomes was available for 71 patients. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among 10 patients without history of BC, one patient did not respond, 5 achieved CHR; 2 patients developed CCR, and CMR was registered in 2 cases. Sixty-nine patients died, more than half of them deceased due to disease progression/relapse.

Figure 2. Four-year OS for allo-HSCT+TKIs and TKIs groups (А), and four-year EFS for allo-HSCT+TKIs and TKIs groups (B)
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; TKI, tyrosine kinase inhibitor

Figure 3. Four-year OS for patients treated in BC who received allo-HSCT+TKIs in CP≥2/AP+TKI, TKIs, and allo- HSCT in BC
Abbreviations: allo-HSCT, allogeneic hematopoietic stem cell transplantation; CP≥2, chronic phase; AP, acceleration phase; TKI, therapy with tyrosine kinase inhibitors; allo-HSCT in BC +TKI, allogeneic hematopoietic stem cell transplantation in blast crisis
Comparisons between allo-HSCT+TKIs and TKIs groups
The CML patients who received allo-HSCT exhibited significantly better 4-year OS compared to the TKI-treated group, i.e., 58% (95% CI 44%-69%) versus 33% (95% CI 23-44%), accordingly (р=0.032), as shown in Fig. 2А). At the same time, no statistically significant differences were found between the 4-year EFS, which was 35% (95% CI 24%-47%) in allo-HSCT, and 17% (95% CI 10%-26%) in TKIs group (р=0.5) (Fig. 2B). Also, there was no statistically significant difference between the 4-year OS receiving allo-HSCT in BC CML. This value was 33% for TKIs group (95% CI 23%-44%), and 23% for allo-HSCT patients (95% CI 3-52%) (р=0.217). However, the 4-year OS reached 63% (95% CI 48%-74%) for the patients transplanted in AP or CP after TKIs therapy, which is significantly better compared to other groups (р=0.005) (Fig. 3).
Discussion
In our study, we compared therapy results for advanced-phase patients with CML who received TKIs with or without allo- HSCT. In allo-HSCT group, the 4-year OS was significantly longer if compared to patients, who were not transplanted (58% versus 33%, accordingly). The 4-year EFS was also longer in allo-HSCT group (35% versus 17%, accordingly), although without statistical significance in this case.
One should note that the relapse rate in allotransplanted patients is still as high as 39%. However, CML is one of the most immunotherapy-responsive malignancies. Kolb et al. have first shown clinical success of DLIs in allo-HSCT recipients with CML [18]. Moreover, development of acute or chronic GvHD was more important for CML patients as a factor able to decrease relapse rate compared to patients with AML, MDS or plasma cell disorders [19, 20].
This may be a reason for higher OS in allo-HSCT group, while EFS rates have not differed significantly. As many relapses were sensitive to immune therapy, they were not invariably fatal, and many patients responded to post-transplant therapy. In our study, 29% of the patients achieved durable CMR upon DLI-containing treatment. However, usage of this method may be limited due to the risk of GvHD, since grade 2-4 acute GVHD develops in 15%, and chronic GvHD occurs in 22% of patients [21]. Donor lymphocyte infusions should not be performed in patients with a history of chronic GvHD. Moreover, donor cells are not available in some cases.
Efficiency of donor lymphocyte infusions depends on many factors. The study by Basak et al. in patients with CML has shown that PBSCs as graft source worsen the long-term OS among DLI recipients. The authors note that there were more patients with advanced stages in PBSC group, and suppose a connection between worse DLI effect and immune response attenuation due to lower immunogenicity of malignant cells in advanced stage patients [22]. Early CML relapse is another potentially negative factor making DLIs less effective [22].
The response to DLI may depend on the type of relapse. In this study we performed DLI for 26 patients who usually presented with hematological relapses. In a study by Radujkovic et al., this method was more effective in treatment of patients with molecular or cytogenetic, but not hematologic relapses. Five-year relapse and GvHD-free survival in patients with cytogenetic and hematological relapses was 40% and 20%, accordingly [22]. In our study, more than a half of relapsed patients developed hematological relapse, which may be a reason for lower response rate observed.
Prophylactic use of TKIs may exert important influence on the allo-HSCT outcomes. The relapses developed only in 38% of HSCT recipients after TKI-based prophylaxis. However, the actual role of prophylactic interventions is yet not quite clear. The largest patient cohort described by DeFilipp et al. included 89 patients [23]. The authors have not found significant differences in OS, which was 61% in recipients with post-transplant TKI prophylaxis versus 57% in the patients without such prophylaxis, and EFS was 42% versus 44%, accordingly (all differences not statistically significant). Hence, the results of our retrospective study may suggest whether prophylactic TKIs improve allo-HSCT results.
Some studies have compared the results of allo-HSCT and TKIs with or without chemotherapy in advanced-stage CML patients. Worth of note, the 1st line therapy was performed in most of these reports. In our study, however, nearly half of patients in each group received 2nd line TKIs; 38% in TKIs and 20% in allo-HSCT+TKIs group received 3 or more lines of TKIs. Allo-HSCT was not performed, due to the lack of potential donor or failure to sign an informed consent. Therefore, the non-transplant group was more likely to receive 3rd line TKIs than the allo-HSCT group. In study by Jiang et al., the results of TKIs with or without allo-HSCT were evaluated in 83 patients with CML BC. The allo-HSCT proved to be advantageous in 4-year OS compared to other treatment modalities, with OS of 46.7% versus 9.7%, and EFS of 47.1% versus 6.7%, accordingly [24]. Jiang et al. analyzed imatinib therapy results compared with allo-HSCT in 132 AP CML cases and found survival advantage for allo-HSCT compared to TKI group [25], with 5-year OS of 100% versus 18% and EFS 67% versus 9%, accordingly. Jain et al. had analyzed survival rates from the moment of BC diagnosis in a group including 104 allo-HSCT recipients and demonstrated that allo-HSCT decreases risk of death [2].
Allo-HSCT in patients with BC lead to the same results as other treatment options, with 4-year OS of 33% and 23% in TKIs and allo-HSCT groups, respectively. It corresponds to the published data from some other studies [24, 26, 27, 28], which makes us to recommend preferential usage of this approach in the patients with therapy-resistant disease.
Of course, the retrospective nature of our study urges us to be cautious for interpretation of its results. Meanwhile, we compared here extensive groups without showing statistically significant differences for the main clinical characteristics that could influence the CML outcomes.
Conclusions
This study has shown allo-HSCT still to be a curative method in many patients with AP and BC of CML in the presence of new-generation TKIs. However, the relapses are probable even after allo-HSCT. Due to good response to immune therapy in CML patients, a long-term remission and even curation may be achieved even after relapse of malignancy following allo-HSCT.
Tyrosine kinase inhibitors may be used as additional method for relapse therapy and prophylaxis. Performing allo-HSCT in patients with BC without achievement of hematological response does not lead to significantly better outcome. The indications for allo-HSCT should be discussed individually for each patient.
Ackhowledgements
No conflicts of interest reported.
References
- Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 2017;31:2398-2406.
- Jain P, Kantarjian HM, Ghorab A, Sasaki K, Jabbour EJ, Nogueras Gonzalez G, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: Cohort study of 477 patients. Cancer 2017;123:4391-4402.
- Bacher U, Klyuchnikov E, Zabelina T, Ottinger H, Beelen DW, Schrezenmeier H, et al. The changing scene of allogeneic stem cell transplantation for chronic myeloid leukemia – A report from the German Registry covering the period from 1998 to 2004. Ann Hematol 2009;88:1237-1247.
- Morozova E, Barabanshchikova M, Mamaev N, Barkhatov I, Alyanskii A, Darskaya E, et al. Chronic myeloid leukemia: Role of allogeneic hematopoietic stem cell transplantation in the era of tyrosine kinase inhibitors. Clin Oncohematology 2020;13:193-198 In Russian).
- Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: Evaluation of its impact within a subgroup of the randomized German CML study IV. Blood 2010;115:1880-1885.
- Calabretta B, Perrotti D. Review in translational hematology The biology of CML blast crisis. Blood 2004;103:4010-4022.
- Gratwohl A. The EBMT risk score. Bone Marrow Transplant 2012;47:749-756.
- Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: A CIBMTR analysis. Bone Marrow Transplant 2012;47:810-816.
- Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020;34:966-984.
- Simons A, Shaffer LG, Hastings RJ. Cytogenetic Nomenclature: Changes in the ISCN 2013 Compared to the 2009 Edition. Cytogenet Genome Res 2013;141:1-6.
- Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of "real time" quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – A Europe Against Cancer Program. Leukemia 2003;17:2318-2357.
- Soverini S, Hochhaus A, Nicolini FE, Gruber F, Lange T, Saglio G, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: Recommendations from an expert panel on behalf of European LeukemiaNet. Blood 2011;118:1208-1215.
- Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Jean Henslee‐Downey P, et al. IBMTR Severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855-864.
- Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant 2015;21:389-401.e1.
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912-2919.
- Radujkovic A, Guglielmi C, Bergantini S, Iacobelli S, van Biezen A, Milojkovic D, et al. Donor lymphocyte infusions for chronic myeloid leukemia relapsing after allogeneic stem cell transplantation: May we predict graft-versus-leukemia without graft-versus-host disease? Biol Blood Marrow Transplant 2015;21:1230-1236.
- Porkka K, Koskenvesa P, Lundán T, Rimpiläinen J, Mustjoki S, Smykla R, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system philadelphia chromosome positive leukemia. Blood 2008;112:1005-1012.
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990;76:2462-2465.
- Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Impact of graft-versus-host disease on relapse after allogeneic hematopoietic stem cell transplantation, an EBMT Megafile Study. Blood 2012;120:469-469.
- Oyekunle AA, Kröger N, Zabelina T, Ayuk F, Schieder H, Renges H, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: A long-term follow-up. Bone Marrow Transplant 2006;37:45-50.
- Chalandon Y, Passweg JR, Guglielmi C, Iacobelli S, Apperley J, Schaap NPM, et al. Early administration of donor lymphocyte infusions upon molecular relapse after allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia: A study by the chronic malignancies working party of the EBMT. Haematologica 2014;99:1492-1498.
- Basak GW, De Wreede LC, Van Biezen A, Wiktor-Jedrzejczak W, Halaburda K, Schmid C, et al. Donor lymphocyte infusions for the treatment of chronic myeloid leukemia relapse following peripheral blood or bone marrow stem cell transplantation. Bone Marrow Transplant 2013;48:837-842.
- DeFilipp Z, Ancheta R, Liu Y, Hu ZH, Gale RP, Snyder D, et al. Maintenance tyrosine kinase inhibitors following allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia: A center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 2020;26:472-479.
- Jiang H, Xu LP, Liu DH, Liu KY, Chen SS, Jiang B, et al. Allogeneic hematopoietic SCT in combination with tyrosine kinase inhibitor treatment compared with TKI treatment alone in CML blast crisis. Bone Marrow Transplant 2014;49:1146-1154.
- Jiang Q, Xu LP, Liu DH, Liu KY, Chen SS, Jiang B, et al. Imatinib mesylate versus allogeneic hematopoietic stem cell transplantation for patients with chronic myelogenous leukemia in the accelerated phase. Blood 2011;117:3032-3040.
- Gratwohl A, Pfirrmann M, Zander A, Kröger N, Beelen D, Novotny J, et al. Long-term outcome of patients with newly diagnosed chronic myeloid leukemia: A randomized comparison of stem cell transplantation with drug treatment. Leukemia 2016;30:562-569.
- Radujkovic A, Dietrich S, Blok H-J, Nagler A, Ayuk F, Finke J, et al. Allogeneic stem cell transplantation for blast crisis chronic myeloid leukemia in the era of tyrosine kinase inhibitors: A retrospective study by the EBMT Chronic Malignancies Working Party. Biol Blood Marrow Transplant 2019;25:2008-2016.
- Hehlmann R. The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA. Cell Ther Transplant. 2020; 9(4): 11-19.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(100) "klinicheskie-iskhody-u-patsientov-s-khronicheskim-mieloidnym-leykozom-prodvinutykh-stadiy-pri-alloge" ["~CODE"]=> string(100) "klinicheskie-iskhody-u-patsientov-s-khronicheskim-mieloidnym-leykozom-prodvinutykh-stadiy-pri-alloge" ["EXTERNAL_ID"]=> string(4) "1938" ["~EXTERNAL_ID"]=> string(4) "1938" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(431) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нееThe outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation " ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(4447) "<p style="text-align: justify;">Клинический прогноз у пациентов с хроническим миелоидным лейкозом (ХМЛ) в развернутой стадии (фаза акселерации – ФА, или бластный криз – БК) все еще остается неблагоприятным в эру применения ингибиторов тирозинкиназ (ИТК). Данное исследование проводилось, чтобы оценить, насколько аллогенная трансплантация гемопоэтических клеток (алло-ТГСК) улучшает их прогноз.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">Общая группа из 162 пациентов с ХМЛ в ФА/БК была разделена на две гомогенные когорты. Первая из них состояла из реципиентов, получавших кондиционирование со сниженной интенсивностью перед алло-ТГСК (n=82). Вторая группа (n=80) включала пациентов, получавших только терапию на базе ИТК (в 85% случаев – препараты 2-го и 3-го поколения), не направленных в центры трансплантации или отказавшихся от нее. Ответ на терапию определяли в соответствии с рекомендациями ELN и NCCN. </p> <h3>Результаты</h3> <p style="text-align: justify;">Медиана сроков наблюдения для всей когорты составляла 44 (1-344) мес. Среди пациентов с БК 36 больных (59%) не отвечали на лечение, в 22 случаях (34%) была установлена полная гематологическая ремиссия (CHR), в одном случае (2%) – полная цитогенетическая ремиссия, и полный молекулярный ответ (ПМО) был достигнут в 2 случаях (3%). Среди реципиентов алло-ТГСК, приживление отмечено в 86% случаев. Кумулятивная безрецидивная смертность на D+100 и через 1 год составила, соответственно, 10% и 18%. У 28 пациентов с посттрансплантационным рецидивом проведена дополнительная терапия, и достигнут ПМО в 9 случаях. Общая 4-летняя выживаемость и бессобытийная выживаемость (ОВ) были лучше после алло-ТГСК по сравнению с группой, леченой ИТК: 58% против 33% (p=0,032) и 35% против 17% (p=0,5), соответственно. Пациенты в БК на момент ТГСК имели значительно более низкие уровни 3-летней ОВ по сравнению с больными, ответившими на лечение: 23% против 63% (p=0,007), соответственно. </p> <h3>Заключение</h3> <p style="text-align: justify;">Хотя алло-ТГСК имеет преимущество у многих больных ХМЛ в развернутых стадиях, результаты ее применения при БК сравнимы с лечением ИТК. Поэтому данные пациенты должны направляться в центры трансплантации по мере достижения ими второй хронической фазы заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, BCR/ABL, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназы, бластный криз, исходы заболевания.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_META_TITLE"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_META_KEYWORDS"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_META_DESCRIPTION"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_PICTURE_FILE_ALT"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_PICTURE_FILE_TITLE"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "klinicheskie-iskhody-u-patsientov-s-khronicheskim-mieloidnym-leykozom-prodvinutykh-stadiy-pri-alloge" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(294) "Клинические исходы у пациентов с хроническим миелоидным лейкозом продвинутых стадий при аллогенной трансплантации гемопоэтических стволовых клеток и без нее" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "klinicheskie-iskhody-u-patsientov-s-khronicheskim-mieloidnym-leykozom-prodvinutykh-stadiy-pri-alloge" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "klinicheskie-iskhody-u-patsientov-s-khronicheskim-mieloidnym-leykozom-prodvinutykh-stadiy-pri-alloge" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "klinicheskie-iskhody-u-patsientov-s-khronicheskim-mieloidnym-leykozom-prodvinutykh-stadiy-pri-alloge" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "170" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27337" ["VALUE"]=> string(22) "07/25/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "07/25/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27338" ["VALUE"]=> string(22) "10/23/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/23/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27323" ["VALUE"]=> array(2) { ["TEXT"]=> string(547) "<p>Елена В. Морозова, Юлия Ю. Власова, Мария В. Барабанщикова, Ксения С. Юровская, Татьяна В. Шнайдер, Татьяна Л. Гиндина, Ильдар М. Бархатов, Евгений А. Бакин, Иван С. Моисеев, Александр Д. Кулагин, Людмила С. Зубаровская, <br><span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(507) "
Елена В. Морозова, Юлия Ю. Власова, Мария В. Барабанщикова, Ксения С. Юровская, Татьяна В. Шнайдер, Татьяна Л. Гиндина, Ильдар М. Бархатов, Евгений А. Бакин, Иван С. Моисеев, Александр Д. Кулагин, Людмила С. Зубаровская,
Борис В. Афанасьев
НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27325" ["VALUE"]=> array(2) { ["TEXT"]=> string(4447) "<p style="text-align: justify;">Клинический прогноз у пациентов с хроническим миелоидным лейкозом (ХМЛ) в развернутой стадии (фаза акселерации – ФА, или бластный криз – БК) все еще остается неблагоприятным в эру применения ингибиторов тирозинкиназ (ИТК). Данное исследование проводилось, чтобы оценить, насколько аллогенная трансплантация гемопоэтических клеток (алло-ТГСК) улучшает их прогноз.</p> <h3>Пациенты и методы</h3> <p style="text-align: justify;">Общая группа из 162 пациентов с ХМЛ в ФА/БК была разделена на две гомогенные когорты. Первая из них состояла из реципиентов, получавших кондиционирование со сниженной интенсивностью перед алло-ТГСК (n=82). Вторая группа (n=80) включала пациентов, получавших только терапию на базе ИТК (в 85% случаев – препараты 2-го и 3-го поколения), не направленных в центры трансплантации или отказавшихся от нее. Ответ на терапию определяли в соответствии с рекомендациями ELN и NCCN. </p> <h3>Результаты</h3> <p style="text-align: justify;">Медиана сроков наблюдения для всей когорты составляла 44 (1-344) мес. Среди пациентов с БК 36 больных (59%) не отвечали на лечение, в 22 случаях (34%) была установлена полная гематологическая ремиссия (CHR), в одном случае (2%) – полная цитогенетическая ремиссия, и полный молекулярный ответ (ПМО) был достигнут в 2 случаях (3%). Среди реципиентов алло-ТГСК, приживление отмечено в 86% случаев. Кумулятивная безрецидивная смертность на D+100 и через 1 год составила, соответственно, 10% и 18%. У 28 пациентов с посттрансплантационным рецидивом проведена дополнительная терапия, и достигнут ПМО в 9 случаях. Общая 4-летняя выживаемость и бессобытийная выживаемость (ОВ) были лучше после алло-ТГСК по сравнению с группой, леченой ИТК: 58% против 33% (p=0,032) и 35% против 17% (p=0,5), соответственно. Пациенты в БК на момент ТГСК имели значительно более низкие уровни 3-летней ОВ по сравнению с больными, ответившими на лечение: 23% против 63% (p=0,007), соответственно. </p> <h3>Заключение</h3> <p style="text-align: justify;">Хотя алло-ТГСК имеет преимущество у многих больных ХМЛ в развернутых стадиях, результаты ее применения при БК сравнимы с лечением ИТК. Поэтому данные пациенты должны направляться в центры трансплантации по мере достижения ими второй хронической фазы заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Хронический миелоидный лейкоз, BCR/ABL, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназы, бластный криз, исходы заболевания.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(4289) "Клинический прогноз у пациентов с хроническим миелоидным лейкозом (ХМЛ) в развернутой стадии (фаза акселерации – ФА, или бластный криз – БК) все еще остается неблагоприятным в эру применения ингибиторов тирозинкиназ (ИТК). Данное исследование проводилось, чтобы оценить, насколько аллогенная трансплантация гемопоэтических клеток (алло-ТГСК) улучшает их прогноз.
Пациенты и методы
Общая группа из 162 пациентов с ХМЛ в ФА/БК была разделена на две гомогенные когорты. Первая из них состояла из реципиентов, получавших кондиционирование со сниженной интенсивностью перед алло-ТГСК (n=82). Вторая группа (n=80) включала пациентов, получавших только терапию на базе ИТК (в 85% случаев – препараты 2-го и 3-го поколения), не направленных в центры трансплантации или отказавшихся от нее. Ответ на терапию определяли в соответствии с рекомендациями ELN и NCCN.
Результаты
Медиана сроков наблюдения для всей когорты составляла 44 (1-344) мес. Среди пациентов с БК 36 больных (59%) не отвечали на лечение, в 22 случаях (34%) была установлена полная гематологическая ремиссия (CHR), в одном случае (2%) – полная цитогенетическая ремиссия, и полный молекулярный ответ (ПМО) был достигнут в 2 случаях (3%). Среди реципиентов алло-ТГСК, приживление отмечено в 86% случаев. Кумулятивная безрецидивная смертность на D+100 и через 1 год составила, соответственно, 10% и 18%. У 28 пациентов с посттрансплантационным рецидивом проведена дополнительная терапия, и достигнут ПМО в 9 случаях. Общая 4-летняя выживаемость и бессобытийная выживаемость (ОВ) были лучше после алло-ТГСК по сравнению с группой, леченой ИТК: 58% против 33% (p=0,032) и 35% против 17% (p=0,5), соответственно. Пациенты в БК на момент ТГСК имели значительно более низкие уровни 3-летней ОВ по сравнению с больными, ответившими на лечение: 23% против 63% (p=0,007), соответственно.
Заключение
Хотя алло-ТГСК имеет преимущество у многих больных ХМЛ в развернутых стадиях, результаты ее применения при БК сравнимы с лечением ИТК. Поэтому данные пациенты должны направляться в центры трансплантации по мере достижения ими второй хронической фазы заболевания.
Ключевые слова
Хронический миелоидный лейкоз, BCR/ABL, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназы, бластный криз, исходы заболевания.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27326" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-20-28" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-20-28" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27329" ["VALUE"]=> array(2) { ["TEXT"]=> string(362) "<p>Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(328) "Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27330" ["VALUE"]=> array(2) { ["TEXT"]=> string(479) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p> <br> <p><b>Correspondence</b><br> Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7(911) 927 8229<br> E-mail: dr_morozova@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(419) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(911) 927 8229
E-mail: dr_morozova@mail.ru
Prognosis of patients with advanced stage CML (accelerated phase, AP, or blast crisis, BC) is still dismal in the era of tyrosine kinase inhibitors (TKIs). This study is aimed to evaluate whether allogeneic hemopoietic stem cell transplantation (allo-HSCT) improves their prognosis.
A total of 162 patients with AP/BC CML were divided into two homogeneous cohorts. The first one consisted of reduced-intensity conditioning allo-HSTC (n=82) recipients. The second (n=80) consisted of patients receiving only TKI-based therapy (in 85% of cases 2nd and 3rd generation TKIs) while not being referred to transplant center or refusing allo-HSCT. The response to therapy was defined according to ELN and NCCN recommendations.
The median follow-up for entire cohort was 44 (1-344) months. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among allo-HST recipients 86% engrafted, the D+100 and 1-year cumulative non-relapse mortality were 10% and 18%, respectively. Twenty eight patients with post-transplant relapse received additional therapy achieving CMR in 9 cases. The 4-year OS and EFS were better in allo-HSCT compared to TKIs group: 58% vs 33% (p=0.032) and 35% vs 17% (p=0.5), accordingly. Patients in BC at the moment of allo-HSCT had significantly worse 4-year OS compared to responders: 23% vs 63% (p=0.007), accordingly. While allo-HSCT has an advantage for many advanced-stage CML patients, in BC its results are comparable to TKIs treatment. Therefore, these patients should be referred to transplant center as soon as the second chronic phase is achieved.
Keywords
Chronic myelogenous leukemia, BCR/ABL, allo-HSCT, tyrosine kinase inhibitors, blast crisis, outcomes.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27327" ["VALUE"]=> string(137) "The outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation " ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(137) "The outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation " ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27328" ["VALUE"]=> string(4) "2311" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2311" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27332" ["VALUE"]=> string(4) "2312" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2312" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27329" ["VALUE"]=> array(2) { ["TEXT"]=> string(362) "<p>Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(328) "Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Boris V. Afanasyev
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(328) "Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Boris V. Afanasyev
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27331" ["VALUE"]=> array(2) { ["TEXT"]=> string(2185) "<p style="text-align: justify;">Prognosis of patients with advanced stage CML (accelerated phase, AP, or blast crisis, BC) is still dismal in the era of tyrosine kinase inhibitors (TKIs). This study is aimed to evaluate whether allogeneic hemopoietic stem cell transplantation (allo-HSCT) improves their prognosis. </p> <p style="text-align: justify;">A total of 162 patients with AP/BC CML were divided into two homogeneous cohorts. The first one consisted of reduced-intensity conditioning allo-HSTC (n=82) recipients. The second (n=80) consisted of patients receiving only TKI-based therapy (in 85% of cases 2nd and 3rd generation TKIs) while not being referred to transplant center or refusing allo-HSCT. The response to therapy was defined according to ELN and NCCN recommendations. </p> <p style="text-align: justify;">The median follow-up for entire cohort was 44 (1-344) months. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among allo-HST recipients 86% engrafted, the D+100 and 1-year cumulative non-relapse mortality were 10% and 18%, respectively. Twenty eight patients with post-transplant relapse received additional therapy achieving CMR in 9 cases. The 4-year OS and EFS were better in allo-HSCT compared to TKIs group: 58% <i>vs</i> 33% (p=0.032) and 35% <i>vs</i> 17% (p=0.5), accordingly. Patients in BC at the moment of allo-HSCT had significantly worse 4-year OS compared to responders: 23% <i>vs</i> 63% (p=0.007), accordingly. While allo-HSCT has an advantage for many advanced-stage CML patients, in BC its results are comparable to TKIs treatment. Therefore, these patients should be referred to transplant center as soon as the second chronic phase is achieved.</p> <h2>Keywords</h2> <p style="text-align: justify;">Chronic myelogenous leukemia, BCR/ABL, allo-HSCT, tyrosine kinase inhibitors, blast crisis, outcomes.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2049) "Prognosis of patients with advanced stage CML (accelerated phase, AP, or blast crisis, BC) is still dismal in the era of tyrosine kinase inhibitors (TKIs). This study is aimed to evaluate whether allogeneic hemopoietic stem cell transplantation (allo-HSCT) improves their prognosis.
A total of 162 patients with AP/BC CML were divided into two homogeneous cohorts. The first one consisted of reduced-intensity conditioning allo-HSTC (n=82) recipients. The second (n=80) consisted of patients receiving only TKI-based therapy (in 85% of cases 2nd and 3rd generation TKIs) while not being referred to transplant center or refusing allo-HSCT. The response to therapy was defined according to ELN and NCCN recommendations.
The median follow-up for entire cohort was 44 (1-344) months. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among allo-HST recipients 86% engrafted, the D+100 and 1-year cumulative non-relapse mortality were 10% and 18%, respectively. Twenty eight patients with post-transplant relapse received additional therapy achieving CMR in 9 cases. The 4-year OS and EFS were better in allo-HSCT compared to TKIs group: 58% vs 33% (p=0.032) and 35% vs 17% (p=0.5), accordingly. Patients in BC at the moment of allo-HSCT had significantly worse 4-year OS compared to responders: 23% vs 63% (p=0.007), accordingly. While allo-HSCT has an advantage for many advanced-stage CML patients, in BC its results are comparable to TKIs treatment. Therefore, these patients should be referred to transplant center as soon as the second chronic phase is achieved.
Keywords
Chronic myelogenous leukemia, BCR/ABL, allo-HSCT, tyrosine kinase inhibitors, blast crisis, outcomes.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2049) "Prognosis of patients with advanced stage CML (accelerated phase, AP, or blast crisis, BC) is still dismal in the era of tyrosine kinase inhibitors (TKIs). This study is aimed to evaluate whether allogeneic hemopoietic stem cell transplantation (allo-HSCT) improves their prognosis.
A total of 162 patients with AP/BC CML were divided into two homogeneous cohorts. The first one consisted of reduced-intensity conditioning allo-HSTC (n=82) recipients. The second (n=80) consisted of patients receiving only TKI-based therapy (in 85% of cases 2nd and 3rd generation TKIs) while not being referred to transplant center or refusing allo-HSCT. The response to therapy was defined according to ELN and NCCN recommendations.
The median follow-up for entire cohort was 44 (1-344) months. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among allo-HST recipients 86% engrafted, the D+100 and 1-year cumulative non-relapse mortality were 10% and 18%, respectively. Twenty eight patients with post-transplant relapse received additional therapy achieving CMR in 9 cases. The 4-year OS and EFS were better in allo-HSCT compared to TKIs group: 58% vs 33% (p=0.032) and 35% vs 17% (p=0.5), accordingly. Patients in BC at the moment of allo-HSCT had significantly worse 4-year OS compared to responders: 23% vs 63% (p=0.007), accordingly. While allo-HSCT has an advantage for many advanced-stage CML patients, in BC its results are comparable to TKIs treatment. Therefore, these patients should be referred to transplant center as soon as the second chronic phase is achieved.
Keywords
Chronic myelogenous leukemia, BCR/ABL, allo-HSCT, tyrosine kinase inhibitors, blast crisis, outcomes.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27326" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-20-28" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-20-28" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-20-28" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27327" ["VALUE"]=> string(137) "The outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation " ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(137) "The outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation " ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(137) "The outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation " } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27330" ["VALUE"]=> array(2) { ["TEXT"]=> string(479) "<p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p> <br> <p><b>Correspondence</b><br> Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br> Phone: +7(911) 927 8229<br> E-mail: dr_morozova@mail.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(419) "RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(911) 927 8229
E-mail: dr_morozova@mail.ru
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(911) 927 8229
E-mail: dr_morozova@mail.ru
Елена В. Морозова, Юлия Ю. Власова, Мария В. Барабанщикова, Ксения С. Юровская, Татьяна В. Шнайдер, Татьяна Л. Гиндина, Ильдар М. Бархатов, Евгений А. Бакин, Иван С. Моисеев, Александр Д. Кулагин, Людмила С. Зубаровская,
Борис В. Афанасьев
Елена В. Морозова, Юлия Ю. Власова, Мария В. Барабанщикова, Ксения С. Юровская, Татьяна В. Шнайдер, Татьяна Л. Гиндина, Ильдар М. Бархатов, Евгений А. Бакин, Иван С. Моисеев, Александр Д. Кулагин, Людмила С. Зубаровская,
Борис В. Афанасьев
Клинический прогноз у пациентов с хроническим миелоидным лейкозом (ХМЛ) в развернутой стадии (фаза акселерации – ФА, или бластный криз – БК) все еще остается неблагоприятным в эру применения ингибиторов тирозинкиназ (ИТК). Данное исследование проводилось, чтобы оценить, насколько аллогенная трансплантация гемопоэтических клеток (алло-ТГСК) улучшает их прогноз.
Пациенты и методы
Общая группа из 162 пациентов с ХМЛ в ФА/БК была разделена на две гомогенные когорты. Первая из них состояла из реципиентов, получавших кондиционирование со сниженной интенсивностью перед алло-ТГСК (n=82). Вторая группа (n=80) включала пациентов, получавших только терапию на базе ИТК (в 85% случаев – препараты 2-го и 3-го поколения), не направленных в центры трансплантации или отказавшихся от нее. Ответ на терапию определяли в соответствии с рекомендациями ELN и NCCN.
Результаты
Медиана сроков наблюдения для всей когорты составляла 44 (1-344) мес. Среди пациентов с БК 36 больных (59%) не отвечали на лечение, в 22 случаях (34%) была установлена полная гематологическая ремиссия (CHR), в одном случае (2%) – полная цитогенетическая ремиссия, и полный молекулярный ответ (ПМО) был достигнут в 2 случаях (3%). Среди реципиентов алло-ТГСК, приживление отмечено в 86% случаев. Кумулятивная безрецидивная смертность на D+100 и через 1 год составила, соответственно, 10% и 18%. У 28 пациентов с посттрансплантационным рецидивом проведена дополнительная терапия, и достигнут ПМО в 9 случаях. Общая 4-летняя выживаемость и бессобытийная выживаемость (ОВ) были лучше после алло-ТГСК по сравнению с группой, леченой ИТК: 58% против 33% (p=0,032) и 35% против 17% (p=0,5), соответственно. Пациенты в БК на момент ТГСК имели значительно более низкие уровни 3-летней ОВ по сравнению с больными, ответившими на лечение: 23% против 63% (p=0,007), соответственно.
Заключение
Хотя алло-ТГСК имеет преимущество у многих больных ХМЛ в развернутых стадиях, результаты ее применения при БК сравнимы с лечением ИТК. Поэтому данные пациенты должны направляться в центры трансплантации по мере достижения ими второй хронической фазы заболевания.
Ключевые слова
Хронический миелоидный лейкоз, BCR/ABL, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназы, бластный криз, исходы заболевания.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(4289) "Клинический прогноз у пациентов с хроническим миелоидным лейкозом (ХМЛ) в развернутой стадии (фаза акселерации – ФА, или бластный криз – БК) все еще остается неблагоприятным в эру применения ингибиторов тирозинкиназ (ИТК). Данное исследование проводилось, чтобы оценить, насколько аллогенная трансплантация гемопоэтических клеток (алло-ТГСК) улучшает их прогноз.
Пациенты и методы
Общая группа из 162 пациентов с ХМЛ в ФА/БК была разделена на две гомогенные когорты. Первая из них состояла из реципиентов, получавших кондиционирование со сниженной интенсивностью перед алло-ТГСК (n=82). Вторая группа (n=80) включала пациентов, получавших только терапию на базе ИТК (в 85% случаев – препараты 2-го и 3-го поколения), не направленных в центры трансплантации или отказавшихся от нее. Ответ на терапию определяли в соответствии с рекомендациями ELN и NCCN.
Результаты
Медиана сроков наблюдения для всей когорты составляла 44 (1-344) мес. Среди пациентов с БК 36 больных (59%) не отвечали на лечение, в 22 случаях (34%) была установлена полная гематологическая ремиссия (CHR), в одном случае (2%) – полная цитогенетическая ремиссия, и полный молекулярный ответ (ПМО) был достигнут в 2 случаях (3%). Среди реципиентов алло-ТГСК, приживление отмечено в 86% случаев. Кумулятивная безрецидивная смертность на D+100 и через 1 год составила, соответственно, 10% и 18%. У 28 пациентов с посттрансплантационным рецидивом проведена дополнительная терапия, и достигнут ПМО в 9 случаях. Общая 4-летняя выживаемость и бессобытийная выживаемость (ОВ) были лучше после алло-ТГСК по сравнению с группой, леченой ИТК: 58% против 33% (p=0,032) и 35% против 17% (p=0,5), соответственно. Пациенты в БК на момент ТГСК имели значительно более низкие уровни 3-летней ОВ по сравнению с больными, ответившими на лечение: 23% против 63% (p=0,007), соответственно.
Заключение
Хотя алло-ТГСК имеет преимущество у многих больных ХМЛ в развернутых стадиях, результаты ее применения при БК сравнимы с лечением ИТК. Поэтому данные пациенты должны направляться в центры трансплантации по мере достижения ими второй хронической фазы заболевания.
Ключевые слова
Хронический миелоидный лейкоз, BCR/ABL, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназы, бластный криз, исходы заболевания.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27324" ["VALUE"]=> array(2) { ["TEXT"]=> string(367) "<p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(355) "НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
" } } } [7]=> array(49) { ["IBLOCK_SECTION_ID"]=> string(3) "171" ["~IBLOCK_SECTION_ID"]=> string(3) "171" ["ID"]=> string(4) "1944" ["~ID"]=> string(4) "1944" ["IBLOCK_ID"]=> string(1) "2" ["~IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["~NAME"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["ACTIVE_FROM"]=> NULL ["~ACTIVE_FROM"]=> NULL ["TIMESTAMP_X"]=> string(22) "02/08/2021 11:15:18 am" ["~TIMESTAMP_X"]=> string(22) "02/08/2021 11:15:18 am" ["DETAIL_PAGE_URL"]=> string(97) "/en/archive/-9-4/eksperimentalnye-issledovaniya/biomimetiki-dlya-terapii-zabolevaniy-endometriya/" ["~DETAIL_PAGE_URL"]=> string(97) "/en/archive/-9-4/eksperimentalnye-issledovaniya/biomimetiki-dlya-terapii-zabolevaniy-endometriya/" ["LIST_PAGE_URL"]=> string(12) "/en/archive/" ["~LIST_PAGE_URL"]=> string(12) "/en/archive/" ["DETAIL_TEXT"]=> string(50111) "Introduction
Depopulation of the indigenous community is among the most urgent problems for Russia with its vast territories. Current total birth rate (TBR) in Russia is 1.3-1.5, which is lower than required for simple reproduction of the population (should be ≈2.12 [1]). It is obvious that the decline in TBR is observed in all industrial countries, where the majority of the population is concentrated in the cities. The depopulation process cannot be stopped completely, but it can be slowed down by socio-economic changes, as well as by reducing secondary infertility of women in their childbearing age.
Primary infertility is the inability to give birth to the first child. This index decreases by 0.1% per year, being about 1.9% [2]. Secondary infertility is the inability to give birth to children after a successful first pregnancy. The prevalence of secondary infertility increases sharply with age – from 2.6% in women aged 20-24 years to 27.1 % in women aged 40-44 years [2]. At the same time, in Russia and other Central and Eastern Europe countries, as well as in Central Asia, secondary infertility is detected in 18% of women aged 20-44 years, compared to only 7.2% in the high-resource countries [2, 3]. Different disorders of uterine endometrium resulting from disturbed pregnancies or various interventions are the main cause of secondary infertility.
Prevention of endometrial disorders
The endometrium is a complex, multicomponent system consisting of the integumentary and glandular epithelium, stroma, basic substance, and blood vessels. The epithelial component of the endometrium consists mainly of secretory and ciliated cells, as well as a few reticular cells, fibroblasts, macrophages, lymphocytes and labrocytes. The human endometrium is a dynamic tissue that undergoes periods of growth and death during the menstrual cycle. Endometrial growth is regulated by the balance between estrogen and progesterone [4]. If this balance is disturbed, hyperplasia (with a deficiency of progesterone) or hypoplasia (with a deficiency of estrogen) of the endometrium may occur.
Both hyperplasia and hypoplasia are causes of infertility. In hyperplasia, there is an overgrowth of the endometrium and uterine stroma, including the release of endometrial cells into the muscle layer and abdominal cavity. With hypoplasia, decreased thickness of the internal uterine mucosa is noted. Thickness of the endometrial layer should range between 7 and 13 mm for successful fertilization. Under the borderline conditions, the attachment of an egg to the endometrium is possible, but miscarriages are more common. Endometrial hyperplasia was found in 70-80% of cases when examining women with infertility. Changes in endometrial thickness may be often caused by metabolic and neuroendocrine disorders. To arrange appropriate management, the major reasons for endometrial disorders should be identified.
Adhesive plaques (synechiae) form in the uterus following surgical interventions (abortions, curettage, complicated pregnancies), the condition known as Asherman’s syndrome (AS) [5]. Synechiae are outgrowths (adhesions) of the sclerotized endometrium that disturb normal anatomy and physiology of the uterine mucosa [6]. The main cause of AS is damage and trauma to the basal layer during gynecological procedures. Less often, intrauterine synechiae are formed after endometritis, the uterine mucosa inflammation caused by schistosomiasis or genital tuberculosis [7]. Synechiae are also formed in intrauterine adhesion (IUA), a disease of the uterus with aberrant occurrence of adhesions within uterus and/or cervix. Patients with IUA often have menstrual irregularities and suffer from pelvic pain. IUA can prevent blastocyst implantation, impair blood supply to the uterus and early fetus, and finally lead to miscarriage or complete infertility in patients.
The leading factor in the formation of synechiae is considered to be mechanical trauma of basal endometrial layer after childbirth or abortion (wound phase). Pathomorphology of intrauterine synechiae is still unclear. A major role in pathogenesis of intrauterine adhesions is assigned to macrophages, the cellular mediators of inflammation. After mechanical damage, macrophages show increased phagocytic and secretory activity and, within 5 days, become the main component of local leukocyte population. Macrophages promote the migration of new mesothelial cells to the damaged surface, which initially form small "islands" on the damaged surface, and then thin layers of mesothelial cells [8]. Certain cytokines, such as fibroblast growth factor (bFGF), platelet growth factor (PDGF), and transforming growth factor β1 (TGF-β1) seem to be involved in the pathogenesis [9]. The role of chemokines and chemokine receptors CXL12/CXCR4/CXCR7 axis in the development of AS was also shown: interaction of the CXCL12 chemokine with the CXCR4 receptor in mouse models caused a decrease in fibrosis and improved fertility [10]. Wang et al. studied the role of nuclear factor-kappaB (NF-KB) in the AS pathogenesis. As a result, NF-KB expression was significantly increased in endometrial samples from the AS patients compared to the control group. The role of NF-KB in the pathogenesis of AS was further confirmed in a rat model [11]. Xue et al. found that expression of TGF-β and connective tissue growth factor (CTGF) in endometrial tissue with adhesions was significantly increased. Moreover, the activity of the NF-KB signaling pathway in endometrial tissue with synechiae was also higher and positively correlated with expression of TGF-β and CTGF. Blocking the NF-KB signaling pathway with a specific inhibitor led to a decrease in TGF-β expression in RL95-2 cells, which confirmed an association between NF-KB and TGF-β signaling pathway in endometrial cells. In addition, the expression of TGF-β and CTGF has been associated with the recurrent IUA, so it can be used as a potential marker of IUA pathology [12].
Chronic activation of cellular and humoral proinflammatory responses is accompanied by increased production of cytokines and other biologically active substances that cause microcirculation disorders, exudation and deposition of fibrin in the endometrial layer, which forms connective tissue fibrinous adhesions in the stroma and/or intrauterine synechia of varying severity. In numerous studies, biopsies obtained from patients with intrauterine adhesions compared to patients with normal endometrium contained 50-80 % of fibrous tissue versus 13-20%, respectively. Due to the fact, that placental tissue fragments can cause fibroblast activation and collagen formation before endometrial regeneration. Occurrence of intrauterine synechiae is more likely in patients with missed abortion than in patients with incomplete abortion. In terms of possible injury to the uterine mucosa, the first 4 weeks after delivery or termination of pregnancy are considered most dangerous. From the histological point of view, endometrial stroma in AS is replaced by fibrous tissue, and the uterine glands are replaced by inactive cubic epithelium that is insensitive to hormonal stimulation. As a result, the normal anatomy and physiology of the uterine mucosa change.
When synechiae are formed, endometrial cells can grow in the muscle layer, which leads to endometriosis. Due to a difficult vaginal discharge of menstrual blood, the endometrium enters abdominal cavity via fallopian tubes. Menstrual blood contains stem cells that may grow in such inappropriate microenvironment.
Hormone therapy is ineffective in AS. The main method of treatment is surgical removal of synechiae, which is problematic in severe cases, due to the inability to locate the adhesions (fusions of the uterine walls). Despite the removal of adhesions, they are re-formed in 25% of women with moderate AS and 75% with severe pathology. Pregnancy occured in 25-75% of operated women, full-term children were born in 26-79% of cases. Different results are reported, due to absence of generally recognized AS classification and lack of common approach to secondary prevention of the disease
Endometrium regeneration using surface-functionalized hydrogels
Recovery of the uterine endometrium is facilitated by introduction of various biological substances that are able to stimulate regeneration of the tissue leading to restoration of reproductive capacity [14]. Type of biomaterial is an important factor in tissue engineering since it may provide structural support mimicking native uterine endometrial tissues [15]. The biomimetic should include a supporting layer and biologically active molecules. Both components should facilitate cellular and extracellular signaling, nutrient transport, stem cell recruitment, proliferation, and differentiation. The biomaterials can release drugs, growth factors, small molecules, and other biologically active compounds in a controlled manner. Recent studies have shown that, in addition to traditional regeneration of uterus promoted by the biomaterials, their combination with modified cells, e.g., cell layers, intercellular boundaries, surface-functionalized frameworks and decellularized biological tissue, may also exhibit functional or structural advantages. These approaches may provide recovery of altered uterine structures to some degree by inducing biomimetic changes and restoring the regenerative microenvironment [16, 17].
Regeneration of endometrium with modified hydrogels
Many endometrial regeneration strategies are focused on modifying the surface or structure of gels for better biocompatibility and stronger adhesion to endometrial surface; delivery of bioactive growth factors, hormones, and extracellular vesicles [18, 19]. Li et al. [19] developed a collagen hydrogel loaded with fibroblast growth factor bFGF conjugated to the collagen-binding domain (CBD). This combination significantly reduced the random bFGF diffusion in vivo and increased the delivery of the factor to the endometrium. Recombinant proteins with CBD were released in the damaged area and maintained an effective concentration for a long time. The complex framework induced high neovascularization, alignment of muscle fibers and thick layers of the endometrium which contributed to effective tissue recovery. However, the incidence of pregnancy in this study was low, indicating that functional recovery of the endometrium was not achieved. Similarly, Lin et al. [20] loaded vascular endothelial growth factor VEGF conjugated with CBD onto a collagen scaffold to improve angiogenesis and endometrial re-epithelization. The authors compared the efficacy of free growth factor, and VEGF included in the gel, aiming for regeneration of a full-layer injury in rat uterus. The resulting growth of vascular tissue provided the damaged areas with nutrients and oxygen. In addition, targeted VEGF release activated matrix metalloproteinases and initiated endometrial remodeling by increasing the number of inflammatory cells at early regeneration stages. The results showed a 31.2% improvement in pregnancy rate when using CBD/VEGF collagen gel (50.0%) compared to only local VEGF injection (18.8%).
Xu et al. [21] used a temperature-sensitive hydrogel loaded with keratinocyte growth factor KGF, which stimulates tissue repair. The hydrogel made it possible to control release and long-term retention of the drug in damaged uterus. The authors found that the modified KGF-hydrogel framework promoted cell autophagy by inhibiting the mammalian rapamycin signaling pathway; improved the expression level of the CD31 stem cell marker; endothelial migration and proliferation of endometrial glandular epithelial cells and luminal epithelial cells. Functional repair of the epithelium was due to the restoration of corresponding microenvironment by reducing inflammation and immune responses [22, 23].
In addition to biologically active proteins some studies aimed at restoring the endometrium have analyzed a role of secreted extracellular vesicles derived from stem cells [17, 22, 24]. A modified stem cell secretome-containing hydrogel was based on hyaluronic acid (HA), which increased the release of a number of regeneration-related growth factors, such as epidermal growth factor EGF, bFGF, insulin-like growth factor IGF-1, and IGF-binding protein IGFBP. The cross-linked gel served as a carrier and increased in vivo retention time for the stem cell secretions, thus been associated with an increase in endometrial thickness and higher number of endometrial glands if compared to the usage of non-modified gel. Nanoscale functionalization of endometrial scaffolds simulates the natural environment, provides stable release of bioactive molecules and transmission of signals from extracellular vesicles during uterine regeneration [25].
Endometrium regeneration on the basis of of gel-cell scaffolds
Endometrial mesenchymal cells
Biomaterials provide structural and mechanical support to help restoration of the architecture and functionality of damaged tissues. However, the scaffold biomaterial is not sufficient enough to repair large uterine defects. Vascularization, recruitment of native cells, and inhibition of scar formation should be considered [26]. Cell culture on the scaffold structures increases biological functions, prolonging cell survival and stimulating cell proliferation, differentiation, and vascularization [27]. E.g., the resident mesenchymal stromal cells (MSCs) could be incorporated into the polymer gels. Kim et al. [28] used endometrial mesenchymal stromal cells (dEMSCs) encapsulated in a HA hydrogel in a mouse model of uterine infertility. Two weeks after the injury, the fibrous tissue decreased and the endometrial thickness increased. The authors showed increased expression of embryonic markers, including desmin, CD44, and platelet endothelial cell adhesion molecules in regenerating endometrium. Successful implantation of the transferred embryos was accompanied by normal development and live birth of offspring after treatment of the damaged uterus with the dEMSC-HA hydrogel. Isotopic analysis of endometrial cell proliferation showed a significant reduction in recovery time with dEMSC-HA compared to isolated mesenchymal stem cells from bone marrow or endometrium. The gels were gradually eliminated from the uterus, due to HA-degrading hyaluronidase activity in uterus, thus allowing the incorporated cells to attach to endometrium in the damaged area and temporarily provide rigidity of the framework necessary for endometrial regeneration.
dEMSCs exert protective effect not only in patients with hypometriosis, but also at the AS. For example, a group of 7 patients suffering from severe AS underwent triple irrigation of uterine cavity with a suspension of autologous menstrual blood-derived endometrial stem cells. In all women, this treatment, along with additional estrogen therapy, was followed by increased endometrium thickness. In 5 out of 7 patients, the thickness reached 7 mm or more, thus being sufficient for implantation. Moreover, one of these women soon became pregnant in natural way, and two more, due to extracorporeal fertilization [29].
Endometrial perivascular cells
These cells could be also applied to promote local vascularization. Their ability to restore angiogenesis and inhibit scar formation when choosing cell types for uterine repair is of fundamental importance. Endometrial perivascular cells (En-PSCs) carrying CD146 markers and platelet growth factor receptor PDGFR-β loaded into a collagen gel had a similar effect upon stem cells in the endometrial layer [30]. En-PSCs, if additionally transfected with the CYR61 angiogenic inducer, were shown to promote vascular formation. Development of CYR61-transfected En-PSC-loaded collagen scaffold significantly increased the density of blood vessels, since it stimulated release of angiogenic factors from extracellular matrix and, generally, accelerated the in vivo neovascularization.
Bone marrow-derived mesenchymal stromal stem cells
A significant number of studies show the effectiveness of stem cells derived from bone marrow (BMSCs) for endometrial and uterine regeneration due to their ease of isolation and reparative potential [31-33]. Administration of BMSCs, supplemented by cauterization (electroacopuncture), improved fertility by activating the CXCR4 chemokine receptor, enhanced expression of cytokeratins and vimentin, VEGF and bFGF [31]. Effect of BMSCs incorporated into polyglycerol sebacinate (PGS) gel was compared with effect of BMSC-loaded collagen, or polylactic and glycolic acid (PLGA) copolymer gels. The authors showed an increase in TGF-β1, bFGF synthesis, and better recovery of endometrial morphology when using PGS gel. However, the fertility rates were comparable to those achieved with collagen-based gel (72%), but higher than with PLGA carrier (42%) [34]. Similar data were reported by Qi et al. [32]. The authors studied ability of BMSCs-PGS scaffolds for restoration of uterine soft tissue deformities under various dynamic conditions without external stimuli. The authors compared efficacy of the materials loaded with different cell types. PGS with BMSCs showed better stimulation of endometrial proliferation and differentiation. Moreover, the in vivo studies have shown longer retention time for BMSCs in situ and more effective vascularization of the PGS scaffold.
Yang et al. [33] used BMSCs encapsulated in a gel based on pluronic F-127 (PF-127) and vitamin C, which resulted in an increased membrane stability. In addition, vitamin C reduced the secretion of TNF-α and interleukin 6 (IL-6) due to its antioxidant activity, maintained redox homeostasis, and promoted a pro-regenerative trend by increasing the IL-10 levels. BMSC/PF-127+vitamin C hydrogel restored endometrial thickness and reduced fibrous areas of endometrial stromal tissues.
Meanwhile, the BMSCs therapy in patients with AS still lacked efficiency in terms of fertility. Indeed, introduction of autologous BMSCs into subendometrial myometrium in 6 women suffering from AS stimulated an increase in endometrial thickness and normalized menstrual cycles, but this biological effect in all patients did not result into successful extracorporeal fertilization attempts [35].
Umbilical cord mesenchymal stromal cells
Wharton’s jelly of the umbilical cord is an alternative source of MSCs, from which stem cells are isolated (UCMSCs). Introduction of UCMSCs as a component of a collagen carrier improved endometrial proliferation, differentiation, and neovascularization after implantation of UCMSCs scaffolds into the endometrium [36]. All 26 patients with AS showed positive dynamics, increased endometrial thickness, and decreased the number of intrauterine adhesions. Ten patients soon became pregnant after completing the treatment. The newborn children were born without any obvious birth defects or placental pathology. UCMSCs mixed with gelatin and collagen fibers stimulated pronounced angiogenesis and reduced scar formation in the damaged area. The composite cell framework destroyed collagen in the scarred areas, probably, by increasing the amount of matrix metalloproteinase 9, FGF-2, and VEGF, and led to angiogenesis and cyclic endometrial regeneration [37]. Xin et al. [38] found that the UCMSC-loaded collagen scaffold reduced cell apoptosis and improved the state of endometrial stromal cells due to eventual paracrine action. The scaffold did not induce inflammation and contributed to collagen remodeling in regenerating endometrium. In addition, the UCMSCs-loaded collagen scaffold induced early rapid re-epithelization by increasing level of cell proliferation and expression of cytokeratin, which is vital for the subsequent endometrial repair after damage.
Growth effects of platelet-rich plasma
Inclusion of platelet-rich plasma (PRP) in the structure of hydrogels is a point for research of uterine endometrial regeneration. In a number of studies, PRP was used to stimulate MSCs obtained from the menstrual blood. Transplantation of these MSCs stimulated thickening of the endometrium [39].
In women who underwent endometrial electroacupuncture for the first time, the effect of autologous PRP administration on endometrial thickness was evaluated. PRP infusion resulted in endometrial thickening up to 7 mm observed 48-72 hours later. Successful pregnancy occurred then after progesterone administration [40]. Similar data were obtained in a randomized clinical trial (n=83) among women with hypometriosis suffering from poor endometrial response to standard hormone replacement therapy. After PRP administration, there was a significant increase in endometrial thickness; in this group, the frequency of implantation and clinical pregnancy per cycle also increased significantly [41].
Application of autologous PRP in rat model with ethanol-induced endometrial injury led to endometrial regeneration. The authors concluded that intrauterine administration of autologous PRP stimulates and accelerates endometrial regeneration, along with reduced formation of fibrosis [42].
There are also studies in which the authors did not show that intrauterine PRP injection improves the results of hysteroscopy after surgical removal of intrauterine adhesions [43].
Hypothetical mechanisms of stem cell actions
Most research groups did not show real engraftment of transplanted mesenchymal cells, which confirms the current opinion about paracrine nature of therapeutic effects caused by allogeneic MSCs [44-48].
Cellular elements can be administered to patients as intravascular infusions [49, 50], intramuscularly [51] or directly into the uterine cavity. Both enriched MSCs or stromal cell suspensions can be used [35, 52, 53]. No serious side effects or signs of cell engraftment were observed in these studies. There is no doubt that further research and clinical trials are needed to optimize the use of cell therapy.
In healthy female body, cytokine environment and immune cellular repertoire depend on the phase of menstrual cycle [44], which are important for reducing fibrosis and restoration of endometrium. It is believed that successful endometrial repair technology is directly related to the activity of local inflammation. To achieve maximal therapeutic effect, it is necessary to ensure optimal mechanism and timely delivery of cellular elements, depending on the cycle phase. It is also necessary to minimize potential risks associated with the delivery method. For example, AS is often associated with disturbed blood flow in uterine spiral arterioles. Therefore, intravascular administration of these cellular elements cannot be used in this case.
Currently, most researchers believe that stem cells can only have a systemic and paracrine therapeutic effect, since they, probably, act as immunomodulators [46, 54, 55]. Immunomodulatory effects result from destruction of these cells or their absorption. This may explain the reparative tissue response during local injection of MSCs [56].
It is also suggested that the release of paracrine factors may represent the main effect of cellular therapy. In this case, the usage of exosomes/microvesicles opens some prospectives for regenerative medicine [57]. E.g., excretory microvesicles (eMVs) isolated from intrauterine fluid also have a protective effect [57-60]. In addition, MSCs have been shown to produce eMVs that have immunomodulatory effect on T cells [61].
During experiments on co-cultivation of mouse embryos and endometrial sMVs, an increase in the number of blastomeres, as well as vascular epithelium and platelets in embryos was observed [62]. These results led to the attempts of intrauterine infusion of PRP which includes eMVs components. In clinical trials, PRP infusion increased epithelial thickness [40, 63, 64], and, as a result, the number of successful implantations and natural pregnancies occurred. The fact that exosomes and other eMVs are an alternative to "direct" cell therapy and allow avoiding its negative consequences is confirmed by the high efficiency of PDGF-α, which is contained in platelets, in tissue repair and regeneration [65].
Clinical usage of antiadhesive polymers to prevent Ascherman’s syndrome
As noted above, the AS adhesions within uterus make it difficult to outflow menstrual blood and reduce women's fertility. Hormone therapy is not effective for AS. The main method of therapy is the removal of adhesions in one way or another. After removing the adhesions, new endometrial damage occurs, which often leads to the formation of the adhesions de novo. To prevent the formation of new adhesions, a number of studies have suggested using copolymers of hyaluronic acid and carboxymethylcellulose etc. for temporary separation of the uterine walls, which can provide the time required for re-epithelization of the wound surface.
For the prevention of AS and IUA in Russia and worldwide, various anti-adhesive materials are used, for example, Antiadhesin (Russia), Seprafilm film (France) based on sodium hyaluronate and sodium salt of carboxymethylcellulose (HA-CMC), Oxiplex/AP gel (USA) based on carboxymethylcellulose (CMC) and polyethylene oxide [8, 66-68], and others.
The use of barrier anti-adhesive agents based on HA-CMC reduces the risk of adhesion formation in the uterine cavity. According to a randomized study [68], intrauterine administration of an anti-adhesive barrier agent containing HA and CMC can not only prevent the formation or reduce the severity of intrauterine adhesions, but also contributes to the preservation of reproductive function. A total of 150 patients with incomplete or missed abortions participated in the clinical study. In the treatment group (Seprafilm) (n=50), membranes were inserted into the endometrial and cervical canal cavity after suction evacuation and/or curettage. The control group (n=100) did not get any treatment. Both groups were divided into two subgroups: patients who had no previous suction or curettage, and those who had at least one previous abortion or curettage. Further fertility was assessed by pregnancy success in all groups. The formation of endometrial synechiae was evaluated using hysterosalpingography in patients of all groups without pregnancy success 8 months after the intervention. The safety of using Seprafilm was evaluated by recording any adverse reactions and performing ultrasound monitoring. Of the subgroups without previous abortions, all 32 patients (100%) who received Seprafilm became pregnant in the next 8 months; in the control group, pregnancy occurred only in 54% of cases. They also showed that patients in the Seprafilm group with one or more previous interventions and no pregnancy after 8 months did not have adhesions in 90% of cases, and only in 50% of cases in the untreated group.
HA performs important physiological functions in the body, including joint lubrication, regulation of blood vessel wall permeability, regulation of protein and electrolyte transport, and wound healing. HA can bind to a large number of water molecules improving tissue hydration, increasing cell resistance to mechanical damage, and reducing post-traumatic formation of granulation and fibrous tissue. Due to its unique biocompatibility and enzymatic biodegradation, HA is often used to prevent postoperative adhesions [69, 70]. HA gel significantly reduces the frequency of IUA after intrauterine surgery, regardless of the type of intervention or the presence of primary diseases. It has been shown that treatment with HA gel increases the frequency of pregnancy after intrauterine surgery [71].
Heat-sensitive matrices based on poly-N-isopropylacrylamide
Poly-N-isopropylacrylamide (PNIPAM) is a polymer that performs a phase transition from a liquid to a gel state when heated above 37°C. As the temperature rises, PNIPAM forms a three-dimensional hydrogel that compresses and pushes the liquid out, releasing the contents into the surrounding tissue. Accordingly, PNIPAM, when administered, provides separation of the uterine walls. At the same time, it creates an enriched environment around itself that stimulates endometrial regeneration. Unlike PNIPAM, HA and CMC swell in the uterus, collecting intrauterine fluid and filling the internal volume. Theoretically, PNIPAM may be more promising for preventing adhesions. Currently, PNIPAM gels are being studied in vitro and in model systems, for example, for the cultivation of chondrocytes. Incubation of chondrocytes with PNIPAM showed that the cells were viable for 24 days, increased in number, and produced type II collagen and glycosaminoglycans [72].
For the functionalization of hydrogels, modification of PNIPAM is possible by including hydrophilic bioactive substances, such as cell culture supernates, eMVs or exosomes of MSCs, PRP, and other molecules. This modification may separate thermosetting and hydrophilic functions and allows each component to act independently. This concept was demonstrated by the Yoshioka group, who developed a tissue culture framework based on PNIPAM-graft-PEG [73-75]. The authors modified PNIPAM by including butyl ether of methacrylic acid in order to shift the phase transition point to a lower temperature. In subsequent studies in cell culture, the authors reported a system with a gelation temperature of 7°C in the cell culture medium [76]. It is known that thermosetting polymers with a hydrophobic component form dispersed gels in aqueous media above the critical point when the concentration exceeds the critical minimum. This phenomenon is associated with particle aggregation due to thermosetting flocculation of Poly(styrene-graft-NIPAM) particles [77, 78]. Poly(styrene-co-NIPAM) particles were used to form core-shell solid particles. Recently, thermosetting dispersed gels with Poly-ε-caprolactone cores and shells consisting of a polyethylene glycol brush were also obtained. Microdispersion procedure allowed to produce gels which were used as a thermally reversible cell culture system for mouse 3T3 fibroblasts [79] or C2C12 myoblasts [80].
Conclusions
The use of biopolymers and biomimetics based on combinations of polymers with various growth factors or live cells opens up new opportunities for the treatment of endometrial disorders. To this purpose, practical clinicians use gels based on hyaluronic acid, carboxymethyl cellulose, collagen, polyethylene oxide and others with certain efficiency. The main task of biopolymers is to separate the walls of the uterus, thus preventing synechiae, e.g., in Asherman’s syndrome. To improve treatment of hypo- and hypermetriosis, the gels should contain appropriate biological factors able to stimulate or inhibit the endometrial growth. Most of such biomimetic substances are currently at the pre-clinical testing stage. Some data from clinical studies have shown promising results of this approach for the treatment of female infertility.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgements
The work was supported by the Ministry of Health of the Russian Federation for 2020-2022 (no. AAAA-A20-120022790039-1).
References
- Borisov VA, Demography, Publishing House NOTABENE; 2001. 272 p. (In Russian).
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. Low N, ed. PLoS Med. 2012;9(12):e1001356. doi:10.1371/journal.pmed.1001356.
- Infertile Marriage. Modern approaches to Diagnosis and Treatment. Textbook (Ed.: GT Sukhykh). Moscow: GEOTAR-Media; 2010 (In Russian).
- Mahajan N, Sharma S. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci. 2016;9(1):3-8.
- Zhu HY, Ge TX, Pan Y Bin, Zhang SY. Advanced Role of Hippo Signaling in Endometrial Fibrosis: Implications for Intrauterine Adhesion. Chin Med J. 2017;130(22):2732-2737.
- Dreisler E, Kjer JJ. Asherman’s syndrome: Current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191-198.
- Engelbrechtsen L, Istre O. Asherman syndrome. In: Minimally Invasive Gynecological Surgery. Springer Berlin Heidelberg; 2015:43-48.
- Arutyunova EE, Buralkina NA, Chuprynin VD, Zhorova VE. Pathogenetic justification and experience in using anti-adhesion gel in patients with intrauterine synechia after surgical hysteroscopy. Medical Council. 2018;(13):160-164. (In Russian)
- Tao Z, Duan H. Expression of adhesion-related cytokines in the uterine fluid after transcervical resection of adhesion. Zhonghua Fu Chan Ke Za Zhi. 2012;47(10):734-737. (In Chinese).
- Krikun G. The CXL12/CXCR4/CXCR7 axis in female reproductive tract disease: Review. Am J Reprod Immunol. 2018;80(5).
- Wang X, Ma N, Sun Q, Huang C, Liu Y, Luo X. Elevated NF-κB signaling in Asherman syndrome patients and animal models. Oncotarget. 2017;8(9):15399-15406.
- Xue X, Chen Q, Zhao G, Zhao JY, Duan Z, Zheng PS. The overexpression of TGF-β and CCN2 in intrauterine adhesions involves the NF-KB signaling pathway. PLoS One. 2015;10(12).
- Makarenko TA, Nikiforova DE. Modern opportunities in the treatment of Asherman syndrome. Mother and child. 2016;(15):1001-1004 (In Russian).
- Cervelló I, Santamaría X, Miyazaki K, Maruyama T, Simón C. Cell Therapy and Tissue Engineering from and toward the Uterus. Semin Reprod Med. 2015;33(05):366-372.
- Zhang SS, Xu XX, Xiang WW, Zhang HH, Lin HL, Shen LE, et al.. Using 17β estradiol heparin poloxamer thermosensitive hydrogel to enhance the endometrial regeneration and functional recovery of intrauterine adhesions in a rat model. FASEB J. 2020;34(1):446-457.
- Liu F, Hu S, Wang S, Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen Biomater. 2019;6(3):141-148.
- Liu F, Hu S, Yang H, Li Z, Huang K, Su T, et al. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv Healthc Mater. 2019;8(14):e1900411.
- Shadish JA, Benuska GM, DeForest CA. Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat Mater. 2019;18(9):1005-1014.
- Li C, Ouyang L, Pence IJ, Moore AC, Lin Y, Winter CW, Armstrong JPK, Stevens MM. Buoyancy-Driven Gradients for Biomaterial Fabrication and Tissue Engineering. Adv Mater. 2019;31(17):e1900291.
- Lin N, Li X, Song T, Wang J, Meng K, Yang J, et al. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials. 2012;33(6):1801-1807.
- Xu HL, Xu J, Zhang SS, Zhu QY, Jin BH, ZhuGe DL, et al.Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017;24(1):867-881.
- Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, Zhang S. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389-402.
- Gargett CE, Chan RWS, Schwab KE. Hormone and growth factor signaling in endometrial renewal: Role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1-2):22-29.
- Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell- based therapy. Biomed Pharmacother. 2018;102:333-343.
- Han Q, Du Y. Advances in the Application of Biomimetic Endometrium Interfaces for Uterine Bioengineering in Female Infertility. Front Bioeng Biotechnol. 2020;8:153.
- Owusu-Akyaw A, Krishnamoorthy K, Goldsmith LT, Morelli SS. The role of mesenchymal-epithelial transition in endometrial function. Hum Reprod Update. 2019;25(1):114-133.
- Frost BA, Sutliff BP, Thayer P, Bortner MJ, Foster EJ. Gradient poly(ethylene glycol) diacrylate and cellulose nanocrystals tissue engineering composite scaffolds via extrusion bioprinting. Front Bioeng Biotechnol. 2019;7:280.
- Kim S, Kim M, Jung S, Kwon K, Park J, Kim S, et al. Co-delivery of therapeutic protein and catalase-mimic nanoparticle using a biocompatible nanocarrier for enhanced therapeutic effect. J Control Release. 2019;309:181-189.
- Reynolds K, Khoury J, Sosnowski J, Thie J, Hofmann G. Comparison of the effect of tamoxifen on endometrial thickness in women with thin endometrium (<7mm) undergoing ovulation induction with clomiphene citrate. Fertil Steril. 2010;93(6):2091-2093.
- Li Z, Yan G, Diao Q, Yu F, Li X, Sheng X, et al. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell Res Ther. 2019;10(1):179.
- Xia L, Meng Q, Xi J, Han Q, Cheng J, Shen J, et al. The synergistic effect of electroacupuncture and bone mesenchymal stem cell transplantation on repairing thin endometrial injury in rats. Stem Cell Res Ther. 2019;10(1):244.
- Qi Y, Lohman J, Bratlie KM, Peroutka-Bigus N, Bellaire B, Wannemuehler M, et al. Vitamin C and B 3 as new biomaterials to alter intestinal stem cells. J Biomed Mater Res Part A. 2019;107(9):1886-1897.
- Yang H, Wu S, Feng R, Huang J, Liu L, Liu F, Chen Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res Ther. 2017;8(1):267.
- Xiao B, Yang W, Lei D, Huang J, Yin Y, Zhu Y, et al. PGS Scaffolds Promote the In Vivo Survival and Directional Differentiation of Bone Marrow Mesenchymal Stem Cells Restoring the Morphology and Function of Wounded Rat Uterus. Adv Healthc Mater. 2019;8(5):1801455.
- Panchal S, Patel H, Nagori C. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci. 2011;4(1):43.
- Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192.
- Xu L, Ding L, Wang L, Cao Y, Zhu H, Lu J, et al. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res Ther. 2017;8(1):1-13.
- Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang Y, et al. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92:160-171.
- Zhang S, Tan J, Li P. Co-transplantation of menstrual stromal cell and platelet-rich plasma improves Asherman’s syndrome in rat model. Fertil Steril. 2017;108(3):e193.
- Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, Liang X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8(1):1286-1290.
- Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol. 2018;57(6):810-813.
- Jang HY, Myoung SM, Choe JM, Kim T, Cheon YP, Kim YM, Park H. Effects of Autologous Platelet-Rich Plasma on Regeneration of Damaged Endometrium in Female Rats. Yonsei Med J. 2017;58(6):1195.
- Javaheri A, Kianfar K, Pourmasumi S, Eftekhar M. Platelet-rich plasma in the management of Asherman’s syndrome; An RCT. Int J Reprod Biomed. 2020;18(2):113-120.
- von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, et al. Analysis of Tissues Following Mesenchymal Stromal Cell Therapy in Humans Indicates Limited Long-Term Engraftment and No Ectopic Tissue Formation. Stem Cells. 2012;30(7):1575-1578.
- Golle L, Gerth HU, Beul K, Heitplatz B, Barth P, Fobker M, et al. Bone marrow-derived cells and their conditioned medium induce microvascular repair in uremic rats by stimulation of endogenous repair mechanisms. Sci Rep. 2017;7(1):9444.
- Lupatov AY, Poltavtseva RA, Bystrykh OA, Yarygin KN, Sukhikh GT. Neural stem/progenitor cells maintained in vitro under different culture conditions alter differentiation capacity of monocytes to generate dendritic cells. J Stem Cells Regen Med. 2017;13(2):54-61.
- Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther. 2019;10(1):257.
- Poltavtseva RA, Poltavtsev A V., Lutsenko G V., Svirshchevskaya E V. Myths, reality and future of mesenchymal stem cell therapy. Cell Tissue Res. 2019;375(3):563-574.
- Singh N, Mohanty S, Seth T, Shankar M, Dharmendra S, Bhaskaran S. Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell based therapy. J Hum Reprod Sci. 2014;7(2):93.
- Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087-1096.
- Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98(10):2900-2908.
- Li X, Sun H, Lin N, Hou X, Wang J, Zhou B, et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials. 2011;32(32):8172-8181.
- Cervelló I, Gil-Sanchis C, Santamaría X, Cabanillas S, Díaz A, Faus A. Human CD133+ bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015;104(6):1552-1560.e3.
- Malhotra N, Bahadur A, Kalaivani M, Mittal S. Changes in endometrial receptivity in women with Asherman’s syndrome undergoing hysteroscopic adhesiolysis. Arch Gynecol Obstet. 2012;286(2):525-530.
- Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416):eaam7828.
- de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells. 2018;36(4):602-615.
- Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, Salamonsen LA. Endometrial Exosomes/Microvesicles in the Uterine Microenvironment: A New Paradigm for Embryo-Endometrial Cross Talk at Implantation. Ward WS, ed. PLoS One. 2013;8(3):e58502.
- Da Silveira J, Andrade GM, Perecin F, Meireles FV, Winger QA, Bouma GJ. Isolation and Analysis of Exosomal MicroRNAs from Ovarian Follicular Fluid. In: Methods in Molecular Biology (Clifton, N.J.). Vol 1733. ; 2018:53-63.
- Da Silveira JC, de Ávila ACFCM, Garrett HL, Bruemmer JE, Winger QA, Bouma GJ. Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J Endocrinol. 2018;236(1):R15-R27.
- Tesfaye D, Gebremedhn S, Salilew-Wondim D, et al. MicroRNAs: tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction. 2018;155(3):R121-R135.
- Álvarez V, Sánchez-Margallo FM, Macías-García B, Gómez-Serrano M, Jorge I, Vázquez J, et al. The immunomodulatory activity of extracellular vesicles derived from endometrial mesenchymal stem cells on CD4+ T cells is partially mediated by TGFbeta. J Tissue Eng Regen Med. 2018;12(10):2088-2098.
- Blázquez R, Sánchez-Margallo FM, Álvarez V, Matilla E, Hernández N, Marinaro F, et al. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. Afink GB, ed. PLoS One. 2018;13(4):e0196080.
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017;21(1):54-56.
- Chang Y, Li J, Wei L-N, Pang J, Chen J, Liang X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltimore). 2019;98(3):e14062.
- Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150-163.
- Molotkov AS, Popov EN, Sudakov DS, Aivazyan TA, Alexandrova LA, Dymarskaya YR. Experience of intrauterine application of anti-adhesive gel based on hyaluronic acid in the prevention of Asherman’s syndrome in patients with the pathology of the uterine cavity and severe forms of endometriosis. J Obstet and Female Dis. 2017;66(6):12-19. (In Russian).
- Tkachenko LV. Method of preventing adhesive disease after myomectomy. J Volgogr State Med Univ. 2019;71(3):70-73. (In Russian).
- Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Mater Res. 2002;63(1):10-14.
- Zheng F, Xin X, He F, Liu J, Cui Y, Meta‑analysis on the use of hyaluronic acid gel to prevent intrauterine adhesion after intrauterine operations, Exp Ther Med. 2020;19(4):2672-2678.
- Hong M-K, Ding D-C. Seprafilm® Application Method in Laparoscopic Surgery. JSLS J Soc Laparoendosc Surg. 2017;21(1):e2016.00097.
- Fei Z, Bin Z, Xin X, Fei H, Yuechong C. Meta-analysis on the use of hyaluronic acid gel to prevent recurrence of intrauterine adhesion after hysteroscopic adhesiolysis. Taiwan J Obstet Gynecol. 2019;58(6):731-736.
- Lapworth JW, Hatton P V, Goodchild RL, Rimmer S. Thermally reversible colloidal gels for three-dimensional chondrocyte culture. J R Soc Interface. 2012;9(67):362-375.
- Yoshioka H, Mikami M, Mori Y, Tsuchida E. A Synthetic Hydrogel with Thermoreversible Gelation. I. Preparation and Rheological Properties. J Macromol Sci Part A. 1994;31(1):113-120.
- Yoshioka H, Mikami M, Mori Y, Tsuchida E. A Synthetic Hydrogel with Thermoreversible Gelation. Ii. Effect of Added Salts. J Macromol Sci Part A. 1994;31(1):121-125.
- Yoshioka H, Mori Y, Cushman JA. A synthetic hydrogel with thermoreversible gelation, III: an NMR study of the sol-gel transition. Polym Adv Technol. 1994;5(2):122-127.
- Yasuda A, Kojima K, Tinsley KW, Yoshioka H, Mori Y, Vacanti CA. In Vitro Culture of Chondrocytes in a Novel Thermoreversible Gelation Polymer Scaffold Containing Growth Factors. Tissue Eng. 2006;12(5):1237-1245.
- Lu Y, Wittemann A, Ballauff M, Drechsler M. Preparation of Polystyrene-Poly(N-isopropylacrylamide) (PS-PNIPA) Core-Shell Particles by Photoemulsion Polymerization. Macromol Rapid Commun. 2006;27(14):1137-1141.
- Li Z, Ngai T. Macroporous Polymer from Core-Shell Particle-Stabilized Pickering Emulsions. Langmuir. 2010;26(7):5088-5092.
- Cheikh Al Ghanami R, Saunders BR, Bosquillon C, Shakesheff KM, Alexander C. Responsive particulate dispersions for reversible building and deconstruction of 3D cell environments. Soft Matter. 2010;6(20):5037.
- W. Wang, H. Liang, R. Cheikh Al Ghanami, L. Hamilton, M. Fraylich, K.M. Shakesheff, et al. Biodegradable Thermoresponsive Microparticle Dispersions for Injectable Cell Delivery Prepared Using a Single-Step Process. Adv Mater. 2009;21(18):1809-1813.
" ["~DETAIL_TEXT"]=> string(50111) "
Introduction
Depopulation of the indigenous community is among the most urgent problems for Russia with its vast territories. Current total birth rate (TBR) in Russia is 1.3-1.5, which is lower than required for simple reproduction of the population (should be ≈2.12 [1]). It is obvious that the decline in TBR is observed in all industrial countries, where the majority of the population is concentrated in the cities. The depopulation process cannot be stopped completely, but it can be slowed down by socio-economic changes, as well as by reducing secondary infertility of women in their childbearing age.
Primary infertility is the inability to give birth to the first child. This index decreases by 0.1% per year, being about 1.9% [2]. Secondary infertility is the inability to give birth to children after a successful first pregnancy. The prevalence of secondary infertility increases sharply with age – from 2.6% in women aged 20-24 years to 27.1 % in women aged 40-44 years [2]. At the same time, in Russia and other Central and Eastern Europe countries, as well as in Central Asia, secondary infertility is detected in 18% of women aged 20-44 years, compared to only 7.2% in the high-resource countries [2, 3]. Different disorders of uterine endometrium resulting from disturbed pregnancies or various interventions are the main cause of secondary infertility.
Prevention of endometrial disorders
The endometrium is a complex, multicomponent system consisting of the integumentary and glandular epithelium, stroma, basic substance, and blood vessels. The epithelial component of the endometrium consists mainly of secretory and ciliated cells, as well as a few reticular cells, fibroblasts, macrophages, lymphocytes and labrocytes. The human endometrium is a dynamic tissue that undergoes periods of growth and death during the menstrual cycle. Endometrial growth is regulated by the balance between estrogen and progesterone [4]. If this balance is disturbed, hyperplasia (with a deficiency of progesterone) or hypoplasia (with a deficiency of estrogen) of the endometrium may occur.
Both hyperplasia and hypoplasia are causes of infertility. In hyperplasia, there is an overgrowth of the endometrium and uterine stroma, including the release of endometrial cells into the muscle layer and abdominal cavity. With hypoplasia, decreased thickness of the internal uterine mucosa is noted. Thickness of the endometrial layer should range between 7 and 13 mm for successful fertilization. Under the borderline conditions, the attachment of an egg to the endometrium is possible, but miscarriages are more common. Endometrial hyperplasia was found in 70-80% of cases when examining women with infertility. Changes in endometrial thickness may be often caused by metabolic and neuroendocrine disorders. To arrange appropriate management, the major reasons for endometrial disorders should be identified.
Adhesive plaques (synechiae) form in the uterus following surgical interventions (abortions, curettage, complicated pregnancies), the condition known as Asherman’s syndrome (AS) [5]. Synechiae are outgrowths (adhesions) of the sclerotized endometrium that disturb normal anatomy and physiology of the uterine mucosa [6]. The main cause of AS is damage and trauma to the basal layer during gynecological procedures. Less often, intrauterine synechiae are formed after endometritis, the uterine mucosa inflammation caused by schistosomiasis or genital tuberculosis [7]. Synechiae are also formed in intrauterine adhesion (IUA), a disease of the uterus with aberrant occurrence of adhesions within uterus and/or cervix. Patients with IUA often have menstrual irregularities and suffer from pelvic pain. IUA can prevent blastocyst implantation, impair blood supply to the uterus and early fetus, and finally lead to miscarriage or complete infertility in patients.
The leading factor in the formation of synechiae is considered to be mechanical trauma of basal endometrial layer after childbirth or abortion (wound phase). Pathomorphology of intrauterine synechiae is still unclear. A major role in pathogenesis of intrauterine adhesions is assigned to macrophages, the cellular mediators of inflammation. After mechanical damage, macrophages show increased phagocytic and secretory activity and, within 5 days, become the main component of local leukocyte population. Macrophages promote the migration of new mesothelial cells to the damaged surface, which initially form small "islands" on the damaged surface, and then thin layers of mesothelial cells [8]. Certain cytokines, such as fibroblast growth factor (bFGF), platelet growth factor (PDGF), and transforming growth factor β1 (TGF-β1) seem to be involved in the pathogenesis [9]. The role of chemokines and chemokine receptors CXL12/CXCR4/CXCR7 axis in the development of AS was also shown: interaction of the CXCL12 chemokine with the CXCR4 receptor in mouse models caused a decrease in fibrosis and improved fertility [10]. Wang et al. studied the role of nuclear factor-kappaB (NF-KB) in the AS pathogenesis. As a result, NF-KB expression was significantly increased in endometrial samples from the AS patients compared to the control group. The role of NF-KB in the pathogenesis of AS was further confirmed in a rat model [11]. Xue et al. found that expression of TGF-β and connective tissue growth factor (CTGF) in endometrial tissue with adhesions was significantly increased. Moreover, the activity of the NF-KB signaling pathway in endometrial tissue with synechiae was also higher and positively correlated with expression of TGF-β and CTGF. Blocking the NF-KB signaling pathway with a specific inhibitor led to a decrease in TGF-β expression in RL95-2 cells, which confirmed an association between NF-KB and TGF-β signaling pathway in endometrial cells. In addition, the expression of TGF-β and CTGF has been associated with the recurrent IUA, so it can be used as a potential marker of IUA pathology [12].
Chronic activation of cellular and humoral proinflammatory responses is accompanied by increased production of cytokines and other biologically active substances that cause microcirculation disorders, exudation and deposition of fibrin in the endometrial layer, which forms connective tissue fibrinous adhesions in the stroma and/or intrauterine synechia of varying severity. In numerous studies, biopsies obtained from patients with intrauterine adhesions compared to patients with normal endometrium contained 50-80 % of fibrous tissue versus 13-20%, respectively. Due to the fact, that placental tissue fragments can cause fibroblast activation and collagen formation before endometrial regeneration. Occurrence of intrauterine synechiae is more likely in patients with missed abortion than in patients with incomplete abortion. In terms of possible injury to the uterine mucosa, the first 4 weeks after delivery or termination of pregnancy are considered most dangerous. From the histological point of view, endometrial stroma in AS is replaced by fibrous tissue, and the uterine glands are replaced by inactive cubic epithelium that is insensitive to hormonal stimulation. As a result, the normal anatomy and physiology of the uterine mucosa change.
When synechiae are formed, endometrial cells can grow in the muscle layer, which leads to endometriosis. Due to a difficult vaginal discharge of menstrual blood, the endometrium enters abdominal cavity via fallopian tubes. Menstrual blood contains stem cells that may grow in such inappropriate microenvironment.
Hormone therapy is ineffective in AS. The main method of treatment is surgical removal of synechiae, which is problematic in severe cases, due to the inability to locate the adhesions (fusions of the uterine walls). Despite the removal of adhesions, they are re-formed in 25% of women with moderate AS and 75% with severe pathology. Pregnancy occured in 25-75% of operated women, full-term children were born in 26-79% of cases. Different results are reported, due to absence of generally recognized AS classification and lack of common approach to secondary prevention of the disease
Endometrium regeneration using surface-functionalized hydrogels
Recovery of the uterine endometrium is facilitated by introduction of various biological substances that are able to stimulate regeneration of the tissue leading to restoration of reproductive capacity [14]. Type of biomaterial is an important factor in tissue engineering since it may provide structural support mimicking native uterine endometrial tissues [15]. The biomimetic should include a supporting layer and biologically active molecules. Both components should facilitate cellular and extracellular signaling, nutrient transport, stem cell recruitment, proliferation, and differentiation. The biomaterials can release drugs, growth factors, small molecules, and other biologically active compounds in a controlled manner. Recent studies have shown that, in addition to traditional regeneration of uterus promoted by the biomaterials, their combination with modified cells, e.g., cell layers, intercellular boundaries, surface-functionalized frameworks and decellularized biological tissue, may also exhibit functional or structural advantages. These approaches may provide recovery of altered uterine structures to some degree by inducing biomimetic changes and restoring the regenerative microenvironment [16, 17].
Regeneration of endometrium with modified hydrogels
Many endometrial regeneration strategies are focused on modifying the surface or structure of gels for better biocompatibility and stronger adhesion to endometrial surface; delivery of bioactive growth factors, hormones, and extracellular vesicles [18, 19]. Li et al. [19] developed a collagen hydrogel loaded with fibroblast growth factor bFGF conjugated to the collagen-binding domain (CBD). This combination significantly reduced the random bFGF diffusion in vivo and increased the delivery of the factor to the endometrium. Recombinant proteins with CBD were released in the damaged area and maintained an effective concentration for a long time. The complex framework induced high neovascularization, alignment of muscle fibers and thick layers of the endometrium which contributed to effective tissue recovery. However, the incidence of pregnancy in this study was low, indicating that functional recovery of the endometrium was not achieved. Similarly, Lin et al. [20] loaded vascular endothelial growth factor VEGF conjugated with CBD onto a collagen scaffold to improve angiogenesis and endometrial re-epithelization. The authors compared the efficacy of free growth factor, and VEGF included in the gel, aiming for regeneration of a full-layer injury in rat uterus. The resulting growth of vascular tissue provided the damaged areas with nutrients and oxygen. In addition, targeted VEGF release activated matrix metalloproteinases and initiated endometrial remodeling by increasing the number of inflammatory cells at early regeneration stages. The results showed a 31.2% improvement in pregnancy rate when using CBD/VEGF collagen gel (50.0%) compared to only local VEGF injection (18.8%).
Xu et al. [21] used a temperature-sensitive hydrogel loaded with keratinocyte growth factor KGF, which stimulates tissue repair. The hydrogel made it possible to control release and long-term retention of the drug in damaged uterus. The authors found that the modified KGF-hydrogel framework promoted cell autophagy by inhibiting the mammalian rapamycin signaling pathway; improved the expression level of the CD31 stem cell marker; endothelial migration and proliferation of endometrial glandular epithelial cells and luminal epithelial cells. Functional repair of the epithelium was due to the restoration of corresponding microenvironment by reducing inflammation and immune responses [22, 23].
In addition to biologically active proteins some studies aimed at restoring the endometrium have analyzed a role of secreted extracellular vesicles derived from stem cells [17, 22, 24]. A modified stem cell secretome-containing hydrogel was based on hyaluronic acid (HA), which increased the release of a number of regeneration-related growth factors, such as epidermal growth factor EGF, bFGF, insulin-like growth factor IGF-1, and IGF-binding protein IGFBP. The cross-linked gel served as a carrier and increased in vivo retention time for the stem cell secretions, thus been associated with an increase in endometrial thickness and higher number of endometrial glands if compared to the usage of non-modified gel. Nanoscale functionalization of endometrial scaffolds simulates the natural environment, provides stable release of bioactive molecules and transmission of signals from extracellular vesicles during uterine regeneration [25].
Endometrium regeneration on the basis of of gel-cell scaffolds
Endometrial mesenchymal cells
Biomaterials provide structural and mechanical support to help restoration of the architecture and functionality of damaged tissues. However, the scaffold biomaterial is not sufficient enough to repair large uterine defects. Vascularization, recruitment of native cells, and inhibition of scar formation should be considered [26]. Cell culture on the scaffold structures increases biological functions, prolonging cell survival and stimulating cell proliferation, differentiation, and vascularization [27]. E.g., the resident mesenchymal stromal cells (MSCs) could be incorporated into the polymer gels. Kim et al. [28] used endometrial mesenchymal stromal cells (dEMSCs) encapsulated in a HA hydrogel in a mouse model of uterine infertility. Two weeks after the injury, the fibrous tissue decreased and the endometrial thickness increased. The authors showed increased expression of embryonic markers, including desmin, CD44, and platelet endothelial cell adhesion molecules in regenerating endometrium. Successful implantation of the transferred embryos was accompanied by normal development and live birth of offspring after treatment of the damaged uterus with the dEMSC-HA hydrogel. Isotopic analysis of endometrial cell proliferation showed a significant reduction in recovery time with dEMSC-HA compared to isolated mesenchymal stem cells from bone marrow or endometrium. The gels were gradually eliminated from the uterus, due to HA-degrading hyaluronidase activity in uterus, thus allowing the incorporated cells to attach to endometrium in the damaged area and temporarily provide rigidity of the framework necessary for endometrial regeneration.
dEMSCs exert protective effect not only in patients with hypometriosis, but also at the AS. For example, a group of 7 patients suffering from severe AS underwent triple irrigation of uterine cavity with a suspension of autologous menstrual blood-derived endometrial stem cells. In all women, this treatment, along with additional estrogen therapy, was followed by increased endometrium thickness. In 5 out of 7 patients, the thickness reached 7 mm or more, thus being sufficient for implantation. Moreover, one of these women soon became pregnant in natural way, and two more, due to extracorporeal fertilization [29].
Endometrial perivascular cells
These cells could be also applied to promote local vascularization. Their ability to restore angiogenesis and inhibit scar formation when choosing cell types for uterine repair is of fundamental importance. Endometrial perivascular cells (En-PSCs) carrying CD146 markers and platelet growth factor receptor PDGFR-β loaded into a collagen gel had a similar effect upon stem cells in the endometrial layer [30]. En-PSCs, if additionally transfected with the CYR61 angiogenic inducer, were shown to promote vascular formation. Development of CYR61-transfected En-PSC-loaded collagen scaffold significantly increased the density of blood vessels, since it stimulated release of angiogenic factors from extracellular matrix and, generally, accelerated the in vivo neovascularization.
Bone marrow-derived mesenchymal stromal stem cells
A significant number of studies show the effectiveness of stem cells derived from bone marrow (BMSCs) for endometrial and uterine regeneration due to their ease of isolation and reparative potential [31-33]. Administration of BMSCs, supplemented by cauterization (electroacopuncture), improved fertility by activating the CXCR4 chemokine receptor, enhanced expression of cytokeratins and vimentin, VEGF and bFGF [31]. Effect of BMSCs incorporated into polyglycerol sebacinate (PGS) gel was compared with effect of BMSC-loaded collagen, or polylactic and glycolic acid (PLGA) copolymer gels. The authors showed an increase in TGF-β1, bFGF synthesis, and better recovery of endometrial morphology when using PGS gel. However, the fertility rates were comparable to those achieved with collagen-based gel (72%), but higher than with PLGA carrier (42%) [34]. Similar data were reported by Qi et al. [32]. The authors studied ability of BMSCs-PGS scaffolds for restoration of uterine soft tissue deformities under various dynamic conditions without external stimuli. The authors compared efficacy of the materials loaded with different cell types. PGS with BMSCs showed better stimulation of endometrial proliferation and differentiation. Moreover, the in vivo studies have shown longer retention time for BMSCs in situ and more effective vascularization of the PGS scaffold.
Yang et al. [33] used BMSCs encapsulated in a gel based on pluronic F-127 (PF-127) and vitamin C, which resulted in an increased membrane stability. In addition, vitamin C reduced the secretion of TNF-α and interleukin 6 (IL-6) due to its antioxidant activity, maintained redox homeostasis, and promoted a pro-regenerative trend by increasing the IL-10 levels. BMSC/PF-127+vitamin C hydrogel restored endometrial thickness and reduced fibrous areas of endometrial stromal tissues.
Meanwhile, the BMSCs therapy in patients with AS still lacked efficiency in terms of fertility. Indeed, introduction of autologous BMSCs into subendometrial myometrium in 6 women suffering from AS stimulated an increase in endometrial thickness and normalized menstrual cycles, but this biological effect in all patients did not result into successful extracorporeal fertilization attempts [35].
Umbilical cord mesenchymal stromal cells
Wharton’s jelly of the umbilical cord is an alternative source of MSCs, from which stem cells are isolated (UCMSCs). Introduction of UCMSCs as a component of a collagen carrier improved endometrial proliferation, differentiation, and neovascularization after implantation of UCMSCs scaffolds into the endometrium [36]. All 26 patients with AS showed positive dynamics, increased endometrial thickness, and decreased the number of intrauterine adhesions. Ten patients soon became pregnant after completing the treatment. The newborn children were born without any obvious birth defects or placental pathology. UCMSCs mixed with gelatin and collagen fibers stimulated pronounced angiogenesis and reduced scar formation in the damaged area. The composite cell framework destroyed collagen in the scarred areas, probably, by increasing the amount of matrix metalloproteinase 9, FGF-2, and VEGF, and led to angiogenesis and cyclic endometrial regeneration [37]. Xin et al. [38] found that the UCMSC-loaded collagen scaffold reduced cell apoptosis and improved the state of endometrial stromal cells due to eventual paracrine action. The scaffold did not induce inflammation and contributed to collagen remodeling in regenerating endometrium. In addition, the UCMSCs-loaded collagen scaffold induced early rapid re-epithelization by increasing level of cell proliferation and expression of cytokeratin, which is vital for the subsequent endometrial repair after damage.
Growth effects of platelet-rich plasma
Inclusion of platelet-rich plasma (PRP) in the structure of hydrogels is a point for research of uterine endometrial regeneration. In a number of studies, PRP was used to stimulate MSCs obtained from the menstrual blood. Transplantation of these MSCs stimulated thickening of the endometrium [39].
In women who underwent endometrial electroacupuncture for the first time, the effect of autologous PRP administration on endometrial thickness was evaluated. PRP infusion resulted in endometrial thickening up to 7 mm observed 48-72 hours later. Successful pregnancy occurred then after progesterone administration [40]. Similar data were obtained in a randomized clinical trial (n=83) among women with hypometriosis suffering from poor endometrial response to standard hormone replacement therapy. After PRP administration, there was a significant increase in endometrial thickness; in this group, the frequency of implantation and clinical pregnancy per cycle also increased significantly [41].
Application of autologous PRP in rat model with ethanol-induced endometrial injury led to endometrial regeneration. The authors concluded that intrauterine administration of autologous PRP stimulates and accelerates endometrial regeneration, along with reduced formation of fibrosis [42].
There are also studies in which the authors did not show that intrauterine PRP injection improves the results of hysteroscopy after surgical removal of intrauterine adhesions [43].
Hypothetical mechanisms of stem cell actions
Most research groups did not show real engraftment of transplanted mesenchymal cells, which confirms the current opinion about paracrine nature of therapeutic effects caused by allogeneic MSCs [44-48].
Cellular elements can be administered to patients as intravascular infusions [49, 50], intramuscularly [51] or directly into the uterine cavity. Both enriched MSCs or stromal cell suspensions can be used [35, 52, 53]. No serious side effects or signs of cell engraftment were observed in these studies. There is no doubt that further research and clinical trials are needed to optimize the use of cell therapy.
In healthy female body, cytokine environment and immune cellular repertoire depend on the phase of menstrual cycle [44], which are important for reducing fibrosis and restoration of endometrium. It is believed that successful endometrial repair technology is directly related to the activity of local inflammation. To achieve maximal therapeutic effect, it is necessary to ensure optimal mechanism and timely delivery of cellular elements, depending on the cycle phase. It is also necessary to minimize potential risks associated with the delivery method. For example, AS is often associated with disturbed blood flow in uterine spiral arterioles. Therefore, intravascular administration of these cellular elements cannot be used in this case.
Currently, most researchers believe that stem cells can only have a systemic and paracrine therapeutic effect, since they, probably, act as immunomodulators [46, 54, 55]. Immunomodulatory effects result from destruction of these cells or their absorption. This may explain the reparative tissue response during local injection of MSCs [56].
It is also suggested that the release of paracrine factors may represent the main effect of cellular therapy. In this case, the usage of exosomes/microvesicles opens some prospectives for regenerative medicine [57]. E.g., excretory microvesicles (eMVs) isolated from intrauterine fluid also have a protective effect [57-60]. In addition, MSCs have been shown to produce eMVs that have immunomodulatory effect on T cells [61].
During experiments on co-cultivation of mouse embryos and endometrial sMVs, an increase in the number of blastomeres, as well as vascular epithelium and platelets in embryos was observed [62]. These results led to the attempts of intrauterine infusion of PRP which includes eMVs components. In clinical trials, PRP infusion increased epithelial thickness [40, 63, 64], and, as a result, the number of successful implantations and natural pregnancies occurred. The fact that exosomes and other eMVs are an alternative to "direct" cell therapy and allow avoiding its negative consequences is confirmed by the high efficiency of PDGF-α, which is contained in platelets, in tissue repair and regeneration [65].
Clinical usage of antiadhesive polymers to prevent Ascherman’s syndrome
As noted above, the AS adhesions within uterus make it difficult to outflow menstrual blood and reduce women's fertility. Hormone therapy is not effective for AS. The main method of therapy is the removal of adhesions in one way or another. After removing the adhesions, new endometrial damage occurs, which often leads to the formation of the adhesions de novo. To prevent the formation of new adhesions, a number of studies have suggested using copolymers of hyaluronic acid and carboxymethylcellulose etc. for temporary separation of the uterine walls, which can provide the time required for re-epithelization of the wound surface.
For the prevention of AS and IUA in Russia and worldwide, various anti-adhesive materials are used, for example, Antiadhesin (Russia), Seprafilm film (France) based on sodium hyaluronate and sodium salt of carboxymethylcellulose (HA-CMC), Oxiplex/AP gel (USA) based on carboxymethylcellulose (CMC) and polyethylene oxide [8, 66-68], and others.
The use of barrier anti-adhesive agents based on HA-CMC reduces the risk of adhesion formation in the uterine cavity. According to a randomized study [68], intrauterine administration of an anti-adhesive barrier agent containing HA and CMC can not only prevent the formation or reduce the severity of intrauterine adhesions, but also contributes to the preservation of reproductive function. A total of 150 patients with incomplete or missed abortions participated in the clinical study. In the treatment group (Seprafilm) (n=50), membranes were inserted into the endometrial and cervical canal cavity after suction evacuation and/or curettage. The control group (n=100) did not get any treatment. Both groups were divided into two subgroups: patients who had no previous suction or curettage, and those who had at least one previous abortion or curettage. Further fertility was assessed by pregnancy success in all groups. The formation of endometrial synechiae was evaluated using hysterosalpingography in patients of all groups without pregnancy success 8 months after the intervention. The safety of using Seprafilm was evaluated by recording any adverse reactions and performing ultrasound monitoring. Of the subgroups without previous abortions, all 32 patients (100%) who received Seprafilm became pregnant in the next 8 months; in the control group, pregnancy occurred only in 54% of cases. They also showed that patients in the Seprafilm group with one or more previous interventions and no pregnancy after 8 months did not have adhesions in 90% of cases, and only in 50% of cases in the untreated group.
HA performs important physiological functions in the body, including joint lubrication, regulation of blood vessel wall permeability, regulation of protein and electrolyte transport, and wound healing. HA can bind to a large number of water molecules improving tissue hydration, increasing cell resistance to mechanical damage, and reducing post-traumatic formation of granulation and fibrous tissue. Due to its unique biocompatibility and enzymatic biodegradation, HA is often used to prevent postoperative adhesions [69, 70]. HA gel significantly reduces the frequency of IUA after intrauterine surgery, regardless of the type of intervention or the presence of primary diseases. It has been shown that treatment with HA gel increases the frequency of pregnancy after intrauterine surgery [71].
Heat-sensitive matrices based on poly-N-isopropylacrylamide
Poly-N-isopropylacrylamide (PNIPAM) is a polymer that performs a phase transition from a liquid to a gel state when heated above 37°C. As the temperature rises, PNIPAM forms a three-dimensional hydrogel that compresses and pushes the liquid out, releasing the contents into the surrounding tissue. Accordingly, PNIPAM, when administered, provides separation of the uterine walls. At the same time, it creates an enriched environment around itself that stimulates endometrial regeneration. Unlike PNIPAM, HA and CMC swell in the uterus, collecting intrauterine fluid and filling the internal volume. Theoretically, PNIPAM may be more promising for preventing adhesions. Currently, PNIPAM gels are being studied in vitro and in model systems, for example, for the cultivation of chondrocytes. Incubation of chondrocytes with PNIPAM showed that the cells were viable for 24 days, increased in number, and produced type II collagen and glycosaminoglycans [72].
For the functionalization of hydrogels, modification of PNIPAM is possible by including hydrophilic bioactive substances, such as cell culture supernates, eMVs or exosomes of MSCs, PRP, and other molecules. This modification may separate thermosetting and hydrophilic functions and allows each component to act independently. This concept was demonstrated by the Yoshioka group, who developed a tissue culture framework based on PNIPAM-graft-PEG [73-75]. The authors modified PNIPAM by including butyl ether of methacrylic acid in order to shift the phase transition point to a lower temperature. In subsequent studies in cell culture, the authors reported a system with a gelation temperature of 7°C in the cell culture medium [76]. It is known that thermosetting polymers with a hydrophobic component form dispersed gels in aqueous media above the critical point when the concentration exceeds the critical minimum. This phenomenon is associated with particle aggregation due to thermosetting flocculation of Poly(styrene-graft-NIPAM) particles [77, 78]. Poly(styrene-co-NIPAM) particles were used to form core-shell solid particles. Recently, thermosetting dispersed gels with Poly-ε-caprolactone cores and shells consisting of a polyethylene glycol brush were also obtained. Microdispersion procedure allowed to produce gels which were used as a thermally reversible cell culture system for mouse 3T3 fibroblasts [79] or C2C12 myoblasts [80].
Conclusions
The use of biopolymers and biomimetics based on combinations of polymers with various growth factors or live cells opens up new opportunities for the treatment of endometrial disorders. To this purpose, practical clinicians use gels based on hyaluronic acid, carboxymethyl cellulose, collagen, polyethylene oxide and others with certain efficiency. The main task of biopolymers is to separate the walls of the uterus, thus preventing synechiae, e.g., in Asherman’s syndrome. To improve treatment of hypo- and hypermetriosis, the gels should contain appropriate biological factors able to stimulate or inhibit the endometrial growth. Most of such biomimetic substances are currently at the pre-clinical testing stage. Some data from clinical studies have shown promising results of this approach for the treatment of female infertility.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgements
The work was supported by the Ministry of Health of the Russian Federation for 2020-2022 (no. AAAA-A20-120022790039-1).
References
- Borisov VA, Demography, Publishing House NOTABENE; 2001. 272 p. (In Russian).
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. Low N, ed. PLoS Med. 2012;9(12):e1001356. doi:10.1371/journal.pmed.1001356.
- Infertile Marriage. Modern approaches to Diagnosis and Treatment. Textbook (Ed.: GT Sukhykh). Moscow: GEOTAR-Media; 2010 (In Russian).
- Mahajan N, Sharma S. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci. 2016;9(1):3-8.
- Zhu HY, Ge TX, Pan Y Bin, Zhang SY. Advanced Role of Hippo Signaling in Endometrial Fibrosis: Implications for Intrauterine Adhesion. Chin Med J. 2017;130(22):2732-2737.
- Dreisler E, Kjer JJ. Asherman’s syndrome: Current perspectives on diagnosis and management. Int J Womens Health. 2019;11:191-198.
- Engelbrechtsen L, Istre O. Asherman syndrome. In: Minimally Invasive Gynecological Surgery. Springer Berlin Heidelberg; 2015:43-48.
- Arutyunova EE, Buralkina NA, Chuprynin VD, Zhorova VE. Pathogenetic justification and experience in using anti-adhesion gel in patients with intrauterine synechia after surgical hysteroscopy. Medical Council. 2018;(13):160-164. (In Russian)
- Tao Z, Duan H. Expression of adhesion-related cytokines in the uterine fluid after transcervical resection of adhesion. Zhonghua Fu Chan Ke Za Zhi. 2012;47(10):734-737. (In Chinese).
- Krikun G. The CXL12/CXCR4/CXCR7 axis in female reproductive tract disease: Review. Am J Reprod Immunol. 2018;80(5).
- Wang X, Ma N, Sun Q, Huang C, Liu Y, Luo X. Elevated NF-κB signaling in Asherman syndrome patients and animal models. Oncotarget. 2017;8(9):15399-15406.
- Xue X, Chen Q, Zhao G, Zhao JY, Duan Z, Zheng PS. The overexpression of TGF-β and CCN2 in intrauterine adhesions involves the NF-KB signaling pathway. PLoS One. 2015;10(12).
- Makarenko TA, Nikiforova DE. Modern opportunities in the treatment of Asherman syndrome. Mother and child. 2016;(15):1001-1004 (In Russian).
- Cervelló I, Santamaría X, Miyazaki K, Maruyama T, Simón C. Cell Therapy and Tissue Engineering from and toward the Uterus. Semin Reprod Med. 2015;33(05):366-372.
- Zhang SS, Xu XX, Xiang WW, Zhang HH, Lin HL, Shen LE, et al.. Using 17β estradiol heparin poloxamer thermosensitive hydrogel to enhance the endometrial regeneration and functional recovery of intrauterine adhesions in a rat model. FASEB J. 2020;34(1):446-457.
- Liu F, Hu S, Wang S, Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen Biomater. 2019;6(3):141-148.
- Liu F, Hu S, Yang H, Li Z, Huang K, Su T, et al. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv Healthc Mater. 2019;8(14):e1900411.
- Shadish JA, Benuska GM, DeForest CA. Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat Mater. 2019;18(9):1005-1014.
- Li C, Ouyang L, Pence IJ, Moore AC, Lin Y, Winter CW, Armstrong JPK, Stevens MM. Buoyancy-Driven Gradients for Biomaterial Fabrication and Tissue Engineering. Adv Mater. 2019;31(17):e1900291.
- Lin N, Li X, Song T, Wang J, Meng K, Yang J, et al. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials. 2012;33(6):1801-1807.
- Xu HL, Xu J, Zhang SS, Zhu QY, Jin BH, ZhuGe DL, et al.Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017;24(1):867-881.
- Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, Zhang S. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389-402.
- Gargett CE, Chan RWS, Schwab KE. Hormone and growth factor signaling in endometrial renewal: Role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1-2):22-29.
- Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell- based therapy. Biomed Pharmacother. 2018;102:333-343.
- Han Q, Du Y. Advances in the Application of Biomimetic Endometrium Interfaces for Uterine Bioengineering in Female Infertility. Front Bioeng Biotechnol. 2020;8:153.
- Owusu-Akyaw A, Krishnamoorthy K, Goldsmith LT, Morelli SS. The role of mesenchymal-epithelial transition in endometrial function. Hum Reprod Update. 2019;25(1):114-133.
- Frost BA, Sutliff BP, Thayer P, Bortner MJ, Foster EJ. Gradient poly(ethylene glycol) diacrylate and cellulose nanocrystals tissue engineering composite scaffolds via extrusion bioprinting. Front Bioeng Biotechnol. 2019;7:280.
- Kim S, Kim M, Jung S, Kwon K, Park J, Kim S, et al. Co-delivery of therapeutic protein and catalase-mimic nanoparticle using a biocompatible nanocarrier for enhanced therapeutic effect. J Control Release. 2019;309:181-189.
- Reynolds K, Khoury J, Sosnowski J, Thie J, Hofmann G. Comparison of the effect of tamoxifen on endometrial thickness in women with thin endometrium (<7mm) undergoing ovulation induction with clomiphene citrate. Fertil Steril. 2010;93(6):2091-2093.
- Li Z, Yan G, Diao Q, Yu F, Li X, Sheng X, et al. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell Res Ther. 2019;10(1):179.
- Xia L, Meng Q, Xi J, Han Q, Cheng J, Shen J, et al. The synergistic effect of electroacupuncture and bone mesenchymal stem cell transplantation on repairing thin endometrial injury in rats. Stem Cell Res Ther. 2019;10(1):244.
- Qi Y, Lohman J, Bratlie KM, Peroutka-Bigus N, Bellaire B, Wannemuehler M, et al. Vitamin C and B 3 as new biomaterials to alter intestinal stem cells. J Biomed Mater Res Part A. 2019;107(9):1886-1897.
- Yang H, Wu S, Feng R, Huang J, Liu L, Liu F, Chen Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res Ther. 2017;8(1):267.
- Xiao B, Yang W, Lei D, Huang J, Yin Y, Zhu Y, et al. PGS Scaffolds Promote the In Vivo Survival and Directional Differentiation of Bone Marrow Mesenchymal Stem Cells Restoring the Morphology and Function of Wounded Rat Uterus. Adv Healthc Mater. 2019;8(5):1801455.
- Panchal S, Patel H, Nagori C. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci. 2011;4(1):43.
- Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192.
- Xu L, Ding L, Wang L, Cao Y, Zhu H, Lu J, et al. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res Ther. 2017;8(1):1-13.
- Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang Y, et al. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019;92:160-171.
- Zhang S, Tan J, Li P. Co-transplantation of menstrual stromal cell and platelet-rich plasma improves Asherman’s syndrome in rat model. Fertil Steril. 2017;108(3):e193.
- Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, Liang X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8(1):1286-1290.
- Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol. 2018;57(6):810-813.
- Jang HY, Myoung SM, Choe JM, Kim T, Cheon YP, Kim YM, Park H. Effects of Autologous Platelet-Rich Plasma on Regeneration of Damaged Endometrium in Female Rats. Yonsei Med J. 2017;58(6):1195.
- Javaheri A, Kianfar K, Pourmasumi S, Eftekhar M. Platelet-rich plasma in the management of Asherman’s syndrome; An RCT. Int J Reprod Biomed. 2020;18(2):113-120.
- von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, et al. Analysis of Tissues Following Mesenchymal Stromal Cell Therapy in Humans Indicates Limited Long-Term Engraftment and No Ectopic Tissue Formation. Stem Cells. 2012;30(7):1575-1578.
- Golle L, Gerth HU, Beul K, Heitplatz B, Barth P, Fobker M, et al. Bone marrow-derived cells and their conditioned medium induce microvascular repair in uremic rats by stimulation of endogenous repair mechanisms. Sci Rep. 2017;7(1):9444.
- Lupatov AY, Poltavtseva RA, Bystrykh OA, Yarygin KN, Sukhikh GT. Neural stem/progenitor cells maintained in vitro under different culture conditions alter differentiation capacity of monocytes to generate dendritic cells. J Stem Cells Regen Med. 2017;13(2):54-61.
- Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther. 2019;10(1):257.
- Poltavtseva RA, Poltavtsev A V., Lutsenko G V., Svirshchevskaya E V. Myths, reality and future of mesenchymal stem cell therapy. Cell Tissue Res. 2019;375(3):563-574.
- Singh N, Mohanty S, Seth T, Shankar M, Dharmendra S, Bhaskaran S. Autologous stem cell transplantation in refractory Asherman’s syndrome: A novel cell based therapy. J Hum Reprod Sci. 2014;7(2):93.
- Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087-1096.
- Körbling M, Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98(10):2900-2908.
- Li X, Sun H, Lin N, Hou X, Wang J, Zhou B, et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials. 2011;32(32):8172-8181.
- Cervelló I, Gil-Sanchis C, Santamaría X, Cabanillas S, Díaz A, Faus A. Human CD133+ bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015;104(6):1552-1560.e3.
- Malhotra N, Bahadur A, Kalaivani M, Mittal S. Changes in endometrial receptivity in women with Asherman’s syndrome undergoing hysteroscopic adhesiolysis. Arch Gynecol Obstet. 2012;286(2):525-530.
- Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416):eaam7828.
- de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells. 2018;36(4):602-615.
- Ng YH, Rome S, Jalabert A, Forterre A, Singh H, Hincks CL, Salamonsen LA. Endometrial Exosomes/Microvesicles in the Uterine Microenvironment: A New Paradigm for Embryo-Endometrial Cross Talk at Implantation. Ward WS, ed. PLoS One. 2013;8(3):e58502.
- Da Silveira J, Andrade GM, Perecin F, Meireles FV, Winger QA, Bouma GJ. Isolation and Analysis of Exosomal MicroRNAs from Ovarian Follicular Fluid. In: Methods in Molecular Biology (Clifton, N.J.). Vol 1733. ; 2018:53-63.
- Da Silveira JC, de Ávila ACFCM, Garrett HL, Bruemmer JE, Winger QA, Bouma GJ. Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J Endocrinol. 2018;236(1):R15-R27.
- Tesfaye D, Gebremedhn S, Salilew-Wondim D, et al. MicroRNAs: tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction. 2018;155(3):R121-R135.
- Álvarez V, Sánchez-Margallo FM, Macías-García B, Gómez-Serrano M, Jorge I, Vázquez J, et al. The immunomodulatory activity of extracellular vesicles derived from endometrial mesenchymal stem cells on CD4+ T cells is partially mediated by TGFbeta. J Tissue Eng Regen Med. 2018;12(10):2088-2098.
- Blázquez R, Sánchez-Margallo FM, Álvarez V, Matilla E, Hernández N, Marinaro F, et al. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. Afink GB, ed. PLoS One. 2018;13(4):e0196080.
- Zadehmodarres S, Salehpour S, Saharkhiz N, Nazari L. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017;21(1):54-56.
- Chang Y, Li J, Wei L-N, Pang J, Chen J, Liang X. Autologous platelet-rich plasma infusion improves clinical pregnancy rate in frozen embryo transfer cycles for women with thin endometrium. Medicine (Baltimore). 2019;98(3):e14062.
- Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150-163.
- Molotkov AS, Popov EN, Sudakov DS, Aivazyan TA, Alexandrova LA, Dymarskaya YR. Experience of intrauterine application of anti-adhesive gel based on hyaluronic acid in the prevention of Asherman’s syndrome in patients with the pathology of the uterine cavity and severe forms of endometriosis. J Obstet and Female Dis. 2017;66(6):12-19. (In Russian).
- Tkachenko LV. Method of preventing adhesive disease after myomectomy. J Volgogr State Med Univ. 2019;71(3):70-73. (In Russian).
- Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Mater Res. 2002;63(1):10-14.
- Zheng F, Xin X, He F, Liu J, Cui Y, Meta‑analysis on the use of hyaluronic acid gel to prevent intrauterine adhesion after intrauterine operations, Exp Ther Med. 2020;19(4):2672-2678.
- Hong M-K, Ding D-C. Seprafilm® Application Method in Laparoscopic Surgery. JSLS J Soc Laparoendosc Surg. 2017;21(1):e2016.00097.
- Fei Z, Bin Z, Xin X, Fei H, Yuechong C. Meta-analysis on the use of hyaluronic acid gel to prevent recurrence of intrauterine adhesion after hysteroscopic adhesiolysis. Taiwan J Obstet Gynecol. 2019;58(6):731-736.
- Lapworth JW, Hatton P V, Goodchild RL, Rimmer S. Thermally reversible colloidal gels for three-dimensional chondrocyte culture. J R Soc Interface. 2012;9(67):362-375.
- Yoshioka H, Mikami M, Mori Y, Tsuchida E. A Synthetic Hydrogel with Thermoreversible Gelation. I. Preparation and Rheological Properties. J Macromol Sci Part A. 1994;31(1):113-120.
- Yoshioka H, Mikami M, Mori Y, Tsuchida E. A Synthetic Hydrogel with Thermoreversible Gelation. Ii. Effect of Added Salts. J Macromol Sci Part A. 1994;31(1):121-125.
- Yoshioka H, Mori Y, Cushman JA. A synthetic hydrogel with thermoreversible gelation, III: an NMR study of the sol-gel transition. Polym Adv Technol. 1994;5(2):122-127.
- Yasuda A, Kojima K, Tinsley KW, Yoshioka H, Mori Y, Vacanti CA. In Vitro Culture of Chondrocytes in a Novel Thermoreversible Gelation Polymer Scaffold Containing Growth Factors. Tissue Eng. 2006;12(5):1237-1245.
- Lu Y, Wittemann A, Ballauff M, Drechsler M. Preparation of Polystyrene-Poly(N-isopropylacrylamide) (PS-PNIPA) Core-Shell Particles by Photoemulsion Polymerization. Macromol Rapid Commun. 2006;27(14):1137-1141.
- Li Z, Ngai T. Macroporous Polymer from Core-Shell Particle-Stabilized Pickering Emulsions. Langmuir. 2010;26(7):5088-5092.
- Cheikh Al Ghanami R, Saunders BR, Bosquillon C, Shakesheff KM, Alexander C. Responsive particulate dispersions for reversible building and deconstruction of 3D cell environments. Soft Matter. 2010;6(20):5037.
- W. Wang, H. Liang, R. Cheikh Al Ghanami, L. Hamilton, M. Fraylich, K.M. Shakesheff, et al. Biodegradable Thermoresponsive Microparticle Dispersions for Injectable Cell Delivery Prepared Using a Single-Step Process. Adv Mater. 2009;21(18):1809-1813.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "20" ["~SORT"]=> string(2) "20" ["CODE"]=> string(48) "biomimetiki-dlya-terapii-zabolevaniy-endometriya" ["~CODE"]=> string(48) "biomimetiki-dlya-terapii-zabolevaniy-endometriya" ["EXTERNAL_ID"]=> string(4) "1944" ["~EXTERNAL_ID"]=> string(4) "1944" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(153) "Биомиметики для терапии заболеваний эндометрияBiomimetics for treatment of endometrial pathologies: an overview" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(3381) "<p style="text-align: justify;">Одной из острых проблем России является вторичное бесплодие женщин детородного возраста. Оно часто вызвано повреждением базального слоя эндометрия при выполнении гинекологических процедур: дилатации полости матки, лечебно-диагностическом выскабливании полости матки, кесаревом сечении, операциях на матке, а также после беременностей, протекающих с осложнениями. Как результат могут развиваться гипо- или гиперметриоз, а также формироваться внутриматочные спайки – синехии, приводящие к развитию синдрома Ашермана. Несмотря на большое количество накопленных клинических данных, отсутствует эффективный способ лечения вторичного бесплодия. В настоящее время с определенным успехом для терапии гипометриоза и синдрома Ашермана используют различные биополимеры и композиты на основе биополимеров с включением активных молекул, генов, их кодирующих, обогащенной тромбоцитами плазмы крови, стволовых клеток или микровезикул/экзосом стволовых клеток. Для использования в клинике сертифицированы гели на основе гиалуроната натрия, карбоксиметилцеллюлозы, полиэтиленоксида, коллагена и другие. Гели биополимеров являются, с одной стороны, разобщающими стенки матки препаратами (барьерная функция), а, с другой стороны, могут работать, как носители биологически активных молекул и клеток. Биомиметики с разной эффективностью способны стимулировать регенерацию и нормализацию эндометрия, что способствует восстановлению репродуктивной способности. Методики лечения на основе биомиметиков находятся в стадии исследований. В обзоре приведены данные по эффективности лечения патологий эндометрия матки с помощью биотерапевтических подходов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Бесплодие, гипометриоз, гиперметриоз, синдром Ашермана, барьерные материалы, биогели, биомиметики.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_META_TITLE"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_META_KEYWORDS"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_META_DESCRIPTION"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_PICTURE_FILE_ALT"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_PICTURE_FILE_TITLE"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_PICTURE_FILE_NAME"]=> string(52) "biomimetiki-dlya-terapii-zabolevaniy-endometriya-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(88) "Биомиметики для терапии заболеваний эндометрия" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(52) "biomimetiki-dlya-terapii-zabolevaniy-endometriya-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(52) "biomimetiki-dlya-terapii-zabolevaniy-endometriya-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(52) "biomimetiki-dlya-terapii-zabolevaniy-endometriya-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "171" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27395" ["VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27396" ["VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27397" ["VALUE"]=> array(2) { ["TEXT"]=> string(278) "<p> Мария В. Коновалова<sup>1</sup>, Дарья С. Царегородцева<sup>1,2</sup>, Римма А. Полтавцева<sup>3</sup>, Елена В. Свирщевская<sup>1,3</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(218) "
Мария В. Коновалова1, Дарья С. Царегородцева1,2, Римма А. Полтавцева3, Елена В. Свирщевская1,3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27398" ["VALUE"]=> array(2) { ["TEXT"]=> string(683) "<p><sup>1</sup> Институт биоорганической химии им. академиков М. М. Шемякина и Ю. А. Овчинникова РАН, Москва, Россия<br> <sup>2</sup> Первый Московский государственный медицинский университет им. И. М. Сеченова Минздрава РФ, Москва, Россия<br> <sup>3</sup> Научный центр акушерства, гинекологии и перинатологии им. акад. В. И. Кулакова Минздрава РФ, Москва, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(623) "1 Институт биоорганической химии им. академиков М. М. Шемякина и Ю. А. Овчинникова РАН, Москва, Россия
2 Первый Московский государственный медицинский университет им. И. М. Сеченова Минздрава РФ, Москва, Россия
3 Научный центр акушерства, гинекологии и перинатологии им. акад. В. И. Кулакова Минздрава РФ, Москва, Россия
Одной из острых проблем России является вторичное бесплодие женщин детородного возраста. Оно часто вызвано повреждением базального слоя эндометрия при выполнении гинекологических процедур: дилатации полости матки, лечебно-диагностическом выскабливании полости матки, кесаревом сечении, операциях на матке, а также после беременностей, протекающих с осложнениями. Как результат могут развиваться гипо- или гиперметриоз, а также формироваться внутриматочные спайки – синехии, приводящие к развитию синдрома Ашермана. Несмотря на большое количество накопленных клинических данных, отсутствует эффективный способ лечения вторичного бесплодия. В настоящее время с определенным успехом для терапии гипометриоза и синдрома Ашермана используют различные биополимеры и композиты на основе биополимеров с включением активных молекул, генов, их кодирующих, обогащенной тромбоцитами плазмы крови, стволовых клеток или микровезикул/экзосом стволовых клеток. Для использования в клинике сертифицированы гели на основе гиалуроната натрия, карбоксиметилцеллюлозы, полиэтиленоксида, коллагена и другие. Гели биополимеров являются, с одной стороны, разобщающими стенки матки препаратами (барьерная функция), а, с другой стороны, могут работать, как носители биологически активных молекул и клеток. Биомиметики с разной эффективностью способны стимулировать регенерацию и нормализацию эндометрия, что способствует восстановлению репродуктивной способности. Методики лечения на основе биомиметиков находятся в стадии исследований. В обзоре приведены данные по эффективности лечения патологий эндометрия матки с помощью биотерапевтических подходов.
Ключевые слова
Бесплодие, гипометриоз, гиперметриоз, синдром Ашермана, барьерные материалы, биогели, биомиметики.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27400" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-68-77" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-68-77" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27403" ["VALUE"]=> array(2) { ["TEXT"]=> string(212) "<p>Maria V. Konovalova<sup>1</sup>, Daria S. Tsaregorodtseva<sup>1,2</sup>, Rimma A. Poltavtseva<sup>3</sup>, Elena V. Svirshchevskaya<sup>1,3</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(152) "Maria V. Konovalova1, Daria S. Tsaregorodtseva1,2, Rimma A. Poltavtseva3, Elena V. Svirshchevskaya1,3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27404" ["VALUE"]=> array(2) { ["TEXT"]=> string(684) "<p><sup>1</sup> Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia<br> <sup>2</sup> Sechenov’s First Moscow State Medical University, Moscow, Russia<br> <sup>3</sup> V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia</p><br> <p><b>Correspondence</b><br> Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia<br> Phone: +7 (910) 464 8760<br> Fax: +7 (495) 330 4011<br> E-mail: esvir@mail.ibch.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(570) "1 Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia
2 Sechenov’s First Moscow State Medical University, Moscow, Russia
3 V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
Correspondence
Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia
Phone: +7 (910) 464 8760
Fax: +7 (495) 330 4011
E-mail: esvir@mail.ibch.ru
Secondary infertility among women in their childbearing age is one of sufficient problems in Russia. It is often caused by damage to the basal layer of endometrium when performing gynecological procedures, e.g., dilatation of uterine cavity, diagnostic curettage, cesarean section, uterine surgey, as well as consequences of complicated pregnancies. As a result, hypo- or hypermetriosis may develop, along with intrauterine adhesions (synechiae), leading to the development of Asherman’s syndrome. Despite large amounts of medical data, there are no quite effective ways to treat secondary infertility. Currently, various biological polymers and composite materials based on biopolymers with incorporated active molecules, genetic substances, platelet-rich plasma, stem cells or microvesicles/exosomes of stem cells are used with some success for treatment of hypometriosis and Asherman’s syndrome. Gel substances based on sodium hyaluronate, carboxymethylcellulose, polyethylene oxide, collagen and others are certified for clinical use. Biopolymer gels serve, on the one hand, as the materials separating the uterine walls (barrier function), and, on the other hand, they work as carriers of biologically active molecules and cells. Biomimetics can stimulate the regeneration and normalization of endometrium at different efficiency rates, thus promoting restoration of reproductive capacity. Biomimetic-based therapies are under investigation. The present review provides data on treatment efficiency of endometrial disorders by means of biotherapeutic approaches.
Keywords
Infertility, hypometriosis, Asherman's syndrome, barrier materials, biogels, biomimetics.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27401" ["VALUE"]=> string(65) "Biomimetics for treatment of endometrial pathologies: an overview" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(65) "Biomimetics for treatment of endometrial pathologies: an overview" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27402" ["VALUE"]=> string(4) "2350" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2350" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27406" ["VALUE"]=> string(4) "2351" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2351" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27403" ["VALUE"]=> array(2) { ["TEXT"]=> string(212) "<p>Maria V. Konovalova<sup>1</sup>, Daria S. Tsaregorodtseva<sup>1,2</sup>, Rimma A. Poltavtseva<sup>3</sup>, Elena V. Svirshchevskaya<sup>1,3</sup></p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(152) "Maria V. Konovalova1, Daria S. Tsaregorodtseva1,2, Rimma A. Poltavtseva3, Elena V. Svirshchevskaya1,3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(152) "Maria V. Konovalova1, Daria S. Tsaregorodtseva1,2, Rimma A. Poltavtseva3, Elena V. Svirshchevskaya1,3
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27405" ["VALUE"]=> array(2) { ["TEXT"]=> string(1811) "<p style="text-align: justify;">Secondary infertility among women in their childbearing age is one of sufficient problems in Russia. It is often caused by damage to the basal layer of endometrium when performing gynecological procedures, e.g., dilatation of uterine cavity, diagnostic curettage, cesarean section, uterine surgey, as well as consequences of complicated pregnancies. As a result, hypo- or hypermetriosis may develop, along with intrauterine adhesions (synechiae), leading to the development of Asherman’s syndrome. Despite large amounts of medical data, there are no quite effective ways to treat secondary infertility. Currently, various biological polymers and composite materials based on biopolymers with incorporated active molecules, genetic substances, platelet-rich plasma, stem cells or microvesicles/exosomes of stem cells are used with some success for treatment of hypometriosis and Asherman’s syndrome. Gel substances based on sodium hyaluronate, carboxymethylcellulose, polyethylene oxide, collagen and others are certified for clinical use. Biopolymer gels serve, on the one hand, as the materials separating the uterine walls (barrier function), and, on the other hand, they work as carriers of biologically active molecules and cells. Biomimetics can stimulate the regeneration and normalization of endometrium at different efficiency rates, thus promoting restoration of reproductive capacity. Biomimetic-based therapies are under investigation. The present review provides data on treatment efficiency of endometrial disorders by means of biotherapeutic approaches. </p> <h2>Keywords</h2> <p style="text-align: justify;">Infertility, hypometriosis, Asherman's syndrome, barrier materials, biogels, biomimetics.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1755) "Secondary infertility among women in their childbearing age is one of sufficient problems in Russia. It is often caused by damage to the basal layer of endometrium when performing gynecological procedures, e.g., dilatation of uterine cavity, diagnostic curettage, cesarean section, uterine surgey, as well as consequences of complicated pregnancies. As a result, hypo- or hypermetriosis may develop, along with intrauterine adhesions (synechiae), leading to the development of Asherman’s syndrome. Despite large amounts of medical data, there are no quite effective ways to treat secondary infertility. Currently, various biological polymers and composite materials based on biopolymers with incorporated active molecules, genetic substances, platelet-rich plasma, stem cells or microvesicles/exosomes of stem cells are used with some success for treatment of hypometriosis and Asherman’s syndrome. Gel substances based on sodium hyaluronate, carboxymethylcellulose, polyethylene oxide, collagen and others are certified for clinical use. Biopolymer gels serve, on the one hand, as the materials separating the uterine walls (barrier function), and, on the other hand, they work as carriers of biologically active molecules and cells. Biomimetics can stimulate the regeneration and normalization of endometrium at different efficiency rates, thus promoting restoration of reproductive capacity. Biomimetic-based therapies are under investigation. The present review provides data on treatment efficiency of endometrial disorders by means of biotherapeutic approaches.
Keywords
Infertility, hypometriosis, Asherman's syndrome, barrier materials, biogels, biomimetics.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1755) "Secondary infertility among women in their childbearing age is one of sufficient problems in Russia. It is often caused by damage to the basal layer of endometrium when performing gynecological procedures, e.g., dilatation of uterine cavity, diagnostic curettage, cesarean section, uterine surgey, as well as consequences of complicated pregnancies. As a result, hypo- or hypermetriosis may develop, along with intrauterine adhesions (synechiae), leading to the development of Asherman’s syndrome. Despite large amounts of medical data, there are no quite effective ways to treat secondary infertility. Currently, various biological polymers and composite materials based on biopolymers with incorporated active molecules, genetic substances, platelet-rich plasma, stem cells or microvesicles/exosomes of stem cells are used with some success for treatment of hypometriosis and Asherman’s syndrome. Gel substances based on sodium hyaluronate, carboxymethylcellulose, polyethylene oxide, collagen and others are certified for clinical use. Biopolymer gels serve, on the one hand, as the materials separating the uterine walls (barrier function), and, on the other hand, they work as carriers of biologically active molecules and cells. Biomimetics can stimulate the regeneration and normalization of endometrium at different efficiency rates, thus promoting restoration of reproductive capacity. Biomimetic-based therapies are under investigation. The present review provides data on treatment efficiency of endometrial disorders by means of biotherapeutic approaches.
Keywords
Infertility, hypometriosis, Asherman's syndrome, barrier materials, biogels, biomimetics.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27400" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-68-77" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-68-77" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-68-77" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27401" ["VALUE"]=> string(65) "Biomimetics for treatment of endometrial pathologies: an overview" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(65) "Biomimetics for treatment of endometrial pathologies: an overview" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(65) "Biomimetics for treatment of endometrial pathologies: an overview" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27404" ["VALUE"]=> array(2) { ["TEXT"]=> string(684) "<p><sup>1</sup> Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia<br> <sup>2</sup> Sechenov’s First Moscow State Medical University, Moscow, Russia<br> <sup>3</sup> V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia</p><br> <p><b>Correspondence</b><br> Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia<br> Phone: +7 (910) 464 8760<br> Fax: +7 (495) 330 4011<br> E-mail: esvir@mail.ibch.ru</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(570) "1 Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia
2 Sechenov’s First Moscow State Medical University, Moscow, Russia
3 V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
Correspondence
Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia
Phone: +7 (910) 464 8760
Fax: +7 (495) 330 4011
E-mail: esvir@mail.ibch.ru
1 Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia
2 Sechenov’s First Moscow State Medical University, Moscow, Russia
3 V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
Correspondence
Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia
Phone: +7 (910) 464 8760
Fax: +7 (495) 330 4011
E-mail: esvir@mail.ibch.ru
Мария В. Коновалова1, Дарья С. Царегородцева1,2, Римма А. Полтавцева3, Елена В. Свирщевская1,3
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(218) "Мария В. Коновалова1, Дарья С. Царегородцева1,2, Римма А. Полтавцева3, Елена В. Свирщевская1,3
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27395" ["VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "10/02/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "10/02/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27396" ["VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/13/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/13/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27399" ["VALUE"]=> array(2) { ["TEXT"]=> string(3381) "<p style="text-align: justify;">Одной из острых проблем России является вторичное бесплодие женщин детородного возраста. Оно часто вызвано повреждением базального слоя эндометрия при выполнении гинекологических процедур: дилатации полости матки, лечебно-диагностическом выскабливании полости матки, кесаревом сечении, операциях на матке, а также после беременностей, протекающих с осложнениями. Как результат могут развиваться гипо- или гиперметриоз, а также формироваться внутриматочные спайки – синехии, приводящие к развитию синдрома Ашермана. Несмотря на большое количество накопленных клинических данных, отсутствует эффективный способ лечения вторичного бесплодия. В настоящее время с определенным успехом для терапии гипометриоза и синдрома Ашермана используют различные биополимеры и композиты на основе биополимеров с включением активных молекул, генов, их кодирующих, обогащенной тромбоцитами плазмы крови, стволовых клеток или микровезикул/экзосом стволовых клеток. Для использования в клинике сертифицированы гели на основе гиалуроната натрия, карбоксиметилцеллюлозы, полиэтиленоксида, коллагена и другие. Гели биополимеров являются, с одной стороны, разобщающими стенки матки препаратами (барьерная функция), а, с другой стороны, могут работать, как носители биологически активных молекул и клеток. Биомиметики с разной эффективностью способны стимулировать регенерацию и нормализацию эндометрия, что способствует восстановлению репродуктивной способности. Методики лечения на основе биомиметиков находятся в стадии исследований. В обзоре приведены данные по эффективности лечения патологий эндометрия матки с помощью биотерапевтических подходов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Бесплодие, гипометриоз, гиперметриоз, синдром Ашермана, барьерные материалы, биогели, биомиметики.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3325) "Одной из острых проблем России является вторичное бесплодие женщин детородного возраста. Оно часто вызвано повреждением базального слоя эндометрия при выполнении гинекологических процедур: дилатации полости матки, лечебно-диагностическом выскабливании полости матки, кесаревом сечении, операциях на матке, а также после беременностей, протекающих с осложнениями. Как результат могут развиваться гипо- или гиперметриоз, а также формироваться внутриматочные спайки – синехии, приводящие к развитию синдрома Ашермана. Несмотря на большое количество накопленных клинических данных, отсутствует эффективный способ лечения вторичного бесплодия. В настоящее время с определенным успехом для терапии гипометриоза и синдрома Ашермана используют различные биополимеры и композиты на основе биополимеров с включением активных молекул, генов, их кодирующих, обогащенной тромбоцитами плазмы крови, стволовых клеток или микровезикул/экзосом стволовых клеток. Для использования в клинике сертифицированы гели на основе гиалуроната натрия, карбоксиметилцеллюлозы, полиэтиленоксида, коллагена и другие. Гели биополимеров являются, с одной стороны, разобщающими стенки матки препаратами (барьерная функция), а, с другой стороны, могут работать, как носители биологически активных молекул и клеток. Биомиметики с разной эффективностью способны стимулировать регенерацию и нормализацию эндометрия, что способствует восстановлению репродуктивной способности. Методики лечения на основе биомиметиков находятся в стадии исследований. В обзоре приведены данные по эффективности лечения патологий эндометрия матки с помощью биотерапевтических подходов.
Ключевые слова
Бесплодие, гипометриоз, гиперметриоз, синдром Ашермана, барьерные материалы, биогели, биомиметики.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3325) "Одной из острых проблем России является вторичное бесплодие женщин детородного возраста. Оно часто вызвано повреждением базального слоя эндометрия при выполнении гинекологических процедур: дилатации полости матки, лечебно-диагностическом выскабливании полости матки, кесаревом сечении, операциях на матке, а также после беременностей, протекающих с осложнениями. Как результат могут развиваться гипо- или гиперметриоз, а также формироваться внутриматочные спайки – синехии, приводящие к развитию синдрома Ашермана. Несмотря на большое количество накопленных клинических данных, отсутствует эффективный способ лечения вторичного бесплодия. В настоящее время с определенным успехом для терапии гипометриоза и синдрома Ашермана используют различные биополимеры и композиты на основе биополимеров с включением активных молекул, генов, их кодирующих, обогащенной тромбоцитами плазмы крови, стволовых клеток или микровезикул/экзосом стволовых клеток. Для использования в клинике сертифицированы гели на основе гиалуроната натрия, карбоксиметилцеллюлозы, полиэтиленоксида, коллагена и другие. Гели биополимеров являются, с одной стороны, разобщающими стенки матки препаратами (барьерная функция), а, с другой стороны, могут работать, как носители биологически активных молекул и клеток. Биомиметики с разной эффективностью способны стимулировать регенерацию и нормализацию эндометрия, что способствует восстановлению репродуктивной способности. Методики лечения на основе биомиметиков находятся в стадии исследований. В обзоре приведены данные по эффективности лечения патологий эндометрия матки с помощью биотерапевтических подходов.
Ключевые слова
Бесплодие, гипометриоз, гиперметриоз, синдром Ашермана, барьерные материалы, биогели, биомиметики.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27398" ["VALUE"]=> array(2) { ["TEXT"]=> string(683) "<p><sup>1</sup> Институт биоорганической химии им. академиков М. М. Шемякина и Ю. А. Овчинникова РАН, Москва, Россия<br> <sup>2</sup> Первый Московский государственный медицинский университет им. И. М. Сеченова Минздрава РФ, Москва, Россия<br> <sup>3</sup> Научный центр акушерства, гинекологии и перинатологии им. акад. В. И. Кулакова Минздрава РФ, Москва, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(623) "1 Институт биоорганической химии им. академиков М. М. Шемякина и Ю. А. Овчинникова РАН, Москва, Россия
2 Первый Московский государственный медицинский университет им. И. М. Сеченова Минздрава РФ, Москва, Россия
3 Научный центр акушерства, гинекологии и перинатологии им. акад. В. И. Кулакова Минздрава РФ, Москва, Россия
1 Институт биоорганической химии им. академиков М. М. Шемякина и Ю. А. Овчинникова РАН, Москва, Россия
2 Первый Московский государственный медицинский университет им. И. М. Сеченова Минздрава РФ, Москва, Россия
3 Научный центр акушерства, гинекологии и перинатологии им. акад. В. И. Кулакова Минздрава РФ, Москва, Россия
Introduction
One of the main lines of development in modern medicine and pharmacology is design of the methods for target delivery of pharmaceutical preparations into the damaged area of a body. This approach enables researchers to (i) increase the dose of a preparation present in the damaged organ; (ii) achieve prolonged action of a drug; (iii) exclude or considerably reduce possibility of toxic action of a drug on healthy organs and tissues. As a rule, conventional treatment involves introduction of a preparation into systemic blood circulation, whereupon the substance is distributed by blood in the organism of a patient. Therefore, in order to reach sufficiently high (i.e., therapeutically effective) concentration of a drug in the damaged area, it is necessary to introduce intentionally high amounts of this drug [1, 2]. The situation is also complicated by the fact that the majority of pharmaceutical preparations possess considerable toxicity; besides, in many cases, multiple administrations (courses of therapy) are necessary. In particular, a major problem of treatment of patients with oncological diseases is related to high or extremely high toxicity of modern chemotherapy drugs [3, 4]. Therefore, development of systems and methods for target drug delivery is an especially important and actual task.
In general, the process of target delivery of medicinal preparations proceeds as follows: (i) the drug-containing carrier is introduced into systemic blood circulation; (ii) the carrier circulates within an organism and is selectively accumulated in the damaged area; (iii) low doses of a drug preparation are gradually released from the carrier [1, 2, 5, 6]. However, the main disadvantage of this approach consists in the inability of carriers to be accumulated in a selected zone. Upon introducing into blood, carriers are distributed in the organism similarly to the conventional drugs [7]. An alternative procedure involves regional administration (for example, injecting drugs directly into the damaged area). This approach allows one to achieve predominant localization of carriers in the target provided that administration is performed with high accuracy.
Drug carriers are commonly designed on the basis of porous (hollow) micro- or nanospheres [8, 9]; however, particles without internal free volume can also be used (including liposomes, polymeric, oxide, metal particles, and the particles based on biocompatible non-toxic salts) [1, 2, 10-14]. Protective shells of various polymers are used to shield carriers and the enclosed drugs from the active internal media of an organism, and to provide prolongation of drug release. The polymers used to create these shells can be divided into two basic groups: synthetic (poly(acrylic acid) [15], polystyrene sulfonate [16], poly(ethylene oxide) [17]) and natural (carboxymethyl chitosan [18], alginate [19], hyaluronic acid [20], and dextran sulfate [21]).
The drug carriers used in the present work were based on porous microparticles of calcium carbonate (СаСО3) covered with a layer of sodium salt of dextran sulfate; these objects meet the biological safety requirements and can be found in living organisms [22]. Spherical СаСО3 porous vaterites (cores) were synthesized. Porous vaterite is one of three calcium carbonate polymorphs; other СаСО3 modifications (cubic calcites and elongated aragonite crystals) do not possess porosity [23].
There are only few research papers concerning in vivo behavior of the СаСО3-based drug carriers. In several works [21, 24], СаСО3 vaterites containing various medicinal preparations were introduced to rats perorally and transdermally; their structures were studied after exposure to rat body for a certain time. The vaterites present in blood and plasma were destructed already in several hours after peroral administration [21, 25]. In the case of transdermal administration, vaterites underwent gradual bioresorption for one week without any morphological transformations [24]. In our earlier works, behavior of native СаСО3-based carriers (without protective shells) in rat muscular tissue has been investigated [26]. It has been demonstrated that in 3 days after implantation of СаСО3 cores into muscular tissue, structural transformation of calcium carbonate (from vaterite into aragonite) occurred; then, aragonite crystals were rapidly resorbed. In 2 weeks after operation, only traces of aragonite were found in tissues, and in 4 weeks, muscular tissue regained its normal state. No toxic action of the carriers on the surrounding tissues and the whole organism was revealed throughout the experiment.
We have found no papers describing in vivo behavior of СаСО3-based carriers covered with dextran sulfate shells in muscular tissue.
The aim of the present work was to study in vivo behavior of porous spherical СаСО3 vaterites (covered with protective shells of sodium salt of dextran sulfate) as components of target drug delivery systems in rat muscular tissue.
Materials and methods
Preparation of objects. Porous spherical vaterites (СаСО3) were obtained by co-precipitation according to the technique described elsewhere [8] with several modifications [27]; namely, equal volumes of 1 M aqueous solutions of СаСl2 × 2H2O and Na2CO3 were poured together at stirring with an RW 20 anchor-type stirrer at 1 000 rpm. The mixture was stirred for 30 s. The suspension formed in 15 min was filtered with a Schott glass filter (#16); the precipitate was washed thrice with distilled water, then with aqueous solutions of acetone of increasing concentrations (30, 60, and 100%). The product was dried in thermostat at 40-50°С until a constant weight was reached. Diameters of the obtained cores varied from 1 to 4 μm. Then the cores were coated with polyanionic sodium salt of dextran sulfate (DexS) with ММ=9-20 kDa (Sigma Aldrich, USA). Calcium carbonate cores (50 mg) were added to 0.001 wt.% aqueous solution of DexS (10 mL). The suspension was stirred using a Multi Bio RS-24 rotor (Biosan, Latvia) for 1 h; the solid fraction was filtered off using a Schott glass filter (#16), washed thrice with distilled water and dried at 20°C.
Scanning electron microscopy. The samples were studied with the aid of a Supra 55VP scanning electron microscope (Carl Zeiss, Germany) using secondary electron imaging. Before measurements, the samples were covered with thin platinum layer.
Experiments with animals. The in vivo experiments involved 25 white 3-month-old male rats of Wistar strain (5 animals per each series of experiments). Weight of the animals varied from 200 to 250 g. For the study of in vivo bioresorption, СаСО3 cores covered with DexS were sterilized in autoclave at 110°C for 1 h. Each weighed amount of СаСО3 (10 mg) was hermetically packed in aluminum foil. The animals were operated under general anesthesia (intraperitoneal injections of Zoletil 100 dissolved in 20 mL of physiological solution and Rometar (20 mg/mL), 0.1 and 0.015 mL of solutions per 0.1 kg of animal body mass, respectively). The samples were placed into thigh great adductor muscle (musculus adductor magnus) of one hind extremity (one sample per animal). Then the wounds in extremities were sutured layer by layer using atraumatic needles and Prolene 4-0 suture. After outer suturing, the rats were caged individually, were fed standard diet, and had free access to water. All animals were active after surgery; no inflammation in the implantation area was observed, which is indicative of the absence of detrimental effects of implantation.
Morphological studies of СаСО3 vaterites covered with DexS implanted in rat muscle tissue. In 3 days, 1, 2, 4 and 12 weeks after operation, samples of muscle tissue containing СаСО3 covered with DexS were removed from animals, fixed with 10% neutral formalin in phosphate buffer (рН=7.4) for not less than 24 hrs, dehydrated using a series of ethanol solutions with increasing concentrations, and enclosed in paraffin blocks according to the standard histological technique. The paraffin cuts (5 μm in width) transverse to muscular fibers were obtained with the use of an Accu-Cut SRT 200 microtome (Sakura, Japan) and stained with Mayer hematoxylin and eosin (BioVitrum, Russia). The connective tissue was visualized according to the Mallory method (BioVitrum, Russia). Microscopic analysis was performed using a Leica DM750 light microscope (Germany) with a 10× ocular and 4, 10, 40, and 100× objectives. Images were recorded with an ICC50 camera (Leica, Germany).
Results and discussion

Figure 1. SEM image of СаСО3 cores covered with DexS
Fig. 1 presents SEM images of surfaces of СаСО3 cores covered with DexS. It is seen that the cores are homogeneous in size; the average diameter of the majority of particles varies from 1 to 4 μm. The core surface is rough; nanometer-sized pores are observed. This structure is convenient for medicinal applications: the loaded substances can freely penetrate into the internal volume of a core, and prolonged release in the damaged area is facilitated. Besides, due to high porosity, transport of high amounts of a preparation is possible, which also contributes to therapeutic effect.
СаСО3 cores covered with DexS in muscular tissue in 3 days after implantation. In 3 days after operation, in 1 of 5 operated animals, round plicated cavity was observed visually; this cavity contained transparent viscous liquid (apparently, DexS) and was surrounded by a thin СаСО3 rim. High amount of leukocytes was found in the formed cavities (mainly neutrophiles and eosinophiles). In 4 of 5 cases, no cavities were revealed, and СаСО3 was localized in muscular tissue in the form of round aggregates and whitish streaks (Fig. 2a). Histological studies showed that calcium carbonate was mainly present in the form of elongated crystals 40-120 μm long and 10-20 μm wide assembled in bundles and surrounded by rather wide cellular shaft. This “mound” or wall consisted of loose lying cells (mainly macrophages, little amounts of segment-nuclear leukocytes (neutrophiles, eosinophiles), single lymphocytes, and few fibroblasts (Fig. 3 a, b)). The vessels surrounding this cellular mound were varicose and plethoric; erythrocyte sludges (stacks of aggregated erythrocytes) were observed. Pronounced edema appeared between muscle fibers near implantation site. High amounts of macrophages were found in endomysium; the vessels were dramatically exaggerated and plethoric. No necrotic damage was revealed in the surrounding tissues.
The proposed mechanism of formation of cavities in muscular tissue involves the reaction between СаСО3 cores and carbonic acid (the product of interaction between carbon dioxide and water, which is present in intercellular fluid). Carbon dioxide, in turn, is formed in the process of cellular respiration; however, it is mainly released by cells in the form of carbonic acid. The acid and the products of its dissociation exist in equilibrium. In addition, carbonic acid is included into the bicarbonate buffer system of blood plasma and intercellular fluid, which accounts for more than 50% of total buffer capacity [28]. The reaction between carbonic acid and СаСО3 cores gives calcium hydrocarbonate Са(HСО3)2, an unstable compound, which dissolves well in water. Decrease in carbonic acid concentration in the reaction zone leads to beginning of decomposition of the salt to СаСО3, carbon dioxide and water already at 15-20°C [29].
The average body temperature of rats is 38°C. Decrease in carbonic acid concentration in the implantation zone is caused by formation of a connective tissue capsule, which forms an impenetrable boundary between the reaction zone and intercellular fluid (containing buffer systems and products of muscle cell respiration). Consequently, СаСО3 precipitates in the form of crystals (and not in the form of cores), and carbon dioxide that is released during decomposition forms a cavity. The most probable morphological modification of the appearing crystals is aragonite, which is formed at similar temperatures [30]. The main reaction equations can be written as follows:
СаСО3 + CO2 + H2O → Са(HСО3)2,
or: СаСО3 + H2CO3 → Са(HСО3)2;
Са(HСО3)2 → СаСО3↓ + CO2 ↑+ H2O
The above reactions can proceed repeatedly and cease gradually as the interacting compounds are absorbed by the surrounding tissues and removed from the reaction zone.
СаСО3 cores covered with DexS in muscular tissue in 1 week after implantation. In one week after operation, round cavities were detected in the implantation site in all cases; their sizes were larger than those formed in 3 days (Fig. 2 b). These cavities were also filled with a transparent viscous liquid; no СаСО3 particles were visible. Microscopy studies revealed low amounts of calcium carbonate crystals accumulated at the periphery, on the inner surface of cavities. High amounts of cellular detritus and neutrophiles were present in the cavities (Fig. 3c). The cavities were surrounded with macrophage wall and a wide connective tissue capsule, in which fibroblasts were localized between young loose collagen fibers; macrophages, single segment-nuclear leukocytes and lymphocytes were also revealed. Many macrophages and lymphocytes penetrated into the cavity and were located inside it. The vessels in the forming capsule were exaggerated and plethoric; erythrocyte sludges were visible in some of them. Muscular tissue around the cavity was edematous; increased amounts of macrophages and lymphocytes were present in endomysium. The vessels in connective tissue interlayers were dramatically expanded and plethoric. No necrotic damage in the surrounding tissues was revealed.

Figure 2. Images of cross-sections of muscles after implantation of СаСО3 cores covered with DexS. a – in 3 days after implantation; b – in 1 week after implantation; c – in 2 weeks after implantation, d – in 4 weeks after implantation. The samples were fixed in neutral 10% formalin for not less than 48 h.

Figure 3. Histologic sections of rat muscular tissues made in 3 days (a, b), 1 week (c), 2 weeks (d), and 4 weeks (e, f) after implantation of СаСО3 cores covered with DexS. The samples were stained with hematoxylin and eosin. Magnification: 10× (а, c, d), 40× (b, e) and 100× (f).
СаСО3 cores covered with DexS in muscular tissue in 2 weeks after implantation. In 2 weeks after implantation, macroscopic cavities were visible in the form of narrow fissures only in 1 animal; no cavities were observed in the remaining rats (Fig. 2 c). Capsules consisting of loosely arranged collagen fibers were revealed around fissure-like cavities. The capsules consisted of fibroblasts, macrophages, and lymphocytes. When a cavity was absent, a wide connective tissue interlayer was found in its place; this interlayer contained high amounts of cells, mainly fibroblasts, macrophages, lymphocytes, and multinucleated foreign body giant cells (MFBGC) arranged around isolated crystals of СаСО3 (Fig. 3 d).
СаСО3 cores covered with DexS in muscular tissue in 4 and 12 weeks after implantation. In 4 weeks after operation, the connective tissue interlayer (Fig. 2d) was revealed visually and with the aid of a microscope in the place where СаСО3 cores have been implanted. Collagen fibers were completely formed; high amounts of cells were found. Fibroblasts, macrophages, MFBGC localized around isolated crystals of СаСО3, and leukocytes were revealed (Fig. 3 e, f). The vessels in the connective tissue interlayer and in endomysium were slightly expanded. The morphology (structures of muscular tissue, endomysium and perimysium) observed in the implantation zone in 12 weeks after the operation was almost similar to normal.
Thus, it was demonstrated that in 3 days after implantation of СаСО3 cores covered with DexS into muscular tissue, they were basically transformed into aragonite crystals, while cavities were formed in muscles in 1 of 5 cases; the cavities contained viscous liquid (obviously, solution of DexS). The cellular composition in the implantation zone indicated aseptic medium-grade inflammation. In 1 week after implantation, cavities filled with viscous liquid were observed in all cases. Note that this liquid is apparently solution of DexS (a natural bioresorbable polymer). СаСО3 cores were not revealed visually. The light microscopy data showed that the amount of calcium carbonate crystals decreased considerably as compared to the case observed in 3 days after operation, which indicates active bioresorption of the cores. Cellular detritus was visible in the cavities, and macrophage shafts were formed around them. The connective tissue started to form; it contained increased numbers of macrophages and leukocytes, which is an indication of a chronic inflammation process. However, bioresorption of vaterites was ahead of formation of the connective tissue capsule, and already in 2 weeks after the beginning of the experiment, only traces of СаСО3 were visible. In 4 weeks after operation, isolated СаСО3 inclusions surrounded with MFBGC were visible. At the same time, the state of muscular tissue became normal again, which is a favorable outcome. In 12 weeks after beginning of the experiment, no СаСО3 inclusions were found. It is important to note that no toxic (damaging) action of СаСО3 cores on the surrounding tissues and the whole organism was revealed at any stages of the experiment. However, appearance of cavities at the early stages of the experiment should be taken into account; tumors should be treated carefully, since the above-mentioned cavities may facilitate formation of metastases.
Conclusion
The obtained data indicate that porous СаСО3 vaterites covered with DexS are safe for medicinal use and capable of bioresorption; thus, they are promising materials for use as target drug delivery systems. The results of this study allow us to recommend the described systems as objects for further in vivo studies.
This work is a continuation of studies on the behavior of targeted drug delivery systems based on СаСО3 vaterites in living systems, published earlier [25, 26].
Financial support
The study was performed within the framework of budget-supported research project №АААА-А20-120022090044-2, Institute of Macromolecular Compounds, RAS.
Compliance with ethical standards
The experiments involving animals were performed according to European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986) and WMA Declaration of Helsinki concerning welfare of laboratory animals (1996).
Conflict of interests
The authors declare no conflict of interests.
References
- Piotrovsky LB. Essays on nanomedicine. SPb.: European House 2013; 204 p. (In Russian).
- Howard KA, Vorup-Jensen T, Peer D. Nanomedicine. Springer 2016; 393.
- O’Brien MER, Borthwick A, Rigg A, Leary A, Assersohn L, Last K, et al. Mortality within 30 days of chemotherapy: a clinical governance benchmarking issue for oncology patients. Br. J. Cancer 2006; 95: 1632-1636.
- Pearce A, Haas M, Viney R, Pearson SA, Haywood P, Brown C, Ward R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS One. 2017; 12: e0184360. https://doi.org/10.1371/journal.pone.0184360.
- Adiseshaiah P, Hall J, McNeil S. Nanomaterial standards for efficacy and toxicity assessment. WIREs Nanomed. Nanobiotechnol. 2010; 2: 99-112.
- Xu C, Song R, Lu P, Chen J, Zhou Y, Shen G, et al. A pH-responsive charge-reversal drug delivery system with tumor-specific drug release and ROS generation for cancer therapy. Int. J. Nanomed. 2020; 15: 65.
- Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J. Control Release 2012; 164: 108.
- Volodkin DV, Petrov AI, Prevot M, Sukhorukov GB. Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir 2004; 20: 3398-3406.
- Zhao Q, Han B, Wang Z, Gao C, Peng C, Shen J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: doxorubicin loading and in vitro and in vivo studies. Nanomed-Nanotechnol Biol Med. 2007; 3: 63-74.
- Matyszewska D. Drug delivery systems in the transport of doxorubicin. Surface Innovations 2014; 2: 201-210.
- Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015; 5: 124-133.
- Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C. 2016; 60: 569-578.
- Mishra V, Kesharwani P, Amin MCM, Iyer A. Nanotechnology-based approaches for targeting and delivery of drugs and genes. 1st Ed. Academic Press 2017; 552.
- Olusanya TOB, Ahmad RRH, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018; 23: 907. https://doi.org/10.3390/molecules23040907
- Huang S, Naka K, Chujo Y. Effect of molecular weights of poly(acrylic acid) on crystallization of calcium carbonate by the delayed addition method. Polymer J. 2008; 40: 154-162.
- Wang C, He C, Tong Z , Liu X, Ren B, Zeng F. Combination of adsorption by porous CaCO3 microparticles and encapsulation by polyelectrolyte multilayer films for sustained drug delivery. Int J Pharm. 2006; 308: 160-167.
- Richardson J, Maina J, Ejima H, Hu M, Guo J, Cho MY, et al. Versatile loading of diverse cargo into functional polymer capsules. Adv Sci. 2015; 2: 1400007. https://doi.org/10.1002/advs.201400007.
- Wang J, Chen J, Zong J, Zhao D, Li F, Zhuo R, Cheng Si. Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. J Phys Chem C. 2010; 114: 18940-18945.
- Zhao D, Zhuo R, Cheng S. Alginate modified nanostructured calcium carbonate with enhanced delivery efficiency for gene and drug delivery. Mol BioSystems. 2012; 8: 753-759.
- Bai J, Xu J, Zhao J, Zhang R. Hyaluronan and calcium carbonate hybrid nanoparticles for colorectal cancer chemotherapy. Mater Res Express. 2017; 4: 095401, https://doi.org/10.1088/2053-1591/aa822d.
- Sudareva N, Suvorova O, Saprykina N, Smirnova N, Bel'tiukov P, Petunov S, Radilov А, Vilesov A. Two-level delivery systems based on CaCO3 cores for oral administration of therapeutic peptides. J Microencapsulation 2018; 35: 619-634.
- Binevski PV, Balabushevich NG, Uvarova VI, Vikulina AS, Volodkin D. Bio-friendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology. Colloids and Surfaces B: Biointerfaces 2019; 181: 437-449.
- Cowan JC, Weintritt DJ. Water-formed scale deposits. Gulf Pub. Co., Book Division 1976; 596.
- Genina EA, Svenskaya YI, Yanina IY, Dolotov LE, Navolokin NA, Bashkatov AN, et al. In vivo optical monitoring of transcutaneous delivery of calcium carbonate microcontainers. Biomed. Opt. Express 2016; 7: 2082-2087.
- Sudareva NN, Popryadukhin PV, Saprykina NN, Suvorova OM, Yukina GY, Galibin OV, Vilesov AD. CaCO3 vaterites as components of target drug delivery systems. Cell Ther Transplant. 2020; 9(2): 13-19.
- Popryadukhin PV, Sudareva NN, Suvorova ОМ, Yukina GYu, Sukhorukova ЕG, Saprykina NN. Morphology of porous CaCO3 vaterites as components of target drug delivery systems in rat muscle tissue. Cytology. 2020; 62: 738-744.
- Sudareva NN, Suvorova ОM, Tarasenko II, Saprykina NN, Smirnova NV, Petunov SG, et al. Hybrid systems for oral delivery of therapeutic neuropeptide. Mendeleev Commun. 2020; 30: 25-27.
- Garrett RH, Grisham CM. Biochemistry. 6th Ed. Cengage Learning. 2017; 1218.
- Lyubomirsky NV, Bakhtin AS, Bakhtina TA, Nikolaenko EYu, Nikolaenko VV. The influence of calcium hydrogen carbonate on the structure formation and properties of materials based on lime carbonization hardening. Int Res J. 2016; 11: 86-93. https://doi.org/10:18454/IRJ.2016.53.146. (In Russian).
- Wray JL, Daniels F. Precipitation of calcite and aragonite. J. Am. Chem. Soc. 1957; 79: 2031-2034.
" ["~DETAIL_TEXT"]=> string(27319) "
Introduction
One of the main lines of development in modern medicine and pharmacology is design of the methods for target delivery of pharmaceutical preparations into the damaged area of a body. This approach enables researchers to (i) increase the dose of a preparation present in the damaged organ; (ii) achieve prolonged action of a drug; (iii) exclude or considerably reduce possibility of toxic action of a drug on healthy organs and tissues. As a rule, conventional treatment involves introduction of a preparation into systemic blood circulation, whereupon the substance is distributed by blood in the organism of a patient. Therefore, in order to reach sufficiently high (i.e., therapeutically effective) concentration of a drug in the damaged area, it is necessary to introduce intentionally high amounts of this drug [1, 2]. The situation is also complicated by the fact that the majority of pharmaceutical preparations possess considerable toxicity; besides, in many cases, multiple administrations (courses of therapy) are necessary. In particular, a major problem of treatment of patients with oncological diseases is related to high or extremely high toxicity of modern chemotherapy drugs [3, 4]. Therefore, development of systems and methods for target drug delivery is an especially important and actual task.
In general, the process of target delivery of medicinal preparations proceeds as follows: (i) the drug-containing carrier is introduced into systemic blood circulation; (ii) the carrier circulates within an organism and is selectively accumulated in the damaged area; (iii) low doses of a drug preparation are gradually released from the carrier [1, 2, 5, 6]. However, the main disadvantage of this approach consists in the inability of carriers to be accumulated in a selected zone. Upon introducing into blood, carriers are distributed in the organism similarly to the conventional drugs [7]. An alternative procedure involves regional administration (for example, injecting drugs directly into the damaged area). This approach allows one to achieve predominant localization of carriers in the target provided that administration is performed with high accuracy.
Drug carriers are commonly designed on the basis of porous (hollow) micro- or nanospheres [8, 9]; however, particles without internal free volume can also be used (including liposomes, polymeric, oxide, metal particles, and the particles based on biocompatible non-toxic salts) [1, 2, 10-14]. Protective shells of various polymers are used to shield carriers and the enclosed drugs from the active internal media of an organism, and to provide prolongation of drug release. The polymers used to create these shells can be divided into two basic groups: synthetic (poly(acrylic acid) [15], polystyrene sulfonate [16], poly(ethylene oxide) [17]) and natural (carboxymethyl chitosan [18], alginate [19], hyaluronic acid [20], and dextran sulfate [21]).
The drug carriers used in the present work were based on porous microparticles of calcium carbonate (СаСО3) covered with a layer of sodium salt of dextran sulfate; these objects meet the biological safety requirements and can be found in living organisms [22]. Spherical СаСО3 porous vaterites (cores) were synthesized. Porous vaterite is one of three calcium carbonate polymorphs; other СаСО3 modifications (cubic calcites and elongated aragonite crystals) do not possess porosity [23].
There are only few research papers concerning in vivo behavior of the СаСО3-based drug carriers. In several works [21, 24], СаСО3 vaterites containing various medicinal preparations were introduced to rats perorally and transdermally; their structures were studied after exposure to rat body for a certain time. The vaterites present in blood and plasma were destructed already in several hours after peroral administration [21, 25]. In the case of transdermal administration, vaterites underwent gradual bioresorption for one week without any morphological transformations [24]. In our earlier works, behavior of native СаСО3-based carriers (without protective shells) in rat muscular tissue has been investigated [26]. It has been demonstrated that in 3 days after implantation of СаСО3 cores into muscular tissue, structural transformation of calcium carbonate (from vaterite into aragonite) occurred; then, aragonite crystals were rapidly resorbed. In 2 weeks after operation, only traces of aragonite were found in tissues, and in 4 weeks, muscular tissue regained its normal state. No toxic action of the carriers on the surrounding tissues and the whole organism was revealed throughout the experiment.
We have found no papers describing in vivo behavior of СаСО3-based carriers covered with dextran sulfate shells in muscular tissue.
The aim of the present work was to study in vivo behavior of porous spherical СаСО3 vaterites (covered with protective shells of sodium salt of dextran sulfate) as components of target drug delivery systems in rat muscular tissue.
Materials and methods
Preparation of objects. Porous spherical vaterites (СаСО3) were obtained by co-precipitation according to the technique described elsewhere [8] with several modifications [27]; namely, equal volumes of 1 M aqueous solutions of СаСl2 × 2H2O and Na2CO3 were poured together at stirring with an RW 20 anchor-type stirrer at 1 000 rpm. The mixture was stirred for 30 s. The suspension formed in 15 min was filtered with a Schott glass filter (#16); the precipitate was washed thrice with distilled water, then with aqueous solutions of acetone of increasing concentrations (30, 60, and 100%). The product was dried in thermostat at 40-50°С until a constant weight was reached. Diameters of the obtained cores varied from 1 to 4 μm. Then the cores were coated with polyanionic sodium salt of dextran sulfate (DexS) with ММ=9-20 kDa (Sigma Aldrich, USA). Calcium carbonate cores (50 mg) were added to 0.001 wt.% aqueous solution of DexS (10 mL). The suspension was stirred using a Multi Bio RS-24 rotor (Biosan, Latvia) for 1 h; the solid fraction was filtered off using a Schott glass filter (#16), washed thrice with distilled water and dried at 20°C.
Scanning electron microscopy. The samples were studied with the aid of a Supra 55VP scanning electron microscope (Carl Zeiss, Germany) using secondary electron imaging. Before measurements, the samples were covered with thin platinum layer.
Experiments with animals. The in vivo experiments involved 25 white 3-month-old male rats of Wistar strain (5 animals per each series of experiments). Weight of the animals varied from 200 to 250 g. For the study of in vivo bioresorption, СаСО3 cores covered with DexS were sterilized in autoclave at 110°C for 1 h. Each weighed amount of СаСО3 (10 mg) was hermetically packed in aluminum foil. The animals were operated under general anesthesia (intraperitoneal injections of Zoletil 100 dissolved in 20 mL of physiological solution and Rometar (20 mg/mL), 0.1 and 0.015 mL of solutions per 0.1 kg of animal body mass, respectively). The samples were placed into thigh great adductor muscle (musculus adductor magnus) of one hind extremity (one sample per animal). Then the wounds in extremities were sutured layer by layer using atraumatic needles and Prolene 4-0 suture. After outer suturing, the rats were caged individually, were fed standard diet, and had free access to water. All animals were active after surgery; no inflammation in the implantation area was observed, which is indicative of the absence of detrimental effects of implantation.
Morphological studies of СаСО3 vaterites covered with DexS implanted in rat muscle tissue. In 3 days, 1, 2, 4 and 12 weeks after operation, samples of muscle tissue containing СаСО3 covered with DexS were removed from animals, fixed with 10% neutral formalin in phosphate buffer (рН=7.4) for not less than 24 hrs, dehydrated using a series of ethanol solutions with increasing concentrations, and enclosed in paraffin blocks according to the standard histological technique. The paraffin cuts (5 μm in width) transverse to muscular fibers were obtained with the use of an Accu-Cut SRT 200 microtome (Sakura, Japan) and stained with Mayer hematoxylin and eosin (BioVitrum, Russia). The connective tissue was visualized according to the Mallory method (BioVitrum, Russia). Microscopic analysis was performed using a Leica DM750 light microscope (Germany) with a 10× ocular and 4, 10, 40, and 100× objectives. Images were recorded with an ICC50 camera (Leica, Germany).
Results and discussion

Figure 1. SEM image of СаСО3 cores covered with DexS
Fig. 1 presents SEM images of surfaces of СаСО3 cores covered with DexS. It is seen that the cores are homogeneous in size; the average diameter of the majority of particles varies from 1 to 4 μm. The core surface is rough; nanometer-sized pores are observed. This structure is convenient for medicinal applications: the loaded substances can freely penetrate into the internal volume of a core, and prolonged release in the damaged area is facilitated. Besides, due to high porosity, transport of high amounts of a preparation is possible, which also contributes to therapeutic effect.
СаСО3 cores covered with DexS in muscular tissue in 3 days after implantation. In 3 days after operation, in 1 of 5 operated animals, round plicated cavity was observed visually; this cavity contained transparent viscous liquid (apparently, DexS) and was surrounded by a thin СаСО3 rim. High amount of leukocytes was found in the formed cavities (mainly neutrophiles and eosinophiles). In 4 of 5 cases, no cavities were revealed, and СаСО3 was localized in muscular tissue in the form of round aggregates and whitish streaks (Fig. 2a). Histological studies showed that calcium carbonate was mainly present in the form of elongated crystals 40-120 μm long and 10-20 μm wide assembled in bundles and surrounded by rather wide cellular shaft. This “mound” or wall consisted of loose lying cells (mainly macrophages, little amounts of segment-nuclear leukocytes (neutrophiles, eosinophiles), single lymphocytes, and few fibroblasts (Fig. 3 a, b)). The vessels surrounding this cellular mound were varicose and plethoric; erythrocyte sludges (stacks of aggregated erythrocytes) were observed. Pronounced edema appeared between muscle fibers near implantation site. High amounts of macrophages were found in endomysium; the vessels were dramatically exaggerated and plethoric. No necrotic damage was revealed in the surrounding tissues.
The proposed mechanism of formation of cavities in muscular tissue involves the reaction between СаСО3 cores and carbonic acid (the product of interaction between carbon dioxide and water, which is present in intercellular fluid). Carbon dioxide, in turn, is formed in the process of cellular respiration; however, it is mainly released by cells in the form of carbonic acid. The acid and the products of its dissociation exist in equilibrium. In addition, carbonic acid is included into the bicarbonate buffer system of blood plasma and intercellular fluid, which accounts for more than 50% of total buffer capacity [28]. The reaction between carbonic acid and СаСО3 cores gives calcium hydrocarbonate Са(HСО3)2, an unstable compound, which dissolves well in water. Decrease in carbonic acid concentration in the reaction zone leads to beginning of decomposition of the salt to СаСО3, carbon dioxide and water already at 15-20°C [29].
The average body temperature of rats is 38°C. Decrease in carbonic acid concentration in the implantation zone is caused by formation of a connective tissue capsule, which forms an impenetrable boundary between the reaction zone and intercellular fluid (containing buffer systems and products of muscle cell respiration). Consequently, СаСО3 precipitates in the form of crystals (and not in the form of cores), and carbon dioxide that is released during decomposition forms a cavity. The most probable morphological modification of the appearing crystals is aragonite, which is formed at similar temperatures [30]. The main reaction equations can be written as follows:
СаСО3 + CO2 + H2O → Са(HСО3)2,
or: СаСО3 + H2CO3 → Са(HСО3)2;
Са(HСО3)2 → СаСО3↓ + CO2 ↑+ H2O
The above reactions can proceed repeatedly and cease gradually as the interacting compounds are absorbed by the surrounding tissues and removed from the reaction zone.
СаСО3 cores covered with DexS in muscular tissue in 1 week after implantation. In one week after operation, round cavities were detected in the implantation site in all cases; their sizes were larger than those formed in 3 days (Fig. 2 b). These cavities were also filled with a transparent viscous liquid; no СаСО3 particles were visible. Microscopy studies revealed low amounts of calcium carbonate crystals accumulated at the periphery, on the inner surface of cavities. High amounts of cellular detritus and neutrophiles were present in the cavities (Fig. 3c). The cavities were surrounded with macrophage wall and a wide connective tissue capsule, in which fibroblasts were localized between young loose collagen fibers; macrophages, single segment-nuclear leukocytes and lymphocytes were also revealed. Many macrophages and lymphocytes penetrated into the cavity and were located inside it. The vessels in the forming capsule were exaggerated and plethoric; erythrocyte sludges were visible in some of them. Muscular tissue around the cavity was edematous; increased amounts of macrophages and lymphocytes were present in endomysium. The vessels in connective tissue interlayers were dramatically expanded and plethoric. No necrotic damage in the surrounding tissues was revealed.

Figure 2. Images of cross-sections of muscles after implantation of СаСО3 cores covered with DexS. a – in 3 days after implantation; b – in 1 week after implantation; c – in 2 weeks after implantation, d – in 4 weeks after implantation. The samples were fixed in neutral 10% formalin for not less than 48 h.

Figure 3. Histologic sections of rat muscular tissues made in 3 days (a, b), 1 week (c), 2 weeks (d), and 4 weeks (e, f) after implantation of СаСО3 cores covered with DexS. The samples were stained with hematoxylin and eosin. Magnification: 10× (а, c, d), 40× (b, e) and 100× (f).
СаСО3 cores covered with DexS in muscular tissue in 2 weeks after implantation. In 2 weeks after implantation, macroscopic cavities were visible in the form of narrow fissures only in 1 animal; no cavities were observed in the remaining rats (Fig. 2 c). Capsules consisting of loosely arranged collagen fibers were revealed around fissure-like cavities. The capsules consisted of fibroblasts, macrophages, and lymphocytes. When a cavity was absent, a wide connective tissue interlayer was found in its place; this interlayer contained high amounts of cells, mainly fibroblasts, macrophages, lymphocytes, and multinucleated foreign body giant cells (MFBGC) arranged around isolated crystals of СаСО3 (Fig. 3 d).
СаСО3 cores covered with DexS in muscular tissue in 4 and 12 weeks after implantation. In 4 weeks after operation, the connective tissue interlayer (Fig. 2d) was revealed visually and with the aid of a microscope in the place where СаСО3 cores have been implanted. Collagen fibers were completely formed; high amounts of cells were found. Fibroblasts, macrophages, MFBGC localized around isolated crystals of СаСО3, and leukocytes were revealed (Fig. 3 e, f). The vessels in the connective tissue interlayer and in endomysium were slightly expanded. The morphology (structures of muscular tissue, endomysium and perimysium) observed in the implantation zone in 12 weeks after the operation was almost similar to normal.
Thus, it was demonstrated that in 3 days after implantation of СаСО3 cores covered with DexS into muscular tissue, they were basically transformed into aragonite crystals, while cavities were formed in muscles in 1 of 5 cases; the cavities contained viscous liquid (obviously, solution of DexS). The cellular composition in the implantation zone indicated aseptic medium-grade inflammation. In 1 week after implantation, cavities filled with viscous liquid were observed in all cases. Note that this liquid is apparently solution of DexS (a natural bioresorbable polymer). СаСО3 cores were not revealed visually. The light microscopy data showed that the amount of calcium carbonate crystals decreased considerably as compared to the case observed in 3 days after operation, which indicates active bioresorption of the cores. Cellular detritus was visible in the cavities, and macrophage shafts were formed around them. The connective tissue started to form; it contained increased numbers of macrophages and leukocytes, which is an indication of a chronic inflammation process. However, bioresorption of vaterites was ahead of formation of the connective tissue capsule, and already in 2 weeks after the beginning of the experiment, only traces of СаСО3 were visible. In 4 weeks after operation, isolated СаСО3 inclusions surrounded with MFBGC were visible. At the same time, the state of muscular tissue became normal again, which is a favorable outcome. In 12 weeks after beginning of the experiment, no СаСО3 inclusions were found. It is important to note that no toxic (damaging) action of СаСО3 cores on the surrounding tissues and the whole organism was revealed at any stages of the experiment. However, appearance of cavities at the early stages of the experiment should be taken into account; tumors should be treated carefully, since the above-mentioned cavities may facilitate formation of metastases.
Conclusion
The obtained data indicate that porous СаСО3 vaterites covered with DexS are safe for medicinal use and capable of bioresorption; thus, they are promising materials for use as target drug delivery systems. The results of this study allow us to recommend the described systems as objects for further in vivo studies.
This work is a continuation of studies on the behavior of targeted drug delivery systems based on СаСО3 vaterites in living systems, published earlier [25, 26].
Financial support
The study was performed within the framework of budget-supported research project №АААА-А20-120022090044-2, Institute of Macromolecular Compounds, RAS.
Compliance with ethical standards
The experiments involving animals were performed according to European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986) and WMA Declaration of Helsinki concerning welfare of laboratory animals (1996).
Conflict of interests
The authors declare no conflict of interests.
References
- Piotrovsky LB. Essays on nanomedicine. SPb.: European House 2013; 204 p. (In Russian).
- Howard KA, Vorup-Jensen T, Peer D. Nanomedicine. Springer 2016; 393.
- O’Brien MER, Borthwick A, Rigg A, Leary A, Assersohn L, Last K, et al. Mortality within 30 days of chemotherapy: a clinical governance benchmarking issue for oncology patients. Br. J. Cancer 2006; 95: 1632-1636.
- Pearce A, Haas M, Viney R, Pearson SA, Haywood P, Brown C, Ward R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS One. 2017; 12: e0184360. https://doi.org/10.1371/journal.pone.0184360.
- Adiseshaiah P, Hall J, McNeil S. Nanomaterial standards for efficacy and toxicity assessment. WIREs Nanomed. Nanobiotechnol. 2010; 2: 99-112.
- Xu C, Song R, Lu P, Chen J, Zhou Y, Shen G, et al. A pH-responsive charge-reversal drug delivery system with tumor-specific drug release and ROS generation for cancer therapy. Int. J. Nanomed. 2020; 15: 65.
- Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J. Control Release 2012; 164: 108.
- Volodkin DV, Petrov AI, Prevot M, Sukhorukov GB. Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir 2004; 20: 3398-3406.
- Zhao Q, Han B, Wang Z, Gao C, Peng C, Shen J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: doxorubicin loading and in vitro and in vivo studies. Nanomed-Nanotechnol Biol Med. 2007; 3: 63-74.
- Matyszewska D. Drug delivery systems in the transport of doxorubicin. Surface Innovations 2014; 2: 201-210.
- Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015; 5: 124-133.
- Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C. 2016; 60: 569-578.
- Mishra V, Kesharwani P, Amin MCM, Iyer A. Nanotechnology-based approaches for targeting and delivery of drugs and genes. 1st Ed. Academic Press 2017; 552.
- Olusanya TOB, Ahmad RRH, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018; 23: 907. https://doi.org/10.3390/molecules23040907
- Huang S, Naka K, Chujo Y. Effect of molecular weights of poly(acrylic acid) on crystallization of calcium carbonate by the delayed addition method. Polymer J. 2008; 40: 154-162.
- Wang C, He C, Tong Z , Liu X, Ren B, Zeng F. Combination of adsorption by porous CaCO3 microparticles and encapsulation by polyelectrolyte multilayer films for sustained drug delivery. Int J Pharm. 2006; 308: 160-167.
- Richardson J, Maina J, Ejima H, Hu M, Guo J, Cho MY, et al. Versatile loading of diverse cargo into functional polymer capsules. Adv Sci. 2015; 2: 1400007. https://doi.org/10.1002/advs.201400007.
- Wang J, Chen J, Zong J, Zhao D, Li F, Zhuo R, Cheng Si. Calcium carbonate/carboxymethyl chitosan hybrid microspheres and nanospheres for drug delivery. J Phys Chem C. 2010; 114: 18940-18945.
- Zhao D, Zhuo R, Cheng S. Alginate modified nanostructured calcium carbonate with enhanced delivery efficiency for gene and drug delivery. Mol BioSystems. 2012; 8: 753-759.
- Bai J, Xu J, Zhao J, Zhang R. Hyaluronan and calcium carbonate hybrid nanoparticles for colorectal cancer chemotherapy. Mater Res Express. 2017; 4: 095401, https://doi.org/10.1088/2053-1591/aa822d.
- Sudareva N, Suvorova O, Saprykina N, Smirnova N, Bel'tiukov P, Petunov S, Radilov А, Vilesov A. Two-level delivery systems based on CaCO3 cores for oral administration of therapeutic peptides. J Microencapsulation 2018; 35: 619-634.
- Binevski PV, Balabushevich NG, Uvarova VI, Vikulina AS, Volodkin D. Bio-friendly encapsulation of superoxide dismutase into vaterite CaCO3 crystals. Enzyme activity, release mechanism, and perspectives for ophthalmology. Colloids and Surfaces B: Biointerfaces 2019; 181: 437-449.
- Cowan JC, Weintritt DJ. Water-formed scale deposits. Gulf Pub. Co., Book Division 1976; 596.
- Genina EA, Svenskaya YI, Yanina IY, Dolotov LE, Navolokin NA, Bashkatov AN, et al. In vivo optical monitoring of transcutaneous delivery of calcium carbonate microcontainers. Biomed. Opt. Express 2016; 7: 2082-2087.
- Sudareva NN, Popryadukhin PV, Saprykina NN, Suvorova OM, Yukina GY, Galibin OV, Vilesov AD. CaCO3 vaterites as components of target drug delivery systems. Cell Ther Transplant. 2020; 9(2): 13-19.
- Popryadukhin PV, Sudareva NN, Suvorova ОМ, Yukina GYu, Sukhorukova ЕG, Saprykina NN. Morphology of porous CaCO3 vaterites as components of target drug delivery systems in rat muscle tissue. Cytology. 2020; 62: 738-744.
- Sudareva NN, Suvorova ОM, Tarasenko II, Saprykina NN, Smirnova NV, Petunov SG, et al. Hybrid systems for oral delivery of therapeutic neuropeptide. Mendeleev Commun. 2020; 30: 25-27.
- Garrett RH, Grisham CM. Biochemistry. 6th Ed. Cengage Learning. 2017; 1218.
- Lyubomirsky NV, Bakhtin AS, Bakhtina TA, Nikolaenko EYu, Nikolaenko VV. The influence of calcium hydrogen carbonate on the structure formation and properties of materials based on lime carbonization hardening. Int Res J. 2016; 11: 86-93. https://doi.org/10:18454/IRJ.2016.53.146. (In Russian).
- Wray JL, Daniels F. Precipitation of calcite and aragonite. J. Am. Chem. Soc. 1957; 79: 2031-2034.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "30" ["~SORT"]=> string(2) "30" ["CODE"]=> string(100) "morfologiya-sistem-adresnoy-dostavki-lekarstvennykh-preparatov-vateritov-caco3-pokrytykh-sulfatom-de" ["~CODE"]=> string(100) "morfologiya-sistem-adresnoy-dostavki-lekarstvennykh-preparatov-vateritov-caco3-pokrytykh-sulfatom-de" ["EXTERNAL_ID"]=> string(4) "1945" ["~EXTERNAL_ID"]=> string(4) "1945" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(349) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крысMorphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(1940) "<p style="text-align: justify;">В настоящей работе изучено поведение пористых сферических ватеритов карбоната кальция (СаСО<sub>3</sub>) покрытых защитной оболочкой из сульфата декстрана – систем адресной доставки лекарственных препаратов – в мышечной ткани крыс на сроках 3 сут., 1, 2, 4 и 12 нед., после имплантации. Было показано, что с течением времени происходит структурная трансформация и биорезорбция изучаемых носителей. Через 3 сут. наблюдается преобразование сферических структур в игольчатые с последующей их биорезорбцией в течение 2 нед. При этом патологического воздействия на окружающие ткани выявлено не было, что подтверждает безопасность применения покрытых защитной оболочкой СаСО<sub>3</sub> ватеритов и позволяет рекомендовать их для проведения дальнейших исследований в качестве систем адресной доставки лекарственных препаратов.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Адресная доставка лекарственных препаратов, карбонат кальция, сульфат декстрана, биорезорбция, мышечная ткань, эксперимент <i>in vivo</i>.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_META_TITLE"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_META_KEYWORDS"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_META_DESCRIPTION"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_PICTURE_FILE_ALT"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_PICTURE_FILE_TITLE"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_PICTURE_FILE_NAME"]=> string(100) "morfologiya-sistem-adresnoy-dostavki-lekarstvennykh-preparatov-vateritov-caco3-pokrytykh-sulfatom-de" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(237) "Mорфология систем адресной доставки лекарственных препаратов (ватеритов CaCO3, покрытых сульфатом декстрана) в мышечной ткани крыс" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "morfologiya-sistem-adresnoy-dostavki-lekarstvennykh-preparatov-vateritov-caco3-pokrytykh-sulfatom-de" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "morfologiya-sistem-adresnoy-dostavki-lekarstvennykh-preparatov-vateritov-caco3-pokrytykh-sulfatom-de" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "morfologiya-sistem-adresnoy-dostavki-lekarstvennykh-preparatov-vateritov-caco3-pokrytykh-sulfatom-de" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "171" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27407" ["VALUE"]=> string(22) "11/05/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/05/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27408" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27409" ["VALUE"]=> array(2) { ["TEXT"]=> string(545) "<p>Павел В. Попрядухин<sup>1</sup>, Наталья Н. Сударева<sup>1,2</sup>, Ольга М. Суворова<sup>1</sup>, Галина Ю. Юкина<sup>2</sup>, Елена Г. Сухорукова<sup>2</sup>, Наталья Н. Сапрыкина<sup>1</sup>, Илья А. Барсук<sup>3</sup>, Олег В. Галибин<sup>2</sup>, Александр Д. Вилесов<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(425) "
Павел В. Попрядухин1, Наталья Н. Сударева1,2, Ольга М. Суворова1, Галина Ю. Юкина2, Елена Г. Сухорукова2, Наталья Н. Сапрыкина1, Илья А. Барсук3, Олег В. Галибин2, Александр Д. Вилесов1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27410" ["VALUE"]=> array(2) { ["TEXT"]=> string(581) "<p> <sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия<br> <sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Военно-медицинская академия им. С. М. Кирова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(521) "
1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Военно-медицинская академия им. С. М. Кирова, Санкт-Петербург, Россия
В настоящей работе изучено поведение пористых сферических ватеритов карбоната кальция (СаСО3) покрытых защитной оболочкой из сульфата декстрана – систем адресной доставки лекарственных препаратов – в мышечной ткани крыс на сроках 3 сут., 1, 2, 4 и 12 нед., после имплантации. Было показано, что с течением времени происходит структурная трансформация и биорезорбция изучаемых носителей. Через 3 сут. наблюдается преобразование сферических структур в игольчатые с последующей их биорезорбцией в течение 2 нед. При этом патологического воздействия на окружающие ткани выявлено не было, что подтверждает безопасность применения покрытых защитной оболочкой СаСО3 ватеритов и позволяет рекомендовать их для проведения дальнейших исследований в качестве систем адресной доставки лекарственных препаратов.
Ключевые слова
Адресная доставка лекарственных препаратов, карбонат кальция, сульфат декстрана, биорезорбция, мышечная ткань, эксперимент in vivo.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27411" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-78-84" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-78-84" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27416" ["VALUE"]=> array(2) { ["TEXT"]=> string(421) "<p>Pavel V. Popryadukhin<sup>1</sup>, Natalia N. Sudareva<sup>1,2</sup>, Оlga М. Suvorova<sup>1</sup>, Galina Yu. Yukina<sup>2</sup>, Еlena G. Sukhorukova<sup>2</sup>, Natalia N. Saprykina<sup>1</sup>, Ilya A. Barsuk<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(301) "Pavel V. Popryadukhin1, Natalia N. Sudareva1,2, Оlga М. Suvorova1, Galina Yu. Yukina2, Еlena G. Sukhorukova2, Natalia N. Saprykina1, Ilya A. Barsuk3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27417" ["VALUE"]=> array(2) { ["TEXT"]=> string(485) "<p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> <sup>2</sup> Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> S.M.Kirov Military Medical Academy, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia<br> E-mail: pavelpnru@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(383) "1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 S.M.Kirov Military Medical Academy, St. Petersburg, Russia
Correspondence
Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia
E-mail: pavelpnru@gmail.com
The present work is focused on the study of target drug delivery systems comprised of porous spherical calcium carbonate vaterites (СаСО3) covered with the dextran sulfate protective shell. Behavior of the objects was investigated in vivo. The samples were implanted into rat muscular tissue and removed after different periods of exposure (3 days, 1, 2, 4, and 12 weeks after operation). It was shown that certain transformations in structure of the implanted carriers occurred over time, after which they underwent bioresorption. In 3 days after implantation, spherical vaterites degraded, and needle-like calcium carbonate objects were formed; during the following two weeks, these objects were completely resorbed in living tissues. Since no pathogenic influence of the samples on the surrounding tissues was revealed, we believe that СаСО3 vaterites covered with protective shells are safe for potential medicinal applications and can be recommended for further studies as target drug delivery systems.
Keywords
Target drug delivery, calcium carbonate, dextran sulfate, bioresorption, muscular tissue, in vivo experiment.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27412" ["VALUE"]=> string(112) "Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(112) "Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27414" ["VALUE"]=> string(4) "2352" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2352" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27419" ["VALUE"]=> string(4) "2353" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2353" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27416" ["VALUE"]=> array(2) { ["TEXT"]=> string(421) "<p>Pavel V. Popryadukhin<sup>1</sup>, Natalia N. Sudareva<sup>1,2</sup>, Оlga М. Suvorova<sup>1</sup>, Galina Yu. Yukina<sup>2</sup>, Еlena G. Sukhorukova<sup>2</sup>, Natalia N. Saprykina<sup>1</sup>, Ilya A. Barsuk<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(301) "Pavel V. Popryadukhin1, Natalia N. Sudareva1,2, Оlga М. Suvorova1, Galina Yu. Yukina2, Еlena G. Sukhorukova2, Natalia N. Saprykina1, Ilya A. Barsuk3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(301) "Pavel V. Popryadukhin1, Natalia N. Sudareva1,2, Оlga М. Suvorova1, Galina Yu. Yukina2, Еlena G. Sukhorukova2, Natalia N. Saprykina1, Ilya A. Barsuk3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27418" ["VALUE"]=> array(2) { ["TEXT"]=> string(1359) "<p style="text-align: justify;">The present work is focused on the study of target drug delivery systems comprised of porous spherical calcium carbonate vaterites (СаСО<sub>3</sub>) covered with the dextran sulfate protective shell. Behavior of the objects was investigated <i>in vivo</i>. The samples were implanted into rat muscular tissue and removed after different periods of exposure (3 days, 1, 2, 4, and 12 weeks after operation). It was shown that certain transformations in structure of the implanted carriers occurred over time, after which they underwent bioresorption. In 3 days after implantation, spherical vaterites degraded, and needle-like calcium carbonate objects were formed; during the following two weeks, these objects were completely resorbed in living tissues. Since no pathogenic influence of the samples on the surrounding tissues was revealed, we believe that СаСО<sub>3</sub> vaterites covered with protective shells are safe for potential medicinal applications and can be recommended for further studies as target drug delivery systems.</p> <h2>Keywords</h2> <p style="text-align: justify;">Target drug delivery, calcium carbonate, dextran sulfate, bioresorption, muscular tissue, <i>in vivo</i> experiment.</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1255) "The present work is focused on the study of target drug delivery systems comprised of porous spherical calcium carbonate vaterites (СаСО3) covered with the dextran sulfate protective shell. Behavior of the objects was investigated in vivo. The samples were implanted into rat muscular tissue and removed after different periods of exposure (3 days, 1, 2, 4, and 12 weeks after operation). It was shown that certain transformations in structure of the implanted carriers occurred over time, after which they underwent bioresorption. In 3 days after implantation, spherical vaterites degraded, and needle-like calcium carbonate objects were formed; during the following two weeks, these objects were completely resorbed in living tissues. Since no pathogenic influence of the samples on the surrounding tissues was revealed, we believe that СаСО3 vaterites covered with protective shells are safe for potential medicinal applications and can be recommended for further studies as target drug delivery systems.
Keywords
Target drug delivery, calcium carbonate, dextran sulfate, bioresorption, muscular tissue, in vivo experiment.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1255) "The present work is focused on the study of target drug delivery systems comprised of porous spherical calcium carbonate vaterites (СаСО3) covered with the dextran sulfate protective shell. Behavior of the objects was investigated in vivo. The samples were implanted into rat muscular tissue and removed after different periods of exposure (3 days, 1, 2, 4, and 12 weeks after operation). It was shown that certain transformations in structure of the implanted carriers occurred over time, after which they underwent bioresorption. In 3 days after implantation, spherical vaterites degraded, and needle-like calcium carbonate objects were formed; during the following two weeks, these objects were completely resorbed in living tissues. Since no pathogenic influence of the samples on the surrounding tissues was revealed, we believe that СаСО3 vaterites covered with protective shells are safe for potential medicinal applications and can be recommended for further studies as target drug delivery systems.
Keywords
Target drug delivery, calcium carbonate, dextran sulfate, bioresorption, muscular tissue, in vivo experiment.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27411" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-78-84" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-78-84" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-78-84" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27412" ["VALUE"]=> string(112) "Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(112) "Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(112) "Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27417" ["VALUE"]=> array(2) { ["TEXT"]=> string(485) "<p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br> <sup>2</sup> Pavlov University, St. Petersburg, Russia<br> <sup>3</sup> S.M.Kirov Military Medical Academy, St. Petersburg, Russia</p><br> <p><b>Correspondence</b><br> Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia<br> E-mail: pavelpnru@gmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(383) "1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 S.M.Kirov Military Medical Academy, St. Petersburg, Russia
Correspondence
Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia
E-mail: pavelpnru@gmail.com
1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 S.M.Kirov Military Medical Academy, St. Petersburg, Russia
Correspondence
Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia
E-mail: pavelpnru@gmail.com
Павел В. Попрядухин1, Наталья Н. Сударева1,2, Ольга М. Суворова1, Галина Ю. Юкина2, Елена Г. Сухорукова2, Наталья Н. Сапрыкина1, Илья А. Барсук3, Олег В. Галибин2, Александр Д. Вилесов1,2
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(425) "Павел В. Попрядухин1, Наталья Н. Сударева1,2, Ольга М. Суворова1, Галина Ю. Юкина2, Елена Г. Сухорукова2, Наталья Н. Сапрыкина1, Илья А. Барсук3, Олег В. Галибин2, Александр Д. Вилесов1,2
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27407" ["VALUE"]=> string(22) "11/05/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/05/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/05/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27408" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/04/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27415" ["VALUE"]=> array(2) { ["TEXT"]=> string(1940) "<p style="text-align: justify;">В настоящей работе изучено поведение пористых сферических ватеритов карбоната кальция (СаСО<sub>3</sub>) покрытых защитной оболочкой из сульфата декстрана – систем адресной доставки лекарственных препаратов – в мышечной ткани крыс на сроках 3 сут., 1, 2, 4 и 12 нед., после имплантации. Было показано, что с течением времени происходит структурная трансформация и биорезорбция изучаемых носителей. Через 3 сут. наблюдается преобразование сферических структур в игольчатые с последующей их биорезорбцией в течение 2 нед. При этом патологического воздействия на окружающие ткани выявлено не было, что подтверждает безопасность применения покрытых защитной оболочкой СаСО<sub>3</sub> ватеритов и позволяет рекомендовать их для проведения дальнейших исследований в качестве систем адресной доставки лекарственных препаратов.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Адресная доставка лекарственных препаратов, карбонат кальция, сульфат декстрана, биорезорбция, мышечная ткань, эксперимент <i>in vivo</i>.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1848) "В настоящей работе изучено поведение пористых сферических ватеритов карбоната кальция (СаСО3) покрытых защитной оболочкой из сульфата декстрана – систем адресной доставки лекарственных препаратов – в мышечной ткани крыс на сроках 3 сут., 1, 2, 4 и 12 нед., после имплантации. Было показано, что с течением времени происходит структурная трансформация и биорезорбция изучаемых носителей. Через 3 сут. наблюдается преобразование сферических структур в игольчатые с последующей их биорезорбцией в течение 2 нед. При этом патологического воздействия на окружающие ткани выявлено не было, что подтверждает безопасность применения покрытых защитной оболочкой СаСО3 ватеритов и позволяет рекомендовать их для проведения дальнейших исследований в качестве систем адресной доставки лекарственных препаратов.
Ключевые слова
Адресная доставка лекарственных препаратов, карбонат кальция, сульфат декстрана, биорезорбция, мышечная ткань, эксперимент in vivo.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1848) "В настоящей работе изучено поведение пористых сферических ватеритов карбоната кальция (СаСО3) покрытых защитной оболочкой из сульфата декстрана – систем адресной доставки лекарственных препаратов – в мышечной ткани крыс на сроках 3 сут., 1, 2, 4 и 12 нед., после имплантации. Было показано, что с течением времени происходит структурная трансформация и биорезорбция изучаемых носителей. Через 3 сут. наблюдается преобразование сферических структур в игольчатые с последующей их биорезорбцией в течение 2 нед. При этом патологического воздействия на окружающие ткани выявлено не было, что подтверждает безопасность применения покрытых защитной оболочкой СаСО3 ватеритов и позволяет рекомендовать их для проведения дальнейших исследований в качестве систем адресной доставки лекарственных препаратов.
Ключевые слова
Адресная доставка лекарственных препаратов, карбонат кальция, сульфат декстрана, биорезорбция, мышечная ткань, эксперимент in vivo.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27410" ["VALUE"]=> array(2) { ["TEXT"]=> string(581) "<p> <sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия<br> <sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br> <sup>3</sup> Военно-медицинская академия им. С. М. Кирова, Санкт-Петербург, Россия</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(521) "
1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Военно-медицинская академия им. С. М. Кирова, Санкт-Петербург, Россия
1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Военно-медицинская академия им. С. М. Кирова, Санкт-Петербург, Россия
Introduction
Cardiovascular diseases are the leading cause of human morbidity worldwide. Systemic atherosclerosis is considered to be one of the most common, severe, and life-threatening conditions [1]. Despite a variety of pharmaceutical and surgical treatment approaches to this pathology, they frequently lack the desired effectiveness. Since the beginning of the 21st century, development of endovascular techniques has changed clinical indications and operative techniques in all the areas of vascular surgery. The classical bypass surgery, which required arterial substitutes, is now less used [2]. Hence, the goal of vascular research should be centered not on the search for an ideal arterial substitute, but on improving minimally invasive techniques such as cell therapy and regenerative medicine, aiming to develop new treatments of atherosclerosis in the near future.
Stem cell therapy is a novel and promising strategy which potentially is more effective than single-agent drug therapies for many diseases [3, 4]. Stem cells function in the repair of injured tissues in two ways: by secretion of related cytokines [5], or by differentiating into the cell types at the site of injury, in order to exert its original function [6].
Multipotent mesenchymal stem cells (MSCs) which possess self-renewal potential and can differentiate into various cell types, such as osteoblasts, chondrocytes or adipocytes [7], may be isolated from adult tissues, including bone marrow, adipose tissue, and birth-associated tissues, such as placenta, umbilical cord, cord blood or amnion. MSCs are identified by three characteristics: (1) adherence to the culture dishes; (2) differentiation into osteoblasts, chondroblasts and adipocytes, and (3) expression of specific surface markers (CD90, CD105, CD73 and CD44), as well as lacking expression of several other markers including CD34 and HLA-DR [8-10].
Wharton`s jelly-derived MSCs (WJ-MSCs) have a high proliferation rate; they do not show any teratogenic, or carcinogenic behavior in case of transplantation [11]. The bone marrow and adipose tissue, among others, are, generally, used as sources of MSCs [7, 12]. Recent findings have shown that MSCs from human umbilical cord have advantages such as large numbers on harvest, strong proliferation and differentiation capacity and low immunogenicity compared to MSCs from the bone marrow [13].
Porous scaffolds prepared from natural polysaccharides are promising matrices for mimicking the in vivo ECM (extracellular matrix), since they resemble glycosaminoglycans (GAGs), which are essential ECM components [14]. Hyaluronic acid (HA) is a natural anionic polymer found in synovial fluid, skin, and cartilage, being among the major GAG components of brain ECM [15]. HA is used for diverse biomedical applications, due to its biocompatibility and water binding capacity [16, 17]. Due to its remarkable hydrodynamic characteristics, particularly in terms of viscosity and ability to retain water, HA plays a significant role in assembly of extracellular and pericellular matrices by regulating their porosity and malleability [18].
The negative charge of HA hinders cell adhesion. Therefore, it is blended with other biomaterials to promote cell attachment [19]. Chitosan (CHI) is a widely used natural cationic polymer derived from crustacean shells that resembles GAGs, and has broad tissue engineering applications in view of its biocompatibility, biodegradability, hydrophilicity, low cost and availability [17]. The cationic nature of chitosan allows it to interact with negatively charged polymers and to form a polyelectrolyte complex (PEC) through ionic bonding [20].
Endothelial cells are one of the major components of the vessel wall, and these cells are important contributors to vascular tissue repair and regeneration [21]. The aim of present study was to explore the potential of WJ-MSCs seeded on HA/CHI multilayers to differentiate into endothelial-like cells, by identifying and evaluating endothelial cell morphology, and studying endothelial cell-specific gene expression at mRNA and protein levels.
Materials and methods
Polyelectrolytes multilayer films and collagen film
Hyaluronic acid solution (0.2 mg/mL in NaCl 0.15 M) and chitosan solution (0.2 mg/mL in NaCl 0.15 M/HCl 2mM) were used to produce the polyelectrolyte multilayers. Reagents were obtained from commercial sources and used without any further purification. Chitosan low-molecular weight and hyaluronan (200 kDa) were obtained from Sigma Aldrich (Germany). Each experiment was preceded by a cleaning step of the cover glasses as follows: 15 min with 1% sodium dodecyl sulfate (Sigma Aldrich, Germany) at 100°C, extensive ultrapure water rinse, 15 min at 100°C with 0.1 M HCl and, finally, cover glasses were thoroughly rinsed with ultrapure water. Coverslips were incubated in CHI solution for 5 min, thoroughly washed in NaCl (0.5 M) and then incubated in HA solution for 5 min. (CHIHA)10 films were built up after 20 alternate depositions of polycation and polynion layers. The type I collagen (100 μg/mL, purchased from BD Biosciences, France) was used as positive control for cellular adhesion. The collagen solution was added to the coverslips and incubated for 1 hour at room temperature. Then, the solution was carefully aspirated and the surface of glasses was rinsed 3 times with serum-free α-MEM.
Stem cell and mature endothelial cell isolation and culture
Fresh human umbilical cords were obtained after full-term births with informed consent using the guidelines approved by the Hanan Hospital. Umbilical cord vessels were removed manually from cord segments, and the exposed Wharton’s jelly was cut into very small pieces or explants. These explants were cultured in α-MEM (Lonza, Belgium) supplemented with 10% decomplemented fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/mL Penicillin/streptomycin and 2.5 mg/mL Fungizon® (Fisher, France) at 37°C and in 5% CO2. At the fourth passage, WJ-MSCs were characterized by flow cytometry (FACSCalibur; BD Bioscience), as previously described [22], by assessing the expression of CD73, CD90, CD44, CD105, CD34, CD45 and HLA-DR, and then used in our experimental procedure.
Human umbilical vein endothelial cells (HUVECs) were isolated according to the method of Jaffe et al. [23]. Briefly, the umbilical cords were washed in HBSS solution and HUVECs were extracted from umbilical cords veins using Trypsin. Then HUVECs were cultured at 37°C in 5% CO2 in 25 cm2 tissue-culture-treated flask (suitable for cell attachment and growth) in complete medium. The medium consisted of an equal mixture of M199/RPMI 1640 media supplemented with 20% human serum albumin, 2 mM L-glutamine, 20 mM HEPES, 100 IU/mL Penicillin, 2.5 mg/mL Fungizon®, being replaced every two days. The cells were used at the second passage culture and were seeded at 3×103 cells/cm2.
Endothelial cell differentiation
WJ-MSCs were seeded in 6-well plates at 3000 cells/cm2 on CHI/HA or on COL-I coated glass substrates in α-MEM for 2 days. The unstimulated cells were then incubated in the complete Endothelial Basal Medium (EBM-2, Lonza®), without growth factor supplements, whereas the stimulated were are incubated in complete Endothelial Growth Medium (EGM2, Lonza® supplemented with EPCs-differentiating medium) for 2 weeks.
endothelial basal medium (EBM-2, Lonza®) supplemented with 0.5% FBS. The culture medium was changed every 2 days. The cells cultured on both surfaces (glass and PEMs architectures) were observed daily by phase contrast microscopy (Leica) to check their morphology.
Evaluation of endothelium-specific mRNA markers
Transcript levels of CD31 (PECAM-1 platelet endothelial cell adhesion molecule), CDH5 (Vascular endothelial Cadherin) and KDR (VEGFR-2 vascular endothelial growth factor 2) genes were quantified by real-time qPCR. Total RNA were isolated with RNeasy mini kit (Qiagen, GmbH, Hilden, Germany) according to the manufacturer’s instructions. Complementary DNA synthesis was performed with 350 ng total RNA using iScript cDNA synthesis kit (Bio-Rad, USA). Real-time qPCR was conducted as described previously [24]. As a positive control, RNA isolated from human umbilical vein endothelial cells (HUVECs) was used. These cells were isolated from the umbilical cord veins and cultured in M199/RPMI medium.
Measurement of gene expression was performed in duplicate; a non-template blank served as a negative control. qPCR was carried out using iQ SYBR® Green Supermix (Bio-Rad®) and in-home designed primers (using Primer3) for human CD-31, VE-cadherin, VEGF-R2 and ribosomal protein. Forward and reverse primers (Eurogenetec) were as follows:
CD31: 5’-ATGATGCCCAGTTTGAGGTC-3’; 5’-ACGTCTTCAGTGGGGTTGTC-3’,
KDR: 5’-GTGACCAACATGGAGTCGTG-3’; 5’-TGCTTCACAGAAGACCATGC-3’;
CDH5: 5’-CCTACCAGCCCAAAGTGTGT-3’; 5’-GACTTGGCATCCCATTGTCT-3’;
RPS29: 5’-TCATCTTCCAGCCCAAATTC-3’; 5’-CTTGAACGGTTACCACCTCA-3’
PCR was performed with MyCycler™ Personal Thermal Cycler (Bio-Rad®). Cycling parameters were 3 min at 95°C; 40 cycles of 3 min at 60°C for CD31, VEGF-R2 and RP29 and 62°C for VE-cadherin and 1 min at 72°C. The results were normalized to the housekeeping gene for S29 ribosomal protein. Analyses and fold differences were determined using the comparative CT method. The fold changes were calculated from the ΔΔCT values using the formula 2-ΔΔCT, and the data were normalized relative to the reference gene values and then expressed as percentage of values obtained in HUVEC for each assayed mRNA.
Detection of endothelium-specific protein markers
Total proteins from cultured cells were prepared as previously described [26]. 25 μg proteins from each sample were heated at 95°C for 5 min in Laemmli sample buffer (BioRad, USA), and the total proteins were separated in acrylamide gel (10% for VEGF-R2, Vascular endothelial growth factor receptor 2, and 7% for CD31 and VE-cadherin. After electrophoresis, the gels were blotted to nitrocellulose membranes. GAPDH was used as loading control. Western blots were performed by using primary antibodies for endothelial VEGFR2 markers with 1/1000 milk/TBST (Tris-Buffered Saline Tween 20 0.5% from Cell Signaling Technology, UK); VE-cadherin with 1/1000 BSA (Bovine Serum Albumin/TBST from Abcam, USA); CD31 with 1/1000 BSA/TBST (Dako, France). The membranes were blocked with the blocking buffer TBS (Tris Buffer Saline) for 1h at room temperature and incubated with primary antibodies under gentle shaking at 4°C overnight. After extensive washing by TBS, the membranes were incubated for 1 h at room temperature with secondary antibodies conjugated to horseradish peroxidase (HRP). HRP activity was detected by enhanced chemiluminescence (ECL, Santa Cruz Biotechnology, USA). Santa Cruz Luminol Reagent A & B associated, will be oxidized by HRP in presence of hydrogen peroxide emitting the light. Densitometry of the obtained bands was estimated by ImageJ software.
Evaluation of endothelial-like cells functionality in terms of LDL-uptaking assay
Low-density lipoprotein (LDL)-uptake assay was performed as described previously. At day 15, WJ-MSCs seeded on collagen and PEMs architectures were incubated for 4 h at 37°C in RPMI 1640 without phenol red supplemented with 0.8 μg/mL Dil-Ac-LDL (Tebu-bio, France) labeled with rhodamine. Cells were washed with RPMI 1640 without phenol red to remove Dil-Ac-LDL. They were then fixed with 4% paraformaldehyde and nuclei were counterstained using 4',6-diamidino-2-phenylindole DAPI. The cells were observed using fluorescence microscopy (Leica microscope, *40) after using the appropriate excitation and emission filters for Rhodamin B (554nmEx/571nmEm).
Von Willebrand Factor (vWF) immunostaining
After 15 days of endothelial differentiation, the WJ-MSCs seeded on collagen and PEMs architectures were analyzed to assess vWF expression. The cells were fixed by 4% paraformaldehyde, permeabilized with PBS/Triton X-100 (0.1%) for 15 min, blocked with 1% BSA and stained by murine anti-vWF (1/100 Dako, France). After two washes with PBS, the appropriate secondary antibody labeled with Alexa-Fluor-488 (diluted at 1/100) was incubated for 30 min at 37°C. The cells were then observed by fluorescence microscopy (Zeiss microscopy, × 630 magnification) using the (485Ex/538Em) spectral line.
Statistical analysis
Data were presented as a mean ± SEM for each condition. Each experiment was repeated independently three times (n=3). Pairwise comparisons were performed using one-factor ANOVA with Fisher correction (Stat view IVs, Abacus Concepts Inc., Berkley, CA). Differences were considered significant for p (rejection level of the null-hypothesis of equal means) values < 0.05.
Results and discussion
Characterization of WJ-MSCs
Morphological characterization of MSCs (4th passage) was performed according to the criteria defined by the International Society for Cellular Therapy [25]. MCSs derived from WJ of three umbilical cords displayed a homogeneous fibroblast-like morphology. Cells were analyzed regarding the expression of specific molecular markers by Fluorescein-Activated Cell Sorting analysis and showed that WJMSCs and BM-MSCs were positive for CD105, CD73, and CD90, and negative for CD45, CD34, CD86, and HLA-DR. These data revealed that WJ-MSCs used in this study showed the typical MSC characteristics (Data not shown).
Evaluation of endothelial markers expression at the mRNA and protein levels
n order to evaluate the effect of different adhesion matrices on differentiation of WJ-MSCs in endothelial-like cells, we measured the expression of endothelial markers by q-PCR and Western Blot. The three endothelial-specific molecules CD31, VE-cadherin and VEGF-R2 (KDR) are known to play an important role in the endothelium maturation during angiogenesis process [26]. CD31, also known as platelet endothelial cell adhesion molecule-1 (PECAM-1), is a type I integral membrane glycoprotein that is expressed at high levels on early and mature endothelial cells, platelets, and most leukocyte subpopulations. PECAM-1 is known to have various roles in vascular biology including angiogenesis, platelet function, and thrombosis [27]. VEGF-R2 is expressed on vascular endothelial cells and lymphatic endothelial cells; it regulates vascular endothelial function. VEGF is an important growth factor for the endothelial differentiation [28]. The endothelial-specific cadherin, vascular endothelial cadherin (VE-cadherin) is required for vascular genesis and the repair of damaged vessels [29].

Figure 1. Investigation of endothelial cell markers at the mRNA level
Expression of the endothelial markers: CD31, CDH5 and KDR at the mRNA level was assessed in HUVECs endothelial-like cells seeded on collagen and CHI/HA in differentiation medium (EGM2) for 15 days. Results show the mRNA level normalized to the reference gene mRNA RP29 level and expressed relative to the mRNA level in HUVECs (set as 100) and non-treated cells (data not shown). Results represent the average of 3 independent experiments ± SEM.

Figure 2. Studies of endothelial markers at the protein level
CD31, VEcadherin and VEGF-R2 blot quantifying for stimulated and unstimulated WJMSCs seeded on different culture surfaces after 15 days. Western blot normalization was performed to the expression of GAPDH. The expression in HUVECs was assumed for 100% (relative protein level=1.0). Results were expressed as the mean of 3 independent experiments ± SEM. ***: p< 0,001.
The mRNA and protein levels of the three endothelial specific markers CD31, VEGF-R2 (KDR) and VE-cadherin were analyzed by real-time qPCR and Western blot (Figures 1 and 2). Relative expression of the three molecules was analyzed at mRNA and protein levels, and expressed relative to appropriate HUVEC values. WJ-MSCs seeded on CHI/HA or collagen and incubated in EBM-2 did not express the three markers at the protein level whereas their expression at the mRNA level was barely detectable (<1% of the levels found in HUVECs for CD31 and CDH5). In WJ-MSCs incubated in EGM-2 for 15 days, mRNA level was higher on CHI/HA than on collagen for CD31 and KDR (increase by 67%, and 79%, respectively). However, only the KDR increase was statistically significant. At the protein level, KDR expression was higher on CHI/HA relative to collagen (45% increase), but this difference was not statistically significant. CD31 protein levels were unchanged between collagen and CHI/HA, whereas CDH5 level was higher on CHI/HA relative to collagen (4% increase), and the difference was statistically significant. The fold change between collagen and CHI/HA at mRNA level was more important than fold change at protein level. After 15 days of differentiation, the transcription of endothelial genes give rise to high levels of mRNA from these genes. The translation process might take more culture time, to produce similar levels of proteins.
Higher mRNA and protein expression of these three markers in differentiated WJ-MSCs seeded on CHI/HA could contribute to more pronounced endothelial differentiation as compared with differentiated WJ-MSCs seeded on collagen. However, collagen is a recommended surface, allowing MSCs chondrogenic and osteogenic differentiation after 21 days [30]. In our report, we have not detected expression of VEGF-R2 protein on the collagen surface; maybe we needed more than 15 days to notice the translation of VEGF-R2 gene.
The capacity to differentiate towards endothelial phenotype is a characteristic of mesenchymal stem cells [30-32], and our results showed that WJ-MSCs express endothelial markers at mRNA and protein levels after 15-day cultures in presence of endothelial growth factors. However, the main purpose of this study was to evaluate endotheliogenic potential of WJ-MSCs seeded onto CHI/HA scaffolds. First, we verified the endothelial potential of WJ-MSCs in monolayer culture conditions both in proliferation (data not shown) and differentiation media. In proliferation medium, no endothelial differentiation was observed during the entire experimental time (15 days), further confirming the stem-cell origin of isolated cells. In differentiation medium, RT-PCR and Western Blot confirmed a better endothelial potential of these cells on CHI/HA.
Our choice of the CHI/HA scaffold was based on some studies that demonstrated the efficacy of this natural scaffold in enhancing hMSCs differentiation into stromal cells. hMSCs were induced to differentiate to chondrogenic, osteogenic, and adipogenic phenotypes [33]. Recent studies have shown the potential of WJ-MSCs to differentiate towards cardiomyocytes using CHI/HA scaffolds [34]. In our study, we have demonstrated that WJ-MSCs are able to differentiate into endothelial cells on CHI/HA films. Therefore, we can deduce that a combination of these cells with this natural scaffold is advantageous for cardio-vascular tissue engineering. Same conclusive results were observed in healing of diabetic skin wound by the proliferation and differentiation of human umbilical cords mesenchymal stem cells (hUCMSCs) on the collagen/chitosan laser drilling acellular dermal matrix (CCLDADM) scaffold, a natural scaffold used in vivo [35].
Evaluation of endothelial-like cells functionality by LDL uptake assay
LDL-uptake assay is applied for detection of functional endothelial cells. WJMSCs, when seeded on CHI/HA and collagen, captured DiIAcLDL to the cytoplasm after 4 hour-incubation in RPMI medium supplemented with DiI-Ac-LDL. However, MSCs were unable to uptake DiI-Ac-LDL after culturing in the growth medium as negative controls (Fig. 3). These results confirm that, after two weeks, WJ-MSCs seeded on CHI/HA and collagen exhibit endothelial cell phenotype. In this respect, Gaffney et al. investigated lipoprotein uptake by means of flow cytometry and showed that the cells in a G2/M (mitosis) phase incorporated about 45% more Dil-Ac-LDL than those in a G1/S (latency) phase [36]. Higher Dil-Ac-LDL uptake of endothelium-like cells on PEMs suggests that more cells are in the G2/M phase on PEMs, a feature of higher proliferation.

Figure 3. LDL-Uptake assay
WJ-MSCs cellular uptake of labeled acetylated LDLs. Double immunofluorescence of nuclei (blue) and for DI-Ac-LDLs (red) for unstimulated (a, b) and stimulated (c, d) WJMSCs after 15 days of culture on collagen and CHI/HA. Internalized labels were detected by Leica fluorescence microscopy (objective *40 oil).
Detection of endothelial-specific marker expression: vWF (von Willebrand Factor) by immunocytochemistry
Von Willebrand adherence factor (vWF) protein contributes to platelet function by mediating the initiation and progression of thrombus formation at the sites of vascular injury. Moreover, novel findings have been obtained on the link between regulation of VWF multimer size and microvascular thrombosis. This progress in basic research has provided critical information to define with greater precision the role of vWF in vascular biology and pathology, including its possible involvement in the onset of atherosclerosis and its acute thrombotic complications. Therefore we have used the expression of vWF as a functionality test of our endothelial-like cells [37]. The cells were examined for expression of endothelial-specific marker (vWF) by immunocytochemistry.
WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to this marker after endothelial differentiation for 15 days. MSCs did not show any positive signal after they were cultured in the growth medium (EBM2) on the same scaffold, as negative controls. On collagen layer, the cells were not marked, that was predicted because they needed more culture time to express vWF protein (Fig. 4).

Figure 4. vWF immunostaining
Double immunofluorescence staining for nuclei (blue), and for prothrombogenic von Willebrand Factor for stimulated (a, b) and unstimulated (d, e) WJ-MSCs after 15 days of culture on collagen and CHI/HA (Zeiss Microscope, objective*63 oil). N=3.
These promising results showed the possibility to combine the use of WJ-MSCs and CHI/HA films aiming for vascular tissue engineering, by evaluating the capacity of WJ-MSCs to differentiate into smooth muscle cells on CHI/HA, coating the surface of alginate hydrogels with CHI/HA films, and enrolling them in order to form a tubular vascular graft.
These promising data showed that the combination of WJ-MSCs and CHI/HA may lead to brilliant results, regarding endothelial differentiation and angiogenesis. One may recommend using of these cells and materials for vascular tissue engineering and regeneration therapy. More studies can be done to evaluate the capacity of WJ-MSCs to differentiate into smooth muscle cells on CHI/HA, to coat the surface of alginate hydrogels with CHI/HA films and to enroll them, in order to form a tubular vascular graft.
Conclusion
These first quite encouraging results showed that it is possible to obtain CEs-like in a non-traumatic way (from Wharton's jelly of human umbilical cords) and in a short time (15 days). Our technique, based on the use of polyelectrolyte films, could therefore be used in the field of vascular engineering for the development of functional vascular substitutes comprising an endothelium resulting from differentiation of mesenchymal stem cells, which would limit the risks of graft rejection and could be applied to patients who require vessel replacement.
Acknowledgements
The authors would like to thank Al Hanan Hospital for providing the umbilical cords used in our researches and Azm & Saadeh society for funding this work.
The authors have no conflicts of interest to declare.
Ethical Statement: The research work was approved by the ethical committee of the Lebanese University, Centre Azm for research in Applied Biotechnology and the ethical Committee of Al Hanan Hospital-Tripoli, Lebanon.
References
- Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J. 2004;25(14):1197-1207.
- Kumar VA, Brewster LP, Caves JM, Chaikof EL. Tissue engineering of blood vessels: functional requirements, progress, and future challenges. Cardiovasc Eng Technol. 2011;2(3):137-148.
- Erler P, Sweeney A, Monaghan JR. Regulation of Injury-Induced Ovarian Regeneration by Activation of Oogonial Stem Cells. Stem Cells. 2017;35(1):236-247.
- Sherman SE, Kuljanin M, Cooper TT, Putman DM, Lajoie GA, Hess DA. High aldehyde dehydrogenase activity identifies a subset of human mesenchymal stromal cells with vascular regenerative potential. Stem Cells. 2017;35(6):1542-1553.
- Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851-858.
- Xu Y, Huang S, Ma K, Fu X, Han W, Sheng Z. Promising new potential for mesenchymal stem cells derived from human umbilical cord Wharton’s jelly: sweat gland cell-like differentiative capacity. J Tissue Eng Regen Med. 2012;6(8):645-654.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
- Fong C-Y, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod Biomed Online. 2007;15(6):708-718.
- Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346(1):53.
- Troyer DL, Weiss ML. Concise review: Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591-599.
- Abediankenari S, Ghasemi M. Generation of immune inhibitory dendritic cells CD4+T regulatory cells inducing by TGF-β. Iran J Allergy Asthma Immunol. 2009;8(1):25-30.
- Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195.
- Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992;6(9):2639-2645.
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528-539.
- Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym Int. 2008;57(3):397-430.
- Muzzarelli RA. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr Polym. 2011;83(4):1433-1445.
- Schanté CE, Zuber G, Herlin C, Vandamme TF. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr Polym. 2011;85(3):469-489.
- Wang X, He J, Wang Y, Cui F-Z. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus. 2012;2(3):278-291.
- Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):35-52.
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343-353.
- Dennaoui H, Chouery E, Rammal H, Abdel-Razzak Z, Harmouch C. Chitosan/hyaluronic acid multilayer films are biocompatible substrate for Wharton’s jelly derived stem cells. Stem Cell Investig. 2018;5.
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973; 52(11):2745-56.
- Rammal H, Harmouch C, Maerten C, Gaucher C, Boulmedais F, Schaaf P, et al. Upregulation of endothelial gene markers in Wharton’s jelly mesenchymal stem cells cultured on polyelectrolyte multilayers. J Biomed Mater Res A. 2017;105(1):292-300.
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393-395.
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581-611.
- Woodfin A, Voisin M-B, Nourshargh S. PECAM-1: a multifunctional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27(12):2514-2523.
- Li L, Liu H, Xu C, Deng M, Song M, Yu X, et al. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res Ther. 2017; 8(1):237.
- Gory-Fauré S, Prandini M-H, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, et al. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126(10):2093-2102.
- Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Oliver DA, Quinn CO, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7(11):581-588.
- Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Begyui R, et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7(3):282-291.
- Seo MJ. Suh SY, Bae YG, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328(1):258-264.
- Lindborg BA, Brekke JH, Scott CM, Chai YW, Ulrich C, Sandquist L, et al. A chitosan-hyaluronan-based hydrogel-hydrocolloid supports in vitro culture and differentiation of human mesenchymal stem/stromal cells. Tissue Eng Part A. 2015;21(11-12):1952-62.
- Rabbani S, Soleimani M, Imani M, Sahebjam M, Ghiaseddin A, Nassiri SM, et al. Regenerating Heart Using a Novel Compound and Human Wharton Jelly Mesenchymal Stem Cells. Arch Med Res. 2017;48(3):228-37.
- Han Y, Sun T, Han Y, Lin L, Liu C, Liu J, et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. Eur J Med Res. 2019;24(1):10.
- Gaffney J, West D, Arnold F, Sattar A, Kumar S. Differences in the uptake of modified low density lipoproteins by tissue cultured endothelial cells. J Cell Sci. 1985;79(1):317-325.
- Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1(7):1335-1342.
" ["~DETAIL_TEXT"]=> string(33999) "
Introduction
Cardiovascular diseases are the leading cause of human morbidity worldwide. Systemic atherosclerosis is considered to be one of the most common, severe, and life-threatening conditions [1]. Despite a variety of pharmaceutical and surgical treatment approaches to this pathology, they frequently lack the desired effectiveness. Since the beginning of the 21st century, development of endovascular techniques has changed clinical indications and operative techniques in all the areas of vascular surgery. The classical bypass surgery, which required arterial substitutes, is now less used [2]. Hence, the goal of vascular research should be centered not on the search for an ideal arterial substitute, but on improving minimally invasive techniques such as cell therapy and regenerative medicine, aiming to develop new treatments of atherosclerosis in the near future.
Stem cell therapy is a novel and promising strategy which potentially is more effective than single-agent drug therapies for many diseases [3, 4]. Stem cells function in the repair of injured tissues in two ways: by secretion of related cytokines [5], or by differentiating into the cell types at the site of injury, in order to exert its original function [6].
Multipotent mesenchymal stem cells (MSCs) which possess self-renewal potential and can differentiate into various cell types, such as osteoblasts, chondrocytes or adipocytes [7], may be isolated from adult tissues, including bone marrow, adipose tissue, and birth-associated tissues, such as placenta, umbilical cord, cord blood or amnion. MSCs are identified by three characteristics: (1) adherence to the culture dishes; (2) differentiation into osteoblasts, chondroblasts and adipocytes, and (3) expression of specific surface markers (CD90, CD105, CD73 and CD44), as well as lacking expression of several other markers including CD34 and HLA-DR [8-10].
Wharton`s jelly-derived MSCs (WJ-MSCs) have a high proliferation rate; they do not show any teratogenic, or carcinogenic behavior in case of transplantation [11]. The bone marrow and adipose tissue, among others, are, generally, used as sources of MSCs [7, 12]. Recent findings have shown that MSCs from human umbilical cord have advantages such as large numbers on harvest, strong proliferation and differentiation capacity and low immunogenicity compared to MSCs from the bone marrow [13].
Porous scaffolds prepared from natural polysaccharides are promising matrices for mimicking the in vivo ECM (extracellular matrix), since they resemble glycosaminoglycans (GAGs), which are essential ECM components [14]. Hyaluronic acid (HA) is a natural anionic polymer found in synovial fluid, skin, and cartilage, being among the major GAG components of brain ECM [15]. HA is used for diverse biomedical applications, due to its biocompatibility and water binding capacity [16, 17]. Due to its remarkable hydrodynamic characteristics, particularly in terms of viscosity and ability to retain water, HA plays a significant role in assembly of extracellular and pericellular matrices by regulating their porosity and malleability [18].
The negative charge of HA hinders cell adhesion. Therefore, it is blended with other biomaterials to promote cell attachment [19]. Chitosan (CHI) is a widely used natural cationic polymer derived from crustacean shells that resembles GAGs, and has broad tissue engineering applications in view of its biocompatibility, biodegradability, hydrophilicity, low cost and availability [17]. The cationic nature of chitosan allows it to interact with negatively charged polymers and to form a polyelectrolyte complex (PEC) through ionic bonding [20].
Endothelial cells are one of the major components of the vessel wall, and these cells are important contributors to vascular tissue repair and regeneration [21]. The aim of present study was to explore the potential of WJ-MSCs seeded on HA/CHI multilayers to differentiate into endothelial-like cells, by identifying and evaluating endothelial cell morphology, and studying endothelial cell-specific gene expression at mRNA and protein levels.
Materials and methods
Polyelectrolytes multilayer films and collagen film
Hyaluronic acid solution (0.2 mg/mL in NaCl 0.15 M) and chitosan solution (0.2 mg/mL in NaCl 0.15 M/HCl 2mM) were used to produce the polyelectrolyte multilayers. Reagents were obtained from commercial sources and used without any further purification. Chitosan low-molecular weight and hyaluronan (200 kDa) were obtained from Sigma Aldrich (Germany). Each experiment was preceded by a cleaning step of the cover glasses as follows: 15 min with 1% sodium dodecyl sulfate (Sigma Aldrich, Germany) at 100°C, extensive ultrapure water rinse, 15 min at 100°C with 0.1 M HCl and, finally, cover glasses were thoroughly rinsed with ultrapure water. Coverslips were incubated in CHI solution for 5 min, thoroughly washed in NaCl (0.5 M) and then incubated in HA solution for 5 min. (CHIHA)10 films were built up after 20 alternate depositions of polycation and polynion layers. The type I collagen (100 μg/mL, purchased from BD Biosciences, France) was used as positive control for cellular adhesion. The collagen solution was added to the coverslips and incubated for 1 hour at room temperature. Then, the solution was carefully aspirated and the surface of glasses was rinsed 3 times with serum-free α-MEM.
Stem cell and mature endothelial cell isolation and culture
Fresh human umbilical cords were obtained after full-term births with informed consent using the guidelines approved by the Hanan Hospital. Umbilical cord vessels were removed manually from cord segments, and the exposed Wharton’s jelly was cut into very small pieces or explants. These explants were cultured in α-MEM (Lonza, Belgium) supplemented with 10% decomplemented fetal bovine serum (FBS), 2 mM L-glutamine, 100 IU/mL Penicillin/streptomycin and 2.5 mg/mL Fungizon® (Fisher, France) at 37°C and in 5% CO2. At the fourth passage, WJ-MSCs were characterized by flow cytometry (FACSCalibur; BD Bioscience), as previously described [22], by assessing the expression of CD73, CD90, CD44, CD105, CD34, CD45 and HLA-DR, and then used in our experimental procedure.
Human umbilical vein endothelial cells (HUVECs) were isolated according to the method of Jaffe et al. [23]. Briefly, the umbilical cords were washed in HBSS solution and HUVECs were extracted from umbilical cords veins using Trypsin. Then HUVECs were cultured at 37°C in 5% CO2 in 25 cm2 tissue-culture-treated flask (suitable for cell attachment and growth) in complete medium. The medium consisted of an equal mixture of M199/RPMI 1640 media supplemented with 20% human serum albumin, 2 mM L-glutamine, 20 mM HEPES, 100 IU/mL Penicillin, 2.5 mg/mL Fungizon®, being replaced every two days. The cells were used at the second passage culture and were seeded at 3×103 cells/cm2.
Endothelial cell differentiation
WJ-MSCs were seeded in 6-well plates at 3000 cells/cm2 on CHI/HA or on COL-I coated glass substrates in α-MEM for 2 days. The unstimulated cells were then incubated in the complete Endothelial Basal Medium (EBM-2, Lonza®), without growth factor supplements, whereas the stimulated were are incubated in complete Endothelial Growth Medium (EGM2, Lonza® supplemented with EPCs-differentiating medium) for 2 weeks.
endothelial basal medium (EBM-2, Lonza®) supplemented with 0.5% FBS. The culture medium was changed every 2 days. The cells cultured on both surfaces (glass and PEMs architectures) were observed daily by phase contrast microscopy (Leica) to check their morphology.
Evaluation of endothelium-specific mRNA markers
Transcript levels of CD31 (PECAM-1 platelet endothelial cell adhesion molecule), CDH5 (Vascular endothelial Cadherin) and KDR (VEGFR-2 vascular endothelial growth factor 2) genes were quantified by real-time qPCR. Total RNA were isolated with RNeasy mini kit (Qiagen, GmbH, Hilden, Germany) according to the manufacturer’s instructions. Complementary DNA synthesis was performed with 350 ng total RNA using iScript cDNA synthesis kit (Bio-Rad, USA). Real-time qPCR was conducted as described previously [24]. As a positive control, RNA isolated from human umbilical vein endothelial cells (HUVECs) was used. These cells were isolated from the umbilical cord veins and cultured in M199/RPMI medium.
Measurement of gene expression was performed in duplicate; a non-template blank served as a negative control. qPCR was carried out using iQ SYBR® Green Supermix (Bio-Rad®) and in-home designed primers (using Primer3) for human CD-31, VE-cadherin, VEGF-R2 and ribosomal protein. Forward and reverse primers (Eurogenetec) were as follows:
CD31: 5’-ATGATGCCCAGTTTGAGGTC-3’; 5’-ACGTCTTCAGTGGGGTTGTC-3’,
KDR: 5’-GTGACCAACATGGAGTCGTG-3’; 5’-TGCTTCACAGAAGACCATGC-3’;
CDH5: 5’-CCTACCAGCCCAAAGTGTGT-3’; 5’-GACTTGGCATCCCATTGTCT-3’;
RPS29: 5’-TCATCTTCCAGCCCAAATTC-3’; 5’-CTTGAACGGTTACCACCTCA-3’
PCR was performed with MyCycler™ Personal Thermal Cycler (Bio-Rad®). Cycling parameters were 3 min at 95°C; 40 cycles of 3 min at 60°C for CD31, VEGF-R2 and RP29 and 62°C for VE-cadherin and 1 min at 72°C. The results were normalized to the housekeeping gene for S29 ribosomal protein. Analyses and fold differences were determined using the comparative CT method. The fold changes were calculated from the ΔΔCT values using the formula 2-ΔΔCT, and the data were normalized relative to the reference gene values and then expressed as percentage of values obtained in HUVEC for each assayed mRNA.
Detection of endothelium-specific protein markers
Total proteins from cultured cells were prepared as previously described [26]. 25 μg proteins from each sample were heated at 95°C for 5 min in Laemmli sample buffer (BioRad, USA), and the total proteins were separated in acrylamide gel (10% for VEGF-R2, Vascular endothelial growth factor receptor 2, and 7% for CD31 and VE-cadherin. After electrophoresis, the gels were blotted to nitrocellulose membranes. GAPDH was used as loading control. Western blots were performed by using primary antibodies for endothelial VEGFR2 markers with 1/1000 milk/TBST (Tris-Buffered Saline Tween 20 0.5% from Cell Signaling Technology, UK); VE-cadherin with 1/1000 BSA (Bovine Serum Albumin/TBST from Abcam, USA); CD31 with 1/1000 BSA/TBST (Dako, France). The membranes were blocked with the blocking buffer TBS (Tris Buffer Saline) for 1h at room temperature and incubated with primary antibodies under gentle shaking at 4°C overnight. After extensive washing by TBS, the membranes were incubated for 1 h at room temperature with secondary antibodies conjugated to horseradish peroxidase (HRP). HRP activity was detected by enhanced chemiluminescence (ECL, Santa Cruz Biotechnology, USA). Santa Cruz Luminol Reagent A & B associated, will be oxidized by HRP in presence of hydrogen peroxide emitting the light. Densitometry of the obtained bands was estimated by ImageJ software.
Evaluation of endothelial-like cells functionality in terms of LDL-uptaking assay
Low-density lipoprotein (LDL)-uptake assay was performed as described previously. At day 15, WJ-MSCs seeded on collagen and PEMs architectures were incubated for 4 h at 37°C in RPMI 1640 without phenol red supplemented with 0.8 μg/mL Dil-Ac-LDL (Tebu-bio, France) labeled with rhodamine. Cells were washed with RPMI 1640 without phenol red to remove Dil-Ac-LDL. They were then fixed with 4% paraformaldehyde and nuclei were counterstained using 4',6-diamidino-2-phenylindole DAPI. The cells were observed using fluorescence microscopy (Leica microscope, *40) after using the appropriate excitation and emission filters for Rhodamin B (554nmEx/571nmEm).
Von Willebrand Factor (vWF) immunostaining
After 15 days of endothelial differentiation, the WJ-MSCs seeded on collagen and PEMs architectures were analyzed to assess vWF expression. The cells were fixed by 4% paraformaldehyde, permeabilized with PBS/Triton X-100 (0.1%) for 15 min, blocked with 1% BSA and stained by murine anti-vWF (1/100 Dako, France). After two washes with PBS, the appropriate secondary antibody labeled with Alexa-Fluor-488 (diluted at 1/100) was incubated for 30 min at 37°C. The cells were then observed by fluorescence microscopy (Zeiss microscopy, × 630 magnification) using the (485Ex/538Em) spectral line.
Statistical analysis
Data were presented as a mean ± SEM for each condition. Each experiment was repeated independently three times (n=3). Pairwise comparisons were performed using one-factor ANOVA with Fisher correction (Stat view IVs, Abacus Concepts Inc., Berkley, CA). Differences were considered significant for p (rejection level of the null-hypothesis of equal means) values < 0.05.
Results and discussion
Characterization of WJ-MSCs
Morphological characterization of MSCs (4th passage) was performed according to the criteria defined by the International Society for Cellular Therapy [25]. MCSs derived from WJ of three umbilical cords displayed a homogeneous fibroblast-like morphology. Cells were analyzed regarding the expression of specific molecular markers by Fluorescein-Activated Cell Sorting analysis and showed that WJMSCs and BM-MSCs were positive for CD105, CD73, and CD90, and negative for CD45, CD34, CD86, and HLA-DR. These data revealed that WJ-MSCs used in this study showed the typical MSC characteristics (Data not shown).
Evaluation of endothelial markers expression at the mRNA and protein levels
n order to evaluate the effect of different adhesion matrices on differentiation of WJ-MSCs in endothelial-like cells, we measured the expression of endothelial markers by q-PCR and Western Blot. The three endothelial-specific molecules CD31, VE-cadherin and VEGF-R2 (KDR) are known to play an important role in the endothelium maturation during angiogenesis process [26]. CD31, also known as platelet endothelial cell adhesion molecule-1 (PECAM-1), is a type I integral membrane glycoprotein that is expressed at high levels on early and mature endothelial cells, platelets, and most leukocyte subpopulations. PECAM-1 is known to have various roles in vascular biology including angiogenesis, platelet function, and thrombosis [27]. VEGF-R2 is expressed on vascular endothelial cells and lymphatic endothelial cells; it regulates vascular endothelial function. VEGF is an important growth factor for the endothelial differentiation [28]. The endothelial-specific cadherin, vascular endothelial cadherin (VE-cadherin) is required for vascular genesis and the repair of damaged vessels [29].

Figure 1. Investigation of endothelial cell markers at the mRNA level
Expression of the endothelial markers: CD31, CDH5 and KDR at the mRNA level was assessed in HUVECs endothelial-like cells seeded on collagen and CHI/HA in differentiation medium (EGM2) for 15 days. Results show the mRNA level normalized to the reference gene mRNA RP29 level and expressed relative to the mRNA level in HUVECs (set as 100) and non-treated cells (data not shown). Results represent the average of 3 independent experiments ± SEM.

Figure 2. Studies of endothelial markers at the protein level
CD31, VEcadherin and VEGF-R2 blot quantifying for stimulated and unstimulated WJMSCs seeded on different culture surfaces after 15 days. Western blot normalization was performed to the expression of GAPDH. The expression in HUVECs was assumed for 100% (relative protein level=1.0). Results were expressed as the mean of 3 independent experiments ± SEM. ***: p< 0,001.
The mRNA and protein levels of the three endothelial specific markers CD31, VEGF-R2 (KDR) and VE-cadherin were analyzed by real-time qPCR and Western blot (Figures 1 and 2). Relative expression of the three molecules was analyzed at mRNA and protein levels, and expressed relative to appropriate HUVEC values. WJ-MSCs seeded on CHI/HA or collagen and incubated in EBM-2 did not express the three markers at the protein level whereas their expression at the mRNA level was barely detectable (<1% of the levels found in HUVECs for CD31 and CDH5). In WJ-MSCs incubated in EGM-2 for 15 days, mRNA level was higher on CHI/HA than on collagen for CD31 and KDR (increase by 67%, and 79%, respectively). However, only the KDR increase was statistically significant. At the protein level, KDR expression was higher on CHI/HA relative to collagen (45% increase), but this difference was not statistically significant. CD31 protein levels were unchanged between collagen and CHI/HA, whereas CDH5 level was higher on CHI/HA relative to collagen (4% increase), and the difference was statistically significant. The fold change between collagen and CHI/HA at mRNA level was more important than fold change at protein level. After 15 days of differentiation, the transcription of endothelial genes give rise to high levels of mRNA from these genes. The translation process might take more culture time, to produce similar levels of proteins.
Higher mRNA and protein expression of these three markers in differentiated WJ-MSCs seeded on CHI/HA could contribute to more pronounced endothelial differentiation as compared with differentiated WJ-MSCs seeded on collagen. However, collagen is a recommended surface, allowing MSCs chondrogenic and osteogenic differentiation after 21 days [30]. In our report, we have not detected expression of VEGF-R2 protein on the collagen surface; maybe we needed more than 15 days to notice the translation of VEGF-R2 gene.
The capacity to differentiate towards endothelial phenotype is a characteristic of mesenchymal stem cells [30-32], and our results showed that WJ-MSCs express endothelial markers at mRNA and protein levels after 15-day cultures in presence of endothelial growth factors. However, the main purpose of this study was to evaluate endotheliogenic potential of WJ-MSCs seeded onto CHI/HA scaffolds. First, we verified the endothelial potential of WJ-MSCs in monolayer culture conditions both in proliferation (data not shown) and differentiation media. In proliferation medium, no endothelial differentiation was observed during the entire experimental time (15 days), further confirming the stem-cell origin of isolated cells. In differentiation medium, RT-PCR and Western Blot confirmed a better endothelial potential of these cells on CHI/HA.
Our choice of the CHI/HA scaffold was based on some studies that demonstrated the efficacy of this natural scaffold in enhancing hMSCs differentiation into stromal cells. hMSCs were induced to differentiate to chondrogenic, osteogenic, and adipogenic phenotypes [33]. Recent studies have shown the potential of WJ-MSCs to differentiate towards cardiomyocytes using CHI/HA scaffolds [34]. In our study, we have demonstrated that WJ-MSCs are able to differentiate into endothelial cells on CHI/HA films. Therefore, we can deduce that a combination of these cells with this natural scaffold is advantageous for cardio-vascular tissue engineering. Same conclusive results were observed in healing of diabetic skin wound by the proliferation and differentiation of human umbilical cords mesenchymal stem cells (hUCMSCs) on the collagen/chitosan laser drilling acellular dermal matrix (CCLDADM) scaffold, a natural scaffold used in vivo [35].
Evaluation of endothelial-like cells functionality by LDL uptake assay
LDL-uptake assay is applied for detection of functional endothelial cells. WJMSCs, when seeded on CHI/HA and collagen, captured DiIAcLDL to the cytoplasm after 4 hour-incubation in RPMI medium supplemented with DiI-Ac-LDL. However, MSCs were unable to uptake DiI-Ac-LDL after culturing in the growth medium as negative controls (Fig. 3). These results confirm that, after two weeks, WJ-MSCs seeded on CHI/HA and collagen exhibit endothelial cell phenotype. In this respect, Gaffney et al. investigated lipoprotein uptake by means of flow cytometry and showed that the cells in a G2/M (mitosis) phase incorporated about 45% more Dil-Ac-LDL than those in a G1/S (latency) phase [36]. Higher Dil-Ac-LDL uptake of endothelium-like cells on PEMs suggests that more cells are in the G2/M phase on PEMs, a feature of higher proliferation.

Figure 3. LDL-Uptake assay
WJ-MSCs cellular uptake of labeled acetylated LDLs. Double immunofluorescence of nuclei (blue) and for DI-Ac-LDLs (red) for unstimulated (a, b) and stimulated (c, d) WJMSCs after 15 days of culture on collagen and CHI/HA. Internalized labels were detected by Leica fluorescence microscopy (objective *40 oil).
Detection of endothelial-specific marker expression: vWF (von Willebrand Factor) by immunocytochemistry
Von Willebrand adherence factor (vWF) protein contributes to platelet function by mediating the initiation and progression of thrombus formation at the sites of vascular injury. Moreover, novel findings have been obtained on the link between regulation of VWF multimer size and microvascular thrombosis. This progress in basic research has provided critical information to define with greater precision the role of vWF in vascular biology and pathology, including its possible involvement in the onset of atherosclerosis and its acute thrombotic complications. Therefore we have used the expression of vWF as a functionality test of our endothelial-like cells [37]. The cells were examined for expression of endothelial-specific marker (vWF) by immunocytochemistry.
WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to this marker after endothelial differentiation for 15 days. MSCs did not show any positive signal after they were cultured in the growth medium (EBM2) on the same scaffold, as negative controls. On collagen layer, the cells were not marked, that was predicted because they needed more culture time to express vWF protein (Fig. 4).

Figure 4. vWF immunostaining
Double immunofluorescence staining for nuclei (blue), and for prothrombogenic von Willebrand Factor for stimulated (a, b) and unstimulated (d, e) WJ-MSCs after 15 days of culture on collagen and CHI/HA (Zeiss Microscope, objective*63 oil). N=3.
These promising results showed the possibility to combine the use of WJ-MSCs and CHI/HA films aiming for vascular tissue engineering, by evaluating the capacity of WJ-MSCs to differentiate into smooth muscle cells on CHI/HA, coating the surface of alginate hydrogels with CHI/HA films, and enrolling them in order to form a tubular vascular graft.
These promising data showed that the combination of WJ-MSCs and CHI/HA may lead to brilliant results, regarding endothelial differentiation and angiogenesis. One may recommend using of these cells and materials for vascular tissue engineering and regeneration therapy. More studies can be done to evaluate the capacity of WJ-MSCs to differentiate into smooth muscle cells on CHI/HA, to coat the surface of alginate hydrogels with CHI/HA films and to enroll them, in order to form a tubular vascular graft.
Conclusion
These first quite encouraging results showed that it is possible to obtain CEs-like in a non-traumatic way (from Wharton's jelly of human umbilical cords) and in a short time (15 days). Our technique, based on the use of polyelectrolyte films, could therefore be used in the field of vascular engineering for the development of functional vascular substitutes comprising an endothelium resulting from differentiation of mesenchymal stem cells, which would limit the risks of graft rejection and could be applied to patients who require vessel replacement.
Acknowledgements
The authors would like to thank Al Hanan Hospital for providing the umbilical cords used in our researches and Azm & Saadeh society for funding this work.
The authors have no conflicts of interest to declare.
Ethical Statement: The research work was approved by the ethical committee of the Lebanese University, Centre Azm for research in Applied Biotechnology and the ethical Committee of Al Hanan Hospital-Tripoli, Lebanon.
References
- Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. Eur Heart J. 2004;25(14):1197-1207.
- Kumar VA, Brewster LP, Caves JM, Chaikof EL. Tissue engineering of blood vessels: functional requirements, progress, and future challenges. Cardiovasc Eng Technol. 2011;2(3):137-148.
- Erler P, Sweeney A, Monaghan JR. Regulation of Injury-Induced Ovarian Regeneration by Activation of Oogonial Stem Cells. Stem Cells. 2017;35(1):236-247.
- Sherman SE, Kuljanin M, Cooper TT, Putman DM, Lajoie GA, Hess DA. High aldehyde dehydrogenase activity identifies a subset of human mesenchymal stromal cells with vascular regenerative potential. Stem Cells. 2017;35(6):1542-1553.
- Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851-858.
- Xu Y, Huang S, Ma K, Fu X, Han W, Sheng Z. Promising new potential for mesenchymal stem cells derived from human umbilical cord Wharton’s jelly: sweat gland cell-like differentiative capacity. J Tissue Eng Regen Med. 2012;6(8):645-654.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
- Fong C-Y, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod Biomed Online. 2007;15(6):708-718.
- Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346(1):53.
- Troyer DL, Weiss ML. Concise review: Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591-599.
- Abediankenari S, Ghasemi M. Generation of immune inhibitory dendritic cells CD4+T regulatory cells inducing by TGF-β. Iran J Allergy Asthma Immunol. 2009;8(1):25-30.
- Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195.
- Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992;6(9):2639-2645.
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528-539.
- Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym Int. 2008;57(3):397-430.
- Muzzarelli RA. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr Polym. 2011;83(4):1433-1445.
- Schanté CE, Zuber G, Herlin C, Vandamme TF. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr Polym. 2011;85(3):469-489.
- Wang X, He J, Wang Y, Cui F-Z. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus. 2012;2(3):278-291.
- Berger J, Reist M, Mayer JM, Felt O, Gurny R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):35-52.
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343-353.
- Dennaoui H, Chouery E, Rammal H, Abdel-Razzak Z, Harmouch C. Chitosan/hyaluronic acid multilayer films are biocompatible substrate for Wharton’s jelly derived stem cells. Stem Cell Investig. 2018;5.
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973; 52(11):2745-56.
- Rammal H, Harmouch C, Maerten C, Gaucher C, Boulmedais F, Schaaf P, et al. Upregulation of endothelial gene markers in Wharton’s jelly mesenchymal stem cells cultured on polyelectrolyte multilayers. J Biomed Mater Res A. 2017;105(1):292-300.
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393-395.
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581-611.
- Woodfin A, Voisin M-B, Nourshargh S. PECAM-1: a multifunctional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27(12):2514-2523.
- Li L, Liu H, Xu C, Deng M, Song M, Yu X, et al. VEGF promotes endothelial progenitor cell differentiation and vascular repair through connexin 43. Stem Cell Res Ther. 2017; 8(1):237.
- Gory-Fauré S, Prandini M-H, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, et al. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126(10):2093-2102.
- Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Oliver DA, Quinn CO, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7(11):581-588.
- Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Begyui R, et al. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7(3):282-291.
- Seo MJ. Suh SY, Bae YG, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328(1):258-264.
- Lindborg BA, Brekke JH, Scott CM, Chai YW, Ulrich C, Sandquist L, et al. A chitosan-hyaluronan-based hydrogel-hydrocolloid supports in vitro culture and differentiation of human mesenchymal stem/stromal cells. Tissue Eng Part A. 2015;21(11-12):1952-62.
- Rabbani S, Soleimani M, Imani M, Sahebjam M, Ghiaseddin A, Nassiri SM, et al. Regenerating Heart Using a Novel Compound and Human Wharton Jelly Mesenchymal Stem Cells. Arch Med Res. 2017;48(3):228-37.
- Han Y, Sun T, Han Y, Lin L, Liu C, Liu J, et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. Eur J Med Res. 2019;24(1):10.
- Gaffney J, West D, Arnold F, Sattar A, Kumar S. Differences in the uptake of modified low density lipoproteins by tissue cultured endothelial cells. J Cell Sci. 1985;79(1):317-325.
- Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1(7):1335-1342.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(100) "endotelialnaya-differentsirovka-mezenkhimnykh-stvolovykh-kletok-iz-soedinitelnoy-tkani-pupoviny-zhel" ["~CODE"]=> string(100) "endotelialnaya-differentsirovka-mezenkhimnykh-stvolovykh-kletok-iz-soedinitelnoy-tkani-pupoviny-zhel" ["EXTERNAL_ID"]=> string(4) "1943" ["~EXTERNAL_ID"]=> string(4) "1943" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(423) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(3935) "<p style="text-align: justify;">Простота заготовки мезенхимных стволовых клеток из желе Вортона (МСК-ЖВ), выраженная пластичность дифференцировки и низкая иммуногенность делают их удобным средством аллогенной клеточной терапии. Целью данного исследования было изучение способности к дифференцировке в эндотелиоподобные клетки МСК-ЖВ, культивированных на гиалуронан-хитозановых (ГХ) многослойных носителях.</p> <h3>Материалы и методы</h3> <p style="text-align: justify;">В этой работе мы проводили дифференцировку МСК-ЖВ в ангиогенном направлении с применением полиэлектролитной многослойной пленки в качестве субстрата. МСК-ЖВ культивировали на ГХ-многослойной пленке и стимулировали факторами культуральной среды EGM-2®. Коллаген типа I использовали в качестве контрольного субстрата. Определяли экспрессию специфических мРНК, а именно: CD31, рецептора фактора роста сосудистого эндотелия типа 2 (VEGF2) и эндотелиального сосудистого кадхерина (VE), наряду с уровнями абсорбции Dil-ацетилированного липопротеина низкой плотности и экспрессией белкового фактора Виллебранда.</p> <h3>Результаты</h3> <p style="text-align: justify;">Выделенные МСК-ЖВ имели типичную морфологию фибробластоподобных клеток. Уровни мРНК, кодирующих CD31 и KDR были выше после культивирования на ГХ-субстрате, нежели на коллагеновом покрытии, при достоверном повышении экспрессии KDR. На уровне белков, показана тенденция к повышению уровней KDR и CDH5 после инкубации на ГХ-субстрате, по сравнению с коллагеном. Кроме того, МСК-ЖВ, культивированные на ГХ, имели высокие уровни экспрессии эндотелиальных маркеров после 15 суток культивирования в среде EGM-2®.</p> <h3>Выводы</h3> <p style="text-align: justify;">В данной работе мы сообщаем о новом биосовместимом субстрате, который способствует дифференцировке МСК-ЖВ в эндотелиоподобные клетки. Разработка этого субстрата является новым подходом в тканевой инженерии для создания аллогенных сосудистых трансплантатов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Мезенхимные стволовые клетки, желе Вортона, дифференцировка, эндотелий, хитозан, гиалуроновая кислота, многослойный субстрат, тканевая инженерия.</p>" ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_META_TITLE"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_META_KEYWORDS"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_META_DESCRIPTION"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_PICTURE_FILE_ALT"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_PICTURE_FILE_TITLE"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_PICTURE_FILE_NAME"]=> string(100) "endotelialnaya-differentsirovka-mezenkhimnykh-stvolovykh-kletok-iz-soedinitelnoy-tkani-pupoviny-zhel" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(297) "Эндотелиальная дифференцировка мезенхимных стволовых клеток из соединительной ткани пуповины (желе Вортона) на многослойных пленках из хитозана/гиалуронинана " ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(100) "endotelialnaya-differentsirovka-mezenkhimnykh-stvolovykh-kletok-iz-soedinitelnoy-tkani-pupoviny-zhel" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(100) "endotelialnaya-differentsirovka-mezenkhimnykh-stvolovykh-kletok-iz-soedinitelnoy-tkani-pupoviny-zhel" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(100) "endotelialnaya-differentsirovka-mezenkhimnykh-stvolovykh-kletok-iz-soedinitelnoy-tkani-pupoviny-zhel" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "171" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27383" ["VALUE"]=> string(22) "08/25/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "08/25/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27384" ["VALUE"]=> string(22) "11/14/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/14/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27385" ["VALUE"]=> array(2) { ["TEXT"]=> string(163) "<p>Хана Деннауи<sup>1</sup>, Элиана Шуэри<sup>2</sup>, Шаза Хармуш<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(115) "
Хана Деннауи1, Элиана Шуэри2, Шаза Хармуш1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27386" ["VALUE"]=> array(2) { ["TEXT"]=> string(631) "<p><sup>1</sup> Лаборатория прикладной биотехнологии: биомолекулы, биотерапия и биопроцессы, научно-прикладной центр биотехнологии AZM, докторская школа науки и технологий, Ливанский университет, Триполи, Ливан <br> <sup>2</sup> Отделение медицинской генетики, факультет медицины, университет Св. Иосифа (USJ), Мар-Микаэл, Бейрут, Ливан</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(589) "1 Лаборатория прикладной биотехнологии: биомолекулы, биотерапия и биопроцессы, научно-прикладной центр биотехнологии AZM, докторская школа науки и технологий, Ливанский университет, Триполи, Ливан
2 Отделение медицинской генетики, факультет медицины, университет Св. Иосифа (USJ), Мар-Микаэл, Бейрут, Ливан
Простота заготовки мезенхимных стволовых клеток из желе Вортона (МСК-ЖВ), выраженная пластичность дифференцировки и низкая иммуногенность делают их удобным средством аллогенной клеточной терапии. Целью данного исследования было изучение способности к дифференцировке в эндотелиоподобные клетки МСК-ЖВ, культивированных на гиалуронан-хитозановых (ГХ) многослойных носителях.
Материалы и методы
В этой работе мы проводили дифференцировку МСК-ЖВ в ангиогенном направлении с применением полиэлектролитной многослойной пленки в качестве субстрата. МСК-ЖВ культивировали на ГХ-многослойной пленке и стимулировали факторами культуральной среды EGM-2®. Коллаген типа I использовали в качестве контрольного субстрата. Определяли экспрессию специфических мРНК, а именно: CD31, рецептора фактора роста сосудистого эндотелия типа 2 (VEGF2) и эндотелиального сосудистого кадхерина (VE), наряду с уровнями абсорбции Dil-ацетилированного липопротеина низкой плотности и экспрессией белкового фактора Виллебранда.
Результаты
Выделенные МСК-ЖВ имели типичную морфологию фибробластоподобных клеток. Уровни мРНК, кодирующих CD31 и KDR были выше после культивирования на ГХ-субстрате, нежели на коллагеновом покрытии, при достоверном повышении экспрессии KDR. На уровне белков, показана тенденция к повышению уровней KDR и CDH5 после инкубации на ГХ-субстрате, по сравнению с коллагеном. Кроме того, МСК-ЖВ, культивированные на ГХ, имели высокие уровни экспрессии эндотелиальных маркеров после 15 суток культивирования в среде EGM-2®.
Выводы
В данной работе мы сообщаем о новом биосовместимом субстрате, который способствует дифференцировке МСК-ЖВ в эндотелиоподобные клетки. Разработка этого субстрата является новым подходом в тканевой инженерии для создания аллогенных сосудистых трансплантатов.
Ключевые слова
Мезенхимные стволовые клетки, желе Вортона, дифференцировка, эндотелий, хитозан, гиалуроновая кислота, многослойный субстрат, тканевая инженерия.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27388" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-59-67" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-59-67" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27391" ["VALUE"]=> array(2) { ["TEXT"]=> string(137) "<p>Hana Dennaoui<sup>1</sup>, Eliane Chouery<sup>2</sup>, Chaza Harmoush<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(89) "Hana Dennaoui1, Eliane Chouery2, Chaza Harmoush1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27392" ["VALUE"]=> array(2) { ["TEXT"]=> string(785) "<p><sup>1</sup> Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon <br> <sup>2</sup> Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon</p><br> <p><b>Correspondence</b><br> Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon<br> E-mail: hana.dennaoui@hotmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(701) "1 Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon
2 Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon
Correspondence
Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon
E-mail: hana.dennaoui@hotmail.com
The ease of harvesting of mesenchymal stem cells from Wharton’s jelly (WJ-MSCs), their great differentiation plasticity and low immunogenicity make them a suitable tool for allogeneic cell therapy. The aim of present study was to explore the potential of WJ-MSCs seeded on chitosan/hyaluronic acid (HA/CHI) multilayers to differentiate into endothelial-like cells.
Methods
In this study, we differentiate WJ-MSCs into an angiogenic lineage using polyelectrolyte multilayer film as a substrate. WJ-MSCs were cultivated on HA/CHI multilayer film and stimulated (or not) with EGM-2® culture medium. Type I collagen was used as control. mRNA and protein expression of CD31, vascular endothelial growth factor-receptor 2 (VEGF2) and vascular endothelial (VE)-cadherin, along with Dil-acetylated low-density lipoprotein-uptake and von Willebrand Factor protein expression were performed.
Results
The isolated MSCs showed typical fibroblast-like morphology. We have shown that WJ-MSCs express endothelial markers after 15 days of culture in EGM-2® medium. The mRNA levels were higher on CHI/HA than on collagen for CD31 and KDR, with only KDR increase being statistically significant. At the protein level, a trend for increase in KDR and CDH5 levels was also shown on CHI/HA relative to collagen. Moreover, the WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to von Willebrand factor after endothelial differentiation for 15 days.
Conclusion
We report here a new biocompatible coating allowing differentiation of WJ-MSCs into endothelial-like cells. This substrate opens new routes in tissue engineering to design allogeneic vascular grafts.
Keywords
Mesenchymal stem cells, Wharton’s jelly, differentiation, endothelial lineage, chitosan, hyaluronic acid, multilayer substrate, tissue engineering.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27389" ["VALUE"]=> string(126) "Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(126) "Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27390" ["VALUE"]=> string(4) "2344" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2344" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27394" ["VALUE"]=> string(4) "2345" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2345" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27391" ["VALUE"]=> array(2) { ["TEXT"]=> string(137) "<p>Hana Dennaoui<sup>1</sup>, Eliane Chouery<sup>2</sup>, Chaza Harmoush<sup>1</sup> </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(89) "Hana Dennaoui1, Eliane Chouery2, Chaza Harmoush1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(89) "Hana Dennaoui1, Eliane Chouery2, Chaza Harmoush1
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27393" ["VALUE"]=> array(2) { ["TEXT"]=> string(2194) "<p style="text-align: justify;">The ease of harvesting of mesenchymal stem cells from Wharton’s jelly (WJ-MSCs), their great differentiation plasticity and low immunogenicity make them a suitable tool for allogeneic cell therapy. The aim of present study was to explore the potential of WJ-MSCs seeded on chitosan/hyaluronic acid (HA/CHI) multilayers to differentiate into endothelial-like cells.</p> <h3>Methods</h3> <p style="text-align: justify;">In this study, we differentiate WJ-MSCs into an angiogenic lineage using polyelectrolyte multilayer film as a substrate. WJ-MSCs were cultivated on HA/CHI multilayer film and stimulated (or not) with EGM-2® culture medium. Type I collagen was used as control. mRNA and protein expression of CD31, vascular endothelial growth factor-receptor 2 (VEGF2) and vascular endothelial (VE)-cadherin, along with Dil-acetylated low-density lipoprotein-uptake and von Willebrand Factor protein expression were performed.</p> <h3>Results</h3> <p style="text-align: justify;">The isolated MSCs showed typical fibroblast-like morphology. We have shown that WJ-MSCs express endothelial markers after 15 days of culture in EGM-2® medium. The mRNA levels were higher on CHI/HA than on collagen for CD31 and KDR, with only KDR increase being statistically significant. At the protein level, a trend for increase in KDR and CDH5 levels was also shown on CHI/HA relative to collagen. Moreover, the WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to von Willebrand factor after endothelial differentiation for 15 days.</p> <h3>Conclusion</h3> <p style="text-align: justify;">We report here a new biocompatible coating allowing differentiation of WJ-MSCs into endothelial-like cells. This substrate opens new routes in tissue engineering to design allogeneic vascular grafts.</p> <h2>Keywords</h2> <p style="text-align: justify;">Mesenchymal stem cells, Wharton’s jelly, differentiation, endothelial lineage, chitosan, hyaluronic acid, multilayer substrate, tissue engineering. </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(2036) "The ease of harvesting of mesenchymal stem cells from Wharton’s jelly (WJ-MSCs), their great differentiation plasticity and low immunogenicity make them a suitable tool for allogeneic cell therapy. The aim of present study was to explore the potential of WJ-MSCs seeded on chitosan/hyaluronic acid (HA/CHI) multilayers to differentiate into endothelial-like cells.
Methods
In this study, we differentiate WJ-MSCs into an angiogenic lineage using polyelectrolyte multilayer film as a substrate. WJ-MSCs were cultivated on HA/CHI multilayer film and stimulated (or not) with EGM-2® culture medium. Type I collagen was used as control. mRNA and protein expression of CD31, vascular endothelial growth factor-receptor 2 (VEGF2) and vascular endothelial (VE)-cadherin, along with Dil-acetylated low-density lipoprotein-uptake and von Willebrand Factor protein expression were performed.
Results
The isolated MSCs showed typical fibroblast-like morphology. We have shown that WJ-MSCs express endothelial markers after 15 days of culture in EGM-2® medium. The mRNA levels were higher on CHI/HA than on collagen for CD31 and KDR, with only KDR increase being statistically significant. At the protein level, a trend for increase in KDR and CDH5 levels was also shown on CHI/HA relative to collagen. Moreover, the WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to von Willebrand factor after endothelial differentiation for 15 days.
Conclusion
We report here a new biocompatible coating allowing differentiation of WJ-MSCs into endothelial-like cells. This substrate opens new routes in tissue engineering to design allogeneic vascular grafts.
Keywords
Mesenchymal stem cells, Wharton’s jelly, differentiation, endothelial lineage, chitosan, hyaluronic acid, multilayer substrate, tissue engineering.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(2036) "The ease of harvesting of mesenchymal stem cells from Wharton’s jelly (WJ-MSCs), their great differentiation plasticity and low immunogenicity make them a suitable tool for allogeneic cell therapy. The aim of present study was to explore the potential of WJ-MSCs seeded on chitosan/hyaluronic acid (HA/CHI) multilayers to differentiate into endothelial-like cells.
Methods
In this study, we differentiate WJ-MSCs into an angiogenic lineage using polyelectrolyte multilayer film as a substrate. WJ-MSCs were cultivated on HA/CHI multilayer film and stimulated (or not) with EGM-2® culture medium. Type I collagen was used as control. mRNA and protein expression of CD31, vascular endothelial growth factor-receptor 2 (VEGF2) and vascular endothelial (VE)-cadherin, along with Dil-acetylated low-density lipoprotein-uptake and von Willebrand Factor protein expression were performed.
Results
The isolated MSCs showed typical fibroblast-like morphology. We have shown that WJ-MSCs express endothelial markers after 15 days of culture in EGM-2® medium. The mRNA levels were higher on CHI/HA than on collagen for CD31 and KDR, with only KDR increase being statistically significant. At the protein level, a trend for increase in KDR and CDH5 levels was also shown on CHI/HA relative to collagen. Moreover, the WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to von Willebrand factor after endothelial differentiation for 15 days.
Conclusion
We report here a new biocompatible coating allowing differentiation of WJ-MSCs into endothelial-like cells. This substrate opens new routes in tissue engineering to design allogeneic vascular grafts.
Keywords
Mesenchymal stem cells, Wharton’s jelly, differentiation, endothelial lineage, chitosan, hyaluronic acid, multilayer substrate, tissue engineering.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27388" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-59-67" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-59-67" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-59-67" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27389" ["VALUE"]=> string(126) "Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(126) "Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(126) "Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27392" ["VALUE"]=> array(2) { ["TEXT"]=> string(785) "<p><sup>1</sup> Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon <br> <sup>2</sup> Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon</p><br> <p><b>Correspondence</b><br> Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon<br> E-mail: hana.dennaoui@hotmail.com</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(701) "1 Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon
2 Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon
Correspondence
Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon
E-mail: hana.dennaoui@hotmail.com
1 Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon
2 Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon
Correspondence
Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon
E-mail: hana.dennaoui@hotmail.com
Хана Деннауи1, Элиана Шуэри2, Шаза Хармуш1
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(115) "Хана Деннауи1, Элиана Шуэри2, Шаза Хармуш1
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27383" ["VALUE"]=> string(22) "08/25/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "08/25/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "08/25/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27384" ["VALUE"]=> string(22) "11/14/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/14/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/14/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27387" ["VALUE"]=> array(2) { ["TEXT"]=> string(3935) "<p style="text-align: justify;">Простота заготовки мезенхимных стволовых клеток из желе Вортона (МСК-ЖВ), выраженная пластичность дифференцировки и низкая иммуногенность делают их удобным средством аллогенной клеточной терапии. Целью данного исследования было изучение способности к дифференцировке в эндотелиоподобные клетки МСК-ЖВ, культивированных на гиалуронан-хитозановых (ГХ) многослойных носителях.</p> <h3>Материалы и методы</h3> <p style="text-align: justify;">В этой работе мы проводили дифференцировку МСК-ЖВ в ангиогенном направлении с применением полиэлектролитной многослойной пленки в качестве субстрата. МСК-ЖВ культивировали на ГХ-многослойной пленке и стимулировали факторами культуральной среды EGM-2®. Коллаген типа I использовали в качестве контрольного субстрата. Определяли экспрессию специфических мРНК, а именно: CD31, рецептора фактора роста сосудистого эндотелия типа 2 (VEGF2) и эндотелиального сосудистого кадхерина (VE), наряду с уровнями абсорбции Dil-ацетилированного липопротеина низкой плотности и экспрессией белкового фактора Виллебранда.</p> <h3>Результаты</h3> <p style="text-align: justify;">Выделенные МСК-ЖВ имели типичную морфологию фибробластоподобных клеток. Уровни мРНК, кодирующих CD31 и KDR были выше после культивирования на ГХ-субстрате, нежели на коллагеновом покрытии, при достоверном повышении экспрессии KDR. На уровне белков, показана тенденция к повышению уровней KDR и CDH5 после инкубации на ГХ-субстрате, по сравнению с коллагеном. Кроме того, МСК-ЖВ, культивированные на ГХ, имели высокие уровни экспрессии эндотелиальных маркеров после 15 суток культивирования в среде EGM-2®.</p> <h3>Выводы</h3> <p style="text-align: justify;">В данной работе мы сообщаем о новом биосовместимом субстрате, который способствует дифференцировке МСК-ЖВ в эндотелиоподобные клетки. Разработка этого субстрата является новым подходом в тканевой инженерии для создания аллогенных сосудистых трансплантатов. </p> <h2>Ключевые слова</h2> <p style="text-align: justify;">Мезенхимные стволовые клетки, желе Вортона, дифференцировка, эндотелий, хитозан, гиалуроновая кислота, многослойный субстрат, тканевая инженерия.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(3777) "Простота заготовки мезенхимных стволовых клеток из желе Вортона (МСК-ЖВ), выраженная пластичность дифференцировки и низкая иммуногенность делают их удобным средством аллогенной клеточной терапии. Целью данного исследования было изучение способности к дифференцировке в эндотелиоподобные клетки МСК-ЖВ, культивированных на гиалуронан-хитозановых (ГХ) многослойных носителях.
Материалы и методы
В этой работе мы проводили дифференцировку МСК-ЖВ в ангиогенном направлении с применением полиэлектролитной многослойной пленки в качестве субстрата. МСК-ЖВ культивировали на ГХ-многослойной пленке и стимулировали факторами культуральной среды EGM-2®. Коллаген типа I использовали в качестве контрольного субстрата. Определяли экспрессию специфических мРНК, а именно: CD31, рецептора фактора роста сосудистого эндотелия типа 2 (VEGF2) и эндотелиального сосудистого кадхерина (VE), наряду с уровнями абсорбции Dil-ацетилированного липопротеина низкой плотности и экспрессией белкового фактора Виллебранда.
Результаты
Выделенные МСК-ЖВ имели типичную морфологию фибробластоподобных клеток. Уровни мРНК, кодирующих CD31 и KDR были выше после культивирования на ГХ-субстрате, нежели на коллагеновом покрытии, при достоверном повышении экспрессии KDR. На уровне белков, показана тенденция к повышению уровней KDR и CDH5 после инкубации на ГХ-субстрате, по сравнению с коллагеном. Кроме того, МСК-ЖВ, культивированные на ГХ, имели высокие уровни экспрессии эндотелиальных маркеров после 15 суток культивирования в среде EGM-2®.
Выводы
В данной работе мы сообщаем о новом биосовместимом субстрате, который способствует дифференцировке МСК-ЖВ в эндотелиоподобные клетки. Разработка этого субстрата является новым подходом в тканевой инженерии для создания аллогенных сосудистых трансплантатов.
Ключевые слова
Мезенхимные стволовые клетки, желе Вортона, дифференцировка, эндотелий, хитозан, гиалуроновая кислота, многослойный субстрат, тканевая инженерия.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(3777) "Простота заготовки мезенхимных стволовых клеток из желе Вортона (МСК-ЖВ), выраженная пластичность дифференцировки и низкая иммуногенность делают их удобным средством аллогенной клеточной терапии. Целью данного исследования было изучение способности к дифференцировке в эндотелиоподобные клетки МСК-ЖВ, культивированных на гиалуронан-хитозановых (ГХ) многослойных носителях.
Материалы и методы
В этой работе мы проводили дифференцировку МСК-ЖВ в ангиогенном направлении с применением полиэлектролитной многослойной пленки в качестве субстрата. МСК-ЖВ культивировали на ГХ-многослойной пленке и стимулировали факторами культуральной среды EGM-2®. Коллаген типа I использовали в качестве контрольного субстрата. Определяли экспрессию специфических мРНК, а именно: CD31, рецептора фактора роста сосудистого эндотелия типа 2 (VEGF2) и эндотелиального сосудистого кадхерина (VE), наряду с уровнями абсорбции Dil-ацетилированного липопротеина низкой плотности и экспрессией белкового фактора Виллебранда.
Результаты
Выделенные МСК-ЖВ имели типичную морфологию фибробластоподобных клеток. Уровни мРНК, кодирующих CD31 и KDR были выше после культивирования на ГХ-субстрате, нежели на коллагеновом покрытии, при достоверном повышении экспрессии KDR. На уровне белков, показана тенденция к повышению уровней KDR и CDH5 после инкубации на ГХ-субстрате, по сравнению с коллагеном. Кроме того, МСК-ЖВ, культивированные на ГХ, имели высокие уровни экспрессии эндотелиальных маркеров после 15 суток культивирования в среде EGM-2®.
Выводы
В данной работе мы сообщаем о новом биосовместимом субстрате, который способствует дифференцировке МСК-ЖВ в эндотелиоподобные клетки. Разработка этого субстрата является новым подходом в тканевой инженерии для создания аллогенных сосудистых трансплантатов.
Ключевые слова
Мезенхимные стволовые клетки, желе Вортона, дифференцировка, эндотелий, хитозан, гиалуроновая кислота, многослойный субстрат, тканевая инженерия.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27386" ["VALUE"]=> array(2) { ["TEXT"]=> string(631) "<p><sup>1</sup> Лаборатория прикладной биотехнологии: биомолекулы, биотерапия и биопроцессы, научно-прикладной центр биотехнологии AZM, докторская школа науки и технологий, Ливанский университет, Триполи, Ливан <br> <sup>2</sup> Отделение медицинской генетики, факультет медицины, университет Св. Иосифа (USJ), Мар-Микаэл, Бейрут, Ливан</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(589) "1 Лаборатория прикладной биотехнологии: биомолекулы, биотерапия и биопроцессы, научно-прикладной центр биотехнологии AZM, докторская школа науки и технологий, Ливанский университет, Триполи, Ливан
2 Отделение медицинской генетики, факультет медицины, университет Св. Иосифа (USJ), Мар-Микаэл, Бейрут, Ливан
1 Лаборатория прикладной биотехнологии: биомолекулы, биотерапия и биопроцессы, научно-прикладной центр биотехнологии AZM, докторская школа науки и технологий, Ливанский университет, Триполи, Ливан
2 Отделение медицинской генетики, факультет медицины, университет Св. Иосифа (USJ), Мар-Микаэл, Бейрут, Ливан
Dear Editor,
I would like to comment a report by Dr. K. Beider "Senescent/exhausted phenotype of CD 19 – targeted CAR-T cells and induction of immuneregulatory environmemt correlate with reduced response to CAR T cell therapy in relapsed/refractory B cell malignancies" presented at the R. Gorbacheva Memorial Meeting in St. Petersburg at September 19, 2020 under Focus on Lymphomas (access: https://rgmm.info/translyacii/auditoriya-5).
The CD19-specific CAR T cells have shown significant antitumor activity in the treatment of high-risk relapsed and refractory leukemias and lymphomas and have revolutionized the treatment landscape for patients with advanced lymphoid malignancies. In 2018 two CAR T cell products were approved by the US and European regulatory authorities. Both Axicabtagene ciloleucel (Yescarta, Gilead/Kite) and tisagenlecleucel (Kymriah, Novartis) are now available for clinical use, based on the results of the ZUMA-1 (Yescarta) and JULIET (Kymriah) trials for refractory/relapsed relapsed/refractory aggressive B-cell lymphomas, and the ELIANA trial (Kymriah) for advanced acute lymphoblastic leukemia in patients up to 25 years of age.
Longer-term follow-up data from the lymphoma trials indicate durable responses for for the 40-60% of patients who achieved a complete response. Studies to better identify predictors of response are needed in order to improve the risk-benefit balance and minimize unnecessary financial burden for individual patients and healthcare systems. This could eventually result in those predicted to respond poorly to chemotherapy but well to CAR T cells receiving them upfront. Among the more commonly cited impediments for effective CAR T cell therapy are loss or modulation of CD 19 expression by the target cells, lack of CAR T cell persistence, as well as product manufacturing failures. Adequate T cell manufacturing is an essential component of CAR T cell therapy. Quantitative and qualitative characteristics of the apheresis product can affect the ability to successfully produce an efficient T cell product. Even if T cell numbers are adequate the starting T cell phenotype can be an important determinant of subsequent clinical activity.
In her presentation Dr. Katia Beider discussed the factors that can preclude durable remissions following CAR T cell therapy with a special focus on the role of T cell senescence and exhaustion phenotype as a cause for reduced response after adoptive cell therapy in patients with relapsed/refractory B cell lymphomas. She presented data on 22 patients (86% with B cell non-Hodgkin’s lymphomas) that were treated at Sheba Medical Center in Tel Hashomer (Israel) with an house-made CAR T cell construct composed of an anti-CD19 single-chain Fv FMC63, CD28 co-stimulatory and CD3-zeta intracellular domain.
For each patient samples were collected after apheresis, but before lymphodepletion, from the CAR T cell infused product and on days 7, 14, 21, 30 and 60 after infusion. Clinical responses were evaluated at day 28, with 11 patients (50%) achieving complete response; 2 patients developed partial response (9%), and 9 patients (41%) had progressive disease. The manufactured product contained high-purity CD3 + T cells, composed with CD4+ and CD8+ T cells. The frequency of exhausted T cells, phenotypically defined as CD28 negative, with upregulation of CD57 and CD39 molecules, was analysed in the CAR T cell products. A significant increase of exhausted CD8 T cells was observed in the product infused in patients with progressive disease when compared to patients with clinical response.
The investigators then asked the question whether the exhausted T cells were already present in the peripheral blood of these patients, or developed following genetic transduction and in vitro expansion. Responding patients had very low levels of exhausted cells in the blood and the final product also had a very low frequency of these cells. In contrast, two groups were seen among the patients with progressive disease: patients who had increased numbers of exhausted T cells in their original pool, that did not change in the final product, and the patients that had low number of these cells in the initial product, but increased during the transduction and expansion procedures, showing that these cells can be induced during the manufacturing process.
Interestingly, the exhausted phenotype in CD8+ T cells was associated with an increase in memory phenotype acquisition in CAR T products; in contrast, naïve T cells were low in non-responders as compared to responders, suggesting that less differentiated phenotypes were associated with better responses.
They also showed an increased expression of the CCR7 chemokine on CD4+ and CD8+ CAR T cells from responding patients, with increased trafficking of these cells to lymphoid tissue. Finally, they studied the PD-1 expression in CD8+ cells in the CAR T product and found a significant correlation of an increased expression with the exhausted phenotype.
These results suggest that the starting T cell phenotype in the apheresis product and after expansion and transduction may influence the subsequent clinical activity of the CAR T cell product and elucidate in part the mechanisms of cell trafficking and efficacy.
The data also suggest that combining CAR T cell therapy with immune checkpoint inhibitors may improve the effectiveness of the treatment, and pre-clinical data support such a synergetic approach [1]. However, strategies that enhance the potency of these products run the risk of inadvertently worsening the already considerable toxic effects.
It would have been interesting to know whether these results have any correlation with the number of previous lines of chemotherapy given to patients before collecting the T cells, and the time from diagnosis to treatment since persistent antigen exposure in cancer has been proposed to lead to cytotoxic T lymphocyte exhaustion.
These results were obtained in patients with B cell lymphomas and contrast with those in patients with B-ALL in whom standard parameters that define T cell potency, such as markers of T cell exhaustion, have been disappointing in terms of predicting clinical efficacy [2-5]. In patients with CLL, however, among whom response rates have been substantially lower than those in patients with ALL or lymphoma, product characteristics, such as enrichment for IL-6-STAT3 signatures, and elevated frequency of CD29+CD45RO-CD8+ T cells before CAR T cell generation, were able to identify a favourable response [6].
Additional data will be required to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.
Conflict of interest
None declared.
References
- Lin AM, Hucks GE, Dinofia AM, Seif AE, Teachey DT, Baniewicz D, et al. Checkpoint inhibitors augment CD19 directed CAR T cell therapy in relapsed B-cell acute lymphoblastic leukemia. Blood 2018; 132 (Suppl 1): 556.
- Shah N, Fry T. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol 2019; 16: 372-385.
- de Wolf C, van den Bovenkamp M, Hoefnagel M. Regulatory perspective on in vitro potency assays for human T cells used in anti-tumor immunotherapy. Cytotherapy 2018; 20: 601-622.
- Xu J, Melenhorst JJ, Fraietta JA. Toward precision manufacturing of immunogene T cell therapies. Cytotherapy 2018; 20: 623-638.
- Kasakovski D, Xu L, Yangqiu L. T cell senescence and CAR T cell exhaustion in hematological malignancies. J Hematol Oncology 2018; 11: 91-99.
- Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici J, Gohil M, Lundh S, et al. Determinants of response and resistence to CD19 CAR T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24(5): 563-571.
" ["~DETAIL_TEXT"]=> string(8652) "
Dear Editor,
I would like to comment a report by Dr. K. Beider "Senescent/exhausted phenotype of CD 19 – targeted CAR-T cells and induction of immuneregulatory environmemt correlate with reduced response to CAR T cell therapy in relapsed/refractory B cell malignancies" presented at the R. Gorbacheva Memorial Meeting in St. Petersburg at September 19, 2020 under Focus on Lymphomas (access: https://rgmm.info/translyacii/auditoriya-5).
The CD19-specific CAR T cells have shown significant antitumor activity in the treatment of high-risk relapsed and refractory leukemias and lymphomas and have revolutionized the treatment landscape for patients with advanced lymphoid malignancies. In 2018 two CAR T cell products were approved by the US and European regulatory authorities. Both Axicabtagene ciloleucel (Yescarta, Gilead/Kite) and tisagenlecleucel (Kymriah, Novartis) are now available for clinical use, based on the results of the ZUMA-1 (Yescarta) and JULIET (Kymriah) trials for refractory/relapsed relapsed/refractory aggressive B-cell lymphomas, and the ELIANA trial (Kymriah) for advanced acute lymphoblastic leukemia in patients up to 25 years of age.
Longer-term follow-up data from the lymphoma trials indicate durable responses for for the 40-60% of patients who achieved a complete response. Studies to better identify predictors of response are needed in order to improve the risk-benefit balance and minimize unnecessary financial burden for individual patients and healthcare systems. This could eventually result in those predicted to respond poorly to chemotherapy but well to CAR T cells receiving them upfront. Among the more commonly cited impediments for effective CAR T cell therapy are loss or modulation of CD 19 expression by the target cells, lack of CAR T cell persistence, as well as product manufacturing failures. Adequate T cell manufacturing is an essential component of CAR T cell therapy. Quantitative and qualitative characteristics of the apheresis product can affect the ability to successfully produce an efficient T cell product. Even if T cell numbers are adequate the starting T cell phenotype can be an important determinant of subsequent clinical activity.
In her presentation Dr. Katia Beider discussed the factors that can preclude durable remissions following CAR T cell therapy with a special focus on the role of T cell senescence and exhaustion phenotype as a cause for reduced response after adoptive cell therapy in patients with relapsed/refractory B cell lymphomas. She presented data on 22 patients (86% with B cell non-Hodgkin’s lymphomas) that were treated at Sheba Medical Center in Tel Hashomer (Israel) with an house-made CAR T cell construct composed of an anti-CD19 single-chain Fv FMC63, CD28 co-stimulatory and CD3-zeta intracellular domain.
For each patient samples were collected after apheresis, but before lymphodepletion, from the CAR T cell infused product and on days 7, 14, 21, 30 and 60 after infusion. Clinical responses were evaluated at day 28, with 11 patients (50%) achieving complete response; 2 patients developed partial response (9%), and 9 patients (41%) had progressive disease. The manufactured product contained high-purity CD3 + T cells, composed with CD4+ and CD8+ T cells. The frequency of exhausted T cells, phenotypically defined as CD28 negative, with upregulation of CD57 and CD39 molecules, was analysed in the CAR T cell products. A significant increase of exhausted CD8 T cells was observed in the product infused in patients with progressive disease when compared to patients with clinical response.
The investigators then asked the question whether the exhausted T cells were already present in the peripheral blood of these patients, or developed following genetic transduction and in vitro expansion. Responding patients had very low levels of exhausted cells in the blood and the final product also had a very low frequency of these cells. In contrast, two groups were seen among the patients with progressive disease: patients who had increased numbers of exhausted T cells in their original pool, that did not change in the final product, and the patients that had low number of these cells in the initial product, but increased during the transduction and expansion procedures, showing that these cells can be induced during the manufacturing process.
Interestingly, the exhausted phenotype in CD8+ T cells was associated with an increase in memory phenotype acquisition in CAR T products; in contrast, naïve T cells were low in non-responders as compared to responders, suggesting that less differentiated phenotypes were associated with better responses.
They also showed an increased expression of the CCR7 chemokine on CD4+ and CD8+ CAR T cells from responding patients, with increased trafficking of these cells to lymphoid tissue. Finally, they studied the PD-1 expression in CD8+ cells in the CAR T product and found a significant correlation of an increased expression with the exhausted phenotype.
These results suggest that the starting T cell phenotype in the apheresis product and after expansion and transduction may influence the subsequent clinical activity of the CAR T cell product and elucidate in part the mechanisms of cell trafficking and efficacy.
The data also suggest that combining CAR T cell therapy with immune checkpoint inhibitors may improve the effectiveness of the treatment, and pre-clinical data support such a synergetic approach [1]. However, strategies that enhance the potency of these products run the risk of inadvertently worsening the already considerable toxic effects.
It would have been interesting to know whether these results have any correlation with the number of previous lines of chemotherapy given to patients before collecting the T cells, and the time from diagnosis to treatment since persistent antigen exposure in cancer has been proposed to lead to cytotoxic T lymphocyte exhaustion.
These results were obtained in patients with B cell lymphomas and contrast with those in patients with B-ALL in whom standard parameters that define T cell potency, such as markers of T cell exhaustion, have been disappointing in terms of predicting clinical efficacy [2-5]. In patients with CLL, however, among whom response rates have been substantially lower than those in patients with ALL or lymphoma, product characteristics, such as enrichment for IL-6-STAT3 signatures, and elevated frequency of CD29+CD45RO-CD8+ T cells before CAR T cell generation, were able to identify a favourable response [6].
Additional data will be required to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.
Conflict of interest
None declared.
References
- Lin AM, Hucks GE, Dinofia AM, Seif AE, Teachey DT, Baniewicz D, et al. Checkpoint inhibitors augment CD19 directed CAR T cell therapy in relapsed B-cell acute lymphoblastic leukemia. Blood 2018; 132 (Suppl 1): 556.
- Shah N, Fry T. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol 2019; 16: 372-385.
- de Wolf C, van den Bovenkamp M, Hoefnagel M. Regulatory perspective on in vitro potency assays for human T cells used in anti-tumor immunotherapy. Cytotherapy 2018; 20: 601-622.
- Xu J, Melenhorst JJ, Fraietta JA. Toward precision manufacturing of immunogene T cell therapies. Cytotherapy 2018; 20: 623-638.
- Kasakovski D, Xu L, Yangqiu L. T cell senescence and CAR T cell exhaustion in hematological malignancies. J Hematol Oncology 2018; 11: 91-99.
- Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici J, Gohil M, Lundh S, et al. Determinants of response and resistence to CD19 CAR T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24(5): 563-571.
" ["DETAIL_TEXT_TYPE"]=> string(4) "html" ["~DETAIL_TEXT_TYPE"]=> string(4) "html" ["PREVIEW_TEXT"]=> string(0) "" ["~PREVIEW_TEXT"]=> string(0) "" ["PREVIEW_TEXT_TYPE"]=> string(4) "text" ["~PREVIEW_TEXT_TYPE"]=> string(4) "text" ["PREVIEW_PICTURE"]=> NULL ["~PREVIEW_PICTURE"]=> NULL ["LANG_DIR"]=> string(4) "/ru/" ["~LANG_DIR"]=> string(4) "/ru/" ["SORT"]=> string(2) "10" ["~SORT"]=> string(2) "10" ["CODE"]=> string(66) "ne-vse-t-kletki-sozdany-odinakovymi-kommentariy-k-dokladu-k-bayder" ["~CODE"]=> string(66) "ne-vse-t-kletki-sozdany-odinakovymi-kommentariy-k-dokladu-k-bayder" ["EXTERNAL_ID"]=> string(4) "1946" ["~EXTERNAL_ID"]=> string(4) "1946" ["IBLOCK_TYPE_ID"]=> string(7) "journal" ["~IBLOCK_TYPE_ID"]=> string(7) "journal" ["IBLOCK_CODE"]=> string(7) "volumes" ["~IBLOCK_CODE"]=> string(7) "volumes" ["IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["~IBLOCK_EXTERNAL_ID"]=> string(1) "2" ["LID"]=> string(2) "s2" ["~LID"]=> string(2) "s2" ["EDIT_LINK"]=> NULL ["DELETE_LINK"]=> NULL ["DISPLAY_ACTIVE_FROM"]=> string(0) "" ["IPROPERTY_VALUES"]=> array(18) { ["ELEMENT_META_TITLE"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["ELEMENT_META_KEYWORDS"]=> string(0) "" ["ELEMENT_META_DESCRIPTION"]=> string(159) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)Not all T cells are created alike!" ["ELEMENT_PREVIEW_PICTURE_FILE_ALT"]=> string(1507) "<p style="text-align: justify;">Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">CAR-T клетки, фенотип истощения, лимфомы, терапия.</p> " ["ELEMENT_PREVIEW_PICTURE_FILE_TITLE"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["ELEMENT_DETAIL_PICTURE_FILE_ALT"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["ELEMENT_DETAIL_PICTURE_FILE_TITLE"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_META_TITLE"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_META_KEYWORDS"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_META_DESCRIPTION"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_PICTURE_FILE_ALT"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_PICTURE_FILE_TITLE"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_PICTURE_FILE_NAME"]=> string(70) "ne-vse-t-kletki-sozdany-odinakovymi-kommentariy-k-dokladu-k-bayder-img" ["SECTION_DETAIL_PICTURE_FILE_ALT"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_DETAIL_PICTURE_FILE_TITLE"]=> string(125) "Не все Т-клетки созданы одинаковыми (комментарий к докладу К. Байдер)" ["SECTION_DETAIL_PICTURE_FILE_NAME"]=> string(70) "ne-vse-t-kletki-sozdany-odinakovymi-kommentariy-k-dokladu-k-bayder-img" ["ELEMENT_PREVIEW_PICTURE_FILE_NAME"]=> string(70) "ne-vse-t-kletki-sozdany-odinakovymi-kommentariy-k-dokladu-k-bayder-img" ["ELEMENT_DETAIL_PICTURE_FILE_NAME"]=> string(70) "ne-vse-t-kletki-sozdany-odinakovymi-kommentariy-k-dokladu-k-bayder-img" } ["FIELDS"]=> array(1) { ["IBLOCK_SECTION_ID"]=> string(3) "172" } ["PROPERTIES"]=> array(18) { ["KEYWORDS"]=> array(36) { ["ID"]=> string(2) "19" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:46:01" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(27) "Ключевые слова" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "KEYWORDS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "19" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "4" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "Y" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "Y" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(27) "Ключевые слова" ["~DEFAULT_VALUE"]=> string(0) "" } ["SUBMITTED"]=> array(36) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27420" ["VALUE"]=> string(22) "11/23/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/23/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL } ["ACCEPTED"]=> array(36) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27421" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL } ["PUBLISHED"]=> array(36) { ["ID"]=> string(2) "22" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Дата публикации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "PUBLISHED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "22" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Дата публикации" ["~DEFAULT_VALUE"]=> NULL } ["CONTACT"]=> array(36) { ["ID"]=> string(2) "23" ["TIMESTAMP_X"]=> string(19) "2015-09-03 14:43:05" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(14) "Контакт" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "CONTACT" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "23" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(14) "Контакт" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHORS"]=> array(36) { ["ID"]=> string(2) "24" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:45:07" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "AUTHORS" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "E" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "Y" ["XML_ID"]=> string(2) "24" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "3" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(13) "EAutocomplete" ["USER_TYPE_SETTINGS"]=> array(9) { ["VIEW"]=> string(1) "E" ["SHOW_ADD"]=> string(1) "Y" ["MAX_WIDTH"]=> int(0) ["MIN_HEIGHT"]=> int(24) ["MAX_HEIGHT"]=> int(1000) ["BAN_SYM"]=> string(2) ",;" ["REP_SYM"]=> string(1) " " ["OTHER_REP_SYM"]=> string(0) "" ["IBLOCK_MESS"]=> string(1) "N" } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> bool(false) ["VALUE"]=> bool(false) ["DESCRIPTION"]=> bool(false) ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> bool(false) ["~DESCRIPTION"]=> bool(false) ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_RU"]=> array(36) { ["ID"]=> string(2) "25" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Авторы" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "25" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27422" ["VALUE"]=> array(2) { ["TEXT"]=> string(521) "<p>Мануэль Абекасис </p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(509) "
Мануэль Абекасис
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_RU"]=> array(36) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27423" ["VALUE"]=> array(2) { ["TEXT"]=> string(526) "<p>Лиссабон, Португалия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(514) "Лиссабон, Португалия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["SUMMARY_RU"]=> array(36) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27424" ["VALUE"]=> array(2) { ["TEXT"]=> string(1507) "<p style="text-align: justify;">Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">CAR-T клетки, фенотип истощения, лимфомы, терапия.</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1451) "Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.
Ключевые слова
CAR-T клетки, фенотип истощения, лимфомы, терапия.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["DOI"]=> array(36) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27425" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-85-87" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-85-87" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" } ["AUTHOR_EN"]=> array(36) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27428" ["VALUE"]=> array(2) { ["TEXT"]=> string(503) "<p>Manuel Abecasis </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(491) "Manuel Abecasis
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["ORGANIZATION_EN"]=> array(36) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27429" ["VALUE"]=> array(2) { ["TEXT"]=> string(276) "<p>Lisboa, Portugal </p><br> <p><b>Correspondence</b><br> Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal<br> E-mail: mabecasis@ipolisboa.min-saude.pt</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(222) "Lisboa, Portugal
Correspondence
Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal
E-mail: mabecasis@ipolisboa.min-saude.pt
The comment concerns discussion of results reported by K. Beider on the clinical response rates in patients with resistant/refractory lymphomas treated with CAR-T cells, and the factors predicting therapeutic success, in particular, distinct role of T cell ageing and their exhaustion phenotype as a reason for decreased response after adoptive cellular therapy. The patients who responded to treatment, had low levels of exhausted cells in blood and final CAR T cell product. Additional studies are needed to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.
Keywords
CAR-T cells, exhaustion phenotype, lymphoma, treatment.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["NAME_EN"]=> array(36) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27426" ["VALUE"]=> string(34) "Not all T cells are created alike!" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(34) "Not all T cells are created alike!" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" } ["FULL_TEXT_RU"]=> array(36) { ["ID"]=> string(2) "42" ["TIMESTAMP_X"]=> string(19) "2015-09-07 20:29:18" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(23) "Полный текст" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(12) "FULL_TEXT_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "42" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(23) "Полный текст" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } } ["PDF_RU"]=> array(36) { ["ID"]=> string(2) "43" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF RUS" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_RU" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "43" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27427" ["VALUE"]=> string(4) "2357" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2357" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF RUS" ["~DEFAULT_VALUE"]=> string(0) "" } ["PDF_EN"]=> array(36) { ["ID"]=> string(2) "44" ["TIMESTAMP_X"]=> string(19) "2015-09-09 16:05:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(7) "PDF ENG" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(6) "PDF_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "F" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "44" ["FILE_TYPE"]=> string(18) "doc, txt, rtf, pdf" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27431" ["VALUE"]=> string(4) "2358" ["DESCRIPTION"]=> NULL ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(4) "2358" ["~DESCRIPTION"]=> NULL ["~NAME"]=> string(7) "PDF ENG" ["~DEFAULT_VALUE"]=> string(0) "" } ["NAME_LONG"]=> array(36) { ["ID"]=> string(2) "45" ["TIMESTAMP_X"]=> string(19) "2023-04-13 00:55:00" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(72) "Название (для очень длинных заголовков)" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "NAME_LONG" ["DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "45" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(80) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> NULL ["VALUE"]=> string(0) "" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(0) "" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(72) "Название (для очень длинных заголовков)" ["~DEFAULT_VALUE"]=> array(2) { ["TYPE"]=> string(4) "HTML" ["TEXT"]=> string(0) "" } } } ["DISPLAY_PROPERTIES"]=> array(10) { ["AUTHOR_EN"]=> array(37) { ["ID"]=> string(2) "37" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(6) "Author" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "AUTHOR_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "37" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27428" ["VALUE"]=> array(2) { ["TEXT"]=> string(503) "<p>Manuel Abecasis </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(491) "Manuel Abecasis
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(6) "Author" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(491) "Manuel Abecasis
" } ["SUMMARY_EN"]=> array(37) { ["ID"]=> string(2) "39" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Description / Summary" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "39" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27430" ["VALUE"]=> array(2) { ["TEXT"]=> string(871) "<p style="text-align: justify;">The comment concerns discussion of results reported by K. Beider on the clinical response rates in patients with resistant/refractory lymphomas treated with CAR-T cells, and the factors predicting therapeutic success, in particular, distinct role of T cell ageing and their exhaustion phenotype as a reason for decreased response after adoptive cellular therapy. The patients who responded to treatment, had low levels of exhausted cells in blood and final CAR T cell product. Additional studies are needed to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.</p> <h2>Keywords</h2> <p style="text-align: justify;">CAR-T cells, exhaustion phenotype, lymphoma, treatment.</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(815) "The comment concerns discussion of results reported by K. Beider on the clinical response rates in patients with resistant/refractory lymphomas treated with CAR-T cells, and the factors predicting therapeutic success, in particular, distinct role of T cell ageing and their exhaustion phenotype as a reason for decreased response after adoptive cellular therapy. The patients who responded to treatment, had low levels of exhausted cells in blood and final CAR T cell product. Additional studies are needed to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.
Keywords
CAR-T cells, exhaustion phenotype, lymphoma, treatment.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Description / Summary" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(815) "The comment concerns discussion of results reported by K. Beider on the clinical response rates in patients with resistant/refractory lymphomas treated with CAR-T cells, and the factors predicting therapeutic success, in particular, distinct role of T cell ageing and their exhaustion phenotype as a reason for decreased response after adoptive cellular therapy. The patients who responded to treatment, had low levels of exhausted cells in blood and final CAR T cell product. Additional studies are needed to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.
Keywords
CAR-T cells, exhaustion phenotype, lymphoma, treatment.
" } ["DOI"]=> array(37) { ["ID"]=> string(2) "28" ["TIMESTAMP_X"]=> string(19) "2016-04-06 14:11:12" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(3) "DOI" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(3) "DOI" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "28" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27425" ["VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-85-87" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-85-87" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(3) "DOI" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(37) "10.18620/ctt-1866-8836-2020-9-4-85-87" } ["NAME_EN"]=> array(37) { ["ID"]=> string(2) "40" ["TIMESTAMP_X"]=> string(19) "2015-09-03 10:49:47" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(4) "Name" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(7) "NAME_EN" ["DEFAULT_VALUE"]=> string(0) "" ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "80" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "40" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "Y" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> NULL ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27426" ["VALUE"]=> string(34) "Not all T cells are created alike!" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(34) "Not all T cells are created alike!" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(4) "Name" ["~DEFAULT_VALUE"]=> string(0) "" ["DISPLAY_VALUE"]=> string(34) "Not all T cells are created alike!" } ["ORGANIZATION_EN"]=> array(37) { ["ID"]=> string(2) "38" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:02:59" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(12) "Organization" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_EN" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "38" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27429" ["VALUE"]=> array(2) { ["TEXT"]=> string(276) "<p>Lisboa, Portugal </p><br> <p><b>Correspondence</b><br> Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal<br> E-mail: mabecasis@ipolisboa.min-saude.pt</p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(222) "Lisboa, Portugal
Correspondence
Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal
E-mail: mabecasis@ipolisboa.min-saude.pt
Lisboa, Portugal
Correspondence
Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal
E-mail: mabecasis@ipolisboa.min-saude.pt
Мануэль Абекасис
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(12) "Авторы" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(509) "Мануэль Абекасис
" } ["SUBMITTED"]=> array(37) { ["ID"]=> string(2) "20" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(21) "Дата подачи" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(9) "SUBMITTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "20" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27420" ["VALUE"]=> string(22) "11/23/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "11/23/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(21) "Дата подачи" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "11/23/2020 12:00:00 am" } ["ACCEPTED"]=> array(37) { ["ID"]=> string(2) "21" ["TIMESTAMP_X"]=> string(19) "2015-09-02 17:21:42" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(25) "Дата принятия" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(8) "ACCEPTED" ["DEFAULT_VALUE"]=> NULL ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "21" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(8) "DateTime" ["USER_TYPE_SETTINGS"]=> NULL ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27421" ["VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> string(22) "12/04/2020 12:00:00 am" ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(25) "Дата принятия" ["~DEFAULT_VALUE"]=> NULL ["DISPLAY_VALUE"]=> string(32) "12/04/2020 12:00:00 am" } ["SUMMARY_RU"]=> array(37) { ["ID"]=> string(2) "27" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(29) "Описание/Резюме" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(10) "SUMMARY_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "27" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27424" ["VALUE"]=> array(2) { ["TEXT"]=> string(1507) "<p style="text-align: justify;">Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.</p> <h2>Ключевые слова</h2> <p style="text-align: justify;">CAR-T клетки, фенотип истощения, лимфомы, терапия.</p> " ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(1451) "Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.
Ключевые слова
CAR-T клетки, фенотип истощения, лимфомы, терапия.
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(29) "Описание/Резюме" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(1451) "Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.
Ключевые слова
CAR-T клетки, фенотип истощения, лимфомы, терапия.
" } ["ORGANIZATION_RU"]=> array(37) { ["ID"]=> string(2) "26" ["TIMESTAMP_X"]=> string(19) "2015-09-02 18:01:20" ["IBLOCK_ID"]=> string(1) "2" ["NAME"]=> string(22) "Организации" ["ACTIVE"]=> string(1) "Y" ["SORT"]=> string(3) "500" ["CODE"]=> string(15) "ORGANIZATION_RU" ["DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["PROPERTY_TYPE"]=> string(1) "S" ["ROW_COUNT"]=> string(1) "1" ["COL_COUNT"]=> string(2) "30" ["LIST_TYPE"]=> string(1) "L" ["MULTIPLE"]=> string(1) "N" ["XML_ID"]=> string(2) "26" ["FILE_TYPE"]=> string(0) "" ["MULTIPLE_CNT"]=> string(1) "5" ["TMP_ID"]=> NULL ["LINK_IBLOCK_ID"]=> string(1) "0" ["WITH_DESCRIPTION"]=> string(1) "N" ["SEARCHABLE"]=> string(1) "N" ["FILTRABLE"]=> string(1) "N" ["IS_REQUIRED"]=> string(1) "N" ["VERSION"]=> string(1) "1" ["USER_TYPE"]=> string(4) "HTML" ["USER_TYPE_SETTINGS"]=> array(1) { ["height"]=> int(200) } ["HINT"]=> string(0) "" ["PROPERTY_VALUE_ID"]=> string(5) "27423" ["VALUE"]=> array(2) { ["TEXT"]=> string(526) "<p>Лиссабон, Португалия </p>" ["TYPE"]=> string(4) "HTML" } ["DESCRIPTION"]=> string(0) "" ["VALUE_ENUM"]=> NULL ["VALUE_XML_ID"]=> NULL ["VALUE_SORT"]=> NULL ["~VALUE"]=> array(2) { ["TEXT"]=> string(514) "Лиссабон, Португалия
" ["TYPE"]=> string(4) "HTML" } ["~DESCRIPTION"]=> string(0) "" ["~NAME"]=> string(22) "Организации" ["~DEFAULT_VALUE"]=> array(2) { ["TEXT"]=> string(0) "" ["TYPE"]=> string(4) "HTML" } ["DISPLAY_VALUE"]=> string(514) "Лиссабон, Португалия
" } } } }12/30/2020

Kulagin A. D. (St. Petersburg, Russia)
Wagemaker G. (Rotterdam, Netherlands)
Zander A. R. (Hamburg, Germany)
Fehse B. (Hamburg, Germany)
Chukhlovin A. B. (St. Petersburg, Russia)
Aleynikova O. V. (Minsk, Republic of Belarus)
Borset M. (Trondheim, Norway)
Chechetkin A. V. (St. Petersburg, Russia)
Fibbe W. (Leiden, Netherlands)
Galibin O. V. (St. Petersburg, Russia)
Hölzer D. (Frankfurt a.M., Germany)
Klimko N. N. (St. Petersburg, Russia)
Kolb H.-J. (München, Germany)
Kröger N. (Hamburg, Germany)
Lange C. (Hamburg, Germany)
Mamaev N. N. (St. Petersburg, Russia)
Mikhailova N. B. (St. Petersburg, Russia)
Moiseev I. S. (St. Petersburg, Russia)
Nagler A. (Tel-Aviv, Israel)
Nemkov A. S. (St. Petersburg, Russia)
Paramonov I. V. (Kirov, Russia)
Roumiantsev A. G. (Moscow, Russia)
Savchenko V. G. (Moscow, Russia)
Smirnov A. V. (St. Petersburg, Russia)
Uss A. L. (Minsk, Republic of Belarus)
Zubarovskaya L. S. (St. Petersburg, Russia)
Dear CTT authors and readers,
Despite great advances made in targeted treatments of blood cancer, we are still facing a lot of cases, in which these methods are not efficient or safe enough. Аllogeneic hematopoietic stem cell transplantation (allo-HSCT), an old method with proven efficacy, is still required to change the course of the disease. For this, chronic myeloid leukemia (CML) may be the most obvious showcase.
For the last 150 years CML has been among most studied hematological malignancies. It was the first hematological disorder with marker chromosomal aberration, later a bcr/abl fusion gene, was identified as the main mechanism of disease development. Imatinib, the first clinically used tyrosine kinase inhibitor (TKI), proved to be remarkably effective for long-term CML control, and now TKIs have largely replaced HSCT, which was the only curative treatment method prior to their introduction into clinical practice. Presently, the survival of most patients with CML is the same as in general population. However, there are still some cases, in which common TKI therapy is ineffective. In some of them, 2nd and 3rd generation TKIs may be a solution. However, in acceleration phase (AP) and blast crisis (BC), HSCT still remain the method of choice.
An evolution of therapeutic approaches to CML is well illustrated by a review of current European LeukemiaNet (ELN) recommendations presented in this issue of CTT by the head of ELN, Prof. Dr. R. Hehlmann, who is one of the persons defining general strategy of CML treatment. This review specifically concerns second and higher-line TKI therapy in CML based on detection of additional chromosomal aberrations, minimal residual disease levels, previous therapies, and age-dependent comorbidities. These factors are also assessed to determine an individual risk for potential allo-HSCT recipients, when this treatment option is considered.
Also, this issue contains a description of single-center experience of HSCT and targeted therapies in patients with advanced-stage CML presented by Dr. Morozova E. et al. In this extensive cohort containing mainly patients with very unfavorable disease allo-HSCT still has an evident advantage over conservative approaches. Thus, it appears to be a positive answer to the key question posed by Prof. Gale: "Is there a future for hematopoietic cell transplantation?"
Transplantation aspects of blood malignancies are in focus of Cellular Therapy and Transplantation. Our potential authors from different clinics worldwide are invited to present their experience in novel treatments in hematological disorders, including immune and targeted therapy, role of HSCT (including genetically modified stem cells) in malignant and nonmalignant disorders.
Professor Alexander D. Kulagin, Editor-in-Chief, Cellular Therapy and Transplantation Journal
Keynote
Robert P. Gale
Review articles
Clinical studies
Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, Boris V. Afanasyev
Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary
Olesya V. Paina1, Irina E. Pavlova1,2, Natalia E. Ivanova1, Alexander L. Alyanskiy1, Tatiana A. Bykova1, Ludmila S. Zubarovskaya1, Alexander D. Kulagin1, Boris V. Afanasyev1
Igor Novitzky-Basso1, Eshrak Al-Shaibani1, Mats Remberger2, Carol Chen1, Wilson Lam1, Arjun D. Law1, Ivan Pasic1, Fotios V. Michelis1, Auro Viswabandya1, Dennis D. Kim1, Jeffrey H. Lipton1, Armin Gerbitz1, Jonas Mattsson1, Rajat Kumar1, Zeyad Al-Shaibani1
Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Boris V. Afanasyev
Experimental studies
Maria V. Konovalova1, Daria S. Tsaregorodtseva1,2, Rimma A. Poltavtseva3, Elena V. Svirshchevskaya1,3
Pavel V. Popryadukhin1, Natalia N. Sudareva1,2, Оlga М. Suvorova1, Galina Yu. Yukina2, Еlena G. Sukhorukova2, Natalia N. Saprykina1, Ilya A. Barsuk3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
Hana Dennaoui1, Eliane Chouery2, Chaza Harmoush1
Letter to the Editor
Manuel Abecasis
Keynote
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27299
[VALUE] => 10/02/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/02/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27300
[VALUE] => 10/23/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/23/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27301
[VALUE] => Array
(
[TEXT] => <p>Роберт П. Гэйл </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Роберт П. Гэйл
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27302
[VALUE] => Array
(
[TEXT] => <p>Центр гематологии, Департамент иммунологии и воспаления, Имперский коледж Лондон, Великобритания</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Центр гематологии, Департамент иммунологии и воспаления, Имперский коледж Лондон, Великобритания
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27303
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен</p>
<p style="text-align: justify;">Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ). </p>
<p style="text-align: justify;">Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.</p>
<p style="text-align: justify;">Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.</p>
<p style="text-align: justify;">Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Нынешние успехи противоопухолевой терапии, таргетной и иммунотерапии поднимают вопрос о том, есть ли будущее у трансплантации гемопоэтических клеток (ТГСК). Я обсуждаю важность этого, но, в итоге, масштаб этих усовершенствований пока скромен
Я подчеркиваю, что эффективность иммунной терапии ограничена, преимущественно, В-клеточными опухолями, и что у многих, если не большинства больных, успешно леченных посредством иммунотерапии, может выполняться аллогенная ТГСК, особенно при остром лимфобластном лейкозе (ОЛЛ).
Я также обсуждаю то, что большинство аллотрансплантаций проводятся по поводу злокачественных заболеваний, которые не лечатся существующей иммунотерапией.
Рандомизированные испытания показывают, что аутотрансплантация – лучше, чем новые препараты при лечении молодых пациентов с плазмаклеточной миеломой. Значительное число данных указывает на то, что эффективность аллотрансплантации обусловлена не опухольспецифическими аллогенными эффектами, которые, очевидно, не связаны с существующей иммунотерапией.
Наконец, я обсуждаю роль применения Т-клеток с донорским химерным антигенным рецептором (CAR-T) у лиц, рецидивирующих после алло-ТГСК при В-клеточных опухолях. В итоге можно предполагать и в последующем значительную роль трансплантации гемопоэтических клеток в различных клинических ситуациях.
Ключевые слова
Трансплантация гемопоэтических стволовых клеток, таргетные препараты, иммунная терапия, CAR-T клетки, эффективность.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27304
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-6-10
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-6-10
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27307
[VALUE] => Array
(
[TEXT] => <p>Robert P. Gale </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Robert P. Gale
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27308
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK</p><br>
<p><b>Correspondence</b><br>
<p style="text-align: justify;">
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK<br>
Phone: +1-908-656-0484<br>
Fax: +1-310-388-1320<br>
E-mail: robertpetergale@alumni.ucla.edu</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Correspondence
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Phone: +1-908-656-0484
Fax: +1-310-388-1320
E-mail: robertpetergale@alumni.ucla.edu
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27309
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Recent advances in anti-cancer chemotherapy and in targeted and immune therapies raise the question whether there is a future for haematopoietic cell transplants. I discuss their importance but in the end the magnitude of these improvements is modest. I point out the efficacy of immune therapy is predominately restricted to B-cell cancers and that many if not most successful immune therapy recipients eventually receive an allogeneic haematopoietic cell transplant, especially those with acute lymphoblastic leukaemia (ALL).</p>
<p style="text-align: justify;">I also discuss most allotransplants are done for cancers not treated with current immune therapy. Randomized trials show an autotransplant is better than new drugs in young persons with plasma cell myeloma. Considerable data indicate much of the efficacy of allotransplants results from a non-cancer-specific allogeneic effect not expected to operate with current immune therapies. Lastly, I discuss a role for donor-derived chimeric antigen receptor (CAR)-T-cells in persons relapsing after an allotransplant for B-cell cancers. The sum of these considerations suggest an ongoing role for haematopoietic cell transplants in diverse settings.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Hematopoietic stem cell transplantation, targeted drugs, immune therapy, (CAR)-T cells, efficiency.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Recent advances in anti-cancer chemotherapy and in targeted and immune therapies raise the question whether there is a future for haematopoietic cell transplants. I discuss their importance but in the end the magnitude of these improvements is modest. I point out the efficacy of immune therapy is predominately restricted to B-cell cancers and that many if not most successful immune therapy recipients eventually receive an allogeneic haematopoietic cell transplant, especially those with acute lymphoblastic leukaemia (ALL).
I also discuss most allotransplants are done for cancers not treated with current immune therapy. Randomized trials show an autotransplant is better than new drugs in young persons with plasma cell myeloma. Considerable data indicate much of the efficacy of allotransplants results from a non-cancer-specific allogeneic effect not expected to operate with current immune therapies. Lastly, I discuss a role for donor-derived chimeric antigen receptor (CAR)-T-cells in persons relapsing after an allotransplant for B-cell cancers. The sum of these considerations suggest an ongoing role for haematopoietic cell transplants in diverse settings.
Keywords
Hematopoietic stem cell transplantation, targeted drugs, immune therapy, (CAR)-T cells, efficiency.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27305
[VALUE] => Is there a Future for Haematopoietic Cell Transplants?
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Is there a Future for Haematopoietic Cell Transplants?
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27306
[VALUE] => 2285
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2285
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27310
[VALUE] => 2286
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2286
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Robert P. Gale
Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Correspondence
Robert Peter Gale MD, PhD, DSc (hc), FACP, FRCPI (hon), FRSM, Haematology Research Centre, Department of Immunology and Inflammation Imperial College, London, UK
Phone: +1-908-656-0484
Fax: +1-310-388-1320
E-mail: robertpetergale@alumni.ucla.edu
Recent advances in anti-cancer chemotherapy and in targeted and immune therapies raise the question whether there is a future for haematopoietic cell transplants. I discuss their importance but in the end the magnitude of these improvements is modest. I point out the efficacy of immune therapy is predominately restricted to B-cell cancers and that many if not most successful immune therapy recipients eventually receive an allogeneic haematopoietic cell transplant, especially those with acute lymphoblastic leukaemia (ALL).
I also discuss most allotransplants are done for cancers not treated with current immune therapy. Randomized trials show an autotransplant is better than new drugs in young persons with plasma cell myeloma. Considerable data indicate much of the efficacy of allotransplants results from a non-cancer-specific allogeneic effect not expected to operate with current immune therapies. Lastly, I discuss a role for donor-derived chimeric antigen receptor (CAR)-T-cells in persons relapsing after an allotransplant for B-cell cancers. The sum of these considerations suggest an ongoing role for haematopoietic cell transplants in diverse settings.
Keywords
Hematopoietic stem cell transplantation, targeted drugs, immune therapy, (CAR)-T cells, efficiency.
Review articles
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27311
[VALUE] => 09/28/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 09/28/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27312
[VALUE] => 11/13/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/13/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27313
[VALUE] => Array
(
[TEXT] => <p>Рюдигер Хельманн </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Рюдигер Хельманн
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27314
[VALUE] => Array
(
[TEXT] => <p>Медицинский факультет Маннгейма, Гейдельбергский университет; Фонд ELN, Вайнхайм, Германия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Медицинский факультет Маннгейма, Гейдельбергский университет; Фонд ELN, Вайнхайм, Германия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27315
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Через 150 лет после, главным образом, паллиативной терапии хронического миелоидного лейкоза (ХМЛ), успехи лечения ингибиторами тирозинкиназы BCR-ABL1 (ИТК) привели к нормальным показателям выживаемости большинства пациентов с ХМЛ. Новой целью лечения является достижение ремиссии без лечения (РБЛ) с хорошим качеством жизни без пожизненной терапии. Европейская организация LeukemiaNet (ELN) учитывает эти разработки в своих свежих рекомендациях. Трансплантация гемопоэтических клеток (ТГСК) сохраняет важную роль в лечении пациентов с резистентностью или непереносимостью ИТК или прогрессированием заболевания в более агрессивную фазу. Данный обзор сосредоточен на рекомендациях ELN-2020 по лечению ХМЛ и ранней ТГСК у пациентов высокого риска.
Ключевые слова
Хронический миелоидный лейкоз, группы высокого риска, ингибиторы тирозинкиназы, трансплантация гемопоэтических стволовых клеток, рекомендации ELN.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27316
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-11-19
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-11-19
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27319
[VALUE] => Array
(
[TEXT] => <p>Rüdiger Hehlmann </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Rüdiger Hehlmann
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27320
[VALUE] => Array
(
[TEXT] => <p>Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany</p><br>
<p><b>Correspondence</b><br>
Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany<br>
E-mail: Hehlmann.eln@gmail.com</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
Correspondence
Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
E-mail: Hehlmann.eln@gmail.com
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27321
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">After 150 years of mostly palliative CML therapy, treatment advances with BCR-ABL1 tyrosine kinase inhibitors (TKI) have resulted in normal survival for most patients with CML. The new treatment goal is treatment-free remission (TFR) with survival at good quality of life without life-long treatment. The European LeukemiaNet (ELN) has accounted for this development with its most recent recommendations. Hematopoietic stem cell transplantation has retained an important role in patients who have become resistant or intolerant to all TKI or progress to advanced phases. This review focuses on the ELN 2020 recommendations for treating CML and on early transplantation in high-risk patients.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Chronic myeloid leukemia, high-risk group, tyrosine kinase inhibitors, hematopoietic stem cell transplantation, ELN recommendations.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => After 150 years of mostly palliative CML therapy, treatment advances with BCR-ABL1 tyrosine kinase inhibitors (TKI) have resulted in normal survival for most patients with CML. The new treatment goal is treatment-free remission (TFR) with survival at good quality of life without life-long treatment. The European LeukemiaNet (ELN) has accounted for this development with its most recent recommendations. Hematopoietic stem cell transplantation has retained an important role in patients who have become resistant or intolerant to all TKI or progress to advanced phases. This review focuses on the ELN 2020 recommendations for treating CML and on early transplantation in high-risk patients.
Keywords
Chronic myeloid leukemia, high-risk group, tyrosine kinase inhibitors, hematopoietic stem cell transplantation, ELN recommendations.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27317
[VALUE] => The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => The new ELN Recommendations for treating CML. Early transplantation in patients with high-risk ACA
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27318
[VALUE] => 2297
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2297
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27322
[VALUE] => 2298
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2298
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Rüdiger Hehlmann
Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
Correspondence
Prof. Dr. Rüdiger Hehlmann, Medical Faculty Mannheim, Heidelberg University; ELN Foundation Weinheim, Germany
E-mail: Hehlmann.eln@gmail.com
After 150 years of mostly palliative CML therapy, treatment advances with BCR-ABL1 tyrosine kinase inhibitors (TKI) have resulted in normal survival for most patients with CML. The new treatment goal is treatment-free remission (TFR) with survival at good quality of life without life-long treatment. The European LeukemiaNet (ELN) has accounted for this development with its most recent recommendations. Hematopoietic stem cell transplantation has retained an important role in patients who have become resistant or intolerant to all TKI or progress to advanced phases. This review focuses on the ELN 2020 recommendations for treating CML and on early transplantation in high-risk patients.
Keywords
Chronic myeloid leukemia, high-risk group, tyrosine kinase inhibitors, hematopoietic stem cell transplantation, ELN recommendations.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27333
[VALUE] => 10/22/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/22/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27334
[VALUE] => 11/13/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/13/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27339
[VALUE] => Array
(
[TEXT] => <p>Иван С. Моисеев, Анна А. Доценко, Анна Г. Смирнова, Юлия Ю. Власова, Елена В. Морозова, Сергей Н. Бондаренко, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Иван С. Моисеев, Анна А. Доценко, Анна Г. Смирнова, Юлия Ю. Власова, Елена В. Морозова, Сергей Н. Бондаренко, Борис В. Афанасьев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27340
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27341
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.</p>
<h3>Пациенты и методы</h3>
<p style="text-align: justify;">В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).</p>
<h3>Выводы</h3>
<p style="text-align: justify;">Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Хроническая реакция «трансплантат против хозяина» (хрРТПХ) является частым осложнением аллогенной трансплантации гемопоэтических стволовых клеток. Частота развития этого осложнения колеблется от 10% до 80% в зависимости от типа профилактики, типа донора и других факторов риска. Хотя хрРТПХ ассоциируется со сниженным риском рецидива, персистенция клинических симптомов связана с долгосрочной летальностью, частыми госпитализациями и инвалидностью. Несмотря на то, что существуют четкие критерии эффективности для клинических испытаний новых препаратов, определение тактики в клинической практике должно включать и долгосрочные цели, как при всех аутоиммунных заболеваниях. Пока нет единого мнения в отношении этих целей терапии. Анализируя результаты терапии хрРТПХ в большой когорте пациентов в рамках одноцентрового исследования, мы попытались сосредоточиться на предикторах долгосрочного прогноза и их связи с терапией.
Пациенты и методы
В исследование были включены 182 пациента с средней тяжестью и тяжелой хрРТПХ. Большинству пациентов была проведена аллогенная трансплантация по поводу злокачественных заболеваний, у 49% была тяжелая форма хрРТПХ, у 51% – проявления средней степени тяжести. Среднее время наблюдения составило 52 месяца. Помимо первой линии, 39,56% пациентов требовали дополнительного лечения.
Результаты
Через пять лет кумулятивная частота полных ремиссии составила 16,9% (95% ДИ 10,5-24,7%), а частота прекращения иммуносупрессивной терапии (ИСТ) без обострения РТПХ составила 51,2% (95% ДИ 40,0-61,2%). Основными предикторами отмены ИСТ были общая тяжесть хрРТПХ (HR 0,45, 95% ДИ 0,25-0,84, p=0,0049) и женщина донор для реципиента мужчины (HR 0,33, 95% CI 0,25-0,81, p=0,0370). Анализ частоты летальности без рецидива (ЛБР) показал, что прекращение ИСТ было основным предиктором ЛБР (2% против 42%, HR 0,03, 95% CI 0,01-0,15, p=0,0005). В конце периода наблюдения пациенты с полным ответом прекратили ИСТ в 91% случаев, с легкой формой РТПХ в 53% случаев, со средней тяжести в 24% случаев и с тяжелой в 2% случаев. Другими значимыми факторами для ЛБР были начало терапии без стероидов (HR 0,25, 95% ДИ 0,08-0,58, p=0,0035) и раннее использование терапии второй линии (HR 0,49, 95% CI 0,25-0,96, p=0,0322).
Выводы
Исследование продемонстрировало, что прекращение системной терапии ИСТ без обострения хронической РТПХ должно быть основной целью терапии. Кроме того, исследование указывает на обоснованность рандомизированных исследований новых методов второй линии не с глюкокортикостероидами в первой линии, а против них.
Ключевые слова
Хроническая реакция трансплантат против хозяина, терапия, долгосрочные результаты, цели терапии.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27335
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-29-36
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-29-36
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27343
[VALUE] => Array
(
[TEXT] => <p>Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, Boris V. Afanasyev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27344
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7(812) 338 6259, +7 (921) 796 1951<br>
Fax: +7(812) 338 6263<br>
E-mail: moisiv@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(812) 338 6259, +7 (921) 796 1951
Fax: +7(812) 338 6263
E-mail: moisiv@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27345
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Chronic graft-versus-host disease (cGvHD) is a common complication of allogeneic hematopoietic stem cell transplantation. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and other risk factors. Although cGvHD is associated with reduced risk of relapse, the persistence of clinical signs is associated with long-term mortality, morbidity and disability. Despite there are clear endpoints for the clinical trials of novel agents, the choices in clinical practice should involve long-term goals like in all autoimmune disease. So far, there is no consensus on these goals. Analyzing the results of cGvHD therapy in the large single-center cohort of patients we tried to focus on predictors of long-term prognosis and their association with therapy.</p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">The study included 182 patients with moderate and severe cGvHD. The majority of patients were allografted for malignant diseases and 49% had severe cGvHD, 51% – moderate disease. Median follow up time was 52 months. Beyond the first line 39.56% of patients required additional treatment.</p>
<h3>Results</h3>
<p style="text-align: justify;">At five years the cumulative incidence of complete responses was 16.9% (95% CI 10.5-24.7%) and immunosuppressive therapy (IST) discontinuation without GvHD flare was 51.2% (95% CI 40.0-61.2%). The major predictors of IST discontinuation were overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The analysis of non-relapse mortality (NRM) demonstrated that discontinuation of IST was the major predictor (2% <i>vs</i> 42%, HR 0.03, 95%CI 0.01-0.15, p=0.0005). At the end of the follow up patients with complete response discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases. The other significant factors for NRM were steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and early use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322). In conclusion, the study demonstrated that discontinuation of systemic IST therapy without the flare of cGvHD should be the goal of therapy. Also the study creates a rationale for randomized studies of novel second-line options not with but against steroids in the first line. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Chronic graft-versus-host disease, therapy, long-term outcomes, goals of therapy.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Chronic graft-versus-host disease (cGvHD) is a common complication of allogeneic hematopoietic stem cell transplantation. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and other risk factors. Although cGvHD is associated with reduced risk of relapse, the persistence of clinical signs is associated with long-term mortality, morbidity and disability. Despite there are clear endpoints for the clinical trials of novel agents, the choices in clinical practice should involve long-term goals like in all autoimmune disease. So far, there is no consensus on these goals. Analyzing the results of cGvHD therapy in the large single-center cohort of patients we tried to focus on predictors of long-term prognosis and their association with therapy.
Patients and methods
The study included 182 patients with moderate and severe cGvHD. The majority of patients were allografted for malignant diseases and 49% had severe cGvHD, 51% – moderate disease. Median follow up time was 52 months. Beyond the first line 39.56% of patients required additional treatment.
Results
At five years the cumulative incidence of complete responses was 16.9% (95% CI 10.5-24.7%) and immunosuppressive therapy (IST) discontinuation without GvHD flare was 51.2% (95% CI 40.0-61.2%). The major predictors of IST discontinuation were overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The analysis of non-relapse mortality (NRM) demonstrated that discontinuation of IST was the major predictor (2% vs 42%, HR 0.03, 95%CI 0.01-0.15, p=0.0005). At the end of the follow up patients with complete response discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases. The other significant factors for NRM were steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and early use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322). In conclusion, the study demonstrated that discontinuation of systemic IST therapy without the flare of cGvHD should be the goal of therapy. Also the study creates a rationale for randomized studies of novel second-line options not with but against steroids in the first line.
Keywords
Chronic graft-versus-host disease, therapy, long-term outcomes, goals of therapy.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27336
[VALUE] => Long-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Long-term goals in the treatment of chronic graft-versus-host disease after matched allogeneic hematopoietic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27342
[VALUE] => 2320
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2320
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27346
[VALUE] => 2321
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2321
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Ivan S. Moiseev, Anna A. Dotsenko, Anna G. Smirnova, Yulia Yu. Vlasova, Elena V. Morozova, Sergey N. Bondarenko, Boris V. Afanasyev
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Ivan S. Moiseev, PhD, MD. RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(812) 338 6259, +7 (921) 796 1951
Fax: +7(812) 338 6263
E-mail: moisiv@mail.ru
Chronic graft-versus-host disease (cGvHD) is a common complication of allogeneic hematopoietic stem cell transplantation. Its incidence varies from 10% to 80% according to the type of prophylaxis, type of the donor and other risk factors. Although cGvHD is associated with reduced risk of relapse, the persistence of clinical signs is associated with long-term mortality, morbidity and disability. Despite there are clear endpoints for the clinical trials of novel agents, the choices in clinical practice should involve long-term goals like in all autoimmune disease. So far, there is no consensus on these goals. Analyzing the results of cGvHD therapy in the large single-center cohort of patients we tried to focus on predictors of long-term prognosis and their association with therapy.
Patients and methods
The study included 182 patients with moderate and severe cGvHD. The majority of patients were allografted for malignant diseases and 49% had severe cGvHD, 51% – moderate disease. Median follow up time was 52 months. Beyond the first line 39.56% of patients required additional treatment.
Results
At five years the cumulative incidence of complete responses was 16.9% (95% CI 10.5-24.7%) and immunosuppressive therapy (IST) discontinuation without GvHD flare was 51.2% (95% CI 40.0-61.2%). The major predictors of IST discontinuation were overall severity of cGvHD (HR 0.45, 95%CI 0.25-0.84, p=0.0049) and female donor for male recipient (HR 0.33, 95%CI 0.25-0.81, p= 0.0370). The analysis of non-relapse mortality (NRM) demonstrated that discontinuation of IST was the major predictor (2% vs 42%, HR 0.03, 95%CI 0.01-0.15, p=0.0005). At the end of the follow up patients with complete response discontinued IST in 91% of cases, with mild cGvHD in 53% of cases, with moderate in 24% of cases and with severe in 2% of cases. The other significant factors for NRM were steroid-free starting therapy (HR 0.25, 95%CI 0.08-0.58, p=0.0035) and early use of second-line therapy (HR 0.49, 95%CI 0.25-0.96, p=0.0322). In conclusion, the study demonstrated that discontinuation of systemic IST therapy without the flare of cGvHD should be the goal of therapy. Also the study creates a rationale for randomized studies of novel second-line options not with but against steroids in the first line.
Keywords
Chronic graft-versus-host disease, therapy, long-term outcomes, goals of therapy.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27359
[VALUE] => 11/09/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/09/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27360
[VALUE] => 12/04/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/04/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27361
[VALUE] => Array
(
[TEXT] => <p>Петр Седлачек, Петра Кеслова, Петр Смишек, Мартина Сукова, Марцела Маликова, Спирос Тавандзис, Ярослав Чермак, Ян Стари</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Петр Седлачек, Петра Кеслова, Петр Смишек, Мартина Сукова, Марцела Маликова, Спирос Тавандзис, Ярослав Чермак, Ян Стари
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27362
[VALUE] => Array
(
[TEXT] => <p>Департамент детской гематологии и онкологии, 2-я медицинская школа Карлова Университета, университетский госпиталь Мотол, Прага, Чешская республика</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Департамент детской гематологии и онкологии, 2-я медицинская школа Карлова Университета, университетский госпиталь Мотол, Прага, Чешская республика
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27363
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.</p>
<h3>Методы</h3>
<p style="text-align: justify;">Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB –
у двух сиблингов.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками. </p>
<h3>Выводы</h3>
<p style="text-align: justify;">ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Анемия Фанкони (АФ) – редкий синдром с гетерогенной картиной, сочетающийся с недостаточностью костного мозга и повышенным риском злокачественных новообразований. Хотя АФ часто характеризуется наличием пороков развития, у некоторых пациентов единственным признаком может быть цитопения. В нашей когорте больных диагностика основывалась на повышенной ломкости хромосом с последующим подтверждением генных мутаций.
Методы
Наша когорта включает 35 пробандов в возрасте от 0 до 24,3 (медиана – 6) лет, у которых была диагностирована АФ в сроки с января 1986 до августа 2020 г. Врожденные аномалии на момент диагноза наблюдались у 5 и цитопения – у 22 пациентов; 8 больных имели семейный анамнез АФ. Генетическое тестирование подтвердило мутации гена FANCA в 24 случаях, FANCG – в 3, FANCD1 – у 4 пациентов и FANCB –
у двух сиблингов.
Результаты
По мере наблюдения, у 7 пациентов развились злокачественные новообразования (в том числе – у 4 больных с мутацией FANCD1). У 17 пациентов развилась костномозговая недостаточность, в связи с чем 15 больным была выполнена трансплантация гемопоэтических стволовых клеток (ТГСК) в возрасте от 4,6 до 24,3 лет (медиана – 9,3 года). У всех трансплантированных пациентов было достигнуто стабильное гемопоэтическое приживление. Однако у 2 больных, в связи с неадекватным восстановлением иммунитета, через 12 и 14 мес. после ТГСК развились, соответственно, цитомегаловирусная пневмония и инвазивный аспергиллез со смертельными исходами. В одном случае мы диагностировали аденокарциному кишечника через 10 лет и сквамозноклеточную карциному языка через 13 лет после ТГСК. Тринадцать пациентов живы при среднем сроке наблюдения после ТГСК 10,6 (0,3-15,1) лет. При сроках наблюдения с медианой 12,6 (0,2-34,4) лет, 28/35 пациентов (80%) живы, 4 погибли от злокачественных новообразований, двое умерли от осложнений ТГСК, и один больной – в связи с тяжелыми врожденными соматическими пороками.
Выводы
ТГСК эффективна у пациентов с АФ и костномозговой недостаточностью и предотвращает дальнейшее развитие гематологических осложнений. Необходимо пожизненное и тщательное мультидисциплинарное наблюдение пациентов с АФ для раннего выявления костномозговой недостаточности или злокачественного заболевания.
Ключевые слова
Анемия Фанкони, соматические аномалии, генные мутации, костномозговая недостаточность, солидные опухоли, трансплантация гемопоэтических стволовых клеток, амбулаторное долгосрочное наблюдение.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27364
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-48-52
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-48-52
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27367
[VALUE] => Array
(
[TEXT] => <p>Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27368
[VALUE] => Array
(
[TEXT] => <p>Department of Pediatric Hematology and Oncology, 2<sup>nd</sup> Medical School at Charles University, University Hospital Motol, Prague, Czech Republic</p><br>
<p><b>Correspondence</b><br>
Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic<br>
Phone: +420 22443 6552<br>
Fax: +420 22443 6519<br>
E-mail: petr.sedlacek@lfmotol.cuni.cz</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, Prague, Czech Republic
Correspondence
Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic
Phone: +420 22443 6552
Fax: +420 22443 6519
E-mail: petr.sedlacek@lfmotol.cuni.cz
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27369
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Fanconi anemia (FA) is a rare and heterogeneous syndrome associated with bone marrow failure and increased risk of cancer. While FA is often characterized by the presence of congenital malformations, in some patients cytopenia may be the only sign. In our cohort diagnosis was based on evidence of increased chromosome fragility with subsequent confirmation by gene mutation detection. </p>
<h3>Methods</h3>
<p style="text-align: justify;">Our cohort includes 35 probands diagnosed with FA aged 0-24.3 (median 6) years between January 1986 and August 2020. Congenital anomalies at diagnosis were seen in 5 and cytopenia in 22 patients, 8 patients had family history of FA. Genetic test confirmed FANCA gene mutations in 24, FANCG in 3, FANCD1 in 4, and FANCB in 2 siblings.</p>
<h3>Results</h3>
<p style="text-align: justify;">During follow-up 7 patients developed malignancy (among them all 4 patients with FANCD1 mutation). Seventeen patients developed marrow failure, for which 15 patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the median age of 9.3 (4.6-24.3) years. All transplanted patients achieved stable hematopoietic engraftment. However, in 2 patients due to inadequate immune reconstitution developed fatal CMV pneumonia and invasive aspergillosis 12 and 14 months post HSCT, accordingly. In one patient we have diagnosed adenocarcinoma of the gut 10 years and squamous cell carcinoma of tongue 13 years after HSCT. Thirteen patients are alive with a median follow-up of 10.6 (0.3 – 15.1 years) years after HSCT. With a median follow-up till the last visit of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died due to HSCT-related complications, and one due to severe congenital somatic defects.</p>
<h3>Conclusions</h3>
<p style="text-align: justify;">HSCT is effective in FA patients with bone marrow failure and prevents further development of hematological malignancies. A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Fanconi anemia, somatic anomalies, gene mutations, bone marrow failure, solid tumors, hematopoietic stem cell transplantation, long-term outpatient observation.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Fanconi anemia (FA) is a rare and heterogeneous syndrome associated with bone marrow failure and increased risk of cancer. While FA is often characterized by the presence of congenital malformations, in some patients cytopenia may be the only sign. In our cohort diagnosis was based on evidence of increased chromosome fragility with subsequent confirmation by gene mutation detection.
Methods
Our cohort includes 35 probands diagnosed with FA aged 0-24.3 (median 6) years between January 1986 and August 2020. Congenital anomalies at diagnosis were seen in 5 and cytopenia in 22 patients, 8 patients had family history of FA. Genetic test confirmed FANCA gene mutations in 24, FANCG in 3, FANCD1 in 4, and FANCB in 2 siblings.
Results
During follow-up 7 patients developed malignancy (among them all 4 patients with FANCD1 mutation). Seventeen patients developed marrow failure, for which 15 patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the median age of 9.3 (4.6-24.3) years. All transplanted patients achieved stable hematopoietic engraftment. However, in 2 patients due to inadequate immune reconstitution developed fatal CMV pneumonia and invasive aspergillosis 12 and 14 months post HSCT, accordingly. In one patient we have diagnosed adenocarcinoma of the gut 10 years and squamous cell carcinoma of tongue 13 years after HSCT. Thirteen patients are alive with a median follow-up of 10.6 (0.3 – 15.1 years) years after HSCT. With a median follow-up till the last visit of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died due to HSCT-related complications, and one due to severe congenital somatic defects.
Conclusions
HSCT is effective in FA patients with bone marrow failure and prevents further development of hematological malignancies. A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease.
Keywords
Fanconi anemia, somatic anomalies, gene mutations, bone marrow failure, solid tumors, hematopoietic stem cell transplantation, long-term outpatient observation.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27365
[VALUE] => Fanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Fanconi Anemia in the Czech Republic: role of HSCT and long-term follow-up
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27366
[VALUE] => 2335
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2335
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27370
[VALUE] => 2336
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2336
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Petr Sedlacek, Petra Keslova, Petr Smisek, Martina Sukova, Marcela Malikova, Spiros Tavandzis, Jaroslav Cermak, Jan Stary
Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, Prague, Czech Republic
Correspondence
Prof. Dr. Petr Sedlacek, Department of Pediatric Hematology and Oncology, 2nd Medical School at Charles University, University Hospital Motol, V Uvalu 84, 150 06 Prague, Czech Republic
Phone: +420 22443 6552
Fax: +420 22443 6519
E-mail: petr.sedlacek@lfmotol.cuni.cz
Fanconi anemia (FA) is a rare and heterogeneous syndrome associated with bone marrow failure and increased risk of cancer. While FA is often characterized by the presence of congenital malformations, in some patients cytopenia may be the only sign. In our cohort diagnosis was based on evidence of increased chromosome fragility with subsequent confirmation by gene mutation detection.
Methods
Our cohort includes 35 probands diagnosed with FA aged 0-24.3 (median 6) years between January 1986 and August 2020. Congenital anomalies at diagnosis were seen in 5 and cytopenia in 22 patients, 8 patients had family history of FA. Genetic test confirmed FANCA gene mutations in 24, FANCG in 3, FANCD1 in 4, and FANCB in 2 siblings.
Results
During follow-up 7 patients developed malignancy (among them all 4 patients with FANCD1 mutation). Seventeen patients developed marrow failure, for which 15 patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) at the median age of 9.3 (4.6-24.3) years. All transplanted patients achieved stable hematopoietic engraftment. However, in 2 patients due to inadequate immune reconstitution developed fatal CMV pneumonia and invasive aspergillosis 12 and 14 months post HSCT, accordingly. In one patient we have diagnosed adenocarcinoma of the gut 10 years and squamous cell carcinoma of tongue 13 years after HSCT. Thirteen patients are alive with a median follow-up of 10.6 (0.3 – 15.1 years) years after HSCT. With a median follow-up till the last visit of 12.6 (0.2-34.4) years 28/35 (80%) patients are alive, 4 died of malignancy, 2 died due to HSCT-related complications, and one due to severe congenital somatic defects.
Conclusions
HSCT is effective in FA patients with bone marrow failure and prevents further development of hematological malignancies. A lifelong and careful multidisciplinary follow-up of patients with FA is essential for early detection of bone marrow failure or any malignant disease.
Keywords
Fanconi anemia, somatic anomalies, gene mutations, bone marrow failure, solid tumors, hematopoietic stem cell transplantation, long-term outpatient observation.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27371
[VALUE] => 09/02/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 09/02/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27372
[VALUE] => 12/04/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/04/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27373
[VALUE] => Array
(
[TEXT] => <p>Олеся В. Паина<sup>1</sup>, Ирина Е. Павлова<sup>1,2</sup>, Наталья Е. Иванова<sup>1</sup>, Александр Л. Алянский<sup>1</sup>, Татьяна А. Быкова<sup>1</sup>, Людмила С. Зубаровская<sup>1</sup>, Александр Д. Кулагин<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев<sup>1</sup></span> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Олеся В. Паина1, Ирина Е. Павлова1,2, Наталья Е. Иванова1, Александр Л. Алянский1, Татьяна А. Быкова1, Людмила С. Зубаровская1, Александр Д. Кулагин1, Борис В. Афанасьев1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27374
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия <br>
<sup>2</sup> Российский научно-исследовательский институт гематологии и трансфузиологии Федерального медико-биологического агентства, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
2 Российский научно-исследовательский институт гематологии и трансфузиологии Федерального медико-биологического агентства, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27375
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Донор-специфические анти-HLA антитела (ДСА) могут являться важным фактором риска недостаточного приживления HLA-гаплосовместимых донорских гемопоэтических клеток (ГСК). Цель настоящего исследования – определить частоту выявления донор-специфических анти-HLA антител у реципиентов перед проведением гаплосовместимой трансплантации ГСК (ТГСК) с помощью метода иммуноферментного анализа и установить взаимосвязь наличия ДСА с первичным неприживлением трансплантата и (или) гипофункцией трансплантата у детей, перенесших ГТГСК.</p>
<p style="text-align: justify;">В работе представлены результаты пилотного ретроспективного одноцентрового исследования. Обследованы 40 пациентов детского возраста, медиана возраста составила 8,5 лет (1 год – 17 лет), которым были выполнены гаплосовместимые трансплантации неманипулированных ГСК в клинике НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой (22-M, 18-ж): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. Средний возраст пациентов составил 8,5 лет (1-17 лет). Медианы общего числа трансплантированных ядросодержащих и CD34+ клеток составляли 8,5×10<sup>8</sup>/кг (2,3-17,9×10<sup>8</sup>/кг) и 7,6×10<sup>6</sup>/кг (2,3-8,2×10<sup>6</sup>/кг) соответственно. Во всех случаях источником CD34+ клеток был стимулированный неманипулированный костный мозг. Определение ДСА в сыворотке крови реципиентов выполнено с использованием коммерческого набора (XMatch®, Protrans), который позволяет определять антитела против донорских антигенов HLA- класса I и класса II отдельно в перекрестном сопоставлении ELISA de facto.
Среди всех обследованных детей у 3-х пациентов (7,5%) выявлены ДСА, в то время как у подавляющего большинства (n=37; 92,5%) ДСА не было, во всех случаях ДСА были направлены против антигенов HLA класса II. Сравнительный анализ результатов показывает, что у пациентов с наличием ДСА до гаплосовместимой ТГСК более вероятно развитие первичной недостаточности трансплантата или гипофункции по сравнению с группой реципиентов без DSA. У всех 3 пациентов (100%) с обнаруженными антителами была диагностирована либо первичная недостаточность трансплантата (n=1, 33%), либо гипофункция трансплантата (n=2, 67%). В группе пациентов без ДСА первичная недостаточность трансплантата или гипофункция трансплантата наблюдались в 5 (13,5%) и 1 (2,7%) случаях соответственно, всего в 6 случаях (16,2%). Внедрение DSA-тестирования в рутинную практику у реципиентов перед гапло-ТГСК оптимизирует выбор донора, а также выберет группу потенциальных реципиентов, которым необходимо пройти курс лечения в предтрансплантационный период для десенсибилизации.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Донор-специфические антитела, трансплантация гемопоэтических стволовых клеток, HLA-гаплосовместимые доноры, HLA-гаплоидентичные доноры.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Донор-специфические анти-HLA антитела (ДСА) могут являться важным фактором риска недостаточного приживления HLA-гаплосовместимых донорских гемопоэтических клеток (ГСК). Цель настоящего исследования – определить частоту выявления донор-специфических анти-HLA антител у реципиентов перед проведением гаплосовместимой трансплантации ГСК (ТГСК) с помощью метода иммуноферментного анализа и установить взаимосвязь наличия ДСА с первичным неприживлением трансплантата и (или) гипофункцией трансплантата у детей, перенесших ГТГСК.
В работе представлены результаты пилотного ретроспективного одноцентрового исследования. Обследованы 40 пациентов детского возраста, медиана возраста составила 8,5 лет (1 год – 17 лет), которым были выполнены гаплосовместимые трансплантации неманипулированных ГСК в клинике НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой (22-M, 18-ж): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. Средний возраст пациентов составил 8,5 лет (1-17 лет). Медианы общего числа трансплантированных ядросодержащих и CD34+ клеток составляли 8,5×108/кг (2,3-17,9×108/кг) и 7,6×106/кг (2,3-8,2×106/кг) соответственно. Во всех случаях источником CD34+ клеток был стимулированный неманипулированный костный мозг. Определение ДСА в сыворотке крови реципиентов выполнено с использованием коммерческого набора (XMatch®, Protrans), который позволяет определять антитела против донорских антигенов HLA- класса I и класса II отдельно в перекрестном сопоставлении ELISA de facto.
Среди всех обследованных детей у 3-х пациентов (7,5%) выявлены ДСА, в то время как у подавляющего большинства (n=37; 92,5%) ДСА не было, во всех случаях ДСА были направлены против антигенов HLA класса II. Сравнительный анализ результатов показывает, что у пациентов с наличием ДСА до гаплосовместимой ТГСК более вероятно развитие первичной недостаточности трансплантата или гипофункции по сравнению с группой реципиентов без DSA. У всех 3 пациентов (100%) с обнаруженными антителами была диагностирована либо первичная недостаточность трансплантата (n=1, 33%), либо гипофункция трансплантата (n=2, 67%). В группе пациентов без ДСА первичная недостаточность трансплантата или гипофункция трансплантата наблюдались в 5 (13,5%) и 1 (2,7%) случаях соответственно, всего в 6 случаях (16,2%). Внедрение DSA-тестирования в рутинную практику у реципиентов перед гапло-ТГСК оптимизирует выбор донора, а также выберет группу потенциальных реципиентов, которым необходимо пройти курс лечения в предтрансплантационный период для десенсибилизации.
Ключевые слова
Донор-специфические антитела, трансплантация гемопоэтических стволовых клеток, HLA-гаплосовместимые доноры, HLA-гаплоидентичные доноры.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27376
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-53-58
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-53-58
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27379
[VALUE] => Array
(
[TEXT] => <p>Olesya V. Paina<sup>1</sup>, Irina E. Pavlova<sup>1,2</sup>, Natalia E. Ivanova<sup>1</sup>, Alexander L. Alyanskiy<sup>1</sup>, Tatiana A. Bykova<sup>1</sup>, Ludmila S. Zubarovskaya<sup>1</sup>, Alexander D. Kulagin<sup>1</sup>, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev<sup>1</sup></span>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Olesya V. Paina1, Irina E. Pavlova1,2, Natalia E. Ivanova1, Alexander L. Alyanskiy1, Tatiana A. Bykova1, Ludmila S. Zubarovskaya1, Alexander D. Kulagin1, Boris V. Afanasyev1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27380
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia<br>
<sup>2</sup> Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2<sup>nd</sup> Sovetskaya St 16, 191024, St. Petersburg, Russia<br>
Phone: +7 (921) 983 6664<br>
E-mail: dr_pavlova_irina@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
2 Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St 16, 191024, St. Petersburg, Russia
Phone: +7 (921) 983 6664
E-mail: dr_pavlova_irina@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27381
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Donor-specific antibodies (DSA) have been recently recognized as an important risk factor for the failing engraftment of HLA-haploidentical donor cells. In this study, we aimed to determine the frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay and establishing an association between the DSA presence and primary graft failure and/or graft hypofunction in children after haplo-HSCT. 40 patients have been tested (22-M, 18-F): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. The median age of the patients was 8.5 years (1-17 years). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×10<sup>8</sup>/kg (2.3-17.9×10<sup>8</sup>/kg) and 7.6×10<sup>6</sup>/kg (2.3-8.2×10<sup>6</sup>/kg) respectively. The donor bone marrow was the source of stem cells in all cases. Detection of DSA has been performed by using the commercial kit (XMatch®, Protrans) that allows determining antibodies against donor's HLA class I and class II separately in the ELISA de facto crossmatching. Among all the examined children, 3 patients (7.5%) were found to be positive for DSA, while the vast majority (n=37; 92.5%) had no detectable DSA. It was also found that in all cases DSA were directed against HLA class II antigens.</p>
<p style="text-align: justify;">Comparative analysis of the results shows that patients with DSA before haplo-HSCT are more likely to develop primary graft failure or hypofunction compared to the group of recipients without DSA. All 3 patients with detected antibodies were diagnosed with either primary graft failure (n=1, 33%) or graft hypofunction (n=2, 67%). In the control group of patients without DSA primary graft failure or graft hypofunction were observed in 5 (13.5%) and 1 (2.7%) cases respectively, with a total of 6 cases (16.2%). Implementation of DSA-testing into routine practice in recipients before haplo-HSCT will optimize the choice of a donor, as well as select a group of potential recipients who need to be treated in the pre-transplant period for desensitization.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Donor-specific antibodies, hematopoietic stem cell transplantation, HLA-haplocompatible donors, HLA-haploidentical donors.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Donor-specific antibodies (DSA) have been recently recognized as an important risk factor for the failing engraftment of HLA-haploidentical donor cells. In this study, we aimed to determine the frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay and establishing an association between the DSA presence and primary graft failure and/or graft hypofunction in children after haplo-HSCT. 40 patients have been tested (22-M, 18-F): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. The median age of the patients was 8.5 years (1-17 years). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×108/kg (2.3-17.9×108/kg) and 7.6×106/kg (2.3-8.2×106/kg) respectively. The donor bone marrow was the source of stem cells in all cases. Detection of DSA has been performed by using the commercial kit (XMatch®, Protrans) that allows determining antibodies against donor's HLA class I and class II separately in the ELISA de facto crossmatching. Among all the examined children, 3 patients (7.5%) were found to be positive for DSA, while the vast majority (n=37; 92.5%) had no detectable DSA. It was also found that in all cases DSA were directed against HLA class II antigens.
Comparative analysis of the results shows that patients with DSA before haplo-HSCT are more likely to develop primary graft failure or hypofunction compared to the group of recipients without DSA. All 3 patients with detected antibodies were diagnosed with either primary graft failure (n=1, 33%) or graft hypofunction (n=2, 67%). In the control group of patients without DSA primary graft failure or graft hypofunction were observed in 5 (13.5%) and 1 (2.7%) cases respectively, with a total of 6 cases (16.2%). Implementation of DSA-testing into routine practice in recipients before haplo-HSCT will optimize the choice of a donor, as well as select a group of potential recipients who need to be treated in the pre-transplant period for desensitization.
Keywords
Donor-specific antibodies, hematopoietic stem cell transplantation, HLA-haplocompatible donors, HLA-haploidentical donors.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27377
[VALUE] => Donor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Donor-specific anti-HLA antibodies detection by de facto crossmatch method in pediatric recipients before haploidentical hematopoetic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27378
[VALUE] => 2339
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2339
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27382
[VALUE] => 2340
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2340
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Olesya V. Paina1, Irina E. Pavlova1,2, Natalia E. Ivanova1, Alexander L. Alyanskiy1, Tatiana A. Bykova1, Ludmila S. Zubarovskaya1, Alexander D. Kulagin1, Boris V. Afanasyev1
1 RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
2 Russian Research Institute of Hematology and Transfusiology, St. Petersburg, Russia
Correspondence
Dr. Irina E. Pavlova, Russian Research Institute of Hematology and Transfusiology, 2nd Sovetskaya St 16, 191024, St. Petersburg, Russia
Phone: +7 (921) 983 6664
E-mail: dr_pavlova_irina@mail.ru
Donor-specific antibodies (DSA) have been recently recognized as an important risk factor for the failing engraftment of HLA-haploidentical donor cells. In this study, we aimed to determine the frequency of anti-HLA DSA detection in recipients before haplo-HSCT using the enzyme-linked immunosorbent assay and establishing an association between the DSA presence and primary graft failure and/or graft hypofunction in children after haplo-HSCT. 40 patients have been tested (22-M, 18-F): 27 ALL, 8 AML, 1 MDS, 1 AA, 3 JMML. The median age of the patients was 8.5 years (1-17 years). Medians of the number of transplanted nucleated and CD34+ cells were 8.5×108/kg (2.3-17.9×108/kg) and 7.6×106/kg (2.3-8.2×106/kg) respectively. The donor bone marrow was the source of stem cells in all cases. Detection of DSA has been performed by using the commercial kit (XMatch®, Protrans) that allows determining antibodies against donor's HLA class I and class II separately in the ELISA de facto crossmatching. Among all the examined children, 3 patients (7.5%) were found to be positive for DSA, while the vast majority (n=37; 92.5%) had no detectable DSA. It was also found that in all cases DSA were directed against HLA class II antigens.
Comparative analysis of the results shows that patients with DSA before haplo-HSCT are more likely to develop primary graft failure or hypofunction compared to the group of recipients without DSA. All 3 patients with detected antibodies were diagnosed with either primary graft failure (n=1, 33%) or graft hypofunction (n=2, 67%). In the control group of patients without DSA primary graft failure or graft hypofunction were observed in 5 (13.5%) and 1 (2.7%) cases respectively, with a total of 6 cases (16.2%). Implementation of DSA-testing into routine practice in recipients before haplo-HSCT will optimize the choice of a donor, as well as select a group of potential recipients who need to be treated in the pre-transplant period for desensitization.
Keywords
Donor-specific antibodies, hematopoietic stem cell transplantation, HLA-haplocompatible donors, HLA-haploidentical donors.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27347
[VALUE] => 09/22/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 09/22/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27348
[VALUE] => 12/04/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/04/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27349
[VALUE] => Array
(
[TEXT] => <p>Игорь Новицки-Бассо<sup>1</sup>, Эшрак Аль-Шайбани<sup>1</sup>, Мата Рембергер<sup>2</sup>, Кэрол Чен<sup>1</sup>, Уилсон Лэм<sup>1</sup>, Арджун Д. Лоу<sup>1</sup>, Айвэн Пазич<sup>1</sup>, Фотиос В. Микелис<sup>1</sup>, Ауро Висвабандья<sup>1</sup>, Деннис Д. Ким<sup>1</sup>, Джеффри Х. Липтон<sup>1</sup>, Армин Гербитц<sup>1</sup>, Ионас Маттсон<sup>1</sup>, Раджат Кумар<sup>1</sup>, Зейяд Аль-Шайбани<sup>1</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Игорь Новицки-Бассо1, Эшрак Аль-Шайбани1, Мата Рембергер2, Кэрол Чен1, Уилсон Лэм1, Арджун Д. Лоу1, Айвэн Пазич1, Фотиос В. Микелис1, Ауро Висвабандья1, Деннис Д. Ким1, Джеффри Х. Липтон1, Армин Гербитц1, Ионас Маттсон1, Раджат Кумар1, Зейяд Аль-Шайбани1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27350
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Программа аллогенных трансплантаций Ханса Месснера, Онкологический Центр принцессы Маргарет, университетская сеть здравоохранения, Университет Торонто, Торонто, Канада<br>
<sup>2</sup> Департамент медицинских наук, университет Уппсала, университетский госпиталь Уппсала, Уппсала, Швеция</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Программа аллогенных трансплантаций Ханса Месснера, Онкологический Центр принцессы Маргарет, университетская сеть здравоохранения, Университет Торонто, Торонто, Канада
2 Департамент медицинских наук, университет Уппсала, университетский госпиталь Уппсала, Уппсала, Швеция
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27351
[VALUE] => Array
(
[TEXT] => <h3>Цель работы</h3>
<p style="text-align: justify;">Недостаточность трансплантата (НТ) после аллогенной трансплантации гемопоэтических клеток (алло-ТГСК) имеет плохой прогноз. Целью данного исследования было установление частоты возникновения, факторов риска (Фри исходов НТ в контингенте из одного центра.</p>
<h3>Пациенты и методы</h3>
<p style="text-align: justify;">В период с 2015 по 2018 гг., алло-ТГСК была выполнена у 557 больных. Первичную НТ (ПНТ) определяли как отсутствие роста абсолютного количества нейтрофилов (АКН) до >0.5×10<sup>9</sup>/л к 28 сут. после алло-ТГСК. Вторичную НТ (ВНТ) характеризовало снижение числа донорских клеток после начального приживления с возвратом КН к уровням <0.5×10<sup>9</sup>/л без рецидива или иных причин цитопении. Конечными результатами исследования была кумулятивная встречаемость НТ и общая выживаемость (ОВ); факторы риска НТ определяли путем многофакторного анализа.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">В 9 случаях выявлена ПНТ, и у 34 больных была ВНТ. Кумулятивная встречаемость НТ была, соответственно, 1,6% ко дню +100 (CI95%; 0,8- 3,0%), и 6,5% – (CI95%; 4,5-8,8%) ко дню +800. Многофакторный анализ показал, что лиагноз (миелодиспластический синдром, миелофиброз, лимфома или неопухолевые заболевания), а также тип донора (HLA-несовместимый неродственный или гаплоидентичный) были достоверно ассоциированы с НТ. Частота НТ составила 3,6% при отсутствии указанных факторов риска, 9.9% при наличии одного ФР и 24.5% при двух ФР. Медиана выживаемости пациентов после ПНТ составила 41 сут., после ВНТ – 144 сут. Общая выживаемость на день +100 при ПНТ была 22%, при ВНТ – 64%. Двухлетняя общая выживаемость в случаях ВНТ была 28%.</p>
<h3>Выводы</h3>
<p style="text-align: justify;">Данное исследование показало наличие повышенного риска недостаточности трансплантата после алло-ТГСК от несовместимого/неродственного или гаплоидентичного донора, или при нелейкемическом диагнозе. Для таких случаев мы предлагаем тщательный мониторинг, ранние диагностические и терапевтические мероприятия и для улучшения клинических исходов. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Аллогенная трансплантация гемопоэтических стволовых клеток, недостаточность трансплантата, встречаемость, факторы риска. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Цель работы
Недостаточность трансплантата (НТ) после аллогенной трансплантации гемопоэтических клеток (алло-ТГСК) имеет плохой прогноз. Целью данного исследования было установление частоты возникновения, факторов риска (Фри исходов НТ в контингенте из одного центра.
Пациенты и методы
В период с 2015 по 2018 гг., алло-ТГСК была выполнена у 557 больных. Первичную НТ (ПНТ) определяли как отсутствие роста абсолютного количества нейтрофилов (АКН) до >0.5×109/л к 28 сут. после алло-ТГСК. Вторичную НТ (ВНТ) характеризовало снижение числа донорских клеток после начального приживления с возвратом КН к уровням <0.5×109/л без рецидива или иных причин цитопении. Конечными результатами исследования была кумулятивная встречаемость НТ и общая выживаемость (ОВ); факторы риска НТ определяли путем многофакторного анализа.
Результаты
В 9 случаях выявлена ПНТ, и у 34 больных была ВНТ. Кумулятивная встречаемость НТ была, соответственно, 1,6% ко дню +100 (CI95%; 0,8- 3,0%), и 6,5% – (CI95%; 4,5-8,8%) ко дню +800. Многофакторный анализ показал, что лиагноз (миелодиспластический синдром, миелофиброз, лимфома или неопухолевые заболевания), а также тип донора (HLA-несовместимый неродственный или гаплоидентичный) были достоверно ассоциированы с НТ. Частота НТ составила 3,6% при отсутствии указанных факторов риска, 9.9% при наличии одного ФР и 24.5% при двух ФР. Медиана выживаемости пациентов после ПНТ составила 41 сут., после ВНТ – 144 сут. Общая выживаемость на день +100 при ПНТ была 22%, при ВНТ – 64%. Двухлетняя общая выживаемость в случаях ВНТ была 28%.
Выводы
Данное исследование показало наличие повышенного риска недостаточности трансплантата после алло-ТГСК от несовместимого/неродственного или гаплоидентичного донора, или при нелейкемическом диагнозе. Для таких случаев мы предлагаем тщательный мониторинг, ранние диагностические и терапевтические мероприятия и для улучшения клинических исходов.
Ключевые слова
Аллогенная трансплантация гемопоэтических стволовых клеток, недостаточность трансплантата, встречаемость, факторы риска.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27352
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-37-47
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-37-47
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27355
[VALUE] => Array
(
[TEXT] => <p>Igor Novitzky-Basso<sup>1</sup>, Eshrak Al-Shaibani<sup>1</sup>, Mats Remberger<sup>2</sup>, Carol Chen<sup>1</sup>, Wilson Lam<sup>1</sup>, Arjun D. Law<sup>1</sup>, Ivan Pasic<sup>1</sup>, Fotios V. Michelis<sup>1</sup>, Auro Viswabandya<sup>1</sup>, Dennis D. Kim<sup>1</sup>, Jeffrey H. Lipton<sup>1</sup>, Armin Gerbitz<sup>1</sup>, Jonas Mattsson<sup>1</sup>, Rajat Kumar<sup>1</sup>, Zeyad Al-Shaibani<sup>1</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Igor Novitzky-Basso1, Eshrak Al-Shaibani1, Mats Remberger2, Carol Chen1, Wilson Lam1, Arjun D. Law1, Ivan Pasic1, Fotios V. Michelis1, Auro Viswabandya1, Dennis D. Kim1, Jeffrey H. Lipton1, Armin Gerbitz1, Jonas Mattsson1, Rajat Kumar1, Zeyad Al-Shaibani1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27356
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada<br>
<sup>2</sup> Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden</p><br>
<p><b>Correspondence</b><br>
Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada<br>
Phone: +1 647 927 8700<br>
Fax: +1 416 946 4407<br>
E-mail: igor.novitzkybasso@uhn.ca</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada
2 Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden
Correspondence
Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada
Phone: +1 647 927 8700
Fax: +1 416 946 4407
E-mail: igor.novitzkybasso@uhn.ca
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27358
[VALUE] => Array
(
[TEXT] => <h3>Objectives</h3>
<p style="text-align: justify;">Graft failure (GF) following allogeneic hematopoietic stem cell transplantation (allo-HCT) has a dismal prognosis. This study assessed incidence, risk factors (RF) and outcome of GF in a single-center population.</p>
<h3>Patients and methods</h3>
<p style="text-align: justify;">Between 2015-2018, 557 patients underwent allo-HCT. Primary GF (PGF) was defined as failure to achieve absolute neutrophil count (ANC) of >0.5×10<sup>9</sup>/L by 28 days after allo-HCT. Secondary GF (SGF) was characterized by loss of donor cells after initial engraftment with recurrence of ANC <0.5×10<sup>9</sup>/L without relapse or other causes of cytopenia. Endpoints of the study were cumulative incidence of GF and overall survival (OS). Risk factors for GF were assessed in multivariate analysis.</p>
<h3>Results</h3>
<p style="text-align: justify;">Nine patients had PGF, and 34 had SGF. The cumulative incidence of GF overall (primary and secondary) is 1.6% (CI95%; 0.8-3.0%) at day 100 and 6.5% (CI95%; 4.5-8.8%) at day 800 respectively. Multivariate analysis showed diagnosis (myelodysplastic syndrome [MDS], myelofibrosis [MF], lymphoma or non-malignant diseases) and donor type (HLA-mismatched-unrelated or haploidentical) were significantly associated with GF. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5% (p=0.002).
Median OS of patients following PGF was 41 days, SGF – 144 days. The D100 OS in PGF was 22%, SGF – 64%; 2-year overall survival for SGF was 28%.</p>
<h3>Conclusions</h3>
<p style="text-align: justify;">This study showed increased risk of GF following mismatched-unrelated or haploidentical donor allo-HCT or for non-leukemia diagnosis, for which we suggest close monitoring for early diagnostic and therapeutic interventions, in order to improve clinical outcomes. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Allogeneic hematopoietic stem cell transplantation, graft failure, incidence, risk factors. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Objectives
Graft failure (GF) following allogeneic hematopoietic stem cell transplantation (allo-HCT) has a dismal prognosis. This study assessed incidence, risk factors (RF) and outcome of GF in a single-center population.
Patients and methods
Between 2015-2018, 557 patients underwent allo-HCT. Primary GF (PGF) was defined as failure to achieve absolute neutrophil count (ANC) of >0.5×109/L by 28 days after allo-HCT. Secondary GF (SGF) was characterized by loss of donor cells after initial engraftment with recurrence of ANC <0.5×109/L without relapse or other causes of cytopenia. Endpoints of the study were cumulative incidence of GF and overall survival (OS). Risk factors for GF were assessed in multivariate analysis.
Results
Nine patients had PGF, and 34 had SGF. The cumulative incidence of GF overall (primary and secondary) is 1.6% (CI95%; 0.8-3.0%) at day 100 and 6.5% (CI95%; 4.5-8.8%) at day 800 respectively. Multivariate analysis showed diagnosis (myelodysplastic syndrome [MDS], myelofibrosis [MF], lymphoma or non-malignant diseases) and donor type (HLA-mismatched-unrelated or haploidentical) were significantly associated with GF. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5% (p=0.002).
Median OS of patients following PGF was 41 days, SGF – 144 days. The D100 OS in PGF was 22%, SGF – 64%; 2-year overall survival for SGF was 28%.
Conclusions
This study showed increased risk of GF following mismatched-unrelated or haploidentical donor allo-HCT or for non-leukemia diagnosis, for which we suggest close monitoring for early diagnostic and therapeutic interventions, in order to improve clinical outcomes.
Keywords
Allogeneic hematopoietic stem cell transplantation, graft failure, incidence, risk factors.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27353
[VALUE] => Risk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Risk factors for graft failure in allogeneic hematopoietic stem cell transplantation: a single-center study
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27354
[VALUE] => 2326
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2326
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27357
[VALUE] => 2327
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2327
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Igor Novitzky-Basso1, Eshrak Al-Shaibani1, Mats Remberger2, Carol Chen1, Wilson Lam1, Arjun D. Law1, Ivan Pasic1, Fotios V. Michelis1, Auro Viswabandya1, Dennis D. Kim1, Jeffrey H. Lipton1, Armin Gerbitz1, Jonas Mattsson1, Rajat Kumar1, Zeyad Al-Shaibani1
1 Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, Canada
2 Department of Medical Sciences, Uppsala University and KFUE, Uppsala University Hospital, Uppsala, Sweden
Correspondence
Igor Novitzky-Basso MD PhD, Hans Messner Allogeneic Transplant Program, Princess Margaret Cancer Centre, University Health Network, University of Toronto, 610 University Avenue, Toronto, ON, M5G 2M9, Canada
Phone: +1 647 927 8700
Fax: +1 416 946 4407
E-mail: igor.novitzkybasso@uhn.ca
Objectives
Graft failure (GF) following allogeneic hematopoietic stem cell transplantation (allo-HCT) has a dismal prognosis. This study assessed incidence, risk factors (RF) and outcome of GF in a single-center population.
Patients and methods
Between 2015-2018, 557 patients underwent allo-HCT. Primary GF (PGF) was defined as failure to achieve absolute neutrophil count (ANC) of >0.5×109/L by 28 days after allo-HCT. Secondary GF (SGF) was characterized by loss of donor cells after initial engraftment with recurrence of ANC <0.5×109/L without relapse or other causes of cytopenia. Endpoints of the study were cumulative incidence of GF and overall survival (OS). Risk factors for GF were assessed in multivariate analysis.
Results
Nine patients had PGF, and 34 had SGF. The cumulative incidence of GF overall (primary and secondary) is 1.6% (CI95%; 0.8-3.0%) at day 100 and 6.5% (CI95%; 4.5-8.8%) at day 800 respectively. Multivariate analysis showed diagnosis (myelodysplastic syndrome [MDS], myelofibrosis [MF], lymphoma or non-malignant diseases) and donor type (HLA-mismatched-unrelated or haploidentical) were significantly associated with GF. For the absence of any of the risk factors (n=279), the incidence of GF was 3.6%. For the presence of one risk factor (n=229), the incidence of GF was 9.9%, while for 2 concurrent risk factors (n=49), the incidence of GF was 24.5% (p=0.002). Median OS of patients following PGF was 41 days, SGF – 144 days. The D100 OS in PGF was 22%, SGF – 64%; 2-year overall survival for SGF was 28%.
Conclusions
This study showed increased risk of GF following mismatched-unrelated or haploidentical donor allo-HCT or for non-leukemia diagnosis, for which we suggest close monitoring for early diagnostic and therapeutic interventions, in order to improve clinical outcomes.
Keywords
Allogeneic hematopoietic stem cell transplantation, graft failure, incidence, risk factors.
Clinical studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27337
[VALUE] => 07/25/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 07/25/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27338
[VALUE] => 10/23/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/23/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27323
[VALUE] => Array
(
[TEXT] => <p>Елена В. Морозова, Юлия Ю. Власова, Мария В. Барабанщикова, Ксения С. Юровская, Татьяна В. Шнайдер, Татьяна Л. Гиндина, Ильдар М. Бархатов, Евгений А. Бакин, Иван С. Моисеев, Александр Д. Кулагин, Людмила С. Зубаровская, <br><span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Борис В. Афанасьев</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Елена В. Морозова, Юлия Ю. Власова, Мария В. Барабанщикова, Ксения С. Юровская, Татьяна В. Шнайдер, Татьяна Л. Гиндина, Ильдар М. Бархатов, Евгений А. Бакин, Иван С. Моисеев, Александр Д. Кулагин, Людмила С. Зубаровская,
Борис В. Афанасьев
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27324
[VALUE] => Array
(
[TEXT] => <p>НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => НИИ детской онкологии, гематологии и трансплантологии им. Р. М. Горбачевой, Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27325
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Клинический прогноз у пациентов с хроническим миелоидным лейкозом (ХМЛ) в развернутой стадии (фаза акселерации – ФА, или бластный криз – БК) все еще остается неблагоприятным в эру применения ингибиторов тирозинкиназ (ИТК). Данное исследование проводилось, чтобы оценить, насколько аллогенная трансплантация гемопоэтических клеток (алло-ТГСК) улучшает их прогноз.</p>
<h3>Пациенты и методы</h3>
<p style="text-align: justify;">Общая группа из 162 пациентов с ХМЛ в ФА/БК была разделена на две гомогенные когорты. Первая из них состояла из реципиентов, получавших кондиционирование со сниженной интенсивностью перед алло-ТГСК (n=82). Вторая группа (n=80) включала пациентов, получавших только терапию на базе ИТК (в 85% случаев – препараты 2-го и 3-го поколения), не направленных в центры трансплантации или отказавшихся от нее. Ответ на терапию определяли в соответствии с рекомендациями ELN и NCCN. </p>
<h3>Результаты</h3>
<p style="text-align: justify;">Медиана сроков наблюдения для всей когорты составляла 44 (1-344) мес. Среди пациентов с БК 36 больных (59%) не отвечали на лечение, в 22 случаях (34%) была установлена полная гематологическая ремиссия (CHR), в одном случае (2%) – полная цитогенетическая ремиссия, и полный молекулярный ответ (ПМО) был достигнут в 2 случаях (3%). Среди реципиентов алло-ТГСК, приживление отмечено в 86% случаев. Кумулятивная безрецидивная смертность на D+100 и через 1 год составила, соответственно, 10% и 18%. У 28 пациентов с посттрансплантационным рецидивом проведена дополнительная терапия, и достигнут ПМО в 9 случаях. Общая 4-летняя выживаемость и бессобытийная выживаемость (ОВ) были лучше после алло-ТГСК по сравнению с группой, леченой ИТК: 58% против 33% (p=0,032) и 35% против 17% (p=0,5), соответственно. Пациенты в БК на момент ТГСК имели значительно более низкие уровни 3-летней ОВ по сравнению с больными, ответившими на лечение: 23% против 63% (p=0,007), соответственно. </p>
<h3>Заключение</h3>
<p style="text-align: justify;">Хотя алло-ТГСК имеет преимущество у многих больных ХМЛ в развернутых стадиях, результаты ее применения при БК сравнимы с лечением ИТК. Поэтому данные пациенты должны направляться в центры трансплантации по мере достижения ими второй хронической фазы заболевания.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Хронический миелоидный лейкоз, BCR/ABL, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназы, бластный криз, исходы заболевания.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Клинический прогноз у пациентов с хроническим миелоидным лейкозом (ХМЛ) в развернутой стадии (фаза акселерации – ФА, или бластный криз – БК) все еще остается неблагоприятным в эру применения ингибиторов тирозинкиназ (ИТК). Данное исследование проводилось, чтобы оценить, насколько аллогенная трансплантация гемопоэтических клеток (алло-ТГСК) улучшает их прогноз.
Пациенты и методы
Общая группа из 162 пациентов с ХМЛ в ФА/БК была разделена на две гомогенные когорты. Первая из них состояла из реципиентов, получавших кондиционирование со сниженной интенсивностью перед алло-ТГСК (n=82). Вторая группа (n=80) включала пациентов, получавших только терапию на базе ИТК (в 85% случаев – препараты 2-го и 3-го поколения), не направленных в центры трансплантации или отказавшихся от нее. Ответ на терапию определяли в соответствии с рекомендациями ELN и NCCN.
Результаты
Медиана сроков наблюдения для всей когорты составляла 44 (1-344) мес. Среди пациентов с БК 36 больных (59%) не отвечали на лечение, в 22 случаях (34%) была установлена полная гематологическая ремиссия (CHR), в одном случае (2%) – полная цитогенетическая ремиссия, и полный молекулярный ответ (ПМО) был достигнут в 2 случаях (3%). Среди реципиентов алло-ТГСК, приживление отмечено в 86% случаев. Кумулятивная безрецидивная смертность на D+100 и через 1 год составила, соответственно, 10% и 18%. У 28 пациентов с посттрансплантационным рецидивом проведена дополнительная терапия, и достигнут ПМО в 9 случаях. Общая 4-летняя выживаемость и бессобытийная выживаемость (ОВ) были лучше после алло-ТГСК по сравнению с группой, леченой ИТК: 58% против 33% (p=0,032) и 35% против 17% (p=0,5), соответственно. Пациенты в БК на момент ТГСК имели значительно более низкие уровни 3-летней ОВ по сравнению с больными, ответившими на лечение: 23% против 63% (p=0,007), соответственно.
Заключение
Хотя алло-ТГСК имеет преимущество у многих больных ХМЛ в развернутых стадиях, результаты ее применения при БК сравнимы с лечением ИТК. Поэтому данные пациенты должны направляться в центры трансплантации по мере достижения ими второй хронической фазы заболевания.
Ключевые слова
Хронический миелоидный лейкоз, BCR/ABL, аллогенная трансплантация гемопоэтических стволовых клеток, ингибиторы тирозинкиназы, бластный криз, исходы заболевания.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27326
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-20-28
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-20-28
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27329
[VALUE] => Array
(
[TEXT] => <p>Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, <span style="border: 1px solid black; margin: 0; padding: 2px 2px;">Boris V. Afanasyev</span></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Boris V. Afanasyev
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27330
[VALUE] => Array
(
[TEXT] => <p>RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia</p>
<br>
<p><b>Correspondence</b><br>
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia<br>
Phone: +7(911) 927 8229<br>
E-mail: dr_morozova@mail.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(911) 927 8229
E-mail: dr_morozova@mail.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27331
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Prognosis of patients with advanced stage CML (accelerated phase, AP, or blast crisis, BC) is still dismal in the era of tyrosine kinase inhibitors (TKIs). This study is aimed to evaluate whether allogeneic hemopoietic stem cell transplantation (allo-HSCT) improves their prognosis. </p>
<p style="text-align: justify;">A total of 162 patients with AP/BC CML were divided into two homogeneous cohorts. The first one consisted of reduced-intensity conditioning allo-HSTC (n=82) recipients. The second (n=80) consisted of patients receiving only TKI-based therapy (in 85% of cases 2nd and 3rd generation TKIs) while not being referred to transplant center or refusing allo-HSCT. The response to therapy was defined according to ELN and NCCN recommendations. </p>
<p style="text-align: justify;">The median follow-up for entire cohort was 44 (1-344) months. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among allo-HST recipients 86% engrafted, the D+100 and 1-year cumulative non-relapse mortality were 10% and 18%, respectively. Twenty eight patients with post-transplant relapse received additional therapy achieving CMR in 9 cases. The 4-year OS and EFS were better in allo-HSCT compared to TKIs group: 58% <i>vs</i> 33% (p=0.032) and 35% <i>vs</i> 17% (p=0.5), accordingly. Patients in BC at the moment of allo-HSCT had significantly worse 4-year OS compared to responders: 23% <i>vs</i> 63% (p=0.007), accordingly.
While allo-HSCT has an advantage for many advanced-stage CML patients, in BC its results are comparable to TKIs treatment. Therefore, these patients should be referred to transplant center as soon as the second chronic phase is achieved.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Chronic myelogenous leukemia, BCR/ABL, allo-HSCT, tyrosine kinase inhibitors, blast crisis, outcomes.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Prognosis of patients with advanced stage CML (accelerated phase, AP, or blast crisis, BC) is still dismal in the era of tyrosine kinase inhibitors (TKIs). This study is aimed to evaluate whether allogeneic hemopoietic stem cell transplantation (allo-HSCT) improves their prognosis.
A total of 162 patients with AP/BC CML were divided into two homogeneous cohorts. The first one consisted of reduced-intensity conditioning allo-HSTC (n=82) recipients. The second (n=80) consisted of patients receiving only TKI-based therapy (in 85% of cases 2nd and 3rd generation TKIs) while not being referred to transplant center or refusing allo-HSCT. The response to therapy was defined according to ELN and NCCN recommendations.
The median follow-up for entire cohort was 44 (1-344) months. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among allo-HST recipients 86% engrafted, the D+100 and 1-year cumulative non-relapse mortality were 10% and 18%, respectively. Twenty eight patients with post-transplant relapse received additional therapy achieving CMR in 9 cases. The 4-year OS and EFS were better in allo-HSCT compared to TKIs group: 58% vs 33% (p=0.032) and 35% vs 17% (p=0.5), accordingly. Patients in BC at the moment of allo-HSCT had significantly worse 4-year OS compared to responders: 23% vs 63% (p=0.007), accordingly.
While allo-HSCT has an advantage for many advanced-stage CML patients, in BC its results are comparable to TKIs treatment. Therefore, these patients should be referred to transplant center as soon as the second chronic phase is achieved.
Keywords
Chronic myelogenous leukemia, BCR/ABL, allo-HSCT, tyrosine kinase inhibitors, blast crisis, outcomes.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27327
[VALUE] => The outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => The outcome of patients with advanced phase chronic myeloid leukemia with and without allogeneic hematopoietic stem cell transplantation
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27328
[VALUE] => 2311
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2311
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27332
[VALUE] => 2312
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2312
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Elena V. Morozova, Yulia Yu. Vlasova, Maria V. Barabanshikova, Ksenia S. Jurovskaya, Tatyana V. Shneider, Tatyana L. Gindina, Ildar M. Barkhatov, Evgenij A. Bakin, Ivan S. Moiseev, Alexander D. Kulagin, Ludmila S. Zubarovskaya, Boris V. Afanasyev
RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, St. Petersburg, Russia
Correspondence
Dr. Elena V. Morozova, RM Gorbacheva Research Institute of Pediatric Oncology, Hematology and Transplantation, Pavlov University, L.Tolstoy St. 6-8, 197022, St. Petersburg, Russia
Phone: +7(911) 927 8229
E-mail: dr_morozova@mail.ru
Prognosis of patients with advanced stage CML (accelerated phase, AP, or blast crisis, BC) is still dismal in the era of tyrosine kinase inhibitors (TKIs). This study is aimed to evaluate whether allogeneic hemopoietic stem cell transplantation (allo-HSCT) improves their prognosis.
A total of 162 patients with AP/BC CML were divided into two homogeneous cohorts. The first one consisted of reduced-intensity conditioning allo-HSTC (n=82) recipients. The second (n=80) consisted of patients receiving only TKI-based therapy (in 85% of cases 2nd and 3rd generation TKIs) while not being referred to transplant center or refusing allo-HSCT. The response to therapy was defined according to ELN and NCCN recommendations.
The median follow-up for entire cohort was 44 (1-344) months. Among the patients with BC, 36 (59%) did not respond to therapy, in 22 cases (34%) CHR was documented, in one case (2%) complete cytogenetic response (CCR) was revealed, and a complete molecular response (CMR) was achieved in two cases (3%). Among allo-HST recipients 86% engrafted, the D+100 and 1-year cumulative non-relapse mortality were 10% and 18%, respectively. Twenty eight patients with post-transplant relapse received additional therapy achieving CMR in 9 cases. The 4-year OS and EFS were better in allo-HSCT compared to TKIs group: 58% vs 33% (p=0.032) and 35% vs 17% (p=0.5), accordingly. Patients in BC at the moment of allo-HSCT had significantly worse 4-year OS compared to responders: 23% vs 63% (p=0.007), accordingly. While allo-HSCT has an advantage for many advanced-stage CML patients, in BC its results are comparable to TKIs treatment. Therefore, these patients should be referred to transplant center as soon as the second chronic phase is achieved.
Keywords
Chronic myelogenous leukemia, BCR/ABL, allo-HSCT, tyrosine kinase inhibitors, blast crisis, outcomes.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27395
[VALUE] => 10/02/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10/02/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27396
[VALUE] => 11/13/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/13/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27397
[VALUE] => Array
(
[TEXT] => <p>
Мария В. Коновалова<sup>1</sup>, Дарья С. Царегородцева<sup>1,2</sup>, Римма А. Полтавцева<sup>3</sup>, Елена В. Свирщевская<sup>1,3</sup>
</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
Мария В. Коновалова1, Дарья С. Царегородцева1,2, Римма А. Полтавцева3, Елена В. Свирщевская1,3
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27398
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Институт биоорганической химии им. академиков М. М. Шемякина и Ю. А. Овчинникова РАН, Москва, Россия<br>
<sup>2</sup> Первый Московский государственный медицинский университет им. И. М. Сеченова Минздрава РФ, Москва, Россия<br>
<sup>3</sup> Научный центр акушерства, гинекологии и перинатологии им. акад. В. И. Кулакова Минздрава РФ, Москва, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Институт биоорганической химии им. академиков М. М. Шемякина и Ю. А. Овчинникова РАН, Москва, Россия
2 Первый Московский государственный медицинский университет им. И. М. Сеченова Минздрава РФ, Москва, Россия
3 Научный центр акушерства, гинекологии и перинатологии им. акад. В. И. Кулакова Минздрава РФ, Москва, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27399
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Одной из острых проблем России является вторичное бесплодие женщин детородного возраста. Оно часто вызвано повреждением базального слоя эндометрия при выполнении гинекологических процедур: дилатации полости матки, лечебно-диагностическом выскабливании полости матки, кесаревом сечении, операциях на матке, а также после беременностей, протекающих с осложнениями. Как результат могут развиваться гипо- или гиперметриоз, а также формироваться внутриматочные спайки – синехии, приводящие к развитию синдрома Ашермана. Несмотря на большое количество накопленных клинических данных, отсутствует эффективный способ лечения вторичного бесплодия. В настоящее время с определенным успехом для терапии гипометриоза и синдрома Ашермана используют различные биополимеры и композиты на основе биополимеров с включением активных молекул, генов, их кодирующих, обогащенной тромбоцитами плазмы крови, стволовых клеток или микровезикул/экзосом стволовых клеток. Для использования в клинике сертифицированы гели на основе гиалуроната натрия, карбоксиметилцеллюлозы, полиэтиленоксида, коллагена и другие. Гели биополимеров являются, с одной стороны, разобщающими стенки матки препаратами (барьерная функция), а, с другой стороны, могут работать, как носители биологически активных молекул и клеток.
Биомиметики с разной эффективностью способны стимулировать регенерацию и нормализацию эндометрия, что способствует восстановлению репродуктивной способности. Методики лечения на основе биомиметиков находятся в стадии исследований. В обзоре приведены данные по эффективности лечения патологий эндометрия матки с помощью биотерапевтических подходов. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Бесплодие, гипометриоз, гиперметриоз, синдром Ашермана, барьерные материалы, биогели, биомиметики.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Одной из острых проблем России является вторичное бесплодие женщин детородного возраста. Оно часто вызвано повреждением базального слоя эндометрия при выполнении гинекологических процедур: дилатации полости матки, лечебно-диагностическом выскабливании полости матки, кесаревом сечении, операциях на матке, а также после беременностей, протекающих с осложнениями. Как результат могут развиваться гипо- или гиперметриоз, а также формироваться внутриматочные спайки – синехии, приводящие к развитию синдрома Ашермана. Несмотря на большое количество накопленных клинических данных, отсутствует эффективный способ лечения вторичного бесплодия. В настоящее время с определенным успехом для терапии гипометриоза и синдрома Ашермана используют различные биополимеры и композиты на основе биополимеров с включением активных молекул, генов, их кодирующих, обогащенной тромбоцитами плазмы крови, стволовых клеток или микровезикул/экзосом стволовых клеток. Для использования в клинике сертифицированы гели на основе гиалуроната натрия, карбоксиметилцеллюлозы, полиэтиленоксида, коллагена и другие. Гели биополимеров являются, с одной стороны, разобщающими стенки матки препаратами (барьерная функция), а, с другой стороны, могут работать, как носители биологически активных молекул и клеток.
Биомиметики с разной эффективностью способны стимулировать регенерацию и нормализацию эндометрия, что способствует восстановлению репродуктивной способности. Методики лечения на основе биомиметиков находятся в стадии исследований. В обзоре приведены данные по эффективности лечения патологий эндометрия матки с помощью биотерапевтических подходов.
Ключевые слова
Бесплодие, гипометриоз, гиперметриоз, синдром Ашермана, барьерные материалы, биогели, биомиметики.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27400
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-68-77
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-68-77
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27403
[VALUE] => Array
(
[TEXT] => <p>Maria V. Konovalova<sup>1</sup>, Daria S. Tsaregorodtseva<sup>1,2</sup>, Rimma A. Poltavtseva<sup>3</sup>, Elena V. Svirshchevskaya<sup>1,3</sup></p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Maria V. Konovalova1, Daria S. Tsaregorodtseva1,2, Rimma A. Poltavtseva3, Elena V. Svirshchevskaya1,3
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27404
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia<br>
<sup>2</sup> Sechenov’s First Moscow State Medical University, Moscow, Russia<br>
<sup>3</sup> V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia</p><br>
<p><b>Correspondence</b><br>
Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia<br>
Phone: +7 (910) 464 8760<br>
Fax: +7 (495) 330 4011<br>
E-mail: esvir@mail.ibch.ru</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia
2 Sechenov’s First Moscow State Medical University, Moscow, Russia
3 V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
Correspondence
Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia
Phone: +7 (910) 464 8760
Fax: +7 (495) 330 4011
E-mail: esvir@mail.ibch.ru
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27405
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Secondary infertility among women in their childbearing age is one of sufficient problems in Russia. It is often caused by damage to the basal layer of endometrium when performing gynecological procedures, e.g., dilatation of uterine cavity, diagnostic curettage, cesarean section, uterine surgey, as well as consequences of complicated pregnancies. As a result, hypo- or hypermetriosis may develop, along with intrauterine adhesions (synechiae), leading to the development of Asherman’s syndrome. Despite large amounts of medical data, there are no quite effective ways to treat secondary infertility. Currently, various biological polymers and composite materials based on biopolymers with incorporated active molecules, genetic substances, platelet-rich plasma, stem cells or microvesicles/exosomes of stem cells are used with some success for treatment of hypometriosis and Asherman’s syndrome. Gel substances based on
sodium hyaluronate, carboxymethylcellulose, polyethylene oxide, collagen and others are certified for clinical use. Biopolymer gels serve, on the one hand, as the materials separating the uterine walls (barrier function), and, on the other hand, they work as carriers of biologically active molecules and cells. Biomimetics can stimulate the regeneration and normalization of endometrium at different efficiency rates, thus promoting restoration of reproductive capacity. Biomimetic-based therapies are under investigation. The present review provides data on treatment efficiency of endometrial disorders by means of biotherapeutic approaches. </p>
<h2>Keywords</h2>
<p style="text-align: justify;">Infertility, hypometriosis, Asherman's syndrome, barrier materials, biogels, biomimetics.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Secondary infertility among women in their childbearing age is one of sufficient problems in Russia. It is often caused by damage to the basal layer of endometrium when performing gynecological procedures, e.g., dilatation of uterine cavity, diagnostic curettage, cesarean section, uterine surgey, as well as consequences of complicated pregnancies. As a result, hypo- or hypermetriosis may develop, along with intrauterine adhesions (synechiae), leading to the development of Asherman’s syndrome. Despite large amounts of medical data, there are no quite effective ways to treat secondary infertility. Currently, various biological polymers and composite materials based on biopolymers with incorporated active molecules, genetic substances, platelet-rich plasma, stem cells or microvesicles/exosomes of stem cells are used with some success for treatment of hypometriosis and Asherman’s syndrome. Gel substances based on
sodium hyaluronate, carboxymethylcellulose, polyethylene oxide, collagen and others are certified for clinical use. Biopolymer gels serve, on the one hand, as the materials separating the uterine walls (barrier function), and, on the other hand, they work as carriers of biologically active molecules and cells. Biomimetics can stimulate the regeneration and normalization of endometrium at different efficiency rates, thus promoting restoration of reproductive capacity. Biomimetic-based therapies are under investigation. The present review provides data on treatment efficiency of endometrial disorders by means of biotherapeutic approaches.
Keywords
Infertility, hypometriosis, Asherman's syndrome, barrier materials, biogels, biomimetics.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27401
[VALUE] => Biomimetics for treatment of endometrial pathologies: an overview
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Biomimetics for treatment of endometrial pathologies: an overview
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27402
[VALUE] => 2350
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2350
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27406
[VALUE] => 2351
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2351
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Maria V. Konovalova1, Daria S. Tsaregorodtseva1,2, Rimma A. Poltavtseva3, Elena V. Svirshchevskaya1,3
1 Shemyakin-Ovchinnikov Institute of Bioorganic Сhemistry RAS, Moscow, Russia
2 Sechenov’s First Moscow State Medical University, Moscow, Russia
3 V. I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia
Correspondence
Elena Svirshchevskaya, PhD, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, 16/10 Miklukho-Maklaya St, Moscow, 117997, Russia
Phone: +7 (910) 464 8760
Fax: +7 (495) 330 4011
E-mail: esvir@mail.ibch.ru
Secondary infertility among women in their childbearing age is one of sufficient problems in Russia. It is often caused by damage to the basal layer of endometrium when performing gynecological procedures, e.g., dilatation of uterine cavity, diagnostic curettage, cesarean section, uterine surgey, as well as consequences of complicated pregnancies. As a result, hypo- or hypermetriosis may develop, along with intrauterine adhesions (synechiae), leading to the development of Asherman’s syndrome. Despite large amounts of medical data, there are no quite effective ways to treat secondary infertility. Currently, various biological polymers and composite materials based on biopolymers with incorporated active molecules, genetic substances, platelet-rich plasma, stem cells or microvesicles/exosomes of stem cells are used with some success for treatment of hypometriosis and Asherman’s syndrome. Gel substances based on sodium hyaluronate, carboxymethylcellulose, polyethylene oxide, collagen and others are certified for clinical use. Biopolymer gels serve, on the one hand, as the materials separating the uterine walls (barrier function), and, on the other hand, they work as carriers of biologically active molecules and cells. Biomimetics can stimulate the regeneration and normalization of endometrium at different efficiency rates, thus promoting restoration of reproductive capacity. Biomimetic-based therapies are under investigation. The present review provides data on treatment efficiency of endometrial disorders by means of biotherapeutic approaches.
Keywords
Infertility, hypometriosis, Asherman's syndrome, barrier materials, biogels, biomimetics.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27407
[VALUE] => 11/05/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/05/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27408
[VALUE] => 12/04/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/04/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27409
[VALUE] => Array
(
[TEXT] => <p>Павел В. Попрядухин<sup>1</sup>, Наталья Н. Сударева<sup>1,2</sup>, Ольга М. Суворова<sup>1</sup>, Галина Ю. Юкина<sup>2</sup>, Елена Г. Сухорукова<sup>2</sup>, Наталья Н. Сапрыкина<sup>1</sup>, Илья А. Барсук<sup>3</sup>, Олег В. Галибин<sup>2</sup>, Александр Д. Вилесов<sup>1,2</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Павел В. Попрядухин1, Наталья Н. Сударева1,2, Ольга М. Суворова1, Галина Ю. Юкина2, Елена Г. Сухорукова2, Наталья Н. Сапрыкина1, Илья А. Барсук3, Олег В. Галибин2, Александр Д. Вилесов1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27410
[VALUE] => Array
(
[TEXT] => <p>
<sup>1</sup> Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия<br>
<sup>2</sup> Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия<br>
<sup>3</sup> Военно-медицинская академия им. С. М. Кирова, Санкт-Петербург, Россия</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] =>
1 Институт высокомолекулярных соединений РАН, Санкт-Петербург, Россия
2 Первый Санкт-Петербургский государственный медицинский университет им. акад. И. П. Павлова, Санкт-Петербург, Россия
3 Военно-медицинская академия им. С. М. Кирова, Санкт-Петербург, Россия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27415
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">В настоящей работе изучено поведение пористых сферических ватеритов карбоната кальция (СаСО<sub>3</sub>) покрытых защитной оболочкой из сульфата декстрана – систем адресной доставки лекарственных препаратов – в мышечной ткани крыс на сроках 3 сут., 1, 2, 4 и 12 нед., после имплантации. Было показано, что с течением времени происходит структурная трансформация и биорезорбция изучаемых носителей. Через 3 сут. наблюдается преобразование сферических структур в игольчатые с последующей их биорезорбцией в течение 2 нед. При этом патологического воздействия на окружающие ткани выявлено не было, что подтверждает безопасность применения покрытых защитной оболочкой СаСО<sub>3</sub> ватеритов и позволяет рекомендовать их для проведения дальнейших исследований в качестве систем адресной доставки лекарственных препаратов.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Адресная доставка лекарственных препаратов, карбонат кальция, сульфат декстрана, биорезорбция, мышечная ткань, эксперимент <i>in vivo</i>.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => В настоящей работе изучено поведение пористых сферических ватеритов карбоната кальция (СаСО3) покрытых защитной оболочкой из сульфата декстрана – систем адресной доставки лекарственных препаратов – в мышечной ткани крыс на сроках 3 сут., 1, 2, 4 и 12 нед., после имплантации. Было показано, что с течением времени происходит структурная трансформация и биорезорбция изучаемых носителей. Через 3 сут. наблюдается преобразование сферических структур в игольчатые с последующей их биорезорбцией в течение 2 нед. При этом патологического воздействия на окружающие ткани выявлено не было, что подтверждает безопасность применения покрытых защитной оболочкой СаСО3 ватеритов и позволяет рекомендовать их для проведения дальнейших исследований в качестве систем адресной доставки лекарственных препаратов.
Ключевые слова
Адресная доставка лекарственных препаратов, карбонат кальция, сульфат декстрана, биорезорбция, мышечная ткань, эксперимент in vivo.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27411
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-78-84
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-78-84
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27416
[VALUE] => Array
(
[TEXT] => <p>Pavel V. Popryadukhin<sup>1</sup>, Natalia N. Sudareva<sup>1,2</sup>, Оlga М. Suvorova<sup>1</sup>, Galina Yu. Yukina<sup>2</sup>, Еlena G. Sukhorukova<sup>2</sup>, Natalia N. Saprykina<sup>1</sup>, Ilya A. Barsuk<sup>3</sup>, Oleg V. Galibin<sup>2</sup>, Aleksandr D. Vilesov<sup>1,2</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Pavel V. Popryadukhin1, Natalia N. Sudareva1,2, Оlga М. Suvorova1, Galina Yu. Yukina2, Еlena G. Sukhorukova2, Natalia N. Saprykina1, Ilya A. Barsuk3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27417
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Institute of Macromolecular Compounds RAS, St. Petersburg, Russia<br>
<sup>2</sup> Pavlov University, St. Petersburg, Russia<br>
<sup>3</sup> S.M.Kirov Military Medical Academy, St. Petersburg, Russia</p><br>
<p><b>Correspondence</b><br>
Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia<br>
E-mail: pavelpnru@gmail.com</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 S.M.Kirov Military Medical Academy, St. Petersburg, Russia
Correspondence
Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia
E-mail: pavelpnru@gmail.com
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27418
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">The present work is focused on the study of target drug delivery systems comprised of porous spherical calcium carbonate vaterites (СаСО<sub>3</sub>) covered with the dextran sulfate protective shell. Behavior of the objects was investigated <i>in vivo</i>. The samples were implanted into rat muscular tissue and removed after different periods of exposure (3 days, 1, 2, 4, and 12 weeks after operation). It was shown that certain transformations in structure of the implanted carriers occurred over time, after which they underwent bioresorption. In 3 days after implantation, spherical vaterites degraded, and needle-like calcium carbonate objects were formed; during the following two weeks, these objects were completely resorbed in living tissues. Since no pathogenic influence of the samples on the surrounding tissues was revealed, we believe that СаСО<sub>3</sub> vaterites covered with protective shells are safe for potential medicinal applications and can be recommended for further studies as target drug delivery systems.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Target drug delivery, calcium carbonate, dextran sulfate, bioresorption, muscular tissue, <i>in vivo</i> experiment.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => The present work is focused on the study of target drug delivery systems comprised of porous spherical calcium carbonate vaterites (СаСО3) covered with the dextran sulfate protective shell. Behavior of the objects was investigated in vivo. The samples were implanted into rat muscular tissue and removed after different periods of exposure (3 days, 1, 2, 4, and 12 weeks after operation). It was shown that certain transformations in structure of the implanted carriers occurred over time, after which they underwent bioresorption. In 3 days after implantation, spherical vaterites degraded, and needle-like calcium carbonate objects were formed; during the following two weeks, these objects were completely resorbed in living tissues. Since no pathogenic influence of the samples on the surrounding tissues was revealed, we believe that СаСО3 vaterites covered with protective shells are safe for potential medicinal applications and can be recommended for further studies as target drug delivery systems.
Keywords
Target drug delivery, calcium carbonate, dextran sulfate, bioresorption, muscular tissue, in vivo experiment.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27412
[VALUE] => Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Morphology of target drug delivery systems (CaCO3 vaterites covered with dextran sulfate) in rat muscular tissue
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27414
[VALUE] => 2352
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2352
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27419
[VALUE] => 2353
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2353
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Pavel V. Popryadukhin1, Natalia N. Sudareva1,2, Оlga М. Suvorova1, Galina Yu. Yukina2, Еlena G. Sukhorukova2, Natalia N. Saprykina1, Ilya A. Barsuk3, Oleg V. Galibin2, Aleksandr D. Vilesov1,2
1 Institute of Macromolecular Compounds RAS, St. Petersburg, Russia
2 Pavlov University, St. Petersburg, Russia
3 S.M.Kirov Military Medical Academy, St. Petersburg, Russia
Correspondence
Dr. Pavel V. Popryadukhin, Institute of Macromolecular Compounds, St. Petersburg, Russia
E-mail: pavelpnru@gmail.com
The present work is focused on the study of target drug delivery systems comprised of porous spherical calcium carbonate vaterites (СаСО3) covered with the dextran sulfate protective shell. Behavior of the objects was investigated in vivo. The samples were implanted into rat muscular tissue and removed after different periods of exposure (3 days, 1, 2, 4, and 12 weeks after operation). It was shown that certain transformations in structure of the implanted carriers occurred over time, after which they underwent bioresorption. In 3 days after implantation, spherical vaterites degraded, and needle-like calcium carbonate objects were formed; during the following two weeks, these objects were completely resorbed in living tissues. Since no pathogenic influence of the samples on the surrounding tissues was revealed, we believe that СаСО3 vaterites covered with protective shells are safe for potential medicinal applications and can be recommended for further studies as target drug delivery systems.
Keywords
Target drug delivery, calcium carbonate, dextran sulfate, bioresorption, muscular tissue, in vivo experiment.
Experimental studies
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27383
[VALUE] => 08/25/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 08/25/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27384
[VALUE] => 11/14/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/14/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27385
[VALUE] => Array
(
[TEXT] => <p>Хана Деннауи<sup>1</sup>, Элиана Шуэри<sup>2</sup>, Шаза Хармуш<sup>1</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Хана Деннауи1, Элиана Шуэри2, Шаза Хармуш1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27386
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Лаборатория прикладной биотехнологии: биомолекулы, биотерапия и биопроцессы, научно-прикладной центр биотехнологии AZM, докторская школа науки и технологий, Ливанский университет, Триполи, Ливан <br>
<sup>2</sup> Отделение медицинской генетики, факультет медицины, университет Св. Иосифа (USJ), Мар-Микаэл, Бейрут, Ливан</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Лаборатория прикладной биотехнологии: биомолекулы, биотерапия и биопроцессы, научно-прикладной центр биотехнологии AZM, докторская школа науки и технологий, Ливанский университет, Триполи, Ливан
2 Отделение медицинской генетики, факультет медицины, университет Св. Иосифа (USJ), Мар-Микаэл, Бейрут, Ливан
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27387
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Простота заготовки мезенхимных стволовых клеток из желе Вортона (МСК-ЖВ), выраженная пластичность дифференцировки и низкая иммуногенность делают их удобным средством аллогенной клеточной терапии. Целью данного исследования было изучение способности к дифференцировке в эндотелиоподобные клетки МСК-ЖВ, культивированных на гиалуронан-хитозановых (ГХ) многослойных носителях.</p>
<h3>Материалы и методы</h3>
<p style="text-align: justify;">В этой работе мы проводили дифференцировку МСК-ЖВ в ангиогенном направлении с применением полиэлектролитной многослойной пленки в качестве субстрата. МСК-ЖВ культивировали на ГХ-многослойной пленке и стимулировали факторами культуральной среды EGM-2®. Коллаген типа I использовали в качестве контрольного субстрата. Определяли экспрессию специфических мРНК, а именно: CD31, рецептора фактора роста сосудистого эндотелия типа 2 (VEGF2) и эндотелиального сосудистого кадхерина (VE), наряду с уровнями абсорбции Dil-ацетилированного липопротеина низкой плотности и экспрессией белкового фактора Виллебранда.</p>
<h3>Результаты</h3>
<p style="text-align: justify;">Выделенные МСК-ЖВ имели типичную морфологию фибробластоподобных клеток. Уровни мРНК, кодирующих CD31 и KDR были выше после культивирования на ГХ-субстрате, нежели на коллагеновом покрытии, при достоверном повышении экспрессии KDR. На уровне белков, показана тенденция к повышению уровней KDR и CDH5 после инкубации на ГХ-субстрате, по сравнению с коллагеном. Кроме того, МСК-ЖВ, культивированные на ГХ, имели высокие уровни экспрессии эндотелиальных маркеров после 15 суток культивирования в среде EGM-2®.</p>
<h3>Выводы</h3>
<p style="text-align: justify;">В данной работе мы сообщаем о новом биосовместимом субстрате, который способствует дифференцировке МСК-ЖВ в эндотелиоподобные клетки. Разработка этого субстрата является новым подходом в тканевой инженерии для создания аллогенных сосудистых трансплантатов. </p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">Мезенхимные стволовые клетки, желе Вортона, дифференцировка, эндотелий, хитозан, гиалуроновая кислота, многослойный субстрат, тканевая инженерия.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Простота заготовки мезенхимных стволовых клеток из желе Вортона (МСК-ЖВ), выраженная пластичность дифференцировки и низкая иммуногенность делают их удобным средством аллогенной клеточной терапии. Целью данного исследования было изучение способности к дифференцировке в эндотелиоподобные клетки МСК-ЖВ, культивированных на гиалуронан-хитозановых (ГХ) многослойных носителях.
Материалы и методы
В этой работе мы проводили дифференцировку МСК-ЖВ в ангиогенном направлении с применением полиэлектролитной многослойной пленки в качестве субстрата. МСК-ЖВ культивировали на ГХ-многослойной пленке и стимулировали факторами культуральной среды EGM-2®. Коллаген типа I использовали в качестве контрольного субстрата. Определяли экспрессию специфических мРНК, а именно: CD31, рецептора фактора роста сосудистого эндотелия типа 2 (VEGF2) и эндотелиального сосудистого кадхерина (VE), наряду с уровнями абсорбции Dil-ацетилированного липопротеина низкой плотности и экспрессией белкового фактора Виллебранда.
Результаты
Выделенные МСК-ЖВ имели типичную морфологию фибробластоподобных клеток. Уровни мРНК, кодирующих CD31 и KDR были выше после культивирования на ГХ-субстрате, нежели на коллагеновом покрытии, при достоверном повышении экспрессии KDR. На уровне белков, показана тенденция к повышению уровней KDR и CDH5 после инкубации на ГХ-субстрате, по сравнению с коллагеном. Кроме того, МСК-ЖВ, культивированные на ГХ, имели высокие уровни экспрессии эндотелиальных маркеров после 15 суток культивирования в среде EGM-2®.
Выводы
В данной работе мы сообщаем о новом биосовместимом субстрате, который способствует дифференцировке МСК-ЖВ в эндотелиоподобные клетки. Разработка этого субстрата является новым подходом в тканевой инженерии для создания аллогенных сосудистых трансплантатов.
Ключевые слова
Мезенхимные стволовые клетки, желе Вортона, дифференцировка, эндотелий, хитозан, гиалуроновая кислота, многослойный субстрат, тканевая инженерия.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27388
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-59-67
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-59-67
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27391
[VALUE] => Array
(
[TEXT] => <p>Hana Dennaoui<sup>1</sup>, Eliane Chouery<sup>2</sup>, Chaza Harmoush<sup>1</sup> </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Hana Dennaoui1, Eliane Chouery2, Chaza Harmoush1
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27392
[VALUE] => Array
(
[TEXT] => <p><sup>1</sup> Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon <br>
<sup>2</sup> Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon</p><br>
<p><b>Correspondence</b><br>
Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon<br>
E-mail: hana.dennaoui@hotmail.com</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => 1 Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon
2 Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon
Correspondence
Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon
E-mail: hana.dennaoui@hotmail.com
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27393
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">The ease of harvesting of mesenchymal stem cells from Wharton’s jelly (WJ-MSCs), their great differentiation plasticity and low immunogenicity make them a suitable tool for allogeneic cell therapy. The aim of present study was to explore the potential of WJ-MSCs seeded on chitosan/hyaluronic acid (HA/CHI) multilayers to differentiate into endothelial-like cells.</p>
<h3>Methods</h3>
<p style="text-align: justify;">In this study, we differentiate WJ-MSCs into an angiogenic lineage using polyelectrolyte multilayer film as a substrate. WJ-MSCs were cultivated on HA/CHI multilayer film and stimulated (or not) with EGM-2® culture medium. Type I collagen was used as control. mRNA and protein expression of CD31, vascular endothelial growth factor-receptor 2 (VEGF2) and vascular endothelial (VE)-cadherin, along with Dil-acetylated low-density lipoprotein-uptake and von Willebrand Factor protein expression were performed.</p>
<h3>Results</h3>
<p style="text-align: justify;">The isolated MSCs showed typical fibroblast-like morphology. We have shown that WJ-MSCs express endothelial markers after 15 days of culture in EGM-2® medium. The mRNA levels were higher on CHI/HA than on collagen for CD31 and KDR, with only KDR increase being statistically significant. At the protein level, a trend for increase in KDR and CDH5 levels was also shown on CHI/HA relative to collagen. Moreover, the WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to von Willebrand factor after endothelial differentiation for 15 days.</p>
<h3>Conclusion</h3>
<p style="text-align: justify;">We report here a new biocompatible coating allowing differentiation of WJ-MSCs into endothelial-like cells. This substrate opens new routes in tissue engineering to design allogeneic vascular grafts.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">Mesenchymal stem cells, Wharton’s jelly, differentiation, endothelial lineage, chitosan, hyaluronic acid, multilayer substrate, tissue engineering. </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => The ease of harvesting of mesenchymal stem cells from Wharton’s jelly (WJ-MSCs), their great differentiation plasticity and low immunogenicity make them a suitable tool for allogeneic cell therapy. The aim of present study was to explore the potential of WJ-MSCs seeded on chitosan/hyaluronic acid (HA/CHI) multilayers to differentiate into endothelial-like cells.
Methods
In this study, we differentiate WJ-MSCs into an angiogenic lineage using polyelectrolyte multilayer film as a substrate. WJ-MSCs were cultivated on HA/CHI multilayer film and stimulated (or not) with EGM-2® culture medium. Type I collagen was used as control. mRNA and protein expression of CD31, vascular endothelial growth factor-receptor 2 (VEGF2) and vascular endothelial (VE)-cadherin, along with Dil-acetylated low-density lipoprotein-uptake and von Willebrand Factor protein expression were performed.
Results
The isolated MSCs showed typical fibroblast-like morphology. We have shown that WJ-MSCs express endothelial markers after 15 days of culture in EGM-2® medium. The mRNA levels were higher on CHI/HA than on collagen for CD31 and KDR, with only KDR increase being statistically significant. At the protein level, a trend for increase in KDR and CDH5 levels was also shown on CHI/HA relative to collagen. Moreover, the WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to von Willebrand factor after endothelial differentiation for 15 days.
Conclusion
We report here a new biocompatible coating allowing differentiation of WJ-MSCs into endothelial-like cells. This substrate opens new routes in tissue engineering to design allogeneic vascular grafts.
Keywords
Mesenchymal stem cells, Wharton’s jelly, differentiation, endothelial lineage, chitosan, hyaluronic acid, multilayer substrate, tissue engineering.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27389
[VALUE] => Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Endothelial differentiation of Wharton’s Jelly-derived mesenchymal stem cells seeded on chitosan/hyaluronan multilayer films
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27390
[VALUE] => 2344
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2344
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27394
[VALUE] => 2345
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2345
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Hana Dennaoui1, Eliane Chouery2, Chaza Harmoush1
1 Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, Tripoli, Lebanon
2 Medical Genetics Unit, Faculty of Medicine, Saint Joseph University (USJ), Mar Mikhaël, Beirut, Lebanon
Correspondence
Dr. Hana Dennaoui, Laboratory of Applied Biotechnology: Biomolecules, Biotherapies and Bioprocesses, AZM Centre for Biotechnology Research and its Applications, Doctoral School of Science and Technology, Lebanese University, El Mitein St, Tripoli, Lebanon
E-mail: hana.dennaoui@hotmail.com
The ease of harvesting of mesenchymal stem cells from Wharton’s jelly (WJ-MSCs), their great differentiation plasticity and low immunogenicity make them a suitable tool for allogeneic cell therapy. The aim of present study was to explore the potential of WJ-MSCs seeded on chitosan/hyaluronic acid (HA/CHI) multilayers to differentiate into endothelial-like cells.
Methods
In this study, we differentiate WJ-MSCs into an angiogenic lineage using polyelectrolyte multilayer film as a substrate. WJ-MSCs were cultivated on HA/CHI multilayer film and stimulated (or not) with EGM-2® culture medium. Type I collagen was used as control. mRNA and protein expression of CD31, vascular endothelial growth factor-receptor 2 (VEGF2) and vascular endothelial (VE)-cadherin, along with Dil-acetylated low-density lipoprotein-uptake and von Willebrand Factor protein expression were performed.
Results
The isolated MSCs showed typical fibroblast-like morphology. We have shown that WJ-MSCs express endothelial markers after 15 days of culture in EGM-2® medium. The mRNA levels were higher on CHI/HA than on collagen for CD31 and KDR, with only KDR increase being statistically significant. At the protein level, a trend for increase in KDR and CDH5 levels was also shown on CHI/HA relative to collagen. Moreover, the WJ-MSCs seeded on CHI/HA showed a high fluorescence specific to von Willebrand factor after endothelial differentiation for 15 days.
Conclusion
We report here a new biocompatible coating allowing differentiation of WJ-MSCs into endothelial-like cells. This substrate opens new routes in tissue engineering to design allogeneic vascular grafts.
Keywords
Mesenchymal stem cells, Wharton’s jelly, differentiation, endothelial lineage, chitosan, hyaluronic acid, multilayer substrate, tissue engineering.
Letter to the Editor
Array
(
[KEYWORDS] => Array
(
[ID] => 19
[TIMESTAMP_X] => 2015-09-03 10:46:01
[IBLOCK_ID] => 2
[NAME] => Ключевые слова
[ACTIVE] => Y
[SORT] => 500
[CODE] => KEYWORDS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 19
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 4
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => Y
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => Y
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Ключевые слова
[~DEFAULT_VALUE] =>
)
[SUBMITTED] => Array
(
[ID] => 20
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата подачи
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUBMITTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 20
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27420
[VALUE] => 11/23/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 11/23/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата подачи
[~DEFAULT_VALUE] =>
)
[ACCEPTED] => Array
(
[ID] => 21
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата принятия
[ACTIVE] => Y
[SORT] => 500
[CODE] => ACCEPTED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 21
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27421
[VALUE] => 12/04/2020 12:00:00 am
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 12/04/2020 12:00:00 am
[~DESCRIPTION] =>
[~NAME] => Дата принятия
[~DEFAULT_VALUE] =>
)
[PUBLISHED] => Array
(
[ID] => 22
[TIMESTAMP_X] => 2015-09-02 17:21:42
[IBLOCK_ID] => 2
[NAME] => Дата публикации
[ACTIVE] => Y
[SORT] => 500
[CODE] => PUBLISHED
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 22
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => DateTime
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Дата публикации
[~DEFAULT_VALUE] =>
)
[CONTACT] => Array
(
[ID] => 23
[TIMESTAMP_X] => 2015-09-03 14:43:05
[IBLOCK_ID] => 2
[NAME] => Контакт
[ACTIVE] => Y
[SORT] => 500
[CODE] => CONTACT
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 23
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Контакт
[~DEFAULT_VALUE] =>
)
[AUTHORS] => Array
(
[ID] => 24
[TIMESTAMP_X] => 2015-09-03 10:45:07
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHORS
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => E
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => Y
[XML_ID] => 24
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 3
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] => EAutocomplete
[USER_TYPE_SETTINGS] => Array
(
[VIEW] => E
[SHOW_ADD] => Y
[MAX_WIDTH] => 0
[MIN_HEIGHT] => 24
[MAX_HEIGHT] => 1000
[BAN_SYM] => ,;
[REP_SYM] =>
[OTHER_REP_SYM] =>
[IBLOCK_MESS] => N
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] =>
)
[AUTHOR_RU] => Array
(
[ID] => 25
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Авторы
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 25
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27422
[VALUE] => Array
(
[TEXT] => <p>Мануэль Абекасис </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Мануэль Абекасис
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Авторы
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_RU] => Array
(
[ID] => 26
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Организации
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 26
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27423
[VALUE] => Array
(
[TEXT] => <p>Лиссабон, Португалия </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Лиссабон, Португалия
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Организации
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_RU] => Array
(
[ID] => 27
[TIMESTAMP_X] => 2015-09-02 18:01:20
[IBLOCK_ID] => 2
[NAME] => Описание/Резюме
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 27
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27424
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.</p>
<h2>Ключевые слова</h2>
<p style="text-align: justify;">CAR-T клетки, фенотип истощения, лимфомы, терапия.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Статья-комментарий посвящена обсуждению результатов К. Байдер относительно частоты клинических ответов у пациентов с резистентными/рефрактерными лимфомами, леченых CAR-T-клетками, и факторов успешности этой терапии, в особенности – роли старения Т-клеток и фенотипа их «истощения» как причины снижения ответа после адоптивной клеточной терапии у больных. Больные, ответившие на лечение, имели низкие уровни «истощенных» клеток в крови и конечном CAR-T-клеточном продукте. Требуются дополнительные исследования для установления требуемых количеств CAR T-клеток, но оптимальные параметры могут различаться в зависимости от генотипа CAR-конструкта и вида злокачественного заболевания.
Ключевые слова
CAR-T клетки, фенотип истощения, лимфомы, терапия.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Описание/Резюме
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[DOI] => Array
(
[ID] => 28
[TIMESTAMP_X] => 2016-04-06 14:11:12
[IBLOCK_ID] => 2
[NAME] => DOI
[ACTIVE] => Y
[SORT] => 500
[CODE] => DOI
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 28
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27425
[VALUE] => 10.18620/ctt-1866-8836-2020-9-4-85-87
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 10.18620/ctt-1866-8836-2020-9-4-85-87
[~DESCRIPTION] =>
[~NAME] => DOI
[~DEFAULT_VALUE] =>
)
[AUTHOR_EN] => Array
(
[ID] => 37
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Author
[ACTIVE] => Y
[SORT] => 500
[CODE] => AUTHOR_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 37
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27428
[VALUE] => Array
(
[TEXT] => <p>Manuel Abecasis </p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Manuel Abecasis
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Author
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[ORGANIZATION_EN] => Array
(
[ID] => 38
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Organization
[ACTIVE] => Y
[SORT] => 500
[CODE] => ORGANIZATION_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 38
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27429
[VALUE] => Array
(
[TEXT] => <p>Lisboa, Portugal </p><br>
<p><b>Correspondence</b><br>
Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal<br>
E-mail: mabecasis@ipolisboa.min-saude.pt</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => Lisboa, Portugal
Correspondence
Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal
E-mail: mabecasis@ipolisboa.min-saude.pt
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Organization
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[SUMMARY_EN] => Array
(
[ID] => 39
[TIMESTAMP_X] => 2015-09-02 18:02:59
[IBLOCK_ID] => 2
[NAME] => Description / Summary
[ACTIVE] => Y
[SORT] => 500
[CODE] => SUMMARY_EN
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 39
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] => 27430
[VALUE] => Array
(
[TEXT] => <p style="text-align: justify;">The comment concerns discussion of results reported by K. Beider on the clinical response rates in patients with resistant/refractory lymphomas treated with CAR-T cells, and the factors predicting therapeutic success, in particular, distinct role of T cell ageing and their exhaustion phenotype as a reason for decreased response after adoptive cellular therapy. The patients who responded to treatment, had low levels of exhausted cells in blood and final CAR T cell product. Additional studies are needed to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.</p>
<h2>Keywords</h2>
<p style="text-align: justify;">CAR-T cells, exhaustion phenotype, lymphoma, treatment.</p>
[TYPE] => HTML
)
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Array
(
[TEXT] => The comment concerns discussion of results reported by K. Beider on the clinical response rates in patients with resistant/refractory lymphomas treated with CAR-T cells, and the factors predicting therapeutic success, in particular, distinct role of T cell ageing and their exhaustion phenotype as a reason for decreased response after adoptive cellular therapy. The patients who responded to treatment, had low levels of exhausted cells in blood and final CAR T cell product. Additional studies are needed to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.
Keywords
CAR-T cells, exhaustion phenotype, lymphoma, treatment.
[TYPE] => HTML
)
[~DESCRIPTION] =>
[~NAME] => Description / Summary
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[NAME_EN] => Array
(
[ID] => 40
[TIMESTAMP_X] => 2015-09-03 10:49:47
[IBLOCK_ID] => 2
[NAME] => Name
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 80
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 40
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => Y
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27426
[VALUE] => Not all T cells are created alike!
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => Not all T cells are created alike!
[~DESCRIPTION] =>
[~NAME] => Name
[~DEFAULT_VALUE] =>
)
[FULL_TEXT_RU] => Array
(
[ID] => 42
[TIMESTAMP_X] => 2015-09-07 20:29:18
[IBLOCK_ID] => 2
[NAME] => Полный текст
[ACTIVE] => Y
[SORT] => 500
[CODE] => FULL_TEXT_RU
[DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 42
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 200
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Полный текст
[~DEFAULT_VALUE] => Array
(
[TEXT] =>
[TYPE] => HTML
)
)
[PDF_RU] => Array
(
[ID] => 43
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF RUS
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_RU
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 43
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27427
[VALUE] => 2357
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2357
[~DESCRIPTION] =>
[~NAME] => PDF RUS
[~DEFAULT_VALUE] =>
)
[PDF_EN] => Array
(
[ID] => 44
[TIMESTAMP_X] => 2015-09-09 16:05:20
[IBLOCK_ID] => 2
[NAME] => PDF ENG
[ACTIVE] => Y
[SORT] => 500
[CODE] => PDF_EN
[DEFAULT_VALUE] =>
[PROPERTY_TYPE] => F
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 44
[FILE_TYPE] => doc, txt, rtf, pdf
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] =>
[USER_TYPE_SETTINGS] =>
[HINT] =>
[PROPERTY_VALUE_ID] => 27431
[VALUE] => 2358
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] => 2358
[~DESCRIPTION] =>
[~NAME] => PDF ENG
[~DEFAULT_VALUE] =>
)
[NAME_LONG] => Array
(
[ID] => 45
[TIMESTAMP_X] => 2023-04-13 00:55:00
[IBLOCK_ID] => 2
[NAME] => Название (для очень длинных заголовков)
[ACTIVE] => Y
[SORT] => 500
[CODE] => NAME_LONG
[DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
[PROPERTY_TYPE] => S
[ROW_COUNT] => 1
[COL_COUNT] => 30
[LIST_TYPE] => L
[MULTIPLE] => N
[XML_ID] => 45
[FILE_TYPE] =>
[MULTIPLE_CNT] => 5
[TMP_ID] =>
[LINK_IBLOCK_ID] => 0
[WITH_DESCRIPTION] => N
[SEARCHABLE] => N
[FILTRABLE] => N
[IS_REQUIRED] => N
[VERSION] => 1
[USER_TYPE] => HTML
[USER_TYPE_SETTINGS] => Array
(
[height] => 80
)
[HINT] =>
[PROPERTY_VALUE_ID] =>
[VALUE] =>
[DESCRIPTION] =>
[VALUE_ENUM] =>
[VALUE_XML_ID] =>
[VALUE_SORT] =>
[~VALUE] =>
[~DESCRIPTION] =>
[~NAME] => Название (для очень длинных заголовков)
[~DEFAULT_VALUE] => Array
(
[TYPE] => HTML
[TEXT] =>
)
)
)
Manuel Abecasis
Lisboa, Portugal
Correspondence
Prof. Manuel Abecasis, MD PhD, Director, Hematology Department, Instituto Português Oncologia, Lisboa, Portugal
E-mail: mabecasis@ipolisboa.min-saude.pt
The comment concerns discussion of results reported by K. Beider on the clinical response rates in patients with resistant/refractory lymphomas treated with CAR-T cells, and the factors predicting therapeutic success, in particular, distinct role of T cell ageing and their exhaustion phenotype as a reason for decreased response after adoptive cellular therapy. The patients who responded to treatment, had low levels of exhausted cells in blood and final CAR T cell product. Additional studies are needed to establish desirable attributes of CAR T cells, but the optimal characteristics might differ depending on the CAR construct and the malignancy being targeted.
Keywords
CAR-T cells, exhaustion phenotype, lymphoma, treatment.

